* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hypothalamus
Neuroeconomics wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Neurotransmitter wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neurophilosophy wikipedia , lookup
Limbic system wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Aging brain wikipedia , lookup
Synaptogenesis wikipedia , lookup
Electrophysiology wikipedia , lookup
Single-unit recording wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Haemodynamic response wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Multielectrode array wikipedia , lookup
Axon guidance wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neural coding wikipedia , lookup
Mirror neuron wikipedia , lookup
Chemical synapse wikipedia , lookup
Neural oscillation wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Central pattern generator wikipedia , lookup
Metastability in the brain wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Channelrhodopsin wikipedia , lookup
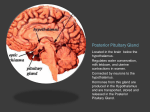
Hypothalamus Introductory article Article Contents J Patrick Card, University of Pittsburgh, Pittsburgh, Pennsylvania, USA . Structural Organization of the Hypothalamus The hypothalamus is a functionally diverse region of the forebrain which exerts profound regulatory influences over physiological and behavioural processes essential for survival. It possesses extensive synaptic connections with other regions of the nervous system and is the region of the brain responsible for controlling the functional activity of the pituitary gland. An essential feature of its function is the ability to convert synaptic information to humoral signals that exert regulatory control over peripheral organ systems and to respond to the functional activity of the peripheral systems that it controls. . Functional Parcellation of the Hypothalamus . Summary Structural Organization of the Hypothalamus The hypothalamus is found at the base of the forebrain, immediately in front of the midbrain flexure (Figure 1a). In the human brain, three distinct superficial landmarks on the ventral surface of the brain define its location (Figure 1b). The most rostral of these is the optic chiasm, formed by the convergence of the two optic nerves. The intermediate portion of the hypothalamus is defined by the pituitary stalk. This structure lies immediately behind the optic chiasm and is one of the most functionally important regions of the hypothalamus. It connects the hypothalamus and pituitary gland, and contains the nerve fibres and blood vessels through which hypothalamic neurons control the secretory activity of this important endocrine gland. The caudal portion of the hypothalamus is defined by two prominent spherical protuberances behind the pituitary stalk, known as the mammillary bodies. These protuberances define the location of cell groups that are the site of termination of a distinct fibre tract, the fornix, which arises from the limbic system and traverses the full longitudinal extent of the hypothalamus. The superficial landmarks noted above also define the location of major subdivisions of the hypothalamus that were initially defined on the basis of cell morphology and packing density (Figure 2). These areas, known as the chiasmatic, tuberal and mammillary subdivisions contain morphologically distinct groups of neurons that were initially visualized in tissue sections stained with basophilic dyes and the Golgi silver impregnation method. The application of contemporary staining methods has shown that many of these neurons are also distinguished by their peptide or neurotransmitter phenotype as well as by their connections. Transverse (frontal) sections through the hypothalamus reveal a central fluid reservoir, the third ventricle, flanked by grey matter containing a mixture of neurons, glia and fibre tracts (Figure 2b). Basophilic stains reveal cytoarchitectural differences in neuronal morphology and packing density that define longitudinal columns extending Figure 1 The location of the hypothalamus in the ventral forebrain is illustrated in midsagittal and ventral exposures of the human brain. (a) The midsagittal exposure reveals the hypothalamus (marked with an asterisk) immediately in front of the midbrain flexure. This same exposure is shown at higher magnification in Figure 2a. (b) The ventral exposure reveals the three major superficial landmarks that mark the position of the hypothalamus in the ventral diencephalon. The most rostral portion is marked by the optic chiasm (OC), which is formed by the convergence of the optic nerves and gives rise to the optic tracts. The pituitary stalk (PS) lies immediately behind the chiasm in the tuberal hypothalamus. The pituitary has been removed from this specimen, revealing the central lumen, which is continuous with the third ventricle. The bilaterally paired mammillary bodies (MB) mark the caudal-most extent of the hypothalamus. ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 1 Hypothalamus Functional Parcellation of the Hypothalamus Although the classical literature defined hypothalamic subdivisions solely on the basis of cell morphology and packing density, it is now known that many of these structural subdivisions also reflect differences in function. A large literature has contributed to the definition of numerous, functionally distinct, cell groups in the hypothalamus that are beyond the scope of this short overview. Instead, a subset of those regions that effectively illustrate the functional diversity of the hypothalamus and the profound influence that it exerts over central and peripheral systems are discussed. The hypothalamus contains a biological clock Figure 2 (a) The rostrocaudal extent of the hypothalamus, defined by the optic chiasm (OC) and mammillary body (MB), is shown in midsagittal exposure. (b) The internal structure of hypothalamus as revealed in a myelin-stained transverse section passing through the level of the pituitary stalk. The homogeneous tissue on either side of the third ventricle (V3) constitutes the bilaterally paired cell masses that form the hypothalamus. The relative position of periventricular (P), medial (M) and lateral (L) columns are defined on the left side of the hypothalamus. OT, optic tract: F, fornix. through the full extent of the hypothalamus. Groups of neurons immediately adjacent to the third ventricle constitute the periventricular column. Prominent among the functions subserved by the small and densely packed neurons of this region are biological timing and neuroendocrine regulation of the pituitary gland. The medial column consists of well defined groups of neurons immediately adjacent to the periventricular subdivision which are involved in the expression of motivated social behaviours. The lateral column consists of diffusely distributed neurons that communicate extensively with other regions of the neuraxis. 2 One of the principal functions of the hypothalamus is to provide temporal organization to the expression of physiological and behavioural processes. This is accomplished by compact cell groups in the rostral hypothalamus, known as the suprachiasmatic nuclei (SCN). SCN neurons exhibit a genetically derived circadian rhythm of neuronal activity in which neuronal activity is high for 12 h of the light dark cycle and low for the remainder. Available evidence supports the conclusion that each SCN neuron is an independent oscillator and that communication between SCN neurons allows them to synchronize their activity and act as an integrated oscillatory unit. The oscillatory activity of SCN neurons is synchronized with the external environment through a ‘retinohypothalamic projection’ which arises from ganglion cells in the retina and synapses in the SCN. Retinal ganglion cells are functionally heterogeneous and one subpopulation is responsible for bringing information on luminance in the external environment to neurons in the SCN. The entrained oscillatory output of the SCN is transmitted to other cells groups through axonal projections of SCN neurons. The synaptic targets of these projections are found largely within the hypothalamus, but the temporal information is distributed to a number of cell groups in the central and peripheral nervous systems through polysynaptic circuits. The temporal information distributed from the biological clock provides important integrating influences necessary for homeostatic regulation, reproduction and behaviour. Regulation of the secretory activity of the pineal gland provides a vivid example of how the SCN exerts it temporal influence upon other areas. Melatonin, the secretory product of the pineal gland, is synthesized and released into the circulation in a circadian manner which persists in constant darkness or in blind individuals with an intact retinohypothalamic tract. Levels of melatonin and its synthetic enzymes are highest during the dark phase of the photoperiod and low during the light phase. However, ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Hypothalamus exposure to light during the dark phase of the photoperiod leads to an abrupt drop in the levels of melatonin synthesis and release. The entrained circadian release of melatonin provides an accurate means of measuring day length that is essential to the regulation of mammalian physiology, particularly in seasonal breeders. Melatonin exerts an influence upon the circadian timing system and endocrine systems through specific binding sites in the brain, most notably within the SCN and pars tuberalis of the pituitary gland. The ability of melatonin to influence the circadian system has led to its use for treatment of disturbances of biological timing that result from jet lag and shift work. Available evidence supports the conclusion that both the circadian and light influences upon melatonin secretion are mediated through a trisynaptic pathway which impinges upon the autonomic outflow to the pineal gland. The first synapse in this circuit is the retinohypothalamic projection to the SCN which, in turn, projects upon neurons in the paraventricular nucleus of the hypothalamus. The subpopulation of PVN neurons receiving this input project to neurons in the thoracic spinal cord that give rise to the dysynaptic autonomic outflow to the pineal gland. Interruption of any component of this circuitry abolishes the circadian and photic regulation of pineal function. Thus, the SCN imposes a temporal influence upon diverse physiological systems through a multisynaptic circuit, which ultimately is converted to a humoral signal exerting feedback regulatory influences upon the brain. The hypothalamus participates in the regulation of sleep It has long been known that the hypothalamus participates in the regulation of sleep. Early studies identified ‘sleep centres’ in different portions of the hypothalamus that were thought to regulate opposite components of the sleep– wake cycle. These studies were based upon the loss of function that resulted from lesions of different portions of the hypothalamus in animals, and suggested that the rostral hypothalamus contained a centre involved in the induction of sleep while the caudal hypothalamus contained a centre responsible for arousal. Recent studies support these basic observations and have begun to reveal the specific neurons and underlying circuitry that provide the basis for this regulation. For example, specific populations of neurons in the rostral preoptic area of the hypothalamus are now known to become functionally active shortly after animals fall asleep, and these same cells inhibit the activity of histaminergic neurons in the caudal hypothalamus thought to be involved in arousal. These compelling observations suggest that the hypothalamus subserves an important role in the larger set of systems involved in the regulation of this complex behaviour. The preoptic hypothalamus influences thermoregulation Numerous observations suggest that multiple systems in the rostral hypothalamus participate in the regulation of temperature. For example, thermosensitive neurons are present in the rostral hypothalamus that are activated by temperature probes. Warming the preoptic area during waking in animals suppresses the activity of arousalassociated neuronal discharge in the caudal hypothalamus, as well as in basal forebrain neurons with diffuse cortical projections. Similarly, this manipulation reduces motor activity, metabolic function and respiratory rate, suggesting that the compensatory responses to thermal stress may be expressed through the same circuitry involved in the initiation of sleep. The well documented circadian rhythm in body temperature also implicates the SCN in thermoregulatory mechanisms, and there is a large literature indicating that fever is mediated by the anterior hypothalamus. Collectively, these observations implicate the rostral hypothalamus as an important site of body temperature regulation. Neuroendocrine regulation is an important hypothalamic function One of the most important and well documented functions of the hypothalamus is to regulate the hormonal outflow from the pituitary gland. This is achieved through an important structural modification of the mediobasal hypothalamus through which synaptic information is converted to bloodborne humoral signals. The pituitary gland contains two distinct lobes, which are distinguished by their developmental origin. The anterior lobe derives from an outpocketing of the ectoderm known as the Rathke pouch. The posterior lobe is an evagination of the portion of the neural tube that gives rise to the hypothalamus. Thus, the posterior lobe is continuous with the floor of the hypothalamus through this stalk-like evagination, whereas a dedicated series of long portal vessels on the surface of the pituitary stalk provides the only connection between the hypothalamus and the anterior lobe of the pituitary. In spite of the structural and developmental differences, the secretory output of both lobes of the pituitary is under the direct control of the hypothalamus. Two general systems of neurons contribute to this regulation. Large ‘magnocellular’ neurons in the hypothalamus give rise to axons that traverse the pituitary stalk to terminate within the posterior lobe. These neurons receive synaptic inputs from other regions of the brain, but release their peptide contents into the peripheral vasculature in the posterior pituitary. In this manner, a synaptic signal is converted to a bloodborne humoral signal that influences peripheral targets. Small ‘parvocellular’ neurons produce releasing ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 3 Hypothalamus hormones and inhibiting hormones that control the secretory activity of cells in the anterior pituitary. However, the parvocellular neurons release their secretory products into fenestrated capillaries that drain into the long portal vessels that drain into the anterior lobe. The magnocellular neurons secrete either vasopressin or oxytocin, and are largely concentrated in the supraoptic (SON) and paraventricular (PVN) hypothalamic nuclei. Although both the PVN and SON contain magnocellular neurons expressing those peptides, the neurons are sequestered within different subdivisions of these two hypothalamic nuclei and participate in different regulatory processes. Oxytocin is best know for its effect on milk letdown in lactating females. Vasopressin, also known as antidiuretic hormone, is involved in the regulation of blood pressure and provides an excellent example of feedback regulation of peripheral systems upon the functional activity of the hypothalamus. In response to low blood pressure, the kidney releases renin into the vasculature which is subsequently metabolized to angiotensin II. Angiotensin II has access to neurons in a region of the nervous system known as the subfornical organ (SFO) that lacks a blood–brain barrier. SFO neurons express angiotensin II receptors and also project to the magnocellular vasopressinergic neurons in the PVN. Interestingly, the SFO neurons involved in this projection also appear to use angiotensin II as a neurotransmitter. The SFO neurons activated by peripherally circulating angiotensin II elicit the release of vasopressin from the magnocellular endings in the posterior pituitary, which acts at the level of the kidney to reduce fluid excretion and thereby increase blood fluid volume. Thus, the hypothalamus is not only an effector system for modulating peripheral functions, but is also subject to feedback regulation by the physiological processes that it controls. As noted above, hypothalamic control of anterior pituitary function also involves conversion of synaptic information to humoral signals. This system draws upon a heterogeneous population of neurochemically distinct, parvocellular neurons in the hypothalamus and a dedicated vascular plexus between the hypothalamus and the pituitary. The long portal vessels that constitute this neurohaemal link arise from fenestrated capillaries in the floor of the hypothalamus and traverse the pituitary stalk to gain access to sinuses in the anterior pituitary gland. Parvocellular neurons project to the circumscribed region of the pituitary stalk, known as the median eminence, where fenestrated vessels drain into the long portal vessels supplying the anterior pituitary. Parvocellular neurons that contribute to anterior pituitary regulation are concentrated in a number of cell groups in the anterior two-thirds of the hypothalamus. The largest concentration of cells is found in the arcuate and paraventricular hypothalamic nuclei. However, substantial numbers of cells are distributed throughout other regions of the 4 hypothalamus, such as the periventricular nucleus and preoptic area. The neurochemical phenotype of parvocellular neurons plays an extremely important role in determining the secretory responses of the anterior pituitary gland. Often, two neurochemically distinct populations of parvocellular neurons will have opposing effects upon the same group of cells in the anterior pituitary. For example, release of growth hormone (GH) from the anterior pituitary is determined by the differential release of two parvocellular populations of hypothalamic neurons. Arcuate neurons that synthesize growth hormonereleasing hormone stimulate the release of GH from the anterior pituitary, and the opposite is achieved when parvocellular neurons in the periventricular nucleus release somatostatin (growth hormone-inhibiting hormone) into the portal plexus. Thus, appropriate regulation of GH release from the pituitary is dependent upon the regulated release of opposing peptides at the level of the median eminence, a complex process that is dependent upon both the hormonal output of the pituitary and feedback from the metabolic products that are driven by GH secretion. Reproductive function is controlled by the hypothalamus The essential role of the hypothalamus in the production of the gametes necessary for reproduction and survival of the species has been documented thoroughly. This is achieved through the aforementioned neuroendocrine regulatory mechanisms within which hypothalamic nuclei control anterior pituitary function. However, it is equally clear that the hypothalamus plays an essential role in the expression of the complex reproductive behaviours necessary for fertilization of ova. Experimental studies in a number of species have demonstrated that different populations of neurons mediate sexual behaviour in males and females, with male sexual behaviour being mediated by the neurons in the preoptic area and female sexual behaviour by neurons in the tuberal hypothalamus. Dimorphism in hypothalamic structure and connectivity in males and females has also been reported in animals and humans. The significance of this dimorphism remains to be determined, but there can be little doubt that the differences in structure are determined by the hormonal milieu present during different stages of brain development. Autonomic function is influenced by the hypothalamus The hypothalamus is also an important integrative centre that modulates the output of the autonomic nervous system (ANS). Dense descending projections of ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Hypothalamus hypothalamic neurons terminate upon preganglionic neurons of both the parasympathetic and sympathetic subdivisions of the ANS in the brainstem and spinal cord. Several hypothalamic cell groups contribute to this projection, but particularly prominent monosynaptic projections from neurons in the PVN and lateral hypothalamic area (LHA) have been demonstrated. Insight into the identity of neurons that modulate the functional activity of sympathetic preganglionic neurons in the intermediolateral cell column of thoracic spinal cord has recently been provided by studies employing neurotropic alpha herpesviruses for transneuronal labelling of synaptically linked circuits. These viruses replicate within permissive neurons and pass transynaptically to infect other neurons in a polysynaptic circuit. Thus, they can be used as self-amplifying transynaptic tracers to identify populations of neurons with a common function. Using this approach. Loewy and colleagues have postulated that neurons in the PVN, LHA and perifornical hypothalamic nucleus are part of a group of ‘central command neurons’ in the diencephalon and brainstem that function under circumstances where it is necessary to coordinate responses in functionally separate components of the sympathetic outflow. Although this interesting hypothesis requires further examination, the findings of this and other studies emphasize the importance of the hypothalamus in the control of autonomic function and identify output pathways through which this regulation is achieved. The hypothalamus influences immune function A large body of evidence now supports the conclusion that the central nervous system influences immune function through both the ANS and neuroendocrine regulation of the pituitary gland. Felten and colleagues have demonstrated that postganglionic noradrenergic neurons of the sympathetic nervous system densely innervate compartments of the spleen and other lymphoid organs involved in immune function. Although the central neurons that modulate the functional activity of this sympathetic outflow remain to be defined, it seems probable that the established descending projections of hypothalamic neurons to the intermediolateral cell columns of thoracic spinal cord contribute to this innervation. Further evidence for a hypothalamic influence upon the immune system can be found in studies of the neuroendocrine system. Work championed by Besedovsky and colleagues in the 1970s, which has been confirmed and expanded by subsequent investigators, revealed that the neuroendocrine outflow of the hypothalamus influences the activity of the immune system which, in turn, exerts a feedback regulatory control on the brain. The hypothalamus influences food and fluid homeostasis The hypothalamus functions within a larger set of central nervous system circuits responsible for homeostatic mechanisms involved in the regulation of food and fluid intake. Neuronal markers of activity have been particularly informative in defining the cell groups that respond to challenges of each of these systems, and have provided considerable insight into the organization of these systems. Stimuli that evoke adaptive changes in fluid osmolarity and blood volume (e.g. dehydration or manipulation of blood sodium levels) have revealed functional parcellation in the regions of the brain responsible for regulating fluid homeostasis. For example, neurons sensitive to fluid osmolarity have been localized to a circumscribed region in the rostral hypothalamus, whereas neurons that respond to changes in blood pressure are found in the SFO and caudal brainstem. Nevertheless, these areas utilize the same effector systems (magnocellular–posterior pituitary system) to elicit compensatory changes in fluid volume. Alterations in food intake are also the product of a balanced interaction between effector pathways that elicit food intake and feedback regulation that inhibits consumption. Sensory feedback to the nervous system after a meal takes the form of excitation of the sensory neurons innervating the viscera as well as the release of neuroactive compounds from the gastrointestinal tract. These sensory stimuli act in the caudal brainstem in regions that maintain dense reciprocal connections with hypothalamic nuclei. In addition, hypothalamic nuclei are the primary target of hormones released into the circulation after a meal. Both of these regulatory pathways have the capacity to alter the functional activity of hypothalamic neurons involved in feeding. The limbic system influences the output of the hypothalamus The functional diversity of the hypothalamus is reflected not only by its many and varied output pathways, but also by the projections that it receives from other regions of the nervous system. This extensive connectivity emphasizes the importance of the hypothalamus as a dynamic integrative centre with profound influences upon both central and peripheral nervous systems. A particularly poignant example of this integrative capacity can be appreciated by considering the projections of the limbic system upon the hypothalamus. Areas that contribute to the limbic system, such as the hippocampus and amygdala, project heavily upon the hypothalamus and there have been considerable advances in our understanding of the function of these connections. The hippocampal projection to the hypothalamus through the fornix is one of the most well described and ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 5 Hypothalamus long acknowledged monosynaptic projections of the limbic system into the diencephalon. In 1937, James Papez proposed that the projection of hippocampal neurons to the mammillary nuclei through the fornix was part of a multisynaptic circuit involved in the expression of emotion. This hypothesis has not been confirmed, and it has subsequently become apparent from contemporary studies that the cells that give rise to this projection are found in the subiculum rather than the hippocampus. Nevertheless, recent work of Risold and Swanson has demonstrated that the hippocampus does exert a profound influence upon hypothalamic function via disynaptic projections through the lateral septal nucleus. This projection is topographically organized such that subpopulations of hippocampal neurons project into subfields of the lateral septum which, in turn, project upon longitudinal columns extending through the rostrocaudal extent of the hypothalamus. The columns that receive these projections are multifunctional, but contain cell groups that can be grouped into general functional categories. Thus, neurons in the periventricular zone are associated with neuroendocrine and autonomic function as well as ingestive behaviours, while those in the rostral medial zone influence motivated reproductive and agonistic behaviours, and those in the mammillary region feed processed information back to the hippocampus. These disynaptic projections into hypothalamus are far more extensive and target many more functionally distinct hypothalamic systems than the monosynaptic projections to the mammillary nuclei that pass through the fornix. The amygdala also gives rise to extensive projections to the hypothalamus that pass through the stria terminalis. There is now strong evidence that the amygdala plays an integral role in the expression of fear and anxiety, and that effector pathways responsible for the expression of behaviours include both the hypothalamus and ANS. Activation of the sympathetic component of the ANS in response to fearful or stressful stimuli is thought to occur through projections of the amygdala and locus coeruleus upon hypothalamic nuclei. These projections also activate neurons in the PVN, which ultimately lead to the increased levels of cortisol in the circulation by virtue of their regulation of anterior pituitary function. This dual activation of hypothalamic systems is a prominent 6 contributor to the stereotypic responses to stress and fear, and vividly illustrates how integration of information by the hypothalamus can activate multiple effector systems that contribute to complex behavioural responses. Summary In conclusion, the hypothalamus is an important integrative centre in the forebrain that subserves a variety of essential functions. Its ability to fulfil effectively the diverse functional demands placed upon it is integrally related to its ability to convert synaptic information to humoral signals and to respond to feedback regulation by the peripheral systems that it controls. Further Reading Alder R (1996) Historical perspectives on psychoneuroimmunology. In: Friedman H, Klein TW and Friedman AL (eds). Psychoneuroimmunology, Stress, and Infection, pp. 1–24. Boca Raton, FL: CRC Press. Charney DS, Grillon C and Bremmer JD (1998) The neurobiological basis of anxiety and fear: circuits, mechanisms, and neurochemical interactions (Part I). Neuroscientist 4: 35–44. Loewy AD (1990) Central autonomic pathways. In: Loewy AD and Spyer KM (eds) Central Regulation of Autonomic Function, pp. 88– 103. New York: Oxford University Press. Martin JB and Reichlin S (1987) Fundamental aspects of neuroendocrinology. In: Clinical Neuroendocrinology, chap. 1, pp. 1–62. Philadelphia: FA Davis. Moore RY (1992) The organisation of the human circadian timing system. Progress in Brain Research 93: 101–117. Ramsay DJ and Thrasher TN (1990) Thirst and water balance. In: Stricker EM (ed.) Handbook of Behavioral Neurobiology, pp. 353–386. New York: Plenum Press. Risold PY and Swanson LW (1997) Connections of the lateral septal complex. Brain Research Reviews 24: 115–195. Saper CB (1990) Hypothalamus. In: Paxinos G (ed.) The Human Nervous System, pp. 389–414. San Diego: Academic Press. Sherin JE, Shiromani PJ, McCarley RW and Saper CB (1996) Activation of ventrolateral preoptic neurons during sleep. Science 271: 216–219. Stellar E (1990) Brain and behaviour. In: Stricker EM (ed.) Handbook of Behavioral Neurobiology, pp. 3–22. New York: Plenum Press. Swaab DF and Fliers E (1985) A sexually dimorphic nucleus in the human brain. Science 228: 1112–1115. ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net