* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ventral Intraparietal Area of the Macaque: Anatomic Location and
Cortical cooling wikipedia , lookup
Environmental enrichment wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroeconomics wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Executive functions wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Visual search wikipedia , lookup
Multielectrode array wikipedia , lookup
Convolutional neural network wikipedia , lookup
Perception of infrasound wikipedia , lookup
Metastability in the brain wikipedia , lookup
Biological neuron model wikipedia , lookup
Neural oscillation wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Development of the nervous system wikipedia , lookup
Central pattern generator wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroanatomy wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Mirror neuron wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Response priming wikipedia , lookup
Biological motion perception wikipedia , lookup
Evoked potential wikipedia , lookup
Time perception wikipedia , lookup
Visual extinction wikipedia , lookup
Nervous system network models wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Optogenetics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Psychophysics wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural coding wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Efficient coding hypothesis wikipedia , lookup
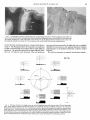
.I~URNALOF Vol. 69. No. Ventral Intraparietal Area of the Macaque: Anatomic Visual Response Properties Location and CAROL L. COLBY, JEAN-RENE DUHAMEL, AND MICHAEL E. GOLDBERG Laboratory e ofSensorimotor Research, National Eve Institute, Bethesda, Mawland 20892 . s 4 SUMMARY AND CONCLUSIONS I. The middle temporal area (MT) projects to the intraparietal sulcus in the macaque monkey. We describe here a discrete area in the depths of the intraparietal sulcus containing neurons with response properties similar to those reported for area MT. We call this area the physiologically defined ventral intraparietal area, or VIP. In the present study we recorded from single neurons in VIP of alert monkeys and studied their visual and oculomotor response properties. 2. Area VIP has a high degree of selectivity for the direction of a moving stimulus. In our sample 72 / 88 ( 80% ) neurons responded at least twice as well to a stimulus moving in the preferred direction compared with a stimulus moving in the null direction. The average response to stimuli moving in the preferred direction was 9.5 times as strong as the response to stimuli moving in the opposite direction, as compared with 10.9 times as strong for neurons in area MT. 3. Many neurons were also selective for speed of stimulus motion. Quantitative data from 25 neurons indicated that the distribution of preferred speeds ranged from 10 to 320” /s. The degree of speed tuning was on average twice as broad as that reported for area MT. 4. Some neurons (22 /4 1 ) were selective for the distance at which a stimulus was presented, preferring a stimulus of equivalent visual angle and luminance presented near (within 20 cm) or very near (within 5 cm) the face. These neurons maintained their preference for near stimuli when tested monocularly, suggesting that visual cues other than disparity can support this response. These neurons typically could not be driven by small spots presented on the tangent screen (at 57 cm). 5. Some VIP neurons responded best to a stimulus moving toward the animal. The absolute direction of visual motion was not as important for these cells as the trajectory of the stimulus: the best stimulus was one moving toward a particular point on the face from any direction. 6. VIP neurons were not active in relation to saccadic eye movements. Some neurons ( 1O/ 17) were active during smooth pursuit of a small target. 7. The predominance of direction and speed selectivity in area VIP suggests that it, like other visual areas in the dorsal stream, may be involved in the analysis of visual motion. INTRODUCTION The parietal cortex of the macaque monkey receives visual input from the middle temporal area (MT) in the superior temporal sulcus. Maunsell and Van Essen ( 1983a) first showed that this projection terminates in the depths of the intraparietal sulcus, in an area that they named the ventral intraparietal area, or VIP. The existence of this projection suggested that the intraparietal sulcus should contain an 902 area with visual properties similar to those in area MT. We report here the existence of such an area and describe its motion-selective response properties. We refer to this area as the physiologically defined VIP. In the present study we examined visual and oculomotor properties of neurons in VIP with stimuli and testing procedures designed to allow direct comparison with results obtained from previous recordings in area MT (Albright 1984; Maunsell and Van Essen 1983a). We found a motion-selective area confined to the floor of the intraparietal sulcus and the adjacent deepest portions of the medial and lateral banks of the sulcus. This physiologically defined area corresponds to a portion of the MT projection zone. Neurons in this area have direction and speed tuning similar to that found in area MT, and some VIP neurons are selective for the distance at which the stimulus is presented. In addition, we found an unusual form of direction selectivity: some neurons are selective for the anticipated point of contact of a stimulus moving toward the animal, regardless of the absolute direction of visual motion. In the course of this study, we discovered that the majority of neurons in VIP have somatosensory as well as visual responses. The somatosensory properties of VIP neurons will be described in a separate paper. Preliminary reports of some of these findings have appeared (Colby and Duhamel 199 1; Colby et al. 1989; Duhamel et al. 1989, 199 1). METHODS Animal preparation Three rhesus monkeys ( A4ctc~~cr mhttu) were surgically prepared for chronic neurophysiological recording by implantation of ocular search coils, head-holding devices, and recording chambers. Surgery was carried out under aseptic conditions by the use of previously described techniques (Goldberg 1983). Briefly, monkeys were pretreated with atropine and sedated with diazepam (Valium) and ketamine hydrochloride. General anesthesia was induced and maintained by isofluorane inhalation. Recording chambers were centered at AP -5.3 and ML 12.2 and placed flat against the skull. This approach produced electrode penetrations parallel to the banks of the intraparietal sulcus rather than in a standard stereotactic plane. All experimental protocols were approved by the National Eye Institute Animal Care and Use Committee and certified to be in compliance with the guidelines set forth in the Public Health Service Guide for the Care and Use of Laboratory Animals. Recordings were made with flexible tungsten electrodes introduced through stainless steel guide tubes placed nearly but not MOTION SELECTIVITY quite through the dura. Guide tubes were stabilized by a nylon grid held rigidly in the recording cylinder (Crist et al. 1988). The grid served as a guide to produce parallel penetrations down the banks ofthe intraparietal sulcus. The rigidity of the system and the atraumatic nature of the electrodes allowed us to perform a large number of penetrations in each hemisphere. Electrode penetrations were made parallel to the cortical surface of the sulcus and extended lO- 15 mm deep. Neuronal response properties were assessed at least every millimeter, beginning at the cortical surface and continuing until the electrode entered the white matter. Adjacent penetrations were made at a l-mm spacing. A systematic mapping of response properties in intraparietal cortex was carried out over the extent of the sulcus accessible from the recording cylinder ( 1.8 cm ID). Electrode penetrations performed several months apart at the same grid locations yielded neurons with similar response types at similar depths. The present report concerns only those neurons identified physiologically as being within area VIP. Visual stimuli were presented on a large, dimly lit, rear-projection tangent screen placed 57 cm from the monkey’s eye. The visual stimuli were typically spots of light, 2” diam, generated by a light-emitting diode ( LED: 0.2 cd/m*) mounted on an optic bench. Stimulus position was set by servo-controlled mirror galvanometers (General Scanner) under computer control. A second optic bench contained a projection lamp and an adjustable slit maker for generating oriented stimuli that were also presented via mirror galvanometers. A third stimulus, the small fixation point (0.25’ ) at the center of the screen, was generated by a stationary LED projector. A fixation point could be placed anywhere on the screen by using one of the optic bench stimuli. The same small spots used for testing direction and speed selectivity were also used for testing saccade and pursuit-related activity. Whole-field random dot stimuli were presented bvd the use of a hand-held projector. Other stimuli were used in additional tests as needed to characterize VIP neurons as completely as possible. For testing distance selectivity, a small (20 x 20 cm) screen could be interposed between the monkey and the tangent screen in such a way that the monkey’s view of the central fixation point was not obscured. The distance of the screen from the monkey’s face was adjustable. An independent projection lamp system, also under computer control, was used to present stimuli on this smaller screen. Hand-held stimuli were also used: an ophthalmoscope was used to generate moving spots and slits displayed on the small screen; small objects (cotton swabs) were used to assess selectivity for motion toward and away from the animal. Quantitative data were obtained with the use of these hand-held stimuli by requiring the monkey to fixate the central point on the tangent screen while the experimenter presented the stimulus and signaled stimulus onset to the computer by a switch closure. This method permitted measurement of response amplitude but not response latency. Stimulus presentation, behavioral monitoring, eye position and unit sampling, and on-line data analysis were controlled by a PDP- 1 l/73 computer (Goldberg 1983). Cells were studied in a series of standard tasks. FIXATION TASK. The monkey gazed at a fixation point and made no eye movement or other discernible response to a second stimulus flashed or moved elsewhere on the tangent screen. In one version of the task, the monkey initiated the trial by grasping a bar. This caused the fixation point to appear, and the monkey had to foveate it to detect a slight dimming that was the signal to release IN AREA VIP 903 the bar. In the other version of the task, the fixation point appeared, and the monkey was rewarded for holding the correct eye position for a certain interval. In both versions the monkey was required to keep his eye within an electronically defined window (2”) centered on the fixation point. The fixation task was used to find the receptive field ofthe cell and to study the visual responsiveness of neurons in a situation where the stimulus had no behavioral significance (Wurtz 1969 ). DELAYED SACCADE TASK. During the fixation period, the stimulus appeared for 5200 ms, and the monkey had to continue to look at the fixation point for at least 500 and up to 1,500 ms after the stimulus had disappeared. The monkey’s task was to make a saccade, after fixation point offset, to the location where the target had been. The target reappeared 200 ms after a correct saccade, and the monkey was rewarded for holding this new eye position. This task was used to dissociate activity related to the appearance of the stimulus from activity associated with the eye movement (Hikosaka and Wurtz 1983). RAMP AND STEP-RAMP TASKS. These tasks were used to measure neural activity during smooth pursuit eye movements. The monkey began by fixating a central point. After a variable period the fixation point began to move, and the monkev had to track it for 800-l ,200 ms (ramp task). In the step-ramp task, the stimulus first jumped to a new position and then began to move (Rashbass 1961). After a neuron had been isolated, the fixation task was used to assess receptive-field properties. The period of fixation per trial varied pseudorandomly from 2,000 to 3,000 ms with a 500-ms intertrial interval. The monkey usually kept his eyes within the electronically defined fixation window even during the inter-trial interval. The receptive field of the neuron was located, and its speed preference was estimated with the use of an ophthalmoscope. The neuron was then tested quantitatively for direction selectivity with computer-generated stimuli. The stimulus was swept through the receptive held in eight standard directions at the approximate best speed. Speed selectivity was then tested quantitatively with the use of a stimulus moving at different speeds in the preferred direction. In these tests for speed and direction selectivity, stimuli were presented in pseudorandom order until data had been collected for 16 trials for each stimulus parameter. Other response properties, such as orientation and distance selectivity, were then assessed by hand and documented quantitatively if appropriate. Finally, eye movement paradigms were run to assess saccadic and pursuit-related activity. Data analysis was begun by measuring the average spike rate in a narrow time window (200 ms for direction, 100 ms for speed ) centered on the peak response. Baseline activity was measured for 200 ms during the fixation period preceding stimulus appearance. After recording was completed, monkeys were anesthetized with an overdose of pentobarbital sodium and perfused through the heart with 10% Formalin. The brain was blocked and equilibrated with 30”/ sucrose. Frozen sections were cut in the plane of the grid used for recording so that complete tracks were visible in single sections. Serial sections, cut at 50 pm and thionine stained, were used to reconstruct the location of each penetration and the depth at which direction-selective neurons were first encountered. A one in five series was reserved for mvelin staining and was used to locate myelin borders in relation to the physiologically determined borders of VIP. Reconstructions of the location and extent of VIP were based on microlesions ( 10 PA for 10 s) made at the end of each experi- 904 C. L. COLBY, J.-R. DUHAMEL, ment. In the first monkey, after having mapped the intraparietal sulcus in both hemispheres, we recorded again at each site and placed a pattern of lesions designed to identify each electrode track and the depths at which specific types of activity were found. In the second monkey, marking lesions were placed at the borders of VIP in one hemisphere. In the other hemisphere an electrolytic lesion was made to destroy all of VIP. Results from this experiment will be reported in a later paper. The third monkey is still being used for recording. Estimates of the size of VIP in this animal were made from the grid recording map. RESULTS Location and borders of area VIP DEFINITION OF VIP. Quantitative data were obtained from 107 single neurons in the ventral intraparietal area of 5 hemispheres in 3 rhesus monkeys. Units were identified as being within VIP on the basis of their response properties and location within the sulcus. The central portion of the medial bank contains neurons with somatosensory responsiveness and no visual activity. The medial border of VIP was identified as the point at which we could first find cells with direction-selective visual responses. The lateral bank of the sulcus contains the lateral intraparietal area (LIP). Neurons in LIP have visual and eye movement-related activity ( Andersen et al. 1990b; Barash et al. 199 1a,b; Colby and Duhamel 199 1; Goldberg et al. 1990). The lateral border of VIP was identified as the point where we first encountered cells with a strong preference for stimuli moving in a specific direction. At the anterior border of VIP, visually responsive neurons gave way to purely somatosensory cells in the fundus. At the posterior border, PHYSIOLOGICAL AND M. E. GOLDBERG motion- and direction-selective neurons gave way to visual cells that were not selective for motion. LOCATION AND sm 0F AREA VIP IN FIVE HEMISPHERES. Area VIP is located in the fundus of the intraparietal sulcus (Fig. I ). In four hemispheres the extent of the zone physiologically identified as VIP was reconstructed histologically. Area VIP occupies the fundus along the middle third of the sulcus. It begins anterior to the end of the annectent gyrus and extends up from the fundus onto both the lateral and medial banks of the sulcus. At its maximum extent, VIP averages 5 mm mediolaterally and 8 mm rostrocaudally. Area VIP is comparable in size with area MT (Gattass and Gross 198 1; Ungerleider and Desimone 1986a; Van Essen et al. 1981). CORRESPONDENCE TO MYELIN-DEFINED REGIONS. Both the lateral and the medial banks of the intraparietal sulcus contain heavily myelinated zones (Blatt et al. 1990; Colby et al. 1988; Seltzer and Pandya 1980; Ungerleider and Desimone 1986). The physiologically defined area VIP described here is confined to the more lightly myelinated zone in the fundus of the sulcus. Histological reconstructions from myelinstained sections show that, in the lateral bank, the ventral border of the densely myelinated zone corresponds to the physiological border between LIP and VIP (Fig. 2). The medial bank of the sulcus also contains a heavily myelinated zone that does not overlap the physiologically defined VIP. Visual response properties Quantitative data on direction selectivity were DIRECTION. obtained for 88 cells. Most VIP neurons are strongly selecA M 5mm FIG. 1. Location of ventral intraparietal area (VIP). The small dorsal view of the hemisphere shows the extent of the recording zone in the opened up intraparietal sulcus, corresponding to sections A through M. Area VIP is the shaded area in the fundus. The 5 sections, spaced 1 mm apart, span VIP from caudal (section I) to rostra] (section M) and show reconstructions, made from serial sections, of electrode tracks that entered VIP. The cross-hatched region in each section shows the extent of cortex containing neurons with the properties defined for VIP. Sections I and J contained a densely myelinated zone (arrows) in the lateral bank of the sulcus adjacent to but not overlapping area VIP. MOTION SELECTIVITY IN AREA VIP F’IG. 2. Correspondence between myeloarchitectonic and physiological borders of ventral intraparietal area (VIP). On the left. the extent ofthe heavily myelinated zone in the lateral bank is indicated by arrows. A microlesion (a) was made at the most dorsal recording site in VIP. The’lesion is more clearly seen in the adjacent thionine-stained section (right). The physiologically defined dorsal border of VIP corresponds to the ventral border of the heavily myelinated zone. tive for direction of stimulus motion. A typical cell is shown in Fig. 3. The histograms show the response to a small spot of light moved through the receptive field in eight different directions. The polar plot shows the average response of the cell over 16 trials for each direction. This cell preferred movement downward and to the right and was very slightly inhibited by movement in the opposite direction. Motion perpendicular to the preferred direction evoked a weaker response. We used two measures to quantify the direction selectivDI = 1.07 EW=l95~ 270° FIG. 3. Direction selectivity of a single neuron in ventral intraparietal area with direction index (DI) and bandwidth (BW). Raster,displays represent trial-by-trial responses to a small spot moving in 8 different directions. The associated histograms show the summed activity over 16 stimulus presentations. Rasters and histograms are aligned on stimulus onset (long vertical line). Vertical calibration mark at the left edge of each histogram indicates a firing rate of 100 spikes/s. Tick marks along the bottom of each histogram are 200 ms apart. Receptive-field location is shown at right. The average response for each direction is shown on the polar plot in the center. Radial axis represents response measured as spikes per second; polar axis represents direction of stimulus motion; small circle at center represents level of spontaneous activity. 906 C. L. COLBY. 20- J.-R. DUHAMEL. N = 82 x = 0.86 SD = 0.46 ul = 158 8 cf ‘O% 22 2 0 0.5 1 .o DIRECTIONALITY 1.5 >1.5 INDEX FIG. 4. Distribution of directionality index for 82 ventral area neurons. This index is based on the difTerence between stimuli moving in the preferred and null directions. intraparietal responses to ity of VIP neurons and compare it with that reported for MT neurons. The first was a standard,direction index (DI) based on the response (average spikes/s - baseline) to a stimulus moving in the preferred direction compared with one moving in the opposite direction (Baker et al. 198 1). This index was calculated by the formula DI T 1 ~ (opposite response/ preferred response) Neurons with a DI near 1 are strongly directional, whereas those with a value near 0 lack direction selectivity. A value greater than 1 reflects inhibition (activity drops below the spontaneous rate) in the nonpreferred direction, as illustrated in Fig. 3. The distribution of DIs for the population is shown in Fig. 4. The average DI for 82 cells was 0.86 t 0.46 (SD). Overall, 80% of the neurons tested had a response that was at least twice as large in the preferred direction as in the null direction. The average response to the preferred direction of motion was 9.53 times that to the opposite direction of motion. The DI is computed from the responses to only two directions of motion. To quantify the sharpness of direction tuning, we used a second measure that is based on the responses to eight directions of motion. For each cell, we constructed a tuning curve by fitting the data with a linear Gaussian function of the following form .\‘l = (1 + /)(, 0.51( ‘, mh,)z/.~l where ~7~is the firing rate in response to a stimulus BW=61 O c89 M. E. GOLDBERG in direction -xi. Four parameters (u, h, -v. and s) are free: u is the minimum firing rate; h is the difference between the maximum and minimum firing rate; &x0is the preferred direction; and s is the standard deviation of the fitted Gaussian. From this Gaussian we measured the full width at half maximum to estimate the tightness of tuning for direction. The mean bandwidth for direction tuning was 129.5” (n = 37). Examples of the variation in direction tuning are illustrated in Fig. 5. For each of three cells, we show a tuning curve fitted to the data obtained for eight different directions of motion. Previous investigations of area MT have noted an asymmetry in the number of neurons with particular direction preferences: for MT neurons recorded in the right hemisphere, there is a significant underrepresentation of rightward and downward directions of stimulus motion (Maunsell and Van Essen 1983a). We examined the distribution of preferred directions for 32 neurons in the left hemisphere that were tested for 8 directions. There was no significant asymmetry in the distribution: all directions of motion were about equally represented (x2 test, P > 0.1). For some cells we tested responsiveness to a large (60 X 60”) random dot stimulus. Out of 23 cells tested, 2 1 responded to the stimulus. For 12 cells, we tested whether the preferred direction of motion using the random dot stimulus matched the preferred direction for a small spot. All of the direction-selective neurons ( 1 1) had matching preferences for the two stimuli; one neuron that responded best to a stationary, flashed spot also responded best to a stationary, flashed random dot stimulus. SPEED. Neurons in VIP are sensitive to speed as well as direction of stimulus motion. Data from a speed-sensitive neuron are shown in Fig. 6. Responses were quantified by measuring average spikes per second during a lOO-ms period centered on the peak response and subtracting the background rate (measured during fixation before onset of the stimulus). The best speed for this neuron was 4O”/s. Across the population of VIP neurons, there was a range of preferred speeds and considerable variability in the degree of selectivity for speed. Representative tuning curves are shown in Fig. 7. Responses of four VIP neurons are shown in which each tuning curve is normalized to its greatest BW=99’ c373 22 sp/sec moving AND -_____I 34 sp/sec BW=l 50° c56 __-_ -____ 26 spkec FIG. 5. Direction tuning curves for 3 representative ventral intraparietal area neurons. Polar plots show average responses to 8 different directions of stimulus motion. Central circles indicate average spontaneous firing rate. Each neuron shows inhibition for stimulus motion in the null direction. Full width at half-maximum measures of tuning bandwidth are indicated for each neuron. MOTION SELECTIVITY IN AREA 907 VIP 1 20"/sec 5”lsec I I 200 msec 80" /set FIG. 6. Responses Raster and histogram on abscissa ) . Degrees/Second ofa ventral intraparietal area neuron to a stimulus moving in its preferred direction at diflerent speeds. displays as in Fig. 3. Tuning curve shows peak firing rate as a function of stimulus speed ( note log scale response. About one-half of the VIP neurons tested ( 13/ 25) were selective for stimulus speed in that the response decreased on either side of the optimum. The remainder ( 11/25 ) were high pass, responding best to the highest speed tested. One neuron was untuned, maintaining a response rate above 0.80 of its best response for all speeds. To compare these results on speed selectivity to previous findings on area MT, we measured both the distribution of preferred speeds and the average speed tuning curve for the 5 IO 20 40 SPEED was) 80 760 population. The distribution of preferred speeds for 25 cells is shown in Fig. 8. This distribution partially overlaps that reported for area MT (Maunsell and Van Essen 1983a), but more neurons in VIP preferred high speeds. To compare the average speed tuning of neurons in MT and VIP, we used Maunsell and Van Essen’s ( 1983a) procedure and plotted normalized response curves for each neuron in VIP and shifted the curves so that the peak responses for all cells were aligned. Points on either side were then averaged to produce a population tuning curve (Fig. 9). The average tuning for speed, measured as full width at half maximum relative to background, is equivalent to a 64-fold change in speed. This average tuning is twice as broad as that found in MT ( Maunsell and Van Essen 1983a). Selectivity for orientation is often tested ORIENTATION. with a moving bar swept across the receptive field. This stimulus confounds orientation and axis of motion and is inappropriate for neurons with strong direction selectivity. To test for orientation selectivity alone, a stationary, flashed bar is the preferred stimulus. VIP neurons generally respond very poorly to such stimuli. Quantitative data on orientation selectivity were taken only for the few neurons 320 ” FIG. 7. Speed tuning curves for 4 representative ventral intraparietal area neurons illustrating the range of tuning for speed. Each curve is normalized to its highest peak firing rate. Neuronal responses range from narrowly tuned to high pass. FIG. 8. intraparietal 10 20 40 80 SPEED (DEGISEC) Distribution of preferred area neurons. 160 320 speeds for the population of ventral 908 C. L. COLBY, J.-R. DUHAMEL, AND 0.9- 0.7- 0 1 I l/64 I l/32 1 l/16 I l/8 l/4 SPEED I I I I I 1 l/2 1 2 4 8 16 RELATIVE TO OPTIMAL wws) FIG. 9. Average speed tuning curve for the population of ventral intraparietal area neurons, including tuned and high-pass neurons. Speed tuning curves for 25 neurons were normalized to their highest peak firing rate, and the peak responses were aligned on 1. Responses on either side of 1 were averaged. Dashed line indicates average normalized background rate of firing. (n = 5) that responded adequately to stationary oriented bars. An example is shown in Fig. 10. This neuron responded to a vertical bar flashed in the receptive field but responded much less to the same bar presented horizontally. Orientation selectivity was independent of stimulus length. For each of these orientation-selective neurons, the 5 5 0 I I II II I I I I I I I II I I I II I I II I II I I II I I I I I I I I I II I I HII I I I II I I I I II II I I I II I II II I I I I II1 I I 62-314-01 111*11 II I Ill1 Ill III IIIIIIII I l I IllIl l I III III11 @I Ill II l IllWrnl II1 rnll l II1 111111 I I 0 *1111II I I II I I Ill IIIII II II 8 I I I I II I I I I I I II II I I I II I I I III II III I to the preferred SELECTIVITY. Some VIP neurons (22/4 1) are selective for the distance at which a visual stimulus is presented. The neuron illustrated in Fig. 1 1 was tested with stationary, flashed stimuli presented at three different distances. In each condition the monkey was required to maintain fixation on a point presented on the tangent screen at 57 cm. The visual stimulus evoked virtually no visual response when presented in the receptive field on the tangent screen. A second, smaller screen was introduced between the monkey and the tangent screen to allow presentation of the same stimulus, equated for luminance and visual angle, at a distance of 10 or 5 cm. These near and ultranear stimuli did evoke a visual response. This neuron’s preference for ultranear stimuli remained even when one eye was patched, suggesting that cues other than disparity were used to determine the location in depth of the stimulus. Forty-one VIP neurons were tested with lights presented on the adjustable screen or with hand-held stimuli (small objects) to determine whether responsiveness could be modulated by bringing the stimulus closer to the animal’s face. Twenty-two neurons showed systematic changes in responsiveness with distance. Of these, 14 preferred ultranear visual stimuli (presented within 5 cm of the monkey’s face), 6 preferred near stimuli (within 20 cm), and 2 responded best to far stimuli. These distance-selective neurons usually could not be driven by any visual stimulus on the tangent screen. Some exhibited a gradient in level of responsiveness, so that a stimulus became increasingly effective in driving the cell when presented at nearer distances, whereas other neurons appeared to have a more sharply delimited WE %z 0.6@ iij cn 0.5 uw u II was perpendicular DISTANCE 0.8- I E. GOLDBERG preferred orientation axis of motion. I- I M. II1 II II I II II I III1 I I I111111 I I I I I I I II11 I I II III Ill II I I I I Ill I II I I II I II I II I II I l 111111 II II II III I II II I II I II II I Ill I I II I II I I I I II RI II11 I I III I II I II I I Ill I I FIG. 10. Orientation selectivity in a ventral intraparietal area neuron. Stimulus was a stationary bar flashed on for 1,500 ms. Vertical calibration bar at left of histograms indicates response rate of 100 spikes/s: tick marks are 200 ms apart. I II I III I I I .&&l, I 62-314-02 MOTION SELECTIVITY 5cm IN AREA 909 VIP 10cm 57 cm I I I I I I I I \ FIG. 1 I. Distance selectivity in a ventral intraparietal area neuron. Each panel shows the response to a stimulus presented at a different distance from the monkey. Stimuli were equated for size and luminance. In each condition the monkey had to maintain fixation on the tangent screen fixation point at 57 cm. Vertical calibration bar indicates 100 spikes/s: tick marks are 50 ms apart. Neuron responds best to the closest stimulus. receptive field. Selectivity for stimulus distance was maintained when cells were tested monocularly. Distance-selective neurons were also direction selective. Many ( 14/22 ) exhibited ordinary, frontoparallel direction selectivity when tested with stimuli presented on a screen close to the animal or with hand-held stimuli. Some neurons, however ( 8 /22), also showed a more complex form of direction selectivity related to stimulus trajectory. These neurons preferred a stimulus moving toward (6) or away (2) from the animal. The critical variable for these neurons was the projected point of contact (or point from which the stimulus was moved away). The neuron shown in Fig. 12A responded to a stimulus moved toward the animal on a trajectory that would (were it continued) contact the chin. It did not respond nearly as well when the same stimulus was moved along a path that would contact the brow. Most VIP neurons have somatosensory as well as visual receptive fields and are readily driven by a somatosensory stimulus applied when the eyes are covered (Duhamel et al. 1989). This neuron had a somatosensory receptive field restricted to the chin. Although this neuron did exhibit planar direction selectivity to a stimulus presented at 20 cm, its best response was to a visual stimulus moving toward its somatosensory receptive field. Changes in the monkey’s eye position did not change this neuron’s preference for stimulus trajectories toward the chin (Fig. 12B). When the monkey was required to fixate a point on the tangent screen either 23’ above or below the center point, the neuron still dis- charged only to stimuli approaching the chin and not to stimuli approaching the brow. Selectivity for stimulus trajectory was not limited to distance-selective neurons. A few neurons ( y1 = 3) that gave good direction- and speed-selective responses to stimuli presented on the tangent screen still showed stronger responses to stimuli approaching the somatosensory receptive field from any direction. The visual response properties described above were assessed by presenting stimuli while the animal maintained fixation. We used saccade and smooth pursuit paradigms to examine neuronal activity in relation to eye movements. SACCADIC EYE MOVEMENTS. The primary paradigm used for testing saccade-related activity was the delayed saccade task in which a stationary target is flashed in the receptive field while the animal fixates (Hikosaka and Wurtz 1983). The offset of the fixation point instructs the monkey to saccade to the location where the target appeared. By introducing a delay between target presentation and performance of the saccade, we can measure separately the cell’s activity in response to target onset ( or offset) and in relation to the saccade. No VIP neuron discharged immediately before a saccade: of 41 neurons tested with this task, 23 gave exclusively visual responses to stimulus onset, and 18 were unresponsive. The small stationary target is a poor 910 C. L. COLBY, J.-R. DUHAMEL, AND M. E. GOLDBERG A Straight Toward Toward From Above - Forehead 84-012-07 Toward \ from Above with Eyes Up ZOOK? Chin from Above with Eyes Up Toward Chin with Eyes Down 20 64-012-08 1A’ FIG. 12. Trajectory selectivity in a ventral intraparietal area neuron. A, tc~pYOU’:stimulus moving toward the brow while monkey fixates central point on tangent screen. Bottom TOW:stimulus moving toward chin while monkey fixates central point. Direction of motion (straight toward vs. down and toward) and portion of visual field stimulated (upper vs. lower) are not as strongly related to response as is projected point of contact of the stimulus. B, tc~p:stimulus moving toward brow while monkey fixates point 23” above central fixation point. Middle: stimulus moving toward chin while monkey fixates above. Bottom: stimulus moving toward chin while monkey fixates point 23” below central fixation point. MOTION MOVING STIMULUS PURSUIT SELECTIVITY IN DARKNESS S E 62-337-03 13. .I: response to a stimulus moving while the monkey tixatcs. i? response during stimulus. FIG;. through the reccptivc field active pursuit of a moving stimulus for VIP neurons, and visual responses were weak compared with those produced by moving stimuli. Two of the 23 visually responsive neurons gave enhanced visual responses to a stationary stimulus when it became the target for a saccade ( Bushnell et al. 198 I). Both of these neurons were also direction selective. PIJRSIJI -1‘. We used ramp and step-ramp parad igms to examme neural activity during smooth pu rsuit. For lo/17 cells tested, neurons were active both during passive stimulation with a moving spot while the animal fixated, and during active pursuit of the moving spot. For six of these neurons, the best d irection for pursui t matched the preferred directi on for stimulus motion. For the re maining four cells, the best pursuit direction was opposite to that preferred for passive stimulus motion. The neuron shown in Fig. 13 had a visu al response to a spot m oved through the receptive field and was driven wh .en the mon key trac ked that stimulus in total darkness. A projection from area MT to parietal cortex was first described in New World monkeys (Spatz and Tigges 1972). In the macaque, Maunsell and Van Essen ( 1983b) observed a projection from area MT to a portion of the intraparietal sulcus, which they called the ventral intraparietal area, or VIP. Their observation was confirmed and extended by Ungerleider and Desimone ( 1986), who showed that MT projects to both the lateral bank and the floor of the intraparietal sulcus. We describe here a physiologically distinct area with receptive-field properties strikingly similar to those of neurons in area MT. This area can be distinguished from neighboring regions on the basis of the prominence of direction selectivity. We have reconstructed the extent of this physiologically defined area and found that it is confined to the fundus of the sulcus and so includes only a part of the region that is connected with MT. Area MT also projects to cortex in the lateral bank where direction and speed tu ning are not prominent. In li ght of the functional hete mrogeneity of the MT-recipient zone in intraparietal sulcus. we propose to restrict the term area VIP to de- IN AREA VIP 911 scribe the physiologically distinct zone in the depths of the sulcus. Area LIP, in the lateral bank, is adjacent to area VIP. Although VIP and LIP share visual inputs from areas MT, MST, FST ( Blatt et al. 1990; Boussaoud et al. 1990; Maunsell and Van Essen 1983b; Ungerleider and Desimone 1986b), and both project to area PO (Colby et al. 1988) there are many other connections that distinguish between the two areas. Area LIP has a large number of visual inputs that have not been observed for area VIP, including inputs from areas V2, V3, V4, PO, MDP, DP, TEO, and TE (Andersen et al. 1990a; Baizer et al. 199 1; Blatt et al. 1990; Cavada and Goldman-Rakic 1989; Morel and Bullier 1990). These extensive visual connections point up an important difference between the two areas: although the visual connections of VIP are exclusively with dorsal stream areas, area LIP is strongly connected with both the dorsal and ventral streams ( Baizer et al. 199 1; Ungerleider and Mishkin 1982). The parietal connections of LIP and VIP also differ. Area LIP projects to, and receives input from, adjacent area 7a, both within the sulcus and on the neighboring convexity cortex (Andersen et al. 1985; Blatt et al. 1990; Seltzer and Pandya 1986). In contrast, area VIP is connected to lateral convexity cortex, closer to the tip of the superior temporal sulcus (STS) and at a distance from the intraparietal sulcus (Seltzer and Pandya 1986). Unlike LIP, area VIP receives input from superior parietal cortex as well, including the adjacent medial bank, caudal area 5 convexity cortex, and parietal cortex on the medial surface of the hemisphere. These superior parietal connections provide a route for somatosensory information to reach VIP, and Seltzer and Pandya ( 1986) predicted that the fundus should contain a region of visual and somatic sensory convergence. These connectional differences between the fundus and the adjacent lateral bank of intraparietal sulcus suggest that the fundus should be considered a separate area. Other anatomic findings also suggest that there is a distinct cortical area limited to the fundus. The intraparietal sulcus has previously been shown to be heterogeneous with regard to myelo- and cytoarchitecture. The border between VIP and LIP can be seen in myelin-stained sections. The lateral bank of the intraparietal sulcus contains a heavily myelinated zone, first identified by Ungerleider and Desimone ( 1986b) or VIP* (VIP-star) to distinguish it from the lightly myelinated zone in the fundus, which they called VIP. In contrast, Andersen and his co-workers (Andersen et al. 1990a,b; Blatt et al. 1990) have included the heavily myelinated zone within area LIP, calling it LIPv. They found that the cytoarchitectonic ventral border of LIP corresponds to the ventral border of the myelin-dense area. We found that the physiologically defined border between LIP and VIP corresponds to the ventral border of the heavily myelinated zone. Further cytoarchitectonic studies of the intraparietal sulcus have identified a distinct cortical area in the fundus. Seltzer and Pandya ( 1986) described area IPd, a small area confined to the depth of the intraparietal sulcus, extending from a rostra1 border with area 2 to a caudal boundary with OA cortex, at the beginning of the annectent gyrus. Mediallv it borders on area PE and laterally it abuts area POa. 912 C. L. COLBY, J.-R. DUHAMEL, The borders of IPd thus correspond by location to those of our physiologically defined area VIP. In contrast, cytoarchitectonic area POa contains within it the physiologically and connectionally distinct area LIP (Blatt et al. 1990). The cytoarchitectonic border between IPd and POa may thus correspond to the physiological border between VIP and LIP. In summary, we have dehned area VIP as an area in the fundus of the intraparietal sulcus with a high concentration of direction-selective neurons. The reconstructed physiological borders correspond to previously identified cyto- and myeloarchitectonic borders. Further, there are substantial connectional differences between the fundus and surrounding parietal cortex. Several lines of evidence thus support the conclusion that there is a distinct area, VIP, confined to the fundus of the sulcus. Area VIP is readily identified by its distinctive visual response properties, including selectivity for the direction and speed of moving visual stimuli. Both the prevalence and the degree of direction selectivity are comparable to that reported for neurons in macaque MT (Albright 1984; Maunsell and Van Essen 1983a; Tanaka et al. 1986). Specifically, we found that the mean directionality index in VIP is 0.86, as compared with 0.93 in MT, and 80% of VIP neurons have best direction responses that are twice as strong as their null direction responses, as compared with 86% of neurons in MT (Maunsell and Van Essen 1983a). The degree of direction selectivity can also be assessed by comparing the ratio between responses in the preferred and opposite directions. In area VIP, responses in the preferred direction average 9.5 times that in the opposite direction. Responses in area MT are 10.9 times as strong in the preferred direction ( Maunsell and Van Essen 1983a). Likewise, the mean direction-tuning bandwidth of 129” in VIP is roughly comparable with the 105” reported for MT (Albright 1984). One apparent difference between MT and VIP is in the range of preferred directions represented. In area MT the distribution of preferred directions shows an underrepresentation of ipsilateral and downward directions of motion (Maunsell and Van Essen 1983a). In our sample there was no significant asymmetry. Neurons in both VIP and MT are selective for stimulus speed. Neurons in VIP on average prefer higher speeds and are more broadly tuned, by a factor of two, than MT neurons. The range of preferred speeds in VIP is a subset of that found in MT. The shift in the average preferred speed reflects the absence of VIP neurons preferring slow speeds and the presence of high-pass neurons. Finally, VIP and MT each contain neurons that are orientation selective, even though stationary, flashed bars are poor stimuli for neurons in both areas. Previous reports on response properties of neurons in parietal cortex have not recognized VIP as a distinct area nor described a sulcal region with these visual properties. Direction selectivity has been noted in neurons in parietal convexity cortex at the border between areas 7a and 7b (Motter et al. 1987; Robinson et al. 1978). Neurons in this 7a/7b border region have very large receptive fields and AND M. E. GOLDBERG complex types of direction selectivity including radial and whole-field motion (Motter and Mountcastle 198 1; Steinmetz et al. 1987). Sensitivity to motion in depth and to disparity change has been observed in neurons in both the intraparietal and superior temporal sulci (Kusunoki et al. 1991). Ew. ~novc-‘lnc’nt-r~~lut~~~ at ivit .1’ Neurons in area VIP are active during smooth pursuit eye movements. The pursuit-related activity of these neurons could be a purely visual response to target motion or to the visual input resulting from the eye movement. Extraretinal input, related to the eye movement itself, may also contribute to these responses, as it does in area MST (Newsome et al. 1988). The only other region of parietal cortex that contains a concentration of neurons active in pursuit is the dorsal bank of the STS, between convexity cortex (area 7a) and MST (Kawano et al. 1984: Sakata et al. 1983). Area VIP neurons do not fire in conjunction with saccadic eye movements. In this regard, area VIP is quite different from the rest of posterior parietal cortex, where cells active in relation to saccadic eye movements predominate (Barash et al. 199 1a,b; Gnadt and Andersen 1988; Goldberg et al. 1990; Mountcastle et al. 1975). Extrastriate visual cortex in the macaque contains over two dozen distinct visual areas. These areas are very heterogeneous, differing widely in their size, retinotopic organization, and visual response properties (Colby and Duhamel 199 1: Felleman and Van Essen 199 1; Gattass et al. 1985; Kaas 1989; Maunsell and Newsome 1987; Van Essen 1985 ). Two basic ideas have been used to describe the organization of these multiple visual areas. The first is that there is an anatomic hierarchy. Maunsell and Van Essen ( 1983) established that visual areas could be ordered by placing any given visual area at a level just above the highest area from which it receives an ascending projection. Area VIP is at least one level above area MT and is generally placed at the same level as areas MST and LIP (Boussaoud et al. 1990; Colby and Duhamel 199 1; Colby et al. 1988; Felleman and Van Essen 199 1; Maunsell and Van Essen 1983; Ungerleider and Desimone 1986b). Our physiological results on complex visual response properties in VIP reinforce its placement at a relatively high level in the hierarchy. A second basic concept in current views of extrastriate cortex organization is that of processing streams. Ungerleider and Mishkin ( 1982) introduced the idea that extrastriate cortex contains two parallel pathways: a dorsal stream leading to posterior parietal cortex and a ventral stream leading to inferior temporal cortex. Area VIP is clearly a member of the dorsal stream on the basis of its prominent connections with other dorsal stream areas. Further, the physiological properties of neurons in area VIP reflect its connectional position in extrastriate cortex: the speedand direction selectivity of VIP neurons is comparable to that seenin other dorsal stream areas like MT. These visual responseproperties are common throughout the dorsal stream, including area V3, and are generally lacking in MOTION SELECTIVITY ventral stream areas such as VP and V4 (Burkhalter et al. 1986; Desimone and Schein 1987; Felleman and Van Essen 1987). Visudfirnct ions of’ . ma WP The predominance of neurons selective for direction and speed in area VIP suggests that this area contributes to the analysis of visual motion. It is of particular interest that some VIP neurons appear to be involved in detection of the trajectory of stimulus motion and anticipation of point of contact for an approaching visual stimulus. For most of these neurons, the visual response was tied to the location of the somatosensory receptive field, although some purely visual neurons were also selective for stimulus trajectory. Previous investigations have identified similar response characteristics in periarcuate cortex ( Rizzolatti et al. 198 1 ), temporal cortex (Mistlin and Perrett 1990) and putamen (Gross and Graziano 1990) and ocular following responses have been shown to depend on viewing distance (Busettini et al. 199 1). Finally,- some VIP neurons were sensitive to the static distance of visual stimuli and were usually most sensitive to stimuli presented very close to the animal. This unusual response could signal the presence of a target that can be acquired by reach ing with th e mouth. One special visual function of area V IP may be to detect and localize stimuli near the face and contribute to eye/ hand and mouth coordination. More generally, area VIP, like the other dorsal stream areas it resembles, may participate in the perception of visual motion. We arc grateful to the stalIof the Laboratory of Sensorimotor Research for their invaluable support: C. Crist and T. Ruffner for machining: A. Hays and B. Zoltick for computer systems support: L. Jensen for electronics; M. Smith for histology: K. Powell and H. MacGruder for animal support: Dr. James Raber for veterinary care: and J. Steinberg and R. Harvey for facilitating everything. Address for reprint requests: C. L. Colby, NIH. Bldg. 10, Rm 1OC 10 1, Bethesda, MD 20892. Received 15 June 1992: accepted in final form 4 November 1992. REFERENCES T. D. Direction and orientation selectivity of neurons in visual area MT of the macaque. .J. N~~z,r.o~,ll.l’.sio/. 52: 1 IO6- 1 130, 1984. ANDERSEN, R. A.. ASANIJMA, C., AND COWAN, M. Callosal and prefrontal associational projecting cell populations in area 7a of the macaque monkey: a study using retrogradely transported fluorescent dyes. ,J. Corny. Ntwrd. 232: 443-455, 1985. ANDERSEN. R. A., ASANUMA, C., ESSI~K, G., AND SIEGEL, R. M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. .J. Corq~. Nczrrd. 296: 65113, 1990a. ANDERSEN, R. A., BRACEWELL, R. M., BARASH, S., GNADT, J. W., AND FOGASSI, L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a of macaque. .I. Nmrosci. 10: 1 176- 1 196, 1990b. BAIZER, J. S., UNGERLEIDER, L. G., AND DESIMONE, R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. .I. Ncurosci. 1 1: 168- 190, 199 1. BAKER, J. F., PETERSEN, S. E., NEWSOME, W. T., AND ALLMAN, J. M. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (htus tri\lir-gutrls): a quantitative comparison of the medial, dorsomedial, dorsolateral, and middle temporal areas. .J. Nwroph~wd. 45: 397-4 16, 198 1. BARASH, S., BRACEWELL, R. M., FOGASSI, L.. GNADT, J. W.. AND ANDERSEN, R. A. Saccade-related activity in the lateral intraparietal area. I. Temporal properties. J. N~~zlr’o~~il!‘.sio/. 66: 1095- 1 108, 199 1a. ALBRIGHT, IN AREA VIP BARASH, S., BRACEWELL, R. M., FOGASSI, L., GNADT, J. W., AND SEN, R. A. Saccade-related activity in the lateral intraparietal 913 ANDER- area. II. properties. J. N~~zrr~)~~l?~*.sio/. 66: 1 109- 1 124, I99 1b. BLATT, G. J.. ANDERSEN, R. A., AND STONER, G. R. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J. C’cvnp. Ncwd. 299: 42 I-445, 1990. BO~SSAOUD, D., UNGERLEIDER, L. G., AND DESIMONE, R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Cwnp. Ntwrol. 296: 462-495, 1990. BURKHALTER, A., FELLEMAN, D. J., NEWSOME, W. T., AND VAN ESSEN, D. C. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. I’isiorz Rtx 26: 63-80, 1986. BUSETTINI, C., MILES, F. A., AND SCHWARZ, U. Ocular responses to translation and their dependence on viewing distance. II. Motion of the scene. .J. Ncr,rc~r~h!~.sio/. 66: 865-878, 199 1. BUSHNELL, M. C., GOLDBERG, M. E., AND ROBINSON, D. L. Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in posterior parietal cortex related to selective visual attention. J. Nclrr(~~~ll!~.siol. 46: 755-772, 198 1. CAVADA, C. AND GOLDMAN-RAKI~, P. S. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. .J. C’omp. Nero/. 287: 393-42 1, 1989. COLBY, C. I,. AND DIJHAMEL, J.-R. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Ilrczrrc~~>s!lc/2oIogicr 29: 497-5 15, 199 1. COLBY, C. L., DUHAMEL, J.-R., AND GOLDBERG, M. E. Visual response properties and attentional modulation of neurons in the ventral intraparietal area (VIP) in the alert monkey. SOCK. Nmrmc~i. Ahstr. 15: 162, 11non YOY. Spatial C. L., GATTASS, R., OLSON, C. R., AND GROSS, C. G. Topographic organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J. <‘orn/~. Nczl~o/. 238: 1257- 1299, 1988. GRIST, C. F., YAMASAKI, D. S. G., KOMATSU, H., AND WURTZ, R. H. A grid system and a microsyringe for single cell recording. J. Nmrosc?. Mctlzd 26: 1 17- 122, 1988. DESIMONE, R. AND SCHEIN. S. J. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. .J. Nczlrc,l.‘h?:sio/. 57: 835868, 1987. DUHAMEL, J.-R., COLBY, C. L., AND GOLDBERG, M. E. Congruent visual and somatosensory response properties of neurons in the ventral intraparietal area ( VIP) in the alert monkey. S’~~c*.Nmrosc~i. Ahstr. 15: 162, 1989. DIJHAMEL, J.-R., COLBY, C. L., AND GOLDBERG, M. E. Congruent representations of visual and somatosensory space in single neurons of monkey ventral intraparietal cortex (area VIP). In: Bruirl cm/ Isj~rc~~, edited by J. Paillard. Oxford. UK: Oxford Univ. Press, 199 1, p. 223-236. FELLEMAN, D. J. AND VAN ESSEN, D. C. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J. N~~z(ro~~h!:sio/. 57: 889-920, 1987. FELLEMAN, D. J. AND VAN ESSEN, D. C. Distributed hierarchical processing in primate cerebral cortex. (‘cj&. Cortc\- 1: l-47, 199 1. GATTASS, R. AND GROSS. C. G. Visual topography of striate projection zone (MT) in posterior superior temporal sulcus of the macaque. .J. N(,lrr’ol,ll!‘.sic,l. 46: 62 I-638, 198 I . GATTASS, R., SOUSA, A. P. B., AND COVEY, E. Cortical visual areas of the macaque: possible substrates for pattern recognition mechanisms. In: Pctttrvw Rcccgrlition Mdurhms, edited by C. Chagas, R. Gattass, and C. Gross. Rome: Pontificae Academiae Scientarium Scripta Varia, 1985, p. l-20. GNADT, J. W. AND ANDERSEN, R. A. Memory related motor planning activity in posterior parietal cortex of macaque. JYxp. Brcrirz Rcs. 70: 216-220, 1988. GOLDBERG, M. E. Studying the neurophysiology of behavior: methods for recording single neurons in awake behaving monkeys. In: Mchods in Cdlzrhr Nczrr~~~~io/~~‘~~~, edited by J. L. Barker and J. F. McKelvy. New York: Wiley. 1983, vol. 3, chapt. 8, p. 225-248. GOLDBERG, M. E.. COLBY, C. L., AND DUHAMEL, J.-R. The representation of visuomotor space in the parietal lobe of the monkey. Cold Spring JImhor S~wp. Qlrmt. Bid. 55: 729-739, 1990. GROSS, C. G. AND GRAZIANO, M. S. A. Bimodal visual-tactile responses in the macaque putamen. Sot*. Nmrosc~i. Ahtr. 16: 1 10, 1990. HIKOSAKA, 0. AND WURTZ, R. H. Visual and oculomotor functions of COLBY, 914 C. L. COLBY, J.-R. DUHAMEL, monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. .J. Ncz(r(~~~lI!‘.si(~/. 49: 1230- 1253, 1983. KAAS, J. H. Changing concepts of visual cortex organization in primates. In: Ncllrop.s~‘~‘llo/og!, of’ Yi.wu/ Pmxyhon, edited by J. W. Brown. Hillsdale, NJ: Erlbaum, 1989, p. 3-32. KAWANO, K., SASAKI, M., AND YAMASHITA, M. Response properties of neurons in posterior parietal cortex of monkey during visual-vestibular stimulation. I. Visual tracking neurons. J. Nczrrophysio/. 5 1: 340-35 1, 1984. KUSUNOKI, M., TANAKA, Y., AND SAKATA, H. Responses of depth movement sensitive neurons to size and disparity changes in the parietal cortex of alert monkeys. Sot*. Nmrosc~i. Ahsir. 17: 44 1, 199 1. MAIJNSELL. J. H. R. AND NEWSOME, W. T. Visual processing in monkey extrastriate cortex. Annrr. Ru. Ncwosci. 10: 363-40 1, 1987. MAUNSELL, J. H. R. AND VAN ESSEN, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. .J. Nc)rrro~>/l!:sic,/. 49: 1127-l 147, 1983a. MAUNSELL. J. H. R. AND VAN ESSEN, D. C. The connections of the middle temporal visual area ( MT) and their relationship to a cortical hierarchy in the macaque monkey. .J. Nmrosci. 3: 2563-2586, 1983b. MISTLIN, A. J. AND PERRETT. D. I. Visual and somatosensory processing in the macaque temporal cortex: the role of ‘expectation.’ h-p. Bruin Rev. 82: 437-450, 1990. MOREL, A. AND BULLIER, J. Anatomical segregation of two cortical visual pathways in the macaque monkey. I ‘iwd Nwrosci. 4: 555-578, 1990. MOTTER, B. C. AND MOUNT~ASTLE. V. B. The functional properties of the light-sensitive neurons of the posterior parietal cortex studied in waking monkeys: fovea1 sparing and opponent vector organization. .I. Nwros~~i. 1: 3-26, 198 1. MOTTER, B. C.. STEINMETZ, M. A., D~JFFY, C. J., AND MOUNTCASTLE, V. B. Functional properties of parietal visual neurons: mechanisms of directionality along a single axis. .J. Nmros~~i. 7: 154- 176, 1987. MOUNT~ASTLE, V. B.. LYNCH, J. C., GEORCJOPOULOS, A.. SAKATA, H., AND ACIJNA, C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. .J. Ncwophysiol. 38: 87 I-908. 1975. NEWSOME, W. T., WURTZ, R. H., AND KOMATSU, H. Relation ofcortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J. Nczrro~~lz!:sio/. 60: 604-620, 1988. RASHBASS, C. The relationship between saccadic and smooth tracking eye movements. .J. Physioi. Lord. 159: 326-338, 196 1. RIZZOLATTI, G., SCANDOLARA, C., MATELLI, M., AND GENTILUCCI, M. AND M. E. GOLDBERG Affcrent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Bduw. Brcrin Rcs. 2: 147- 163, 198 1. ROBINSON, D. L.. GOLDBERG, M. E., AND STANTON, G. B. Parietal association cortex in the primate: sensory mechanisms and behavioral modulations. .I. N~~ilr-o~~lr!~.sic,/. 4 1: 9 1O-932, 1978. SAKATA, H., SHIBUTANI, H., AND KAWANO, K. Functional properties of visual tracking neurons in posterior parictal association cortex of the monkey. .J. hl’crrrt~I,ll!~.si(,/. 49: 1364- 1380, 1983. SELTZER, B. AND PANDYA, D. N. Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Bruin Rcs. 192: 339-35 1, 1980. SELTZER, B. AND PANDYA, D. N. Posterior parictal projections to the intraparietal sulcus of the rhesus monkey. /~.Y/x Bruirl Rev. 62: 459-469, 1986. SPATZ, W. B. AND TICXES, J. Experimental-anatomical studies on the “middle temporal visual arca (MT)” in primates. I. Ell’erent corticocortical connections on the marmoset Cd/i//vi.\,jlrc~h~r.s. ,J. Camp. Nwrol. 146: 45 l-464, 1972. STEINMETZ, M. A.. MOTTER, B. C., D~JFFY, C. J., AND MOUNT~ASTLE, V. B. Functional properties of pnrietal visual neurons: radial organization of directionalities within the visual held. .J. Nowosci. 7: 177- 19 1, 1987. TANAKA, K., HIKOSAKA, K.. SAITO, H.-A.. YUKIE, M.. FUKADA, Y.. AND IWAI. E. Analysis of local and wide-held movements in the superior temporal visual areas of the macaque monkey. .J. N~wosci. 6: 134- 144, 1986. UNCJERLEIDER, L. G. AND DESIMONE, R. Projections to the superior temporal sulcus from the central and peripheral field representations of V 1 and V2. .J. Camp. Ncrrrd. 248: 147- 163, 1986a. UNGERLEIDER, L. G. ANII DESIMONE, R. Cortical connections of visual area MT in the macaque. .J. Camp. Nero/. 248: 190-222, 1986b. UNGERLEIDER, L. G. AND MISHKIN, M. Two cortical visual systems. In: .hw!\xi.s (?/’ I ‘iwd Bdm~ior, edited by D. J. Inglc, M. A. Goodale, and R. J. W. Mansfield. Cambridge. MA: MIT Press, 1982, p. 549-586. VAN ESSEN, D. C. Functional organization of primate visual cortex. In: Ccw/wd Cortm, edited by A. Peters and E. G. Jones. New York: Plenum, 1985, vol. III. p. 259-329. VAN ESSEN, D. C., MAUNSELL, J. H. R., AND BIXBY. J. L. The middle temporal visual area in the macaque: mycloarchitecturc. connections, functional properties and topographic organization. .J. Camp. Ncwd. 199: 293-326, 1981. WURTZ, R. H. Visual receptive fields of striate cortex neurons in awake monkeys. .J. Ncltr.c~~>/l!~.sio/. 32: 727-742. 1969.