* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cellular mechanisms underlying network synchrony in the medial
Long-term depression wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Limbic system wikipedia , lookup
Memory consolidation wikipedia , lookup
Neurotransmitter wikipedia , lookup
Development of the nervous system wikipedia , lookup
Theta model wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroplasticity wikipedia , lookup
Apical dendrite wikipedia , lookup
Biological neuron model wikipedia , lookup
Synaptogenesis wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Recurrent neural network wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural coding wikipedia , lookup
Chemical synapse wikipedia , lookup
Hippocampus wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Central pattern generator wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Nervous system network models wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptic gating wikipedia , lookup
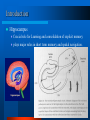
Ch.2 Cellular mechanisms underlying network synchrony in the medial temporal lobe Information Processing by Neuronal Populations Edward O. Mann and Ole Paulsen 2008-12-17 Heo, Min-Oh One-slide Summary How does brain (especially Hippocampus) make oscillation signals as constant frequency clock? Element level - from intrinsic cellular properties: Frequency preference Self-sustained oscillation in a single neuron Structural level Recurrent feedback loop Interaction between local network and global rhythm So they can make theta-frequency, gamma-frequency, delta-frequency and sharp wave – ripple complexes in Hippocampus using mechanisms described above. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 2 Outline 1. Introduction 2. Basic cellular mechanisms contributing to cortical network oscillations Intrinsic cellular properties : self-sustained oscillation Electrical synaptic coupling : Gap junction coupling Chemical synaptic coupling : combination of excitation and inhibition 3. Specific mechanisms underlying entorhinal and hippocampal network oscillations Slow oscillations Gamma-frequency oscillations Sharp wave-ripple complexes Theta-frequency oscillations 4. Functional implications 5. Conclusion © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 3 Rat hippocampal EEG and CA1 neural activity - the theta (awake/behaving) - LIA (slow-wave sleep) 4 Introduction Hippocampus Crucial role for Learning and consolidation of explicit memory plays major roles in short term memory and spatial navigation. 5 The entorhinal cortex The entorhinal cortex (EC) forms the main input to the hippocampus and is responsible for the pre-processing (familiarity) of the input signals. On Medial surface, EC approximately maps to areas 28 and 34, at lower left. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 6 Introduction STDP (Spike Timing-Dependent Plasticity) Increasing or decreasing in the efficacy of synaptic transmission (known as synaptic plasticity) The timing sensitivities are on the order of milliseconds. pre-post spiking: long-term potentiation (LTP) post-pre spiking: long-term depression (LTD) pre-post spiking by >40 ms may also lead to LTD 7 Brain Oscillation STDP fails ? The inherent spike jitter affects converging inputs in polysynaptic pathways The behaviorally relevant temporal associations Oscillation in the cortical network Providing a mechanism to organize spike times Not yet resolved whether network oscillation serve temporal association of behavior. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 8 Basic cellular mechanisms contributing to cortical network oscillations Hippocampal subfields follow a stereotypic organizational principle Excitatory cells: ~80% Inhibitory cells: ~20% Information Storing Mainly on the synaptic connections between excitatory neurons Cortical interneurons control the precision of spike timing within cortical network oscillations. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 9 Basic cellular mechanisms : 1. Intrinsic cellular properties A Neuron’s frequency preference Iin: injecting sinusoidal input current (linearly increasing freq. 0 to 100Hz) Vm: membrane potential Ih: hyperpolarization-activated non-specific cation current INaP: persistent sodium current The neuron act as a band-pass filter. Sub- and suprathreshold oscillations There may be a range of Vm in which the neuron displays self-sustained oscillation Rhythmic bursting The inactivation and activation processes of the low-threshold Ca2+ current can mediate amplified resonance. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 10 Basic cellular mechanisms : 2. Electrical synaptic coupling Ephaptic interactions Resulting from current flow in the extracellular space Gap junction coupling Stronger and more reliable coupling than ephaptic interactions Bidirectional electrical connections preferentially Tend to act as low-pass filters Occur almost exclusively between interneurons belonging to the same subtype. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 11 Basic cellular mechanisms : 3. Chemical synaptic coupling Neuronal communication via neurotransmitter release Enables coupling over more distributed areas More diverse and dynamic Coupling through excitatory synapses Can act to synchronize intrinsic oscillators Enable the emergence of rhythmic bursting Positive feedback requires a mechanism to reduce activity Not easy to explain the millisecond precision of spike timing Synaptic inhibition Many interneurons coupled by mutual inhibition are capable of selfsynchronizing their output. Negative feedback loop can generate fast oscillations Temporal control on time scales relevant for STDP Mediated through Inhibitory GABAergic transmission © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 12 Specific mechanisms underlying entorhinal and hippocampal network oscillations Some stereotypical patterns of activity have provided the basis for understanding some of the cellular mechanisms underlying network synchrony. Theta-frequency oscillations: Pacemaker Higher-frequency oscillations: Recurrent feedback loops, Interneuronal network © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 13 Specific mechanisms underlying entorhinal and hippocampal network oscillations : 1. Slow oscillations States UP states: periods of sustained activity Down states: relative quiescence Delta-frequency range ( < 4Hz ) During slow-wave sleep (non-dreaming sleep) In the neocortex Rhythmic bistable oscillation Intrinsically generated through recurrent synaptic excitation Hippocampal pyramidal neurons Not display rhythmic bistability Influenced by propagated oscillations in the superficial layers of the entorhinal cortex © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 14 Specific mechanisms underlying entorhinal and hippocampal network oscillations : 2. Gamma-frequency oscillations (30-80Hz) In many sleeping and awake states Driven by two separate gamma generators in the superficial layers of the entorhinal cortex and hippocampal CA3 respectively. Depends on synaptic feedback loops between pyramidal neurons and perisomatic-targeting interneurons with the oscillation propagated via feedforward inhibition. Irrespective of the precise mechanism of generation, there fast network oscillations could control principal cell spike timing with a precision appropriate for STDP. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 15 Specific mechanisms underlying entorhinal and hippocampal network oscillations : 3. Sharp wave-ripple complexes Sharp wave Randomly-timed large deflections of the EEG signal lasting for 200-300 msec During slow-wave sleep and awake immobility Generated by recurrent excitation in the CA3 Ripple oscillations (150-250Hz) ride on sharp waves in CA1 The frequency of ripple oscillations in vivo is sensitive to benzodiazepines, which modulate the kinetics of GABAA– receptor-mediated inhibition. – On GABA-receptor-blocking situation, another cases are shown… Ripple-frequency oscillations are associated with the rapid replay of spike sequences previously observed during exploratory behavior and could therefore enable information stored in the hippocampus to be transferred to the neocortex. 16 Specific mechanisms underlying entorhinal and hippocampal network oscillations : 4. Theta-frequency oscillations (4-12 Hz) The medium septum Lesions of the medial septum - the central node of the theta system - cause severe disruptions of memory. projects to all of the regions that show theta rhythmicity, and destruction of it eliminates theta throughout the brain. During exploratory behavior and REM sleep The interaction between a local network oscillator and the global theta activity offer the opportunity to control the local spike timing relative to that of the external afferents at the millisecond timescale. The spiking of principal neurons throughout hippocampus is phase-coupled to the global theta rhythm. While global theta-frequency oscillations in vivo depend on subcortical structures, individual cortical neurons, as well as the local networks, appear tuned to participate in the theta-frequency rhythm. Subthreshold resonance in the theta-frequency range is observed in many neuronal types. Phase precession: cells fire selectively in discrete regions of the animal’s environment © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 17 Functional implications STDP depends on postsynaptic action potentials STDP mechanisms would be activated maximally during the replay of spike sequences during sharp wave-ripple complexes. Encoding interference problem Spike time variability represented phase precession or spike sequences within gamma cycles. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 18 Functional implications The reported phenomenon of phase precession cells with overlapping place fields along an animal’s trajectory could fire at progressively earlier phases of the theta oscillation. © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 19 Conclusion Network oscillations observed during different behaviors clearly reflect which neuronal populations are active, and how they communicate with each other. It remains unclear whether this rhythmic coordination of spiking activity has an independent functional role. The intrinsic and synaptic properties of neurons seem tuned to embrace network rhythmicity © 2008, SNU Biointelligence Lab, http://bi.snu.ac.kr/ 20