* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
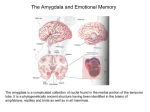
Download Plasticity-related genes in brain development and amygdala
Single-unit recording wikipedia , lookup
Neurotransmitter wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Donald O. Hebb wikipedia , lookup
State-dependent memory wikipedia , lookup
Environmental enrichment wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroanatomy wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroplasticity wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Signal transduction wikipedia , lookup
Chemical synapse wikipedia , lookup
Memory consolidation wikipedia , lookup
Nervous system network models wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Synaptogenesis wikipedia , lookup
Traumatic memories wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Emotional lateralization wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Limbic system wikipedia , lookup
Genes, Brain and Behavior (2016) 15: 125–143 doi: 10.1111/gbb.12255 Review Plasticity-related genes in brain development and amygdala-dependent learning D. E. Ehrlich†,‡,∗ and S. A. Josselyn§,¶,∗∗,†† † Department of Neuroscience and Physiology, Neuroscience Institute, NYU Langone Medical Center, ‡ Department of Otolaryngology, NYU Langone School of Medicine, New York, NY, USA, § Program in Neurosciences & Mental Health, Hospital for Sick Children, ¶ Department of Psychology, ∗∗ Institute of Medical Sciences, and †† Department of Physiology, University of Toronto, Toronto, ON, Canada *Corresponding author: D. E. Ehrlich, Department of Neuroscience and Physiology, Neuroscience Institute, NYU Langone Medical Center, New York, NY, USA. E-mail: [email protected] Learning about motivationally important stimuli involves plasticity in the amygdala, a temporal lobe structure. Amygdala-dependent learning involves a growing number of plasticity-related signaling pathways also implicated in brain development, suggesting that learning-related signaling in juveniles may simultaneously influence development. Here, we review the pleiotropic functions in nervous system development and amygdala-dependent learning of a signaling pathway that includes brain-derived neurotrophic factor (BDNF), extracellular signaling-related kinases (ERKs) and cyclic AMP-response element binding protein (CREB). Using these canonical, plasticity-related genes as an example, we discuss the intersection of learning-related and developmental plasticity in the immature amygdala, when aversive and appetitive learning may influence the developmental trajectory of amygdala function. We propose that learning-dependent activation of BDNF, ERK and CREB signaling in the immature amygdala exaggerates and accelerates neural development, promoting amygdala excitability and environmental sensitivity later in life. Keywords: Amygdala, BDNF, CREB, critical period, development, ERK, excitability, GABA, learning and memory, plasticity Received 7 July 2015, revised 12 September 2015, accepted for publication 14 September 2015 Cell signaling molecules involved in brain development have long been recognized to contribute later in life to learning and memory (Kandel & O’Dell 1992). Throughout life, neural plasticity is necessary to provide adaptive and enduring refinement of the brain and behavior. Brain structure and function must be permanently altered in the face of developmental cues, and comparable long-term alterations are thought to be the physical substrate of learning. Adaptation on such time scales is afforded by alterations of neuronal gene expression, meaning neurons require signal transduction mechanisms to relay external developmental guidance and learning cues to the cytosol and nucleus. Classic examples of signal transduction molecules that promote both developmental and learning-related plasticity include brain-derived neurotrophic factor (BDNF) and the extracellular signaling-related kinases (ERKs). Although originally described for their roles in cell proliferation, maturation and survival (Cohen-Cory et al. 2010; Lonze et al. 2002; Riccio et al. 1999), these molecules were subsequently shown to contribute to plasticity underlying learning and memory in adulthood (Poo 2001; Sweatt 2001). A wealth of literature has since identified BDNF and ERK as key mediators of the plasticity necessary for learning in the amygdala, a brain region important in mediating learning about motivationally important stimuli (Rattiner et al. 2004b; Schafe et al. 1999). More recently, additional signal transduction molecules classically implicated in brain development were found to play roles in amygdala-dependent learning; these molecules include Wnt (Maguschak & Ressler 2011), Notch (Dias et al. 2014) and CREB (cyclic AMP response element binding protein), a transcription factor acting downstream of BDNF and ERK (Josselyn 2010). Given the pleiotropic function of signal transduction pathways in both development and learning, an important question arises: to what extent does learning-induced plasticity influence ongoing development? Early in life, the amygdala encodes learning about motivational stimuli by engaging molecules that may also regulate development. When immature organisms with developing brains learn about their environments, intracellular signaling pathways that mediate this learning may also alter developmental trajectories by virtue of shared molecular pathways. More specifically, learning-dependent activation of amygdala BDNF, ERK and CREB may influence amygdala development and alter subsequent behavioral outcomes. Here, we review the diverse functions of the BDNF–ERK– CREB signaling cascade in brain development and amygdala-dependent learning. First, we describe the physiological conditions that activate these molecules and identify the pathways that link them. Next, we outline the contributions of BDNF, ERK and CREB to amygdala-dependent © 2015 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society 125 Ehrlich and Josselyn Figure 1: Intracellular signaling pathways linking BDNF, ERK, and CREB. For a detailed description of the signaling pathways linking these molecules, see text. BDNF from paracrine sources including afferent axon terminals and dendrites in target tissue, as well as autocrine pools, binds to TrkB receptors and causes receptor dimerization and subsequent downstream signaling. Autophosphorylation of TrkB at specific tyrosine residues leads to recruitment of adaptor complexes and distinct intracellular cascades. Phosphorylation of Y515 leads to Ras-ERK and PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase) signaling, while phosphorylation of Y816 leads to signaling via PLC𝛾. Through IP3 (inositol trisphosphate) activation and subsequent elevation of cytosolic calcium levels, PLC𝛾 induces signaling to the nucleus via CaM and CaM-dependent protein kinases. Synapses can also supplement TrkB-dependent signaling by activating neurotransmitter receptors and ion channels that elevate intracellular calcium. These complementary signaling pathways are each capable of phosphorylating nuclear CREB and promoting transcription of CREB target genes. AC, adenylyl cyclase; DAG, diacylglycerol; ECP, extracellular protease; Glu, glutamate; mBDNF, mature BDNF; NMDAR, N-methyl-D-aspartate receptor; PKA, protein kinase A; Rap1, Ras-related protein 1; RSK, ribosomal S6 kinase; VGCC, voltage-gated calcium channel. associative learning that occurs early in life while the amygdala is still developing. Based on the canonical roles of BDNF, ERK and CREB in neural development, we discuss the potential for crosstalk between development and learning-related plasticity in juveniles, when motivational learning may alter developmental trajectories of amygdala function. Finally, we consider the impact of juvenile learning on amygdala function and speculate on implications for behavioral outcomes and psychiatric disease. Intracellular signaling pathways linking BDNF, ERK and CREB Signal transduction pathways convey information regarding extracellular stimuli to the nucleus to regulate gene expression, enabling adaptive plasticity utilized throughout biology. These signal transduction pathways are not linear or discrete, but involve many molecules with convergent and divergent 126 interactions. The interconnectedness and overlap of signaling pathways not only provides adaptability but also affords the potential for crosstalk. While distinct extracellular stimuli give rise to cellular changes underlying neural development and memory encoding, such stimuli elicit convergent intracellular signaling through BDNF, ERK and CREB. Below, we describe the major constituents of this signaling pathway and their interactions (Fig. 1). Brain-derived neurotrophic factor secretion and downstream signaling Brain-derived neurotrophic factor is a secreted molecule capable of conveying signals to the nucleus to influence gene expression. It is tonically released from neuron somata via the ‘constitutive secretion’ pathway to provide a continuous survival signal, and phasic release from neurites can be triggered via the ‘regulated secretion’ pathway (Brigadski et al. 2005; Chen et al. 2005). Brain-derived neurotrophic factor expression and signaling tend to correlate positively with Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning neural activity (Tongiorgi et al. 1997; for review, see West et al. 2014), affording a useful signal for activity-dependent synaptic modulation. Brain-derived neurotrophic factor may be released from either axons or dendrites (Kohara et al. 2001; Kolarow et al. 2007; Matsuda et al. 2009) and act in both a paracrine and autocrine fashion (Cheng et al. 2011). In addition to mature protein, immature pro-BDNF may be released from axons and bind receptors or cleaved by extracellular proteases into the mature peptide (Gottmann et al. 2009; Lu et al. 2005; Yang et al. 2009). Brain-derived neurotrophic factor dimers bind the extracellular domain of the tropomyosin-related kinase B (TrkB) receptor, causing receptor dimerization and autophosphorylation. Subsequent binding of intracellular adaptor proteins leads to the activation of three major signaling cascades: ERK, phosphatidylinositol 3-kinase (PI3K) and phospholipase C-𝛾 (PLC𝛾) (Segal & Greenberg 1996). Autophosphorylation of TrkB receptors at specific tyrosine residues leads to distinct signaling pathways that include the ERK family of kinases and CREB (Minichiello et al. 2002). For instance, phosphorylation of TrkB at tyrosine 515 (Y515) provides a docking site for Shc (Src homology 2 domain-containing adaptor protein), which recruits the adaptor protein, Grb2 (growth factor receptor-bound protein 2) complexed with Sos (son of sevenless). Sos is an exchange factor for Ras, which sits upstream of ERK in a canonical mitogen-activated protein kinase cascade (Huang & Reichardt 2003). On the other hand, phosphorylation of TrkB at Y816 leads indirectly to nuclear CREB signaling via the calcium/calmodulin (CaM) kinase pathway (Minichiello et al. 2002). The ERK pathway Extracellular signaling-related kinases are a family of effectors for a plasticity-related intracellular signaling cascade, activated not only by BDNF but also by neurotransmitter-dependent calcium signaling (Sweatt 2001). The ERK pathway consists of three kinases linked in series via sequential phosphorylation. Ras phosphorylates a Ras-associated factor (Raf) kinase family member, c-Raf, which in turn phosphorylates the mitogen-activated protein kinase kinases, MEK1 and/or MEK2. The MEKs directly phosphorylate ERK1 and ERK2. Importantly, ERK indirectly influences transcription via the CREB signaling, and TrkB phosphorylation may induce translocation of activated ERK to the nucleus (Pizzorusso et al. 2000; Ying et al. 2002). For example, ligand-induced endocytosis of neurotrophin receptors drives activation and nuclear translocation of ERK5, which itself phosphorylates CREB (Watson et al. 2001). In addition, ERK1, ERK2 and ERK5 phosphorylate the ribosomal S6 kinase family of protein kinases that can also mediate activation of CREB (Huang & Reichardt 2003; Xing et al. 1996). Transcriptional regulation by CREB activation Cyclic AMP-response element binding protein acts as an effector of multiple signaling cascades to transduce signals from synapses to the nucleus, regulating transcription of plasticity-related genes (Deisseroth & Tsien 2002). Cyclic AMP-response element binding protein is a member of a family of basic-leucine zipper transcription factors that Genes, Brain and Behavior (2016) 15: 125–143 bind as dimers to a cAMP-response element (CRE) in the promoter of numerous genes (Shaywitz & Greenberg 1999; Sheng et al. 1991). It is canonically sensitive to activation of the cAMP-dependent protein kinase, protein kinase A (Gonzalez & Montminy 1989). Cyclic AMP-response element binding protein also serves as a point of convergence for the three major pathways activated by BDNF, and CREB is activated by a variety of extracellular signals including hormones, growth factors and synaptic activity (Finkbeiner et al. 1997; Ma et al. 2011), as well as by activity-dependent calcium influx (Kornhauser et al. 2002). For instance, TrkB receptor activation can elicit ERK signaling not only via Ras but also through PLC𝛾, which drives MEK phosphorylation through diacylglycerol pathway and the Raf kinase, b-Raf (Schinelli et al. 2001). Complementarily, calcium entry via the PLC𝛾 pathway can activate the calcium sensor CaM, which is shuttled to the nucleus by 𝛾-CaM kinase II (𝛾CaMKII) to phosphorylate the CREB kinase, CaMKIV (Ma et al. 2014). In addition, CREB is a phosphorylation target of Akt (also known as protein kinase B), which is activated by BDNF and TrkB receptors via the PI3K pathway (Du & Montminy 1998; Yamada et al. 1997). Phosphorylation of CREB at a key serine residue, Ser133, allows it to interact with transcriptional coactivators (Mayr & Montminy 2001) to promote transcription of genes enabling structural and functional plasticity of neurons (Barco et al. 2003; Bourtchuladze et al. 1994; Josselyn & Nguyen 2005; Martin & Kandel 1996; Silva et al. 1998). Cyclic AMP-response element binding protein can also activate transcription via a phosphorylation-independent pathway involving the co-factor CRTC1 (CREB-regulated transcription coactivator 1) (Sekeres et al. 2012). The BDNF–ERK–CREB cascade is not unidirectional, as ERK and CREB also provide feedback regulation of BDNF expression. Cyclic AMP-response element binding protein is thought to promote transcription of the Bdnf gene at promoter IV, which contains a CRE regulatory component (Zheng & Wang 2009). When applied to neuronal cultures, for instance, BDNF drives transcription of the Bdnf gene in a CREB-dependent fashion (Shaywitz & Greenberg 1999; Tao et al. 1998). Activity-dependent transcription of BDNF also requires ERK activity (Zheng & Wang 2009). Together, these findings suggest that BDNF, ERK and CREB work in concert to adapt neuronal gene expression and function to developmental and environmental demands. The various interactions of these signaling molecules creates a point of convergence in neural function, meaning a variety of extracellular factors can similarly influence signal transduction and stimulate neuronal plasticity. Brain-derived neurotrophic factor, ERK and CREB signaling in amygdala-dependent learning Amygdala function in learning and memory The BDNF signaling pathway has been long recognized as a regulator of synaptic strength whose experience-dependent secretion underlies learning (Kang & Schuman 1995; Korte et al. 1995; Kovalchuk et al. 2002; Minichiello 2009; Pang 127 Ehrlich and Josselyn et al. 2004; Patterson et al. 1996), and more recent work has implicated this pathway specifically in memory encoding in the amygdala (Andero et al. 2014; Leal et al. 2014; Minichiello 2009). The amygdala is a critical component of the circuit mediating Pavlovian fear conditioning (Davis & Whalen 2001; Fanselow & Gale 2003; LeDoux 2007; Maren & Quirk 2004; Pape & Pare 2010). In this paradigm, a motivationally neutral cue (such as a tone or light) is paired with a noxious stimulus (such as a footshock). Subsequent presentation of the tone or light alone elicits conditioned fear responses (typically measured by freezing behavior or fear-potentiated startle). The formation of these learned associations regarding threatening stimuli are thought to promote survival by enabling protective behavioral responses. The amygdala is required for many forms of fear conditioning, and synaptic plasticity in amygdala circuits is thought to encode these memories (Davis et al. 2003; LeDoux 2007; Pape & Pare 2010). Through fear conditioning, subsets of amygdala neurons become necessary (Han et al. 2007, 2009) and sufficient (Kim et al. 2014; Redondo et al. 2014; Yiu et al. 2014) for recall of memories for fear-inducing experiences, suggesting that this collection of neurons form a critical hub in the physical representation of associative fear learning, known as the ‘memory trace’ or ‘engram’. Much of the learning-related plasticity in the amygdala occurs in its basolateral complex, comprised of the lateral and basolateral (or basal) nuclei, which acts as a thoroughfare that receives sensory input and projects to amygdala output nuclei (Davis 1992; Fanselow & LeDoux 1999; McDonald 1998). The majority of experiments described below focused specifically on this subregion. In addition to its role in mediating fear conditioning, the amygdala, in particular its basolateral complex, is important for learning about rewarding stimuli (Baxter & Murray 2002; Costafreda et al. 2008; Hennenlotter et al. 2005; Maren 2003; Namburi et al. 2015). For instance, the amygdala is required for learning in the conditioned place preference paradigm, in which an experimental context becomes preferred via association with a rewarding drug (Everitt et al. 2003; Fuchs et al. 2002; Heldt et al. 2014; Hiroi & White 1991; LeDoux 2000). Amygdala neurons become activated during appetitive Pavlovian conditioning (Cole et al. 2013). By a similar mechanism as that observed for aversive conditioning, a subset of neurons in the amygdala also constitutes a hub in a rewarding engram (Hsiang et al. 2014). The BDNF–TrkB signaling promotes amygdala plasticity and learning Amygdala-dependent learning requires BDNF signaling (Musumeci & Minichiello 2011; for review, see Rattiner et al. 2005). Both BDNF (Conner et al. 1997; Yan et al. 1997a) and TrkB (Fryer et al. 1996; Yan et al. 1997b) are expressed highly in the amygdala, and BDNF in the amygdala is activated by fear conditioning. For instance, presentation of paired neutral and aversive stimuli, as during Pavlovian fear conditioning, elicits an increase in BDNF expression and Trk receptor activation in the amygdala 2 h later. However, unpaired presentation of these stimuli, in a configuration not capable of producing learning, does not elicit BDNF signaling (Rattiner 128 et al. 2004a). Corroborating evidence has since identified a positive correlation of amygdala BDNF expression and fear memory (Yee et al. 2007). Fear conditioning involves selective upregulation in the amygdala of two specific Bdnf transcripts, with mRNA levels for exons I and III (but not II, IV or V) showing significant elevation following training (Rattiner et al. 2004b). Importantly, Exon I of the Bdnf gene is preferentially transcribed in an activity-dependent fashion by CREB (Tabuchi et al. 2002). Brain-derived neurotrophic factor expression in the amygdala is elevated as much as 12 h after fear conditioning, and TrkB receptor blockade during this time course interferes with memory at 7 days but not 1 day after training; these data suggest protracted amygdala BDNF signaling following learning plays a role in consolidation of fear memory (Ou et al. 2010). Manipulation of BDNF expression and function interferes with several forms of amygdala-dependent learning. Intra-amygdala infusions of nonspecific Trk receptor antagonists or expression of dominant-negative, truncated TrkB protein interferes with acquisition of Pavlovian fear conditioning (Rattiner et al. 2004a). The contribution of BDNF to fear learning is likely conserved across species, as the Bdnf Val66Met single nucleotide polymorphism in humans, which reduces activity-dependent BDNF release, correlates with reduced fear learning and episodic memory (Egan et al. 2003; Lonsdorf et al. 2010). In addition, BDNF is required for the learned suppression of previously acquired fear associations known as ‘extinction’ (Chhatwal et al. 2006). Tropomyosin-related kinase B receptor agonists enhance extinction (Andero et al. 2011), and humans and rodents with the Val66Met mutation exhibit deficits in extinction learning that may be instantiated over the course of development, downstream of BDNF expression (Psotta et al. 2013; Soliman et al. 2010). Recent research has also shown a role for BDNF and TrkB activation in the amygdala during appetitive learning (Heldt et al. 2014). Brain-derived neurotrophic factor release and TrkB activation are thought to contribute to learning by enhancing synaptic plasticity in the amygdala. For instance, BDNF is required for the enduring strengthening of synapses known as ‘long-term potentiation’ (LTP) of amygdala afferents, and exogenous BDNF application reduces the stimulation threshold for LTP (Li et al. 2011). Interestingly, LTP of amygdala afferents was also blocked using an inhibitor of extracellular protease activity, suggesting conversion of synaptically released pro-BDNF to mature BDNF may be a critical step in amygdala LTP (ibid.). Blockade of postsynaptic TrkB receptors in amygdala neurons was subsequently found to prevent LTP specifically in thalamic inputs, which are thought to convey sensory representations of aversive cues (Meis et al. 2012). Extracellular signaling-related kinase is necessary for fear learning and amygdala plasticity Brain-derived neurotrophic factor activation of TrkB receptors promotes learning via activation of the ERK signaling pathway. The first evidence for a role of ERK signaling in amygdala-dependent learning was observed after blockade of fear conditioning by systemic inhibition of MEK (Atkins et al. 1998). Later studies corroborated this effect (Di Benedetto Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning (a) (b) (c) Priming Neurons for Allocation Priming Requires Elevated Excitability LA Typical Associative Memory Trace LA Neuron CREB or excitability Neuron in memory trace (d) (e) Primed Neurons Sufficient for Fear CREB & excitability (f) Primed Neurons Required for Memory New Learning Post-Erasure LA et al. 2009; Schafe et al. 2000; Tarpley et al. 2009) and identified an essential role for ERK in fear memory consolidation (Schafe et al. 1999). The ERKs are critical mediators of the effects of BDNF on fear learning (Ou & Gean 2006) and synaptic plasticity in the amygdala (Li et al. 2011), and ERK phosphorylation is increased throughout the amygdala following Pavlovian fear conditioning (Besnard et al. 2014). ERK signaling is specifically required for the late phase of LTP of synapses in the amygdala (Huang et al. 2000), potentially explaining the memory consolidation deficits caused by TrkB Y515 deletion (Minichiello et al. 2002). In addition, the ERK pathway is required for extinction of fear learning (Herry et al. 2006) and reconsolidation following fear memory reactivation (Duvarci et al. 2005). ERK signaling in the amygdala is not restricted to Pavlovian fear conditioning, as post-training infusion of an MEK inhibitor into the amygdala also prevents consolidation of inhibitory avoidance learning (Walz et al. 2000). X X X X X X X Cyclic AMP-response element binding protein shapes memory traces in the amygdala Similar to BDNF and ERK, CREB is also implicated in learning and synaptic plasticity. The first studies that identified a role for CREB in memory formation were performed in invertebrates (Dash et al. 1990; Kaang et al. 1993; Yin et al. 1994, 1995). Cyclic AMP-response element binding protein was soon after recognized as an essential contributor to synaptic plasticity and long-term memory formation in rodents, including in the amygdala for aversive learning (Bourtchuladze et al. 1994; Josselyn et al. 2001, 2004; Kogan et al. 1997). Cyclic AMP-response element binding protein may contribute to memory allocation in the mouse amygdala by promoting dendritic spine growth (Sargin et al. 2013). Cyclic AMP-response element binding protein shapes memory encoding in the amygdala by regulating competition among neurons, by virtue of varying expression levels (Fig. 2). Memory traces encoding Pavlovian fear conditioning include approximately 15% of pyramidal (excitatory) neurons in the lateral nucleus of the amygdala (LA). Importantly, LA neurons can be primed for recruitment to a memory trace through overexpression of CREB, resulting in their incorporation into memory traces at much higher rates than neighboring neurons (Han et al. 2007). During memory recall, this small subset of LA neurons becomes preferentially re-activated (ibid.) and was shown to be necessary and sufficient for recall of conditioned fear; selective ablation of this population causes specific memory erasure without disrupting other memories or subsequent learning (Han et al. 2009) and specific reactivation of this population elicits fear responses (Kim et al. 2014). This mechanism of memory allocation in the amygdala appears to be employed broadly, as amygdala neurons expressing higher levels of CREB are selectively recruited to memory traces for both aversive (Han et al. 2007, 2009; Zhou et al. 2009) and appetitive experiences (Hsiang et al. 2014). The contribution of CREB to amygdala-dependent learning is likely mediated by its actions as a transcription factor, promoting expression of plasticity-related target genes. Genes, Brain and Behavior (2016) 15: 125–143 CREB, then reactivate CREB, then ablate Neuron in new trace Figure 2: The role of CREB in memory allocation in the amygdala. (a) Approximately 15% of neurons in the LA are incorporated into memory traces for aversive and appetitive experiences (Han et al. 2007; Hsiang et al. 2014). (b) LA neurons overexpressing CREB or with artificially elevated excitability are preferentially recruited to these memory traces (Han et al. 2007, 2009; Hsiang et al. 2014; Yiu et al. 2014; Zhou et al. 2009). (c) CREB-mediated increases in amygdala neuron excitability are required for preferential memory allocation, as reducing excitability by co-expression of a potassium channel blocks the effect of CREB (Yiu et al. 2014). (d) CREB-primed neurons recruited to a memory trace are sufficient for fear expression when activated post-learning, as illustrated by co-expressing CREB with an inhibitory cation channel sensitive to an exogenous ligand (Kim et al. 2014). (e, f) CREB-overexpressing neurons in a memory trace are necessary for recall. Ablation of this population erases memories encoded when CREB levels were high (e) but does not prevent subsequent learning (f) (Han et al. 2009). The effects of CREB on memory consolidation may be mediated by transcription of Bdnf (Suzuki et al. 2011), but CREB targets also include immediate early genes, calcium-binding proteins and ion channels (Impey et al. 2004). More recently, a genomic study in the nematode identified potential memory-promoting targets of CREB that were upregulated specifically in neurons that mediate learning. These CREB downstream targets include a wide variety of intracellular signaling molecules, neurotransmitter receptor subunits, synaptic scaffolds, synaptogenic proteins, axon guidance cues and regulators of neuronal adhesion and migration (Lakhina et al. 2015). Similar classes of genes were identified as targets of CREB specifically in the amygdala, based on a transcriptomic comparison 129 Ehrlich and Josselyn from CREB-deficient transgenic and wild-type mice (Ecke et al. 2011). CREB-dependent transcription is supported by CRTC1, which also acts in the amygdala and hippocampus to promote fear memory formation (Nonaka et al. 2014; Sekeres et al. 2012). Complementary functions of ERK and CREB in amygdala-dependent learning Downstream of BDNF activation of TrkB receptors, ERK and CREB make divergent contributions to amygdala-dependent learning. As described above, phosphorylation of TrkB at distinct tyrosine residues leads to activation of ERK (Y515) and CREB (Y816) (Minichiello et al. 2002). Knock-in mice containing single mutations in the ERK signaling site of TrkB exhibit specific deficits in cued fear consolidation (Musumeci et al. 2009). In contrast, comparable mutation of the CREB signaling site of TrkB causes a distinct constellation of effects: deficits in acquisition, not consolidation, of cued fear, reduced postsynaptic calcium signaling and deficits in intra-amygdalar LTP (ibid.). Importantly, these data suggest TrkB-dependent ERK and CREB signaling act somewhat independently and are both required for proper associative memory formation in the amygdala. Amygdala excitability regulates learning and is sensitive to intracellular signaling Cyclic AMP-response element binding protein signaling indirectly contributes to learning by promoting amygdala excitability. Persistent elevation of neuronal excitability is long known to contribute to memory encoding (Byrne et al. 1991), and learning classically enhances the excitability of amygdala neurons. Fear conditioning specifically elevates the excitability of individual amygdala neurons (Rosenkranz & Grace 2002; Sehgal et al. 2014), and comparable changes were recently observed in the amygdala following reward learning (Motanis et al. 2014). Amygdala neurons more intrinsically excitable than their neighbors have a competitive advantage for recruitment to memory traces (Yiu et al. 2014). Importantly, CREB may promote memory allocation in the amygdala by enhancing amygdala neuron excitability, as CREB potently elevates the excitability of amygdala neurons (Viosca et al. 2009; Yiu et al. 2014; Zhou et al. 2009), and artificially reducing neuronal excitability in CREB-overexpressing neurons negates their competitive advantage for recruitment to fear memory traces (Yiu et al. 2014). Therefore, experimentally increasing excitability in a subset of neurons at the time of training may mimic and amplify endogenous processes that occur during normal memory encoding. During natural engram formation, amygdala neurons that happen to be more excitable at the time of training are preferentially allocated to the resulting engram (Gouty-Colomer et al. 2015), an effect predicted by in silico studies (Kim et al. 2013, 2015). Extracellular signaling-related kinase may also promote learning via effects on neural excitability. While manipulations of ERK signaling have no observable effect on cortical neuron excitability at baseline, when applied after learning, MEK inhibitors abolish the learning-induced increase in intrinsic 130 excitability. ERK signaling is therefore thought to play a role in maintaining excitability changes following learning (Cohen-Matsliah et al. 2007). Elevated excitability may in turn promote CREB-dependent transcription by enhancing depolarization-induced calcium entry via voltage-gated calcium channels and the N-methyl-D-aspartate glutamate receptor, which stimulate CaM-dependent signaling pathways that promote CREB phosphorylation (Dolmetsch et al. 2001; Ma et al. 2011). Amygdala excitability and memory encoding are gated by the inhibitory neurotransmitter 𝛾-aminobutyric acid (GABA), which in turn is modulated by BDNF, ERK and CREB. 𝛾-Aminobutyric acid is the primary source of synaptic inhibition in the vertebrate brain, and suppression of GABAergic inhibition is a common mechanism used throughout the nervous system (Froemke 2015) and specifically in the amygdala (for review, see Ehrlich et al. 2009) to elevate excitability and promote learning. GABAergic inhibition typically opposes learning and synaptic plasticity in the amygdala, and GABAergic agonists suppress the acquisition of both fear and extinction learning (Ehrlich et al. 2009), although recent evidence highlights the heterogeneous functions of GABA in the amygdala (Ryan et al. 2012; Wolff et al. 2014). Excessive excitability of the amygdala caused by loss of GABAergic tone is suggested to underlie psychiatric disorders involving excessive amygdala reactivity and memory encoding (Grace & Rosenkranz 2002; Quirk & Gehlert 2003; Rainnie et al. 2004). Indirect effects of BDNF and CREB on neural excitability are mediated by suppression of GABAergic transmission. For instance, BDNF reduces GABAergic neuron excitability and inhibitory synaptic transmission, providing net disinhibition to neural circuits (Frerking et al. 1998; Holm et al. 2009; Tanaka et al. 1997). In the amygdala, activation of TrkB receptors by BDNF can elicit GABA receptor internalization, dampening GABAergic signaling (Mou et al. 2011). In addition, BDNF knockout mice exhibit increased GABAergic synaptic transmission in the adult hippocampus. This effect is mimicked by acutely blocking BDNF signaling with scavenger proteins, suggesting BDNF constitutively suppresses GABAergic neuron excitability (Olofsdotter et al. 2000). The effects of BDNF on the GABA system may rely on CREB signaling, as activity-dependent transcription of Bdnf at promoter IV, where CREB acts to stimulate BDNF expression, plays a key role in regulating GABAergic synaptic transmission and plasticity (Sakata et al. 2009). In contrast, ERK signaling serves to promote GABA release, and excessive ERK signaling has been linked to GABA-dependent deficits in learning and synaptic plasticity (Cui et al. 2008). Temporal overlap of amygdala development and amygdala-dependent learning The juvenile amygdala participates in motivational learning Given the well-established role of BDNF–ERK–CREB signaling in amygdala-dependent learning, these molecules may contribute to the effects of early experience on amygdala Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning Week 1 2 3 With age... dendrites expand; dendritic spines emerge; excitability decreases, firing is more regular GABAergic currents are larger and faster. development. If memory is encoded at early developmental stages when signaling molecules still regulate neuron maturation, learning-induced signaling may alter ongoing development. Such an interaction may occur following amygdala-dependent learning, as Pavlovian conditioning emerges as early as infancy and maturation of the amygdala proceeds throughout childhood. Below, we address this temporal overlap by outlining the ontogeny of fear learning in relation to the trajectory of amygdala development. Several recent reviews have thoroughly outlined the maturation of associative learning, including Pavlovian fear conditioning, so we provide a brief survey (Callaghan & Richardson 2013; King et al. 2013; Landers & Sullivan 2012; Wiedenmayer 2009). In humans, associative fear learning is observed in childhood and becomes more pronounced with age (Gao et al. 2010). Infant rodents also exhibit the capacity for fear conditioning well before puberty, which occurs around postnatal day 30 (P30) in rats. When presented with an adult conspecific, infant rats as young as P12 innately respond with defensive freezing behavior and corresponding amygdala activation (Moriceau et al. 2004; Takahashi 1992). Rodents also become capable of associative fear learning at this stage (Akers et al. 2012; Vogt & Rudy 1984). Pavlovian fear conditioning elicits the mature phenotype – avoidance of the originally neutral cue – in infant rats as young as P10. However, before this age, training leads to a paradoxical approach to the cue (Sullivan et al. 2000). Fear-induced enhancement of startle responses, known as ‘fear-potentiated startle’, also emerges at various points in infancy, depending on the modality of the conditioned stimulus (Barnet & Hunt 2006; Hunt 1999). Developmental regulation of motivational learning proceeds beyond infancy, as adolescent rodents and humans exhibit temporary suppression of fear expression and extinction learning (Pattwell et al. 2011, 2012). Genes, Brain and Behavior (2016) 15: 125–143 4 Figure 3: A summary of early postnatal development of amygdala neurons. Amygdala development progresses throughout infancy and into early adolescence, with pronounced maturation of neuron structure and function. Across the first postnatal month, changes include (from top to bottom): expansion of dendritic arbors, illustrated with representative dendrite reconstructions; dendritic spine emergence from relatively aspinous dendrites at 1 week of age; reduced intrinsic excitability, with more depolarizing input required to drive action potential production, and a concurrent increase in maximal action potential frequency; more regularity of firing, with a loss of calcium-dependent burst discharges and increased synaptic strength, depicted with currents elicited by application of GABA (adapted with permission from Ehrlich et al. 2012, 2013; Ryan et al. 2014). Childhood development of amygdala structure, function and connectivity The emergence and refinement of fear learning in infancy parallels the structural and functional maturation of the amygdala. In children, the amygdala already contributes to motivationally relevant behavior, as this region is specifically activated in response to viewing faces that depict emotional states (Baird et al. 1999; Thomas et al. 2001). The amygdala is also activated during fear conditioning in human children (Monk et al. 2003). Studies on humans and nonhuman primates have shown protracted structural development of the amygdala well into childhood (Giedd et al. 1996; Payne et al. 2010). In rodents, the age at which Pavlovian avoidance emerges directly corresponds with training-induced activation of the amygdala, further suggesting that the amygdala begins to contribute to learning in infancy (Sullivan et al. 2000). Rodent research has identified maturation of amygdala neurons throughout infancy and into adolescence (Fig. 3). The amygdala emerges during gestation in rats (Berdel et al. 1997a) and undergoes volumetric changes soon after birth (Berdel et al. 1997b; Chareyron et al. 2012). As the region grows individual amygdala neurons mature, exhibiting pronounced dendritic expansion and emergence of dendritic spines between birth and adolescence (Ryan et al. 2014). Concomitant with structural maturation are pronounced electrophysiological changes in the amygdala. During infancy, amygdala neurons become an order of magnitude less excitable and lose their propensity to fire bursts of action potentials (Ehrlich et al. 2012). Synaptic transmission in the amygdala is also refined during this period (Bosch & Ehrlich 2015; Ehrlich et al. 2013), and adult-like LTP of amygdala afferents emerges during infancy when the amygdala begins to contribute to fear learning (Thompson et al. 2008). 131 Ehrlich and Josselyn Infant amygdala development also includes refinement of GABAergic function. Individual GABAergic neurons develop more widespread connectivity, as GABAergic axons increasingly collateralize with age while cell bodies decrease in density (Brummelte et al. 2007). Early in infancy, GABA in the amygdala is not inhibitory, but rather elicits excitatory responses in amygdala projection neurons (Ehrlich et al. 2013). This switch to mature GABAergic inhibition in the amygdala coincides with the emergence of LTP of amygdala synapses and the expression of fear conditioning (Sullivan et al. 2000; Thompson et al. 2008). There is a concurrent shift in the complement of GABA receptor expression in the amygdala, influencing the kinetics of GABA receptor-mediated responses (Ehrlich et al. 2013; Zhang et al. 1992). As intrinsic connectivity of the amygdala is refined, its neurons also become better integrated with distant brain regions. For instance, relative to adults, children have less functional connectivity of the amygdala with cerebral cortex, including prefrontal and association cortices (Qin et al. 2012). Prefrontal cortical and thalamic afferents in the amygdala undergo growth and subsequent pruning during infancy and adolescence (Bouwmeester et al. 2002a; Cressman et al. 2010). One indication of synapse number suggests that synaptic density increases in the amygdala threefold from infancy to adolescence (Morys et al. 1998). Amygdala projections to distant target regions also mature during this developmental window, as efferents to the prefrontal cortex become refined during infancy (Bouwmeester et al. 2002b; Verwer et al. 1996). Brain-derived neurotrophic factor, ERK and CREB regulation of neural development We propose that learning-induced BDNF–ERK–CREB signaling in juveniles alters amygdala development by exaggerating and accelerating the developmental functions of these molecules. Below, we describe the contributions of BDNF, ERK and CREB to neural development, which include promoting proliferation and survival, enhancing neurite growth, stimulating synapse and dendritic spine formation and regulating developmental synaptic plasticity. Given the breadth of developmental functions and stages under regulation of BDNF–ERK–CREB signaling, identifying specific processes and temporal windows of potential regulation will be key for determining mechanisms of crosstalk between development and learning. Signal transduction pathways for cell proliferation, differentiation and survival Early in development, BDNF, ERK and CREB promote neuron proliferation and survival. For instance, BDNF signaling at the TrkB receptor opposes apoptosis and promotes survival of developing neurons via an ERK-dependent mechanism (Hetman et al. 1999). Similarly, ERKs 1 and 2 were first identified as regulators of cell division and differentiation that are sensitive to mitogens (for review, see Sweatt 2001). Deletion of ERK early in development limits neural progenitor populations and causes precocious neurogenesis, altering 132 developmental trajectories and outcomes for neuron number (Pucilowska et al. 2012). It is now known that neurotrophin signaling activates ERK5 in axons and stimulates its translocation to the nucleus, where it acts to promote survival (Watson et al. 2001). Similar to ERK5, CREB provides an axonally-derived signal for survival, being trafficked to the soma following local translation in developing axons (Cox et al. 2008). Nearly every dividing neural cell, in neurogenic brain regions in both the embryonic and adult brain, contains activated CREB (Dworkin et al. 2007; Nakagawa et al. 2002), and loss of CREB in neural progenitor cells reduces BDNF expression and limits neuron survival and growth (Dworkin et al. 2009). CREB regulation of neural proliferation is bidirectional; while overexpression of dominant negative CREB limits neuronal proliferation, constitutively active mutant CREB causes excessive proliferation (Dworkin et al. 2007). Signal transduction pathways for neurite growth and synapse formation Brain-derived neurotrophic factor, ERK and CREB also regulate wiring of developing brain circuits. Consistent with its membership in the neurotrophin family, BDNF acts as a guidance cue for growth cones (Song et al. 1998) and potentiates transmitter release from developing axons (Zhang & Poo 2002). Axon guidance also relies on ERK signaling, as ERK1 and ERK2 mediate responses of developing axons to the guidance cue, Netrin (Forcet et al. 2002). Complementarily, BDNF helps provide sites of synaptic contact by promoting outgrowth of dendrites in developing cortical neurons (McAllister et al. 1995). The effects of BDNF on dendritogenesis may act via CREB signaling, as targeted ablation of CREB restricts dendrite growth (Herold et al. 2011). Cyclic AMP-response element binding protein may also support nascent communication by promoting proliferation and differentiation of oligodendrocytes, the myelinating glia of the central nervous system (Afshari et al. 2001; Sato-Bigbee et al. 1999). Brain-derived neurotrophic factor, ERK and CREB also regulate neuron morphogenesis, shaping dendrite patterning and the growth of dendritic spines. For instance, BDNF application to developing neurons in culture promotes spinogenesis in a neural activity- and TrkB-dependent manner (Shimada et al. 1998; Tyler & Pozzo-Miller 2003). Brain-derived neurotrophic factor acts via TrkB to enable structural plasticity, destabilizing dendrites and dendritic spines of cortical neurons (Horch et al. 1999) and elevating spine density of dendrites (Sanchez et al. 2006). Consistent with its role in signal transduction, BDNF promotes robust dendritic outgrowth from developing cortical neurons in a neural activity-dependent manner (McAllister et al. 1996), and BDNF is required for dendritic spine enlargement following paired pre- and post-synaptic stimulation at individual synapses (Tanaka et al. 2008). Such BDNF-mediated increases in dendritic spine density also require ERK signaling (Alonso et al. 2004). Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning Brain-derived neurotrophic factor, ERK and CREB regulation of developmental ‘critical periods’ Brain-derived neurotrophic factor and CREB play key roles in coordinating ‘critical periods’ of plasticity in development, when re-wiring exclusively occurs during specific developmental stages. Critical periods have been observed for several amygdala-dependent behaviors, with critical period timing likely determined by amygdala plasticity. For example, amygdala development is thought to trigger critical period closure for the paradoxical appetitive conditioning to aversive stimuli observed in infancy (Sullivan et al. 2000; Thompson et al. 2008). In addition, although extinction training typically suppresses the original association and permits recovery of fear, extinction learning in infancy occurs by a distinct mechanism that causes memory erasure (Kim & Richardson 2007, 2008). Despite the robustness and stereotyped timing of these transitions, a major knowledge gap remains regarding the contribution of signal transduction pathways to critical period plasticity in the amygdala. Insight on the potential role of BDNF, ERK and CREB in amygdala critical period plasticity must therefore proceed from evidence gleaned from other developing neural circuits. Molecular regulation of critical period plasticity has been elucidated largely through work focused on the critical period for ocular dominance (OD) plasticity in the visual cortex. Selectively during this developmental critical period, visual experience can shift the balance of visual cortical sensitivity to sensory input from each eye. Early in life, blockade of input from one eye, termed ‘monocular deprivation’, leads to synaptic remodeling that affords heightened sensitivity to visual input from the unaffected eye at the expense of sensitivity to the deprived eye (for review, see Hensch 2005). It is now known that BDNF and CREB play a key role in establishing the critical period for OD plasticity. For instance, overexpression of BDNF in transgenic mice leads to precocious closure of the critical period, suggesting BDNF–TrkB signaling promotes maturation in these circuits (Hanover et al. 1999; Huang et al. 1999). In support of this notion, while sensory deprivation typically delays visual cortical development and critical period onset, overexpression of BDNF blocks such effects (Gianfranceschi et al. 2003). Trk receptor activation is reduced in visual cortex by sensory deprivation, which may thereby suppress BDNF-dependent maturation (Viegi et al. 2002). As a potential mechanism for BDNF-induced critical period closure, BDNF prevents synaptic plasticity typically observed in immature visual cortical neurons in response to low-frequency electrical stimulation (Kinoshita et al. 1999). On the other hand, infusion of either BDNF or a TrkB antagonist into primary visual cortex of developing cats blocks OD plasticity near the infusion site, suggesting optimal levels of BDNF are necessary to enable critical period plasticity (Cabelli et al. 1995, 1997). Optimal expression of plasticity-related genes may not simply promote brain development but may actively oppose critical period plasticity in adulthood, as expression of constitutively active CREB can reintroduce OD plasticity to the adult visual cortex (Pham et al. 2004). Brain-derived neurotrophic factor, ERK and CREB effects on critical period timing may occur indirectly via effects on neuronal excitability, allowing for potential interaction Genes, Brain and Behavior (2016) 15: 125–143 with learning-induced changes to excitability. Extracellular signaling-related kinase and CREB are thought to promote learning by elevating neuron excitability (Cohen-Matsliah et al. 2007; Yiu et al. 2014), and increasing neuronal excitability accelerates critical period closure. Changes in visual cortical representation during the critical period for OD plasticity are mediated by changes to intrinsic neuronal excitability (Lambo & Turrigiano 2013; Nataraj et al. 2010), and intrinsic excitability typically increases during closure of the critical period for sensory map formation in barrel cortex. Importantly, sensory deprivation delays the maturation of neuronal excitability and prolongs critical period plasticity for sensory map formation (Maravall et al. 2004). Elevating intrinsic excitability may promote critical period closure by negatively regulating signal transduction pathways; in immature hippocampal neurons, BDNF–TrkB–ERK signaling typically promotes synapse maturation, but elevating the excitability of cultured hippocampal cells blocks the capacity of BDNF and ERK to stimulate synapse development (Suzuki et al. 2005). Brain-derived neurotrophic factor, ERK and CREB accelerate GABAergic development Brain-derived neurotrophic factor, ERK and CREB indirectly regulate development, including critical period timing, by affecting GABAergic function and maturation. 𝛾-Aminobutyric acid is implicated in a variety of developmental processes including cell proliferation, migration and differentiation, synapse maturation and stabilization and the wiring of neural networks (Huang & Scheiffele 2008; Le Magueresse & Monyer 2013; Owens & Kriegstein 2002). 𝛾-Aminobutyric acid receptor activation is necessary and sufficient to close critical period plasticity (for review, see Hensch 2005), and transplantation of immature GABAergic neurons reopens critical period plasticity in adulthood, suggesting immature GABAergic neurons imbue circuits with plasticity during development (Southwell et al. 2010). Furthermore, GABAergic transmission is typically modulated at critical period onset, and appropriate levels of inhibition are required to enable critical period plasticity (Katagiri et al. 2007; Toyoizumi et al. 2013); given that levels of excitatory synaptic transmission are largely stable during development, GABAergic synaptic structure and strength must undergo experience-dependent regulation to balance excitation and inhibition (Dorrn et al. 2010; Zhang et al. 2011). While adult BDNF–ERK–CREB signaling directly influences GABAergic transmission, during development this pathway accelerates GABA circuit maturation (Huang et al. 1999). Chronic BDNF application to immature hippocampal cultures facilitates GABA release and increases expression of GABA receptors and the GABA-synthesizing enzyme, glutamic acid decarboxylase (Yamada et al. 2002). Brain-derived neurotrophic factor is required for dendritic expansion of GABAergic neurons as they develop in culture (Kohara et al. 2003; Jin et al. 2003). Furthermore, BDNF application to developing cultures elevates expression of parvalbumin, a marker for a subtype of GABAergic interneurons that emerges during critical period closure, in a TrkB- and ERK-dependent manner (Patz et al. 2004). In isolated cultures of parvalbumin-expressing GABAergic neurons, BDNF 133 Ehrlich and Josselyn application promotes cellular growth and mature spike patterning while elevating expression of markers for GABAergic synapses (Berghuis et al. 2004). Cyclic AMP-response element binding protein promotes GABAergic circuit development indirectly via Bdnf transcription. Interfering with Bdnf expression by manipulating promoter IV, one of the two sites at which CREB acts to increase transcription, or directly influencing CREB binding to this promoter, reduces the density of GABAergic interneurons and the number, strength and release rates for GABAergic synapses (Hong et al. 2008; Sakata et al. 2009). Cyclic AMP-response element binding protein activity at Bdnf promoter IV is also required for reorganization of GABAergic circuitry in the developing cortex following sensory deprivation (Jiao et al. 2011). Interestingly, while BDNF–ERK–CREB signaling promotes GABAergic maturation, neurotransmission provides positive feedback for this signal transduction pathway. Specifically, in immature neurons when GABA is excitatory, GABA release promotes ERK activation, downstream CREB phosphorylation, and Bdnf transcription (Fukuchi et al. 2014; Obrietan et al. 2002). Excitatory GABA in the immature brain is also capable of stimulating release of BDNF protein (Fiorentino et al. 2009). In turn, BDNF can provide further feedback, promoting GABA release by increasing the excitability of GABAergic neurons and preventing GABA receptor endocytosis (Obrietan et al. 2002; Porcher et al. 2011). Effects of learning-dependent intracellular signaling on amygdala development and behavioral outcomes Given the myriad contributions of BDNF, ERK and CREB to neural development, dysregulation of their activity during development may influence brain function later in life. A wealth of studies described above have shown that BDNF–ERK–CREB signaling during development influences structural and functional outcomes including cell density, neuron morphology, synaptic connectivity, neuron excitability and GABAergic neurotransmission. Based on this complement of developmental functions, we predict how learning-induced activation of BDNF, ERK and CREB in the juvenile amygdala may influence the maturation of its neurons. Furthermore, we relate these proposed effects to clinical and preclinical observations of adverse consequences of early motivational learning. Proposed effects of excessive BDNF–ERK–CREB signaling in the developing amygdala We hypothesize that learning-related activation of BDNF, ERK and CREB in the immature amygdala exaggerates and accelerates amygdala development (Fig. 4). Intracellular signaling typically promotes development and wiring of the immature amygdala, and motivationally relevant experiences that elicit amygdala-dependent learning may cause excessive signaling. Specific predictions about the consequences of excessive signaling follow from the typical function of these molecules in development. 134 Brain-derived neurotrophic factor, ERK and CREB promote neuron proliferation and survival (Cohen-Cory et al. 2010; Riccio et al. 1999), and excessive signaling in the developing amygdala may increase amygdala volume and neuron density. Amygdala volume and neuron density continue to increase into childhood (Giedd et al. 1996; Morys et al. 1999; Payne et al. 2010), suggesting that the proliferative and pro-survival effects of the BDNF–ERK–CREB pathway could act in childhood to enlarge amygdala volume. Increased volume of the amygdala may result in greater amygdala activation and downstream signaling, potentially yielding exaggerated motivational learning. In support of this notion, stress exposure in juveniles, which likely stimulates BDNF–ERK–CREB signaling, results in greater amygdala volume and emotional dysfunction in humans and non-human primates (Howell et al. 2014; Tottenham et al. 2010). Brain-derived neurotrophic factor, ERK and CREB promote dendrite expansion and spinogenesis (Alonso et al. 2004; Herold et al. 2011; McAllister et al. 1995), and excessive activation of this pathway may alter amygdala neuron morphology. Dendrite growth and spine emergence proceed into early adolescence in the amygdala (Ryan et al. 2014). In juveniles, amygdala-dependent learning and BDNF–ERK–CREB signaling may cause enlarged or more spine-dense dendrites later in life. As dendrites constitute the major site for synaptic input onto amygdala neurons, dendritic expansion may result in greater synaptic input to amygdala neurons and more capacity for learning related to motivationally relevant stimuli. Interestingly, stress exposure during adolescence causes enlargement of amygdala neuron dendritic arbors (Eiland et al. 2012), which has been suggested to mediate some adverse behavioral effects of chronic stress (McEwen & Chattarji 2004). Signal transduction by BDNF, ERK and CREB promotes neural circuit maturation and triggers critical period closure, and learning-dependent activation of this pathway may restrict the formation of new synaptic connections in the amygdala. Brain-derived neurotrophic factor signaling is directly linked to critical period closure (Hanover et al. 1999; Huang et al. 1999), and BDNF–ERK–CREB effects to elevate neural excitability and stimulate GABAergic circuit development may indirectly promote critical period closure (Hensch 2005; Huang et al. 1999). In the cortex, critical period plasticity enables drastic remodeling of connectivity; amygdala remodeling also occurs in adolescence, as afferent and efferent synapses with neurons in the prefrontal cortex are formed (Bouwmeester et al. 2002a, 2002b; Cressman et al. 2010; Verwer et al. 1996). If critical period plasticity were prematurely closed in the amygdala before the establishment of connections with the prefrontal cortex, amygdala neurons may be less sensitive to arriving cortical axons and form fewer synapses. Given that the prefrontal cortex provides top–down inhibition of the amygdala, excessive amygdala activation and behavioral reactivity may result from deficits in amygdala–prefrontal cortical connectivity (Callaghan et al. 2014; Casey et al. 2008; Correll et al. 2005). Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning Developing Amygdala F proliferation & survival BDN motivationally relevant experience dendritogenesis ERK greater amygdala volume synaptogenesis GABA circuit maturation CREB elevated excitability memory encoding enlarged dendritic arbors Key: preferential connection with early synaptic w partners enhanced motivational learning diminished top-down regulation of emotion critical period closure developmental function amygdala outcome behavioral impact Figure 4: Predicted effects of learning-induced BDNF–ERK–CREB signaling on amygdala development. Learning about motivationally relevant experience is encoded in the amygdala via BDNF–ERK–CREB signaling. Given the additional contributions of BDNF, ERK and CREB to neural development, learning-induced signaling in juveniles may exaggerate or accelerate typical development of the amygdala. The BDNF–ERK–CREB pathway promotes neuron proliferation and survival, so learning-dependent signaling may promote larger amygdala volume later in life. This pathway also promotes dendritic arborization, so learning-dependent signaling may cause excessive expansion of dendritic arbors. In addition, by promoting synaptogenesis and premature closure of critical period plasticity, juvenile BDNF–ERK–CREB signaling may bias amygdala connectivity toward early over late-developing synaptic partners. Motivational learning and intracellular signaling in juveniles promote risk for psychiatric disease Given the prominent role for BDNF, ERK and CREB in brain development, early associative learning that activates this pathway in the amygdala may perturb trajectories of development and behavioral outcomes. A wealth of literature has identified effects of motivational learning during infancy on amygdala development. For instance, cued fear conditioning in infant rat pups at P8 causes deficits in fear learning and amygdala activation later in life (Moriceau et al. 2009). On the other hand, contextual fear conditioning later in infancy, at P17, causes subsequent lifelong enhancement of fear learning (Quinn et al. 2014). In addition, when an expected reward is withheld from pups at P10, the amygdala is acutely activated and those same subjects in adulthood exhibit enhanced fear learning (Stamatakis et al. 2013). Models of early-life stress have also identified long-term changes to amygdala structure and function (Ehrlich & Rainnie 2015; Ehrlich et al. 2015; for review, see Tottenham & Sheridan 2009) that may emerge because of the altered critical period timing (Callaghan et al. 2013, 2014). However, no study to date has established BDNF, ERK or CREB as a mediator of the effects of early experience on amygdala development or emotional outcomes. Genes, Brain and Behavior (2016) 15: 125–143 Intracellular signaling pathways may provide a means of direct interaction between genetic and environmental risk for psychiatric illness. Owing to its breadth of function, this signaling pathway constitutes a point of vulnerability to insult for the developing brain. Aberrant expression of BDNF, ERK and CREB has been linked to numerous psychiatric disorders, including autism spectrum disorders (Almeida et al. 2014; Castren & Castren 2014; Correia et al. 2010; Kalkman 2012; Yin et al. 2014), mood disorders (Geller et al. 2004; Kerner et al. 2013; Strauss et al. 2004) and schizophrenia (Chiaruttini et al. 2009; Green et al. 2011; Ho et al. 2007; Kawanishi et al. 1999; however, see Crisafulli et al. 2012). Given the sensitivity of BDNF–ERK–CREB signaling to environmental factors, this signaling pathway may provide a substrate through which gene-by-environment interactions can act on genetic predisposition for illness. Defining the consequences of motivational learning on intracellular signaling in the developing amygdala therefore holds promise for identifying the sequelae underlying psychiatric disorder pathogenesis. Conclusions Brain-derived neurotrophic factor, ERK and CREB work in concert to promote plasticity by transducing extracellular signals 135 Ehrlich and Josselyn to the nucleus to regulate gene expression. This intracellular signaling pathway underlies plasticity essential not only for neural development but also for learning. Infants learn associations regarding motivationally relevant stimuli utilizing the amygdala, suggesting that learning-related signaling by the BDNF–ERK–CREB pathway may perturb ongoing amygdala development. We suggest that amygdala-dependent learning may exaggerate and accelerate amygdala development, negatively affecting later amygdala function and motivational behavior. More specifically, we suggest that accelerated closure of critical period plasticity in the amygdala may diminish connectivity with late-developing inputs that inhibit amygdala reactivity. If adverse behavioral consequences of early life experience are instantiated throughout development as amygdala connections form, promoting critical period plasticity in the precocious amygdala may provide a novel avenue for intervention. However, several key knowledge gaps remain; a critical next step in this line of inquiry will be to determine the contribution of intracellular signaling cascades to learning-related plasticity in the immature amygdala. Given the potency of early life experience in regulating behavioral outcomes, we predict that BDNF–ERK–CREB signaling in the immature amygdala is sensitive to experiential factors, but this notion remains largely unexplored. References Afshari, F.S., Chu, A.K. & Sato-Bigbee, C. (2001) Effect of cyclic AMP on the expression of myelin basic protein species and myelin proteolipid protein in committed oligodendrocytes: differential involvement of the transcription factor CREB. J Neurosci Res 66, 37–45. Akers, K.G., Arruda-Carvalho, M., Josselyn, S.A. & Frankland, P.W. (2012) Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn Mem 19, 598–604. Almeida, L.E., Roby, C.D. & Krueger, B.K. (2014) Increased BDNF expression in fetal brain in the valproic acid model of autism. Mol Cell Neurosci 59, 57–62. Alonso, M., Medina, J.H. & Pozzo-Miller, L. (2004) ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem 11, 172–178. Andero, R., Heldt, S.A., Ye, K., Liu, X., Armario, A. & Ressler, K.J. (2011) Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry 168, 163–172. Andero, R., Choi, D.C. & Ressler, K.J. (2014) BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122, 169–192. Atkins, C.M., Selcher, J.C., Petraitis, J.J., Trzaskos, J.M. & Sweatt, J.D. (1998) The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1, 602–609. Baird, A.A., Gruber, S.A., Fein, D.A., Maas, L.C., Steingard, R.J., Renshaw, P.F., Cohen, B.M. & Yurgelun-Todd, D.A. (1999) Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry 38, 195–199. Barco, A., Pittenger, C. & Kandel, E.R. (2003) CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets 7, 101–114. Barnet, R.C. & Hunt, P.S. (2006) The expression of fear-potentiated startle during development: integration of learning and response systems. Behav Neurosci 120, 861–872. Baxter, M.G. & Murray, E.A. (2002) The amygdala and reward. Nat Rev Neurosci 3, 563–573. 136 Berdel, B., Morys, J. & Maciejewska, B. (1997a) Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci 15, 755–765. Berdel, B., Morys, J., Maciejewska, B. & Dziewiatkowski, J. (1997b) Volume and topographical changes of the basolateral complex during the development of the rat’s amygdaloid body. Folia Morphol 56, 1–11. Berghuis, P., Dobszay, M.B., Sousa, K.M., Schulte, G., Mager, P.P., Hartig, W., Gorcs, T.J., Zilberter, Y., Ernfors, P. & Harkany, T. (2004) Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking GABAergic interneurons. Eur J Neurosci 20, 1290–1306. Besnard, A., Laroche, S. & Caboche, J. (2014) Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Struct Funct 219, 415–430. Bosch, D. & Ehrlich, I. (2015) Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. J Physiol. Advance online publication. doi:10.1113/JP270645. Bourtchuladze, R., Frenguelli, B., Blendy, J., Cioffi, D., Schutz, G. & Silva, A.J. (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79, 59–68. Bouwmeester, H., Smits, K. & Van Ree, J.M. (2002a) Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol 450, 241–255. Bouwmeester, H., Wolterink, G. & van Ree, J.M. (2002b) Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol 442, 239–249. Brigadski, T., Hartmann, M. & Lessmann, V. (2005) Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci 25, 7601–7614. Brummelte, S., Witte, V. & Teuchert-Noodt, G. (2007) Postnatal development of GABA and calbindin cells and fibers in the prefrontal cortex and basolateral amygdala of gerbils (Meriones unguiculatus). Int J Dev Neurosci 25, 191–200. Byrne, J.H., Baxter, D.A., Buonomano, D.V., Cleary, L.J., Eskin, A., Goldsmith, J.R., McClendon, E., Nazif, F.A., Noel, F. & Scholz, K.P. (1991) Neural and molecular bases of nonassociative and associative learning in Aplysia. Ann N Y Acad Sci 627, 124–149. Cabelli, R.J., Hohn, A. & Shatz, C.J. (1995) Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science 267, 1662–1666. Cabelli, R.J., Shelton, D.L., Segal, R.A. & Shatz, C.J. (1997) Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron 19, 63–76. Callaghan, B.L. & Richardson, R. (2013) Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord 3, 8. Callaghan, B.L., Graham, B.M., Li, S. & Richardson, R. (2013) From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front Psychiatry 4, 90. Callaghan, B.L., Sullivan, R.M., Howell, B. & Tottenham, N. (2014) The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Dev Psychobiol 56, 1635–1650. Casey, B.J., Jones, R.M. & Hare, T.A. (2008) The adolescent brain. Ann N Y Acad Sci 1124, 111–126. Castren, M.L. & Castren, E. (2014) BDNF in fragile X syndrome. Neuropharmacology 76, 729–736. Chareyron, L.J., Lavenex, P.B. & Lavenex, P. (2012) Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol 520, 3745–3763. Chen, Z.Y., Ieraci, A., Teng, H., Dall, H., Meng, C.X., Herrera, D.G., Nykjaer, A., Hempstead, B.L. & Lee, F.S. (2005) Sortilin controls Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25, 6156–6166. Cheng, P.L., Song, A.H., Wong, Y.H., Wang, S., Zhang, X. & Poo, M.M. (2011) Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A 108, 18430–18435. Chhatwal, J.P., Stanek-Rattiner, L., Davis, M. & Ressler, K.J. (2006) Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci 9, 870–872. Chiaruttini, C., Vicario, A., Li, Z., Baj, G., Braiuca, P., Wu, Y., Lee, F.S., Gardossi, L., Baraban, J.M. & Tongiorgi, E. (2009) Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A 106, 16481–16486. Cohen-Cory, S., Kidane, A.H., Shirkey, N.J., & Marshak, S. (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70, 271–288. Cohen-Matsliah, S.I., Brosh, I., Rosenblum, K. & Barkai, E. (2007) A novel role for extracellular signal-regulated kinase in maintaining long-term memory-relevant excitability changes. J Neurosci 27, 12584–12589. Cole, S., Powell, D.J. & Petrovich, G.D. (2013) Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learn Mem 20, 295–299. Conner, J.M., Lauterborn, J.C., Yan, Q., Gall, C.M. & Varon, S. (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17, 2295–2313. Correia, C.T., Coutinho, A.M., Sequeira, A.F., Sousa, I.G., Lourenco Venda, L., Almeida, J.P., Abreu, R.L., Lobo, C., Miguel, T.S., Conroy, J., Cochrane, L., Gallagher, L., Gill, M., Ennis, S., Oliveira, G.G. & Vicente, A.M. (2010) Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav 9, 841–848. Correll, C.M., Rosenkranz, J.A. & Grace, A.A. (2005) Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry 58, 382–391. Costafreda, S.G., Brammer, M.J., David, A.S. & Fu, C.H. (2008) Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58, 57–70. Cox, L.J., Hengst, U., Gurskaya, N.G., Lukyanov, K.A. & Jaffrey, S.R. (2008) Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol 10, 149–159. Cressman, V.L., Balaban, J., Steinfeld, S., Shemyakin, A., Graham, P., Parisot, N. & Moore, H. (2010) Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol 518, 2693–2709. Crisafulli, C., Chiesa, A., Han, C., Lee, S.J., Shim, D.S., Balzarro, B., Andrisano, C., Sidoti, A., Patkar, A.A., Pae, C.U. & Serretti, A. (2012) Possible influence of CREB1, CREBBP and CREM variants on diagnosis and treatment outcome in patients with schizophrenia. Neurosci Lett 508, 37–41. Cui, Y., Costa, R.M., Murphy, G.G., Elgersma, Y., Zhu, Y., Gutmann, D.H., Parada, L.F., Mody, I. & Silva, A.J. (2008) Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560. Dash, P.K., Hochner, B. & Kandel, E.R. (1990) Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345, 718–721. Davis, M. (1992) The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15, 353–375. Davis, M. & Whalen, P.J. (2001) The amygdala: vigilance and emotion. Mol Psychiatry 6, 13–34. Davis, M., Walker, D.L. & Myers, K.M. (2003) Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci 985, 218–232. Deisseroth, K. & Tsien, R.W. (2002) Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron 34, 179–182. Genes, Brain and Behavior (2016) 15: 125–143 Di Benedetto, B., Kallnik, M., Weisenhorn, D.M., Falls, W.A., Wurst, W. & Holter, S.M. (2009) Activation of ERK/MAPK in the lateral amygdala of the mouse is required for acquisition of a fear-potentiated startle response. Neuropsychopharmacology 34, 356–366. Dias, B.G., Goodman, J.V., Ahluwalia, R., Easton, A.E., Andero, R. & Ressler, K.J. (2014) Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron 83, 906–918. Dolmetsch, R.E., Pajvani, U., Fife, K., Spotts, J.M. & Greenberg, M.E. (2001) Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294, 333–339. Dorrn, A.L., Yuan, K., Barker, A.J., Schreiner, C.E. & Froemke, R.C. (2010) Developmental sensory experience balances cortical excitation and inhibition. Nature 465, 932–936. Du, K. & Montminy, M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273, 32377–32379. Duvarci, S., Nader, K. & LeDoux, J.E. (2005) Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 21, 283–289. Dworkin, S., Heath, J.K., deJong-Curtain, T.A., Hogan, B.M., Lieschke, G.J., Malaterre, J., Ramsay, R.G. & Mantamadiotis, T. (2007) CREB activity modulates neural cell proliferation, midbrain-hindbrain organization and patterning in zebrafish. Dev Biol 307, 127–141. Dworkin, S., Malaterre, J., Hollande, F., Darcy, P.K., Ramsay, R.G. & Mantamadiotis, T. (2009) cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 27, 1347–1357. Ecke, L.E., Cleck, J.N., White, P., Schug, J., Mifflin, L. & Blendy, J.A. (2011) CREB-mediated alterations in the amygdala transcriptome: coordinated regulation of immune response genes following cocaine. Int J Neuropsychopharmacol 14, 1111–1126. Egan, M.F., Kojima, M., Callicott, J.H., Goldberg, T.E., Kolachana, B.S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., Lu, B. & Weinberger, D.R. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269. Ehrlich, D.E. & Rainnie, D.G. (2015) Prenatal stress alters the development of socioemotional behavior and amygdala neuron excitability in rats. Neuropsychopharmacology 40, 2135–2145. Ehrlich, I., Humeau, Y., Grenier, F., Ciocchi, S., Herry, C. & Luthi, A. (2009) Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. Ehrlich, D.E., Ryan, S.J. & Rainnie, D.G. (2012) Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol 590, 4819–4838. Ehrlich, D.E., Ryan, S.J., Hazra, R., Guo, J.D. & Rainnie, D.G. (2013) Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol 110, 926–941. Ehrlich, D.E., Neigh, G.N., Bourke, C.H., Nemeth, C.L., Hazra, R., Ryan, S.J., Rowson, S., Jairam, N., Sholar, C.A., Rainnie, D.G., Stowe, Z.N. & Owens, M.J. (2015) Prenatal stress, regardless of concurrent escitalopram treatment, alters behavior and amygdala gene expression of adolescent female rats. Neuropharmacology 97, 251–258. Eiland, L., Ramroop, J., Hill, M.N., Manley, J. & McEwen, B.S. (2012) Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37, 39–47. Everitt, B.J., Cardinal, R.N., Parkinson, J.A. & Robbins, T.W. (2003) Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci 985, 233–250. Fanselow, M.S. & Gale, G.D. (2003) The amygdala, fear, and memory. Ann N Y Acad Sci 985, 125–134. Fanselow, M.S. & LeDoux, J.E. (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232. 137 Ehrlich and Josselyn Finkbeiner, S., Tavazoie, S.F., Maloratsky, A., Jacobs, K.M., Harris, K.M. & Greenberg, M.E. (1997) CREB: a major mediator of neuronal neurotrophin responses. Neuron 19, 1031–1047. Fiorentino, H., Kuczewski, N., Diabira, D., Ferrand, N., Pangalos, M.N., Porcher, C. & Gaiarsa, J.L. (2009) GABA(B) receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J Neurosci 29, 11650–11661. Forcet, C., Stein, E., Pays, L., Corset, V., Llambi, F., Tessier-Lavigne, M. & Mehlen, P. (2002) Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 417, 443–447. Frerking, M., Malenka, R.C. & Nicoll, R.A. (1998) Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol 80, 3383–3386. Froemke, R.C. (2015) Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci 38, 195–219. Fryer, R.H., Kaplan, D.R., Feinstein, S.C., Radeke, M.J., Grayson, D.R. & Kromer, L.F. (1996) Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol 374, 21–40. Fuchs, R.A., Weber, S.M., Rice, H.J. & Neisewander, J.L. (2002) Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res 929, 15–25. Fukuchi, M., Kirikoshi, Y., Mori, A., Eda, R., Ihara, D., Takasaki, I., Tabuchi, A. & Tsuda, M. (2014) Excitatory GABA induces BDNF transcription via CRTC1 and phosphorylated CREB-related pathways in immature cortical cells. J Neurochem 131, 134–146. Gao, Y., Raine, A., Venables, P.H., Dawson, M.E. & Mednick, S.A. (2010) The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev Sci 13, 201–212. Geller, B., Badner, J.A., Tillman, R., Christian, S.L., Bolhofner, K. & Cook, E.H. Jr. (2004) Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 161, 1698–1700. Gianfranceschi, L., Siciliano, R., Walls, J., Morales, B., Kirkwood, A., Huang, Z.J., Tonegawa, S. & Maffei, L. (2003) Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A 100, 12486–12491. Giedd, J.N., Vaituzis, A.C., Hamburger, S.D., Lange, N., Rajapakse, J.C., Kaysen, D., Vauss, Y.C. & Rapoport, J.L. (1996) Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol 366, 223–230. Gonzalez, G.A. & Montminy, M.R. (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–680. Gottmann, K., Mittmann, T. & Lessmann, V. (2009) BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res 199, 203–234. Gouty-Colomer, L.A., Hosseini, B., Marcelo, I.M., Schreiber, J., Slump, D.E., Yamaguchi, S., Houweling, A.R., Jaarsma, D., Elgersma, Y. & Kushner, S.A. (2015) Arc expression identifies the lateral amygdala fear memory trace. Mol Psychiatry. Advance online publication. doi:10.1038/mp.2015.18. Grace, A.A. & Rosenkranz, J.A. (2002) Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav 77, 489–493. Green, M.J., Matheson, S.L., Shepherd, A., Weickert, C.S. & Carr, V.J. (2011) Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry 16, 960–972. Han, J.H., Kushner, S.A., Yiu, A.P., Cole, C.J., Matynia, A., Brown, R.A., Neve, R.L., Guzowski, J.F., Silva, A.J. & Josselyn, S.A. (2007) Neuronal competition and selection during memory formation. Science 316, 457–460. 138 Han, J.H., Kushner, S.A., Yiu, A.P., Hsiang, H.L., Buch, T., Waisman, A., Bontempi, B., Neve, R.L., Frankland, P.W. & Josselyn, S.A. (2009) Selective erasure of a fear memory. Science 323, 1492–1496. Hanover, J.L., Huang, Z.J., Tonegawa, S. & Stryker, M.P. (1999) Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci 19, RC40. Heldt, S.A., Zimmermann, K., Parker, K., Gaval, M., Weinshenker, D. & Ressler, K.J. (2014) BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci 34, 2444–2450. Hennenlotter, A., Schroeder, U., Erhard, P., Castrop, F., Haslinger, B., Stoecker, D., Lange, K.W. & Ceballos-Baumann, A.O. (2005) A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage 26, 581–591. Hensch, T.K. (2005) Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 69, 215–237. Herold, S., Jagasia, R., Merz, K., Wassmer, K. & Lie, D.C. (2011) CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol Cell Neurosci 46, 79–88. Herry, C., Trifilieff, P., Micheau, J., Luthi, A. & Mons, N. (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24, 261–269. Hetman, M., Kanning, K., Cavanaugh, J.E. & Xia, Z. (1999) Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 274, 22569–22580. Hiroi, N. & White, N.M. (1991) The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci 11, 2107–2116. Ho, B.C., Andreasen, N.C., Dawson, J.D. & Wassink, T.H. (2007) Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry 164, 1890–1899. Holm, M.M., Nieto-Gonzalez, J.L., Vardya, I., Vaegter, C.B., Nykjaer, A. & Jensen, K. (2009) Mature BDNF, but not proBDNF, reduces excitability of fast-spiking interneurons in mouse dentate gyrus. J Neurosci 29, 12412–12418. Hong, E.J., McCord, A.E. & Greenberg, M.E. (2008) A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 60, 610–624. Horch, H.W., Kruttgen, A., Portbury, S.D. & Katz, L.C. (1999) Destabilization of cortical dendrites and spines by BDNF. Neuron 23, 353–364. Howell, B.R., Grand, A.P., McCormack, K.M., Shi, Y., LaPrarie, J.L., Maestripieri, D., Styner, M.A. & Sanchez, M.M. (2014) Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev Psychobiol 56, 1735–1746. Hsiang, H.L., Epp, J.R., van den Oever, M.C., Yan, C., Rashid, A.J., Insel, N., Ye, L., Niibori, Y., Deisseroth, K., Frankland, P.W. & Josselyn, S.A. (2014) Manipulating a "cocaine engram" in mice. J Neurosci 34, 14115–14127. Huang, E.J. & Reichardt, L.F. (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72, 609–642. Huang, Z.J. & Scheiffele, P. (2008) GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol 18, 77–83. Huang, Z.J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M.F., Maffei, L. & Tonegawa, S. (1999) BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. Huang, Y.Y., Martin, K.C. & Kandel, E.R. (2000) Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci 20, 6317–6325. Hunt, P.S. (1999) A further investigation of the developmental emergence of fear-potentiated startle in rats. Dev Psychobiol 34, 281–291. Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning Impey, S., McCorkle, S.R., Cha-Molstad, H., Dwyer, J.M., Yochum, G.S., Boss, J.M., McWeeney, S., Dunn, J.J., Mandel, G. & Goodman, R.H. (2004) Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119, 1041–1054. Jiao, Y., Zhang, Z., Zhang, C., Wang, X., Sakata, K., Lu, B. & Sun, Q.Q. (2011) A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A 108, 12131–12136. Jin, X., Hu, H., Mathers, P.H. & Agmon, A. (2003) Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci 23, 5662–5673. Josselyn, S.A. (2010) Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci 35, 221–228. Josselyn, S.A. & Nguyen, P.V. (2005) CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord 4, 481–497. Josselyn, S.A., Shi, C., Carlezon, W.A. Jr., Neve, R.L., Nestler, E.J. & Davis, M. (2001) Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 21, 2404–2412. Josselyn, S.A., Kida, S. & Silva, A.J. (2004) Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol Learn Mem 82, 159–163. Kaang, B.K., Kandel, E.R. & Grant, S.G. (1993) Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron 10, 427–435. Kalkman, H.O. (2012) Potential opposite roles of the extracellular signal-regulated kinase (ERK) pathway in autism spectrum and bipolar disorders. Neurosci Biobehav Rev 36, 2206–2213. Kandel, E.R. & O’Dell, T.J. (1992) Are adult learning mechanisms also used for development? Science 258, 243–245. Kang, H. & Schuman, E.M. (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662. Katagiri, H., Fagiolini, M. & Hensch, T.K. (2007) Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53, 805–812. Kawanishi, Y., Harada, S., Tachikawa, H., Okubo, T. & Shiraishi, H. (1999) Novel variants in the promoter region of the CREB gene in schizophrenic patients. J Hum Genet 44, 428–430. Kerner, B., Rao, A.R., Christensen, B., Dandekar, S., Yourshaw, M. & Nelson, S.F. (2013) Rare genomic variants link bipolar disorder with anxiety disorders to CREB-regulated intracellular signaling pathways. Front Psychiatry 4, 154. Kim, J.H. & Richardson, R. (2007) A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem 88, 48–57. Kim, J.H. & Richardson, R. (2008) The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci 28, 1282–1290. Kim, D., Pare, D. & Nair, S.S. (2013) Assignment of model amygdala neurons to the fear memory trace depends on competitive synaptic interactions. J Neurosci 33, 14354–14358. Kim, J., Kwon, J.T., Kim, H.S., Josselyn, S.A. & Han, J.H. (2014) Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci 17, 65–72. Kim, D., Samarth, P., Feng, F., Pare, D. & Nair, S.S. (2015) Synaptic competition in the lateral amygdala and the stimulus specificity of conditioned fear: a biophysical modeling study. Brain Struct Funct. Advance online publication. doi:10.1007/s00429-015-1037-4. King, E.C., Pattwell, S.S., Sun, A., Glatt, C.E. & Lee, F.S. (2013) Nonlinear developmental trajectory of fear learning and memory. Ann N Y Acad Sci 1304, 62–69. Kinoshita, S., Yasuda, H., Taniguchi, N., Katoh-Semba, R., Hatanaka, H. & Tsumoto, T. (1999) Brain-derived neurotrophic factor prevents low-frequency inputs from inducing long-term depression in the developing visual cortex. J Neurosci 19, 2122–2130. Genes, Brain and Behavior (2016) 15: 125–143 Kogan, J.H., Frankland, P.W., Blendy, J.A., Coblentz, J., Marowitz, Z., Schutz, G. & Silva, A.J. (1997) Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol 7, 1–11. Kohara, K., Kitamura, A., Morishima, M. & Tsumoto, T. (2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291, 2419–2423. Kohara, K., Kitamura, A., Adachi, N., Nishida, M., Itami, C., Nakamura, S. & Tsumoto, T. (2003) Inhibitory but not excitatory cortical neurons require presynaptic brain-derived neurotrophic factor for dendritic development, as revealed by chimera cell culture. J Neurosci 23, 6123–6131. Kolarow, R., Brigadski, T. & Lessmann, V. (2007) Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci 27, 10350–10364. Kornhauser, J.M., Cowan, C.W., Shaywitz, A.J., Dolmetsch, R.E., Griffith, E.C., Hu, L.S., Haddad, C., Xia, Z. & Greenberg, M.E. (2002) CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron 34, 221–233. Korte, M., Carroll, P., Wolf, E., Brem, G., Thoenen, H. & Bonhoeffer, T. (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92, 8856–8860. Kovalchuk, Y., Hanse, E., Kafitz, K.W. & Konnerth, A. (2002) Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295, 1729–1734. Lakhina, V., Arey, R.N., Kaletsky, R., Kauffman, A., Stein, G., Keyes, W., Xu, D. & Murphy, C.T. (2015) Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron 85, 330–345. Lambo, M.E. & Turrigiano, G.G. (2013) Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J Neurosci 33, 8810–8819. Landers, M.S. & Sullivan, R.M. (2012) The development and neurobiology of infant attachment and fear. Dev Neurosci 34, 101–114. Le Magueresse, C. & Monyer, H. (2013) GABAergic interneurons shape the functional maturation of the cortex. Neuron 77, 388–405. Leal, G., Comprido, D. & Duarte, C.B. (2014) BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76, 639–656. LeDoux, J.E. (2000) Emotion circuits in the brain. Annu Rev Neurosci 23, 155–184. LeDoux, J. (2007) The amygdala. Curr Biol 17, R868–R874. Li, C., Dabrowska, J., Hazra, R. & Rainnie, D.G. (2011) Synergistic activation of dopamine D1 and TrkB receptors mediate gain control of synaptic plasticity in the basolateral amygdala. PLoS One 6, e26065. Lonsdorf, T.B., Weike, A.I., Golkar, A., Schalling, M., Hamm, A.O. & Ohman, A. (2010) Amygdala-dependent fear conditioning in humans is modulated by the BDNF val66met polymorphism. Behav Neurosci 124, 9–15. Lonze, B.E., Riccio, A., Cohen, S. & Ginty, D.D. (2002) Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34, 371–385. Lu, B., Pang, P.T. & Woo, N.H. (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6, 603–614. Ma, H., Groth, R.D., Wheeler, D.G., Barrett, C.F. & Tsien, R.W. (2011) Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation. Neurosci Res 70, 2–8. Ma, H., Groth, R.D., Cohen, S.M., Emery, J.F., Li, B., Hoedt, E., Zhang, G., Neubert, T.A. & Tsien, R.W. (2014) gammaCaMKII shuttles Ca(2)(+)/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294. Maguschak, K.A. & Ressler, K.J. (2011) Wnt signaling in amygdala-dependent learning and memory. J Neurosci 31, 13057–13067. 139 Ehrlich and Josselyn Maravall, M., Stern, E.A. & Svoboda, K. (2004) Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol 92, 144–156. Maren, S. (2003) What the amygdala does and doesn’t do in aversive learning. Learn Mem 10, 306–308. Maren, S. & Quirk, G.J. (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5, 844–852. Martin, K.C. & Kandel, E.R. (1996) Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron 17, 567–570. Matsuda, N., Lu, H., Fukata, Y., Noritake, J., Gao, H., Mukherjee, S., Nemoto, T., Fukata, M. & Poo, M.M. (2009) Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci 29, 14185–14198. Mayr, B. & Montminy, M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2, 599–609. McAllister, A.K., Lo, D.C. & Katz, L.C. (1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803. McAllister, A.K., Katz, L.C. & Lo, D.C. (1996) Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17, 1057–1064. McDonald, A.J. (1998) Cortical pathways to the mammalian amygdala. Prog Neurobiol 55, 257–332. McEwen, B.S. & Chattarji, S. (2004) Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol 14(Suppl. 5), S497–S502. Meis, S., Endres, T. & Lessmann, V. (2012) Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents. J Physiol 590, 193–208. Minichiello, L. (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10, 850–860. Minichiello, L., Calella, A.M., Medina, D.L., Bonhoeffer, T., Klein, R. & Korte, M. (2002) Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36, 121–137. Monk, C.S., McClure, E.B., Nelson, E.E., Zarahn, E., Bilder, R.M., Leibenluft, E., Charney, D.S., Ernst, M. & Pine, D.S. (2003) Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 20, 420–428. Moriceau, S., Roth, T.L., Okotoghaide, T. & Sullivan, R.M. (2004) Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci 22, 415–422. Moriceau, S., Raineki, C., Holman, J.D., Holman, J.G. & Sullivan, R.M. (2009) Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci 3, 22. Morys, J., Berdel, B., Kowianski, P. & Dziewiatkowski, J. (1998) The pattern of synaptophysin changes during the maturation of the amygdaloid body and hippocampal hilus in the rat. Folia Neuropathol 36, 15–23. Morys, J., Berdel, B., Jagalska-Majewska, H. & Luczynska, A. (1999) The basolateral amygdaloid complex--its development, morphology and functions. Folia Morphol 58, 29–46. Motanis, H., Maroun, M. & Barkai, E. (2014) Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cereb Cortex 24, 1075–1087. Mou, L., Heldt, S.A. & Ressler, K.J. (2011) Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience 176, 72–85. Musumeci, G. & Minichiello, L. (2011) BDNF-TrkB signalling in fear learning: from genetics to neural networks. Rev Neurosci 22, 303–315. Musumeci, G., Sciarretta, C., Rodriguez-Moreno, A., Al Banchaabouchi, M., Negrete-Diaz, V., Costanzi, M., Berno, V., Egorov, A.V., von Bohlen Und Halbach, O., Cestari, V., Delgado-Garcia, J.M. & Minichiello, L. (2009) TrkB modulates fear learning and 140 amygdalar synaptic plasticity by specific docking sites. J Neurosci 29, 10131–10143. Nakagawa, S., Kim, J.E., Lee, R., Chen, J., Fujioka, T., Malberg, J., Tsuji, S. & Duman, R.S. (2002) Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci 22, 9868–9876. Namburi, P., Beyeler, A., Yorozu, S., Calhoon, G.G., Halbert, S.A., Wichmann, R., Holden, S.S., Mertens, K.L., Anahtar, M., Felix-Ortiz, A.C., Wickersham, I.R., Gray, J.M. & Tye, K.M. (2015) A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678. Nataraj, K., Le Roux, N., Nahmani, M., Lefort, S. & Turrigiano, G. (2010) Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron 68, 750–762. Nonaka, M., Kim, R., Fukushima, H., Sasaki, K., Suzuki, K., Okamura, M., Ishii, Y., Kawashima, T., Kamijo, S., Takemoto-Kimura, S., Okuno, H., Kida, S. & Bito, H. (2014) Region-specific activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron 84, 92–106. Obrietan, K., Gao, X.B. & Van Den Pol, A.N. (2002) Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol 88, 1005–1015. Olofsdotter, K., Lindvall, O. & Asztely, F. (2000) Increased synaptic inhibition in dentate gyrus of mice with reduced levels of endogenous brain-derived neurotrophic factor. Neuroscience 101, 531–539. Ou, L.C. & Gean, P.W. (2006) Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology 31, 287–296. Ou, L.C., Yeh, S.H. & Gean, P.W. (2010) Late expression of brain-derived neurotrophic factor in the amygdala is required for persistence of fear memory. Neurobiol Learn Mem 93, 372–382. Owens, D.F. & Kriegstein, A.R. (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3, 715–727. Pang, P.T., Teng, H.K., Zaitsev, E., Woo, N.T., Sakata, K., Zhen, S., Teng, K.K., Yung, W.H., Hempstead, B.L. & Lu, B. (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491. Pape, H.C. & Pare, D. (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90, 419–463. Patterson, S.L., Abel, T., Deuel, T.A., Martin, K.C., Rose, J.C. & Kandel, E.R. (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145. Pattwell, S.S., Bath, K.G., Casey, B.J., Ninan, I. & Lee, F.S. (2011) Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A 108, 1182–1187. Pattwell, S.S., Duhoux, S., Hartley, C.A., Johnson, D.C., Jing, D., Elliott, M.D., Ruberry, E.J., Powers, A., Mehta, N., Yang, R.R., Soliman, F., Glatt, C.E., Casey, B.J., Ninan, I. & Lee, F.S. (2012) Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A 109, 16318–16323. Patz, S., Grabert, J., Gorba, T., Wirth, M.J. & Wahle, P. (2004) Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an early period of postnatal development. Cereb Cortex 14, 342–351. Payne, C., Machado, C.J., Bliwise, N.G. & Bachevalier, J. (2010) Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus 20, 922–935. Pham, T.A., Graham, S.J., Suzuki, S., Barco, A., Kandel, E.R., Gordon, B. & Lickey, M.E. (2004) A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn Mem 11, 738–747. Pizzorusso, T., Ratto, G.M., Putignano, E. & Maffei, L. (2000) Brain-derived neurotrophic factor causes cAMP response Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci 20, 2809–2816. Poo, M.M. (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2, 24–32. Porcher, C., Hatchett, C., Longbottom, R.E., McAinch, K., Sihra, T.S., Moss, S.J., Thomson, A.M. & Jovanovic, J.N. (2011) Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J Biol Chem 286, 21667–21677. Psotta, L., Lessmann, V. & Endres, T. (2013) Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiol Learn Mem 103, 34–38. Pucilowska, J., Puzerey, P.A., Karlo, J.C., Galan, R.F. & Landreth, G.E. (2012) Disrupted ERK signaling during cortical development leads to abnormal progenitor proliferation, neuronal and network excitability and behavior, modeling human neuro-cardio-facial-cutaneous and related syndromes. J Neurosci 32, 8663–8677. Qin, S., Young, C.B., Supekar, K., Uddin, L.Q. & Menon, V. (2012) Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A 109, 7941–7946. Quinn, J.J., Skipper, R.A. & Claflin, D.I. (2014) Infant stress exposure produces persistent enhancement of fear learning across development. Dev Psychobiol 56, 1008–1016. Quirk, G.J. & Gehlert, D.R. (2003) Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci 985, 263–272. Rainnie, D.G., Bergeron, R., Sajdyk, T.J., Patil, M., Gehlert, D.R. & Shekhar, A. (2004) Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci 24, 3471–3479. Rattiner, L.M., Davis, M., French, C.T. & Ressler, K.J. (2004a) Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci 24, 4796–4806. Rattiner, L.M., Davis, M. & Ressler, K.J. (2004b) Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem 11, 727–731. Rattiner, L.M., Davis, M. & Ressler, K.J. (2005) Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist 11, 323–333. Redondo, R.L., Kim, J., Arons, A.L., Ramirez, S., Liu, X. & Tonegawa, S. (2014) Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430. Riccio, A., Ahn, S., Davenport, C.M., Blendy, J.A. & Ginty, D.D. (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286, 2358–2361. Rosenkranz, J.A. & Grace, A.A. (2002) Dopamine-mediated modulation of odour-evoked amygdala potentials during Pavlovian conditioning. Nature 417, 282–287. Ryan, S.J., Ehrlich, D.E., Jasnow, A.M., Daftary, S., Madsen, T.E. & Rainnie, D.G. (2012) Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS One 7, e35320. Ryan, S.J., Ehrlich, D.E. & Rainnie, D.G. (2014) Morphology and dendritic maturation of developing principal neurons in the rat basolateral amygdala. Brain Struct Funct. Advance online publication. doi:10.1007/s00429-014-0939-x. Sakata, K., Woo, N.H., Martinowich, K., Greene, J.S., Schloesser, R.J., Shen, L. & Lu, B. (2009) Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A 106, 5942–5947. Sanchez, A.L., Matthews, B.J., Meynard, M.M., Hu, B., Javed, S. & Cohen Cory, S. (2006) BDNF increases synapse density in dendrites of developing tectal neurons in vivo. Development 133, 2477–2486. Sargin, D., Mercaldo, V., Yiu, A.P., Higgs, G., Han, J.H., Frankland, P.W. & Josselyn, S.A. (2013) CREB regulates spine density of lateral Genes, Brain and Behavior (2016) 15: 125–143 amygdala neurons: implications for memory allocation. Front Behav Neurosci 7, 209. Sato-Bigbee, C., Pal, S. & Chu, A.K. (1999) Different neuroligands and signal transduction pathways stimulate CREB phosphorylation at specific developmental stages along oligodendrocyte differentiation. J Neurochem 72, 139–147. Schafe, G.E., Nadel, N.V., Sullivan, G.M., Harris, A. & LeDoux, J.E. (1999) Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6, 97–110. Schafe, G.E., Atkins, C.M., Swank, M.W., Bauer, E.P., Sweatt, J.D. & LeDoux, J.E. (2000) Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci 20, 8177–8187. Schinelli, S., Zanassi, P., Paolillo, M., Wang, H., Feliciello, A. & Gallo, V. (2001) Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways. J Neurosci 21, 8842–8853. Segal, R.A. & Greenberg, M.E. (1996) Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19, 463–489. Sehgal, M., Ehlers, V.L. & Moyer, J.R. Jr. (2014) Learning enhances intrinsic excitability in a subset of lateral amygdala neurons. Learn Mem 21, 161–170. Sekeres, M.J., Mercaldo, V., Richards, B., Sargin, D., Mahadevan, V., Woodin, M.A., Frankland, P.W. & Josselyn, S.A. (2012) Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 32, 17857–17868. Shaywitz, A.J. & Greenberg, M.E. (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68, 821–861. Sheng, M., Thompson, M.A. & Greenberg, M.E. (1991) CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252, 1427–1430. Shimada, A., Mason, C.A. & Morrison, M.E. (1998) TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci 18, 8559–8570. Silva, A.J., Kogan, J.H., Frankland, P.W. & Kida, S. (1998) CREB and memory. Annu Rev Neurosci 21, 127–148. Soliman, F., Glatt, C.E., Bath, K.G., Levita, L., Jones, R.M., Pattwell, S.S., Jing, D., Tottenham, N., Amso, D., Somerville, L.H., Voss, H.U., Glover, G., Ballon, D.J., Liston, C., Teslovich, T., Van Kempen, T., Lee, F.S. & Casey, B.J. (2010) A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327, 863–866. Song, H., Ming, G., He, Z., Lehmann, M., McKerracher, L., Tessier-Lavigne, M. & Poo, M. (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518. Southwell, D.G., Froemke, R.C., Alvarez-Buylla, A., Stryker, M.P. & Gandhi, S.P. (2010) Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148. Stamatakis, A., Diamantopoulou, A., Panagiotaropoulos, T., Raftogianni, A. & Stylianopoulou, F. (2013) Effects of an early experience involving training in a T-maze under either denial or receipt of expected reward through maternal contact. Front Endocrinol 4, 178. Strauss, J., Barr, C.L., George, C.J., King, N., Shaikh, S., Devlin, B., Kovacs, M. & Kennedy, J.L. (2004) Association study of brain-derived neurotrophic factor in adults with a history of childhood onset mood disorder. Am J Med Genet B Neuropsychiatr Genet 131B, 16–19. Sullivan, R.M., Landers, M., Yeaman, B. & Wilson, D.A. (2000) Good memories of bad events in infancy. Nature 407, 38–39. Suzuki, K., Sato, M., Morishima, Y. & Nakanishi, S. (2005) Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci 25, 9535–9543. 141 Ehrlich and Josselyn Suzuki, A., Fukushima, H., Mukawa, T., Toyoda, H., Wu, L.J., Zhao, M.G., Xu, H., Shang, Y., Endoh, K., Iwamoto, T., Mamiya, N., Okano, E., Hasegawa, S., Mercaldo, V., Zhang, Y., Maeda, R., Ohta, M., Josselyn, S.A., Zhuo, M. & Kida, S. (2011) Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci 31, 8786–8802. Sweatt, J.D. (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76, 1–10. Tabuchi, A., Sakaya, H., Kisukeda, T., Fushiki, H. & Tsuda, M. (2002) Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem 277, 35920–35931. Takahashi, L.K. (1992) Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav 52, 493–498. Tanaka, T., Saito, H. & Matsuki, N. (1997) Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17, 2959–2966. Tanaka, J., Horiike, Y., Matsuzaki, M., Miyazaki, T., Ellis-Davies, G.C. & Kasai, H. (2008) Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319, 1683–1687. Tao, X., Finkbeiner, S., Arnold, D.B., Shaywitz, A.J. & Greenberg, M.E. (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726. Tarpley, J.W., Shlifer, I.G., Birnbaum, M.S., Halladay, L.R. & Blair, H.T. (2009) Bilateral phosphorylation of ERK in the lateral and centrolateral amygdala during unilateral storage of fear memories. Neuroscience 164, 908–917. Thomas, K.M., Drevets, W.C., Whalen, P.J., Eccard, C.H., Dahl, R.E., Ryan, N.D. & Casey, B.J. (2001) Amygdala response to facial expressions in children and adults. Biol Psychiatry 49, 309–316. Thompson, J.V., Sullivan, R.M. & Wilson, D.A. (2008) Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res 1200, 58–65. Tongiorgi, E., Righi, M. & Cattaneo, A. (1997) Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci 17, 9492–9505. Tottenham, N. & Sheridan, M.A. (2009) A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci 3, 68. Tottenham, N., Hare, T.A., Quinn, B.T., McCarry, T.W., Nurse, M., Gilhooly, T., Millner, A., Galvan, A., Davidson, M.C., Eigsti, I.M., Thomas, K.M., Freed, P.J., Booma, E.S., Gunnar, M.R., Altemus, M., Aronson, J. & Casey, B.J. (2010) Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 13, 46–61. Toyoizumi, T., Miyamoto, H., Yazaki-Sugiyama, Y., Atapour, N., Hensch, T.K. & Miller, K.D. (2013) A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron 80, 51–63. Tyler, W.J. & Pozzo-Miller, L. (2003) Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 553, 497–509. Verwer, R.W., Van Vulpen, E.H. & Van Uum, J.F. (1996) Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol 376, 75–96. Viegi, A., Cotrufo, T., Berardi, N., Mascia, L. & Maffei, L. (2002) Effects of dark rearing on phosphorylation of neurotrophin Trk receptors. Eur J Neurosci 16, 1925–1930. Viosca, J., Lopez de Armentia, M., Jancic, D. & Barco, A. (2009) Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem 16, 193–197. 142 Vogt, M.B. & Rudy, J.W. (1984) Ontogenesis of learning: IV. Dissociation of memory and perceptual-altering processes mediating taste neophobia in the rat. Dev Psychobiol 17, 601–611. Walz, R., Roesler, R., Quevedo, J., Sant’Anna, M.K., Madruga, M., Rodrigues, C., Gottfried, C., Medina, J.H. & Izquierdo, I. (2000) Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol Learn Mem 73, 11–20. Watson, F.L., Heerssen, H.M., Bhattacharyya, A., Klesse, L., Lin, M.Z. & Segal, R.A. (2001) Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci 4, 981–988. West, A.E., Pruunsild, P. & Timmusk, T. (2014) Neurotrophins: transcription and translation. Handb Exp Pharmacol 220, 67–100. Wiedenmayer, C.P. (2009) Plasticity of defensive behavior and fear in early development. Neurosci Biobehav Rev 33, 432–441. Wolff, S.B., Grundemann, J., Tovote, P., Krabbe, S., Jacobson, G.A., Muller, C., Herry, C., Ehrlich, I., Friedrich, R.W., Letzkus, J.J. & Luthi, A. (2014) Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458. Xing, J., Ginty, D.D. & Greenberg, M.E. (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273, 959–963. Yamada, M., Ohnishi, H., Sano, S., Nakatani, A., Ikeuchi, T. & Hatanaka, H. (1997) Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine-phosphorylated and associated with phosphatidylinositol 3-kinase in response to brain-derived neurotrophic factor in cultured cerebral cortical neurons. J Biol Chem 272, 30334–30339. Yamada, M.K., Nakanishi, K., Ohba, S., Nakamura, T., Ikegaya, Y., Nishiyama, N. & Matsuki, N. (2002) Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 22, 7580–7585. Yan, Q., Rosenfeld, R.D., Matheson, C.R., Hawkins, N., Lopez, O.T., Bennett, L. & Welcher, A.A. (1997a) Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78, 431–448. Yan, Q., Radeke, M.J., Matheson, C.R., Talvenheimo, J., Welcher, A.A. & Feinstein, S.C. (1997b) Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol 378, 135–157. Yang, J., Siao, C.J., Nagappan, G., Marinic, T., Jing, D., McGrath, K., Chen, Z.Y., Mark, W., Tessarollo, L., Lee, F.S., Lu, B. & Hempstead, B.L. (2009) Neuronal release of proBDNF. Nat Neurosci 12, 113–115. Yee, B.K., Zhu, S.W., Mohammed, A.H. & Feldon, J. (2007) Levels of neurotrophic factors in the hippocampus and amygdala correlate with anxiety- and fear-related behaviour in C57BL6 mice. J Neural Transm 114, 431–444. Yin, J.C., Wallach, J.S., Del Vecchio, M., Wilder, E.L., Zhou, H., Quinn, W.G. & Tully, T. (1994) Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79, 49–58. Yin, J.C., Del Vecchio, M., Zhou, H. & Tully, T. (1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115. Yin, A., Qiu, Y., Jia, B., Song, T., Yu, Y., Alberts, I. & Zhong, M. (2014) The developmental pattern of the RAS/RAF/Erk1/2 pathway in the BTBR autism mouse model. Int J Dev Neurosci 39, 2–8. Ying, S.W., Futter, M., Rosenblum, K., Webber, M.J., Hunt, S.P., Bliss, T.V. & Bramham, C.R. (2002) Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22, 1532–1540. Yiu, A.P., Mercaldo, V., Yan, C., Richards, B., Rashid, A.J., Hsiang, H.L., Pressey, J., Mahadevan, V., Tran, M.M., Kushner, S.A., Woodin, M.A., Frankland, P.W. & Josselyn, S.A. (2014) Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83, 722–735. Genes, Brain and Behavior (2016) 15: 125–143 Plasticity-related genes in brain development and amygdala-dependent learning Zhang, X. & Poo, M.M. (2002) Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 36, 675–688. Zhang, J.H., Sato, M., Araki, T. & Tohyama, M. (1992) Postnatal ontogenesis of neurons containing GABAA alpha 1 subunit mRNA in the rat forebrain. Brain Res Mol Brain Res 16, 193–203. Zhang, Z., Jiao, Y.Y. & Sun, Q.Q. (2011) Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience 174, 10–25. Zheng, F. & Wang, H. (2009) NMDA-mediated and self-induced bdnf exon IV transcriptions are differentially regulated in cultured cortical neurons. Neurochem Int 54, 385–392. Genes, Brain and Behavior (2016) 15: 125–143 Zhou, Y., Won, J., Karlsson, M.G., Zhou, M., Rogerson, T., Balaji, J., Neve, R., Poirazi, P. & Silva, A.J. (2009) CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 12, 1438–1443. Acknowledgments The authors declare no conflicts of interest. This work was supported by Canadian Institutes of Health Research grant MOP-74650 to SAJ. 143