* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download THESIS D - Krishikosh
Activity-dependent plasticity wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Electrophysiology wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Biological neuron model wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neural oscillation wikipedia , lookup
Time perception wikipedia , lookup
Single-unit recording wikipedia , lookup
Mirror neuron wikipedia , lookup
Multielectrode array wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural coding wikipedia , lookup
Neuroeconomics wikipedia , lookup
Convolutional neural network wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroplasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Apical dendrite wikipedia , lookup
Haemodynamic response wikipedia , lookup
Environmental enrichment wikipedia , lookup
Subventricular zone wikipedia , lookup
Human brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Nervous system network models wikipedia , lookup
Metastability in the brain wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
“HISTOLOGICAL AND HISTOCHEMICAL STUDIES ON
CEREBRUM, CEREBELLUM, PONS AND MEDULLA
OBLONGATA IN GOAT (Capra hircus)”
THESIS
Submitted
in partial fulfillment of the requirements for the degree of
DOCTOR OF PHILOSOPHY
IN
VETERINARY ANATOMY AND HISTOLOGY
BY
AMOL MADHUKAR SALANKAR
Enrollment No: V/02/1828
NAGPUR VETERINARY COLLEGE, NAGPUR
MAHARASHTRA ANIMAL AND FISHERY SCIENCES
UNIVERSITY, NAGPUR- 440006
(INDIA)
2017
DECLARATION OF STUDENT
I hereby declare that the experimental research work and interpretation
of the thesis entitled “HISTOLOGICAL AND HISTOCHEMICAL STUDIES
ON CEREBRUM, CEREBELLUM, PONS AND MEDULLA OBLONGATA IN
GOAT (Capra hircus)” or part thereof has not been submitted for any other
degree or diploma of any University nor the data have been derived from any
thesis / publication of any University or scientific organization. The sources of
materials used and assistance received during the course of investigation
have been duly acknowledged.
Date:
Signature
(AMOL MADHUKAR SALANKAR)
Enroll. No. V/02/1828
Dr. R. S. DALVI
Chairman,
Advisory Committee
DECLARATION OF ADVISORY COMMITTEE
AMOL MADHUKAR SALANKAR has satisfactorily prosecuted his
course of research for a period of not less than two semester and that the
thesis entitled “HISTOLOGICAL AND HISTOCHEMICAL STUDIES ON
CEREBRUM, CEREBELLUM, PONS AND MEDULLA OBLONGATA IN
GOAT (Capra hircus)” submitted by him is the result of student’s bonafide
research work is sufficient to warrant its presentation to the examination in the
subject of VETERINARY ANATOMY AND HISTOLOGY for the award of
DOCTOR OF PHILOSOPHY degree by Maharashtra Animal and Fishery
Sciences University, Nagpur.
We also certify that the thesis or part thereof has not been previously
submitted by him for a degree or diploma of any other University.
Date:
Place:
Dr. R.S. DALVI
Chairman / Advisor,
Professor and Head
Veterinary Anatomy and Histology
ADVISORY COMMITTEE
Name
Designation
Signature
Chairman
………………..
2) Dr. S. B. Banubakode
Member
………………..
3 Dr. N. C. Nandeshwar
Member
………………..
4) Dr. M. G. Thorat
Member
………………...
5) Dr. S. K. Sahatpure
Member
1) Dr. R. S. Dalvi
………………...
CERTIFICATE
This is to certify that the thesis entitled “HISTOLOGICAL AND
HISTOCHEMICAL STUDIES ON CEREBRUM, CEREBELLUM, PONS AND
MEDULLA OBLONGATA IN GOAT (Capra hircus)” submitted by AMOL
MADHUKAR SALANKAR to the Maharashtra Animal and Fishery Sciences
University in partial fulfillment of the requirement for the degree of DOCTOR
OF PHILOSOPHY has been approved by the student’s Advisory Committee
after examination in collaboration with the External Examiner.
Name & Signature of
External Examiner
Dr. R. S. Dalvi
Dr. R. S. Dalvi
Professor & Head
Advisor / Guide
Dept. of Anatomy and Histology
N.V.C., Nagpur.
Professor & Head
Dept. of Anatomy and Histology
N.V.C., Nagpur.
ADVISORY COMMITTEE
Name
Designation
Signature
1) Dr. R. S. Dalvi
Chairman
………………..
2) Dr. S. B. Banubakode
Member
………………..
3) Dr. N. C. Nandeshwar
Member
…...................
4) Dr. M. G. Thorat
Member
………………...
5) Dr. S. K. Sahatpure
Member
………………...
(Dr. N. P. DAKSHINKAR)
Associate Dean
Nagpur Veterinary College,
Nagpur.
ACKNOWLEDGEMENT
Undertaking this Ph.D. has been a truly life-changing experience for me and it
would not have been possible to do without the support and guidance that I
received from many people.
I would like to first say a very big ‘thank you’ to my research guide cum
chairman, advisory committee Dr. R. S. Dalvi, Professor and Head, Department of
Veterinary Anatomy & Histology, Nagpur Veterinary College, Nagpur for his
invaluable guidance, constant encouragement and keen interest which immensely
helped me throughout the study period.
Many thanks also to Dr. S. B. Banubakode, Associate Professor, Department of
Veterinary Anatomy & Histology, Nagpur Veterinary College, Nagpur. Without his
guidance and constant feedback this Ph.D. would not have been achievable.
I greatly appreciate the support received through the members of my
advisory committee Dr. N. C. Nandeshwar, Associate Professor, Department of
Veterinary Anatomy & Histology, Nagpur Veterinary College, Nagpur, Dr. M. G.
Thorat, Associate Professor, Department of Veterinary Surgery & Radiology, PGIVS,
Akola and Dr. S. K. Sahatpure, Associate Professor, Department of Animal
Reproduction, Obstetrics & Gynecology, Nagpur Veterinary College, Nagpur for their
valuable support, suggestions, help and advice from time to time.
I am very grateful to Dr. N. P. Dakshinkar, Associate Dean, Nagpur Veterinary
College, Nagpur for his support and provisions of all facilities in the college to
undertake and complete the research work.
I also extend my sincere thanks to Dr. Rupali Y. Charjan and Dr. Umesh P.
Mainde, Assistant Professors, Department of Veterinary Anatomy & Histology,
Nagpur Veterinary College, Nagpur for their constant guidance, keen interest and
valuable suggestions throughout the work.
I express my sincere thanks to Mr. S. N. Gawande. University Librarian,
MAFSU, Nagpur. I also offer my sincere thanks to Mr. Dinesh Patil, Assistant
Professors of Statistics, Department of Veterinary Genetics, Nagpur Veterinary
College, Nagpur for their suggestions and guidance as and when required.
I was fortunate to get devoted and selfless help from my departmental
colleagues Dr. Sirsikar, Dr. Gedam, Dr. Sukhdeve, Sriniwas, Khandate, Pawan Kawareti
and Jigyasa Rana at various stages of my research work and my post graduate
studies too. I express my gratitude to all of them.
There are no words at my commands to pay regards my father Mr. Madhukar
and mother Mrs. Vibha, who took pains to bring me up this stage, without their love,
inspiration and blessing this Ph. D. could not have been accomplished.
I shall be failing in my duties if do not express my gratitude towards my
younger brother Dr. Prashant and his wife Dr. Shweta and nephew Sanvi, my father in
law Mr. Bhimraoji Chaudhari and mother in law Mrs. Kamal Chaudhari, brother in law
Dr. Praful, his wife Dr. Ritu and Dr. Amol Chaudhari for their love, constant support
and well wishes, I want to preserve a special love for my relatives and well wishers,
which enable me to complete the entire research program successfully.
I would always remember the invaluable help, active cooperation and
constant inspiration of my wife Dr. Sanjivani and my lovely son Arjun who makes my
life happy and memorable and for rendering help during the course of study and
making it possible for me to complete what I started.
During my study in this esteemed institute I was fortunate to receive
the kind of cooperation from almost everyone in one way or other. It is extremely
difficult for me to thank all of them individually by names. This short coming may
please be pardoned.
(AMOL MADHUKAR SALANKAR)
TABLE OF CONTENTS
CHAPTERS
NO.
PARTICULARS
PAGE NO.
1
INTRODUCTION
1-3
2
REVIEW OF LITERATURE
4-30
3
MATERIALS AND METHODS
31-35
4
RESULTS AND DISCUSSION
36-73
5
SUMMARY AND CONCLUSIONS
74-81
A
BIBLIOGRAPHY
I-XI
B
APPENDICES
C
VITA
D
THESIS ABSTRACT
I-XXIV
A
a-b
LIST OF TABLES
Sr. No.
Table
Page No.
1
Biometrical observation showing of brain in group I and
group II.
1
2
Biometrical showing different indices of brain in group I
and group II.
2
3
4
5
6
7
8
9
10
11
12
Micrometrical observation showing thickness of different
layers of frontal lobe of cerebral cortex in group I and
group II (µm).
Micrometrical observation showing thickness of different
layers of temporal lobe of cerebral cortex in group I and
group II (µm).
Micrometrical observation showing thickness of different
layers of occipital lobe of cerebral cortex in group I and
group II (µm).
Micrometrical observation showing neuronal count of
different layers of frontal lobe of cerebral cortex in group I
and group II.
Micrometrical observation showing neuronal count of
different layers of temporal lobe of cerebral cortex in group
I and group II.
Micrometrical observation showing neuronal count of
different layers of occipital lobe of cerebral cortex in group
I and group II.
Micrometrical observation showing neuron density of
different layers of frontal lobe of cerebral cortex in group I
and group II.
Micrometrical observation showing neuron density of
different layers of temporal lobe of cerebral cortex in group
I and group II.
Micrometrical observation showing neuron density of
different layers of occipital lobe of cerebral cortex in group
I and group II.
Total neuron count of different lobes of cerebral cortex in
group I and group II.
3
4
5
6
7
8
9
10
11
12
13
Layer thickness of cerebellar cortex in group I and group II
(µm).
13
14
Neuronal count of different layers of cerebellar cortex in
group I and group II
14
15
Neuronal density of different layers of cerebellar cortex in
group I and group II
15
16
Neuron count, total neuron count and neuronal density in
Pons in group I and group II.
16
17
Neuron count, total neuron count and neuronal density in
medulla oblongata in group I and group II.
17
LIST OF FIGURE
No.
Particulars
Stain
Mag
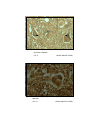
1
Photomicrograph of showing cerebral cortex with
piamater (P) and blood vessel (B) (Gr.I).
HE
100 X
2
Photomicrograph of showing cerebral cortex with
six layers (Gr.- I, Frontal lobe).
Gallocyanine
50 X
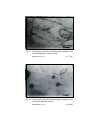
3
Photomicrograph of showing cerebral cortex with
six layers (Gr.- II, Frontal lobe).
Gallocyanine
50 X
4
Photomicrograph of showing cerebral cortex with
six layers (Gr.- I, Temporal lobe).
Gallocyanine
50 X
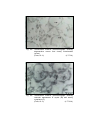
5
Photomicrograph of showing cerebral cortex with
six layers (Gr.- II, Temporal lobe).
Gallocyanine
50 X
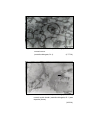
6
Photomicrograph of showing cerebral cortex with
six layers in group I (Occipital lobe).
HE
50 X
7
Photomicrograph of showing different layers of
cerebral cortex (Gr.- II, Occipital lobe).
Gallocyanine
100 X
9
Photomicrograph of showing small neuron (M)
and area calculated by Q-imaging software (Gr.II cerebral cortex).
Toluidine
blue
1000 X
Photomicrograph of showing Large neuron (L)
and area
calculated
by
Q-imaging
software (Gr.-II cerebral cortex).
Toluidine
blue
1000 X
11
Photomicrograph of showing spindle shape
neuron (N) (Gr.- I cerebral cortex).
Toluidine
blue
1000 X
12
Photomicrograph of showing pyramidal shape
neuron (N) (Gr.- II cerebral cortex).
Toluidine
blue
1000 X
13
Photomicrograph of showing stellate shape
neuron (N) (Gr. -II cerebral cortex).
Toluidine
blue
1000 X
14
Photomicrograph of showing molecular layer of
cerebral cortex (Gr.- I, Occipital lobe).
Gallocyanine
100X
15
Photomicrograph of showing molecular layer of
cerebral cortex (Gr.- II, Frontal lobe).
Toluidine
blue
200X
16
Photomicrograph of showing cells of Cajal (C)
(Gr. - I, cerebral cortex).
HE
200 X
10
17
Photomicrograph of showing external granular
layer of cerebral cortex (Gr.- I, Frontal lobe).
Toluidine
blue
200X
18
Photomicrograph of showing external pyramidal
layer of cerebral cortex (Gr.- II, temporal lobe).
Toluidine
blue
200X
19
Photomicrograph of showing internal granular
layer(arrow) of cerebral cortex (Gr.- I, Frontal
lobe).
Gallocyanin
200X
20
Photomicrograph of showing internal pyramidal
layer of cerebral cortex (Gr.- II, temporal lobe)
Toluidine
blue
200 X
21
Photomicrograph of showing internal pyramidal
layer and Betz cells (arrow) of cerebral cortex
(Gr.- I, frontal lobe).
Toluidine
blue
200 X
Photomicrograph of showing multiform layer of
cerebral cortex and white matter (arrow) (Gr.- II,
temporal lobe).
Toluidine
blue
200 X
Photomicrograph of showing molecular layer
(M), Purkinje cell layer (P) and Granule cell layer
(G) (Gr.-I, cerebellar cortex).
HE
200 X
Photomicrograph of showing molecular layer
(M), Purkinje cell layer (P) and Granule cell layer
(G) (Gr.-I, cerebellar cortex).
HE
100 X
Photomicrograph showing molecular layer (M),
Purkinje cell layer (P) and Granule cell layer (G)
(Gr.-II, cerebellar cortex).
Silver
Impregnation
100X
26
Photomicrograph showing arborisation (arrow)
from Purkinje cell (Gr.-II, cerebellar cortex).
Luxol fast
200 X
27
Photomicrograph of showing Basket
(arrow) (Gr.-II, cerebellar cortex).
Toluidine
blue
400 X
28
Photomicrograph of showing Purkinje cells
(arrow) (Gr.-I, cerebellar cortex).
HE
200 X
29
Photomicrograph of showing Golgi cells (arrow)
(Gr.- I, cerebellar cortex).
HE
200 X
30
Photomicrograph of showing Epineurium (E),
Perinurium (P) and Oligodendrocytes (arrow)
(Gr.-II, Pons).
Toluidine
blue
200 X
Toluidine
blue
100 X
22
23
24
25
31
cells
Photomicrograph of showing large (L), medium
(M) and small (S) neurons (Gr.-I, Pons).
32
Photomicrograph of showing nerve fibres and
oligodendrocyte
(arrow)
(Gr.-II,
medulla
oblongata).
Toluidine
blue
200 X
33
Photomicrograph showing large cells (Gr.- II,
medulla oblongata).
HE
200X
34
Photomicrograph of cerebrum (frontal lobe Gr. I)
showing small neuron (N), blood vessel (B) and
neuropil (P).
PAS reaction
200X
Photomicrograph of cerebrum (temporal lobe Gr.
I) showing small neuron (N), blood vessel (B)
and neuropil (P).
PAS reaction
200X
Photomicrograph of cerebrum (occipital lobe Gr.
I) showing small neuron (N), blood vessel (B)
and neuropil (P).
PAS reaction
200X
Photomicrograph of cerebrum (frontal lobe Gr. II)
showing small neuron (N), blood vessel (B) and
neuropil (P).
PAS reaction
200X
Photomicrograph of cerebrum (temporal lobe Gr.
II) showing small neuron (N), blood vessel (B)
and neuropil (P).
PAS reaction
200X
Photomicrograph of cerebrum (occipital lobe Gr.
II) showing small neuron (N), blood vessel (B)
and neuropil (P).
PAS reaction
40
Photomicrograph of cerebrum (Gr. I) showing
PAS activity in large neuron (L).
PAS reaction
400X
41
Photomicrograph of cerebrum (Gr. II) showing
PAS activity in large neuron (L).
PAS reaction
200X
42
Photomicrograph of cerebellum (Gr.I) showing
PAS activity in molecular layer(M), Purkinje cell
layer (P),Granular layer and Neuropil (PL).
PAS reaction
100X
Photomicrograph of cerebellum (Gr. II) showing
PAS activity in molecular layer(M), Purkinje cell
layer (P), Granular layer and Neuropil (PL).
PAS reaction
200X
44
Photomicrograph of pons (Gr. I) showing PAS
activity in large neuron (L).
PAS reaction
100X
45
Photomicrograph of pons (Gr. II) showing PAS
activity in large neuron (L).
PAS reaction
200X
46
Photomicrograph of medulla oblongata (Gr. I)
showing PAS activity.
PAS reaction
100X
35
36
37
38
39
43
200X
47
Photomicrograph of medulla oblongata (Gr. II)
showing PAS activity.
PAS reaction
100X
Photomicrograph of cerebrum (Gr.I) showing
acid phosphatase activity in blood vessel (B),
neuron (N) and neuropil (P)
Acid
phosphatase
200X
Photomicrograph of cerebrum from (Gr. II)
showing acid phosphatase activity in blood
vessel (B), neuron (N)
and neuropil (P).
Acid
phosphatase
200X
Photomicrograph of cerebellum (Gr. I) showing
acid phosphatase activity in molecular layer (M),
Purkinje cell layer (P), Granular layer and
Neuropil (PL).
Acid
phosphatase
200X
Photomicrograph of cerebellum (Gr. II) showing
acid phosphatase in molecular layer (M),
Purkinje cell layer (P), Granular layer (G) and
Neuropil (PL).
Acid
phosphatase
100X
Photomicrograph of pons from (Gr. I) showing
acid
phosphatase activity.
Acid
phosphatase
100X
53
Photomicrograph of pons (Gr. II) showing acid
phosphatase activity.
Acid
phosphatase
100X
54
Photomicrograph of medulla oblongata from (Gr.
I) showing acid phosphatase activity.
Acid
phosphatase
100X
55
: Photomicrograph of medulla oblongata (Gr. II)
showing
acid phosphatase activity.
Acid
phosphatase
100X
Alkaline
phosphatase
200X
Alkaline
phosphatase
200X
Alkaline
phosphatase
200X
Alkaline
phosphatase
200X
48
49
50
51
52
56
57
Photomicrograph of cerebrum (Gr.I) showing
alkaline phosphatase activity.
Photomicrograph of cerebrum (Gr. II) showing
alkaline phosphatase activity.
58
Photomicrograph of cerebellum (Gr. I) showing
alkaline phosphatase activity.
59
Photomicrograph of cerebrum (Gr. II) showing
alkaline phosphatase activity.
60
Photomicrograph of pons (Gr.I) showing alkaline
phosphatase activity.
Alkaline
phosphatase
100X
61
Photomicrograph of pons (Gr. II) showing
alkaline phosphatase activity.
Alkaline
phosphatase
100X
Photomicrograph of medulla oblongata from
group I showing alkaline phosphatase activity.
Alkaline
phosphatase
200X
62
63
Photomicrograph of medulla oblongata (Gr. II)
showing alkaline phosphatase activity.
Alkaline
phosphatase
200X
64
Photomicrograph of cerebrum (Gr. I) showing
lipofuscin deposits.
Sudan black
B
200X
65
Photomicrograph of cerebrum (Gr. II) showing
lipofuscin deposits.
Sudan black
B
200X
66
Photomicrograph of cerebrum (Gr. II) showing
lipofuscin deposits and displaced nucleus.
Sudan black
B
1000X
67
Photomicrograph of cerebellum (Gr. I) showing
lipofuscin deposits.
Sudan black
B
100X
68
Photomicrograph of cerebellum (Gr. II) showing
lipofuscin deposits.
Sudan black
B
1000X
69
Photomicrograph of
lipofuscin deposits.
showing
Sudan black
B
400X
70
Photomicrograph of pons (Gr. II) showing
lipofuscin deposits.
Sudan black
B
1000X
71
Photomicrograph of medulla oblongata (Gr. I)
showing lipofuscin deposits.
Sudan black
B
200X
72
: Photomicrograph of medulla oblongata (Gr. II)
showing lipofuscin deposits.
Sudan black
B
200X
73
Transmission electron photomicrograph showing
normal neuron from cerebrum (Gr. I) with fatty
deposit (arrow)
Uranyl
acetate and
lead citrate
X 9.6 M
Transmission electron photomicrograph showing
normal neuron (cerebrum Gr. I) with electron
dense material (arrow).
Uranyl
acetate and
lead citrate
X 5.7 M
Transmission
electron
photomicrograph
(cerebrum Gr.II) showing granulation (G),
pigmentation (P) and myelin degeneration (M)
Uranyl
acetate and
lead citrate
X 13.5M
Transmission electron photomicrograph showing
nerve fiber (M) (cerebrum Gr. II) bulging (B)
and dense cytoplasm (C).
Uranyl
acetate and
lead citrate
X 12 M
Transmission electron photomicrograph showing
nerve cell (cerebrum, Gr. II) and displaced
nucleus (arrow)
Uranyl
acetate and
lead citrate
X 7.7 M
Transmission electron photomicrograph showing
nerve cell (cerebellum, Gr. I) and fat deposites
(arrow)
Uranyl
acetate and
lead citrate
X 4.8 M
74
75
76
77
78
pons
(Gr.
I)
79
80
81
82
83
84
85
86
87
Transmission electron photomicrograph showing
nerve cell (cerebellum, Gr. I) and fat deposits
(arrow)
Uranyl
acetate and
lead citrate
X 4.8 M
Transmission electron photomicrograph showing
myeline sheath (cerebellum, Gr. I) and deposits
(arrow)
Uranyl
acetate and
lead citrate
X 57 M
Transmission electron photomicrograph showing
degenerating neuron (cerebellum, Gr. II) and
shrunken mitochondria (arrow)
Uranyl
acetate and
lead citrate
X 9.6 M
Transmission electron photomicrograph showing
degenerating neuron (cerebellum, Gr. II) and
shrunken mitochondria (arrow) with dense rim
Uranyl
acetate and
lead citrate
X 7.7 M
Transmission electron photomicrograph showing
apoptic neuron, shrunken mitochondria (M) and
regressed axon (arrow), (cerebellum, Gr. II)
Uranyl
acetate and
lead citrate
X 6.7 M
Transmission electron photomicrograph showing
normal neuron (Pons, Gr. I) and very less
deposits (arrow).
Uranyl
acetate and
lead citrate
X 7.7 M
Transmission electron photomicrograph showing
degenerative neuron (Pons, Gr. II) and cloudy
mitochondria (arrow).
Uranyl
acetate and
lead citrate
X 7.7 M
Transmission electron photomicrograph showing
vesicular appearance of myelin (M) (Pons, Gr. II)
and scanty cytoplasm (C).
Uranyl
acetate and
lead citrate
X 77 M
Transmission electron photomicrograph showing
normal neuron (medulla oblongata, Gr. I).
Uranyl
acetate and
lead citrate
X 7.7 M
LIST OF GRAPHS
Sr. No.
I
II
III
IV
V
VI
VII
VIII
IX
X
XI
XII
XIII
Graph
Showing different indices of brain
Showing thickness of different layers of frontal lobe
Showing thickness of different layers of temporal lobe
Showing thickness of different layers of occipital lobe
Showing neuronal density of different layers of frontal lobe
Showing neuronal density of different layers of temporal lobe
Showing neuronal density of different layers of occipital lobe
Showing total neuron count in different lobes.
Showing neuronal density of different layers of cerebellar cortex
Showing thickness of different layers of cerebellar cortex
Showing neuronal count in different layers of cerebellar cortex
Showing neuronal density in pons.
Showing neuronal density in medulla oblongata.
1. INTRODUCTION
The goat (Capra hircus) is an important livestock species in India. They
are the most adaptable and geographically widespread livestock species. Goat is
one of the earliest domesticated animals by human‟s around 10,000 years ago at
the dawn of the Neolithic period in the Fertile Crescent (Porter, 1996 and Pringle
1998). Goats played a central role in the Neolithic agricultural revolution and the
spread of human civilization around the globe (Legge, 1996 and Zeder and
Hesse, 2000). Goats are gaining acceptance as an established model for
biomedical research and for surgical training and teaching. They are used in
medical, orthopaedic, psychological, chemotherapeutic and physiologic research
(Lincicome and Hall, 1984). Goats are easy to transport and they appear to be
handier than other members of the ruminant family. Compared with cattle, their
small size permits goats to be maintained in a relatively small area.
Goats are extensively studied for structural information being considered
as a suitable model in small ruminant category. The visceral systems of animals
of veterinary importance are extensively studied however the nervous system is
scantily studied by the scientist in contrast to human subject. The complex well
secured expression of brain is one of the restrictions to easy availability of
samples. The present study is undertaken to explore the gross as well as
microscopic details of chief components of this vital system. The system is
peculiar in the sense, it is lodged in limited well secured place but governs total
activity of the body. The system is highly evolved with clear-cut defined functions,
which makes imperative to study the structural details of each component of
brain. Even the slight change may lead to significant morbid changes in body
function. The present study would be helpful to understand the finer details of
major brain components with reference to morphology, microstructure,
histochemistry and ultrastructure.
The cerebrum is the largest part of the brain, associated with higher brain
function such as thought and action. The cerebrum is divided into four lobes;
these are frontal lobe, parietal lobe, occipital lobe, and temporal lobe. Frontal
lobe is associated with reasoning, planning, parts of speech, movement,
emotions, and problem solving. Parietal lobe is associated with movement,
1
orientation, recognition, perception of stimuli. Occipital lobe is associated with
visual processing, however the temporal lobe is associated with perception and
recognition of auditory stimuli, memory, and speech.
The cerebral cortex is highly corrugated structure. This makes the brain
more efficient, because it can increase the surface area of the brain and the
amount of neurons within it. The importance of the cerebral cortex in various
motor and cognitive functions has drawn scientist‟s attention to the study of its
age-related modifications in the last few decades. Decreases in the functional
capacity of the central nervous system with age occur universally in all living
organisms. For instance, significant alteration in the gait control, sleeping cycle,
and learning and memory with age are the three commonest neural impairments
in aged humans.
The cerebellum has alike surface configuration to that of cerebrum with
finer structure. The cerebellum receives information from the sensory systems,
the spinal cord, and other parts of the brain and then regulates motor
movements. The cerebellum coordinates voluntary movements such as posture,
balance, coordination, and speech, resulting in smooth and balanced muscular
activity.
The brain stem is responsible for regulation of basic vital life functions
such as breathing, heartbeat, and blood pressure. The brain stem is made of the
midbrain, pons, and medulla oblongata. The pons is involved in the transmission
of signals to and from other structures in the brain, such as the cerebrum or the
cerebellum. The pons is also involved in sensations such as hearing, taste, and
balance. Medulla oblongata is the caudal most part of the brain stem, between
the pons and spinal cord. It is responsible for maintaining vital body functions,
such as breathing and heart rate. The medulla oblongata helps to regulate
breathing, heart and blood vessel function, digestion, sneezing, and swallowing.
Age is an important factor for most common neurodegenerative diseases,
including Mild cognitive impairment, Alzheimer's disease, cerebrovascular
disease, Parkinson's disease and Lou Gehrig's disease. While much research
has focused on diseases of aging, there are few informative studies available on
the molecular biology of the aging brain in the absence of neurodegenerative
disease. However, research does suggest that the aging process is associated
2
with several structural, chemical, and functional changes in the brain.
Considering the importance of brain in the neuroscience, the present study is
undertaken with reference to structural details. The objectives of present study
are
1. To study the histological changes in the cerebrum, cerebellum, pons and
medulla oblongata in young and adult goat.
2. To study the histochemical changes in the cerebrum, cerebellum, pons
and medulla oblongata in young and adult goat.
3
2. REVIEW OF LITERATURE
2.1. Brain morphology
Olopade et al. (2005) carried out morphometric study on the brains of
twenty West African Dwarf (WAD) sheep. The mean brain weight was 69.14g,
while the mean brain length and depth were 7.48cm and 4.17cm respectively.
The mean length of the cerebrum and cerebellum were 5.08 cm and 2.27cm,
respectively. There was a significant difference in the weight of head, weight of
brain, brain length and depth and in the relative brain weight. Animals aged one
year and above, had significantly heavier body weights and longer cerebrum
(p<0.05), than those below this age mark, the latter however had significantly
lower relative brain weight. They concluded that the study will be useful in
comparative Neuroanatomy and as baseline research data in neuropathology,
pharmacology, anaesthesiology and neurophysiology.
Olopade et al. (2007) studied neurometrics of the brain of Sahel goat.
They found that the mean brain weight was 85.13g. The mean brain length,
depth, cerebral length, depth and cerebellar length and depth were 9.38cm,
4.34cm, 5.78cm, 4.34cm, 2.79cm and 2.43cm respectively, while relative brain
weight was 0.004. Animals below one year of age and 20kg body weight had
significantly higher relative brain weight than animals at and above this age and
body weight groups. Female goats had a lower mean brain weight than males.
They concluded that there was a strong positive correlation between body weight
and brain depth while a strong negative correlation existed between body weight
and relative brain weight.
Byanet et al. (2008) studied morphometry of brain of 10 African
grasscutter. They found that the mean brain weight was 10.5±0.31 gm and mean
brain length was 5.95±0.12 cm. The mean brain height was 1.59±0.10 cm. The
mean cerebellar weight and length were found to be 1.26±0.05 and 3.48±0.45 cm
respectively. They also reported that the ratio of the brain to body weight was
0.01, which was bigger than Red Sokoto sheep, but smaller than of man. They
concluded that the increase in the body weight did not directly affect the brain
weight.
Byanet et al. (2014) recorded the morphometric parameters of brain of
male and female grasscutters. They found that the mean brain weights were
4
9.80±0.50 g and 10.27±0.45 g for males and females respectively. The cerebral
and cerebellar mean lengths of 3.14±0.04 cm and 1.34±0.04 cm for males, 6.26
±0.10 cm and 3.80±0.32 cm for females were observed. The mean brain lengths
were 5.63±0.07 cm and 6.26±0.1 cm for males and females respectively. They
found significant differences in the body and olfactory bulb weights and also, in
the whole brain and cerebral lengths between the males and females.
Kigir et al. (2010) studied the nerometrics of the sahel goats using a total
of 14 goats. They recorded brain weight, weight of the head, length of cerebrum,
depth of cerebrum, length of cerebellum and depth of cerebellum were 96.14 kg,
1.19 kg, 7.18 cm, 3.81 cm, 3.42 cm and 2.77 cm respectively. They concluded
that the animals more than 2 to 3 years age have slightly higher brain values than
those less than 1½ years. The females had lower brain weight than males.
Further they stated that the location had no effect on the neurometrical data of
the Sahel goats.
Olopade
et
al.
(2011)
studied
the
craniofacial
indices
and
neuromorphometrics of the Nigerian local pig. They reported that the mean brain
weight, mean brain length, cerebrum and cerebellum lengths, brain and
cerebellar heights were 84±12 g, 6.9±1.5 cm, 4.9±1.7 cm, 2.2±1.0 cm, 5.2±0.88
cm and 3.0±1.1 cm respectively. There was a negative correlation between the
weights of the animal and the height of the cerebellum, the length of cerebrum
and length of the cerebellum and between the weight of the head and height of
the cerebellum. They observed a positive correlation between the length of brain
and the weight of brain, and between the length of the cerebrum and weight of
brain. The cerebral length was statistically longer in the males than the females.
They concluded that the data obtained from this study will provide added
information in the field of comparative anatomy and porcine neuroanatomy
research.
Byanet and Dzenda (2014) studied 12 cerebella (6 males and 6 females)
to determine the effect of sex and also its relationship to other body variables in
adult African giant pouched rats using a quantitative morphometric method. They
reported that mean weight of cerebellum was slightly higher in females (0.82 ±
0.03 g) than males (0.76 ± 0.02 g). In females, the cerebellum weight was
positively correlated with the brain and the head weights. In males, the
cerebellum weight was positively correlated with the body, the head and brain
5
weights. They concluded that the cerebellum in females may be estimated
accurately from the brain mass, while in males, it may be used to estimate the
body mass.
2.2. Neuronal density and neuron number
Shefer (1972) studied absolute number of neurons and thickness of the
cortex of areas 6, 10, 18, 21, 21/38 and 40 healthy persons of different ages and
in patients with senile and vascular dementia or with Pick's or Alzheimer's
disease. He reported that in old age the mean absolute number of neurons in
mentally healthy persons was reduced by 20%, while the thickness of the cortex
remain unchanged. In persons with senile and vascular dementia, the number of
nerve cells was reduced by 35-38 %, but there was no decrease in the thickness
of the cortex on its free surface. In Alzheimer's disease the number of nerve cells
were reduced by half and the thickness of the cortex by 6% in Pick's disease and
was characterized by mass death of nerve cells in the affected areas, leading to a
reduction of 14-30 in their number, and there was decrease in thickness of the
cortex by half.
Rockel et al. (1980) counted number of neuronal cell bodies in a narrow
strip (30 micrometers) through the depth of the neocortex in mouse, rat, cat,
monkey and man. They reported that in mammalian evolution the area of the
neocortex increases in larger brains but the number of neurons through the depth
remains constant. They suggested that the intrinsic structure of the neocortex
was basically more uniform.
Terry et al. (1987) studied changes occurring in neocortical cell
populations of normal aging brains. They reported that the total number of
neurons, neuronal density and percentage of cell area remain unchanged. They
concluded that the aging affects the frontal and temporal lobes more than the
parietal. They stated that constant neuronal density coupled with diminished
cortical volume (decreased brain weight and cortical thinning) was indicative of
some neuronal loss with age.
Vincent et al. (1989) studied light and electron microscopic examination of
cerebral cortex in well-fixed young (5-6 years) and old (25-35 years) rhesus
6
monkeys to determine the effects of age on neurons. Light microscopic
measurements of the mean cortical depth in vertically oriented 1-micron-thick
sections revealed no obvious thinning with age, and the mean diameter of
neuronal nuclei did not change with age. On the basis of counts of neuronal
profiles containing nuclei in 250-microns-wide strips of 1-micron-thick sections
passing through the entire depth of the cortex, no significant neuronal loss could
be detected.
Tigges et al. (1990) studied right or left area 4 in 19 rhesus monkeys,
ranging in age from 1 day to 35 years, and assessed age-related changes in the
neuronal population. Approximately one-third loss was observed in the total
number of neurons in maturing monkeys. They reported no age-associated loss
of neurons.
Beaulieu (1993) used dissector method to estimate the numerical density
of neurons and their actual number per column in the occipital, the parietal and
the frontal cortex of adult rat. The numerical density of neurons in the frontal
cortex (34,000/mm3) was significantly lower than in the two other neocortical
areas (occipital: 52,000; parietal: 48,000/mm3).
He also found an alternate distribution of low and high density of neurons
from layers II-III to VI in the three cortical areas, with the highest density in layer
IV of the two sensory areas. There were more neurons under 1 mm2 of surface in
the parietal (90,000) than the occipital or the frontal cortex (71,000). He
compared these values from the rat with those previously obtained in cat and
monkey, and found that the number of neurons per cortical column was the
highest in the sensory area preferentially used by each species.
Sholl (1993) made a quantitative investigation of samples under standard
conditions from different well-defined anatomical regions of the cerebral cortex in
several mammals. He computed the total numbers of neurons in a cylinder of
cortex with cross-sectional area 400 µ2. He reported that total number of neuron
were about 80-60 in man and cat. The number of perikarya in a similar cylinder of
mouse cortex was about 14.
Heinsen et al. (1994) counted total nerve cell numbers in the right and left
human entorhinal areas by cell density determinations with the optical dissector.
They stated that the laminar composition of gallocyanin (Nissl)-stained sections
7
could easily be compared. They observed that the human entorhinal area was
quantitatively characterized by an age-related nerve cell loss in pre as well as pri
layers.
Witelson et al. (1995) studied cytoarchitectonic area in the cortex of the
planum temporale in men and women. Cortical depth, the number of neurons
through the depth of cortex and the number of neurons per unit volume were
obtained for the total cortex and for each of the six layers in each hemisphere.
For total cortex in both hemispheres, depth and number of neurons were similar,
but neuronal density was greater by 11% in women, with no overlap of scores
between the sexes. The sex difference in neuron number was attributable to
layers II and IV; in contrast, neuronal density did not differ between the sexes in
layers Ill, V, and VI. They suggested that the cortical functional unit had a
different ratio of input and output components in men and women which had
implications for the sex differences in cognition and behavior.
Gazzaley et al. (1997) examined cortex of 17 macaque monkeys,
consisting of 3 juvenile (1–2 years old), 8 young adult (8–12 years old) and 6
aged (25–32 years old) monkeys. The results of the quantitative study
demonstrated that there were no significant differences in the total number of
cortex layer II neurons among juvenile, young adult, and aged monkeys and
there was no correlation between neuron number and the age of the animals.
They concluded that a significant source of variability in the adult and aged
groups was likely to be biological in origin.
Peinado et al. (1997) evaluated the quantitative and cytomorphometric
effects of aging on neuronal and glial populations in cortex of the rat. They
recorded cortical volume, neuronal density, glial density, neuronal area and
shapes of the soma and nucleus in cortical layers I, II–IV, V, and VI. They found
no changes with age in volume of the cortex or neuronal density. They concluded
that the stability of neuronal density together with the increased number of glial
cells and the changes in neuronal soma size was suggestive of aged-related
cognitive impairment which had a consequence of neuronal dysfunction rather
than actual neuronal losses.
Greferath et al. (2000) investigated age-related changes in the number
and size of neurons in female Dark Agouti rats. There was a 13% reduction in the
number of neurons at 17 months compared to six months, and a 30% reduction
8
at 26 months. They recorded 8091±125 neurons at six months of age, 8187±223
at 17 months, 8203±353 at 20 months and 7066±853 at 26 months. They noted
no significant differences between any of the four age-groups (six, 17, 20 and 26
months) with regard to the number of neurons. They observed strong correlation
between the presence of spatial learning impairment and a reduction in the
number of neurons.
Peters (2002) stated that human and non-human primates showed
cognitive decline during normal aging. This decline was attributed to a loss of
cortical neurons, but recent studies have shown that there was no significant
cortical neuronal loss with age. Neurons were found to acquire pigment, but the
only other obvious changes were in layer 1 of neocortex. Layer 1 became thinner
as apical tufts of pyramidal cells lose branches, as well as synapses, and at the
same time the glial limiting membrane was found thickened. That might be
contributing to cognitive decline because it would cause a slowing of conduction
along nerve fibers and disrupting the timing in neuronal circuits.
Davanlou and Smith (2004) estimated the total numbers of neurons, glial
cells, and endothelial cells in rat cerebral cortex by using unbiased stereological
counting techniques and systematic sampling. They devised a method for
reducing problems associated with the uncertainties those arise when
distinguishing between various types of cells. In a sample of brains, the mean
total number of cells (neurons, glial and endothelial) in the syncortex of the rat
brain was 128 x 106. These numbers of cells were categorized as 47% neurons,
24% glial cells, 17% endothelial cells, and 11% uncertain cell types.
Cullen et al. (2006) suggested that neuronal density in left dorsolateral
prefrontal cortex was increased in schizophrenia. They estimated neuronal
density, size and shape in the prefrontal cortex of the left and right hemispheres
of brains. They found that the mean total neuronal density for the schizophrenia
series was 35,000 (s.d.= 6500) per mm3 on the left and 38,200 (s.d.=3600) per
mm3 on the right.
The corresponding values in control group were 41,500
(s.d.=8600) per mm3 and 36,200 (s.d.=5700) per mm3 respectively. Total
neuronal density in control group brain was generally greater in the left
hemisphere, while the reverse pattern was observed in the schizophrenia brains.
This loss or reversal of asymmetry was most significant in cortical layer 3.
Pyramidal neurons in this cell layer were significantly larger on the left and more
9
spherical in shape than on the right side in control brains, but size and shape did
not differ between the two sides in schizophrenia.
Jelsing et al. (2006a) estimated neocortical cell numbers from the
developing pig brain. The postnatal development of neocortical neurons and glial
cells from the experimental Gottingen minipig was compared with the postnatal
development of neocortical neurons in the domestic pig. A significant postnatal
development was observed in the Gottingen minipig brain for both neuronal
(28%; P=0.01) and glial cells (87%; P<0.01). A corresponding postnatal
development of neurons was not detected in the domestic pig brain. The mean
total number of neocortical neurons was 324 million in the adult Gottingen minipig
compared with 432 million in the domestic pig. They concluded that the domestic
pig seemed to be a more suitable model for evaluating the effects of
developmental insults on human brain growth and neuronal development than
the Gottingen minipig.
Yates et al. (2008) studied neuron number in the medial prefrontal cortex
and primary visual cortex of young adult (85–90 days of age) and aged (19–22
months old) male and female rats in order to investigate any age-related losses.
Possible sex differences in aging were also examined since sexually dimorphic
patterns of aging have been seen in other measures. An age-related loss of
neurons (18–20%), which was mirrored in volume losses, was found to occur in
the primary visual cortex in both sexes in all layers except IV. They reported that
there was loss of neurons (15 %) from layer V/VI of the ventral medial prefrontal
cortex and observed decrease in volume of this region in male but not in female.
In contrast, dorsal medial prefrontal cortex showed no age-related changes. They
stated that the effects of aging clearly differ among regions of the rat brain and to
some degree, between the sexes.
Diao et al. (2009) examined the density of Nissl-stained neurons in the
primary visual cortex of four young adult cats. The results of their study showed
that there was no significant difference in the density of Nissl-stained neurons
between young and old cats (P>0.05). They concluded that the effect of
excitatory transmitter system in the old visual cortex was increased relative to the
inhibitory transmitter system, which might cause an imbalance between cortical
excitation and inhibition and might be an important factor mediating the visual
function decline during aging.
10
Meyer et al. (2010) reported the number and distribution of NeuN-positive
neurons within the C2, D2, and D3 TC projection columns in P27 rat
somatosensory barrel cortex. They noted a single column with 19,109 ± 444
neurons (17 560 ± 399 when normalized to a standard-size projection column).
Neuron
density differences
along
the
vertical
column
axis
delineated
„„cytoarchitectonic‟‟ layers. The resulting neuron numbers per layer in the average
column were 63 ± 10 (L1), 2039 ± 524 (L2), 3735 ± 905 (L3), 4447 ± 439 (L4),
1737 ± 251 (L5A), 2235 ± 99 (L5B), 3786 ± 168 (L6A), and 1066 ± 170 (L6B).
These data were then used to derive the layer-specific action potential (AP)
output of a projection column. They confirmed that the ensembles of spiny L4 and
thick-tufted pyramidal neurons emit the major fraction of APs of a column. The
number of APs evoked in a column by a sensory stimulus (principal whisker
deflection) was estimated as 4441 within 100 ms post-stimulus.
Fischer et al. (2012) quantified the neuronal density in human cerebral
cortex categories into young and old age group of equal size of either below or
above 60 years of age. They found that the neuronal density in parietal, temporal,
occipital, frontal and entorhinal cortex showed a tendency of age-related decline
in each area. There was, however, no significant correlation between decreasing
neuronal number and increasing age. Age-related changes were most
pronounced in the temporal cortex and least obvious for the entorhinal cortex,
these regional differences, however, were statistically not significant.
Walloe et al. (2014) estimated total number of neurons in 94 normal
Danish individuals between 18 and 93 years of age using stereological methods.
They found that the total number of neocortical neurons in females was 19 × 109,
where as in males it was 23 × 109, which accounts for a sex related difference of
16%. However, the total number of neocortical neurons varies among individuals
by more than a factor of two, with a range of 118% (14.7–32.0 × 109 neurons),
there was considerable overlap between men and women. They reported
reductions in neocortical volume, surface area, white matter, archicortex volume,
and brain weight with advancement of age. There were no changes in gray
matter volume or neocortical thickness. The change in total number of neocortical
neurons from age 18 to 93 years was 9.5%, resulting in an average “loss” of
about 85,000 neurons per day. This age-dependent neuronal decrease was
equivalent for both sexes. They found that the total neocortical neuron number in
11
individuals between 94 and 105 years of age (seven females, one male) was the
same in very old females compared with younger women, group.
2.3. Cerebellar cortex
Fox and Barnard (1957) studied cerebellar cortex in the adult monkey
(Maccaca mulatta). They recorded quantitative observations and measurements
in Golgi preparations and from cell counts and measurements in cresyl-violet
preparations. In fixed preparations, there were an average of 510 Purkinje cells
per mm2 of Purkinje cell layer and 2.4 million granule cells per mm 3 of granular
layer. The ratio of the cross-sectional area of the molecular layer to the granular
layer was 1.5 to 1. The average thickness of the granular layer was 0.2 mm.
Nandy (1981) studied cerebellar cortices in 4, 10 and 20 year of Macaca
nemestrina for the number of Purkinje (P) and granule cells and the deposition of
lipofuscin in P cells in relation to aging. Lipofuscin distribution significantly
increased within the P cells in these animals. The number of P cells was
significantly reduced, while there were no changes in the number of granule cells.
It appears from this and other studies that the Purkinje cells were more prone to
aging changes than the granule cells of the cerebellum both in lipofuscin
formation and cell loss. Although the precise functional significance of these
changes in P cells was not clear, their vulnerability may be related to changes in
motor function in old age
Mwamengele et al. (1993) counted Purkinje neurons in mammalian
cerebella. Nucleoli were chosen as the counting unit and numbers were
estimated from uniform random samples of wax-embedded tissue sections in the
cerebella of rat, rabbit, cat, dog, goat, sheep, pig, ox, horse and human. There
was a significant linear relationship between log number and log weight. In Goat
Cerebellar Purkinje cells number were 2.691 x 106. Mean cerebellar weight
varied from 0.22 g (rat) to 91 g (human) and corresponding numbers of PC
nucleoli from 0.25 to 15.7 millions. They analysed brains of females and males
separately (cat, goat, pig, ox, horse, human) and found that there were no
significant differences between the regression lines. They concluded that, for any
given cerebellar weight, females and males had similar numbers of neurons.
12
Renovell et al. (1996) measured the number and volume of granule
neurons (GC) in the cerebellar cortex in the human cerebellum for agedependent changes. The total number of GC decline significantly during the
aging process. The human cerebellum aging involved a decrease of the number
of GC in the granular layer. The group I showed a GC densities of 388±103 x10 3
mm3. While in the group II, this cellular type reduces to 23% (299 ±130 x103 mm3)
and in the group III the decrease was greater to 38% (241 ± 66 x103 mm3)
respect to the group I. Also the volume of GC was smaller in the group III.
Furthermore they demonstrated that there exists a great variability in the GC
densities in the II group 43% as compared to a 26% in the group I and 27% in the
group III.
Sjobeck et al. (1999) studied cerebellum and inferior olive of individuals of
very high age. The study group included 15 non demented and basically healthy
cases aged 32-104 years. Linear neuronal density was expressed as number of
PC per millimeter tissue measured in the vermis and as neuronal numbers per
square millimeter tissue in the inferior olive. The linear PC density clearly
decreased with increasing age. In a comparison between the centenarian group
and non centenarians, the mean PC density in the vermis of the former group
was 6.09/mm and of the latter 2.85/mm. The difference between the two groups
was significant. They concluded that aging results in reduced PC density in the
vermis cerebelli, further accentuated in the very late stages of life.
Pal et al. (2003) studied the gross anatomy and histology of the
cerebellum of man and fowl. The shape, size, weight, volume, lobar and folial
arrangement were considered under gross observation. The cerebellum of fowl
presented a well-developed lobe, which represented the vermiform lobe of
human cerebellum. They also studied histomorphology of the organ with the help
of slides stained with H&E and Golgi-cox method. Large flask shaped Purkinje
cells in single row were observed in both the species. The sizes of the Purkinje
cells were greater but the population per unit volume was lesser in case of
human cerebellum. Purkinje cell numbers in 1mm length in man was found to be
6.6, while Purkinje cell numbers in fowl was 18.9. However both the species
presented a common type of histological organization.
Jelsing et al. (2006b) estimated total number and perikaryon volume of
cerebellar Purkinje cells, in the cerebellar cortex of the Gottingen minipig during
13
postnatal development. The total number of Purkinje cells in neonate to adult
ranged from 1.83x106 to 2.82x106. The volume of the cerebellum increased
almost four-fold from a mean of 2.45 cm3 (0.048) in neonates to 9.34 cm3 (0.094)
in adults. The study demonstrated that a pronounced postnatal neurogenesis in
Purkinje cell number and perikaryon volume was part of the growth and
development of the cerebellum in the Gottingen minipig. The Purkinje cells of the
Gottingen minipig were found to be substantially large compared with human and
represents the largest cells described hitherto from mammalian cerebella.
Zhang et al. (2006) studied age related changes in the structures of the
cerebellar cortex of young adult and old cats. They measured thickness of the
cerebellar cortex and the density of neurons in all the layers. There was a
significant decrease in the thickness of molecular layer and total cerebellar cortex
and significantly increased in the granular layer in old cats. They noted that the
Purkinje cells (PCs) showed much fewer NF-IR dendrites in old cats than those in
young adults. They further reported that there was loss of neurons and decrease
in the number of dendrites of the PCs in the aged cerebellar cortex and
correlated it to the functional decline of afferent efficacy and information
integration in the senescent cerebellum.
Agashiwala et al. (2008) developed a new method for stereologic
sampling of the cerebellar cortex in an effort to quantify Purkinje cells in
association with certain neurodegenerative disorders. Using this approach, they
counted Purkinje cells in the right cerebella of four human male control
specimens, aged 41, 67, 70 and 84 years, and estimated the total Purkinje cell
number for the four entire cerebella to be 27.03, 19.74, 20.44 and 22.03 million
cells, respectively. By this method they compared the density of the cells within
the tissue as 266,274, 173,166, 167,603 and 183,575 cells/cm3 respectively.
They stated that data demonstrated the accuracy of their approach which offers
an improvement over previous methodologies. This approach could be applied to
morphometric studies of other tissues.
Axelrad et al. (2008) quantitatively assessed the number of Purkinje cells
in brains of essential tremor ET patients and similarly aged controls. Calbindin
immunohistochemistry was performed on paraffin sections of the cerebellum.
Images were digitally recorded and blinded measurements of the number of
Purkinje cells per millimeter of cell layer (linear density) were made. Purkinje cell
14
linear density was inversely correlated with age (r=-0.53, P=.006) and number of
torpedoes (r=-0.42, P=.04). They demonstrated a reduction in Purkinje cell
number in the brains of patients with ET who do not have Lewy bodies. These
data further support the view that the cerebellum was anatomically, as well as
functionally, abnormal in these ET cases.
Whitney et al. (2008) studied autistic and normal brain of 13-54 years of
age in human, irrespective of sex. They reported that Purkinje cell counts in
normal human were 4.7±0.8 PCs/mm and in autistic brains it was 3.5±1.8
PCs/mm. The decrease in PC density was evident in some cases, the role of PCs
in the clinical manifestations of autism remains obscure, since there was no
apparent correlation between the density of PCs and the clinical severity of
autism.
Woodruff-Pak et al. (2010) used unbiased stereology to estimate the total
number of Purkinje neurons in cerebellar cortex of mice. Study indicated
significant loss of Purkinje neurons in the 18 and 24 month groups. There was a
significant effect of age. They reported that the processes of aging impact brain
structures and associated behaviors differentially, with cerebellum. They further
noted that Post hoc comparisons of the significant age effect using the Turkey
HSD test indicated significantly fewer Purkinje neurons in the age groups of 18
and 24 months in comparison with the age groups of 4, 8, and 12 months.
Yesmin et al. (2011) calculated age wise change in the number of
Purkinje cells in Bangladeshi people. They reported Purkinje cell as 160.71 ±
24.47 in group A (Age 20-29 years) and 152.20 ± 6.49 in group D (age> 50
years), where the mean reduction was 2.5% per decade. Histological studies
revealed that the number of Purkinje cell per square mm decreased with age
which was statistically significant.
Maseko et al. (2012) studied the cerebellar cortex of the African elephant
by using a combination of basic neuroanatomical and immunohistochemical
stains with Golgi and stereologic analysis. Stereologic analysis revealed that the
volume of the somata of the Purkinje cells averaged between 8,186 and 8,903
µm3 across the regions. The Nissl-stained material determined that the density of
Purkinje cells varied between 5,894 and 8,812 cells/mm3. The posterior
hemispheric and vermal blocks had higher densities than the anterior
15
hemispheric and vermal blocks. The stereologic analysis confirmed that neuronal
density was low in the elephant cerebellar cortex, providing for a larger volume
fraction of the neuropil. They concluded that quantitatively larger and more
complex cerebellar cortex likely represents part of the neural machinery required
to control the complex motor patterns involved in movement of the trunk and the
production of infrasonic vocalizations.
Viswasom et al. (2013) studied the number of Granule cells in the human
cerebellar cortex and its quantitative variation with respect to age was studied in
seventy human cerebellums using light microscopy. The study revealed a
progressive decrease in number of granule cells with increase in the fibre
components. They concluded that the number of granule cells showed
statistically significant negative correlation with age and the study provides more
information regarding the quantitative histological structure of human cerebellar
cortex.
Louis et al. (2014) quantified cerebellar molecular layer and cellular
density in 15 essential tremor (ET) cases, 15 controls, and 7 spinocerebellar
ataxia (SCA) cases. They stated that the Purkinje cell count differed across the
three groups (p < 0.001), with the highest counts in controls, intermediate counts
in ET cases and lowest counts in SCA cases. ET cases and controls had similar
molecular layer cellular density (p = 0.79) but SCA cases had higher values than
both groups (p < 0.01). A robust inverse correlation between Purkinje cell count
and molecular layer cellular density (i.e., brains with more Purkinje cell loss had
higher molecular layer cellular density), observed in SCA and controls (r =-0.55, p
= 0.008), was not observed in ET cases. Although Purkinje cell counts were
reduced in ET cases compared to controls, an increase in molecular layer cellular
density was not evident in ET. The increase in molecular layer cellular density,
observed in SCA cases, may require a more marked loss of PCs than occurs in
ET.
2.4. Size and shape of cell body
Games and Winer (1988) studied brain of adult male albino rats. They
found that the layer II-III boundary showed a sharp decrease in packing density
and larger somata. The layer III had both pyramidal and nonpyramidal neurons
the somatic area of which was 149 μm2. There was a slight increase in the
16
packing density of the small stellate cells and the chief neuronal population of
layer IV. The average somatic area of layer IV was 111 μm2 which was much
smaller than that of cells in layers III or V. Layer V had a lower neuronal packing
density and large cell rise than layer IV. The large pyramidal cells were more
numerous in the layer (Vb) than in the layer (Va). The average somatic area of
layer V was 182 μm2. Layer VI contained closely packed, flattened neural somata
with an average perikaryal area of 122 μm2. They concluded that the data would
serve as a basis for the subsequent identification of experimentally labeled cells
on the basis of their somatic shape and dendritic profiles.
Krimer et al. (1997) studied enterorrhinal cortex in 14 postmortem
schizophrenic brains and 14 matched controls. They noted that total neuronal
numbers in the schizophrenic group compared with controls were: 10185 ± 1730
versus 10105 ± 3144 in Ir, 6923 ± 2276 versus 6631 ± 1575 in Ic and 7303 ±
1125 versus 7746 ± 1330 in S. They found pyramidal shaped neurons in third
layer of cerebral cortex in schizophrenic patients. They concluded that mild
quantitative abnormalities might exist in the ERCr and might possibly be revealed
in a larger sample of schizophrenic brains.
Peinado et al. (1997) evaluated the quantitative and cytomorphometric
effects of aging on neuronal and glial populations in cortex of the rat. They
recorded cortical volume, neuronal density, glial density, and neuronal area, and
shapes of the soma and nucleus in cortical layers I, II–IV, V, and VI using serial
sections stained with cresyl-fast violet. They found age-related decrease in the
area of the neuronal soma in layers II–IV, V, and VI. They concluded that the
stability of neuronal density together with the increased number of glial cells and
the changes in neuronal soma size was suggestive of aged-related cognitive
impairment which had a consequence of neuronal dysfunction rather than actual
neuronal losses.
Zecevic and Rakic (2001) studied the layer I of the cerebral cortex and
found that, in the macaque monkey, neurons of this layer were generated during
the entire 2 month period of corticogenesis. The large, classical Cajal-Retzius
cells were generated first and that processes of these cells form a stereotyped,
rectangular network oriented parallel to the pial surface.
Rabinowicz et al. (2002) investigated gender differences in size of
neuronal somata. They reported that the neuronal soma size was found to be
17
838.7±10.5 μm3 in case of male while in female it was 871.2±14.9 μm 3. The
female group showed significantly larger neuropil volumes than males, whereas
neuronal soma size and astrocytic volumes did not differ. The expanded data
confirmed higher neuronal densities in males than in females without a gender
difference in cortical thickness.
Srivastava et al. (2009) studied cyto-architecture and morphology of the
neuronal types of the dorsomedial cortex of the lizard, Hemidactylus flaviviridis.
Bitufted neurons had vertically arranged fusiform somata, which were long,
slender, or short thick (20 x 9 μm, mean size), but they always had their cell
bodies located in the center of the cell layer. Pyramidal neurons had conical to
pyramidal shaped somata (19 x 13 μm, mean size) situated in the inner rim of the
cell layer. The length of soma ranged from 15 to 23 μm while width ranged from
10 to 16 μm. Inverted pyramidal neurons had conical to pyramidal shaped
somata (21 x 12 μm, mean size), whose apical dendrite oriented towards the
inner plexiform layer. The length of soma ranged from 13 to 30 μm while width
ranged from 8 to 12 μm. Bipyramidal neurons had biconical or bipyramidal shape
cell body (20 x 12 μm, mean size), whose length varied from 14 to 26 μm and
width ranged from 6 to 15 μm. Multipolar neurons had polygonal or ovoid cell
bodies (19 x 12 μm, mean size). The length of soma ranged from 15 to 25 μm
while width ranged from 10 to 16 μm.
Sur et al. (2011) compared characteristics of silver stained nucleolus of
the Purkinje cells and structural differences of the cortex, in turkeys, ducks,
pigeons, and starlings. The thickness of the molecular and granular layer at the
summit of the folia was the highest in pigeons, whereas the highest value of the
molecular and granular layer was determined at the deep of the folia in turkeys.
The highest number of Purkinje cells per unit scale was observed in pigeons and
starlings. The mean sizes of the Purkinje cells were greater in turkeys than in
other species. The mean area of the Purkinje cell nucleus and counts were found
to be higher in turkeys. However, there was no difference in the mean ratio of
Purkinje cell area to Purkinje cell nucleolus area among the species. They
concluded that the results obtained from this study could be of particular interest
to comparative biologists and physiologists.
18
2.5. Cortex width
Shefer (1972) studied thickness of the cortex in areas 6, 10, 18, 21, 21/38
and 40 and in the healthy persons of different ages and in patients with senile
and vascular dementia with Pick's or Alzheimer's disease. In old age, the mean
thickness of the cortex on the free surface of the gyri in healthy persons found
unchanged. In persons with senile and vascular dementia, there was no
decrease in the thickness of the cortex on its free surface. In Alzheimer's
disease, the thickness of the cortex reduced by 6%.
Pick's disease was
characterized by mass death of nerve cells in the affected areas, leading to
decrease in thickness of the cortex by half. The subicular cortex thickness in old
age was reduced by 28 % and in diseases leading to dementia by 47-71%.
Games and Winer (1988) studied adult male albino rats. Layer I had few
neurons and extended some 140 μm below the pial surface. It formed 13% of the
total thickness of the cortex (averaging 1100 μm). Layer II was 125 μm thick,
represented 11% of the cortical thickness. Layer III was 190 μm thick and formed
17% of the cortical thickness. Layer IV was 105 μm thick, represented 10% of the
cortical depth. Layer V was 270 μm thick, formed 26% of the cortical thickness.
Layer VI was 245 μm thick, represented 22% of the cortical depth.
Witelson et al. (1995) analysed of nine brain from five men and four
women. They found that the average cortical depth did not differ between the
sexes for either total cortex or for any layer. There was a tendency for layer III to
be greater in men (902 µm) than women (810 µm) by 11%. In contrast, layers II
and IV showed virtually no difference between the sexes. The total cortical width
was found to be 2735 µm in women and 2923 µm in men. The minimum cortical
width was found to be 275 µm in women and 277 µm in men in cortical layer II.
Semendeferi et al. (2001) studied values for the relative width of the
supragranular, granular, and infragranular layers. In the human, layer I had a
mean value of 11% of the total cortical depth, layers II and III make up 43% of the
cortical thickness and infragranular layers V and VI was found to constitute 40%.
Layer IV showed to make up 6% of the total depth. The size of the infragranular
layers was almost half the total thickness of the cortex of the frontal pole, while
the size of layers II and III was reduced. With regard to the gibbon and the
macaque, the values were similar, with the exception of layer IV, which was much
wider in the gibbon brain (11% vs. 6% in the macaque).
19
Rabinowicz et al. (2002) investigated gender differences in cortical
thickness. The histo-morphometric study included brains of 6 males and 5
females, 12 to 24 yr old. The cortical thickness was found to be 2.580± 0.036 mm
in case of male, while in female it was 2.656±0.034 mm. The female group
showed significantly larger neuropil volumes than males, whereas neuronal soma
size and astrocytic volumes did not differ. The expanded data confirmed higher
neuronal densities in males than in females without a gender difference in cortical
thickness.
Cullen et al. (2006) suggested that neuronal density in left dorsolateral
prefrontal cortex was increased in schizophrenia. They estimated neuronal
density, size and shape in the prefrontal cortex of the left and right hemispheres
of brains. They found marked decrease in layer 3 thickness. This loss or reversal
of asymmetry was most significant in cortical layer 3. Pyramidal neurons in this
cell layer were significantly larger on the left and more spherical in shape than on
the right side in control brains, but size and shape did not differ between the two
sides in schizophrenia.
Altamura et al. (2007) assessed the thickness and neuronal cell density of
various cerebral cortical areas in mice. The thickness of layer IV was decreased
in aged mice. Overall cortical thickness was decreased in many cortical areas of
5-HTT ko mice with increased in supragranular and infragranular layers, which
compensate entirely for decreased layer IV thickness, resulting in unchanged or
even enhanced cortical thickness.
Srivastava et al. (2009) studied cyto-architecture and morphology of the
neuronal types of the dorsomedial cortex of the lizard & Hemidactylus flaviviridis.
They recorded three neuronal layers in dorsomedial cerebral cortex. The
thickness of outermost layer-I ranged from 28-210 μm. This layer had only few
neuronal somas and also the dendrites which were ascending from subjacent
layer-II. The Layer-II was characterized by densely packed neuronal cell bodies.
It also contained dendrites descending from outer layer-I and ascending from
inner layer-III. The thickness of layer-II ranged from 19-93 μm. Layer-III was 28269 μm thick and had loosely packed neuronal cell bodies. It also contained
dendrites descending from layer-I and II and ascending processes from
ependymal layer. The ependymal layer was observed in all the cortical areas
except the lateral cortex.
20
Sur et al. (2011) compared characteristics of silver stained nucleolus of
the Purkinje cells and structural differences of the cortex, in turkeys, ducks,
pigeons, and starlings. The thickness of the molecular and granular layer at the
summit of the folia was the highest in pigeons, whereas the highest value of the
molecular and granular layer was determined at the deep of the folia in turkeys.
They concluded that the results obtained from this study could be of particular
interest to comparative biologists and physiologists.
Smiley et al. (2012) measured neuron density and width of the cortex in
planum temporal and also in prefrontal area 9 of the same brains. They reported
smaller volume and width of the outer cortex (layers I-III) in bilateral planum
temporal in the left hemisphere of schizophrenic patients. There was no
significant effect of schizophrenia on neuronal density or width of cortex in both
the cortical regions.
2.6. Histochemistry and histoenzymology
Manocha (1970) studied distribution of some phosphatases (alkaline and
acid phosphatase and ATPase) in the various regions and nuclei of the aged
squirrel monkey brain. The alkaline phosphatase activity was concentrated in the
blood vessels and the peripheral part of the neurons of cerebrum and cerebellum.
Acid phosphatase (AC) was concentrated in the pyramidal cells of cerebral cortex
and the Purkinje cells of cerebellar cortex. They concluded that AC activity was
more related to static maintenance metabolism of cells than to dynamic functional
metabolism.
Sohal and Sharma (1972) compared fine structure and population density
of neurons in the brains of young and senescent male houseflies to elucidate the
role of the nervous system in the physiological aging of multicellular organisms.
Statistical comparisons of neuronal populations in 3 day old flies with those of 30
day old flies showed no significant difference in the number of neurons. Neurons
of senescent flies showed several significant deteriorative changes in their fine
structure including loss of ribosomes, focal cytoplasmic degeneration, autophagy
and accumulation of acid-phosphatase-positive dense residual bodies. The
observations made in this study suggest that the age-related impairment in
function of the nervous system may be due to intraneuronal alterations rather
than to the loss of nerve cells.
21
Sood and Mulchandani (1977) studied the histochemical mapping of the
distribution of acid and alkaline phosphatases and succinic dehydrogenase in the
medulla oblongata and spinal cord of mouse. The acid phosphatase was
observed in neurons of all the nuclei with activity varying from intense to
moderate. In alkaline phosphatase preparations most of the nuclei were either
intensely positive or moderately positive. In case of succinic dehydrogenase out
of 36 nuclei studied, 9 nuclei were intensely positive, 11 areas were moderately
positive, 7 were mildly positive and 9 were completely devoid of enzymatic
activity. They correlated this distribution of the above enzyme with the functional
significance of the various nuclei.
Hafiza and Sood (1979) studied the distribution of alkaline phosphatase
and 5-nucleotidase in the medulla oblongata of Taphozous melanopogon (Bat).
They found that the activity of alkaline phosphatase was stronger in neurons than
in neuropil and that of 5-nucleotidase was stronger in neuropil than in neurons.
Blood capillaries were completely negative for alkaline phosphatase and
intensely positive for 5-nucleotidase.
Mohanakumar and Sood (1979) comparatively studied the acid and
alkaline phosphatases within the medulla oblongata and pons of the hedgehog
(Paraechinus micropus). They stated that the cellular elements were positive for
acid phosphatase, irrespective of their sensory or motor nature and the large
neurons of all the nuclei were more strongly positive than the small cells.
Furthermore, the nuclei which contain a dense population of neurons, appeared
more strongly positive than areas containing scattered neurons. Alkaline
phosphatase preparations showed a strong activity in the walls of blood
capillaries of the medulla oblongata and pons. Reaction of this enzyme in the
neuropil varies from moderate to strong.
Sood and Sinha (1983) studied distribution of alkaline phosphatase (GPAII) and acetylcholinesterase (CHO-A) in the hind brain of Channa punctatus and
Heteropneustes. They stated that CHO-A was localized only in the neurons. The
motor neurons show a stronger staining than the sensory neurons. In
Heteropneustes the sensory nuclei of the VIIth and Xth nerve are better
developed and show stronger activity of CHO-A than Channa. In Channa, motor
nuclei were better developed and show stronger activity of CHO-A than in
Heteropneustes. Irrespective of their sensory or motor nature, all the cranial
22
nerve nuclei exhibit a strong GP-AII activity. Purkinje cells of Heteropneustes
showed stronger activity of GP-AII than Channa.
Khan and Sood (1984) studied histoenzymological architecture and
location of phosphatases (acid and alkaline phosphatases, 5-nucleotidase,
adenosine triphosphatase) of the rhombencephalon and mesencephalon of a
fresh water turtle. They reported that concentration of acid phosphatase was
higher in large neurons. Alkaline phosphatase predominates in blood vessels.
Neuropil and neuronal activity of this enzyme was restricted to limited nuclei,
only. 5-nucleotidase was localized in all the cells as well as in the neuropil.
Adenosine triphosphatase activity was quite strong in all the brain areas
irrespective of their sensory and motor nature. In turtle brain it was not been
possible to distinguish sensory and motor areas on the basis of phosphatases
distribution as in fishes and mammals by several workers.
Ng and Tam (1986) studied agewise changes in acid and alkaline
phosphatase activities of cerebrum, cerebellum and brainstem in mouse. Acid
and alkaline phosphatase activities in the mouse cerebrum, brainstem and
cerebellum increased during the late fetal and perinatal period and reached a
maximum on postnatal day 28 (P28). Myelination was most extensive on P28–
P45 in the brainstem and in the cerebellum, and phosphatidic acid phosphatase
activity concomitantly decreased to a low level after birth. They correlated the
changes in acid and alkaline phosphatase activities with the extent of myelination
than with the overall brain tissue growth.
Nakamura et al. (1989) studied age-dependent change in activities of
seven lysosomal enzymes was studied in four brain regions (cerebrum,
hippocampus, pons and cerebellum) of Wistar rats. The activity of cathepsin D
was significantly increased with aging in the four regions. The age-dependent
change in activities of acid and alkaline DNases showed the characteristic
regional difference, and the ratio of acid to alkaline DNases was increased with
aging in all regions. Acid RNase showed the lowest activity in 18-month-old rats,
and alkaline RNase activity was decreased with aging. Acid phophatase showed
no significant age-dependent change except in pons. They demonstrated that all
of the lysosomal enzyme activities do not change in parallel with aging and the
age-dependent change showed the characteristic regional difference.
23
Capucchio et al. (2010) studied systematically characterize lesions in the
brains of 60 horses aged from 7 to 23 years. They reported that the PAS positive
activity increases with age in horse brain. Lipofuscin storage chiefly affected the
large cortical neurons and numerous neurons of the brainstem nuclei in 57
horses (95%). Lipofuscin was observed mostly in the perikaryon, between the
nucleus and the axon hillock. They could not found lipofuscin pigment in the
brains of the young control animals.
Gerhauser et al. (2012) studied 520 Syrian golden hamsters to detect
background lesions observed in the CNS. They reported PAS positive activity in
few large neurons in cerebellum but they did not find any agewise difference.
Hamsters (92.1%) of the oldest age group showed a mild lipofuscinosis
characterised by LFB and PAS positivity in few large neurons. These cells were
localised predominantly in the spinal cord ventral horns, the medulla oblongata
including the facial and cochlear nuclei.
Kellett et al. (2011) investigated Memory and Aging cohort (121 AD
patients, 89 mild cognitive impairment (MCI) patients and 180 control subjects) in
human brain. They reported increasing alkaline phosphatase activity. Alkaline
phosphatase was present on neuronal membranes that reflect the neuronal loss.
Alkaline phosphatase activity was significantly higher in the AD patients relative
to the controls. Plasma alkaline phosphatase activity inversely correlated with
cognitive function in controls, MCI and AD patients. These data indicated that
plasma alkaline phosphatase activity was increased in AD and inversely
correlates with cognitive function.
Vardy et al. (2012) measured the activity of alkaline phosphatase in
human brain in Alzheimer‟s disease and appropriate age-matched controls, and
in an ageing series of brains. In addition, they also measured TNAP activity in
plasma from 110 AD and 110 non-demented control participants. They reported
that there was no increase in alkaline phosphatase activity with the advancement
of age, but the activity was found increased in brain of Alzheimer‟s disease.
Manich et al. (2016) studied age related changes in hippocampus,
piriform, entorhina and cerebral cortices of mice brain. They reported progressive
appearance and expansion of degenerative granular structures frequently
referred as "PAS granules" because of their positive staining with periodic acidSchiff (PAS). They reported the presence of a neo-epitope in mice hippocampal
24
PAS granules and the existence of natural IgM auto-antibodies directed against
the neo-epitope in the plasma of the animals. They suggested that neo-epitopes
may turn into a useful brain-ageing biomarker and that autoimmunity could
become a new focus in the study of age-related degenerative processes.
2.7. Lipofuschin pigment
Whiteford (1964) conducted an experiment to determine the occurrence,
distribution, and significance of lipofuscin in the central nervous systems of the
dog and pig. Tissue blocks representing the medulla oblongata, pons,
cerebellum, mesencephalon, and diencephalon were embedded in paraffin,
sectioned and stained for lipofuscin pigment. He reported that lipofuscin was
widely distributed throughout the central nervous systems of the aged canine and
porcine specimens. The intracytoplasmlc pigment granules were seen varied in
pattern of distribution with advancement of age than to the functional type of
neuron in which it occurred. He concluded that the amount of pigment contained
within the individual cell, and the number of cells containing pigment increased
with age and appeared to be independent of breed and sex.
Heinsen (1981) studied the distribution of lipofuscin in the perikarya of
Purkinje cells of vermal and hemispheric lobules in 7 rats of 30-38 months old, by
the point-counting method. He found that the Purkinje cells of lobule VIa were
extremely lipofuscin-rich. The Purkinje cells of the hemispheres, lobules V, Vlb+c
and VII contain considerable amounts of a finely granular lipofuscin while lobules
I-III and VIII- IXa contain globular type. The Purkinje cells of sublobule XI d c and
X were lipofuscin-poor cells. The Purkinje cells immediately rostral and caudal to
the primary fissure were rich in lipofuscin and there was 2 to 2½ times as much
lipofuscin in these cells as in the Purkinje cells of lobules X and IX d c. The
Purkinje cells in lobules II-IV were found to accumulate increasing amounts of
lipofuscin as one approaches the primary fissure. He concluded that the Purkinje
cells of lobule I and IX were not fit into this pattern, because the cells in lobule I
had more lipofuscin than the cells in lobule II.
Nandy (1981) studied cerebellar cortices in 4, 10 and 20 year of Macaca
nemestrina for the number of Purkinje (P) and granule cells and the deposition of
lipofuscin in P cells in relation to aging. Lipofuscin distribution significantly
increased within the P cells in these animals. The number of P cells was
25
significantly reduced, while there were no changes in the number of granule cells.
It appears from this and other studies that the Purkinje cells are more prone to
aging changes than the granule cells of the cerebellum both in lipofuscin
formation and cell loss. Although the precise functional significance of these
changes in P cells is not clear, their vulnerability may be related to changes in
motor function in old age.
Newsholme et al. (1985) studied paresis afflicted 85 Merino ewes kept on
old wheat lands in the Western Cape, where vegetation was sparse but
dominated by Trachyandra divaricata. They found abundant, yellowish-brown
pigment granules in the cytoplasm of most of the larger neurons and in some
non-nervous tissues. Shrinkage and loss of a few randomly scattered axons were
observed in the white matter of the spinal cord in 2 sheep. Histochemical and
ultrastructural features of the pigment were consistent with those of lipofuscin.
They concluded that the Trachyandra poisoning appears to be the first
documented example in farm animals of an acquired lipofuscin storage disease
involving nervous and non-nervous tissues.
Sturrock (1989) carried quantitative histological study sections of brains
from mice upto 31 months of age. The average diameter of large neurons in the
lateral vestibular nucleus was 12.2±0.2 µm at 25 months of age which increased
to 14.3±0.2 at 31 months. The average diameter of small neurons was 11.02±0.2
µm at 25 months of age, which was 10.9±0.1 at 31 months. Large neurons were
found to accumulate lipofuscin from 25 months and their mean nuclear diameter
increased significantly between 25 and 28 months of age. Small neurons showed
very little lipofuscin even at 31 months of age and their mean nuclear diameter
remained constant between 6 and 31 months.
Tigges et al. (1990) studied age related changes in neuronal population of
area 4 of 19 rhesus monkeys, ranging in age from 1 day to 35 years. They found
that lipofuscin granules were dissemble in Betz cells beginning at the age of 5
years and their number increased with increasing age. In the older rhesus
monkeys, the lipofuscin granules were so large and numerous that in some Betz
cell soma, they displaced the nucleus from its usual location in the center of the
cell. They found no age-related change in thickness of area 4.
Monteiro (1991) studied quantitative age related changes occurring in
somatic organelles of the neocerebellar Purkinje cells using female rats aged 2 to
26
24 months. He calculated the somatic volumes and volumetric fractions. He
stated that the dense bodies having lipofuscin were found the display the most
expressive ultrastructural age changes.
Sharma and Singh (1994) examined the alteration in the number of
lipofuscin containing neurons in the parietal cortex of brain rat at 4,8,16 and 24
months of age. They noted in the neuron. They found increase in number of
neurons with lipofuscin with advancement of age. Similarly they observed
decreased PAS positive neurons and increased NBS positive neurons with
advancement of age. The statistical interrelations between the neuron changes
indicated that the scattered lipofuscin gets aggregated with the advancement of
age.
Mochizuki et al. (1995) compared the autofluorescence features of
lipofuscin in the brain and adrenal of rat. They reported that in 18 -21 months old
rats, the brain lipofuscin was granular and its autofluorescence was bright whitish
yellow to bright orange. On the contrary, the adrenal lipofuscin was not
demarcated as granules, and its autofluorescence was subdued orange. They
concluded that the present results showed that the autofluorescence features of
the bright whitish yellow brain lipofuscin and the adrenal lipofuscin were quite
different.
Borras et al. (1999) compared brains of 20 old dogs, ranging in age from
8 to 18 years, with those of 10 young dogs using routine staining techniques.
They noted age-related changes with respect to lipofuscin, polyglucosan bodies,
and β-amyloid. Narrowing of gyri and widening of sulci were also evident in some
of the oldest dogs. They recorded mild to moderate ventricular enlargement in
aged animals. Meningeal calcification was observed in dog‟s dorsal hemispheric
leptomeninges and concentric basophilic structures resembling plammoma
bodies. They observed small localized foci of satellitosis and neuronophagia in 10
dogs (50%) particularly in frontal cerebral cortex and dorsal thalamus section
which was supposed to play a role in the pathogenesis of the canine cognitive
dysfunction syndrome.
Gilissen et al. (1999) compared aged dwarf (Cheirogaleus medius) and
mouse (Microcebus murinus) lemurs by autofluorescence for lipofuscin and iron
distribution in brain sections. Lipofuscin accumulation was observed in the aged
animals, but not in the young ones. Affected regions where no iron accumulation
27
was observed include the hippocampus (granular and pyramidal cells), the
olfactory nucleus and the olfactory bulb (mitral cells), the basal forebrain, the
hypothalamus, the cerebellum (Purkinje cells), the neocortex (essentially in the
pyramidal cells), and the brainstem. They concluded that the different
biochemical and morphological cellular compartments might be involved in iron
and lipofuscin deposition. The nonuniform distribution of lipofuscin indicated that
brain structures were not equally sensitive to the factors causing lipofuscin
accumulation.
Vila et al. (2000) carried out quantitative study of the lipofuscin content by
image analysis in brains of known-age, pond-reared prawns Penaeus japonicas.
Three distinct measurements of lipofuscin levels (% area fraction, granule density
and mean granule size) were recorded in ten sections of the olfactory lobe cell
mass (OLCM) per animal. The concentration of lipofuscin increased significantly
with age and was independent of sex. The relationship between age and
lipofuscin concentration was best described by a seasonalized von Bertalanffy
function, since the accumulation rate of the pigment dramatically slowed down in
fall-winter, and ascribed it to a result of reduced seasonal metabolism. They
confirmed the potential of the lipofuscin method in the estimation of physiological
age in penaeids and suggested that the application of this methodology could be
useful in studies of age structure in wild populations.
Sobrino et al. (2001) developed a new method on basis of the gradual
deposition of lipofuscin in post-mitotic tissues for calculating age of animals. The
identification of the age-pigment realized on transversal sections of the olfactory
lobe cells mass in brain of shrimp (Aresteus antennatus). Three different
measurements of lipofuscin levels (%area fraction, granule density and granule
mean size) were recorded in ten distinct sections. Relationship between body
size and lipofuscin concentration (% area fraction and density granule) increased
significantly. They found that the age calculation from the lipofuscin distributions
was more successful than for body size distribution. Growth parameters using
Von Bertalanffy function were derived for this species on basis of the lipofuscin.
They confirmed the potential of the lipofuscin method for the resolution of cohorts
in deep-water pennies and suggested that the method could be useful in studies
of the age structure in wild populations.
28
Nesic et al. (2013) studied brain samples from 59 dogs from four groups
according to age ( group A, up to 5 years; group B, 5-10 years; group C, 10-15
years; and group D, >15 years). Parts of the brain (frontal cortex, parietal cortex,
hippocampus,
cerebellum
and
medulla
oblongata)
were
stained
with
haematoxylin and eosin, periodic acid Schiff and Ziehl Neelsen techniques. They
found that the pigment was detected in 80% of the dogs in group D, while this
percentage was 30% in group A. In groups A and B, lipofuscin accumulated only
in neurons of the medulla oblongata. In group C and D lipofuscin was detected in
various percentages in neurons of all brain sections. They concluded that the
lipofuscin was found mostly accumulated in large neurons of the nuclei of the
medulla oblongata. The accumulation of lipofuscin pigment in neurons increased
with the age and progressively involved neurons of different brain regions.
2.8. Electron microscopy:
Vincent et al. (1989) studied light and electron microscopic examination of
cerebral cortex in well-fixed young (5-6 years) and old (25-35 years) rhesus
monkeys to determine the effects of age on neurons. They found that in old
monkeys the neurons showed little cytological evidence of advanced age beyond
the presence of a few lipofuscin granules, although the neuropil contains some
profiles of degenerating small-caliber dendrites, myelinated axons, and a few
axon terminals. Large vacuoles, some 10 microns or more in diameter, were
present in the neuropil of the old animals. Some of these vacuoles appeared to
represent a late stage in the degeneration of myelinated axons, for that they were
bounded by a thin, laminated sheath. Other large vacuoles, of unknown origin,
often contain membranous debris and had an attenuated limiting membrane.
They concluded that the cell bodies of neurons in area 17 of old rhesus monkeys
did not show significant structural changes due to age, although some of the
neuronal processes in the neuropil were affected.
Peters et al. (2000) studied myelin sheaths in young and old monkeys.
They reported local splitting of the major dense line to accommodate dense
cytoplasm derived from the oligodendrocytes. Other alterations were the
formation of redundant myelin so that formation of double sheaths, in which one
layer of compact myelin was surrounded by another one. These alterations in
myelin increase in frequency with the ages of the monkeys, and there was a
29
significant correlation between the breakdown of the myelin and the impairments
in cognition exhibited by individual monkeys. They suggested that the breakdown
of myelin could impair cognition by leading to a change in the conduction rates
along axons, resulting in a loss of synchrony in cortical neuronal circuits.
Peters (2002) stated that human and non-human primates showed
cognitive decline during normal aging. He stated that some myelin sheath exhibit
degenerative changes such as splitting of myelin sheath and formation of
balloons with the advancement of age. He further suggested that such
degenerative changes lead to cognitive decline since they cause change in
conduction velocity, which results in dysfunction of normal timing in neuronal
circuits. Neurons were found to acquire pigment, but the only other obvious
changes were in pyramidal cells lose branches, as well as synapses, and at the
same time the glial limiting membrane was found thickened. That might be
contributing to cognitive decline because it would cause a slowing of conduction
along nerve fibers and disrupting the timing in neuronal circuits.
30
3. MATERIALS AND METHODS
Collection of samples:
The present study was conducted on the samples of cerebrum,
cerebellum, pons and medulla oblongata of brain from 20 goats irrespective of
sex. The Group-I comprised of goats between 9 to 12 months of age and GroupII comprised of goats of 13 months and above age. The brain samples were
procured from the slaughter house, Municipal Corporation and Ahillyabai Holkar
sheli-mendhi Vikas Mahamandal Bondri, immediately after the slaughter.
The fresh samples thus collected were brought to laboratory in the
container containing ice cubes. Samples were preserved in 10% neutral buffered
formalin for 48 hours for routine processing by paraffin technique. Unfixed fresh
samples were also utilized for cryostat sectioning.
Gross observations:
The biometrical / morphometrical observations of the brain were recorded
as follows.
1. Weight of the brain (Wt): The weight of brain on digital balance before treating
with 10% neutral buffered formalin for 48 hours. The measurements were
recorded in Gram (gm).
2. Length of brain (LB): Distance from the most rostral point of the olfactory bulb
to the medulla oblongata, recorded in centimetres (cm).
3. Width of brain (WB): Largest distance between lateral borders of two
hemispheres, recorded in centimetres (cm).
4. Depth of brain (DB): Distance from the dorsal aspect of the cerebrum to the
ventral aspect of the cerebrum, recorded in centimetres (cm).
5. Length of cerebrum (LC): Distance from the rostral pole of the cerebrum to the
caudal pole, caudal to the occipital gyrus, recorded in centimetres (cm).
31
6. Width of cerebrum (WC): Largest distance between lateral borders of two
cerebral hemispheres, recorded in centimetres (cm).
7. Depth of cerebrum (DC): Distance from the dorsal aspect of the cerebrum to
the ventral aspect of the cerebrum, recorded in centimetres (cm).
8. Length of cerebellum (LCB): Distance from the most rostral end of the
cerebellum where it is in contact with the cerebrum, to the most caudal point
or far extremity, rostral to the medulla oblongata, recorded in centimetres
(cm).
9. Width of cerebellum (WCB): Widest distance between two lateral lobes,
recorded in centimeters (cm).
10. Depth of cerebellum (DCB): Distance from the highest point of the median
vermis
to the roof of the fourth ventricle, recorded in centimetres (cm).
11. Brain Index= (length of brain x 100) / width of brain.
12. Cerebral Index = (length of cerebrum x 100) / width of cerebrum.
13. Cerebellar Index = (Width of cerebellum x 100) / length of cerebellum.
Processing and preparing samples for Histological study:
The samples were fixed in 10% neutral buffered formalin for 48 hours at
room temperature. After fixation, the tissues were washed in running tap water
for overnight, dehydrated in ascending grades of alcohol (70%, 80%, 90%, 95%
and absolute alcohol), cleared in xyline, infiltrated in three changes of melted
paraffin at 600 C for 4 hours and embedded in fresh melted paraffin by employing
manual tissue schedule as per the method of Drury and Wallington (1980). The
paraffin blocks were prepared and stored in refrigerator. Sections were cut at ten
micron thickness with help of rotary microtome, mounted on glass slides, dried at
370 C and preserved carefully for different staining as follows.
1. The Haematoxylin and Eosin staining methods as per Drury and
Wallington (1980).
2. Toluidine blue as per Bancroft & Stevens (1982)
32
3. Luxol fast blue as per Bancroft & Stevens (1982)
4. Silver impregnation method as per Bancroft & Stevens (1982)
The stained slides were observed under light microscope to study the
histoarchitecture of the cerebrum, cerebellum, pons and medulla oblongata.
Micrometrical parameters:
The following micrometrical parameters were carried out for the various
components of the brain and recorded in micrometer (µm) as per the method of
Culling (1969). The micrometrical parameters were recorded by calculating
averages of 3 to 4 fields for each prepared slides.
1. The Neuron numbers per square mm in cerebrum at different
lobes.(Frontal, temporal and occipital lobes)
2. The Neuron numbers per square mm in cerebrum at different layers.
3. Thickness of cerebral cortex (µm).
4. Thickness of each layers of cerebral cortex (µm).
5. Cell packing density for total cerebral cortex (per cubic mm).
6. Cell packing density for each cerebral layer (per cubic mm).
7. The Neuron numbers in cerebellum at different layers (per square mm).
8. Thickness of each layers of cerebellar cortex (µm).
9. Cell packing density for each cerebellar layer (per cubic mm).
10. The Neuron numbers in pons (per square mm).
11. Cell packing density for pons (per cubic mm).
12. The Neuron numbers in medulla oblongata (per square mm).
13. Cell packing density for medulla oblongata (per cubic mm).
All the measurements recorded were analysed statistically as per the
standard method given by Snedecor and Cochran (1994). Finally, the slides were
microphotographed.
1. The Neuron numbers in cerebrum and cerebellum in different layers.
33
The method used for neuronal count was based on a computer assisted
manual method involving cell differentiation in Nissl-stained sections under the
microscope,
marking
neurons
and
defining
layer
thickness
using
photomicrography. Low power photomicrographs of the sites chosen for
measurement for each slide were used to delineate the six cortical layers. The
number of neurons was counted for each layer from the tracings with the aid of a
computer. This neuron count is used for calculating the neuron density by using
following formula (Witelson et al. 1995).
Nv = n/(d x w x t)
Where,
Nv = the number of neurons per 1 mm3 of cortical tissue (cell packing density)
n = the mean number of neurons from two traverses,
d = the mean cortical or laminar depth of the two traverses (in mm)
w = the width of the traverse (in mm)
t = the mean section thickness (in mm).
Number of neurons under 1 mm2 of cortical surface for each layer
Nc = n/(wxt),
Where
Nc = the number of neurons under 1 mm2 of cortical surface,
n = the mean number of neurons from two traverses,
w = the width of the traverse (in mm),
t = the mean section thickness (in mm).
2. Thickness of cerebral and cerebellar cortex
Thickness of cerebral and cerebellar cortex was calculated by
photomicrography using Haematoxylin and Eosin staining method and Toludine
blue staining method.
34
Processing and preparing samples for histochemical study:
Fresh tissue samples were cut at 10 µ thickness on cryostat and stained
for glycogen, lipid, alkaline phosphatase and acid phosphatase by staining with
the following procedures:
1. Periodic Acid Schiff‟s (PAS) reaction method for glycogen (Singh and
Sulochana,1996).
2. Standard Sudan Black B method for lipids (Singh and Sulochana,1996).
3. Acid phosphatase activity as per Bancroft & Stevens (1982).
4. Alkaline phosphatase activity as per Bancroft & Stevens (1982).
Transmission Electron Microscopy (TEM) Protocol :
Samples were fixed in 2.5% gluteraldehyde in 0.1 M phosphate buffer (pH
7.2) for 24 hrs at 40c and washed with PBS for 2 times each 45 minutes, then
post fixed in 1% aqueous Osmium Tetroxide for 2 hrs later washed with
deionised distilled water for 4 times each 45 minutes, dehydrated in series of
graded alcohols, infiltrated and embedded in araldite 6005 resin or spur resin
(Spurr 1969). Incubated at 800C for 48 hrs for complete polymerization. Ultra
thin (60 nm) sections were made with a glass knife on ultra microtone (Lieca
Ultra cut UCT-GA-D/E-1/00), mounted on copper grids and stained with
saturated aqueous Uranyl acetate (UA) and counter stained with Reynolds lead
citrate (LC). Viewed under TEM (Model: Hitachi, H-7500 from Japan) at required
magnifications as per standard procedure at RUSKA Lab‟s College of Veterinary
Sciences, SPVNRTSUVAFS, Rajendranagar, Hyderabad, India.
35
4. RESULTS AND DISCUSSION
In the present study the fresh samples of goat brain were collected on ice.
The biometrical observations of whole brain were recorded. Pieces of cerebrum,
cerebellum, pons and medulla oblongata were cut separately for histological,
histochemical, histoenzymic and electron microscopic study. The general
histological structures of these parts of brain were studied from the sections
stained with hematoxylin and eosin method. Various tissue components were
studied from the sections stained with Toluidine blue, Gallocyanin, Luxal fast and
silver impregnation stains. Age wise histochemical and histoenzymic changes in
the cerebrum, cerebellum, pons and medulla oblongata were observed by
performing PAS reaction for glycogen, Azo dye coupling method for acid
phosphatase and alkaline phosphatase and lipofuscin deposited by Sudan black
B. Age wise changes pertaining to myelin degeneration, pigment deposition and
mitochondrial changes if any, were studied by Electron microscopic method.
4.1. Biometrical observations:
The gross morphology indicated that the cerebrum of goat is oblong to
oval in shape. The two hemispheres were separated by a mid sagittal cleft. The
olfactory lobe was blunt. The gyri and sulci were prominent. The gyri were fine
and sulci were deeper. The cerebellum was oval with wider capacity along
transverse axis. Because of longer width the cerebellum appears more globular
in shape. Pons was transversely elongated structure located on ventral aspect of
brain stem with faint median furrow. While the medulla oblongata was distal
continuation of brain and continued as spinal cord. It was dorsolaterally flattened
cord like structure. The different measurements of various components of brain
are recorded as follows.
4.1.1. Whole brain:
Weight of the brain in Group I was recorded as 120.30±2.85 gm which
ranged between 104 gm to 134 gm while in Group II, it ranged between 125 gm
to 140 gm with mean value of 131.60±1.75 gm (Table 1). A highly significant
difference was observed in the weight of whole brain in Group I and Group II with
the advancement of age. In agreement with the present findings, Olopade et al.
36
(2011) in Nigerian pig and Olopade et al. (2005) in African dwarf sheep reported
positive correlation between the weight of brain and weight of head. However,
this observation of the present study is not in accordance with that reported by
Byanet et al. (2008). They stated that there is no proportional increase in the
brain weight with increase in body weight in African grasscutter. Kigir et al. (2010)
also reported that there was no significant difference between weights of brain
with the advancement of age in Sahel goat. Olopade et al. (2007) reported that
the weight of brain in the Nigerian goats were 85.13 gm in Sahel, 85.85 gm in
Red Sokoto and 56.89 gm in the West African Dwarf goat. These values were
much lesser than the current findings, this variation in weight of brain might be
due to breed difference. The variation in the weight of the whole brain in Group I
and Group II with the advancement of age found during present study might be
attributed to the fact that brain is the site for deposition of fat which is more during
the advanced age or might be due to proliferation of neuronal supporting tissuemicroglia.
The Length of whole brain in Group I ranged between 9.20 cm to 10.80
cm with mean value of 9.91±0.15 cm and in Group II, it ranged between 9.20 cm
to 10.60 cm with mean value of 9.77±0.13 cm. The Width of the brain in Group I
ranged between 5.6 cm to 6.9 cm with mean value of 6.33±0.16 cm and for
Group II, it ranged between 5.6 cm to 6.7 cm with mean value of 6.23±0.10 cm.
Depth of the brain in Group I ranged between 3.7 cm to 4.3 cm with mean value
of 3.98±0.06 cm and for Group II, it ranged between 3.6 cm to 4.4 cm with mean
value of 3.96±0.09 cm. These observations of the present study showed that
there was linear decrease in the length, width and depth of brain with
advancement of age. However, no significant difference in the length, width and
the depth of brain was observed during present study. These observations of the
present study are in accordance with those reported by Olopade et al. (2005) and
Olopade et al. (2007) who reported similar decrease in measurements of whole
brain in goat with advancement of age. This decrease in the measurements of
various parameters of the brain could be ascribed to age associated shrinkage of
neuronal tissues or elements.
37
4.1.2. Cerebrum
During the present study, it was noted that the length of the cerebrum in
Group I ranged between 7.60 cm to 8.50 cm with mean value of 7.82±0.15 cm
and in Group II, it ranged between 7.60 cm to 8.60 cm with mean value of
8.13±0.11 cm (Table 1). Similarly the width of the cerebrum in Group I ranged
between 5.10 cm to 6.90 cm with mean value of 6.04±0.18 cm and in Group II, it
ranged between 5.60 cm to 6.80 cm with mean value of 6.26±0.13 cm. Although,
there was no significant difference between the length and width of cerebrum with
the advancement of age, but the length as well as width of cerebrum was found
increased with the advancement of age. In contrast with the observations of the
present study, Olopade et al. (2007) and Kigir et al. (2010) in goat reported
decrease in length and width of the cerebrum with the advancement of age.
Depth of the cerebrum in Group I ranged between 3.70 cm to 4.30 cm
with mean value of 3.98±0.06 cm and in Group II, it ranged between 3.60 cm to
4.40 cm with mean value of 3.96±0.09 cm. Although, the depth of cerebrum
showed slight decrease from Group I to Group II, but there was no significant
difference in the depth of cerebrum with the advancement of age. Kigir et al.
(2010) reported similar decrease in the depth of the cerebrum with the
advancement of age in goat and they correlated this decrease to the fact that as
the animal begin to grow, a rostrocaudal compression occurs in the brain
development.
4.1.3. Cerebellum
The average length of the cerebellum in Group I and Group II was
recorded as 3.12±0.10 cm and 3.15±0.10 cm respectively. The average width of
the cerebellum in Group I and Group II was recorded as 4.70±0.21 cm and
4.42±0.15 cm respectively (Table 1). There was no significant difference between
the length and width of cerebellum with advancement of age. However, depth of
the cerebellum in Group I was recorded as 3.01±0.05 cm and in Group II, it was
3.11±0.06 cm. These values indicated that there was increase in depth of
cerebellum with the advancement of age. This might be attributed to the
progressive growth of brain tissues. These findings of the present study are in
agreement with those reported by Olopade et al. (2007) in Sahel goat. They
38
stated that this occurrence of increase in length and depth of the cerebellum with
the advancement of age may translate to early independence and cognitive
function for young goats.
The measurement of various components of brain thus recorded were
utilised for calculations of different indices such as brain index, cerebral index,
cerebellar index. (Table 2). The brain index in Group I was recorded as
157.21±3.65, while in Group II it was 157.38±4.31. There was no significant
difference in the brain index of Group I and Group II. Similarly, the cerebral index
also did not show any significant difference between Group I and Group II. The
cerebral index of the brain in Group I ranged between 104.41 to 152.94 with
mean value of 130.54±4.68 and for Group II it ranged between 117.91 to 153.57
with mean value of 130.40±3.32. The cerebellar index of the brain in Group I
ranged between 131.25 to 192.85 with mean value of 151.80±6.79 and for Group
II it ranged between 111.76 to 161.51 with mean value of 140.79±4.27. There
was no significant difference between Group I and II with respect to cerebellar
index.
4.2. Histomorphology:
The histomorphology of the cerebrum, cerebellum, pons and medulla
oblongata was studied by staining sections with routine Haematoxylin and eosin,
Toluidine blue, Gallocyanine, Luxol fast and Bielschwosky silver staining
methods.
4.2.1. Cerebrum:
The cerebrum was microscopically found to be made up of mainly the
gray matter, which forms the cortex, while the inner part of cerebrum was made
up of white matter, which was composed of myelinated axons. The grey matter
externally showed the presence of very thin layer of pia matter, which was
projected inside the cerebral cortex at sulci and afforded pathways for blood
vessels (Fig. 1).
39
4.2.1.1. Cerebral cortex
The microstructure of cerebral cortex in almost all the regions of the
cerebrum viz. frontal, temporal and occipital lobes in Group I as well as in Group
II, showed six layers with scattered neurons supported by varying amount of glial
tissue viz. the molecular layer, the external granular layer, the external pyramidal,
the internal granular, the internal pyramidal and the multiform layer (Fig. 2 to 7).
The thickness of these layers was however, not uniform in all the lobes. The total
cortical depth in Group I at frontal, temporal and occipital lobes was recorded as
1785.77±12.75 µm, 1745.59±11.57 µm and
1781.85±10.33 µm respectively,
while in Group II, it was recorded as 1666.82±18.13 µm, 1669.10±16.20 µm and
1565.50±23.36 µm respectively. These values indicated that the cortical depth at
all the three lobes under study decreased with the advancement of age. Similarly,
it was also noted that there was highly significant difference in the cortical depth
of the frontal, temporal and occipital lobes (table 3 to 5).
In contrast with the findings of the present study, Shefer (1972) reported
that thickness of the cerebral cortex remain unchanged during old age, while in
Alzheimer's disease the thickness of the cerebral cortex reduced by 6%.
Similarly, in Pick's disease, thickness of the cortex reduced by half. He correlated
this reduction in cortical depth to mass death of nerve cells in the affected areas
with the advancement of age. Tigges et al. (1990) reported no change in
thickness of cortical depth of frontal lobe in monkey. In contrast with the findings
of the present study regarding significant difference in cortical depth of temporal
lobe with advancement of age, Smiley et al. (2012) reported that there was no
significant effect of schizophrenia on neuronal density in temporal lobe with
advancement of age in human. Sholl (1993) compared total cortical thickness of
cerebrum in human, cat and mouse. His findings regarding total thickness of
cerebral cortex in human and cat are in agreement with the present observations,
but he did not report any age dependent changes in the cortical thickness. The
variations in the reduction of the cortical depth at frontal, temporal and occipital
lobes in the present study might be attributed to the species difference as regards
to development of cerebral cortex, which is highly evolved in human beings as
compared to animals. This can also be attributed to the decrease in neuronal
elements along with supporting tissue with the advancement of age.
40
Three types of neurons were identified in the cerebral cortex based on the
size. These neurons are categorised according to the area of the soma as small
(1 to 100 µm3), medium (101 to 200 µm3) and large (more than 200 µm3) (Fig. 8,
9 and 10). Different shapes of neurons were recorded during the present study
viz. spindle, pyramidal and stellate (Fig 11, 12 and 13). Similar shaped neurons
were observed by Srivastava et al. (2009) in lizard and Sur et al. (2011) in turkey,
duck, pigeon and starling.
In Group I, the number of small neurons in frontal lobe, temporal lobe and
occipital lobe were recorded as 645.4±44.29, 669.3±42.70 and 665.5±34.60
respectively; whereas in Group II, these numbers were recorded as 563.78±
52.70, 523.90± 35.20 and 531.58± 42.00 respectively. The number of medium
size neurons in frontal lobe, temporal lobe and occipital lobe of Group I were
recorded as 335.15±22.80, 329.22±27.90
and 285.53±24.93 respectively,
whereas in Group II, these numbers were recorded as 225.55±24.80,
265.22±27.90 and 285.53±24.93 respectively. Similarly, the large sized neuron
were 335.15±22.80, 329.22±27.90 and 285.53±24.93 in frontal lobe, temporal
lobe and occipital lobe of Group I while in Group II, the number of neurons was
225.55±24.80, 265.22±27.90 and 285.53±24.93 respectively. The total number of
neurons in frontal lobe of cerebral cortex irrespective of their size in Group I was
661.4±9.12 and in Group II, it was 612.8±8.19. The total number of neurons at
temporal lobe was 687.3±15.99 and 664.8±9.73 in Group I and Group II
respectively. The overall number of neurons irrespective of their size in occipital
lobe was 681.5±14.79 and 615.4±5.47 in Group I and Group II respectively
(Table 12). These observations regarding total number of neuron in cerebral
cortex in almost all the lobes indicated that there was linear decrease with the
advancement of age. These observations of the present study regarding total
neuron count in frontal and temporal lobes of cerebral cortex showed highly
significant difference between Group I and Group II with the advancement of age.
However occipital lobe of cerebral cortex did not show any significant difference
between Group I and Group II with respect to total neuron number in cerebral
cortex.
In agreement with the present observations, Shefer (1972) reported that
the total neuron number in different areas of cerebrum decreases by 20% with
the advancement of age in human. Greferath et al. (2000) reported 30%
41
reduction in number of neurons in caudal basal forebrain of female Dark Agouti
rats at 26 months. In contrast to current findings Davanlou et al. (2004) reported
the mean total number of cells was 128 x 106 in the cortex of the rat brain, which
was found to be very high in number. Sholl (1993) reported that total numbers of
neuron were about 80-60 in man and cat, which was very less in number
compared to the present findings. Heinsen et al. (1994) reported that the total
number of nerve cell loss was 50% in the human entorhinal areas characterized
by an aging.
The neuronal density was calculated based on the number of neurons in 1
3
mm area. The neuronal density in frontal lobe of cerebral cortex in Group I and
Group II was recorded as 37074.73±605.76 and 36775.42±415.51 respectively.
In
temporal
lobe,
the
neuronal
density
was
38896.97±720.07
and
38571.99±292.39 in Group I and Group II respectively. The density of neurons in
occipital lobe of cerebral cortex was 40053.80±809.46 and 39842.70±575.80 in
Group I and Group II respectively (Table 9, 10 and 11). These observations
regarding neuronal density in cerebral cortex in almost all the lobes indicated that
there was a linear decrease with the advancement of age. The neuronal density
differed non significantly in all the lobes of cerebral cortex in Group I and Group
II. Similar findings were reported by Fischer et al. (2012) regarding numerical
neuronal density in the cerebral cortex showed a tendency of age-related decline.
There was however, no significant correlation between decreasing neuronal
number and increasing age in frontal, temporal and occipital lobes of cerebrum.
In agreement with the present observations, Tigges et al. (1990) reported that the
neuronal density decreases with the advancement of age in monkey; however
they reported significant difference in neuronal density with the advancement of
age, which was not significant in present study. Similar observations were also
recorded by Beaulieu (1993) regarding numerical density of neurons in the
occipital, the parietal and the frontal cortex of adult rat. Smiley et al. (2012)
reported that there was no significant effect of schizophrenia on neuronal density
in temporal lobe with advancement of age in human.
In contrast to present study, Rockel et al. (1980) reported that there were
147,000 neurons present beneath 1 mm2 of cortical surface of area 4, which was
much more in numbers than the findings of the present study. In contrast with the
findings of present study Vincent et al. (1989) in rhesus monkeys and Terry et al.
42
(1987) in human reported that the neuronal density did not change significantly in
any of the three cortical regions with advancement of age. Diao et al. (2009)
reported no significant difference in the density of Nissl-stained neurons between
young and old cats in primary visual cortex. Peinado et al. (1997) reported no age
wise changes in neuronal density along with the increased number of glial cells.
This was suggestive of aged-related cognitive impairment which had a
consequence of neuronal dysfunction rather than actual neuronal losses.
4.2.1.2. The molecular layer:
The uppermost layer of cerebral cortex showed the molecular
layer. It was the outer most layer of cerebral cortex. It was thickest layer amongst
all six layers. It contributed 22.40% of the total cortical depth. The thickness of
this layer in frontal lobe of Group I varied from 381.99 µm to 430.42 µm with an
average thickness of 405.05±18.66 µm. The thickness of this molecular layer in
frontal lobe of Group II varied from 375.18 µm to 429.29 µm, with average
thickness of 403.32±19.10 µm. The thickness of molecular layer in temporal lobe
of Group I varied from 381.47 µm to 427.21 µm with average thickness of
400.42±14.44 µm. whereas in temporal lobe of Group II, it varied from 390.88 µm
to 463.17 µm, with an average of 418.50±21.76 µm. Similarly, the thickness of
molecular layer in occipital lobe of Group I was calculated as 383.22 µm to
436.34 µm with an average of 403.69±16.49 µm. While it ranged between 259.74
µm to 332.44 µm, in occipital lobe of Group II with an average of 300.46±22.89
µm (Table 3, 4 and 5). These observations indicated that there was linear
decrease in the thickness of molecular layer in almost all the lobes under study
with the advancement of age. Although, there was no significant decrease in the
thickness of molecular layer of cerebral cortex in frontal lobe of Group I and
Group II, but significant decrease was recorded in molecular layer of cerebral
cortex between Group I and Group II in temporal as well as in occipital lobe with
the advancement of age. Peters (2002) stated that the Layer 1 became thinner
as apical tufts of pyramidal cells lose branches. That might be contributing to
cognitive decline because it would cause a slowing of conduction along nerve
fibers and disrupting the timing in neuronal circuits.
The molecular layer of the cerebral cortex during present study
showed that the number of small sized neurons in frontal lobe of Group I were in
43
the range of 22 to 59 with mean value 38.60 ±12.29. However, small sized
neurons in Group II, varied from 24 to 59, with mean of 38.90±11.36. The small
size neurons in temporal lobe of Group I varied between 28 to 58 with mean of
42.20±11.86, while in Group II, it varied from 29 to 55 with mean of 38.90±7.50.
The small size neurons in occipital lobe of Group I ranged between 27 to 63 with
the mean of 42.90±12.83. While, the small size neurons in Group II, varied
between 34 to 68, with mean of 46.40±10.51. The medium size neurons in frontal
lobe of Group I vary in-between 6 to 16, while the average number was 8.9±3.31.
In Group II the medium size neurons vary in-between 3 to 13, while the average
number was 8.0±2.82. The medium size neurons in temporal lobe of Group I vary
in-between 06 to 14 with mean of 10.40±2.59 while in Group II it varies inbetween 07 to 14 with mean value of 10.40±2.31. The medium size neurons in
occipital lobe of Group I ranged between 07 to 14 with mean of 10.20±2.40 while
in Group II, it ranged between 06 to 14 with mean of 10±2.50 neurons (Table 6, 7
and 8). During the present study, it was noted that the small sized neuron as well
as medium sized neuron in frontal, temporal, and occipital lobes of cerebral
cortex did not show any significant difference between Group I and Group II with
the advancement of age. It was noted that the large sized neurons were absent
in almost all the lobes of cerebral cortex in Group I as well as in Group II. (Fig.
14 and 15). Available literature indicated that the molecular layer did not show
any considerable large sized neuron in cerebral cortex in human beings.
The molecular layer was found to be lodged with the small size spindle
shaped neurons, which were scattered but their number was comparatively very
less. The soma of the molecular neuron was stellate shaped and showed
numerous cerebral vesicles. The Nissl substance was finely granular with
centrally placed nuclei. Some neurons showed parallel orientation to surface and
are called as „Cells of Cajal‟ (Fig. 16). The deeper layer of the molecular layer
had some medium size scattered neurons. This peculiar arrangement might be
attributed to focus on the maximum reception of stimuli from the surface and act
as relay centres. In agreement with the findings of the present study, Zecevic and
Rakic (2001) reported that remarkable perpendicular orientation of C-R cells
processes in the plane parallel to the pial surface may be related to the cortical
patterning and specification and a more precise geometrical pattern of laminar
and columnar organization in the primate cortex. They further reported that only a
subset of C-R cell survives in adulthood and many of them change their
44
morphology. They stated that the identification of several sub populations of layer
I cell with selective persistence in primates may help in elucidating their possible
role in congenital malformation of human cereberal cortex such as lissencephalic.
The neuronal density in molecular layer of frontal lobe of cerebral cortex
in Group I and Group II was recorded as 11769±1229 and 11565±790
respectively. In temporal lobe, the neuronal density was 13119±1006 and
11781±396 in Group I and Group II respectively. The density of neurons in
occipital lobe of cerebral cortex was 13188±1100 and 18771±1149 in Group I and
Group II respectively (Table 9, 10 and 11). These observations of the present
study regarding neuronal density in molecular layer of frontal and temporal lobes
of cerebral cortex in Group I and Group II showed linear decrease but there was
no significant difference with the advancement of age. The neuronal density in
molecular layer of occipital lobe of cerebral cortex showed significant linear
increase. These observations are in agreement with those reported by Witelson
et al. (1995) in human who recorded similar count with respect to neuronal
density in molecular layer of temporal lobe of cerebral cortex. Greferath et al.
(2000) in rat reported the similar number of neurons in layer I of cerebral cortex
but reported much less neuronal density of small, medium and large size neurons
in layer I.
4.2.1.3. The external granular layer:
This layer was below the outer layer and had densely packed neurons
and glial cells. This layer was found to contribute nearly 13.33% of the total
cortical depth. The thickness of this layer in frontal lobe in Group I varied from
213.70 µm to 269.23 µm with an average thickness of 238.65±19.17 µm. The
thickness of this layer in Group II showed variation from 203.97 µm to 249.23 µm,
with an average thickness of 225.73±15.49 µm. The thickness of external
granular layer in temporal lobe of Group I ranged from 247.18 µm to 213.43 µm
with average thickness of 231.12±11.92 µm. The thickness of this layer in
temporal lobe of Group II ranged from 276.33 µm to 223.13 µm, with the average
of 248.60±21.01 µm. The thickness of external granular layer in occipital lobe of
Group I ranged from 231.88 µm to 267.38 µm with average thickness of
251.24±11.59 µm. The thickness of this layer in occipital lobe of Group II ranged
from 229.35 µm to 193.87 µm, with the average thickness of 212.97±12.30 µm
45
(Table 3, 4 and 5). Although no significant difference was noticed in thickness of
external granular layer of cerebral cortex in frontal lobe, but significant difference
was found in thickness of external granular layer of cerebral cortex in temporal
and occipital lobes with advancement of age from Group I to Group II.
The neurons in the external granular layer were stellate shaped with
centrally located nuclei. These neurons were arranged perpendicular to surface.
The cell size was found to increase towards the bottom of the layer (Fig. 17).
Beaulieu (1993) observed ovoid and triangular neurons in second layer. He
further stated that layer II showed marked increase in neuronal density. However,
he did not mention any agewise change in the number of neurons. Walloe et al.
(2014) reported that they observed granular cells in second layer of cerebrum in
human.
The number of small size neurons in frontal lobe of Group I and Group II
were 120.70±18.40 and 129.30±13.20 respectively. The number of small size
neurons in temporal lobe of Group I and Group II were 124±17.22 and
118.20±11.80 respectively. The number of small size neurons in occipital lobe of
Group I and Group II 118.20±18.27 and 120.80±15.25 respectively. No significant
difference was noticed in the number of small sized neurons in frontal, temporal
and occipital lobes of granular layer of cerebral cortex with advancement of age.
The number of medium size neurons in frontal, temporal and occipital
lobes of external granular layer of Group I was 49.60±12.59, 47.50±9.36 and
42.10±8.86 respectively whereas in Group II these numbers were 44.0±11.20,
40.40±8.79. and 46.30±8.40 respectively. No significant difference was recorded
during present study with respect to number of medium size neurons in all the
regions of Group I and Group II with the advancement of age (Table 6, 7 and 8).
Unlike first layer i.e. molecular layer of cerebral cortex, exhibited few, but
scanty number of large neurons in external granular layer (Fig. 17). The number
of large size neurons in frontal lobe of Group I ranged between 05 to 17 with a
mean of 10.70±3.94, while in Group II it ranged between 08 to 23, with a mean of
13.80±4.84. The temporal lobe of Group I showed 07 to 16 number of large size
neurons with a mean of 11.00±2.86, while in Group II it ranged from 08 to 16 with
mean of 11.90±2.72. In occipital lobe of Group I, the number of large size
46
neurons ranged in-between 04 to 16 with mean of 10.40 ±3.43, while in Group II
it ranged from 08 to 18 with mean of 12.20±3.36 neurons. The number of large
size neurons in Group I and Group II did not show any significant difference with
the advancement of age.
The neuronal density in external granular layer of frontal lobe of cerebral
cortex in Group I and Group II was recorded as 76102±3331 and 83175±2146
respectively. In temporal lobe, the neuronal density was 79140±2841 and
69098±3333 in Group I and Group II respectively. The density of neurons in
occipital lobe of cerebral cortex was 68080±2738 and 84310±2609 in Group I and
Group II respectively (Table 9, 10 and 11). These observations of the present
study regarding neuronal density in frontal lobe of external granular layer of
cerebral cortex did not show any significant difference between Group I and
Group II with the advancement of age, however the neuronal density in temporal
and occipital lobes showed significant difference. These observations recorded in
present study regarding neuronal density in temporal and occipital lobes of
granular layer of cerebral cortex are in agreement with those recorded by Walloe
et al. (2014) in human. In contrast with the current findings, Gazzaley et al.
(1997) reported that there was no significant difference in the total number of
layer II neurons among juvenile, young adult, and aged monkeys.
4.2.1.4. The external pyramidal layer:
This layer was nearly similar to the external granular layer in thickness. It
contributed nearly 14% of the total cortical depth. The neurons had pyramidal
shape. During the present study, it was noticed that the small pyramidal neurons
were present towards the top of the layer, while the large pyramidal neurons were
located at bottom part of the layer. These neurons were more densely packed.
This layer was very easily demarcated from external granular and internal
granular layer (Fig. 18). Similar observations were recorded by Games and Winer
(1988) in albino rats measured thickness of third layer of cerebral cortex and
stated that it constitutes about 16% of the total cortical depth. This observation is
in concurrence with the present findings. In contrast with present observations,
Cullen et al. (2006) in human noted that third layer constitutes about 31% of total
cortical depth. Krimer et al. (1997) reported pyramidal shaped neurons in third
layer of cerebral cortex in schizophrenic patients. In agreement with the
47
observations of the present study regarding shape of the neuronal cell body in
third layer of cerebral cortex, Srivastava et al. (2009) described that the neuronal
pyramidal cells were characterised by dendritic skirts immerging from triangular
shape cell body.
The thickness of external pyramidal layer in frontal lobe of Group I varied
from 234.42 µm to 268.25 µm with average thickness of 249.93±12.24 µm. The
thickness of this layer in Group II varied from 203.91 µm to 249.19 µm, with an
average thickness of 221.70±15.91 µm. The thickness of external pyramidal layer
in temporal lobe of Group I varied from 207.37 µm to 249.87 µm with an average
thickness of 231.08±13.40 µm and in Group II, it varied from 167.43 µm to 199.21
µm, with an average thickness of layer was 184.47±11.47 µm. The thickness of
external pyramidal layer in occipital lobe of Group I varied from 206.19 µm to
249.28 µm with average thickness of 228.91±16.28 µm, and in Group II it varied
from 191.05 µm to 227.38 µm, with an average of 213.51±12.88 µm (Table 3, 4
and 5). A significant difference was noticed in the thickness of external pyramidal
layer in frontal, temporal and occipital lobes of cerebral cortex in Group I and
Group II with the advancement of age during the present study. Cullen et al.
(2006) reported reduction in thickness of external pyramidal layer in
schizophrenia.
The external pyramidal layer showed more population of medium and
large size neurons than small size neurons in comparison to other layers. The
number of small size neurons in frontal lobe of Group I ranged between 43 to 86
with a mean of 67.40±12.66. In Group II, small size neurons varied from 39 to 67
with a mean of 54.60±8.79. The small size neurons in temporal lobe of Group I
varied from 46 to 70 with mean of 58.90±7.70 while Group II, it varied from 40 to
69 with mean of 53.30±8.60. The small size neurons in occipital lobe of Group I,
ranged between 51 to 78 with a mean of 65.20±9.00, while in Group II, it varied
between 44 to 73, with a mean of 56.10±8.60. The population of small size
neuron in frontal and occipital lobes showed significant difference between Group
I and Group II. But no significant difference was noted in the population of small
size neuron in temporal lobe. Rabinowicz et al. (2002) reported that average
neuron size in the third layer of cerebral cortex was 185 sq. um. The size of this
neuron is nearly similar to the small sized neuron of the present study.
48
The medium size neurons in frontal lobe of Group I varied from 41 to 71,
with average number of 56.80±11.06. In Group II, the medium size neuron
ranged in between 37 to 63 with an average of 47.30±9.04. The medium size
neurons in temporal lobe of Group I varied from 39 to 60 with a mean of
48.50±7.90, while in Group II, it varied in between 32 to 63 with a mean value of
48.60±9.33. The medium size neurons in occipital lobe of Group I ranged
between 38 to 65 with a mean of 50.90±9.37, while in Group II it ranged between
37 to 62 with mean of 49.70±9.32 neurons. The medium size neuron in all the
three lobes of cerebrum did not show any significant difference with the
advancement of age from Group I to Group II.
The large size neurons in frontal lobe of Group I ranged in between 14 to
28 neurons with mean of 20.80±4.09, while in Group II, it ranged in between 15 to
24, with mean of 17.80±2.86. Similarly, in temporal lobe of Group I, it varied from
16 to 25 with a mean of 20.10±3.24, while in Group II, it was in the range of 13 to
23 with a mean of 18.10±3.63. In occipital lobe of Group I, large size neurons
ranged in between 16 to 28 with a mean of 21.80±4.02, while in Group II, it varied
from 11 to 24 with mean of 17.70±4.00 neurons. No significant difference in the
number of large size neurons in frontal and temporal lobes of cerebrum was
recorded with advancement of age, while occipital lobe showed significant
difference. (Table 6, 7 and 8).
The neuronal density in external pyramidal layer of frontal lobe of cerebral
cortex in Group I and Group II was recorded as 55368±2031 and 56164±2808
respectively. In temporal lobe, the neuronal density was 55266±1850 and
65383±2842 in Group I and Group II respectively. The density of neurons in
occipital lobe of external pyramidal layer in cerebral cortex was 60328±1555 and
57875±1645 in Group I and Group II respectively. These observations of the
present study regarding neuronal density in frontal and temporal lobes of external
pyramidal layer of cerebral cortex showed there was linear increase in the
neuronal density from Group I to Group II. A significant difference was observed
in temporal lobe between Group I and Group II with the advancement of age. In
agreement with the findings of the present study, Meyer et al. (2010) in rat
reported that the neuronal density in frontal lobe increases with the advancement
of age, but there was no significant difference. However, in contrast with the
findings of the present study, Cullen et al. (2006) reported that the overall
49
neuronal density in external pyramidal layer of cerebral cortex did not differ with
advancement of age. They further stated that there was no correlation between
neuronal density and age. In contrast with the findings of the present study,
Yates et al. (2009) stated that although there was decrease in neuronal density in
frontal and temporal lobes of cerebral cortex of rat, but there was no significant
difference with the advancement of age
4.2.1.5. The internal granular layer:
This layer is compressed in between external and internal pyramidal
layer. It contributes nearly 19.32% of the total cortical depth. This layer consists
of less number of large sized neurons compared to small and medium sized
neurons. The cells found in this layer were surrounded by abundant glial tissue
(Fig 19). In contrast with the findings of the present study, Semendeferi et al.
(2001) reported that distribution of layer four makes up to 6% of total thickness of
cerebral cortex in human, Chimpanzee and Bonobo, while in Gibbon it was up to
11%. Similarly, Games and Winer (1988) recorded thickness of layer four of
cerebral cortex and stated that it constitutes about 10% of total thickness of
cerebral cortex.
During the present study, it was observed that the thickness of internal
granular layer in frontal lobe of cerebral cortex in Group I varied from 321.76 µm
to 365.23 µm with average thickness of 344.49±5.18 µm. The thickness of this
layer in frontal lobe of Group II varied from 223.67 µm to 267.03 µm, with an
average thickness of 244.50±4.70 µm. The thickness of internal granular layer in
temporal lobe of Group I varied from 324.21 µm to 358.93 µm with an average
thickness of 341.59±3.72 µm. The thickness of this layer in temporal lobe of
Group II varied from 217.06 µm to 269.13 µm, with an average thickness of
241.55±5.36 µm. The thickness of internal granular layer in occipital lobe of
Group I varied from 323.43 µm to 357.36 µm with an average of 338.50±3.95 µm.
The thickness of this layer in occipital lobe of Group II varied from 208.63 µm to
253.17 µm, with an average of 231.20±5.10 µm (Table 3, 4 and 5). A significant
difference was noted in the thickness of internal granular layer in frontal, temporal
and occipital lobes of cerebral cortex in Group I and Group II with the
advancement of age. There was linear decrease in the thickness of internal
granular layer of cerebral cortex from Group I to Group II with the advancement
50
of age. Altamura et al. (2007) reported that the thickness of internal granular layer
of cerebral cortex decreases with the advancement of age but they did not
observed any significant difference with the advancement of age in mice.
The small size neurons in frontal lobe of Group I ranged in-between 39 to
77 with the mean of 57.50±4.00. The small size neurons in Group II varied from
38 to 68 with the mean of 49.50±3.24. The small size neurons in temporal lobe of
Group I varied in-between 43 to 69 with mean of 56.80±2.66, while in Group II, it
varied from 48 to 72 with mean of 57.80±2.40. The small size neurons in occipital
lobe of Group I ranged between 42 to 77, with the mean of 58.50±3.69. The small
size neurons in Group II, varied from 43 to 70, with the mean of 56.20±2.48.
There is no significant difference between the number of small size neuron in
Group I and Group II.
The medium size neurons in frontal lobe of Group I varied in-between 21
to 50, with the average number of 52.10±3.01. In Group II, the medium size
neurons varied in-between 13 to 41, with an average number was 45.40±2.75.
The medium size neurons in temporal lobe of Group I vary in-between 24 to 50
with mean of 36.50±2.68 while in Group II it varies in-between 19 to 44 with mean
value of 29.40±2.30. The medium size neurons in occipital lobe of Group I ranged
between 24 to 53 with mean of 38.30±3.18, while in Group II it were ranged
between 25 to 47 with a mean of 36.00±2.57 neurons (Table 6, 7 and 8). These
values indicated that the number of medium size neurons in temporal lobe of
cerebral cortex showed significant difference with the advancement of age.
However, the medium size neurons in temporal and occipital lobes of cerebral
cortex did not show any significant difference between Group I and Group II.
In this layer large size neurons in frontal lobe of Group I ranged inbetween 04 to 16 neurons with a mean of 8.30±1.2, while in Group II, it ranged
in-between 06 to 13, with mean of 8.70±0.70. Similarly in temporal lobe of Group
I, it varied from 07 to 14 with mean of 10.70±0.79, while in Group II, it varied from
07 to 14 with mean of 10.60±0.77. In occipital lobe of Group I, large size neurons
ranged in-between 07 to 18 with mean of 11.60±1.31 while in Group II, it varied
from 07 to 16 with mean of 11.50±1.65 neurons. No significant difference was
noted during the present study in the large size neurons in frontal, temporal and
51
occipital lobes of cerebral cortex with the advancement of age from Group I to
Group II.
The neuronal density in internal granular layer of frontal lobe of cerebral
cortex in Group I and Group II was recorded as 34199±1819 and 42331±2386
respectively. In temporal lobe, the neuronal density was 35210±1672 and
40664±1991 in Group I and Group II respectively. The density of neurons in
occipital lobe of internal granular layer in cerebral cortex was 37366±1432 and
51792±2228 in Group I and Group II respectively. These observations of the
present study regarding neuronal density in frontal and occipital lobes of internal
granular layer of cerebral cortex showed significant difference between Group I
and Group II with the advancement of age. In contrast with the findings of the
present study, Beaulieu (1993) counted the Neuronal density in frontal, temporal
and occipital lobes of cerebral cortex in adult rats, but their number was much
less than that recorded during present study. Similarly, Witelson et al. (1995) in
human recorded neuronal density in internal granular layer of cerebral cortex,
which was also less than that recorded during present study.
4.2.1.6. The internal pyramidal layer:
This layer was thick layer and contributed 18.87% of the total cortical
thickness. In this layer, moderate quantities of large and medium size pyramidal
cells were present surrounded by large quantity of glial tissue. This layer was
densely packed due to larger size cells. This layer was found to contain more
number of large size pyramidal cells compared to any other layer (Fig. 20). Some
giant pyramidal neurons were also found in this layer called as Betz cell (Fig. 21).
Similar observations were reported by Fischer et al. (2012) in human. They noted
densely packed large pyramidal neurons in the internal pyramidal layer. Davanlou
et al. (2004) reported similar findings and observed large conical cell bodies
internal pyramidal layer of cerebral cortex in mouse. In agreement with the
findings of the present study, Semendeferi et al. (2001) reported large darkly
stained pyramidal cells in internal pyramidal layer of cerebral cortex in
chimpanzee, gibbon and bonobo. Cullen et al. (2006) reported the thickness of
internal pyramidal layer as 16%, which was reduced to 15% with the
advancement of age in human. Games and Winer (1988) reported thickness of
internal pyramidal layer as 26% of total thickness of cerebral cortex in albino rats.
52
The thickness of internal pyramidal layer in frontal lobe of cerebral cortex
in Group I varied from 311.72 µm to 359.44 µm with an average of 337.66±5.9
µm. The thickness of this layer in frontal lobe of Group II varied from 305.78 µm
to 349.15 µm, with an average thickness of 332.92±4.61 µm. The thickness of
internal pyramidal layer in temporal lobe of Group I varied from 303.27 µm to
339.08 µm with an average of 322.32±3.91 µm. The thickness of this layer in
temporal lobe of Group II varied from 318.32 µm to 377.81 µm, with an average
thickness of 342.32±6.8 µm. The thickness of this layer in occipital lobe of Group
I varied from 314.29 µm to 359.39 µm with an average thickness of 332.69±5.01
µm. The thickness of this layer in occipital lobe of Group II varied from 281.39 µm
to 349.24 µm, with an average thickness of 314.21±8.10 µm. No significant
difference was noted in the thickness of internal pyramidal layer of frontal and
occipital lobes of cerebral cortex with the advancement of age from Group I to
Group II during the present study. However, a significant difference was recorded
in the thickness of internal pyramidal layer of temporal lobe in Group I and Group
II. Games and Winer (1988) in albino rat reported that thickness of internal
pyramidal layer was 270 µm which is less than the observations made in the
present study. This variation in the observation could be attributed to the species
difference and degree of motor activity of the portion of innervations concern.
Beaulieu (1993) recorded much higher thickness of the internal pyramidal layer at
frontal and occipital lobes of cerebral cortex in adult rat than that recorded during
present study.
The small size neurons in frontal lobe of Group I ranged in-between 41 to
73 with the mean of 60.40±3.34. The small size neurons in Group II varied from
39 to 61, while the mean numbers of neuron were 52.10±2.29. The small size
neurons in temporal lobe of Group I ranged in between 49 to 74 with a mean of
61.00±2.65, while in Group II, it varied from 40 to 63 with a mean of 51.50±2.58.
The small size neurons in occipital lobe of Group I ranged between 47 to 79 with
a mean of 61.60±3.60. The small size neurons in Group II varied in-between 39
to 72 with the mean of 55.10±3.50. These figures recorded during present study
indicated that there is linear decrease in the number of small size neurons in
frontal, temporal and occipital lobes of cerebral cortex from Group I and Group II.
But there was no significant difference between the numbers of small size
neurons in frontal and occipital lobes of the cerebral cortex under study with the
advancement of age.
53
The medium size neurons in frontal lobe of Group I varied from 29 to 52,
with the average of 41.30±2.57. In Group II, the medium size neurons varied inbetween 33 to 50, while the average was 41.10±1.63. The medium size neurons
in temporal lobe of Group I varied in between 41 to 63 with mean of 53.10±2.40
while in Group II, it varied in-between 31 to 59 with a mean value of 45.60±2.53.
The medium size neurons in occipital lobe of Group I ranged between 36 to 63
with a mean of 51.90±3.11, while in Group II it ranged between 32 to 64 with a
mean of 48.20±3.13 neurons (Table 6, 7 and 8). Although no significant
difference was noticed in the number of medium size neurons in frontal and
occipital lobes of cerebral cortex between Group I and Group II, but there was
linear decrease in the number of medium size neurons in all the lobes of cerebral
cortex with the advancement of age.
In these layer large size neurons in frontal lobe of Group I ranged inbetween 14 to 29 neurons with a mean of 22.00±1.49, while in Group II, it ranged
in-between 13 to 22, with mean of 17.70±0.98. Similarly, in temporal lobe of
Group I, it ranged from 22 to 34 with a mean of 28.00±1.22, while in Group II, it
was in the range of 21 to 31 with mean of 27.00±1.06. In occipital lobe of Group I,
large size neurons ranged in-between 21 to 36 with mean of 28.90±1.41, while in
Group II, it varied from 22 to 39 with a mean of 28.50±1.55 neurons. A significant
difference was noticed in the number of large size neurons in frontal lobe, but
temporal and occipital lobes of cerebral cortex did not show any significant
difference in Group I and Group II with the advancement of age. Since no
references are available in literature on number of small, medium and large sized
neuron present in internal pyramidal layer of cerebral cortex, the values could not
be compared.
The neuronal density in internal pyramidal layer of frontal lobe of cerebral
cortex in Group I and Group II was recorded as 36640±744 and 33401±915
respectively. In temporal lobe, the neuronal density was 44084±978 and
36295±896 in Group I and Group II respectively. The density of neurons in
occipital lobe of internal pyramidal layer in cerebral cortex was 42694±791 and
39680±1289 in Group I and Group II respectively. These observations of the
present study regarding neuronal density in frontal and temporal lobes of internal
pyramidal layer of cerebral cortex showed significant difference between Group I
and Group II with the advancement of age. However occipital lobe of cerebral
54
cortex did not show any significant difference between Group I and Group II with
respect to neuronal density in internal pyramidal layer of cerebral cortex.
4.2.1.7. The multiform layer:
This was the innermost layer of the cerebral cortex and somewhat less in
thickness. This layer contributed nearly 12.88% of cortical depth. In this layer,
mixture of pyramidal, stellate and large spindle shaped neurons was present
which were surrounded by large numbers of glial cells. At the bottom of this layer,
the spindle shaped neurons were present which demarcated this layer from
deeper white matter zone (Fig. 22). Vincent et al. (1989) observed similar cells
but described it as pyramidal and spiny stellate shaped cells in multiform layer of
cerebral cortex in Macaque Monkey. However, in contrast with the findings of the
present study, Games and Winer (1988) in albino rat observed closely packed
flattened neuronal soma in multiform layer of cerebral cortex. They also reported
that this layer constitutes about 22% of total cortical thickness. Cullen et al.
(2006) however, reported that multiform layer constituted about 18% of total
cortical thickness in human.
During the present work, it was observed that the thickness of multiform
layer in frontal lobe of Group I varied from 211.06 µm to 249.58 µm with an
average thickness of 230.77±4.14 µm. The thickness of this layer in frontal lobe
of Group II varied from 229.27 µm to 269.23 µm, with the average of 248.93±4.26
µm. The thickness of this layer in temporal lobe of Group I varied from 203.99 µm
to 249.53 µm with an average of 227.43±5.68 µm. The thickness of multiform
layer in temporal lobe of Group II varied from 312.19 µm to 279.13 µm, with an
average of 240.20±6.67 µm. The thickness of this layer in occipital lobe of Group
I varied from 214.48 µm to 259.31 µm with an average of 236.37±4.66 µm. The
thickness of this multiform layer in occipital lobe of Group II varied from 273.43
µm to 328.46 µm, with an average of 298.05±6.62 µm. These observations of the
present study showed that there is progressive increase in the thickness of
multiform layer at frontal, temporal and occipital lobes of cerebral cortex with the
advancement of age from Group I to Group II. There was a significant difference
between increase in thickness of multiform layer at frontal and occipital lobes of
cerebral cortex. But the temporal lobe of cerebral cortex did not show any
significant difference between Group I and Group II with respect to increase in
55
thickness of multiform layer, although there was linear increase in thickness with
the advancement of age.
The number of small size neurons in frontal lobe of cerebral cortex in
Group I ranged in between 23 to 44 with a mean of 34.70±2.21. The number of
small size neurons in Group II varied from 22 to 49 with a mean of 38.80±2.62.
The number of small size neurons in temporal lobe of Group I ranged in between
34 to 56 with a mean of 43.50±2.07, while in Group II, it varied from 31 to 53 with
a mean of 40.40±2.08. The small size neurons in occipital lobe of Group I ranged
in between 29 to 58, with a mean number of 40.50±2.96. The number of small
size neurons in Group II ranged in between 27 to 52, with a mean of 38.70±2.19.
The medium size neurons in frontal lobe of Group I varied in between 06
to 15, with an average of 10.20±0.97. In Group II, the number of medium size
neuron varied in between 06 to 13, with an average of 9.2±0.68. The number of
medium size neurons in temporal lobe of Group I varied in between 07 to 18 with
a mean of 11.70±1.6, while in Group II, it varied in between 08 to 16 with mean
value of 11.80±0.86. The medium size neurons in occipital lobe of Group I ranged
between 07 to 21 with mean of 13.80±1.54, while in Group II it ranged between
08 to 20 with a mean of 13.80±1.18 neurons (Table 5, 6 and 7).
In this layer large size neurons in frontal lobe of Group I ranged in
between 0 to 4 neurons with a mean of 1.40±0.40, while in Group II, it ranged in
between 0 to 4 with a mean of 1.60±0.45. Similarly in temporal lobe of Group I, it
varied from 0 to 3 with a mean of 1.60±0.37, while in Group II, it varied from 0 to
3 with a mean of 1.60±0.26. In occipital lobe of Group I, large size neurons
ranged in between 1 to 6 with a mean of 2.50±0.54, while in Group II, it varied
from 0 to 4 with a mean of 1.60±0.40 neurons.
These observations of the present study regarding number of small,
medium and large size neurons in all the three lobes i.e. frontal, temporal and
occipital lobes of cerebral cortex did not show any significant difference in Group
I and Group II with the advancement of age.
The neuronal density in multiform layer of frontal lobe of cerebral cortex in
Group I and Group II was recorded as 20195±1456 and 17994±1247
respectively. In temporal lobe, the neuronal density was 24829±1352 and
56
22552±102 in Group I and Group II respectively. The density of neurons in
occipital lobe of multiform layer in cerebral cortex was 24047±1344 and
18321±3808 in Group I and Group II respectively (Table 9, 10 and 11). These
observations of the present study regarding neuronal density in multiform layer of
occipital lobe of cerebral cortex showed significant difference between Group I
and Group II with the advancement of age. While frontal and temporal lobe of
cerebral cortex did not show any significant difference between Group I and
Group II. In agreement with the findings of the present study, Yates et al.(2009)
reported that although the neuronal density in multiform layer of cerebral cortex
increases with the advancement of age in rats, but this increase was non
significant. Witelson et al. (1995) recorded neuronal density in multiform layer of
cerebral cortex in human which was much less than that recorded during the
present study. This variation in the neuronal density could be attributed to the
species difference. Cullen et al. (2006) in human reported that the neuronal
density of cerebral cortex was reduced with the advancement of age. However,
they stated that there was no correlation between neuronal density and age.
4.2.2. Cerebellum:
The cerebellum was found to have a thin cortex of grey matter that
overlies white matter. The cerebellum was found to have three layers from
outside to inside as molecular layer, Purkinje cell layer and granular layer (Fig.
23, 24 and 25)
4.2.2.1. Molecular layer:
The molecular layer was the outer most layer of cerebellar cortex, which
consisted of neuropil, with medium to small sized scattered stellate and spindle
shaped neurons. It also consisted of capillaries, that travels throughout molecular
layer and become enlarged on entering the middle layer. This layer had a
compact arborisation of dendrites originating from purkinje cells (Fig. 26).
Molecular layer also contains axons of neurons of granular innermost layer of
cerebellum which are believed to maintain continuity for propagation of signal
throughout cerebellar tissue. Few basket cells were encountered at the deeper
part of this layer (Fig. 27). Similar observations were recorded by Maseko et al.
(2012) reported that stellate cells were present in upper 2/3rd and basket cells
57
were found in lower 1/3rd of cerebellar cortex in elephant, which is in agreement
with the findings of the present study.
During the present study, the thickness of molecular layer of cerebellar
cortex in group I varied from 176.85 µm to 398.51 µm with an average thickness
of 318.27±25.95 µm. The thickness of this layer in group II showed variation from
194.53 µm to 371.44 µm, with an average thickness of 303.72±17.46 µm. A
gradual reduction in the thickness of molecular layer was noticed during present
study from group I to group II. (Table 13) However, no significant difference was
noticed in the thickness of molecular layer of cerebellar cortex in group I and
group II with the advancement of age. The reduction in the thickness of molecular
layer observed during the present study with the advancement of age could be
attributed to loss of dendritic fibres of Purkinje cells entered in the molecular layer
from the Purkinje cell layer as is being described by Zhang et al. (2006) who
reported reductions in the thickness of molecular layer with the advancement of
age in cat; however, in contrast with the findings of the present study, they
reported that there was significant difference in the reduction of thickness of
molecular layer with advancement of age in cat. In agreement with the findings of
the present study, Pal et al. (2003) recorded the thickness of molecular layer in
man and fowl as 343.5 µm and 294 µm respectively. Both these values recorded
by them fall within the range of the present study. Similar observations regarding
thickness of molecular layer of cerebellum were recorded by Fox and Barnard
(1957) in adult monkey.
The neuron number in molecular layer of cerebellar cortex in group I and
group II was recorded as 68.9±2.48 and 63.4±2.65 respectively (Table 14). A non
significant difference was noted in neuron number of molecular layer with
advancement of age from group I to group II. Louis et al. (2014) reported neuron
number as 25.6±4.53 in human, which is much less than that recorded during
present study. This variation in the number of neuron may be attributed to the
species difference.
During the present work, the neuronal density in molecular layer of
cerebellar cortex in group I and group II was recorded as 23204.84±2396 and
21920.35±2232 respectively (Table 15). A gradual decrease in the neuronal
density was observed with the advancement of age. But there was no significant
58
difference in neuronal density of group I and group II. In agreement with the
findings of the present study, Zhang et al. (2006) reported that the neuronal
density in molecular layer of cerebellum decreases with the advancement of age
in cat. However, they recorded significant decrease in neuronal density of
molecular layer, which is in contrast with the current findings.
4.2.2.2. Purkinje cell layer :
During the present study, it was observed that Purkinje cell layer was the
second layer which was interposed in between the molecular layer and the
granular layer of cerebellum. It was thinnest layer and consisted of chiefly
Purkinje cells. These Purkinje cells were found scattered throughout this layer.
These Purkinje cells were arranged in two ways, i.e. vertical and obliquely
horizontal. At certain places, Purkinje cells were arranged in two rows, of which
the upper cells were smaller in size than the lower cells (Fig. 27). Few Purkinje
cells were flask shaped and of larger size with vertical orientation. These cells
were found to dominate the middle layer.
During the present work, it was noted that the thickness of Purkinje cell
layer of cerebellar cortex in group I varied from 34.63 µm to 74.63 µm with an
average thickness of 58.31±4.41 µm. In group II, the thickness of this layer was
found to vary from 37.98 µm to 72.06 µm, with an average thickness of
55.66±4.14 µm. (Table 13) These values indicated that there was reduction in the
thickness of Purkinje cell layer from group I to group II. But no significant
difference was noticed in the decrease of thickness of Purkinje cell layer of
cerebellar cortex with advancement of age from group I to group II. This decrease
in thickness of Purkinje cell layer observed during the present study could be due
to atrophic changes of dendritic arborisation of Purkinje cell during advancement
of age.
The number of neurons in Purkinje cell layer of cerebellar cortex in group I
and group II were recorded as 10±0.55 and 9.8±0.41 respectively (Table 14).
These values indicated that there was linear decrease in the number of neurons
from group I to group II with the advancement of age. But there was no significant
difference between the number of neurons in group I and group II. However, the
neuronal density in Purkinje cell layer of cerebellar cortex in group I and group II
was recorded as 18670.59±2325 and 18690.59±1770 respectively (Table 15),
59
which indicated that the neuronal density increased with the advancement of age
from group I to group II. This increase is density of Purkinje cell might be the
result of reduction in layer thickness. Woodruff-Pak et al. (2010) indicated
significant loss of Purkinje neurons in aged mice. They reported that the
processes of aging impact brain structures. The observations recorded by
Viswasom et al. (2013) in human regarding decrease in number of Purkinje cells
with advancement of age are in agreement with the findings recorded in the
present study, however they reported significant difference in number of Purkinje
cell with the advancement of age, which was not significant in present study.
Nandy (1981) reported reduction in Purkinje cells significantly with age in Macaca
nemestrina. Yesmin et al. (2011) reported that the number of Purkinje cell per
square mm decreased with age which was significant in Bangaladesh people.
They correlated this with changes in motor function in old age. In contrast to the
observations recorded during present study, Whitney et al. (2008) reported non
significant difference in reduction in density of Purkinje cells in control and
acoustic brain in human. Similar findings regarding reduction in neuronal density
of Purkinje cells with advancement of age was reported by Zhang et al. (2006) in
cat.
4.2.2.3. Granular layer :
The granular layer of cerebellum was the innermost layer. It lies below the
Purkinje cell layer. This layer consisted of abundant neurons of small size
granular cells. These cells were spherical in shape. The neurons were denser in
upper layer, while towards bottom the cells become scantier. Few Golgi cells
were found at the superficial part of this layer. The Golgi cells were larger than
the granular cell. The Golgi cells were spindle shaped with centrally located
nucleus (Fig. 29). These observations of the present study are in agreement with
the findings reported by Maseko et al. (2012) in African Elephant. They observed
Golgi cells within upper half of the granule cell layer.
During the present work, it was observed that the thickness of granular
layer of cerebellar cortex in group I was in the range of 311.85 µm to 549.58 µm
with an average of 452.35±26.01 µm. The thickness of this layer in group II
showed variation from 389.19 µm to 548.27 µm, with an average thickness of
471.46±19.63 µm (Table 13). Although there was linear increase in the thickness
60
of granular layer from group I to group II, but no significant difference was noticed
in the thickness of granular layer of cerebellar cortex with advancement of age.
These findings of the present study regarding increase in thickness of granular
layer corroborates with the observations reported by Zhang et al. (2006) in cat.
However, in contrast with the findings of the present study, they reported
significant increase in thickness of granular layer with advancement of age. The
measurements regarding thickness of granular layer reported by Pal et al. (2003)
in fowl and man were less than that recorded during present study and do not
corroborate with observations of the present study. This variation could be
attributed to the species variation.
The number of neuron in Granular cell layer of cerebellar cortex in group I
and group II was recorded as 288.40±9.01 and 286.90±5.71 respectively (Table
14). There was no significant difference between group I and group II regarding
number of neurons in granular layer with advancement of age. However, the
values recorded showed that there was progressive decrease in the number of
neuron from group I to group II. This observation of the present study is in
agreement with the findings recorded by Viswasom et al. (2013) in human. They
also noted progressive decrease in number of granular cells with the
advancement of age. Renovell et al. (1996) reported significant decline in number
of granular cells with advancement of age in human. Although similar decline was
noted in the number of granular cell during present study, but there was no
significant difference in group I and group II with advancement of age.
The neuronal density in Granular cell layer of cerebellar cortex in group I
and group II was recorded as 65379.71±3716 and 61750.72±2714 respectively
(Table 15). This observation of the present study showed that there was
progressive decrease in the density of granular cells from group I to group II with
the advancement of age. However, no significant difference was noted in group I
and group II. In accordance with the findings of the present study, Zhang et al.
(2006) reported that density decreased by 27% with advancement of age in cat,
but in contrast with the present findings they reported that there was significant
difference in the neuronal density with advancement of age. Renovell et al.
(1996) reported that the total number of Granular cells decline significantly during
the aging process in human. Nandy (1981) found no age related changes in
granular density in Macaca –nemestrina.
61
4.2.3. Pons
During the present study, it was observed that the pons contain large
number of nerve fibres with few neurons. The fibre tracks were noted to be well
organized in the form of nerve fascicles. In cross section, the nerve fibres were
surrounded by distinct epineurium, which send its septa and divide it into small
fascicles by perinurium and each axon was covered by endoneurium and myelin
sheath. Oligodendrocyte cells with distinct nucleoli were adjacent to axon (Fig.
30). In between the fibres, large number of glial cells were found which were of
round to stellate shaped with centrally located nuclei. Very few large multipolar
neurons were also found at deep layer. With appreciable perineuronal spaces
especially around the large neurons were observed.
During the present work, three types of neuron were identified in the Pons
based on their size. These neurons were categorised according to the perimeter
of the soma as small (1 to 100 µm), medium (101 to 200 µm) and large (more
than 200 µm) (Fig 31).
In group I, the number of small neurons was recorded as 20±0.93;
whereas in group II, these numbers were recorded as 17.8±0.813. The number of
medium size neurons of group I were recorded as 9.1±0.525, whereas in group II,
these numbers were recorded as 9±0.614. Similarly, the large sized neuron were
3.1±0.433 in pons of group I while in group II, the number of neurons were
2.6±0.33 (Table 16). From the values regarding number of small, medium and
large neurons recorded during present study, it was observed that the number of
neurons decreased from group I to group II with the advancement of age.
However, this decrease in the number of neurons from group I to group II was
statistically non significant. The overall number of neurons in pons irrespective of
size also did not show any significant difference between group I (32.2 ±1.152)
and group II (29.4±0.90), although the number was found reduced with the
advancement of age (Table 16).
The neuronal density of neurons in Pons was recorded as 3220±115.27 in
group I, while in group II it was recorded as 2940±90.921. This figure indicated
that there was reduction in density with the advancement of age from group I to
group II (Table 16). The neuronal density of neuron in pons did not show any
significant difference between group I and group II. This reduction in the number
62
as well as density of neuron in pons may be related to the loss of neuron with the
age factor. These observations of the present study could not be compared with
the observations of the other scientists since very little literature is available
regarding this parameter.
4.2.4. Medulla oblongata
During the present study, the medulla oblongata in all the age groups was
noted to be made up of chiefly the broad bands of nerve fibres, which were
longitudinally placed and had some oligodendrocyte cells scattered amongst
them (Fig. 32). In between these fibres, neurons of spindle and stellate type were
observed. Although all size of neurons were found in the superficial layer, but
only medium to large sized neuronal cells were observed in deeper layer of
medulla oblongata (Fig. 33).
In the present study, the number of small neurons was 14±0.73 in group I,
whereas in group II, these numbers were noted as 13.4±1.00. The number of
medium size neurons in group I and group II were recorded as 6±0.39 and
4.9±0.37 respectively. Similarly, the large sized neuron were 1.8±0.29 in medulla
oblongata of group I, while in group II, the numbers were 1.7±0.21 (Table 17).
From the values regarding number of small, medium and large neurons recorded
during present study, it was observed that the number of neurons decreased from
group I to group II with the advancement of age. However, this decrease in the
number of neurons from group I to group II was statistically non significant. The
overall number of neurons in medulla oblongata irrespective of size was
21.8±0.69 and 20±1.14 in group I and group II respectively (Table 17). These
values also did not show any significant difference between group I and group II,
although the number was found reduced with the advancement of age.
The neuronal density of neurons in medulla oblongata was recorded as
2180±69.60 in group I, while in group II it was recorded as 2000±114.50 (Table
17). These figures indicated that there was reduction in density with the
advancement of age from group I to group II. The neuronal density of neuron in
medulla oblongata did not show any significant difference between group I and
group II.
63
This reduction in the number as well as density of neuron in medulla
oblongata may be related to the loss of neuron with the age factor. These
observations of the present study could not be compared with the observations of
the other scientists since very little literature is available regarding this parameter.
From the observations of the present study regarding reduction in number of
neurons as well as reduction in density of neuron from group I to group II with
advancement of age, it can be concluded that there is permanent loss of neuron
with the advancement of age.
4.3. Histochemistry and histoenzymology
4.3.1. Glycogen:
4.3.1.1. Cerebrum:
In the present study, the PAS activity for presence of glycogen in small
neurons of almost all the layers of cerebrum at frontal, temporal and occipital
lobes in group I showed weak activity, but the blood vessels and neuropil showed
mild PAS positive activity (Fig.34, 35 and 36) while in group II with the
advancement of age, the PAS activity was recorded as mild to moderate in small
neurons of almost all layers of cerebrum, blood vessels and neuropil of frontal,
temporal and occipital lobes (Fig. 37, 38 and 39). The large neurons in various
layers of cerebrum at frontal, temporal and occipital lobes however showed mild
PAS positive activity in group I. The activity for presence of glycogen in large
neurons of group II exhibited mild to moderate activity (Fig. 40 and 41). These
observations regarding presence of glycogen in small neurons, large neurons,
blood vessels and neuropil of cerebrum indicated that the PAS activity increases
with the advancement of age from group I to group II. These observations of
present study regarding increasing trend in PAS activity with the advancement of
age are in agreement with the findings reported by Manich et al. (2016) who
reported progressive increase in PAS activity in brain of aged mice. In agreement
with findings of present study Capucchio et al. (2010) reported that the PAS
positive activity increases with age in horse brain.
The presence of glycogen is related with the energy requirement for
metabolism. The continuous decrease in number of neurons with the
advancement of age observed during present study might lead to additional
64
metabolic burden on the available neurons, blood vessels and neuropil and may
require more energy to carry out its normal function.
4.3.1.2. Cerebellum :
During the present study it was noted that there was mild to moderate
PAS positive activity for the presence of glycogen in neuropil, molecular cells,
purkinje cells and granular cells of cerebellum in group I (Fig. 42). With the
advancement of age in group II the PAS activity for the presence of glycogen was
observed as moderate to intense in neuropil, molecular cells, purkinje cells and
granular cells of cerebellum (Fig. 43). Observations of the present study
corroborates with the findings reported by Gerhauser et al. (2012) in hamster,
who reported PAS positive activity in few large neurons in cerebellum but they did
not report any agewise difference. The increase PAS positive activity for
presence of glycogen in cerebellum with advancement of age observed during
present study might be ascribed to the more energy requirement for carrying out
cerebellar function during advanced age.
4.3.1.3. Pons :
The neuronal cells of the pons showed weak PAS positive activity in
group I where as in group II mild PAS positive activity was noted in the neuronal
cells of pons with the advancement of age (Fig. 44 and 45). This increase in PAS
positive activity could be attributed to the deposition of lipofuscin granules during
advancement of age.
4.3.1.4. Medulla oblongata:
During the present study weak PAS positive activity was observed in
cellular component of medulla oblongata in group I, where as weak to mild PAS
positive activity was noticed in the medulla oblongata in group II with the
advancement of age (Fig. 46 and Fig. 47). Similar observations were reported by
Gerhauser et al. (2012) in hamster. They reported PAS positive activity in few
large neurons of medulla oblongata in oldest group. The presence of PAS activity
during advanced age recorded during present study could be due to deposition of
lipofuscin granules.
65
4.3.2. Acid phosphatase:
4.3.2.1. Cerebrum:
During the present work, it was observed that the acid phosphatase
activity in small as well as large neurons, blood vessels and neuropil of different
layers at frontal, temporal and occipital lobes of cerebrum was mild in group I,
while in group II, this activity was moderate in all the components of cerebrum in
all the lobes with the advancement of age (Fig. 48 and 49). Similar findings were
reported by Sohal and Sharma (1972) in aged flies brain. They recorded
accumulation of dense residual bodies of acid phosphatase. However, in contrast
with the findings of the present study, Nakamura et al. (1989) found no significant
age dependent changes in rat.
The findings of the present study regarding increasing trend of acid
phosphatase activity with the advancement of age may be indicative of
continuous alterations occurring in cerebrum due to degeneration of neurons.
Although, the exact physiological function of acid phosphatase in cerebrum is not
known, the fundamental significance of phosphatase was their ability to catalyse
the hydrolases of phosphate esters.
4.3.2.2. Cerebellum:
The acid phosphatase activity in neurons, neuropils and blood vessels of
cerebellum in group I exhibited mild activity, while in group II, the acid
phosphatase activity was moderate (Fig. 50 and 51). There was gradual increase
in the activity of acid phosphatase with the advancement of age from group I to
group II. Similar findings were also recorded by Manocha (1970) in aged squirrel
monkey. They relate this activity to static maintenance metabolism of cells than to
dynamic functional metabolism. The report of occurrence of acid phosphatase
activity with the advancement of age in present study was contrary to the
statements made by Nakamura et al. (1989) in rat. They stated that although acid
phosphatase activity was noted in cerebellum, but there was no significant age
dependent change in the activity of acid phosphatase. Release of lysosomal
hydrolases associated with modifications in neuronal cells occurring during
ageing might be the probable reason for increase in the acid phosphatase activity
with the advancement of age observed during present study.
66
4.3.2.3. Pons:
In the present study, the acid phosphatase activity in all the cellular
elements of pons was moderate in group I, whereas it was intense in group II with
the advancement of age (Fig. 52 and 53). In agreement with the observations of
the present study, Mohankumar and Sood (1979) reported that all the cellular
elements in pons of Hedgehog were positive for acid phosphatase activity with
the advancement of age. Similarly, Nakamura et al. (1989) reported significant
age dependent changes in pons of rat. The increased acid phosphatase activity
with the advancement of age observed during present study could be due to
accumulation of acid phosphatase positive dense residual bodies over the pons
during advanced age.
4.3.2.4. Medulla oblongata:
The acid phosphatase activity in all the cellular elements of medulla
oblongata during present work was found to be moderate in group I, whereas it
was intense in group II with the advancement of age (Fig. 54 and 55). This
observation of the present study is in agreement with that reported by
Mohankumar and Sood (1979) in medulla oblongata of Hedgehog. They reported
positive activity for acid phosphatase in all the components of medulla oblongata
with the advancement of age. Similar observations were also reported by Hafiza
and Sood (1979) in bat. They noted strong activity in the large neurons of
medulla oblongata.
The findings of the present study regarding increasing trend of acid
phosphatase activity with the advancement of age from group I to group II in all
the components of medulla oblongata may be attributed to the alterations caused
due to degeneration of neurons in medulla oblongata or accumulation of dense
residual bodies or may be ascribed to the phagocytosis which is likely to occur
after degeneration of neuronal cells. However, the exact cause of intense acid
phosphatase activity remained unknown.
67
4.3.3. Alkaline phosphatase :
4.3.3.1. Cerebrum :
During the present work, it was observed that the alkaline phosphatases
activity in small as well as large neurons, blood vessels and neuropils of different
layers at frontal, temporal and occipital lobe of cerebrum was weak in group I
where as in group II it was mild (Fig. 56 and 57). There was increase in the
activity of alkaline phosphatase with the advancement of age. Manocha (1970)
reported that the alkaline phosphatase activity was concentrated in the blood
vessels and the peripheral part of the neurons of cerebrum. In agreement with
the findings of the present study, Kellett et al. (2011) reported increasing alkaline
phosphatase activity on neuronal membrane in human brain injury and
Alzheimer‟s disease. Vardy et al. (2012) in human reported that there was no
increase in alkaline phosphatase activity with the advancement of age, but the
activity was found increased in brain of Alzheimer‟s disease.
4.3.3.2. Cerebellum:
The alkaline phosphatase activity in neurons, neuropils and blood vessels
of cerebellum in group I as well as group II exhibited weak to mild activity (Fig. 58
and 59). In agreement with the findings of the present study, Ng and Tam (1986)
stated that alkaline phosphates activity increases with advancement of age in
mouse.
4.3.3.3. Pons:
In the present study, the alkaline phosphatase activity in all the cellular
elements of pons was mild and moderate in group I and group II respectively
(Fig. 60 and 61). In agreement with the observations of the present study,
Mohankumar and Sood (1979) reported that all the cellular elements in pons of
Hedgehog were positive for alkaline phosphatase activity with the advancement
of age.
68
4.3.3.4. Medulla oblongata:
The alkaline phosphatase activity in all the cellular elements of medulla
oblongata during present work was found to be moderate in group I as well as in
group II (Fig. 62 and 63). There was no difference in the intensity of activity with
the advancement of age. In contrast with the findings of the present study,
Mohankumar and Sood (1979) reported that all the cellular elements in medulla
oblongata of Hedgehog were positive for alkaline phosphatase activity during
advanced age.
4.3.4. Lipofuscin:
4.3.4.1. Cerebrum :
During the present work, mild lipofuscin pigmentation was observed in
small neurons and large neuron of different layers at frontal, temporal and
occipital lobes of cerebrum in group I where as in group II, moderate to intense
lipofuscin pigmentation was noted with the advancement of age (Fig. 64 and 65).
The lipofuscin pigmentation in general was found more towards the axon hillock
and the periphery of the neuron in small as well as large neurons of group I and
group II. It was observed that the number of cells containing pigment increased
with age. It was also noted that due to heavy lipofuscin pigmentation, the nucleus
of the large neuron was shifted toward periphery with the advancement of age
(Fig. 66). Similar observations were reported by Whiteford (1964).
In agreement with the findings of the present study, Tigges (1990) stated
that the lipofuscin granules were discernible in large neurons in cerebrum of
monkey. He further mentioned that these lipofuscin granules increased with the
advancement of age. Findings of the present study were also supported by Nesic
et al. (2013), who reported that there was agewise progression in deposition of
lipofuscin in large neurons of cerebrum in human. Whiteford (1964) stated that
although agewise increase in deposition of lipofuscin in neuron was observed in
dog and pig, but the deposition was independent of breed and sex. These
findings corroborates with the observations of the present study. Gilissen et al.
(1999) observed accumulation of lipofuscin in cerebrum of dwarf lemur and gray
lemur only in aged animals but not in young animals. These findings are also in
accordance with the observations of the present study.
69
The formation or deposition of lipofuschin, although is not clearly
understood, but it is felt that agewise increase in number of lipodal granules in
neurons with advancement of age observed during present study may due to the
fact that the homogenous distribution of the granules might be getting gradually
lost and may gathered in clusters in the cell body to form finally a localised mass,
which might continue to increase in size with the advancement of age. Similarly,
the accumulation of lipofuscin deposition even in young age cannot be eliminated
either by degradation or by exocytosis and get accumulated in cell and since
oxidative reactions are obligatory for life, they act as age dependent enhancer of
lipofuscin accumulation with the advancement of age.
4.3.4.2. Cerebellum:
During the present work, weak to mild pigmentation was observed in
Purkinje cells, molecular cells and granule cells of cerebellum in group I, whereas
in group II, moderate lipofuscin pigmentation was recorded in Purkinje cells,
molecular cells and granule cells of cerebellum. This indicated that the
pigmentation increased with the advancement of age from group I to group II
(Fig. 67 and 68). Similar findings were reported by Heinsen (1981) and Monteiro
(1991) in rats. They recorded increased lipofuscin pigment in the perikarya of
Purkinje cells. Gilissen et al. (1999) investigated lipofuscin deposits in large
Purkinje cells of dwarf lemur with advancement of age. Findings of the present
study regarding increased lipofuscin pigmentation with advancement of age also
corroborates with the findings reported by Nesic et al. (2013) and Gilissen et al.
(1999) who reported age related progressive lipofuscin accumulation in
cerebellum of aged cheirogaleids lemur. Nandy (1981) reported deposition of
lipofuscin in Purkinje cells in relation to aging in Macaca nemestrina. These
observations of the present study as well as findings of other scientists related
with progressive accumulation of lipofuscin in cerebellum with the advancement
of age are a basic biological ageing process. The lipofuscin pigments, which were
present in the region of dendritic attachment, are likely to cause interference with
the propagation of nerve impulse. It can also be concluded that the lipofuscin
pigment may be detrimental to the cells by virtue of being a rigid mass leading to
disturbance in plasticity of the cells.
70
4.3.4.3. Pons :
Pons in general showed weak to mild lipofuscin pigmentation in neurons of group
I, while in group II, there was moderate pigmentation of lipofuschin. This indicated
that the pigmentation increased with the advancement of age from group I to
group II (Fig. 69 and 70). In agreement with the findings of the present study,
Gilissen et al. (1999) reported that accumulation of lipofuscin was observed only
in aged cheirogaleids lemur but not in young ones. The findings of the present
study are also supported by Whiteford (1964) in dog and pig. He stated that the
amount of pigment contained within the individual cells and the number of cells
containing pigment increased with age, but were independent of breed and sex.
4.3.4.4. Medulla oblongata:
During the present study, a weak to mild lipofuscin pigmentation in neurons of
medulla oblongata was observed in group I, while in group II, moderate
pigmentation of lipofuscin was recorded. There was linear increase in lipofuscin
pigmentation in medulla oblongata with the advancement of age from group I to
group II (Fig. 71 and 72). These findings of the present study are in accordance
with those reported by Whiteford (1964) in dog and pig. He reported that the
amount of pigmentation of lipofuscin within the individual cells increased with
age. Nesic et al. (2013) observed progressive agewise increase in accumulation
of lipofuscin in neurons of medulla oblongata. This observation is also in
agreement with the findings of the present study. The findings of the Gilissen et
al. (1999) regarding lipofuscin accumulation in medulla oblongata of aged lemur
also corroborates with the findings of the present study.
The age wise increasing trend of lipofuscin accumulation of neuron of
cerebrum, cerebellum, pons and medulla oblongata observed during present
study could be justified by considering different modalities operating within the
nerve cells such as
Lipofuscin is deposited in cell as a result of metabolic process.
Initial pigment deposition occurs in relation to level of activity
characteristic of a nuclear group.
Within a cell group lipofuscin accumulates as a function of time.
71
Accumulation of lipofuscin in neuron may be a criterion of basic biological
aging processes.
4.4. Electron microscopy
4.4.1. Cerebrum :
During the present study, cerebrum of young goats, at 10 months of age
showed mild fatty deposits scattered in almost all the organelles of cell. These
fatty deposits were electron dense material and appeared as black coloured
spots (Fig. 73 and 74). With the advancement of age there was progressive
granulation followed by mild pigmentation on myelin sheath (Fig. 75). The myelin
sheath was found to undergo splitting and cytoplasm of oligodendrocyte
appeared as electron dense, which subsequently appeared as projected myelin
balloons (Fig. 76). The myelin balloon was found to increase in size and caused
disruption of topography of neuronal cells. Due to increase in size of myelin
balloons, the vacuoles are formed and nucleus gets pushed towards one side
(Fig. 77). These observations of the present study are in agreement with the
findings reported by Peters (2002) in human and non human primates. He stated
that some myelin sheath exhibit degenerative changes such as splitting of myelin
sheath and formation of balloons with the advancement of age. He further
suggested that such degenerative changes lead to cognitive decline since they
cause change in conduction velocity, which results in dysfunction of normal
timing in neuronal circuits. He further mentioned that due to degeneration, other
changes such as formation of redundant myelin and increase thickness of myelin,
which were observed, could be suggestive of continuation of some myelin
formation with advancement of age. Similar observations were also recorded by
Peters et al. (2000) and Vincent et al. (1989), they reported that in old monkey
the vacuoles appeared to represent a late stage in the degeneration of
myelinated axons, for that they were bounded by a thin, laminated sheath.
4.4.2. Cerebellum:
The electron microscopy of cerebellum in group I, showed electron dense
material in the cytoplasm of the neuron, which was considered as fatty deposits
(Fig. 78). The purkinje cells of cerebellum showed presence of electron dense
material of fatty bodies (Fig. 79). The deposits of fat, which appeared as electron
72
dense material, were found in the myelin sheath of the young goats (Fig. 80).
During the present study, it was observed that the neurons of the cerebellum
undergo degenerative changes with the advancement of age. Initially, the
degenerative process was found to start with shrinkage of mitochondria (Fig. 81)
and regression of axon making the cell non functional. Further the process of
apoptosis continued and nucleus became narrow and fat deposits appeared in
nucleoplasm (Fig. 82). Finally the neurons of the cerebellum undergo complete
degeneration with total shrinkage of mitochondria and cell was considered as
apoptic (Fig. 83).
4.4.3 Pons:
In young goats of group I, the pons showed less fatty deposits with no
granulation as well as no degenerative changes in the organelles of neuronal
cells (Fig. 84). However, with the advancement of age in group II, almost all the
organelles of neuronal cells of pons exhibited disruption. The mitochondria
appeared hedgy (Fig. 85). Similarly, myelin showed large vesicular appearance
with very little cytoplasm (Fig. 86).
4.4.4. Medulla oblongata:
The electron microscopic structure of medulla oblongata in young goats of
group I showed normal structure of all the cell organelle. There were no fatty
deposits in neuropil of medulla oblongata (Fig. 87). However, little granulation
was present in myelin sheath of medulla oblongata of young goats (Fig. 88). In
group II, with the advancement of age, myelin sheath of the medulla oblongata
showed electron dense material of fatty deposits. The degenerated mitochondria
in the form of vesicles showed electron lucent areas and were found gathered at
one place (Fig. 89).
73
5. SUMMARY AND CONCLUSIONS
The present work was carried on 20 samples each of cerebrum,
cerebellum, pons and medulla oblongata of goat divided into two groups as 9 to
12 months and 13 months and above. The biometrical observation of whole
brain, were recorded and samples of cerebrum, cerebellum, pons and medulla
oblongata were cut and processed for histological, histochemical, histoenzymic
and electron microscopy. The tissue sections were stained with hematoxylin and
eosin, Toluidine blue, Gallocyanin, Luxol fast and silver impregnation stains for
histological observations. The tissues were also stained to verify the presence of
glycogen, acid phosphatase, alkaline phosphatase and lipofuscin. Agewise
changes in myelin degeneration, pigment deposition and mitochondrial changes,
if any, were studied by Electron microscopic method.
The gross morphology indicated that the cerebrum of goat was oblong to
oval in shape. The cerebellum was oval with wider dimension along transverse
axis. Pons was transversely elongated structure located on ventral aspect of
brain stem with faint median furrow. The medulla oblongata was distal
continuation of brain and continued as spinal cord.
A highly significant difference was observed in the weight of whole brain
in Group I and Group II with the advancement of age. There was linear decrease
in the length, width and depth of brain with advancement of age. However, no
significant difference in the length, width and the depth of brain was observed.
There was no significant difference between the length and width of
cerebrum with the advancement of age, but the length as well as width of
cerebrum was found increased with the advancement of age. Although, the depth
of cerebrum showed slight decrease from Group I to Group II, but there was no
significant difference in the depth of cerebrum with the advancement of age. No
significant difference between the length and width of cerebellum was observed
with advancement of age.
There was no significant difference in the brain index of Group I and
Group II. Similarly, the cerebral index also did not show any statistical difference
74
between Group I and Group II. There was no significant difference between
Group I and II with respect to cerebellar index.
The microstructure of cerebral cortex in almost all the regions of the
cerebrum viz. frontal, temporal and occipital lobes in Group I as well as in Group
II, showed six layers with scattered neurons supported by varying amount of glial
tissue viz. the molecular layer, the external granular layer, the external pyramidal,
the internal granular, the internal pyramidal and the multiform layer.
The cortical depth at all the three lobes of cerebrum under study
decreased with the advancement of age. There was significant difference in the
cortical depth of the frontal, temporal and occipital lobes of cerebrum in Group I
and Group II.
Three types of neuron were identified in the cerebral cortex based on the
size as small, medium and large. Different shapes of neurons were recorded
during the present study viz. spindle, pyramidal and stellate.
The total number of neuron in frontal and temporal lobes of cerebral
cortex showed highly significant difference between Group I and Group II with the
advancement of age. However, occipital lobe of cerebral cortex did not show any
significant difference between Group I and Group II with respect to total number
of neuron in cerebral cortex.
These observations regarding neuronal density in cerebral cortex in
almost all the lobes indicated that there was linear decrease with the
advancement of age. The neuronal density differed non significantly in all the
lobes of cerebral cortex in Group I and Group II.
The uppermost layer of cerebral cortex showed the molecular layer.
Although, there was no significant decrease in the thickness of molecular layer of
cerebral cortex in frontal lobe of Group I and Group II, but significant decrease
was recorded in thickness of molecular layer of cerebral cortex between Group I
and Group II in temporal as well as in occipital lobe with the advancement of age.
The small sized neuron as well as medium sized neuron in frontal,
temporal, and occipital lobes of cerebral cortex did not show any significant
75
difference between Group I and Group II with the advancement of age. It was
noted that the large sized neurons were absent in almost all the lobes of cerebral
cortex in Group I as well as in Group II.
The soma of the molecular neuron was stellate shaped and showed
numerous cerebral vesicles. Some neurons showed parallel orientation to surface
and are called as „Cells of Cajal‟.
The neuronal density in molecular layer of frontal and temporal lobes of
cerebral cortex in Group I and Group II showed linear decrease but there was no
significant difference with the advancement of age. The neuronal density in
molecular layer of occipital lobe of cerebral cortex showed significant linear
increase from Group I and Group II.
No significant difference was noticed in thickness of external granular
layer of cerebral cortex in frontal lobe, but significant difference was found in
thickness of external granular layer of cerebral cortex in temporal and occipital
lobes with advancement of age from Group I to Group II.
The neurons in the external granular layer were stellate shaped with
centrally located nuclei. These neurons were arranged perpendicular to surface.
No significant difference was noticed in the number of small sized neurons in
frontal, temporal and occipital lobes of granular layer of cerebral cortex with
advancement of age.
No significant difference was recorded during present study with respect
to number of medium size neurons in all the regions of Group I and Group II with
the advancement of age. The numbers of large size neurons in Group I and
Group II did not show any significant difference with the advancement of age.
The neuronal density in frontal lobe of external granular layer of cerebral
cortex did not show any significant difference between Group I and Group II with
the advancement of age, however, the neuronal density in temporal and occipital
lobe showed significant difference.
A significant difference was noticed in the thickness of external pyramidal
layer in frontal, temporal and occipital lobes of cerebral cortex in Group I and
76
Group II with the advancement of age. The external pyramidal layer showed
more population of medium and large size neurons than small size neurons in
comparison to other layers.
The population of small size neuron in frontal and occipital lobe showed
significant difference between Group I and Group II. But no significant difference
was noted in the population of small size neuron in temporal lobe. The number of
medium size neuron in all the three lobes of cerebrum did not show any
significant difference with the advancement of age from Group I to Group II. No
significant difference in the number of large size neurons in frontal and temporal
lobes of cerebrum was recorded with advancement of age, while occipital lobe
showed significant difference. A significant difference was observed in neuronal
density of temporal lobe between Group I and Group II with the advancement of
age.
A significant difference was noted in the thickness of internal granular
layer in frontal, temporal and occipital lobes of cerebral cortex in Group I and
Group II with the advancement of age. The neuronal density in frontal and
occipital lobes of internal granular layer of cerebral cortex showed significant
difference between Group I and Group II with the advancement of age.
The internal pyramidal layer was densely packed due to larger size cells.
This layer was found to contain more number of large size pyramidal cells
compared to any other layer. No significant difference was noted in the thickness
of internal pyramidal layer of frontal and occipital lobes of cerebral cortex with the
advancement of age from Group I to Group II during the present study. However,
a significant difference was recorded in the thickness of internal pyramidal layer
of temporal lobe in Group I and Group II.
There was no significant difference between the number of small size
neurons in frontal and occipital lobes of the internal pyramidal layer of the
cerebral cortex with the advancement of age. No significant difference was
noticed in the number of medium size neurons in frontal and occipital lobes of
cerebral cortex between Group I and Group II, but there was linear decrease in
the number of medium size neurons in all the lobes of cerebral cortex with the
advancement of age. A significant difference was noticed in the number of large
77
size neurons in frontal lobe but temporal and occipital lobes of cerebral cortex did
not show any significant difference in Group I and Group II with the advancement
of age.
Neuronal density in frontal and temporal lobes of internal pyramidal layer
of cerebral cortex showed significant difference between Group I and Group II
with the advancement of age. Occipital lobe of cerebral cortex did not show any
significant difference between Group I and Group II with respect to neuronal
density in internal pyramidal layer of cerebral cortex.
A significant difference was noticed between Group I and Group II with
respect to increase in thickness of multiform layer at frontal and occipital lobe of
cerebral cortex. But the temporal lobe of cerebral cortex did not show any
significant difference between Group I and Group II with respect to increase in
thickness of multiform layer. The number of small, medium and large size
neurons in all the three lobes i.e. frontal, temporal and occipital lobe of cerebral
cortex did not show any significant difference in Group I and Group II with the
advancement of age.
The neuronal density in multiform layer of occipital lobe of cerebral cortex
showed significant difference between Group I and Group II with the
advancement of age. While, frontal and temporal lobe of cerebral cortex did not
show any significant difference between Group I and Group II.
No significant difference was noticed in the thickness of molecular layer of
cerebellar cortex in group I and group II with the advancement of age. There was
no significant difference in neuronal density of group I and group II.
No significant difference was noticed in the decrease of thickness of
Purkinje cell layer of cerebellar cortex with advancement of age from group I to
group II. The neuronal density increased with the advancement of age from group
I to group II.
No significant difference was noticed in the thickness of granular layer of
cerebellar cortex with advancement of age. There was progressive decrease in
the density of granular cells from group I to group II with the advancement of age.
However, no significant difference was noted in group I and group II.
78
It was observed that the pons contain large number of nerve fibres with
few neurons. The number of small, medium and large neurons in pons decreased
from group I to group II with the advancement of age. The decrease in the
number of neurons from group I to group II was statistically non significant. The
overall number of neurons in pons irrespective of size also did not show any
significant difference between group I and group II. The neuronal density of
neuron in pons did not show any significant difference between group I and group
II.
The medulla oblongata at all the age groups was noted to be made up of
chiefly the broad bands of nerve fibres, which were longitudinally placed. The
numbers of small, medium and large neurons were decreased from group I to
group II with the advancement of age. However, this decrease in the number of
neurons from group I to group II was statistically non significant.
The neuronal density of neuron in medulla oblongata did not show any
significant difference between group I and group II. Regarding reduction in
number of neurons as well as reduction in density of neuron from group I to group
II with advancement of age, it can be concluded that there is permanent loss of
neuron with the advancement of age.
The presence of glycogen in small neurons, large neurons, blood vessels
and neuropil of cerebrum increases with the advancement of age from group I to
group II. It was noted that there was mild to moderate PAS positive activity for the
presence of glycogen in neuropil, molecular cells, purkinje cells and granular cells
of cerebellum in group I. With the advancement of age in group II the PAS activity
for the presence of glycogen was observed as moderate to intense in neuropil,
molecular cells, purkinje cells and granular cells of cerebellum. The neuronal
cells of the pons showed weak PAS positive activity in group I, where as, in group
II mild PAS positive activity was noted. The PAS positive activity was not
observed in any cellular component of medulla oblongata in group I, where as
weak to mild activity was noticed in group II with the advancement of age.
The acid phosphatase activity in small as well as large neurons, blood
vessels and neuropil of different layers at frontal, temporal and occipital lobes of
cerebrum was mild in group I, while in group II, it was moderate. The acid
phosphatase activity in neurons, neuropils and blood vessels of cerebellum in
79
group I exhibited mild activity, while in group II, it was moderate. The acid
phosphatase activity in all the cellular elements of pons was moderate in group I,
whereas it was intense in group II. The acid phosphatase activity in all the cellular
elements of medulla oblongata was found to be moderate in group I, whereas it
was intense in group II with the advancement of age.
The alkaline phosphatases activity in small as well as large neurons,
blood vessels and neuropils of different layers at frontal, temporal and occipital
lobe of cerebrum was weak in group I, where as in group II it was mild. The
alkaline phosphatase activity in neurons, neuropils and blood vessels of
cerebellum in group I as well as group II exhibited weak to mild activity. The
alkaline phosphatase activity in all the cellular elements of pons was mild and
moderate in group I and group II respectively. The alkaline phosphatase activity
in all the cellular elements of medulla oblongata during present work was found to
be moderate in group I as well as in group II.
Mild lipofuscin pigmentation was observed in small and large neuron of
different layers at frontal, temporal and occipital lobes of cerebrum in group I
whereas in group II, moderate to intense lipofuscin pigmentation was noted with
the advancement of age. It was also noted that due to heavy lipofuscin
pigmentation, the nucleus of the large neuron was shifted toward periphery with
the advancement of age. Weak to mild pigmentation was observed in Purkinje
cells, molecular cells and granule cell of cerebellum in group I, where as in group
II, moderate lipofuscin pigmentation was recorded. Pons in general showed weak
to mild lipofuscin pigmentation in neurons of group I, while in group II, there was
moderate pigmentation of lipofuschin. A weak to mild lipofuscin pigmentation in
neurons of medulla oblongata was observed in group I, while in group II,
moderate pigmentation was recorded.
Cerebrum of young goats, at 10 months of age showed mild fatty deposits
scattered in almost all the organelles of cell. Electron microscopically cerebrum
showed progressive granulation followed by mild pigmentation on myelin sheath.
The myelin sheath was found to undergo splitting and cytoplasm of
oligodendrocyte appeared as electron dense, which subsequently appeared as
projected myelin balloons. Due to increase in size of myelin balloons, the
vacuoles are formed and nucleus gets pushed towards one side. The neurons of
80
the cerebellum were found to undergo complete degeneration with total
shrinkage of mitochondria and cell was considered as apoptic with the
advancement of age.
Electron microscopically almost all the organelles of the neuronal cells of
pons exhibited disruption. The mitochondria appeared hedgy and myelin showed
large vesicular appearance with very little cytoplasm with the advancement of
age. Electron microscopically the degenerated mitochondria in the form of
vesicles showed electron lucent areas and were found gathered at one place
during advancement of age in medulla oblongata.
81
Fig. 1: Photomicrograph showing cerebral cortex with piamater
(P) and blood vessel (B) (Gr. I).
(Haematoxylin and Eosin X 100)
Fig. 2: Photomicrograph showing cerebral cortex with six layers
viz. molecular, outer granular, outer pyramidal, inner
granular, inner pyramidal and multiform layer (Gr.- I,
Frontal lobe)
(Gallocyanine X 50)
Fig. 3: Photomicrograph showing cerebral cortex with six layers
viz. molecular, outer granular, outer pyramidal, inner
granular, inner pyramidal and multiform layer (Gr.- II,
Frontal lobe)
(Gallocyanine X 50)
Fig. 4: Photomicrograph showing cerebral cortex with six layers
viz. molecular, outer granular, outer pyramidal, inner
granular, inner pyramidal and multiform layer (Gr.I,Temporal lobe)
(Gallocyanine X 50)
Fig. 5: Photomicrograph showing cerebral cortex with six layers
viz. molecular, outer granular, outer pyramidal, inner
granular, inner pyramidal and multiform layer (Gr.- II,
Temporal lobe)
(Gallocyanine X 50)
Fig. 6: Photomicrograph showing cerebral cortex with six layers
viz. molecular, outer granular, outer pyramidal, inner
granular, inner pyramidal and multiform layer (Occipital
lobe, Gr. I)
(Haematoxylin & Eosin X 50)
Fig. 7: Photomicrograph showing different layers of cerebral
cortex viz. molecular, outer granular, outer pyramidal,
inner granular, inner pyramidal and multiform layer (Gr.II, Occipital lobe)
(Gallocyanine X 100)
Fig. 8: Photomicrograph showing small neuron (S) and area
calculated by Q-imaging software.
(Gr.-I cerebral cortex)
(Toluidine blue X 1000)
Fig. 9: Photomicrograph showing medium neuron (M) and area
calculated by Q-imaging software.
(Gr.-II cerebral cortex)
(Toluidine blue X 1000)
Fig. 10: Photomicrograph showing Large neuron (L) and area
calculated
by
Q-imaging
software.
(Gr.-I cerebral cortex) (Toluidine blue X 1000)
Fig. 11: Photomicrograph showing spindle shape neuron (N).
(Gr.- I cerebral cortex)
(Toluidine blue X 1000)
Fig.12: Photomicrograph showing pyramidal shape neuron (N)
(Gr.- II cerebral cortex)
(Toluidine blue X 1000)
Fig. 13: Photomicrograph showing stellate shape neuron (N)
(Gr. -II cerebral cortex)
(Toluidine blue X 1000)
Fig. 14: Photomicrograph showing molecular layer of cerebral
cortex.
(Gr.- I, Occipital lobe)
(Gallocyanine X 100)
Fig. 15: Photomicrograph showing molecular layer of cerebral
cortex.
(Gr.- II, Frontal lobe)
(Toluidine blue X 200)
Fig. 16: Photomicrograph showing cells of Cajal (C) in layer I.
(Gr. -I, cerebral cortex) (Haematoxylin & Eosin X 200)
Fig. 17: Photomicrograph showing external granular layer of
cerebral cortex.
(Gr.- I, Frontal lobe)
(Toluidine blue X 200)
Fig. 18: Photomicrograph showing external pyramidal layer of
cerebral cortex.
(Gr.- II, temporal lobe)
(Toluidine blue X 200)
Fig. 19 Photomicrograph showing internal granular layer
(arrow) of cerebral cortex.
(Gr.- I, Frontal lobe)
(Gallocyanin X 200)
Fig. 20: Photomicrograph showing internal pyramidal layer of
cerebral cortex.
(Gr.- II, temporal lobe)
(Toluidine blue X 200)
Fig. 21: Photomicrograph showing internal pyramidal layer and
Betz cells (arrow) of cerebral cortex.
(Gr.- I, frontal lobe)
(Toluidine blue X 200)
Fig. 22 : Photomicrograph showing multiform layer of cerebral
cortex and white matter (arrow).
(Gr.- II, temporal lobe)
(Toluidine blue X 200)
Fig. 23: Photomicrograph showing molecular layer (M), Purkinje
cell layer (P) and Granule cell layer (G).
(Gr.-I, cerebellar cortex) (Haematoxylin & Eosin X 200)
Fig. 24: Photomicrograph showing molecular layer (M), Purkinje
cell layer (P) and Granule cell layer (G).
(Gr.-II, cerebellar cortex) (Haematoxylin & Eosin X 100)
Fig. 25: Photomicrograph showing molecular layer (M),
Purkinje cell layer (P) and Granule cell layer (G)
(Gr.-II, cerebellar cortex) (Silver impregnation X 100)
Fig. 26: Photomicrograph showing arborisation (arrow) from
Purkinje cell.
(Gr.-II, cerebellar cortex)
(Luxol fast X 200)
Fig. 27 : Photomicrograph showing Basket cells (arrow).
(Gr.-II, cerebellar cortex)
(Toluidine blue X 400)
Fig. 28: Photomicrograph showing Purkinje cells (arrow).
(Gr.-I, cerebellar cortex) (Haematoxylin & Eosin X 200)
Fig. 29: Photomicrograph showing Golgi cells (arrow).
(Gr.- I, cerebellar cortex) (Haematoxylin & Eosin X 200)
Fig. 30: Photomicrograph showing Epineurium (E), Perinurium
(P) and Oligodendrocytes (arrow).
(Gr.-II, Pons)
(Toluidine blue X 200)
Fig. 31: Photomicrograph showing large (L), medium (M) and
small (S) neurons (Gr.-I, Pons)
(Toluidine blue X 100)
Fig.
32:
Photomicrograph showing nerve fibres and
oligodendrocyte (arrow) (Gr.-II, medulla oblongata)
(Toluidine blue X 200)
Fig. 33: Photomicrograph showing large cells (Gr.- II, medulla
oblongata )
Haematoxylin & Eosin X 200)
Fig. 34: Photomicrograph of cerebrum showing weak PAS
activity in small neuron (N) and mild activity in blood
vessel (B) and neuropil (P).
(frontal lobe, Gr. I)
(PAS reaction x 200)
Fig. 35: Photomicrograph of cerebrum showing weak PAS
activity in small neuron (N) and mild activity in blood
vessel (B) and neuropil (P).
(temporal lobe, Gr. I)
(PAS reaction x 200)
Fig. 36: Photomicrograph of cerebrum showing weak PAS
activity in small neuron (N) and mild activity in blood
vessel (B) and neuropil (P).
(occipital lobe, Gr. I)
(PAS reaction x 200)
Fig. 37: Photomicrograph of cerebrum showing mild to
moderate PAS activity in small neuron (N), blood
vessel (B) and neuropil (P).
(frontal lobe, Gr. II)
(PAS reaction x 200)
Fig. 38: Photomicrograph of cerebrum showing mild to
moderate PAS activity in small neuron (N), blood
vessel (B) and neuropil (P).
(temporal lobe, Gr. II)
(PAS reaction x 200)
Fig. 39: Photomicrograph of cerebrum showing mild to
moderate PAS activity in small neuron (N), blood
vessel (B) and neuropil (P).
(occipital lobe Gr. II)
(PAS reaction x 200)
Fig. 40: Photomicrograph of cerebrum showing mild PAS
activity in large neuron (L).
(Gr. I)
(PAS reaction x 400)
Fig. 41: Photomicrograph of cerebrum showing mild to
moderate PAS activity in large neuron (L).
(Gr. II)
(PAS reaction x 200)
Fig. 42: Photomicrograph of cerebellum showing mild to
moderate PAS activity in molecular cell (M),
Purkinje cell (P), Granular cell and Neuropil (PL).
(Gr.I)
(PAS reaction x 100)
Fig. 43: Photomicrograph of cerebellum showing moderate to
intense PAS activity in molecular cell (M), Purkinje cell
(P), Granular cell and Neuropil (PL).
(Gr. II)
(PAS reaction x 200)
Fig. 44: Photomicrograph of pons showing weak PAS activity in
large neuron (L).
(Gr. I)
(PAS reaction x 100)
Fig. 45 : Photomicrograph of pons showing mild PAS activity
in large neuron (L).
(Gr. II)
(PAS reaction x 200)
Fig. 46: Photomicrograph of medulla oblongata showing weak
PAS positive activity in neuronal cell.
(Gr. I)
(PAS reaction x 100)
Fig.47: Photomicrograph of medulla oblongata showing weak to
mild PAS positive activity.
(Gr. II)
(PAS reaction x 200)
Fig. 48: Photomicrograph of cerebrum showing mild acid
phosphatase activity in blood vessel (B), neuron (N)
and neuropil (P).
(Gr. I)
(Acid phosphatase x 200)
Fig. 49: Photomicrograph of cerebrum showing mild acid
phosphatase activity in blood vessel (B), neuron (N)
and neuropil (P).
(Gr. II)
(Acid phosphatase x 200)
Fig. 50: Photomicrograph of cerebellum showing moderate acid
phosphatase activity in molecular cell (M), Purkinje cell
(P), Granular cell and Neuropil (PL).
(Gr. I)
(Acid phosphatase x 200)
Fig. 51: Photomicrograph of cerebellum showing moderate acid
phosphatase activity in molecular cell (M), Purkinje cell
(P), Granular cell (G) and Neuropil (PL).
(Gr. II)
(Acid phosphatase x 100)
Fig. 52: Photomicrograph of pons showing moderate acid
phosphatase activity in cellular elements.
(Gr. I)
(Acid phosphatase x 100)
Fig. 53:
Photomicrograph of pons showing intense acid
phosphatase activity.
(Gr. II)
(Acid phosphatase x 100)
Fig. 54: Photomicrograph of medulla oblongata showing
moderate acid phosphatase activity in large neuron
(arrow).
(Gr. I)
(acid phosphatase x 100)
Fig. 55: Photomicrograph of medulla oblongata showing
moderate acid phosphatase activity in large neuron.
(Gr. II)
(Acid phosphatase x 100)
Fig. 56: Photomicrograph of cerebrum showing weak alkaline
phosphatase activity in neurons (arrow).
(Gr. I)
(Alkaline phosphatase x 200)
Fig. 57 : Photomicrograph of cerebrum showing mild alkaline
phosphatase activity.
(Gr. II)
(Alkaline phosphatase x 200)
Fig. 58 : Photomicrograph of cerebellum showing weak to mild
alkaline phosphatase activity.
(Gr. I)
(Alkaline phosphatase x 200)
Fig. 59 : Photomicrograph of cerebrum showing weak to mild
alkaline phosphatase activity.
(Gr. II)
Fig. 60:
(Alkaline phosphatase x 200)
Photomicrograph of pons showing mild alkaline
phosphatase activity.
(Gr. I)
(Alkaline phosphatase x 100)
Fig. 61: Photomicrograph of pons showing moderate alkaline
phosphatase activity.
(Gr. II)
(Alkaline phosphatase x 100)
Fig. 62 : Photomicrograph of medulla oblongata showing
moderate alkaline phosphatase activity.
(Gr. I)
(Alkaline phosphatase x 200)
Fig. 63:
Photomicrograph of medulla oblongata showing
moderate alkaline phosphatase activity.
(Gr. II)
(Alkaline phosphatase x 200)
Fig. 64: Photomicrograph of cerebrum showing mild lipofuscin
deposits in large and small neuron (arrow).
(Gr. I)
(Sudan black B x 200)
Fig. 65: Photomicrograph of cerebrum showing moderate to
intense lipofuscin deposits in neurons (arrow).
(Gr. II)
(Sudan black B x 200)
Fig. 66: Photomicrograph of cerebrum showing moderate to
intense lipofuscin deposits (arrow) and displaced
nucleus (arrow yellow).
(Gr. II)
(Sudan black B x 1000)
Fig. 67: Photomicrograph of cerebellum showing weak to mild
lipofuscin deposits.
(Gr. I)
(Sudan black B x 100)
Fig. 68: Photomicrograph of cerebellum showing moderate
lipofuscin deposits in Purkinje cell (arrow).
(Gr. II)
(Sudan black B x 1000)
Fig. 69: Photomicrograph of pons showing weak to mild
lipofuscin deposits.
(Gr. I)
(Sudan black B x 400)
Fig. 70 : Photomicrograph of pons showing moderate lipofuscin
deposits.
(Gr. II)
(Sudan black B x 1000)
Fig. 71: Photomicrograph of medulla oblongata showing weak
to mild lipofuscin deposits.
(Gr. I)
(Sudan black B x 200)
Fig. 72 : Photomicrograph of medulla oblongata showing
moderate lipofuscin deposits.
(Gr. II)
(Sudan black B x 200)
Fig. 73: Transmission electron photomicrograph showing
normal neuron from cerebrum with fatty deposit
(arrow). (Gr. I)
(X 9.6 M)
Fig. 74: Transmission electron photomicrograph showing
normal
neuron
with
electron
dense material
(arrow).
(cerebrum Gr. I)
(X 5.7 M)
Fig. 75: Transmission electron photomicrograph showing
granulation (G), pigmentation (P) and myelin
degeneration (M)
(cerebrum Gr. II)
(X13.5 M)
Fig. 76: Transmission electron photomicrograph showing nerve
fiber (M) bulging (B) and dense cytoplasm (C).
(cerebrum Gr. II)
(X 12 M)
Fig. 77: Transmission electron photomicrograph showing nerve
cell and displaced nucleus (arrow).
(cerebrum, Gr. II)
(X 7.7 M)
Fig. 78: Transmission electron photomicrograph showing nerve
cell and fat deposites (arrow).
(cerebellum, Gr. I)
(X 4.8 M)
Fig. 79: Transmission electron photomicrograph showing nerve
cell and fat deposits (arrow).
(cerebellum, Gr. I)
(X 4.8 M)
Fig. 80: Transmission electron photomicrograph showing
myelin
sheath
(cerebellum, Gr. I)
and
deposits
(arrow)
(X 57.9 M)
Fig. 81: Transmission electron photomicrograph showing
degenerating neuron and shrunken mitochondria
(arrow).
(cerebellum, Gr. II)
(X 9.6 M)
Fig. 82: Transmission electron photomicrograph showing
degenerating neuron and shrunken mitochondria
(arrow) with dense rim.
(cerebellum, Gr. II)
(X 7.7 M)
Fig. 83: Transmission electron photomicrograph showing
apoptic neuron, shrunken mitochondria (M) and
regressed axon (arrow).
(cerebellum, Gr. II)
(X 6.7 M)
Fig. 84: Transmission electron photomicrograph showing
normal neuron and very less deposits (arrow).
(Pons, Gr. I)
(X 7.7 M)
Fig. 85: Transmission electron photomicrograph showing
degenerative neuron and cloudy mitochondria
(arrow).
(Pons, Gr. II)
(X 7.7 M)
Fig. 86: Transmission electron photomicrograph showing
vesicular appearance of myelin (M) and scanty
cytoplasm (C).
(Pons, Gr. II)
(X 77.2 M)
Fig. 87: Transmission electron photomicrograph showing
normal neuron.
(medulla oblongata, Gr. I).
(X 7.7 M)
Fig. 88: Transmission electron photomicrograph showing
normal myelin sheath (medulla oblongata, Gr. I) and
deposits (arrow).
(X 23 M)
Fig. 89: Transmission electron photomicrograph showing
myelin sheath mitochondria (M) and deposits
(arrow).
(medulla oblongata, Gr. II)
(X 15.5 M)
Table 1 : Biometrical observations of brain in group I and group II
Group I
Observations
Group II
Sample mean
SD
SE
Sample
mean
SD
SE
T –stat
F-cal
P
Weight of brain (gm)
120.30
8.87
2.85
131.60
5.50
1.75
3.41
2.87*
0.003
Length of brain (cm)
9.91
0.50
0.15
9.77
0.43
0.13
0.66
2.10
0.51
Width of brain (cm)
6.33
0.50
0.16
6.23
0.32
0.10
0.52
2.13
0.60
Depth of brain (cm)
3.98
0.19
0.06
3.96
0.30
0.09
0.17
2.13
0.86
Length of cerebrum (cm)
7.82
0.50
0.15
8.13
0.34
0.11
1.60
2.11
0.12
Width of cerebrum (cm)
6.04
0.57
0.18
6.26
0.44
0.13
0.96
2.10
0.34
Depth of cerebrum (cm)
3.98
0.19
0.06
3.96
0.30
0.09
0.17
2.13
0.86
Length of cerebellum
(cm)
Width of cerebellum
(cm)
Depth of cerebellum
(cm)
* significant at 1%
3.12
0.31
0.10
3.15
0.31
0.10
0.21
2.10
0.83
4.70
0.69
0.21
4.42
0.48
0.15
1.12
2.11
0.27
3.01
0.16
0.05
3.11
0.21
0.06
1.18
2.10
0.25
Table 2: Showing different indices of brain in group I and group II
Group I
Group II
Observations
Sample mean
SD
SE
Sample
mean
SD
SE
T –stat
F-cal
P
Brain Index
157.21
11.57
3.65
157.38
13.64
4.31
0.03
2.10
0.97
Cerebral Index
130.54
14.8
4.68
130.4
10.52
3.32
0.02
2.11
0.98
Cerebellar
Index
151.8
21.49
6.79
140.79
13.51
4.27
1.37
2.13
0.190
Table 3: Micrometrical observations showing thickness of different layers of frontal lobe of cerebral cortex in group I and group II. (µm)
Group I
Layers
Group II
SD
SE
Total Depth
Sample
mean
1785.77
SD
SE
T –stat
F-cal
P
12.75
Sample
mean
1666.82
40.32
57.36
18.13
5.31
2.92*
6.94
Layer I
405.05
18.66
5.90
403.32
19.10
6.04
0.20
2.10
0.83
Layer II
238.65
19.17
6.60
225.73
15.49
4.9
1.65
2.10
0.11
Layer III
249.93
12.24
3.87
221.70
15.91
5.03
4.43
2.89*
0.00
Layer IV
344.49
16.38
5.18
244.50
15.14
4.78
15.16
2.87*
1.06
Layer V
337.66
18.77
5.90
332.92
14.61
4.62
0.63
2.10
0.53
Layer VI
230.77
13.10
4.14
248.93
13.48
4.26
3.05
2.87*
0.00
* significant at 1%
Table 4 : Micrometrical observations showing thickness of different layers of temporal lobe of cerebral cortex in group I and group II.
Group I
Layers
Group II
SD
SE
Total Depth
Sample
mean
1745.5
SD
SE
T –stat
F-cal
P
11.57
Sample
mean
1669.10
36.61
51.29
16.2
3.832
2.920**
0.0015
Layer I
400.42
14.44
4.56
418.50
21.76
6.88
2.188
2.119*
0.043
Layer II
11.92
13.40
3.77
4.23
248.60
21.01
6.64
2.288
2.144*
0.03
Layer III
231.12
231.08
184.47
11.47
3.63
8.351
2.87**
1.32
Layer IV
341.59
11.79
3.72
241.55
16.97
5.36
15.304
2.920**
5.65
Layer V
322.32
12.39
3.91
342.32
21.53
6.8
2.54
2.144*
0.023
Layer VI
227.43
17.98
5.68
240.20
21.09
6.67
0.909
2.100
0.374
**significant at 1% , *significant at 5% level
Table 5 : Micrometrical observations of thickness of different layers of occipital lobe of cerebral cortex in group I and group II.
Group I
Layers
Sample mean
SD
Group II
SE
Total Depth
1781.8
32.66
10.33
Sample
mean
1565.5
Layer I
403.69
16.49
5.21
Layer II
251.24
11.59
3.66
Layer III
228.91
16.28
Layer IV
338.5
Layer V
Layer VI
SD
SE
T –stat
F-cal
P
73.87
23.36
8.47
3.05**
2.09
300.46
22.89
7.23
11.56
2.92**
3.48
12.3
3.89
7.159
2.878**
1.14
5.15
212.97
213.51
12.88
2.34
2.10*
0.03
12.51
3.95
231.2
16.15
4.07
5.1
16.63
2.10**
5.90
332.69
15.86
5.01
314.21
25.64
1.93
2.13
0.07
236.37
14.75
4.66
298.05
20.96
7.60
2.92**
1.05
** significant at 1% , * significant at 5% level
8.1
6.62
Table 6 : Micrometrical observations showing neuronal count of different layers of frontal lobe of cerebral cortex of group I and group II.
* significant at 5% level
/ ** significant at 1% level
Layer I
Group I
Group II
Statistic
Layer II
Layer III
Layer IV
Layer V
Layer VI
Size
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
Mean
38.6
8.9
-
120.7
49.60
10.7
67.40
56.80
20.80
57.50
52.1
8.30
60.40
41.30
22.0
34.70
10.20
1.4
SD
12.2
3.31
-
18.40
12.59
3.94
12.66
11.06
4.09
12.65
9.53
3.91
10.58
8.15
4.73
6.99
3.08
1.26
SE
3.88
1.04
-
5.82
3.98
1.24
4.00
3.49
1.65
4.00
3.01
1.20
3.34
2.57
1.49
2.21
0.97
0.4
Mean
38.9
8.0
-
129.3
44
13.8
54.60
47.30
17.80
49.50
45.4
8.70
52.10
41.10
17.70
33.80
9.2
1.6
SD
11.3
2.82
-
13.20
11.20
4.84
8.79
9.04
2.86
10.24
8.69
2.21
7.24
5.17
3.129
8.31
2.15
1.43
SE
3.59
0.89
-
4.17
3.54
1.53
2.78
2.86
0.90
3.24
2.75
0.70
2.29
1.63
0.98
2.62
0.68
0.45
T –stat
0.05
0.65
1.20
2.10
2.10
2.11
2.10
1.54
1.55
1.24
0.28
2.04
0.06
2.39
0.26
0.84
0.33
F-cal
2.10
2.10
-
2.11
2.10
2.10
2.62*
2.10
2.10
2.10
2.11
2.14
2.11
2.13
2.11*
2.10
2.11
2.10
P
value
0.95
0.52
-
0.24
0.30
0.13
0.01
0.05
0.13
0.13
0.23
0.78
0.05
0.94
0.02
0.79
0.41
0.74
Table 7: Micrometrical observations showing neuronal count of different layers of temporal lobe of cerebral cortex in group I and group II.
Layer I
Group I
Group II
Statistic
Layer II
Layer III
Layer IV
Layer V
Layer VI
Size
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
Mean
42.2
10.40
-
124
47.50
11.0
58.9
48.5
20.1
56.8
36.50
10.7
61.00
53.10
28.0
43.5
11.70
1.60
SD
11.86
2.59
-
17.22
9.36
2.86
7.7
7.90
3.24
8.41
8.47
2.49
8.38
7.59
3.88
6.57
3.36
1.17
SE
3.75
0.81
-
5.44
2.96
0.96
2.45
2.56
1.02
2.66
2.68
0.79
2.65
2.40
1..22
2.07
1.065
0.371
Mean
38.90
10.40
-
118.2
40.40
11.9
53.3
48.6
18.1
57.8
29.40
10.6
51.50
45.60
27.0
40.4
11.80
1.6
SD
7.5
2.31
-
11.8
8.79
2.72
8.6
9.33
3.63
8.27
7.94
2.45
8.16
8.03
3.36
6.60
2.74
0.83
SE
2.3
0.73
-
3.75
2.78
0.86
2.74
2.9
1.14
2.40
2.30
0.77
2.58
2.53
1.06
2.08
0.86
0.26
T –stat
0.74
0.0
-
0.87
0.41
0.71
1.52
0.02
1.29
0.26
5.45
0.09
2.56
2.14
0.67
1.05
0.07
0.00
F-cal
2.13
2.10
-
2.11
2.11
2.10
2.10
2.10
2.10
2.10
2.10**
2.10
2.10*
2.10*
2.10
2.10
2.10
2.11
P
value
0.46
1.0
-
0.39
0.68
0.48
0.14
0.97
0.21
0.79
4.30
0.92
0.01
0.04
0.50
0.30
0.94
1.00
* significant at 5% level
/ ** significant at 1% level
Table 8: Micrometrical observations showing neuronal count of different layers of occipital lobe of cerebral cortex of group I and group I.
* significant at 5% level
/ ** significant at 1% level
Layer I
Group I
Group II
Statistic
Layer II
Layer III
Layer IV
Layer V
Layer VI
Size
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
S
M
L
Mean
42.90
10.20
-
118.2
42.10
10.40
65.20
50.90
21.80
58.50
38.30
11.60
61.60
51.90
28.90
40.50
13.80
2.50
SD
12.83
2.40
-
18.27
8.68
3.43
9.00
9.37
4.02
11.69
10.06
4.14
11.39
9.86
4.48
9.36
4.89
1.71
SE
4.05
0.77
-
5.70
2.74
1.08
2.84
2.96
1.27
3.69
3.18
1.31
3.60
3.11
1.41
2.96
1.54
0.54
Mean
46.40
10
-
120.80
46.30
12.20
56.10
49.70
17.70
56.20
36.0
11.5
55.10
48.20
28.50
38.70
13.80
1.60
SD
10.51
2.5
-
15.25
8.40
3.36
8.60
9.32
4.00
7.80
8.13
3.13
11.26
9.89
4.97
6.92
3.73
1.26
SE
3.3
0.81
-
4.80
2.60
1.06
2.70
2.94
1.26
2..48
2.57
1.65
3.5
3.13
1.55
2.19
1.18
0.40
T –stat
0.66
0.17
-
0.34
1.09
1.18
2.30
0.28
2.28
0.51
0.86
0.06
1.28
0.83
0.18
0.48
0.0
1.33
F-cal
2.10
2.10
-
2.10
2.10
2.10
2.10*
2.10
2.10*
2.11
2.10
2.10
2.10
2.10
2.10
2.10
2.10
2.10
P
value
0.51
0.86
-
0.73
0.28
0.25
0.03
0.77
0.03
0.61
0.39
0.95
0.21
0.41
0.85
0.63
1.00
0.19
Table 9: Neuron density of different layers of frontal lobe of cerebral cortex in group I and group II.
Group I
Group II
Layers
Sample
mean
SD
SE
SD
SE
T –stat
F-cal
P
605
Sample
mean
36775
Total
Depth
37075
1915
1314
415
0.34
2.11
0.68
Layer I
11769
3889
1229
11565
2498
790
0.140
2.131
0.89
Layer II
76102
10536
3331
83175
6789
2146
1.784
2.131
0.09
Layer III
55368
6424
2031
56164
8880
2808
0.22
2.11
0.82
Layer IV
34199
5752
1819
42331
7546
2386
2.71
2.10*
0.01
Layer V
36640
2354
744
33401
2895
915
2.74
2.10*
0.01
Layer VI
20195
4607
1456
17994
3943
1247
1.14
2.10
0.26
* significant at 5% level
Table 10 : Neuron density of different layers of temporal lobe of cerebral cortex in group I and group II.
Group I
Layers
Group II
Sample
mean
38896
SD
SE
SD
SE
T –stat
F-cal
P
720
Sample
mean
38571
2277
924
292
0.418
2.17
0.68
13119
3182
1006
11781
1252
396
1.23
2.17
0.23
Layer II
79140
8984
2841
69098
10540
3333
2.29
2.10*
0.03
Layer III
55266
5850
1850
65383
8989
2842
2.98
2.94**
0.009
Layer IV
35210
5289
1672
40664
6297
1991
2.10
2.10
0.05
Layer V
44084
3094
978
36295
2835
896
5.86
2.87**
1.49
Layer VI
24829
4278
1352
22552
3237
1023
1.34
2.10
0.19
Total
Depth
Layer I
** significant at 1% , * significant at 5% level
Table 11 : Neuron density of different layers of occipital lobe of cerebral cortex in group I and group II.
Group I
Layers
Group II
Sample
mean
40054
SD
SE
SD
SE
T –stat
F-cal
P
809
Sample
mean
39843
2559
1820
575
0.212
2.11
0.83
13188
3481
1100
18771
3636
1149
3.507
2.87**
0.002
Layer II
68080
8658
2738
84310
8252
2609
4.290
2.87**
0.0004
Layer III
60328
4919
1555
57875
5204
1645
1.08
2.10
0.292
Layer IV
37366
4529
1432
51792
7047
2228
5.44
2.94**
6.80
Layer V
42694
2501
791
39680
4077
1289
1.99
2.13
0.06
Layer VI
24047
4250
1344
18321
3808
1204
3.17
2.87**
0.005
Total
Depth
Layer I
** significant at 1% , * significant at 5% level
.
Table 12: Total neuron count of different lobes of cerebral cortex in group I and group II
Total
Depth
Lobes
Group I
Sample
mean
661.4
Frontal
lobe
Temporal
681.5
lobe
Occipital
687.3
lobe
** significant at 1%
Group II
SD
SE
T –stat
F-cal
P
9.12
Sample
mean
612.8
25.92
8.19
3.96
2.87**
0.00
46.77
14.79
615.4
17.30
5.47
4.19
3.10**
0.00
50.58
15.99
664.8
30.79
9.73
1.20
2.13
0.24
SD
SE
28.86
Table 13: Layer thickness of cerebellar cortex in group I and group II (µm).
Group I
Group II
Layers
Sample mean
SD
SE
Sample mean
SD
SE
T –stat
F-cal
P
Molecular
cell layer
Purkinje
318.27
82.06
25.95
303.72
55.24
17.46
0.46
2.11
0.64
58.31
13.95
4.41
55.66
13.12
4.14
0.43
2.10
0.66
452.35
82.26
26.01
471.46
62.09
19.63
0.28
2.10
0.56
cell layer
Granular
cell layer
Table 14: Neuronal count of different layers of cerebellar cortex in group I and group II.
Group I
Group II
Layers
Sample mean
SD
SE
Sample mean
SD
SE
T –stat
F-cal
P
Molecular
cell layer
Purkinje
68.90
7.86
2.48
63.40
8.39
2.65
1.51
2.10
0.14
10.00
1.76
0.55
9.8
1.31
0.41
0.28
2.10
0.77
288.40
28.50
9.01
286.90
18.05
5.71
0.14
2.13
0.89
cell layer
Granular
cell layer
Table 15: Neuronal density of different layers of cerebellar cortex in group I and group II.
Group I
Group II
Layers
Sample mean
SD
SE
Sample mean
SD
SE
T –stat
F-cal
P
Molecular
cell layer
Purkinje
23204.84
7577
2396
21920
7058
2232
0.39
2.10
0.69
18670
7352
2325
18690.59
5598
1770
0.006
2.10
0.99
65379.71
11752
3716
61750.72
8585
2714
0.784
2.11
0.44
cell layer
Granular
cell layer
Table 16: Neuron count, total neuron count and neuronal density in Pons in Group I and Group II
Neuron count
Group I
Group II
Statistic
Total Neuron
count
Neuronal
density
Size
S
M
L
Mean
20
9.10
3.10
32.20
3220
SD
2.94
1.66
1.37
3.64
364.53
SE
0.93
0.52
0.43
1.15
115.27
Mean
17.80
9.00
2.60
29.40
2940
SD
2.57
1.94
1.07
2.87
287.51
SE
0.81
0.61
0.33
0.90
90.92
T –stat
1.77
0.12
0.90
1.90
1.90
F-cal
2.10
2.10
2.10
2.10
2.10
P value
0.09
0.90
0.37
0.07
0.07
Table 17: Neuron count, total neuron count and neuronal density in medulla oblongata in Group I and Group II
Neuron count
Group I
Group II
Statistic
Total Neuron
count
Neuronal
density
Size
S
M
L
Mean
14.00
6.0
1.18
21.80
2180
SD
2.30
1.24
0.91
2.20
220.10
SE
0.73
0.39
0.29
0.69
69.60
Mean
13.40
4.90
1.70
20.00
2000
SD
3.16
1.19
0.67
3.62
362.09
SE
1.00
0.37
0.21
1.14
114.50
T –stat
0.48
2.01
0.27
1.34
1.34
F-cal
2.11
2.10
2.10
2.13
2.13
P value
0.63
0.05
0.78
0.19
0.19
Graph 1: Showing different indices of brain
160
140
120
100
Group I
80
Group II
60
40
20
0
Brain Index
Cerebral Index
Cerebellar Index
Index
Graph II: Showing thickness of different layers of frontal lobe
1800
1600
Thickness (µm)
1400
1200
1000
800
Group I
600
Group II
400
200
0
Total
Depth
I
II
III
Cortical Layers
IV
V
VI
Graph III: Showing thickness of different layers of temporal lobe
1800
1600
Thickness (µm)
1400
1200
1000
800
Group I
600
Group II
400
200
0
Total
Depth
I
II
III
IV
V
VI
Cortical Layers
Graph IV: Showing thickness of different layers of occipital lobe
1800
1600
Thickness (µm)
1400
1200
1000
800
Group I
600
Group II
400
200
0
Total
Depth
I
II
III
Cortical Layers
IV
V
VI
Graph V: Showing neuronal density of different layers of frontal
cortex
90000
80000
70000
50000
40000
Group I
30000
Group II
20000
10000
0
Total
Depth
I
II
III
IV
V
VI
Cortical Layers
Graph VI: Showing neuronal density of different layers of temporal
lobe
80000
70000
60000
Density
Density
60000
50000
40000
30000
Group I
20000
Group II
10000
0
Total
Depth
I
II
III
Cortical Layers
IV
V
VI
Graph VII: Showing neuronal density of different layers of occipital
lobe
90000
80000
Neuronal density
70000
60000
50000
40000
Group I
30000
Group II
20000
10000
0
Total
Depth
I
II
III
IV
V
VI
Cortical layers
Graph VIII: Showing total neuron count in different lobes
700
Total neuron count
680
660
640
Group I
620
Group II
600
580
560
Frontal lobe
Temporal lobe
Cerebral Lobes
Occipital lobe
Graph IX: Showing neuronal density of different layers of cerebellar
cortex
70000
Neuron Density
60000
50000
40000
Group I
30000
Group II
20000
10000
0
Molecular cell
layer
Purkinje cell
layer
Granular cell
layer
Cerebellar cortex layer
Graph X: Showing thickness of different layers of cerebellar cortex
500
450
Thickness (µm)
400
350
300
250
Group I
200
Group II
150
100
50
0
Molecular cell
layer
Purkinje cell layer Granular cell layer
Cortical layers
Graph XI: Showing neuron count in different layers of cerebellar cortex
300
200
150
Group I
Group II
100
50
0
Molecular cell
layer
Purkinje cell layer Granular cell layer
Cortical layers
Graph XII: Showing neuronal density in Pons
3250
3200
Neuronal Density
Neuron number
250
3150
3100
3050
Group I
3000
Group II
2950
2900
2850
2800
Group I
Group II
Graph XIII: Showing neuronal density in medulla oblongata
2200
Neuronal Density
2150
2100
Group I
2050
Group II
2000
1950
1900
Group I
Group II
BIBLIOGRAPHY
Agashiwala, A. M., E. D. Louis, P. R. Hof and Perla D. P. (2008) A novel approach to
non-biased systematic random sampling: A stereologic estimate of
Purkinje cells in the human cerebellum Brain Research 1236 : 73-78.
Altamura, C., M. L. Dell’Acqua, R. Moessner, D. L. Murphy, K. P. Lesch and A. M.
Persico (2007) Altered neocortical cell density and layer thickness in
serotonin transporter knockout mice: a quantitation study. Cerebral
Cortex 17 : 1394-1401.
Axelrad, J. E., E. D. Louis, L. S. Honig, I. Flores, G. W. Ross, R. Pahwa, K. E. Lyons,
P. L. Faust and J. P. Vonsattel (2008) Reduced Purkinje cell number in
essential tremor: a postmortem study. Arch. Neurol. 65(1) : 101–107.
Bancroft, J. D. and A. Stevens (1982) Theory and Practice of Histological
Techniques, 2nd ed. Churchill Livingstone, Edinburgh.
Beaulieu, C. (1993) Numerical data on neocortical neurons in adult rat with special
reference to the GABA population. Brain Research 609 : 284-292.
Borras, D., I. Ferrer and M. Pumarola (1999) Age related changes in the brain of the
dog. Vet. Pathol. 36 : 202-211.
Byanet, O., J. O. Nzalak, S. O. Salami, A. D. Umosen, S. A. Oja and B. A. Onoja
(2008) Morphometric observations of the brain of the African Grasscutter
in Nigeria. Veterinary Research 2(2) : 22-24.
Byanet, O. and T. Dzenda (2014) Quantitative biometry of body and brain in the
grasscutter (Thryonomys swinderianus) and African giant rat (Cricetomys
gambianus)
:
Encephalization
Neuroscience 3(1) : 1-6.
i
quotient
implication.
Research
in
Byanet, O., T. Dzenda, M. Sulaiman (2014) Dimensional sex differences in
cerebellum of the African giant pouched rat (Cricetomys gambianus –
waterhouse, 1840). International Journal of Biosciences 5(8) : 1-10.
Capucchio, M. T., M. Marquez, P. Pregel, L. Foradada, M. Bravo and G. Mattutino
(2010) Parenchymal and vascular lesions in ageing equine brains :
histological and immunohistochemical studies. Journal of Comparative
Pathology 142 : 61–73.
Cullen, T. J., M. A. Walker, S. L. Eastwood, M. M. Esiri, P. J. Harrison and T. J. Crow
(2006) Anomalies of asymmetry of pyramidal cell density and structure in
dorsolateral prefrontal cortex in schizophrenia. British Journal of
Psychiatry 188 : 26 – 31.
Culling, C. F. A. (1969) A Handbook of Histopathological and Histochemical
Techniques 3rd Edn. Butterworths, London. (pp:192:196).
Davanlou, M. and D. F. Smith (2004) Unbiased stereological estimation of different
cell types in rat. cerebral cortex. Image Anal. Stereol. 23 : 1-11.
Diao, J., J. Xu, G. Li, C. Tang and T. Hua (2009) Age-related Changes of Glu/GABA
Expression in the Primary Visual Cortex of Cat. Zoological Research
30(1) : 38−44.
Drury, R. A. B. and E. A. Wallington (1980) Carleton’s Histological Technique, 5th
ed. Oxford University Press, New York / Toranto. (pp:450:465)
Fischer, H. G., M. Morawski, M. K. Bruckner, A. Mittag, A. Tarnok and A. Arendt
(2012) Changes in neuronal DNA content variation in the human brain
during aging. Aging Cell 11 : 628–633.
Fox, C. A. and J. A. Barnard (1957) A quantitative study of the purkinje cell dendritic
branchlets and their relationship to afferent fibres. Journal of Anatomy
91(3) : 299-313.
ii
Games K. D. and J. A. Winer (1988) Layer V in rat auditory cortex: Projections to the
inferior colliculus and contralateral cortex. Hearing Research 34 : 1-26.
Gazzaley, A. H., M. M. Thakker, P. R. Hof and J. H. Morrison (1997) Preserved
number of entorhinal cortex layer II neurons in aged macaque monkeys.
Neurobiol Aging 18(5) : 549–553.
Gerhauser, I., P. Wohlsein, H. Ernst, P. G. Germann and W. Baumgartner (2012)
Vacuolation and mineralisation as dominant age-related findings in
hamster brains. Experimental and Toxicologic Pathology. In press, www.
Sciencedirect.com/j.etp.2011.12.001.
Gilissen, E. P., R. E. Jacobs, E. R. Mcguinness and J. M. Allman (1999)
Topographical localization of lipofuscin pigment in the brain of the aged
fat-tailed dwarf lemur (cheirogaleus medius) and grey lesser mouse lemur
(microcebus murinus) : comparison to iron localization division of biology.
American Journal of Primatology 49 : 183–193.
Greferath, U., A. Bennie, A. Kourakis and G. L. Barrett (2000) Impaired spatial
learning in aged rats is associated with loss of p75-positive neurons in the
basal forebrain. Neuroscience 100(2) : 363–373.
Hafiza, B. and P. P. Sood (1979) Histoenzymological mapping of alkaline
phosphatase and 5-nucleotidase in the medulla oblongata of a
microchiropteran
bat
(Taphozous
melanopogon
temminck).
Brain
Research Bulletin 4(4) : 467-74.
Heinsen, H. (1981) Regional differences in the distribution of lipofuscin in purkinje
cell perikarya a quantitative pigmentarchitectonic study of the cerebellar
cortex of senile albino rats. Anat. Embryo. 161 : 453-464.
Heinsen, H., R. Henn, W. Eiscnmenger, M. Gotz, J. Bohl, B. Bethke, U. Lockemann
and K. Puschel (1994) Quantitative investigations on the human
iii
entorhinal area: left-right asymmetry and age-related changes. Anatomy
and Embryology, 190 : 181-194.
Jelsing, J., R. Nielsen, A. K. Olsen, N. Grand, R. Hemmingsen and B. Pakkenberg
(2006a) The postnatal development of neocortical neurons and glial cells
in the Gottingen minipig and the domestic pig brain. The Journal of
Experimental Biology 209 : 1454-1462.
Jelsing, J., H. Jorgen, G. Gundersen, R. Nielsen, R. Hemmingsen and B.
Pakkenberg (2006b) The postnatal development of cerebellar Purkinje
cells in the Gottingen minipig estimated with a new stereological sampling
technique – the vertical bar fractionators. J. Anat. 209 : 321–33.
Kellett, K. A., J. Williams, E. R. Vardy, D. A. Smith and N. M. Hooper (2011) Plasma
alkaline phosphatase is elevated in Alzheimer’s disease and inversely
correlates with cognitive function. International Journal of Molecular
Epidemiology 2(2) : 114-121.
Khan, A.H. and P. P. Sood (1984) A comparative study of phosphatases in the
rhombencephalon and mesencephalon of fresh water turtle (Lissemys
punctata granosa). Journal of Hirnforsch 25(6) : 633-45.
Kigir, E. S., H. D. Kwari and I. B. Thilza (2010) Some aspects of neurometrics in
sahel goats in Maiduguri, Nigeria. Academia Arena. 2(8) : 44:47.
Krimer, L. S., M. M. Herman, R. C. Saunders, J. C. Boyd, T. M. Hyde, J. M. Carter, J.
E. Kleinman and D. R. Weinberger (1997) A qualitative and quantitative
analysis of the entorhinal cortex in schizophrenia. Cerebral Cortex 7 :
732–739.
Legge, T. (1996) The Beginning of Caprine Domestication in Southwest Asia, 2nd
ed. Smithsonian Institute, Washington D. C. (236–262).
iv
Lincicome, P. P. and A. Hall (1984) The pygmy in Extension Goat Handbook,
George F. W. Haenlein and Donald L. Ace, eds. Newark, Delaware:
Cooperative Extension Service, University of Delaware. B-7 : 1-4.
Louis, R. J., M. Lee, S. H. Kuo, J. Paul, G. Vonsattel and E. D. Louis (2014)
Cellular density in the cerebellar molecular layer in essential tremor,
spinocerebellar ataxia and controls. Parkinsonism and Related Disorders
20 : 1270-1273.
Manich, G., I. Cabezon, E. Auge, C. Pelegri and J. Vilaplana (2016) Periodic acidSchiff granules in the brain of aged mice: From amyloid aggregates to
degenerative structures containing neo-epitopes. Ageing Research
Review 27 : 42-55.
Manocha, S. L. (1970) Histochemical distribution of alkaline and acid phosphatase
and adenosine triphosphatase in the brain of squirrel monkey (Saimiri
sciureus). Histochemie. 21(3) : 221.
Maseko, B. C., B. Jacobs, M. A. Spocter, C. C. Sherwood, P. R. Hof and P. R.
Manger (2012) Qualitative and quantitative aspects of the microanatomy
of the african elephant cerebellar cortex. Brain Behaviour Evolution 10 :
1-16.
Meyer, H. S., V. C. Wimmer, M. Oberlaender, C. P. de Kock, B. Sakmann and M.
Helmstaedter (2010) Number and laminar distribution of neurons in a
thalamocortical projection column of rat vibrissal cortex. Cerebral Cortex.
20 : 2277-2286.
Mochizuki, Y., M. K. Park, T. Mori and S. Kawashima (1995) The difference in
autofluorescence features of lipofuscin between brain and adrenal.
Zoological Science 12 : 283-288.
v
Mohanakumar and P. P. Sood (1979) Histological and histoenzymological studies on
the medulla oblongata and pons of hedgehog (Paraechinus micropus).
Acta Morphologica Neerlando-Scandinavica 18(4) : 291-304.
Monteiro, R. A. F. (1991) Age-related quantitative changes in the organelles of rat
neocerebellar Purkinje cells. Histol. Histopath. 6 : 9-20.
Mwamengele, G. L. M., T. M. Mayhew
and V. Dantzer (1993) Purkinje cell
complements in mammalian cerebella and the biases incurred by
counting nucleoli. Journal of Anat. 183 : 155-160.
Nakamura, Y., M. Takeda, H. Suzuki, H. Morita, K. Tada, S. Hariguchi and T.
Nishimura (1989) Age-dependent change in activities of lysosomal
enzymes in rat brain. Mechanisms of Ageing and Development 50(3) :
215–225.
Nandy K. (1981) Morphological changes in the cerebellar cortex of aging Macaca
nemestrina. Spring 2(1) : 61–64.
Nesic, S., B. Risti and M. Jovanovi (2013) Aged-related accumulation of lipofuscin in
the brain of dogs. ESVP/ECVP Proceedings 148 : 1:97.
Newsholme, S. J., D. J. Schneider and C. Reid (1985) A suspected lipofuscin
storage disease of sheep associated with ingestion of the plant,
TRACHYANDRA DIVARICATA (JACQ.) Kunth. Onderstepoort J. vet.
Res. 52 : 87-92.
Ng, T. B. and P. P. Tam (1986) Changes of acid and alkaline phosphatase activities
in the developing mouse brain. Biology of Neonate 50 : 107–113.
Olopade, J. O., S. K. Onwuka, B. A. Balogun, and B. O. Oke (2005) Morphometric
investigation of the brain of West African dwarf sheep in Nigeria. Int. J.
Morphol. 23(2) : 99-104.
vi
Olopade, J.O., H.D. Kwari, J.C. Shawulu, M. Ofua and S.K. Onwuka (2007) Some
aspects of the neurometrics of the Sahel goat in Nigeria. Europian
Journal of Anatomy 11(2) : 101-104.
Olopade, J. O., O. O Igado., C. I. Nwafor, A. O. Alamu and S. K. Onwuka (2011)
Some aspects of the craniofacial indices and macro neurometrics of the
Nigerian local pig (Sus scrofa). Italian Journal of Anatomy and
Embryology. 116(1) : 38-44.
Pal, B., S. Chowdhury and R. K. Ghosh (2003) Comparative anatomical study of the
cerebellum of man and fowl. J. Anat. Soc. India 52(1) : 32-37.
Peinado, M. A., A. Quesada, J. A. Pedrosa, M. Martinez, F. J. Esteban, M. L. Del
Moral and J. M. Peinado (1997) Light microscopic quantification of
morphological changes during aging in neurons and glia of the rat parietal
cortex. The Anatomical Record 247 : 420–425.
Peters, A., M. B. Moss and C. Sethares (2000) Effects of aging on myelinated nerve
fibers in monkey primary visual cortex. Journal of Comparative
Neurology. 419(3) : 364-76.
Peters, A. (2002) Structural changes that occur during normal aging of primate
cerebral hemispheres Neuroscience and Biobehavioral Reviews 26 :
733–741.
Porter, V. (1996) Goats of the world, Farming Press, Ipswich, UK.
Pringle, H. (1998) Neolithic agriculture: reading the signs of ancient animal
domestication. Science 282 :1448.
Rabinowicz, T., M. C. Petetot and P. S. Gartside (2002) Structure of the Cerebral
Cortex in Men and Women. Journal of Neuropathology and Experimental
Neurology 61(1) : 46 57.
vii
Renovell, A., J. Giner and M. Portoles (1996) Loss of granule neurons in the aging
human cerebellar cortex. Aging and cell death 193-194.
Rockel, A. J., R. W. Hiorns and T. P. S. Powell (1980) The basic uniformity in
structure of the neocortex. Brain 103 : 221-244.
Semendeferi, K., E. Armstrong, A. Schleicher, K. Zilles and G. W. Van Hoesen
(2001) Prefrontal cortex in humans and apes : a comparative study of
area 10 american. Journal of Physical Anthropology 114 : 224–241.
Sharma, D. and R. Singh (1994) Age related changes in the number of lipofuscin
containing neurons and neuronal histochemistry of lipofuscin and the
effect of ageing reversal drug centrophenoxine on senile neurons in the
parietal cortex of rat. Proc. Indian. Sci. Acad. 60B(6) : 523-532.
Shefer, V.F. (1972) Absolute number of neurons and thickness of the cerebral cortex
during aging, senile and vascular dementia, and pick's and alzheimer's
diseases. Zhurnal Nevropatologii I Psikhiatrii Imeni Korsakova 72(7) :
1024-1029.
Sholl, D. A. (1993) A comparative study of the neuronal packing density in the
cerebral cortex. Journal of Anatomy 10 : 143-158.
Singh, U. B. and S. Sulochana (1996) Handbook of Histological and Histochemical
Technique, 1st ed. Primier Publishing House, Hyderabad : (20-94).
Sjobeck, M., S. Dahlen and E. Englund (1999) Neuronal loss in the brainstem and
cerebellum part of the normal aging process? a morphometric study of
the vermis cerebelli and inferior olivary nucleus. Journal of Gerontology:
Biological sciences 54(9) : B363-B368.
Smiley, J.F., G. Rosoklija, B. Mancevski and D. Pergolizzi (2012) Hemispheric
comparisons of neuron density in the planum temporale of schizophrenia
and nonpsychiatric brains. Psychiatry Res. 192(1) : 1–11.
viii
Snedecor, G. W. and W. G. Cochran (1994) Statistical Methods, 7th ed. Oxford and
IBH Publishing Co; Calcutta.
Sobrino, Y., F. Vila, B. Ramos and A. Medina (2001) Identification and quantification
of the age-pigment, lipofuscin, in brains of the deep-water rose shrimp
aristeus antennatus. Scientific council meeting september 2001. Nafo scr.
doc. 01/98.
Sohal, R. S. and S. P. Sharma (1972) Age-related changes in the fine structure and
number of neurons in the brain of the housefly, Musca domestica.
Experimental Gerontology 7(4) : 243-249.
Sood,
P.
P.
and
M.
M.
Mulchandani
(1977)
A
comparative
study
of
histoenzymological mapping on the distribution of acid and alkaline
phosphatases and succinic dehydrogenase in the spinal cord and
medulla oblongata of mouse. Acta histochemica 60(2) : 180-203.
Sood, P. P. and S. P. Sinha (1983) A comparative histochemical study of alkaline
phosphatase and acetylcholinesterase in the hind brain of Channa
punctatus and heteropneustis fossilis. Folia Histoche. Cytochem.
(Krakow). 21(2) : 107-114.
Spurr, A.R. (1969) journal of Ultrastructural Research, 26 : 311.
Srivastava, C. V., R. C Maurya and P. Chand (2009) Cyto-architecture and neuronal
types of the dorsomedial cerebral cortex of the common Indian wall lizard,
Hemidactylus flaviviridis . Archives Italiennes de Biologie.147 : 21-35.
Sturrock, R. R. (1989) Age related changes in neuron number in the mouse lateral
vestibular nucleus J. Anat. 166 : 227-232.
Sur, E., Y. Oznurlu, T. Ozaydin, F. Colakoglu, S. Unsal, and Y. Yener (2011)
Comparative histometrical study of the cerebellum and the determination
of some agnor parameters in different avian species. Bull. Vet. Inst.
Pulawy. 55 : 261-265.
ix
Terry, R.D., R. DeTeresa, and L.A. Hansen (1987) Neocortical cell counts in normal
human adult aging. Annals of Neurology. 21(6) : 530–539.
Tigges, J., J. G. Herndon and A. Peters (1990) Neuronal population of area 4 during
the life span of the rhesus monkey. Neurobiol aging 11(3) : 201-208.
Vardy, E. R., K. A. Kellett, S. L. Cocklin and N. M. Hooper (2012) Alkaline
phosphatase is increased in both brain and plasma in Alzheimer's
disease. Neurodegenerative Disease 9(1) : 31-37.
Vila, Y., A. Medina, C. Megina, F. Ramos and I. Sobrino (2000) Quantification of the
age-pigment lipofuscin in brains of known-age, pond-reared prawns
(Penaeus japonicas). Journal of experimental zoology 286 : 120–130.
Vincent, S. L., A. Peters and J. Tigges (1989.) The effects of aging on the neurons
within area 17 of rhesus monkey cerebral cortex. Anatomical Records
223 : 329-341.
Viswasom, A. A., S. Sivan and A. Jobby (2013) age related changes in the granule
cell number in the human cerebellar cortex Journal of Evolution of
Medical and Dental Science. 2(16) : 2699.
Walloe, S., B. Pakkenberg and K. Fabricius (2014) Stereological estimation of total
cell numbers in the human cerebral and cerebellar cortex. Frontiers in
Human Neuroscience 8 : 1-9.
Whiteford, R.D. (1964) Distribution of lipofuscin as related to aging in the canine and
porcine brain Iowa State University. Digital Repository @ Iowa State
University ,Retrospective Theses and Dissertations.
Whitney, E. R., T.L. Kemper, M. L. Bauman, D. L. Rosene and G.L. Blatt (2008)
Cerebellar Purkinje Cells are Reduced in a Subpopulation of Autistic
Brains: A Stereological Experiment Using Calbindin-D28k. Cerebellum 7 :
406–416.
x
Witelson, S. F., I. I. Glezer and D. L. Kigar (1995) Women have greater density of
neurons in posterior temporal cortex. The Journal of Neuroscience 15(5) :
3418-3428.
Woodruff-Pak, D.S., M. R. Foy, G. G. Akopian, K. H. Leed, J. Zacha, K. P. Nguyena,
D. M. Comallia, J. A. Kennarda,
A. Agelane and
R. F. Thompsonc
(2010) Differential effects and rates of normal aging in cerebellum and
hippocampus PNAS. 107(4) :1624–1629.
Yates, M. A., J. A. Markham, S. E. Anderson, J. R. Morris and J. M. Juraska (2008)
Regional variability in age-related loss of neurons from the primary visual
cortex and medial prefrontal cortex of male and female rats. Brain Res.
1218 : 1–12.
Yesmin, T., Ara S., B. U. Umar, M. Rahman, H. Afroz, K. Sultana and A. Begum
(2011) Numbers of Purkinje cell with increasing age-a post mortem study.
Faridpur Med. Coll. J. 6(2) : 92-94.
Zecevic, N. and P. Rakic (2001) Development of layer I neurons in the primate
cerebral cortex. Journal of Neuroscience 21(15) : 5607-5619.
Zeder, M. A. and B. Hesse (2000) The initial domestication of goats (Capra hircus) in
the Zagros Mountain 10,000 years ago. Science 287 : 2254–2257.
Zhang, C., T. Hua, Z. Zhu and X. Luo (2006) Age-related changes of structures in
cerebellar cortex of cat. Journal of Bioscience 31 : 55–60.
xi
THESIS ABSTRACT
a)
:
Title of the thesis
“Histological and histochemical studies
on cerebrum, cerebellum, pons and
medulla
oblongata
in
goat
(Capra
hircus)”
b)
Full name of student
c)
Name
and
:
of :
address
Salankar Amol Madhukar
Dr. R. S. Dalvi
Professor and Head
Dept. of Anatomy and Histology,
Nagpur Veterinary College, Nagpur
Advisor / Guide
d)
Degree to be awarded
:
Doctor of Philosophy (Ph.D.)
e)
Year of award of degree
:
2017
f)
Major subject
:
Veterinary Anatomy and Histology
g)
Total number of pages in :
81
the thesis
h)
Number of words in the :
887
abstract
i)
Signature of student
j)
Signature,
address
Name
of
:
and :
forwarding
authority.
Dr. N. P. Dakshinkar
Associate Dean,
Nagpur Veterinary College,
Nagpur
ABSTRACT
The present work is carried on 20 samples each of cerebrum, cerebellum,
pons and medulla oblongata of goat. The biometrical, histological, histochemical,
histoenzymic and electron microscopic observation of cerebrum, cerebellum,
pons and medulla oblongata were recorded.
The gross morphology indicated that cerebrum of goat was oblong to oval
in shape. The cerebellum was oval along transverse axis. Pons was transversely
elongated. The medulla oblongata continued as spinal cord. A highly significant
statistical difference was observed in weight of whole brain with advancement of
age.
The microstructure of cerebral cortex, showed six layers. There was
significant statistical difference in the cortical depth of cerebrum in Group I and
Group II. Different shapes of neurons in cerebral cortex were recorded viz.
spindle, pyramidal and stellate. The total number of neuron in frontal and
temporal lobes of cerebral cortex showed highly significant statistical difference
between Group I and Group II, but significant statistical decrease was recorded in
Group I and Group II in temporal as well as in occipital lobe. It was noted that
large sized neurons were absent in cerebral cortex in Group I as well as in Group
II.
Some neurons in molecular layer showed parallel orientation to surface
and are called as ‘Cells of Cajal’. Significant statistical difference was found in
thickness of external granular layer of cerebral cortex with advancement of age
from Group I to Group II.
The neurons in external granular layer were stellate shaped with centrally
located nuclei. These neurons were arranged perpendicular to surface.
A significant statistical difference was noticed in thickness of external
pyramidal layer of cerebral cortex with advancement of age. The external
pyramidal layer showed more population of medium and large size neurons than
small size neurons in comparison to other layers.
A significant statistical difference was observed in neuronal density of
temporal lobe with advancement of age.
A significant statistical difference was noted in the thickness of internal
granular layer in cerebral cortex with advancement of age. The number of
medium size neurons in internal granular layer of temporal lobe of cerebral cortex
showed significant difference with advancement of age. The neuronal density in
frontal and occipital lobes of internal granular layer of cerebral cortex showed
significant statistical difference with advancement of age.
A significant difference was recorded in thickness of internal pyramidal
layer of temporal lobe in Group I and Group II. A significant statistical difference
was noticed in number of large size neurons of internal pyramidal layer in frontal
lobe of cerebral cortex with advancement of age. Neuronal density in frontal and
temporal lobes of internal pyramidal layer of cerebral cortex showed significant
statistical difference with advancement of age.
A significant statistical difference was noticed between increase in
thickness of multiform layer at frontal and occipital lobe of cerebral cortex with
advancement of age. The neuronal density in multiform layer of occipital lobe of
cerebral cortex showed significant statistical difference with advancement of age.
The neuronal density in Purkinje cell layer of cerebellum increased with
advancement of age from group I to group II. There was progressive decrease in
density of granular cells of cerebellum from group I to group II with the
advancement of age. There was reduction in number of neurons as well as
density of neurons in medulla oblongata from group I to group II with
advancement of age.
The presence of glycogen in small neurons, large neurons, blood vessels
and neuropil of cerebrum as well as in neuropil, molecular cells, Purkinje cells
and granular cells of cerebellum increases with advancement of age from group I
to group II. Pons and medulla oblongata showed weak to mild glycogen activity
with advancement of age.
The neurons, blood vessels and neuropil of cerebrum as well as
cerebellum exhibited gradual increase in the acid phosphatase activity with the
advancement of age. Pons and medulla oblongata showed intense activity in all
cellular components.
The alkaline phosphatases activity in small and large neurons, blood
vessels and neuropil of cerebrum as well as cerebellum showed weak to mild
activity whereas pons and medulla oblongata showed moderate alkaline
phosphatase activity in their cellular components.
Moderate to intense lipofuscin pigmentation was noted with advancement
of age in cerebrum. It was also noted that due to heavy lipofuscin pigmentation,
the nucleus of the large neuron was shifted toward periphery with advancement
of age. Lipofuscin pigmentation was found to increase from weak to moderate in
Purkinje cells, molecular cells and granular cells of cerebellum and also in
neurons of pons and medulla oblongata.
Electron microscopically cerebrum showed progressive granulation
followed by mild pigmentation on myelin sheath. The myelin sheath was found to
undergo splitting and cytoplasm of oligodendrocyte appeared as electron dense,
which subsequently appeared as projected myelin balloons. Due to increase in
size of myelin balloons, the vacuoles are formed and nucleus gets pushed
towards one side.
The neurons of the cerebellum were found to undergo complete
degeneration with total shrinkage of mitochondria and cell was considered as
apoptic with advancement of age. Electron microscopically, almost all the
organelles of neuronal cells of pons exhibited disruption. The mitochondria
appeared cloudy and myelin showed large vesicular appearance with very little
cytoplasm
with
advancement
of
age.
In
medulla
oblongata,
electron
microscopically the degenerated mitochondria in the form of vesicles showed
electron lucent areas and were found gathered at one place during advancement
of age.
izca/k lkjka’k
v- izca/kkps f'k"kZd
% ^^cdjhP;k izefLr”d] vuqefLr”d] vuwlrs q vkf.k
yaceTtk ;kaPkk mfrjpuk‘kkL=h; o mrhjlk;fu;
vH;kl**
c-
fo|kF;kZps iq.kZ ukao
d- ekxZn'kZdkps ukao o iRrk
% lkyudj veksy e/kqdj
% MkW- vkj- ,l- nGoh
izk/;kid o foHkkx izeq[k
'kjhjjpuk 'kkL= o mfr'kkL= foHkkx]
ukxiwj i’kqqoS|d egkfo|ky;] ukxiwj
M- iznku dj.;kr ;s.kkjh
inoh
% vkpk;Z inoh
b-
% 2017
inoh iznku dj.;kps o"kZ
Q- eq[; fo"k;
% 'kjhjjpuk 'kkL= o mfr’kkL=
x-
izca/kkrhy ,dw.k i`"Bs
% 81
gbZ-
lkjka'kkrhy ,dw.k 'kCn
fo|kF;kZph lgh
% 569
%
t- vxzsf"kr dj.kkÚ;k
vf/kdkÚ;kph lgh] ukao
vkf.k iRrk
%
¼MkW- ,u- ih- nf{k.kdj½
lg;ksxh vf/k"Bkrk
ukxiwj i’kqoS|d egkfo|ky;]
ukxiwj
lkjka'k
lnj vH;kl ohl cdÚ;kaP;k izefLr”d] vuqefLr”d] vuwlrs q vkf.k yaceTtk ;kaP;koj
dj.;kr vkyk- ;k vH;klk njE;ku ojhy vo;okaps mfrjpuk‘kkL=h;] mrhjlk;fu; o vrhlq{e
lajpuh; fufj{k.k dj.;kr vkys-
ckg~;jpuk’kkL=h; vH;klkr cdjhpk izefLRk”d vk;rkd`rh rs yacorqZGdkj vlY;kps
fnlys- vuqefLr”d gk vkMO;k js”ksr yacorqZGdkj gksrk- vuqlsrq gk vkMO;k js”ksr ykacqGdk
vlY;kps fnlwu vkys- yaceTtk gk eTtkjTtw Eg.kwu iw<s >kykiw.kZ eans qP;k otukr ok<R;k o;kuqlkj vfry{kf.k; ok< >kY;kps vk<Gys- eans q funsZ’kad
izefLr”d funsZ’kkad rlsp vuqefLRk”d funsZ’kakd ;ke/;s o;ksekukuqlkj dks.kRkkgh cny >kY;kps
vk<Gys ukghlq{en’kZdh; vH;klkr izefLr”d ckg~;kaxkps ,dq.k 6 Fkj vlY;kps fnlys- lnj
ckg;axkP;k [kksyhr o;ksekukuqlkj y{k.kh; Qjd vlY;kps vk<Gys- izefLr”d ckg~;ax Fkjkrhy
psrkis’kh g~;k rdZq] rkjkdkj o ‘kadq ;k vkdkjkps vlY;kps vk<Gys- izefLr”d ckg;axkrhy
yykV[akM o ‘ka[k[kaMkrhy psrkis’khrhy la[ksr o;ksekukuqlkj y{k.kh; cny vlY;kps fnlysjs.kqFkjkrhy dkgh psrkis’kh g;k i`”VHkkxkyk lekarj vlrkr o R;kauk ^dkty is’kh* g~;k
ukokus lacks/kY;k tkrs- izefLr”d ckg;akxkrhy ckg; d.kh; FkjkP;k tkMhe/;s o;ksekukuqlkj
y{kf.k; cny vlY;kps vk<Gys- ;k Fkjkrhy psrkiks’khpk vkdkj gk rkjdkj vlwu R;krhy
dsaUnzd gs is’khP;k e/kkse/k vlqu lnj Pksrki’kh g;k i`”BHkkxkyk dkVdks.k vlY;kPks vk<GysizefLr”d ckg~;axkarhy ckg~;a’kadqdkj FkjkP;k tkMhe/;s o;ksekukuqlkj y{kf.k; cny gksr vlY;kps
vk<Gys- lnj Fkjke/;s e/;e o eksB;k vkdkjkP;k psrkis’kahPa kh la[;k g~;k ygku vkdkjkP;k
psrhis’khis{kk tkLr vlY;kps vk<Gys- ckg~;kaxkrhy vkarj d.kh; FkjkP;k tkMhe/;s o;ksekukuqlkj
y{kf.k; cny vlY;kps fnlys- ;k Fkjkrhy ‘ka[k[kaMke/khy e/;e vkdkjkP;k psrhis’khaP;k
la[;se/;s o;ksekukuqlkj y{kf.k; cny gksr vlY;kps fnlys- lnj Fkjkrhy yykV[kaM o
i’pdjksVh[kaM ;karhy psrkis’khP;k ?kurse/;s o;ksekukuqlkj y{kf.k; cny >kY;kps vk<Grsvkrhy ‘kadqdkj FkjkP;k tkMhe/khy ‘ka[k[kaMke/;s nksuhgh o;ksxVkr y{k.kh;
cny vlY;kps fnlys- ;kp Fkjke/khy yykV[kaMkrhy eksB~;k vkdkjkP;k psrkis’khP;k la[;sse/;s
ok<R;k o;ksekukuqlkj Yk{kf.k; cny >kY;kps vk<Gwu vkys- ;k Fkjkrhy yykVpkaM o
‘ka[k[kaMkrhy psris’khP;k ?kurse/;s o;ksekukuqlkj y{kf.k; cny >kY;kps vk<Gys- izefLr”d
ckg~;axkrhy yykV[kaM o i’pdjksVh[kaMkrhy fofo/kkdkfj; FkjkP;k psrkis’khrhy ?kurse/;s
o;ksekUkkuqlkj y{kf.k; cny >kY;kps vk<Gwu vkysvuqefLRk”dkrhy ijdhuts is’khFkjkrhy psrkis’khrhy ?kurse/;s ok<R;k o;ksxVkuqlkj
ok< >kY;kps vk<Gwu vkys-
vuqefLr”d Fkjkrhy psrkis’khrhy ?kurse/;s ok<R;k
o;ksxVkuqlkj ?kV >kY;kps vk<Gwu vkYksyaceTtse/khy psrkis’khla[;k o psrkis’kh ?kUkrk ;ke/;s ok<Y;k o;ksxVkuqlkj ?kV >kY;kps
vk<Gwu vkys
izefLr”dke/khy ygku psrkis’kh] eksB;k psrkis’kh] jDrokgh.;k vkf.k U;qjksihy rlsp
vuqefLr”dkrhy js.kqis’kh] ijdhuts is’kh] d.kh;is’kh vkf.k U;qjksihy ;ke/khy Xyk;dkstups
vfLrRo ok<R;k o;ksekukuqlkj ok<Y;kps vk<Gy- vuqlsrq o yaceTtk ;ke/khy Xyk;dkstuph
fdz;kf’kyrk ok<R;k o;ksekukuqlkj v’kDr rss lkSE; >kY;kps vk<Gwu vkysizefLr”d rlsp vuqefLr”d ;ke/khy psrkis’kh jDrokghU;k vkf.k U;qjksihy ;ke/;s
vWflM QkWLQsVst ;k fodjkph fdz;kf’kyrk ok<R;k o;ksekukuqlkj ok<ysyh vk<Gwu vkyhizefLr”d o vuqefLr”dkrhy ygku o eksB~;k psrkis’kh jDrokgh.;k vkf.k
U;qjksihy ;ke/;s v’kDr rs lkSE; v’kh vYdykbu QkWLQsVst fodjkph fdz;kf’kyrk vk<Gwu
vkyhvuqlsrw o yaceTtk ;kaP;k is’kh?kVdke/;s lkSE; v’kh vYdykbZu QkWLQsVst fodjkph
fdz;kf’kyrk vk<Gwu vkyh- izefLr”dke/;s ok<R;k o;kuqlkj lkSE; rs rhoz vls yk;iksiq’phu
o.kZd.k vk<Gwu vkysr- ;ke/;s vlsgh vk<Gwu vkys dh eksB~;k psrkis’kh e/khy dssna zd ijh?kkdMs
LFkkykarjhr >kyk- vuqekfLr”dkrhy ijdhuts is’kh] js.kqi’s kh o df.kdkis’kh rlsp vuqlsrw o
yaceTtk ;ke/khy psrkis’khe/;s v’kDr rs lkSE; vls yk;iksi’q phu o.kZd.k vk<Gwu vkysrvrhlq{e lajpuh; fujh{k.kkr izefLr”dke/khy ekbZyhu vkoj.kkoj ok<ho df.kdk rlsp
lkSE; o.kZd.k vk<Gwu vkys- ekbZyhu vkoj.kkr foHkktu >kysys vk<Gys rlsp
vYio`f{kdkis’khe/khy
is’khnzO;
bYksDVªku
xnZ
>kY;kps
fnlys
rs
iq<s
ekbyhu
QqX;ke/;s :ikarfjr >kys- ekbyhu QqX;kPkk vkdkj ok<Y;keqGs R;kr fjDrhdk r;kj >kY;k o
R;keqGs dasUnzd ,dk cktqyk <dyyk xsykv.kqefLr”dke/khy
psrkis’kh
iq.kZi.ks
Úgkl
ikowu
R;krhy
rarfq udsP;k
vkdkjkr ?kV >kyh vkf.k ok<R;k o;ksekukuqlkj is’kh u”V >kYksY;k vk<Gwu vkY;k- vfrlq{e
nf’kZds[kkyh vuqlsrw e/khy cgqrsd loZ psrkis’khrhy vaxd Hkax ikoY;kps vk<Gwu vkysok<R;k o;kskekukuqlkj rarfq udk /kqlq j >kYksY;k vk<Gwu vkY;k o ekbyhu e/;s eksB~;k
iqVhdk fnlwu vkY;k o R;kr Qkj deh izek.kkr is’khnzO; vk<Gwu vkysvrhlq{en’khZds[kkyh vls fnlys dh yaceTtk e/khy Úgkl >kYksY;k rarfq udsP;k iqVhdseqGs
bYksDVªku pdkdh {ks= rS;kj >kyss o ok<R;k o;ksekukuqlkj rs loZ ,dk fBdk.kh tek >kysys
vk<Gwu vkys-