* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Morphological Basis of Learning and Memory: Vertebrates
Apical dendrite wikipedia , lookup
Time perception wikipedia , lookup
Memory consolidation wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neurophilosophy wikipedia , lookup
Limbic system wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Haemodynamic response wikipedia , lookup
Long-term potentiation wikipedia , lookup
Brain morphometry wikipedia , lookup
Human brain wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neuropsychology wikipedia , lookup
Neurotransmitter wikipedia , lookup
State-dependent memory wikipedia , lookup
Long-term depression wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Adult neurogenesis wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Brain Rules wikipedia , lookup
Neuroesthetics wikipedia , lookup
Development of the nervous system wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Dendritic spine wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Neuroeconomics wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Aging brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroplasticity wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Synaptogenesis wikipedia , lookup
Environmental enrichment wikipedia , lookup
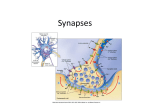
Morphological Basis of Learning and Memory: Vertebrates The central issue in morphology of learning and memory is how such constructs are stored in the nervous system. The basic shape of neurons and synapses is illustrated in Figure 1. In the late nineteenth century, Santiago Ramón y Cajal suggested that learning might involve changes in the synaptic connections through which neurons communicate. Such synaptic change could take at least four possible forms. First, the pattern of functional connections could be altered by forming new synapses or removing existing synapses. Second, the pattern of functional connections could be altered by selectively strengthening or weakening some synapses. There is very strong evidence for both of these possibilities during learning, and in models of learning such as Long-Term Potentiation. Third, neuronal circuits could be modified by forming new neurons (neurogenesis), for which there is growing support suggesting that neurogenesis is possible in the mature brain. Fourth, changes could occur in both the nonsynaptic regions of neurons and in nonneural elements of the brain such as glial cells and the vascular system subserving the brain, for which there is evidence as well. Changes in Synapse Number Important roots of memory research lie in studies of the effects of experience upon brain development. For example, visual experience is necessary to develop normal visual ability in mammals. Searching for a basis for this in brain anatomy, Cragg (1975), and others, noted that animals deprived of visual experience had fewer synaptic connections per nerve cell in visual cortex. These studies profoundly influenced thinking about the processes by which the brain stores information, because they showed that (1) brain structure is malleable; (2) synaptic organization can be orchestrated into different configurations by behavioral experience; (3) both forming new connections and pruning existing connections are involved in altering brain organization; (4) differential experience can modify the structure of synapses, suggesting that synaptic efficacy (or strength) also can change. These effects of experience on synaptic connections during development led to proposals that such changes might underlie adult learning as well. A separate developmental approach that was very fruitful in understanding brain substrates of learning and memory involved enriching young animals’ lives with additional stimulation. Donald Hebb proposed ways in which synaptic change could be incorporated meaningfully into functional circuitry. With his students, he showed that enriching the rearing environment of rats with cagemates and toys improved the animals’ ability to solve complex problems. Hebb concluded that behavior, and by implication brain organization, was permanently altered by this early experience. Subsequently, Rosenzweig, Bennett and Diamond (1972) found that regions of the cerebral cortex were thicker and heavier in rats reared in enriched environments, compared with rats reared in solitary or group cages. Volkmar and Greenough (1972) followed up these findings, reporting that visual cortical neurons of rats reared in enriched environments had larger dendritic fields than did those of cage housed controls. Dendrites of neurons receive the bulk of the synaptic input (see Figure 1), so the implication was that new synapses formed. Similar findings were subsequently reported in other areas of the cerebral cortex and in brain regions such as hippocampus, superior colliculus, and cerebellum. Of particular importance to learning and memory was that the enriched environment changed brain anatomy in adult rats. Turner and Greenough (1985) found that rats reared in enriched environments had more synapses per neuron in the visual cortex, compared with rats reared alone or in pairs in standard laboratory cages. Moreover, similar changes occur in the striatum as well (Comery, Shah and Greenough, 1995), suggesting that the experience-dependent changes in neuronal morphology influence multiple levels/systems in the brain. The general conclusion from the enriched environment studies is that when animals are placed in an environment in which they store information that affects later behavior, they form new synapses. (The term “enriched” is used in comparison with the typical laboratory environment and does not imply superiority to the natural environment.) Follow-up studies have explored the effects of specific learning tasks upon these same measures. There is compelling evidence that many forms of learning change both the amount of dendrite per neuron and the number of synapses per neuron. For example, dendritic branching is increased in visual cortical neurons following 25 days’ exposure to a series of maze problems (Greenough, Juraska and Volkmar, 1979). Subsequent work used “split-brain” rats, severing the nerve fibers that allow the right and left hemispheres to communicate, and opaque contact lenses that restricted visual input from training to one eye. Neurons on the “trained” side of the brain selectively exhibited dendritic field size increases; thus these changes were not of the general sort that might be due to stress or arousal associated with the task, which should affect both sides of the brain equally. These studies, and others, indicated that the altered dendritic fields were associated with neural input and output related to the training. Synaptogenesis is also implicated in associative learning. Tsukahara (1981) investigated associative limb flexion conditioning, using stimulation to the cerebral peduncle as the conditioned stimulus and electric shock to the forelimb as the unconditioned stimulus. In this paradigm, red nucleus lesions abolish the conditioned response, implicating the involvement of this structure. Electrophysiological studies following conditioning indicated enhanced input to the red nucleus from the cerebral cortex. Subsequently, Tsukahara’s coworkers Murakami et al. (1987) reported morphological evidence for formation of new corticorubral synapses in conditioned animals. Similar anatomical effects of training have been observed in other behavioral tasks. Stewart (1991) examined dayold chicks that learned to avoid pecking a bad-tasting food particle and found an elevated number of synapses in a forebrain region previously shown to be involved in the learning. In another involved brain region, there were also increases in the number of spines (see Figure l), the dendritic component of one type of synapse. Likewise, the density of spines was reported to be elevated in hippocampal area CA1 in adult rats following spatial learning (Moser, Trommald, and Andersen, 1994). Comparable changes have been observed in numerous other paradigms (e.g., Bird Song Learning and Imprinting in birds). Finally, while this discussion is confined to vertebrates; there is excellent evidence for comparable synaptic number changes in invertebrate plasticity paradigms (see “Invertebrates,” above). Neurogenesis presents yet another mechanism whereby brain organization could be modified in response to experience. For example, increased numbers of new neurons have been reported in the dentate gyrus of the hippocampus of animals exposed to a complex environment (Kempermann, Kuhn and Gage, 1998) or permitted access to an exercise wheel (van Praag et al., 1999). More specific to learning and memory, Gould et al. (1999a) reported that the number of “new” neurons in rat dentate gyrus dramatically increased following associative conditioning. While most reports of neurogenesis have been limited to rodents and to relatively unique areas in the brain such as hippocampus or olfactory bulb, neurogenesis has been reported in primate cortex (Gould et al., 1999b) and disputed (Kornack and Rakic, 2001). While the issues of the extent, location, and functional relevance of neurogenesis has yet to be resolved, the mere possibility of such a mechanism in the mature central nervous system has spurred a great deal of excitement and hope for many areas of neural research. An issue that affects all of these studies is whether anatomical changes that are seen following training result merely from increased neural activity involved in performing the learned task. Muscles grow larger in response to exercise; perhaps neurons do too, such that these structural changes have nothing to do with learning or memory per se. (This issue is, of course, not unique to morphological studies; proposed molecular and other aspects of the cellular mechanisms of memory may similarly be artifacts of activity; see Protein Synthesis in Long-Term Memory in Vertebrates.) There have been direct tests of the effects of neural activity versus learning on synapse change. For example, Black et al. (1990) compared a cerebellar cortical region in rats that had learned a complex series of motor tasks to that of rats that performed one of two forms of physical exertion involving little learning: running on a treadmill or in an activity wheel. Exercise alone had no effect on synapse number whereas rats that had learned increased the number of synapses per neuron in the cerebellar cortex. Similar effects have been observed in areas such as motor cortex (Kleim et al., 1996). In contrast, blood vessel density was elevated in affected regions in rats that had exercised, whereas the motor learning rats had the same blood vessel density as control animals. These results indicate that activity and learning have very different effects on brain tissue. Additional support for the role of synapse formation in plastic neural change has come from studies of long-term potentiation. LTP involves an increase in the response of postsynaptic neurons following high-frequency bursts of presynaptic firing. LTP induction has been shown to increase spine density and modify dendritic morphology in motor cortex (Ivanco, Racine and Kolb, 2000) and in hippocampal subfield CA1 (Lee et al., 1981). Chang and Greenough (1984) included a type of high-frequency stimulation that did not induce LTP, and subsequently did not alter synapse formation, suggesting that LTP-induced synaptogenesis was not caused by high-frequency stimulation alone. In contrast to CA1, induction of LTP in the hippocampal dentate gyrus does not cause synapse formation but changes synapse structure (Geinisman, de Toledo-Morrell, and Morrell, 1991). Recent advances in microscopy have permitted near realtime evaluation of changes in morphology of living neurons. Using these tools, Engert and Bonhoeffer (1999) reported that postsynaptic spines are formed de novo in response to stimulation. Taken together, these findings reinforce the view that numerous different cellular changes may be involved in learning, and other forms of neural plasticity as well. Changes in Synapse Structure: Indications of Synapse Efficacy Change Several structural features of synapses have been found to be altered by behavioral experience. One of the most obvious features is the size of synapses. Larger synapses may release more neurotransmitter or have more receptors, such that a size change could indicate a strength change. Early findings indicated smaller synapses in visual cortex of animals visually deprived during development, and Tieman (1985) reported smaller geniculocortical synapses in monocularly deprived cats. Conversely, synapse size increased following imprinting in day-old chicks, and similar size changes were found after avoidance learning. Larger synapses were also observed in layer IV of visual cortex of rats reared in enriched environments, compared with individually caged controls. Likewise, changes in the size of synaptic spine heads and necks (see Figure 1) have been described by Van Harreveld and Fifkova (1975) following LTP induction in dentate gyrus. It has been suggested that larger spine components, and the associated spine neck restriction, may permit activation of focalized intracellular cascades (Koch and Zador, 1993) and reduce electrical resistance, thereby facilitating the passage of synaptic current into the dendrite (Harris and Kater, 1994). Synaptic vesicles are believed to contain neurotransmitters, and changes in their numbers could indicate changes in synapse strength. Synaptic vesicle numbers have been reported to decrease with visual deprivation (e.g., Tieman, 1985) and there have are reports of both decreased vesicle density and altered vesicle location within the presynaptic terminal following LTP induction (e.g., Fifkova and Van Harreveld, 1977; Applegate, Kerr, and Landfield, 1987). In contrast, vesicle numbers have been shown to increase in rats reared in enriched environments (Sirevaag and Greenough, 1991; Nakamura et al., 1999). At least three other synapse features appear to be sensitive to experience. First, small discontinuities in the postsynaptic density, termed perforations, have been found to increase in number following complex environment exposure and to decrease in affected synapses subsequent to sensory deprivation (Greenough, West, and DeVoogd, 1978). Moreover, Vrensen and Cardozo (1981) found that the number of perforated synapses increased in the visual cortex following visual discrimination learning. The function of these perforations is unknown. Second, the incidence of multiple synaptic boutons (presynaptic elements that synapse with multiple postsynaptic components) is elevated in numerous brain areas following exposure to a complex environment and in animals that have been trained in an associative learning paradigm (Jones et al., 1997; Geinisman et al., 2001). Third, the cellular organelles that synthesize protein, polyribosomal aggregates, are frequently found in the heads and necks of spines during periods of synapse formation (Steward and Falk, 1985). They are also found more frequently in spines of animals in complex environments, possibly reflecting greater rates of synapse formation. Protein synthesis at the synapse has been proposed as a memory mechanism (Steward and Schuman, 2001). Changes in Nonneural Elements The enriched environment work indicated from its earliest days that morphological changes were not restricted to neurons. Glial cells, supportive elements that maintain ionic, metabolic, and neurotransmitter homeostasis, respond similarly to environmental complexity. Sirevaag and Greenough (1991) reported that astrocytes grow larger and extend additional processes into the tissue during the first phase of their response to the animal’s housing in an enriched environment. In a second phase, astrocytes divide, increasing their numbers, and shrink, on average, toward their preexposure size. These stages are qualitatively comparable with those of gliosis, the glial reaction to injury, yet protracted. Moreover, Anderson et al. (1994) have shown an increase in the volume of glia per Purkinje cell in the cerebellum of animals that learned a complex motor skill, but not those who simply exercised. Likewise, blood vessel density increased in rats placed in an enriched environment at the age of weaning. In animals that are older at the time they are first exposed to enrichment, this blood vessel response diminishes with increasing age. Conclusions Morphological research has provided strong evidence for both forms of synaptic change that have been proposed to underlie learning and memory. Formation, and occasionally loss, of synapses occurs both during periods of development when the brain is storing information and during exposure to specific learning tasks. Various control procedures have largely ruled out the possibility that these synaptic changes are artifactual results arising from factors other than learning. Changes in the structure of synapses, such as in the size or shape of synaptic components, also occur during learning and in other situations in which functional brain organization is altered, such as LTP. Many of these structural changes have been associated with synapse strength differences in other research. Recent, still developing research implicates addition of new neurons via neurogenesis and changes in non-neuronal elements of the brain. Thus the weight of the evidence indicates that both synapse formation/removal and synapse strength changes are involved in learning and memory, but additional mechanisms are also likely. REFERENCES Andersen, B. J., Li, X., Alcantara, A. A., Isaacs, K. R., Black, J. E., and Greenough, W. T. (1994). Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia 11, 73-80. Applegate, M. D., Kerr, D. S., and Landfield, P. W. (1987). Redistribution of synaptic vesicles during long-term potentiation in the hippocampus. Brain Research 401, 401–406. Black, J. E., Isaacs, K. R., Anderson, B. J., Alcantara, A. A., and Greenough, W. T. (1990). Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences 87, 5568–5572. Chang, F.-L. F., and Greenough, W. T. (1984). Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Research 309, 35–46. Comery, T. A., Shah, R., and Greenough, W. T. (1995). Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for the association of morphological plasticity with early response gene expression. Neurobiology of Learning and Memory 63, 217-219. Cragg, B. G. (1975). The development of synapses in kitten visual cortex during visual deprivation. Experimental Neurology 46, 445–451. Engert, F., and Bonhoeffer, T. (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66-70. Fifkova, E., and Van Harreveld, A. (1977). Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. Journal of Neurocytology 6, 211–230. Geinisman, Y., Berry, R. W., Disterhoft, J. F., Power, J. M., and Van der Zee, E. A. (2001). Associative learning elicits the formation of multiple-synapse boutons. Journal of Neuroscience 21, 5568-73. Geinisman, Y., de Toledo-Morrell, L., and Morrell, F. (1991). Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Research 566, 77–88. Gould, E., Beylin, A., Tanapat, P., Reeves, A., and Shors, T. J. (1999a). Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience 2, 260-265. Gould, E., Reeves, A. J., Graziano, M. S., and Gross, C. G. (1999b). Neurogenesis in the neocortex of adult primates. Science 286, 548-552. Greenough, W. T., Juraska, J. M., and Volkmar, F. R. (1979). Maze training effects on dendritic branching in occipital cortex of adult rats. Behavioral and Neural Biology 26, 287–297. Greenough, W. T., West, R. W., and DeVoogd, T. J. (1978). Sub-synaptic plate perforations: Changes with age and experience in the rat. Science 202, 1096–1098. Harris, K. M., and Kater, S. B. (1994). Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annual Review of Neuroscience 17, 341-371. Ivanco, T. L., Racine, R. J., and Kolb, B. (2000). Morphology of layer III pyramidal neurons is altered following induction of LTP in sensorimotor cortex of the freely moving rat. Synapse 37, 16-22. Jones, T. A., Klintsova, A. Y., Kilman, V. L., Sirevaag, A. M., and Greenough, W. T. (1997). Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiology of Learning and Memory 68, 13-20. Kempermann, G., Kuhn, H. G., and Gage, F. H. (1998). Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience 18, 3206-3212. Kleim, J. A., Lussnig, E., Schwarz, E. R., Comery, T. A., and Greenough, W. T. (1996). Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience 16, 4529-4535. Koch, C., and Zador, A. (1993). The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. Journal of Neuroscience 13, 413-422. Kornack, D. R., and Rakic, P. (2001). Cell proliferation without neurogenesis in adult primate neocortex. Science 294, 2127-2130. Lee, K. S., Oliver, M., Schottler, F., and Lynch, G. (1981). Electron microscopic studies of brain slices: The effects of high-frequency stimulation on dendritic ultrastructure. In G. A. Kerkut, and H. V. Wheal, eds., Electrophysiology of isolated mammalian CNS preparations, pp. 189–211. New York: Academic Press. Moser, M.-B., Trommald, M., and Andersen, P. (1994). An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proceedings of the National Academy of Sciences 91, 12673-12675. Murakami, F., Higashi, S., Katsumaru, H., and Oda, Y. (1987). Formation of new corticorubral synapses as a mechanism for classical conditioning in the cat. Brain Research 437, 379–382. In this article Tsukahara’s coworkers offer definitive evidence for the association of new synapse formation with learning. Nakamura H., Kobayashi, S., Ohashi, Y., and Ando, S. (1999). Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. Journal of Neuroscience Research 56, 307-315. Rosenzweig, M. R., Bennett, E. L., and Diamond, M. C. (1972). Chemical and anatomical plasticity of brain: Replications and extensions. In J. Gaito, ed., Macromolecules and behavior, 2nd ed., pp. 205–278. New York: Appleton-Century-Crofts. This is one of the best summaries of the work of this group, which began in the early 1960s. Sirevaag, A. M., and Greenough, W. T. (1991). Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Research 540, 273-278. Steward, O., and Falk, P. M. (1985). Polyribosomes under developing spine synapses: Growth specializations of dendrites at sites of synaptogenesis. Journal of Neuroscience Research 13, 75–88. Steward O., and Schuman E. M. (2001). Protein synthesis at synaptic sites on dendrites. Annual Review of Neuroscience 24, 299-325. Stewart, M. G. (1991). Changes in dendritic and synaptic structure in chick forebrain consequent on passive avoidance learning. In R. J. Andrew, ed., Neural and behavioral plasticity, pp. 305–328. London: Oxford University Press. Thorough review of this occasionally overlooked body of work. Tieman, S. B. (1985). The anatomy of geniculocortical connections in monocularly deprived cats. Cellular and Molecular Neurobiology 5, 35–45. A very thorough and careful piece of work. Tsukahara, N. (1981). Sprouting and the neuronal basis of learning. Trends in Neurosciences 4, 234–237. Turner, A. M., and Greenough, W. T. (1985). Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research 329, 195–203. Van Harreveld, A.., and Fifkova, E. (1975). Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Experimental Neurology 49, 736–749. A classic that inspired both rapid freezing studies and much theoretical biophysical modeling regarding LTP mechanisms and the functions of dendritic spines. van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences 96, 13427-13431. Volkmar, F. R., and Greenough, W. T. (1972). Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 176, 1445–1447. Vrensen, G., and Cardozo, J. N. (1981). Changes in size and shape of synaptic connections after visual training: An ultrastructural approach of synaptic plasticity. Brain Research 218, 79–97. William T. Greenough James D. Churchill Word Count: 2415