* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Transmitters and Modulation of Sleep
Rapid eye movement sleep wikipedia , lookup
Sleep paralysis wikipedia , lookup
Apical dendrite wikipedia , lookup
Mirror neuron wikipedia , lookup
Neuroscience of sleep wikipedia , lookup
Metastability in the brain wikipedia , lookup
Subventricular zone wikipedia , lookup
Neural coding wikipedia , lookup
Sleep and memory wikipedia , lookup
Synaptogenesis wikipedia , lookup
Axon guidance wikipedia , lookup
Electrophysiology wikipedia , lookup
Sleep medicine wikipedia , lookup
Central pattern generator wikipedia , lookup
Neural oscillation wikipedia , lookup
Multielectrode array wikipedia , lookup
Spike-and-wave wikipedia , lookup
Sleep deprivation wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Nervous system network models wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Non-24-hour sleep–wake disorder wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Effects of sleep deprivation on cognitive performance wikipedia , lookup
Hypothalamus wikipedia , lookup
Development of the nervous system wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Start School Later movement wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroanatomy wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Circumventricular organs wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Chemical Transmitters and Modulation of Sleep vs.
Wake Promoting Neurons
Mandana Modirrousta M.D.
Department ofNeurology and Neurosurgery
Neuroscience Program
McGiIl University, Montreal, Quebec, Canada
December 2005
Supervisor
Barbara E. Jones Ph.D.
A thesis submitted to the
Faculty of Graduate Studies and Research
In partial fulfillment of the requirements for the degree
Doctor ofPhilosophy
© Mandana Modirrousta, 2005
1+1
Library and
Archives Canada
Bibliothèque et
Archives Canada
Published Heritage
Branch
Direction du
Patrimoine de l'édition
395 Wellington Street
Ottawa ON K1A ON4
Canada
395, rue Wellington
Ottawa ON K1A ON4
Canada
Your file Votre référence
ISBN: 978-0-494-25212-3
Our file Notre référence
ISBN: 978-0-494-25212-3
NOTICE:
The author has granted a nonexclusive license allowing Library
and Archives Canada to reproduce,
publish, archive, preserve, conserve,
communicate to the public by
telecommunication or on the Internet,
loan, distribute and sell th es es
worldwide, for commercial or noncommercial purposes, in microform,
paper, electronic and/or any other
formats.
AVIS:
L'auteur a accordé une licence non exclusive
permettant à la Bibliothèque et Archives
Canada de reproduire, publier, archiver,
sauvegarder, conserver, transmettre au public
par télécommunication ou par l'Internet, prêter,
distribuer et vendre des thèses partout dans
le monde, à des fins commerciales ou autres,
sur support microforme, papier, électronique
et/ou autres formats.
The author retains copyright
ownership and moral rights in
this thesis. Neither the thesis
nor substantial extracts from it
may be printed or otherwise
reproduced without the author's
permission.
L'auteur conserve la propriété du droit d'auteur
et des droits moraux qui protège cette thèse.
Ni la thèse ni des extraits substantiels de
celle-ci ne doivent être imprimés ou autrement
reproduits sans son autorisation.
ln compliance with the Canadian
Privacy Act some supporting
forms may have been removed
from this thesis.
Conformément à la loi canadienne
sur la protection de la vie privée,
quelques formulaires secondaires
ont été enlevés de cette thèse.
While these forms may be included
in the document page count,
their removal does not represent
any loss of content from the
thesis.
Bien que ces formulaires
aient inclus dans la pagination,
il n'y aura aucun contenu manquant.
•••
Canada
q'o my lius6ana, Œelizaâ
ana
my ûttfe son, (JJarsa
wlio inspirea me witli tlie passion of l'ove ana Eife
ana
to my supervisor
wlio tauglit me tlie meanino ofpatience ana e~ce{fent researcli.
II
Abstract
Aithough evidence has suggested a dual role of the basal forebrain (BF) in arousai
and sleep generation, the neurotransmitter identity and activity ofBF neurons serving
these different functions have remained uncertain. Furthermore, few studies have been
done to clarify how the wake vs. sleep promoting neurons may be modulated to generate
their differential activity and sleep-wake cycle. Using a paradigm of sleep deprivation
and sleep recovery and examining c-Fos expression as an indicator of cell activity, we
found that across the BF and the adjacent preoptic area, more cells including cholinergie
neurons were active during waking than during sleep and thus contribute to generating a
waking state. On the other hand, the proportion of c-Fos expressing neurons that were
GABAergic was higher during sleep recovery than sleep deprivation, indicating that
particular GABAergic cells are involved in generating sleep.
Aithough the posterior hypothalamus has long been known to play a critical role
in the maintenance ofwaking, the neurotransmitter identity ofneurons fulfilling this role
was not known. Here using c-Fos, we show that Orexin (Orx) neurons are active during
waking. On the other hand, we show that co-distributed cells containing melanin
concentrating hormone (MCH) are more active during sleep recovery than sleep
deprivation, suggesting an opposite role ofMCH to that of Orx neurons in sleep vs.
waking regulation.
Sleep vs. wake promoting cells could be differently modulated by noradrenaline
(NA) and accordingly would bear different adrenergic receptors. We found that the
majority ofGABAergic BF cells expressing c-Fos during sleep bear alpha2 adrenergic
III
receptors (U2AAR). They would accordingly be inhibited during waking through these
receptors. We also found that many Orx cells in the hypothalamus bear UIAAR and thus
would be excited by NA during waking. Like the BF GABAergic cells, many MeR
neurons were endowed with U2AAR and thus would be inhibited during waking.
Activation vs. inhibition of sleep or wake-active cells could also be modulated by
changes in the availability of cell surface receptors across behavioral states. We show
that following sleep deprivation, the presence and the intensity of GABAARs on the BF
cholinergie cell membrane were increased. Such activity dependent changes of
GABAARs could underlie homeostatic regulation ofwake promoting cells across the
sleep-waking cycle.
These studies identify clearly for the first time the specific neurotransmittercontaining neurons that are active during and thus generative for waking vs. sleep
through the BF, the preoptic area and the posterior hypothalamus. They moreover
illuminate how by bearing different receptors or changing membrane receptor
availability, different cells can be modulated to generate the sleep-waking cycle.
IV
Résumé
Bien qu'il ait été suggéré que le télencéphale basal joue un double rôle dans la
génération de l'éveil et du sommeil, l'identité des neurotransmetteurs ainsi que l'activité
des neurones de cette structure permettant l'établissement de ces différentes fonctions
restent à éclaircir. En outre, peu d'études ont été conduites pour clarifier comment les
divers neurones impliqués peuvent être modulés pour permettre leurs activités
différentielles pour générer le cycle veille-sommeil. Utilisant un modèle de privation et
de rétablissement de sommeil et en examinant l'expression de c-Fos comme un indicateur
d'activité cellulaire, nous avons trouvé que dans l'ensemble du télencéphale basal et
l'aire préoptique adjacente plus de cellules, incluant les neurones cholinergiques, étaient
actives durant l'éveil que pendant le sommeil et ainsi contribuent à la génération de l'état
d'éveil. D'autre part, parmi les neurones exprimant c-Fos, la proportion des cellules
GABAergiques était plus grande pendant le rétablissement que durant la privation de
sommeil, indiquant que les cellules GABAergiques sont particulièrement impliquées dans
la génération du sommeil.
Bien que l'hypothalamus postérieur ait longtemps été reconnu pour jouer un rôle
critique dans le maintien de l'éveil, l'identité du neurotransmetteur des neurones
remplissant ce rôle n'était pas connue. En utilisant c-Fos, nous avons montré que les
neurones exprimant les peptides Orexine (Orx) sont actifs durant l'éveil. Par ailleurs,
nous avons montré que les neurones co-distribués contenant 1'hormone de mélanoconcentration (MeR) sont plus actifs durant le rétablissement du sommeil que pendant la
v
privation de sommeil, suggérant un rôle opposé des neurones à MCH à celui des
neurones à Orx dans la régulation du sommeil et de l'éveil.
Les cellules à l'origine du sommeil et celles promouvant l'éveil pourraient être
différemment modulées par la noradrénaline et porteraient par conséquent des récepteurs
adrénergiques différents. Nous avons trouvé que la majorité des neurones
GABAergiques du télencéphale basal exprimant c-Fos durant le sommeil porte des
récepteurs adrénergiques alpha2 (Œ2AAR). Ces cellules seraient donc inhibées durant
l'éveil à travers ces récepteurs. Nous avons aussi trouvé que beaucoup de neurones à Orx
dans l'hypothalamus portent ŒIAAR et ainsi seraient excités par noradrénaline pendant
l'éveil. Comme les cellules GABAergiques du télencéphale basal, beaucoup de neurones
à MCH sont dotés de Œ2AAR et seraient donc inhibés durant l'éveil.
L'activation ou l'inhibition des cellules pendant le sommeil et l'éveil pourraient
aussi être modulées par des changements de disponibilité des récepteurs membranaires
lors des différents états. Nous avons montré que la présence ou le nombre des GABAARs
sur la membrane des cellules cholinergiques du télencéphale basal sont augmentés après
une période de privation de sommeil. De tels changements de GABAARs qui dépendent
de l'activité pourraient être à la base de la régulation des cellules générant l'éveil pendant
le cycle veille-sommeil.
Ces études identifient clairement pour la première fois, dans le télencéphale basal,
l'aire préoptique et l'hypothalamus postérieur, des neurones contenant un
neurotransmetteur spécifique et qui favorisent l'éveil ou le sommeil. De plus, elles
révèlent comment, en portant des récepteurs différents ou en changeant la disponibilité
VI
des récepteurs membranaires, des cellules différentes peuvent être modulées pour générer
le cycle veille-sommeil.
VII
Note to examiners
This thesis consists of 4 manuscripts, 3 published, 1 in preparation for
submission. The author ofthis thesis is the main researcher and first author in 3, and coauthor in 1 manuscript. The original publisher and co-authors have given their written
consents that these manuscripts be included in this thesis. The manuscripts citations are:
1. Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE
Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain
neurons. Eur J Neurosci. 2003 Aug;18(3):723-7.
2. Modirrousta, M, Mainville L, Jones BE
Gabaergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area
express c-Fos during sleep. Neuroscience.2004;129(3):803-10.
3. Modirrousta, M, Mainville L, Jones BE
Orexin and MeH neurons express c-Fos differently after sleep deprivation vs. recovery
and bear different adrenergic receptors. Eur J Neurosci. 2005 May;21(10):2807-16.
4. Modirrousta, M, Mainville L, Jones BE
GABAA receptor modifications on basal forebrain cholinergie cells across the sleepwaking cycle (In preparation).
VIII
Contribution of authors
My contribution to the first manuscript was mapping and estimating proportions
of GABAergic cens labeled for the U2AAR in the BF and making comments on the text of
the manuscript.
1 was the principal investigator of the next three manuscripts. My contributions
were designing and performing the experimental paradigm of sleep deprivation and sleep
recovery in rats, executing the experiments inc1uding experimental manipulation and
observation, immunohistochemical processing ofbrains, image analysis and data analysis
using statistics. 1 was also the main author of the three manuscripts. The results in an of
the four manuscripts were original and novel and consisted of new findings that have
illuminated several important areas in the sleep field.
Lynda Mainville, the coauthor in the three manuscripts and the laboratory
technician, performed sorne of immunohistochemical staining.
IX
Acknowledgements
Throughout my graduate studies, 1 have had the privilege of the company,
support and friendship of a number of individuals.
1 first would like to express my sincerest gratitude to my exceptional supervisor,
Dr. Barbara Jones, who with her vast knowledge, experience, kindness and patience
guided me through a fruitful and successful graduate pro gram. 1 am especially grateful to
her as she taught me that acquiring broad knowledge from different aspects around our
theories is the pivotai principles of qualified research. Dr. Jones was always very
encouraging whenever 1 asked a question. She not only answered each of my simplest
questions with her endless patience, but she also opened new windows of other
fascinating and interesting ideas and concepts. Thank you Dr. Jones, my appreciation is
boundless.
1 must also thank Lynda Mainville, who, with her excellent skills and knowledge
in immunohistochemistry, trained me in a way where 1 finally felt confident in the
accuracy ofwhat 1 was doing. 1 thank my colleagues in the lab, the soon to be Dr. Pablo
Henny, and Drs Maan Gee Lee, Oum Hassani and Frederic Brischoux, who were always
generously available whenever 1 sought help. They also added the flavour of exciting
and stimulating scientific discussions in the lab and were always eager to hear about my
new results and findings. 1 especially thank Dr. Frederic Brischoux for his great
assistance in the French translation of the abstract.
1 extremely thank Naomi Takeda who has been a precious asset in the Complex
Neural System Unit. She always found the best possible solutions for my problems and
x
always kindly provided me with her great help and information. Her presence brings
relief to everyone who works with her.
1 thank my advisory committee members, Dr. Edith Hamel, the late Dr. Angel
Alonso and my mentor Dr. Andrea Leblanc who provided excellent advice and helped me
to obtain a broader view in my field of research.
1 deeply thank my husband, Dr. Behzad Mansouri, who was always there for me,
in life and in research as well. 1 thank him for his great help in editing and organizing my
thesis.
1 would also like to sincerely thank my parents for their eternal kindness, love and
support.
XI
Abbreviations
ACh, acetylcholine
AR, adrenergic receptor
uIAR, alphal adrenergic receptor
u2AR, alpha2 adrenergic receptor
BF, basal forebrain
ChAT, choline acetyltransferase
EEG, e1ectroencephalogram
DBB, diagonal band of Broca nuclei
DMH, dorsomedial hypothalamic nucleus
Dopamine, DA
GABA gamma amino butyric acid
GAD, glutamic acid decarboxylase
GP, globus pallidum
Interleukin l, III
LC, locus coeruleus
LH, lateral hypothalamus
LPO, lateral preoptic area
MCH, melanin concentrating hormone
MCPO, magnocellular preoptic area
mIPSCs, miniature Inhibitory Post-Synaptic Currents
MnPO, median preoptic nucleus
XII
MPO, medial preoptic area
MS, medial septum
NA, noradrenaline
Nb, Neurobiotin
NDS, nonnal donkey serum
Non-REMS, non-rapid eye movement sleep
Orx,Orexin
PF, perifomical area
POA, preoptic area
Prostoglandine D2, PGD2
PS, paradoxical sleep
REMS, rapid eye movement sleep
Rs, receptors
SC, sleep control
SCN, suprachiasmatic nucleus
SD, sleep deprivation
SI, substantia innominata
SIa, substantia innominata, anterior part
SIp, substantia innominata, posterior part
SR, sleep recovery
SWA, slow wave activity
SWS, slow wave sleep
TMN, tuberomammillary nucleus
VLPO, ventrolateral preoptic area
XIII
ZI, zona incerta
XIV
Table of contents
Abstract •....••.•.•................................................•........................................................................ III
Résumé .............••..............••..•................•.•.•.•.........•.•.••.•...........•........•.•.•.•.•.......•.•...•........•.•....... V
Note to examiners ...•.••....•.•.•.•..•.•.•.•.......•.•.•.•.......•.•.•.•........•.•.•.•.•.•....•.•.•..•....•..•.•••.•..•.....•.•.• VIII
Contribution of authors •..........••.•.•..............•.•.••.......•.•.•..........•••.•.•........••.•.•.....•.•.•.••.•.•.•.•.•.•. IX
A cknowledgements••••••••••••••••••••••.•••••••••••••••••••••••••••.•••••••••••••••••••••••••••••••••••••••••••••••••••••••••• x
Abbreviations ..................................•...•...........•...•...•........•.•.•..........••..........................•.•....... XII
Table of contents .............................•.•............•.•.•..•.........•.••••...........•............................••....... XV
1. General introduction ...•.••.•.•...•.•..••••••••••.•...•.••••••......••••.••.......••••.•.......•.•••.•...•..•.•.•........ 1
1.1 Sleep vs. wake promoting neurons in the basal forebrain and adjacent preoptic area 5
1.2 VLPO, an area of sleep active cells ........................•...........•..•...........•..••.....•...•••........•.•.•.. 7
1.3 The posterior hypothalamus and sleep vs. wake promoting neurons ........................... 9
1.4 c-Fos expression as an indicator of neuronal activity ................................................... 10
1.5 c-Fos expression in the brain across the sleep-waking cycle ........................................ 12
1.6 Modulation of sleep vs. wake promoting neurons by noradrenaline•.....•.•.•.••.•.....•.•••. 14
1.7 The dynamics of GABAA receptors during sleep vs. waking on cholinergie ceUs of the
basal forebrain ............•.•.............................................••.••.........•.•.•••......•...•.•.•.•.•••••••.•..•.•.•.•••.• 15
1.8 The first experiment......................................................................................................... 17
1.9 The second experiment ........•.•.•.......................•.•.•..•.•...•.•.•.•.•••..•..••.•.•.•.•.••••••.••.•..•...•.•.•.• 18
1.10 The third experiment ..................................................................................................... 19
1.11 The fourth experiment .....•.....•.•.•............•••.......................................................•••.......•.• 19
1.12 Preface to chapter 1 •.................•...••......•...•............•.•....................•.................•.•..•......... 21
2. Chapter 1: Alpha 2 Adrenergic Receptors on GABAergic Putative Sleep-Promoting
Basal Forebrain Neurons .•......................................................................•.•.......•....•........ 22
2.1 Abbreviations ................................................................................................................... 23
2.2 Abstract •................................•.•...........•...............•.•..........•...•.•.....•....•.......•..•.................... 24
2.3 Introduction ............•..............•...........•............................•.•.•••..........•.•.•.....•.•.•.•••......•••..... 26
2.4 Materials and Methods .........................................•..........•.•........................•...•........•.•..... 28
2.5 Results .....................•.........•....•...............•......................•.•.•..•.....•.•.•.•.•.•.•...•.•......•............. 31
2.6 Discussion...........•.•.•........•.•...•...•..••.•...............•..•........•.•.•................•.........•.........•............ 39
2. 7 Acknowledgements ................................................................•.•....................•.•.•.•......•.•.•.• 42
2.8 Preface to chapter 2 ......................................................................................................... 43
xv
3. Chapter 2: GABAergic Neurons with Alpha 2- Adrenergic Receptors in Basal
Forebrain and Preoptic Area Express c-Fos during Sieep •••••••••••••••••••••••••••••••••••••••••••• 45
3.1 Abbreviations ................................................................................................................... 46
3.2 Abstract ............................................................................................................................. 47
3.3 Introduction ...................................................................................................................... 48
3.4 Experimental procedures ................................................................................................ 51
3.5 Results ............................................................................................................................... 55
3.6 Discussion.......................................................................................................................... 68
3.7 Acknowledgments ............................................................................................................ 72
3.8 Preface to chapter 3 ......................................................................................................... 73
4. Chapter 3: Orexin and MCH Neurons Express c-Fos Differently After Sleep
Deprivation vs. Recovery and Bear Different Adrenergic Receptors••••••.•.•••••••••••••••••••• 75
4.1 Abbreviations ................................................................................................................... 76
4.2 Abstract ............................................................................................................................. 77
4.3 Introduction ...................................................................................................................... 78
4.4 Material and methods ...................................................................................................... 80
4.5 Results ............................................................................................................................... 84
Sleep deprivation and recovery ........................................................................................................... 84
c-Fos expression in Orx and MCH neurons after sleep deprivation and recovery .............................. 84
Adrenergic receptors on Orx and MCH, including c-Fos expressing, neurons after sleep deprivation
and recovery ........................................................................................................................................ 94
4.6 Discussion ........................................................................................................................ 101
c-Fos differentially expressed in Orx and MCH neurons as a function of sleep ............................... 101
Adrenergic receptors differentially distributed on Orx and MCH including c-Fos expressing neurons
.......................................................................................................................................................... 104
4. 7 Acknowledgements ......................................................................................................... 106
4.8 Preface to chapter 4 ............................................................................•••.........••.•.•.....•.•• 107
5. Chapter 4: GABAA Receptor Modifications on Basal Forebrain Cholinergie Cells
across the Sleep-Waking Cycle ...................................................................................... 109
5.1 Abbreviations ................................................................................................................. 110
5.2 Abstract .......................................•............................•...................................................... 111
5.3 Introduction .................................................................................................................... 112
5.4 Materials and Methods .......................................................•.•.•........•...•........•.•........•.•.•. 114
5.5 Results ............................................................................................................................. 119
Sleep amount in the three experimental groups ................................................................................ 119
Cholinergie cells immunostained with GABAAR ............................................................................. 119
Luminance ofGABAAR membrane staining across conditions ........................................................ 121
5.6 Discussion .................•................•..............•...............................................................•...... 127
5.7 Acknowledgements ........•...............................•.........•.•.•..................•..........•...•................ 131
XVI
6. General Discussion ...............•.............•............•......•.•..••..•.....•.....•....•.•..•....•..........•.. 132
7. Append"ix...................•....................................................•...........•.•.......•..•..•.•............. 152
7.1 Ethics approval •.••.•.•.•.••.•.•.•.••••..•••.•...••.•.•....•.•.•......•.....................••.•..•.•••.••.••••.•••.••.•...... 153
7.2 Consent letters •.•.•..•.•.•.•.•.•••.•••••••••.•••.•.••••.................................•..•••.••••••.•.•.•...•.•............. 154
7.
References •.•.•...•.•.•....•.•.•.•.•.....•.....•.•..•.•......•..........•.•.•.•.•...•.•..•••.••••.•..•.•..•............• 159
8.
Articles hard prints..............•...•...................•.•...•......•.....•.•.•...•...•.•.•.••••.•........•.•..•.• 190
XVII
1. General introduction
Sleep and wakefulness are two different behavioral states that occur in all mammals
in a cyc1ical manner. Sleep is characterized by behavioral unresponsiveness together
with slow activity in the electroencephalogram (EEG) whereas wakefulness is defined by
behavioral responsiveness together with fast activity in the EEG. Since early studies,
several regions in the brain have been shown to play roles in the generation of waking.
Multiple ascending arousal systems are located in the brainstem. They project forward
through a dorsal pathway to the thalamic nuc1ei that form the non-specifie thalamocortical projection system. This system stimulates in tum cortical activation and arousal
in a widespread manner (Jones, 2000). In addition, through a second ventral
extrathalamic relay pathway, the brainstem arousal systems project through the
hypothalamus up to the basal forebrain (BF) from where the basalo-cortical system also
projects to the entire cortex and stimulates cortical activation (Starzl et al., 1951, Jones,
2000). In addition to the BF, a critical role of the posterior hypothalamus in maintenance
ofwaking has long been known (Jones, 2000). Indeed, the role ofthe posterior
hypothalamus in maintenance of waking was first recognized during the large European
epidemic of encephalitis lethargica (1916-1927) when von Economo observed that
encephalitic lesions in the posterior hypothalamus resulted in hypersomnia, and
conversely similar lesions in the anterior hypothalamus were accompanied by insomnia
(von Economo, 1931). Since then, it has been suggested that the posterior hypothalamus
induces wakefulness whereas the anterior hypothalamus is more important for sleep
promotion. Later unit recording revealed that the majority of posterior hypothalamic
1
cells fire during waking (Szymusiak et al., 1989). It appears thus that in addition to the
brainstem, the BF and the posterior hypothalamus play important roles in regulation of
arousal.
With regard to sleep generation, initially it was believed that sleep occurs when the
arousal systems are be10w the threshold to become activated. However, studies later
suggested that sleep per se is an active process. Early studies showed that lesions in
sorne brain regions inc1uding the BF produced insomnia (McGinty and Sterman, 1968).
These studies led to the hypothesis that in addition to wake promoting areas, there are
specifie nuc1ei in the brain that are involved in sleep generation. Indeed, it was later
shown that sorne cells in the BF and the adjacent preoptic areas (POA) are active during
sleep and not during waking and therefore could participate in sleep generation and
maintenance (Detari et al., 1984, Szymusiak and McGinty, 1986a, Koyama and Hayaishi,
1994).
From several pharmacologie al and lesion studies as well as unit recordings, it is
now known that different neurotransmitters as well as endogenous factors act on multiple
regions to promote and maintain sleep or wakefulness. Accordingly the major ascending
arousal systems consist of fibres utilizing different transmitters inc1uding reticular
formation fibres that like1y use glutamate (Jones, 1995), locus coeruleus (LC) neurons
that contain noreadrenaline (NA), mesencephalic tegmental neurons that synthesize
dopamine (DA), acetylcholine (ACh)-containing pontomesencephalic neurons and
midline raphe nuc1ei that synthesize and release 5HT. While the re1ease of glutamate is
considered to be the major component of cortical activation, it is shown that other
neurotransmitters contribute each in a different way to maintain or enhance wakefulness.
Pharmacological studies have shown that drugs which increase the NA transmission such
2
as amphetamine either prolong or enhance waking (Hartmann and Cravens, 1976).
Consistently, LC noradrenergic neurons discharge maximally during waking and
minimally during sleep (Aston-Jones and Bloom, 1981). Similar to LC neurons, 5HT
containing cells tire maximally during waking, decrease their discharge during slow wave
sleep (SWS) and cease tiring during paradoxical sleep (PS) (Jacobs and Fornal, 1991).
However, the role of 5HT in sleep and waking regulation appears to be paradoxical as the
raphe neurons have also inhibiting influence on other activating systems including AChcontaining cells (Leonard and Llinas, 1990, Luebke et al., 1992, Cape et al., 1997).
Furthermore, tricyclic antidepressant drugs that selective1y inhibit 5HT reuptake have
sedative effects (Mayers and Baldwin, 2005). The cholinergie pontomesencephalic
neurons of the brainstem can act on different target cell populations to promote cortical
activation (Jones, 2005). Finally, DA-containing neurons also participate in the
formation of the major ascending arousal projections and their activity is associated with
enhanced arousal as well as positive1y rewarding states.
In addition to the chemical transmitters of ascending arousal systems, a number of
endogenous factors such as adenosine, prostaglandin-D2 (PGD2) and interleukin 1(I11)
have been shown to be regulators of the sleep-waking cycle (Lue et al., 1988, Satoh et al.,
1996, Hayaishi and Urade, 2002). Both adenosine and PGD2 accumulate in the brain
during arousal and sleep deprivation (Ram et al., 1997, Porkka-Heiskanen et al., 2000).
The stimulant effect of coffee is large1y due to the antagonistic effect of caffeine on
adenosine receptors (Yanik et al., 1987, Virus et al., 1990). Subarachnoid infusion of
adenosine A2 receptor agonists promotes sleep (Satoh et al., 1996, Satoh et al., 1998).
Similarly, infusion ofPGD2 in the subarachnoid space promotes sleep with a dosedependent increase oflocal adenosine (Matsumura et al., 1994). The involvement of III
3
in the regulation of non-rapid eye movement sleep (non-REMS) has been now suggested
by multiple lines of evidence (Obal and Krueger, 2003, Alam et al., 2004). It has been
shown that ILl concentrations in the cerebrospinal fluid peak at the onset of sleep (Lue et
al., 1988). The sleep modulatory role of III is proposed to be mediated by suppressing
the wake-active and activating the sleep-active neurons of the BF and POA (Alam et al.,
2004). These factors are likely synthesized and released by glial cells or multiple
neurons as a function of activity and diffuse through the cerebrospinal to act upon
multiple systems.
Despite considerable evidence showing the involvement of particular chemical
transmitters or modulators in the generation of sleep-wake states, little has been done to
chemically identify neurons of the BF, the POA and the posterior hypothalamus that are
specifically involved in sleep or wake generation. Lesion studies in these regions for
instance, mostly reveal the significance of the area rather than of a specific cell type
within the area in the regulation ofbehavioral states. In most lesion studies, multiple
types of cells and fibres are destroyed. Therefore, it would be extremely inappropriate to
relate and interpret only a specific neuron or neurotransmitter of that region as due to the
consequence oflesions. Unit recording across sleep-wake states bears also sorne
limitations; the majority of these studies record the discharge profiles of neurons in a
region without chemically identifying them. Furthermore, only a small number of cells
are recorded at a time and the activity pattern of all cell types across states can not be
easily studied. Understanding the chemical transmitters that regulate sleep vs. waking
not only improves our knowledge about the neurophysiology of sleep but it could
potentially also illuminate the underlying causes of sleep-wake disorders.
4
1.1 Sleep vs. wake promoting neurons in the basal forebrain and
adjacent preoptic area
The BF neurons lie in the ventral path of the ascending arousal systems and receive
inputs from brainstem noradrenergic and cholinergie cells (Semba et al., 1988, Jones and
Cuello, 1989). The BF therefore acts as a re1ay centre for cortical activation. In addition
however, electrical stimulations in the BF and adjacent POA also e1icit cortical slow
wave activity (SWA) and SWS (Sterman and Clemente, 1962a). Recording studies
moreover revealed that in the BF while many cells fire maximally in association with
cortical activation and waking, many cells fire maximally during cortical SWA and SWS
(Lee et al., 2004). It appears thus that the BF could have a dual role in the regulation of
sleep-waking cycle. As for the POA, it has been shown that many neurons in the POA
increase their overall rate of discharge during SWS (Findlay and Hayward, 1969,
McGinty and Szymusiak, 1988). In paralle1, lesions in the BF and the adjacent POA
were found to produce insomnia (Szymusiak and McGinty, 1986b, Sallanon et al., 1989).
Lesions in the lateral and the medial preoptic areas (LPO and MPO respective1y) resulted
in a long-lasting insomnia, characterized by a significant increase in wakefulness and
decrease in SWS (Schmidt et al., 2000, Thomas and Kumar, 2000).
The BF consists of different cell types including cholinergie cells that contain ACh,
GABAergic cells that contain gamma amino butyric acid (GABA) and glutamatergic
cells that contain glutamate (Gritti et al., 1993, Manns et al., 2001). It is thus like1y that
the dual role of the BF in sleep vs. waking regulation is performed by neurons containing
different neurotransmitters.
5
BF cholinergie cells are distributed through the globus pallidum (GP),
magnocellular proptic area (MCPO), substantia innominata (SI), medial septum (MS) and
diagonal band of Broca (DBB). Lesions of the BF neurons cause deficits in cortical
activation (Stewart et al., 1984). BF cholinergic cells project to the entire neocortex and
limbic te1encephalon, exert excitatory effects on target cells and thereby elicit activated
EEG patterns characteristic of waking and rapid eye movement sleep REMS (Szymusiak,
1995). Unit recording together with juxtacellular labeling in anesthetized and
unanesthetized rats further revealed that all cholinergic cells dis charge in bursts and have
their maximal activity during cortical activation characterized by fast waves in the EEG
(Lee et al., 2003). Pharmacological and microdialysis studies have moreover e1ucidated
the significance of ACh in cortical activation and arousal (Marrosu et al., 1995, Jones,
2004).
GABA is the most frequent inhibitory neurotransmitter of the brain. This
neurotransmitter is found in the BF and POA neurons projecting to the cortex and the
neurons projecting to the posterior hypothalamus (Szymusiak et al., 1989, Gritti et al.,
1994, Gritti et al., 1997). In addition, there are sorne other locally projecting presumed
GABAergic cells in the BF that may have a local inhibitory role on the BF cholinergic
cells (Manns et al., 2000a). In unit recording studies in anesthetized rats the majority of
identified or presumably GABAergic cells of the BF and the POA fired maximally in
association with cortical SWA (Szymusiak and McGinty, 1986a, Manns et al., 2000a,
Lee et al., 2004). Altogether, it is like1y that GABAergic neurons located within the BF
and POA mediate sleep promotion. However, no study showed whether the GABAergic
cell populations across the BF and POA are active in naturally sleeping animaIs.
6
Moreover, it was not clarified whether the GABAergic cells are the only sleep-active
neurons in these regions.
Both the BF and POA are comprised of several nuclei including the MCPO, SI, MS,
DBB, LPO, ventrolateral preoptic area (VLPO), MPO and median preoptic area (MnPO).
The GABAergic cells are intermingled among other cell types across the BF and POA
nuclei. Single unit recording also showed that sleep-active neurons are also present and
distributed in the nuclei of the BF including the MCPO and SI (Lee et al., 2002) (above).
However, sorne studies have shown and argued that sleep-active neurons are mainly
confined to specifie nuclei in these areas including most importantly the VLPO and the
MnPO rather than being more widely distributed (Scammell et al., 1998, Gong et al.,
2000, Gong et al., 2004).
1.2 VLPO, an area of sleep active cells
The VLPO was recently found as a small triangular shaped group of cells located
lateral to the optic chiasm and rostral to the suprachiasmatic nucleus (SCN) (Sherin et al.,
1996). It was first shown that cells in the VLPO cluster expressed c-Fos after sleep and
the number of c-Fos expressing cells was positive1y corre1ated with the amount of sleep
(Sherin et al., 1996). c-Fos is an immediate early gene whose expression is known to be
correlated with neuronal activity. Subsequently by unit recording, it has been observed
that the frring rate ofthese neurons increased during both REMS and non-REMS
(Szymusiak et al., 1998). Later studies showed that the VLPO lesions caused longlasting and prolonged insomnia whereas the lesions in the area with scattered VLPO
7
neurons medial or dorsal to the VLPO cluster caused smaller changes in non-REMS time
and were more close1y associated with loss of REMS (Lu et al., 2000).
The majority ofVLPO neurons provide robust innervations to the nuclei ofthe
major arousal systems including the hypothalamic tuberomammillary nucleus (TMN),
(Sherin et al., 1996, Sherin et al., 1998) and the noradrenergic LC cells (Steininger et al.,
2001). The VLPO is dense1y innervated by histaminergic, noradrenergic and
serotonergic fibers (Chou et al., 2002). In vitro studies have shown that the majority of
VLPO neurons are inhibited by noradrenaline (NA) and ACh, two major transmitters of
arousal systems (Gallopin et al., 2000). The VLPO receives moderate or heavy inputs
from hypothalamic regions including the MPO, lateral hypothalamic area, and
dorsomedial hypothalamic nucleus (DMH), autonomic regions including the infralimbic
cortex and parabrachial nucleus, and limbic regions including the lateral septal nucleus
and ventral subiculum (Chou et al., 2002). There are light to moderate projections from
the posterior hypothalamic orexin (Orx) and me1anin concentrating hormone (MCH)
neurons to the VLPO and almost no projection from the BF or the brainstem cholinergic
neurons (Chou et al., 2002). In addition, the circadian pacemaker, the SCN, directlyor
indirectly, via the DMH projects to the VLPO and might underlie the influence of
circadian rhythmicity on sleep.
Nonethe1ess, severallines of evidence including lesion and in vivo studies have
suggested that the influence of the BF and POA regions on sleep is not exclusively
restricted to the VLPO cell cluster (Szymusiak and McGinty, 1986b, Szymusiak and
McGinty, 1989, Manns et al., 2000a, Gong et al., 2004). With a precise1y designed
paradigm of sleep deprivation (SD) and sleep recovery (SR), we tried to answer whether
8
sleep-active cells are only located in specific nuclei including the VLPO or the MnPO or
rather are distributed across the BF and POA.
1.3 The posterior hypothalamus and sleep vs. wake promoting neurons
Similar to the BF, the posterior hypothalamus contains different cell types including
cells containing the peptides Orx and MCR. These two cell types have been recently
found to be exclusively located in the posterior hypothalamus and perifomical areas
(Bittencourt et al., 1992, de Lecea et al., 1998). They both have widespread projections
to the entire brain including the cortex and the spinal cord (Broberger et al., 1998, Peyron
et al., 1998). Since the discovery of Orx neurons, the hypothalamus has been
rediscovered as a key regulator of sleep and wakefulness. Many studies have been done
to investigate the role of Orx in behavioral states. Quite interestingly, it was found that
mice lacking the Orx gene showed syndromes of narcolepsy, a disorder of sleep
characterized by an inability to maintain waking and by sudden onset of cataplexy with
muscle atonia (Chemelli et al., 1999). They also became obese despite being hypophagic
due to a decrease in basal metabolism. Genetic canine narcolepsy was found to be caused
by mutations in the Orx2 receptor gene (Lin et al., 1999). Later, it was discovered that
human narcolepsy is associated with deficient Orx neurons or peptide (Peyron et al.,
2000, Thannickal et al., 2000). Orx neurons have dense projections to all monoaminergic
and cholinergic cell groups responsible for arousal (Marcus et al., 2001, Beuckmann and
Yanagisawa, 2002, Taheri et al., 2002). These findings brought the intriguing possibility
that the critical role of posterior hypothalamus in maintenance of waking is due to the
role of Orx neurons.
9
MeR is the other peptide that is found in neurons codistributed in the same region
as the Orx cells, yet in distinct cell populations. It has been shown that mice lacking the
gene for MeR, in contrast to Orx knockout mice, became lean despite being hyperphagic
due to an increase in basal metabolic rate (Shimada et al., 1998). Electrophysiological
studies moreover showed that whereas Orx had consistently excitatory effects on
postsynaptic targets, MeR had hyperpolarizing effects (Bayer et al., 2001, Eggermann et
al., 2001, Gao and van den Pol, 2001, Bayer et al., 2002a, Bayer et al., 2004). Like the
opposite roles of Orx vs. MeR on basal metabolism and postsynaptic effects, we thought
that Orx and MeR could exert opposite influences in sleep-waking regulation as well.
In addition to its pivotaI role in the maintenance of waking, the posterior
hypothalamus is involved in the regulation of different neuroendocrine functions as a part
ofhypothalamic pituitary axis as well as in the control offeeding and energy expenditure.
Thus to find and distinguish the hypothalamic peptide/s or neuronls that might
specifically control the sleep-waking cycle would help to functionally differentiate the
neurons of the posterior hypothalamus.
1.4 c-Fos expression as an indicator of neuronal activity
In order to study the activity of different cell types in the BF, POA and the posterior
hypothalamus across the sleep-waking cycle, we used c-Fos expression, together with
immunohistochemical techniques for enzymes, neurotransmitters or peptides in a
paradigm of sleep deprivation and sleep recovery in rats. c-Fos is expressed in a limited
number oftissues, including the central nervous system (Johnson et al., 1992). The
expression of c-Fos, an immediate eady gene, induces Fos protein synthesis that goes
10
back to the nucleus after synthesis and acts as a transcription factor. It is known that cFos expression occurs in association with action potentials and Ca++ entry into the cells
and can thus reflect cell activation (Morgan and Curran, 1986, Dragunow and Faull,
1989, Fields et al., 1997).
c-Fos can be induced by many different stimuli such as classical neurotransmitters,
trophic factors, thermal, visual and somatosensory stimuli (Cirelli and Tononi, 2000). cFos expression can be induced within 20 minutes of stimulus onset, whereas the
accumulation of Fos protein needs a period of approximately 90 minutes (Sheng and
Greenberg, 1990, Morgan and Curran, 1991, Cirelli and Tononi, 2000). High leve1s of
Fos protein are generally observed for hours and then decline (Chaudhuri, 1997).
Following a light pulse of 5 minutes, Fos protein peaks in the SCN after one to two hours
and disappears within six hours (Shiromani and Schwartz, 1995). However, c-Fos data
do not always match well with single unit recording data. Many wake-on cells in the BF,
for example, did not express c-Fos after sleep deprivation or spontaneous waking
(Pompeiano et al., 1992, Pompeiano et al., 1994). During PS rebound after sleep
deprivation, only small numbers of cholinergic cells in the dorsal pontine tegmentum
expressed c-Fos (Maloney et al., 1999). These observations indicate that increase in
firing rate per se may not be enough to induce c-Fos in many neurons. Sorne studies
emphasized the role ofCa++ entry as a key factor for c-Fos expression (Ginty, 1997). It
has been shown that, glutamate for instance, can induce c-Fos expression in a Ca++
dependent manner (Lerea et al., 1992). Thus, we took into consideration that what we
observed as c-Fos expression would like1y reflect only those neuronal discharges that
were associated with strong and substantial changes in intracellular Ca++ level.
11
1.5 c-Fos expression in the brain across the sleep-waking cycle
The expression of c-Fos together with sorne other genes ofthe immediate early
gene family have been shown to be enhanced during spontaneous waking or after sleep
deprivation (Pompeiano et al., 1992, Grassi-Zucconi et al., 1993, O'Hara et al., 1993).
The pattern of c-Fos expression during total sleep deprivation in the brain is similar to the
pattern observed after spontaneous wakefulness (Cirelli et al., 1995). In addition, it has
been shown that most genes including c-Fos that are up-regulated by 3-8 hours of sleep
deprivation are also up-regulated by spontaneous wakefulness (Cirelli and Tononi, 2000).
Moreover, c-Fos expression in the paraventricular hypothalamic nucleus, a key structure
known to induce c-Fos in response to stress was minimal after sleep deprivation showing
a minimal amount of stress with sleep deprivation (Semba et al., 2001). These findings
suggest that the change in gene expression in response to sleep deprivation is probably
related to the arousal per se and not to the non-specific effects of stress. Accordingly, to
perform our study we applied a paradigm of sleep deprivation to reliably represent a quiet
non-stressed waking state in rats.
During waking, there is strong c-Fos expression in sorne areas of the forebrain
including the MPO, the LPO, the posterior hypothalamus, the DBB, and the intralaminar
nuclei of the thalamus (central and lateral) (Cirelli and Tononi, 2000). In most ofthese
areas, the highest levels of c-Fos were seen after 3 hours of sleep deprivation showing
that the amount of c-Fos expression is not proportional to the amount ofprior
wakefulness (Cirelli et al., 1995).
With regard to sleep, it was shown that the expression of c-Fos is low throughout
the brain during sleep as compared to the waking state (Grassi-Zucconi et al., 1993).
12
There is a negative correlation between the numbers of c-Fos expressing neurons in most
brain regions and the amount of total sleep during one to two hours preceding sacrifice
(Grassi-Zucconi et al., 1994). As for REMS, it is known that physiological REMS is not
associated with significant c-Fos accumulation (Grassi-Zucconi et al., 1994), since, as
mentioned before, at least 20 minutes is required for the induction of c-Fos gene and 90
minutes for Fos protein synthesis. Nevertheless, pharrnacological and nonpharrnacological techniques that induced longer REMS like behavior could also result in
increased c-Fos in sorne brain regions such as the SeN, dorsal hypothalamus, laterodorsal
and pedunculopontine tegrnental nuclei (LDT and PPT respectively) and pontomedullary
reticular formation (Merchant-Nancy et al., 1992, Maloney and Jones, 1999, Maloneyet
al., 2000).
Despite previous studies showing lower amount of c-Fos in almost all brain areas
during sleep than during waking, recently it was reported that Fos irnrnunoreactivity in
the VLPO was increased during sleep, and the number oflabeled cells was positively
correlated with the time spent in sleep (Sherin et al., 1996). Although since the discovery
of the VLPO, several other studies have been performed to confirm the original results
and the VLPO was later considered by sorne as the sleep nucleus of the brain, we
believed that the original and many subsequent studies, using c-Fos expression in the
VLPO, were not precisely designed. Particularly in the original experiments, rats were
spontaneously asleep for the majority oftime only in the one hour (~>60%) prior to
sacrifice (Sherin et al., 1996, Lu et al., 2002) or they were deprived of sleep for 9 or 12
hours and were subsequently allowed to recover from sleep deprivation for 45, 90 and
180 minutes before sacrifice (Sherin et al., 1996). In either case, considering the time
needed for Fos protein synthesis, what was observed as an increase in c-Fos in the VLPO
13
could not unequivocally be attributed to prior sleep as opposed to the previous behavioral
state ofwaking or deprivation. Moreover, all ofthose studies were based on c-Fos
expression alone without chemical identification of the neurotransmitter phenotype of the
cells.
1.6 Modulation of sleep vs. wake promoting neurons by noradrenaline
Multiple brain regions will probabely utilize different neurotransmitter to regulate
sleep wake cycle.Finding the chemical transmitters of individual cells in each region that
are responsible for sleep-waking cycle regulation is the first step toward understanding
the physiology of sleep. The second step is to know the mechanism of cyclical activation
of sleep vs. wake-active cells. It is possible that different cells respond differently to the
neurotransmitters of sleep or waking, most importantly to the NA released from the LC
brainstem neurons projecting to the BF and POA (Jones and Moore, 1977, Jones and
Cuello, 1989, Chou et al., 2002). The release of NA is highest during waking, decreases
during non-REMS and complete1y ceases during REMS (Jones, 1989) .. Through alphal
adrenergic receptor (aIAR) NA depolarizes, whereas through alpha2 adrenergic receptor
(a2AR), NA hyperpolarizes postsynaptic target neurons. Both alAR and a2AR are G
protein coupled receptors that act through closing or opening ofK+ channels respectively
(McCormick, 1992, Williams and Reiner, 1993, Liu and Alreja, 1998). Indeed, in vitro
studies have shown that the BF cholinergie neurons are excited by NA through uIARs
(Fort et al., 1995), whereas non-cholinergie cells ofthe BF and the majority of cells in the
VLPO are inhibited by NA through u2ARs (Fort et al., 1998, Gallopin et al., 2000). We
assumed thus that sleep vs. wake-active cells through bearing different adrenergic
14
receptors become inhibited or disinhibited as the re1ease of NA fluctuates during the
sleep-waking cycle. Accordingly, during waking, sleep-active cells would be inhibited
whereas wake-active cells would be excited by NA. Reciprocally during sleep, wakeactive cells would no longer be excited whereas sleep-active cells would be disinhibited
as the release of NA is lowest. By applying a paradigm of sleep deprivation and recovery
in rats we studied the differential adrenergic receptor expression in sleep vs. wake-active
neurons across the BF, POA and posterior hypothalamus
1.7 The dynamics ofGABAAreceptors during sleep vs. waking on
cholinergie cells of the basal forebrain
The concept that sleep vs. wake promoting neurons bear different receptors for the
major neurotransmitters of arousal systems could explain how different neurons
cyclically become active or inactive during the sleep-waking cycle. Another mechanism
however, could potentially be due to differential expression or mobilization of receptors
on sleep vs. wake-active neurons depending on the behavioral conditions. In other
words, cells might alter the availability of their functional receptors across different
conditions in a homeostatic manner and therefore become more or less responsive to a
specific neurotransmitter. Indeed, according to the theory of synaptic homeostasis,
neurons in order to maintain their functions alter their responses to neurotransmitters
partly dependant upon their prior amount of activity (Turrigiano, 1999, Marder and Prinz,
2002). This change occurs in part through changes in receptors associated with
depolarizing vs. hyperpolarizing currents. After a period of neuronal activity, cells tend
to shift into a more silent state through enhancing the amount of inhibitory synapses. In
15
contrast, after blockade of activity for a short time, cens tend to start up and enhance their
dis charge through decreasing inhibitory synapses and/or through increasing excitatory
synapses (Turrigiano, 1999, Marder and Prinz, 2002, Mody, 2005). The majority of
inhibitory synapses throughout the brain utilize GABA as a neurotransmitter. Through
ion-gated channel receptors (Rs), GABAARs and GABAcRs, or G protein coupled
receptors, GABABRs, GABA opens cr or K+ channels and consequently hyperpolarizes
cens. GABAARs are present and expressed ubiquitously in most neurons of the brain.
Severallines of study have found that neuronal susceptibility to the effects of
neurotransmitters changes under different circumstances. Activity blockade in rat
cultured visual cortex for 2 days, reduced miniature inhibitory post-synaptic currents
(mIPSCs), the intensity GABAAR immunostaining and the numbers of synaptic sites that
expressed GABAAR (Kilman et al., 2002). Moreover, it has been shown that after 24
hours ofwhisker stimulation in rats, inhibitory synapses in the corresponding barrel field
that were detected by e1ectron microscopy increased (Knott et al., 2002). Similarly,
pharmacological studies revealed that the application ofbicucunine, a GABAAR
antagonist, in hippocampal cultured slices, increased the density of GABAARs (Marty et
al., 2004).
With regard to sleep and waking, nobody has yet shown whether the phenomenon
of synaptic homeostasis happens in a daily and cyc1ical manner. Many cens in order to
maintain waking, dis charge in bursts continuously and therefore could be suitable
candidates for this study. Accordingly, as it occurs after a period of prolonged neuronal
activity, there might be functional changes in GABAARs on activated cells after a period
ofwaking. As will be shown in the second chapter, the BF cholinergic cens express cFos during sleep deprivation but not during sleep recovery. Most recently in the current
16
lab, it has been similarly shown that identified BF cholinergic neurons discharge in
bursts during waking associated with fast activity in the EEG, and they become silent
during sleep associated with slow wave activity (Lee et al., 2003). We thought that
numbers, or intensity of GABAARs on the BF cholinergic cells might consequently
change during sleep vs. waking.
In summary, prior to my research project, few studies had been done to characterize
the activity of chemically identified neurons in association with sleep and waking across
the BF, POA and the posterior hypothalamus. Moreover, little was known about the
mechanism of cyclical regulation of sleep vs. wake promoting neurons. With the goal of
understanding chemically identified neurons and their modulation across the sleepwaking cycle we designed several experiments.
1.8 The first experiment
In the first chapter, with the aim of identifying putative sleep-active cells, the
activity of GABAergic neurons of the BF was studied in urethane-anesthetized rats. By
juxtacellular recording, neurobiotin (Nb) labeling and subsequent immunohistochemical
identification for glutamic acid decarboxylase (GAD), the dis charge profiles of identified
GABAergic neurons in the Mepo and SI was examined in relation to EEG activity. In
addition, by using dual-immunostaining for GAD and U2AAR we looked at the
distribution of GAD immunoreactive (+) neurons that were also labeled for U2 AAR.
Finally in urethane-anesthetized rats, we examined whether Nb-Iabeled, GAD+ cortical
activation 'off neurons that discharged maximally in association with cortical SWA,
17
were immunopositive for the a2AAR. This part of study revealed that the majority of
GABAergic cells in the MCPO fired maximally in association with SWA and decreased
their firing in association with evoked cortical activation. By further labeling for a2AAR,
first we found that the majority of GAD+ neurons through the MCPO and SI were
labeled for the a2AAR. Second, we found that ail the Nb-Iabe1ed GAD+ cortical
activation 'off cells were labe1ed for a2AAR. These results suggested that significant
population of GABAergic cells of the BF might be sleep-active cells. It moreover
suggested that the common phenotype ofthese sleep-on cells is GABA-containing and
a2AAR-bearing. To answer the question ofwhether in naturally sleeping animaIs
GABAergic cells of the BF still play roles in sleep promotion, the second experiment was
designed.
1.9 The second experiment
In the second chapter by using dual-immunostaining for c-Fos and choline acetyl
transferase (ChAT) or GAD, we studied the activity of cholinergie and GABAergic cells
through the BF and the adjacent POA across conditions of sleep deprivation and sleep
recovery. c-Fos study brought the possibility oflooking at different types ofneurons and
in different regions at the same time. Moreover, it gave us the chance to study the
activity of populations of cells, rather than a few cells, under different conditions. We
found that the numbers of cholinergie cells that expressed c-Fos were significantly higher
during SD as compared with the SR condition, whereas the proportions of c-Fos
expressing cells that were GABAergic were significantly higher after SR as compared
18
with the SD condition. Moreover, consistent with our results in anesthetized rats, we
showed that the majority ofGABAergic cens in the BF and POA that expressed c-Fos
after SR also bore U2AARs.
1.10 The third experiment
In the third chapter, we studied c-Fos expression in Orx and MCR cens of the
posterior hypothalamus across conditions of sleep deprivation vs. sleep recovery. We
also looked at the incidence of UIAAR vs. U2AAR on Orx and MCR neurons. We found
that the numbers of c-Fos expressing Orx cens were significantly higher after SD as
compared with SR condition. In contrast, the numbers ofMCR cens that expressed c-Fos
were significantly higher after SR as compared with SD condition. In addition, while
Orx cens were immunostained with both types of UIAAR and U2AAR, MCR neurons were
only immunostained with U2AAR.
DifferentiaI adrenergic receptor expression on GABAergic sleep vs. wake-active
cens and Orx vs. MCR neurons suggested that bearing different receptors for NA could
be one mechanism for cyc1ical activations of sleep vs. wake promoting neurons.
1.11 The fourth experiment
In the last chapter, we investigated the possibility of dynamic changes of receptors
under conditions of sleep or waking. To test this possibility, by applying
immunohistochemistry for ChAT and GABAARs (J32,3) subunits, we quantified and
19
analyzed the intensity of membrane GABAARs on the BF cholinergie cells across
different conditions of sleep deprivation and sleep recovery. Since the large majority of
GABAARs are localized on somatodendritic membrane and initial segments of axons
(Lus cher and Keller, 2004), here we analyzed GABAARs that were located on the soma
representing the major GABAARs compartment. We found that both the numbers and the
immunofluorescent GABAAR intensity on cholinergie cells were increased after SD as
compared with the SR condition. This finding suggested another novel mechanism for
modulation of wake promoting cholinergie neurons that in tum could regulate the sleepwaking cycle.
20
1.12 Preface to chapter 1
As reviewed in the Introduction, multiple lines of evidences have suggested that
the GABAergic cells might be important for sleep generation andlor maintenance. In the
current laboratory, it has been previously shown that the majority of GABAergic neurons
in the MCPO nucleus of the BF, fire maximally in association with slow cortical activity
whereas their firing is attenuated in association with evoked cortical activation. To test
the possibility that these GABAergic putative sleep promoting cells are inhibited by NA
during cortical activation and thus perhaps waking, we examined the presence of a2AR
on the BF cortical activation-off GABAergic neurons.
21
2. Chapter 1: Alpha 2 Adrenergic Receptors on GABAergic
Putative Sleep-Promoting Basal Forebrain Neurons 1
Ian D. Manns, Maan Gee Lee, Mandana Modirrousta, Yiping P. Hou and Barbara E.
Jones
1
Eur J Neurosci. 2003 Aug; 18(3 ):723-7
22
2.1 Abbreviations
ACh, acetylcholine
u2AR, alpha2 adrenergic receptor
EEG, electroencephalogram
GAD, glutamic acid decarboxylase
LC, locus coeruleus
MCPO, magnocellular preoptic nucleus
NA, noradrenaline
Nb, Neurobiotin
PS, paradoxical sleep
SI, substantia innominata
SWS, slow wave sleep
VLPO, ventrolateral preoptic region
23
2.2 Abstract
The basal forebrain plays an important role in the modulation of cortical activity
and sleep-wake states. Yet its role must be multivalent since lesions reportedly diminish
cortical fast activity and also cortical slow activity along with slow wave sleep (SWS).
Basal forebrain cholinergie versus GABAergic cell groups could differentially influence
these processes. By labeling recorded neurons with Neurobiotin (Nb) using the
juxtacellular technique and identifying them by immunostaining, we previously found
that whereas aIl cholinergic cells increased their firing, the majority of GABAergic
neurons decreased their firing in association with evoked cortical activation in urethaneanesthetized rats. Here, we examined the possibility that such GABAergic, cortical
activation 'off cells might bear alpha 2 adrenergic receptors (a2AR) through which
noradrenaline (NA) could inhibit them during cortical activation.
Pirst using simple dual-immunostaining for glutamic acid decarboxylase (GAD)
and the a2AAR, we found that the majority (-60%) ofGAD-immunopositive (+) neurons
through the magnocellular preoptic nucleus (MCPO) and substantia innominata (SI) were
labe1ed for the U2AAR. Second, in urethane-anesthetized rats, we examined whether Nblabeled, GAD+ cortical activation 'off neurons that discharged maximally in association
with cortical slow wave activity, were immunopositive for U2AAR. We found that all the
Nb+/GAD+ 'off cells were labe1ed for the U2AAR. Such cells could be inhibited in
association with cortical activation and waking when noradrenergic locus coeruleus (LC)
neurons discharge and be disinhibited with cortical slow waves and SWS when these
24
neurons become inactive. We thus propose that Œ2AR-bearing GABAergic basal
forebrain neurons constitute sleep-active and sleep-promoting neurons.
25
2.3 Introduction
The basal forebrain plays an important role in modulating cortical activity and
sleep-wake states (Jones, 2000). Lesions or inactivation ofthe basal forebrain reportedly
diminish cortical fast activity during waking (Stewart et al., 1984, Cape and Jones, 2000)
but also diminish cortical slow wave activity and slow wave sleep (SWS) (Szymusiak
and McGinty, 1986b). The basal forebrain may thus be comprised of different cell
groups that promote different cortical activities and states. In addition to cholinergie
neurons that are known to stimulate cortical activation, the basal forebrain contains large
numbers ofGABAergic neurons (Gritti et al., 1993). By employingjuxtacellular labeling
and immunohistochemical identification of recorded neurons in urethane-anesthetized
rats, we recently discovered that GABAergic cells behave very differently as a group than
cholinergie cells. Whereas all cholinergie neurons increased their firing with evoked
cortical activation, the majority of GABAergic neurons decreased their firing in
association with cortical activation and discharged at their highest rate in association with
cortical slow wave activity (Manns et al., 2000a). We proposed that such GABAergic
cells could be sleep-active neurons. Neurons that discharge at their highest rate during
natural SWS have indeed been recorded in the basal forebrain and in the adjacent
preoptic area of naturally sleeping-waking rats and cats (Szymusiak and McGinty, 1986a,
Koyama and Hayaishi, 1994, Szymusiak et al., 1998). Such SWS-active neurons were
inhibited by stimulation of the locus coeruleus (LC) or iontophoretic application of
noradrenaline (NA) in vivo (Osaka and Matsumura, 1994, Osaka and Matsumura, 1995).
In vitro studies also identified non-cholinergie neurons in the basal forebrain and
26
GABAergic neurons in the ventrolateral preoptic area (VLPO) that are commonly
hyperpolarized and inhibited by NA (Fort et al., 1998, Gallopin et al., 2000). Such
inhibitory effects of NA are mediated by alpha 2 adrenergic receptors (a2AR) (Osaka and
Matsumura, 1995, Bai and Renaud, 1998). The presence of a2AR on basal forebrain
neurons might thus identify them as sleep-active cells. The aim of the present study was
to determine immunohistochemically whether GABAergic and potentially sleeppromoting, basal forebrain neurons possess a2AR.
In a preliminary investigation, we employed dual-immunostaining for glutamic
acid decarboxylase (GAD) and the a2AAR to assess if a signiticant proportion of GADimmunopositive (GAD+) neurons in the magnocellular preoptic nucleus (MCPO) or
overlying substantia innominata (SI) of the rat were labe1ed for the a 2AAR. We then
examined using urethane-anesthetized rats, whether neurons that tire maximally in
association with cortical slow wave activity and are GAD+ are labeled for the a2AAR.
Recorded within the MCPO or overlying SI, such characterized units were labe1ed with
Neurobiotin (Nb) using the juxtacellular technique and subsequently triple-stained for
Nb, GAD and the a2AAR.
27
2.4 Materials and Methods
For the immunohistochemical study of Œ2AAR on GAD-immunoreactive neurons,
3 male Wistar rats (weighing 200-250 g) were employed for simple perfusion-fixation of
the brain under barbiturate anesthesia (Somnotol, 120 mg/kg, i.p.). For the study ofthe
receptors on GAD-immunoreactive physiologically characterized celIs, 7 male Long
Evans rats (weighing 200-250 g) were employed for acute e1ectrophysiological recording
prior to perfusion-fixation ofthe brain. He1d in a stereotaxic frame, they were
anesthetized with urethane for the duration of the recording (1.4 g/kg initial dose
followed by boosters of .14 g/kg as needed, i.p.) and for the subsequent perfusionfixation (with an additional booster if needed). AlI procedures were approved by the
McGill University Animal Care Committee and the Canadian Council on Animal Care.
In the electrophysiological experiments, units were recorded in the MCPO or SI
using glass micropipettes and characterized in association with electroencephalogram
(EEG) or field potentials recorded with surface or deep e1ectrodes from retrosplenial or
prefrontal cortex and olfactory bulb, as described in previous publications (Manns et al.,
2000a, Manns et al., 2000b). Following isolation of a single unit, its discharge was
characterized during baseline cortical irregular slow wave activity and during stimulated
cortical activation evoked by tail pinch. Cells that decreased their rate of discharge with
cortical activation, previously described as 'off celIs, were se1ected. One such cell per
animal was then labe1ed with Neurobiotin (Nb, Vector Laboratories, Burlingame, CA) by
applying the 'juxtacellular' technique. As established in our previous publications,
28
modulating the discharge of one recorded unit using 200 ms CUITent pulses over a period
of 5 to 30 minutes resulted in labeling of only one neuron.
Brains were fixed by intra-aortic perfusion of a paraformaldehyde solution (4% in
0.1 M phosphate buffer, PB, pH 7.4, ~500 ml) preceded by saline (0.9% NaCI, ~50 ml).
After removal, aIl brains were placed in a sucrose solution (30% in PB at 4° C) for ~2
days and then frozen. Sections were cut at 25 J.lm thickness in the coronal plane on a
freezing microtome.
For dual-immunostaining of GAD and Œ2AAR, sections were colIected at 400 J.lm
intervals through the basal forebrain. They were incubated for 3 nights at 4° C with an
affinity-purified goat polyclonal antibody for the <l2AAR (C-19, sc-1478, Santa Cruz
Biotechnology, Santa Cruz, CA) as employed previously (Hou et al., 2002), together with
a rabbit polyclonal antibody for GAD67 (1 :3000, Chemicon International, Temecula,
CA). The Œ2AAR was revealed using Cy3-conjugated donkey anti-goat antiserum and
GAD revealed simultaneously using Cy2-conjugated donkey anti-rabbit antiserum
(Jackson Immuno Research, West Grove, PA). GAD-immunoreactive neurons in the
MCPO and overlying SI were examined by fluorescence microscopy for the presence of
Œ2AAR .
For triple staining of recorded celIs for Nb, GAD and Œ2AAR, aIl sections were
colIected through the basal forebrain for staining ofthe Nb-Iabe1ed celI using AMCAconjugated streptavidin (Jackson ImmunoResearch Laboratories). After locating the Nblabeled celI, the section containing it was subsequently incubated with the Œ2AAR and
GAD antibodies for 3 nights and revealed with Cy2- and Cy3-conjugated secondary
antibodies as described above. Nb-Iabe1ed celIs that were also GAD+ were examined by
29
fluorescence microscopy for the presence of a2AAR. To provide images of double
labeling, they were superimposed using Adobe Photoshop (v9, Adobe Systems, San Jose,
CA).
30
2.5 Results
In dual-immunostained series, GAD+ cells within the MCPO and overlying SI
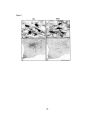
were often immunopositive for the a2 AAR (GAD+/a2AAR+, triangles in Fig. 1; filled
arrowheads in Fig. 2A'and 2B'). In these cells, the receptor labeling was punctate and
distributed over the cell body and proximal dendrites. In many cases, it appeared to be
within the cytoplasm, since it surrounded the nucleus of the cell. Punctate or diffuse
receptor labeling was also present over blood vessels in the region (bv, Fig. 2A' and B').
In the same area, sorne GAD+ cells were immunonegative for the a2AAR
(GAD+/U2AAR-, empty arrowheads, Fig. 2A' and B'). The GAD+/U2AAR+ and
GAD+/a2AAR- cells were present in the same vicinity (Fig. 2A and A') and sometimes
situated immediately adjacent to one other (Fig. 2B and B'). In a quantitative sampling, a
majority of the GAD+ neurons in the MCPO (average of 66%) and a substantial
proportion ofthose in the SI (average of38%) or an overall majority in the MCPO-SI
(average of 59% in three brains) were judged positively labeled for u2AAR.
31
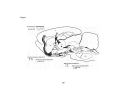
Figure 1
GAD+/0'2AAR+
....
Nb+/GAD+/0'2AAR+ ..
CPu
ac
32
Figure 1. GAD+ cells that were labeledfor the a2AAR (gray triangles) are
plotted in the BF cholinergic cell area, including the magnocellular preoptic nucleus
(MCPO) and substantia innominata (SI) (Gritti et al., 1993). In this same area, units
were recorded and selected for juxtacellular labeling and triple-staining for Nb, GAD,
and a2AAR. Ali Nb+/GAD+ cortical activation 'off' cells were located in the MCPO and
were positively labeledfor a2AAR (black stars). Scale bar = 1 mm. Other abbreviations:
ac, anterior commissure; CPu, caudate putamen; GP, globus pallidus; LPO, lateral
preoptic area; MPO, medial preoptic area; oc, optic chiasm, OTu, olfactory tubercle.
33
Figure 2
34
Figure 2. Photomicrographs showing GAD immunostaining with Cy2 by green
fluorescence emission (A, B, C and D) and Œ2AAR immunostaining with Cy3 by red
fluorescence of the same view (A " B', C' and D '). In the left panels, dualimmunostaining reveals that some GAD+ cells (filled arrowhead, A and B) are labeled
for the Œ2AAR+ (filled arrowheads, A' and B '), and others are not (open arrowheads, A'
and B '). Blood vessels (bv) are also immunostained for the Œ2AAR+. In the right panels,
triple-staining reveals that the GAD+ cells (filled arrowheads, C and D) that were
physiologically characterized as cortical activation 'off' cells and labeled with Nb in blue
fluorescence by AMCA-conjugated streptavidin (C' and D') are also immunopositivefor
Œ2AAR (solid arrows, C' and D '). Photomicrographs show a simple image with single
green fluorescence emission for GAD immunostaining (left) and superimposed images
with blue and red fluorescence for Nb and Œ2AAR immunostaining (right, C' and D '). All
Nb+/ GAD+/Œ2AAR+ neurons were located in the MCPO (Fig. 1). Scale bars = 20,Llln.
35
In recording experiments performed under urethane anesthesia, cells were
se1ected that decreased their firing with stimulation-evoked cortical activation for
labeling with Nb (Manns et al., 2000a). Generally discharging in a tonic irregular
manner in association with the baseline irregular cortical slow wave activity, these 'off
cells decreased or ceased firing in association with cortical activation (Fig. 3). They
subsequently increased their discharge following cessation of the stimulation as cortical
slow wave activity retumed to pre-stimulation leve1s (Fig. 3). Across cells, the average
discharge rate decreased significantly during stimulation as compared to pre-stimulation
(mean ± sem: 3.36 ± 1.25 vs. 9.8 ± 2.27; t = 3.44, df= 4, P = .03) and subsequently
retumed to pre-stimulation levels (9.28 ± 2.56) within one to two minutes. Of the 'off
cells labeled with Nb in the current study, the majority were immunopositive for GAD
(517), as was also the case in our previous study. The Nb+/GAD+ 'off cells identified
here were alliocated in the MCPO (n = 5; Fig. 1). All ofthese Nb+/GAD+ 'off cells
(Fig. 2C and D) appeared to be labe1ed for the Œ2AAR by the presence ofpunctate
staining over the cell body and cytoplasm surrounding the nucleus (Fig. 2C' and D').
36
Figure 3
Nb+/GAD+/a2A+ Off cell
A. EEG
Somalie Stimulation
B. Unit Discharge Rate
5
o
o
1
Il
1
,
Il
1
60,
- - _
20 sec
, 260
120
C.EEG
~
D. Unit Discharge
W
2 sec
~11mv 1111111111111111
37
Figure 3. Unit activity together with simultaneously recorded EEG from
prefrontal cortex showing a typical decrease or cessation offiring in association with
stimulation-evoked cortical activation and recovery offiringfollowing stimulation in a
urethane-anesthetized rat. This cortical activation 'off' cell was labeled with Nb and
immunopositive for GAD and the a2.AAR (Fig. 2, C and C 'J. The compressed EEG
recording (A) is expanded (C) and shown together with the unit recording (D) below.
The unit discharge rate plot (spikes per second in B) shows the decrease and cessation in
firing that occurs as the EEG changes from irregular slow wave activity to fast activity
during stimulation and the return to pre-stimulation rates that occurs as the EEG
resumes irregular slow wave activity following stimulation.
38
2.6 Discussion
This study documents by immunohistochemistry combined with
electrophysiology that the majority (59%) ofbasal forebrain GABAergic neurons possess
u2AR and that the GABAergic neurons that fire maximally with cortical slow wave
activity bear these receptors.
In our previous recording studies of GABAergic neurons in urethane-anesthetized
rats (Manns et al., 2000a), the majority (57%) ofNb+/GAD+ neurons through the
MCPO-SI were found to discharge at a higher rate with cortical slow wave activity than
with cortical activation. No Nb+/ChAT+ cells did so (Manns et al., 2000b), and only a
minority (18%) ofNb+/GAD- cells did so (Manns et al., 2000a). These results suggested
that a substantial group of GABAergic basal forebrain neurons would be most active in
association with cortical slow wave activity during SWS and thus correspond in large
part to previously identified sleep-active neurons in this region (Szymusiak and McGinty,
1986b). These neurons could correspond to the major proportion (59%) of GAD+
neurons that bear U2AAR documented here. The u2AR is responsible for
hyperpolarization of the membrane through opening ofK+ channels, as shown on
preoptic area neurons as on locus coeruleus neurons (Williams et al., 1985, Bai and
Renaud, 1998). Although other subtypes of u2AR have been shown to be present in the
basal forebrain (Rosin et al., 1996), the U2A was previously shown to be distributed
densely therein (Talley et al., 1996) and using the same antibody as that in the present
study, to be present upon locus coeruleus neurons (Hou et al., 2002). It cannot be
39
excluded in the present study performed using epifluorescence microscopy that u2AR
labeling could also be present on presynaptic terminaIs of the noradrenergic fibers as
documented in other areas by electron microscopy (Glass et al., 2001) or of other fibers
as indicated in the preoptic area by electrophysiological evidence (Matsuo et al., 2003).
However, the u2AR labeling over the cell body and proximal dendrites of GAD+ cells
here was predominantly associated with the postsynaptic cells since it often appeared to
be located within the cytoplasm surrounding the cell nucleus, as also described in other
neuronal perikarya (Talley et al., 1996).
Examination for triple labeling by Nb, GAD and U2AAR of physiologically
characterized cells showed here that cortical activation 'off GABAergic cells do bear
U2AR. These GAD+/u2 AAR+ neurons could be inhibited byNA in association with
cortical activation and waking when LC neurons discharge at their highest rate (AstonJones and Bloom, 1981). Reciprocally they would be disinhibited during cortical slow
wave activity and SWS when LC neurons decrease firing. They could also be active
during paradoxical sleep (PS) when LC neurons are silent and many sleep-active neurons
in the forebrain continue to discharge (Szymusiak and McGinty, 1986a, Koyama and
Hayaishi, 1994, Szymusiak et al., 1998). However, in view of the present results, they
are more likely to be silent in association with the cortical activation that occurs during
PS as well as waking. Silence by these cells during PS could be effected by acetylcholine
(ACh), which also inhibits the non-cholinergic cells inhibited by NA (Fort et al., 1998,
Gallopin et al., 2000) and which is released by neighboring cholinergic cells during both
PS and waking (Vazquez and Baghdoyan, 2001). The GABAergic u2AR-bearing
neurons identified here thus most likely correspond to sleep-active cells that are
40
maximally active during SWS. Given the evidence from lesion studies that destruction of
cells in the basal forebrain leads to a loss of sleep (Szymusiak and McGinty, 1986b),
these sleep-active neurons must also function importantly as sleep-promoting neurons.
They may do so via projections to and inhibitory effects on local cholinergie neurons, the
cortex (Gritti et al., 1997) or the posterior hypothalamus (Gritti et al., 1994). In this role,
they may function in synergy with GABAergic neurons of the preoptic region, inc1uding
the VLPO (Sherin et al., 1996, Szymusiak et al., 1998), that project in paralle1 to the
posterior hypothalamus (Gritti et al., 1994, Sherin et al., 1998) and are similarly inhibited
by NA (Fort et al., 1998, Gallopin et al., 2000).
41
2.7 Acknowledgements
This research was supported by grants from the Canadian Institutes ofHealth
Research (CIHR, 13458) and United States National Institute of Mental Health (NIMH,
ROI MH60119":OIAI). Y.P. Hou was a Visiting Scientist from the Lanzhou Medical
College, Lanzhou, China. I.D. Manns held a graduate student fellowship from the
Canadian Natural Science and Engineering Research Council (NSERC). Lynda
Mainville is greatly appreciated for her technical assistance.
42
2.8 Preface to chapter 2
The first chapter revealed that the majority of identified GABAergic neurons
decreased their firing in association with evoked cortical activation in urethaneanesthetized rats. It moreover showed that GABAergic cortical activation "off' neurons
that discharged maximally in association with cortical SWA, were labeled for the o,2AAR.
These GABAergic neurons could thus be distinguished from other cortical activation
"on" GABAergic cells by their differential response to NA. Accordingly, the
GABAergic "off' neurons that also bear o,2AAR would be inhibited by NA during waking
whereas they would be removed from inhibition during sleep when release of NA is
lowest and thus could take part in sleep promotion and maintenance. Based on these
results, we proposed that O,2AAR-bearing GABAergic basal forebrain neurons constitute
sleep-active and sleep-promoting cells.
In the next chapter in order to verify our results in naturally sleeping animaIs, we
designed a paradigm of sleep deprivation and sleep recovery in rats and by applying
immunohistochemistry for GAD, the enzyme needed for GABA synthesis, ChAT and cFos, as an indicator for cell activity, we test the possibility ofwhether 1) the basal
forebrain and adjacent preoptic area are comprised of GABAergic sleep-active cells and
2) these sleep-active cells are not cholinergic. Finally by applying triple labeling for cFos, GAD and O,2AAR across different conditions, we examined whether GABAergic
sleep-active cells bear in common O,2AARs and thus could be distinguished from other
GABAergic cells of the region. In addition, the study in the next chapter provided us the
43
opportunity to study simultaneously the activity of populations of cells across the
widespread regions of the basal forebrain and the preoptic area.
44
3. Chapter 2: GABAergic Neurons with Alpha 2- Adrenergic
Receptors in Basal Forebrain and Preoptic Area Express c-Fos
during Sleep2
Mandana Modirrousta, Lynda Mainville and Barbara E. Jones
2
Neuroscience.2004;129(3):803-10
45
3.1 Abbreviations
AR, adrenergic receptor
BF, basal forebrain
ChAT, choline acetyltransferase
DBB, diagonal band of Broca nuclei
GAD, glutamic acid decarboxylase
LPO, lateral preoptic area
MCPO, magnocellular preoptic area
MnPO median preoptic nucleus
MPO medial preoptic area
NA, noradrenaline
NDS, normal donkey serum
non-REMS, non-rapid eye movement sleep
POA, preoptic area;
REMS, rapid eye movement sleep
SC, sleep control
SD, sleep deprivation
SIa, substantia innominata, anterior part
SIp, substantia innominata, posterior part
SR, sleep recovery
SWS, slow wave sleep;
VLPO, ventrolateral preoptic area
46
3.2 Abstract
The basal forebrain (BF) contains cholinergie neurons that stimulate cortical
activation during waking. In addition, both the BF and adjacent preoptic area (POA)
contain neurons that promote sleep. We examined c-Fos expression in cholinergie and
GABAergic neurons in the BF and POA to determine whether they are differentially
active following sleep deprivation versus recovery and whether the GABAergic neurons
are active during sleep. Whereas the numbers of c-Fos+ cells and proportions of c-Fos+
cells that were cholinergie were decreased, the proportions that were GABAergic were
increased following sleep recovery across BF and POA nuclei. Moreover, the sleepactive GABAergic neurons were immunostained for a2A-adrenergic receptors. We
conclude that GABAergic neurons that commonly bear a2-adrenergic receptors comprise
sleep-active cells of the BF and POA. These GABAergic cells would be inhibited by
noradrenaline (NA) released from locus coeruleus neurons during waking; they would be
disinhibited through diminished NA release during drowsiness and thus become active to
promote sleep by inhibiting in tum wake-promoting neurons.
47
3.3 Introduction
Since early studies, the BF has been known to play an important role in cortical
activation as the ventral extrathalamic re1ay to the cerebral cortex from the brainstem
ascending activating system (Starzl et al., 1951, Jones, 2000). Yet, e1ectrical stimulation
in the region of the BF and adjacent POA also elicited cortical slow wave activity with
slow wave sleep (SWS) (Sterman and Clemente, 1962b, Sterman and Clemente, 1962a),
and lesions therein produced insomnia (McGinty and Sterman, 1968). From single unit
recording studies, it became apparent that whereas many neurons in the BF and POA
increase their firing with cortical activation or waking, others increase their firing with
cortical slow wave activity or SWS (Detari et al., 1984, Szymusiak and McGinty, 1986a,
Koyama and Hayaishi, 1994, Alam et al., 1996). More recentlyby examining c-Fos
expression as a reflection of neural activity, a restricted collection of neurons was
identified within the ventrolateral preoptic area (VLPO) that was active during sleep and
thus proposed to be the principal sleep-promoting cell group of the POA and forebrain
(Sherin et al., 1996). In immunohistochemical studies, many cells in the VLPO, LPO and
BF, inc1uding projection neurons, were shown to contain glutamic acid decarboxylase
(GAD), the synthetic enzyme for GABA (Gritti et al., 1993, Gritti et al., 1994, Sherin et
al., 1998). The possibility was thus raised that GABAergic neurons in both POA and BF
could comprise sleep-active and promoting cells. Indeed, in studies employing
juxtacellular labeling of recorded neurons in urethane-anesthetized rats, a majority of
immunohistochemically identified GABAergic BF neurons were found to fire maximally
48
with cortical slow wave activity, whereas all cholinergie neurons fired maximally with
stimulation-induced cortical activation (Manns et al., 2000b, Manns et al., 2000a).
Wake-active versus sleep-active neurons most likely respond differently to the
major neurotransmitters of the ascending arousal systems, most notably noradrenaline
(NA) contained in locus coeruleus neurons projecting to the BF and POA (Jones and
Moore, 1977, Jones and Cuello, 1989, Chou et al., 2002). Indeed, it was first found in
vivo that NA excited wake-active neurons, whereas it inhibited sleep-active neurons
through u2_adrenergic receptors (u2AR) in the POA and adjacent BF (Osaka and
Matsumura, 1995). It was also found in vitro that whereas all cholinergie neurons were
excited by NA in the BF, sorne co-distributed non-cholinergie neurons
(~15%)
were
inhibited by NA (Fort et al., 1995, Fort et al., 1998). It was subsequently discovered that
the majority of neurons in the VLPO
(~60%)
were inhibited by NA and also contained
mRNA for GAD (Gallopin et al., 2000). Most recently, we found that
immunohistochemically identified GABAergic BF neurons which fired maximally with
slow wave activity in urethane-anesthetized rats were immunostained for U2AAR (Manns
et al., 2003). These results suggested that GABA and u2AR may reflect a common
phenotype as well as mechanism for a population of sleep-promoting neurons distributed
across the BF and POA.
In the present study, c-Fos expression was examined in sleep deprived versus
sleep recovery groups of rats to test the hypotheses that sleep-promoting neurons 1) are
distributed across nuc1ei ofthe BF and POA, 2) are not comprised of cholinergie neurons
and 3) are comprised of GABAergic neurons that are inhibited by NA and thus bear
49
a2AR. A portion of these results was presented in abstract form (Modirrousta et al.,
2003, Modirrousta et al., 2004a).
50
3.4 Experimental procedures
Male Wistar rats (Charles River Canada, St. Constant, Quebec, Canada)
weighing between 200-250 g were housed individualIy in cages with free access to food
and water. Three different groups of four rats per group were treated in their home cages
for: 1) total sleep deprivation (SD) by gentle touching for 3 hours (1200 to 1500 h), 2)
total sleep deprivation for 3 hours (900 to 1200 h) folIowed by sleep recovery (SR) for 3
hours (1200 to 1500 h), or 3) undisturbed sleep and waking as sleep control (SC) for 3
hours (1200 to 1500 h). Visual observation was used to record the behavioral state every
20 sec as wake, non-rapid eye movement sleep (non-REMS) or REMS, whereby nonREMS was scored when the animal was recumbent with eyes closed and showing little or
no movement and REMS when the animal was recumbent with eyes closed and showing
rapid movements or twitches ofthe eyes, whiskers, ears or paws (as previously
determined to correspond to polygraphicalIy scored sleep states (Maloney et al., 1997».
AnimaIs were prevented from sleeping during SD by gentle touching (with a small soft
paint brush inserted through the bars of the cage top) upon eye closure. The deprivation
procedure resulted in the total absence of sleep during 3 hours for the SD and SR groups
and was folIowed in the SR group by an increase in total sleep relative to both the SD
group and SC group. At the end of the experimental period (1500 h), the rats were
immediately killed under pentobarbital anesthesia (100 mglkg, i.p.) by intra-aortic
perfusion with a fixative solution of3% paraformaldehyde. AlI procedures were
approved by the McGill University Animal Care Committee and the Canadian Council on
Animal Care.
51
Following immersion in a 30% sucrose solution, the brains were frozen and stored at -80 0
C. They were cut in coronal sections at 20 Jlm, which was determined to be the greatest
thickness that would allow full penetration and staining with the antibodies employed.
Adjacent series of sections were collected and processed for double
immunohistochemical staining using peroxidase-antiperoxidase (P AP) for 1) c-Fos
(rabbit antiserum,1:10,000, Ab-5, PC38, Oncogene Research Products, La Jolla, CA)
with DAB-Ni as chromogen and 2) a) at 400 Jlm intervals, choline acetyltransferase
(ChAT, mouse antibody, 1:2000, MAB5270, Chemicon International, Temecula, CA) or
b) at 200 Jlm intervals, GAD (mouse antibody, detecting both GAD65 and GAD67
isoforms, 1: 100, MSA-225, Stressgen Biotechnologies, Victoria, BC) with pink, alphanaphthol pyronin B. Other series taken at 800 Jlm intervals were processed for triple
immunostaining for 1) c-Fos in the first position using DAB-Ni, 2) U2AAR (goat purified
antiserum, 1:50, sc-1478, Santa Cruz Biotechnology, Santa Cruz, CA) in the second
position using red Cy3-conjugated donkey antigoat antiserum (Jackson ImmunoResearch
Laboratories, West Grove, PA), and 3) GAD (rabbit antiserum, 1:1000, AB5992;
Chemicon) using green Cy2-conjugated donkey antirabbit antiserum (Jackson) in the
third position. Incubations with primary antibodies were performed at room temperature
overnight for c-Fos, ChAT and GAD antibodies and three nights at 4 oC for u2AAR
antibody using a Tris-saline solution (0.1 M) containing 1% normal donkey serum (NDS)
(following initial blocking with 3% NDS).
Sections were viewed by light and fluorescence microscopy (Leica DMLB
microscope equipped with an x/ylz movement-sensitive stage and video camera attached
to a computer). Cells immunopositive (+) for single c-Fos+, double c-Fos+/ChAT+ or c-
52
Fos+/GAD+ and triple c-Fos+/GAD+/u2AAR+ were counted by applying stereology
using the Optical Fractionator program of Stereo Investigator (2003, MicroBrightFie1d,
Williston, VT). Cells were counted within the contours of 8 nuclei in the BF and POA,
respectively: SIa, SIp, MCPO, DBB and LPO, VLPO, MPO, MnPO. The nuclei were
delineated in a computer resident atlas (modified from (Gritti et al., 1993» that was
placed in register with each section. For each nucleus, labe1ed cells were counted
bilaterally in at least three sections (3 - 14, depending upon the length of each nucleus) at
400 /lm intervals for the c-Fos/ChAT-immunostained series through the BF region nuclei
or 200 /lm for the c-Fos/GAD-immunostained series through the BF and POA regions.
Through all nuclei, cells were counted under a 63x oïl objective (with 1.4 numerical
aperture) within a counting frame of 125 x 125 /lffi. In most nuclei, cells were counted
through 25% of the area at each level by setting the grid size (within which the counting
frame is automatically inserted) to 250 x 250 /lm. For the smaller nuclei (VLPO and
MnPO), cells were counted through the entire area of the nucleus by setting the grid size
to 125 x 125 /lm. Counts oftriple-Iabeled cells immunostained for c-Fos/GAD/u2AAR
were combined across nuclei for estimates of total cell numbers within the BF and POA
regions. By counting nuclei that came into focus beneath the surface of the section, cFos+ nuclei were counted within a counting block of 8 /lm in depth (in the dehydrated,
delipidated, mounted and coverslipped sections that were on average 10 /lm thick).
Sleep and cell counts were analyzed between groups and across nuclei or regions using
one and two way ANOVA in Systat (v10.2, Richmond, CA). All main effects were
confirmed by nonparametric rank order tests (K.ruskal-Wallis, p < .05) to insure that the
distribution of variance in groups (containing zeros as a result of the experimental
53
condition) did not distort the parametric statistics. Figures were composed using Adobe
Photoshop 6 and Illustrator 9 (Adobe, San Jose, CA).
54
3.5 Results
Rats submitted to sleep deprivation (SD) by gentle touching did not sleep during
the 3 hours prior to sacrifice at 1500 h, whereas those permitted sleep recovery (SR)
during the same period after 3 hours deprivation in the moming (900 to 1200 h) slept
~91 %
of the time. The amount oftotal sleep differed significantly across conditions of
SD, SR and sleep control (SC, Table 1). Recovery and control sleep were comprised
predominantly ofbehaviorally quiet, non-REMS (non-REMS: 77.23 ± 2.94% in SR and
68.68 ± 0.10% in SC with REMS: 13.58 ± 1.30% in SR and 7.10 ± 0.66% in SC, mean ±
S.E.M. of total time).
55
Table 1. Average Percent Sleep and c-Fos expression across nuc1ei (Nuc) of the basal forebrain (BF) and preoptic area (POA)
under conditions (Cond) ofsleep control (SC), sleep deprivation (SD) and sleep recovery (SR) in three groups ofratsa
sc
Variable
% Sleep
75.75 ± 0.61
650.53 ± 86.29
Total Number c-Fos+ Cells
Percent c-Fos+/ChAT+
Percent c-Fos+/GAD+
3.57 ± 1.61
-
44.00 ± 3.66
SD
F (Cond)
SR
F (Nuc)
1095.40
***
2462.70 ± 341.40 (SC,SR) 575.80 ± 95.02 (SD)
70.72
***
0.00 ± 0.00
1
(SC,SR)
90.80 ± 2.47
(SC, SD)
1
16.67
F (CondxNuc)
***
11.68 ± 2.64
(SC, SR)
0.00 ± 0.00
(SD)
8.95
***
0.33 ns
17.16 _:±: ~.7L_
~C,SR)
41.16 ± 4.04
(SD)
25.86
***
6.53
56
***
4.77
***
0.16 ns
1.35 ns
Table 1.
aMean ± S.E.M values from 4 rats per group. % Sleep was calculated as % of3 br
observation period prior to killing and was examined across groups by a one-way
ANOVA. For number or percent of c-Fos expressing cens, two-way ANOVAs were
performed with Cond (3) and Nuc (8) as factors. For each variable, there was a
significant main effect of condition. Post-hoc paired comparisons with Bonferroni
correction were performed to examine differences between conditions (indicated in
parentheses, p < .05). For % Sleep, SD and SR both differed from SC and from each
other; for c-Fos+ cen variables, SD differed from SC and SR but SR did not differ from
Sc. For total number of c-Fos+ cens (ca1culated as c-Fos+ plus c-Fos+/GAD+ cens in
the c-Fos/GAD dual-immunostained series), there was a significant difference across
nuclei and a significant interaction of condition with nuclei. Post-hoc comparisons
between conditions in each nucleus revealed a main effect of condition in every nucleus
except the MnPO. For percent of c-Fos+ cens (as c-Fos+/ChAT+ or c-Fos+/GAD+ of
the total), there was a significant difference for GAD+ cens between nuc1ei, but there was
no significant interaction between condition and nuclei for GAD+ or ChAT+ cells, thus
contraindicating post-hoc comparisons for condition in each nucleus. (*** indicates Fratio withp < .001; ns means not significant.)
57
In SR and/or SD rats, c-Fos was expressed in ChAT-immunopositive (+) and
GAD+ cens as wen as in ChAT-immunonegative (-) and GAD- cens within the BF and
POA (Fig. lA-D). The numbers of c-Fos+ cens varied significantly across conditions,
differing between SR or SC and SD but not between SR and SC (Table 1), According to
a two-way ANOVA with condition and nuc1ei as factors, c-Fos+ cens were significantly
decreased in SR (like SC) as compared to SD (see Figs. 2A-B and 3B, Table 1). Given a
significant interaction between condition and nuc1ei, post-hoc comparisons were
performed to determine if the decrease was significant in an nuc1ei (Table 1). The
difference was found to be significant in an nuc1ei, except the MnPO. The proportions of
c-Fos+ cens that were ChAT+ were also significantly decreased in SR (like SC) as
compared to SD (Figs. 2C-D and 3C, Table 1). In this case, there was no significant
interaction between condition and nuc1ei, indicating that the effect of condition was not
different across individual nuc1ei ofthe BF. In contrast to those being ChAT+, the
proportions of c-Fos+ cens that were GAD+ were significantly increased in SR (like SC)
as compared to SD (Figs. 2E-F and 3D; Table 1). In this case, there was also no
significant interaction between condition and nuc1ei, indicating that the main effect of
condition did not differ across the nuc1ei of the BF and POA.
58
Figure 1
59
Figure 1. Labeled cells in the nuc/ei of the BF or POA. A. Single-Iabeled cFos+ cell (stained black with DAB-Ni, black arrowhead), single-Iabeled ChAT+ cell
(stained pink with alpha-naphthol pyronin B, white arrowhead) and double-Iabeled cFos+/ChAT+ cell (double arrowhead) in the MCPo. B-D. Single-Iabeled c-Fos+ cells
(stained black, black arrowheads), single-Iabeled GAD+ cells (stained pink, white
arrowheads) and double-Iabeled c-Fos+/GAD+ cells (double arrowheads) in the MCPO
(B), MPO (C) and VLPO (D). E and F. A triple-Iabeled c-Fos+/GAD+/a2AAR+ cell
shown in images of c-Fos staining (stained black with DAB-Ni, black arrowhead in E)
and a2AAR (stained by redfluorescence with Cy3) together with GAD staining (stained
by green fluorescence with Cy2, white arrowhead in F). The a2AAR labeling is evident
over the GAD+ cell body (in orange or yellow). Magnification bars = 20 j.lm (for A-D
shown in D and E-F shown in F).
60
Figure 2
E
.t.
c.-Fos+/GAD+
SD
SR
5D
61
SR
Figure 2. Distribution ofc-Fos+ cells in one SD and one SR representative brain
through the BF and POA (at ~ A8.6 in A, C and E and ~A9.0 in B, D and F, (Gritti et al.,
1993)). A-B. c-Fos+ cells (black dots) within BF and POA nuclei, C-D. Double-labeled
c-Fos+/ChAT+ cells (red circles) distinguishedfrom single-labeled c-Fos+ cells (black
dots) within the BF, and E-F. Double-labeled c-Fos+/GAD+ cells (blue triangles)
distinguishedfrom single-labeled c-Fos+ cells (black dots) within the BF and POA
nuclei. Atlas images show cells plotted in one 20 pm thick section per level (~A 8.6 on
left and ~A 9.0 on right) from adjacent series (separated by 200 f1Jn) dual-immunostained
for c-Fos/ChAT (C-D) or c-Fos/GAD (A-B and E-F). Abbreviations: SIa, substantia
innominata, anterior part; (SIp, substantia innominata, posterior part, not shown);
MCPO, magnocellular preoptic area; DBB, diagonal band ofBroca nuclei; LPO, lateral
preoptic area; VLPO, ventrolateral preoptic area; MPO, medial preoptic area; MnPO,
median preoptic nucleus. (Note that all nuclei examined are contained within the
sections illustrated in Fig. 2, except SIp, which is located in the same relative position as
SIa at more caudallevels.).
62
Figure 3
A
B
Sla
~
~û
'5
1
~
~
~
Ù
'5
1iE
g
~
1
C ...
8
DBB
Sla
~
l
6
".
SR
D
SR
~
ô
~
~
>fi
SO
SR
Condition
SO
SR
Condition
63
Figure 3. Bar charts showing the percentage ofsleep (in A) and the total
numbers of c-Fos+ cells (in B) or proportions of c-Fos+ cells that were cholinergic or
GABAergic (in Cor D) within each nucleus of the BF and/or POA in SD and SR groups.
A. The % sleep (representing % total sleep of 3 hour period prior to sacrifice) was
significantly increased in SR as compared to SD. B. The total numbers of c-Fos+ cells
(obtainedfrom c-Fos/GAD dual-immunostained series as the total of c-Fos+ and cFos+/GAD+ cells) were significantly decreased across nuclei of the BF and POA in SR
as compared to SD (with a significant difference across nuclei and a significant
interaction between condition and nuclei, due to a significant decrease in ail nuclei
except MnPO, Table 1). C. The percentages of c-Fos+ cells that were ChAT+ (obtained
from the c-Fos/ChAT dual-immunostained series as the % of c-Fos+ plus c-Fos+/ChAT+
cells that were c-Fos+/ChAT+) were significantly decreased across nuclei of the BF in
SR as compared to SD (with no significant difference across nuclei or interaction
between condition and nuclei, Table 1). D. The percentages of c-Fos+ cells that were
GAD+ (calculatedfrom total numbers shown in B) were significantly increased in SR as
compared to SD (with a significant difference across nuclei but no significant interaction
between condition and nuclei, contraindicating post-hoc comparisons in individual
nuclei, Table 1). For each group (n
=
4), mean rS.E.M are presented. For
abbreviations see Fig. 2.
64
The incidence of Œ2AAR labeling was subsequently examined on c-Fos+/GAD+
cells in the SD and SR groups by triple-immunostaining (Fig. lE and F). The proportions
of c-Fos+/GAD+ cells that were Œ2AAR+ were small in the SD group and significantly
higher in the SR group across the BF and POA regions (with no significant difference
across the regions or interaction of condition with region, Fig. 4). On average across
regions, 3.88 ± 1.73% of c-Fos+/GAD+ cells were Œ2AAR+ in the SD group and 77.70 ±
22.07% in the SR group.
65
Figure 4
BF
fi)
A ~
+
~
..
~
+
Cl
~
!!l
u..
+"
<)
'*
SO
SR
Condition
POA
fi)
B ~
+
et:
..
<f
....
~
+
~'"
0
~
'*
SO
SR
Condition
66
Figure. 4. Bar charts showing the proportions of c-Fos+/GAD+ cells that bear
a2AAR in the BF (A) and POA (B) in SD and SR groups. There was a significant
increase in c-Fos+/GAD+/a2AAR+ cells in SR as compared to SD (ANOVAfor main
effect of condition, F = 7.168, p < 0.05 with no significant difference across the two
regions and no significant interaction between condition and region, p > 0.05).
67
3.6 Discussion
The present results indicate that across the BF and POA fewer neurons are active
during sleep than during waking, and that of those active during sleep, none are
cholinergic and a substantial proportion are GABAergic. Moreover, the majority of the
sleep-active GABAergic neurons appear to bear <X2AAR.
In aIl nuclei of the BF and aIl but the MnPO of the POA, more neurons
accumulated c-Fos protein following 3 hours ofwaking produced by gentle deprivation
of sleep than following 3 hours of sleep either during recovery from deprivation or
control condition. These results suggest that the majority of neurons in the BF and POA
are more active during waking than during sleep. Similar conclusions had been reached
by other studies employing c-Fos immunohistochemistry or in situ hybridization in the
past (Pompeiano et al., 1992, Cirelli et al., 1993, Ledoux et al., 1996, Sastre et al., 2000).
Only recently was it reported tirst for the VLPO and then for the MnPO in the POA, that
more neurons expressed c-Fos during sleep than during waking (Sherin et al., 1996, Gong
et al., 2000). It is possible that the particular experimental conditions in those studies,
such as the housing, surgery or method of sleep deprivation, which can be associated with
certain degrees of stress and associated c-Fos expression at particular times (Dragunow
and Faull, 1989, Cullinan et al., 1995, Maloney et al., 1999), resulted in relatively higher
c-Fos expression in those nuclei during sleep than in the present study. Here, we
employed conditions that would minimize stress prior to and during the experimental
period by studying the animaIs in their home cages, using behavioral observation to
record sleep so as to avoid prior surgery and applying gentle touching with a soft brush
68
for preventing sleep during a re1ative1y short (3 hour) period. Vnder these conditions, an
increase in the total number ofneurons expressing c-Fos was not observed in any nucleus
ofBF or POA during recovery or control sleep as compared to enforced waking. Indeed,
the number ofneurons active in SR was on average <25% ofthat active in SD. The
proportions did differ however across nuclei with the highest proportions being in the
MnPO
(~75%)
(~20%),
and VLPO
(~50%)
and lower proportions in the BF and LPO nuclei
suggesting different concentrations of sleep- versus wake-active neurons with
the highest concentrations in MnPO and VLPO. These results are supported by both in
vivo and in vitro e1ectrophysiological studies showing re1atively high concentrations of
putative sleep-promoting neurons in these POA nuclei (Szymusiak et al., 1998, Ga1lopin
et al., 2000, Suntsova et al., 2002).
Single unit recording studies have also found relative1y small proportions of
neurons that are maximally active specifically during non-REMS or SWS in BF and POA
nuclei (Detari et al., 1984, Szymusiak and McGinty, 1986a, Koyama and Hayaishi, 1994,
Szymusiak et al., 2000, Suntsova et al., 2002, Lee et al., 2004). On the other hand in the
same studies, a large and varying proportion of neurons has been characterized through
these regions as active during REMS, sorne as non-REMS/REMS-active, sorne
se1ective1y REMS-active and others as Wake/REMS-active. In a study examining c-Fos
in pharmacologically produced predominant REMS versus predominant non-REMS
conditions, it was found that REMS was associated with increased c-Fos expression in
many forebrain regions (Sastre et al., 2000). We assume that in the present study the
major percent oftime spent in non-REMS
time spent in REMS
(~14%)
(~77%)
as compared to the small percent of
in the 3 hours preceding sacrifice served as the major
determinant in c-Fos expression and accumulation during recovery as well as control
69
conditions. We thus conclude that the c-Fos+ neurons seen following sleep recovery or
controllargely represent non-REMS-active neurons (which would include nonREMS/REMS-active but not selective REMS-active or Wake/REMS-active neurons) that
are distributed across BF and POA as a small population though with relatively greater
representation in certain nuclei of the POA.
In parallel with the total number ofneurons expressing c-Fos, the proportions of
those neurons that were cholinergie across BF nuclei were diminished during sleep. In
fact, no c-Fos+/ChAT+ neurons were detected in the sleep recovery condition. These
results confirm electrophysiological studies indicating that presumed cholinergie neurons
are silent during SWS (Jones, 2004, Lee et al., 2004). They also confirm that cholinergie
neurons are active during waking. According to release of acetylcholine and firing of
presumed cholinergie BF neurons, however, they should also be active during REMS
(Marrosu et al., 1995, Jones, 2004). As for aIl c-Fos+ cells (above), we assume that the
lack of c-Fos immunostaining in cholinergie neurons in the recovery condition, as weIl as
control, is due to the predominance of non-REMS in the 3 hours prior to sacrifice and the
small amount oftime spent in REMS (14%) as weIl as waking
(~9%)
that would be
inadequate for accumulation of the Fos protein during that period (Dragunow and FauIl,
1989).
In contrast to the proportion of cholinergie neurons, the proportion of GABAergic
neurons expressing c-Fos increased significantly with SR as compared to SD across BF
and POA nuclei. These results indicate that many GABAergic neurons distributed across
these regions remain or become active in association with sleep following waking. They
support claims that neurons in the BF and POA including the VLPO that project to the
posterior hypothalamus and contain GAD may be sleep-active and promoting neurons
70
and may act by inhibiting wake-promoting neurons ofthat region (Gritti et al., 1994,
Sherin et al., 1998). They also support the hypothesis that identified GABAergic BF
neurons that discharge in association with cortical slow wave activity in urethaneanesthetized rats and project either to posterior hypothalamus or cortex, if not locally, as
determined by antidromic activation, could be sleep-active and promoting as well (Manns
et al., 2000a). Finally, they confirm similar results most recently reported by others for cFos expression in GAD+ neurons of the MnPO and VLPO (Gong et al., 2004).
GABAergic neurons of different BF and POA nuc1ei could thus exert an inhibitory
influence upon wake-active and promoting systems in the posterior hypothalamus, cortex
and/or basal forebrain. However, in single unit recording studies, it was also found that
another physiologically distinct group of GABAergic neurons discharged maximally in
association with cortical activation (Manns et al., 2000a). Therefore, different groups of
GABAergic neurons in the BF and POA are likely active in association with cortical
activation of waking and others in association with SWS.
The major proportion (~78%) ofGAD+ neurons that expressed c-Fos with SR in
the present study were immunostained for the a2AAR, whereas only a minimal
percentage (~4%) ofthose that did so with SD were so labe1ed. These results confirm the
hypothesis that GABAergic neurons in the BF and POA that are active during sleep bear
a2AR and would accordingly be inhibited by NA (Osaka and Matsumura, 1995, Bai and
Renaud, 1998, Manns et al., 2003, Saint-MIeux et al., 2004). This particular group of
GABAergic neurons would be held under inhibition during waking by re1ease of NA
from afferent locus coeruleus neurons (Jones and Cuello, 1989) that fire maximally
during active waking, decrease firing during quiet waking, and further diminish firing
71
during SWS to cease firing during REMS (McCarley and Hobson, 1975, Aston-Jones and
Bloom, 1981). With decremental re1ease of NA during drowsiness, these GABAergic
neurons would be disinhibited to become active and promote sleep by inhibiting in tum
the noradrenergic neurons together with other neurons of the brainstem and forebrain
activating systems (Gritti et al., 1994, Luppi et al., 1995, Sherin et al., 1998, Steininger et
al., 2001, Chou et al., 2002).
3.7 Acknowledgments
The research was supported by a grant from the Canadian Institutes of Health Research
(13458).
72
3.8 Preface to chapter 3
The study in the second chapter revealed that across the BF and POA, higher
numbers of cens s expressed c-Fos during sleep deprivation than they did during sleep
recovery. Similarly, the proportions of c-Fos expressing cens that were cholinergic were
higher after SD than they were after SR. In contrast, the proportions of c-Fos expressing
cens that were GABAergic were higher after SR as compared with the SD condition.
Moreover, the majority of sleep c-Fos expressing GABAergic cens bore a2AAR, whereas
few wake c-Fos expressing bore a2AAR. These results revealed that the BF and POA are
comprised of many GABAergic cens that are active during sleep and thus might be
important in sleep promotion and maintenance. We showed that these sleep-active
GABAergic cens are distributed across the BF and POA yet they are more concentrated
in sorne nuc1ei inc1uding the VLPO and the MnPO. Our study also explained one
potential mechanism being able to explain how sleep promoting cens become active in a
cyc1ical manner. These particular GABAergic cens are under NA inhibition through
a2AAR during waking when the release of NA is highest. They subsequently become
disinhibited during sleep, when the release of NA is lowest, to become active to promote
and perhaps maintain sleep.
These studies chemicany identified the neurons of the BF and the anterior
hypothalamic POA that are involved in sleep-wake regulation. As has been long known,
in addition to the importance of the forebrain, the posterior hypothalamus plays a critical
role in the regulation ofbehavioral states, particularly arousal and waking. With the goal
of identifying the chemical transmitters of neurons in the posterior hypothalamus that
73
play roI es in the sleep-waking cycle, the next study was devoted to investigate the
potential roles of the two hypothalamic peptides, Orx and MeR. We hypothesized that
Orx neurons are active during waking whereas MeR cens are active during sleep. Using
the same paradigm of sleep deprivation and sleep recovery in rats and by applying
immunohistochemistry for antibodies against Orx and MeR and by studying c-Fos
expression, the activity of these two cen populations across behavioral conditions were
studied.
Furthermore in order to understand the mechanisms of cyclical regulation of Orx
vs. MeR cens, the incidence of alAAR vs. a2AAR on these cens were studied to test the
hypothesis that during waking Orx cens would be excited by NA and therefore might
bear alAAR whereas MeR cens would be inhibited by NA during waking and thus might
beara2AAR.
74
4. Chapter 3: Orexin and MCR Neurons Express c-Fos
Differently After Sleep Deprivation vs. Recovery and Bear
Different Adrenergic Receptors 3
Mandana Modirrousta, Lynda Manville and Barbara E. Jones
3
Eur J Neurosci. 2005 May;21(10):2807-16
75
4.1 Abbreviations
AR, adrenergic receptor
DMH, dorsomedial hypothalamus
LH, lateral hypothalamus
MCH, melanin concentrating hormone
NA, noradrenaline
non-REMS, non-rapid eye movement sleep
Orx,orexin
PF, perifomical area
REMS, rapid eye movement sleep
SC, sleep control
SD, sleep deprivation
SR, sleep recovery
ZI, zona incerta
76
4.2 Abstract
Though overlapping in distribution within the posterior hypothalamus, neurons
containing orexin (Orx) and melanin concentrating hormone (MeR) may play different
roi es in the regulation ofbehavioral state. In the present study in rats, we tested whether
they express c-Fos differently after total sleep deprivation (SD) vs. sleep recovery (SR).
Whereas c-Fos expression was increased in Orx neurons after SD, it was increased in
MeR neurons after SR. We reasoned that Orx and MeR neurons could be differently
modulated by noradrenaline (NA) and accordingly bear different adrenergic receptors
(ARs). Of aIl Orx neurons (estimated at ~6700), substantial numbers were
immunostained for the 0, 1AAR, including ceIls expressing c-Fos after SD. Yet,
substantial numbers were also immunostained for the o,2AAR, also including ceIls
expressing c-Fos after SD. Of aIl MeR neurons (estimated at ~ 12,300), rare neurons
were immunostained for the 0, 1AAR, whereas significant numbers were immunostained
for the o,2AAR, including cells expressing c-Fos after SR. We conclude that Orx neurons
may act to sustain waking during sleep deprivation, whereas MeR neurons may act to
promote sleep foIlowing sustained waking. Sorne Orx neurons would participate in the
maintenance ofwaking during deprivation when excited by NA through o,lARs, whereas
MeR neurons would participate in sleep recovery after deprivation when released from
inhibition by NA through o,2ARs. On the other hand under certain conditions, Orx
neurons may also be submitted to an inhibitory influence by NA through o,2ARs.
77
4.3 Introduction
The posterior hypothalamus has long been known to play a critical role in the
maintenance ofwakefulness (Jones, 2000). Recently two peptides, orexin (Orx, also
ca11ed hypocretin) and melanin concentrating hormone (MCR) have been found to be
contained in distinct populations of magnocellular neurons that partially overlap in their
distribution through the mid to posterior hypothalamus and commonly give rise to
widespread projections through the central nervous system (Bittencourt et al., 1992,
Broberger et al., 1998, de Lecea et al., 1998, Peyron et al., 1998). They were both
initially found to have a stimulatory influence upon eating and thus to be considered as
contingents of the hypothalamic 'orexigenic' systems (Williams et al., 2004). Yet, as
became evident in knock out experiments e1iminating the peptides or their receptors, their
normal roles appeared to extend beyond influences upon eating and to differ with regard
to energy homeostasis. Most notably, mice lacking the gene for orexin or dogs lacking
the gene for its receptor manifested a syndrome ofnarcolepsy (Chemelli et al., 1999, Lin
et al., 1999) and humans with narcolepsy were found to lack the gene, peptide or neurons
containing Orx (Peyron et al., 2000, Thannickal et al., 2000). In addition, Orx knock out
mice became obese despite being hypophagic, attributed to a decrease in activity and
basal metabolic rate. As evidenced by c-Fos expression and Orx re1ease, Orx cells also
appeared to be most active during the night, when animaIs were awake and engaged in
motor activity (Estabrooke et al., 2001, Yoshida et al., 2001). Orx thus appeared to
stimulate arousal with motor activity and increased energy expenditure. In contrast,
78
although MeR knoek out miee were also hypophagie, they manifested a decrease in body
weight attributed to an increase in aetivity and basal metabolic rate (Shimada et al., 1998,
Marsh et al., 2002). MeR thus appeared to decrease arousal and energyexpenditure.
Aecording to these results, Orx and MeR neurons may perform opposite roles in
wake vs. sleep promotion and/or maintenance. Rere, we examined whether Orx and
MeR cells respond differently to total sleep deprivation (SD) vs. sleep recovery (SR) by
using c-Fos expression as an indieator of neuronal aetivity. Different activity profiles
eould be due to different responses of the Orx and MeR neurons to the major
neurotransmitters of the ascending arousal systems inc1uding most importantly
noradrenaline (NA). In the basal forebrain and preoptic areas, cholinergie wake-aetive
neurons are exeited by NA through al adrenergic reeeptors (aIARs), whereas
GABAergic putative sleep-aetive neurons are inhibited by NA through a2ARs (Fort et
al., 1995, Fort et al., 1998, Gallopin et al., 2000, Manns et al., 2003, Modirrousta et al.,
2004b). We thus examined whether the Orx and MeR neurons bear different adrenergic
receptors while possibly expressing c-Fos under different conditions of sustained waking
vs. reeovery sleep.
79
4.4 Material and methods
AlI procedures were approved by the McGill University Animal Care Committee
and the Canadian Council on Animal Care, whose standards meet those of the
Association for Assessment and Accreditation of Animal Care International. Male
Wistar rats (200-250 g) were used in minimal numbers to make up three test groups. The
rats were housed individually with free access to food and water at all times and
maintained on a 12:12lightldark schedule (with lights on from 700 to 1900 hours). As
previously described (Modirrousta et al., 2004b), the experiment was designed to deprive
rats of sleep under non-stressful conditions and was thus performed in the home cages.
The three different groups (n = 4 per group) were submitted to 1) total sleep deprivation
(SD) for 3 hours (1200 to 1500 h), 2) total sleep deprivation for 3 hours (900 to 1200 h)
followed by sleep recovery (SR) for 3 hours (1200 to 1500 h), or 3) undisturbed sleep and
waking as sleep control (SC) for 3 hours (1200 to 1500 h). The experimenter (MM)
brought each rat in its cage (in groups of 3) to an experimental room in the animal facility
and habituated each rat to her presence and to a paint brush inserted through the cage top
for 3 days prior to the experimental day. During the experiment for the SD and SR
groups, she deprived each rat of sleep by gently touching it with the brush when it c10sed
its eyes. She deprived and/or observed one rat at a time (on one day) and scored by
visual observation its behavioral state every 20 sec. as comprised in the majority by
wake, non-rapid eye movement sleep (non-REMS) or REMS. The behavioral scoring
was based upon previous studies in the current lab (Maloney et al., 1997) together with
80
demonstrations in other labs (Bergmann et al., 1987, Espana et al., 2003), showing that
behavior can be used in nonnal rats to reliably score the major states ofW, non-REMS
and REMS. Thus, as previously detennined to correspond to polygraphically scored
sleep states in our lab (Maloney et al., 1997), non-REMS was scored when the animal
was recumbent with eyes closed and showing little or no movement and REMS when the
animal was recumbent with eyes closed and showing rapid movements or twitches of the
eyes, whiskers, muzzle, ears or paws. The deprivation procedure resulted in the total
absence of sleep during 3 hours for the SD and SR groups and was followed in the SR
group by an increase in total sleep relative to both the SD and SC groups. At the end of
the experimental period (1500 h), the rats were immediately killed under pentobarbital
anesthesia (100 mg/kg, i.p.) by intra-aortic perfusion with a fixative solution of3%
parafonnaldehyde.
Following immersion in a 30% sucrose solution, brains were frozen and stored at
-80 0 C. They were cut in corona! sections at 20 /Jm, which was detennined to be the
greatest thickness that would allow full penetration and staining with the antibodies
employed. Adjacent series of sections were collected at 400 /Jm intervals and processed
for double immunohistochemical staining using peroxidase-antiperoxidase (P AP) for 1)
c-Fos (rabbit antiserum, 1:10,000, Ab-5, PC38, Oncogene Research Products, La Jolla,
CA) with DAB-Ni as chromogen and 2) Orexin (Orexin-A (C-19), goat antibody, 1:500,
sc-8070, R230, Santa Cruz Biotechnology, Santa Cruz, CA) or MCR (MCR (E-16) goat
antibody, 1:500, sc-14507, L281, Santa Cruz Biotechnology) with pink, alpha-naphthol
pyronin B. Other series were processed for triple immunostaining for 1) c-Fos in the first
position using DAB-Ni, 2) a 1AAR (goat purified antiserum, 1:50, sc-14 75, R31 0, Santa
81
Cruz Biotechnology) or U2AAR (goat purified antiserum, 1:50, sc-1478, K0702, Santa
Cruz Biotechnology) in the second position using Cy3-conjugated donkey anti-goat
antiserum (Jackson ImmunoResearch Laboratories, West Grove, PA), and 3) Orx (Orexin
A, rabbit antiserum, 1:2000, R261-3, Phoenix Pharmaceuticals, Belmont, CA) or MCR
(rabbit antiserum, 1:5000, R177-6, Phoenix Pharmaceuticals) in the third position using
Cy2-conjugated donkey anti-rabbit antiserum (Jackson). Incubations with primary
antibodies wereperformed at room temperature ovemight for c-Fos, Orx and MCR
antibodies and two nights at 4° C for UIAAR and U2AAR antibodies using a Tris-saline
solution (0.1 M) containing 1% normal donkey serum (NDS, fonowing initial blocking
with 3% NDS).
Sections were viewed by light and fluorescence microscopy with a Leica DMLB
microscope equipped with an x/y/z movement-sensitive stage and video camera attached
to a computer. Single-, double- and triple-immunostaining were evaluated for c-Fos, Orx
or MCR and UIAAR or U2AAR. Single-, double- and triple-Iabeled cens were counted by
applying stereology using the Optical Fractionator program of Stereo Investigator (2003,
MicroBrightField, Williston, VT). cens were sampled and counted within delineated
contours at appropriate levels of a computer resident atlas (expanded from (Gritti et al.,
1993)). On each side, a large contour was employed to inc1ude the nuc1ei in which the
Orx neurons were located (lateral hypothalamus (LR), perifomical area (PF), and
dorsomedial hypothalamus (DMR)) or those in which the MCR neurons were located
(LR, PF, DMR and zona incerta (ZI)). Partially overlapping through the hypothalamus,
the Orx and MCR cens were distributed within these contours and nuc1ei over
approximately 1600-1800 !lm from anterior to posterior. Accordingly, cens single-,
82
double- or triple-Iabe1ed for c-Fos and/or peptide (Orx or MCR) and/or receptor (a,lAAR
or a,2AAR) were sampled and counted bilaterally within the contours in three sectionslevels at 400
~m
intervals from anterior to posterior. Within the stereology program, the
sizes of the counting frame, within which cells are counted, and the grid, within which
the counting frames are automatically placed, were optimized for each single-, double- or
triple-Iabeled series so that a suitable number of cells were counted per level in the
deprived or recovery condition. Accordingly, the counting frame (125 x 125
grid size (300 x 300
~m
or 350 x 3 50
~m)
~m)
and
were set to sample ~ 13 or 17% of the area for
single-Iabe1ed c-Fos, Orx and MCR cell counts; the counting frame (140 x 140 ~m) and
grid size (200 x 200 ~m) were set to sample 49% of the area for counting double-Iabeled
c-Fos/Orx or c-Fos/MCR celIs; and the counting frame and grid size (both at 140 x 140
/lm) were set to sample 100% ofthe area for double-Iabe1ed Orx or MCR and a,lAAR or
a,2AAR cell counts as well as triple-Iabe1ed profiles with c-Fos. The cells were counted
under a 63x oil objective (with 1.4 numerical aperture). Nuc1ei or cells that came into
focus beneath the surface of the section were counted within a counting block of 8 /lm in
depth (in the dehydrated, de1ipidated, mounted and coverslipped sections that were on
average 10 /lm thick).
Sleep and cell counts were analyzed between groups and across nuc1ei or regions
using one and two way ANOVAs in Systat (v10.2, Richmond, CA). AlI main effects
were confirmed by nonparametric rank order tests (Kruskal-Wallis, p < .05) to insure that
the distribution of variance in groups (containing zeros as a result of the experimental
condition) did not distort the parametric statistics. Figures were composed using Adobe
Photoshop Creative Suite (CS) and Adobe Illustrator CS (Adobe, San Jose, CA).
83
4.5 Results
Sleep deprivation and recovery
Rats in the SD group did not sleep during the 3 h in the aftemoon prior to
sacrifice (at 1500 h), whereas rats in the SR group that were allowed to recover sleep in
the aftemoon after 3 h of sleep deprivation in the moming (900-1200 h) slept ~91 % of
the time prior to sacrifice (at 1500 h). The amount of sleep differed significantly across
conditions of SD, SR and SC (Table 1). The major proportion of time for the SR and SC
groups was spent in non-REMS (77.23 ± 2.94% in SR and 68.68 ± 0.10% in SC) and a
minor proportion in REMS (13.58 ± 1.30% in SR and 7.10 ± 0.66% in SC, mean ±
S.E.M. of total time).
c-Fos expression in Orx and MCH neurons after sleep deprivation and
recovery
c-Fos was expressed in neurons ofthe hypothalamus inc1uding Orx, MCH, nonOrx and non-MCH cells (Fig. lA, B). According to ANOVA with condition (and peptide
series) as factors, total numbers of c-Fos-immunopositive (+) cells varied significantly
across conditions (independent of peptide series) being highest in the SD condition
(Table 1). Total numbers of Orx- and MCH-immunopositive neurons did not vary
significantlyas a function of condition. The Orx+ neurons, which are distributed across
the LH, PF and DMH (Figs. 1C and 2A) numbered on average ~6700 (Table 1). MCH
neurons, which are distributed across LH, PF, DMH and ZI (Figs. ID and 2D) numbered
84
on average ~ 12,300 and were significantly more numerous than the Orx cells (Table 1).
The numbers of c-Fos+/Orx+ and c-Fos+/MCH+ cells varied significantly across
conditions but also varied significantly as a function of an interaction between condition
and peptide (Table 1). Numbers of c-Fos+/Orx+ cells were significantly decreased in SR
as compared to SD (Figs. 2B, C and 3C; Table 1), whereas numbers of c-Fos+/MCH+
cells were significantly increased in SR as compared to SD (Figs. 2E, F and 3F; Table 1).
Numbers of c-Fos+/MCH+ cells were also significantly higher in SR as compared to SC
(Table 1). Across the SD and SR groups, the numbers of c-Fos+/Orx+ cells were
negatively correlated with the numbers of c-Fos+/MCH+ cells (correlation coefficient for
pairwise values, r = -0.82, n = 8, p = 0.01). Relative to all c-Fos+ cells in SD, the cFos+/Orx+ cells represented a small proportion (~6%, Figs. 2B and 3B, C). Theyalso
represented a small proportion of the Orx cell population (~9%, Figs. 2A, B and 3A, C).
Similarly, relative to ail c-Fos+ cells in SR, the c-Fos+/MCH+ cells represented a small
proportion (~6%, Figs. 2F and 3E, F). They also represented a small proportion of the
MCH cell population (3%, Figs. 2D, F and 3D, F).
85
Table 1. Average percent sleep and numbers of Orx and MCR cells expressing c-Fos under conditions ofSleep Control (SC),
Sleep Deprivation (SD) and Sleep Recovery (SR)
Variable
SC
% Sleep
75.75 ± 0.61
0.00 ± 0.00
.(sC, SR)
c-Fos+
2535.67 ± 424.00
11924.38 ± 1382.56
(SC,SR)
Orx+
4888.75 ± 536.98
8042.00 ± 1421.88
25.50 ± 25.50
675.75 ± 123.84
10906.67 ± 1120.81
12711.75 ± 1430.25
25.50 ± 14.72
38.25 ± 12.75
c-Fos+/Orx+
MCH+
c-Fos+IMCH+
F (Coud)
SR
SD
86
(SC, SR)
90.80 ± 2.47
(SC, SD 1095.40
5187.88 ± 1112.65 (SD)
19.57
***
7227.25 ± 397.04
3.26
us
20.54
***
1.21
us
30.28
***
142.50 ± 39.54
(SD)
13212.00 ± 592.98
(SR)
***
382.50 ± 60.70
(SC,_~I>
Table 1.
Values represent Mean ± S.E.M. for 3 groups of 4 rats (n = 12). Cell numbers
were obtained from stereological estimates for total numbers on left and right sides.
Values across conditions (Cond) were compared by one or two-way ANOVAs (with
condition and peptide series as factors), followed by one-way ANOVA for condition
(with p < 0.001 indicated by *** in table) and post hoc pair-wise comparisons between
conditions (using Fisher's LSD with p < 0.05 indicated for SD and SR with respect to
SC, SR or SD). The estimated total numbers of c-Fos+ cells differed across conditions in
both the Orx and MCH series and did not differ between (or in interaction with) the
peptide series (df = 2, 1, 2, 18) and are thus presented as the mean values for the two
peptide series (n = 24). The numbers of Orx and MCH cells did not differ across (or in
interaction with) conditions but did differ between each other, Orx cells (grand mean ±
S.E.M., 6719 ± 622) being significantly fewer than MCH cells (12,277 ± 649; F = 18.26,
df= 1,2,9; P = 0.002). In a two-way ANOVA, the numbers of c-Fos+/Orx+ and cFos+/MCH+ neurons differed significantly as a function of condition (F = 18.3; df= 2, 9;
p < 0.001), differed between the two peptide series (F = 6.56; df= 1,9; p = 0.03) and
differed as a function of an interaction between condition x peptide (F = 25.62; df = 2, 9;
p < 0.001). Given the significant interaction, one-way ANOVAs were performed for
each series and presented in the table along with post hoc pair-wise comparisons.
87
Figure 1
88
Figure 1. c-Fos expression in Orx and MCH neurons. A. Single-Iabeled c-Fos+
cell (stained black with DAB-Ni, black arrowhead), single-Iabeled Orx+ cell (stained
pink with alpha-naphthol pyronin B, white arrowhead) and double-Iabeled c-Fos+/Orx+
cells (double arrowheads). B. Single-Iabeled c-Fos+ cell (stained black, black
arrowhead), single-Iabeled MCH+ cells (stained pink, white arrowheads) and doublelabeled c-Fos+/MCH+ cell (double arrowhead). C Orx cell distribution in the LH, PF
and DMH (stained brown with DAB). D. MCH cell distribution in the LH, PF, DMH
and ZI (stained brown). Abbreviations: f, fornix. Magnification bars
and 1 mm in C, D.
89
=
20 pm in A, B
Figure 2
SD
SR
. .~~~~-------,--. . ~~~~--~------,.~~~~~~-------,--~ Â
Orx+
• c-Fos+
.. c-Fos+/Orx+
~~~~~----~--~~~~~~------.,--~~~r-~----~~~o MCH+
• c-Fos+
• c-Fos+/MCH+
MCH
90
Figure2: Distribution of Orx and MCH neurons expressing c-Fos after SD or SR.
A. Orx ce/l distribution in the LH, PF and DMH (open triangles). B, C. Double-Iabeled
c-Fos+/Orx+ ce/ls (fi/led triangles) distinguishedfrom single-Iabeled c-Fos+ ce/ls (black
dots) in one SD (B) and one SR (C) representative brain. D. MCH ce/l distribution in
the LH, PF, DMH and Z1 (open squares). E, F. Double-Iabeled c-Fos+/MCH+ ce/ls
(fi/led squares) distinguishedfrom single-Iabeled c-Fos+ ce/ls (black dots) in one SD (E)
and one SR (F) representative brain. Abbreviations: f, fornix; DMH, dorsomedial
hypothalamus; LH, lateral hypothalamus; PF, perifornical area; ZL zona incerta.
Magnification bar = 1 mm.
91
Figure 3
A
B
§
t..
D
1
~
1
~
<f
~
'1
(,)
D
~
E
111
B
fi,
t
,.,.â
:t
0
..
i!
&
.:.
~
~6
MCH
92
Figure 3. Numbers of Orx and MCH neurons expressing c-Fos after SD or SR.
A. Total numbers of Orx + ceUs counted bilateraUy and estimated by stereological
analysis in the hypothalamus including LH, PF and DMH B. Total numbers ofc-Fos+
ceUs (obtainedfrom c-Fos/Orx dual-immunostained series as the total ofsingle c-Fos+
and double c-Fos+/Orx+ ceUs) were significantly decreased in SR as compared to SD
(Table 1). C Total numbers ofc-Fos+/Orx+ ceUs were significantly decreased in SR as
compared to SD (Table 1). D. Total numbers ofMCH+ ceUs counted and estimated by
stereological analysis in the hypothalamus including LH, PF, DMH and ZL E. Total
numbers ofc-Fos+ ceUs (obtainedfrom c-Fos/MCH dual-immunostained series as the
total ofsingle c-Fos+ and double c-Fos+/MCH+ ceUs) were significantly decreased in
SR as compared to SD (Table 1). F. Total numbers of c-Fos+/MCH+ ceUs were
significantly increased in SR as compared to SD (Table 1). Note same scale in A, B, D
and E (0 - 20,000) for single-labeled ceUs and smaUer scale in C and F (0 - 1000) for
double-labeled ceUs.
93
Adrenergic receptors on Orx and MeR, including c-Fos expressing,
neurons after sleep deprivation and recovery
Immunostaining for adrenergic receptors (UIAAR or U2AAR), peptide (Orx or
MCR) and c-Fos was performed to examine the incidence of UIAAR or U2AAR on Orx
and MCR cens and also on Orx and MCR cens expressing c-Fos in the different
conditions. Orx cens were immunostained for both types of adrenergic receptors, ~ 1100
for UIAAR (Fig. 4A) and ~1400 for U2 AAR (Fig. 4B; Table 2). Orx cens bearing the
different ARs were distributed across the LR, PF and DMR with no ostensible
segregation (Fig. 5A, B). Very few MCR neurons were immunostained for UIAAR (~160
having minimal positive staining, Fig. 4C), whereas a large number were immunostained
for U2AAR (~1800, Table 2; Fig. 4D). MCR cens immunopositive for U2AAR were
distributed across the LR, PF, DMR and ZI (Fig. 5D). The numbers of Orx and MCR
AR-immunopositive cens differed significantly between the two peptide cen types (Table
2). There was a significantly higher prevalence of Orx+/UIAAR+ cens than
MCR+/UIAAR+ cens. Moreover, whereas there was no significant difference between
the numbers of UIAAR+ vs. U2AAR+ Orx cens, there was a significant difference between
the numbers OfUIAAR+ vs. U2AAR+ MCR cens (Table 2). Indeed,
~16%
of Orx cens
bore UIAAR and ~21 % bore U2AAR, whereas only ~1.5% ofMCR cells bore UIAAR and
~15%
bore U2AAR. The numbers of AR-immunopositive Orx and MCR cells did not
vary significantly as a function of condition (Table 2). Analysis of the tripleirnrnunostaining revealed sorne c-Fos expressing Orx cells in SD which bore UIAAR and
94
sorne which bore U2AAR (Fig. 4E, F). No c-Fos expressing MeR neurons in SR were
found to be immunopositive for UIAAR, whereas sorne were found to be irnrnunopositive
for U2AAR (Fig. 40).
95
Table 2. Average number of Orx or MCH cells that were immunostained for UIAAR or U2AAR under conditions of SC, SD and
SR.
Variable
SC
SD
Orx+/alAR+
1102.75 ± 122.74
1058.33 ± 456.97
1325.00 ± 450.69
1084.56
± 179.41
Orx+/a2AR+
1531.75 ± 297.58
1133.33 ± 475.29
1735.00 ± 761.07
1406.33
± 290.04
MCH+/alAR+
213.25 ± 48.48
166.67 ± 87.00
141.67 ± 117.56
MCH+/a2AR+
1479.25 ± 170.93
2300.00 ± 478.93
1933.33 ± 147.43
Mean
SR
F (Cond)
F (AR)
F (CondxAR)
0.29 ns
1.95ns
0.27 ns
2.4 ns
81.64**
1.86 ns
161.44 ± 44.38
1818.56
± 196.29
1
Values represent Mean ± S.E.M. for 3 groups of 3 or 4 rats (n = 10). Cell numbers were obtained from stereological estimates
for total numbers on left and right sides. Values of each peptide series (Orx and MCH) were analyzed by two-way ANOVA for
condition (Cond) and adrenergic receptor subtype (AR). Total numbers of Orx and MCH neurons with UIAAR or U2AAR did not vary
significantlyas a function of condition (ns, not significant). Whereas the number of neurons immunostained for UIAAR vs. U2AAR did
not differ for the Orx neurons, it did differ for the MCH neurons (**, p < 0.01).
96
Figure 4
97
Figure 4. Adrenergic receptors (AR) on Orx and MCH neurons including those
expressing c-Fos. A. Cell immunostainedfor Orx (Al) and
aJ~R
(Al and in
superimposed image inA3). B. Cell immunostainedfor Orx (Bl) and
a2~R
B3). C Cell immunostainedfor MCH (Cl) that was minimally stainedfor
(Bl and
aJ~R
(Cl
and C3). D. Cells immunostainedfor MCH (Dl) ofwhich one was also stainedfor
a2~R
(Dl and D3). E. Labeled Orx+ cell (El) that was
(E3). F. Labeled Orx+ cell (Fl) that was
MCH+ cell (Gl) that was
a2~R+
a2~R+
aJ~R+
(El) and c-Fos+
(Fl) and c-Fos+ (F3). G. Labeled
(Gl) and c-Fos+ (G3). Peptides were stained by
green fluorescence with Cy2; receptors were stained by redfluorescence with Cy3 and cFos by black DAR-Ni. Magnification bar = 20 !lm.
98
Figure 5
<dAR
r~~'-"",--~=""'-""'~~""'-T;;:';';:;';"-~-- * Orx+/aIAR+
o Orx+/a2AR+
* MCH+/alAR+
o MCH+/a2AR+
99
Figure 5. Distribution of al vs. a2AR immunopositive Orx and MCH neurons.
A. Distribution of al~R+/Orx+ ceUs (stars) and B.
a2~R+/Orx+
cells (circ/es)
across LH, PF and DMH. C Distribution of al~R+/MCH+ ceUs (stars) and D.
a2~R+/MCH+
ceUs (circ/es) across LH, PF, DMH and ZI. For abbreviations see Fig.
2. Magnification bar = 1 mm.
100
4.6 Discussion
Paralle1 with a large population of neurons in the posterior hypothalamus, more
Orx cells express c-Fos after sleep deprivation than after sleep recovery, whereas more
MCH cells express c-Fos after sleep recovery. This reciprocal pattern of activity could
be explained in part by different adrenergic receptors and thus response to NA in the Orx
and MCH neurons.
c-Fos differentially expressed in Orx and MeR neurons as a function of
sleep
A large population of neurons in the posterior hypothalamus expressed c-Fos
following 3 hours oftotal sleep deprivation. As a reflection ofneural activity, the c-Fos
expression thus confirms unit recordings showing the maximal discharge rate by the vast
majority ofneurons in this region to be during waking as compared to non-REMS
(Steininger et al., 1999, Alam et al., 2002, Koyama et al., 2003).
In paralle1 with the major population ofhypothalamic neurons, more Orx neurons
also expressed c-Fos following sustained waking than following recovery or control
sleep. These results confirm microdialysis studies showing that Orx re1ease is high in
association with waking and low in association with sleep (Fujiki et al., 2001, Yoshida et
al., 2001, Zeitzer et al., 2003). However, the percentage of c-Fos+ cells that were Orx+
in the present study was very small
(~5%)
indicating that the Orx cells were the not major
contributors in this region to the maintenance of the waking state. Moreover, the
percentage ofOrx+ cells that were c-Fos+ was also very small (~10%), indicating that the
101
Orx cells were not maximally activated for the maintenance of the waking state under the
present conditions. In fact, the experimental conditions were intended to deprive rats of
sleep without stress and accordingly employed behavioral observations for sleep-wake
state scoring to avoid the surgery and tethering needed for polygraphic recording,
maintained the animaIs in their home cages with food and water ad libitum to avoid any
alimentary deprivation and used gentle touching with a soft brush when necessary to
avoid continuous or stressful stimulation and evoked activity during the day. The results
suggest that although other neurons in the posterior hypothalamus are active in
association with quiet waking and thus potentia11y involved in maintaining cortical
activation of the waking state, the Orx neurons may only be active with more aroused or
stressful waking. In another study, it was found that Orx neurons expressed c-Fos in
large numbers during the day only in association with high arousal and stressful waking
induced by continuous auditory stimulation (Espana et al., 2003). Selective deprivation
of REM sleep was also found to be associated with increased c-Fos expression in Orx
neurons (Verret et al., 2003), perhaps due to stress that may be associated with that
procedure. Orx re1ease has also been found to be maximal in association with motor
activity (Kiyashchenko et al., 2002, Zeitzer et al., 2003). Effects of
intracerebroventricular (leV) administration of Orx and loss offunction in Orx knock out
mice moreover indicate that Orx can stimulate energy metabolism along with motor
activity by positive influences upon the sympathetic nervous system (stimulating
increased heart rate, blood pressure, temperature and thermogenesis), the hypothalamopituitary thyroid axis (HPT, stimulating increased basal metabolic rate) and the
hypothalamo-pituitary adrenal axis (HP A, stimulating increased corticosteroid levels)
(Lubkin and Stricker-Krongrad, 1998, Shirasaka et al., 1999, Ida et al., 2000, Hara et al.,
102
2001, Espana et al., 2002, Monda et al., 2003, Yamanaka et al., 2003). An integral role
in stimulating arousal, activity and metabolism can be mediated by excitatory influences
of Orx upon multiple central arousal systems, inc1uding noradrenergic locus coeruleus
neurons and cholinergic brainstem and basal forebrain neurons (Horvath et al., 1999,
Bayer et al., 2001, Eggermann et al., 2001, BurIet et al., 2002), upon central motor and
sympathetic systems (Peyron et al., 1998, van den Pol, 1999, Krout et al., 2003, Yamuy
et al., 2004) and upon hypothalamic neurons (van den Pol et al., 1998, Ferguson and
Samson, 2003).
In contrast to the Orx cells, MCH cells showed more c-Fos activation after sleep
recovery than after sleep deprivation. c-Fos expression was specifica1ly associated with
recovery sleep, when animaIs slept ~90% of the time and not with normal sleep in the
control condition, when animaIs slept ~75% of the time. These results suggest that the cFos activation is associated with the recovery pro cess and not simply with natural sleep.
Nonetheless, the percentage ofMCH neurons that expressed c-Fos during recovery
following 3 hours of gentle sleep deprivation was very small «5%). In another study
examining c-Fos expression following 72 hours ofparadoxical sleep (PS) deprivation
upon inverted flower pots, it was found that a large proportion ofMCH neurons (>50%)
expressed c-Fos during recovery, when animaIs slept 77% ofthe time, comprised by 33%
SWS and 44% PS (Verret et al., 2003). In our paradigm, the recovery sleep was
comprised in greatest proportion by non-REMS sleep (estimated at ~ 77% as compared to
14% REMS), and the percentages ofboth non-REMS and REMS were significantly
increased above control (estimated at ~69% and 7% respective1y). Accordingly, the
increased c-Fos expression in MCH cells could be explained by the predominance of
non-REMS in SR or by a recovery process afforded by total sleep that inc1udes
103
unimpeded non-REMS and REMS along with the loss ofpostural muscle tone. MCR
cells may thus become activated only when there is an increased need as well as
possibility for the behavioral inactivity and rest that is permitted by non-REMS and
REMS sleep. Based upon evidence ofMCR administration or MCR knock out, it has
been shown that MCR decreases energy metabolism along with activity through a
negative influence upon the sympathetic nervous system, the RPT axis and the RP A axis
(Shimada et al., 1998, Kennedy et al., 2001, Marsh et al., 2002, Ito et al., 2003, Shearman
et al., 2003, Zhou et al., 2005). MCR neurons could thus play a role in opposition to Orx
neurons by decreasing activity and energy metabolism in association with sleep through
inhibition of transmission in the hypothalamus and other arousal systems (Bittencourt et
al., 1992, van den Pol et al., 1998, Gao and van den Pol, 2001).
Adrenergic receptors differentially distributed on Orx and MCH
including c-Fos expressing neurons
C-Fos expression was inverse1y re1ated in Orx and MCR neurons across the
deprived and recovery conditions in the present study suggesting a reciprocal re1ationship
in their activity and roles. Orx and MCR cells are partially overlapping in their
distribution within the hypothalamus and have reciprocal synaptic re1ationships, which
could mediate reciprocal profiles of activity (Bayer et al., 2002b, Guan et al., 2002). It is
also possible that they would be under reciprocal influences by other activating systems
involved in behavioral state and energy regulation, such as the noradrenergic systems,
including importantly the locus coeruleus neurons (Jones and Yang, 1985). It couid thus
be expected that Orx neurons would bear alAR, associated with depolarizing responses
to NA as present upon cholinergie basal forebrain neurons, whereas MCR neurons would
104
bear a2AR, associated with hyperpolarizing responses to NA as present upon GABAergic
sleep-active basal forebrain neurons (Fort et al., 1995, Fort et al., 1998, Modirrousta et
al., 2004b).
Many Orx neurons were found to be immunostained for the alAAR, although
unexpectedly many were also immunostained for the a2AAR. In contrast, MCH cens
were almost exclusive1y immunostained for the a2AAR. The difference in the incidence
of alAAR on Orx vs. MCH cens explains one mechanism by which Orx neurons may be
stimulated, whereas MCH neurons would be inhibited during waking when noradrenergic
locus coeruleus neurons are active (Aston-Jones and Bloom, 1981). The MCH neurons
may become active when released from inhibition by noradrenergic input with sleep
onset. These conclusions were recently substantiated in electrophysiological studies
using slices from rat brain, in which NA was found to have an excitatory effect upon
Neurobiotin-Iabeled Orx cens and to have an inhibitory effect upon Neurobiotin-Iabe1ed
MCH cens (Bayer et al., 2005). On the other hand, the present finding that sorne Orx
neurons bear a2AARs suggests that sorne Orx cens may be inhibited by NA. In fact, in
transgenic mice expressing GFP in Orx neurons, NA was found to have a hyperpolarizing
effect upon the GFP-Iabeled Orx cens in vitro (Li et al., 2002). And most recently it was
discovered that the excitatory effect of NA on Orx cens in the rat slice was transformed
into a hyperpolarizing or biphasic effect after two hours of sleep deprivation (Grivel et
al., 2004). It is thus possible that under certain circumstances in mi ce and rats, Orx cens
may mobilize or express a2ARs. In the present experiment, the numbers of cens bearing
alAARs or a2AARs did not vary as a function of sleep-wake condition, thus indicating
that mild sleep deprivation or recovery in adult rats does not appear to alter the
105
expression of these receptors. On the other hand, sleep deprivation in younger rats
(~15
- 20 days), as employed in the in vitro studies, would be associated with stress, as
indicated by e1evated corticosterone (Hairston et al., 2004), and stress or corticosteroids
have been shown to be associated with changes in the expression of Œ2ARs (JhanwarUniyal et al., 1986, Flugge et al., 2003). Thus, the influence ofnoradrenergic input from
locus coeruleus or other brainstem cell groups may under normal conditions be
predominantly excitatory upon Orx neurons through ŒIARs, however change following
stress to become predominantly inhibitory upon them through increased expression or
mobilization of Œ2ARs. This dual potential may allow adaptive changes under conditions
of stress, when sleep along with energy conservation, instead of sustained waking along
with energy expenditure, could enhance survival.
In summary, the present results show that Orx and MCH neurons are active in a
reciprocal manner during sleep deprivation and sleep recovery such as to suggest that
they play opposing roles in regulating sleep-wake states along with activity and energy
metabolism. These opposing roles may in part be mediated by reciprocal relationships or
by differential responses to afferent inputs inc1uding importantly noradrenergic inputs.
4.7 Acknowledgements
The research was supported by a grant from the Canadian Institutes ofHealth
Research (13458).
106
4.8 Preface to chapter 4
The study in the third chapter revealed that within the posterior hypothalamus,
higher numbers of cens expressed c-Fos during sleep deprivation than they did during
sleep recovery. Similarly, it showed that the numbers of c-Fos expressing Orx cens were
higher after SD as compared with the SR condition. In contrast, the numbers of c-Fos
expressing MeR cens were higher after SR as compared with the SD condition. In
addition, many Orx cens were immunostained with UIAAR including those that expressed
c-Fos during SD while few MeR cens were immunostained with UIAAR. Many MeR
cens on the other hand, were found to bear U2AAR including those that expressed c-Fos
during sleep recovery. Sorne Orx neurons were also immunostained with U2AAR. This
study substantiates the role of Orx as one of the posterior hypothalamic peptides
important in waking regulation. The presence of UIAAR on Orx cens could moreover
explain how Orx cens are under further excitation during waking when the release of NA
is highest. These results also identified MeR cens as a population of neurons that play
roI es in opposition to Orx neurons. Under certain situations ofhigh pressure for sleep,
they become active during sleep to facilitate rest behaviors. The presence of U2AAR on
MeR cens could explain how these cens might be inhibited during waking when release
of the NA is highest and how they would be removed from inhibition during sleep when
NA release is at its lowest. The fact that sorne Orx cens also bear u2AAR brings the
possibility that sorne Orx neurons might be inhibited by NA. It moreover suggests the
possibility that under certain circumstances, such as prolonged sleep deprivation or stress,
107
Orx neurons might express or mobilize U2AAR to their surface to dampen their own
activity.
In the first, second and third chapter, we showed that sleep vs. wake promoting
neurons might respond differentially to NA as one of the main neurotransmitters of
waking and therefore might bear different adrenergic receptors. This could be one
mechanism to explain how cells are modulated during sleep vs. waking in a cyclical
manner. Another possible mechanism is that sleep or wake promoting neurons show
different amount of receptor expression or mobilization on their membranes. As shown
in the first and second chapters, GABAergic cell activity and therefore GABA release is
important for sleep promotion and maintenance. We moreover showed, in the second
chapter, that BF cholinergie cells are active during waking but not during sleep. With the
goal ofunderstanding the interaction of sleep promoting GABAergic cells with the wake
promoting cholinergie neurons, we designed our last experiment to test the hypothesis
that cholinergie cells differentially respond to GABA across behavioral states by changes
in the availability of GABAARs on their membrane.
In a paradigm of sleep deprivation and sleep recovery in rats we studied possible
changes that might occur both in the presence and/or intensity of GABAARs on
cholinergie cell membranes across different conditions.
108
5. Chapter 4: GABAA Receptor Modifications on Basal
Forebrain Cholinergie Cells across the Sleep-Waking Cycle4
Mandana Modirrousta, Lynda Mainville and Barbara E. Jones
4
In preparation
109
5.1 Abbreviations
ACh, acetylcholine
ChAT, choline acetyl transfrase
MCPO, magnocellular preoptic nucleus
mIPSCs, miniature Inhibitory Post Synaptic Currents
NDS, normal donkey serum
non-REMS, non-rapid eye movement sleep
REMS, rapid eye movement sleep
Rs, receptors
SC, sleep control
SD, sleep deprivation
SR, sleep recovery
SWA, slow wave activity
SWS, slow wave sleep
110
5.2 Abstract
The basal forebrain cholinergie cells play a critical role in cortical activation and
arousai. These cells discharge in bursts during waking with cortical activation and
become silent during slow wave sleep (SWS) with cortical slow wave activity. We
hypothesized that the activity of cholinergie cells across the sleep-waking cycle could be
homeostatically regulated by changes in GABAA receptors (Rs). In a paradigm of sleep
deprivation (SD) and sleep recovery (SR) in rats, we tirst counted the numbers of
cholinergie cells in the magnocellular preoptic nucleus of the BF that were
immunostained for GABAAR (P2-3 chain). We subsequently measured in fluorescent
microscopy the luminance of GABAAR over the cell membrane. We found that both the
numbers of cholinergie cells that were positively labe1ed for the GABAAR and the
intensity of the GABAAR fluorescence labeling over the cell membrane were
signiticantly increased after SD as compared to SR and sleep control (SC) conditions.
We conclude that the expression or mobilization of GABAARs on cholinergie cell
membranes increases after sustained waking. This increase in membrane GABAAR
could be associated with higher susceptibility to inhibition of the cholinergie neurons by
GABA and consequent decrease in cortical activation with a greater propensity for SWS.
These results suggest a mechanism involving homeostatic regulation of GABAARs and
GABA inhibition of cholinergie neurons that could underlie the sleep-waking cycle.
111
5.3 Introduction
The cholinergie cells of the basal forebrain (BF) play an important role in cortical
activation as the ventral extra thalamic relay from the brainstem arousal systems to the
cerebral cortex (Starzl et al., 1951, Jones, 2000). Recently, extracellularrecording and
juxtacellular labeling in the unanesthetized head fixed rats revealed that identified BF
cholinergie neurons dis charge in bursts with cortical activation (Lee et al., 2005). In
parallel, microdialysis studies have shown that acetylcholine (ACh) release in the cortex
is highest with cortical activation and attentive behaviors (Marrosu et al., 1995,
Himmelheber et al., 2000). Similarly, pharmacological block of ACh receptors
diminishes cortical activation (Vanderwolf, 1975). We have recently shown that
cholinergie BF neurons express c-Fos during sleep deprivation (SD) and not sleep
recovery (SR) during the day (Modirrousta et al., 2004b). All together these studies
demonstrate that the cholinergie BF neurons are active in association with cortical
activation during waking and inactive in association with slow wave activity (SWA)
during SWS. Their relative silence during SWS is likely imposed by GABAergic
inhibitory input (Khateb et al., 1998). We considered the possibility that the effect of
GABA might be under homeostatic regulation across the sleep-waking cycle.
Accordingly, cholinergie neurons would be regulated by synaptic homeostasis such that
after a period of activity, they would decrease their activity in order to preserve stability
and survive (Turrigiano, 1999). It has been shown that the amount of inhibitory vs.
excitatory synaptic activity varies depending upon prior activity (Shatz, 1990).
Application ofTTX on pyramidal neurons decreased miniature Inhibitory Post Synaptic
112
Currents (mIPSCs), the number of open GABAA receptor (GABAAR) channe1s and the
intensity of GABAARs immunostained at synaptic sites (Kilman et al., 2002). Moreover,
application ofbicuculline in rat hippocampal slices increased the density and size of
GABAAR (a. subunits) clusters (Marty et al., 2004). These studies suggest that prolonged
activity elicits more inhibition on neurons, whereas activity blockade elicits more
excitation. Whether the same process occurs during the sleep-waking cycle remains to be
investigated. We thus hypothesized that after a prolonged waking period, those cells
which are active like the cholinergie BF cells would undergo certain synaptic or other
changes to reduce their activity in association with SWA and SWS. This process could
occur through decreasing the excitatory/inhibitory balance by an increase in GABAARs
on the active cholinergie cells.
Here, by applying a paradigm of SD and SR in rats, we investigated the incidence
of cholinergie cells of the magnocellular preoptic nucleus (MCPO) that appeared to be
immunostained for the GABAAR in association with c-Fos under the different conditions.
We then analyzed the intensity ofGABAAR (132-3 chain subunits) on cholinergie cell
membranes across conditions.
113
5.4 Materials and Methods
AlI procedures were approved by the McGill University Animal Care Committee
and the Canadian Council on Animal Care. These procedures also conform to those of
the Association for Assessment and Accreditation of Animal Care International.
Sleep deprivation. Male Wistar rats (200-250 g) were housed individually with
free access to food and water at aIl times with a 12:12 lightldark schedule (lights on from
700 to 1900 hours). As previously described (Modirrousta et al., 2004b), the rats were
deprived of sleep in home cages. Therefore, the experimenter (MM) deprived and/or
observed one rat at a time (on one day) and scored its behavioral state every 20 sec by
visual observation as comprised ofwake, non-rapid eye movement sleep (non-REMS) or
rapid eye movement sleep (REMS). In this manner, the stress of surgery and cage
displacement necessary for recording which can affect c-Fos expression were avoided
(Maloneyet al., 1999). Non-REMS was scored when the animal was recumbent with
eyes closed, showing little or no movement and REMS when the animal was recumbent
with eyes closed, showing rapid movements or twitches of the whiskers, ears or paws.
Rats were submitted to 1) total sleep deprivation for 3 hours (1200 to 1500 h, n=3) as the
SD group, 2) total sleep deprivation for 3 hours (900 to 1200 h,) followed by sleep
recovery for 3 hours (1200 to 1500 h, n=3) as the SR group, or 3) undisturbed sleep and
waking as sleep control (SC) for 3 hours (1200 to 1500 h, n=4). Rats were habituated to
the experimenter's presence and to a paint brush (for sleep deprivation) inserted through
the cage top for 3 days prior to the experimental day. During the experiment, the
deprivation for the SD and SR groups was done by gentle touching using the brush upon
114
dosure of the eyes. Sleep deprivation resulted in the total absence of sleep during 3
hours for the SD and SR groups which was followed by an increase in total sleep in the
SR group relative to both the SD and SC group. At the end of the experiment (1500 h),
rats were immediate1y killed under pentobarbital anesthesia (100 mglkg, i.p.) by intraaortic perfusion with a fixative solution of 3% paraformaldehyde.
Immunohistochemistry. Following immersion in a 30% sucrose solution, brains
were frozen and stored at -80° C. They were cut in coronal sections at 20 Jlm, the
maximal allowable thickness that was determined to allow full penetration and staining
with the antibodies employed. Adjacent series of sections were collected at 800 Jlm
intervals and processed for triple or double immunohistochemical staining for c-Fos
(rabbit antiserum, 1:10,000, Ab-5, PC38, Oncogene Research Products, La Jolla, CA)
using DAB-Ni, choline acetyl transfrase (ChAT) (rabbit antiserum, 1:1000, AB143,
Chemicon International, Temecula, CA, USA) using Cy2-conjugated anti-rabbit
antiserum (Jackson ImmunoResearch Laboratories, West Grove, PA and GABAAR
(mouse antiserum, 1: 100, MAB341, Chemicon) using Cy3-conjugated anti-mouse
antiserum (Jackson). In order to examine whether GABAARs were present upon
cholinergic neurons that expressed c-Fos following SD, SR or SC, triple immunostaining
was performed for c-Fos in the first position incubated over night at room temperature
and ChAT and GABAAR in the second position co-incubated for three nights at 4° C.
Subsequently, other series were processed for double immunostaining of ChAT and
GABAAR co-incubated for three nights at 4° C. Incubations with antibodies were
performed using a Tris-saline solution (0.1 M) containing 1% normal donkey serum
(NDS), following initial blocking with 3% NDS.
115
Microscopie analysis. Sections were viewed by light and fluorescence
microscopy with a Nikon Eclipse E800 microscope equipped with an x/ylz movementsensitive stage and video camera (Optronics, S99808) attached to a computer. Cell
counts and image acquisition were performed by systematic random sampling using
Stereo Investigator (2003, MicroBrightField). Luminance measurement on acquired
images was performed by using Neurolucida software (2003, MicroBrightField,
Williston, VT).
Cell counts. In triple labeled series, single-, double- and triple-immunostained
cells were evaluated in the MCPO for ChAT, ChAT/GABAAR and cFos/ChAT/GABAAR immunostained cells respectively. Single-, double- and triplelabeled cells were counted by applying stereology using the Optical Fractionator program
of Stereo Investigator. Cells were sampled and counted within a delineated contour of
the left side for the M CPO at appropriate levels of a computer resident atlas (expanded
from (Gritti et al., 1993)). Labeled cells were sampled and counted in three sections at
800 !lm intervals from anterior to posterior. Within the stereology pro gram, the size of
the counting frame, within which cells were counted, was set at 125 x 125 !lm and the
grid, within which the counting frames were automatically randomly placed, was set at
250 x 250 !lm. Accordingly, 25% of the MCPO area at each level was sampled. The
cells were counted under a 60x oil objective (with 1.4 numerical aperture, NA). Cells
were counted within a counting block of 8 !lm in depth (in the dehydrated, delipidated,
mounted and coverslipped sections that were on average 10 !lm thick) when the cell
bodies came into focus beneath the surface of each section
116
Luminance measurement. In double labeled series stained for ChAT and
GABAARs, images were sampled and acquired by using random sampling through Stereo
Investigator. In each sampling site, images were acquired using at 60x oil objective (1.4
NA) and filters for Cy2 and Cy3. For images acquired with both filters and across aIl
sections, the exposure time was consistently set at 100 ms with a gain of 4 and target
intensity of 70%. Within Stereo Investigator, the size of the counting frame used to
sample cells was set at 50 x
50~m
and the grid at 250 x 250
~m.
In each frame, the Cy2-
labeled ChAT+ cell which was the closest to the centre of the counting frame was
selected for acquisition and brightness measurement of the membrane Cy3-labeled
GABAAR. For luminance measures, a measurement box was employed with a length of
5
~m
and a width of 1 ~m, which was determined to be the maximal width observed for
membrane GABAAR staining. For each selected ceIl, boxes were positioned over the
nucleus and the portions of the cell membrane that had the minimal distance from the
nucleus and were most paralIel in orientation on each si de of the cell. One other box was
placed over the cytoplasm adjacent to the box over the upper membrane. Luminance
information was colIected as the average luminance of pixels, based on luminance values
ofNeurolucida software within each box (Fig. lB). The overalI membrane luminance in
each condition was obtained by averaging the values per animal and then per condition.
Statistical analysis. Sleep, celI counts and luminance data from celIs were
analyzed between groups using one way ANOVAs in Systat (vI 0.2, Richmond, CA).
When there was a significant main effect, Fisher's LSD post-hoc paired comparisons
were applied between groups. AlI main effects were confirmed by nonparametric rank
order tests (Kruskal-WalIis, p < .05) to insure that the distribution of variance in groups
117
(containing zeros in sorne cases as a result of the experirnental condition) did not distort
the parametric statistics. Figures were cornposed using Adobe Photoshop Creative Suite
(CS) and Adobe Illustrator CS (Adobe, San Jose, CA).
118
5.5 Results
Sleep amount in the three experimental groups
Rats in the sn group did not sleep during the 3 hours in the afternoon prior to
sacrifice (at 1500 h), whereas rats in the SR group that were allowed to recover sleep in
the afternoon after 3 hours of sleep deprivation in the morning (900-1200 h) slept -91 %
ofthe time prior to sacrifice (at 1500 h). Similarly, rats in SC that had undisturbed sleep
or waking for 3 hours before sacrifice spent the majority oftime, -75%, in sleep prior to
sacrifice (1200-1500 h). The amount of sleep differed significantly across conditions of
SC,
sn and SR (Table 1).
spent in non-REMS (77.23
The major proportion of time for the SR and SC groups was
± 2.94% in SR and 68.68 ± 0.10% in SC) and a minor
proportion in REMS (13.58 ± 1.30% in SR and 7.10 ± 0.66% in SC, mean ± S.E.M. of
total time).
Cholinergie eeUs immunostained with GABAAR
According to one way ANOVA with condition as the main factor, the total
number of cholinergie cells (ChAT+) in the MCPO did not differ across conditions
(Table 1), whereas the proportion of the cholinergie cells that were immunostained for
GABAAR (ChAT+/GABAAR+) significantly varied across conditions (F=7.49, df=2,
df=7, p<O.05) with higher proportions in sn as compared with both SC and SR (p=0.006
and p=0.05 respectively) and with no significant difference between SC and SR
119
conditions (Fig. 2, 3A, Table 1). In addition, analysis ofthe triple labe1ed cells revealed
that in the SD group sorne ChAT+/GABAAR+ cells expressed c-Fos whereas no c-Fos
was expressed in the ChAT+/GABAAR+ cells in either SC or SR groups (Fig. lA).
Table 1. Average percent sleep and numbers ofChAT+ and ChAT+/GABAAR+
cells under conditions of Sleep Control (SC), Sleep Deprivation (SD) and Sleep Recovery
(SR)
Variable
SD'
SC
% Sleep
75.75 ±
ChAT+
12750 ±
ChAT+/GABAAR+
2350
±
Membrane GABAAR
luminance
2.23
±
9:!?J~~,~~,QA~~:,:~ 2.32
±
F (Cond)
SR
±
0
90.8
±
2.47
1975.29***
2418.50 15000
±
3500.48
14400
±
871.78
0.228 ns
298.608 78°ot
±
1833
4666.67 ±
1387.2
5.437 *
±
0.91
4.25tt
0.79
14.102***,.,.
0.38
0.604
0.61
0.35
0.27
ott
7.~lt
t
2.91
~-~'"'
±
±
~~mm
0.41
3.1
±
luminance
Values represent Mean ± S.E.M. for 3 groups ofrats. Cell numbers were obtained
from stereological estimates for total numbers on the left side. Values across conditions
(Cond) were compared by one-way ANOVAs (with condition as the main factor), and
post hoc pair-wise comparisons between conditions (using Fisher's LSD). The estimated
total numbers ofChAT+ cells did not differ across conditions (df= 2; dferror=7). Total
numbers ofChAT+/GABAAR+ cells increased significantly across conditions (df=2; df
error=7). Average membrane GABAAR luminance significantly differed (df=2; df
error=222) while average cytoplasmic GABAAR luminance did not differ across
conditions (df=2; df error=223) (ns indicates non-significant; *, p<0.05; ***, p<O.OOl;
120
t,
significant differences between SD and SC;
t between SD and SR and tt between SR
and SC).
Luminance of GABAAR membrane staining across conditions
In each brain, 19-27 ChAT+ cells in the MCPO across three sections were
selected for luminance measures of GABAAR immunofluorescent staining by systematic
random sampling using Stereo Investigator. The luminance value of membrane staining
for each cell was calculated as the average between the two boxes located on two sides
over the membrane and minus that of the nucleus which was considered to represent the
background staining (Fig. lB). According to one way ANOVA with condition as the
main factor, the luminance ofGABAAR membrane labeling (-nucleus) significantly
differed across conditions (F=14.10, df=2, dferror=222, p<O.OOl) (Table 1). Post-hoc
paired comparisons with Fisher's LSD correction revealed that membrane labeling was
significantly higher in SD as compared with both SC and SR (p<O.OOl and p<0.05
respectively) and it was significantly higher in SR as compared to the SC condition (p<
0.05) (Figs. 1C-E and 3B) (Table1). In contrast, the luminance over the cytoplasm (nucleus) did not differ across conditions (F=1.45; df=2; df error=223; p>0.05) (Table 1).
121
Figure 1
122
Figure 1. c-Fos expression and GABAAR on cholinergie cells of the BF. A. A
triple labeled cell for c-Fos (Al) and ChAT( stained green with cy2) bearing GABAAR
(stained red with cy3) (A2). B. A sampIe cholinergie cell in green (B1) stainedfor
GABAAR in red (B2) and the location ofboxes (in yellow) that were usedfor luminance
measurements of the two membrane sides, cytoplasm and nucleus. C Cell
immunostainedfor ChAT (Cl) and GABAAR (C2) in one SC representative brain. D.
Cel! immunostainedfor ChAT (Dl) and GABAAR (D2) in one SD representative brain.
E. Cel! immunostainedfor ChAT (El) and GABAAR (E2) in one SR representative brain.
Arrow heads indicate the locations ofboxes on each membrane sides. Magnification bar
=20J.ll1t
123
Figure 2
sc
SD
SR
Fig.2
Figure 2. Distribution ofChAT+ neurons that were (filled circ/es) or were not
(open circ/es) positively immunostainedfor GABAAR in SC (A), SD (B) and SR (C)
representative brains. Magnification bar
=
1 mm.
124
Figure 3
0.6
J!l 0.5
f3
0:::: 0.4
<l
~
0.3
~
0.2
Ü
'#. 0.1
0.0
<IJ
v
c:
SC
SD
SR
SD
SR
10
<IJ
u
~
0
::1
q:
0::
<
CX)
«~
<IJ
5
C
~
.0
E
111
:2
>-
.'!.:
VI
C
~
.E
0
SC
Cordtion
125
Figure 3. A. Percent ChAT+ ceUs that were also GABAAR+ in the MCPO afier
SC, SD and SR conditions. B. Luminance of GABAAR membrane immunostaining on
ChAT+ ceUs in the MCPO afier Sc, SD and SR conditions. Membrane luminance
represents the mean values per animal per condition. For each ceU, the membrane
luminance was obtained by averaging over two membranes minus that over the nue/eus
of each ceU. (* indicates comparisons with the SD group;*, p= <O. 05; **, p<O.Ol; ***,
p<O.OOl.
t indicates the comparison between the SC and SR groups with p<0.05).
126
5.6 Discussion
We show that the proportion of cholinergic cells with detectable GABAARs
increased at the end of SD, when the animaIs were awake continuously, relative to the
proportions after SC and SR conditions when the animaIs slept the vast majority of time.
We also show that the immunostaining of membrane GABAARs was higher after SD as
compared with both SC and SR conditions. It is then possible that a period ofwaking
enhances expression or mobilization of GABAAR to the cell surface, whereas sleep
reduces the expression or mobilization ofthe GABAAR. From our c-Fos study, we
recently found that sleep active neurons of the BF and the preoptic area are comprised of
GABAergic cells. These GABAergic cells by their local or descending projections
(Sherin et al., 1998, Henny and Jones, 2003) can inhibit neurons of the arousal systems
and promote sleep. Here we show that the numbers or intensity of membrane GABAARs
was altered as a function ofbehavioral state.
From pharmacological studies, it has been known that many anesthetics and
hypnotic drugs act through GABAARs(Lancel and Steiger, 1999). Although sedatives
that induce sleep or anesthesia are physiologically distinct from naturally occurring sleep,
certain EEG and behavioral similarities do exist. Studies in rats showed that after 24
hours of sleep deprivation with a 6 hour period of either ad libitum sleep or propofol
anesthesia, recovery for sleep was similar in timing and persisted not more than 12 hours
in both groups. This study suggests that recovery pro cesses that occur during natural
sleep also take place during anesthesia and suggest that sleep and anesthesia share
common neuroregulatory mechanisms (Tung et al., 2004). Moreover, both circadian
127
rhythmicity and sleep deprivation affect anesthetic actions implying a link: between
factors which regulate natural sleep and drug induced sedation, hypnosis or anesthesia
(Munson et al., 1970, Tung et al., 2002). It is likely then that increases in GABAAR
availability on neurons of the arousal systems including the BF cholinergic cens would
enhance inhibitory responses and consequently sleep promotion.
The profile of GABAAR changes on cholinergic BF cens is comparable to the
homeostatic changes of SWA during sleep. It has been known that SWA is directly
correlated with the duration of prior waking and that SWA is highest during the first
hours and lowest during the last hours of sleep (Borbely et al., 1984). Interestingly,
application of GABA agonists including muscimol and TRIP, enhance SWA (Lancel,
1997, Lancel et al., 1997, Vyazovskiy et al., 2005). It is assurnable thus that increased
numbers of GABAARs on cholinergic cens and likelyon other wake promoting neurons
lead to higher SWA in the beginning of sleep and as sleep pressure reduces, the
GABAAR expression attenuates leading to disinhibition of arousal systems and
preparation of animaIs for the fonowing waking period.
Although there was not any significant difference in the nurnber of cholinergic
cens irnrnunostained for GABAAR between SC and SR groups, the intensity of
membrane GABAAR staining was significantly higher in SR when compared with the SC
condition. This difference could be explained by the prior waking during deprivation of
the SR group in contrast to undisturbed sleep of the SC group during the same moming
period (900-1200 h). The difference in the amount of time spent in sleep, ~ 91 % and
~ 75%
in SR and SC respectively, indicates that SR animaIs needed to sleep more and
recovered from the sleep deficit which would explain the somewhat enhanced GABAAR
on the cholinergic cens. It is thus possible that during 3 hours of sleep recovery, the
128
GABAAR membrane staining appears to decrease progressively retuming to control
levels, although not entirely given the slightly higher staining.
We show that after 3 hours of sustained waking, BF cholinergic cells show
increased immunofluorecent staining for GABAAR. Although previous experiments
showed a time course of days for the increases or decreases of GABAAR as a result of
inactivity (TTX) or activity (bicuculline) in cultured visual or hippocampal cortex
respectively(Kilman et al., 2002, Marty et al., 2004), other studies have demonstrated a
shorter time scale for trafficking of membrane GABAARs. Application of insulin caused
a rapid increase within minutes the expression of GABAARs on the postsynaptic and
dendritic membranes through translocation of intracellular GABAARs to the plasma
membrane (Wan et al., 1997). Similarly, brief stimulation of rat hippocampal cultured
neurons by a dopamine agonist significantly enhanced protein synthesis and surface
expression of GluI receptor within 60 minutes (Smith et al., 2005). In our paradigm
therefore, it appears that 3 hours of waking could be enough for either rapid translocation
ofGABAARs to the post-synaptic domain or translational protein synthesis.
Consequently, enhancement of membrane GABAAR immunofluorecence after the SD
condition could be due to more expression, more translation of GABAAR or it could be
due to more recruitment of subsynaptic GABAAR to surface synaptic zones.
The differential expression or mobilization of GABAARs to cell membranes can
be attributed to several plausible mechanisms. Many studies have shown that higher
neural activity leads to increase in GABA transmission (Marty et al., 2000, Marty et al.,
2004). In parallel, after activity deprivation in cultured rat visual cortex for 2 days, the
number of synaptic sites that expressed detectable levels of GABAARs was decreased by
approximately 50% and the amplitude of mIPSCs similarly diminished (Kilman et al.,
129
2002). AIl together, these studies suggest that in general, stimulation leads to
enhancement of inhibitory synapses. In our study, cholinergie ceIls as shown previously
by c-Fos expression (Modirrousta et al., 2003) are active during waking and inactive
during sleep that represented mainly non-REMS either in SC or SR groups with minimal
amount of REMS. Although unit recording has shown that the activity of cholinergie
ceIls is highest during both arousal and REMS (Lee et al., 2005), the lack of c-Fos
expression in cholinergie ceIls during SC or SR conditions could reflect minimal amount
of REMS to be able to influence on sustained cholinergie activity. The increase in both
numbers and density of GABAAR on the cholinergie ceIls after SD condition and
significant decrease after SC or SR condition could thus be related to their higher activity
during waking and lower activity during sleep.
The trafficking of GABAARs could be regulated by various mechanisms. It has
been shown that application ofbrain derived neurotrophic factor (BDNF), a member of
the neurotrophin family of peptides, in rat visual cortex induced a rapid increase in the
total number of ceIl surface GABAARs, through the activation ofTrk B receptor tyrosine
kinases. BDNF also rapidly induced a sustained potentiation of GABAAR-mediated
currents and se1ective1y increased the mean mIPSC current (Mizoguchi et al., 2003). In
another study, BDNF application prevented the decrease in GABA-mediated inhibition
onto pyramidal neurons that was induced by activity blockade of cultured rat visual
cortex (Rutherford et al., 1997). Interestingly, the expression ofBDNF is regulated by
neuronal activity (Castren et al., 1992, Tabuchi et al., 2000). BDNF is in addition one of
the genes of which expression is upregulated by waking independent of the time of day
(Cirelli et al., 2004). Considering that BDNF mRNA is expressed in the BF (Conner et
al., 1997), it is possible that neuronal activity of cholinergie ceIls during waking, drives
130
expression of more GABAAR on cholinergie cells and this change could be partly
regulated by release of BDNF from active, likely, glutamatergic neurons.
In summary, these results suggest a novel mechanism through which cholinergie
cells, as part of arousal systems, are self regulated in a homeostatic manner during
waking and thus by accumulation of more inhibitory receptors as waking continues, they
will be prepared to be inhibited to allow sleep occurrence and maintenance. Whether or
not the same mechanism occurs in other neurons of the arousal systems is a subject for
future study.
5.7 Acknowledgements
The research was supported by a grant from the Canadian Institutes ofHealth
Research (13458).
131
6. General Discussion
Experiment one
The results of the first chapter revealed that in anesthetized rats, a substantial
proportion of the BF GABAergic cens (57%) discharged at higher rates during cortical
SWA and their firing rates decreased during evoked cortical activation. This finding
suggested that those identified GABAergic cens in the BF that are maximany active
during cortical SWA are putative sleep promoting cens. In the next step, by dual
immunohistochemical staining for GAD and a2AAR, we first found that approximately
60% ofGABAergic cens distributed through the MCPO and SI were immunostained for
a2AAR. Second in urethane anesthetized rats, we found that an Nb-Iabe1ed GAD+ cells
that were maximally active during cortical SWA bore a2AAR. These cells can
correspond in a large part to previously identified sleep-active neurons in this region
(Szymusiak and McGinty, 1986a). Moreover, this study proposed that these neurons
could be differentiated from other GABAergic SWA-off cells perhaps by their inhibitory
response to NA through a2AAR. The a2AR, through opening ofK+ channe1s, causes
hyperpolarization of the membrane as shown on the neurons of the POA and locus
coeruleus (Williams et al., 1985, Bai and Renaud, 1998). Consequently sleep promoting
GABAergic cells could be inhibited by NA during waking when LC neurons discharge at
their highest rate (Aston-Jones and Bloom, 1981). Reciproca1ly, they would be
disinhibited during cortical SWA and SWS when LC neurons decrease their firing rate.
These results indicated a captivating possibility that particular BF GABAergic cells
132
might be important for sleep promotion and maintenance. In order to confirm these
results in natural sleep and waking behaviors we designed our next experiment that is
presented in chapter two.
Experiment two
The study in chapter two looked at the pattern of c-Fos activation, as an indicator
for neuronal activity in the cholinergie and GABAergic cells of the BF and/or POA after
different conditions. Consistent with our results in the first chapter and in anesthetized
rats, these results for the first time revealed that substantial proportions of neurons that
were active during sleep across both the BF and POA were GABAergic and none were
cholinergie. Furthermore, our results for the first time showed that GABAergic sleepactive cells are not confined to only one or two nuclei but are distributed across these two
regions and are intermingled among other wake-active cells. These GABAergic neurons
by their ascending projections to the cortex (Szymusiak et al., 1989, Gritti et al., 1997),
descending projections to the other arousing systems such as the posterior hypothalamus
(Gritti et al., 1994, Gong et al., 2004) or also likely by their local projections to the BF
cholinergie cells (Manns et al., 2000a) could exert an inhibitory influence and thus lead to
cortical dampening and inhibition of arousing systems in the posterior hypothalamus and
BF (Fig. 1). In addition, our results showed that across both the BF and POA more
neurons, being either GABAergic or non-GABAergic, expressed c-Fos during waking
than during sleep. This finding is consistent with previous c-Fos and in situ hybridization
studies (Pompeiano et al., 1992, Cirelli et al., 1993, Ledoux et al., 1996, Sastre et al.,
133
2000). However, it is in opposition with recent reports demonstrating that in the VLPO
and MnPO more cells expressed c-Fos during sleep than during waking. This apparent
discrepancy could result from different experimental conditions, such as housing, surgery
and method of sleep deprivation which could be associated with more stress and therefore
more c-Fos expression. Furthermore, in most previous studies, animaIs were allowed to
recover from sleep deprivation or spontaneously sleep not more than two hours. Taking
into consideration that almost 90 minutes are required for Fos protein synthesis and
considering the Fos halflife of ~2-3 hours, it is possible that those results reporting
higher numbers of c-Fos in the VLPO during sleep, in fact mostly reflected activity from
the prior waking state. In the current study by keeping the animaIs in their home cages
during the experiment, by using behavioral observation instead of electrode implantation
for sleep-waking scoring and finally by applying gentle touching when needed for sleep
deprivation, the amount of imposed stress was minimized. It is thus considered that 3
hours of sleep deprivation is short enough for minimization of sleep deprivation induced
stress and long enough for accumulation of Fos protein (Sheng and Greenberg, 1990,
Morgan and Curran, 1991). Vnder these conditions, an increase in the total number of
neurons expressing c-Fos was not observed in any nucleus ofBF or POA during the
recovery or control sleep as compared with sleep deprivation. In parallel with increased
c-Fos expression during waking, higher numbers of cholinergic cells expressed c-Fos
after SD as compared with both SR and SC conditions. Indeed, no c-Fos was observed in
cholinergic cells after SC or SR conditions. Although it has been known that in addition
to arousal, the cholinergic cells discharge during REMS (Lee et al., 2004), we assume
that small proportion of REMS in either SC or SR conditions, 14% vs. 9% respectively,
was inadequate to allow accumulation of c-Fos in these groups.
134
In addition to those GABAergic cens that expressed c-Fos during sleep, we
showed that across the BF and POA, many GABAergic cens expressed c-Fos also after
sleep deprivation. This finding is in accordance with unit recording that showed
physiologicany distinct population of GABAergic cens discharged at higher rate during
cortical activation (Manns et al., 2000a). In the first chapter, we showed that an SWA-on
GABAergic cells bore <l2AAR. Similarly in the second chapter, we showed that on
average ~80% vs. ~4% of c-Fos expressing GABAergic cells were immunostained for
<l2AAR after sleep recovery vs. sleep deprivation respective1y. It confirms the hypothesis
that particular GABAergic neurons of the BF and POA that are active during sleep have
the common phenotype ofbearing <l2ARs and differ from those GABAergic cells that are
active during waking. In paralle1, it has been shown that GABAergic neurons of the
VLPO were inhibited by application of NA (Gallopin et al., 2000). Consistent with
adrenergic receptor results, evidence from autoradiographie and retro grade tracing studies
have suggested projections to the BF from the locus coeruleus noradrenergic neurons that
could be a source of NA to act on <l2AARs (Jones and Moore, 1977, Semba et al., 1988,
Jones and Cuello, 1989). A recent e1ectron microscopie study has shown that an
individual LC axon can establish synaptic contacts with both cholinergie and noncholinergie neuronal e1ements (Hajszan and Zaborszky, 2002). The <l2AAR bearing
GABAergic cens of the BF and the POA thus appear to be under inhibition during
waking by NA. Accordingly, when re1ease of NA is maximal during waking, particular
sleep-active GABAergic cells would be inhibited through <l2AAR and with decremental
re1ease of NA during sleep, they would be disinhibited. They in tum by inhibiting the
monaminergic neurons of the brainstem and cholinergie neurons of the BF could initiate
135
sleep (Gritti et al., 1994, Luppi et al., 1995, Sherin et al., 1998, Steininger et al., 2001,
Chou et al., 2002).
Experiment three
In the third chapter, in order to chemically identify neurons of the posterior
hypothalamus that exert roles in sleep vs. wake promotion, we looked at c-Fos expression
in Orx and MCR cells after conditions of sleep deprivation vs. sleep recovery. The
results indicated that in paralle1 with neurons of the BF and POA, more cells in the
posterior hypothalamus expressed c-Fos after sustained waking than after recovery sleep.
These findings reflect and confirm the participation of the posterior hypothalamus in the
maintenance ofwaking (Fig. 1). They also confirm electrophysiological studies showing
that in the posterior hypothalamus the majority of cells fire in association with waking
(Steininger et al., 1999, Alam et al., 2002, Koyama et al., 2003). Similarly, higher
numbers of Orx cens expressed c-Fos after sleep deprivation than after sleep recovery.
This result verifies microdialysis studies showing that re1ease of Orx is high during
waking and low during sleep (Fujiki et al., 2001, Yoshida et al., 2001, Zeitzer et al.,
2003). Yet, only 5% of total c-Fos expressing cens during waking in the posterior
hypothalamus were Orx+ meaning that under quiet waking states, other posterior
hypothalamic cens might be more important than Orx cens in the maintenance ofwaking.
The role of Orx cens, on the other hand, might be more prominent when animaIs are in
transition from sleep to waking state (Lee and Jones, 2004) or when they have more
locomotor activity or need more energy expenditure and thus increased food seeking
(Sakurai et al., 1998, Wu et al., 2002). Orx neurons may be particularly important to
136
maintain a continuous, stable wakefulness and to avoid rapid fluctuations in behavioral
state (Mieda et al., 2004). Orx cells project to multiple neuronal systems including the
cholinergie neurons in the BF, laterodorsal tegmental (LDTg) and pedunculopontine
tegmental (PPTg) nuclei, monoaminergic neurons including histaminergic in
tuberomammillary nucleus, noradrenergic neurons in locus coeruleus, serotoninergic
neurons in raphe nuclei and dopaminergic neurons in the ventral tegmental area, as well
as other neurons in the brainstem and the spinal cord regions (Peyron et al., 1998,
Horvath et al., 1999, Marcus et al., 2001, Beuckmann and Yanagisawa, 2002, Taheri et
al., 2002).
The widespread projections of Orx neurons to the two main components of the
ascending arousal system in the brainstem and in the BF suggest a central role of Orx in
promoting wakefulness. It is possible that activation of Orx cells during sleep
deprivation or waking could drive more excitation of other arousing systems for further
maintenance of an aroused state. The orexinergic system together with other
monoaminergic and cholinergie arousing systems may thus work in concert to regulate
sleep and wakefulness. In addition, the link between the sleep-wake regulatory role of
the hypothalamus and other hypothalamic functions such as metabolism, hormone
release, regulation of food and water intake and temperature is now becoming more
evident(Gong et al., 2000, Rechtschaffen et al., 2002). Interestingly, it is shown that Orx
neurons, in addition to their important role in arousal, stimulate energy metabolism
through the hypothalamo-pituitary thyroid axis (HPT) (Lubkin and Stricker-Krongrad,
1998) and the hypothalamo-pituitary adrenal axis (HP A, stimulating increased
corticosteroid leve1s) (Ida et al., 2000). They are also sensitive to metabolic eues such as
glucose, leptin and ghrelin (Beuckmann and Yanagisawa, 2002, Taheri et al., 2002) and
137
have a positive influence on the sympathetic nervous system by increasing heart rate,
blood pressure and temperature (Shirasaka et al., 1999). It is therefore more likely that
Orx cells play an integrative role to enhance arousal whenever there is a higher demand
for increasing basal metabolism, energy expenditure or locomotion by their excitatory
influence upon other arousing systems (Rorvath et al., 1999, Bayer et al., 2001,
Eggermann et al., 2001, Burlet et al., 2002) or upon other hypothalamic neurons (van den
Pol et al., 1998, Ferguson and Samson, 2003). Furthermore, the direct projections from
the circadian pacemaker, SeN, to Orx and MeR containing cells (Abrahamson et al.,
2001) suggests the interaction between sleep control in the posterior hypothalamus and
circadian rhythm.
In contrast to Orx cells, MeR cells that are distributed in almost the same area as
the Orx cells, expressed more c-Fos after recovery for sleep than after sleep deprivation.
Increase of c-Fos expression in MeR cells was only observed in association with
recovery for sleep when the animaIs slept more than 90% of the 3 hours and it was not
seen after the control natural sleep condition in which the animaIs slept -75% ofthe total
time. This means that in naturally sleeping animaIs and under normal conditions,
hypothalamic MeR cells might not participate in sleep promotion and/or maintenance
but only during a recovery process when there is a high demand for sleep, these cells
could become active perhaps to take part in sleep facilitation and associated recovery
processes. It has been shown that MeR decreases energy metabolism and activity
through a negative influence on the sympathetic nervous system, the RPT and the RP A
axes (Shimada et al., 1998, Kennedy et al., 2001, Marsh et al., 2002, Ito et al., 2003,
Shearman et al., 2003, Zhou et al., 2005). In this contex, MeR neurons could play an
138
opposite role to that of Orx neurons by decreasing activity and energy metabolism in
association with sleep through inhibition of transmission in the hypothalamus and other
arousal systems (Fig. 1) (Bittencourt et al., 1992, van den Pol et al., 1998, Gao and van
den Pol, 2001)
We found that many Orx neurons were immunostained for u lAARs, although
some were also immunostained for u2AARs. In contrast, MeR cens were almost
exclusively immunostained for u2AARs. The incidence of UIAARs on Orx cens that
induces depolarization could explain one mechanism by which Orx neurons would be
stimulated by locus coeruleus noradrenergic neurons during waking when the release of
NA is high (Aston-Jones and Bloom, 1981). In fact it is known that noradrenergic locus
coeruleus neurons send ascending projections to the posterior hypothalamus (Jones and
Moore, 1977, Jones and Yang, 1985). Although it is not known whethernoradrenergic
cens from the Le make synapses on Orx neurons, in vitro studies have shown that NA
can directly depolarize and excite Orx cells (Bayer et al., 2005). Orx cells in turn by their
excitatory projections to the locus coeruleus are able to further enhance or maintain
release of NA. On the other hand, the MeR neurons would not be excited by NA during
waking rather they would be inhibited through u2AAR. Rowever, they may become
active when released from the inhibitory influence of NA with sleep ons et and decreasing
NA release. The finding that some Orx neurons bear u2AARs suggests that populations
of Orx cens are likely inhibited by NA. It is possible that under certain circumstances
such as stress, or prolonged excitation by NA, Orx cells mobilize or express u2AARs on
their plasma membrane to be inhibited and consequently permit sleep initiation. In fact,
in vitro studies on neonatal rat slices have shown that the depolarizing effect of NA on
139
Orx cens changes into a hyperpolarizing response after 2 hours of sleep deprivation
(Grivel et al., 2005). In other studies, it is shown that stress or corticosteroids are
associated with changes in expression of u2ARs (Jhanwar-Uniyal et al., 1986, Flugge et
al., 2003). Knowing that sleep deprivation in neonatal rats increases corticosterone levels
and is thus stress fui (Hairston et al., 2004), we assume that in response to the
corticosterone and stress, Orx cens could potentiany express U2AAR. Here, we did not
find any significant difference on the numbers of Orx cens bearing UIAAR vs. U2AAR
across conditions, and since the receptors were not judged as being in cytoplasm or on the
membrane, we could not judge whether those receptors were functional. However,
changes in receptor expression or mobilization leading to changes in their functions could
be partly responsible for excitation vs. inhibition of different sleep vs. wake-active cens
across behavioral states. This phenomenon might be another potential mechanism for
sleep-wake regulation. To test this possibility we designed our last experiment to study
receptor plasticity under different conditions of sleep deprivati~n vs. sleep recovery.
Experiment four
In the fourth and last chapter, we analyzed the numbers and the intensity of
membrane GABAARs on BF cholinergie cens. We found that the proportion of
cholinergie cens that were immunostained for GABAARs was increased after sleep
deprivation as compared with either control or recovery sleep. Moreover, we showed
that fluorescence GABAAR immunostaining was increased after sustained waking. These
results showed that a period of sleep vs. a period of sleep deprivation have contrary
140
effects on the expression or mobilization of the GABAARs on cholinergic ceIls. During
waking, the BF cholinergic cells discharge in bursts and in the meantime accumulate
more membrane GABAARs. It is known that the strength of inhibitory synaptic currents
is directly corre1ated with the number of synaptic GABAARs (Otis et al., 1994) (Nusser et
al., 1997, Nusser et al., 1998). Therefore, this build up ofGABAARs on cholinergic ceIl
surfaces would make them more susceptible to GABA release that could occur with sleep
onset. Consequently, after a prolonged waking period along with an increase in sleep
pressure, the cholinergic cells may respond to even small amounts of GABA because of
their enhanced membrane GABAARs. This in tum would dampen cholinergic activity
and facilitate sleep promotion by decreasing cortical activation. Moreover in the tirst and
second chapters, we showed that the GABAergic sleep-active ceIls are intermingled with
the BF cholinergic neurons and other ceIls in the adjacent POA. These GABAergic ceIls,
in paraIle1 with the attenuation of arousal and cortical activation, would be removed from
inhibition and begin their activity which then by local projections to the cholinergic cells
and acting through expressed or mobilized GABAARs lead to stronger inhibition of
cholinergic neurons.
The fact that many anesthetics and hypnotic drugs, inc1uding barbiturates and
benzodiazepines, act through GABAARs suggests the importance of this receptor in sleep.
Although the EEG during anesthesia is distinct from naturaIly occurring sleep, recent
studies suggested that there are behavioral and perhaps neurophysiological similarities
between sleep and anesthesia. Studies in rats revealed that recovery from sleep
deprivation can occur during propofol anesthesia (Tung et al., 2004).
141
We showed that the immunofluorecent intensity of GABAARs was higher after
sleep deprivation relative to sleep recovery and control conditions and it was higher after
recovery for sleep relative to control conditions. It appears thus that during 3 hours of
sleep recovery the membrane GABAARs staining decreases to return to controllevels,
although not entirely and still would be higher than after 3 hours of control sleep. This
difference could possibly be related to the condition prior to the experiment. Whereas the
animaIs in sleep recovery group were deprived of sleep for 3 hours in the morning and
before the experiment, the animaIs in the control group were aIlowed to sleep at the same
time. In other words, after sleep recovery condition the animaIs were probably still under
more pressure for sleep than after sleep control condition. The significantly higher
GABAAR intensity after sleep recovery, could explain higher demand for maintaining
sleep in those animaIs.
Several studies have shown that neuronal activity leads to enhancement of
membrane GABAAR c1usters. Activity blockade of cultured visual cortex by TTX after 2
days reduced the intensity of GABAAR labeling (Kilman et al., 2002). Similarly, the
activity driven by the application ofbicuculline on cultured hippocampal cells after 13
days increased the density of GABAARs (Marty et al., 2004). These results suggest that
cellular activity for a period of time is followed by synaptic changes toward a direction
that leads to cellular inactivity or inhibition. In reverse, when neurons are under
inhibition for a certain period, their synaptic changes prepare them for subsequent robust
excitation. These findings are aIso consistent with the theory of synaptic homeostasis
stating that in complex systems homeostatic plasticity has to take place to prevent
destabilization during various physiological processes (Turrigiano, 1999, Marder and
142
Prinz, 2002, Mody, 2005). During waking, many cells fire continuously in order to
sustain wakefulness. Yet, no one investigated whether the same phenomenon happens
during waking on the arousing activating neurons. Here, for the first time we showed
that the numbers and the intensity of GABAARs on cholinergie cells as part of the
arousing systems were increased after 3 hours of sleep deprivation. As we showed in the
second chapter, the BF cholinergie neurons are active during sleep deprivation
represented by c-Fos expression. A recent study in the CUITent lab moreover revealed that
identified BF cholinergie cells discharge in bursts during cortical activation and arousal
in unanesthetized head-fixed rats (Lee et al., 2003). The enhanced membrane GABAARs
on the cholinergie neurons at the end of the waking period could be thus the consequence
of their dis charge activity during waking suggesting a homeostatic mechanism for the
activity regulation of cholinergie cells as a part of the arousing systems.
We showed that 3 hours of sleep deprivation was enough to induce significant
changes in the amount and the intensity of fluorescence GABAARs staining. Other
studies indeed have shown that insulin could provoke rapid translocation of intracellular
GABAARs to the membrane (Wan et al., 1997). In paralle1, the application of dopamine
agonists on cultured hippocampal cells induced glutamate receptor 1 (GluR1) synthesis
within 60 minutes (Smith et al., 2005). This evidence thus shows that even for protein
synthesis, changes could occur in a short time scale. Here, we did not assess the mRNA
for GABAARs and therefore what we recorded as the increase in numbers or intensity of
detectable membrane GABAARs could be either due to recruitment of
intracellular/subsynaptic pools or it could reflect more receptor synthesis.
143
The insertion of GABAARs into cell surface membrane appears to be regulated by
diverse signaling pathways. Application ofBDNF, a neurotrophin factor, on rat visual
cortex, through activation ofTrkB receptors resulted in increased mIPSC amplitude in
pyramidal cell neurons (Mizoguchi et al., 2003). In paraIlel, BDNF prevented the
decrease in GABA-mediated inhibitory currents that was induced by activity blockade in
rat visual cortex cultures (Rutherford et al., 1997). Besides, both neuronal activity and
wakefulness are known to increase the expression ofBDNF (Castren et al., 1992, Cirelli
et al., 2004). One potential signaling mechanism of regulating the cell surface GABAARs
thus could be expression ofBDNF (Conner et al., 1997)that might occur following the
activity of cholinergie cells and therefore through activating TrkB receptors and
intracellular Ca++ cascades (Mizoguchi et al., 2003) would be able to recruit more
GABAARs to the plasma membrane.
144
Summary
In summary, multiple brain regions and neurotransmitters play roi es to regulate
the sleep-waking cycle. During waking, beside the glutamatergic, monoaminergic and
cholinergic arousing systems in the brainstem, we showed that the cholinergic BF
neurons and the Orx cens of the posterior hypothalamus take part in cortical activation
and behavioral arousal (Fig. 1). These findings show that different aspects ofwaking are
regulated by activation of different neurons. Accordingly, the BF cholinergic cens by
their cortical projections stimulate cortical activation. Orx neurons on the other hand, by
their widespread excitatory projections to the cortex, the spinal cord as wen as to the
multiple arousing systems including the BF cholinergic cens and the brainstem
monoaminergic and cholinergic neurons would maintain continuous waking. They are in
addition, of paramount importance during those arousal periods that are associated with
increased motor activity, high demand for metabolism, energy expenditure and increased
sympathetic drive. While exciting arousing systems, these cens receive reciprocal
excitatory inputs from other elements ofthe arousal systems including most importantly
from the Le noradrenergic fibres. As shown for Orx cens, theyare endowed by ŒIAAR
and would be further excited during waking when release of NA is highest.
Similar to waking states, several neuronal systems act in coordination to facilitate
sleep promotion and maintenance. We showed that particular GABAergic cens of the BF
and the adjacent POA become active during sleep and thus might be important for sleep
generation and maintenance (Fig. 1). These particular GABAergic cens through their
145
long cortical projections (Gritti et al., 1997) might be involved in cortical dampening and
SWA (Szymusiak et al., 1989, Manns et al., 2000a). In addition, locally projecting
GABAergic neurons could inhibit the BF cholinergie cells during SWA (Manns et al.,
2000a, Szymusiak et al., 2000) and finally by descending projections to the posterior
hypothalamus (Gritti et al., 1994, Henny and Jones, 2003), they could inhibit the activity
of Orx neurons. These findings c1arify how the arousal systems inc1uding the BF
cholinergie cells or the posterior hypothalamic Orx neurons could become and remain
inactive during sleep. Consistently, it has been shown that the GABA release is highest
during SWS in the posterior hypothalamus (Nitz and Siegel, 1996). Due to the intrinsic
depolarization state of Orx neurons (Eggermann et al., 2003), by which wakefulness may
be maintained over long periods, their inhibition through GABAergic inputs from the BF
and POA is crucial for sleep initiation. The inhibition of Orx cells in turn attenuates the
sympathetic nervous system drive, motor activity, energy metabolism and feeding
behaviors and thus facilitates a restful behavioral quiescence and sleep. Our results could
provide a physiological basis for the hypnotic and sedative effects of many drugs that act
through enhancement of GABA transmission.
We found that the sleep-active GABAergic cells are distributed across the BF and
the POA yet are more concentrated in sorne nuc1ei inc1uding the VLPO and the MnPO.
These particular GABAergic cells bear U2AAR and thus could be distinguished from other
wake-active GABAergic neuron across the BF and POA. These findings show how
sleep-active cells are reciprocally influenced by arousal systems such as noradrenergic
LC neurons. Consequently, they are under inhibition through U2AAR during waking
when the release of NA is greatest whereas with decremental release of NA that occurs
146
during sleep, they become activated to dampen arousal systems and bring on sleep. In
addition to the activity of GABAergic neurons, GABA transmission could be moreover
enhanced postsynaptically through increases in expression or mobilization of GABAARs
on arousal systems including the BF cholinergic cells at the end of waking phase to
promote sleep. During waking, the BF cholinergic cells are active to maintain cortical
activation. As a consequence of their activity, we showed that the cholinergic cells
accumulate more GABAAR on their surface. The accumulated membrane GABAARs in
tum leads to robust inhibition of cholinergic cells to allow sleep occurrence. These results
explain a novel and intriguing mechanism for homeostatic inactivation or activation of
the BF cholinergic cells across the sleep-waking cycle.
We found that under certain situations such as high pressure for sleep, the
hypothalamic MCH neurons in paralle1 with the GABAergic cells of the BF and POA
become active so that by their widespread projections and their negative influence on
energy metabolism, activity and sympathetic nervous systems, in opposition to Orx cells,
facilitate recovery for sleep (Fig. 1). We showed that similar to the waking state, sleep is
regulated by different cell types. Depending on the prior state and whether it imposes
high demand of sleep or energy compensation, additional systems such as MCH neurons
become active to aid the GABAergic sleep promoting cells. We found that MCH
neurons bear uzAAR. Accordingly during waking, MCH cells are inhibited by NA
through uzAAR, whereas during sleep recovery, they would be disinhibited and thus could
exert a role in the recovery process.
These studies e1ucidated different chemically identified neurons that play roles in
sleep-waking cycle regulation. Moreover, they explained in part how sleep and wake
147
promoting neurons interact with each other to create a cycle and to modulate their
activity. They also revealed that as heing part of complex systems, sleep or wake
promoting neurons could show dynamic plasticity of their receptors to regulate their own
activity in a homeostatic manner. The results have enriched our understanding of the
sleep-waking cycle that could he important for the understanding and treatment of sleep
or waking disorders.
148
Figure 1
Fast EEG (W & PS):
!~liW!"il~
Slow EEG (SWS):
1\V1'1\j ,J\,Vt\VrI\\
Fast EEG active (Gamma+/Delta-; W-PSl
.. ACh
A GABA
Slow EEG active (Gamma-/Delta+; SWSl
À
GABA (a2-ARJ
Behavioral wake active (EMG+;W)
o
NA
1iji(- Orx
Behavioral sleep active ŒMG-; SWS-PS)
.... GABA (IÛ-AR)
,. MCH
149
Figure 1. Sleep vs. wake promoting systems. Schematic sagittal view ofrat brain
(modifiedfrom (Jones, 2005)) showing the major neuronal systems and their major
pathways (red arrows) and transmitters (red symbols) involved in waking. The major
ascending pathway emerges from the brainstem reticular formation (RF) to ascend along
a dorsal pathway into the thalamus (Th) from where the second thalamo-cortical
projection fibres in turn reach the cerebral cortex (Cx) in a widespread manner. They
also through a ventral pathway project to the hypothalamus and then up to the basal
forebrain (BF) neurons where they terminate and the second basal-cortical fibres again
in a wide spread manner project to the cerebral cortex. Noradrenergic neurons of the
locus coeruleus (LC) send axons along the major ascending arousal systems to project in
a diffuse manner to the cerebral cortex, hypothalamus and the BF. The basal forebrain
cholinergie neurons which contain acetylcholine (ACh) in addition to laterodorsal and
pedunculopontine tegmental (LDTg and PPTg) nuclei of the brainstem are located in the
BF from where they project to the cortex. Orexinergic neurons (Orx) in the mid- and
posterior hypothalamus (PH) project diffusely through the forebrain, brainstem and
spinal cord to exert an excitatory influence at multiple levels. Some specifie GABAergic
neurons may be on during waking perhaps to project to cortical interneurons and induce
an indirect excitation ofpyramidal cells. The major sleep promoting systems and their
major pathways (blue arrows) together with their chemical transmitters (blue symbols)
are also shown. Particular cortical/y projecting GABAergic cel/s of the BF and preopticanterior hypothalamic areas (POAH) may have the capacity to dampen cortical
activation during sleep. Local/y projecting GABAergic cel/s may inhibit the BF
cholinergie cel/s. Other GABAergic neurons of the BF and POA project caudally to the
PH where they may inhibit multiple arousing systems including the hypothalamic Orx
150
neurons. MCH cells with their widespread inhibitory projections to the en tire brain can
influence multiple systems. Along with sleep-on GABAergic neurons they could
potentially send projections to the cortex to inhibit cortical activation. They may also
inhibit their neighbouring Orx cells by local projections or even by proximal cell to cell
contacts. Abbreviations: 7g, genu 7th nerve; ac, anterior commissure; CPu, caudate
putamen; Cx, cortex; EEG, electroencephalogram; EMG, electromyogram; Gi,
gigantocellular RF; GiA, gigantocellular, a part RF; GiV, gigantocellular, ventral part
RF; GP, globus pallidus; Hi, hippocampus; ic, internaI capsule; LDTg, laterodorsal
tegmental nucleus; Mes RF, mesencephalic RF; opt, optic tract; PH, posterior
hypothalamus; PnC, pontine, caudal part RF; PnO, pontine, oral part RF; POA, preoptic
area; PPTg, pedunculopontine tegmental nucleus; Rt, reticularis nucleus of the
thalamus; s, solitary tract; sep, superior cerebellar peduncle; SL substantia innominata;
SN, substantia nigra; Sol, solitary tract nucleus; Th, thalamus; TM, tuberomammillary
nuclei; VTA, ventral tegmental area.
151
7. Appendix
152
7.1 Ethics approval
'.
Vo/WN.mcgiltcafrgolanfmaU
Prolocol 1#:
Approval end date: ~M.u.\I., ~(, ~O()<O
RENEWAL
Faellity Commluee: \-t}..) 1..
of Animal Use Protocol
Researeh l8J Teaehing 0
For:
RenewalN:
p~oject
Principallnvestigalor:
Dr. Barbara E. Jones
Prolocol Tille:
Neuml BnsÎs orthe Slcee·Wakins Cycle
Mhl
NcurolollY & Neurosurgery, Montreal Neurologieal
Institllte. 3801 University St" #896. Montreal, Quebec HlA
284
Unit, Depl. & Address:
Emoil:
t~ li '1
MeGiIl University Animal Care Committee
barbnra.joncs!Cllmcgîll.ca
Slart of Funding:
Prolocoll#
Level:
April 1. 20DI '
'1.
Phone:
loi
1943·0
--~~-----------. . ::;39:..::8:.. . :. :19:.:.1;::.3_______
. .::c39:..::8:..;.S::.;:8:..:.7.:.1_ _ _ _ __
Fax:
Funding source:
0
~
CIHR
q ')..1\.<.-
End ol Fundlng: _r.:.:;I"'nr:.::c:.;.h.:;.3.;.,1.c.:;2:..:.0.:;.06=--_ _ _ _ _ _ _ _ _ __
Emergency contact III + phone Ils Dr. Barbara E. Jones· 514·937·5550
Emergcncy COntact 1#2 + phone 115 -=Lyn=d=a..:.:r.:::la:::in.:..:v..:.:iI:.:le:....•...:4.::.S0;::.-4:.:.5~8:....•.:. :IS:;.: 8:.:.4_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __
1. Personnel and
ualifications
List the bames of the Principal Investlgalor and orau individunis who will bc ln Clmlaet wlth anlmab in this sludy and thclr
emptoymenl classification (investigator, technicÎnn, research assistant, undergradualeJ graduale student, fellow). Ir an
undergraduale student!s involved, the role of the sludent and the supervision received must be described. Training Is
mandalory for ail personnellisted here. Reler to www.lln;malca.e.mcglll.cl/for detalls. ElIch person l!sled ln thls seelion must
51 n. (S lice will .... and as needetl.
Oceupntional Helllth
Name
Classification
Animal Related
Signature
~ lias rClld Ih, I1rlglnal
Training Information
Program •
1411 rolol:ol"
Dr. Barbara E. Jones
PI
Lynda Mainville
Technician
Initial training with
> 30 years experience
Theory course + rat workshop
no
Mandana Modirroust.'1
PhO Student
Theory course + tat workshop
no
Frédéric Brischoux
POF.
Theory course + rat workshop
no
no
3. Summary (in language that will be understood by members of the general public)
AIMS AND BENEFITS: Oescrlbe. in a short paragraph, the overallnim oC the study and Ils potentinl bene lit 10 humnnlllnimnl
henUb or to Ihe ndvtmtemellt of sclenlilit knowlcdlte (Iflas sectloll 5a 1/1 maill protoc:ol).
Understand howan attentive wakeful stale is maintaincd and how normal sleep occurs 50 as to know why such
O.~FEV. 200~
153
MONTREAL
NEUROLOGICAL
INSTITUTE
AND HOSPITAL
INSTITUT ET
HÔPITAL
NEUROLOGIQUES
DE MONTRÉAL
MeCi/! University
Université McCil/
Barabara E. Jones, Ph..D.
Professor
Departmmt ofNeuro/ogy and
Neurosurg"y
Oecember 7, 2005
To Whom It May Concern,
This letter authorizes Mandana Modirrousta to reprint a manuscript in her Ph.D. thesis
of which we are co-authors. The manuscript is:
Modirrousta M, Mainville L, Jones, BE. GABAp, Receptor Modifications on Basal
Forebrain Cholinergie CeUs across the Sleep-Waking Cycle, in preparation.
Thank you.
Sincerely,
~~~
Lyn Mainville
Research Technician
Int
3801 University Street, #896
Montreal, Quebee
Canada H3A 2B4
Telephone: (514) 3981913
Fax: (514) 398 5871
E-mail: barbarajones@mcgill.
154
-~---
Original Message -----
Ft:qm: eilams"Zôe'; :"'"
To: Chalifour Kristin; [email protected]
Sent: Monday, July 04,20058:10 AM
Subject: RE: Permission for publishing my pa pers in my Ph.D. thesis
Dear Mandana
Thank you for yOuf email request. Permission is granted for you to use the mate rial below for
yOuf thesis subject to the usual acknowledgements and on the understanding that you will reapply
for permission ifyou wish to distribute or publish yOuf thesis commercially.
Goodluck!
Best wishes,
Zoë Ellams (Miss)
Permissions Co-ordinator
Blackwell Publishing
9600 Garsington Road
Oxford
0X42DQ
Tel: 00441865476149
Fax: 00441865471149
[email protected]
AlI future permission requests should be sent to
mailto:[email protected]
From: Mandana Modirrousta [mailto:[email protected]]
Sent: Thursday, June 30, 2005 10:49 AM
To: Customer Service Requests - Oxford; Customer Service Requests - USA
Subject: Permission for publishing my papers in my Ph.D. thesis
155
Eur J Neurosci. 2005 May;21(10):2807-16.
Orexin and MCH neurons express c-Fos differently aCter sleep deprivation
vs. recovery and bear different adrenergic receptors.
Modirrousta M, Mainville L, Jones BE.
Eur J Neurosci. 2003 Aug;18(3):723-7.
Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting
basal forebrain neurons.
Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE.
156
TRADITION j EXCEUENCE
,
fO!·the~{
of the ~CQmpmyin 18;80.
Our ref: HG/smc/July 2005.j1238
22 July 2005
Dr Mandana Modirrousta
[email protected]
Dear Dr Modirrousta
NEUROSCIENCE, Vol 129, No 3, 2004, Pages 803-10, 'Gabaergic neurons with .. .'
As per your letter dated 30 June 2005, we hereby grant you permission to reprint the
aforementioned material at no charge in your thesis subject to the following conditions:
1.
If any part of the material to be used (for example, figures) has appeared in our
publication with credit or acknowledgement to another source, permission must also be
sought from that source. If such permission is not obtained then that material may not be
included in your publication/copies.
2.
Suitable acknowledgment to the source must be made, either as a footnote or in a
reference list at the end of your publication, as follows:
"Reprinted from Publication title, Vol number, Author(s), Title of article, Pages No.,
Copyright (Year), with permission from Elsevier".
3.
Reproduction of this material is confined to the purpose for which permission is
hereby given.
4.
This permission is granted for non-exclusive world English rights only. For other
languages please reapply separately for each one required. Permission excludes use in an
157
electronic form. Should you have a specific electronic project in mind please reapply for
permlSSlOn.
5.
This includes permission for the National Library of Canada to supply single
copies, on demand, of the complete thesis. Should your thesis be published
commercially, please reapply for permission.
Helen Gainford
Rights Manager
Your future requests will be handled more quickly if you complete the
online form at www.elsevier.com/locate/permissions
158
7.
References
159
Abrahamson, E. E., Leak, R. K. and Moore, R. Y., 2001. The suprachiasmatic
nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 12,435440.
Alam, M. N., Gong, H., Alam, T., Jaganath, R., McGinty, D. and Szymusiak, R.,
2002. Sleep-waking discharge patterns of neurons recorded in the rat perifornical
lateral hypothalamic area. J Physiol. 538,619-631.
Alam, M. N., McGinty, D., Bashir, T., Kumar, S., Imeri, L., Opp, M. R. and
Szymusiak, R., 2004. Interleukin-lbeta modulates state-dependent discharge
activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur
J Neurosci. 20,207-216.
Alam, M. N., McGinty, D. and Szymusiak, R., 1996. Preoptic/anterior
hypothalamic neurons: thermosensitivity in wakefulness and non rapid eye
movement sleep. Brain Res. 718, 76-82.
Aston-Jones, G. and Bloom, F. E., 1981. Activity ofnorepinephrine-containing
locus coeruleus neurons in behaving rats anticipates fluctuations in the sleepwaking cycle. J Neurosci. 1,876-886.
Bai, D. and Renaud, L. P., 1998. Median preoptic nucleus neurons: an in vitro
patch-clamp analysis of their intrinsic properties and noradrenergic receptors in
the rat. Neuroscience. 83,905-916.
160
Bayer, L., Eggennann, E., Saint-MIeux, B., Machard, D., Jones, B. E.,
Muhlethaler, M. and Serafin, M., 2002a. Selective action of orexin (hypocretin)
on nonspecific thalamocortical projection neurons. J Neurosci. 22, 7835-7839.
Bayer, L., Eggennann, E., Serafin, M., Grivel, J., Machard, D., Muhlethaler, M.
and Jones, B. E., 2005. Opposite effects ofnoradrenaline and acetylcholine upon
hypocretinlorexin versus melanin concentrating honnone neurons in rat
hypothalamic slices. Neuroscience. 130,807-811.
Bayer, L., Eggennann, E., Serafin, M., Saint-MIeux, B., Machard, D., Jones, B.
and Muhlethaler, M., 2001. Orexins (hypocretins) directlyexcite
tuberomammillary neurones. Eur J Neurosci. 14, 1571-1575.
Bayer, L., Mairet-Coello, G., Risold, P. Y. and Griffond, B., 2002b.
Orexinlhypocretin neurons: chemical phenotype and possible interactions with
melanin-concentrating honnone neurons. Regul Pept. 104, 33-39.
Bayer, L., Serafin, M., Eggennann, E., Saint-MIeux, B., Machard, D., Jones, B. E.
and Muhlethaler, M., 2004. Exclusive postsynaptic action ofhypocretin-orexin on
sublayer 6b cortical neurons. J Neurosci. 24, 6760-6764.
Bergmann, B. M., Winter, J. B., Rosenberg, R. S. and Rechtschaffen, A., 1987.
NREM sleep with low-voltage EEG in the rat. Sleep. 10, 1-11.
Beuckmann, C. T. and Yanagisawa, M., 2002. Orexins: from neuropeptides to
energy homeostasis and sleep/wake regulation. J Mol Med. 80, 329-342.
Bittencourt, J. C., Presse, F., Arias, C., Peto, C., Vaughan, J., Nahon, J. L., Vale,
W. and Sawchenko, P. E., 1992. The melanin-concentrating honnone system of
161
the rat brain: an immuno- and hybridization histochemical characterization. J
Comp Neurol. 319,218-245.
Borbely, A. A., Tobler, 1. and Hanagasioglu, M., 1984. Effect ofsleep deprivation
on sleep and EEG power spectra in the rat. Behav Brain Res. 14, 171-182.
Broberger, C., De Lecea, L., Sutcliffe, J. G. and Hokfelt, T., 1998.
Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form
distinct populations in the rodent lateraI hypothalamus: relationship to the
neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 402,
460-474.
BurIet, S., Tyler, C. J. and Leonard, C. S., 2002. Direct and indirect excitation of
laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for
wakefulness and narcolepsy. J Neurosci. 22, 2862-2872.
Cape, E. G. and Jones, B. E., 2000. Effects of glutamate agonist versus pro caine
microinjections into the basal forebrain cholinergic cell area upon gamma and
theta EEG activity and sleep-wake state. Eur J Neurosci. 12,2166-2184.
Cape, E. G., Nouel, D. and Jones, B. E., 1997. DifferentiaI modulation ofhigh
frequency gamma EEG activity by microinjection of noradrenaline and serotonin
into the basal forebrain. Soc Neurosci Abst. 23, 2388.
Castren, E., Zafra, F., Thoenen, H. and Lindholm, D., 1992. Light regulates
expression ofbrain-derived neurotrophic factor mRNA in rat visual cortex. Proc
Nat! Acad Sci USA. 89, 9444-9448.
Chaudhuri, A., 1997. Neural activity mapping with inducible transcription factors.
Neuroreport. 8, iii-vii.
162
Chemelli, R. M., Willie, J. T., Sinton, C. M., Elmquist, J. K., Scammell, T., Lee,
C., Richardson, J. A., Williams, S. C., Xiong, Y., Kisanuki, Y., Fitch, T. E.,
Nakazato, M., Hammer, R. E., Saper, C. B. and Yanagisawa, M., 1999.
Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell.
98,437-451.
Chou, T. C., Bjorkum, A. A., Gaus, S. E., Lu, J., Scammell, T. E. and Saper, C.
B., 2002. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 22, 977-990.
Cirelli, C., Gutierrez, C. M. and Tononi, G., 2004. Extensive and divergent effects
ofsleep and wakefulness on brain gene expression. Neuron. 41, 35-43.
Cirelli, C., Pompeiano, M. and Tononi, G., 1993. Fos-like immunoreactivity in
the rat brain in spontaneous wakefulness and sleep. Arch Ital Biol. 131,327-330.
Cirelli, C., Pompeiano, M. and Tononi, G., 1995. Sleep deprivation and c-fos
expression in the rat brain. J Sleep Res. 4, 92-106.
Cirelli, C. and Tononi, G., 2000. On the functional significance of c-fos induction
during the sleep-waking cycle. Sleep. 23, 453-469.
Conner, J. M., Lauterbom, J. C., Yan, Q., Gall, C. M. and Varon, S., 1997.
Distribution ofbrain-derived neurotrophic factor (BDNF) protein and mRNA in
the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci.
17,2295-2313.
Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H. and Watson, S. J., 1995.
Pattern and time course of immediate early gene expression in rat brain following
acute stress. Neuroscience. 64,477-505.
163
de Lecea, L., Kilduff, T. S., Peyron, C., Gao,
Fukuhara,
x., Foye, P. E., Danielson, P. E.,
c., Battenberg, E. L., Gautvik, V. T., Bartlett, F. S., 2nd, Frankel, W.
N., van den Pol, A. N., Bloom, F. E., Gautvik, K. M. and Sutcliffe, J. G., 1998.
The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity.
Proc Nad Acad Sci USA. 95, 322-327.
Detari, L., Juhasz, G. and Kukorelli, T., 1984. Firing properties of cat basal
forebrain neurones during sleep-wakefulness cycle. Electroencephalogr Clin
Neurophysiol. 58, 362-368.
Dragunow, M. and Faull, R., 1989. The use of c-fos as a metabolic marker in
neuronal pathway tracing. J Neurosci Meth. 29, 261-265.
Eggermann, E., Bayer, L., Serafin, M., Saint-Mieux, B., Bernheim, L., Machard,
D., Jones, R E. and Muhlethaler, M., 2003. The wake-promoting hypocretinorexin neurons are in an intrinsic state of membrane depolarization. J Neurosci.
23, 1557-1562.
Eggermann, E., Serafin, M., Bayer, L., Machard, D., Saint-Mieux, R, Jones, R E.
and Muhlethaler, M., 2001. Orexins/hypocretins excite basal forebrain cholinergic
neurones. In: N euroscience), vol. 108, pp. 177-181.
Espana, R. A., Plahn, S. and Berridge, C. W., 2002. Circadian-dependent and
circadian-independent behavioral actions ofhypocretinlorexin. Brain Res. 943,
224-236.
Espana, R. A., Valentino, R. J. and Berridge, C. W., 2003. Fos immunoreactivity
in hypocretin-synthesizing and hypocretin-l receptor-expressing neurons: effects
of diurnal and noctumal spontaneous waking, stress and hypocretin-1
administration. Neuroscience. 121,201-217.
164
Estabrooke,I. V., McCarthy, M. T., Ko, E., Chou, T. C., Chemelli, R. M.,
Yanagisawa, M., Saper, C. B. and Scammell, T. E., 2001. Fos expression in
orexin neurons varies with behavioral state. J Neurosci. 21, 1656-1662.
Ferguson, A. V. and Samson, W. K., 2003. The orexinlhypocretin system: a
critical regulator of neuroendocrine and autonomie function. Front
Neuroendocrinol. 24, 141-150.
Fields, R. D., Eshete, F., Stevens, B. and Itoh, K., 1997. Action potentialdependent regulation of gene expression: temporal specificity in Ca2+, cAMPresponsive element binding proteins, and mitogen-activated protein kinase
signaling. J Neurosci. 17, 7252-7266.
Findlay, A. L. R. and Hayward, J. N., 1969. Spontaneous activity of single
neurones in the hypothalamus ofrabbits during sleep and waking. J Physiol. 201,
237-258.
Flugge, G., van Kampen, M., Meyer, H. and Fuchs, E., 2003. Alpha2A and
alpha2C-adrenoceptor regulation in the brain: alpha2A changes persist after
chronic stress. Eur J Neurosci. 17, 917-928.
Fort, P., Khateb, A., Pegna, A., Muhlethaler, M. and Jones, B. E., 1995.
Noradrenergic modulation of cholinergie nucleus basalis neurons demonstrated by
in vitro pharmacological and immunohistochemical evidence in the guinea pig
brain. Eur J Neurosci. 7, 1502-1511.
Fort, P., Khateb, A., Serafin, M., Muhlethaler, M. and Jones, B. E., 1998.
Pharmacological characterization and differentiation of non-cholinergie nucleus
basalis neurons in vitro. NeuroReport. 9, 1-5.
165
Fujiki, N., Yoshida, Y., Ripley, B., Honda, K., Mignot, E. and Nishino, S., 2001.
Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in
response to food deprivation. Neuroreport. 12,993-997.
Gallopin, T., Fort, P., Eggennann, E., Cauli, B., Luppi, P. H., Rossier, J., Audinat,
E., Muhlethaler, M. and Serafin, M., 2000. Identification of sleep-promoting
neurons in vitro. Nature. 404, 992-995.
Gao, X. B. and van den Pol, A. N., 2001. Melanin concentrating honnone
depresses synaptic activity of glutamate and GABA neurons from rat lateral
hypothalamus. J Physiol. 533,237-252.
Ginty, D. D., 1997. Calcium regulation of gene expression: isn't that spatial?
Neuron. 18, 183-186.
Glass, M. J., Huang, J., Aicher, S. A., Milner, T. A. and Pickel, V. M., 2001.
Subcellular localization of alpha-2A-adrenergic receptors in the rat medial
nucleus tractus solitarius: regional targeting and relationship with catecholamine
neurons. J Comp Neurol. 433, 193-207.
Gong, H., McGinty, D., Guzman-Marin, R., Chew, K. T., Stewart, D. and
Szymusiak, R., 2004. Activation of c-fos in GABAergic neurones in the preoptic
area during sleep and in response to sleep deprivation. J Physiol. 556,935-946.
Gong, H., Szymusiak, R., King, J., Steininger, T. and McGinty, D., 2000. Sleeprelated c-Fos protein expression in the preoptic hypothalamus: effects of ambient
wanning. Am J Physiol Regul Integr Comp Physiol. 279, R2079-2088.
Grassi-Zucconi, G., Giuditta, A., Mandile, P., Chen, S., Vescia, S. and
Bentivoglio, M., 1994. c-fos spontaneous expression during wakefulness is
166
reversed during sleep in neuronal subsets of the rat cortex. J Physiol Paris. 88,9193.
Grassi-Zucconi, G., Menegazzi, M., Carcereri De Prati, A., Bassetti, A.,
Montagnese, P., Mandile, P., Cosi, C. and Bentivoglio, M., 1993. c-fos mRNA is
spontaneously induced in the rat brain during the activity period of the circadian
cycle. Eur J Neurosci. 5, 1071-1078.
Gritti, 1., Mainville, L. and Jones, B. E., 1993. Codistribution ofGABA- with
acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp
Neuro!. 329,438-457.
Gritti, 1., Mainville, L. and Jones, B. E., 1994. Projections ofGABAergic and
cholinergie basal forebrain and GABAergic preoptic-anterior hypothalamic
neurons to the posterior lateral hypothalamus of the rat. J Comp Neuro!. 339,251268.
Gritti, 1., Mainville, L., Mancia, M. and Jones, B. E., 1997. GABAergic and other
non-cholinergic basal forebrain neurons project together with cholinergie neurons
to meso- and iso-cortex in the rat. J Comp Neuro!. 383, 163-177.
Grive1, J., Cvetkovic, V., Bayer, L., Machard, D., Tobler, 1., Muhlethaler, M. and
Serafin, M., 2005. The wake-promoting hypocretin/orexin neurons change their
response to noradrenaline after sleep deprivation. J Neurosci. 25,4127-4130.
Grivel, J., Tobler, 1., Muhlethaler, M. and Serafin, M., 2004. Following sleep
deprivation the excitation ofhypocretin/orexin neurons by noradrenaline reverses
to inhibition. Soc Neurosci Abst Online.
167
Guan, J. L., Uehara, K., Lu, S., Wang, Q. P., Funahashi, H., Sakurai, T.,
Yanagizawa, M. and Shioda, S., 2002. Reciprocal synaptic relationships between
orexin- and melanin-concentrating hormone-containing neurons in the rat lateral
hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat
Metab Disord. 26, 1523-1532.
Hairston,1. S., Peyron, C., Denning, D. P., Ruby, N. F., Flores, J., Sapolsky, R.
M., Heller, H. C. and O'Hara, B. F., 2004. Sleep deprivation effects on growth
factor expression in neonatal rats: a potential role for BDNF in the mediation of
delta power. J Neurophysiol. 91, 1586-1595.
Hajszan, T. and Zaborszky, L., 2002. Direct catecholaminergic-cholinergic
interactions in the basal forebrain. III. Adrenergic innervation of choline
acetyltransferase-containing neurons in the rat. J Comp Neurol. 449, 141-157.
Hara, J., Beuckmann, C. T., Nambu, T., Willie, J. T., Chemelli, R. M., Sinton, C.
M., Sugiyama, F., Yagami, K., Goto, K., Yanagisawa, M. and Sakurai, T., 2001.
Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and
obesity. Neuron. 30, 345-354.
Hartmann, E. and Cravens, J., 1976. Sleep: effects of d- and l-amphetamine in
man and in rat. Psychopharmacology (Berl). 50, 171-175.
Hayaishi,
o. and Urade, Y., 2002. Prostaglandin D2 in sleep-wake regulation:
recent progress and perspectives. Neuroscientist. 8, 12-15.
Henny, P. and Jones, B. E., 2003. Vesicular transporter proteins for glutamate
(VgluT), GABA (VGAT) and acetylcholine (VAChT) in terminal varicosities of
basal forebrain neurons projecting to the posterior lateral hypothalamus. Sleep.
26, A9.
168
Himmelheber, A. M., Sarter, M. and Bruno, J. P., 2000. Increases in cortical
acetylcholine release during sustained attention performance in rats. Brain Res
Cogn Brain Res. 9,313-325.
Horvath, T. L., Peyron, C., Diano, S., Ivanov, A., Aston-Jones, G., Kilduff, T. S.
and van den Pol, A. N., 1999. Hypocretin (orexin) activation and synaptic
innervation ofthe locus coeruleus noradrenergic system. J Comp Neurol. 415,
145-159.
Hou, Y. P., Manns, I. D. and Jones, B. E., 2002. Immunostaining of cholinergic
pontomesencephalic neurons for alphal versus alpha 2 adrenergic receptors
suggests different sleep-wake state activities and roles. Neuroseience. 114, 517521.
Ida, T., Nakahara, K., Murakami, T., Hanada, R., Nakazato, M. and Murakami,
N., 2000. Possible involvement of orexin in the stress reaction in rats. Biochem
Biophys Res Commun. 270, 318-323.
Ito, M., Gomori, A., Ishihara, A., Oda, Z., Mashiko, S., Matsushita, H., Yumoto,
M., Sano, H., Tokita, S., Moriya, M., Iwaasa, H. and Kanatani, A., 2003.
Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol
Metab. 284, E940-945.
Jacobs, B. L. and Fomal, C. A., 1991. Activity ofbrain serotonergic neurons in
the behaving animal. Pharmacol Rev. 43, 563-578.
Jhanwar-Uniyal, M., Roland, C. R. and Leibowitz, S. F., 1986. Diurnal rhythm of
alpha 2-noradrenergic receptors in the paraventricular nucleus and other brain
areas: relation to circulating corticosterone and feeding behavior. Life Sei. 38,
473-482.
169
Johnson, R. S., Spiegelman, B. M. and Papaioannou, V., 1992. Pleiotropic effects
of a null mutation in the c-fos proto-oncogene. Cell. 71, 577-586.
Jones, B. E., 1989. Basic mechanisms of sleep-wake states. In: Kryger, M. R. et
al. (Eds.), Principles and Practice of Sleep Medicine. Saunders, Philadelphia, pp.
121-138.
Jones, B. E., 1995. Reticular formation. Cytoarchitecture, transmitters and
projections. In: Paxinos, G. (Ed.), The Rat Nervous System. Academic Press
Australia, Sydney, pp. 155-171.
Jones, B. E., 2000. Basic Mechanisms ofSleep-Wake States. In: Kryger, M. H. et
al. (Eds.), Principles and Practice ofSleep Medicine. Saunders, Philadelphia, pp.
134-154.
Jones, B. E., 2004. Activity, modulation and role ofbasal forebrain cholinergic
neurons innervating the cerebral cortex. Progr Brain Res. 145, 157-169.
Jones, B. E., 2005. From waking to sleeping: neuronal and chemical substrates.
Trends Pharmacol Sci. 26, 578-586.
Jones, B. E. and Cuello, A. C., 1989. Afferents to the basal forebrain cholinergic
cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine-neurons. Neuroscience. 31, 37-61.
Jones, B. E. and Moore, R. Y., 1977. Ascending projections of the locus coeruleus
in the rat. II. Autoradiographic study. Brain Res. 127,23-53.
170
Jones, B. E. and Yang, T.-Z., 1985. The efferent projections from the reticular
formation and the locus coeruleus studied by anterograde and retro grade axonal
transport in the rat. J Comp Neurol. 242, 56-92.
Kennedy, A. R., Todd, J. F., Stanley, S. A., Abbott, C. R., Small, C. J., Ghatei, M.
A. and Bloom, S. R., 2001. Melanin-concentrating hormone (MCH) suppresses
thyroid stimulating hormone (TSH) release, in vivo and in vitro, via the
hypothalamus and the pituitary. Endocrinology. 142,3265-3268.
Khateb, A., Fort, P., Williams, S., Serafin, M., Muhlethaler, M. and Jones, B. E.,
1998. GABAergic input to cholinergie nucleus basalis neurons. Neuroscience. 86,
937-947.
Kilman, V., van Rossum, M. C. and Turrigiano, G. G., 2002. Activity deprivation
reduces miniature IPSC amplitude by decreasing the number of postsynaptic
GABA(A) receptors clustered at neocortical synapses. J Neurosci. 22, 1328-1337.
Kiyashchenko, L.I., Mileykovskiy, B. Y., Maidment, N., Lam, H. A., Wu, M. F.,
John, J., Peever, J. and Siegel, J. M., 2002. Release ofhypocretin (orexin) during
waking and sleep states. J Neurosci. 22, 5282-5286.
Knott, G. W., Quairiaux, C., Genoud, C. and Welker, E., 2002. Formation of
dendritic spines with GABAergic synapses induced by whisker stimulation in
adult mice. Neuron. 34,265-273.
Koyama, Y. and Hayaishi, O., 1994. Firing ofneurons in the preoptic/anterior
hypothalamic areas in rat: its possible involvement in slow wave sleep and
paradoxical sleep. Neurosci Res. 19,31-38.
171
Koyama, Y., Takahashi, K., Kodama, T. and Kayama, Y., 2003. State-dependent
activity of neurons in the perifornical hypothalamic area during sleep and waking.
Neuroscience. 119, 1209-1219.
Krout, K. E., Mettenleiter, T. C. and Loewy, A. D., 2003. Single CNS neurons
link both central motor and cardiosyrnpathetic systems: a double-virus tracing
study. Neuroscience. 118,853-866.
Lancel, M., 1997. The GABA(A) agoni st THIP increases non-REM sleep and
enhances non-REM sleep-specific delta activity in the rat during the dark period.
Sleep. 20, 1099-1104.
Lancel, M., Faulhaber, J., Schiffelholz, T., Mathias, S. and Deisz, R. A., 1997.
Muscimol and midazolam do not potentiate each other's effects on sleep EEG in
the rat. J Neurophysiol. 77, 1624-1629.
Lancel, M. and Steiger, A., 1999. Sleep and Its Modulation by Drugs That Affect
GABA(A) Receptor Function. Angew Chern Int Ed Engl. 38, 2852-2864.
Ledoux, L., Sastre, J. P., Buda, C., Luppi, P. H. and Jouvet, M., 1996. Alterations
in c-fos expression after different experimental procedures of sleep deprivation in
the cat. Brain Res. 735, 108-118.
Lee, M. G., Hassani, O. K., Alonso, A. and Jones, B. E., 2005. Cholinergie basal
forebrain neurons burst with theta during waking and paradoxical sleep. J
Neurosci. 25, 4365-4369.
Lee, M. G., Henny, P. and Jones, B. E., 2003. Sleep-wake dis charge properties of
juxtacellularly labeled and immunohistochemically identified cholinergie basal
forebrain neurons in head-restrained rats. Soc Neurosci Abst Online, 932.936.
172
Lee, M. G. and Jones, B. E., 2004. Discharge ofidentified orexin neurons across
the sleep-waking cycle. Abstract Viewer/Itinerary Planner Washington, DC:
Society for Neuroscience, 2004 Online, Program No. 841.841.
Lee, M. G., Manns, 1. D., Alonso, A. and Jones, B. E., 2002. Sleep-wake
discharge profile ofbasal forebrain neurons recorded and labeled by the
juxtacellular method in head-restrained rats. Soc Neurosci Abst Online, 672.674.
Lee, M. G., Manns, 1. D., Alonso, A. and Jones, B. E., 2004. Sleep-wake related
dis charge properties ofbasal forebrain neurons recorded with micropipettes in
head-fixed rats. J Neurophysiol. 92, 1182-1198.
Leonard, C. and Llinas, R. R., 1990. Serotonin (5-HT) inhibits mesopontine
cholinergie neurons in vitro. Soc Neurosci Abst. 16, 1233.
Lerea, L. S., Butler, L. S. and McNamara, J. O., 1992. NMDA and non-NMDA
receptor-mediated increase of c-fos mRNA in dentate gyms neurons involves
calcium influx via different routes. J Neurosci. 12,2973-2981.
Li, Y., Gao, X. B., Sakurai, T. and van den Pol, A. N., 2002. HypocretiniOrexin
excites hypocretin neurons via a local glutamate neuron-A potential mechanism
for orchestrating the hypothalamic arousal system. Neuron. 36, 1169-1181.
Lin, L., Faraco, J., Li, R., Kadotani, H., Rogers, W., Lin,
x., Qiu, x., de Jong, P.
J., Nishino, S. and Mignot, E., 1999. The sleep disorder canine narcolepsy is
caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 98, 365376.
Liu, W. and Alreja, M., 1998. Norepinephrine inhibits neurons of the intermediate
subnucleus of the lateral septum via alpha2-adrenoceptors. Brain Res. 806,36-54.
173
Lu, J., Bjorkum, A. A., Xu, M., Gaus, S. E., Shiromani, P. J. and Saper, C. B.,
2002. Selective activation of the extended ventrolateral preoptic nucleus during
rapid eye movement sleep. J Neurosci. 22, 4568-4576.
Lu, J., Greco, M. A., Shiromani, P. and Saper, C. B., 2000. Effect oflesions of the
ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 20, 38303842.
Lubkin, M. and Stricker-Krongrad, A., 1998. Independent feeding and metabolic
actions of orexins in mice. Biochem Biophys Res Commun. 253, 241-245.
Lue, F. A., Bail, M., Jephthah-Ochola, J., Carayanniotis, K., Gorezynski, R. and
Moldofsky, H., 1988. Sleep and eerebrospinal fluid interleukin-l-like activity in
the eat. Int J Neurosci. 42, 179-183.
Luebke, J.I., Greene, R. W., Semba, K., Kamondi, A., McCarley, R. W. and
Reiner, P. B., 1992. Serotonin hyperpolarizes cholinergic low-threshold burst
neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Nad Acad Sei. 89,
743-747.
Luppi, P. H., Aston-Jones, G., Akaoka, H., Chouvet, G. and Jouvet, M., 1995.
Afferent projections to the rat locus coeruleus demonstrated by retro grade and
anterograde traeing with eholera-toxin B subunit and Phaseolus vulgaris
leueoagglutinin. Neuroseience. 65, 119-160.
Luscher, B. and Keller, C. A., 2004. Regulation of GABAA receptor traffieking,
channel aetivity, and functional plasticity of inhibitory synapses. Pharmaeol Ther.
102, 195-221.
174
Maloney, K. J., Cape, E. G., Gotman, J. and Jones, B. E., 1997. High frequency
gamma electroencephalogram activity in association with sleep-wake states and
spontaneous behaviors in the rat. Neuroscience. 76, 541-555.
Maloney, K. J. and Jones, B. E., 1999. C-Fos expression in neurons of the
pontomedullary reticular formation and raphe, including GABAergic and
serotonergic, neurons during paradoxical sleep deprivation and recovery. Soc
Neurosci Abst. 25, 26.
Maloney, K. J., Mainville, L. and Jones, B. E., 1999. DifferentiaI c-Fos
expression in cholinergie, monoaminergic and GABAergic cell groups of the
pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery.
J Neurosci. 19,3057-3072.
Maloney, K. J., Mainville, L. and Jones, B. E., 2000. c-Fos expression in
GABAergic, serotonergic and other neurons of the pontomedullary reticular
formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci.
20,4669-4679.
Manns, 1. D., Alonso, A. and Jones, B. E., 2000a. Discharge profiles of
juxtacellularly labeled and immunohistochemically identified GABAergic basal
forebrain neurons recorded in association with the electroencephalogram in
anesthetized rats. J Neurosci. 20, 9252-9263.
Manns, 1. D., Alonso, A. and Jones, B. E., 2000b. Discharge properties of
juxtacellularly labeled and immunohistochemically identified cholinergie basal
forebrain neurons recorded in association with the electroencephalogram in
anesthetized rats. J Neurosci. 20, 1505-1518.
175
Manns, 1. D., Lee, M. G., Modirrousta, M., Hou, Y. P. and Jones, B. E., 2003.
Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal
forebrain neurons. Eur J N eurosci. 18, 723-727.
Manns, 1. D., Mainville, L. and Jones, B. E., 2001. Evidence for glutamate, in
addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain
neurons projecting to the entorhinal cortex. Neuroscience. 107,249-263.
Marcus, J. N., Aschkenasi, C. J., Lee, C. E., Chemelli, R. M., Saper, C. B.,
Yanagisawa, M. and Elmquist, J. K., 2001. DifferentiaI expression oforexin
receptors 1 and 2 in the rat brain. J Comp Neurol. 435, 6-25.
Marder, E. and Prinz, A. A., 2002. Modeling stability in neuron and network
function: the role of activity in homeostasis. Bioessays. 24, 1145-1154.
Marrosu, F., Portas, C., Mascia, S., Casu, M. A., Fa, M., Giagheddu, M.,
Imperato, A. and Gessa, G. L., 1995. Microdialysis measurement of cortical and
hippocampal acetylcholine release during sleep-wake cycle in free1y moving cats.
Brain Res. 671, 329-332.
Marsh, D. J., Weingarth, D. T., Novi, D. E., Chen, H. Y., Trumbauer, M. E.,
Chen, A. S., Guan, X. M., Jiang, M. M., Feng, Y., Camacho, R. E., Shen, Z.,
Frazier, E. G., Yu, H., Metzger, J. M., Kuca, S. J., Shearman, L. P., Gopal-Truter,
S., MacNeil, D. J., Strack, A. M., MacIntyre, D. E., Van der Ploeg, L. H. and
Qian, S., 2002. Me1anin-concentrating hormone 1 receptor-deficient mice are
lean, hyperactive, and hyperphagic and have altered metabolism. Proc Nat! Acad
Sci USA. 99, 3240-3245.
176
Marty, S., Wehrle, R., Fritschy, J. M. and Sotelo, C., 2004. Quantitative effects
produced by modifications of neuronal activity on the size of GABAA receptor
clusters in hippocampal slice cultures. Eur J Neurosci. 20, 427-440.
Marty, S., Wehrle, R. and Sotelo, C., 2000. Neuronal activity and brain-derived
neurotrophic factor regulate the density of inhibitory synapses in organotypic slice
cultures of postnatal hippocampus. J Neurosci. 20, 8087-8095.
Matsumura, H., Nakajima, T., Osaka, T., Satoh, S., Kawase, K., Kubo, E.,
Kantha, S. S., Kasahara, K. and Hayaishi, O., 1994. Prostaglandin D2-sensitive,
sleep-promoting zone defined in the ventraI surface of the rostral basal forebrain.
Proc Nad Acad Sci USA. 91, 11998-12002.
Matsuo, S., Jang, 1. S., Nabekura, J. and Akaike, N., 2003. alpha 2-AdrenoceptorMediated Presynaptic Modulation of GABAergic Transmission in Mechanically
Dissociated Rat Ventrolateral Preoptic Neurons. J Neurophysiol. 89, 1640-1648.
Mayers, A. G. and Baldwin, D. S., 2005. Antidepressants and their effect on
sleep. Hum Psychophannacol. 20, 533-559.
McCarley, R. W. and Hobson, J. A., 1975. Neuronal excitabilitymodulation over
the sleep cycle: a structural and mathematicaI model. Science. 189,58-60.
McCormick, D. A., 1992. Cellular mechanisms underlying cholinergic and
noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal
lateral geniculate nucleus. J Neurosci. 12,278-289.
McGinty, D. and Szymusiak, R., 1988. Neuronal unit activity patterns in behaving
animaIs: brainstem and limbic system. Annu Rev Psychol. 39, 135-168.
177
McGinty, D. J. and Stennan, M. B., 1968. Sleep suppression after basal forebrain
lesions in the cat. Science. 160, 1253-1255.
Merchant-Nancy, H., Vazquez, J., Aguilar-Roblero, R. and Drucker-Colin, R.,
1992. c-fos proto-oncogene changes in relation to REM sleep duration. Brain Res.
579, 342-346.
Mieda, M., Willie, J. T., Hara, J., Sinton, C. M., Sakurai, T. and Yanagisawa, M.,
2004. Orexin peptides prevent cataplexy and improve wakefulness in an orexin
neuron-ablated model ofnarcolepsy in mice. Proc Natl Acad Sci USA. 101,
4649-4654.
Mizoguchi, Y., Kanematsu, T., Hirata, M. and Nabekura, J., 2003. A rapid
increase in the total number of cell surface functional GABAA receptors induced
by brain-derived neurotrophic factor in rat visual cortex. J Biol Chem. 278,
44097 -44102.
Modirrousta, M., Mainville, L. and Jones, B. E., 2003. c-Fos expression in
cholinergic and GABAergic neurons ofthe basal forebrain and/or preoptic area
following sleep deprivation and recovery. Soc Neurosci Abst Online, 341.312.
Modirrousta, M., Mainville, L. and Jones, B. E., 2004a. GABAergic neurons of
basal forebrain and preoptic area that express c-fos with sleep recovery bear
alpha2 adrenergic receptors. Sleep. 27, A5.
Modirrousta, M., Mainville, L. and Jones, B. E., 2004b. GABAergic neurons with
alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos
during sleep. Neuroscience. 129,803-810.
178
Mody, L, 2005. Aspects of the homeostaic plasticity ofGABAA receptormediated inhibition. J Physiol. 562, 37-46.
Monda, M., Viggiano, A. and De Luca, V., 2003. Paradoxical [correction of
parodoxical] effect of orexin A: hypophagia induced by hyperthermia. Brain Res.
961,220-228.
Morgan, J. 1. and Curran, T., 1986. Role ofion flux in the control of c-fos
expression. Nature. 322, 552-555.
Morgan, J. 1. and Curran, T., 1991. Stimulus-transcription coupling in the nervous
system: involvement of the inducible proto-oncogenes fos andjun. Annu Rev
Neurosci. 14,421-451.
Munson, E. S., Martucci, R. W. and Smith, R. E., 1970. Circadian variations in
anesthetic requirement and toxicity in rats. Anesthesiology. 32, 507-514.
Nitz, D. and Siegel, J. M., 1996. GABA release in posterior hypothalamus across
sleep-wake cycle. Am J Physiol. 271, RI707-RI712.
Nusser, Z., CuH-Candy, S. and Farrant, M., 1997. Differences in synaptic
GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron.
19,697-709.
Nusser, Z., Hajos, N., Somogyi, P. and Mody, L, 1998. Increased number of
synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory
synapses. Nature. 395,172-177.
179
O'Hara, B. F., Young, K. A., Watson, F. L., Heller, H. C. and Kilduff, T. S., 1993.
Immediate early gene expression in brain during sleep deprivation: pre1iminary
observations. Sleep. 16, 1-7.
Obal, F., Jr. and Krueger, J. M., 2003. Biochemical regulation ofnon-rapid-eyemovement sleep. Front Biosci. 8, d520-550.
Osaka, T. and Matsumura, H., 1994. Noradrenergic inputs to sleep-re1ated
neurons in the preoptic area from the locus coeruleus and the ventrolateral
medulla in the rat. Neurosci Res. 19, 39-50.
Osaka, T. and Matsumura, H., 1995. Noradrenaline inhibits preoptic sleep-active
neurons through Œ2-receptors in the rat. Neurosci Res. 21, 323-330.
Otis, T. S., De Koninck, Y. and Mody, 1., 1994. Lasting potentiation of inhibition
is associated with an increased number of gamma-aminobutyric acid type A
receptors activated during miniature inhibitory postsynaptic currents. Proc Nat!
Acad Sci USA. 91, 7698-7702.
Peyron, C., Faraco, J., Rogers, W., Ripley, B., Overeem, S., Charnay, Y.,
Nevsimalova, S., Aldrich, M., Reynolds, D., Albin, R., Li, R., Hungs, M.,
Pedrazzoli, M., Padigaru, M., Kucherlapati, M., Fan, J., Maki, R., Lammers, G. J.,
Bouras, C., Kucherlapati, R., Nishino, S. and Mignot, E., 2000. A mutation in a
case of early onset narcolepsy and a generalized absence ofhypocretin peptides in
human narcoleptic brains. Nat Med. 6, 991-997.
Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutc1iffe,
J. G. and Kilduff, T. S., 1998. Neurons containing hypocretin (orexin) project to
multiple neuronal systems. J Neurosci. 18,9996-10015.
180
Pompeiano, M., Cirelli, C. and Tononi, G., 1992. Effects ofsleep deprivation on
fos-like immunoreactivity in the rat brain. Arch Ital Biol. 130, 325-335.
Pompeiano, M., Cirelli, C. and Tononi, G., 1994. Immediate-early genes in
spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and
protein. J Sleep Res. 3, 80-96.
Porkka-Heiskanen, T., Strecker, R. E. and McCarley, R. W., 2000. Brain sitespecificity of extracellular adenosine concentration changes during sleep
deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience.
99,507-517.
Ram, A., Pandey, H. P., Matsumura, H., Kasahara-Orita, K., Nakajima, T.,
Takahata, R., Satoh, S., Terao, A. and Hayaishi, O., 1997. CSF levels of
prostaglandins, especially the level of prostaglandin D2, are correlated with
increasing propensity towards sleep in rats. Brain Res. 751, 81-89.
Rechtschaffen, A., Bergmann, B. M., Everson, C. A., Kushida, C. A. and
Gilliland, M. A., 2002. Sleep deprivation in the rat: X. Integration and discussion
of the findings. 1989. Sleep. 25, 68-87.
Rosin, D. L., Talley, E. M., Lee, A., Stometta, R. L., Gaylinn, B. D., Guyenet, P.
G. and Lynch, K. R., 1996. Distribution of alpha 2C-adrenergic receptor-like
immunoreactivity in the rat central nervous system. J Comp Neurol. 372, 135165.
Rutherford, L. C., DeWan, A., Lauer, H. M. and Turrigiano, G. G., 1997. Brainderived neurotrophic factor mediates the activity-dependent regulation of
inhibition in neocortical cultures. J Neurosci. 17,4527-4535.
181
Saint-MIeux, B., Eggennann, E., Bisetti, A., Bayer, L., Machard, D., Jones, B. E.,
Muhlethaler, M. and Serafin, M., 2004. Nicotinic enhancement of the
noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic
area. J Neurosci. 24, 63-67.
Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, L, Chemelli, R. M., Tanaka, H.,
Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., Arch, J. R.,
Buckingham, R. E., Haynes, A. C., Carr, S. A., Annan, R. S., McNulty, D. E.,
Liu, W. S., Terrett, J. A., Elshourbagy, N. A., Bergsma, D. J. and Yanagisawa,
M., 1998. Orexins and orexin receptors: a family ofhypothalamic neuropeptides
and G protein-coupled receptors that regulate feeding behavior. Cell. 92, 573-585.
Sallanon, M., Denoyer, M., Kitahama, K., Aubert, C., Gay, N. and Jouvet, M.,
1989. Long-lasting insomnia induced by preoptic neuron lesions and its transient
reversaI by museimol injection into the posterior hypothalamus in the cat.
Neuroscience. 32, 669-683.
Sastre, J. P., Buda, C., Lin, J. S. and Jouvet, M., 2000. DifferentiaI c-fos
expression in the rhinencephalon and striatum after enhanced sleep-wake states in
the cat. Eur J Neurosci. 12, 1397-1410.
Satoh, S., Matsumura, H. and Hayaishi, O., 1998. Involvement of adenosine A2A
receptor in sleep promotion. Eur J Pharmacol. 351, 155-162.
Satoh, S., Matsumura, H., Suzuki, F. and Hayaishi, O., 1996. Promotion ofsleep
mediated by the A2a-adenosine receptor and possible involvement of this receptor
in the sleep induced by prostaglandin D2 in rats. Proc Nad Acad Sei USA. 93,
5980-5984.
182
Scammell, T., Gerashchenko, D., Urade, Y., Onoe, H., Saper, C. and Hayaishi,
O., 1998. Activation of ventrolateral preoptic neurons by the somnogen
prostaglandin D2. Proc Nad Acad Sci USA. 95, 7754-7759.
Schmidt, M. H., Valatx, J. L., Sakai, K, Fort, P. and Jouvet, M., 2000. Role ofthe
lateral preoptic area in sleep-related erectile mechanisms and sleep generation in
the rat. J Neurosci. 20, 6640-6647.
Semba, K, Pastorius, J., Wilkinson, M. and Rusak, B., 2001. Sleep deprivationinduced c-fos and junB expression in the rat brain: effects of duration and timing.
Behav Brain Res. 120, 75-86.
Semba, K, Reiner, P. B., McGeer, E. G. and Fibiger, H. C., 1988. Brainstem
afferents to the magnocellular basal forebrain studied by axonal transport,
immunohistochemistry and electrophysiology in the rat. J Comp Neurol. 267,
433-453.
Shatz, C. J., 1990. Impulse activity and the patterning of connections during CNS
development. Neuron. 5, 745-756.
Shearman, L. P., Camacho, R. E., Sloan Stribling, D., Zhou, D., Bednarek, M. A.,
Hreniuk, D. L., Feighner, S. D., Tan, C. P., Howard, A. D., Van der Ploeg, L. H.,
MacIntyre, D. E., Hickey, G. J. and Strack, A. M., 2003. Chronic MCH-l receptor
modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol.
475,37-47.
Sheng, M. and Greenberg, M. E., 1990. The regulation and function of c-fos and
other immediate early genes in the nervous system. Neuron. 4,477-485.
183
Sherin, J. E., Elmquist, J. K., Torrealba, F. and Saper, C. B., 1998. Innervation of
histaminergic tuberomammillary neurons by GABAergic and galaninergic
neurons in the ventrolateral preoptic nucleus ofthe rat. J Neurosci. 18,4705-4721.
Sherin, J. E., Shiromani, P. J., McCarley, R. W. and Saper, C. B., 1996.
Activation ofventrolateral preoptic neurons during sleep. Science. 271, 216-219.
Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S. and Maratos-Flier, E., 1998.
Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature.
396, 670-674.
Shirasaka, T., Nakazato, M., Matsukura, S., Takasaki, M. and Kannan, H., 1999.
Sympathetic and cardiovascular actions of orexins in conscious rats. Am J
Physiol. 277, R1780-1785.
Shiromani, P. J. and Schwartz, W. J., 1995. Towards amolecularbiologyofthe
circadian clock and sleep ofmammals. Adv Neuroimmunol. 5,217-230.
Smith, W. B., Starck, S. R., Roberts, R. W. and Schuman, E. M., 2005.
Dopaminergic stimulation of local protein synthesis enhances surface expression
ofGluRl and synaptic transmission in hippocampal neurons. Neuron. 45, 765779.
Starzl, T. E., Taylor, C. W. and Magoun, H. W., 1951. Ascending conduction in
reticular activating system, with special reference to the diencephalon. J
Neurophysiol. 14,461-477.
Steininger, T. L., Alam, M. N., Gong, H., Szymusiak, R. and McGinty, D., 1999.
Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the
albino rat. Brain Res. 840, 138-147.
184
Steininger, T. L., Gong, H., McGinty, D. and Szymusiak, R., 2001. Subregional
organization of preoptic area/anterior hypothalamic projections to arousal-related
monoaminergic cell groups. J Comp Neurol. 429, 638-653.
Sterman, M. B. and Clemente, C. D., 1962a. Forebrain inhibitorymechanisms:
Cortical synchronization induced by basal forebrain stimulation. Exp Neurol. 6,
91-102.
Sterman, M. B. and Clemente, C. D., 1962b. Forebrain inhibitory mechanisms:
Sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp
Neurol. 6, 103-117.
Stewart, D. J., MacFabe, D. F. and Vanderwolf, C. H., 1984. Cholinergie
activation of the electrocorticogram: Role of the substantia innominata and
effects of atropine and quinuclidinyl benzilate. Brain Res. 322, 219-232.
Suntsova, N., Szymusiak, R., Alam, M. N., Guzman-Marin, R. and McGinty, D.,
2002. Sleep-waking dis charge patterns of median preoptic nucleus neurons in rats.
J Physiol. 543, 665-677.
Szymusiak, R., 1995. Magnocellular nuclei of the basal forebrain: substrates of
sleep and arousal regulation. Sleep. 18,478-500.
Szymusiak, R., Alam, N. and McGinty, D., 2000. Discharge patterns of neurons
in cholinergie regions of the basal forebrain during waking and sleep. Behav
Brain Res. 115, 171-182.
Szymusiak, R., Alam, N., Steininger, T. L. and McGinty, D., 1998. Sleep-waking
dis charge patterns ofventrolateral preoptic/anterior hypothalamic neurons in rats.
Brain Res. 803, 178-188.
185
Szymusiak, R., lriye, T. and McGinty, D., 1989. Sleep-waking dis charge of
neurons in the posterior lateral hypothalamic area of cats. Brain Res Bull. 23, 111120.
Szymusiak, R. and McGinty, D., 1986a. Sleep-re1ated neuronal dis charge in the
basaI forebrain of cats. Brain Res. 370, 82-92.
Szymusiak, R. and McGinty, D., 1986b. Sleep suppression following kainic acidinduced lesions ofthe basal forebrain. Exp Neurol. 94, 598-614.
Szymusiak, R. and McGinty, D., 1989. Sleep-waking discharge ofbasal forebrain
projection neurons in cats. Brain Res Bull. 22, 423-430.
Tabuchi, A., Nakaoka, R., Amano, K., Yukimine, M., Andoh, T., Kuraishi, Y. and
Tsuda, M., 2000. DifferentiaI activation ofbrain-derived neurotrophic factor gene
promoters 1 and III by Ca2+ signaIs evoked via L-type voltage-dependent and Nmethyl-D-aspartate receptor Ca2+ channe1s. J Biol Chem. 275, 17269-17275.
Taheri, S., Zeitzer, J. M. and Mignot, E., 2002. The role ofhypocretins (orexins)
in sleep regulation and narcolepsy. Annu Rev Neurosci. 25, 283-313.
Talley, E. M., Rosin, D. L., Lee, A., Guyenet, P. G. and Lynch, K. R., 1996.
Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the rat
central nervous system. J Comp Neurol. 372, 111-134.
ThannickaI, T. C., Moore, R. Y., Nienhuis, R., Ramanathan, L., Gulyani, S.,
Aldrich, M., Comford, M. and Siegel, J. M., 2000. Reduced number ofhypocretin
neurons in human narcolepsy. Neuron. 27, 469-474.
186
Thomas, T. C. and Kumar, V. M., 2000. Effect of ambient temperature on sleepwakefulness in normal and medial preoptic area lesioned rats. Sleep Res Online.
3, 141-145.
Tung, A., Bergmann, B. M., Herrera, S., Cao, D. and Mendelson, W. B., 2004.
Recovery from sleep deprivation occurs during propofol anesthesia.
Anesthesiology. 100, 1419-1426.
Tung, A., Szafran, M. J., Bluhm, B. and Mendelson, W. B., 2002. Sleep
deprivation potentiates the onset and duration ofloss ofrighting reflex induced by
propofol and isoflurane. Anesthesiology. 97, 906-911.
Turrigiano, G. G., 1999. Homeostatic plasticity in neuronal networks: the more
things change, the more they stay the same. Trends Neurosci. 22, 221-227.
van den Pol, A. N., 1999. Hypothalamic hypocretin (orexin): robust innervation of
the spinal cord. J Neurosci. 19,3171-3182.
van den Pol, A. N., Gao, X. B., Obrietan, K., Kilduff, T. S. and Belousov, A. B.,
1998. Presynaptic and postsynaptic actions and modulation of neuroendocrine
neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 18, 79627971.
Vanderwolf, C. H., 1975. Neocortical and hippocampal activation in relation to
behavior: effects of atropine, eserine, phenothiazines and amphetamine. J Comp
Physiol Psyc. 88,300-323.
Vazquez, J. and Baghdoyan, H. A., 2001. Basal forebrain acetylchoIine release
during REM sleep is significantly greater than during waking. Am J Physiol
Regul Integr Comp Physiol. 280, R598-601.
187
Verret, L., Goutagny, R., Fort, P., Cagnon, L., Salvert, D., Leger, L., Boissard, R.,
Salin, P., Peyron, C. and Luppi, P. H., 2003. A role ofme1anin-concentrating
hormone producing neurons in the central regulation of paradoxical sleep. BMC
Neurosci.4, 19.
Virus, R. M., Ticho, S., Pilditch, M. and Radulovacki, M., 1990. A comparison of
the effects of caffeine, 8-cyclopentyltheophylline, and alloxazine on sleep in rats.
Possible roles of central nervous system adenosine receptors.
Neuropsychopharmacology. 3,243-249.
von Economo, C., 1931. Encephalitis Lethargica. Hs Seque1ae and Treatment.
Oxford University Press, London.
Vyazovskiy, V. V., Kopp, C., Bosch, G. and Tobler, I., 2005. The GABAA
receptor agonist THIP alters the EEG in waking and sleep of mice.
Neuropharmacology. 48, 617-626.
Wan, Q., Xiong, Z. G., Man, H. Y., Ackerley, C. A., Braunton, J., Lu, W. Y.,
Becker, L. E., MacDonald, J. F. and Wang, Y. T., 1997. Recruitment offunctional
GABA(A) receptors to postsynaptic domains by insulin. Nature. 388, 686-690.
Williams, G., Cai, X. J., Elliott, J. C. and Harrold, J. A, 2004. Anabolic
neuropeptides. Physiol Behav. 81, 211-222.
Williams, J. A and Reiner, P. B., 1993. Noradrenaline hyperpolarizes identified
rat mesopontine cholinergie neurons in vitro. J Neurosci. 13,3878-3883.
Williams, J. T., Henderson, G. and North, R. A, 1985. Characterization of alpha
2-adrenoceptors which increase potassium conductance in rat locus coeruleus
neurones. Neuroscience. 14,95-101.
188
Wu, M. F., John, J., Maidment, N., Lam, H. A. and Siegel, J. M., 2002.
Hypocretin release in normal and narcoleptic dogs after food and sleep
deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol.
283, RI079-1086.
Yamanaka, A., Beuckmann, C. T., Willie, J. T., Hara, J., Tsujino, N., Mieda, M.,
Tominaga, M., Yagami, K., Sugiyama, F., Goto, K., Yanagisawa, M. and Sakurai,
T., 2003. Hypothalamic orexin neurons regulate arousal according to energy
balance in mice. Neuron. 38, 701-713.
Yamuy, J., Fung, S. J., Xi, M. and Chase, M. H., 2004. Hypocretinergic control of
spinal cord motoneurons. J Neurosci. 24, 5336-5345.
Yanik, G., Glaum, S. and Radulovacki, M., 1987. The dose-response effects of
caffeine on sleep in rats. Brain Res. 403, 177-180.
Yoshida, Y., Fujiki, N., Nakajima, T., Ripley, B., Matsumura, H., Yoneda, H.,
Mignot, E. and Nishino, S., 2001. Fluctuation of extracellular hypocretin-l
(orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake
activities. Eur J Neurosci. 14, 1075-1081.
Zeitzer, J. M., Buckmaster, C. L., Parker, K. J., Hauck, C. M., Lyons, D. M. and
Mignot, E., 2003. Circadian and homeostatic regulation ofhypocretin in a primate
model: implications for the consolidation ofwakefulness. J Neurosci. 23, 35553560.
Zhou, D., Shen, Z., Strack, A. M., Marsh, D. J. and Shearman, L. P., 2005.
Enhanced running wheel activity ofboth Mchlr- and Pmch-deticient mice. Regul
Pept. 124, 53-63.
189
8. Articles hard prints
190
European Journal of Neuroscience, Vol. 18, pp. 723-727,2003
© Federation of European Neuroscience Societies
SHORT COMMUNICATION
Alpha 2 adrenergic receptors on GABAergic, putative
sleep-promoting basal forebrain neurons
tan D. Manns, Maan Gee Lee, Mandana Modirrousta, Yiping P. Hou* and Barbara E. Jones
Department of Neurology and Neurosurgery, McGill University, Montreal Neurologicallnstitute Montreal, Quebec, Canada H3A 284
Keywords: cholinergie, cortical activation, EEG, noradrenergic, rat
Abstract
The basal forebrain plays an important role in the modulation of cortical activity and sleep-wake states. Vet its role must be multivalent
as lesions reportedly diminish cortical fast activity and also cortical slow activity along with slow wave sleep (SWS). Basal forebrain
cholinergie vs. GABAergic cell groups could differentially influence these processes. By labelling recorded neurons with Neurobiotin
(Nb) using the juxtacellular technique and identitying them by immunostaining, we previously found that whereas ail cholinergie cells
increased their firing, the majority of GABAergic neurons decreased their firing in association with evoked cortical activation in
urethane-anaesthetized rats. Here, we examined the possibility that such GABAergic, cortical activation 'off' cells might bear alpha 2
adrenergic receptors (u2AR) through which noradrenaline (NA) could inhibit them during cortical activation. First using simple dualimmunostaining for glutamic acid decarboxylase (GAD) and the u2AAR, we found that the majority (~60%) of GAD-immunopositive
(GAD+) neurons through the magnocellular preoptic nucleus (MCPO) and substantia innominata (SI) were labelled for the u2AAR.
Second, in urethane-anaesthetized rats, we examined whether Nb-Iabelled, GAD+ cortical activation 'off' neurons that discharged
maximally in association with cortical slow wave activity, were immunopositive for u2AAR. We found that ail the Nb+/GAD+ 'off' cells
were labelled for the u2AAR. Such cells could be inhibited in association with cortical activation and waking when noradrenergic locus
coeruleus (LC) neurons discharge and be disinhibited with cortical slow waves and SWS when these neurons become inactive. We thus
propose that u2AR-bearing GABAergic basal forebrain neurons constitute sleep-active and sleep-promoting neurons.
Introduction
The basal forebrain plays an important role in modulating cortical
activity and sleep-wake states (Jones, 2000). Lesions or inactivation of
the basal forebrain reportedly diminish cortical fast activity during
waking (Stewart et al., 1984; Cape & Jones, 2000) but also diminish
cortical slow wave activity and slow wave sleep (SWS) (Szymusiak &
McGinty, 1986a). The basal forebrain may thus be comprised of
different cell groups that promote different cortical activities and
states. In addition to cholinergic neurons that are known to stimulate
cortical activation, the basal forebrain contains large numbers of
GABAergic neurons (Gritti et al., 1993). By employing juxtaceUular
labelling and immunohistochemical identification of recorded neurons
in urethane-anaesthetized rats, we recently discovered that GABAergic
ceUs behave very differently as a group than cholinergic cells. Whereas
aU cholinergic neurons increased their firing with evoked cortical
activation, the majority of GABAergic neurons decreased their firing in
association with cortical activation and discharged at their highest rate
in association with cortical slow wave activity (Manns et al., 2000a).
We proposed that such GABAergic ceUs could be sleep-active neurons.
Correspondence: Dr Barbara E. Jones, as above.
E-mail: [email protected]
.1
'Present address: Department of Anatomy, Lanzhou Medical College, Lanzhou, P. R.
China 730000.
Received 21 March 2003, revised 15 May 2003, accepted 20 May 2003
doi: \ 0.\ O46Ij.\460-9568.2003.02788.x
Neurons that discharge at their highest rate during natural SWS have
indeed been recorded in the basal forebrain and in the adjacent preoptic
area of naturally sleeping-waking rats and cats (Szymusiak &
McGinty, 1986b; Koyarna & Hayaishi, 1994; Szymusiak et al.,
1998). Such SWS-active neurons were inhibited by stimulation of
the locus coeruleus (Le) or iontophoretic application of noradrenaline
(NA) in vivo (Osaka & Matsumura, 1994, 1995). In vitro studies also
identified noncholinergic neurons in the basal forebrain and GABAergic neurons in the ventrolateral preoptic area (VLPO) that are commonly hyperpolarized and inhibited by NA (Fort et al., 1998; GaUopin
et al., 2000). Such inhibitory effects of NA are mediated by alpha 2
adrenergic receptors (U2AR) (Osaka & Matsumura, 1995; Bai &
Renaud, 1998). The presence of U2AR on basal forebrain neurons
might thus identify them as sleep-active cells. The aim of the present
study was to determine immunohistochemicaUy whether GABAergic
and potentially sleep-promoting, basal forebrain neurons possess
U2AR.
In a preliminary investigation, we employed dual-immunostaining
for glutamic acid decarboxylase (GAD) and the U2AAR to assess if a
significant proportion of GAD-immunopositive (GAD+) neurons in
the magnoceUular preoptic nucleus (MCPO) or overlying substantia
innominata (SI) of the rat were labelled for the u2AAR. We then
examined using urethane-anaesthetized rats, whether neurons that fire
maximaUy in association with cortical slow wave activity and are
GAD+ are labeUed for the u2AAR. Recorded within the MCPO or
overlying SI, such characterized units were labelled with Neurobiotin
724 1. D. Manns et al.
(Nb) using the juxtacellular technique and subsequently triple-stained
Results
for Nb, GAD and the a2AAR.
Materials and methods
For the immunohistochemical study of a2AAR on GAD-immunoreactive neurons, three male Wistar rats (weighing 200-250 g) were
employed for simple perfusion-fixation of the brain under barbiturate
anaesthesia (Somnotol, 120 mglkg, Lp.). For the study of the receptors
on GAD-immunoreactive physiologically characterized cells, seven
male Long Evans rats (weighing 200-250 g) were employed for acute
electrophysiological recording prior to perfusion-fixation of the brain.
Held in a stereotaxic frame, they were anaesthetized with urethane for
the duration of the recording (1.4 glkg initial dose followed by boosters
of 0.14 glkg as needed, Lp.) and for the subsequent perfusion-fixation
(with an additional booster if needed). Ail procedures were approved
by the McGill University Animal Care Committee and the Canadian
Council on Animal Care.
ln the electrophysiological experiments, units were recorded in the
MCPO or SI using glass micropipettes and characterized in association
with electroencephalogram (BEG) or field potentials recorded with
surface or deep electrodes from retrosplenial or prefrontal cortex and
olfactory bulb, as described in previous publications (Manns et al.,
2000b, 2000a). Following isolation of a single unit, its discharge was
characterized during baseline cortical irregular slow wave activity and
during stimulated cortical activation evoked by tail pinch. Cells that
decreased their rate of discharge with cortical activation, previously
described as 'off' ceIls, were selected. One such cell per animal was
then labelled with Neurobiotin (Nb, Vector Laboratories, Burlingame,
CA) by applying the 'juxtacellular' technique. As established in our
previous publications, modulating the discharge of one recorded unit
using 200 ms current pulses over a period of 5-30 min resulted in
labelling of only one neuron.
Brains were fixed by intra-aortic perfusion of a paraformaldehyde
solution (4% in 0.1 M phosphate buffer, PB, pH7.4, ~500mL) preceded by saline (0.9% NaCI, ~50 mL). After removal, all brains were
placed in a sucrose solution (30% in PB at 4 oC) for ~2 days and then
frozen. Sections were cut at 25-J.lID thickness in the coronal plane on a
freezing microtome.
For dual-immunostaining of GAD and a2AAR. sections were collected at 400 ~m intervals through the basal forebrain. They were
incubated for three nights at 4 oC with an affinity-purified goat polyclonaI antibody for the a2AAR (C-19, sc-1478, Santa Cruz Biotechnology, Santa Cruz, CA) as employed previously (Hou et al., 2002),
together with a rabbit polyclonal antibody for GAD67 (1: 3000,
Chemicon International, Temecula, CA). The a2AAR was revealed
using Cy3-conjugated donkey antigoat antiserum and GAD revealed
simultaneously using Cy2-conjugated donkey antirabbit antiserum
(Jackson Immuno Research, West Grove, PA). GAD-immunoreactive
neurons in the MCPO and overlying SI were exarnined by fluorescence
microscopy for the presence of a2AAR.
For triple staining of recorded cells for Nb, GAD and a2AAR, aIl
sections were collected through the basal forebrain for staining of the
Nb-Iabelled cell using AMCA-conjugated streptavidin (Jackson
ImmunoResearch Laboratories). After locating the Nb-Iabelled cell,
the section containing it was subsequently incubated with the a2AAR
and GAD antibodies for three nights and revealed with Cy2-conjugated and Cy3-conjugated secondary antibodies as described above.
Nb-Iabelled cells that were also GAD+ were exarnined by fluorescence microscopy for the presence of a2AAR. To provide images of
double labelling, they were superimposed using Adobe Photoshop (v9,
Adobe Systems, San Jose, CA).
In dual-immunostained series, GAD+ cells within the MCPO and
overlying SI were often immunopositive for the a2AAR (GAD+/
a2AAR+, triangles in Fig. 1; filled arrowheads in Fig.2A' and B').
In these cells, the receptor labelling was punctate and distributed over
the cell body and proximal dendrites. In many cases, it appeared to be
within the cytoplasm, as it surrounded the nucleus of the cell. Punctate
or diffuse receptor labelling was also present over blood vessels in the
region (bv, Fig. 2A' and B'). In the same area, sorne GAD+ cells were
immunonegative for the a2AAR (GAD+/a2AAR-, empty arrowheads,
Fig.2A' and B'). The GAD+/a2AAR+ and GAD+/a2AAR- cells were
present in the same vicinity (Fig. 2A and A') and sometimes situated
immediately adjacent to one other (Fig. 2B and B'). In a quantitative
sampling, a majority of the GAD+ neurons in the MCPO (average of
66%) and a substantial proportion of those in the. SI (average of 38%)
or an overall majority in the MCPO-SI (average of 59% in three
brains) were judged positively labelled for a2AAR.
In recording experiments performed under urethane anaesthesia,
cells were selected that decreased their firing with stimulationevoked cortical activation for labelling with Nb (Manns et al.,
2000a). Generally discharging in a tonic irregular manner in association with the baseline irregular cortical slow wave activity, these
'off' cells decreased or ceased firing in association with cortical
activation (Fig. 3). They subsequently increased their discharge
following cessation of the stimulation as cortical slow wave activity
returned to prestimulation levels (Fig. 3). Across cells, the average
1. GAD+ cells that were labelled for the <X2AAR (grey triangles) are
plotted in the BF cholinergie cell ares, including the magnocellular preoptic
nucleus (MCPO) and substantia innominata (SI) (Gritti et al., 1993). In this
same area, units were recorded and selected for juxtacellular labelling and
·triple-staining for Nb, GAD, and <X2AAR. Ali Nb+/GAD+ cortical activation
'off' cells were located in the MCPO and were positively labelled for <X2AAR
(black stars). Scale bar, 1 mm. Other abbreviations: ac, anterior commissure;
CPU, caudate putamen; GP, globus pallidus; LPO, lateral preoptic area; MPO,
medial preoptic area; oc, optic chiasm, OTu, olfactory tubercle.
FIG.
© 2003 Federation of European Neuroscience Societies, European Journal of Neuroscience, 18,723-727
Alpha 2 adrenergic receptors on GABAergic neurons
725
FIG.2. Photomicrographs showing GAD irnrnunostaining withCy2 by green fluorescence emission (A, B, C and D) and a2AAR irnrnunostaining with Cy3 by red
fluorescence of the same view (A', B', C'! and D') ..In the left panels, dual-irnrnunostaining reveals that some GAD+ cells (filled arrowhead, A and B) are labelled for
the azAAR+ (filled arrowheads, A' and B'), and others are not (open arrowheads, ft( and B'). Blood vessels (bv) are also irnrnunostained for the a2AAR+. In the right
panels, triple-staining reveals that the GAD+ cells (filled arrowheads, C and D) that were physiologically characterized as cortical activation 'off' cells and labelled
with Nb in blue fluorescence by AMCA-conjugated streptavidin (C' and D') are also irnrnunopositive for a2AAR (solid arrows, C' and D'). Photomicrographs show a
simple image with single green fluorescence emission for GAD irnrnunostaining (left) and superimposed images with blue and red fluorescence for Nb and azAAR
irnrnunostaining (right, C' and D'). Ali Nb+/GAD+/a2AAR+ neurons were located in the MCPO (Fig. 1). Scale bars, 20 Iffil.
discharge rate decreased significantly during stimulation as compared to prestimulation (mean ± SEM: 3.36 ± 1.25 vs. 9.8 ± 2.27;
t = 3.44, d.f. = 4, P = 0.03) and subsequently retumed to prestimulation levels (9.28 ± 2.56) within one to two minutes. Of the 'off' ceUs
labeUed with Nb in the current study, the majority were immunopositive for GAD (5n), as was also the case in our previous study.
The Nb+/GAD+ 'off' ceUs identified here were aU located in the
MCPO (n = 5; Fig. 1). AU of these Nb+/GAD+ 'off' ceUs (Fig.2C
and D) appeared to he lahelled for the a2AAR by the presence of
punctate staining over the celI body and cytoplasm surrounding the
nucleus (Fig. 2C' and D').
Discussion
This study documents by immunohistochemistry combined with electrophysiology that the majority (59%) of basal forebrain GABAergic
neurons possess <X2AR and that the GABAergic neurons that fire
maximally with cortical slow wave activity bear these receptors.
© 2003 Federation of European Neuroscience Societies, European Journal of Neuroscience, 18, 723-727
726
1. D. Manns et al.
Nb+/GAD+/a2A+ Off œil
A. EEG
Somalie Stimulation
~", ,
B. Unit Discharge Rate
5
1
Il
1
1
Il
1
1
Il
1
1
lit
60,
20 sec
120
160
1/
•
200
':
, ,
, ,
, ,
'260
C.EEG
~
D. Unit Discharge
-
2 sec
-..-.I,mv IIIIIIIIIIIIII~
FIG. 3. Unit activity together with simultaneously recorded EEG from prefrontal cortex 'showing a typical decrease or cessation of firing in association
with stimulation-evoked cortical activation and recovery of firing following
stimulation in a urethane-anaesthetized rat This cortical activation 'off' œIl
was labelled with Nb and inununopositive for GAD and the <l2AAR (Fig. 2C and
C'). The compressed EEG recording (A) is expanded (C) and shown together
with the unit recording (D) below. The unit discharge rate plot (spikes per
second in B) shows the decrease and cessation in firing that occurs as the EEG
changes from irregular slow wave activity to fast activity during stimulation and
the retum to prestimulation rates that occurs as the EEG resumes irregular slow
wave activity following stimulation.
In our previous recording studies of GABAergic neurons in
urethane-anaesthetized rats (Manns et al., 2000a), the majority
(57%) of Nb+/GAD+ neurons through the MCPO-SI were found
to discharge at a higher rate with cortical slow wave activity than with
cortical activation. No Nb+/ChAT+ cells did so (Manns et al., 2000b),
and only a minority (18%) of Nb+/GAD- cells did so (Manns et al.,
2000a). These results suggested that a substantial group of GABAergic
basal forebrain neurons would he most active in association with
cortical slow wave activity during SWS and thus correspond in a large
part to previously identified sleep-active neurons in this region (Szymusiak & McGinty, 1986a). These neurons could correspond to the
major proportion (59%) of GAD+ neurons that bear <lzAAR documented here. The <lzAR is responsible for hyperpolarization of the
membrane through opening of K+ channels, as shown on preoptic area
neurons as on locus coeruleus neurons (Williams et al., 1985; Bai &
Renaud, 1998). Although other subtypes of <lzAR have been shown to
be present in the basal forebrain (Rosin et al., 1996), the <lZA was
previously shown to he distributed densely therein (Talley et al., 1996)
and using the same antibody as that used in the present study, found to
he present upon locus coeruleus neurons (Hou et al., 2(02). In the
present study, performed using epifluorescence microscopy, it cannot
be excluded that <lzAR labelling could also be present on presynaptic
terminaIs of the noradrenergic fibers as documented in other areas by
electron rnicroscopy (Glass et al., 2001) or of other fibers as indicated
in the preoptic area by electrophysiological evidence (Matsuo et al.,
2(03). However, the <l2AR labelling over the cell body and proximal
dendrites of GAD+ cells here, was predominantly associated with the
postsynaptic cells as it often appeared to he located within the
cytoplasm surrounding the cell nucleus, as also described in other
neuronal perikarya (Talley et al., 1996).
Examination for triple labelling by Nb, GAD and <lzAAR of
physiologically characterized cells showed here that cortical activation
'off' GABAergic cells do hear <l2AR. These GAD+/<l2AAR+ neurons
could be inhibited by NA in association with cortical activation and
waking when LC neurons discharge at their highest rate (Aston-Jones
& Bloom, 1981). Reciprocally they would be disinhibited during
cortical slow wave activity and SWS when LC neurons decrease
firing. They could also he active during paradoxical sleep (PS) when
LC neurons are silent and many sleep-active neurons in the forebrain
continue to discharge (Szymusiak & McGinty, 1986b; Koyama &
Hayaishi, 1994; Szymusiak et al., 1998). However, in view of the
present results, they are more likely to be silent in association with the
cortical activation that occurs during PS as weIl as waking. Silence by
these cells during PS could he effected by acetylcholine, which also
inhibits the noncholinergic cells inhibited by NA (Fort et al., 1998;
Gallopin et al., 2(00) and which is released by neighbouring choli. nergic cells during both PS and waking (Vazquez & Baghdoyan, 2(01).
The GABAergic <lzAR-hearing neurons identified here, thus, most
likely correspond to sleep-active cells that are maximally active during
SWS. Given the evidence from lesion studies that destruction of cells
in the basal forebrain leads to a loss of sleep (Szymusiak & McGinty,
1986a), these sleep-active neurons must also function irnportantly as
sleep-promoting neurons. They may do so via projections to and
inhibitory effects on local cholinergic neurons, the cortex (Gritti
et al., 1997) or the posterior hypothalamus (Gritti et al., 1994). In
this role, they may function in synergy with GABAergic neurons of the
preoptic region, including the VLPO (Sherin et al., 1996; Szymusiak
et al., 1998), that project in parallel to the posterior hypothalamus
(Gritti et al., 1994; Sherin et al., 1998) and are similarly inhibited by
NA (Fort et al., 1998; Gallopin et al., 2000).
Acknowledgements
This research was supported by grants from the Canadian Institutes of Health
Research (CIHR, 13458) and United States National Institute of Mental Health
(NIMH, ROl MH60119-01Al). Y.P. Hou was a Visiting Scientist from the
Lanzhou Medical College, Lanzhou, China. 1.0. Manns held a graduate student
fellowship from the Canadian Natural Science and Engineering Research
Council (NSERC). Lynda Mainville is greatly appreciated for her technical
assistance.
Abbreviations
<l2AR, alpha2 adrenergic receptor; GAD, glutarnic acid decarboxylase; LC,
locus coeruleus; MCPO, magnocellular preoptic nucleus; NA, noradrenaline;
Nb, Neurobiotin; PS, paradoxical sleep; SI, substantia innominata; SWS, slow
wave sleep; VLPO, ventrolateral preoptic region.
References
Aston-Jones, G. & Bloom, F.E. (1981) Activity of norepinephrine-containing
locus coeruleus neuronsin behaving rats anticipates fluctuations in the sleepwaking cycle. J. Neurosci., 1, 87~886.
Bai, D. & Renaud, L.P. (1998) Median preoptic nucleus neurons: an in vitro
patch-clamp analysis of their intrinsic properties and noradrenergic receptors
in the rat. Neuroscience, 83, 905-916.
Cape, E.G. & Jones, B.E. (2000) Effects of glutamate agonist versus procaine
microinjections into the basal forebrain cholinergic œil area upon
gamma and theta EEG activity and sleep-wake state. Eur. 1. Neurosci.,
12, 216~2184.
Fort, P., Khateb, A., Serafin, M., Muhlethaler, M. & Jones, B.E. (1998)
Pharmacological characterization and differentiation of non-cholinergie
nucleus basalis neurons in vitro. Neuroreport, 9, 1-5.
© 2003 Federation of European Neuroscienee Societies, European Journal of Neuroscience, 18, 723-727
Alpha 2 adrenergic receptors on GABAergic neurons
Gallopin, T., Fort, P., Eggennann, E., Cauli, B., Luppi, P.H., Rossier, J.,
Audinat, E., Muhlethaler, M. & Serafin, M. (2000) Identification of sleeppromoting neurons in vitro. Nature, 404, 992-995.
Glass, M.J., Huang, J., Aicher, S.A., Milner, T.A. & Pickel, V.M. (2001)
Subcellular localization of alpha-2A-adrenergie receptors in the rat medial
nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J. Comp. Neurol., 433, 193-207.
Gritti, L, Mainville, L. & Jones, B.E. (1993) Codistribution of GABA- with
acety\choline-synthesizing neurons in the basal forebrain of the rat. J. Comp.
Neurol., 329, 438-457.
Gritti, 1., Mainville, L. & Jones, B.E. (1994) Projections of GABAergie and
cholinergie basal forebrain and GABAergie preoptic-anterior hypothalamic
neurons to the posterior lateral hypothalamus of the rat. J. Comp. Neurol.,
339,251-268.
Gritti, L, Mainville, L., Mancia, M. & Jones, B.E. (1997) GABAergic and other
non-cholinergie basal forebrain neurons project together with cholinergic
neurons to meso- and iso-cortex in the rat. J. Comp. Neurol., 383,163-177.
Hou, Y.P., Manns, I.D. & Jones, B.E. (2002) Immunostaining of cholinergie
pontomesencephalic neurons for alphal versus alpha 2 adrenergie receptors
suggests different sleep-wake state activities and roles. Neuroscience, 114,
517-52\.
Jones, B.E. (2000) Basie Mechanisms ofSleep-Wake States. In Kryger, M.H.,
Roth, T., & Dement, W.C. (Eds), Princip/es and Practice of Sleep
Medicine. Saunders, Philadelphia, pp. 134-154.
Koyama, Y. & Hayaishi, O. (1994) Firing of neurons in the preopticJanterior
hypothalamic areas in rat: its possible involvement in slow wave sleep and
paradoxical sleep. Neurosci. Res., 19, 31-38.
Manns, 1.0., Alonso, A. & Jones, B.E. (2000a) Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal
forebrain neurons recorded in association with the electroencephalogram in
anesthetized rats. 1. Neurosci., 20, 9252-9263.
Manns, 1.0., Alonso, A. & Jones, B.E. (2000b) Discharge properties of
juxtacellularly labeled and immunohistochemically identified cholinergie
basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J. Neurosci., 20, 1505---1518.
Matsuo, S., Jang, I.S., Nabekura, J. & Akaike, N. (2003) alpha 2-adrenoceptormediated presynaptic modulation of GABAergic transmission in mechani-
727
cally dissociated rat ventrolateral preoptic neurons. J. Neurophysiol., 89,
1640---1648.
Osaka, T. & Matsumura, H. (1994) Noradrenergie inputs to sleep-related
neurons in the preoptic area from the locus coeruleus and the ventrolateral
medulla in the rat. Neurosci. Res., 19,39-50.
Osaka, T. & Matsumura, H. (1995) Noradrenaline inhibits preoptic sleep-active
neurons through Clrreceptors in the rat. Neurosci. Res., 21, 323-330.
Rosin, D.L., Talley, E.M., Lee, A., Stometta, R.L., Gaylinn, B.D., Guyenet, P.G.
& Lynch, K.R. (1996) Distribution of alpha 2C-adrenergic receptor-like
immunoreactivity in the rat central nervous system. J. Comp. Neurol., 372,
135---165.
Sherin, J.E., Elmquist, J.K., Torrealba, F. & Saper, C.B. (1998) Innervation of
histaminergic tuberomammillary neurons by GABAergic and galaninergic
neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci., 18,
4705-472\.
Sherin, J.E., Shiromani, P.J., McCarley, R.W. & Saper, C.B. (1996) Activation
of ventrolateral preoptic neurons during sleep. Science, 271, 216-219.
Stewart, D.1., MacFabe, D.F. & Vanderwolf, C.H. (1984) Cholinergie
activation of. the electrocorticogram: Role of the substantia innominata
and effects of atropine and quinuclidinyl benzilate. Brain Res., 322,
219-232.
Szymusiak, R, Alam, N., Steininger, T.L. & McGinty, D. (1998) Sleep-waking
discharge patterns of ventrolateral preopticJanterior hypothalamic neurons in
rats. Brain Res., 803, 178-188.
Szymusiak, R. & McGinty, D. (1986a) Sleep suppression following kainic
acid-induced lesions of the basal forebrain. Exp. Neurol., 94, 598-614.
Szymusiak, R & McGinty, D. (l986b) Sleep-related neuronal discharge in the
basal forebrain of cats. Brain Res., 370, 82-92.
Talley, E.M., Rosin, D.L., Lee, A., Guyenet, P.G. & Lynch, K.R (1996)
Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the
rat central nervous system. 1. Comp. Neurol., 372,111-134.
Vazquez, J. & Baghdoyan, H.A. (2001) Basal forebrain acetylcholine release
during REM sleep is significantly greater than during waking. Am. 1. Physiol.
Regul. Integr. Comp. Physiol., 280, R598-R60\.
Williams, J.T., Henderson; G. & North, RA. (1985) Characterization of alpha
2-adrenoceptors which increase potassium conductance in rat locus coeruleus
neurones. Neuroscience, 14,95---10\.
© 2003 Federation of European Neuroscience Societies, European Journal of Neuroscience, 18, 723-727
Neuroscience 129 (2004) 803-810
GABAERGIC NEURONS WITH a2-ADRENERGIC RECEPTORS IN
BASAL FOREBRAIN AND PREOPTIC AREA EXPRESS C-FOS DURING
SLEEP
M. MOOIRROUSTA, L. MAINVILLE AND B. E. JONES·
SWS (Oetari et al., 1984; Szymusiak and McGinty, 1986;
Koyama and Hayaishi, 1994; Alam et al., 1996). More recently by examining c-Fos expression as a reflection of neural activity, a restricted collection of neurons was identified
within the ventrolateral POA (VLPO) that was active during
sleep and thus proposed to be the principal sleep-promoting
cell group of the POA and forebrain (Sherin et al., 1996). In
immunohistochemical studies, many cells in the VLPO, lateral POA (LPO) and BF, including projection neurons, were
shown to contain glutamic acid decarboxylase (GAD), the
synthetic enzyme for GABA (Gritti et al., 1993, 1994; Sherin
et al., 1998). The possibility was thus raised that GABAergic
neurons in both POA and BF could comprise sleep-active
and promoting cells. Indeed, in studies employing juxtacellular labeling of recorded neurons in urethane-anesthetized
rats, a majority of immunohistochemically identified GABAergic BF neurons were found to tire maximally with cortical slow
wave activity, whereas ail cholinergie neurons tired maximally
with stimulation-induced cortical activation (Manns etaI.,
2OOOa,b).
Wake-active versus sleep-active neurons most likely
respond differently to the major neurotransmitters of the
ascending arousal systems, most notably noradrenaline
(NA) contained in locus coeruleus neurons projecting to
the BF and POA (Jones and Moore, 1977; Jones and
Cuello, 1989; Chou et al., 2002). Indeed, it was tirst found
in vivo that NA excited wake-active neurons, whereas it
inhibited sleep-active neurons through <l2_adrenergic receptors (<l2-AR) in the POA and adjacent BF (Osaka and
Matsumura, 1995). It was also found in vitro that whereas
ail cholinergie neurons were excited by NA in the BF, sorne
co-distributed non-cholinergie neurons (approximately
15%) were inhibited by NA (Fort et al., 1995, 1998). It was
subsequently discovered that the majority of neurons in the
VLPO (approximately 60%) were inhibited by NA and also
contained mRNA for GAD (Gallopin et al., 2000). Most
recently, we found that immunohistochemically identified
GABAergic BF neurons which fired maximally with slow
wave activity in urèthane-anesthetized rats were immunestained for <l2A-AR (Manns et al., 2003). These results
suggested that GABA and <l2-AR may reflect a corn mon
phenotype as weil as mechanism for a population of sleeppromoting neurons distributed across the BF and POA.
ln the present study, c-Fos expression was examined
Montreal Neurological Institute, McGill University, 3801 University
Street, Room 897, Montreal, Quebec, Canada H3A 284
Abstract-The basal forebrain (BF) contains cholinergie neu·
rons that stimulate cortical activation during waking. In addi·
tion, both the BF and adjacent preoptic area (POA) contain
neurons that promote sleep. We examined c:-Fos expression in
cholinergie and GABAergic neurons in the BF and POA to
detennine whether they are differentially active following sleep
deprivation versus recovery and whether the GABAerglc neu·
rons are active during sleep, Whereas the numbers of c-Fos+
cells and proportions of c:-Fos+ cells that were cholinergie were
decreased, the proportions that were GABAergic were in·
creased following sleep recovery across BF and POA nuclei.
Moreover, the sleep-active GABAergic neurons were immunostained for <lzA-adrenergic receptors. We conclude that
GABAergic neurons that c:ommonly bear ~-adrenergic receptors comprise sleep-active cells of the BF and POA. These
GABAergic cells would be inhibited by noradrenaline (NA) raleased from locus coeruleus neurons during waking; they
wou Id be disinhibited through diminished NA release during
drowsiness and thus become active to promote sleep by inhibiting in tum wake-promotlng neurons. © 2004 IBRO. Published
by Elsevier Ltd. Ali rights reserved.
Key words: cholinergie, waking, slow wave sleep, REM sleep,
noradrenaline, rat.
Since early studies, the basal forebrain (BF) has been known
to play an important role in cortical activation as the ventral
extrathalamic relay to the cerebral cortex tram the brainstem
ascending activating system (Starzl et al., 1951; Jones,
20(0). Yet, electrical stimulation in the region of the BF and
adjacent preoptic area (POA) also elicited cortical slowwave
activity with slow wave sleep (SWS; Sterman and Clemente,
1962a,b), and lesions therein produced insomnia (McGinty
and Sterman, 1968). From single unit recording studies, it
became apparent that whereas many neurons in the BF and
POA increase their firing with cortical activation or waking,
others increase their firing with cortical slow wave activity or
*Corresponding author. Tel: +1-514-398-1913; fax: +1-514-398-5871.
E-mail address:[email protected] (B. E. Jones).
Abbreviations: AR, adrenergic receptor; BF, basal forebrain; ChAT,
choline acetyltransferase; OBB, diagonal band of Broca nuclei; GAD.
glutamic acid decarboxylase; LPO, lateral preoptic area; MCPO, magnocellular preoptic area; MnPO, median preoptic nucleus; MPO, medial preoptic area; NA, noradrenaline; NOS, normal donkey serum;
non-REMS, non-rapid eye movement sleep; POA, preoptic area;
REMS, rapid eye movement sleep; SC, sleep control; 50, sleep
deprivation; Sla, substantia innominata, anterior part; Slp, substantia
innominata, posterior part; SR, sleep recovery; SWS, slow wave sleep;
VLPO, ventrolateral preoptic area.
in sleep deprived versus sleep recovery groups of rats to
test the hypotheses that sleep-promoting neurons 1) are
distributed across nuclei of the BF and POA, 2) are not
composed of cholinergie neurons and 3) are composed of
GABAergic neurons that are inhibited by NA and th us bear
0306-4522/04$30.00+0.00 © 2004 IBRO. Published by Elsevier Ltd. Ali rights reserved.
doi: 10.1 016fJ.neuroscience.2004.07.028
803
804
M. Modirrousta et al. 1 Neuroscience 129 (2004) 803-810
a2-AR. A portion of these results was presented in abstract
form (Modirrousta et aL, 2003, 2004).
EXPERIMENTAL PROCEDURES
Male Wistar rats (Chari es River Canada, St. Constant, Quebec,
Canada) weighing between 200 and 250 9 were housed individually in cages with free access to food and water. Three different
groups of four rats per group were treated in their home cages for:
1) total sleep deprivation (SO) by gentle touching for 3 h (12:0015:00 h), 2) total SO for 3 h (09:00-12:00 h) followed by sleep
recovery (SR) for 3 h (12:00-15:00 h), or 3) undisturbed sleep and
waking as sleep control (SC) for 3 h (12:00-15:00 h). Visual
observation was used to record the behavioral state every 20 s as
wake, non-rapid eye movement sleep (non-REMS) or REMS,
whereby non-REMS was scored when the animal was recumbent
with eyes closed and showing little or no movement and REMS
when the animal was recumbent with eyes closed and showing
rapid movements or twitches of the eyes, whiskers, ears or paws
(as previously determined to correspond to polygraphically scored
sleep states (Maloney et al., 1997». Animais were prevented from
sleeping during SO by gentle touching (with a small soft paint
brush inserted through the bars ofthe cage top) upon eye closure.
The deprivation procedure resulted in the total absence of sleep
during 3 h for the SO and SR groups and was followed in the SR
group by an increase in total sleep relative to both the SO group
and SC group. At the end of the experimental period (15:00 h), the
rats were immediately killed under pentobarbital anesthesia
(100 mgfkg, Lp.) by intra-aortic perfusion with a fixative solution of
3% paraformaldehyde. Ali procedures were approved by the
McGiII University Animal Care Committee and the Canadian
Council on Animal Care. Our experiments meet or exceed ail
guidelines of the Association for Assessment and Accreditation of
Lab Animal Case International.
Following immersion in a 30% sucrose solution, the brains
were frozen and stored at -80 oC. They were cut in coronal
sections at 20 J1.m, which was determined to be the greatest
thickness that would allow full penetration and staining with the
antibodies employed. Adjacent series of sections were collected
and processed for double immunohistochemical staining using
peroxidase-antiperoxidase for 1) c-Fos (rabbit antiserum;1:
10,000; Ab-5; PC38; Oncogene Research Products, La Jolla, CA,
USA) with OAB-Ni as chromogen and 2) a) at 400 J1.m intervals,
choline acetyltransferase (ChAT; mouse antibody; 1:2000;
MAB5270; Chemicon International, Temecula, CA, USA) or b) at
200 J1.m intervals, GAD (mouse antibody, detecting both GA065
and GA067 isoforms; 1:100;. MSA-225; Stressgen Biotechnologies, Victoria, BC, Canada) with pink, a-naphthol pyronin B. Other
series taken at 800 J1.m intervals were processed for triple immunostaining for 1) c-Fos in the first position using OAB-Ni, 2)
<X2A-AR (goat-purified antiserum; 1:50; sc-1478; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in the second position using
red Cy3-conjugated donkey antigoat antiserum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and 3) GAO
(rabbit antiserum; 1:1000; AB5992; Chemicon) using green Cy2conjugated donkey antirabbit antiserum (Jackson) in the third
position. Incubations with primary antibodies were performed at
room temperature ovemight for c-Fos, ChAT and GAO antibodies
and three nights at 4 oC for <X2A-AR antibody using a Tris-saline
solution (0.1 M) containing 1% normal donkey serum (NOS; following initial blocking with 3% NOS).
Sections were viewed by light and fluorescence microscopy
(Leica OMLB microscope Leica Microsystems (Canada, Inc.,
Richmond Hill, Ontario, Canada) equipped with an x/y/z movement-sensitive stage and video camera at!ached to a computer).
Cells immunopositive (+) for single c-Fos+, double c-Fos+1
ChAT + or c-Fos+/GAO+ and triple c-Fos+/GAO+/<X2A-AR+
were counted by applying stereology using the Optical Fractiona-
tor program of Stereo Investigator (2003; MicroBrightField, Williston, VT, USA). Cells were counted within the contours of eight
nuclei in the BF and POA, respectively: substantia innominata,
anterior part (Sla), substantia innominata, posterior part (Slp),
magnocellular POA (MCPO), diagonal band of Broca nuclei (OBB)
and LPO, VLPO, medial POA (MPO), median preoptic nucleus
(MnPO). The nuclei were delineated in a computer resident atlas
(modified from Gritti et al., 1993) that was placed in register ~ith
each section. For each nucleus, labeled cells were counted bllaterally in at least three sections (three to 14, depending upon the
length of each nucleus) at 400 J1.m intervals for the c-FosfChATimmunostained series through the BF region nuclei or 200 J1.m for
the c-Fos/GAO-immunostained series through the BF and POA
regions. Through ail nuclei, cells were counted under a 63x oil
objective (with 1.4 numerical aperture) within a counting frame of
125x125 J1.m. In most nuclei, cells were counted through 25% of
the area at each level by setting the grid size (within which the
counting frame is automatically inserted) to 250x250 J1.m. For the
smaller nuclei (VLPO and MnPO), cells were counted through the
entire area of the nucleus by setting the grid size to 125x125 IJ.m.
Counts of triple-Iabeled cells immunostained for c-Fos/GADI
<X2A-AR were combined across nuclei for estimates of total cell
numbers within the BF and POA regions. By counting nuclei that
came into focus beneath the surface of the section, c-Fos+ nuclei
were counted within a counting block of 8 IJ.m in depth (in the
dehydrated, delipidated, mounted and coverslipped sections that
were on average 10 J1.m thick).
Sleep and cell counts were analyzed between groups and
across nuclei or regions using one and two way ANOVA in Systat
(v10.2, Richmond, CA, USA). Ali main effects were confirmed by
nonparametric rank order tests (Kruskal-Wallis, P<0.05) to insure
that the distribution of variance in groups (containing zeros as a
result of the experimental condition) did not distort the parametric
statistics. Figures were composed using Adobe Photoshop 6 and
lIIustrator 9 (Adobe, San Jose, CA, USA).
RESULTS
Rats submitted to SD by gentle touehing did not sleep
du ring the 3 h prior to kil! at 15:00 h, whereas those
perrnitted SR during the same period after 3 h deprivation
in the moming (09:00-12:00 h) slept approximately 91 % of
the time. The amount of total sleep differed significantly
aeross conditions of SD, SR and SC (Table 1). Recovery
and control sleep were comprised predominantly of behaviorally quiet, non-REMS (non-REMS: 77.23:±:2.94% in SR
and 68.68:±:0.10% in SC with REMS: 13.58:±:1.30% in SR
and 7.10:±:0.66% in SC, mean:±:S.E.M. of total time).
ln SR and/or SD rats, c-Fos was expressed in ChATimmunopositive (+) and GAD+ cells as weil as in ChATimmunonegative (-) and GAD- cells within the BF and
POA (Fig. 1A-O). The numbers of c-Fos+ cells varied
significantly aeross conditions, differing between SR or SC
and SD but not between SR and SC (Table 1), According
to a two-way ANOVA with condition and nuelei as factors,
c-Fos+ cells were significantly deereased in SR (like SC)
as compared with SD (see Fig. ZA, Band 3B, Table 1).
Given a significant interaction between condition and nuclei, post hoc comparisons were perforrned to deterrnine if
the deerease was significant in ail nuelei (Table 1). The
difference was found to be significant in ail nuelei, exeept
the MnPO. The proportions of e-Fos+ eells that were
ChAT + were also signifieantly deereased in SR (Iike SC)
as eompared with SD (Fig. 2C, D and 3C, Table 1). In this
805
M. Modirrousta et al. / Neuroseience 129 (2004) 803-810
Table 1. Average percent sleep and e-Fos expression aeross nuelei (Nue) of the BF and POA under conditions (Cond) of SC, SO and SR in three
groups of ratsa
Variable
SC
SO
% Sleep
Total number c-Fos+ ceUs
Percent c-Fos+/ChAT +
Percent c-Fos+/GAO+
75.75:!:0.61
650.53:!: 86.29
3.57:!:1.61
44.00:!:3.66
O.OO:!:O.OO
2462.70:!:341.40
11.68:!:2.64
17.16:!:2.71
SR
(SC,SR)
(SC,SR)
(SC. SR)
(SC,SR)
90.80:!:2.47
575.80:!:95.02
O.OO:!:O.OO
41.16:!:4.04
(SC, SO)
(SO)
(SO)
(SO)
F (Cond)
F (Nue)
F (CondxNue)
1095.40'"
70.72'"
8.95'"
25.86'"
16.67'"
0.33 ns
6.53'"
4.77'"
0.16 ns
1.35 ns
• Mean:!:S.E.M. values from four rats per group. % Sleep was calculated as percentage of 3 h observation period prior to killing and was examined
across groups by a one-way ANOVA. For number or percent of c-Fos expressing ceUs, two-way ANOVAs were performed with Cond (3) and Nue (8)
as factors. For each variable, there was a significant main effect of condition. Post hoc paired comparisons with Bonferroni correction were peIformed
to examine differences between conditions (indicated in parentheses, P<.05). For % Sleep, SO and SR both differed from SC and from each other;
for c-Fos+ ceU variables, SO differed from SC and SR but SR did not differ from SC. For total number of c-Fos+ ceUs (calculated as c-Fos+ plus
c-Fos+/GAD+ ceUs in the c-Fos/GAO dual-immunostained series), there was a significant difference across nuclei and a significant interaction of
condition with nuclei. Post hoc comparisons between conditions in each nucleus revealed a main effect of condition in every nucleus except the MnPO.
For percent of c-Fos+ ceUs (as c-Fos+/ChAT + or c-Fos+/GAO+ of the total), there was a significant difference for GAD + ceUs between nuclei, but
there was no significant interaction between condition and nuclei for GAO+ or ChAT + cells, thus contraindicating post hoc comparisons for condition
in each nucleus. ('" indicates F-ratio with P<.001; ns means not significant.)
case, there was no significant interaction between condition and nuclei, indicating that the effect of condition was
not different across individual nuclei of the BF. In contrast
to those being ChAT +, the proportions of c-Fos + cells that
were GAD + were significantly increased in SR (like SC) as
compared with SD (Fig. 2E, F and 3D; Table 1). In this
case, there was also no significant interaction between
condition and nuclei, indicating that the main effect of
condition did not differ across the nuclei of the BF and
POA.
The incidence of <XzA-AR labeling was subsequently examined on c-Fos+/GAD+ cells in the SD and SR groups by
triple-immunostaining (Fig. 1E and F). The proportions of
c-Fos+/GAD+ cells that were <XzA-AR+ were small in the SD
group and significantly higher in the SR group across the BF
and POA regions (with no significant difference across the
regions or interaction of condition with region, Fig. 4). On
average across regions, 3.88:±: 1.73% of c-Fos+/GAD+ cells
were Ot2A-AR+ in the SD group and 77.70:±:22.07% in the SR
group.
DISCUSSION
Fig. 1. Labeled ceUs in the nuclei of the BF or POA. (A) Single-labeled
c-Fos+ cell (stained black with OAB-Ni, black arrowhead), single-labeled
ChAT + cell (stained pink with cr.-naphthol pyronin B, white arrowhead)
and double-labeled c-Fos+/ChAT+ cell (double arrowhead) in the
MCPO. (8-0) Single-Iabeled c-Fos+ cells (stained black, black arrowheads), single-Iabeled GAO+ cells (stained pink, white arrowheads) and
double-labeled c-Fos+/GAO+ cells (double arrowheads) in the MCPO
(B), MPO (C) and VLPO (0). (E, F) A triple-labeled c-Fos+/GAD+/~
AR+ cell shown in images of c-Fos staining (stained black with OAB-Ni,
black arrowhead in E) and ~-AR (stained by red fluorescence with Cy3)
together with GAD staining (stained by green fluorescence with Cy2,
white arrowhead in F). The ~-AR labeling is avident over the GAD + cell
body (in orange or yellow). Scale bars=2Q ....m (for A-O, shown in D, and
E-F, shown in F).
The present results indicate that across the BF and POA
fewer neurons are active during sleep than during waking,
and that of those active during sleep, none are cholinergic
and a substantial proportion are GABAergic. Moreover, the
majority of the sleep-active GABAergic neurons appear to
bear Ot 2A-AR.
ln ail nuclei of the BF and ail but the MnPO of the
POA, more neurons accumulated c-Fos protein following 3 h of waking produced by gentle deprivation of
sleep than following 3 h of sleep either during recovery
from deprivation or control condition. These results suggest that the majority of neurons in the BF and POA are
more active during waking than during sleep. Similar
conclusions had been reached by other studies employing c-Fos immunohistochemistry or in situ hybridization
in the past (Pompeiano et al., 1992; Cirelli et al., 1993;
Ledoux et aL, 1996; Sastre et aL, 2000). Only recently
was it reported first for the VLPO and then for the MnPO
in the POA, that more neurons expressed c-Fos during
sleep than during waking (Sherin et aL, 1996; Gong et
al., 2000). It is possible that the particular experimental
conditions in those studies, such as the housing, surgery
or method of SD, which can be associated with certain
degrees of stress and associated c-Fos expression at
806
M. Modirrousta et al./ Neuroscience 129 (2004) 803-810
..... A8.6
E ..
..... A9.0
F
(·Fos+/GAD+
SO
SR
SO
SR
Fig. 2. Distribution of c-Fos+ cells in one SD and one SR representative brain through the BF and POA (at approximately A8.6 in A, C and E and
approximately A9.0 in B, 0 and F; Gritti etat, 1993). (A, B) C-Fos+ cells (blaekdots)within BF and POA nuelei. (C, 0) Double-Iabeled c-Fos+/ChAT+
cells (red circ/es) distinguished from single-Iabeled c-Fos+ cells (black dots) within the BF. (E, F) Double-Iabeled c-Fos+/GAD+ cells (blue triangles)
distinguished from single-Iabeled c-Fos+ cells (black dots) within the BF and POA nue/ei. Atlas images show cells plotted in one 20 ""m thick section
per level (approximately A 8.6 on left and approximately A 9.0 on right) from adjacent series (separated by 200 ""m) dual-immunostained for
c-Fos/ChAT (C, 0) or c-Fos/GAD (A, Band E, F). Sip not shown. (Note that ail nuelei examined are contained within the sections iIIustrated in Fig.
2, except Slp, which is located in the same relative position as Sla at more caudal levels.)
particular times (Oragunow and Faull, 1989; Cullinan et
al., 1995; Maloney et aL, 1999), resulted in relatively
higher c-Fos expression in those nuclei during sleep
than in the present study. Here, we employed conditions
that would minimize stress prior to and during the experimental period by studying the animais in their home
cages, using behavioral observation to record sleep so
as to avoid prior surgery and applying gentle touching
with a soft brush for preventing sleep during a relatively
short (3 h) period. Under these conditions, an increase
in the total number of neurons expressing c-Fos was not
observed in any nucleus of BF or POA during recovery
or control sleep as compared with enforced waking.
Indeed, the number of neurons active in SR was on
average <25% of that active in SO. The proportions did
differ however across nuclei with the highest proportions
being in the MnPO (approximately 75%) and VLPO (approximately 50%) and lower proportions in the BF and
LPO nuclei (approximately 20%), suggesting different
concentrations of sleep- versus wake-active neurons
with the highest concentrations in MnPO and VLPO.
These results are supported by both in vivo and in vitro
electrophysiological studies showing relatively high concentrations of putative sleep-promoting neurons in these
POA nuclei (Szymusiak et aL, 1998; Gallopin et aL,
2000; Suntsova et aL, 2002).
Single unit recording studies have also found relatively smaH proportions of neurons that are maximaHy
active specifically during non-REMS or SWS in BF and
POA nuclei (Oetari et aL, 1984; Szymusiak and McGinty,
1986; Koyama and Hayaishi, 1994; Szymusiak et aL,
2000; Suntsova et aL, 2002; Lee et al., 2004). On the
M. Modirrousta et al. / Neuroscience 129 (2004) 803-810
807
A
Bt
1
~
1
!
i
~
~
1
!
C
i
!
+
~
'#
0
Fig. 3. Bar charts showing the percentage of sleep (in A) and the total numbers of e-Fos+ ceUs (in B) or proportions of o-Fos+ ceUs that were
cholinergie or GABAergic (in C or 0) within each nucleus of the BF and/or POA in SO and SR groups. (A) The percentage of sleep (representing
percentage of total sleep of 3 h period prior to kiU) was significantly inereased in SR as compared with SO. (B) The total numbers of e-Fos+ ceUs
(obtained trom c-Fos/GAO dual-immunostained series as the total of o-Fos+ and e-Fos+/GAO+ ceUs) were significantly deereased across nuelei of
the BF and POA in SR as compared with SO (with a significant differenee across nuclei and a significant interaction between condition and nuelei, due
to a significant decrease in ail nuelei except MnPO, Table 1). (C) The percentages of e-Fos+ cells that were ChAT + (obtained trom the o-Fos/ChAT
dual-immunostained series as the percentage of e-Fos+ plus o-Fos+/ChAT + cells that were e-Fos+/ChAT +) were significantly decreased aeross
nuclei of the BF in SR as compared with SO (with no significant difference across nuelei or interaction betwèen condition and nUclei, Table 1). (0) The
percentages of o-Fos+ cells that were GAD + (caleulated from total numbers shown in B) were significantly inereased in SR as compared with SO (with
a significant difference across nuelei but no significant interaction between condition and nuelei, contraindicating post hoc comparisons in individual
nuelei, Table 1). For each group (n=4), mean±S.E.M. are presented.
M. Modirrousta et al. 1 Neuroscience 129 (2004) 803-810
808
BF
A ~~
cr:+
oc:(
1
oc:(
~+
0
oc:(
~
~
~
6
~
0
SO
SR
Condition
POA
B
!Il.
~
+
cr:
oc:(
1
oc:(
~+
0
oc:(
~
~
~
ô
~
0
SO
SR
Condition
Fig.4. Bar charts showing the proportions of c-Fos+/GAO+ ceUs that
bear «2A-AR in the BF (A) and POA (B) in SO and SR groups. There
was a significant increase in c-Fos+/GAO+/«2A-AR+ ceUs in SR as
compared with SO (ANOVA for main effect of condition. F=7.168.
P<O.05 with no significant difference across the two ragions and no
significant interaction between condition and region, P>O.05).
other hand in the sa me studies, a large and varying
proportion of neurons has been characterized through
these regions as active during REMS, some as nonREMS/REMS-active, some selectively REMS-active and
others as Wake/REMS-active. In a study examining cFos in pharmacologically produced predominant REMS
versus predominant non-REMS conditions, it was found
that REMS was associated with increased c-Fos expression in many forebrain regions (Sastre et al., 2000). We
assume that in the present study the major percent of
time spent in non-REMS (approxirhately 77%) as compared with the small percent of time spent in REMS
(approximately 14%) in the 3 h preceding kHI served as
the major determinant in c-Fos expression and accu mu-
lation during recovery as weil as control conditions. We
thus conclude that the c-Fos+ neurons seen following
SR or control largely represent non-REMS-active neu~
rons (which would include non-REMS/RE MS-active but
not selective REMS-active or Wake/REMS-aetive neurons) that are distributed aeross BF and POA as a sm ail
population though with relatively greater representation
in certain nuelei of the POA.
ln parallel with the total number of neurons expressing
e-Fos, the proportions of those neurons that were eholinergic aeross BF nuelei were diminished during sleep. In
fact, no c-Fos+/ChAT + neurons were detected in the SR
condition. These results confirm electrophysiological studies indicating that presumed cholinergie neurons are silent
during SWS (Jones, 2004; Lee et aL, 2004). They also
confirm that cholinergie neurons are active during waking.
Aecording to release of aeetyleholine and firing of presumed cholinergie BF neurons, however, they should also
be active during REMS (Marrosu et aL, 1995; Jones,
2004). As for ail e-Fos+ ce"s (above), we assume that the
laek of e-Fos immunostaining in cholinergie neurons in the
recovery condition, as weil as control, is due to the predominance of non-REMS in the 3 h prior to kill and the
small am ou nt of time spent in REMS (14%) as weil as
waking (approximately 9%) that would be inadequate for
accumulation of the Fos protein during that period (Dragunowand Faull, 1989).
ln contrast to the proportion of cholinergie neurons, the
proportion of GABAergic neurons expressing c-Fos increased significantly with SR as compared with SD across
BF and POA nuclei. These results indicate that many
GABAergic neurons distributed across these regions remain
or become active in association with sleep following waking.
They support claims that neurons in the BF and POA including the VLPO that project to the posterior hypothalamus and
contain GAD may be sleep-active and promoting neurons
and may act by inhibiting wake-promoting neurons of that
region (Gritti et aL, 1994; Sherin et aL, 1998). They also
support the hypothesis that identified GABAergie BF neurons
that discharge in association with cortical slow wave activity
in urethane-anesthetized rats and project either to posterior
hypothalamus or cortex, if not locally, as determined by antidromic activation, could be sleep-active and promoting as
weIl (Manns et aL, 2OOOa). Finally, they confirm similar results
most recently reported by others for e-Fos expression in
GAD+ neurons of the MnPO and VLPO (Gong et al., 2004).
GABAergic neurons of different BF and POA nuclei could
thus exert an inhibitory influence upon wake-active and promoting systems in the posterior hypothalamus, cortex and/or
BF. However, in single unit recording studies, it was also
found that another physiologically distinct group of GABAergic neurons discharged maximally in association with cortical
activation (Manns et aL, 2000a). Therefore, different groups
of GABAergic neurons in the BF and POA are likely active in
association with cortical activation of waking and others in
association with SWS.
The major proportion (approximately 78%) of GAD+
neurons that expressed c-Fos with SR in the present study
were immunostained for the Cl2A-AR, whereas only a min-
M. Modirrousta et al. 1 Neuroscience 129 (2004) 803-810
imal percentage (approximately 4%) of those that did so
with SO were so labeled. These results confirm the hypothesis. that GABAergic neurons in the BF and POA that are
active during sleep bear <x2 -AR and would accordingly be
inhibited by NA (Osaka and Matsumura, 1995; Bai and
Renaud, 1998; Manns et al., 2003; Saint-Mieux et al.,
2004). This particular group of GABAergic neurons would
be he Id under inhibition during waking by release of NA
from afferent locus coeruleus neurons (Jones and Cuello,
1989) that fire maximally during active waking, decrease
firing during quiet waking, and further diminish firing during
SWS to cease firing during REMS (McCarley and Hobson,
1975; Aston-Jones and Sloom, 1981). With decremental
release of NA during drowsiness, these GABAergic neurons would be disinhibited to become active and promote
sleep by inhibiting in tum the noradrenergic neurons together with other neurons of the brainstem and forebrain
activating systems (Gritti et al., 1994; Luppi et al., 1995;
Sherin et al., 1998; Steininger et al., 2001; Chou et al.,
2002).
Acknowledgments-The research was supported by a grant From
the Canadian Institutes of Health Research (13458).
REFERENCES
Alam MN, MeGinty D, Szymusiak R (1996) PreopticJanterior hypothalamie neurons: therm05ensitivity in wakefulness and non rapid eye
movement sleep. Brain Res 718:76-82.
Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing
locus coeruleus neurons in behaving rats antieipates fluctuations in
the sleep-waking cycle. J Neurosci 1:876-886.
Bai D, Renaud LP (1998) Median preoptic nucleus neurons: an in vitro
patch-clamp analysis of their intrinsic properties and noradrenergic
receptors in the rat. Neur05cience 83:905-916.
Chou TC, Bjorkum M, Gaus SE, Lu J, Scammell TE, Saper CB (2002)
Afferents to the ventrolateral preoptic nucleus. J Neurosci 22:
977-990.
Cirelli C, Pompeiano M, Tononi G (1993) Fos-like immunoreactivity in
the rat brain in spontaneous wakefulness and sleep. Arch Ital Biol
131:327-330.
Cullinan WE, Herman JP, Battaglia OF, Akil H, Watson SJ (1995)
Pattern and time course of Immediate early gene expression in rat
brain following acute stress. Neuroscience 64:477-505.
Detari L, Juhasz G, Kukorelli T (1984) Firing properties of cat basal
forebrain neurones during sleep-wakefulness cycle. Electroencephalogr Clin Neurophysiol 58:362-368.
Dragunow M, Faull R (1989) The use of c-f05 as a metabolic marker in
neuronal pathway tracing. J Neur05ci Methods 29:261-265.
Fort p, Khateb A, Pegna A, Muhlethaler M, Jones BE (1995) Noradrenergic modulation of cholinergie nucleus basalis neurons
demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea pig brain. Eur J Neurosci
7:1502-1511.
Fort P, Khateb A, Serafin M, Muhlethaler M, Jones BE (1998)
Pharmacological characterization and differentiation of noncholinergie nucleuS basalis neurons in vitro. Neuroreport
9:1-5.
Galtopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J,
Audinat E, Muhlethaler M, Serafin M (2000) Identification of sleeppromoting neurons in vitro. Nature 404:992-995.
Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R (2004) Activation of c-f05 in GABAergic neurones in the
preoptic area during sleep and in response to sleep deprivation.
J Physiol 556:935-946.
809
Gong H, Szymusiak R, King J, SteiningerT, McGinty 0 (2000) Sleeprelated c-Fos protein expression in the preoptic hypothalamus:
effects of ambient warming. Am J Physiol Regul Integr Comp
Physiol 279:R2079-R2088
Gritti l, Mainville L, Jones BE (1993) Codistribution of GABA- with
acetylcholine-synthesizing neurons in the basal forebrain of the rat.
J Comp Neurol 329:438-457.
Gritti l, Mainville L, Jones BE (1994) Projections of GABAergie and
cholinergie basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat.
J Comp Neurol 339:251-268.
Jones BE (2000) Basic mechanisms of sleep-wake states. In: Principies and practice of sleep medicine (Kryger MH, Roth T, Dement
WC, eds), pp 134-154. Philadelphia: Saunders.
Jones BE (2004) Activity, modulation and role of basal forebrain
cholinergie neurons innervating the cerebral cortex. Prog .Brain
Res 145:157-169.
Jones BE, Cuelto AC (1989) Afferents to the basal forebrain cholinergie cell aree from pontomesencephalic-catecholamine, serotenin, and acetylcholine-neurons. Neuroscience 31:37-61.
Jones BE, Moore RY (1977) Ascending projections of the locus
coeruleus in the rat: II. Autoradiographie study. Brain Res
127:23-53.
Koyama y, Hayaishi 0 (1994) Firing of neurons in the preopticJanterior
hypothalamic areas in rat: its possible involvement in slow wave
sleep and paradoxical sleep. Neur05ci Res 19:31-38.
Ledoux L, Saslre JP, Buda C, Luppi PH, Jouvet M (1996) Alterations
in c-f05 expression after different experimental procedures of sleep
deprivation in the cat. Brain Res 735:108-118.
Lee MG, Manns ID, Alonso A, Jones BE (2004) Sleep-wake related
discharge properties of basal forebrain neurons recorded with
micropipettes in head-fixed rats. J NeurophysioI92:1182-1198.
Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M (1995)
Afferent projections to the rat locus coeruleus demonstrated by
retrograde and anterograde tracing with cholera-toxin B subunit
and Phaseolus vulgaris leucoagglutinin. Neuroscience
65:119-160.
Maloney KJ, Cape EG, Gotrnan J, Jones BE (1997) High frequency
gamma electroencephalogram activity in association with sleepwake states and spontaneous behaviors in the rat. Neuroscience
76:541-555.
Maloney KJ, Mainville L, Jones BE (1999) Differentiai c-Fos expression in cholinergie, monoaminergic and GABAergic cell groups of
the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci 19:3057-3072.
Manns ID, Alonso A, Jones BE (2000a) Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal
forebrain neurons recorded in association with the electroencephalegram in anesthetized rats. J Neurosci 20:9252-G263.
Manns ID, Alonso A, Jones BE (2000b) Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergie basal
forebrain neurons recorded in association with the electroencephalegram in anesthetized rats. J Neurosci 20:1505-1518.
Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE (2003) Alpha 2
adrenergic receptors on GABAergic, putative sleep-promoting
basal forebrain neurons. Eur J Neur05ci 18:723-727.
Marr05u F, Portas C, Mascia S, Casu MA, Fa M, Giagheddu M,
Imperato A, Gessa GL (1995) Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake
cycle in freely moving cats. Brain Res 671:329-332.
McCariey RW, Hobson JA (1975) Neuronal excitability modulation
over the sleep cycle: a structural and mathematical model. Science
189:58-60.
McGinty DJ, Sterman MB (1968) Sleep suppression after basal forebrain lesions in the cat. Science 160:1253-1255.
Modirrousta M, Mainville L, Jones BE (2003) e-Fos expression in
cholinergie and GABAergic neurons of the basal forebrain and/or
810
M. Modirrousta et al. 1 Neuroscience 129 (2004) 803-810
preoptic area following sleep deprivation and recovery. Soc
Neurosci Abst Online:341.312.
Modirrousta M, Mainville L, Jones BE (2004) GABAergic neurons of
basal forebrain and preoptic area that express o-fos with sleep
recovery bear alpha2 adrenergic receptors. Sleep 27:A5
Osaka T, Matsumura H (1995) Noradrenaline inhibits preoptic sleepactive neurons through a2-receptors in the rat. Neurosci Res
21:323-330.
Pompeiano M, Cirelli C, Tononi G (1992) Effects of sleep deprivation
on fos-like immunoreactivity in the rat brain. Arch Ital Biol
130:325-335.
Saint-Mieux B, Eggermann E, Bisetti A. Bayer L, Machard D, Jones
BE, Muhlethaler M, Serafin M (2004) Nicotinic enhancernent of the
noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci 24:63-67.
Sastre JP, Buda C, Lin JS, Jouvet M (2000) Differentiai c-fos
expression in the rhinencephalon and striatum after enhanced
sleep-wake states in the cat. Eur J Neurosci 12:1397-1410.
Sherin JE, Elmquist JI<. Torrealba F, Saper CB (1998) Innervation of
hlstaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolaleral preoptic nucleus of the ral J Neurosci 18:4705-4721.
Sherin JE, Shiromani PJ, McCariey RW, Saper CB (1996) Activation of
ventrolateral preoptic neurons during sleep. Science 271:216-219.
Starzl TE, Taylor CW, Magoun HW (1951) Ascending conduction in
reticular activating system, with special reference to the diencephalon. J NeurophysioI14:461-477.
Steininger TL, Gong H, McGinty D, Szymusiak R (2001) Subregional
organization of preoptic area/anterior hypothalamic projections to
arousal-related monoaminergic cell groups. J Comp Neurol 429:
638-653.
Sterman MB, Clemente CD (1962a) Forebrain inhibltory mechanisrns:
Cortical synchronization induced by basal forebrain stimulation.
Exp Neurol 6:91-102.
Sterman MB, Clemente CD (1962b) Forebrain inhibitory mechanisms:
sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp NeuroI6:103-117.
Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D
(2002) Sleep-waking discharge patterns of rnedian preoptic nucleus neurons in rats. J Physiol 543:665-677.
Szymusiak R, A1am N, McGinty D (2000) Discharge patterns of neurons in cholinergie regions of the basal forebrain during waking and
sleep. Behav Brain Res 115:171-182.
Szymusiak R, A1am N, Steininger TL, McGinty D (1998) Sleep-waking
discharge patterns of ventrolateral preopticlanterior hypotihalarnic
neurons in rats. Brain Res 803:178-188.
Szymusiak R, McGinty D (1986) Sleep-related neuronal discharge in
the basal forebrain of cats. Brain Res 370:82.,.92.
(Acœpted 14 July 2004)
(Avai/able online 18 Oatober 2004)
European Journal of Neuroscience, Vol. 21, pp. 2807-2816, 2005
© Federation of European Neuroscience Societies
Orexin and MCH neurons express c-Fos differently after
sleep deprivation vs. recovery and bear different adrenergic
receptors
Mandana Modirrousta, Lynda Mainville and Barbara E. Jones
Montreal Neurological Institute, McGili University, Montreal, Quebec, Canada H3A 284
Keywords: hypocretin, hypothalamus, noradrenaline, rat, waking
Abstract
Though overlapping in distribution within the posterior hypothalamus, neurons containing orexin (Orx) and melanin concentrating
hormone (MCH) may play different roles in the regulation of behavioural state. In the present study in rats, we tested whether they
express c-Fos differently after total sleep deprivation (SO) vs. sleep recovery (SR). Whereas c-Fos expression was increased in Orx
neurons after SO, it was increased in MCH neurons after SR. We reasoned that Orx and MCH neurons could be differently modulated
by noradrenaline (NA) and accordingly bear different adrenergic receptors (ARs). Of ail Orx neurons (estimated at R:l6700),
substantial numbers were immunostained for the <X1KAR, including ceUs expressing c-Fos after SO. Yet, substantial numbers were
also immunostained for the <X2A-AR, also including cells expressing c-Fos after SO. Of ail MCH neurons (estimated at R:l12 300), rare
neurons were immunostained for the <X1A-AR, whereas significant numbers were immunostained for the <X2A-AR, including cells
expressing c-Fos after SR. We conclude that Orx neurons may act to sustain waking during sleep deprivation, whereas MCH neurons
may act to promote sleep following sustained waking. Sorne Orx neurons would participate in the maintenance of waking during
deprivation when excited by NA through ()(1-ARs, whereas MCH neurons would participate in sleep recovery after deprivation when
released from inhibition by NA through <X2-ARs. On the other hand, under certain conditions, Orx neurons may also be submitted to an
inhibitory influence by NA through <X2-ARs.
Introduction
The posterior hypothalamus has long been known to play a critical
role in the maintenance of wakefulness (Jones, 2000). Recently two
peptides, orexin (Orx, also called hypocretin) and melanin concentrating hormone (MCH) have been found to be contained in distinct
populations of magnocellular neurons that partially overlap in their
distribution through the mid to posterior hypothalamus and commonly
give rise to widespread projections through the central nervous system
(Bittencourt et al., 1992; Broberger et al., 1998; de Lecea et al., 1998;
Peyron et al., 1998). They were both initially found to have a
stimulatory influence upon eating and thus to be considered as
contingents of the hypothalamic 'orexigenic' systems (Williams et al.,
2004). Yet, as became evident in knock out experiments eliminating
the peptides or their receptors, their normal roles appeared to extend
beyond influences upon eating and to differ with regard to energy
homeostasis. Most notably, mice lacking the gene for orexin or dogs
lacking the gene for its receptor manifested a syndrome of narcolepsy
(Chemelli et al., 1999; Lin et al., 1999) and humans with narcolepsy
were found to 1ack the gene, peptide or neurons containing Orx
(Peyron et al., 2000; Thannickal et al., 2000). In addition, Orx knock
out mice became obese despite being hypophagic, attributed to a
decrease in activity and basal metabolic rate. As shown by c-Fos
expression and Orx release, Orx cells also appeared to be most active
during the night, when animaIs were awake and engaged in motor
Correspondence: Dr Barbam E. Jones, as above.
E-mail: [email protected]
Received 22 December 2004. revised 18 February 2005. accepted 14 March 2005
doi:IO.llll/j.l460-9568.2005.04104.x
activity (Estabrooke et al., 2001; Yoshida et al., 2001). Orx thus
appeared to stimulate arousal with motor activity and increased energy
expenditure. In contrast, although MeH knock out mice were also
hypophagic, they manifested a decrease in body weight attributed to
an increase in activity and basal metabolic rate (Shimada et al., 1998;
Marsh et al., 2002). MCH thus appeared to decrease arousal and
energy expenditure.
According to these results, Orx and MCH neurons may perform
opposite roles in wake vs. sleep promotion and/or maintenance. Here,
we examined whether Orx and MCH ceUs respond differently to total
sleep deprivation (SD) vs. sleep recovery (SR) by using c-Fos
expression as an indicator of neuronal activity. Different activity
profiles could he due to different responses of the Orx and MCH
neurons to the major neurotransmitters of the ascending arousal
systems including most importantly noradrenaline (NA). In the basal
forebrain and preoptic areas, cholinergic wake-active neurons are
excited by NA through <Xl adrenergic receptors (<XI-ARs), whereas
GABAergic putative sleep-active neurons are inhibited by NA through
<xrARs (Fort et al., 1995; Fort et al., 1998; Gallopin et al., 2000;
Manns et al., 2003; Modirrousta et al., 2004). We thus examined
whether the Orx and MCH neurons bear different adrenergic receptors
while possibly expressing c-Fos under different conditions of
sustained waking vs. recovery sleep.
Materials and methods
AH procedures were approved by the McGill University Animal
Care Committee and the Canadian Council on Animal Care, whose
2808 M. Modirrousta et al.
standards meet those of the Association for Assessment and
Accreditation of Animal Care International. Male Wistar rats
(200-250 g) were used in minimal numbers to make up three test
groups. The rats were housed individual!y with free access to food
and water at al! times and maintained on a 12-h light : 12-h dark
schedule (with lights on from 07:00 to 19:00 h). As previously
described (Modirrousta et al., 2004), the experiment was designed
to deprive rats of sleep under non-stressful conditions and was thus
performed in the home cages. The three different groups (n = 4 per
group) were submitted to: (i) total sleep deprivation (SD) for 3 h
(12:00-15:00 h); (ii) total sleep deprivation for 3 h (09:00-12:00 h)
followed by sleep recovery (SR) for 3 h (12:00-15:00 h) or (iii)
undisturbed sleep and waking as sleep control (SC) for 3 h (12:0015:00 h). The experimenter (MM) brought each rat in its cage (in
groups of three) to an experimental room in the animal facility and
habituated each rat to her presence and to a paint brush inserted
through the cage top for 3 days prior to the experimenta1 day.
During the experiment for the SD and SR groups, she deprived
each rat of sleep by gently touching it with the brush when it
closed its eyes. She deprived and/or observed one rat at a time (on
one day) and scored by visua1 observation its behavioura1 state
every 20 s as comprised in the majority by wake, non-rapid eye
movement sleep (non-REMS) or REMS. The behavioura1 scoring
was based upon previous studies in the current lab (Maloney et al.,
1997) together with demonstrations in other labs (Bergmann et al.,
1987; Espana et al., 2003), showing that behaviour can be used in
normal rats to reliab1y score the major states of W, non-REMS and
REMS. Thus, as previously determined to correspond to polygraphically scored sleep states in our 1aboratory (Maloney et al.,
1997), non-REMS was scored when the animal was recumbent with
eyes closed and showing little or no movement and REMS when
the animal was recumbent with eyes closed and showing rapid
movements or twitches of the eyes, whiskers, muzzle, ears or paws.
The deprivation procedure resulted in the total absence of sleep
during 3 h for the SD and SR groups and was followed in the SR
group by an increase in total sleep relative to both the SD and SC
groups. At the end of the experimental period (15:00 h), the rats
were immediately killed under pentobarbital anaesthesia
(100 mglkg, Lp.) by intra-aortic perfusion with a fixative solution
of 3% paraformaldehyde.
Following immersion in a 30% sucrose solution, brains were
frozen and stored at -80 oC. They were cut in coronal sections at
20 Ilm, which was determined to be the greatest thickness that
would allow full penetration and staining with the antibodies
employed. Adjacent series of sections were collected at 400-llm
intervals and processed for double immunohistochemical staining
using peroxidase-anti-peroxidase (PAP) for: (i) c-Fos (rabbit antiserum, 1 : 10 000, Ab-5, PC38, Oncogene Research Products, La
JolIa, CA) with DAB-Ni as chromogen and (ii) Orexin (Orexin-A;
C-19, goat antibody, 1 : 500, sc-8070, R230, Santa Cruz Biotechno10gy, Santa Cruz, CA) or MCR [MCR (E-16) goat antibody,
1 : 500, sc-14507, L281, Santa Cruz Biotechno10gy] with pink,
a1pha-naphthol pyronin B. Other series were processed for triple
immunostaining for: (i) c-Fos in the first position using DAB-Ni;
(ii) (l(IKAR (goat purified antiserum, 1: 50, sc-1475, R310,
Santa Cruz Biotechnology) or (l(2A-AR (goat purified antiserum,
1 : 50, sc-1478, K0702, Santa Cruz Biotechnology) in the second
position using Cy3-conjugated donkey anti-goat antiserum (Jackson
ImmunoResearch Laboratories, West Grove, PA) and (iii) Orx
(Orexin A, rabbit antiserum, 1 : 2000, R261-3, Phoenix Pharmaceuticals, Belmont, CA) or MCR (rabbit antiserum, 1: 5000,
Rl77-6, Phoenix Pharmaceuticals) in the third position using
Cy2-conjugated donkey anti-rabbit antiserum (Jackson). Incubations
with primary antibodies were performed at room temperature
overnight for c-Fos, Orx and MCR antibodies and two nights at
4 oC for (l(IKAR and (l(2KAR antibodies using a Tris-saline
solution (0.1 M) containing 1% normal donkey serum (NOS,
following initial blocking with 3% NDS).
Sections were viewed by light and fluorescence microscopy with
a Leica DMLB microscope equipped with an x/y/z movementsensitive stage and video camera attached to a computer. Single-,
double- and triple-immunostaining were evaluated for c-Fos, Orx or
MCR and (l(IKAR or 1X2A-AR. Single-, double- and triple-laheUed
cel!s were counted by applying stereology using the Optical
Fractionator program of Stereo Investigator (2003, MicroBrightField, Williston, VT). Cells were sampled and counted within
delineated contours at appropriate levels of a computer resident
atlas (expanded from Gritti et al., 1993). On each side, a large
contour was employed to include the nuclei in which the Orx
neurons were located [lateral hypothalamus (LR), perifornical area
(PF), and dorsomedial hypothalamus (DMR») or those in which the
MCR neurons were located [LR, PF, DMH and zona incerta (ZI»).
Partially overlapping through the hypothalamus, the Orx and MCR
cells were distributed within these contours and nuclei over
approximately 1600-1800 Ilm from anterior to posterior. Accordingly, celIs, single-, double- or triple-iabelled for c-Fos and/or
peptide (Orx or MCR) and/or receptor «(l(IA-AR or a2A-AR) were
sampled and counted bilaterally within the contours 'in three
section-levels at 400-llm intervals from anterior to posterior. Within
the stereology program, the sizes of the counting frame, within
which cells are counted, and the grid, within which the counting
frames are automatically placed, were optimized for each single-,
double- or triple-labeUed series so that a suitable number of cells
were counted per level in the deprived or recovery condition.
Accordingly, the counting frame (125 x 125 Jlffi) and grid size
(300 x 300 Jlffi or 350 x 350 Ilm) were set to sample ~13 or 17%
of the area for single-labeIled c-Fos, Orx and MCR cell counts; the
counting frame (140 x 140 Ilm) and grid size (200 x 200 Jlffi) were
set to sample 49% of the area for counting double-labeIled
c-Fos/Orx or c-FosIMCR ceUs; and the counting frame and grid
size (both at 140 x 140 Ilm) were set to sample 100% of the area
for double-labelled Orx or MCR and aIA-AR or a2A-AR cell
counts as weIl as triple-labeUed profiles with c-Fos. The cells were
counted under a 63x oil objective (with 1.4 numerical aperture).
Nuclei or cells that came into focus beneath the surface of the
section were counted within a counting block of 8 Jlffi in depth (in
the dehydrated, delipidated, mounted and coverslipped sections that
were on average 10 Jlffi thick).
Sleep and cell counts were analysed between groups and across
nuclei or regions using one- and two-way ANOVAS in Systat (vl0.2,
Richmond, CA). AlI main effects were confirmed by non-parametric
rank order tests (Kruskal-Wallis, P < 0.05) to insure that the
distribution of variance in groups (containing zeros as a result of the
experimental condition) did not distort the parametric statistics.
Figures were composed using Adobe Photoshop Creative Suite (CS)
and Adobe Illustrator CS (Adobe, San Jose, CA).
Results
Sleep deprivation and recovery
Rats in the SD group did not sleep during the 3 h in the afternoon prior
to kiIIing (at 15:00 h), whereas rats in the SR group that were allowed
to recover sleep in the afternoon after 3 h of sleep deprivation in the
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816
Orx and MCR neurons 2809
moming (09:00-12:00 h) slept ~91% of the time prior to killing (at
15:00 h). The amount of sleep differed significantly across conditions
of SD, SR and SC (Table 1). The major proportion of time for the SR
and SC groups was spent in non-REMS (77.23 ± 2.94% in SR and
68.68 ± 0.10% in SC) and a minor proportion in REMS
(13.58 ± 1.30% in SR and 7.10 ± 0.66% in SC, mean ± SEM of
total time).
c-Fos expression in Orx and MCH neurons after sleep deprivation and recovery
c-Fos was expressed in neurons of the hypothalamus including Orx,
MCR, non-Orx and non-MCR cells (Fig. lA and B). According to
ANOVA with condition (and peptide series) as factors, total numbers
of c-Fos-immunopositive (+) cells varied significantly across
TABLE 1. Average percentage sleep and numbers of Orx and MCH cells expressing c-Fos under conditions of sleep control (SC), sleep deprivation (SD) and sleep
recovery (SR)
Variable
SC
Sleep (%)
75.75
c-Fos+ cells (n)
Orx+ cells (n)
c-Fos+/Orx+ cells (n)
MCH+ cells (n)
c-Fos+/MCH+ cells (n)
2535.67 of; 424.00
4888.75 of; 536.98
25.50 of; 25.50
10906.67 of; 1120.81
25.50 of; 14.72
SD
of;
0.61
0.00
of;
0.00
11924.38 of; 1382.56
8042.00 of; 1421.88
675.75 of; 123.84
12711.75 of; 1430.25
38.25 of; 12.75
SD
comparisons
SR
(SC,SR)
90.80
(SC,SR)
5187.88 of; 1112.65
7227.25 of; 397.04
142.50 of; 39.54
13212.00 of; 592.98
382.50 of; 60.70
(SC,SR)
(SR)
of;
2.47
SR
comparisons
F (Cond)
(SC, SD)
1095.40"·
(SD)
19.57***
3.26 (ns)
20.54·"
1.21 (ns)
30.28***
(SD)
(SC, SD)
Values represent mean of; SEM for three groups of four rats (n = 12). Cell numbers were obtained from stereologica1 estimates for total numbers on left and right
sides. Values across conditions (Cond) were compared by one- or two-way ANOVAS (with condition and peptide series as factors), followed by one-way ANOVAS for
condition (with P < 0.001 indicated by ... in the table; ns, non significant) and posthoc pair-wise comparisons between conditions (using Fisher's LSD with
p < 0.05 indicated for SD and SR with respect to SC, SR or SD). The estimated total numbers of c-Fos+ cells differed across conditions in both the Orx and MCH
series and did not differ between (or in interaction with) the peptide series (df = 2, 1,2, 18) and are thus presented as the mean values for the two peptide series
(n = 24). The numbers of Orx and MCH cells did not differ across (or in interaction with) conditions but did differ between each other, Orx cells (grand
mean of; SEM, 6719 of; 622) being significantly fewerthan MCH cells (12 277 of; 649; F = 18.26, df = 1,2,9; P = 0.002). In a two-way ANOVA, the numbers of
c-Fos+/Orx+ and c-Fos+/MCH+ neurons differed significantly as a function of condition (F = 18.3; df = 2, 9; P < 0.001), differed between the two peptide series
(F = 6.56; df = l, 9; P = 0.03) and differed as a function of an interaction between condition x peptide (F = 25.62; df = 2, 9; P < 0.001). Given the significant
interaction, one-way ANOVAS were performed for each series and presented in the table along with posthoc pair-wise comparisons.
MCH
Orx
"
,-,
,
~.
f
.
'.
f
.-
FIG. 1. c-Fos expression in Orx and MCH neurons. (A) Single-Iabelled c-Fos+ cell (stained black with DAB-Ni, black arrowhead), single-Iabelled Orx+ cell
(stained pink with alpha-naphthol pyronin B, white arrowhead) and double-Iabelled c-Fos+/Orx+ cells (double arrowheads). (8) Single-Iabelled c-Fos+ cell
(stained black, black arrowhead), single-Iabelled MCH+ cells (stained pink, white arrowheads) and double-Iabelled c-Fos+/MCH+ cell (double arrowhead).
(C) Orx cell distribution in the LH, PF and DMH (stained brown with DAB). (D) MCH cell distribution in the LH, PF, DMH and ZI (stained brown). f, fornix.
Magnification bars, 20 !lm (A and B); 1 mm (C and D).
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21,2807-2816
2810 M. Modirrousta et al.
conditions (independent of peptide series) being highest in the SD
condition (Table 1). Total numbers of Orx- and MCH-immunopositive neurons did not vary significantly as a function of
condition. The Orx+ neurons, which are distributed across the
LH, PF and DMH (Figs IC and 2A) numbered on average ::::::6700
(Table 1). MCH neurons, which are distributed across LH, PF,
DMH and ZI (Figs ID and 2D) numbered on average R::12 300 and
were significantly more numerous than the Orx cells (Table 1). The
numbers of c-Fos+/Orx+ and c-Fos+/MCH+ cells varied significantly across conditions but also varied significantly as a function
of an interaction between condition and peptide (Table 1). Numbers
of c-Fos+/Orx+ cells were significantly decreased' in SR as
compared to SD (Figs 2B and C, and 3C; Table 1), whereas
numbers of c-Fos+/MCH+ cells were significantly increased in SR
as compared to SD (Figs 2E and F, and 3F; Table 1). Numbers of
c-Fos+/MCH+ cells were also significantly higher in SR as
compared to SC (Table 1). Across the SD and SR groups, the
numbers of c-Fos+/Orx+ cells were negatively correlated with the
numbers of c-Fos+/MCH+ cells (correlation coefficient for pairwise
values, r = -0.82, n = 8, P = 0.01). Relative to all c-Fos+ cells in
SD, the c-Fos+/Orx+ cells represented a small proportion (R::6%,
Figs 2B, and 3B and C). They also represented a small proportion
of the Orx cell population (R::9%, Figs 2A and B, and 3A and C).
Similarly, relative to all c-Fos+ cells in SR, the c-Fos+IMCH+ cells
represented a small proportion (R::6%, Figs 2F, and 3E and F). They
also represented a small proportion of the MCH cell population
(3%, Figs 2D and F, and 3D and F).
SO
Adrenergic receptors on Orx and MCH, including c-Fos
expressing, neurons after sleep deprivation and recovery
Immunostaining for adrenergic receptors (cxIA-AR or CX2KAR),
peptide (Orx or MCH) and c-Fos was performed to examine the
incidence of cxIA-AR or CX2A-AR on Orx and MCH cells and also on
Orx and MCH cells expressing c-Fos in the ditTerent conditions. Orx
cells were immunostained for both types of adrenergic receptors,
R:: 1100 for CXIA-AR (Fig. 4A) and R:: 1400 for <X2A-AR (Fig. 4B;
Table 2). Orx cells bearing the ditTerent ARs were distributed across
the LH, PF and DMH with no ostensible segregation (Fig. 5A and B).
Very few MCH neurons were immunostained for <xIKAR (R::160
having minimal positive staining, Fig. 4C), whereas a large number
were immunostained for <X2A-AR (R::1800, Table 2; Fig. 4D). MCH
cells immunopositive for <X2A-AR were distributed across the LH, PF,
DMH and ZI (Fig. 5D). The numbers of Orx and MCH ARimmunopositive cells ditTered significantly between the two peptide
cell types (Table 2). There was a significantly higher prevalence of
Orx+/<XIA-AR+ cells than MCH+/<xIKAR+ cells. Moreover, whereas
there was no significant ditTerence between the numbers of <XIAAR+ vs. CX2KAR+ Orx cells, there was a significant ditTerence
between the numbers of <XIA-AR+ vs. CX2A-AR+ MCH cells (Table 2).
Indeed, R::16% of Orx cells bore <XIA-AR and R::21 % bore <X2A-AR,
whereas only R::l.5% ofMCH cells bore <XIA-AR and R::15% bore CX2AAR. The numbers of AR-immunopositive Orx and MCH cells did not
vary significantly as a firnction of condition (Table 2). Analysis of the
triple-immunostaining revealed sorne c-Fos expressing Orx cells in SD
SR
Orx+cells
• c-Fos+ cells
• c-Fos+/Orx+ cells
-Orx
MCH+celis
• c-Fos+ cells
• c-Fos+/MCH+ cells
MCH
FIG. 2. Distribution of Orx and MCH neurons expressing c-Fos after SD or SR. (A) Orx cell distribution in the LH, PF and DMH (open triangles). (B and C)
Double-Iabelled c-Fos+/Orx+ cells (filled triangles) distinguished from single-labelled c-Fos+ cells (black dots) in one SD (B) and one SR (C) representa-'
tive brain. (0) MCH cell distribution in the LH, PF, DMH and ZI (open squares). (E and F) Double-Iabelled c-Fos+/MCH+ cells (filled squares) distinguished
from single-Iabelled c-Fos+ cells (black dots) in one SD (E) and one SR (F) representative brain. f, fomix; DMH, dorsomedial hypothalamus; LH, lateral
hypothalamus; PF, perifornical area; ZI, zona incerta. Maguification bar, 1 mm.
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816
Orx and MCH neurons
A
2811
B
t
t
lJ
c;;
:;,
!
+
8
'il
~
0
o
+
~
6
sc
SR
SO
SR
E
MCH
Condition
SO
SR
Condition
FIG. 3. Numbers of Orx and MCH neurons expressing c-Fos after SD or SR. (A) Total numbers ofOrx+ ceUs counted bilaterally and estimated by stereological
analysis in the hypothalamus including LH, PF and DMH. (8) Total numbers of c-Fos+ ceUs (obtained from c-Fos/Orx dual-immunostained series as the total of
single c-Fos+ and double c-Fos+/Orx+ cells) were significantly decreased in SR as compared to SD (Table 1). (C) Total numbers of c-Fos+/Orx+ ceUs were
significantly decreased in SR as compared to SD (Table 1). (0) Total numbers of MCH+ ceUs counted and estimated by stereological analysis in the hypothalamus
including LH, PF, DMH and ZI. (E) Total numbers ofc-Fos+ ceUs (obtained from c-Fos/MCH dual-immunostained series as the total of single c-Fos+ and double
c-Fos+/MCH+ cells) were significantly decreased in SR as compared to SD (Table 1). (F) Total numbers ofc-Fos+/MCH+ cells were significantly increased in SR
as compared to SD (Table 1). Note the sarne scale in A, B, D and E (0-20000) forsingle-labeUed ceUs and the smallerscale in C and F (0-1000) for double-labeUed
cells.
that bore (XIKAR and some that bore (X2A-AR (Fig. 4E and F). No
c-Fos expressing MCH neurons in SR were found to be immunopositive for (XIA-AR, whereas sorne were found to be immunopositive
for (X2A-AR (Fig. 4G).
Discussion
Parallel with a large population of neurons in the posterior hypothalamus, more Orx cells express c-Fos after sleep deprivation than after
sleep recovery, whereas more MCH ceUs express c-Fos after sleep
recovery. This reciprocal pattern of activity could be explained in part
by different adrenergic receptors and thus response to NA in the Orx
and MCH neurons.
c-Fos differentially expressed in Orx and MCH neurons as a
function of sleep
A large population ofneurons in the posterior hypothalamus expressed
c-Fos following 3 h of total sleep deprivation. As a refiection of neural
activity, the c-Fos expression thus confirms unit recordings showing
the maximal discharge rate by the vast majority of neurons in this
region to be during waking as compared to non-REMS (Steininger
et al., 1999; Alam et al., 2002; Koyama et al., 2003).
In parallel with the major population of hypothalamic neurons,
more Orx neurons also expressed c-Fos followirtg sustained waking
than following recovery or control sleep. These results confirm
microdialysis studies showing that Orx release is high in association
with waking and 10W in association with sleep (Fujiki et al., 2001;
Yoshida et al., 2001; Zeitzer et al., 2003). However, the percentage of
c-Fos+ cells that wete Orx+ in the present study was very small
(~5%) indicating that the Orx cells were not the major contributors in
this region to the maintenance of the waking state. Moreover, the
percentage of Orx+ ceUs that were c-Fos+ was also very small
(~10%), indicating that the Orx cells were not maximally activated for
the maintenance of the waking state under the present conditions. In
fact, the experimental conditions were intended to deprive rats of sleep
without stress and accordingly employed behavioural obseIVations for
sleep-wake state scoring to avoid the surgery and tethering needed for
polygraphic recording, maintained the animais in their home cages
with food and water ad libitum to avoid any alimentary deprivation
and used gentle touching with a soft brush when necessary to avoid
continuous or stressful stimulation and evoked activity during the day.
The results suggest that although other neurons in the posterior
hypothalamus are active in association with quiet waking and thus
potentially involved in maintaining cortical activation of the waking
state, the Orx neurons may only be active with more aroused or
stressful waking. In another study, it was found that Orx neurons
expressed c-Fos in large numbers during the day only in association
with high-arousal and stressful waking induced by continuous
auditory stimulation (Espana et al., 2003). Selective deprivation of
REM sleep was also found to be associated with increased c-Fos
expression in Orx neurons (Verret et al., 2003), perhaps due to stress
that may be associated with that procedure. Orx release has also been
© 2005 Federation of European Neuroscience Societies, European Journal ofNeuroscience, 21, 2807-2816
2812 M. Modirrousta et al.
FIG. 4. Adrenergic receptors (AR) on Orx and MCH neurons including those expressing c-Fos. (A) Cell immunostained for Orx (AI) and IXIA-AR (A2 and in
superimposed image in A3). (B) Cell immunostained for Orx (BI) and 1X2A-AR (B2 and B3). (C) Cell immunostained for MCH (CI) that was minima1ly stained
for IXIA"AR (C2 and C3). (D) Cells immunostained for MCH (DI) ofwhich one was also stained for 1X2A-AR (D2 and D3). (E) Labelled Orx+ cell (El) that was
IXIA-AR+ (E2) and c-Fos+ (E3). (F) Labelled Orx+ cell (FI) that was 1X2A-AR+ (F2) and c-Fos+ (F3). (G) Labelled MCH+ cell (G 1) that was 1X2A-AR+ (G2) and
c-Fos+ (G3). Peptides were stained by green fluorescence with Cy2; receptors were stained by red fluorescence with Cy3 and c-Fos by black DAB-Ni. Magnification
bar, 20 Iffil.
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816
Orx and MCH neurons
TABLE 2. Average number of Orx or MCH cens that were immunostained for
~IKAR
or
~2A-AR
under conditions of Sc., SD and SR
Variable
SC
SD
SR
Mean
Orx+/~I-AR+
1102.75 ± 122.74
1058.33 ± 456.97
1325.00 ± 450.69
1084.56 ± 179.41
1531.75 ± 297.58
1133.33 ± 475.29
1735.00 ± 761.07
1406.33 ± 290.04
213.25 ± 48.48
166.67 ± 87.00
141.67 ± 117.56
161.44 ± 44.38
1479.25 ± 170.93
2300.00 ± 478.93
1933.33 ± 147.43
1818.56 ± 196.29
Orx+/~-
AR+
MCH+htl- AR+
MCH+/~-
AR+
2813
F-value
(Condition)
F-value
(AR)
F-value
(Condition
0.29 (ns)
1.95 (ns)
0.27 (ns)
2.40 (ns)
81.64**
1.86 (ns)
X
AR)
Values represent mean ± SEM for three groups of three or four rats (n = 10). Cell numbers were obtained from stereological estimates for total numbers on left and
right sides. Values of each peptide series (Orx and MCH) were analyzed by two-way ANOVA for condition and adrenergic receptor subtype (AR). Total numbers of
Orx and MCH neurons with IXIA-AR or OI2A-AR did not vary significantly as a function of condition (ns, not significant). Whereas the number of neurons
immunostained for OIIA-AR vs. OI2A-AR did not differ for the Orx neurons, it did differ for the MCH neurons (**P < 0.01).
alAR
a2AR
'* Orx+/alAR+ cells
Orx+/a2AR+ cells
Orx
'* MCH+/alAR+ cells
MCH+/a2AR+ cells
MCH
FIG. 5. Distribution of OII-AR vs. 0I2-AR immunopositive Orx and MCH neurons. (A) Distribution of OIIA -AR+/Orx+ cens (stars) and (B) OI2A-AR+/Orx+ cens
(circles) across LH, PF and DMH. (C) Distribution of OIIA-AR+/MCH+ cens (stars) and (D) OI2A-AR+/MCH+ cells (circles) across LH, PF, DMH and ZI. For
abbreviations see Fig. 2. Magnification bar, 1 mm.
found to be maximal in association with motor activity (Kiyashchenko
et al., 2002; Zeitzer et al., 2003). Effects of intracerebroventricular
(lCV) administration of Orx and loss of function in Orx knock out
mice moreover indicate that Orx can stimulate energy metabolism
along with motor activity by positive influences upon the sympathetic
nervous system (stimulating increased heart rate, blood pressure,
temperature and thermogenesis), the hypothalamo-pituitary thyroid
axis (HPT, stimulating increased basal metabolic rate) and the
hypothalamo-pituitary adrenal axis (HPA, stimulating increased
corticosteroid levels) (Lubkin & Stricker-Krongrad, 1998; Shirasaka
et al., 1999; Ida et al., 2000; Hara et al., 2001; Espana et al., 2002;
Monda et al., 2003; Yamanaka et al., 2003). An integral role in
stimulating arousal, activity and metabolism can be mediated by
excitatory influences of Orx upon multiple central arousal systems,
including noradrenergic locus coeruleus neurons and cholinergic
brainstem and basal forebrain neurons (Horvath et al., 1999; Bayer
et al., 2001; Eggerrnann et al., 2001; Burlet et al., 2002), upon central
motor and sympathetic systems (peyron et al., 1998; van den Pol,
1999; Krout et al., 2003; Yamuy et al., 2004) and upon hypothalamic
neurons (van den Pol et al., 1998; Ferguson & Samson, 2003).
In contrast to the Orx ceUs, MCH ceUs showed more c-Fos activation
after sleep recovery than after sleep deprivation. c-Fos expression was
specifically associated with recovery sleep, when animais slept ~90%
of the time and not with normal sleep in the control condition, when
animais slept ~75% of the time. These results suggest that the c-Fos
activation is associated with the recovery process and not simply with
natural sleep. Nonetheless, the percentage of MCH neurons that
expressed c-Fos during recovery foUowing 3 h of gentle sleep
deprivation was very small « 5%). In another study examining
c-Fos expression following 72 h ofparadoxical sleep (PS) deprivation
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816
2814 M. Modirrousta et al.
upon inverted flower pots, it was found that a large proportion ofMCR
neurons (> 50%) expressed c-Fos during recovery, when animaIs slept
77% of the time, comprised by 33% SWS and 44% paradoxical sleep
(Verret et al., 2003). In our paradigm, the recovery sleep was comprised
in greatest proportion by non-REMS sleep (estimated at ~77% as
compared to 14% REMS), and the percentages ofboth non-REMS and
REMS were significantly increased above control (estimated at ~69%
and 7%, respectively). Accordingly, the increased c-Fos expression in
MCR cells could be explained by the predominance of non-REMS in
SR or by a recovery process afforded by total sleep that includes
unimpeded non-REMS and REMS along with the loss of postural
muscle tone. MCR cells may thus become activated only when there is
an increased need as well as possibility for the behavioural inactivity
and rest that is pennitted by non-REMS and REMS sleep. Based upon
evidence ofMCR administration or MCR knock out, it has been shown
that MCR decreases energy metabolism along with activity through a
negative influence upon the sympathetic nervous system, the hypothalamo-pituitary thyroid (HPT) axis and the hypothalamo-pituitary
adrenal (RPA) axis (Shimada et al., 1998; Kennedy et al., 2001; Marsh
et al;, 2002; Ito et al., 2003; Shearman et al., 2003; Zhou et al., 2005).
MCR neurons could thus play a role in opposition to Orx neurons by
decreasing activity and energy metabolism in association with sleep
through inhibition of transmission in the hypothalamus and other
arousal systems (Bittencourt et al., 1992; van den Pol et al., 1998; Gao
& van den Pol, 2001).
Adrenergic receptors differentia/ly distributed on Orx and MCH
including c-Fos expressing neurons
c-Fos expression was inversely related in Orx and MCR neurons
across the deprived and recovery conditions in the present study
suggesting a reciprocal relationship in their activity and roles. Orx and
MCR cells are partially overlapping in their distribution within the
hypothalamus and have reciprocal synaptic relationships, which could
mediate reciprocal profiles of activity (Bayer et al., 2002; Guan et al.,
2002). It is also possible that they would be under reciprocal
influences by other activating systems involved in behavioural state
and energy regulation, such as the noradrenergic systems, including
importantly the locus coeruleus neurons (Jones & Yang, 1985). It
could thus be expected that Orx neurons would bear <Xl-AR, associated
with depolarizing responses to NA as present upon cholinergic basal
forebrain neurons, whereas MCR neurons would bear <xrAR,
associated with hyperpolarizing responses to NA as present upon
GABAergic sleep-active basal forebrain neurons (Fort et al., 1995;
Fort et al., 1998; Modirrousta et al., 2004).
Many Orx neurons were found to be immunostained for the <XIAAR, although unexpectedly many were also inImunostained for the
<X2A-AR. In contrast, MCR ceUs were almost exclusively immunostained for the <X2A-AR. The difference in the incidence of <XIA-AR on
Orx vs. MCR cells explains one mechanism by which Orx neurons
may be stimulated, whereas MCR neurons would be inhibited during
waking when noradrenergic locus coeruleus neurons are active
(Aston-Jones & Bloom, 1981). The MCR neurons may become
active when released from inhibition by noradrenergic input with sleep
onset. These conclusions were recently substantiated in electrophysiological studies using slices from rat brain, in which NA was found
to have an excitatory effect upon Neurobiotin-labeUed Orx ceUs and to
have an inhibitory effect upon Neurobiotin-labeUed MCR ceUs (Bayer
et al., 2005). On the other hand, the present finding that sorne Orx
neurons bear <X2A"ARs suggests that sorne Orx ceUs may be inhibited
by NA. In fact, in transgenic mice expressing GFP in Orx neurons, NA
was found to have a hyperpolarizing effect upon the GFP-IabeUed Orx
ceUs in vitro (Li et al., 2002) and most recently it was discovered that
the excitatory effect of NA on Orx ceUs in the rat slice was
transfonned into a hyperpolarizing or biphasic effect after two hours
of sleep deprivation (Grivel et al., 2004). It is thus .possible that under
certain circumstances in mice and rats, Orx ceUs may mobilize or
express <Xz-ARs. In the present experiment, the numbers of ceUs
bearing <XlA"ARs or <X2A"ARs did not vary as a function ofsleep-wake
condition, thus indicating that mild sleep deprivation or recovery in
adult rats does not appear to alter the expression of these receptors. On
the other hand, sleep deprivation in younger rats (~15-20 days), as
employed in the in vitro studies, would be associated with stress, as
indicated by elevated corticosterone (Rairston et al., 2004), and stress
or corticosteroids have been shown to be associated with changes in
the expression of <xrARs (Jhanwar-Uniyal et al., 1986; Flugge et al.,
2003). Thus, the influence ofnoradrenergic input from locus coeruleus
or other brainstem cell groups may under nonnal conditions be
predominantly excitatory upon Orx neurons through <XI-ARs, however,
change foUowing stress to become predominàntly inhibitory upon
them through increased expression or mobilization of <XrARs. This
dual potential may aUow adaptive changes under conditions of stress,
when sleep along with energy conservation, instead of sustained
waking along with energy expenditure, could enhance survival.
ln summary, the present results show that Orx and MCH neurons
are active in a reciprocal manner during sleep deprivation and sleep
recovery such as to suggest that they play opposing roles in regulating
sleep-wake states along with activity and energy metabolism. These
opposing roles may in part be mediated by reciprocal relationships or
by differential responses to afferent inputs including importantly
noradrenergic inputs.
Acknowledgements
The research was supported by a grant from the Canadian Institutes of Health
Research (13458).
Abbreviations
AR, adrenergic receptor; DMH, dorsomedial hypothalamus; LH, lateral
hypothalamus; MCH, melanin concentrating hormone; NA, noradrenaline;
non-REMS, non-rapid eye movement sleep; Orx, orexin; PF, perifomical area;
REMS, rapid eye movement sleep; SC, sleep control; SD, sleep deprivation;
SR, sleep recovery; ZI, zona incerta.
References
Alam, M.N., Gong, H., Alam, T., Jaganath, R., McGinty, D. & Szymusiak, R.
(2002) Sleep-waking discharge patterns of neurons recorded in the rat
perifomicallateral hypothalamic area. J. Physiol. (Land.), 538, 619-631.
Aston-Jones, G. & Bloom, F.B. (1981) Activity of norepinephrlne-containing
locus coeruleus neurons in behaving rats anticipates fluctuations in the sleepwaking cycle. J. Neurosci., 1, 87<H!86.
Bayer, L., Bggermann, B., Serafin, M., Grivel, J., Machard, D., Muhlethaler, M.
& Jones, B.B. (2005) Opposite effects of noradrenaline and acetylcholine
upon hypocretin/orexin versus melanin concentrating hormone neurons in
rat hypothalamic slices. Neuroscience, 130, 807-811.
Bayer, L., Bggermann, B., Serafin, M., Saint-MIeux, B., Machard, D., Jones, B.
& Muhlethaler, M. (2001) Orexins (hypocretins) directly excite tuberomammillary neurones. Eur. J. Neurosci., 14, 1571-1575.
Bayer, L., Mairet-Coello, G., Risold, P.Y. & Griffond, B. (2002) Orexin/hypocretin neurons: chemical phenotype and possible interactions with melaninconcentrating hormone neurous. Regul. Pept., 104,33-39.
Bergmann, B.M., Winter, lB., Rosenberg, R.S. & Rechtschaffen, A. (1987)
NREM sleep with low-voltage BBG in the rat. Sleep, 10, 1-11.
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816
Orx and MeR neurons 2815
Bittencourt, J.C., Presse, F., Arias, C., Peto, C., Vaughan, l, Nahon, J.L., Vale,
W. & Sawchenko, P.E. (1992) The melanin-concentrating hormone system of
the rat brain: an immuno- and hybridization histochemical characterization.
J. Comp. Neurol., 319, 218-245.
Broberger, C., De Lecea, L., Sutcliffe, J.G. & Hokfelt, T. (1998)
Hypocretin/orexin- and melanin-concentrating hormone-expressing cells
form distinct populations in the rodent lateral hypothalamus: relationship to
the neuropeptide Y and agouti gene-related protein systems. J. Comp.
Neurol., 402, 460--474.
Burlet, S., Tyler, C.J. & Leonard, C.S. (2002) Direct and indirect excitation of
laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications
for wakefulness and narcolepsy. J. Neurosei., 22, 2862-2872.
Chemelli, R.M., Willie, J.T., Sinton, C.M., Elmquist, J.K., Scammell, T., Lee,
C., Richardson, lA., Williams, S.C., Xiong, Y., Kisanuki, Y., Fitch, T.E.,
Nakazato, M., Hammer, R.E., Saper, C.B. & Yanagisawa, M. (1999)
Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation.
Cel/, 98, 437-451.
Eggennann, E., Serafin, M., Bayer, L., Machard, D., Saint-Mieux, B., Jones,
B.E. & Muhlethaler, M. (2001) Orexins/hypocretins excite basal forebrain
cholinergie neurones. Neuroscience, 108, 177-181.
Espana, R.A, Plahn, S. & Berridge, C.W. (2002) Circadian-dependent and
circadian-independent behavioral actions of hypocretin/orexin. Brain Res.,
943, 224-236.
Espana, R.A., Valentino, R.J. & Berridge, C.W. (2003) Fos immunoreactivity in
hypocretin-synthesizing and hypocretin-I receptor-expressing neurons:
effects of diurnal and noctumal spontaneous waking, stress and hypocretin-I administration. Neuroseience, 121,201-217.
Estabrooke, LV., McCarthy, M.T., Ko, E., Chou, T.C., Chemelli, R.M.,
Yanagisawa, M., Saper, C.B. & Scammell, T.E. (2001) Fos expression in
orexin neurons varies with behavioral state. J. Neurosci., 21, 1656-1662.
Ferguson, A.V. & Samson, W.K. (2003) The orexin/hypocretin system: a
critical regulator of neuroendocrine and autonomic function. Front. Neuroendocrinol., 24, 141-150.
Flugge, G., van Kampen; M., Meyer, H. & Fuchs, E. (2003) Alpha2A and
alpha2C-adrenoceptor regulation in the brain: alpha2A changes persist after
chronic stress. Eur. J. Neurosci., 17,917-928.
Fort, P., Khateb, A., Pegna, A., Muhlethaler, M. & Jones, B.E. (1995)
Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in
the guinea pig brain. Eur. J. Neurosci., 7,1502-1511.
Fort, P., Khateb, A., Serafin, M., Muhlethaler, M. & Jones, B.E. (1998)
Pharmacological characterization and. differentiation of non-cholinergie
nucleus basalis neurons in vitro. Neuroreport, 9, 1-5.
Fujiki, N., Yoshida, Y., Ripley, B., Honda, K, Mignot, E. & Nishino, S. (2001)
Changes in CSF hypocretin-I (orexin A) leveis in rats across 24 hours and in
response to food deprivation. Neuroreport, 12, 993-997.
Gallopin, T., Fort, P., Eggennann, E., Cauli, B., Luppi, P.H., Rossier, J.,
Audinat, E., Muhlethaler, M. & Serafin, M. (2000) Identification of sleeppromoting neurons in vitro. Nature, 404, 992-995.
Gao, X.B. & van den Pol, A.N. (2001) Melanin concentrating hormone
depresses synaptic activity of glutamate and GABA neurons from rat lateral
hypothalamus. J. Physio/. (Lond.), 533, 237-252.
Gritti, L, Mainville, L. & Jones, B.E. (1993) Codistribution of GABA with
acetylcholine-synthesizing neurons in the basal forebrain of the rat. J. Comp.
Neurol., 329, 438-457.
Grivel, J., Tobler, L, Muhlethaler, M. & Serafin, M. (2004) Following sleep
deprivation the excitation of hypocretin/orexin neurons by noradrenaline
reverses to inhibition. Soc. Neurosci. Abstr., 318.8.
Guan, J.L., Uehara, K, Lu, S., Wang, Q.P., Funahashi, H., Sakurai, T.,
Yanagizawa, M. & Shioda, S. (2002) Reciprocal synaptic relationships
between orexin- and melanin-concentrating hormone-containing neurons in
the rat lateral hypothalamus: a novel circuit implicated in feeding regulation.
lnt. J. Obes. Re/at. Metab. Disord., 26, 1523-1532.
Hairston, I.S., Peyron, C., Denning, D.P., Ruby, N.F., Flores, J., Sapolsky,
R.M., Helier, H.C. & O'Hara, B.F. (2004) Sleep deprivation effects on
growth factor expression in neonatal rats: a potential role for BDNF in the
mediation of delta power. J. Neurophysio/., 91,1586-1595.
Hara, J., Beuckmann, C.T., Nambu, T., Willie, J.T., Chemelli, R.M., Sinton,
C.M., Sugiyama, F., Yagami, K., Goto, K., Yanagisawa, M. & Sakurai, T.
(2001) Genetic ablation of orexin neurons in mice results in narcolepsy,
hypophagia, and obesity. Neuron, 30, 345-354.
Horvath, T.L., Peyron, C., Diano, S., Ivanov, A., Aston-Jones, G., Kilduff, T.S.
& van den Pol, A.N. (1999) Hypocretin (orexin) activation and synaptic
innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol.,
415, 145-159.
Ida, T., Nakahara, K, Murakami, T., Hanada, R., Nakazato, M. & Murakami,
N. (2000) Possible involvement of orexin in the stress reaction in rats.
Biochem. Biophys. Res. Commun., 270,318-323.
lto, M., Gomori, A., Ishihara, A., Oda, Z., Mashiko, S., Matsushita, H.,
Yumoto, M., Sano, H., Tokita, S., Moriya, M., lwaasa, H. & Kanatani, A.
(2003) Characterization of MCH-mediated obesity in mice. Am. J. Physiol.
Endocrinol. Metab., 284, E940-E945.
Jhanwar-Uniyal, M., Roland, C.R. & Leibowitz, S.F. (1986) Diurnal rhythm of
alpha 2-noradrenergic receptors in the paraventricular nucleus and other
brain areas: relation to circulating corticosterone and feeding behavior. Life
Sei., 38, 473-482.
Jones, B.E. (2000) Basic mechanisms of sleep-wake states. In Kryger, M.H.,
Roth, T., & Dement, W.C., (Eds), Principles and Practice ofSleep Medicine.
Saunders, Philadelphia, pp. 134-154.
Jones, B.E. & Yang,.T.-Z. (1985) The efferent projections from the reticular
formation and the locus coeruleus studied by anterograde and retrograde
axonal transport in the rat. J. Comp. Neurol., 242, 56-92.
Kennedy, A.R., Todd, J.F., Stanley, S.A., Abbott, C.R., Small, C.J., Ghatei,
M.A. & Bloom, S.R. (2001) Melanin-concentrating hormone (MCH)
suppresses thyroid stimulating hormone (TSH) release, in vivo and in vitro,
via the hypothalamus and the pituitary. Endocrin%gy, 142, 3265-3268.
Kiyashchenko, L.I., Mileykovskiy, B.Y., Maidment, N., Lam, H.A., Wu, M.F.,
John, J., Peever, J. & Siegel, J.M. (2002) Release of hypocretin (orexin)
during waking and sleep states. J. Neurosci., 22, 5282-5286.
Koyama, Y., Takahashi, K., Kodama, T. & Kayama, Y. (2003) State-dependent
activity of neurons in the perifomical hypothalarnic area during sleep and
waking. Neuroscience, 119, 1209-1219.
Krout, K.E., Mettenleiter, T.C. & Loewy, A.D. (2003) Single CNS neurons link
both central motor and cardiosympathetic systems: a double-virus tracing
study. Neuroscience, 118, 853-866.
de Lecea, L., Kilduff, T.S., Peyron, C., Gao, X., Foye, P.E., Danielson, P.E.,
Fukuhara, C., Battenberg, E.L., Gautvik, V.T., Bartlett, F.S., 2nd, Frankel,
W.N., van den Pol, AN., Bloom, F.E., Gautvik, K.M. & Sutcliffe, J.G.
(1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Nat/ Acad. Sci. USA, 95, 322-327.
Li, Y., Gao, X.B., Sakurai, T. & van den Pol, A.N. (2002) Hypocretin/Orexin
excites hypocretin neuronsvia a local glutamate neuron-A potential
mechanism for orchestrating the hypothalamic arousai system. Neuron, 36,
1169-1181.
Lin, L., Faraco, J., Li, R., Kadotani, H., Rogers, W., Lin, X., Qiu, X., de Jong,
P.J., Nishino, S. & Mignot, E. (1999) The sleep disorder canine narcolepsy is
caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cel/, 98,
365-376.
Lubkin, M. & Stricker-Krongrad, A. (1998) Independent feeding and
metabolic actions of orexins in rnice. Biochem. Biophys. Res. Commun.,
253,241-245.
Maloney, K.l, Cape, E.G., Gotrnan, J. & Jones, B.E. (1997) High frequency
gamma electroencephalogram activity in association with sleep-wake states
and spontaneous behaviors in the rat Neuroscience, 76, 541-555.
Manns, LD., Lee, M.G., Modirrousta, M., Hou, Y.P. & Jones, B.E. (2003)
Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal
forebrain neurons. Eur. J. Neurosei., 18, 723-727.
Marsh, D.J., Weingarth, D.T., Novi, D.E., Chen, H.Y., Trumbauer, M.E., Chen,
AS., Guan, X.M., Jiang, M.M., Feng, Y., Camacho, R.E., Shen, Z. &
Frazier, E.G., Yu, H., Metzger, J.M., Kuca, S.J., Shearman, L.P., GopalTruter, S., MacNeil, D.J., Strack, AM., MacIntyre, D.E., Van der Ploeg, L.H.
& Qian, S. (2002) Melanin-concentrating hormone 1 receptor-deficient mice
are lean, hyperactive, and hyperphagic and have altered metabolism. Proc.
Natl Acad. Sei. USA, 99, 3240-3245.
Modirrousta, M., Mainville, L. & Jones, B.E. (2004) GABAergic neurons with
alpha2-adrenergic receptors in basal forebrain and preoptic area express
c-Fos during sleep. Neuroscience, 129,803-810.
Monda, M., Viggiano, A. & De Luca, V. (2003) Paradoxical [correction of
parodoxical] effect of orexin A: hypophagia induced by hyperthennia. Brain
Res., 961, 220-228.
Peyron, C., Faraco, J., Rogers, W., Ripley, B., Overeem, S., Charnay, Y.,
Nevsimalova, S., Aldrich, M., Reynolds, D., Albin, R., Li, R., Hungs, M.,
Pedrazzoli, M., Padigaru, M., Kucherlapati, M., Fan, J., Maki, R., Lammers,
G.J., Bouras, C., Kucherlapati, R., Nishino, S. & Mignot, E. (2000) A
mutation in a case of early onset narcolepsy and a generalized absence
of hypocretin peptides in human narcoleptic brains. Nature Med., 6,
991-997.
Peyron, C., Tighe, O.K., van den Pol, A.N., de Lecea, L., Helier, H.C.,
Sutcliffe, J.G. & Kilduff, T.S. (1998) Neurons containing hypocretin (orexin)
project to multiple neuronal systems. J. Neurosei., 18, 9996-10015.
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 2J, 2807-2816
2816 M. Modirrousta et al.
van den Pol, A.N. (1999) Hypothalamic hypocretin (orexin): robust innervation
of the spinal cord. J. Neurosci., 19, 3171-3182.
van den Pol, A.N., Gao, X.B., Obrietan, K., Kilduff, T.S. & Belousov, A.B.
(1998) Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretinlorexin. J. Neurosei., 18, 7962-7971.
Sheannan, L.P., Camacho, R.E., Sioan Stribling, D., Zhou, D., Bednarek, MA,
Hreniuk, D.L., Feighner, S.D., Tan, C.P., Howard, A.D., Van der Ploeg, L.H.,
Maclntyre, D.E., Hickey, G.1. & Strack, A.M. (2003) Chronic MCH-I
receptor modulation alters appetite, body weight and adiposity in rats. Eur. J.
Pharmacol., 475, 37-47.
Shimada, M., Tritos, N.A., Lowell, B.B., Flier, J.S. & Maratos-Flier, E. (1998)
Mice lacking melanin-concentrating hormone are hypophagic and lean.
Nature, 396, 670-674.
Shirasaka, T., Nakazato, M., Matsukura, S., Takasaki, M. & Kannan, H. (1999)
Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J.
Physiol., 277, R1780--R1785.
Steininger, T.L., Alam, M.N., Gong, H., Szymusiak, R. & McGinty, D. (1999)
Sleep-waking discharge of neurons in the posterior lateral hypothalamus of
the albino rat. Brain Res., 840, 138-147.
Thannickal, T.C., Moore, R.Y., Nienhuis, R., Ramanathan, L., Gulyani, S.,
Aldrich, M., Comford, M. & Siegel, J.M. (2000) Reduced number of
hypocretin neurons in human narcolepsy. Neuron, 27, 469-474.
Verret, L., Goutagny, R., Fort, P., Cagnon, L., Salvert, D., Leger, L., Boissard,
R., Salin, P., Peyron, C. & Luppi, P.H. (2003) A role of melaninconcentrating hormone producing neurons in the central regulation of
paradoxical sleep. BMC Neurosci., 4, 19.
Williams, G., Cai, X.1., Elliott, J.C. & Harrold, J.A. (2004) Anabolic
neuropeptides. Physiol. Behav., 81, 211-222.
Yamanaka, A., Beuckmann, C.T., Willie, J.T., Hara, J., Tsujino, N., Mieda, M.,
Tominaga, M., Yagami, K., Sugiyama, F., Goto, K., Yanagisawa, M. &
Sakurai, T. (2003) Hypothalamic orexin neurons regulate arousal according
to energy balance in mice. Neuron, 38, 701-713.
Yamuy, J., Fung, S.1., Xi, M. & Chase, M.H. (2004) Hypocretinergic control of
spinal cord motoneurons. J. Neurosci., 24, 5336-5345.
Yoshida, Y., Fujiki, N., Nakajima, T., Ripley, 8., Matsumura, H., Yoneda, H.,
Mignot, E. & Nishino, S. (2001) Fluctuation of extracellular hypocretin-I
(orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake
activities. Eur. J. Neurosci., 14, 1075-1081.
Zeitzer, J.M., Buckmaster, C.L., Parker, K.J., Hauck, C.M., Lyons, D.M. &
Mignot, E. (2003) Circadian and homeostatic regulation of hypocretin in a
primate model: implications for the consolidation of wakefulness.
J. Neurosci., 23, 3555-3560.
Zhou, D., Shen, Z., Strack, A.M., Marsh, D.1. & Shearman, L.P. (2005)
Enhanced running wheel activity ofboth Mchlr- and Pmch-deficient mice.
Regul. fept., 124, 53-63.
© 2005 Federation of European Neuroscience Societies, European Journal of Neuroscience, 21, 2807-2816