* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Suzuki and Eichenbaum, 2000
Aging brain wikipedia , lookup
Nervous system network models wikipedia , lookup
Executive functions wikipedia , lookup
Psychophysics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Development of the nervous system wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Environmental enrichment wikipedia , lookup
Metastability in the brain wikipedia , lookup
Memory consolidation wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Optogenetics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Hippocampus wikipedia , lookup
Childhood memory wikipedia , lookup
Limbic system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Holonomic brain theory wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
State-dependent memory wikipedia , lookup
Music-related memory wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Emotion and memory wikipedia , lookup
Prenatal memory wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Time perception wikipedia , lookup
Neural coding wikipedia , lookup
Misattribution of memory wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
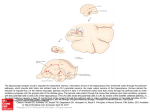
The Neurophysiology of Memory WENDY A. SUZUKIa,c AND HOWARD EICHENBAUMb aCenter for Neural Science, New York University, 4 Washington Place, Room 809, New York, New York 10003, USA bLaboratory of Cognitive Neurobiology, Department of Psychology, Boston University, 64 Cummington Street, Boston, Massachusetts 02215, USA ABSTRACT: How do the structures of the medial temporal lobe contribute to memory? To address this question, we examine the neurophysiological correlates of both recognition and associative memory in the medial temporal lobe of humans, monkeys, and rats. These cross-species comparisons show that the patterns of mnemonic activity observed throughout the medial temporal lobe are largely conserved across species. Moreover, these findings show that neurons in each of the medial temporal lobe areas can perform both similar as well as distinctive mnemonic functions. In some cases, similar patterns of mnemonic activity are observed across all structures of the medial temporal lobe. In the majority of cases, however, the hippocampal formation and surrounding cortex signal mnemonic information in distinct, but complementary ways. INTRODUCTION The landmark study of the amnesic patient H.M. first showed that bilateral damage to the medial temporal lobe severely impairs the ability to form and retain new long-term declarative memories (i.e., memories for facts and events).1 Since that description, the development of animal models of medial temporal lobe amnesia in both monkeys and rats, together with detailed neuroanatomical studies of this region, have helped identify the individual areas important for normal declarative memory.2,3 These structures include the hippocampal formation (defined here as the dentate gyrus, areas CA3, CA1, and the subiculum) and the entorhinal, perirhinal, and parahippocampal cortices. While these studies have contributed substantially to our understanding of the brain basis of memory, neuropsychological studies on braindamaged patients or experimental animals can provide only indirect insights into the specific patterns of neural activity that allow us to form and retain new long-term declarative memories. Instead, characterizations of behavior-related neural activity offer the most direct insight into the neural processes that underlie memory. How do medial temporal lobe neurons signal the acquisition, retention, or recall of a particular stimulus, fact, or event? Do neurons throughout the medial temporal lobe signal memory in a similar way or are different regions of the medial temporal lobe specialized for different aspects of memory? Findings from neurophysiological studies in monkeys, rats, and humans have begun to provide answers to these questions. cAddress for correspondence: Wendy A. Suzuki, Ph.D., Center for Neural Science, New York University, 4 Washington Place, Room 809, New York, NY 10003. Phone: (212) 998-3734; fax: (212) 995-4011. e-mail: [email protected] 175 176 ANNALS NEW YORK ACADEMY OF SCIENCES Although the neurophysiological correlates of memory in rats and monkeys have been studied for over 20 years, early comparisons in the two species provided few points of contact. Comparisons in the two species were limited by an emphasis on studies of spatial learning in rats and by a focus on visual recognition memory in monkeys. More recently, however, a broader range of tasks, especially those designed for rats, has allowed more direct comparisons of the patterns of neural responses both across species and across different medial temporal lobe areas. These comparative neurophysiological findings make two main points. First, the mnemonic signals observed throughout the medial temporal lobe are largely conserved across species. Second, neurons throughout the medial temporal lobe can perform both similar as well as distinctive mnemonic functions that usually operate cooperatively in signaling declarative memory. In most cases, the hippocampal formation and surrounding cortex signal mnemonic information in different but complementary ways. New evidence indicates that in some situations the same recognition memory signals are apparent throughout the medial temporal lobe. In this chapter, we review the neurophysiological findings that address these two main points. We focus on findings from neurophysiological studies of recognition and associative memory in both monkeys and rats. We also discuss relevant findings from studies on neuronal activity and functional imaging in humans. These mnemonic signals can be further illuminated by considering the patterns of parallel and serial connections between the structures of the medial temporal lobe. Thus, we begin with a brief overview of the neuroanatomical organization of this region. ANATOMY OF THE MEDIAL TEMPORAL LOBE A large body of tract tracing studies in monkeys and rats have provided detailed information about the neuroanatomical organization of the medial temporal lobe.4– 11 Although some notable differences exist in the details of the cortical and subcortical connections of these structures across species, the general principles of organization of medial temporal lobe connections are highly conserved. FIGURE 1 illustrates two major organizing principles of the medial temporal lobe. First, the connections of the medial temporal lobe are organized in a hierarchy of increasing amounts of sensory convergence. At the lowest “rung” of the hierarchy are the perirhinal (areas 35 and 36) and parahippocampal (areas TH and TF) cortices. The parahippocampal cortex in monkeys and humans is considered homologous to the postrhinal cortex in rats.12 The perirhinal and parahippocampal/postrhinal cortices are distinguished by receiving not only inputs from other higher order association areas (including regions of the prefrontal cortex and other polymodal areas within the temporal and parietal lobes), but also unimodal sensory input from visual, somatosensory, olfactory, and auditory association areas. Although the perirhinal and parahippocampal/postrhinal cortices in monkeys and rats receive a similar convergence of unimodal sensory inputs, the two species differ in the relative proportions of the various sensory modalities they receive. For example, the perirhinal cortex in monkeys receives the strongest projections from visual area TE, reflecting the prominent role of visual information processing in this species. By contrast, the perirhinal cortex of rats receives a more even distribution of information from visual, SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 177 FIGURE 1. Summary of the sensory connections and interconnections of the structures of the medial temporal lobe. The lower half of the figure illustrates the pattern and relative strength of projections from various unimodal and polymodal sensory areas to the perirhinal cortex, parahippocampal/postrhinal cortex, and entorhinal cortex. Projections in the monkey are shown in black and the projections in rats are shown in gray. The thickness of the lines corresponds to the relative strength of the projections, where the thickest line represents approximately 30% of all cortical inputs or higher, the medium thick line represents between approximately 10 and 29% of projections, and the thinnest line represents less than 10% of inputs. All projections between the perirhinal, parahippocampal/postrhinal, entorhinal, and hippocampus are similar in monkey and in rats and are shown in black (except for one instance described in the text). All projections are reciprocal except those between subfields of the hippocampus, which are denoted with an arrowhead. Quantitative estimates for monkey data were taken from Suzuki et al.18 and those from the rat were taken from Burwell et al.12 178 ANNALS NEW YORK ACADEMY OF SCIENCES somatosensory, as well as auditory and olfactory areas, reflecting the more prominent role of these other sensory modalities in this species. This difference across species can be appreciated by comparing the patterns of cortical inputs to the perirhinal cortex in monkeys (black lines) to those in rats (gray lines) in the lower portion of FIGURE 1. Projections from other unimodal and polymodal sensory areas to the parahippocampal cortex in monkeys and the postrhinal cortex in rats are roughly similar. The perirhinal and parahippocampal/postrhinal cortices then provide the major input to the next “rung” of the hierarchy, the entorhinal cortex. A second notable difference between monkeys and rats is the organization of the direct olfactory inputs to the entorhinal cortex. In monkeys, for example, the entorhinal cortex receives only a small direct projection from olfactory areas.13 The entorhinal cortex in rats is distinguished by receiving more extensive olfactory inputs.11,14 Consistent with these differences in the pattern and strength of visual and olfactory inputs to the medial temporal lobe, behavioral studies of memory in monkeys have most often used visual stimuli, whereas studies in rats have commonly used olfactory stimuli. The entorhinal cortex then provides the major cortical input to the hippocampal formation via a series of well-described serial and parallel projections.15 In addition to the prominent projections from the entorhinal cortex to various components of the hippocampal formation, there is also a direct, though weaker, projection from both the perirhinal and parahippocampal cortices to the CA1/subicular border region in monkeys.16 In rats, while evidence indicates that the perirhinal cortex provides a similar direct projection to both area CA1 and the subiculum,12 postrhinal projections terminate only in the subiculum (Naber and Witter, personal communication). A second prominent feature of the connections of the medial temporal lobe is that all projections (except those between the different subregions of the hippocampal formation) are strongly reciprocal. For example, the perirhinal and parahippocampal cortices not only have strong and reciprocal connections with the entorhinal cortex, but also have weaker interconnections with the CA1/subicular border region. Thus, the medial temporal lobe is made up of a complex series of both serial and parallel projections in which any level of the hierarchy has either direct or indirect access to all other levels of the system. What do these neuroanatomical data tell us about the functional organization of these structures? These data make at least two predictions. On the one hand, the fact that each of these structures differs markedly in the amount and content of convergent cortical input suggests that each area may be able to process or combine information to encode, store, or retrieve memory in different ways. This view suggests that each area may be characterized by distinct patterns of mnemonic activity. On the other hand, the strong parallel projections from the perirhinal and parahippocampal/ postrhinal cortices to both the entorhinal cortex and area CA1 suggest that neurons in all of these regions have access to similar kinds of sensory/mnemonic information. This raises the possibility that in certain situations, each of these structures may encode or store memory in similar ways. The available neurophysiological data support both functional predictions. For example, evidence is strong that the hippocampal formation combines information in memory in ways not observed in the adjacent cortex. In other situations, similar patterns of mnemonic activity have been observed across many medial temporal lobe structures. These patterns of mnemonic activity will be described in greater detail below. SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 179 NEURAL SIGNALS OF RECOGNITION MEMORY Perhaps the largest body of work on the neurophysiology of memory has been done using variants of the delayed match to sample task. This classic task of recognition memory typically consists of three phases. First, a to-be-remembered sample stimulus is presented. The sample stimulus is then followed by a delay period and finally by a “test” or recognition phase in which one or more items are presented. Animals are rewarded for responding to either the stimulus that matches the sample (delayed match to sample) or the stimulus that does not match the sample (delayed nonmatch to sample). Another commonly used version of this task is the “continuous” delayed nonmatch to sample task.17 In this task, stimuli are presented to the animal sequentially, separated by delay intervals. Animals are rewarded for responding if the stimulus is different from the preceding stimulus. In this version of the task, each stimulus is both the sample for the following item and the test stimulus for the preceding item. Other variations of the task include the use of either highly familiar or novel stimuli, the use of varying numbers of “test” stimuli presented between the sample stimulus and the final match, or variations in the length of the delay intervals used. How do medial temporal lobe neurons signal recognition memory? To approach this question, it is useful to ask what the animal must do to solve a typical recognition memory task. To perform such a task, the animal must first successfully discriminate between the stimuli being used. Second, the animal must maintain a representation of the to-be-remembered sample stimulus throughout the trial including during the delay interval(s). Third, the animal must determine whether the current “test” stimulus matches the stimulus held in memory. Neurons throughout the medial temporal lobe exhibit firing properties consistent with each of these three key task requirements. The most extensively characterized medial temporal lobe regions have been the perirhinal and the entorhinal cortices. We begin with a description of the recognition memory signals observed in these cortical areas. The patterns of neural activity underlying each of the three recognition task requirements just outlined will be discussed in turn. We will then compare the patterns of activity observed in the perirhinal and entorhinal cortices to the patterns seen in the hippocampal formation. Much less is known about the patterns of mnemonic activity in the parahippocampal/postrhinal cortex, and therefore this region will not be discussed. Recognition Memory in Perirhinal and Entorhinal Cortices An important requirement to solve any recognition memory task is the ability to discriminate between the different sensory stimuli being shown. A defining characteristic of neurons throughout the entorhinal and perirhinal cortices of rats and monkeys is that they respond with a high firing rate to certain sensory stimuli and not to others (i.e., sensory-selective response). For example, in monkeys, 47% of the visually responsive neurons in the entorhinal cortex18 and 94% of the visually responsive neurons in the perirhinal cortex19 respond selectively to particular visual stimuli. In rats, 35% of the sampled lateral entorhinal neurons and 11% of the sampled perirhinal neurons exhibit odor-specific firing properties.20 Visually responsive neurons have been reported in the rat perirhinal and entorhinal cortices,21 although the selectivity of these responses has not been extensively characterized. 180 ANNALS NEW YORK ACADEMY OF SCIENCES A second requirement of a typical recognition memory task is the ability to maintain the representation of the to-be-remembered sample stimulus throughout the duration of the trial including the delay interval. Consistent with this task requirement, some neurons in the perirhinal and entorhinal cortices either sustain or reactivate their stimulus-selective response for the to-be-remembered sample stimulus during the delay interval. For example, in rats, some neurons in the perirhinal and entorhinal cortices respond robustly during the delay interval of a delayed nonmatching to sample task if the to-be- remembered stimulus is one particular odor (i.e., odor A), but not if it is any other odor from a set of alternative stimuli.20 Other cells in these same areas appear to reactivate their selective response only at the very end of the delay period immediately before the choice phase of the task.20 In monkeys, some perirhinal or entorhinal neurons exhibit stimulus-selective activity during the delay period that begins immediately following the sample stimulus of a delayed match to sample task.18,22 In the entorhinal cortex, this selective activity was maintained in the delay intervals following multiple intervening test stimuli.18 By contrast, selective delay activity in the perirhinal cortex was disrupted by the presentation of even a single intervening stimulus.22 The effect of intervening stimuli on the selective delay activity in the perirhinal or entorhinal cortices of rats has not been examined. A third requirement of a typical delayed match to sample task is the ability to determine if the current stimulus matches or does not match the stimulus held in memory. In other words, is a neuron’s response to a particular stimulus influenced by whether that stimulus is held in memory or not? To address this question, comparisons have been made between the response of a neuron to its preferred stimulus when it was presented as a match and the response to the same stimulus when it was presented as a nonmatch in other trials.18–20 Importantly, the physical characteristics of the stimulus were identical in both the “match” and the “nonmatch” conditions. The only difference between the two stimulus presentations was that in the match condition (and not in the nonmatch condition), the animals carried a memory of having seen the stimulus at the beginning of the trial. This analysis showed that neurons in the entorhinal and perirhinal cortices signaled the occurrence of a matching stimulus with either a suppressed (decreased) or an enhanced (increased) response. These so-called match-suppression or match-enhancement signals convey information about the previous occurrence of a particular stimulus on a given trial. The match-suppression response cannot be explained by sensory fatigue or habituation, because the suppression disappears when the same stimulus is shown as a sample on the following trial.19 The match-suppression or match-enhancement effects occurred very early in the sensory response and well before the animal's behavioral response, suggesting that these suppressed or enhanced sensory signals could be used by the animals to perform the task. Studies conducted in the entorhinal and perirhinal cortex in both monkeys and rats typically find varying proportions of neurons with selectively suppressed or enhanced responses.18,19,23–26 In general, more neurons tend to respond to a repeated stimulus with a suppressed response. One intriguing question concerns which factors determine whether a neuron signals a remembered stimulus with an enhanced or a suppressed response. One study found that a seemingly minor change in the task demands resulted in a significant shift in the ratio of enhanced versus suppressed responses observed in the perirhinal cortex.25 This study compared the pattern of neu- SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 181 ral activity in two different versions of a delayed match to sample task. In the “standard” version of the task, a sample stimulus was followed by a variable number of unique “nonmatching” test items, and the animals were required to respond to the repetition of the sample stimulus (i.e., the match). If A, B, and C refer to different visual stimuli, a typical trial in the standard version of the task would be A…B…C…A. The animals were always rewarded for responding to the second A, and none of the intervening “test” stimuli (i.e., B and C) were ever repeated. In the second version of the task, the sample stimulus was also followed by a variable number of nonmatching test items, but in this case the nonmatching test items could also repeat. This was termed the “ABBA” version of the task, where BB refers to the repeated nonmatch. In this version of the task, the animals had to learn to ignore the repeated nonmatch and respond only when the sample was repeated. Match suppression and match enhancement were observed as animals performed both versions of the task. However, the proportion of neurons exhibiting match enhancement was substantially higher during performance of the ABBA version of the task compared to the standard version. Moreover, an important difference was noted in the pattern of responses of the match-suppression and match-enhancement neurons on the ABBA version of the task. That is, neurons exhibiting match suppression showed suppressed responses to any repeated stimulus including the repeated nonmatch. By contrast, the match-enhancement neurons were enhanced only when the behaviorally relevant stimulus was repeated. Thus, match enhancement may represent an “active” form of working or recognition memory that is associated with signaling the behaviorally relevant stimulus.25 If match enhancement signals active or working memory, then what does match suppression represent? One possibility is that match suppression also represents a form of active memory in that it resets between trials of the standard version of the DMS task.19 This recognition memory signal may be used in situations different from those that engage the match-enhancement signal. An alternative possibility, however, is that match suppression does not reflect a form of active memory, but instead reflects a passive or implicit form of memory. Priming is one possible candidate. Priming is defined as an improvement in the ability to detect or identify stimuli as the result of previous experience with the stimuli. The improvement in performance is observed independent of whether subjects are consciously aware of the learning experience (i.e., it is a form of implicit memory). Consistent with the idea that match suppression may be associated with a priming-like memory signal, functional imaging studies have shown that priming is associated with decreased brain activation.27,28 Although many forms of priming are intact following medial temporal lobe lesions, a recent study showed that one form of implicit memory was impaired in patients with medial temporal lobe amnesia.29 This finding raises the possibility that the medial temporal lobe can participate in certain forms of priming or implicit memory. If match suppression was a form of implicit or unconscious memory, one would expect the mnemonic signal to occur with any repeated stimulus irrespective of whether the animal consciously recognized the stimulus as familiar or repeated (i.e., whether the animal got the trial correct or not). On the other hand, if match suppression represents a more active or “conscious” form of memory, the prediction would be that match suppression should be robust on correct trials and diminished on error trials. Unfortunately, this prediction has not been extensively test- 182 ANNALS NEW YORK ACADEMY OF SCIENCES ed, because animals typically perform these recognition tasks at very high levels of accuracy. Therefore, few if any error trials have been available for analysis. Additional studies are needed to examine the relationship between match suppression, match enhancement, and conscious or unconscious forms of memory. While the studies just described used familiar sets of stimuli to test recognition memory, robust patterns of match suppression are also observed when novel visual stimuli are used. In general, neurons in the entorhinal and perirhinal cortex respond with their highest firing rate to particular novel (or rarely seen) stimuli, and these stimulus-selective responses continue to diminish gradually as the novel stimulus becomes more familiar with repeated exposure. For some neurons, the firing rate is suppressed when the novel stimulus is repeated, even after a delay interval of 24 hours.24,26 This pattern of activity (referred to as the familiarity effect) is seen as animals passively view repeated stimuli24 or when animals perform a simple novel/ familiar discrimination task.26 This kind of robust stimulus-selective response suppression could be used to perform one widely used recognition memory task in which novel or rarely seen objects are used for each trial (i.e., the trial unique version of the delayed nonmatching to sample task). Performance of the trial-unique version of the delayed nonmatching to sample task is impaired in monkeys following lesions that include the perirhinal and entorhinal cortices.30–32 Recognition Memory in the Hippocampal Formation Early neurophysiological studies of recognition memory in the hippocampal formation of rats and monkeys reported no evidence of the stimulus-selective recognition signals just described for perirhinal and entorhinal neurons. Recordings in the monkey hippocampal formation tended to include neurons throughout the dentate gyrus, CA3, CA1, and the subiculum,23,26,33–35 whereas recordings in rats tended to be limited to area CA1.17,36,37 Although the neurons of the hippocampal formation do not convey robust stimulus-selective recognition signals, they do indeed signal mnemonic information during the performance of various recognition memory tasks. The nature of these mnemonic signals, however, is distinctly different from the patterns of activity typically observed in the adjacent cortex. Two different kinds of memory signals have been described in the hippocampal formation. First, some neurons in this region respond differentially to stimuli when they are shown as matches compared to when the same stimuli are shown as nonmatches (15 of 120 or 12.5% of the responsive neurons).17 Unlike neurons in the entorhinal or perirhinal cortex that signal this match-nonmatch difference for particular stimuli (i.e., stimulus-selective match-nonmatch responses), neurons in the hippocampal formation signaled this match-nonmatch difference for all stimuli.17 This pattern of activity has been referred to as an “abstracted” recognition signal in the sense that these hippocampal neurons do not convey specific information about particular stimuli but, instead, appear to signal the outcome of the match-nonmatch comparison (i.e., the answer) for all stimuli. Importantly, control experiments showed that these abstracted matchnonmatch signals could be clearly dissociated from activity related to the behavioral (i.e., motor) response the animals made.17 Like the selective match-suppression and match-enhancement cells observed in the cortex, these abstracted match-nonmatch signals observed in the hippocampus could be used by the animal to signal stimulus recognition. Although direct comparisons are difficult because of differences in SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 183 analyses, similar patterns of activity have also been described in other studies of recognition memory in monkeys.35,36 A second pattern of mnemonic activity was observed in hippocampal (areas CA3 and CA1) neurons as rats performed an olfactory recognition task (continuous delayed nonmatching to sample) in which odors were presented in cups of odorized sand at many different locations on an open platform.37 Animals learned to dig in a cup of odorized sand to retrieve a food reward if the odor did not match the previous sample stimulus. If the odor matched the previous sample, no reward was present and the rats learned to turn away from the cup. The location of the odor presentation changed on each trial but did not predict whether the trial was a nonmatch or a match. All recordings in this study were limited to areas CA1 and CA3 of the hippocampus. Consistent with the study described above, some neurons signaled the match-nonmatch status of an odor irrespective of the odor identity (“abstracted” match-nonmatch signal; 13 of 91 or 14% of the responsive neurons). A second subset of neurons in this task signaled the abstracted match-nonmatch signals in conjunction either with the position in which it occurred (18 of 91 or 20% of the responsive neurons) or with both position as well as odor identity (4 of 91 or 4%). The pattern of activity exhibited by the second subset of neurons has been referred to as “conjunctive coding” and can be considered a form of associative memory (see below). Similar patterns of conjunctive coding in hippocampal neurons were also reported by Hampson et al.38 in a spatial delayed match to sample task and by Wiebe and Staubli39 in an olfactory delayed nonmatching to sample task. Conjunctive coding neurons in the hippocampal formation that carry abstract information about the match-nonmatch status of a stimulus could also be used by the animal to perform a recognition memory task. Conjunctive coding is consistent with a recently outlined theoretical framework in which hippocampal activity is characterized as reflecting a sequence of episodic representations including the many complex conjunctions or associations unique to a particular episode.40 Although the majority of neurons in the hippocampal formation signal recognition memory either with the abstracted match-nonmatch signal or in conjunction with other aspects of the task, two recent studies37,39 report a small number of stimulus-selective match-suppression or match-enhancement neurons in the rat hippocampal formation. Although these neurons were a minority, these findings suggest that in certain situations, hippocampal neurons can also signal “cortex-like” recognition memory signals. This kind of selective recognition memory signal has also been observed in the human hippocampus (see below). In no case has selective delay activity been reported in the hippocampal formation. Contrasting Recognition Memory Signals in Cortex and Hippocampal Formation The neurophysiological data described herein show that the entorhinal and perirhinal cortex, on the one hand, and the hippocampal formation, on the other, both signal recognition memory in different but complementary ways. The patterns of mnemonic activity observed in the entorhinal and perirhinal cortices can be summarized by two general principles. First, some cortical neurons signal recognition memory by either suppressing or enhancing their sensory-selective responses. Thus, if a neuron responds selectively to odor A, it might signal the repetition of odor A (i.e., a “match”) with an enhanced response. Second, neurons in these cortical medi- 184 ANNALS NEW YORK ACADEMY OF SCIENCES al temporal lobe areas signal memory by sustaining or reactivating their activity in the delay period associated with a remembered stimulus. Thus, an entorhinal neuron might respond with an increased firing rate in the delay interval when odor A was presented as the sample, but not if any other odor was presented as the sample. In contrast to the stimulus-selective recognition signals observed in cortex, neurons of the hippocampal formation convey abstracted recognition memory signals. For example, hippocampal neurons might signal any matching stimulus with a significantly enhanced or suppressed response. In other cases, hippocampal neurons signal stimulus recognition in conjunction with other aspects or elements of the task (i.e., conjunctive coding). It has been suggested that conjunctive coding may represent elements of unique episodes held in memory (e.g., an odor sampled in a particular place).40 By this account, the “abstract” representations may provide access to all the episodic memories that include that abstraction. Thus, cells whose activity reflects, for example, a nonmatch, may provide a link to recent episodic memories of nonmatch trials. The more abstracted or conjunctive nature of the signal in the hippocampal formation compared to the cortex can be understood in terms of the connectivity of these areas. Whereas the cortex has prominent access to unimodal sensory information, the hippocampus is characterized by receiving input from other polymodal association areas. The hippocampus is in a privileged position to either combine the stimulus-selective mnemonic information in cortex into more complex conjunctions or to abstract, from the stimulus-selective information, a more general “output” signal. In certain task situations, a small number of neurons in the hippocampal formation signal the stimulus-selective match suppression or match enhancement similar to that observed in the entorhinal or perirhinal cortex. These “cortexlike” recognition signals may be conveyed via the direct projections from both the perirhinal and the entorhinal cortices to area CA1. Taken together, these findings suggest that all these medial temporal lobe areas work cooperatively to signal many forms of recognition memory. Recognition Memory Signals in the Human Medial Temporal Lobe While the majority of the information concerning neuronal activity in the medial temporal lobe during memory tasks comes from experimental studies in animals, there are also relevant data from the human literature. For example, a single unit physiology study in human neurological patients reported prominent recognition signals in the hippocampus (subregions of the hippocampus not specified), entorhinal cortex, and amygdala. In this study, individual neurons in all three areas were recorded during the encoding and retrieval phases of a yes-no recognition task in which faces and objects were used as stimuli.41 Unlike most experimental studies in animals in which stimuli are only remembered for a maximum of several seconds, the retrieval phase in this task occurred between 1 and 10 hours after the encoding phase. Importantly, this is a delay interval known to challenge amnesic patients with selective hippocampal damage.42 Despite the relatively small number of neurons examined (74 neurons in 3 regions), the overall pattern of activity was strikingly similar to the findings described for the perirhinal and entorhinal neurons in rats and monkeys. That is, neurons in the human hippocampus and entorhinal cortex responded selectively during the encoding phase of the task. Moreover, neural responses during the recognition phase were influenced not only by stimulus identity but also by SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 185 whether the stimulus had been seen before. The responses of 35% (10 of 29) of cells recorded in the human hippocampus and 40% (8 of 20) of cells recorded in the entorhinal cortex in the retrieval phase were influenced by whether a stimulus was new or old. The majority of these cells exhibited enhanced activity in response to previously seen stimuli. These findings support the idea that neurons in the human hippocampus and entorhinal cortex signal long-term stimulus recognition for objects or faces. These findings are also consistent with findings from human neuropsychological studies42 showing that discrete lesions limited to the hippocampus can impair performance on a wide variety of recognition tasks. Another relevant set of findings derives from human functional imaging literature. A growing body of studies has shown that the hippocampal formation signals the relative novelty or familiarity of stimuli during the encoding phase of various tasks. Several studies compared responses during the encoding phase of recognition memory tasks of items that had been seen before (OLD conditions) to novel items (NEW). Irrespective of whether the to-be-remembered stimuli were pictures,43,44 auditory sentences,45 common words,46,47 or nonpictorial visual stimuli,47 significant activations were observed in the hippocampal formation and parahippocampal gyrus when the OLD condition was subtracted from the NEW. These findings are reminiscent of the patterns of activity of entorhinal and perirhinal neurons that respond best to particular novel stimuli and give increasingly suppressed responses to the same stimulus as it becomes familiar. Based on these findings, Tulving and colleagues43 have argued that the medial temporal lobe is part of a novelty detection network. These patterns of activity could participate in many different forms of recognition memory, including the kind of recognition memory required to perform delayed nonmatching to sample with novel stimuli.48 In this task, animals must discriminate a novel stimulus from one that has been seen once before. Indeed, lesions limited to the hippocampus produce a significant recognition memory impairment on this task that is most striking at the longest delay intervals.48–50 In another study, however, no recognition impairment was observed following selective lesions of both the hippocampus and the amygdala.51 Taken together, findings from the human functional imaging literature support the idea that the hippocampus signals information useful for certain forms of recognition memory. NEURAL SIGNALS OF ASSOCIATIVE MEMORY A second form of memory that has been examined at a neurophysiological level is associative memory. We define associative memory in a broad sense to include any memory about the relationship between two or more items. Unlike recognition memory, very little exists in the way of direct comparisons between firing patterns of neurons in the perirhinal and entorhinal cortex versus those in the hippocampal formation in tasks of associative memory. In a study explicitly designed to evaluate associative responses, Sakai and Miyashita52 examined responses of anterior inferotemporal and perirhinal neurons to 24 fractal stimulus patterns. These stimuli were arbitrarily paired so that on each trial one stimulus of a pair was presented as a sample cue, and after a delay period was followed by a choice between the assigned paired cue and one of the other stimuli. After acquisition of this paired associate task over a series of training sessions, two different associative correlates were observed 186 ANNALS NEW YORK ACADEMY OF SCIENCES in the firing of these neurons. “Pair-coding” neurons fired maximally associated with presentations of either of the two cues that were paired associates, more so than for any of several other cues involved in different paired associates. “Pair-recall” neurons increased their firing rate during the delay period following presentation of the associate of the optimal cue, leading up to a robust response to that cue. Thus, within these cortical areas, stimulus-stimulus associations may be represented by the acquired capacity of a stimulus to generate the neural representation of another stimulus with which it has been associated. In addition, in the study by Fried et al.41 described above, single entorhinal and hippocampal neurons were recorded as human subjects viewed different faces in the encoding phase of the task. They found that some cells responded during the presentation of faces in both areas and that many of these neurons were activated differentially associated with the conjunction of particular facial expressions and gender and between facial expression and the identity of the face. These conjunctive (i.e., associative) responses were observed in both the entorhinal cortex and the hippocampus. Other studies on humans using brain imaging methods have also shed light on neural activity in the hippocampus and neighboring parahippocampal cortex during associative learning. Henke et al.53 measured activation of the medial temporal lobe using PET in subjects learning about pictures of people and houses. Subjects were trained in two conditions. In one condition they learned separately about a person and about a house, while making independent judgments about the gender of a person (single item learning condition) or whether the view of a house was inside or outside. In the other condition they were encouraged to learn an association between a person and a house while making a judgment about whether a particular person was likely to live in a particular house (associative learning condition). Participation in the associative learning condition led to better subsequent recognition of previously presented person-house pairings, suggesting this kind of processing indeed resulted in learning associations between particular persons and houses. In addition, both the parahippocampal gyrus and the hippocampus showed increased PET activation in the associative learning condition as compared to the single item learning condition, showing selective activation of both areas during this type of associative learning. On the other hand, Gabrieli et al.54 dissociated parahippocampal from hippocampal activation using fMRI. They found that the parahippocampal gyrus, but not the hippocampus, was selectively activated during the encoding of novel pictures. Conversely, the hippocampus (specifically the subiculum), but not the parahippocampal gyrus, was selectively activated during retrieval of a previously seen picture cued by the name of the pictured item or during retrieval of previously seen names of items cued by a picture of the item. Thus, under these conditions, activation of the parahippocampal cortex was differentially associated with encoding novel materials, whereas activation of the hippocampus was differentially associated with retrieval based on name-and-picture associations. Findings from single unit recording studies in rodents also shed light on the issue of associative learning.40 A common finding in this large literature is that hippocampal principal neurons fire when an animal is in a particular location in its environment. The firing patterns of many of these so-called place cells are dependent on the spatial configuration of multiple environmental cues and therefore are an example of conjunctive (i.e., associative) encoding. In addition, as described above, recent work SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 187 has shown that the firing patterns of hippocampal principal neurons also incorporate a broad variety of nonspatial stimuli and behavioral events into their representations37 and encode conjunctions of spatial and nonspatial information.55 Thus, conjunctive/associative coding is particularly prominent in rodent hippocampal neurons. Quirk et al.56 directly compared the spatial firing properties of entorhinal neurons with those of principal cells of the hippocampus in rats foraging for food in each of two different environments. One environment was a circular open field surrounded by a plain cylinder with a contrasting vertical stripe that provided a salient orienting cue. The other environment was a square open field of the same area surrounded by a rectangular set of walls, one of which had the same contrasting color and therefore provided a similar orienting cue. Place cells were observed in both the entorhinal cortex and the hippocampus. In addition, many of the entorhinal place cells had similar spatial firing patterns in the two environments, so that translation of the “place field” from the circular to the square produced a close correspondence in the activity pattern of the cells in the two environments. By contrast, hippocampal neurons nearly always showed different and independent patterns of spatial activity in the two environments. These findings were interpreted as evidence that entorhinal cells were more strongly controlled by particular salient sensory cues (e.g., the orienting stripe), whereas hippocampal neurons were more strongly controlled by differences in the spatial geometry of the environments. To summarize, consistent with the studies of recognition memory, evidence exists both for similarities in the types of associative memory coding apparent in the perirhinal and entorhinal cortex and the hippocampal formation under some testing conditions and for differences under other conditions. A compelling direct comparison that reveals the critical aspects of these different testing conditions has yet to be made. Yet the evidence showing differences between these areas suggests that parahippocampal cortical neurons may participate in associative memory by developing representations of complex perceptual stimuli that can be activated by their associates (i.e., pair-coding neurons of Sakai and Miyashita52), whereas neurons of the hippocampal formation participate in associative memory by representing conjunctions of independent perceptual stimuli as well as behavioral events (i.e., conjunctive coding neurons of Wood et al.37). SUMMARY AND CONCLUSIONS How do the structures of the medial temporal lobe signal the formation, retention, and recall of declarative memories? Far from a simple division of labor within the medial temporal lobe, the available neurophysiological evidence suggests that all medial temporal lobe areas can signal information relevant for all forms of declarative memory, from recognition to association. There are key differences, however, in the level or character of those signals, suggesting evidence for certain functional dissociations. These findings are also consistent with a large body of results from experimental lesion studies. For example, numerous studies have shown that damage including the entorhinal and perirhinal cortices produces impairment on tasks of recognition memory detectable even at short delay intervals (on the order of several sec- 188 ANNALS NEW YORK ACADEMY OF SCIENCES onds). This memory impairment is exacerbated as the delay intervals increase.32,57,58 Importantly, the severe memory impairment following damage to the entorhinal or perirhinal cortex does not appear to be a perceptual deficit, because animals with large medial temporal lobe lesions including these structures can perform normally on recognition tasks when there is no delay interval between the sample and choice phases (zero-second delay interval).59 Consistent with these findings from lesion studies, robust match-suppression and match-enhancement signals are observed with short delay intervals (on the order of several seconds), and these recognition signals persist for intervals as long as 24 hours.26 Thus, these stimulus-selective recognition signals appear to be used at both short as well as longer delay intervals. In contrast to lesion studies involving the cortical medial temporal lobe areas, damage limited to the hippocampus produces the most striking recognition impairments when delay intervals are long (many minutes to hours).48-50 At longer delay intervals, when interference can play a detrimental role, information about the context or episode in which the stimulus was seen could be particularly helpful to successful performance. By this view, the conjunctive signals conveyed by the hippocampus may be particularly useful when contextual or episodic information is needed to solve recognition tasks with long delay intervals. These findings support the view that the prominent selective recognition signals observed in the cortex as well as the robust conjunctive signals observed in the hippocampus contribute to successful recognition performance. Depending on the particular mnemonic demands of the task (long- vs. short-term memory or high vs. low interference), one or the other mnemonic signal may be preferentially used. Whereas less is known about the neural correlates of associative memory, differential patterns of associative coding have been described in the cortex on the one hand (pair-coding and pair-recall neurons) and in the hippocampus on the other (conjunctive signals). Additional comparative studies are needed to determine the full scope of neural signals underlying associative memory across the medial temporal lobe. REFERENCES 1. SCOVILLE, W.B. & B. MILNER. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20: 11–21. 2. EICHENBAUM, H. 1992. The hippocampal system and declarative memory in animals. J. Cognit. Neurosci. 4: 217–231.50. 3. SQUIRE, L.R. & S.M. ZOLA. 1996. Structure and function of declarative and nondeclarative memory systems. Proc. Natl. Acad. Sci. USA 93: 13515–13522. 4. VAN HOESEN, G.W. et al. 1972. Cortical afferents to the entorhinal cortex of the rhesus monkey. Science 175: 1471–1473. 5. VAN HOESEN, G.W. et al. 1975. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 95: 25–38. 6. VAN HOESEN, G.W. & D.N. PANDYA. 1975. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 95: 1–24. 7. VAN HOESEN, G.W. & D.N. PANDYA. 1975. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 95: 48–67. 8. DEACON, T.W. et al. 1983. Afferent connections of the perirhinal cortex in the rat. J. Comp. Neurol. 220: 168–190. SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 189 9. MARTIN-ELKINS, C.L. & J.A. HOREL. 1992. Cortical afferents to behaviorally defined regions of the inferior temporal and parahippocampal gyri as demonstrated by WGAHRP. J. Comp. Neurol. 321: 177–192. 10. SUZUKI, W.A. & D.G. AMARAL. 1994. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J. Comp. Neurol. 350: 497–533. 11. BURWELL, R.D. & D.G. AMARAL. 1998. Cortical afferents of the perirhinal, postrhinal and entorhinal cortices. J. Comp. Neurol. 398: 179–205. 12. BURWELL, R.D. et al. 1995. The perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5: 390–408. 13. INSAUSTI, R. et al. 1987. The entorhinal cortex of the monkey: II. Cortical afferents. J. Comp. Neurol. 264: 356–395. 14. BECKSTEAD, R.M. 1978. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 152: 249–264. 15. AMARAL, D.G. & M.P. WITTER. 1995. Hippocampal Formation. In The Rat Nervous System. G. Paxinos, Ed. Academic Press Inc. New York. 16. SUZUKI, W. & D.G. AMARAL. 1990. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neurosci. Lett. 115: 43–48. 17. OTTO, T. & H. EICHENBAUM. 1992. Neuronal activity in the hippocampus during delayed nonmatch to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus 2: 323–334. 18. SUZUKI, W.A. et al. 1997. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 78: 1062–1081. 19. MILLER, E.K. et al. 1993. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J. Neurosci. 13: 1460–1478. 20. YOUNG, B.J. et al. 1997. Memory representation within the parahippocampal region. J. Neurosci. 17: 5183–5195. 21. ZHU, X.O. et al. 1995. Neuronal signaling of information important to visual recognition memory in rat rhinal and neighboring cortices. Eur. J. Neurosci. 7: 753–765. 22. MILLER, E.K. et al. 1996. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 16: 5154–5167. 23. RICHES, I.P. et al. 1991. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J. Neurosci. 11: 1763–1779. 24. FAHY, F.L. et al. 1993. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp. Brain Res. 96: 457–472. 25. MILLER, E.K. & R. DESIMONE. 1994. Parallel neuronal mechanisms for short-term memory. Science 263: 520–522. 26. XIANG, J.Z. & M.W. BROWN. 1998. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology 37: 657–676. 27. UNGERLEIDER, L.G. 1995. Functional brain imaging studies of cortical mechanisms for memory. Science 270: 769–775. 28. SCHACTER, D.L. & R.L. BUCKNER. 1998. On the relations among priming, conscious recollection, and intentional retrieval: evidence from neuroimaging research. Neurobiol. Learn. Mem. 70: 284–303. 29. CHUN, M.M. & E.A. PHELPS. 1999. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neurosci. 2: 844–847. 30. GAFFAN, D. & E.A. MURRAY. 1992. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav. Neurosci. 106: 30–38. 31. MEUNIER, M. et al. 1993. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J. Neurosci. 13: 5418–5432. 190 ANNALS NEW YORK ACADEMY OF SCIENCES 32. MEUNIER, M. et al. 1996. Effects of rhinal cortex lesions combined with hippocampectomy on visual recognition memory in rhesus monkeys. J. Neurophys. 75: 1190–1205. 33. BROWN, M.W. et al. 1987. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 409: 158–162. 34. COLOMBO, M. & C.G. GROSS. 1994. Responses of inferior temporal cortex and hippocampal neurons during delayed matching to sample in monkeys (Macaca fascicularis). Behav. Neurosci. 108: 443–455. 35. WILSON, F.A. et al. 1990. Hippocampus and medial temporal cortex: neuronal activity related to behavioural responses during the performance of memory tasks by primates. Behav. Brain Res. 40: 7–28. 36. SAKURAI, Y. 1990. Hippocampal cells have behavioral correlates during the performance of an auditory working memory task in the rat. Behav. Neurosci. 104: 253–263. 37. WOOD, E.R. et al. 1999. The global record of memory in hippocampal neuronal activity. Nature 397: 613–616. 38. HAMPSON, R.E. et al. 1993. Hippocampal cell firing correlates of delayed-matching-tosample performance in the rat. Behav. Neurosci. 107: 715–739. 39. WIEBE, S. & U. STAUBLI. 1999. Dynamic filtering of recognition memory codes in the hippocampus. J. Neurosci. 19: 10562–10574. 40. EICHENBAUM, H. et al. 1999. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23: 209–226. 41. FRIED, I. et al. 1997. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 18: 753–765. 42. REED, J.M. & L.R. SQUIRE. 1997. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav. Neurosci. 111: 667–675. 43. TULVING, E. et al. 1996. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb. Cortex 6: 71–79. 44. STERN, C.E. et al. 1996. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 93: 8660–8665. 45. TULVING, E. et al. 1994. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc. Natl. Acad. Sci. USA 91: 2012–2015. 46. KAPUR, S. et al. 1995. Functional role of the prefrontal cortex in memory retrieval: a PET sutdy. NeuroReport 6: 1880–1884. 47. MARTIN, A. et al. 1997. Modulation of human medial temporal lobe activity by form, meaning and experience. Hippocampus 7: 587–593. 48. ZOLA, S.M. et al. 2000. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 20: 451–463. 49. BEASON-HELD, L. et al. 1999. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 9: 562–574. 50. HAMPSON, R.E. et al. 1999. Effects of ibotenate hippocampal and extrahippocampal destruction on delayed-match and -nonmatch-to-sample behavior in rats. J. Neurosci. 19: 1492–1507. 51. MURRAY, E.A. & M. MISHKIN. 1998. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 18: 6568. 52. SAKAI, K. & Y. MIYASHITA. 1991. Neural organization for the long-term memory of paired associates. Nature 354: 152–155. 53. HENKE, K. et al. 1997. Human hippocampus establishes associations in memory. Hippocampus 7: 249–256. 54. GABRIELI, J.D.E. et al. 1997. Separate neural basis of two fundamental memory processes in the human medial temporal lobe. Science 276: 264–266 55. DEADWYLER, S.A. et al. 1996. Hippocampal ensemble activity during spatial delayednonmatch-to-sample performance in rats. J. Neurosci. 16: 354–372. 56. QUIRK, G.J. et al. 1992. The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J. Neurosci. 12: 1945–1963. 57. ZOLA-MORGAN, S. et al. 1989. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J. Neurosci. 9: 4355–4370. SUZUKI & EICHENBAUM: NEUROPHYSIOLOGY OF MEMORY 191 58. SUZUKI, W.A. et al. 1993. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J. Neurosci. 13: 2430–2451. 59. OVERMAN, W.H. et al. 1990. Picture recognition vs. picture discrimination learning in monkeys with medial temporal removals. Exp. Brain Res. 79: 18–24.