* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Existence of a Layer IV in the Rat Motor Cortex
Executive functions wikipedia , lookup
Affective neuroscience wikipedia , lookup
Binding problem wikipedia , lookup
Single-unit recording wikipedia , lookup
Subventricular zone wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Brain Rules wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Time perception wikipedia , lookup
Neuroesthetics wikipedia , lookup
Multielectrode array wikipedia , lookup
Neural oscillation wikipedia , lookup
Neural coding wikipedia , lookup
Convolutional neural network wikipedia , lookup
Central pattern generator wikipedia , lookup
Mirror neuron wikipedia , lookup
Apical dendrite wikipedia , lookup
Embodied language processing wikipedia , lookup
Neuroanatomy wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Development of the nervous system wikipedia , lookup
Metastability in the brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Human brain wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroplasticity wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Synaptic gating wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
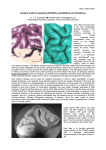
The Existence of a Layer IV in the Rat Motor Cortex T. S. Skoglund, R. Pascher and C.-H. Berthold We have reconstructed the laminar pattern of rat primary motor cortex (Fr1) using a computerized analysis system based on the so-called ‘optical dissector’. Data were visualized on a graphics terminal. In contrast to current views, which state that there is no prominent layer IV in the motor cortex of the rat, our method of analysis revealed a genuine layer IV consisting of densely packed small neurons. We used perfusion fixed cerebral cortex (5% glutaraldehyde) from seven adult male Sprague–Dawley rats (400–500 g). The brains were removed, kept in phosphate buffer and divided by a median cut. Each brain was glued with Histoacryl® (Braun, Melsungen, Germany) to a support and the cortical area Fr1 (Zilles, 1985) (primary motor cortex; Fig. 1) was sectioned from its lateral side in sagital sections (six brains) or from its anterior pole in coronal sections (one brain) in a consecutive series of 50-µm-thick sections using an Oxford Vibratome®. Three to four sections from each series were used for the counting. In brief, the staining and the counting methods were as follows (see Skoglund et al., 1997): the sections were stained in Richardson’s solution (Richardson et al., 1960) and mounted in 90% glycerol in 10% PBS on glass slides. This procedure avoids shrinkage and highlights the neurons (Skoglund et al., 1997). A computersupported system was used to digitize the sections and to count and measure the neurons. The system consisted of a CCD camera (DAGE-MTI CCD72E) mounted on a light microscope (Leitz Aristoplan) equipped with a motorized object stage (Positioniersystem MCL, Märzhäuser Wetzlar, Germany). The three axes, x, y and z, of the motorized object stage were controlled by a computer (Toshiba T1000). The CCD camera was connected to a Silicon Graphics Indy workstation equipped with a 24-bit Indy Video card. The reconstructions of the distribution of the neurons were visualized on a Megatek 9300 graphic terminal (Skoglund et al., 1993). Images were digitized at a number of sequential positions from the pial surface down to the white matter along a vector parallel to radial elements of the cortex (i.e. to neuronal microcolumns, dendritic bundles and blood vessels). At each position along the vector, seven optical sections were digitized, each with a width of 200 µm and a distance of 5 µm between their focal planes. A compound image, referred to as a cortical image, reaching from the pial surface down to the white matter was constructed for each of the seven optical sections. Together the seven cortical images formed what we will refer to as a ‘3-D mosaic’, measuring 200 µm × 30 µm × cortical thickness. Our program ‘Mark’ was then used to count the number of neurons in a counting frame (i.e. counting box) applied within the ‘3-D mosaic’. The counting frame measured 170 µm × 25 µm × cortical thickness. ‘Mark’ is a program developed by us and based on the optical dissector (Gundersen, 1986), which is an unbiased method not dependent on assumptions of cell size and cell shape. The technique means, in practice, that the neurons are counted when they first come into focus as one focuses through a known distance of a thick section. Since large neurons have a greater probability of being counted, cells that are in focus in the first focal plane are excluded. Neurons crossing the left side or the bottom of the counting frame are also excluded. The position of each neuron was stored in a database. The organization of neuronal cell bodies in six layers parallel to the pial surface is one of the most conspicuous features of the mature mammalian neocortex. The lamination is due to variations in the packing density and the shapes and sizes of the neurons as traced from the pial surface down to the white matter. The first systematic descriptions of the cortical layering were provided by Meynert (Meynert, 1867, 1869, 1872). The concept of a six-layered neocortex developed as a result of Brodmann’s studies (Brodmann, 1903, 1909, 1912; Jones, 1984; Kemper and Galaburda, 1984). An exception to the six-layer pattern was found in the adult primate motor cortex, which Lewis (Lewis, 1878; Kemper and Galaburda, 1984) described as being five-layered, with the conventional fourth layer missing. A similarly five-layered motor cortex has also been described as relevant for the rat and for the mouse, and is, according to the current literature, accepted as the norm for these species as well (Donoghue and Wise, 1982; Zilles, 1985; Beaulieu, 1993; Zilles and Wree, 1995). However, in an often overlooked paper, Krieg (Krieg, 1946) describes the motor cortex of the rat as being six-layered including a conventional lamina IV. Because the rat is one of the most commonly used species in neuroscience, the confusion with regard to whether its motor cortex contains or lacks a conventional lamina IV should receive some further illumination. In order to study the cytoarchitecture of the cerebral cortex and to examine, for example, the cortical lamination, we have developed a computer-based system for the reconstruction of neuronal organization (Skoglund et al., 1997). The system creates a database which includes the coordinates and sizes of neurons, and these can be visualized either by displaying the neurons as spheres or by plotting the neuronal distribution as a function of the distance from the pial surface. In this way, laminar borders are detected as changes in the packing density of the neurons — a method used earlier to detect both neuronal (Ryzen, 1956) and nerve fiber stratification (Sanides, 1972; Wagner et al., 1986). When we used our system to study rat neocortex we found the distribution of neurons in the motor cortex inconsistent with the current view of five layers but in line with Krieg’s findings (Krieg, 1946) of a six-layered pattern. This paper presents our results indicating the existence of a layer IV in the motor cortex of the adult rat. Institute of Anatomy and Cell Biology and MEDNET-laboratory, University of Göteborg, Medicinaregatan 3, S-413 90 Göteborg, Sweden Cerebral Cortex Mar 1997;7:178–180; 1047–3211/97/$4.00 Figure 1. Micrograph from a sagital section stained in Richardson’s solution. Area Fr1 (primary motor cortex) used for counting. When the sections were examined under light microscope without computer assistance, the motor cortex appeared five-layered, i.e. lacking layer IV. A computer-assisted reconstruction of the distribution of neurons was then made by displaying the position of each neuron as a sphere in a prism of the cortex measuring 170 µm × 75 µm × cortical thickness (Fig. 2b). With this approach, a distinct increase in the density of neurons became apparent at the bottom of layer III, ∼600 µm from the pial surface (Fig. 2b). The increase became even more evident when the distribution of the neurons was plotted versus the distance from the pial surface. As shown in Figure 2c, layer I contains few neurons, whereas layer II is very cell rich. The neuronal density falls when entering layer III but increases again before entering the relatively low density layer V. The location of the borders was obtained by derivating the density data. In the derived density data the maximum changes could be found, thus indicating a lamina border. The size (diameter) of the neuronal perikarya was measured during the counting procedure and the volume was calculated by assuming that the cell bodies were spherical. The mean volumes of the neuronal somas along an axis from the pial surface to the white matter are plotted in Figure 2d. A decrease in the variable is seen at the bottom of layer III. The same pattern was detected in all investigated animals. Layer IV is usually described as containing densely packed neurons of small size (Braak, 1980). This description matches the neurons in the layer we found at ∼600–800 µm from the pial surface. Here, the density is higher than in layers III and V (Fig. 2c), and the neurons are relatively small (Fig. 2d). We interpret this layer in the adult rat motor cortex as being a genuine layer IV, and our results thus support the findings of Krieg (1946). Differences in the fundamental organization between the mature neocortex of the rat and that of more advanced species have been described earlier (Krieg, 1963; Wagner and Wolff, 1982; Marín-Padilla, 1992) and have been interpreted as being part of the evolution towards increasingly more ‘complicated’ brains (Innocenti and Kaas, 1995; Northcutt and Kaas, 1995). However, studies of fetal monkey (Huntley and Jones, 1991) and human (Ramón y Cajal, 1900; Brodmann, 1903; Marín-Padilla, 1970) brains have shown a six-layered motor cortex. In the postnatal stage there is an increase in the size of large pyramidal neurons of the relatively low-density layers III and V and an increase in the degree to which they become intermingled at the expense of layer IV. This leads to a progressive reduction in Figure 2. (a) Micrograph and (b, c and d) reconstructions of area Fr1. (a) Micrograph from 50-µm-thick section of Fr1 stained in Richardson’s solution. (b) Reconstruction of the positions of the neurons within a cortical prism from Fr1. Each neuron is shown as a sphere (all spheres are of the same size). A layer IV can be detected ∼600–800 µm from the pial surface. (c) Linear plot showing the relative distribution of neurons versus the distance from the pial surface. The high density region 600–800 µm from the pial surface is what we refer to as layer IV. (d) Linear plot showing the medium size (volume) of the neurons versus the distance from the pial surface. The layer IV is here recognizable as a decrease in the mean size of the neurons. neuronal packing density and finally to an apparent obliteration of layer IV. We do not know whether our computer-assisted method would resolve a fourth layer in adult monkey and human motor cortex when sorting out the neurons according to size. In conclusion, we have confirmed the presence of a genuine though somewhat elusive lamina IV in the adult rat motor cortex. According to our results, the mature rat motor cortex is subdivided into the conventional six layers of the following approximate thicknesses as estimated for lamina I through lamina VI: 210, 230, 180, 190, 520 and 550 µm respectively. We would like to emphasize the importance of recognizing the presence of a lamina IV in forthcoming descriptions of the mature rat motor cortex. Notes This work was supported by the Medical Faculty in Göteborg, by the Swedish MRC proj. no. 3157, by the Göteborg Medical Society, by Lundberg’s Research Foundation, by Anna Ahrenberg’s Foundation and by Rådman Ernst Colliander’s Foundation. We thank Marieanne Eriksson Cerebral Cortex Mar 1997, V 7 N 2 179 and Rita Grandér for excellent technical assistance. The facilities of the MEDNET laboratory were used. Address correspondence to Dr Thomas S. Skoglund, Institute of Anatomy and Cell Biology, University of Göteborg, Medicinaregatan 3, S-413 90 Göteborg, Sweden. References Beaulieu C (1993) Numerical data on neocortical neurons in adult rat, with special reference to the GA BA population. Brain Res 609:284–292. Braak H (1980) Architectonics of the human telencephalic cortex. Berlin: Springer-Verlag. Brodmann K (1903) Beiträge zur histologischen Lokalisation der Grosshirnrinde: Die Regio Rolandica. J Psychol Neurol 2:79–132. Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth. Brodmann K (1912) Neue Ergebnisse über die vergleichende Lokalisation der Grosshirnrinde mit besonderer Berücksichtigung des Stirnhirns. Anat Anz Suppl 41:157–216. Donoghue JP, Wise SP (1982) The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol 212: 76–88. Gundersen HJG (1986) Stereology of arbitrary particles. J Microsc 143:3–45. Huntley GW, Jones EG (1991) The emergence of architectonic field structure and areal borders in developing monkey sensorimotor cortex. Neuroscience 44:287–310. Innocenti GM, Kaas JH (1995) The cortex. Trends Neurosci 18:371–372. Jones EG (1984) History of cortical cytology. In: Cerebral cortex (Peters A, Jones EG, eds), Vol 1, pp. 1–32. New York: Plenum Press. Kemper TLB, Galaburda AM (1984) Principles of cytoarchitectonics. In: Cerebral cortex (Peters A, Jones EG, eds), Vol 1, pp. 35–57. New York: Plenum Press. Krieg WJS (1946) Connections of the cerebral cortex. J Comp Neurol 84:277–323. Krieg WJS (1963) Connections of the cerebral cortex. Evanston, IL: Brain Books. Lewis WB (1878) On the comparative structure of the cortex cerebri. Brain 1:79–86. Marín-Padilla M (1970) Prenatal and early postnatal ontogenesis of the 180 The Existence of Layer IV in Rat Motor Cortex • Skoglund et al. human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res. 23:167–183. Marín-Padilla M (1992) Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol 321:223–240. Meynert T (1867) Der Bau der Grosshirnrinde und seine örtlichen Verschiedenheiten, nebst einen patologisch-anatomischen Corollarium. Vierteljahressch Psychiatr 1:77. Meynert T (1869) Der Bau der Grosshirnrinde und seine örtlichen Verschiedenheiten, nebst einen patologisch-anatomischen Corollarium. Vierteljahressch Psychiatr 2:88. Meynert R (1872) The brain of mammals. In Manual of histology (Stricker SA, ed), Vol 2, pp 650–766. New York: Wood. Northcutt RG, Kaas JH (1995) The emergence and evolution of mammalian cortex. Trends Neurosci 18:373–379. Ramón y Cajal S (1900) Estudios sobre la corteza cerebral humana II: corteza motriz (conclusión). Rev Trim Micrográf 5:1–11. Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35: 313–323. Ryzen M (1956) A microphotometric method of cell enumeration within the cerebral cortex of man. J Comp Neurol 104:233–245. Sanides F (1972) Representation in the cerebral cortex and its areal lamnination patterns. In: The structure and function of the nervous tissue (Bourne GH, ed), Vol V, pp 329–453. New York: Academic Press. Skoglund TS, Pascher R, Berthold C-H, Rydmark M, Jansson T, Gustavsson T (1993) 3D reconstruction of biological objects from sequential image planes — applied on cerebral cortex from cat. Comput Med Imag Graph 17:165–174. Skoglund TS, Pascher R, Berthold C-H (1997) Aspects of the quantitative analysis of neurons in the cerebral cortex. J Neurosci Methods (in press). Wagner GP, Wolff JR (1982) Topography, orientation, and density of intracortical connections in the adult rat. Verh Anat Ges 76:451–452. Wagner GP, Eins S, Wolff JR (1986) Tangential organization of the infragranular fiber plexus in rat cerebral cortex. Acta Anat 126:1–12. Zilles K (1985) The cortex of the rat — a stereotaxic atlas. Berlin: Springer-Verlag. Zilles K, Wree A (1995) Cortex: areal and laminar structure. In: The rat nervous system (Paxinos G, ed), pp 649–685. San Diego, CA: Academic Press.