* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Feeding Stimulants Activate an Identified Dopaminergic Interneuron
Multielectrode array wikipedia , lookup
Neural modeling fields wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neurocomputational speech processing wikipedia , lookup
Environmental enrichment wikipedia , lookup
Time perception wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Electrophysiology wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Microneurography wikipedia , lookup
End-plate potential wikipedia , lookup
Neuroeconomics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Neural coding wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural oscillation wikipedia , lookup
Mirror neuron wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Muscle memory wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Single-unit recording wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neurotransmitter wikipedia , lookup
Embodied language processing wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Biological neuron model wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feeding Stimulants Activate an Identified Dopaminergic Interneuron
That Induces the Feeding Motor Program in Helisoma
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
Department of Biological Sciences, Neuroscience Group, University of Illinois at Chicago, Chicago, Illinois 60607
INTRODUCTION
Central pattern generators (CPGs) are neuronal ensembles
that produce the motor neuron activity patterns responsible
for many life-sustaining rhythmic behaviors. Although capable of producing rhythmic motor patterns in the absence of
afferent input, most CPGs are highly regulated by sensory
and modulatory influences. Such modulatory pathways must
transduce information about the animal’s external environment and internal physiological state and transmit relevant
information to the interneurons of the CPG, adapting patterned motor activity to suit immediate demands.
Monoaminergic pathways that initiate and/or modulate
CPG activity have been demonstrated in many species. For
example, the initiation of alternating activity in the flexor
and extensor nerves of curarized spinal rabbits is induced
by injection of the catecholamine presurser L-dopa (Viala
and Buser 1969). Similarly, bath application of L-dopa initiates patterned motor activity in the locomotory CPGs of the
cat (Grillner 1986) and lamprey (Poon 1980). Applications
of the monoamines dopamine and serotonin evoke distinct
motor patterns in CPGs controlling the hindlimbs of neonate
812
rats (Kiehn and Kjaerulff 1996) and controlling the stomatogastric system of the lobster (for reviews see Harris-Warrick
1988; Selverston 1995). Serotonin application can evoke
feeding (Lent 1985; but see also Wilson et al. 1996) or
swimming (Brodfuehrer et al. 1995) in the leech, and aspects
of these behaviors can be triggered by stimulation of identified serotonergic neurons (Lent 1985; Nusbaum and Kristan
1986). In the snail Helisoma, superfusion of the buccal ganglia with serotonin evokes a biphasic motor pattern (Granzow and Kater 1977) that mediates repetitive swallowing
(Arnett 1996). This effect of serotonin can be mimicked by
stimulation of the giant serotonergic neuron C1 (Granzow
and Kater 1977; Murphy et al. 1985a). In some cases modulatory monoaminergic interneurons are intrinsic components
of the CPG (Katz and Frost 1995; Katz et al. 1994).
Thus modulation of CPGs by monoaminergic pathways
is phylogenetically widespread. However, rarely has it been
possible to show in a single system 1) that natural stimuli
activate both a specific behavior and its underlying motor
pattern in intact or semi-intact animals; 2) that application
of a monoaminergic neurotransmitter to the CNS mimics the
effects of natural stimuli; 3) that the natural stimuli also
activate an identified monoaminergic modulatory interneuron; 4) that depolarization of the identified modulatory
interneuron evokes a motor pattern similar to that activated
by natural stimuli; and 5) that the effects of the applied
neuromodulator and of stimulation of the modulatory interneuron are blocked by the same monoaminergic antagonist(s). A behavioral, electrophysiological, pharmacological, morphological, and histochemical analysis of the neuroeffector system mediating feeding in the snail Helisoma
afforded this opportunity.
In gastropod mollusks a CPG in the buccal ganglia controls a variety of oral behaviors (e.g., procurement, swallowing, rejection, or regurgitation of food) that are mediated
by similar but slightly different patterns of motor neuron
activity (Arnett 1996; Audesirk and Audesirk 1985; McClellan 1982a,b; Morton and Chiel 1993a,b). Dopamine has
been implicated in the initiation or modulation of rhythmic
buccal motor patterns in several gastropod species (Kabotyanski et al. 1994; Kyriakides and McCrohan 1989; Rosen et
al. 1991; Teyke et al. 1993; Trimble and Barker 1984; Wieland and Gelperin 1983). However, elucidation of the modulatory role(s) of dopamine in molluscan feeding has been
confounded by several factors. First, the functional consequences of dopamine-induced motor patterns have not been
thoroughly investigated. Most of these electrophysiological
analyses of dopaminergic effects have been restricted to
studies of ‘‘fictive feeding motor patterns’’ in the buccal
0022-3077/97 $5.00 Copyright q 1997 The American Physiological Society
/ 9k17$$au17 J721-6
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
Quinlan, E. M., B. C. Arnett, and A. D. Murphy. Feeding stimulants activate an identified dopaminergic interneuron that induces
the feeding motor program in Helisoma. J. Neurophysiol. 78: 812–
824, 1997. The neurotransmitter dopamine is shown to play a
fundamental role in the generation of the feeding motor pattern and
resultant feeding behavior in Helisoma. Application of exogenous
dopamine triggered the fictive feeding motor pattern in the isolated
CNS and triggered feeding movements in semi-intact preparations.
Application of feeding stimulants to the oral cavity excited the
putatively dopaminergic buccal interneuron N1a, and depolarization of interneuron N1a triggered the production of the fictive
feeding motor pattern. The ability of dopamine superfusion and of
interneuron N1a stimulation to activate the fictive feeding motor
pattern was blocked by the dopamine antagonist sulpiride. The
phase of the fictive feeding motor pattern was reset by brief hyperpolarization of interneuron N1a, demonstrating that interneuron
N1a is an integral component of the buccal central pattern generator
(CPG). During spontaneous fictive feeding patterns, prolonged
hyperpolarizations of interneuron N1a inhibited the production of
patterned activity. Exogenous dopamine maintained the fictive
feeding motor pattern in the absence of interneuron N1a activity.
Interneuron N1a was labeled by the formaldehyde-glutaraldehyde
histochemical technique, which is indicative of the presence of
dopamine in mollusks. These data suggest that interneuron N1a is
an endogenous source of the neuromodulator dopamine, intrinsic
to the buccal CPG, and that interneuron N1a has a prominent
role in the sensory-motor integration triggering the consummatory
response.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
METHODS
recording dish with a ball-and-socket joint, was used to stabilize
the buccal ganglia for intracellular recordings during feeding movements. The microplatform was insulated with Paraplastic embedding medium and dipped in black ink to enhance visibility.
A more reduced preparation was required to determine the effects of food stimuli on interneuron N1a, because of the relatively
small size ( õ40 mm) and lateral position of its soma. The buccal
mass, salivary glands, and esophagus were extracted while innervation from the buccal ganglia was preserved. The buccal mass was
split ventrally, from the radular sac to the mouth, and reflected
outward. A stabilizing silicone rubber platform was placed beneath
the buccal ganglia. The esophagus was cannulated with polyethylene tubing ( õ1 mm OD) attached to a tuberculin syringe filled
with watermelon that had been homogenized and strained through
cheesecloth. Approximately 0.1 ml of watermelon extract, delivered to the anterior proesophagus, spread over the surface of the
buccal cavity in each experiment.
Electrophysiology, intracellular staining, and video
microscopy
Standard electrophysiological techniques were used. Glass microelectrodes with internal fibers were filled with 3 M potassium
acetate (DC resistance 20–40 MV ) or 3% Lucifer yellow CH (in
distilled and filtered water, DC resistance 100–200 MV ). The
Lucifer yellow staining procedures have been previously described
(Murphy et al. 1983). Reactive red No. 4 was pressure injected into
interneuron N1a via a pneumatic injection system (Picospritzer,
General Valve) under visual control.
For simultaneous video microscopic and electrophysiological
analyses, movements of the buccal mass were recorded with a
Hitachi KP-140 solid-state video camera attached to a Wild M5a
dissection microscope with a trinocular head. A Panasonic WVCD20 closed-circuit TV camera simultaneously recorded oscilloscope traces of intracellular electrophysiological neuronal activity.
The two camera signals were digitally mixed via a Panasonic AV
mixer, and the neural activity was placed as an inset into the images
of the buccal mass. The combined images were sent to a Panasonic
AG-7300 video recorder and stored on video cassettes for subsequent analyses. Photographs were taken of individual video frames
(33 ms) with a Polaroid freeze-frame video recorder.
Unless otherwise noted, intracellular recordings were made in
normal physiological saline containing (in mM) 51.3 NaCl, 1.7
KCl, 1.5 MgCl2 , 4.1 CaCl2 , and 5.0 N-2-hydroxyethylpiperazineN *-2-ethanesulfonic acid buffer, pH 7.3. All chemicals, including
neurotransmitters and antagonists, were obtained from Sigma.
Animals and experimental preparations
All experiments were performed on an albino strain of the planorbid pond snail, H. trivolvis, descended from stocks originally
established in the laboratory of S. B. Kater. Adult snails with a
vertical shell diameter of 10–12 mm were used. The general dissection has been previously described (Kater and Kaneko 1972).
Three types of experimental preparations were used. Most experiments employed an isolated CNS preparation, in which the CNS
(paired buccal, cerebral, pedal, pleural, and parietal ganglia, and
the visceral ganglion) and the salivary glands were removed and
stabilized in a recording dish with a silicone rubber base
(GE:RTV616).
Simultaneous analyses of feeding movements and their underlying neural patterns employed semi-intact preparations. A midsagittal incision was made in the dorsal body wall, from the mantle to
the outer lip, to expose the CNS. Part of the lateral body wall
was cut away to facilitate visualization of the buccal mass. The
esophagus was severed and deflected forward to raise the buccal
ganglia (attached to the caudal surface of the buccal mass) into
a dorsal position. A spear-shaped microplatform, attached to the
/ 9k17$$au17 J721-6
Formaldehyde-glutaraldehyde histochemistry
The formaldehyde-glutaraldehyde (FaGlu) histochemical procedure was performed on acutely dissected CNS following the methods of Goldstein and Schwartz (1989). Briefly, preparations were
incubated in FaGlu mixture (4% formaldehyde/0.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4) for 24 h at 47C.
Preparations were dehydrated via an ascending ethanol sequence,
then cleared and mounted for microscopy in methyl salicylate.
RESULTS
Dopamine elicits the fictive feeding motor pattern from the
buccal CPG in the isolated CNS
Multiple patterns of motor neuron activity responsible for
the expression of distinct rhythmic oral behaviors are produced by the buccal CPG in Helisoma (Arnett 1996; Quinlan
and Murphy 1996). The CPG is composed of three interactive interneuronal subunits, named S1, S2, and S3, that
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
ganglia, in the presence or absence of sensory afferents or
connections with the rest of the CNS (but see Kabotyanski et
al. 1994). Second, dopaminergic neurons, whose activation
mimics the effects of dopamine superfusion, have rarely
been identified. Third, there is diversity of dopamine receptors both within and across species (Ascher 1972; Berry
and Cottrell; 1975; Green et al. 1996; Lo and Weiss 1994;
Magoski et al. 1995). Thus comparative analyses of dopaminergic modulation and of the roles of putatively dopaminergic neurons in feeding in mollusks remain problematic.
In Helisoma trivolvis, we have previously analyzed feeding behavior in intact semitransparent newly hatched snails
by video microscopy. Similar video microscopic analyses of
feeding in semi-intact animals were made simultaneously
with intracellular recordings from identified buccal neurons
and with extracellular myograms. These multidisciplinary
analyses have demonstrated that the standard triphasic pattern of activity in buccal motor neurons (Quinlan and Murphy 1991, 1996; Quinlan et al. 1995) mediates the typical
feeding behavior (Arnett 1996; Arnett and Murphy, unpublished data). Here we demonstrate that dopamine plays a
fundamental role in the generation of the feeding motor pattern and consequent feeding behavior. We identify and characterize a dopaminergic interneuron, named N1a, that is
stimulated by natural stimulants that evoke feeding. Depolarization of interneuron N1a in quiescent preparations is sufficient to trigger the generation of the feeding motor program.
In addition, brief hyperpolarization of interneuron N1a resets
the phase of ongoing feeding motor patterns. This indicates
that interneuron N1a is an intrinsic part of the buccal CPG,
and suggests that monoaminergic modulation of CPGs by
interneurons intrinsic to the CPGs may be a common regulatory mechanism (cf. Katz and Frost 1995; Katz et al. 1994).
Such dopaminergic modulation of the buccal CPG is fundamental to the sensory-motor integration that selects typical
feeding behavior from the behavioral repertoire of the multifunctional buccal CPG.
A preliminary report of some of these data was presented
previously in abstract form (McLean et al. 1989).
813
814
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
are schematically represented in Fig. 1A. Each subunit consists of one or more pairs of interneurons and provides the
primary excitation to a corresponding set of effector motor
neurons (Murphy and Lu 1987; Quinlan and Murphy 1996;
/ 9k17$$au17 J721-6
Dopamine triggers the feeding motor pattern and
consequent feeding behavior in semi-intact snails
Dopamine previously has been shown to evoke buccal
motor patterns, thought to represent fictive feeding, in a
number of gastropods (e.g., Kabotyanski et al. 1994; Kyrakides and McCrohan 1989; Teyke et al. 1993; Wieland and
Gelperin 1983). To determine whether dopamine triggers
feeding behavior, the effects of dopamine were examined
in semi-intact preparations capable of generating feeding
movements while intracellular recordings were made from
buccal neurons. Movements of the pharyngeal buccal mass,
which contains the muscles responsible for feeding movements of the odontophore, were videotaped with a camera
mounted on the dissecting microscope. Simultaneously, the
activity of each CPG subunit was ascertained by recording
from selected pairs of identified motor neurons. A second
camera, focused on the oscilloscope screen, videotaped the
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
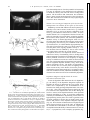
FIG . 1. Dopamine (DA) modulates multifunctional buccal central pattern generator (CPG) to generate feeding motor pattern. A: schematic diagram of buccal CPG. Each of the 3 CPG subunits (S1, S2, and S3) is an
independent conditional oscillator comprising ¢1 pairs of interneurons.
Membrane properties of individual CPG interneurons, and interactions between CPG subunits, can be modulated (diagonal arrows) to permit production of multiple motor neuron activity patterns. Chemosensory afferents
stimulated by food in buccal cavity, and DA, either extrinsic to CPG or
released by S1 interneurons, induce CPG to be active in feeding mode.
Depolarization of S1 interneurons stimulates S1 follower motor neurons
(MNs) and S2 interneurons. Depolarization of S2 interneurons stimulates
S2 follower neurons and simultaneously inhibits S1 and S3 activity. S3
activity is generated by postinhibitory rebound following termination of S2
inhibition. Lines ending in horizontal bars: excitatory pathways. Lines ending in filled circles: inhibitory pathways. B: bath application of DA (10
mM) triggered production of triphasic (S1-S2-S3) feeding motor pattern.
No buccal CPG activity was observed in physiological saline before application of DA. Phase 1 motor neuron B6 is excited by S1 and inhibited by
S2. Phase 2 motor neuron B27 is excited by S2. Phase 3 motor neuron B19
is inhibited by S2 and excited by S3.
Quinlan et al. 1995). There are several mechanisms by
which plasticity of motor output of the CPG can arise. Each
CPG subunit is a conditional neuronal oscillator that can be
independently rhythmically active. The subunits also can be
functionally linked in different combinations and in different
temporal patterns. Additional motor plasticity can arise from
variability in the rate of rhythmic activity (i.e., cycle period)
and in the intensity of action potential bursts (i.e., graded
changes in intraburst action potential number and frequency)
in subunits 1 and 3 (Quinlan and Murphy 1991, 1996; Quinlan et al. 1995) (see below).
A triphasic buccal motor pattern, with the CPG subunits
activated in the sequence S1-S2-S3, was previously described (Quinlan and Murphy 1991, 1996; Quinlan et al.
1995) and has been shown to mediate functional feeding
movements (Arnett 1996; Arnett and Murphy 1991; Arnett
and Murphy, unpublished data). When the CPG is active in
this feeding mode, bursts of action potentials in S1 interneurons simultaneously evoke excitatory postsynaptic potentials
(EPSPs) in phase 1 motor neurons and S2 interneurons.
Depolarization of S2 interneurons, beyond the threshold for
the production of plateau potentials, evokes excitation in
phase 2 motor neurons and inhibition in both S1 and S3
interneurons. The inhibitory feedback from S2 terminates
the activity of S1, and postinhibitory rebound, following
the termination of S2 inhibition, activates S3 (Quinlan and
Murphy 1996). S1 interneurons slowly repolarize on termination of S2 inhibition. In the presence of continuous sensory
stimulation or endogenous modulation, a subsequent burst
of action potentials will be generated in S1 interneurons to
initiate a new feeding cycle.
Dopamine superfusion (1–10 mM) of the isolated CNS
triggered the production of the triphasic fictive feeding motor
pattern (Fig. 1B). During phase 1 of each cycle, motor neuron B6, which is involved in protraction of the odontophore,
generated a burst of action potentials. Both neuron B6 and
phase 3 motor neuron B19 were inhibited during phase 2,
whereas motor neuron B27, which is involved in retraction
of the odontophore, was excited and generated a burst of
action potentials. Motor neuron B19, which is involved in
hyperretraction of the odontophore, generated bursts of action potentials during phase 3.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
815
Dopamine induces multiple patterns of buccal neuronal
activity in a concentration-dependent manner
During initial examinations of the effects of dopamine
concentrations on feeding behavior in semi-intact preparations, we observed that 10 mM dopamine routinely evoked
feeding movements, 1 mM dopamine had variable behavioral
effects, and õ1 mM dopamine did not induce feeding movements. Therefore the effects of various dopamine concentrations on the activities of the three CPG subunits were investigated in previously quiescent preparations (i.e., no CPG
activity was observed ¢30 s before application). Simultaneous recordings were made from phase 1 motor neurons that
are excited by S1 and inhibited by S2, and from phase 3
motor neurons that are inhibited by S2 and excited by S3
(Fig. 3). A dopamine concentration of 0.1 mM was subthreshold for activation of the buccal CPG (Fig. 3A). At
0.5 mM, dopamine application stimulated activity in all three
CPG subunits (Fig. 3B). However, S1 and S3 were coactivated, as indicated by simultaneous bursts of action potentials in the phase 1 and phase 3 motor neurons. S2 was also
activated, indicated by the S2-induced inhibitory postsynaptic potentials (IPSPs) observed in both motor neurons. Thus
an S1/S3-S2 motor pattern was produced by 0.5 mM dopamine. We have previously observed similar ‘‘spontaneous’’
buccal motor patterns (i.e., in physiological saline) with S1
and S3 interneurons coactive (Quinlan and Murphy 1996).
Applications of dopamine ranging from 1 to 3 mM induced
variable effects. The S1-S2-S3 fictive feeding motor pattern
/ 9k17$$au17 J721-6
FIG . 2. Superfusion of semi-intact preparations with DA induced feeding motor pattern and consequent feeding movements. Movements of buccal
mass triggered by 5 mM DA were videotaped through dissecting microscope
during intracellular recording from phase 1 protraction motor neuron (B6/
B7/B8, morphology not confirmed) and from phase 3 hyperretraction motor
neuron B19. Electrophysiological recordings on oscilloscope screen were
simultaneously videotaped with second camera and images were electronically merged. Esophagus, deflected anteriorly, lies between salivary gland
ducts at tops of photographs. Buccal ganglia, supported by microplatform,
is visible immediately above top left corner of oscilloscope screen. A:
photograph of videotape frame depicts broadened contours of buccal mass
during protraction of odontophore. This form and position of buccal mass
resulted from muscle contractions driven by last action potential burst in
phase 1 motor neuron recorded in top trace in inset and in synergistic
protractor motor neurons. tip of arrowhead points to edge of laterally protruded buccal mass. B: contours of buccal mass are depicted during hyperretraction of odontophore. Note diagnostic narrowing of buccal mass, with
its lateral edge (arrowhead) drawn away from fixed minuten pin. This form
and position of buccal mass resulted from muscle contractions driven by
bursts of action potentials in phase 3 motor neuron B19 ( bottom trace in
inset) and in other synergistic protraction motor neurons.
(Fig. 3C) was induced in 20% of the experiments ( n Å 15)
and an S1/S3-S2-S3 pattern (i.e., simultaneous bursts in S1
and S3, followed by S2 activation and a subsequent S3 burst)
was observed in 40%. A mixture of S1-S2-S3 cycles interspersed with S1/S3-S2-S3 cycles occurred in 40% of these
experiments. Dopamine concentrations of 5–10 mM evoked
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
intracellular recordings. Superfusion of semi-intact preparations with dopamine elicited functional feeding movements
similar to those evoked by application of food to the oral
cavity.
The external contours of the buccal mass can be used to
identify each phase of the feeding cycle (Arnett 1996; Arnett
and Murphy 1991; Smith 1988, 1991). During phase 1, the
odontophore is rotated forward and protracted downward
through the mouth. These odontophore movements result
largely from contractions of the posterior jugalis (pj) muscle,
and cause a lateral bulging of the buccal mass (Fig. 2A).
The pj is innervated by motor neurons B6 and B8, and bursts
of action potentials in these neurons trigger pj contractions
during phase 1 of the feeding motor pattern (Arnett 1996).
During phase 2 of the feeding cycle, the odontophore is
retracted back to the rest position, in part because of contractions of the anterior jugalis (aj) muscle. This movement
causes a narrowing of the buccal mass as the aj contracts
and the pj relaxes. A more pronounced narrowing of the
buccal mass is seen during phase 3 as the odontophore is
hyperretracted toward the esophageal opening (Fig. 2B).
This movement is due largely to intense contractions of the
aj and supralateral radular tensor muscles. The aj is innervated by both phase 2 motor neuron B27 and phase 3 motor
neuron B19. Neuron B19 also innervates the supralateral
radular tensor. Thus dopamine superfusion of semi-intact
preparations configures the CPG to generate the triphasic
feeding motor pattern and movements of the buccal mass
diagnostic for feeding. This suggested that dopaminergic
modulation may mediate the feeding response induced by
chemosensory stimulation.
816
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
application. The cycle period observed in 5–10 mM dopamine was similar to feeding rates observed in intact snails
(Arnett 1996).
Dopamine antagonist sulpiride specifically blocks the
dopamine-induced feeding pattern
the S1-S2-S3 fictive feeding pattern in 100% (n Å 18) of
the experiments (Fig. 3D). Thus the higher concentrations
of dopamine entrained S3 activity to follow S2 inhibition,
apparently because of a dopamine-induced enhancement of
postinhibitory rebound in S3 interneurons (Quinlan and
Murphy 1996; M. Zoran, personal communication).
Increasing dopamine concentrations not only changed the
qualitative nature of the patterns but also increased the frequency of cyclic activity and the intensity of burst generation. Dopaminergic effects on the cycle frequency were
quantified as the number of cycles of S2 activity evoked in
the first 30 s after application of dopamine to quiescent
preparations. 0.1 mM dopamine evoked 0.60 { 0.89 (SD)
cycles (n Å 5); 0.3–0.5 mM dopamine evoked 1.75 { 1.71
cycles (n Å 4); 1.0 mM dopamine evoked 6.37 { 5.71 cycles
(n Å 19); and 5–10 mM dopamine (n Å 18) evoked
9.39 { 5.23 cycles of S2 activity during the first 30 s after
/ 9k17$$au17 J721-6
Identification and morphological characterization of
‘‘dopaminergic’’ neuron N1a
Dopamine initiates the feeding response in Helisoma. Putatively dopaminergic neurons were localized in the buccal
ganglia to identify candidate feeding modulatory neurons.
Immunocytochemistry employing antibodies to dopamine,
or to enzymes involved in dopamine synthesis, has had
mixed efficacy in molluscan preparations, and often fails to
label identified dopaminergic neurons (Croll and Chiasson
1990) (see DISCUSSION ). Therefore dopaminergic buccal
neurons were localized with a fluorescent histochemical
method that employs a mixture of 4% FaGlu to induce fluorophore production in catecholaminergic neurons (Furness
et al. 1977; Goldstein and Schwartz 1989). FaGlu histochemistry consistently induced yellow-green fluorescence in
Ç50 pairs of neurons in the buccal ganglia (Fig. 5A, n Å
10). Yellow-green fluorescence also was observed in the
giant pedal dopaminergic neuron, but no fluorescence was
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
FIG . 3. Concentration-dependent effects of DA on buccal motor patterns. Activities of phase 1 motor neuron B8 ( bottom traces) and phase 3
motor neuron B19 (top traces) were recorded simultaneously as concentration of DA in bathing medium was varied. No CPG activity was observed
before application of DA. A: superfusion of low concentration (0.1 mM)
of DA was subthreshold for activation of buccal CPG. B: superfusion of
0.5 mM DA induced nonfeeding pattern of rhythmic activity in buccal motor
neurons. Action potential bursts were generated simultaneously by phase 1
motor neuron B8 and phase 3 motor neuron B19, indicating coactivation
of CPG subunits 1 and 3. These rhythmic depolarizations were followed
by simultaneous S2-evoked inhibitory postsynaptic potentials (IPSPs), indicating that CPG was active in S1/S3-S2 sequence. C: superfusion of 1.0
mM DA elicited fictive feeding (S1-S2-S3) motor pattern. S3 activity, monitored by action potential bursts in phase 3 motor neuron B19, was entrained
to follow phase 2 IPSPs. Cycle frequency was also increased over that seen
at lower DA concentrations. D: superfusion of 10 mM DA further increased
cycle frequency and intraburst action potential frequency during production
of feeding motor pattern. Vertical calibration: 20 mV ( bottom traces in C
and D); 40 mV (all other traces).
In the basommatophoran snail, Lymnaea, the dopamine
receptor antagonist sulpiride blocked both EPSPs and IPSPs
at identified dopaminergic synapses, and blocked the effects
of exogenous dopamine (Magoski et al. 1995). Application
of sulpiride also blocked the effects of dopamine superfusion
on the buccal CPG of Helisoma (Fig. 4). A feeding motor
pattern, evidenced by phase 1 bursts of action potentials
and phase 2 IPSPs in motor neuron B7, was activated by
application of dopamine. The addition of 100 mM sulpiride
(Fig. 4A), in the continuous presence of dopamine, inhibited
activity in both S1 and S2 of the CPG. Motor neuron B7
generated action potentials tonically, and neither S1-induced
bursts of action potentials nor S2-induced IPSPs were observed. Sulpiride also blocked the dopamine-induced activity
in phase 3 motor neuron B19 (Fig. 4B). In the presence of
dopamine, neuron B19 displayed S3-driven bursts of action
potentials alternating with S2-induced IPSPs. The addition
of 100 mM sulpiride, in the continuous presence of dopamine, first eliminated S2 inhibition, and then S3 excitation,
in motor neuron B19.
To determine whether sulpiride was specifically antagonizing the effects of dopamine on the CPG, rather than causing a general disruption of CPG function, we tested the
effects of sulpiride on the response to the neuromodulator
serotonin. Serotonin application initiates an S2-S3 motor
pattern by activating rhythmic plateau potentials in S2 interneurons and by enhancing postinhibitory rebound in phase
3 interneurons (Quinlan and Murphy 1996; Quinlan et al.
1995). Application of serotonin to preparations made quiescent by the continuous presence of sulpiride initiated
S2-evoked IPSPs followed by S3 excitation in buccal motor
neuron B19. This suggests that the inhibitory effects of sulpiride on buccal CPG activity are due to the specific antagonism of modulatory dopamine receptors.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
817
FIG . 4. The DA receptor antagonist sulpiride specifically
blocks DAergic stimulation of buccal CPG. A: sulpiride
blocked DA-induced rhythmic S1-S2 activity in phase 1 motor neuron B7. Application of 0.1 mM sulpiride (F ) blocked
S1-induced action potential bursts and S2-evoked IPSPs.
Three seconds were excised to eliminate solution change artifact. B: sulpiride blocked DA-induced rhythmic S2-evoked
IPSPs and S3-evoked action potential bursts in phase 3 motor
neuron B19. Sulpiride application (F ) 1st eliminated rhythmic
S2-evoked IPSPs, then S3-induced burst generation gradually
dampened into tonic action potentials. C: sulpiride does not
antagonize serotonergic effects on buccal CPG. Serotonin
application (F ) triggered rhythmic phase 2 IPSPs and phase
3 action potentials in motor neuron B19 in continuous presence of sulpiride. B and C are discontinuous traces from same
neuron. Horizontal calibration bar: 2 s (A); 5 s (B and C).
Feeding stimulants evoke the feeding motor pattern in
part by activating phase 1 interneuron N1a
Watermelon extract is a potent feeding stimulant in both
intact and semi-intact snails (Arnett 1996). In reduced preparations, lacking the circumesophageal ring ganglia, superfusion of the anterior esophagus and the surface of the buccal
cavity with watermelon extract initiated the feeding motor
pattern (Fig. 6). Neuron N1a generated bursts of action
potentials during phase 1 that were terminated by inhibition
during phase 2. Thus chemosensory stimulation of the buccal
cavity can activate neuron N1a and trigger feeding, via afferents in buccal nerves, in the absence of descending afferents
or neuromodulatory inputs from the cerebral ganglia. Chemosensory afferents may have multiple targets in the buccal
CPG. In the example shown in Fig. 6, watermelon extract
/ 9k17$$au17 J721-6
triggered a large IPSP, similar to those evoked by S2, before
the first burst of action potentials in neuron N1a, suggesting
a direct chemosensory activation of S2. This observation is
consistent with the fact that semitransparent juvenile snails,
observed and videotaped feeding sporadically, typically retract the odontophore before the first protraction at the beginning of a feeding bout (Arnett 1996).
Neuron N1a can evoke the feeding motor pattern
To determine whether depolarization of neuron N1a is
sufficient to induce the production of the fictive feeding
motor pattern, the electrophysiological activity of identified
buccal motor neurons was monitored simultaneously with
that of interneuron N1a in quiescent preparations. Depolarization of interneuron N1a excited phase 1 motor neurons
and triggered recurrent inhibition by S2 (Fig. 7, n ú 10).
Therefore activation of interneuron N1a is sufficient to evoke
an S1-S2 pattern of CPG subunit activity. To determine
whether interneuron N1a could evoke the full triphasic fictive feeding pattern, it was necessary to monitor S3 of the
CPG while depolarizing interneuron N1a. Neuron N1a often
accommodates during depolarizing current injection before
an S1-S2-S3 fictive feeding pattern can be initiated. However, penetration of interneuron N1a with a microelectrode
often triggered the fictive feeding motor pattern in previously
quiescent preparations (Fig. 8). This was confirmed by
phase 1 action potential bursts in neuron N1a, phase 2 IPSPs
in both neuron N1a and phase 3 motor neuron B19, and
phase 3 bursts of action potentials in neuron B19. Hyperpolarization of interneuron N1a eliminated rhythmic activity
in the CPG. The left and right homologues of neuron N1a
are electrotonically coupled (data not shown) and thus the
activity of both neurons N1a can be inhibited by hyperpolarizing current injected into either the left or right soma. On
termination of the hyperpolarizing current, the fictive feeding
pattern was reinitiated. Rhythmic activity in S1 and S2 resumed first, and S3 activity appeared after several cycles of
S1-S2 activitiy. This sequence of activation of CPG subunits
by interneuron N1a is similar to the sequence observed with
increasing concentrations of exogenous dopamine. This sug-
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
seen in identified serotonergic neurons of Helisoma (e.g.,
neuron C1) (Granzow and Kater 1977). It has been reported
previously that epinephrine and norepinephrine levels are
low or insignificant in Helisoma ganglia (Trimble et al.
1984). Therefore most, if not all, of the neurons exhibiting
FaGlu-induced fluorescence in the buccal ganglia of Helisoma are hypothesized to be dopaminergic.
FaGlu histochemistry consistently induced yellow-green
fluorescence in a moderately sized ( Ç40 mm diam) bilaterally symmetrical pair of neuronal somata on the lateral edges
of the dorsal surface of the buccal ganglia. A mirror-image
pair of neurons similar in size, shape, and relative somata
positions was morphologically and electrophysiologically
characterized and named neuron N1a. (Fig. 5, A and B).
Iontophoretic injection of the fluorescent dye Lucifer yellow
CH revealed the unique morphology of neuron N1a (Fig. 5,
C and D). Neuron N1a is a true buccal interneuron with
extensive neuritic arbors in both of the paired buccal ganglia
but no processes traversing buccal nerve roots or connectives. It has a single unipolar axon that crosses the buccal
commissure and terminates in the contralateral buccal ganglion. All physiological studies of neuron N1a were accompanied with dye injections to confirm morphological identity
(n ú 100).
818
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
gests that multiple bursts of action potentials in interneuron
N1a may be required to raise dopamine levels sufficiently
to entrain S3 activity and evoke the fictive feeding motor
pattern. The observation that interneuron N1a stimulates
phase 1 motor neurons and activates S2 of the CPG demonstrates that interneuron N1a fulfills the major physiological
criteria for an S1 interneuron.
Neuron N1a is an integral component of the buccal CPG
and dopamine can substitute for the effects of N1a activity
Dopamine antagonist sulpiride blocks the neuron
N1a-induced activation of S2
FIG . 5. Localization of formaldehyde-glutaraldehyde (FaGlu)-stained
buccal neurons and morphological characterization of interneuron N1a. A:
distribution of Ç50 pairs of neurons in buccal ganglia localized with DAsensitive FaGlu histochemical procedure. Somata of prominent pair of neurons on medioposterior edge of giant buccal neuron B4 are similar in
position to interneuron N1a. Calibration bar: 100 mm. B: diagram of identified neurons on dorsal surface of buccal ganglion. Cerebrobuccal connectives (CBCs) emerge from posterior corners and esophageal trunks (ETs)
emerge from anterior corners of buccal ganglia. HBN, heterobuccal nerve;
VBN, ventrobuccal nerve; PBN, posterior buccal nerve. C: intracellular
injection with Lucifer yellow CH revealed unique morphology of interneuron N1a. D: composite tracing of interneuron N1a, made from projections of ektachrome slides photographed at several different focal planes.
/ 9k17$$au17 J721-6
To test the hypothesis that interneuron N1a is dopaminergic, the efficacy of the dopamine antagonist sulpiride for
blocking the effects of interneuron N1a was tested. These
studies were complicated by the accommodation of interneuron N1a during prolonged depololarization. For example, in Fig. 7 the action potential frequency during the first
0.5-s interval of the depolarization was 24 Hz. During the
last 0.5-s interval of the depolarization the action potential
frequency had fallen to 16 Hz. To avoid the complication
caused by accommodation to depolarizing current, trains of
hyperpolarizing current pulses were used to generate bursts
of action potentials in neuron N1a on anode break, in the
absence and presence of the dopamine antagonist sulpiride
(Fig. 10). The activation of interneuron N1a in physiological
saline triggered activity in S2 of the CPG, as indicated by
the recurrent S2-evoked IPSPs in neuron N1a. Application
of sulpiride blocked the ability of interneuron N1a to activate
S2 (n Å 5 preparations). In an exemplary preparation (Fig.
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
Modulatory neurons that influence the motor pattern produced by a CPG can be extrinsic to the CPG (e.g., Granzow
and Kater 1977) or can be an intrinsic component of the
CPG (e.g., Katz and Frost 1995; Katz et al. 1994). To assess
whether neuron N1a is a component of the buccal CPG, the
ability of neuron N1a to reset the phase of ongoing rhythmic
buccal motor neuron patterns was examined. Spontaneous
rhythmic activity in normal physiological saline was observed in neuron N1a and an S1 motor neuron, with a regular
cycle frequency of 0.2 Hz (Fig. 9A). Inhibition of the activity of neurons N1a, via a short-duration hyperpolarizing current pulse injected into the soma of a single neuron N1a,
reset the phase of the ongoing rhythmic activity (n Å 4).
The interburst interval exhibited by the S1 motor neuron
was similar before and after the current injection.
Dopamine, however, can compensate for the absence of
neuron N1a activity in maintaining a fictive feeding motor
pattern. Dopamine superfusion of a rhythmically active preparation produced an increase in the frequency of ongoing
activity recorded from neuron N1a and an S1 motor neuron
(Fig. 9B). Hyperpolarization of interneuron N1a in the presence of exogenous dopamine had little, if any, effect on the
pattern of rhythmic activity. The ability of hyperpolarizations of neuron N1a to reset the phase of ongoing buccal
motor neuron activity in the absence, but not the presence,
of dopamine suggests that neuron N1a is an integral component of S1 of the buccal CPG, and that the modulatory effects
of neuron N1a on the buccal CPG are mediated by dopamine.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
819
FIG . 6. Chemosensory stimulation of oral cavity triggered rhythmic activity in interneuron N1a and activated feeding
motor program. Watermelon extract, a potent feeding stimulant, was superfused over epithelial lining of buccal cavity of
quiescent reduced preparation (arrowhead). Feeding motor pattern was induced, as evidenced by production of rhythmic
bursts of action potentials in interneuron N1a during phase 1, phase 2 IPSPs in both neurons N1a and B19, and bursts of
action potentials in neuron B19 during phase 3. Note that S2-evoked IPSPs occurred before 1st burst of action potentials in
interneuron N1a, indicating multiple sites of chemosensory input to CPG.
FaGlu-induced fluorescence characteristic of dopamine in
the physiologically characterized interneurons N1a
To further demonstrate that buccal interneuron N1a is
dopaminergic, the FaGlu histochemical staining procedure
for catecholamines was combined with intracellular dye
injections into electrophysiologically characterized interneurons N1a ( n Å 4 ) . Following intracellular recordings,
the dye reactive red No. 4 was pressure injected into the
somata of interneurons N1a, and buccal ganglia were sub-
FIG . 7. Depolarization of interneuron N1a excited phase 1 motor neurons and activated subunit 2 of CPG. In quiescent preparation, depolarizing
current pulse injected into interneuron N1a evoked bursts of action potentials that excited S1 follower motor neuron (B6 or B7, morphology not
confirmed). Stimulation of interneuron N1a also triggered rhythmic S2
activity, as evidenced by phase 2 IPSPs observed in both neuron N1a and
phase 1 motor neuron. Vertical calibration bar: 20 mV (top trace); 40 mV
(bottom trace).
/ 9k17$$au17 J721-6
jected to the FaGlu histochemical staining procedure ( Fig.
11 ) . FaGlu-induced flourescence, indicative of the presence of dopamine, was revealed in the dye-injected interneurons N1a.
DISCUSSION
These data demonstrate that dopamine plays a major role
in the organization of the consummatory feeding response
in Helisoma. The dopaminergic interneuron N1a is stimulated by chemosensory afferents arriving from the oral cavity
and anterior esophagus via buccal nerve roots. Interneuron
N1a is a component of S1 of the buccal CPG and modulates
the multifunctional CPG to generate the fictive feeding motor
pattern. A comparative analysis of the literature on gastropod
feeding suggests that a homologous dopaminergic modulatory pathway may be a general feature in the organization
of the consummatory feeding response.
Dopamine may be a common regulator of gastropod
feeding
Dopamine has been implicated in the control of feeding
in basommatophoran and stylomatophoran pulmonates, as
FIG . 8. Interneuron N1a activated feeding motor pattern. Activity of
buccal CPG was inhibited by injection of hyperpolarizing DC (1 nA) into
interneuron N1a, before onset of record shown. On release from current
injection, interneuron N1a generated rhythmic bursts of action potentials
during phase 1, which were terminated by phase 2 IPSPs. Simultaneous
S2-evoked IPSPs also were observed in phase 3 motor neuron B19. Generation of triphasic fictive feeding pattern, with distinct burst of action potentials in motor neuron B19 during phase 3, occurred only following sustained
activity in interneuron N1a. Injection of hyperpolarizing current (H) into
interneuron N1a again eliminated rhythmic CPG activity. Burst of action
potentials in neuron B19 at beginning of record was initiated before neuron
N1a was released from hyperpolarization and was similar to other spontaneous bursts of unknown origin that occurred in this neuron B19 with intervals
of 18–25 s. One minute was excised from record ( < ).
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
10), in physiological saline anode-break-induced bursts of
action potentials in neuron N1a activated S2 in 89% of the
trials (n Å 9). Application of sulpiride (100 mM) completely
blocked the ability of anode-break-induced action potential
bursts in neuron N1a to activate S2 following termination of
identical current pulses (n Å 12). On return to physiological
saline, the ability of interneuron N1a to activate S2 was
immediately restored, demonstrating that the activation of
S2 by interneuron N1a is dependent, either directly or indirectly, on the activation of dopamine receptors.
820
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
novine maleate. In the opisthobranch Aplysia, application of
dopamine or the metabolic precursor L-DOPA to the cerebral
(Rosen et al. 1991; Teyke et al. 1993) or buccal ganglia
(Kabotyanski et al. 1994) activated patterned buccal motor
activity. It is unclear whether these dopamine-induced motor
patterns mediate feeding, but semi-intact preparations perfused with L-DOPA produced rhythmic radular movements
suggestive of feeding (Kabotyanski et al. 1994).
Dopamine application evokes the feeding motor pattern
and consequent feeding behavior in Helisoma
well as opisthobranchs. In addition to its effects in Helisoma,
dopamine application reversibly stimulated rhythmic activity
in buccal motor neurons, or increased the rate of spontaneous
activity in the buccal ganglia, in the related basommatophoran snail Lymnaea stagnalis (Kyriakides and McCrohan
1989). The pattern of motor neuron activity induced by
dopamine appeared similar to the triphasic buccal motor
pattern reported to be associated with feeding in Lymnaea.
However, some motor neurons active during the feeding
motor pattern were inhibited by dopamine application. In the
stylomatophoran terrestrial slug, Limax maximus, dopamine
applied to the cerebral and buccal ganglia triggered a fictive
feeding motor program measured by extracellular suction
electrodes on peripheral buccal nerve roots (Wieland and
Gelperin 1983). The motor program elicited by superfusion
of dopamine was indistinguishable from the pattern elicited
by chemostimulation of the lips in a semi-intact preparation
(intact afferent neural connections between the lips and the
CNS, but no buccal mass). The effects of dopamine in Limax
maximus were mimicked by the vertebrate D1 dopamine
receptor agonist 2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronapthalene and blocked by the dopamine antagonist ergo-
/ 9k17$$au17 J721-6
Localization of dopamine and putatively dopaminergic
neurons in the CNS of Helisoma and other gastropods
Several techniques have been used to localize catecholamines to molluscan ganglia and to specific gastropod neu-
FIG . 10. The DA antagonist sulpiride blocked ability of interneuron N1a
to stimulate activity in S2 of buccal CPG. In quiescent preparations in
normal physiological saline, trains of hyperpolarizing current pulses (2.5
s, 2 nA) were used to generate bursts of action potentials in interneuron N1a
via postinhibitory rebound (i.e., anode break). Bursts of action potentials
generated in neuron N1a stimulated S2 activity, evidenced by recurrent S2evoked IPSPs (2) observed in interneuron N1a. After superfusion with
100 mM sulpiride (bottom trace), identical hyperpolarizing current pulses
continued to trigger similar bursts of action potentials but these bursts no
longer activated S2. In this preparation action potential frequency (measured
during 1.0-s interval on termination of hyperpolarizing pulse) ranged from
26 to 30 Hz in physiological saline and from 23 to 27 Hz in presence of
sulpiride.
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
FIG . 9. Interneuron N1a is component of buccal CPG and its effects
are mimicked by exogenous DA. A: spontaneous rhythmic phase 1 action
potentials and phase 2 IPSPs were observed in interneuron N1a and S1
motor neuron (B6/7/8, morphology not confirmed) in normal physiological
saline (NS). Injection of brief hyperpolarizing current (0.3 nA) into interneuron N1a reset phase of ongoing rhythmic activity. B: exogenous DA
substituted for effects of interneuron N1a on buccal CPG. Following DA
superfusion (10 mM), injection of same hyperpolarizing current into same
interneuron N1a had little effect on rhythmic activity observed in S1 motor
neuron. Vertical calibration bar: 20 mV (top traces); 40 mV (bottom
traces).
The large repertoire of motor neuron activity patterns produced by the multifunctional buccal CPG of Helisoma is
mirrored by the diversity in observed oral behaviors (Arnett
1996; Quinlan and Murphy 1996). Functional feeding movements (i.e., the cyclic protraction, retraction, and hyperretraction of the buccal odontophore) are produced when the
CPG subunits are active in the S1-S2-S3 sequence (Arnett
1996). Dopamine initiates the fictive feeding motor pattern
in the isolated CNS and evokes feeding behavior in semiintact preparations. Previous reports had demonstrated that
dopamine increased the firing rate of a phase 3 motor neuron,
and increased the frequency of spontaneously occurring
rhythmic activity in isolated buccal ganglia of Helisoma
(Trimble and Barker 1984; Trimble et al. 1984). At that
time, phase 1 protractor motor neurons had not been identified, and the feeding motor pattern was only partially characterized.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
821
FIG . 11. FaGlu histochemical technique indicated presence of DA in interneuron N1a. Electrophysiologically characterized
neuron N1a was injected with dye, reactive red No. 4, before processing preparation for FaGlu histochemistry. Left: FaGlu
labeling of presumptive DAergic neuronal somata in left buccal ganglion. Prominent bright soma near left edge is interneuron
N1a. Preparation was photographed with the use of the filter combination designed for Lucifer yellow. Right: FaGlu-sensitive
soma displayed dye that was injected into electrophysiologically characterized interneuron N1a. Soma of only neuron N1a
was visible when preparation was photographed with filter combination designed for rhodamine. Calibration bar: 100 mm.
/ 9k17$$au17 J721-6
produced green-yellow fluorescence in the same cells that
fluoresced when the glyoxylic acid technique was used to
localize catecholaminergic neurons. Occasionally, FaGlu
induced fluorescence in known serotonergic neurons (identified by immunocytochemical or other histochemical methods), but the yellow-brown fluorescence induced in serotonergic neurons was easily distinguished from the green-yellow fluorescence induced in catecholaminergic neurons (R.
Croll, personal communication).
In Helisoma, the FaGlu histochemical method did not
label identified serotonergic neurons (e.g., the giant cerebral
neuron C1) but did label the giant pedal dopaminergic neuron. Because epinephrine and norepinephrine levels have
been reported to be low or insignificant (Trimble et al.
1984), it is presumed that most, if not all, of the buccal
neurons that exhibit FaGlu-induced fluorescence are dopaminergic. In addition, FaGlu histochemistry was more sensitive than other methods in Helisoma. FaGlu induced fluorescence in Ç50 pairs of buccal neurons, more than were
localized when the glyoxylic acid procedure (Trimble et
al. 1984) or antidopamine immunocytochemistry (Murphy,
unpublished data) was used. In addition, FaGlu induced fluorescence in axons in the cerebrobuccal connectives that had
not been detected with the glyoxylate staining procedure.
In contrast, FaGlu histochemistry induced autofluorescence
in only four pairs of neurons on the dorsal surface of the buccal
ganglia in Lymnaea (Croll and Chiasson 1990). A similar
staining pattern was obtained with the glyoxylic acid histochemical procedure and with antidopamine immunocytochemistry (Elekes et al. 1991). In our hands, the FaGlu histochemical technique labeled a similarly small number of cells of the
buccal ganglia of Lymnaea in comparison with Helisoma.
FaGlu also stained relatively few neurons in the buccal ganglia
of Aplysia (Teyke et al. 1993). In Helisoma a number of small,
FaGlu histofluorescent somata often were observed in buccal
nerve roots, especially the esophageal nerve trunk that innervates the foregut (data not shown). Ganglionic neurons migrate
into the ganglia from the periphery during gastropod development. Thus Helisoma buccal ganglia may contain a population
of small dopaminergic neurons whose homologues in Lymnaea
and Aplysia may remain in the peripheral nerve plexus. Consistent with this suggestion, it has been demonstrated that the
buccal ganglia of Aplysia receive an abundant catecholaminergic input from the foregut (Susswein et al. 1993).
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
rons. Monoamines were first identified in molluscan CNS
by Sweeney (1963) with the use of fluorimetric and paper
chromatographic techniques. Substantial amounts of dopamine, but little or no trace of norepinephrine or epinephrine,
were found in ganglia of 10 different molluscan species.
More recently, high-performance liquid chromatographic
analysis yielded measurements of 8 pmol dopamine per
paired buccal ganglia and 90 pmol dopamine per circumesophageal ring ganglia of Helisoma (Gadotti et al. 1986),
10 pmol dopamine/buccal ganglia in Limax (Wieland and
Gelperin 1983), and 25 pmol dopamine/buccal ganglia in
Lymnaea (Elekes et al. 1991). Radioactive precursors used
to examine the synthesis of dopamine in the CNS of Helisoma demonstrated that neurons of the buccal, cerebral, and
pedal ganglia synthesized 3H-dopamine but not 3H-norepinephrine after incubation in 3H-tyrosine (Trimble et al.
1984). Tritiated dopamine synthesis was revealed in all buccal nerve roots except the cerebrobuccal connectives, the
only connections between the buccal ganglia and the central
circumesophageal ganglia. This suggested the possibility of
a dopaminergic system intrinsic to the buccal ganglia.
Immunocytochemistry (e.g., Elekes et al. 1991), as well
as the Falck-Hillarp (Falck et al. 1962; Trimble et al. 1984),
glyoxylate (DeLaTorre and Surgeon 1976; Kabotyanski et
al. 1994; Trimble et al. 1984; Wieland and Gelperin 1983),
and FaGlu (Croll and Chiasson 1990; Furness et al. 1977;
Goldstein and Schwartz 1989; Teyke et al. 1993) histochemical techniques, has indicated the presence of catecholaminergic neurons in the buccal ganglia of several gastropods. We
employed the FaGlu histochemical technique, which induces
autofluorescence in catecholaminergic neurons after incubation in a combination of 4% paraformaldehyde and 0.5%
glutaraldehyde, because it is both highly sensitive and quite
specific for dopamine in Helisoma. Because the tissue is
fixed by the same mixture that produces the fluorescence,
there is good preservation both of the aldehyde-induced fluorescence and of staining produced by procedures preceding
exposure to the FaGlu mixture.
In an early study in which FaGlu histochemistry was used
in whole mounts of guinea pig myenteric and submucosal
plexuses, relatively nonspecific fluorescence was found in
adrenergic, noradrenergic, dopaminergic, and serotonergic
neurons, but not in neurons known to contain histamine
(Furness et al. 1977). In Lymnaea, FaGlu histochemistry
822
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
Evidence that interneuron N1a in Helisoma is
dopaminergic
Although it is difficult to rigorously demonstrate all the
original criteria for the identification of a neurotransmitter
at a specific synapse (Paton 1958), we present the following
substantial evidence that interneuron N1a is dopaminergic.
1) The presence of dopamine in interneuron N1a was indicated by observing FaGlu-induced fluorescence in electrophysiologically characterized, dye-injected interneurons
N1a. 2) The effects of depolarization of interneuron N1a on
the buccal CPG were mirrored by exogenous dopamine. 3)
The effects both of depolarization of interneuron N1a and
of exogenous dopamine were blocked by the dopamine antagonist sulpiride. Together these data suggest that interneuron N1a is dopaminergic.
We have previously suggested that the neural basis of
feeding is fundamentally similar in gastropods that feed by
grasping or rasping movements of a radula (Quinlan and
Murphy 1996). In support of this hypothesis, a buccal interneuron similar in many respects to Helisoma interneuron
N1a has been described in Lymnaea (Yeoman et al. 1995).
Depolarization of this neuron, called neuron N1L, stimulated
the production of the triphasic fictive feeding motor pattern
in Lymnaea. Interneuron N1L also excited some identified
phase 1 interneurons and motor neurons in the Lymnaea
buccal ganglion. Neuron N1L exists as a bilateral pair of
mirror-image homologues with somata near the lateral edges
of the buccal ganglia. They project a single unipolar axon
to the contralateral buccal ganglion. Thus neurons N1a of
Helisoma and N1L of Lymnaea are very similar in morphology and physiology.
Despite these similarities, it is still unclear whether neuron
N1L in Lymnaea and neuron N1a in Helisoma are true homologues, in part because of differences in their reported neurotransmitters. Action potentials in neuron N1L in Lymnaea
evoked one-for-one fast EPSPs superimposed on a slow depolarization in follower cells. The fast EPSPs were inhibited
by the ‘‘cholinergic antagonists’’ d-tubocurarine and hexamethonium (Vehovszky and Elliott 1995). However, d-tubocurarine also blocks some identified dopaminergic synapses in gastropods (Ascher 1972; Berry and Cottrell 1975).
No effective antagonists for the slow depolarization were
reported, but two dopamine antagonists, fluphenazine and
ergometrine (i.e., ergonovine), were ineffective at blocking
the slow depolarization. This observation prompted the authors to conclude that neuron N1L in Lymnaea was not
dopaminergic. However, there is considerable diversity in
the pharmacology of molluscan dopamine receptors, and neither ergometrine nor fluphenazine were tested as antagonists
against the effects of exogenous dopamine in the buccal
ganglia of Lymnaea. Ergometrine blocks dopaminergic effects in the buccal ganglia of Limax (Wieland and Gelperin
1983) and Aplysia (Teyke et al. 1993), but is less effective
than sulpiride in Helisoma (data not shown). Sulpiride, but
not ergometrine or fluphenazine, was an effective antagonist
at identified synapses of the giant pedal dopamine cell
(RpeD1) in Lymnaea (Magoski et al. 1995).
/ 9k17$$au17 J721-6
Roles of interneuron N1a in mediation of feeding in
Helisoma
Our data suggest that interneuron N1a couples sensory
information provided by feeding stimulants in the buccal
cavity to the generation of the feeding consummatory response. Food applied to the oral cavity and esophagus in
semi-intact preparations activates neuron N1a and the feeding response. Activation of interneuron N1a by food in the
buccal cavity must occur via afferents in buccal nerves, because the response is seen in the absence of the remainder
of the CNS. Because the lip afferents indirectly affect the
buccal CPG via pathways through the cerebral ganglia, sensory inputs from the external lips are not required for the
consummatory response. Sensory afferents from the external
lips stimulate identified giant serotonergic cerebral neurons
that are apparently homologous in many gastropods (e.g.,
Croll 1986; Granzow and Rowell 1981). Activation of these
serotonergic neurons has been associated with behavioral
arousal and an enhancement of the feeding neuromuscular
system at many levels (e.g., Kupfermann and Weiss 1982;
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
Comparison of interneuron N1a with potential
homologues in other gastropod buccal CPGs
We have shown that sulpiride blocks the effects both of
exogenous dopamine and of depolarization of interneuron
N1a on the buccal CPG of Helisoma. In addition, electrophysiologically characterized neurons N1a exhibited yellowgreen fluorescence following FaGlu histochemistry, indicative of the presence of dopamine. Dopamine-immunoreactive somata have also been located in the position of
interneuron N1L in the Lymnaea buccal ganglia (Elekes et
al. 1991). It remains possible that interneuron N1a in Helisoma and interneuron N1L in Lymnaea are true homologues
that utilize both dopamine and acetylcholine as neurotransmitters. Analysis of cholinergic antagonists in Helisoma and
of other dopaminergic antagonists (i.e., sulpiride) in Lymnaea are necessary to resolve this issue.
Interneurons involved in feeding that are potentially homologous to interneuron N1a of Helisoma also have been
identified in opisthobranchs. In Aplysia, two putatively dopaminergic buccal interneurons (B20 and B65) can evoke buccal motor patterns (Kabotyanski et al. 1994; Teyke et al.
1993). Neuron B20 seems unlikely to be homologous to
Helisoma neuron N1a on the basis of its morphology and
soma position (Teyke et al. 1993). The soma of neuron B20
is adjacent to the buccal commissure and it projects an axon
into the contralateral cerebrobuccal connective. On the other
hand, the somata of interneurons B65 are on the lateral edges
of the buccal ganglia, and they project axons that arborize
and terminate in the contralateral buccal ganglion (Kabotyanski et al. 1994). Stimulation of interneuron B65 evoked
patterned buccal neuronal activity similar to that evoked by
dopamine application. Thus the morphology, relative soma
position, and physiological effects of Aplysia interneuron
B65 appear similar to those of interneuron N1a in Helisoma.
A buccal protractor interneuron with similar morphology to
Helisoma neuron N1a, Lymnaea neuron N1L, and Aplysia
neuron B65 also has been identified in Clione (Arshavsky
et al. 1989). We hypothesize that these buccal interneurons
are homologous, and that similar dopaminergic neurons with
major roles in organizing the consummatory response will
be found in other gastropods.
DOPAMINERGIC INTERNEURON MODULATES FEEDING CPG
McCrohan and Audesirk 1987). In Lymnaea, stimulation of
the external lip afferents with sucrose in lip-brain preparations activated a nitric-oxide-dependent pathway that evoked
variable buccal motor patterns, in part by stimulating the
large serotonergic cerebral neuron C1 (Elphick et al. 1995).
This suggests that in rasping and grasping radular-feeding
gastropods, external lip afferents trigger ‘‘feeding arousal’’
and some components of the feeding response via nitricoxide- and serotonin-dependent pathways through the cerebral ganglia. Subsequently, food stimuli in the buccal cavity
activate the full consummatory response via afferents reaching the buccal ganglia through buccal nerve roots and acting,
in part, by stimulating dopaminergic interneurons, such as
interneuron N1a in Helisoma and homologous neurons in
other gastropods.
Received 10 September 1996; accepted in final form 17 April 1997.
REFERENCES
ARNETT, B. C. Feeding and Regurgitation: Two Modes of Operation of the
Buccal Central Pattern Generator in Helisoma (PhD thesis). Chicago,
IL: Univ. of Illinois at Chicago, 1996.
ARNETT, B. C. AND MURPHY, A. D. Correlations of specific neural patterns
and buccal muscle contractions with dynamic stages of the feeding cycle
of Helisoma trivolvis. Soc. Neurosci. Abstr. 17: 1391, 1991.
ARSHAVSKY, Y. I., DELIAGINA, T. G., ORLOVSKY, G. N., AND PANCHIN,
Y. V. Control of feeding movements in the pteropod mollusc, Clione
limacina. Exp. Brain Res. 78: 387–397, 1989.
ASCHER, P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J. Physiol. Lond. 225: 173–209, 1972.
AUDESIRK, T. AND AUDESIRK, G. Behavior of gastropod molluscs. In: The
Mollusca, Neurobiology and Behavior, edited by A. O. D. Willows. Orlando, FL: Academic, 1985, vol. 8, part 1, p. 2–94.
BERRY, M. S. AND COTTRELL, G. A. Excitatory, inhibitory and biphasic
synaptic potentials mediated by an identified dopamine-containing neurone. J. Physiol. Lond. 244: 589–612, 1975.
BRODFUEHRER, P. D., DEBSKI, E. A., O’GARA, B. A., AND FRIESSEN, W. O.
Neuronal control of leech swimming. J. Neurobiol. 27: 403–418, 1995.
CROLL, R. P. Identified neurons and cellular homologies. In: Nervous Systems in Invertebrates, edited by M. A. Ali. New York: Plenum, 1986, p.
41–59.
CROLL, R. P. AND CHIASSON, B. J. Distribution of catecholamines and of
immunoreactivity to substances like vertebrate enzymes for the synthesis
of catecholamines within the central nervous system of the snail, Lymnaea
stagnalis. Brain Res. 525: 101–114, 1990.
DELATORRE, J. C. AND SURGEON, J. W. Histochemical fluoresence of tissue
and brain monoamines: results in 18 minutes using the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience 1: 451–453, 1976.
ELEKES, K., KEMENES, G., HIRIPI, L., GEFFARD, M., AND BENJAMIN, P. R.
Dopamine-immunoreactive neurones in the central nervous system of the
pond snail Lymnaea stagnalis. J. Comp. Neurol. 307: 214–224, 1991.
ELLIOTT, C.J.H. AND BENJAMIN, P. R. Interactions of pattern generating
interneurons controlling feeding in Lymnaea stagnalis. J. Neurophysiol.
54: 1396–1411, 1985.
ELPHICK, M. R., KEMENES, G., STARAS, K., AND O’SHEA, M. Behavioral
role for nitric oxide in chemosensory activation of feeding in a mollusc.
J. Neurosci. 15: 7653–7664, 1995.
FALCK, B., HILLARP, N. A., THIENE, G., AND TORP, A. Fluorescence of
catecholamines and related compounds condensed with formaldehyde. J.
Histochem. Cytochem. 10: 348–354, 1962.
/ 9k17$$au17 J721-6
FURNESS, J. B., COSTA, M., AND WILSON, A. J. Water-stable fluorophores,
produced by reaction with aldehyde solutions, for histochemical localization of catechol- and indolethylamines. Histochemistry 52: 159–170,
1977.
GADOTTI, D., BAUCE, L., LUKOWIAK, K., AND BULLOCH, A. Transient depletion of serotonin in the nervous system of Helisoma. J. Neurobiol. 17:
431–447, 1986.
GOLDSTEIN, R. S. AND SCHWARTZ, J. H. Catecholamine neurons in Aplysia:
improved light-microscopic resolution and ultrastructural study using
paraformaldehyde and glutaraldehyde (FaGlu) histochemsitry. J. Neurobiol. 20: 203–218, 1989.
GRANZOW, B. AND KATER, S. B. Identified higher-order neurons controlling
the feeding motor program of Helisoma. Neuroscience 2: 1049–1063,
1977.
GRANZOW, B. AND ROWELL, C.H.F. Further observations on the serotonergic
cerebral neurones of Helisoma (Mollusca, Gastropoda): the case for
homology with the metacerebral giant cells. J. Exp. Biol. 90: 283–305,
1981.
GREEN, K. A., HARRIS, S. J., AND COTTRELL, G. A. Dopamine directly activates a ligand-gated channel in snail neurones. Pfluegers Arch. Eur. J.
Physiol. 431: 639–644, 1996.
GRILLNER, S. The effect of L-DOPA on the spinal cord—relation to locomotion and the half center hypothesis. In: Neurobiology of Vertebrate Locomotion, edited by S. Grillner, P.S.G. Stein, D. G. Stuart, H. Forssberg,
and R. M. Herman. London: Macmillian, 1986, p. 269–277.
HARRIS-WARRICK, R. M. Chemical modulation of central pattern generators.
In: Neural Control of Rhythmic Movements in Vertebrates, edited by
A. H. Cohen, S. Rossignol, and S. Grillner. New York: Wiley, 1988, p.
285–331.
KABOTYANSKI, E. A., ZIV, I., BAXTER, D. A., AND BYRNE, J. H. Experimental and computational analyses of a central pattern generator underlying
aspects of feeding behavior of Aplysia. Neth. J. Zool. 44: 357–373, 1994.
KATER, S. B. AND KANEKO, C.R.S. An endogenously bursting neuron in
the gastropod mollusc, Helisoma trivolvis. J. Comp. Physiol. 79: 1–14,
1972.
KATZ, P. S. AND FROST, W. N. Intrinsic neuromodulation in the Tritonia
swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J. Neurophysiol. 74: 2281–2294,
1995.
KATZ, P. S., GETTING, P. A., AND FROST, W. N. Dynamic modulation of
synaptic strength intrinsic to a central pattern generator circuit. Nature
Lond. 361: 729–731, 1994.
KIEHN, O. AND KJAERULFF, O. Spatiotemporal characteristics of 5-HT and
dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat.
J. Neurophysiol. 75: 1472–1482, 1996.
KUPFERMANN, I. AND WEISS, K. R. Activity of an identified serotonergic
neuron in free moving Aplysia correlates with behavioral arousal. Brain
Res. 241: 334–337, 1982.
KYRIAKIDES, M. A. AND MCCROHAN, C. R. Effect of putative neuromodulators on rhythmic buccal motor output in Lymnaea stagnalis. J. Neurobiol.
20: 635–650, 1989.
LENT, C. M. Serotonergic modulation of the feeding behavior of the medicinal leech. Brain Res. Bull. 14: 643–655, 1985.
LO, X. AND WEISS, K. R. Cloning, expression and characterization of two
dopamine receptors from Aplysia californica. Soc. Neurosci. Abstr. 20:
89, 1994.
MAGOSKI, N. S., BAUCE, L. G., SYED, N. I., AND BULLOCH, A.G.M. Dopaminergic transmission between identified neurons from the mollusk, Lymnaea stagnalis. J. Neurophysiol. 74: 1287–1300, 1995.
MCCLELLAN, A. D. Re-examination of presumed feeding motor activity in
the isolated nervous system of Pleurobranchea. J. Exp. Biol. 98: 213–
228, 1982a.
MCCLELLAN, A. D. Movements and motor patterns of buccal mass of Pleurobranchea during feeding, regurgitation and rejection. J. Exp. Biol. 108:
257–272, 1982b.
MCCROHAN, C. R. AND AUDESIRK, T. E. Initiation, maintenance and modification of patterned buccal motor output by the cerebral giant cells of
Lymnaea stagnalis. Comp. Biochem. Physiol. 87A: 969–977, 1987.
MC LEAN, E. M., BEHJATNIA, B., AND MURPHY, A. D. An identified ‘‘dopaminergic’’ buccal interneuron activates patterned motor activity in Helisoma. Soc. Neurosci. Abstr. 15: 737, 1989.
MORTON, D. W. AND CHIEL, H. J. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transition in Aplysia. J. Comp. Physiol. A Comp. Physiol. 172: 17–32, 1993a.
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
We thank A. Carol, J. Gibbons, and C. Comer for technical help with
figure preparation. J. E. Richmond did the first intracellular recording of
interneuron N1a in A. D. Murphy’s laboratory in 1986.
This work was supported by National Science Foundation Grant BNS
91-21374 to A. D. Murphy.
Present address of E. M. Quinlan: Dept. of Neuroscience, Brown University, Box 1953, Providence, RI 02912.
Address for reprint requests: A. D. Murphy, Dept. of Biological Sciences,
M/C 066, University of Illinois at Chicago, Chicago, IL 60607.
823
824
E. M. QUINLAN, B. C. ARNETT, AND A. D. MURPHY
/ 9k17$$au17 J721-6
I. Identification and characterization of cerebral-to-buccal interneurons
implicated in the control of motor programs associated with feeding in
Aplysia. J. Neurosci. 11: 3630–3655, 1991.
SELVERSTON, A. Modulation of circuits underlying rhythmic behaviors. J.
Comp. Physiol. A Sens. Neural Behav. Physiol. 176: 139–147, 1995.
SMITH, D. A. Radular kinetics during grazing in Helisoma trivolvis (gastropoda: pulmonata). J. Exp. Biol. 136: 89–102, 1988.
SMITH, D. A. Comparative buccal anatomy in Helisoma (Mollusca, pulmonata, basommatophora). J. Morphol. 203: 107–116, 1991.
SUSSWEIN, A. J., KABOTYANSKI, E. A., HURWITS, I., AND SAKHAROV, D. A.
Catecholaminergic neurons in the esophagus of Aplysia: a peripheral
brain? Soc. Neurosci. Abstr. 19: 350, 1993.
SWEENEY, D. Dopamine: its occurrence in Molluscan ganglia. Science
Wash. DC 139: 1051, 1963.
TEYKE, T., ROSEN, S. C., WEISS, K. R., AND KUPFERMAN, I. Dopaminergic
neruon B20 generates rhythmic neuronal activity in the feeding motor
circuitry of Aplysia. Brain Res. 630: 226–237, 1993.
TRIMBLE, D. L. AND BARKER, D. L. Activation by dopamine of patterned
motor output from the buccal ganglia of Helisoma trivolvis. J. Neurobiol.
15: 37–48, 1984.
TRIMBLE, D. L., BARKER, D. L., AND BULLARD, B. J. Dopamine in a molluslcan nervous system: synthesis and fluorescence histochemistry. J. Neurobiol. 15: 27–36, 1984.
VEHOVSZKY, A. AND ELLIOTT, C.J.H. The hybrid modulatory/pattern generating N1L interneuron in the buccal feeding system of Lymnaea is cholinergic. Invertebr. Neurosci. 1: 67–74, 1995.
VIALA, D. AND BUSER, P. The effects of L-dopa and 5-HT on rhythmic
efferent discharges in hind-limb nerves in the rabbit. Brain Res. 12: 437–
443, 1969.
WIELAND, S. J. AND GELPERIN, A. Dopamine elicits feeding motor program
in Limax maximus. J. Neurosci. 3: 1735–1745, 1983.
WILSON, R.J.A., KRISTAN, W. B., JR ., AND KLEINHAUS, A. L. An increase
in activity of serotonergic Retzius neurons may not be necessary for the
consummatory phase of feeding in the leech, Hirudo medicinalis. J. Exp.
Biol. 199: 1405–1414, 1996.
YEOMAN, M. S., VEHOVSZKY, A., KEMENES, G., ELLIOTT, C.J.H., AND BENJAMIN, P. R. A novel interneuron having hybrid modulatory-central pattern generator properties in the feeding system of the snail, Lymnaea. J.
Neurophysiol. 73: 112–124, 1995.
08-05-97 14:29:11
neupa
LP-Neurophys
Downloaded from http://jn.physiology.org/ by 10.220.33.4 on June 17, 2017
MORTON, D. W. AND CHIEL, H. J. The timing and activity in motor neurons
that produce radula movements distinguishes ingestion from rejection in
Aplysia. J. Comp. Physiol. A Comp. Physiol. 173: 519–536, 1993b.
MURPHY, A. D., BARKER, D. L., LORING, J. F., AND KATER, S. B. Sprouting
and functional regeneration of an identified serotonergic neuron following
axotomy. J. Neurobiol. 16: 137–151, 1985a.
MURPHY, A. D., HADLEY, R. D., AND KATER, S. B. Axotomy-induced parallel increases in electrical and dye coupling between identified neurons
of Helisoma. J. Neurosci. 3: 1422–1429, 1983.
MURPHY, A. D. AND LU, M. M. Neural control of feeding in basommatophoran snails. Soc. Neurosci. Abstr. 13: 822, 1987.
MURPHY, A. D., LUKOWIAK, K., AND STELL, W. K. Peptidergic modulation
of patterned motor activity in identified neurons of Helisoma. Proc. Natl.
Acad. Sci. USA 82: 7140–7144, 1985b.
NUSBAUM, M. P. AND KRISTAN, W. B., JR . Swim initiation in the leech by
serotonin-containing interneurons, cells, 21 and 61. J. Exp. Biol. 122:
277–302, 1986.
OSBORNE, N. N. Phenylethanolamine-N-methyltransferase and dopamineB-hydroxylase. Immunoreactivity and the occurence of noradrenaline and
adrenaline in the nervous system of the snail Helix aspera. Cell Tissue
Res. 237: 605–608, 1984.
PATON, W.D.M. Central and synaptic transmission in the nervous system
(pharmacological aspects). Annu. Rev. Physiol. 20: 431–470, 1958.
POON, M. Induction of swimming in lamprey by L-DOPA and amino acids.
J. Comp. Physiol. 136: 337–344, 1980.
QUINLAN, E. M., GREGORY, K., AND MURPHY, A. D. An identified glutamatergic interneuron patterns feeding motor activity via both excitation
and inhibition. J. Neurophysiol. 73: 945–956, 1995.
QUINLAN, E. M. AND MURPHY, A. D. Glutamate as a putative neurotransmitter in the buccal central pattern generator of Helisoma trivolvis. J. Neurophysiol. 66: 1264–1271, 1991.
QUINLAN, E. M. AND MURPHY, A. D. Plasticity in the multifunctional buccal
central pattern generator of Helisoma illuminated by the identification of
phase 3 interneurons. J. Neurophysiol. 75: 561–574, 1996.
RICHMOND, J. E., MURPHY, A. D., LUKOWIAK, K., AND BULLOCH, A.G.M.
GABA regulates the buccal motor output of Helisoma by two pharmacologically distinct actions. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 174: 593–600, 1994.
ROSEN, S. C., TEYKE, T., MILLER, M. W., WEISS, K. R., AND KUPFERMANN,