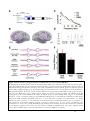
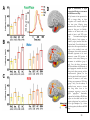
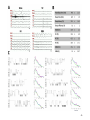
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electrophysiological markers of Rapid Eye Movements in
Haemodynamic response wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Electroencephalography wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain–computer interface wikipedia , lookup
Affective neuroscience wikipedia , lookup
Lunar effect wikipedia , lookup
Emotional lateralization wikipedia , lookup
Human brain wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Process tracing wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Aging brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroplasticity wikipedia , lookup
Time perception wikipedia , lookup
Brain Rules wikipedia , lookup
Neural oscillation wikipedia , lookup
Single-unit recording wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroesthetics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroscience in space wikipedia , lookup
Biology of depression wikipedia , lookup
Delayed sleep phase disorder wikipedia , lookup
Metastability in the brain wikipedia , lookup
Sleep apnea wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroscience of sleep wikipedia , lookup
Sleep paralysis wikipedia , lookup
Sleep deprivation wikipedia , lookup
Obstructive sleep apnea wikipedia , lookup
Sleep medicine wikipedia , lookup
Sleep and memory wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Effects of sleep deprivation on cognitive performance wikipedia , lookup
Rapid eye movement sleep wikipedia , lookup
Rapport de Stage CogMaster – Master en Sciences Cognitives 2ère année - Spéc. Neurosciences Electrophysiological markers of Rapid Eye Movements in wake and REM sleep: insights from intracranial recordings in Humans Thomas Andrillon sous la direction de Giulio Tononi, MD PhD en collaboration avec Yuval Nir, PhD au sein du Center for Sleep and Consciousness Department of Psychiatry, University of Wisconsin-Madison, 6001 Research Park Blvd, Madison, WI, 53719, USA 1 “We are such stuff. As dreams are made on; and our little life Is rounded with a sleep.” (Shakespeare, The Tempest) Abstract When awake, rapid eye movements (REMs) direct our fixation to specific parts of the visual scene and constantly guide our visual perception and behavior. Since the discovery of Rapid Eye Movement (REM) sleep, it became clear that REMs are also prevalent during certain periods of sleep, but the neuronal activity underlying such events and their relation to visual dream experiences remains unclear. Here we used a unique opportunity to examine and compare neuronal activity associated with REMs in wakefulness and sleep by examining simultaneously recorded electro-oculogram (EOG) along with intracerebral EEG, and single-unit firing in multiple brain regions of neurosurgical epilepsy patients. We find that REMs share similar properties across wakefulness and REM sleep. Neuronal activity was robustly modulated with ocular movements in both wakefulness and sleep, advocating for a functional continuity of REMs across vigilance states. Many neurons showed a bi-phasic pattern of activity including a decrease just before REMs onsets, possibly related to behavioral saccadic suppression, and a large increase immediately after REMs onsets. During wakefulness, the activity increase mainly followed a feedforward propagation with a progression from entorhinal cortex to hippocampus. By contrast, REMs in sleep were associated with a reversal of signal propagation suggesting a top-down propagation mode. We conclude that REMs and underlying neuronal activities share many similarities across wakefulness and sleep but the directionality of signal propagation may differ. Keywords: REM sleep, rapid eye movements, wakefulness, Humans, intracranial recordings, information directionality Words count1: 10,168 words, 51 pages 1 Without the cover page, table of contents, references and acknowledgements (Total: 12,544). 2 TABLE OF CONTENTS INTRODUCTION ..................................................................................................4 Rapid eye movements (REMs), a key feature shaping our perception .................................................................... 4 REMs also occur during certain stages of sleep ..................................................................................................... 5 Are REMs functionally equivalent during sleep and wakefulness? ....................................................................... 7 Are REMs in REM sleep a bottom-up or top-down phenomenon? ...................................................................... 8 A unique dataset to explore REM sleep and dreams ........................................................................................... 9 METHODS ............................................................................................................ 10 Subjects .............................................................................................................................................................. 10 Polysomnographic sleep studies............................................................................................................................ 10 Visual recognition studies................................................................................................................................... 12 Data Acquisition............................................................................................................................................... 12 Detection of Rapid-Eye Movements (REMs) ..................................................................................................... 13 Evoked Potentials (EPs) in depth EEG ........................................................................................................... 14 Spiking activities during REMs......................................................................................................................... 15 Statistical Analysis ............................................................................................................................................ 17 RESULTS ............................................................................................................... 19 Data overview and analysis of sleep parameters................................................................................................... 19 Eye movement properties are similar in wakefulness and REM sleep ................................................................. 20 Modulation of neuronal activity during eye movements ........................................................................................ 23 Directionality of signal propagation following eye movements changes between wakefulness and sleep .................... 28 DISCUSSION ......................................................................................................... 31 The persistence of saccadic suppression and the nature of REMs in REM sleep.................................................. 31 Ponto-geniculo-occipital (PGO) waves and REMs ............................................................................................. 32 Signal propagation during wakefulness and REM sleep ..................................................................................... 33 Study limitations ................................................................................................................................................ 35 CONCLUSION: ..................................................................................................... 36 ACKNOWLEDGEMENTS ................................................................................... 37 REFERENCES: ..................................................................................................... 38 APPENDICES:....................................................................................................... 45 3 Introduction Rapid eye movements (REMs), a key feature shaping our perception Rapid eye movements can be defined as fast rotatory ocular movements. This definition embraces a great variety of eye movements including saccades performed by awake individuals, a phenomenon extensively studied and one of the hallmarks of cognitive sciences. Saccades permit individuals to rapidly move the fovea, acquiring high quality visual information from a limited portion of the visual scene (Henderson 2003). Therefore REMs enable to scan in detail the environment and orientate attention to a limited portion of the visual space (Findlay 2004). As a consequence, saccades are omnipresent in primates' life. For example, humans produce about 3 saccades per second (Schiller and Tehovnik 2005). By determining what you see and when you see it, ocular movements actively shape our perception of the world (Ballard, Hayhoe et al. 1997). Despite their apparent simplicity, fast REMs are precise and highly complex movements. Numerous studies have dissected the neural circuits implementing the execution and control of REMs (Tehovnik, Sommer et al. 2000; PierrotDeseilligny, Milea et al. 2004) . Saccades involve various neocortical and limbic areas and reflect various cognitive processes including memory, decision-making, and attention allocation (Henderson 2003). Saccades are classically separated into two groups entailing different generators: (i) reflexive saccades (eye movements triggered externally by the sudden apparition of a new feature in the visual scene), (ii) voluntary saccades (ocular movements triggered internally after a cognitive decision process) (Mort, Perry et al. 2003; Schraa-Tam, van Broekhoven et al. 2009). In real-world perception, both types of saccades occur. REMs affect not only what we see but also how we see. Indeed, each saccade is accompanied by a so-called saccadic suppression: visual information is processed fully only during periods of gaze stability and smeared pictures caused by fast ocular movements are wiped out (Henderson 2003; Burr and Morrone 2005). Thus, although we have the phenomenological impression of continuity, our 4 visual perception actually consists of a sequence of snapshots disrupted by REMs (VanRullen and Koch 2003). REMs represent therefore an interesting index of ongoing visual processing. Do such ‘snapshots’, such resets in visual flow also occur in REM sleep? And are they accompanied by saccadic suppression? REMs also occur during certain stages of sleep Ocular movements are not only a predominant features of wakefulness, they are also observed in sleep (Aserinsky and Kleitman 1953) and are indeed used to define and categorize sleep stages: Rapid Eye Movements (REM) and Non-Rapid Eye Movement (NREM) sleep (see Box 1)2. Michel Jouvet’s work highlighted the importance of this distinction, REM and NREM sleep referring to different cognitive states and brain activity (Jouvet 1992). While NREM is classically defined as a state during which the brain has a broadly synchronized and stereotypical activity, REM sleep is characterized by EEG activity similar to wakefulness coupled to a generalized postural muscle atonia (Iber, Ancoli-Israel et al. 2007; Kryger, Roth et al. 2011). This paradox of a sleep stage for which brain and ocular activity are similar to wake led Michel Jouvet to designate REM sleep as paradoxical sleep (Jouvet 1992). REM and NREM sleep are also characterized by differences in consciousness. Reports of conscious experiences (dreams) are more frequent and vivid following awakening from REM sleep compared with NREM sleep (Tononi and Massimini 2008; Nir and Tononi 2010). Despite being one of the prominent features of REM sleep, REMs are not present continuously throughout REM sleep. In fact, REM sleep can be divided into phasic REM sleep during which REMs are observed (14-27% of REM sleep) and tonic REM sleep (73-86% of REM sleep) characterized by an absence of REMs. REMs during REM sleep typically occur in bursts of 5-6 consecutive ocular movements, which is not usual in wakefulness (Arnulf 2011). REMs density in sleep is highly variable (5-35 REMs/min) and shows great interindividual variability (Takahashi and Atsumi 1997), whereas density during However, REMs are not present in REM sleep in all species. For example, an absence of REMs was demonstrated in owls during REM sleep (Susic, V. T. and R. M. Kovacevic (1973). "Sleep patterns in the owl Strix aluco." Physiol Behav 11(3): 313-317.). 2 5 wakefulness is more consistent. Despite their weaker prevalence during REM sleep compared to wake, REMs share kinematic parameters with eyes closed voluntary saccades (Sprenger, Lappe-Osthege et al. 2010). Box 1: Sleep in a few words Polysomnography: multi-parametric recordings used to assess sleep and classically including scalp EEG (electroencephalogram), EOG (electrooculogram) and EMG (electromyogram). Sleep scoring: Sleep is usually divided in 4 stages (REM, NREM1, NREM2, NREM3) defined by precise electrophysiological and behavioral criteria (Iber, Ancoli-Israel et al. 2007) Rapid Eye Movement (REM) sleep: sleep stage characterized by an electric activity resembling wakefulness (low amplitude EEG, theta activity (4-7Hz)) coupled with postural muscle atonia and rapid eye movements. Dreams usually occur during REM sleep (Nir and Tononi 2010), which is predominant late at night. REM sleep is also called paradoxical, desynchronized or active sleep. REM sleep represents about 20% of sleep time in adults. Non Rapid Eye Movement (NREM) sleep: sleep phase divided in 3 stages (NREM1-3). NREM1 is a stage of transition between wakefulness and sleep characterized by a reduction in alpha (8-10 Hz) rhythm in parietal regions and a loss of responsiveness. Slow eye movements are typical of the transition into sleep. NREM2 and NREM3 are deeper sleep stages characterized by the presence of wellidentified sleep rhythms (slow-waves (0.5-4 Hz) and sleep spindles (11-16Hz), see (Andrillon, Nir et al. 2011; Nir, Staba et al. 2011) for a description in our data). Slowwaves sleep (SWS) refers to NREM3 and is defined by the predominance of slowwaves synchronized throughout the cortex. Sleep cycles: Classically, NREM and REM sleep alternate across the night, each night comprising ~3-5 NREM-REM cycles. The ratio of REM to NREM sleep within a cycle gradually increases throughout the night. Hypnogram: diagram describing the occurrence of sleep stages and wakefulness throughout the night (see Fig. 1D and App. 1C for examples). As for their genesis, REMs were observed in close relation with PontoGeniculo-Occipital (PGO) waves described by Mircea Steriade as “the internal activation of the brain during REM sleep” (Steriade 2003) suggesting that REMs could be under the control of the brainstem. Many studies eventually observed PGO waves and REMs as occurring simultaneously along visual and limbic areas with a common generator in the Ventro-Medial part of the Caudal Pontine Tegmentum (Pace-Schott and Hobson 2002; Arnulf 2011) as demonstrated by 6 Carbachol microinjections in this structure (Vanni-Mercier and Debilly 1998). In addition, an absence of readiness-potential was shown during sleep REMs compared to voluntary saccades in wake (Klostermann, Kompf et al. 1994; Abe, Ogawa et al. 2004) advocating for a cortical disengagement during REM sleep REMs. Another study exploring the time course of blood oxygenation leveldependent responses locked to REMs in REM sleep (Miyauchi, Misaki et al. 2009) supported the role of the brainstem in triggering REMs. However, due to the spatial and temporal limitations of non-invasive electrophysiological or imaging studies, it is hard to be conclusive. In addition, other imaging or neurosurgical studies challenged the idea that only brainstem was involved in REMs generation (Doricchi, Iaria et al. 2007; Hong, Harris et al. 2009). The question of the function of REMs in REM sleep (if any) is a long-lasting debate. REMs have been linked to brain maturation since they appear early in development and increase during the first semester of life (Roffwarg, Muzio et al. 1966) in parallel with visual ontogenesis. REMs have also been thought to play a role in brain plasticity (Datta 1999) and sensorimotor gating (Bowker and Morrison 1976). The fact that REMs and dreams occur during the same phase of sleep gave rise to the hypothesis that ocular movements during sleep are related to dream content (scanning hypothesis, see (Arnulf 2011) for a review). According to this hypothesis, the occurrence and direction of REMs would be correlated to the dreamer’s actions or visual experience (Dement and Kleitman 1957). Another influential view regards REMs as the mere reflection of brainstem activity, occurring independently of dreams just as penile erections happen independently of sexual dreams (see (Arnulf 2011) for a review). Are REMs functionally equivalent during sleep and wakefulness? Previous studies have explored the properties of REMs in REM sleep compared to wakefulness to unravel whether they can be seen as similar events. The absence of REMs in NREM sleep when reports of conscious experience are less frequent and simpler (Hobson 1990; Massimini, Ferrarelli et al. 2005) supports the notion that in REM sleep they may relate to visual experiences and 7 exploration of visual scenes (Dement and Kleitman 1957; Arnulf 2011). Moreover, the activation of primary visual cortex during REMs in REM sleep contrary to self-paced saccades in complete darkness (Miyauchi, Takino et al. 1987; Peigneux, Laureys et al. 2001) may outline the relation of REMs with a visual experience in REM sleep as in wake. There is behavioral evidence of a relation between REMs and dream content: (i) dream reports are usually more vivid during bursts of REMs (Berger and Oswald 1962); (ii) suppression of postural muscle atonia in cats (lesions in the Dorsolateral Pontine Tegmentum (DT)) results in the expression, during REM sleep, of a repertoire of actions similar to those performed during waking. Notably, limb movements coincide with REMs (Sastre and Jouvet 1979); (iii) in DT-lesioned cats visual stimulation during REM sleep does not affect the direction of REMs; (iv) more recently, a correspondence, albeit not perfect, has been reported between REMs direction and limb movements acted out during REM sleep in REM Behavior Disorder (RBD) patients, a pathology characterized by the enactment of dreams due to an absence of muscle atonia (LeclairVisonneau, Oudiette et al. 2010). Are REMs in REM sleep a bottom-up or top-down phenomenon? One influential theory on dreams consisted in seeing dreams as generated through bottom-up processes (Hobson and McCarley 1977) close to visual perception during wakefulness. In this view, dream scenery would start from activity in low-level sensory areas and would then be interpreted in higher-order visual areas (Nir and Tononi 2010). The brainstem, sending simultaneously corollary discharges to the whole cortex (i.e. visual, occulomotor and motor cortices) would insure the timely coordination between visual images, eye movements and body movements within the dream. In other words, the cortex would be the spectator of a 4-dimensions movie (Arnulf 2011) driven by brainstem activation: “the stuff dreams are made off” as described by Mircea Steriade (Steriade 2003). On the contrary, in line with the Freudian tradition, dreams could originate from memories and psychic motives. Thus, the dream 8 scenery would be formed following a top-down fashion as in imagination, abstract thoughts being represented as a scene within the dream. Understanding how the information flow is directed (top-down vs. bottom-up) during REM sleep and wakefulness could shed some light on this debated question of the nature of dreams (Nir and Tononi 2010). Our approach will consist in using REMs as triggers to explore the directionality of information in these different stages. A unique dataset to explore REM sleep and dreams We propose to analyze intracranial recordings during REMs in wakefulness and sleep to investigate and contrast their neurophysiological correlates. This unique dataset has been recorded in UCLA in epileptic patients resistant to pharmacological treatment and implanted with electrodes for potential surgical treatment (18 patients, 129 regions recorded and hundreds of units, see Methods). The dataset includes brain recordings at different scales (scalp EEG, depth EEG, LFP and single-unit recordings), which gives us the possibility to bridge the gap between human and animal studies and may provide a better comprehension of the mechanisms involved. Compared to previous electrophysiological or imaging studies in humans, we benefit here from a good spatial and temporal resolution. In our analysis, we will focus on two main questions: (i) Are REMs equivalent in wake and REM sleep? In particular, can we see a saccadic suppression associated to REMs in REM sleep? (ii) Can we observe changes in the information flow associated to REMs in REM sleep and wakefulness? 9 Methods Subjects Eighteen patients with pharmacologically intractable epilepsy (ages 19-52) underwent monitoring with depth electrodes for seizure foci identification and potential surgical treatment (Fried, Wilson et al. 1999). Patients provided written informed consent prior to participation in the research study, under the approval of the Medical Institutional Review Board at the University of California, Los Angeles, USA. Electrode location was based only on clinical criteria, and Itzhak Fried performed all surgery. For each subject, localization of the seizure onset zone was based on recordings during hospital monitoring, in combination with prior functional and anatomical neuroimaging (see appendix table 1 for patient information). The data were recorded at UCLA prior to the start of my internship. Thirteen patients participated in a full overnight sleep study and nine patients participated in a visual object recognition paradigm (four of these individuals participated in both experiments). Polysomnographic sleep studies Sleep recordings were conducted at a minimal interval of 12 hours from identifiable seizures, 48-72 hr after surgery, and lasted for about 7 hours between 23:00 and 06:00. Recordings were performed by Yuval Nir3. In addition to continuous video monitoring4, a classical polysomnographic montage was used, including two electrooculogram (EOG), two electromyogram (EMG), and four scalp electrodes (positioned at C3, C4, Pz and Fz) as well as two earlobe electrodes used as references. Sleep-wake stages (wakefulness, NREM sleep stages N1-N3 and REM sleep) were scored according to established guidelines (Iber, Ancoli-Israel et al. 2007) (see Box 1 for further details and App. 1A for examples). I participated to one recording session during a previous internship in Dr Tononi’s research center. Because of legal restrictions pertaining to patient confidentiality, I could not have access to the continuous video monitoring stored at UCLA. 3 4 10 Figure 1: Data overview, sleep parameters and detection of eye movements (REMs). A. Illustration of flexible probes used for recording depth EEG (blue: platinum contact) and unit activity (green: microwires). B. Medial view of 129 depth electrode locations (purple dots) spanning multiple brain regions in the 13 individuals having participated in the sleep study. SM, Supplementary motor; PC/P, posterior cingulate/parietal cortex; PH, parahippocampal gyrus; HC, hippocampus; E, entorhinal cortex; Am, amygdala; LH, left hemisphere; RH, right hemisphere. C. Power spectra of scalp EEG in one representative individual in sleep stages N2 (blue), N3 (red), and REM sleep (green). These power spectra are typical signature of REM and NREM sleep. D. Hypnogram in the same individual. W: Wake; R: REM sleep; N1–N3: NREM sleep stages 1–3. E. REMs detection. 1st row: raw EOG showing 2 typical REMs in REM sleep. 2nd row: Band-pass filtered (0.1-3Hz) EOG with mean+2SD associated threshold (black lines). 3rd row: Detection of epochs over threshold for one EOG channel (blue curve, green dots). 4th row: Detection is confirmed for the second EOG channel (red curve, yellow dot). 5th row: Detection of potential epileptic artifacts in high-pass filtered trace. 6th row: Visual confirmation and confirmation of onset. The second REM displayed would be then similarly detected. F. Occurrence of REMs across different vigilance states. Note the near-absence of REMs in NREM sleep. Error bars represent SEM across subjects (REM sleep: 11 patients, Wakefulness: 12 patients, NREM: 13 patients). 11 Visual recognition studies Eight patients participated in a visual object recognition study. These data are subject to a separate analysis but they were used here as a control to elucidate the directionality of signal propagation along the visual hierarchy upon visual processing in wakefulness in a well-controlled situation. The experimental procedure was as follows. Before each session, 2 sets of 6 pictures each were chosen based on their effectiveness in eliciting responses in the recorded neurons in a 'visual screening' experiment performed earlier that day, as in (Quiroga, Reddy et al. 2005). Each session included two blocks, lasting 12 minutes each. During each block, subjects were presented with 4 face and 2 place images (see App. 3 for examples) for 200ms on a laptop computer, and subjects performed a face/place task by pressing two different buttons. Each picture was presented 24 times in a pseudo-randomized order, and trials were separated by a random interval between 2 and 8 seconds (uniform distribution). Behavioral responses were recorded along with intracranial and scalp electrophysiological data. Overall 22 such sessions were recorded in 8 individuals. Data Acquisition For each patient, 8 to 12 flexible polyurethane depth electrodes (1.25 mm diameter, see Fig. 1A) were placed in the following regions: hippocampus, amygdala, entorhinal cortex, parahippocampal gyrus, temporal gyrus, fusiform gyrus, temporo-occipital junction; anterior, middle and posterior cingulate; supplementary motor area, inferior frontal gyrus, orbitofrontal cortex, and parietal cortex. Electrode location varied between patients based on their clinical profiles (see Fig. 1B for an overview and App. Table 1). Subsequently, I confirmed the position of each electrode using post-implant computed tomography (CT) coregistered with pre-implant magnetic resonance (MR) imaging (Brain Navigator, Grass-Telefactor Corp., Philadelphia, PA) based on a Talairach atlas (Lancaster, Rainey et al. 1997; Lancaster, Woldorff et al. 2000). 12 Scalp and intracranial depth EEG data were continuously recorded sampled at 2 kHz, bandpass-filtered in hardware between 0.1Hz and 500Hz and rereferenced offline to the mean signal of the earlobes electrodes. Scalp EEG, EOG, EMG and behavioral data were collected and preprocessed according to established guidelines for sleep study polysomnography (Iber, Ancoli-Israel et al. 2007). Specifically, two EOG electrodes were pasted below the left and above the right canthi, and referenced to contralateral reference electrodes. In addition, each depth electrode terminated in a set of eight insulated 40-μm platinum-iridium microwires (impedances 200 to 500 kΩ, see Figure 1A) (Fried, Wilson et al. 1999). Microwire signals were simultaneously recorded continuously (Cheetah Recording System; Neuralynx, Tucson, AZ for 10 patients; Neuroport Recording System; Blackrock, Salt Lake City, UT for 3 patients), sampled at 28 kHz (10 patients) or 30 kHz (3 patients), band-pass filtered in hardware between 1Hz and 9kHz, and referenced locally to a ninth non-insulated microwire. Detection of Rapid-Eye Movements (REMs) REMs were detected in a semi-automatic manner as illustrated in Fig. 1E. The two EOG signals recorded during sleep studies (Fig. 1E, 1st row, blue and red curves) were first band-pass filtered between 0.1 and 3 Hz using a zero-phase 2ndorder Butterworth filter, to attenuate fast background activity (Fig. 1E, 2nd row). A detection threshold was then set at mean + 2 SD of the EOG signals across the whole night (epochs with absolute amplitude over 1,000µV were discarded from this computation so that such abnormal voltage would not influence our estimation) (Fig. 1E, 2nd row). Events crossing this threshold were considered REMs candidates (Fig. 1E, 3rd row) and further inspected so that (1) the segments above threshold had a duration of less than 1.5s; (2) they corresponded to an amplitude exceeding an equivalent threshold in the other EOG trace and of opposite sign; (3) their maximal slope (temporal 1st derivative) was above a threshold of 1µV/ms (Fig. 1E, 4th row). We discarded REMs too close from each other (<0.5s) as well as REMs in close vicinity (within 0.5s) with detected epileptiform interictal spikes. Epileptiform interictal spikes were identified in the EOG by searching for abnormal increase (absolute amplitude superior to mean + 13 10 SD) in the 100-150 Hz band (Fig. 1E, 5th row). We converged on these parameters after careful visual examination of the detected REMs and rejected candidates. We selected only the EOGs deflections with opposite phases since they correspond, in our EOG montage, to horizontal eye movements. Signal deflections that were in-phase across the two EOG traces were not analyzed since these may reflect both vertical eye movements as well as cortical potentials (e.g. slow-waves or K-complexes in NREM sleep) leaking to EOG measurements. REM onsets were defined automatically as the first crossing of the 2 raw EOGs traces before detected REMs (Fig. 1E, 6th row). Finally, to ensure the quality of this automatic detection and since our study needed a high precision in determining the exact onset of REMs, I visually reviewed all the automatically detected REMs and rejected candidates, as well as their onset times. It was then decided to reject or include the REMs and fine-tune their time of onset. REMs onsets were used as reference time points throughout our analysis after a comparison with other REMs parameters (REMs peak amplitude or maximum slope). In addition to this detection, random epochs were selected to be used as a control data set. For each detected REMs, we detected within the same 10s segments, 2s epochs during which there was no significant increase in either EOG channel and no traces of epileptiform interictal spikes. To this end, we detected epochs presenting an increase within the [100, 150] Hz band (zero-phase 2nd-order Butterworth filter) superior to a threshold set as 10 SD. These “random” events matched REMs detection in terms of density, sleep stages and occurrence within the ultradian cycle (i.e. time within sleep) for each patient. Evoked Potentials (EPs) in depth EEG EPs for depth EEG channels were computed as follows. Depth EEG channels were segmented in 10s segments, notch-filtered (2nd-order IIR notch filter at 60 Hz and its harmonic) to eliminate electrical noise and corrected for baseline activity by subtracting the mean amplitude across 10s segments. Epochs around REMs onsets were selected ([-1000, 800] ms). Epileptic-spikes free epochs were 14 then averaged by EEG channel and vigilance stage. Figure 5 show the average EPs at times of REMs occurrence for depth EEG across subjects. The exact same procedure was repeated for random epochs to assess the amplitude level of random fluctuations with this extent of data. Statistical deviance between REMs and random EPs was assessed at each sample across subjects for each depth electrode and stage using a paired Mann-Whitney U-test (alpha=0.05, n=11 patients). Simes’ method was used to correct for multiple comparisons (Rodland 2006). Only the most medial recording sites (blue contact in Fig. 1A) were analyzed, although each electrode included other EEG contacts along the shaft of the electrode. Spiking activities during REMs Unit identification and spike sorting. Units were identified using the 'wave_clus' software package (Quiroga, Nadasdy et al. 2004) as follows: (i) extracellular microwire recordings were highpass filtered above 300Hz, (ii) a 5 SD threshold above the median noise level was computed, (iii) detected events were clustered using superparamagnetic clustering and (iv) classed as single-unit, multi-unit clusters or noise based on the reliability of action potential waveforms and by the presence of a refractory period for single units (see App. 2A-B and (Nir, Mukamel et al. 2008)). The enduring nature of unit recordings throughout hours of continuous recordings (sleep studies: ~7h) was assessed by ensuring that action potential waveforms, and inter-spike-interval distributions were conserved in 1hour intervals and that clusters remained separable throughout the recording session. Multiunit activity (MUA) traces, used in Figure 3 (green curves) were extracted from each microwire by filtering the recorded signal offline between 300Hz and 3000Hz. Overall, for sleep recordings, 600 units were identified across 129 regions and 1,334 units for the visual recognition sessions (see App. 2C). Analysis of neuronal discharges around REMs. A triggered-averaging analysis was performed for each unit separately as follows. Spike data were time-locked to REMs onsets and averaged for each unit across a [-600, 800] ms window around REMs onsets. The post-event-time histogram was smoothed using a Gaussian 15 kernel (σ = 60ms) and normalized by its minimum and maximum values, facilitating the comparisons between low- and high-firing rate neurons. The same procedure was repeated for random epochs (see above) and statistical deviance between triggered-averages for REMs and random events was assessed using a paired Mann-Whitney U-test (α=0.05). Simes’ method was used to correct for multiple comparisons. After carefully inspecting the results for each neuron separately, two patterns were identified in line with previous studies (Schall 1991; Schall 1991): (i) Group A: units showing a decrease in activity just before REMs onset and a larger increase shortly after REMs onsets, (ii) Group B: units with increased firing rates preceding REMs. In order to quantitatively distinguish between these profiles and categorize units based on these groups, we analyzed the relation between spike times and the phase of the EOG signal taking inspiration from my prior work on this very dataset (Andrillon, Nir et al. 2011). Thus, EOG channels were band-pass filtered (0.1-3Hz, a range used and optimized for REMs detection) and the instantaneous phase of the filtered EOG was computed via the Hilbert transform. Then, for each unit, we extracted the distribution of EOG phases corresponding to spikes occurrence within a [-600, 800] ms interval around REMs onsets. The nonuniformity of phase distributions was assessed using Rayleigh’s test for nonuniformity (circ_mean function, Circular Statistics Toolbox for Matlab). Since EOG skewness can introduce nonuniformity above and beyond units’ preference for a given phase (Siapas, Lubenov et al. 2005), we compared the p-value obtained with the Rayleigh’s test to surrogate data. For each unit separately, we shuffled spikes occurrence for the different REMs across the same [-600, 800] ms window, extracted EOG phases for each spikes and computed a new p-value using Rayleigh’s test. This operation was repeated 500 times. We then extracted the position of the original p-value within the distribution of bootstrapped p-values. This process was repeated for both EOG channels and results were averaged for each unit. Finally we split the data in 2 groups by gathering units whose phase original p-value was superior to the median of bootstrapped p-values (units with phase preference) and units whose original p-value was inferior to this median value (units without phase preference). On the whole, units showing a tendency to fire spikes at specific phases of the 16 REMs waveform (as in Figure 4A) showed a firing pattern of group A, while units without a particular phase preference typically showed a firing pattern of group B. Finally, neuronal activity around REMs was averaged by group (Fig. 4C) and across regions (see Figure 4B) for each group separately (Fig. 4D). Latencies of neuronal activity following REMs. We computed the latency of such activity increases as follows. For each unit in group A (see above), we extracted spikes occurring within 1s after REMs onsets (for wake and REM sleep separately). Then, spikes were binned across fifteen 100ms windows (50ms overlap). For each window and across REMs, we compared the firing rate to the baseline activity (computed on a [-500 0] ms window before REMs onsets) with a Mann-Whitney U-test. The timing of the first window showing a significant increase in firing rate compared to baseline (α=0.05, Simes correction) was defined as the latency of increased activity following REMs for this particular unit and vigilance state. We focused on group A units since they show an increase in their firing rate after REMs onsets. Therefore, for this group of units, REMs trigger an increase in activity, allowing us to analyze its propagation between brain regions. The same procedure was repeated for the object recognition paradigm, using the time of image presentation as time zero (instead of REMs onsets). Only units showing responses to pictures (96 out of 1334 neurons, 18% of MTL neurons) were selected for this analysis as for the sleep study (see App. 3 for examples). To this end, standard post-stimulus-time-histograms (PSTH) were computed with 100ms bins across 144 trials and the firing rate in the time interval 300-500ms after image presentation was compared via a one-tail Student t-test to the baseline firing rate (α=0.001). Statistical Analysis Error bars in figures denote standard error of the mean (SEM = SD/√(n-1), where n is the number of data points). Student T-tests were performed after confirming normal distributions via Kolmogorov-Smirnov tests or the MannWhitney U-test (non-parametric) when the normality was not confirmed. When 17 necessary, corrections for multiple comparisons were performed using Simes’ correction5 (Rodland 2006). 5 Simes correction is an optimized version of Bonferroni correction. 18 Results Data overview and analysis of sleep parameters Thirteen neurosurgical patients with intractable epilepsy underwent polysomnographic sleep studies. Recordings were conducted overnight (421 ± 20 min (mean ± SEM)) and included scalp EEG, EOG, EMG and continuous video monitoring. Sleep–wake stages were scored following established guidelines (Iber, Ancoli-Israel et al. 2007) as wakefulness, NREM sleep (3 stages) and REM sleep (see Box 1 for a brief description of sleep scoring and App. 1A for examples). One patient did not show any REM sleep before the sleep recording was terminated. Overall, sleep parameters including sleep efficiency and the time spent in different sleep stages (App. 1B) were in general agreement with typical findings in healthy young adults (Riedner, Vyazovskiy et al. 2007). The reduction in the amount of REM sleep (13% compared to 20% usually in adults) probably reflects the fact that sleep recordings were usually terminated around 6AM and REM sleep is predominant in the morning hours (McCarley 2007). Hypnograms (timecourse of sleep-wake stages, see Box 1) showed typical sleep architecture and NREM-REM sleep cycles (Fig. 1D and App. 1C). Power spectra of scalp EEG (Fig. 1C for an example and App. 1C) resembled those found during sleep of healthy individuals, including robust slow-wave activity (0.5-4 Hz) and spindle (9– 16 Hz) power that was specific to NREM sleep stages. These results indicate that sleep measures could be assimilated to those of natural sleep in individuals without epilepsy, which makes us confident that it is reasonable to generalize our results to the healthy population. During sleep studies, we recorded brain signals from 129 medial brain regions bilaterally within the medial temporal lobe, frontal and parietal cortices (Fig. 1B). Electrodes were implanted based on clinical criteria and spanned mostly medial brain areas. It should be noted that some regions that are implicated in controlling and generating eye movements (REMs), such as the Frontal Eye Field and the occipital lobe (Pierrot-Deseilligny, Milea et al. 2004), were not monitored in these neurosurgical patients. We simultaneously recorded scalp EEG, depth EEG, and 19 spiking activity from a total of 600 units (355 putative single units, 245 multi-unit clusters (see Methods and App. S2C)). Eye movement properties are similar in wakefulness and REM sleep We detected REMs in EOG channels in a semiautomatic fashion (see Methods and Fig. 1E) across all vigilance states (Fig. 1F). Epochs of wakefulness and REM sleep with at least 50 detected eye movements (n=12 and 11 individuals, respectively) were used for further analysis (Fig. 2). On average, 2.6 ± 0.5 REMs per minute (mean ± SEM) were detected in wakefulness (range: 0.6-5.1 REMs/min). During REM sleep, REMs occurred with an average density of 4.4 ± 1.6 REMs per minute (range: 1.0-8.7). This rate is at the very low range of that reported in previous studies (Arnulf Figure 2: Eye movement properties are similar in wakefulness and REM sleep. A. Average traces of REMs for each subject in wakefulness (black, n=12) and REM sleep (red, n=11). B. Comparison of eye movement characteristics in wakefulness and REM sleep including their density, amplitude, slope, duration and inter-eye-movementinterval (IMI). P-values denote statistical significance (paired Mann-Whitney test, n=11 pairs). Note that apart from REMs amplitude being significantly higher in wakefulness, all other REMs parameters are comparable across wake and REM sleep. 2011), and likely reflects the fact that with our recording montage, only horizontal REMs could be observed, thus leading to less detected events. Contrary to previous studies (Schiller and Tehovnik 2005; Arnulf 2011), in our data REMs were significantly more numerous in REM sleep than wake (p<0.05, paired MannWhitney U-test, n=11 pairs of patients). This is probably a sign of the conditions 20 in which our recordings were obtained during wakefulness – typically, subjects while awake in bed were not performing a visual task, or may have eyes closed. Importantly, the rate of REMs detection in NREM sleep was not significantly different from zero (Fig. 1F; p=0.17, Mann-Whitney U-test, n=13 patients), attesting to a low rate of false detections of our algorithm. Except for a larger amplitude of REMs in wakefulness compared to REM sleep (p<0.001, paired Mann-Whitney U-test, n=11 pairs), other parameters (initial slope, duration and time between REMs (IMI)) were not significantly different between the two stages (Fig. 2B). Moreover, the shape of REMs as recorded by EOGs signals was highly similar between REM sleep and wakefulness (Fig. 2A). Overall, these results support the notion that properties of eye movement are similar in wakefulness and REM sleep (Sprenger, Lappe-Osthege et al. 2010). 21 Figure 3: Example of neuronal activity during eye movements Example of neuronal activities across multiple medial temporal lobe regions during 15sec of wakefulness in one individual. Rows (top to bottom) depict EOG recordings (red), and unit activity in right Amygdala (rAm), right Hippocampus (rHC), right parahippocampal gyrus (rPHG), left Amygdala (lAm), left Hippocampus (lHC) and left parahippocampal gyrus. Black lines: single-unit spikes; blue curves: multi-unit activity; green lines: onset of detected REMs. Note that bursts of activity often follow REMs in different MTL regions. 22 Modulation of neuronal activity during eye movements Neurons often exhibited clear bursts of activity around REMs occurrence. Figure 3 provides an example of single- and multi-unit activities across multiple medial temporal lobe (MTL) regions during REMs in wakefulness. To quantify the modulations in neuronal firing across the entire dataset (n=437 wake and 349 in sleep in 12 individuals), we performed a triggered-averaging analysis of spiking activity around REMs onsets. Inspection of the results for each neuron separately revealed complex firing rate modulations, in line with previous reports (Schall 1991; Schall 1991; Timofeev, Grenier et al. 2001). Overall, modulations could be readily divided in two categories based on the tendency of neurons to fire at a specific instantaneous phase of the EOG signal (Fig. 4A). In wakefulness, neurons in group A (63% of units) showed a bi-phasic pattern of activity including a decrease just before REMs onsets (down to 29% of maximal firing rate at -105ms, p<10-10 uncorrected Mann-Whitney U-test) and a large increase immediately after REMs onsets (66% of maximal firing rate at +315ms, p<0.05 corrected MannWhitney U-test). In REM sleep, a similar pattern was observed, although the proportion of units in group A was slightly reduced (52% of units) and so was the amplitude of their average firing rate modulations (down to 37% of maximum firing rate at -224ms and up to 57% at +348 ms). The robust decrease immediately prior to REMs in the activity of group A neurons is reminiscent of effects often associated with saccadic suppression (see Discussion). By contrast, neurons in group B (37% and 48% of units in wakefulness and REM sleep, respectively) showed a slight increase in firing rate before REMs (54% at -176ms in wake, p<0.05 corrected Mann-Whitney U-test) followed by a decrease after REMs onsets (38% at +366ms in wake, p<0.05 corrected). In REM sleep the proportion of units in group B as well as their increase in firing rate before REMs was slightly larger (57% at -187ms, p<0.05 corrected). The two patterns of responses resemble previous results obtained with intracellular recordings in cats (Timofeev, Grenier et al. 2001), regular-spiking neurons showing a decrease of activity after REMs and fast-spiking neurons an increase. In our study, a significant difference was found between group A and B 23 based on units’ average firing rate in wakefulness (p=0.03, two-tailed Student ttest across units in group A and B) but not in REM sleep (p=0.14). A better characterization of our recorded units’ cell types would be needed here, rather than the mere average firing rate. To better understand the neuronal activity around REMs, we examined the firing rate modulations in each brain region separately. Units were grouped into 9 regional clusters, as illustrated in Figure 4B, based on electrodes location (PHG: parahippocampal gyrus; Am: amygdala; EC: entorhinal cortex; HC: hippocampus; OFC: orbito-frontal cortex; AC: anterior cingulate; SMA: supplementary motor area; Par: parietal cortex; Temp: temporal cortex). Regional analysis of firing rate modulation (Fig. 4D) revealed that group A neurons in all regions showed consistent increase in firing rate right after REMs onset, preceded by a decrease in firing rate before REMs onsets, and this modulation was more robust in wakefulness than in REM sleep. Neurons in group B showed similar modulations across regions and vigilance states, albeit with more variability. Interestingly, neurons in frontal regions including AC and SMA exhibited particularly robust firing rate modulations well before REMs, in line with their possible role in motor preparation (Shibasaki and Hallett 2006; Fried, Mukamel et al. 2011) and REMs generation during wakefulness (Pierrot-Deseilligny, Milea et al. 2004). Finally, biphasic changes in neuronal activity around REMs onsets in both wakefulness and REM sleep were accompanied by evoked potentials in depth intracranial EEG in the MTL (Fig. 5). REMs were associated with sharp positive potentials highly synchronized across all MTL regions and electrodes often referred to as the spike positivity in the literature (Berchicci, Stella et al. 2012) and possibly associated with “reset” of activity at those times (see Discussion). Overall, REMs onsets are accompanied by robust modulations in neuronal activity that is similar across wakefulness and REM sleep. 24 25 Figure 4: Quantitative analysis of unit activity underlying REMs in wakefulness and REM sleep A. Example of relation between the firing of one unit and the instantaneous phase of EOG during wakefulness. Bar graph (black) shows number of spikes around REMs onsets with a 60ms moving average. Red curve shows the average instantaneous phase for one EOG channel extracted via the Hilbert transform. Note the preference of this unit to fire at a given phase of the EOG signal (~0 rad). B. Medial view of the brain with regional clusters used in panel D and the position of the corresponding electrodes. (PHG, parahippocampal gyrus; HC, hippocampus; Am, amygdala; EC, entorhinal cortex; OFC, orbitofrontal cortex; AC, anterior cingulate; SMA, supplementary motor; Par, parietal cortex; Temp, temporal cortex) C. Categorization of units based on their preferred phase. Left: wakefulness; Right: REM sleep. Bar graphs (black) represent average normalized units’ activity around REMs onsets. Red curves, superimposed smoothed activity patterns (60ms Gaussian kernel). Yellow, proportion of units in each category. Green horizontal bars, time points where firing rate modulation is significantly different than chance (p<0.05, corrected MannWhitney U-test). D. Unit activities during REMs by vigilance state (left: wakefulness; right: REM sleep), by category (top: group A; bottom: group B) and by region (colors as in panel B). Note that robust and similar firing rate modulations can be seen in the activities of each region and state separately. 26 Figure 5: Intracranial evoked potentials during eye movements Evoked potentials (EPs) in MTL depth intracranial EEG recordings triggered around REMs onsets during wakefulness (panel A : PHG (n=11 electrodes), HC (n=19), Am (n=15), EC (n=8)) and REM sleep (panel B PHG (n=9 electrodes), HC (n=12), Am (n=12), EC (n=7)). EPs were computed by averaging depth EEG in [1000, 800]ms windows around REMs onsets after subtraction of mean voltage. Gray shade highlights the presence of a significant spike positivity (see Methods for the statistical analysis) across all MTL regions in wakefulness and REM sleep. 27 Directionality of signal propagation following eye movements changes between wakefulness and sleep Next, we set out to explore the fine temporal dynamics of signal propagation along different brain structures in the activity immediately following REMs in wakefulness and REM sleep (neurons in group A). We hypothesized that in wakefulness the latency of activity monotonically increases along the visualmnemonic pathway in the MTL, thereby reflecting feedforward processes. We analyzed latencies of activity during spontaneously generated REMs during wakefulness. In order to match these results to well-established results on visual processing (Felleman, Burkhalter et al. 1997; Mormann, Kornblith et al. 2008), we examined in parallel the latencies in response to visual stimulation in the morecontrolled paradigm of an object recognition task (see Methods). The results indicate that during visual stimulation (Fig. 6A) unit activity showed a sequence of activation matching the anatomical hierarchy of information processing (Felleman and Van Essen 1991), consistent with a previous study using intracranial recordings (Mormann, Kornblith et al. 2008). Latencies increase from the fusiform gyrus (295 ± 25 ms, mean ± SEM, 31 units), to PHG (337 ± 35 ms, 32 units), EC (385 ± 25 ms, 19 units) and to HC (421 ± 49 ms, 10 units). A similar direction of signal propagation was observed during spontaneous REMs in wakefulness (Fig. 6B) with the following latencies (PHG: 286 ± 29 ms, 48 units; Am: 305 ± 35 ms, 32 units; EC: 378 ± 60 ms, 13 units; HC: 432 ± 37 ms, 41 units). Interestingly, an anterior-posterior propagation was also noted in parietal and frontal cortices (Parietal: 304 ± 63 ms, 12 units compared with OFC: 330 ± 66 ms, 9 units; AC: 362 ± 67 ms, 13 units; and SMA: 401 ± 69 ms, 13 units), in line with previous findings (Felleman and Van Essen 1991). These results confirm the prediction that in wakefulness, as observed during the visual object recognition task, the latency of neuronal activity triggered by REMs primarily follows a feedforward direction. Next, we examined the latency of activity around REMs in REM sleep (Fig. 6C). A partial reversal of latencies was observed within the MTL where activity was seen earlier in the HC (264 ± 38 ms, 25 units) compared with EC (287 ± 46 28 ms, 20 units). While the sequence of activity was not a perfect mirror-image of that in wakefulness (e.g. PHG was active on average at 275 ± 39 ms following REMs (24 units)), the reverse order between EC and HC is particularly relevant since only unidirectional anatomical pathways link these two structures (Felleman, Burkhalter et al. 1997). Although during REMs the differences in latencies between regions and within each state reflected a trend rather than a statistically significant effect (visual stimulation: p<0.05; wake: p=0.2; REM: p=0.7), a comparison of latencies in EC and HC between wakefulness and sleep revealed a significant difference (Mann-Whitney U-test, α = 0.05), supporting the notion that the directionality of signal propagation in REM sleep may reflect a top-down mode of signal propagation at least with respect to the hippocampal formation (see also Discussion). 29 Figure 6: Directionality of signal propagation following eye movements in wakefulness and sleep A. Left: Latencies of unit responses in the MTL to images during an object recognition task. Latencies reflect the first time point following image presentation that showed a significant increase in firing rate (see Methods). Numbers on the abscissa refer to the number of units in each MTL region. Right: Post-stimulus-time-histogram (PSTH) analysis of unit responses to stimuli by region (smoothed with 60ms Gaussian kernel). Note that both types of analysis show that response latencies are longer in the entorhinal cortex and hippocampus, in line with the anatomical hierarchical organization of the visualmnemonic systems. B. Latencies of increases in unit activity following eye movements in wakefulness (group A only). Note that during spontaneous REMs in wakefulness the directionality of signal propagation resembles that found during visual object processing, and that posterior-anterior gradients are also found in parietal and frontal regions. C. Latencies of increases in unit activity following eye movements in REM sleep (group A only). Note the tendency of entorhinal cortex neurons to increase their firing before those in the hippocampus, suggesting a reversal of signal propagation directionality compared with wakefulness. D. Different regions are color-coded based on their latencies and projected on a medial view of the right hemisphere. Top to bottom: visual recognition, wakefulness and REM sleep. 30 Discussion The persistence of saccadic suppression and the nature of REMs in REM sleep Saccadic suppression is a behavioral phenomenon consisting of a transient increase in visual perception thresholds during saccadic eye movements (Dodge 1905). Although this phenomenon can be readily observed (e.g. one cannot see one’s own eyes moving in a mirror but can perfectly see somebody else’s eyes moving), its neural underpinnings remain unclear. Initially, saccadic suppression was regarded as a side effect of the eye movement itself (Volkmann 1962) but later studies established that not all visual features are equally suppressed (Melcher 2005; Watson and Krekelberg 2009) and that behavioral suppression starts before REMs onsets and proprioceptive feedback (Latour 1962; Matin, Clymer et al. 1972) thus pointing to a more complex neuronal process. The most robust pattern of neuronal activity accompanying REMs observed in visual regions of the monkey thalamus and cortex is a bi-phasic modulation of firing rates - with decreased activity just before saccade onset followed by a larger post-saccade burst (Reppas, Usrey et al. 2002), and such activity persists in the absence of visual input (Kayama, Riso et al. 1979). Activity suppression is believed to relate to a corollary discharge accompanying the production of saccades in the brainstem (Wurtz 2008; Wurtz, Joiner et al. 2011) and affecting high-level visual regions through the colliculus-pulvinar-frontal pathway (Kleiser, Seitz et al. 2004). However, activity suppression is not seen in all brain regions (e.g. V1) (Wurtz 2008). The differential neuronal effects along the visual pathway could reflect the fact that parallel mechanisms evolved to implement different information processing tasks during saccades (Bremmer, Kubischik et al. 2009). Modulation of firing rates at times of saccadic suppression may be further mediated by attentional shifts involving parietal cortex (Duhamel, Colby et al. 1992; Hamker, Zirnsak et al. 2011). Interestingly, reflexive and voluntary saccades may involve exogenous and endogenous attentional processes respectively (Wurtz, Joiner et al. 2011). Overall, underlying neuronal activity patterns are highly variable across 31 units and regions and suggest a complex interplay between systems of motor preparation, corollary discharge, visual perception, and attentional modulation (Super, van der Togt et al. 2004; Wurtz 2008; Watson and Krekelberg 2009; Cloherty, Mustari et al. 2010). The present results confirm the presence in humans of a bi-phasic modulation of unit activity, accompanied by evoked field potentials, across multiple regions of the human brain during eye movements in wakefulness (Figures 4 and 5). A more novel finding is that a similar profile of neuronal activity around REMs is also found during REM sleep, in the absence of visual input. This underscores the similarity between cortical activity in wakefulness and REM sleep (Nir and Tononi 2010), and supports the notion that saccadic suppression-related activity may be linked to perceptual and attentional processes, above and beyond its possible relation to retinal input. It has been proposed that cortical activity may follow a succession of quasi-stable patterns of activity or "micro-states" (Lehmann 1971; Jones, Fontanini et al. 2007). Conceivably, eye movements and underlying neuronal activity could play a role in "resetting" such states of cortical activity. More generally, given the prevalence of dreaming in REM sleep, it is tempting to speculate that eye movements in REM sleep reflect transitions between perceptual "snapshots" in dream content. Ponto-geniculo-occipital (PGO) waves and REMs Ponto-geniculo-occipital (PGO) waves are sharp field potentials occurring concomitantly with eye movements in REM sleep (Morrison and Pompeiano 1966). They often appear during the transition from NREM to REM sleep and alongside muscle atonia and are therefore considered a hallmark characteristic of REM sleep (Jouvet 1992; Steriade 2003; Kryger, Roth et al. 2011). PGO waves were first observed in the pons, lateral geniculate nucleus of the thalamus and in the occipital cortex of cats (Jouvet and Michel 1959; Brooks and Bizzi 1963) and later described in other species (Gucer and Viernstein 1979; Farber, Marks et al. 1980) and across multiple brain regions (Amzica and Steriade 1996). PGO waves are generated in the brainstem (Sastre and Jouvet 1979) and induce broad 32 synchronized waves through thalamic relays (Amzica and Steriade 1996). Due to their widespread effects on brain activity, PGO waves have been implicated in the putative functions of REM sleep: brain plasticity (Datta 1999), sensory gating (Bowker and Morrison 1976), sensorimotor processing (Callaway, Lydic et al. 1987) and dream generation (Hobson 1990). However, some evidence suggests that PGO waves are akin to lambda responses triggered by visual stimulation in wakefulness (Barlow and Ciganek 1969). According to this view, PGO waves reflect the corollary discharges and saccadic suppression associated with REMs and may not be exclusive to REM sleep (Brooks 1968; Brooks 1968). There are a few reports documenting intracerebral PGO waves in humans (Lim, Lozano et al. 2007; Fernandez-Mendoza, Lozano et al. 2009) as well as their non-invasive correlates (Peigneux, Laureys et al. 2001). In our study, none of the patients were implanted with electrodes in the pons, LGN or the occipital cortex, so the current data cannot confirm or dismiss the existence of human PGO waves unequivocally. However, we observed time-locked potentials associated with REMs during REM sleep across multiple neocortical and MTL limbic regions, which may support a candidate for PGO waves observed in animals. Moreover, similar potentials associated with REMs were found in wake and REM sleep (Mccarley, Winkelman et al. 1983), supporting the notion that such events reflect similar processes across wakefulness and sleep (Brooks 1968; Brooks 1968). Signal propagation during wakefulness and REM sleep When we are awake with our eyes open, every saccade invariably leads to a new image impinging on the retina. The new information is processed by the nervous system, leading to a barrage of neuronal activity along visual pathways. Although far from being well understood, the process of visual object recognition is believed to start with a feedforward sequence of activations along hierarchical processing nodes in the ventral occipitotemporal cortex (Hochstein and Ahissar 2002; Riesenhuber and Poggio 2002). Activity in initial stages of this process (e.g. occipital cortex) is more closely linked to features of the visual image while later processing better reflects abstract representations that transcend retinal location, 33 size, viewing angle etc (Liu, Agam et al. 2009). In the medial temporal lobe, both the latency and selectivity of responses is higher in entorhinal cortex and hippocampus than in the parahippocampal gyrus (Mormann, Kornblith et al. 2008) suggesting a ‘feed-forward’ mode of signal propagation during passive viewing. Interestingly, a recent study in primates shows that feed-forward visual propagation reverses to a ‘top-down’ mode during mnemonic processes (Takeuchi, Hirabayashi et al. 2011). In dreaming, our internally-generated visual experience resembles that of wakefulness (Nir and Tononi 2010). However, it remains unclear whether dream imagery originates in activity of low-level visual areas and it is later processed by higher-level areas as in visual perception (Nir and Tononi 2010; Arnulf 2011) or whether dreams originate as memories or abstract thoughts “objectified in the dream, and represented as a scene” (Freud 1931) as during imagination. According to one influential view, dream imagery is largely ‘bottom-up’: it is triggered by PGO waves that excite the visual cortex and this activity is then interpreted and synthesized by high-order areas (Hobson 1990; Nir and Tononi 2010). Indeed, the absence of aminergic neuromodulation in REM sleep may facilitate feed-forward processing (Hobson 1990; Hasselmo 1999). However, various lines of evidence including lesion studies, cognitive and developmental studies, suggest that dreaming may be more closely related to imagination than it is to perception (Nir and Tononi 2010). Accordingly, dreams may originate from memories and psychic motives as originally proposed by Freud (Freud 1931). In terms of signal propagation, this may be associated with a reversal of the information flow where memory representations supported by the hippocampus may give rise to visual representations supported by the visual system, reflecting ‘top-down’ signal propagation. Our data, collected during REMs in both wakefulness and REM sleep and recorded simultaneously in multiple regions in the medial temporal lobe, offer a rare opportunity to explore this question in humans. The current results show signs of possible reversal of signal propagation in the medial temporal lobe immediately following REMs in REM sleep compared to wakefulness (see Fig. 6). During wakefulness, REMs burst-activity is observed in 34 the parahippocampal gyrus before it is seen in entorhinal cortex and hippocampus, suggesting that saccades recruit feed-forward visual processing of successive retinal inputs. By contrast, during REM sleep, REMs burst-activity is observed first in the hippocampus and later in the entorhinal cortex, as may be the case during ‘top-down’ mental imagery. This result is at odd with a previous fMRI study (Miyauchi, Misaki et al. 2009), however our recordings benefit from a largely better temporal resolution. In addition, a larger proportion of units increase their firing rates before saccades and notably within regions implicated in motor preparation (e.g. SMA or AC), which would fit with an involvement of frontal cortex in dream generation (see Fig. 4). Study limitations As mentioned before, our study bears some limitations notably due to the very peculiar nature of our data. For example, electrode location being determined exclusively by clinical criteria resulted in the absence of electrodes in critical regions for identifying PGO waves (e.g. the occipital lobe). This constraint limits our ability to unequivocally bridge a gap between PGO waves recorded in animals and the neuronal markers of REMs observed in our data. Similarly, the EOG montage used in our sleep studies did not permit us to record and analyze vertical eye movements. Lastly, we did not explore the question of the lateralization of brain responses to REMs in link with gaze direction (right vs. left eye movements), a phenomenon observed in animals (Nelson, McCarley et al. 1983; Steriade 2003). In the same line, it would have been interesting to better characterize REMs produced in wakefulness by analyzing the videos recorded during sleep studies. However, I could not access these sensitive recordings. 35 Conclusion: “And the goddess stirred in him unwearying strength: sleep never fell upon his eyes; but he kept sure watch always.” (Hesiod, Aegimius) We provide here an analysis of the neuronal activity underlying REMs in different vigilance states, obtained in a unique dataset of intracranial recordings in intractable epileptic patients. Our findings underscore many similarities in the properties of rapid ocular movements in wakefulness and REM sleep. Not only do REMs share similar mechanical properties, but they affect neuronal activity in a similar way. More specifically, REMs seem to "reset" brain activity as evident by robust evoked potentials across the brain and an inhibition of a large portion of recorded neurons prior to REMs onsets, which could be related to the phenomenon of saccadic suppression. The presence of this suppression in REM sleep is consistent with the idea that REMs serve the same role of exploring the visual scene in our dreams as when we are awake. Interestingly, we also observed a reversal of the information flow along entorhinal-hippocampal pathways between REM sleep and wakefulness, which could reflect differences in the processes involved in dreams compared to visual perception. It is tempting indeed to link our findings with the idea that our dreams do not reflect the mere activation of the visual system by brainstem signals, but may originate from memories in the sleeper’s mind that are then represented as a dream image. Future work should be able to further quantify the neural dynamics following REMs using approaches such as Granger causality. 36 Acknowledgements I would like to express my deepest gratitude to Yuval Nir for his friendly supervision and daily patience at every single step of this project as well as to Giulio Tononi and Chiara Cirelli. I want to thank Aaron Nelson, Chadd Funk, Michele Bellesi, Luisa de Vivo and all the members of the laboratory for their help, comments and occasional empathy. My life stretching across the Atlantic, I also thank Sid Kouider for his supervision during the first semester, the administrative staff of the CogMaster for making our lives easier, Gecia Bravo Hermsdorff for her never-ending enthusiasm and, last but not least, Chiara Varazzani for everything and beyond. I am grateful to all the persons that showed interest in my work and who gave me the needed feeling that this was, after all, a decent occupation for an adult. Finally, my gratitude goes to all the anonymous patients who accepted to participate in this study. 37 References: Abe, T., K. Ogawa, et al. (2004). "Lack of presaccadic positivity before rapid eye movements in human REM sleep." Neuroreport 15(4): 735-738. Amzica, F. and M. Steriade (1996). "Progressive cortical synchronization of ponto-geniculo-occipital potentials during rapid eye movement sleep." Neuroscience 72(2): 309-314. Andrillon, T., Y. Nir, et al. (2011). "Sleep Spindles in Humans: Insights from Intracranial EEG and Unit Recordings." Journal of Neuroscience 31(49): 17821-17834. Arnulf, I. (2011). "The 'scanning hypothesis' of rapid eye movements during REM sleep: a review of the evidence." Archives Italiennes De Biologie 149(4): 367-382. Aserinsky, E. and N. Kleitman (1953). "Regularly occurring periods of eye motility, and concomitant phenomena, during sleep." Science 118(3062): 273-274. Ballard, D. H., M. M. Hayhoe, et al. (1997). "Deictic codes for the embodiment of cognition." Behav Brain Sci 20(4): 723-742; discussion 743-767. Barlow, J. S. and L. Ciganek (1969). "Lambda responses in relation to visual evoked responses in man." Electroencephalogr Clin Neurophysiol 26(2): 183-192. Berchicci, M., A. Stella, et al. (2012). "Spatio-temporal mapping of motor preparation for self-paced saccades." Biol Psychol 90(1): 10-17. Berger, R. J. and I. Oswald (1962). "Eye movements during active and passive dreams." Science 137(3530): 601. Bowker, R. M. and A. R. Morrison (1976). "The startle reflex and PGO spikes." Brain Res 102(1): 185-190. Bremmer, F., M. Kubischik, et al. (2009). "Neural dynamics of saccadic suppression." J Neurosci 29(40): 12374-12383. Brooks, D. C. (1968). "Localization and characteristics of the cortical waves associated with eye movement in the cat." Exp Neurol 22(4): 603-613. Brooks, D. C. (1968). "Waves associated with eye movement in the awake and sleeping cat." Electroencephalogr Clin Neurophysiol 24(6): 532-541. Brooks, D. C. and E. Bizzi (1963). "Brain Stem Electrical Activity during Deep Sleep." Archives Italiennes De Biologie 101: 648-665. Burr, D. and M. C. Morrone (2005). "Eye movements: building a stable world from glance to glance." Curr Biol 15(20): R839-840. Callaway, C. W., R. Lydic, et al. (1987). "Pontogeniculooccipital waves: spontaneous visual system activity during rapid eye movement sleep." Cell Mol Neurobiol 7(2): 105-149. Cloherty, S. L., M. J. Mustari, et al. (2010). "Effects of saccades on visual processing in primate MSTd." Vision Res 50(24): 2683-2691. 38 Datta, S. (1999). PGO wave generation: Mecahnism and functional significance. Rapid Eye Movement Sleep. B. N. M. a. S. Inoue. New Dehli, Narosa Publishing House: 91-106. Dement, W. and N. Kleitman (1957). "The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming." J Exp Psychol 53(5): 339-346. Dodge, R. (1905). "The Illusion of Clear Vision during Eye Movement." Psychological Bulletin 2(6): 193-199. Doricchi, F., G. Iaria, et al. (2007). "The "ways" we look at dreams: evidence from unilateral spatial neglect (with an evolutionary account of dream bizarreness)." Experimental Brain Research 178(4): 450-461. Duhamel, J. R., C. L. Colby, et al. (1992). "The Updating of the Representation of Visual Space in Parietal Cortex by Intended Eye-Movements." Science 255(5040): 90-92. Farber, J., G. A. Marks, et al. (1980). "Rapid eye movement sleep PGO-type waves are present in the dorsal pons of the albino rat." Science 209(4456): 615617. Felleman, D. J., A. Burkhalter, et al. (1997). "Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex." Journal of Comparative Neurology 379(1): 21-47. Felleman, D. J. and D. C. Van Essen (1991). "Distributed Hierarchical Processing in the Primate Cerebral Cortex." Cerebral Cortex 1(1): 1-47. Fernandez-Mendoza, J., B. Lozano, et al. (2009). "Evidence of Subthalamic PGOlike Waves During REM Sleep in Humans: A Deep Brain Polysomnographic Study." Sleep 32(9): 1117-1126. Findlay, J. M. (2004). Eye scanning and visual search. The Interface of Language, Vision, and Action: Eye Movements and the Visual World. J. M. a. F. Henderson, F., Psychology Press. Freud, S. (1931). The Interpretation of Dreams. New-York, The Modern Library. Fried, I., R. Mukamel, et al. (2011). "Internally Generated Preactivation of Single Neurons in Human Medial Frontal Cortex Predicts Volition." Neuron 69(3): 548-562. Fried, I., C. L. Wilson, et al. (1999). "Cerebral microdialysis combined with singleneuron and electroencephalographic recording in neurosurgical patients. Technical note." J Neurosurg 91(4): 697-705. Gucer, G. and L. J. Viernstein (1979). "Intracranial pressure in the normal monkey while awake and asleep." J Neurosurg 51(2): 206-210. Hamker, F. H., M. Zirnsak, et al. (2011). "Computational models of spatial updating in peri-saccadic perception." Philos Trans R Soc Lond B Biol Sci 366(1564): 554-571. Hasselmo, M. E. (1999). "Neuromodulation: acetylcholine and memory consolidation." Trends Cogn Sci 3(9): 351-359. 39 Henderson, J. M. (2003). "Human gaze control during real-world scene perception." Trends Cogn Sci 7(11): 498-504. Hobson, J. A. (1990). The dreaming brain. Harmondsworth, Penguin. Hobson, J. A. and R. W. McCarley (1977). "The brain as a dream state generator: an activation-synthesis hypothesis of the dream process." Am J Psychiatry 134(12): 1335-1348. Hochstein, S. and M. Ahissar (2002). "View from the top: hierarchies and reverse hierarchies in the visual system." Neuron 36(5): 791-804. Hong, C. C. H., J. C. Harris, et al. (2009). "fMRI Evidence for Multisensory Recruitment Associated With Rapid Eye Movements During Sleep." Human Brain Mapping 30(5): 1705-1722. Iber, C., S. Ancoli-Israel, et al. (2007). AASM Manual for the Scoring of Sleep and Associate Events. Rules, terminology and technical specifications. . Westchester, IL, American Association of Sleep Medicine. Jones, L. M., A. Fontanini, et al. (2007). "Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles." Proc Natl Acad Sci U S A 104(47): 18772-18777. Jouvet, M. (1992). Le sommeil et le rêve. Paris, Odile Jacob. Jouvet, M. and F. C. Michel, J. (1959). "L'activite electrique du rhinencephale au cours dusommeil chez le chat." C.R. Soc. Biol.(153): 101–105. Kayama, Y., R. R. Riso, et al. (1979). "Luxotonic Responses of Units in Macaque Striate Cortex." Journal of Neurophysiology 42(6): 1495-1517. Kleiser, R., F. J. Seitz, et al. (2004). "Neural correlates of saccadic suppression in humans." Current Biology 14(5): 386-390. Klostermann, W., D. Kompf, et al. (1994). "The Presaccadic Cortical Negativity Prior to Self-Paced Saccades with and without Visual Guidance." Electroencephalography and Clinical Neurophysiology 91(3): 219-228. Kryger, M. H., T. Roth, et al. (2011). Principles and practice of sleep medicine. St. Louis, Mo. ; London, Saunders. Lancaster, J. L., L. H. Rainey, et al. (1997). "Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method." Hum Brain Mapp 5(4): 238-242. Lancaster, J. L., M. G. Woldorff, et al. (2000). "Automated Talairach atlas labels for functional brain mapping." Hum Brain Mapp 10(3): 120-131. Latour, P. L. (1962). "Visual threshold during eye movements." Vision Research 2(3): 261-262. Leclair-Visonneau, L., D. Oudiette, et al. (2010). "Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model." Brain 133(Pt 6): 1737-1746. Lehmann, D. (1971). "Multichannel Topography of Human Alpha Eeg Fields." Electroencephalography and Clinical Neurophysiology 31(5): 439-&. 40 Lim, A. S., A. M. Lozano, et al. (2007). "Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons." Sleep 30(7): 823827. Liu, H., Y. Agam, et al. (2009). "Timing, timing, timing: fast decoding of object information from intracranial field potentials in human visual cortex." Neuron 62(2): 281-290. Massimini, M., F. Ferrarelli, et al. (2005). "Breakdown of cortical effective connectivity during sleep." Science 309(5744): 2228-2232. Matin, E., A. B. Clymer, et al. (1972). "Metacontrast and saccadic suppression." Science 178(4057): 179-182. McCarley, R. W. (2007). "Neurobiology of REM and NREM sleep." Sleep Medicine 8(4): 302-330. Mccarley, R. W., J. W. Winkelman, et al. (1983). "Human Cerebral Potentials Associated with Rem-Sleep Rapid Eye-Movements - Links to Pgo Waves and Waking Potentials." Brain Research 274(2): 359-364. Melcher, D. (2005). "Spatiotopic transfer of visual-form adaptation across saccadic eye movements." Curr Biol 15(19): 1745-1748. Miyauchi, S., M. Misaki, et al. (2009). "Human brain activity time-locked to rapid eye movements during REM sleep." Exp Brain Res 192(4): 657-667. Miyauchi, S., R. Takino, et al. (1987). "Electrophysiological evidence for dreaming: human cerebral potentials associated with rapid eye movement during REM sleep." Electroencephalogr Clin Neurophysiol 66(4): 383-390. Mormann, F., S. Kornblith, et al. (2008). "Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe." J Neurosci 28(36): 8865-8872. Morrison, A. R. and O. Pompeiano (1966). "Vestibular influences during sleep. IV. Functional relations between vestibular nuclei and lateral geniculate nucleus during desynchronized sleep." Archives Italiennes De Biologie 104(4): 425-458. Mort, D. J., R. J. Perry, et al. (2003). "Differential cortical activation during voluntary and reflexive saccades in man." Neuroimage 18(2): 231-246. Nelson, J. P., R. W. McCarley, et al. (1983). "REM sleep burst neurons, PGO waves, and eye movement information." J Neurophysiol 50(4): 784-797. Nir, Y., R. Mukamel, et al. (2008). "Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex." Nat Neurosci 11(9): 1100-1108. Nir, Y., R. J. Staba, et al. (2011). "Regional slow waves and spindles in human sleep." Neuron 70(1): 153-169. Nir, Y. and G. Tononi (2010). "Dreaming and the brain: from phenomenology to neurophysiology." Trends Cogn Sci 14(2): 88-100. 41 Pace-Schott, E. F. and J. A. Hobson (2002). "The neurobiology of sleep: genetics, cellular physiology and subcortical networks." Nat Rev Neurosci 3(8): 591-605. Peigneux, P., S. Laureys, et al. (2001). "Generation of rapid eye movements during paradoxical sleep in humans." Neuroimage 14(3): 701-708. Pierrot-Deseilligny, C., D. Milea, et al. (2004). "Eye movement control by the cerebral cortex." Curr Opin Neurol 17(1): 17-25. Quiroga, R. Q., Z. Nadasdy, et al. (2004). "Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering." Neural Comput 16(8): 1661-1687. Quiroga, R. Q., L. Reddy, et al. (2005). "Invariant visual representation by single neurons in the human brain." Nature 435(7045): 1102-1107. Reppas, J. B., W. M. Usrey, et al. (2002). "Saccadic eye movements modulate visual responses in the lateral geniculate nucleus." Neuron 35(5): 961974. Riedner, B. A., V. V. Vyazovskiy, et al. (2007). "Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans." Sleep 30(12): 1643-1657. Riesenhuber, M. and T. Poggio (2002). "Neural mechanisms of object recognition." Curr Opin Neurobiol 12(2): 162-168. Rodland, E. A. (2006). "Simes' procedure is 'valid on average'." Biometrika 93(3): 742-746. Roffwarg, H. P., J. N. Muzio, et al. (1966). "Ontogenetic development of the human sleep-dream cycle." Science 152(3722): 604-619. Sastre, J. P. and M. Jouvet (1979). "[Oneiric behavior in cats]." Physiol Behav 22(5): 979-989. Schall, J. D. (1991). "Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields." J Neurophysiol 66(2): 559-579. Schall, J. D. (1991). "Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys." J Neurophysiol 66(2): 530-558. Schiller, P. H. and E. J. Tehovnik (2005). "Neural mechanisms underlying target selection with saccadic eye movements." Prog Brain Res 149: 157-171. Schraa-Tam, C. K., P. van Broekhoven, et al. (2009). "Cortical and cerebellar activation induced by reflexive and voluntary saccades." Exp Brain Res 192(2): 175-187. Shibasaki, H. and M. Hallett (2006). "What is the Bereitschaftspotential?" Clinical Neurophysiology 117(11): 2341-2356. Siapas, A. G., E. V. Lubenov, et al. (2005). "Prefrontal phase locking to hippocampal theta oscillations." Neuron 46(1): 141-151. 42 Sprenger, A., M. Lappe-Osthege, et al. (2010). "Eye movements during REM sleep and imagination of visual scenes." Neuroreport 21(1): 45-49. Steriade, M. (2003). Neuronal Substrates of Sleep and Epilepsy. Cambridge, UK, Cambridge University Press. Super, H., C. van der Togt, et al. (2004). "Correspondence of presaccadic activity in the monkey primary visual cortex with saccadic eye movements." Proceedings of the National Academy of Sciences of the United States of America 101(9): 3230-3235. Susic, V. T. and R. M. Kovacevic (1973). "Sleep patterns in the owl Strix aluco." Physiol Behav 11(3): 313-317. Takahashi, K. and Y. Atsumi (1997). "Precise measurement of individual rapid eye movements in REM sleep of humans." Sleep 20(9): 743-752. Takeuchi, D., T. Hirabayashi, et al. (2011). "Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex." Science 331(6023): 1443-1447. Tehovnik, E. J., M. A. Sommer, et al. (2000). "Eye fields in the frontal lobes of primates." Brain Research Reviews 32(2-3): 413-448. Timofeev, I., F. Grenier, et al. (2001). "Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study." Proc Natl Acad Sci U S A 98(4): 1924-1929. Timofeev, I., F. Grenier, et al. (2001). "Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study." Proceedings of the National Academy of Sciences of the United States of America 98(4): 1924-1929. Tononi, G. and M. Massimini (2008). "Why does consciousness fade in early sleep?" Ann N Y Acad Sci 1129: 330-334. Vanni-Mercier, G. and G. Debilly (1998). "A key role for the caudoventral pontine tegmentum in the simultaneous generation of eye saccades in bursts and associated ponto-geniculo-occipital waves during paradoxical sleep in the cat." Neuroscience 86(2): 571-585. VanRullen, R. and C. Koch (2003). "Is perception discrete or continuous?" Trends Cogn Sci 7(5): 207-213. Volkmann, F. C. (1962). "Vision during voluntary saccadic eye movements." J Opt Soc Am 52: 571-578. Watson, T. L. and B. Krekelberg (2009). "The relationship between saccadic suppression and perceptual stability." Curr Biol 19(12): 1040-1043. Wurtz, R. H. (2008). "Neuronal mechanisms of visual stability." Vision Res 48(20): 2070-2089. Wurtz, R. H., W. M. Joiner, et al. (2011). "Neuronal mechanisms for visual stability: progress and problems." Philos Trans R Soc Lond B Biol Sci 366(1564): 492-503. 43 44 Appendices: Appendix Table 1: Patient information Clinical information and sleep study details for all patients who participated in the sleep studies. P415, P422, P423, P424 have also participated in the visual recognition paradigm. Information not available for the 5 patients who participated in the visual paradigm only. 45 46 Appendix 1: Sleep parameters are similar to natural sleep in healthy individuals A. Representative examples of 30s epochs extracted from one patient depicting sleep stages used in our scoring: wakefulness, NREM2, NREM3 and REM sleep. B. Sleep parameters computed across the entire night and expressed as mean ± SEM (n=13 patients). Sleep efficiency corresponds to total sleep time per time spent in bed. Sleep latency is calculated to stage NREM2. WASO refers to Waking After Sleep Onset. C. Hypnograms and power spectra of scalp EEG in all derivations for 10 individuals (top to bottom: W: wake; R: REM; NREM stages: N1, N2, and N3). Colors code in power spectra is: Blue, NREM2; Red, NREM3; Green, REM sleep as in Fig. 1C. 47 48 Appendix 2: Unit identification and stability of the recordings A. Illustration of unit identification: LFP was high-pass filtered (top row) and a threshold was set at 5 SDs above the biological noise level (red line). Action potential waveforms were then clustered (middle row, 3 representative examples). Inter-spike-intervals (ISIs) distribution was computed to help separate clusters and to determine wetter clusters were single or multi-units (bottom row). Based on parameters, clusters were categorized as: multi-unit clusters (example in blue), single-unit clusters (red) or noise clusters (black). B. Waveforms and ISI distributions are conserved throughout sleep and remain separable. Mean action potential waveforms for 5 distinct units recorded in the same patient. Each color denotes a different unit and each trace denotes the mean waveform in separate 1h intervals throughout sleep. Note the stability of our unit recordings throughout long (~7h) recordings. Inset depicts ISI distributions for two units (red and green curves) separated by1h intervals. C. A full description of the brain structures and number of units identified in our sleep recordings. Grey boxes mark implanted brain regions and numbers show detected units. Total number of implanted regions and units are displayed by patients. (LH, left hemisphere; RH, right hemisphere; H, hippocampus; A, amygdala; EC, entorhinal cortex; PH, parahippocampal gyrus; TG, temporal gyrus; AC, anterior cingulate; MC, middle cingulate; PC, posterior cingulate; OF, orbitofrontal and medial prefrontal cortex; SM, supplementary motor area; P, parietal cortex; TO, temporo-occipital, PT, posterior temporal cortex; FG, fusiform gyrus; IFG, inferior frontal gyrus) 49 50 Appendix 3: Examples of units responsive to pictures Two examples of units responsive to pictures in the object recognition task for a high-firing responsive unit (panel A) and a low-firing unit (panel B) in one individual. In each panel, all stimuli (4 face, 2 place images) used during the session are displayed. Below each picture, raster plots depicting units’ activity in all 24 trials are shown. Post-event histograms are also displayed and were used to detect responsive units for each visual stimulus. As described in the Methods, spikes were binned (100ms) and statistical deviance from baseline was computed. Significant bins are colored in red (one-tail Student t-test, α=0.001). Responsive units are highlighted with a green box. In our analysis only trials for which units were declared responsive are analyzed. Note that units can have different pattern of responses to stimuli: the unit in panel A responds to most pictures displayed while the unit in panel B is more selective. 51