* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cystatin C prevents degeneration of rat nigral dopaminergic neurons
Neuroplasticity wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Single-unit recording wikipedia , lookup
Environmental enrichment wikipedia , lookup
Electrophysiology wikipedia , lookup
Axon guidance wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural oscillation wikipedia , lookup
Neural coding wikipedia , lookup
Synaptogenesis wikipedia , lookup
Mirror neuron wikipedia , lookup
Haemodynamic response wikipedia , lookup
Subventricular zone wikipedia , lookup
Central pattern generator wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Metastability in the brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Multielectrode array wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Nervous system network models wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Circumventricular organs wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroanatomy wikipedia , lookup
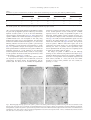
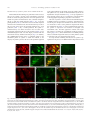
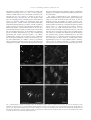
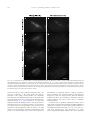
www.elsevier.com/locate/ynbdi Neurobiology of Disease 18 (2005) 152 – 165 Cystatin C prevents degeneration of rat nigral dopaminergic neurons: in vitro and in vivo studies Lei Xu,a,b Jiansong Sheng,a,b Zhongshu Tang,a Xuefei Wu,a Yi Yu,a Hong Guo,a Yan Shen,a Changfu Zhou,b,c Luminita Paraoan,d and Jiawei Zhoua,b,* a Key Laboratory of Proteomics, Institute of Biochemistry and Cell Biology, Shanghi Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, 200031, P.R. China b Graduate School of the Chinese Academy of Sciences, Shanghai, 200031, P. R. China c Research Center of Life Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, 200031, P.R. China d Department of Medicine, University of Liverpool, Liverpool, L69 3GA, UK Received 28 December 2003; revised 11 June 2004; accepted 24 August 2004 Available online 24 November 2004 Destruction of nigrostriatal dopaminergic (DA) pathway triggers various persistent responses, such as inflammation and increased synthesis of neural growth factors, both in striatum and in substantia nigra. The pathological processes involved in such responses are poorly characterized and could contribute to secondary damage and/or regeneration in the central nervous system (CNS). Cystatin C was previously implicated in the process of neurodegeneration. However, its biological role during neurodegeneration is not understood and remains controversial. The present study identified an increased cystatin C mRNA level in the DA-depleted rat striatum, starting from the second week following a 6-OHDA-induced lesion. Immunohistochemical analysis confirmed the increase in cystatin C protein level in the striatum following DA depletion. Double-labeled fluorescence immunohistochemistry revealed that nigrostriatal neurons, astrocytes, and microglia contributed to the elevated level of cystatin C. Exposure to 6hydroxydopamine, a DA-specific neurotoxin, resulted in DA neurons loss in the fetal mesencephalic cultures, an effect which could be partially reversed by treatment with cystatin C. Moreover, in vivo DA neurons survival study showed that administration of cystatin C in rats with 6OHDA-induced lesion partially rescued the nigral DA neurons. The results indicate that the 6-OHDA lesioning induced a relatively slow but sustained up-regulation of cystatin C expression and suggest that the inhibitor may exert a neuroprotective action on DA neurons. The findings raise the possibility that cysteine proteinase inhibitors may be new candidates for neuroprotective treatment of Parkinson’s disease. * Corresponding author. Key Laboratory of Proteomics, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Building 23, Room 316, 320 Yueyang Road, Shanghai, 200031, P.R. China. Fax: +86 21 5492 1073. E-mail address: [email protected] (J. Zhou). Available online on ScienceDirect (www.sciencedirect.com). 0969-9961/$ - see front matter D 2004 Elsevier Inc. All rights reserved. doi:10.1016/j.nbd.2004.08.012 Cystatin C may be useful therapeutically in limiting neuropathy in Parkinson’s disease. D 2004 Elsevier Inc. All rights reserved. Keywords: Cystatin C; Dopaminergic; Cathepsin; Parkinson’s disease; Cell culture Introduction Parkinson’s disease, a common neurodegenerative disease, is characterized by degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) (Dawson and Dawson, 2003). The characteristic features of the disease can be reproduced to some extent in animals through the administration of various neurotoxic agents disturbing the DA neurotransmission. For example, intraparenchymal injections of the neurotoxin 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle in rats destroy catecholamine-containing neuronal cell bodies and nerve terminals (Ungerstedt and Arbuthnott, 1970). The 6-OHDA animal model is widely used to examine the function of neural transplants and the alterations in the expression of receptors and their sensitivity to agonist drugs (Betarbet et al., 2002). The lesion causes robust biochemical and structural changes in the SN and its target striatum leading to apparent reorganization of the nigrostriatal pathway. Specifically, the lesion differentially affects mRNA expression levels of subtypes of DA receptor, cannabinoid CB1 receptor, and nicotinic acetylcholine receptor subunit (Elliott et al., 1998; Romero et al., 2000). The lesion also initiates inflammation by elevating the tumor necrosis factor-a in the DA-denervated striatum, which can be suppressed by the immunosuppressant FK506 (Mogi et al., 2000). These processes appear to be accompanied by neural growth-associated activity as evidenced by increased expression of a number of factors that are known to L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 stimulate the growth of central neurons in vitro, such as the neurotrophins brain-derived neurotrophic factor (including its specific receptor trkB) and members of glial cell line-derived neurotrophic factor family (Hida et al., 2003; Numan and Seroogy, 1997; Zhou et al., 1996, 2000). Consistent with these changes, it was shown that the striatal extracts from patients with Parkinson’s disease promotes growth of DA neurons in fetal mesencephalic cultures (Carvey et al., 1993), suggesting a trophic activity of the striatum on DA neurons. Thus, the striatum appears to play a role in self-neuronal protection when neurodegeneration of midbrain DA neurons occurs. However, the molecular mechanisms underlying the neural plasticity of the DA-denervated striatum are far from being elucidated. Cystatin C, a cysteine protease inhibitor, was recently identified by an approach based on suppression subtractive hybridization (SSH) as being differentially expressed in DA-depleted striatum of the 6-OHDA-lesioned rats (Xu et al., 2000). Cystatin C is a member of family 2 of the cystatin superfamily (for reviews, see Akopyan, 1991; Barrett and Kirschke, 1981; Barrett et al., 1984), widely expressed by various tissues (Abrahamson et al., 1990; Lofberg et al., 1982, 1983; Paraoan et al., 2001) and present in various biological fluids including urine, blood, and cerebrospinal fluid (Abrahamson et al., 1986; Lofberg et al., 1980; Sickmann et al., 2000). Involvement of cystatin C in various degenerative processes in the central nervous system (CNS) was only relatively recently described. Delayed expression of cystatin C by CA1 pyramidal cells and reactive astrocytes of rat hippocampus was reported in transient forebrain ischemia (Ishimaru et al., 1996; Palm et al., 1995). However, cystatin C was persistently upregulated in neurons and glia in a rat model for medial temporal lobe epilepsy (Aronica et al., 2001). In addition, cystatin C protein level was found increased in neurons cultured in the high oxygen atmosphere, suggesting that oxidative stress stimulates the expression of cystatin C in cultured neurons and that cystatin C might therefore have a role in regulation of apoptosis elicited by oxidative stress (Nishio et al., 2000). Recent studies showed that, in patients with Alzheimer’s disease (AD), cystatin C is colocalized with amyloid beta-protein (Abeta) in parenchymal and vascular amyloid deposits (Levy et al., 2001). The role of cystatin C in the neurodegeneration associated with PD is unknown. The present study demonstrates that the expression of cystatin C is up-regulated, both at mRNA and protein level, in the DA-depleted striatum following 6-OHDA treatment. Moreover, the evidence provided by in vitro and in vivo studies suggests a role of cystatin C in the prevention of degeneration of mesencephalic DA neurons, thus implying that cystatin C might be a potential neuroprotectant of nigral DA neurons. Materials and methods Animals All animal experiments were carried out in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Female Sprague–Dawley rats (180–220 g) provided by the Animal House, Shanghai Institutes for Biological Sciences, were caged in groups of three with food and water given ad libitum. The animals were kept in a temperature controlled environment at 218C on a 12:12-h light–dark cycle. Timed pregnant rats were used as the cell culture source. 153 Induction of unilateral lesion in medial forebrain bundle (MFB) by 6-OHDA and animal behavioral tests All surgery was performed under Equithesin anesthesia (0.3 ml/ 100 g) and adequate measures were taken to minimize pain or discomfort. Unilateral DA degeneration of the striatum was achieved by stereotaxic injections of 6-OHDA (Sigma, St. Louis, MO, USA) into the ascending medial forebrain bundle as described previously (Ungerstedt and Arbuthnott, 1970). Briefly, 4 Al of 6OHDA (2.5 Ag/Al in 0.2 mg/ml ascorbate–saline) were injected at 4.4 mm caudal to bregma, 1.2 mm lateral to midline, 7.8 mm below dura. Sham-lesioned animals received administration of 4 Al of ascorbate vehicle. The lesion parameters used in the present study result in the selective destruction of virtually all dopaminergic neurons in the SN (N95%) and the ventral tegmental area (N80%), as indicated by reduction in mRNA level of tyrosine hydroxylase (TH) (Zhu et al., 1993), DA contents, and TH immunoreactivity (Zhou et al., 1996). One week after lesioning, rotational behavior was assessed with apomorphine (0.05 mg/kg). Animals that exhibited adequate turning (i.e., at least six full body turns per min for rats and three full body turns per min for mice contralateral to the lesion side) were used in the study. Isolation of total RNA and Northern blot analysis The animals were sacrificed by decapitation at various time points after the lesion was induced and the brains were removed within 5 min postmortem. For each time point, four to five 6OHDA-lesioned animals were used. The lesion-side striata were dissected and stored at 808C until used. The tissues were homogenized and total RNA was isolated using a Totally RNA isolation kit (Ambion, Austin, TX). Polyadenylated RNA was further purified with Oligotex mRNA Mini Kit (Qiagen, Germany). Northern blot analysis followed standard procedures (Sambrock et al., 1989) with a few modifications. Thirty micrograms of total RNA isolated from DA-depleted and sham-lesioned striata of rats at 1, 2, and 5 weeks postlesion were size fractionated by 1.0% formalin-denatured agarose gel electrophoresis and transferred to a nylon membrane (Amersham). A 32P-labeled cystatin C-specific cDNA probe was produced using random primer DNA Labeling Kit (TaKaRa Biotechnology, Dalian, China) followed by EcoRI digestion. The specific activity of the probe was approximately 109 dpm/Ag DNA. After hybridization with the labeled probes at 428C, the filters were sequentially washed with 2 SSC, 0.1% SDS at room temperature followed by 0.2 SSC, 0.1% SDS at 428C, and 0.1 SSC, 0.1% SDS at 658C. Autoradiography was performed using an intensifying screen at 808C and the exposure time was varied so that the band intensity was kept within the linear range. Densitometric scanning of blots allowed the calculation of the ratio of signal obtained with cystatin C transcript and 28S ribosome in both denervated striatum and control. The results were expressed as percentage of the control (100%). Semiquantitative reverse transcriptase (RT)-PCR Single strand (ss) cDNA was synthesized from 1 Ag total RNA in a volume of 20 Al containing 50 pmol random hexamers (Gibco BRL, UK), 0.2 mM each dATP, dCTP, dGTP, and dTTP (Promega, Madison, WI, USA), 50 mM Tris–HCl pH 8.3, 75 mM KCl, 3 mM MgCl2, 50 mM DTT, 0.75 U RNasin (Promega), and either 200 U 154 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 or no MMLV reverse transcriptase (Promega). Reactions were incubated for 1 h at 378C, terminated by heating 5 min at 958C, and stored at 808C. PCR primers were designed to specifically amplify a 394-bp fragment of cathepsin H (between nucleotides 505–958, GenBank accession number NM_012939; forward primer: 5V-GTGGATTGTGCCCAGAACTT-3V; reverse primer: 5V-TGTTCTTTCCACGCTCAATG-3V), and a 405-bp fragment of cathepsin L (between nucleotides 196–600, GenBank accession number NM_013156; forward primer: 5V-ATGGCACGAATGAGGAAGAG-3V; reverse primer: 5V-TGCCTTGATCGTGAGAACAG-3V). Primers were also designed to amplify regions of coding sequence from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Negative controls containing no template were performed for each set of PCR reactions. Amplification was performed with Mastercycler GradientR (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany) in a volume of 20 Al containing 1–3 Al template from the ss cDNA synthesis reaction, 25 pmol each primer, 0.2 mM each dATP, dGTP, dCTP, and dTTP, 1.5 mM MgCl2, 0.5 ACi {a-32P}-dATP, and 1.25 U Taq polymerase (Promega). PCR reactions were subjected to 5 min denaturation at 948C and 25 cycles of (948C for 3 min, 948C for 1 min, 558C for 1 min, 728C for 1 min), followed by a final amplification at 728C for 7 min. The concentration of GAPDH cDNA of each sample was adjusted to the same level before PCR amplification. Seven-microliter aliquots from the amplification reactions was analyzed on 1.5% agarose gel electrophoresis. The gels were fixed with 6% trichloric acid for 30 min, vacuum-dried, and then exposed to X-ray film at 808C for 5 h. All PCR products obtained were sequenced using automated PCR sequencer (ABI Prism System, Perkin-Elmer, Shelton, CT, USA). In situ hybridization Cystatin C mRNA in situ hybridization was performed according to the procedure described previously (Ying et al., 2002). A pBluescript II SK( ) construct containing a 670-bp fulllength cDNA of mouse cystatin C (GenBank accession number M59470) was linealized and digoxigenin-labeled antisense and sense riboprobes were synthesized using an in vitro transcription kit (Roche Diagnostics GmbH, Mannheim, Germany). The animals were sacrificed 4 weeks postlesion. Following the pretreatments, the brain sections were prehybridized for 3 h at 508C in the hybridization buffer containing 50% deionized formamide, 10% dextran sulfate, 10 mM Tris–HCl (pH 7.5), 600 mM NaCl, 0.25% SDS, 1 Denhardt’s solution and 400 Ag/ml salmon sperm DNA, and hybridized in the same buffer containing 0.2 Ag/ml of either antisense or sense cystatin C probe at 508C overnight. Following hybridization, sections were washed and the hybridization signals were detected by incubation with antidigoxigenin antibody conjugated with alkaline phosphatase using 4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as substrates. In vitro DA neuron survival assay Fetal ventral mesencephalons were obtained at day 14 (E0 = day of vaginal plug) of gestation immediately after the rats were sacrificed by decapitation. The rat embryos were stored in icecold Hank’s balanced saline solution (Ca2+- and Mg2+-free, HBSS) until dissection. After removal of meninges, the embryos were dissected out in HBSS and cut into 0.5 mm sections. Following washing, the tissue was incubated in HBSS containing 1 mg/ml trypsin and 0.5 mg/ml DNase at 378C for 10 min. Tissue was then sequentially washed in HBSS containing DNase. Rat mesencephalic cells were seeded into 96-well plates (Nunc, Denmark) precoated with poly-l-lysine (10 Ag/ml) as described previously (Zhou et al., 1994). Cell viability in suspensions of dissociated cells was determined by the ability of viable neurons to exclude the dye trypan blue. The cells were plated at 105 cells/cm2 in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) and Ham’s F12 (1:1), supplemented with 10% fetal calf serum. Four hours later, cultures were switched to serum-free conditions, that is, DMEM/Ham’s F12 (1:1) with addition of B27 (1: 50) and streptomycin/penicillin. Human cystatin C (Calbiochem, San Diego, CA, USA) was added immediately after the medium was changed. Cells were incubated at 378C in a 95% air/5% CO2 humidified atmosphere. Twenty-four hours after switching to serum-free medium, the cultures were fixed in 4% paraformaldehyde and immunostained for TH. In vivo DA neurons survival study Retrograde labeling and unilateral lesions induced by nigral 6OHDA infusion were performed as described previously (Rosenblad et al., 2000; Sauer and Oertel, 1994). Briefly, under Equithesin anesthesia (0.3 ml/100 g), 21 rats were injected bilaterally with 0.2 Al of a 2% saline solution of the retrograde tracer fluorogold (Fluorochrome, Inc., Englewood, CO, USA). Injections were made at the following coordinates: AP, +0.5 mm; ML, F3.4 mm relative to bregma; DV, 5.0 mm relative to the dura and incisor bar set to 0.0 mm. One week after the fluorogold injections, the animals were randomly divided into three groups (I, II, and III) with seven animals in each group and reanesthetized for the placement of 6-OHDA lesioning in the SN of right side. The administration of cystatin C or saline containing 0.1% bovine serum albumin as vehicle was performed 6 h before the 6-OHDA challenge. Group I received the vehicle to test whether the vehicle was protective in itself. Groups II and III received 5 or 15 Ag human cystatin C in 0.1% BSA containing PBS, respectively. The injections of vehicle, cystatin C, or 6-OHDA were administered to animals anesthetized as described above, placed in a stereotaxic frame (ASI Instrument, USA) with the incisor bar set at 3.3 mm, directly into the right substantia nigra pars compacta (SNc) using the following coordinates: AP, 5.4 mm; L, 2.2 mm; and V, 8.5 mm from skull (Kearns et al., 1997; Paxinos and Watson, 1986). All injections were performed using a Hamilton 10-Al syringe at a rate of 0.4 Al/min. At the completion of each injection, the needle was left in place for 5 min and then withdrawn at a rate of 1 mm/min. One animal from each group died after the surgery because of infection. Four weeks after surgery, the rats were anesthetized with an overdose of sodium pentobarbital and perfused with precooled saline followed by 4% paraformaldehyde. Immunohistochemistry Immunohistochemistry was used to characterize the cystatin Cpositive cell population in the brain of 6-OHDA-treated rats. From each experimental animal, five sets of 30 Am thick, frozen brain L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 sections were collected in cryoprotectant solution (0.1 M PBS, 30% sucrose, and 30% ethylene glycol) and stored at 208C until stained. Immunohistochemical staining of cell type-specific markers was performed on sequential sections using the following antibodies: mouse anti-RIP (1:1000; kind gift of Drs. S. R. Whittemore and X. M. Xu), mouse anti-GFAP (glial fibrillary acidic protein, 1:400, Sigma), and mouse anti-CD11b (1:100, Chemicon, Temecula, CA, USA). After washing with PBS containing 0.1% Triton X-100, the sections were incubated for 1–3 h with appropriate secondary antibodies (1:300; FITCconjugated horse anti-mouse or goat anti-rabbit IgG; Vector Laboratories, USA). Following fixation in 4% paraformaldehyde, cystatin C immunoreactivity was assayed using an anti-cystatin C antibody (Dako, Denmark) and visualized using a Texas redconjugated goat anti-rabbit IgG antibody (1:500; Molecular Probe, USA). Our Western blot analysis on lysis of cultured 293 cells transfected with pcDNA3.1-cystatin C-eGFP showed a single band at right size. This suggests that the antibody we used has high specificity. Moreover, the same antibody has been used to characterize distribution of cystatin C in the retinal pigmental cells (Paraoan et al., 2001). Every fifth section was incubated with the primary antibody against TH (1:5,000; Sigma) using an ABC kit (Vector Laboratories). TH immunoreactivity was visualized using 3,3V-diaminobenzidine as the chromogen. 155 Morphometric analysis Cell numbers in vitro The number of TH-positive neurons in fetal mesencephalic cell cultures treated with or without cystatin C protein was counted on the entire surface area of a culture well. The results were expressed as either real numbers or a percentage of TH-positive cells counted in a paired culture treated with H2O. Cell numbers in vivo The numbers of fluorogold-labeled and TH-positive neurons in the SNc were assessed as described previously (Rosenblad et al., 2000; Sauer and Oertel, 1994). In brief, three consecutive sections centered around the level of the medial terminal nucleus of the accessory optic tract (MTN; 5.3 mm in the atlas of Paxinos and Watson, 1986) were used and all labeled/stained neurons lateral to the MTN were counted at 400 magnification. Fluorogold-labeled neurons were included if they were brightly fluorescent under epiillumination at 330 nm, displayed a neuronal profile, and extended at least one neuritic process. TH-positive neurons (excited at 530 nm) were counted when displaying a nucleus surrounded by TH-positive cytoplasm. The localization of the oculomotor nerve rootlets was the criteria for delineating the SN from the ventral tegmental area. The ventral tegmental area was considered to be within and medial to the rootlets, whereas the SN was considered to be located laterally. The extent of DA neuronal loss was Fig. 1. The 6-OHDA lesion increased expression of cystatin C mRNA in the denervated striatum (A, C) and the lesioned substantia nigra (B, D). Total RNA from 6-OHDA-lesioned nigrostriata and sham-operated rats, 1, 2, and 5 weeks postsurgery, was extracted and subjected to Northern blot analysis using a cDNA fragment of cystatin C as a probe. The cystatin C mRNA band at 0.7–0.8 kb is indicated with the arrow. (A, B) Northern blots show that the mRNA levels of cystatin C were significantly increased in both the striatum (A) and the substantia nigra (B) of the lesioned side compared to that in sham-operated group at 1, 2 and 5 weeks following the lesion. CTL, control (sham operated); LES, 6-OHDA lesioned. (C, D) The optical density of the bands (A, B) was expressed as a percentage of that of the control group at each time point (mean F SEM). *P b 0.05 compared with the control group. Two to five samples were analyzed at each time points. 156 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 estimated by the loss of TH-positive SN neurons on the lesioned side with respect to the control side of the brain. immunostained for both cystatin C and TH and visualized as described above. Semiquantitation of immunoreactive signals in the caudate–putamen and the globus pallidus For the purpose of a general survey of the expression of cystatin C in disparate regions of the rat brain, a semiquantitative analysis was performed. The relative levels of signals in all the sections immunostained were visually inspected and assigned a grade, between b+++Q (strongest) and bFQ (weakest) immunoreactivity. Statistical analysis Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining Some of the brain sections at both striatal and nigral levels from the 6-OHDA-lesioned rats were analyzed for evidence of apoptosis by using in situ cell death detection kit (Roche, USA). The staining was performed according to manufacturer’s protocol. The sections were analyzed under a fluorescence microscope using an excitation wavelength in the range of 450–500 nm and detection in the range of 515–565 nm. The brain sections were then sequentially Statistical analysis used the GraphPad Prism v4.0 software (GraphPad Software Inc., USA). The data were submitted to either a one- or two-way analysis of variance (ANOVA) as indicated in the Results section. Either the Dunnet test or the Student– Newman–Keul’s test (as a post hoc test) was used to compare data samples from the control group with the different treatment groups or between pairs of groups. Differences were considered significant when P values were less than 0.05. Results Up-regulation of cystatin C mRNA level in the denervated striatum and SN following 6-OHDA lesioning Up-regulation of a variety of genes by DA-depletion in the striatum might contribute to the neural plasticity of the striatal Fig. 2. In situ hybridization of cystatin C mRNA in the rat brain following 6-hydroxydopamine lesioning. The brain sections were prepared from the lesioned animals 4 weeks postsurgery and were hybridized with a digoxigenin-labeled cystatin C cRNA probe. More prominent dotlike hybridization products in the caudate–putamen (B), the globus pallidus (D), and the subventricle zone (F) of the lesioned side were seen compared to those in the contralateral side (A, C, and E). LV, lateral ventricle. Scale bar, 100 Am. L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 157 Table 1 Distribution of cystatin C immunoreactivity in both the striatum and the substantia nigra at various time points following 6-OHDA lesioning Time (weeks postlesion) 1 2 5 CPu Pallidum SNc SNr Contralateral side Lesion side Contralateral side Lesion side Contralateral side Lesion side Contralateral side Lesion side + + + + ++ +++ F + + F ++ ++ + + + + ++ ++ + + + + ++ ++ Data were from at least three animals in each group. +++, ++, +, and F indicate strong, moderate, weak, and very weak immunoreactive signals, respectively. CPu, caudate putamen; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata. cells. A previous suppressional subtractive hybridization screening for genes with up-regulated expression induced by DA neurons depletion revealed cystatin C as one of the most differentially expressed genes in injured striatum (Xu et al., 2000). The level of cystatin C mRNA in rat striatum denervated as a consequence of a 6-OHDA-induced lesion was investigated in this study using Northern blot analysis and in situ hybridization. The denervated striatum were taken for Northern blot analysis at 1, 2, and 5 weeks following induction of lesion. The rat cystatin C gene transcript was identified as a single transcript of approximately 0.7–0.8 kb (Fig. 1A). Quantitative analysis of the signals in lesioned and sham-operated striatum revealed the level of cystatin C mRNA was moderately increased at 1 week postlesion in the denervated striatum compared to the sham control (1.2-fold; P b 0.05), and by 5 weeks postlesion the increase became more prominent (1.57-fold of the control; P b 0.05) (Figs. 1A and C). In situ hybridization analysis revealed that cystatin C mRNA was expressed in vast regions of the rat adult brain, such as the cerebrocortex, the lateral septum, the hippocampus, and the striatum (data not shown). In the striatum, the hybridization signals were scattered in the entire nucleus, in particular the head of the caudate–putamen nucleus. Following the 6-OHDA injection, however, the density of hybridization signals in the denervated caudate–putamen nucleus appeared elevated at 4 weeks postlesion compared to that in the striatum of contralateral side (Figs. 2A and B). Increased hybridization signals were also detected in the globus pallidus (Figs. 2C and D) and subventricular zone of the lesion side (Figs. 2E and F). The expression levels of cystatin C mRNA in other brain regions, such as the cerebrocortex, the lateral septum, were not significantly altered following the lesion. In parallel control experiments, brain sections hybridized with the sense probe were completely devoid of signals at all samples (data not shown). The change of cystatin C mRNA in the SN following lesioning was also examined using Northern blot analysis (Figs. 1B and D). Modest increase in the level of cystatin C mRNA was observed at 1 week postlesion in the lesioned SN compared to the sham control (1.26-fold; P b 0.05). The levels were further elevated in 2 and 5 weeks postlesion (1.4- and 1.6-fold, respectively, P b 0.05). Fig. 3. Photomicrographs showing expression of cystatin C protein in the caudate–putamen following 6-OHDA lesioning. The rats were sacrificed 5 weeks after the lesion, their brains were cryosectioned, and immunohistochemistry using cystatin C antibody was performed as described in Materials and methods. Semiquantitative evaluation of cystatin C expression showed prominent increase in 6-OHDA-treated rats compared with control. Panels A and B illustrate low magnification views, while panels C and D represent high magnification views of the same respective fields. A and C, contralateral side; B and D, lesioned side. Scale bars: A and C, 400 Am; C and D, 50 Am. 158 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 Elevated levels of cystatin C protein in the striatum and the SN Immunohistochemical staining was performed in order to assess the level of cystatin C protein in the nigrostriatum of lesioned animals at various time point postlesion. At 2 weeks postlesion, the number of cystatin C-immunoreactive cells, evaluated semiquantitatively, in both the caudate–putamen (Table 1, Fig. 3) and the globus pallidus (Table 1) of the lesion side was elevated compared with that in the contralateral side. The immunoreactivity of cells in the two areas of the lesion side appeared unchanged at 1 week postlesion (Table 1). By 5 weeks postlesion, the cystatin Cimmunoreactivity was further elevated in the two brain areas investigated compared to that at 2 weeks postlesion (Table 1). The immunohistological results were thus largely consistent with the observations made by Northern blot analysis (Fig. 1). In addition, the 6-OHDA-induced lesion led to a significant change in the distribution pattern of cystatin C within the striatum, as the majority of cystatin C-positive cells at 5 weeks postlesion appeared in the globus pallidus and the dorsal part of the caudate–putamen. This pattern was very similar to that demonstrated by the in situ hybridization analysis illustrated in Fig. 2. The up-regulations in these particular regions may be a consequence of the denervation of the topographic dopaminergic projection in the striatum. The level of cystatin C was also altered in the SN following the 6-OHDA lesioning. The intensity of cystatin C immunoreactivity in both the pars compacta and reticulata of SN of the lesion side was increased at 2 and 5 weeks postlesion compared with that in the contralateral side (Table 1). In the contralateral side of the SN, the cystatin C expression was localized in microglial- and neuronal-like cells. The latter appeared to include some nigral DA neurons as evidenced by colocalization of TH and cystatin C staining (Figs. 4A–C). Since administration of 6-OHDA primarily induces cell death of nigral DA neurons, it is likely that the cystatin C-expressing cells were degenerating DA neurons. As a step towards examining the relevance of cystatin C upregulation in the process of 6-OHDA-induced degeneration of the Fig. 4. Photomicrographs of brain sections in the midbrain of the rats following 6-OHDA-induced lesion. The rats were sacrificed 2 weeks postlesion and the brain tissue was fixed and double immunostained for cystatin C and either tyrosine hydroxylase (TH), glial fibrillary acid protein (GFAP), or CD11b. Some brain sections were also TUNEL stained. Colocalization of cystatin C and TH was observed in nigral DA neurons of contralateral side (A–C). The 6-OHDA treatment resulted in sustained up-regulation of cystatin C in the SNc where apoptotic cells were detected (E–G) and no TH immunoreactivity was observed (H), while no TUNEL-positive cells were observed in the SN of contralateral side (D). (A, E, I, and L) Immunostaining using anti-cystatin C antibody; (B, F, J, and M) same fields immunostained with anti-TH (B), TUNEL (F), anti-GFAP (J), and anti-CD11b (M) antibodies, respectively. Colocalization is illustrated in merged images shown in C, G, K, and N. Micrographs A–D were from the substantia nigra of contralateral side; the rest of micrographs were from the substantia nigra of lesion side. SNc, substantia nigra pars compacta. Arrows indicate double-labeled cells. Scale bars: A–C: 10 Am; D: 120 Am; E–H, 60 Am; I– K, 30 Am; L–N: 10 Am. L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 nigrostriatum, TUNEL staining was performed on animals with unilateral nigrostriatal lesion. At 1 and 2 weeks postlesion, numerous TUNEL-stained cells were detected in the SNc of the lesioned side (Fig. 4F), whereas no staining in the SN of contralateral side was observed (Fig. 4D). The striatum of both sides was completely devoid of TUNEL-positive signals (data not shown). To determine whether the degenerating nigral cells expressed cystatin C, TUNEL staining was combined with double immunohistochemical labeling for cystatin C and TH. A significant portion of cystatin C-expressing cells in the lesion side of SNc were labeled with TUNEL 1 week postlesion (data not shown). By 2 weeks after the lesion, the majority of cystatin C-positive cells in the SNc were TUNEL-positive (Figs. 4E–G) and they were completely devoid of TH-like immunoreactivity (Fig. 4H), indicating that degenerating DA neurons expressed cystatin C. In contrast, TUNEL-positive staining was not observed in the contralateral side where numerous TH-positive neurons were detected (Figs. 4A– D). Since administration of 6-OHDA is known to specifically induce cell death of nigral DA neurons, these results indicate that up-regulation of cystatin C is associated with degeneration of nigral 159 DA neurons following the injury. Whether cystatin C contributed to apoptosis as a secondary action following 6-OHDA exposure needs to be determined. The cystatin C-immunoreactive cells contributing to the increased immunoreactivity described above appeared morphologically to be of both neuronal and glial cell origin. In order to determine precisely the identities of cystatin C-positive cells, double-labeling immunohistochemical staining of brain sections of striatum and SN was performed. A significant proportion of striatal neurons (35.6%, n = 200) showed strong cystatin C immunoreactivity (Fig. 5A). GFAP colocalized with cystatin C in the striatum of both contralateral and ipsilateral sides (Fig. 5B), and more than 49.7% of cystatin C-positive cells were GFAP-positive cells. Moreover, microglial cells identified with CD11b antibody also presented strong cystatin C-immunoreactivity in the same brain area (14.7%, n = 200) where cystatin C-expressing astrocytes were found (Figs. 5E and F). However, no RIP-positive, mature oligodendrocytes (Yan et al., 2003) exhibited cystatin C immunoreactivity (Figs. 5C and D). Conversely, no cystatin C-expressing astrocytes were observed in the SN of the lesion side (Figs. 4I–K). Fig. 5. Characterization of cystatin C-expressing cells in the DA-depleted striatum following 6-OHDA-induced lesion. The rats were sacrificed 2 weeks postlesion and the brain tissue was fixed and double immunostained for cystatin C and cell markers, that is, glial fibrillary acid protein (GFAP), RIP, and CD11b. (A, C, and E) Immunostaining using anti-cystatin C antibody; (B, D, and F) immunostaining of the same field with anti-GFAP (B), anti-RIP (D), and anti-CD11b (F) antibodies. A typical neuronal-like cystatin C-expressing cell is marked with an arrowhead in panel A. There was no colocalization of cystatin C and RIP (C and D). Arrows indicate double-labeled cells. 160 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 However, microglial cells in the SN expressed cystatin C (Figs. 4L–N), suggesting that elevated cystatin C in the microglia may modulate interaction between neurons and microglia in the SN following lesioning. Increased mRNA levels of cathepsin H but not cathepsin L in the SN following 6-OHDA lesion Cathepsins H and L are among the target cysteine proteinases for the inhibitory action of cystatin C (Anastasi et al., 1983). To investigate a potential link between cathepsins H and L and degenerative events of nigrostriatal cells following administration of 6-OHDA, their expression level in the SN following the 6OHDA treatment was analyzed using RT-PCR. The analysis indicated that the mRNA level of cathepsin H, but not cathepsin L, was increased at all time points examined (1, 2, and 5 weeks postlesion), compared to the contralateral side (Fig. 6). The increase coincided with the up-regulation of cystatin C mRNA in the SN following the lesion (Figs. 1B and D). Cystatin C promoted the survival of the mesencephalic DA neurons in cultures Our findings in the cystatin C immunohistochemical analysis of the present study indicated that the expressions of cystatin C were up-regulated in both the striatum and the SN, the two brain areas were at two distinct states. The striatum had no apparent cell apoptosis in this model, whereas the SN underwent severe apoptosis following lesioning. Thus, the question was raised whether the cystatin C was beneficial or deleterious to nigral DA neurons. To investigate the potential roles of cystatin C in this setting, we examined the effect of cystatin C on the survival of rat fetal mesencephalic neurons in cultures. Addition of cystatin C into the cultures markedly increased the number of surviving TH-positive neurons in the rat mesencephalic cultures in a dose-dependent manner (Fig. 7). The number of TH-positive neurons in the cultures was increased up to 2.2-fold in the presence of cystatin C at 20 ng/ml, compared with the untreated (Fig. 7A). The effect of leupeptin, another cysteine protease inhibitor, on the survival of DA neurons in vitro was also examined. As shown in Fig. 7B, treatment with leupeptin promoted the survival of DA neurons in dosedependent manner yielding similar magnitude as cystatin C. These results indicate the survival-promoting effect of cystatin C on DA neurons. The scale of survival was similar to that promoted by leupeptin. The protective contribution of cystatin C on DA neurons against apoptosis triggered by the 6-OHDA, a DA-specific neurotoxin, was investigated in primary cultures of fetal ventral mesencephalic neurons. The cultures were treated with 6-OHDA for 24 h in the presence or absence of cystatin C. The number of TH-positive neurons was quantified in at least three independent, entire wells of 96-well plates. Treatment with 6-OHDA (0.3–100 Am) reduced the number of TH-positive neurons in a dose-dependent manner (Fig. 7C). The number of TH-positive neurons was reduced by 56.7% following addition of 30 AM 6-OHDA (136.3 F 8.1 TH-positive neurons/well) compared with the untreated cultures (315.0 F 16.1 TH-positive neurons/well, P b 0.05). However, the 6-OHDA toxicity could be reversed by addition of cystatin C, as coadministration of 6-OHDA and cystatin C significantly reduced the death of TH-positive neurons (Fig. 7C). In the presence of 20 ng/ml cystatin C, the average number of TH-positive neurons was reduced by 30.0% when the cultures were treated with 30 AM 6-OHDA (i.e., from 315.0 F 16.1 per well to 220.7 F 10.3 per well). These results strongly suggest a neuroprotective action of cystatin C on DA neurons against toxicity of 6-OHDA. Nigral infusion of cystatin C reduced the cell lose induced by striatal injection of 6-OHDA In order to investigate the neuroprotective action of cystatin C in vivo, the experimental animals received nigral infusion of cystatin C followed 6 h later by a selective lesion of nigral DA neurons with intrastriatal injection of 6-OHDA. To evaluate the effect of the treatment independent of TH expression, nigral DA neurons were retrogradely labeled by bilateral injection with fluorogold 1 week prior to the lesion. Control 6-OHDA-lesioned animals received nigral infusion of BSA. The lesioning induced a marked loss of both retrogradely labeled and TH-positive neurons in the SNc, with only 7.4% of fluorogold-labeled neurons and 6.9% of TH-positive neurons remaining undegenerated, in comparison with the contralateral side (Figs. 8C–D, respectively, and Table 2). This observation was consistent with a previous report by Kearns et al. (1997), which demonstrated that approximately 94% of nigral TH-positive neurons degenerated in the same model. The degenerating fluorogold-labeled neurons appeared surrounded by small brightly fluorescent dots, which may represent phagocytic cells that engulfed fluorogold-positive debris (Crews and Wigston, 1990; Rosenblad et al., 2000). In contrast, in rats that had received an infusion of 5 Ag cystatin C prior to lesioning, 28% of fluorogoldpositive neurons and 27.7% of TH-positive neurons in the SNc were observed (Figs. 8E and F, respectively). The quantification of the results is presented in Table 2. Similarly, the treatment with 15 Ag cystatin C significantly increased the number of both Fig. 6. Semiquantitative RT-PCR analysis of cathepsin H and cathepsin L mRNA in the substantia nigra ipsilateral to the lesion side following 6-OHDA infusion. Specific PCR products were amplified as described in Materials and methods. L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 161 Discussion Fig. 7. Neuroprotective action of cystatin C on nigral dopaminergic neurons in vitro. The ventral mesencephalic cells plated at cell density of 105/cm2 were either untreated or treated with various concentrations of cystatin C or leupeptin and were maintained in serum-free conditions for 24 h. Dose– response curves to cystatin C (A) and to leupeptin (B) on the survival of mesencephalic DA neurons in vitro. (C) The cultures were treated with 6hydroxydopamine ranging from 0.3 to 100 AM. The treatment led to a decline in the number of TH-positive neurons. Coadministration of cystatin C (20 ng/ml) and 6-hydroxydopamine partially restored the number of THpositive neurons. Data represent the mean F SEM of at least triplicates from three independent experiments. *P b 0.05, compared with control (A and B). *P b 0.05, compared with the corresponding cultures treated with 6-OHDA only (C). fluorogold- and TH-positive neurons in the SNc (27.1% and 28.2% of that in the contralateral side, respectively, P b 0.01; Table 2) (Figs. 8G and H). These in vivo findings, together with the results of the in vitro dopamine neuron assay, suggest that cystatin C is a neuroprotective agent capable of rescuing DA neurons from neurotoxicity. The present study revealed that the expression of cystatin C, a cysteine protease inhibitor, was up-regulated in response to 6OHDA lesions of nigrostriatal pathway. The spatiotemporal characteristics of the expression indicate that cystatin C could be part of the cascades of degeneration and/or regeneration triggered by the 6-OHDA-induced lesion. The finding that cystatin C has a significant survival promoting effect on mesencephalic DA neurons, both in vitro and in vivo, suggests that cystatin C may play an important role in brain self-protection following injury. The up-regulation of cystatin C mRNA and protein in the striatum was observed at 2 and 5 weeks postsurgery, suggesting that 6-OHDA-induced lesions can change gene expression in denervated target tissue, and that mRNA levels of cystatin C in striatal cells may be modulated by afferent dopaminergic input in a slow-rising and long-lasting fashion. The latter characteristic is similar to cystatin C spatial and temporal distribution in other disease models. For instance, increased cystatin C expression in the hippocampus and entorhinal cortex is detectable 24 h after onset of status epilepticus and persists for at least 3 months thereafter (Aronica et al., 2001). In transient forebrain ischemia, a delayed expression of cystatin C was reported in rat hippocampus (Palm et al., 1995). Cystatin C expression was previously correlated with diverse CNS diseases or injuries and it was documented in degenerating neural cells. In transient forebrain ischemia, expression of cystatin C was reported in degenerating pyramidal neurons of the CA1 region except for reactive astrocytes of rat hippocampus (Palm et al., 1995). Lee et al. (2002) assumed that cystatin C may contribute to apoptosis since following toxicity induced by 50 AM 6-OHDA cystatin C immunoreactivity, cathepsin B immunoreactivity, and cathepsin D immunoreactivity were localized in degenerating PC12, while TUNEL-positive staining was also observed in the same cells at 24, 48, and 72 h. In agreement with these findings, we observed in the present study that cystatin C was localized in apoptotic nigral neurons in the 6-OHDA-treated animals. These observations raised the possibility that the elevated expression of cystatin C may contribute to apoptosis following exposure to deleterious stimuli. The results presented in this study, however, demonstrated that cystatin C is able to counteract progression of apoptosis of nigral DA neurons induced by specific neurotoxins 6-OHDA. Moreover, elevated expression of cystatin C was observed in other regions, such as subventricle subventricular zone (Fig. 2) and lateral septum, apart from the striatum. The fact that these brain areas, where cell apoptosis is not evident, are not directly involved in degeneration of nigrostriatal pathway suggests that up-regulation of cystatin C in the adult brain after lesion does not necessarily lead to cell death. Indeed, overexpression of cystatin C in mammalian cells does not induce cell apoptosis (Paraoan et al., 2001). The most important finding of the present study is the protective action of cystatin C on the nigral DA neurons both in vitro and in vivo. In the in vitro DA neuron survival assay using dissociated cultures of fetal ventral mesencephalons, it was found that cystatin C (20 Ag/ml) increased the number of surviving THpositive neurons by more than twofold. In the in vivo study, it was observed that the infusion of cystatin C in the SN prior to lesioning provided partial protection of the nigral DA neurons from cell death induced by intrastriatally administered 6-OHDA. Up to 27– 28% of either the retrogradely labeled or the TH-positive nigral 162 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 Fig. 8. In vivo neuroprotective action of cystatin C on nigral dopaminergic neurons. The infusion of cystatin C protein, unilateral nigrostriatal lesioning, and immunohistochemistry were performed as described in the Materials and methods. (A, C, E, and G) Coronal sections through the midbrain showing retrogradely labeled (fluorogold-positive) nigral neurons. (B, D, F, and H) The same sections stained with anti-TH antibody. Compared to contralateral side (A and B), the majority of fluorogold-positive and TH-immunoreactive neurons in the lesioned side were lost in the BSA-only-treated control animals (C and D). In contrast, cystatin C-treated animals (in both doses of 5 and 15 Ag) had significantly more remaining fluorogold-positive or TH-immunoreactive neurons in the substantia nigra in the lesioned side. Scale bar, 200 Am. neurons survived by 4 weeks postlesion compared to only 7.4% and 6.9%, respectively, in the control group. The extent of protection in the cystatin C-treated animals was considerably lower than that in the GDNF-treated animals in which more than 80% of nigral DA neurons were rescued from cell death induced by neurotoxin (Kearns et al., 1997; Rosenblad et al., 2000). This difference indicates that although cystatin C has a potent survivalpromoting action in vitro, its protective effect in vivo is limited to some extent, at least in our experimental system. Since the exact mechanism of the neuroprotective effect of cystatin C on nigral neurons is not yet understood, the mode of administration of the protein to the animal models may not have been optimal for demonstrating its maximum protective capacity. Alternative delivery procedures, such as delayed infusion or virus-based gene therapy, may result in a better protective effect and should be studied in the future. Also treatment in combination with other protease inhibitors may achieve better effect in combating neurodegeneration. At present there is significant experimental evidence for the involvement of cysteine protease inhibitors (including inhibitors of cathepsins and calpains) as well of other protease inhibitors in neuroprotection against brain injury (Lee et al., 1991; Ohe et al., 1996; Rami and Krieglstein, 1993; Yamashima et al., 1998). Various cell-permeable calpain inhibitors have been synthesized L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 163 Table 2 The number of retrogradely fluorogold-labeled neurons and TH-immunoreactive neurons in the substantia nigraa Group n Controlb 5 Ag 15 Ag 6 6 6 Fluorogold labeled TH immunoreactive Contralateral Lesioned Percentage Contralateral Lesioned Percentage 226.3 F 11.6 224.8 F 7.5 239.3 F 6.3 16.7 F 2.3 63.0 F 5.9c 65.2 F 6.1c 7.4 F 0.9 28.0 F 2.4c 27.1 F 2.1c 572.2 F 15.9 558.0 F 15.1 563.0 F 10.4 39.8 F 4.2 154.4 F 12.5c 158.5 F 12.7c 6.9 F 0.6 27.7 F 2.2c 28.2 F 2.4c a The table presents the average number of labeled neurons counted in three consecutive sections from six animals. Only the neurons localized laterally from the medial terminal nucleus of the accessory optic tract were counted (see Materials and methods). Percentages indicate the proportion of neurons remaining in the substantia nigra of lesioned side compared to respective contralateral side and are presented as mean F SEM. b Control group was treated with bovine serum albumin (as described in Materials and methods). c Values for cystatin C-treated groups are statistically different from control group (P b 0.001, ANOVA followed by post hoc Newman–Keul’s test). and have shown significant neuroprotection in animal models of the CNS injuries and diseases (reviewed in Ray and Banik, 2003). However, the information on the neuroprotective effects of cysteine protease inhibitors directly on the DA neurons is still limited. Recent studies provided evidence to support a role for the involvement of the calpains in the loss of DA neurons in a mouse model of PD. Inhibition of calpain proteolysis using either a calpain inhibitor MDL-28170 or adenovirus-mediated overexpression of the endogenous calpain inhibitor protein calpastatin significantly attenuated 1-methyl-4-pheynyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced loss of nigral DA neurons (Crocker et al., 2003). In addition, we have previously demonstrated that amyloidprecursor-like protein 2 (APLP2) modified with chondroitin sulphate, which has structural/functional homology to the Kunitz-type serine protease inhibitors, but not the form lacking the modification, is able to promote the survival of fetal mesencephalic DA neurons in culture (Shen et al., 2002); the study indicated that the serine protease inhibitory characteristic of APLP2 plays an important role in the process. The present findings provide evidence supporting the role of cysteine proteinase inhibitor cystatin C in combating negative impact of neurotoxicity on nigral DA neurons. How might the cysteine protease inhibitory activity of cystatin C protect DA neurons? The cysteine protease class comprises cathepsins B, H, L, K, S, and O (Barrett and Kirschke, 1981). Most cathepsins are lysosomal and are involved in normal cellular metabolism, participating in various processes such as peptide biosynthesis and protein degradation. Cathepsins may also cleave some protein precursors, thereby releasing regulatory peptides. It has been shown that cathepsins could leak out of lysosomes in neurodegenerative conditions, for example, in ischemia (Nitatori et al., 1995; Sun, 1989). Members of this cathepsin family, for example, cathepsin B, are able to induce neuronal apoptosis when secreted by microglia (Kingham and Pocock, 2001; Yamashima, 2000). Controlled proteolysis is crucial in the plasticity of the CNS (Lynch and Baudry, 1984), whereas excessive proteolysis contributes to various neuropathologies (Crocker et al., 2003; SierraParedes et al., 1999). Taken together, these observations suggest that cysteine proteases are involved in neurodegeneration-associated pathology and that their inhibitors may be instrumental in regulating their deleterious effects. Noteworthy, in the present study, not only cystatin C was up-regulated but also one of its substrates, cathepsin H. It is therefore possible that cystatin C protects nigral neurons against apoptosis by inhibiting the activity of cathepsin H. Inhibition of other cysteine proteinases present at sites of injury in the brain could also not be excluded. Moreover, cystatin C may inhibit activities of caspases that have a cysteine residue at their active site and cleave the substrate after aspartate residues (Yamashima, 2000), which were also shown to participate in the process of neuronal death. Prevention of procathepsin B processing by cystatin C, for example, may be beneficial in neuronal death protection (Ryan et al., 1995). There is evidence suggesting that activity and/or distribution of the lysosomal proteases cathepsins B and D may be implicated in abnormal protein processing resulting in formation of the neurotoxic amyloid A4 peptide, which is associated with Alzheimer’s disease. Comparative studies of cathepsin protease activities in normal frontal cortex versus frontal cortex from patients with Alzheimer’s disease, Lewy body dementia, PD, and Huntington’s disease revealed a significant increase in the activity of cathepsins H, D, and dipeptidyl aminopeptidase II, specifically in cases with Huntington’s disease, but not those with PD (Mantle et al., 1995). However, the potential role of cathepsins in the pathogenesis of PD could not be ruled out as the two regions that are severely affected in PD, the SN and the striatum, were not examined by the abovementioned study. Nevertheless, the data from the present study indicate that the level of cathepsin H mRNA was elevated in the lesioned SN, and moreover the presence of a specific cysteine protease inhibitor (cystatin C) effectively reduced cell death of nigral DA neurons. The inferred link between cathepsins and nigral DA neurons death raises the possibility for novel strategies for protection of nigral DA neurons in PD. Oxidative stress contributes to DA cell death in PD and the neurotoxin 6-OHDA, which is easily oxidized to reactive oxygen species (ROS), appears to induce neuronal death by a free radical-mediated mechanism (Ferger et al., 2001; Lotharius et al., 1999). The 6-OHDA toxicity may be primarily mediated by apoptotic pathways (Lotharius et al., 1999). The neuroprotective effect of cystatin C on nigral DA neurons challenged with 6-OHDA demonstrated by this study suggests that release of cysteine proteases could be one of feature of the toxicity induced by 6-OHDA. This step leading to neuronal cell death could be blocked, at least in part, by treatment with cystatin C protein. In the present study, cystatin C, a cysteine protease inhibitor, was able to partially protect DA neurons. This could be due to fact that multiple death pathways are involved in degeneration of nigral DA neurons following treatment of 6-OHDA and cysteine proteases are only part of these pathways. Moreover, the upregulation of endogenous cystatin C protein level was elicited in relatively late stage (1 week after lesioning). Thus, the increased level of cystatin C, which occurred in delayed manner, cannot completely reverse the degenerative process that have already 164 L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 started. This may explain why cystatin C-expressing cells in the nigra are also TUNEL-positive after 6-OHDA treatment. Our result that earlier administration of cystatin C partially protected nigral DA neurons in vivo is consistent with this notion. In summary, the robust changes in the expression of cystatin C following 6-OHDA/-lesioning suggest that an imbalance of cysteine proteases and their inhibitors may contribute to the pathophysiological processes leading to cell death during the neurodegeneration in the nigrostriatum. The protective action of cystatin C on DA neurons against 6-OHDA-induced neurotoxicity suggests that cystatin C is one of the contributors to neuronal survival. Cystatin C may therefore be useful therapeutically in limiting neuropathy in Parkinson’s disease. Acknowledgments We thank Drs. S.R. Whittemore and XM Xu, Louisville University, for kindly providing RIP antibody. This work was supported by grants from the Chinese Academy of Sciences (No. KSCX2-SW-209; KSCX1-SW-11), the National Natural Science Foundation of China (No. 30000047 to L.X.), the National Basic Research Program of China (G1999054000), the Chinese Ministry of Science and Technology (No. 2001AA221221), and Shanghai Metropolitan Fund for Research and Development (04DZ14005). The support of the Royal Society, UK, is also gratefully acknowledged (grant no. 15429 to L.P.). References Abrahamson, M., Barrett, A.J., Salvesen, G., Grubb, A., 1986. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J. Biol. Chem. 261, 11282 – 11289. Abrahamson, M., Olafsson, I., Palsdottir, A., Ulvssback, M., Lundwall, A., Jensson, O., Grubb, A., 1990. Structure and expression of the human cystatin C gene. Biochem. J. 268, 287 – 294. Akopyan, T., 1991. Protein inhibitors of proteinases from brain. Neurochem. Res. 16, 513 – 517. Anastasi, A., Brown, M.A., Kembhavi, A.A., Nicklin, M.J., Sayers, C.A., Sunter, D.C., Barrett, A.J., 1983. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem. J. 211, 129 – 138. Aronica, E., van Vliet, E.A., Hendriksen, E., Troost, D., Lopes da Silva, F.H., Gorter, J.A., 2001. Cystatin C, a cysteine protease inhibitor, is persistently up-regulated in neurons and glia in a rat model for mesial temporal lobe epilepsy. Eur. J. Neurosci. 14, 1485 – 1491. Barrett, A.J., Kirschke, H., 1981. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 80 (Pt C), 535 – 561. Barrett, A.J., Davies, M.E., Grubb, A., 1984. The place of human gammatrace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem. Biophys. Res. Commun. 120, 631 – 636. Betarbet, R., Sherer, T., Greenamyre, J.T., 2002. Animal models of Parkinson’s disease. BioEssays 24, 308 – 318. Carvey, P.M., Ptak, L.R., Nath, S.T., Sierens, D.K., Mufson, E.J., Goetz, C.G., Klawans, H.L., 1993. Striatal extracts from patients with Parkinson’s disease promote dopamine neurons growth in mesencephalic cultures. Exp. Neurol. 120, 149 – 152. Crews, L.L., Wigston, D.J., 1990. The dependence of motorneurons on their target muscle during postnatal development of the mouse. J. Neurosci. 10, 1643 – 1653. Crocker, S.J., Smith, P.D., Jackson-Lewis, V., Lamba, W.R., Hayley, S.P., Grimm, E., Callaghan, S.M., Slack, R.S., Melloni, E., Przedborski, S., Robertson, G.S., Anisman, H., Merali, Z., Park, D.S., 2003. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J. Neurosci. 23, 4081 – 4091. Dawson, T.M., Dawson, V.L., 2003. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302, 819 – 822. Elliott, K.J., Jones, J.M., Sacaan, A.I., Lioyd, G.K., Corey-Naeve, J., 1998. 6-Hydroxydopamine lesion of rat nigrostriatal dopaminergic neurons differentially affects nicotinic acetylcholine receptor subunit mRNA expression. J. Mol. Neurosci. 10, 251 – 260. Ferger, B., Rose, S., Jenner, A., Halliwell, B., Jenner, P., 2001. 6Hydroxydopamine increases hydroxyl free radical production and DNA damage in rat striatum. NeuroReport 12, 1155 – 1159. Hida, H., Jung, C.G., Wu, C.Z., Kim, H.J., Kodama, Y., Masuda, T., Nishino, H., 2003. Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur. J. Neurosci. 17, 2127 – 2134. Ishimaru, H., Ishikawa, K., Ohe, Y., Takahashi, A., Maruyama, Y., 1996. Cystatin C and apolipoprotein E immunoreactivities in CA1 neurons in ischemic gerbil hippocampus. Brain Res. 709, 155 – 162. Kearns, C.M., Cass, W.A., Smoot, K., Kryscio, R., Gash, D.M., 1997. GDNF protection against 6-OHDA: time dependence and requirement for protein synthesis. J. Neurosci. 17, 7111 – 7118. Kingham, P.J., Pocock, J.M., 2001. Microglial secreted cathepsin B induces neuronal apoptosis. J. Neurochem. 76, 1475 – 1484. Lee, K.S., Frank, S., Vanderklish, P., Arai, A., Lynch, G., 1991. Inhibition of proteolysis protects hippocampal neurons from ischemia. Proc. Natl. Acad. Sci. U. S. A. 88, 7233 – 7237. Lee, D.C., Palm, D.E., Jackson, I.M., Womble, T., Wight, C., 2002. Cystatin C expression in apoptotic PC12 cells following 6-hydroxydopamine toxicity. Abstr. - Soc. Neurosci. 194.112. Levy, E., Sastre, M., Kumar, A., Gallo, G., Piccardo, P., Ghetti, B., Tagliavini, F., 2001. Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer disease patients. J. Neuropathol. Exp. Neurol. 60, 94 – 104. Lofberg, H., Grubb, A.O., Sveger, T., Olsson, J.E., 1980. The cerebrospinal fluid and plasma concentrations of gamma-trace and beta2-microglobulin at various ages and in neurological disorders. J. Neurol. 223, 159 – 170. Lofberg, H., Nilsson, K.E., Stromblad, L.G., Lasson, A., Olsson, S.O., 1982. Demonstration of gamma-trace in normal endocrine cells of the adrenal medulla and in phaeochromocytoma. An immunohistochemical study in monkey, dog and man. Acta Endocrinol. (Copenh) 100, 595 – 598. Lofberg, H., Grubb, A., Davidsson, L., Kjellander, B., Stromblad, L.G., Tibblin, S., Olsson, S.O., 1983. Occurrence of gamma-trace in the calcitonin-producing C-cells of simian thyroid gland and human medullary thyroid carcinoma. Acta Endocrinol. (Copenh) 104, 69 – 76. Lotharius, J., Dugan, L.L., O’Malley, K.L., 1999. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 19, 1284 – 1293. Lynch, G., Baudry, M., 1984. The biochemistry of memory: a new and specific hypothesis. Science 224, 1057 – 1063. Mantle, D., Falkous, G., Ishiura, S., Perry, R.H., Perry, E.K., 1995. Comparison of cathepsin protease activities in brain tissue from normal cases and cases with Alzheimer’s disease, Lewy body dementia, Parkinson’s disease and Huntington’s disease. J. Neurol. Sci. 131, 65 – 70. Mogi, M., Togari, A., Tanaka, K.-I., Ogawa, N., Ichinose, H., Nagatsu, T., 2000. Increase in level of tumor necrosis factor-a in 6-hydroxydopamine-lesioned striatum in rats is suppressed by immunosuppressant FK506. Neurosci. Lett. 289, 165 – 168. Nishio, C., Yoshida, K., Nishiyama, K., Hatanaka, H., Yamada, M., 2000. Involvement of cystatin C in oxidative stress-induced apoptosis of cultured rat CNS neurons. Brain Res. 873, 252 – 262. Nitatori, T., Sato, N., Waguri, S., Karasawa, Y., Araki, H., Shibanai, K., Kominami, E., Uchiyama, Y., 1995. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. 15, 1001 – 1011. L. Xu et al. / Neurobiology of Disease 18 (2005) 152–165 Numan, S., Seroogy, K.B., 1997. Increased expression of trkB mRNA in rat caudate–putamen following 6-OHDA lesions of the nigrostriatal pathway. Eur. J. Neurosci. 9, 489 – 495. Ohe, Y., Ishikawa, K., Itoh, Z., Tatemoto, K., 1996. Cultured leptomeningeal cells secrete cerebrospinal fluid proteins. J. Neurochem. 67, 964 – 971. Palm, D.E., Knuckey, N.W., Primiano, M.J., Spangenberger, A.G., Johanson, C.E., 1995. Cystatin C, a protease inhibitor, in degenerating rat hippocampal neurons following transient forebrain ischemia. Brain Res. 691, 1 – 8. Paraoan, L., White, M.R.H., Spiller, D.G., Grierson, I.B., Maden, E.H., 2001. Precursor cystatin C in cultured retinal pigment epithelium cells: evidence for processing through the secretory pathway. Mol. Membr. Biol. 18, 229 – 236. Paxinos, G., Watson, C., 1986. The Rat Brain in Stereotaxic Coordinates. Academic, Sydney, Australia. Rami, A., Krieglstein, J., 1993. Protective effects of calpain inhibitors against neuronal damage caused by cytotoxic hypoxia in vitro and ischemia in vivo. Brain Res. 609, 67 – 70. Ray, S.K., Banik, N.L., 2003. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr. Drug Target CNS Neurol. Disord. 2, 173 – 189. Romero, J., Berrendero, F., Perez-Rosado, A., Manzanares, J., Rojo, A., Fernandez-Ruiz, J.J., de Yebenes, J.G., Ramos, J.A., 2000. Unilateral 6hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate–putamen. Life Sci. 2000, 485 – 494. Rosenblad, C., Gronborg, M., Hansen, C., Blom, N., Meyer, M., Johansen, J., Dago, L., Kirik, D., Patel, U.A., Lundberg, C., Trono, D., Bjorklund, A., Johansen, T.E., 2000. In vivo protection of nigral dopamine neurons by lentiviral gene transfer of the novel GDNF-family member neublastin/artemin. Mol. Cell. Neurosci. 15, 199 – 214. Ryan, R.E., Sloane, B.F., Sameni, M., Wood, P.L., 1995. Microglial cathepsin B: an immunological examination of cellular and secreted species. J. Neurochem. 65, 1035 – 1045. Sambrock, J., Fritsch, E.F., Maniatis, T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. Sauer, H., Oertel, W.H., 1994. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59, 401 – 415. Shen, Y., Yu, Y., Tang, Z., Guo, H., Yu, F.-S.X., Zhou, J., 2002. Identification and comparative analysis of differentially expressed proteins in rat striatum following 6-OHDA lesions of nigrostriatal 165 pathway: up-regulation of amyloid precursor like-protein 2 expression. Eur. J. Neurosci. 16, 896 – 906. Sickmann, A., Dormeyer, W., Wortelkamp, S., Woltalla, D., Kuhn, W., Meyer, H.E., 2000. Identification of proteins from human cerebrospinal fluid, separated by 2-dimensional polyacrylamide gel electrophoresis. Electrophoresis 21, 2127 – 2128. Sierra-Paredes, G., Cornes, J.M., Sierra-Marcuno, G., 1999. Calpain inhibitor I retards seizure offset in the hippocampus of freely moving rats. Neurosci. Lett. 263, 165 – 168. Sun, Q., 1989. Growth stimulation of 3T3 fibroblasts by cystatin. Exp. Cell Res. 180, 150 – 160. Ungerstedt, U., Arbuthnott, G.W., 1970. Quantitative recording of rotational behaviour in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res. 24, 485 – 493. Xu, L., Shen, Y., Tang, Z.-S., Yu, Y., Zhou, J., 2000. Striatal extracts promote the survival and phenotype expression of fetal dopaminergic neurons in vitro. Abstr. - Soc. Neurosci. 26, 1363. Yamashima, T., 2000. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62, 273 – 295. Yamashima, T., Kohda, Y., Tsuchiya, K., Ueno, T., Yamashita, J., Yoshioka, T., Kominami, E., 1998. Inhibition of ischemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on dcalpain–cathepsin hypothesisT. Eur. J. Neurosci. 10, 1723 – 1733. Yan, P., Liu, N., Kim, G.-M., Xu, J., Xu, J., Li, Q., Hsu, C.Y., Xu, X.M., 2003. Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp. Neurol. 183, 286 – 297. Ying, G.X., Huang, C., Jiang, Z.H., Jing, N.H., Zhou, C.F., 2002. Up-regulation of cystatin C expression in the murine hippocampus following perforant path transections. Neuroscience 112, 289 – 298. Zhou, J., Bradford, H.F., Stern, G.M., 1994. The response of human and rat fetal ventral mesencephalon in culture to the brain-derived neurotrophic factor treatment. Brain Res. 656, 147 – 156. Zhou, J., Pliego-Rivero, B., Bradford, H.F., Stern, G.M., 1996. The BDNF content of postnatal and adult rat brain: the effects of 6-hydroxydopamine lesions. Dev. Brain Res. 97, 297 – 303. Zhou, J., Yu, Y., Tang, Z.-S., Xu, L., 2000. Differential expression of mRNAs of GDNF family in the striatum following 6-OHDA-induced lesion. NeuroReport 11, 3289 – 3293. Zhu, Y.S., Jones, S.B., Burke, R.E., Franklin, S.O., Inturrisi, C.E., 1993. Quantitation of the levels of tyrosine hydroxylase and preproenkephalin mRNAs in nigrostriatal sites after 6-hydroxydopamine lesions. Life Sci. 52, 1577 – 1584.