* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ventral Medial Nucleus Neurons Send Thalamocortical Afferents
Human brain wikipedia , lookup
Bird vocalization wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Node of Ranvier wikipedia , lookup
Neuroregeneration wikipedia , lookup
Embodied language processing wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Aging brain wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Convolutional neural network wikipedia , lookup
Neurotransmitter wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Biological neuron model wikipedia , lookup
Environmental enrichment wikipedia , lookup
Apical dendrite wikipedia , lookup
Neuroplasticity wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Single-unit recording wikipedia , lookup
Multielectrode array wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Neural oscillation wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neural coding wikipedia , lookup
Synaptogenesis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Mirror neuron wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Axon guidance wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Nervous system network models wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
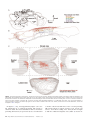
Cerebral Cortex January 2015;25:221–235 doi:10.1093/cercor/bht216 Advance Access publication August 22, 2013 Ventral Medial Nucleus Neurons Send Thalamocortical Afferents More Widely and More Preferentially to Layer 1 than Neurons of the Ventral Anterior–Ventral Lateral Nuclear Complex in the Rat Eriko Kuramoto1, Sachi Ohno1,2, Takahiro Furuta1, Tomo Unzai1, Yasuhiro R. Tanaka1, Hiroyuki Hioki1 and Takeshi Kaneko1 1 Department of Morphological Brain Science, Graduate School of Medicine, Kyoto University, Kyoto 606–8501, Japan and Department of Dental Anesthesiology, Graduate School of Medicine and Dentistry, Kagoshima University, Kagoshima 890-8544, Japan 2 Address correspondence to: Prof. Takeshi Kaneko, Department of Morphological Brain Science, Graduate School of Medicine, Kyoto University, Kyoto 606-8501, Japan. Email: [email protected] Not only inhibitory afferent-dominant zone (IZ) of the ventral anterior–ventral lateral thalamic complex (VA-VL) but also the ventral medial nucleus (VM) is known to receive strong inputs from the basal ganglia and send axons to motor areas. We previously reported differences in axonal arborization between IZ neurons and the other VA-VL neurons in rats by single-neuron tracing with viral vectors. In the present study, the axonal arborization of single VM neurons was visualized by the same method, and compared with that of IZ neurons. VM neurons formed fewer axon collaterals in the striatum, but sent axon fibers more widely and more preferentially (79% of fibers) to layer 1 of cortical areas than IZ neurons. Furthermore, the VM seemed to contain at least 2 types of neurons; a major population of VM neurons sent axon fibers principally to motor-associated areas as VA-VL neurons did, and the other population projected mainly to orbital or cingulate areas. Although both VM and IZ neurons receive strong basal ganglia inputs, these results suggest that VM neurons, at a single neuron level, innervate the apical dendrites of cortical pyramidal neurons more intensely and more widely than IZ neurons. Keywords: layer 1, motor thalamic neurons, sindbis viral vector, singleneuron tracing, ventral medial thalamic nucleus Introduction The ventral medial thalamic nucleus (VM) receives afferents from the basal ganglia (Carter and Fibiger 1978; Hendry et al. 1979; Di Chiara et al. 1979; Herkenham 1979; Deniau and Chevalier 1992; Sakai et al. 1998; Kha et al. 2001; Sakai and Bruce 2004; Kuramoto et al. 2011) and relays basal ganglia information to the cerebral cortex including motor areas. Thus, the VM is at least partly associated with the motor function as well as with other cortical functions. In addition to the VM, the ventral anterior and ventral lateral nuclear complex (VA-VL), the motor thalamic nuclei, accepts abundant afferents not only from the cerebellum but from the basal ganglia (for review, see Groenewegen and Witter 2004; Jones 2007). Those basal ganglia afferents to the VM and VA-VL are emitted in rats by the entopeduncular nucleus (Ep; internal segment of the globus pallidus) and substantia nigra pars reticulata (SNr), and have been suggested to be GABAergic by several lines of evidence. The destruction of the Ep and/or SNr has been reported to cause the reduction of GABA content, glutamic acid decarboxylase (GAD) activity, and GAD immunoreactivity in the motor thalamic nuclei and VM (Di Chiara et al. 1979; Kilpatrick et al. 1980; Penney and Young 1981; Kuramoto et al. 2011); nigrothalamic transmission is blocked by GABA antagonist (MacLeod et al. © The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: [email protected] 1980); and GABA immunoreactivity has frequently been observed in the axon terminals derived from the Ep/SNr (Ilinsky et al. 1997; Bodor et al. 2008). These inhibitory afferents principally enter the VM and inhibitory input-dominant zone (IZ) of the VA-VL (Kuramoto et al. 2009, 2011), the latter being located in the rostroventral portion of the VA-VL. On the other hand, the caudodorsal portion of the VA-VL receives glutamatergic excitatory afferents mainly from the deep cerebellar nuclei (Schwarz and Schmitz 1997; Kuramoto et al. 2011), and has been named excitatory subcortical afferent-dominant zone (EZ) because of the abundance of axon terminals immunoreactive for vesicular glutamate transporter 2 (VGluT2) (Kuramoto et al. 2009). It has been proposed that thalamic projection neurons are classified into core-type and matrix-type neurons mainly on the basis of projection targets in the cerebral cortical layers (for review, see Jones 1998, 2001). Core-type neurons are located in the specific relay nuclei and send axon fibers mainly to the middle layers of the cerebral cortex, that is, L4 and the deep part of L3, whereas matrix-type neurons are distributed throughout the thalamic nuclei and project preferentially to L1 of the widespread cortical areas. Although the core- or matrix-type neurons are also characterized by parvalbumin or calbindin immunoreactivity, respectively, in cat and monkey thalamic nuclei (Jones 1998, 2001), this chemical differentiation only partially works in rodent thalamic nuclei. In the rat thalamus, no projection neurons are immunopositive for parvalbumin, but many neurons are positive for calbindin (Rubio-Garrido et al. 2007; Kuramoto et al. 2009, 2011; Ohno et al. 2012). Neurons in the VM are well known to project mainly to L1 of widespread cortical areas including motor-associated areas in the rat (Krettek and Price 1977; Herkenham 1979, 1980; Arbuthnott et al. 1990; Mitchell and Cauller 2001; Rubio-Garrido et al. 2009) and cat brains (Glenn et al. 1982), although a similar projection of VM neurons has not been reported in the monkey brain (Jones 2007). We recently developed a replication-deficient Sindbis viral vector which was designed to express palmitoylation siteattached green fluorescent protein (palGFP) (Furuta et al. 2001), and used the vector for anterograde tracing of neurons (Nakamura et al. 2002, 2004, 2005; Ito et al. 2007; Furuta et al. 2008). The vector worked as a highly sensitive anterograde tracer, because infected neurons produced an enormous amount of palGFP under the strong subgenomic promoter of the virus, and the palGFP was targeted to plasma membrane including axonal membrane by the addition of 2 long fatty acyl residues, palmitoyl residues, to the protein (Moriyoshi et al. 1996). We first applied this vector to single neuron labeling of mesencephalic dopamine neurons, and were surprised to observe far denser and more widespread axon fibers in the striatum than expected (Matsuda et al. 2009). We then started to apply this single neuron-labeling technique to visualization of thalamic projection neurons including motor and sensory thalamic nuclei (Kuramoto et al. 2009; Ohno et al. 2012). In the VA-VL, IZ neurons were classified as matrix-type neurons from the observation that more than half of their projecting axon fibers were distributed in L1 of the cerebral cortex (Kuramoto et al. 2009). VM neurons had similar input–output organization to those of IZ neurons; both the neuron groups received inhibitory afferents from the basal ganglia and sent efferents preferentially to L1 of the cerebral cortex including motor-associated areas. However, we noticed some differences between the 2 groups of matrix-type neurons during a series of single neuronlabeling studies in the motor thalamic nuclei and their surrounding nuclei. In the present study, we first tried to reveal the quantitative properties of axonal arborization of single VM neurons and then compare them with those of single IZ neurons. Materials and Methods Animals Adult male Sprague-Dawley rats (Shizuoka Laboratory Animal Center, Shizuoka, Japan), weighing 300–400 g, were used in the present study. The experiments were approved by the Committees for Animal Care (Med Kyo 12013, 12014) and for Recombinant DNA Study (120093) in Kyoto University. All efforts were made to minimize the suffering and number of animals used in the present study. Injection of Sindbis Viral Vectors and Fixation Seventy rats were anesthetized by intraperitoneal injection of chloral hydrate (30 mg/ 100 g body weight). For single neuron labeling, a mixture of palGFP Sindbis viral vectors (1–2 × 102 infectious units (IU); Furuta et al. 2001) and Sindbis viral vectors expressing palmitoylation site-attached monomeric red fluorescent protein ( pal-mRFP) (1–2 × 102 IU; Nishino et al. 2008) in 0.3 µl of 5 mM sodium phosphate ( pH 7.4)-buffered 0.9% saline (PBS) containing 0.5% bovine serum albumin (BSA) was injected into the VM (2.3–3.0 mm posterior to the bregma, 1.2–1.6 mm lateral to the midline, and 6.3–6.7 mm deep from the brain surface) by pressure through a glass micropipette attached to Picospritzer II (General Valve Corporation, East Hanover, NJ, USA). The virus-injected rats survived for 51–54 h after the injection. The 70 virus-injected rats were anesthetized again with chloral hydrate (70 mg/100 g), and perfused transcardially with 200 mL of PBS, followed by 200 mL of 3% formaldehyde, 75% saturated picric acid, and 0.1 M Na2HPO4 (adjusted with NaOH to pH 7.0). The brains were then removed and postfixed for 4 h at room temperature with the same fixative. After cryoprotection with 30% sucrose in PBS, the brains were cut into 40-µm-thick parasagittal or 50-µm-thick frontal sections on a freezing microtome, and the sections were collected serially in PBS. Characterization of palGFP- or pal-mRFP-Expressing Thalamic Neurons The sections including the injection site were observed under epifluorescent microscope Axiophot (Zeiss, Oberkochen, Germany) to find thalamic neurons infected with the viruses. All the following incubations were performed at room temperature and followed by a rinse with PBS containing 0.3% Triton X-100 (PBS-X). The sections containing palGFP- or pal-mRFP-positive thalamic neurons were incubated overnight with 2 µg/mL mouse monoclonal IgG2a to recombinant GAD of 67 kDa (GAD67; MAB5406, EMD Millipore, Temecula, CA, USA) in PBS-X containing 0.12% lambda-carrageenan, 0.02% sodium azide, and 1% donkey serum (PBS-XCD), and then for 4 h with 1 µg/mL AlexaFluor 647-conjugated anti-[mouse IgG] goat antibody (A21236; Life Technologies, Gaithersburg, MD, USA) in PBS-XCD. Under an LSM5 PASCAL confocal laser-scanning microscope (Zeiss), the location of 222 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. palGFP- or pal-mRFP-labeled neurons was examined in reference to GAD67 immunoreactivity as described in the Results section. Further, the sections containing palGFP- or pal-mRFP-labeled neurons were incubated 2 h with 10 µg/mL of propidium iodide or 1/200-diluted NeuroTrace 500/525 green fluorescent Nissl stain (N-21480; Life Technologies), respectively, in PBS-X, and the location of the labeled neurons was re-examined in reference to Nissl-like staining. Immunoperoxidase Staining for GFP or mRFP GFP or mRFP immunoreactivity was visualized by combining the avidinbiotinylated peroxidase complex (ABC) method with the biotinylated tyramine (BT)-glucose oxidase (GO) amplification (Furuta et al. 2009; Kuramoto et al. 2009). All the serial sections containing one palGFP- or pal-mRFP-labeled VM neuron in a hemisphere were incubated overnight with 0.5 µg/mL affinity-purified rabbit antibody to GFP (Tamamaki et al. 2000; Nakamura et al. 2008) or 0.5 µg/mL affinity-purified rabbit antibody to mRFP (Hioki et al. 2010), respectively, in PBS-XCD. After a rinse with PBS-X, the sections were incubated for 2 hrs with 10 µg/mL biotinylated anti-[rabbit IgG] goat antibody (BA-1000; Vector, Burlingame, CA, USA) and then for 1 hr with ABC (1:100; Elite variety, Vector) in PBS-X. After a rinse in 0.1 M sodium phosphate buffer (PB; pH 7.4), the sections were incubated for 30 min in the BT-GO reaction mixture containing 1.25 µM BT, 3 µg/mL of glucose oxidase (16831-14; nacalai tesque, Kyoto, Japan; 259 U/mg), 2 mg/mL of β-D-glucose and 1% BSA in 0.1 M PB (pH 7.4), followed by a wash with PBS. Subsequently, the sections were again incubated for 1 h with ABC in PBS-X, and the bound peroxidase was finally developed brown by reaction for 30–60 min with 0.02% diaminobenzidine-4HCl (DAB), and 0.0001% H2O2 in 50 mM Tris–HCl, pH 7.6. All the above incubations and reactions were performed at room temperature. All the stained sections were serially mounted onto gelatinized glass slides, dried up, dehydrated in an ethanol series, cleared in xylene, and finally coverslipped. After reconstruction of palGFP- or pal-mRFP-labeled neurons, the sections were counterstained for Nissl with 0.2% Cresyl violet to determine cytoarchitecture of the cerebral cortex. The cytoarchitectonic areas of the cerebral cortex were mainly determined after Nissl staining according to the atlas of Paxinos and Watson (2007), and, specifically in case of orbital areas, to Van De Werd and Uylings (2008). Cortical lamination was delineated according to Swanson (2004) and Zilles (1985) with an appropriate reference to Vogt et al. (2004) for cingulate areas. Reconstruction and Morphological Analysis of Single VM Neurons The cell body and dendrites of stained VM neurons were reconstructed onto a sagittal plane under a microscope attached with camera lucida apparatus or with Neurolucida 10 (MicroBrightField, Williston, VT, USA). The reconstructed figures were captured and digitized by a conventional scanner. The axonal arborization was reconstructed as described previously (Ohno et al. 2012). Briefly, a whole parasagittal or frontal section was automatically captured into a large color image with a spatial resolution of 1.038 µm/pixel using digital slide scanner TOCO (CLARO, Aomori, Japan), and at most 174 parasagittal or 270 frontal images (file size = 50–150 MB/image) were obtained from a hemisphere. On the images, we traced and digitized the axon fibers with a pen tablet (Bamboo Tablet; Wacom, Saitama, Japan) and software CANVAS X (ACD Systems International, Inc., Victoria, Canada). The axon fibers were thereby reconstructed 2-dimensionally to a collection of many short Bézier curves section by section onto a sagittal plane, and the digitized fibers from all the sections were superimposed in the computer. The length and color code of Bézier curves in a Canvas file was automatically determined curve by curve and written out to an Excel file by using an Applescript macro, which was programmed by Y.R.T. For 3D reconstruction, we needed to change the Bézier curves of axon fibers into a TIFF image temporarily, and to re-trace the axon fibers on the TIFF image in software Neurolucida, because the Neurolucida did not have a software module to directly transform the Bézier curve information to the Neurolucida file format (DAT file). More specifically, the Bézier curves were exported section by section to a black-and-white TIFF file (file size of 15–20 MB/section) with the maximum resolution of software CANVAS X, and were re-traced using submodule AutoNeuron (settings: bright field, max process diameter = 3 µm, and seed detection sensitivity = 100). Black-and-white images were used because of the file size limitation (≤20 MB/image) in the Neurolucida, and the maximum resolution was required to re-trace the image of Bézier curves smoothly. All the re-traced lines were arranged 3-dimensionally in the Neurolucida, and the 3D image was rotated, when necessary, and visualized with enhancement of depth perception using the Depth Cueing option in submodule Explorer. The cerebral cortex was outlined in the Neurolucida at every 200 µm distance by tracing the contour on black-and-white TIFF images (1.42 µm/pixel) made from original photo-images (1.038 µm/pixel) of every fifth serial sections. The fine morphological indices, such as intervaricosity interval, were measured with an ×100 objective lens (PlanApo100; NA = 1.4; Nikon, Tokyo, Japan). For statistical analysis, such as Bonferroni post hoc multiple comparison test following 2-way analysis of variance (ANOVA), Mann–Whitney’s U test and Student’s t-test, softwares GraphPad Prism 4 (Graphpad Software, Inc., San Diego, CA, USA) and Excel (Microsoft, Redmond, WA, USA) were used. proximal sites of their axons were well labeled without any pathological signs, we suspected that the infection of these neurons was delayed for substantial hours after the injection, and the remaining survival time was not enough for sufficient production or transport of the reporter protein. Excluding these 5 neurons from 19 neurons, we reconstructed the axonal arborization of the remaining 14 VM neurons as far as possible. The axons of the 14 neurons were clearly stained up to the exact end of fine axon fibers without any fading away, and the ends always formed small terminal varicosities. This suggests that the axonal arborization of the 14 neurons was mostly, if not completely, reconstructed. The 14 neurons were numbered according to the location of their cell bodies in the VM from the lateral to the medial portion (Fig. 2). Results Selection of palGFP- or pal-mRFP-Labeled Neurons for Single-Cell Tracing Because almost all thalamic neurons projected to ipsilateral hemispheres (Donoghue and Parham 1983), a mixture of palGFP- and pal-mRFP-expressing Sindbis viral vectors was injected at an adequate dilution into both hemispheres of 70 rat brains (140 hemispheres) as reported previously (Ohno et al. 2012). The survival time was as short as possible, that is, 51–54 h, to avoid possible effects of viral infection on the axonal morphology of the infected neurons. Fifty-five hemispheres containing only one palGFP- or pal-mRFP-labeled neuron were selected under an epifluorescent microscope and further processed. We previously found that immunoreactivity for GAD67 was clearly more intense in the VM and the IZ of the VA-VL than in the EZ, and that these intensely GAD67-immunoreactive region mainly received GABAergic inputs from the basal ganglia (Kuramoto et al. 2011). In addition, GAD67 immunoreactivity was useful for demarcating the border between the VM/IZ and submedius thalamic nucleus, because the submedius nucleus shows clearly weaker immunoreactivity than the VM and IZ (Kuramoto et al. 2011). Thus, immunofluorescence staining for GAD67 immunoreactivity revealed that 27 of the 55 labeled neurons were located in the VM or IZ showing intense immunoreactivity (Fig. 1A′,C′). Subsequently, the fluorescent Nissl-like staining showed that the 27 neurons in the intensely GAD67-immunoreactive region were further differentiated into 19 VM and 8 IZ neurons by the cytoarchitecture (Fig. 1A,C) in reference to the atlas of Paxinos and Watson (2007). The sections of the 19 hemispheres containing singlelabeled VM neurons were then processed for immunoperoxidase staining for GFP or mRFP by the ABC method with the BT-GO amplification (Fig. 1B′,D′). Thus, the axonal arborizations of 10 palGFP-labeled and 9 pal-mRFP-labeled VM neurons were visualized. Of the 19 VM neurons, 10 neurons were revealed to mainly send axons to the primary (M1) and secondary motor areas (M2), whereas 9 neurons did not chiefly project to the motor areas, but to the orbital and/or cingulate areas. Furthermore, when reconstructing the axonal arborization, we noticed that the axon labeling of 5 VM neurons faded away at the distal sites of their arborization. Because the Figure 1. Identification of VM neurons infected with palGFP- or pal-mRFP-expressing Sindbis viral vectors. The parasagittal sections which contained palGFP- or pal-mRFP-labeled neuronal cell bodies (arrowheads in A–D) were immunostained for GAD67 (A′,C′). Under the confocal laser-scanning or fluorescent microscope, GFP was detected in 488 nm or 450–490 nm excitation and 505–530 nm emission condition, mRFP was in 543 nm or 540–552 nm excitation and 590–625 or 575–625 nm emission condition, and GAD67 immunoreactivity (AlexaFluor647) was in 633 nm excitation and ≥650 nm emission condition. The sections were further stained with propidium iodide (PI; A, B, excitation 540–552 nm, emission 590–625 nm) or NeuroTrace 500/525 green (C, D, excitation 450–490 nm, emission 505–530 nm). After the identification, the labeled neurons were visualized by immunoperoxidase staining with the anti-GFP (B′) or anti-mRFP antibody (D′). AD, anterodorsal thalamic nucleus; AM, anteromedial thalamic nucleus; AV, anteroventral thalamic nucleus; CL, central lateral thalamic nucleus; LD, laterodorsal thalamic nucleus; LHb, lateral habenula; LP, lateral posterior thalamic nucleus; MD, mediodosal thalamic nucleus; Pc, paracentral thalamic nucleus; Pf, parafascicular thalamic nucleus; Rt, thalamic reticular nucleus; VPpc, parvocellular part of the ventral posterior thalamic nuclei. Scale bar in C′ applies to A, A′, C, C′; that in D′ applies to B, B′, D, D′. Cerebral Cortex January 2015, V 25 N 1 223 Axonal Arborization of VM Neurons All the 14 VM neurons reconstructed in the present study extensively projected axon fibers to the cerebral cortex (Fig. 3A,B), but no neurons possessed axon collaterals inside of the dorsal thalamus. When the axons exited from the thalamus, VM neurons always emitted a few axon collaterals to the thalamic reticular nucleus (Fig. 3C). In the course to the cerebral cortex, 12 of the 14 VM neurons sent axon collaterals to the neostriatum (Fig. 3D,E), although neurons 10 and 13 mainly projected to the orbital and cingulate areas (Table 1) without any axon collaterals emitted to the neostriatum. Thus, the main targets of VM neurons were the cerebral cortex, neostriatum, and thalamic reticular nucleus with exceptions that some neurons formed a tiny axon collateral in the zona incerta (Fig. 4A), claustrum (Fig. 5A), or external segment of the globus pallidus (Fig. 6D, Supplementary Figs 1A and 2A). Every axon fiber in the cerebral cortex, striatum, and thalamic reticular nucleus was clearly labeled, and its axon varicosities were easily detectable (Fig. 3B,E) by the combination of the ABC method with the BT-GO amplification. The target cortical areas of single VM neurons were listed in Table 1, and their axonal arborization was almost completely reconstructed as shown in Figures 4–7 and Supplementary Figures 1 and 2. Although the axon fibers of each VM neuron were widely distributed to many cortical fields in the present reconstruction, 8 VM neurons sent axon fibers mainly to the M1, M2, forelimb (FL), and/or hindlimb areas (HL), and 6 neurons projected chiefly to the orbital and/or cingulate areas (Table 1). The M1 and M2 corresponded to the lateral and medial agranular fields of Donoghue and Wise (1982), whereas the HL and FL were granular fields, which were often included in the primary somatosensory area. However, because the HL and a medial part of the FL were reported to share the electrophysiological and morphological characteristics of the M1 (Hall and Lindholm 1974; Donoghue et al. 1979; Donoghue and Wise 1982), the 8 VM neurons (neurons 1, 3–7, 9, and 14) were considered to innervate motor-associated areas principally, and were tentatively named “motor-preferring VM neurons.” In addition, the VM contained the second population of neurons (6/14 = 43 or 9/19 = 47%), named “nonmotor-preferring VM neurons” here, which did not seem to principally innervate for the motor-associated areas but mainly headed for the orbital and/or cingulate areas (neurons 2, 8, 10–13). However, the latter population of nonmotor-preferring VM neurons did not appear to be a single entity, because the population contained the neurons without projection to the orbital area (neuron 2) and without projection to the cingulate area (neuron 12). Orbital areas were divided into the medial, ventral, dorsolateral, lateral, and ventrolateral orbital areas in the present study according to a recent cytoarchitectonic and chemoarchitectonic work of Van De Werd and Uylings (2008). These orbital subareas were reported to be identified in frontal sections by the cytoarchitecture with Nissl staining. Because of the difficulty in delineating these subareas on parasagittal planes, a complete series of parasagittal Nissl-stained sections of a rat brain was made, and the orbital subareas in parasagittal planes were determined in reference to another complete series of Figure 2. The somal location of VM neurons reconstructed in the present study. VM neurons were projected onto the nearest parasagittal plane of Nissl-stained sections, and serially numbered from the lateral to medial portions of the VM. The broken lines indicate the border of the VM, which was determined in the Nissl-stained sections with the aid of GAD67 immunoreactivity in the adjacent sections. APt, anterior pretectal nucleus; ml, medial lemniscus; mt, mammillothalamic tract; Po, posterior thalamic nuclei; Sm, submedius thalamic nucleus; VPM, ventral posteromedial thalamic nucleus; ZI, zona incerta. For the other abbreviations, see the legends of Figure 1. Scale bar in D applies to A–D. 224 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. Figure 3. Axon fibers and varicosities of VM neurons. VM neurons projected axon fibers to the superficial layer of the M1 (A, B), thalamic reticular nucleus (Rt; C) and neostriatum (D, E). Arrowheads in B and E indicate axon varicosities which were located at a focus plane of the microscope. To determine cortical areas and layers, we counterstained sections for Nissl with Cresyl violet after the reconstruction. The counterstaining was not clear in the figure because the Cresyl violet color was photographically suppressed with a 450-nm-centered band path filter. frontal Nissl-stained sections (Supplementary Fig. 3). On the other hand, cingulate areas were the same as Cg1 and Cg2 of Paxinos and Watson (2007), which mostly corresponded to the dorsal and ventral subdivisions of the anterior cingulate area (Krettek and Price 1977) or to areas 24b and 24a (Vogt and Peters 1981). When VM neurons projected axons to the cingulate areas, the axons were mainly distributed in the Cg1 or dorsal subdivision of the anterior cingulate area. As the lateral portion of the VM was reported to receive nociceptive information from the brainstem reticular formation (Villanueva et al. 1996, 1998), the location of cell bodies might be functionally segregated within the VM according to their roles or projection targets. Thus, the somal location of motorpreferring VM neurons was statistically compared with that of nonmotor-preferring neurons. As shown in Figure 2, where the circled and boxed numbers indicated 8 motor-preferring and 6 nonmotor-preferring VM neurons, respectively, the nonmotor-preferring neurons were seemingly located more medially than the motor-preferring ones. However, no statistically significant difference in the mediolateral or rostrocaudal location was noticed between the 2 groups (P = 0.18 or P = 0.28, respectively, by the 2-tailed unpaired U test). Although the main targets of motor-preferring VM neurons were M1, M2, FL, and HL (motor-associated areas), they sent axon fibers to many other cortical areas. For example, the fine varicose axon fibers of VM neuron 3 were distributed in very wide cortical fields over M2, M1, HL, primary somatosensory, cingulate, medial parietal association, prelimbic, secondary visual, frontal association, FL, lateral parietal association, primary visual, and retrosplenial dysgranular areas (Fig. 4A,B; Table 1). The cortical fields covered by this single neuron were spread more than 7 mm rostrocaudally and more than 3 mm mediolaterally (Fig. 4C). A similarly widespread distribution of axon fibers was observed in all the motor-preferring VM neurons (Figs. 5, 7A–F, Supplementary Fig. 1). Most of the neurons sent axon fibers to cortical regions spreading more than 5 mm rostrocaudally. Even when the list of cortical projection targets was limited to the Table 1 Target cortical areas of reconstructed VM and IZ neurons Neurons VM neuron 1 2 3 4 5 6 7 8 9 10 11 12 13 14 IZ neuronc 1 2 3 4 5 Target areas listed in a descending order of the total axonal length FL a, M1, S1b, M2, HL, LPtA a Cg, V1, V2, Au, M2, PtP, IL, MPtA, M1, LPtA, S1, PL, HL M2, M1, HL, S1, Cg, MPtA, PL, V2, FrA, FL, LPtA, V1, RSD M1, FL, LO, FrA, S1, VLO, DLO, DI, M2, AI M1, S1, S2, M2, VLO, FL, LPtA, FrA, LO, Cg, PtP, DLO, VO, MO, AI M2, M1, S2, VLO, LO, FrA, Cg, FL, S1, LPtA, MO, DLO, VO M2, Cg, M1, FrA, VLO, MO, Ent, Ect, LO, VO, PL, MPtA, AI, RSD VO, MO, Cg, VLO, LO, FrA, PL, Fr3, DLO, M2, AI M1, M2, Cg, FL, HL, VLO, FrA, PL, S1, MO, DLO MO, VO, PL, M2, VLO, Cg, FrA, LO, AI, DLO, IL, M1 Cg, VO, PL, M2, MO, FrA, VLO, Fr3 VLO, LO, M1, AI, VO, M2, MO, FrA, DLO, DI, S1 VO, LO, PL, MO, Cg, VLO, M2, M1, DLO, FrA, AI M2, M1, LO, VLO, S1, LPtA, FrA, PL, V1, MPtA, AI, HL, DLO, VO, V2, PtP, FL M1, M2, HL, Cg, V1, RSD, PL FL, M1, S1, FrA, HL, M2, Fr3, LO, DLO FL, M1, S1, HL M1, M2, FL, FrA, S1, DLO, LO, AI, VLO, Fr3 M2, Cg, M1, HL, S1, MPtA, FL, PL, FrA, V2, RSD Note: AI, agranular insular area; Au, auditory area; Cg, cingulate area; DI, dysgranular insular area; DLO, dorsolateral orbital area; Fr3, frontal cortex, area 3; Ect, ectorhinal area; Ent, entorhinal area; FrA, frontal association area; IL, infralimbic area; LO, lateral orbital area; LPtA, lateral parietal association area; MO, medial orbital area; MPtA, medial parietal association area; PL, prelimbic area; PtP, parietal cortex, posterior area; RSD, retrosplenial dysgranular area; S2, secondary somatosensory area; V1, primary visual area; V2, secondary visual area; VLO, ventrolateral orbital area; VO, ventral orbital area. For the other abbreviations, see text. a Underline, boldface, and italic fonts indicate the target area containing varicose axon fibers of ≥60, ≥30, and <5 mm, respectively. The axon length of VM neurons in each cortical area is listed in Supplementary Table 1. b FL and HL are excluded from the primary somatosensory area (S1). c The data of IZ neurons were obtained from the IZ neurons published in Kuramoto et al. (2009). areas receiving axon fibers of more than 30 mm (underlined and/or bold letters in Table 1), a large variety of cortical areas including the primary somatosensory, secondary somatosensory, cingulate, orbital, and parietal association areas received an axonal projection from the motor-preferring VM neurons. Cerebral Cortex January 2015, V 25 N 1 225 Figure 4. Reconstructed axon fibers of VM neuron 3. The main axon of neuron 3 sent a few axon collaterals to the thalamic reticular nucleus when it exited from the thalamus, and then provided several collaterals with the striatum during the course to the cerebral cortex (A). After entering the subcortical white matter or cerebral cortex, the main axon formed branches and the branches were distributed to widespread cortical field including the motor-associated areas and their surrounding areas. The colors of axon fibers indicate fine varicose axon fibers in different cortical layers (B). The varicose axon fibers were preferentially distributed in L1 of widespread cortical areas. In (C), the cortical distribution of varicose axon fibers is shown in a 3D manner. Gray figures in C are the outlines of cerebral cortex of the rat. St, striatum; ZI, zona incerta. For the other abbreviations, see text and the legends of Table 1 and Figure 1. In Figures 6, 7G–J, and Supplementary Figure 2, the axonal arborization of 6 nonmotor-preferring VM neurons is shown in detail. The axonal arborization of these nonmotorpreferring VM neurons (except neuron 2) was concentrated in 226 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. a smaller cortical field than that of the 8 motor-preferring VM neurons. Of the 6 neurons, neurons 8, 10, and 13 sent varicose axon fibers totaling more than 30 mm in length to both the orbital and cingulate areas (Table 1). Neuron 12 Figure 5. Axonal arborization of 4 VM neurons projecting principally to the motor-associated areas. The axon fibers were widely distributed in the motor-associated areas (A, E, I, K) and especially in their L1 (red colors in B–D, F–H, J, L, M). Compared with neurons 5, 6, and 14, neuron 1 sent many axon collaterals to the striatum (I). Cl, claustrum; St, striatum; for the other abbreviations, see text and the legends of Table 1 and Figure 1. projected mainly to the orbital areas and M1 (Supplementary Fig. 2C,D), whereas neurons 2 and 11 chiefly projected to the cingulate area (Table 1). Neuron 2 further projected to the visual, auditory, and secondary motor areas widely (Fig. 6G–J). Thus, the present results clearly show that the individual VM neurons innervated widespread cortical areas as the Cerebral Cortex January 2015, V 25 N 1 227 Figure 6. Axonal arborization of 3 VM neurons projecting mainly to the orbital and/or cingulate areas. Neurons 8 and 13 sent axon fibers mainly to L1 of the orbital areas (A–F), where the spread of axon fibers appeared smaller than those of motor preferring VM neurons in Figures 4 and 5. Of the 14 reconstructed VM neurons, the most abundant striatal axon collaterals were observed in neuron 8 (A). In contrast, no striatal axon collaterals were provided by neuron 13 (D). The axon fibers of neuron 2 were widely spread (G) and 97.0% of varicose axon fibers were distributed in L1 (H–J). Dense distribution of axon fibers in the visual areas was another characteristic of this neuron. GPe, external segment of the globus pallidus; St, striatum; for the other abbreviations, see text and the legends of Table 1 and Figure 1. 228 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. Figure 7. Examples of single VM neurons reconstructed from the frontal sections. Neuron 7 projected mainly to the motor areas (A–F), whereas neuron 10 projected chiefly to the orbital areas (G–J). Both the neurons sent axon fibers preferentially to L1 of the cortical areas. St, striatum; for the other abbreviations, see text and the legends of Table 1 and Figure 1. nucleus as a whole is well known to innervate wide areas (Herkenham 1979; Glenn et al. 1982; Arbuthnott et al. 1990; Desbois and Villanueva 2001). However, each VM neuron did not send their axons throughout the cerebral cortex, but preferred the motor-associated, orbital, and/or cingulate areas to the other areas. Cerebral Cortex January 2015, V 25 N 1 229 Quantitative Data and Laminar Distribution of Varicose Axon Fibers of VM Neurons In Table 2, the length of varicose axon fibers and estimated number of axon varicosities of each VM neuron were described. The axon varicosity was defined by the localized swelling of axon fibers with ≥1.5-fold larger diameters than the thickness of intervaricosity axon fibers (Fig. 3B). Intervaricosity interval was measured by random sampling, and the mean interval was calculated area by area and layer by layer (Supplementary Table 2). The number of axon varicosities was estimated by dividing the axon length by the mean intervaricosity interval. The total length of varicose axon fibers ranged from 200 to 579 mm with the mean ± SD = 351 ± 107 mm, and the estimated total number of axon varicosities was from 34 360 to 101 480 with 60 264 ± 18 997, showing a large variation neuron by neuron. When compared with VA-VL neurons (Kuramoto et al. 2009), the total axon length of VM neurons was longer than IZ (206 ± 48 mm; P = 0.011 by unpaired 2-tailed t-test) or EZ neurons (234 ± 92 mm; P = 0.046). The varicose axon fibers emitted from the EZ and IZ of the VA-VL were targeted at middle cortical layers and L1, respectively (Kuramoto et al. 2009). In contrast, all the VM neurons reconstructed in the present study preferred the superficial part of L1 to the other layers. Furthermore, all but one of the VM neurons sent more abundant varicose axon fibers to L1 than IZ neurons. In Figures 4B, 5–7, and Supplementary Figures 1 and 2, fine varicose axon fibers were labeled with different colors. As indicated by red color in these figures, most VM neurons sent a high percentage of varicose axon fibers to L1; the percentage of L1 axon length in total cortical axon length was 78.6 ± 14.4% in mean ± SD (Table 2), being significantly larger (P = 0.002 by the 2-tailed unpaired t-test) than that of IZ neurons (53.5 ± 7.3%; Kuramoto et al. 2009). All the VM neurons except neuron 12 sent more than 70% of varicose axon fibers and varicosities to L1, whereas IZ neurons projected at most 66% of varicose axon fibers to L1. This finding suggests that VM neurons constitute a different population from IZ neurons, although both the VM and IZ neurons receive strong inhibitory inputs from the basal ganglia. VM neuron 12 was located in the center of the VM (Fig. 2C) and showed no clear difference in the dendritic arborization from the other VM neurons (Fig. 8A), but the neuron was different in cortical axonal arborization from the surrounding VM neurons, sending only 40% of axon fibers to L1 of the orbital areas (Supplementary Fig. 2C,D). As reported previously (Herkenham 1979; Arbuthnott et al. 1990), the axon fibers of the VM neurons were sometimes ramified moderately in L2/3 (neurons 1, 5 and 6 in Fig. 5; neuron 9 in Supplementary Fig. 1; neuron 12 in Supplementary Fig. 2; neuron 13 in Fig. 6). Varicose axon fibers of the VM neurons were distributed in L2/3 and L5, ranging 2.7–128.9 mm and 4.1–71.2 mm, respectively, although the length of axon fibers in these layers was much shorter than that in L1 (Supplementary Table 3). In Table 3, the morphological parameters of axons were compared between motor-preferring and nonmotor-preferring VM neurons, the latter of which projected mainly to the orbital and/or cingulate areas. Neither the total length of varicose axon fibers in the cortex or striatum nor the relative axon length in L1 differed significantly between the 2 populations. Quantitative Comparison of Axon Fibers of Motor-Preferring VM Neurons with Those of IZ Neurons in Motor-Associated Areas and Striatum Because both VM and IZ neurons preferred L1 of motorassociated areas (M1, M2, FL, and HL) as their cortical target, we compared the morphological data of axon fibers between VM and IZ neurons, using the recalculated data of the 5 IZ neurons reported before (Kuramoto et al. 2009). In addition, to compare functionally related neurons, we selected the 8 VM neurons projecting mainly to the motor-associated areas, and collated them with the IZ neurons (Table 4). In the striatum, the mean axon length of the 8 motor-preferring VM neurons was less than half of the length of IZ neurons, being significantly shorter than that of IZ neurons (P < 0.05 by the 2-tailed unpaired t-test; Table 4), although the difference of striatal collaterals was not significant between all the 14 VM neurons (27.5 ± 31.1 mm) and the 5 IZ neurons (P = 0.056). On the other hand, the varicose axon fibers of the motor-preferring VM neurons in the motor-associated areas were longer than Table 2 Fine varicose axon fibers of VM neurons in the cerebral cortex VM neuron Total axon length in cortex (mm)a Estimated number of varicosities in cortexb Relative axon length in L1 (%) Estimated number of varicosities in L1b 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Mean ± SD 378.9 579.4 428.6 265.7 478.6 438.3 364.2 200.3 234.3 362.9 221.6 364.1 311.0 283.8 350.8 ± 107.4 65 772 101 480 75 631 44 602 82 605 75 655 61 737 34 360 40 158 62 815 37 897 58 037 52 291 50 663 60 264 ± 18 997 69.8 97.0 78.0 78.6 75.2 71.6 76.9 95.1 71.6 92.7 89.8 40.3 76.0 88.4 78.6 ± 14.4 47 618 (72.4%) 98 872 (97.4%) 61 332 (81.1%) 36 579 (82.0%) 64 407 (78.0%) 56 648 (74.9%) 49 781 (80.6%) 32 814 (95.5%) 30 223 (75.3%) 58 729 (93.5%) 34 445 (90.9%) 24 975 (43.0%) 40 858 (78.1%) 45 630 (90.1%) 48 779 ± 18 948 (80.9 ± 13.6%) Note: aThe length of axon fibers was estimated by multiplying the length of varicose axons projected onto parasagittal planes by π/2. The length in each layer was presented in Supplementary Table 2. The measured axons did not contain thick straight axon fibers which might be myelinated portions of thalamocortical axons and make no synaptic contacts. b The estimated number of varicosities in each cortical layer of each area was calculated by dividing the axon length by the mean intervaricosity interval (Supplementary Table 3). The mean intervaricosity interval was measured with 30 nearby varicosity pairs on the axon fibers which ran parallel to the section surface. 230 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. Table 3 Comparison of varicose axon fibers between motor-preferring and nonmotor-preferring VM neurons Number of neurons Total axon length in striatum (mm) Total axon length in cortex (mm) Axon length in L1 (mm) Relative axon length in L1 (%) Motor-preferring Nonmotor-preferring P valuea VM neurons VM neurons U test t-test 8 24.3 ± 24.6 359.1 ± 89.3 272.6 ± 64.1 76.3 ± 5.9 6 31.8 ± 40.3 339.9 ± 136.2 278.4 ± 153.0 81.8 ± 21.7 0.950 0.414 0.573 0.142 0.672 0.755 0.923 0.500 Note: aCalculated by the 2-tailed unpaired U- or t-test. Table 4 Comparison of fine varicose axon fibers between motor-preferring VM and IZ neurons VM neuron 1 3 4 5 6 7 9 14 Mean ± SD IZ neuronb 1 2 3 4 5 Mean ± SD P valuec Total axon length in the striatum (mm)a Total axon length in motor-associated areas (mm)a Relative axon length in L1 of motor-associated areas (%) 32.6 11.4 37.2 4.1 6.4 15.3 77.5 10.1 24.3 ± 24.6 355.9 251.8 202.2 269.2 315.1 253.2 187.8 170.4 250.7 ± 63.6 68.8 77.5 83.1 73.2 71.9 73.6 67.6 89.9 75.7 ± 7.6 47.1 97.8 67.9 43.6 39.4 59.2 ± 24.2 0.0295 152.9 151.2 171.1 269.5 117.4 172.4 ± 57.6 0.0473 44.0 50.0 53.1 47.3 75.7 54.0 ± 12.6 0.0024 Note: aThe length of axon fibers was estimated by multiplying the length of varicose axons projected onto parasagittal plane by π/2. Thus, the measured axons did not contain thick straight axon fibers which might be myelinated portions of thalamocortical axons and make no synaptic contacts. b The data were measured and calculated from the samples in the previous report (Kuramoto et al. 2009) for comparison with the present data. c P value between motor-preferring VM and IZ neurons was calculated by the 2-tailed unpaired t-test. those of IZ neurons (P < 0.05). It was more impressive that the relative axon length (76 ± 8%) of VM neurons in L1 of the motor-associated areas was clearly greater than that of IZ neurons (54 ± 13%; P < 0.005). This suggests that the VM neurons are more specialized for activating the apical dendritic tufts of pyramidal neurons in the motor-associated areas than IZ neurons. Cell Bodies and Dendrites of VM Neurons The somal size of VM neurons was 230 ± 24 µm2 (mean ± SD; Table 5), being similar to that of IZ or EZ neurons (Kuramoto Figure 8. Dendrites and cell bodies of VM neurons. Extents of dendritic processes of VM neurons (1–14; A) were elongated in rostrocaudal direction. Circled and boxed numbers indicate motor-preferring and nonmotor-preferring VM neurons, respectively. The dendrites of neurons 7 and 10 were traced in the Neurolucida 3-dimensionally, rotated, and projected to a parasagittal plane. The Sholl analysis revealed that the differences between the VM and IZ neurons were statistically significant at distances of 140–220 µm from the cell body (B). In addition, the dendritic arborization was significantly different between motor-preferring and nonmotor-preferring VM neurons at distances of 40–120 µm away from the cell body (C). Circles and bars in B and C indicate mean and SD, respectively, and asterisks point to the statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001 by Bonferroni multiple comparison test). The data of IZ neurons were taken from Kuramoto et al. (2009). Cerebral Cortex January 2015, V 25 N 1 231 Table 5 Cell body and dendrites of single-labeled VM and IZ neurons Number of neurons Somal area (μm2) Dendritic spread Rostrocaudal (μm) Dorsoventral (μm) Mediolateral (μm) VM neurons IZ neuronsa P valueb 14 229.7 ± 24.2c 5 245.2 ± 24.3 0.2356 648.3 ± 164.2 330.4 ± 58.5 556.4 ± 119.4 339.3 ± 39.8 329.7 ± 47.1 432.0 ± 99.6 0.0008 0.9813 0.0534 Note: aThe somal area of IZ neurons was obtained from the previous report (Kuramoto et al. 2009), and the dendritic spread was measured using the same samples. b P value was calculated by the 2-tailed unpaired t-test. c Mean ± SD. et al. 2009). The region around each palGFP- or pal-mRFP-positive neuron was darkly immunostained (Fig. 1B′,D′) probably because of extracellularly leaked palGFP or pal-mRFP, suggesting an extremely strong expression of protein by the subgenomic promoter of Sindbis viral vectors. However, neither spread of the fluorescent proteins to nor uptake by adjacent cells was found. The reconstructed VM neurons were multipolar with many dendrites (Fig. 8A) as reported previously in rat thalamus (Sawyer et al. 1989, 1994; Yamamoto et al. 1991). When the reconstructed dendrites were projected to a parasagittal plane (Fig. 8A), it was noticed that the dendritic arborization of VM neurons was elongated rostrocaudally. When compared with 5 IZ neurons sampled in the previous report (Kuramoto et al. 2009), only the rostrocaudal extent of dendritic arborization was significantly larger in VM neurons than in IZ neurons (Table 5). This difference was also reflected in the Sholl analysis (Sholl 1953); the dendrites of VM neurons were significantly longer than those of IZ neurons, although the branching of both the VM and IZ neurons was in a similar range up to 100 µm apart from the cell body (Fig. 8B). The dendritic arborization of 6 nonmotor-preferring VM neurons was denser than that of 8 motor-preferring neurons (Fig. 8A). This is confirmed quantitatively by the Sholl analysis showing that the intersections of the nonmotor-preferring neurons were significantly more numerous at 40–120 µm from the cell body than those of the motor-preferring neurons (Fig. 8C). Discussion We here showed the morphological detail of axonal arborization of VM neurons at a single-neuron level using viral vectors. The present results indicate that VM neurons are different from IZ neurons of the VA-VL, although both the neuronal groups receive strong GABAergic inputs from the basal ganglia and mainly send axon fibers to L1 of the motor-associated cortical areas. This is because 1) the relative distribution of axon fibers in L1 was much higher in VM neurons than in IZ neurons, 2) VM neurons sent fewer axon collaterals to the striatum than IZ neurons, and 3) the dendritic arborization of VM neurons was more extensive rostrocaudally than that of IZ neurons. Furthermore, VM neurons were seemingly divided into at least 2 types, “motor-preferring” and “nonmotorpreferring” neurons according to their cortical projection targets (Fig. 9), although the nonmotor-preferring VM neurons might be heterogenous. 232 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. Figure 9. Summary diagram of axonal projections of VM neurons in comparison with IZ and EZ neurons of the VA-VL. Although the 2 populations of VM neurons receive basal ganglia inputs, one population sends axon fibers principally to motor-associated areas and the other projects mainly to orbital and/or cingulate areas. Almost all the VM neurons innervate L1 of the cerebral cortex more preferentially than IZ neurons. See the text for more detail. Cortical Projection of VM Neurons The VM has been shown, in previous morphological studies with anterograde tracers, to innervate the superficial part of L1 of widespread cortical areas, including the motor-associated, orbital, cingulate, and visual areas, in the rat (Krettek and Price 1977; Herkenham 1979; Arbuthnott et al. 1990; Desbois and Villanueva 2001; Mitchell and Cauller 2001; Rubio-Garrido et al. 2009) and cat brains (Glenn et al. 1982). The projection of VM neurons to L1 has been confirmed by the retrograde tracing technique with superficial application of horseradish peroxidase or fluorescent tracers (Rieck and Carey 1985; Desbois and Villanueva 2001; Monconduit and Villanueva 2005; Rubio-Garrido et al. 2007, 2009). Furthermore, widespread L1 distribution of axon fibers at a single-neuron level has been suggested by the double retrograde tracer technique, which detects doubly retrogradelabeled neurons in the VM after the 2 tracers are separately applied into the superficial portions of 2 distant cortical areas (Monconduit and Villanueva 2005). The present study clearly showed that all the VM neurons examined sent axon fibers to widespread cortical areas even at a single-neuron level, and that they highly preferred L1 as the target layer, irrespective of target areas. Thus, the present results on VM neurons, which are in good agreement of those previous findings, indicate that VM neurons are classified as matrix-type neurons (Jones 1998, 2001). Recently, a further distinction has been made between matrix-type neurons with widely ramified axons targeting multiple, distant cortical areas (multiareal subtype) and those with an axon that arborizes within a single or few adjacent areas (focal subtype) (Clascá et al. 2012). VM neurons are obviously classified as the multiareal subtype, because all the examined VM neurons projected to multiple distant cortical areas. Furthermore, we noticed the presence of 2 comparable populations of VM neurons in the present study (Fig. 9); a major population of VM neurons sent axon fibers mainly to the motor-associated cortical areas, and the other population projected chiefly to the orbital and/or cingulate areas. Although, from the present sampling of VM neurons, we did not detect a clear spatial grouping of somal locations for the 2 populations within the VM, nonmotor-preferring VM neurons had more abundant dendritic arborization than motorpreferring neurons (Fig. 8A,C). This suggests that the VM contained 2 distinct groups of neurons that differ in dendritic and axonal arborizations. Functional Implication of VM Neurons in the Motor Systems As described in the Introduction section, IZ and EZ neurons in the VA-VL receive strong inputs from the basal ganglia and cerebellum, respectively (Kuramoto et al. 2011), and project mainly and widely to the motor-associated cortical areas (Fig. 9). In addition, VM neurons have been reported to accept strong basal ganglia inputs as IZ neurons (Di Chiara et al. 1979; Deniau and Chevalier 1992; Sakai et al. 1998; Bodor et al. 2008; Kuramoto et al. 2011), and send axon fibers to L1 of widespread cortical areas including motor-associated areas in the rat brain (Krettek and Price 1977; Herkenham 1979, 1980; Arbuthnott et al. 1990; Mitchell and Cauller 2001; Rubio-Garrido et al. 2009). The present study further showed that a major population of VM neurons sent axon fibers principally to the motor-associated areas. Thus, the motor-associated areas receive 3 kinds of information: 1) cerebellar information through the EZ to their middle cortical layers, 2) basal ganglia information through the IZ mainly to L1, and 3) basal ganglia information through the VM highly preferentially to L1. Taking the shape of pyramidal neurons into consideration, the basal dendrites of L2/3 and L5 pyramidal neurons receive cerebellar information through the EZ and L4 spiny neurons, and the apical dendritic tufts of those pyramidal neurons admit information from the basal ganglia through the IZ and VM. As the axonal arborizations of single EZ, IZ, and VM neurons are widely distributed in the motor-associated areas, these 3 kinds of inputs are considered to converge on single pyramidal neurons in the motor-associated areas. Thus, the cerebellar and basal ganglia information would be integrated in those pyramidal neurons and used for an appropriate motor control. These findings hence raise a question of why the basal ganglia information is transferred by the 2 routes through the IZ and VM. One possible answer is that the 2 routes convey different lines of information, which are produced in the basal ganglia. Actually, the IZ or rostroventral portion of the VA-VL receives afferents mainly from the ventral edge of the SNr, whereas the VM accepts afferents chiefly from the central and dorsal portion of the SNr in the rat brain (Deniau and Chevalier 1992; Nishimura et al. 1997; Sakai et al. 1998). Thus, the basal ganglia information transferred by motor-preferring VM neurons might be different from that conveyed by IZ neurons. However, to our knowledge, no functional difference has been reported between the 2 portions of the rat SNr, except for the proposal that the ventral SNr–IZ pathway is concerned with oculomotor function (Sakai et al. 1998). The main target areas (receiving ≥30-mm-long varicose axon fibers) of single IZ neurons included not only the motorassociated areas but also the cingulate area (Table 1). However, when compared with the target areas of motorpreferring VM neurons, the target areas of IZ neurons were less widespread, and motor-preferring VM neurons innervated, in addition to the areas listed above, many cortical fields such as medial orbital, ventrolateral orbital, cingulate, medial parietal association, and primary and secondary somatosensory areas (Table 1). Considering this wide distribution of axon fibers, we suppose that VM neurons are in association with general arousal mechanism as suggested repeatedly in the literature (Glenn et al. 1982; Arbuthnott et al. 1990; Sakai et al. 1998). The present study indicates that, even when a small number of VM neurons are activated, many L2/3 and L5 pyramidal neurons in widespread cortical areas would be directly activated through the apical dendritic tufts. Actually, a lesion of the VM has been reported to result in a large reduction of glucose utilization of widespread cortical areas (Girault et al. 1985). Thus, this system is efficient in recruiting many pyramidal neurons in wide cortical areas, and may thus be associated with general arousal or attentional mechanism. Function of VM Neurons in the Orbital and Cingulate Areas For more than 30 years, neurons in the rat VM are known to project densely not only to the dorsolateral frontoparietal areas but also to the prefrontal areas including the orbital and anterior cingulate areas (Krettek and Price 1977; Herkenham 1979). In the present study, 6 of 14 VM neurons sent many axon fibers principally to L1 of the orbital and/or cingulate areas, but many fewer fibers to the medial prefrontal area composed of the infralimbic and prelimbic areas. This finding is compatible with the previous findings that, when the injection site of anterograde tracer was confined to the VM, the labeled terminals were distributed densely in the dorsolateral frontoparietal, cingulate, or orbital areas, but less frequently in the medial prefrontal area (Herkenham 1979; Arbuthnott et al. 1990). In contrast, when the injection site included the submedius nucleus, many terminals were found in L1 of the medial prefrontal area (Krettek and Price 1977; Herkenham 1979; Arbuthnott et al. 1990). It is thus likely that, in the prefrontal areas, VM neurons mainly innervate L1 of the orbital and cingulate areas, whereas the submedius nucleus primarily supplies L1 of the medial prefrontal area with axon fibers. Although all 6 nonmotor-preferring VM neurons also projected to the motor-associated areas, the present finding differentiates VM neurons from IZ neurons since the main target fields of IZ neurons were almost constantly the motor-associated areas (Kuramoto et al. 2009). This indicates much wider function of VM neurons beyond the motor control task in the motorassociated areas. Because the dorsal division of the anterior cingulate area is associated with visuomotor integration and nocifensive processing in rats (Vargo et al. 1988; Pastoriza et al. 1996; Yamamura et al. 1996; Donahue et al. 2001), and because neurons in rat orbital areas are activated in association with the odor discrimination task and in the odor-based conditioned associative task (Schoenbaum and Eichenbaum 1995; Yonemori et al. 2000), VM neurons are considered to contribute to these higher-order functions. The thalamocortical axon fibers of these VM neurons were mostly distributed in L1 of those orbital and cingulate areas. However, the thalamocortical axon terminals, which are specifically characterized by VGluT2 immunoreactivity in the cerebral cortex (Fujiyama et al. 2001; Kaneko and Fujiyama 2002), are densely distributed in middle cortical layers as well as in the superficial part of L1. Thus, these areas may receive afferents at their middle layers from thalamic nuclei other than the VM (Fig. 9). The mediodorsal thalamic nucleus (MD) is generally known to project to the “prefrontal areas,” which consists of 1) infralimbic and prelimbic areas, 2) orbital areas, 3) anterior cingulate areas, and 4) agranular insular areas in rat brain (Krettek and Price 1977; Divac et al. 1978; Vogt et al. 1981; Groenewegen Cerebral Cortex January 2015, V 25 N 1 233 1988; Zeng and Stuesse 1991; Ray and Price 1992; Reep et al. 1996). The MD sends axon fibers to the middle layers as well as to L1 of cingulate and orbital areas (Krettek and Price 1977; Vogt et al. 1981; Groenewegen 1988), and thus their thalamocortical fibers may collaborate with the afferents from VM neurons that mainly project to orbital and/or cingulate areas. This relation of MD axons to VM axons in the cingulate and orbital areas might be similar to that of EZ axons to IZ/VM axons in the motor-associated areas. Thus, a further singleneuron-labeling study on the axon fibers of MD neurons is necessary to understand the structural organization of VM afferents to these higher-order prefrontal areas. Supplementary Material Supplementary Material can be found at http://www.cercor.oxfordjournals.org/. Funding This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) for Young Scientists (23700413 and 25830034 to E.K.); for Scientific Research (22300113 and 25250006 to T.K., 24500409 to T.F., and 24500408 to H.H.); for Exploratory Research (23650175 to T.K.); for Research Activity Start-up (24890179 to S.O.); and for Scientific Research on Innovative Areas, “Mesoscopic Neurocircuitry” (23115101 to T.K.), “Brain and Information Science on Material Perception” (23135519 to T.F.), “Neuronal Diversity and Neocortical Organization” (23123510 and 25123709 to H.H.) and “Foundation of Synapse and Neurocircuit Pathology” (22110007 to H.H.). Notes Conflict of Interest: None declared. References Arbuthnott GW, MacLeod NK, Maxwell DJ, Wright AK. 1990. Distribution and synaptic contacts of the cortical terminals arising from neurons in the rat ventromedial thalamic nucleus. Neuroscience. 38:47–60. Bodor ÁL, Giber K, Rovó Z, Ulbert I, Acsády L. 2008. Structural correlates of efficient GABAergic transmission in the basal gangliathalamus pathway. J Neurosci. 28:3090–3102. Carter DA, Fibiger HC. 1978. The projections of the entopeduncular nucleus and globus pallidus in rat as demonstrated by autoradiography and horseradish peroxidase histochemistry. J Comp Neurol. 177:113–124. Clascá F, Rubio-Garrido P, Jabaudon D. 2012. Unveiling the diversity of thalamocortical neuron subtypes. Eur J Neurosci. 35:1524–1532. Deniau JM, Chevalier G. 1992. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience. 46:361–377. Desbois C, Villanueva L. 2001. The organization of lateral ventromedial thalamic connections in the rat: a link for the distribution of nociceptive signals to widespread cortical regions. Neuroscience. 102:885–898. Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. 1979. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 176:273–284. Divac I, Kosmal A, Björklund A, Lindvall O. 1978. Subcortical projections to the prefrontal cortex in the rat as revealed by the horseradish peroxidase technique. Neuroscience. 3:785–796. Donahue RR, LaGraize SC, Fuchs PN. 2001. Electrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in rats. Brain Res. 897:131–138. 234 Single Thalamocortical Axon Fibers of Ventral Medial Nucleus • Kuramoto et al. Donoghue JP, Kerman KL, Ebner FF. 1979. Evidence for two organizational plans within the somatic sensory-motor cortex of the rat. J Comp Neurol. 183:647–664. Donoghue JP, Parham C. 1983. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 217: 390–404. Donoghue JP, Wise SP. 1982. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 212:76–88. Fujiyama F, Furuta T, Kaneko T. 2001. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 435:379–387. Furuta T, Kaneko T, Deschênes M. 2009. Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J Neurosci. 29:4089–4095. Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschênes M. 2008. Inhibitory gating of vibrissal inputs in the brainstem. J Neurosci. 28:1789–1797. Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T. 2001. In vivo transduction of central neurons using recombinant sindbis virus: Golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem. 49:1497–1507. Girault JA, Savaki HE, Desban M, Glowinski J, Besson MJ. 1985. Bilateral cerebral metabolic alterations following lesion of the ventromedial thalamic nucleus: mapping by the 14C-deoxyglucose method in conscious rats. J Comp Neurol. 231:137–149. Glenn LL, Hada J, Roy JP, Deschênes M, Steriade M. 1982. Anterograde tracer and field potential analysis of the neocortical layer I projection from nucleus ventralis medialis of the thalamus in cat. Neuroscience. 7:1861–1877. Groenewegen HJ. 1988. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 24:379–431. Groenewegen HJ, Witter MP. 2004. Thalamus. In: Paxinos G, editor. The rat nervous system. 3rd ed. San Diego: Elsevier. p. 407–453. Hall RD, Lindholm EP. 1974. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 66:23–38. Hendry SHC, Jones EG, Graham J. 1979. Thalamic relay nuclei for cerebellar and certain related fiber systems in the cat. J Comp Neurol. 185:679–714. Herkenham M. 1979. The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol. 183:487–518. Herkenham M. 1980. Laminar organization of thalamic projections to the rat neocortex. Science. 207:532–535. Hioki H, Nakamura H, Ma Y, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. 2010. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 518:668–686. Ilinsky IA, Yi H, Kultas-Ilinsky K. 1997. Mode of termination of pallidal afferents to the thalamus: a light and electron microscopic study with anterograde tracers and immunocytochemistry in Macaca mulatta. J Comp Neurol. 386:601–612. Ito T, Hioki H, Nakamura K, Tanaka Y, Nakade H, Kaneko T, Iino S, Nojyo Y. 2007. gamma-Aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion. J Comp Neurol. 502:113–125. Jones EG. 2001. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24:595–601. Jones EG. 2007. The Thalamus. 2nd ed. Cambridge: Cambridge University Press. Jones EG. 1998. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 85:331–345. Kaneko T, Fujiyama F. 2002. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 42:243–250. Kha HT, Finkelstein DI, Tomas D, Drago J, Pow DV, Horne MK. 2001. Projections from the substantia nigra pars reticulata to the motor thalamus of the rat: single axon reconstructions and immunohistochemical study. J Comp Neurol. 440:20–30. Kilpatrick IC, Starr MS, Fletcher A, James TA, MacLeod NK. 1980. Evidence for a GABAergic nigrothalamic pathway in the rat. Exp Brain Res. 40:45–54. Krettek JE, Price JL. 1977. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 171:157–192. Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T. 2011. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci. 33:95–109. Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T. 2009. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb Cortex. 19:2065–2077. MacLeod NK, James TA, Kilpatrick IC, Starr MS. 1980. Evidence for a GABAergic nigrothalamic pathway in the rat. Exp Brain Res. 40:55–61. Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. 2009. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 29:444–453. Mitchell BD, Cauller LJ. 2001. Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res. 921:68–77. Monconduit L, Villanueva L. 2005. The lateral ventromedial thalamic nucleus spreads nociceptive signals from the whole body surface to layer I of the frontal cortex. Eur J Neurosci. 21:3395–3402. Moriyoshi K, Richards LJ, Akazawa C, O’Leary DDM, Nakanishi S. 1996. Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron. 16:255–260. Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R et al. 2004. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 24:5370–5380. Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. 2002. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 22:4600–4610. Nakamura KC, Kameda H, Koshimizu Y, Yanagawa Y, Kaneko T. 2008. Production and histological application of affinity-purified antibodies to heat-denatured green fluorescent protein. J Histochem Cytochem. 56:647–657. Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. 2005. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 22:3137–3146. Nishimura Y, Takada M, Mizuno N. 1997. Topographic distribution and collateral projections of the two major populations of nigrothalamic neurons–a retrograde labeling study in the rat. Neurosci Res. 28:1–9. Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. 2008. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci. 28:7153–7164. Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, Sonomura T, Uemura M, Sugiyama K, Kaneko T. 2012. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex. 22:2840–2857. Pastoriza LN, Morrow TJ, Casey KL. 1996. Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain. 64:11–17. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. 6th ed. London: Academic Press. Penney JB Jr, Young AB. 1981. GABA As the pallidothalamic neurotransmitter: implications for basal ganglia function. Brain Res. 207:195–199. Ray JP, Price JL. 1992. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol. 323:167–197. Reep RL, Corwin JV, King V. 1996. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 111:215–232. Rieck RW, Carey RG. 1985. Organization of the rostral thalamus in the rat: evidence for connections to layer I of visual cortex. J Comp Neurol. 234:137–154. Rubio-Garrido P, Pérez-De-Manzo F, Clascá F. 2007. Calcium-binding proteins as markers of layer-I projecting vs. deep layer-projecting thalamocortical neurons: a double-labeling analysis in the rat. Neuroscience. 149:242–250. Rubio-Garrido P, Pérez-De-Manzo F, Porrero C, Galazo MJ, Clascá F. 2009. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 19:2380–2395. Sakai ST, Bruce K. 2004. Pallidothalamocortical pathway to the medial agranular cortex in the rat: a double labeling light and electron microscopic study. Thalamus Relat Syst. 2:273–286. Sakai ST, Grofova I, Bruce K. 1998. Nigrothalamic projections and nigrothalamocortical pathway to the medial agranular cortex in the rat: single- and double-labeling light and electron microscopic studies. J Comp Neurol. 391:506–525. Sawyer SF, Tepper JM, Groves PM. 1994. Cerebellar-responsive neurons in the thalamic ventroanterior-ventrolateral complex of rats: light and electron microscopy. Neuroscience. 63:725–745. Sawyer SF, Young SJ, Groves PM. 1989. Quantitative Golgi study of anatomically identified subdivisions of motor thalamus in the rat. J Comp Neurol. 286:1–27. Schoenbaum G, Eichenbaum H. 1995. Information coding in the rodent prefrontal cortex. I. single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 74:733–750. Schwarz C, Schmitz Y. 1997. Projection from the cerebellar lateral nucleus to precerebellar nuclei in the mossy fiber pathway is glutamatergic: a study combining anterograde tracing with immunogold labeling in the rat. J Comp Neurol. 381:320–334. Sholl DA. 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 87:387–406. Swanson LW. 2004. Brain maps: structure of the rat brain. 3rd ed. London: Academic Press. Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T. 2000. Neurons in golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res. 38:231–236. Van De Werd HJJM, Uylings HBM. 2008. The rat orbital and agranular insular prefrontal cortical areas: a cytoarchitectonic and chemoarchitectonic study. Brain Struct Funct. 212:387–401. Vargo JM, Corwin JV, King V, Reep RL. 1988. Hemispheric asymmetry in neglect produced by unilateral lesions of dorsomedial prefrontal cortex in rats. Exp Neurol. 102:199–209. Villanueva L, Bouhassira D, Le Bars D. 1996. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain. 67:231–240. Villanueva L, Desbois C, Bars DL, Bernard J-F. 1998. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis and the adjacent cuneate nucleus: a retrograde and anterograde tracer study in the rat. J Comp Neurol. 390:133–160. Vogt BA, Peters A. 1981. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 195:603–625. Vogt BA, Rosene DL, Peters A. 1981. Synaptic termination of thalamic and callosal afferents in cingulate cortex of the rat. J Comp Neurol. 201:265–283. Vogt BA, Vogt L, Farber NB. 2004. Cingulate cortex and disease model. In: Paxinos G, editor. The rat nervous system. London: Elsevier Academic Press. p. 705–777. Yamamoto T, Kishimoto Y, Yoshikawa H, Oka H. 1991. Intracellular recordings from rat thalamic VL neurons: a study combined with intracellular staining. Exp Brain Res. 87:245–253. Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R. 1996. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res. 735:83–92. Yonemori M, Nishijo H, Uwano T, Tamura R, Furuta I, Kawasaki M, Takashima Y, Ono T. 2000. Orbital cortex neuronal responses during an odor-based conditioned associative task in rats. Neuroscience. 95:691–703. Zeng D, Stuesse SL. 1991. Morphological heterogeneity within the cingulate cortex in rat: a horseradish peroxidase transport study. Brain Res. 565:290–300. Zilles K. 1985. The cortex of the rat. a stereotaxic atlas. Berlin, Heidelberg: Springer-. Cerebral Cortex January 2015, V 25 N 1 235