* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sleep/Neurology-The Orexin System
Activity-dependent plasticity wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Environmental enrichment wikipedia , lookup
Central pattern generator wikipedia , lookup
Brain Rules wikipedia , lookup
Lunar effect wikipedia , lookup
Neural oscillation wikipedia , lookup
Circadian rhythm wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroscience in space wikipedia , lookup
Aging brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Biology of depression wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroanatomy wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Circumventricular organs wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Delayed sleep phase disorder wikipedia , lookup
Neuroscience of sleep wikipedia , lookup
Sleep apnea wikipedia , lookup
Sleep and memory wikipedia , lookup
Sleep paralysis wikipedia , lookup
Rapid eye movement sleep wikipedia , lookup
Sleep deprivation wikipedia , lookup
Sleep medicine wikipedia , lookup
Effects of sleep deprivation on cognitive performance wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
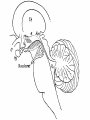
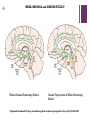
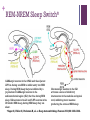
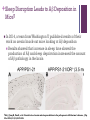
+ Sleep/Neurology-The Orexin System Todd J. Swick, M.D. FAAN, FAASM Assistant Clinical Professor of NeurologyUniversity of Texas Health Sciences CenterHouston + Speaker Disclosures Grants/Research Support Consultant Jazz Pharmaceuticals, UCB, Vanda Pharmaceuticals, XenoPort, Aerial Pharmaceuticals Jazz Pharmaceuticals, Vanda Pharmaceuticals, XenoPort, Aerial Pharmaceuticals, Merck Pharmaceuticals Honorarium Jazz Pharmaceuticals, Vanda Pharmaceuticals, XenoPort, Merck Pharmaceuticals + Sleep/Neurology-The Orexin/Hypocretin System Objectives Historical Overview of Sleep-Wake Signaling 1998 Discovery of the Orexin/HCRT Neuropeptides Neurophysiologic Effects of Orexin/HCRT peptides Control of Sleep/Wake mechanisms Clinical aspects of orexin/HCRT activity and loss Possible role of Orexin/HCRT in the pathogenesis of MCI/Alzheimer’s Possible answer as to “Why we need sleep” von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71(3):249-259. Original Hypocretin/Orexin Papers + Sleep/Neurology-The Orexin System Two papers were published within 3 weeks of one another in early 1998 The San Diego/Stanford groups were looking for genes with expressed selectivity in the control of appetite, thirst and other autonomic and arousal functions Two peptides derived from a single gene were identified and named Hypocretins for their hypothalamic location and sequence homology to secretin + Sleep/Neurology-The Orexin/HCRT System Team from Dallas led by Masashi Yanagisawa was looking for ligands for “orphan receptors” with strong homologies to known G protein receptors but no identified endogenous ligands They identified two novel peptides which were synthesized only in the hypothalamus When injected into the 3rd ventricle they induced feeding They named the peptides Orexins, after the Greek word for appetite + Sleep/Neurology-The Orexin System* Orexin/HCRT (ORX) neurons originate in the posterior and lateral hypothalamus as a paired set of nuclei comprising a total of 50,00080,000 neurons The ORX system is comprised of neurons producing two ORX neuropeptides (ORX-A and ORX-B or HCRT-1 and HCRT-2) with projections throughout the CNS Preprohypocretin (prepro-orexin) the precursor polypeptide is composed of 130 residues, undergoes proteolytic cleaving to produce ORX-A and ORX-B with 33 and 28 amino acids respectively *Chemelli R, Willie J, Sinton C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437-451. + Sleep/Neurology-The Orexin System ORX-A is highly lipophilic, crosses the BBB via simple diffusion and is stable in the CSF ORX-B is a linear peptide that is not stable in the CSF, has a short biological half-life due to rapid metabolism and clearance Both peptides bind to two G-protein coupled receptors (GPCRs) The OX1 receptor is a selective receptor with a high affinity for ORX-A The OX2 receptor is a non-selective receptor with equal affinity for both ORX-A and ORX-B + Orexin and Orexin Receptors* (Preprohypocreti n) (HCRT-1) (HCRT-2) *Inutsuka A, Yamanaka A. The Regulation of Sleep and Wakefulness by the Hypothalamic Neuropeptide Orexin/Hypocretin. Nagoya J. Med. 2013;75:25-36. + Knock-Out Mice* Yanagisawa’s group used transgenic techniques to construct a null mutant mouse that did not produce either orexin peptide The mice had reduced food intake but not as much as had been expected and had no effect on weight It was observed that the mice would often abruptly cease movement (cataplectic attack) *Chemelli R, Willie J, Sinton C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437-451. + Knock-Out Mice They then created mice without the OX2 receptor These animals exhibited slowness in movements consistent with sleep attacks Mice without the OX1 receptor Exhibited disrupted sleep but showed fewer signs of narcolepsy than the OX2 receptor knockouts + Orexin/Hypocretin and Genetic Narcolepsy In 1985, Dement et al showed that canine genetic narcolepsy was caused by an autosomal recessive gene1 In 1999, Mignot identified the gene responsible for canine narcolepsy as a mutated, non-functional version of the OX2 receptor gene1 Human narcolepsy cannot be explained by genetic mutations in that human narcolepsy is NOT a genetic disease (human narcolepsy is discordant in identical twins)2 In 2000, Siegel et al demonstrated that there is massive reduction (>90%) of postero-lateral hypothalamic orexin neurons in human narcolepsy/cataplexy pointing to a secondary loss of these cells while at the same time sparing co-localized neurons (i.e. melanin concentrating hormone)-raising the possibility of an auto-immune attack against orexin neurons exclusively2 1Mignot E. History of narcolepsy at Stanford University. Immunol Res. 2014;58:315-339. J, Moore R, Thannickal T, al. e. A brief history of hypocretin/orexin and narcolepsy. Neuropsychopharmacology. 2001;25 (Suppl 5): S14-20. 2Siegel + Sleep/Neurology-The Orexin System Orexins are excitatory neurotransmitters They act to sustain wakefulness and stabilize sleep Facilitatory role in the regulation of muscle tone Promote arousal responses to homeostatic challenges Drive motivated behavior such as seeking food Excite neurons of the mesolimbic reward pathways ORX antagonists have been shown to reduce the motivation to seek drugs of abuse Activated by humoral indicators of hunger such as low glucose or high levels of ghrelin + Integrative Physiologic Roles of Orexin/Hypocretin Peptides* 5-HT, serotonin; ACh, Acetylcholine; Arc, arcuate nucleus; BST, bed nucleus of the stria terminalis; DA, dopamine; DR, dorsal raphe nucleus; GABA, gamma-aminobutyric acid; HA, histamine; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; NA, noradrenaline; NPY, neuropeptide Y; POA, preoptic area; POMC, proopiomelanocortin; PPN, pedunculopontine tegmental nucleus; TMN, tuberomammillary nucleus; VTA, ventral tegmental area *Inutsuka A, Yamanaka A. The Regulation of Sleep and Wakefulness by the Hypothalamic Neuropeptide Orexin/Hypocretin. Nagoya J. Med. 2013;75:25-36. + Wake-Promoting Neurochemical Systems Acetylcholine (ACh) Basal Forebrain (BF) contain ACh neurons that promote wakefulness and REM sleep Also contains GABA which increases cortical activation by inhibiting cortical interneurons Pons Laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT) contain ACh neurons which project to subcortical regions (active during wakefulness and REM sleep) Thalamus Lateral Hypothalamus Basal Forebrain + Wake-Promoting Neurochemical Systems Norepinephrine (NE) Locus coeruleus (LC) Fire most rapidly during wakefulness Less active during NREM sleep Virtually silent during REM sleep Histamine (HA) Tuberomammillary nucleus (TMN) Fire most rapidly during wakefulness Less active during NREM sleep Least active during REM sleep + Wake-Promoting Neurochemical Systems Serotonin (5-HT) [Binds to at least 15 different receptors with varied effects] Neurons in the dorsal raphe (DR) nucleus and other raphe nuclei scattered along the midline of the brainstem Promote wakefulness and reduces REM sleep Fire most rapidly during wakefulness Less active during NREM sleep Least active during REM sleep + Wake-Promoting Neurochemical Systems* Dopamine (DA) Ventral (and ventral-lateral)periaqueductal gray (vPAG/vlPAG) of the pons Potent wake-promoting effects Sleep promoting effects of DA antagonists Orexin/Hypocretin ORX neurons are located exclusively in the lateral and posterior hypothalamus Project widely and heavily innervate all arousal regions with particular dense innervation of the LC and TMN ORX neurons fire mainly during wakefulness and are silent during NREM and REM sleep *Swick T. The Neurology of Sleep 2012. In: Teofilo Lee-Chiong J, MD, ed. Biology of Sleep. Vol 7. Philadelphia, PA: W.B. Saunders; 2012:399-415. + WAKE/AROUSAL and OREXIN NUCLEI* Wake/Arousal Promoting Nuclei Orexin Projections to Wake Promoting Nuclei *Espana R, Scammell T. Sleep neurobiology from a clinical perspective. Sleep. 2011;34:845-858. + REM-NREM Sleep Switch Two populations of mutually inhibitory neurons in the upper pons form a switch for controlling transitions between REM and NREM sleep GABAergic neurons in the vlPAG and the adjacent LPT fire during non-REM states to inhibit entry into REM sleep During REM sleep the vlPAG and LPT neurons are inhibited by GABAergic neurons in the sublaterodorsal regions (SLD) that fire during REM sleep This mutually inhibitory relationship produces a REM-NREM flip-flop switch, promoting rapid and complete transitions between these states + REM-NREM Sleep Switch The core REM switch is modulated by other neurotransmitter systems NA neurons in the LC and 5-HT neurons in the DR inhibit REM sleep work on both sides of the flip-flop switch (exciting REM-off and inhibiting REM-on neurons During REM sleep these monoaminergic amine neurons are silent whereas the ACh neurons (LDT/PPT) promote REM sleep by having opposite actions on the same two neuronal populations (vlPAG and the LPT) + REM-NREM Sleep Switch Orexin neurons inhibit entry into REM sleep by exciting neurons in the REM-off population (vlPAG/LPT) VLPO neurons promote entry into REM sleep by inhibiting the vlPAG/LDT During REM sleep, glutamatergic neurons in the sublaterodorsal (SLD) nuclei activate a series of inhibitory interneurons in the medulla and spinal cord, inhibiting motor neurons producing the atonia of REM sleep + REM-NREM Sleep Switch* GABAergic neurons in the vlPAG and the adjacent LPT fire during non-REM to inhibit entry into REM sleep. During REM sleep they are inhibited by a Glutamatergic neurons in the SLD population of GABAergic neurons in the activates a series of inhibitory sublaterodorsal region (SLD) that fire during REM interneurons in the medulla and spinal sleep. NA neurons in the LC and 5-HT neurons in the cord, inhibiting motor neurons DR inhibit REM sleep, during REM sleep they are producing the atonia of REM sleep silent. *Saper C, Fuller C, Pedersen N, al. e. Sleep state switching. Neuron. 2010;68:1023-1042. + State-Specific Firing Rates of Brainstem and Cortical Neuronal Groups* *Swick T. The Neurology of Sleep 2012. In: Teofilo Lee-Chiong J, MD, ed. Biology of Sleep. Vol 7. Philadelphia, PA: W.B. Saunders; 2012:399-415. + Narcolepsy/Cataplexy Pentad of symptoms [REM sleep characteristics that intrude into wakefulness/sleep] Excessive daytime sleepiness Cataplexy Hypnogogic hallucinations Sleep paralysis Disrupted nocturnal sleep + Narcolepsy/Cataplexy Canine narcolepsy caused by an exon-skipping mutation in the OX2 receptor gene (1999) Murine narcolepsy found after the deletion of the gene coding for both orexin peptides (severe sleepiness and cataplexy) (1999) Humans with narcolepsy/cataplexy had a >90% loss of orexin producing neurons (2000) with loss of other markers of the orexin neurons (dynorphin and pentraxin) while sparing intermingled MCH neurons High association with HLA DQB1*06:02 leading to the hypothesis that orexin loss is secondary to an autoimmune process + Narcolepsy/Cataplexy Impaired orexin signaling causes behavioral states to become unstable Patients with narcolepsy/cataplexy have normal amounts of wake and sleep but they have many more transitions between states (accounts for disrupted nocturnal sleep) The loss of orexin neurons permits more frequent transitions into and out of REM sleep throughout the day (pathognomonic of narcolepsy) Can be partial REM sleep states, e.g. cataplexy (abrupt onset of loss of muscle tone); hypnogogic hallucinations (onset of dream mentation in the transition between wakefulness and sleep); sleep paralysis (sleep onset muscle atonia) + Insomnia Characterized by Affects approximately 70 million Americans (one or more symptoms) Difficulty falling asleep Difficulty staying asleep Early AM awakenings Poor quality of nocturnal sleep Daytime consequences (psycho-motor complaints, memory deficits, etc.) 23.5 million (~10% of US adult population) have symptoms consistent with the diagnosis of insomnia 8 million have had prescriptions written and 10 million use OTC or other sleep aids (alcohol or herbal products) Chronic insomnia is associated with Hyperarousal (hypermetabolic) changes (as seen on PET scans from chronic insomnia patients*) in all the wake-promoting neural centers of the brain stem, diencephalon and cortex suggesting orexin overdrive *Nofzinger E, Buysse D, Germain A, Price J, Miewald J, Kupfer D. Functional Neuroimaging Evidence for Hyperarousal in Insomnia. Am J Psychiatr. 2004;161:2126-2129. + Insomnia Prescription hypnotics typically work on augmenting sleep promoting centers or blocking specific wake-promoting centers GABA (enhance GABA signaling via GABAA receptors) Benzodiazepines (diazepam, clonazepam, lorazepam, triazolam) Non-Benzodiazepine receptor agonists [NBZAs] (zolpidem, eszoplicone, zaliplon) Histamine (Blocks HA H1 receptors in TMN) Central antihistamines (diphenhydramine, doxylamine, hydroxyzine) Block DA receptors (mainly D2) Typical antipsychotics (chlorpromazine, haloperidol, thioridazine) + Insomnia Orexin Receptor Antagonists* Evidence from null ORX knock-out mice showed that sleep propensity was greatest in those animals where there was absence of both ORX subtypes If chronic insomnia is due to an overactive wake system then dampening of the entire wake-promoting system should facilitate sleep by blocking orexin activity This led to the development of dual orexin receptor antagonists (DORA) Suvorexant (Belsomra®) was approved by the FDA in 2015 for the treatment of insomnia (sleep onset and sleep maintenance) in patients ages 18 and above Almorexant (another DORA) was withdrawn from consideration before it got to the FDA approval stage *Ruoff C, Guilleminault C. Hypocretin receptor antagonists for insomnia: rationale and clinical data. Clin Invest. 2012;2(6):623-637. + β-Amyloid and Orexin* In 2009 Holtzman’s group from Washington University in St. Louis showed that the amount of interstitial fluid (ISF) levels of Aβ correlated with wakefulness ISF Aβ significantly increased during acute sleep deprivation (in mice) ISF Aβ significantly increased during orexin infusion ISF Aβ decreased with infusion of a DORA Chronic sleep restriction significantly increased Aβ plaque formation in amyloid precursor protein transgenic mice DORA infusion decreased Aβ plaque formation in amyloid precursor protein transgenic mice *Kang J, Lim M, Bateman R, et al. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005-1007. + Sleep-Disordered Breathing Advances Cognitive Decline in the Elderly† In 2015, Osorio et. al. from NYU reported on patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) looked at the following questions Is sleep-disordered breathing (SDB) associated with an earlier age of onset of mild cognitive impairment (MCI) or Alzheimer’s disease (AD) onset? †Osorio SDB is associated with sleep fragmentation and cyclical hypoxemia/hypercarbia Does treatment with CPAP delay the onset of cognitive decline? R, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1-8. + Sleep-Disordered Breathing Advances Cognitive Decline in the Elderly Results SDB patients had a younger age at onset of MCI and AD-dementia CPAP treated patients appeared to delay progression of cognitive impairment + Survival Curves of Age at MCI or ADDementia Onset* Excluded patients with missing data Excluded patients with missing data Excluded pts with ambiguous group allocation Excluded pts with ambiguous group allocation Documented MCI or AD based on F/U assessment Documented MCI or AD based on F/U assessment *Osorio, RS, Gumb, T, Pirraglia, E, et al. Sleep disordered breathing advances cognitive decline in the elderly. Neurology, 2015;84:1-8. + Associations of Brain Lesions at Autopsy with Polysomnography Features Before Death Honolulu-Asia Aging Study (prospective cohort study of Japanese American men in Honolulu) Neuropathologic analysis Braak stage Neurofibrillary tangle and neuritic plaque counts Microinfarcts Generalized brain atrophy Lacunar infarcts Lewy body (LBs) Neuronal loss and gliosis in the locus coeruleus + Associations of Brain Lesions at Autopsy with Polysomnography Features Before Death 167 patients (all males) were included in the analysis who underwent polysomnography in 1999-2000 (mean age=84 years) and died through 2010 (mean 6.4 years to death) Results SpO2<95% was associated with higher numbers of microinfarcts (OR=3.88; CI=1.10-13.76) Greater SWS (Stage N3) duration was associated with less generalized brain atrophy (OR=0.32; CI=0.10-1.03) and slower cognitive score reductions + Associations of Brain Lesions at Autopsy with Polysomnography Features Before Death‡ Discussion Men with lower SpO2 during sleep were more likely to have higher levels of microinfarcts Men with less SWS (slow wave sleep) a presumed marker of poor sleep quality and strongly associated with fragmented sleep had more brain atrophy at autopsy Men with greater hypoxemia during REM sleep exhibited more gliosis and neuronal loss in the LC (locus coeruleus) AHI (a measure used in standard definitions of OSA and by Medicare to determine CPAP eligibility) and # of arousals were not associated with any of the lesions whereas O2 saturation level was associated with microinfarcts, the major lesion of vascular dementia ‡Gelber R, Redline S, Ross G, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84(10.1212/WNL.001163):296-303. + Alzheimer’s disease: sleep, orexin and cognitive decline‡‡ Liguori’s group from U. of Rome looked at 48 consecutive untreated AD patients and 29 healthy controls (CSF ORX levels and PSG findings) AD patients were divided into two groups Mild AD (MMSE>21; 21 subjects) Moderate-severe AD (MMSE<21; 27 patients) Results Controls and mild AD patients had no significant difference in CSF Orexin levels Moderate to severe AD patients showed significantly increased orexin levels compared to normal controls and mild AD patients Moderate-severe AD patients exhibited more impaired nocturnal sleep compared to normal controls and mild AD patients The global AD group had orexin levels that were positively correlated with total tau proteins and strictly related to sleep impairment Cognitive impairment (as measured by MMSE) was correlated with sleep structure deterioration (reduction in SWS and increased amounts of WASO) ‡‡Liguori C, Romigi A, Nuccetelli M, et al. Orexinergic System Dysregulation, Sleep Impairment, and Cognitive Decline in Alzheimer Disease. JAMA Neuro. 2014;71(12):1498-1505. + Correlations between CSF tau and MMSE (WASO, and SE) in Patient’s with Moderate to Severe Alzheimer’s Disease (Total tau protein) (Phosphorylated tau protein) (WASO) (SE) + Correlations between CSF Orexin Levels and PSG Data in Patient’s with AD (WASO) (SE) (SOL) + Sleep Disruption Leads to Aβ Deposition in Mice¶ In 2014, a team from Washington U published results of their work on orexin knock-out mice looking at Aβ deposition Results showed that increase in sleep time slowed the production of Aβ and sleep deprivation increased the amount of Aβ pathology in the brain ¶Roh J, Jiang H, Finn B, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med. 2014;211(13):2487-2496. + Why Do We Need Sleep? Why is sleep restorative? Why does lack of sleep impair brain function? Sleep deprivation Reduces learning Impairs performance in cognitive and motor tests Prolongs reaction time Common cause of seizures + Why Do We Need Sleep? Proteins linked to neurodegenerative diseases (β-amyloid, αsynuclein and tau) are present in the interstitial space surrounding cells of the brain The brain lacks a conventional lymphatic system to remove excess interstitial proteins into the general circulation for degradation in the liver CSF recirculates through the brain interchanging with interstitial fluid (ISF) to remove interstitial proteins including β-amyloid + Why Do We Need Sleep? The convective exchange of CSF and ISF is organized around the cerebral vasculature CSF influx occurs around arteries ISF efflux occurs along the veins These pathways were named “glymphatic system” on the basis of their dependence on astrocytic aquaporin-4 (AQP4) water channels with results equivalent to the peripheral lymphatic removal of interstitial metabolic byproducts and toxins Removal of AQP4 channels reduce clearance of Aβ by 65% The interstitial concentration of Aβ is higher in the awake state compared to the sleep states in rodents and humans + Why Do We Need Sleep? Nedergaard and associates* from U of Rochester in 2013 hypothesized that Aβ clearance increased with sleep and the sleep-wake cycle regulates glymphatic clearance of metabolic “neurotoxins” that Using real-time assessments of tetramethylammonium (TMA) diffusion and two-photon imaging in live mice it was demonstrated Natural sleep or anesthesia was associated with a 60% increase in interstitial space Increasing the convective fluxes of ISF which in turn increased the rate of β-amyloid clearance during sleep *Xie L, Kang H, Xu Q, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373-377. +Sleep Drives Metabolite Clearance from the Brain *P<0.05 compared with awake A) Time-disappearance curves of I-Aβ after its injection into the frontal cortex in awake (orange triangles), sleeping (green diamonds) and anesthetized (red squares) B) Rate constants derived from the clearance curves for I-Aβ infusion C) Time-disappearance curves of C-inulin after its injection into the frontal cortex of awake (orange triangles), sleeping (green diamonds) and anesthetized (red squares) D) Rate constants derived from the clearance curves for inulin infusion + Summary The orexin system acts as the master conductor of the wake-promoting system of the sleep/wake and REM/NREM states The absence of orexin creates an unstable sleep/wake condition and clinically is cause of narcolepsy/cataplexy Sleep fragmentation, a common component of such varied conditions as sleep apnea, insomnia, RLS, PLMDs, and AD is thought to be secondary to, or associated with, orexin over activity With an increase in orexin activity, the normal physiologic function of the glymphatic system is perturbed, potentially leading to a decrease in Aβ and Tau protein elimination and the development of MCI/AD Treatment of sleep disordered breathing (and potentially other sleep and neurologic conditions) with resultant fragmented sleep might decrease the onset of vascular dementia as well as the development of other degenerative neurologic disorders