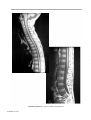
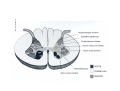
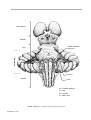
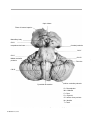
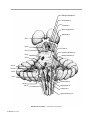
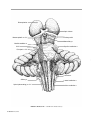
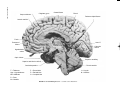
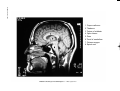
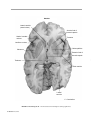
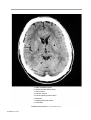
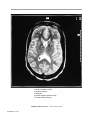
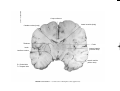
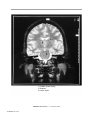
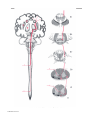
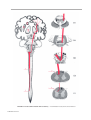
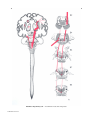
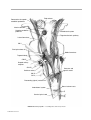
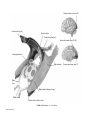
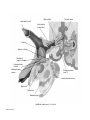
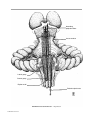
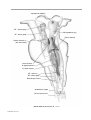
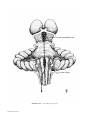
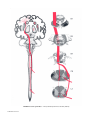
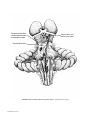
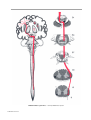
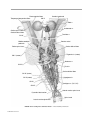
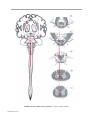
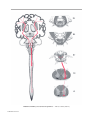
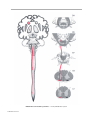
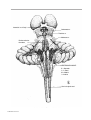
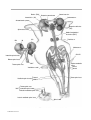
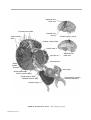
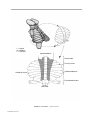
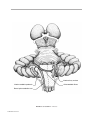
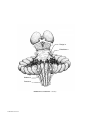
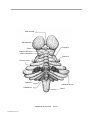
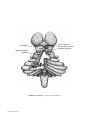
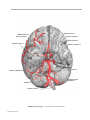
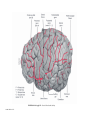
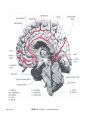
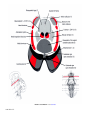
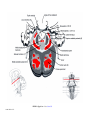
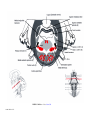
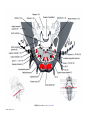
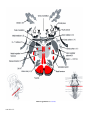
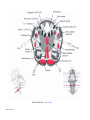
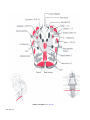
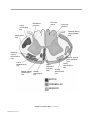
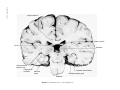
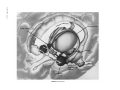
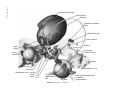
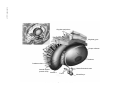
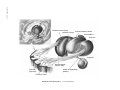
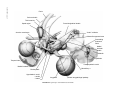
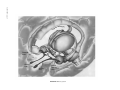
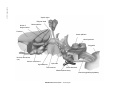
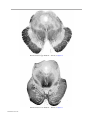
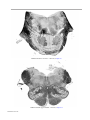
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ATLAS OF FUNCTIONAL NEUROANATOMY
Cognitive neuroscience wikipedia , lookup
Environmental enrichment wikipedia , lookup
Central pattern generator wikipedia , lookup
Metastability in the brain wikipedia , lookup
Embodied language processing wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroregeneration wikipedia , lookup
Time perception wikipedia , lookup
Development of the nervous system wikipedia , lookup
Human brain wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Aging brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Synaptic gating wikipedia , lookup
Microneurography wikipedia , lookup
Neuroanatomy wikipedia , lookup
Limbic system wikipedia , lookup
Evoked potential wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Hypothalamus wikipedia , lookup
Basal ganglia wikipedia , lookup
Superior colliculus wikipedia , lookup
ATLAS OF FUNCTIONAL NEUROANATOMY By WALTER J. HENDELMAN, M.D., C.M. Professor Department of Cellular and Molecular Medicine Faculty of Medicine University of Ottawa Ottawa, Canada ©2000 CRC Press LLC Library of Congress Cataloging-in-Publication Data Hendelman, Walter. Atlas of functional neuroanatomy/by Walter J. Hendelman. p. cm. Includes bibliographical references and index. ISBN 0-8493-1177-2 (alk. paper) 1. Neuroanatomy--Atlases. I. Title. [DNLM: 1. Central Nervous System--anatomy & histology-Atlases. WL17 H495a 2000] QM451 .H347 2000 611.8’022’2--dc21 99-087837 This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. The consent of CRC Press LLC does not extend to copying for general distribution, for promotion, for creating new works, or for resale. Specific permission must be obtained in writing from CRC Press LLC for such copying. Direct all inquiries to CRC Press LLC, 2000 N.W. Corporate Blvd., Boca Raton, Florida 33431. Trademark notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation, without intent to infringe. ©2000 by CRC Press LLC No claim to original U.S. Government works International Standard Book Number 0-8493-1177-2 Library of Congress Card Number 99-087837 Printed in the United States of America 1 2 3 4 5 6 7 8 9 0 Printed on acid-free paper ©2000 CRC Press LLC DEDICATION To my wife, Teena, and our children, Lisanne and Devra To my many teachers and mentors, particularly in remembrance of Dr. Donald Hebb Dr. Richard Bunge Dr. Malcolm Carpenter each an inspiring tutor at a particular point in my growth and development as a neuroscientist And finally, to all students of the Brain … ©2000 CRC Press LLC PREFACE The instructional goal of this Atlas is to assist the student of the brain to achieve an understanding and a three-dimensional visualization of the human central nervous system (CNS). The Atlas of Functional Neuroanatomy is written for medical students who are studying the CNS for the first time, students in allied health fields, and professionals-in-training (physicians, nurses, physical and occupational therapists) who require a visual reference to the structures of the CNS, as well as students in certain undergraduate courses, particularly neuroscience and psychology. Regardless of the student, the challenge to the teaching faculty is the same — how could we improve, enhance, and facilitate the learning process? We, as teachers, must see the learning task from the perspective of the student — how can he/she learn, understand, and assimilate this very complex subject matter, particularly with the volume of material to be learned and the short period of time typical of new curricula? Clearly the challenge to any author is to try to organize and reduce the information load and present core material with adequate explanation. The Atlas of Functional Neuroanatomy contains both diagrams and text, an optimum way of guiding the student through the complexity of the structure and function of the CNS, with some clinical references to make the information relevant to the real world of people with diseases. The illustrations include diagrams and photographs, each labeled to the degree necessary for a student learning the material for the first time, or for a professional requiring a resource review of the CNS. The focus is on the illustrations, each of which is accompanied by explanatory text on the facing page, supplemented by a brief introduction to various sections (e.g., brainstem, motor systems, limbic). This Atlas is built upon three previous editions [titled Student’s Atlas of Neuroanatomy] which began with some illustrations, then photographic material, and subsequently added air brush diagrams on the basal ganglia, thalamus, and limbic system. After use in our course here and much feedback from students, the material of the Atlas has been significantly reorganized and rethought, and much of the text rewritten. The approach of this revised Atlas is to integrate the structure and function so that a student can understand the neurological approach to disease of the nervous system, with a focus on where the information has been interrupted. The Atlas starts with an Orientation to the various parts of the nervous system, presented from the spinal cord upward to the brain. Radiographic material has been added, since this is the way the CNS will be viewed and investigated by all our students in clinics. The second section, Functional Systems, presents the sensory and motor pathways as they traverse the nervous system. The addition of color to these diagrams contributes substantially to their visual impact. The third section, Neurological Neuroanatomy, has both an anatomical and neurological orientation. Sufficient information is given to allow the student to work through the neurological question — where is the disease process occurring (i.e., neurological localization)? The emphasis in this section is on the brainstem. To assist in this goal, a select series of cross sections of the human brainstem is included. In addition, new illustrations have been added on the blood supply to the brain, using color and graphic overlays, since vascular lesions are still most common and relate closely to the functional neuroanatomy. This section is supplemented with cross sections of the human brainstem. The section on the Limbic System has been completely revised and much reduced in content from the previous edition. It is placed as the last section of the Atlas because it can be taught at various points in the medical curriculum, e.g., as part of “mind” or psychiatry. Other courses might not include this specialized topic. Consistent with the computer/digital revolution, many of the illustrations were converted into computer graphics, with tones of shading. Color has been added to facilitate the visualization of the CNS pathways, in both system-based (Section B) and cross-sectional diagrams (Section C). Some students might still want to add color to the illustrations; ©2000 CRC Press LLC coloring the illustrations has assisted many students by adding an active component to the learning process. (A guide to color coding is included after the list of illustrations.) Much of the subject matter’s difficulty is terminology — complex, difficult to spell, sometimes inconsistent, with a Latin base, and sometimes with names of individuals (used often by neurologists, neurosurgeons, and neuroradiologists). A glossary of terms is appended to help the student through this task. Students might wish to consult more complete texts on the anatomy and physiology of the nervous system and certainly some neurology books. A guide to this reference material is included in the annotated bibliography. Added to this are suggestions for material available on CD-ROMs, as well as the Internet. Students are encouraged to seek out additional resources of this nature. The digital revolution has led to the expectation of a visual presentation with clear graphics on the screen! Therefore, this edition of the Atlas is being published with an accompanying CD-ROM, allowing the use of full-color illustrations, where relevant. As in the book, each graphic has a brief explanatory text. We hope that students will have easy access to view this CD and that this additional resource will enhance learning! The author is grateful to CRC Press for agreeing to publish the Atlas with the CD. Many individuals have contributed to the Atlas. Their efforts are deeply appreciated. We have worked collaboratively to try to present a clear understandable view of the structure and function of the CNS. All have worked under my direction and therefore the ultimate value of the Atlas, whether favorable or unfavorable, rests on the shoulders of the author. You, the learner, will be our best judge. Comments are welcome and can be e-mailed to the author ([email protected]). Special thanks are extended to the members of CRC Press LLC without whose help this project would not have been completed. Walter J. Hendelman, M.D., C.M. ©2000 CRC Press LLC AUTHOR BIOGRAPHY Dr. Walter Hendelman is a Canadian, born and raised in Montreal. He completed his undergraduate studies at McGill University with an honors program in psychology. His first experimental work was with rats that had lesions of the hippocampus, which was then a little-known area of the brain. At that time, Professor Donald Hebb was the chair of the Psychology Department and was gaining prominence for his theory known as “cell assembly” (how the brain functions). Dr. Hendelman then proceeded to do his medical studies at McGill, in the shadow of the world-famous Montreal Neurological Institute (MNI) where Dr. Wilder Penfield and colleagues were forging a new frontier in the understanding of the brain. Dr. Hendelman then completed an internship and a year of pediatric medicine, again in Montreal. Dr. Hendelman’s next decision was between clinical (pediatiric) neurology or brain research — he chose the latter and completed four years of postgraduate studies in the U.S., in the emerging field of developmental neuroscience, using what were then the new techniques of nerve tissue culture and electron microscopy. His research mentor at Columbia Medical Center in New York was Dr. Richard Bunge, and his neuroanatomy mentor was Dr. Malcolm Carpenter, the author of a neuroanatomy textbook. Ottawa, Canada has been the site for Dr. Hendelman’s entire academic career, at the Faculty of Medicine of the University of Ottawa. The Department of Anatomy became the Department of Anatomy and Neurobiology, which then merged with physiology and pharmacology, forming what is now the Department of Cellular and Molecular Medicine. Dr. Hendelman continued his research, using nerve tissue culture and studying the development of the cerebellum; more recently, he has been involved in studies on the development of the cerebral cortex. He is a member of various neuroscience and anatomy professional organizations, has attended and presented at their meetings, and has numerous research publications, often in collaboration with other scientists. In addition to research, teaching, and the usual academic “duties,” Dr. Hendelman was involved with a University committee on research ethics. He has also been very active in curriculum planning and teaching matters in the Faculty. In addition, after some further training, Dr. Hendelman participated in a clinic for the assessment of children with learning problems at the Children’s Hospital of Eastern Ontario. Dr. Hendelman is a student of the brain and has been deeply engaged as a teacher of the subject throughout his career. He is dedicated to assisting those who wish to learn functional neuroanatomy – he has produced teaching videotapes and three previous editions of this Atlas. More recently, he has authored (with collaborators) two computer learning modules, one on the spinal cord based upon the disease syringomyelia, and the other on voluntary motor pathways. Both modules have original graphics to assist the learning of this challenging and fascinating subject matter, the human brain. ©2000 CRC Press LLC ACKNOWLEDGMENTS The illustrations in the Atlas have evolved and could not have been created without the efforts of the following individuals: Illustrations Mr. Jean-Pierre Morrissey, a medical student at the time of the first edition of Student’s Atlas of Neuroanatomy (line drawings of pathways and cerebellum) Dr. Andrei Rosen (the airbrush diagrams of the basal ganglia, thalamus, limbic system) Photography Mr. Stanley Klosovych, formerly director of the Health Sciences Communication Services, University of Ottawa (all photographs of the brain) Medical Artist Mr. Emil Purgina, of the same service, now retired (Figure 21 and improvements to the third edition of Student’s Atlas of Neuroanatomy) Medical Illustrator Mr. Gordon Wright (all digital enhancements) Radiographs Colleagues and staff at the Ottawa Hospital (General Campus) and the Children’s Hospital of Eastern Ontario Brainstem Sections Colleagues and staff of the Department of Pathology, Children’s Hospital of Eastern Ontario Parts of the Student’s Atlas of Neuroanatomy , First, Second, and Third Editions which also appear in this book, were supported by grants from Teaching Resources Services of the University of Ottawa. Also very much appreciated and acknowledged are the contributions of the University of Ottawa Press and W.B. Saunders to the previous editions. The support of my home department and its members at the Faculty of Medicine of the University of Ottawa, including secretaries and other support staff, is gratefully acknowledged; originally called Anatomy, then Anatomy and Neurobiology, the department is now the Department of Cellular and Molecular Medicine. Finally, I gratefully acknowledge the many classes of students who have provided their comments, suggestions, and feedback over the years. With thanks, Walter J. Hendelman, M.D., C.M. ©2000 CRC Press LLC TABLE OF CONTENTS Preface Acknowledgements List of Illustrations Suggestions for Color Coding Section A: Orientation Spinal Cord Brainstem and Cranial Nerves Cerebellum Diencephalon (Thalamus) Cerebral Hemispheres Cortex Corpus Callosum and White Matter Ventricles and CSF Basal Ganglia Internal Capsule Section B: Functional Systems Part I: Sensory Systems Somatosensory and Trigeminal Systems Special Senses (Audition and Vision) Part II: Reticular Formation and Pain Part III: Motor Systems Cortical Brainstem Basal Ganglia Cerebellum Section C: Neurological Neuroanatomy Blood Supply Thalamus Brainstem Histology Midbrain Pons Medulla Spinal Cord Brainstem (Human) Cross Sections ©2000 CRC Press LLC Section D: The Limbic System Limbic Lobe Limbic Structures Hippocampus Amygdala Limbic Diencephalon Hypothalamus and Septal Region Olfactory System Basal Forebrain Annotated Bibliography Glossary of Terms ©2000 CRC Press LLC LIST OF ILLUSTRATIONS Section A: Orientation FIGURE 1A: Spinal Cord I — Spinal Cord: Longitudinal View FIGURE 1B: Spinal Cord — Spinal Cord MRI: Longitudinal View FIGURE 2A: Spinal Cord II — Spinal Cord Cross Section: C8 Level FIGURE 2B: Spinal Cord — Spinal Cord MRI: Axial View FIGURE 3: Brainstem I — Brainstem and Diencephalon: Ventral View FIGURE 4: Brainstem II — Brainstem and Diencephalon: Ventral (Photographic)view FIGURE 5: Brainstem III — Cranial Nerve Nuclei: Motor FIGURE 6: Brainstem IV — Cranial Nerve Nuclei: Sensory FIGURE 7: Brainstem V — Brainstem: Dorsal View (Cerebellum Removed) FIGURE 8: The Cerebellum — Cerebellum and Brainstem (Photographic View) FIGURE 9: The Diencephalon — Thalamus: Orientation FIGURE 10: Thalamus — Thalamus: Nuclei FIGURE 11: Cerebral Hemispheres I — Cerebral Cortex: Dorsal View FIGURE 12: Cerebral Hemispheres II — Cerebral Cortex: Dorsolateral View FIGURE 13: Cerebral Hemispheres III —Cerebral Cortex: Inferior View FIGURE 14: Cerebral Hemispheres IV — Inferior Surface: Brainstem Removed FIGURE 15: Cerebral Hemispheres V — Corpus Callosum: Dorsal View FIGURE 16: Cerebral Hemispheres VI — Cerebral Cortex: Medial View FIGURE 17: Radiologic View of Hemispheres — MRI: Sagittal View FIGURE 18: Corpus Callosum — Cerebral Hemispheres: Dissected View FIGURE 19A: White Matter — Cerebral Hemispheres: Association Fibers FIGURE 19B: White Matter — Cerebral Hemispheres: Association Fibers FIGURE 20A: Ventricles — Ventricles: Lateral View FIGURE 20B: Ventricles — Ventricles: Anterior View FIGURE 21: Cerebrospinal Fluid — Schematic of CSF Circulation FIGURE 22: Basal Ganglia I — Basal Ganglia: Orientation FIGURE 23: Basal Ganglia II — Basal Ganglia: Nuclei A FIGURE 24: Basal Ganglia III — Basal Ganglia: Nuclei B FIGURE 25: Basal Ganglia IV — Basal Ganglia and Ventricles FIGURE 26: Internal Capsule I — Internal Capsule: White Matter FIGURE 27: Internal Capsule II — Horizontal Section of Hemispheres (Photographic View) FIGURE 28A: Horizontal View — Horizontal View: CT FIGURE 28B: Horizontal View — Horizontal View: MRI FIGURE 29: Coronal View — Coronal Section of Hemispheres (Photographic View) FIGURE 30: Coronal View — Coronal View: MRI ©2000 CRC Press LLC Section B: Functional Systems Part I: Sensory Systems FIGURE 31: Anterolateral System — Pain, Temperature, Crude Touch FIGURE 32: Dorsal Column — Medial Lemniscus Pathway — Discriminative Touch, Joint Position, Vibration FIGURE 33: Trigeminal System — Discriminative Touch, Pain, Temperature FIGURE 34: Sensory Systems— Somatosensory and Trigeminal Pathways FIGURE 35: Audition: Hearing — Auditory Pathway I FIGURE 36: Auditory System — Auditory Pathway II FIGURE 37: Auditory System — Auditory Gyri (Photographic View) FIGURE 38: Sensory Systems — Ascending Tracts and Sensory Nuclei FIGURE 39A:Visual System: A — Visual Pathway FIGURE 39B: Visual System: B — Visual Reflexes Part II: Reticular Formation FIGURE 40A: Reticular Formation I — Organization FIGURE 40B: Reticular Formation II — Nuclei FIGURE 41: Pain — Descending Control System Part III : Motor Systems FIGURE 42: Cortico-spinal Tract: The Pyramidal System — Direct Voluntary Pathway FIGURE 43: Cortico-bulbar (and Cortico-pontine) Fibers — Brainstem Motor System FIGURE 44: Rubro-spinal Tract — Non-Pyramidal Motor System FIGURE 45: Descending Tracts and Motor Nuclei — Motor Pathways and Nuclei FIGURE 46: Pontine (Medial) Reticulo-spinal Tract — Indirect Voluntary Pathway FIGURE 47: Medullary (Lateral) Reticulo-spinal Tract — Indirect Voluntary Pathway FIGURE 48: Lateral Vestibulo-spinal Tract — Non-pyramidal Motor System FIGURE 49A: Vestibular System — Vestibular Nuclei FIGURE 49B: Medial Longitudinal Fasciculus — MLF and Associated Tracts FIGURE 50: Basal Ganglia: Circuitry — Motor Regulatory Systems FIGURE 51: Thalamus: Motor Circuits — Motor Regulatory Systems FIGURE 52: Cerebellum I — Functional Lobes FIGURE 53: Cerebellum II — Afferents FIGURE 54A: Cerebellum III — Circuitry FIGURE 54B: Cerebellum IV — Efferents FIGURE 55: Cerebellum V — Superior Cerebellar Peduncle Section C: Neurological Neuroanatomy FIGURE 56: Blood Supply I — The Arterial Circle of Willis (Overlay) FIGURE 57: Blood Supply II — MR Angiogram (MRA) FIGURE 58: Blood Supply III — Cortical: Dorsolateral (Overlay) FIGURE 59: Blood Supply IV — Cortical: Medial (Overlay) ©2000 CRC Press LLC FIGURE 60: Blood Supply V — Internal Capsule and Basal Ganglia FIGURE 61: Thalamus — Nuclei: Histological FIGURE 62: Brainstem: Histology — Ventral View: Schematic FIGURE 63: Brainstem: Histology — Sagittal View: Schematic FIGURE 64: Upper Midbrain — Cross Section (B1) FIGURE 65: Lower Midbrain — Cross Section (B2) FIGURE 66: Upper Pons — Cross Section (B3) FIGURE 67: Mid Pons — Cross Section (B4) FIGURE 68: Lower Pons — Cross Section (B5) FIGURE 69: Upper Medulla — Cross Section (B6) FIGURE 70: Mid Medulla — Cross Section (B7) FIGURE 71: Lower Medulla — Cross Section (B8) FIGURE 72: Spinal Cord Tracts — C8 level FIGURE 73: Spinal Cord — Cross-sectional Views Brainstem Cross-Sections (Human) Section D : The Limbic System FIGURE 74: Limbic Lobe FIGURE 75: Limbic Structures FIGURE 76: Hippocampus (Photographic View) FIGURE 77A: Hippocampal Formation FIGURE 77B: Hippocampal Formation: 3 Parts FIGURE 78: Coronal Brain Section (Photographic View) FIGURE 79A: Amygdala FIGURE 79B: Amygdala — Connections FIGURE 80: Limbic Structures and the Lateral Ventricle FIGURE 81: Limbic Diencephalon — Anterior Nucleus FIGURE 82: Limbic Diencephalon — Dorsomedial Nucleus FIGURE 83A: Hypothalamus and Limbic Midbrain FIGURE 83B: Septal Region and Medial Forebrain Bundle FIGURE 84: Olfactory System FIGURE 85A: Basal Forebrain — Basal Nucleus. FIGURE 85B: Basal Forebrain — Basal Ganglia ©2000 CRC Press LLC SUGGESTIONS FOR COLOR CODING Learning styles vary from person to person, but for many people color seems to add a significant beneficial dimension to the learning of neuroanatomy. Although color has been added to several diagrams in the current edition, particularly the pathways (in Sections B and C), many students have been helped in their learning by adding color to the illustrations. The process adds information and depth to the diagrams and adds an active component to the learning process. We have consistently used various gray tones in the cross sections of the brainstem and spinal cord (Section C) to unify — visually — the functional aspects of the nuclei and tracts. While this presentation scheme might suffice for some students, others might want to use color. For convenience, numbers have been inserted beside certain structures to indicate which color to use (e.g., Figure 64). The same color coding has been used in the accompanying CD-ROM. Note to student: It is recommended that you view the colors used on the CD-ROM and select your coloring choices to match. The following color scheme is suggested: SENSORY Dorsal column – medial lemniscus #7 royal blue Anterolateral #7B blueberry Trigeminal #4 jade green Special senses #11 dark brown #10 orange MOTOR Voluntary (cortico-spinal) Reticular formation #1 yellow Vestibular (nuclei and tracts) #5 salmon Cerebellum (nuclei and tracts) #9 light blue Other motor pathways #8 green Parasympathetic #13 violet Substantia Nigra #12 black Red Nucleus (and tract) #3 red Locus Ceruleus #6 dark blue Other #2 peach SPECIAL NUCLEI Background pencil shading Some students may even wish to add color to some of the airbrush diagrams, including the basal ganglia, thalamus, and limbic system. It’s your choice! ©2000 CRC Press LLC Section A ORIENTATION An understanding of the central nervous system — the CNS — requires a knowledge of its component parts and their specialized function. This section introduces the student to the CNS from an anatomical and functional viewpoint; subsequent sections use these components to build the functional units that are the sensory and motor systems. FUNCTIONAL NEUROHISTOLOGY The CNS is composed of neurons and supporting cells, the glia. The neuron has a cell body (also called soma, or perikaryon), dendrites which extend a short distance from the soma, and an axon which connects one neuron with others, either close by (for interneurons) or at a distance. Neuronal membranes are specialized for electrochemical events, which allow these cells to receive and transmit messages to other neurons. The dendrites and cell bodies of the neurons receive information, and the axons transmit the firing pattern of the cell to the next neuron. Communication between neurons occurs almost exclusively at specialized junctions called synapses, using biological molecules called neurotransmitters. These modify ion movements across the neuronal membranes of the synapse and alter neurotransmission — they may be excitatory, inhibitory, or modulatory in their action. The post-synaptic neuron will modify its firing pattern depending upon the summative effect of all the synapses acting upon it at any moment in time. Within the CNS, neurons that share a common function are often grouped together; such collections are called nuclei. In other parts of the brain, the neurons are positioned at the surface, forming a cortex. In the cortical organization, cells are arranged in layers, and the neurons in each layer are functionally distinct. This arrangement enhances, so it seems, the complexity of information processing. Older cortical areas have three layers (e.g., the cerebellum); more recently evolved cortices have six layers (the cerebral cortex) and ©2000 CRC Press LLC sometimes sublayers. Much of the remainder of the brain consists of axons which connect one part of the brain with other areas. These fibers serve to link the various parts of the brain with each other. Many of the axons are myelinated, which serves to increase the speed of axonal conduction; the thicker the myelin sheath the faster the conduction. Axons originating from one area (cortex or nucleus) and destined for another are usually grouped together and form a tract, also called a pathway (or fasciculus). There are two types of glial cells. Astrocytes are involved in supportive structural and metabolic events. Oligodendrocytes are responsible for the formation and maintenance of the myelin which ensheaths the axons. Some of the early maturation that we see in infants and children can be accounted for by the progressive myelination of the various pathways within the CNS. ORGANIZATION OF THE CNS One approach to an understanding of the nervous system is to conceptualize a number of functional modules, starting with simpler ones and moving to the higher primates and humans with a more complex organizational network of cells and connections. The basic unit of the CNS is the spinal cord (see Figures 1A and 1B), which connects the central nervous system with the periphery. It receives sensory information from the skin and body wall and sends motor commands to the muscles. Reflex circuits and other motor patterns are organized in the spinal cord. These are under the influence of motor areas in other parts of the brain. Afferent and efferent information concerning the autonomic nervous system is also part of the functioning of the spinal cord. The incoming sensory nerves and the outgoing motor nerves organize the spinal cord into segments (e.g., cervical, lumbar) and levels (see Figure 73). As the systems of the brain become more complex, new control “centers” have evolved. These are often spoken of as higher centers. The first set of these is located in the brainstem, which is situated above the spinal cord and within the skull (in humans). The brainstem includes three distinct areas — the medulla, pons, and midbrain (see Figures 3 and 4). Some nuclei within the brainstem are concerned with essential functions, such as the regulation of blood pressure, pulse, and respiration. Other nuclei within the brainstem are involved in setting our level of arousal and play an important role in maintaining our state of consciousness. Special nuclei in the brainstem are responsible for some basic types of movements in response to gravity or sound. Many nuclei in the brainstem are related to the cerebellum. In addition, most of the cranial nerves and their nuclei (which supply the structures of the head and neck) are anchored in the brainstem. The cerebellum is situated behind the brainstem (see Figure 8). This “little brain” is involved in motor coordination and also in the planning of movements. How this is accomplished will be understood once the input/output connections of the various parts of the cerebellum are studied. Parts of the cerebellum are quite old in the evolutionary sense, and other parts are relatively new. The cerebellum has a simpler form of cortex which consists of only three layers. Next in the hierarchy of the development of the CNS is the area of the brain called the diencephalon (see Figures 3 and 9). Its largest part, the thalamus, develops in conjunction with the cerebral hemispheres and acts as the gateway to the cerebral cortex. The thalamus consists of a group of nuclei which project to the cortex and receive reciprocal connections from the cortex. The hypothalamus, a much smaller part, serves mostly to control the neuroendocrine system and also organizes the activity of the autonomic nervous system. Parts of the hypothalamus are intimately connected with the expression of basic drives (e.g., hunger and thirst), the regulation of water in our bodies, and emotional behavior as part of the limbic system (see below). With the continued evolution of the brain there is encephalization, which has culminated in the development ©2000 CRC Press LLC of the cerebral hemispheres. Buried within the cerebral hemispheres are the basal ganglia, large collections of neurons (see Figure 22) which are involved mainly in the initiation and organization of motor movements. These neurons affect motor activity through their influence on the cerebral cortex. The surface of the cerebral hemispheres is occupied by cortex, the cerebral cortex (see Figures 11 and 12), most of which is six-layered (also called the neocortex). We need our cerebral cortex for thinking, consciousness, and language, and many other functions related to the sensory and motor systems. In the human, the cerebral cortex is thrown into ridges (gyri; singular, gyrus) and valleys (sulci; singular, sulcus). The expansion of the cerebral cortex in the human, both in terms of size and complexity, has resulted in this part of the brain becoming the dominant controller of the CNS, being capable, so it seems, of overriding most of the other regulatory systems. A number of areas of the brain are involved in behavior which is characterized by the reaction of the animal or person to situations. This reaction is often termed “emotional,” and in humans it consists of both psychological and physiological changes. Various parts of the brain are involved with these activities, and collectively they have been named the limbic system. This network of neurons includes those found in the cortex, various subcortical areas, parts of the basal ganglia, the hypothalamus and parts of the brainstem. (The limbic system is described in the final section of the Atlas.) In summary, the nervous system has evolved so that its various parts have assigned tasks. In order for the nervous system to function properly, there must be communication between the various parts. Some of these links are the major sensory and motor pathways, called tracts (or fascicles). Much of the mass of tissue in our brain is made up of these pathways (e.g., see Figures 32 and 42). One of the major puzzles in the growth and development of the nervous system involves understanding the mechanisms whereby the various parts of the nervous system link with each other in a seemingly precise manner. STUDY OF THE CNS Early studies of the normal brain were generally descriptive. Brain tissue does not have a firm consistency and the brain needs to be fixed for gross and microscopic examination. One of the most common fixatives used to preserve the brain for study is formalin; after being preserved in formalin, the brain can be handled and sectioned. Areas containing predominantly neuronal cell bodies (and their dendrites and synapses) become grayish in appearance after formalin fixation and are traditionally called gray matter. Tracts containing myelinated axons become white in color with formalin fixation and such areas are likewise simply called the white matter (see Figures 27 and 29). We have learned much about the normal function of the human CNS through study of diseases and injuries to the nervous system. Diseases of the nervous system can ©2000 CRC Press LLC involve the neurons, either directly (e.g., metabolic disease) or by reducing the blood supply which is critical for the viability of nerve cells. Neurons are highly specialized cells and do not have the ability to regenerate; once lost, they cannot be replaced. Some degenerative diseases affect a particular group of neurons. Other diseases can affect the cells supporting the myelin sheath, thereby disrupting neurotransmission. Biochemical disturbances may disrupt the balance of neurotransmitters and cause functional disease states. The recent introduction of functional imaging of the nervous system is revealing fascinating information about the functional organization of the CNS. We are slowly beginning to piece together an understanding of what is considered by many as the last and most important frontier of human knowledge, an understanding of the brain. FIGURE 1A SPINAL CORD I SPINAL CORD: LONGITUDINAL VIEW The spinal cord is an elongated part of the CNS that is located in the vertebral canal. It consists of gray matter (neurons), organized as nuclei (see Figure 2A), and white matter, the various pathways (see Section B; also Figures 72 and 73). Nerve roots enter and exit from the spinal cord, giving the appearance of a segmented structure. The spinal cord is covered with meninges — dura, arachnoid, and pia. The four vertebral levels — cervical, thoracic, lumbar, and sacral — are indicated (on the right side). The spinal cord ends at the level of L2 (second lumbar vertebra) in the adult. Hence, the levels of the spinal cord do not match the vertebral levels. One must be very aware of which reference point is being used when discussing spinal cord injuries, the vertebral or spinal. Macroscopically, there are two enlargements of the cord: at the cervical level is the brachial plexus (for the upper limb), and at the lumbosacral level are the lumbar and sacral plexuses (for the lower limb). The cord’s lowermost portion tapers and is called the conus medullaris. Below that level of L2/vertebral in the adult, inside the vertebral canal, are numerous nerve roots collectively called the cauda equina; these are found within the lumbar cistern (see MRI in next illustration; also discussed with Figure 21), an enlargement of the subarachnoid space. This is where a lumbar puncture (LP) is performed to obtain a cerebrospinal fluid (CSF) sample. The spinal cord, notwithstanding its relatively small size (compared with the rest of the brain), is absolutely essential for our normal function. It is the connector between the CNS and the body (other than the head and neck). On the sensory side, the information arriving from the skin, muscles, and viscera informs the CNS about what is occurring in the periphery; this information then ascends to higher centers in the brain. On the motor side, the nerves leaving the spinal cord control our muscles (acting through the anterior horn cells and their axons). Although the spinal cord has a functional ©2000 CRC Press LLC organization within itself, the neurons of the spinal cord receive their instructions from higher centers, including the cerebral cortex, via several descending tracts. This enables us to carry out normal movements, including walking and voluntary activities. The spinal cord also has a motor output to the viscera and glands, part of the autonomic nervous system. The area of the conus is responsible for the autonomic control of bowel and bladder, subject to commands from higher centers (including the cortex). Developmental Aspects Embryologically, the spinal cord commences as a tube of uniform size. In those segments that innervate the limbs (muscles and skin), all the neurons reach maturity. However, in the intervening portions, there is massive programmed cell death during development because there is less peripheral tissue to be supplied. In the adult, therefore, the spinal cord has two enlargements: the cervical for the upper limb, and the lumbo-sacral for the lower limb, each giving rise to the nerve plexuses for the upper and lower limbs, respectively. In the fetus, the spinal cord and vertebral column are the same length. After birth, the vertebral column continues to grow while the spinal cord does not. In the adult, the spinal cord ends at the level of the second lumbar vertebra. Therefore, the exiting roots for the lower extremity travel within the subarachnoid space (the lumbar cistern) for a fair distance before exiting, forming the cauda equina. The incoming sensory and outgoing motor roots which innervate the periphery allow us to discuss the spinal cord in terms of segments. This “segmentation” has an embryological explanation: areas of skin are supplied by certain nerve segments, called dermatomes (e.g., umbilical region = T10), and various muscles are supplied by certain segments called myotomes (e.g., biceps of the upper limb = C5 & 6; quadriceps of the lower limb = L3 & 4). Clinical Aspects The segmental organization of the spinal cord and the known pattern of innervation allow a knowledgeable practitioner, after performing a detailed neurological examination, to develop an accurate idea of the location — the spinal cord segmental level — of a lesion of the spinal cord. Dura mater Cervical Arachnoid mater Subarachnoid space Thoracic Pia mater Conus medullaris Lumbar Cauda equina Lumbar cistern Sacral FIGURE 1A: Spinal Cord I — Spinal Cord: Longitudinal View ©2000 CRC Press LLC FIGURE 1B SPINAL CORD SPINAL CORD MRI: LONGITUDINAL VIEW This is an MRI — magnetic resonance image — of the vertebral column and spinal cord, viewed in a mid-sagittal plane. It is a TI-weighted image, in which the cerebrospinal fluid (CSF) is dark. (The various radiological techniques used to image the nervous system are discussed with Figure 17.) This image is from an older child, in which no pathology was found in the spinal cord radiological examination. Because of the length of the spinal cord, it is shown in two parts, upper and lower. The upper portion (image on the left) shows the spinal cord to be a continuation of the medulla of the brainstem, at the lowermost border of the skull. A white asterisk has been placed in the enlargement of the subarachnoid space — the cisterna magna (see Figure 20A) — below the cerebellum. The spinal cord tissue is located in the middle of the vertebral column, surrounded by the meninges (which cannot be visualized), with the dura separating the subarachnoid space containing CSF from the space outside the meninges, the epidural space (between the meninges and vertebra themselves). The epidural space in the lower thoracic region and in the lumbar and sacral regions often contains fat, which appears bright in the image. ©2000 CRC Press LLC The lower portion of the spinal cord (image on the right) shows the spinal cord itself tapering and terminating as the conus medullaris, around the level of vertebra L1-L2. Below that level is the enlarged subarachnoid space, called the lumbar cistern within which are the nerve roots (dorsal and ventral) — the cauda equina — for the lower extremity. The nerve roots exit the spinal cord at the appropriate intervertebral level. The roots to the lower extremity, particularly those exiting between L4–L5 and L5–S1 are the ones most commonly involved in everyday back injuries that affect many adults. The student should be familiar with the signs and symptoms that accompany degenerative disc disease in the lumbar region. The spinal cord can be affected by tumors, either within the cord (intramedullary) or outside the cord (extramedullary). There is a large plexus of veins on the outside of the dura of the spinal cord and this is a site for metastases from pelvic (including prostate) tumors. These tumors press upon the spinal cord as they grow and cause symptoms as they interfere with the various pathways. Clinical Aspects Sampling of CSF is done by placing a needle between the vertebra, below the level of the L2, into the lumbar cistern. This procedure is called a lumbar puncture (LP)(further discussed with Figure 1A). FIGURE 1B: Spinal Cord — Spinal Cord MRI: Longitudinal View ©2000 CRC Press LLC FIGURE 2A SPINAL CORD II SPINAL CORD CROSS SECTION: C8 LEVEL This diagram is a cross section of the spinal cord at the C8 level, the eighth cervical segmental level of the spinal cord, not the vertebral level. The gray matter is said to be arranged in the shape of a butterfly or somewhat like the letter “H.” The gray matter of the spinal cord contains a variety of cell groups, i.e., nuclei, which subserve different functions. Although hard to visualize, these groups are continuous longitudinally throughout the length of the spinal cord. The dorsal region of the gray matter, called the dorsal or posterior horn, is associated with the incoming dorsal root and is thus related to sensory functions. The ventral gray matter, called the ventral or anterior horn, is the motor portion of the gray matter and has the large motor neurons, the anterior horn cells. An anterior horn cell and its axon, along with all the muscle fibers innervated, forms the functional motor unit. The area between is usually called the intermediate gray and has a variety of cell groups with some association-type functions. The neurons controlling the autonomic nervous system (sympathetic and parasympathetic) are found in this region. The nuclei of the spinal cord gray matter have both names and numbers. The names are older and descriptive. A newer classification of these nuclei is based upon the functional aspect of these nuclei and uses a numbering system: these are the so-called Rexed lamina. This cross section of the spinal cord shows the descriptive names on the right side and the Rexed lamina on the left; these are listed below with their associated function: Name Lamina Function Posteromarginal nucleus Substantia gelatinosa Proper sensory nucleus Intermediate gray Dorsal nucleus (of Clarke) Ventral horn Anterior horn cells I II III, IV V, VI, VII Part of VII Sensory Sensory Sensory Association Relay to cerebellum VIII IX medial = proximal lateral = distal X Motor (axial muscles) Limb musculature Commissural neurons ©2000 CRC Press LLC Unknown The central canal of the spinal cord (see Figures 20A and 20B) is located in the center of the commissural gray matter. It represents the remnant of the neural tube and is filled with CSF. In the adult, the central canal of the spinal cord is probably not patent throughout the whole spinal cord. Physiologically, the spinal cord is the basis of a number of simple and complex reflexes. The basic motor reflex is the stretch reflex, also called the myotatic reflex — tapping on a tendon stretches the tendon, activates the muscle spindle, and causes a reflex contraction of the muscle. This is a monosynaptic reflex and perhaps one of the most important for a neurological examination (also called a deep tendon reflex, DTR). Other reflexes, such as the withdrawal from a painful stimulus, are multisynaptic. All these reflexes involve hard-wired circuits of the spinal cord but are influenced by information descending from higher levels of the nervous system. Recent studies indicate that complex motor patterns are present in the spinal cord, such as stepping movements with alternating movements of the limbs, and that influences from higher centers provide the organization for these built-in patterns of activity. The integrity of the spinal cord is needed for normal bowel and bladder function. Clinical Aspects Lesions of the spinal cord in humans are usually devastating in their effects. Often these occur as a result of diving accidents (into shallow water) or following car accidents. The immediate effect of a spinal cord transection in the human is a complete shut-down of all spinal cord activity. This is referred to as spinal shock. After a period of a few weeks, intrinsic spinal reflexes appear, now no longer modified from higher control centers. (The details of the pathways involved are discussed in Section B.) The result is a dramatic increase in muscle tone (spasticity) and hyperactive deep tendon reflexes (discussed with Figure 47). Thereafter, a number of abnormal or excessive reflex responses occur. Such patients require exceptional care by the nursing staff. ©2000 CRC Press LLC FIGURE 2A: Spinal Cord II — Spinal Cord Cross Section: C8 Level FIGURE 2B SPINAL CORD SPINAL CORD MRI: AXIAL VIEW There are two axial MRI views of the spinal cord — the upper image at the C7 cervical cord level and the lower image at the T1 thoracic cord level. The CSF is again dark. The spinal cord can be easily visualized within the vertebral canal. The size difference between the C7 level and the T1 level should be noted. The C7 spinal cord is at the level of the brachial plexus enlargement (discussed with Figure 1A). The T1 level is the high thoracic level of the spinal cord where the cord is smaller (see also Figure 73). Not much detail can be seen in this normal radiograph. The dorsal (indicated by a white arrow) and ventral roots can be seen in the upper image (but not in the lower one), with the emerging spinal nerve. ©2000 CRC Press LLC Clinical Aspects Any abnormal protrusion of a vertebra or disc could be visualized, as well as tumors within the vertebral canal or of the cord itself. A small arterio-venous (A-V) malformation can also be visualized with MRI. Syringomyelia, an uncommon disease, involves a pathological cystic enlargement of the central canal. The enlargement often interrupts the pain and temperature fibers in their crossing anterior to the central canal (see Figures 31 and 72). Usually this occurs in the cervical region and the patients complain of the loss of these modalities in both upper limbs. If the spinal cord is completely transected (i.e., cut through completely), all the tracts are interrupted. For the ascending pathways, this means that sensory information from the periphery is no longer available to the brain. On the motor side, all the motor commands cannot be transmitted to the anterior horn cells, the final common pathway for the motor system. The person therefore is completely cut off on the sensory side and loses all voluntary control below the level of the lesion. Bowel and bladder control are also lost. C7 level T1 level FIGURE 2B: Spinal Cord — Spinal Cord MRI: Axial View ©2000 CRC Press LLC FIGURE 3 BRAINSTEM I BRAINSTEM AND DIENCEPHALON: VENTRAL VIEW The brainstem is the lowermost part of the brain, situated inside the skull and above the spinal cord. This region of the brain is responsible for key vital functions including blood pressure, pulse, and respiration (lower portions), as well as consciousness (upper portions). Many motor activities are organized in the brainstem. The brainstem and cerebellum occupy the posterior cranial fossa of the skull. The brainstem is a relatively small mass of tissue that is packed with various nuclei and tracts. Firstly, it is the site of origin or termination of ten of the cranial nerves (CN III to CN XII). Of great clinical significance is the fact that all the ascending (sensory) pathways pass through the brainstem, and all the motor (descending) pathways either originate in or go through the brainstem (see Section B). In addition, many of the connections to the cerebellum, including pathways and nuclei, are found in the brainstem. The brainstem is divided anatomically into three parts — the narrow midbrain, the pons with its ventral bulge, and the medulla lowest. Each of these parts is recognizably distinct when one sees a gross brain specimen or a microscopic cross section. The specimen in Figure 3 was obtained by isolating the brainstem from the remainder of the brain. The diencephalon (to be discussed with Figure 9) is also included. A photographic view of such a specimen is presented in the next illustration. The cerebellum is located behind the brainstem (to be described with Figure 8). pontine nuclei). • The medulla has two distinct elevations known as the pyramids on each side of the midline. The direct voluntary motor pathway from the cortex to the spinal cord — the cortico-spinal tract — is located within the pyramid. Behind each is a prominent bulge, the olive, which in fact represents a major nucleus of the medulla, the inferior olivary nucleus. One of the keys to understanding the brainstem is identifying the cranial nerves. In almost all cases the attachment site of each cranial nerve (CN) to the brainstem is a marker to the location of the cranial nerve nucleus within the brainstem (see Figures 4, 5, 6 and 7; discussed in Section C). Knowledge of this is critical in determining the clinical location of a lesion of the brainstem region. The cranial nerves are presented in numerical order, starting at the midbrain level. (Details concerning their function will be discussed with Figures 4, 5 and 6.) Midbrain Level • CN III, the oculomotor nerve, emerges ventrally between the cerebral peduncles. • CN IV, the trochlear nerve, which exits posteriorly, is a thin nerve that wraps around the lowermost border of the cerebral peduncle. Pontine Level • CN V, the trigeminal nerve, is a massive nerve attached along the middle cerebellar peduncle. • CN VI, the abducens nerve, is seen exiting at the junction between the pons and medulla. • CN VII, the facial nerve, and CN VIII, the vestibulocochlear nerve, are both attached to the brainstem at the ponto-cerebellar angle. Medullary Level The distinguishing features of the three parts of the brainstem visualized on this ventral view include the following (from above downwards): • CN IX, the glossopharyngeal, and CN X, the vagus, are attached to the lateral margin of the medulla, behind the inferior olive. • The midbrain region (mesencephalon). It has two large “pillars” anteriorly, called the cerebral peduncles, which consist of millions of axons descending from the cerebral cortex to various levels of the brainstem and spinal cord. • CN XI, the spinal accessory nerve, is the part that exits from the uppermost region of the spinal cord, enters the skull, and then exits from the skull as if it were a cranial nerve. • The pons portion is distinguished by its anterior bulge, an area which is composed of nuclei (the ©2000 CRC Press LLC • CN XII, the hypoglossal nerve, emerges by a series of rootlets between the inferior olive and the pyramid. Diencephalon Midbrain Middle cerebellar peduncle Cerebellum Pons Flocculus Medulla Tonsil CP = Cerebral peduncle P = Pons Py = Pyramid O = Inferior olive FIGURE 3: Brainstem I — Brainstem and Diencephalon: Ventral View ©2000 CRC Press LLC FIGURE 4 BRAINSTEM II BRAINSTEM AND DIENCEPHALON: VENTRAL (PHOTOGRAPHIC) VIEW The specimen in Figure 4 was obtained by isolating the brainstem and diencephalon from the remainder of the brain. It is the same specimen as in Figure 3. The three parts of the brainstem can be differentiated on this ventral view (from above downwards): • The midbrain region has the two large “pillars” anteriorly called the cerebral peduncles. The fossa between the cerebral peduncles is the interpeduncular fossa, with the emerging CN III. • The pontine portion is distinguished by its bulge anteriorly, the pons proper, an area composed of nuclei (the pontine nuclei); • The medulla is distinguished by the pyramids, two distinct elevations on each side of the midline, within which is located the direct voluntary motor pathway from the cortex to the spinal cord — the corticospinal tract (see Figure 42). Behind each pyramid is the olive (inferior olivary nucleus). As mentioned previously, one of the keys to understanding the brainstem is locating the cranial nerves. In almost all cases, knowledge of the attachment site of each cranial nerve (CN) to the brainstem is a marker to the location of the cranial nerve nucleus within the brainstem (see Figures 5 and 6). Unfortunately, not all of these nerves have been preserved on this specimen because stripping of the meninges often also removes these nerves. Midbrain Level • CN III, the oculomotor nerve, emerges ventrally from the interpeduncular fossa. It supplies several extraocular muscles, and includes parasympathetic innervation to the pupil. • CN IV, the trochlear nerve, exits posteriorly (see Figure 7). It is a thin nerve that wraps around the lowermost border of the cerebral peduncle (on the right side in the photograph). It supplies one extraocular muscle. Pontine Level • CN V, the trigeminal nerve (not labeled), is a massive ©2000 CRC Press LLC nerve attached along the middle cerebellar peduncle (see Figures 5 and 7). It is the major sensory nerve of the head and also has a motor component. • CN VI, the abducens nerve, exits at the junction between the pons and medulla (it is preserved only on the left side on the photograph). It supplies one extraocular muscle. • CN VII, the facial nerve, has been torn; it is attached to the brainstem just above CN VIII (the acoustic nerve), both at the ponto-cerebellar angle. The facial nerve supplies the facial muscles and has other components. CN VIII is a special sense nerve for balance and hearing. Medullary Level • CN IX, the glossopharyngeal, and CN X, the vagus, cannot be seen on this view of the brainstem. (They are attached to the lateral margin of the medulla, behind the inferior olive. See Figure 7).They innervate the muscles of the pharynx and larynx; the vagus is the major parasympathetic nerve to the viscera. • CN XI, the spinal accessory nerve, is not clearly visible on this specimen. It innervates the large muscles of the neck and is not a true cranial nerve. • CN XII, the hypoglossal nerve, emerges by a series of rootlets between the inferior olive and the pyramid (preserved only on the left side in the photograph). It supplies the muscles of the tongue. The fibers projecting from the cerebral cortex to the spinal cord (the cortico-spinal tract) traverse the whole brainstem and are located in the cerebral peduncles, the pons proper, and the pyramids (see Figure 42). The medulla ends where these cortico-spinal fibers cross the midline as the pyramidal decussation. Below this is the cervical spinal cord (not labeled). Knowledge of the brainstem is necessary during the study of almost all parts of the CNS. As would be expected, this information is essential for the diagnosis of clinical syndromes that involve this part of the brain. A lesion might interrupt either one or more sensory and/or motor pathways. Because of the close relationship with the cerebellum, there may be cerebellar signs as well. The accompanying cranial nerve deficits would assist a neurologist to pinpoint the brainstem level involved. Optic chiasm Fibers of internal capsule Mammillary body CN III Interpeduncular fossa Cerebral peduncle CN IV CN VI CN VIII Middle cerebellar peduncle Flocculus CN XII Pyramidal decussation Inferior cerebellar peduncle D = Diencephalon Md = Midbrain P = Pons Py = Pyramid O = Inferior olivary nucleus M = Medulla T = Tonsil FIGURE 4: Brainstem II — Brainstem and Diencephalon: Ventral (Photographic) View ©2000 CRC Press LLC FIGURE 5 BRAINSTEM III CRANIAL NERVE NUCLEI: MOTOR The cranial nerves are the peripheral nerves that supply the head and neck region (except CN I and CN II). Each cranial nerve is unique and does not follow a general pattern as with the spinal nerves; each of the cranial nerves may have one or more functional components, either sensory or motor, or both, and some also have an autonomic (parasympathetic) component. The motor aspects are reviewed in this diagram; the sensory ones are considered in the next diagram. On the motor side, there are three kinds of motor functions: (i) The motor supply to the muscles derived from somites, including CN III, IV, VI, and XII; (ii) The motor supply to the muscles derived from the branchial arches, branchiomotor, including CN V, VII, IX, and X (and the cranial part of XI); (iii) The parasympathetic supply to smooth muscles and glands of the head and viscera, including CN III, VII, IX, and X. Figure 5 shows the location of the motor nuclei of the cranial nerves, superimposed upon the ventral view of the brainstem. These nuclei are also shown in Figure 45, in which the brainstem is presented from a dorsal perspective. Detailed information regarding the cranial nerve nuclei within the brainstem is presented in Section C (Figures 64–71). Midbrain Level • CN III, the oculomotor nerve, has both motor and autonomic fibers. The somatic motor nucleus of the oculomotor nerve, which supplies most of the muscles of the eye, is found at the upper midbrain level. This is the level of the superior colliculus. The parasympathetic nucleus known as the Edinger-Westphal nucleus, is associated with the nucleus of III. These fibers supply the pupillary constrictor muscle and the muscle that controls the curvature of the lens. • CN IV, the trochlear nerve, is a motor nerve to one eye muscle, the superior oblique muscle. The trochlear nucleus is found at the lower midbrain level, the level of the inferior colliculus. ©2000 CRC Press LLC Pontine level: • CN V, the trigeminal nerve, is the major sensory nerve of the head region and also has a motor component; this branchiomotor nucleus supplies the muscles of mastication. The nucleus is located at the mid-pontine level; the small motor nerve is attached to the brainstem at the mid-pontine level, along the middle cerebellar peduncle, with the sensory root. • CN VI, the abducens nerve, is a motor nerve which supplies one extraocular muscle, the lateral rectus muscle. The somatic motor nucleus is located in the lower pontine region. • CN VII, the facial nerve, is a mixed cranial nerve. The branchiomotor nucleus, which supplies the muscles of facial expression, is found at the lower pontine level. The parasympathetic fibers (to salivary and lacrimal glands) come from the superior salivatory nucleus. Medullary Level • CN IX, the glossopharyngeal nerve, and CN X, the vagus nerve, are mixed cranial nerves attached to the medulla along its lateral margin, behind the inferior olive. Both have a branchiomotor component, which supplies the muscles of the pharynx (IX) and larynx (X), originating from the nucleus ambiguus. In addition, each has a parasympathetic component: the fibers of IX come from the inferior salivatory nucleus (to the parotid gland); those of X from the dorsal motor nucleus of the vagus supply the organs of the thorax and abdomen. Both nuclei are found throughout the mid and lower portions of the medulla. • CN XI, the spinal accessory nerve, has two portions. The cervical or spinal component, which originates from a cell group in the upper 4–5 segments of the cervical spinal cord, supplies the large muscles of the neck (the sternomastoid and trapezius). • CN XII, the hypoglossal nerve, innervates all the muscles of the tongue. It has an extended nucleus in the medulla situated alongside the midline. Its fibers exit between the pyramid and the olive. (Note: In the diagram it appears that the nucleus ambiguus is supplying the fibers to CN XII. This is merely a visualization problem.) Edinger-Westphal n. Oculomotor n. Trochlear n. Motor trigeminal n. Abducens n. CN II CN III Facial n. CN IV Superior salivatory n. CN V Inferior salivatory n. CN VI CN VII CN VIII CN IX CN X CN XII CN XI Dorsal motor n. Hypoglossal n. Ambiguus n. Spinal accessory n. FIGURE 5: Brainstem III — Cranial Nerve Nuclei: Motor ©2000 CRC Press LLC FIGURE 6 BRAINSTEM IV CRANIAL NERVE NUCLEI: SENSORY The cranial nerve nuclei with sensory functions are presented in this diagram. Sensory information from the region of the head and neck includes the following: 1. 2. 3. General sensations, consisting of touch (both discriminative and crude touch), pain, and temperature. These come from the skin, the surface of the eye (cornea and conjunctiva), the lips, and the mucous membranes of the mouth (including the tongue) and the nose, via branches of the trigeminal nerve. These are classified as somatic afferents. Sensory input from the pharynx and other homeostatic receptors of the neck (e.g., for blood pressure) and from the organs of the thorax and abdomen. This afferent input is carried mainly by the vagus but also by the glossophayngeal nerve. These are also called visceral afferents. Special senses, consisting of auditory (hearing) and vestibular (balance) afferents, as well as the special sense of taste. The sensory nuclei are also shown in Figure 38, which presents the brainstem from a dorsal (posterior) perspective. It should be noted that the olfactory nerve (CN I) and the optic nerve (CN II) are not attached to the brainstem and not considered at this stage. CN V Trigeminal Nerve The major sensory nerve of the head region is the trigeminal nerve through its three divisions. The sensory components of the trigeminal nerve are found at several levels of the brainstem. The principal nucleus, which is responsible for the discriminative aspects of touch, is located at the mid-pontine level, adjacent to the motor nucleus of CN V. From this region extending caudally is ©2000 CRC Press LLC a long column of cells that relay pain and temperature information from the teeth, oral mucosa, and skin of the face. This cell group, known as the spinal nucleus of V, or the descending trigeminal nucleus, reaches the upper cervical levels of the spinal cord. Another group of cells extends into the midbrain region, the mesencephalic nucleus of V. It is an unusual cell type for the CNS in that its cells appear to be morphologically similar to neurons of the dorsal root ganglia. These neurons are thought to be the sensory proprioceptive neurons for the muscles of mastication. CN VIII, the vestibulocochlear nerve, consists of: Cochlear nuclei: The auditory fibers from the spiral ganglion (in the cochlea) are carried to the CNS, in the VIIIth nerve, and form their first synapses in the cochlear nuclei. These nuclei are situated along the course of the nerve, as it enters the brainstem at the uppermost level of the medulla (see Figure 7). Tonotopic localization is maintained in these nuclei. Vestibular nuclei: Vestibular afferents enter the CNS as part of CN VIII. The vestibular nuclei are located in the upper medullary level as well as the lower pontine level. There are four nuclei: the medial and inferior located in the medulla, the lateral located at the ponto-medullary junction, and the small superior nucleus located in the lower pontine region. The vestibular afferents terminate in these nuclei. (The vestibular nuclei are further discussed with Figure 49A.) Visceral Afferents and Taste: The nucleus that receives the visceral afferents from CN IX and X is the solitary nucleus, found in the medulla. The special sense of taste, mainly carried in CN VII, and also in nerves IX and X, also terminates in the solitary nucleus. Note: Additional sensory nuclei of the brainstem are discussed with Figure 7, in which the brainstem is viewed from the posterior (dorsal) perspective. Diencephalon Optic chiasm Pituitary stalk Mesencephalic n. of V Mammillary n. Medial vestibular n. Superior vestibular n. CN V Principal n. of V Cochlear n. Solitary n. Lateral vestibular n. Spinal (descending) n. of V FIGURE 6: Brainstem IV — Cranial Nerve Nuclei: Sensory ©2000 CRC Press LLC Inferior vestibular n. FIGURE 7 BRAINSTEM V BRAINSTEM: DORSAL VIEW (CEREBELLUM REMOVED) The view of the brainstem that is used for some of the diagrams is an oblique perspective of the brainstem from behind — a dorsal view — with the cerebellum removed. This dorsal perspective is useful for presenting the combined visualization of many of the cranial nerve nuclei and the various pathways of the brainstem (e.g., Figures 38 and 45). Additional structures of the brainstem are seen from this perspective: • The dorsal part of the midbrain has four elevations, the superior and inferior colliculi (see also Figure 8). These colliculi form the quadrigeminal plate, also called the tectal plate or tectum. The upper ones are the superior colliculi, and they are functionally part of the visual system, a center for visual reflexes. The lower ones are the inferior colliculi, and these are relay nuclei in the auditory pathway. (The brachium of the inferior colliculus and the medial geniculate body will be discussed with the auditory pathway; see Figure 36.) This view also shows the back edge of the cerebral peduncle, the most anterior structure of the midbrain (see Figures 3 and 4). The trochlear nerves (CN IV) emerge at the lower level of the midbrain, below the inferior colliculi (as in Figure 8). ©2000 CRC Press LLC • The dorsal aspect of the pons is represented, with most of the fourth ventricle “unroofed.” (The ventricles of the brain are discussed with Figures 20A and 20B.) The roof of the upper portion of the fourth ventricle is shown and bears the name superior medullary velum; more relevant, it contains an important connection of the cerebellum, the superior cerebellar peduncles (which are discussed with Figure 55). As seen from this perspective, the fourth (IVth) ventricle has a “floor”; noteworthy are two large bumps, called the facial colliculus (discussed with the pons in Section C of the Atlas). Because the cerebellum has been removed, the cut edges of the middle and inferior cerebellar peduncles, the other cerebellar connections, are seen. CN V emerges through the middle cerebellar peduncle. • The posterior aspect of the medulla (including the floor of the fourth ventricle) has some special structures, including the entering CN VIII and the inferior cerebellar peduncle. Below the fourth ventricle, two large protuberances are seen on either side of the midline — the gracilis and cuneatus nuclei, which belong to the ascending somatosensory pathway (discussed with Figure 32). More anteriorly, from this oblique view, are the fibers of the glossopharyngeal (CN IX) and vagus (CN X) nerves, as these emerge from the lateral aspect of the medulla, behind the inferior olive. A representative cross section of the spinal cord is also shown from this posterior perspective. Brachium of inferior colliculus Red n. Medial geniculate n. Superior colliculus Inferior colliculus Cerebral peduncle CN IV Superior cerebellar peduncle Superior medullary velum CN V Fourth ventricle Middle cerebellar peduncle Inferior cerebellar peduncle Facial colliculus CN VIII Acoustic stria CN IX CN X Inferior olive Cuneatus n. Gracilis n. Cervical spinal cord FIGURE 7: BRAINSTEM V — Brainstem: Dorsal View (Cerebellum Removed) ©2000 CRC Press LLC FIGURE 8 THE CEREBELLUM CEREBELLUM AND BRAINSTEM (PHOTOGRAPHIC VIEW) This specimen of the brainstem, with the cerebellum attached, is shown from the dorsal or posterior perspective, as in the previous figure. The paired diencephalon are again seen (discussed with Figure 9), separated from each other by the third ventricle. The colliculi of the midbrain are in view, with CN IV exiting posteriorly. The cerebellum, sometimes called the “little brain,” is easily recognizable by its surface which is composed of narrow ridges of cortex, called folia (singular is folium). The cerebellum is located beneath a thick sheath of the meninges, the tentorium cerebelli, inferior to the occipital lobe of the hemispheres (see Figure 16), in the posterior cranial fossa of the skull. The cerebellum is involved with motor control and is part of the motor system, influencing posture, gait, and voluntary movements (discussed in more detail with the motor system). Its function is to facilitate the performance of movements by coordinating the action of the various participating muscle groups. This is often spoken of simply as “smoothing out” motor acts. Although it is rather difficult to explain in words what the cerebellum does in motor control, damage to the cerebellum leads to quite dramatic alterations in ordinary movements (discussed with Figure 55). Lesions of the cerebellum result in the decomposition of the activity, or fractionation of movement, so that the action is no longer smooth and coordinated. Certain cerebellar lesions also produce a tremor which is seen when performing voluntary acts, better known as an intention tremor. There are two distinct ways of dividing the cerebellum: 1. an anatomical approach, which is outlined below, and 2. a functional approach, which is explained in the discussion of the cerebellum as part of the motor system (Section B). Anatomically, the cerebellum can be described by looking at its appearance in a number of ways. The human cerebellum in situ has an upper or superior surface, as seen in this photograph, and a lower or inferior surface (see Figure 4). The central portion is known ©2000 CRC Press LLC as the vermis. The lateral portions are called the cerebellar hemispheres. Sulci separate the folia, and some of the deeper sulci are termed fissures. The horizontal fissure is located at the margin between the superior and inferior surfaces. Using these sulci and fissures, the cerebellar cortex has traditionally been divided into a number of different lobes, but most of these do not have a distinctive functional or clinical importance, so only a few are mentioned. The primary fissure, located on the superior surface of the cerebellum, separates the anterior lobe of the cerebellum — part of the functional spinocerebellum (discussed with Figure 52). The other functional area that can be visualized — on the ventral view of the cerebellum (see Figure 4) — is the flocculus (also discussed with Figure 52). The cerebellar peduncles are the connections between the brainstem and the cerebellum, and there are three pairs of them. In the inferior view of the brainstem and cerebellum (see Figure 4), two of them can be seen: the inferior cerebellar peduncle attaching the medulla and the cerebellum, and the prominent middle cerebellar peduncle from the pons to the cerebellum. (These are also shown from the dorsal perspective in Figure 7.) Details of the information carried in these pathways is outlined in the discussion of the functional aspects of the cerebellum with the motor system. The superior cerebellum peduncle is located in Figure 7 on the dorsal aspect of the brainstem, in the roof of the fourth ventricle. Clinical Aspect The cerebellar lobule adjacent to the medulla is known as the cerebellar tonsil (see ventral view of the cerebellum, Figure 4). The tonsils are found just inside the foramen magnum of the skull. Should there be an increase in the mass of tissue occupying the posterior cranial fossa (e.g., a tumor or hemorrhage), the cerebellum would be pushed downward. This pressure may force the cerebellar tonsils into the foramen magnum, thereby compressing the medulla. The compression, if severe, may lead to a compromising of function of the vital centers located in the medulla (discussed with Figure 40A). The complete syndrome is known as tonsillar herniation, or coning, and is a life-threatening situation which can cause cardiac and/or respiratory arrest. The pineal is discussed with Figure 9. Third ventricle Fibers of internal capsule Superior colliculus Pineal Inferior colliculus CN IV Primary fissure Horizontal fissure Vermis of cerebellum D = Diencephalon FIGURE 8: The Cerebellum — Cerebellum and Brainstem (Photographic View) ©2000 CRC Press LLC FIGURE 9 THE DIENCEPHALON THALAMUS: ORIENTATION The diencephalon translates as “between brain.” The diencephalon is composed of both thalamus and hypothalamus as well as some other subparts. It is situated between the brainstem and the cerebral hemispheres, deep within the brain. As shown photographically (see Figure 4) and also diagrammatically (see Figure 3), the diencephalon sits “atop” the brainstem. During development of the human brain the enormous growth of the cerebral hemispheres has virtually hidden, or “buried,” the diencephalon (somewhat like a weeping willow tree) so that it can no longer be visualized from the outside, except from the inferior view (see hypothalamus in Figure 13). It is important to note that there are two thalami; these are often connected across the midline by the massa intermedia (as seen in Figure 3). The thalamus makes up the bulk of the diencephalon. It has many nuclei which are strongly linked with the cerebral cortex, even during development. This feature becomes clearer in one of the principles of thalamic function, namely that most thalamic nuclei that project to the cerebral cortex also receive input from that area — these are called reciprocal connections. This principle does not apply, however, to all of the nuclei (see below). The major function of the thalamic nuclei is to process information before forwarding it to the select area of the cerebral cortex. This is especially true for all the sensory systems, except the olfactory. Crude forms of sensation, including pain, may possibly be “appreciated” in the thalamus, but localization of the sensation requires the involvement of the cortex. Likewise, two subsystems of the motor system, the basal ganglia and the cerebellum, relay in the thalamus before sending their information ©2000 CRC Press LLC to the motor areas of the cortex. In addition, the limbic system also has circuits that involve the thalamus. Other thalamic nuclei are related to association areas of the cerebral cortex (explained below — these are areas not specifically related either to sensory or motor functions of the cortex). Parts of the thalamus play an important role in the maintenance and regulation of the state of consciousness, alertness, and also possibly attention. The hypothalamus and other nuclear areas of the diencephalon are discussed with the limbic system (Section D). The pineal (visible in Figure 8) is sometimes considered a part of the diencephalon. This gland is thought to be involved with the regulation of circadian rhythm. Many people now take melatonin, which is produced by the pineal, to regulate their sleep cycle and to overcome jetlag. The subthalamus is an area between the thalamus and midbrain; the subthalamic nucleus, located in this small zone, is an important nucleus involved with the circuitry of the basal ganglia and substantia nigra and is discussed with those structures (see Figure 24). Additional Detail The following topographic information will be understood only after studying the hemispheres (see Figures 11–16). It is recommended that students review this material at that time. As shown in the diagram, the diencephalon is situated within the brain below the level of the body of the lateral ventricles (see also Figures 29 and 30). In fact, the thalamus forms the “floor” of this part of the ventricle (see Figure 16). In a horizontal section of the hemispheres, the two thalami are located at the same level as the lentiform nucleus (see Figures 27, 28A, and 28B); on each side, the thalamus forms one of the boundaries of the posterior limb of the internal capsule. Between the two thalami is the third ventricle (see Figures 8 and 20B). Lateral ventricle (body) Corpus callosun Caudate nucleus (body) Thalmus (paired) Brainstem FIGURE 9: The Diencephalon — Thalamus: Orientation ©2000 CRC Press LLC FIGURE 10 THALAMUS THALAMUS: NUCLEI There are two ways of dividing up the nuclei of the thalamus: topographically and functionally. 1. 2. Topographically, the thalamus is subdivided by bands of white matter into a number of component parts. The main white matter band that runs within the thalamus is called the internal medullary lamina and it is shaped like the letter “Y” (see also the previous illustration). It divides the thalamus into a lateral mass, a medial mass, and an anterior group of nuclei. Functionally, the thalamus has three different types of nuclei: • The specific relay nuclei relay incoming sensory information to specific sensory areas of the cerebral cortex. Included with these are the medial and lateral geniculate bodies, relay nuclei for the auditory and visual systems. In addition, motor regulatory information from the basal ganglia and cerebellum is also relayed in the thalamus as part of this set of nuclei. These nuclei are located in the lateral nuclear mass. • The association nuclei are connected to broad areas of the cerebral cortex known as the association areas. One of the most important nuclei of this group is the dorsomedial nucleus, located in the medial mass of the thalamus. • The non-specific nuclei are scattered nuclei that have other and/or multiple connections. Some of these nuclei are located within the internal (medullary) lamina and are often referred to as the intralaminar nuclei. This functional group of nuclei does not have the strong reciprocal connections with the cortex like the other nuclei. Some of these nuclei form part of the ascending reticular activating system which is involved in the regulation of our state of consciousness and arousal (discussed with Figure 40A). The reticular nucleus which lies on the outside of the thalamus is also part of this functional system. ©2000 CRC Press LLC The nuclei are organized as follows. Specific relay nuclei (and function) These nuclei are reciprocally connected to specific primary sensory or motor areas of the cerebral cortex. VA ventral anterior (motor) VL ventral lateral (motor) VPL ventral posterolateral (somatosensory) VPM ventral posteromedial (trigeminal) MG medial geniculate (body) nucleus (auditory) LG lateral geniculate (body) nucleus (vision) Association nuclei (and association cortex) These nuclei are reciprocally connected to association areas of the cerebral cortex. DM dorsomedial nucleus (prefrontal cortex) AN anterior nucleus (limbic lobe) P pulvinar (visual cortex) LP lateral posterior (parietal lobe) Nonspecific nuclei These nuclei relay to widespread areas of the cerebral cortex. IL intralaminar CM centromedian (not illustrated — see Figures 51 and 61) R reticular (not illustrated — see Figure 61) For schematic purposes, this presentation of the thalamic nuclei, which is also shown in a number of textbooks, is quite usable. A more histological view of the thalamus is shown in Section C (see Figure 61). Note to student: The thalamus is introduced at this point because it is involved in all the functional systems. The student should learn the names and understand the general organization of the various nuclei at this point. The student should also consult this diagram as the cerebral cortex is described (see the following figures). Each of the specific relay nuclei involved in one of the pathways is discussed again with the functional system (Section B), and at that point the student should return to this and the previous illustration. Various nuclei are involved with the limbic system (Section D). After studying all of these systems, it is worthwhile returning again to Figures 9 and 10 for better understanding and integration of the thalamus. AN DM IL VA VL LP P VP(L)(M) LG FIGURE 10: Thalamus — Thalamus: Nuclei ©2000 CRC Press LLC MG FIGURE 11 CEREBRAL HEMISPHERES I CEREBRAL CORTEX: DORSAL VIEW When people talk about the brain, they are generally referring to the cerebral hemispheres, also called the cerebrum. The brains of the higher apes and humans are dominated by the cerebral hemispheres. The nervous tissue of the hemispheres, particularly the outer layer of neurons — the cerebral cortex — is responsible for consciousness, language, thinking, memory, movements, sensory perceptions, and certain aspects of emotion. In short, neuronal activities in the cerebral cortex determine to a large extent our capabilities. It is not that the other parts of the CNS are not important, but working in and adapting to our complex modern world depends upon proper functioning of the cerebral hemispheres. The hemispheres are organized in the following way: billions of neurons and their dendrites (and synapses) are located at the surface, forming a cortex, the cerebral cortex. Most of the cerebral cortex is organized in six layers, the neocortex, with the neurons of each layer having a different function. In formalin-fixed material, the neuronal cortex takes on a grayish appearance and is often referred to as the gray matter. The surface of the hemispheres in humans and some other species is thrown into irregular folds. These ridges are called gyri (singular, gyrus) and the intervening crevices are called sulci (singular, sulcus). A very deep sulcus is called a fissure. This arrangement allows for a greater surface area to be accommodated within a confined space, the skull. The cerebral hemispheres occupy the interior of the skull, the cranial cavity, which is divided into the three cranial fossa. The surface of the cerebral hemispheres can be visualized from a number of perspectives: from above (dorsal view, as seen in Figure 11), from the side (the dorsolateral view, as in Figure 12), and from below (inferior view, as in Figures 13 and 14 ). In addition, dividing the two hemispheres along the interhemispheric fissure (in the midline) shows the hemispheres have a medial surface as well (see Figure 16). The photograph in Figure 11 shows the cerebral hemispheres from above, a dorsal view. ©2000 CRC Press LLC Different parts of the cortex have different functions. Some parts have a predominantly motor function, whereas other parts are receiving areas for one of the major sensory systems. Most of the cerebral cortex in humans has an association function, a term that can perhaps be explained functionally as interrelating the various activities in the different parts of the brain. Each of the hemispheres is divided into four lobes: frontal, parietal, temporal, and occipital. Two prominent fissures allow this subdivision to be made — the central fissure and the lateral fissure. The central fissure, which is easier to identify on the right side of the specimen in Figure 11, divides the area anteriorly, the frontal lobe, from the area posteriorly, the parietal lobe. The parietal lobe extends posteriorly to the parieto-occipital fissure, which is more easily seen on other views (see Figure 16). The brain area behind the parieto-occipital fissure is the occipital lobe. The temporal lobe and the lateral fissure cannot be seen on this view of the brain (see Figure 12). Large areas of the frontal lobes have a predominantly motor function. The most anterior parts of the frontal lobe are the newest in evolution and are known as the prefrontal cortex. This broad cortical area seems to be the chief “executive” part of the brain. The parietal areas are connected to sensory inputs and have a major role in integrating sensory information from the various modalities. The occipital lobe is concerned with the processing of visual information (see Figure 16). The meningeal layers (arachnoid and pia) have not been removed from this specimen, which means that the blood vessels are also present. Under the arachnoid membrane is the subarachnoid space, which is collapsed in this fixed specimen; it is normally filled with cerebrospinal fluid (CSF) (which is discussed with Figure 20A). This photographic view shows some coral-like whitish material lying adjacent to the interhemispheric fissure; this material is collectively called the arachnoid granulations and is part of the CSF circulation, returning the CSF to the venous circulation (discussed with Figure 21). Anterior Arachnoid granulations Central fissure Interhemispheric fissure Parieto-occipital fissure F = Frontal lobe P = Parietal lobe O = Occipital lobe FIGURE 11: Cerebral Hemispheres I — Cerebral Cortex: Dorsal View ©2000 CRC Press LLC FIGURE 12 CEREBRAL HEMISPHERES II CEREBRAL CORTEX: DORSOLATERAL VIEW With the meninges removed, it is possible to identify the sulci and fissures with more certainty. The central fissure (often called the fissure of Rolando) is now seen more completely, dividing the frontal lobe anteriorly from the parietal lobe posteriorly. Some cortical areas are directly connected functionally with either a sensory or motor system; these are known as the primary areas. The gyrus in front of the central fissure is called the precentral gyrus (see also Figure 51) and is the primary motor area, specialized for the control of voluntary movements. The frontal eye field, an area in the frontal lobe (outlined), has a motor function related to eye movements. The gyrus behind the central fissure, the postcentral gyrus (see also Figure 34), has a somatosensory function for information from the skin (and joints). (Other sensory primary areas are identified where appropriate.) Those cortical areas that are not directly linked to either a sensory or motor function are called association cortex. The area in front of the frontal eye fields previously mentioned, the prefrontal cortex, is a typical example of an association area. Large parts of the parietal and temporal lobes are association cortex. The cortex has been studied by many people using different techniques. It is possible to recognize distinct histological (microscopic) features between different cortical areas, and these might reflect the differing functions of each particular area. One of the most commonly used sub-parcelations of the cerebral cortex is that of Brodmann who divided brain areas numerically. Some of these numbers are sometimes used interchangeably with the names, such as area 4 for the precentral gyrus (the motor strip), area 8 for the frontal eye field, area 6 for the lateral premotor cortex (in-between) (see also Figure 51), and areas 3, 1, and 2 which are synonymous for the postcentral somatosensory gyrus. Some cortical functions are not equally divided between the two hemispheres. One hemisphere is therefore said to be dominant for that function. This is the case for ©2000 CRC Press LLC language ability, which, in most right-handed people, is located in the left hemisphere. The photograph in Figure 12 shows the left hemisphere, and the two language areas are indicated: Broca’s area for the motor aspects of speech, and Wernicke’s area for the comprehension of written and verbal language. The lateral fissure (fissure of Sylvius) divides the temporal lobe below from the frontal and parietal lobes above. Extending the line of the lateral fissure posteriorly continues the demarcation between the temporal and parietal lobes. In the parietal lobe there are two gyri whose association type of function is known; they have been labeled the supramarginal and angular gyri. These areas, particularly on the nondominant side, seem to be involved in visuo-spatial activities. The temporal lobe is a large area of association cortex whose function is still being defined. The areas exposed on this dorsolateral view — other than the portions involved with the auditory system and language (on the dominant side) — are, in fact, still to be assigned a functional role. Other portions of the temporal lobe include the inferior parts (to be discussed with the subsequent figures) and the medial portion which is part of the limbic system (see Section D). The specialized cortical areas for audition are located within the lateral fissure (as shown in Figure 37). It should be noted that the lateral fissure has a large number of blood vessels within it, branches of the middle cerebral artery (discussed with Figure 58). They have been removed from the specimen in the figure. The location of the parieto-occipital fissure is indicated on this photograph. This fissure separates the parietal lobe from the occipital lobe, which can be seen in Figure 16. The cerebellum lies below the occipital lobe, with the large dural sheath — the tentorium cerebelli — separating these parts of the brain (see Figure 16). It is most important to delineate anatomically the various functional areas of the cortex. This forms the basis for understanding the possible clinical implications of lesions in the various parts of the brain, a task becoming more sophisticated with the help of modern imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI — see, for example, Figure 17). Central fissure Supramarginal gyrus Postcentral Angular gyrus gyrus (areas 3, 1, 2) Parieto-occipital fissure Anterior Frontal eye field (area 8) Precentral gyrus (area 4) Broca’s area Auditory gyri Lateral fissure Wernicke’s area Cerebellum P = Parietal lobe F = Frontal lobe T = Temporal lobe O = Occipital lobe (areas 18, 19) FIGURE 12: Cerebral Hemispheres II — Cerebral Cortex: Dorsolateral View ©2000 CRC Press LLC FIGURE 13 CEREBRAL HEMISPHERES III CEREBRAL CORTEX: INFERIOR VIEW Figure 13 is a photographic view of the intact brain, including the brainstem and the cerebellum, seen from below. The medulla and pons, parts of the brainstem, can be identified, but the midbrain is mostly hidden from view. The cranial nerves are still attached to the brainstem, and the arteries to the brainstem and cerebellum are also present. The inferior surface of the frontal lobe extends from the frontal pole to the anterior tip of the temporal lobe (and the beginning of the lateral fissure). These gyri rest on the roof of the orbit and are sometimes referred to as the orbital gyri. This cortex is an association type and has strong connections with the limbic system (discussed in Section D). The next area is the inferior surface of the temporal lobe. The temporal lobe extends medially towards the midbrain and ends in a blunt knob of tissue which is known as the uncus. Moving laterally from the uncus, the first sulcus visible is the collateral sulcus/fissure (seen clearly on the right side of the photograph in Figure 13). It demarcates the parahippocampal gyrus, part of the limbic system (discussed with Figure 74), which is the most medial gyrus of the temporal lobe. It should be noted that the uncus is the most medial protrusion of this gyrus. (The clinical significance of the uncus and the discussion of uncal herniation are discussed with Figure 14.) Parts of the olfactory and visual sensory afferent systems are seen on this view; both, in fact, are CNS tracts and ©2000 CRC Press LLC are not peripheral cranial nerves although they are generally called CN I and CN II. The true olfactory nerves penetrate the roof of the nose (the cribriform plate) as a number of filaments and then synapse in the olfactory bulb (discussed with Figure 84). Olfactory information is then carried in the olfactory tract to the uncal region of the temporal lobe. The optic nerves exit from the orbit and continue to the optic chiasm where there is a partial crossing of visual fibers (see Figures 39A and 39B). Posterior to the chiasm is the area of the hypothalamus, part of the diencephalon, which can be seen more clearly in Figure 14. The brainstem and cerebellum occupy the posterior part (the posterior cranial fossa) and obscure the visualization of the occipital lobe (shown in Figure 14). Various cranial nerves can be identified (as in Figure 4). The oculomotor nerve, CN III, exits from the midbrain. The trigeminal nerve, CN V, exits along the middle cerebellar peduncle. Cranial nerves VII and VIII are seen attached to the brainstem at the ponto-cerebellar angle. The arterial system is also seen in this illustration. The two vertebral arteries unite to form the basilar artery (which is displaced from the midline of the pons in this specimen). The arterial supply to the brainstem and cerebellum comes from these arteries. There are three pairs of cerebellar arteries — posterior inferior, anterior inferior, and superior. The basilar artery gives off the two superior cerebellar arteries at the upper level of the pons, and ends by dividing into the posterior cerebral arteries to supply the occipital regions of the brain. Parts of the arterial Circle of Willis are also seen on this specimen. The arterial supply to the hemispheres is fully described in Section C (see Figure 56). Anterior Olfactory bulb Olfactory tract Lateral fissure Optic chiasm Hypothalamus Uncus CN III Parahippocampal gyrus Posterior cerebral artery Basilar artery Collateral sulcus Superior cerebellar CN V CN VII and CN VIII Middle cerebellar peduncle Flocculus Anterior inferior cerebellar artery Posterior inferior cerebellar artery Vertebral artery Tonsil F = Frontal lobe T = Temporal lobe C = Cerebellum P = Pons M = Medulla FIGURE 13: Cerebral Hemispheres III —Cerebral Cortex: Inferior View ©2000 CRC Press LLC FIGURE 14 CEREBRAL HEMISPHERES IV INFERIOR SURFACE: BRAINSTEM REMOVED In Figure 14, the brainstem has been sectioned at the level of the midbrain, and most of the brainstem itself and the attached cerebellum have been removed. The cut surface of the midbrain exposes for view the pigmented cells of the substantia nigra; since this specimen has not been processed for microscopy, the pigment is retained (discussed with the basal ganglia and see Figure 24; also shown with the cross sections of the midbrain in Figure 64). This dissection reveals the inferior surface of both the temporal and the occipital lobes. It is not possible to define the boundary between these two lobes on this view. Some of these gyri are involved with the processing of visual information, including color, as well as facial recognition. The parahippocampal gyri should be noted on both sides, with the collateral sulcus demarcating the lateral border of this gyrus (discussed with Figure 13). The optic nerves lead to the optic chiasm. Behind the optic chiasm is the median eminence and the mammillary bodies, both of which belong to the hypothalamus. The median eminence is an elevation of tissue that contains some hypothalamic nuclei. The pituitary stalk is attached to the median eminence and connects the hypothalamus to the pituitary gland. (The pituitary stalk is not present in this photograph.) Behind are the paired mammillary bodies, two nuclei of the hypothalamus ©2000 CRC Press LLC (discussed with the limbic system, see Figure 83A). A thick sheath of dura separates the occipital lobe from the cerebellum below — the tentorium cerebelli (as it covers the cerebellum, it is not present on this specimen). The tentorium divides the cranial cavity into an area above it, the supratentorial space, and an area below, the infratentorial space, which is the posterior cranial fossa. The sheath of dura splits to allow the brainstem to pass through; this split in the tentorium is called the tentorial notch (hiatus). The uncus is clearly seen, with its blunted tip pointed medially. Note that it lies just above the free edge of the tentorium cerebelli. The occurrence of a tumor or a large cerebral hemorrhage in the cerebral hemispheres, or swelling of the brain for any reason, will lead to increased tissue mass in the cranial cavity above the tentorium, accompanied by increased intracranial pressure (ICP). As the volume of brain tissue increases, the hemispheres are forced out of their supratentorial space and the only avenue is in a downward direction, through the tentorial notch. The uncus becomes the leading edge of this pathological event. The whole process is clinically referred to as uncal herniation. Since the edges of the tentorium cerebelli are very rigid, the extra tissue in the area causes a compression of the brain matter, leading to brainstem compression and a progressive loss of consciousness. CN III is usually compressed as well, damaging it and causing a fixed and dilated pupil on that side, an ominous sign in any lesion of the brain. (The pupillary light reflex is discussed with the introduction to the midbrain in Section C.) Anterior Mammillary bodies Optic nerve Median eminence Substantia nigra Midbrain Parahippocampal gyrus T = Temporal lobe O = Occipital lobe FIGURE 14: Cerebral Hemispheres IV — Inferior Surface: Brainstem Removed ©2000 CRC Press LLC FIGURE 15 CEREBRAL HEMISPHERES V CORPUS CALLOSUM: DORSAL VIEW In the photograph in Figure 15, the brain is again viewed from above, as in Figure 11. The interhemispheric fissure is opened. The dura between the hemispheres, the falx cerebri, has been removed from the interhemispheric fissure. This thick sheath of dura holds the two halves of the hemispheres in place within the cranial cavity. The superior sagittal venous sinus has also been removed. A whitish structure is seen in the depths of the fissure — the corpus callosum. One of the other major features of the cerebral cortex is the number of neurons devoted to communicating with other neurons of the cortex. These interneurons are essential for the processing and elaboration of information, whether generated in the external world or internally by our thoughts. This intercommunicating network is reflected in the vast interconnections between cortical areas. Therefore, one would expect to find various bundles of axons that course within the hemispheres (further discussed with Figures 19A and 19B). These interconnecting axons are located within the depths of the hemispheres. They have a white coloration after fixed in formalin, and these regions are usually called the white matter (see Figures 27 and 29). There are three kinds of white matter bundles within the hemispheres: those connecting cortical areas across the ©2000 CRC Press LLC midline (commissural bundles), those interconnecting the various cortical areas on the same side (association bundles), and those connecting the cerebral cortex with various subcortical structures (called projection fibers). The corpus callosum is the largest of the commissural bundles, as well as the latest in evolution. This is the anatomic structure required for each hemisphere to be kept informed of the activity of the other hemisphere. Functionally, the axons connect to and from the lower layers of the cerebral cortex, and in most cases the connections are between homologous areas and are reciprocal. If the brain is sectioned in the sagittal plane along this interhemispheric fissure, the corpus callosum will be divided (see Figure 16). This sectioning will reveal the medial aspect of the brain. It is difficult from the view in Figure 15 to appreciate the depth of the corpus callosum. In fact, there is a considerable amount of cortical tissue on the medial surface of the hemispheres, as represented by the frontal, parietal, and occipital lobes. It should be noted that the cerebral ventricles are located below (i.e., inferior to) the corpus callosum (see Figures 9 and 16). Note on the safe handling of brain tissue: Figure 15 shows a rather old photograph of a brain specimen. Current guidelines recommend the use of disposable gloves when handling any brain tissue (as is seen in Figures 37 and 76). Anterior Corpus callosum FIGURE 15: Cerebral Hemispheres V — Corpus Callosum: Dorsal View ©2000 CRC Press LLC FIGURE 16 CEREBRAL HEMISPHERES VI CEREBRAL CORTEX: MEDIAL VIEW The view in Figure 16 is one of the most important for understanding the gross anatomy of the brain, brainstem, and ventricles. In this figure, the brain has been sectioned in the midline, mid-sagittally, through the corpus callosum and brainstem. The medial aspects of the lobes of the brain are in view. The central fissure does extend onto this part of the brain (although not as deep, usually, as on the dorsolateral surface). The medial surface of the frontal lobe is situated anteriorly, with the parietal lobe behind. Moving posteriorly, the parieto-occipital fissure has been opened. The occipital lobe is visible, divided by a deep fissure, the calcarine fissure, into upper and lower portions. The primary visual area, the cortical area where the visual fibers first arrive in the cerebral cortex, is located along the banks of the calcarine fissure. This area is commonly called area 17 (described with Figures 39A and 39B). The adjacent areas of the occipital lobe are visual association areas, also known as areas 18 and 19. The corpus callosum in this particular specimen does not have the usual white matter appearance that would be expected. The septum pellucidum, a membrane which divides the lateral ventricle of one hemisphere from that of the other side (see Figure 28A), has been removed, thereby exposing one of the lateral ventricles which is seen to be situated inferior to the corpus callosum. Above the corpus callosum is an important gyrus which is part of the limbic system, the cingulate gyrus (see Figures 74 and 75). This medial view of the brain exposes one-half of the paired diencephalon (see Figures 3, 4, and 9). The thalamic portion is separated from the hypothalamic part by ©2000 CRC Press LLC a groove, the hypothalamic sulcus. This sulcus starts at the foramen of Monro (the interventricular foramen, discussed with the ventricles; see Figure 20B) and ends at the aqueduct of the midbrain. The optic chiasm is found at the anterior aspect of the hypothalamus (see also Figures 13 and 14). The pineal body (sectioned) is located off the posterior aspect of the diencephalon (see also Figure 8). The three parts of the brainstem can be distinguished from this view — the midbrain, the pons (with its bulge anteriorly), and the medulla (refer to the ventral view shown in Figure 4). Through the midbrain is a narrow channel for CSF, the cerebral aqueduct, also known as the aqueduct of the midbrain or the aqueduct of Sylvius (see Figure 20B). The posterior aspect of the midbrain (behind the aqueduct) consists of the superior and inferior colliculi (see Figure 8).This aqueduct opens into the fourth ventricle, which separates the pons and medulla from the cerebellum. The fourth ventricle is said to have a floor, which is the brainstem, and a roof (see Figure 20A). The roof is divided into an upper and lower portion. The upper part consists of a band of white matter known as the superior medullary velum (see also Figure 7). The lower part of the roof of this ventricle is occupied by choroid plexus, which has not been preserved on this specimen. The cerebellum lies behind (or above) the fourth ventricle. It has been sectioned through its midline portion, the vermis. Although it is not necessary to name all of the various parts of the vermis, it is useful to know two of them: the lingula and the nodulus. (The reason for knowing these is evident when describing the cerebellum; see Figure 52). The lingula is that part of the vermis lying immediately above the superior medullary velum. The nodulus is that part of the vermis lying adjacent to the lower portion of the roof of the fourth ventricle. ©2000 CRC Press LLC Corpus callosum Cingulate gyrus Central fissure Pineal Parieto-occipital fissure Lateral ventricle Fornix Foramen of Monro Area 17 Calcarine fissure Hypothalamic sulcus Optic chiasm Superior medullary Superior and inferior colliculi Cerebral aqueduct T = Thalamus Hyp = Hypothalamus Md = Midbrain P = Pons M = Medulla Fourth ventricle F = Frontal lobe P = Parietal lobe O = Occipital lobe FIGURE 16: Cerebral Hemispheres VI — Cerebral Cortex: Medial View L = Lingula N = Nodulus FIGURE 17 RADIOLOGIC VIEW OF HEMISPHERES MRI: SAGITTAL VIEW The radiological image in Figure 17 was obtained by magnetic resonance imaging (MRI), which shows the brain as clearly as the actual brain itself. MRI imaging is the way the brain is seen in clinical settings. The view presented in this figure is a T1-weighted image (see below). Note that the cerebrospinal fluid is dark in this image, including the subarachnoid space and cisterns (see Figure 21). The bones (tables) of the skull are visible as dark lines; bone marrow, including its replacement by fatty tissue, and layers of soft tissue and fatty tissue of the scalp are well demarcated. By comparing this view with the photographic view of the brain shown in Figure 18, the various structures of the brain can be easily identified: various fissures (e.g., parieto-occipital, calcarine), cortical gyri (e.g., area 17, cingulate), the corpus callosum, lateral ventricle, and thalamus. The three parts of the brainstem — midbrain, pons and medulla — can be identified, with the tectum (colliculi) seen behind the aqueduct of the midbrain. The fourth ventricle separates the cerebellum from the brainstem. The location of the cerebellar tonsil(s) should be noted, adjacent to the medulla and immediately above the foramen magnum, the “opening” at the base of the skull (see discussion on tonsillar herniation with Figure 8). The location of the cerebello-medullary cistern (the cisterna magna) behind the medulla and just above the foramen magnum is easily seen (see Figure 20A; also Figure 1B). The remaining structures are those of the nose and mouth, which are not within the subject matter in this Atlas. Note on radiological imaging Ordinary x-rays show the skull and its bony structures ©2000 CRC Press LLC but not the brain. A remarkable revolution occurred in clinical neurology and our understanding of the brain when imaging techniques were developed that allowed for visualization of the brain. These techniques now include computed tomography (CT) and magnetic resonance imaging (MRI): • CT (often pronounced as a “cat” scan — see Figure 28A) uses x-rays and is a computer reconstruction of the brain after a series of views are taken from many perspectives. In this view the bones of the skull are bright, the CSF is dark, and the brain tissue gray, not clear. This image can be obtained in several seconds, even with a very sick patient. • MRI does not use x-rays; the image is created by capturing the energy of the hydrogen ions of water on their return to a steady state. It uses an extremely strong magnet, and requires more time. Again there is a computer reconstruction of the images. The brain itself looks “anatomic.” The view can be weighted during acquisition of the image to produce a TI image, in which the CSF is dark (as in Figure 17), or a T2 image in which the CSF is white (see Figure 30). An intermediate setting allows the structures of the interior of the brain to be seen; this method produces a proton density image. With MRI, the bones of the skull are dark, while fatty tissue (including bone marrow) is bright. As imaging and technology improve, we are able to visualize the brain during functional activity. Functional MRIs allow us to see which areas of the brain are particularly active during a certain task, based upon the metabolic rate (oxygen saturation). They are becoming more widely available. Other techniques also visualize the living brain and its activity, such as the positron emission tomography (PET) scan. PET uses a very short-acting radioactive compound which is injected into the arterial system. Its use is usually restricted to specialized neurologic centers involved in research on the human brain. ©2000 CRC Press LLC 1. Corpus callosum 2. Thalamus 3. Tectum of midbrain 4. Optic chiasm 5. Pons 6. Tonsil of cerebellum 7. Cisterna magna 8. Spinal cord FIGURE 17: Radiologic View Of Hemispheres — MRI: Sagittal View FIGURE 18 CORPUS CALLOSUM CEREBRAL HEMISPHERES: DISSECTED VIEW Structures within the depths of the cerebral hemispheres include the white matter, cerebral ventricles, and basal ganglia, all of which are described in the following figures. The white matter consists of the myelinated axonal fibers connecting brain regions. In the spinal cord these are called tracts; in the hemispheres these bundles are classified in the following way (also discussed with Figure 15): • association bundles which connect cortical areas on the same side; • projections fibers, connecting the cortex with subcortical structures in the diencephalon, brainstem, and spinal cord; and • commissural connections, across the midline — the largest of these is the corpus callosum. In the dissection of this specimen, the brain is again seen from the medial view. Its anterior aspect is on the right side of the photograph. Cortical tissue has been removed (as shown in Figure 16), using blunt dissection techniques. If done successfully, the fibers of the corpus callosum can be followed to the cerebral cortex. These fibers intermingle with the other fiber bundles which make up the mass of white matter in the depth of the hemisphere. ©2000 CRC Press LLC The dissection in Figure 18 shows the white matter of the corpus callosum, followed to the cortex. The corpus callosum is the massive commissure of the forebrain, connecting homologous regions of the two hemispheres of the cortex across the midline (see also Figure 15). In a sagittal section, the thickened anterior aspect of the corpus callosum is called the genu, and the thickened posterior portion the splenium (neither has been labeled in this figure). Clinical Aspects In a clinical setting, the corpus callosum is sectioned surgically in individuals with intractable epilepsy (epilepsy that could not be controlled with anti-convulsant medication). The idea behind the surgery is to stop the spread of the abnormal discharges from one hemisphere to the other. Studies of these individuals have helped to clarify the role of the corpus callosum in normal brain function. Generally, the surgery has been helpful in well selected cases and there is apparently no noticeable change in the person, or his or her level of brain function. Under laboratory conditions, it has been possible to demonstrate in these individuals how the two hemispheres of the brain function independently, after the sectioning of the corpus callosum. These studies show how each hemisphere responds differently to various stimuli, and the consequences of information not being transferred from one hemisphere to the other. Corpus callosum FIGURE 18: Corpus Callosum — Cerebral Hemispheres: Dissected View ©2000 CRC Press LLC FIGURE 19A WHITE MATTER CEREBRAL HEMISPHERES: ASSOCIATION FIBERS The dorsolateral aspect of the brain is shown in Figure 19A. Under the cerebral cortex is the white matter of the brain. Various fiber bundles can be dissected (not easily) using a blunt instrument (e.g., a wooden tongue depressor). Some of these, functionally, are the association bundles, fibers that interconnect different parts of the cerebral cortex on the same side. ©2000 CRC Press LLC The specimen in Figure 19A has been dissected to show one of the association bundles within the hemispheres. There are also shorter association fibers between adjacent gyri. These association bundles are extremely important in informing different brain regions of ongoing neuronal processing, allowing for integration of activities (for example, sensory with motor and limbic). The various names of these association bundles are usually not of much importance in a general introduction to the CNS (except as in Figure 19B). White matter association bundle FIGURE 19A: White Matter — Cerebral Hemispheres: Association Fibers ©2000 CRC Press LLC FIGURE 19B WHITE MATTER CEREBRAL HEMISPHERES: ASSOCIATION FIBERS The arcuate bundle is a specific group of association fibers of some importance, particularly on the side dominant for language (the left hemisphere in most people). This fiber bundle connects the two cortical areas for language (see Figure 12), Broca’s anteriorly with Wernicke’s posteriorly. ©2000 CRC Press LLC Damage to these fibers in humans causes a disruption of language, called conduction aphasia. Aphasia is a general term for a disruption or disorder of language. In conduction aphasia, the person has normal comprehension (intact Wernicke’s area) and fluent speech (intact Broca’s area). The only deficit is an inability to repeat what has been heard. This is usually tested by asking the patient to repeat single words or phrases whose meaning cannot be readily understood (e.g., the phrase “no ifs, ands, or buts”). Arcuate bundle FIGURE 19B: White Matter — Cerebral Hemispheres: Association Fibers ©2000 CRC Press LLC FIGURE 20A VENTRICLES that the “hole” in the middle of the third ventricle represents the massa intermedia, linking the two thalami across the midline (see Figure 3; described with Figure 9). VENTRICLES: LATERAL VIEW The ventricular system then narrows considerably as it goes through the midbrain and is now called the aqueduct of the midbrain, the cerebral aqueduct, or the aqueduct of Sylvius (see Figures 16 and 17). In the hindbrain region, the area consisting of pons, medulla, and cerebellum, the ventricle widens again to form the fourth ventricle (see Figures 16 and 17). The channel continues within the CNS and becomes the very narrow central canal of the spinal cord (Figure 2A). The ventricles are cavities within the brain filled with CSF, cerebrospinal fluid. The formation, circulation, and locations of the CSF are explained with Figure 21. The ventricles of the brain are what remain of the original neural tube, the tube that was present during development. The cells of the nervous system, both neurons and glia, originated from a germinal matrix adjacent to the lining of this tube. The cells multiply and migrate away from the walls of the neural tube, forming the nuclei and cortex. As the nervous system develops, the mass of tissue grows and the size of the tube diminishes, leaving various spaces in different parts of the nervous system. The parts of the tube that remain in the hemispheres are called the cerebral ventricles, or the lateral ventricles. In Figure 20A, the lateral ventricle of one hemisphere is shown from the lateral perspective. It is shaped like a reversed letter “C”; it curves posteriorly to enter into the temporal lobe. Its various parts are • the anterior horn, which lies deep to the frontal lobes; • the central portion or body, which lies deep to the parietal lobes; • the atrium or trigone where the ventricle widens and curves into the inferior horn, which goes into the temporal lobes. In addition, there may be an extension into the occipital lobes, called the occipital or posterior horn. These lateral ventricles are also called ventricles I and II (assigned arbitrarily). Each lateral ventricle is connected to the midline third ventricle by an opening, the foramen of Monro (interventricular foramen — seen in the medial view of the brain, Figure 16). The third ventricle is a narrow, slitlike ventricle between the thalamus on either side and is also called the ventricle of the diencephalon (see also Figure 8). Sectioning through the brain in the midline (as in Figure 16) passes through the third ventricle. Note ©2000 CRC Press LLC Within the ventricles is specialized tissue, the choroid plexus. It is made up of the lining cells of the ventricles, called the ependyma, and pia with blood vessels (discussed with Figure 21). The choroid plexus is the tissue responsible for the formation of most of the CSF. It is found in the body and inferior horn of the lateral ventricle, in the roof of the third ventricle, and in the lower half of the roof of the fourth ventricle. The tissue forms large invaginations into the ventricles in each of these locations (unfortunately, none of these are visible in the photographic views). CSF flows through the ventricular system, from the lateral ventricles, through the interventricular foramina into the third ventricle, then through the narrow aqueduct and into the fourth ventricle. At the bottom of the fourth ventricle, CSF exits from the ventricular system and enters the subarachnoid space. The exit points are foramina in the fourth ventricle. The major exit is the foramen of Magendie in the midline. There are two additional exits of the CSF laterally from the fourth ventricle, the foramina of Luschka (seen in Figure 20B). From the foramen of Magendie, the CSF then enters one of the enlargements of the subarachnoid space, called a cistern, in this case the cisterna magna, the cerebellomedullary cistern. It lies below the cerebellum and is found inside the skull, just above the foramen magnum (see Figure 1B). The CSF then flows in the subarachnoid space downwards around the spinal cord and upwards around the brain (discussed with Figure 21). LATERAL VENTRICLE Anterior horn Body Inferior horn Posterior horn Cisterna magna Foramen of Monro Third ventricle Aqueduct of Sylvius Fourth ventricle Foramen of Magendie Central canal FIGURE 20A: Ventricles — Ventricles: Lateral View ©2000 CRC Press LLC FIGURE 20B VENTRICLES VENTRICLES: ANTERIOR VIEW The ventricular system is viewed from the anterior perspective in Figure 20B. Both lateral ventricles and the short interventricular foramen (of Monro) are visible on both sides, connecting each lateral ventricle with the midline third ventricle. It is important to note that the thalamus (diencephalon) is found on each side of the third ventricle (see also Figure 8). Sectioning of the brain in the coronal (frontal) plane, if done at the appropriate plane, reveals the spaces of the lateral ventricles within the hemispheres (see Figure 29). Likewise, sectioning of the brain in the horizontal axis, if done at the appropriate level, shows the ventricular spaces of the lateral and third ventricles (see Figure 27). These can also be visualized with radiographic imaging (CT and MRI; see Figures 28A , 28B, and 30). The ventricular channel continues through the aqueduct of the midbrain. CSF then enters the fourth ventricle, which also straddles the midline. The fourth ventricle is diamond-shaped, and the lateral recesses carry CSF into the cisterna magna through the foramina of Luschka, the lateral apertures, one on each side. Clinical Aspects The flow of CSF can be interrupted or blocked at various key points within the ventricular system. One of the most common is the cerebral aqueduct, the aqueduct ©2000 CRC Press LLC of the midbrain (Sylvius). Since most of the CSF is formed upstream, in the lateral (and third) ventricles, a blockage at the narrowest point at the level of the aqueduct of the midbrain will create a damming effect. In essence, this causes a marked enlargement of the ventricles. The CSF flow can be blocked in a variety of ways, such as developmentally, following a meningitis, or by a tumor in the region. The result is an enlargement of the ventricles, called hydrocephalus, which can be seen with imaging techniques (e.g., CT scan). Not uncommonly, hydrocephalus in infancy occurs spontaneously, for unknown reasons. Since the sutures of the infant’s skull are not yet fused, hydrocephalus leads to an enlargement of the head. Clinical assessment of normal infants should include measuring the size of the head and charting this in the same way as one charts height and weight. Untreated hydrocephalus eventually leads to a compression of the nervous tissue of the hemispheres and damage to the developing brain. Clinical treatment of this condition, after evaluation of the causative factor, includes shunting the CSF out of the ventricles into one of the body cavities. In adults, hydrocephalus caused by a blockage of the CSF flow leads to an increase in intracranial pressure, since the sutures are fused and skull expansion is not possible. The cause in adults is usually a tumor, and, in addition to experiencing the specific symptoms, the patient will usually complain of headache, often in the early morning. Lateral ventricle Foramina of Luschka Foramen of Monro Third ventricle Central canal Aqueduct of Sylvius Fourth ventricle T = Thalamus FIGURE 20B: Ventricles — Ventricles: Anterior View ©2000 CRC Press LLC FIGURE 21 CEREBROSPINAL FLUID SCHEMATIC OF CSF CIRCULATION Figure 21 presents a schematic representation of the relationship between the brain tissue, CSF, and cerebral blood vessels. The CSF is formed within the ventricles, flows through the various channels, exits from within the brain, circulates in the subarachnoid space and cisterns around the brain and spinal cord, and is finally reabsorbed into the venous system (the venous sinuses). The physiological barriers between the various compartments is also indicated in the illustration. The ventricles of the brain are lined with a layer of cells known as the ependyma. In certain loci within each of the ventricles the ependymal cells and the pia meet, thus forming the choroid plexus, which invaginates into the ventricle. Functionally, the choroid plexus has a vascular layer, i.e., the pia, on the inside, and the ependymal layer on the ventricular side. The blood vessels of the choroid plexus are freely permeable, but there is a cellular barrier between the interior of the choroid plexus and the ventricular space — the blood-CSF barrier (labeled B-CSFB in the diagram). The barrier consists of tight junctions between the ependymal cells that line the choroid plexus. CSF is actively secreted and an enzyme is involved. The ionic and protein composition of CSF is different from that of serum. CSF leaves the ventricular system from the fourth ventricle, as indicated schematically in the diagrams. In the intact brain, this occurs via the medially placed foramen of Magendie and the two laterally placed foramina of Luschka (described in the previous illustrations). CSF ©2000 CRC Press LLC flows through the subarachnoid space (SAS), between the pia and arachnoid. The CSF fills the enlargements of the subarachnoid spaces around the brainstem — the various cisterns — and also flows downwards around the spinal cord to fill the lumbar cistern (see Figures 1A and 1B). This slow circulation is completed by the return of CSF to the venous system. The return is through the arachnoid villi which protrude into the venous sinuses of the brain, particularly the superior sagittal sinus (located in the interhemispheric fissure). These can be seen as collections of villi, called arachnoid granulations, on the surface of the brain (shown diagrammatically; see also Figure 11). There is no real barrier between the intercellular tissue of the brain and the CSF, either through the ependyma (at all sites other than the choroid plexus) or the pia. This lack of barrier is depicted by the arrows, which indicate a free communication between these compartments. Therefore, substances found in detectable amounts in the intercellular spaces of the brain may be found in the CSF. On the other hand, there is a real barrier, both structural and functional, between the blood vessels and the brain tissue. This is called the blood-brain barrier (BBB) and is situated at the level of the brain capillaries. Only oxygen, carbon dioxide, glucose, and other select, small molecules are normally able to cross the BBB. Sampling of CSF for clinical evaluation, including inflammation of the meninges (meningitis), is performed almost always in the lumbar cistern (discussed with Figures 1A and 1B). A SAS Pia Brain Ependyma B Ventricle B-CSF-B B B Choroid plexus Vein BBB = Blood-brain barrier A = Artery B-CSF-B = Blood-CSF barrier SAS = Subarachnoid space FIGURE 21: Cerebrospinal Fluid — Schematic of CSF Circulation ©2000 CRC Press LLC FIGURE 22 BASAL GANGLIA I BASAL GANGLIA: ORIENTATION The basal ganglia are large collections of neurons belonging to the forebrain. This neuronal mass is found deep within the cerebral hemispheres. Our understanding of the functional role of the basal ganglia is derived largely from disease states affecting these neurons. In general, humans with lesions in the basal ganglia have some form of motor dysfunction (movement disorder) — dyskinesia. This large group of neurons is now thought to be involved in the control of complex patterns of motor activity, such as skilled movements (e.g., writing). There are two aspects to this involvement. The first concerns the initiation of the movement, and the second concerns the quality of the performance of the motor task. It seems that different parts of the basal ganglia are concerned with the speed and magnitude of a movement. In addition, some of the structures that make up the basal ganglia are thought to influence cognitive aspects of motor control, helping to plan the sequence of tasks needed for purposeful activity. This process is sometimes referred to as the selection of motor strategies. From the strictly anatomical point of view, the basal ganglia are collections of neurons located within the hemispheres. Traditionally, this would include the caudate nucleus, the putamen, the globus pallidus, and the amygdala. Although the caudate and putamen are separated from each other anatomically, they are histologically the same neurons, and are known as the striatum. The putamen and globus pallidus are anatomically found together and are called the lentiform or lenticular nucleus. From the functional point of view and based upon the complex pattern of interconnections, two other nuclei should be included with the description of the basal ganglia: the subthalamic nucleus (part of the diencephalon) and the substantia nigra (located in the midbrain). The amygdala is now included with the limbic system (see Section D). Clinical Aspects Overall, the basal ganglia receive much of their input from the cortex, the motor areas of the cortex, as well as wide areas of association cortex. There are intricate connections between the various parts of the system (involving different neurotransmitters), and the output is directed via the thalamus mainly to premotor, supplementary motor, and frontal cortical areas. Therefore, the basal ganglia act as a subloop of the motor system by altering cortical activity (to be fully discussed with Figures 50 and 51). Note on terminology: The term ganglia refers to a collection of cells in the peripheral nervous system. Therefore, the anatomically correct name for this group of neurons should be the basal nuclei. Few texts use this term. Most clinicians would be hard-pressed to change to the use of this term, so the traditional name remains in use. ©2000 CRC Press LLC The functional role of this large collection of neurons is best illustrated by clinical conditions affecting this neuronal system — abnormal movements, such as chorea (jerky movements), athetosis (writhing movements), and tremors (rhythmic movements). The most common condition that affects this group of neurons is Parkinson’s disease. A person with this disease has difficulty initiating movements, loses facial expressiveness taking on a mask-like appearance, and has muscular rigidity, slowing of movements (bradykinesia), and a tremor of the hands when at rest that goes away with purposeful movements (and in sleep). The other major disease that affects the basal ganglia is Huntington’s Chorea, an inherited degenerative condition. Corpus callosum Caudate nucleus Cerebellum Lenticular nucleus Brainstem FIGURE 22: Basal Ganglia I — Basal Ganglia: Orientation ©2000 CRC Press LLC FIGURE 23 BASAL GANGLIA II BASAL GANGLIA: NUCLEI A The basal ganglia, from the point of view of strict neuroanatomy, are located within the cerebral hemispheres. There are three major nuclei in the hemispheres: • The caudate nucleus has three portions: the head, located deep within the frontal lobe; the body, located deep in the parietal lobe; and the tail, which goes into the temporal lobe. • The lentiform or lenticular nucleus, so-named because it is lens-shaped. In fact, it is composed of two nuclei (see Figure 24): putamen and globus pallidus. It is situated laterally and is deep in the hemispheres within the central white matter. Sectioning of the brain in the horizontal plane (see Figure 27) and in the coronal (frontal) plane (see Figure 29) shows the location of the lentiform nucleus in the depths of the hemispheres. Both the caudate and the lentiform nuclei are found below the level of the corpus callosum. In Figure 23 the basal ganglia are shown in isolation from the lateral perspective, as well as from above, allowing a view of the caudate nucleus of both sides. The various parts of the caudate nucleus are easily recognized — head, body and tail. The head of the caudate nucleus is large and actually intrudes into the space of the anterior horn of the lateral ventricle (see Figures 27 and 28A). The body of the caudate nucleus is considerably smaller and lies above the thalamus (see Figure 29). ©2000 CRC Press LLC The tail follows the inferior horn of the lateral ventricle into the temporal lobe (see Figure 25). In a coronal section (Figure 80), the tail of the caudate is found above the inferior horn of the lateral ventricle. The lentiform (lenticular) nucleus is only a descriptive name, meaning lens-shaped. The nucleus is in fact composed of two distinct parts — the putamen, laterally, and the globus pallidus, medially (see Figures 24 and 27). When viewing the basal ganglia from the lateral perspective, one sees only the putamen part. Strands of tissue are seen connecting the caudate nucleus with the lentiform nucleus; in fact, they connect the caudate with the putamen (as discussed with Figure 26). The caudate and the putamen are histologically alike and are collectively called the neostriatum, or simply the striatum. The amygdala, though part of the basal ganglia by definition, has its functional connections with the limbic system and is discussed with it (see Figures 79A and 79B). The inferior portions of the putamen and globus pallidus are found at the level of the anterior commissure. These ventral parts of the lentiform nucleus may have a limbic connection (discussed with Figure 85B). The anterior commissure connects the amygdala and other temporal lobe structures of the two sides (discussed with Figure 74). The other two nuclei of the functional basal ganglia — the subthalamus and substantia nigra — are not shown in this illustration. Caudate nucleus (body) Lenticular nucleus (putamen) Caudate nucleus (tail) Caudate nucleus (head) Amygdala Anterior commissure FIGURE 23: Basal Ganglia II — Basal Ganglia: Nuclei A ©2000 CRC Press LLC FIGURE 24 BASAL GANGLIA III BASAL GANGLIA: NUCLEI B The view in Figure 24 has been obtained by removing all parts of the basal ganglia of one hemisphere, except the tail of the caudate and the amygdala. This view exposes the lentiform nucleus of the “distal” side; the lentiform nucleus is thus visualized from a medial perspective. The two portions of this nucleus are seen: the putamen laterally and the globus pallidus, which is medially placed. The caudate nucleus and the putamen receive the inputs into the basal nuclei. As has been explained previously, the caudate and putamen are, in fact, the same neurons embryologically. Together they are known as the neostriatum. (In some texts they are simply called the striatum.) Strands of cells may be seen connecting the various portions of the caudate with the putamen. This nuclear structure has been separated into two distinct components by groups of axons, which collectively are called the internal capsule (see Figure 26). These fiber bundles “fill the spaces” between the cellular strands. ©2000 CRC Press LLC The globus pallidus is one of the efferent nuclei of the basal ganglia. It is composed of two segments — the medial and lateral segments, also known as internal and external segments, respectively. (These segments can also be seen in the horizontal section of the brain, Figure 27). This subdivision of the globus pallidus is quite important functionally. This view exposes the two additional nuclei of the basal ganglia — the subthalamic nucleus (part of the diencephalon) and the substantia nigra (of the midbrain). The functional connections of these nuclei are discussed as part of the motor system (see Figures 50 and 51). A distinct collection of neurons is also found in the ventral region, composed in part of neurons belonging to the basal nuclei — the nucleus accumbens. The nucleus accumbens is unique in that it seems to consist of a mix of neurons from the basal ganglia and from the limbic structures in the region (discussed with the limbic system, Figure 85B). Putamen Caudate nucleus Globus pallidus (external segment) Subthalamic nucleus Globus pallidus (internal segment) Substantia nigra Red nucleus Caudate nucleus (tail) N. accumbens Midbrain Amygdala Anterior commissure FIGURE 24: Basal Ganglia III — Basal Ganglia: Nuclei B ©2000 CRC Press LLC FIGURE 25 BASAL GANGLIA IV BASAL GANGLIA AND VENTRICLES In humans, the three forebrain nuclei of the basal ganglia have a complex arrangement in space, and visualization of their location is made easier by understanding their relationship with the cerebral ventricles (also illustrated in Figure 80). In Figure 25 the basal ganglia are visualized deep in the hemisphere, from the lateral perspective. The various parts include the caudate nucleus, the lentiform nucleus, and also the amygdala. Included in this view is the ventricular system (as in Figure 20A) and the thalamus, which lies adjacent to the third ventricle (see Figures 16 and 20B). All three parts of the caudate nucleus — the head, body, and tail — are situated adjacent to the lateral ventricle. In fact, the head protrudes into the anterior horn of the lateral ventricle (see Figure 27). The body of the caudate lies adjacent to the body of the ventricle (see Figure 9), with the tail following the ventricle into the temporal lobe (see Figure 80). The lentiform nucleus (which includes the putamen and globus pallidus), is located deep within the hemispheres, ©2000 CRC Press LLC not adjacent to the ventricle. This “nucleus” is found lateral to the thalamus, which locates the lentiform nucleus in a horizontal section as lateral to the third ventricle. In the view provided by Figure 25, one can see that the caudate and the lentiform nuclei are connected by strands of tissue between them; in fact, these neurons are the same embryologically. (These connecting strands are shown in the previous diagrams.) As fiber systems develop, namely the internal capsule (see Figure 26), these nuclei become separated from each other (by the anterior limb of the internal capsule). Some connecting strands of tissue can still be found in the adult brain on a few horizontal sections through the lowermost parts of these nuclei. The internal capsule, which consists of a collection of fibers (described with Figure 26) is situated between the lentiform nucleus and the head of the caudate, and between the lentiform nucleus and the thalamus. A horizontal section through the brain at the level of the lateral fissure would reveal all these structures (see Figure 27). The amygdala is located in front of the tip of the inferior horn of the lateral ventricle, under the uncus (see Figures 13 and 14). Connection between lentiform and caudate nn. CAUDATE NUCLEUS Head Body Tail Amygdala Lentiform nucleus Third ventricle Lateral ventricle Thalamus FIGURE 25: Basal Ganglia IV — Basal Ganglia and Ventricles ©2000 CRC Press LLC FIGURE 26 INTERNAL CAPSULE I INTERNAL CAPSULE: WHITE MATTER The white matter bundles that course between parts of the basal ganglia and the diencephalon are collectively grouped together and called the internal capsule. The internal capsule is a group of fibers located at a specific location within the cerebral hemispheres — between the diencephalon (the thalamus) and the various parts of the basal ganglia. These so-called projection fibers are axons going to and coming from the cerebral cortex, linking the cortex with the diencephalon (thalamus), brainstem, and spinal cord. The internal capsule has 3 parts: • As was noted earlier, a group of fibers has separated off the two parts of the neostriatum from each other, the caudate from the putamen. This fiber system forms its anterior limb; • At the level of the lentiform nucleus, situated medially, is the thalamus, part of the diencephalon (see Figure 27). The fiber system that runs between the thalamus (medially) and the lentiform nucleus (laterally) is the posterior limb of the internal capsule. The posterior limb carries sensory information from thalamus to cortex, the reciprocal connections from cortex to thalamus, and most of the descending fibers to the brainstem (cortico-bulbar, Figure 43) and spinal cord (cortico-spinal, Figure 42). • The internal capsule can be seen in a horizontal section of the brain (see Figure 27), and with neuroradiological imaging (CT, see Figure 28A, and MRI, see Figure 28B). In this view, the internal capsule (of each side) is seen to be V-shaped. The anterior portion between the caudate and lentiform is the anterior limb. The portion between the lentiform nucleus and the thalamus is the posterior limb. The bend of the “V” is called the genu and it is situated medially. In addition, there are numerous axons descending from the cortex destined for the cerebellum (and that will synapse first in the pontine nuclei; discussed with Figure 53). These cortico-pontine fibers descend in both the anterior and posterior limbs of the internal capsule. ©2000 CRC Press LLC Other fiber systems emanating from the thalamus are often described in relation to the internal capsule. For example, the visual radiation is situated posterior to the internal capsule, yet functionally is part of it (see Figures 39A and 39B). The auditory radiation, which runs inferiorly (see Figure 36), is similar. Below the level of the internal capsule is the midbrain. The descending fibers of the internal capsule are continuous with those found in the cerebral peduncle of the midbrain. A parcellation of the descending fibers occurs in the cerebral peduncle, where the cortico-pontine fibers are found in the outer and inner thirds of the peduncle, and the cortico-bulbar and cortico-spinal fibers are located in the middle third (see also Figure 43). In summary, at the level of the internal capsule, there are both the ascending fibers from thalamus to cortex, as well as the descending fibers from widespread areas of the cerebral cortex to the thalamus and the rest of the neuraxis. These ascending and descending fibers are all called projection fibers (discussed with Figure 18). This fiber system is sometimes likened to a funnel, in which the top of the funnel is the cerebral cortex and the stem is the cerebral peduncle. The base of the funnel would be the internal capsule. The main point is that the various fiber systems, both ascending and descending, are condensed together in the region of the internal capsule. Clinical Aspects The area of the internal capsule is clinically important because of the frequency of lesions here. The blood vessels that supply the internal capsule come from the middle cerebral artery, as it courses in the lateral fissure; they are known as the striate arteries (see Figure 60). For reasons that are not clearly understood, these blood vessels are prone to occlusion which destroys the surrounding axons. Because of the high packing density of the axons in this region, a small lesion can create extensive disruption of descending and/or ascending pathways; this is a common form of “stroke.” These small lesions are seen as small holes with neuroimaging and called “lacunes” (discussed with Figure 60). Posterior limb Genu Visual radiation Anterior limb Descending fibers Midbrain Thalamus Caudate nucleus (head) Lenticular nucleus Cortico-pontine fibers Anterior commissure FIGURE 26: Internal Capsule I — Internal Capsule: White Matter ©2000 CRC Press LLC Cortico-pontine fibers Cortico-spinal fibers Cortico-bulbar fibers FIGURE 27 INTERNAL CAPSULE II HORIZONTAL SECTION OF HEMISPHERES (PHOTOGRAPHIC VIEW) The brain specimen in Figure 27 has been sectioned in the horizontal plane, at the level of the lateral fissure. This view exposes the white matter of the hemispheres and the basal ganglia, as well as parts of the ventricular system. Understanding the topography of the structures seen in this view is immeasurably important when the student enters the clinical setting. The basal ganglia are observable when the brain is sectioned at this level. The head of the caudate nucleus is seen, protruding into the lateral ventricle. The lentiform nucleus is shaped somewhat like a lens and is demarcated by white matter. The outer part, the putamen, has neurons that are identical to the caudate nucleus, and, therefore, the two nuclei look the same. The inner portion, the globus pallidus, is functionally different and contains many more fibers and therefore is lighter in color. Depending upon the level of the section, it is sometimes possible (as in the right side of the photograph in Figure 27) to see the two subdivisions of the globus pallidus, the internal and external segments (see Figure 24). The white matter medial to the lentiform nucleus is the ©2000 CRC Press LLC internal capsule. It is divisible into an anterior portion, the anterior limb, and a posterior limb; the base of the “V” is known as the genu. The anterior limb separates the lentiform nucleus from the head of the caudate nucleus. This portion of the caudate nucleus is related to the anterior horn of the lateral ventricle, which is cut through its lowermost part and is represented in this photograph by a very small cavity. Some strands of gray matter located within the anterior limb represent the tissue that unites the caudate nucleus with the putamen (as shown in Figure 25). The posterior limb of the internal capsule separates the lentiform nucleus from the thalamus. The major ascending sensory tracts and the descending motor tracts from the cerebral cortex are found in the posterior limb. Lateral to the lentiform nucleus is another thin strip of tissue, the claustrum. The functional contribution of this small strip of tissue is not really known. Lateral to this is the cortex of the insula (see Figure 37). Posteriorly, behind the thalamus, the cerebellum is visible. It is also possible to see the atrium portion of the lateral ventricle, deep within the parietal lobe. The ventricle is sectioned at this level as it enters into the temporal lobe and is becoming the inferior horn of the lateral ventricle (see Figure 20A). The third ventricle can be seen situated between the thalamus of both sides. A view similar to this is commonly presented in brain scans of patients — CTs and MRIs — as shown in the following figures. Anterior Lateral ventricle (anterior horn) Anterior limb of internal capsule Head of caudate nucleus Putamen Lentiform nucleus Globus pallidus Claustrum Posterior limb of internal capsule Thalamus Third ventricle Lateral ventricle C = Cerebellum FIGURE 27: Internal Capsule II — Horizontal Section of Hemispheres (Photographic View) ©2000 CRC Press LLC FIGURE 28A HORIZONTAL VIEW HORIZONTAL VIEW: CT The radiological view of the brain is not in the exact horizontal plane as the anatomical specimen shown in the previous illustration. The radiological images of the brain are done at an angle in order to minimize the dense bones of the posterior cranial fossa, which impair the viewing of the structures (brainstem and cerebellum) of the posterior cranial fossa. A CT image (refer to Figure 17) shows the skull bones and the relationship of the brain to the skull. The outer cortical tissue can be seen, with gyri and sulci. The structures seen in the interior of the brain include the white matter and the ventricular spaces. Note that the CSF is dark (black) in the ventricles (#2 and #3 in the illustration) and in the cisterns (#7 in the illustration). The ventricular space in the frontal lobes is the anterior horn of the lateral ventricle on each side (see Figure 27, also Figures 20A and 20B), separated in the midline by the septum pellucidum. (The septum has been removed from the specimen shown in Figure 16, a mid-sagittal view of the brain and brainstem.) Although the basal ganglia and thalamus can be identified (see #1, #4, and #6 in the illustration), there is little ©2000 CRC Press LLC tissue definition. Note that the head of the caudate nucleus protrudes, or bulges, into the anterior horn of the lateral ventricle (see also Figure 27). The area of the internal capsule can be seen as well (#5 in the illustration; compare with the MRI in the next illustration, Figure 28B). The cerebellum can be recognized with its characteristic folia (#8 in the illustration). The CSF cistern, called the quadrigeminal plate cistern, behind the tectal plate (the colliculi; also known as the quadrigeminal plate; discussed with Figure 7) is a very important landmark for the neuroradiologist (seen also in mid-sagittal views, but not labeled, in Figures 16 and 17). Clinical Aspects A regular CT can show areas of increased density (e.g., fresh hemorrhage) or of decreased density (e.g., an infarct), as well as changes in the size and shifting of the ventricles. Tumors may be seen as an abnormal area of either increased or decreased density. A CT can also be enhanced by injecting an iodinated compound into the blood circulation and noting whether it escapes into the brain tissue because of leakage in the blood-brainbarrier (BBB; see Figure 21). This examination is invaluable in the assessment of a neurological patient in the acute stage of illness (e.g., tumor, head injury), and it is most frequently used because the image can be captured in seconds. 1. Head of caudate nucleus 2. Lateral ventricle (anterior horn) 3. Third ventricle 4. Lentiform nucleus 5. Internal capsule (posterior limb) 6. Thalamus 7. Quadrigeminal plate cistern 8. Cerebellum FIGURE 28A: Horizontal View — Horizontal View: CT ©2000 CRC Press LLC FIGURE 28B HORIZONTAL VIEW HORIZONTAL VIEW: MRI Figure 28B is a view of the brain taken in the same plane as used for Figure 28A, but with MRI adjusted for a T2weighted image (see explanation with Figure 17). In this view, the CSF of the ventricles is white. The lateral ventricle posteriorly (see number 5) is cut at the level of its widening — the atrium — as it curves into the temporal lobe (see Figure 20A). ©2000 CRC Press LLC This MRI shows the brain as if it were an anatomical specimen (compare with Figure 28A), showing the cortex and white matter. There is a clear visualization of the basal ganglia and its subdivisions (head of the caudate, putamen), as well as of the thalamus. In addition, the area of the internal capsule is also seen. Clinical Aspects The MRI has proved to be invaluable in assessing lesions of the CNS — infarcts, tumors, plaques of multiple sclerosis, and numerous other lesions. 1. Head of caudate nucleus 2. Lentiform nucleus 3. Thalamus 4. Internal capsule (posterior limb) 5. Lateral ventricle (atrium) FIGURE 28B: Horizontal View — Horizontal View: MRI ©2000 CRC Press LLC FIGURE 29 CORONAL VIEW CORONAL SECTION OF HEMISPHERES (PHOTOGRAPHIC VIEW) The photographic view of the brain in Figure 29 is sectioned in the coronal plane and shows the internal aspect of the hemispheres. The plane of this section is somewhat asymmetric, and includes the parietal and temporal lobes. The external aspect of the hemispheres is composed of the cerebral cortex, seen as the gray matter. It is thrown into various gyri with sulci between. The deep interhemispheric fissure is seen (not labeled; see Figure 15), along with the lateral fissure (also not labeled), with the insula within the depths of this fissure (see Figure 37). The white matter is seen internally; it is not possible to separate the various fiber systems of the white matter. The fibers of the corpus callosum are seen crossing the midline at the bottom of the interhemispheric fissure (see also Figure 18). Below the corpus callosum are the two spaces, the cavities of the lateral ventricle, represented at this plane by the body of the ventricles. Because the section was not cut symmetrically, the inferior horn of the lateral ventricle is found only on the right side of ©2000 CRC Press LLC the photograph, in the temporal lobe. The brain is sectioned in the coronal plane through the diencephalic region. At this plane of section, the third (midline) ventricle is not present between the thalami, except for a rather small space, likely because the section passes through the connecting link between the two thalami — the massa intermedia (discussed with Figures 9 and 20A). Various parts of the basal ganglia are seen in this view, as has been described with the illustration of the ventricles (see Figure 25). At the outer margins of the ventricle (body) is a dark nuclear structure, the body of the caudate nucleus. More laterally is the lentiform nucleus; because the brain has not been sectioned symmetrically, more of this nucleus is found on the left side of the photograph. Lateral to the thalamus and medial to the lentiform nucleus is the internal capsule, its posterior limb. (The student should refer to the horizontal section in Figure 27 and note why this is the posterior limb of the internal capsule.) The structures noted in this section should be compared with a similar (coronal) view of the brain taken more posteriorly (see Figure 78). The fornix is explained with the limbic system (Section D). ©2000 CRC Press LLC Corpus callosum Caudate nucleus (body) Thalamus Lateral ventricle (body) Fornix Insula Internal capsule (posterior limb) Lentiform nucleus Lateral ventricle (inferior horn) P = Parietal lobe T = Temporal lobe FIGURE 29: Coronal View — Coronal Section of Hemispheres (Photographic View) FIGURE 30 CORONAL VIEW CORONAL VIEW: MRI Figure 30 presents the same coronal view of the brain as shown in Figure 29, but with MRI. It shows the cortex, white matter, a little of the basal ganglia, the thalamus, and the ventricular spaces. This is a T2-weighted view, with the CSF white. The cortex and white matter can be easily differentiated in the figure. The plane of this section includes the body of the caudate nucleus (compare with the coronal section of the brain shown in Figure 78). ©2000 CRC Press LLC The region of the thalamus is seen adjacent to the midline third ventricle, interrupted by the massa intermedia. This view of the ventricles is similar to that presented in Figure 20B. The area of the internal capsule (posterior limb) is lateral to the thalamus, with the lentiform nucleus lateral to it. This view also includes the brainstem (the pontine region). 1. Caudate nucleus (body) 2. Thalamus 3. Pontine region FIGURE 30: Coronal View — Coronal View: MRI ©2000 CRC Press LLC Section B FUNCTIONAL SYSTEMS INTRODUCTION The functioning nervous system requires a hierarchical organization to carry out its activities. A simple reflex (e.g., touching a hot surface) involves incoming information from the periphery, some central processing, and a response. The incoming fibers are the afferent (sensory) fibers, the outgoing are the efferent (motor) fibers, and the central processing portions are the interneurons of the spinal cord (see Figure 2A). In order to go beyond the simple reflex state, more elaborate central processing stations have evolved, creating functional systems — both sensory and motor. These involve nuclei of the CNS at the level of the brainstem and forebrain, and the connections between these nuclei. The axons connecting various nuclei usually run together, forming a distinct bundle of fibers, called a tract or pathway. Along their way, these axons may distribute information to several other parts of the CNS by means of axon collaterals. In almost all functional systems in humans, the cerebral cortex is involved. Part I of this section discusses the sensory tracts or pathways and their connections in the CNS. Part II introduces the reticular formation which has sensory, motor, and other functions. Part III discusses the pathways and brain regions concerned with motor control. PART I: SENSORY SYSTEMS Sensory systems, also called modalities (singular, modality), share many features. All sensory systems begin with receptors, some of which are highly specialized, such as those in the skin for touch and vibration sense, and the hair cells in the cochlea for hearing, as well as the rods and cones in the retina. These receptors are hard-wired to activate the peripheral sensory fibers appropriate for that sensory system. The peripheral nerves have their cell bodies in sensory ganglia that ©2000 CRC Press LLC belong to the peripheral nervous system (PNS). For the body (neck down), these are the dorsal root ganglia located in the intervertebral spaces. The trigeminal ganglion serves the sensory fibers of the head. The central process of these peripheral neurons enters the CNS and synapses in the nucleus appropriate for that sensory system (again hard-wired). Generally speaking, the older systems both peripherally and centrally involve axons that are thinly myelinated or unmyelinated, with a slow rate of conduction. In general, these pathways consist of fibers-synapses-fibers, creating a multisynaptic chain with many opportunities for spreading the information, but thereby making transmission slow and quite insecure. The newer pathways that have evolved have axons that are more thickly myelinated and that conduct at a more rapid speed. These form rather direct connections with few, if any, collaterals. The latter type of pathway transfers information more securely and is more specialized functionally. Because of the upright posture of humans, the sensory systems go upwards, or ascend, to the cortex — the ascending systems. The sensory information is processed by various nuclei along the pathway. Three systems are concerned with sensory information from the skin, two from the body region, and one (with subparts) from the head: 1. The anterolateral system, an older system which carries pain and temperature and some less discriminative forms of touch sensations (formerly called the lateral spino-thalamic and anterior (ventral) spino-thalamic tracts, respectively). 2. The dorsal column-medial lemniscus pathway, a newer system for the somatosensory modalities of discriminative touch, joint position, and “vibration.” 3. The trigeminal pathways, carrying sensations from the face area (including discriminative touch, pain, and temperature), involve both older and newer components. The central nuclei for the afferents from the body region are located in the spinal cord. The trigeminal nuclei are found in the brainstem. The auditory and visual systems are two special senses that will be studied in some detail. Each has some unique features which are described. Other sensory pathways, such as vestibular and taste, are reviewed below. All these pathways relay in the thalamus before going to the cerebral cortex (refer to Figure 10). The olfactory system (smell) is considered with the limbic system (see Figure 84). Reticular Formation Interspersed with the consideration of the pathways is the reticular formation, located in the core of the brainstem. This group of nuclei comprises a rather old system with multiple functions — some generalized, and some involving both sensory and motor systems. Some sensory pathways have collaterals to the reticular formation, some do not. The explanation of the reticular formation is presented after the sensory pathways; the motor aspects are discussed with the motor systems. Clinical Aspects Destruction of the nuclei and pathways due to disease or injury leads to a neurological loss of function. How does the physician or neurologist diagnose what is wrong? He or she does so on the basis of a detailed knowledge of the pathways and their position within the central nervous system; this knowledge is a prerequisite for the part of the diagnosis that locates where the disease is occurring in the nervous system localization. The disease can sometimes be recognized by experienced physicians because of the pattern of the disease process; at other times, specialized investigations are needed to make the disease-specific diagnosis. There is an additional caveat — almost all of the pathways cross the midline, each at a unique and different ©2000 CRC Press LLC location; this is called a decussation. The important clinical correlate is that destruction of a pathway may affect the opposite side of the body, depending upon the location of the lesion. Learning Plan Studying pathways in the CNS necessitates visualizing them, a challenging task for many. The pathways studied here extend longitudinally through the CNS, going from spinal cord to thalamus and cortex. Various texts and atlases attempt to facilitate this visualization exercise for the student by presenting diagrams; color adds to the ability to visualize these pathways. The tracts are presented in two ways. On the left side of the page is a schematic of the CNS, with the spinal cord, brainstem, and forebrain including the internal capsule. (Note: T = thalamus, C = head of caudate nucleus, L = lentiform nucleus.) This diagram is used to convey the overall course of the tract, and particularly at what level the fibers cross in the CNS. This information will assist the student in correlating the anatomy of the pathway with the clinical symptomatology. On the right side, for each pathway, are cross sections through the brainstem and spinal cord. The course of the pathway is followed through the various levels on one side; the exact position of the tract within the brainstem or spinal cord is indicated (in black) in the cross sections on the other side. These brainstem sections are similar to those shown in Section C, and the level of each is identified. That section is titled “Neurological Neuroanatomy” because it allows precise localization of an injury or disease. At that stage, the student is presented with details of the histological anatomy of the spinal cord and brainstem. In this overview of the pathways, the student is advised to return to the description of the thalamus and the various specific relay nuclei (see Figures 9 and 10). Likewise, reference to the cortical illustrations (see Figures 11–16) will inform the student which areas of the cerebral cortex are involved in the various sensory modalities and will assist in integrating the anatomical information presented in the previous section. FIGURE 31 ANTEROLATERAL SYSTEM PAIN, TEMPERATURE, CRUDE TOUCH Figure 31 shows the pathway that carries the modalities of pain and temperature and a form of touch sensation called crude or light touch. Also conveyed in this fiber system are the sensations of itch and tickle, and other forms of sensation (e.g., sensations of a sexual nature). In the periphery the receptors are usually simply free nerve endings, without any specialization. The nerves are unmyelinated or thinly myelinated and conduct slowly. The nerve cell bodies for these peripheral fibers are located in the dorsal root ganglia (sometimes referred to as the first order neuron). These fibers enter the spinal cord and synapse in the dorsal horn (see Figure 2A). There are many collaterals within the spinal cord that are the basis of several protective reflexes. The number of synapses formed is variable and somewhat uncertain, but eventually a neuron is reached that will project its axon up the spinal cord (sometimes referred to as the second order neuron). This axon will cross — decussate — in the spinal cord, usually within two to three segments above the level of entry of the peripheral fibers. This crossing occurs in the white matter in front of the central canal and commissural neurons (see Figures 2A and 72) and is called the anterior (ventral) white commissure of the spinal cord. These axons then form the anterolateral tract, located in that portion of the white matter of the spinal cord. In fact, it was traditional to speak of two pathways, that for pain and temperature, the lateral spino-thalamic tract, and that for light (crude) touch, the anterior spinothalamic tract. These are now considered together under one name. The tract ascends in the same position through the spinal cord (see also Figure 72). As fibers are added from the upper regions of the body, they are positioned medially, pushing the fibers from the lower body more laterally. Thus, there is a topographic organization to this pathway in the spinal cord. In the brainstem collaterals are given off to the reticular formation which are thought to be quite significant for the function of the nervous system. ©2000 CRC Press LLC Further consideration of the connections and pathways for pain sensation have led to the notion of an older and a newer pain system. The older pathway (also called the paleospinothalamic) involves the reported sensation of an ache, or diffuse pain that is poorly localized. The fibers underlying this pain system are likely unmyelinated both peripherally and centrally, and the central connections are probably very diffuse; most likely these fibers terminate in the nonspecific thalamic nuclei (see Figure 10) and influence the cortex widely. The newer pathway, sometimes called the neospinothalamic system, involves thinly myelinated fibers in the PNS and CNS, likely ascends to the VPL nucleus of the thalamus, and from there is relayed to the postcentral (sensory) gyrus. Therefore, the sensory information in this pathway can be well localized. The common example for these different pathways is a paper cut: immediately one knows exactly where the cut has occurred; this is followed many seconds later by a diffuse poorly localized aching sensation. Clinical Aspects Lesions of the anterolateral pathway from the point of crossing in the spinal cord upward result in a loss of the modalities of pain, temperature, and crude touch on the opposite side of the body. The exact level of the lesion can be quite accurately ascertained because the sensation of pain can be quite simply tested at the bedside by using the end of a pin. (The tester should be aware that this is a very uncomfortable or unpleasant sensation for the patient being tested.) Neurological Neuroanatomy The cross-sectional levels for this pathway include spinal cord levels L3 (lumbar) and C8 (cervical), and brainstem levels B7 (mid-medulla), B4 (mid-pons), and B1 (upper midbrain). In the spinal cord, this pathway is found amongst the various pathways in the anterolateral region of the white matter (see Figure 72), hence its name. In the brainstem, the tract is small and cannot usually be seen as a distinct bundle of fibers. In the medulla, this pathway is situated dorsal to the inferior olivary nucleus. In the pons, it is again located laterally. In the uppermost pons and certainly in the midbrain, the fibers join the medial lemniscus (to be described with the next pathway in Figure 32). FIGURE 31: Anterolateral System — Pain, Temperature, Crude Touch ©2000 CRC Press LLC FIGURE 32 DORSAL COLUMN: MEDIAL LEMNISCUS PATHWAY DISCRIMINATIVE TOUCH, JOINT POSITION, VIBRATION The dorsal column–medial lemniscus pathway, shown in Figure 32, carries the following modalities from the body: discriminative touch sensation, joint position, and the somewhat artificial “sense” of vibration. Discriminative touch is the ability to discriminate the distance between two points touched simultaneously; it is usually tested by asking the patient to identify objects placed in the hand (with the eyes closed). Joint position is tested by moving a joint and asking the patient to report the direction of the movement (again with the eyes closed). Vibration is tested by placing a tuning fork which has been set into motion onto a bony prominence (e.g., the wrist, the ankle). In the periphery, the sensory receptors are quite specialized, and the fibers are thickly myelinated. The neurons in the dorsal root ganglia are also large in size. The axons enter the spinal cord but do not synapse immediately; instead they ascend, after giving off local collaterals which form the basis of various reflexes. Those fibers entering below the level of about T6 form the fasciculus (another word for tract) gracilis, the gracile tract; those entering above T6, particularly those from the upper limb, form the fasciculus cuneatus, the cuneate tract, which is situated more laterally. These tracts ascend the spinal cord in the dorsal area, between the two dorsal horns, forming the dorsal column (see Figures 2A and 72). Their first synapse occurs in the lowermost brainstem, in the nuclei that have the same names — the nuclei gracilis and cuneatus (see Figures 7 and 38). Topographical organization, called somatotopic, is maintained in this well-myelinated pathway. After neurophysiological processing in these nuclei, which includes sharpening the focus of the sensations, axons emanate and cross the midline. This stream of fibers can be recognized in suitably stained sections of the lower medulla; they are called the internal arcuate fibers (see Figures 38 and 71). After crossing in the lower medulla, the fibers then group to form the medial lemniscus, which ascends through the brainstem. ©2000 CRC Press LLC It should be noted that this pathway does not give off collaterals to the reticular formation in the brainstem. The medial lemniscus terminates in the ventral posterolateral nucleus of the thalamus, usually called the VPL (see Figures 10 and 34). After synapsing there, fibers enter the internal capsule, its posterior limb, and travel to the somatosensory cortex, terminating along the postcentral gyrus (see Figures 12, 16, and 34). The postcentral gyrus has a representation of the body called the sensory homunculus. The face and hand are represented on the dorsolateral surface (see Figure 12), with the lower limb on the medial aspect of the hemisphere (see Figure 16). The hand, particularly the thumb, has an extensive area of representation on the post-central gyrus. Clinical Aspects Lesions involving this tract will result in the loss of the sensory modalities carried in this pathway. A lesion of the dorsal column in the spinal cord will cause a loss on the same side; after the synapse and the crossing in the lower brainstem, any lesion of the medial lemniscus (or internal capsule) will result in the loss occurring on the opposite side of the body. With cortical lesions, the area of the body affected by a lesion will be determined by the area of the post-central gyrus involved. Neurological Neuroanatomy The cross-sectional levels for this pathway include spinal cord levels L3 and C8, and brainstem levels B8 (lower medulla), B4 (mid-pons), and B1 (upper midbrain). In the spinal cord, this dorsal column pathway is found in the dorsal region, between the two dorsal horns, as a well-myelinated bundle of fibers. The medial lemniscus tract is a heavily myelinated tract that is easily seen in myelin-stained sections of the brainstem (see sections of the brainstem, Figures 64–71). It is located initially between the inferior olivary nuclei and is oriented in the dorsal-ventral position (see Figures 70 and 71). In the pons, it changes to a mediallateral orientation (see Figure 68); in the upper part of the pons, the tract moves more laterally (see Figure 66). This shift of position continues in the midbrain.The fibers in the medial lemniscus are topographically organized, with the leg represented laterally and the upper limb medially. FIGURE 32: Dorsal Column: Medial Lemniscus Pathway — Discriminative Touch, Joint Position, Vibration ©2000 CRC Press LLC FIGURE 33 TRIGEMINAL SYSTEM terminate in the VPM nucleus of the thalamus and other thalamic nuclei, and follow the same course as those of the anterolateral system (discussed with Figure 34). DISCRIMINATIVE TOUCH, PAIN, TEMPERATURE Clinical Aspects The sensory fibers from the face include all the modalities. The fibers enter the brainstem at the level of the middle cerebellar peduncle (see Figures 3 and 4). Within the CNS there is a differential handling of the modalities, which allows us to describe the system using the analogy of the dorsal column-medial lemniscus and the anterolateral pathways. Those fibers carrying the sensations of discriminative touch synapse in the principal (main) nucleus of CN V, in the mid-pons, at the level of entry of the nerve (see Figure 6). The axons from this nucleus cross and join the medial lemniscus. These fibers terminate in the ventral posteromedial (VPM) nucleus of the thalamus (see Figures 10 and 34), and are relayed to the post-central gyrus via the posterior limb of the internal capsule. The face area is located on the dorsolateral surface and is very well represented on the post-central gyrus (see Figure 12). Some sensory input from the midline area of the face ascends on the same side (ipsilaterally), without crossing (this is not shown; see Figure 34). The fibers carrying the modalities of pain and temperature, including those from the teeth and gums, and the mucous membranes of the eyes, nose, and mouth, descend within the brainstem. They form a tract that starts at the mid-pontine level, descends through the medulla, and reaches the upper level of the spinal cord (see Figure 6). The tract is called the descending or spinal tract of V (also known as the spinal trigeminal tract). Immediately medial to this tract is a nucleus, with the same name. The fibers terminate in this nucleus and, after synapsing, cross to the other side and ascend (see Figure 38). Therefore, these fibers decussate over a wide region, from the lower pons to the spinal cord, and do not form a compact bundle of crossing fibers. This system will give off collaterals to the reticular formation (not shown), just like those of the anterolateral system. Just as the anterolateral pathway joins the medial lemniscus in the pons, so do the trigeminal fibers join the medial lemniscus (see Figures 34 and 38). The fibers ©2000 CRC Press LLC Lesions of the lateral medulla, such as an infarct or tumor, will disrupt the descending pain and temperature fibers and result in a loss of these sensations on the same side of the face. Such a lesion will leave the fibers for discriminative touch sensation (the medial lemniscus) intact. This vascular lesion can and does occur in the lateral medullary (Wallenberg) syndrome (discussed with the introduction to the medulla in Section C). A lesion of the medial lemniscus above the mid-pontine level will involve all trigeminal sensations from the opposite side. Internal capsule and cortical lesions will also involve trigeminal sensations of the opposite side (with some midline representation maintained), as well as other pathways. There is a particular affliction of the trigeminal nerve called trigeminal neuralgia, also known as tic douloureux. The patients report that they have “electriclike” sensations of intense pain in the distribution of one of the branches of the trigeminal nerve. The cause of this disorder is sometimes viral (like shingles, which is caused by the virus of chicken pox) but often unknown. The painful sensations can be brought on by any object touching the skin or even by air currents. As one can imagine, this is an extremely unpleasant and disabling condition. Treatment, involving drugs or surgery, is fraught with difficulties. Neurological Neuroanatomy The cross-sectional levels for this pathway include brainstem levels B8, B7, and B6 (medulla), B4 (mid-pons), and B2 (lower midbrain). The principal nucleus of CN V is seen at the midpontine level (see also Figure 6). The descending trigeminal tract is found in the lateral aspect of the medulla, with the nucleus situated immediately medially (see Figures 69–71). The crossing pain and temperature fibers join the medial lemniscus over a wide area and are thought to have completely crossed by the lower pontine region (see Figure 38). The collaterals of these fibers to the reticular formation are not shown. FIGURE 33: Trigeminal System — Discriminative Touch, Pain, Temperature ©2000 CRC Press LLC FIGURE 34 SENSORY SYSTEMS SOMATOSENSORY AND TRIGEMINAL PATHWAYS The diagram in Figure 34 presents all the somatosensory and trigeminal pathways through the midbrain, into the thalamus and to the cortex. The view is a dorsal perspective (as in Figure 7); the cross-sectional representations through the midbrain are also shown. The pathway that carries discriminative touch sensation and information about joint position (as well as vibration) from the body is the medial lemniscus (Figure 32). The equivalent pathway for the face comes from the principal nucleus of the trigeminal, which is located at the pontine level (see Figures 7 and 33). Most of the trigeminal system is crossed (TTc); there are also ascending trigeminal fibers that come from midline portions of the face that remain ipsilateral (TTi). By the level of the midbrain, all of these sensory pathways merge together, including also the anterolateral system (see Figure 31). This merging is shown in a series of cuts (in the lower portion of the diagram) through the midbrain region. At the level of the lower midbrain, these pathways are located near to the surface, dorsal to the substantia nigra; as they ascend they are found deeper within the midbrain, dorsolateral to the red nucleus (shown in cross section in Figures 64 and 65). The two pathways carrying the modalities of fine touch and position sense (and vibration) terminate in different specific relay nuclei of the thalamus (see Figure 10): • the medial lemniscus from the body, in the VPL, ventral posterolateral nucleus; • the trigeminal pathways from the face, in the VPM, ventral posteromedial nucleus. Sensory modality and topographic information is retained in these nuclei. There is physiologic processing of the sensory information and some type of sensory perception occurs at the thalamic level. Precise localization and two-point discrimination are cortical functions. ©2000 CRC Press LLC After the synaptic relay, the pathways continue as the superior thalamo-cortical radiation through the posterior limb of the internal capsule, between the thalamus and lenticular nucleus (see Figures 26 and 27). The fibers are then found within the white matter of the hemispheres. The somatosensory information is distributed to the cortex along the postcentral gyrus (see the small diagrams of the brain) areas 3, 1, and 2, also called SI. The information from the face and hand is topographically located on the dorsolateral aspect of the hemispheres (see Figure 12). The information from the lower limb is localized along the continuation of this gyrus on the medial aspect of the hemispheres (see Figure 16). This cortical representation is called the sensory homunculus, a distorted representation of the body and face with the trunk and lower limbs having very little area, whereas the face and fingers receive considerable representation. Further elaboration of the sensory information occurs in the parietal association areas adjacent to the postcentral gyrus (known as areas 5 and 7). This allows us to learn to recognize objects by tactile sensations (e.g., food in the mouth, coins in the hand). The pathways carrying pain and temperature from the body (the anterolateral system) and the face (descending trigeminal) terminate in part in the specific relay nuclei, ventral posterolateral and posteromedial (VPL and VPM), respectively, but mainly in the intralaminar nuclei. These latter terminations may well be involved with the emotional correlates that accompany many sensory experiences (e.g., pleasant or unpleasant). Lesions of the thalamus may sometimes give rise to pain syndromes. Axons projecting from the specific relay nucleus of the thalamus enter the posterior limb of the internal capsule and are conveyed to several cortical areas, including the post-central gyrus and area SII (a secondary sensory area) which is located in the lower portion of the parietal lobe, as well as other cortical regions. The output from the intralaminar nuclei of the thalamus goes to widespread cortical areas. Thalamo-cortical radiation Caudate nucleus (head) Ventral posterolateral n. Ventral posteromedial n. Putamen Trigeminal lemniscus (TL) Red nucleus (RN) Globus pallidus RN SN Trigeminal-thalamic tract — ipsilateral (TTi) Trigeminal-thalamic tract — crossed (TTc) Substantia nigra (SN) ML TL Medial lemniscus (ML) ML Anterolateral system TTi TTc FIGURE 34: Sensory Systems— Somatosensory and Trigeminal Pathways ©2000 CRC Press LLC FIGURE 35 AUDITION: HEARING AUDITORY PATHWAY I The auditory pathway is more complex than the other sensory pathways. Firstly, the pathway is bilateral; secondly, there are more synaptic stations (nuclei) along the way, with numerous connections across the midline. It also has a unique feature — a feedback pathway from the CNS to cells in the cochlea. The specialized hair cells in the cochlea respond maximally to certain frequencies (pitch) in a tonotopic manner. The peripheral ganglion for these sensory fibers is the spiral ganglion. The afferent fibers from the ganglion project to the first brainstem nuclei at the level of entry of the eighth nerve at the ponto-medullary junction — the dorsal and ventral cochlear nuclei. After this, the pathway can follow a number of different routes. In an attempt to make some semblance of order, these routes are discussed in sequence, even though an axon may or may not synapse in each of these nuclei. At the level of the lower pons is the superior olivary complex, which consists of a number of nuclei (see Figure 68). Most of the fibers leaving the cochlear nuclei will synapse in the superior olivary complex, either on the same side or on the opposite side. Crossing fibers are found in a structure known as the trapezoid body (see Figures 38 and 68). The main function of the superior olive is sound localization; sound coming from one side does not reach each ear at the same moment. This differential is processed by the dendrites of the neurons in the superior olive. Fibers from the superior olivary complex either ascend on the same side or cross (in the trapezoid body) and ascend on the other side. They form a tract, the lateral lemniscus, which begins just above the level of these nuclei (see Figure 38). The lateral lemniscus carries the auditory information upwards to the inferior colliculus of the midbrain. There are nuclei scattered along the way, interspersed with the lateral lemniscus, and some fibers terminate or relay in these nuclei. The lateral lemnisci are interconnected across the midline throughout the brainstem. ©2000 CRC Press LLC Almost all the axons of the lateral lemniscus terminate in the inferior colliculus. Next, the fibers ascend to the medial geniculate nucleus of the thalamus (a specific relay nucleus; see Figure 10) and project to the auditory cortex (shown in the following illustrations). In summary, audition involves a complex pathway, has bilateral representation, with numerous opportunities for synapses along its course. The name “lemniscus” is rather unfortunate because it does not transmit information in the efficient manner of the medial lemniscus. It is important to note that although the pathway is predominantly a crossed system, there is also a significant ipsilateral component. There are also numerous interconnections between the two sides. Therefore, a lesion of the auditory pathway on one side will not lead to a total loss of hearing on the opposite side. The auditory pathway has a feedback system, from the higher levels to lower levels (e.g., from the inferior colliculus to the superior olivary complex). The final link in this feedback is unique in the mammalian CNS, for it influences the cells in the receptor organ itself. This pathway, known as the olivo-cochlear bundle, has its cells of origin in the vicinity of the superior olivary complex. It has both a crossed and an uncrossed component. Its axons reach the hair cells of the cochlea by traveling in the eighth nerve. This system changes the responsiveness of the peripheral hair cells. Neurological Neuroanatomy The auditory system is shown at various levels of the brainstem, including B6 (upper medulla), B5, B4, and B3 (all pontine), and B2 (lower midbrain, inferior collicular level). The cochlear nuclei are the first CNS synaptic relay for the auditory fibers from the peripheral spiral ganglion of the internal ear; these nuclei are found along the incoming eighth nerve at the level of the upper medulla (B6 level; see Figure 69). The superior olivary complex, consisting of several nuclei, is located in the lower pontine level (B5; see Figure 68). The trapezoid body, containing the crossing auditory fibers, is also found at this level. By mid-pons (level B4; see Figure 67), the lateral lemniscus can be recognized. These fibers move towards the outer margin of the upper pons (level B3) and terminate in the inferior colliculus (level B2; see Figure 65). FIGURE 35: Audition: Hearing — Auditory Pathway I ©2000 CRC Press LLC FIGURE 36 AUDITORY SYSTEM AUDITORY PATHWAY II The illustration in Figure 36 shows the projection of the auditory system fibers from the level of the inferior colliculus (lower midbrain) to the thalamus and then to the cortex. Auditory information is carried via the lateral lemniscus to the inferior collicular level (see Figures 35 and 38) after several synaptic relays. There is another synapse in this nucleus, making the auditory pathway overall different and more complex than the medial lemniscal and visual pathways. The inferior colliculi are connected to each other by a small commissure (not labeled in the illustration). The auditory information is next projected to a specific relay nucleus of the thalamus, the medial geniculate (MG) body (nucleus; see Figure 10). The tract that connects the two, the brachium of the inferior colliculus, can be seen on the dorsal aspect of the midbrain (see Figure 7; Figure 8 not labeled) and this is shown diagrammatically in Figure 36. From the medial geniculate nucleus the auditory pathway continues to the cortex. This projection, which courses beneath the lenticular (lentiform) nucleus of the basal ganglia (see Figure 22), is called the sublenticular pathway, the inferior limb of the internal capsule, or simply the auditory radiation. The cortical areas involved with receiving this information are the transverse gyri of Heschl, situated on the superior temporal gyrus, within the lateral fissure. The location of these gyri is shown in the inset as the primary auditory areas (a photographic view is shown in Figure 37). ©2000 CRC Press LLC The medial geniculate nucleus is likely involved with some analysis and integration of the auditory information. More exact analysis occurs in the cortex. Further elaboration of auditory information is carried out in the adjacent cortical areas. On the dominant side for language, these cortical areas overlap Wernicke’s area (see Figure 12). Sound frequency, known as tonotopic organization, is maintained all along the auditory pathway, starting in the cochlea. This can be depicted as a musical scale with high and low notes. The auditory system localizes the direction of a sound in the superior olivary complex (discussed with Figure 35) by analyzing the difference in the timing that information reaches each ear and by the difference in sound intensity reaching each ear. The loudness of a sound would be represented physiologically by the number of receptors stimulated and by the frequency of impulses, as in other sensory modalities. Neurological Neuroanatomy This view of the brain includes the body of the lateral ventricle (cut) and adjoining structures. The thalamus is seen to form the floor of the ventricle; the body of the caudate nucleus lies above the thalamus and on the lateral aspect of the ventricle. This diagram also includes the lateral geniculate body (nucleus) which subserves the visual system and its projection, the optic radiation (to be discussed subsequently). The temporal lobe structures are also shown, including the inferior horn of the lateral ventricle, the hippocampus proper, and adjoining structures (relevant to the limbic system, which is discussed in Section D). Caudate nucleus (body) Lateral ventricle (body) Putamen (lenticular n.) Superior temporal gyrus Lateral fissure Thalamus Primary auditory areas Medial geniculate n. Association auditory areas Lateral geniculate n. Brachium of inferior colliculus Stria terminalis Inferior colliculus Lateral lemniscus Midbrain Caudate nucleus (tail) Optic radiation Lateral ventricle (inferior horn) Auditory radiation Hippocampus proper FIGURE 36: Auditory System — Auditory Pathway II ©2000 CRC Press LLC FIGURE 37 AUDITORY SYSTEM AUDITORY GYRI (PHOTOGRAPHIC VIEW) In Figure 37 the photographic view of the right hemisphere is shown from the lateral perspective. The lateral fissure has been opened slightly, exposing two gyri oriented transversely. These gyri are the areas of the cortex that receive the incoming auditory sensory information first. They are named the transverse gyri of Heschl (see also Figure 36). The lateral fissure (see Figure 12) completely separates this part of the temporal lobe and the frontal and parietal lobes above. The auditory gyri occupy the superior aspect of the temporal lobe, namely, the superior temporal gyrus. Further opening of the lateral fissure reveals some cortical tissue which is normally completely hidden from view. This area is the insula, and a small part of it is seen in this photograph in the depth of the lateral fissure. It is important not to confuse the auditory gyri and insula. ©2000 CRC Press LLC The position of the insula in the depth of the lateral fissure is also shown in the coronal slice of the brain (see Figure 29). The function of this area of cortex is still not clear. Cortical representation of sensory systems reflects the particular sensation (modality). The auditory gyri are organized according to pitch, giving rise to the term tonotopic localization. This representation is similar to that of the somatosensory system on the postcentral gyrus (somatotopic localization; the sensory homunculus — see Figure 34). The precentral strip has the equivalent, a motor homunculus, again with large areas devoted to the hand and face, reflecting the fine control possible with these muscles. It should be noted that the lateral fissure usually has within it a large number of blood vessels, branches of the middle cerebral artery. These branches emerge and then distribute to the cortical tissue seen on the dorsolateral surface, including the frontal, temporal, parietal, and occipital cortex (discussed with Figure 58). Small branches are distributed to the internal capsule and basal ganglia within the lateral fissure (discussed with Figure 60). Insula Auditory gyri (transverse gyri of Heschl) FIGURE 37: Auditory System — Auditory Gyri (Photographic View) ©2000 CRC Press LLC FIGURE 38 SENSORY SYSTEMS olivary complex. From this point, the tract known as the lateral lemniscus is formed. It terminates in the inferior colliculus. ASCENDING TRACTS AND SENSORY NUCLEI The other sensory cranial nerves in this illustration are CN VII, the facial nerve (sensory afferents and taste) and CN IX and X, the glossopharyngeal and vagus nerves (sensory and visceral afferents, and taste). Figure 38 is a diagrammatic presentation of the internal structures of the brainstem shown from the dorsal perspective (as in Figures 7 and 8). The information concerning the major sensory systems is presented in an abbreviated manner, as most of this has been reviewed with previous figures. The orientation of the cervical spinal cord representation should be noted. Dorsal column-medial lemniscus — The dorsal columns (cuneate and gracile tracts) of the spinal cord terminate (synapse) in the nuclei gracilis and cuneatus in the lowermost medulla. Axons from these nuclei then cross the midline (decussate) as the internal arcuate fibers (not labeled; see Figure 71), forming a new bundle called the medial lemniscus. These fibers ascend through the medulla, change orientation in the pons, and move laterally, occupying a lateral position in the midbrain. Anterolateral system — Having already crossed, this tract ascends from the spinal cord through the brainstem. In the medulla it is posterior to the inferior olive. At the upper pontine level, it becomes associated with the medial lemniscus, and the two lie adjacent to each other in the midbrain region. Some of its fibers enter the superior colliculus (not labeled). Trigeminal system — The sensory afferents for discriminative touch synapse in the principal nucleus of V; the fibers then cross at the level of the mid-pons and form the trigemino-thalamic tract, which joins the medial lemniscus. The pain and temperature fibers descend and form the descending trigeminal tract through the medulla with the nucleus medial to it. These fibers synapse and cross, over a wide area, eventually joining the trigemino-thalamic tract and medial lemniscus in the uppermost pons. Lateral lemniscus — The auditory fibers (CN VIII) enter the brainstem at the uppermost portion of the medulla. After the initial synapse in the cochlear nuclei, many of the fibers cross the midline, forming the trapezoid body. Some of the fibers synapse in the superior ©2000 CRC Press LLC Facial nerve — Sensory fibers entering in the facial nerve (CN VII) include some afferents from the ear lobe and taste fibers from the anterior two thirds of the tongue. The sensory afferents join those of the descending tract and nucleus of V. The taste fibers synapse in the solitary nucleus (not shown) which lies adjacent to the descending tract and nucleus of V (see Figure 6; also seen in cross section in Figure 70) in the medulla. Glossopharyngeal and vagus nerves — These nerves convey mainly visceral afferents, as well as taste fibers from the posterior one third of the tongue (IX) and also the epiglottis region (X). These fibers synapse in the solitary nucleus. The few sensory fibers from the ear and meninges join with the descending tract and nucleus of V. Clinical Aspects The diagram in Figure 38 allows the visualization of all the pathways together which assists in understanding lesions of the brainstem. The cranial nerve nuclei affected help locate the level of the lesion. One of the classic lesions of the brainstem is an infarct of the lateral medulla (see Figure 33), known as the Wallenberg syndrome. (The blood supply of the brainstem is reviewed with Figure 56.) This lesion affects the lateral pathways including the anterolateral tract and the lateral lemniscus, but not the medial lemniscus. The descending trigeminal system is also involved, as are the nuclei of CN IX and X. Neurological Neuroanatomy The red nucleus is one of the prominent structures of the midbrain. The superior cerebellar peduncles are shown, located within the superior medullary velum, the roof of the fourth ventricle (see Figure 7). This cerebellar efferent pathway decussates in the lower midbrain (shown in cross section, Figure 65). Red nucleus Decussation of superior cerebellar peduncles Inferior colliculus Superior cerebellar peduncle Anterolateral system Trigemino-thalamic pathway Lateral lemniscus CN V Principal nucleus of V Medial lemniscus Trapezoid body CN VII Superior olivary complex CN VIII Cochlear nuclei Cuneatus and gracilis nuclei CN IX CN X Descending (spinal) nucleus of V Anterolateral system Dorsal column tracts Cervical spinal cord FIGURE 38: Sensory Systems — Ascending Tracts and Sensory Nuclei ©2000 CRC Press LLC FIGURE 39A VISUAL SYSTEM: A VISUAL PATHWAY The visual image exists in the visual fields; because of the lens of the eye, the visual information from the upper visual field is seen in the lower retina (likewise the lower visual field is seen in the upper retina). The visual fields are also divided into temporal (lateral) and nasal (medial) portions. Again, because of the lens of the eye, the temporal visual field is projected onto the nasal part of the retina of the ipsilateral eye, and onto the temporal part of the retina of the contralateral eye. The primary purpose of the visual apparatus — muscles, cornea, lens — is to have the visual image fall on corresponding points on the retina of both eyes. The central portion of the visual field is seen by the macular area of the retina, composed of cones; it is the area for discriminative (e.g., reading) and color vision. Visual processing begins in the retina with the photoreceptors (rods and cones). The bipolar neurons, the next neurons in the chain, are functionally equivalent to cells in the dorsal root ganglion in the somatosensory system. The axons of the next neuron in any sensory system (the second order neuron) cross the midline and project to the thalamus (compare to the nuclei gracilis and cuneatus, Figure 32). In the visual system, these neurons are the ganglion cells of the retina whose axons are carried in the optic nerve (CN II). After exiting from the orbit, these nerves undergo only a partial crossing (decussation) in the optic chiasm, and the axons continue as the optic tract. Some fibers go to the superior colliculus and other nuclei of the midbrain (discussed with Figure 39B), but most terminate in the lateral geniculate nucleus (LG). In fact, this entire pathway, from ganglion cells to geniculate, is a pathway of the CNS, with the oligodendrocyte being the myelin-forming cell (as for the CNS). As indicated, there is a partial crossing of fibers in the optic chiasm. The fibers from the nasal retina, representing the temporal visual fields, cross in the optic chiasm. Therefore, the information from the visual field of one side — the temporal visual field of one eye and the nasal visual field of the other — is brought together in the ©2000 CRC Press LLC optic tract on the opposite side. (The best way of learning this is to sketch the visual pathway.) The fibers that terminate in the lateral geniculate nucleus, a specific relay nucleus of the thalamus (see Figures 9 and 10) synapse in specified layers, and after processing they project to the primary visual cortex, area 17. The projection is unusual (shown also in the next illustration), with some of the fibers sweeping forward alongside the inferior horn of the lateral ventricle in the temporal lobe, called Meyer’s loop, while others project directly posteriorly (see also Figure 36). The geniculo-calcarine (optic) radiation is arranged in the following manner: • The fibers representing the lower retinal field sweep forward into the temporal lobe, as Meyer’s loop. Destruction of only these fibers results in a loss of vision in the upper visual field of both eyes on the side opposite the lesion, specifically the upper quadrant of both eyes. • Those fibers representing the upper retinal field project posteriorly without this unusual looping, passing deep within the parietal lobe. Destruction of only these fibers results in the loss of the lower visual field of both eyes on the side opposite the lesion, specifically the lower quadrant of both eyes. The visual information goes to area 17 (shown in black in the insets), the primary visual area, also called the calcarine cortex. The visual area located at the occipital pole (looking at the external aspect of the brain; see Figure 12) is where macular vision is represented; the visual cortex on the medial surface along the banks of the calcarine fissure (see Figure 16) represents the peripheral areas of the retina. The adjacent cortical areas of the occipital lobe are association areas for vision, usually called areas 18 and 19. Clinical Aspects The visual pathway is easily testable, even at the bedside. Students should be able to draw the visual field defect in both eyes that would follow a lesion (e.g., homonymous hemianopsia). Visual loss can occur for many reasons, including a lesion in the retina, the temporal lobe, or the loss of blood supply to the cortical areas. Primary visual area (area 17) Lateral ventricle (cut) Stria terminalis Caudate nucleus (tail) Association visual areas (18, 19) Lateral geniculate n. Optic radiation Optic tract Optic radiation (temporal loop) Optic chiasm Lateral ventricle (inferior horn) FIGURE 39A:Visual System: A — Visual Pathway ©2000 CRC Press LLC Primary visual area (area 17) FIGURE 39B VISUAL SYSTEM: B VISUAL REFLEXES The vast majority of fibers of the optic tract project to the lateral geniculate nucleus, the LG. This nucleus has a six-layered structure, and each of the eyes projects to three of the layers. After synapsing, these fibers relay to the primary visual area (area 17) The illustration in Figure 39B shows some fibers from the optic tract which project to the superior colliculus (bypassing the lateral geniculate) via the brachium of the superior colliculus. This nucleus serves as an important center for visual reflex behavior, particularly that involving eye movements. Fibers leave this nucleus and connect with the nuclei of the extra-ocular and neck muscles via the medial longitudinal fasciculus (the MLF, discussed with Figures 49A and 49B). There is also a projection to the spinal cord via a small pathway (the tecto-spinal tract), which is found incorporated with the MLF throughout the brainstem and the upper spinal cord. Other fibers are illustrated emerging from the pulvinar, the visually related association nucleus of the thalamus (see Figure 10). These are carried in the optic radiations and go to areas 18 and 19, the visual association areas of the cortex (shown in Figure 39A, alongside area 17). Some other fibers terminate in the suprachiasmatic nucleus of the hypothalamus (located above the optic chiasm), which is involved in the control of diurnal (day-night) rhythms. A small but extremely important group of fibers from the optic tract (not shown) project to the pretectal area situated in the upper portion of the midbrain. The reflex adjustment of the diameter of the pupil — the pupillary light reflex — is coordinated in the pretectal nucleus. Reflex adjustments of the visual system are also required for seeing objects nearby (such as for reading), known as the accommodation reflex. Pupillary light reflex — Some of the visual information (from certain ganglion cells in the retina) is carried in the optic nerve and tract to the midbrain. A nucleus located in the area in front of the colliculi, called the pretectal area (the other name for the colliculi is the ©2000 CRC Press LLC tectal area; see Figures 7 and 8) is the site of synapse for the pupillary light reflex. Shining light on the retina causes a constriction of the pupil on the same side; this is the direct pupillary light reflex. Fibers also cross to the nucleus on the other side and the pupil there reacts as well; this is the consensual light reflex. The efferent part of the reflex involves the parasympathetic nucleus (Edinger-Westphal) of the oculomotor nucleus (discussed with the midbrain and also Figure 64). The efferent fibers course in CN III, synapsing in the ciliary ganglion in the orbit before innervating the smooth muscle of the iris. Clinical Aspects The student is encouraged to draw this pathway and to work out the clinical picture of a lesion involving the afferent fibers and the efferent fibers. In addition, diseases such as multiple sclerosis can diminish the number of fibers in the optic nerve and can lead to a condition called a relative afferent pupillary defect; a specific test for this condition is the swinging light test. In this condition, because of the diminished afferent input to the pretectal nucleus, the pupil on the affected (lesion) side reacts less to the light stimulus than does the normal eye, and it therefore dilates paradoxically. CN III is oftentimes involved in brain herniation syndromes, particularly uncal herniation (discussed with Figure 14). This results in a fixed dilated pupil on one side, a critical sign when one is concerned about increased intracranial pressure from any cause. The significance and urgency of this situation must be understood by everyone involved in critical care. Accommodation reflex — The second reflex associated with incoming visual fibers is the accommodation reflex, involving looking at a nearby object, such as in reading. Three events occur simultaneously: convergence of both eyes (involving both medial recti muscles), a change (rounding) of the curvature of the lens, and pupillary constriction. This reflex requires the visual information to be processed at the cortical level. The descending (cortico-bulbar) fibers go to the oculomotor nucleus and influence both the motor portion (the medial recti muscles), and the parasympathetic portion (via the ciliary ganglion) to the smooth muscle of the lens and the pupil. Optic radiation Calcarine fissure Lateral ventricle (cut) Lateral ventricle (occipital horn) Pulvinar Superior colliculus Brachium of superior colliculus Lateral geniculate nucleus Medial geniculate nucleus Primary visual area (area 17) Lateral geniculate nucleus Optic tract Red nucleus Substantia nigra FIGURE 39B: Visual System: B — Visual Reflexes ©2000 CRC Press LLC PART II: RETICULAR FORMATION FIGURE 40A RETICULAR FORMATION I ORGANIZATION The reticular formation is the name for a group of neurons found throughout the brainstem. Using the ventral view of the brainstem, the reticular formation occupies the central portion or core area of the brainstem from midbrain to medulla (shown on the right portion of this illustration; see also cross sections in Figures 64–71). This collection of neurons is a phylogenetically old set of neurons that functions like a network or reticulum, from which it derives its name These nuclei receive afferents from most of the sensory systems and project to virtually all parts of the nervous system. Functionally, it is possible to localize different subgroups within the reticular formation: • Cardiac and respiratory “centers”: Subsets of neurons within the medullary reticular formation are responsible for the control of the vital functions of heart rate and respiration. • Motor areas: Both the pontine and medullary nuclei of the reticular formation contribute to motor control via the cortico-reticulo-spinal system (discussed with Figures 46 and 47). In addition, these nuclei exert a very significant influence on muscle tone. • Ascending projection system: Fibers from the reticular formation ascend to the thalamus and project to various nonspecific thalamic nuclei. From these nuclei, fibers are distributed diffusely to the cerebral cortex. This whole system is concerned with consciousness and has been called the ascending reticular activating system (ARAS). • Pre-cerebellar nuclei: Numerous nuclei in the brainstem located within the boundaries of the reticular formation project to the cerebellum. These nuclei are not always included in discussions of the reticular formation. ©2000 CRC Press LLC It is also possible to describe the reticular formation topographically. The neurons appear to be arranged in three longitudinal sets (these are shown on the left hand side of this illustration): • The lateral group consists of neurons that are small in size. These are the neurons that receive the various inputs to the reticular formation, including those from the anterolateral (pain and temperature) and trigeminal systems, as well as auditory and visual input. • The next group of neurons (medially) is called the central group. These cells are larger in size and project their axons upwards and downwards. The ascending projection from the midbrain area is particularly involved with the consciousness system. Within this group are the well-known nucleus gigantocellularis of the medulla and the pontine reticular nuclei, caudal (lower) and oral (upper) portions, which give origin to the two reticulo-spinal tracts (discussed with Figure 40B, see also Figures 46 and 47). • A set of neurons, called the raphe nuclei, occupies the midline region of the brainstem. The best-known nucleus of this group is the nucleus raphe magnus, which plays an important role in the descending pain system (discussed with Figure 41). In addition, both the locus ceruleus and the periaqueductal gray are considered part of the reticular formation (discussed with Figure 40B). In summary, the reticular formation is connected with almost all parts of the CNS. Although it has a generalized influence within the CNS, it also contains subsystems that are directly involved in specific CNS functions. Ascending projection fibers Locus ceruleus Lateral group Central group Raphe nuclei Reticulo-spinal tracts FIGURE 40A: Reticular Formation I — Organization ©2000 CRC Press LLC FIGURE 40B RETICULAR FORMATION II NUCLEI In this diagram, the reticular formation is viewed from the dorsal (posterior) perspective. Various nuclei of the reticular formation — RF — which have a known significant functional role are depicted, as well as the descending tracts emanating from some of these nuclei. There are afferent and efferent nuclei in the reticular formation and groups of neurons that are distinct because of the catecholamine neurotransmitter used, either noradrenaline or serotonin. This understanding of the reticular formation overlaps with the topographical description, as being arranged in three longitudinal sets of neurons, as discussed with Figure 40A. • The neurons that receive the various inputs to the RF are found in the lateral group. In Figure 40B, these neurons are shown receiving collaterals (or terminal branches) from the ascending anterolateral system, carrying pain and temperature (see Figure 31). Similar information is received from the descending fibers of the trigeminal nerve (see Figure 33). The RF also receives visual and auditory information. The medial lemniscus, though, does not give off collaterals to the RF. • The central group of neurons are larger and are the output neurons of the reticular formation, at various levels. These cells project their axons upwards and/or downwards. The nucleus gigantocellularis of the medulla, and the pontine reticular nuclei, caudal and oral portions, give rise to the descending tracts that emanate from these nuclei — the medial and lateral reticulo-spinal pathways, part of the indirect voluntary motor system (discussed in the introduction to the motor system and with Figures 46 and 47). • A set of neurons, called the raphe nuclei, occupies the midline region of the brainstem. All the neurons of this group use the neurotransmitter serotonin. The serotonergic raphe nuclei project to all parts of the CNS. Recent studies seem to indicate that serotonin plays a significant role in emotional equilibrium, as well as in the regulation of sleep. One nucleus of this group, the nucleus raphe magnus, located in the upper part of the medulla, plays a special role in the ©2000 CRC Press LLC descending pain pathway (described with Figure 41). Other nuclei in the brainstem seem to functionally belong to the reticular formation yet are not located within the core region. These include the periaqueductal gray and the locus ceruleus. The periaqueductal gray of the midbrain (for its location see Figures 64 and 65) includes neurons that are found around the aqueduct of the midbrain (see also Figure 20B). This area also receives input (illustrated but not labeled) from the ascending sensory systems conveying pain and temperature, the anterolateral and trigeminal. As shown in Figure 41, some of the neurons of the periaqueductal gray as well as the nucleus raphe magnus of the medulla are part of a descending pathway to the spinal cord that is concerned with pain control. The locus ceruleus is a small nucleus in the upper pons region (see Figure 66). In some species (including humans), the neurons of this nucleus accumulate a pigment that can be seen when the brain is sectioned (prior to histological processing). Output from this small nucleus is distributed widely throughout the brain to virtually every part of the CNS, including all cortical areas, subcortical structures, the brainstem and cerebellum, and the spinal cord. The neurotransmitter involved is noradrenaline. Although the functional and electrophysiogical role of this nucleus is still not clear, the locus ceruleus has been thought to act like an “alarm system” in the brain; it has also been implicated in a wide variety of CNS activities, such as mood, reaction to stress, and various autonomic activities. The cerebral cortex sends fibers to the RF nuclei, forming part of the so-called cortico-bulbar fibers (see Figure 43). Those nuclei that give off the pathways to the spinal cord form part of an indirect motor system — the cortico-reticulo-spinal pathways. These pathways are known to have an important role in the voluntary control of the muscles of the spine (axial musculature) and those of the large joints (proximal joints of the shoulder and hip). In addition, this system is known to play an extremely important role in the control of muscle tone. Lesions of the cortical input to the reticular formation have a very significant impact on muscle tone and reflexes (discussed with Figure 47). Aqueduct of midbrain RF - central group Periaqueductal gray RF - lateral group Fourth ventricle Pontine reticular nn. (oral and caudal) Locus ceruleus N. gigantocellularis N. raphe magnus RF - raphe nn. RF - lateral group Reticulo-spinal tracts Anterolateral system Cervical spinal cord FIGURE 40B: Reticular Formation II — Nuclei ©2000 CRC Press LLC FIGURE 41 PAIN DESCENDING CONTROL SYSTEM Pain is a unique sensory modality — it both warns of tissue injury and can distract the organism from performing essential functions. Pain may be perceived at several points, and there is good evidence that some of this occurs at the thalamic level. Localization of pain requires the cortex of the post-central gyrus (SI); SII is also likely involved in the perception of pain (discussed with Figure 31). Widespread areas of the limbic system and association cortex of the frontal lobe are involved with the human reactions to pain, particularly to chronic pain (e.g., such as from cancer or arthritis). Humans have a built-in system for dampening the influences of pain — the descending pain modulation pathway(s). Some of this modulation occurs at the brainstem level and some in the spinal cord. Substantial evidence suggests that the transmission of pain from the periphery can be influenced by nuclei located in the brainstem. Those areas that have been found to exert the major influence include the periaqueductal gray of the midbrain and one of the midline serotonergic nuclei of the medulla, the nucleus raphe magnus. The system apparently functions as follows. The neurons of the periaqueductal gray can be activated in a number of ways. In terms of physiology, many ascending fibers from the anterolateral system (and trigeminal system) activate neurons in this area (see Figure 38). These are either collaterals of ascending pain fibers or direct endings of these fibers in the midbrain. This area is also rich in opiate receptors, and it seems that neurons of this region can be activated by circulating endorphins. In experiments, one can activate these neurons by direct stimulation or by a local injection of morphine. In addition, descending cortical fibers (cortico-bulbar) may also be available to activate these neurons. The axons of some of the neurons of the periaqueductal gray descend and terminate in one of the raphe nuclei in the medulla — the nucleus raphe magnus. This nucleus is one of the serotonin-containing raphe system of nuclei. From here, there is a descending, crossed, ©2000 CRC Press LLC pathway which is located in the dorsolateral funiculus of the spinal cord. The serotonergic fibers terminate in the substantia gelatinosa of the spinal cord (see Figure 2A), a nuclear area of the dorsal horn of the spinal cord. The axons are thought to terminate on small interneurons that contain enkephalin. It is postulated that these enkephalin-containing spinal neurons inhibit the transmission of the pain afferents in the spinal cord. Thus, descending influences are thought to activate a local circuit. This idea is based upon the proposed mechanism that these same interneurons can be activated by stimulation of other sensory afferents, particularly those conveying information from the mechanoreceptors; these are anatomically large, well-myelinated peripheral nerve fibers. This is the physiological basis for the gate control theory of pain. It should be pointed out that the same circuitry is thought to be operative in the descending nucleus of the trigeminal nerve, the lower portion of which is responsible for pain (and also temperature) transmission. This nucleus is in fact continuous with the substantia gelatinosa. Clinical Aspects Some of the current treatments for pain are based upon the structures and neurotransmitters being discussed here. The gate control theory of pain underlies the use of transcutaneous stimulation which is one of the current therapies offered for the relief of pain. More controversial and certainly less certain is the postulated mechanism(s) for the use of acupuncture in the treatment of pain. The role of the limbic (emotional) system is discussed in Section D. Most discussions concerning pain refer to acute pain. Chronic pain, a particularly tragic state of being for many people, should be thought of very differently. The pain of chronic arthritis or cancer is extremely difficult to treat. Many of these people are now referred to pain clinics, where a team of physicians (such as anesthetists and neurologists) and others (such as social workers and psychologists) try to assist people, using a variety of therapies, to alleviate their disabling condition. Periaqueductal gray N. raphe magnus FIGURE 41: Pain — Descending Control System ©2000 CRC Press LLC PART III: MOTOR SYSTEMS INTRODUCTION The motor system is a complex subject to understand because of the multiple centers involved in motor control. All of our voluntary motor output is controlled by the motor regions of the cortex. Other motor activity may be controlled from several brainstem nuclei, some of which are under the control of the motor cortices. In addition, there are various spinal motor activities and reflexes. Finally, there is the regulation of muscle tone and reflexes, some of which depends upon the afferents from the muscle spindle and some of which is regulated by descending influences from higher centers. In addition, there are two important large areas of the brain, the cerebellum and basal ganglia, devoted to motor regulation, “working behind the scene.” Voluntary motor control involves both direct and indirect pathways: • the direct voluntary pathway includes the corticospinal fibers which are found within the pyramids and the lateral cortico-spinal tract of the spinal cord, mainly for the control of fine motor movements; and • the indirect voluntary pathway, an older system for control of proximal and axial musculature, involving the reticular formation of the brainstem. The pathway goes from the motor areas of the cerebral cortex to the reticular formation (via cortico-bulbar fibers), and then from the reticular formation to the spinal cord (via the reticulo-spinal pathways). Other pathways from the brainstem (from the reticular formation, vestibular nuclei, possibly the red nucleus) also control proximal joint movements and axial musculature, as well as muscle tone and reflex responsiveness. Each of these gives rise to a descending pathway. Some but not all of these nuclei are under the influence of the cortex. A typical human lesion of the brain usually affects several of the descending systems in addition to the cortico-spinal tract, and results in a more profound weakness or paralysis of movement. Most important, because of the involvement of other parts of the motor control system, there is in most cases an eventual increase in muscle tone (spasticity) and reflexes (discussed with Figures 46 and 47). The control of the ©2000 CRC Press LLC muscles of the head and neck (eye, tongue, facial, mastication, swallowing, phonation) is discussed separately. The basal ganglia and cerebellum, which might be called motor regulatory systems, calibrate the output from the motor cortex. They are discussed after the pathways are described. The motor pathways (tracts) are called descending because they commence in the cortex or brainstem and influence motor cells lower in the neuraxis, either in the brainstem or spinal cord. Those neurons in the cortex or brainstem (including the reticular formation) giving rise to these pathways are collectively called the upper motor neurons. The motor neurons in the spinal cord or brainstem that give rise to the peripheral efferent fibers (spinal and cranial nerves) are often collectively called the lower motor neuron. In the spinal cord, these neurons are located in the ventral or anterior horn and are (histologically) the anterior horn cells. Physiologists call these neurons the alpha motor neurons. In the brainstem, these neurons include the motor neurons of the cranial nerves (see Figure 5). Since all of the descending influences converge upon the lower motor neurons, these neurons have also been called, in a functional sense, the final common pathway. There are a number of descending tracts or pathways: • Cortico-spinal tract originates in motor areas of the cerebral cortex and travels from cortex to spinal cord. It is a relatively new tract and one of the most important for voluntary motor control in humans, particularly for fine movements of the hand and digits — the direct voluntary motor pathway. • Cortico-bulbar fibers is a descriptive term that is poorly defined and includes all fibers going to the brainstem, both cranial nerve nuclei and other brainstem nuclei. The fibers going to the reticular formation include those that form part of the indirect voluntary motor pathway. • Rubro-spinal tract originates from the red nucleus of the midbrain. Its connections are such that it may play a role in voluntary motor activity; this may be the case in higher primates, but its precise role in humans is not clear. • Reticulo-spinal tracts are involved in the voluntary (indirect) pathways, as well as in the underlying control of muscle tone and reflex responsiveness. Two tracts descend (on each side) from the reticular formation, one from the pontine region (the medial reticulo-spinal tract) and one from the medulla (the lateral reticulo-spinal tract). • Lateral vestibulo-spinal tract comes from the lateral vestibular nucleus in the pons. This nucleus plays an important role in the regulation of our responses to gravity (vestibular afferents). It is under control of the cerebellum, not the cerebral cortex. • Medial longitudinal fasciculus (usually called MLF) is a complex pathway of the brainstem and upper spinal cord which serves to coordinate various eye and neck reflexes. Descending vestibular influences from other vestibular nuclei join this pathway. There are also ascending fibers within the MLF. Descending fibers from the superior colliculus (the tecto-spinal pathway) form part of the MLF. ©2000 CRC Press LLC In humans, there is one abnormal reflex that indicates there has been a lesion interrupting the cortico-spinal pathway, at any level (cortex, white matter, internal capsule, brainstem, spinal cord). Normally stroking the bottom of the foot (a most uncomfortable sensation for most people) results in flexion of the toes, called a plantar response, and often an attempt to withdraw the limb. After a lesion interrupting the cortico-spinal pathway, stroking of the bottom of the foot results in an upward movement of the big toe (extension), and a fanning apart of the other toes. The whole response is called a Babinski reflex and it is found almost immediately after any lesion that interrupts any part of the cortico-spinal pathway, from cortex through to spinal cord. Most interestingly, the Babinski reflex is normally present in infants and disappears somewhere in the second year of life, concurrent with the myelination that occurs in this pathway. FIGURE 42 CORTICO-SPINAL TRACT — THE PYRAMIDAL SYSTEM DIRECT VOLUNTARY PATHWAY The cortico-spinal tract is a direct pathway linking the cortex with the spinal cord. From the evolutionary point of view, it is one of the newer pathways. The corticospinal tract is the most important one for voluntary motor movements in humans. It is also known as the pyramidal system, particularly in the clinical setting. The neurons giving rise to this pathway are mostly located in the motor areas of the cerebral cortex (see Figures 12 and 16; discussed below and with Figure 51). The axons are well myelinated and descend through the white matter of the hemispheres, through the posterior limb of the internal capsule (see Figures 26 and 27). After descending through the midbrain and pons, the fibers are found within the medullary pyramids (see Figures 3 and 4). Hence, the cortico-spinal pathway is often called the pyramidal tract. At the lowermost part of the medulla, most (90%) of the cortico-spinal fibers decussate (cross) in the pyramidal decussation, and form the lateral cortico-spinal tract in the spinal cord (see Figure 72). Many end directly on the anterior horn cells. The entire pathway is involved with controlling the individualized fine movements, particularly of our fingers and hands — the distal limb musculature. Those fibers that do not cross in the pyramidal decussation form the anterior (or ventral) cortico-spinal tract. The ventral pathway is found in the ventral portion of the white matter of the spinal cord (see Figure 72). Many of these axons will cross before terminating, while others supply motor neurons on both sides. The ventral pathway is concerned with movements of the proximal limb joints and axial movements. Other pathways are also involved in the control of this musculature. Other areas of the cortex, including the sensory cortical areas (e.g., the postcentral gyrus), contribute to the cortico-spinal pathway. This part of the pathway presumably carries “instructions” from the cortex to the dorsal horn of the spinal cord (its sensory portion; see Figure 2A) that may modify the transmission of sensory ©2000 CRC Press LLC information at the spinal cord level (also discussed with Figure 43). Clinical Aspects Lesions involving the cortico-spinal tract in humans are quite devastating because they rob the individual of voluntary motor control, particularly fine (skilled) motor movements. This pathway is quite commonly involved in strokes, as a result of vascular lesions of the cerebral arteries or of the deep arteries to the internal capsule (reviewed with Figures 58 and 60). These lesions result in a weakness or paralysis of the muscles on the opposite side. A Babinski reflex would be found almost immediately after interruption of this pathway. Damage to the tract in the spinal cord is seen after traumatic injuries (e.g., automobile and diving accidents). Amyotrophic lateral sclerosis (ALS) (also known as Lou Gehrig’s disease) involves a degeneration of both upper (cortical) and lower (spinal and brainstem) motor neurons. Neurological Neuroanatomy The cross-sectional levels for following this pathway through the brainstem include B1 (upper midbrain), B4 (mid-pons), B7 (mid-medulla), and spinal cord levels C8 and L3. After emerging from the internal capsule, the corticospinal tract is found in the midportion of the cerebral peduncles (see Figures 26, 43, and 64) in the midbrain. The cortico-spinal fibers are then dispersed in the pontine region and are seen as bundles of axons among the pontine nuclei (see Figure 67). The fibers collect again in the medulla as a single tract, one on each side of the midline. These fibers are located within the elevations known as the pyramids (see Figures 3 and 4). At the lowermost level of the medulla, 90% of the fibers decussate and form the lateral cortico-spinal tract, situated in the lateral aspect of the entire spinal cord (see Figure 72). The ventral cortico-spinal tract is found in the anterior portion of the white matter of the spinal cord (see Figure 72). FIGURE 42: Cortico-spinal Tract — The Pyramidal System-Direct Voluntary Pathway ©2000 CRC Press LLC FIGURE 43 CORTICO-BULBAR (AND CORTICO-PONTINE) FIBERS weakness, not paralysis, of the muscles supplied. For example, a lesion on one side might result in difficulty in swallowing or phonation, and often these problems dissipate in time. There are two exceptions to this rule, which are very important in the clinical setting. BRAINSTEM SYSTEM 1. The major exception is the cortical input to the facial nucleus. In humans, there is a most important difference between the innervation to the upper and lower face. The part of the facial nucleus controlling the lower facial muscles receives only a crossed input from the cortex. The cortical input to the part of the facial nucleus controlling the upper facial muscles is supplied from both hemispheres. Therefore, a patient with a lesion of the appropriate area of the motor cortex or of the cortico-bulbar fibers on one side will be able to wrinkle his/her forehead normally on both sides, but will not be able to show the teeth or smile symmetrically on the side opposite the lesion; often there will be a drooping of the lower face. This will also affect the muscle of the cheek (the buccinator muscle) and cause some difficulties with drinking, eating, and chewing (the food gets stuck in the cheek and often has to be manually removed); sometimes there is also drooling. This functional loss must be distinguished from a lesion of the facial nerve itself (most often seen with Bell’s palsy, a lesion of the facial nerve as it emerges from the skull) which affects the muscles of both the upper and lower face, on one side. 2. The cortical innervation to the hypoglossal nucleus is not always bilateral. In some individuals, there is a predominantly crossed innervation. The term cortico-bulbar is a descriptive one and does not indicate a single pathway. The word bulb (i.e., bulbar) refers to the brainstem and is borrowed from an older descriptive literature. Many of the fibers descending from the cerebral cortex to all parts of the neuraxis are involved in motor control. These axons, part of the so-called projection fibers of the hemispheres, course via the internal capsule (see Figure 26) and continue into the cerebral peduncles of the midbrain. Some of these are part of a distinct pathway to spinal cord (cortico-spinal, described with Figure 42); the cortico-bulbar fibers are those that end in the brainstem and include several functional components. These, along with the cortico-spinal tract, occupy the middle one-third of the cerebral peduncle of the midbrain (see also Figure 42). A subgroup of fibers — cortico-pontine — go to the nuclei of the pons (described fully with Figure 53; also discussed with Figure 55). These occupy the remainder of the cerebral peduncle. The cortico-bulbar fibers include those to brainstem motor control nuclei (including the reticular formation) and cranial nerve nuclei: • Brainstem motor control nuclei — Cortical fibers likely influence all the brainstem motor nuclei, particularly the reticular formation and including the red nucleus, with the exception of the lateral vestibular nucleus (see Figure 45). The cortico-reticular fibers are extremely important for some voluntary movements (indirect pathway) and for muscle tone (discussed with Figure 47). • Cranial nerve nuclei — The motor neurons of the cranial nerves of the brainstem (see Figures 5 and 6) are functionally lower motor neurons; the cortical motor cells are the upper motor neuron. These motor nuclei are generally innervated by fibers from both sides, i.e., each nucleus receives input from both hemispheres. Therefore, loss of cortical innervation to the cranial nerve motor nuclei is usually associated with a ©2000 CRC Press LLC The cortical input to the sensory nuclei of the brainstem, including the somatosensory nuclei, nuclei cuneatus and gracilis (see Figure 38), is similar to the cortical input to the dorsal horn of the spinal cord (discussed with Figure 42). • Cortico-pontine fibers — The cortical input to the pontine nuclei, located in the outer and inner thirds of the cerebral peduncle (see also Figures 45 and 64), is discussed with the cerebellum (see Figures 53 and 55). Temporo-pontine fibers Occipito-pontine fibers Parieto-pontine fibers Cortico-bulbar (and cortico-spinal) fibers Fronto-pontine fibers FIGURE 43: Cortico-bulbar (and Cortico-pontine) Fibers — Brainstem Motor System ©2000 CRC Press LLC FIGURE 44 RUBRO-SPINAL TRACT NON-PYRAMIDAL MOTOR SYSTEM The red nucleus is a prominent nucleus of the midbrain. It gets its name from a reddish color seen in fresh dissections of the brain, presumably due to its high vascularity. The nucleus (see Figure 64) has two portions: a small-celled upper division and a lower portion with large neurons, more ventrally located. The rubrospinal pathway originates, at least in humans, from the larger cells. The red nucleus receives its input from the motor areas of the cerebral cortex and from the cerebellum. The cortical input is directly onto the projecting cells, thus forming a potential two-step pathway from motor cortex to spinal cord. The rubro-spinal tract is also a crossed pathway, with the decussation occurring in the ventral part of the midbrain (see also Figure 45). The tract descends within the tegmentum (the central part of the brainstem) and is not clearly distinguishable from other fiber systems. The fibers then course in the lateral portion of the white matter of the spinal cord, just anterior to and intermingled with the lateral cortico-spinal tract (see Figure 72). ©2000 CRC Press LLC The rubro-spinal tract is a well-developed pathway in some animals. In monkeys it seems to be involved in flexion movements of the limbs. Stimulation of this tract in cats produces an increase in tone of the flexor muscles. The functional significance of this pathway in humans is not well known. The number of large cells in the red nucleus in the human is significantly less than in the monkey. Motor deficits associated with a lesion involving only the red nucleus or only the rubro-spinal tract have not been adequately described. Although the rubro-spinal pathway may play a role in some flexion movements, it seems that the cortico-spinal tract predominates in the human. Neurological Neuroanatomy The location of this tract within the brainstem is shown at the cross-sectional levels B1 (upper midbrain), B4 (mid-pons), B7 (mid-medulla), and spinal cord levels C8 and L3. The tract is said to continue throughout the length of the spinal cord in primates but probably extends only into the cervical spinal cord in humans. The fibers of CN III (oculomotor) exit through the medial aspect of this nucleus at the level of the upper midbrain (see Figure 64). FIGURE 44: Rubro-spinal Tract — Non-Pyramidal Motor System ©2000 CRC Press LLC FIGURE 45 DESCENDING TRACTS AND MOTOR NUCLEI MOTOR PATHWAYS AND NUCLEI Various descending pathways are shown in Figure 45, along with those cranial nerve nuclei that have a motor component, using the somewhat oblique posterior view of the brainstem (see Figure 7). The explanations presented below are in summary form, as most have been discussed with previous figures. The major motor pathways include the following: • Cortico-spinal tract courses in the middle third of the cerebral peduncle. The tract fibers disperse in the pontine region between the pontine nuclei, and regroup as a compact bundle in the medulla where they are frequently called the pyramidal tract. At the lowermost part of the medulla (Figure 4), most of the fibers decussate to form the lateral cortico-spinal tract of the spinal cord. A small portion of the tract continues ipsilaterally, mostly into the cervical spinal cord region, as the anterior (ventral) cortico-spinal tract. • Cortico-bulbar fibers that project to the cranial nerve nuclei of the brainstem are shown in this diagram. Cortico-bulbar fibers also include those cortical fibers that project to the reticular formation and other brainstem nuclei. These fibers are also located in the middle third of the cerebral peduncle and are given off at various levels within the brainstem. • Cortico-pontine fibers — The descending cortical fibers from various parts of the cerebral cortex to the pontine nuclei are found in the outer and inner thirds of the cerebral peduncles. After synapsing in the pontine nuclei, the fibers cross and project to the cerebellum via the middle cerebellar peduncle. • Rubro-spinal tract fibers, which originate from the lower portion of the red nucleus, decussate in the midbrain region. The tract descends through the brainstem. In the spinal cord, the fibers are located anterior to the lateral cortico-spinal tract. The cranial nerve nuclei include (see also Figure 5) the following: • Oculomotor (to most extra-ocular muscles and parasympathetic) — This large nucleus, located at the level of the superior colliculus, sends its fibers to most ©2000 CRC Press LLC of the extraocular muscles. These fibers traverse through the medial portion of the red nucleus, before exiting in the fossa between the cerebral peduncles, the interpeduncular fossa (see Figure 64). The parasympathetic fibers associated with CN III originate from the Edinger-Westphal nucleus. • Trochlear (to the superior oblique muscle) — fibers cross before exiting from the midbrain posteriorly (see Figure 7). They then wrap around the cerebral peduncles in their course anteriorly. • Trigeminal (to muscles of mastication) — The motor fibers pierce the middle cerebellar peduncle as they exit in the pontine region. (The major part of this nerve is sensory.) • Abducens (to the lateral rectus muscle) — The anterior course of the exiting fibers could not be depicted from the perspective used in Figure 45. • Facial (to muscles of facial expression) — The fibers to the muscles of facial expression have an internal loop before exiting. The nerve loops over the abducens nucleus, forming a bump called the facial colliculus in the floor of the fourth ventricle (see Figure 7). It should be noted that the nerve of only one side is shown in this illustration. • Glossopharyngeal and vagus (branchiomotor and parasympathetic) — The fibers exit behind the inferior olive, on the lateral aspect of the medulla. These fibers include those from the nucleus ambiguus (branchiomotor to muscles of the pharynx and larynx) and the parasympathetic fibers from the dorsal motor nucleus of the vagus that supply the structures in the neck, thorax, and abdomen. The fibers of CN XI, the spinal accessory, that originate from the nucleus ambiguus, will join CN X immediately after exiting. This joining is not shown in the illustration. • Spinal accessory (to neck muscles) — The fibers that supply the large muscles of the neck (sternomastoid and trapezius) originate in the upper spinal cord and ascend into the skull before exiting. When referring to the spinal accessory nerve, one usually has in mind only this component. • Hypoglossal (to muscles of the tongue) — These fibers actually course anteriorly, exiting from the medulla between the inferior olive and the corticospinal (pyramidal) tract. Fronto-pontine fibers Temporoparieto-pontine fibers Cerebral peduncle CN III Red n. Oculomotor n. Cortico-spinal fibers Cortico-bulbar fibers Trochelar n. CN IV Pontine nuclei Middle cerebellar peduncle Rubro-spinal tract Cortico-bulbar fibers Trigeminal n. (motor) CN V (motor) Abducens n. CN VII Facial n. Cortico-bulbar fibers CN IX (motor) Hypoglossal n. CN X (motor) CN XI Ambiguus n. (IX, X, XI) CN XII Anterior cortico-spinal tract Pyramidal decussation Spinal cord Lateral cortico-spinal tract FIGURE 45: Descending Tracts and Motor Nuclei — Motor Pathways and Nuclei ©2000 CRC Press LLC FIGURES 46 AND 47 RETICULO-SPINAL TRACTS INDIRECT VOLUNTARY PATHWAY As noted with Figures 40A and 40B, the reticular formation is a collection of nuclei that participates in a number of functions, some quite general (e.g., “arousal”) and others more specific (e.g., respiratory control). These nuclei are also part of the indirect voluntary motor pathway (see Introduction to Motor Systems). The indirect voluntary pathway — the cortico-reticulospinal pathway — is apparently an older pathway for the control of movements, particularly of proximal joints and the axial musculature. Therefore, some voluntary movements can still be performed after destruction of the cortico-spinal pathway (discussed with Figure 42). The reticular formation receives input from many sources, including most sensory pathways (anterolateral, trigeminal, auditory, and visual). At this point, the focus is on the input from the cerebral cortex. These axons form part of the so-called cortico-bulbar system of fibers (discussed with Figure 43). Muscle tone is greatly influenced by activity in the reticular formation, and cortical input to the reticular formation is part of this regulation. There are two pathways (on each side) from the reticular formation to the spinal cord: one originates in the pontine region (Figure 46) and one in the medullary region (Figure 47). ©2000 CRC Press LLC FIGURE 46 PONTINE (MEDIAL) RETICULO-SPINAL TRACT The pontine (medial) reticulo-spinal tract originates in the pontine reticular formation from two nuclei: the upper one is the oral portion of the pontine reticular nuclei (nucleus reticularis pontis oralis), and the lower part is the caudal portion (see Figure 40B). The tract descends to the spinal cord and is located in the medial region of the white matter (see Figure 72); therefore, this pathway is called the medial reticulo-spinal tract. In terms of function, this pathway exerts its action on the extensor muscles, in both movements and tone. The area in the pons is known as the reticular extensor facilitatory area. The fibers terminate on the anterior horn cells controlling the axial muscles, likely via interneurons — not directly. This system is complementary to that of the lateral vestibular nucleus (see Figure 48). Lesions involving the cortico-bulbar fibers are discussed with the medullary reticular formation (Figure 47). Neurological Neuroanatomy The location of the tract in the brainstem is shown at cross-sectional levels B4 (mid-pons), B5 (lower pons), B7 (mid-medulla), and spinal cord levels C8 and L3. FIGURE 46: Pontine (Medial) Reticulo-spinal Tract — Indirect Voluntary Pathway ©2000 CRC Press LLC FIGURE 47 MEDULLARY (LATERAL) RETICULO-SPINAL TRACT in tone is velocity dependent and involves the antigravity muscles. In humans, for reasons difficult to explain, these are the flexors of the upper limb and the extensors of the lower limb. This tract originates in the medullary reticular formation, mainly from the nucleus known as the nucleus gigantocellularis (see Figures 40B and 70). The tract descends more laterally in the spinal cord than the pontine pathway and is named the lateral reticulo-spinal tract (Figure 72). Some of the fibers are crossed. The tract lies beside the (lateral) vestibulo-spinal pathway. The increase in reflex responsiveness — hyperreflexia — is tested with the monosynaptic stretch reflex (deep tendon reflex, DTR; discussed with Figure 2A). Another feature of hyperreflexia is clonus, usually elicited at the ankle by a rapid jerking of the ankle upwards. This tract also has its greatest influence on axial musculature, and its functional contribution has been classified as the reticular extensor inhibitory area. In this way, its influence is opposite to that of the pontine reticular formation. This area depends for its normal activity on influences coming from the cortex. Hyperreflexia also develops over a period of a few days or weeks. This development period is thought to be due to the decrease in the descending influences to the spinal cord. There are two hypotheses for the increase in reflex responsiveness: A. One possibility is a change of responsiveness of neurotransmitter receptors of the motor neurons or the interneurons. This is called denervation supersensitivity, leading to an increase in the responsiveness of the lower motor neuron. B. An alternate possibility is sprouting of the incoming (1A) muscle afferents, from the muscle spindles to “vacated” synaptic sites (due to the loss of descending fibers). This is called collateral sprouting. Hence, this increased input to the motor neurons causes the increased responsiveness of the motor neurons or interneurons. Neurological Neuroanatomy The location of the tract in the brainstem is shown at cross-sectional levels B4 (mid-pons), B5 (lower pons), B7 (mid-medulla), and spinal cord levels C8 and L3. Clinical Aspects: Spasticity Lesions involving the motor system, particularly affecting the function of the reticular formation, are often very confusing, mostly because of the lack of agreed upon terminology and the lack of a clear understanding of what is commonly seen clinically. The activity of the reticular formation has a profound effect on muscle reactivity to passive stretch and on deep tendon reflexes. It is extremely important for the clinician or neurologist to be able to detect changes in muscle tone and reflex activity and to differentiate between spasticity and rigidity. Destruction of the cortical input to the reticular formation results in a disruption of their descending influences by disturbing the balance between the two parts of the reticular formation. The result is an increase in the tone of the anti-gravity muscles, which develops over a period of several days. This condition is called an upper motor neuron lesion and any injury of the motor cortex or cortico-bulbar system above the level of the pons may give rise to this syndrome. The increase in tone — spasticity — is tested by passive flexion and extension of a limb. In spasticity, the change ©2000 CRC Press LLC Lesions involving large parts of the motor areas of the cerebral cortex and lesions of the descending fibers (particularly in the internal capsule) may lead to this clinical state in which a patient is paralyzed or has marked weakness, with spasticity and hyperreflexia (with or without clonus) on the contralateral side. With a lesion of the spinal cord which involves all the descending motor pathways of one half of the spinal cord, the clinical deficit would be ipsilateral to the lesion. In a Parkinsonian patient, the change of muscle tone is called rigidity (discussed with Figure 50). In contrast, a lower motor neuron lesion of the anterior horn cell (e.g., polio) leads to weakness, a decrease in muscle tone, and hyporeflexia. FIGURE 47: Medullary (Lateral) Reticulo-Spinal Tract — Indirect Voluntary Pathway ©2000 CRC Press LLC FIGURE 48 LATERAL VESTIBULOSPINAL TRACT NON-PYRAMIDAL MOTOR SYSTEM This pathway is very important in that it provides a link between the vestibular influences (i.e., gravity and balance) and the control of axial musculature, via the spinal cord. The main function is to provide corrective muscle activity when the body (and head) tilt or change orientation in space (activation of the vestibular system, CN VIII). This tract originates in the lateral vestibular nucleus, which is located in the lower pontine region (see Figures 6 and 49). The nucleus is found at the lateral edge of the fourth ventricle (see Figure 68) and is characterized by extremely large neurons. (This nucleus is also called Deiter’s nucleus in some texts and the large neurons are often called by the same name.) The lateral vestibular nucleus receives its major inputs from the vestibular system and from the cerebellum; there is no cortical input. This tract descends through the medulla and traverses the entire spinal cord (see Figure 72). It does not decussate. The fibers terminate in the medial portion of the anterior horn, namely on those motor cells that control the axial musculature (see Figure 2A). ©2000 CRC Press LLC In terms of function, this pathway increases extensor muscle tone and activates extensor muscles. It is easier to think of these muscles as anti-gravity muscles in a fourlegged animal; in humans, one must translate these muscles as functionally the extensors of the lower extremity and the flexors of the upper extremity. A lesion of this pathway would occur with spinal cord injuries and would involve one of the “upper motor neuron” pathways, leading to spasticity and hyperreflexia. Neurological Neuroanatomy The same cross-sectional levels have been used as with the reticular formation, starting at B4 (mid-pons). The vestibular nuclei are found at the B5 (the lower pontine level) and are seen through B7 (mid-medulla); the tract descends through the spinal cord, as seen at C8 and L3. In the spinal cord the tract is positioned anteriorly, just in front of the ventral horn (see Figure 72). Other Vestibular Connections The other vestibular nuclei — inferior and medial — contribute to the MLF (medial longitudinal fasciculus, discussed with Figures 49A and 49B). Some of these fibers descend to the cervical spinal cord and are named the medial vestibulo-spinal tract (see Figure 72), and others ascend to the midbrain. They form part of this interconnecting fiber system that coordinates movements of the eyes and the head and neck with vestibular input. These descending fibers also influence mainly the “axial” muscles. FIGURE 48: Lateral Vestibulo-spinal Tract — Non-Pyramidal Motor System ©2000 CRC Press LLC FIGURE 49A VESTIBULAR SYSTEM VESTIBULAR NUCLEI The vestibular system carries information about our position and changes in that position in relation to gravity. The sensory system is located in the inner ear and consists of three semicircular canals and other sensory organs in a bony and membranous labyrinth. There is a peripheral ganglion and the central processes of these cells, CN VIII, enter the brainstem at the cerebellarmedullary angle, just above the cerebellar flocculus (see Figures 3 and 4). The vestibular information is carried to four vestibular nuclei which are located in the upper medulla and lower pons: superior, lateral, medial, and inferior (see also Figure 6). The lateral vestibular nucleus gives rise to the lateral vestibulo-spinal tract (as described with Figure 48; see also Figure 49B). It serves to adjust the posture to changes in position in relation to gravity. The medial and inferior vestibular nuclei give rise to both ascending and descending fibers which join a conglomerate bundle called the medial longitudinal fasciculus (MLF, described more fully with Figure 49B). The descending fibers from the medial vestibular nucleus, if considered separately, could be named the medial vestibulo-spinal tract and likely participate in postural adjustments to positional changes. The ascending fibers adjust the position of the eyes and coordinate movements of the two eyes by interconnecting the three cranial nerve nuclei involved in the control of eye movements, CN III (oculomotor), CN IV (trochlear), and CN VI (abducens), all at different levels of the brainstem (see also Figure 5). Lateral gaze, a ©2000 CRC Press LLC movement of the eyes to the side (in the horizontal plane), requires the coordination of the lateral rectus muscle (abducens nucleus) of one side and the medial rectus (oculomotor nucleus) of the other side. These fibers for coordinating the eye movements are carried in the MLF. Clinical Aspects A lesion of the MLF interferes with the normal conjugate movements of the eyes. When a person is asked to follow an object (e.g., the tip of a pencil) with the head steady, the two eyes move together, usually in the horizontal plane. With a lesion of the MLF (such as demyelination in multiple sclerosis), the abducting eye moves normally (intact abducens nucleus) but the adducting eye fails to follow; yet, adduction is preserved on convergence. This condition is known as internuclear ophthalmoplegia. Sometimes there is monocular horizontal nystagmus of the abducting eye. There is a “gaze center” within the pontine reticular formation for saccadic eye movements; these are extremely rapid (ballistic) movements of both eyes, yoked together, usually in the horizontal plane. The cortical fibers originate from the frontal eye field (see Figure 12) and also likely course in the MLF. There is a small nucleus in the periaqueductal gray region of the midbrain which is associated with the visual system and is involved in the coordination of eye and neck movements. This nucleus is called the interstitial nucleus (of Cajal). It is located near the oculomotor nucleus. This nucleus (see IN in Figure 49B) receives input from various sources and contributes fibers to the MLF. Some may actually name this the interstitiospinal tract. Interstitial n. of Cajal Oculomotor n. Trochlear n. Abducens n. Pontine reticular formation VESTIBULAR NUCLEI S = Superior L = Lateral M = Medial I = Inferior Cervical spinal cord FIGURE 49A: Vestibular System — Vestibular Nuclei ©2000 CRC Press LLC FIGURE 49B MEDIAL LONGITUDINAL FASCICULUS MLF AND ASSOCIATED TRACTS The MLF (medial longitudinal fasciculus) is a tract within the brainstem and upper spinal cord which links the visual world and vestibular events with the movements of the eyes and the neck, as well as links the nuclei that are responsible for eye movements. The tract runs from the midbrain level to the upper thoracic level of the spinal cord. It has a rather constant location near the midline, dorsally, just anterior to the cerebral aqueduct and the fourth ventricle (see brainstem cross sections, e.g., Figures 65 and 69). Several tracts together form the actual MLF. Each of the component parts of the system can be considered separately: • Vestibular fibers — Of the four vestibular nuclei (see Figure 49A), descending fibers originate from the medial vestibular nuclei and become part of the MLF; this can be named the medial vestibulo-spinal tract. There are also ascending fibers which come from the medial, inferior, and superior vestibular nuclei that also are carried in the MLF. Therefore, the MLF carries both ascending and descending vestibular fibers. • Visuomotor fibers — The interconnections between the various nuclei concerned with eye movements are carried in the MLF. • Vision-related fibers — Visual information is received by various brainstem nuclei. • The superior colliculus is a nucleus for the coordination of visual-related reflexes, including eye movements. It also receives input from the visual association cortical areas (areas 18 and 19, see ©2000 CRC Press LLC Figure 39A). The descending fibers from the superior colliculus, called the tecto-spinal tract, are very closely associated with the MLF and can be considered part of this system (although in most books it is discussed separately). The superior colliculus coordinates the movements of the eyes and the turning of the neck, in response to visual information. As shown in the upper inset of Figure 49B, these fibers cross in the midbrain. (Note that the superior colliculus (SC) of only one side is shown in order not to obscure the crossing fiber systems at that level.) • The small interstitial nucleus and its contribution were noted in Figure 49A. The lower inset of Figure 49B shows the MLF in the ventral funiculus of the spinal cord at the cervical level (see also Figure 72). The three components of the tract are identified — those coming from the medial vestibular nucleus, the fibers from the interstitial nucleus, and the tecto-spinal tract. These fibers are mingled together in the MLF. In summary, the MLF is a complex fiber bundle which is necessary for the proper functioning of the visual apparatus. The MLF interconnects the three cranial nerve nuclei responsible for movements of the eyes with the motor cells controlling the movements of the head and neck. It allows the visual movements to be influenced by vestibular, visual, and other information and carries fibers (upwards and downwards) that coordinate the eye movements with the turning of the neck. The diagram also shows the posterior commissure. This small commissure carries fibers connecting the superior colliculi. In addition, it carries the important fibers for the consensual pupillary light reflex (discussed with Figure 39B). The role of the commissural nuclei is not known. Red n. (RN) Posterior commissure Commissural n. Oculomotor n. Interstitial n. (IN) Anterolateral system Superior colliculus (SC) Spino-tectal tract Medial longitudinal fasciculus (MLF) IN RN SC Trochlear n. Abducens n. CN VIII Interstitio-spinal tract Rubro-spinal tract Tecto-spinal tract Spino-cerebellar tracts Dorsal Ventral MLF Vestibular nuclei Vestibulo-spinal tracts { MLF { Lateral Medial Tecto-spinal tract Tecto-spinal tract Interstitio-spinal tract Medial vestibulo-spinal tract Lateral vestibulo-spinal tract Spinal cord FIGURE 49B: Medial Longitudinal Fasciculus — MLF and Associated Tracts ©2000 CRC Press LLC FIGURE 50 BASAL GANGLIA: CIRCUITRY MOTOR REGULATORY SYSTEMS Many years ago it was commonplace to refer to the basal ganglia as part of the extrapyramidal motor system (in contrast to the pyramidal motor system - discussed with Figure 42, the cortico-spinal tract). It is now known that the basal ganglia exert their influence through the appropriate parts of the cerebral cortex, which then acts either directly, i.e. using the cortico-spinal (pyramidal) tract, or indirectly, via certain brainstem nuclei, to alter motor activity. The term extrapyramidal should probably be abandoned, but it is still frequently encountered in a clinical setting. Other terms could be used, such as “non-pyramidal” or simply basal ganglia. At best one could perhaps consider this system in the same way as one is accustomed to viewing the influence of the cerebellum on motor control (to be discussed as part of the motor regulatory systems). The basal ganglia are introduced in Section A (see Figures 22–25). In this illustration, the removal of the head of the caudate nucleus, and some of its body, exposes the putamen more completely. The two parts of the globus pallidus are also seen. The illustration also includes the two other parts of the functional basal ganglia — the subthalamic nucleus and the substantia nigra. • The subthalamic nucleus is situated in a small region below the level of the diencephalon. This nucleus is connected with the globus pallidus, both receiving fibers from and sending fibers to different parts of that nucleus. The motor abnormality associated with a lesion of the subthalamic nucleus is called hemiballismus. The person (or animal) with this abnormality has sudden flinging movements of one or both limbs, on the opposite side of the body. • The substantia nigra is located in the midbrain region, as a sheet-like nucleus (see also Figure 24). It is composed of two parts (see Figure 64): 1. The pars reticulata is situated more ventrally. It receives fibers from the basal ganglia and is involved in the output from the basal ganglia to ©2000 CRC Press LLC the thalamus. 2. The pars compacta has the pigment-containing cells. These neurons project their fibers to the caudate and putamen (the striatum or neostriatum). This is called the nigro-striatal “pathway”; the neurotransmitter involved is dopamine. Clinical Aspects It is the degeneration of these dopamine-containing neurons, with the consequent loss of their dopamine input to the basal ganglia, the striatum, that leads to the clinical entity Parkinson’s disease. Those afflicted with this disease have slowness of movement (bradykinesia), reduced facial expressiveness (“mask-like” facies), and a tremor at rest (typically a “pill-rolling” type of tremor). On examination, there is rigidity, which is an increased resistance to passive movement of both flexors and extensors (contrast with spasticity, discussed with Figure 47) which is not velocity-dependent, and there is no change in reflexes. Basal Ganglia Circuitry Information flows into the caudate and putamen from all areas of the cerebral cortex (in a topographic manner), from the substantia nigra (pars compacta), and from the centromedian nucleus of the thalamus (see Figure 51). This information is processed and passed to the globus pallidus (internal segment) and the pars reticulata of the substantia nigra of the midbrain. (In addition, there is a sub-circuit involving the external segment of the globus pallidus and the subthalamic nucleus.) The most important output from the basal ganglia is from the internal segment of the globus pallidus. Most of this information is relayed to the thalamus, to the specific relay nuclei, ventral anterior (VA) and ventral lateral (VL) nuclei (see Figure 10). These project to the supplementary motor and premotor cortical areas (see Figures 12 and 51). These are the cortical areas concerned with motor planning and motor regulation. (Contrast this with the projection of the cerebellum to the cortex — to be discussed subsequently.) Caudate nucleus Putamen Globus pallidus (external segment) Subthalamic nucleus Substantia nigra Red nucleus Globus pallidus (internal segment) Midbrain Anterior commissure Amygdala FIGURE 50: Basal Ganglia: Circuitry — Motor Regulatory Systems ©2000 CRC Press LLC FIGURE 51 THALAMUS: MOTOR CIRCUITS MOTOR REGULATORY SYSTEMS The specific relay nuclei of the thalamus that are linked with the motor system are the ventral lateral and the ventral anterior nuclei. These project to different cortical areas involved in motor control. These nuclei also receive input from these cortical areas, in line with the reciprocal connections of the thalamus and cortex. One of the intralaminar nuclei, the centromedian nucleus, is also linked with the motor system. Basal Ganglia Input to the basal ganglia, the striatal parts, comes from all parts of the cortex — the cortico-striatal fibers. After processing, the striatum sends its information to the globus pallidus (striato-pallidal fibers). The major outflow from the basal ganglia is from the internal (medial) segment of the globus pallidus. Two slightly different pathways project to the thalamus — pallido-thalamic fibers — one passing around and the other passing through the fibers of the internal capsule (represented on the diagram by large stippled arrows). These merge and end in the ventral anterior (VA) and ventral lateral (VL) nuclei of the thalamus. (The ventral anterior nucleus is not seen on this section through the thalamus; see Figure 10.) These nuclei also receive input from the substantia nigra, pars reticulata (not shown). The cortical projection is to the premotor and supplementary motor areas, as shown in the small insets. The functional contribution of the basal ganglia to the motor system is discussed in Section A. It is still not known what role these thalamic nuclei play in this pathway. Clinically, in a person with Parkinson’s disease, ©2000 CRC Press LLC a lesion in the thalamic region that interrupts this pathway and/or destroys part of these nuclei has been shown to alleviate some of the symptoms. To date, the theory has been that the surgical removal of impulses restores the balance between the various thalamic influences to the cortical areas involved in motor control. The pathway involving the centromedian nucleus (CM) is rather distinct. The afferents come from the medial segment of the globus pallidus. Efferents from CM go to the caudate-putamen, hence forming a feedback loop within the basal ganglia. Cerebellum Note to student: Review after study of the cerebellum. The other part of the motor regulatory system, the cerebellum, also projects to the cortex via the thalamus. The dentate nucleus, the largest of the deep cerebellar nuclei and the one that receives input from the neocerebellum, projects its fibers via the superior cerebellar peduncle (see Figures 38 and 55). The major projection is to the ventral lateral (VL) nucleus, but a different portion of it than receives from the basal ganglia. From here, the fibers project to the motor areas of the cerebral cortex, predominantly the motor area of the precentral gyrus as well as the premotor area (areas 4 and 6, respectively). With regard to function, the neocerebellum seems to be involved in motor planning (discussed with the cerebellum, Figure 55). Again, it is not easy to understand what role the thalamus plays in integrating this information, prior to the projection to the cerebral cortex. The motor areas of the cerebral cortex that receive input from these two subsystems of the motor system are shown diagrammatically in Figure 51 — both on the dorsolateral surface and on the medial surface of the hemispheres. Supplementary motor area Thalamo-cortical fibers Premotor area (area 6) Cortico-striatal fibers Precentral gyrus (area 4) Caudate nucleus (body) Ventral lateral n. Intralaminar n. Supplementary motor area Centromedian n. Globus pallidus Putamen Red nucleus Striato-pallidal fibers Internal capsule (fibers) Pallido-thalamic fibers Cerebello-thalamic fibers Decussation of superior cerebellar peduncles Substantia nigra FIGURE 51: Thalamus: Motor Circuits — Motor Regulatory Systems ©2000 CRC Press LLC FIGURE 52 CEREBELLUM I FUNCTIONAL LOBES The anatomical approach to the cerebellum is introduced in the Orientation section (see Figure 8). In order to understand the functional anatomy of the cerebellum and its contribution to the regulation of motor control, it is necessary to subdivide the cerebellum into operational units. The three functional lobes of the cerebellum are the vestibulocerebellum, the spinocerebellum, and the neo- or cerebrocerebellum. These lobes of the cerebellum are defined by the areas of the cerebellar cortex involved, the related deep cerebellar nucleus, and the connections (afferents and efferents) with the rest of the brain. There is a convention of portraying the surface of the cerebellum as if it were found in a single plane. The best analogy to use is a book, with the binding towards you. The binding is the hinge around which the book will open, and the landmark for this on the cerebellum is the horizontal fissure, which demarcates the superior and inferior surfaces. If you place the fingers of your right hand on the edge of the front cover (the superior surface of the cerebellum) and the fingers of your left hand on the edges of the back cover (the inferior surface of the cerebellum), you can gently open the book to expose both the front and back covers simultaneously. Both are now laid out in a single plane. Using the lingula and the nodulus of the vermis as fixed points (see Figure 16), the lingula is at the “top” of the cerebellum and the nodulus is at the bottom of the diagram. This same portrayal can be done with an isolated cerebellum and attached brainstem in the following way: the brain knife is introduced at the ponto-medullary junction and directed towards the horizontal fissure. Before reaching there, the superior and inferior surfaces are pulled very gently apart, thereby making it possible to place the cerebellum on a flat surface. Having done this, it is possible to discuss the three functional lobes of the cerebellum. The vestibulocerebellum is composed of two cortical components, the flocculus and the nodulus; hence it is ©2000 CRC Press LLC also called the flocculonodular lobe. This is the functional part of the cerebellum responsible for balance and gait. The flocculus is a small lobule of the cerebellum located on its inferior surface and oriented in a transverse direction, below the middle cerebellar peduncle (see Figures 3 and 4). The flocculus is connected to the nodulus of the vermis (see Figure 16), the two together forming the flocculonodular lobe. The vestibulocerebellum sends its fibers to the fastigial nucleus, one of the deep cerebellar nuclei which connect the cerebellum with other parts of the brain (discussed with Figures 54A and 54B). The functional lobe called the spinocerebellum is concerned with coordinating the activities of the limb musculature. Part of its role is to act as a comparator between the intended and the actual movements. It is made up of three areas. The anterior lobe of the cerebellum is an anatomical division of the cerebellum found on the superior surface, in front of the primary fissure (see Figure 8). Most of the vermis (other than the parts mentioned above — see Figure 16) comprises the second part of the spinocerebellum. The third portion is a strip of tissue on either side of the vermis called the intermediate zone (or paravermis) — there is no anatomical fissure demarcating this functional area. The output deep cerebellar nuclei for this functional part of the cerebellum is in part the fastigial nucleus and mostly the intermediate nuclei, the globose and emboliform nuclei (see Figures 54A and 54B). With the exception of the vermis and the adjacent strip (the intermediate zone), the tissue behind the primary fissure comprises the neocerebellum. This continues onto the inferior surface of the cerebellum, until the dorsal aspect of the medulla is reached. This is the largest part of the cerebellum and the newest from an evolutionary point of view. It is usually called the neocerebellum, and also the cerebrocerebellum, since most of its connections are with the cerebral cortex. This part of the cerebellum is involved with the overall coordination of voluntary motor activities and is also involved in motor planning. The output nucleus of this part of the cerebellum is the dentate nucleus. L F N F L = Lingula N = Nodulus F = Flocculus Spinocerebellum L Anterior lobe Primary fissure Cerebrocerebellum Horizontal fissure F N F Vermis Intermediate FIGURE 52: Cerebellum I — Functional Lobes ©2000 CRC Press LLC Flocculonodular lobe FIGURE 53 CEREBELLUM II AFFERENTS Information relevant to the role of the cerebellum in motor regulation comes from the cerebral cortex, the brainstem, and the muscle receptors in the periphery. The information is conveyed to the cerebellum mainly via the middle and inferior cerebellar peduncles. Only one afferent tract enters via the superior cerebellar peduncle. Inferior Cerebellar Peduncle The inferior cerebellar peduncle goes from the medulla to the cerebellum. It lies behind the inferior olivary nucleus and can sometimes be seen on the ventral view of the brainstem (as in Figure 4). This peduncle conveys a number of fiber systems to the cerebellum. These are shown schematically in Figure 53 with the ventral view of the brainstem and cerebellum. The fiber systems include the posterior (dorsal) spino-cerebellar pathway, the cuneo-cerebellar tract, olivo cerebellar tract, and other cerebellar afferents: • The posterior (dorsal) spino-cerebellar pathway is conveying proprioceptive information from most of the body. This is one of the major tracts of the inferior peduncle. These fibers, carrying information from the muscle spindles as well as from cutaneous sources, relay in the dorsal nucleus of Clarke in the spinal cord (see Figure 2A). They ascend ipsilaterally in a tract which is found at the edge of the spinal cord (see Figure 72). The dorsal spino-cerebellar fibers terminate ipsilaterally. These fibers are distributed to the spino-cerebellar areas of the cerebellum. • The homologous tract for the upper limb is the cuneo-cerebellar tract. These fibers relay in the accessory (external) cuneate nucleus in the lower medulla (see Figures 70 and 71). This pathway is not shown in the diagram. ©2000 CRC Press LLC • The olivo-cerebellar tract is also carried in this peduncle. The fibers originate from the inferior olivary nucleus (see Figures 4 and 70), cross in the medulla, and are distributed to all parts of the cerebellum. These axons have been shown to be the climbing fibers to the main dendritic branches of the Purkinje neuron. • Other cerebellar afferents from other nuclei of the brainstem, including the reticular formation, are conveyed to the cerebellum via this peduncle. Most important are those from the vestibular nuclei to the vestibulocerebellum. Afferents from the visual and auditory system are also known to be conveyed to the cerebellum. Middle Cerebellar Peduncle All parts of the cerebral cortex contribute to the massive cortico-pontine system of fibers (described with Figures 43 and 45). These descend via the anterior and posterior limbs of the internal capsule, then the inner and outer parts of the cerebral peduncle and terminate in the pontine nuclei (shown in Figure 67). The fibers synapse, cross, and go to all parts of the cerebellum via the middle cerebellar peduncle (not labeled in this diagram; see Figure 3). This input provides the cerebellum with the cortical information relevant to motor commands and the intended motor activities. Superior Cerebellar Peduncle One group of cerebellar afferents, those carried in the ventral (anterior) spino-cerebellar tract, enters the cerebellum via the superior cerebellar peduncle. These fibers cross in the spinal cord, ascend (see Figure 72), enter the cerebellum, and cross again, thus terminating on the same side from which they originated. All afferent fibers provide excitatory influences to the deep cerebellar nuclei via collaterals and end in the cerebellar cortex. Inferior olivary nucleus Olivo-cerebellar fibers Inferior cerebellar peduncle Dorsal spino-cerebellar tract FIGURE 53: Cerebellum II — Afferents ©2000 CRC Press LLC FIGURE 54A CEREBELLUM III CIRCUITRY The cerebellum is organized with cortical tissue on the outside, the cerebellar cortex. The cortex consists of three layers and all areas of the cerebellum are histologically alike. The most important cell of the cortex is the Purkinje neuron, which forms a single layer; their massive dendrites receive the various cerebellar afferents. Various interneurons are also located in the cortex. The axon of the Purkinje neuron is the only axonal system to leave the cerebellar cortex. Deep within the cerebellum are masses of gray matter which are part of the cerebellar circuitry. These are called the intracerebellar nuclei or the deep cerebellar nuclei; both names are used. There are four pair of deep cerebellar nuclei — the fastigial nucleus (most medially), the intermediate group (named the globose and emboliform), and the lateral or dentate nucleus. Each belongs to a different functional part of the cerebellum. These nuclei are the output nuclei of the cerebellum to other parts of the central nervous system. The position of the deep cerebellar (intracerebellar) nuclei, which are located within the cerebellum, is indicated in Figure 54A. Their location is superimposed upon the ventral view of the cerebellum. (The intracerebellar nuclei are shown from a posterior perspective in Figure 54B.) The nuclei are arranged in the following manner: 1. The fastigial (medial) nucleus is located next to the midline. 2. The globose and emboliform nuclei are slightly more lateral; often these are grouped together and called the intermediate or interposed nucleus. 3. The dentate nucleus, with its irregular margin, is most lateral. This nucleus is sometimes called the lateral nucleus and is by far the largest. The nuclei are located within the cerebellum at the level of the junction of the medulla and the pons. Therefore, the cross sections shown at this level (see Figure 68) may include these deep cerebellar nuclei. Usually, only the dentate nucleus can be identified in the real sections. ©2000 CRC Press LLC The same holds true for sections of the gross brainstem and cerebellum done at this level. Overall, the circuitry is as follows: input (excitatory) to the cerebellum goes to both the deep cerebellar nuclei and the cerebellar cortex. After processing in the cortex, the Purkinje neuron influences (inhibitory) the activity of the neurons of the deep cerebellar nuclei. Their output (excitatory) exits the cerebellum to the brainstem and to the cerebral cortex via the thalamus, which modulates motor activity. Details of Cerebellar Circuitry The cerebellum receives information from many parts of the nervous system, including the spinal cord, vestibular system, brainstem, and cerebral cortex. Most of this input is related to motor function, but some is also sensory. These afferents are excitatory in nature and influence the ongoing activity of the neurons in the intracerebellar nuclei, as well as projecting to the cerebellar cortex. The incoming information to the cerebellar cortex is processed by various interneurons of the cerebellar cortex and eventually influences the Purkinje neuron, which leads to either increased or decreased firing of this neuron. Its axon is the only one to leave the cerebellar cortex and these axons project (in an organized manner) to the deep cerebellar nuclei. The Purkinje neurons are an inhibitory neuron and their influence modulates the activity of the deep cerebellar nuclei. Increased firing of the Purkinje neuron increases the ongoing inhibition onto these deep cerebellar nuclei, while decreased Purkinje cell firing results in a decrease in the inhibitory effect on the deep cerebellar cells, i.e., it results in the increased firing of the deep cerebellar neurons (called disinhibition). Axons from the deep nuclei neurons project from the cerebellum to many areas of the CNS, including brainstem motor nuclei (e.g., vestibular, reticular formation) and thalamus (to motor cortex). In this way the cerebellum exerts its influence on motor performance. This topic is discussed with Figure 54B. Fastigal n. Emboliform n. Globose n. Dentate n. FIGURE 54A: Cerebellum III — Circuitry ©2000 CRC Press LLC FIGURE 54B CEREBELLUM IV cent to the inferior cerebellar peduncle. The name of this particular bundle is the juxtarestiform body*, a rather awkward name for a bundle of fibers. EFFERENTS It is interesting to note that the cerebellar cortex projects fibers directly to the lateral vestibular nucleus. As would be anticipated, these are inhibitory. The lateral vestibular nucleus (see Figure 49A) could therefore, in some sense, be considered one of the intracerebellar nuclei. This nucleus also receives input from the vestibular system then projects to the spinal cord (review with Figure 48). Figure 54B is a dorsal view of the diencephalon, midbrain, and cerebellum. The superior surface of the cerebellum is visualized, and the position of the deep cerebellar (intracerebellar) nuclei within the cerebellum has been added. This view of the cerebellum is similar to the photograph of the brain in Figure 8. The third ventricle is situated between the two diencephala, with the interconnecting massa intermedia. The pineal gland is seen attached to the posterior aspect of the thalamus. Below are the colliculi, superior, and inferior. The vermis includes a group of midline folia which are elevated. In front of the primary fissure is the anterior lobe, part of the spinocerebellum. The location of the horizontal fissure is also indicated, separating the superior surface from the inferior one. The perspective of this diagram is such as to include several folia belonging to the inferior surface of the cerebellum (see Figure 52). The intracerebellar nuclei are depicted within the cerebellum, as if they could be seen from the outside (similar to the view presented from the ventral perspective, in Figure 54A). Their location within the cerebellum will be correlated with the projection they receive from the cerebellar cortex (refer also to Figure 52). The fastigial nuclei are centrally located and receive fibers from the vermis. These nuclei are connected with the vestibulocerebellum and, to some degree, with the spinocerebellum. From the fastigial nuclei, efferent fibers go to brainstem motor nuclei (e.g., vestibular nuclei and reticular formation), influencing balance and gait. They exit from the cerebellum in a bundle that is found adja- ©2000 CRC Press LLC The emboliform and globose are the intermediate nuclei and they receive fibers from the intermediate zone of the cerebellum and the anterior lobe. These nuclei are functionally part of the spinocerebellum. These fibers also project to brainstem nuclei, including the red nucleus of the midbrain and to the appropriate limb areas of the motor cortex. The cortical fibers project via the thalamus (see below) to the motor areas of the cerebral cortex and are involved in the comparator function of this part of the cerebellum. The dentate nucleus is most lateral of the intracerebellar nuclei and it receives input from the neocerebellum. Its connections are described with Figure 55. *The name is derived from an older terminology — the inferior cerebellar peduncle used to be called the restiform body. It is unlikely that a student will be exposed to this terminology in a clinical setting except, perhaps, in neuroradiology. Third ventricle Diencephalon Fastigial n. Pineal Superior colliculus Inferior colliculus Dentate n. Primary fissure Globose n. Horizontal fissure Emboliform n. Vermis FIGURE 54B: Cerebellum IV — Efferents ©2000 CRC Press LLC FIGURE 55 CEREBELLUM V SUPERIOR CEREBELLAR PEDUNCLE The superior cerebellar peduncle is the major outflow tract of the cerebellum. This peduncle connects the cerebellum with the midbrain; the fibers are on their way to the thalamus and cortex. It is difficult to visualize this pathway and even more difficult to locate it on the actual specimen (see Figures 7, 8, and 38). The outflow fibers originate mainly from the dentate nucleus, with some from the intermediate nucleus (not shown). The axons start laterally and converge towards the midline. In this part of their course they are located in the roof of the upper half of the fourth ventricle (see Figure 7). Some fibers that form a bridge between the superior cerebellar peduncles in this area are named the superior medullary velum (discussed with Figure 7; see also Figure 38). From this dorsal perspective it is possible to visualize the superior cerebellar peduncles in a gross brain specimen. The superior cerebellar peduncles continue to “ascend” and enter the upper part of the pons (see the cross section in Figure 66). In the lower midbrain (see Figure 65) there is a complete decussation of fibers. Some of the fibers may terminate in the red nucleus of the midbrain, particularly those from the interposed nucleus. The majority of the fibers, particularly those from the dentate nucleus, terminate in the ventral lateral nucleus (VL) of the thalamus (see Figure 51). Note to student: At this point it is important to return to Figure 10 and review the specific thalamic relay — ventral lateral nucleus of the thalamus to the motor cortex. From here they are relayed to the motor cortex, predominantly area 4, and also to the premotor cortex, area 6. It is thought the neocerebellum is also involved in motor planning. Therefore, the neocerebellum is linked to the cerebral cortex by a circuit which forms a loop. Fibers are relayed from the cerebral cortex via the pons (the pontine nuclei) to the cerebellum. The ponto-cerebellar fibers ©2000 CRC Press LLC cross and go to the neocerebellum of the opposite side. After cortical processing, the neocerebellar fibers project to the dentate nucleus. The efferents project to the thalamus, after crossing (decussating) in the lower midbrain. From the thalamus, fibers are relayed mainly to the motor areas of the cerebral cortex. Because of the two crossings, the messages are returned to the same side of the cerebral cortex from which the circuit began. Clinical Aspects Lesions of the neocerebellum of one side cause motor deficits to occur on the same side of the body, that is ipsilaterally for the cerebellum. The explanation for this lies in the fact that the cortico-spinal tract is also a crossed pathway (see Figure 42). For example, the errant messages from the left cerebellum which are delivered to the right cerebral cortex cause the symptoms to appear on the left side — contralateral for the cerebral cortex but ipsilaterally from the point of view of the cerebellum. The cerebellar symptoms associated with lesions of the neocerebellum (or the superior cerebellar peduncle) are collectively called dyssynergia, in which the range, direction, and amplitude of voluntary muscle activity are disturbed. The specific symptoms include the following: • distances are improperly gauged when pointing, called dysmetria, and include pastpointing; • rapid alternating movements are poorly performed, called dysdiadochokinesis; • complex movements are performed as a series of successive movements, called decomposition of movement; • tremor is seen during voluntary movement, an intention tremor (in contrast to Parkinsonian tremor which is present at rest and disappears during voluntary movement); • disturbances occur in the normally smooth production of words, resulting in slurred and explosive speech. In addition, cerebellar lesions in humans are often associated with hypotonia and sluggish deep tendon reflexes. Red nucleus Fibers to thalamus Decussation of superior cerebellar peduncles Superior cerebellar peduncle FIGURE 55: Cerebellum V — Superior Cerebellar Peduncle ©2000 CRC Press LLC Section C NEUROLOGICAL NEUROANATOMY Clinical neurology is based upon a detailed understanding of the structure and function of the nervous system, in health and disease. The task of the neurologist is to establish • whether the disease process is neurological; • where the nervous system is involved, i.e., localization; and • what the pathophysiological mechanisms are, i.e., the disease. The clinical decision-making process involves taking a detailed history, doing a complete neurological examination, and establishing a differential diagnosis. Select investigations are usually necessary to confirm the diagnosis. Appropriate therapy can then be instituted and the patient (and family) can be counseled regarding the likely prognosis. Skilled and knowledgeable clinicians can recognize diseases based upon their presentation (for example, vascular lesions have a sudden onset versus slow onset for tumors), the patient’s age, the parts of the nervous system involved, and the evolution of the disease process. The learning objective of this section is to enable the student to localize the disease process within the nervous system. The emphasis is on the brainstem. In addition, the vascular supply of the brain and spinal cord need to be studied at this point. Vascular Supply The CNS is totally dependent upon a continuous supply of blood; viability of the neurons depends upon the immediate and constant availability of both oxygen and glucose. Interruption of this lifeline causes sudden loss of function. Study of the nervous system must include a complete knowledge of the blood supply and structures ©2000 CRC Press LLC (nuclei and tracts) situated in the vascular territory of the various arteries. Failure of the blood supply to a region, either because of occlusion or hemorrhage, will lead to death of the neurons and axons, resulting in functional deficits. Areas of gray matter, where the neurons are located, have a greater blood supply than white matter. Loss of oxygen and glucose supply to these neurons will lead to loss of electrical activity after a few minutes (in the adult) and, if continued, to neuronal death. The white matter requires less blood supply; loss leads to destruction of the axons in the area of the infarct and an interruption of pathways. The axonal portions (and the synapses) which are separated from the cell body will degenerate, leading to a loss of function. Visualization of the arterial (and venous) branches can be accomplished using MR angiogram and arteriogram: • MR Angiogram — Using neuroradiology imaging with MRI (discussed with Figure 17), the major blood vessels (such as the Circle of Willis) can be visualized; this is called a magnetic resonance angiogram, or MRA (see Figure 57). • Arteriogram — By injecting a radiopaque substance into the arteries (a procedure done by a neuroradiologist) and following its course through a rapid series of x-rays (called an arteriogram), a detailed view of the vasculature of the brain is obtained; either the carotid or vertebral artery is usually injected, according to which arterial tree is under investigation. Histological Neuroanatomy In addition to a detailed look at the blood supply as a basis for analyzing the clinical consequences of vascular lesions, this section also presents the detailed neuroanatomy that is needed for localization of lesions in the brainstem and also the spinal cord. A series of illus- trations is presented through the brainstem with details of the tracts, cranial nerve nuclei, and other nuclei of importance. Accompanying these schematics are photographs of the brainstem from the human brain at the same levels. Intracranial Pressure (ICP) Note to student: Consult other texts for a visual understanding of these structures. In addition to knowledge of the brain and the function of the various parts, many disease processes exert their effect because of a rise in intracranial pressure (ICP) causing a shifting of structures within the skull. The adult skull is a rigid container filled with the brain, the cerebrospinal fluid (CSF), and blood. The interior of the skull is divided into compartments by folds of dura: the falx cerebri in the midline between the hemispheres and the tentorium cerebelli, which separates the hemispheres from the contents of the posterior cranial fossa (brainstem + cerebellum). Any increase in volume inside the skull — due to brain swelling, tumor, abscess, hemorrhage, abnormal amount ©2000 CRC Press LLC of CSF — causes a rise in pressure inside the skull (increased ICP). Although brain tissue itself has no pain fibers, the blood vessels and meninges do, hence any pulling on the meninges may give rise to a headache. A rise in ICP can be seen in due course by examining the optic disc; the margins become blurred and the disc itself engorged, called papilledema, with a pathological increase of ICP. Depending upon the lesion, sooner or later a displacement of brain tissue from one compartment to another occurs. This pathological displacement itself causes damage to the brain. This is called a brain herniation syndrome, and typically occurs • through the tentorial notch, also called uncal herniation (discussed with Figure 14); • through the foramen magnum, also known as tonsillar herniation (discussed with Figure 8); and • under the falx cerebri itself. These shifts are life-threatening and require emergency management. FIGURE 56 BLOOD SUPPLY I THE ARTERIAL CIRCLE OF WILLIS (OVERLAY) The arterial circle of Willis is a set of interconnecting arteries of the vertebral and common carotid arteries. It is located at the base of the brain, surrounding the optic chiasm and the hypothalamus (the mammillary nuclei; review Figures 13 and 14). Within the skull it is situated above the pituitary fossa (and gland). The major arteries to the hemispheres — the cerebral cortex — are branches of this arterial circle. Figure 56 is a photographic view of the inferior aspect of the brain, including brainstem and cerebral hemispheres (as in Figure 13), with the blood vessels presented as an overlay (created with Photoshop) onto this illustration. The cut end of the internal carotid arteries is a starting point. Each artery divides into the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). The middle cerebral artery courses within the lateral fissure. (It is shown lightly shaded on the left side, as if it could be visualized in its course through the lateral fissure.) Within the fissure, small arteries are given off to the basal ganglia, called the striate arteries (not labeled; see Figure 60). The artery continues and further branches are distributed onto the dorsolateral surface of the brain (see Figure 58). By removing the optic chiasm, the anterior cerebral arteries can be followed anteriorly. A very short artery connects the two of them, the anterior communicating artery. This anterior cerebral artery supplies the medial surface of the brain (see Figure 59). The vertebro-basilar system supplies the brainstem and cerebellum, and the posterior part of the hemispheres. The two vertebral arteries unite to form the midline basilar artery, which courses in front of the pons. The basilar artery terminates at the midbrain level by dividing into two posterior cerebral arteries. These supply the inferior aspect of the brain and particularly the occipital lobe. ©2000 CRC Press LLC The arterial circle is completed by the posterior communicating artery (one on each side), which connects the internal carotid or middle cerebral artery (the anterior circulation) with the posterior cerebral artery (from the posterior circulation). Small arteries directly from the circle (a few are shown) provide the blood supply to the diencephalon (thalamus and hypothalamus), some parts of the internal capsule, and part of the basal ganglia. The major blood supply to these regions is from the striate arteries (see Figure 60). The branches from the vertebral and basilar artery supply the brainstem. There are three major branches from this part of the arterial tree to the cerebellum — the posterior inferior cerebellar artery (PICA), the anterior inferior cerebellar artery (AICA), and the superior cerebellar artery. All supply the lateral aspects of the brainstem en route to the cerebellum as the circumferential branches. Small branches directly from the vertebral and basilar arteries (a few are shown) supply the medial structures of the brainstem, known as paramedian arteries (further discussed with Figure 62). The blood supply to the spinal cord is discussed with Figure 72. Clinical Aspects The most common clinical lesion involving the cerebral blood vessels is occlusion, often due to an embolus originating from the heart or the carotid bifurcation in the neck. This results in infarction of the nervous tissue supplied by that branch and typically a sudden loss of function; the clinical deficit will depend upon where the occlusion occurs. If there were an occlusion of one of the major blood vessels, it is possible that one of the major branches of the Circle would be large enough to provide sufficient blood to the area deprived. Usually this is not the case when there is a sudden occlusion (e.g., an embolus), but, given some time, these connecting channels may become large enough to prevent the death of the part of the brain deprived of blood. Middle cerebral a. Anterior comm. a. Anterior cerebral a. Internal carotid a. Middle cerebral a. Posterior comm. a. Posterior cerebral a. Basilar a. Superior cerebellar a. Posterior inferior cerebellar a. Vertebral a. Anterior spinal a. FIGURE 56: Blood Supply I — The Arterial Circle of Willis (Overlay) ©2000 CRC Press LLC FIGURE 57 BLOOD SUPPLY II MR ANGIOGRAM (MRA) Recent advances in technology have allowed for a visualization of the arterial system without injecting a radiopaque substance directly into an artery, a rather invasive procedure with some risk. The image obtained is called an MR angiogram (MRA). Although the quality of such images cannot match the detail seen after an angiogram of select blood vessels, the noninvasive nature of this procedure and the fact that the patient is not exposed to any risk clearly establishes this investigation as desirable to provide some information about the state of the cerebral vasculature. Figure 57 shows the arterial Circle of Willis as if looking at the brain from below (as in Figure 56). The carotid artery and its branches can be followed. The basilar artery is seen at its termination, as it divides into the posterior cerebral arteries. The communicating arteries — anterior and posterior — can also be seen. ©2000 CRC Press LLC Clinical Aspects One of the characteristic vascular lesions in the vicinity of the arterial circle of Willis is a Berry aneurysm. It is caused by a weakness of part of the artery wall, causing a local ballooning of the artery. Often these rupture spontaneously, particularly if there is accompanying hypertension. This sudden rupture occurs into the subarachnoid space and may also involve nervous tissue of the base of the brain. The whole event is known as a subarachnoid hemorrhage and must be considered when one is faced clinically with an acute major cerebrovascular accident, CVA, without trauma. An MRA will provide sufficient information if an aneurysm is suspected, or when a screening examination is necessary because of a positive family history of cerebral aneurysm. 1. Carotid siphon 2. Middle cerebral a. 3. Anterior cerebral a. 4. Anterior communicating a. 5. Posterior communicating a. 6. Basilar a. 7. Posterior cerebral a. FIGURE 57: Blood Supply II — MR Angiogram (MRA) ©2000 CRC Press LLC FIGURE 58 BLOOD SUPPLY III CORTICAL: DORSOLATERAL (OVERLAY) Figure 58 shows the blood supply to the dorsolateral aspect of the hemispheres; it has been created (with Photoshop) by superimposing blood vessels on this view of the brain (see Figure 12). After coursing through the depths of the lateral fissure (see Figure 56), the middle cerebral artery emerges and breaks into a number of branches that supply different parts of the dorsolateral cortex — the frontal, parietal, and temporal areas of cortex. Each branch supplies a different territory. The branches of the middle cerebral artery extend towards the midline sagittal fissure, where branches from the other cerebral vessels are found, coming from the medial aspect of the hemispheres (see Figure 59). A zone, the arterial borderzone region (a watershed area), remains between the various arterial territories. This area is poorly perfused and prone to infarction, particularly if there is a loss of blood pressure (e.g., with cardiac arrest or after a major hemorrhage). Clinical Aspects The most common clinical lesion involving these cerebral blood vessels is occlusion, often caused by an embolus originating from the heart or the carotid bifurcation in the neck. This results in infarction of the nervous tissue supplied by that branch; the clinical deficit will depend upon which branches are involved. For example, loss of sensory and/or motor function to ©2000 CRC Press LLC the arm and face region is seen after the blood vessel to the central region is occluded. The type of language loss depends upon the branch affected in the dominant hemisphere: a motor deficit with problems in expression is seen with a lesion affecting Broca’s area, whereas a comprehension deficit is found with a lesion affecting Wernicke’s area. Recent studies indicate that the core of tissue that has lost its blood supply is surrounded by a region that has a marginal blood supply — the penumbra, as it is called. In this penumbra, the blood supply is reduced below the level of nervous tissue functionality and the area is therefore “silent,” but the neurons are still viable and might be rescued! These studies have led to a rethinking of the therapy of strokes: • In the acute stage, if the patient can be seen quickly and investigated immediately, the site of the lesion might be identified. It is then possible for a neuroradiologist (with specialized training) to insert a catheter into the artery and to inject a drug that could dissolve the clot. If done soon enough after the “stroke,” it might be possible to avert any clinical deficit! • There may be an additional period beyond this initial timeframe when damaged neurons in the penumbra can be rescued through the use of neuroprotective agents, specific pharmacological agents that protect the neurons from the damaging consequences of loss of blood supply. Because strokes cause a loss of function and diminished quality of life, and since our population is aging, research on stroke and on how neurons die (e.g., by apoptosis), and how this process could be arrested is one of the most active areas of neuroscience research. FIGURE 58: Blood Supply III — Cortical: Dorsolateral (Overlay) ©2000 CRC Press LLC FIGURE 59 BLOOD SUPPLY IV CORTICAL: MEDIAL (OVERLAY) In Figure 59, the blood supply to the medial aspect of the hemispheres has been superimposed (using Photoshop) onto this view of the brain (see Figure 16). Two arteries supply this part: the anterior cerebral artery and the posterior cerebral artery. Anterior Cerebral Artery (ACA) The anterior cerebral artery (ACA) is a branch of the internal carotid artery (see Figures 56 and 57) from the Circle of Willis. It runs in the interhemispheric fissure, above the corpus callosum, and supplies the medial aspects of the frontal lobe and the parietal lobe; this includes the cortical areas responsible for sensory-motor function of the lower limb. Clinical Aspects The deficit most characteristic of an occlusion of the ACA is selective loss of function of the lower limb. Clinically, the control of micturition seems to be located on this medial area of the brain, perhaps in the supplementary motor area (see Figure 51), and symptoms related to bladder control may also occur. ©2000 CRC Press LLC Posterior Cerebral Artery (PCA) The posterior cerebral artery (PCA), a branch of the vertebro-basilar system, supplies the occipital lobe and the visual areas of the cortex (area 17). Clinical Aspects The clinical deficit found after occlusion of this blood vessel on one side is a loss of one-half of the visual field of both eyes — a contralateral homonymous hemianopsia. (Note to student: This is an opportune time to review the optic pathway and to review the visual field deficits that are found after a lesion in different parts of the system.) Both sets of arteries have branches that spill over to the dorsolateral surface. As noted in Figure 58, there is a gap between these and the territory supplied by the middle cerebral artery, known as the arterial borderzone region. The visual cortex is supplied by the posterior cerebral artery (from the vertebro-basilar system). Part of the occipital pole, with the representation of the macular area of vision, is supplied by the middle cerebral artery (from the internal carotid system, see Figure 58). In some fortunate cases, macular sparing is found after occlusion of the posterior cerebral artery, presumably because of the other blood supply to this area. ©2000 CRC Press LLC FIGURE 59: Blood Supply IV — Cortical: Medial (Overlay) FIGURE 60 BLOOD SUPPLY V INTERNAL CAPSULE AND BASAL GANGLIA In Figure 60, a coronal section of the brain (see Figures 29 and 78), the middle cerebral artery, is shown schematically traversing the area of the lenticular nucleus and the internal capsule. The artery begins as a branch of the Circle of Willis (see Figure 56; also Figure 57). It then emerges after passing through the lateral fissure to supply the dorsolateral cortex (see Figure 58). One of the most important sets of branches of the middle cerebral artery — within the lateral fissure — is the group of arteries that supply much of the internal structures of the hemispheres. These are known as the striate arteries, also called lenticulo-striate arteries (discussed with Figure 26; see also Figures 27 and 29). These small blood vessels are the major source of blood supply to the internal capsule and the adjacent portions of the basal ganglia (head of caudate nucleus and putamen), as well as the thalamus. Additional blood supply to these structures comes directly from small branches of the Circle of Willis (discussed with Figure 56) and from other blood vessels. Clinical Aspects These small caliber arteries are functionally different from the cortical (cerebral) vessels. Firstly, they are endarteries and do not anastomose. Secondly, they react to a chronic increase of blood pressure (hypertension) by a ©2000 CRC Press LLC necrosis of the muscular wall of the blood vessels, called fibrinoid necrosis. Two possibilities follow: • These blood vessels may occlude, causing small infarcts in the region of the internal capsule. As these small infarcts resolve, they leave small “holes” called lacunes (lakes), which can be visualized radiographically. Hence, they are known as lacunar infarcts, a common form of a stroke. The extent of the clinical deficit with this type of infarct depends upon its location and size in the internal capsule (see Figure 26). A relatively small lesion may cause major motor and/or sensory deficits on the contralateral side, resulting in a devastating incapacity of the person, with contralateral paralysis. (Note: The student should review the major ascending and descending tracts at this time and their course through the internal capsule.) • The other possibility is that these weakened blood vessels can rupture, leading to hemorrhage deep in the hemispheres. Although the blood supply to the white matter of the brain is significantly less (because of the lower metabolic demand), this nervous tissue is also dependent upon a continuous supply of oxygen and glucose. A loss of blood supply to the white matter will result in the loss of the axons (and myelin) and hence interruption of the transmission of information. This type of stroke may result in a more extensive clinical deficit, because the hemorrhage itself causes a loss of brain tissue, as well as a loss of the blood supply to areas distal to the site of the hemorrhage. Brain hemorrhage can be visualized by CT (computed tomography; reviewed with Figure 17). FIGURE 60: Blood Supply V — Internal Capsule and Basal Ganglia ©2000 CRC Press LLC FIGURE 61 THALAMUS NUCLEI : HISTOLOGICAL The thalamus is introduced initially in Section A (Orientation) with a schematic perspective (see Figures 9 and 10). As noted in that section, there are two ways of classifying the nuclei of the thalamus: functionally and topographically (review text with Figure 10). In the schematic diagram, the various nuclei of the thalamus are arranged in a somewhat orderly manner. In reality, however, sections taken at different planes through the thalamus will show different nuclei of varying size in a continuously changing configuration. In the present diagram, the thalamus is being shown from the histological perspective at three different planes of section. In the main diagram, the thalamus is opened in its middle to show some of the nuclei, as well as the internal medullary lamina with its intralaminar nuclei, including the important centromedian nucleus (see Figure 51). This view is at the level of the ventral lateral nucleus and the ventral posterolateral nucleus; a portion of the ventral posteromedial nucleus is also seen (all specific relay nuclei). The dorsomedial nucleus (an association nucleus) is also visible. Exterior to the main thalamus is the reticular nucleus, one of the nonspecific thalamic nuclei that is part of the ascending reticular activating system (discussed with Figure 40A). ©2000 CRC Press LLC Two other cuts have been made, one more anteriorly and the other posteriorly, showing the configuration of the different nuclei. The anterior cut includes the mammillary nucleus of the hypothalamus and the mammillothalamic tract that goes to the anterior nucleus of the thalamus; this is an association nucleus (actually a group of nuclei) which belongs to the limbic system. It is discussed in that section (see Figure 81A). In the posterior cut, the section goes through the lateral geniculate nucleus (the latter is a laminated nucleus; see also Figures 39A and 39B), the medial geniculate nucleus, and the pulvinar. In addition, the diagram shows the relationship of the thalamus with adjacent structures. Above the thalamus is the body of the lateral ventricle (see Figure 20B), with the choroid plexus intervening. At the lateral edge of the ventricle is the body of the caudate nucleus and the stria terminalis (see also Figure 36). Near the midline is the fornix, just below the corpus callosum (see Figures 77A and 80). Lateral to the thalamus is the internal capsule; the posterior limb is seen in the anterior cut (see Figures 26 and 27) with the lentiform nucleus and the so-called inferior limb, which is actually the auditory radiation (see Figure 36) in the posterior cut. Both cuts include also the temporal lobe, with the inferior horn of the lateral ventricle and the tail of the caudate nucleus in its upper aspect. Protruding into the ventricle is the hippocampus proper and the dentate gyrus (reviewed with the limbic system in Section D). Stria terminalis Anterior n. Caudate nucleus (body) Choroid plexus Lateral ventricle (body) Stria terminalis Septum pellucidum Reticular n. Fornix Lentiform nucleus Corpus callosum Internal capsule (posterior limb) Mammillo-thalamic tract Ventral lateral n. Fornix Cistern Third ventricle Pulvinar Mammillary n. Ventral anterior n. Medial geniculate n. Lateral geniculate n. Internal medullary lamina Lateral dorsal n. Caudate nucleus (tail) Internal capsule (inferior limb) Dorsomedial n. Hippocampus proper Ventral lateral n. Lateral ventricle (inferior horn) Reticular n. Ventral anterior n. Intralaminar nuclei Pulvinar Ventral posterolateral n. Ventral posteromedial n. Centromedian n. Medial geniculate body Lateral geniculate body FIGURE 61: Thalamus — Nuclei: Histological ©2000 CRC Press LLC FIGURE 62 BRAINSTEM: HISTOLOGY B5 — CN VI, VII, and part of VIII, the lowermost pons • three through the medulla B6 — CN VIII (some parts), the uppermost medulla VENTRAL VIEW: SCHEMATIC Figure 62 uses the diagrammatic of the brainstem (see Figure 3) which is similar to the photographic view of the brainstem (see Figure 4). Study of the brainstem is continued here by examining its histological neuroanatomy through a series of cross sections. Since it is well beyond the scope of the nonspecialist to know all the details, salient points have been selected: • cranial nerve nuclei, • ascending and descending tracts, • specific brainstem nuclei that belong to the reticular formation, • and other select special nuclei. As has been indicated, comprehending the attachment of the cranial nerves to the brainstem is one of the keys to understanding this part of the brain (see Figures 5 and 6). Wherever one sees a cranial nerve attached to the brainstem, one knows that its nucleus, or some of its nuclei, will be located at that level. Therefore, if one visually recalls or memorizes the attachment of the cranial nerves to the brainstem, one has a key to its understanding. Conversely, in the clinical setting, knowledge of the cranial nerve(s) involved is the main clue for localizing a lesion in the brainstem. Since the focus here is on the cranial nerves, only a limited number of cross sections are studied. This diagram shows the ventral view of the brainstem, with the attached cranial nerves, and indicates the sections that will be depicted in the subsequent series. The letter “B” refers to the brainstem level. Eight cross sections are taken at the following levels: • two through the midbrain B1 — CN III, upper midbrain B2 — CN IV, lower midbrain • three through the pons B3 — uppermost pons (level for a special nucleus; there is no cranial nerve attachment at this level) B4 — CN V (through the principal and motor nuclei), mid-pons ©2000 CRC Press LLC B7 — CN IX, X, and XII, the mid-medullary level B8 — lowermost medulla, with some special nuclei. The information presented in this series should be sufficient for a student to recognize the clinical signs that would accompany a lesion at a particular level, espcially as they involve the cranial nerves. Such lesions would interrupt ascending and/or descending tracts, and this information would assist in localizing the lesion. Note: The student using this Atlas should note the following points: 1. A small figurine of this view of the brainstem will be shown with each cross-sectional level, with the plane of section indicated. 2. Reference to these cross sections is by either the figure number or the cross-sectional level (e.g., B2). 3. These cross-sectional levels are the ones shown alongside the pathways in Section B – Functional Systems. Blood Supply The vertebro-basilar system supplies the brainstem in the following pattern (see Figures 13 and 56). Penetrating branches from the basilar artery supply nuclei and tracts which are adjacent to the midline. These are known as the paramedian branches. The lateral aspects of the brainstem, both tracts and nuclei, are supplied by one of the cerebellar circumferential arteries (posterior inferior, anterior inferior, superior). Specific lesions are discussed with the cross-sectional levels. B1 B2 B3 B4 B5 B6 B7 B8 FIGURE 62: Brainstem: Histology — Ventral View: Schematic ©2000 CRC Press LLC FIGURE 63 BRAINSTEM: HISTOLOGY SAGITTAL VIEW: SCHEMATIC Figure 63 is a schematic of the brainstem from a midsagittal view (see Figure 16 for a photograph of the brain from this view). This view is presented because it is one that is commonly used to portray the brainstem. It should be correlated with the ventral view shown in Figure 62. This schematic is also shown in each of the cross section diagrams, with the exact level indicated, in order to orient the student to the plane of section through the brainstem. The location of some nuclei of the brainstem can be easily visualized using this sagittal view, including the red nucleus in the midbrain, the pontine nuclei which form the bulging “pot-belly” of the pons, and the inferior olivary nucleus of the medulla. Some of the cranial nerve attachments are shown as well, but not labeled. other nuclei, including the red nucleus and the inferior olive, as well as the remaining tracts. • The ventricular system (which has been represented by stippling) is found throughout the brainstem (see Figures 20A and 20B). Sections can be oriented according to the parts of the ventricular system that pass through this region, namely the aqueduct in the midbrain region and the fourth ventricle (note shape) lower down. • The tectum — with the four colliculi — is located behind (dorsal to) the aqueduct of the midbrain; likewise, the fourth ventricle separates the pons and medulla from the cerebellum. The upper part of the roof of the fourth ventricle is called the superior medullary velum (see Figure 7). The location of the choroid plexus in the inferior aspect of the roof of the fourth ventricle is also shown (see also Figure 20A). Note: The brainstem description starts from the midbrain and goes downwards to the medulla for two reasons: Using this orientation, one can approach the description of the brainstem cross sections systematically: 1. This order follows the numbering of the cranial nerves, from above downwards. • The most anterior portion of each area of the brainstem contains some representation of the descending cortical fibers, specifically the cortico-bulbar, corticopontine, and cortico-spinal pathways (see Figures 42 and 43). In the midbrain, the cerebral peduncles include all these axon systems. The cortico-bulbar fibers are distributed to the various brainstem and cranial nerve nuclei. In the pons, the cortico-pontine fibers terminate in the pontine nuclei, which form the bulge known as the pons proper; the cortico-spinal fibers are dispersed amongst the pontine nuclei. In the medulla, the cortico-spinal fibers form the pyramids. The medulla ends at the point where these fibers decussate (see Figure 4). 2. This sequence has been described for the fibers descending from the cortex. • The central portion of the brainstem is the tegmentum. The reticular formation occupies the core region of the tegmentum (see Figures 40A and 40B). This area contains virtually all the cranial nerve nuclei and ©2000 CRC Press LLC Others may prefer to start the description of the cross sections from the medulla upwards. Note to the student: The various nuclei of the brainstem have been shaded differently, so that sensory and motor nuclei can be distinguished. Cerebellar-related and vestibular-related nuclei and tracts have been assigned other shadings. This visual cataloging is maintained uniformly throughout the brainstem cross sections. The numbers beside the various nuclei are there as guides to color-coding; those students who wish to color the various nuclei should assign a different color to each (as suggested in the COLOR CODE, found on p. xviii ). The nuclei are shown in color on the accompanying CD-ROM. B1 B2 B3 B4 Choroid plexus B5 B6 Foramen of Magendie B7 B8 R = Red nucleus P = Pontine nuclei IO = Inferior olivary n. FIGURE 63: Brainstem: Histology — Sagittal View: Schematic ©2000 CRC Press LLC THE MIDBRAIN FIGURES 64 AND 65 The midbrain is the smallest of the three parts of the brainstem. Often, it is not actually seen on an inferior view of the brain because the temporal lobes of the hemispheres tend to obscure its presence (see photograph of the inferior view of the brain, Figure 13). The midbrain area is easily recognizable from the anterior view in a dissected specimen of the brainstem (see photograph in Figure 4). Most anteriorly is the massive cerebral peduncles. The peduncles contain axons that are a direct continuation of the fiber systems of the internal capsule (see Figure 26). Within them are found the pathways descending from the cerebral cortex to the brainstem (cortico-bulbar), the cerebellum via the pons (cortico-pontine), and the spinal cord (corticospinal tracts) (see also Figures 42 and 43). When viewing a mid-sagittal section of the brain and brainstem (Figure 16), one can easily identify the midbrain area as the part containing the cerebral aqueduct. Posterior to the aqueduct are the two pair of colliculi, which can also be seen on the dorsal view of the isolated brainstem (Figure 7). The four nuclei together form the tectal plate, or tectum. The superior colliculus is a subcortical center for certain visual reflexes. These nuclei give rise to a fiber tract — the tecto-spinal tract — that descends to the cervical spinal cord as part of the medial longitudinal fasciculus (see Figure 49B). The system is involved in the coordination of the movements of the eyes with those of the head and neck, both of these responding to the visual image. The inferior colliculus is a relay nucleus in the auditory pathway and is discussed with this system (see Figures 35 and 36). There are two special nuclei in the midbrain region — the substantia nigra and the red nucleus, as well as the superior colliculus and the pretectal region. The substantia nigra is found throughout the midbrain and is located behind the cerebral peduncles (see also Figure 50). It derives its name from the dark melaninlike pigment found within its neurons in a freshly dissected specimen (see Figure 14; the nucleus has been ©2000 CRC Press LLC color-coded black). This nucleus is important in the regulation of movements. The substantia nigra is functionally part of the basal ganglia, with which it has interconnections. In fact, it consists of two parts: the pars compacta and the pars reticulata. The neurons of the pars compacta produce dopamine, a distinct neurotransmitter. Loss of these neurons results in the clinical entity, Parkinson’s disease (discussed with Figure 50). The pars reticulata is one of the output nuclei of the basal ganglia to the thalamus (the other portion is the internal segment of the globus pallidus). It is important to realize that this nuclear area, despite its name, is clear (white) in most photographs in atlases because the pigment is not retained when the tissue is processed for sectioning. With myelin-type stains, the area will appear “empty”; with cell stains, the neurons will be visible. The pigment is not present in all species. The red nucleus is located in the tegmentum, the inner region of the brainstem. The label “red” is derived from the fact that this nucleus has a reddish color in a freshly dissected specimen, presumably due to its marked vascularity. (In the figure, this nucleus has been color-coded red.) The red nucleus is found at the superior colliculus level. It also gives rise to a fiber tract that descends to the spinal cord, the rubro-spinal tract (see Figure 44 ). The functional role of the red nucleus in humans is not clearly known. The substantia nigra, the red nucleus, and the superior colliculus are all involved with more integrative aspects of motor control. The pretectal region is located in front of and somewhat above the superior colliculus. This region is the center for the reflex response of the pupil to light, the pupillary light reflex (discussed with Figure 39B). The reflex requires input from receptors in the retina, and the output occurs via the parasympathetic neurons in the EdingerWestphal nucleus (see Figure 5) and the oculomotor nerve (CN III), with a synapse in the ciliary ganglion in the orbit. Light shone on one eye will normally lead to a rapid constriction of the pupil on the same side — the direct response, and a similar response on the other side — the consensual response. The connection of the responses of the two eyes is via commissural fibers (in the posterior commissure, see Figure 49B). The pupillary light reflex is one of the most important reflexes to test clinically, particularly in head-injured and comatose patients, so knowledge of this pathway and the areas involved is essential. The reticular formation is found in the core area of the tegmentum. The reticular formation of the midbrain is particularly important for the maintenance of consciousness. A rather special part of the reticular formation at this level is the periaqueductal gray surrounding the aqueduct. This region forms part of the descending control system for pain modulation (see Figure 41). Some lesions might destroy much of the brainstem yet ©2000 CRC Press LLC leave the midbrain intact (e.g., a thrombosis of the basilar artery), which might allow the patient to survive in a rather tragic state known as locked-in syndrome. Usually, all voluntary movements are gone, except for some vertical eye movements; usually there is no loss of sensation. The patient is left in a state of consciousness with intellectual functions generally intact. The midbrain is examined at two levels: 1. the upper (rostral) one passes through the third nerve nucleus and the superior colliculus; 2. the lower (caudal) one is at the level of the IVth nerve nucleus and the inferior colliculus, and the decussation of the superior cerebellar peduncles. FIGURE 64 UPPER MIDBRAIN CROSS SECTION (B1) In a cross-section of the midbrain, the most ventral (anterior) structure is the cerebral peduncle. Posterior to it is the substantia nigra. The superior colliculus is located dorsally, behind the aqueduct. The region surrounding the aqueduct of the midbrain (the aqueduct of Sylvius) is the periaqueductal gray. The remainder of the midbrain is the tegmentum, with nuclei and tracts. Within the cerebral peduncles, the fiber systems are segregated: the cortico-bulbar and particularly the corticospinal pathways occupy its middle one-third area; the outer and inner portions carry the cortico-pontine fibers (see Figures 26, 42, 43, and 45). The substantia nigra has two parts which are functionally quite distinct: the pars compacta and pars reticulata. The pars reticulata lies adjacent to the cerebral peduncle and contains some widely dispersed neurons; these neurons connect the basal ganglia to the thalamus as one of the output nuclei of the basal ganglia (similar to the globus pallidus). The pars compacta is a cell-rich region, located more dorsally, whose neurons contain the melanin-like pigment (see Figure 14). These are the dopaminergic neurons which project to the neostriatum (discussed with Figure 50). The oculomotor nucleus (CN III) is quite large and occupies the region in front of the periaqueductal gray, near the midline; this is the typical location for all nuclei that are efferent to somatic muscles. These motor neurons are quite large in size and easily recognizable. The parasympathetic portion of this nucleus is incorporated within it and is known as the Edinger-Westphal (EW) nucleus. The fibers of CN III exit anteriorly between the cerebral peduncles, in the interpeduncular fossa. The red nucleus is located within the tegmentum. With a cell-type stain, one can discern the outline of the nucleus; large neurons are typical of the ventral part of the nucleus. With a section that has been stained for myelin, the nucleus is seen as a clear zone. The fibers of CN III exit through the medial portion of this nucleus. ©2000 CRC Press LLC The red nucleus gives origin to a descending pathway, the rubro-spinal tract (see Figures 44 and 45) which is involved in motor control. This pathway is not thought to play as important a role in the overall functioning of the motor system in humans, compared to other mammals and higher apes. The superior colliculus gives rise to a descending pathway that is involved in the control of eye and neck movements. Functionally these can be considered part of the medial longitudinal fasciculus — the MLF (discussed with Figure 49B). In fact, this descending pathway travels with the MLF throughout the brainstem and upper spinal cord. The MLF stains heavily with a myelin-type stain and is found anterior to the somatic motor nucleus, next to the midline, at this level as well as the other levels of the brainstem. The ascending (sensory) tracts present in the midbrain are a continuation of those present throughout the brainstem. The medial lemniscus, the ascending trigeminal pathways, and the fibers of the anterolateral system incorporated with them are on their way to the thalamus (see Figure 34). These ascending pathways are located in the outer part of the tegmentum, on their way to the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei of the thalamus. Also to be noted at this level is the brachium of the inferior colliculus, a part of the auditory pathway. This fiber bundle connects the inferior colliculus to the medial geniculate nucleus of the thalamus. It is situated close to the surface on the dorsal aspect (see Figures 7 and 36). The nuclei of the reticular formation are found in the central region of the brainstem (the tegmentum); they are functionally part of the ascending reticular activating system and play a significant role in consciousness (discussed also with Figure 40A). The periaqueductal gray surrounding the cerebral aqueduct is involved with pain and also with a descending pathway for the modulation of pain (see Figure 41). FIGURE 64: Upper Midbrain — Cross Section (B1) ©2000 CRC Press LLC FIGURE 65 LOWER MIDBRAIN CROSS SECTION (B2) The cerebral peduncles are still located anteriorly. Often, the section is done at the level of the lowermost midbrain and includes the beginning of the pontine nuclei. Therefore, one may see a somewhat confusing mixture of structures. The substantia nigra is located immediately behind the fibers of the cerebral peduncle. The unique feature in the lower midbrain is the decussation (crossing) of the superior cerebellar peduncles. Dorsal to the cerebral aqueduct is the inferior colliculus. The nucleus of CN IV, the trochlear nucleus, is located in front of the periaqueductal gray, next to the midline. Because it supplies only one extra-ocular muscle, it is a smaller nucleus than the oculomotor. CN IV heads dorsally and exits from the brainstem below the inferior colliculus (see Figure 7), on the posterior aspect of the brainstem. The MLF lies just anterior to the trochlear nucleus. The medial lemniscus, the trigeminal fibers, and the anterolateral fibers (system) are situated at the lateral edge of the tegmentum (see Figures 34 and 38). In fact, they are found at this level at the surface of the midbrain. In very select cases, particularly with cancer patients who are suffering from intractable pain, it is possible to surgically sever the sensory ascending pathways at this level. Obviously, this is a rather dangerous and difficult neurosurgical procedure; today it would be considered only as a measure of last resort. Pain control is usually accomplished through the use of drugs, often in a pain clinic. However, infarcts of this area would interrupt all the ascending sensory fibers from the head and body of the opposite side. ©2000 CRC Press LLC In sections through the lower levels of the midbrain, there is a brief appearance of a massive fiber system (as seen with a myelin-type stain) occupying the central region of the midbrain, the decussation of the superior cerebellar peduncles. These fibers are the continuation of the superior cerebellar peduncles found in the pons, which are crossing (decussating) at this level. The fibers are coming from the deep cerebellar nuclei (the intracerebellar nuclei), mainly the dentate nucleus, and are headed for the ventral lateral (VL) nucleus of the thalamus, and then to the motor cortex (discussed with Figures 38 and 55 ). Some of the fibers that come from the intermediate nucleus synapse in the red nucleus. The nuclei of the reticular formation of this level are in the central region of the brainstem (the tegmentum); they are functionally part of the ascending reticular activating system and play a significant role in consciousness. The periaqueductal gray surrounding the cerebral aqueduct is involved with pain and also with a descending pathway for the modulation of pain (see Figure 41). Some unusually large round cells are often seen at the edges of the periaqueductal gray; these cells are part of the mesencephalic nucleus of the trigeminal (CN V) nerve (see Figure 6). Between the cerebral peduncles is a small nucleus, the interpeduncular nucleus, which belongs with the limbic system. The inferior colliculus is a relay nucleus in the auditory pathway (see Figures 35 and 36). The ascending auditory fibers, the lateral lemniscus, are still present at this level and are often seen terminating in this nucleus. After synapsing here, the fibers relay to the medial geniculate nucleus via the brachium of the inferior colliculus, seen at the upper midbrain level (Figure 64). FIGURE 65: Lower Midbrain — Cross Section (B2) ©2000 CRC Press LLC THE PONS FIGURES 66, 67, AND 68 The pons, medulla, and cerebellum form the so-called hindbrain. These structures are located in the posterior cranial fossa of the skull, along with the fourth ventricle. This ventricle separates the pons and medulla anteriorly from the cerebellum posteriorly (see Figures 16 and 20A). The pons is characterized by its protruding anterior portion, the pons proper. This consists of a large group of nuclei, the pontine nuclei. The cortico-pontine fibers, which have descended through the cerebral peduncles, terminate in these nuclei. From here, they are relayed to the cerebellum (see Figure 53) via the middle cerebellar peduncle. The pons (proper) forms a bridge-like structure between these two prominent middle cerebellar peduncles. Also found intermingled with the pontine nuclei are the fiber bundles that belong to the cortico-spinal system. These continue through this region (without synapsing) and emerge at the medullary level to form the pyramids (see Figure 42). Behind the pons proper is the tegmentum, the region of the brainstem that contains the cranial nerve nuclei, most of the ascending and descending tracts, and the nuclei of the reticular formation. The cranial nerves attached to the pons include the trigeminal (CN V), the abducens (CN VI), the facial (CN VII), and part of CN VIII (the vestibulo-cochlear). The various nuclei of these cranial nerves are located within the pontine tegmentum. CN V: Parts of the trigeminal nerve nuclei are found at all levels of the pons (see Figures 6 and 33). The mid-pontine section is taken at the level of the attachment of CN V, and both the principal sensory nucleus and the motor nucleus are found at this cross-sectional level. CN VI: CN VI is a typical somatic motor nucleus, innervating the lateral rectus muscle of the eye. The nucleus is located in the lowermost pons and its fibers exit anteriorly and at a slightly lower level, at the junction between the pons and medulla (see Figures 4 and 5). ©2000 CRC Press LLC CN VII: CN VII has a most unusual course within the brainstem. The fibers of VII form an internal loop over the abducens nucleus (see Figure 45; also note the facial colliculus in Figure 7); they ascend, loop, and then descend to exit laterally at the junction between the pons and the cerebellum, the cerebello-pontine angle. CN VIII: The fibers of the cochlear and vestibular divisions of CN VIII enter the brainstem adjacent to CN VII, at the cerebello-pontine angle (see Figure 3). • Cochlear portion — The auditory fibers synapse in the dorsal and ventral cochlear nuclei, which can be seen in the medulla in a section just below this level (review with the auditory system, Figures 35 and 36). After this, there will be a synapse in the nuclear group called the superior olivary complex, which is found in the lowermost pons. Some of the fibers cross the midline before synapsing, and some cross after synapsing. The crossing fibers form a structure known as the trapezoid body. After one or more synapses, the fibers then ascend and, in so doing, form a new tract, the lateral lemniscus which actually commences at this level. • Vestibular portion — The vestibular nuclei are found in the lowermost pontine region and at the upper levels of the medulla (see Figures 6 and 49A). The lateral vestibular nucleus gives rise to the lateral vestibulo-spinal tract (see Figure 48). The medial and superior vestibular nuclei contribute fibers to the medial longitudinal fasciculus (MLF, discussed with Figure 49B), relating the vestibular sensory information to eye movements. A not uncommon tumor, called an acoustic neuroma, can occur along the course of the acoustic nerve, usually at the cerebello-pontine angle. This is a slow-growing benign tumor, composed of Schwann cells, the cell responsible for myelin in the peripheral nervous system. Initially, there will be a complaint of hearing loss, and/or perhaps a ringing noise in the ear (called tinnitus). Because of its location, as the tumor grows it begins to compress the adjacent nerves (including VII). Eventually, if left unattended, there are additional symptoms due to further compression of the brainstem. Detection of this tumor has been made much easier with modern imaging techniques. Surgical removal, though, still requires considerable skill so as not to damage adjacent structures, including CN VII. The ascending tracts present in the tegmentum are those conveying sensory information from the body and face. These include the medial lemniscus and the anterolateral fibers (system). The medial lemniscus shifts its position in its course through the brainstem (see Figure 38), moving from a central to a lateral position. The fibers of the trigeminal system that have crossed in the pons (discriminative touch from the principal nucleus of V) and those of pain and temperature (from the descending nucleus of V) that crossed in the medulla join together in the upper pons with the medial lemniscus. The anterolateral system, which is too small to be identified, also eventually becomes incorporated with the medial lemniscus (see Figure 38) in the mid and upper pons. The lateral lemniscus (auditory) also is located in the tegmentum. One of the distinctive nuclei of the pons is the locus ceruleus, a pigment-containing nucleus located in the upper pontine region and discussed with Figure 66. The nuclei of the reticular formation of the pons have their typical location in the tegmentum (see Figures 40A and 40B). These nuclei play a particular role in the ©2000 CRC Press LLC voluntary control of movements of the proximal joints and axial musculature, as well as the regulation of muscle tone (discussed with Figures 46 and 47). The fourth ventricle begins in the pontine region (see Figure 20A). It starts as a widening of the aqueduct then continues to enlarge so that it is widest at about the level of the junction between the pons and medulla. There is no pontine nucleus dorsal to the fourth ventricle. The cerebellum is located above (posterior to) the roof of the ventricle. It is at the level of the mid- and lower pontine cross sections that one finds the deep cerebellar nuclei (presented in Figures 54A and 54B). The pons is represented and discussed in three sections: • Uppermost pons — This has been taken at the level of a distinctive nucleus, the locus ceruleus. There are features here that are important in making the transition between the pons and the midbrain. • Mid (middle) pons — This is at the level of the attachment of the trigeminal nerve. It includes the massive middle cerebellar peduncles. • Lowermost pons — This cross section is taken just above the junction with the medulla. This lowermost level is one of the most complex sections of the brainstem, because it has the nuclei of cranial nerves VI, VII, and parts of both divisions of CN VIII. FIGURE 66 UPPER PONS CROSS SECTION (B3) The upper pons is presented mainly to allow an understanding of the transition of midbrain to pons. This particular section is taken at the uppermost pontine level, where the trochlear nerve, CN IV, exits (below the inferior colliculus; see Figure 7). It is the only cranial nerve that exits posteriorly; its fibers cross (decussate) before exiting. Anteriorly, the pontine nuclei are beginning to be found. Cortico-pontine fibers terminate in the pontine nuclei. From these cells, a new tract is formed which crosses and projects to the cerebellum, the middle cerebellar peduncle. The cortico-spinal fibers become dispersed between these nuclei and course in bundles between them (without synapsing). Centrally, the cerebral aqueduct is beginning to enlarge, becoming, by definition, the fourth ventricle. The MLF is found in its typical location ventral to the fourth ventricle, next to the midline. Nuclei of the reticular formation are present as they are throughout the brainstem. The ascending tracts include the lateral lemniscus (auditory), the medial lemniscus and anterolateral system (somatosensory from the body), and the ascending trigeminal fibers (see Figure 34). The auditory fibers are located dorsally, just before terminating in the inferior colliculus (in the lower midbrain, which is just above this level). The medial lemniscus, with the inclusion of fibers of the anterolateral system, as well as most of the ascending trigeminal fibers, is located midway between its more ©2000 CRC Press LLC central position inferiorly and the lateral position found in the midbrain (see Figure 38). In sections stained for myelin, it has a typical “comma-shaped” configuration. There is a rather special nucleus, the locus ceruleus, located at this level. The nucleus derives its name from its bluish color in fresh specimens. (It has therefore been color-coded blue.) The locus ceruleus is considered part of the reticular formation (as discussed with Figure 40B). This nucleus is unique because of its widespread connections with virtually all parts of the brain and because it has noradrenaline as its catecholamine neurotransmitter substance. The nucleus is located in the dorsal part of the tegmentum, not too far from the edges of the fourth ventricle. Nearby are some of the very large neurons belonging to the mesencephalic nucleus of the trigeminal (see Figure 6). This small cluster of cells might not be found in every cross section of this particular region. The superior cerebellar peduncle is found within the tegmentum of the pons. These fibers carry information from the cerebellum to the red nucleus and the thalamus. The fibers, which are the axons from the deep cerebellar nuclei, leave the cerebellum and course in the roof of the fourth ventricle (superior medullary velum; see Figures 7, 38, and 55). They then enter the pontine region and move towards the midline, finally decussating in the lower midbrain (see cross section B2, Figure 65). The uppermost part of the cerebellum is found at this level. One of the parts of the vermis, the midline portion of the cerebellum, is identified — the lingula. This particular lobule is a useful landmark in the study of the cerebellum and is identified in the discussion of anatomy of the cerebellum in Figure 52. FIGURE 66: Upper Pons — Cross Section (B3) ©2000 CRC Press LLC FIGURE 67 MID PONS CROSS SECTION (B4) The cross section presented in Figure 67 is taken through the level of the attachment of the trigeminal nerve. Anteriorly, the pontine nuclei and the bundles of cortico-spinal fibers are easily recognized. The pontine cells (nuclei), whose axons cross and then become the middle cerebellar peduncle, are particularly numerous at this level. The cortico-spinal fibers are also very dispersed at this level. The trigeminal nerve is attached along the course of the middle cerebellar peduncle. The CN V nerve has several nuclei with different functions (see Figures 5, 6, and 33). This level contains only two of its four nuclei: the principal (or main) sensory nucleus and the motor nucleus. The principal sensory nucleus subserves discriminative (e.g., two-point) touch sensation and accounts for the majority of fibers. The motor nucleus supplies the muscles of mastication and sometimes exits as a separate nerve, alongside the major sensory root. Within the pons, these nuclei are separated by the fibers of CN V; the sensory nucleus (with smaller cells) is found more laterally, and the motor nucleus (with larger cells) more medially. The ascending fiber systems are easily located at this cross-sectional level. The medial lemniscus has moved away from the midline, as it ascends (see Figure 38). ©2000 CRC Press LLC The anterolateral fiber system becomes associated with it by this level. The lateral lemniscus is seen as a distinct tract, lying just lateral to the medial lemniscus. The MLF is found in its typical location anterior to the fourth ventricle. The core area of the tegmentum is occupied by the nuclei of the reticular formation. Some of the nuclei here are called the oral portion of the pontine reticular formation (see Figure 46). This “nucleus” contributes fibers to a descending reticulo-spinal tract that is involved in motor control and plays a major role in the regulation of muscle tone (discussed with Figures 46 and 47). The fourth ventricle is quite wide at this level. At its edges are found the superior cerebellar peduncles, exiting from the cerebellum and heading towards the midbrain (red nucleus) and thalamus. The thin sheet of white matter that connects these peduncles is called the superior medullary velum. These peduncles and the superior medullary velum can be located in a specimen (such as the one shown in Figure 7), a dorsal view of the isolated brainstem. These structures are found dorsally just below the exiting fibers of CN IV. The cerebellum, which is quite large at this level, is situated behind the ventricle. The lingula of the cerebellum is again labeled and is seen sometimes actually intruding into the ventricular space. FIGURE 67: Mid Pons — Cross Section (B4) ©2000 CRC Press LLC FIGURE 68 LOWER PONS CROSS SECTION (B5) This cross section of the lowermost pons is very complex because of the number of nuclei related to the cranial nerves located in the tegmental portion, including CN V, VI, VII, and VIII. In this section some of the tracts are shifting in position or forming. Anteriorly, the pontine nuclei have all but disappeared, and the fibers of the cortico-spinal tract are regrouping into a more compact bundle that will become the pyramids in the medulla (below). Cranial Nerve V The fibers of the trigeminal nerve carrying pain and temperature, which entered at the mid-pontine level, descend into the lower pons and continue through the medulla into the upper level of the spinal cord (see Figure 33). This pathway is the descending trigeminal tract, also called the spinal tract of V because it reaches to the level of the spinal cord. Cranial Nerve VI The abducens nucleus is a somatic motor nucleus to the lateral rectus muscle of the eye. It is located (as expected) in front of the ventricular system. The MLF, also as usual, is found just anteriorly. Some of the exiting fibers of CN VI may be seen at this level. The nerve emerges anteriorly at the junction of pons and medulla (see Figures 4 and 5). Cranial Nerve VII The facial nerve nucleus is located in the ventrolateral portion of the tegmentum, where the branchiomotor nucleus is supposed to be located. The motor cells (to the muscles of facial expression) are large. (The parasympathetic portion of this nucleus is rarely identifiable.) As explained previously, the fibers of CN VII form an internal loop (see Figure 45). It is common to see only parts of the course of this nerve on any one section through this level of the pons, and one must know the course of the nerve to be able to identify it. The diagram is drawn as if the whole course of this nerve were present in a single section. Cranial Nerve VIII: Cochlear Division CN VIII enters the brainstem slightly lower, at the ©2000 CRC Press LLC ponto-cerebellar angle (see Figures 3 and 4). The two distinctive parts of this nerve at this level are the crossing fibers, which form the so-called trapezoid body, and the superior olivary complex. The superior olivary complex includes a group of nuclei that subserve the function of sound localization; they also give rise to a unique bundle of fibers, the olivo-cochlear bundle, which goes from the CNS to the outer hair cells of the cochlea (discussed with Figure 35). Cranial Nerve VIII: Vestibular Division Of the four vestibular nuclei (see Figures 6 and 49A), three are found at this level. The lateral vestibular nucleus, with its giant-sized cells, is located at the lateral edge of the fourth ventricle. The large neurons are dispersed through the nuclear area. This nucleus gives rise to the lateral vestibulo-spinal tract (see Figure 48). The medial vestibular nucleus is also present at this level, an extension from the medullary region. There is also a small superior vestibular nucleus in this region. The latter two nuclei contribute fibers to the MLF, relating the vestibular sensory information to eye movements (discussed with Figure 49B). The tegmentum of the pons also includes the ascending sensory tracts and the reticular formation. The medial lemniscus is often somewhat obscured by the fibers of the trapezoid body. It is situated close to the midline but here has changed its orientation from that seen in the medullary region (see Figure 38 and cross sections of the medulla). The anterolateral system is too small to be identified. The nuclei of the reticular formation include the caudal portion of the pontine reticular formation which also contributes to the pontine reticulo-spinal tract (see Figure 46). The fourth ventricle is very large but often seems smaller because the lobule of the cerebellar vermis, called the nodulus (part of the flocculonodular lobe; refer to Figure 52), impinges upon its space. The intracerebellar (deep cerebellar) nuclei are also found at this cross-sectional level. They are located within the white matter of the cerebellum (discussed with Figures 54A and 54B). Usually only the most lateral and largest nucleus, the dentate, can be identified. FIGURE 68: Lower Pons — Cross Section (B5) ©2000 CRC Press LLC THE MEDULLA FIGURES 69, 70, AND 71 This part of the brainstem has a different appearance from the midbrain and pons because of the presence of two new distinct structures — the pyramids and the inferior olivary nucleus: • The pyramids are an elevated pair of structures located on either side of the midline (see Figures 3 and 4). They contain the cortico-spinal fibers that have descended from the motor (and sensory) areas of the cortex, funneled via the internal capsule (posterior limb), and then continued through the cerebral peduncles of the midbrain and the pontine region, and now emerge as a distinct bundle (see Figure 42). The cortico-spinal tract is in fact often called the pyramidal tract because its fibers form the pyramids. Most of its fibers cross (decussate) at the lowermost part of the medulla (see Figure 4). • The inferior olive is a prominent nuclear structure that has a distinct scalloped profile when seen in cross section. It is so large that it forms a prominent bulge on the lateral surface of the medulla. Its fibers are distributed to the cerebellum (discussed with Figure 53). The tegmentum is the area of the medulla that contains the inferior olivary nucleus, the cranial nerve nuclei, and the nuclei of the reticular formation. Cranial nerves VIII, IX, X (including cranial part of XI), and XII are attached to the medulla and have their nuclei here. The most prominent nucleus of the reticular formation in this region has very large cells and is called the nucleus gigantocellularis (see Figure 40B). Also included in the tegmentum are the various tracts. The ascending fibers include the large bundle, the medial lemniscus, carrying discriminative touch sensation, joint position, and the “sense” of vibration from the body (see Figure 32). This tract is formed in the lowermost medulla from the dorsal column nuclei (cuneate and gracile; see Figure 38), and the fibers cross and ascend. The anterolateral system, a smaller tract carrying pain and temperature information from the body, is not usually identifiable in the cross sections, but knowledge of its location is important for vascular lesions that occur at this level. The spinal trigeminal tract (and ©2000 CRC Press LLC nucleus), conveying the modalities of pain and temperature from the face and teeth, is also found throughout the medulla (see Figure 33). One of the most important sensory systems in the medulla is the solitary nucleus and tract, which subserves both taste and visceral afferents (discussed with Figure 6). The MLF is still a distinct tract in its usual location (see Figure 49B). The fourth ventricle lies behind the tegmentum, separating the medulla from the cerebellum. The ventricle tapers and becomes quite narrow in the lowest part of the medulla (see Figures 20A and 20B); eventually it is continuous with the central canal of the spinal cord. The roof of this lower part of the ventricle has choroid plexus (see Figure 63). CSF escapes from the fourth ventricle via the various foramina located here, and flows into the subarachnoid space (discussed with Figure 21). The medulla is represented by three sections: • the uppermost section typically includes the CN VIII nerve (both parts) and its nuclei; • the section through the middle of the medulla is at the mid-olivary level and includes the nuclei of cranial nerves IX, X, and XII; • the lowermost section is at the level of the dorsal column nuclei, the nuclei gracilis and cuneatus, and the decussating fibers of the medial lemniscus. Clinical Aspects Vascular lesions in this area of the brainstem are not uncommon. The midline area is supplied by the paramedian branches from the vertebral artery (see Figure 56). The structures included in this territory are the corticospinal fibers, the medial lemniscus, and the hypoglossal nerve and its nucleus. The lateral portion is supplied by the posterior inferior cerebellar artery, a branch of the vertebral artery (see Figure 56), often called PICA by the neuroradiologists. This artery is apparently quite prone to infarction, for some unknown reason. Included in its territory are the cranial nerve nuclei and fibers of IX and X, the descending trigeminal nucleus and tract, fibers of the anterolateral system, and the solitary nucleus and tract, as well as descending autonomic fibers. The whole clinical picture is called the lateral medullary syndrome (Wallenberg syndrome). Interruption of the descending autonomic fibers gives rise to a clinical condition called Horner’s syndrome in which there is loss of the autonomic sympathetic supply to one side of the face, ipsilaterally. This leads to drooping of the (upper) eyelid, dry skin, and constriction of the pupil. The pupillary change is due to the competing influences of the parasympathetic fibers, which are still intact. Lesions elsewhere can also give rise to a Horner’s syndrome. It is instructive for a student to work out the clinical symptomatology that would be seen following a vascular lesion affecting each of these branches (medial vs. lateral). ©2000 CRC Press LLC FIGURE 69 UPPER MEDULLA CROSS SECTION (B6) This cross section has the characteristic features of the medullary region, namely the pyramids anteriorly with some remaining parts of the inferior olivary nucleus situated just behind. The medial lemniscus is the most prominent ascending (sensory) tract throughout the medulla. The tracts are located next to the midline, oriented in the anteroposterior (ventrodorsal) direction, just behind the pyramids (see Figure 38). Dorsal to them, also along the midline, are the paired tracts of the MLF, situated in front of the fourth ventricle. The anterolateral tract lies dorsal to the olive, although it is not of sufficient size to be clearly identified. Both the medial lemniscus and the anterolateral system are carrying fibers from the other side of the body at this level. The descending tract of CN V is present more laterally, carrying fibers (pain and temperature) from the ipsilateral face (see Figure 33). Medial to this tract, along its full extent, is a corresponding nucleus which is called by the same name. The descending fibers synapse in this nucleus, cross, and then ascend (see Figure 38) eventually joining the medial lemniscus in the upper pons. The eighth nerve enters the medulla at its uppermost level, at the cerebello-pontine angle, passing over the inferior cerebellar peduncle. The nerve has two nuclei along its course, the ventral and dorsal cochlear nuclei. The auditory fibers synapse in these nuclei and then go on to the superior olivary complex in the lower pons region. The crossing fibers are seen in the lowermost pontine region as the trapezoid body (see Figures 35 and 36, also Figure 68). ©2000 CRC Press LLC The vestibular part of the eighth nerve is represented at this level by two nuclei, the medial and inferior vestibular nuclei. Both these nuclei lie in the same position as the vestibular nuclei in the pontine section, adjacent to the lateral edge of the fourth ventricle. The inferior vestibular nucleus is rather distinct because of the many axon bundles that course through it. The vestibular nuclei contribute fibers to the MLF (discussed with Figure 49B). The solitary nucleus is found at this level, surrounding a tract of the same name. This nucleus is the synaptic station for incoming taste fibers and for visceral afferents entering with CN IX and X. The solitary nucleus and tract are situated just beside (anterior to) the vestibular nuclei. The core area is occupied by the cells of the reticular formation. The most prominent of its nuclei at this level is the gigantocellular nucleus, which gives rise to the medullary (lateral) reticulo-spinal tract (see Figure 47). The other functional aspects of the reticular formation should be reviewed (discussed with Figures 40A and 40B). The other prominent tract in the medullary region is the inferior cerebellar peduncle (see Figure 4). This tract is conveying fibers to the cerebellum, both from the spinal cord and the medulla, including the inferior olivary nucleus (discussed with Figure 53). The fourth ventricle is still quite large at this level. Its roof (lower portion) has choroid plexus (see Figure 63). Behind the ventricle is the cerebellum, with the vermis (midline) portion; the lateral lobule that is present at this juncture is the cerebellar tonsil (see Figures 4 and 13). FIGURE 69: Upper Medulla: Cross Section (B6) ©2000 CRC Press LLC FIGURE 70 MID MEDULLA CROSS SECTION (B7) This is a classic level for descriptive purposes. The inferior olive and pyramids are easily recognized. Cranial nerves IX, X, and XII are attached to the medulla at this level with the many nuclei associated with these nerves. The hypoglossal nucleus (CN XII) is a somatic motor nucleus occupying the same location — near the midline, and in front of the ventricle — as the nuclei of CN III, IV, and VI. The fibers of CN XII exit anteriorly, between the pyramid and the olive (see Figures 3 and 4). The MLF lies in front of the nucleus of CN XII, and the medial lemniscus lies in front of that, both situated adjacent to the midline. Vascular lesions of the brainstem in this region (supplied by the small paramedian arteries — see Figure 56) might involve all of these structures, namely the pyramids, the medial lemniscus, and CN XII. (The student should try to work out the clinical deficits that would be found following a lesion of this nature.) The other ascending sensory systems are found in the lateral aspect of the medulla. The fibers of the anterolateral system are situated dorsal to the olive. The descending nucleus and tract of the trigeminal system have the same location in the lateral aspect of the tegmentum. Therefore, a lesion here (e.g., occlusion of the posterior inferior cerebellar artery) will produce a different pattern of sensory loss (as discussed above). CN IX (glossopharyngeal) and CN X (vagus) are attached at the lateral aspect of the medulla (see Figures 3 and 7). Their efferent fibers are derived from two nuclei: the dorsal motor nucleus, which is parasympathetic, and the nucleus ambiguus, which is branchiomotor (see ©2000 CRC Press LLC Figure 5). The dorsal motor nucleus lies adjacent to the fourth ventricle just lateral to the nucleus of XII. The nucleus ambiguus lies dorsal to the olivary nucleus; in a single cross section only a few cells of this nucleus are usually seen, making the identification of this nucleus difficult (i.e., “ambiguous”) in actual sections. The taste and visceral afferents that are carried in these nerves synapse in the solitary nucleus which is located in the posterior aspect of the tegmentum. The other nuclei in this lateral region of the medulla are sometimes difficult to sort out. Sometimes the vestibular nuclei are present at this level. In some sections the accessory cuneate nucleus may be found. This nucleus is a relay for some of the cerebellar afferents from the upper extremity (see Figure 53). The fibers then go to the cerebellum via the inferior cerebellar peduncle, which is found along the dorsal margin of the medulla (see Figure 4). The reticular formation occupies the central core of the tegmentum, as usual. Some large cells are present at this level, and these are said to form a “nucleus,” the nucleus gigantocellularis, in this part of the reticular formation (see Figure 40B). These cells give rise to a descending tract, the lateral reticulo-spinal tract (see Figure 47) and are involved in the voluntary control of proximal joints as well as with the regulation of muscle tone. The fourth ventricle is still a rather large space, behind the tegmentum, with the choroid plexus attached to its roof (in this area). Very often these structures are absent in sections taken at this level and the ventricle appears “open.” There is no cerebellar tissue posteriorly since the section is below the level of the cerebellum (see the schematic accompanying this figure). FIGURE 70: Mid Medulla — Cross Section (B7) ©2000 CRC Press LLC FIGURE 71 LOWER MEDULLA CROSS SECTION (B8) The medulla seems significantly smaller in size at this level, approaching the size of the spinal cord below. The section is still easily recognized as medullary because of the presence of the pyramids and the inferior olivary nucleus. The dorsal aspect of the medullary tegmentum is occupied by two large nuclei: the nucleus cuneatus (cuneate nucleus, lateral) and the nucleus gracilis (gracile nucleus, more medial). These are found on the dorsal aspect of the medulla (see Figures 7 and 38). These nuclei are the synaptic terminations of the tracts of the same name that have ascended the spinal cord (see Figure 32). The fibers relay in these nuclei and then move anteriorly to form the medial lemniscus. In so doing, they pass through the tegmentum and are seen as a stream of axons called the internal arcuate fibers. These axons actually cross the midline (decussate) to form the medial lemniscus of the other side. At this level, the medial lemniscus is situated between the olivary nuclei and dorsal to the pyramids and is oriented anteroposteriorly. The nuclei of CN X as well as CN XII are present as before, as is the descending nucleus and tract of V (CN XI is discussed with Figure 3). The MLF and anterolateral fibers are in the same position. The solitary tract and nucleus are also still found in the same location. The internal arcuate fibers may obscure the exact location of ©2000 CRC Press LLC the nucleus ambiguus. Finally, the reticular formation is still present. The accessory cuneate nucleus would definitely be found at this level (as discussed with Figure 70). The inferior cerebellar peduncle has not yet been formed. Cross sections through the lowermost part of the medulla may include the decussating cortico-spinal fibers, i.e., the pyramidal decussation (seen in Figure 4). This would therefore alter significantly the appearance of the structures in the actual section. Posteriorly, the fourth ventricle is tapering in size, giving a “V-shaped” appearance to the dorsal aspect of the medulla (see Figure 7). It is usual for the ventricle roof to be absent at this level. This is likely accounted for by the presence of the foramen of Magendie, where the CSF escapes from the ventricular system into the subarachnoid space (see Figure 20A). Posterior to this area is the cerebello-medullary cistern, otherwise known as the cisterna magna (see Figure 20A; also Figure 63 — not labeled). One special nucleus is found in the “floor” of the ventricle at this level, the area postrema. This forms a little bulge that can be appreciated on some sections. The nucleus is part of the system that controls vomiting, and it is often referred to as the vomiting “center.” It is interesting to note that this region lacks a blood-brain barrier, allowing this particular nucleus to be “exposed” directly to whatever is circulating in the blood stream. FIGURE 71: Lower Medulla — Cross Section (B8) ©2000 CRC Press LLC FIGURE 72 SPINAL CORD TRACTS C8 LEVEL The major tracts of the spinal cord are shown on this diagram, the descending tracts on the left side and the ascending ones on the right side. (Note the different shading intensity of the two systems; the cerebellarrelated tracts have a different shading.) In fact, both sets are present on both sides. The functional aspects of each of these tracts should be reviewed by noting the loss of function that would be found following a lesion of the various pathways. tive touch sensation, joint position and vibration. The somatotopic arrangement of the fibers should be reviewed. • Anterolateral system, consisting of the anterior (ventral) spino-thalamic and lateral spino-thalamic tracts (see Figure 31) — These pathways carry pain and temperature, as well as crude touch information. There is also a somatotopic arrangement of fibers in this system. • Spino-cerebellar tracts, anterior (ventral) and posterior (dorsal); (see Figure 49B, and Figure 53) — These tracts convey information from the muscle spindles as part of the “comparator” function of the cerebellum. Descending tracts: Special tract: • Lateral cortico-spinal, from the cerebral (motor) cortex — This pathway crosses in the lowermost medulla (see Figure 42). These fibers supply mainly the lateral motor neurons that control fine motor movements of the hand and fingers. The dorsolateral fasciculus, better known as the tract of Lissauer, carries intersegmental information, particularly relating to pain afferents. • Anterior (ventral) cortico-spinal, also from the motor cortex — These fibers, which do not cross in the pyramidal decussation (see Figure 42), go to the motor neurons that supply the proximal and axial musculature. • Rubro-spinal, from the red nucleus — This tract crosses at the level of the midbrain (see Figures 44 and 45). Its role in motor function in humans is not certain. • Lateral and medial reticulo-spinal tracts, from the medullary and pontine reticular formation, respectively (see Figures 46 and 47) — These pathways are the additional ones for voluntary control of the proximal joints and for posture, as well as being important for the control of muscle tone. • Lateral vestibulo-spinal, from the lateral vestibular nucleus — Its important function is participating in the response of the axial muscles to changes in gravity. This pathway remains ipsilateral (see Figure 48). • Medial longitudinal fasciculus (MLF) — This pathway is involved in the response of the eye and muscles of the neck to vestibular and visual input (see Figure 49A). It likely descends only to the cervical spinal cord level. Ascending tracts: • Dorsal column tracts, consisting at this level of both the fasciculus cuneatus and fasciculus gracilis (see Figure 32) — These are the pathways for discrimina©2000 CRC Press LLC Clinical Aspects Traumatic lesions of the spinal cord occur following car and bicycle accidents and still occur because of diving accidents in shallow water (swimming pools!). Protruding discs can impinge upon the spinal cord. Other traumatic lesions involve gunshot and knife wounds. A classic lesion of the spinal cord is the BrownSequard syndrome, a lesion of one-half of the spinal cord on one side. Although rare, review of the various deficits helps a student learn which side of the body is affected because of the various crossing of the pathways (ascending and descending) at different levels. The vascular supply of the spinal cord is notoriously poor. Clinically, it is known that the blood supply to the mid-thoracic region is marginally adequate. The main blood supply to the spinal cord comes from two branches, one from each vertebral artery (see Figure 56). These two small arteries join and form the anterior spinal artery, which descends in the midline. The anterior spinal artery supplies the ventral horn and the anterior and lateral group of tracts, including the lateral corticospinal pathway. This artery receives supplementary branches from the aorta along its way, which follow the nerve roots and are called radicular arteries. There are two very small posterior spinal arteries; the posterior spinal artery supplies the dorsal horn and the dorsal columns. Lateral cortico-spinal tract Dorsolateral fasciculus Fasciculus gracilis Fasciculus cuneatus Posterior (dorsal) spino-cerebellar tract Rubro-spinal tract Medullary (lateral) reticulo-spinal tract Anterior (ventral) spino-cerebellar tract Lateral vestibulo-spinal tract Pontine (medial) reticulo-spinal tract Anterior cortico-spinal tract Lateral Medial spino-thalamic longitudinal tract fasciculus Anterior (MLF) spino-thalamic tract MOTOR CEREBELLAR SENSORY FIGURE 72: Spinal Cord Tracts — C8 level ©2000 CRC Press LLC FIGURE 73 SPINAL CORD CROSS-SECTIONAL VIEWS The spinal cord is introduced in Section A; see Figures 1A, 1B, 2A, and 2B. The tracts that ascend from the spinal cord (sensory and cerebellar) and the various motor pathways that descend to the spinal cord have likewise been reviewed in Section B. The white matter surrounds the gray matter and is divided by it into three areas: the dorsal, lateral, and anterior areas. These zones are sometimes referred to as funiculi (single is funiculus). Various tracts are located in each of these three zones, some ascending and some descending (see Figure 72). Cross Section of Spinal Cord: Cervical Level — C8 This cross section of the spinal cord has been made at the cervical enlargement (C8). This level is shown in many of the illustrations of the various pathways in Section B. amount of gray matter. There are fewer muscles and less dense innervation of the skin in the thoracic region. The gray matter also has a lateral horn, which represents the sympathetic neurons. This cell group is the pre-ganglionic sympathetic portion of the autonomic nervous system. The lateral horn is present throughout the thoracic region of the spinal cord and also the upper segments of the lumbar region (T1–L2). Cross Section of Spinal Cord: Lumbar Level — L3 This cross-sectional level of the spinal cord is shown in the various illustrations of the pathways in Section B. At this level the spinal cord is similar in appearance to the cervical section because both are innervating the limbs. There is, however, proportionately less white matter at the lumbar level. The descending tracts are smaller because many of the fibers have terminated at higher levels. The ascending tracts are smaller because they are conveying information only from the lower regions of the body. This level contributes to the formation of the brachial plexus to the upper limb. The gray matter ventrally is very large because of the number of neurons involved in the innervation of the upper limb, particularly the muscles of the hand. The dorsal horn is likewise large, because of the number of afferents coming from the skin of the fingers and hand. By this level of the spinal cord, in the adult human, the spinal cord segments do not match the vertebral level. This is because the spinal cord in the human usually ends, in fact, at the vertebral level L2 in the adult. Below this level the vertebral canal is filled with nerve roots — the cauda equina (like a horse’s tail; see Figure 1A). The CSF space in that vertebral region is the lumbar cistern. The white matter is very large in comparison with lower regions because Cross Section of Spinal Cord: Sacral Level — S3 1. all the ascending tracts are present and are carrying information from the lower parts of the body as well as the upper limb; and 2. the descending tracts are fully represented, as many of the fibers will terminate in the cervical region of the spinal cord. In fact, some of them do not descend to lower levels. Cross Section of Spinal Cord: Thoracic Level — T6 The thoracic region of the spinal cord presents an altered morphology because of the decrease in the ©2000 CRC Press LLC The sacral region of the spinal cord is the smallest in size. The white matter is quite reduced in size. There is still a fair amount of gray matter because of the innervation of the pelvic musculature. The cord tapers and ends as the conus medullaris (see Figure 1B). This region of the spinal cord also contains the pre-ganglionic parasympathetic neurons of the autonomic nervous system , which innervate the bowel and the bladder. Injury to this region of the spinal cord results in a serious problem in terms of regulation of bowel and bladder function. Cervical Thoracic Lumbar Sacral FIGURE 73: Spinal Cord — Cross-Sectional Views ©2000 CRC Press LLC Section D THE LIMBIC SYSTEM INTRODUCTION The term limbic is almost synonymous with the term emotional brain — the parts of the brain involved with our emotional state. In 1937, Dr. James Papez initiated the limbic era by proposing that a number of limbic structures in our brain formed the anatomical substratum for emotion. EVOLUTIONARY PERSPECTIVE This section begins with a brief overview of theories of brain function in the lower animal kingdom, then extends the discussion to mammals and finally to humans. Dr. Paul MacLean has postulated that there are in fact three separable “brains” that have evolved. The premammalian (reptilian) brain has the capacity to look after the basic life functions and, behaviorally, has organized ritualistic stylized patterns of behavior. In higher species, including mammals, forebrain structures have evolved which relate to the external world (e.g., visual input). These are adaptive, allowing for a modification of behavior depending upon the situation. MacLean has also suggested that the limbic system arises in early mammals to link these two brain functions. According to this scheme, the limbic system relates the reptilian brain, which monitors the internal milieu, with the newer forebrain areas of mammals, which are responsible for analyzing the external environment. Hence, we now view the limbic system as those parts of the brain that are involved in regulating the internal state of the animal in relation to the external world. DEFINITION Most of us are quite aware or have a general sense of what we mean when we use the terms emotion or feelings, yet it is somewhat difficult to explain or define precisely. A medical dictionary (Stedman’s) defines emotion ©2000 CRC Press LLC as “a strong feeling, aroused mental state, or intense state of drive or unrest directed toward a definite object and evidenced in both behavior and in psychologic changes.” Thus, emotions involve the following: • Physiologic changes — These changes include basic drives involving thirst, sexual behavior, and appetite. They are often manifested as involving the autonomic nervous system and/or endocrine system. • Behavior — The animal or human performs some type of motor activity, for example fighting, fleeing, displaying anger, mating behavior; in humans, it might include facial expression. • Alterations in the mental state — This alteration can be understood as a subjective change in the way the organism “feels” or reacts to the events occurring in the outside world. In humans, we use the term psychological reaction. It is clear, at least in humans, that some of these functions and behaviors must involve the cerebral cortex. In addition, many of these alterations are conscious and involve association areas. In fact, humans are sometimes able to describe and verbalize their reactions or the way they feel. Both cortical and subcortical areas (e.g., basal ganglia) might be involved in the behavioral reactions associated with emotional responses. The hypothalamus, along with brainstem nuclei, controls the autonomic changes as well as the activity of the pituitary gland underlying the endocrine responses. Therefore, we can finally arrive at a definition of the limbic system as an inter-related group of cortical and subcortical (noncortical) structures that are involved in the regulation of the internal/emotional state, with the accompanying physiological, behavioral, and psychological responses. In summary, limbic functions can be summarized by the use of a rather simple mnemonic using Fs — feeding, fighting, fleeing, fornicating, feeling. NEURAL STRUCTURES In neuroanatomical terms, the limbic system now is thought to include cortical and noncortical (subcortical, diencephalic, and brainstem) structures. The following is a listing of the structures. Core structures are those definitely associated with the limbic system; those listed as extended are closely connected with limbic functions: Cortical core — parahippocampal gyrus, cingulate gyrus, hippocampal formation (three subparts which are “buried” in the medial temporal lobe in humans) extended — parts of the prefrontal and orbitofrontal cortex (the limbic forebrain) Noncortical forebrain core — amygdala, septal region, ventral portions of the basal ganglia (including the nucleus accumbens) extended — the basal forebrain diencephalic and brainstem core — certain nuclei of the thalamus, the hypothalamus extended — parts of the midbrain (the limbic midbrain) These structures are collectively called the limbic system. The particular role of the olfactory system and its connections are discussed in the context of the limbic system. OVERVIEW OF “KEY” LIMBIC STRUCTURES Key structures of the limbic system integrate information and relate the external and internal worlds — the parahippocampal gyrus, the hippocampal formation, the amygdala, and the hypothalamus. The parahippocampal gyrus has widespread connections with many cortical (particularly sensory) areas and is probably the source of the most significant afferents to the hippocampal information. The hippocampal formation is an older cortical region that is involved with integrating information (its role in the formation of ©2000 CRC Press LLC memory for facts and events is discussed below). The amygdala is in part a subcortical nucleus involved with internal (visceral afferent) information as well as sensory input about olfaction (our sense of smell). The hypothalamus oversees autonomic and hormonal regulation. Both areas control motor and autonomic activities of the organism, (the amygdala in part via the hypothalamus), and both are involved in generating emotional reactions. Certain basic drives (as they are known in the field of psychology), such as hunger, thirst, sex, and temperature regulation, are regulated through the limbic system. For example, the reaction to a cold environment or dehydration leads to a complex series of motor activities, autonomic adjustments, as well as hormonal changes. It is not difficult to show how the hypothalamus regulates the pituitary gland and that it is the master comptroller of autonomic functions, but clearly additional connections are required for the behavioral (motor) activities. In humans, there is also an internal state of discomfort to being cold, which we call an emotional reaction — where is this “feeling” generated in the brain? It is generally assumed that other mammals also have “feelings,” which can be assessed in other ways, when faced with similar situations. Certainly, it is hard to deny that higher apes behave and react to these events in terms that can only be described as emotional. It has also been suggested that some mammalian behavior associated with caring for its young is associated with the limbic structures, such as recognizing and responding to the vocalizations of the “pups” in mice and rats, and the particular tone of a baby’s crying. Some of this may involve the cingulate gyrus. This notion of rearing and “family” would add another “F” to our list of limbic functions. It is also interesting to speculate that the elaboration of limbic functions is closely associated with the development of self-awareness, consciousness of the self (not an “F” word). Limbic Connections The limbic system has internal circuits involving the key structures; these link the amygdala, the hippocampal formation, and the hypothalamus, as well as other struc- tures of the limbic system. There are multiple interconnections within and between these structures, and knowledge of the circuits of the limbic system, which are quite complex, allows one to trace pathways within it. Only some of these pathways are presented here. The best known of these in terms of function (and for historical reasons) is the Papez Circuit (discussed with Figure 81). Additional pathways connect the limbic structures to the remainder of the nervous system and through which the limbic system influences the activity of the nervous system. Memory Unfortunately, the definition and description of the limbic system does not include one aspect of brain function that seems to have evolved in conjunction with the limbic system — memory. Memory systems are now thought of as comprising two types: • memory for facts and events — declarative or episodic memory, and • memory for skills and procedures — procedural memory. Some parts of the hippocampal formation are specifically necessary for the initial formation of declarative ©2000 CRC Press LLC memories. It is critical to understand that this initial step is an absolute prerequisite to the formation of any memory trace. It is interesting to speculate (as we see below) that forgetting may be theoretically more appropriate for this unique aspect of limbic function. Once encoded by the hippocampal formation, the memory trace is then transferred to other parts of the brain for short and long-term storage. The limbic system is not involved in the storage and retrieval of long-term memories. It is interesting to speculate that part of the function of the limbic system is to undo or unlock the fixed behavioral patterns of the old reptilian brain. In order to do this, one needs to remember what happened the last time when faced with a similar situation. Hence, the development of memory functions of the brain in association with the evolution of the limbic system. The availability of stored memories makes it possible for mammals to override or overrule the stereotypical behaviors of the reptile, allowing for more flexibility and adaptiveness when faced with a changing environment or altered circumstances. Therefore, we suggest that another “F” mnemonic — forgetting — may be applicable for this “memory” function. FIGURE 74 LIMBIC LOBE The limbic lobe refers to cortical areas of the limbic system that form a border (limbus) around the inner structures of the diencephalon and midbrain. These cortical areas include the cingulate gyrus, parahippocampal gyrus, and cortical components in the hippocampal formation. One of the distinguishing features of the limbic cortical areas is the fact that, for the most part, these are older cortical areas consisting of three to five layers (allocortex), in contrast to the six-layered neocortex typical of the dorsolateral areas of the cerebral cortex. The cingulate gyrus lies above the corpus callosum. Some parts of this gyrus consist of a five-layered cortex, as well as neocortex. The cingulate gyrus is connected reciprocally with the parahippocampal gyrus via by a bundle of fibers in the white matter, known as the cingulum bundle. This connection unites the various portions of the limbic lobe and is discussed as part of the limbic circuit known as the Papez circuit (discussed with Figure 81). It also has widespread connections with the frontal lobe. MacLean’s studies have indicated that the development of this gyrus is correlated with the evolution of the mammalian species. He has postulated that this gyrus is important for nursing and play behavior, characteristics associated with the rearing of young in mammals. It is this cluster of behavioral patterns that forms the basis for including “family” in the list of functions of the limbic system. The cingulate gyrus also seems to have an important role in attention. The parahippocampal gyrus, situated on the inferior aspect of the brain (see Figures 13 and 14), is a critical area of the limbic lobe. It is also composed of five- and six-layered cortex. This gyrus has widespread connections with many areas of the cerebral cortex, including all the sensory cortical regions, as well as the cingulate gyrus. It is also heavily connected (reciprocally) with the hippocampal formation. It is thought to play a key role in limbic function and memory. ©2000 CRC Press LLC A number of cortical areas located in the most medial aspects of the temporal lobe, deep to the parahippocampal gyrus, form part of this limbus — these are collectively called the hippocampal formation. One of its three major components, the hippocampus proper, is not at the surface of the hemisphere, as would be expected for a cortical area. Understanding the development of the region explains this fact (discussed with Figure 77B). The other parts of the hippocampal formation are the dentate gyrus, part of which can be found at the surface, and the subicular region (a cortical area). The hippocampus proper and the dentate cortex are three-layered cortical areas while the subicular region has four to five layers (see Figures 77A and 77B). Of the many tracts of the limbic system, two major tracts have been included in this diagram: the fornix and the anterior commissure. The fornix is one of the more visible tracts and is often seen during dissections of the brain (e.g., see Figure 16). This fiber bundle connects the hippocampal formation with other areas (discussed with Figure 77B). The anterior commissure is an older commissure than the corpus callosum and connects several structures of the limbic system on the two sides of the brain. These include the amygdala, the hippocampal formation, and parts of the parahippocampal gyrus. The anterior commissure will be seen on many of the limbic diagrams and is also a convenient reference point. The details of these various limbic structures, their important connections, and the functional aspects of these cortical components of the limbic system are discussed with the relevant diagram. Other areas of the brain are now known to be involved in limbic functions, and they are included in the limbic system. This includes large parts of the so-called prefrontal cortex, particularly cortical areas lying above the orbit, the orbitofrontal cortex (not labeled in the figure). A small cortical region under the anterior part (the rostrum) of the corpus callosum is discussed with the septal region. ©2000 CRC Press LLC Corpus callosum “area” Fornix Cingulate gyrus Diencephalon Midbrain Septal region Anterior commissure Cerebellum Mammillary n. Pons Amygdala Medulla Parahippocampal gyrus FIGURE 74: Limbic Lobe Hippocampal formation FIGURE 75 LIMBIC STRUCTURES The diagram in Figure 75 focuses on the noncortical components of the limbic system — forebrain, diencephalon, and midbrain. Each of the structures, including the connections, are discussed in greater detail in subsequent illustrations. In those discussions, this diagram will sometimes be used as a locator map, where the parts of the limbic system being described will be indicated appropriately. The noncortical areas shown in this diagram include • amygdala • septal region • thalamus • hypothalamus • limbic midbrain The olfactory system is also discussed in this section. The amygdala (amygdaloid nucleus), as discussed (with Figure 23), is anatomically one of the basal ganglia. Functionally, and through its connections, it is part of the limbic system. Therefore, it is considered in this section. Parts of the basal ganglia, namely the ventral portions of the putamen and globus pallidus (not shown on this diagram), might also have limbic functions and these are discussed as well (with Figure 85B). The septal region includes two components, the cortical gyri below the rostrum of the corpus callosum and some nuclei deep to them. These nuclei are not located within the septum pellucidum in the human. The nucleus accumbens (see Figures 24 and 85B) is a specific nucleus adjacent to these septal nuclei which has recently been found to have important functions in reward and punishment. It might be the critical area of the brain involved in addiction. Two of the nuclei of the thalamus, the anterior group of nuclei and the dorsomedial nucleus (see Figures 81 and 82), are part of the pathways of the limbic system, relaying information from subcortical nuclei to limbic parts of the cortex (the cingulate gyrus and areas of the prefrontal cortex). ©2000 CRC Press LLC The hypothalamus lies below and somewhat anterior to the thalamus. Only a few of the nuclei are shown, and amongst these is the prominent mammillary nucleus, visible on the inferior view of the brain (seen in Figure 14). The connection of the hypothalamus to the pituitary gland is not shown. The limbic system also includes nuclei of the midbrain, the limbic midbrain. Some of the descending limbic pathways terminate in this region and it is important to consider the role of this area in limbic functions. An important limbic pathway, the medial forebrain bundle (see Figure 83B) interconnects the septal region, the hypothalamus, and the limbic midbrain. The olfactory system is described with the limbic system, because many of its connections are directly with limbic areas. Years ago, it was commonplace to think of various limbic structures as part of the “smell brain,” the rhinencephalon. We now know that this is not correct, and that the limbic system has many other functional capabilities. Not represented in this diagram is the region known as the basal forebrain. This is a subcortical region, which is composed of a group of structures, located beside the hypothalamus and below the anterior commissure (see Figures 85A and 85B). This somewhat obscure region has connections with several limbic areas and the prefrontal cortex. It may play a major role in memory. The various pathways shown in Figure 75 — fornix, stria terminalis, ventral amygdalofugal — are discussed with the relevant structures. ©2000 CRC Press LLC Fornix Cingulate gyrus a rpus c llosum Co Stria terminalis Thalamus Septal nuclei Midbrain Pons Hypothalamic nuclei Mammillary n. Medulla Lateral olfactory stria Olfactory bulb Hippocampal formation Amygdala Ventral amygdalofugal pathway Olfactory tract Parahippocampal gyrus FIGURE 75: Limbic Structures FIGURE 76 HIPPOCAMPUS (PHOTOGRAPHIC VIEW) Figure 76 shows the brain from the dorsolateral aspect. The left hemisphere is partially obscured by the (gloved) hand holding the structures. The right hemisphere has been dissected by cutting away all of the hemisphere above the corpus callosum, and then removing some cortical tissue of the temporal lobe, thereby exposing the inferior horn of the lateral ventricle (see Figure 20A). This dissection exposes a large mass of tissue which is actually protruding into this part of the ventricle, the hippocampus, as it is often called as a gross brain structure. In fact, the correct term is hippocampal formation, as it is composed of various parts (discussed below). The choroid plexus tissue has been removed from this part of the ventricle in order to improve visualization of the structures. The protrusion of the hippocampus into the inferior horn of the lateral ventricle can also be seen in coronal sections through this region (see Figures 29, 36, 78, and 80). The hippocampal formation is composed of three distinct regions — the hippocampus proper (Ammon’s horn), the dentate gyrus and the subicular region; these are explained in the next diagrams. The fiber bundle which arises from the visible hippocampus, the fornix, can be seen along its innermost aspect. The fornix receives fibers from some of the parts of the hippocampal formation. Its course and connections are considered with Figure 77. Memory We now know that the hippocampal formation is one of the critical structures for memory. This function of the hippocampal formation is known because of a patient called H.M. in the literature, who has been extensively studied by neuropsychologists. H.M. had surgery several decades ago for sound therapeutic reasons — removal of an epileptic area in the temporal lobe of one side — but unfortunately before the functional contribution of this area was known. Most importantly, the surgeons did not know (and could not have known according to the methods available at that time) that the contralateral hippocampal area was also severely damaged. ©2000 CRC Press LLC We now know that bilateral damage or removal of the anterior temporal lobe structures, including the amygdala and the hippocampal formation, leads to a unique condition in which the person can no longer form new declarative or episodic memories, although older memories are intact. The individual cannot remember what occurred moments before. Therefore, the individual is unable to learn — to acquire new information — and is not able to function independently. Apparently, the full-blown syndrome results from damage to the hippocampus on both sides. Today, if surgery is to be performed in this region, special testing is done to ascertain that the side contralateral to the surgery is intact and functioning. This area is prone to damage for a variety of reasons, including trauma and vascular conditions. The key neurons for this memory function are located in the hippocampus proper and these neurons are extremely sensitive to anoxic states. An acute hypoxic event, such as occurs in a cardiac arrest, is thought to trigger a delayed death of these neurons, several days later. This is termed apoptosis, programmed cell death. Much research is now in progress to try to understand this cellular phenomenon and to devise methods to stop this reaction of these neurons. Currently, studies indicate that in certain forms of dementia, particularly Alzheimer’s, there is a loss of neurons in this same region of the hippocampus proper. This loss is due to involvement of these neurons in the disease process. Again, this correlates with the memory deficit seen in this condition, although the disease clearly involves other areas which correlate with the other deficits typical of the early stages of this disease. ©2000 CRC Press LLC Fornix Lateral ventricle (inferior horn) Hippocampus Temporal lobe FIGURE 76: Hippocampus (Photographic View) FIGURE 77A HIPPOCAMPAL FORMATION The diagram in Figure 77 (which is the same one as Figure 75) highlights the “hippocampus,” i.e. the hippocampal formation, and the pathway known as the fornix. The hippocampal formation refers to older cortical regions, all consisting of less than six layers, which are located in the medial aspect of the temporal lobe in humans. Much of the difficulty of understanding these structures is their anatomical location deep within the medial portions of the temporal lobe. In the rat, the hippocampal formation is located dorsally, above the thalamus. During the evolution of the temporal lobe, these structures have migrated into the temporal lobe, leaving behind a fiber pathway, the fornix, which is located above the thalamus. In fact, a vestigial part of the hippocampal formation is still located above the corpus callosum, as shown in this illustration (not labeled). ©2000 CRC Press LLC The hippocampal formation includes: • the hippocampus proper, a three-layered cortical area which during development becomes “rolled-up” and is no longer found at the surface of the hemispheres (as is the case for all other cortical regions); • the dentate gyrus, a three-layered cortical area which is partly found on the surface of the brain, although its location is so deep as to present a challenge to nonexperts to actually locate and visualize this thin ridge of cortex; • the subicular region, a transitional cortical area of three to five layers which becomes continuous with the parahippocampal gyrus (located on the inferior aspect of the brain). Clinical Aspect The term medial temporal (mesiotemporal) sclerosis is a general term for damage to the hippocampal region located in this part of the brain. It is now possible to view this area in detail on MRI and to assess the volume of tissue. Bilateral damage here apparently correlates with the loss of memory function in humans, for the formation of new memories for facts or events. ©2000 CRC Press LLC Cingulate gyrus Fornix Septal region Co s callosum u p r Mammillary n. Hippocampal formation FIGURE 77A: Hippocampal Formation FIGURE 77B HIPPOCAMPAL FORMATION 3 PARTS The hippocampal formation is one of the most important components of the limbic system in humans. It is certainly the most complex. One expects a cortical area to be found at the surface of the brain, even if this surface is located deep within a fissure. During the evolution and development of the hippocampal formation, these areas became rolled up within the brain. (The student is advised to consult Williams and Warwick for a detailed visualization and understanding of this phenomenon.) Of the three parts, the hippocampus proper is found completely “within the brain.” Hippocampus Proper — The hippocampus proper consists of a three-layered cortical area forming a large mass, which actually intrudes into the ventricular space of the inferior horn of the lateral ventricle (see Figures 76 and 78). In a coronal section through this region, there is a resemblance of the hippocampal structures to the shape of a seahorse. It is from this shape that the name “hippocampus” is derived, from the French word for seahorse. The other name for this area is Ammon’s horn or cornu ammonis (abbreviated as CA), named after an Egyptian deity with ram’s horns because of the curvature of the placement of the hippocampus in the brain. This cortical region has been divided into a number of subportions (CA 1–4, usually studied in more advanced courses). Dentate Gyrus — The dentate gyrus is also a phylogenetically older cortical area consisting of only three layers. During the formation discussed above, the leading edge of the cortex detaches itself and becomes the dentate gyrus. Parts of it remain visible at the surface of the brain. Since this small surface is buried on the most medial aspect of the temporal lobe and is located deep within a fissure, it is rarely located in studies of the gross brain. Once visualized, it is seen to have ridges or a serrated surface, which seemed to suggest tooth marks, giving it the name dentate (referring to teeth; see also Figure 80). ©2000 CRC Press LLC The appearance of the dentate gyrus is shown on the view of the medial aspect of the temporal lobe (on the far side of Figure 77B). A coronal section through the temporal lobe, as seen in the lower part of the diagram (see also Figure 36), indicates that the dentate gyrus is more extensive than its visible medial portion. Subicular Region — The next part of the cortically rolled-in structures that makes up the hippocampal formation is the subicular region (see also Figure 80). The cortical thickness is transitional, starting from the threelayered hippocampal formation to the six-layered parahippocampal gyrus. (Again, there are a number of subparts of this area which are rarely studied in an introductory course.) In the temporal lobe, the hippocampal formation is adjacent to the six-layered parahippocampal gyrus, with which it has extensive connections. Connections and Function The hippocampal formation receives its major input from the adjacent parahippocampal gyrus, as well as the amygdala. There are extensive interconnections within the component parts of the hippocampal formation itself. Part of the output of this cortical region is directed back towards the parahippocampal gyrus, which itself has extensive connections with other cortical areas of the brain, particularly sensory areas. This is analogous to cortical association pathways described earlier. The other major output of the hippocampal formation is through the fornix. Only the hippocampus proper and the subicular region project fibers into the fornix. This can be regarded as a subcortical pathway which terminates in the septal region (via the precommissural fibers, discussed with Figure 83B) and in the mammillary nucleus of the hypothalamus (via the post-commissural fibers, discussed with Figure 83A). There are also reciprocal connections in the fornix. The dentate gyrus connects only with other parts of the hippocampal formation and does not project beyond. Corpus callsoum (splenium) Dentate gyrus Fornix Precommisural fibers Hippocampus proper Mammillary n. Dentate gyrus Subicular region Parahippocampal gyrus Collateral fissure FIGURE 77B: Hippocampal Formation — 3 Parts ©2000 CRC Press LLC Temporal lobe FIGURE 78 CORONAL BRAIN SECTION PHOTOGRAPHIC VIEW The section shown in Figure 78 is taken posterior to the one shown in Figure 29 and includes part of the lateral ventricle as it begins to curve into the temporal lobe (see Figures 20A and 20B). The basal ganglia are no longer present. The most posterior portion of the thalamus, the pulvinar, is seen (see Figures 10 and 61). Between the thalamus and the corpus callosum is the fornix. The space between the thalamic areas is not the third ventricle (it is also too large to be the normal third ventricle) because this coronal section has been taken so posteriorly; it is, in fact, outside the brain, posterior to the diencephalic region (see Figure 16). It is located behind the pineal and the colliculi, and in front of the cerebellum (see also Figure 8). The quadrigeminal plate cistern is found in this location (see Figure 28A). It also contains some important cerebral veins which drain the interior of the brain (these have been removed from this specimen). The section also includes the brainstem. The inferior horn of the lateral ventricles is found in the temporal lobes on both sides and is seen as only a small crescent-shaped cavity (shown also in Figure 36). The inferior horn of the lateral ventricle is reduced to a narrow slit because a mass of tissue protrudes into this part of the ventricle from its medial-inferior aspect. Closer inspection of this tissue reveals that it is gray matter; this gray matter is the hippocampus proper. This plane of viewing allows one to follow the gray matter from the hippocampus proper medially and through an intermediate zone, known as the subiculum or subicular region (see Figure 77B), until it becomes continuous with the gray matter of the parahippocampal gyrus. The hippocampus proper has only three cortical layers. The subicular region consists of four to five layers; the parahippocampal gyrus is mostly a six-layered cortex. The subicular region, as part of the hippocampal formation, both receives from and sends fibers to the other parts of the hippocampal formation, as well as contributing the majority of fibers to the fornix. ©2000 CRC Press LLC This view allows us to see that the parahippocampal gyrus is so named because it lies beside the hippocampus. The collateral sulcus (fissure), seen previously on the inferior aspect of the temporal lobe (see Figures 13 and 14), is indicated. Memory Recent studies in humans have indicated that the neurons located in one portion of the hippocampus proper (CA3 region) are critical for the formation of new memories — declarative or episodic types of memories (not procedural). This means that in order for the brain to remember some new fact or event, the new information must be registered within the hippocampal formation. This information is processed through some complex circuitry in these structures and is retained for a brief period. In order for it to be remembered for longer periods, some process not yet understood occurs so that the transient memory trace is transferred to other parts of the brain and stored in working memory or as a long-term memory. (An analogy to computers might be useful here.) In the study of the function of the hippocampus in animals, there is considerable evidence that the hippocampal formation is involved in constructing a spatial map. According to this literature, the hippocampal formation is needed for orientation in a complex environment (such as a maze). It is not quite clear whether this is a memory function, or whether this spatial representation depends upon the connections of the hippocampal formation and parahippocampal gyrus with other parts of the brain. ©2000 CRC Press LLC Corpus callosum Lateral ventricle Fornix Thalamus Lateral ventricle (inferior horn) Hippocampal formation Collateral sulcus/fissure Subicular region Parahippocampal gyrus Brainstem FIGURE 78: Coronal Brain Section — (Photographic View) FIGURE 79A AMYGDALA The diagram in Figure 79A (which is the same one as Figure 75) highlights a functional portion of the limbic system: the amygdala and its pathways (the stria terminalis and the ventral amygdalofugal pathway), the septal region, as well as functionally connected portions of the midbrain and medulla. The amygdala (amygdaloid nucleus) is a subcortical nuclear structure located in the temporal lobe (in humans). As a subcortical nucleus of the forebrain, it belongs by definition with the basal ganglia, but is now usually described with the limbic system. The amygdala is located between the temporal pole and the “end” of the inferior horn of the lateral ventricle (in the temporal lobe; see Figures 25 and 80). Its mass is responsible for the elevation known as the uncus, which is seen on the inferior aspect of the brain (see Figures 13 and 14) as a large medial protrusion of the anterior aspect of the temporal lobe. The amygdala receives input from the olfactory system as well as from visceral structures. Two fiber tracts are shown connecting the amygdala to other limbic structures, a dorsal one (the stria terminalis) and a ventral one (the ventral amygdalofugal pathway, consisting of two parts). These are described in detail with the following diagram. Stimulation of the amygdaloid nucleus produces a variety of vegetative responses, including licking and chewing movements. Functionally, in animal experimentation stimulation of the amygdala might produce a “rage” response, whereas removal of the amygdala (bilaterally) results in docility. These responses are also seen with stimulation or lesions in the hypothalamus. ©2000 CRC Press LLC In monkeys, bilateral removal of the anterior parts of the temporal lobe (including the amygdala) produces a number of effects which are collectively called the Kluver-Bucy syndrome. The monkeys evidently become tamer after the surgery, put everything into their mouths, and display inappropriate sexual behavior. In rather unusual circumstances, bilateral destruction of the amygdala is recommended in humans for individuals whose violent behavior cannot be controlled by other means. This type of treatment is called psychosurgery. The amygdala is known to have a low threshold for electrical discharges, which may make it prone to be the focus for seizure development. This seems to occur in kindling, an experimental model of epilepsy. In humans, epilepsy from this part of the brain (anterior and medial temporal regions) usually gives rise to complex partial seizures in which oral and licking movements are often seen, along with a loss of conscious activity. The amygdala is also known to contain a high amount of enkephalins. It is not clear why this is so and what is the functional significance. The role of the amygdala in the formation of memory is not clear. Bilateral removal of the anterior portions of the temporal lobe in humans for the treatment of severe cases of epilepsy, results in a memory disorder, which has been described with the hippocampal formation (discussed with Figure 76). It is possible that the role of the amygdala in the formation of memories is mediated either through the connections of this nuclear complex with the hippocampus or with the dorsomedial nucleus of the thalamus. ©2000 CRC Press LLC callosum pus r Co Stria terminalis Septal region Midbrain Medulla Ventral amygdalofugal pathway Amygdala FIGURE 79A: Amygdala FIGURE 79B AMYGDALA — CONNECTIONS One of the other major differences between the amygdala and the other parts of the basal ganglia is that the amygdala is not a homogeneous nuclear structure but is in fact composed of different parts. (These parts are not usually studied in an introductory course.) The amygdala receives a variety of inputs from other parts of the brain, including the adjacent parahippocampal gyrus (not illustrated). It receives olfactory input directly (via the lateral olfactory stria; see also Figure 84) and indirectly from the cortex of the uncal region (as shown on the left side of the diagram). The amygdaloid nuclei are connected to the hypothalamus, thalamus (mainly the dorsomedial nucleus), and the septal region. These connections, which are reciprocal, travel through two routes: • a dorsal route, known as the stria terminalis, which follows the ventricular curve and is found on the upper aspect of the thalamus (see Figures 61 and 75). ©2000 CRC Press LLC The stria terminalis lies adjacent to the body of the caudate nucleus in this location (see Figure 80) and connects the amygdala with the hypothalamus and the septal region. • a ventral route, known as the ventral pathway or the ventral amygdalofugal pathway. This pathway, which goes through the basal forebrain region (see Figure 85B), connects to the hypothalamus (as shown) and to the thalamus (the fibers are shown “en route”), particularly the dorsomedial nucleus (see Figure 82). Further possible connections of the amygdala with other limbic structures and other parts of the brain can occur via the hypothalamus (discussed with that structure, Figure 83A), via the septal region (see Figure 83B), and via the dorsomedial nucleus of the thalamus (see Figure 82). The amygdala also has connections with autonomicrelated nuclei in the midbrain (the limbic midbrain) and possibly also in the medulla. These influences may be direct and indirect (as shown). The anterior commissure conveys connections between the nuclei of the two sides (discussed also with Figure 74). ©2000 CRC Press LLC Caudate nucleus (body) Stria terminalis Thalamus Hypothalamic-midbrain fibers Periaqueductal gray “Limbic” midbrain Midbrain Septal region Descending autonomic fibers Anterior commissure Medulla Uncal cortex Parasympathetic nuclei Optic tract Hypothalamic nuclei – preoptic – medial – lateral Lateral olfactory stria Amygdala FIGURE 79B: Amygdala — Connections Ventral amygdalofugal pathway – to thalamus – to hypothalamus FIGURE 80 LIMBIC STRUCTURES AND THE LATERAL VENTRICLE The temporal lobe is a more recent addition in the evolution of the hemispheres and develops later in the formation of the brain. During the development of the temporal lobe, a number of structures migrate into it — the lateral ventricle, hippocampal formation, caudate nucleus, as well as various tracts, the fornix and stria terminalis. It is easiest to visualize these structures in the temporal lobe in relation to the lateral ventricle (refer to Figures 20A, 20B, and 25). Caudate Nucleus (see Figure 23) — The various parts of the caudate nucleus are shown in Figure 80. The large head is found in relation to the anterior horn of the lateral ventricle, where it in fact bulges into this part. It is also seen in a horizontal section through the brain (see Figure 27). The body of the caudate nucleus is coincident with the body of the lateral ventricle, being found on its lateral aspect (see Figures 25 and 61). In this location it is situated above the thalamus (see Figure 79B). The fibers of the fornix pass in front of the interventricular foramen of Monro (see medial view of brain in Figure 16). At this point some of the fibers are given off to the septal region and pass in front of the anterior commissure. These are the precommissural fibers (see Figure 77B). Others continue (behind the anterior commissure; postcommissural fibers) through the hypothalamus and terminate in the mammillary nucleus (which is not portrayed in this diagram; see Figure 77B). Stria Terminalis — The stria terminalis (connecting amygdala with septal region and hypothalamus) follows essentially the same course as the fornix (see Figure 79B). Its fibers lie slightly more medially and are found on the dorsal aspect of the thalamus, in the floor of the body of the lateral ventricle. In the temporal lobe, the stria is found in the roof of the inferior horn of the lateral ventricle (see also Figure 36). This view of the relationship of these various structures is augmented by a number of sections at various points: • the first section is through the anterior horn of the ventricle, in front of the interventricular foramen (of Monro); As the caudate nucleus curves into the temporal lobe, it becomes the tail of the caudate nucleus. In the temporal lobe it is found on the upper aspect of the inferior horn of the ventricle, its roof (see Figure 61). • the following section is over the dorsal aspect of the thalamus and above the third ventricle; Amygdala • the last section is through the temporal lobe, including the hippocampal formation. The amygdala is clearly seen to be situated anterior to the temporal horn of the lateral ventricle and in front of the hippocampal formation. Fornix — The fornix is easily found in studies of the gross brain (e.g., Figure 16). Its fibers can be seen as a continuation of the hippocampal formation (see Figures 76 , 77A, and 77B), and these fibers course on the inner aspect of the ventricle as they sweep forward above the thalamus. In the area above the thalamus and below the corpus callosum (see coronal section, Figure 29), the fornix is found at the lower edge of the septum pellucidum. Here, the fornix of one side is in fact adjacent to that of the other side (see also Figure 74). There are some interconnections between the two sides in this area (see Figure 75). ©2000 CRC Press LLC • the next section shows the ventricle at its curvature (the atrium) into the temporal lobe; Note: The initials used in these sections to identify structures are found in brackets after the labeled structure in the main part of the diagram. ©2000 CRC Press LLC LV LV CB CT Caudate nucleus – body (CB) ST ST F Lateral ventricle (LV) F Stria terminalis (ST) Fornix (F) ST CH Occipital horn LV Caudate nucleus – head (CH) Temporal horn Dentate gyrus Amygdala Hippocampus proper Caudate nucleus – tail (CT) FIGURE 80: Limbic Structures and the Lateral Ventricle F ST CT LV FIGURE 81 LIMBIC DIENCEPHALON ANTERIOR NUCLEUS LIMBIC DIAGRAM (INSET) The inset diagram of the structures of the limbic system (see Figure 75) has certain parts accentuated, as these are the areas shown in the detailed diagram. The thalamus on the far side is the one seen in the detailed diagram, as are the two mammillary nuclei, and a small portion of the cingulate gyrus. (Note: The student is advised to refer to the diagram and classification of the thalamic nuclei, Figure 10.) ANTERIOR NUCLEUS — CINGULATE GYRUS The detailed diagram shows some of the major connections of the limbic system. A major tract leaves the mammillary nuclei — the mammillo-thalamic tract — and its fibers are headed for a group of association nuclei of the thalamus called the anterior nuclei. The fibers from this nuclear group project to the cingulate gyrus. Axons leave the anterior nuclei of the thalamus and course through the anterior limb of the internal capsule. These fibers course between the caudate nucleus (head and body) and the lentiform nucleus (which is just visible in the background). The axons terminate in the cortex of the cingulate gyrus after passing through the corpus callosum. PAPEZ CIRCUIT About 60 years ago James Papez described a circuit involving some limbic and cortical structures and ©2000 CRC Press LLC pathways. He postulated that this was the anatomical substrate for emotional experiences. This pathway forms a loop and is known as the Papez circuit. If one commences with the hippocampal formation and proceeds through the fornix, some of the fibers terminate in the mammillary nuclei of the hypothalamus. From there, the mammillo-thalamic tract ascends to the anterior group of thalamic nuclei. This group of nuclei project to the cingulate gyrus (discussed with Figure 74). From the cingulate gyrus there is an association bundle, the cingulum, that connects the cingulate gyrus (reciprocally) with the parahippocampal gyrus as part of the limbic lobe (refer to Figure 74). The parahippocampal gyrus projects to the hippocampal formation, which processes the information and sends it via the fornix to the septal region and mammillary nuclei of the hypothalamus. Hence the circuit is formed. We now have a broader view of the limbic system and the precise functional role of the Papez circuit is not completely understood. It should be realized that although the circuitry forms a loop, the various structures have connections with other parts of the limbic system and other areas of the brain and can thus influence other neuronal functions. Clinical Aspect In the days of psychosurgery, bilateral removal of the cingulate gyrus was done for certain psychiatric disorders. One of these was extreme obsessive-compulsive behavior. This type of surgery is rarely performed now because new and powerful drugs have been found to help treat these disorders. ©2000 CRC Press LLC Cingulate projections Cingulate gyrus Corpus callosum Thalamus Caudate nucleus Mammillo-thalamic tract Internal capsule (anterior limb) Anterior n. Fornix FIGURE 81: Limbic Diencephalon — Anterior Nucleus Mammillary n. FIGURE 82 LIMBIC DIENCEPHALON DORSOMEDIAL NUCLEUS anterior limb of the internal capsule (between the head of the caudate nucleus and the lentiform nucleus) is seen on the left side of the diagram. The fibers course in the white matter of the frontal lobes. LIMBIC DIAGRAM (INSET) Psychosurgery The areas of the thalamus indicated in the inset of Figure 82 are portrayed somewhat differently and are those shown in the more detailed diagram. These areas include the thalamus on the distal side, as well as the thalamus situated nearer the viewer. The focus here is on the medial nuclear mass of the thalamus, the dorsomedial nucleus. DORSOMEDIAL NUCLEUS — PREFRONTAL CORTEX The cortical area known as the prefrontal cortex, anterior to the motor regions (discussed with Figure 11), is thought to be the most recently evolved portion of the cerebral cortex. This area becomes progressively larger as one ascends through the mammalian kingdom and the higher apes. Our expanded view of the limbic system now includes its extension to the prefrontal cortex, specifically the infraorbital and medial portions of the frontal lobe — the limbic forebrain. The thalamic nucleus that connects to this region of cortex is the dorsomedial nucleus, an association nucleus (see Figure 10). It collects information from a variety of sources. Some information comes from the amygdala, via the ventral pathway (see also Figure 79B), and other information comes from other thalamic nuclei. It also collects information from various hypothalamic nuclei, as well as the ventral parts of the basal ganglia that are connected with the limbic system (see Figure 85B). The dorsomedial nucleus projects heavily to the prefrontal cortex. The course of the fibers through the ©2000 CRC Press LLC This connection between the dorsomedial nucleus of the thalamus and the prefrontal cortex has been extensively studied in humans, because surgical interruption of these fibers (bilaterally) used to be done as psychosurgery for some psychiatric disorders, including schizophrenia. This operation, called a frontal lobotomy, was frequently performed throughout North America prior to the availability of drugs to treat psychiatric disorders. The surgical approach was probably abandoned in the 1950s. Long-term studies of individuals who have had frontal lobotomies have shown profound personality changes in these individuals. These people become emotionally “flat” and lose some hard-to-define human quality in their interpersonal interactions. In addition, such an individual might perform socially inappropriate acts not in keeping with his or her personality prior to surgery. Because the long-term effects of this surgery, which eventually became clear, and because powerful and selective drugs are now widely available for various psychiatric conditions, this surgery is never performed now. This surgical procedure had also been recommended for the treatment of pain in terminal cancer patients, as part of the palliative care of an individual. After the surgery, the individual is said still to have the pain but no longer to suffer from it; that is, the psychic aspect of the pain has been removed. There may even be a reduced demand for pain medication such as morphine. Again, other approaches to pain management are now used. ©2000 CRC Press LLC Caudate nucleus (head) Internal medullary lamina Lentiform nucleus Dorsomedial n. Thalamus Amygdala Internal capsule (anterior limb) Ventral amygdalofugal pathway Prefrontal projections Prefrontal cortex FIGURE 82: Limbic Diencephalon — Dorsomedial Nucleus FIGURE 83A HYPOTHALAMUS AND LIMBIC MIDBRAIN One of the core structures of the limbic system is the hypothalamus. The (prominent) mammillary nuclei are part of the hypothalamus (see Figures 13 and 14). The hypothalamus is closely connected to the septal region (labeled but not marked in Figure 83A). It is also connected directly with nuclei of the midbrain which are collectively called the limbic midbrain. There are also some indirect connections to nuclei of the medulla via descending autonomic fibers. Both parts of the brainstem are therefore highlighted in this illustration. HYPOTHALAMUS The hypothalamus is primarily responsible for the control of homeostatic mechanisms, including water balance, temperature regulation, and food intake (also discussed as part of the introduction to the limbic system). It accomplishes these tasks in two ways — as a neural structure linked into the limbic system, and as a neuroendocrine structure controlling the activities of the pituitary gland. In its neural role, it acts as the head ganglion of the autonomic nervous system, influencing both sympathetic and parasympathetic activities. Some of the major inputs to the hypothalamus come from limbic structures, including the amygdala (via the stria terminalis and the ventral pathway; see Figure 79B) and the hippocampal formation (via the fornix ; see Figure 77B). Stimulation of particular small areas of the hypothalamus can lead to a variety of behaviors (e.g., sham rage) similar to that which occurs following stimulation of other parts of the limbic system (e.g., the amygdala). The hypothalamus is usually divided (see Figure 83B) into a medial and lateral group of nuclei, with the third ventricle between the two sides. A number of nuclei that control the anterior pituitary gland are located in the medial group. This occurs via the median eminence (see Figure 14) and the portal system of veins along the pituitary stalk. Other nuclei in the supraoptic region connect directly with the posterior pituitary via the pituitary stalk. ©2000 CRC Press LLC Running through the lateral mass of the hypothalamus is a prominent fiber tract — the medial forebrain bundle (see Figure 83B). This tract interconnects the hypothalamus with two areas — the septal region of the forebrain (discussed with Figure 83B), and certain midbrain nuclei associated with the limbic system, the limbic midbrain. All of the connections are reciprocal in this system. Other fiber bundles connect the hypothalamus with the limbic midbrain. The mammillary nuclei, large nuclei of the hypothalamus, are of special importance as part of the limbic system. They receive a direct input from the hippocampal formation (via the fornix) and give rise to fibers that connect directly to the limbic midbrain. In addition, a major fiber tract, the mammillo-thalamic tract, connects the mammillary nuclei with the thalamic anterior group of nuclei as part of the Papez circuit (discussed with Figure 81). LIMBIC MIDBRAIN A number of limbic pathways terminate within the reticular formation of the midbrain (see Figures 40B, 64, and 65), including the periaqueductal gray, leading to the notion that this area be incorporated in the structures that comprise the limbic system – thus use of the term limbic midbrain. Two limbic pathways — the medial forebrain bundle, and the descending tract from the mammillary nuclei — terminate in the midbrain reticular formation. From there, apparently, descending pathways convey the “commands” to the parasympathetic and other nuclei of the pons and medulla (e.g., the dorsal motor nucleus of the vagus, the facial nucleus for emotional facial responses) and areas of the reticular formation of the medulla concerned with cardiovascular and respiratory control mechanisms (discussed with Figure 40A). Other connections are apparently made with autonomic neurons in the spinal cord (e.g., for sympathetic-type responses). ©2000 CRC Press LLC callosum pus r Co Septal region Midbrain Hypothalamic nuclei Medulla Mammillary n. FIGURE 83A: Hypothalamus and Limbic Midbrain FIGURE 83B SEPTAL REGION AND MEDIAL FOREBRAIN BUNDLE SEPTAL REGION The septal region includes both cortical and subcortical areas that belong to the forebrain. The cortical areas are found under the rostrum of the corpus callosum and include the subcallosal gyrus (see Figures 74 and 75). Nuclei lying deep in this region are called the septal nuclei and in some species (not humans) are in fact located within the septum pellucidum (the septum that separates the anterior horns of the lateral ventricles; see Figure 28A). Several decades ago experiments were done in rats with a small electrode implanted in the septal region; pressing a bar completed an electrical circuit that resulted in a tiny (harmless) electric current going through the brain tissue. It was shown that rats will quickly learn to press a bar to deliver a small electric current to the septal region. In fact, the animals will continue pressing the bar, virtually nonstop, even in preference to food! From this it has been inferred that the animals derive some type of pleasant sensation from stimulation of this region. Some call this septal region the “pleasure center”; it has since been shown that other areas can produce similar behavior. However, this type of positive effect is not seen in all parts of the brain, and, in fact, in some areas an opposite (negative) reaction is seen. The septal region receives input from the amygdala (via the stria terminalis; see Figure 79B) and the hippocampal formation (via the fornix; see Figure 77B). The major connection of the septal region with the hypothalamus and the limbic midbrain occurs via the medial forebrain bundle. MEDIAL FOREBRAIN BUNDLE Knowledge of the medial forebrain bundle of fibers is necessary if one is to understand the circuitry of the limbic system and how the limbic system influences the activity of the nervous system. The medial forebrain bundle (MFB) interconnects the septal region, the hy- ©2000 CRC Press LLC pothalamus, and the limbic midbrain; it is a two-way pathway. Part of its course is through the lateral part of the hypothalamus where the fibers become somewhat dispersed (as illustrated). The MFB connects the septal region with the hypothalamus and extends into the limbic midbrain. It is relatively easy to understand how the septal region and the hypothalamus can influence the behavior of the animal (e.g., autonomic activity via descending autonomic fibers). Note to student: Visualization of the location of this pathway is a definite challenge. The Habenula (not illustrated) In many texts, the habenular region of the diencephalon is labeled and supplementary information is provided about these structures. The habenular nuclei are a group of small nuclei which are part of the diencephalon. They are situated at the posterior end of the thalamus, on its upper surface. The pineal gland is attached to the brain in this region. There is another circuit whereby septal influences are conveyed to the midbrain. The first part of the pathway is the stria medullaris (note that confusion of terminology is definitely a possibility) which connects the septal nuclei (region) with the habenular nuclei. The stria medullaris is found on the medial surface of the thalamus. The tract is seen in Figure 16, a view of the medial aspect of the brain. It is not labeled but can be located above the letter “T” on the diagram. From the habenular nuclei, a tract descends to the midbrain reticular formation, mainly to a nucleus located between the cerebral peduncles, the interpeduncular nucleus (see cross section B2, Figure 65). This tract is best called the habenulo-interpeduncular tract. In some texts it is labeled the fasciculus retroflexus. The further connections of the interpeduncular nucleus are unclear but are thought to be similar to those of other midbrain reticular formation nuclei which have a limbic function. ©2000 CRC Press LLC Fornix Stria terminalis Third ventricle Dorsal longitudinal bundle Septal region “Limbic” midbrain Anterior commissure Mammillo-tegmental tract Descending autonomic fibers Medial forebrain bundle Medulla Midbrain Temporal lobe Parasympathetic nuclei Medial forebrain bundle Mammillary n. Pituitary stalk Hypothalamic nuclei – medial – lateral Amygdala Ventral amygdalofugal pathway FIGURE 83B: Septal Region and Medial Forebrain Bundle FIGURE 84 OLFACTORY SYSTEM The olfactory system, our sense of smell, is a sensory system that inputs directly to the limbic system, and does not have a thalamic nucleus. The olfactory system is a phylogenetically older sensory system. Its size depends somewhat on the species, being large in animals which have a highly developed sense of smell; in humans, the olfactory system is small. Its component parts are the olfactory bulb and tract, and various areas where the primary olfactory fibers terminate, including the amygdala and the cortex over the uncal region. Olfactory Nerve, Bulb, and Tract The sensory cells in the nasal mucosa project their axons into the CNS. These tiny fibers, which constitute the actual peripheral olfactory nerve (CN I), pierce the cribriform plate in the roof of the nose and terminate in the olfactory bulb, part of the CNS. There is a complex series of interactions in the olfactory bulb, and one cell type then projects its axon into the olfactory tract, a CNS pathway. The olfactory tract runs posteriorly along the inferior surface of the frontal lobe (see Figures 13 and 14) and terminates by dividing into lateral and medial tracts, called stria. At this dividing point there are a number of small holes for the entry of several blood vessels to the interior of the brain, the anterolateral striate arteries (discussed with Figure 60; see also Figures 85A and 85B). This triangular area is known as the anterior perforated space. It is best to remember only the lateral tract as the principal tract of the olfactory system. It is said to have cortical tissue along its course for the termination of some olfactory fibers. The lateral tract ends in the cortex of the uncal area (see Figures 13 and 14), with some of the fibers terminating in an adjacent part of the amygdaloid nucleus (see also Figure 79B). It is interesting to note that the olfactory system terminates directly in primary olfactory areas of the cortex without a thalamic relay. Olfactory Connections The connections of the olfactory system involve limbic cortex. These are called secondary olfactory areas and ©2000 CRC Press LLC include the cortex in the anterior portion of the parahippocampal gyrus, an area that has been referred to as entorhinal cortex. The term rhinencephalon refers to the olfactory parts of the CNS, the “smell brain.” This input of olfactory information into the limbic system makes sense if one remembers that one of the functions of the limbic system is procreation of the species. Smell is important in many species for mating behavior and for identification of the nest and territory. Olfactory influences may spread to other parts of the limbic system, including the amygdala and the septal region. Through these various connections, information can reach the dorsomedial nucleus of the thalamus. Smell is an interesting sensory system. We have all had the experience of a particular smell evoking a flood of memories, often associated with strong emotional overtones. This simply demonstrates the extensive connections that the olfactory system has with components of the limbic system and therefore with other parts of the brain. One form of epilepsy often has a significant olfactory aura that precedes the seizure itself. In such cases, the “trigger” area is often orbitofrontal cortex. This particular form of epilepsy has unfortunately been known as uncinate fits. The name is derived from a significant association bundle which interconnects this part of the frontal lobe and the anterior parts of the temporal lobe — the uncinate bundle. Diagonal Band This obscure fiber bundle and an associated nucleus are additional olfactory connections, some of which interconnect the amygdala with the septal region (see also Figure 85B). ©2000 CRC Press LLC callosum pus r Co Diagonal band Olfactory bulb Olfactory tract Lateral olfactory stria FIGURE 84: Olfactory System FIGURE 85A BASAL FOREBRAIN BASAL NUCLEUS Figure 85A shows the basal forebrain using the same diagram of the limbic system as in Figure 75. This area, previously called the substantia innominata, contains a variety of neurons. It is located below the anterior commissure and lateral to the hypothalamus. On the gross brain, this region can be found by viewing the inferior surface of the brain where the olfactory tract ends and divides into medial and lateral stria (see Figures 13, 14, and 84). This particular spot is the location where a number of blood vessels, the striate arteries, penetrate the brain substance, hence, it is called the anterior perforated space. The basal forebrain region is found above this area. The basal forebrain contains a group of diverse structures: • clusters of large cells that are cholinergic, and which have been collectively called the basal nucleus (of Meynert); • groups of cells that are continuous with the amygdala, now called the extended amygdala; • the ventral portions of the putamen and globus pallidus, namely the ventral striatum and ventral pallidum; and • the nucleus accumbens, which may include a number of diverse neurons within its boundaries. Cholinergic Neurons These are rather large neurons found in clusters throughout this region. The clusters of cells are collectively called the basal nucleus (of Meynert).These cells project to widespread areas of the prefrontal cortex, providing that area with cholinergic innervation. Several years ago it was reported that there was a depletion of acetylcholine in the frontal lobe areas in Alzheimer patients. Subsequent reports indicated that this depletion was accompanied by a loss of these cholinergic cells in the basal forebrain. Many thought that the “cause” of Alzheimer’s disease had been uncovered: a cellular degeneration of a unique group of cells and a neurotransmitter deficit. (The model for this way of thinking is Parkinson’s disease.) These reports were ©2000 CRC Press LLC followed immediately by several therapeutic trials using medication to boost the acetylcholine levels of the brain. It is currently thought that cortical degeneration is the primary event in Alzheimer’s disease, starting often in the parietal areas of the brain. We now know that several other neurotransmitters are depleted in the cortex in Alzheimer’s disease. This information would lead us to postulate that the loss of the target neurons in the prefrontal cortex, the site of termination for the cholinergic neurons, would be followed, or accompanied, by the degeneration of the cholinergic cells of the basal forebrain. Notwithstanding this current state of our knowledge, therapeutic intervention to boost the cholinergic levels of the brain is currently considered a valid therapeutic approach, particularly in the early stages of this tragic human disease. New drugs that maintain or boost the level of acetylcholine in the brain are currently undergoing evaluation and apparently some improvement in memory function has been reported, which may last for a short while. Extended Amygdala A group of cells extends medially from the amygdaloid nucleus and follows the ventral pathway (the ventral amygdalofugal pathway; Figures 75, 79A, 79B, 82, and 83B) through this basal forebrain region. These neurons receive a variety of inputs from the limbic cortical areas and from other parts of the amygdala. Its output projects to the hypothalamus and to autonomic-related areas of the brainstem, thereby influencing neuroendocrine, autonomic, and somatomotor activities. ©2000 CRC Press LLC callosum pus r Co Basal forebrain FIGURE 85A: Basal Forebrain — Basal Nucleus FIGURE 85B BASAL FOREBRAIN BASAL GANGLIA Figure 85B presents a somewhat schematic view of the various nuclei located in the basal forebrain area. The hypothalamus is shown in the midline, with the third ventricle. The penetrating striate arteries are seen in the anterior perforated area. This view shows the ventral pathway emerging from the amygdala with some of the fibers going to the hypothalamus and the others on their way to the dorsomedial nucleus of the thalamus. The anterior commissure demarcates the upper boundary of this area. The cell clusters that form the basal (cholinergic) nucleus are contained within this area but are not portrayed. Ventral Striatum and Pallidum The lowermost portion of the putamen and globus pallidus are found in the basal forebrain area; they are referred to as the ventral striatum and ventral pallidum. The ventral part of the striatum (the putamen) receives input from limbic cortical areas, as well as a dopaminergic pathway from a group of dopamine-containing cells in the midbrain. The information is then relayed to the ventral pallidum (both parts of the globus pallidus are seen on the left side of the diagram). This area has a significant projection to the dorsomedial nucleus of the thalamus, hence to prefrontal cortex. ©2000 CRC Press LLC The overall organization is therefore quite similar to that of the dorsal parts of the basal ganglia, although the sites of relay and termination are different. Just as the amygdala is now considered a limbic nucleus, many now argue that the ventral striatum and pallidum should be included with the limbic system. Nucleus Accumbens This group of cells contains neurons that are part of the basal ganglia and in part other possibly limbic neurons (see also Figure 24). It has many of the connections of the ventral striatum as well as those of the extended amygdala. Its functional contribution is still unknown, although it might be the neural area that becomes activated in situations that involve reward and punishment, integrating certain cognitive aspects of the situation with the emotional component. There is strong evidence that this area is involved in addiction behavior in animals and likely in humans. In summary, the region of the basal forebrain has important links with other parts of the limbic system. There is a major output to the prefrontal cortex, which is considered by some to be the forebrain component of the limbic system. The basal forebrain is thus thought to have a strong influence on drives and emotions, as well as higher cognitive functions that have an emotional component. The cholinergic neurons in this area might have a critical role in memory. ©2000 CRC Press LLC Septal region Diagonal band Nuclei of diagonal band Globus pallidus Putamen Ventral pallidum Ventral putamen Amygdala Nucleus accumbens (cut) Anterior commissure Hypothalamus Optic tract Third ventricle Striate branches Middle cerebral artery FIGURE 85B: Basal Forebrain — Basal Ganglia Ventral amygdalofugal pathway LIMBIC SYSTEM: SYNTHESIS After studying the structures and connections of the limbic system in some detail, a synthesis of the anatomical information with the notion of an “emotional” part of the brain seems appropriate. It is not easy to understand how the limbic system is responsible for the reactions required by the definition of “emotion” proposed in the introduction to this section. The key structures of the limbic system are the limbic lobe (the cortical regions, including the hippocampal formation), the amygdala, the hypothalamus, and the septal region. The limbic pathways interconnect these limbic areas (e.g., the Papez circuit). In many ways it seems that the limbic structures communicate with each other. What is not clear is how activity in these structures influences the rest of the brain. How does the limbic system influence changes in the physiological systems, motor activity (behavior), and mental state (psychological reactions)? The following discussion is presented as a way of understanding the outcome or output of limbic function. Hormonal and Homeostatic Responses Hormonal changes, as regulated by the hypothalamus, are part of the physiological responses to emotional states, both acute and chronic. The work of Dr. Hans Selye, for example, has shown how chronic stress influences our body and mind. Complex motor actions are often associated with responses to homeostatic changes. Consider, for example, the motor activities associated with thirst, temperature regulation, and satisfying other basic drives. The amygdala and septal region are likely involved in the motor patterns associated with these basic drives. Physiological Responses Some of the responses to the emotional states involve the autonomic nervous system which is regulated in the hypothalamus. Limbic activity involves areas of the midbrain reticular formation and other brainstem nuclei in specific ways. The best examples are perhaps the facial expressions associated with emotions, the responses to pain that are generated in part in the brainstem, and the various parasympathetic nuclei controlling the pupil, salivation, respiration, blood pressure, pulse, and various ©2000 CRC Press LLC gastrointestinal functions. The basic “fight or flight” response to emergency situations involves a considerable number of physiological reactions. Motor and Psychological Responses The ventral parts of the basal ganglia and various cortical areas are likely the areas of the CNS involved with the motor activities associated with emotional reactions. Neocortical areas that are involved in limbic function include portions of the prefrontal cortex, the cingulate gyrus, and the parahippocampal gyrus. Activity in these limbic cortices (and the associated thalamic nuclei) are clearly candidates for the psychological reactions of emotion. In summary, the limbic system has many connections outside itself through which it influences the hormonal, autonomic, motor, and psychological functions of the brain. The older cortical regions of the hippocampal formation seem to have an additional function related to the formation of new episodic memories, specifically related to events and factual information. Why this is so and how this evolved is a matter of speculation. One hopes that the basic activities of the limbic system that are involved in preservation of the self and species can be controlled and tamed by higher-order cortical influences, leading humankind to a more human and hopefully a more humane millennium. BRAINSTEM (HUMAN) CROSS SECTIONS The following figures are photographs of sections of the human brainstem. Because of the complexity of this part of the brain, including them seemed the best way to assist the student to visualize the structures labeled in the diagrams. Each section is indentified with • the part of the brainstem (e.g., upper midbrain), • the section level (e.g., B1), as in Figures 62 and 63, and • the corresponding illustration in Section C (e.g., Figure 64). The eight cross sections were matched as closely as possible with the diagrammatic illustrations of the brainstem shown in Figures 64–71 (from upper midbrain to lower medulla). Brains are removed at autopsy, usually for medical or legal reasons. This specimen was selected because the brain itself was not the primary reason for the death. Preservation of the brain tissue is invariably less than ©2000 CRC Press LLC perfect in the following figures because of the delay between the time of death and the autopsy. The brains are usually stored in 20% formalin. The brainstem was subdivided at approximately the levels indicated in Figures 62 and 63, embedded in paraffin, and sectioned at 15 µm. The sections were then stained with the Kluver-Barrera stain which used luxolfast blue to stain the myelinated fibers blue. The sections were counterstained with cresyl violet, resulting in a violet-reddish color of the neuronal cell bodies, the perikaryon. (The Nissl bodies are violet at higher magnification, with the cell cytoplasm taking on a reddish hue). In a black and white illustration, the areas with myelinated tracts are black, whereas the areas with cells and virtually no myelin are “clear” or white. These sections are not labeled here, and the student should refer to the corresponding level in the Atlas to identify the structures. These same sections are on the accompanying CD, in color, and are labeled there. Human brainstem: Upper Midbrain — B1 level; see Figure 64 Human brainstem: Lower Midbrain — B2 level; see Figure 65 ©2000 CRC Press LLC Human brainstem: Upper Pons — B3 level; see Figure 66 Human brainstem: Mid Pons — B4 level; see Figure 67 ©2000 CRC Press LLC Human brainstem: Lower Pons — B5 level; see Figure 68 Human brainstem: Upper Medulla — B6 level; see Figure 69 ©2000 CRC Press LLC Human brainstem: Mid Medulla — B7 level; see Figure 70 Human brainstem: Lower Medulla — B8 level; see Figure 71 ©2000 CRC Press LLC ANNOTATED BIBLIOGRAPHY This select list of references with commentary is designed to help the student learn about the structure, function, and diseases of the human brain. Recent publications have usually been preferentially selected. The listing includes texts, atlases, and videotapes, as well as CD-ROMs and web sites. This edited large text, with many color illustrations, is an excellent reference book, mainly for neuroanatomical detail. TEXTS A thorough presentation of the functional (physiological) aspects of the nervous system, which is suitable as a reference book and for graduate students. Adams, R. D., Victor, M., and Ropper, A. H., Principles of Neurology, 6th ed., McGraw-Hill, New York, 1997. A comprehensive neurology text — part devoted to cardinal manifestations of neurologic diseases and part to major categories of diseases. Asbury, A. K., McKhann, G. M., and McDonald, W. I., Diseases of the Nervous System: Clinical Neurobiology, 2nd ed., W. B. Saunders, Philadelphia, 1992. A complete neurology text, in two volumes, on all aspects of basic and clinical neurology and the therapeutic approach to diseases of the nervous system. Carpenter, M. B., Core Text of Neuroanatomy, 4th ed., Williams and Wilkins, Baltimore, 1991. A detailed presentation of the subject matter by a highly respected author. Also available is the full reference text [Carpenter’s Human Neuroanatomy (1995), now with A. Parent as the author]. Crossman, A. R. and Neary, D., Neuroanatomy, Churchill Livingstone, Edinburgh, 1995. A concise, well-illustrated (with color) presentation of the nervous system with many clinical correlations. This slim book contains much essential information. Guyton, A. C., Basic Neuroscience: Anatomy and Physiology, 2nd ed., W. B. Saunders, Philadelphia, 1991. This is a sufficient explanation of the physiological aspects of the nervous system, with handy diagrams. Haines, D. E., Fundamental Neuroscience, Churchill Livingstone, New York, 1997. ©2000 CRC Press LLC Kandel, E. R., Schwartz, J. H., and Jessell, T. M., Principles of Neural Science, 3rd ed., Elsevier, New York, 1991. Kiernan, J. A., The Human Nervous System, 7th ed., Lippincott-Raven, Philadelphia, 1998. This is the book previously authored by Dr. M. Barr. Clearly written and clearly presented neuroanatomical information, with a glossary and accompanying diskette. Kolb, B. and Whishaw, I. Q., Fundamentals of Human Neuropsychology, 4th ed., W. H. Freeman and Co., New York, 1996. A classic in the field and highly recommended for a good understanding of the human brain in action. Topics discussed include memory, attention, language, and the limbic system. Martin, J. H., Neuroanatomy: Text and Atlas, 2nd ed., Appleton & Lange, Stamford, CT, 1996. A very complete text with some fine illustrations, written as the neuroanatomical companion to Kandel et al. Includes an atlas section. Merritt, H. H., Merritt’s Textbook of Neurology, 9th ed., Williams and Wilkins, Baltimore, 1997. A well-known, complete and trustworthy neurology textbook, now edited by L. P. Rowland. Nolte, J., The Human Brain, 4th ed., Mosby, St. Louis, 1999. An excellent neuroscience text with anatomical and functional (physiological) information on the nervous system. Includes several hundred illustrations in full color, along with three-dimensional brain reconstructions by John Sundsten. Williams, P. and Warwick, R., Functional Neuroanatomy of Man, W. B. Saunders, Philadelphia, 1975. This is the “neuro” section from Gray’s Anatomy. Although somewhat dated, there is excellent reference material on the central nervous system, as well as on the nerves and autonomic parts of the peripheral nervous system. Wilson-Pauwels, L., Akesson, E. J., and Stewart, P.A., Cranial Nerves: Anatomy and Clinical Comments, B. C. Decker, Toronto, 1988. A handy resource on the cranial nerves, with some very nice illustrations. Relatively complete and easy to follow. Young, P.A. and Young, P. H., Basic Clinical Neuroanatomy, Williams and Wilkins, Baltimore, 1997. A clearly presented and well-illustrated (no color) text that integrates structure, function, and clinical aspects. Includes questions and answers, an appendix on cranial nerves, and a glossary, as well as a small atlas. ATLASES DeArmond, S. J., Fusco, M. M. and Dewey, M. M., Structure of the Human Brain: A Photographic Atlas, 2nd ed., Oxford University Press, New York, 1976. An excellent and classic reference to the neuroanatomy of the human CNS. No explanatory text and no color. England, M. A. and Wakely, J., Color Atlas of the Brain and Spinal Cord, Mosby Year Book, St. Louis, 1991. A very well-illustrated atlas, with most photographs and sections in color. Little in the way of explanatory text. Haines, D., Neuroanatomy: An Atlas of Structures, Sections and Systems, 4th ed., Williams and Wilkins, Baltimore, 1995. A popular atlas that has some fine photographs of the brain, some color illustrations of the vascular supply, a radiologic section, a very detailed atlas portion, and limited (schematic) presentation with text of functional systems. Hanaway, J., Woolsey, T. A., Gado, M. H., and Roberts, M. P., The Brain: A Visual Guide to the Human Central Nervous System, Fitzgerald Science Press, Maryland, 1998. ©2000 CRC Press LLC A complete pictorial atlas of the human brain, with some color illustrations and radiographic material. Netter, F. H., The CIBA Collection of Medical Illustrations, Volume 1: Nervous System, Part 1, Anatomy and Physiology, CIBA, Summit, NJ, 1983. A classic. Excellent illustrations of the nervous system, as well as of the skull, autonomic and peripheral nervous systems, and embryology. The text is interesting but might be dated. Nolte, J. and Angevine, J. B., The Human Brain in Photographs and Diagrams, Mosby, St. Louis, 1995. A well-illustrated, color atlas, with text, and neuroradiology. Functional systems are drawn both onto the sections and separately. Quite detailed. VIDEOTAPES BY THE AUTHOR These are edited video presentations on the skull and the brain as the material would be shown to students in the anatomy laboratory. They have been prepared with the same teaching orientation as the Atlas and are particularly useful for selfstudy or small groups. These videotapes of actual specimens are particularly useful for students who have limited or no access to actual brain specimens. The videotapes are fully narrated, and each lasts approximately 20–25 minutes. Interior of the Skull — A detailed look at the bones of the skull, the cranial fossa, and the various foramina for the cranial nerves and other structures. Includes views of the meninges and venous sinuses. The Gross Anatomy of the Human Brain Part I: The Hemispheres — A presentation on the hemispheres, the functional areas of the cerebral cortex, including the basal ganglia. Part II: Diencephalon, Brainstem and Cerebellum — A detailed look at the brainstem, with a focus on the cranial nerves, and a functional presentation of the cerebellum. Part III: Cerebrovascular System and Cerebrospinal Fluid — A presentation of these two subjects. Part IV: The Limbic System — A quite detailed presenta- tion on the various aspects of the limbic system, with much explanation and special dissections. Note: It is suggested that these videotapes be purchased by the library or by an institutional (or departmental) media or instructional resource center. Information regarding the purchase of these and other videotapes may be obtained from Health Sciences Consortium (a nonprofit publishing cooperative for instructional media), 201 Silver Cedar Ct., Chapel Hill, NC, USA, 27514-1517. Phone: (919) 942-8731. Fax: (919) 942-3689. CD-ROMS Numerous CDs are appearing on the market and their evaluation by the teaching faculty is critical before recommending them to the students. It is a difficult task indeed to review all the CDs now available and perhaps one that can be shared with the students after they have completed their program of study on the nervous system. Sundsten, John W., The Digital Anatomist - Interactive Brain Atlas, University of Washington. An excellent visual resource to the nervous system, using computer graphic reconstructions of the brain. Sundsten, John W. and Mulligan, K. A., The Digital Anatomist — Neuroanatomy Interactive Syllabus, University of Washington. A more functional presentation of the nervous system, with some explanatory text, using many of the same images. Both CDs are available from University of Washington, Health Sciences Center for Educational Resources Box 357161, Seattle, WA 98195-7161 Phone: (206) 685-1186. Fax: (206) 543-8051. e-mail: [email protected] Coppa, Gary and Tancred, Elizabeth, Brainstorm: Interactive Neuroanatomy, Stanford University. A highly interactive and well-integrated cross-linked presentation of the anatomy and some functional aspects of the nervous system. Published by Mosby, 11830 Westline Industrial Drive, P.O. Box 46908, St. Louis, MO., USA, 63146-9934 ©2000 CRC Press LLC WEB SITES Web sites are recommended to students only after the sites have been critically evaluated by the teaching faculty. If keeping up with various teaching texts and CD-ROMs is difficult enough, a critical evaluation of the various web sites is an impossible task for any single person. This is indeed a task to be shared with colleagues and students, and perhaps by a consortium of teachers. At this point, only a limited number of sites can be suggested. For the time being, it is still an open playing field. General Neuroscience http://faculty.washington.edu/chudler/ehc.html This site is maintained by Eric H. Chudler, Ph.D., Research Associate Professor, Dept. of Anesthesiology, University of Washington, Box 356540, Seattle, WA 98195-6540, [email protected]. This site focuses on systems neuroscience and includes numerous excellent Internet resources concerning all other aspects of neuroscience. Links on this page are limited to those that the author has found to be the most interesting and useful. Examples of links: • Neuroscience Education • Neuroscience Journals • Neuroscience, Psychology • Neurological Disorders (Alzheimer’s Disease, Huntington’s) • Searching the Web • Search Engines • Miscellaneous Links • Reference, Science • Neuroscience for Kids Neurological Disorders http://www.dana.org/brainweb/ This site has links to information on brain diseases and disorders maintained by the Dana Foundation. The Dana Alliance for Brain Initiatives, an independent nonprofit organization of more than 175 pre-eminent neuroscientists, including six Nobel laureates, recommends the Internet sites reviewed below as helpful resources for people concerned about brain diseases and disorders. Send your comments and questions to [email protected]. The links include the following diseases: • Alcohol and drug abuse • Alzheimer’s disease • ALS (Lou Gehrig’s disease) • Anxiety • Autism • Blindness/vision impairment • Brain tumors • Cerebral palsy • Deafness/hearing impairment • Depression/manic depression • Epilepsy Biological Structure, University of Washington, Seattle, WA. The Atlas is available on CD-ROM and there are similar movie materials on the videodisc from the University of Washington Health Sciences Center for Educational Resources (see CD-ROMS above). Neuroanatomy Interactive Syllabus Authors: John W. Sundsten and Kathleen A. Mulligan Content: This Syllabus uses the images in the Neuroanatomy Atlas above, and many others. It is organized into functional chapters suitable as a laboratory guide, with an instructive text accompanying each image. It contains 3-D computer graphic reconstructions of brain material, MRI scans, tissue sections (some enhanced with pathways) gross brain specimens and dissections; and summary drawings. • Headache • Head injury • Huntington’s disease • Learning disabilities • Multiple sclerosis • Pain (chronic) • Parkinson’s disease • Schizophrenia • Sleep disorders • Spinal cord injury • Stroke • Tourette syndrome • General health and neuroscience sites Inclusion of any particular organization does not imply endorsement by the Charles A. Dana Foundation or the Dana Alliance for Brain Initiatives. The information provided is not a substitute for medical advice; be sure to consult your doctor for diagnosis and treatment. Digital Anatomist Project http://www9.biostr.washington.edu/ Interactive Brain Atlas Author: John W. Sundsten Content: 2-D and 3-D views of the brain from cadaver sections, MRI scans, and computer reconstructions. Institution: Digital Anatomist Program, Dept. of ©2000 CRC Press LLC Chapters include: • Topography and development • Vessels and ventricles • Spinal cord, brainstem, and cranial nerves • Sensory and motor systems • Cerebellum and basal ganglia • Eye movements • Hypothalamus and limbic system • Cortical connections and forebrain • MRI scan serial sections Institution: Digital Anatomist Program, Department of Biological Structure, University of Washington, Seattle,WA. Note: The Neuroanatomy Interactive Syllabus is available on CD-ROM (Java program running on Mac and PC platforms) from the University of Washington Health Sciences Center for Educational Resources. See above under CD-ROMs. E-mail: [email protected] The Whole Brain Atlas http://www.med.harvard.edu:80/AANLIB/home.html Authors: K. Johnson (Harvard) and J. Becker (MIT) This presents a gallery of images on the normal and diseased brain(cerebrovascular, tumor, degenerative conditions). GLOSSARY Abducens nerve Sixth cranial nerve (CN VI); to lateral rectus muscle of the eye. Accessory nerve Eleventh cranial nerve (CN XI); see Spinal accessory nerve. Afferent Toward (sensory if toward the CNS). Agnosia Lack of ability to recognize the significance of sensory stimuli (auditory, visual, tactile). Agraphia Inability to express thoughts in writing because of a central lesion. Akinesia Absence, loss, or weakness of motor function; lack of spontaneous movement (as in Parkinson’s disease). Alexia Word blindness; inability to read due to a central lesion. Allocortex The phylogenetically older cerebral cortex, consisting of less than six layers. Includes paleocortex (e.g., subicular region = 3–5 layers) and archicortex (e.g., hippocampus proper = 3 layers). Ammon’s horn Amygdala Angiogram Anopsia ©2000 CRC Press LLC The hippocampus, which has an outline in cross section suggestive of a ram’s horn. Also known as the cornu ammonis (CA). Amygdaloid nucleus or body in the temporal lobe of the cerebral hemisphere. It is a nucleus of the limbic system. Display of blood vessels, for diagnostic purposes, by using contrast medium injected into the vascular system and x-rays or using MRI. A defect of vision (e.g., hemianopsia — loss of one half of visual field). Antidromic Relating to the propagation of an impulse along an axon in a direction that is the reverse of the normal or usual direction. Aphasia A disruption or disorder of language; specifically a deficit of expression by speech or of comprehending spoken or written language. Apraxia Inability to carry out purposeful or skilled movements despite the preservation of power, sensation, and coordination. Arachnoid The middle meningeal layer, forming the outer boundary of the subarachnoid space. Archicerebellum A phylogenetically old part of the cerebellum, functioning in the maintenance of equilibrium. Includes flocculonodular lobe. Archicortex Three-layered cortex included in the limbic system; located mainly in the hippocampus proper and dentate gyrus of the temporal lobe. Area postrema An area in the caudal part of the floor of the fourth ventricle, with no blood-brain-barrier, involved in vomiting. Areflexia Loss of reflexes (usually tested using the stretch/deep tendon reflex). Ascending tract Central sensory pathway, usually from spinal cord to brainstem, cerebellum, or thalamus. Association fibers Fibers connecting parts of the cerebral hemisphere, on the same side. Astereognosis Loss of ability to recognize objects or to appreciate their form by touching or feeling them. Astrocyte A type of neuroglial cell. Asynergy Disturbance of the proper sequencing in the contraction of muscles, at the proper moment, and of the proper degree, so that the act is not executed accurately or smoothly. Ataxia Athetosis Autonomic A loss of coordination of voluntary movements. An affliction of the nervous system, caused by degenerative changes in the striatum, characterized by bizarre, writhing movements of the fingers and toes. Autonomic system; usually taken to mean the efferent or motor innervation of viscera (smooth muscle and glands). the subthalamus, and the substantia nigra. Basilar artery The major artery supplying the brainstem and cerebellum, formed by the two vertebral arteries. Brachium With regard to the CNS, denotes a large bundle of fibers connecting one part with another (e.g., brachia associated with the colliculi of the midbrain). Bradykinesia Abnormal slowness of movements (seen usually in Parkinson’s disease). Brainstem In the mature human brain, usually denotes the medulla, pons, and midbrain. Brodmann areas Numerical subdivisions of the cerebral cortex on the basis of histological differences between different functional areas (e.g., area 4 is the motor cortex; area 17 is the primary visual area). Bulb Referred at one time to the medulla oblongata but, in the context of “cortico-bulbar tract,” refers to the brainstem, in which motor nuclei of cranial nerves are located. CAT or CT scan Computerized axial tomography — a diagnostic imaging technique that uses x-rays and computer reconstruction of the brain. Carotid siphon Hairpin bend of the internal carotid artery within the skull. Cauda equina Translates as horse’s tail; the lower lumbar, sacral, and coccygeal spinal nerves as they lie in the subarachnoid space within the lumbar (CSF) cistern. Caudal Towards the tail, or hindmost part, of neuraxis. Caudate nucleus Part of the neostriatum, consists of a head, body, and tail (which extends into the temporal lobe). Autonomic nervous Visceral innervation; sympathetic system and parasympathetic divisions. Axon Efferent process of a neuron conducting impulses to other neurons or to muscle fibers (striated and smooth) and gland cells. Babinski reflex Actually an incorrect term — should be a Babinski response. Stroking the outer border of the sole of the foot in an adult normally results in a plantar (downgoing) of the toes. The Babinski response indicates a lesion of the Pyramidal tract and consists of an upgoing of the first toe and a fanning of the other toes. Ballismus Basal ganglia (nuclei) ©2000 CRC Press LLC Violent jerking or flinging movements of a limb, usually on one side (hemiballismus) or of one limb, due to a lesion of the subthalamic nucleus. Nuclei involved in motor control, the caudate, putamen and globuspallidus (the lentiform nucleus), Central nervous system Cerebellar peduncle Inferior, middle, and superiorfiber tracts linking cerebellum and brainstem. Cerebellum Cerebral aqueduct (of Sylvius) Cerebral peduncle (The little brain) An older part of the brain with motor functions, dorsal to the brainstem, situated in the posterior cranial fossa. Passage carrying CSF through midbrain; part of ventricular system. Descending cortical fibers in the basal portion of the midbrain; sometimes includes the substantia nigra (located immediately behind). Cerebrospinal fluid CSF; fluid in ventricles and in subarachnoid space (and cisterns). Cerebrum The principal portion of the brain, including the diencephalon and cerebral hemispheres, but not the brainstem and cerebellum. Cervical Referring to the neck region; the part of the spinal cord that supplies the structures of the neck. Chorda tympani Part of the seventh cranial nerve (CN VII) (see Facial nerve) ; carrying taste from anterior two thirds of tongue and parasympathetic innervation. Chordotomy Cutting of the spinothalamic tract for intractable pain (tractotomy). Also spelled cordotomy. Chorea A disorder characterized by irregular, spasmodic, involuntary movements of the limbs or facial muscles. Attributed to degenerative changes in the neostriatum. Choroid A delicate membrane; choroid plexuses are found in the ventricles of the brain. Choroid plexus Vascular structures “secreting” CSF into ventricles. Cingulum A bundle of association fibers in ©2000 CRC Press LLC the white matter under cingulate gyrus; part of Papez (limbic) circuit. CNS; brain and spinal cord. Circle of Willis Anastomosis between internal carotid and basilar arteries around pituitary. Cistern(a) Expanded portion of subarachnoid space containing CSF, e.g., cisterna magna (cerebellomedullary cistern); lumbar cistern. Claustrum A thin sheet of gray matter, of unknown function, situated between the lentiform nucleus and the insula. CNS Abbreviation for central nervous system. Colliculus A small elevation or mound. Superior and inferior colliculi comprising the tectum of the midbrain; facial colliculus in the floor of the fourth ventricle. Commissure A bundle of nerve fibers connecting structures on one side to the other in the hemispheres (e.g., corpus callosum). Conjugate eye movements Movement of both eyes together (so that image falls on the corresponding points of both retinas). Contralateral On the opposite side. Corona radiata Fibers radiating from the internal capsule to various parts of the cerebral cortex. A term often used by neuroradiologists. Corpus callosum The main (largest) neocortical commissure of the cerebral hemispheres. Corpus striatum Caudate, putamen, and globus pallidus nuclei inside cerebral hemisphere, with motor function; part of the basal ganglia. Cortex Outer layer of gray matter (neurons and neuropil) of the cerebral hemispheres (mostly six layers) and cerebellum (three layers). Cortico-bulbar tract Descending tract connecting motor cortex with motor cranial nerve nuclei and other nuclei of brainstem (including reticular formation). Cortico-fugal fibers Axons carrying impulses away from the cerebral cortex. Cortico-petal fibers Axons carrying impulses towards the cerebral cortex. Cortico-spinal tract Descending tract, from motor cortex to anterior (ventral) horn cells of the spinal cord (sometimes direct); also called Pyramidal tract. Cranial nerve nuclei Collections of cells in brainstem giving rise to or receiving fibers from cranial nerves (CN III–XII); may be sensory, motor, or autonomic. Cranial nerves Twelve pairs of nerves arising from the brain and innervating structures of the head and neck (CN I and II are actually CNS tracts). CSF Cerebrospinal fluid in ventricles, subarachnoid space, and cisterns. Cuneatus (Cuneate) Sensory tract (fasciculus cuneatus) of the dorsal column of spinal cord, from the upper limbs and body; cuneate nucleus of medulla. Decerebrate posturing Characterized by extension of the upper and lower limbs; lesion at the brainstem level between the vestibular nuclei and the red nucleus. Decorticate posturing Characterized by extension of the lower limbs and flexion of the upper; lesion above the level of the red nucleus. Decussation The point of crossing of paired tracts. Decussations of the pyramids, medial lemnisci, and superior cerebellar peduncles are examples. ©2000 CRC Press LLC Dendrite Receptive process of a neuron. Dendritic spine Cytoplasmic excrescence of a dendrite and site of an excitatory synapse (can be visualized using special stains at the light microscopic level). Dentate (Toothed) Dentate nucleus of the cerebellum (intracerebellar nucleus); dentate gyrus of the hippocampal formation. Descending tract Central motor pathway (e.g., from cortex to brainstem or spinal cord). Diencephalon Consisting of the thalamus, epithalamus (pineal), subthalamus, and hypothalamus. Diplopia Double vision. Dominant hemisphere The hemisphere responsible for language; this is the left hemisphere in about 85–90% of people. Dorsal column Fasciculus (tract) gracilis and fasciculus cuneatus of spinal cord, pathways for fine touch and conscious proprioception and vibration. Dorsal root Afferent sensory component of spinal nerve. Dorsal root ganglion A group of peripheral neurons whose axons carry afferent information from the periphery; their central process enters the spinal cord. Dura Dura mater, the thick external layer of the meninges (brain and spinal cord). Dural venous sinuses Large venous channels for draining blood from the brain; run within dura mater of the skull. Dyskinesia Abnormality of motor function, characterized by involuntary, purposeless movements. Fasciculus A large tract or bundle of nerve fibers. Disturbance of the ability to control the range of movement in muscular action. Fasciculus cuneatus Part of dorsal column; ascending tract for conscious proprioception and discriminative touch (from upper body and upper limb). Dysphagia Difficulty in swallowing. Fasciculus gracilis Efferent Away from the central nervous system; usually means motor to muscles. Part of dorsal column; ascending tract for conscious proprioception and discriminative touch (from lower body and lower limb). Emboliform Emboliform nucleus of the cerebellum, one of the intracerebellar (deep cerebellar) nuclei. Fastigial nucleus One of the deep cerebellar (intracerebellar) nuclei. Fiber Synonymous with an axon (either peripheral or central). Fimbria A band of nerve fibers along the medial edge of the hippocampus, continuing as the fornix. Flaccid paralysis Muscle paralysis with hypotonia due to a lower motor neuron lesion. Flocculus Lateral part of flocculonodular lobe (vestibulocerebellum). Folium/folia A flat, leaflike fold of the cerebellar cortex. Plural is folia. Foramen An opening between spaces (e.g., Monro and Magendie). Foramen of Luschka Lateral foramen of fourth ventricle. Foramen of Magendie Median foramen of fourth ventricle. Foramen of Monro Between each lateral ventricle and third ventricle. Forebrain Anterior division of embryonic brain; cerebrum and diencephalon. Fornix The efferent (noncortical) tract of hippocampal formation, arching over the thalamus and terminating in the mammillary nucleus of the hypothalamus and in the septal region. Fourth ventricle Cavity between brainstem and cerebellum, containing CSF. Dysmetria Entorhinal Ependyma The entorhinal area is the anterior part of the parahippocampal gyrus of the temporal lobe adjacent to the uncus. It is involved with olfaction (smell). Lining epithelium of the ventricles of brain, choroid plexus (with specialized junctions here), and also central canal of spinal cord. Epithalamus A region of the diencephalon above the thalamus; includes the habenula and pineal body. Extrapyramidal system In broadest terms, consists of all motor parts of the central nervous system except the Pyramidal motor system. “extrapyramidal system” is subject to various interpretations and is most often used clinically to mean basal ganglia. Facial nerve Falx ©2000 CRC Press LLC Seventh cranial nerve (CN VII); Motor to muscles of facial expression; carries taste from anterior two thirds of tongue (see also chorda tympani); also parasympathetic to two salivary glands, lacrimal, and nasal glands. Two of the dural partitions in the cranial cavity: the large falx cerebri between the cerebral hemispheres, and the small falx cerebelli. Frontal lobe Part of cerebral hemisphere, in front of central fissure. Funiculus A large aggregation of white matter in the spinal cord, can contain several tracts. Ganglion/ganglia Geniculate bodies Genu Glial cell Globus pallidus A swelling composed of nerve cells, as in dorsal root and sympathetic ganglion. Also used inappropriately for certain regions of gray matter in the brain (e.g., basal ganglia of the cerebral hemisphere). Plural is ganglia. Medial and lateral, specific relay nuclei of thalamus, for auditory (medial) and visual (lateral) pathways. Knee or bend; middle part of internal capsule; genu of facial nerve. Also geniculate nuclei of thalamus, and geniculate ganglion of facial nerve. Supporting cell in central nervous system (astrocyte and oligodendrocyte); also called neuroglial cell. Medial part of lentiform nucleus of corpus striatum; efferent part of basal ganglia. Glossopharyngeal nerve Ninth cranial nerve (CN IX); to muscles of swallowing and carries taste from posterior one third of tongue. Needed for gag reflex. Gracilis (Gracile) Sensory tract (fasciculus gracilis) of the dorsal column of spinal cord; nucleus gracilis of medulla. Granule Used to denote small neurons, such as granule cells of cerebellar cortex and stellate cells of cerebral cortex. Hence granular cell layers of both cortices. Gray matter Nerve tissue, mainly nerve cell bodies and adjacent neuropil; looks grayish after fixation in formalin. ©2000 CRC Press LLC Gyrus/gyri A convoluted fold of a cerebral hemisphere; includes cortex and white matter. Plural is gyri. Habenula A small swelling in the epithalamus, adjacent to the posterior end of the roof of the third ventricle; part of the limbic system. Hemiballismus A violent form of motor restlessness involving one side of the body, caused by a destructive lesion involving the subthalamic nucleus. Hemiparesis Muscular weakness affecting one side of the body. Hemiplegia Paralysis of one side of the body. Hindbrain Posterior division of the embryonic brain; pons, medulla, and cerebellum of adult. Hippocampus/ hippocampus proper Specialized area of phylogenetically old (three-layered) cortex; located in medial part of temporal lobe; part of hippocampal formation of limbic system. Hydrocephalus Excessive accumulation of cerebrospinal fluid. Hyperreflexia Abnormal increase in muscle (deep tendon/stretch) reflexes; usually seen with spasticity as a result of upper motor neuron lesion. Hypertonia Increased tone of muscles manifested by increased resistance to passive stretch or movements. Hypoglossal nerve Twelfth cranial nerve (CN XII); to muscles of the tongue. Hypothalamus A region of the diencephalon that serves as the main controlling center of the autonomic nervous system and is involved in several limbic circuits. Also involved in regulation of the pituitary gland. Infarction Local death of tissue because of loss of blood supply. Infundibulum (Funnel) Infundibular stem of the neurohypophysis (posterior pituitary). Innervation Nerve supply, sensory or motor. Insula (Island) Cerebral cortex concealed from surface view and lying at the bottom of the lateral fissure (also called the island of Reil). Internal capsule White matter between lentiform nucleus and head of caudate nucleus, and thalamus; consists of anterior limb, genu, and posterior limb. Internal carotid artery One of the pair of arteries supplying the brain. Interventricular foramen (of Monro); two openings from each lateral ventricle into third ventricle. Ipsilateral On the same side. Isocortex Cerebral cortex having six layers (neocortex). Kinesthesia The sense of perception of movement. Lacune Irregularly shaped venous “lake” or channel draining into the superior sagittal sinus; also the pathological “hole” after an infarct in the internal capsule. Lateral foramen (Foramen of Luschka) Openings in lateral edges of fourth ventricle for escape of CSF into subarachnoid space (cerebello-medullary cistern). Lateral ventricle Cavity, one in each cerebral hemisphere, containing CSF; consists of anterior horn, body, atrium (or trigone), posterior horn, and inferior horn (in temporal lobe). Lemniscus Used to designate a bundle of nerve fibers (pathway) in the central nervous system (e.g., medial lemniscus and lateral lemniscus). ©2000 CRC Press LLC Lentiform Lens-shaped; lentiform nucleus, a component of the basal ganglia. Also called lenticular nucleus; composed of putamen and globus pallidus. Leptomeninges Arachnoid and pia mater, part of meninges. Lesion Any injury or damage — vascular, tumor, traumatic, etc. Limbic system Part of brain associated with emotional behavior. Limbus (Border) Limbic lobe; a C-shaped configuration of cortex on the medial surface of the cerebral hemisphere, consisting of the cingulate and parahippocampal gyri, and the hippocampal formation. Locus ceruleus A small nucleus of the uppermost pons on each side of the floor of the fourth ventricle; contains pigment in freshly sectioned brain. Lower motor neurons Anterior horn cells (and their axons) of spinal cord, or cells in motor cranial nerve nucleus; also alpha motor neuron. Lumbar Referring to the lower back region. Mammillary Mammillary bodies; nuclei of the hypothalamus which are seen as small swellings on the ventral surface of diencephalon (also spelled mamillary). Massa intermedia A bridge of gray matter connecting the thalami of the two sides across the third ventricle; present in 70% of human brains. Also called the inter-thalamic adhesion. Medial lemniscus Brainstem portion of sensory pathway for fine touch and conscious proprioception, after synapse in nucleus gracilis and nucleus cuneatus. Medial longitudinal A tract throughout the brainstem fasciculus (MLF) and cervical spinal cord which interconnects visual and vestibular input with movements of the eyes and the head and neck. Neocortex Six-layered cortex, characteristic of mammals and constituting most of the cerebral cortex in humans. Neostriatum The phylogenetically newer part of the basal ganglia consisting of the caudate nucleus and putamen; the striatum. Medulla Caudal portion of the brainstem; in current usage, “medulla” refers to the medulla oblongata. Meninges Covering layers of the CNS — dura, arachnoid, and pia. Nerve fiber Axonal cell process, plus sheathing cells, plus myelin if present. Mesencephalon The midbrain (upper part of the brainstem). Neuraxis Metathalamus The medial and lateral geniculate bodies (nuclei). The straight longitudinal axis of the embryonic or primitive neural tube, bent in later evolution and development. Midbrain The middle division of the embryonic brain, part of the adult brainstem. Also known as mesencephalon. Neuroglia Accessory or interstitial cells of the CNS; includes astrocytes, oligodendrocytes, microglial cells, and ependymal cells. Mnemonic Pertaining to memory. Neuron Motor Having to do with movement or response. MRI/NMR Magnetic resonance imaging, a diagnostic imaging technique that does not use x-rays (uses extremely strong magnet). The morphological unit of the nervous system, consisting of the nerve cell body and its processes (dendrites and axon). Neuropil A complex net of nerve cell processes — axon terminals and dendrites and synapses — occupying the intervals between cell bodies in gray matter. NMR/MRI Nuclear magnetic resonance, also known as magnetic resonance image (MRI); a diagnostic imaging method for brain tissue and other organs. Nociceptive Refers to an injurious stimulation. Node of Ranvier Gap in myelin sheath between two successive Schwann cells or oligodendrocytes; necessary for saltatory (rapid) conduction. Nucleus/nuclei An aggregation of nerve cells within the CNS. In histology, the nucleus of a cell. Plural is nuclei . Nystagmus An involuntary oscillation of the eye(s). Occipital lobe Part of cerebral hemisphere, mostly related to vision. Oculomotor nerve Third cranial nerve (CN III); to most muscles of the eye, and to iris and lens. Myelin Myelin sheath Myotatic reflex Neocerebellum ©2000 CRC Press LLC The layers of lipid and protein substances forming a sheath around nerve fibers which are important for rapid nerve conduction. Covering of nerve fiber, formed and maintained by Schwann cell (PNS) or oligodendrocyte (CNS). A deep tendon reflex which causes a reflex contraction of the same muscle; monosynaptic from muscle spindle afferents to anterior horn cell. Also spelled myotactic reflex. The phylogenetically newest part of the cerebellum, present in mammals and especially well developed in humans. Ensures smooth muscle action in the finer voluntary movements and involved in motor planning. Olfactory nerve First cranial nerve (CN I); special sense of smell. Oligodendrocyte A neuroglial cell; forms and maintains myelin sheath in the CNS in the same manner as the Schwann cell in peripheral nerves. PET Positron emission tomography; a neuroimaging technique used to visualize areas of the living brain which become “activated” under certain task conditions. Pia Pia mater; the thin innermost layer of the meninges, attached to the surface of the brain and spinal cord. Forms the inner boundary of the subarachnoid space. Optic chiasm(a) Partial crossing of optic nerves (nasal half of retina representing the temporal visual fields), after which the optic tracts are formed. Optic nerve Second cranial nerve (CN II); for special sense of vision (actually a tract of the CNS). Pineal Pertaining to the pineal body; also called the pineal gland (part of epithalamus). Paleocortex Phylogenetically older cerebral cortex consisting of three to five layers (e.g., subicular region). Plexus An arrangement of interwoven vessels or nerves that form a network. Papilledema Edema of the optic disc, visualized with an ophthalmoscope (also called a choked disc); usually a sign of abnormal increased intracranial pressure. PNS Peripheral nervous system. Pons (Bridge) That part of the brainstem that lies between the medulla and the midbrain; appears to constitute a bridge between the right and left halves of the cerebellum. Proprioception The sense of body position (conscious or unconscious). Proprioceptor One of the specialized sensory endings in muscles, tendons, and joints; provides information concerning movement and position of body parts (proprioception). Ptosis Drooping of the upper eyelid. Pulvinar The posterior nucleus of the thalamus; involved with vision. Putamen The larger and lateral part of the lentiform nucleus; part of the neostriatum (with the caudate nucleus) of the basal gangla. Pyramidal system Called such because the corticospinal tracts occupy the pyramidshaped areas on the ventral surface of the medulla. Pyramidal tract refers specifically to the cortico-spinal tract. Quadrigeminal (plate) Referring to the tectum of the Paralysis Loss of voluntary action. Paraplegia Paralysis of both legs and lower part of trunk. Paresis Muscle weakness. Paresthesia Abnormal sensation, tingling (pins and needles). Pathway A chain of functionally interconnected neurons (nuclei) and their axons, making a connection between one region of CNS and another; a tract, e.g., visual pathway, sensory pathway (dorsal column — medial lemniscus). Peduncle A thick stalk or stem; bundle of nerve fibers. (Note cerebral, from the cerebral cortex, in midbrain; also three cerebellar). Perikaryon The cytoplasm surrounding the nucleus. Sometimes refers to the cell body of a neuron. Peripheral nervous system Nerve roots, peripheral nerves, and ganglia (motor, sensory, and autonomic) outside the CNS. ©2000 CRC Press LLC midbrain with the four colliculi (also called the tectum). Quadriplegia Paralysis affecting the four limbs (also called tetraplegia). Raphe An anatomical structure in the midline; in the brainstem, several nuclei of the reticular formation are in the midline of the medulla, pons, and midbrain (these nuclei use serotonin as the neurotransmitter). Septum pellucidum A triangular double membrane separating the frontal horns of the lateral ventricles. Situated in the median plane, it fills in the interval between the corpus callosum and the fornix. Somatic Nucleus in the midbrain (reddish color in fresh material). Used in neurology to denote the body, exclusive of the viscera (as in somatic efferent neurons supplying the skeletal musculature). Somatic senses Reticular formation of brainstem; pertaining to or resembling a net. Touch, pain, temperature, pressure, proprioception, vibration. Somatotopic Reticular formation Diffuse nervous tissue nuclei and connections in brainstem; quite old phylogenetically. The orderly representation of the body parts in CNS pathways, nuclei, and cortex Somesthetic The consciousness of having a body. Somesthetic senses are the general senses of pain, temperature, touch, pressure, position, movement, and vibration. Spasticity Increased resistance to passive stretch of the antigravity muscles, usually flexors of the upper limb and extensors of the lower limb in humans. Special senses Sight, hearing, balance, taste (gustatory), and smell (olfactory). Spinal accessory nerve Eleventh cranial nerve (CNXI);usually refers to the part of the nerve that originates in the upper spinal cord (C1–5) and in nervates the sternomastoid and trapezius. Spinal shock Complete “shut down” of all spinal cord activity (in humans) below a lesion, following a sudden interruption of cortical input (e.g., severed cord after a diving or motor vehicle accident). Spino-cerebellar tracts Ascending tracts, anterior and posterior, for “unconscious” proprioception to cerebellum. Spino-thalamic tracts Ascending tracts for pain and temperature (lateral), and nondiscriminative or light touch and pressure (anterior). Red nucleus Reticular Rhinencephalon Refers in humans to structures related to the olfactory system. Rigidity Stiffness; usually applied to muscles in Parkinson’s disease in which there is increased resistance to passive movement of both flexors and extensors. Rostral Towards the nose, or the most anterior end of the neuraxis. Rubro- Red; pertaining to the red nucleus, as in rubro-spinal tract and cortico-rubral. Saccadic To jerk; extremely quick movements of both eyes together (conjugate movement) in altering direction of gaze. Sacral Referring to the pelvic region. Schwann cell Sheathing cell of peripheral nerve fibers; responsible for formation and maintenance of myelin. Secretomotor Motor nerve supply to a gland. Sensory Having to do with receiving information, usually from the environment. Septal region ©2000 CRC Press LLC A cortical area ventral to the anterior end of the corpus callosum on the medial aspect of the frontal lobe that includes the septal nuclei. Split brain A brain in which the corpus callosum has been severed in the midline, usually as a therapeutic measure for intractable epilepsy. Subthalamus Region of the diencephalon beneath the thalamus, containing fiber tracts and the subthalamic nucleus; part of basal ganglia. Strabismus A squint; deviation of the eyes; lack of parallelism of the visual axes of the eyes. Sulcus/sulci Stria A slender strand of fibers (e.g., stria terminalis from amygdala). Groove between adjacent gyri of the cerebral cortex (a deep sulcus may be called a fissure). Plural is sulci. Synapse Area of structural and functional specialization between neurons where transmission occurs (excitatory, inhibitory, or modulation) using neurotramsmitter substances (e.g., glutamate, GABA). Syringomyelia A condition characterized by central cavitation of the spinal cord and gliosis around the cavity. Tectum Roof of the midbrain (behind the aqueduct) consisting of the paired superior and inferior colliculi; also called the quadrigeminal plate. Tegmentum The “core area” of the brainstem, between the ventricle (or aqueduct) and the cortico-spinal tract. Contains the reticular formation, cranial nerve and other nuclei, and various tracts. Telencephalon Rostral part of embryonic forebrain; primarily cerebral hemisphere of adult brain. Tentorium The tentorium cerebelli is a dural partition between the occipital lobes of the cerebral hemispheres and the cerebellum; a hiatus or notch allows passage of brainstem (midbrain). Thalamus A major portion of the diencephalon with sensory, motor, and integrative functions; consists of several nuclei with connections to areas of the cerebral cortex. Third ventricle Cavity (midline) in diencephalon, containing CSF. Striatum The phylogenetically more recent part of the basal ganglia (neostriatum) consisting of the caudate nucleus and the putamen (lateral portion of the lentiform nucleus). Stroke A sudden severe attack; usually refers to a sudden loss of neurologic function. Mostly this is due to a vascular lesion, either infarct (embolus, occlusion) or hemorrhage. Subarachnoid space Space between arachnoid and pia mater, containing CSF. Subcortical Not in the cerebral cortex, i.e., at a functionally or evolutionary lower level in the central nervous system; also refers to white matter of the cerebral hemispheres. Subicular region Transitional cortex (3–5 layers) between that of the parahippocampal gyrus and the hippocampus proper; part of limbic system. Substantia gelatinosa A cluster (nucleus) of small neurons at the apex of the dorsal gray horn throughout the spinal cord; receives pain and temperature afferents. Substantia nigra ©2000 CRC Press LLC A large nucleus with motor functions in the midbrain consisting of two parts; many of the constituent cells in the pars compacta contain dark melanin pigment. These neurons degenerate in Parkinson’s disease; the pars reticulata is an output nucleus of the basal ganglia. Vagus Tenth cranial nerve (CN X); supplies motor fibers to larynx; the major parasympathetic nerve to organs of the thorax and abdomen. Velum A membranous structure; The superior medullary velum forming the roof of the fourth ventricle. Transverse crossing fibers of the auditory pathway situated in the ventral portion of the tegmentum of the lower pons. Ventral root Efferent (motor) component of mixed spinal nerve. Ventricles CSF-filled cavities inside the brain. Fifth cranial nerve (CN V). The major sensory nerve of the head (face, eye, tongue, nose, sinuses); also supplies muscles of mastication. Vermis Unpaired midline portion of cerebellum between hemispheres. Vertebral artery An artery (one of a pair) supplying spinal cord and brainstem. Vestibulocochlear Eighth cranial nerve (CN VIII); special senses of hearing and balance (acoustic nerve is not correct). Visceral Referring to internal organs. White matter Nerve tissue made up of nerve fibers (axons), some of which are myelinated; appears whitish after fixation in formalin. Tomography Sectional roentgenography. Computerized tomography (CT scan) is a valuable diagnostic technique. Tract A bundle of nerve fibers within the CNS, with a common origin and termination, e.g., optic tract, cortico-spinal tract. Trapezoid body Trigeminal nerve Trochlear nerve Fourth cranial nerve (CN IV); to the superior oblique muscle of the eye. Uncus The hooked-back portion of the rostral end of the parahippocampal gyrus of the temporal lobe, constituting a landmark (e.g., uncal herniation) the amygdaloid nucleus lies deep to this area. Upper motor neuron Cell in motor cortex or other motor areas in the brain or brainstem connected by descending tract to lower motor neurons in brainstem (for cranial nerves) or spinal cord (for body and limbs). Upper motor neuron lesion Disorder characterized by spasticity and hyperreflexia seen a few weeks following a lesion of the brain (cortex, white matter of hemisphere, or spinal cord) involving descending motor influences to lower motor neuron (of the spinal cord). ©2000 CRC Press LLC