* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The role of neuronal signaling in controlling cerebral blood flow
Cortical cooling wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Subventricular zone wikipedia , lookup
Neural oscillation wikipedia , lookup
Neural engineering wikipedia , lookup
Perivascular space wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Brain morphometry wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Environmental enrichment wikipedia , lookup
Synaptic gating wikipedia , lookup
Brain Rules wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neurolinguistics wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroinformatics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuropsychology wikipedia , lookup
Nervous system network models wikipedia , lookup
Neurophilosophy wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Intracranial pressure wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Human brain wikipedia , lookup
Selfish brain theory wikipedia , lookup
Optogenetics wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neuroplasticity wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
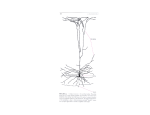
ARTICLE IN PRESS Brain and Language xxx (2006) xxx–xxx www.elsevier.com/locate/b&l The role of neuronal signaling in controlling cerebral blood Xow Carrie T. Drake ¤, Costantino Iadecola Department of Neurology and Neuroscience, Division of Neurobiology, Weill Medical College of Cornell University, 411 East 69th St., New York, NY 10128, USA Accepted 1 August 2006 Abstract Well-regulated blood Xow within the brain is vital to normal function. The brain’s requirement for suYcient blood Xow is ensured by a tight link between neural activity and blood Xow. The link between regional synaptic activity and regional cerebral blood Xow, termed functional hyperemia, is the basis for several modern imaging techniques that have revolutionized the study of human brain activity. Here, we review the mechanisms of functional hyperemia and their implications for interpreting the blood oxygen level-dependent (BOLD) contrast signal used in functional magnetic resonance imaging (fMRI). © 2006 Elsevier Inc. All rights reserved. Keywords: Functional hyperemia; Cerebral blood Xow; Neurovascular unit; BOLD 1. Introduction Although it is only 2% of total body weight, the brain uses 20% of the total energy consumed by the body (SokoloV, 1989). Well-regulated blood Xow within the brain is vital to maintain energy-dependent processes and to clear metabolic byproducts produced by neuronal activity, such as CO2, excess lactate, other metabolites and heat. The dependence of the brain on blood Xow is highlighted by the fact that even relatively small reductions in cerebral blood Xow (CBF) can have deleterious eVects on the brain. SpeciWcally, a 20% reduction in CBF inhibits cerebral protein synthesis (Hossmann, 1994). A 50% CBF reduction leads to extracellular accumulation of glutamate and lactate, and water shift from intra- to extra-cellular compartments (Hossmann, 1994). CBF reductions greater than 50% impair ATP synthesis and decrease the ability of neurons to Wre action potentials. If Xow is reduced by 80%, neurons lose ionic gradients, a process termed anoxic depolarization, and undergo irreversible damage (Hossmann, 1994). * Corresponding author. Fax: +1 212 988 3672. E-mail address: [email protected] (C.T. Drake). Thus, even small reductions in CBF negatively aVect neuronal function, and large CBF reductions, such as are seen in cerebral ischemia, can produce massive damage to the brain. Moreover, cerebrovascular dysregulation is associated with Alzheimer’s disease and other neurodegenerative conditions (see (Iadecola, 2004) for review). The brain’s requirement for suYcient blood Xow is ensured by a tight link between neural activity and blood Xow. This link between regional synaptic activity and regional CBF, termed functional hyperemia, is the basis for several modern imaging techniques that have revolutionized the study of human brain activity (Logothetis & Wandell, 2004; Raichle, 1998). Here, we review the mechanisms of functional hyperemia and their implications for interpreting functional magnetic resonance imaging (fMRI) data. 1.1. Neuroanatomical basis of functional hyperemia: the neurovascular unit In both normal and injury conditions, neurons, glia and blood vessels exhibit a close anatomical and functional coupling (del Zoppo & Mabuchi, 2003; Woolsey et al., 1996). These cells can be considered a functional entity, 0093-934X/$ - see front matter © 2006 Elsevier Inc. All rights reserved. doi:10.1016/j.bandl.2006.08.002 Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 2 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx termed the neurovascular unit (Lo, Dalkara, & Moskowitz, 2003). As shown in Fig. 1, the cellular constituents of the neurovascular unit vary with the type, size and location of blood vessels. On the surface of the brain, large cerebral arteries give rise to smaller arteries and arterioles known as pial arteries (Jones, 1970). Pial arteries are composed of an endothelial cell layer, a smooth muscle cell layer and an outer layer of leptomeningeal cells (adventitia), which is separated from the brain by the Virchow-Robin space (Peters, Palay, & Webster, 1991). Pial arteries are densely innervated by perivascular nerves that originate from autonomic and sensory ganglia, mainly the sphenopalatine, trigeminal and superior cervical ganglia (Edvinsson & Hamel, 2002). These nerves contain several neurotransmitters and neuropeptides that can either constrict or dilate arteries and arterioles (Edvinsson & Hamel, 2002). Because of the diVuse nature of the innervation and the relatively large size of the vessels innervated, perivascular innervation by peripheral autonomic nerves is unlikely to play a role in the highly localized changes in Xow initiated by functional brain activity. Rather, these neurons are likely to modulate global increases in CBF that occur during epileptic seizures, hypertension, or following transient interruption of CBF (Iadecola, 1998). As pial arteries and arterioles penetrate deeper into the brain parenchyma, they are still separated from the substance of the brain by a virtual space (the Virchow-Robin space) (Fig. 1). However, recent work (Takano et al., 2006) has shown that arterioles are closely associated with astrocytic end-feet, which can inXuence arteriole diameter. Cerebral endothelial cells are specialized to form the blood–brain barrier: they lack fenestrations and are connected by small adhesions known as tight junctions. Endothelial cells are further connected by gap junctions, which allow transmission of intracellular responses between adjacent cells (Segal, 2000; Sokoya et al., 2006). Endothelial cells can release a variety of vasoactive Virchow-Robin space Perivascular nerves Smooth muscle cells Smooth muscle cell Central neuron Endothelial cell Pial artery Glial end-foot Interneuron Intraparenchymal arteriole Glial end-feet Central neuron Glial end-foot Projections from: Locus coeruleus Raphe Ventral tegmental area Nucleus basalis Interneuron Pericyte Capillary Fig. 1. The neurovascular unit consists of functionally coupled neurons, glia, smooth muscle cells and endothelial cells. Its cellular constituents vary with the type, size and location of blood vessels. Cross-sections (dashed lines) are shown for pial arteries, intraparenchymal arterioles, and capillaries. Notably, innervation of pial arteries is provided by peripheral nerves, while arterioles and capillaries are innervated by central neurons (local interneurons and projection neurons) and are closely apposed by glial end-feet. Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx substances (Busse & Fleming, 2003). These include the vasodilators nitric oxide, reactive oxygen species (which can cause vascular dysregulation at high concentrations (Faraci & Heistad, 1998)) prostacyclin, and endotheliumderived hyperpolarizing factor, and the vasoconstrictors endothelin and endothelium-derived constrictor factor (Faraci & Heistad, 1998; Golding, Marrelli, You, & Bryan, 2002). In addition, carbon monoxide, possibly derived from the endothelium, has been proposed to produce vasodilation in the piglet cerebral microcirculation (Kanu, WhitWeld, & LeZer, 2006). Astrocytes also can release a factor derived from lipoxygenase metabolism that produces endothelium-dependent relaxation of cerebral arteries (Murphy, Rich, Orgren, Moore, & Faraci, 1994). The Wnal targets of these vasoactive substances, whether released from endothelial cells, astrocytes, or neurons, are the smooth muscle cells and pericytes. Studies in the retinal microcirculation suggest that pericytes respond to vasoactive signals by constricting or relaxing to alter vascular diameter (Kawamura et al., 2003; Wu, Kawamura, Sakagami, Kobayashi, & Puro, 2003). Smooth muscle cells also respond directly to increased intravascular pressure with constriction (Prewitt, Rice, & Dobrian, 2002). This property allows smooth muscle cells to counter pressure-induced changes in the rate of blood Xow, and contributes to the ability of cerebral blood vessels to maintain Xow in the face of changes in arterial pressure (cerebrovascular autoregulation). Additionally, gap junctions between smooth muscle cells facilitate propagation of vascular signals along the vessel (Kawamura et al., 2003; Lagaud, Karicheti, Knot, Christ, & Laher, 2002). As vessels penetrate deeper into the brain, the Virchow-Robin space is no longer found and vessels become intraparenchymal arterioles and capillaries. At this level the vascular basal lamina, and to a lesser extent smooth muscle cells and pericytes, directly contact neural processes and astrocytic end-feet (Cohen, Bonvento, Lacombe, & Hamel, 1996; Rennels & Nelson, 1975) (Fig. 1). Intracerebral arterioles and capillaries are likely to be involved in the local regulation of microvascular Xow, and chemical signals to these vessels from astrocytes and neurons are important inXuences on local Xow. Astrocytic end-feet occupy a much greater proportion of the vascular surface than do neural processes (Jones, 1970; Maynard, Schultz, & Pease, 1957), and evidence has emerged for a direct role of astrocytes in inXuencing vascular diameter (Hirase, 2005; Mulligan & MacVicar, 2004; Zonta & Angulo et al., 2003a). Neuronal processes that contact intraparenchymal blood vessels originate from local interneurons or from neurons whose cell bodies are located in subcortical regions and project to neocortex (Fig. 1). To date, subcortical regions known to contribute to cortical perivascular innervation include the basal forebrain, raphe, ventral tegmental area and locus coeruleus (Van Lieshout, Wieling, Karemaker, & Secher, 2003). 3 Thus, the close anatomical and functional associations between microvessels, neurons, and astrocytes in the brain suggest the importance of conceptualizing them as a neurovascular unit. The ability of this unit to regulate CBF involves several types of interactions between all of the components. 2. Mechanisms of functional hyperemia 2.1. DiVusion of synaptically released neurotransmitters More than a century ago, Roy and Sherrington (Roy & Sherrington, 1890) proposed that working neurons release vasoactive agents in the extracellular space, and these agents reach blood vessels by diVusion and produce relaxation of vascular smooth muscles. Considerable evidence has since accumulated supporting vasoactive consequences of neurotransmitter release, in particular for the synaptically released fast transmitters glutamate and GABA, but the mechanisms are far more complex and indirect than simple diVusion to vascular targets. 2.1.1. Glutamate There is substantial evidence from studies of cerebellum, hippocampus and neocortex that glutamate inXuences blood Xow. In cerebellar cortex, the Purkinje cells, which are the only cerebellar output neurons, receive their major excitatory synaptic inputs from parallel Wbers and climbing Wbers. The parallel Wbers are known to be glutamatergic (Ito, 1991), and the climbing Wbers utilize an excitatory amino acid such as glutamate, aspartate or N-acetyl-aspartyl-glutamate (Ross, Bredt, & Snyder, 1990). Focal activation of these excitatory inputs leads to an increased blood Xow that is blocked by antagonists at non-NMDA-type glutamate receptors, and is mimicked by exogenous glutamate application (Yang & Iadecola, 1996; Yang & Iadecola, 1997) . Glutamate also has vasodilatory actions in neocortical and hippocampal slice preparations. Exogenous glutamate or selective glutamate receptor agonists dilate pial arterioles and/or precapillary microvessels. In contrast to cerebellum, this eVect in hippocampus and neocortex does involve NMDA-type glutamate receptors (Fergus & Lee, 1997a; Lovick, Brown, & Key, 1999). Glutamate does not act directly on smooth muscle cells to produce vasodilation (Faraci & Breese, 1993). Rather, this neurotransmitter increases CBF by inducing the release of vasoactive factors from other cells through several calcium-dependent mechanisms (Fig. 2). In all brain areas studied, the vasoactive actions of glutamate are attenuated by blocking nitric oxide synthase (NOS) (Akgören, Fabricius, & Lauritzen, 1994; Faraci & Breese, 1993; Fergus & Lee, 1997a; Li & Iadecola, 1994; Lovick et al., 1999; Yang, Chen, Ebner, & Iadecola, 1999) or in NOS null mice (Yang, Zhang, Ross, & Iadecola, 2003). Neuronal NOS (NOS I) is present in neurons and glia, and is the synthetic enzyme for nitric oxide (NO), a potent vasodilator that is released during synaptic activity (Strijbos, 1998). Several region-speciWc Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 4 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx NOS COX-2 Ca++ ATP Glu Ado GJ Glu mGluR K+ H+ NO PGs Ado Ca++ waves Ca++ Ca++ PGs COX-2 Central neuron EETs P450 ATP Ado K+ siphoning NO GABA 5HT NE ACh DA SP NT VIP SOM NPY Fig. 2. Neuronal activity releases vasoactive mediators. Glutamate (Glu) increases the intracellular concentration of Ca++ in neurons and glia, which activates the synthesis of nitric oxide (NO) from NO synthase (NOS), prostaglandins (PGs) from cyclooxygenase-2 (COX-2), and epoxyeicosatrienoic acids (EETs) from cytochrome P450 epoxygenase. Calcium elevations in astrocytes are produced by activation of metabotropic glutamate receptors (mGluRs) and by propagation of Ca++ waves from adjacent astrocytes via gap junctions (GJ). Neurons release H+ and K+ ions during synaptic transmission and astrocytes release K+ (K+ siphoning) from perivascular end-feet during spatial buVering. Neurovascular terminals originating from cortical aVerents and local interneurons release vasoactive neurotransmitters and neuromodulators including NO, GABA, serotonin (5-HT), norepinephrine (NE), acetylcholine (ACh), dopamine (DA), substance P (SP), neurotensin (NT), vasoactive intestinal peptide (VIP), somatostatin (SOM), and neuropeptide Y (NPY). eVects of glutamate are also seen. In cerebellum, pharmacological and genetic studies have shown that NOS-dependent CBF increases are responsible for the vast majority of the response to functional activation (Li & Iadecola, 1994; Yang et al., 1999; Yang et al., 2003), whereas in somatosensory cortex, NOS-related mechanisms are less prominent (Lindauer, Megow, Matsuda, & Dirnagl, 1999). Cyclooxygenase-2 (COX-2), an enzyme that synthesizes prostaglandins from arachidonic acid and is associated with glutamatergic synapses (Kaufmann, Worley, Pegg, Bremer, & Isakson, 1996), is also involved in the regulation of CBF during synaptic activity (Niwa, Araki, Morham, Ross, & Iadecola, 2000). In neocortex, glutamate-induced increases in intracellular calcium also activate the synthesis of epoxyeicosatrienoic acids from cytochrome P450 epoxygenase (Alkayed et al., 1997). Recent evidence suggests a prominent role for astrocytes in glutamate-mediated vasoactivity. Astrocytes are known to possess metabotropic glutamate receptors (mGluRs) and to respond to glutamate with a transient increase in intracellular Ca++ that spreads to more distant portions of the cell (Kang, Jiang, Goldman, & Nedergaard, 1998; Pasti, Volterra, Pozzan, & Carmignoto, 1997; Porter & McCar- thy, 1996). Zonta et al. (Zonta & Angulo et al., 2003a) found that in somatosensory cortex slices, neuronal activity or certain subtype-selective mGluR agonists trigger astrocytic Ca++ waves that correspond temporally with activityinduced vasodilation of local arterioles. Antagonism of the same mGluR subtypes diminishes astrocytic Ca++ waves without aVecting neurons, and direct stimulation of individual astrocytes produces rapid vasodilation of nearby arterioles. The astrocytic eVect is largely dependent on a COX product, perhaps the powerful vasodilator prostaglandin E2, which is released from somatosensory cortical astrocytes by Ca++ waves (Zonta & Sebelin et al., 2003b). It was suggested that synaptically released glutamate spills over from the synapse and activates nearby astrocytes; if the resulting intracellular Ca++ wave is suYcient to reach an end-foot contacting a microvessel, vasodilation will occur (Zonta & Angulo et al., 2003a). On the other hand, others have shown that increases in Ca++ in astrocytes produce vasoconstriction rather than vasodilation by releasing P450 metabolites (Mulligan & MacVicar, 2004). The discrepancy could be explained by the fact that Zonta et al. preconstricted the vessels in the slice with L-NAME, a blocker of NO synthesis (Zonta & Angulo et al., 2003a). Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx Although the observed eVects in slices may not completely mimic in vivo responses, given the limitations inherent in the slice model, these studies clearly show that glutamate can have direct eVects on astrocytes that inXuence vascular tone. 2.1.2. GABA Exogenous GABA, acting via GABAA receptors, dilates precapillary microvessels in hippocampus and neocortex (Fergus & Lee, 1997b). Furthermore, local neurons mediate the neocortical vasodilation resulting from cerebellar stimulation (Iadecola, Arneric, Baker, Tucker, & Reis, 1987). However, in the cerebellar cortex itself GABA is not involved in activation-induced increases in Xow (Li & Iadecola, 1994; Mathiesen, Caesar, Akgoren, & Lauritzen, 1998). The vasoactive eVects of GABA in the forebrain are interesting in light of continued controversy over whether inhibitory neurotransmission contributes to brain energy use or to functional imaging signals. In cerebral cortex, GABAergic interneurons substantially innervate microvessels, suggesting they may act as integrators of local vascular responses (Vaucher, Tong, Cholet, Lantin, & Hamel, 2000). Interestingly, some GABAergic interneurons also contain NOS (Cauli et al., 2004), and NOS-containing neurons are known to contact large cerebral arteries and microvessels (Estrada & De, 1998; Iadecola et al., 1993) 2.1.3. Other vasoactive mediators released by neural activity During neurotransmission, potassium ions are released during neuronal repolarization and astrocytic spatial buVering; the latter process particularly involves perivascular endfeet, where K+ conductance is greatest (Iadecola, Li, Yang, & Xu, 1996). Activity-evoked rises in extracellular K+ have been shown to moderately increase blood Xow (Caesar, Akgoren, Mathiesen, & Lauritzen, 1999; Iadecola & Kraig, 1991), an eVect in part mediated by NO (Dreier et al., 1995). Neural activity also produces extracellular increases in hydrogen ions, as well as in adenosine produced by ATP catabolism (IliV, D’Ambrosio, Ngai, & Winn, 2003). 2.2. Vascular and glial innervation In addition to diVusion of synaptically released vasoactive agents, central neurons could inXuence vascular tone through direct contacts with arterioles, capillaries, and perivascular glial processes (Fig. 2). These neurons contain neuromodulators and neuropeptides, which contrast with GABA and glutamate in that they can be released from non-synaptic portions of the neuron to act on nearby cellular targets. The neuromodulators identiWed in terminals forming perivascular contacts have been shown to inXuence vascular diameter to regulate CBF, suggesting important roles in controlling blood Xow in the normal brain. Many studies have suggested vasoactive roles for monoaminergic and cholinergic neurons in the brain. Serotonergic axons and terminals contact parenchymal microvessels (Reinhard, Liebmann, Schlosberg, & Moskowitz, 1979). 5 These neurons originate in the brainstem raphe and project diVusely throughout the brain. Serotonin has a pronounced vasoconstrictor eVect, although it can be vasodilatory under some conditions. This dual eVect is consistent with the complex distribution of serotonin receptors to both neurons and astrocytes, the large number of serotonin receptor subtypes, and the demonstrated ability of serotonin to aVect vessels directly as well as indirectly through astrocytes (Cohen et al., 1996). Norepinephrine-containing terminals from locus coeruleus contact scattered cortical microvessels (Cohen, Molinatti, & Hamel, 1997; Raichle, Hartman, Eichling, & Sharpe, 1975), particularly capillaries (Cohen et al., 1997), and norepinephrine applied in vivo produces vasoconstriction and a reduced CBF (Raichle et al., 1975). These terminals more frequently contact astrocytic end-feet that are associated with microvessels (Cohen et al., 1997; Paspalas & Papadopoulos, 1996). The functional relevance of this association is suggested by recent evidence that norepinephrine can produce vasoconstriction that is tightly associated with direct norepinephrineinduced eVects on astrocytes (Mulligan & MacVicar, 2004). Dopaminergic projections from ventral tegmental area contact penetrating arterioles and cerebral capillaries in cerebral cortex. These aVerents terminate either directly on the vascular basal lamina or on perivascular astrocytic endfeet, or are near capillary pericytes (Krimer, Muly, Williams, & Goldman-Rakic, 1998). In cortical slice preparations, iontophoretic microapplication of dopamine near cerebral microvessels produces vasoconstriction in approximately 50% of the microvessels studied (Krimer et al., 1998). Similarly, in isolated cerebral arteries and in pial arterioles in situ, dopamine produces vasoconstriction (Sharkey & McCulloch, 1986). Finally, axons and terminals containing choline acetyl transferase, the acetylcholine synthetic enzyme, are apposed to the basal laminae of capillaries and small arterioles, and to capillary endothelial cells (Arneric et al., 1988; Parnavelas, Kelley, & Burnstock, 1985). Neurons in the basal forebrain are a major source of this cholinergic perivascular innervation, which is particularly prominent in cerebral cortex (Arneric, 1989; Arneric et al., 1988). The functional signiWcance of cholinergic innervation is suggested by Wndings that stimulation of basal forebrain neurons produces vasodilation and increases blood Xow in the cerebral cortex (reviewed in (Sato & Sato, 1992)). This increase in CBF is not associated with increased cortical energy metabolism (Sato & Sato, 1992) and is likely to involve production of NO in endothelial cells (Zhang, Xu, & Iadecola, 1995). More recently, evidence has also emerged for direct actions of acetylcholine on pericytes in retinal microvessels (Wu et al., 2003). Additionally, several types of neuropeptide-containing terminals have been found to make perivascular associations. For example, the brainstem parabrachial nuclei contain substance P and neurotensin in perivascular processes (Milner & Pickel, 1986a, 1986b). Vasoactive intestinal peptide (VIP) is in perivascular terminals in cortex (Paspalas & Papadopoulos, 1998), and application of VIP dilates cortical Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 6 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx vessels (Yaksh, Wang, & Go, 1987). Moreover, stimulation of individual cortical GABAergic neurons containing VIP and NOS produces local vasodilation, and vasodilation can be evoked by application of VIP to slices (Cauli et al., 2004). Somatostatin and neuropeptide Y (NPY) appear to play the opposite role: vasoconstriction is produced following exogenous application of either peptide (Cauli et al., 2004; Long, Rigamonti, Dosaka, Kraimer, & Martinez-Arizala, 1992) or by stimulation of individual GABAergic somatostatin-containing neurons (Cauli et al., 2004). In addition to microvessels, some major cerebral arteries are surrounded by perivascular NPY-containing terminals, and constrict in response to NPY application (Tuor, Kelly, Edvinsson, & McCulloch, 1990). However, it must be noted that the vasoactive eVects of neurotransmitters and neuropeptides vary in diVerent animal species (Faraci & Heistad, 1998). Therefore, the species needs to be taken into account in the interpretation of their physiological eVect. The function of the innervation of intracerebral microvessels has been debated extensively (Edvinsson & Hamel, 2002). One possible function of neuromodulatory innervation of blood vessels may be to focus the vascular response to regions experiencing neural activity. That is, when local Xow increases, more distant pial arteries that supply the activated region must also increase Xow. This raises the possibility of increasing Xow in other microvessels supplied by the arteries. In order to restrict the increase in Xow to the area of increased activity, microvascular adjustments involving both the activated area and surrounding areas must be made. Although there is certainly a role for intravascular control of upstream blood supply (see below), it is possible that perivascular neuromodulators also play a role. Additionally, as has been suggested (Krimer et al., 1998; Raichle et al., 1975), innervation of endothelial cells could modulate the transfer of substances across the blood–brain barrier. 2.3. Energy deWcit as the stimulus for functional hyperemia Increases in CBF have been correlated with local energy use during activity, although the extent to which energy deWcit drives the increase in CBF is still a subject of active inquiry (see reviews by (Attwell & Laughlin, 2001; Shulman, Rothman, Behar, & Hyder, 2004)). Certainly, understanding functional hyperemia requires addressing how the brain uses energy, and which processes are most energy demanding. This question has been recently addressed by several studies. Attwell and Laughlin (Attwell & Laughlin, 2001) used a “bottom-up” approach in which speciWc cellular functions were given an energy cost based on published data, and then summed up to construct an “energy budget” for the rodent and primate brain. The results of this analysis (Fig. 3) indicate that over 80% of the energy in the primate brain is used for processes related to glutamate signaling at synapses, a conclusion also reached by Ames (Ames, 2000) and Shulman and colleagues (Sibson et al., 1998). The energy used by inhibition has remained controversial. Some reports suggest that inhibitory synapses use less energy than excitatory synapses (Waldvogel et al., 2000), while others have noted that increased glucose usage was associated with inhibition of hippocampal pyramidal and auditory cells (Ackermann, Finch, Babb, & Engel, 1984; Nudo & Masterton, 1986). Although the ion Xuxes that generate resting and action potentials are the same in inhibitory neurons and excitatory neurons, the electrochemical gradient down which Cl¡ moves postsynaptically at inhibitory synapses is less than that down which Na+ moves at excitatory synapses, implying less energy expenditure to pump the ions back. Furthermore, cortical inhibitory neurons and synapses are only one tenth as numerous as excitatory neurons (Abeles, 1991; Braitenberg & Schuz, 1998). Most of the energy used by the brain derives from oxidative metabolism. Energy can also be produced, albeit less eYciently, by the anaerobic metabolism of glucose or glycolysis. The telltale sign of glycolysis is lactate production, which increases with activation (Frahm, Kruger, Merboldt, & Kleinschmidt, 1996; Prichard et al., 1991; Ueki, Linn, & Hossmann, 1988). Why does the brain need to use glycolysis? Gusnard and Raichle (Gusnard & Raichle, 2001) have recently examined this issue. One possibility is that, because glycolysis generates energy faster than glucose oxidation, the rapid increase in energy demands at the onset of activation is best met by anaerobic metabolism of glucose released from glycogen. With sustained activation, the increase in CBF has time to develop fully, resulting in an increase in the tissue delivery of oxygen and glucose. At this point the energy metabolism switches from anaerobic to aerobic glucose metabolism. This view is supported by the following observations. First, brain activation increases the utilization of glycogen, which in brain is present mainly in glial cells (Brown, Tekkok, & Ransom, 2003; Swanson, Morton, Sagar, & Sharp, 1992). Second, during sustained activation lactate production is maximal at the onset of activation and then decreases with time (Prichard et al., 1991). Third, oxygen consumption increases only minimally at the onset of activation and then rises progressively as the activation continues (Fox & Raichle, 1986; Fox, Raichle, Mintun, & Dence, 1988; Mintun, Vlassenko, Shulman, & Snyder, 2002). Thus, it would seem that the brain uses anaerobic glycolysis at the onset of activation and, if energy demands are sustained, oxidative metabolism. Magistretti and colleagues have proposed a model that Wts well with the idea of a shift between anaerobic and aerobic glucose metabolism during activation (Magistretti, Pellerin, Rothman, & Shulman, 1999; Pellerin & Magistretti, 2003). Glutamate released during synaptic activity is taken up by astrocytes through speciWc glutamate transporters (GT), GLAST and GLT-1, coupled to Na+, so that for each glutamate transported 3 Na+ enter the cell (Fig. 3). To reestablish the ionic gradient, Na+ is pumped out of the cell by activation of the Na/K ATPase, which is fueled by glycolytically derived ATP. The lactate so produced is passed on to neurons that metabolize it aerobically, generating ATP. ATP is then used to fuel the ion pumps that reestablish ionic gradients after depolarization. Therefore, it is conceivable that the Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx 7 tate shuttle” idea remains a useful working hypothesis that Wts well with the available data on glucose-oxygen coupling in the activated brain. A recent study indicates that NADH, produced during energy metabolism by transfer of electrons and protons from glucose to cytosolic free NAD(+), augments blood Xow (Ido, Chang, & Williamson, 2004). In retina and visual cortex of rats, visual stimulation produces an elevated blood Xow that is enhanced by the injection of lactate and diminished by injection of pyruvate. This photostimulation-induced Xow is prevented by inhibition of NOS. The authors suggest that lactate provided additional electrons and protons for transfer to NAD(+), converting it to NADH, while pyruvate received transferred electrons and protons from NADH, converting it to NAD(+). These data led to the suggestion that cytosolic free NADH fuels a signaling cascade that increases nitric oxide production, which in turn augments blood Xow in photostimulated retina and visual cortex. The signiWcance and generality of this Wnding is still unclear. increase in lactate observed at the onset of activation is derived from astroglial glycolysis. The subsequent reduction in lactate and rise in oxygen consumption reXects the aerobic metabolism of lactate by neurons. This hypothesis, which has generated considerable interest and controversy (Chih & Roberts, 2003; Pellerin & Magistretti, 2003), is supported by a growing body of evidence indicating that: (1) glutamate stimulates anaerobic glycolysis in astrocyte cultures leading to lactate release into the medium (Pellerin & Magistretti, 1994; Takahashi, Driscoll, Law, & SokoloV, 1995); (2) neurons and astrocytes in culture have the potential of metabolizing both glucose and lactate, but neurons prefer extracellular lactate and astrocytes favor glucose (BouzierSore, Voisin, Canioni, Magistretti, & Pellerin, 2003; Itoh et al., 2003); (3) MCT2, the monocarboxylic acid transporter that carries lactate, is preferentially located in neurons (Pierre, Magistretti, & Pellerin, 2002); (4) downregulation of glial glutamate transporters attenuates cerebral glucose utilization (Cholet et al., 2001; Voutsinos-Porche et al., 2003). Because the critical evidence supporting the astrocytes-toneurons lactate shuttle hypothesis is based on in vitro experiments, the biological relevance of the model has been questioned (Chih & Roberts, 2003). Therefore, there is a need for in vivo evidence supporting the cellular compartmentalization of these metabolic processes. In the meantime, the “lac- 2.4. Propagation of responses along cerebral blood vessels During functional hyperemia, local vasodilation increases Xow most eVectively if the upstream arterioles also dilate (Duling et al., 1987). This has been observed in Resting potential 2% Na+ K+ Astrocyte 6% lactate glycolysis ATP Glu Action potentials 10% K+ Na+ Glutamine Glu K+ K+ MCAT2 lactate+O2 Na+ Presynaptic 7% ATP Ca++ Na+ Na+ Postsynaptic 75% Fig. 3. Energy budget of the brain. The predicted expenditure of energy by gray matter in the primate brain. Postsynaptic processes make up the majority of energy use. Action potentials and neurotransmitter release use approximately 17% of the energy, while maintaining the resting potential uses 2%. The activity of astrocytes uses about 6%. The ion pumps (Na+/K+ and Na+/Ca++) are shown to indicate that most signaling energy is used to restore transmembrane ionic gradients. Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 8 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx several brain areas, for example, activation of whisker barrel cortex increases vascular diameter in pial arterioles that are several hundred micrometers away from the site of activation (Cox, Woolsey, & Rovainen, 1993; Erinjeri & Woolsey, 2002; Ngai, Ko, Morii, & Winn, 1988). In cerebellum, direct electrical stimulation of parallel Wbers increases vascular diameter of local arterioles and of the upstream branches from which these arterioles originate (Iadecola, Yang, Ebner, & Cheng, 1997). Several possible mechanisms have been proposed for controlling upstream vasodilation, including widespread neurovascular innervation and “intramural” signaling within the vascular wall. The former possibility seems increasingly unlikely: the main innervation of larger pial arterioles originates from outside the brain (Fig. 1), and is not well linked to the localized neural pathways involved in activation. Moreover, cutting the nerves innervating pial arterioles does not attenuate their dilation in response to somatosensory stimulation (Ibayashi et al., 1991). Intramural vascular signaling, in contrast, is supported by several lines of evidence. Endothelial cells and smooth muscle cells in the brain are connected by homocellular gap junctions, and can propagate vasodilation in a retrograde fashion (Dietrich, Kajita, & Dacey, 1996; Sokoya et al., 2006). Flow-mediated vasodilation also could contribute to intramural vascular signaling (Fujii, Faraci, & Heistad, 1991). Local vasodilation increases Xow velocity in upstream branches, which, due to increased shear stress, leads to the local release of endothelium-dependent vasodilators (Busse & Fleming, 2003). These vasodilators relax the larger arteries and amplify the increase in Xow. However, it is unclear to what extent shear stress induces vasodilation in cerebral vessels (Bryan, Marrelli, Steenberg, Schildmeyer, & Johnson, 2001a; Bryan, Steenberg, & Marrelli, 2001b; Madden & Christman, 1999; Ngai & Winn, 1996; Wilkerson et al., 2005) and the mechanisms of retrograde propagation of vasodilation need to be explored further. 3. Functional brain imaging Changes in CBF are measured as a proxy for neural activity in several modern imaging techniques, including positron-emission tomography (PET), single photon emission computed tomography (SPECT), and fMRI. One of the most widely utilized is fMRI, and the most common technique used in fMRI is the blood oxygen level-dependent (BOLD) contrast signal. When hemoglobin (Hb) in red blood cells loses oxygen, its iron becomes paramagnetic, which generates magnetic Weld gradients between the deoxyHb-containing compartments and protons of neighboring water molecules (Ogawa, Lee, Kay, & Tank, 1990). This oxygen-dependent Weld contrast can be detected with magnetic resonance imaging, and confers the dual advantages of non-invasive imaging and high spatiotemporal resolution. The BOLD contrast signal depends on CBF, cerebral blood volume, and blood oxygenation (Ogawa et al., 1993). Several uncertainties remain as to the mechanisms underlying the BOLD signal. For example, as detailed above, both neurotransmitter-related signaling and energy demand are correlated with increased CBF, but controversy remains over the relative contribution of each process to the BOLD signal (reviewed in (Attwell & Laughlin, 2001; Shulman et al., 2004)). Another issue is the extent to which the BOLD signal accurately reXects the location and magnitude of the neural events that produce it. Clearly, this is important in determining the degree to which fMRI data yields quantitative information about particular brain regions, and the spatial precision of this information. 3.1. Correlation between the BOLD signal and neural activity Accurate interpretation of the BOLD signal requires understanding what sort of neural activity produces it (Logothetis & Wandell, 2004). Increasing evidence supports a linkage between CBF changes and local somatic and dendritic activity, rather than output spikes (synchronized action potentials). In the cerebellar cortex, stimulation of the excitatory parallel Wbers increases CBF, but inhibits Purkinje cell spiking (Lauritzen, 2001; Mathiesen et al., 1998). In cerebral cortex, the BOLD signal correlates well with local Weld potentials, which reXect inputs, local-circuit activity, and somatodendritic processing and integration of these inputs in cortical neurons (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). In contrast, cortical neuron output spikes are less well correlated with the BOLD signal (Logothetis et al., 2001). An important question is whether the spatial extent of the BOLD signal exactly matches that of neuronal activity. As discussed in the preceding sections, local neural activity produces an increase in blood Xow that propagates to a larger area (Iadecola et al., 1997; Malonek & Grinvald, 1996). Potentially, contributions from both upstream vasodilation and from downstream draining vessels carrying away deoxyHb could enlarge the BOLD signal beyond the area of neural activity. However, these factors can be minimized by knowing local vasoarchitecture and by optimizing the scanning conditions (Logothetis, 2003). Indeed, in paired physiological recordings and fMRI, there is a linear correlation between electrical activity and the BOLD signal using a voxel size of approximately 3–5 mm2, suggesting good spatial accuracy at this level of resolution (Kim et al., 2004; Logothetis et al., 2001). At smaller voxel sizes, the correlation between BOLD contrast and physiological activity varies considerably, with false positives occurring outside the area of neural activity (Kim et al., 2004). Interpretation of BOLD signals can also be confounded by regional variations in the BOLD response. It has been widely observed in visual cortex that an initial negative “dip” in the BOLD signal precedes the increase in CBF and the secondary increase in the BOLD signal (Logothetis & Wandell, 2004; Ugurbil, Toth, & Kim, 2003). This transient dip has been attributed to a brief local increase in deoxyHb Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx before the increase in CBF brings an oversupply of oxyHb (Fox & Raichle, 1986; Fox et al., 1988). The initial dip has been observed across species and in awake as well as anesthetized animals. However, in the rat somatosensory cortex, the initial “dip” was not seen, rather CBF changes occur before BOLD signal (Silva, Lee, Iadecola, & Kim, 2000). This discrepancy suggests that regional and/or species diVerences may exist in the coupling between deoxyHb levels and CBF. In conclusion, neurotransmission alters blood Xow through several processes working in concert. Synaptic activity triggers neurons and astrocytes to release vasoactive mediators. These mediators act on local blood vessels to produce vasodilation of arterioles, and perhaps capillaries, at the site of activation. Concurrent activation of neurovascular projections and local interneurons can restrict the Xow response to the activated area through the coordinated release of vasodilator and vasoconstrictor agents. Vascular endothelial cells and smooth muscle cells further communicate among themselves to propagate vasodilation upstream and Wne tune the local distribution of blood Xow. These factors together contribute to the neural activity-induced alterations in CBF that form the basis of functional brain mapping techniques. There is still much debate concerning the speciWc sources of the BOLD signal used in fMRI studies. Although the neurovascular mechanisms of BOLD are not completely understood, this technique remains a powerful tool for exploring the function of the behaving human brain. References Abeles, M. (1991). Corticonics: neural circuits of the cerebral cortex. Cambridge: Cambridge University Press. Ackermann, R. F., Finch, D. M., Babb, T. L., & Engel, J., Jr. (1984). Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. Journal of Neuroscience, 4, 251– 264. Akgören, N., Fabricius, M., & Lauritzen, M. (1994). Importance of nitric oxide for local increases of blood Xow in rat cerebellar cortex during electrical stimulation. Proceedings of the National Academy of Sciences of the United States of America, 91, 5903–5907. Alkayed, N., Birks, E. K., Narayanan, J., Petrie, K. A., Kholer-Cabot, A. E., & Harder, D. R. (1997). Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood Xow to glutamate in rats. Stroke, 28, 1066–1072. Ames, A., 3rd (2000). CNS energy metabolism as related to function. Brain Research Brain Research Review, 34, 42–68. Arneric, S. A. (1989). Cortical cerebral blood Xow is modulated by cholinergic basal forebrain neurons: eVects of ibotenic acid lesions and electrical stimulation. In J. Seylaz & E. T. MacKenzie (Eds.), Neurotransmission of cerebrovascular function (pp. 381–384). Amsterdam: Elsevier. Arneric, S. P., Honig, M. A., Milner, T. A., Greco, S., Iadecola, C., & Reis, D. J. (1988). Neuronal and endothelial sites of acetylcholine synthesis and release associated with microvessels in rat cerebral cortex: ultrastructural and neurochemical studies. Brain Research, 454, 11–30. Attwell, D., & Laughlin, S. B. (2001). An energy budget for signaling in the grey matter of the brain. Journal of Cerebral Blood Flow and Metabolism, 21, 1133–1145. Bouzier-Sore, A. K., Voisin, P., Canioni, P., Magistretti, P. J., & Pellerin, L. (2003). Lactate is a preferential oxidative energy substrate over glucose 9 for neurons in culture. Journal of Cerebral Blood Flow and Metabolism, 23, 1298–1306. Braitenberg, V., & Schuz, A. (1998). Cortex: statistics and geometry of neuronal connectivity. Berlin: Springer. Brown, A. M., Tekkok, S. B., & Ransom, B. R. (2003). Glycogen regulation and functional role in mouse white matter. Journal of Physiology, 549, 501–512. Bryan, R., Jr., Marrelli, S., Steenberg, M., Schildmeyer, L., & Johnson, T. (2001a). EVects of luminal shear stress on cerebral arteries and arterioles. American Journal of Physiology Heart and Circulatory Physiology, 280, H2011–H2022. Bryan, R., Jr., Steenberg, M., & Marrelli, S. (2001b). Role of endothelium in shear stress-induced constrictions in rat middle cerebral artery. Stroke, 32, 1394–1400. Busse, R., & Fleming, I. (2003). Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends in Pharmacological Sciences, 24, 24–29. Caesar, K., Akgoren, N., Mathiesen, C., & Lauritzen, M. (1999). ModiWcation of activity-dependent increases in cerebellar blood Xow by extracellular potassium in anaesthetized rats. Journal of Physiology (London), 520(Pt. 1), 281–292. Cauli, B., Tong, X., Rancillac, A., Serluca, N., Lambolez, B., Rossier, J., et al. (2004). Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. Journal of Neuroscience, 24, 8940–8949. Chih, C. P., & Roberts, E. L., Jr. (2003). Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. Journal of Cerebral Blood Flow and Metabolism, 23, 1263–1281. Cholet, N., Pellerin, L., Welker, E., Lacombe, P., Seylaz, J., Magistretti, P., et al. (2001). Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. Journal of Cerebral Blood Flow and Metabolism, 21, 404–412. Cohen, Z., Bonvento, G., Lacombe, P., & Hamel, E. (1996). Serotonin in the regulation of brain microcirculation. Progress in Neurobiology, 50, 335–362. Cohen, Z., Molinatti, G., & Hamel, E. (1997). Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. Journal of Cerebral Blood Flow and Metabolism, 17, 894–904. Cox, S. B., Woolsey, T. A., & Rovainen, C. M. (1993). Localized dynamic changes in cortical blood Xow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. Journal of Cerebral Blood Flow and Metabolism, 13, 899–913. del Zoppo, G. J., & Mabuchi, T. (2003). Cerebral microvessel responses to focal ischemia. Journal of Cerebral Blood Flow and Metabolism, 23, 879–894. Dietrich, H. H., Kajita, T., & Dacey, R. G. Local and conducted vasomotor responses in isolated rat cerebral arterioles. American Journal of Physiology, 271 (Heart Circ. Physiology 40), H1109–H1116. Dreier, J. P., Körner, K., Görner, A., Lindauer, U., Weih, M., Villringer, A., et al. (1995). Nitric oxide modulates the CBF response to increased extracellular potassium. Journal of Cerebral Blood Flow and Metabolism, 15, 914–919. Duling, B. R., Hogan, R. D., Langille, D. H., Lelkes, P., Segal, S. S., Vatner, S. F., et al. (1987). Vasomotor control: functional hyperemia and beyond. Federation Proceedings, 46, 251–263. Edvinsson, L., & Hamel, E. (2002). Perivascular nerves in brain vessels. In L. Edvinsson & D. N. Krause (Eds.), Cerebral blood Xow and metabolism (pp. 43–67). Philadelphia: Lippincott, Williams and Wilkins. Erinjeri, J. P., & Woolsey, T. A. (2002). Spatial integration of vascular changes with neural activity in mouse cortex. Journal of Cerebral Blood Flow and Metabolism, 22, 353–360. Estrada, C., & De, F. J. (1998). Nitric oxide-producing neurons in the neocortex: morphological and functional relationship with intraparenchymal microvasculature. Cerebral Cortex, 8, 93–203. Faraci, F. M., & Breese, K. R. (1993). Nitric oxide mediates vasodilation in response to activation of N-methyl-D-aspartate receptors in brain. Circulation Research, 72, 476–480. Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 10 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx Faraci, F. M., & Heistad, D. D. (1998). Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiology Review, 78, 53–97. Fergus, A., & Lee, K. (1997a). Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Research, 754, 35–45. Fergus, A., & Lee, K. S. (1997b). GABAergic regulation of cerebral microvascular tone in the rat. Journal of Cerebral Blood Flow and Metabolism, 17, 992–1003. Fox, P. T., & Raichle, M. E. (1986). Focal physiological uncoupling of cerebral blood Xow and oxidative metabolism during somatosensory stimulation in human subjects. Proceedings of the National Academy of Sciences of the United States of America, 83, 1140–1144. Fox, P. T., Raichle, M. E., Mintun, M. A., & Dence, C. (1988). Nonoxidative glucose consumption during focal physiological neural activity. Science, 241, 462–464. Frahm, J., Kruger, G., Merboldt, K. D., & Kleinschmidt, A. (1996). Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magnetic Resonance Medicine, 35, 143–148. Fujii, K., Faraci, F., & Heistad, D. D. (1991). Flow-mediated vasodilation of the basilar artery in vivo. Circulation Research, 69, 697–705. Golding, E. M., Marrelli, S. P., You, J., & Bryan, R. M., Jr. (2002). Endothelium-derived hyperpolarizing factor in the brain: a new regulator of cerebral blood Xow? Stroke, 33, 661–663. Gusnard, D. A., & Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience, 2, 685–694. Hirase, H. (2005). A multi-photon window onto neuronal-glial-vascular communication. Trends in Neuroscience, 28, 217–219. Hossmann, K.-A. (1994). Viability thresholds and the penumbra of focal ischemia. Annals of Neurology, 36, 557–565. Iadecola, C. (1998). Cerebral circulatory dysregulation in ischemia. In M. D. Ginsberg & J. Bogousslavsky (Eds.), Cerebrovascular diseases (pp. 319–332). Cambridge, MA: Blackwell Science. Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Reviews Neuroscience, 5, 347–360. Iadecola, C., Arneric, S., Baker, H., Tucker, L., & Reis, D. (1987). Role of local neurons in cerebrocortical vasodilation elicited from cerebellum. American Journal of Physiology, 252, R1082–R1091. Iadecola, C., Beitz, A., Renno, W., Xu, X., Mayer, B., & Zhang, F. (1993). Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Research, 606, 148–155. Iadecola, C., & Kraig, R. P. (1991). Focal elevations in neocortical interstitial K+ produced by stimulation of the fastigial nucleus in rat. Brain Research, 563, 273–277. Iadecola, C., Li, J., Yang, G., & Xu, S. (1996). Neural mechanisms of blood Xow regulation during synaptic activity in cerebellar cortex. Journal of Neurophysiology, 75, 940–950. Iadecola, C., Yang, G., Ebner, T., & Cheng, G. (1997). Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. Journal of Neurophysiology, 78, 651–659. Ibayashi, S., Ngai, A. C., Howard, M. A., Meno, J. R., Mayberg, M. R., & Winn, R. (1991). Lack of sympathetic and cholinergic inXuences on the cerebral vasodilation caused by sciatic nerve stimulation in the rat. Journal of Cerebral Blood Flow and Metabolism, 11, 678–683. Ido, Y., Chang, K., & Williamson, J. R. (2004). NADH augments blood Xow in physiologically activated retina and visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101, 653–658. IliV, J. J., D’Ambrosio, R., Ngai, A. C., & Winn, H. R. (2003). Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. American Journal of Physiology Heart Circulation Physiology, 284, H1631–H1637. Ito, M. (1991). The cellular basis of cerebellar plasticity. Current opinions in neurobiology, 1, 616–620. Itoh, Y., Esaki, T., Shimoji, K., Cook, M., Law, M. J., Kaufman, E., et al. (2003). Dichloroacetate eVects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proceedings of the National Academy of Sciencesof the United States of America, 100, 4879–4884. Jones, E. G. (1970). On the mode of entry of blood vessels into the cerebral cortex. Journal of Anatomy, 106, 507–520. Kang, J., Jiang, L., Goldman, S., & Nedergaard, M. (1998). Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature Neuroscience, 1, 683–692. Kanu, A., WhitWeld, J., & LeZer, C. (2006). Carbon monoxide contributes to hypotension-induced cerebrovascular vasodilation in piglets. American Journal of Physiology Heart Circulation Physiology, in press. Kaufmann, W. E., Worley, P. F., Pegg, J., Bremer, M., & Isakson, P. (1996). COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America, 93, 2317–2321. Kawamura, H., Sugiyama, T., Wu, D. M., Kobayashi, M., Yamanishi, S., Katsumura, K., et al. (2003). ATP: a vasoactive signal in the pericytecontaining microvasculature of the rat retina. Journal of Physiology, 551.3, 787–799. Kim, D., Ronen, I., Olman, C., Kim, S., Ugurbil, K., & Toth, L. (2004). Spatial relationship between neuronal activity and BOLD functional MRI. Neuroimage, 21, 876–885. Krimer, L. S., Muly, E. C., 3rd, Williams, G. V., & Goldman-Rakic, P. S. (1998). Dopaminergic regulation of cerebral cortical microcirculation. Nature Neuroscience, 1, 286–289. Lagaud, G., Karicheti, V., Knot, H. J., Christ, G. J., & Laher, I. (2002). Inhibitors of gap junctions attenuate myogenic tone in cerebral arteries. American Journal of Physiology Heart Circulation Physiology, 283, H2177–H2186. Lauritzen, M. (2001). Relationship of spikes, synaptic activity, and local changes of cerebral blood Xow. Journal of Cerebral Blood Flow and Metabolism, 21, 1367–1383. Li, J., & Iadecola, C. (1994). Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology, 33, 1453–1461. Lindauer, U., Megow, D., Matsuda, H., & Dirnagl, U. (1999). Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. American Journal of Physiology, 277, H799– H811. Lo, E. H., Dalkara, T., & Moskowitz, M. A. (2003). Mechanisms, challenges and opportunities in stroke. Nature Reviews Neuroscience, 4, 399–415. Logothetis, N. (2003). MR imaging in the non-human primate: studies of function and of dynamic connectivity. Current Opinions in Neurobiology, 13, 630–642. Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412, 150–157. Logothetis, N. K., & Wandell, B. A. (2004). Interpreting the BOLD signal. Annual Review Physiology, 66, 735–769. Long, J., Rigamonti, D., Dosaka, K., Kraimer, J., & Martinez-Arizala, A. (1992). Somatostatin causes vasoconstriction, reduces blood Xow and increases vascular permeability in the rat central nervous system. Journal of Pharmacology and Experimental Therapeutics, 260, 1425–1432. Lovick, T. A., Brown, L. A., & Key, B. J. (1999). Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying Xow-metabolism coupling in intraparenchymal microvessels. Neuroscience, 92, 47–60. Madden, J., & Christman, N. (1999). Integrin signaling, free radicals, and tyrosine kinase mediate Xow constriction in isolated cerebral arteries. American Journal of Physiology, 277, H2264–H2271. Magistretti, P. J., Pellerin, L., Rothman, D. L., & Shulman, R. G. (1999). Energy on demand. Science, 283, 496–497. Malonek, D., & Grinvald, A. (1996). Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science, 272, 551–554. Mathiesen, C., Caesar, K., Akgoren, N., & Lauritzen, M. (1998). ModiWcation of activity-dependent increases of cerebral blood Xow by excit- Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx atory synaptic activity and spikes in rat cerebellar cortex. Journal of Physiology, 512, 555–566. Maynard, E. A., Schultz, R. L., & Pease, D. C. (1957). Electron microscopy of the vascular bed of rat cerebral cortex. American Journal of Anatomy, 100, 409–433. Milner, T., & Pickel, V. (1986a). Neurotensin in the rat parabrachial region: ultrastructural localization and extrinsic sources of immunoreactivity. Journal of Comparative Neurology, 247, 326–343. Milner, T., & Pickel, V. (1986b). Ultrastructural localization and aVerent sources of substance P in the rat parabrachial region. Neuroscience, 17, 687–707. Mintun, M. A., Vlassenko, A. G., Shulman, G. L., & Snyder, A. Z. (2002). Time-related increase of oxygen utilization in continuously activated human visual cortex. Neuroimage, 16, 531–537. Mulligan, S., & MacVicar, B. (2004). Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature, 431, 195–199. Murphy, S., Rich, G., Orgren, K., Moore, S., & Faraci, F. (1994). Astrocytederived lipoxygenase product evokes endothelium-dependent relaxation of the basilar artery. Journal of Neuroscience Research, 38, 314–318. Ngai, A. C., Ko, K. R., Morii, S., & Winn, H. R. (1988). EVect of sciatic nerve stimulation on pial arterioles in rats. American Journal of Physiology, 254, H133–H139. Ngai, A., & Winn, H. (1996). Estimation of shear and Xow rates in pial arterioles during somatosensory stimulation. American Journal of Physiology Heart and Circulatory Physiology, 270, H1712–H1717. Niwa, K., Araki, E., Morham, S. G., Ross, M. E., & Iadecola, C. (2000). Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. Journal of Neuroscience, 20, 763–770. Nudo, R., & Masterton, R. (1986). Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. Journal of Comparative Neurology, 245, 553–565. Ogawa, S., Lee, T. M., Kay, A. R., & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87, 9868–9872. Ogawa, S., Menon, R. S., Tank, D. W., Kim, S. G., Merkle, H., Ellermann, J. M., et al. (1993). Functional brain mapping by blood oxygenation leveldependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophysical Journal, 64, 803–812. Parnavelas, J., Kelley, W., & Burnstock, G. (1985). Ultrastructural localization of choline acetyltransferase in vascular endothelial cells in rat brain. Nature, 316, 724–725. Paspalas, C., & Papadopoulos, G. (1996). Ultrastructural relationships between noradrenergic nerve Wbers and non-neuronal elements in the rat cerebral cortex. Glia, 17, 133–146. Paspalas, C. D., & Papadopoulos, G. C. (1998). Ultrastructural evidence for combined action of noradrenaline and vasoactive intestinal polypeptide upon neurons, astrocytes, and blood vessels of the rat cerebral cortex. Brain Research Bulletin, 45, 247–259. Pasti, L., Volterra, A., Pozzan, T., & Carmignoto, G. (1997). Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. Journal of Neuroscience, 17, 7817–7830. Pellerin, L., & Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America, 91, 10625–10629. Pellerin, L., & Magistretti, P. J. (2003). Food for thought: challenging the dogmas. Journal of Cerebral Blood Flow and Metabolism, 23, 1282– 1286. Peters, A., Palay, S., & Webster, H. D. (1991). The Wne structure of the nervous system. New York: Oxford University Press. Pierre, K., Magistretti, P. J., & Pellerin, L. (2002). MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. Journal of Cerebral Blood Flow and Metabolism, 22, 586–595. Porter, J., & McCarthy, K. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. Journal of Neuroscience, 16, 5073–5081. 11 Prewitt, R. L., Rice, D. C., & Dobrian, A. D. (2002). Adaptation of resistance arteries to increases in pressure. Microcirculation, 9, 295–304. Prichard, J. W., Rothman, D. L., Novotny, E. J., PetroV, O. A. C., Kuwabara, T., Avison, M., et al. (1991). Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proceedings of the National Academy of Sciences of the United States of America, 88, 5829–5831. Raichle, M. E. (1998). Behind the scenes of functional brain imaging: a historical and physiological perspective. Proceedings of the National Academy of Sciences of the United States of America, 95, 765–772. Raichle, M. E., Hartman, B. K., Eichling, J. O., & Sharpe, L. G. (1975). Central noradrenergic regulation of cerebral blood Xow and vascular permeability. Proceedings of the National Academy of Sciences of the United States of America, 72, 3726–3730. Reinhard, J., Jr., Liebmann, J., Schlosberg, A., & Moskowitz, M. (1979). Serotonin neurons project to small blood vessels in the brain. Science, 206, 85–87. Rennels, M., & Nelson, E. (1975). Capillary innervation in the mammalian central nervous system: an electron microscope demonstration (1). American Journal of Anatomy, 144, 233–241. Ross, C. A., Bredt, D., & Snyder, S. H. (1990). Messenger molecules in the cerebellum. Trends in Neuroscience, 13, 216–222. Roy, C., & Sherrington, C. (1890). On the regulation of the blood-supply of the brain. Journal of Physiology (London), 11, 85–108. Sato, A., & Sato, Y. (1992). Regulation of regional cerebral blood Xow by cholinergic Wbers originating in the basal forebrain. Neuroscience Research (NY), 14, 242–274. Segal, S. S. (2000). Integration of blood Xow control to skeletal muscle: key role of feed arteries. Acta Physiologica Scandinavia, 168, 511–518. Sharkey, J., & McCulloch, J. (1986). Dopaminergic mechanisms in the regulation of cerebral blood Xow and metabolism: role of diVerent receptor subtypes. In C. Owman & J. E. Hardebo (Eds.), Neural regulation of brain circulation (pp. 111–127). New York: Elsevier Science Pub.. Shulman, R. G., Rothman, D. L., Behar, K. L., & Hyder, F. (2004). Energetic basis of brain activity: implications for neuroimaging. Trends in Neuroscience, 27, 489–495. Sibson, N. R., Dhankhar, A., Mason, G. F., Rothman, D. L., Behar, K. L., & Shulman, R. G. (1998). Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proceedings of the National Academy of Sciences of the United States of America, 95, 316– 321. Silva, A. C., Lee, S. P., Iadecola, C., & Kim, S. G. (2000). Early temporal characteristics of cerebral blood Xow and deoxyhemoglobin changes during somatosensory stimulation. Journal of Cerebral Blood Flow and Metabolism, 20, 201–206. SokoloV, L. (1989). Circulation and energy metabolism of the brain. In G. Segal, B. AgranoV, R. W. Albers, & P. MolinoV (Eds.), Basic neurochemistry (pp. 565–590). New York: Raven Press. Sokoya, E., Burns, A., Setiawan, C., Coleman, H., Parkington, H., & Tare, M. (2006). Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. American Journal of Physiology Heart Circulation Physiology, 291, H385–H393. Strijbos, P. (1998). Nitric oxide in cerebral ischemic neurodegeneration and excitotoxicity. Critical Reviews in Neurobiololgy, 12, 223–243. Swanson, R. A., Morton, M. M., Sagar, S. M., & Sharp, F. R. (1992). Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience, 51, 451–461. Takahashi, S., Driscoll, B. F., Law, M. J., & SokoloV, L. (1995). Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proceedings of the National Academy of Sciences of the United States of America, 92, 4616–4620. Takano, T., Tian, G., Peng, W., Low, N., Libionka, W., Han, X., et al. (2006). Astrocyte-mediated control of cerebral blood Xow. Nature Neuroscience, 9, 159–161. Tuor, U., Kelly, P., Edvinsson, L., & McCulloch, J. (1990). Neuropeptide Y and the cerebral circulation. Journal of Cerebral Blood Flow and Metabolism, 10, 591–601. Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002 ARTICLE IN PRESS 12 C.T. Drake, C. Iadecola / Brain and Language xxx (2006) xxx–xxx Ueki, M., Linn, F., & Hossmann, K.-A. (1988). Functional activation of cerebral blood Xow and metabolism before and after global ischemia of rat brain. Journal of Cerebral Blood Flow and Metabolism, 8, 486–494. Ugurbil, K., Toth, L., & Kim, D. S. (2003). How accurate is magnetic resonance imaging of brain function? Trends in Neuroscience, 26, 108–114. Van Lieshout, J. J., Wieling, W., Karemaker, J. M., & Secher, N. H. (2003). Syncope, cerebral perfusion, and oxygenation. Journal of Applied Physiology, 94, 833–848. Vaucher, E., Tong, X., Cholet, N., Lantin, S., & Hamel, E. (2000). GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood Xow. Journal of Comparative Neurology, 421, 161–171. Voutsinos-Porche, B., Bonvento, G., Tanaka, K., Steiner, P., Welker, E., Chatton, J. Y., et al. (2003). Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron, 37, 275–286. Waldvogel, D., van, G. P., Muellbacher, W., Ziemann, U., Immisch, I., & Hallett, M. (2000). The relative metabolic demand of inhibition and excitation. Nature, 406, 995–998. Wilkerson, M., Lesniewski, L., Golding, E., Bryan, R., Jr., Amin, A., Wilson, E., et al. (2005). Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism. American Journal of Physiology Heart and Circulatory Physiology, 288, H1652–H1661. Woolsey, T. A., Rovainen, C. M., Cox, S. B., Henegar, M. H., Liang, G. E., Liu, D., et al. (1996). Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cerebral Cortex, 6, 647–660. Wu, D. M., Kawamura, H., Sakagami, K., Kobayashi, M., & Puro, D. G. (2003). Cholinergic regulation of pericyte-containing retinal microvessels. American Journal of Physiology Heart Circulation Physiology, 284, H2083–H2090. Yaksh, T., Wang, J., & Go, V. (1987). Cortical vasodilatation produced by vasoactive intestinal polypeptide (VIP) and by physiological stimuli in the cat. Journal of Cerebral Blood Flow and Metabolism, 7, 315–326. Yang, G., & Iadecola, C. (1996). Glutamate microinjections in cerebellar cortex reproduce cerebral vascular eVects of parallel Wber stimulation. American Journal of Physiology, 271 (Regulatory Integrative Comp. Physiol. 40), R1568–R1575. Yang, G., & Iadecola, C. (1997). Obligatory role of NO in glutamatedependent hyperemia evoked from cerebellar parallel Wbers. American Journal of Physiology, 272 (Regulatory Integrative Comp. Physiol. 41), R1155–R1161. Yang, G., Chen, G., Ebner, T.J., & Iadecola, C. (1999). Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. American Journal of Physiology, 277 (Regulatory Integrative Comp. Physiol. 46), R1760–R1770. Yang, G., Zhang, Y., Ross, M. E., & Iadecola, C. (2003). Attenuation of activity-induced increases in cerebellar blood Xow in mice lacking neuronal nitric oxide synthase. American Journal of Physiology Heart Circulation Physiology, 285, H298–H304. Zhang, F., Xu, X., & Iadecola, C. (1995). Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience, 69, 1195–1204. Zonta, M., Angulo, M., Gobbo, S., Rosengarten, B., Hossmann, K., Pozzan, T., et al. (2003a). Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience, 6, 43–50. Zonta, M., Sebelin, A., Gobbo, S., Fellin, T., Pozzan, T., & Carmignoto, T. (2003b). Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. Journal of Physiology, 553.2, 407–414. Please cite this article as: Carrie T. Drake, Costantino Iadecola, The role of neuronal signaling in controlling cerebral blood Xow, Brain and Language (2006), doi:10.1016/j.bandl.2006.08.002