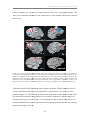
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Csercsa Richárd
Cognitive neuroscience wikipedia , lookup
Environmental enrichment wikipedia , lookup
Human brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Electrophysiology wikipedia , lookup
Time perception wikipedia , lookup
Neuroscience in space wikipedia , lookup
Aging brain wikipedia , lookup
Emotion and memory wikipedia , lookup
Limbic system wikipedia , lookup
Single-unit recording wikipedia , lookup
Biology of depression wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain Rules wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Nervous system network models wikipedia , lookup
Optogenetics wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neuroplasticity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neural oscillation wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Delayed sleep phase disorder wikipedia , lookup
Spike-and-wave wikipedia , lookup
Synaptic gating wikipedia , lookup
Rapid eye movement sleep wikipedia , lookup
Memory consolidation wikipedia , lookup
Sleep apnea wikipedia , lookup
Sleep paralysis wikipedia , lookup
Neuroscience of sleep wikipedia , lookup
Sleep deprivation wikipedia , lookup
Metastability in the brain wikipedia , lookup
Sleep medicine wikipedia , lookup
Sleep and memory wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Effects of sleep deprivation on cognitive performance wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Laminar analysis of the slow wave activity in humans Doctoral thesis Csercsa Richárd Semmelweis University János Szentágothai School of Neurosciences Functional Neurosciences Supervisor: Dr. Ulbert István Ph.D. Official opponents: Dr. Kamondi Anita C.Sc. Dr. Barthó Péter Ph.D. President of committee: Members of committee: Dr. Szirmai Imre Ph.D., D.Sc. Dr. Bódizs Róbert Ph.D. Dr. Gál Viktor Ph.D. Budapest 2010 Csercsa Richárd TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................... 2 LIST OF ABBREVIATIONS ........................................................................................ 4 INTRODUCTION .......................................................................................................... 6 Sleep – general introduction ......................................................................................... 6 Sleep patterns ................................................................................................................ 7 Sleep stages .............................................................................................................. 7 Development ............................................................................................................. 9 Neural basis of arousal ............................................................................................... 10 Passive or active ..................................................................................................... 11 Deafferentation ....................................................................................................... 11 Ascending reticular activating system .................................................................... 12 Ventrolateral Preoptic Nucleus .............................................................................. 14 Sleep regulatory substances (SRS) ......................................................................... 16 Local sleep .............................................................................................................. 17 Sleep deprivation ........................................................................................................ 21 Functions of sleep ....................................................................................................... 22 Recovery and restoration........................................................................................ 22 Energy conservation ............................................................................................... 22 Plasticity, memory, learning................................................................................... 23 Memory consolidation during sleep ........................................................................... 24 Memory categories ................................................................................................. 24 Memory formation .................................................................................................. 26 Synaptic consolidation............................................................................................ 26 System consolidation .............................................................................................. 28 Slow wave sleep ......................................................................................................... 29 Slow oscillation ...................................................................................................... 29 Slow oscillation generation .................................................................................... 31 Propagating wave and source localization ............................................................ 34 Stimulation evoked slow oscillation ....................................................................... 36 2 Laminar analysis of the slow wave actvity in humans Modeling of slow oscillation .................................................................................. 37 Memory consolidation during SWS ........................................................................ 40 Slow waves and K-complexes ................................................................................. 47 OBJECTIVES ............................................................................................................... 49 METHODS .................................................................................................................... 50 Patients and electrodes................................................................................................ 50 Histology .................................................................................................................... 50 Recordings .................................................................................................................. 52 Slow wave activity detection ...................................................................................... 54 Current source density analysis .................................................................................. 56 Statistical analysis of electrophysiology and histology .............................................. 57 Single and multiple unit activity analysis ................................................................... 58 RESULTS ...................................................................................................................... 60 Slow oscillation in humans ......................................................................................... 60 Supragranular activity................................................................................................. 65 Up-state onset ............................................................................................................. 69 Frequency of slow oscillation ..................................................................................... 71 Sparse firing................................................................................................................ 72 DISCUSSION ................................................................................................................ 75 Epilepsy and slow waves ............................................................................................ 75 Supragranular activity in SWS ................................................................................... 77 Frequency of the slow waves ..................................................................................... 81 Role of calretinin positive neurons ............................................................................. 82 Firing rate in SWS ...................................................................................................... 83 SUMMARY ................................................................................................................... 87 BIBLIOGRAPHY......................................................................................................... 88 PUBLICATIONS ........................................................................................................ 107 ACKNOWLEDGEMENTS ....................................................................................... 108 3 Csercsa Richárd LIST OF ABBREVIATIONS 5-HT Serotonin (5-Hydroxytryptamine) Ach Acetylcholine AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ANOVA Analysis Of Variance ARAS Ascending Reticular Activating System BOLD signal Blood Oxygenation Level Dependent signal CEM Competitive Expectation-Maximization CR Calretinin CSD Current Source Density DAB 3,3'-Diaminobenzidine ECoG Electrocorticography EEG Electroencephalography EPSP Excitatory Postsynaptic Potential FFT Fast Fourier Transform fMRI Functional Magnetic Resonance Imaging GABA Gamma-Aminobutyric Acid GFAP Glial Fibrillar Acidic Protein IPSP Inhibitory Postsynaptic Potential LFP Local Field Potential LFPg Local Field Potential gradient 4 Laminar analysis of the slow wave actvity in humans LTD Long-Term Depression LTP Long-Term Potentiation ME Multielectrode MEG Magnetoencephalography MRI Magnetic Resonance Imaging MUA Multiunit Activity NeuN Neuronal Nuclei staining NMDA N-methyl-D-aspartic acid NREM Non Rapid Eye Movement PB Phosphate Buffer PET Positron Emission Tomography PGO wave Ponto-Geniculo-Occipital wave REM Rapid Eye Movement SD Standard Deviation SO Slow Oscillation STDP Spike-Timing-Dependent Plasticity SUA Single Unit Activity SWA Slow Wave Activity SWS Slow Wave Sleep TMS Transcranial Magnetic Stimulation VLPO Ventrolateral Preoptic Nucleus 5 Csercsa Richárd INTRODUCTION SLEEP – GENERAL INTRODUCTION Sleep seems to be essential for every living creature with a nervous system, though its function is not clear yet. It can be observed in most mammals and birds, and also in many insects (e.g. cockroach, bee, scorpion, or Drosophila), reptiles (e.g. turtle), amphibians (e.g. tree frog), and fish (e.g. zebrafish or perch) (Cirelli and Tononi, PLoS Biol, 2008). There are species that do not seem to meet the criteria of sleeping, e.g. bullfrog, or coral reef fish (Siegel, Trends Neurosci, 2008). Sleep is a condition during which animals are resting and their responses to external sensory stimuli (sounds, touch, smell) and processing of the information these stimuli carry are altered compared to the waking state. Most of these stimuli don’t reach the conscious level, they fade in the neuronal silence of the brain. Opposing this function that maintains sleep, there is need for waking, when external stimuli represent for example imminent danger. The brain needs to uphold a state that protects the physiologically important sleep but aborts it when necessary. Circadian rhythmicity and homeostatic regulation are both important criteria for determining sleep (Borbely, Hum Neurobiol, 1982). Circadian rhythmicity means the sleep need is elevated in certain phases of the day. In diurnal animals it is increased during the night, while nocturnal animals tend to sleep during the day. In humans, besides the elevation of sleep need during the night, there is an increase of sleep willingness in the early afternoon also. A behavioral state cannot be called sleep if there is no homeostatic regulation, i.e. the amount of sleep should be proportional to the amount of waking and this is maintained by the nervous system itself. It is signaled by the compensatory rebound after sleep deprivation, when subjects tend to sleep more. (Bódizs, Kognitív idegtudomány, 2003) 6 Laminar analysis of the slow wave actvity in humans SLEEP PATTERNS Sleep can be divided into different stages, which follow a specific pattern in animals and humans alike, though the duration and properties of these stages may vary from species to species. SLEEP STAGES In humans the two main types of sleep are Rapid Eye Movement (REM) and NonRapid Eye Movement (NREM) sleep. NREM can be further divided into three or four stages which go from light to deep sleep. During the night, these stages follow each other in a more or less determined manner, which can be described on a hypnogram (Figure 1). The hypnogram shows the cyclic alternations of sleep stages during the whole night. Stages are characterized by specific brain electrical activity, muscle tone, etc., that became the main criteria for determining them. These criteria were standardized in 1968 by Rechtschaffen and Kales. Their classification contained four stages of NREM sleep. They were used for sleep staging until 2007, when the American Academy of Sleep Medicine (AASM) issued a manual for the scoring of sleep, in which NREM stages 3 and 4 were merged into stage 3 (Iber et al., 2007). Figure 1. Sleep hypnogram. Change in sleep stages (vertical axis) during the night (horizontal axis). It shows that the first half of the night consists mostly of NREM sleep, while the second half is rich in REM sleep. Modified from (Siegel, 2002) 7 Csercsa Richárd NREM SLEEP The dominant sleep stage during the first half of a night’s sleep is the NREM sleep that can be further divided to three stages according to the new AASM guidelines that correspond to the levels of awakability, with stage 1 being the lightest and stage 3 the deepest stage (Figure 2). • Stage 1 is the onset of sleep, a transition from waking that takes 510% of total sleep time. Alpha waves (8-12 Hz) that are characteristic for the drowsy waking state are replaced with theta waves (4-8 Hz). Sudden twitches and hypnic jerks may also occur. • Stage 2 occupies 45-55% of total sleep and it is characterized by sleep spindles (around 12 Hz) generated by thalamocortical loops and K-complexes. Muscle tone decreases and conscious awareness of the external environment disappears. • Stage 3 (15-25% of sleep time) is commonly referred to as deep sleep or slow wave sleep (SWS), because during this stage delta frequency (1-4 Hz) and slow (below 1 Hz) oscillations appear. Deep sleep is considered to be a state when the mind is detached from the external world, and sensory stimuli are rarely able to reach the cortex for further processing. Recent findings show a more complex image of the processes taking place during deep sleep. This is also the stage when night terrors and sleepwalking occur. 8 Laminar analysis of the slow wave actvity in humans Figure 2. The EEG graphoelements of sleep stages. The awake EEG consists of highfrequency, low-amplitude waves, while during deep sleep low-frequency, high-amplitude oscillations can be observed. REM sleep EEG pattern is similar to waking. Modified from (Pace-Schott and Hobson, Nat Rev Neurosci, 2002). REM SLEEP Rapid Eye Movement (REM) sleep was first described in 1953 by Aserinsky and Kleitman (Aserinsky and Kleitman, Science, 1953). It accounts for 20-25% of total sleep time and it is mainly characterized by fast movements of the eyes, desynchronized high-frequency EEG activity, muscle atonia, PGO waves (recorded from the pons, the geniculatum, and the occipital cortex), and theta frequency (4-10 Hz) oscillation in the hippocampal formation. Brain activity largely resembles that of the waking activity, thus this stage is also called paradoxical sleep. REM occurs more often during the second half of sleep. It is thought to have important function in spatial and procedural (implicit) memory consolidation, and dreaming occurs during REM sleep. DEVELOPMENT Sleep patterns also change with the life span. In humans, sleep time decreases with age. Infants sleep up to 13-14 hours a day, which decreases to 7-8 hours in adolescence, and further to 5-6 hours at the age of 70. The proportion of REM sleep to NREM also decreases from 50% in infants to 20% in adolescents and 15% in old age (Détári, 2008). 9 Csercsa Richárd Slow wave activity (SWA) during NREM sleep also undergoes prominent changes, it increases during the first years of life, reaches its peak around the age of 8 and after that it declines, forming an inverted U-shape curve. It has been suggested that SWA decrease is correlated with thinning of gray matter, therefore decline in synaptic density (Campbell and Feinberg, Proc Natl Acad Sci U S A, 2009). At the age of 2-5, SWA is dominant in the occipital cortex and shifts to frontal cortex by the age of 20 (Kurth et al., J Neurosci, 2010). This shift is also consistent with studies of cortex maturation. These findings support the hypothesis of a strong connection between SWA and synaptic strength (Tononi and Cirelli, Sleep Med Rev, 2006). Interestingly, genetics seem to largely influence the individual sleep profile. It has been shown in a study of monozygotic and dizygotic twins that the electroencephalographic “fingerprint” (power spectra between 8 and 16 Hz during NREM sleep, which allows a correct discrimination between individuals) is genetically determined. It shows a heritability estimate of 96%, making the sleep EEG profile one of the most heritable traits of humans (De Gennaro et al., Ann Neurol, 2008). NEURAL BASIS OF AROUSAL Despite sleep is an everyday phenomenon, we still don’t entirely understand its functional significance. Neural elements and processes related to sleep may give us a clue for its functions. Unfortunately, the neural mechanisms underlying the generation, maintenance, and termination of sleep are still not clear. Theories and the current conceptions of these mechanisms are described in this section. 10 Laminar analysis of the slow wave actvity in humans PASSIVE OR ACTIVE There are several theories of what could cause the change in arousal as well as in cortical excitability during sleep. There are two classical conceptions: the passive and the active hypothesis. According to the passive hypothesis, the default arousal state is sleep, and it is a result of a decrease in sensory stimulation. The active hypothesis states that sleep is the result of an active process mediated by some sleep-promoting factors in the nervous system. According to our present knowledge, sleep is far too complex to be regarded as a passive state. There are indeed chemical substances that induce sleep such as sedatives or cytokines (e.g. TNF and IL-1) (Krueger et al., Nat Rev Neurosci, 2008), supporting the active hypothesis. On the other hand, both sleep-promoting (von Economo, Ergebn. Physiol. (München), 1929) and wake-promoting centers (Moruzzi and Magoun, Electroencephalogr Clin Neurophysiol, 1949) have been described. DEAFFERENTATION An important milestone in the history of sleep research is the experiment by Frederick Bremer in 1935 (Bremer, C R Soc Biol, 1936). He surgically isolated the brain from the body by cutting the neural axis between the brain stem and the spinal cord at the level of the first or second cervical vertebra. This preparation is called the encéphale isolé (Figure 3, left). After the lesion he experienced normal sleep-wake cycles. Furthermore, his second surgical preparation, the cerveau isolé (Figure 3, right), when he isolated the forebrain from the brain stem, resulted in constant sleep. From these experiments, Bremer concluded that waking requires sensory stimulation of the brain and sleep is the result of sensory deafferentation. This indicates that sleep is the default state of arousal and the nervous system reverts to the sleep mode as a passive process after sensory stimuli cease to reach the forebrain. Despite recent theories think of sleep rather as an active process generated by complex mechanisms in distributed systems, the importance of deafferentation cannot be neglected. When sleeping time 11 Csercsa Richárd comes, all animals and humans look for a dark, silent place with as little external stimuli as possible. Another interpretation of these findings could be that brain areas necessary for waking are below the lesion of cerveau isolé, but this was not suggested at that time. Figure 3. The surgically isolated brain experiment setups by Bremer. Left: encephalé isolé (cut between the brain stem and the spinal cord, normal sleep-wake cycles), right: cerveau isolé (lesion above the brain stem, results in constant sleep). From (Bremer, C R Soc Biol, 1936) ASCENDING RETICULAR ACTIVATING SYSTEM The next big step in understanding sleep processes was the discovery of the Ascending Reticular Activating System (ARAS) by Moruzzi and Magoun in 1949 (Moruzzi and Magoun, Electroencephalogr Clin Neurophysiol, 1949). They showed that sleep activity induced by anesthesia or the encéphale isolé preparation can be turned into waking activity by stimulating the reticular formation. Interestingly, this waking activity could be observed throughout the cortex, and lasted as long as the stimulation period (Figure 4). Figure 4. Waking activity due to brainstem stimulation. Electrical stimulation of the reticular formation (horizontal bar) turns sleeping EEG activity into waking. From (Moruzzi and Magoun, Electroencephalogr Clin Neurophysiol, 1949) 12 Laminar analysis of the slow wave actvity in humans The ARAS consists of pathways ascending from the brain stem to the thalamus and the neocortex, regulating the release of different neurotransmitters. The cholinergic pathway originates in the nucleus pedunculopontine tegmentum and the nucleus laterodorsalis tegmentum, the noradrenergic pathway in the locus coeruleus in the pons, the serotonergic system in the raphe nuclei of the medulla, pons, and midbrain, and the histaminergic system in the tuberomammillary nucleus in the hypothalamus (Figure 5). The interaction of these distributed systems is thought to promote arousal. During waking, both the cholinergic and the monoaminergic (noradrenergic, serotonergic and histaminergic) systems are active. During NREM sleep, the influence of both decreases, while during REM sleep, the monoaminergic system is totally inactive. These nuclei project to the thalamus and excite the cortex broadly. Figure 5. Ascending reticular activating system. The origins of neuromodulators affecting arousal and their projections. From (Saper et al., Trends Neurosci, 2001) In addition, a very small population of cells in the lateral hypothalamus that produce orexin (or hypocretin as it is also called) is also thought to promote wakefulness. These neurons provide excitatory input to the arousal system as well as to the cerebral cortex. Orexin plays a critical role in preventing abnormal behavioral state transitions, particularly into REM sleep, thus being a major factor in narcolepsy. In 13 Csercsa Richárd experiments in which the gene for orexin was knocked out in mice, the animals became narcoleptic (Chemelli et al., Cell, 1999). In humans with narcolepsy, the orexin level in the cerebrospinal fluid is abnormally low (Nishino et al., Lancet, 2000). It has been found that histamine-synthesizing cells are excited by orexin and orexin cells are synapsed upon by histaminergic cells (Eriksson et al., J Neurosci, 2001), so it seems that there are two cooperating neuronal populations in the hypothalamus regulating arousal level. (Siegel, 2002) VENTROLATERAL PREOPTIC NUCLEUS Besides the distributed activating system, a sleep-promoting center situated in the posterior hypothalamus has been described. The preoptic area of hypothalamus was proposed to play a critical role in sleep by von Economo in 1929 (von Economo, Ergebn. Physiol. (München), 1929). He examined patients with encephalitis lethargica and found that lesions in the preoptic area caused insomnia (Triarhou, Brain Res Bull, 2006). This finding was verified in rats by Nauta in 1946 (Nauta, J Neurophysiol, 1946) and in cats by Sterman and Clemente in 1962 (Wyrwicka et al., Science, 1962). Sleep promoting neurons were not localized precisely until 1996, when Sherin et al. found in rats that after long sleep, c-fos, an indirect marker of neural activity was densely packed in the Ventrolateral Preoptic nucleus (VLPO), a small group of neurons in the preoptic area. It has also been demonstrated that the density of neurons stained for c-fos in VLPO positively correlates with the amount of sleep, and that the activity of VLPO and suprachiasmatic nucleus (critical in circadian regulation) is synchronized (Sherin et al., Science, 1996). Electrophysiological recordings revealed that VLPO neurons start firing seconds before the onset of SWS and remain active, with their firing rate increasing when sleep gets deeper. Furthermore, the lesion of VLPO neurons in rats caused long-lasting insomnia. These results point to the VLPO as a sleep-promoting center in the brain. 14 Laminar analysis of the slow wave actvity in humans VLPO is reciprocally connected to histaminergic, orexinergic, serotoninergic, noradrenergic, and cholinergic neurons of the reticular activating system (Figure 6). These reciprocal connections are inhibitory in both directions, i.e. the sleep-promoting VLPO inhibits the wake-promoting systems, and vice versa. During sleep, VLPO neurons increase their activity, inhibiting wake-promoting neurons, thus disinhibiting themselves. During waking, VLPO neurons are inhibited by the activating system, reinforcing the firing of wake-promoting neurons. Figure 6. VLPO projections. The sleep-promoting VLPO reciprocally innervates the wakepromoting ARAS with inhibitory connections, thus forming a ’flip-flop’. From (Saper et al., Trends Neurosci, 2001) This structure of mutual negative feedback resembles a piece of electrical circuitry, called the ‘flip-flop’ (Figure 7). This bistable switch ensures that any time during the day, only one of the two arousal states is present, and there are no overlaps between the two (Saper et al., Trends Neurosci, 2001). Studies with narcolepsy, when sleep suddenly breaks into waking, point to orexin as a regulating factor of the switch. A recent study indicates that the ‘flip-flop’ can be switched by the high-frequency bursting of a single cortical neuron (Li et al., Science, 2009). This activity of a single neuron may affect arousal states through cortico-cortical connections, the thalamo- 15 Csercsa Richárd cortico-reticular loop, or by descending projections from the cortex to the reticular activating system. (Fort et al., Eur J Neurosci, 2009) Figure 7. The ’flip-flop’ of sleep and waking. Several neuromodulators play roles in its operation, with orexin as the main switch. From (Saper et al., Trends Neurosci, 2001) SLEEP REGULATORY SUBSTANCES (SRS) The theory that sleep is induced by humoral factors rather than neural networks was first proposed in 1909 by Kuniomi Ishimori in Japan, and in the same time, but independently, by Henri Piéron in France. They both took samples from the cerebrospinal fluid of sleep-deprived dogs and injected it in the brain of dogs with normal sleep-wave cycle, and the dogs fell asleep for 2-6 hours after the injection. In 1967, J. R. Pappenheimer repeated the experiment, injecting the cerebrospinal fluid of sleep-deprived goats in cats and rats, inducing sleep-like behavior that could be broken by noise or handling, but reverted back to sleep if the animal was left undisturbed (Pappenheimer et al., Proc Natl Acad Sci U S A, 1967). 16 Laminar analysis of the slow wave actvity in humans Many substances have been proposed as sleep regulatory. The main criteria that a viable candidate should fulfill include: the substance should enhance sleep, it should reduce spontaneous sleep if inhibited, its brain levels should vary with sleep-wake history, and it should act on sleep-regulatory circuits (Krueger et al., Nat Rev Neurosci, 2008). For example adenosine, nitric oxide, prostaglandin D2, tumor necrosis factor (TNF), interleukin-1 (IL1), and growth hormone releasing hormone (GHRH) meet these criteria and have been proposed as SRSs. The idea is that ATP, released during neurotransmission, acting on glia releases IL1 and TNF that in turn act on neurons to change their electrical properties. These events happen locally and thereby alter the state of the neural network (Krueger, Curr Pharm Des, 2008). Cytokines like IL1 and TNF have long evolutionary history, dating back to invertebrates. This may explain the universality of sleep among mammals. LOCAL SLEEP Sleep is traditionally seen as a global process, based on that changes in behavior and physiological properties are present in the whole organism. The systemic nature and top-down control of sleep seems to be obvious if we consider that the animal’s posture, hormone secretion, metabolic rate, and brain activity all change during the transition from waking to sleep and from sleep to waking. But if we examine it on a finer scale of time and space, at the level of milliseconds and cortical columns, we find a bottom-up organization of ‘sleeping’ local networks. SLEEP IN GROUPS OF NEURONS There are several observations that conflict with the global nature of sleep. First, it was shown by Mukhametov in 1977 (Mukhametov et al., Brain Res, 1977), that marine mammals like dolphins, whales, and manatees sleep unihemispherically, i.e. they almost 17 Csercsa Richárd never exhibit slow wave activity in both hemispheres at the same time (they are the only mammals without REM sleep, too). The eye contralateral to the hemisphere with the slow waves is closed, while the other is open. The immobility, associated with the sleep of terrestrial mammals, is not a definitive criterion for sleep in marine mammals, since many of them keep moving during their whole life. (Siegel, Nature, 2005). It has been proposed that human subjects during sleep walking are awake and asleep simultaneously (Mahowald and Schenck, Nature, 2005). Furthermore, no case of survivable brain lesion has been reported that caused complete insomnia. This suggests that sleep is a fundamental self-organizing property of any group of neurons (Krueger et al., Nat Rev Neurosci, 2008). SLEEP IS USE-DEPENDENT/EXPERIENCE-DEPEDENT Another observation that points to the emergence of sleep in local networks is that the activation of specific brain regions during wakefulness leads to an increase in slow wave activity during sleep in the same brain areas. In 1993, Krueger and Obál suggested that sleep begins as a local neuronal group event, serving to preserve a constancy of a synaptic superstructure by the use of synapses unstimulated during waking (Krueger and Obal, J Sleep Res, 1993). According to their theory, neural activity leads to the enhanced production of sleep factors acting locally, modifying the neural activity that results in the amplification of synaptic strength (increase in postsynaptic potentials) of active synapses. They also hypothesized the existence of higher order neuronal groups that globally coordinate sleep either through their extensive projections or via humoral agents. One of the consequences of this theory was justified when Rector et al. showed that a sleep-like state can exist in local networks in the brain, specifically in cortical columns, that are thought to be the basic processing neuronal assemblies (Rector et al., Brain Res, 2005). The response evoked by afferent sensory stimulation depends on the state of the column that can be either waking or sleeping. During sleep escalated to the 18 Laminar analysis of the slow wave actvity in humans whole organism, responses from most columns indicate that it is sleeping, while during waking most columns are in waking mode. However, some columns are in waking mode during global sleep and some are sleeping while the animal is awake. This suggests that the basic element of sleep is a neuronal assembly and they can go into sleeping or waking state independently from each other. Moreover, this local sleep seems to be homeostatically regulated, which is another direct consequence of Krueger and Obál’s theory. The likelihood a column goes to sleep depends on the amount of time it spent awake (Rector et al., Brain Res, 2005). Another crucial consequence of the theory is that the amount of sleep in a column or a brain area measured by the slow wave activity seems to be dependent on the level of activity it was exposed to during waking. This property has been tested in several configurations. Kattler et al. applied vibratory stimuli to a subject’s left or right hand for 6 hours. They found that during subsequent sleep, EEG power in the delta frequency range was shifted to the hemisphere contralateral to the stimulated hand (Kattler et al., J Sleep Res, 1994). In a rat experiment, sensory inputs were reduced to one side by cutting the contralateral whiskers. After 6 hours spent awake in an enriched environment, spectral power in the NREM sleep EEG in the 0.75-6 Hz range exhibited an interhemispheric shift towards the side opposite to the intact whiskers (Vyazovskiy et al., J Sleep Res, 2000). In a human study using 256-channel EEG recordings, the subject had to perform a visuomotor task either with rotation of the target (learning task) or without rotation (no learning). A local increase in slow wave activity (1-4 Hz) appeared during the first half hour of sleep immediately after the task. This increase was confined to brain areas associated with spatial tasks and spatial coordinate transformation and could be observed only after the learning task. The same group also found a correlation between local increase in slow wave activity and post-sleep task performance improvement (Huber et al., Nature, 2004). 19 Csercsa Richárd If an increase in synaptic strength due to learning leads to an increase in slow wave activity, the decrease in synaptic strength should do the opposite. A subject’s arm was immobilized during the day, reducing synaptic strength locally as indicated by an amplitude reduction in somatosensory evoked potentials (electrical median nerve stimulation) and in motor evoked potentials (transcranial magnetic stimulation). This resulted in a drop in motor performance. During subsequent sleep, slow wave activity was reduced over the same cortical area (contralateral to the immobilized arm). Thus, local sleep regulation can be linked to cortical plasticity even without learning (Huber et al., Nat Neurosci, 2006). These results show that the amount of slow wave activity is correlated with synaptic weights. The bigger the synaptic strength, the more local slow waves exist, and vice versa. Tononi and Cirelli suggested that the purpose of slow waves is to depress the synapses, thus maintaining a homeostatic regulation of synaptic strength (Tononi and Cirelli, Sleep Med Rev, 2006). Synaptic downscaling is thought to have a beneficial effect on memory that is described in a further chapter. Other hypothesized advantages of downscaling include energy and space saving. The gray matter is the most energydemanding part of the brain, and the majority of the energy requirement is due to the repolarization following postsynaptic potentials and spike generation. The number of spikes increases with stronger synaptic weights, so downscaling would significantly reduce energy demand. Synaptic strengthening probably increases the number and the size of terminal boutons. Therefore, downselection is crucial in maintaining the space requirements of synapses (Tononi and Cirelli, Sleep Med Rev, 2006). Cytokines have been proposed to play an important role in promoting sleep in local circuits (Krueger, Curr Pharm Des, 2008). Furthermore, the role of cytokines in synaptic plasticity is crucial. It has been shown that TNF-α mediates synaptic scaling through changes in the number of AMPA receptors and that glia cells are the source of this form of plasticity (Stellwagen and Malenka, Nature, 2006). This might link the theory of synaptic downscaling as the cause of local sleep to the theory of cytokines provoking sleep in neuronal assemblies. The state-of-the-art idea is thus that on macroscopic scale sleep is global, with physiological and behavioral changes having impact on the whole organism, while on 20 Laminar analysis of the slow wave actvity in humans the level of neuronal assemblies, cortical columns can exhibit slow wave activity individually, depending on past activity. SLEEP DEPRIVATION Sleep is essential and executes numerous vital functions. We can come to that conclusion if we notice that sleep deprivation can be harmful. Rats kept awake for a prolonged time developed an increase in metabolic rate and decrease in body weight, which culminated in death. It can lead to death in flies and cockroaches, too. Pigeons, however, appear capable of surviving prolonged sleep deprivation (Cirelli and Tononi, PLoS Biol, 2008). In humans, staying awake for longer than usual leads to a feeling of sleepiness, microsleep episodes (intrusions of sleep into waking), cognitive impairment, tremors, hallucinations, and even death. A well-documented case is an experiment from 1965, where the condition of Randy Gardner, a 17-year old high school student from San Diego, was monitored by sleep researcher William Dement during sleep deprivation for 11 days. Except for a few illusions he demonstrated no unusual behavior and his physiological conditions remained within healthy limits (Siegel, 2002) showing large individual differences in sleep demand. Sleeping too much may also be harmful. People who sleep too much have a more unstable mood, they are more anxious and distressed than short sleepers. In contrast, staying awake during the second half of the night can induce a positive change in the mood of depressed patients. There is also a correlation between average daily sleep time and death rate. People who sleep very short or very long have higher death rates than people who sleep the average, daily 7-8 hours (Bódizs, Kognitív idegtudomány, 2003). 21 Csercsa Richárd FUNCTIONS OF SLEEP There has been extensive search for the roles of sleep, but they still remain elusive. Sleep is supposed to serve fundamental functions, since it occurs very frequently, despite putting the sleeper in danger, and deprivation of sleep may have lethal consequences. Functions sleep may execute include recovery, conserving energy, and recent findings indicate that it plays an important role in memory consolidation. RECOVERY AND RESTORATION The theory that recovery is a function of sleep comes from the observation that we cannot execute either physical or mental tasks after a considerable amount of waking as well as immediately after sleep. This means that sleep recovers the body and restores the same state of its functionality as the day before. One of the conditions thought to be restored during sleep is the synaptic weights between neurons. In their synaptic downscaling theory, Tononi and Cirelli proposed that synaptic weights are strengthened during waking due to learning and downscaled during deep sleep in order to keep newly formed memories, and make place for even newer ones (Tononi and Cirelli, Sleep Med Rev, 2006). ENERGY CONSERVATION During deep sleep, metabolism is decreased with about 10% compared to waking, but the energy conservation at lower temperatures may be even higher. The metabolic reduction in an unclothed human subject at the room temperature of 21 °C may reach 40% after sleep onset. (Velluti, 2008) 22 Laminar analysis of the slow wave actvity in humans Energy conservation may be particularly important in newborns. They have a high ratio of surface area to body mass, thus they need more energy to maintain their body temperature. Because their sensory-motor system is immature, they also benefit from the less exposure to danger during sleep, since they are not moving around exposing themselves, but lie quietly in a protective environment (Siegel, Nature, 2005). Furthermore, the high amount of sleep in infants can be explained by sleep’s role in memory consolidation and learning. However, energy conservation cannot be the only function of sleep since quite wakefulness is also a state with reduced energy consumption without the danger that is due to the loss of consciousness. Moreover, arctic ground squirrels periodically interrupt their hibernation and consistently sleep during these arousals with their EEG showing a progressive decrease in slow wave activity, indicating a declining requirement for sleep (Daan et al., Neurosci Lett, 1991). Since hibernation is already an energy conserving state, this points to that sleep serves more functions than simply conserving energy, functions that are important enough to interrupt hibernation from time to time. PLASTICITY, MEMORY, LEARNING It is a widely accepted theory that sleep benefits learning (Maquet, Science, 2001), (Frank and Benington, Neuroscientist, 2006). Several studies show that performance of diverse memory tasks is significantly improved after sleep. But it is still debated which sleep stage contributes to the consolidation of which type of memories and what the underlying mechanisms are. Some researchers question this theory (Siegel, Science, 2001), (Vertes and Siegel, Sleep, 2005). They argue that there is no clear evidence for a major role of REM sleep in memory consolidation, instead they bring the example of patients with REM sleep deprivation induced by drugs (serotonin re-uptake inhibitors) or lesion who did not develop learning deficit (Rasch et al., Nat Neurosci, 2009). 23 Csercsa Richárd MEMORY CONSOLIDATION DURING SLEEP Memory consolidation could well be the reason for the loss of consciousness during sleep, making it the main candidate in the search for the function sleep is essential for. The same neuronal networks in the brain are used for storing and processing information, two processes that may not be executed in the same time. Thus two modes of operation seem to be necessary: an 'online' mode for information processing (waking) and an 'offline' mode for memory formation (sleep). The idea that sleep benefits memory dates back to 1801, when David Hartley, a British psychologist proposed that dreaming might change the association links of memory. However, the first study examining the connection between sleep and learning was performed only in 1924 by Jenkins and Dallenbach (Jenkins and Dallenbach, American Journal of Psychology, 1924). They showed that memory recall improved after a night of sleep. They theorized that this improvement resulted from a lack of sensory interference during sleep. Instead of this passive process theory, the modern conception is that sleep actively modifies synaptic structure, thus consolidates memory traces (Walker and Stickgold, Annu Rev Psychol, 2006). MEMORY CATEGORIES Memory is the collective term of processes, not a unitary entity. In humans it can be classified in two main categories: declarative and non-declarative (Figure 8) (Cohen and Squire, Science, 1980). Non-declarative memory is regarded as non-conscious, implicitly learned, including procedural memory that is the learning of perceptual and motor skills (‘knowing how’). They are acquired gradually by repeated practice, but remain fairly stable. Their consolidation is dependent on cortico-striatal circuitry. 24 Laminar analysis of the slow wave actvity in humans Declarative memories can be considered as explicitly learned, consciously accessible memories of facts and events (‘knowing what’). It can be further divided to episodic memory (autobiographical memory for events in the past) and semantic memory (memory for general knowledge not related to specific events). They are rapidly encoded, but sensitive for attenuation and interference. Their encoding and short-term retrieval essentially rely on structures in the medial temporal lobe, especially the hippocampus. In 1957, Scoville and Milner reported the case of HM, a patient with epilepsy, whose hippocampus on both sides was removed surgically and as a result he developed anterograde amnesia. He could not store long-term declarative memories, but his working memory and procedural skill learning remained intact (Scoville and Milner, J Neurol Neurosurg Psychiatry, 1957). According to the standard model, the retrieval of declarative memories becomes independent of hippocampus over time, possibly due to a hypothesized information transfer to the neocortical stores. However, the multiple memory trace theory suggests that while semantic information can be established in the neocortex, hippocampus is always necessary for the retrieval of episodic and spatial information (Nadel et al., Hippocampus, 2000). (Walker and Stickgold, Annu Rev Psychol, 2006), (Stickgold, Nature, 2005), (Marshall and Born, Trends Cogn Sci, 2007), (Born et al., Neuroscientist, 2006) Figure 8. Memory categories. Memory consists of two main categories, declarative and nondeclarative, which can be further distinguished. From (Stickgold, Nature, 2005) 25 Csercsa Richárd MEMORY FORMATION The formation of memory consists of three fundamental stages: acquisition, consolidation, and retrieval. Acquisition is the learning process when new information is encoded into a neural trace, its representation as a neural ensemble. Consolidation is the stabilization and enhancement of memories. Stabilization means that memory becomes resistant to interference, while enhancement is the active maintenance of a memory. During consolidation, the brain has to integrate newly encoded memories with pre-existing long-term memories. In this sense, we can differentiate two levels of consolidation. ‘Synaptic consolidation’ is accomplished within hours after learning and involves mainly the stabilization of synaptic changes. ‘System consolidation’ means that the memory trace undergoes a reorganization so it becomes represented by different neural networks, coming into context with related memories. This process can take days or months to complete. Retrieval refers to the recall of stored memories. The act of memory recall is believed to destabilize the memory representation therefore reconsolidation is needed to prevent degradation. (Walker and Stickgold, Annu Rev Psychol, 2006), (Born et al., Neuroscientist, 2006) SYNAPTIC CONSOLIDATION Since the recognition that the number of neurons in the adult brain is more or less constant, it became obvious that learning and forming new memories cannot be attributed to new neurons arising. At the end of the 19th century, Santiago Ramón y Cajal suggested that learning is the result of a change in neuronal connections, modifying their communication. In 1949, Donald Hebb postulated that if a cell fires persistently soon enough after another cell’s firing, then there will be a change in their 26 Laminar analysis of the slow wave actvity in humans connection due to some growth process or metabolic change so that the efficiency of their communication will be increased (‘cells that fire together, wire together’) (Hebb, 1949). The microbiological basis of Hebbian learning was discovered by Lømo in 1966. He experienced that excitatory postsynaptic potentials elicited by single electrical pulses applied to presynaptic cells were increased if preceded by the application of a highfrequency spike train to the presynaptic cell, and that the increase was long-lasting (Lomo, Acta Physiologica Scandinavia, 1966). This effect is called long-term potentiation (LTP). LTP can be induced not only by high-frequency spiking but by the precise timing of pre- and postsynaptic action potentials (spike-time dependent plasticity, STDP). If the firing of the presynaptic cell precedes the firing of the postsynaptic cell, the connection between them is strengthened. However, if the postsynaptic cell fires first, the connection becomes weaker (long-term depression, LTD) (Figure 9). Figure 9. Time dependency of firing during STDP. If presynaptic firing precedes postsynaptic firing, it causes LTP, while the opposite order results in LTD. From (Nevian and Sakmann, J Neurosci, 2006) LTP has an early phase, when the synapses become stronger, and a late phase, when this conformation is maintained. Early LTP depends on the calcium concentration of the postsynaptic cell. The concentration has to exceed a certain threshold, which makes the postsynaptic N-methyl-d-aspartate (NMDA) receptors essential. Late LTP requires gene transcription and protein synthesis. 27 Csercsa Richárd It was shown that the time and threshold necessary for LTP induction vary on different synapses, but once it is established, the response is rapid and digital. It means that there is a step increase in postsynaptic currents after the LTP and stays at this elevated level even for further potentiation. The advantage of this digital type of memory storage may be its robustness in a noisy environment. If there is a slight change in the synaptic weight due to noise, it might affect the graded value greatly, but it would take a considerable amount of noise to affect the digital value. Even if there is variability (as there often is in biological systems), it will not lead to errors (Petersen et al., Proc Natl Acad Sci U S A, 1998) (Lynch, Physiol Rev, 2004). SYSTEM CONSOLIDATION Two types of memory stores is thought to be present in the brain: a short term storage for keeping information from seconds to hours, and a long term storage from hours to months or even the whole lifetime. The theory that new learning takes some time dates back to the 19th century. But it was Atkinson and Shiffrin who proposed a model that included short term and long term memory stores (Atkinson and Shiffrin, The psychology of learning and motivation: II, 1968). The basic idea is that newly acquired, labile memory traces are converted into long-lasting forms during different modes of operation. Marr suggested that the archicortex (hippocampus) and neocortex are ideal candidates for these two stores (Marr, Proc R Soc Lond B Biol Sci, 1970), (Marr, Philos Trans R Soc Lond B Biol Sci, 1971), (Buzsaki, Neuroscience, 1989), (Squire, Psychol Rev, 1992). The state-of-the-art concept of the model is that the synaptic weight structure is reorganized due to learning during waking (online mode), by the sequential firing of neurons in an assembly, forming memory traces (encoding). These traces are re-activated during sleep (offline mode) in order to strengthen the new structure of connections (synaptic consolidation) and transfer the new information from temporary storage to long term storage, from the hippocampus to the neocortex (system consolidation) (McClelland et al., Psychol Rev, 1995). 28 Laminar analysis of the slow wave actvity in humans The neurobiological mechanisms underlying memory consolidation during sleep is still not known, though several theories exist. A widely accepted theory states that declarative, hippocampus-dependent memories are mainly consolidated during SWS, while consolidation of procedural memories independent of hippocampus is supported mainly by REM sleep (Born et al., Neuroscientist, 2006), (Diekelmann et al., Sleep Med Rev, 2009), (Marshall and Born, Trends Cogn Sci, 2007), though the latter seems to be inconsistent with the finding that antidepressant drugs (selective serotonin reuptake inhibitors) suppressing REM sleep slightly improved skill memory consolidation (Rasch et al., Nat Neurosci, 2009), (Vertes and Siegel, Sleep, 2005), and studies showing that sleep does not benefit implicit sequence-specific motor learning (Song et al., J Neurosci, 2007), (Nemeth et al., Exp Brain Res, 2010). Other studies suggest that proper memory consolidation takes place only when the whole night’s sleep is undisturbed, meaning that REM sleep follows SWS (Gais et al., Nat Neurosci, 2000). SLOW WAVE SLEEP Slow wave sleep is considered the deepest phase of sleep, dominant during the first half of the night. It is accompanied by maintained muscle tone, lower awakability, and high-amplitude, low-frequency (< 2Hz) waves on the EEG. SLOW OSCILLATION Delta waves (0-4 Hz) during sleep were reported in 1937 (Blake and Gerard, Am J Physiol, 1937), but slow oscillations (SO, < 1 Hz), the main characteristic electrophysiological features of deep sleep were first described in anesthetized cats by Mircea Steriade in 1993 (Steriade et al., J Neurosci, 1993a). They were later detected in other mammals and humans, too (Achermann and Borbely, Neuroscience, 1997), during various arousal states like anesthesia, natural sleep (Destexhe et al., J Neurosci, 1999), 29 Csercsa Richárd and quiet wakefulness (Petersen et al., Proc Natl Acad Sci U S A, 2003). Furthermore, SO can be induced in cortical slices in vitro (Sanchez-Vives and McCormick, Nat Neurosci, 2000). SO consist of the alternation of two states in the membrane potential of the neurons: a depolarized state, during which the membrane potential is closer to the firing threshold and neurons generate action potentials (also called active or up-state), and a hyperpolarized state, during which the membrane potential is more negative with virtually no firing (passive or down-state) (Steriade and Timofeev, Neuron, 2003). The cortical local field potential recorded in cats with a laminar extracellular electrode is positive superficially during up-states that turns into negativity in deep layers. During down-states a superficial negativity and deep positivity can be detected. During upstates, fast oscillations emerge, while during down-states they disappear. In human SWS, the alpha and beta power is increased during the surface positive half-wave (up-state) compared to the surface negative half-wave (down-state), suggesting that their basic neurophysiology may be similar to animal findings (Molle et al., J Neurosci, 2002), (Massimini et al., J Neurosci, 2004). While the SO in animals is limited to below 1 Hz (Steriade et al., J Neurosci, 1993a), the recent AASM guidelines suggest the 0.5-2 Hz range for slow wave activity (SWA) in humans (Iber et al., 2007). The fine scale laminar structure of neuronal activity was analyzed in ferret cortical slice preparations, revealing that firing during the up-state is the earliest in the infragranular layers and spread towards the superficial layers with a long ~100ms interlaminar delay (Sanchez-Vives and McCormick, Nat Neurosci, 2000). In intact animals the up-state onset related initial firing, intracellular membrane potential and LFP changes could be detected in any layer in a probabilistic manner, with a short interlaminar delay (~10ms), however, on average, the earliest activity was found in the infragranular layers (Sakata and Harris, Neuron, 2009), (Chauvette et al., Cereb Cortex, 2010). Current source density (CSD) analysis of the low frequency (<1 Hz) components of the anesthesia induced SO in cats (Steriade and Amzica, Proc Natl Acad Sci U S A, 1996), revealed sink in the middle layers during the up-state and source during the 30 Laminar analysis of the slow wave actvity in humans down-state. This indicates current flow during up-states into the cell, probably in the somatic region (positively charged ions traveling through the membrane into the cell or negative ions to the outside), and current flow out of the cell during down-states (positive ions flowing out to the extracellular space or negative ions flowing into the cell). The most prominent up-state related sinks were localized in the middle and deepest cortical layers (most probably layer III-VI). In contrast, the fast (30-40 Hz) components were more distributed, composed of “alternating microsinks and microsources” along the whole cortical depth (Steriade and Amzica, Proc Natl Acad Sci U S A, 1996). During spontaneous and evoked K-complexes, a massive up-state related sink was reported in layers II-III besides weaker ones in the deeper layers (Amzica and Steriade, Neuroscience, 1998). In still another cat study, the maximal sink during the up-states in natural sleep was located in the middle and deep layers (Chauvette et al., Cereb Cortex, 2010). In the rat primary auditory cortex, the laminar distribution of the major up-state related sink was variable (Sakata and Harris, Neuron, 2009). On average across animals, it was located in middle and deep layers (most probably layer III-V) in natural sleep, whereas it was located in superficial layers (most probably layer II-III) under urethane anesthesia (Sakata and Harris, Neuron, 2009). Although the cellular and synaptic/trans-membrane mechanisms of slow waves during natural sleep are thus under intense investigation in animals, these mechanisms have not previously been studied in humans. Up-states are proposed to be micro-wake ‘fragments’, similar to the activated state of waking (Destexhe et al., Trends Neurosci, 2007), while down-states are considered silent states filtering stimuli to the cortex. SLOW OSCILLATION GENERATION Slow oscillation can be detected in several brain structures: • all neocortical areas (in all types of neurons and glia cells), • thalamus (in thalamocortical and reticular neurons as well), 31 Csercsa Richárd • hippocampus (Moroni et al., PLoS One, 2007), • basal ganglia (Tseng et al., J Neurosci, 2001), • cerebellum (Ros et al., J Neurosci, 2009). Despite of the above findings, SO is traditionally considered an oscillation generated by neocortical networks. The main arguments for its neocortical origin are that it is present in the neocortex after thalamectomy (Steriade et al., J Neurosci, 1993b), but absent in the thalamus after decortication (Timofeev and Steriade, J Neurophysiol, 1996), and the disconnection of intracortical synaptic linkages resulting in the disruption of its long-range synchronization (Amzica and Steriade, J Neurosci, 1995). Recently, the pure neocortical origin of SO has been disputed. Crunelli and Hughes pointed out the crucial role of thalamus in the generation of SO (Crunelli and Hughes, Nat Neurosci, 2010). They argue that SO in the isolated neocortex was not identical to those present when all cortical connections were intact. In fact, SO-like activity can be detected in thalamic slices if the metabotropic glutamate receptors (mGluR) of thalamocortical or reticular neurons were activated. They suggest that SO can be generated in three structures: • cortical circuits (due to synaptic network properties), • thalamocortical neurons (intrinsically, depending on glutamatergic input from the cortex), • reticular neurons (intrinsically, depending on glutamatergic input from the cortex), and the interplay between these oscillators is necessary for the full manifestation of the slow oscillation. Furthermore, they insist that high-frequency (150-300 Hz) bursts of thalamocortical neurons mediated by a low-threshold Ca2+ potential consequently precede the firing of cortical cells and the depth-negative peak of the LFP in the cortex, pointing out that cortical up-state generation may be triggered by thalamic input. 32 Laminar analysis of the slow wave actvity in humans The activation of persistent sodium current that is essential for up-state initiation (Mao et al., Neuron, 2001) may be achieved by thalamic input, spontaneously occurring coincidence of mini EPSPs, or the action of some neurons that have a slightly lower spiking threshold. But subthreshold membrane potential fluctuations clearly precede neuronal firing at up-state onset, thus, firing may be the consequence rather than the cause of up-state initiation (Chauvette et al., Cereb Cortex, 2010). Mechanisms generating and maintaining down-states are also debated. Input resistance was found to be the highest during the down-states and lowest during the early part of up-states, increasing until its end (Destexhe et al., Nat Rev Neurosci, 2003). Furthermore, during down-states, inhibitory neurons are silent, too. Thus, instead of an active inhibition, the hyperpolarization is thought to be the result of disfacilitation i.e. the lack of firing of input neurons (Contreras et al., J Physiol, 1996), or the buildup of activity-dependent potassium conductances (Sanchez-Vives and McCormick, Nat Neurosci, 2000) or both (Hill and Tononi, J Neurophysiol, 2005). K+ currents are believed to be the main factor in generating down-states since the application of Cs+, blocker of K+ currents, reduces hyperpolarization (Timofeev et al., Proc Natl Acad Sci U S A, 2001). However, it has been shown that GABAB has an important role in terminating up-states (Mann et al., J Neurosci, 2009). Indeed, the observation that the onset of down-states is synchronized more precisely than the onset of up-states suggests a long-range synaptic mechanism (Volgushev et al., J Neurosci, 2006). 33 Csercsa Richárd PROPAGATING WAVE AND SOURCE LOCALIZATION Unlike oscillations during waking or REM sleep, slow oscillations during slow wave sleep are correlated over larger cortical areas (7 mm distance in cats) (Destexhe et al., J Neurosci, 1999). Slow oscillation was described as a traveling wave in rats (Luczak et al., Proc Natl Acad Sci U S A, 2007) and humans (Massimini et al., J Neurosci, 2004). It was shown that in humans it can start on any recording site of the scalp EEG and travel in arbitrary direction, though the most frequent site of origin is the frontal region and the most probable direction of propagation is anteroposterior (Figure 10). Using high-density EEG recordings (256 electrodes) and source localization methods, slow waves in general were found to travel through definite cortical structures, despite the unique localization of the individual waves (Murphy et al., Proc Natl Acad Sci U S A, 2009). Waves with multiple peaks may have multiple origins (Riedner et al., Sleep, 2007). The hot spots of wave initiation are the lateral sulcus (including insular, temporal, frontal, and parietal cortices) and the medial cingulate gyrus. The main areas involved in the propagation include the anterior cingulate, the cingulate, the posterior cingulate, the precuneus, and the left inferior frontal gyrus and insula. Although most of the waves travel in the anterioposterior direction, propagation in the opposite direction also occurs (Murphy et al., Proc Natl Acad Sci U S A, 2009). The same structures were found to be activated by simultaneous EEG and fMRI recordings, with the addition of the pontine tegmentum, the cerebellum, and the parahippocampal gyrus (Dang-Vu et al., Proc Natl Acad Sci U S A, 2008). Cerebral structures active during SWS have been associated with the so called default-mode network (medial prefrontal cortex, anterior cingulate, posterior cingulate, precuneus, left inferior parietal cortex) (Raichle et al., Proc Natl Acad Sci U S A, 2001), (Greicius et al., Proc Natl Acad Sci U S A, 2003), (Raichle and Snyder, Neuroimage, 2007). The default system is a network of functionally connected brain structures that show decreased activity in PET and fMRI images during goal-oriented cognitive tasks compared to resting. The energy requirement of the brain’s responses to external stimuli is only a small fraction of the amount required for ongoing activity. It is not probable that the human body can afford expending this vast amount of energy (20% of the 34 Laminar analysis of the slow wave actvity in humans body’s total energy consumption for an organ weighing 2% of total body weight) on idle behavior. The purpose of this expenditure may be maintaining balance of excitability, generating predictions about the future or spontaneous cognition (Raichle, Science, 2006). Tasks activating regions in the default network include internal mentation, theory of mind, or self-referential decision making. Recent experiments suggest that the default network has two subsystems with distinct functions: a medial temporal lobe subsystem that shows increased activity when participants made decisions based on episodic memories, and a dorsal medial prefrontal cortex subsystem preferentially active when they considered the mental states of themselves or others (Andrews-Hanna et al., Neuron, 2010). Figure 10. Slow oscillation as a traveling wave. Upper traces show superimposed recordings from EEG channels during the same time period. Below, propagation of individual slow waves. Red asterisk signals the initiation of the wave and blue streamlines indicate the path of propagation. From (Massimini et al., J Neurosci, 2004) The significance of the overlap between the structures involved in slow oscillation generation and propagation and the structures assigned to the default network is not yet known. The similarity between introspection during wakefulness and the brain’s dissociation from external stimuli during sleep may explain this overlap. But activation of similar regions does not imply that similar neural processes take place. Furthermore, spontaneous fluctuations in the BOLD signal have a frequency of < 0.1 Hz, while slow oscillations are < 1 Hz, which is a substantial difference. Though, BOLD fluctuations 35 Csercsa Richárd may reflect infraslow EEG oscillations that modulate excitability and group slow oscillations (Vanhatalo et al., Proc Natl Acad Sci U S A, 2004). STIMULATION EVOKED SLOW OSCILLATION Slow oscillations occur spontaneously during anesthesia and deep natural sleep and they can be evoked with external stimulation, too. Solitary SO-like events, called Kcomplexes can be evoked during sleep by sensory stimuli, and though the mechanisms underlying their generation are likely similar to those of the hyperpolarized phase of SO (Cash et al., Science, 2009), they are isolated phenomena not constituting an ongoing oscillation that may distinguish them from the fully developed slow oscillation (see in section Slow waves and K-complexes). It has been shown that sound stimuli can entrain the slow oscillation in the thalamus (He, J Neurosci, 2003), (Gao et al., J Neurosci, 2009), furthermore, SO can be induced by transcranial magnetic stimulation (TMS) in humans (Massimini et al., Proc Natl Acad Sci U S A, 2007), and by electrical stimulation in rats (Vyazovskiy et al., J Neurophysiol, 2009), (Ozen et al., J Neurosci, 2010) and humans (Entz et al., Society for Neuroscience, 2009). Massimini et al. showed that slow waves triggered by transcranial magnetic stimulation resemble spontaneous waves in their morphology and propagation properties (Massimini et al., Proc Natl Acad Sci U S A, 2007). Every TMS pulse evoked a slow wave that started under the stimulator and spread over the scalp. Interestingly, while slow waves could be evoked in any phase of NREM sleep, responses during waking had a low-amplitude, complex wave shape. Sleep TMS pulses triggered a stereotyped down-state, either local (indicating the breakdown of connectivity during sleep) or global (an aspecific response spreading away from the stimulation site). On the other hand, awake responses were long-range, spatially, and temporally differentiated. These findings suggest that the sleeping brain, despite being active and reactive, loses its ability of entering states that are both integrated and differentiated. Both features may be important to account for the fading of 36 Laminar analysis of the slow wave actvity in humans consciousness during the early phase of sleep (Massimini et al., Proc Natl Acad Sci U S A, 2007). Entz et al. showed that slow waves can be evoked in humans by cortical electrical stimulation and characterized the laminar profile of evoked waves (Entz et al., Society for Neuroscience, 2009). They showed that the local field potential, multiunit activity, spectrogram, and current-source density attributes of intracortical responses are similar to those measured during natural SO. Responses triggered in different vigilance states (awake, sleeping, and anesthetized) differed only in the amplitude and latency of evoked potential components derived from subdural electrodes (Entz et al., FENS Forum, 2008). MODELING OF SLOW OSCILLATION Computational models and computer simulations are useful complementary approaches to traditional experimental techniques in understanding information processing in neural systems (Dayan and Abbott, 2001). Several models have been developed in order to understand the cellular and network properties of the sleep slow oscillation. The model by Bazhenov et al. (Bazhenov et al., J Neurosci, 2002) includes Hodgkin-Huxley-type principal cells and interneurons from the thalamus and the cortex (100 pyramidal cells, 25 interneurons, 50 thalamocortical, 50 reticular cells) (Figure 11). They found that down-up transitions are mainly caused by mini EPSPs and downup transitions by Ca-dependent K current, synaptic depression, and inhibition from interneurons. 37 Csercsa Richárd Figure 11. Thalamocortical model by Bazhenov et al. PY: pyramidal cells, IN: interneurons, RE: reticular cells, TC: thalamocortical neurons. Lines indicate connections, filled circle: excitatory, empty circle: inhibitory. From (Bazhenov et al., J Neurosci, 2002). The network of Compte et al. (Compte et al., J Neurophysiol, 2003) consists of 1024 pyramidal neurons and 256 interneurons placed equidistantly on a line of ~5 mm (Figure 12). Pyramidal cells are two-compartment, interneurons single compartment Hogkin-Huxley models. They analyzed the propagation of evoked slow waves and found that the firing of inhibitory neurons precedes the firing of pyramidal cells. The membrane resistance is the lowest at the onset of the up-state and increases until the end of the down-state. The inhibitory and excitatory synaptic conductances change over time, but their ratio remains constant, around 4:1. They theorized that spontaneous firing causes up-states, and the accumulating Na-dependent K current causes downstates. Wave propagation in the model was similar to propagation observed in experiments only if weak, long-range, ‘patchy’ connections were established. There is example for this type of connectivity in the neocortex, especially in the supragranular layers (Chalupa and Werner, 2001). 38 Laminar analysis of the slow wave actvity in humans Figure 12. Model of Compte et al. Empty circles: pyramidal cells, filled circles: interneurons. Triangle: excitatory, circle: inhibitory connections. Distribution of inhibitory connections is narrower than distribution of excitatory connections. From (Compte et al., J Neurophysiol, 2003). Hill et al. constructed a large-scale model of the thalamocortical system (Hill and Tononi, J Neurophysiol, 2005) with three-layered cortex (supragranular, granular, infragranular) and two-segment thalamus (reticular nucleus and dorsal thalamus). The model in total consists of about 53,000 cortical and 10,000 thalamic single compartment Hodgkin-Huxley neurons (Figure 13). They found that up-state generation depends on the activation of persistent Na current that can be produced via either mini EPSPs, synaptic input, or a hyperpolarization-activated cation current. Up-states can be terminated by depolarization-dependent K current and synaptic depression. Furthermore, network connections are crucial in synchronizing the oscillation. The important role of horizontal connections in synchronization is in accordance with the finding that the frequency of the slow oscillation depends on the size of the corticocortical network (Timofeev et al., Cereb Cortex, 2000). 39 Csercsa Richárd Figure 13. Large-scale thalamocortical model of Hill et al. Vp: three-layered primary visual cortex, Vs: three-layered secondary visual cortex, R: reticular nucleus, T: dorsal thalamus. White circles indicate exciatatory, black circles inhibitory cells. From (Hill and Tononi, J Neurophysiol, 2005). MEMORY CONSOLIDATION DURING SWS Oscillations during SWS like SO, spindles, sharp waves, and ripples may have a critical role in memory consolidation. Several studies show correlation between the amount of slow wave sleep and learning performance. Cortical areas activated during learning exhibit larger slow wave activity during SWS and the increase in task performance was correlated with the amount of slow wave activity (Huber et al., Nature, 2004), (Tucker and Fishbein, J Sleep Res, 2009). 40 Laminar analysis of the slow wave actvity in humans Presenting odors while learning hippocampus-dependent declarative memories and re-exposure to them during subsequent slow wave sleep improved memory retention (Rasch et al., Science, 2007). In contrast, re-exposure during REM sleep or presenting odors during SWS without prior association did not result in memory improvement. In case of hippocampus-independent procedural memories, cuing during either SWS or REM sleep did not benefit memory consolidation. Not only spontaneous but induced slow oscillations may benefit memory. The transcranial application of potentials oscillating at 0.75 Hz enhanced the retention of declarative memories in humans (Marshall et al., Nature, 2006). Another recent concept that gained wide acceptance is the synaptic downscaling model by Tononi and Cirelli (Tononi and Cirelli, Sleep Med Rev, 2006). According to this theory, the global downscaling of synaptic strengths during slow oscillations improves signal to noise ratio and thus enhances important memory traces while eliminating the weaker ones. It also provides a baseline state of synaptic strengths after sleep, preparing the brain for the acquisition of new memories. Re-activation theory assumes memory consolidation is an active process, while synaptic downscaling results in consolidation passively. Though these two theories can be combined (Diekelmann and Born, Nat Rev Neurosci, 2010). Re-activation might select memories worth keeping and transfer them to long-term storage, while downscaling creates an environment necessary for the acquisition of new memories. MEMORY TRANSFER (SYSTEM CONSOLIDATION) Following the online mode of acquisition, an offline mode of operation is necessary for memory consolidation to avoid interference between new and existing memories. In the offline mode, memories are placed in context and transferred to the long-term store. This concept is corroborated by the observations that after lesion of the hippocampus, memories acquired just before the lesion were forgotten, while memories 41 Csercsa Richárd learned earlier could be recalled (Zola-Morgan and Squire, Science, 1990). It suggests that the retrieval of hippocampus-dependent memories gradually became hippocampusindependent over time. Neuroimaging studies showed that recall of new declarative memories depended on hippocampus, but over time BOLD activity during recall exponentially decreased in hippocampus and increased in the medial prefrontal cortex (Takashima et al., Proc Natl Acad Sci U S A, 2006). The expression of immediate early genes required for synaptic plasticity increased significantly 30 days after memory acquisition in prefrontal and anterior cingulate cortices in mice, compared to control subjects, while their expression in the hippocampus showed a decrease (Maviel et al., Science, 2004). These results point to the hippocampus as short-term storage, that is active in the early stages of encoding and recall, like a librarian that searches for the right information, and neocortex as long-term storage like the library itself that stores the books (Buzsáki, 2006). Theoretical models also support the idea of memory transfer from hippocampus to neocortex (Kali and Dayan, Nat Neurosci, 2004). This model also predicts that retrieval of episodic memories should become impaired without hippocampus-initiated replay, due to plasticity in the neocortex. Semantic knowledge, on the other hand, would not be disrupted by neocortical plasticity, since it remains consistent with the observed statistical structure of the world. This is in contrast with episodes, whose instability arises because they correspond to extremely peaked probability distributions that are not statistically representative. RE-ACTIVATION Re-activation of memory traces is necessary for synaptic consolidation, since the co-activation of neurons strengthens the synapses between them (‘fire together – wire together’, Hebbian-rule). It is also required for system consolidation, since for the transfer of memories they need to be re-activated again. 42 Laminar analysis of the slow wave actvity in humans An experiment in rats supporting re-activation showed that hippocampal cell pairs whose activity was correlated when the animal was awake will be correlated during subsequent sleep (Wilson and McNaughton, Science, 1994) (Figure 14). The same was found in macaque neocortex, within and across all examined brain regions (sensory, motor, association areas), but not the dorsal prefrontal cortex (Hoffman and McNaughton, Science, 2002). Figure 14. Replay of cortical activity associated with learning. Each dot represents a cell and lines indicate correlation between cell pairs. On the left (PRE) shows correlations during sleep before learning task, in the middle (RUN) during task, and on the right (POST) during sleep after learning. Cell pairs active during learning task are active during post-learning sleep. From (Wilson and McNaughton, Science, 1994) Not only the firing of cell pairs, but spike sequences of neuron assemblies are repeated during sleep, too (Nadasdy et al., J Neurosci, 1999). Cell assembly sequences are thought to represent episodic memories with a temporal order and support planning actions and goals (Pastalkova et al., Science, 2008). Beyond correlation, the replay of the temporal order of cell ensemble firing sequences has been shown in rat hippocampus (Lee and Wilson, Neuron, 2002) and neocortex (Ji and Wilson, Nat Neurosci, 2007). Neuron ensembles repeated the firing sequence of awake running experience during subsequent slow wave sleep frames (upstates) (Ji and Wilson, Nat Neurosci, 2007) (Figure 15). About 8 percent of all examined sleep frames contained firing sequences similar to prior awake frames, which is significantly above chance level. Furthermore, cortical and hippocampal replaying 43 Csercsa Richárd frames occurring together could be observed. Their number was also above chance level. Such replays possibly occur more frequently, but the number of electrodes, hence the number of recordable cells is limited. The repeated pattern of activation during sleep is compressed in time by a factor of 20 in the hippocampus (Lee and Wilson, Neuron, 2002) and of 6 to 7 in the neocortex (Euston et al., Science, 2007). Fast replay may reflect the speed of the brain’s intrinsic dynamics (e.g., conduction speeds, synaptic delays, etc.) when not constrained by behavioral events. Figure 15. Re-activation of cell assemblies during sleep. The temporal order of cell firing was preserved from learning task (RUN) to post-learning sleep (POST), in the cortex (left, CTX) and in the hippocampus (right, HP). From (Ji and Wilson, Nat Neurosci, 2007) HIPPOCAMPUS-NEOCORTEX INTERACTION The transfer of memory traces from hippocampus to neocortex for long-term storage requires functional connection between the two structures during SWS. Siapas and Wilson found a temporal correlation between hippocampal ripples and neocortical spindles (Siapas and Wilson, Neuron, 1998), but the direction of information flow was not clarified. Buzsáki suggested that information flows from cortex to hippocampus during wakefulness and from hippocampus to cortex during sleep (Buzsaki, J Sleep Res, 1998). Several studies indicate that neocortex initiates the communication during SWS. Ji and Wilson showed that neocortical MUA precede hippocampal neuronal population activity by about 40 ms (Ji and Wilson, Nat Neurosci, 2007). Mölle et al. came to a similar conclusion, finding that prefrontal MUA precedes hippocampal sharp waves by about 30 ms (Molle et al., J Neurophysiol, 2006). In vivo whole-cell recordings from 44 Laminar analysis of the slow wave actvity in humans hippocampal CA1 interneurons and parietal cortex neurons in urethane-anesthetized mice showed a consistent lag of hippocampal activity with a latency of 160 ± 50 ms (Hahn et al., Nat Neurosci, 2006). Wierzynski et al., on the other hand, found that prefrontal cells fire in a 100 ms window following hippocampal cell firing (Wierzynski et al., Neuron, 2009), which might be the response of the hippocampus to the call of the neocortex. All these studies were conducted in rats. Human intracranial EEG examinations revealed a bidirectional coupling, with the influence of neocortex on hippocampus significantly increased during sleep compared to waking (Wagner et al., Cortex, 2010). These results altogether indicate that the two structures are synchronized by neocortical slow oscillations, during which memory traces are transferred. The exact mechanisms underlying the memory transfer are unclear, but special oscillations seem to be involved. High-frequency sharp wave-ripple complexes in the hippocampus (Chrobak and Buzsaki, J Neurosci, 1996), present during waking and SWS and thalamocortical spindles during NREM sleep are thought to play a critical role. A temporal correlation between hippocampal ripples and cortical spindles was observed (Siapas and Wilson, Neuron, 1998), indicating that a link is established between the two structures during these episodes. It was also shown that neocortical slow oscillation groups spindles (Contreras and Steriade, J Neurosci, 1995), (Molle et al., J Neurosci, 2002) and sharp waves (Molle et al., J Neurophysiol, 2006), (Clemens et al., Brain, 2007) in animals and humans alike. Furthermore, neocortical replay of neural patterns that appeared during learning, preferentially occur during hippocampal sharpwaves (Peyrache et al., Nat Neurosci, 2009). The importance of these oscillations in memory consolidation is indicated by several findings: the density of spindles is increased after learning (Gais et al., J Neurosci, 2002), memory retention performance is correlated with the number of spindles (Clemens et al., Neuroscience, 2005), and on the molecular level, spindle stimulation patterns consisting of spike trains extracted from a slow oscillation up-state induced LTP (Rosanova and Ulrich, J Neurosci, 2005). Hippocampal ripples are correlated with memory performance (Axmacher et al., Brain, 2008) and their elimination results in memory impairment (Girardeau et al., Nat Neurosci, 2009). They occur most frequently during waking (Axmacher et al., Brain, 45 Csercsa Richárd 2008), which corroborates the idea that events experienced during waking replay during sleep. DOWNSCALING According to the model of Tononi and Cirelli (Tononi and Cirelli, Sleep Med Rev, 2006), the processes of synaptic downscaling are: (1) synapses are potentiated due to learning during waking, (2) slow wave activity increases due to potentiated synapses, (3) synapses are depressed due to slow wave activity, (4) slow wave activity decreases as synaptic strengths return to a ‘baseline’ due to depression, leading to the beneficial effects of sleep. They hypothesize that downscaling depresses all synapses in the region proportionally, thus eliminating weaker ones therefore improving the signal-to-noise ratio of others (Figure 16). Figure 16. Synaptic downscaling. Synapses are strengthened due to experiences during waking, and downscaled during sleep. Downscaling causes weak synapses to disappear and improves signal-to-noise ratio. From (Diekelmann and Born, Nat Rev Neurosci, 2010) There are experiments supporting processes (1), (2) (Kattler et al., J Sleep Res, 1994), (Vyazovskiy et al., J Sleep Res, 2000), (Huber et al., Nature, 2004), and (4) (Huber et al., Nat Neurosci, 2006), though it is not easy to prove point (3) directly. It has been shown that protein levels of key components of synapses are high after waking and low after sleep in the rat (Vyazovskiy et al., Nat Neurosci, 2008), (Faraguna et al., J Neurosci, 2008) and in Drosophila (Gilestro et al., Science, 2009), independent of the 46 Laminar analysis of the slow wave actvity in humans time of the day. Furthermore, the number of synaptic terminals is reduced after sleep (Donlea et al., Science, 2009). In addition, mini EPSCs are increased after waking and decreased after sleep (Liu et al., J Neurosci, 2010), also indicating synaptic depression during SWS, supported by a computational model too (Olcese et al., J Neurophysiol, 2010). SLOW WAVES AND K-COMPLEXES K-complexes are large graphoelements of the sleep EEG, occurring mostly in stage 2 of NREM sleep. They are isolated events consisting of two or three phases: first, a short surface-positive transient, then a slow and large surface-negative complex with peaks at 350 and 550 ms (also called N550), which may be followed by a positivity at 900 ms. K-complexes are sometimes followed by 10-14 Hz spindles. They can be evoked by stimuli of multiple modalities or occur spontaneously (maybe due to internal stimuli, like changes in respiration, limb movement, gastric noises, or because of spontaneous neuronal activity). (Loomis et al., J Neurophysiol, 1938), (Halasz, Sleep Med Rev, 2005), (Colrain, Sleep, 2005) K-complexes have been suggested to represent either arousal response or sleep protecting mechanisms. In the first case, K-complexes are hypothesized to be the cortical responses to potentially arousing stimuli, accompanied by peripheral sympathetic activation, and elevation in heart rate and blood pressure. On the other hand, Amzica and Steriade suggested that they are the expressions of the sleep slow oscillation (Amzica and Steriade, Neuroscience, 1998). Recent experiments revealing the laminar profile of K-complexes in humans (Cash et al., Science, 2009) support this idea. During the main component (i.e. the slow and prominent surface-negative wave) there was an active source in layer III and a passive sink close to the surface. The multiunit activity in all cortical layers decreased, which, together with the CSD results, indicate a hyperpolarizing current in layer III. Spectral power in the gamma frequency range (20-100 Hz) was also decreased during the surface negativity. These findings altogether largely resemble the laminar profile of the hyperpolarized phase (down-state) 47 Csercsa Richárd of the slow oscillation. These findings support the sleep protecting function of the Kcomplex, because it suppresses neuronal activity and arousal to sensory stimuli (Cash et al., Science, 2009). 48 Laminar analysis of the slow wave actvity in humans OBJECTIVES Sleep is a general behavioral state in the animal kingdom, and also a unique mode of brain operation. Brain waves during sleep are markedly different from awake oscillations, as visible on scalp EEG recordings. Unfortunately, until recently, EEG was the only way of studying brain activity in humans. While in animal models, microelectrodes with multiple contacts could be inserted into the cortex, in vivo electrophysiological experiments were restricted to scalp recordings in humans. Slow wave sleep is the deepest stage of sleep, named after the high-amplitude, low-frequency waves that characterize it on scalp derivations in humans and intracortical recordings in animals, called slow oscillations. This stage is thought to underlie essential restorative processes and facilitate memory consolidation. Thus, understanding the mechanisms generating and maintaining slow oscillations is of great importance in sleep research as well as in other areas of neuroscience. Our objectives were to derive local field potentials and action potentials from within the cortex of humans during slow wave sleep, and give a description of slow wave activity in humans on the level of cortical cells and layers. Animal models show increased firing, increased wide-band spectral power and inward transmembrane currents during surface positive LFP (up-state), while decreased firing, decreased spectral power and outward currents during surface negative waves (down-state). Current-source density analysis localized the most prominent up-state related sinks to middle or deep cortical layers in animals and showed deep layers to initiate firing at the onset of an up-state. Since the human prefrontal cortex is more than a hundred times larger than the prefrontal cortex of cats and rats, with a striking increase in pyramidal cell dendritic complexity, some differences were expected in the cellular and laminar properties of human deep sleep compared to animals. We implanted laminar multielectrodes chronically in the frontal and parietal cortical areas of epileptic patients in order to examine the electrophysiological properties of deep sleep in humans. 49 Csercsa Richárd METHODS PATIENTS AND ELECTRODES Five patients with intractable epilepsy underwent chronic clinical subdural grid and strip electrode implantation as a standard procedure for localization of the seizure focus and eloquent areas. Fully informed consent was obtained from each subject under the auspices of the Hungarian Medical Scientific Council and local ethical committee; National Institute of Neuroscience, Budapest, Hungary according to the World Medical Association Declaration of Helsinki. Conventional clinical subdural electrocorticography (ECoG) electrode strips and grids were implanted to cover the frontal, temporal and parietal gyri. In addition to the surface electrodes, a 350 µm diameter, 24 contact experimental laminar multichannel microelectrode array (ME) was implanted perpendicular to the cortical surface, underneath the clinical grids (Ulbert et al., J Neurosci Methods, 2001), (Ulbert et al., Hum Brain Mapp, 2001), (Ulbert et al., Epilepsia, 2004), (Cash et al., Science, 2009), (Keller et al., J Neurosci Methods, 2009). The 40 µm diameter Pt/Ir contacts were spaced evenly at 150 µm providing LFP recordings from a vertical, 3.5mm long cortical track, spanning from layer I to layer VI. A silicone sheet attached to the top of the ME shank prevented the first contact from sliding more than 100 µm below the pial surface (Ulbert et al., J Neurosci Methods, 2001). In each case, the explanted ME was visually inspected under a microscope for structural damage, and we did not find any alteration, indicating intact structure throughout the recordings. The location and duration of the clinical electrode implantation were determined entirely by clinical considerations, the ME was placed in cortex that was likely to be removed at the definitive surgery. HISTOLOGY The positions of the electrodes were confirmed by intraoperative navigation, colocalization of intraoperative photographs, pre- and postoperative MRI scans and 3D MRI reconstructions. Photographs were also taken during the resective surgery to 50 Laminar analysis of the slow wave actvity in humans confirm that the surface electrodes did not shift during monitoring. The brain tissue containing the electrode track in Patients 4 and 5 was removed en-bloc for further anatomical analysis (Ulbert et al., Exp Neurol, 2004), (Fabo et al., Brain, 2008). It was cut into 2-5 mm blocks and immersed into a fixative containing 4% paraformaldehyde, 0.1% glutaraldehyde and 0.2% picric acid in 0.1 M phosphate buffer (PB, pH 7.4). The fixative was changed every hour to a fresh solution during constant agitation for 6 hours, and then the blocks were post-fixed in the same fixative overnight. Vibratome sections (60 µm thick) were cut from the blocks, and photographs were taken from the electrode tracks. Following washing in PB, sections were immersed in 30% sucrose for 1–2 days, and then frozen three times over liquid nitrogen. Endogenous peroxidase was blocked by 1% H2O2 in PB for 10 min. Sections containing the electrode track were processed for immunostaining against the neuron marker NeuN (Figure 17A), calretinin (CR) (Figure 17B, reconstructed from camera lucida), SMI-32 (Figure 17C), glial fibrillar acidic protein (GFAP) (Figure 17D) to stain every neuron, a subset of interneurons, pyramidal cells and glia respectively. PB was used for all the washes (3x3-10 min between each step) and dilution of the antisera. Non-specific immunostaining was blocked by 5% milk powder and 2% bovine serum albumin. Monoclonal mouse antibodies against NeuN (1:3000, Chemicon, Temecula, CA, USA), SMI-32 (1:3000, Covance, Princeton, NJ, USA), GFAP (1:2000, Novocastra, Newcastle, upon Tyne, UK) and CR (1:5000, SWANT, Bellinzona, Switzerland) were used for 2 days at 4 °C. Specificity of the antibodies has been thoroughly tested by the manufacturers. For visualization of immunopositive elements, biotinylated anti-mouse immunoglobulin G (1:300, Vector) was applied as secondary antiserum followed by avidin-biotinylated horseradish peroxidase complex (ABC; 1:300, Vector). The immunoperoxidase reaction was developed by 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma), as a chromogen. Sections were then osmicated (0.25% OsO4 in PB, 30 min) and dehydrated in ethanol (1% uranyl acetate was added at the 70% ethanol stage for 30 min) and mounted in Durcupan (ACM, Fluka). Layers of the neocortex were outlined using all of the above stains and a shrinkage correction factor published earlier (Turner et al., J Comp Neurol, 1995), (Wittner et al., Eur J Neurosci, 2006). 51 Csercsa Richárd Figure 17. Electrode track histology, representative examples from Patient 4. (A) The ME electrode track (black contour line) and inferred contact locations (black dots) are shown relative to cortical layers (Roman numbers) revealed by the NeuN stain. Laminarization appears to be intact. (B) Camera lucida reconstruction of CR+ cell bodies and processes next to the electrode track. (C) Well preserved pyramidal (SMI-32 stain) and (C) glial cells (GFAP stain) next to the electrode track. Cell counting was performed in Patients 4 and 5 using camera lucida drawing (Figure 17B) of CR immunopositive (CR+) cells (two sections per patient). The normalized (between 0 and 1) CR+ cell density laminar depth profile (number of cells over unit area of cortex) was calculated in each consecutive 150 µm wide and variable length (1-3 mm) horizontal cortical stripes to match the depth structure of the electrophysiology measurements. RECORDINGS After electrode placement, the patients were transferred to the intensive monitoring unit for 5-7 days, where continuous 24 hour video-EEG observation took place in order to localize the seizure focus. ECoG from clinical strip and grid electrodes (32-92 channels, mastoid reference) was recorded concurrently with patient video using the standard hospital system (band-pass: 0.1-200 Hz, acquisition rate: 400-5000 Hz / 16 52 Laminar analysis of the slow wave actvity in humans bit). Video-EEG data for the duration of monitoring were stored on hard disks for later analysis. Spatial LFP gradient (LFPg), the voltage difference between consecutive laminar electrode contacts was provided by a special preamplifier placed inside the head bandage of the patient (Ulbert et al., J Neurosci Methods, 2001). For simplicity, throughout the text the spatial potential gradient is expressed in µV unit rather than the formally correct µV per inter-contact distance (150 µm). This reference independent measurement method was proven to be effective in minimizing the motion related and electro-magnetic artefacts (Ulbert et al., J Neurosci Methods, 2001). The LFPg was split to EEG range (0.1-300 Hz) and single (SUA), multiple unit activity (MUA) frequency range (300-5000 Hz) by analogue band-pass filtering at the level of a custom made main amplifier (Ulbert et al., J Neurosci Methods, 2001). EEG range signal was sampled at 2 kHz / 16 bit; MUA range was sampled at 20 kHz / 12 bit and stored on a hard drive. Figure 18. Histology and representative continuous traces of all 23 channels of LFPg in SWS from Patient 4. One minute long raw data without additional filtering on the right. Histology slide from the vicinity of the electrode track on the left. Numbers from 1 to 23 on the slide represent the LFPg channels from the surface to the depth of the cortex. Roman numerals represent the cortical layers. 53 Csercsa Richárd SLOW WAVE ACTIVITY DETECTION We have analyzed the LFPg, MUA, SUA and ECoG data acquired from each patient during one to three nocturnal recording sessions. Since the sleep of the patients was fragmented due to medical care, distress from the hospitalization and head wound, we cannot provide standard hypnograms that are usually obtained from healthy subjects without a recent craniotomy. Craniotomies may also distort the scalp distribution of the EEG due to lack of bone and excessive fluid accumulation below the scalp, furthermore scalp electrodes if placed close to the frontal craniotomy wounds may induce infection, this why we avoided placing more than two frontal scalp EEG electrodes. Partial sleep staging was performed based on readings of the available scalp EEG and ECoG electrodes by expert neurologists. In this study, we have analyzed electrophysiological data obtained only from the deepest stage of NREM sleep (N3, or SWS) (Iber et al., 2007). Behavioral sleep was confirmed by the video recording, while SWS was electrographically identified in accordance with the recent AASM guidelines (Iber et al., 2007). SWS periods were identified when 20% or more of an epoch consisted of SWA (waves in the 0.5–2 Hz frequency range with peak-to-peak amplitude larger than 75 µV, measured over the frontal regions) (Iber et al., 2007). Data containing interictal spikes (within 1 min) and seizures (within 60 min) were excluded from the study to avoid epileptic contamination. In addition to spectral (Figure 23A) and autocorrelation analyses (Figure 23B), SWA cycle detection was based on phase and amplitude information, extracted from the narrow-band filtered (0.3-3 Hz, 24 dB/octave, zero phase shift) layer II LFPg (Figure 19A) and ECoG (Figure 19B) data. Instantaneous phase of the filtered signal was calculated by the Hilbert transformation. The discrete-time analytic signal was computed with the help of Hilbert transform, then the instantaneous phase was calculated as the arctangent of the quotient of the imaginary and real part of the analytic signal. In our implementation, a single SWA cycle was defined between -180 ° and +180 ° phase. The -180 ° phase value corresponded to the trough of the negative halfwave (down-state) preceding the 0 ° phase, which corresponded to the peak of the positive half-wave (up-state) and finally the +180 ° phase corresponded to the following 54 Laminar analysis of the slow wave actvity in humans negative half-wave trough (down-state). At each +180 ° crossing the phase was wrapped -360 ° for better visualization. To avoid the detection of higher frequency (e.g.: theta) oscillations, waves with shorter than 500 ms cycle lengths (corresponding to higher than 2 Hz frequency) were excluded from the analysis. Waves with non-monotonic phase runs were also excluded, since phase inversions may also indicate higher frequency contamination. In addition to phase constraints, valid SWA cycles had to fulfil the following amplitude criteria: the up-state peak amplitude had to be more positive than +50 µV and the preceding or following down-state trough amplitude had to be more negative than -50 µV. The SWA detection algorithm parameters were tuned and carefully validated by expert electroencephalographers. Similar algorithmic parameters were used for all of the patients. To facilitate comparison of our results with previous animal studies, the threshold level was set to +50 µV on the filtered (0.3-3 Hz, 24 dB/octave, zero phase shift) upper layer III LFPg, and the wave triggered (up-state locked) averages were calculated on the un-filtered LFPg and MUA (Figure 24). Figure 19. Similarity between LFPg recorded with ME within the cortex and ECoG recorded from macrocontacts subdurally. (A) Upper: ‘raw’ traces of single sweeps containing SWA; broadband (0.1-300 Hz) LFPg from layer II. Middle: ‘filtered’ traces after band pass (0.3-3 Hz) filtering. Lower: ‘phase’ traces showing the instantaneous phase of the ‘filtered’ trace above derived by the Hilbert transform. Grey rectangles indicate automatically detected up- 55 Csercsa Richárd states (surface positive half-waves). Patient 4. (B) Same as (A), but recorded from neighbouring ECoG contacts. (C) Colour map of LFPg and (D) ECoG spectral power during the SWA shown in (A) and (B). X-axis: time, Y-axis: frequency, Z-axis: colour coded relative spectral power in dB, compared to the mean of the entire interval in each frequency band (relative spectrogram). To quantify and compare SWA parameters with other studies, the frequency of SWA occurrence (detected valid cycles per minute), the interdetection interval histogram (Figure 23C) and the cycle length histogram (Figure 23D) were calculated (Massimini et al., J Neurosci, 2004). The single sweep time-frequency content of the SWA signal (Figure 19C-D) was also computed using wavelet transforms (Delorme and Makeig, J Neurosci Methods, 2004). In addition, we attempted to describe the laminar distribution of the SWA in more detail using the LFPg FFT power spectrum depth profile (Figure 27), the pairwise linear coherence between each LFPg trace in the SWA (0.3-3 Hz) frequency range (Figure 28), and the depth profile of the LFPg autocorrelation (Figure 29). CURRENT SOURCE DENSITY ANALYSIS CSD analysis identifies synaptic/trans-membrane generators of LFP in laminated neural structures (Nicholson and Freeman, J Neurophysiol, 1975), (Freeman and Nicholson, J Neurophysiol, 1975). According to Poisson’s equation, ߲ଶΦ ߲ଶΦ ߲ଶΦ ܫ = − ቆߪ௫ ∙ + ߪ௬ ∙ + ߪ௭ ∙ ቇ ߲ ݔଶ ߲ ݕଶ ߲ ݖଶ the current source density equals the negative of the second spatial derivative of the potential. Under the assumptions that cortical activity is constant in planes parallel to the surface, and the extracellular conductivity is homogeneous, this equation reduces to the second spatial derivative of the local field potentials in the z direction (perpendicular to the surface), which we can record with the laminar multielectrode. The result will closely approximate the macroscopic current density over unity cell membrane area. 56 Laminar analysis of the slow wave actvity in humans Since LFPg (Figure 30A) is the first spatial derivative of LFP, one additional spatial derivation resulted in the CSD (Figure 30C) for the EEG range (0.1-300 Hz) data. Inhomogeneous conductivity and electrode spacing were not taken into account (both were substituted by the dimensionless number 1 in the calculations); high spatial frequency noise and boundary effects were reduced by Hamming-window smoothing and interpolation (Ulbert et al., J Neurosci Methods, 2001) thus CSD was expressed in µV units. It was shown previously that our recording and analysis techniques can reliably detect CSD activity in each layer of the human cortex and hippocampus (Ulbert et al., J Neurosci Methods, 2001), (Ulbert et al., Hum Brain Mapp, 2001), (Ulbert et al., Epilepsia, 2004), (Ulbert et al., Exp Neurol, 2004), (Wang et al., J Neurosci, 2005), (Halgren et al., Neuroimage, 2006), (Knake et al., Neuroimage, 2007), (Fabo et al., Brain, 2008), (Cash et al., Science, 2009), (Steinvorth et al., Hippocampus, 2010), (Wittner et al., Brain, 2009). STATISTICAL ANALYSIS OF ELECTROPHYSIOLOGY AND HISTOLOGY ANOVA with Tukey’s HSD (honestly significant difference) test were applied to the normalized values (LFPg and CSD: between -1 and +1; MUA, gamma band LFPg and CSD: between 0 and 1). Normalized values were grouped by layers (I-VI), and the grand average (across all patients) of LFPg, MUA, CSD, gamma band (30-150 Hz) LFPg and gamma band CSD power depth profile at the up-state peak were tested to determine statistically significant differences (p<0.01) between electrophysiological activations in different layers of the cortex (Figure 30E-H). Results are depicted on boxwhisker plots; small box: mean, big box: standard error (SE), whisker: standard deviation (SD). In Patients 4 and 5 (with available histology), the averaged, normalized CR+ cell density (Figure 38A) and averaged, normalized electrophysiology depth profiles (consecutive values at each cortical depth) were constructed. Average depth profiles of LFPg (Figure 38B) and CSD (Figure 38C) at the peak of the up-state were normalized between -1 and +1, while average depth profiles of MUA (Figure 38D) and spectral measures (Figure 38E-F) (gamma band LFPg and CSD) at the peak of the up-state were 57 Csercsa Richárd normalized between 0 and 1. CR+ cell density depth profile was normalized between 1 and 0. SE is marked by whisker in this case. The normalized cell density and electrophysiology measures were compared using the Pearson r correlation method with p<0.01 significance level criterion. SINGLE AND MULTIPLE UNIT ACTIVITY ANALYSIS A continuous estimate of population neuronal firing rate was calculated from the MUA range (300-5000 Hz) data. The signal was further filtered (500-5000 Hz, zero phase shift, 48 dB/octave), rectified and decimated at 2 kHz, applying a 0.5 ms sliding average rectangular window, followed by a final, smoothing low-pass filter (20 Hz, 12 dB/octave) (Figure 30B). Putative single units (Figure 20, Figure 34B) were analyzed by conventional threshold detection and clustering methods using Dataview and Klustawin (Heitler, 2006) and custom made Matlab software. Putative single units from Patients 1, 4 and 5 were included in the analyses, which were recorded stably for at least 600-1000 sec in SWS. In Patient 2, MUA was not recorded for technical reasons, while Patient 3 showed no discriminable single units. After threshold recognition (mean ±3-5 SD) (Csicsvari et al., Neuron, 1998) at a given channel, three representative amplitude values were assigned to each un-clustered spike waveform. These triplets constituted the features by which spikes were categorized into different clusters. They were projected into 3D space, and a CEM (competitive expectation-maximization) based algorithm (Harris et al., J Neurophysiol, 2000) was used for cluster cutting (Heitler, 2006). If the autocorrelogram of the resulting clusters contained spikes within the 2 ms refractory interval, it was re-clustered. If re-clustering did not yield a clean refractory period, the cell was regarded as multiple units and omitted from the single cell analysis. Given the gradient recording, spikes at neighbouring traces appeared as mirror images, thus from adjacent channels (150 µm apart) only one channel (the one that yielded the better signal to noise ratio) was included in the analysis. To reveal double detection, the crosscorrelogram was constructed (Staba et al., Hippocampus, 2002) for next to adjacent (300 µm apart) pairs of putative single cells. No coincident interactions (99% confidence limit at 0 ms (Staba et al., Hippocampus, 2002)) were found. A spike train 58 Laminar analysis of the slow wave actvity in humans was determined as a burst, if at least three consecutive spikes occurred within a maximum 20 ms long interval, which was preceded and followed by at least 20 ms long intervals with neuronal silence (Staba et al., J Neurosci, 2002). Phase dependence of single cell firing rate (Figure 36) was computed for 30° phase bins; the total number of firing in a given bin was divided by the total time that the cortex spent in that phase bin (thus producing a phase histogram). The Rayleigh test (p<0.01) was used to judge if the resulting circular distribution was significantly different from the uniform distribution. We have shown previously that our SUA, MUA recording and analysis techniques can reliably detect task or epilepsy related modulation of neuronal firing from each layer of the human cortex and hippocampus (Ulbert et al., J Neurosci Methods, 2001), (Ulbert et al., Hum Brain Mapp, 2001), (Ulbert et al., Epilepsia, 2004), (Ulbert et al., Exp Neurol, 2004), (Wang et al., J Neurosci, 2005), (Halgren et al., Neuroimage, 2006), (Fabo et al., Brain, 2008), (Cash et al., Science, 2009), (Wittner et al., Brain, 2009). Figure 20. Spike sorting. (A) Upper trace shows band pass filtered (500-5000 Hz, 24 dB/oct) MUA from Pt. 1 with high signal to noise ratio. Besides the prominent single unit activity, some less clearly differentiated action potentials are also evident. Lower left inset (B) shows three well separated unit clusters, with single action potential waveforms (C) from Pt. 4. 59 Csercsa Richárd RESULTS Previous studies of SWA in humans (Achermann and Borbely, Neuroscience, 1997), (Massimini et al., J Neurosci, 2004), (Molle et al., Proc Natl Acad Sci U S A, 2004), (Massimini et al., Science, 2005), (Marshall et al., Nature, 2006), (Massimini et al., Proc Natl Acad Sci U S A, 2007) have been limited to macroelectrode recordings that superimpose activity from several cm2 of cortex. These recordings are ambiguous as to the circuits involved, are not sensitive to neuronal firing, and do not distinguish between excitatory and inhibitory mechanisms. We used laminar multichannel microelectrode array recordings (Ulbert et al., J Neurosci Methods, 2001), (Ulbert et al., Hum Brain Mapp, 2001), (Ulbert et al., Epilepsia, 2004), (Ulbert et al., Exp Neurol, 2004), (Wang et al., J Neurosci, 2005), (Halgren et al., Neuroimage, 2006), (Knake et al., Neuroimage, 2007), (Fabo et al., Brain, 2008), (Cash et al., Science, 2009), (Steinvorth et al., Hippocampus, 2010), (Wittner et al., Brain, 2009) to estimate neuronal firing and synaptic/trans-membrane currents in different cortical layers. Since cortical neuronal populations and synaptic inputs are organized into distinct layers, these recordings allowed us to resolve the cortical generators underlying SWA in humans. SLOW OSCILLATION IN HUMANS I. Human slow wave activity reflects a rhythmic oscillation in the extracellular local field potential: the surface positive half-wave corresponds to the depolarized up-state with increased cell firing and the surface negative half-wave corresponds to the hyperpolarized down-state with neuronal silence. Clinical subdural strip and grid electrodes and MEs were implanted into the frontal and parietal cortices of patients (n=5) with intractable epilepsy (Figure 21) in 60 Laminar analysis of the slow wave actvity in humans order to identify the seizure focus and eloquent cortex prior to surgical therapy. The focus was eventually localized to the frontal lobe in four patients, aand nd to the temporal lobe in one. Figure 21. Grid, strip and multichanne multichannell microelectrode array (ME) locations in all patients. Locations are superimposed on 3D reconstructions of MRIs taken with the electrodes in place, aided by intraoperative navigation and photographs. Grid and strip electrode contacts are depicted in red and d blue colors; the first grid contact is marked with G1. ME locations of Patient 1 (blue): left Brodmann area (BA) 9, Patient 2 (red): right BA 2, Patient 3 (green): left BA 46, Patient 4 (black): right BA 9, Patient 5 (orange): right BA 8 are marked with circles. Grid and superficial ME recordings showed great similarity. LFPg recorded in layer II closely resembled to thee locally recorded ECoG ECoG, with Pearson r>0.9 (p<0.01) <0.01) in all patients (Figure 22). Time-locked locked averages to the peak of the surface positive half-wave half (up-state) state) showed similar LFPg and ECoG waveforms regardless if time locking was based on the LFPg or ECoG ((Figure 27A A red and green traces). Both LFPg and ECoG showed broadband (10-200 200 Hz) spectral increases during up up-states states and decreases during down-states (Figure 19C-D). D). 61 Csercsa Richárd Figure 22. Similarity between SWA recorded simultaneously within the cortex by microcontacts (LFPg) and subdurally with macrocontacts (ECoG). ECoG and LFPg traces during SWS in three patients (Patients 3, 4 and 5). Upper, colour SWA traces mark ECoG from four grid channels (e.g.G14 etc…) surrounding the ME. Black SWA traces below show layer II local field potential gradient (LFPg) from each ME. Overlay in the bottom panel indicates good correspondence between ECoG and LFPg. Positive is depicted upwards on all figures. Positive in potential gradient recordings indicates relative positivity in the more superficial lead. Histology of the ME penetration track was recovered in two patients. It showed intact laminarization (Figure 17A), well preserved interneurons, pyramidal cells and glia (Figure 17B-D), indicating no discernable epilepsy or implantation related damage of the examined cortex. None of the patients had pre-operative pathological MRI findings in the 1-2 cm vicinity of the ME implantation site. From the histology we could determine the layers each channel was recording from (Figure 18). Automatic SWA cycle detection was based on amplitude and phase information using LFPg (Figure 19A) and ECoG (Figure 19B) recorded during SWS. On average, 20 SWA cycles (mean=20 1/min, range=12-26 1/min, SD=7 1/min) were detected per minute (Figure 25, Figure 26). Cycle length peaked on average at 0.8 sec (mean=0.8 sec, range=0.6-1.4 sec, SD=0.3 sec) (Figure 23D). Interdetection interval (Figure 23B) peaked on average at 1.1 sec (mean=1.1 sec, range=0.8-1.2 sec, SD=0.4 sec), all comparable to healthy subjects (Massimini et al., J Neurosci, 2004). LFPg (Figure 23A, C) and ECoG (not shown) FFT power spectrum and autocorrelation (Figure 23A-B, Figure 27G-H) also corresponded well to previous human (Achermann and Borbely, Neuroscience, 1997) and animal (Isomura et al., Neuron, 2006) findings, indicating correct SWA cycle identification and relatively normal SWA production. 62 Laminar analysis of the slow wave actvity in humans Figure 23. Spectral and temporal properties of SWA cycles. (A) Representative examples of FFT power spectrum and (B) autocorrelation of supragranular LFPg in Patients 3 and 5. (C) entative example Wave triggered SWA averages of LFPg and MUA show increased neuronal activity during up-states and decreased firing during down-states, similar to animal models (Figure 24). Figure 24. Wave triggered SWA averages of LFPg and MUA in upper layer III in Patient 4. To facilitate comparison of our results with animal studies, the threshold level was set to +50 µV on the filtered (0.3-3 Hz, 24 dB/octave, zero phase shift) upper layer III LFPg, and the wave triggered (up-state locked) averages were calculated on the un-filtered LFPg (upper trace) and MUA (lower trace). Note, that in our case the threshold crossing was calculated as a triggering point instead of peak detection, this explains why the up-states in our analysis have a less sharply contoured peak as compared to the up-states in cats, where peak-locked averages were calculated. 63 Csercsa Richárd Layer II LFPg (lower row of Figure 25) and the closest neighbour grid ECoG trace (upper row of Figure 25) were used with similar parameters to test the SWA detection algorithm. The average number of SWA cycles was calculated, which clearly showed deepening sleep as the detection frequency for both ECoG and LFPg increases towards the end (Figure 25, first column). For the average SWA cycle length, similar values were found for ECoG and LFPg, with consistent duration across the 600 s epoch (Figure 25, second column). Both the interdetection interval and the cycle length distribution were very similar between LFPg and ECoG, further indicating strong coupling between surface and intracortical potentials (Figure 25, third and fourth column). In addition, the distribution of cycle lengths and interdetection intervals shown here are similar to those found in human scalp recordings during SWS. We also found good temporal coincidence between ECoG and LFPg based detection as reflected in the cross-correlogram between ECoG and LFPg detected up-state time stamps (Figure 25, on the right). Figure 25. Uniformity of SWA cycle detection in ECoG and LFPg data. Representative sample from Patient 3. Layer II LFPg (lower row of figures) and the closest neighbour grid ECoG trace (upper row). Detection frequency (Y-axis) was calculated in cycles/minute as indicated in the first column. Average number of SWA cycles was calculated over a 30 sec interval bin, with 15 sec overlap for a total duration of 600 sec (X-axis). The second column shows the average SWA cycle length (Y-axis) in ms versus elapsed time in seconds (X-axis). Third column illustrates the interdetection interval (Y-axis: counts, X-axis: interval, in 250 ms bins) distribution. Fourth column shows the distribution of cycle lengths (Y-axis: counts, X-axis: duration, in 62.5 ms bins). The fifth column depicts the cross-correlogram between ECoG and LFPg detected up-state time stamps (Y-axis: counts, X-axis: lag between ECoG and LFPg, 50 ms bins). 64 Laminar analysis of the slow wave actvity in humans The uniformity of SWA detection is illustrated on Figure 26. The detection frequency, the interdetection interval, and the cycle lengths were similar in all patients. Figure 26. Representative samples of detection parameters of SWA cycles in Patients 1, 2 and 3. Upper row: SWA detection frequency in a 240 sec interval (divided into 30 bins) during SWS (X-axis: time, Y-axis detected cycles per minute, 1/min). Middle row: histogram of the interdetection intervals (X-axis: time between valid SWA cycle detections, in 166 ms bins, Yaxis: counts). Lower row: cycle length histogram (X-axis: valid cycle lengths, in 33 s bins, Yaxis: counts). SUPRAGRANULAR ACTIVITY II. The increase in wideband (0.3-200 Hz) spectral power, multiunit and single unit activity, and inward transmembrane currents, associated with the up-state; and the decrease in spectral power, unit activity, and outward transmembrane currents, associated with the down-state, are mainly localized to the supragranular layers. 65 Csercsa Richárd To estimate the laminar contribution of various activities, ME channels were assigned into six putative layers (I-VI) based on the histological findings (Figure 17A) when available, and cortical depth when not. This analysis revealed substantial concentration of the 0.3-3 Hz band LFPg FFT power within layers I-III (Figure 27) in each patient, indicating strong supragranular synaptic/trans-membrane activity. The SWA shape similarities between electrode contacts were significantly greater in supragranular versus infragranular layers in each patient, (0.634 versus 0.423, grand average pairwise coherence, Kruskal-Wallis ANOVA, p<0.01) (Figure 28), while autocorrelation profiles revealed a more precisely paced rhythm supragranularly (Figure 29) in each patient. Figure 27. Depth distribution profile of LFPg FFT power spectrum in Patient 3 and 5 (EEG range: 0.1-300Hz data, no additional digital filtering was used, X-axis: frequency, Y-axis: cortical depth, with corresponding layers, Z-axis: colour coded FFT power). Figure 28. Depth distribution profile of pairwise coherence of LFPg channels in different cortical layers in Patient 3 and 5. X-axis: cortical depth, with corresponding layers, Y-axis: cortical depth, with corresponding layers, Z-axis: colour coded pairwise coherence of band pass (0.3-3 Hz) LFPg. 66 Laminar analysis of the slow wave actvity in humans Figure 29. Depth distribution profile of LFPg autocorrelation in Patient 4. X-axis: time, Yaxis: cortical depth, with corresponding layers, Z-axis: colour coded autocorrelation of the LFPg. Several measurements, both in individual patients (Figure 30A-D) and in grand averages (Figure 30E-H), reflecting different aspects of population synaptic/transmembrane and firing activity, were maximal in supragranular at the up-state peak. Normalized, grand average depth profiles of LFPg (Figure 30E, Figure 38B) were marked by maximally positive deflections in layers I-III, inverting in layers V-VI into a small negativity. MUA was also maximal in layer III (Figure 30F, Figure 38D). The CSD depth profile at the peak of the SWA up-state showed a maximal source (outward current) in layer I and maximal sink (inward current) in layers II-III, only very small CSD deflections were observed infragranularly (Figure 30G, Figure 38C). In contrast, the CSD depth profile of a population of interictal spikes detected manually and locked to the surface positive LFPg (similarly as in the case of the up-state), were markedly different, exhibiting a large sink-source pair in the infragranular layers (Figure 37). Significant increases (bootstrap analysis (Delorme and Makeig, J Neurosci Methods, 2004), p<0.01) in LFPg spectral power were detected in all layers at 10-100 Hz frequencies during up-states (Figure 30D). Gamma power of LFPg and CSD was maximal in layer III (Figure 30H, Figure 38E-F). Detailed statistical analysis of normalized grand averages is shown on Table 1. 67 Csercsa Richárd Figure 30. Role of supragranular layers in SWA generation. Representative depth profile map examples from Patient 4 (A-D) and grand averages of all patients (E-H). (A) LFPg, (B) MUA, and (C) CSD depth profile maps. X-axis: time, Y-axis: cortical depth, with corresponding laminarization, Z-axis: colour coded amplitude of LFPg, MUA and CSD units. Positive values are red, negative are blue, except for CSD, where sink is depicted in red and source in blue. (D) LFPg spectrograms from nine representative channels in layers I-VI. X-axis: time, Y-axis: frequency, Z-axis: colour coded averaged relative spectral power in dB. Box-whisker plots of (E) LFPg, (F) MUA, (G) CSD, (H) LFPg (red) and CSD (blue) gamma power (30-150Hz); normalized grand average of all patients at the peak of the up-state in each layer. Mean: small box, standard error (SE): large box, standard deviation (SD): whisker. 68 Laminar analysis of the slow wave actvity in humans UP-STATE ONSET III. Action potentials at up-state onset are synchronized within ±10 ms across all cortical layers, suggesting that any layer could initiate firing. The time courses of MUA were examined to determine if one layer may lead others. It was shown in ferret slices, (Sanchez-Vives and McCormick, Nat Neurosci, 2000) that layer V MUA consistently led layers II-III by an average of over 100 ms. In our study, the up-state associated MUA peak-locked averages indicated no evident timing difference in any of the patients, between layers III and V, regardless of whether peak alignment was based on layer III or layer V activity (Figure 31). Figure 31. Simultaneity of MUA response in supra- and infragranular layers of Patient 4 and Patient 5. MUA from layers III (red trace) and V (blue) are shown when aligned and averaged on the up-state associated MUA peak detected in layer III and in layer V. There is no visible MUA delay between layers III and V regardless of which layer is used for time locking. 69 Csercsa Richárd To further characterize the MUA timing between different layers, it was crosscorrelated (3 SD threshold, 10 ms bin size) between each pair of channels, within 200ms of every up-state onset. In agreement with animal studies (Sakata and Harris, Neuron, 2009), (Chauvette et al., Cereb Cortex, 2010), delay maps and histograms (Figure 32) indicated a short inter-laminar MUA timing difference at up-state onset; most of the delays were within the ±10 ms bin. We also calculated how often (in percentage of all sweeps) any given MUA channel shows the earliest firing at up-state onset. In all patients (where MUA was available), the initial firing was quite uniformly distributed across cortical depths. Unlike in a ferret in vitro study (Sanchez-Vives and McCormick, Nat Neurosci, 2000), we found no evidence for long (~100 ms) lead or lag times between different layers (Figure 32). Figure 32. (A) MUA cross-correlation peak latencies (X-axis versus Y-axis) between each pair of channels in Patient 1. Positive latencies (red) indicate that X channel leads over Y channel, while negative latencies (blue) represent lagging. (B) Histogram of leading and lagging values from (A). (C-D) Percentage of a given MUA channel showing the earliest firing at up-state onset, representative data from Patients 1 and 3. 70 Laminar analysis of the slow wave actvity in humans FREQUENCY OF SLOW OSCILLATION IV. The laminar mechanisms generating slow oscillation are similar within frequency bands 0.6-2 Hz. Separate averages of different SWA cycle lengths corresponding to appropriate (0.6-0.8 Hz, 0.8-1 Hz, 1-1.3 Hz and 1.3-2 Hz) oscillation frequencies also yielded qualitatively similar LFPg, spectral LFPg, MUA and CSD distribution (Figure 33). We have found no statistically significant differences in any layers (ANOVA, Tukey’s HSD post-hoc test, p>0.3) in the CSD or MUA at the peak of the up-state between any of the four frequency bands indicating similar cortical generator mechanisms above (up to 2 Hz) and below 1 Hz (down to 0.6 Hz). Figure 33. Depth profiles at different SWA frequencies. Up-state locked averages of LFPg, LFPg spectrogram, MUA and CSD in Patient 3 and Patient 4 at four different SWA frequencies. Frequencies 1.3-2Hz, correspond to a cycle length of: 500-750 ms; 1-1.3 Hz to 750-1000 ms; 0.8-1 Hz to 1000-1250 ms; and 0.6-0.8 Hz to 1250-1500 ms. Roman numerals mark putative cortical layers. Colour calibrations are on the bottom. CSD sink is depicted in red, source in blue. Each spectrogram (SPC) window shows the spectral content (Z-axis, colour coded) versus time (X-axis) of a representative LFPg channel from a given layer from 1-100 Hz (Y-axis), measures are expressed in dB relative to a distant baseline (-2500 to 1500 ms). 71 Csercsa Richárd SPARSE FIRING V. Neuronal firing in the up up-state state is sparse compared to extracellular llular recordings in animals. Recordings from three patients yielded good quality single unit activity (Figure ( 20, Figure 34, Figure 35). ). Epochs (~1000 sec) showin showing g the largest SWA detection frequencies were selected for analysis from the first sleep cycle. Overall 33 single units were clustered (9, 12 and 12 from Patients 1, 4 and 5) with mean firing rate of 0.66 Hz (range=0.12-2.0 2.0 Hz, SD=0.48) and mean burst frequ frequency ency of 3.1 1/minute (range=0-14 (range=0 1/minute, SD=3.6). Both the average firing rate and the spontaneous burst rate were well below the reported epileptic threshold found in cortical and hippocampal structures (Staba Staba et al., J Neurosci, 2002 2002). Figure 34.. Single unit firing in SWA. (A) Superimposed (40 consecutive sweeps) and (B) individual single sweeps of simultaneously recorded supragranular LFPg and MUA/SUA (Patient 5). Solid and dashed red lines represent LFPg mean and standard deviation. Nearly all cells (31 of 33) showed si significantly non-uniform uniform spiking (Figure ( 34, Figure 35, Figure 36)) over the SWA cycle (Rayleigh test, p<0.01), with peak up-state up firing rate mean of 1.63 Hz (range=0.45 (range=0.45-4.6 4.6 Hz, SD=0.96). We found no significant differences between patients in mean firing rates (Kruskal (Kruskal-Wallis Wallis ANOVA, p>0.2), 72 Laminar analysis of the slow wave actvity in humans indicating homogeneous distribution. Although mean firing rates grouped by supraversus infragranular layers showed no significant differences (p>0.1), supragranular peak up-state firing rates were significantly higher (2.2 Hz versus 1.2 Hz, KruskalWallis ANOVA, p<0.01) than the same measure for infragranular layers. We found the proportion of firing cells and the rate at which they fire in any given up-state (Figure 35, Figure 39) remarkably low. On average, only 27% of the clustered cells were active (firing at least one spike) during any given up-state (20%, 25% and 36% in each patient). Thus, an average neuron fired in every third to fifth up-state. Moreover, on average, each neuron fired only 0.32 spikes per up-state (0.44, 0.2 and 0.32 in each patient). As an example, out of the 12 clustered neurons in Patient 4, the most probable number of active cells in a given up-state was 2 (Figure 39C), and the most probable number of overall spikes the 12 cells fired within a given up-state was also 2 (Figure 39B). Figure 35. Representative single unit firing in SWA. (A) Phase raster plots of two well separated units (cell1, cell2) in SWS. Columns represent individual SWA cycles (1-385), rows represent phase from -180° to +180° (top to bottom) in 30° bin increments. Blue: no firing in the given phase bin, green: one, yellow: two, red: three or more discharges. (B) Phase histograms of action potentials for cell 1 (upper) and 2 (lower), firing rate in Hz versus phase, in 30° bins. 73 Csercsa Richárd Figure 36. Representative normalized (from 0 to 1) firing rate versus phase histograms (from 180° to +180°, in 30° bins) of clustered cells from different layers and patients. Red line: positive half-wave (up-state), green line: negative half-wave (down-state). 74 Laminar analysis of the slow wave actvity in humans DISCUSSION Our results establish a close similarity between the human SWA and the animal SO at the level of field potential, cellular firing activity and spectral measurements (Steriade, Neuroscience, 2006), but also reveal a number of novel, unexpected findings. Consistent with prior studies in animals, we have shown in humans that the up-state was associated with increased firing and elevated spindle, alpha, beta, gamma and ripple power during the surface positive LFP half-wave, while the down-state was characterized by the widespread surface negative LFP half-wave with decreased firing, and oscillatory activity (Cash et al., Science, 2009). Differences from prior studies were found in the laminar distribution of the up-state, average firing rates during the up-state, and the consistency of generators for oscillations above versus below one Hertz. These contrasts could reflect cortical cytoarchitectonic differences or they could be due to the circumstances of the recordings, including natural sleep versus different types of anesthesia, or in vivo versus in vitro preparations. They could also be due to epileptic pathology or to phylogenetic differences. EPILEPSY AND SLOW WAVES Epilepsy is a multi causal disease with diverse etiology. Focal epilepsies have circumscribed seizure initiating regions without wide spread pathological alterations in other areas. Surgical candidates are selected exclusively from this patient group during the careful pre-operative evaluation, based on several diagnostic findings (CT, MRI, fMRI, PET, SPECT, video-EEG, MEG, functional neurophysiological tests, Wada-test and seizure semiology). In the present study we included only patients with the focal disease and carefully avoided to analyze data from the spatial and temporal proximity of the seizure focus and epileptiform events. The laminar LFPg, CSD, MUA and spectral profile of interictal activity in vivo and in vitro has already been established by our group (Ulbert et al., Epilepsia, 2004), 75 Csercsa Richárd (Ulbert et al., Exp Neurol, 2004), (Fabo et al., Brain, 2008), (Wittner et al., Brain, 2009). We have shown that the initial phase of the interictal discharges are large amplitude brief events characterized by substantial action potential, LFPg, CSD, MUA and spectral surges, often emerging from the granular and infragranular layers of the cortex. These events are clearly distinctive from the background activity and exquisitely visible in single sweeps (Figure 37). Based on our prior knowledge we carefully excluded any suspicious pathological events from the analysis presented in here. Figure 37. CSD depth profile of the up-state and a population of interictal spikes in Patient 4. Boxes indicate the mean normalized (between +1 and -1) CSD in layers I-VI, whiskers indicate the standard error. Up-state depth profile is depicted in blue, interictal spike depth profile in red. Interictal spikes were detected manually, such a way that the peak of the surface positivity was designated as time zero, such as with the up-states. While up-states exhibit a major sink-source pair in the superficial layers, the calculated population of interictal spikes exhibit a large sink-source pair in the deep layers. Several other considerations suggest that the current findings on the neuronal mechanisms underlying SWA, although recorded in epileptic patients, might also apply to healthy subjects. Our SWA morphology corresponded well to those collected from standard scalp EEG recordings from healthy subjects. Similarities included not only the SWA frequency and rhythmicity (Achermann and Borbely, Neuroscience, 1997), but 76 Laminar analysis of the slow wave actvity in humans the asymmetric shape, the briefer and sharper deflection in the down-state (Massimini et al., J Neurosci, 2004) and higher beta power content in the up-state (Molle et al., J Neurosci, 2002). Our results of detection frequency, cycle length and inter-detection interval histograms are all comparable with previous findings from healthy subjects (Massimini et al., J Neurosci, 2004), despite that both the recording and analysis methodology were different. Minor deviations in the exact numbers are therefore natural and may reflect methodological differences rather than disease related alterations. In addition, neither the firing rate, nor the burst rate exceeded the pathological criteria found for single neurons in SWS (Staba et al., J Neurosci, 2002), (Staba et al., Hippocampus, 2002). Finally, the lack of any MRI abnormalities and intact laminarization of the excised tissue strongly suggests that we recorded from structurally intact regions, free of gross functional alterations. Nevertheless, there are some observations in our study that may be related to the pathology. Out of the 33 clustered units in 3 patients, 2 single cells in one patient (Patient 1) showed uniform firing during the SWA cycle (layer III unit #3, average firing rate=1.17 Hz, layer V unit #6, average firing rate=0.54 Hz) . While it was expected due to biological (short up-states in a long down-state) and/or methodological reasons (inaccuracy of the state detection algorithm) that some of the detected downstates may contain firing activity, it was unexpected that cells did not show significant modulation during the two-state oscillation at all. We are not aware of similar effects in humans or in animal models. This finding may reflect an excessive resistance of a small subgroup of cortical neurons to the network-wide hyperpolarisation occurring in the down-state and needs further investigation. SUPRAGRANULAR ACTIVITY IN SWS A consistent CSD pattern of our study was the prominent sink-source pair in the supragranular layers compared to the weak infragranular activation. This localization was true for both the low (0.5-2 Hz) and the high frequency (10-200 Hz) oscillations in all patients, in frontal and parietal areas. 77 Csercsa Richárd In our interpretation, the prominent layer II-III sink during the up-state reflects the large active inward currents flowing across the distal dendritic membrane compartments of layer V-VI pyramidal cells and distal, proximal and basal dendritic membrane compartments and perhaps on the somatic membrane of layer II-III pyramidal cells. The corresponding passive, return, source currents are flowing in layer I, across the most superficial apical dendritic membrane compartments of the pyramidal cells. The spatial CSD pattern in the down-state is inverted, exhibiting a large active current source in layer II-III due to hyperpolarizing currents (likely outward potassium flows from the pyramidal cells) and a passive return sink in layer I (Cash et al., Science, 2009). A I II III IV V VI 0.766085 0.000124 0.000124 0.000124 0.000124 0.001666 0.000124 0.000124 0.000124 0.688070 0.000124 0.000124 0.000124 0.949348 0.957987 I II III IV V VI 0.766085 0.000124 0.000124 0.000124 0.000124 0.000124 0.000124 0.000124 0.000124 0.001666 0.000124 0.000124 0.688070 0.949348 0.957987 B I II III IV V I II III IV V VI C I II III IV V VI 0.000125 0.000125 0.000125 0.000125 0.000125 0.000125 I 0.999849 0.000126 0.000125 0.000125 II 0.085258 0.085258 0.000143 0.095084 0.002729 0.341979 0.435127 1.000000 0.971067 0.786468 0.000125 0.999849 0.000125 0.000125 0.000125 III 0.000143 0.435127 0.400887 0.778203 0.000478 78 0.000125 0.000126 0.000125 0.607915 0.180558 IV 0.095084 1.000000 0.400887 0.961539 0.816120 0.000125 0.000125 0.000125 0.607915 VI 0.000125 0.000125 0.000125 0.180558 0.927437 0.927437 V 0.002729 0.971067 0.778203 0.961539 0.090742 VI 0.341979 0.786468 0.000478 0.816120 0.090742 Laminar analysis of the slow wave actvity in humans D I II III IV V VI E I II III IV V VI I II 0.023491 0.023491 0.000135 0.079675 0.988682 0.879812 I 0.701007 0.972408 0.023824 0.000159 II 0.012708 0.012708 0.000226 0.432125 0.999551 0.965169 0.980461 0.424561 0.004422 0.000248 III 0.000135 0.701007 0.105680 0.000124 0.000123 IV 0.079675 0.972408 0.105680 0.087117 0.000210 III 0.000226 0.980461 0.026647 0.000126 0.000125 IV 0.432125 0.424561 0.026647 0.376114 0.027556 V 0.988682 0.023824 0.000124 0.087117 VI 0.879812 0.000159 0.000123 0.000210 0.185144 0.185144 V 0.999551 0.004422 0.000126 0.376114 VI 0.965169 0.000248 0.000125 0.027556 0.697058 0.697058 Table 1. Detailed statistical tables of (A) LFPg, (B) CSD, (C) MUA, (E) LFPg gamma power (30-150Hz) and (F) CSD gamma power. Normalized grand average of all patients at the peak of the up-state, grouped by layers I-VI. Significant differences (ANOVA with Tukey HSD test, p<0.01) are depicted in red. Our CSD findings from the frontal and parietal areas in natural sleep are in contrast with a study in the cat suprasylvian area, albeit under ketamine/xylazine anesthesia (Steriade and Amzica, Proc Natl Acad Sci U S A, 1996). At low frequencies, (~1 Hz) the maximal up-state related sink in the cat was located in the middle rather than in the superficial layers, surrounded by not only a superficial but also a large deep source. In addition, a substantial up-state related sink in the deepest layer was present in the cat, which was practically invisible in our human recordings. The same authors also showed a massive supragranular (layer II-III) up-state related sink besides one or two deeper and weaker sinks, during the spontaneous and evoked K-complex (Amzica and Steriade, Neuroscience, 1998). Moreover, at higher frequencies, (~35 Hz) and during the K-complex, a series of “alternating microsinks and microsources” was found throughout the depth of the cat suprasylvian area (Steriade and Amzica, Proc Natl Acad Sci U S A, 1996), (Amzica and Steriade, Neuroscience, 1998). Such alternating patterns are best explained by insufficient spatial resolution in the LFP sampling (8 contact, 250 µm spacing electrode array) and corresponding spatial aliasing error, and not by neuronal sources (Tenke et al., Exp Brain Res, 1993). 79 Csercsa Richárd A recent study in the cat suprasylvian area in natural sleep with adequate spatial sampling (100 µm) revealed alternation free middle and deep layer sinks and a superficial source during the up-state (Chauvette et al., Cereb Cortex, 2010). Thus, besides cytoarchitectonic differences between different types of cortices (e.g.: frontal and parietal areas in humans versus suprasylvian area in the cat), and methodological errors, it is also plausible to assume that neuronal mechanisms of natural sleep and ketamine/xylazine anesthesia may be different, further accounting for the divergent findings. Another recent study on the rat primary auditory cortex using high spatial resolution (50 µm) CSD mapping showed the maximal up-state related sink to be in the presumed supragranular layers under urethane anesthesia, while in natural sleep it is rather the presumed granular and probably infragranular layers that exhibited the largest up-state related sink (see average data, Supplementary Figures S10 and S16 in (Sakata and Harris, Neuron, 2009)). Our CSD results in natural sleep are quite close to the results of Sakata and Harris obtained under urethane anesthesia, except for the large source deep in the infragranular layers. Some differences between humans and cats or rats might also be expected given that our prefrontal cortex is more than hundred times larger, with a striking increase in pyramidal cell dendritic complexity (Elston, Cereb Cortex, 2003), (Elston et al., Anat Rec A Discov Mol Cell Evol Biol, 2006). Our finding that the SWA and corresponding high frequency rhythms including spindle, alpha, beta, gamma and ripple oscillations may involve supragranular layers is consistent with this massive cortical expansion of neuronal number (Herculano-Houzel et al., Proc Natl Acad Sci U S A, 2007), inasmuch as increased dendritic complexity and cortico-cortical association fibres predominantly target these layers (Gonzalez-Burgos et al., Cereb Cortex, 2000). The strong supragranular oscillatory activity in sleep may be beneficial for the local, higher order processing of sensory experience and perhaps memory consolidation, since these layers are interconnected by dense cortico-cortical projections forming finescale functional networks to perform integrative functions (Yoshimura et al., Nature, 2005). The weaker infragranular activity may reflect the relatively suppressed cortical executive, output functions, which may prevent effective connectivity between distant cortical areas from developing in SWS (Massimini et al., Science, 2005). 80 Laminar analysis of the slow wave actvity in humans FREQUENCY OF THE SLOW WAVES According to our observations, the SWA depth profile, represented by the upstate peaks, was similar between the four investigated frequency ranges including the slow (<1 Hz) and delta band (up to 2 Hz). In our opinion, these frequency bands are thus substantially overlapping, hence a less strict distinction should be applied between activities above versus below 1 Hz. We agree that the slow activity and thalamic delta have obviously different neuronal mechanisms, but it seems that these waves cannot be distinguished using exclusively a frequency band criterion. We have found similar signs of elementary hierarchical organization of low and high frequency oscillations in humans as it was shown in animal models (Lakatos et al., J Neurophysiol, 2005). The organizing substrate was the up-state of the SWA, which gave rise to a wide variety of higher frequency activity including spindle, alpha, beta, gamma and ripple oscillations. Each of these high frequency oscillatory bursts was quite different from sweep to sweep, showing for example occasional spindle sequences or marked gamma or ripple band enhancements at various peak frequencies. These observations suggest that each SWA cycle with unique oscillatory signature reflect individual information content coded differently in the oscillatory process. Given the variability of the high frequency oscillatory activity during up-states, it is plausible to assume that different underlying neuronal populations might be responsible for the generation of each specific oscillatory pattern. This strategy might be beneficial in the configuration of functional connectivity between neurons to form stable ensembles that may promote the consolidation of memory in sleep. Paroxysmal activity is known to emerge more often from NREM sleep compared to REM (Steriade and Timofeev, Neuron, 2003). Animal studies revealed that cortical hyperexcitability associated with ripple oscillations are often result in pathological synchronization leading to epileptic seizures (Grenier et al., J Neurophysiol, 2003). We have shown that up-states are characterized by a large increase in cortical excitability reflected in the increased power of gamma and ripple oscillations. Thus, we hypothesize that the active state of the slow oscillation may play 81 Csercsa Richárd an important role in the generation of seizures and other paroxysmal signs in the cortical epileptic network. ROLE OF CALRETININ POSITIVE NEURONS Calretinin positive (CR+) cell density depth profiles (Figure 38A) were calculated in two patients and correlated with the depth profile at up-state peak of LFPg, CSD, MUA, LFPg and CSD gamma power (Figure 38B-F). CR+ cell density between Patient 4 and 5 showed high similarity (r=0.95, p<0.01). The highest positive correlation was found between CR+ cell density and CSD gamma power (r=0.85, p<0.01). Figure 38. Depth profiles of CR+ cell density and SWA. (A) Averaged normalized CR+ cell density profile of Patients 4 and 5, whiskers represent standard errors. (B) LFPg, (C) CSD, (D) MUA, (E) LFPg gamma power and (F) CSD gamma power of averaged normalized depth profile of up-state in Patients 4 and 5 with standard error (whisker). Number in the upper right corner indicates the Pearson r correlation between CR+ density and (B-F). The relatively high correlation in laminar location between LFPg, CSD, MUA and gamma power during up-states and CR+ cell density may provide additional insights into the mechanism for the predominance of oscillatory activity in supragranular layers. CR+ cells are relatively numerous for inhibitory cells, comprising ~8% of the total number of prefrontal neurons, and ~14.2-17.6% of layer II-III neurons, 82 Laminar analysis of the slow wave actvity in humans in human prefrontal cortex (Gabbott et al., J Comp Neurol, 1997). CR+ cell density in the human, monkey, cat, and rat cortex is highest in the supragranular layers (Fonseca and Soriano, Brain Res, 1995), (Gabbott et al., J Comp Neurol, 1997), (Schwark and Li, Brain Res Bull, 2000). The layer II-III population of CR+ cells (78% of all CR+ cells) is significantly more numerous (+31%) in humans than in the rat (Gabbott et al., J Comp Neurol, 1997), and the supragranular CR+ predominance in humans is not affected by epilepsy (Barinka et al., Epilepsy Res, 2010). The relatively high density and remarkable vertically oriented dendritic alignment (Gabbott et al., J Comp Neurol, 1997) of layer II-III CR+ cells (Figure 17B) suggest that this population on its own may contribute significantly to the CSD. Unlike basket cells that target principal cells (Somogyi et al., Neuroscience, 1983) establishing local negative-feedback circuits, layer II-III CR+ interneurons preferentially target other inhibitory cells locally in layer II-III and target pyramidal cells in layer V (Meskenaite, J Comp Neurol, 1997) forming local positive-feedback circuits (Dantzker and Callaway, Nat Neurosci, 2000) in the supragranular layers and negative-feedback circuits between supra- and infragranular layers. The negative-feedback imposed by the population of CR+ cells may relatively attenuate the infragranular activation, while the positive-feedback disinhibition (Tamas et al., J Neurosci, 1998) may amplify the supragranular synaptic/trans-membrane oscillations in the gamma band and cellular activity (Whittington et al., Nature, 1995). In addition, GABAB receptors are more concentrated in the upper layers of the cortex, at least in rodents (Lopez-Bendito et al., Eur J Neurosci, 2002), (Tamas et al., Science, 2003), which might also contribute to the potassium current that may play an important role in the down-state generation (Timofeev et al., Proc Natl Acad Sci U S A, 2001). FIRING RATE IN SWS In vitro slice studies in animals found that firing in infragranular layers consistently lead supragranular layers by over 100 ms at the onset of the up-state (Sanchez-Vives and McCormick, Nat Neurosci, 2000). In contrast, we found that the onset of activity during up-states differs less than ±10 ms between layers. Although the 83 Csercsa Richárd slice preparation is a powerful tool, it severs connections that are present in the intact animal, removing background synaptic input and placing the cell in an artificial medium. In healthy humans, it is believed that each individual slow wave cycle has distinct origin and propagates uniquely across a number of brain areas (Murphy et al., Proc Natl Acad Sci U S A, 2009). Similar patterns were also found in animal models (Ferezou et al., Neuron, 2007), (Mohajerani et al., J Neurosci, 2010). Thus, variable projections may be involved in its propagation, terminating in variable cortical layers making the laminar distribution of the initial unit firing also variable, as it was shown in our study. Figure 39. Single unit data from Patients 1, 4 and 5 indicating sparse firing in up-states. (A) Colour raster plot illustrates the firing of clustered neurons in up-states. Columns represent individual SWA cycles (221 in Patient 1, 252 in Patient 4, and 222 in Patient 5), rows represent clustered neurons (cell 1-7 in Patient 1, cell 1-12 in Patient 4 and 5), colour represents the firing of a given cell. Blue: no firing in the given up-state for the given cell, green: one, yellow: two, red: three or more action potentials. (B) Histogram of the total number of fired action potentials (spike number count) from all clustered cells during a given up-state (X-axis: total number of action potentials fired by all cells, Y-axis: number of upstates with that number of action potentials, count). (C) Histogram of the number of active clustered cells (active cell count) during the up-states (X-axis: number of cells that fired at least one action potential, Y-axis: number of up-states, count). These data illustrate sparse firing in up-states, only a small fraction of the clustered cells fire and these cells together generate only a few action potentials. 84 Laminar analysis of the slow wave actvity in humans The low average firing rate (0.6 Hz) found in here is consistent with a prior human report (Ravagnati et al., Sleep, 1979). Animal studies using extracellular silicon probes and intracellular sharp electrodes report average firing rates in the 2-20 Hz range from the entire depth of the cortex (Steriade et al., J Neurophysiol, 2001), (Isomura et al., Neuron, 2006), (Luczak et al., Proc Natl Acad Sci U S A, 2007), cell-attached and whole-cell patch-clamp studies from layer II-III neurons report average firing rates in the 0.01-0.3 Hz range (Margrie et al., Pflugers Arch, 2002), (Waters and Helmchen, J Neurosci, 2006), (Hromadka et al., PLoS Biol, 2008). Some of these differences may be related to the laminar location of the neurons and to ‘collateral damage’ inherent to the different techniques. Extracellular recordings might be biased towards higher average firing rates, because of the use of a minimum spontaneous firing rate (1-2 Hz) constraint (Luczak et al., Proc Natl Acad Sci U S A, 2007). We did not use such correction in our unit analysis, thus slower firing cells were also included. Sharp electrode intracellular recordings (Steriade et al., J Neurophysiol, 2001), (Isomura et al., Neuron, 2006) disrupt the cell membrane and introduce leakage current, which may also alter the firing rate. In cell-attached recordings (Margrie et al., Pflugers Arch, 2002), (Hromadka et al., PLoS Biol, 2008) the membrane is partially covered by the recording pipette causing substantial mechanical stress, receptor, ion channel masking and membrane capacitance changes, while the whole-cell configuration (Waters and Helmchen, J Neurosci, 2006) disrupts the membrane and causes cell dialysis. Indeed, when establishing the wholecell configuration the spontaneous firing rate may double compared to the cell-attached state (Margrie et al., Pflugers Arch, 2002). Given these apparently strong effects of recording methodology on cell firing, it is hard to definitively relate the present findings to animal experiments. However, it seems reasonable to expect that any technique which physically contacts the cell would alter the firing rate to a greater extent than techniques which do not. To further elucidate these differences, unbiased extracellular action potential techniques need to be implemented in different animal models. The apparently lower firing rates of human cortical neurons during up-states may also reflect an adaptation to prevent runaway excitation of our larger and more densely interconnected neocortex, or it may better support the sparse representation of our extensive long term memories (Quiroga et al., Nature, 2005). 85 Csercsa Richárd Finally we conclude, that it is extremely important to further characterize the above discussed influences and establish an integrated view that takes into account that the neuronal mechanisms underlying slow wave generation may be depending on several parameters including for example the neurological condition, the observed cortical areas, the experimental preparation, the type of sleep induction and perhaps species differences. 86 Laminar analysis of the slow wave actvity in humans SUMMARY The cortical slow wave activity emerging during the deepest stages of non-rapid eye movement sleep is thought to underlie essential restorative processes and facilitate the consolidation of declarative memories. In animals the slow oscillation consists of two rhythmically recurring phases: one of them is characterized by widespread, increased cellular and synaptic activity, referred to as active- or up-state, followed by cellular and synaptic inactivation, referred as silent- or down-state. However, its neural mechanisms in humans are poorly understood since the traditional intracellular techniques used in animals are inappropriate for investigating the cellular and synaptic/trans-membrane events in humans. For the examination of the neuronal properties of the sleep slow oscillation, we recorded intracortical laminar local field potential gradient, multiple and single unit activities with laminar multichannel microelectrodes and simultaneous surface potentials with subdural grid electrodes chronically implanted into the cortex of patients with drug resistant focal epilepsy undergoing cortical mapping for seizure focus localization. We also analyzed the current source density and spectral features of the recorded signals. We found that slow wave activity in humans reflects a rhythmic oscillation (0.6 – 2 Hz) between neuronal activation and silence. Similar to animal studies, cortical activation was demonstrated as increased wideband (0.3-200 Hz) spectral power, increased multiple and single unit activity, and powerful inward transmembrane currents, all mainly localized to the supragranular layers. Neuronal firing was sparse and the average discharge rate of single cells was less than expected from animal studies. The latency of firing at up-state onset across all layers was in the range of 10 ms, suggesting close inter-laminar coupling at up-state onset. Here we provide strong direct experimental evidences that slow wave activity in humans is characterized by hyperpolarizing currents associated with suppressed cell firing, alternating with high levels of oscillatory synaptic activity associated with increased cell firing. Our results emphasize the major involvement of supragranular layers in the genesis of slow wave activity. 87 Csercsa Richárd BIBLIOGRAPHY Achermann, P. and Borbely, A.A. (1997) Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience, 81, 213-222. Amzica, F. and Steriade, M. (1995) Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. J Neurosci, 15, 4658-4677. Amzica, F. and Steriade, M. (1998) Cellular substrates and laminar profile of sleep Kcomplex. Neuroscience, 82, 671-686. Andrews-Hanna, J.R., Reidler, J.S., Sepulcre, J., Poulin, R., Buckner, R.L. (2010) Functional-anatomic fractionation of the brain's default network. Neuron, 65, 550-562. Aserinsky, E. and Kleitman, N. (1953) Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science, 118, 273-274. Atkinson, R.C. and Shiffrin, R.M. (1968) Human memory: a proposed system and its control processes. In Spence, K.W. (ed) The psychology of learning and motivation: II. Academic Press, Oxford. Axmacher, N., Elger, C.E., Fell, J. (2008) Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain, 131, 1806-1817. Barinka, F., Druga, R., Marusic, P., Krsek, P., Zamecnik, J. (2010) Calretinin immunoreactivity in focal cortical dysplasias and in non-malformed epileptic cortex. Epilepsy Res, 88, 76-86. Bazhenov, M., Timofeev, I., Steriade, M., Sejnowski, T.J. (2002) Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J Neurosci, 22, 8691-8704. Blake, H. and Gerard, R. (1937) Brain potentials during sleep. Am J Physiol, 119, 692703. Bódizs, R. (2003) Az alvás és jelenségköre. In Pléh, C., Kovács, G., Gulyás, B. (eds) Kognitív idegtudomány. Osiris Kiadó, Budapest, pp. 601-618. 88 Laminar analysis of the slow wave actvity in humans Borbely, A.A. (1982) A two process model of sleep regulation. Hum Neurobiol, 1, 195204. Born, J., Rasch, B., Gais, S. (2006) Sleep to remember. Neuroscientist, 12, 410-424. Bremer, F. (1936) Nouvelles recherches sur le mécanisme du sommeil. C R Soc Biol, 460-464. Buzsaki, G. (1989) Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience, 31, 551-570. Buzsaki, G. (1998) Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res, 7 Suppl 1, 17-23. Buzsáki, G. (2006) Rhythms of the brain. Oxford University Press. Campbell, I.G. and Feinberg, I. (2009) Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A, 106, 5177-5180. Cash, S.S., Halgren, E., Dehghani, N., Rossetti, A.O., Thesen, T., Wang, C., Devinsky, O., Kuzniecky, R., Doyle, W., Madsen, J.R., Bromfield, E., Eross, L., Halasz, P., Karmos, G., Csercsa, R., Wittner, L., Ulbert, I. (2009) The human K-complex represents an isolated cortical down-state. Science, 324, 1084-1087. Chalupa, L.M. and Werner, J.S. (2001) The Visual Neurosciences. The MIT Press. Chauvette, S., Volgushev, M., Timofeev, I. (2010) Origin of Active States in Local Neocortical Networks during Slow Sleep Oscillation. Cereb Cortex. Chemelli, R.M., Willie, J.T., Sinton, C.M., Elmquist, J.K., Scammell, T., Lee, C., Richardson, J.A., Williams, S.C., Xiong, Y., Kisanuki, Y., Fitch, T.E., Nakazato, M., Hammer, R.E., Saper, C.B., Yanagisawa, M. (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell, 98, 437-451. Chrobak, J.J. and Buzsaki, G. (1996) High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci, 16, 3056-3066. 89 Csercsa Richárd Cirelli, C. and Tononi, G. (2008) Is sleep essential? PLoS Biol, 6, e216. Clemens, Z., Fabo, D., Halasz, P. (2005) Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience, 132, 529-535. Clemens, Z., Molle, M., Eross, L., Barsi, P., Halasz, P., Born, J. (2007) Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain, 130, 2868-2878. Cohen, N.J. and Squire, L.R. (1980) Preserved learning and retention of patternanalyzing skill in amnesia: dissociation of knowing how and knowing that. Science, 210, 207-210. Colrain, I.M. (2005) The K-complex: a 7-decade history. Sleep, 28, 255-273. Compte, A., Sanchez-Vives, M.V., McCormick, D.A., Wang, X.J. (2003) Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol, 89, 2707-2725. Contreras, D. and Steriade, M. (1995) Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci, 15, 604-622. Contreras, D., Timofeev, I., Steriade, M. (1996) Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol, 494 ( Pt 1), 251-264. Crunelli, V. and Hughes, S.W. (2010) The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci, 13, 9-17. Csicsvari, J., Hirase, H., Czurko, A., Buzsaki, G. (1998) Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron, 21, 179-189. Daan, S., Barnes, B.M., Strijkstra, A.M. (1991) Warming up for sleep? Ground squirrels sleep during arousals from hibernation. Neurosci Lett, 128, 265-268. Dang-Vu, T.T., Schabus, M., Desseilles, M., Albouy, G., Boly, M., Darsaud, A., Gais, S., Rauchs, G., Sterpenich, V., Vandewalle, G., Carrier, J., Moonen, G., Balteau, E., Degueldre, C., Luxen, A., Phillips, C., Maquet, P. (2008) Spontaneous neural 90 Laminar analysis of the slow wave actvity in humans activity during human slow wave sleep. Proc Natl Acad Sci U S A, 105, 1516015165. Dantzker, J.L. and Callaway, E.M. (2000) Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci, 3, 701-707. Dayan, P. and Abbott, L.F. (2001) Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. The MIT Press, Cambridge, Massachusetts. De Gennaro, L., Marzano, C., Fratello, F., Moroni, F., Pellicciari, M.C., Ferlazzo, F., Costa, S., Couyoumdjian, A., Curcio, G., Sforza, E., Malafosse, A., Finelli, L.A., Pasqualetti, P., Ferrara, M., Bertini, M., Rossini, P.M. (2008) The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol, 64, 455-460. Delorme, A. and Makeig, S. (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods, 134, 9-21. Destexhe, A., Contreras, D., Steriade, M. (1999) Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci, 19, 4595-4608. Destexhe, A., Rudolph, M., Pare, D. (2003) The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci, 4, 739-751. Destexhe, A., Hughes, S.W., Rudolph, M., Crunelli, V. (2007) Are corticothalamic 'up' states fragments of wakefulness? Trends Neurosci, 30, 334-342. Détári, L. (2008) Biológiai ritmusok, Lecture notes. Diekelmann, S., Wilhelm, I., Born, J. (2009) The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev, 13, 309-321. Diekelmann, S. and Born, J. (2010) The memory function of sleep. Nat Rev Neurosci, 11, 114-126. Donlea, J.M., Ramanan, N., Shaw, P.J. (2009) Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science, 324, 105-108. 91 Csercsa Richárd Elston, G.N. (2003) Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex, 13, 1124-1138. Elston, G.N., Benavides-Piccione, R., Elston, A., Zietsch, B., Defelipe, J., Manger, P., Casagrande, V., Kaas, J.H. (2006) Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol, 288, 26-35. Entz, L., Fabo, D., Eross, L., Halasz, P., Wittner, L., Karmos, G., Ulbert, I. (2008) Cortical electrical stimulation induced slow oscillation in different vigilance states in humans. FENS Forum. Geneva, Switzerland. Entz, L., Fabo, D., Eross, L., Halasz, P., Wittner, L., Csercsa, R., Dombovari, B., Karmos, G., Ulbert, I. (2009) Detection of slow oscillation elicited by cortical electrical stimulation in different vigilance states in humans. Society for Neuroscience. Chicago, USA. Eriksson, K.S., Sergeeva, O., Brown, R.E., Haas, H.L. (2001) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci, 21, 9273-9279. Euston, D.R., Tatsuno, M., McNaughton, B.L. (2007) Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science, 318, 1147-1150. Fabo, D., Magloczky, Z., Wittner, L., Pek, A., Eross, L., Czirjak, S., Vajda, J., Solyom, A., Rasonyi, G., Szucs, A., Kelemen, A., Juhos, V., Grand, L., Dombovari, B., Halasz, P., Freund, T.F., Halgren, E., Karmos, G., Ulbert, I. (2008) Properties of in vivo interictal spike generation in the human subiculum. Brain, 131, 485-499. Faraguna, U., Vyazovskiy, V.V., Nelson, A.B., Tononi, G., Cirelli, C. (2008) A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci, 28, 4088-4095. Ferezou, I., Haiss, F., Gentet, L.J., Aronoff, R., Weber, B., Petersen, C.C. (2007) Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron, 56, 907-923. Fonseca, M. and Soriano, E. (1995) Calretinin-immunoreactive neurons in the normal human temporal cortex and in Alzheimer's disease. Brain Res, 691, 83-91. 92 Laminar analysis of the slow wave actvity in humans Fort, P., Bassetti, C.L., Luppi, P.H. (2009) Alternating vigilance states: new insights regarding neuronal networks and mechanisms. Eur J Neurosci, 29, 1741-1753. Frank, M.G. and Benington, J.H. (2006) The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist, 12, 477-488. Freeman, J.A. and Nicholson, C. (1975) Experimental optimization of current sourcedensity technique for anuran cerebellum. J Neurophysiol, 38, 369-382. Gabbott, P.L., Jays, P.R., Bacon, S.J. (1997) Calretinin neurons in human medial prefrontal cortex (areas 24a,b,c, 32', and 25). J Comp Neurol, 381, 389-410. Gais, S., Plihal, W., Wagner, U., Born, J. (2000) Early sleep triggers memory for early visual discrimination skills. Nat Neurosci, 3, 1335-1339. Gais, S., Molle, M., Helms, K., Born, J. (2002) Learning-dependent increases in sleep spindle density. J Neurosci, 22, 6830-6834. Gao, L., Meng, X., Ye, C., Zhang, H., Liu, C., Dan, Y., Poo, M.M., He, J., Zhang, X. (2009) Entrainment of slow oscillations of auditory thalamic neurons by repetitive sound stimuli. J Neurosci, 29, 6013-6021. Gilestro, G.F., Tononi, G., Cirelli, C. (2009) Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science, 324, 109-112. Girardeau, G., Benchenane, K., Wiener, S.I., Buzsaki, G., Zugaro, M.B. (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci, 12, 1222-1223. Gonzalez-Burgos, G., Barrionuevo, G., Lewis, D.A. (2000) Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex, 10, 82-92. Greicius, M.D., Krasnow, B., Reiss, A.L., Menon, V. (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A, 100, 253-258. Grenier, F., Timofeev, I., Steriade, M. (2003) Neocortical very fast oscillations (ripples, 80-200 Hz) during seizures: intracellular correlates. J Neurophysiol, 89, 841852. 93 Csercsa Richárd Hahn, T.T., Sakmann, B., Mehta, M.R. (2006) Phase-locking of hippocampal interneurons' membrane potential to neocortical up-down states. Nat Neurosci, 9, 1359-1361. Halasz, P. (2005) K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev, 9, 391-412. Halgren, E., Wang, C., Schomer, D.L., Knake, S., Marinkovic, K., Wu, J., Ulbert, I. (2006) Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage, 30, 1401-1413. Harris, K.D., Henze, D.A., Csicsvari, J., Hirase, H., Buzsaki, G. (2000) Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol, 84, 401-414. He, J. (2003) Slow oscillation in non-lemniscal auditory thalamus. J Neurosci, 23, 8281-8290. Hebb, D.O. (1949) Organization of Behavior: a Neuropsychological Theory. John Wiley & Sons. Heitler, J.W. (2006) DataView v5: software for the display and analysis of digital signals in neurophysiology. Herculano-Houzel, S., Collins, C.E., Wong, P., Kaas, J.H. (2007) Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A, 104, 3562-3567. Hill, S. and Tononi, G. (2005) Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol, 93, 1671-1698. Hoffman, K.L. and McNaughton, B.L. (2002) Coordinated reactivation of distributed memory traces in primate neocortex. Science, 297, 2070-2073. Hromadka, T., Deweese, M.R., Zador, A.M. (2008) Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol, 6, e16. Huber, R., Ghilardi, M.F., Massimini, M., Tononi, G. (2004) Local sleep and learning. Nature, 430, 78-81. 94 Laminar analysis of the slow wave actvity in humans Huber, R., Ghilardi, M.F., Massimini, M., Ferrarelli, F., Riedner, B.A., Peterson, M.J., Tononi, G. (2006) Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci, 9, 1169-1176. Iber, C., Ancoli-Israel, S., Chesson, A., Quan, S.F. (2007) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine, Westchester. Isomura, Y., Sirota, A., Ozen, S., Montgomery, S., Mizuseki, K., Henze, D.A., Buzsaki, G. (2006) Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron, 52, 871-882. Jenkins, J.G. and Dallenbach, K.M. (1924) Obliviscence during sleep and waking. American Journal of Psychology, 35, 605-612. Ji, D. and Wilson, M.A. (2007) Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci, 10, 100-107. Kali, S. and Dayan, P. (2004) Off-line replay maintains declarative memories in a model of hippocampal-neocortical interactions. Nat Neurosci, 7, 286-294. Kattler, H., Dijk, D.J., Borbely, A.A. (1994) Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res, 3, 159-164. Keller, C.J., Cash, S.S., Narayanan, S., Wang, C., Kuzniecky, R., Carlson, C., Devinsky, O., Thesen, T., Doyle, W., Sassaroli, A., Boas, D.A., Ulbert, I., Halgren, E. (2009) Intracranial microprobe for evaluating neuro-hemodynamic coupling in unanesthetized human neocortex. J Neurosci Methods, 179, 208218. Knake, S., Wang, C.M., Ulbert, I., Schomer, D.L., Halgren, E. (2007) Specific increase of human entorhinal population synaptic and neuronal activity during retrieval. Neuroimage, 37, 618-622. Krueger, J.M. and Obal, F. (1993) A neuronal group theory of sleep function. J Sleep Res, 2, 63-69. Krueger, J.M. (2008) The role of cytokines in sleep regulation. Curr Pharm Des, 14, 3408-3416. 95 Csercsa Richárd Krueger, J.M., Rector, D.M., Roy, S., Van Dongen, H.P., Belenky, G., Panksepp, J. (2008) Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci, 9, 910-919. Kurth, S., Ringli, M., Geiger, A., LeBourgeois, M., Jenni, O.G., Huber, R. (2010) Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci, 30, 13211-13219. Lakatos, P., Shah, A.S., Knuth, K.H., Ulbert, I., Karmos, G., Schroeder, C.E. (2005) An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol, 94, 1904-1911. Lee, A.K. and Wilson, M.A. (2002) Memory of sequential experience in the hippocampus during slow wave sleep. Neuron, 36, 1183-1194. Li, C.Y., Poo, M.M., Dan, Y. (2009) Burst spiking of a single cortical neuron modifies global brain state. Science, 324, 643-646. Liu, Z.W., Faraguna, U., Cirelli, C., Tononi, G., Gao, X.B. (2010) Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci, 30, 8671-8675. Lomo, T. (1966) Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiologica Scandinavia, 68, 128. Loomis, A.L., Harvey, E.N., Hobart, G. (1938) Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. J Neurophysiol, 1, 413-430. Lopez-Bendito, G., Shigemoto, R., Kulik, A., Paulsen, O., Fairen, A., Lujan, R. (2002) Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur J Neurosci, 15, 1766-1778. Luczak, A., Bartho, P., Marguet, S.L., Buzsaki, G., Harris, K.D. (2007) Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A, 104, 347-352. Lynch, M.A. (2004) Long-term potentiation and memory. Physiol Rev, 84, 87-136. 96 Laminar analysis of the slow wave actvity in humans Mahowald, M.W. and Schenck, C.H. (2005) Insights from studying human sleep disorders. Nature, 437, 1279-1285. Mann, E.O., Kohl, M.M., Paulsen, O. (2009) Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci, 29, 7513-7518. Mao, B.Q., Hamzei-Sichani, F., Aronov, D., Froemke, R.C., Yuste, R. (2001) Dynamics of spontaneous activity in neocortical slices. Neuron, 32, 883-898. Maquet, P. (2001) The role of sleep in learning and memory. Science, 294, 1048-1052. Margrie, T.W., Brecht, M., Sakmann, B. (2002) In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch, 444, 491-498. Marr, D. (1970) A theory for cerebral neocortex. Proc R Soc Lond B Biol Sci, 176, 161234. Marr, D. (1971) Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci, 262, 23-81. Marshall, L., Helgadottir, H., Molle, M., Born, J. (2006) Boosting slow oscillations during sleep potentiates memory. Nature, 444, 610-613. Marshall, L. and Born, J. (2007) The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci, 11, 442-450. Massimini, M., Huber, R., Ferrarelli, F., Hill, S., Tononi, G. (2004) The sleep slow oscillation as a traveling wave. J Neurosci, 24, 6862-6870. Massimini, M., Ferrarelli, F., Huber, R., Esser, S.K., Singh, H., Tononi, G. (2005) Breakdown of cortical effective connectivity during sleep. Science, 309, 22282232. Massimini, M., Ferrarelli, F., Esser, S.K., Riedner, B.A., Huber, R., Murphy, M., Peterson, M.J., Tononi, G. (2007) Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A, 104, 8496-8501. 97 Csercsa Richárd Maviel, T., Durkin, T.P., Menzaghi, F., Bontempi, B. (2004) Sites of neocortical reorganization critical for remote spatial memory. Science, 305, 96-99. McClelland, J.L., McNaughton, B.L., O'Reilly, R.C. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev, 102, 419-457. Meskenaite, V. (1997) Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol, 379, 113-132. Mohajerani, M.H., McVea, D.A., Fingas, M., Murphy, T.H. (2010) Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci, 30, 3745-3751. Molle, M., Marshall, L., Gais, S., Born, J. (2002) Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci, 22, 10941-10947. Molle, M., Marshall, L., Gais, S., Born, J. (2004) Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A, 101, 13963-13968. Molle, M., Yeshenko, O., Marshall, L., Sara, S.J., Born, J. (2006) Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol, 96, 62-70. Moroni, F., Nobili, L., Curcio, G., De Carli, F., Fratello, F., Marzano, C., De Gennaro, L., Ferrillo, F., Cossu, M., Francione, S., Lo Russo, G., Bertini, M., Ferrara, M. (2007) Sleep in the human hippocampus: a stereo-EEG study. PLoS One, 2, e867. Moruzzi, G. and Magoun, H.W. (1949) Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol, 1, 455-473. Mukhametov, L.M., Supin, A.Y., Polyakova, I.G. (1977) Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res, 134, 581584. 98 Laminar analysis of the slow wave actvity in humans Murphy, M., Riedner, B.A., Huber, R., Massimini, M., Ferrarelli, F., Tononi, G. (2009) Source modeling sleep slow waves. Proc Natl Acad Sci U S A, 106, 1608-1613. Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J., Buzsaki, G. (1999) Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci, 19, 9497-9507. Nadel, L., Samsonovich, A., Ryan, L., Moscovitch, M. (2000) Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus, 10, 352-368. Nauta, W.J. (1946) Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol, 9, 285-316. Nemeth, D., Janacsek, K., Londe, Z., Ullman, M.T., Howard, D.V., Howard, J.H., Jr. (2010) Sleep has no critical role in implicit motor sequence learning in young and old adults. Exp Brain Res, 201, 351-358. Nevian, T. and Sakmann, B. (2006) Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci, 26, 11001-11013. Nicholson, C. and Freeman, J.A. (1975) Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol, 38, 356-368. Nishino, S., Ripley, B., Overeem, S., Lammers, G.J., Mignot, E. (2000) Hypocretin (orexin) deficiency in human narcolepsy. Lancet, 355, 39-40. Olcese, U., Esser, S.K., Tononi, G. (2010) Sleep and synaptic renormalization: A computational study. J Neurophysiol. Ozen, S., Sirota, A., Belluscio, M.A., Anastassiou, C.A., Stark, E., Koch, C., Buzsaki, G. (2010) Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci, 30, 11476-11485. Pace-Schott, E.F. and Hobson, J.A. (2002) The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci, 3, 591-605. 99 Csercsa Richárd Pappenheimer, J.R., Miller, T.B., Goodrich, C.A. (1967) Sleep-promoting effects of cerebrospinal fluid from sleep-deprived goats. Proc Natl Acad Sci U S A, 58, 513-517. Pastalkova, E., Itskov, V., Amarasingham, A., Buzsaki, G. (2008) Internally generated cell assembly sequences in the rat hippocampus. Science, 321, 1322-1327. Petersen, C.C., Malenka, R.C., Nicoll, R.A., Hopfield, J.J. (1998) All-or-none potentiation at CA3-CA1 synapses. Proc Natl Acad Sci U S A, 95, 4732-4737. Petersen, C.C., Hahn, T.T., Mehta, M., Grinvald, A., Sakmann, B. (2003) Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A, 100, 13638-13643. Peyrache, A., Khamassi, M., Benchenane, K., Wiener, S.I., Battaglia, F.P. (2009) Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci, 12, 919-926. Quiroga, R.Q., Reddy, L., Kreiman, G., Koch, C., Fried, I. (2005) Invariant visual representation by single neurons in the human brain. Nature, 435, 1102-1107. Raichle, M.E., MacLeod, A.M., Snyder, A.Z., Powers, W.J., Gusnard, D.A., Shulman, G.L. (2001) A default mode of brain function. Proc Natl Acad Sci U S A, 98, 676-682. Raichle, M.E. (2006) Neuroscience. The brain's dark energy. Science, 314, 1249-1250. Raichle, M.E. and Snyder, A.Z. (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage, 37, 1083-1090; discussion 1097-1089. Rasch, B., Buchel, C., Gais, S., Born, J. (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315, 1426-1429. Rasch, B., Pommer, J., Diekelmann, S., Born, J. (2009) Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci, 12, 396-397. Ravagnati, L., Halgren, E., Babb, T.L., Crandall, P.H. (1979) Activity of human hippocampal formation and amygdala neurons during sleep. Sleep, 2, 161-173. 100 Laminar analysis of the slow wave actvity in humans Rector, D.M., Topchiy, I.A., Carter, K.M., Rojas, M.J. (2005) Local functional state differences between rat cortical columns. Brain Res, 1047, 45-55. Riedner, B.A., Vyazovskiy, V.V., Huber, R., Massimini, M., Esser, S., Murphy, M., Tononi, G. (2007) Sleep homeostasis and cortical synchronization: III. A highdensity EEG study of sleep slow waves in humans. Sleep, 30, 1643-1657. Ros, H., Sachdev, R.N., Yu, Y., Sestan, N., McCormick, D.A. (2009) Neocortical networks entrain neuronal circuits in cerebellar cortex. J Neurosci, 29, 1030910320. Rosanova, M. and Ulrich, D. (2005) Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci, 25, 9398-9405. Sakata, S. and Harris, K.D. (2009) Laminar structure of spontaneous and sensoryevoked population activity in auditory cortex. Neuron, 64, 404-418. Sanchez-Vives, M.V. and McCormick, D.A. (2000) Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci, 3, 1027-1034. Saper, C.B., Chou, T.C., Scammell, T.E. (2001) The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci, 24, 726-731. Schwark, H.D. and Li, J. (2000) Distribution of neurons immunoreactive for calciumbinding proteins varies across areas of cat primary somatosensory cortex. Brain Res Bull, 51, 379-385. Scoville, W.B. and Milner, B. (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20, 11-21. Sherin, J.E., Shiromani, P.J., McCarley, R.W., Saper, C.B. (1996) Activation of ventrolateral preoptic neurons during sleep. Science, 271, 216-219. Siapas, A.G. and Wilson, M.A. (1998) Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron, 21, 1123-1128. Siegel, J. (2002) The Neural Control of Sleep and Waking. Springer-Verlag, New York. 101 Csercsa Richárd Siegel, J.M. (2001) The REM sleep-memory consolidation hypothesis. Science, 294, 1058-1063. Siegel, J.M. (2005) Clues to the functions of mammalian sleep. Nature, 437, 1264-1271. Siegel, J.M. (2008) Do all animals sleep? Trends Neurosci, 31, 208-213. Somogyi, P., Kisvarday, Z.F., Martin, K.A., Whitteridge, D. (1983) Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience, 10, 261-294. Song, S., Howard, J.H., Jr., Howard, D.V. (2007) Sleep does not benefit probabilistic motor sequence learning. J Neurosci, 27, 12475-12483. Squire, L.R. (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev, 99, 195-231. Staba, R.J., Wilson, C.L., Bragin, A., Fried, I., Engel, J., Jr. (2002) Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci, 22, 5694-5704. Staba, R.J., Wilson, C.L., Fried, I., Engel, J., Jr. (2002) Single neuron burst firing in the human hippocampus during sleep. Hippocampus, 12, 724-734. Steinvorth, S., Wang, C., Ulbert, I., Schomer, D., Halgren, E. (2010) Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus, 20, 166-173. Stellwagen, D. and Malenka, R.C. (2006) Synaptic scaling mediated by glial TNFalpha. Nature, 440, 1054-1059. Steriade, M., Nunez, A., Amzica, F. (1993a) A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci, 13, 3252-3265. Steriade, M., Nunez, A., Amzica, F. (1993b) Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci, 13, 3266-3283. 102 Laminar analysis of the slow wave actvity in humans Steriade, M. and Amzica, F. (1996) Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci U S A, 93, 2533-2538. Steriade, M., Timofeev, I., Grenier, F. (2001) Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol, 85, 1969-1985. Steriade, M. and Timofeev, I. (2003) Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron, 37, 563-576. Steriade, M. (2006) Grouping of brain rhythms in corticothalamic systems. Neuroscience, 137, 1087-1106. Stickgold, R. (2005) Sleep-dependent memory consolidation. Nature, 437, 1272-1278. Takashima, A., Petersson, K.M., Rutters, F., Tendolkar, I., Jensen, O., Zwarts, M.J., McNaughton, B.L., Fernandez, G. (2006) Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A, 103, 756-761. Tamas, G., Somogyi, P., Buhl, E.H. (1998) Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci, 18, 42554270. Tamas, G., Lorincz, A., Simon, A., Szabadics, J. (2003) Identified sources and targets of slow inhibition in the neocortex. Science, 299, 1902-1905. Tenke, C.E., Schroeder, C.E., Arezzo, J.C., Vaughan, H.G., Jr. (1993) Interpretation of high-resolution current source density profiles: a simulation of sublaminar contributions to the visual evoked potential. Exp Brain Res, 94, 183-192. Timofeev, I. and Steriade, M. (1996) Low-frequency rhythms in the thalamus of intactcortex and decorticated cats. J Neurophysiol, 76, 4152-4168. Timofeev, I., Grenier, F., Bazhenov, M., Sejnowski, T.J., Steriade, M. (2000) Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex, 10, 11851199. Timofeev, I., Grenier, F., Steriade, M. (2001) Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A, 98, 1924-1929. 103 Csercsa Richárd Tononi, G. and Cirelli, C. (2006) Sleep function and synaptic homeostasis. Sleep Med Rev, 10, 49-62. Triarhou, L.C. (2006) The percipient observations of Constantin von Economo on encephalitis lethargica and sleep disruption and their lasting impact on contemporary sleep research. Brain Res Bull, 69, 244-258. Tseng, K.Y., Kasanetz, F., Kargieman, L., Riquelme, L.A., Murer, M.G. (2001) Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci, 21, 6430-6439. Tucker, M.A. and Fishbein, W. (2009) The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J Sleep Res, 18, 304-312. Turner, D.A., Li, X.G., Pyapali, G.K., Ylinen, A., Buzsaki, G. (1995) Morphometric and electrical properties of reconstructed hippocampal CA3 neurons recorded in vivo. J Comp Neurol, 356, 580-594. Ulbert, I., Halgren, E., Heit, G., Karmos, G. (2001) Multiple microelectrode-recording system for human intracortical applications. J Neurosci Methods, 106, 69-79. Ulbert, I., Karmos, G., Heit, G., Halgren, E. (2001) Early discrimination of coherent versus incoherent motion by multiunit and synaptic activity in human putative MT+. Hum Brain Mapp, 13, 226-238. Ulbert, I., Heit, G., Madsen, J., Karmos, G., Halgren, E. (2004) Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia, 45 Suppl 4, 48-56. Ulbert, I., Magloczky, Z., Eross, L., Czirjak, S., Vajda, J., Bognar, L., Toth, S., Szabo, Z., Halasz, P., Fabo, D., Halgren, E., Freund, T.F., Karmos, G. (2004) In vivo laminar electrophysiology co-registered with histology in the hippocampus of patients with temporal lobe epilepsy. Exp Neurol, 187, 310-318. Vanhatalo, S., Palva, J.M., Holmes, M.D., Miller, J.W., Voipio, J., Kaila, K. (2004) Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A, 101, 5053-5057. 104 Laminar analysis of the slow wave actvity in humans Velluti, R.A. (2008) The Auditory System in Sleep. Elsevier Academic Press. Vertes, R.P. and Siegel, J.M. (2005) Time for the sleep community to take a critical look at the purported role of sleep in memory processing. Sleep, 28, 1228-1229; discussion 1230-1223. Volgushev, M., Chauvette, S., Mukovski, M., Timofeev, I. (2006) Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected]. J Neurosci, 26, 5665-5672. von Economo, C. (1929) Schlaftheorie. Ergebn. Physiol. (München), 312-339. Vyazovskiy, V., Borbely, A.A., Tobler, I. (2000) Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res, 9, 367-371. Vyazovskiy, V.V., Cirelli, C., Pfister-Genskow, M., Faraguna, U., Tononi, G. (2008) Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci, 11, 200-208. Vyazovskiy, V.V., Faraguna, U., Cirelli, C., Tononi, G. (2009) Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol, 101, 1921-1931. Wagner, T., Axmacher, N., Lehnertz, K., Elger, C.E., Fell, J. (2010) Sleep-dependent directional coupling between human neocortex and hippocampus. Cortex, 46, 256-263. Walker, M.P. and Stickgold, R. (2006) Sleep, memory, and plasticity. Annu Rev Psychol, 57, 139-166. Wang, C., Ulbert, I., Schomer, D.L., Marinkovic, K., Halgren, E. (2005) Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci, 25, 604-613. Waters, J. and Helmchen, F. (2006) Background synaptic activity is sparse in neocortex. J Neurosci, 26, 8267-8277. 105 Csercsa Richárd Whittington, M.A., Traub, R.D., Jefferys, J.G. (1995) Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature, 373, 612-615. Wierzynski, C.M., Lubenov, E.V., Gu, M., Siapas, A.G. (2009) State-dependent spiketiming relationships between hippocampal and prefrontal circuits during sleep. Neuron, 61, 587-596. Wilson, M.A. and McNaughton, B.L. (1994) Reactivation of hippocampal ensemble memories during sleep. Science, 265, 676-679. Wittner, L., Henze, D.A., Zaborszky, L., Buzsaki, G. (2006) Hippocampal CA3 pyramidal cells selectively innervate aspiny interneurons. Eur J Neurosci, 24, 1286-1298. Wittner, L., Huberfeld, G., Clemenceau, S., Eross, L., Dezamis, E., Entz, L., Ulbert, I., Baulac, M., Freund, T.F., Magloczky, Z., Miles, R. (2009) The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro. Brain, 132, 3032-3046. Wyrwicka, W., Sterman, M.B., Clemente, C.D. (1962) Conditioning of induced electroencephalographic sleep patterns in the cat. Science, 137, 616-618. Yoshimura, Y., Dantzker, J.L., Callaway, E.M. (2005) Excitatory cortical neurons form fine-scale functional networks. Nature, 433, 868-873. Zola-Morgan, S.M. and Squire, L.R. (1990) The primate hippocampal formation: evidence for a time-limited role in memory storage. Science, 250, 288-290. 106 Laminar analysis of the slow wave actvity in humans PUBLICATIONS Relevant to thesis ’Laminar analysis of the slow wave activity in humans’, Csercsa R., Dombovári B., Fabó D., Wittner L., Erőss L., Entz L., Sólyom A., Rásonyi Gy., Szűcs A., Kelemen A., Jakus R., Juhos V., Grand L., Magony A., Halász P., Freund T. F., Maglóczky Zs., Cash S. S., Papp L., Karmos Gy., Halgren E., Ulbert I., Brain, 133(9):2514-5., 2010., IF: 9.490 ’The human K-complex represents an isolated cortical down-state', Cash S. S., Halgren E., Dehghani N., Rossetti A., Thesen T., Wang C., Devinsky O., Kuzniecky R., Doyle W., Madsen J. R., Bromfield E., Erőss L., Halász P., Karmos Gy., Csercsa R., Wittner L., Ulbert I., Science, 324(5930):1084-7., 2009., IF: 29.747 Non-relevant to thesis ’Short and long term biocompatibility of NeuroProbes silicon probes’, Grand L., Wittner L., Herwik S., Göthelid E., Ruther P., Oscarsson S., Neves H., Dombovári B., Csercsa R., Karmos G., Ulbert I., J Neurosci Methods, 189(2):216-29., 2010., IF: 2.295 ’Control and data acquisition software for high-density CMOS-based microprobe arrays implementing electronic depth control’, Seidl K., Torfs T., De Mazière P. A., Van Dijck G., Csercsa R., Dombovári B., Nurcahyo Y., Ramirez H., Van Hulle M. M., Orban G. A., Paul O., Ulbert I., Neves H., Ruther P., Biomedizinische Technik, 55(3):183-91., 2010., IF: 0.525 107 Csercsa Richárd ACKNOWLEDGEMENTS First, I would like to thank my supervisor, Dr. István Ulbert for his guidance throughout my undergraduate and graduate years, for his inexhaustable enthusiasm and support in all aspects of work. I am much obliged to Professor György Karmos for his outstanding lectures that directed my interest to neuroscience, and for his sponsorship. I thank Balázs Dombovári, László Grand, and Andor Magony for their friendly support professionally as well as personally. I also thank Richárd Fiáth, Domonkos Horváth, Bálint Kerekes, Lucia Wittner, and all of my co-workers at the Institute for Psychology for the good atmosphere and inspiring collaboration. I learned a lot from their interesting experiments and the interdisciplinary topics of our conversations. I owe Dr. Loránd Erőss, Dr. László Entz, and Dr. Dániel Fabó for their invaluable cooperation and for providing the data for my work, and Dr. Zsófia Maglóczky for the histology analysis. I would like to express my thanks to Péter Kottra for his assistance in the lab. I thank Dr. István Czigler, the director of the Institute for Psychology of the Hungarian Academy of Sciences for providing the facilities for my work. I am grateful to Dr. Patrick Ruther, Professor Oliver Paul, and Karsten Seidl of the Laboratory for Microsystem Materials of the Institute for Mikrosystemtechnik (IMTEK) in Freiburg, Germany, for having me at their institute and giving me the opportunity to get acquainted with electrode design and fabrication. Last but not least, I would like to thank my family for their encouragement and support. 108