* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neural Oscillations
Signal transduction wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Recurrent neural network wikipedia , lookup
Neurotransmitter wikipedia , lookup
End-plate potential wikipedia , lookup
Haemodynamic response wikipedia , lookup
Subventricular zone wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Theta model wikipedia , lookup
Patch clamp wikipedia , lookup
Resting potential wikipedia , lookup
Neural engineering wikipedia , lookup
Synaptogenesis wikipedia , lookup
Central pattern generator wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Circumventricular organs wikipedia , lookup
Development of the nervous system wikipedia , lookup
Chemical synapse wikipedia , lookup
Binding problem wikipedia , lookup
Multielectrode array wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroanatomy wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural coding wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Electrophysiology wikipedia , lookup
Spike-and-wave wikipedia , lookup
Biological neuron model wikipedia , lookup
Single-unit recording wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Nervous system network models wikipedia , lookup
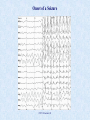
CN510: Principles and Methods of Cognitive and Neural Modeling Neural Oscillations Lecture 24 Instructor: Anatoli Gorchetchnikov <[email protected]> Teaching Fellow: Rob Law <[email protected]> It Is Much Easier to Have Oscillations CN 510 Lecture 24 Importance of Oscillating Models It is much easier to have oscillations in the model than not to have them: – Delayed feedback inhibition is one of the main causes of oscillations, and there is no instantaneous feedback in vivo Oscillations allow to synchronize neurons across multiple brain regions: – Modulatory systems that set oscillatory patterns project to many brain areas simultaneously Oscillation-based models allow to consider individual spikes rather than firing rates: – Randomness is reduced or eliminated by synchronization – Phase coding can be used in addition to rate coding Last and most important: oscillations are present all over the brain, we will not be able to model brain accurately without them CN 510 Lecture 24 What Is Oscillation? Neural network or neuron is said to be oscillatory if there is a variable (membrane potential, Ca concentration, some current) that is periodic with a well defined period The system has to be stable to perturbations, meaning that it will return to the original amplitude and frequency (period) with a possible phase shift The absence of this shift usually means that the perturbed network is not the source of oscillation, but rather is driven by external source CN 510 Lecture 24 What Is Synchrony? Neurons are essentially coincidence detectors We talked about spatial and temporal summation of neuronal inputs: signals have more effect on the membrane potential if they arrive close in time How close depends on the receptor we use: for AMPA simultaneous means within 20ms, for NMDA within 300ms Neurons below fire phase-locked to each other with 5ms delay, but if our time resolution is 10ms, then they fire synchronously CN 510 Lecture 12 Multiple Scales of Oscillations and Synchrony Membrane potential oscillations in individual cells (intracellular recordings) Local field potentials (extracellular recordings, EEG, etc): combined activity of many cells, usually mixing individual oscillations with network-driven oscillations CN 510 Lecture 24 Extracellular Recordings What extracellular recordings actually record? The current through the cell membrane consists of the capacitive and resistive component dV Cm Ii dt i dV I m Cm Ir dt From the extracellular point of view: – If this current flow in the cell then it creates a current sink – If it flows outside of the cell it creates the current source in this particular part of the membrane If the electrode is placed at a distance twice as large as the distance between parts of the cell with major currents, then the field on this electrode will be similar to the field generated by the current dipole (Humphrey, 1979) CN 510 Lecture 24 Extracellular Recordings Assuming the extracellular fluid has constant conductance, the potential on the electrode is 1 I m I m Ve 4 r r where – I-s and r-s are currents and distances, respectively, – + and – mark the attributes of source and sink, respectively, – σ is the extracellular conductivity Note, that currents have opposite signs: I+ is positive, while I- is negative Therefore, if r+ > r- then Ve < 0, and if r+ < r- then Ve > 0 CN 510 Lecture 24 Extracellular Recordings In the case of more complex cell with many possible sources and sinks Ii Ve 4 i ri 1 Finally, for the continuous neuron the sum can be replaced with the integral (Humphrey, 1979) So extracellular recordings reflect the sum of all transmembrane currents for all neurons in the vicinity of the electrode tip weighted by the distances CN 510 Lecture 24 Conditions for Simulation of LFP Need to have sources and sinks: model neurons have to have at least two compartments, otherwise by Kirchhoff's law the total current is zero for each neuron Need to have some spatial distribution of these neurons, otherwise distances from the electrode are meaningless How to measure a total current that goes across the membrane if you have an arbitrary number of these currents? dV Cm I s IV I a I g dt You can measure the currents that don’t go across: axial and gap junctions only! Note that both of these are computed from voltages: g V i 1 (t ) V i (t ) g V i 1 (t ) V i (t ) so we can compute them off-line after the simulation is done! CN 510 Lecture 24 SMART Diagram for LFP Simulation CN 510 Lecture 23 Examples of Simulated LFP CN 510 Lecture 24 Oscillations in Extracellular Recordings CN 510 Lecture 24 EEG Frequency Bands delta (1–4 Hz) theta (4–8 Hz) alpha (8–12 Hz) beta (13–30 Hz) gamma (30–70 Hz) Note that these are human frequencies, animals differ slightly CN 510 Lecture 12 Membrane Potential Oscillations Many neurons produce rhythmic subthreshold membrane potential oscillations Intrinsic electrical properties of neurons, primarily voltage gated channels lead to generation of these oscillations The functional relevance of these oscillations is based on increased and decreased excitability of the neuron and can lead to noticeable firing changes: – Giocomo et al (2008) found a gradient of properties of Ih current in the entorhinal cells, which fit nicely into the interference model of grid cells Resonate-and-Fire neuronal model by Izhikevich is a simple approach to modeling subthreshold oscillations CN 510 Lecture 24 Membrane Potential Oscillations Subthreshold oscillation frequency can vary, from few Hz to over 40Hz They are often similar in frequency to LFP oscillations Why is that? CN 510 Lecture 24 Measuring Synchrony Word of caution: certain number of synchronous events will always happen by random coincidence Need to compare to some non-zero baseline – Calculate expected number of synchronous events based on firing rates and interspike interval distributions, then calculate the number of actual events and take the ratio as a measure of synchrony – Do spike shuffling: shuffle the spike train preserving firing rates and interspike interval distributions and compare number of events for unshuffled vs shuffled case Advantage of this method comparing to correlation methods is that synchronous events do not have to be reoccurring or periodic CN 510 Lecture 23 Measuring Oscillations Spike train autocorrelation: – Align signal with itself, calculate correlation coefficient (gotta get 1 here) – Delay signal relative to itself by Δt, calculate correlation coefficient again – Repeat. You get a value for each Δt. Plot these values in a histogram: autocorrelation histogram Note that for autocorrelation the plot is symmetrical White noise autocorrelation is flat CN 510 Lecture 24 Measuring Oscillations Similar technique can be used for LFPs You can also compare different signals by building crosscorrelation diagram CN 510 Lecture 24 Measuring Oscillations and Synchrony Two aperiodic and synchronous signals will have a single peak at zero Two aperiodic phase-locked signals will have single peak shifted from zero Two periodic synchronous signals will have wavy crosscorrelogram if their frequencies are similar: the further away the frequencies the faster the waviness will decay Two periodic phase-locked signals will have wavy crosscorrelogram with peak shifted from zero CN 510 Lecture 12 What Causes Oscillations? In the individual neuron any kind of delayed inhibitory feedback will lead to oscillations: – In Hodgkin-Huxley there is a fast Na and slow K currents – Adding more currents with different time courses will manipulate the frequency Similar, in the network any kind of delayed interactions will also lead to oscillations – Delayed inhibition in RCF leads to oscillation – Delayed excitation can also lead to oscillatory patterns, but most of the time there is some form of inhibition that does the job Combining individually oscillating neurons into a feedback network leads to most interesting oscillatory patterns You can overlay neuromodulation on top to further regulate the dynamics CN 510 Lecture 24 Interactions Between Delay, Oscillations, and Synchrony Conductance delays in the brain lead to oscillatory patterns Cells within or across brain areas synchronize despite these delays In the 90-es Nancy Kopell and Bard Ermentrout looked in detail on how conductance delays interact with oscillations and can lead to synchrony Another group that looked at the similar issues were Eugene Izhikevich and Frank Hoppensteadt CN 510 Lecture 24 Neuronal Types Type I: – Based on saddle-node bifurcation – All-or-none action potential – Long latency to fire after transient stimulus – Square root or linear FI curve (firing rate from input current) – Arbitrary long oscillation period Type II: – Based on Hopf bifurcation – Graded action potential amplitude – Limited range of frequencies – Short firing latencies CN 510 Lecture 24 Weakly-coupled Systems Only timing of firing is changed by inputs Approximate the system (neuron) by a periodic function and analyze interactions between these functions Most of the analysis deals with phase shifts of the oscillators For Type I neurons: – If coupled with inhibition at low frequencies both synchronous and anti-phase solutions are stable, at high frequency only the synchronous one is stable – If coupled with excitation – synchrony is stable at low frequencies, anti-phase at high frequencies More on these systems in Izhikevich (2006) and his references CN 510 Lecture 24 Strongly-coupled Systems Inputs can make neuron fire but do not influence the next oscillation cycle Reduction of the system to a map (Ermentrout and Kopell, 1998) Example of a system: E-I circuit is an oscillator: – tonically active E cell drives I cell that spikes only once – I cell spikes sufficiently fast to prevent a second E spike until the inhibition wears off – inhibition recovery time constant sets the frequency of this oscillation (realistic GABAA leads to γ frequencies) CN 510 Lecture 24 Strongly-coupled Systems Conductance delay in cross-circuit connections is – slower than I recovery period, so I will fire one spike in response to local excitation and another for remote excitation if both E cells spike simultaneously – faster than wearing off of the inhibition, so this second I spike will arrive at local E cell before it recovers from the first spike So basically the circuit can be fully symmetric with E cells firing synchronously at a frequency that is determined by four inhibitory events: – – – – Local I spike in response to local E spike Local I spike in response to remote E spike Remote I spike in response to remote E spike Remote I spike in response to local E spike CN 510 Lecture 24 Strongly-coupled Systems From the circuit E&K proceed to map representation in terms of spike timings and derive a map function F in terms of time difference between two E spikes Δ and the conduction delay δ This function was analyzed for stability of oscillations for a complete system as well as for system with E-to-I or I-to-E cross circuit projections removed Network with both projections synchronizes over larger range of conductance delays and does it faster than network with just Eto-I projections CN 510 Lecture 24 Strongly-coupled Systems There are two independent effects in the model – The response of the inhibitory (I) cells to excitation from more than one local circuit – The response of the excitatory (E) cells to the multiple inhibitory spikes they receive Together, they give the network synchronization properties that are not intuitively clear from the properties of either alone – For the weaker cross-circuit E-I coupling the synchrony fails if the delay in these projections is too small – Reducing the I refractory periods or increasing cross-circuit I-E coupling reduces the minimal delay needed for stability CN 510 Lecture 24 Strongly-coupled Systems In the network with no I-to-E cross-circuit projections: E cells receive only local inhibition (two spikes instead of four) – Here the timing of I spikes does not affect the range of delays over which the synchrony is stable – System in general is more tricky and can have some weird aperiodic or high frequency solutions – On the other hand non-homogeneous networks synchronize faster and better with just E-to-I projections Network with just I-to-E cross-circuit projections: I cells fire single spikes rather then doublets, so E cells again receive two I spikes – Synchrony is always stable – Can also form stable anti-phase solution To prove that map reduction works they also ran full scale simulations with the same results CN 510 Lecture 24 Slow Coupling Coupling is strong and slow so that inputs can make neuron fire on a different oscillation cycle Coupled leaky integrate-and-fire behave similar to coupled conductance based (HH-style) Type I neurons – Slow inhibition or fast excitation is beneficial for synchronizing neurons – Fast inhibition or slow excitation is beneficial for locking them in anti-phase Izhikevich proved that for one parameter regime the system of identical slow coupled oscillators converges to stable oscillations independently of the sign of projections, but the convergence to this state is oscillatory by itself: – Phase difference oscillates on its way to 0 For a different parameter setting the phase difference can oscillate indefinitely (converges to a limit cycle) CN 510 Lecture 24 Example of Simple Cases Two identical integrate-and-fire neurons will – Synchronize when coupled through inhibitory connections – Phase-lock in anti-phase when coupled with excitatory connections CN 510 Lecture 24 What Drives You? Three regimes of network oscillations: Tonic – when period of oscillation is small comparing to membrane and synaptic time constants; here the oscillation is driven by some fast network interactions and both membrane constant and inhibition work like damping Phasic – when membrane constant is short comparing to the period of oscillation and synaptic constant; here synaptic activity drives the frequency Fast – when synaptic constant is short comparing to period and membrane constant; here membrane properties drive the frequency It is especially important to know these when you attempt to synchronize a heterogeneous network CN 510 Lecture 24 What Is the Function of Oscillations? Is there a function? Some people believe that oscillations per se have a stabilizing function Others believe that interplay between different oscillations leads to some interesting effects (interference model of grid cells) Yet others believe that oscillations that we observe are just a byproduct of neuronal activity, which also leads to all effects attributed to oscillations Suggestion: If you model a structure that is a source of a certain oscillation then make sure that these oscillations are created by your network in the process of solving whatever task you model CN 510 Lecture 24 Brief Summary Research on oscillations and synchrony addresses two main questions: function and mechanism It is not clear to date if oscillations are a necessary byproduct of neural interactions, or if they serve a specific function More is known about how oscillations arise in neural circuits; in fact, it seems quite difficult not to have oscillations to emerge when neurons are connected together Oscillations are not always a sign of healthy brain function, indeed, when oscillations become dominant one can often see seizures during which large areas of the brain oscillate together in an undamped fashion CN 510 Lecture 24 Onset of a Seizure CN 510 Lecture 24 Next Time Rob’s lecture CN 510 Lecture 24