* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Properties of Primary Sensory (Lemniscal) Synapses in the
Biology of depression wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Convolutional neural network wikipedia , lookup
Environmental enrichment wikipedia , lookup
Sensory substitution wikipedia , lookup
Apical dendrite wikipedia , lookup
Electrophysiology wikipedia , lookup
Metastability in the brain wikipedia , lookup
Multielectrode array wikipedia , lookup
Psychophysics wikipedia , lookup
Long-term depression wikipedia , lookup
Neural oscillation wikipedia , lookup
Perception of infrasound wikipedia , lookup
Mirror neuron wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Biological neuron model wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Single-unit recording wikipedia , lookup
Optogenetics wikipedia , lookup
Neural coding wikipedia , lookup
Neurotransmitter wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Circumventricular organs wikipedia , lookup
Synaptogenesis wikipedia , lookup
Evoked potential wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Nervous system network models wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Central pattern generator wikipedia , lookup
Spike-and-wave wikipedia , lookup
Efficient coding hypothesis wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
End-plate potential wikipedia , lookup
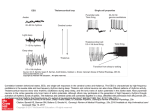
J Neurophysiol 87: 946 –953, 2002; 10.1152/jn.00426.2001. Properties of Primary Sensory (Lemniscal) Synapses in the Ventrobasal Thalamus and the Relay of High-Frequency Sensory Inputs MANUEL A. CASTRO-ALAMANCOS Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Quebec H3A 2B4, Canada Received 23 May 2001; accepted in final form 14 September 2001 Castro-Alamancos, Manuel A. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of highfrequency sensory inputs. J Neurophysiol 87: 946 –953, 2002; 10.1152/jn.00426.2001. The main role of the thalamus is to relay sensory inputs to the neocortex. In the primary somatosensory thalamus (ventrobasal thalamus), sensory inputs deliver tactile information through the medial lemniscus tract. The transmission of sensory information through this pathway is affected by behavioral state. For instance, the relay of high-frequency somatosensory inputs through the thalamus is suppressed during anesthesia or quiescent states but allowed during behaviorally activated states. This change may be due to the effects of modulators on the efficacy of lemniscal synapses. Here I show that lemniscal synapses of adult rodents studied in vitro produce large amplitude-highly secure unitary excitatory postsynaptic potentials (EPSPs), which depress in response to repetitive stimulation at frequencies ⬎2 Hz. Acetylcholine and norepinephrine, which are important thalamic modulators, have no effect on the efficacy of lemniscal EPSPs but reduce evoked inhibitory postsynaptic potentials and corticothalamic EPSPs. Although acetylcholine and norepinephrine do not affect lemniscal synapses, the postsynaptic depolarization they produce on thalamocortical neurons serves to warrant the relay of lemniscal inputs at high-frequency rates by bringing the depressed lemniscal EPSPs close to firing threshold. In conclusion, acetylcholine and norepinephrine released during activated states selectively enhance sensory transmission through the lemniscal pathway by depolarizing thalamocortical neurons and simultaneously depressing the other afferent pathways. The rodent ventroposterior medial thalamus (VPM) receives sensory information about the whiskers from the principal trigeminal nucleus through the medial lemniscus tract. Lemniscal terminals form glutamatergic synaptic contacts with the soma and proximal dendrites of VPM neurons (Chiaia et al. 1991; Diamond 1995; Feldman and Kruger 1980; Liu et al. 1995; Spacek and Lieberman 1974; Veinante and Deschenes 1999; Williams et al. 1994b). Although the whisker system of rodents is among the most widely investigated sensory systems, the electrophysiological properties of lemniscal synapses have not been investigated in vitro. In addition to lemniscal synapses, thalamocortical neurons in the ventrobasal thalamus receive a massive excitatory input from the neocortex via corticothalamic synapses and an inhibitory input from the nucleus reticularis of the thalamus (nRt). Moreover, several neuromodulatory systems from the brain stem and basal forebrain project to the ventrobasal thalamus; cholinergic and noradrenergic fibers innervate the rodent ventrobasal thalamus to different degrees depending on the species and nucleus (Bennett-Clarke et al. 1999; Hallanger et al. 1990; Simpson et al. 1997). Cholinergic and noradrenergic cell populations discharge vigorously during activated states (Aston-Jones et al. 1991; Buzsaki et al. 1988) and thus the levels of these modulators increase in the thalamus during activated states (Williams et al. 1994a). We have recently shown that cholinergic and noradrenergic inputs from the brain stem regulate corticothalamic synapses (Castro-Alamancos and Calcagnotto 2001). The extent to which these neuromodulatory inputs regulate lemniscal and inhibitory synapses is unknown. Studies conducted in vivo suggest that the lemniscal pathway may be regulated by neuromodulators because the relay of high-frequency sensory inputs through this pathway is affected by behavioral state. For instance, Poggio and Mountcastle (1963) demonstrated that the capacity for frequency following of tactile stimuli is dramatically different for thalamic cells in the waking as compared with the anesthetized monkey. More recent work in the freely behaving rat using electrical stimulation of the infraorbital nerve has shown that VPM sensory responses at frequencies ⬎10 Hz were suppressed during quiescent states but not during active exploration (Fanselow and Nicolelis 1999). These results indicate that during quiescent states, the relay of high-frequency sensory inputs is impeded but allowed during activated states. This may be due to the effects of neuromodulators released in the thalamus during behavioral activation, such as acetylcholine and norepinephrine. Thus the lemniscal pathway may be regulated by these neuromodulators so that the relay of high-frequency inputs is possible during behaviorally activated states. The present study explored the properties of lemniscal synapses in slices of rodent tissue and how acetylcholine and norepinephrine affect these synapses and the relay of highfrequency lemniscal inputs. Address for reprint requests: Montreal Neurological Institute, 3801 University St., Rm. WB210, Montreal, Quebec H3A 2B4, Canada (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. INTRODUCTION 946 0022-3077/02 $5.00 Copyright © 2002 The American Physiological Society www.jn.org LEMNISCAL SYNAPSES METHODS Horizontal slices were prepared from adult (ⱖ7 wk) BALB/C mice. Slices were cut in ice-cold buffer using a vibratome and kept in a holding chamber for ⱖ1 h. Experiments were performed in an interface chamber at 32°C. The slices were perfused constantly (1–1.5 ml/min) with artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 MgSO4 7H2O, 10 dextrose, 2.5 CaCl2 2H2O. The ACSF was bubbled with 95% O2-5% CO2. Synaptic responses were induced using a concentric stimulating electrode placed in the medial lemniscus (Fig. 1). Another stimulating electrode was placed in some cases in the thalamic radiation to evoke corticothalamic responses onto the same neuron. The stimulus consisted of a 200-s pulse of ⬍70 A unless otherwise indicated. The 947 ventrobasal thalamus and medial lemniscus were easily and clearly identifiable with a dissecting microscope. Intracellular recordings were performed using sharp electrodes (60 – 80 M⍀) filled with potassium-acetate (3 M) or with cesium-acetate (1 M) and QX-314 (50 –100 mM). In some experiments, the GABAA receptor antagonist, bicuculline methobromide (BMI; 10 –20 M), was included in the ACSF. Inhibitory postsynaptic potentials (IPSPs) were isolated with bath application of 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 20 M) and 2-amino-5-phosphonovaleric acid (AP5, 50 M) and evoked by stimulating nRt fibers within the ventrobasal thalamus. In some experiments, a cut was produced to excise the nRt from the slice. RESULTS Properties of lemniscal EPSPs FIG. 1. Intracellular responses evoked in ventrobasal thalamic neurons by stimulating the medial lemniscus in vitro. A: diagram of the slice preparation used in the present study. The typical locations for simulating electrodes in the medial lemniscus and in the thalamic radiation are shown. Also shown is the placement of the recording electrode in the ventrobasal thalamus. B: excitatory postsynaptic potential (EPSP) triggered by stimulating the medial lemniscus (below) and the action potential it triggers when firing threshold is reached (above). Note the different y axis scales. C: comparison of lemniscal and corticothalamic EPSPs evoked on the same neuron by a pair of stimuli at 50-ms interstimulus interval. D: effect of bath-applied 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 10 M) on a lemniscal EPSP. Neurons were recorded using K⫹-acetate-filled pipettes. J Neurophysiol • VOL Figure 1A shows a schematic representation of the horizontal slice preparation used in the present study. Stimulation of the medial lemniscus produced a very short-latency (⬃1 ms), fast-rising EPSP that peaked at ⬃2 ms. When the EPSP reaches firing threshold it produces an action potential at a latency of ⬃2 ms (Fig. 1B). Thus lemniscal synapses are extremely fast (Sabatini and Regehr 1999). Corticothalamic synapses formed onto neurons of the ventrobasal thalamus display paired-pulse facilitation (Castro-Alamancos and Calcagnotto 1999). The next experiments (n ⫽ 10 neurons) explored the frequency-dependent properties of the lemniscal response and compared it with the corticothalamic response. Figure 1C illustrates the effect of a pair of stimuli delivered to the medial lemniscus and to the thalamic radiation to activate corticothalamic fibers onto the same cell. The lemniscal response shows paired-pulse depression while the corticothalamic response shows facilitation. When both responses are overlaid, several characteristic differences are apparent. The lemniscal response to the first stimulus has a larger amplitude and shorter latency and rises faster than the corticothalamic response. As a consequence of facilitation and depression, the excitatory postsynaptic potential (EPSP) amplitudes for both pathways become similar after the first stimulus (see Fig. 1C overlay), but the difference in latency remains. In every case tested (n ⫽ 4), the lemniscal response was completely abolished by bath application of 10 M CNQX (Fig. 1D). To further investigate the properties of the lemniscal EPSP, recordings were performed from ventrobasal neurons (n ⫽ 8) filled with CS⫹ acetate and QX-314, which suppress K⫹ and Na⫹ currents. The intensity, frequency, and voltage dependency of the lemniscal response was investigated. Manipulation of the intensity of the stimulus revealed that the lemniscal response is all or none (Fig. 2A). Threshold stimulation produces a unitary event that always has the same amplitude. When stimulation was delivered above threshold intensity, the probability of occurrence of this event was 100% and its amplitude was unchanged (i.e., between trials it varied ⬍5% at 0.1 Hz). The average amplitude of the unitary event across cells was 11.87 ⫾ 2 mV (mean ⫾ SD; n ⫽ 8). As mentioned in the preceding text, the lemniscal unitary EPSP depressed slightly but significantly at frequencies ⬎2 Hz, and the amount of depression increased with frequency and was particularly strong at ⱖ10 Hz (Fig. 2B). Manipulation of the voltage with current injection revealed that the unitary event occurred at both hyperpolarized and depolarized potentials and was able to trigger low-threshold potentials (presumably low-threshold 87 • FEBRUARY 2002 • www.jn.org 948 M. A. CASTRO-ALAMANCOS FIG. 2. Intensity, frequency, and voltage dependency of lemniscal EPSPs in vitro. A: effects of varying the stimulus intensity on the evoked lemniscal response. Note that the response does not increase steadily with current intensity. Instead, it is an all-or-none event. In this experiment, the maximum current used was 250 A, which produced the same effect as 65 A. Superimposed are 10 stimulus trials at each intensity. B: effects of varying the frequency of the applied stimuli on the lemniscal EPSPs. The graph below displays the percentage of change in amplitude between the 1st stimulus and the following 3 stimuli (average of 3 neurons; 5– 40 Hz are significantly depressed as compared with 1 Hz, P ⬍ 0.0001). C: voltage dependency of lemniscal EPSPs. Three trials are superimposed at each voltage. Neurons were recorded using Cs⫹- and QX-314-filled pipettes. calcium currents) when hyperpolarized and high-threshold potentials (presumably high-threshold calcium currents) when depolarized (Fig. 2C). The low- and high-threshold potentials were also triggered with the application of current injection and thus they did not require synaptic input to be evoked (not shown). In the present study, stimulation of the medial lemniscus did not usually evoke inhibitory postsynaptic potentials. Moreover, a GABAA receptor antagonist (BMI) was bath applied in several experiments (n ⫽ 4) with no significant effects on the evoked lemniscal response or its characteristic frequency-dependent depression (not shown). This was expected because the lemniscal pathway does not produce feed-forward inhibition due to the lack of inhibitory interneurons in the rodent ventrobasal thalamus (Ohara and Lieberman 1993; Spacek and Lieberman 1974). The only source of inhibition is the nRt, which provides feedback inhibition when activated by collaterals from thalamocortical fibers on their way to the neocortex. To avoid stimulating directly nRt fibers, special care was taken to place the lemniscal-stimulating electrode outside of the ventrobasal thalamus. Thus in this preparation, feedback inhibition would only occur subsequent to the firing of sufficient thalamocortical neurons. The reason why recurrent IPSPs are rare could also be attributed to the orientation of the slice preparation so that recurrent fibers between ventrobasal neurons and nRt neurons are not in the same slice plane as lemniscal fibers. To avoid any potential confounding effects of IPSPs, the following experiments that studied lemniscal EPSPs were performed in the presence of BMI in the bath. Effects of acetylcholine The next question was whether the lemniscal response was affected by acetylcholine or norepinephrine, which are important thalamic modulators (Bennett-Clarke et al. 1999; Hallanger et al. 1990; McCormick 1992; Simpson et al. 1997; Steriade et al. 1997). Neurons in the ventrobasal thalamus were recorded with J Neurophysiol • VOL Cs⫹ and QX-314, which suppress the postsynaptic actions of acetylcholine and norepinephrine by reducing Na⫹ and K⫹ currents (Castro-Alamancos and Calcagnotto 2001; Gil et al. 1997). BMI (10 –20 M) was bath applied. After ⬎1 h of recording to allow the intracellular diffusion of the drugs, acetylcholine was applied in the bath (10 mM; n ⫽ 5 neurons) for 10 min (Fig. 3A). In every neuron tested, application of acetylcholine had no significant effects on the size (mean ⫾ SE; 97 ⫾ 3% of baseline; n ⫽ 5) or shape of the lemniscal EPSP and its frequency-dependent depression. However, corticothalamic EPSPs on the same neurons were significantly depressed (Fig. 3A), as previously described (Castro-Alamancos and Calcagnotto 2001). Also, input resistance and membrane potential were not significantly affected under these recording conditions (Castro-Alamancos and Calcagnotto 2001). The dose of acetylcholine used is effective because it depolarizes neurons recorded using K⫹ acetate and significantly depresses corticothalamic synapses on the same neurons. The effect of acetylcholine was tested on IPSPs recorded from ventrobasal neurons impaled with Cs⫹- and QX-314filled electrodes (n ⫽ 4). Isolated IPSPs were evoked by stimulating nRt fibers in the presence of bath-applied CNQX (20 M) and AP5 (50 M). Acetylcholine (10 mM) significantly reduced the amplitude of isolated IPSPs (81 ⫾ 4% reduction; P ⬍ 0.0001; Fig. 3B). As indicated in the preceding text, under these recording conditions, acetylcholine did not significantly affect the input resistance of thalamocortical neurons, which was monitored by the application of a negative current pulse (Fig. 3B). In several experiments, a cut was produced to excise the nRt from the slice so that acetylcholine application would only affect the fiber terminals and not the cell bodies of nRt neurons. Under these conditions, IPSPs were also depressed by acetylcholine (80 ⫾ 5% reduction; P ⬍ 0.0001; n ⫽ 3). This indicates that acetylcholine acts at the terminals of nRt neurons to depress IPSPs in the ventrobasal thalamus. 87 • FEBRUARY 2002 • www.jn.org LEMNISCAL SYNAPSES 949 Like acetylcholine, norepinephrine depressed isolated IPSPs (76 ⫾ 6% reduction; P ⬍ 0.0001; n ⫽ 4) evoked by stimulating nRt fibers. In several experiments, a cut was produced to excise the nRt from the slice so that norepinephrine application would only affect the fiber terminals and not the cell bodies of nRt neurons. Under these conditions, IPSPs were also depressed by norepinephrine (70 ⫾ 7% reduction; P ⬍ 0.0001; n ⫽ 4). There was a major difference however between the experiments performed with the nRt intact and those where the nRt had been excised. When the nRt was intact, application of norepinephrine produced a large increase in the frequency of spontaneous IPSPs, which bombarded the recorded thalamocortical neuron. This effect was expected because norepineph- FIG. 3. Effect of acetylcholine on lemniscal EPSPs and on isolated inhibitory postsynaptic potentials (IPSPs) in vitro. A: lack of effect of bath-applied acetylcholine (Ach, 10 mM) on lemniscal EPSPs, while depressing corticothalamic EPSPs onto the same neuron. The overlaid traces correspond to before and during the application of Ach for a single lemniscal stimulus (top left traces), for 4 lemniscal stimuli delivered at 10 Hz (bottom traces) and for 2 corticothalamic stimuli delivered at 20 Hz (top right traces). B: depression of isolated IPSPs in the ventrobasal thalamus by bath application of Ach (10 mM). The overlaid traces show an IPSP induced by stimulating nRt fibers, before (control) and during (Ach) the application of acetylcholine (10 mM). Following the IPSP is the effect of a negative current pulse (0.1 nA, 50 ms), which was used to monitor input resistance. Two traces are overlaid corresponding to before and during Ach. The intracellular recordings were performed using Cs⫹- and QX-314-filled pipettes. IPSPs were isolated using bath-applied CNQX and 2-amino-5-phosphonovaleric acid (AP5). EPSPs were isolated using bath-applied bicuculline methobromide (BMI). Data are presented as the percentage of the baseline EPSP (A) and as the absolute IPSP amplitudes (B). Effects of norepinephrine The next experiments explored the effects of norepinephrine on lemniscal synapses. Like acetylcholine, norepinephrine (100 M) had no significant effect on the size (95 ⫾ 4% of baseline; n ⫽ 7) or shape of the lemniscal EPSP and its frequency-dependent depression (Fig. 4A). Also, input resistance and membrane potential were not significantly affected by norepinephrine under these recording conditions (Fig. 4B), but corticothalamic EPSPs were significantly depressed (Fig. 4A), as previously described (Castro-Alamancos and Calcagnotto 2001). J Neurophysiol • VOL FIG. 4. Effect of norepinephrine on lemniscal EPSPs and on isolated IPSPs in vitro. A: lack of effect of bath-applied norepinephrine (NE, 100 M) on lemniscal EPSPs, while depressing corticothalamic EPSPs onto the same neuron. The overlaid traces correspond to before and during the application of NE for a single lemniscal stimulus (top left traces), for 4 lemniscal stimuli delivered at 10 Hz (bottom traces), and for 2 corticothalamic stimuli delivered at 20 Hz (top right traces). B: depression of isolated IPSPs in the ventrobasal thalamus by bath application of NE (100 M). The overlaid traces show an IPSP induced by stimulating nRt fibers, before (control) and during (NE) the application of norepinephrine. Following the IPSP is the effect of a negative current pulse (0.3 nA, 50 ms), which was used to monitor input resistance. Two traces are overlaid corresponding to before and during NE. The intracellular recordings were performed using Cs⫹- and QX-314-filled pipettes. IPSPs were isolated using bath-applied CNQX and AP5. Data are presented as the percentage of the baseline EPSP (A) and as the absolute IPSP amplitudes (B). 87 • FEBRUARY 2002 • www.jn.org 950 M. A. CASTRO-ALAMANCOS rine depolarizes nRt neurons increasing their firing rates (Kayama et al. 1982; McCormick and Wang 1991). This increase in spontaneous IPSPs was never observed when the nRt was excised. However, depression of evoked IPSPs was present in both conditions (with or without nRT cell bodies), indicating that norepinephrine acts at the terminals of nRt neurons to depress IPSPs in the ventrobasal thalamus, despite depolarizing and increasing the firing rate of nRt neurons. In conclusion, lemniscal responses consist of a short-latency fast-rising unitary EPSP, which is all or none, depresses at frequencies ⬎2 Hz, and is insensitive to the modulators acetylcholine and norepinephrine. In contrast, IPSPs evoked by stimulation of nRt fibers and corticothalamic EPSPs are strongly reduced by acetylcholine and by norepinephrine in the ventrobasal thalamus. Relay of lemniscal inputs The main function of the thalamus is to relay sensory inputs to the neocortex. The transfer of high-frequency sensory inputs may be jeopardized by the depression of lemniscal synapses. However, sensory relay is functionally relevant during information processing states in which the thalamus is activated due to the effects of modulators. The main consequence of these modulators, such as acetylcholine and norepinephrine, is to depolarize thalamocortical neurons (McCormick 1992; Steriade et al. 1997). Thus the next experiments explored in slices how postsynaptic depolarization and synaptic depression interact to relay lemniscal inputs at different frequencies. Intracellular recordings (n ⫽ 5) were performed using K⫹-acetatefilled electrodes. Figure 5 shows the effect of stimuli applied to the medial lemniscus at different frequencies for a neuron in the bursting mode or in the tonic mode. In the bursting mode, neurons responded robustly to the first stimulus with a burst of action potentials but were unable to follow at frequencies ⬎2 Hz. In contrast, in the tonic mode, the firing of thalamic neurons was highly reliable for every stimulus delivered at frequencies ⱕ40 Hz (Fig. 5A). Thus in the tonic mode, the lemniscal input seems to be able to overcome the synaptic depression because the membrane potential is placed close to firing threshold. Thalamocortical neurons are unable to follow high-frequency inputs in the burst mode because of the properties of the currents involved in burst generation (McCormick and Feeser 1990; Sherman 1996). However, the suppression of high-frequency lemniscal inputs does not require thalamocortical neurons to be in the bursting mode. Indeed, synaptic depression is effective at suppressing the transfer of lemniscal inputs when neurons are sufficiently depolarized so they are no longer in the bursting mode (Fig. 5B). Within the tonic mode, the suppression of lemniscal inputs is expressed when the neuron is sufficiently hyperpolarized. If the neuron is depolarized closer to the firing threshold, it can overcome the depression and relay sensory inputs at high frequency (Fig. 5B). Thus because of synaptic depression, the relay of sensory information through the lemniscal pathway requires sufficient postsynaptic depolarization to overcome synaptic depression. When these two events coincide (lemniscal release and postsynaptic depolarization), the relay of sensory information is warranted for high-frequency inputs. This was further quantified by calculating a correlation between the set membrane potential of thalamic neurons and the probability of firing to the fourth lemniscal stimulus delivered at 10 Hz. This relation was found to be significant (r ⫽ 0.78, P ⬍ 0.0001; n ⫽ 3 neurons). The last experiments were performed to test if indeed the depolarization produced by acetylcholine and norepinephrine FIG. 5. Voltage dependency of lemniscal suppression. A: stimuli were applied to the medial lemniscus and the neuron was held hyperpolarized in the bursting mode (left) or it was depolarized to the tonic mode (right). Note the ability of thalamic neurons to follow high-frequency lemniscal inputs in the tonic mode. The intracellular recordings were performed using K⫹-acetate-filled pipettes. B: 4 lemniscal stimuli are applied at 10 Hz. The neuron is placed in the bursting mode (bottom) or in the tonic mode (top 3 traces) by the application of current injection. Within the tonic mode, the neuron is unable to follow the lemniscal inputs with action potentials because of synaptic depression. However, as the neuron is further depolarized it can then follow the synaptic input. The intracellular recordings were performed using K⫹-acetate-filled pipettes. J Neurophysiol • VOL 87 • FEBRUARY 2002 • www.jn.org LEMNISCAL SYNAPSES is able to facilitate the relay of high-frequency lemniscal inputs. The results shown in the preceding text reveal that norepinephrine and acetylcholine do not affect the efficacy of lemniscal synapses, but the depolarization these modulators produce may suffice to overcome synaptic depression and the suppression of high-frequency lemniscal inputs. Intracellular recordings (n ⫽ 6) were performed using K-acetate-filled electrodes to monitor the relay of lemniscal inputs and BMI was present in the bath. Figure 6A shows a typical example from a thalamocortical neuron at resting membrane potential. Notice 951 the suppression of high-frequency lemniscal inputs when the neuron is at resting membrane potential. Application of acetylcholine (10 mM) or norepinephrine (100 M) was accompanied by an increase in the neurons input resistance and depolarization to around spiking threshold, where spontaneous tonic firing could occur (McCormick 1992). Moreover, under these conditions, lemniscal suppression was not apparent and thalamocortical neurons were able to relay high-frequency lemniscal inputs. When the neurons are hyperpolarized with current injection, in the presence of acetylcholine or norepinephrine, lemniscal suppression is again revealed. This indicates that the postsynaptic depolarization produced by these neuromodulators suffices to eliminate the suppression of highfrequency lemniscal inputs. Figure 6B shows population data corresponding to the effects of acetylcholine and norepinephrine on the relay of lemniscal inputs at different frequencies (n ⫽ 6 neurons). The results for acetylcholine and norepinephrine were pooled together because they did not differ significantly. Note that when neurons are at resting membrane potential, lemniscal suppression occurs at frequencies ⬎2 Hz, and is quite significant at frequencies ⬎10 Hz. In contrast, in the presence of acetylcholine or norepinephrine, lemniscal inputs can be relayed at ⱕ40 Hz. DISCUSSION FIG. 6. Lemniscal suppression is eliminated due to the depolarization caused by acetylcholine and norepinephrine. A: stimuli were applied to the medial lemniscus when the neurons were at resting membrane potential (control). Note that application of stimuli at 10 or 20 Hz produces lemniscal suppression. Application of acetylcholine (10 mM) depolarizes the ventrobasal neuron so that lemniscal suppression is no longer present (Ach). Hyperpolarizing the neuron back to resting membrane potential using intracellular current application reinstates lemniscal suppression (Ach ⫹ Hyperpol.). B: the effect of acetylcholine on the cell’s input resistance is shown by comparing the voltage deflection to a negative current pulse (0.3 nA; 50 ms) applied before and after the drug. C: population data corresponding to the probability of firing to four lemniscal stimuli delivered at 10 Hz when the neurons are at resting membrane potential under control conditions (control) and in the presence of acetylcholine (n ⫽ 4) or norepinephrine (n ⫽ 2; Ach-NE). D: population data corresponding to the probability of firing to the last of 4 lemniscal stimuli delivered at 0.1, 1, 5, 10, 20, and 40 Hz. Data correspond to 5–10 trials per frequency before and after the application of acetylcholine (n ⫽ 4 cells) or norepinephrine (n ⫽ 2 cells). The data for both drugs were pooled together because they were not significantly different. The intracellular recordings were performed using K⫹-acetate-filled pipettes. J Neurophysiol • VOL The present experiments reveal that lemniscal responses consist of a short-latency fast-rising unitary EPSP that is all or none, depresses at frequencies ⬎2 Hz, and is insensitive to the modulators acetylcholine and norepinephrine, which selectively depress corticothalamic responses and thalamic IPSPs. The relay of lemniscal activity through the thalamus at frequencies ⬎2 Hz requires sufficient postsynaptic depolarization to overcome synaptic depression. In conclusion, lemniscal synaptic depression and postsynaptic depolarization combine to gate the flow of sensory inputs to the cortex. Differences between the rise time and onset latency of sensory and corticothalamic synaptic responses have been previously described in the visual system (Turner and Salt 1998) and may be attributed to the dendritic locations of both inputs, the conduction velocity of the fibers and the properties of the underlying currents. Corticothalamic terminals are located at distal dendrites, while lemniscal synapses occur more proximal to the cell body (Liu et al. 1995; Spacek and Lieberman 1974; Williams et al. 1994b), and consequently corticothalamic EPSPs can be low-pass filtered by the dendritic cable (Spruston et al. 1994). Also, corticothalamic fibers have smaller diameters than lemniscal fibers and consequently conduct slower (Sherman and Guillery 1996; Steriade et al. 1997). In addition, to achieve their high-speed, lemniscal synapses must have optimized the steps for synaptic transmission (Sabatini and Regehr 1999). Rodent lemniscal synapses have been described at the electron microscope level as large size terminals with numerous closely spaced synaptic contacts (Spacek and Lieberman 1974; Williams et al. 1994b). The unitary events may result from synchronous release at these multiple contacts, and depression may be a consequence of vesicle depletion (Thomson 2000). It was interesting to find that application of two important thalamic neuromodulators in vitro, acetylcholine and norepinephrine, did not directly affect the efficacy of lemniscal synapses, although under the same conditions, they depress IPSPs and the 87 • FEBRUARY 2002 • www.jn.org 952 M. A. CASTRO-ALAMANCOS efficacy of corticothalamic synapses. Acetylcholine and norepinephrine have opposite effects on the firing properties of nRt neurons (McCormick 1992). Acetylcholine hyperpolarizes nRt neurons (Ben Ari et al. 1976; McCormick and Prince 1986), while norepinephrine depolarizes them (Kayama et al. 1982; McCormick and Wang 1991). This was clearly apparent in our recordings from ventrobasal neurons when the nRt was intact because application of norepinephrine, but not of acetylcholine, caused an increase in spontaneous IPSPs, indicating the depolarization and firing of nRt neurons. Increased firing in nRt neurons has been shown to suppress the background activity of ventrobasal neurons, with no significant effect on the relay of lemniscal inputs (Warren and Jones 1994). Despite the differential effects of these modulators on the firing of nRt neurons, the present study demonstrates that both acetylcholine and norepinephrine can depress IPSPs within the ventrobasal thalamus at the terminals of nRt neurons. This is an important consideration because intracellular recordings from the rodent ventrobasal thalamus show that tactile stimulation produces an EPSP-IPSP sequence (Salt and Eaton 1990); because of the lack of interneurons in the ventrobasal thalamus, the longlatency IPSP is a result of feedback inhibition from the nRt. The present results indicate that neuromodulators released within the ventrobasal thalamus can further regulate the amplitude of feedback IPSPs coming from the nRt and thus impact the regulation that nRt exerts on ventrobasal neurons. Stimulation of the medial lemniscus in the horizontal slices used here does not reliably produce feedback IPSPs, especially when care is taken to make sure that the stimulating electrode is placed outside of the ventrobasal thalamus; if placed inside the ventrobasal thalamus, it could directly activate nRt fibers or collaterals of thalamocortical fibers projecting to the nRt. The lack of robust feedback inhibition in the slice is likely due to recurrent fibers between the nRt and the ventrobasal thalamus not being in the same plane as the lemniscal fibers. Nonetheless, the present results demonstrate that feedback inhibition is not required to produce lemniscal suppression of sensory inputs in the ventrobasal thalamus because lemniscal suppression was robust when feedback inhibition was blocked. Although not required, feedback IPSPs in vivo contribute to sensory suppression (Lee et al. 1994). Thus the reduction of IPSPs produced by acetylcholine and norepinephrine in the ventrobasal thalamus will further facilitate the relay of high-frequency sensory inputs during activated states. Although acetylcholine and norepinephrine do not affect lemniscal EPSPs, the postsynaptic depolarization they cause on thalamocortical neurons is sufficient to overcome the suppression of lemniscal inputs. This is due to the fact that the depressed lemniscal EPSPs generated by high-frequency stimulation retain considerable amplitude, which can reach firing threshold if aided by postsynaptic depolarization. Thus during behaviorally activated states, when acetylcholine and norepinephrine are released in the thalamus, feedback IPSPs will be depressed and thalamocortical neurons depolarize to facilitate the relay of high-frequency sensory information. The present results serve to explain previous results obtained in vivo such as those reported by Poggio and Mountcastle (1963). These authors found that the capacity for frequency following of tactile stimuli is dramatically different for thalamic cells in the waking as compared with the anesthetized monkey. Also, in anesthetized rats some studies have shown that thalamocortical neurons can follow whisker stimulation ⱕ12 Hz (Hartings and Simons 1998; Simons 1985; Simons and Carvell 1989), while J Neurophysiol • VOL other studies report strong frequency-dependent depression at frequencies ⬎5 Hz (Diamond et al. 1992) or 2 Hz (Ahissar et al. 2000). The present study indicates that these discrepancies could be explained by the anesthetic state of the preparation and in particular the membrane potential of thalamocortical neurons. The more efficient frequency following of lemniscal sensory inputs during activated states as compared with quiescent states would result from the depolarization of thalamocortical neurons during that state caused by the release of neuromodulators in the thalamus, which serves to bring depressed lemniscal EPSPs close to firing threshold. Interestingly, the same modulators that enhance the relay of lemniscal sensory inputs also filter corticothalamic inputs so that only high-frequency activity (⬎5 Hz) from the cortex can reach the thalamus (Castro-Alamancos and Calcagnotto 2001). Thus acetylcholine and norepinephrine produce a generalized depolarization of thalamocortical neurons that should enhance all inputs to these neurons. However, the same modulators that depolarize thalamocortical neurons selectively depress corticothalamic and nRt inputs, so that postsynaptic depolarization results in a selective enhancement of the sensory input. During behavioral activation, the thalamus will function as an effective relay of sensory information from the periphery to the neocortex, but it will be disconnected from the neocortex unless the neocortex sends high-frequency activity. The neocortex would only be able to influence thalamic activity and the flow of sensory inputs when high-frequency cortical activity is present, perhaps through corticothalamic oscillations that occur during certain behavioral states in waking animals (Nicolelis et al. 1995; Rougeul-Buser and Buser 1997). Previous work has emphasized the thalamus as the first stage for gating sensory information (Steriade and McCarley 1990), and the capacity of thalamic activation to enhance sensory transmission (Eysel et al. 1986; Francesconi et al. 1988; Humphrey and Saul 1992; Pare et al. 1990; Singer 1977; Steriade and Demetrescu 1960; Uhlrich et al. 1995). The present study highlights the interplay between synaptic depression and postsynaptic depolarization as a gating mechanism of sensory information flow. Particularly interesting is that sensory transfer by lemniscal inputs is not only controlled by a simple change between the burst-to-tonic modes of thalamic relay neurons. The degree of depolarization within the tonic mode seems to be an important variable. This suggests important functional consequences in relation to information processing; cells will relay high-frequency sensory inputs only if sufficiently depolarized within the tonic mode. On a speculative note, this may reflect the difference between being awake and being attentive. This work was supported by the Medical Research Council of Canada, Natural Sciences and Engineering Council of Canada, Fonds de la Reserche en Sante du Quebec, Canadian Foundation for Innovation, and Savoy Foundation. REFERENCES AHISSAR E, SOSNIK R, AND HAIDARLIU S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302–306, 2000. ASTON-JONES G, CHIANG C, AND ALEXINSKY T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88: 501–520, 1991. BEN ARI Y, DINGLEDINE R, KANAZAWA I, AND KELLY JS. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. J Physiol (Lond) 261: 647– 671, 1976. 87 • FEBRUARY 2002 • www.jn.org LEMNISCAL SYNAPSES BENNETT-CLARKE CA, CHIAIA NL, AND RHOADES RW. Differential expression of acetylcholinesterase in the brainstem, ventrobasal thalamus and primary somatosensory cortex of perinatal rats, mice, and hamsters. Somatosens Mot Res 16: 269 –279, 1999. BUZSAKI G, BICKFORD RG, PONOMAREFF G, THAL LJ, MANDEL R, AND GAGE FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8: 4007– 4026, 1988. CASTRO-ALAMANCOS MA AND CALCAGNOTTO ME. Presynaptic long-term potentiation in corticothalamic synapses. J Neurosci 19: 9090 –9097, 1999. CASTRO-ALAMANCOS MA AND CALCAGNOTTO ME. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: in vitro and in vivo. J Neurophysiol 85: 1489 –1497, 2001. CHIAIA NL, RHOADES RW, BENNETT-CLARKE CA, FISH SE, AND KILLACKEY HP. Thalamic processing of vibrissal information in the rat. I. Afferent input to the medial ventral posterior and posterior nuclei. J Comp Neurol 314: 201–216, 1991. DIAMOND ME. Somatosensory thalamus of the rat. Cereb Cortex 11: 189 –220, 1995. DIAMOND ME, ARMSTRONG-JAMES M, AND EBNER FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462– 476, 1992. EYSEL UT, PAPE HC, AND VAN SCHAYCK R. Excitatory and differential disinhibitory actions of acetylcholine in the lateral geniculate nucleus of the cat. J Physiol (Lond) 370: 233–254, 1986. FANSELOW EE AND NICOLELIS MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci 19: 7603–7616, 1999. FELDMAN SG AND KRUGER L. An axonal transport study of the ascending projection of medial lemniscal neurons in the rat. J Comp Neurol 192: 427– 454, 1980. FRANCESCONI W, MULLER CM, AND SINGER W. Cholinergic mechanisms in the reticular control of transmission in the cat lateral geniculate nucleus. J Neurophysiol 59: 1690 –1718, 1988. GIL Z, CONNORS BW, AND AMITAI Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679 – 686, 1997. HALLANGER AE, PRICE SD, LEE HJ, STEININGER TL, AND WAINER BH. Ultrastructure of cholinergic synaptic terminals in the thalamic anteroventral, ventroposterior, and dorsal lateral geniculate nuclei of the rat. J Comp Neurol 299: 482– 492, 1990. HARTINGS JA AND SIMONS DJ. Thalamic relay of afferent responses to 1- to 12-Hz whisker stimulation in the rat. J Neurophysiol 80: 1016 –1019, 1998. HUMPHREY AL AND SAUL AB. Action of brain stem reticular afferents on lagged and nonlagged cells in the cat lateral geniculate nucleus. J Neurophysiol 68: 673– 691, 1992. KAYAMA Y, NEGI T, SUGITANI M, AND IWAMA K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience 7: 655– 666, 1982. LEE SM, FRIEDBERG MH, AND EBNER FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. J Neurophysiol 71: 1716 –1726, 1994. LIU XB, HONDA CN, AND JONES EG. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. J Comp Neurol 352: 69 –91, 1995. MCCORMICK DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39: 337–388, 1992. MCCORMICK DA AND FEESER HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience 39: 103–113, 1990. MCCORMICK DA AND PRINCE DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature 319: 402– 405, 1986. MCCORMICK DA AND WANG Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol (Lond) 442: 235–255, 1991. J Neurophysiol • VOL 953 NICOLELIS MA, BACCALA LA, LIN RC, AND CHAPIN JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 268: 1353–1358, 1995. OHARA PT AND LIEBERMAN AR. Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. J Neurocytol 22: 815– 825, 1993. PARE D, STERIADE M, DESCHENES M, AND BOUHASSIRA D. Prolonged enhancement of anterior thalamic synaptic responsiveness by stimulation of a brain-stem cholinergic group. J Neurosci 10: 20 –33, 1990. POGGIO GF AND MOUNTCASTLE VB. The functional properties of ventrobasal thalamic neurons studied in unanesthetized monkeys. J Neurophysiol 26: 775– 806, 1963. ROUGEUL-BUSER A AND BUSER P. Rhythms in the alpha band in cats and their behavioural correlates. Int J Psychophysiol 26: 191–203, 1997. SABATINI BL AND REGEHR WG. Timing of synaptic transmission. Annu Rev Physiol 61: 521–542, 1999. SALT TE AND EATON SA. Postsynaptic potentials evoked in ventrobasal thalamus neurones by natural sensory stimuli. Neurosci Lett 114: 295–299, 1990. SHERMAN SM. Dual response modes in lateral geniculate neurons: mechanisms and functions. Vis Neurosci 13: 205–213, 1996. SHERMAN SM AND GUILLERY RW. Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–1395, 1996. SIMONS DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol 54: 615– 635, 1985. SIMONS DJ AND CARVELL GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989. SIMPSON KL, ALTMAN DW, WANG L, KIRIFIDES ML, LIN RC, AND WATERHOUSE BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol 385: 135–147, 1997. SINGER W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev 57: 386 – 420, 1977. SPACEK J AND LIEBERMAN AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat 117: 487–516, 1974. SPRUSTON N, JAFFE DB, AND JOHNSTON D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci 17: 161–166, 1994. STERIADE M AND DEMETRESCU M. Unspecific systems of inhibition and facilitation of potentials evoked by intermittent light. J Neurophysiol 23: 602– 617, 1960. STERIADE M, JONES EG, AND MCCORMICK DA. Thalamus. New York: Elsevier, 1997. STERIADE M AND MCCARLEY RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum, 1990. THOMSON AM. Molecular frequency filters at central synapses. Prog Neurobiol 62: 159 –196, 2000. TURNER JP AND SALT TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. J Physiol (Lond) 510: 829 – 843, 1998. UHLRICH DJ, TAMAMAKI N, MURPHY PC, AND SHERMAN SM. Effects of brain stem parabrachial activation on receptive field properties of cells in the cat’s lateral geniculate nucleus. J Neurophysiol 73: 2428 –2447, 1995. VEINANTE P AND DESCHENES M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci 19: 5085–5095, 1999. WARREN RA AND JONES EG. Glutamate activation of cat thalamic reticular nucleus: effects on response properties of ventroposterior neurons. Exp Brain Res 100: 215–226, 1994. WILLIAMS JA, COMISAROW J, DAY J, FIBIGER HC, AND REINER PB. Statedependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci 14: 5236 –5242, 1994a. WILLIAMS MN, ZAHM DS, AND JACQUIN MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur J Neurosci 6: 429 – 453, 1994b. 87 • FEBRUARY 2002 • www.jn.org