* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Differential Spatial Organization of Otolith Signals in Frog Vestibular
Artificial general intelligence wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Axon guidance wikipedia , lookup
Neural engineering wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Multielectrode array wikipedia , lookup
Neural oscillation wikipedia , lookup
Mirror neuron wikipedia , lookup
Neuroregeneration wikipedia , lookup
Single-unit recording wikipedia , lookup
Neural coding wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroscience in space wikipedia , lookup
Development of the nervous system wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Evoked potential wikipedia , lookup
Central pattern generator wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Optogenetics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuroanatomy wikipedia , lookup
Microneurography wikipedia , lookup
Synaptic gating wikipedia , lookup
Circumventricular organs wikipedia , lookup
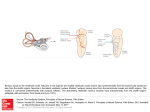
J Neurophysiol 90: 3501–3512, 2003. First published July 9, 2003; 10.1152/jn.00372.2003. Differential Spatial Organization of Otolith Signals in Frog Vestibular Nuclei Hans Straka,1 Stefan Holler,1 Fumiyuki Goto,1 Florian P. Kolb,1 and Edwin Gilland2 1 Physiologisches Institut, 80336 München, Germany; and 2Department of Physiology and Neuroscience, New York University Medical Center, New York, New York 10016 Submitted 15 April 2003; accepted in final form 5 July 2003 Afferent terminations from individual otolith organs in frog overlap in many vestibular subnuclei with those of semicircular canal afferent fibers (Birinyi et al. 2001). This overlap of canal and otolith afferent terminations is paralleled by a highly specific convergence of afferent signals from particular canal and otolith organs in ⬃50% of the second-order vestibular neurons (2°VN) as evidenced from intracellular recordings after electrical stimulation of individual canal and otolith nerve branches (Straka et al. 2002b). Similar proportions of convergent otolith⫹canal neurons have been recorded in mammalian vestibular nuclei (Dickman and Angelaki 2002). Detailed comparisons, however, between anuran and mammalian vestibular pathways are complicated by the presence in frogs and most nonmammalians of three otolith organs (de Burlet 1929) that are sensitive to vestibular and/or auditory stimuli (Lewis and Narins 1999). In frogs, both utricle and lagena function primarily as graviceptors, whereas the saccule is primarily an auditory organ sensing low-frequency substrate vibrations (Blanks and Precht 1976; Caston et al. 1977; Harada et al. 2001; Lewis and Narins 1999). In mammals other than monotremes, the lagena is absent, and the saccule primarily serves equilibrium functions. In frog and mammals, the saccule, however, also has a lesser role in transducing, vestibular (Gallé and Clemens 1973; Lannou and Cazin 1976) and auditory (McCue and Guinan 1994) signals, respectively. The variation in otolith functions between different taxa contrasts with the essentially complete conservation of semicircular canal function in all jawed vertebrates. Similarities between mammalian and frog semicircular canal pathways extend from general patterns of afferent fiber projections down to precise locations within vestibular nuclei of canal-specific premotor projection neurons (Baker 1998; Birinyi et al. 2001; Büttner-Ennever 1992; Straka et al. 2000a). In frog, afferent nerve fibers from individual semicircular canals terminate differentially in the four major vestibular subnuclei (Birinyi et al. 2001) in patterns that quantitatively match the spatial distribution of semicircular canal nerve-evoked monosynaptic responses as well as the locations of identified 2° canal neurons (Straka et al. 2000a). Intracellular recordings demonstrated that monosynaptic responses of 2°VN predominately originated from only one ipsilateral semicircular canal in frog (Holler and Straka 2001; Straka et al. 1997, 2002b) as in pigeon (Wilson and Felpel 1972) and cat (Kasahara and Uchino 1974; Sans et al. 1972; Sato et al. 2002). The restricted convergence of afferent signals from the three semicircular canals at the first central synapse is paralleled by a partial segregation of clusters of 2° horizontal canal (HC), 2° anterior vertical canal (AC), and 2° posterior vertical canal (PC) neurons (Straka et al. 2000a). Because the locations of 2°HC, 2°AC and 2°PC neurons were not restricted to particular vestibular subnuclei (Straka et al. 2000a), the possibility exists that the different clusters of 2°VN were organized with respect to their developmental origins. In fact, all classical vestibular subnuclei have been shown to originate from similar rhombomeres in frog (Straka et al. 2001) and chicken (Cambronero and Puelles 2000). Furthermore, vestibular neurons projecting to different targets occupy unique and rather Address for reprint requests and other correspondence: H. Straka, Dept. of Physiology, Pettenkoferstr. 12, 80336 Munich, Germany (E-mail: straka@ wifomail.med.uni-muenchen.de). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. INTRODUCTION www.jn.org 0022-3077/03 $5.00 Copyright © 2003 The American Physiological Society 3501 Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 Straka, Hans, Stefan Holler, Fumiyuki Goto, Florian P. Kolb, and Edwin Gilland. Differential spatial organization of otolith signals in frog vestibular nuclei. J Neurophysiol 90: 3501–3512, 2003. First published July 9, 2003; 10.1152/jn.00372.2003. Activation maps of pre- and postsynaptic field potential components evoked by separate electrical stimulation of utricular, lagenar, and saccular nerve branches in the isolated frog hindbrain were recorded within a stereotactic outline of the vestibular nuclei. Utricular and lagenar nerveevoked activation maps overlapped strongly in the lateral and descending vestibular nuclei, whereas lagenar amplitudes were greater in the superior vestibular nucleus. In contrast, the saccular nerveevoked activation map coincided largely with the dorsal nucleus and the adjacent dorsal part of the lateral vestibular nucleus, corroborating a major auditory and lesser vestibular function of the frog saccule. The stereotactic position of individual second-order otolith neurons matched the distribution of the corresponding otolith nerve-evoked activation maps. Furthermore, particular types of second-order utricular and lagenar neurons were clustered with particular types of second-order canal neurons in a topology that anatomically mirrored the preferred convergence pattern of afferent otolith and canal signals in second-order vestibular neurons. Similarities in the spatial organization of functionally equivalent types of second-order otolith and canal neurons between frog and other vertebrates indicated conservation of a common topographical organization principle. However, the absence of a precise afferent sensory topography combined with the presence of spatially segregated groups of particular second-order vestibular neurons suggests that the vestibular circuitry is organized as a premotor map rather than an organotypical sensory map. Moreover, the conserved segmental location of individual vestibular neuronal phenotypes shows linkage of individual components of vestibulomotor pathways with the underlying genetically specified rhombomeric framework. 3502 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND METHODS In vitro experiments were performed on the isolated brains of 29 grass frogs (Rana temporaria) in compliance with the “Principles of animal care,” publication No. 86-23, revised 1985 of the National Institutes of Health. Permission for these experiments was granted by Regierung von Oberbayern (211-2531-31/95). Grass frogs were deeply anesthetized (0.1% 3-aminobenzoic acid ethyl ester; MS-222) and perfused transcardially with iced Ringer solution [containing (in mM) 75 NaCl, 25 NaHCO3, 2 CaCl2, 2 KCl, 0.5 MgCl2, and 11 glucose; pH 7.4) (Straka and Dieringer 1993). The skull and the bony labyrinth were opened by a ventral approach, the three semicircular canals on either side were sectioned, and the brain with all labyrinthine endorgans attached to the J Neurophysiol • VOL VIIIth nerve was removed. The isolated brain, with the forebrain disconnected, was submerged in iced Ringer solution, and the dura, the labyrinthine endorgans, and the choroid plexus covering the IVth ventricle were removed. Brains were stored overnight at 6°C in oxygenated Ringer solution with a pH of 7.5 ⫾ 0.1 and were used ⱕ4 days after their isolation. For the experiments, the brain stem was fixed to the sylgard floor of a chamber (volume: 2.4 ml) that was continuously perfused with oxygenated Ringer solution at a rate of 1.3–2.1 ml/min. The temperature of the bath was electronically controlled and maintained at 14 ⫾ 0.1°C. For electrical stimulation of individual otolith or semicircular canal nerve branches, single constant current pulses (0.2 ms; 8 –12 A) across suction electrodes (120 –150 m ID) were used. The use of suction electrodes facilitated the isolation of individual nerve branches and minimized false negative or false positive results (see Straka et al. 1997, 2002b). Constant current pulses were produced by a stimulus isolation unit (WPI A 360) at a repetition rate of 0.5 Hz. For extracellular recordings, glass microelectrodes were fabricated with a horizontal puller (P-87 Brown/Flaming), beveled (30°, 20-m tip diameter) and filled with 2 M sodium chloride (⬃1 M⍀). Electrodes for intracellular recordings were filled with a mixture of 2 M potassium acetate and 3 M potassium chloride (10:1) which gave final resistances of ⬃90 –120 M⍀. Vertical displacements of the recording electrodes were controlled by a nanostepper. Horizontal displacements were performed with a two-axis micromanipulator. At the beginning of every experiment, field potentials evoked by separate stimulation of labyrinthine nerve branches were recorded at common reference locations to optimize the positions of the stimulus electrodes and to determine the stimulus thresholds for each nerve branch. The recording site for utricular and lagenar stimulation was 0.4 mm caudal to the VIIIth nerve at a depth of 0.4 mm below the top of the brain stem, while the saccular nerve reference site was at the same depth but at the caudal end of the VIIIth nerve. The stimulus threshold for the postsynaptic N1 field potential component (Precht et al. 1974) was similar for each of the three otolith nerves and ranged between 1.5 and 2.6 A. Stimulus intensities were indicated as multiples of these threshold values (⫻T). N1 field potential components evoked at 4⫻T were used for the construction of the activation maps. The spatial distribution of the evoked field potential components was systematically mapped with recording tracks from the surface of the brain stem to a depth of 0.8 mm in a parasagittal plane and three frontal planes (Fig. 1). Distances in the rostrocaudal direction were in reference to the caudal end of the entry of the VIIIth nerve, while dorsoventral and mediolateral distances were in reference to the convex apex of the brain stem at the particular rostrocaudal level (Fig. 1, B and D). Distances rostral to the caudal end of the VIIIth nerve root and medial to the apex of the brain stem were denoted with a negative sign (see Fig. 1, B and D). Frontal planes were positioned 0, ⫹0.4, and ⫹0.7 mm caudal to the VIIIth nerve (Fig. 1C, - - -) and comprised five depth tracks separated by 0.15 mm in laterality (Fig. 1B, 2). In the parasagittal plane, seven depth tracks explored the dorsal brain stem at a laterality of 0 mm (Fig. 1A, - - -). In this parasagittal plane, the recording sites were located ⫺0.7 and ⫺0.4 rostral and 0, 0.4, 0.7, 1.0, and 1.5 mm caudal to the entry of the VIIIth nerve (Fig. 1D, 2). Records were taken every 0.1 mm in depth. Second-order VN were recorded in depth tracks separated by 0.05 mm throughout most of the vestibular nuclei, i.e., between ⫺0.4 mm rostral and 1 mm caudal to the zero reference point. The recording area included the superior, lateral, and descending vestibular nuclei and excluded the most medial parts of the medial vestibular nuclei. The electrophysiological results were mapped onto a normalized geometry of the vestibular nuclei using the same reference coordinate system as in earlier studies (Birinyi et al. 2001; Straka et al. 2000a). This reference frame was obtained by reconstruction of the borders of the different vestibular subnuclei (Kuruvilla et al. 1985; Matesz 1979) in different frontal planes and a parasagittal plane based on serially sectioned specimens corrected for distortion and shrinkage (Straka et al. 2000a). The brain stem of each frog in the present study was stereotactically measured. The mean distance between the caudal end 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 conserved positions along the underlying rhombomeric scaffold (Straka et al. 2001). Thus it is possible that 2° canal and 2° otolith neurons of all types are segmentally organized (Baker 1998; Straka et al. 2001; Suwa et al. 1999). Although rhombomeres generally are evident only during embryonic stages, all cranial nerve efferent neurons in ranid frogs reside in their original embryonic rhombomeric locations not only in larvae (Straka et al. 1998) but also in adults (Straka et al. 2000b). This unique positional stability was exploited to create a stereotactic map in adult frogs that mirrors the embryonic and larval rhombomeric framework and that can be used to assign the location of particular subgroups of 2°VN within the underlying hindbrain segmental units (Straka et al. 2000b). The differences in function of specific otolith organs in frogs and mammals may provide a test case for examining the determinants of central vestibular organization. Although utricular function seems roughly comparable between these groups, lagenar and saccular functions diverge significantly in ways that should be reflected in both afferent projections and central organization. The detailed convergence pattern of afferent signals originating from individual labyrinthine nerve branches in frog was described elsewhere (Straka et al. 2002b). The present study focuses on the spatial distribution of field potentials evoked from individual otolith nerve branches and the locations of identified second-order otolith neurons within the vestibular and auditory nuclei. Correlation of these data allows further inferences about the segmental organization of functional pathways and the dendritic geometry of vestibular neuropile zones. Pre- and postsynaptic field potentials evoked from separate electrical stimulation of the utricular, lagenar, and saccular nerve branches were mapped in the isolated frog brain. Systematic recording of monosynaptic afferent excitatory postsynaptic potentials (EPSPs) in 2°VN from the utricular, lagenar, or saccular nerves, as well as from individual canal nerves, was used to determine the positions of particular types of 2° otolith and 2° canal neurons. Superimposing the activation maps and the positions of the identified 2°VN on a stereotactic anatomical map of the vestibular nuclei (Straka et al. 2000a) as well as on a reconstructed map of adult frog rhombomere boundaries (Straka et al. 2000b) allowed specification of otolith pathways both in terms of their anatomical organization within the vestibular subnuclei and in terms of their possible ontogenetic segmental origins. The results show that the locations of specific classes of 2°VN are highly conserved between frogs and other vertebrates. Two closely related explanations for this are discussed, the role of segmentation in spatial specification of neuronal phenotypes and the organization of central vestibular pathways as premotor maps for postural and gaze control. SPATIAL ORGANIZATION OF OTOLITH SIGNALS A C 3503 B D of the cerebellum and the obex was 3.62 ⫾ 0.08 mm (mean ⫾ SD; n ⫽ 29). The distance between the left and right top of the brain stem at the level of the VIIIth nerve was 2.57 ⫾ 0.05 mm (n ⫽ 29), and the distance between the top of the brain stem and the midline (floor of the IVth ventricle at the level of the VIIIth nerve) was 0.74 ⫾ 0.03 mm (n ⫽ 29). These values were very similar to those obtained in an earlier study (Straka et al. 2000a) and were used to define a standard brain size to which the brains of different individuals were normalized. The normalized locations of the vestibular nuclei in the frontal planes (e.g., Fig. 1, A and B) and in the parasagittal plane (Fig. 1, B and C) were used to specify the search areas for this mapping study. In addition to being mapped within the boundaries of the vestibular nuclei, the locations of particular types of 2° canal and 2° otolith neurons were also correlated to a stereotactic map of adult rhombomere borders (Straka et al. 2000b). The adult frog rhombomeric map was generated from measurements on a large series of horizontally sectioned brains that showed retrogradely labeled neurons in all cranial nerve efferent nuclei at essentially the same positions as in larval and embryonic frogs (Straka et al. 2000b). Averages from 20 single sweeps of the evoked field potentials at a given recording site were digitized at 20 kHz (CED 1401, Cambridge Electronic Design), stored on computer and analyzed off-line (Signal, Cambridge Electronic Design). From the averaged field potentials evoked from individual otolith nerves, separate depth profiles of N0 and N1 components were constructed for each recording track. Maximal response amplitudes of N0 and N1 field potentials evoked by stimulation of a particular otolith nerve was determined for the three frontal planes and for the parasagittal plane, respectively. These maximal N0 and N1 field potential amplitudes served to normalize all other responses evoked from the same nerve branch for all planes in a particular experiment. Isopotential surface plots were calculated through linear interpolation between maximal response amplitudes after averaging the results from all corresponding experiments (Straka et al. 2000a). Four groups of relative response amplitudes (ⱕ25%, 50, 75, or 100%) were represented by different gray tones (Origin, Microcal Software). Synaptic responses in intracellularly recorded 2°VN after electrical stimulation of individual labyrinthine nerve branches were analyzed from averages of 20 –30 single sweeps. Graphical presentations were made using Corel Draw (Corel). J Neurophysiol • VOL RESULTS Field potentials after stimulation of individual otolith nerve branches Field potentials recorded in the vestibular nuclei after separate electrical stimulation of otolith nerve branches consisted of early (N0) and late (N1) response components (Fig. 2). The mean latencies of lagenar (LA) nerve-evoked N0 and N1 response components (N0: 1.2 ⫾ 0.2 ms; N1: 3.0 ⫾ 0.3 ms; n ⫽ 18) were significantly shorter (P ⱕ 0.05) than those of saccular (SA; N0: 1.5 ⫾ 0.3 ms; N1: 3.2 ⫾ 0.4 ms; n ⫽ 15) or utricular (UT) nerve-evoked responses (N0: 1.7 ⫾ 0.2 ms; N1: 3.5 ⫾ 0.4 ms; n ⫽ 16). These differences corresponded to the distances between the recording sites in the brain stem and the stimulation electrodes on the three nerves. N0 and N1 response components were separated by ⬃1.8 ms, which represents the delay of one synapse at a bath temperature of 14°C as in earlier studies (Straka and Dieringer 1993; Straka et al. 1997, 2002b). Thus the N1 field potential component represents the monosynaptic excitation of 2°VN as described by Precht et al. (1974). N0 and N1 field potential components showed similar spatial amplitude distributions along the rostrocaudal extent of the recording area, whereas in frontal planes the N0 field potential components were shifted laterally by ⬃50 –100 m with respect to the N1 field potential components (not shown). This lateral shift of the N0 field potential components is similar to that observed for canal nerve-evoked N0 field potentials (Straka et al. 2000a) and reflects the entry of afferent fibers from the lateral direction. Because other differences were not evident, only the distribution of N1 field potential components will be used to describe the topography of otolith nerve-evoked responses in the brain stem. Topography of otolith nerve-evoked field potentials The spatial distributions of the N1 field potential components evoked after stimulation of the UT, LA, and SA nerve branches 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 FIG. 1. Schematic diagram of the vestibular nuclei and distribution of the recording sites in the dorsal hindbrain. A and C: frontal (A) and parasagittal (C) view of the vestibular nuclei as reconstructed from data published by Matesz (1979) and Kuruvilla et al. (1985). Inset: schematic outline of the transverse section at 0.4 mm caudal to the VIIIth nerve. - - - in A, the laterality of the parasagittal plane shown in C; - - - in C, the rostrocaudal positions of the three frontal planes. B and D: electrode tracks (from surface to 0.8 mm in depth) through the vestibular nuclei. 2, the laterality (B) or the rostrocaudal position (D) of electrode tracks. Zero laterality in B refers to the top of the brain stem, 0 rostrocaudal position in D refers to the caudal end of the entry of the VIIIth nerve. Calibrations are the same for A and B and C and D, respectively. CB, cerebellum; DN, dorsal nucleus; DVN, descending vestibular nucleus; LVN, lateral vestibular nucleus; MVN, medial vestibular nucleus; SVN, superior vestibular nucleus. Figure modified from Straka et al. (2000a). 3504 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND B C A FIG. 2. Field potentials in the vestibular nuclei evoked by separate stimulation of individual otolith nerve branches. A–C: pre- (N0) and postsynaptic (N1) field potential components evoked by stimulation of the utricular (UT) nerve (A), lagenar (LA) nerve (B), and saccular (SA) nerve (C). Utricular and lagenar responses were recorded 0.4 mm caudal to the entry of the VIIIth nerve, and saccular responses at the entry of the VIIIth nerve at a depth of 0.4 mm. Each record represents an average of 20 responses. - - -, baseline and arrowheads, the stimulus onset. Calibration bar in C applies also for A and B. are shown in a parasagittal plane (Fig. 3, A–C) and a series of frontal planes (Fig. 4) with reference to the outlines of the vestibular and auditory subnuclei. A substantial otolith nerveevoked N1 field potential component was encountered in all vestibular subnuclei except for the medial vestibular nucleus (MVN) where amplitudes rarely reached 25% of the maximal amplitudes (Fig. 4, A–C). After selective stimulation of the UT nerve (n ⫽ 5) the largest relative amplitudes (75–100%, black area in Figs. 3A and 4A, 1–3) of the N1 field potential component were recorded in a broad region ⱕ0.3 mm wide located ventrally in the vestibular nuclei. This region coincided with the lateral vestibular nucleus (LVN) and more caudally with the descending vestibular nucleus (DVN). In the LVN, the hotspot shifted from a more ventral position rostrally (Fig. 4A1) to a more central and dorsal position caudally (Fig. 4A, 2 and 3). Selective stimulation of the LA nerve (n ⫽ 5) produced maximal amplitudes of the N1 field potential component in a region about 0.2 mm wide, between the entry of the VIIIth nerve and 0.4 mm more caudal, corresponding to the dorsal portion of the central LVN and the rostral tip of the DVN (Figs. 3B and 4B, 1–3). An area with 50 –75% of the maximal amplitude (dark gray area in Fig. 3B) extended rostrally and dorsally from the nerve entry and coincided with the dorsal parts of the rostral LVN and adjacent superior vestibular nuclei (SVN; Figs. 3B and 4B1). Overall, the LA nerve-evoked N1 field potential component was located slightly more dorsal than that of the UT nerve but overlapped with the latter in the central part of the LVN (Figs. 3, A and B, and 4, A1–A3 and B1–B3). The maximal amplitudes of the N1 field potential component J Neurophysiol • VOL B C FIG. 3. Amplitude distribution plots of otolith nerve-evoked postsynaptic N1 field potential components and location of 2nd-order otolith neurons in a parasagittal plane through the vestibular nuclei at a laterality of 0 mm. A–C: correlation of normalized amplitudes of the N1 field potential components with outlines of the average location (⫾SD) of identified 2nd-order otolith neurons in each recording track and with reconstructed color-coded anatomical outlines of the vestibular nuclei. Second-order vestibular neurons were monosynaptically activated from the UT (A), the LA (B), or the SA (C; data obtained from Fig. 6D). Data from 5 animals, respectively. Calibration arrows indicate dorsal and caudal and correspond to 0.2 mm. Coordinates are the same as in Fig. 1. Relative amplitude magnitudes are represented by gray tones. 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 after selective stimulation of the SA nerve (n ⫽ 5) formed a narrow band ⬃0.1– 0.2 mm wide oriented from rostral and ventral to caudal and dorsal in parts of both the vestibular nuclei and auditory nuclei (Figs. 3C and 4C, 1–3). The more dorsal part of this distribution coincided with the ventral and caudal part of the dorsal nucleus (DN), an auditory relay center in frogs, and with the dorsal edge of the rostral DVN (Figs. 3C and 4C, 2 and 3). The rostroventral region of maximal amplitudes corresponded to the dorsal part of the LVN at the level of the VIIIth nerve (Figs. 3C and 4C1). Thus except for an overlap with UT and LA nerve-evoked N1 field potential component in the LVN, the topography of SA nerve-evoked responses differed considerably from those of the UT and LA nerves. Although the otolith nerve-evoked field potentials shown here do not represent tightly closed fields, regions with high amplitudes closely matched the topographical distribution of monosynaptically activated 2° otolith neurons. This is shown by superposition on a parasagittal plane of the stereotactically determined location of second-order UT (2°UT), LA (2°LA), and SA (2°SA) neurons (see following text) onto the respective N1 field potential distributions (Fig. 3, A–C). The upper and lower ranges of cell locations outline areas that closely match A SPATIAL ORGANIZATION OF OTOLITH SIGNALS A2 A3 B1 B2 B3 C1 C2 C3 the field potential areas with ⬎50% of maximal amplitude for LA and SA nerves (Fig. 3, B and C) and ⬎75% of maximal amplitude for the central region of UT nerve activation (Fig. 3A). Such close correspondence suggests that the evoked responses initiated mostly near soma, likely at proximal dendrites. Thus when sampled within a normalized stereotactic framework, the topography of otolith nerve-evoked postsynaptic N1 field potentials provides good evidence for differential spatial organization of otolith signals in the vestibular nuclei. Differential spatial organization of second-order vestibular neurons identified by afferent otolith or canal signals Vestibular neurons (n ⫽ 874) were identified by their afferent input after stimulation of combinations of individual otolith and semicircular canal nerves in different sets of experiments. Identified cells were classified according to the origin of the monosynaptic input from a particular labyrinthine endorgan (Fig. 5, A1, B1, and C1). The remaining vestibular nerve branches evoked either small di- or polysynaptic excitatory responses or no responses (Fig. 5, A2–A4, B2–B4, and C2–C4). According to this definition, 2°VN were subdivided into 2°UT (Fig. 5A), 2°LA (Fig. 5B), or 2°SA neurons (Fig. 5C), into 2° anterior vertical canal (AC), 2° posterior vertical canal (PC), and 2° horizontal canal (HC) neurons or into particular 2°otolith⫹canal neurons. The percentages of specific monoJ Neurophysiol • VOL FIG. 4. Amplitude distribution plots of N1 field potential components evoked by separate stimulation of the 3 otolith nerve branches in frontal planes with reference to the vestibular subnuclei. A–C: normalized amplitudes of the N1 field potential component evoked by separate stimulation of the UT (A, 1–3), LA (B, 1–3), and SA (C,1–3) recorded in frontal planes at 3 different rostrocaudal levels (0 mm, A1–C1; 0.4 mm, A2–C2; 0.7 mm, A3–C3 caudal to the VIIIth nerve; see Fig. 1, C and D). N1 field potential distribution maps were superimposed on reconstructed color-coded anatomical outlines of the vestibular nuclei. Calibration arrows indicate dorsal and lateral and correspond to 0.3 mm. Mediolateral coordinates are the same as Fig. 1B. Relative amplitude magnitudes are represented by gray tones. Abbreviations are the same as in Fig. 1. synaptic canal and otolith signal convergence in 2°VN are indicated in Table 1. The predominant convergences were between UT and HC signals and between LA and either AC or PC signals. Very few 2°SA⫹canal neurons were observed. In a few 2°VN a convergence of mono- and disynaptic inputs from two labyrinthine nerve branches were encountered. However, neither the specific convergence patterns nor the spatial locations of these neurons differed markedly from those that exhibited convergent monosynaptic signals. The location of each identified 2°VN was stereotactically mapped in the same coordinate system used for the field potential maps in the parasagittal plane (Fig. 6) (Straka et al. 2000a). The spatial distribution of particular classes of 2°VN were then correlated with the anatomical outlines of the vestibular subnuclei and with the boundaries of ontogenetically defined hindbrain segments (Fig. 7, A–C). Second-order VN with monosynaptic afferent input from individual otolith nerve branches were distributed across the entire recording area (Fig. 6, A–C), and exhibited a crude clustering into three general zones that were dominated by particular afferent inputs (Fig. 6D). The degree of clustering was more pronounced for 2° otolith neurons than for 2° canal neurons (Fig. 6, A–C). Specifically, most 2°UT and 2°UT⫹canal neurons were located ventrally and caudally in the recording area, coinciding with the peak of the activation 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 A1 3505 3506 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND map of the UT nerve-evoked N1 field potential component (Figs. 3A and 6, A and D). Moreover, this area also coincided with the area of largest concentration of 2°HC neurons (Fig. 6A). This cluster of 2°UT and UT⫹canal neurons was located in the central and ventral part of the LVN and in the DVN, whereas only a few neurons with an afferent UT nerve input were encountered in the ventral part of the SVN (Fig. 7, A and C). In contrast, the majority of 2°LA and LA⫹canal neurons were distributed in a relatively broad band from rostral and dorsal to caudal and ventral (Fig. 6, B and D), corresponding largely to the SVN and the dorsal part of the LVN/DVN (Fig. 7, A and C). This distribution coincided very closely with the region in which the LA nerve-evoked N1 field potential component exceeded 50% of the maximal amplitude (Fig. 3B). In the SVN, 2°LA and 2°LA⫹canal neurons were concentrated with 2°AC neurons (Fig. 7B) and in the LVN/DVN with 2°UT and 2°UT⫹canal neurons (Fig. 7, A and C). The population of 2°SA and the few 2°SA⫹canal neurons formed a more restricted cluster than the other classes of neurons and extended caudally and dorsally beginning from the caudal end of the VIIIth nerve (Fig. 6, C and D). Second-order SA neurons were localized with 2° canal, 2°UT, and 2°LA neurons only at the level of the VIIIth nerve (Fig. 6, A–D), while at more caudal levels 2°SA neurons were located distinctly more dorsal than the other types. This distribution coincided largely with the area of maximal SA nerve-evoked N1 amplitudes, although 2°SA neurons were located slightly more dorsal relative to the N1 field potential component (Fig. 3C). With respect to nuclear borders, 1. Distribution of second-order (2°) vestibular neurons with non-convergent and convergent monosynaptic afferent inputs from individual otolith and semicircular canal nerve branches TABLE Utricular-Canal Convergence 2° otolith neurons 2° canal neurons AC PC HC 2° otolith ⴙ canal neurons otolith ⫹ AC otolith ⫹ PC otolith ⫹ HC 2° multi. canal neurons 2° otolith ⴙ mult. canal neurons Total Lagenar-Canal Convergence 23 (10) 122 (50) 56 (23) 50 (20) 16 (7) 28 (12) 98 (43) 19 (8) 39 (17) 40 (18) 65 (27) 8 (3) 9 (4) 48 (20) Saccular-Canal Convergence 41 (25) 110 (67) 38 (23) 39 (24) 33 (20) 71 (31) 45 (20) 26 (11) 0 (0) 19 (8) 13 (5) 242 (100) 5 (3) 3 (2) 2 (1) 0 (0) 17 (8) 14 (6) 228 (100) 9 (5) 0 (0) 164 (100) Numbers indicate recorded neurons. Percentages in parentheses. Major categories are in bold type; canal-specific subcategories are in plain type. In 3 different sets (utricular, lagenar, and saccular canals) 1 otolith nerve branch and all 3 canal nerve branches, respectively, were electrically stimulated (modified from Straka et al. 2002b). AC, PC, and HC, anterior vertical, posterior vertical, and horizontal canal, respectively. J Neurophysiol • VOL 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 FIG. 5. Convergence of otolith and canal nerve-evoked inputs in 3 different 2nd-order vestibular neurons. A, 1– 4: 2nd-order UT neuron identified by a monosynaptic excitatory postsynaptic potential (EPSP) from the UT nerve (A1). This neuron received in addition a disynaptic excitation from the horizontal canal (HC) nerve (A4), a very weak long-latency excitatory input from the anterior vertical canal (AC) nerve (A2) and no input from the posterior vertical canal (PC) nerve (A3). B, 1– 4: 2nd-order LA neuron identified by a monosynaptic EPSP from the LA nerve (B1). This neuron received in addition an oligosynaptic excitation from the AC (B2) and PC (B3) nerve, respectively, and no input from the HC nerve (B4). C, 1– 4: 2nd-order SA neuron identified by a monosynaptic EPSP from the SA nerve (C1). This neuron received no input from any 1 of the 3 semicircular canal nerves (C, 2– 4). Stimulus intensity is expressed in multiples of the stimulus threshold intensity (⫻T) of the N1 field potential component. o, the mean ⫾ SD of the onset latency of the N1 field potential after stimulation of the respective nerve branch. - - -, baselines; , stimulus onset. Each record represents the average of 24 responses. SPATIAL ORGANIZATION OF OTOLITH SIGNALS 3507 B C A D B FIG. 6. Stereotactic position of 2nd-order vestibular neurons identified from individual otolith and semicircular canal nerve branches plotted in a parasagittal coordinate system. A–C: scatter plots showing the coordinates of vestibular neurons monosynaptically activated from the UT nerve (A), from the LA nerve (B), from the SA nerve (C) and/or from 1 of the 3 semicircular canal nerves. D: scatter plot showing the differential distribution of all 2nd-order vestibular neurons that received a monosynaptic input from the UT, LA, or SA nerve, respectively. Neurons in A–D were from multiple sets of experiments. Coordinates and abbreviations are the same as in Fig. 1. C 2°SA neurons were located in the dorsal part of the LVN and throughout the adjacent ventral part of the DN (Fig. 7A). Rhombomeric organization of second-order otolith and canal neurons Although rhombomeres (r) are not visible in adult frogs, a segmental map of adult cranial nerve efferent nuclei can be inferred because of the complete retention of the larval segmental efferent neuronal topography in adults (Straka et al. 2000b, 2002a). Precise measurements of the borders of adjacent efferent nuclei within a coordinate system based on exJ Neurophysiol • VOL FIG. 7. Differential location of 2nd-order otolith and 2nd-order canal neurons with respect to vestibular subnuclei and the adult frog rhombomeric framework in a parasagittal plane. A–C: mapping of the spatial position of vestibular neurons with a monosynaptic EPSP from a particular otolith nerve (A), from a particular semicircular canal nerve (B), or from an otolith nerve and a semicircular canal nerve (C) onto anatomical outlines of the vestibular nuclei (black) and onto reconstructed rhombomere boundaries (red). Vestibular subnuclei in C apply also to A and B. Calibration arrows in A indicating dorsal and lateral correspond to 0.2 mm and apply also to B and C. Hindbrain segmental boundaries for adult frogs at the level of the vestibular nuclei were obtained from published data (Straka et al. 2000b). r2–r7, adult rhombomeric borders. Other abbreviations as in Fig. 1. 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 ternal landmarks were used to create a quantitative segmental map in adult frogs that mirrors the organization of the larval rhombomeric framework (Straka et al. 2000b). Plotting of physiologically identified hindbrain neurons onto this map provides an initial estimate of the linkage between the physiological properties of 2°VN in the adult frog hindbrain with the underlying genetically specified segmental framework (Fig. 7, A–C). Correlation with the rhombomeric borders indicated that the majority of 2°VN with an afferent UT nerve input were located in r3 and, especially, in r5, the same segments in which 2°HC neurons were particularly abundant (Fig. 7B). Correspondingly, most 2°UT⫹HC neurons that comprised the largest subpopulation of convergent 2°UT⫹canal neurons (see Table 1) also were located in r5 (Fig. 7C). In contrast, 2°VN with an afferent lagenar input were distributed from r3 to r6, with a particularly large number in r3 (Fig. 7A). This segment also contained most of the 2°AC neurons as well as many 2°PC neurons (Fig. 7B). This matches the exclusive convergence of afferent AC and PC signals in 2°LA⫹canal neurons (see Table 1), the majority of which were likewise located in r3 (Fig. 7C). Finally, the rhombomeric plots indicated that r4 contained few 2°otolith or 2°canal neurons except for 2°SA neurons, which A 3508 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND spanned from r4 to r6, with a preponderance in r4 (Fig. 7A). The 2°SA neurons in r5 and r6 occupied a far dorsal position as indicated earlier. DISCUSSION Specificity of otolith nerve-evoked response topography in the hindbrain The N1 field potential components evoked by electrical stimulation of the vestibular nerve or of individual branches to labyrinthine endorgans reflects the monosynaptic activation of 2°VN (Precht et al. 1974; Straka et al. 1997). Di- and polysynaptic connections were evident in intracellular recordings, including, in a few cases, convergence with monosynaptic responses in 2°VN. However, disynaptic field potential components are more variable in amplitude than monosynaptic responses and have not yielded to reliable generation and analysis. Notably, neither the convergence pattern nor the spatial location of neurons with disynaptic responses differed much from neurons that received convergent inputs with monosynaptic latencies. For that reason, the study was restricted to the topography of the N1 field potential amplitudes and to the topography of 2°VN. Due to the large and complex dendrites of 2°VN, the vestibular nucleus represents less of a closed field than, for example, the abducens nucleus. However, afferent labyrinthine nerve inputs have been shown to terminate largely on the soma and proximal dendrites of frog 2°VN (Dieringer and Precht 1979; Ozawa et al. 1974). This is reflected by the close correlation between the otolith nerve-evoked N1 field potential component maps and the weighted locations of respective 2° otolith neurons (Fig. 3, A–C). A similar close spatial correlation was evident in a similar analysis of semicircular canal nerve field responses and 2°VN locations (Straka et al. 2000a). Minor differences between the N1 field potential map and the J Neurophysiol • VOL Organization of afferent otolith signals in the vestibular nuclei of frogs and other vertebrates Selective nerve branch labelings in frog have shown that both UT and LA afferent fibers terminate differentially throughout most of the ipsilateral vestibular subnuclei (Birinyi et al. 2001). Both nerves project strongly to the central LVN and rostral DVN. UT nerve afferents also terminate densely in the lateral and ventral parts of the LVN, more caudal portions of the DVN and in the lateral portion of the abducens complex, whereas LA fibers are rare or absent in these regions (Birinyi et al. 2001). Conversely, the LA terminal distribution was especially dense in the SVN, where UT terminations were less dense (Birinyi et al. 2001). The MVN contains very few UT or LA terminal endings. The activation maps of both pre- and postsynaptic response components for UT and LA nerve stimulation were highly consistent with these anatomical data, including the absence of field potential components from the MVN. Likewise, the large number of 2°UT neurons recorded in the ventral part of the LVN and DVN, and of 2°LA neurons recorded more dorsal in these subnuclei as well as in the SVN, closely matched the anatomical distribution of UT and LA afferent fibers (Fig. 3, A and B) (Birinyi et al. 2001). The predominant termination of LA and UT afferent fibers in vestibular but not in auditory nuclei, as well as the colocalization of 2°LA and 2°UT neurons in particular vestibular areas, is consistent with their main functional role being vestibular signal processing (Baird and Lewis 1986; Lewis and Narins 1999). This is further corroborated by the pronounced convergence of specific canal and LA or UT signals (Straka et al. 2002b). The spatial overlap of UT and LA afferent fibers and of pre- and postsynaptic response components is not however mirrored at the single cell level because a given 2°VN may exhibit afferent UT or afferent LA signals but generally not both (Straka et al. 2002b). In addition to their role as linear gravitoinertial sensors, a second function of the frog UT and LA in detection of substrate vibrations (i.e., high-frequency vertical acceleration) is suggested by the presence of a narrow band of specialized hair cells along the striola of both otolith organs (Lewis and Li 1975) and by the presence of a small subpopulation of afferent fibers with high-frequency tuning properties that innervate these hair cells in the LA (Cortopassi and Lewis 1996). Central projections and second-order neurons of these higher frequency substrate vibration pathways remain to be investigated. In mammalian and avian species for which comparable data are available, the pattern of UT afferent terminations, evoked field potentials and identified 2°UT neurons are strikingly similar to the frog. UT afferent fibers terminate mainly in the DVN and to a lesser degree in the ventral part of the LVN in pigeon (Dickman and Fang 1996; Schwarz and Schwarz 1986), 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 Systematic mapping of field potentials evoked by separate stimulation of UT, LA, and SA nerve branches in the in vitro frog brain revealed the topography of pre- and postsynaptic otolith-specific activity in the ipsilateral vestibular and auditory nuclei. Both UT and LA nerve-evoked field potentials extended through large portions of the vestibular nuclei, with greatest overlap in the ventral part of the LVN and in the rostral DVN. In contrast, SA nerve-evoked field potentials occupied the dorsal LVN and adjacent ventral auditory nucleus. The segregation of afferent otolith signals was paralleled by the differential locations of corresponding types of 2° otolith neurons. These were localized with particular types of 2° canal neurons in functional patterns related to vestibular endorgan and motor plant spatial axes. Mapping the 2°VN types onto the adult rhombomeric framework allowed estimation of the segmental positions for the main sites of otolith-canal convergence. The present results complete an initial anatomical and physiological survey of afferent terminations and second-order neurons for each of the main vestibular endorgans in frog. Therefore after a note on specificity of the methods, the results will be discussed in the context of related frog studies and in comparison with other vestibular models. Finally, the topographical stability of second-order vestibular groups in different species will be examined from both functional and developmental views. average location of the respective 2°VN, e.g., SA nerve signals in the DN (Fig. 3C), reflect the likelihood that some afferent nerve fiber inputs may extend farther out along the proximal dendrites than others. Nonetheless, the topography of the N1 component largely mirrors the location of respective 2°VN. Thus combining the N1 field potential maps with the topography of stereotactically localized types of 2° otolith neurons gives reliable evidence for a differential organization of postsynaptic canal and otolith signals in the vestibular nuclei. SPATIAL ORGANIZATION OF OTOLITH SIGNALS J Neurophysiol • VOL portion of the SVN in cat (Gacek 1969; Stein and Carpenter 1967), monkey (Naito et al. 1995), and pigeon (Dickman and Fang 1996). In the cat, 2°SA neurons were mainly located in the LVN (35%) and DVN (42%) and to a lesser extent in the SVN (5%) and MVN (18%) (Imagawa et al. 1998; Kushiro et al. 2000; Ono et al. 2000; Sato et al. 2000; Zhang et al. 2001). In addition, a few SA afferent fibers were reported to be sensitive to high-frequency auditory stimuli and to terminate in the cochlear nucleus in cat (McCue and Guinan 1994) and gerbil (Kevetter and Perachio 1989). The spatial distribution of 2°SA neurons in cat thus differs considerably from frog, where most are located in the auditory nuclei and only a minor population in the rostral part of the LVN (Fig. 7A). This difference parallels the termination pattern of SA afferents in mammals versus frog and corresponds to a mainly vestibular role of the saccule in mammals and a mainly auditory role in frogs. Because the saccule is considered homologous between amphibians and mammals, this is an example of divergent change in both primary functional role and central terminations. Such functional changes, or changes in weighting among multiple functional roles, have occurred for other otolith organs as well (Fritzsch 1992). It is notable that the termination pattern of SA afferents and the locations of 2°SA neurons in cat are quite similar to those of the frog lagena, especially in the LVN and DVN. Likewise, strong convergence of PC and AC signals on 2°SA neurons and a predominance of spinal projections have been reported for cat (Sato et al. 2000; Zhang et al. 2002), strongly paralleling the frog LA pathways. This apparent evolutionary analogy is not too surprising because the mammalian saccule and the frog lagena are both primarily involved in sensing of vertical linear acceleration (Harada et al. 2001; Wilson and Melvill Jones 1979). Spatial organization and convergence of otolith and semicircular canal signals Comparing the spatial distribution of otolith signals with those from semicircular canals (Figs. 6, A–C and 7) shows that UT and LA but not SA inputs overlap a great deal with canal inputs. When colocalization of second-order canal and otolith neurons are compared (Fig. 7), the segregation of second-order vestibular and auditory centers are evident. Second-order UT neurons located mainly in the ventral LVN and DVN, were mixed predominantly with 2°HC neurons. On the other hand, 2°LA neurons, located mainly in the SVN and in the dorsal LVN, cluster mainly with 2°AC and 2°PC neurons. Individual neurons with monosynaptic inputs from both canal and otolith organs lie within the clusters of single-input 2°VN and show the same specific canal and otolith convergence patterns (Fig. 7C) (Straka et al. 2002b). The absence of overlap of canal and SA nerve inputs reflects the mainly auditory function of the saccule and is matched by the minimal degree of convergence of monosynaptic canal and SA signals in 2°VN (Fig. 7) (see also Straka et al. 2002b). Convergence between a putative minor SA tilt component and individual canal inputs might be represented by the few 2°SA⫹vertical canal neurons (Fig. 6C), but this has not been directly tested. The specific convergence patterns of canal and otolith signals on 2°VN show another detailed similarity between frog and mammals. The proportions of canal-only, otolith-only (⬃25% each) and canal⫹otolith neurons (⬃50%) recorded in primate VN 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 cat (Gacek 1969; Imagawa et al. 1995; Siegborn et al. 1991), and monkey (Naito et al. 1995; Newlands et al. 2002; Stein and Carpenter 1967). Descriptions of UT afferent terminations in the SVN vary from considerable (Dickman and Fang 1996; Imagawa et al. 1995; Naito et al. 1995) to negligible even within the same species (Gacek 1969; Stein and Carpenter 1967). In the cat, most 2°UT neurons were located in the ventral and central part of the LVN (45%) and in the DVN (33%), whereas fewer neurons were encountered in the SVN (9%) and MVN (13%) (Kushiro et al. 2000; Ono et al. 2000; Sato et al. 2000; Zakir et al. 2000; Zhang et al. 2001). The location of the majority of 2°UT neurons in cat is thus similar to that in frog and matches the major termination areas of UT afferents in both species. UT pathways thus present extensive similarities between frogs, mammals and birds with the exception of the limited number of high-frequency sensitive hair cells on the UT macula in the frog (Lewis and Li 1975) that might form part of a general substrate-to-bone sound conduction system primitively present in amphibians and amniotes (Clack 1997; Hetherington et al. 1986). Although a LA macula is present in monotremes (Ladhams and Pickles 1996), it is absent in therian mammals (de Burlet 1929), therefore a comparison of LA pathways between mammalian and frog vestibular models is not possible. The avian lagena is reported as the source of nonauditory signals in the statoacoustic nerve with central projections in the lateral portions of almost all vestibular subnuclei (Manley et al. 1991). LA projections to the cochlear nuclei have been reported in some studies (Boord and Karten 1974; Dickman and Fang 1996) but denied in others (Kaiser and Manley 1996) with the possibility that tracer spread to the adjacent basilar papilla might be the source of the cochlear nuclei projections. As with the utricle, no higher frequency LA pathways have been reported in birds. SA nerve afferent fibers terminate mainly in the DN and SA nuclei in frogs (Birinyi et al. 2001; Matesz 1988; Suarez et al. 1985; Will et al. 1985). These nuclei mediate signals to the superior olive and to the classical midbrain auditory nuclei (Kulik et al. 1994; Wilczynski 1981). This predominance of SA afferent termination in auditory nuclei is compatible with the exquisite substrate vibration sensitivity of SA nerve fibers (Christensen-Dalsgaard and Jørgensen 1988). Likewise, a large percentage of neurons recorded in this area in vivo exhibited tuning characteristics similar to SA afferents (ChristensenDalsgaard and Walkowiak 1999). The presence of maximal SA nerve-evoked activity in the ventral part of the DN and the location of many 2°SA neurons in an area dorsal to the vestibular nuclei (Figs. 3C and 4C) agree with a predominant role of the saccule as an acoustic organ sensing substrate vibration in frog (Lewis and Narins 1999; Lewis et al. 1982). SA activity does not extend into the dorsal part of the DN where afferent fibers from the basilar and amphibian papillae terminate (Straka, unpublished results). A secondary vestibular function for the frog saccule (Gallé and Clemens 1973; Lannou and Cazin 1976) is supported by the termination of afferent SA fibers (Birinyi et al. 2001), the extension of maximal saccular nerveevoked activity (Figs. 3C and 4C) and the presence of a small population of 2°SA neurons (Fig. 7A) all in a small area within the LVN. SA afferent fibers terminate densely in the LVN, in the rostral portion of the DVN, and to a lesser degree in the central 3509 3510 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND during alert three-dimensional angular and linear head acceleration (Dickman and Angelaki 2002) were similar to those of frog 2°VN recorded after separate electrical stimulation of nerves to the three canals, the utricle and the lagena (Straka et al. 2002b). Segmental organization of second-order vestibular neurons in frogs and other vertebrates J Neurophysiol • VOL 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 Specific vestibular subnuclei develop from similar combinations of rhombomeres in frog (Straka et al. 2001, 2002a), chicken (Cambronero and Puelles 2000) and mouse (Auclair et al. 1999). Likewise, vestibular relay neurons mediating specific canal and otolith reflexes develop from similar rhombomeres in frog (Straka et al. 2001, 2002a), fish (Baker 1998; Suwa et al. 1999) and chicken (Dı́az and Glover 2002; Glover 2000, 2001). Among these species, frogs are unique in retaining the segmental arrangement of these particular types of vestibular relay neurons and also of cranial nerve motoneurons throughout ontogeny (Straka et al. 2000b). This allowed construction of a stereotactic segmental map of the adult frog hindbrain based on the persistent embryonic and larval segmental framework (Straka et al. 2002a) (see also Fig. 7, A–C). Second-order canal neurons are spread throughout the region from r2 to r6 (Fig. 7B) with greater numbers of all three canal types found in r3 and r5 and lesser in r4 and r6. Also, 2°PC neurons are generally more dorsal than 2°HC neurons in r5– 6. Cells with monosynaptic input from single otolith organs form a more distinctly segregated pattern (Fig. 7A) with 2°UT and 2°LA neurons heavily represented in r3 and r5, whereas 2°SA neurons are concentrated dorsally in r4– 6. Mapping of 2°otolith⫹canal neurons (Fig. 7C) in effect shows the intersection of the separate otolith and canal patterns because cells with convergent monosynaptic inputs appear generally to be mingled with their respective single-input neighbors. Combined with retrograde labeling from oculomotor and spinal targets in larval frogs (Straka et al. 2001), the second-order convergence pattern seems readily interpretable in terms of distinct otolith pathways with unique canal convergences and efferent targets. Second-order UT neurons are especially numerous in the r5 region, extending both rostrocaudally and mediolaterally through all of r5 and the rostral part of r6, whereas a second smaller concentration of 2°UT neurons is seen in r3 (Fig. 7, A and C). In the large r5– 6 domain, three major groups stand out based on their specific canal convergences and locations within vestibular subnuclei and rhombomeric segments. First, a large cluster of 2°UT⫹canal neurons lying laterally and ventrally in r5 forms a possible tangential nucleus (Tan). Next, a smaller 2°UT⫹HC cluster located more medially in r5 likely projects to the adjacent abducens complex. Finally, a broader, more diffuse group extends dorsally and medially from the Tan group through caudal r5 and much of r6. The latter neurons comprise mixed 2°UT⫹canal neurons and match topographically with known ipsi- and contralateral oculomotor and spinal projections from the DVN (Straka et al. 2001). The putative tangential group is a dense clustering of 2°UT, 2° canal, and 2°UT⫹canal neurons of all three axes located in ventral parts of the caudal LVN and central DVN in r5 (Fig. 7). This group coincides with a proposed larval frog Tan (Straka et al. 2001) and with the teleost r5 Tan that serves as a combined linear and angular gravitoinertial center projecting to oculomotor and spinal targets (Suwa et al. 1999). The more medial r5 population of 2°HC and 2°UT neurons in the DVN are likely vestibular neurons projecting to the ipsiand contralateral abducens nuclei mediating the angular horizontal vestibuloocular reflex (Rohregger and Dieringer 2002; Straka et al. 1998, 2001). An additional origin of such a projection from the MVN at this segmental level is unlikely because HC nerve-evoked pre- and postsynaptic field potentials were absent from the MVN (Straka et al. 2000a). These neurons in the frog DVN would thus be homologous to the classical 2°HC neurons located in the mammalian MVN (Büttner-Ennever 1992). Such a comparison probably reflects differences in defining nuclear boundaries in frogs and mammals more than it does any genuine evolutionary modification. The smaller number of mixed 2°UT and 2° canal neurons in caudal r5– 6 might represent the origin of a separate vestibulospinal projection that relays canal and otolith signals from the DVN as suggested from larval dye mapping (Straka et al. 2001). Similarly, the r5 hindbrain segment in chicken (Cambronero and Puelles 2000) and most likely mouse (Auclair et al. 1999), gives rise to the DVN which along with the LVN is a major source of vestibulospinal projections mediating UT reflexes in mammals (see Büttner-Ennever 1999; Ono et al. 2000; Zakir et al. 2000; Zhang et al. 2001). Two groups receiving UT signals are located in the r2–3 region. A group of mixed 2°UT and 2°HC neurons is located in r3 (Fig. 7C) and may represent the origin of an ascending tract of Deiters’ (ATD) projecting to ipsilateral medial rectus motoneurons as suggested from larval dye labelings (Straka et al. 2001). The mammalian ATD arises from cells in the LVN (Highstein and Reisine 1981), an appropriate match for the r3 location in frogs. A second group of 2°UT neurons intermingled with 2°AC and 2°PC neurons (Fig. 7, B and C) coincides with the expected location of vestibular neurons that relay vertical canal and otolith signals to the oculomotor and trochlear nuclei. The small number of neurons in r2 is an underestimation because recordings at these hindbrain positions were restricted by the cerebellar peduncle. Second-order AC excitatory and inhibitory vestibuloocular neurons were suggested to develop mainly from r2–3 in fish (Baker 1998; Suwa et al. 1999), frog (Straka et al. 2001), and chicken (Dı́az and Glover 2002; Glover 2000), a location that coincides with the origin of the SVN in frog (Straka et al. 2001) and chicken (Cambronero and Puelles 2000). A predominant location of 2°AC vestibuloocular neurons in the SVN was also reported for pigeon (Wilson and Felpel 1972), rabbit (Highstein 1973; Highstein et al. 1971), cat (Hirai and Uchino 1984; Uchino et al. 1981), and monkey (Abend 1977; see Büttner-Ennever 1992). Overall therefore most of the vestibular neurons receiving UT input in frogs fall into groups with detailed segmental and projection similarities to UT pathways in fish and amniotes. Most 2°LA neurons lie interspersed with 2°AC and 2°PC neurons in r2–3 and r5– 6 (Fig. 7, A–C). This is not surprising because many 2°VN that received LA nerve input also received AC or PC nerve input and thus represent the same subpopulations (Straka et al. 2002b). The rostral group of 2°LA neurons in r2–3, however, in contrast to adjacent 2°AC and 2°PC neurons should not project to oculomotor and trochlear targets because lagenar signals do not contribute to linear vestibuloocular reflexes in frogs (Hess and Precht 1984; Rohregger and Dieringer 2002). The caudal group of 2°LA neurons in r5– 6 should be part of the spinal pathway mediating LA SPATIAL ORGANIZATION OF OTOLITH SIGNALS Thanks are due to L. Schindler for technical assistance. DISCLOSURES This research was supported by Sonderforschungsbereich 462 (Sensomotorik) der Deutschen Forschungsgemeinschaft and by the Friedrich-BaurStiftung 44/95. J Neurophysiol • VOL REFERENCES Abend WK. Functional organization of the superior vestibular nucleus of the squirrel monkey. Brain Res 132: 65– 84, 1977. Auclair FR, Marchand R, and Glover JC. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of mouse and rat embryos. J Comp Neurol 411: 288 –300, 1999. Baird RA and Lewis ER. Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena. Brain Res 369: 48 – 64, 1986. Baker R. From genes to behavior in the vestibular system. Otolaryngol Head Neck Surg 119: 263–275, 1998. Birinyi A, Straka H, Matesz C, and Dieringer N. Location of dye-coupled second-order and of efferent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain Res 921: 44 –59, 2001. Blanks RHI and Precht W. Functional characterization of primary vestibular afferents in the frog. Exp Brain Res 25: 369 –390, 1976. Boord RL and Karten HJ. The distribution of primary lagenar fibers within the vestibular nuclear complex of the pigeon. Brain Behav Evol 10: 228 – 235, 1974. Büttner-Ennever JA. Patterns of connectivity in the vestibular nuclei. Ann NY Acad Sci 656: 363–378, 1992. Büttner-Ennever JA. A review of otolith pathways to brainstem and cerebellum. Ann NY Acad Sci 871: 51– 64, 1999. De Burlet HM. Zur vergleichenden Anatomie der Labyrinthinnervation. J Comp Neurol 47: 155–169, 1929. Cambronero F and Puelles L. Rostrocaudal nuclear relationships in the avian medulla oblongata: a fate map with quail chick chimeras. J Comp Neurol 427: 522–545, 2000. Caston J, Precht W, and Blanks RHI. Response characteristics of frog⬘s lagena afferents to natural stimulation. J Comp Physiol 118: 273–289, 1977. Christensen-Dalsgaard J and Jørgensen MB. The response characteristics of vibration-sensitive saccular fibers in the grassfrog, Rana temporaria. J Comp Physiol 162: 633– 638, 1988. Christensen-Dalsgaard J and Walkowiak W. In vitro and in vivo responses of saccular and caudal nucleus neurons in the grassfrog (Rana temporaria). Eur J Morphol 37: 206 –210, 1999. Clack JA. The evolution of tetrapod ears and the fossil record. Brain Behav Evol 50: 198 –212, 1997. Cortopassi KA and Lewis ER. High-frequency tuning properties of bullfrog lagenar vestibular afferent fibers. J Vestib Res 6: 105–119, 1996. Dı́az C and Glover JC. Comparative aspects of the hodological organization of the vestibular nuclear complex and related neuron populations. Brain Res Bull 57: 307–312, 2002. Dickman JD and Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518 –3533, 2002. Dickman JD and Fang Q. Differential central projections of vestibular afferents in pigeons. J Comp Neurol 367: 110 –131, 1996. Dieringer N and Precht W. Mechanisms of compensation for vestibular deficits in the frog. I. Modification of the excitatory commissural system. Exp Brain Res 36: 311–328, 1979. Fritzsch B. The water-to-land transition: evolution of the tetrapod basilar papilla, middle ear, and auditory nuclei. In: The Evolutional Biology of Hearing, edited by Webster DB, Fay RR, and Popper AN. New York: Springer, 1992, p. 351–375. Gacek RR. The course and central termination of first order neurons supplying vestibular endorgans in the cat. Acta Otolaryngol Suppl 254: 1– 66, 1969. Gallé H and Clemens A. The sacculus of rana: an equilibrium organ. Equilib Res 3: 33– 47, 1973. Glover JC. Neuroepithelial “compartments” and the specification of vestibular projections. Prog Brain Res 124: 3–21, 2000. Glover JC. Correlated patterns of neuron differentiation and Hox gene expression in the hindbrain: a comparative analysis. Brain Res Bull 55: 683– 693, 2001. Harada Y, Kasuga S, and Tamura S. Comparison and evolution of the lagena in various animal species. Acta Otolaryngol 121: 355–363, 2001. Hess BJM and Precht W. Identification of vestibular sense organs responsible for maculo-ocular reflexes in the frog. Exp Brain Res 55: 570 –573, 1984. Hetherington TE, Jaslow AP, and Lombard RE. Comparative morphology of the amphibian opercularis system. I. General design features and functional interpretation. J Morphol 190: 43– 61, 1986. Highstein SM. The organization of the vestibulo-oculomotor and trochlear reflex pathway in rabbit. Exp Brain Res 17: 285–300, 1973. 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 signals that contribute to postural reflexes in frogs (MacNaughton and McNally 1946). The majority of 2°SA neurons were located in the auditory nuclei in r4 – 6 except for a few neurons in the r4 portion of the LVN. This part of the LVN generally contains very few 2°VN (Fig. 7A-C) (Straka et al. 2000a) and is likely to be homologous to the mammalian dorsal LVN, which likewise receives little or no direct vestibular nerve input (Büttner-Ennever 1992). Some of the convergent 2°LA⫹vertical canal neurons in frog, in particular the r5– 6 group, correspond closely to the 2°SA⫹vertical canal neurons in the mammalian LVN/DVN that are implicated in vestibulocollic reflexes (Sato et al. 2000; Zhang et al. 2002) and that may represent some of the vestibulospinal neurons seen in the r5– 6 region in mouse embryos (Auclair et al. 1999). It thus appears that the segmental positions of most vestibular neurons performing similar computational tasks are the same in frog, fish (Baker 1998; Suwa et al. 1999), chicken (Dı́az and Glover 2002; Glover 2000, 2001), and mouse (Auclair et al. 1999). This conserved central pattern appears to hold even when the peripheral sources of similar functional signals are different as with the vertical acceleration signals transduced by the frog lagena and mammalian saccule. As more studies linking segmental and connectional aspects of vestibular circuitry in tetrapods become available, a general vertebrate organizational pattern for the vestibular neuronal network, as initially proposed for teleost fish (Baker 1998), becomes apparent. Overall, the distinct spatial segregation of premotor functional groups, their phylogenetic conservation and the overlapping rather than discrete topographical arrangement of the afferent input suggests that the hindbrain vestibular circuitry is spatially organized according to the output of the vestibular nuclei more than to the sensory input. In effect, the spatial arrangement of central vestibular neurons, shown especially by the clustering of 2°canal⫹otolith neurons with common projection targets, seems to reflect basic directional commands for oculomotor and postural movements. This concept of the vestibular nuclei being organized as a premotor map and not as an organotypical map with a precise sensory topography from individual labyrinthine endorgans contrasts strongly with the organization of the visual and auditory systems. It more closely resembles the modular representation of limb muscle synergies or torque vectors suggested for spinal interneuron networks (Nichols 1994; Saltiel et al. 2001). The proposed segmental premotor topography relating vestibular endorgans to specific oculomotor and spinal subnuclei can be tested by intracellular recording and labeling of ortho- and antidromically identified vestibular neurons in larval and adult frogs. In addition to supporting or rejecting the segmental assignments for numerous second-order vestibular groups, such experiments will allow detailed evaluation of the proposed tangential and ATD homologs in frogs and should provide further insights into the strong functional parallels between frog LA and mammalian SA pathways. 3511 3512 H. STRAKA, S. HOLLER, F. GOTO, F. P. KOLB, AND E. GILLAND J Neurophysiol • VOL Rohregger M and Dieringer N. Principles of linear and angular vestibuloocular reflex organization in the frog. J Neurophysiol 87: 385–398, 2002. Saltiel P, Wyler-Duda K, D’Avella A, Tresch MC, and Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J Neurophysiol 85: 605–19, 2001. Sans A, Raymond J, and Marty R. Projections des crêtes ampullaires et de l‘utricle dans les noyaux vestibulaires primaires. Étude microphysiologique et corrélations anatomo-fonctionelles. Brain Res 44: 337–355, 1972. Sato H, Imagawa M, Kushiro K, Zakir M, and Uchino Y. Convergence of posterior semicircular canal and saccular inputs in single vestibular nuclei neurons in cats. Exp Brain Res 131: 253–261, 2000. Sato H, Imagawa M, Meng H, Zhang X, Bai R, and Uchino Y. Convergence of ipsilateral semicircular canal inputs onto single vestibular nucleus neurons in cats. Exp Brain Res 145: 351–364, 2002. Schwarz DWF and Schwarz IE. Projection of afferents from individual vestibular sense organs to the vestibular nuclei in the pigeon. Acta Otolaryngol 102: 463– 473, 1986. Siegborn J, Yingcharoen K, and Grant G. Brainstem projections of different branches of the vestibular nerve: an experimental study by transganglionic transport of horseradish peroxidase in the cat. Anat Embryol 184: 291–299, 1991. Stein BM and Carpenter MB. Central projections of portions of the vestibular ganglia innervating specific parts of the labyrinth in the rhesus monkey. Am J Anat 120: 281–317, 1967. Straka H, Biesdorf S, and Dieringer N. Canal-specific excitation and inhibition of frog second-order vestibular neurons. J Neurophysiol 78: 1363– 1372, 1997. Straka H, Baker R, and Gilland E. Rhombomeric organization of vestibular pathways in larval frogs. J Comp Neurol 437: 42–55, 2001. Straka H, Baker R and Gilland E. The frog as a unique vertebrate model for studying the rhombomeric organization of functionally identified hindbrain neurons. Brain Res Bull 57: 301–305, 2002a. Straka H, Biesdorf S, and Dieringer N. Spatial distribution of semicircular canal nerve evoked monosynaptic response components in frog vestibular nuclei. Brain Res 880: 70 – 83, 2000a. Straka H and Dieringer N. Electrophysiological and pharmacological characterization of vestibular inputs to identified frog abducens motoneurons and internuclear neurons in vitro. Eur J Neurosci 5: 251–260, 1993. Straka H, Gilland E, and Baker R. Rhombomeric organization of brainstem motor neurons in larval frog. Biol Bull 195: 220 –222, 1998. Straka H, Gilland E and Baker R. Rhombomeric pattern of hindbrain efferent neurons is retained in adult frogs. Soc Neurosci Abstr 26: 310, 2000b. Straka H, Holler S, and Goto F. Patterns of canal and otolith afferent input convergence in frog second-order vestibular neurons. J Neurophysiol 88: 2287–2301, 2002b. Suarez C, Kuruvilla A, Sitko S, Schwartz I, and Honrubia V. Central projections of primary vestibular fibers in the bullfrog. II. Nerve branches from individual receptors. Laryngoscope 95: 1238 –1250, 1985. Suwa H, Gilland E, and Baker R. Otolith ocular reflex function of the tangential nucleus in teleost fish. Ann NY Acad Sci 871: 1–14, 1999. Uchino Y, Hirai N, Suzuki S, and Watanabe S. Properties of secondary vestibular neurons fired by stimulation of ampullary nerve of the vertical, anterior or posterior semicircular canals in the cat. Brain Res 223: 273–286, 1981. Will U, Luhede G, and Görner P. The area octavo-lateralis of Xenopus laevis. I. The primary afferent projections. Cell Tissue Res 239: 147–161, 1985. Wilczynski W. Afferents to the midbrain auditory center in the bullfrog Rana catesbeiana. J Comp Neurol 198: 421– 433, 1981. Wilson VJ and Felpel LP. Specificity of semicircular canal input to neurons in the pigeon vestibular nuclei. J Neurophysiol 35: 253–254, 1972. Wilson VJ and Melvill Jones G. Mammalian Vestibular Physiology. New York: Plenum, 1979. Zakir M, Kushiro K, Ogawa Y, Sato H, and Uchino Y. Convergence patterns of the posterior semicircular canal and utricular inputs in single vestibular neurons in cats. Exp Brain Res 132: 139 –148, 2000. Zhang X, Sasaki M, Sato H, Meng H, Bai RS, Imagawa M, and Uchino Y. Convergence of the anterior semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 147: 407– 417, 2002. Zhang X, Zakir M, Meng H, Sato H, and Uchino Y. Convergence of the horizontal semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 140: 1–11, 2001. 90 • NOVEMBER 2003 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.1 on June 15, 2017 Highstein SM and Ito M. Differential localization within the vestibular nuclear complex of the inhibitory and excitatory cells innervating IIIrd nucleus oculomotor neurons in the rabbit. Brain Res 29: 358 –362, 1971. Highstein SM and Reisine H. The ascending tract of Deiters and horizontal gaze. Ann NY Acad Sci 374: 102–111, 1981. Hirai N and Uchino Y. Superior vestibular nucleus neurones related to the excitatory vestibulo-ocular reflex of anterior canal origin and their ascending course in the cat. Neurosci Res 1: 73–79, 1984. Holler S and Straka H. Plane-specific brainstem commissural inhibition in frog second-order semicircular canal neurons. Exp Brain Res 137: 190 –196, 2001. Imagawa M, Isu N, Sasaki M, Endo K, Ikegami H, and Uchino Y. Axonal projections of utricular afferents to the vestibular nuclei and the abducens nucleus in cats. Neurosci Lett 186: 87–90, 1995. Imagawa M, Graf W, Sato H, Suwa H, Isu N, Izumi R, and Uchino Y. Morphology of single afferents of the saccular macula in cats. Neurosci Lett 240: 127–130, 1998. Kaiser A and Manley GA. Brainstem connections of the macula lagenae in the chicken. J Comp Neurol 374: 108 –117, 1996. Kashara M and Uchino Y. Bilateral semicircular canal inputs to neurons in cat vestibular nuclei. Exp Brain Res 20: 285–296, 1974. Kevetter GA and Perachio AA. Projections from the sacculus to the cochlear nuclei in the Mongolian gerbil. Brain Behav Evol 34: 193–200, 1989. Kulik Á, Matesz C, and Székely G. Mesencephalic projections of the cochlear nucleus in the frog Rana esculenta. Acta Biol Hung 45: 323–335, 1994. Kuruvilla A, Sitko S, Schwartz IR, and Honrubia V. Central projections of primary vestibular fibers in the bullfrog. I. The vestibular nuclei. Laryngoscope 95: 692–707, 1985. Kushiro K, Zakir M, Sato H, Ono S, Ogawa Y, Meng H, Zhang X, and Uchino Y. Saccular and utricular inputs to single vestibular neurons in cats. Exp Brain Res 131: 406 – 415, 2000. Ladhams A and Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol 366: 335–347, 1996. Lannou J and Cazin L. Responses to tilting of fibers of the frog⬘s saccular nerve. Pflüger’s Arch 366: 143–146, 1976. Lewis ER, Baird RA, Leverenz EL, and Koyama H. Inner ear: dye injection reveals peripheral origins of specific sensitivities. Science 215: 1641–1643, 1982. Lewis ER and Li CW. Hair cell types and distributions in the otolithic and auditory organs of the bullfrog. Brain Res 83: 35–50, 1975. Lewis ER and Narins PM. The acoustic periphery of amphibians: anatomy and physiology. In: Comparative Hearing: Fish and Amphibians. Springer Handbook of Auditory Research, edited by Fay RR and Popper AN. New York: Springer, 1999, p. 101–154. MacNaughton IPJ and McNally WJ. Some experiments which indicate that the frog⬘s lagena has an equilibrial function. J Laryngol Otol 61: 204 –214, 1946. Manley GA, Haeseler C, and Brix J. Innervation patterns and spontaneous activity of afferent fibres to the lagenar macula and apical basilar papilla of the chick’s cochlea. Hear Res 6: 211–226, 1991. Matesz C. Central projection of the VIIIth cranial nerve in the frog. Neuroscience 4: 2061–2071, 1979. Matesz C. Fine structure of the primary afferent vestibulocochlear terminals in the frog. Acta Biol Hung 39: 267–277, 1988. McCue MP and Guinan JJ Jr. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci 14: 6058 – 6070, 1994. Naito Y, Newman A, Lee WS, Beykirch K, and Honrubia V. Projection of the individual end-organs in the brain stem of the squirrel monkey. Hear Res 87: 141–156, 1995. Newlands SD, Purcell IM, Kevetter GA, and Perachio AA. Central projections of the utricular nerve in the gerbil. J Comp Neurol 452: 11–23, 2002. Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat 151: 1–13, 1994. Ono S, Kushiro K, Zakir M, Meng H, Sato H, and Uchino Y. Properties of utricular and saccular nerve-activated vestibulocerebellar neurons in cats. Exp Brain Res 134: 1– 8, 2000. Ozawa S, Precht W, and Shimazu H. Crossed effects on central vestibular neurons in the horizontal canal system of the frog. Exp Brain Res 19: 394 – 405, 1974. Precht W, Richter A, Ozawa S, and Shimazu H. Intracellular study of frog’s vestibular neurons in relation to the labyrinth and spinal cord. Exp Brain Res 19: 377–393, 1974.