* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Passive Properties of Swimmeret Motor Neurons
Microneurography wikipedia , lookup
Apical dendrite wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Environmental enrichment wikipedia , lookup
Metastability in the brain wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Convolutional neural network wikipedia , lookup
Bird vocalization wikipedia , lookup
End-plate potential wikipedia , lookup
Neurotransmitter wikipedia , lookup
Multielectrode array wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Electrophysiology wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Axon guidance wikipedia , lookup
Neural oscillation wikipedia , lookup
Single-unit recording wikipedia , lookup
Synaptogenesis wikipedia , lookup
Muscle memory wikipedia , lookup
Neural coding wikipedia , lookup
Chemical synapse wikipedia , lookup
Mirror neuron wikipedia , lookup
Development of the nervous system wikipedia , lookup
Embodied language processing wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Circumventricular organs wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Optogenetics wikipedia , lookup
Nervous system network models wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Central pattern generator wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Passive Properties of Swimmeret Motor Neurons
CAROLYN M. SHERFF AND BRIAN MULLONEY
Section of Neurobiology, Physiology and Behavior, Division of Biological Sciences, University of California,
Davis, California 95616-8755
INTRODUCTION
Motor neurons in many systems are not only the final
common paths through which the products of central patterngenerating circuits are exported, but also contribute directly
to the generation of motor patterns by synaptic interactions
with other components of these circuits. In crayfish, some
motor neurons that innervate the swimmerets—limbs that
occur in pairs on several abdominal segments—perform
both tasks (Heitler 1978, 1983; Sherff and Mulloney 1996).
When crustaceans swim forward by beating their swimmerets, each limb moves rhythmically through cycles of power
strokes and return strokes that propel the animal forward.
These movements are produced by alternating contractions
of power-stroke (PS) and return-stroke (RS) muscles that
are innervated by separate sets of PS and RS motor neurons.
About half of the Ç70 swimmeret motor neurons that control
the movements of each swimmeret (Mulloney et al. 1990; B.
Mulloney and W. M. Hall, unpublished data) are PS motor
neurons, and the other half are RS motor neurons. Within
each of these sets, motor neurons can be further subdivided
by their peripheral functions: glutamatergic excitors cause
contraction, and GABAergic inhibitors prevent contraction
(Atwood 1976; Mulloney and Hall 1990; Sherff and Mulloney 1996). Thus we can distinguish four kinds of swimmeret
motor neurons in the pool that innervates each swimmeret
(Davis 1969; Stein 1971): PS excitors (PSEs), RS excitors
(RSEs), PS inhibitors (PSIs), and RS inhibitors (RSIs).
Given these four kinds of motor neurons, do differences
in their passive properties play some role in producing the
swimmeret motor pattern?
Because excitatory and inhibitory fast-flexor motor neurons that innervate the trunk musculature differ in their input
resistances (Rins) and membrane time constants ( tms) (Edwards and Mulloney 1987), and because these differences
permit them to integrate synaptic currents in ways that affect
their functions, we began with the hypothesis that the passive
properties of excitatory swimmeret motor neurons would
differ from those of inhibitory motor neurons, and that the
passive properties of PS neurons might also differ from those
of RS neurons.
In the crayfish Pacifastacus leniusculus, axons that innervate RS and PS muscles of each swimmeret are segregated
into two branches of the swimmeret nerve, N1, that runs
from the ganglion to the swimmeret. The anterior branch of
N1 contains RS axons; the posterior branch contains PS
axons (Mulloney et al. 1990). We exploited this anatomic
segregation to identify different kinds of swimmeret motor
neurons by the N1 branch that contained their axons and by
the phase of the motor pattern in which they fired (Stein
1971). To visualize their cell bodies and processes within
the ganglion, we backfilled neurons that had axons in these
different branches. We also filled individual motor neurons
by injecting a marker from a microelectrode. The cell bodies
of PS and RS neurons were clustered on different sides of
the base of each N1, but all four kinds had similar structures
within the ganglion.
We measured membrane potentials (Vms), Rins, and tms of
0022-3077/97 $5.00 Copyright q 1997 The American Physiological Society
92
/ 9k16$$jy07
J658-6
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
Sherff, Carolyn M. and Brian Mulloney. Passive properties of
swimmeret motor neurons. J. Neurophysiol. 78: 92–102, 1997.
Four different functional types of motor neurons innervate each
swimmeret: return-stroke excitors (RSEs), power-stroke excitors
(PSEs), return-stroke inhibitors (RSIs), and power-stroke inhibitors (PSIs). We studied the structures and passive electrical properties of these neurons, and tested the hypothesis that different types
of motor neurons would have different passive properties that influenced generation of the swimmeret motor pattern. Cell bodies of
neurons innervating one swimmeret were clustered in two anatomic
groups in the same ganglion. The shapes of motor neurons in both
groups were similar, despite the differences in locations of their
cell bodies and in their functions. Diameters of their axons in the
swimmeret nerve ranged from õ2 to Ç35 mm. Resting membrane
potentials, input resistances, and membrane time constants were
recorded with microelectrodes in the processes of swimmeret motor
neurons in isolated abdominal nerve cord preparations. Membrane
potentials had a median of 059 mV, with 25th and 75th percentiles
of 066.0 and 053 mV. The median input resistance was 6.4 MV,
with 25th and 75th percentiles of 3.4 and 13.7 MV. Membrane time
constants had a median of 9.3 ms, with 25th and 75th percentiles of
5.7 and 15.0 ms. Excitatory and inhibitory motor neurons had
similar passive properties. RSE motor neurons were typically more
depolarized than the other types, but the passive properties of RSE,
PSE, RSI, and PSI neurons were not significantly different. Membrane time constants measured from cell bodies were briefer than
those measured from neuropil processes, but membrane potentials
and input resistances were not significantly different. The relative
sizes of different motor neurons were measured from the sizes of
their impulses recorded extracellularly from the swimmeret nerve.
Smaller motor neurons had lower membrane potentials and were
more likely to be active in the motor pattern than were large motor
neurons. Motor neurons of different sizes had similar input resistances and membrane time constants. Motor neurons that were
either oscillating or oscillating and firing in phase with the swimmeret motor pattern had lower average membrane potentials and
longer time constants than those that were not oscillating. When
the state of the swimmeret system changed from quiescence to
continuous production of the motor pattern, the resting potentials,
input resistances, and membrane time constants of individual swimmeret motor neurons changed only slightly. On average, both input
resistance and membrane time constant increased. These similarities are considered in light of the functional task each motor neuron
performs, and a hypothesis is developed that links the brief time
constants of these neurons and graded synaptic transmission by
premotor interneurons to control of the swimmeret muscles and
the performance of the swimmeret system.
PROPERTIES OF SWIMMERET MOTOR NEURONS
neurons in each of the four kinds, and found little difference
between them. To explore possible causes of the variability
we observed in each of these parameters, we measured the
relative sizes of different swimmeret motor neurons and
noted whether they were firing in phase with the motor pattern or whether their Vm oscillated with the motor pattern.
Preliminary descriptions of these passive properties have
been published in abstract form (Sherff and Mulloney
1992).
These similarities in passive properties contradict the idea
that differences in the passive properties of these different
neurons contribute to pattern generation. We propose that
this similarity results from design constraints imposed by the
motor neurons’ task of exporting to their peripheral targets
a motor pattern that must vary smoothly in period and
intensity.
Crayfish (P. leniusculus) were obtained from local suppliers and
kept in aerated freshwater aquaria. Animals were anesthetized by
cooling on ice, then exsanguinated by removing the claws and
perfusing the hemocoel with physiological saline (Sherff and Mulloney 1996) through a hypodermic needle inserted into one of the
wounds.
Backfills of swimmeret motor neurons
Selected N1s were backfilled according to the procedure of Leise
et al. (1986). Abdominal nerve cords were pinned flat in Sylgardlined (Dow-Corning) petri dishes. N1s were placed in a Vaseline
well filled with 250 mM CoCl2 . The CoCl2 was allowed to diffuse
through the nerves overnight. The nerve cords were then washed
in saline and incubated in 0.1 M sodium cacodylate. Cobalt sulfide
was precipitated by adding ammonium sulfide. The nerve cords
were washed in saline and then fixed in 2.7% glutaraldehyde overnight. Staining was intensified with the use of Timm’s solution
(Leise et al. 1986). The nerve cords were dehydrated, cleared in
methyl salicylate, and photographed as whole mounts.
Filling individual motor neurons
Neurobiotin (Vector Labs) was injected with the use of /3- to
/5-nA current pulses 250 ms in duration at 2 Hz for Ç30 min.
Nerve cords were fixed overnight in 4% paraformaldehyde, rinsed
in 0.1 M glycine in phosphate-buffered saline (PBS) (Sigma) and
in straight PBS, dehydrated to 95% EtOH to increase their permeability, and rehydrated back to PBS. The tissue was washed in
wash buffer [0.3% reduced Triton X-100 (Aldrich), 5% goat serum (BRL) in PBS] and in no-serum wash buffer (3 30-min
washes in each buffer) and incubated in Texas-Red Streptavidin
(Amersham), diluted 1:100 in no-serum wash buffer, for 18–20
h. Finally, the tissue was washed in PBS, dehydrated in ethanol,
and cleared in methyl salicylate.
Preparations were viewed and photographed with a fluorescence
microscope. Drawings of filled neurons in cleared ganglia were
made with the use of a camera lucida, or from projections of slides
of photographed ganglia.
Plastic sections
In preparation for sectioning, ganglia were stained with osmiumethyl gallate, embedded in Spurr’s plastic (Electron Microscopy
Sciences), and sectioned (Leise and Mulloney 1986; Mulloney
and Hall 1991). The diameters of axons were measured from
2-mm cross sections of N1s from a different set of nerve cords.
/ 9k16$$jy07
J658-6
Electrophysiology
Experiments were performed on isolated abdominal nerve cords.
The N1s, which project bilaterally from each of the first five abdominal ganglia, were cut as far distally as possible to allow us to
record from them with extracellular pin electrodes. In electrophysiology experiments, the last two thoracic ganglia were left attached
to the abdominal cord to increase the stability of expression of the
swimmeret motor pattern. Nerve cords were pinned in Sylgardcoated dishes. Recordings were made from 168 motor neurons in
83 nerve cords.
The swimmeret motor pattern was recorded extracellularly from
the RS and PS branches of N1 with stainless steel pin electrodes
(Mulloney and Selverston 1974). Intracellular recordings were
made from processes of motor neurons in the lateral neuropil LN
(Skinner 1985). Microelectrodes were filled either with 2.5 M
KCl, or, if the motor neurons were to be filled for anatomic studies,
with 10 mM KH2PO4 and 1 M KCl with 5% Neurobiotin in the
tip. Microelectrode resistances were between 20 and 30 MV. Most
measurements of Rin and tm were made in bridge mode with either a
Getting M5 preamplifier or an Axoclamp-2A (Axon Instruments).
Both extracellular and microelectrode recordings were collected
on video cassette recorded tape with the use of a Neuro-Corder
886 (Neurodata Instruments). Records were later transferred to
computer for analysis with pClamp programs (Axon Instruments)
or played back onto a Gould ES1000 electrostatic recorder. Recordings displayed in this paper were played onto a Gould 2400
pen recorder or were collected in Axotape files (Axon Instruments)
and printed in SigmaPlot (Jandel Scientific).
Measuring Rin and tm
To measure Rin , responses to 50 200-ms pulses of 00.5-nA
current were averaged with the use of the Clampfit program (Axon
Instruments) and the averaged response was measured in the
TableCurve program (Jandel Scientific). In a few cases, Rin was
also measured as the slope of the current-voltage relation, measured
in discontinuous current-clamp mode.
To calculate tm , responses to 50 2-ms pulses of 05.0-nA current
were recorded and averaged. The recovery of the Vm to its rest
level after the termination of the current, represented by the absolute values of the averaged data, was then fitted directly with
one-, two-, and three-term exponential equations with the use of
TableCurve (Jandel Scientific). The longest time constant in the
equation that best fit the data was considered tm , whereas briefer
time constants were considered equalizing constants (Rall 1969;
Rall et al. 1992).
Statistical analysis
The SigmaStat program (Jandel Scientific) was used to calculate
statistics of the different parameters we measured and to compare
parameters from different types of neurons. Normally distributed
data were summarized by mean { SD; other data were described
by median, 25th, and 75th percentiles. Deviations from normality
were estimated with Kolmogorov-Smirnov tests. Student’s t-tests
were used to assess differences of normally distributed populations;
Mann-Whitney rank sum tests were used to assess differences between populations that were not normally distributed. To compare
parameters of more than two groups, we used one-way analysis of
variance (ANOVA) if data were normally distributed or a KruskalWallis one-way ANOVA on ranks if data were not normally
distributed.
RESULTS
Motor neuron morphology
The cell bodies of all the motor neurons that innervate a
swimmeret are located in the same abdominal ganglion. Backfills
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
METHODS
93
94
C. M. SHERFF AND B. MULLONEY
Physiological identification of swimmeret motor neurons
During spontaneous production of the swimmeret motor
pattern, large bursts of excitatory RS action potentials alter-
nate with bursts of excitatory PS action potentials (Fig. 2).
Smaller bursts of impulses in axons of peripheral inhibitory
motor neurons often occur between the larger, excitatory
bursts. A cell was identified as a motor neuron if it had an
axon in one of the N1 branches, which was determined by
injecting current into the neuron to elicit orthodromic spikes
in one of the N1 branches time-locked to intracellularly recorded action potentials, and by stimulating branches of N1
to elicit an antidromic action potential. RS motor neurons
had axons in the anterior branch of N1; PS motor neurons
had axons in the posterior branch. Neurons were classified
as excitatory or inhibitory by the phases of their potentials’
oscillations relative to the major bursts of impulses in the
nerve that contained their axons (Fig. 3) (Stein 1971).
The identifications of 18 neurons as PS or RS motor neurons with the use of these criteria were tested anatomically
by filling the neurons with Neurobiotin. We observed no
contradictions between the physiological identifications and
the structures of the filled neurons. Motor neurons could be
distinguished from those sensory neurons that also have
axons in N1 by their central cell bodies; all known sensory
neurons except the two NSSRs have peripheral cell bodies.
One other neuron with an axon in N1, the segmental giant
interneuron (Heitler and Darrig 1986; Roberts et al. 1982),
also has a central cell body. Both this interneuron and the
NSSRs could be identified by their characteristic electrical
activity and by their shape (Fig. 1A).
Passive properties of swimmeret motor neurons
To ensure that errors in identification of motor neurons
were not biasing these physiological results, we compared
the cumulative frequency distributions of Vm , Rin , and tm
measured in physiologically identified motor neurons with
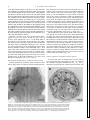
FIG . 1. A: whole mount of abdominal ganglion 3 that contained backfills of a few swimmeret motor neurons. Their cell
bodies (cb) were clustered near the base of the swimmeret nerve (N1). Each motor neuron sent a process into the lateral
neuropil (LN) and an axon out the ipsilateral N1. Within the LN, these neurons had many secondary branches. Large, lightly
stained processes in posterior median region are parts of the segmental giant interneuron, not of a motor neuron (see RESULTS ).
This is a frontal view from the ventral side; anterior is at top. B: cross section of an N1 proximal to division into powerstroke (PS) and return-stroke (RS) branches. Diameters of axons were measured from 2-mm cross sections like this one.
The 2 large axons ( ∗ ) are those of nonspiking stretch receptors. Smallest axons ( z ) are probably sensory afferents.
/ 9k16$$jy07
J658-6
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
of the RS and PS branches of the nerve, N1, that innervates
each swimmeret, revealed that cell bodies of RS motor neurons
were clustered together on the ventral surface of the ganglion,
just anterior to the base of N1, whereas PS cell bodies formed
their own ventral cluster just posterior to the base of N1. Regardless of whether they were RS or PS neurons, the shapes of most
swimmeret motor neurons were similar (Fig. 1A). The primary
neurite extended from the cell body toward LN (Skinner 1985)
and divided into two primary branches: one branch projected
anteriorly and medially to the midline of the ganglion toward
the contralateral LN, the other branch projected laterally to exit
the ganglion as the axon in N1. Each of these primary branches
had many fine secondary branches in the LN, where they synapse
with other swimmeret motor neurons and with interneurons of
the local swimmeret pattern-generating circuit (Murchison et al.
1993; Paul and Mulloney 1985a,b).
Diameters of axons in N1 were measured from photographs of 2-mm cross sections of four N1s (Fig. 1B). In
each N1, axons ranged from õ2 to Ç35 mm diam. Most of
the axons in N1 are not axons of motor neurons. The numerous small axons are probably sensory hair afferents (Killian
and Page 1992a,b; Nordlander and Singer 1973); and the
two largest axons belong to the nonspiking stretch-receptor
neurons (NSSRs) (Heitler 1982; McDonald 1981). Thus
most of the motor neuron axons are probably in the middle
of the observed range, between 2 and 25 mm diam. These
values may underestimate the actual sizes because of shrinkage that occurs during processing of the tissue. If our tissue
shrank by 20%, as observed by Edwards et al. (1994), axon
diameters would range from õ3 to Ç44 mm.
PROPERTIES OF SWIMMERET MOTOR NEURONS
95
the distributions of these parameters measured in a separate
set of 16 motor neurons whose identities we confirmed by
filling them with Neurobiotin (Fig. 4). For each parameter,
these distributions were not significantly different (Vm : P Å
0.112; Rin : P Å 0.360; tm : P Å 0.715, Mann-Whitney rank
sum test). We conclude that the observed distributions of
these parameters from our larger set of physiologically iden-
tified motor neurons were not distorted by inclusion of neurons with properties different from those of anatomically
identified swimmeret motor neurons, so in the rest of this
paper we will focus on the larger set of results from physiologically identified neurons.
Vm was measured during stable neuropil recordings. If the
motor neuron’s Vm was oscillating with the swimmeret motor
FIG . 3. The 4 functional types of swimmeret motor neurons (mn) were identified
physiologically by phases of oscillations of
their membrane potentials in the motor pattern and by the branch of N1 in which their
axons occurred. One example of each type
of swimmeret motor neuron is illustrated.
Experimental depolarization caused action
potentials in each motor neuron that were
matched 1:1 with spikes recorded from 1
of the branches of N1. PSI, power-stroke
inhibitor.
/ 9k16$$jy07
J658-6
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
FIG . 2. Examples of activity in the swimmeret system from 3 preparations, including extracellular recordings of spikes
in RS and PS branches of N1 and an intracellular recording from a swimmeret motor neuron. In active preparations, bursts
of action potentials in RS excitor (RSE) axons alternate with bursts in PS excitor (PSE) axons. In RS record in B, bursts
of impulses in an inhibitory motor neuron (RS inhibitor, RSI) alternate with RSEs. In active preparations, membrane potentials
of most motor neurons oscillated in phase with the expressed motor pattern (A and B). Some motor neurons also fired action
potentials (A). When preparation was quiescent (C), motor neurons did not oscillate; a few motor neurons fired action
potentials tonically, but most were quiet.
96
C. M. SHERFF AND B. MULLONEY
pattern, Vm was recorded as the midpoint in the oscillation.
Vms of the swimmeret motor neurons were not normally
distributed, but skewed toward less polarized values (Fig.
4). Median Vm was 059.0 mV (n Å 168), with 25th and
75th percentiles of 066.0 and 053.0 mV.
The Rins of five motor neurons were measured in two
ways: as the slope of the current-voltage relation near resting
potential (measured in discontinuous current-clamp mode),
and as the steady-state response to a small hyperpolarizing
current (measured in bridge mode). Within Ç20 mV of
resting potential, the measured current-voltage relations of
these five neurons were linear (Fig. 5A). For each cell, the
two methods yielded the same result, so we measured Rin in
most cells by averaging steady-state voltage responses to 50
00.5-nA current pulses (Fig. 5). Measured Rins of swimmeret motor neurons were not normally distributed, but were
skewed toward lower values (Fig. 4). The median Rin was
6.4 MV (n Å 152), with 25th and 75th percentiles of 3.4
and 13.7 MV.
To measure the tm of swimmeret motor neurons, we injected brief pulses of hyperpolarizing current through a
bridge circuit and recorded the return of Vm to rest. The
absolute values of the averaged transient response were fit
with one-, two-, and three-exponential equations of the form
Vm Å C0 / ∑ Cx exp( 0t/ tx )
where C0 normalizes the steady-state Vm to 0 mV, Cx is a
portion of the total voltage response, tx is time constant, and
t is the time in milliseconds. The abilities of these different
equations to fit the measured data were compared, and the
equation that best fit the data was selected as the best description of the cell’s properties. If two equations gave equally
good fits but predicted different time constants, the cell was
excluded from further analysis.
The longest of these tx recovered from the equation that
best fit the measured response was taken to be tm (Rall
1969). An example of a transient response recorded in one
motor neuron is shown in Fig. 5B. This cell was fit with the
three-exponential equation
Vm Å 0.20 / 7.08 exp( 0t/0.33) / 3.28 exp( 0t/1.96)
/ 4.00 exp( 0t/8.84)
/ 9k16$$jy07
J658-6
yielding a tm value of 8.84 ms, and two equalizing constants
of 0.33 ms and 1.96 ms. The graph of this equation is superimposed on the measured data in Fig. 5 Bi, and the residual
differences between this curve and the measured points are
plotted in Fig. 5Bii; the coefficient of regression of these
data to this equation was 0.9998.
The cumulative distribution of measured tms of swimmeret motor neurons was not normally distributed, but
skewed toward lower values (Fig. 4). The median tm was
9.2 ms (n Å 49), with 25th and 75th percentiles at 5.7 ms
and 14.5 ms. All cells were fit with equations that yielded
regression coefficients between 0.997 and 1.00, with a median regression coefficient of 0.999.
Effects of recording site on these results
The cell body of a swimmeret motor neuron (Fig. 1) is
at the end of a cable whose diameter is larger than that of
many secondary processes, but smaller than that of the primary neurite in the neuropil, and smaller than the diameter
of the axon. Because most of the synaptic contacts onto
swimmeret motor neurons are located on their processes in
the LN, we usually recorded intracellularly from their major
processes in this neuropil. To see how the recording site
influenced our measurements of passive properties, we compared the distributions of tm and Rin recorded from neuropil
with those recorded from cell bodies (n Å 17). tms measured
in the cell body were shorter than those measured in the
neuropil (median: 5.2 vs. 10.2 ms, Mann-Whitney rank sum
test, P Å 0.05). This difference resembles those seen in
other crayfish neurons (Czernasty et al. 1989; Takahashi et
al. 1995), and might be due to an elevated resting potassium
current in the cell bodies (Chrachri 1995).
Rin is determined not only by local membrane resistance but
also by the axial resistances through which currents flow to
other parts of the neuron (Edwards and Mulloney 1987; Rall
et al. 1992), so we expect that Rin would change if we measured
it at different locations in a cell with a complex branching
structure. The median Rin measured in cell bodies was 16 MV,
whereas the median measured in the neuropil was 6.4 MV
(Mann-Whitney rank sum test, P Å 0.14). This difference
probably reflects the structure of these cells (Fig. 1A); although
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
) and
FIG . 4. Cumulative frequency distributions of passive properties of all measured swimmeret motor neurons (
of the subset whose identities were confirmed anatomically ( ●; n Å 16). Number of measurements of each parameter made
from unfilled neurons is shown on left ordinate of each graph.
PROPERTIES OF SWIMMERET MOTOR NEURONS
97
the diameter of the cell body is relatively large, only one process leaves it, so injected currents encounter fewer conductive
pathways than they would in a major process in the neuropil.
Passive properties of different kinds of swimmeret motor
neurons
To see whether different kinds of swimmeret motor neurons had different passive properties, we compared Vms, Rins,
and tms of excitors and inhibitors. These data were not normally distributed (cf. Fig. 4). Mann-Whitney rank sum tests
of each parameter showed that excitors did not differ significantly from inhibitory motor neurons (P ú 0.46).
Comparisons of the properties of PSE, RSE, PSI, and
RSI motor neurons revealed that RSE motor neurons had a
slightly lower average Vm than the others (Table 1). The
/ 9k16$$jy07
J658-6
mean Vm of RSE neurons was 052.1 mV; a one-way
ANOVA indicated that the distributions of RSE potentials
were not different from the others (P Å 0.122). The Rins
and tms of these four kinds of neurons also differed twofold
(Table 1). However, a Kruskal-Wallis ANOVA on ranks
for Rin indicated that they were probably not different (P Å
0.283), and a one-way ANOVA of tm indicated that they
too were not different (P Å 0.461). Thus differences in the
resting membrane conductances of PSE and RSE neurons,
which would cause differences in tm , can offer only a partial
explanation of their observed differences in Vm .
Influence of cell size on integrative properties
In lobsters, the size of a swimmeret motor neuron’s extracellularly recorded action potential is correlated with the
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
FIG . 5. Ai: single response to a 00.5-nA pulse of current, and below, it averaged response to 50 such pulses. This neuron
had an input resistance of 6.0 MV. Aii: current-voltage relations of 1 RS motor neuron ( Rin Å 23.6 MV ) and 1 PS motor
neuron (Rin Å 6.7 MV ), plotted as deviations from resting potential. Bi: decay of a transient voltage response recorded in
a swimmeret motor neuron ( h ) plotted as absolute value of response vs. time, and graph of 3-exponential equation fitted to
these data (
). For clarity, only every 4th point is plotted. Time 0: time at which 05-nA current pulse stopped. Bii:
residual differences between data points and fitted curve in Bi.
98
C. M. SHERFF AND B. MULLONEY
1. Passive properties of different kinds of swimmeret
motor neurons
TABLE
Membrane
Potential, mV
PSE
RSE
PSI
RSI
059.8
052.1
059.8
060.0
{
{
{
{
1.4
3.8
5.5
3.2
(45)
(18)
(5)
(7)
Input Resistance,
MV
8.0,
9.0,
4.8,
17.7,
4.0–13.3 (41)
3.5–25.9 (16)
2.0–8.8 (4)
7.1–43.8 (7)
Membrane
Time Constant,
ms
14.2
9.7
10.8
8.3
{
{
{
{
2.3
1.0
1.1
3.5
(15)
(5)
(3)
(3)
For each kind of motor neuron, the distributions of membrane potential
and time constant were normal; statistics for these parameters are means {
SE, with number of motor neurons tested in parentheses. The distributions
of input resistances were not normal; statistics are medians, 25th and 75th
percentiles, with number of motor neurons tested in parentheses. PSE,
power-stroke excitor; RSE, return-stroke excitor; PSI, power-stroke inhibitor; RSI, return-stroke inhibitor.
/ 9k16$$jy07
J658-6
Changes in passive properties of swimmeret motor
neurons associated with changes in the state of the
swimmeret system
Both in the intact crayfish and in vitro, the state of the
swimmeret system can change from quiescent to active, a
change marked by the expression of coordinated bursts of
impulses in the nerves that innervate the swimmerets (Fig.
2). In a few isolated ventral nerve cords, these transitions
occurred spontaneously and, in some preparations, frequently. When the state of the system changed from quiet
to active, the Vms of most motor neurons began to oscillate
in phase with the motor pattern expressed in their own ganglion. The oscillations of swimmeret motor neurons are not
due to intrinsic cellular properties, but are caused by synaptic
input from the local pattern-generating circuit (Murchison
et al. 1993). These oscillations ceased when the system
stopped producing coordinated swimmeret activity (Fig. 2).
From these observations it seemed possible that synaptic
input from the local circuit might cause major changes in
the passive properties of swimmeret motor neurons, changes
that would alter the way they integrated synaptic information
from sources other than the local pattern-generating circuit.
To examine the plausibility of this idea, we first compared
the passive properties of motor neurons whose potentials
oscillated when the system was active with those of neurons
whose potentials did not oscillate. Neurons whose potentials
oscillated were slightly more depolarized ( /3 mV, P Å
0.104) and had higher Rins ( /2.5 MV, P Å 0.146) and longer
tms ( /4.0 ms, P Å 0.020) than neurons whose potentials did
not oscillate. The same trend occurred when we compared
neurons measured in active preparations with those measured in quiet preparations. Motor neurons that fired during
the depolarizing phase of their oscillations were also more
depolarized ( /7 mV, P Å 0.006) and had higher mean Rins
( /4.5 MV, P Å 0.100) and longer mean tms ( /9.5 ms,
P Å 0.034) than neurons that did not fire.
The magnitudes of these mean difference were less than
the ranges of values measured under these different conditions, so state changes do not explain all the observed variability of passive properties of swimmeret motor neurons
(Fig. 4). When transitions from quiescence to active firing
occurred during a continuous record from one neuron, we
observed only small changes in the neuron’s passive properties: Rin changed by an average of 3%, tm increased on
average by 35%.
DISCUSSION
Passive properties and motor neuron function
Within each set of swimmeret motor neurons, we recognize four functional types (PSE, RSE, PSI, and RSI) that
have different targets, different neurotransmitters, and different functions. Because excitor motor neurons and inhibitor
motor neurons of the fast-flexor muscles differ in their passive properties in ways that contribute to the performance
of the tail-flip escape circuit (Edwards and Mulloney 1987),
we thought that passive properties of these swimmeret neurons would differ in ways that might reveal something of
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
order in which the neuron is recruited during each burst of
impulses in synergist motor neurons (Davis 1971). This
observation suggested that some passive properties of swimmeret motor neurons might differ systematically with their
size. To test this hypothesis, swimmeret motor neurons were
classified as small, medium, or large on the basis of the size
of their impulses (Figs. 3 and 6). Each neuron was stimulated by injecting current with a bridge circuit, and its impulses recorded by the microelectrode were matched with
extracellular spikes in a branch of N1 (Figs. 3 and 6). The
size of each neuron’s extracellular spike was measured and
normalized to the smallest unit recorded from that nerve in
the same experiment. With the use of these measurements,
we divided the data from motor neurons into three size categories: small motor neurons had spikes between 1 and 4
times larger than the smallest spike, medium neurons had
spikes 7–20 times larger, and large motor neurons had spikes
25–80 times larger than the smallest unit (Fig. 6).
Different sizes of motor neurons received qualitatively
similar synaptic drive, but as a group, small swimmeret motor neurons were more likely to fire impulses than were
larger ones ( x 2 test, P Å 0.01). The Vms of between 75%
and 89% of the small, medium, and large neurons oscillated
in phase with the motor pattern. However, although 48% of
these small motor neurons also fired impulses, only 8% of
the medium motor neurons, and none of the large motor
neurons, fired action potentials during the depolarizing phase
of these spontaneous oscillations. The swimmeret motor patterns expressed spontaneously in our experiments did not
cover the full range of periods and intensities of which the
system is capable in the intact crayfish (Braun and Mulloney
1993; Davis and Kennedy 1972); this spontaneous activity
was relatively weak and slow. When the system is producing
stronger activity, it seems likely that these larger motor neurons would be systematically recruited (Davis 1971).
Different sizes of motor neurons differed significantly in
their Vms (Fig. 7). Small motor neurons had, on average,
less polarized Vms than medium or large motor neurons
(P õ 0.05). Mean Vms for small, medium, and large motor
neurons were 057, 063, and 069 mV. Eighty-three percent
of the RS motor neurons from which we recorded were
classified as small, whereas only 50% of the PS motor neurons were small; this size-related difference in Vm might
explain why RSE neurons also had a lower average Vm (Ta-
ble 1). The Rins and tms of neurons in these different size
categories were not significantly different (Fig. 7).
PROPERTIES OF SWIMMERET MOTOR NEURONS
99
the mechanisms that generate their normal patterned firing.
Therefore it was surprising that these different types had
similar passive properties (Table 1). In particular, the similarity of their tms suggests that these neurons have similar
membrane current densities, and integrate synaptic currents
in similar ways. It follows that differences in the passive
properties of these four types of motor neuron are unlikely
to be important factors in generating the motor pattern that
drives normal swimmeret movements.
The tms we measured in these neurons were quite brief
(Table 1) compared with those of some other crustacean
motor neurons (e.g., Golowasch and Marder 1992). Given
these brief time constants, we conclude that their membrane
resistances are low. The neurons’ space constants are small,
too, because the space constant is proportional to the square
root of membrane resistance. This combination of small
space constants and brief time constants implies that postsynaptic potentials and retrograde action potentials invading the
central processes of swimmeret motor neurons would attenuate quickly as they spread passively through the neurite to
the soma. It is characteristic of recordings from the cell
bodies of these motor neurons, even when the system is
/ 9k16$$jy07
J658-6
vigorously producing coordinated activity in every ganglion,
that action potentials are õ5 mV high (Heitler 1983; Stein
1977).
Passive properties and motor neuron size
In preparations that spontaneously produced a weak motor
pattern, small motor neurons oscillated and often fired action
potentials, but larger motor neurons either oscillated with a
very low amplitude or did not oscillate and were silent. In
preparations with a stronger motor pattern, small, medium,
and large cells oscillated and some of the small and medium
motor neurons fired action potentials. Both in lobster swimmeret system (Davis 1971; Davis and Kennedy 1972) and
in cat spinal cords (Henneman and Mendell 1977), motor
neurons that innervate the same muscle are recruited during
spontaneous movements or graded nerve stimulation in order
of increasing size. In the lobster, stimulation of certain axons
in the nerve cord elicits firing from motor neurons with small
extracellular spikes. Stronger stimulation recruits larger units
in addition to the small ones. Similarly, in the cat, increasing
muscle tension was associated with the recruitment first of
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
FIG . 6. Sizes of impulses in motor neuron, measured relative to smallest active N1 unit, plotted as a histogram. Motor
neurons between 1 and 4 times the size of the smallest unit were classified as small (S); those between 7 and 20 times
smallest unit were medium (M); and those 25–80 times smallest unit were large (L). These ranges are marked by shaded
boxes. Recordings from motor neurons in each of these categories, and their corresponding action potentials recorded from
N1 when current pulses were injected into them, are shown below.
100
C. M. SHERFF AND B. MULLONEY
small, then of larger a-motor neurons (Henneman et al.
1965). In recordings of PS and RS activity driving a whole
crayfish swimmeret, we also saw systematic recruitment of
larger units when the level of excitation given to the system
increased (Braun and Mulloney 1993).
Although some small swimmeret motor neurons had
higher Rins than did larger ones (Fig. 7), a feature of motor
neurons in other systems (Burke 1968; Burke et al. 1982;
Kernell and Zwaagstra 1981; Zengel et al. 1985; and Henneman et al. 1965), Zucker (1973) has shown that a strict
scaling of Rin with the surface area of a neuron is not sufficient to account for a size principle, the orderly recruitment
reviewed above, because a proportional change in Rin and
surface area would preserve the densities of all kinds of
channels; simply increasing the area of the cell’s membrane
would lower Rin but increase the absolute number of synaptic
receptors, and so increase the synaptic currents. The simplest
sort of scaling of neurons with similar time constants leads
to bigger neurons with the same thresholds for both injected
currents and synaptic excitation. However, given a case
where small motor neurons receive a higher density of excitatory synapses, or have lower voltage thresholds for firing
action potentials, or where large neurons have a disproportionately larger soma size than small motor neurons, a size
principle could result (Zucker 1973). In the swimmeret system, the small motor neurons had more depolarized resting
Vms, which might act to keep them closer to firing threshold
than the large motor neurons. In our experiments, large motor neurons never spontaneously fired action potentials even
when the swimmeret system was active. We did observe that
RSE motor neurons were normally less polarized than PSE
motor neurons, a difference that might reflect differences in
the synaptic drive to neurons of each type. However, all of
our sample of RSE motor neurons consisted of small neurons, but half of our PSE neurons were either medium or
large, so this difference in mean potential might be incidental
to this difference in sizes of the PSE and RSE neurons we
sampled (Fig. 7).
/ 9k16$$jy07
J658-6
Synaptic influences on the integrative properties of
swimmeret motor neurons
External influences like neuromodulators or synaptic input
can alter passive membrane properties by gating ionic conductances (Golowasch and Marder 1992; Moore and Buchanan 1993). We saw little evidence that such factors had
significant effects on the passive properties of swimmeret
motor neurons. The distributions of Vm , Rin , and tm recorded
from neurons in quiescent preparations were similar to those
recorded from neurons in active preparations that were receiving phasic synaptic input from the pattern-generating
circuit. There were some differences between motor neurons
that were themselves in different activity states. Time constants of oscillating cells were longer than those in cells that
were not oscillating. Cells that were firing action potentials
had longer tms and lower Vms than cells that were not firing.
These trends indicate that synaptic drive from the active
pattern-generating circuits is associated with a change in
membrane conductance in these motor neurons. Quiet, nonoscillating motor neurons seem to be tonically inhibited by
currents that hyperpolarize them, and when the swimmeret
system changes to an active state, these tonic currents disappear. The magnitudes of these changes were smaller, however, than the differences between tm of flexor excitor and
flexor inhibitor motor neurons that contribute to effective
performance of the escape circuit (Edwards and Mulloney
1987).
Changes on this scale might be functionally important
because they are consistent with measured changes from
swimmeret motor neurons exposed to g-aminobutyric acid
(GABA) and glutamate (Sherff and Mulloney 1996). Both
of these neurotransmitters inhibit swimmeret motor neurons,
often hyperpolarizing them and decreasing Rin by amounts
similar to those seen during the transitions in state described
here. It seems unlikely that changes this small would significantly alter the integrative characteristics of the motor neurons, but without modeling the neurons in detail, it is prema-
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
FIG . 7. Box plots comparing passive properties of small, medium, and large motor neurons. Solid lines inside boxes:
means. Dotted lines: medians. Top and bottom boundaries of boxes: 25th and 75th percentiles. Bars: 10th and 90th percentiles.
Numbers: number of neurons in sample. Asterisks and daggers: distributions are significantly different ( P õ 0.05).
PROPERTIES OF SWIMMERET MOTOR NEURONS
ture to conclude that these changes do not have a functional
significance.
/ 9k16$$jy07
J658-6
We thank W. Hall for help with the histology and archeological excavations in the data books. D. Edwards, A. Ishida, and K. Sigvardt read drafts
of this manuscript and made important suggestions.
This work was supported by National Science Foundation Grants BNS
87-19397, IBN 92-22470, and IBN 95-14889.
Address for reprint requests: C. M. Sherff, Psychology, Yale, PO Box
208205, New Haven CT 06520-8205.
Received 14 August 1996; accepted in final form 5 March 1997.
REFERENCES
ATWOOD, H. L. Organization and synaptic physiology of crustacean neuromuscular systems. Prog. Neurobiol. 7: 291–391, 1976.
BRAUN, G. AND MULLONEY, B. Cholinergic modulation of the swimmeret
system in crayfish. J. Neurophysiol. 70: 2391–2398, 1993.
BRAUN, G. AND MULLONEY, B. Coordination in the crayfish swimmeret
system: differential excitation causes changes in intersegmental phase.
J. Neurophysiol. 73: 880–885, 1995.
BURKE, R. E. Group la synaptic input to fast and slow twitch motor units
of cat triceps surae. J. Physiol. Lond. 196: 605–630, 1968.
BURKE, R. E., DUM, R. P., FLESHMAN, J. W., GLENN, L. L., LEV -TOV, A.,
O’DONOVAN, M. J., AND PINTER, M. J. An HRP study of the relation
between cell size and motor unit type in cat ankle extensor motoneurons.
J. Comp. Neurol. 209: 17–28, 1982.
CHRACHRI, A. Ionic currents in identified swimmeret motor neurones of the
crayfish Pacifastacus leniusculus. J. Exp. Biol. 198: 1483–1492, 1995.
CHRACHRI, A., NEIL, D., AND MULLONEY, B. State-dependent responses of
two motor systems in the crayfish, Pacifastacus leniusculus. J. Comp.
Physiol. A Sens. Neural Behav. Physiol. 175: 371–380, 1994.
CZTERNASTY, G., KADO, R. T., AND BRUNER, J. Analysis of mechanisms of
spiking in normally ‘‘non-spiking’’ motoneurone somata in crayfish. J.
Exp. Biol. 147: 91–110, 1989.
DAVIS, W. J. Neural control of swimmeret beating in the lobster. J. Exp.
Biol. 50: 99–117, 1969.
DAVIS, W. J. Functional significance of motoneuron size and soma position
in swimmeret system of the lobster. J. Neurophysiol. 34: 274–288, 1971.
DAVIS, W. J. AND KENNEDY, D. Command interneurons controlling swimmeret movements in the lobster. I. Types of effects on motorneurons. J.
Neurophysiol. 35: 1–12, 1972.
DE RUYTER VAN STEVENINCK, R. R. AND LAUGHLIN, S. B. The rate of information transfer at graded-potential synapses. Nature Lond. 379: 642–
645, 1996.
EDWARDS, D. H. AND MULLONEY, B. Synaptic integration in excitatory and
inhibitory crayfish motoneurons. J. Neurophysiol. 57: 1425–1445, 1987.
EDWARDS, D. H., YEH, S.-R., BARNETT, L. D., AND NAGAPPAN, P. R.
Changes in synaptic integration during the growth of the lateral giant
neuron of crayfish. J. Neurophysiol. 72: 899–908, 1994.
GOLOWASCH, J. AND MARDER, E. Proctolin activates an inward current
whose voltage dependence is modified by extracellular Ca 2/. J. Neurosci.
12: 810–817, 1992.
HEITLER, W. J. Coupled motoneurones are part of the crayfish swimmeret
central oscillator. Nature Lond. 275: 231–234, 1978.
HEITLER, W. J. Non-spiking stretch receptors in the crayfish swimmeret
system. J. Exp. Biol. 96: 355–366, 1982.
HEITLER, W. J. The control of rhythmic limb movements in crustacea. Symp.
Soc. Exp. Biol. 37: 351–382, 1983.
HEITLER, W. J. AND DARRIG, S. The segmental giant neurone of the signal
crayfish, Pacifastacus leniusculus, and its interactions with abdominal
fast flexor and swimmeret motoneurones. J. Exp. Biol. 121: 55–75, 1986.
HENNEMAN, E. AND MENDELL, L. M. Functional organization of motoneuron
pool and its inputs. In: Handbook of Physiology. The Nervous System.
Motor Control. Bethesda, MD: Am. Physiol. Soc., 1977, sect. 1, vol. II,
p. 423–507.
HENNEMAN, E., SOMJEN, G., AND CARPENTER, D. O. Functional significance
of cell size in spinal motoneurons. J. Neurophysiol. 28: 560–580, 1965.
HILLE, B. Ionic Channels of Excitable Membranes. Sunderland MA: Sinauer, 1992.
HODGKIN, A. L. AND RUSHTON, W.A.H. The electrical constants of a crustacean nerve fiber. Proc. R. Soc. Lond. B Biol. Sci. 133: 444–479, 1946.
KERNELL, D. AND ZWAAGSTRA, B. Input conductance, axonal conduction
velocity, and cell size among hindlimb motoneurones of the cat. Brain
Res. 203: 311–326, 1981.
KILLIAN, K. A. AND PAGE, C. H. Mechanosensory afferents innervating the
swimmerets of the lobster. I. Afferents activated by cuticular deformation.
J. Comp. Physiol. A Sens. Neural Behav. Physiol. 170: 491–500, 1992a.
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
Why do different types of swimmeret motor neurons have
such similar integrative properties?
Swimmeret motor neurons that are active in different
phases of the motor pattern, and those that have different
peripheral functions, nonetheless are similar in their Rins and
tms (Table 1). These similarities imply that they integrate
synaptic currents in similar ways. Because measured values
of tms in other types of neurons range from õ2 ms to ú1,000
ms (Hille 1992), and other motor neurons in these same
ganglia have significantly different time constants that affect
their performance (Edwards and Mulloney 1987), the narrow ranges of these parameters observed in these swimmeret
neurons suggest that some requirement of the system has
selected for a narrow window of passive properties, despite
difference in the neurons’ peripheral targets and functions.
The function common to all swimmeret motor neurons is
to transform periodic synaptic currents from the premotor
pattern-generating module into bursts of impulses that will
trigger effective movements of the required period and
power. Periods of these movements in intact animals range
from õ0.2 to Ç2 s. The power produced also varies widely,
although its range has not been quantitatively described.
Unlike the extensor and flexor motor neurons of the tail-flip
system (Wine and Krasne 1982), which transform a brief
synaptic input into a brief, relatively invariant synchronized
discharge, swimmeret neurons fire periodic bursts that vary
in intensity. These bursts last about half the period of the
cycle (Fig. 2), and both the number and frequency of impulses in each burst can be regulated to control the force of
the resulting movements (Braun and Mulloney 1993; Davis
1969).
On the basis of these considerations, we suggest that two
features of the swimmeret system—brief tms of motor neurons and graded synaptic transmission by the local interneurons that drive them (Paul and Mulloney 1985a,b) —are
design constraints imposed by the system’s need for periodic
bursts of impulses that can be graded in intensity. The tms
of these local interneurons are ú40 ms (D. H. Paul and B.
Mulloney, unpublished data), 4 times longer than those of
the motor neurons they drive. According to our hypothesis,
the force of contraction in swimmeret muscles is regulated
through a continuous range by continuous variation in firing
frequency of the motor axon (Atwood 1976). High impulse
frequencies during each burst require high densities of sodium and potassium channels in the axons (cf. Hodgkin and
Rushton 1946), and the presence of these channels produces
brief tms because some minor fraction of these channels will
be open, even at rest potential. However, in neurons with
brief tms, summation of transient synaptic currents triggered
by presynaptic action potentials would produce rapidly fluctuating changes in Vm and impulse frequency, discontinuous
transitions in force of the motor unit, and therefore a more
restricted range of power for the system (cf. de Ruyter van
Steveninck and Laughlin 1996). Graded synaptic transmission by premotor interneurons avoids these fluctuations because it causes sustained changes in synaptic conductances
and synaptic currents. These features of graded transmission
permit these motor neurons to change their impulse frequency continuously through a wide range.
101
102
C. M. SHERFF AND B. MULLONEY
/ 9k16$$jy07
J658-6
pattern generator for the crayfish swimmeret. J. Neurophysiol. 54: 28–
39, 1985b.
RALL, W. Time constants and electrotonic length of membrane cylinders
and neurons. Biophys. J. 9: 1483–1508, 1969.
RALL, W., BURKE, R. E., HOLMES, W. R., JACK, J.J.B., REDMAN, S. J., AND
SEGEV, I. Matching dendritic neuron models to experimental data. Physiol. Rev. 72, Suppl.: S159–S186, 1992.
ROBERTS, A., KRASNE, F. B., HAGIWARA, G., WINE, J. J., AND KRAMER,
A. P. Segmental giant: evidence for a driver neuron interposed between
command and motor neurons in the crayfish escape system. J. Neurophysiol. 47: 761–781, 1982.
SHERFF, C. M. AND MULLONEY, B. Motor neurons of the swimmeret system:
membrane properties of antagonists. Soc. Neurosci. Abstr. 1992.
SHERFF, C. M. AND MULLONEY, B. Tests of the motor neuron model of the
local pattern-generating circuits in the swimmeret system. J. Neurosci.
16: 2839–2859, 1996.
SKINNER, K. The structure of the fourth abdominal ganglion of the crayfish,
Procambarus clarkii. II. Synaptic neuropils. J. Comp. Neurol. 234: 182–
191, 1985.
STEIN, P.S.G. Intersegmental coordination of swimmeret motor neuron activity in crayfish. J. Neurophysiol. 34: 310–318, 1971.
STEIN, P.S.G. Application of the mathematics of coupled oscillator systems
to the analysis of the neural control of locomotion. Federation Proc. 36:
2056–2059, 1977.
TAK AHASHI, M., TAK ASHIMA, A., AND TAK AHATA, M. Regional characteristics of the membrane response of an identified crayfish nonspiking interneuron to intracellularly injected current. J. Neurophysiol. 74: 2242–
2250, 1995.
WINE, J. J. The structural basis of an innate behavior. J. Exp. Biol. 112:
283–319, 1984.
WINE, J. J. AND KRASNE, F. B. The cellular organization of crayfish escape
behavior. In: The Biology of Crustacea. Neural Integration and Behavior,
edited by D. C. Sandeman and H. L. Atwood. New York: Academic,
1982, vol. 4, p. 241–292.
ZENGEL, J. E., REID, S. A., SYPERT, G. W., AND MUNSON, J. B. Membrane
electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J. Neurophysiol. 53: 1323–1344, 1985.
ZUCKER, R. S. Theoretical implications of the size principle of motoneurone
recruitment. J. Theor. Biol. 38: 587–596, 1973.
08-05-97 13:23:07
neupal
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.32.247 on June 18, 2017
KILLIAN, K. A. AND PAGE, C. H. Mechanosensory afferents innervating the
swimmerets of the lobster. II. Afferents activated by hair deflection. J.
Comp. Physiol. A Sens. Neural Behav. Physiol. 170: 501–508, 1992b.
LEISE, E. M., HALL, W. M., AND MULLONEY, B. Functional organization of
crayfish abdominal ganglia. I. The flexor systems. J. Comp. Neurol. 253:
25–45, 1986.
LEISE, E. M. AND MULLONEY, B. The osmium-ethyl gallate procedure is
superior to silver impregnations for mapping neuronal pathways. Brain
Res. 367: 265–272, 1986.
MC DONALD, V. N. Central Effects of Motor Neurons on Swimmeret Motor
Output (M.A. thesis). Davis, CA: Univ. of California, Davis, 1981.
MOORE, L. E. AND BUCHANAN, J. T. The effects of neurotransmitters on the
integrative properties of spinal neurons in the lamprey. J. Exp. Biol. 175:
89–114, 1993.
MULLONEY, B., ACEVEDO, L. D., CHRACHRI, A., HALL, W. M., AND SHERFF,
C. M. A confederation of neural circuits: control of swimmeret movements by a modular system of pattern generators. In: Frontiers in Crustacean Neurobiology, edited by K. Wiese, W. D. Krenz, J. Tautz, H.
Reichert, and B. Mulloney. Basel: Birkhäuser, 1990, p. 439–447.
MULLONEY, B. AND HALL, W. M. GABAergic neurons in the crayfish nervous system: an immunocytochemical census of the segmental ganglia
and stomatogastric system. J. Comp. Neurol. 291: 383–394, 1990.
MULLONEY, B. AND HALL, W. M. Neurons with histamine-like immunoreactivity in the segmental and stomatogastric nervous system of the crayfish,
Pacifastacus leniusculus, and the lobster, Homarus americanus. Cell Tissue Res. 266: 197–207, 1991.
MULLONEY, B. AND SELVERSTON, A. I. Organization of the stomatogastric
ganglion of the spiny lobster. I. Neurons driving the lateral teeth. J.
Comp. Physiol. A Sens. Neural Behav. Physiol. 91: 1–32, 1974.
MURCHISON, D., CHRACHRI, A., AND MULLONEY, B. A separate local pattern-generating circuit controls the movements of each swimmeret in
crayfish. J. Neurophysiol. 70: 2620–2631, 1993.
NORDLANDER, R. H. AND SINGER, M. Degeneration and regeneration of
severed crayfish sensory fibers: an ultrastructural study. J. Comp. Neurol.
152: 175–192, 1973.
PAUL, D. H. AND MULLONEY, B. Local interneurons in the swimmeret system of the crayfish. J. Comp. Physiol. A Sens. Neural Behav. Physiol.
156: 489–502, 1985a.
PAUL, D. H. AND MULLONEY, B. Nonspiking local interneuron in the motor