* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ARIEL LEVINE Postdoctoral Associate, The Salk Institute for
Neuroplasticity wikipedia , lookup
Bird vocalization wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Binding problem wikipedia , lookup
Axon guidance wikipedia , lookup
Neurocomputational speech processing wikipedia , lookup
Biological neuron model wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Environmental enrichment wikipedia , lookup
Multielectrode array wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Neural engineering wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural coding wikipedia , lookup
Neural oscillation wikipedia , lookup
Mirror neuron wikipedia , lookup
Metastability in the brain wikipedia , lookup
Evoked potential wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Muscle memory wikipedia , lookup
Development of the nervous system wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Embodied language processing wikipedia , lookup
Central pattern generator wikipedia , lookup
Optogenetics wikipedia , lookup
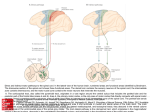
ARIEL LEVINE Postdoctoral Associate, The Salk Institute for Biological Studies, La Jolla, CA, USA EDUCATION/TRAINING INSTITUTION AND LOCATION Brandeis University, Waltham, MA Rockefeller University, New York, NY Weill Medical College of Cornell University, New York, NY The Salk Institute for Biological Studies, La Jolla, CA DEGREE YEAR FIELD OF STUDY B.S. 2000 Biology PhD 2008 Developmental Biology MD 2009 Medicine Postdoc 2009 present Neuroscience Ariel Levine is an MD/PhD postdoctoral associate in the laboratory of Dr. Samuel Pfaff at the Salk Institute, studying how the central nervous system controls movement. In particular, I am working to uncover how the neurons of the spinal cord receive motor commands from the brain and sensory systems and integrate these into functional motor outputs. Building on the expertise of the Pfaff lab and developing new strategies, I approach this question with a variety of techniques, including cutting-edge viral tracing techniques, neuronal and synaptic labeling, optogenetics, large-scale gene expression database analysis, knock-in mouse genetics, and behavioral testing. Most recently, I identified a molecularly-defined population of neurons in the medial deep dorsal horn of the spinal cord that receive direct inputs from the motor cortex and sensory pathways, and whose activation is sufficient to drive coordinated multi-joint motor activity. Ongoing research is focused on examining the strategy by which these neurons ‘encode’ for movements, how they learn to code for new movements, and the role of these neurons during behavior and following injury. Identification of a Cellular Node for Motor Control Pathways Ariel J. Levine; Christopher A. Hinckley; Kathryn L. Hilde, Shawn P. Driscoll, Samuel L. Pfaff The rich behavioral repertoire of animals is encoded within the central nervous system as a set of motorneuron activation patterns. However, the neurons that orchestrate motor programs, as well as their cellular properties and connectivity are poorly understood. We have identified a population of premotor spinal neurons that may provide the cellular basis for encoding coordinated motor output programs. These molecularly-defined “motor synergy encoder” (MSE) neurons represent a central node in neural pathways for volitional and reflexive movement. Direct optical stimulation of MSE neurons is sufficient to drive reliable patterns of activity in multiple motor groups, and we found that the evoked motor patterns vary based on the rostrocaudal location of the stimulated MSE. Thus, the spatial organization of MSE neurons may simplify the computational challenge of coordinating muscle group activity by employing a circuit structure that translates MSE neuron position into specific motor programs. In clinical cases of spinal cord injury and upper motor neuron degeneration, spinal neuronal networks are effectively isolated from descending input and voluntary movement of the body is compromised. We propose that it may be useful to directly activate MSE cells to facilitate controlled movement in patients.