* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Larry M. Jordan, Urszula Sławińska
Environmental enrichment wikipedia , lookup
Neuroregeneration wikipedia , lookup
Aging brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Axon guidance wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Convolutional neural network wikipedia , lookup
Synaptogenesis wikipedia , lookup
Multielectrode array wikipedia , lookup
Neural coding wikipedia , lookup
Mirror neuron wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Apical dendrite wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Neuroeconomics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neural oscillation wikipedia , lookup
Metastability in the brain wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Development of the nervous system wikipedia , lookup
Hypothalamus wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Basal ganglia wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
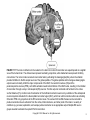
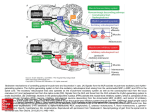
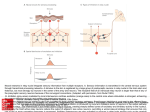
Chapter 17 The Brain and Spinal Cord Networks Controlling Locomotion Larry M. Jordan, Urszula Sławińska Copyright © 2014 Elsevier Inc. All rights reserved. 1 FIGURE 17.1 The main contributors to the network in the brain for control of locomotion are superimposed on a sagittal view of the human brain. The white arrows represent excitatory projections, while shaded arrows represent inhibitory connections. The motor cortex can select a locomotor task by activating the basal ganglia (BG), where the striatum provided inhibition to the BG output neurons of the globus pallidus. The globus pallidus and homologous basal ganglia output neurons tonically inhibit the major components of the MLR, the cuneiform nucleus (CN) and the pedunculopontine nucleus (PPN), so that BG activation leads to disinhibition of the MLR nuclei, resulting in the initiation of locomotion through a relay in reticulospinal (RS) neurons. The BG output is monitored and fed back to the cortex via the thalamus (Th). Another route for activation of the midbrain locomotor neurons is by excitation of the widespread neuronal systems included in the diencephalic locomotor region (DLR), which can elicit locomotion either via activating CN and/or PPN or by projections to the RS locomotor areas. The various DLR and MLR areas can be recruited to produce locomotion due to activation from the cortex, limbic structures, and other parts of the brain in a variety of conditions (e.g. arousal, exploration, and escape) where locomotion is an appropriate output. Multiple RS neuron groups are able to activate the spinal CPG for locomotion. Copyright © 2014 Elsevier Inc. All rights reserved. 2 FIGURE 17.2 Diagram showing the main components of the reticulospinal (RS) projections to the spinal cord to activate the locomotor CPG. The RS systems that are effective for eliciting locomotion are distinguishable based upon their transmitter content. Pathways containing excitatory amino acids (EAA) such as glutamate project from magnocellular and gigantocellular parts of the RS system to the spinal cord. Other RS pathways arise in the 5-hydroxytryptamine (5HT) and noradrenergic (NA) regions of the medulla. The RS systems are known to be activated from the lateral hypothalamus (LH), which includes an orexinergic (Ox) pathway, and from the components of the MLR, including the cuneiform nucleus (CN) and the pedunculopontine nucleus (PPN). CN and PPN provide glutamatergic (excitatory amino acid, or EAA) input to RS neurons, and PPN also produces RS activation due to a cholinergic (acetylcholine, or ACh) projection. There is a newly described orexinergic input to neurons of the CN for initiation of locomotion. Another putative component of the MLR is the A7 noradrenergic group of neurons found at the junction of the midbrain and the pons, in a site where electrical stimulation elicits locomotion. These cells project directly to the spinal cord. A dopaminergic (DA) pathway, thought to arise from the All group of dopamine-containing neurons of the hypothalamus, may also be an important descending pathway for the initiation of locomotion in some species. Copyright © 2014 Elsevier Inc. All rights reserved. 3 FIGURE 17.3 Models of the CPG. (A) The half-center model of Graham-Brown, as modified by Lundberg to explain the findings in LDOPA-treated spinal cats. Stimulation of ipsilateral flexor-reflex afferents (iFRAs) produced late-long lasting excitation of flexor motoneurons, while stimulation of contralateral flexor reflex afferents (co-FRAs) produced similar excitation of extensor motoneurons. The L-DOPA treatment produced locomotion in the spinal cat preparation, and the half-center model was proposed as a plausible organization to explain these findings. (B) A computational model of spinal locomotor circuitry with a two-level CPG.31 Rhythm generator (RG) and pattern formation (PF) networks represent the two levels of the CPG. The excitatory RGE-E (rhythm generator—extensor) and RGE-F (rhythm generator—flexor) populations reciprocally inhibit each other via the inhibitory RG populations RGI. The PF excitatory populations (PFE) reciprocally inhibit each other through the PF inhibitory populations (PFI). The RGE-E and RGE-F populations have recurrent excitatory connections. Locomotion is initiated by a tonic excitatory drive (from MLR and/or MRF) to both the RG and PF populations. The locomotor rhythm and the durations of the flexor and extensor phases are determined by the RG network that controls the activity of the PF network by direct excitation of PFE neurons (and inhibition to PFE neurons mediated by the RGI populations—not shown). PFE population activity produces a phase-specific activation of the corresponding group of synergist motoneuron (Mn-E and Mn-F) pools. Phase-dependent inhibition of motoneurons is produced by the MnI-E and MnI-F populations whose activity is regulated by excitation from the PF network and inhibition from MnI neurons. IaINs are included in the MnI population, and inhibition from Renshaw cells (not shown) as well as mutual inhibitory connections between the MnI populations can also control MnI rhythmicity. Copyright © 2014 Elsevier Inc. All rights reserved. 4 FIGURE 17.4 Diagram representing the progenitor domains (p0–p3) that give rise to ventral spinal interneuron groups (V0–V3). The transcription factors that characterize the progenitor domains and the postmitotic interneuron subgroups are illustrated. Where possible the transmitter phenotypes of the interneuron subgroups are given, along with their known sites of termination. Motoneuron progenitors and their associated transcription factors are also illustrated. Interneurons that express Hb9 (similar to motoneurons) are also illustrated, although the cardinal progenitor group that produces them is not known. V0d: dorsal V0 interneurons; V0v: ventral V0 interneurons; V0c: cholinergic V0 interneurons; RCs: Renshaw cells; IaIN: Ia inhibitory interneurons; V2a: excitatory V2 interneurons; V2b: inhibitory V2 interneurons; MN: motoneurons; V3s: V3 interneurons producing synchrony; V3r: V3 interneurons with connections expected of rhythm-generating layer neurons. Copyright © 2014 Elsevier Inc. All rights reserved. 5