* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Physiological Plasticity of Single Neurons in Auditory Cortex of the
Functional magnetic resonance imaging wikipedia , lookup
Mirror neuron wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Aging brain wikipedia , lookup
Executive functions wikipedia , lookup
Haemodynamic response wikipedia , lookup
Electrophysiology wikipedia , lookup
Multielectrode array wikipedia , lookup
Time perception wikipedia , lookup
Central pattern generator wikipedia , lookup
Perception of infrasound wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuroeconomics wikipedia , lookup
Nervous system network models wikipedia , lookup
Neural coding wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Conditioned place preference wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neural oscillation wikipedia , lookup
Development of the nervous system wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroplasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Spike-and-wave wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Classical conditioning wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Behavioral Neuroscience
1984, Vol. 98, No 2,171-188
Copyright 1984 by the
American Psychological Association, Inc
Physiological Plasticity of Single Neurons
in Auditory Cortex of the Cat During Acquisition
of the Pupillary Conditioned Response:
I. Primary Field (AI)
Norman M. Weinberger, William Hopkins, and David M. Diamond
Center for the Neurobiology of Learning and Memory
and Department of Psychobiology
University of California, Irvine
The effects of conditioning on the discharges of single neurons in primary
auditory cortex (AI) were determined during acquisition of the pupillary
conditioned response in chronically prepared cats. Acoustic stimuli (1-s white
noise or tone) were presented with electrodermal stimulation unpaired during
a sensitization control phase followed by pairing during a subsequent conditioning phase. Stimulus constancy at the periphery was ensured by the use of
neuromuscular blockade. Discharge plasticity developed rapidly for both
evoked and background activity, the former attaining criterion faster than the
latter. The pupillary dilation conditioned response was acquired at the same
rate as were changes in evoked activity (i.e., 10-15 trials) and faster than
background activity (i.e., 20-25 trials). Increases in background activity were
correlated with increasing level of tonic arousal, as indexed by pretrial size of
the pupil.
Behavioral adaptation requires accurate
information about the environment. That
sensory systems provide such information
has never been in serious question. However, the mechanisms involved have not yet
been elucidated fully. This problem appears
to be further complicated by the fact that
electrophysiological responses to environmental stimuli within sensory systems are
modified by learning. Numerous studies of
classical and instrumental conditioning in
animals have demonstrated that responses
to conditioned and discriminative stimuli
in sensory systems are altered systematically by associative processes (for reviews,
This research was funded by a grant from the
Monsanto Company, and by National Institute of
Neurological and Communicative Disorders and
Stroke Grant NS16108 and National Science Foundation Grant BNS76-81924 to N. M. Weinberger.
The authors gratefully acknowledge Cathy Bennett
and Thomas McKenna for helpful discussion during
the preparation of the manuscript, Herman Birch for
writing the computer programs, and Lisa Weinberger
for secretarial assistance.
Requests for reprints should be sent to Norman M.
Weinberger, Center for the Neurobiology of Learning
and Memory, University of California, Irvine, California 92717.
see John, 1961; Sokolov, 1977; Thompson,
Patterson, & Teyler, 1972). Such response
plasticity is particularly evident in sensory
cortex; it has been documented most extensively in auditory cortex (e.g., Buchwald,
Halas, & Schramm, 1966; Cassady, Cole,
Thompson, & Weinberger, 1973; Galambos, Sheatz, & Vernier, 1955; Oleson, Ashe,
& Weinberger, 1975) and has been reported
as well in olfactory (Freeman, 1980), somatosensory (e.g., Voronin, Gerstein, Kudryashov, & Ioffe, 1975), and visual (e.g.,
Shinkman, Bruce, & Pfingst, 1974) cortices. Thus, sensory responses are affected
by two types of variables: (a) the physical
parameters of stimuli and (b) the meaning
or cue value of stimuli. This situation suggests a paradox because the requirement
for veridical responses to the physical parameters of stimuli appears to be compromised by the effects of stimulus meaning.
In recent years, this issue has been investigated most thoroughly within the auditory system, and there now appears to be
a partial resolution of the paradox. Anatomical and physiological studies have revealed that the auditory system contains
both lemniscal and non-lemniscal or "lem171
172
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
niscal adjunct" ascending pathways (Graybiel, 1972; Herkenham, 1980; Ryugo & Killackey, 1974; J. Winer, Diamond, & Raczkowski, 1977). At the level of the thalamic auditory system, these subsystems
engage different subdivisions of the medial
geniculate body (Morest, 1964, 1965). The
lemniscal line projects to the ventral medial
geniculate nucleus (MGv), the neurons of
which are tonotopically organized, with
narrow tuning functions (Aitkin & Webster, 1972), whereas the nonlemniscal line
projects to the magnocellular medial geniculate nucleus (MGm), the neurons of which
are not tonotopically organized and have
very broad tuning functions (Aitkin, 1973).
Analysis of the physical parameters of
sound and the meaning of sound is also
compartmentalized at this level of the auditory system. Neurons in the lemniscal
MGv respond to the physical parameters of
the acoustic environment, but these responses are unaffected by learning. In contrast, neurons in the nonlemniscal MGm
are not particularly sensitive to changes in
the physical parameters of sound, but they
develop discharge plasticity rapidly during
learning. These findings have been obtained in cat during classical defensive conditioning (Ryugo & Weinberger, 1976,
1978), rabbit during instrumental avoidance conditioning (Gabriel, Miller, & Saltwick, 1976), and rat during hybrid classical-instrumental appetitive conditioning
(Birt, Nienhuis, & Olds, 1979; Birt & Olds,
1981).
unit activity in this cortical field are modified by associative processes. Insofar as
both the ventral and the magnocellular medial geniculate nuclei project upon primary
auditory cortex, we have suggested that the
former, being nonplastic, is not a source of
associative effects on AI whereas the latter
might be involved in learning-induced cortical discharge plasticity (Weinberger, in
press). Additional analysis of this and many
related issues requires investigation of the
effects of learning on the discharge properties of single neurons in auditory cortex,
because of the inherent limitations of
evoked potentials and multiple-unit data.
Single-unit studies during the acquisition
of behavioral conditioned responses are
technically difficult, requiring that adequate recordings be obtained continuously
during a prolonged period of training, and
they yield data from only one cell per training session. Nonetheless, such studies are
important because they provide a link between the fields of single-unit auditory
physiology and learning and they can reveal
the changes underlying neurophysiological
plasticity as indexed by evoked potentials
and particularly by multiple-unit recordings (Kraus & Disterhoft, 1982).
The present experiment is concerned
with the discharges of single cells in primary auditory cortex (AI) during the acquisition of a behavioral conditioned response. A companion report is concerned
with the effects of classical conditioning on
the secondary auditory cortical field (All)
At the level of auditory cortex, the situ- which is prototypical of nonlemnical audiation has not yet been clarified, but it is tory cortex (D. Diamond & Weinberger,
probably more complex than at the thala- 1984). Such comparative information may
mus. Auditory cortex consists of several be important for understanding the funcfields, which have either lemniscal or non- tional role of multiple sensory cortical
lemniscal characteristics. The primary au- fields (I. Diamond, 1979; Merzenich &
ditory cortex (AI) and the anterior (AAF) Kass, 1980) as well as bearing more directly
and posterior (P) fields are tonotopically on issues regarding the neuronal bases of
organized; in contrast, the secondary audi- learning. Further, it is hoped that the data
tory cortex (AH) and insular (I) and tem- so obtained will promote the rapprocheporal (T) fields are not so organized and ment of the fields of sensory neurobiology
thus seem to be nonlemniscal in nature and learning, as both areas 'are critically
(e.g., Reale & Imig, 1980). Almost all pre- concerned with how the brain acquires invious studies of learning in the auditory formation about the environment.
cortex have investigated AI, and, as pointed
A preliminary report of some of these
out above, there is abundant documenta- results has been presented (Hopkins &
tion that evoked potentials and multiple- Weinberger, 1980).
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
Method
Surgical Preparation
The subjects were 8 adult cats, 3.2-5.0 kg, in good
health. The animals were anesthetized with sodium
pentobarbital (Nembutal, 40 mg/kg, ip) and placed in
a stereotaxic frame, with care taken to preserve the
integrity of the external auditory meatus and tympanic membrane. The scalp was incised and reflected,
and the calvarium was cleared of connective tissue. A
pedestal containing metal fixtures was built with dental acrylic and secured to the skull with stainless steel
screws. The pedestal allowed for immobilization of the
head during subsequent training sessions without direct pressure on the animal. Body temperature was
maintained by a thermostatically controlled warm
water pad during the surgery and recording sessions.
Ophthalmic ointment (Terramycin) was applied to
prevent corneal drying. Antibiotics (Panalog and Delagon) were applied locally to exposed skin surfaces,
and Bicillin (300,000 U im) was administered for 2-3
days following the surgery. Training began after a
recovery period of 1-2 weeks.
Experimental Design and Procedures
At the beginning of a training session, the animals
underwent neuromuscular blockade induced by gallamine triethiodide (Flaxedil, 10 mg/kg, ip). The trachea was intubated with a pediatnc catheter, coated
with a local anesthetic (Xylocaine), under laryngoscopic control, and the animal was artificially respired
with a Harvard respirator. Following immobilization,
the animal was positioned in a modified stereotaxic
frame fitted with bars which attached to the pedestal.
All procedures were carried out with the animals enclosed in an acoustically isolated chamber (IAC 1202).
Neuromuscular blockade was maintained with intravenous infusion of Flaxedil (20 mg/hr, iv). Expired
CO2 levels were not monitored because we had found
in previous experiments that pupillary size and motility in response to sensory stimulation are more sensitive indexes of the animal's condition. At the end of
a training session, the animals were recovered with
the assistance of Tensilon (0.6 ml im).
Acoustic stimulation was delivered to the ear contralateral to the recording electrode by a Beyer earphone which was attached to plastic tubing that fitted
into a mold of the external auditory meatus. The mold
was sealed with plasticine to provide a closed acoustic
delivery system. Acoustic conditioned stimuli (CS)
were either 1-3 kHz tones (n = 4) produced by a
Wavetek oscillator (70-85 dB) or white noise (n = 17)
produced by a Grason-Stadler generator (bandwidth
0.2-20 kHz, 80 dB) and were 1 s in duration. Stimulus
intensities are expressed as decibels above a reference
of 20 jjN/m2 and were controlled by Hewlett-Packard
attenuators. Sound intensity level was measured with
a Bruel and Kjaer sound lever meter, condenser microphone, and probe tube inserted into the external
auditory meatus through a sealable port in the sound
delivery tube. The unconditioned stimulus (US) was
173
electrodermal stimulation (EDS) consisting of a 375ms train of 50-Hz pulses (5.0 ms) produced by a Grass
S-44 constant-current stimulator via an optical isolation transformer. Stimulus intensity (2-9 mA) was set
at the beginning of a session to produce a brief (2-5
s) pupillary dilation. The US was delivered to the
subcutaneous tissue of the forepaw contralateral to
the recording site via a pair of fine wire electrodes.
Pupillary size was monitored by an infrared pupillometer (Cassady, Farley, Weinberger, & Kitzes, 1982)
positioned in front of the one eye. Care was taken to
avoid possible discomfort due to drying of the corneas
by covering them with ophthalmic ointment. In some
sessions, pupillary stability was enhanced by a low
level of illumination presented to the eye contralateral
to the pupillometer, but there were no differences in
behavioral or neuronal results related to the use of
such illumination. The output of the pupillometer was
amplified by a dc amplifier and written out on a Grass
Model 7 polygraph. The level of drift of the pupillometric recording system was negligible.
The skin area surrounding the pedestal was locally
anesthetized with Marcaine (Breon Labs, Inc.) and
then gently retracted to expose the lateral surface of
the skull. A small burr hole was drilled and a slit was
made in the dura. These procedures were not stressful
to the animal as indicated by the lack of pupil dilation.
Single-unit discharges were recorded with tungsten
electrodes (1-ftm diameter tips) insulated with Epoxylite (2-4 Mfi impedance at 1 kHz). Electrodes were
attached to a Narashige stepping microdrive controlled from outside the acoustic chamber and advanced through the dural slit until the discharges of
single neurons were encountered (see below). Discharges were amplified with a conventional differential preamplifier (bandwidth 80 Hz-10 kHz) and led
to an active high-pass filter (0.4-6 kHz), the output of
which was displayed on a storage oscilloscope and
recorded on a direct channel of a Hewlett-Packard
3964A tape recorder. A single unit was identified by
visual inspection of the spike waveform. Neuronal
data were considered acceptable if the record consisted
of a clearly distinguishable unit whose waveform exhibited no notches or other signs of injury, with a
signal-to-noise ratio of at least 3:1. Data reported here
are only for units meeting these criteria.
A training session consisted of two parts, a sensitization phase and a conditioning phase. During sensitization, 15 CSs and USs each were presented in an
unpaired fashion, at pseudorandom intervals at an
average density of two per minute with the restriction
that the stimuli not occur within 10 s of each other.
During conditioning, the CS and US were always
paired, the US being presented at CS offset. Stimulus
density was maintained at two per minute, the average
mtertrial interval was 60 s (range, 30-90 s). The
sensitization phase served as a control for nonassociative factors. This phase used unpaired rather than
randomized presentation of the CS and US because
conditioned inhibition does not develop during the
presentation of small numbers of unpaired stimuli
(Furedy, 1971; Furedy, Poulos, & Schiffman, 1975).
Backward pairing was not used because it can produce
conditioned inhibition to the CS due to its cue value
as a "safety" signal of the termination of the US (e.g.,
174
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
Segundo, Galeano, Sommer-Smith, & Roig, 1961).
Conditioning trials were initiated without break after
the last sensitization trial and were continued for up
to 60 trials or until acceptable recordings from a single
cell could not be obtained. Animals received only one
training session on a single day, and at least 7 days
intervened between successive training sessions.
Data Analysis
The effects of training on pupillary behavior were
assessed as described previously (Ashe, Cassady, &
Weinberger, 1976; Oleson et al., 1975;, Oleson, Vododnick, & Weinberger, 1973; Oleson, Westenberg, &
Weinberger, 1972; Ryugo & Weinberger, 1978).
Briefly, the pupillometer write-out was measured immediately preceding the presentation of the CS
throughout training and preceding the US for sensitization trials on which EDS was given alone. The
peak amplitude of dilations to these stimuli was measured and the pretnal level was subtracted, which
yielded a difference score hereafter referred to as a
pupillary response. The scores and baseline levels were
determined for every trial throughout training. They
were normalized for comparison across animals by
expressing each value as a percentage change from the
average of the last five-trial block of the sensitization
phase. The number of trials to criterion for pupillary
conditioning was defined as five consecutive trials
during the conditioning phase all of which had responses greater than the average of the last five trials
of the sensitization phase. The probability of this
occurring by chance is .03 (Feller, 1968).
Neuronal discharges were analyzed with the assistance of an LSI/11 computer. Single-unit discharges
were passed through a voltage detector or window that
produced a single pulse for each discharge detected.
The output of the trigger source was led to a pulse
input in the computer which recorded the occurrence
of spikes during 0.5-4 s immediately preceding a trial
and during the trial. Spike counts were stored in
consecutive bins of 2 or 3 ms The average number of
counts per bin was determined for every pretrial period
and every dunng-tnal period. The pretrial average was
substracted from the during-tnal average for each
trial; this yielded a difference score. The pretrial scores
are hereafter referred to as background activity, and
the difference scores as evoked activity. Each background and each evoked score were normalized by
expressing it as a percentage change from the average
pretrial and evoked scores, respectively, for the last
five-trial block of the sensitization period. The number
of trials to criterion of discharge plasticity was defined
as five consecutive trials during the conditioning phase
all of which had greater or smaller values than the
average of the last five trials during the sensitization
phase (p = .03). All data were evaluated by parametric
(B Winer, 1971) or nonparametric (Siegel, 1956) statistics.
Histology
Following a training session, a small electrolytic
lesion was produced by passing anodal current through
the recording electrode. After the final session, the
animal was given an overdose of Nembutal and perfused through the carotid arteries with 0.9% saline
followed by 10% formalin; the brain was removed and
stored in formalin. Frozen Berial sections (50 /urn) were
taken throughout auditory cortex and stained with
cresyl violet. Recording sites were reconstructed according to the cytoarchitectural distinctions of the
various subfields of auditory cortex described by Rose
(1949) and Sousa-Pinto (1973).
Results
Pupillary Behavior and Conditioning
Pupillary dilation responses were recorded for all experimental sessions. At the
beginning of sensitization, the acoustic
stimulus generally elicited brief, low-amplitude dilations. By the end of the sensitization phase, dilation responses were reduced
in both amplitude and duration. The EDS
(US) produced consistently large dilation
responses throughout the experimental session. During conditioning, acoustically
evoked dilations increased rapidly over
trials, as has been reported previously
(Ashe et al., 1976; Oleson et al., 1972, 1973,
1975; Ryugo & Weinberger, 1978; Weinberger, Oleson, & Haste, 1973). The average
conditioned dilations typically exceeded
the largest acoustically elicited responses
of the preceding sensitization period during
Trials 6-10, attained asymptote by Trials
21-25, and maintained high values thereafter (Figure 1). The pupillary dilation conditioned response attained criterion in 16
of 21 sessions. For these sessions, the mean
trials to criterion was 11.81 (SD = 5.76,
range, 6-27 trials).
Of the 8 animals, 7 received more than
one, and some as many as four, training
sessions at weekly intervals. In order to
assess the cumulative effect of earlier training sessions, each animal was assigned a
savings score based on a sequential comparison of values for the trials-to-criterion
measure from session to session. Thus, a
subject would receive a net positive savings
score if the number of all possible session
comparisons for which there was a reduction in the trials-to-criterion measure were
greater than the number of comparisons
that yielded an increase in this measure. In
this manner it was determined that 4 of 7
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
175
tioned response. However, this index of
behavioral learning is useful as a sign that
the subject is "adequate," in order to permit
unambiguous interpretation of "negative"
neuronal findings. The failure of a neuron
to develop a systematic change in its discharges during conditioning might be due
to its presumptive membership in a group
of "nonplastic" neurons, at least nonplastic
5
6
1
2
3
for the circumstances of a given experiStNSITIZAIlON
CONDITIONING
BLOCKS OF FIVE TRIALS
ment. Although this is the usual interpretation of negative findings, an alternative
explanation is that the subject was "inadequate," that is, a "substandard" preparaFigure 1. Group pupillary learning curve for the 17
cases in which conditioned pupillary dilation re- tion. Thus, a neuron in question might have
sponses attained the criterion of learning. (Each point the capacity to develop plasticity but be
is the mean percentage change in maximal pupillary unable to express it because of the inadedilation during presentation of the conditioned stim- quacy of the subject. In short, if the subject
ulus for blocks of five trials relative to the last fivetrial block during the sensitization period In some is unable to acquire a conditioned response
cases, recording was terminated after the fourth block and a neuron in that animal also fails to
of conditioning [Trial 20] due to deterioration of iso- express discharge plasticity, no conclusions
lation of discharges from single units; the numbers of can be drawn about the functional plasticsubjects for Blocks 5-10 were 12,11, 11, 10, 10, and 9, ity of the cell. Accordingly, the data from
respectively. Vertical bars denote ±1 SE.)
such neurons should be set aside. In contrast, a cell that does not develop discharge
plasticity in an animal that does develop a
animals had net positive savings scores, 1 behavioral conditioned response can be said
had a zero savings score, and 2 had negative to be functionally nonplastic for that situsavings scores.
ation because the alternative explanation
of an inadequate preparation can be rejected.
Location of Neurons
,
As noted above, data were obtained from
Data were obtained from 21 neurons with 21 neurons in primary auditory cortex. The
recording sites verified histologically in pri- data from 2 neurons were eliminated from
mary auditory cortex. The laminar sites of the total sample because the animals failed
recording could be determined in 15 cases. to acquire the pupillary dilation condiThirteen sites were in infragranular layers tioned response and the neurons developed
V and VI, and two sites were in layer IV. neither background nor evoked discharge
This distribution is insufficient for corre- plasticity. In all other cases in which dislating anatomical lamina with neurophysi- charge plasticity did not develop, there was
ological data. The results presented here independent evidence of the adequacy of
should be regarded as representing mainly the preparation.
the deep lamina of primary auditory cortex.
Neuronal Data—Interpretation
of Negative Findings
The establishment of behavioral conditioned responses provides a framework
within which to interpret neuronal activity
during conditioning procedures. As explained previously (Weinberger, 1982a,
1982b), we do not seek the neural circuit
underlying the pupillary dilation condi-
Evoked Activity
For the 19 cells in the analysis set, 14
attained the criterion of discharge plasticity. However, we excluded any neurons
that, although meeting this criterion, were
simply continuing a trend existent during
sensitization. This was accomplished by
computing the slopes of functions for sensitization and conditioning, on a trial-by-
176
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
Table 1
Effects of Conditioning on Evoked Discharges
Increase
Cell no.
IB
5B
6C
7E
8A
Pupil
6
17
7
11
8D
Decrease
Evoked
25
9
7
12
11
7
No change
Cell no.
Pupil
3B
5D
5E
7C
16
6
11
6
10A
27
6
27
10B
38
10
Evoked
6
6
32
Cell no.
Pupil
1C
1D2
3E*
6A1
12
8
(-)
18
_—
(-)
16
7
10
(-)
—
—
—
6
11.83
4.40
7
—
—
6B
7B*
8B
8C
9A
n
M
SD
4
10.25
3.20
6
11.83
6.77
n
M
SD
6
17.33
12.80
6
14.50
11.83
n
M
SD
Evoked
—
—
(-)
—
* Deleted from analysis because neither pupillary learning nor neuronal plasticity developed.
trial basis. Cells that had the same slope
during conditioning as during sensitization
were considered not to be plastic. Two neurons failed the slope test and are hereafter
classed as nonplastic with respect to evoked
activity. Thus, 12 cells (63%) were classified as plastic.
Changes in evoked discharges developed
rapidly and were evident during the first
block of 5 trials. Statistical criterion was
attained on the average in 13.17 trials. Six
neurons developed increased responses (M
= 11.83), and six developed decreased responses (M = 14.50; Table 1). Functions
for evoked activity are presented in Figure
2. These functions are significantly different from each other (Mann-Whitney U
tests: increases vs. decreases, p < .001; increases vs. no change, p < .001; decreases
vs. no change, p < .001). Examples of increases in evoked discharges are given in
Figures 3B and 4 (cell 18D) and decreases
in Figures 3A and 4 (cell 6C).
Kraus and Disterhoft (1982) reported
that some portions of the evoked discharge
of neurons in auditory association cortex of
the rabbit are affected differently than others, during conditioning of the nictitating
membrane response. Therefore, we analyzed separately various portions of evoked
activity, in particular discharges having a
latency less than 50 ms and longer latency
250 -
a. -150
-200
-250
•300
•—•INCREASE n-6
o - - o DECREASE n= 7
A — A NO CHANGE n=7
-350
t
1 2 3
1 2 3 4 5 6 7 8 9
SENSITIZATION
CONDITIONING
10
BLOCKS OF FIVE TRIALS
Figure 2 Group functions for changes in evoked
discharges, sorted according to whether the criterion
of discharge plasticity attained was for increases or
decreases, or failure to meet the criterion ("no
change"). (Each point is the mean percentage change
in evoked discharges for blocks of five trials relative
to the last five-trial block during the sensitization
period. Number of cells for each block during conditioning:
increases,
6,6,6,5,4,3,3,3,3;
decreases,
7,7,7,7,7,7,7,6,6,4; no change, 7,7,7,7,6,5,5,4,4,4. Note
that the development of increased and decreased
evoked discharges is evident during the first block of
conditioning [Trials 1-5]. Vertical bars denote ±1SE.)
177
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
A
48-
B
SENSITIZATION
TR 11-15
1-3E
36-
1000
129630
SENSITIZATION
TR 6-15
1-6
10 ms bin
•II.IAII.JI,!! . . , ....
0
129630
..l. r
1000
CONDITIONING
TR 1-10
-fcj^ ..AI L I . .,.. .. . . . f l y , \','
1000
500
2963-
1000
f
500
o- ... In . A . a
,
1000
TR 11-20
,,L
\
' , 1,—i ' > " M ».
msec
1000
msec
Figure 3. Representative poststinuulus histograms for two neurons that developed discharge plasticity during conditioning. (In this, and Figures 4 and 7, the horizontal bars below the abscissa of each
graph denote presentation of the acoustic conditioned stimulus. A: Cell I-3E—each histogram is the
sum of discharges for 5 consecutive trials, as designated. Background discharges were unaffected by
conditioning, whereas discharges evoked by the conditioned stimulus decreased. B: Cell 1-6—each
histogram is the sum of 10 consecutive trials, as indicated. Background discharges decreased during
conditioning, whereas evoked discharges did not develop a statistically significant change relative to
background activity.)
activity. We were unable to find consistent
effects of conditioning on various portions
of the evoked response.
The probability of developing discharge
plasticity was unrelated to whether the cell
was recorded during the first training session for an animal or a later session, x2(l>
N = 18) = 0.078, p > .05.
Comparisons of the pupillary and neuronal evoked data indicated that there was
no significant difference in trials to criterion for the entire group (pupil M = 11.81;
neuronal M = 13.17), £(24) = 0.89, p > .05.
Within subjects, the pupil reached criterion
(if at all) more rapidly than did changes in
neural activity (if at all) in 9/19 cases, more
slowly in 8/19, and at the same time in 3/
19 cases. There was no significant difference between the rates of change for this
group (pupil M = 12.25; evoked M = 15.75,
Mann-Whitney U test, p < .05). For those
12 cells attaining criterion, the pupil also
attained criterion in 8 cases. There was no
significant difference in trials to criterion
for this group (pupil = 12.25; evoked =
15.75, Mann-Whitney Utest,p> .05). Also,
within subjects, the pupil attained criterion
more rapidly than evoked activity in 4/12,
more slowly in 7/12, and at the same time
in 1/12 cases. Overall, then, there was no
statistically significant difference between
the rate of pupillary learning and the rate
at which evoked discharge plasticity developed.
SENSITIZftTION
SENSITIZATION
12
</> 9
I-6B
10 ms bin
SENSITIZATION
TR 1-15
TR 1-15
TR 1-15
UJ
* 6a.
<" 3-
500
1000
1000
CONDITIONING
CONDITIONING
CONDITIONING
12-
TR 1-15
TR 1-15
1000
TR 1-15
<n 9UJ
* 6O_
<" 3-
oil
JJUJJUL
500
I2i
1000
12-
TR 16-30
1000
jOOO
1000
1000
TR 16-30
in 9
<n
UJ
UJ
5£ 6-
* 6
<" 3 - LJILJLI
u> 3
0
12
i
CL
500
1000
500
p
TR 31-45
oo 9
UJ
D
¥ 6
8>3
500
1000
Figure 4 Representative poststimulus histograms for three neurons that developed discharge plasticity during conditioning. (Each histogram is the
sum of 15 consecutive trials, as indicated. Left: Cell I-8B—background discharges increased over the course of conditioning, whereas evoked activity
was not significantly altered. Middle: Cell I-6C—background activity was not altered by conditioned, whereas evoked discharges decreased. Right: Cell
I-18D—background discharges were not changed, whereas evoked activity increased during conditioning.)
1000
179
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
Background Activity
Twelve neurons attained the criterion for
discharge plasticity. Data from one cell
were discarded because the slopes of its
changes in discharges from sensitization
and conditioning were equal. The 11 cells
remaining attained criterion on the average
in 22.91 trials. Seven neurons developed
increased background activity (M = 23.00
trials; e.g., Figure 4, cell 8B). Four neurons
developed decreased discharges (M = 21.25
trials; e.g., Figure 3B). Changes in background activity typically were initiated during the first block of five conditioning
trials. The cells that were classified as unchanged (n = 8) tended toward increases in
background discharges, but these were not
maintained consistently at a level sufficient
to attain the statistical criterion. Those
cells that developed decreases had a small
(20%-50%) but highly consistent change.
Functions for background activity are presented in Figure 5. These functions are
significantly different from each other
(Mann-Whitney U tests), increases vs. decreases, p < .001; increases vs. no change,
p < .01; decreases vs. no change, p < .001).
Overall, the direction of change during
conditioning was opposite to that during
sensitization. Thus, neurons that developed
increased background discharges during
CS-US pairing exhibited decreased background activity during the preceding sensitization control period, and vice versa for
neurons that developed decreased background activity.
The pupil reached criterion more rapidly
than did changes in background activity in
13/20 cases, more slowly in 4/20 cases, with
3 ties. This outcome is statistically significant (p < .05, two-tailed binomial test). In
8 cases, both pupil and background attained
criterion, the former being significantly
faster than the latter (pupil M = 11.39,
background = 22.38, Mann-Whitney U
test, p < .001). In fact, the pupil reached
criterion more rapidly than did background
activity in all 8 cases (p < .002, binomial
test). Thus, across-subjects and withinsubjects comparisons indicated that the development of the pupillary conditioned
response preceded the development of
changes in background activity.
1 2 3
1 2 3 4 5 6 7 8 9
SENSITIZATION
CONDITIONING
BLOCKS OF FIVE TRIALS
10
Figure 5. Group functions for changes in background
discharges, sorted according to whether the criterion
attained was for increases or decreases, or failure to
meet the criterion. (Each point is the mean percentage
change in background discharges for blocks of five
trials relative to the last five-trial block during the
sensitization period. Numbers of cells for each block
during conditioning: increases, 7,7,7,7,7,6,6,6,6,5; decreases, 4,4,4,4,3; no change, 8,8,8,7,6,6,6,4,4,4. Neurons designated as "no change" did develop increased
background activity, but in each case these cells failed
to attain the criterion of five consecutive trials in
which background activity was greater than the mean
of the last five-trial block during sensitization. Note
that changes in background activity developed rapidly,
being evident during the first block [Trials 1-5] of
conditioning This effect is particularly clear because
the direction of change during conditioning is opposite
to the direction that occurred during the sensitization
period. Vertical bars denote ± 1 SE.)
Relation Between Background and Evoked
Activity
Of the 19 cells in the primary auditory
field, 15 (79%) developed discharge plasticity during conditioning, either for background or for evoked activity, or for both.
Inspection of the data suggested that for
any given neuron, the type of activity
(background or evoked) and the effect of
training (increase, decrease, no change)
were independent (Table 2). This was validated statistically, x 2 ( l , N = 19) = 0.88, p
< .05. However, for the 8 cells that attained
criterion for both background and evoked
activity, the direction of change was different for the two aspects of neuronal activity
180
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
Table 2
Effect of Conditioning on Background Activity
Increase
Cell no.
Pupil
1D2
8
16
6
11
3B
5D
5E
6B
9A
10B
10
11
Decrease
Background
32
32
16
32
7
14
28
Cell no.
Pupil
5B
6C
7E
8A
No change
Background
10
21
43
17
17
7
11
Cell no.
Pupil
IB
1C
6
12
(-)
18
(-)
6
16
7
—
—
(-)
(-)
—
—
—
27
—
7
13.14
7.80
8
—
—
3Ea
6A1
7B*
7C
8B
8C
8D
10A
n
M
SD
6
10.33
3.39
7
23.00
10.44
n
M
SD
3
11.67
5.03
4
21.25
19.60
n
M
SD
Background
* Deleted from analysis because neither pupillary learning nor neuronal plasticity developed.
in all cases (Table 2), which is statistically
significant x 2 ( l , N = 8) = 4.50, p < .05.
The rates of change for evoked and background activity were found to be significantly different: Evoked activity attained
criterion in fewer trials than did background activity, £(21) = 2.27, p < .05.
Relation Between Arousal Level and
Neuronal Discharge Plasticity
The measurement of pupillary diameter
during conditioning provides an opportunity to investigate the relation between
arousal level and neuronal discharge plasticity. The relation between pupillary diameter and arousal level is well established:
increased diameter, increased arousal level,
and vice versa (e.g., Nunnally, Knott,
Duchnowski, & Parker, 1967). It is useful
to distinguish between transient or phasic
arousal and enduring or tonic arousal (Sokolov, 1963). In the present study and its
companion study on secondary auditory
cortex (D. Diamond & Weinberger, 1984),
unconditioned phasic arousal is operationally defined as the dilation that is evoked
by the unconditioned stimulus and attains
peak within 1.5 s of its onset. Tonic arousal
is defined as the baseline (pretrial) level of
the pupil.
To assess the effects of phasic arousal on
neurons in AI, we compared cellular discharges for the 1.5 s immediately preceding
the EDS with discharges for the 1.5 s immediately following this stimulus for every
US trial during the sensitization phase.
This yielded 15 pairs of values which were
then evaluated by the Mann-Whitney U
test for each cell separately. EDS produced
pupillary dilation indicative of phasic
arousal on every trial. Only 6 of 19 cells
were responsive to EDS (Mann-Whitney U
tests, p < .05 or less); 5 cells responded with
increased firing, and 1 cell with decreased
discharges. There were no significant relations between the effects of phasic arousal
and the effects of training, for either background or evoked activity.
Tonic arousal was indexed by the size of
the pupil immediately preceding the onset
of each acoustic stimulus, hereafter referred
to as pupillary baseline. These data were
averaged for consecutive blocks of five
trials and are expressed as percentage
change from the mean value of the last five
CS trials of the sensitization period for each
case. In order to determine the relation of
pupillary baseline to background or evoked
activity, the data were assigned to groups
on the basis of the effects of training on
background and evoked activity, and averaged. Thus, the average pupillary baseline
data were computed for six groups: in-
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
creases, decreases, and no change for background discharges and for evoked discharges, respectively. These findings are
presented in Figure 6.
Two sets of findings emerged from this
analysis. First, with respect to background
60 -
_ i
i
1
2
3
1
2
3
4
5
6
7
SENSITIZATION
CONDITIONING
BLOCKS OF FIVE TRIALS
i
8
i
9
Figure 6. The level of tonic arousal for the various
outcomes of training upon background (A) and evoked
(B) discharges (Tonic arousal is defined as pupillary
baseline, i.e., the level of dilation during intertrial
intervals, preceding presentation of the conditioned
stimulus. Each point is the mean percentage change
of the average level of the pupillary baseline for blocks
of five trials, relative to the last five-trial block during
sensitization. Each function is the change in pupillary
baseline for those neurons that developed increases
[solid circles], decreases [open circles], or no significant change [open triangles] in background and
evoked discharges during conditioning. A: Background
discharges—neurons that developed increased background activity occurred in subjects whose level of
tonic arousal increased throughout CS-US pairing.
Neurons that either developed decreased or no change
in background activity were obtained in subjects whose
level of tonic arousal did not increase. B: Evoked
discharges—cells whose evoked activity failed to
change during conditioning were recorded in animals
whose level of tonic arousal increased during conditioning. Such increased tonic arousal was not associated with neurons that developed evoked discharge
plasticity during conditioning. Vertical bars denote ±
1SE.)
181
discharges, those neurons that developed
increased background activity during CSUS pairing were in subjects that developed
increased tonic arousal during pairing
(Mann-Whitney U test, p < .001). Those
cells that developed decreased background
activity or failed to change in background
activity were in subjects that tended toward
lower, but not significant, levels of tonic
arousal (Figure 6A).
Second, with respect to evoked activity,
those neurons that failed to develop discharge plasticity were in subjects in which
tonic arousal increased during CS-US pairing (Mann-Whitney Utest,p < .02). There
was no statistically significant relation between pupillary baseline and neurons that
developed either increases or decreases in
evoked discharges (Mann-Whitney U test,
p > .05; Figure 6B).
Thus, increasing tonic arousal during
conditioning was compatible with the development of increased background discharges but was incompatible with the development of plasticity of evoked discharges.
Single-Unit Data and Multiple-Unit Data
One reason that the present data for
single neurons were obtained is that multiple-unit data, that is, unsorted discharges
recorded simultaneously from more than
one neuron by a single electrode, may be
insensitive to different types of discharge
plasticity; for example, such data may mask
divergent changes in various neurons. Although this experiment did not involve the
recording of multiple-unit data, it was feasible to construct composite "multipleunit" histograms by combining the records
of all individual neurons. Because this had
to be done laboriously by hand on a binby-bin basis, we undertook this process
only for selected blocks of trials, specifically
for the last 5 CS trials of sensitization and
Trials 16-20 during conditioning. The latter were selected because most discharge
plasticity was present during this part of
training. These compilations yielded the
"multiple-unit" histograms shown in Figure 7. They reveal an effect of conditioning.
182
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
Note that there is an increase in evoked
discharges following onset of the CS during
Trials 16-20 of conditioning relative to the
last 5 trials of sensitization. Decreases that
are evident in single unit data are masked,
as are the changes in background activity
of single neurons.
Discussion
Acoustically evoked pupillary responses
developed progressive increases during the
conditioning phase of training. Such
changes never occurred during sensitization during which the CS and US were not
paired. Further, increments in the pupillary
response developed rapidly, reaching crite-
1000
1000
Figure 7. "Multiple-unit" poststimulus histograms
constructed by summing the number of discharges of
all 21 neurons from which data were recorded during
training. (The numbers of spikes were determined for
500 ms immediately preceding presentation of the
conditioned stimulus and for the 1,000 ms during
presentation of the conditioned stimulus. Histograms
depicted are for the last five trials of the sensitization
period [SENS.] and for a representative five-trial
block [COND.] during conditioning [Trials 16-20].
Notice the increase in the evoked discharge following
onset of the conditioned stimulus during conditioning
relative to sensitization.)
rion in an average of 12 trials. In the present case, a discrimination paradigm, to
demonstrate stimulus-specific pupillary
conditioning, was not employed because it
would have prolonged the duration of training and thus greatly reduced the probability
of obtaining continuous recordings from
single cells during the development of conditioned responses. We have reported that
pupillary conditioning exhibits all of the
major characteristics of Pavlovian conditioned responses: systematic increase in
magnitude due to stimulus pairing, discrimination both within and between modalities, discrimination reversal, conditioned
inhibition, and inhibition of delay (Oleson
et al., 1972, 1973, 1975; Ryugo & Weinberger, 1978; Weinberger et al., 1973). Therefore, the pupillary dilation conditioned response can serve as a framework for the
analysis of the effects of associative processes on the discharges of neurons in auditory cortex. Further, as discussed in Results, it provides a basis for the interpretation of "negative" neuronal data, that is,
instances in which discharge plasticity for
background or evoked activity did not develop during the pairing of the conditioned
and unconditioned stimuli.
A major finding is that discharge plasticity is prevalent in Al neurons during classical conditioning. Of 19 cells, 15 developed
statistically significant changes in either
evoked or background activity, or both.
These effects are attributable to associative
processes, for several reasons. The effects
of CS-US pairing were assessed relative to
a sensitization control period. Stimulus
constancy was assured by neuromuscular
blockade, which eliminates movement of
the head or pinna with respect to the sound
source (Marsh, Worden, & Hicks, 1962;
Wiener, Pfeiffer, & Backus, 1966), contraction of the middle ear muscles (Starr, 1964),
and movement-induced masking noise
(Imig & Weinberger, 1970). Finally, neuronal changes due to alleged sensory feedback from conditioned responses, that is,
the pupillary dilation conditioned response,
can be ruled out because the pupillary musculature does not contain proprioceptors
(Lowenstein & Loewenfeld, 1969).
Although these effects are associative,
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
Kitzes, Farley, and Starr (1978) argued that
such discharge plasticity in sensory systems
may be unrelated to the cue value of the
conditioned stimulus. These experimenters
presented brief tones
continuously
throughout defensive Pavlovian training in
which the CS was white noise. They reported changes in AI single-unit activity to
the tones and periods of silence present
during CS-US intervals relative to intertrial intervals. They concluded that training causes merely a general change in cortical excitability so that evoked discharge
plasticity is not directly related to the significance of the conditioned stimulus. However, this conclusion rests on the assumption that the tone pips were devoid of significance during the CS-US interval, but
Kitzes et al. failed to provide independent
evidence to support this assumption. Quite
the contrary, these stimuli may have had
CS properties because of the particular
training regimen employed. The subjects
were trained initially with a 5-s white noise
CS, followed by electrodermal stimulation.
After establishment of the pupillary conditioned response, they were shifted from a
delay to a trace paradigm, which resulted
in a 0.5-s CS (white noise) followed by 4.5
s of silence during which "neutral" tone
pips could be presented. But the original
training caused conditioning to 5 s of acoustic stimulation, and the pips filling the CSUS interval could have acquired CS value
due to within-modality stimulus generalization, that is, the effective CS was actually
white noise followed by tone pips. A more
severe problem is that the control group
received only tones, not tones unpaired
with EDS, hence the effects were not demonstrably associative. Finally, the relevance of these findings to the acquisition
of response plasticity, as in the present
case, is unknown. Resolution of this particular issue must await more definitive experiments.
Relation Between Pupillary and Neural
Conditioned Responses
The present study replicates and extends
to single neurons two findings reported previously (Oleson et al., 1975): (a) The rates
183
of acquisition of pupillary conditioned responses (CRs) and evoked discharge plasticity are rapid and do not differ from each
other and (b) development of pupillary CRs
and evoked discharge plasticity were not
closely related, for example, conditioned
responses developed during some sessions
in which neuronal plasticity did not appear,
and vice versa. These findings suggest that
the neuronal changes are not causal to the
pupillary CRs. That both develop at the
same rapid rate during associative learning
suggests that they are related to a common
process which has not yet been delineated.
Although neuronal changes did not precede acquisition of the pupillary dilation
conditioned reflex, it is likely that they
precede the acquisition of several other
conditioned responses because the rate of
pupillary conditioning is among the fastest
of any response system (Weinberger, 1982a,
1982b). During defensive conditioning, responses that are not specific to the nature
of the unconditioned stimulus develop conditioned responses more rapidly than do
those systems in which the conditioned response is determined by the specific characteristics of the unconditioned response
(Schneiderman, 1972). Among the former
are pupil, cardiovascular, respiration, and
general skeletal movement. Responses that
are specific to the nature of the US include
limb flexion, eye blink, and extension of
the nictitating membrane. The present
findings indicate that discharge plasticity
for single neurons in primary auditory cortex develops as rapidly as does pupillary
conditioning. Such rapidly developing behavioral and neuronal plasticity may comprise part of the first stage of a two-stage
(Konorski, 1967) or three-stage (Thompson
et al., in press) conditioning process in
which the second stage is the elaboration
of a somatic conditioned response which is
specific to the nature of the unconditioned
stimulus (Weinberger, 1982a, 1983).
The functional role of associatively induced discharge plasticity in primary auditory cortex, and for that matter in other
sensory systems, is unknown. Evoked plasticity may reflect changes in the threshold
functions or in the receptive field properties
of sensory neurons, but appropriate exper-
184
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
iments have not yet been reported It is
known that ablations of auditory cortex
produce deficits in the discrimination of
complex acoustic stimuli (Neff, Diamond,
& Cassady, 1975), and evidence suggests
that auditory cortex is essential for the
appreciation of stimulus constancy or
equivalence (Whitfield, 1979). The fact
that discharge plasticity develops rapidly in
auditory cortex during Pavlovian conditioning suggests that even apparently simple learning situations may be involved in
such complex processes.
Relation to Previous Studies
The present report appears to be the first
in which the discharges of single neurons
were recorded in the primary auditory cortex during the acquisition of a behavioral
conditioned response. Issues relating to auditory cortical unit discharges during the
performance of previously acquired responses are considered in a companion article on auditory cortical field All (D. Diamond & Weinberger). However, there are
several previous reports in which multipleunit activity was recorded during conditioning.
With respect to evoked discharges, there
is consistent evidence that responses in
auditory cortex to an acoustic conditioned
stimulus are augmented during conditioning. This has been found in the cat during
Pavlovian defensive conditioning both in
freely moving and in muscle-blocked subjects (Buchwald et al., 1966; Halas et al.,
1970; Oleson et al., 1975) and during instrumental conditioning (Halas et al.,
1970), in the rat during a hybrid classical-instrumental appetitive task (Disterhoft & Olds, 1972; Disterhoft & Stuart,
1976; Olds, Disterhoff, Segal, Kornblith, &
Hirsh, 1972) and in the rabbit during instrumental avoidance learning (Foster,
Orona, Lambert, & Gabriel, 1980). This
concordance is noteworthy, given the variations in subjects, tasks, and conditions of
training, plus the fact that the data reported for the rat include activity from
cortical sites beyond the limits of auditory
cortex. The present results for single neurons reveal that decreases in evoked dis-
charges also develop and that they do so at
the same rapid rate as do increases in
evoked discharges. Thus it appears likely
that multiple-unit recordings mask decreases in discharge plasticity during learning. This conclusion is underscored by the
analysis in which we constructed "multipleunit" histograms by combining the data
from single neurons (Figure 7).
In regard to background multiple-unit
activity, there is less agreement. Disterhoft
and co-workers reported the development
of decreases in background activity (Disterhoft & Olds, 1972; Disterhoft & Stuart,
1976), whereas we found a pronounced increase in background discharges during differential conditioning and reversal of differential conditioning (Oleson et al., 1975).
A further difference is that those experimenters reported that background activity
changed before evoked activity whereas we
found the opposite relation. The present
study extends this finding to single units:
Evoked activity attained criterion significantly earlier than did background discharges. It is difficult to reconcile the contrary findings, particularly because the concordance regarding evoked activity in studies of multiple-unit activity suggests that
differences in subjects and training conditions may still yield similar findings for
evoked activity. It may be that changes in
background activity are more tightly linked
to the cytoarchitectonically delimited region of primary auditory cortex than are
changes in evoked activity. In any event, it
is now evident that previous studies have
reported either increases or decreases in
background activity whereas the present
study of single neurons has revealed both
increases and decreases under the same
conditions of training and acquisition of a
behavioral conditioned response. Studies,
such as the current investigation, that undertake finer grain analyses than previous
experiments are likely to find complexities
heretofore not discovered. Determination
of the functional role indexed by such findings remains a challenge.
Single-Unit and Multiple-Unit Data
Kraus and Disterhoft (1982) argued for
the importance of obtaining discharge data
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
from single neurons because of the limitations of multiple-unit recordings. We are
in complete agreement with this view, and
the present findings offer empirical support
for the position that multiple-unit records
are not adequate representations of the
changes that develop for single cells; for
example, as pointed out above, multipleunit histograms indicative of response plasticity indicate an increase in evoked discharges during learning. In this study, we
constructed "multiple-unit" histograms by
adding the histograms of single neurons.
These histograms are not identical to standard multiple-unit records because they
were obtained from separate experimental
sessions and various loci and their composition is known. Nonetheless, they also provided a picture of an increase in evoked
response to the CS during conditioning
(Figure 7). Yet the histograms of the individual neurons are heterogeneous, including decreases and no changes as well as
increases during CS-US pairing. Thus,
multiple-unit records are not necessarily
comprised of the discharges of a homogeneous population of neurons, all or most of
which develop an increase in response.
Also, the "masking" of unit heterogeneity
holds for background as well as evoked
activity (Figure 7). Therefore, it would be
incorrect to conclude that the general excitability of sensory cortex simply increases
during conditioning.
Previously, we reported similar results
for discharge plasticity in the magnocellular medial geniculate nucleus (MGm).
Poststimulus histograms of multiple-unit
activity exhibit the same general pattern of
activity and the same sort of increase in
evoked activity during conditioning as reported for the primary auditory cortex
(Ryugo & Weinberger, 1976, 1978). However, changes in the discharges of individual neurons in the MGm also are heterogeneous for both background and evoked
activity (Weinberger, 1982a). Thus, multiple-unit activity overshadows certain aspects of single-unit discharge plasticity in
the thalamus as well as in the cortex. There
is no reason to doubt that the same problem
may exist for multiple-unit recordings from
other parts of the brain, as well. Therefore,
185
appropriate caution should be exercised in
the interpretation of multiple-unit records.
Discharge Plasticity, Conditioning, and
Tonic Level of Arousal
Plasticity of background and evoked discharges was a common event in this study.
Given the caveat that any microelectrode
study may be limited by sampling bias and
that classes of neurons not yet described
may exist in primary auditory cortex, these
findings are in essential agreement with the
observations of Kraus and Disterhoft
(1982), who emphasized the heterogeneity
of discharge plasticity in the auditory association cortex of the rabbit. This can be
seen in the highly variable shapes of poststimulus histograms and in the fact that
conditioning is accompanied by both increases and decreases in discharges as well
as lack of change.
However, one facet of this heterogeneity
may be explicable. With the pretrial size of
the pupil used as an index of the level of
tonic arousal, it was found that increased
tonic arousal was accompanied by the failure of neurons to develop discharge plasticity in evoked activity. Although no causal
roles can be assigned yet, it does seem that
increased tonic arousal level is not conducive to evoked discharge plasticity. This
seems to be a neurophysiological expression
of the well-known Yerkes-Dodson law that
high levels of arousal impair learning and
performance (e.g., for review, see Eysenck,
1982).
Possible Mechanisms of Discharge
Plasticity in Primary Auditory Cortex
The discharge properties of neurons in
AI during conditioning may be, in part, a
function of the combined influences of their
thalamic sources of input, that is, the ventral and magnocellular medial geniculate
nuclei. As the ventral medial geniculate
does not develop discharge plasticity during
conditioning whereas the magnocellular
does (see introduction), the plastic properties of AI neurons might relate to input
from the latter region. In addition, there
may be mechanisms of plasticity intrinsic
186
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
to the cortex and other extrinsic sources as
well. However, there is a striking similarity
between the effects of conditioning on
MGm and the effects on AI neurons. Using
an identical preparation and training regimen, we reported that evoked discharge
plasticity developed at the same rate as for
AI cells, that is, 10-20 trials.
Although the present picture is very incomplete, it is now possible to propose a
preliminary hypothesis of how associative
evoked discharge plasticity may develop in
the primary auditory cortex. The lemniscal,
tonotopic projection from the ventral medial geniculate body (MGv) terminates
densely in the middle layers of AI (I. Diamond, 1979; Herkenham, 1980; Jones &
Rockel, 1971). However, the discharges of
the MGv are not altered by learning, so
that the predominant input to the middle
layers of AI probably reflects only the physical parameters of stimuli. It should be
noted that the rates of discharges of individual neurons in MGv can vary widely in
response to a physically constant stimulus
when background activity changes in the
unanesthetized cat (Humphrey & Orman,
1977; Imig & Weinberger, 1973). However,
the pattern of response is conserved despite
fluctuations in the actual number of discharges (Imig & Weinberger, 1973). Therefore, it is not necessary to hold that primary
auditory cortex always receives the same
number of discharges to the same acoustic
stimulus via the MGv but only that there
is some constant relation between the physical parameters of an acoustic stimulus and
the input from the MGv to primary auditory cortex. In contrast, the magnocellular
medial geniculate body projects to layer I
of AI (Burton & Jones, 1976; Herkenham,
1980; Jones & Rockel, 1971; Ryugo & Killackey, 1974). This arrangement suggests
that the MGm regulates the responses to
acoustic information of neurons whose
soma are in lower layers, perhaps by effects
on apical dendrites that ascend to the upper
lamina.
The failure to develop evoked discharge
plasticity in primary auditory cortex has
been linked in the present study to high
levels of tonic arousal, as discussed above.
The possible mechanisms cannot be con-
sidered yet because there are essentially no
pertinent data on this point. Rather, it
would seem that future inquiry into the
mechanisms of response plasticity in auditory cortex, and perhaps in many other
areas as well, also will have to attempt to
account for such failures to develop plasticity.
Finally, the present findings were obtained from single neurons located mainly
in the infragranular lamina (V and VI)
which are the major sources of subcortical
projections from AI (I. Diamond, 1979;
Kelly & Wong, 1981). It is therefore noteworthy that these "output" neurons are
altered by associative processes. The consequences of associative discharge plasticity in these neurons, and the intracortical
mechanisms that may lead to these
changes, will require extensive detailed
study, including laminar analyses during
learning.
References
Aitkin, L. M. (1973). Medial geniculate body of the
cat: Responses to tonal stimuli of neurons in medial
division. Journal of Neurophysiology, 36, 275-283.
Aitkin, L. M., & Webster, W. R. (1972). Medial geniculate body of the cat: Organization and responses to
tonal stimuli of neurons in the ventral division.
Journal of Neurophysiology, 35, 365-380.
Ashe, J. H., Cassady, J. M., & Weinberger, N. M.
(1976). The relationship of the cochlear microphonic potential to the acquisition of a classically
conditioned pupillary dilation response. Behavioral
Biology, 16, 45-62.
Birt, D., Nienhuis, R., & Olds, M. (1979). Separation
of associative from non-associative short latency
changes in medial geniculate and inferior colhculus
during differential conditioning and reversal in rats.
Brain Research, 167, 129-138.
Birt, D., & Olds, M. (1981). Associate response
changes in lateral midbrain tegmentum and medial
geniculate during differential appetitive conditioning. Journal of Neurophysiology, 46, 1039-1055.
Buchwald, J. S., Halas, E. S., & Schramm, S. (1966).
Changes in cortical and subcortical unit activity
during behavioral conditioning. Physiology and Behavior, 1, 11-22.
Burton, H., & Jones, E. G. (1976). The posterior
thalamic region and its cortical projection in New
World and Old World monkeys. Journal of Comparative Neurology, 168, 249-302.
Cassady, J. M., Cole, M., Thompson, R. F., & Weinberger, N. M. (1973). Neural correlates of asymptotic avoidance and classical conditioned leg flexion.
Experimental Neurology, 40, 207-215.
Cassady, J. M., Farley, G. R., Weinberger, N. M., &
PLASTICITY OF SINGLE AI NEURONS DURING LEARNING
Kitzes, L. M. (1982). Pupillary activity measured by
reflected infra-red light. Physiobgy and Behavior,
28, 851-854.
Diamond, D. M , & Weinberger, N. M. (1984). Physiological plasticity of single neurons in auditory
cortex of the cat during acquisition of the pupillary
conditioned response: II. Secondary field (All). Behavioral Neuroscience, 98, 189-211.
Diamond, I. (1979). The subdivisions of neocortex: A
proposal to revise the traditional view of sensory,
motor, and association areas. In J. M. Sprague & A.
N. Epstein (Eds.), Progress in psychobiobgy and
physiological psychology (Vol. 8, pp. 1-43). New
York: Academic Press.
Disterhoft, J., & Olds, J. (1972). Differential development of conditioned unit changes in thalamus and
cortex of rat. Journal of Neurophysiology, 35, 665679.
Disterhoft, J., & Stuart, D. (1976). Trial sequence of
changed unit activity in auditory system of alert rat
during conditioned response acquisition and extinction. Journal of Neurophysiology, 39, 266-281.
Eysenck, M. W. (1982). Attention and arousal. New
York: Springer.
Feller, W. (1968). An introduction to probability theory
and its applications. New York: Wiley.
Foster, K., Orona, E., Lambert, R., & Gabriel, M.
(1980). Neuronal activity in the auditory system
during differential conditioning in rabbits. Society
for Neuroscience Abstracts, 6, 424.
Freeman, W. J. (1980). Evidence for an olfactory
search image or representation in the EEG of conditioned cats and rabbits. Advancement in Physiological Sciences, 16, 421-429.
Furedy, J. J. (1971). Explicitly-unpaired and trulyrandom CS- controls in human classical differential
autonomic conditioning. Psychophysiology, 8, 497503.
Furedy, J. J., Poulos, C. X., & Schiffman, K. (1975).
Contingency theory and classical autonomic excitatory and inhibitory conditioning: Some problems
of assessment and interpretation. Psychophysiology,
12, 98-105.
Gabriel, M., Miller, J. D., & Saltwick, S. E. (1976).
Multiple unit activity of the rabbit medial geniculate
nucleus in conditioning, extinction, and reversal.
Physiological Psychology, 4, 124-134.
Galambos, R., Sheatz, G. C , & Vernier, B. (1955).
Electrophysiological correlates of a conditioned response in cats. Science, 123, 376-377.
Graybiel, A. (1972). Some fiber pathways related to
the posterior thalamic region in the cat. Brain,
Behavior and Evolution, 6, 363-393.
Halas, E. S., Beardsley, J. V., & Sandlie, M. E. (1970).
Conditioned neuronal responses at various levels in
conditioning paradigms.
Electroencephalography
and Clinical Neurophysiology, 28, 468-477.
Herkenham, M. (1980). Laminar organization of thalamic projections to the rat neocortex. Science, 207,
532-535.
Hopkins, W., & Weinberger, N. M. (1980). Modification of auditory cortex single unit activity during
pupillary conditioning [Abstract]. Society for neuroscience: Proceedings of the Tenth Annual Meeting,
6, 424.
187
Humphrey, G. L., & Orman, S. S. (1977). Activity of
the auditory system related to arousal. Experimental
Neurology, 55, 520-537.
Imig, T. J., & Weinberger, N. M. (1970). Auditory
system multi-unit activity and behavior in the rat.
Psychonomic Science, 18, 164-165.
Imig, T. J., & Weinberger, N. M. (1973). Relationships
between the rate and pattern of unitary discharges
in the medial geniculate body of the cat in responses
to click and amplitude-modulated white noise stimulation. Journal of Neurophysiology, 36, 385-397.
John, E. R. (1961). High nervous functions: Brain
functions and learning. Annual Review of Physiology, 23, 451-484.
Jones, E. G., & Rockel, A. J. (1971). The synaptic
organization in the medial geniculate body of afferent fibres ascending from the inferior colliculus.
Zeitschrift fur Zellforschung und Mikroskopische
Anatomie, 113, 44-66.
Kelly, J. P., & Wong, D. (1981). Laminar connections
of the cat's auditory cortex Brain Research, 212, 115.
Kitzes, L. M., Farley, G. R., & Starr, A. (1978). Modulation of auditory cortex unit activity during the
performance of a conditioned response. Experimental Neurology, 62, 678-697.
Konorski, J. (1967). Integratiue activity of the brain.
Chicago: University of Chicago Press.
Kraus, N., & Disterhoft, J. F. (1982). Response plasticity of single neurons in rabbit auditory association cortex during tone-signalled learning. Brain
Research, 246, 205-215.
Lowenstein, O., & Loewenfeld, I. E. (1969). The Pupil.
In H. Davson (Ed.), The eye (pp. 255-337). New
York: Academic Press.
Marsh, J. T., Worden, F. G., & Hicks, L. (1962). Some
effects of room acoustics on evoked auditory potentials. Science, 137, 280-282.
Merzenich, M., & Kaas, J. (1980). Principles of organization of sensory-perceptual systems in mammals.
In J. M. Sprague & A. N. Epstein (Eds.), Progress
In Psychobiology and Physiological Psychology (Vol.
9, pp. 1-42). New York: Academic Press.
Morest, D. K. (1964). The neuronal architecture of
the medial geniculate body of the cat. Journal of
Anatomy, 98, 611-630.
Morest, D. K (1965). The laminar structure of the
medial geniculate body of the cat. Journal of Anatomy, 99, 611-634.
Neff, W. D., Diamond, I. T., & Cassady, J. H. (1975).
Behavioral studies of auditory discrimination: central nervous system. In W. D. Keibel & W. D. Neff
(Eds.), Handbook of sensory physiology (Vol. 5, Pt.
2, pp. 307-400). New York: Springer Verlag.
Nunnally, J. C , Knott, P. D., Duchnowski, A., &
Parker, R. (1967). Pupillary response as a general
measure of activation. Perception & Psychophysics,
2, 149-155.
Olds, J., Disterhoft, J. F., Segal, M., Kornblith, C , &
Hirsh, R. (1972). Learning centers of the brain
mapped by measuring latencies of conditioned unit
responses. Journal of Neurophysiology, 35, 202-219.
Oleson, T. D., Ashe, J. H., & Weinberger, N. M. (1975).
Modification of auditory and somatosensory system
activity during pupillary conditioning in the para-
188
N. WEINBERGER, W. HOPKINS, AND D. DIAMOND
lyzed cat. Journal of Neurophysiology, 38, 11141139.
Oleson, T. D., Vododnick, D. S., & Weinberger, N. M.
(1973). Pupillary inhibition of delay during Pavlovian conditioning in paralyzed cat. Behavioral Biology, 8, 337-346.
Oleson, T. D., Westenberg, I. S., & Weinberger, N. M.
(1972). Characteristics of the pupillary dilation response during Pavlovian conditioning in paralyzed
cats. Behavioral Biology, 7, 829-840.
Reale, R. A., & Imig, T. J. (1980). Tonotopic organization in auditory cortex of the cat. Journal of
Comparative Neurology, 192, 265-294.
Rose, J. E. (1949). The cellular structure of the auditory region of the cat. Journal of Comparative Neurology, 21, 409-440.
Ryugo, D. K., & Killackey, H. P. (1974). Differential
telencephalic projections of the medial and ventral
divisions of the medial geniculate body of the rat.
Brain Research, 82, 173-177.
Ryugo, D. K., & Weinberger, N. M. (1976). Differential plasticity of morphologically distinct neuron
populations in the medial geniculate body of the cat
during classical conditioning [Abstract]. Society for
Neuroscience- Proceedings of the Sixth Annual Meeting 2, 435.
Ryugo, D. K., & Weinberger, N. M. (1978). Differential plasticity of morphologically distinct neuron
populations in the medial geniculate body of the cat
during classical conditioning. Behavioral Biobgy,
22, 275-301.
Schneiderman, N. (1972). Response system divergencies in aversive classical conditioning, In A. H.
Black & W. F. Prokasy (Eds.), Classical conditioning
II: Current research and theory (pp. 341-376). New
York: Appleton-Century-Crofts.
Segundo, J. P., Galeano, C , Sommer-Smith, J. A., &
Roig, J. A. (1961). Behavioural and EEG effects of
tones 'reinforced' by cessation of painful stimuli. In
J. F. Delafresnaye (Ed.), Brain mechanisms and
teaming (pp. 265-291). Oxford: Blackwell Scientific
Publications.
Shinkman, P. G., Bruce, C. G., & Pfingst, B. E. (1974)
Operant conditioning of single-unit response patterns in visual cortex. Science, 184, 1194-1196.
Siegel, S. (1956). Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill.
Sokolov, E. N. (1963). Perception and the conditioned
reflex. Oxford: Pergamon Press.
Sokolov, E. N. (1977). Brain function: Neuronal mechanisms of learning and memory. Annual Review of
Psychology, 28, 85-112.
Sousa-Pinto, A. (1973). The structure of the first
auditory cortex (AI) in the cat. I. Light microscopic
observations on its organization. Archives of Italian
Biology, 111, 112-137.
Starr, A. (1964). Influence of motor activity on clickevoked responses in the auditory pathway of waking
cats. Experimental Neurology, 10, 191-204.
Thompson, R. F., Clark, G. A., Donegan, N. H., Lavond, D. G., Lincoln, J. S., Madden, J., Mamounas,
L. A., Mauk, M. D., McCormick, D. A., & Thompson, J. K. (in press). Neuronal substrates of learning
and memory: A "multiple-trace" view. In G. S.
Lynch, J. L. McGaugh, & N. M. Weinberger (Eds.),
Neurobiology of learning and memory. New York:
Guilford Press.
Thompson, R. F., Patterson, M. M., & Teyler, T. J.
(1972). The neurophysiology of learning. Annual
Review of Psychology, 23, 73-104.
Voronin, L. L., Gerstein, G. L., Kudryashov, I. E., &
Ioffe, S. V. (1975). Elaboration of a conditioned
reflex in a single experiment with simultaneous
recording of neural activity. Brain Research, 92,
385-^03.
Weinberger, N. M. (1982a). Effects of conditioned
arousal on the auditory system. In A. L. Beckman.
(Ed.), The neural basis of behavior (pp. 63-91). New
York: Spectrum Publications.
Weinberger, N. M. (1982b). Sensory plasticity and
learning: The magnocellular medial geniculate nucleus of the auditory system. In C. D. Woody, (Ed.),
Conditioning: Representation of involved neural
function (pp. 697-710). New York: Plenum Publishing.
Weinberger, N. M. (in press). The neurophysiology of
learning: A view from the sensory side. In N. Butters
& L. Squire (Eds.), The neuropsychology of memory.
New York: Guilford Press.
Weinberger, N. M., Oleson, T. D., & Haste, D. (1973).
Inhibitory control of conditional pupillary dilation
response in the paralyzed cat. Behavioral Biology, 9,
307-316.
Whitfield, I. C. (1979). The object of sensory cortex.
Brain, Behavior and Evolution, 16, 129-154.
Wiener, J. M., Pfeiffer, R. R., & Backus, A. S. M.
(1966). On the sound pressure transformation by
the head and auditory meatus of the cat. Acta Otolaryngolica, 61, 255-269.
Winer, B. J. (1971). Statistical principles in experimental design (2nd ed.). New York: McGraw-Hill.
Winer, J. A., Diamond, I. T., & Raczkowski, D. (1977).
Subdivisions of the auditory cortex of the cat: Retrograde transport of horseradish peroxidase to the
medial geniculate body and posterior thalamic nuclei. Journal of Comparative Neurology, 176, 387418.
Received August 17,1983
Revision received November 29,1983







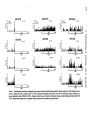




















![Classical Conditioning (1) [Autosaved]](http://s1.studyres.com/store/data/001671088_1-6c0ba8a520e4ded2782df309ad9ed8fa-150x150.png)
