* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download A soft-wired hypothalamus
Neural coding wikipedia , lookup
Biological neuron model wikipedia , lookup
Apical dendrite wikipedia , lookup
Single-unit recording wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Aging brain wikipedia , lookup
Multielectrode array wikipedia , lookup
Subventricular zone wikipedia , lookup
Haemodynamic response wikipedia , lookup
Development of the nervous system wikipedia , lookup
Central pattern generator wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neuroplasticity wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Nervous system network models wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Selfish brain theory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Chemical synapse wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Synaptic gating wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Circumventricular organs wikipedia , lookup
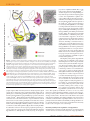
© 2005 Nature Publishing Group http://www.nature.com/natureneuroscience F E E D I N G R E G U L AT I O N A N D O B E S I T Y PERSPECTIVE The hardship of obesity: a soft-wired hypothalamus Tamas L Horvath Food intake and energy expenditure are determinants of metabolic phenotype and are regulated by the CNS. Although humans have a well-balanced homeostatic feedback loop, obesity and metabolic disorders are spreading rapidly and carry a heavy toll of morbidity and mortality. The past decade has witnessed major advances in the understanding of basic metabolic processes, the brain circuitry that determines appropriate and, but, inappropriate behavioral and humoral responses to changing metabolic cues remains largely ill defined. This review summarizes current knowledge of the brain anatomy that supports food intake and energy expenditure and discusses cellular mechanisms such as synaptic plasticity that may provide clues toward the development of successful central therapies to combat metabolic disorders, including obesity and diabetes. on leptin-deficient obese ob/ob mice8–11. Subsequently, receptors for leptin were cloned and localized to structures in the hypothalamus12,13, some of which had been implicated in the regulation of metabolism by lesion studies. However, leptin bound and its receptors accumulated predominantly in a ventromedial hypothalamic structure, the arcuate nucleus14, which was not specifically suggested by the lesion studies. Further support for a role for this small hypothalamic nucleus in energy homeostasis came when the melanocortin system was found to be involved in obesity15,16. Because melanocortin is found in neurons of the arcuate nucleus17, it was logical to test whether leptin affected metabolism mediated by the arcuate nucleus melanocortin system—as indeed was found to be the case18. An attractive model came from further analysis of components of the central melanocortin system19, which today is considered to be the key component in the regulation of feeding and energy expenditure. Obesity is a metabolic state in which excess fat is accumulated in peripheral tissues, including the white adipose tissue, muscle and liver. Sustained obesity has profound consequences on one’s life, spanning from superficial psychological symptoms to serious co-morbidities that may markedly diminish both the quality and length of life. Paradoxically, compelling evidence has been gathered during the last 100 years suggesting that the underlying cause of fat accumulation in peripheral tissues arises, at least in part, from the CNS1–4. Since the dawn of modern neurobiology at the end of the nineteenth century, both intellectual and experimental attempts have been made to identify and isolate the brain’s role in energy metabolism regulation. For example, based on the emerging experimental observations on brainstem regulation of respiration at the time, Sherrington suggested that a similar blood link between the periphery and brain should exist for the regulation of food intake5. Others also concluded that there must be feeding regulation signals from the periphery to the brain, implicating gut-born humoral signals as responsible for appropriate brain responses to changing metabolic needs6,7. Of the various brain regions, the hypothalamus emerged as one of the critical sites for the regulation of energy homeostasis during degeneration studies2 in the 1940s and 1950s. Destruction of the hypothalamic ventromedial (VMH), paraventricular (PVH) and dorsomedial (DMH) nuclei induced hyperphagia. In contrast, discrete lesions placed in the lateral hypothalamus reduced food intake. A fundamental breakthrough came in 1994 and 1995 when the gene encoding the adipose signal leptin was discovered and its product tested Hypothalamic regulators of feeding and energy expenditure A simple and appealing model of the ‘heart’ of the central feeding center followed from two discoveries: that the arcuate nucleus melanocortin system is critical in mediating leptin’s effect on metabolism and that there are distinct local counterparts of the pro-opiomelanocortin (POMC) cells: agouti-related protein– and neuropeptide Y–producing cells (NPY/AgRP cells) in the arcuate nucleus20. In this model, activation of POMC neurons by leptin21,22 triggers the release of α-melanocyte–stimulating hormone (α-MSH) from POMC axon terminals, which in turn activates melanocortin receptor 4 (MC4R), leading to suppressed food intake and increased energy expenditure. Simultaneously, leptin suppresses the activity of arcuate nucleus NPY/ AgRP neurons21,22, which otherwise would antagonize the effect of α-MSH on MC4Rs through the release of AgRP19. Not only does the NPY/AgRP system antagonize anorexigenic melanocortin cells at their target sites, where MC4Rs are located, but it also very robustly and directly inhibits POMC perikarya through both NPY and the inhibitory neurotransmitter GABA22, which acts through basket-like synaptic innervation of POMC cells by NPY/AgRP cell terminals23. This apparent unidirectional anatomical interaction between the NPY/AgRP and POMC perikarya23 is of potential significance, as it provides a tonic inhibition of the melanocortin cells whenever the NPY/AgRP neurons are active. Because there is no constitutive direct feedback mechanism from the POMC cells to disengage the NPY/AgRP neurons, this simple anatomical constellation (Fig. 1) may provide the easiest explanation of why the baseline blueprint of feeding circuits is more likely to promote feeding than satiety. Although this bias toward positive energy balance is necessary from an evolutionary perspective, it is also likely to contribute to the etiology of metabolic disorders, such as obesity. The majority of both NPY/AgRP and POMC neurons are small cells characterized by infolded nuclei with a 1:1 ratio of inhibitory to excitatory Tamas L. Horvath is in the Department of Obstetrics, Gynecology & Reproductive Sciences and the Department of Neurobiology, Yale University School of Medicine, 333 Cedar Street, New Haven, Connecticut 06510, USA. e-mail: [email protected] Published online 6 April 2005; doi:10.1038/nn1453 NATURE NEUROSCIENCE VOLUME 8 | NUMBER 5 | MAY 2005 561 © 2005 Nature Publishing Group http://www.nature.com/natureneuroscience PERSPECTIVE projections of AgRP and POMC fibers to the nucleus of the solitary tract in the hindbrain26,27. Another exception is a set of larger POMC perikarya that also express the anorexigenic peptide cocaine and amphetamine–regulated transcript (CART) in the arcuate nucleus and retrochiasmatic area and that provide innervation of the sympathetic ganglia at thoracic segments of the spinal cord28. This projection, which does not contact by NPY/AgRP fibers, is most likely to be important in the activation of the sympathetic nervous system. An important common target of NPY/AgRP and POMC projections is the hypothalamic parvicellular paraventricular nucleus (PVN). The interplay between AgRP and α-MSH fibers on MC4R-expressing neurons in the PVN is suggested to be the most critical interaction for metabolism regulation. Signals from here may then travel in various directions, including to brainstem nuclei, to spinal cord, to cortex (through various routes), to the thalamus and to portal vessels connecting the hypothalamus to the anterior pituitary. It is intriguing that in this case, once again, an interaction between the arcuate nucleus NPY and POMC neurons in food intake regulation had been proposed years before leptin was discovered or the significance of MC4Rs recognized in metabolism regulation23. The simplicity of the putative hypothalamic feeding center is remarkable, and it suggests Figure 1 Schematic of three hypothalamic peptidergic systems, which show rapid synaptic perikaryal remodeling in response to peripheral metabolic changes. In the arcuate nucleus (ARC), the orexigenic that manipulation of either component of the NPY/AgRP neurons (yellow) have a greater ratio of excitatory (red) to inhibitory (green) input during melanocortin system should shift energy balnegative energy balance (low leptin, high ghrelin in the circulation) than during satiety. Arrows ance. More importantly, if this system is the indicate the direction of synaptic movement after leptin levels increase and ghrelin levels diminish primary regulator of energy homeostasis, then as satiety emerges. In contrast, neighboring anorexigenic α-MSH cells (blue) have a higher ratio of it should be possible to correct metabolic disinhibitory to excitatory input during negative energy balance than during satiety. These two systems orders such as obesity by altering either the have overlapping projections, targeting neurons that express melanocortin receptor 4 (MC4R) in various regions, including the hypothalamic paraventricular nucleus (PVN). MC4Rs (brown membranes NPY/AgRP or POMC circuit. Although genetic in the PVN) may be localized postsynaptically or presynaptically in close proximity to AgRP- and αmanipulations of either component can lead MSH–containing fibers. A substantial proportion of the inhibitory inputs on melanocortin perikarya to an altered metabolic phenotype, it is reasonin the arcuate nucleus arise from local NPY/AgRP neurons. A large proportion of the excitatory able to think that this arcuate nucleus system inputs on NPY/AgRP neurons are from neurons expressing hypocretin/orexin originating in the lateral does not stand alone as the backbone for the hypothalamus (LH). These lateral hypothalamic cells are also orexigenic, and their perikarya are adequate regulation of daily energy balance, as, dominated by excitatory inputs both during negative energy balance and during satiety, although the level of excitatory inputs is blunted during satiety. As shown by the electron micrographs, all three of for example, no successful medical strategies these cell types are frequently in close proximity to capillary vessels (v), indicating a likelihood that have emerged that target this system for the they are directly regulated by circulating leptin or ghrelin. treatment of either positive (obesity) or negative (cachexia) energy balance. Indeed, other hypothalamic systems have also been closely synaptic inputs24. Both neuronal systems are directly targeted by leptin tied to this regulatory mechanism (for review, see refs. 3,4). These and can also be affected by other peripheral metabolic signals, such as include two distinct lateral hypothalamic orexigenic neuronal popughrelin, glucose, insulin and peptide YY, a putative satiety signal released lations producing either melanin-concentrating hormone (MCH)29 from the gastrointestinal tract postprandially in proportion to the calorie and hypocretin/orexin30. The interaction of the melanocortin system content of a meal25. Their localization in ventromedial aspects of the arcu- with other hypothalamic and extrahypothalamic neuronal systems is ate nucleus close to the median eminence, a region with no blood-brain redundant, so that alternative signaling modalities can be used once a barrier between vessels and the parenchyma, means that these neurons pathway is blocked. In addition, the flexibility of the hypothalamic cenmight be reached by circulating metabolic signals in the most effective ter of metabolism may be due not only to redundant interconnectivity manner. Indeed, NPY/AgRP or POMC perikarya or dendrites are often but also to the consequence of its soft wiring24. seen in direct contact with capillaries (Fig. 1), thus increasing the likelihood that these neurons are direct targets of peripheral signals. The pro- Neuronal plasticity in the regulation of energy balance jections of these two systems grossly overlap both within and outside the Underlying this connectivity and the integration of peripheral signals hypothalamus26,27, although they are rarely very long. An exception is of brain circuits associated with feeding and energy expenditure is 562 VOLUME 8 | NUMBER 5 | MAY 2005 NATURE NEUROSCIENCE © 2005 Nature Publishing Group http://www.nature.com/natureneuroscience PERSPECTIVE an intriguing redundancy. This is reflected by the ability of a feeding circuit to rearrange itself to maintain its prior level of output even if one of the components of the normal signaling modality is removed. This indicates that the interactions of hypothalamic neuronal circuits have substantial plasticity, some of which is retained in adulthood. Synapses in the magnocellular system are rearranged during changes in water homeostasis31, and they are rearranged in the arcuate nucleus interneuronal system as a result of variations in the gonadal steroid milieu32. Such synaptic plasticity has not previously been considered to be a critical component in the regulation of daily energy homeostasis. However, our observations24,33 now suggest34 that synaptic plasticity is a key component in the physiological regulation of energy homeostasis, and that under pathological conditions, the synaptic constellation and its plasticity may be impaired. Mice that lack the gene encoding circulating leptin (ob/ob mice) and their wild-type littermates were used in these experiments. The phenotype of ob/ob mice resembles human morbid obesity. Replacement of leptin in ob/ob mice rapidly decreases food intake and triggers weight loss9–11. Thus, ob/ob mice and their wild-type littermates provide an excellent model in which to determine the effects of leptin on the wiring pattern of the hypothalamic peptidergic circuits. Two distinct populations of neurons of the melanocortin system were analyzed: the NPY/AgRP and POMC neurons. Although these studies focused on the arcuate nucleus melanocortin system, it is important to emphasize that regulation of energy metabolism by the brain is organized from various sites that are not limited to the hypothalamus but include other regions, most notably the brainstem35. To more easily and precisely identify the two populations of cells (NPY/AgRP and POMC), we used transgenic mice in which τ-sapphire green fluorescence protein (GFP) is expressed under the transcriptional control of the NPY genomic sequence, or in which τ-topaz GFP is expressed under the transcriptional control of the POMC genomic sequence. We examined the afferent inputs to these neuronal populations of the arcuate nucleus using patch-clamp electrophysiological recording in slice preparations together with stereology to quantify the synaptic density onto these cells. The results showed that leptin-deficient ob/ob mice differed from wild-type mice in the numbers of excitatory and inhibitory synapses and postsynaptic currents onto NPY and POMC neurons. When leptin was delivered systemically to ob/ob mice, the synaptic density rapidly normalized24; this effect could be detected within 6 h—several hours before leptin’s effect on food intake (Fig. 2). These observations clearly indicate that rapid synaptic rearrangement is induced by leptin, and most likely by other metabolic signals as well. In support of the latter, we found that the orexigenic peripheral hormone ghrelin rapidly (within 2 h) shifts input organization of POMC perikarya to support decreased cellular activity24. Because of these rapid synaptic changes and because circulating ghrelin and leptin levels show circadian rhythms in which the peak of one coincides with the nadir of the other36, it is very plausible that the synaptic organization of the melanocortin system undergoes circadian alterations in line with circadian feeding and metabolic patterns. An important question is whether these synaptic alterations influence neuronal activity and, consequently, metabolic phenotype. Although this remains to be proven, these changes occur both before and during the various behavioral and endocrine outputs24. Even if it does not directly affect action potentials, the changing input organization of NPY/AgRP and POMC perikarya should influence the setpoint of these cells under various circumstances. For example, a trans-synaptic excitatory signal is more likely to trigger an action potential if this input is located proximally with few surrounding inhibitory connections than if it is bordered by numerous inhibitory synapses. If the synaptic wiring NATURE NEUROSCIENCE VOLUME 8 | NUMBER 5 | MAY 2005 Figure 2 Schematic showing relative changes in body weight and food intake in wild-type (yellow), ob/ob PBS-replaced (red) and ob/ob leptinreplaced (purple) mice. Leptin replacement induced statistically significant changes in food intake and adiposity within 2 d, and by 12 d, food intake and adiposity of leptin-replaced mice were lower than those of PBS-treated ob/ob mice and closely resembled values for wild-type controls. Synaptic measurements were done 6 h, 48 h and 12 d into leptin or PBS replacement (blue arrows). By 6 h, significant changes in the wiring of the melanocortin system were detected preceding the behavioral and metabolic changes. By 48 h, synaptic organization of leptin-treated ob/ob mice resembled that of wild-type mice, and food intake was already significantly decreased. This close temporal relationship between synaptic and behavioral changes establishes a correlation between synaptic changes and behavioral and endocrine alterations induced by leptin. indeed participates in determining the setpoint of the central feeding circuit rather than acutely determining postsynaptic activity, then it is possible that subjects who are sensitive to diet-induced obesity have a wiring and plasticity of the melanocortin system different from those who are resistant to obesity, even before obesity develops. If this is the case, then one could argue that developmental programming of hypothalamic circuits37 contributes not to obesity per se but to predisposing individuals to the development of obesity. There is a critical developmental period during which the development of efferents from the melanocortin system is affected by leptin37. This event may affect the hardwiring of at least some parts of the feeding circuits, thereby determining the set point of this system. The melanocortin system is not the sole regulator of energy balance. Thus, it is important to determine whether synaptic plasticity is characteristic of other systems in the adult brain that are associated with metabolic regulation. The lateral hypothalamic hypocretin-orexin system is important in establishing orexigenic drive associated with arousal38,39. As mentioned above, our unpublished pilot studies found that this system also seems to be softwired in nonhuman primates. To further investigate this, we used a transgenic mouse model in which neurons that express hypocretin/orexin are visualized using GFP40. Hypocretin neurons showed an unusual perikaryal synaptic input organization, in which excitatory inputs dwarfed inhibitory contacts by several orders of magnitude (Fig. 1), as seen in both ultrastructural and electrophysiological analyses33. This domination of excitatory inputs was further increased, in a leptin-dependent manner, by fasting33. Normally, such long-projecting neurons have an excess of inhibitory inputs on their perikarya to appropriately filter out noise41. In the case of the hypocretin neurons, however, the proximal localization of stimulatory inputs suggests that these cells are easily excited by various stimuli. From an evolutionary perspective, this paradoxical input organization makes perfect sense in the case of the hypocretin neurons, as subjects need to be easily aroused when in danger or in need of food. Of course, there is another side to the coin: when incom- 563 © 2005 Nature Publishing Group http://www.nature.com/natureneuroscience PERSPECTIVE ing signals increase, perhaps because of emotional or physical stress, easy activation of the hypocretin system will trigger insomnia and an orexigenic drive. Thus, the unorthodox input organization and plasticity of hypocretin perikarya may improve understanding of how insomnia leads to obesity42–44. To gain further insight into the role of synaptic plasticity in the regulation of feeding and energy expenditure, it will be critical first to determine regulatory elements and intracellular correlates of synaptic plasticity triggered by the changing metabolic state. Without these basics, it will be almost impossible to answer the ultimate question: what contribution does synaptic plasticity make to physiological and pathological metabolic conditions? When this question is addressed, it will be important to keep in mind the experience with the most studied plasticity phenomena, long-term potentiation (LTP) and long-term depression (LTD), in relation to learning and memory. LTP and LTD by now are generally accepted as building blocks for learning and memory. Notably, however, although both LTP and LTD and their morphological correlates are very closely associated with behavioral output, no causal direct evidence exists that LTP is mandatory for learning and memory in the mammalian brain45–47. This work suggests that a similar approach to hypothalamic synaptic plasticity, with mechanistic and correlative studies of its physiological role in energy metabolism, may hold promise. The lack of therapies for obesity: an apparent paradox After the discovery of leptin in 1994 and 1995, the assumption that medical approaches for metabolic disorders would soon follow seemed to be well founded. These medical breakthroughs have yet to occur, however, and this is unlikely to change in the immediate future, despite the amount of intellectual talent and monetary resources being directed at this issue. To emphasize the complexity of the peripheral tissue functions that are governed by the hypothalamus, it is logical to draw parallels between the regulation of food intake and energy expenditure and that of the pituitary gonadal axis. The discovery of hypophyseotropic hormones in the 1960s rapidly helped uncover regulation by the brain of various peripheral tissues, including the ovaries and testes, and helped clarify how the feedback from these tissues influences the brain. For example, the characterization and synthesis of luteinizing hormone–releasing hormone (LHRH) and its influence on the pituitary had immediate academic as well as clinical implications for issues associated with ovarian cycles and infertility48. This rapid translation of newfound principles for clinical medicine occurred at a time when genetics and molecular biology were not in common practice. The argument can be made that, from an evolutionary perspective, the regulation of energy balance is at least as important as reproduction. Thus, if the regulation of metabolism turns out to be redundant, a method could be found to successfully shortcut its CNS component, similar to what was done in reproductive medicine. It is worthwhile to note that although the principal hypothalamic neurons in the regulation of ovarian and testicular function, the LHRH cells, have been extensively studied49, the blueprint of gonadal feedback on the hypothalamus remains largely ill-defined. Whether or not it is reasonable to anticipate similar breakthroughs in metabolism regulation (the dream of patients and the pharmaceutical industry!) will largely depend upon our ability to understand the fine points of the brain’s role in this regulatory process and how these brain processes couple with the activity of peripheral tissues. ACKNOWLEDGMENTS The work of T.L.H. on the relationship between synaptic plasticity and energy metabolism has been supported by National Institutes of Health grants DK-060711 and RR-014451. 564 COMPETING INTERESTS STATEMENT The author declares that he has no competing financial interests. Received 12 January; accepted 11 March 2005 Published online at http://www.nature.com/natureneuroscience/ 1. Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998). 2. Elmquist, J.K., Elias, C.F. & Saper, C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232 (1999). 3. Kalra, S.P. et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20, 68–100 (1999). 4. Schwartz, M.W., Woods, S.C., Porte, D. Jr, Seeley, R.J. & Baskin, D.G. Central nervous system control of food intake. Nature 404, 661–671 (2000). 5. Sherrington, C. Sir. Cutaneous sensation. in Textbook of Physiology (ed. SharpeySchaefer, E.A.) 920–1001 (Pentland, Edinburgh, 1900). 6. Cannon, W.B. & Washburn, A.L. An explanation of hunger. Am. J. Physiol. 29, 441 (1912). 7. Carlson, A.J. in Control of Hunger in Health and Disease 2nd impr. (Univ. of Chicago Press, Chicago, 1916). 8. Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). 9. Halaas, J.L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995). 10. Pelleymounter, M.A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995). 11. Campfield, L.A., Smith, F.J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995). 12. Tartaglia, L.A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995). 13. Mercer, J.G. et al. Localization of leptin receptor mRNA and the long splice variant (O-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 (1996). 14. Schwartz, M.W., Seeley, R.J., Campfield, L.A., Burn, P. & Baskin, D.G. Identification of leptin action in rat hypothalamus. J. Clin. Invest. 98, 1101–1106 (1996). 15. Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997). 16. Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J. & Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997). 17. Pelletier, G. & Dube, D. Electron microscopic immunohistochemical localization of α-msh in the rat brain. Am. J. Anat. 150, 201–205 (1977). 18. Seeley, R.J. et al. Melanocortin receptors in leptin effects. Nature 390, 349 (1997). 19. Ollmann, M.M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997). 20. Hahn, T.M., Breininger, J.F., Baskin, D.G. & Schwartz, M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998). 21. Elias, C.F. et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786 (1999). 22. Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001). 23. Horvath, T.L., Naftolin, F., Kalra, S.P. & Leranth, C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131, 2461–2467 (1992). 24. Pinto, S. et al. Rapid re-wiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115 (2004). 25. Cowley, M.A. Hypothalamic melanocortin neurons integrate signals of energy state. Eur. J. Pharmacol. 480, 3–11 (2003). 26. Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 95, 15043–15048 (1998). 27. Mezey, E. et al. Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, α-melanocyte-stimulating hormone and β-endorphin (ACTH, α-MSH, β-END) in the rat hypothalamus. Brain Res. 328, 341–347 (1985). 28. Elias, C.F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998). 29. Qu, D. et al. A role for melanin-concentrating hormone in the central regulation of feeding behavior. Nature 380, 243–247 (1996). 30. Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998). 31. Theodosis, D.T. & Poulain, D.A. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience 11, 183–193 (1984). 32. Garcia-Segura, L.M., Baetens, D. & Naftolin, F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res. 366, 131–136 (1986). VOLUME 8 | NUMBER 5 | MAY 2005 NATURE NEUROSCIENCE © 2005 Nature Publishing Group http://www.nature.com/natureneuroscience PERSPECTIVE 33. Horvath, T.L. & Gao, X.B. Input organization and plasticity of hypocretin neurons: possible clues for obesity’s association with insomnia. Cell Metabolism 1, 279–286 (2005). 34. Horvath, T.L. & Diano, S. The floating blueprint of hypothalamic feeding circuits. Nat. Rev. Neurosci. 5, 662–667 (2004). 35. Grill, H.J. & Kaplan, J.M. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 23, 2–40 (2002). 36. Bodosi, B. et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 (2004). 37. Bouret, S.G., Draper, S.J. & Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004). 38. Chemelli, R.M. et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 (1999). 39. Yamanaka, A. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713 (2003). 40. Li, Y., Gao, X.B., Sakurai, T. & van den Pol, A.N. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36, 1169–1181 (2002). 41. Shepherd, G.M. Introduction to synaptic circuits. in The Synaptic Organization of the Brain. (ed. Shepherd, G.M.) 1–38 (Oxford Univ. Press, New York, 2004). NATURE NEUROSCIENCE VOLUME 8 | NUMBER 5 | MAY 2005 42. Taheri, S., Lin, L., Austin, D., Young, T., Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med., published online 7 December 2004 (doi: 10.1371/journal.pmed.0010062). 43. Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004). 44. Flier, J.S. & Elmquist, J.K. A good night’s sleep: future antidote to the obesity epidemic? Ann. Intern. Med. 141, 885–886 (2004). 45. Pittenger, C. & Kandel, E.R. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Phil. Trans. R. Soc. Lond. B 358, 757–763 (2003). 46. Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004). 47. Cooke, S.F. & Bliss, T.V. Long-term potentiation and cognitive drug discovery. Curr. Opin. Investig. Drugs 6, 25–34 (2005). 48. Schally, A.V. et al. Basic and clinical studies with luteinizing hormone-releasing hormone (LH-RH) and its analogues. J. Reprod. Fertil. Suppl. 20, 119–136 (1973). 49. Pfaff, D.W. et al. GnRH neurons and other cellular and molecular mechanisms for simple mammalian reproductive behaviors. Recent Prog. Horm. Res. 49, 1–25 (1994). 565