* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Brain days-Part V-Limbic
Brain Rules wikipedia , lookup
Neurophilosophy wikipedia , lookup
Memory consolidation wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Emotion and memory wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Cortical cooling wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Human brain wikipedia , lookup
Time perception wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Environmental enrichment wikipedia , lookup
Emotion perception wikipedia , lookup
Hypothalamus wikipedia , lookup
Neuroplasticity wikipedia , lookup
Executive functions wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Hippocampus wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Biology of depression wikipedia , lookup
Aging brain wikipedia , lookup
Affective neuroscience wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroeconomics wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Insular cortex wikipedia , lookup
Prefrontal cortex wikipedia , lookup
Basal ganglia wikipedia , lookup
Emotional lateralization wikipedia , lookup
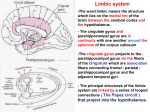
kaan yücel m.d.,ph.d. Outline 1. Introduction to the Limbic System 2. History of the Limbic System 3. Main Players of the Limbic System 4. Functional Anatomy of the Limbic System 5. Limbic System Dysfunction 5.1. Anatomy of major depressive disorder 5.2. Anatomy of some neuropsychiatric disorders 1.1. What does limbus mean? 1.1. What does limbus mean? Limbo Lat. Border Edge Circle Ring 1.2. What is limbic system? survival YOUR MISSION IN LIFE? Two main functions Emotional processing Motivation The Anatomy of Behaviour Medial Temporal Lobe Hippocampus Parahippocampal gyrus Amygdala Emotion Memory The limbic system works to process our emotions and is related to motivation and with its connections with the cognitive parts of the brain helps us to “use our mind” a.k.a. accomplish mental processes. The limbic system structures are telencephalic & subcortical structures. The complex network for the process of emotions and is also related to memory and learning in addition to hippocampus, amygdala and parahippocampus includes: • Cingulate gyrus • Hypothalamus • Major areas in the prefrontal cortex • Striatum • Some thalamic nuclei • Orbitofrontal cortex • Septal area • Some medial components of the midbrain (e.g. VTA) • Habenula … + white matter tracts H.M. Henry Gustav Molaison (1926 –2008) Dr. William Beecher Scoville EPILEPSY Treatment Bilateral removal of medial temporal lobe hippocampus, amygdala & parahippocampal gyrus Names Phone numbers SHORT- TERM MEMORY Great Limbic Lobe Le Grand Lobe Limbique Pierre Paul Broca Cingulate gyrus (L. cingulated=belt) & parahippocampal gyrus form a ring on the medial surface of the mammalian brain. James Papez Papez Circuit A list of structures in the brain and a closed circuit related to emotions Hippocampal formation (Subiculum) → fornix → mammillary bodies Mammillary bodies → mammillothalamic tract → anterior thalamic nucleus Anterior thalamic nucleus → genu of the internal capsule → cingulate gyrus Cingulate gyrus → cingulum → parahippocampal gyrus Parahippocampal gyrus → entorhinal cortex → perforant pathway → hippocampus. Limbic System added Amygdala Septum Pre-frontal cortex to the Papez circuit Paul D. MacLean Klüver-Bucy Syndrome bilateral removal of amygdala and hippocampal formation What happens if we remove the medial temporal lobe of an animal, a monkey? Became docile;”good monkeys”. A tendency towards oral behaviour such as attempting to ingest inedible objects. Hypersexualized behaviour by mounting females of the same and different species. A compulsion to attend and react to every visual stimulus No fear. Change in dietary habits 1848 The patient Phineas Gage 1919 Pneumoencephalography was first introduced. 1936 First “frontal lobotomy” operation was performed on a Kansas housewife. 1958 Nauta added midbrain structures in the limbic system, such as habenula 1967 Freeman operated his last frontal lobotomy and after that this operation was banned. 1974 Ingvar and Franzen first reported frontal lobe hypoperfusion using Single Photon Emission Computed Tomography (SPECT) in a series of chronic schizophrenic patients. 1974 Siemens Medical Solutions (then known as Siemens Medical Engineering) presented the first commercially available computed tomography system. 1975 Ventral striatum concept defined by Heimer 1977 Limbic striatum concept defined by Nauta and Domesick 1977 First MRI scan in history was accomplished. 1982 "Cortical atrophy in schizophrenia and mania: a comparative CT study” published in Journal of Clinical Psychiatry by Nasrallah and his colleagues. 1983 “Ventricular enlargement in child psychiatric patients: a controlled study with planimetric measurements” published in American Journal of Psychiatry by Reiss and his colleagues. 1988 The article of Robert Sapolsky from Stanford University titled as “Glucocorticoid toxicity in the hippocampus: in vitro demonstration” published in the journal Brain Research. 1992 “Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging” published in the journal “Neurology” by Craig Watson and his colleagues. 1994 “Functional magnetic resonance imaging at 1.5 T: activation pattern in schizophrenic patients receiving neuroleptic medication.” published in the journal ”Magnetic Resonance Imaging” by Wenz and his colleagues. the most famous two guys of the limbic system hippocampus & amygdala 3.1. Hippocampal formation/Hippocampus Temporal horn of lateral ventricle HC TERMINOLOGY HIPPOCAMPAL FORMATION VS. HIPPOCAMPUS 1. Hippocampus (proper) Cornu Ammonis (CA) CA1-CA4 2. Dentate gyrus 3. Subicular complex Fornix [Arch] The road/white matter tract from the hippocampus Precommissural fibers 25% Septal area Postcommmissural fibers originate from the subicular complex Mamillary bodies Fornix 3.2. Amygdaloid complex/Amygdala Two main pathways emerging from the amygdala Stria terminalis terminate in the septal area, the medial preoptic area of the hypothalamus, and the bed nucleus of stria terminalis Ventral amygdalahypothalamic tract (Ventral amygdalofugal pathway) main projection pathway from the amygdala to the hypothalamus 3.3. Parahippocampal gyrus 3.4. Cingulate gyrus 3.4.1. Anterior cingulate cortex (ACC) 1=BA25 (subcallosal gyrus) 2=BA24sg (SGPFC) 3=BA32 (paracingulate gyrus) 3.4.2. Posterior cingulate cortex (PCC) Right Cingulate Cortex Subgenual prefrontal cortex (SGPFC) Rostral ACC Caudal ACC PCC Röthlisberger M, Riecher-Rössler A, Aston J, Fusar-Poli P, Radü EW, Borgwardt S. Cingulate volume abnormalities in emerging psychosis. Curr Pharm Des. 2012;18:495-504. 3.5. Limbic structures in the Prefrontal cortex (PFC) 3.5. Limbic structures in the Prefrontal cortex (PFC) • • • • • • DLPFC, dorsolateral prefrontal cortex VLPFC, ventrolateral prefrontal cortex FP, frontopolar cortex OFC, orbitofrontal cortex DMPFC dorsomedial prefrontal cortex VMPFC, ventromedial prefrontal cortex 3.6. Hypothalamus Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for healthNat Rev Immunol. 2005 Mar;5(3):243-51. 3.7. Thalamus Limbic thalamic nuclei Anterior thalamic nuclei Mediodorsal thalamic nuclei 3. 8. Striatum 4 major nuclei (1) corpus striatum Caudate nucleus Putamen Ventral striatum –nuc.accumbens (2) globus pallidus internal and external segments (3) substantia nigra pars compacta pars reticulata (4) subthalamic nucleus 3.1.9. Basal forebrain nuclei 1. Septal nuclei (medial and lateral) 2. Nucleus of the diagonal band of Broca 3. Basal nucleus of Meynert (a group of neurons in the substantia innominata of the basal forebrain) 4. Bed nucleus of stria terminalis (BNST) 5. Medial ventral pallidum 3.10. Structures related to the limbic system in the brainstem Ventral tegmental area (VTA) midbrain dopamine Periaqueductal gray matter (PAG) midbrain Locus coeruleus pons noradrenaline Dorsal raphe nuclei brainstem serotonin Reticular formation brainstem VENTRAL TEGMENTAL AREA (VTA) modulates the processing of memory via dopamingergic fibers that terminate in cortical areas related to the limbic system 5 dopaminergic pathways 1. Mesostriatal pathway Origin: Substantia nigra (and also some from VTA) to the anteromedial and ventral neostriatum 2. Mesolimbic pathway Origin: VTA (and also some from substantia nigra) to the nuc.accumbens, amygdala, ACC, entorhinal cortex, hippocampus 3. Mesocortical pathway Origin: VTA (and also some from substantia nigra) to the neocortex (sensory, motor, and association cortices, such as prefrontal, temporal and insular cortices) 4. Mesodiencephalic pathway Origin: VTA and Substantia nigra to the several thalamic and hypothalamic nuclei. 5. Mesorhombencephalic pathway Origin: VTA- Substantia nigra to the superior collicilus, reticular formation,PAG, and spinal cord Movement, cognition, emotion, and positive reinforcement are influenced by mesostriatal, mesocortical, and mesolimbic dopamine systems. There is nigral degeneration attending Parkinson's disease and Parkinson’s disease is related to deficit in the nigrastriatal (mesostriatal) pathway and lower tone of dopamine as a result. PERIACQUEDUCTAL GRAY MATTER (PAG) o Anatomic & functional interface between forebrain &lower brainstem o A major role in integrated behavioral responses to internal (e.g., pain) or external (e.g., threat) stressors o A critical component of “emotional motor system.” PERIACQUEDUCTAL GRAY MATTER (PAG) o Involvement in affective and motivational processes o Suggested that PAG is a core region involved in human emotion. o A role in emotional coping strategies. o Receives prefrontal and hypothalamic afferents. 3.11.1. Habenula • A pair of small nuclei located above the thalamus at its posterior end • Rostral to the posterior commissure • Habenular commissure runs between them 3.11.1. Habenula medial habenular nucleus (MHb) lateral habenular nucleus (LHb) involved in motivational control of behavior wide range of behaviors including • Olfaction • Ingestion • Mating • Endocrine and reward function • Pain and analgesia 3.11.1. Habenula Stria medullaris thalami Connects hypothalamus and other limbic system structures with habenula Dorsomedial surface of the thalamus Limbic system influences the brainstem reticular formation. Habenulointerpeduncular tract (fasciculus retroflexus) From habenula to the interpedincular nucleus which is located between the cerebral peduncles in the midbrain. Dorsal diencephalic conduction (DDC) system Limbic forebrain –midbrain /hindbrain connection The other is Medial Forebrain Bundle 3 components Habenula Stria medullaris Fasciculus retroflexus • Cognitive processes, in particular relating to spatial learning and attention • Circadian rhythms • Involvement of the LHb in defence responses to stressful stimuli Dorsal diencephalic conduction (DDC) system DDC circuitry is implicated in various psychological conditions depression anxiety schizophrenia neuropathological responses to addictive drugs Dorsal diencephalic conduction (DDC) system LHb, serves as a convergence point for limbic and basal ganglia circuits. Given the broad involvement across numerous functions and behaviors, the region seems to have a basic but vital role as an evaluator, and acts as a major point of convergence where external stimuli is received, evaluated, and redirected for motivation of appropriate behavioral response. 3.11.2. Medial Forebrain Bundle In addition to the dorsal diencephalon conduction system/circuit, there is the medial forebrain bundle carrying information from the limbic forebrain to the midbrain. As you might guess, (VTA, dopamine) we are talking about the reward and pleasure. Actually the street drugs like MFB runs from medial regions of cocaine stimulate MFB. the mesencephalon to forebrain regions, especially the ventral striatal nuclei (e.g., nucleus accumbens) and medial frontal cortices. 3.12. Insula o A cortical structure with extensive connections to many areas of the cortex and limbic system. o Integrates external sensory input with the limbic system and o Integral to the awareness of the body’s internal state (interoception). SELF o Processing emotional and sensory stimuli Anterior insula Extensive and reciprocal connections to limbic areas, higherorder visual areas, olfactory areas and to the posterior insula Processing the emotional component of interoceptive awareness , the anticipation and evaluation of emotional stimuli, and self-awareness. Involved in extending self-awareness to other ‘selves’ through action. EMPATHY Posterior insula Reciprocal connections from higher-order visual areas, auditory processing areas, somatosensory areas and to the anterior insula. Directly experienced, multimodal sensory processing; particularly somatosensory processing 1) Topographical-functional organization 2) Functions of the structures within the circuits of the limbic system 4. 1. Hippocampus and amygdala: Emotions & Memory Right hippocampus Left hippocampus Spatial memory Verbal memory Anterior hippocampus rostral hypothalamus and amygdala HPA-axis control Stress Posterior hippocampus spatial memory Multiple functions of the hippocampus Learning and memory Mood regulation - Affect - Emotional Behavior Regulation of HPA axis Pain Erectile function Attention When you meet someone you know at the street Hippocampus...... Context Amygdala..... Emotions Emotional Memory Fear Amygdala 4.2. Reward-Punishment System Reward is a central component for driving incentive based learning, appropriate responses to stimuli, and the development of goaldirected behaviors. T he reward circuit comprises several cortical and subcortical regions forming a complex network that mediates different aspects of incentive-based learning, leading to control circuits. Emotions have been defined as a group of interrelated superior cerebral functions, resulting from states of reward and punishment. Behavioural rewarding conditions reinforce certain reactions in a quest to experience a favourable result, which brings satisfaction, comfort, or wellbeing. Main neurotransmitter: Dopamine Released @ VTA in midbrain Serotonin modulates dopamine release. Glutaminergic inputs from PFC, hippocamps and amygdala to VTA and nucleus accumbens influence the mesocortical dopamine system. Medial forebain bundle: An important tract between VTA and forebrain ACC attention, emotional processing and self monitoring OFC behavioral inhibition, signaling of expected outcomes and reward/punishment sensitivity. Understanding the roles of key brain areas in reward requires analysis of network interactions between subareas of the amygdala, nucleus accumbens, prefrontal cortex, ventral tegmental area and other structures involved in reward and motivation. The projections descending from the PFC to nucleus accumbens, amygdala and other limbic brain regions have regulatory control over reward-seeking behavior. Here we have four main cores in the reward-punishment systems. 1) VTA in the midbrain 2) Basal ganglia 3) Prefrontal cortex 4) Hippocampus and amygdala * * Reward circuit embedded within Cortico-basal ganglia circuit central component for developing and monitoring motivated behaviors ACC, OFC, ventral striatum, ventral pallidum, midbrain + dorsal prefrontal cortex, amygdala, hippocampus, thalamus, and lateral habenular nucleus, and specific brainstem structures such as the pedunculopontine nucleus, and the raphe nucleus key components in regulating the reward circuit Ventral striatum receives its main cortical input from OFC and ACC, (1) Fibers from and a massive dopaminergic input from the midbrain . different prefrontal areas converge within subregions of the striatum. (2) Through the organization of striato-nigro-striatal projections, the VS can influence the dorsal striatum. (3) The cortico-thalamic projection carries information from rewardrelated regions, through cognitive, and motor controls. 4.3. Memory & Learning: Anatomy Learning acquisition of new information Memory keeping it Long-term memory •Declerative memory Medial temporal lobe •Non-declerative (procedural memory) Striatum 4.3.3. Anatomy of working memory The network of cortical and subcortical areas typically includes posterior brain regions (eg, visual association areas) that are linked with prefrontal regions to form a circuit. Particularly, the dorsolateral prefrontal cortex (DLPFC) is involved with working memory. 4.3.4. PFC & memory Whereas the medial temporal lobe has traditionally been associated with the encoding, storage and retrieval of long-term memories, the prefrontal cortex has been linked with cognitive control processes such as selection, engagement, monitoring and inhibition. 4.4. Anatomy of cognition & emotion Two circuits & Crossing roads cognition means thinking and emotion means feeling TWO MAIN CIRCUITS IN THE BRAIN COGNITIVE CIRCUIT EMOTION CIRCUIT DORSAL CIRCUIT VENTRAL CIRCUIT The cognitive networks inhibit the ventral circuit. Dorsal (cognitive) circuit o o o o o Hippocampus Dorsolateral prefrontal cortex (DLPFC) Dorsal regions of the anterior cingulate cortex (ACC) Parietal cortex Posterior insular region Modulates selective attention, planning and effortful regulation of affective state. Ventral (limbic) circuit structures: o Amygdala o Insula (Particularly, anterior insula) o Ventral striatum o Ventral regions of the anterior cingulate cortex (ACC) o Orbitofrontal cortex (OFC) and medial PFC We can also add “hippocampus” in the limbic circuit. It is possible that the altered emotional regulation or cognition found in all of these syndromes involves aberrant function of these circuits, but perhaps with different patterns on a molecular level. Phillips et al. 2003 MPFC (medial prefrontal cortex) and related limbic and striato-pallido-thalamic structures organize emotional expression. 4.4. 1. Cerebellum & vermis The cerebellum is a great modulator of neurologic function, and that whatever it does to motor control, it also does the same thing to other kinds of behaviors. 4.4. 1. Cerebellum & vermis For many years, functions related only to movement, gait, posture, and balance were attributed to the cerebellum. However, some studies have suggested a possible involvement of the cerebellum in cognition, emotion processing and behavior. 4.4. 1. Cerebellum & vermis 4.4. 1. Cerebellum & vermis Vermis or posterior vermis Limbic cerebellum Anterior 4.4.1. Loops through the basal ganglia Functional anatomy of basal ganglia 5 LOOPS THROUGH THE BASAL GANGLIA 1. LIMBIC CIRCUIT Anterior part of ACC- Ventral striatum (nuc.accumbens) behavior control and adaptation of behaviours after making a mistake. The damage of this circuit results in emotional disorders especially deep apathy and lack of spontaneity. Lowered mood is accompanied by weakening of affect and motor adynamy. It was shown that the anterior cingulate loop is responsible for correcting behavior following a mistake. 4.4.1. Loops through the basal ganglia Functional anatomy of basal ganglia 5 LOOPS THROUGH THE BASAL GANGLIA 2. PREFRONTAL (ASSOCIATIVE LOOP) Between DLPFC- head of nuc.caudatus choice of aims, planning, programming of the sequence of mental actions and behaviours, switching between sentences (the ability to change attitude flexibly), verbal and spatial working memory, selfcontrol and metacognition (self-consciousness) 4.4.1. Loops through the basal ganglia Functional anatomy of basal ganglia 3. LATERAL OFC LOOP Between lateral OFC aand head of nuc.caudatus initiating social behaviours motivated by an award and in inhibiting behaviours, which can trigger punishment. Incorrect functioning of this circuit may result in disinhibition of behaviours, personality changes, lack of control and emotional liability, as well as irritability and gaiety. The damage of this loop can cause perseverations, which make it difficult to process information from external environment and adaptation of behaviours to a particular situation. Orbito-frontal circuit socially adjusted behaviour and hampering not socially accepted one important for the estimation of the risk of the behavior chosen 4.5. White Matter Tracts of the Limbic System • • • • • • • • • • • • • Alveus, fimbria and fornix Perforant pathway Cingulum Septohippocampal tract Ventral amygdalohypothalamic tract Mammillothalamic tract (mammillary fasciculus) Mammillointerpeduncular tract Mammillotegmental tract Stria terminalis Stria medullaris (thalami) Anterior commissure Diagonal band of Broca Habenulointerpeduncular tract 4.5. Default Network Must have a total of 5 symptoms for at least 2 weeks. One of the symptoms must be depressed mood or loss of interest. 1. Depressed mood. 2. Markedly diminished interest or pleasure in all or almost all activities. 3. Significant (>5% body weight) weight loss or gain, or increase or decrease in appetite. 4. Insomnia or hypersomnia. 5. Psychomotor agitation or retardation. 6. Fatigue or loss of energy. 7. Feelings of worthlessness or inappropriate guilt. 8. Diminished concentration or indecisiveness. 9. Recurrent thoughts of death or suicide. Response to stress Response to stress is about “fight” or “flight”. In any case, you need some energy to run or have the battle (or go back to sleep- you wake up early and study- or study all night). Response to stress The sympathetic-adrenomedullary (SAM) system implicated in releasing adrenaline which facilitates the flight/fight response and the HPA axis are regulated by “limbic brain circuits that involve the amygdala, hippocampus and orbital/medial prefrontal cortex. Depressed brain brain networks that normally regulate the evaluative, expressive and experiential aspects of emotional behavior in the pathophysiology of mood disorder Limbic-cortical-striatal-pallidal-thalamic circuits (LCSPT) formed by connections between o Orbital and medial prefrontal cortex (OMPFC) o Amygdala o Hippocampus o Striatum o Thalamic nuclei o Ventral pallidum Depressed brain o The most prominent volumetric abnormality reported to date has been a reduction in gray matter in the left anterior cingulate cortex (ACC) ventral to the corpus callosum genu (i.e., ‘‘subgenual’’). o Reductions in the size of genu and splenium of corpus callosum also have been observed in patients with MDD. Depressed brain Dysregulation Activity Ventral prefrontal areas; ventrolateral prefrontal cortex (vlPFC), in subgenual anterior cingulate cortex (sgACC) and in the amygdala Activity DLPFC, dorsomedial PFC History of abuse in childhood a significant factor in depression and suicidal diathesis Hippocampal volume loss Decrease in ACC volume with increase in amygdala volume in suicidal patients with MDD 5.2.1. Anorexia Nervosa (AN) Common in adolescent girls Affects 0.3% to 0.7% of the population Symptoms on eating behavior and body image distortions 5.2.1. Anorexia Nervosa (AN) Disturbances of limbic and cognitive neural circuits -perhaps related to altered serotonin and dopamine metabolismImpaired balance between the ventral (limbic) circuit and dorsal (cognitive) circuit 5.2.1. Anorexia Nervosa (AN) ACC; processing the conflict between available behaviors and outcomes e.g., “shall I eat this cake and satisfy my hunger now or shall I not eat this cake and stay thin?” OFC; adjusts reward valuation based on the current body state of the individual. DPLFC switches between competing behavioral programs based on the error signal it receives from the ACC. Insula; interoceptive dysfunction Striatum; altered reward modulation 5.2.2. Obsessive Compulsive Disorder (OCD) Characterized by intrusive anxiety-provoking thoughts (obsessions) and subsequent repetitive time-consuming ritualistic behaviours (compulsions) Imbalance between the dorsal and ventral frontal– striatal circuits in the symptoms and cognitive deficits. Ventral frontal–striatal impairment contributes to the inability to inhibit cognitions and behaviours. Dorsal frontal–striatal failure contributes to impaired executive performance and enhanced error monitoring processes, corresponding to the patients' frequent feeling that ‘something is wrong’. 5.2.2. Obsessive Compulsive Disorder (OCD) Cortico-basal ganglia-thalamo-cortical loops; deficits observed in the orbitofrontal cortex (OFC), PFC, anterior cingulate cortex (ACC), thalamus, and caudate nucleus Orbitofrontal-striatal-thalamic circuits; associated with implicit processing deficit and intrusive symptoms. Other structures with abnormalities in OCD: insula, amygdala and the white matter tract; cingulum 5.2.3. Posttraumatic Stress Disorder (PTSD) Developed by some individuals after exposure to emotionally severe traumatic events such as childhood abuse, war traumas, and natural accidents, among others. A circuit of brain regions, including the hippocampus, prefrontal cortex (including anterior cingulate), and amygdala, in the symptoms of PTSD. During emotion regulation, an increase in vmPFC activity is associated with a decrease in amygdala activity as well as a decrease in the experience of negative affect. The relation between vmPFC and amygdala in emotion regulation is suggested to be abnormal in patients with PTSD as well as in MDD. Insula is also found abnormal in PSTD. 5.2.4. Social Phobia Fear of social or performance situations when the person feels they are under scrutiny by others and fears that they will do something embarrassing or humiliating. Right DLPFC & left parietal cortex Greater activation in these areas may be related to planning affective responses and to awareness of body position. A neural circuit involving the striatum, thalamus, amygdala and cortical structures may provide a framework for integrating much of the current knowledge on the neurobiology of social phobia. 5.2.5. Autism spectrum disorders (ASD) Characterized by deficits in social interaction, communication, and stereotyped or repetitive behaviors Increased total brain volume Most popular structures investigated in ASD: cerebellum (vermis) & amygdala Also deficits in Hippocampus Striatum. Fusiform face area (FFA) Cingulate cortex Thalamus Insula Corpus callosum Brainstem Default network 5.2.6. Pathological Gambling (PG) Inappropriate, persistent, and maladaptive gaming behavior VTA- OFC Dysfunction in the cortico-striatal networks from the prefrontal cortex to midbrain Dysfunction in the DLPFC and ventral striatal networks