* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell-Type Specific Properties of Pyramidal
Neural coding wikipedia , lookup
Single-unit recording wikipedia , lookup
Biological neuron model wikipedia , lookup
Central pattern generator wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Axon guidance wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroplasticity wikipedia , lookup
Subventricular zone wikipedia , lookup
Multielectrode array wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Synaptogenesis wikipedia , lookup
Chemical synapse wikipedia , lookup
Electrophysiology wikipedia , lookup
Circumventricular organs wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Cerebral cortex wikipedia , lookup
Synaptic gating wikipedia , lookup
Optogenetics wikipedia , lookup
Apical dendrite wikipedia , lookup
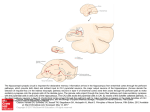
Cerebral Cortex April 2010;20:826--836 doi:10.1093/cercor/bhp152 Advance Access publication July 30, 2009 Cell-Type Specific Properties of Pyramidal Neurons in Neocortex Underlying a Layout that Is Modifiable Depending on the Cortical Area To understand sensory representation in cortex, it is crucial to identify its constituent cellular components based on cell-type--specific criteria. With the identification of cell types, an important question can be addressed: to what degree does the cellular properties of neurons depend on cortical location? We tested this question using pyramidal neurons in layer 5 (L5) because of their role in providing major cortical output to subcortical targets. Recently developed transgenic mice with cell-type--specific enhanced green fluorescent protein labeling of neuronal subtypes allow reliable identification of 2 cortical cell types in L5 throughout the entire neocortex. A comprehensive investigation of anatomical and functional properties of these 2 cell types in visual and somatosensory cortex demonstrates that, with important exceptions, most properties appear to be celltype--specific rather than dependent on cortical area. This result suggests that although cortical output neurons share a basic layout throughout the sensory cortex, fine differences in properties are tuned to the cortical area in which neurons reside. Keywords: BAC transgenic, connectivity, dendrite morphology, neocortex, pyramidal cell Introduction The organization of the cortex into layers of different cell-types is a stereotypic attribute with functional consequences. For example, sensory responses in somatosensory cortex and visual cortex (VC) are layer and cell-type specific (Gilbert and Wiesel 1979; Armstrong-James et al. 1992; Brecht and Sakmann 2002; Martinez et al. 2005; de Kock et al. 2007) and a correlation between morphology and response property can be drawn. In somatosensory (vibrissal) cortex (BC), in rodents, the thicktufted and the slender-tufted pyramidal neurons in layer 5 (L5) respond differently to tactile (whisker deflection) stimuli (Manns et al. 2004; de Kock et al. 2007). The functional difference between slender- and thick-tufted pyramidal neurons raises several questions. First, what are the defining characteristics of these neurons in terms of cell-type--specific properties in dendrite morphology, intrinsic excitability, axon projection target, gene expression, and properties of synaptic transmission? Second, are these properties unique for a cortical area or retained in different cortical areas? Classification of L5 pyramidal neurons has relied on a combination of soma--dendrite morphology, intrinsic electrophysiological properties, gene expression pattern, as well as projection targets (Hevner et al. 2003; Molnar and Cheung 2006; Morishima and Kawaguchi 2006; Nelson et al. 2006; Sugino et al. 2006; Le Be et al. 2007; Watakabe et al. 2007). A scheme that has emerged is the correlation between soma--dendrite morphology and projection Ó The Author 2009. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: [email protected] Alexander Groh1, Hanno S. Meyer2, Eric F. Schmidt3, Nathaniel Heintz3, Bert Sakmann1,4 and Patrik Krieger5 1 Institute for Neuroscience of the Technical University Munich, Biedersteiner Strasse 29, 80802 Munich, Germany, 2 Max-Planck-Institut für medizinische Forschung, Jahnstrasse 29, D-69120 Heidelberg, Germany, 3Laboratory of Molecular Biology, The Rockefeller University, New York, NY 10065, USA, 4 Max Planck Institute for Neurobiology, Am Klopferspitz 18, 82152 Martinsried, Germany and 5Department of Neuroscience, Karolinska Institutet, SE-17177 Stockholm, Sweden targets (Hübener and Bolz 1988; Hübener et al. 1990; Larkman and Mason 1990; Mason and Larkman 1990; Hattox and Nelson 2007). Uncertainties, however, of cell identities remain when correlating parameters from individual studies. The identification of genetically labeled neurons throughout the neocortex enabled in the present work the combined study of many parameters and their dependency on the sensory modality. We have characterized 2 populations of L5 pyramidal neurons genetically labeled with enhanced green fluorescent protein (EGFP) and compared each cell type in somatosensory cortex with the corresponding cell type in VC. We used 2 mouse lines for the 2 L5 cell types expressing EGFP under the control of either a promoter for a transcription factor (ets variant gene [etv] 1) or the promoter for a glycosyltransferase (glycosyltransferase 25 domain containing [glt] 2). Each is specifically expressed in a subpopulation of the slender- and thick-tufted pyramidal neurons, respectively. We show that in both somatosensory cortex and VC, cell-type-specific properties define 2 populations of tufted L5 pyramidal neurons. Each subpopulation retains its basic properties between cortices. In both cortices, the 2 different populations can be clearly distinguished based on anatomical and functional parameters. However, when comparing a given cell-type between the 2 cortices, some parameters are characteristically tuned to the cortical area. These differences suggest that tactile and visual stimuli are processed by similar local neuronal circuits that, however, show variations depending on cortical region. Materials and Methods Experiments were performed on Glt25d2 bacterial artificial chromosome (BAC)-EGFP transgenic mice and Etv1 BAC-EGFP transgenic mice obtained from the GENSAT project. Information on the genes and expression data at the GENSAT Web site (http://www.gensat.org). See also Gong et al. (2007) and Doyle et al. (2008) for a brief description of the Etv1and Glt25d2 BAC-EGFP lines and other BAC lines. Experiments were performed on mice with both parents BAC-EGFP mice and mice with one parent BAC-EGFP and one wild-type C57Bl/6. Experiments were conducted in accordance with the German animal welfare guidelines. Stereotaxic Injection Stereotaxic injections, using isofluorane anesthesia, were done at postnatal day 60--120. Cholera toxin subunit B (Molecular Probes) conjugated to Alexa 555 (pseudo color red) or Alexa 647 (pseudo color blue) for retrograde labeling was mixed with a solution containing adeno-associated viral particles (AAVs) encoding for dTomato (AAVdTomato) for anterograde labeling. In total, 50- to 100-nL solution, containing tracers, was slowly injected into target sites (pontine nucleus, caudate putamen, and posterior medial thalamic nucleus [POm]) with the following coordinates (mm) relative to bregma, midline, and dura: caudate putamen: 0.8, 1.8, –3.6; pontine nucleus: –3.6, 0.55, –5.3; and POm: –2.0, 1.15, –2.9. To avoid inadvertent diffusion of tracer from the intended target area, the tracer injection volume was kept to a minimum. The resulting fraction of retrogradely labeled cells is, thus, unlikely to reflect all cortical cells projecting into the target. The relevant comparison is the relative fraction of retrogradely labeled etv- versus glt-pyramids from injection into different targets and the depth distribution of EGFP and retrogradely labeled cells. Slice Preparation Coronal slices containing primary somatosensory (barrel) cortex or VC were prepared by placing the anterior part of the brain on a 10-deg slope and then cutting perpendicular to the brain surface, discarding the tissue caudal to the cut. The brain was subsequently glued with the cut surface facing down, and 300-lm thick slices were cut. In a few experiments, parasagittal slices (300 lm) containing VC were also used. Slices were maintained at room temperature before recording. All experiments were performed close to physiological temperatures (32--34 °C). Electrophysiology Patch pipettes for whole-cell recordings were filled with (in mM) 105 K-gluconate, 30 KCl, 10 phosphocreatine-Na2, 10 N-2-hydroxyethylpiperazine-N#-2-ethanesulfonic acid (HEPES), 4 ATP-Mg, 0.3 GTP, with pH adjusted to 7.3 with KOH (osmolarity 300 mOsm). Biocytin (2 mg/ml; FLUKA) was included in the pipette to allow the morphology of the neurons to be reconstructed in the fixed tissue. For cell-attached stimulation, the pipette contained the following solution (in mM): 105 Na-gluconate, 30 NaCl, 10 HEPES, 10 phosphocreatine, 4 ATP-Mg, and 0.3 GTP (adjusted to pH 7.3 with NaOH). The extracellular solution contained (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 25 glucose, 2 CaCl2, and 1 MgCl2, continuously bubbled with 95% O2 and 5% CO2. Membrane potential was not corrected for junction potential. Dual whole-cell recordings to investigate synaptic connectivity 1) in barrel cortex (BC) were made on 19 Etv1 BAC-EGFP transgenic mice with an average age of 22 days (range: 18--25) days and on 14 Glt25d2 BAC-EGFP transgenic mice, average age 25 days (range: 16--39 days) 2) in VC (including both primary and secondary VC) were performed on 7 Etv1 BAC-EGFP transgenic mice, average age 23 days (range: 19--25 days) and 2 Glt25d2 BAC-EGFP transgenic mice (age 21 and 22 days). One pipette was used to obtain whole-cell recordings from one cell and the second pipette was used to search for synaptically connected neurons. The search pipette was used for cell-attached stimulation or whole-cell recording. Only cell pairs located within 20 lm of one another in the z-plane were tested. Cell pairs with no synaptic connection in either direction were counted as 2 unconnected pairs, pairs with a unidirectional connection were counted as one connected pair and one unconnected pair, and pairs with a bidirectional connection were counted as 2 connected pairs. The horizontal distance (x-axis) between neurons was measured from the center of the soma, with the vertical y-axis aligned to the apical dendrite. Recordings were made with an Axoclamp2B amplifier (Axon Instruments, Union City, CA), filtered at 3 kHz and sampled at 10 kHz using custom written software (Igor; WaveMetrics, Lake Oswego, OR). Histological Procedures Slices were fixed in 100 mM PBS containing 4% paraformaldehyde and kept at 4 °C for 1--2 days. Slices were stained for cytochrome oxidase, to visualize barrels, and biocytin to visualize cell morphology. Biocytin-labeled neurons were visualized using the avidin:biotinylated horseradish peroxidase complex (Vectastain ABC kit, Vector Laboratories, CA). The location of the neurons within the BC could, thus, be confirmed, and neurons were reconstructed in 3D with the Neurolucida software (MicroBrightField, Colchester, VT) using an Olympus (BX 51) or Zeiss microscope with an oil immersion 3100/1.4 numerical aperture (NA) objective. Quantification of Dendrite Morphology, Intrinsic Membrane Properties, and Cell Counting Apical tuft dendrite was defined in BC as the dendritic profiles in the upper 10% of the pia--white matter distance and upper 15% of the pia-- white matter distance in VC. This corresponds approximately to layer 1. Apical dendrite tuft width was the maximum horizontal width. Primary obliques were dendrites bifurcating from the main apical dendrite and located between the soma and the major apical dendrite bifurcation. ‘‘Nodes’’ are bifurcation or trifurcation points. Soma and dendrite parameters were calculated with Neurolucida Explorer (MicroBrightField). Fluorescently labeled cells were counted manually using the AMIRA (Visage Imaging, Germany version 4.0; Mercury Computer Systems) software. Threshold current was the minimum current to elicit at least one action potential at resting membrane potential. Frequency adaptation was calculated by measuring the average time interval between successive spikes elicited using three 1-s pulses with current in the range 200--400 pA, in steps of 20 pA. The average interspike time intervals (ISIs), excluding the 2 first ISIs, was normalized to the average of the third ISI and plotted as a function of the ISI number (ISI #). Subsequently, a linear regression line was fitted. The slope of the line is taken as the ‘‘adaptation index.’’ An adaptation index of ‘‘10,’’ thus, means that on average the time interval increases with 10 percentage points between successive spikes. Two-Photon Imaging EGFP-labeled cells were identified in the living slice using two-photon microscopy. Two-photon excitation was made by a Ti:Sa-Laser (MIRA 900F; Coherent, Santa Clara, CA) set at 820--840 nm and pumped by a solid-state laser (Verdi 5W; Coherent). Scanning was performed using a resonant scanning unit (TCS-SP2RS; Leica Microsystems, Mannheim, Germany) mounted on an upright microscope (DMLFS; Leica Microsystems) equipped with a 340 objective (HCX APO, 340, NA 0.8; Leica). A dichroic mirror (560DCXR) split the fluorescence to one detector (bandpass filter HQ525/50M) for green fluorescence from EGFPexpressing L5 pyramidal neurons and to one detector (bandpass filter, HQ610/75M) recording red fluorescence from Alexa Fluor 594. Online superposition of fluorescence and infrared contrast-enhanced image allowed targeted recordings from fluorescently labeled neurons. Statistical Analysis Two-tailed unpaired t-tests, and one-way analysis of variance (ANOVA) using Bonferroni posttests, were done with GraphPad Prism 4 (GraphPad Software, CA). Significant differences in the morphological data and the intrinsic properties were determined with one-way ANOVA and Bonferroni posttests unless otherwise stated. Data are reported as mean ± standard deviation (SD), unless stated otherwise. Numerical Analysis of Cell Densities in a Barrel Column The distribution of L5 etv-expressing pyramidal neurons (etv-pyramids) and L5 glt-expressing pyramidal neurons (glt-pyramids) was determined by manual cell counting of all EGFP-labeled cells in 2 barrel columns in one brain slice from each of 3 etv-mice and 2 glt-mice (age 25--28 days). No correction was done for tissue shrinkage. GAD67 and NeuN immunohistochemistry was used to estimate column and layer borders. A detailed description of the immunocytochemistry and analytical tools developed for the numerical analysis of cells in a barrel column will be presented elsewhere (Meyer HS, Wimmer VC, Schwarz D, Sakmann B, and Helmstaedter M, unpublished data). Results Soma Depth Location and Dendrite Morphology of 2 Genotypically Defined L5 Pyramidal Neurons in Primary Somatosensory (Barrel) Cortex Genetic labeling of cortical cells using the BAC method (Gong et al. 2002, 2003, 2007; Doyle et al. 2008) has generated several lines of transgenic mice with fluorescent labeling of potentially specific classes of neurons. To investigate subpopulations of L5 pyramidal neurons in different cortical areas, we have used 2 BAC transgenic mouse lines where expression of Glt25d2 (glt) or Etv1 (etv) was reported with EGFP expression. Expression of Cerebral Cortex April 2010, V 20 N 4 827 these 2 genes is shown to identify L5 pyramidal neurons in the primary somatosensory (barrel) cortex (Fig. 1A,B), VC (Fig. 3), and other neocortical areas (data not shown). For each mouse line, an average density map was calculated showing the 2D soma distribution in 2 neighboring barrel columns (Fig. 1C). L5 etv-pyramids were located predominately in L5A (Fig. 1C; median depth from pia 610 lm) and glt-pyramids in L5B (Fig. 1C; median depth from pia 690 lm). In the horizontal column axis, the highest density was located in the center of the barrel columns (Fig. 1C). In somatosensory cortex, etv- and glt-pyramids are, thus, expressed in 2 distinct sublayers. The peak cell densities are approximately 80 lm apart. A profile in 1D representing the ratio of etv- and glt-pyramids to all excitatory neurons in a barrel column shows that at the peak cell density, the etv- and glt-pyramids represent approximately 55% of the excitatory neurons (Fig. 1D). The fraction of etv-pyramids and glt-pyramids to the total number of neurons was estimated to 29.0 ± 3.1% in L5A (etv) and 12.3 ± 3.5% in L5B (glt). The average neuron density in each layer was higher (Supplementary Table 1) than previously reported for mouse somatosensory cortex (White and Peters 1993). In the acute brain slice preparation, EGFP-labeled etv- and gltpyramids were filled and subsequently stained for biocytin, and their dendrite morphology was reconstructed and quantified. Figure 2 (and Supplementary Fig. 1) illustrates examples of reconstructed etv- (Fig. 2A) and glt-pyramids (Fig. 2B). In both populations, the apical dendrites extended to layer 1. Compared with the glt-pyramids, the etv-pyramids have a narrower apical dendrite tuft width (etv-pyramids: 157 ± 54 lm, n = 29; gltpyramids 356 ± 84 lm, n = 19) and had fewer primary apical oblique dendrites (etv-pyramids: 3.7 ± 2.2 primary branches; gltpyramids: 8.9 ± 2.6 primary branches). The apical dendrite Figure 1. EGFP-labeled etv-pyramids and glt-pyramids in L5 of the somatosensory BC. Confocal images of EGFP-labeled pyramidal cells in brain slices (coronal section) from transgenic mice carrying the BAC constructs for the (A) etv1 or (B) glt2. Red dotted line added for comparison of the relative depth of the 2 populations. Data in (C) and (D) was obtained from 50-lm thick coronal slices from 3 etv-mice and 2 glt-mice. (C) The soma positions of EGFP-labeled cells in the 2 most medial barrel columns per slice were marked manually in 3D and their average density distribution (bin size: 50 3 50 lm2) in the plane of slicing was plotted. Layer borders (outlined with black dots and lines) were derived independently of soma distribution using GAD67 and NeuN immunohistochemistry. The 2D cell-density maps show that etv-pyramids were located in L5A and glt-pyramids in L5B. Contours (black lines superimposed on the cell-density distribution) show isodensity lines at 80% (for etv) or 70% (for glt) of the respective peak density. Along the horizontal column axis, both cell populations had their peak density in the center of the barrel column. (D) Excitatory neuron density was estimated by subtracting GAD67-positve neuron density from NeuN-positive neuron density. The 1D etv- and glt-pyramid density profiles were then divided by the respective excitatory neuron density profiles, yielding the ratio of etv- and glt-pyramids to all excitatory neurons at different depth (etv, black line; glt, blue line). The distances were normalized to the pia--white matter distance and registered to a mean column height to plot etv- and glt-pyramid ratio profiles from different animals on the same axis. 828 Genetically Labeled L5 Pyramidal Neurons d Groh et al. diameter at the base was for glt-pyramids 4.3 ± 1.3 lm, which was wider (P < 0.0001, unpaired t-test) than for etv-pyramids (2.7 ± 1.2 lm). Furthermore, for glt-pyramids, the average total apical dendrite length (4990 ± 1052 lm) was longer than the average total basal dendrite length (2585 ± 1038 lm; Fig. 2C). In contrast, for etv-pyramids, the average total apical dendrite length (2363 ± 823 lm) was shorter (P = 0.0044, unpaired t-test) than the average total basal dendrite length (3006 ± 994 lm; Fig. 2C). The apical tuft dendrite is the most obvious discriminating morphological parameter for the 2 cell-types with etv-pyramids having a shorter total length, fewer nodes, and a smaller width compared with the glt-pyramids. For the basal dendrite, etvpyramids have more nodes compared with glt-pyramids (Table 1). glt- and etv-pyramids are, thus, morphologically different cell types corresponding to thick-tufted and slender-tufted pyramidal neurons, respectively (Markram et al. 1997; Schubert et al. 2001, 2006; Larsen and Callaway 2006; de Kock et al. 2007). L5 in VC was further confirmed by crossing etv-mice with gltmice (Fig. 4). In the glt/etv-hybrid mice, the 2 cell types can be separated because glt-pyramids have EGFP fluorescence coupled to ribosomal proteins in the nucleolus (visible as a bright spot in the soma; Fig. 4B), whereas etv-pyramids have a homogenously labeled soma and brighter EGFP-labeled apical dendrites. In the glt/etv-hybrid mice, the layer separation in BC (Fig. 4A,D) and intermingling in VC was evident (Fig. 4C,E). This raises the possibility that synaptic inputs to the basal dendrites of these 2 cell types are coming from different sources in BC, whereas in VC, glt- and etv-pyramids share inputs (e.g., from the pulvinar). Similar to BC for glt-pyramids (Fig. 3E), the total apical dendrite length (3867 ± 1288 lm, n = 15) was significantly longer than the total basal dendrite length (2640 ± 792), and for the etv-pyramids (Fig. 3E), the total apical dendrite length (2264 ± 647 lm, n = 21) was shorter than the total basal dendrite length (2730 ± 773. Compared with BC, the etvpyramids in VC had on average the same number (4) of oblique dendrites (P = 0.39, unpaired t-test). glt-Pyramids in VC and BC both had on average 9 oblique dendrites. etv-Pyramids appear as slender-tufted (apical dendrite tuft width: 182 ± 50 lm; apical dendrite diameter: 2.7 ± 0.7 lm) and glt-pyramids as thick-tufted pyramidal neurons (apical dendrite tuft width: 290 ± 92 lm; apical dendrite diameter: 2.9 ± 1.1 lm). Similar to BC, the dendritic tuft is Soma Depth Location and Dendrite Morphology of etvand glt-Pyramids in VC Specific EGFP labeling of 2 pyramid populations in L5 was also observed in VC (Fig. 3). In contrast to the somatosensory cortex, the somata of etv- and glt-pyramids are more intermingled in L5 (Compare Fig. 1 and Fig. 3). Intermingling of the soma position in Figure 2. Morphology of etv- and glt-pyramids in BC; etv- and glt-pyramids were filled with biocytin in the acute slice and reconstructed. (A) Slender-tufted etv-pyramids in layer 5A have a narrow apical dendrite tuft and few oblique dendrites. A part of the axon is shown to illustrate that projections were ipsilateral and contralateral. (B) Thick-tufted gltpyramids have a broad apical tuft and relatively more oblique dendrites. A part of the axon is shown to illustrate that the glt-pyramids had an ipsilateral projection only. (C) On average, the total apical dendrite length was longer than total basal dendrite length for glt- but not for etv-pyramids. See also Table 1 and supplementary figure 2. Table 1 Summary of intrinsic properties and morphology of etv- and glt-pyramids in BC and VC Membrane potential (mV) BC etv 68 ± 5 glt 64 ± 4 VC etv 68 ± 5 glt 66 ± 4 Comparison between cell types and cortical etv, BC vs. VC glt, BC vs. VC glt vs. etv in BC glt vs. etv in VC Input resistance (MX) Threshold current (pA) Adaptation index Total apical tuft length (lm) No. of nodes in apical dendrite (excluding obliques) Tuft width (lm) Total basal length (lm) No. of nodes in basal dendrite No. of primary basal dendrites No. of oblique dendrites 86 ± 25 53 ± 17 109 ± 25 116 ± 44 regions 158 158 131 103 24 ± 25 1±1 17 ± 15 3±3 944 2323 1021 1784 12 19 11 14 157 356 182 290 3006 2585 2730 2640 29 19 22 21 7 8 8 7 3.7 8.9 4.1 9.3 ± ± ± ± 9 11 73 16 ± 366 ± 757 ± 421 ± 725 ± ± ± ± 7 6 5 5 ± ± ± ± 54 84 50 92 ± ± ± ± 994 1038 773 792 ± ± ± ± 11 8 6 7 ± ± ± ± 2 2 2 2 ± ± ± ± 2.2 2.6 1.6 3.9 Note: Numbers are derived from pooled data and are given as mean ± SD. Graphical display indicating whether parameters are significantly different (n) or not different (h) for the compared groups. Cerebral Cortex April 2010, V 20 N 4 829 the most obvious discriminator between the 2 cell types: etvpyramids have a shorter total length and narrower width compared with glt-pyramid tufts. In contrast to BC, the basal dendrites of glt- and etv-pyramids are indistinguishable for all parameters tested here (Table 1). Taken together, the morphological properties show that glt- and etv-pyramids are clearly 2 different cell types in both cortical areas. Thalamocortical Innervation Fields To determine the thalamic inputs to etv- and glt-pyramids in BC, we expressed dTomato via AAV injections into POm and ventral posterior medial thalamic nucleus (VPM) in order to map thalamocortical innervation fields. POm axons densely overlapped with basal dendrites and soma of etv-pyramids (Fig. 5A). Potentially also the apical tufts of both etv- and gltpyramids were densely innervated in L1 (Fig. 5B). VPM axons were predominately located in layer 4 and lower L5B. They more sparsely overlapped with basal dendrites and somata of glt-pyramids (Fig. 5C). The preferential innervation of different populations of L5 pyramidal neurons by VPM and POm axons confirms that the different thalamic nuclei can be part of separate thalamocortical circuits (Ahissar and Kleinfeld, 2003; Bureau et al. 2006). Projection Fields of Somatosensory L5 etv- and gltPyramids The knowledge of cortical long-range projections to subcortical targets is relevant for elucidating the possible function of corticofugal projections in, for example, driving behaviors that depend on sensory input. The subcortical targets of the 2 populations of pyramidal neurons in BC were determined by injections of retrograde tracers (in 5 animals) into striatum, POm, and pons (Fig. 6). Gltpyramids projected to ipsilateral pons (Fig. 6A) and POm (data not shown) but not to striatum (Fig. 6A). The depth distribution of glt-pyramids overlapped with retrogradely labeled corticopontine cells but was well separated from corticostriatal cells (Fig. 6E). Glt-pyramids (9 of 9 cells) at the level of the white matter had only an ipsilaterally projecting main axon, thus, projecting to thalamus and pons and also likely to the spinal cord and trigeminal nucleus (Wise and Jones 1977). Etv-pyramids projected to ipsilateral striatum, and to some extent to contralateral striatum (1%, 2 of 156 cells), but not to pons (Fig. 6C) or POm (data not shown). In 5 etvpyramids, the axon could be traced to the white matter, where 4 of 5 cells had a bifurcating main axon (Figs. 2 and 8), indicating both an ipsilateral and contralateral projection. The majority of etv-pyramids in BC, thus, appear to project both ipsilaterally and contralaterally. The retrograde labeling indicated, however, that a minority of the etv-pyramids project to contralateral striatum and, thus, the contralateral projection, presumably, targets mainly contralateral cortex. The depth distribution of etv-pyramids overlapped with retrogradely labeled corticostriatal cells but was largely separated from corticopontine cells (Fig. 6F); etv is, thus, inferred to be expressed in a subpopulation of L5A slender-tufted pyramidal Figure 3. Layer distribution and dendrite morphology of EGFP-labeled etv-pyramids and glt-pyramids in L5 of the VC. Confocal images of EGFP-labeled pyramidal neurons in brain slices (coronal sections) of transgenic mice carrying the BAC constructs for (A) etv or (B) glt. (C) In the acute brain slice, EGFP-labeled cells in L5 were filled with biocytin and reconstructed. Slender-tufted etv-pyramids had a relatively narrow apical 830 Genetically Labeled L5 Pyramidal Neurons d Groh et al. dendrite tuft and few oblique dendrites. (D) Thick-tufted glt-pyramids had a broad apical dendrite tuft and relatively more oblique dendrites. (E) On average, the total apical dendrite length was larger than total basal dendrite length for glt- but not for etv-pyramids. See also Table 1. Figure 4. Layer distribution of etv- and glt-pyramids in glt--etv chimera mice. (A) Confocal images of EGFP-labeled pyramidal neurons in brain slices (coronal sections) showing the layer separation in BC. (B) Enlarged section of the cells marked in (A); etv-pyramids are homogeneously EGFP labeled (triangles), whereas glt-pyramids have a labeled nucleolus and more faintly labeled cell cytoplasm compared with the cell membrane (circles with dot). This difference was used to identify the different cells types. (C) In VC cells were less well separated. (D) Symbols mark the position of etv- (triangle) and glt-pyramids (circle with dot) derived from the confocal image in (A). (E) Symbols mark the position of etv- (triangle) and glt-pyramids (circle with dot) derived from the confocal image in (D). Scale bar in (A) applies to (A), (C), (D), and (E). Figure 5. Thalamocortical innervation of etv- and glt-pyramids in BC. (A) POm axons (red) innervate L5A with an extensive overlap with somata and basal dendrites of etvpyramids (green). (B) POm axons (red) also densely project to layer 1. Blue cells are retrogradely labeled POm projection neurons. Note that POm axons are restricted to the upper layer 5 (L5A), whereas cortical neurons projecting to POm are located in deep L5 and layer 6. (C) VPM axons (red) innervate L4 and glt-pyramids (green) in L5B, predominately the deeper cells. Scale bars, 100 lm. neurons projecting to the ipsilateral striatum and contralateral cortex, with a minority projecting also to contralateral striatum and with no projections to pons. The target specificity of etvand glt-pyramids is furthermore indicated by EGFP labeling in striatum in etv-mice and in pons in glt-mice (Supplementary Fig. 3). Similarly, in VC, the glt-pyramids project to pons (Fig. 6B), but not striatum (Fig. 6B), and etv-pyramids project to striatum (Fig. 6D) but not to pons (Fig. 6D). Compared, however, with BC, there were relatively fewer retrogradely labeled etvpyramids (Fig. 6C,D), and the depth distribution of etvpyramids did not overlap to the same degree with retrogradely labeled corticostriatal cells (Fig. 6H). This difference could indicate that, in VC, the etv-pyramids are a relatively small subpopulation of the corticostriatal cells. We conclude that in VC and BC slender-tufted L5 etv-pyramids are corticostriatal and that the majority of thick-tufted L5 glt-pyramids are corticopontine and thalamic projection neurons. In BC, the depth distribution of retrogradely labeled corticostriatal and corticopontine cells was well separated (Fig. 6E,F), whereas in VC, there was a larger overlap (Fig. 6G,H). This difference indicates that, at least in some parts of the VC, the corticostriatal pyramidal neurons are to a higher degree than in BC located at the same depth as Cerebral Cortex April 2010, V 20 N 4 831 Figure 6. Retrograde labeling of cortical projection neurons. Retrograde tracer cholera toxin subunit B (CT-B) Alexa 555 (red) was injected in striatum, and CT-B Alexa 647 (blue) was injected into pons in glt-mice (glt) and etv-mice (etv). Confocal images showing the distribution of labeled cells in BC and VC. All images are ipsilateral to the injection site. Cell count numbers refer to the total and not only to the depicted image. The total number of cells counted depended on the size of the cell counting frame. Scale bars, 100 lm. (A) In BC, glt-pyramids were double labeled by pons (blue) injection (16%, 202 of 1271 cells). Corticostriatal cells (red) were predominately found in the upper L5 and did not label glt-pyramids (green). (B) In VC, the glt-pyramids (green) were intermingled with corticostriatal cells (red) but were only double labeled by the pons injection (13%; 44 of 337 cells). (C) etv-Pyramids in BC were double labeled by the striatum (red) injection (11%, 38 of 336 cells) but not by the pons injection (blue). (D) In VC, retrogradely labeled corticopontine and corticostriatal cells were to a higher degree intermingled. etv-Pyramids were double labeled by the striatum (red) injection (5%, 9 of 188 cells) but not by the pons injection (blue). (E--H) Distribution in VC and BC of etv-pyramids (etv), glt-pyramids (glt), retrogradely labeled corticostriatal (striatum), and corticopontine (pons) cells plotted as a function of soma depth from the pia. Filled circle shows the median, and error bars show 25--75 percentiles. corticopontine cells. This finding supports the observation that somata of etv- and glt-pyramids are less well separated in VC (Fig. 4). This is, thus, potentially an anatomical substrate for a less specific thalamocortical innervation of infragranular neurons. The projection patterns of etv- and glt-pyramids are in agreement with those inferred from EGFP antibody staining (http://www.gensat.org). The layer localization of retrogradely labeled corticostriatal and corticopontine cells is similar to previously reported results (Wise and Jones 1977; Wiesendanger and Wiesendanger 1982; Akintunde and Buxton 1992; Jinno and Kosaka 2004; Hattox and Nelson 2007). Intrinsic Membrane Properties of etv- and glt-Pyramids in BC and VC Cell-type and area specificity of intrinsic membrane properties was then investigated. The resting membrane potential was –68 ± 1 mV for etv-pyramids in both BC and VC. glt Neurons had a resting membrane potential of –64 ± 1 mV in BC and –66 ± 1mV in VC. Thus, in contrast to VC, in BC, etv- and glt-pyramids have significantly different membrane potentials. The input resistance (MOhm) for etv-pyramids was cortex specific with 86 ± 4 (n = 45) in BC and 110 ± 4 (n = 33) in VC (unpaired two-tailed t-test, P < 0.0001). Furthermore, input resistances of etv- and glt-pyramids were indistinguishable in VC but were different in BC (VC: etv 110 ± 4 MOhm and glt 116 ± 13 MOhm; BC: etv 86 ± 4 MOhm and glt 53 ± 3 MOhm). Threshold current was dependent on the cortical area for the glt-pyramids but not for etv-pyramids and was furthermore not significantly different between glt-pyramids and etv-pyramids in both areas (in pA) etv-pyramids 158 ± 9 in BC and 131 ± 12 in VC and 2) glt-pyramids 158 ± 11 in BC and 103 ± 5 in VC). Spike frequency adaptation was quantified by measuring the average time interval between successive spikes to calculate an ‘‘adaptation index’’ (see Materials and Methods). Adaptation index was for: 1) etv-pyramids 23 ± 27 in BC and 17 ± 17 in VC. 832 Genetically Labeled L5 Pyramidal Neurons d Groh et al. The relatively high adaptation index indicates that the interspike interval increased between successive spikes (Fig. 7A,B; Supplementary Fig. 4A,B) and 2) glt-pyramids 1 ± 2 in BC and 2 ± 2 in VC, indicating a close to constant interspike interval (Fig. 7C,D; Supplementary Fig. 4C,D); etv- and glt-pyramids can, thus, be referred to as adapting and nonadapting, respectively. Among the glt-pyramids, approximately 50% and 20% of the cells were intrinsically bursting in BC and VC, respectively (Fig. 7E,F; Supplementary Fig. 4E,F). glt-Pyramids responding with bursts of action potential (Fig. 7E,F; Supplementary Fig. 4E,F) were not included in the calculation of the adaptation index. A comparison between etv- and glt-pyramids showed that etv-pyramids in BC were more hyperpolarized and had a higher input resistance than glt-pyramids. The adaptation index was higher for etv-pyramids in both areas. Threshold current was cortex specific only for glt-pyramids, but input resistance was cortex specific for both cell types. Intrinsic electrical properties are subject to both, region, and cell-type specificity: etvand glt-pyramids have cell-type--specific properties in both sensory cortices, concomitantly showing a region specificity. Monosynaptic Connections between etv-pyramids and gltpyramids Paired whole-cell recordings were made to investigate cell-type and area dependency of synaptic properties. A monosynaptic connection between etv-pyramids in BC (Fig. 8, Supplementary Fig. 5A) was found in 13 of 76 recorded pairs (17%; 9 unidirectional pairs and 2 bidirectional pairs). The average monosynaptic excitatory postsynaptic potential (EPSP) amplitude was 0.39 ± 0.26 mV (range: 0.09--0.82 mV, n = 13 pairs), with a coefficient of variation (CV) of 0.46 ± 0.11. For EPSP amplitudes larger than 0.23 mV, the failure rate was low (range: 0--3%, n = 6), but increased for smaller EPSP amplitudes (failure range: 8--28%, n = 3). The paired-pulse ratio at an interstimulus Figure 7. Intrinsic electrical properties of etv-pyramids and glt-pyramids in BC and VC. Examples of action potential trains evoked by current injection in 6 different cells (A--F). etv-Pyramids in BC (A) and VC (B) showed spike frequency adaptation. glt-Pyramids in BC (C) and VC (D) were either regular spiking with no frequency adaptation or intrinsically bursting (E, F). Inset in (E, F) shows with a higher magnification that burst of spikes were elicited in intrinsically bursting cells. Scale bar in (A) applies to all figures, except time scale for insets in (E) and (F). Square pulse shows current injection. Current was 300 pA in (A), (C), and (E) and 240 pA in (B), (D), and (F). See summary table 1 for numbers. Figure 8. Synaptic transmission between slender-tufted etv-pyramids in BC and VC. (A) Two-photon image in the acute slice of EGFP-labeled etv-pyramids (upper panel) in BC. The same field of view in differential interference contrast (lower panel). Star marks the same cells in both panels. (B) Reconstruction of 2 etv-pyramids. (C) The etv-pyramids reconstructed in (B) were bidirectionally connected. Calibration: EPSP trace 0.2 mV, 100 ms; action potential (AP) trace 40 mV, 100 ms. (D) Amplitude ratios for etv-pyramid pairs in BC between successive EPSPs and the first EPSP at different stimulation frequencies (EPSP2/EPSP1 5 2/1). Average of all tested connections (mean, SD for data points: 10 Hz: 2/1: 0.91 ± 0.19, 3/1: 0.66 ± 0.33, 4/1: 0.60 ± 0.16, 5/1: 0.53 ± 0.29; 20 Hz: 2/1: 0.79 ± 0.52, 3/1: 0.68 ± 0.44, 4/1: 0.54 ± 0.60, 5/1: 0.42 ± 0.39; 40 Hz: 2/1: 0.82 ± 0.38, 3/1: 0.74 ± 0.72, 4/1: 0.50 ± 0.59, 5/1: 0.35 ± 0.39). (E) Reconstruction of 2 connected etv-pyramids. (F) Eliciting action potentials in cell-1--evoked EPSPs in cell-2 that decreased in amplitude with successive APs. Calibration: EPSP trace 1 mV, 200 ms; AP trace 100 mV, 200 ms. (G) Amplitude ratios for etv-pyramid pairs in VC between successive EPSPs and the first EPSP at different stimulation frequencies (mean, SD for data points: 10 Hz: 2/1: 0.80 ± 0.36, 3/1: 0.75 ± 0.348, 4/1: 0.53 ± 0.27, 5/1: 0.55 ± 0.38; 20 Hz: 2/1: 0.62 ± 0.34, 3/1: 0.46 ± 0.28, 4/1: 0.36 ± 0.31, 5/1: 0.28 ± 0.21; 40 Hz: 2/1: 0.55 ± 0.32, 3/1: 0.35 ± 0.23, 4/1: 0.35 ± 0.35, 5/1: 0.30 ± 0.26). Cerebral Cortex April 2010, V 20 N 4 833 interval of 100 ms was 0.91 ± 0.19. The connection was depressing in the 10- to 40-Hz stimulation range (Fig. 8C,D). Synaptic connectivity between glt-pyramids in BC (Supplementary Fig. 6) was less extensively investigated, but the data suggest that these cells are connected with a lower connection probability (6%; 2 connected pairs of 35) compared with etvpyramids. To compare synaptic properties within a pyramidal cell population in different cortical areas, we also made whole-cell recordings from pairs of etv-pyramids in the VC (Fig. 8E--G, Supplementary Fig. 5B). A monosynaptic connection was found in 6 of 33 (18%) recorded pairs. The average monosynaptic amplitude was 0.82 ± 0.94 mV (range: 0.08--2.3 mV, n = 6 pairs), with a CV of 0.43 ± 0.20. For EPSP amplitudes larger than 0.23 mV, the failure rate was low (range 0--3.8%, n = 4) but increased for smaller EPSP amplitudes (failure: 9 and 18%, n = 2). Similar to the BC, the etv--etv pyramid connection was depressing in the 10- to 40-Hz stimulation range (Fig. 8G). The paired-pulse ratio at an interstimulus interval of 100 ms was 0.80 ± 0.36. The similar synaptic properties and connectivity (chi-square test 0.89) for connected etv-pyramids in BC and VC suggest that within a population of cells synaptic properties may be retained across different cortical areas. Furthermore, corticostriatal etvpyramids appeared more interconnected than corticopontine/ thalamic glt-pyramids. Comparison between Cortices The majority of properties of etv- and glt-pyramids were similar in VC compared with the properties of the corresponding cell type in BC, although with some notable differences outlined below. A given cell type shows variations in dendrite geometry depending on the cortical region. Compared with BC, etv cells in VC have fewer basal dendrite nodes, but the same total length. The basal dendrite tree of glt-pyramids is similar in BC and VC, but in VC, the tuft dendrite is shorter and has fewer nodes (Table 1). The soma depth was also characteristic for the cortical region with a clear separation of glt- and etv-pyramids in BC and the tendency to share the layer in VC. Taken together, the anatomical data suggest that etv- and glt-pyramids are more similar to each other in VC and more distinct in BC. Differences between the cortical regions could also be observed for intrinsic electrical properties. Both cell types had a lower input resistance and needed larger currents to reach spiking threshold in BC. In summary, the differences for a given cell type in different areas suggests that a basic cell layout is fine tuned to the cortical area and by the sensory system, respectively. Discussion Untangling the diversity of cortical cell types requires the combination of genetic targeting, quantification of cell anatomy and physiology, and eventually the molecular characterization of the different cell types (Luo et al. 2008). We have undertaken this approach to provide a detailed histological and physiological characterization of L5 neocortical cell types using BAC-transgenic mice. This allows evaluation of the extent of conservation of cellular properties in different cortical areas and the influence of the sensory modality on L5 neurons. In both VC and somatosensory cortex, the Etv1 line had selective EGFP expression in ‘‘slender-tufted’’ L5 pyramidal neurons, and the Glt25d2 line had selective EGFP-expression in ‘‘thick834 Genetically Labeled L5 Pyramidal Neurons d Groh et al. tufted’’ L5 pyramidal neurons. Etv-pyramids in both somatosensory cortex and VC share characteristic properties compared with glt-pyramids, being more hyperpolarized, having a higher input resistance, an adapting spiking pattern, and being more densely interconnected. In BC, the slender-tufted corticostriatal etv-pyramids were predominately found in upper L5 and the thick-tufted corticopontine/thalamic gltpyramids in lower L5. In contrast, in VC, the 2 populations were not well separated, suggesting a more common thalamic innervation of these cell types in VC but not in BC. A cell classification in L5 based on soma--dendrite morphology, intrinsic electrical excitability, properties of synaptic transmission and gene expression can, thus, differentiate between cells with different projection targets. The 2 genetically defined L5 pyramidal neuron types were found to have in general conserved properties in BC and VC, with the notable anatomical exceptions and, thus, a dependency on the sensory modality (touch or vision), in apical dendrite shape and layer organization. Subpopulations of Pyramidal Neurons in L5 In BC, 16% of all neurons located in L5 were etv-pyramids and 9% were glt-pyramids. Within the layer, however, the local density of labeled pyramidal neurons was significantly higher (Fig. 1C,D). By means of retrograde labeling, the fraction of corticostriatal or corticothalamic cells in L5 of mouse somatosensory cortex has been estimated to 26% and 8%, respectively, of the total number of neurons (Hattox and Nelson 2007). Because fewer etv-pyramids are labeled than this estimated total fraction of corticostriatal cells, it is likely that Etv1 labels a subpopulation of corticostriatal (slender-tufted) pyramidal neurons. Because etv-pyramids have callosally projecting axons, they are likely to be part of the intratelencephalically projecting type of corticostriatal neurons (Wilson 1987; Reiner et al. 2003). The thalamic POm projection of glt-pyramids is, most likely, a collateral of the main axon projecting predominately to pons and POm, but also to other brainstem nuclei and the spinal cord (Veinante et al. 2000), suggesting an anatomical substrate for efference copy of motor instructions to POm. In comparison to related work on the classification of pyramidal neurons in L5, the dendrite morphology and spike frequency adaptation of etv- and glt-pyramids are similar to those found in retrogradely labeled corticostriatal and corticothalamic pyramidal neurons, respectively (Hattox and Nelson 2007). Furthermore, potassium channel Kv3.1-expressing L5 pyramidal neurons (Akemann et al. 2004) which, with respect to their dendrite morphology and spike frequency adaptation, are similar to the etv-pyramids are described here, with one noticeably difference. The Kv3.1-expressing cells made local intracortical connections only and did not have axons projecting to subcortical targets. Taken together with our results, this comparison suggests that slender-tufted pyramidal neurons in L5 are a very heterogeneous group of neurons. It may consist of at least 2 subpopulations, corticocortical and corticofugal projecting neurons (Ivy and Killackey 1981; Hattox and Nelson 2007; Le Be et al. 2007). According to the anatomical classification by Tsiola et al. (2003), the L5 gltpyramids in VC appear to fall in the class denoted ‘‘subgroup 1A’’ and the L5 etv-pyramids in the class denoted ‘‘subgroup 1B.’’ Pyramidal cells in L5 that are organized in cell clusters (Krieger et al. 2007) are similar to the glt-pyramids; these clustered pyramidal neurons were located in lower L5 and projected to POm and pons. The less significant bundling of apical dendrites originating from glt-pyramids suggests, however, at most a partial overlap between the cell types. Notably, the glt- and etv-pyramids project to pons/POm or striatum, respectively, but not to both areas. There were, however, non-EGFP-labeled cells in L5 that were double labeled by tracer injections into pons and striatum (data not shown; compare Levesque et al. 1996). A possible difference in the cell-type-specific properties of etv- and glt-pyramids in other cortices, notably the motor cortex (Miller et al. 2008), remains to be investigated. Genetically defined cell types, thus, enable a detailed investigation of epigenetic factors controlling the formation of local cortical circuits in different cortical areas. Using Genetic Markers to Understand the Contribution of Different Cell Types to the Animal’s Behavioral Repertoire Genetic markers that define specific cell-types can be used to selectively manipulate cortical circuits. This will aid to understand the contribution of a particular pyramidal cell type in driving behaviors. Conditional silencing, for example, of pyramidal neurons in L5 could unravel their contribution to learning and execution of behaviors. The transcription factor Etv1 (also referred to as ER81) has been shown to participate in neurogenesis in the mouse olfactory bulb (Stenman et al. 2003) and circuit formation in the spinal cord (Arber et al. 2000). Taken together with the present results, this indicates that etvdependent proteins can participate in the formation of neuronal circuits in different areas of the central nervous system. The restricted expression of etv in L5 is consistent with previous studies (Xu et al. 2000; Sugitani et al. 2002; Beggs et al. 2003; Hasegawa et al. 2004; Watakabe et al. 2007) and with the in situ hybridization data documented in the Allen Brain Atlas (http://www.brain-map.org). However, expression differs in essential aspects from previous reports of dendrite morphology, functional properties, and projection targets of etvexpressing cells. Yoneshima et al. (2006) reported that ER81 was expressed in non-tufted cells, was equally distributed in L5, and was expressed in neurons projecting to the spinal cord or superior colliculus, whereas Sugino et al. (2006) report that ER81 mRNA was detected in non-adapting L5 and L6 cells. The reasons for these discrepancies remain unclear. We note that the restricted expression to L5 reported for the Etv1 EGFPreporter mice used in the present study, and that reported for an independently generated Etv1 BAC Cre recombinase driver line (Gong et al. 2007) agrees well. Cell-Type Specific Properties in Somatosensory and VC In both somatosensory cortex and VC, etv-pyramids were distinct from glt-pyramids on the basis of the soma--dendrite morphology, intrinsic membrane properties, and projection target. Furthermore, the properties of synaptic transmission between etv-pyramids in the 2 cortices were similar, with a connectivity ratio of approximately 20%. The connectivity for glt-pyramids in BC was estimated, based only on a limited sample, to 6% which is similar to the 10% estimated for clustered thick-tufted pyramidal neurons in L5 (Krieger et al. 2007). In rat VC, the connectivity ratio for thick-tufted L5 pyramidal neurons has also been estimated to approximately 10% (Song et al. 2005). A similar difference in connection probability for L5 pyramidal neurons projecting to different targets has been observed in rat neocortex (Morishima and Kawaguchi 2006; Le Be et al. 2007). Comparing etv and glt pyramidal neurons, we find that they are clearly distinguishable based on morphology, intrinsic, and synaptic properties and long-range circuitry. However, when comparing individual cell types in BC and VC, we find that the difference between cell types (etv vs. glt) is much more pronounced than the differences of these cell types between different cortical areas. The individual cell types are, thus, influenced by the cortical region they belong to, but the overall layout of etv- and glt-pyramids is retained. Our results, thus, raise the intriguing possibility that genes can be identified that characterize cell types which not only share morphology but are also incorporated in comparable local circuits with a stereotypic layout in different cortical areas. It can, thus, be concluded that for a genetically defined neuron population, cell-type--specific properties such as soma--dendrite morphology, intrinsic electrical excitability, projection target, and properties of synaptic transmission are not necessarily unique for a cortical area. They may be retained, with some noted modification, between sensory cortical areas, and possibly other neocortical areas. Supplementary Material Supplementary Table 1 and Figs 1--6 can be found at: http:// www.cercor.oxfordjournals.org/. Notes We thank Randy Bruno and Gilad Silberberg for comments on the manuscript, Rolf Sprengel for AAV-dTomato, and Marlies Kaiser for excellent technical assistance. This work was supported by Åke Wibergs stiftelse to P.K. Author contributions: Conceived and designed the experiments: A.G., H.S.M., B.S., and P.K. Performed the experiments: A.G., H.S.M., and P.K. Analyzed the data: A.G., H.S.M., B.S., and P.K. Contributed reagents/materials/analysis tools: H.S.M., E.S., and N.H. Wrote the paper: A.G., B.S., and P.K. Conflict of Interest: None declared. Address correspondence to Patrik Krieger, Nobel Institute for Neurophysiology, Department of Neuroscience, Karolinska Institutet, SE-171 77 Stockholm, Sweden. Email: [email protected]. References Ahissar E, Kleinfeld D. 2003. Closed-loop neuronal computations: focus on vibrissa somatosensation in rat. Cereb Cortex. 13:53--62. Akemann W, Zhong YM, Ichinohe N, Rockland KS, Knopfel T. 2004. Transgenic mice expressing a fluorescent in vivo label in a distinct subpopulation of neocortical layer 5 pyramidal cells. J Comp Neurol. 480:72--88. Akintunde A, Buxton DF. 1992. Origins and collateralization of corticospinal, corticopontine, corticorubral and corticostriatal tracts: a multiple retrograde fluorescent tracing study. Brain Res. 586:208--218. Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. 2000. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 101:485--498. Armstrong-James M, Fox K, Das-Gupta A. 1992. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 68:1345--1358. Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. 2003. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 40:501--514. Brecht M, Sakmann B. 2002. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol. 543:49--70. Cerebral Cortex April 2010, V 20 N 4 835 Bureau I, von Saint Paul F, Svoboda K. 2006. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4:e382. de Kock CP, Bruno RM, Spors H, Sakmann B. 2007. Layer- and cell-typespecific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 581:139--154. Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. 2008. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 135:749--762. Gilbert CD, Wiesel TN. 1979. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 280:120--125. Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. 2007. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 27:9817--9823. Gong S, Yang XW, Li C, Heintz N. 2002. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 12:1992--1998. Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. 2003. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 425:917--925. Hasegawa H, Ashigaki S, Takamatsu M, Suzuki-Migishima R, Ohbayashi N, Itoh N, Takada S, Tanabe Y. 2004. Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci. 24:8711--8719. Hattox AM, Nelson SB. 2007. Layer v neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 98:3330--3340. Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. 2003. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 25:139--151. Hübener M, Bolz J. 1988. Morphology of identified projection neurons in layer 5 of rat visual cortex. Neurosci Lett. 94:76--81. Hübener M, Schwarz C, Bolz J. 1990. Morphological types of projection neurons in layer 5 of cat visual cortex. J Comp Neurol. 301:655--674. Ivy GO, Killackey HP. 1981. The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol. 195:367--389. Jinno S, Kosaka T. 2004. Parvalbumin is expressed in glutamatergic and GABAergic corticostriatal pathway in mice. J Comp Neurol. 477:188--201. Krieger P, Kuner T, Sakmann B. 2007. Synaptic connections between layer 5B pyramidal neurons in mouse somatosensory cortex are independent of apical dendrite bundling. J Neurosci. 27:11473--11482. Larkman A, Mason A. 1990. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 10:1407--1414. Larsen DD, Callaway EM. 2006. Development of layer-specific axonal arborizations in mouse primary somatosensory cortex. J Comp Neurol. 494:398--414. Le Be JV, Silberberg G, Wang Y, Markram H. 2007. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex. 17:2204--2213. Levesque M, Gagnon S, Parent A, Deschenes M. 1996. Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cereb Cortex. 6:759--770. Luo L, Callaway EM, Svoboda K. 2008. Genetic dissection of neural circuits. Neuron. 57:634--660. Manns ID, Sakmann B, Brecht M. 2004. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J Physiol. 556:601--622. Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. 1997. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 500(Pt 2):409--440. 836 Genetically Labeled L5 Pyramidal Neurons d Groh et al. Martinez LM, Wang Q, Reid RC, Pillai C, Alonso JM, Sommer FT, Hirsch JA. 2005. Receptive field structure varies with layer in the primary visual cortex. Nat Neurosci. 8:372--379. Mason A, Larkman A. 1990. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 10:1415--1428. Miller MN, Okaty BW, Nelson SB. 2008. Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by kv1 subunits. J Neurosci. 28:13716--13726. Molnar Z, Cheung AF. 2006. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 55:105--115. Morishima M, Kawaguchi Y. 2006. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 26:4394--4405. Nelson SB, Hempel C, Sugino K. 2006. Probing the transcriptome of neuronal cell types. Curr Opin Neurobiol. 16:571--576. Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. 2003. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol. 457:420--440. Schubert D, Kotter R, Luhmann HJ, Staiger JF. 2006. Morphology, electrophysiology and functional input connectivity of pyramidal neurons characterizes a genuine layer va in the primary somatosensory cortex. Cereb Cortex. 16:223--236. Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. 2001. Layerspecific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 21:3580--3592. Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. 2005. Highly nonrandom features of synaptic connectivity in local cortical circuits. PloS Biol. 3:e68. Stenman J, Toresson H, Campbell K. 2003. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 23:167--174. Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. 2006. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 9:99--107. Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. 2002. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 16:1760--1765. Tsiola A, Hamzei-Sichani F, Peterlin Z, Yuste R. 2003. Quantitative morphologic classification of layer 5 neurons from mouse primary visual cortex. J Comp Neurol. 461:415--428. Veinante P, Lavallee P, Deschenes M. 2000. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. J Comp Neurol. 424:197--204. Watakabe A, Ichinohe N, Ohsawa S, Hashikawa T, Komatsu Y, Rockland KS, Yamamori T. 2007. Comparative analysis of layerspecific genes in Mammalian neocortex. Cereb Cortex. 17:1918--1933. White EL, Peters A. 1993. Cortical modules in the posteromedial barrel subfield (Sml) of the mouse. J Comp Neurol. 334:86--96. Wiesendanger R, Wiesendanger M. 1982. The corticopontine system in the rat. I. Mapping of corticopontine neurons. J Comp Neurol. 208:215--226. Wilson CJ. 1987. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 263:567--580. Wise SP, Jones EG. 1977. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol. 175:129--157. Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. 2000. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 26:233--245. Yoneshima H, Yamasaki S, Voelker CC, Molnar Z, Christophe E, Audinat E, Takemoto M, Nishiwaki M, Tsuji S, Fujita I, et al. 2006. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 137:401--412.