* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download - Columbia University Medical Center
Types of artificial neural networks wikipedia , lookup
Convolutional neural network wikipedia , lookup
Biological neuron model wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Electrophysiology wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Bird vocalization wikipedia , lookup
Environmental enrichment wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Single-unit recording wikipedia , lookup
Neurogenomics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Multielectrode array wikipedia , lookup
Axon guidance wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neural oscillation wikipedia , lookup
Embodied language processing wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural coding wikipedia , lookup
Mirror neuron wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroanatomy wikipedia , lookup
Circumventricular organs wikipedia , lookup
Central pattern generator wikipedia , lookup
Synaptic gating wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
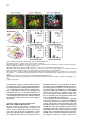
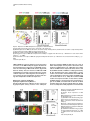
Cell, Vol. 109, 205–216, April 19, 2002, Copyright 2002 by Cell Press Regulation of Motor Neuron Pool Sorting by Differential Expression of Type II Cadherins Stephen R. Price,1 Natalia V. De Marco Garcia,1 Barbara Ranscht,2 and Thomas M. Jessell1,3 1 Howard Hughes Medical Institute Department of Biochemistry and Molecular Biophysics Columbia University 701 West 168th Street New York, New York 10032 2 Neurobiology Program The Burnham Institute 10901 N. Torrey Pines Road La Jolla, California 92037 Summary During spinal cord development, motor neurons with common targets and afferent inputs cluster into discrete nuclei, termed motor pools. Motor pools can be delineated by transcription factor expression, but cell surface proteins that distinguish motor pools in a systematic manner have not been identified. We show that the developmentally regulated expression of type II cadherins defines specific motor pools. Expression of one type II cadherin, MN-cadherin, regulates the segregation of motor pools that are normally distinguished by expression of this protein. Type II cadherins are also expressed by proprioceptive sensory neurons, raising the possibility that cadherins regulate additional steps in the development of sensory-motor circuits. Introduction Many hundreds of neuronal cell types are generated during the development of the vertebrate central nervous system (CNS)—a diversity essential to the formation of selective neuronal circuits. Soon after their generation, neurons of a particular class segregate from those of other classes—a process that is linked to their later patterns of connectivity. The organization of neurons in the developing CNS proceeds along two major schemes (Ramon y Cajal, 1894). In some regions of the CNS, such as the cerebral cortex and dorsal spinal cord, neurons are assembled into stratified layers, or laminae (Rexed, 1952; Rakic, 1972). However, the prevalent strategy of neuronal organization is based on nuclear subdivisions (Ramon y Cajal, 1911): neurons with similar functions are clustered into discrete nuclei. Advances have been made in defining the cellular and molecular basis of neuronal lamination (Ross and Walsh, 2001), but little is known of the developmental mechanisms used to segregate CNS neurons into discrete nuclei. The nuclear organization of neurons is prominent in the ventral spinal cord. Functional subsets of motor neurons in the lateral motor column (LMC) are clustered into small groups, termed motor pools. The existence 3 Correspondence: [email protected] of motor pools has long been appreciated (Elliott, 1942; Romanes, 1942), and detailed motor pool maps have been compiled in the developing and adult spinal cord (Romanes, 1964; Landmesser, 1978a, 1978b; Hollyday, 1980). Motor pools have been linked to three main features of motor organization. First, all neurons within a motor pool project to a single muscle target in the limb (Landmesser, 1978a, 1978b). Second, all receive monosynaptic input from proprioceptive sensory neurons that supply the same muscle target (Frank et al., 1988). Third, neurons within an individual motor pool are electrically coupled (Brenowitz et al., 1983), a feature that is thought to coordinate the patterns of firing of functionally related motor neurons in response to afferent, segmental, and supraspinal inputs (Chang and Balice-Gordon, 2000). The segregation of LMC motor neurons into discrete pools begins soon after their exit from the cell cycle and appears to occur in two phases. In one phase, motor neurons segregate by means of an “inside-out” migration, during which prospective lateral LMC neurons migrate through neurons of the medial LMC to reach their final settling position (Hollyday and Hamburger, 1977; Sockanathan and Jessell, 1998). Superimposed on this program, motor neurons within both the medial and lateral subdivisions of the LMC segregate to form discrete pools (Lin et al., 1998). Many of the physiological features that characterize motor pools arise during early spinal cord development (Milner and Landmesser, 1999; Landmesser, 2001), implying molecular distinctions in motor pool identity. Different motor pools can be distinguished by their profile of expression of ETS and LIM homeodomain transcription factors (Lin et al., 1998; Arber et al., 2000; J. Livet et al., submitted), and the expression of two of these ETS genes, Er81 and PEA3, is matched in functionally interconnected proprioceptive sensory and motor neurons in the chick spinal cord (Lin et al., 1998). The mechanisms that direct the segregation of motor neurons into specific pools, however, remain unknown. Several classes of cell surface proteins, notably Trks, Ephrins, Eph kinases, and Neuropilins are expressed by subsets of spinal motor neurons (Eberhart et al., 2000; VarelaEchavarria et al., 1997; Iwamasa et al., 1999; Feng et al., 2000), but these proteins have not been shown to segregate with specific motor pools in any systematic manner. Another family of vertebrate recognition molecules is the classic cadherins (Nollet et al., 2000), and their potential involvement in defining motor pools is suggested by two observations. First, the differential expression of classic cadherins demarcates distinct regions of the developing brain (Suzuki et al., 1997; Arndt et al., 1998; Yoon et al., 2000). More tellingly, a divergent type I cadherin, T cadherin, is expressed by certain motor pools in the developing chick spinal cord (Fredette and Ranscht, 1994). Nevertheless, the contribution of cadherins to the functional organization of neurons within the developing CNS has not been defined. We have explored the role of cadherins in the organization of neuronal nuclei through an analysis of the development of motor pools in the chick spinal cord. We Cell 206 find that the expression of classic type II cadherins defines specific motor pools and provide in vivo evidence for their role in motor pool segregation. Type II cadherins are also expressed by subsets of proprioceptive neurons, raising the possibility that these proteins have additional roles in the formation of sensory-motor connections. Results Expression of Type II Cadherins by Embryonic Motor Neurons To identify cadherin genes expressed by developing spinal motor neurons, we performed a degenerate PCR screen on cDNA isolated from HH (Hamburger and Hamilton, 1951) stage 35 chick spinal cord. We cloned 15 cadherin genes that were dispersed throughout the major cadherin subfamilies (Nollet et al., 2000; see Supplementary Figure S1 at http://www.cell.com/cgi/content/ full/109/2/205/DC1). Analysis of the expression of these cadherins in HH stage 35 spinal cord, a stage when motor pool segregation approximates the mature state (Hollyday, 1980), showed that all 15 cadherins were expressed at detectable levels by spinal cord cells (Figure 1; data not shown). We focused on the type II cadherins, since members of this subfamily were prominently expressed in subsets of motor neurons in the LMC, both at forelimb and hindlimb levels (Figures 1E–1L; see Supplemental Figure S2; data not shown). Of the type I cadherins analyzed, only the divergent member, T-cad, was expressed by selected motor pools (Figure 1I; data not shown; Fredette and Ranscht, 1994). To determine whether type II cadherins are expressed by specific motor pools within the LMC, we examined lumbosacral (LS) segments 1–3, levels of the spinal cord for which detailed motor pool maps are available (Landmesser, 1978a, 1978b; Hollyday, 1980). In addition, we used the motor pool-specific expression of Er81 and PEA3 (Lin et al., 1998) as a molecular guide to pool identity (Figures 1A–1D and 1M). The expression of each type II cadherin appeared to conform to identified motor pools (Figures 1E–1L; data not shown). The Adductor (A) pool expressed high levels of MN-cad, T-cad, cad-6b, and cad-8 (Figures 1E and 1G–1J); the external Femorotibialis (eF) pool expressed high levels of T-cad, cad-6b, and cad-8 (Figures 1H–1J); the internal Femorotibialis (iF) pool selectively expressed cad-8 (Figure 1J); the Iliotrochanterici (ITR) pool expressed cad-7 and cad-6b (Figures 1K and 1H) and a low level of cad-8 (Figure 1J); the anterior Iliotibialis (ITB) pool expressed cad-6b (Figure 1H) and a low level of MN-cad (Figure 1G); the Hip-Retractor (HR) motor pools expressed cad-8 and a low level of cad-12 (Figures 1J and 1L); and the Sartorius (S) motor pool selectively expressed cad-10 (Figure 1F; data not shown). We also performed dual color fluorescence in situ hybridization with probes for selected cadherin and ETS genes and found coincident expression of Er81 and MN-cad in essentially all A neurons (Supplemental Figures S2E–S2G at http://w ww.cell.com/cgi /content/full/109/2 /205/ DC1). Similar findings were made with cad-6b and cad-8 in A and eF neurons (data not shown). Thus, all motor neurons within a pool appear to express a given cadherin. We conclude that a combinatorial profile of classic cadherin genes distinguishes individual motor pools within the lumbar LMC. At rostral lumbar levels, individual motor pools are typically defined by the expression of more than one cadherin gene, and no two motor pools exhibit an identical profile of cadherin expression (Figure 1N). Developmental Regulation of Cadherin Expression in Motor Neurons Motor pool segregation occurs between HH stages 24 and 29, and we therefore addressed when the poolrestricted patterns of cadherin expression become evident. Two contrasting developmental profiles of cadherin expression were detected in rostral lumbar LMC motor neurons. For one set of cadherins, MN-cad, cad-12, and cad-8, expression appeared to be initiated in most or all LMC neurons at the time of their generation and was subsequently extinguished from subsets of neurons to produce the mature pool-restricted pattern (Figures 2A–2C; data not shown). Thus, at HH stage 21, soon after the generation of postmitotic motor neurons, MN-cad expression was evident in most or all LMC motor neurons (Figure 2A). By HH stage 24, many LMC motor neurons still expressed MN-cad, but laterally positioned motor neurons had extinguished expression of the gene (Figure 2B), and by HH stage 27 only the more medially located neurons within the LMC expressed detectable levels of MN-cad (Figures 2C and 2J). The lateral, presumptive eF motor neurons expressed Er81 but no longer expressed MN-cad (Figures 2C and 2I). For a second set of cadherins, T-cad, cad-6b, and cad-7, motor pool-specific gene expression was evident well after the exit of motor neurons from the cell cycle (Figures 2D–2F; data not shown). The onset of expression of T-cad, cad-6b, and cad-7 expression by LMC pools occurred only around HH stages 26–27 and was restricted to a subset of LMC neurons (Figures 2D–2F and 2J; data not shown). The temporal profile of expression of these cadherins by LMC neurons appeared to parallel the pool-specific profile of ETS gene expression (Figures 2G–2J; data not shown; Lin et al., 1998). For both programs, the restriction in cadherin expression to subsets of motor neurons appeared to coincide with the segregation of motor neurons into discrete pools (Figure 2J). The coincidence in expression of cadherin and ETS genes by motor pools led us to examine whether there is a functional link between the expression of ETS and cadherin genes. The onset of expression of ETS genes in motor pools is dependent on the limb target (Lin et al., 1998), and we therefore examined whether the poolspecific profile of classic cadherin expression is subject to a similar regulation. To assess this, the expression of Er81, MN-cad, and T-cad was examined in embryos in which the hindlimb had been ablated unilaterally at stage 18 (Lin et al., 1998). Analysis of operated embryos at stage 29 revealed that the absence of Er81 expression in motor neurons on the side of the spinal cord lacking a limb bud (Figure 2K) was accompanied by the loss of a pool-specific pattern of MN-cad and T-cad expression (Figures 2L and 2M). In contrast, analysis of MN-cad expression at stage 24, prior to the pool-specific extinction of MN-cad Cadherins and Motor Neuron Sorting 207 Figure 1. Expression of Classic Cadherins in HH Stage 35 Chick Spinal Cord (A) ETS gene Er81 expression at LS2. (B) Er81 and Isl1 expression defines three motor pools at LS2: A, Er81⫹ Isl1⫹; eF, Er81⫹ Isl1⫺; and HR, Er81⫺ Isl1⫹. (C) PEA3 expression at LS2. (D) Er81 and PEA3 expression A and eF, and ITR/ITB pools. (E) MN-cad expression at LS1. (F) Cad-10 expression at LS1. (G–L) Comparison of MN-cad, cad-6b T-cad, cad-8, cad-7, and cad-12 expression at LS2. Arrow in (K) indicates expression of cad-7 by cells at the ventral root exit point. (M) Expression of LIM-HD and ETS proteins in motor pools at LS2 level. (N) Cadherin expression in motor pools at LS2. Cadherins indicated in brackets are expressed at low levels. The scale bar equals 150 m. expression, revealed similar levels of MN-cad expression on the operated and control sides of the spinal cord (Figure 2N). These findings indicate that the poolspecific profile of classic cadherin expression depends on peripheral signals. To examine whether cadherin expression in motor neurons is regulated by ETS genes, we ectopically expressed Er81 by electroporation in stage 16 chick spinal cord and analyzed the resulting pattern of MN-cad expression at stage 29. Misexpression of Er81 resulted in ectopic expression of MN-cad within the ventral spinal cord, both at hindlimb and thoracic levels (see Supplementary Figures S3A–S3C at http://www.cell.com/cgi/content/full/109/2/205/DC1). Together, these findings support the idea that cadherin expression in motor pools is regulated by ETS proteins. MN-cad Expression Regulates Motor Pool Segregation To test the possibility that the differential expression of type II cadherins is involved in the segregation of LMC neurons into pools, we focused on the eF and A motor pools because at rostral lumbar levels these are the only two motor pools that can be distinguished by the expression of a single cadherin: MN-cad is expressed by A but not by eF neurons (Figures 1E, 1G, and 1N). If the differential expression of MN-cad contributes to the segregation of eF and A motor neurons, we reasoned that the expression of MN-cad in eF neurons and/or the elimination of MN-cad activity from A neurons might be expected to perturb the normal segregation of these two motor pools. MN-cad expression becomes restricted to subsets of LMC neurons after stage 24, and thus, ectopic expression of MN-cad is likely to perturb the normal pattern of MN-cad expression only from this stage onward. Moreover, since the eF pool resides within the lateral LMC and the A pool within the medial LMC, a perturbation in motor pool segregation by ectopic expression of MN-cad is likely to reflect interactions between eF and A neurons during the inside-out phase of neuronal migration. We used in ovo electroporation to express three cadherins—MN-cad, E-cad, and Cad-6b—in mosaic Cell 208 Figure 2. Developmental Regulation Cadherin Expression in LMC Neurons of (A–C) MN-cad expression at LS2. (D–F) T-cad expression at LS2. (G–I) Er81 expression at LS2. (J) MN-cad and T-cad expression in the eF motor pool. (K–M) Er81, MN-cad, and T-cad expression at LS1 in stage 29 embryos, after unilateral (left side) ablation of limb bud at HH stage 18. Similar findings were obtained in five embryos. (N) Motor neuron expression of MN-cad at HH stage 24 is not affected by unilateral (left side) ablation of the limb bud at HH stage 18. The scale bar equals 75 m in (A) and (G), 100 m in (B), (D), (H), and (N), and 150 m in (C), (F), (I), and (K–M) fashion in motor neurons located at rostral lumbar levels of the spinal cord, and we examined the impact on motor pool segregation (Figure 3A). Chick embryos were electroporated unilaterally at HH stages 15–18 and analyzed at HH stages 29–30, at which time motor pool segregation is essentially complete (Figure 2J; Lin et al., 1998). Expression was analyzed with antibodies directed against native cadherin proteins or through the use of cad-GFP fusions (Figures 3B–3E). Electroporation resulted in high levels of expression of MN-cad, E-cad, and Cad-6b on the cell bodies, dendrites, and axons of motor neurons (Figures 3B–3E; data not shown). In functional experiments, we expressed cadherin cDNAs in a bicistronic vector, from which nuclear-targeted LacZ was also expressed, permitting the identification of neurons that expressed cadherins. Our analysis was restricted to embryos in which over 30% of LMC neurons expressed cadherins (mean incidence of ectopic MN-cad expression in LMC neurons was 46%; assessed by LacZ expression; n ⫽ 18 embryos). The consequence of cadherin expression on eF and A motor pool segregation was assessed by analysis of Isl1 and Er81 expression, and in all instances the identity of individual neurons expressing ectopic cadherins was determined by LacZ expression, using triple-label immunocytochemistry (Figures 4A–4C). We first examined whether ectopic expression of MN-cad altered the specification of motor pool identity, as assessed by the number of Er81⫹, Isl1⫹ A and Er81⫹, Isl1⫺ eF neurons present at rostral lumbar levels of HH stage 30 spinal cord. Ectopic expression of MN-cad did not change the total number of A or eF motor neurons (control side, 292 A neurons and 307 eF neurons at LS1/LS2 levels; electroporated side, 314 A neurons and 304 eF neurons at LS1/ LS2 levels; mean values from five embryos 2 : p ⫽ 0.5, 1 df). Thus, the profile of ETS protein expression in these two motor pools appears not to be affected by MN-cad expression. This finding permitted us to examine the influence of MN-cad misexpression on the segregation of A and eF motor neurons through an analysis of Er81 and Isl1 expression. Ectopic expression of MN-cad did not alter the position or integrity of the LMC itself but did result in a marked increase in the extent of intermixing of eF and A neurons within the LMC (Figures 4A and 4B). After MN-cad misexpression, A neurons were found in ectopic lateral positions, and conversely eF neurons were found in ectopic medial positions (Figure 4B). To quantify this effect, we devised an index of neuronal mixing. Individual motor neurons were assigned to specific motor pools on the basis of Er81 and Isl1 expression, and the pool identity of each of the five motor neurons that were located adjacent to the motor neuron under study was scored. This index permits an analysis of the spatial relationship between two motor neuron subtypes that is independent of the medio-lateral position of these neurons, and thus provides a quantitative measure of the segregation of the two neuronal populations. In control spinal cord analyzed at stage 29, eF neurons were surrounded almost exclusively by other eF neurons (Figure 4A), resulting in a neuronal mixing index that is dominated by zero scores (Figure 4E). After MN-cad expression, eF neurons were sometimes surrounded by two to three A neurons (Figures 4B, 4C, and 4E), a situation rarely, if ever, encountered on the control side of the spinal cord (Figure 4E), and the mixing of eF and A neurons was highly significant (2: p ⬍ 0.001; Figure 4E; data not shown). In addition, the degree of intermixing of A and eF neurons was strictly dependent on the status of MN-cad expression by eF neurons. All eF neurons that intermixed with A neurons expressed MN-cad, whereas eF neurons that lacked MN-cad expression showed no increase in intermixing with A neurons over controls (2: p ⬎ 0.05; Figure 4F). Thus, expression of MN-cad in eF neurons promotes their intermixing with A neurons in a cell-autonomous manner. Since electroporation directed mosaic MN-cad expression in all LMC motor pools, two populations of A neurons were generated in these experiments: nonelectroporated A neurons that exhibited wild-type levels of MN-cad and electroporated A neurons that presumably had elevated levels of MN-cad expression (Figure 4G). Cadherins and Motor Neuron Sorting 209 Figure 3. Mosaic Expression of Cadherin Transgenes in LMC Neurons (A) Strategy for expression of cadherins by in ovo electroporation. (B–E) Ectopic expression of an MN-cad-GFP fusion (B and C), E-cad (D), and cad-6b (E) results in labeling of axons, somata, and dendrites of spinal cord neurons. The level of ectopic cad-6b expression (E) is ⵑ5- to 10-fold that of endogenous cad-6b expression in motor neurons. Scale bar equals 150 m. We examined whether the intermixing of A and eF neurons varied according to the status of MN-cad expression in A neurons. As predicted, this analysis revealed that A neurons were intermixed with eF neurons, but there was no significant difference in the extent to which electroporated and nonelectroporated A neurons mixed with eF neurons (2: p ⬎ 0.05; comparison of data in Figures 4H and 4I). We also observed that after MN-cad misexpression, Isl1⫹ Er81⫺ HR neurons (green neurons in Figure 4B) were interspersed within A neurons, a finding that we return to in more detail below. Expression of a Truncated MN-cad Isoform in A Neurons Promotes Intermixing with eF Neurons Since ectopic MN-cad expression in eF neurons promotes their intermixing with A neurons, we considered whether a reduction in MN-cad function in A neurons might similarly promote their intermixing. Truncated forms of classical cadherins that lack carboxy-terminal regions of their cytoplasmic domain have been shown to function in vivo in a dominant-negative manner, selectively blocking the function of their cognate cadherins (Levine et al., 1994; Ozawa and Kemler, 1998). A truncated version of MN-cad, termed ⌬MN-cad, which lacks 114 residues at its carboxyl terminus but retains the membrane-proximal cytoplasmic domain, was expressed in the developing spinal cord. Since MN-cad appears initially to be expressed by all postmitotic motor neurons (Figures 2A and 2J), the expression of ⌬MN-cad could, in principle, perturb the normal profile of MN-cad expression in A neurons as soon as they have left the cell cycle. The expression of ⌬MN-cad in A neurons promoted their mixing with eF neurons (Figures 5A–5E). In contrast, A neurons that lacked ⌬MN-cad expression did not mix with eF neurons (Figure 5F). The degree of intermixing of ⌬MN-cad-electroporated A neurons with eF neurons was comparable to that obtained by electroporation of MN-cad within eF neurons (compare Figures 4E and 5E). As predicted, analysis of the position of eF neurons after ⌬MN-cad expression revealed a significant mixing with A neurons, but the intermixed eF neurons were comprised of those that expressed and lacked ⌬MN-cad at equal incidence (data not shown). Together, these findings provide evidence that the mixing of neurons in the A and eF motor pools can be elicited either by expression of MN-cad in eF neurons or by expression of ⌬MN-cad in A neurons. As a control for the specificity of action of ⌬MN-cad, we expressed a version of MN-cad that lacks the entire extracellular domain but retains the transmembrane and cytoplasmic domains (⌬EC-MN-cad). With other classic cadherins, deletion of the extracellular domain has been shown to generate proteins that inhibit the function of multiple cadherins (Kintner, 1992; Fujimori and Takeichi, 1993). Expression of ⌬EC-MN-cad did not result in the intermixing of neurons in the A and eF pools (see Supplemental Figures S4C and S4D at http://www.cell.com/ cgi/content/full/109/2/205/DC1) but did result in an apparent disruption of the segregation of motor columns. Motor neurons with a median motor column (MMC) identity were found interspersed with LMC neurons (Supplemental Figure S4E). A similar intermixing of MMC and LMC neurons was not observed after expression of MN-cad or ⌬MN-cad (data not shown). These findings provide evidence for the specificity of ⌬MN-cad function and also raise the possibility that certain classic cadherins function in the segregation of motor neurons into columns as well as pools. Selectivity of Motor Pool Mixing after MN-cad Expression As a further test of the specificity of MN-cad in eliciting A and eF pool mixing, we examined the actions of two other classic cadherins, E-cad and cad-6b. Between HH stages 24 and 30, E-cad is not expressed by LMC neurons (data not shown), and cad-6b comes to be expressed in both A and eF neurons (Figures 1H and 1N). Thus, ectopic expression of either of these cadherins is Cell 210 Figure 4. Misexpression of MN-cad in eF Neurons Promotes Mixing with A Neurons (A) eF and A neurons are segregated on the control side of the spinal cord. (B) After misexpression of MN-cad, eF neurons are found in ectopic medial positions, and A neurons are found in ectopic lateral positions. (C) Neuronal MN-cad expression, assessed by LacZ expression. (D) Diagram indicating that expression of MN-cad generates a mosaic of electroporated and wild-type eF neurons. (E) eF neurons that express MN-cad mix with A neurons (n ⬎ 450 neurons in each class from nine embryos; 2 , p ⬍ 0.001). The histogram plots the incidence with which eF neurons are surrounded by A neurons, indicated as neuronal mixing index. (F) eF neurons that do not express MN-cad remain laterally positioned and segregated from A neurons (n ⬎ 500 neurons in each class from nine embryos; 2 , p ⬎ 0.05). Data in (E) and (F) indicate that eF neurons that express MN-cad are segregated from nonelectroporated eF neurons. (G) Expression of MN-cad generates a mosaic of electroporated and wild-type A neurons. (H and I) Both electroporated and wild-type A neurons mix with electroporated eF neurons (nine embryos, comparison of [H] and [I]; 2 , p ⬎ 0.05). Scale bar equals 60 m. not predicted to equate the profile of cadherin expression of eF and A neurons (Figure 1N). Misexpression of E-cad did not promote intermixing of A and eF neurons (2: p ⬎ 0.05; Figures 6A–6C). Similarly, ectopic expression of cad-6b did not influence the segregation of A and eF motor pools (2: p ⬎ 0.05; Figures 6D–6F). Thus, the regulation of A and eF motor pool mixing by MN-cad is not mimicked by two other classic cadherins, despite their high-level expression by motor neurons (Figures 3D and 3E). Specificity of Motor Pool Desegregation after MN-cad and ⌬MN-cad Misexpression We also examined the influence of MN-cad and ⌬MN-cad on the segregation of eF or A neurons from neurons in other motor pools present at the LS1-LS3 level—pools that would not be predicted to acquire equivalent cadherin profiles by manipulation of expression of MN-cad alone. We first assayed the effect of MN-cad misexpression on the segregation of eF neurons from neurons in the ITR/ITB motor pools, two laterally positioned pools adjacent to the eF pool that express PEA3 (Figures 7A and 7B; Lin et al. 1998). Ectopic expression of MN-cad or ⌬MN-cad in eF and ITR/ITB neurons did not perturb the segregation of these two motor pools (Figures 7C–7E; data not shown). Thus, there is selectivity in the desegregation of LMC motor pools elicited by MN-cad misexpression. As noted above, we also examined the segregation of A neurons from Er81⫺, Isl1⫹ neurons in the HR pool, a motor pool complex that normally forms ventral to A neurons and expresses cad-8 and cad-12 (Figure 1N). After MN-cad expression, a significant intermixing of A and HR neurons was detected (Figures 4B and 7H). The intermixing of HR and A neurons, however, occurred independent of the status of MN-cad expression by HR neurons. Thus, nonelectroporated as well as electroporated HR neurons showed increased mixing with A neurons (Figures 7I and 7J). In contrast, expression of Cadherins and Motor Neuron Sorting 211 Figure 5. Expression of ⌬MN-cad Impairs the Segregation of A and eF Neurons (A) Segregation of A and eF neurons on the control side of the spinal cord. (B) After misexpression of ⌬MN-cad, eF neurons are found in ectopic medial positions, and A neurons are found in ectopic lateral positions. (C) LacZ expression indicates ⌬MN-cad electroporated neurons. (D) Analysis of A-eF neuronal mixing after ⌬MN-cad misexpression. (E) A neurons that express ⌬MN-cad are impaired in their ability to segregate from eF neurons (n ⬎ 200 neurons in each class from five embryos; 2 , p ⬍ 0.001). (F) A neurons that do not express ⌬MN-cad segregate normally from eF neurons. (n ⬎ 200 neurons in each class from five embryos; 2, p ⬎ 0.05). Scale bar equals 60 m. ⌬MN-cad did not result in a similar increase in the mixing of neurons in the HR and A pools (Figure 5B; data not shown). Furthermore, expression of cad-6b did not promote the intermixing of HR and A neurons (Figure 6E; data not shown). Thus, the influence of MN-cad on HR and A mixing is not simply a general response to ectopic cadherin expression. Taken together, these findings reveal a largely selective perturbation of motor pool sorting after manipulation of MN-cad expression. Expression of Classic Cadherins by Proprioceptive Sensory Neurons We observed that type II cadherins are also expressed by proprioceptive sensory neurons in the embryonic dorsal root ganglion (DRG). At HH stage 36, a time at which monosynaptic sensory-motor connections have just been established (Lee et al., 1988; Davis et al., 1989), subsets of DRG neurons expressed cad-8, cad-12, cad6b, and MN-cad and, in addition, T-cad (Figures 8C–8H). At this developmental stage, proprioceptive sensory neurons are located in a lateral domain of the DRG and can be identified by expression of Er81 and PEA3 (Figures 8A and 8B; Lin et al., 1998). We found that DRG neurons that expressed cad-8, T-cad, cad-12, cad-6b, and MN-cad were concentrated in the ventrolateral region of the DRG, coincident with the domain of PEA3 and Er81 expression (Figures 8A–8G; data not shown; Lin et al., 1998). Of the total population of rostral lumbar Figure 6. E-cad and cad-6b Do Not Promote the Mixing of A-eF Neurons (A) Ectopic E-cad expression in LMC neurons. (B) A and eF neurons segregate normally after E-cad misexpression. (C) Quantitation of A and eF neuronal segregation after E-cad misexpression (2, p ⬎ 0.05; n ⬎ 150 neurons in each class from four embryos). (D) Ectopic cad-6b expression. Electroporated neurons are marked by LacZ expression. (E) A and eF neurons segregate normally after cad-6b misexpression. (F) Quantitation of A and eF neuron segregation after cad-6b misexpression (2, p ⬎ 0.05; n ⬎ 200 neurons in each class from five embryos). Scale bar equals 60 m. Cell 212 Figure 7. Selectivity of Motor Pool Sorting after MN-cad Expression (A–E) The lateral ITR/ITB pool (labeled I) segregates from eF neurons after MN-cad expression. (A) Analysis of eF-I neuron mixing after MN-cad expression. (B) PEA3⫹ I neurons are found lateral to eF neurons on the control side of the spinal cord. (C) I neurons are in a normal position after MN-cad expression. (D) MN-cad expression in I neurons, detected by Lac-Z expression. (E) Quantitation of eF-I mixing after MN-cad expression (n ⬎ 70 neurons in each class from three embryos; 2 , p ⫽ 0.9; in order to detect a significant difference, ⬎4000 neurons from each class would have to be counted). (F) Analysis of HR-A neuron sorting after MN-cad expression. (G) Normal segregation of A and HR neurons. (H) Mixing of A and HR neurons after MN-cad expression. (I and J) Both electroporated and wild-type HR neurons mix with A neurons after MN-cad expression. (n ⬎ 200 neurons in each class from five embryos; 2, p ⬍ 0.001). Scale bar equals 60 m. level proprioceptive neurons, defined by the sum of Er81 and PEA3 expression (see Lin et al., 1998), 22% expressed cad-8, 21% expressed cad-12, 18% expressed cad-6b, 15% expressed T-cad, and 13% expressed MN-cad (Figure 8). The expression of Er81 and PEA3 is linked in proprioceptive sensory and motor neurons that supply the same muscle (Lin et al., 1998), prompting us to examine whether there might be a similar linkage in cadherin expression. To test this, we injected HRP into identified muscles in the hindlimb of HH stage 35 or 36 chick embryos and examined cadherin mRNA or protein expression in HRP-labeled proprioceptive neurons. This analysis focused on the profile of MN-cad and T-cad expression in DRG neurons that innervated A, F, and S muscles, since the motor neurons that supply these muscles differ in their profile of expression of these two cadherins (Figure 1N). We detected a significant, albeit imperfect, correlation in cadherin expression by proprioceptive sensory and motor neurons that supplied the same muscle target. A motor neurons express both MN-cad and T-cad, and we found that 62% of A DRG neurons expressed MN-cad and 93% expressed T-cad (Figures 8K, 8L, and 8O). eF motor neurons express T-cad but not MN-cad, and we found that 62% of the total population of F DRG neurons expressed T-cad, whereas only 29% expressed MN-cad (Figures 8M–8O). S motor neurons express neither MN-cad nor T-cad, and we found that only 19% of S DRG neurons expressed MN-cad and only 29% expressed T-cad (Figures 8I, 8J, and 8O). A 2 analysis of pair-wise muscle combinations for each cadherin indicates that the sensory expression of cadherins is significantly correlated with motor neuron expression of the same cadherin (p ⬍ 0.01; correlation coefficient R ⫽ 0.91). Discussion The segregation of spinal motor neurons into discrete motor pools typifies the nuclear organization of neuronal cell groups evident throughout the vertebrate CNS. Type II cadherins are differentially expressed by neurons in specific motor pools over the period of motor pool sorting. Perturbation of the expression of one type II cadherin, MN-cad, impairs the normal program of segregation of motor pools that differ selectively in the expression of this gene. We consider the implication of these findings to the contribution of cadherin-mediated recognition to nuclear organization within the developing CNS. A Role for Cadherins in Neuronal Segregation Classic cadherins are a large class of vertebrate cell surface proteins and have been implicated in cell adhe- Cadherins and Motor Neuron Sorting 213 Figure 8. Expression of Classic Cadherins in Proprioceptive Sensory Neurons (A and B) Expression of ER81 and PEA3 in DRG at LS1-LS3 in HH stage 36 embryos. (C–H) Cadherin expression in DRG at LS1-LS3 in HH stage 36 embryos. (I–O) Expression of MN-cad and T-cad in proprioceptive DRG neurons, defined by retrograde transport of HRP from specific muscles. The cadherin expression status of corresponding motor neurons is indicated under each panel. (I) MN-cad is largely excluded from sensory neurons that project to the F muscle. (J) T-cad is expressed in eF sensory neurons. (K and L) MN-cad (K) and T-cad (L) label A proprioceptive sensory neurons. (M and N) MN-cad (M) and T-cad (N) expression is largely excluded from S sensory neurons. (O) Quantitation of proprioceptive sensory neuron expression of MN-cad and T-cad. 2 analysis indicates that sensory neuron expression of MN-cad and T-cad is positively correlated with motor neuron expression. (For each muscle, ⬎100 HRP-labeled neurons were counted in ⬎5 embryos, p ⬍ 0.01; correlation coefficient of regression analysis R ⫽ 0.91.) Scale bar equals 150 m in (A–H) and 200 m in (I–N). sion and recognition in many tissue types (Shapiro and Colman, 1998; Luo et al., 2001). Cadherins exhibit regional patterns of expression in the developing brain, notably in the cerebral cortex, cerebellum, and thalamus (Suzuki et al., 1997). Studies on cad-6b and cad-7 have invoked a role for these type II cadherins in the delamination of neural crest cells from the dorsal neural tube and in the establishment of compartment boundaries in the early forebrain (Inoue et al., 2001; Nakagawa and Takeichi, 1998). In the Drosophila and vertebrate visual systems, the type I cadherin, N-cad, has been implicated in the targeting of retinal axons (Lee et al., 2001; Inoue and Sanes, 1997). In addition, type I cadherins have been localized to synaptic sites in the mature CNS (Uchida et al., 1996). A role for type II cadherins in the segregation of CNS neurons into discrete nuclei is supported by two sets of observations. First, the combinatorial profile of type II cadherin expression delineates neurons within a functional motor pool in the developing spinal cord. Second, for motor pools that differ by expression of a single cadherin, changing their profile of cadherin expression to one in which both pools are predicted to express the same combination markedly impairs their normal program of segregation (see Supplemental Figure S5 at http://www.cell.com/cgi/content/full/109/2/205/DC1). The activity of cadherins revealed by these findings raises three specific issues. First, what is the contribution of cadherin-based recognition to the normal segregation of neurons during motor pool development? Second, how selective are type II cadherin-mediated interactions between developing motor neurons? Third, what steps in motor pool formation are regulated by differential type II cadherin expression? The Contribution of MN-cad to eF and A Pool Mixing The known profile of type II cadherin expression by A and eF motor neurons differs solely in the expression of MN-cad. Expression of MN-cad in eF neurons, or ⌬MN-cad in A neurons, results in an intermixing of the neurons that populate these two motor pools (see Supplemental Figure S5 at http://www.cell.com/cgi/content/ full/109/2/205/DC1). Yet the mixing of eF and A neurons is incomplete after both of these manipulations—a finding that has several possible explanations. First, fewer than half of the motor neurons within a pool ectopically express transgenic cadherins, and thus some electroporated eF neurons may simply not come into proximity with A neurons. Second, the levels of cell surface MN-cad expression achieved after misexpression of MN-cad or ⌬MN-cad may vary between neurons within an individual motor pool, with the consequence that a significant change in the level of functional MN-cad may be achieved in only a fraction of electroporated neurons. Third, the subtype diversity of motor neurons known to exist within an individual pool—␣ and ␥ (Eccles and Sherrington, 1930) and fast and slow (Rafuse et al., 1996)—may limit the actions of ectopic MN-cad to a subset of neurons within a pool. Finally, additional cadherin genes could distinguish eF and A neurons, with the consequence that manipulation of MN-cad expression does not completely equate their profile of cadherin expression. The Selectivity of Cadherin-Mediated Pool Mixing The actions of MN-cad on eF and A mixing are not mimicked by two other classic cadherins, E-cad and cad-6b. Thus, the desegregation of eF and A motor neurons cannot simply be accounted for by a nonspecific elevation of cadherin expression on motor neurons. Cell 214 Moreover, manipulation of MN-cad expression does not promote the intermixing of eF neurons with other motor pools that exhibit additional distinctions in cadherin expression profile, notably the ITR/ITB pools (see Supplemental Figure S5 at http://www.cell.com/cgi/content/ full/109/2/205/DC1). Nevertheless, predictions based on the known profile of cadherin expression by LMC neurons do not readily explain the intermixing of HR and A neurons observed after MN-cad expression (see Supplemental Figure S5). The mixing of HR and A neurons is unlikely to be a secondary consequence of A and eF mixing, since HR and A neuron mixing does not occur after a similar disruption of eF-A neuron segregation by ⌬MN-cad expression. The aberrant mixing of electroporated HR and A neurons could mean that MN-cad expression in HR neurons results in a cell surface cadherin profile that, although distinct from A neurons, still promotes interactions between these two neuronal classes. Since heterophilic interactions have been demonstrated in vitro between classic cadherins (Shimoyama et al., 2000; Shan et al., 2000), MN-cad expressed on HR neurons could interact with other type II cadherins expressed on A neurons, thus promoting their intermixing. However, this scenario fails to explain the mixing of nonelectroporated HR neurons with A neurons. An alternative, and perhaps more likely, possibility is that HR neurons that express MN-cad acquire the ability to mix with both A neurons and nonelectroporated HR neurons such that the resultant dispersal of electroporated HR neurons leads to a secondary mixing of A and nonelectroporated HR neurons. More generally, our findings emphasize the fact that the molecular basis of cadherin-mediated recognition has traditionally been considered under conditions of interaction between single cadherins (Steinberg and Takeichi, 1993). Our results reveal that developing CNS neurons typically express more than one, and in some cases as many as four, classic cadherins. The rules of cadherin-based interaction under these more complex, but perhaps more developmentally relevant, conditions remain to be delineated. The Cellular Basis of Cadherin Action during Motor Pool Formation How does the differential expression of MN-cad influence the segregation of eF and A neurons? The downregulation of MN-cad expression by eF neurons is evident from stage 24 onward. At this stage, eF neurons, as other lateral LMC neurons, are beginning to migrate past A neurons to reach their lateral setting position (Tsuchida et al., 1994; Sockanathan and Jessell, 1998). Thus, the early downregulation of MN-cad expression by eF neurons may ensure that they do not interact with A neurons during the inside-out phase of neuronal migration. In this view, a reduction in MN-cad activity in A neurons or expression of MN-cad by eF neurons would result in an aberrant interaction between these two sets of motor neurons, perturbing their segregation. We note that the degree of intermixing of eF and A neurons achieved by perturbing the cadherin profile of A neurons at early stages (through ⌬MN-cad expression) and of eF neurons at later stages (through MN-cad expression) appear similar. This finding implies that the distinction in profile of cadherin expression that emerges after stage 24 is relevant to the segregation of neurons in these two motor pools. The formation of motor pools depends not only on the early segregation of the lateral and medial subdivisions of the LMC, but also on the subsequent segregation of neurons within each of these subdivisions (Lin et al., 1998). Our studies have focused on the function of MN-cad, a protein that appears to perturb the segregation of motor neurons during the inside-out phase of migration, but the differential expression of other type II cadherins clearly distinguishes each of the motor pools that form within the medial and lateral subdivisions of the LMC. Cadherins are therefore plausible mediators of the secondary phase of motor pool segregation. Correlated Expression of Type II Cadherins by Proprioceptive Sensory and Motor Neurons Several findings suggest that the expression of type II cadherins and ETS genes in motor pools is linked. First, both ETS and cadherin expression in motor pools is dependent of limb-derived signals. Second, ectopic expression of Er81 results in the deregulation of MN-cad expression in the chick spinal cord. Third, the analysis of PEA3 mutant mice indicates that the expression of type II cadherins in specific motor pools within the LMC is regulated by this ETS gene (J. Livet et al., submitted). Nevertheless, the functional link between ETS and cadherin gene expression in motor neurons is not simple. The multiple type II cadherin genes expressed by motor neurons define many more motor pools than are revealed by PEA3 and Er81 (Lin et al., 1998). Secondly, the early and widespread expression of MN-cad, cad-8, and cad-12 by developing LMC neurons indicates that the known ETS genes are not involved in the initial onset of expression of these cadherins. Physiological studies have suggested that motor neurons in the LMC acquire an initial pool identity prior to the segregated expression of ETS or cadherin genes (Milner and Landmesser, 1999). Defining the molecular basis of this early phase of motor pool specification may clarify the link between ETS and cadherin gene expression in developing LMC neurons. The expression of Er81 and PEA3 is linked in interconnected proprioceptive sensory and motor neurons (Lin et al., 1998). The present studies reveal a significant correlation in type II cadherin expression in proprioceptive sensory and motor neurons that project to the same target muscle. A matching in cadherin expression on the axons of proprioceptive sensory neurons and on the dendrites of motor neurons could provide a basis for the selectivity with which monosynaptic connections are formed in this neuronal circuit (Frank et al., 1988). Motor Pool Formation and the Nuclear Organization of CNS Neurons The organization of neuronal subtypes in the CNS is based in large part on the segregation of neurons into discrete nuclei. Since the formation of motor pools captures many of the essential features of neuronal nuclear organization, the role of cadherin function in motor pool segregation demonstrated in this study suggests a more widespread role for cadherins in the organization of neuronal nuclei in the developing CNS. The patterns of Cadherins and Motor Neuron Sorting 215 cadherin expression in many areas of the developing brain (Inoue et al., 2001; Suzuki et al., 1997) are consistent with an involvement in neuronal clustering. The functional rationale for organizing motor neurons into specific pools remains enigmatic, however. Motor pools have been correlated with electrical coupling, a feature that has been suggested to coordinate the phasic firing of functionally related motor neurons (Chang and Balice-Gordon, 2000). Physiological studies have also suggested distinctions in local interneuronal connections with motor pools (Ritter et al., 1999), and such specificity in local circuitry could depend on the clustered organization of neurons into motor pools. Motor pool clustering might also be involved in late steps in the establishment of motor axon projections (J. Livet et al., submitted). The role of cadherins in motor pool sorting revealed in these studies should permit a dissociation between motor neuron position and identity. In turn, defining how the organization of motor neurons into discrete nuclei influences the function of motor circuits may provide insights pertinent to neuronal organization in other regions of the CNS. Health grant HD25938. T.M.J. is supported by a grant from the Harold and Leila Mathers Foundation and is a Howard Hughes Medical Institute Investigator. Experimental Procedures Eberhart, J., Swartz, M., Koblar, S.A., Pasquale, E.B., Tanaka, H., and Krull, C.E. (2000). Expression of EphA4, ephrin-A2 and ephrinA5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev. Neurosci. 22, 237–250. Chick Embryo Preparation Chick eggs (Spafas, Truslow Farms) were incubated and staged as in Hamburger and Hamilton (1951). Cloning of Cadherin Genes Details of the cloning of chicken cadherin genes are available as Supplemental Data at http://www.cell.com/cgi/content/full/109/2/ 205/DC1. In Situ Hybridization Histochemistry Digoxigenin (DIG)-labeled cRNA probes were used for in situ hybridization histochemistry as in Schaeren-Wiemers and Gerfin-Moser (1993). Dual color fluorescence in situ hybridization histochemistry was performed as described in the Supplemental Data. Immunohistochemistry Antibodies used in this study are included in the Supplemental Data at http://www.cell.com/cgi/content/full/109/2/205/DC1. Retrograde Labeling of DRG Neurons HRP labeling of DRG neurons was performed essentially as described in Lin et al. (1998). Generation of Cadherin Expression Constructs Details of cloning and expression of cadherin cDNAs are provided as Supplemental Data. In Ovo Electroporation Expression of cDNAs was achieved by in ovo electroporation using an ECM830 electro-squareporator (BTX Inc.). Pulses were of 50 ms duration, 5 times at 30V. Embryos were electroporated at HH stages 15–18 and analyzed at HH stages 28–30. Modeling Neuronal Mixing Details of quantitation of neural mixing are provided in the Supplemental Data at http://www.cell.com/cgi/content/full/109/2/205/DC1. Acknowledgments We are grateful to M. Takeichi for providing reagents. We thank S. Arber, R. Axel, K. Baldwin, J. de Nooij, C. Henderson, J. Kaltschmidt, P. Scheiffele, and L. Shapiro for critical comments on the manuscript and K. MacArthur and I. Schieren for help in preparing the text and figures. S.R.P. is indebted to the Helen Hay Whitney Foundation, of which he is a fellow. B.R. is supported by National Institutes of Received: December 14, 2001 Revised: February 20, 2002 References Arber, S., Ladle, D.R., Lin, J.H., Frank, E., and Jessell, T.M. (2000). ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101, 485–498. Arndt, K., Nakagawa, S., Takeichi, M., and Redies, C. (1998). Cadherins define segments and parasagital cell ribbons in the developing chick cerebellum. Mol. Cell. Neurosci. 10, 211–228. Brenowitz, G.L., Collins, W.F.D., and Erulkar, S.D. (1983). Dye and electrical coupling between frog motor neurons. Brain Res. 274, 371–375. Chang, Q., and Balice-Gordon, R.J. (2000). Gap junctional communication among developing and injured motor neurons. Brain Res. Brain Res. Rev. 32, 242–249. Davis, B.M., Frank, E., Johnson, F.A., and Scott, S.A. (1989). Development of central projections of lumbosacral sensory neurons in the chick. J. Comp. Neurol. 279, 556–566. Eccles, J.C., and Sherrington, C. (1930). Numbers and contraction values of individual motor-units examined in some muscles of the limb. Proc. R. Soc. Lond. B Biol. Sci. 106, 326–357. Elliott, H.C. (1942). Studies on the motor cells of the spinal cord. I. Distribution in the normal human cord. Am. J. Anat. 70, 95–117. Feng, G., Laskowski, M.B., Feldheim, D.A., Wang, H., Lewis, R., Frisen, J., Flanagan, J.G., and Sanes, J.R. (2000). Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 25, 295–306. Frank, E., Smith, C., and Mendelson, B. (1988). Strategies for selective synapse formation between muscle sensory and motor neurons in the spinal cord. In From Message to Mind, S.S. Easter, K.F. Barald, and B.M. Carlson, eds. (Sunderland, MA: Sinauer Associates Inc.), pp. 180–202. Fredette, B.J., and Ranscht, B. (1994). T-cadherin expression delineates specific regions of the developing motor axon-hindlimb projection pathway. J. Neurosci. 14, 7331–7346. Fujimori, T., and Takeichi, M. (1993). Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated non-functional N-cadherin. Mol. Biol. Cell 4, 37–47. Hamburger, V., and Hamilton, H. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 160, 535–546. Hollyday, M. (1980). Organization of motor pools in the chick lumbar lateral motor column. J. Comp. Neurol. 194, 143–170. Hollyday, M., and Hamburger, V. (1977). An autoradiographic study of the formation of the lateral motor column in the chick embryo. Brain Res. 132, 197–208. Inoue, A., and Sanes, J.R. (1997). Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science 276, 1428–1431. Inoue, T., Tanaka, T., Takeichi, M., Chisaka, O., Nakamura, S., and Osumi, N. (2001). Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 128, 561–569. Iwamasa, H., Ohta, K., Yamada, T., Ushijima, K., Terasaki, H., and Tanaka, H. (1999). Expression of Eph receptor tyrosine kinases and their ligands in chick embryonic motor neurons and hindlimb muscles. Dev. Growth Differ. 41, 685–698. Kintner, C. (1992). Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell 69, 225–236. Cell 216 Landmesser, L. (1978a). The distribution of motoneurones supplying chick hind limb muscles. J. Physiol. 284, 371–389. man, D.R., and Shapiro, L. (2000). Functional cis-heterodimers of N- and R-cadherins. J. Cell Biol. 148, 579–590. Landmesser, L. (1978b). The development of motor projection patterns in the chick hind limb. J. Physiol. 284, 391–414. Shapiro, L., and Colman, D.R. (1998). Structural biology of cadherins in the nervous system. Curr. Opin. Neurobiol. 8, 593–599. Landmesser, L.T. (2001). The acquisition of motoneuron subtype identity and motor circuit formation. Int. J. Dev. Neurosci. 19, 175–182. Shimoyama, Y., Tsujimoto, G., Kitajima, M., and Natori, M. (2000). Identification of three human Type-II classic cadherins and frequent heterophilic interactions between different subclasses of Type-II classic cadherins. Biochem. J. 349, 159–167. Lee, M.T., Koebbe, M.J., and O’Donovan, M.J. (1988). The development of sensorimotor synaptic connections in the lumbosacral spinal cord of the chick embryo. J. Neurosci. 8, 2530–2543. Lee, C.H., Herman, T., Clandinin, T.R., Lee, R., and Zipursky, S.L. (2001). N-cadherin regulates target specificity in the Drosophila visual system. Neuron 30, 437–450. Levine, E., Lee, C.H., Kintner, C., and Gumbiner, B.M. (1994). Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development 120, 901–909. Lin, J.H., Saito, T., Anderson, D.J., Lance-Jones, C., Jessell, T.M., and Arber, S. (1998). Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell 95, 383–407. Luo, Y., Ferreira-Cornwell, M.C., Baldwin, H.S., Kostetski, I., Lenox, J.M., Lieberman, M., and Radice, G.L. (2001). Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development 128, 459–469. Milner, L.D., and Landmesser, L.T. (1999). Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J. Neurosci. 19, 3007–3022. Nakagawa, S., and Takeichi, M. (1998). Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125, 2963–2971. Nollet, F., Kools, P., and van Roy, F. (2000). Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 299, 551–572. Ozawa, M., and Kemler, R. (1998). The membrane proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J. Cell Biol. 142, 1605–1613. Rafuse, V.F., Milner, L.D., and Landmesser, L.T. (1996). Selective innervation of fast and slow muscle regions during early chick neuromuscular development. J. Neurosci. 16, 6864–6877. Rakic, P. (1972). Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–84. Ramon y Cajal, S. (1894). La fine structure des centres nerveux. Proc. R. Soc. Lond 55, 444–468. Ramon y Cajal, S. (1911). Histologie du system nerveux de l’home et des vertebras, Volumes 1 and 2. (Paris: A Maloine). Reprinted by Consejo Superior de Investigaciones Cientificas, Inst. Ramon y Cajal, Madrid, 1955. Rexed, B. (1952). The cytoarchitectonic organization of the spinal cord in the cat. J. Comp. Neurol. 96, 415–495. Ritter, A., Wenner, P., Ho, S., Whelan, P.J., and O’Donovan, M.J. (1999). Activity patterns and synaptic organisation of ventrally located interneurons in the embryonic chick spinal cord. J. Neurosci. 19, 3457–3471. Romanes, G.J. (1942). The development and significance of the cell columns in the ventral horn of the cervical and upper thoracic spinal cord of the rabbit. J. Anat. Lond. 76, 112–130. Romanes, G.J. (1964). The motor pools of the spinal cord. In Organization of the Spinal Cord, Volume 11, J.C. Eccles, and J.P. Schade, eds. (Amsterdam: Elsevier Publishing), pp. 93–119. Ross, M.E., and Walsh, C.A. (2001). Human brain malformations and their lessons for neuronal migration. Annu. Rev. Neurosci. 24, 1041–1070. Schaeren-Wiemers, N., and Gerfin-Moser, A. (1993). A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridisation using digoxygenin labelled cRNA probes. Histochemistry 100, 431–440. Shan, W.S., Tanaka, H., Phillips, G.R., Arndt, K., Yoshida, M., Col- Sockanathan, S., and Jessell, T.M. (1998). Motor neuron-derived retinoid signals determine the number and subtype identity of motor neurons in the developing spinal cord. Cell 94, 503–514. Steinberg, M.S., and Takeichi, M. (1993). Experimental specification of cell sorting, tissue spreading and specific patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA 91, 206–209. Suzuki, S.C., Inoue, T., Kimura, Y., Tanaka, T., and Takeichi, M. (1997). Neuronal circuits are subdivided by differential expression of Type-II classic cadherins in postnatal mouse brains. Mol. Cell. Neurosci. 9, 433–447. Tsuchida, T., Ensini, M., Morton, S.B., Baldassare, M., Edlund, T., Jessell, T.M., and Pfaff, S.L. (1994). Topographic organisation of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79, 957–970. Uchida, N., Honjo, Y., Johnson, K.R., Wheelock, M.J., and Takeichi, M. (1996). The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 135, 767–779. Varela-Echavarria, A., Tucker, A., Puschel, A.W., and Guthrie, S. (1997). Motor axon subpopulations respond differentially to the chemorepellents netrin-1 and semaphorin D. Neuron 18, 193–207. Yoon, M.S., Puelles, L., and Redies, C. (2000). Formation of cadherinexpressing brain nuclei in diencephalic alar plate divisions. J. Comp. Neurol. 427, 461–480. Accession Numbers The sequence of MN-cad has been submitted to GenBank with the accession number AF459439.