* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Imaging the premotor areas Nathalie Picard* and Peter L Strick
Microneurography wikipedia , lookup
Neuropsychology wikipedia , lookup
Response priming wikipedia , lookup
Brain–computer interface wikipedia , lookup
Mirror neuron wikipedia , lookup
Broca's area wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Biology of depression wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Optogenetics wikipedia , lookup
Neurolinguistics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Time perception wikipedia , lookup
Cortical cooling wikipedia , lookup
Environmental enrichment wikipedia , lookup
Human multitasking wikipedia , lookup
Executive functions wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
History of neuroimaging wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Human brain wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Emotional lateralization wikipedia , lookup
Affective neuroscience wikipedia , lookup
Muscle memory wikipedia , lookup
Aging brain wikipedia , lookup
Posterior cingulate wikipedia , lookup
Neuroesthetics wikipedia , lookup
Cerebral cortex wikipedia , lookup
Neuroeconomics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
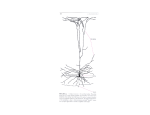
663 Imaging the premotor areas Nathalie Picard* and Peter L Strick† Recent imaging studies of motor function provide new insights into the organization of the premotor areas of the frontal lobe. The pre-supplementary motor area and the rostral portion of the dorsal premotor cortex, the ‘pre-PMd’, are, in many respects, more like prefrontal areas than motor areas. Recent data also suggest the existence of separate functional divisions in the rostral cingulate zone. Addresses Department of Neurobiology, University of Pittsburgh School of Medicine, W1640 Biomedical Science Tower, 200 Lothrop Street, Pittsburgh, PA 15261, USA *e-mail: [email protected] † e-mail: [email protected] Current Opinion in Neurobiology 2001, 11:663–672 0959-4388/01/$ — see front matter © 2001 Elsevier Science Ltd. All rights reserved. Abbreviations CCZ caudal cingulate zone CMAd dorsal cingulate motor area CMAr rostral cingulate motor area CMAv ventral cingulate motor area FEF frontal eye field fMRI functional magnetic resonance imaging M1 primary motor cortex PMd dorsal premotor cortex PMv ventral premotor cortex RCZ rostral cingulate zone RCZa anterior rostral cingulate zone RCZp posterior rostral cingulate zone SMA supplementary motor area VCA line level of the anterior commissure Introduction Large regions of the brain located on the lateral surface and on the medial wall of each hemisphere participate in the generation and control of movement. The premotor areas in the frontal lobe have the anatomical substrate to influence motor output, both through connections with the primary motor cortex (M1) and through direct projections to the spinal cord (e.g. [1]). In monkeys, the frontal lobe contains six well-defined premotor areas (Figure 1a). The presence of analogous areas in humans has been inferred from functional imaging studies (Figure 1b) [2,3]. However, the definition of premotor areas in humans is still evolving. Some associations between anatomy and function proposed in the past [2] have been validated by recent imaging data, and other associations are emerging. In this review, we present the results of salient imaging studies that have helped to increase our understanding of the underlying anatomical and functional organization of the premotor areas. Much recent effort in the field of functional imaging has been to examine brain regions involved in cognitive operations. The interpretation of activations in premotor cortex during cognitive operations depends critically on a clear definition of the location and boundaries of these cortical areas. Thus, one focus of our review synthesizes the new data that is relevant to this issue. Medial wall Pre-supplementary motor area and supplementary motor area In monkeys, it is now established that area 6 on the medial wall of the brain contains two separate areas: the supplementary motor area proper (SMA) in the caudal portion of area 6, and the pre-SMA in the rostral portion (Figure 1a; reviewed in [2,4]). The SMA and pre-SMA are equivalent to fields F3 and F6 described by Matelli et al. [5]. In humans, the level of the anterior commissure (VCA line) [6] marks the border between the two areas. The division of medial area 6 into two distinct fields is based on a collection of anatomical and functional data [2,4]. Because the pre-SMA was born out of a premotor area — the traditional SMA of Woolsey et al. [7] — the common perception is that the pre-SMA is also a motor area. However, the connectivity and physiology of the pre-SMA suggest that it is more like a prefrontal area than a motor area. Prefrontal areas provide cognitive, sensory or motivational inputs for motor behavior, whereas the motor areas are concerned with more concrete aspects of movement (e.g. muscle patterns). Two important differences in the anatomical connections of the SMA and pre-SMA support this view. First, only the SMA is directly connected to M1 and to the spinal cord [1,8–11]. Second, only the pre-SMA is interconnected with the prefrontal cortex [12–14]. These anatomical features, among others, are reflected by differential patterns of activity in the SMA and pre-SMA [4]. In neuroimaging studies, the pre-SMA/SMA distinction is the clearest example of an anatomical and functional dissociation that emerged from the primate model [2]. In the following, we describe recent findings that provide further support for this dissociation. Initially, studies in monkeys suggested that the pre-SMA has involved in learning sequential movements [15–17]. This motor view of pre-SMA function was supported by the observation that, in humans, activation of the pre-SMA increased in parallel to acquisition of a motor sequence task [18]. In contrast, SMA activation during the same task was related simply to aspects of motor execution. More recent studies by the same group have resulted in a modified view of pre-SMA function. Sakai et al. [19] recently compared pre-SMA activation in closely matched tasks that all required visuo–motor associations, but varied in motor and perceptual sequence components. Activation of the pre-SMA occurred in all tasks related to visuo–motor association demands rather than to sequential components. In fact, pre-SMA activation was greatest in the conditional task, in which non-sequential responses were arbitrarily 664 Motor systems Figure 1 (a) (b) PreSMA SMA Pre- SMA M1 SMA CCZ RCZp RCZa M1 CMAd CMAr CMAv PMd PrePMd PMd F4 PMv PMv F1 FEF M1 FEF M1 F7 F2 Motor areas of the frontal lobe in monkeys (a) and homologous areas in the human (b). In humans, the border between areas 6 and 4 on the lateral surface is located in the anterior bank of the central sulcus [65]. For illustration, the border is drawn on the surface of the hemisphere along the central sulcus (bottom, white dotted line). Except for the most medial portion, M1 does not occupy the precentral gyrus. Pictures of the monkey brain courtesy of Richard P Dum; pictures of the human brain reproduced with permission from [93]. 45 44 F5 Current Opinion in Neurobiology determined by the color of visual cues, and showed a relative decrease in sequential paradigms. This important study shows conclusively that activation of the pre-SMA in these tasks has little to do with motor sequence learning per se. Instead, pre-SMA activation occurs in association with establishing or retrieving visuo–motor associations (see also [20,21]). The activity in the pre-SMA in association with visually cued changes between motor sequences [22,23] may also be due to the role of the pre-SMA in retrieving visuo–motor associations. motor preparation, during working memory delays of a match to sample task [26] (Figure 2c). In addition, the pre-SMA showed transient activation associated with shifts of attention to visual object features (shape, color, location) in a card sorting task [27]. Attending to various features of visual stimuli was found to activate the pre-SMA equally in another context [28]. These results suggest that pre-SMA function is more closely related to processing or maintenance of relevant sensory information than response selection or production. Additional studies have extended the involvement of the pre-SMA to associations based on auditory stimuli [24••,25] (Figure 2a). Visual and auditory versions of a conditional choice reaction time paradigm generated pre-SMA activations of equal magnitude [25]. Thus, the contribution of the pre-SMA to sensory–motor associations is modality-independent. In addition, the contribution of the pre-SMA appears to be effector-independent. Similar regions are activated in conditional manual motor [24••,25] and oculomotor tasks [21] (Figure 2b). This is consistent with the poor somatotopic organization of the pre-SMA [2] and supports the view that the pre-SMA operates at a more abstract level. In contrast, SMA activation displayed only movement-related activation in visual or auditory tasks. Overall, neuroimaging studies leave no doubt that the pre-SMA is fundamentally different from the SMA. Activation of the SMA, caudal to the VCA line, is observed primarily in relation to aspects of movement behavior. In contrast, pre-SMA activation, rostral to the VCA line, is associated with cognitive aspects of a variety of tasks (see also [29–34]). These findings, together with anatomical results, suggest that the pre-SMA might be functionally considered a region of prefrontal cortex. It must be emphasized that pre-SMA activation during conditional motor behaviors was not due to response selection or preparation [19,24••]. In fact, activation patterns of the pre-SMA during non-conditional behavior suggest that its contribution to sensory–motor association is detached from motor aspects of the task. For example, the pre-SMA displayed sustained activation, independent of Cingulate motor areas The cingulate sulcus contains three separate motor areas in primates: the rostral cingulate motor area (CMAr), the caudal cingulate motor area in the ventral bank of the sulcus (CMAv) and the caudal cingulate motor area in the dorsal bank of the sulcus (CMAd; Figure 1a). We have proposed [2] that corresponding areas are also present in humans: a rostral cingulate zone (RCZ) with two subdivisions (anterior: RCZa, and posterior: RCZp) and a caudal cingulate zone (CCZ; Figure 1b). The anatomical variability of the cingulate sulcus in humans complicates functional analysis of this region [35–37]. As a consequence, a degree of Imaging the premotor areas Picard and Strick 665 Figure 2 Anatomical and functional distinction of the SMA and the pre-SMA in three different tasks. (a) Top: activation in the SMA and pre-SMA during an auditory conditional task; bottom: selective activation in the pre-SMA during an auditory–motor association task. Reproduced with permission from [24••]. (b) Top: Activation in the supplementary eye field (SEF) during visually guided saccades; bottom: selective activation of the pre-SMA for a mixed condition of compatible and incompatible (Stroop-like) conditional saccades. Reproduced with permission from [21]. (c) Top: motor-related activations in the SMA and CCZ; bottom: sustained activation during working memory delay in the pre-SMA and RCZ. Reproduced with permission from [26]. (a) PreSMA (b) SMA (c) SMA SEF CCZ PreSMA uncertainty in the definition of the cingulate motor areas remains. However, on the basis of results from recent experiments, consistent patterns of activation are found in the CCZ, and a new view of the RCZ is emerging. The CCZ, which appears to be comparable to the CMAd of monkeys, is activated primarily in relation to movement execution ([2,3,26,38••], see also [39]). Painful stimuli activate an area in or near the CCZ ventral to the area associated with movement execution [38••]. Thus, different functional areas may exist for movement and for painful stimuli. The site of movement-related activation in the CCZ is remarkably similar across studies. For example, in two different studies, finger movements activate virtually identical sites in the CCZ [26,38••] (Figure 2c). In addition, activation in the CCZ is consistently separable from that in the SMA. These observations reinforce the view that the CCZ is distinct from the SMA, despite the proximity of the two areas and their tendency to be coactivated during manual tasks [40]. In the monkey, the functional dissociation of the CMAd from the SMA occurs under specific behavioral conditions [41]. Thus, future studies are likely to find important functional differences between the human CCZ and SMA. Recent studies confirm that movement leads to activation in the RCZ, rostral to the VCA line [26,38••]. Movementrelated activation in this region is best demonstrated in analyses of single subjects [3,37,38 ••], presumably because of anatomical variability. The activation in these studies is clustered near the cingulate sulcus or its ramifications. Similarly, the RCZ activation associated with a word generation task is located in or near the cingulate or paracingulate sulci and never extends onto the cingulate gyrus [37]. Thus, some motor and cognitive functions may share a common substrate within the RCZ. In contrast, activations related to attention or arousal are located at more rostral and ventral locations [38••,42], which are probably outside the RCZ on the cingulate gyrus proper. PreSMA PreSMA RCZ Current Opinion in Neurobiology Two theories predominate about the overall function of this region of cortex: ‘conflict monitoring’ [43] and ‘attention/selection for action’ [44]. The first theory emphasizes the evaluative function of the RCZ, whereas the second emphasizes its motor function ([45•], but see also [46]). How do these two theories and functions relate to the motor area(s) in the RCZ? We propose that they reflect the properties of two different functional regions: conflict monitoring within the RCZa and selection for action within the RCZp. For example, a series of imaging studies that were carefully designed to control conflict monitoring, found activation in the ‘anterior cingulate’ cortex [43,45•,47,48•]. On average, the sites of these activations were located 24 mm ± 7 mm (mean ± standard deviation) anterior to the VCA line (Table 1). This location corresponds to the RCZa, which may correspond to the CMAr of monkeys [2]. In contrast, when some of the same investigators used a similar paradigm that did not specifically dissociate conflict monitoring from response selection, they found response-related activation in the area of the ‘anterior cingulate’ cortex, located 1 mm anterior to the VCA line [49]. This site corresponds to the RCZp, which may correspond to the CMAv of monkeys [2]. The results of other imaging studies can be interpreted within the same anatomical–functional framework. Two groups of investigators, using single subject analysis, found multiple foci of activation in the ‘anterior cingulate’ cortex during word generation tasks that involved response selection [37,42] (Figure 3a). In both instances, the major site of activation was located largely within the RCZp. Tasks that can be interpreted as involving both conflict processing and response selection (versions of Go/NoGo and STOP tasks) evoked distinct sites of activation in the ‘anterior cingulate’ cortex [50•,51]. In a Go/NoGo task, different cues (e.g. green or red stimuli) each determine a particular response (Go or NoGo). Thus, this task is similar to a conditional visuo–motor association task. Because only one cue is presented for each response to produce (no interference) and because a cue is always associated with a single response, 666 Motor systems Figure 3 Table 1 Talairach coordinates [6] of activation sites in the RCZ. (a) PreSMA Ref Task Type x [43] Flanker task: previous trial type effect Conflict –2 31 29 0 15 41 3 28 29 [48•] Flanker task: congruency x expectancy Conflict –8 22 32 [92] Delayed recognition: high interference vs no interference Conflict 6 22 43 [50•] STOP task vs control Conflict 6 25 37 Conflict & 3 selection 3 31 14 37 42 [45•] RCZ Stroop task: high conflict & low control Conflict vs low conflict & high control [47] Verb generation: high vs low response competition VCA (b) (STOP task vs control) & (Go/NoGo vs control) RCZa Anatomical and functional division of the RCZ. (a) Activation during free word generation. The dashed line separates activations located in the preSMA and in the RCZ. Reproduced with permission from [37]. (b) Activation related to conflict monitoring. Brain images from the two studies were matched in size in the anterior–posterior axis. Dotted lines in (b) represent the estimated locations of levels indicated in (a). Activations related to conflict monitoring are located at the rostral edge of activations found during word generation. This topography suggests that the RCZa is involved in conflict monitoring (evaluative function) whereas the RCZp is involved in response selection. Note that all cingulate activations are in sulci, not on the cingulate gyrus, supporting the view that they overlap with cingulate motor areas. Reproduced with permission from [43]. conflict is negligible in Go/NoGo tasks in which response selection is required. Activation in the RCZ during the Go/NoGo task was located, on average, 7.5 ± 9 mm in front of the VCA line (Table 1). This site falls within the RCZp [2]. In addition, activation in the RCZp was larger during the Go/NoGo task than during the STOP task. In STOP tasks, a cued Go response is prevented by the delayed presentation of a second cue signaling NoGo. Thus, STOP tasks present a high degree of response conflict because contradictory instructions determine the subject’s response. The RCZ activation in STOP tasks, like that in other high conflict tasks, was located in a separate focus 25 mm rostral to the VCA line (in the RCZa) [50•] (Table 1). In sum, these data support the existence of at least three functional areas in the anterior portion of the cingulate z [50•] Go/NoGo vs control Selection –3 0 42 [50•] Selection –6 0 42 Selection 4 46 Go/NoGo vs STOP task [51] Go/NoGo vs Go Current Opinion in Neurobiology Conflict y 16 sulcus: the CCZ and two subdivisions within the RCZ. The CCZ appears to be activated during simple motor tasks, whereas the RCZa and the RCZp appear to be differentially involved in conflict monitoring and response selection. Overall, proposals about the RCZ are founded on a relatively small number of studies. Further research is necessary to fully define the function(s) of these cortical regions. Lateral surface The dorsal part of the lateral premotor cortex In monkeys, the dorsal part of the lateral premotor cortex (PMd) has been divided into rostral (F7, PMdr) and caudal (F2, PMdc) subdivisions [5,52], on the basis of anatomical and physiological differences (Figure 1a). These differences are analogous to those that generate the pre-SMA/SMA split. In fact, in a number of important respects, the caudal portion of the PMd has much in common with the SMA proper. Both areas project to the primary motor cortex and directly to the spinal cord [1,8,52,53]. Neither area has substantial interconnections with prefrontal cortex [14]. Neurons in both regions are primarily involved in aspects of motor control [4]. In contrast, the rostral portion of the PMd has much in common with the pre-SMA. Neither of these areas projects to the primary motor cortex or to the spinal cord [1,8,52,53]. Instead, both regions are interconnected with areas of prefrontal cortex and with the reticular formation [4,14,54]. Interestingly, neither the pre-SMA nor the PMdr appears to have substantial interconnections with the SMA proper or with the caudal portion of the PMd [11,13,55]. Furthermore, the results of neuronal recording and functional imaging studies suggest that the pre-SMA and the rostral portion of the PMd are more involved in cognitive than in motor processes (see below). On the basis of these findings, we believe that there is heuristic value in adopting a new terminology to refer to these cortical areas. Imaging the premotor areas Picard and Strick 667 Figure 4 Anatomical and functional division of dorsolateral area 6. (a) Top: activation of the PMd proper during execution of finger flexion/extension movements; bottom: activation of the pre-PMd during imagined movements of the fingers. Reproduced with permission from [31]. (b) Activation of the pre-PMd related to spatial attention/memory is shown in red, activation of the PMd on the precentral gyrus related to movement preparation is shown in yellow, and the overlap is shown in green. Reproduced with permission from [59••]. (a) PMd (b) PrePMd PrePMd PMd Current Opinion in Neurobiology We suggest that the rostral portion of the PMd (F7) be termed the pre-PMd, and that the caudal portion of the PMd (F2) be termed the PMd proper. This terminology more clearly reflects the parallels between these areas and the pre-SMA and SMA proper. Recent studies in monkeys further support the distinction between the pre-PMd and the PMd proper. Parietal cells that project to the pre-PMd convey eye movement signals, whereas those that project to the PMd convey hand movement signals [56,57]. The two subdivisions can be further characterized on the basis of their differential involvement in oculomotor control [58]. Eye movements are evoked by stimulation of pre-PMd, whereas predominantly limb and body movements are evoked from the PMd. Similarly, neurons in the pre-PMd are more active during a saccade task than during a limb movement task. In contrast, the reverse is the case for neurons in the PMd. Thus, there is ample evidence that the pre-PMd and the PMd are anatomically and physiologically distinct [59••]. The functional distinction between the pre-PMd and the PMd has not always been obvious in imaging studies of human subjects. However, we have found that a consistent pattern emerges when M1 activation related to hand movement is used as an ‘anchor point’ and activations in premotor cortex, relating to other more cognitive types of movement in the same subjects, are measured in relation to this point. The data in Table 2 illustrates that, in a recent group of studies, movement performance tasks produced activations more caudally in the PMd than more cognitively demanding tasks. Movement-related activations were located on average 8.1 mm rostral to the center of M1 activation in the same subjects [31,32,60–64]. This value is strikingly similar to the location of the hand representation on the precentral gyrus. In humans, the precentral gyrus corresponds to the premotor cortex (area 6) [65], which is 8.8 mm anterior to the hand representation in the central sulcus [66]. Over a relatively large number of studies, the average of the absolute coordinates for movement-related activation in the PMd and the location of the hand representation on the precentral gyrus [66] are very similar (Table 2). Conversely, activations related to higher-order processing (e.g. conditional visuo–motor associations, response selection or motor imagery) are located in the prePMd an average of 22.9 mm anterior to movement-related activation in M1 [25,31,61,67]. The rostro-caudal gradient of activation across the pre-PMd–PMd is most convincingly demonstrated within subjects [24••,25,31,59••,61,67–69] (Figure 4). For example, activation that specifically relates to sensory–motor association in an auditory conditional task is restricted to the rostral edge of area 6 [24••]. The site of this activation is likely to be in the pre-PMd, rather than in the PMd. Similarly, activation in other conditional motor tasks is located in the pre-PMd, 18–26 mm anterior to movementrelated activation in M1 [25,67] (Table 2). In addition, rostral portions of area 6 in the pre-PMd are activated during the presentation of visual cues or movement imagination, whereas caudal portions of area 6 in the PMd are activated during movement preparation or execution [59••,61,69]. This interpretation is compatible with the response properties of neurons seen in recording studies of 668 Motor systems Table 2 Talairach coordinates [6] of activation sites in the PMd relative to movement-related activations in M1*. Refs Contrast Type [25] Choice RT (auditory mode): (control-rest)∩(response uncertainty-rest)∩ (time uncertainty-rest)∩(dual uncertainty-rest) Movement-related PMd x, y, z M1 x, y, z ∆y ∆z –2 –42,–18,48 Choice RT (auditory mode): response uncertainty-control Higher-order –54,6,46 24 Choice RT (auditory mode): time uncertainty-control Higher-order –50,4,42 22 –6 Choice RT (auditory mode): dual uncertainty-control Higher-order –52,6,48 24 0 0 Choice RT (visual mode): (control-rest)∩(response uncertainty-rest)∩ (time uncertainty-rest)∩(dual uncertainty-rest) Movement-related Choice RT (visual mode): response uncertainty-control Higher-order –50,8,50 24 Choice RT (visual mode): time uncertainty-control Higher-order –44,2,50 18 0 Choice RT (visual mode): dual uncertainty-control Higher-order –52,10,46 26 –4 15 12 0 –3 21 21 –15 –6 12 6 0 12 21 24 –15 –15 [31] Finger movement-rest –36,–16,50 Movement-related –36,–3,66 42,–3,60 Imagined finger movement-rest Higher-order (finger movement-rest)-(imagined movement-rest) Movement-related –36,–6,69 24,–9,75 (imagined movement-rest)-(finger movement-rest) Higher-order –42,–18,66 39,–15,63 –42,3,51 42,6,57 –42,–18,69 39,–15,63 –42,3,51 48,9,48 [32] Self-paced finger movement-rest Movement-related –12,–14,60 –34,–18,56 4 4 [60] Object manipulation-rest Movement-related –40,–16,52 –46,–32,50 16 2 [61] Event-related: finger movement period Movement-related –38,–4,60 –36,–8,54 36,–20,58 16 12 2 –4 26 12 [62] Conditional visual-perceptual control Event-related: instruction & delay periods Movement-related –34,–14,62 Higher-order –18,6,70 –42,–28,52 14 10 [63] Hand movement-rest Movement-related 32,–29,64 32,–29,54 0 10 –39,–29,63 –35,–27,53 6 –2 –1 –3 Movement-related –41,–23,62 –57,–21,48 –36,–23,53 0 2 9 –5 Higher-order –33,–3,48 –33,–27,55 24 –7 Movement-related 37.3,–14.4, 60.3 38.2,–22.2, 56.9 8.1 2.4 4.0,5.9,6.5 6.5 5.8 [64] Hand reaching: (eye-arm-jump & eye-arm-stationary)-(eye-jump & eye-stationary) Movement-related –41,–23,62 –53,–29,50 Hand reaching: (eye-arm-stationary)-(eye-stationary) [67] Conditional grip-mandatory grip Average† (n=14) Standard deviation† Average† (n=13) Standard deviation† [66] High resolution fMRI & 3D reconstruction: finger movement-rest Average (n=5) Standard deviation 10.9,9.3,7.4 Higher-order 43.9,5.0,50.6 22.9 –4.8 10.1,3.5,7.1 2.4 7.9 Movement-related –35.5,–14.6, –36.6,–23.4, 8.8 8.5 65.3 6.0,4.5,5.6 56.8 7.6,4.1,9.4 *Only hand movement tasks were considered in this comparison. † Absolute values used for calculation of x averages and standard deviations. ∩, intersection. awake trained monkeys [59••,70,71]. The PMd contains a high proportion of neurons that display set- and movement-related activity. In contrast, neurons in the pre-PMd are more responsive to sensory cues, and fewer are active in relation to movement. Thus, neuroimaging studies and neuron properties indicate that the PMd is primarily involved in aspects of movement preparation or generation (see also [72,73]), whereas the functions of the pre-PMd are more closely related to cognitive processes than to motor processes. The ventral part of the lateral premotor cortex In monkeys, the ventral part of the lateral premotor cortex (PMv) lies in area 6 below the spur of the arcuate sulcus (Figure 1a). The PMv, like the PMd proper, is densely interconnected with M1. In addition, the portion of the PMv in and adjacent to the caudal bank of the arcuate sulcus contains neurons that project directly to the spinal cord [1,53]. There is general agreement that the PMv in humans lies ventral to the frontal eye field (FEF). However, the precise location and boundaries of the PMv in humans is uncertain [3,74•]. Imaging the premotor areas Picard and Strick Matelli et al. [75] suggest that the PMv of monkeys consists of two cytoarchitectonic fields, F4 and F5 (Figure 1a). The connections of F4 with posterior parietal cortex and the responses properties of F4 neurons have led Rizzolatti et al. [76] to suggest that F4 is involved in ‘transforming object locations into appropriate movements towards them’ (see also [77]). This hypothesis has not been explored by imaging studies in humans. Furthermore, the location or even the existence of a human equivalent of the monkey F4 remains to be established. The F5 portion of the PMv has received more attention. Rizzolatti et al. [76] have described two types of neurons in monkey F5 that have unique responses to visual stimuli: ‘canonical’ and ‘mirror’ neurons [4,76,78,79]. Canonical neurons respond to the visual presentation of three-dimensional objects of different size and shape. The motor responses of these neurons are limited to specific goal-directed actions towards these three-dimensional objects. Canonical neurons are largely found in the portion of the PMv that is buried in the bank of the arcuate sulcus. Mirror neurons are active when a monkey watches someone else perform a specific action, and when the monkey executes a similar action. Mirror neurons are largely found in the portion of the PMv that is on the cortical surface, caudal to the arcuate sulcus. The unique properties of mirror neurons have prompted the search for the equivalent of F5 in human subjects. Neuroimaging studies have generated conflicting results on this issue. In a recent meta-analysis, Grèzes and Decety [74•] failed to find conclusive evidence for neuronal activity in inferior frontal areas, including ventral portions of area 6, during action observation. In contrast, other studies have reported activation of area 44 during imitation and observation of finger movements [80,81], as well as when objects are gripped and manipulated [60,82,83]. This has led some investigators to suggest that area 44, which has traditionally been considered part of Broca’s area, is the human equivalent of the monkey F5 [76,84]. Other results raise doubts over this conclusion. Amunts et al. [85] found considerable variation in the location of the border between area 44 and rostro-ventral area 6, and noted that this border cannot be determined solely on the basis of gyral and sulcal landmarks. Area 44 is strongly activated during tasks that involve silent or covert speech (e.g. [86–88]). Thus, it is possible that activation of area 44 during action observation, in some cases, reflects internal verbalization of the observed action (e.g. [74•]). This view is compatible with the absence of activation in Broca’s area during the observation of meaningless hand gestures that cannot be named [89,90]. However, verbalization alone cannot account for all activations related to action observation, which are also found in parts of premotor cortex clearly outside of Broca’s area [74•,89–91]. A recent report by Buccino et al. [91] indicates that observation of recognizable face, hand or foot actions resulted in 669 a somatotopically organized pattern of activation that involved all of the premotor cortex on the lateral surface (i.e. both the PMd and the presumed PMv). These authors also observed activation in or near Broca’s area in the action observation tasks. Thus, the relationship between the activation in area 44 and that observed in portions of area 6 is unclear. Importantly, these results indicate that the human PMv can’t be identified solely by the presence of activation during movement observation tasks. In summary, on the basis of previous data, it is not possible to establish a definite correspondence between the functional subdivisions of the monkey PMv and the inferior frontal areas in humans. Conclusions The data presented in this review help to clarify the location and boundaries of the premotor areas in the frontal lobe of humans. For many of these areas, there is a clear correspondence with a specific premotor area in the monkey. For other regions, such as the motor fields in inferior frontal cortex, the correspondence with areas in the monkey brain remains to be established. Two regions have generally been considered to be motor fields: the rostral part of area 6 on the medial wall of the hemisphere (pre-SMA), and the rostral part of area 6 on the dorsal surface (pre-PMd). However, both these areas display activation that is more closely related to cognitive than to motor processes. Thus, it might be more appropriate to consider the pre-SMA and the pre-PMd as functional components of prefrontal cortex, rather than as premotor areas. In other cases, cognitive and motor functions appear to have a common anatomical substrate. The RCZa, which may correspond to the CMAr of monkeys, is involved in ‘conflict monitoring’. The RCZp, which may correspond to the CMAv of monkeys, appears to be involved in response selection. Other premotor areas, including the SMA proper, the PMd proper and the CCZ are involved in various aspects of movement generation and control. Overall, we feel that it is important to continue to seek correspondences between motor areas in the monkey and human cortex, as this will result in important insights into the functional organization of the frontal lobe. Acknowledgements Our work is supported by the Veterans Administration Medical Research and Rehabilitation Research and Development Services (PL Strick), and United States Public Health Service grant NS24328 (PL Strick). References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: • of special interest •• of outstanding interest 1. Dum RP, Strick PL: The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 1991, 11:667-689. 2. Picard N, Strick PL: Medial wall motor areas: a review of their location and functional activation. Cereb Cortex 1996, 6:342-353. 3. Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE: Multiple nonprimary motor areas in the human cortex. J Neurophysiol 1997, 77:2164-2174. 4. Geyer S, Matelli M, Luppino G, Zilles K: Functional neuroanatomy of the primate isocortical motor system. Anat Embryol 2000, 202:443-474. 670 Motor systems 5. Matelli M, Luppino G, Rizzolatti G: Architecture of superior and medial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 1991, 311:445-462. 6. Talairach J, Tournoux P: Coplanar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. 7. Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM: Patterns of localization in precentral and ‘supplementary’ motor areas and their relation to the concept of a premotor area. Assoc Res Nerv Ment Disord 1952, 30:238-264. 8. Muakkassa KF, Strick PL: Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res 1979, 177:176-182. 9. Dum RP, Strick PL: Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 1996, 16:6513-6525. 10. He SQ, Dum RP, Strick PL: Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci 1995, 15:3284-3306. 11. Wang Y, Shima K, Sawamura H, Tanji J: Spatial distribution of cingulate cells projecting to the primary, supplementary, and presupplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neurosci Res 2001, 39:39-49. 12. Bates JF, Goldman-Rakic PS: Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 1993, 336:211-228. 13. Luppino G, Matelli M, Camarda R, Rizzolatti G: Corticocortical connections of area F3 (SMA-proper) and area F6 (Pre-SMA) in the macaque monkey. J Comp Neurol 1993, 338:114-140. 14. Lu MT, Preston JB, Strick PL: Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol 1994, 341:375-392. and a response selection control task. A similar dissociation is found in the dorsal premotor cortex. 25. Sakai K, Hikosaka O, Takino R, Miyachi S, Nielsen M, Tamada T: What and when: parallel and convergent processing in motor control. J Neurosci 2000, 20:2691-2700. 26. Petit L, Courtney SM, Ungerleider LG, Haxby JV: Sustained activity in the medial wall during working memory delays. J Neurosci 1998, 18:9429-9437. 27. Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J et al.: Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage 1999, 10:193-199. 28. Schubotz RJ, von Cramon DY: Functional organization of the lateral premotor cortex: fMRI reveals different regions activated by anticipation of object properties, location and speed. Cogn Brain Res 2001, 11:97-112. 29. Deiber MP, Honda M, Ibañez V, Sadato N, Hallett M: Medial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 1999, 81:3065-3077. 30. Lee KM, Chang KH, Roh JK: Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 1999, 9:117-123. 31. Gerardin E, Sirigu A, Lehéricy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D: Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 2000, 10:1093-1104. 32. Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ: Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 2000, 123:1216-1228. 15. Nakamura K, Sakai K, Hikosaka O: Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol 1998, 80:2671-2687. 33. Crosson B, Sadek JR, Maron L, Gökçay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW: Relative shift of activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci 2001, 13:272-283. 16. Nakamura K, Sakai K, Hikosaka O: Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol 1999, 82:1063-1068. 34. Schubotz RI, von Cramon DY: Interval and ordinal properties of sequences are associated with distinct premotor areas. Cereb Cortex 2001, 11:210-222. 17. 35. Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D’Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC: In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 1996, 376:664-673. Shima K, Tanji J: Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol 1998, 80:3247-3260. 18. Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Pütz B: Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 1996, 76:617-621. 19. Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Pütz B: Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci 1999, 19:RC1-6. 20. Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J: The effect of stimulus-response compatibility on cortical motor activation. Neuroimage 2001, 13:1-14. 21. Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA: Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage 2001, 13:794-800. 22. Shima K, Mushiake H, Saito N, Tanji J: Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 1996, 93:8694-8698. 23. Jäncke L, Himmelbach M, Shah NJ, Zilles K: The effect of switching between sequential and repetitive movements on cortical activation. Neuroimage 2000, 12:528-537. 24. Kurata K, Tsuji T, Naraki S, Seino M, Abe Y: Activation of the dorsal •• premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J Neurophysiol 2000, 84:1667-1672. The anatomical and functional dissociation of the SMA and pre-SMA is clearly shown in this study of conditional motor behavior. The results extend the involvement of the pre-SMA to sensory–motor associations of auditory stimuli. Only the pre-SMA showed activation specific to conditional associations. In contrast, the SMA was activated equally for the conditional task 36. Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC: Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex 1996, 6:207-214. 37. Crosson B, Sadek JR, Bobholz JA, Gökçay D, Mohr CM, Leonard CM, Maron L, Auerbach EJ, Browd SR, Freeman AJ, Briggs RW: Activity in the paracingulate and cingulate sulci during word generation: an fMRI study of functional anatomy. Cereb Cortex 1999, 9:307-316. 38. Kwan CL, Crawley AP, Mikulis DJ, Davis KD: An fMRI study of the •• anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 2000, 85:359-374. Activations on the medial wall of the hemisphere related to the processing of painful or innocuous thermal stimuli and during a motor task (finger to thumb opposition) were evaluated with functional magnetic resonance imagine (fMRI). The single subject analyses of this study reveal a level of functional organization of the medial wall that is difficult to see in most group analyses. The results confirm the motor role of the CCZ, as well as that of the SMA. An important observation is that activations related to noxious stimuli in the caudal portion of the ‘anterior cingulate’ (behind the VCA line) were located ventral to the area of CCZ activation related to hand movements and, thus, may be located in a different functional area. Innocuous thermal stimulations activated primarily a rostral and ventral cingulate region near the genu of the corpus callosum. It is argued that these activations are nonspecifically related to arousal or attention. 39. Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE: Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 1999, 19:6134-6144. Imaging the premotor areas Picard and Strick 671 40. Koski L, Paus T: Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain mapping metaanalysis. Exp Brain Res 2000, 133:55-65. 54. Keizer K, Kuypers HG: Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 1989, 74:311-318. 41. Picard N, Strick PL: Activation on the medial wall during remembered sequences of reaching movements in monkeys. J Neurophysiol 1997, 77:2197-2201. 55. Kurata K: Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res 1991, 12:263-280. 42. Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ: Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 1997, 77:3370-3380. 56. Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R: Eye–hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex 2001, 11:513-527. 43. Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD: Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999, 402:179-181. 57. 44. Petersen SE, Fox PT, Posner MI, Mintum M, Raichle ME: Positron emission tomographic studies of the cortical anatomy of singleword processing. Nature 1988, 331:585-589. 45. Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger A, Noll D, • Cohen JD: Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000, 97:1944-1948. The evaluative function of the RCZa in conflict monitoring was demonstrated using the Stroop task and event-related fMRI. Strategic and evaluative functions were dissociated by manipulating stimulus-response compatibility and expectancy on a trial-by-trial basis. The RCZa showed patterns of activation that reflected the degree of conflict present on individual trials. However, the RCZa was less activated when strategic processes were engaged and conflict reduced. 46. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001, 2:417-424. 47. Barch DM, Braver TS, Sabb FW, Noll DC: Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci 2000, 12:298-309. 48. Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, • Jennings JR, Crone EA: Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA 2000, 97:8728-8733. The authors show that attentional conflict and selection are separate components, by examining the patterns of activation in distributed brain systems, using fMRI and the Flanker task. Activation in the RCZa and in the dorsolateral prefrontal cortex was correlated with response conflict. Other regions of the brain had activity patterns corresponding to simple violation of expectations (basal ganglia, insula) or selective visuo-spatial attention (area 8, superior parietal lobule, cerebellum). 49. MacDonald AW III, Cohen JD, Stenger VA, Carter CS: Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288:1835-1838. 50. Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, • Sharma T, Simmons A, Williams SCR, Giampietro V, Andrew CM, Taylor E: Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 2001, 13:250-261. An interesting finding of this study is the differential location of activation within the RCZ for Go/NoGo and STOP tasks. The tasks differ in terms of response conflict. Go/NoGo tasks are similar to conditional motor association tasks, in which motor responses are determined by arbitrary cues. Thus, these tasks engage response selection processes and present relatively low conflict. STOP tasks present a higher conflict situation because of the direct competition between Go and NoGo cues for the production of a single response. Activation in the RCZp was greater for the Go/NoGo task. This observation supports the proposed role of the RCZp in response selection. A separate focus of activation was found in the RCZa during both tasks. This location corresponds to the region of the anterior cingulate cortex associated with conflict monitoring. Consistent with its role in establishing or retrieving sensory–motor associations, the pre-SMA was activated in all tasks. Areas of prefrontal and parietal cortex were also activated during Go/NoGo or STOP tasks. 51. Menon V, Adleman NE, White CD, Glover GH, Reiss AL: Errorrelated brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 2001, 12:131-143. 52. Barbas H, Pandya DN: Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 1987, 256:211-228. 53. He SQ, Dum RP, Strick PL: Topographic organization of corticospinal projections from the fontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 1993, 13:952-980. Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R: Eye–hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 2001, 11:528-544. 58. Fuji N, Mushiake H, Tanji J: Rostrocaudal distinction of the dorsal premotor area based on oculomotor involvement. J Neurophysiol 2000, 83:1764-1769. 59. Boussaoud D: Attention versus intention in the primate premotor •• cortex. Neuroimage 2001, 14:S40-S45. Here, the author presents anatomical and physiological evidence for the presence of distinct functional subdivisions in dorsolateral area 6. In trained monkeys, neurons in the caudal subdivision (PMd proper) are primarily active in relation to movement preparation. Conversely, neurons in the rostral subdivision (pre-PMd) are primarily active in relation to spatial attention/memory cues. Interestingly, the same dissociation was shown in a parallel fMRI study in humans using a similar task. 60. Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ: A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci 1999, 11:3276-3286. 61. Toni I, Schluter ND, Josephs O, Friston K, Passingham RE: Signal-, set- and movement-related activity in the human brain: an eventrelated fMRI study. Cereb Cortex 1999, 9:35-49. 62. Toni I, Passingham RE: Prefrontal-basal ganglia pathways are involved in the learning of arbitrary visuomotor associations: a PET study. Exp Brain Res 1999, 127:19-32. 63. Ehrsson HH, Naito E, Geyer S, Amunts K, Zilles K, Forssberg H, Roland PE: Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: a PET study. Eur J Neurosci 2000, 12:3385-3398. 64. Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST: Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci 2001, 21:2919-2928. 65. White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D: Structure of the human sensorimotor system. I. Morphology and cytoarchitecture of the central sulcus. Cereb Cortex 1997, 7:18-30. 66. Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM: Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 2000, 84:558-569. 67. Grafton ST, Fagg AH, Arbib MA: Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J Neurophysiol 1998, 79:1092-1097. 68. Iacoboni M, Woods RP, Mazziotta JC: Bimodal (auditory and visual) left frontoparietal circuitry for sensorimotor integration and sensorimotor learning. Brain 1998, 121:2135-2143. 69. Pochon JB, Levy R, Poline JB, Crozier S, Lehéricy S, Pillon B, Deweer B, Le Bihan D, Dubois B: The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex 2001, 11:260-266. 70. Di Pellegrino G, Wise SP: A neurophysiological comparison of three distinct regions of the primate frontal lobe. Brain 1991, 114:951-978. 71. Johnson PB, Ferraina S, Bianchi L, Caminiti R: Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 1996, 6:102-119. 72. van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE: Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol 1998, 80:2177-2199. 672 Motor systems 73. Horwitz B, Deiber MP, Ibáñez V, Sadato N, Hallett M: Correlations between reaction time and cerebral blood flow during motor preparation. Neuroimage 2000, 12:434-441. 83. Ehrsson HH, Fagergren A, Forssberg H: Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 2001, 85:2613-2623. 74. Grèzes J, Decety J: Functional anatomy of execution, mental • simulation, observation, and verb generation of actions: a metaanalysis. Hum Brain Mapp 2001, 12:1-19. This meta-analysis shows the involvement of the dorsal and ventral premotor cortex in movement execution and imagination. However, the data reviewed does not support the involvement of inferior frontal areas in movement observation. Three hypotheses regarding PMv location are examined [3]: area 6 on the precentral gyrus adjacent and inferior to the FEF; opercular area 6; area 44. The review suggests that activation in Broca’s area is primarily related to overt or covert verbal processes. On the other hand, inferior area 6 was frequently activated during motor execution or imagery. 84. Rizzolatti G, Arbib MA: Language within our grasp. Trends Neurosci 1998, 21:188-194. 75. Matelli M, Luppino G, Rizzolatti G: Pattern of cytochrome oxidase activity in frontal agranular cortex of the macaque monkey. Behav Brain Res 1985, 18:125-136. 76. Rizzolatti G, Luppino G, Matelli M: The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 1998, 106:283-296. 77. Graziano MSA, Gross CG: Spatial maps for the control of movement. Curr Opin Neurobiol 1998, 8:195-201. 78. Fadiga L, Fogassi L, Gallese V, Rizzolatti G: Visuomotor neurons: ambiguity of the discharge or ‘motor’ perception? Int J Psychophysiol 2000, 35:165-177. 79. Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G: Cortical mechanism for the visual guidance of hand grasping movements in the monkey. A reversible inactivation study. Brain 2001, 124:571-586. 80. Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G: Cortical mechanisms of human imitation. Science 1999, 286:2526-2528. 81. Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, Zilles K, Seitz RJ: Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp 2000, 11:273-285. 82. Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H: Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 2000, 83:528-536. 85. Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K: Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 1999, 421:319-341. 86. Grafton ST, Fadiga L, Arbib MA, Rizzolatti G: Premotor cortex activation during observation and naming of familiar tools. Neuroimage 1997, 6:231-236. 87. Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, Jesberger JA, Lewin JS: Brain activation during silent word generation evaluated with functional MRI. Brain Lang 1998, 64:231-256. 88. Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, TzourioMazoyer N: A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage 2000, 11:347-357. 89. Grèzes J, Costes N, Decety J: The effects of learning and intention on the neural network involved in the perception of meaningless actions. Brain 1999, 122:1875-1887. 90. Hermsdörfer J, Goldenberg G, Wachsmuth C, Conrad B, CeballosBauman AO, Bartenstein P, Schwaiger M, Boecker H: Cortical correlates of gesture processing: clues to the cerebral mechanisms underlying apraxia during the imitation of meaningless gestures. Neuroimage 2001, 14:149-161. 91. Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese R, Seitz RJ, Rizzolatti G, Freund HJ: Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 2001, 13:400-404. 92. Herrmann M, Rotte M, Grubich C, Ebert AD, Schiltz K, Münte TF, Heinze HJ: Control of semantic interference in episodic memory retrieval is associated with an anterior cingulate-prefrontal activation pattern. Hum Brain Mapp 2001, 13:94-103. 93. Duvernoy HM: The Human Brain. Surface, Three-dimensional Anatomy and MRI. New York: Springer-Verlag; 1991.