* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The human brain has on average 100 billion neurons, to each
Lateralization of brain function wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Limbic system wikipedia , lookup
Evolution of human intelligence wikipedia , lookup
Neuromarketing wikipedia , lookup
Electroencephalography wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neural modeling fields wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Human multitasking wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Single-unit recording wikipedia , lookup
Neurogenomics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Time perception wikipedia , lookup
Neuroesthetics wikipedia , lookup
Brain–computer interface wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuroinformatics wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Magnetoencephalography wikipedia , lookup
Mind uploading wikipedia , lookup
Synaptic gating wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Neurophilosophy wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain morphometry wikipedia , lookup
Neurolinguistics wikipedia , lookup
Selfish brain theory wikipedia , lookup
Neurotechnology wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Human brain wikipedia , lookup
Brain Rules wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Nervous system network models wikipedia , lookup
Aging brain wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
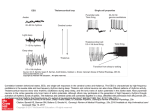
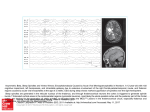
Issue 1 VERY Oct 04 SCIENTIFIC AUSTRALIAN Probing the hidden secrets of the Brain… … A physical investigation into the underlying physiology of the brain by Elie Matar Did you know? The human brain has, on average, 100 billion neurons, and to each neuron there exists between 10 and 10000 connections to other neurons. Therefore theoretically, the minimum number of activation patterns possible in the brain is about 10 raised to the power of 100 billion – more than the estimated number of atoms in the entire universe! The problem of ‘unravelling’ what is arguably the most complex system in the universe – the human brain - has been plaguing scientists for centuries. However, with the prevalence of new technological advancements and modern scientific tools, more has become understood of that which is the source of our cognitive and physical behaviour. Elie Matar reports. A team of scientists at the University of Sydney have taken a step back to focus on an observation made by Richard Caton in 1875 and since has become a widely known fact – that the brain generates electrical activity. Many of the approaches to understanding human brain function and structure in recent years has involved the mathematical modelling of the complex neural networks or ‘circuits’ that constitute the major proportion of the brain. It has been understood that these are also directly associated with its organisation and operation. Such studies have been aided by the emergence of many new and viable diagnostic methods that have allowed different aspects of brain behaviour to be analysed and measured. From the examination of oxygen in the flow of blood in the brain to the metabolic processes, terms such as fMRI (functional Magnetic Resonance Imaging) and PET (Positron Emission Tomography) have become commonplace in the realms of neuroscience. However, it is renewed exploration of a method discovered more than a century ago that has attracted the interest of Scientific Australian. Through a device known as an Electroencephalogram, (EEG), a spectrum of this electrical activity is resolved and then analysed. What are EEGs? Electroencephalograms (EEGs) are measured scalp potentials as a result of cortical electrical activity amassed over scales larger than single neurons. They are obtained via the placement of electrodes at specific points over the surface area of the scalp. While it has spatially rough scales of about 2cm, the time scale is superior to most other methods, with accuracy to the scale of milliseconds! Associations between brain function and EEG spectra have been used extensively in diagnostic methods; however the specific links between these spectra and the underlying 1. physiology have not been clearly understood. Despite this obstacle, Professor Peter Robinson and a team of researchers at the University of Sydney, in conjunction with the Westmead Centre for Brain Dynamics, developed a continuum model, based on experimentally derived parameters, that was able to reproduce the spectra observed from EEG’s. The success, predominance and efficacy of the model relied on its adherence to a strict framework (below) that aimed to provide sufficient but detailed mathematical rationalisation of the most crucial aspects of the brain in terms of its electrical activity. Framework 1. Foundations in real physiology with the most crucial structures and their properties taken into account. 2. Development of a model that relates global dynamics of EEG to small scale physiology 3. Description of process in terms of mathematical steady-state equations with independently determined parameters. 4. Reproduce observable EEG spectra from theory. A discussion of the model, according to these criteria, will follow; along with a sample of results obtained that demonstrate its usefulness and application. Issue 1 VERY SCIENTIFIC AUSTRALIAN Oct 04 Physiological foundations The critical physiology of the brain represented in this model includes the neuronal populations of the cortex and the thalamus (see across). Why these in particular? It seems quite obvious why the cerebral cortex should be included. Not only does it comprise the greatest volume of the brain, but it is the structure that lies closest to the recording electrodes of an EEG. The reason for the thalamus is no less obvious, as the primary receiver of sensory input to the brain, sensory information from external stimuli comes through the thalamus to be then transmitted to other structures in the brain. In the process of reading this article, your vision is transformed into a series of electrical pulses that travel to the thalamus and are then sent primarily to the cerebral cortex where they are further processed. This is what leads to the conceptualization of the key interactions in the brain as being a series of circuits or loops between populations within the thalamus to the cortex and vice versa. The thalamus is broken up into two main divisions, the relay nuclei, which function to transmit the signals to the cortex, and the reticular nucleus, which has an inhibitory effect on these signals. The thalamus also receives feedback from the cortex thus closing what is called a ‘corticothalamic loop’. The model treats the populations of neurons in the brain as a continuum rather than as individual cells. The properties of a specific neuron are averaged over about a tenth of a millimetre which is a sufficient approximation, considering an EEG measures roughly to about 2 cm! Guide to the Human Brain The field of neuroscience and Brain Dynamics is fast emerging in the scientific world and a working knowledge of the brain is important to keep up with the field. The aspects of the brain that are fundamental to this model include the neuron, the thalamus and the cortex. Fig. 3 - A Pyramidal Cell The neuron (pictured above) is the most fundamental unit of the brain and nervous system. These cells communicate to each other via connections from axons to dendrites across a gap called a synapse. Impulses propagate through these via electrochemical gradients (action potentials). The pulses arrive at the dendrites, and are carried down to the cell body (soma). They then travel down the axon hillock to the axonal tree where they are then imparted to other neurons. There are different types of neurons in the brain. Pyramidal cells are of particular importance, comprising 90% of the cerebral cortex and play an essential part in most neuronal circuits. There are also inhibitory cells that inhibit signals by chemical means, the action of these are highly localised. As we can see here, the model was based on real life physiology that would account for the theoretical reproduction of EEG spectra 2. Fig. 4 – Four lobes of the cerebral cortex The cortex (pictured above) is the outermost layer of the brain; it is the main contributor to the scalp potentials as it is the closest to the scalp and the site of termination of many of the electrical signals that arrive to the brain. The cortex receives most of the sensory information from the thalamus. With a relative thickness of 2-4 mm, it is often treated in its unfolded state as a 2D sheet with a total length of 1m! Fig. 5 – Position of thalamus in a medial cross-section of the brain The thalamus (above) is the main gating station for all sensory input received from the peripheral nervous system (except smell) and relays this information to other parts of the brain, namely the cortex. It also receives feedback from the cortex and forms closed corticothalamic loops. Populations (nuclei) within the thalamus act to either relay the signals (excitatory) or inhibit them (Inhibitory). In the next sections we will investigate how the model works to relate large-scale dynamics (EEG) to this small-scale physiology. Issue 1 VERY SCIENTIFIC AUSTRALIAN Oct 04 Developing the model So now we deal with the question of how we break down this information into a set of mathematical equations that will help us extract an EEG from the basic physiological principles we just discussed. The Approach immeasurably complex neurocircuitry in the brain into a viable and mathematically flexible system can be a difficult task in the least. However, these researchers at the University of Sydney have achieved such a model that adequately depicts these circuits in an accessible form. “...Simplifying the…complex neurocircuitry in the brain…can be a difficult task…” Most modelling theory emanates from the basic instinct to get from A to B. Ok, so what is B? Well, we want a model that gives us the output of an EEG at the cortical surface, therefore our endpoint has to be a power spectrum that is the sum of all the populations on the surface of the cortex (remember this is a continuum model) And that’s our B! The elemental features of this representation include treating the thalamus as two functioning nuclei the relay nucleus and the reticular nucleus (refer to previous page). What about A? As mentioned in our framework, we want to derive this model from basics; i.e., from small-scale physiology. So A has to be the source of our power output at a basic structural level. And what is the primary source of the impulses in the brain? Casting our minds to the previous section, we realize that the thalamus, which receives almost all the external input, is an ideal starting point. So in this model, the thalamus is our A. The sum of all the fields over the cortical loop ultimately affects the power output. And stepping up the scale, the sum of all the loops over the continuum of neuronal populations over the area covered by the electrodes determines the final EEG output spectra. How do we get from A to B? Herein lies the ingenuity. In previous section, we discussed significance of certain loops in brain between the cortex in thalamus. Simplifying the the the the the Neuro-circuitry: Any of the complex series of biological circuits present in the human brain that are activated in several patterns cohesively to perform a given function. Model Makeover! Fig. 6 – Schematic interactions between the thalamus and the cortex The impulses are defined by the pulse density field Φ. The inhibitory and excitatory action of the respective nuclei in the cortex and the thalamus are included. Parameters, Parameters… And of course no biophysical model would be complete without biophysical parameters. Usually, the more complex a system is, the more parameters involved. Fortunately in this model, they have been restricted sufficiently without sacrifice of detail to the model. Among these include the propagation delays, projection ranges, and damping rates. Most are independently measurable and have fixed anatomical values. These are direct physiological links in the model. 3. Fig. 7 – The simplified representation of the Corticothalamic loop Both figures stress the primary structures of the brain – the cortex and the thalamus as well as the corticothalamic loop that connects them. The Subscript ‘e’ denotes excitatory action and ‘i’ inhibitory. Φ represents the average of the field potential of each population. The loop begins with the external impulse Φn feeding into the relay nuclei (s). The signal is then relayed to the cortex (e) via Φs. This signal is then processed in the cortex with regulatory action Φi accounted for by inclusion of inhibitory populations within the cortex itself. The corticothalamic circuit is completed by cortical feedback Φe to the thalamus. An EEG measures Φe at the cortical surface. Issue 1 VERY Oct 04 SCIENTIFIC AUSTRALIAN The Physics of the model Fundamentally, the physics is based on these corticothalamic loops being the driving force behind the EEG spectra. This article will now only discuss the theory at a superficial level and leave it to the reader’s own curiosity to indulge in the rigorous details of the proofs. (See Further Reading) 2. Pulse Generation The action potentials generated at the axon hillock are averaged over a population of neurons. The properties of the population are statistically analysed and, along with deviation between different populations, are taken into account in a formula describing the firing rate. A theoretical perspective 3. Propagation The main aspects of theory: The propagation of the pulses throughout the neural matrix are analysed using the mathematical concept of propagators, (outlined below). Linear propagation is assumed as an approximation in this model and describes the transfer of the impulses across the axonal tree and the dendrites of the receiving neuron. The expression for the propagators takes into consideration the arborisation (tree-like branching) of these respective structures. 1. Soma potential, synaptic and dendritic dynamics 2. Pulse generation at the axon 3. Propagation of the pulses within and between neuron populations 4. Parameters involved 5. Relation to corticothalamic loops 1. Soma potential and synaptodendritic dynamics In accordance with the continuum model, the mean soma potential is calculated for a particular population of neurons. This was done by making a linear summation over all the potential contributions arriving at the soma from the dendrites of the cell. The dynamics are in terms of spatiotemporal coordinates. The spatial coordinates are derived from a primary topographic mapping between the thalamus and cortex, treating the cortex as a 2D sheet in its unfolded state. The corresponding responses of the subpotentials in a cell due to the synaptodendritic dynamics are approximated by a differential equation. (One of many!) Thus, the power output observed in an EEG of the brain begins at the scale of subpotentials. The theory then goes on to show the way a signal travels is approximately the same as a damped wave! So a second order differential equation (an equation that governs wave motion) is substituted. This is in terms of projection ranges and damping rates. A simplified and rearranged equation for propagator Γab (denoting Propagators: Propagators are essentially functions that allow you to determine where a particular subject (such as an impulse) will be given its spatial coordinates at an earlier time. Here is the expression for the propagator Γab(0) after simplification in terms of angular frequency ‘’ and the wave vector ‘k’ . Note: q2r2 is a function of 4. propagation between population ‘b’ to ‘a’ is shown in terms of the wavenumber ‘k’, the damping coefficient γab, and projection ranges rab. 4. Parameters The equations describing the propagation of the pulses throughout the brain rely on roughly 40 physiologically dependent parameters. In this article, we detail the investigation of one such parameter rab denoting the projection range (explained later). Other parameters include the already mentioned damping and synaptodendritic rates, but also propagation delays, gains and projection velocities. The Transfer function: The equation below is called the ‘transfer function’ and is the ratio of the averaged impulses Φe at the cortex to the input Φn at the thalamus. The magnitude of this expression squared yields the power spectra relating to that observed by an EEG in terms of and k. 5. Corticothalamic loops All parameters above are given for each set of populations – intracortical, intrathalamic and corticorthalamic shown on the schematic diagram on the previous page. All these terms appear in the final treatment of the mathematics exemplified in the transfer function evaluated and manipulated to obtain the power spectrum at the cortex, as we will see next. Issue 1 VERY Oct 04 SCIENTIFIC AUSTRALIAN EEG spectra – General Results Now comes some reward for all that ‘brain strain’ - the muchanticipated results! But before you switch your brain waves into alpha (a stable resting mode), a tiny bit more theory is required to understand the added twist included in this section. Some general results Fig. 7 (left) – Spectra obtained from an adult subject (solid) against model predictions (dotted) Fig. 8 (bottom left) – The isolated model predictions for power spectrum in terms of the frequency Over the next couple of pages, we will see some general results proving the validity of the model and outlining generic features of the spectra for the untrained eye. Then we will follow an exploration conducted on the effect of the projection range parameter (rab) in the model. Fig. 9 (below) – The power spectrum in terms of the wave number The general results Two-dimensional integration resolves the power spectra as a function of ‘’ or ‘k’, which is used in this section to analyse the results. All parameters and observed readings have been obtained from an adult in relaxed, eyes closed state. All graphs presented in this article have been calculated and plotted using IDL (Interactive Data Language). Before we continue, we shall first confirm the validity of the model using Figure 7. In this figure, the input signal Φn is approximated by white noise across all frequencies. As we can see, there is a strong correlation between the theoretical predictions and the observed data. Most of the results to follow will be reproduced in terms of frequency-dependent power spectra to allow for a more straightforward interpretation. The spectrum in terms of the wave number in Fig. 9 is only included for the interest of completeness The general features of the curves in normal steady state mode such as that shown in Figures 7 and 8 include alpha and beta peaks, which are simply resonance phenomena occurring within the 8-12 Hz and 13+ Hz range respectively. There is a predominant alpha peak at about 10 Hz and is a dominant feature in these corticothalamic loops. It has come to be realized that the stronger these loops, the greater the resonance at 10 Hz by which they have thus come to be defined by. It can also be noted that there is a dominance of the spectra at low frequencies with a leveling effect occurring in the range of 0.1-4 Hz. This is due to an effect known as ‘low pass filtering’, which occurs at the synaptodendritic scale, allowing only the lowest frequencies to dominate in the power spectra. Also as a result, there is a sharp decline in 5. the power spectrum after a cut-off frequency occurring at about 15 Hz. While this model accounts for all the features of an EEG, there remains some uncertainty shrouding the exact physiological basis of some of the features, and in particular, their dependence on the individual factors in the transfer function. Terminology: White Noise: A random signal with equal power across all frequencies. Alpha Range: Frequency band in the range 8-12 Hz Beta Range: Frequency band in the range 13 Hz and above Resonance: The prominence of a particular frequency or band in response to a stimulus. Cut-off frequency: A frequency that marks the transition of a signal or spectra towards zero. Issue 1 VERY Oct 04 SCIENTIFIC AUSTRALIAN EEG spectra – An exploration of the projection range parameter Scientific curiosity coupled with man’s thirst for knowledge has always been a driving force that leads scientists to explore, analyse and draw meaning out of any phenomenon with as much depth as possible. In trying not to break such a fundamental law, we include some details of a project that gives greater insight into the structure of the brain and its corresponding observed electrical phenomena. The essence of the project relies on varying the projection ranges in the model and interpreting the EEG output. But first - a little bit more on projection ranges. The Results (a) (b) (c) (d) What are projection ranges? The parameter rab refers to the projection range from population b to a. It is defined as the spatial range over which the signal is transmitted. In other words it is a measure of the dispersion of the signal throughout the brain. Fig. 11 a) Varying relay-cortex b) Varying cortex-thalamus c) Varying intrathalamic ranges d) Varying intracortical excitatory e) Varying intracortical inhibitory (e) A decrease in the range would correspond to an increase in the specificity of the signal. Conversely, an increase in the range would lead to a decrease of the specificity. The diagram below summarises this in terms of the corticothalamic interactions. The solid lines in the graphs indicate the original value of parameters for a normal steady state of a typical adult and the others are the result of changing one or more values of ranges res, rse, rre, rsr rrs, ree and rei Projection Ranges Fig. 10 - Difference between specific and diffuse projection ranges for signals – ‘es’ (blue) and ‘se’ (red). Left has small projection range while the right a large range. 6. The results above look similar at first glance, but on further inspection, one sees that there are subtle but noticeable differences owing to changing different parameters in different populations with either inhibitory or excitatory roles. By brief mathematical analysis, we can guess what the trends should appear like and then look at them in further detail. Issue 1 VERY SCIENTIFIC AUSTRALIAN Oct 04 EEG spectra – An exploration of the projection range – (Cont’d) In the mathematical model, most of the range terms exist in the denominator of the transfer function. Therefore by mere inspection we can roughly assume that by increasing the ranges the spectral power density will decrease. Some of the more specific features of the results can also be inferred by realising that increasing the specificity of the impulse further emphasises the corticothalamic loops in the model. The stronger and more defined that these loops become, the more the features that are dependent on the loop - such as the peaks - are accentuated. Fig. 11 (a) represents the spectra when range res - from the thalamus to the cortex - is increased from 0.3 mm (solid line), to 0.5 m. We can note the obvious shift downward in the power spectrum, confirming our hypothesis. In fact, increasing the ranges of all the excitatory populations such as in Fig. 11 (b), and (d), display that property of a decreasing power output. An interesting note can be made when observing the degree to which the output spectra decline. The shift up or down in the spectra refers to its normalization. In (a) which denotes variations in the relay to cortical ranges, there is a 10-fold decline in the output spectra. Meanwhile there is only a barely noticeable (25%) decline in the cortex to the thalamus spectrum. This means that the cortical feedback loops play a much less important role in the normalization, and thus the magnitude, of the output power spectrum at the cortical surface. In harmony with this decline in power spectra, there is also a noticeable smearing effect on the features of the curve. The alpha and beta peaks become much less prominent in all cases (a), (b) and (d). So it can be added that while the cortical feedback loops do not strongly affect the normalization, they have a significant effect on the fine resonance features of the graphs. In the case of all of the figures, the smearing effect on the output can be attributed to a smearing effect occurring at a physiological scale, whereby the connections in the corticothalamic loops are ‘smeared’ or spread out and deviate from the loops. Consequently, decreased weighting on the loops by increasing range results in the loss of power emitted at the resonant frequency by these loops - recalling the dependency relationship of these peaks with respect to the corticothalamic loop. Parallels may also be drawn between the intrathalamic and the intracortical spectra in Fig 11. (c), (e). For (c) the ranges rsr and rrs were increased together. While for the intracortical diagram, only the inhibitory ranges rei were increased. But here, the power spectrum increases with increasing range! While this may appear to contradict our earlier argument, remember that the action of these populations or connections is inhibitory. And so by increasing the diffuseness of the connections we are decreasing their action on the corticothalamic circuits, which is analogous to increasing the excitatory impulses. Thus the loops become less inhibited, more defined and lead to a greater power output at the cortical surface. 7. The last main thing to note in the diagrams is the variance in the low pass filtering effects. Essentially, by looking at the graphs that have main effects on EEG – (a) and (d) we can see the cut off frequency, before the spectrum begins to decline, decreases so that the effect of low pass filtering is stronger. How might this correspond to what is actually happening according to the physiology? As we know, increasing the projection ranges increases the dispersal of the signals. On a physiological level, this corresponds to the spreading out of a signal across the axons and their reception across different lengths of the dendrites. This changes the temporal profile of the signals when they reach the soma and are then relayed. This is what causes the observed low pass filtering in the first place, remembering that the dispersal of the signal happens anyway but not to the same extent. We shall leave the discussion at that, but the ‘take-home lesson’ is evident in the scope of skills employed and the results obtained by analysing only a small part of the model as a whole. Like a fractal image of the brain, the closer we look, the more we find out. Simple circuits: A useful analogy It may help to think of the corticothalamic loop as a simple, ideal electrical circuit. A light bulb connected in series to a fixed power source (input stimulus) can be the EEG output. The circuit represents a corticothalamic loop. Increasing the projection ranges is analogous to sequentially adding resistors to the circuit in parallel. The current is divided and decreases in the main loop, thus the power running through that section of the circuit will also decrease along with the intensity of the light. Issue 1 VERY SCIENTIFIC AUSTRALIAN Oct 04 Implications – What can we do with this? At this point, the implications of this work should have become self-evident. Just by exploring a single parameter, we have been able to infer what happens in the brain at a real physical scale. There are numerous implications of this model with respect to further research as well as in the pragmatic sense of practical application. The model’s explanation of the basis for EEG spectra will allow us to ‘probe’ underlying physiological parameters from observed output spectra of subjects. It also follows that we can work backwards so that we start from an EEG reading and from that, infer the values of different states and projection ranges. This paves the way for EEGs being used as diagnostic tools for finding abnormalities in patients, tracing specific features of EEGs to their physiological source. In fact, the model is currently being used to analyze the spectra of people suffering from epilepsy, tumors and attention deficit hyperactivity disorder (ADHD) as well as gauging the effects of the presently available treatments. (See Further Reading) Stepping up to a more general scale, the model may even shed light on why certain structures of the brain are organized the way they are - taking us one step closer to the ultimate goal of a complete understanding of the human brain. Future work - Where do we go from here? research into the effects of other parameters, for example the, propagation delays or projection velocities would increase its precision. Fig 12 – Electron micrograph of a neural network The model in its entirety leaves a lot of facets to be explored, an example being the case study of the effect of the projection range variation within the model. Further The inclusion of other subcortical structures such as the basal ganglia or the brain stem would improve the accuracy of the model by further accounting for the subtle but complex contributions of these structures outside the corticothalamic loops. The inclusion of modulating dynamics in and between these extra structures with the extra feedback loops would serve as a definite enhancement to the model. On the same idea of physiological truthfulness, extra populations in the thalamus may be included greater as there are really 12 nuclei in the thalamus. These are simply some of the possible future investigations that will yield greater resolution of the EEG spectra in terms of specific coordinates. Conclusion The human brain, while aweinspiring, was and still remains an enormous source of frustration as an obstacle to all who thirst to understand the inner workings of the mind. However through the growing wealth of scientific knowledge demonstrated by this article, the ever expanding frontier of technological advancement, and through the persistence and innovation of dedicated scientists such as those at the University of Sydney - we are slowly, but surely…unravelling the secrets of the human brain. ■ An Updat VERY SCIENTIFIC AUSTRALIAN Oct 04 e ofIssue 1 the Acknowledgements Huma 8. n I thank my Supervisor, Prof. Peter Robinson for his tutelage throughout this TSP project. The time and patience Brain spent on us (especially the times when we were eating away at your schedule!) was greatly appreciated. The field of Thanks also to Peter Robens for his collaborative effort on this project. neuroscien ce and Brain Dynamics is Further Reading fast emerging in the Robinson PA, (2004). Propagator theory of brain dynamics. Physical Review E, 72: 011904 scientific world Robinson and PA, (2003). Brain Waves. The Physicist, Vol 40, No. 5. pp 132-137 a working knowledge Robinson PA (2003). Interpretation of scaling properties of electroencephalographic fluctuations via of the brain spectral analysis and underlying physiology. Physical Review E, 67: 032902. is important to keep up! The Robinson PA, Rennie, CJ, Rowe, DL (2003). Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Human Brain Mapping, 23: 53-72. aspects of the brain that are Robinson PA, Rennie CJ, Rowe DL (2003). Neurophysical modelling of brain dynamics. fundament Neuropsychopharmacology, 28: S74-S79. al to this model Robinson PA, Rennie CJ, Wright JJ, Bahramali H, Gordon E, Rowe DL (2001). Prediction of include the electroencephalographic spectra from neurophysiology. Physical Review E, 63: 021903. neuron, the thalamus Rowe DL, Robinson PA, Gordon E. (2004). Stimulant drug action in attention deficit hyperactivity and the cortexdisorder all of (ADHD): inference of neurophysiological mechanisms via quantitative modelling. Clinical whichNeurphysiology, are 2004: 1-12. discussed below.O’Connor SC, Robinson PA (2005) Analysis of electroencephalographic activity associated with thalamic tumors. Journal of Theoretical Biology, 233 (2005) 271 - 286 The neuron is the most Wright JJ, Rennie fundament al unitand of global scales. the brain and nervous system, these cells communic ate to each other via connection s from axons to dendrites across a gap called a synapse. Impulses propagate CJ, Robinson PA (2003). Simulated electrocortical activity at microscopic, mesoscopic Neuropsychopharmacology, 28: S80-S93. 9.