* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF of this article
Neurophilosophy wikipedia , lookup
Perivascular space wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neurotransmitter wikipedia , lookup
Haemodynamic response wikipedia , lookup
Environmental enrichment wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neural oscillation wikipedia , lookup
Neuroanatomy wikipedia , lookup
Central pattern generator wikipedia , lookup
Neuroplasticity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroeconomics wikipedia , lookup
Nervous system network models wikipedia , lookup
Development of the nervous system wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Metastability in the brain wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Aging brain wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Optogenetics wikipedia , lookup
Hypothalamus wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
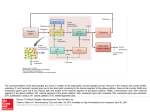
THE ROLE OF THE SUBTHALAMIC NUCLEUS IN THE PATHOPHYSIOLOGY OF PARKINSON’S DISEASE Fabio Blandini Laboratory of Functional Neurochemistry, IRCCS C. Mondino Institute of Neurology, Pavia, Italy Reprint requests to: Dr Fabio Blandini, Laboratory of Functional Neurochemistry, C.Mondino Institute of Neurology, Via Palestro, 3 - 27100 Pavia, Italy. E-mail: [email protected] Many environmental and occupational chemicals are known to affect the central and/or peripheral nervous system, causing changes that may result in neurological and psychiatric disorders. Because of the limited accessibility of the mammalian nervous tissue, new strategies are being developed to identify biochemical parameters of neuronal cell function, which can be measured in easily obtained tissues, such as blood cells, as potential markers of the chemically-induced alterations occurring in the nervous system. This review includes a comparative analysis of the effects of mercurials on calcium signalling in the neuroadrenergic PC12 cells and rat splenic T lymphocytes in an attempt to characterize this second messenger system as KEY WORDS: Basal ganglia, dopamine, glutamate, movement disorders, neuroprotection, substantia nigra. FUNCT NEUROL 2001;16 (SUPPL.): 99-106 INTRODUCTION Parkinson’s disease (PD) is a progressive, neurodegenerative disorder, in which the capacity to execute voluntary movements is gradually lost. The clinical picture of PD includes tremor, rigidity and bradykinesia. The pathologic hallmark of the disease is the degeneration of melanine-containing, dopaminerg i c neurons of the substantia nigra pars compacta, which leads to severe dopaminergic denervation of the corpus striatum. The dopaminergic deficit of the nigrostriatal system is accompanied by functional modifications of the other basal ganglia nuclei, which constitute the neural substrate for the expression of PD motor symptoms. In the context of this alteration of the basal ganglia circuitry activity, the subthal- amic nucleus – the only excitatory nucleus of the circuit – plays a prominent role. THE BASAL GANGLIA CIRCUITRY Proper execution of voluntary movements results from the correct processing of sensorimotor information by a complex neural network, which includes the cerebral cortex, the motor thalamus and the basal ganglia nuclei. The basal ganglia circuit, which is functionally interposed between the cortex and the thalamus, plays the major role in this process. The basal ganglia nuclei process the inputs flowing from the cortex to produce an output signal that returns – via the motor thalamus – to the cortex, to modulate movement execution (1). FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 99 F. Blandini The basal ganglia circuit consists of four nuclei: corpus striatum (or striatum), globus pal lidus, substantia nigra and subthalamic nucleus (Fig. 1). The basal ganglia nuclei are interconnected, and their functional organization has been extensively investigated over the past decade. According to a very popular model (2,3), the striatum – the main input nucleus of the circuit – transmits the flow of information received from the cortex to the basal ganglia o u t p u t nuclei, substantia nigra pars reticulata and medial globus pallidus, via a direct and an indirect pathway originating from different subsets of striatal neurons. In the direct pathway, striatal GABAergic neurons containing dynorphin as a co-trans- mitter and expressing D1 dopamine receptors, project monosynaptically to the substantia nigra pars reticulata and medial globus pallidus. In the indirect pathway, a subset of GABAergic neurons containing enkephaline and expressing D2 receptors project to the lateral globus pallidus, which sends GABAergic projections to the STN. In turn, the STN sends its glutamatergic (excitatory) efferents to the output nuclei and to the lateral globus pallidus. From the output nuclei, inhibitory, GABAergic projections reach the ventral lateral and ventral anterior nuclei of the motor thalamus. Thalamic nuclei then send glutamatergic projections to the motor cortex, thus closing the loop (Fig. 1). Output Nuclei Fig. 1 - Simplified representation of the functional organization of the basal ganglia nuclei. SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulata; STN: subthalamic nucleus; LGP: lateral globus pallidus; MGP: medial globus pallidus. 100 FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 Subthalamic nucleus and Parkinson’s disease From the model, it follows that the subthalamic nucleus occupies a strategic position in the circuitry. As repeatedly demonstrated by experimental studies, the subthalamic nucleus can modulate the neuronal activity of both basal ganglia output nuclei (4-6). In addition, recent studies have shown that the subthalamic nucleus receives direct excitatory projections from the primary motor cortex (7) and, more importantly, that the nucleus receives dopaminergic projections from the substantia nigra pars compacta (8-10). The description of this additional connectivity has further increased the importance of the subthalamic nucleus in the functional architecture of the basal ganglia, suggesting a more independent role for this nucleus with respect to the other components of the circuitry. Functional changes associated with Parkinson’s disease: role of the subthalamic nucleus As mentioned above, the degeneration of nigrostrial neurons that underlies PD causes a cascade of changes, leading to a functional rearrangement of the basal ganglia circuitry. The ultimate consequence of this phenomenon is the increased activity of the subthalamic nucleus and/or the basal ganglia output nuclei, which receive subthalamic projections. Enhanced activity of the output nuclei results in increased inhibitory control over the motor thalamus and subsequent reduction of the thalamic, glutam a t e rgic output to the motor cortex. These changes are likely to constitute the neural substrate for parkinsonian motor symptoms (1). Numerous experimental studies have shown that the subthalamic nucleus is central to the PDrelated enhancement of the basal ganglia output. In animals, it is possible to replicate the anatomical damage of PD using pharmacological agents. The two most popular techniques involve the intracerebral injection of 6-hydroxydopamine (6OHDA), in rodents (11), and the systemic (intracarotid) administration of 1-methyl-4-phenyl1,2,3,6-tetrahydropiridine (MPTP) to monkeys (12). Both toxins produce, via different mechanisms, selective lesions of the nigrostriatal pathway. Using these animal models of PD, various groups have found increased levels of neuronal firing rate (13,14), glucose metabolism (15), and mitochondrial enzyme activity (16,17) in the subthalamic nucleus and in its projection nuclei. In rodents, the metabolic and electrophysiological changes that affect the output nuclei, as a consequence of the nigrostriatal lesion, are prevented by lesioning the subthalamic nucleus (18,19). Human studies also support the primary role of subthalamic hyperactivity in PD pathophysiology. For example, it has been reported that the occurrence of a subthalamic hematoma caused the disappearance of parkinsonian symptoms in a PD patient (20). Furthermore, Lange et al. (21) have reported down-regulation of NMDA receptors in the medial globus pallidus of PD patients, which has been interpreted as a consequence of the increased activity of subthalamic projections to the medial globus pallidus. All these findings have led to the introduction of a new, electrophysiological technique in the therapy of PD, known as “deep brain stimulation” (22). This technique relieves PD motor symptoms by stimulating subthalamic neurons at high frequencies through locally implanted electrodes. The procedure induces the functional blockade (depolarization) of the nucleus and, as shown recently, consistent changes in residual dopaminergic transmission at nigrostriatal level (23,24). Hypotheses on PD-related subthalamic overactivity: the role of dopaminergic mechanisms According to the classical model of basal ganglia organization, subthalamic hyperactivity results from reduction of the inhibitory control exerted by the lateral globus pallidus, possibly inhibited by overactive striato-pallidal, GABAergic, neurons (2). This is one of the controversial points of the model. In fact, instead of showing a reduction, recent studies have found increased pallidal activity in both rodent (16,25) and pri- FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 101 F. Blandini mate models of PD (17). In addition, Hassani et al. (26) have demonstrated that complete lesioning of the globus pallidus causes a slight increase in the firing rate of subthalamic neurons, which is far less pronounced than the increase observed in animals with nigrostriatal damage. These data suggest that an additional, if not alternative, explanation for the PD-related subthalamic hyperactivity should be considered. As mentioned above, emerging evidence shows that dopamine plays an important role at subthalamic level. In addition to receiving dopaminergic projections from the substantia nigra pars compacta (8-10), subthalamic neurons have dopamine receptors that mediate their response to a variety of dopaminergic drugs (14,2731). Consequently, it is likely that subthalamic dopaminergic mechanisms are directly involved in the basal ganglia functional changes associated with PD. Indeed, the density of subthalamic, dopamine D2 receptors increases in rats bearing a nigrostriatal lesion (28,32), while marked loss of dopaminergic innervation has been reported in MPTP-treated monkeys (33). As a consequence, neuronal and motor responses to intra-subthalamic administration of dopaminergic agonists change dramatically after a nigrostriatal lesion (14,30,34). Moreover, Mukhida et al. have recently shown that the use of intra-subthalamic, dopaminergic transplants enhances the sensorimotor behavioral recovery in hemiparkinsonian rats (35). Taken together, these data indicate that dopamine plays a central role in the regulation of subthalamic activity and that degeneration of the dopamine neurons of the substantia nigra affects subthalamic activity directly, inducing a dopaminergic “denervation” of the nucleus. SUBTHALAMIC OVERACTIVTY AND NIGRAL DEGENERATION: IS THERE A LINK? Although the substantia nigra pars compacta is not considered a typical target of subthalamic projections, there is solid evidence that subthala- 102 FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 mic neurons send glutamatergic fibers to nigral dopaminergic neurons, too (36). Through this excitatory pathway, the subthalamic nucleus is able to affect the activity of nigral neurons (37). Therefore, the subthalamic hyperactivity occurring in PD also has repercussions on residual neurons of the substantia nigra pars compacta. In addition to being a neurotransmitter, glutamate can also be a toxin. Excessive stimulation of the glutamate receptor, particularly the N-methylD-aspartate subtype, causes cell death, which can be of the necrotic (38) or apoptotic (39) type, or both (40). Glutamate toxicity has been evoked in the pathogenesis of PD, although via an indirect mechanism. In fact, although glutamate is likely to play a direct role in acute neurological disorders, such as hypoxic/ischemic brain damage – which is accompanied by massive release of glutamate (41) – this is unlikely to happen in a chronic, slowly evolving disorder, such as PD. However, glutamate can become neurotoxic – even at physiological concentrations – in the presence of a state of cellular energetic impairment, through a mechanism known as indirect excitotoxicity (42,43). This mechanism may very well occur in the nigral neurons of PD patients, which are exceedingly vulnerable to toxic insults due to a number of specific conditions, the most important being mitochondrial enzyme complex I deficiency (44). The increase in glutamatergic inputs to the subtantia nigra pars compacta, originating from the subthalamic nucleus, may therefore interact with the bioenergetic defects, thus triggering or – more likely – aggravating the progression of the degenerative process (45). A vicious circle may ensue, with the nigrostriatal damage causing subthalamic hyperactivity that, in turn, could sustain nigrostriatal degeneration. Indeed, in rats, a subthalamic nucleus lesion protects neurons of the substantia nigra pars compacta against the toxicity of 6-OHDA (46) and prevents transneuronal degeneration of the substantia nigra pars reticulata (47). More recently, Nakao et al. (48) have shown that previous ablation of the subthalamic nucleus attenuates the loss of nigral dopaminergic neurons Subthalamic nucleus and Parkinson’s disease by intrastriatal administration of neurotoxin 3-nitropropionic acid. Thus, in addition to the role it plays in the development of PD motor symptoms, the subthalamic nucleus may also be directly involved in the mechanisms underlying the progression of the neurodegenerative process. PHARMACOLOGICAL BLOCKADE OF SUBTHALAMIC OVERACTIVITY: A NEUROPROTECTIVE TOOL? From what has been discussed so far, it follows that, in addition to the symptomatic effects (amelioration of PD motor symptoms), the blocking of PD-related subthalamic hyperactivity might also have a neuroprotective effect. Reduction of enhanced glutamatergic transmission has been recently suggested as an alternative approach to the treatment of PD (49,50). Indeed, the use of glutamate antagonists – as both symp- tomatic and neuroprotective agents – has proved beneficial in numerous experimental studies (49,50). However, the general limitation of the use of these drugs is that glutamate receptors are widespread in the brain. Thus, if a glutamate antagonist is given systemically, it will affect glutamatergic transmission outside the basal ganglia circuitry as well (even if the drug targets a specific glutamate receptor subtype), which is not desirable. For these reasons, we have recently studied the effects of selective blockade of glutamatergic transmission, at subthalamic level, on the development of a nigrostriatal lesion and the associated functional changes in the basal ganglia nuclei (25). Using permanent cannulas connected to subcutaneous pumps, we infused – directly into the subthalamic nucleus – two glutamate antagonists acting on different receptor subtypes, MK-801 (NMDA antagonist) and NBQX (AMPA antagonist), in rats with an evolving nigrostriatal lesion induced by 6OHDA (Fig. 2). Subthalamic infusion of MK- Fig. 2 - Drawing illustrating the experimental design followed in the study (ref. 25). Six-hydroxydopamine (2.5 µg/µl) was injected into the striatum, so as to induce a progressive, retrograde lesion in the substantia nigra pars compacta (SNc), which is connected to the striatum by the medial forebrain bundle (mfb). At the same time, a permanent cannula connected to a sub-cutaneous, osmotic mini-pump, was lowered into the subthalamic nucleus (STN). Pumps were loaded with MK-801 (NMDA antagonist), or NBQX (AMPA antagonist), or saline and delivered the solutions – continuously – for four weeks. After three weeks, animals were tested with systemic amphetamine (3 mg/kg, i.p.) for the presence of rotational behavior. At the fourth week, animals were sacrificed and brains were analyzed to determine cytochrome oxidase activity (functional evaluation) and Nissl staining (anatomical evaluation). FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 103 F. Blandini 801, but not of NBQX, prevented the increase in metabolic activity of basal ganglia output nuclei and reduced the amphetamine-induced rotational behavior associated with nigrostriatal lesions. In addition, animals treated with MK-801 showed a marked reduction of the nigral cell loss caused by the toxin. 16. 17. CONCLUDING REMARKS The subthalamic nucleus plays a central role in the functional changes affecting the basal ganglia circuitry in PD. The pathological hyperactivity of subthalamic neurons, which develops as a consequence of the nigrostriatal degeneration, constitutes the neural substrate for PD motor symptoms. Subthalamic hyperactivity is also likely to contribute to the progression of the degenerative process. Therefore, manipulation of the subthalamic activity – possibly through selective pharmacological agents – may constitute a valuable tool to achieve both symptomatic improvement and neuroprotection in PD patients. REFERENCES 11. Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol 2000;62:63-88 12. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989;12:366-375 13. Alexander GE, Crutcher ME. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13:266-271 14. Tzagournissakis M, Dermon CR, Savaki HE. Functional metabolic mapping of the rat brain during unilateral electrical stimulation of the subthalamic nucleus. J Cereb Blood Flow Metab 1994;14:132-144 15. Blandini F, Greenamyre JT. Effect of sub- 104 FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 18. 19. 10. 11. 12. 13. 14. 15. thalamic nucleus lesion on mitochondrial enzyme activity in rat basal ganglia. Brain Res 1995;669:59-66 Blandini F, Porter RHP, Greenamyre JT. Autoradiographic study of mitochondrial complex I and glutamate receptors in the basal ganglia of rats after unilateral subthalamic lesion. Neurosci Lett 186:99-102 Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 1998;86:353-387 Cossette M., Levesque M, Parent A. Extrastriatal dopaminergic innervation of human basal ganglia. Neurosci Res 1999;34:51-54 Hassani OK, Feger J, Yelnik J, Francois C. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Res 1997;749:88-94 Hedreen JC. Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. J Comp Neurol 1999;409:400-410 Ungerstedt U. Six-hydroxydopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 1968;5:107-110 DeLong MR. Primate models of movement disorders of basal ganglia origin Trends Neurosci 1990;13:281-285 Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 1994;72:507520 Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson’s disease. J Neurosci 1997;17:6807-6819 Mitchell IJ, Clarke CE, Boyce S et al. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4 phenyl 1,2,3,6-tetrahydropyridine. Neuroscience 1989;32:213-226 Subthalamic nucleus and Parkinson’s disease 16. Porter RHP, Greene JG, Higgins DS, Greenamyre JT. Polysynaptic regulation of glutamate receptors and mitochondrial enzyme activities in the basal ganglia of rats with unilateral dopamine depletion. J Neurosci 1994;14:7192-7199 17. Vila M, Levy R, Herrero MT et al. Metabolic activity of the basal ganglia in parkinsonian syndromes in human and non-human primates: a cytochrome oxidase histochemistry study. Neuroscience 1996;71:903-912 18. Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Exp Brain Res 1995;105:48-58 19. Blandini F, Garcia-Osuna M, Greenamyre JT. Subthalamic ablation reverses changes in basal ganglia oxidative metabolism and motor response to apomorphine induced by nigrostriatal lesion in rats. Eur J Neurosci 1997;9:1407-1413 20. Sellal F, Hirsh E, Lisovoski F, Mutshler V, Collard M, Marescaux C. Contralateral disappearance of parkinsonian signs after subthalamotomic hematoma. Neurology 1992; 42:255-256 21. Lange KW, Kornhuber J and Riederer P. Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev 1997; 21:393-400 22. Pollak P, Benabid AL, Limousin P, Benazzouz A. Chronic intracerebral stimulation in Parkinson’s disease. Adv Neurol 1997;345: 213-220 23. Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology 2000;55:S13-6 24. Meissner W, Reum T, Paul G et al. Striatal dopaminergic metabolism is increased by deep brain stimulation of the subthalamic nucleus in 6-hydroxydopamine lesioned rats. Neurosci Lett 2001;303:165-168 25. Blandini F, Nappi G, Greenamyre JT. Subthalamic infusion of an NMDA antagonist prevents basal ganglia metabolic changes and nigral degeneration in a rodent model of Parkinson’s disease. Ann Neurol 2001;49: 525-529 26. Hassani OK, Mouroux M, Feger J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience 1996;72:105-115 27. Dawson TM, Barone P, Sidhu A, Wamsley JK, Chase TN. The D1 dopamine receptor in the rat brain: quantitative autoradiographic localization using an iodinated ligand. Neuroscience 1988;26:83-100 28. Flores G, Liang JJ, Sierra A et al. Expression of dopamine receptors in the subthalamic nucleus of the rat: characterization using reverse transcriptase-polymerase chain reaction and autoradiography. Neuroscience 1999;91:549-556 29. Kreiss DS, Anderson LA, Walters JR. Apomorphine and dopamine D1 receptor agonists increase the firing rate of subthalamic nucleus neurons. Neuroscience 1996;72:863-876 30. Hassani OK, Feger J. Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: an electrophysiological and c-Fos study. Neuroscience 1999;92:533-543 31. Ruskin DN, Marshall JF. D1 dopamine receptors influence Fos immunoreactivity in the globus pallidus and subthalamic nucleus of intact and nigrostriatal-lesioned rats. Brain Res 1995;703:156-164 32. Murer MG, Ferrario J, Delfino M, Dziewczapolski G, Gershanik OS, Raisman-Vozari R. Increased [I125] sulpiride binding in the subthalamic nucleus of rats with nigrostriatal lesions. Neuroreport 1999;14:15011505 33. Francois C, Savy C, Jan C, Tande D, Hirsch EC, Yelnik J. Dopaminergic innervation of FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 105 F. Blandini 34. 35. 36. 37. 38. 39. 40. 41. 42. 106 the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkins o n ’s disease patients. J Comp Neurol 2000;425:121-129 Mehta A, Thermos K, Chesselet MF. Increased behavioral response to dopaminergic stimulation of the subthalamic nucleus after nigrostriatal lesions. Synapse. 2000;37:298307 Mukhida K, Baker KA, Sadi D, Mendez I. Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants. J Neurosci 2001;21:3521-3530 Smith Y, Charara A, Parent A. Synaptic innervation of mid-brain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J Comp Neurol 1996;364:231253. Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse 1992;12:287-303 Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689-695 Kure S, Tominaga T, Yoshimoto T, Tada K, Narisawa K. Glutamate triggers internucleosomal DNA cleavage in neuronal cells. Biochem Biophys Res Commun 1991;179: 39-45 Tan S, Wood M and Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem 1998;71:95-105. Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol 1986;19:105111 Albin RL, Greenamyre JT. Alternative exci- FUNCTIONAL NEUROLOGY (16) SUPPL. 4 2001 43. 44. 45. 46. 47. 48. 49. 50. totoxic hypotheses. Neurology 1992;42:733738 Beal MF, Hyman BT, Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci 1993;16:125-131 Schapira A.H.V., Cooper J.M., Dexter D., Clark J.B., Jenner P., Marsden C.D. Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem 1990;54:823-827 Rodriguez M.C., Obeso A, Olanow W. Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 1998;44 (Suppl 1):S175S188 Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408-1414 Saji M, Blau AD, Volpe BT. Prevention of transneuronal degeneration of neurons in the substantia nigra reticulata by ablation of the subthalamic nucleus. Exp Neurol 1996;141: 120-129 Nakao N, Ekini N, Nakai K, Itakura T. Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced by the mitochondrial toxin 3-nitropropionic acid. Ann Neurol 1999;45:640-651 Blandini F, Greenamyre JT, Nappi G. The role of glutamate in the pathophysiology of Parkinson’s disease. Funct Neurol 1996;1:315 Blandini F, Greenamyre JT. Prospects of glutamate antagonists in the therapy of Parkinson’s disease. Fundam Clin Pharmacol 1998; 12:4-12