* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download By ON THE ROLE OF THE SUPERIOR COLLICULUS IN THE CONTROL... VISUALLY-GUIDED SACCADES
Brain–computer interface wikipedia , lookup
Multielectrode array wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Neuroethology wikipedia , lookup
Recurrent neural network wikipedia , lookup
Biological neuron model wikipedia , lookup
Embodied language processing wikipedia , lookup
Neurocomputational speech processing wikipedia , lookup
Binding problem wikipedia , lookup
Neuroscience in space wikipedia , lookup
Optogenetics wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural engineering wikipedia , lookup
Sensory cue wikipedia , lookup
Neural modeling fields wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neural oscillation wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nervous system network models wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Central pattern generator wikipedia , lookup
Neural coding wikipedia , lookup
Convolutional neural network wikipedia , lookup
Development of the nervous system wikipedia , lookup
Visual search wikipedia , lookup
Time perception wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Visual memory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Visual extinction wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Evoked potential wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Response priming wikipedia , lookup
Neuroesthetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Visual servoing wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
ON THE ROLE OF THE SUPERIOR COLLICULUS IN THE CONTROL OF
VISUALLY-GUIDED SACCADES
By
Robert Anthony Marino
A thesis submitted to the Centre for Neuroscience Studies
in conformity with the requirements for
the degree of Doctor of Philosophy
Queen's University
Kingston, Ontario, Canada
March 2011
Copyright © Robert Anthony Marino, 2011
Abstract
The ability to safely react to dangerous situations, or exploit opportunities within
a dynamically changing world is fundamental for our survival. In order to respond to
such changes in the environment, sensory information must first be received and
processed by the nervous system before an appropriate motor response can be planned
and executed. However, relatively little is known about how the central nervous system
computes such sensory to motor transformations that are so critical for guiding efficient
behavior. This thesis explores some of the neural mechanisms that underlie the
visuomotor transformations that guide eye movements. Specifically, this thesis studied
saccades (rapid eye movements critical for visual orienting in primates) and examined the
relationships between visual and motor signals in the primate Superior Colliculus (SC, a
midbrain structure located at the nexus between visual input and motor output that is
critical for visual orienting). I recorded extracellular action potentials (spikes) from single
neurons related to: 1) the appearance of visual saccade targets; 2) saccade planning and
preparation; and 3) the execution of precise saccades that orient to visual targets.
In this thesis I present four studies that examine the relationships between visual
and motor related responses in the SC during visually guided saccades. In chapter 2 I
examined the alignment between visual and motor response fields and concluded that
they were well aligned. In chapters 3 and 4 I explored how visual responses were
modulated by stimulus intensity and how this modulation influenced saccade behavior. I
concluded that luminance modulated multiple properties of the visual response including
the timing and maximum discharge rate and these changes were highly correlated to
changes in saccade latency and metrics. In the fifth chapter I applied some of the
ii
knowledge gained from the previous chapters to develop a neural network model of the
SC that was capable of simulating saccadic sensory to motor transformations and predict
saccadic reaction time. I concluded that saccade latency was strongly dependant on the
spatial interactions of visual and saccade related signals in the SC. Together, these
findings provide novel insight into the neural mechanisms underlying saccadic
sensorimotor transformations.
iii
Abstract
The ability to safely react to dangerous situations, or exploit opportunities within
a dynamically changing world is fundamental for our survival. In order to respond to
such changes in the environment, sensory information must first be received and
processed by the nervous system before an appropriate motor response can be planned
and executed. However, relatively little is known about how the central nervous system
computes such sensory to motor transformations that are so critical for guiding efficient
behavior. This thesis explores some of the neural mechanisms that underlie the
visuomotor transformations that guide eye movements. Specifically, this thesis studied
saccades (rapid eye movements critical for visual orienting in primates) and examined the
relationships between visual and motor signals in the primate Superior Colliculus (SC, a
midbrain structure located at the nexus between visual input and motor output that is
critical for visual orienting). I recorded extracellular action potentials (spikes) from single
neurons related to: 1) the appearance of visual saccade targets; 2) saccade planning and
preparation; and 3) the execution of precise saccades that orient to visual targets.
In this thesis I present four studies that examine the relationships between visual
and motor related responses in the SC during visually guided saccades. In chapter 2 I
examine the alignment between visual and motor response fields and concluded that they
were well aligned. In chapters 3 and 4 I explored how visual responses were modulated
by stimulus intensity and how this modulation influenced saccade behavior. I concluded
that luminance modulated multiple properties of the visual response including the timing
and maximum discharge rate and these changes were highly correlated to changes in
saccade latency and metrics. In the fifth chapter I applied some of the knowledge gained
ii
from the previous chapters to develop a neural network model of the SC that was capable
of simulating saccadic sensory to motor transformations. This model was designed to
predict how the spatial interactions between neural signals related to visual processing
and saccadic preparation interact within the SC to influence saccadic reaction time. I
concluded that saccade latency was strongly dependant on the spatial representation and
interaction of visual and saccade related signals in the SC. Together, these findings
provide novel insight into the neural mechanisms underlying saccadic sensorimotor
transformations.
iii
Co-Authorship
The research in this thesis was conducted by Robert Marino under the supervision
of Dr. Douglas P. Munoz. Robert Marino conducted the majority of the neural data
collection and the entirety of the data analysis. Data collection for Chapter 2 was
collected primarily by Kip Rogers with assistance from Dr. Ron Levy and Robert
Marino. Portions of the data from Chapter 5 were collected by Dr. Michael Dorris.
Robert Marino conceived and designed Chapter 3, and 4. Chapter 2 was conceived by
Dr. Doug Munoz and Chapter 5 was co-conceived by Robert Marino, Dr. Doug Munoz
and Dr. Michael Dorris. The computational model in Chapter 5 was based on designs by
Dr. Thomas Trappenberg. Robert Marino wrote the first draft of each chapter of this
thesis, however Kip Rogers wrote an alternate draft of Chapter 2. All subsequent drafts
involved contributions from the respective co-authors.
iv
Acknowledgements
I would like to express my heartfelt gratitude to my supervisor and mentor Doug
Munoz for his countless hours of support and guidance. I hope that he has enjoyed the
many hours of heated discussions about the superior colliculus as much as I have. I think
that I have learned more from these arguments than I have from any of the resulting
resolutions. Thank you for recognizing my potential and always pushing me to become a
better scientist. Through Doug, I have also been given the opportunity to travel and learn
from gifted scientists from around the world. Such opportunities are rare and I am truly
privileged and thankful to have been granted these experiences.
Where Doug has supervised my scientific training, Ann Lablans has taught me the
finer points of animal training and care. Her many lessons have taught me how to work
with monkeys competently, responsibly and compassionately (as well as taught me how
to gain their respect).
Special thanks to: Susan Boehnke for her ongoing support and friendship. Her
generosity over the years has provided me with everything from home furnishings to
clothing. Brian Coe for his technical and graphical wizardry that has dramatically
improved the calibre of my presentations. Ron Levy for sharing his expertise in the
development of new electrophysiological techniques and technologies. My scientific
siblings Alicia Peltsch, Ian Cameron and Rebecca Hakvoort for their good-natured rivalry
and friendship that continually trigger my competitive instincts to keep up with them. The
Munoz lab for fostering a family-like atmosphere of encouragement and support that
always pushes you to be the best that you can be.
v
I must thank my wife Megan for her ongoing love and support. As a successful
professional, mother, wife and fellow graduate student her dedication and capabilities far
exceed my own and never cease to amaze me. And finally, my beautiful daughter Nora
whom I adore. I am blessed and honoured to be your Dad and look forward to watching
you grow up, and seeing where your aptitudes and interests take you.
I would also like to thank my parents, Susan Birnie Marino and Dr. Robert (Bob)
Marino. Thank you for your love and support, and for showing me the inherent value of
education. Thank you also for demonstrating through your long and successful careers
that work should be a vocation, and serve a greater purpose. Thank you to my brother,
Samuel Marino. You have been one of my most ardent supporters through the years and
your own successes encourage me to strive to do better. Thank you also to Heather and
Brian Kerrigan, my parents-in-law. I truly appreciate your generosity, kindness and
support over the years. Finally, thank you to my extended family, Paulette, Daniel, Rae,
Toni, Rob, and my wonderful nieces and nephew. You enrich my life greatly.
This thesis represents the completion of my fourth and final degree from Queen’s
University. It has been a long road spanning more than a decade but I am very gratified to
be finally finishing this chapter of my life and moving on to the next adventure with hope
and optimism.
This work was funded by an operational research grant from the Canadian
Institutes of Health Research (CIHR) to Dr. Doug Munoz and by a grant from the
National Science Foundation to Dr. Laurent Itti and Dr. Doug Munoz. I was personally
supported by graduate fellowships from Queen’s University and the Centre for
Neuroscience.
vi
Table of Contents
Abstract ............................................................................................................................... ii
Co-Authorship.................................................................................................................... iv
Acknowledgements ............................................................................................................. v
Table of Contents .............................................................................................................. vii
List of Tables .................................................................................................................... xii
List of Figures and Illustrations ....................................................................................... xiii
List of Abbreviations and Symbols.................................................................................. xvi
Chapter 1: General Introduction ......................................................................................... 1
1.1 Processes Underlying Saccadic Sensorimotor Transformations ............................... 5
1.2 Express Saccades ...................................................................................................... 5
1.2 Brainstem Control of Saccadic Eye Movements ...................................................... 9
1.2.1 Eye Position Signals ........................................................................................ 10
1.2.2 Brainstem Premotor Neuron Types ................................................................. 11
1.3 Higher Brain Regions Involved in Saccade Control .............................................. 14
1.4 Superior Colliculus ................................................................................................. 17
1.5 Visuomotor Transformations in the Superior Colliculus ........................................ 21
1.6 Neural Network Models of the Saccadic System ................................................... 23
1.7 Thesis Objectives .................................................................................................... 25
1.8 General Overview ................................................................................................... 27
Chapter 2: The Spatial Relationships of Visuomotor Transformations in the Superior
Colliculus Map .................................................................................................................. 29
2.1 Abstract ................................................................................................................... 30
2.2 Introduction ............................................................................................................. 31
2.3 Methods................................................................................................................... 35
2.3.1 Animal Preparation .......................................................................................... 35
vii
2.3.2 Behavioral Paradigms ...................................................................................... 37
2.3.3 Recording Techniques ..................................................................................... 39
2.3.4 Data Collection ................................................................................................ 39
2.3.5 Average Spike Density Functions and Neuron Classification ......................... 40
2.3.6 Calculation of Individual Neuron Response Fields and Peak Response Vectors
................................................................................................................................... 41
2.3.7 Calculation of Individual Neuron Response Field Peak and Asymmetry ....... 42
2.3.8 Calculation of Neural Population Point Image Response Fields and Peak
Activity ..................................................................................................................... 47
2.4 Results ..................................................................................................................... 48
2.4.1 Discharge Characteristics of Single-Neurons .................................................. 48
2.4.2 Spatial Comparison of Visual and Motor Response Fields ............................. 49
2.4.3 Comparison of Peak Visual and Motor Response Locations .......................... 49
2.4.4 Comparison of Visual and Motor Point Images .............................................. 50
2.4.5 Changes in Response Field Size ...................................................................... 52
2.4.6 Response Field Shape Symmetry..................................................................... 55
2.5 Discussion ............................................................................................................... 58
2.5.1 Spatial Relationships Between Visual and Motor RFs .................................... 58
2.5.2 Alignment of Peak Visual and Motor Responses ............................................ 59
2.5.3 Changes in Response Field Size ...................................................................... 61
2.5.4 Field Shape Asymmetry................................................................................... 62
2.5.5 Conclusions ...................................................................................................... 64
Chapter 3: Linking Visual Response Properties in the Superior Colliculus to Saccade
Behavior ............................................................................................................................ 65
3.1 Abstract ................................................................................................................... 66
3.2 Introduction ............................................................................................................. 67
3.3 Materials and Methods ............................................................................................ 68
3.3.1 Animal preparation .......................................................................................... 68
3.3.2 Experimental tasks and behavioral stimuli ...................................................... 69
3.3.3 Recording Techniques ..................................................................................... 72
3.3.4 Data Collection ................................................................................................ 72
3.3.5 Neuron classification ....................................................................................... 73
3.3.6 Behavioral Analyses ........................................................................................ 74
3.3.7 Neuronal Analyses ........................................................................................... 75
viii
3.4 Results ..................................................................................................................... 77
3.4.1 Target Luminance Modulates Saccade Behavior ............................................ 78
3.4.2 Target Luminance Modulates SC Visual Activity ........................................... 80
3.4.3 Relationships Between Visual Response Properties ........................................ 85
3.4.4 Linking Visual Response Properties to Saccade Behavior .............................. 85
3.4.5 Target Luminance Modulates the Saccade Motor Response ........................... 88
3.4.6 Linking Motor Response Properties to Saccade Behavior .............................. 90
3.5 Discussion ............................................................................................................... 90
3.5.1 Relation to Previous Work .............................................................................. 92
3.5.2 Stages of Visuomotor Processing .................................................................... 93
3.5.3 Evidence for Visual Inhibitory Feedback ........................................................ 95
3.5.4 Implications for Modelling of the Visual System ............................................ 96
Chapter 4: The Timing and Magnitude of Visual and Preparatory Responses in the
Superior Colliculus Influences Express Saccade Latency and Prevalence ....................... 98
4.1 Abstract ................................................................................................................... 99
4.2 Introduction ........................................................................................................... 100
4.3 Methods................................................................................................................. 105
4.3.1 Animal preparation ........................................................................................ 105
4.3.2 Experimental tasks and behavioral stimuli .................................................... 106
4.3.3 Averaged spike density functions and neuron classification ......................... 109
4.3.4 Classification of build-up neurons. ................................................................ 110
4.3.5 Behavioral Analyses ...................................................................................... 112
4.3.6 Neuronal Analyses ......................................................................................... 114
4.3.7 Calculation of Express Saccade Ranges ........................................................ 115
4.3.8 Expanded Express Saccade Model ................................................................ 115
4.4 Results ................................................................................................................... 116
4.4.1 Target Luminance Modulates Express Saccade Latency............................... 116
4.4.2 Target Luminance Modulates SRT Bimodality ............................................. 119
4.4.3 Merging of Sensory and Motor Bursts During Express Saccades ................. 119
4.4.4 Target Luminance Modulates the Likelihood of Producing an Express Saccade
................................................................................................................................. 120
4.4.5 Target Luminance Modulates Peak Visual Response and Accumulated Buildup in the gap task .................................................................................................... 121
ix
4.4.6 Predicting the Relative Influence of the Visual Response and Build-up
Activity for Express Saccade Production ............................................................... 123
4.5 Discussion ............................................................................................................. 124
4.5.1 Neural Correlates of Express Saccades in the SCi......................................... 125
4.5.2 Top-Down and Bottom-Up Influences on Express Saccades ........................ 127
4.5.3 Expanding Express Saccade Models ............................................................. 129
4.5.4 Other Potential Neural Properties Influencing Express Saccade Production 129
4.5.5 Conclusions .................................................................................................... 132
Chapter 5: Spatial Interactions in the Superior Colliculus Predict Saccade Behavior in a
Neural Field Model ......................................................................................................... 134
5.1 Abstract ................................................................................................................. 135
5.2 Introduction ........................................................................................................... 137
5.3 Methods................................................................................................................. 142
5.3.1 Animal preparation ........................................................................................ 142
5.3.2 Experimental tasks and behavioral stimuli .................................................... 143
5.3.3 Neural Classification ...................................................................................... 145
5.3.4 Calculation of Neural Population Point Images on the SC Map ................... 148
5.3.5 2-Dimensional Continuous Attractor Neural Field Model ............................ 152
5.3.6 External Inputs ............................................................................................... 153
5.3.7 Model Architecture ........................................................................................ 157
5.3.8 Model Parameters .......................................................................................... 158
5.3.9 Model simulations.......................................................................................... 159
5.4 Results ................................................................................................................... 160
5.4.1 Spatial Properties of Visual, Preparatory, and Motor Point Images on the SC
................................................................................................................................. 160
5.4.2 Simulation 1. Spatial Interactions Within Single Input Signals .................... 162
5.4.3 Simulation 2. Effects of Spatial Signal Distance ........................................... 168
5.4.4 Simulation 3. The Effects of the Number of competing BU Signals............. 170
5.4.5 Simulation 4. Effects of number of competing TD Signals ........................... 174
5.5 Discussion ............................................................................................................. 177
5.5.1 An Expansion of Linear Saccade Accumulator Models ................................ 179
5.5.2 Oculomotor Violations of Sensorimotor Transformation Laws .................... 180
5.5.3 Intrinsic versus extrinsic sources of inhibition in the SC .............................. 181
5.5.4 Conclusions .................................................................................................... 183
x
Chapter 6: General Discussion........................................................................................ 184
6.1 Summary of Objectives and Major Findings ........................................................ 185
6.2 Unanswered Questions......................................................................................... 188
6.3 Future Directions ................................................................................................. 191
6.3.1 Testing the Neural Field model of the SCi ................................................... 191
6.3.2 Is Preparatory Activity Preceding Express Saccades TD or BU?.................. 192
6.3.3 Moving Towards Understanding Visuomotor Transformations During Real
World Free Viewing ............................................................................................... 194
Reference List ................................................................................................................. 197
Appendix: Supplementary Analysis and Figures ............................................................ 216
3.A1 Supplemental Experimental Procedures for Chapter 3 ...................................... 217
3.A1.1 Characterization of Visual Response Field ................................................. 217
3.A2 Supplemental Results for Chapter 3................................................................... 219
3.A2.1 Comparison of the visual response between V and VM neurons ............... 219
xi
List of Tables
3.1
Neuron breakdown by monkey, task and subtype.................................................82
4.1
SCi neuron breakdown by monkey and subtype..................................................111
5.1
SCi Neuron breakdown by monkey and subtype.................................................147
xii
List of Figures and Illustrations
1.1
A simple visually guided saccade task................................................................... 6
1.2
Sensory and motor brain areas involved in saccade generation............................. 8
1.3
Schematic of the brainstem burst generator circuit for saccades.......................... 12
1.4
Location and retinotopic mapping of the superior colliculus............................... 19
1.5
Schematic of visuomotor transformations in the superior colliculus for visually
guided saccades..................................................................................................... 22
1.6
Techniques for sorting neural waveforms corresponding to action potentials
(spikes) from individual neurons.......................................................................... 26
2.1
Logarithmic spatial transformation from visual field space to the topographic SC
map....................................................................................................................... 32
2.2
Schematic representation of the visually guided saccade task used in this
study...................................................................................................................... 38
2.3
Relationships between visual and motor RF areas and visual and motor peak
response locations in visual and SC space............................................................ 46
2.4
Simultaneous population activity (point image) from all VM cells plotted on the
SC for a 6° right horizontal saccade..................................................................... 51
2.5
Relationship between RF area and peak response eccentricity in visual space and
the SC map............................................................................................................ 54
2.6
Analysis of visual and motor RF asymmetry in both visual and SC space.......... 57
3.1
Schematic representation of temporal events in the delay and gap
tasks....................................................................................................................... 70
3.2
Effect of target luminance on mean SRT, peak velocity, endpoint error and
percent error rate................................................................................................... 79
3.3
Population spike density functions aligned on target onset and saccade onset for
all V and VM neurons recorded in the delay and gap tasks with seven randomly
interleaved target luminance levels....................................................................... 81
xiii
3.4
Effects of target luminance on the signal properties of the visual sensory response
in the delay and gap tasks collapsed across all V and VM neurons..................... 84
3.5
Cumulative distributions of correlation coefficients and median r2 values for each
behavioral and visual sensory neural variable measured...................................... 87
3.6
Effects of target luminance on the properties of the saccade motor response in the
gap task collapsed across all VM neurons............................................................ 89
3.7
Cumulative distributions of correlation coefficients and median r2 values between
each behavioral and saccade motor neural variable measured............................ 91
4.1
A pre-existing model of express saccade generation based on neural trigger
thresholds in the SCi........................................................................................... 104
4.2
Schematic representation of temporal events in the delay and gap tasks for the
fixation point, target and eye position................................................................. 107
4.3
Effects of target luminance on express saccades in the gap task........................ 117
4.4
Population spike density functions (Gaussian kernel σ = 5ms) for all VMBNs
and all BUNs aligned on target appearance in the gap task................................ 122
4.5
Our proposed extension of the pre-existing express saccade model................... 130
5.1
Architecture of the 2-dimensional neurofield model (developed from Trappenberg
et. al 2001).......................................................................................................... 139
5.2
Schematics of spatial competition and lateral interaction that can occur from
individual or multiple activation signals within the retinotopic, topographical SCi
map as predicted by our model........................................................................... 141
5.3
Schematic representation of temporal events in the delay and gap tasks for the
fixation point, target and eye position................................................................. 144
5.4
Population spike density functions of VMBNs in the delay task and BUNs in
the gap task aligned on target appearance and saccade onset............................. 150
5.5
Spatial point images of BU visual, TD preparatory, and saccadic motor activity in
the SCi map......................................................................................................... 151
5.6
Implementation and functionality of the 2-dimensional neurofield model........ 154
5.7
Simulations of the effects of magnitude and width of a single input signal on
modeled SRT...................................................................................................... 164
xiv
5.8
Simulations of the effects of distance between two single input signals on
modeled SRT...................................................................................................... 169
5.9
Simulations of the effects of number of competing BU inputs both nearby and far
from central fixation on modeled SRT............................................................... 172
5.10
Simulations of the effects of number of competing TD inputs on modeled SRT
for signals located nearby and far from central fixation (1 BU signal representing
the appearance of the visual target was always presented at the resulting saccade
location).............................................................................................................. 176
3.S1
Schematic representation of temporal events in the visual response field mapping
task for the fixation point, target and eye position.............................................. 218
3.S2
Effects of neuron V and VM subtype on visual sensory response properties
recorded at the brightest luminance (42 cd/m2) in the delay and gap tasks........ 220
xv
List of Abbreviations and Symbols
Units of Measurement:
cd
candela
Hz
hertz
kHz
kilohertz
m
meter
mm
millimeter
ms
millisecond
Oculomotor Neuron Types:
BUN
build-up neuron in the superior colliculus
EBN
excitatory burst neuron
IBN
inhibitory burst neuron
LLBN
long-lead burst neuron
MLBN
medium-lead burst neuron
MN
motoneuron
OPN
omnipause neuron
V
visual neuron
VM
visual-motor neuron
M
motor neuron
FN
fixation neuron
Experimental Terms:
ANOVA
statistical analysis of variance
xvi
fMRI
functional magnetic resonance imaging
FP
fixation point
ITI
inter trial interval
LATER
linear approach to threshold with ergodic rate model
SRT
saccadic reaction time
T
target
Oculomotor Brain regions and Subregions:
CD
caudate nucleus
CNS
central nervous system
DLPFC
dorsolateral prefrontal cortex
FEF
frontal eye fields
GPe
globus palidus external segment
LGN
lateral geniculate nucleus of the thalamus
LIP
lateral intraparietal sulcus
MVN
medial vestibular nucleus
NIC
interstitial nucleus of cajale
NPH
nucleus propositus hypoglossi
PPRF
paramedian pontine reticular formation
SAI
stratum album intermedium
SAP
stratum album profundum
SC
superior colliculus
SCi
superior colliculus intermediate layers
SCs
superior colliculus superficial layers
xvii
SEF
supplementary eye fields
SGI
stratum griseum intermedium
SGP
stratum griseum profundum
SGS
stratum griseum superficiale
SNr
substantia nigra pars reticulate
SO
stratum opticum
STN
subthalamic nucleus
SZ
stratum zonal
V1
primary visual cortex
xviii
Chapter 1: General Introduction
1
The ability to produce behaviours that effectively react to or exploit the constantly
changing external environment is crucial for survival. In order to accomplish this,
external sensory stimuli must be processed by the nervous system and then used to guide
an appropriate motor response - whether reflexively withdrawing from a hot stimulus or
volitionally reaching for a conspicuous piece of low hanging fruit. Thus, generating a
movement in response to external sensory stimuli is a critically important function of the
central nervous system (CNS) for interacting with and responding to changes in the
external world. In the visual system, such sensory to motor transformations are utilized
for both goal driven eye movements like looking for a tasty snack in an overcrowded
refrigerator, or for more automatic/reflex-like movements such as quickly looking toward
a bright flash of lightning during a thunderstorm (Munoz et al., 2000; Sparks, 2002).
Many of the details regarding how the nervous system computes these sensory to motor
transformations are poorly understood and are actively researched by neuroscientists. In
this thesis, I explore some of the neural mechanisms underlying such sensorimotor
transformations in the eye movement system. The eye movement system provides an
excellent model for such explorations due to its relative simplicity (only 3 synergistic
pairs of orbital muscles, a single point of rotation, and nearly inertialess eyeball) that is
free from the complicated mass and intersegmental dynamics associated with peripheral
limb and body movement. Furthermore, eye movement parameters (i.e. latency and
metrics) can be measured relatively simply in the laboratory and much of the underlying
neural circuitry forming the visual sensory input and the motor output is already known
(Wurtz and Goldberg, 1989; Sparks, 2002; Krauzlis, 2005).
2
In the primate eye, the most detailed visual information is received by the fovea, a
small area near the center of the retina where cone photoreceptor cells are most densely
packed (Perry and Cowey, 1985a).
The fovea represents the center of the visual field
that spans approximately one degree of visual angle where visual acuity is greatest (Perry
and Cowey, 1985a). In order for the many important visual aspects of the external
environment to be perceived and processed by the nervous system, the eyes must be
moved around until all relevant features of the visual scene have been foveated (Yarbus,
1961). Primates have developed 5 different types of eye movements that align the fovea
onto objects of interest in the visual scene including: saccades, smooth pursuit, vergence
(moving the eyes in opposite directions to view nearby objects), vestibular ocular reflexes
(to compensate for head perturbations), and optokinetic shifts (to compensates for
movements of full field images) (Sparks, 2002). This thesis will focus on saccades, which
are rapid eye movements (execution time < 100 ms) that move the fovea to locations or
objects of interest within the visual world (Leigh and Zee, 1999).
Once a saccade has moved the fovea to a new location, a period of fixation
follows that allows the details of the newly fixated location to be processed and analyzed.
Such patterns of movement (saccade-fixate-saccade) occur in rapid succession and can
typically be repeated up to hundreds of thousands of times each day (Yarbus, 1967;
Rayner, 1998; Land et al., 1999; Liversedge and Findlay, 2000; Land, 2009). Saccades
are critical for enabling the performance of complex visually guided behaviours that are
often taken for granted such as riding a bike or watching a movie (Yarbus, 1967; Rayner,
1998; Land et al., 1999; Liversedge and Findlay, 2000; Hayhoe and Ballard, 2005; Land,
2009). During saccades to specific visual targets, a direct sensory to motor transformation
3
occurs whereby a visual target is first detected and then a precise eye movement is
generated to move the fovea toward it.
The time required to perform this movement (i.e. saccadic reaction time: SRT)
can last from dozens to hundreds of milliseconds and reflects the underlying neural
chronometry of these processes in the brain (Posner, 2005). The precise neural
computations underlying such sensorimotor transformations remain unclear, but, it can be
presumed that some neural mechanism must link incoming visual sensory signals to the
subsequent performance of motor behavior. This thesis explores these links by
examining how visual sensory responses in the brain are related to the motor commands
that ultimately drive saccadic behavior.
The basic principle underlying this thesis is that anything affecting visual
response properties in the nervous system should have consequences on the neural
mechanisms that compute sensorimotor transformations and thus affect behaviour in a
predictable and measurable way. In this thesis I study awake behaving monkeys
performing simple saccade tasks while simultaneously recording from neurons in the
midbrain Superior Colliculus (SC, a sensory and motor structure critical for saccades).
Monkeys possess a visual and brainstem control system that is very similar (and
phylogenetically well preserved) to humans, thus they provide an excellent animal model
in which to study the neural mechanisms underlying saccades (Felleman and Van Essen,
1991; Van Essen et al., 1992; Van Essen, 2004; Leigh and Zee, 2006).
4
1.1 Processes Underlying Saccadic Sensorimotor Transformations
The temporal components underlying saccadic reaction time (SRT) are composed
of a combination of top-down (TD) and bottom-up (BU) processes (Carpenter, 2004;
Marino and Munoz, 2009). BU processes are related to the external properties of visual
stimuli that define the salience of sensory signals (Itti and Koch, 2001; Dehaene et al.,
2006; Fecteau and Munoz, 2006). BU processes include retinal transduction and
conduction delays as well as the time to decide that a task dependant target is present
(Reddi and Carpenter, 2000; Itti and Koch, 2001). Examples of some pre-potent BU
properties in the saccadic system include motion (Treue and Martinez Trujillo, 1999),
colour (Chaparro et al., 1993; D'Zmura et al., 1997; White et al., 2006), and luminance
(Boch et al., 1984; Bell et al., 2006). TD processes are related to volitional task-related
goals and objectives and can be influenced by previous experience and expectation
(Dehaene et al., 2006; Fecteau and Munoz, 2006). TD processes affect SRT by altering
the neural prediction or preparation states of oculomotor structures during saccade tasks
independent of the properties of the sensory stimulus (Basso and Wurtz, 1998; Dorris and
Munoz, 1998; Marino and Munoz, 2009). Examples of some pre-potent TD processes in
the saccadic system include temporal or spatial predictability of when or where a task
related saccade target goal will appear (Marino and Munoz, 2009).
1.2 Express Saccades
Saccades to supra threshold visual targets typically average around 150 - 250ms,
(Fischer, 1986; Schall, 1995; Thompson et al., 1996), however SRT distributions from
repetitions of these tasks are variable and yield skewed distributions over well
5
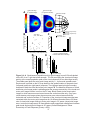
A
Visually Guided Saccade Task
FP
T
T
B
express
Occurances
150
regular
100
50
0
0
100
SRT (ms)
150
200
Figure 1.1. A. Simple visually guided saccade task: monkey is instructed to look from
the fixation point (FP) to a target (T) when it appears. B. Distribution of SRT latencies
from 2 monkeys performing visually guided saccades in the gap condition (200ms
temporal gap introduced between the disappearance of the FP and the appearance of
the T). Red bars denote express saccades and blue bars denote regular latency
saccades.
6
characterized temporal ranges (Fig. 1.1). Under certain circumstances, SRT distributions
can be multi-modal. In these cases, the fastest mode has been defined as express
saccades, which represent the minimum afferent (~50ms) and efferent (~20ms)
conduction delays between the retina and the extra-ocular muscles (Fischer and Boch,
1983; Fischer and Weber, 1993). The later mode(s) in these distributions have been
described as regular latency saccades (Fischer and Boch, 1983; Fischer et al., 1984;
Fischer, 1986). Both regular and express saccades describe different mechanisms by
which the oculomotor system performs visuomotor transformations. Express saccades
represent the fastest visually triggered saccades that can be performed. These fast
saccades are triggered when the visual sensory signal is directly transformed into the
saccadic motor command that triggers the eyes to move (Edelman and Keller, 1996;
Dorris et al., 1997). Thus express saccades represent a direct reflex like sensorimotor
transformation. The specific latency ranges for express saccades vary across studies,
laboratories, primate species tested, and task properties, however average latency ranges
generally are around 60-90 ms in monkeys (Paré and Munoz, 1996; Bell et al., 2006) and
70-140 ms in humans (Weber et al., 1992; Weber et al., 1993; Munoz et al., 2003; Chan
et al., 2005). Regular saccades have longer latencies that are increased relative to
express saccades (Fig. 1.1). These longer latencies reflect an indirect sensorimotor
transformation and result when the sensory response does not directly trigger a saccade,
but instead undergoes additional processing by higher order mechanisms that are required
to decide that the target is present and that a saccade should be made toward it (Reddi and
Carpenter, 2000; Carpenter, 2004). These higher order saccadic decision mechanisms
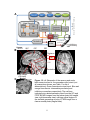
have been shown to involve cortical regions that include the FEF and LIP (Fig. 1.2,
7
Cortex
A
Direct pathway
Basal Ganglia
GPe
CD
STN
SEF
Anterior
Thalamus
Posterior
Thalamus
SCs
Cerebellum
PPRF
B
LGN
SNr
SCi
Human
DLPFC
VC
PEF
PEF
FEF
SEF
FEF
retinotectal
pathway
Retino-geniculo-cortical
pathway
indirect pathway
DLPFC
Retina
Inhibitory
Excitatory
PEF
Thalamus
SC
V1
Cerebellum
C
Basal Ganglia
(CD Head)
Monkey
STN
SNr
PPRF
Figure 1.2. A. Schematic of the sensory and motor
brain areas involved in the saccade control circuit (not
all connections shown; see Table 1 for list of
abbreviations). Green lines denote visual input. Blue and
orange lines denote intermediary excitatory and
inhibitory connections respectively. The red lines
indicate motor related saccade output from the SCi and
PPRF. B. MRI images form the human brain (left sagital
view, right transverse view) outlining critical regions of
the saccade generating circuitry. C. MRI image from a
rhesus monkey brain (sagital view).
8
Schall, 1995; Wurtz et al., 2001a). Thus express and regular saccades reflect two
different neural mechanisms for sensorimotor transformations that determine whether a
saccadic response is as rapid as possible or more carefully controlled.
A limited set of carefully controlled experimental conditions are typically
required in order to reliably elicit express saccades in the laboratory. Several variables
have been identified that influence the probability of generating an express saccade.
These include: imposing a temporal gap between the offset of the fixation point and the
onset of a visual target (Saslow, 1967), presenting only a limited number (1 or 2) of
nearby suddenly appearing target stimuli at a time (McPeek and Schiller, 1994; Weber
and Fischer, 1994), limiting the spatial and temporal predictability of when or where the
visual target can appear (Rohrer and Sparks, 1993; Paré and Munoz, 1996; Basso and
Wurtz, 1998), and repetitively overtraining subjects on specific visually guided saccade
tasks (Kowler, 1990; Paré and Munoz, 1996). Express saccades can also be elicited
during scanning tasks where multiple stable objects are present, however, the abrupt
onset of a single target in an anticipated location is still required (Sommer, 1994;
Sommer, 1997).
1.2 Brainstem Control of Saccadic Eye Movements
Eye movements are controlled by three synergistic pairs of muscles for each eye.
Vertical rotational components are controlled by the superior and inferior recti and the
superior and inferior oblique muscle pairs. Horizontal rotational components are
controlled by the medial and lateral rectus muscle pairs. Three cranial nerves control the
innervation of these muscle pairs: cranial nerve III (oculomotor), IV (trochlear) and VI
9
(abducens).
The brainstem burst generator contains a neurally coded representation of
current eye position (fixation) and contains mechanisms for generating both goal driven
and stimulus driven movements of the eyes to any orbital location reachable by the
oculomotor muscles. A combination of electrical stimulation studies, lesion studies,
histology, and single unit electrophysiology has supplied a great deal of information
regarding the different brain areas and cell types that make up the brainstem burst
generator circuit (Moschovakis et al., 1996; Scudder et al., 2002; Sparks, 2002).
1.2.1 Eye Position Signals
Periods of visual fixation are imposed between saccadic eye movements so that
the visual system can perform a detailed analysis of the retinal image. These periods of
fixation require balanced contraction of agonist and antagonist muscle pairs. Motor
neurons achieve this control via tonic activity (step signal) that is linearly related to the
absolute position of the eyes in their orbits (Munoz, 2002; Scudder et al., 2002; Sparks,
2002). The vertical and horizontal components of eye position are controlled
synchronously but independently from different regions of the brainstem. Tonic vertical
eye position signals are supplied by neurons in the interstitial nucleus of Cajal (NIC) and
the vestibular nucleus (Crawford et al., 1991; Moschovakis et al., 1996; Doubell et al.,
2003). Horizontal eye position signals are controlled separately by the nucleus prepositus
hypoglossi (NPH) and the medial vestibular nucleus (MVN) (Moschovakis et al., 1996).
Together these nuclei project to motoneurons (MN) in the oculomotor, trochlear, and
abducens nuclei to provide the vertical and horizontal components of the signal that hold
the eyes in a given position of their orbit between saccades.
10
Motoneurons discharge a high frequency burst (pulse signal) of activity to drive
saccades to predetermined orbital positions. (Munoz, 2002; Scudder et al., 2002; Sparks,
2002). This burst composes a pulse signal that must be sufficient to overcome the
relatively small inertia of the eye and the elastic properties of the oculomotor muscles in
order to move the eyes ballisticly (Munoz, 2002). The horizontal and vertical components
of these saccadic bursts scale with target eccentricity and are tightly coupled to the
metrics of the saccade such that the peak firing rate, duration of the burst, and total
number of spikes generated are proportional to the velocity, duration, and amplitude of
the movement (Sparks et al., 2000; Scudder et al., 2002).
1.2.2 Brainstem Premotor Neuron Types
Although the vertical and horizontal components of saccades are controlled
separately, they are composed of identical neuron subtypes and contain some projections
from common brainstem nuclei (Fig. 1.3). Both vertical and horizontal saccades are
generated by premotor neurons located in three different areas of the brainstem reticular
formation. Horizontal saccades are generated in the pontine and medullary regions,
vertical saccades are generated in the pons and the mesencephalon (Munoz, 2002).
Premotor cells (Fig. 1.3) were first classified based on their activity and modulation with
saccade generation. The three basic premotor saccade neuron types are
excitatory/inhibitory burst neurons (EBN/IBN), omipause neurons (OPN), and long lead
burst neurons (LLBN) (Moschovakis et al., 1996; Munoz, 2002; Scudder et al., 2002;
Sparks, 2002) (Fig. 1.3). The EBNs are excitatory to agonist motoneurons and the IBNs
are inhibitory to antagonist motoneurons on the opposite side. EBNs are silent during
visual fixation, but discharge a high frequency burst of action potentials for ipsiversive
11
A
SN
SCi
FN
OPN
LLBN
PPRF
EBN/IBN
MN
Eye Position
SCi
SN
LLBN
EBN
PPRF
Midline
B
SCi
FN
FN
OPN
OPN
IBN
IBN
MN
MN
Agonist Antagonist
SN
LLBN
EBN
MN
MN
Antagonist Agonist
PPRF
Figure 1.3. Schematic of the brainstem burst generator circuit for saccades with
example activity from each structure. Adapted from Munoz (2002). A. Example timing
of neural spike action potentials from the SC and brainstem nuclei controlling
saccadic eye movements. B. The excitatory and inhibitory connections existing
between the SCi and neurons in the brainstem controlling saccades. A high frequency
burst of action potentials is sent from the deeper motor layers of the SC to the
contralateral LLBN and EBNs of the brainstem burst generator. The OPNs gate the
motor signals sent from the EBN and LLBNs to the extra ocular muscles and also
receive monosynaptic input from the contralateral SC.
12
saccades (Fig. 1.3A). EBNs connect monosynaptically with agonist MNs and
characteristically fire approximately 10ms prior to saccade onset and shut off their
activity a few milliseconds before saccade end (Moschovakis et al., 1996; Scudder et al.,
2002; Sparks, 2002). IBNs have an identical firing pattern; they receive direct input from
EBNs in order to inhibit the activation of antagonist muscles during the saccade
(Moschovakis et al., 1996; Scudder et al., 2002; Sparks, 2002).
A second basic type of premotor cells are the omnipause neurons (OPNs) in the
pons. OPNs have opposite discharge patterns to EBNs and IBNs: they discharge tonic
activity usually over 100 spikes per second during fixation and shut off during saccades
in all directions (Moschovakis et al., 1996; Scudder et al., 2002; Sparks, 2002). The
OPNs project directly to all EBNs and IBNs and fulfill a critical inhibitory role to in the
oculomotor circuit. Saccades are triggered by EBNs only at the instant when OPNs stop
discharging. Saccades then terminate when OPN activity resumes and another period of
fixation begins (Moschovakis et al., 1996; Scudder et al., 2002; Sparks, 2002). Thus
OPNs represent a critical latch that controls when a saccade is launched.
LLBNs are similar to EBN/IBNs in that they exhibit a burst of activity for
ipsiversive saccades, but also include a lower frequency build up of activity that precedes
the saccade. Although some LLBNs have burst characteristics similar to EBNs with
activity proportional to all saccades in the same direction (Hepp and Henn, 1983), some
LLBNs have more precisely tuned movement fields that only respond to more specific
saccade vectors and amplitudes (Hepp et al., 1982; Sparks, 2002). LLBNs appear to have
multiple functions, not all of which have been classified. Three potential subclassifications of LLBNs are relay, trigger/latch, and precerebellar neurons (Scudder et
13
al., 2002). Because the vertical and horizontal components of saccades are separate and
spread out across three cranial nerve nuclei and six oculomotor muscles, a central
command signal must be relayed synchronously to the horizontal and vertical EBNs.
Subclasses of LLBNs that synapse directly on EBNs provide this relay signal from
projections from saccade related neurons in the superior colliculus (SC) in the midbrain
(Raybourn and Keller, 1977). Both the horizontal and vertical components of the
saccadic command are represented retinotopically in the SC, and are relayed
simultaneously by the LLBNs to the appropriate pools of EBNs and IBNs (Sparks et al.,
2000; Scudder et al., 2002). Evidence for trigger/latch LLBN subtypes is not yet
conclusive (Sparks, 2002). A trigger/latch signal must inhibit OPNs in order to trigger a
saccade. Some research has shown that stimulation of LLBN regions in cats
monosynaptically inhibits OPNs but this does not yet clearly establish a trigger/latch
function for LLBNs (Kamogawa et al., 1996). Finally, some LLBNs project from the
nucleus reticularis tegmenti pontis and the paramedian pontine reticular formation to the
cerebellum and provide a feedback signal that will flow through a cerebellar component
of the circuit and ultimately influence saccade termination in the brainstem burst
generator (Scudder et al., 2002).
1.3 Higher Brain Regions Involved in Saccade Control
Saccades are controlled by a distributed network of brain regions spanning the
cortex to the brainstem (Fig. 1.2). These regions project to the different layers of the SC
(functionally defined as the superficial (SCs) and intermediate (SCi) layers) and
brainstem premotor circuitry to influence saccade control. Some of the most important of
14
these structures include regions of the cerebellum, basal ganglia and cerebral cortex (Fig.
1.2). Some of the most important regions within the cortex include the dorsolateral
prefrontal cortex (DLPFC), frontal and supplementary eye fields (FEF, SEF), parietal
areas (LIP), as well as primary visual cortex (VC). Together, these interconnected and
regions generate the BU stimulus-driven and TD goal-directed saccadic command signals
that are sent to the SC to drive saccades. (Munoz, 2002; Sparks, 2002; Hall and
Moschovakis, 2003; Leigh and Zee, 2006; Johnston and Everling, 2008). Although
several details of how these distributed regions cooperatively combine to control
saccades are unknown, much of the circuitry is already understood at a significantly
detailed level.
Before the saccadic system can align the fovea upon locations of interest within
the visual field, visual input must be available. In cortical areas this visual input is
relayed from VC to parietal and frontal regions as well as to the SC (Fig. 1.2A green
pathways) (Schiller et al., 1974; Schiller and Malpeli, 1977; Schiller et al., 1979; Pare
and Wurtz, 2001; Isa, 2002). BU visual input arriving into parietal and frontal cortices
are combined with TD goal-driven signals (including attention, decision making, and
working memory related signals) to influence saccadic sensorimotor transformations
(Fig. 1.2A blue pathways). In parietal cortex, the lateral intraparietal area (LIP) projects
to the SCi and has been shown to be involved in both sensorimotor transformations as
well as attention related processing for saccades (Andersen et al., 1997; Colby and
Goldberg, 1999; Glimcher, 2001). In frontal cortex, the DLPFC and SEF also project to
the SCi and have been shown to play a role in working memory and decision making.
The FEF is a critical hub that is interconnected with multiple saccade related areas
15
including the SEF, DLPFC, LIP, and SCi (Schall, 1997; Schall and Thompson, 1999).
Neurons in LIP, FEF and the SC exhibit increases to their firing rate that is time locked to
both stimulus appearance (sensory events) and saccade initiation (motor events)
indicating that these structures are involved in sensorimotor transformations (Pare and
Wurtz, 2001; Wurtz et al., 2001b; Munoz and Schall, 2003). Furthermore, direct
electrical stimulation of the FEF and SC also reliably evoke saccades (Robinson and
Fuchs, 1969), which indicates that these structures in particular are important for relying
the motor command to the brainstem to drive saccades.
The oculomotor basal ganglia (BG) are composed of an interconnected network
of subcortical muclei which include the: caudate (CD), globus pallidus external segment
(GPe), substantia nigra pars reticulata (SNr) and subthalamic nuclei (STN) (Fig. 1.2A
Orange Pathways). Together the BG nuclei influences saccade initiation and modulation
of TD and BU saccade control (Hikosaka, 1989; Hikosaka et al., 2000; Hikosaka et al.,
2006). The CD is the main input nucleus of the BG and receives input directly from the
DLPFC, FEF and SEF (Hikosaka et al., 1989; Ford and Everling, 2009). The SNr is the
main output nucleus of the oculomotor basal ganglia (Hikosaka et al., 2000; Hikosaka et
al., 2006; Shires et al., 2010) that projects inhibitory GABAergic input to the SCi
(Jayaraman et al., 1977; Chevalier et al., 1984; Kaneda et al., 2008). The SNr modulates
activity in the SCi by increasing or decreasing its tonic inhibitory signal. This allows the
BG to selectively suppress (inhibit) or facilitate (disinhibit) saccades by increasing or
decreasing this tonic inhibition signal that is sent to the SCi (Hikosaka and Wurtz, 1983a;
Hikosaka and Wurtz, 1983b; Hikosaka and Wurtz, 1983c; Handel and Glimcher, 1999;
Handel and Glimcher, 2000; Basso and Wurtz, 2002). There are multiple pathways
16
through the BG that control SNr activation to either facilitate or suppress/inhibit saccades
(Fig. 1.2A). The direct pathway (CD to SNr) facilitates saccades when CD excitation
directly inhibits the SNr and reduces its inhibitory effects on saccades (Hikosaka and
Wurtz, 1983a). The indirect pathway (CD to GPe to STN to SNr) is believed to suppress
saccades because disinhibition of the STN (double inhibitory synapses from CD to GPe
to STN) leads to an increase in excitation within the SNr. This increase in inhibitory SNr
output likewise increases saccadic supression/inhibition (Jiang et al., 2003). For normal
TD and BU driven saccades to be performed, the activity between these pathways (direct
and indirect) must be properly balanced. Multiple disorders of the BG can cause
imbalances between these pathways and results in multiple impairments to normal
saccade control (Winograd-Gurvich et al., 2003; Chan et al., 2005; Peltsch et al., 2008;
Peltsch et al., 2008; Chambers and Prescott, 2010).
Lastly, the cerebellum is also interconnected with the PPRF, SCi and FEF
(Moschovakis et al., 1996; Scudder et al., 2002; Sparks, 2002). The cerebellum has been
shown to be involved in online feedback control which is important for ensuring saccadic
movement accuracy within the limited portion of the visual field that is covered by the
fovea.
1.4 Superior Colliculus
The SC is a sensorimotor integration node that is critical for visual orienting
(Moschovakis et al., 1996) and occupies the nexus point where visual-sensory input
converges with saccadic-motor output. The SC is located in the anterior midbrain and
spans approximately 5 mm along the rostro-caudal axis on either side of the midline
17
(Robinson, 1972) (Fig. 1.4A,B). The SC is a laminated structure possessing 7 distinct
anatomical layers: stratum zonal (SZ), stratum griseum superficiale (SGS), stratum
opticum (SO), stratum giseum intermedium (SGI), stratum album intermedium (SAI),
stratum griseum profumdum (SGP) and the stratum album profundum (SAP)
(Moschovakis et al., 1996, Fig. 1.4B). In this thesis these 7 anatomical layers have been
grouped into superficial (SCs) and intermediate/deeper (SCi) layers based on sensory
only (SCs: SZ, SGS, SO) or sensorimotor/motor (SCi: SGI, SAI, SGP, SAP) function
(Munoz, 2002). Each functional SC layer receives afferent input from different visual and
oculomotor structures within the brain (Fig. 1.2).
The SCs is solely a sensory structure that receives visual input from the retina
both directly (via the retinotectal pathway) as well as indirectly from VC and other early
extrastriate areas (via the retino-geniculo-cortical pathway) (Robinson and McClurkin,
1989; Sherman, 2007, Fig. 1.2A).
The SCi is both a sensory and a saccadic motor structure. The SCi receives
indirect visual input from visual (Schiller et al., 1974; Schiller et al., 1979) and parietal
cortices (Pare and Wurtz, 2001) as well as from the SCs (Isa and Hall, 2009). The SCi
also receives saccadic-motor related input from parietal and frontal cortices as well as the
cerebellum and basal ganglia (Munoz et al., 2000; Munoz, 2002) and relays saccadic
commands directly to the brainstem burst generator to drive saccades (Rodgers et al.,
2006).
Both the SCs and SCi are organized into a retino-topic map of the visual field that
has been defined physiologically (Fig. 1.4C,D, Robinson, 1972) and mathametically
(Fig. 1.4. E,F, Van Gisbergen et al., 1987). This map contains an organized
18
Brainstem (Dorsal View)
A
Midbrain Slice (Sagital View)
B
Superficial Layers
(SZ-SO)
Intermediate/Deep Layers
(SGI-SAP)
Superior
Colliculi
C
Visual Field Space
D
B
E
C
E
A
D
A
A
B
C
Anatomical SC Map
D
E
Mathametically Transformed SC Map
E
D
A
B
C
F
R 2 + A 2 + 2AR cos(Φ )
u = B u ln
A
R sin (Φ )
v = B v arctan
R cos(Φ ) + A
Van Gisbergen et al. 1987
Figure 1.4. A. Location of the Superior colliculus. B Anatomical and Functional
subdivisions of the primate SC. C,D. Schematic of the coordinate frame transformation
from projected 2 dimensional visual space to the retino-topic anatomical SC map. E. The
mathematical transformation from the anatomical SC map (3D) to an idealized
mathematical map. F. The formulas used to calculate direct transformations from visual
space to the idealized mathematical SC map. Brain Images adapted from
www9.biostr.washington.edu/cgi-bin/DA/imageform .
19
representation of the contralateral visual hemi-field where the fovea is represented at the
rostral pole and peripheral regions are located more caudally. In the SCs, organized
populations of visually responsive neurons within the map encode the retino-topic
locations of visual stimuli in the field of view (Schiller and Koerner, 1971; Wurtz and
Goldberg, 1972b). In the SCi, populations of sensory and motor neurons encode both the
retino-topic locations of visual stimuli in addition to the target location of a planned or
executed saccade vector (Krauzlis, 2005).
The SC is critical for saccade production because: 1) Its removal eliminates the
ability to produce express saccades (Schiller et al., 1987); 2) Removal of both the SC and
Frontal Eye Fields (FEF) eliminates the ability to make any saccade (Schiller et al.,
1980); and 3) Deactivation of the SCi (via injection of the GABAA agonist muscimol)
leads to the inability to evoke a saccade from the FEF with microstimulation (Hanes and
Wurtz, 2001). Furthermore, the SCi is a critical node for saccadic sensorimotor
transformations because it contains neurons that are close to both the sensory input
(visual responses have been recorded as early as 40ms in SCs and SCi (Guitton, 1992a;
White et al., 2009)) and the motor output (saccades can be evoked via electrical
stimulation with latencies as short as 20ms in SCi (Robinson, 1972; Stanford et al.,
1996)). Thus the layers of the SC contain neurons that span early (visual only SCs
neurons close to retinal input) to late (visuomotor SCi neurons project directly to the
brainstem) stages of visual processing (Sparks, 1986; Munoz et al., 2000; Hall and
Moschovakis, 2003; Krauzlis, 2005; Rodgers et al., 2006).
20
1.5 Visuomotor Transformations in the Superior Colliculus
The precise neural mechanisms responsible for transforming visual input into
saccadic motor commands are not known. The SC, however, is an ideal structure in
which to study these visuomotor transformations because it is situated between the visual
input and the saccadic motor output (Hall and Moschovakis, 2003, Fig. 1.5A). During a
regular latency saccade, the visual and motor responses within SCi neurons are
temporally separate and distinct (Mohler and Wurtz, 1976; Sparks, 1978) (Fig. 1.5B).
Here the time between these responses reflects the integration and processing delays
necessary for a subject to detect that a target is present, decide that it is appropriate to be
saccaded to, and then plan and execute an appropriate response (Reddi and Carpenter,
2000; Carpenter, 2004). How is the visuomotor transformation accomplished during this
interval? Several modeling approaches can help to solve this problem and bridge the gap
between the distinct visual and motor responses that are observed physiologically within
the SCi. One simple and popular hypothesis asserts that the underlying neural mechanism
involves the accumulation of a saccade signal toward a threshold that triggers a saccade
once it is crossed. This accumulating signal has been successfully modeled as a simple
linear signal (Carpenter and Williams, 1995; Munoz and Schall, 2003; Nakahara et al.,
2006a). Such models have been successfully used to describe both variations in SRT
behaviour as well as saccade related neural activity within the SC (Paré and Hanes, 2003)
and FEF (Hanes and Schall, 1996) (linear version of model illustrated in figure 1.5B).
The question that arises when applying such models to the neural activity within the SCi
is how does any saccadic decision signal accumulate between these two apparently
distinct visual and motor responses if at all? In many SCi neurons there is little or no
21
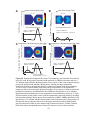
Saccadic Visuomotor Transformation in the Superior Colliculus
A
Visual
Input
SCs
SCi
Saccade
Output
B
Superior Colliculus (Sagital View)
Target Appearance
Eye position
Saccade
Target Detection
Transduction +
Afferent Conduction
Delays
Integration + Processing
Delays
Saccade Trigger Threshold
Accumulating Saccade Decision Signal
SCs (neural activity)
Regular Saccade
Express Saccade
Preparatory Build-up
SCi (neural activity)
Sustained Visual Response
Visual Response
Motor Response
Figure 1.5. A. Schematic of the functional subdivisions of the primate SC showing
visual input (green arrow) to the superficial and intermediate layers and the saccade
motor response output (red arrow) from the intermediate/deeper layers to the brain
stem burst generator. B. Schematic of the saccadic visuomotor transformation
demonstrating simplified temporal profiles of averaged neural firing rates for a phasic
visual response (green) in the SCs and SCi and a saccadic motor response (red) in the
SCi. This visuomotor transformation can be modeled as the accumulation of a neural
sacccadic decision signal (orange line) to a saccadic threshold (orange dotted line) that
(once crossed) triggers a saccadic motor command to move the eyes. During an
express saccade (red dotted line) the visual response combines with pre-target buildup
activity enabling it to be transformed directly into the motor command to move the eyes.
22
activity linking these individual phasic bursts as would be predicted by such accumulator
models (Fig. 1.5B). Some SCi neurons do, however, exhibit a lower frequency
sustainment of visual activity when a visual target stimulus remains within its response
field (McPeek and Keller, 2002; Li and Basso, 2008)(Fig. 1.5B dotted green line), but, it
is unclear whether this is in any way related to threshold accumulation.
During short latency express saccades, there is only a single response that is time
locked to both target appearance and saccade execution (Edelman and Keller, 1996;
Dorris and Munoz, 1998). In this case, many SCi neurons also demonstrate an increase in
pre-target activation that combines with the visual response (Dorris and Munoz, 1998).
This combined activity may boost it above threshold and thus enable it to be transformed
directly into the motor command during an express saccade (Fig. 1.5B dotted red line).
How does the visual response alone trigger a saccade in some instances (express saccade)
and a separate motor response trigger a saccade in others (regular saccade)? Furthermore,
how does the visual response link to the motor response if not via some accumulation to
threshold mechanism? Not only is it uncertain how the visual response is transformed
into the motor response, but the specific links between the visual and motor responses are
also poorly understood. Thus, in order to better understand visuomotor transformations,
we must first understand the relationships that link visual-sensory input and saccadicmotor output signals within critical structures like the SC.
1.6 Neural Network Models of the Saccadic System
Neural networks (also referred to as neural fields) can be powerful tools for
helping to solve some of these outstanding questions by simulating biologically plausible
23
computational mechanisms that may underlie these visuomotor transformations. The
predictions made by such models can be used to guide future physiological research
through testing if they are reflected in the underlying neural circuitry. Thus neural
networks have the potential to provide an important computational framework for
bridging the gap between neural activity and behavioral responses.
Neural field models have several advantages that make them ideally suited to
describing visuomotor transformations in the saccadic system. Firstly, the neural nodes in
such models are spatially organized into a map that can capture the spatial organization of
visual objects and saccadic vectors in the physiological SCi map (Arai et al., 1994;
Trappenberg et al., 2001). Secondly, neural field models describe visuomotor
transformations continuously in space and time which can be readily computed
mathematically and allows for a continuous transformation (i.e. accumulating saccadic
decision signal) between the sensory input and motor output. Thus conceptually, neural
field models can be easily incorporated into existing mathematical and conceptual
saccadic accumulator models.
Previous work has shown that these neural field models of the SC during saccades
are especially powerful as they can reflect both the activity patterns of physiologically
recorded neurons as well as important aspects of the resulting behavior (Trappenberg et
al., 2001; Trappenberg, 2008). Neural field models of the SC have already been able to
successfully account for a large variety of saccadic behaviors, including: 1) differences in
SRT due to endogeneously and exogeneously triggered saccades (Trappenberg et al.,
2001; Trappenberg, 2008); 2) saccade trajectory variations produced by focal SCi lesions
(Badler and Keller, 2002); and 3) competing visual stimuli (Arai and Keller, 2005).
24
1.7 Thesis Objectives
The overall goal of this thesis is to gain a greater insight into the neural
mechanisms that underlie how visual sensory signals are transformed into saccadic motor
commands. I employed modern electrophysiological techniques to record extra-cellular
neural activity in the SC of monkeys trained to perform visually guided saccade tasks that
enabled the examination of the relationships between visual and motor signals to saccadic
behavior. Neural waveforms corresponding to spiking action potentials were recorded
with Plexon data acquisition software and hardware (Plexon Inc.). The activity
corresponding to individual neurons was sorting using cluster sorting techniques (See
Appendix.). I then utilized the knowledge gained from these experiments to develop a
computational neural network model of the SC that simulates sensorimotor
transformations and makes predictions for guiding future physiological research. I
approached this question with three primary objectives:
The first objective was to assess the correspondence between visual and
motor maps within the SCi. The SCi contains a retinotopically organized visual sensory
and saccadic-motor map. However, the relationships between these maps has never been
fully described. This is the goal of Chapter 2. I recorded from neurons in the SCi that
discharged both visual and motor responses and compared their response fields. This
study revealed the correspondence between visual and motor maps in the SCi as well as
the computational consequences of transforming response fields onto the logarithmic SC
map.
25
Recorded Waveforms From an Isolated Single Neuron
A
B
Cluster Sorting Analysis
Figure 1.6. Techniques for sorting neural waveforms corresponding to action potentials
(spikes) from individual neurons. A. Neural waveforms (750-1000 μsec,digitally sampled
at 40 kHz) were triggered in real time from amplified and filtered (150 Hz to 9 kHz)
neural activity recorded with a microelectrode via Plexon data acquisition hardware
(Plexon Inc.). A combination of waveform template matching criteria (derived from the
mean of a sample of waveforms) and manual sorting techniques (cluster sorting of
waveform principal components or other characteristic features) were used to accurately
isolate the action potentials from individual neurons. B. Example of cluster sorted
waveforms from a single neuron (first principle component of the waveform plotted
against second principle component) forming a discrete cluster (yellow dots) from the
background noise (grey dots).
26
The second objective was to examine how external stimulus properties
modulate visual processing in the SC and to determine how these modulations
influence saccade behavior. I recorded from neurons in the SCs and SCi while monkeys
performed visually guided saccades. The luminance of the saccade targets was
manipulated systematically to influence both visual response properties and saccade
behavior. These studies revealed how luminance can alter multiple properties of the
visual response which carry through the sensorimotor transformation to impact saccade
latency and metrics. Furthermore, such modulations to the visual response also
influenced the neural mechanisms underlying the generation of express saccades. These
results are described in Chapters 3 and 4.
The third objective was to apply our findings to the development of a neural
network model of the SC that simulates visuomotor transformations. I developed a
two-dimensional neural field model that simulates saccadic sensorimotor transformations
in the SCi via a non-linear rise to threshold mechanism. This model exploits lateral
interactions between competing TD and BU signals within the SCi map to predict
saccade latency. This model can account for many contradictory findings of how TD and
BU factors influence SRT. These results are described in Chapter 5.
1.8 General Overview
The following chapters provide novel insight into how visual stimuli are
transformed into saccadic targets. I follow a logical progression whereby first I show that
the spatial representation of visual and motor maps are aligned in the SCi thus
demonstrating a critical link between visual input and motor output. I then demonstrate
27
how the size and timing of visual responses can be manipulated by stimulus intensity and
explore the corresponding consequences on saccade behavior. Finally I use the
knowledge gained from the studies in chapters 2 through 4 to develop a model that
simulates saccadic sensorimotor transformations within the SCi map. I show that
modeling the spatial interactions over a two dimensional map yields increased
computational power for predicting many complex relationships between SRT and BU
and TD factors. This model provides a new interpretive framework with specific
predictions that can be used to guide future physiological studies. The testing of these
predictions may reveal novel neural mechanisms influencing visually guided saccades.
The results of each study are discussed in detail at the end of each chapter with a broad
overview of the entire thesis provided in Chapter 6.
28
Chapter 2: The Spatial Relationships of Visuomotor Transformations in the
Superior Colliculus Map
29
2.1 Abstract
The oculomotor system is well understood compared to other motor systems,
however, we do not yet know the spatial details of sensory to motor transformations. This
study addresses this issue by quantifying the spatial relationships between visual and
motor responses in the superior colliculus (SC), a midbrain structure involved in the
transformation of visual information into saccadic motor command signals. We collected
extra-cellular single-unit recordings from 150 visual-motor (VM) and 28 motor (M)
neurons in two monkeys trained to perform a non-predictive visually-guided saccade task
to 110 possible target locations. Motor related discharge was greater than visual related
discharge in 94% (141/150) of the VM neurons. Across the population of VM neurons,
the mean locations of the peak visual and motor responses were spatially aligned. The
visual response fields (RFs) were significantly smaller than and usually contained within
the motor RFs. Converting RFs into the SC coordinate system significantly reduced any
misalignment between peak visual and motor locations. RF size increased with increasing
eccentricity in visual space but remained invariant on the SC map beyond 1 mm of the
rostral pole. RF shape was significantly more symmetric in SC map coordinates
compared to visual space coordinates. These results demonstrate that VM neurons specify
the same location of a target stimulus in the visual field as the intended location of an
upcoming saccade with minimal misalignment to downstream structures. The
computational consequences of spatially transforming visual field coordinates to the SC
map resulted in increased alignment and spatial symmetry during visual-sensory to
saccadic-motor transformations.
30
2.2 Introduction
Multiple sensory and motor related topographic maps have been identified in both
cortical and subcortical regions of the brain. One benefit of these maps regardless of
modality, is to allow for the precise spatial localization of the sensory input signal and the
appropriate motor response (Talbot and Masson, 1941; Kaas, 1997). Understanding how
these maps encode sensory and motor information spatially and facilitate accurate
sensorimotor transformations is a fundamental question in neuroscience. For example, in
the oculomotor system, a visually guided saccade requires a precise eye movement in
order to align the fovea directly onto a specific visual stimulus. It is generally assumed
that this action requires a spatially precise transformation from visual-sensory to
saccadic-motor commands. However, the relations between sensory and motor mappings
and their interactions during sensorimotor transformations have never been
systematically quantified. How these neural maps spatially represent and relay different
sensorimotor transformations is important to understanding what the underlying neural
mechanisms are computing and establishes a link between visual input and motor output.
The superior colliculus (SC) is an ideal structure to study these spatial
transformations in the oculomotor system because it serves as a sensorimotor integration
node with a well understood topographically organized retinotopic sensorimotor map
(Schiller and Koerner, 1971; Robinson, 1972; Wurtz and Goldberg, 1972b; Sparks et al.,
1976), that has been defined mathematically (Ottes et al., 1986; Van Gisbergen et al.,
1987) (Fig. 2.1A). Individual visuomotor (VM) neurons in the intermediate layers of the
SC (SCi) contain organized response fields (RF), whereby individual neurons discharge a
burst of action potentials for both saccades and the appearance of visual targets to a
31
A
Visual Space
-60o
50o
-30o
C
0o
30o
D
B
A
E
10o 20o
50o
90o
Left
20o 10o
5o
90o
Right
D
2o
60o
30o
0o
A
C
B
E
D
60o
B
SC Space
-90o
-30o
-60o
Rostral
Caudal
1mm
-90o
Response Field Size
Invariant in SC Space
C
Invariant in Visual Space
Response Field Shape Symmetry
Symmetric in SC Space
E
Symmetric in Visual Space
Figure 2.1. A. Logarithmic spatial transformation from visual field space to the
topographic SC map using the transformations defined by equations 1 and 2 (Ottes,
Van Gisbergen and Eggermont, 1986, Van Gisbergen, Van Opstal and Tax, 1987).
B,C: Potential field size relationships between target eccentricity (relative to fovea
position) and RF size across visual and SC space. B. As RF eccentricity increases, RF
size increases in visual space but remains invariant in SC space. C. As RF eccentricity
increases RF size decreases in SC space but remains invariant in visual space. D,E.
Potential relationships between RF shape across visual and SC space. RF shape is
symmetrical in SC space and asymmetrical in visual space (D), or RF shape is
asymmetrical in SC space and symmetrical in visual space (E).
32
restricted region of the visual field (Wurtz and Goldberg, 1972a; Sparks and Mays,
1980). The SC plays a pivotal role in integrating afferent sensory information into
efferent motor signals for controlling saccadic eye movements (Sparks, 1986; Munoz et
al., 2000; Hall and Moschovakis, 2003; Krauzlis, 2005).
However, the precise spatial
relationships of these sensory and motor signals within the SC map has not been
described and is fundamental to understanding sensorimotor transformations in the
oculomotor system.
In this study we quantify visuomotor transformations in the SCi in
order to better understand the computational consequences of representing these signals
within an organized spatial map. These computational consequences determine how
sensory to motor transformations are simplified, or spatial discrepancy is reduced by
transforming signals into SC space (Fig. 2.1A). Previous studies exploring spatial
sensorimotor activity in the SC exhibit two major limitations: 1) neural responses to
sensory or motor activity was only assessed from a limited spatial density of target
locations (Goldberg and Wurtz, 1972b; Sparks et al., 1976; Anderson et al., 1998) or
along a single dimension of possible locations (Munoz and Wurtz, 1995b); and 2)
predictability of target location or time of target appearance elicited preparatory activity
which can contaminate the pure sensorimotor responses (Munoz and Wurtz, 1995a;
Basso and Wurtz, 1997; Basso and Wurtz, 1998; Dorris and Munoz, 1998). Here, we
avoided these limitations by employing a visually guided saccade task with high spatial
resolution that minimizes spatial and temporal prediction.
One corollary to the logarithmic mapping function between visual and SC space is
that if RF size is invariant in one space, then it must change with eccentricity in the other
(Fig. 2.1B,C). The size and extent of population activity in the SC is an important signal
33
parameter believed to represent a spatially weighted average of the underlying activity in
the SC map coding both the visual target and saccade vector (Sparks et al., 1976; Lee et
al., 1988; Trappenberg et al., 2001) locations. Thus, the spatial change of visual and
motor signals over the SC map can have computational consequences that include an
increased recruitment of neural tissue in order to represent larger areas of the map. If any
systematic invariance exists then either: RF size will remain constant across SC space
(McIlwain, 1986; Munoz and Wurtz, 1995a) and increase with increasing eccentricity
across visual space (Fig. 2.1B), or RF size will decrease with increasing eccentricity
across SC space and remain constant across visual space (Fig. 2.1C).
Because of the logarithmic transformation between visual and SC space (Ottes et
al., 1986; Van Gisbergen et al., 1987), most spatial models assume that the underlying
circuitry is evenly distributed (Fig. 2.1B) and symmetrical (Fig. 2.1D) across the SC map
(Arai et al., 1994; Optican, 1995; Gancarz and Grossberg, 1999; Short and Enderle, 2001;
Trappenberg et al., 2001). In these models, the symmetrical and evenly distributed
circuitry represents visual and motor activity identically and uniformly across the map.
Visual to motor transformations are therefore simplified in these models because they are
computing signals of the same shape and size everywhere in the map. However, this
simplified view has been challenged (Nakahara et al., 2006b) by suggesting that if the
underlying circuitry is asymmetric in the SC (Fig. 2.1E), then the activity would
inherently exhibit previously observed caudal-to-rostral (Munoz et al., 1991; Munoz and
Wurtz, 1995b) and lateral (Anderson et al., 1998) spread during execution of gaze shifts.
As this spread has been observed physiologically and interpreted controversially as being
related to gaze control (Munoz et al., 1991; Guitton, 1992b; Wurtz and Optican, 1994;
34
Guitton et al., 2003), it is important to assess whether RF symmetry could account for it
and whether simple uniform and symmetric models of the SC should be revised.
Because VM neurons project to premotor circuitry in the brainstem reticular
formation (Scudder et al., 1996; Kato et al., 2006; Rodgers et al., 2006) and are thought
to shape oculomotor (Dorris et al., 1997; Dorris et al., 2007) and head motor commands
(Corneil et al., 2004), understanding the visuo-motor transformation in the SC is a critical
link between sensory input and motor output. The specific aims of this study are to assess
the computational consequences of spatially transforming sensorimotor activity from
visual space into SC space by: 1) determine the spatial correspondence between visual
and motor RFs in visual and SC space; 2) test whether RF size is invariant across either
visual or SC space (Fig. 2.1 B,C); and 3) test whether RF shape is symmetric across
either visual or SC space (Fig. 2.1D,E).
Our data reveal a tight spatial alignment between visual- and motor-related
information within the intermediate layer of the SC and show that transforming activity
into SC coordinates improves alignment between sensory and motor signals.
Furthermore, transforming from visual space to SC space produces more symmetrical
RFs (Fig. 2.1D) with sizes that are relatively invariant across eccentricity (Fig. 2.1B).
2.3 Methods
2.3.1 Animal Preparation
All animal care and experimental procedures were in accordance with the
Canadian Council on Animal Care policies on the use of laboratory animals and approved
by the Queen’s University Animal Care Committee. Two male monkeys (Macaca
35
mulata, 4 kg and 12 kg) were trained to perform oculomotor tasks. One day prior to
surgery, the animal was placed under Nil per Os (NPO, water ad lib) and a prophylactic
treatment of antibiotics was initiated (5.0 mg/kg Baytril). On the day of the surgery,
anesthesia was induced by ketamine (6.7 mg/kg IM). A catheter was placed intravenously
to deliver fluids (lactated ringer) at a rate of 10 ml/kg/hr throughout the duration of the
surgical procedure. Glycopyrolate (0.013 mg/kg im) was administered to control
salivation, bronchial secretions, and to optimize heart rate (HR). An initial dose was
delivered at the start of surgery followed by a second dose 4 hours into the surgery.
General anesthesia was maintained with gaseous isofluorene (2-2.5%) after an
endotracheal tube was inserted (under sedation) induced by an intravenous bolus of
propofol (2.5 mg/kg). HR, pulse, pulse oximetry saturation (SpO2), respiration rate, fluid
levels, circulation, and temperature were monitored throughout the surgical procedure.
To allow access of microelectrodes to the SC, a craniotomy was made based on
stereotaxic coordinates of the SC, centered on the midline and angled 38º posterior of
vertical. The stereotaxic apparatus (KOPF Instruments) stabilized the animal’s head
during implantation of stainless steel screws, which served to anchor the explant
(recording chambers and head post stabilized with acrylic cement) to the skull. A
stainless steel recording chamber (Crist Inst.) allowed for mounting of a microdrive and a
stainless steel head holder were embedded in the acrylic explant. The chamber was tilted
38° posterior to vertical to allow for access to the SC. Sterilized scleral search coils (1920 mm diameter) were inserted subconjunctivally into each eye (Judge et al., 1980) and
were used to measure eye position with the search coil technique (Robinson, 1963). The
search coil leads had a Powell connector attached at termination that was embedded in
36
the acrylic explant. The analgesic Buprenorphine (0.01-0.02 mg/kg im) was administered
throughout the surgery and during recovery (8-12 hours). The anti-inflammatory agent
Anafen (2.0 mg/kg first dose, 1.0 mg/kg additional doses) was administered at the end of
the surgery (prior to arousal), the day after the surgery, and every day thereafter (as
required). Monkeys were given at least 2 weeks to recover prior to onset of behavioral
training.
2.3.2 Behavioral Paradigms
Behavioral paradigms and visual displays were under the control of two Dell 8100
computers running UNIX-based real-time data control and presentation systems (Rex
6.1)(Hays et al., 1982). Monkeys were seated in a primate chair with their heads
restrained for the duration of an experiment (2-4 hours). They faced a display cathode ray
tube monitor that provided an unobstructed view of the central visual area 54° x 44°. For
this study, each monkey was required to perform a visually-guided saccade task (Fig.
2.2A). Experiments were performed in total darkness with individual trials lasting ~1-2
seconds. Each trial required a single saccade to a specific visual target located some
distance away from the central fixation point. The display screen was diffusely
illuminated during the inter-trial interval (800 - 1500 ms), to prevent dark adaptation. At
the start of each trial, the screen turned black and, after a period of 250 ms, a central
fixation point (0.5° diameter spot, 25cd/m2) appeared at the center of the screen. The
monkeys had 1000 ms to initiate fixation and they were required to maintain fixation for
300-500 ms. Following fixation, the fixation point was extinguished and a visual target
(0.5° diameter spot, 25cd/m2) was presented simultaneously in the periphery. The
37
B
C
5500
Total Trials
Baseline
(100ms) Saccade Onset
300 - 500 ms
Eye
Position
00
E
D
200
800
Visual Response
Field
Firing Rate (spikes/s)
Firing Rate (spikes/s)
10°
400
0
0 40 80 120 160 -80 -40 0 40 80
Time from Target Onset Time from Saccade Onset
(ms)
(ms)
F
100
200 300
SRT (ms)
G Visual Peak Locations 3 H
2
100
1
0
Visual Field Boundary
Visual Peak
Motor Field Boundary
Motor Peak
Mediolateral Axis (mm)
VM
500
Motor Response
Field
100
Horizontal Position
0
400
100
Motor Peak Locations
3
M
VM
2
1
0
-1
-1
-2
-2
-3
4
3
2
1
0
Rostrocaudal Axis (mm)
-3
4
3
2
1
0
Rostrocaudal Axis (mm)
Vertical Position
Fixation
Point
Target
Mediolateral Axis (mm)
A
Figure 2.2. A. Schematic representation of the visually guided saccade task used in
this study. Baseline activity was calculated from the last 100ms of fixation before the
appearance of the target. B. 110 target locations (black dots) presented at 12 different
directions and 8-10 different eccentricities. C. Saccadic reaction time (SRT) distribution
histogram for all saccades (N = 45,633 trials). The dashed vertical line denotes the 120
ms cut-off time whereby early anticipatory responses and express saccades were
removed (11.7% of all trials were removed). D. Rasters and spike density function of a
representative VM neuron for target-aligned (left panel) and saccade-aligned (right
panel) responses to the optimal target location, 9° along the horizontal meridian in the
left visual hemifield. E. Visual (left panel) and motor (right panel) RFs for representative
neuron shown in D. F. Visual and motor RF boundaries defining area with activity 3
standard deviations above baseline for representative neuron shown in D and E. The
cross and triangle represent visual and motor peak response vectors calculated from
the location of maximal activity in part E. G,H. Neuron locations on the SC map. The
anatomical location of each neuron recorded was determined by the spatial location of
its peak visual response (G) and peak motor response (H) converted onto the SC map.
Black closed circles and gray open circles denote VM and M neurons, respectively.
38
monkeys had 500 ms to initiate a saccade to the target and were required to fixate on the
target for an additional 300 ms. Each correct trial was rewarded with a drop of fruit juice
or water. Luminance was measured with an optometer (UDT instruments, model S471)
that was positioned directly against the screen of the monitor and centered on the
stimulus. Targets were presented along one of 12 directions at amplitudes of 1, 2, 4, 6, 9,
12, 16, 20, 25 or 30° from central fixation (Fig. 2.2B). Purely vertical saccades could not
be tested beyond the 20° locations and purely horizontal saccades could not be tested
beyond 25° due to the shape of the monitor. This gave a total of 110 possible target
locations that were randomly interleaved.
2.3.3 Recording Techniques
Extracellular recording was performed with tungsten microelectrodes (0.5-5 MΩ
impedance, Frederick Haer) inserted through guide tubes (23 gauge) that were anchored
in delrin grids with 1 mm hole separations inside the recording chamber (Crist C.F. et al.,
1988). Electrodes were advanced with a microdrive to the dorsal surface on the SC,
distinguished clearly by large increases in multiunit activity following each saccade or
change in visual stimuli. The electrodes were then lowered approximately 1000 μm into
the intermediate layers of the SC to a location with saccade-related activity, and we
proceeded to isolate single neurons.
2.3.4 Data Collection
Multi-unit waveforms (800 μsec, sampled at 40 kHz) were triggered in real time
from amplified and filtered (150 Hz to 9 kHz) neural activity recorded with Plexon data
acquisition hardware (Plexon Inc.). Neural and behavioral data were viewed online with
39
commercially available software (Sort Client 2.1, Plexon Inc.) that made it possible to
isolate single or multiple neurons from an individual electrode. Confirmation of accurate
isolation and modifications to neural sorting was performed offline (Offline Sorter 2.5,
Plexon Inc.). Horizontal and vertical eye position was collected from one eye and
sampled at 1 kHz. The precise onset and end of each saccadic eye movement was
identified off-line. Figure 2.2C illustrates the saccadic reaction time (SRT: the time from
target appearance to saccade initiation) distribution of correct saccades collapsed across
all 110 target locations from both monkeys. The shape of the distribution indicated a
single modal skewed normal distribution (mean: 166ms) that is characteristic of SRT
distributions (Carpenter and Williams, 1995). Trials with SRTs of less than 120 ms
(11.7% of all trials) were excluded from analysis in order to remove anticipatory
responses (saccades made before target perception) and express saccades (in which visual
and motor related discharges overlap); (Edelman and Keller, 1996; Dorris et al., 1997;
Sparks et al., 2000) from further analysis. This ensured that visual responses were
analyzed separately from motor related activity. This was confirmed by the timing of the
peak visual responses of all VM neurons, which occurred with latencies between 70 and
100 ms after target appearance. The distinct separation of visual and motor activity was
also confirmed within each VM neuron by the experimenter during offline analysis.
2.3.5 Average Spike Density Functions and Neuron Classification
Neuron activity associated with target locations that had 4 or more repeated trials
to each of the 110 possible target locations were included in the analysis. Trains of action
potentials for each trial were convolved into spike density functions using a Gaussian
40
kernel (σ = 5ms) for each spike. Spike density functions were aligned on target onset
when analyzing visual responses and saccade onset when analyzing motor responses (Fig.
2.2D). Neural activity from repeated trials with the same target location were summed
together and averaged to produce a spike density function of a neuron’s visual and motor
responses to each target. Neurons were classified as either VM or motor (M) based on the
presence of saccadic motor with or without visual sensory related activity, respectively. A
significant visual response was classified based on an increase of activity with a distinct
peak of greater than 60 spikes/s during an epoch of greater than 60 ms following target
presentation but prior to the onset of saccade-related activity. A significant motor
response was classified based on an increase in activity greater than 80 spikes/s +/-1ms
around saccade onset. Baseline activity was calculated as the average discharge from all
correct trials during the last 100 ms of active central fixation prior to target appearance
(Fig. 2.2A).
2.3.6 Calculation of Individual Neuron Response Fields and Peak Response
Vectors
For each neuron, visual-related discharge rate was calculated from target-aligned
average spike density functions at ± 1 ms around the time of peak visual activity
occurring over a time interval of 60-100 ms following target presentation. Motor-related
discharge was calculated from saccade-aligned spike density functions ± 1 ms around
saccade onset for saccades with SRT greater than 120ms. The spatial extent of the visual
and motor RFs were calculated with a cubic spline (de Boore, 1978) that interpolated
between the 110 possible target locations across the visual field (Fig. 2.2E). Visual and
41
motor RFs were defined spatially as the area in visual or SC space (within the viewable
area of the display monitor) that contained activity greater than 3 standard deviations
(SD) above baseline activity (e.g., Fig. 2.2F).
Data obtained in visual field coordinates were also transformed into SC
coordinates based on the following transformations described by Van Gisbergen and
colleagues (Ottes et al., 1986; Van Gisbergen et al., 1987):
R 2 A2 2 AR cos
u Bu ln
A
(1)
R sin
v Bv arctan
R cos A
(2)
Where u denotes the anatomical distance from the rostral pole (mm) along the horizontal
position axis, v is the distance (mm) along the vertical axis, R is the retinal eccentricity,
and Ф is the meridional direction of the target (deg). The constants are Bu = 1.4 mm; Bv
= 1.8 mm/rad; and A = 3°.
2.3.7 Calculation of Individual Neuron Response Field Peak and Asymmetry
The location of the peak response vector was determined from the point of
maximal activity in the visual and motor RFs plotted in visual or SC space. The
eccentricity of the peak activity in the visual field was defined as the distance from the
peak of the visual and motor RFs to the central fixation point. The eccentricity of this
peak response in SC space was defined as the distance from the peak of the visual and
motor RFs to the rostral pole of the SC. We chose to measure distances in SC space
linearly in order to be consistent and comparable with visual space. In order to confirm
42
that the linear distances measured in SC space did not differ significantly from the
corresponding logarithmic distances measured along the curved isolines, we compared
the percent difference in eccentricity between linear and logarithmic measures
(logarithmic distance / linear distance). The mean percent difference in the eccentricity
of the peak visual and motor responses were 2.35% (standard error = 0.18) and 2.26%
(standard error = 0.19) respectively across the population of 150 VM neurons when
measured logarithmically rather than linearly. This difference between linear and
logarithmic measures was therefore very small (2%) and did not significantly change any
of the findings of this paper.
13 of 150 neurons were removed from the analysis of peak visual and motor
locations in SC space because they exhibited peak visual and motor responses that were
nearly vertical and located on opposite sides of the vertical meridian. (This mapped the
peak visual and motor responses of these neurons to opposite sides of the SC and made it
impossible to compare them within the same colliculus.)
Asymmetry was defined as the distance between the location of peak visual or
motor activity and the center of each neuron’s RF. The center of each RF was calculated
in both SC and visual space as the center of mass, where mass was assumed to be equally
distributed over the entire significant area above 3 standard deviations. The normalized
proportional asymmetry between visual space and SC space was determined by dividing
the overall asymmetry by the mean eccentricity of the peak visual and motor responses.
The 13 of 150 neurons that had peak visual and motor locations in opposite SC were
eliminated from the asymmetry analysis in SC space only. Although Nakahara et. al.
2006 analyzed asymmetry based on the center of mass, we utilized the centre of the RF
43
(as defined by the statistically significant spatial signal) in order to capture the maximal
asymmetry as defined by the full extent of the shape of the RF. Since the centre of mass
is influenced by the peak, and the peak varied in our physiological data relative to
Nakahara’s modeled data, we did not want our measure of asymmetry to be artificially
influenced by physiological variation in the magnitude of the peak. However, the same
trends were confirmed when the asymmetry analysis was repeated utilizing the centre of
mass, i.e. the correlation between asymmetry and eccentricity was reduced in the SC map
relative to visual space, and overall the proportional asymmetry of the visual and motor
response was significantly reduced in the SC map relative to the visual map. When
computed using the centre of mass, the overall measured asymmetry was reduced
because the centre of mass was biased closer to the peak than the centre of the active area
of the RF.
In order to determine whether the observed asymmetry was directed rostrally in
visual space (Fig. 2.1D) or caudally in SC space (Fig. 2.1E) we analyzed the direction of
the asymmetry for all neurons that expressed a minimum of 3% normalized asymmetry
(Fig. 2.6E). We defined rostral and caudal asymmetry as any shift of the peak activity
relative to the centre of the RF within a direction of +/- 45º of either the rostral pole or
the caudal SC. Unimodal Rayleigh tests were used to determine whether the distributions
of the asymmetric directions were statistically unimodal relative to a uniform distribution
(Batschelet, 1981). This statistic is based on the mean vector length which describes
similarity across a sample of angles (e.g., directions of asymmetry). For the unimodal
test, a mean vector length of 0 is obtained if all angles are uniformly distributed and a
value of 1 is obtained if all angles are identical. The value of a mean vector length along
44
this continuum provides an index of unimodality that is compared with a Rayleigh
distribution. The average orientation of angles in circular coordinates provides the
preferred direction of a unimodal distribution.
For 99/150 of VM neurons and 19/28 of M-only neurons, the motor RFs extended
beyond the outer edge of the visual display. Some of these neurons may have had
unbounded (or open-ended) movement fields (Munoz and Wurtz, 1995a) and some may
have had a distinct distal border beyond the edge of the display. In SC space these
neurons had a peak motor response vector distal of 2 mm or more from the rostral pole.
This limitation of display size indicates that some portion of the RF area was
underestimated in these cells; however this underestimation does not invalidate our
results as visual RFs were found to be smaller and contained within motor RFs (Fig. 2.3
A,B). We would expect a theoretically perfect measure of RF area to increase the
significant correlation we observed between RF area and eccentricity in visual space, but
would have very little impact in SC space due to logarithmic compression which
increases with distance from the rostral pole.
Our underestimation of RF area correspondingly decreased our estimation
asymmetry in both SC and visual space, but reduced asymmetry most significantly in
visual space. As we found that asymmetry was significantly greater in visual space (Fig.
2.6E), an increase in the testable area will only magnify this finding. Furthermore, in
order to account for observed caudal-rostral spread of activity during saccades, the
asymmetry in the SC that the Nakahara model (Nakahara et al., 2006b) predicted was
directed towards the rostral pole. Because we have underestimated asymmetry that is
45
B
60
40
50
20
D
30
R = 0.58
10
20
30
Mean Eccentricity of Peak Response (deg)
Overall Misalignment (mm)
F2
1.6
0.3
1.2
*
40
30
20
10
0
R = 0.28
0.8
0.4
0
0
1.5
-1.5
Misalignment (mm)
50
ua
l
SC Sp
a
Sp ce
ac
e
E0.6
Normalized Percent Misalignment
60
10
0
-20
0
20
Misalignment (deg)
SC Space
G
20
0.3
Percent of Neurons
0
Vi
s
Visual Space
0.6
Percent of Neurons
C
10 20 30 40 50 60 70 80 90 100
Percent of Visual Response Field
Contained within Motor Response Field
Overall Misalignment (deg)
0
0
100
% Size of Visual RF
Relative to Motor RF
Visual Space
SC Space
ua
SCl S
p
Sp ac
ac e
e
Percent of VM Neurons
80
Vi
s
A
1
2
3
4
Mean Eccentricity of Peak Response (mm)
Figure 2.3. Relationships between visual and motor RF areas and visual and motor
peak response locations in visual and SC space. A. The proportion of the visual RF
area which was contained within the area of the motor RF for all VM neurons (N = 150)
in visual (black bars) and SC (gray bars) space. B. Mean percent size of visual RF
relative to motor RF across all neurons in both visual field space and SC space (visual
RF area / motor RF area). t-test p > 0.4. C. Histogram of the difference in alignment
between peak visual and motor response locations in visual space. D. Absolute
difference of visual and motor peak response locations plotted against the average
eccentricity of the visual and motor peak locations in degrees in visual space. Solid line:
regression line for all data points (R = 0.58, m=0.48, p<0.01). E. Histogram of the
difference in alignment between peak visual and motor response locations in SC space.
F. The absolute difference of visual and motor peak response locations is plotted
against the average eccentricity of the visual and motor peak response locations in mm
on the SC map. Solid line: regression line for all data points (R = 0.28, m=0.13, p
<0.01). G. Normalized overall proportional misalignment difference between peak visual
and motor responses in both SC and visual space (Overall Difference / Mean
Eccentricity of Peak Response) (t-test p <0.01).
46
directed away from the rostral pole only, our analysis of the Nakahara model’s prediction
is not compromised.
2.3.8 Calculation of Neural Population Point Image Response Fields and
Peak Activity
To confirm independently the relationships between visual and motor RFs
observed within individual neurons, we also analyzed the simultaneous population
activity or “point images” (McIlwain, 1986) of visual and motor activity across the SC in
our task. The activity of all neurons was plotted simultaneously on the SC map in order to
analyze the visual and motor-related population responses. The position of each neuron
on the SC map was determined from the location of its peak visual response (for the
visual population point image, Fig. 2.2G) and peak motor response (for the motor
population point image, Fig. 2.2H). An interpolation with a cubic spline fit was used to
create a point image (McIlwain, 1986) on the SC map. Population activity was analyzed
independently using two different techniques; first by mirroring all neurons across the
vertical meridians (to ensure that all neurons were plotted in the same SC) and separately
by mirroring across both the horizontal and vertical meridians. Neurons were mirrored
across the horizontal meridian because we found no bias in eccentricity or alignment of
the visuomotor responses from the upper and lower visual field. Mirroring neurons into
the same SC improved spatial resolution by exploiting the underlying symmetry of the
SC map. All neurons were plotted in the left SC, while visual and motor point images
were calculated for 18 different rightward saccade vectors with eccentricities ranging
from 1 to 12 degrees within +/-30º visual angle from the horizontal meridian. Limiting
47
the amplitude range to a maximum of 12º eccentricity ensured that we had sufficient
neuron data points on the SC map to close the distal border of the point image within the
visual range of the monitor (Fig. 2.2B,G,H). Furthermore by limiting the visual angle to
+/-30º from horizontal likewise ensured sufficient enough data points to close the distal
border of the point image within the upper and lower vertical limits of the SC map. The
population activity corresponding to each target location and saccade vector were
analyzed separately. Visual and motor point image fields were defined as the area of
activity greater than 3 SD above the average baseline for the population of 150 VM
neurons.
2.4 Results
2.4.1 Discharge Characteristics of Single-Neurons
We collected complete RFs (at least 4 correct trials for each of 110 target
locations) from 150 VM and 28 M neurons located throughout the intermediate layers of
the SCi (Fig. 2.2G,H). The activity of a typical VM neuron is shown in Fig. 2.2D. This
neuron had a maximal visual (351 spikes/s) and motor (804 spikes/s) response to its
optimal target location on the horizontal meridian 9° into the contralateral visual
hemifield. Figure 2.2E shows the interpolated cubic spline maps of the peak visual- and
motor-related discharge and Fig. 2.2F plots the 3 standard deviation borders of the peak
visual and motor RFs from the same neuron. Higher peak motor than visual related
discharge was observed in 94% (141/150) of the VM neurons analyzed. The range of
peak visual response latencies, calculated as the time from target appearance to the peak
visual-related discharge at the peak target location, was 71-99 ms.
48
2.4.2 Spatial Comparison of Visual and Motor Response Fields
Across the population, visual RFs were significantly smaller than motor RFs in
both visual and SC space (Fig. 2.3A). Figure 2.3B shows the proportion of the visual RF
that was contained within the motor RF for all VM neurons. In visual space, 72.7 % of
neurons (108 of 150) had visual RFs that were at least 90% contained within their motor
RFs (Fig. 2.3B, black bars). In SC space, 80 % of neurons (120 of 150) had visual RFs
that were at least 90% contained within their motor RFs (Fig. 2.3B, gray bars).
2.4.3 Comparison of Peak Visual and Motor Response Locations
The cross and triangle in Fig. 2.2F represent the peak visual (black) and motor
(gray) response for the representative neuron. To compare the relative positions of the
peak visual and motor response locations across the population of VM neurons, the
difference between visual and motor peak locations and eccentricity was compared.
There were no systematic differences in eccentricity between visual and motor peak
response locations in visual space (Fig. 2.3C) and SC space (Fig. 2.3E), respectively.
However, many individual neurons did exhibit some misalignment between the visual
and motor peak locations as the median misalignment difference was 3.2 deg in visual
space and 0.34 mm in SC space.
There was a significant correlation between the difference in visual and motor
peak locations and eccentricity of VM neurons in both visual (Fig. 2.3D, r =0.58; p<0.01)
and SC space (Fig. 2.3F, r =0.28; p<0.01), however this correlation was significantly
reduced in SC space (z-test for two correlations, z=3.14; p<0.05). That is, as the
49
eccentricity of the peak response increased, so too did the difference between peak visual
and motor response locations in both visual and SC space. However, the increasing
logarithmic compression of visual space with increasing distance across SC space caused
large differences between visual and motor peaks at distal target locations to be reduced.
When normalized to the mean eccentricity of the peak visual and motor response
locations, the proportional percent difference between visual and motor peaks was
significantly higher in visual space (49.3%) than in SC space (27.3%) (Fig. 2.3G, paired
t-test p<0.01). This indicates that the mean misalignment between the visual and motor
peaks was reduced when transformed into SC space. As a consequence, the
transformation of visual signals into the SC map reduced the misalignment of sensory
and motor signals.
2.4.4 Comparison of Visual and Motor Point Images
In order to independently confirm the alignment of visual and motor activity in
SC space, we also analyzed the simultaneous population activity or point images from all
150 VM neurons to 18 specific saccade vectors. Figure 2.4A shows an example visual
(left panel) and motor (middle panel) point image from a 6° rightward horizontal saccade.
The rightmost panel shows the boundary and peak location of each of the visual and
motor responses. The visual point image was smaller and completely contained within
the motor point image. The visual and motor peak locations were misaligned by 0.3mm.
Across the population of visual and motor point images to each of the 18 saccade vectors
analyzed, there was no correlation between the eccentricity of the visual and motor point
images and the misalignment of the visual and motor peak locations (Fig. 2.4B). Thus,
50
Visual Point Image
(6o Rightward Target)
Motor Point Image
(6o Rightward Saccade)
150
Visuomotor Overlap
Firing Rate (spikes/s)
A
Visual Point Image Boundary
Visual Peak
Motor Point Image Boundary
Motor Peak
1mm
C
Vert. Mirror
Vert., Horiz. Mirror
Vert. Mirror
Vert., Horiz. Mirror
100
Percent of Point Image
Overall Misalignment (mm)
B1
0
0.8
80
0.6
60
0.4
40
0.2
20
0
1
2
3
Mean Eccentricity of Peak Response (mm)
50
40
30
20
10
E
50
Normalized
Percent Misalignment
Size of Visual Point Image
Relative to Motor Point Image
D
75
90
95
80
85
100
Percent of Visual Point Image
Contained within Motor Point Image
40
30
20
*
10
Vert. Mirror
Vert., Horiz. Mirror
Vert. Mirror
Vert., Horiz. Mirror
Figure 2.4. A. Simultaneous population activity (point image) from all VM cells plotted
on the SC for a 6° right horizontal saccade. The left panel plots the visual point image
activity at the overall population peak of the visual response (90 ms after target onset).
The middle panel plots the motor point image activity +/- 1ms around saccade onset.
Each neuron is plotted on the SC map based on the spatial location of the peak visual
(left panel) and motor (right panel) response. The rightmost panel plots the boundary
and peak of both the visual and motor point images. B. The absolute difference of visual
and motor point image peaks is plotted against the average eccentricity of the visual and
motor point image location in mm on the SC map. Black closed circles denote point
images in which neurons were mirrored across the vertical meridian only. Gray open
circles denote point image values where neurons were mirrored across both the
horizontal and vertical meridians. C. Proportion of the visual point image area that was
contained within the motor point image area for 18 different saccade vectors. D. Mean
size of visual point image relative to motor point image in SC space (visual point image
area / motor point image area). E. Normalized overall proportional misalignment between
peak visual and motor point images in SC space (Overall Misalignment / Mean
Eccentricity of Peak Response) (t-test p <0.03).
51
the misalignment of visual and motor point image peak responses did not scale with
eccentricity. The visual point image was at least 95% contained within the motor point
image 83% of the time when neurons were mirrored across the vertical meridian only
(Fig. 2.4C). When we mirrored neurons across both the vertical and horizontal meridians,
100% of all visual point images were at least 95% contained within the motor point
images. Across the population, the visual point images were smaller than the motor point
images (Fig. 2.4D). Thus consistent with the single neuron data, the visual point images
were smaller and tended to be contained completely within the motor point image. Figure
2.4E plots the normalized misalignment between the visual and motor point images. The
normalized visuomotor misalignment of the point images (36% vert mirror; 16%, vertical
and horizontal mirror) was not significantly different from the normalized misalignment
calculated from the single cell analysis (27%) in SC space (t-test; p>0.05). However, the
decrease in the normalized misalignment between the vertical and the horizontal and
vertical mirroring was significant (t-test; p<0.003). This indicates that the increased data
resolution gained from mirroring across both the horizontal and vertical meridians
yielded visual and motor point images with reduced misalignment. Overall, the point
image analysis confirmed the alignment and spatial relationships between visual and
motor RFs that was independently calculated from the population of individual neurons.
2.4.5 Changes in Response Field Size
In order to spatially assess and compare the computational consequences of
representing visual and motor signals in both visual and SC space, we quantitatively
assessed the relationships between RF size and eccentricity (Fig. 2.1B,C). Figure 2.5
52
plots the relationships between RF size and eccentricity in visual and SC space for VM
and M-only neurons. There was a strong positive correlation in visual space between the
eccentricity of the peak visual response and the visual RF area for visual (Fig. 2.5A,
r=0.51; p<0.01) and motor (Fig. 2.5B, r=0.65; p<0.01) responses in VM neurons. This
correlation was also present among M-only (Fig. 2.5B, r=0.58; p<0.01). This indicates
that in visual space, RF size increases with increasing eccentricity. When mapped into SC
space, there was no relationship between RF area and the eccentricity of peak response
vectors for visual responses in VM neurons (Fig. 2.5C, r=0.08; p>0.3). A significant
correlation remained between motor RF area and eccentricity in SC space for the motor
response of both VM (Fig. 2.5D black line r =0.46; p<0.01) and M-only (Fig. 2.5D gray
line r =0.48; p<0.01) neurons.
Previous studies have shown that neurons located in the rostral pole of the of the
SC are active during visual fixation (Munoz and Wurtz, 1993a) and possess very small
RFs (Munoz and Wurtz, 1993a; Krauzlis et al., 2000; Krauzlis, 2003). Antidromic
identification has revealed that these neurons project more densely to the OPN region of
the brainstem (Gandhi and Keller, 1997). Fixation activity modulates with task epochs
and there is likely greater activation during the baseline epoch for these neurons (see Fig.
2.2A). Therefore, we analyzed the population of neurons located below 1mm eccentricity
separately. 24 of 150 VM and 6 of 28 M-only neurons had motor peak locations that
placed them within 1mm of the rostral pole. When neurons below 1mm were removed,
the correlation between RF size and eccentricity in SC coordinates was eliminated for
both VM (r=0.17; p>0.05) and M-only (r=0.09; p>0.6) populations. These data indicate
that RF field size increased across the visual field with increasing eccentricity, but
53
200
600
4
0
25
10
R = 0.46
R = 0.48
6
2
0
0
1
2
3
4
Eccentricity of Peak Motor Reponse (mm)
8
*
20
6
*
15
Area of Motor RF
*
Visuomotor Neurons
*
Motor Neurons
*
4
10
2
5
m
>1 m
m
<1 m
m
>1 m
m
m
*
<1
>1mm
m
m
<1
>1mm
m
m
10
20
30
Eccentricity of Peak Motor Reponse (deg)
Baseline Variability
Baseline
*
0
D14
0
1
2
3
4
Eccentricity of Peak Visual Reponse (mm)
35
30
25
20
15
10
5
R = 0.58
200
Area of Motor RF (sq. mm)
8
R = 0.65
<1
Area of Visual RF (sq. mm)
0
10
20
30
Eccentricity of Peak Visual Reponse (deg)
E 40
Spikes/Sec
Visuomotor Neurons
Motor Neurons
<1
Area of Motor RF (sq. mm)
m
<1 m
m 3
>1 m S
m 1 D
<1 m 3 SD
m S
<1 m D
3
>1mm S
m 1 D
m S
3 D
S
D
R = 0.51
0
Motor Reponse
1000
400
C12
Area of Motor RF (sq. deg)
Visuomotor Neurons
600
0
SC Space
B1400
Visual Reponse
Spikes/Sec
Visual Space
Area of Visual RF (sq. deg)
A800
Figure 2.5. Relationship between RF area and peak response eccentricity in visual
space and the SC map. A. Visual RF area of all VM neurons is plotted against the
eccentricity of the peak visual response vector in visual space. Solid line: regression
line (R = 0.51, m=12, p<0.01) through all data points. B. Motor RF area of all VM
neurons (black circles) an M neurons (gray circles) plotted against the eccentricity of the
peak motor response vector in visual space. Black line: regression line (R = 0.65,
m=31.3, p <0.01) through VM neurons. Gray line: regression line (R = 0.58, m=19.9, p
<0.01) through M neurons. C. Visual RF area plotted against the eccentricity of the
eccentricity of the peak visual response in SC coordinates. Regression analysis was not
significant for VM neurons (R =0.08, p>0.3). D. Motor RF area of all VM neurons (black
circles) or M neurons (gray circles) plotted against the eccentricity of the peak motor
response vector in SC coordinates. Black line: regression line (R = 0.46, m=1.7, p
<0.01) through VM neurons. Gray line: regression line (R = 0.48, m=1.5, p =0.01)
through M neurons. Regression analyses were not significant for VM neurons (R =0.17,
p>0.05) or M-only neurons (R =0.09, p>0.6) when cells with eccentricities below 1 mm
were removed. E. Comparison of baseline activity (left panel), baseline variability (middle
panel), and motor RF area (right panel) for all VM (black bars <=1mm N=24, >1mm
N=126) and M (gray bars <=1mm N=6, >1mm N=22) neurons above and below 1mm
eccentricity on the SC map. Asterisks denote statistical significance between paired
means. (Bonferroni corrected Univariate ANOVA Pairwise comparisons p <0.05).
54
remained invariant in SC space beyond 1mm of the rostral pole, consistent with the
hypothesis in Fig. 2.1B.
To further explore the differences between neurons within and beyond 1mm
eccentricity in SC space, we analyzed differences in baseline activity, baseline variability,
and area of the motor RF (Fig. 2.5E). Both VM and M-only neurons located < 1mm of
the rostral pole had significantly higher baseline activity (Fig. 2.5E left panel) and
baseline variability (Fig. 2.5E middle panel) relative to those located > 1mm of the rostral
pole (t-test; p <0.01). The motor RF area of both VM and M-only neurons was also
significantly smaller for neurons with peak response locations within 1mm of the rostral
pole, compared to neurons with peak response locations beyond 1mm eccentricity (Fig.
2.5E right panel). In order to confirm that the motor RF area below 1mm was smaller we
recalculated the area using a reduced cut off threshold of 1 standard deviation from
baseline. This reduced threshold compensated for the increased baseline variability
recorded from neurons below 1mm eccentricity. With this reduced cut-off threshold, the
mean motor RF area of VM neurons below 1mm remained significantly smaller than the
mean motor RF areas above 1mm (Fig. 2.5E right panel). The mean motor RF area of Monly neurons also remained smaller, however the small number of M-only cells below
1mm (N=6) was not enough to achieve statistical significance. (Bonferroni corrected
Univariate ANOVA Pairwise comparisons p <0.05).
2.4.6 Response Field Shape Symmetry
The final goal of this study was to test whether RFs are symmetric in visual space
and asymmetric in SC space (Fig. 2.1D), or asymmetric in visual space and symmetric in
55
SC space (Fig. 2.1E) in order to test a specific model prediction (Nakahara et al., 2006b).
We tested this hypothesis by comparing the symmetry of VM and M- only neuron RFs in
both visual and SC space for both visual and motor responses. Figure 2.6 shows that there
was a significant correlation between motor and visual asymmetry with increasing target
eccentricity in visual (Fig. 2.6 A,B) and SC space (Fig. 2.6 C,D), however, this
correlation was reduced in SC space for the VM visual (black, z= 1.065; p<0.15), VM
motor (black, z= 2.58; *p<0.01), and M-only (gray, z= 0.8; p<0.22) populations (z-test
for two correlations). This indicates that there was some asymmetry in visual and motor
RFs within both visual and SC space. When the normalized proportional asymmetry was
compared, the degree of asymmetry was significantly greater (paired t-test p<0.01) in
visual space relative to SC space for VM visual (visual space 49.5%, SC space 13.8% )
and motor responses (visual space 67.6%, SC space 26.1%) and M-only motor responses
(visual space 47.6%, SC space 17.8%) (Fig. 2.6E). These data indicate that significantly
greater asymmetry exists in visual field space relative to SC space (Fig. 2.1D); however,
some reduced asymmetry still remains within the SC. When we analyzed the direction of
the resulting asymmetry in visual and SC space for both the single cell and the population
point images, we found that across all conditions there was some asymmetry directed
both rostrally and caudally, however we only found significant asymmetry in visual space
biased in the rostral direction (Fig. 2.1D) for both the visual (unimodal Rayleigh test,
preferred direction=4.6º of rostral pole, r=0.34, p<0.001 ) and motor responses (unimodal
Rayleigh test, preferred direction=1.5º of rostral pole, r=0.43, p<0.001 ).
56
0
10
20
Eccentricity of Peak
Visual Reponse (deg)
SC Space
Visual Asymmetry (mm)
C
30
1.6
1.2
1.4
1
R = 0.31
0.6
0.4
0.2
0
0
0.5
1 1.5 2 2.5 3
Eccentricity of Peak
Visual Reponse (mm)
R = 0.68
0
10
20
Eccentricity of Peak
Motor Reponse (deg)
3.5
4
30
1.2
1
R = 0.30
0.8
0.6
R = 0.54
0.4
0.2
0
0
0.5
1
1.5 2 2.5 3 3.5
Eccentricity of Peak
Motor Reponse (mm)
4
Normalized Percent Asymmetry
R = 0.55
D
1.4
0.8
Visuomotor Neurons
Motor Neurons
Magnitude of Asymmetry
R = 0.42
20
18
16
14
12
10
8
6
4
2
0
E
Motor Reponse
70
60
*
50
40
Point Image
(SC Space)
30
20
10
0
ce e Mir Mirr
pa ac
l S C Sp I VH PI V
a
P
u S
Vis
Visual Response
Normalized Percent Asymmetry
Visuomotor Neurons
Motor Asymmetry (deg)
20
18
16
14
12
10
8
6
4
2
0
B
Visual Reponse
Motor Asymmetry (mm)
Visual Space
Visual Asymmetry (deg)
A
70
60
50
40
*
Visuomotor Neurons
Motor Neurons
Visuomotor Neuron
Point Image
*
Point Image
(SC Space)
30
20
10
0
ir irr ace ce
ce e
pa pac M M
p pa
l S C S I VH PI V al S C S
a
u S
u S
P
s
s
i
i
V
V
Motor Response
Figure 2.6. Analysis of visual and motor RF asymmetry in both visual and SC space. A.
Visual asymmetry plotted against the eccentricity of the peak visual response in visual
space (deg). Solid line: regression line for all data points (N = 150 neurons, R =0.42,
m=0.18, p<0.01). B. Motor asymmetry (difference between motor RF peak and center
locations) plotted against the eccentricity of the peak motor response in visual space
(deg.). Black line: regression line (N = 150 neurons, R =0.55, m=0.3, p<0.01) through
VM neurons. Gray line: regression line (N = 28 neurons, R = 0.68, m=0.32, p <0.01)
through M-only neurons. C. Visual asymmetry (difference between visual RF peak and
center locations) plotted against the eccentricity of the peak visual response in SC
space (mm). Solid line: regression line for all data points (N = 137, R =0.31, m=0.08,
p<0.01). D. Motor asymmetry (difference between motor RF peak and center
locations) plotted against the eccentricity of the peak motor response in SC space
(mm). Black line: regression line (N = 137 neurons, R =0.3, m=0.12, p<0.01) through
VM neurons. Gray line: regression line (N = 28 neurons, R = 0.54, m=0.19, p <0.01)
through M-only neurons. E. Normalized overall proportional asymmetry (Asymmetry /
Eccentricity of Peak Response) in both SC and visual space from VM neurons (black
bars), M neurons (light gray bars) and population point images (dark gray bars) mirrored
horizontally and vertically and vertically only. Asterisks denote statistical significance
between paired means (t-test p <0.05).
57
2.5 Discussion
This study has shown that transformations from visual space into SC space result
in several consequences which include: extensive overlap between visual and motor RFs
of individual neurons and population point images (Fig. 2.3A, Fig. 2.4C), reduced
misalignment between peak visual and motor locations (Fig. 2.3G), decreased asymmetry
(Fig. 2.6E), and spatial RFs that remain size invariant over the SC beyond 1mm (Fig.
2.5C,D). Neurons located within 1mm of the rostral pole in SC possess different
discharge properties including: significantly higher baseline activity (average discharge
during the last 100ms of active central fixation), higher baseline variability, and smaller
RF areas (Fig. 2.5E). These findings are consistent with the discharge properties of
previously recorded neurons located in the intermediate layers of the rostral SC (Munoz
and Wurtz, 1993a; Munoz and Wurtz, 1995a; Krauzlis et al., 2000; Krauzlis, 2003).
2.5.1 Spatial Relationships Between Visual and Motor RFs
There was a close alignment of the peak visual and motor vectors of VM neurons
(Fig. 2.3C,E , Fig. 2.4E), confirming general assumptions that the visual and motor RFs
and point images of VM neurons are aligned closely within the visual field with no
systematic biases. Wurtz and Goldberg, (1972) reported a congruence between visual and
motor RFs, commenting that they lie in the same general area but are not necessarily
continuous. Sparks and colleagues (1976) noted a rough overlap but not a complete coextension of visual and motor RFs. Our results demonstrate that either visual or motor
peak response vectors can be used as an accurate representation of a neuron’s location on
the SC map. This congruence between the location of the visual and motor responses
58
supports previous studies that have employed either visual (Anderson et al., 1998) or
motor peak response vectors (Munoz and Wurtz, 1995b; Port et al., 2000; Soetedjo et al.,
2002) to map visual and motor activity onto the SC.
Although neurons displayed RF areas that remained relatively constant across all
locations when transformed into SC coordinates, about two-thirds of the motor RFs had
distal borders that extended beyond the area of visual space that was tested. Two caveats
of this limitation are that the measurement was dependent on the statistical method used
to define the boundaries of the RFs (i.e., boundary defined for discharge that were greater
than 3 SD) and that there may be an underestimation of RF size because the task was
designed to test eccentricities of less than 30°. This underestimation, however, has a
much more significant effect in visual space rather than SC space. For example, for a
hypothetical neuron with a RF diameter that extends from 15° to 45° diagonally along the
longest viewable target direction in the display, its diameter would be measured as 15°
(to the edge of the screen) which underestimates 15° of the RF that extends beyond the
30° corner display boundary. In this hypothetical example, the RF diameter would be
underestimated by 50% (15/30 deg) in visual space but only by 38.6% (0.53/1.37 mm) in
SC space. Thus, utilizing a larger theoretical viewing area would only increase the
differences in RF area and asymmetry between visual and SC space that we reported.
2.5.2 Alignment of Peak Visual and Motor Responses
Our data show that visual stimuli activate the same area of the SC that discharges
to specify an upcoming saccade vector to that stimulus. Action potentials of VM neurons
elicited by visual stimuli travel along the predorsal bundle to influence neurons in the
59
brainstem saccade generating circuit (Moschovakis et al., 1990; Munoz et al., 1991;
Moschovakis et al., 1996; Kato et al., 2006; Rodgers et al., 2006). The spatial
organization of the SC is conserved in projections to downstream structures in the
brainstem including: rostral SC neurons projecting to omnipause neurons in the raphe
interpositus nucleus (Buttner-Ennever et al., 1988; Buttner-Ennever et al., 1999), caudal
SC neurons projecting to long lead burst neurons in the nucleus reticularis tegmentum
pontis (Grantyn and Grantyn, 1982; Scudder et al., 1996), and SC projections to the
abducens nucleus (Grantyn and Grantyn, 1982; Grantyn and Berthoz, 1985; Olivier et al.,
1993). Therefore, an alignment of sensory and motor RFs among individual neurons
within the SC provides oculomotor structures in the brainstem with equivocal spatial
information with regard to both target and intended saccade location. This alignment of
visual and motor information is likely useful for both oculomotor (Edelman and Keller,
1996; Dorris et al., 1997; Sparks et al., 2000) and head premotor circuits during saccades
or orienting gaze shifts that involve combined motion of the eyes and head (Corneil et al.,
2004).
Spatially distinct point images resulting from confined population activity on the
SC map during visually guided saccades has been demonstrated in both single unit data
(Munoz and Wurtz, 1995b; Anderson et al., 1998) as well as functional imaging
(Moschovakis et al., 2001). In a model of the sensory-motor transformation, McIlwain
(1986) postulated that visual point images on the SC motor map shape the saccade
response by “activating the appropriate combination” of output neurons. These sensory
signals code the location of a stimulus that can be transformed into motor signals in the
SC that are thought to specify the intended location of an upcoming saccade via vector
60
averaging (Van Opstal and Munoz, 2004). Our results indicate that such vector
averaging in SC space may be simplified due to the symmetry and size invariance of
visual and motor RFs. Thus, theoretical vector averaging algorithms relaying desired
saccade endpoints could be uniformly implemented across the SC map. This reduction in
the difference between visual and motor peak locations during sensorimotor
transformations in the SC acts to reduce the biological noise that may be occurring when
the visual and motor peak locations do not align perfectly. Anderson and colleagues
(1998) reported that a peak visual response was a more reliable measurement of a VM
neuron’s location in SC coordinates. Our findings support this claim as: 1) visual and
motor peaks tended to be aligned (Fig. 2.3); 2) visual RFs were smaller then motor RFs
(Fig. 2.3B); and 3) visual RFs tended to be entirely contained within motor RFs (Fig.
2.3A). These results suggest that VM neurons may themselves be involved in sensorymotor transformations occurring in the SC where the overlap of single-unit RFs may
combine to focus population activity. As such, it would appear that the sensory RFs of
SC neurons may serve to influence and possibly shape motor RFs.
2.5.3 Changes in Response Field Size
An increase in RF size with increasing eccentricity was observed in visual space
for both visual and motor responses with increasing eccentricity. This finding is
consistent with previous studies which showed the same relationship for visual (Goldberg
and Wurtz, 1972a; McIlwain, 1986) and motor RFs (Sparks et al., 1976; Munoz and
Guitton, 1991; Munoz and Wurtz, 1995a). When transformed into SC space, visual RFs
were invariant across the SC map. Motor RFs from both VM and M-only populations
61
were size invariant beyond 1mm from the rostral pole, indicating that neurons within
1mm of the rostral pole possessed different discharge characteristics related to behavior.
The small RFs and increased tonic baseline activity observed in this subpopulation of
cells below 1mm is consistent with these previous studies (Munoz and Wurtz, 1993a;
Krauzlis et al., 2000). The decreased area in SC space of neurons below 1mm indicate
that these neurons have smaller RFs in SC space despite the overrepresentation of visual
space at the rostral pole. However, if the modeled equations defining the SC map by Van
Gisbergen and collegues (Van Gisbergen et al., 1987) are somewhat inaccurate near the
rostral pole, then neurons below 1 mm could potentially maintain the same size
invariance with eccentricity that was observed beyond 1mm.
2.5.4 Field Shape Asymmetry
RF asymmetry was significantly greater in visual space relative to SC space (Fig.
2.6E) for both the single cell and population point image data. This indicates that the
underlying circuitry in SC space is more equally distributed and symmetrically connected
than it would be if projected into visual space. This finding validates many previously
reported SC models which were based on assumptions of symmetrical and evenly
distributed connections in the SC and asymmetric connections in visual space (Arai et al.,
1994; Optican, 1995; Gancarz and Grossberg, 1999; Short and Enderle, 2001;
Trappenberg et al., 2001). Nakahara’s model, which is able to account for previously
observed rostral spread of activity during saccades (Nakahara et al., 2006b), requires
asymmetrical connections in SC space that shift the peak activity caudally away from the
rostral pole (Fig. 2.1E). Although our findings demonstrated significant rostrally directed
62
asymmetry in visual space, some reduced asymmetry was still observed in SC space (Fig.
2.6E) in both the single cell and point image data. This reduced asymmetry in SC space
was observed in both the rostral direction (where the peak activity was shifted rostral of
the RF centre, which occurred most often in neurons with open movement fields) and the
caudal direction, but these were not significant in either the single cell or point image
population data. The observed rostrally directed asymmetry in SC space is unrelated to
the caudally directed SC asymmetry that was predicted by the Nakahara model (Fig.
2.1E). Therefore it is unclear whether the remaining caudal asymmetry that was observed
in SC space would be sufficient to result in the rostral spread of activity predicted by this
model and previously observed physiologically (Guitton and Munoz, 1991; Munoz and
Guitton, 1991; Munoz et al., 1991; Munoz and Wurtz, 1995b; Anderson et al., 1998).
Although these physiological data provide primarily contrary evidence against the
Nakahara et al. 2006 model, it is important to acknowledge that the behaviour in this
model is primarily dependant on the lateral connection field intrinsic to the local circuitry
since only a simple circular input was used. The RFs that we recorded were shaped by a
combination of intrinsic lateral connections as well as external inputs that originate from
many cortical and subcortical areas. However as previous physiological evidence for a
rostral spreading of activity during saccades has also been recorded in the SC in vivo
where all these external inputs were active (Munoz et al., 1991; Guitton, 1992b; Wurtz
and Optican, 1994; Guitton et al., 2003), it is unlikely (based on the current evidence)
that the asymmetric connections proposed by Nakahara et. al. are solely responsible for
this spread. These results lead to some important questions for future study. How strongly
does SC input shape the resulting visual and motor activity across the map? Perhaps
63
future studies of the isolated SC local circuit in vitro may be useful to examine RF and
point image shape in isolation of external input signals. Does the shape of the RF and
point image change dynamically over time course of the visual response, or the saccadic
movement? Because this study only examined motor activity at saccade onset and visual
activity at the time of the peak, it may be important to examine how spatial SC activity
changes dynamically. It is possible that the shape of the external inputs or the local
interactions within the SC map could change at different times during the visual response
or the movement and result in better fits to the Nakahara et. al. 2006 model.
2.5.5 Conclusions
Transforming visual to motor activity in SC space resulted in the following
computational consequences: 1) increased RF overlap and decreased differences between
visual and motor peak locations thereby simplifying vector averaging and spatial
sensorimotor transformations; and 2) increased RF symmetry resulting in symmetrical
point images across the SC map beyond 1mm such that the area of tissue integrated over
remains the same for all target vectors. Our data demonstrate that there is a close
alignment of visual and motor RFs and point images in VM neurons in the SCi.
Accordingly, during sensorimotor transformations in the SC, we suggest that the spatial
component of an efferent SC signal is preserved and downstream structures receive
equivocal information as to target location and the intended location for an upcoming
saccade. Furthermore, our data support the use of both visual and motor peak vector
locations in future studies to define a neuron’s peak response vector as well as its
subsequent position coordinates on the mathematical SC map.
64
Chapter 3: Linking Visual Response Properties in the Superior Colliculus to
Saccade Behavior
65
3.1 Abstract
The basic properties of a sensory stimulus influence the neural coding of, and
therefore the behavioral response to that stimulus. Here, we examined the influence of the
visual response in the Superior Colliculus (SC) (an oculomotor control structure
integrating sensory, motor, and cognitive signals) on the development of the motor
command that drives saccadic eye movements. We varied stimulus luminance to alter the
timing and magnitude of visual responses in the SC and examined how these changes
correlated with resulting saccade behavior. Increasing target luminance resulted in
multiple modulations of the visual response including increased magnitude and decreased
response onset latency. These signal modulations correlated strongly with changes in
saccade latency and metrics, indicating that these signal properties carry through to the
neural computations that determine when, where, and how fast the eyes will move. Thus,
components of the earliest part of the visual response in the SC provide important
building blocks for the neural basis of the sensory-motor transformation, highlighting a
critical link between the properties of the visual response and saccade behavior.
66
3.2 Introduction
Crucial to survival is the ability to optimally extract ongoing information about
the environment through sensory channels in order to guide the appropriate behavioral
responses. One of the simplest of such sensory-motor transformations to investigate is the
visual guidance of saccadic eye movements (Wurtz and Goldberg, 1989; Wurtz, 2008).
The superior colliculus (SC), a layered structure in the midbrain, is a critical sensorimotor
integration node for saccade control, located at the interface between sensory input and
motor output (Hall and Moschovakis, 2003). The SC receives visual input directly from
the retina as well as indirectly from visual cortex (Fries, 1984; Cusick, 1988; Robinson
and McClurkin, 1989; Lock et al., 2003). Following the appearance of a visual stimulus,
neurons in both the superficial and intermediate SC discharge a phasic burst of action
potentials (visual response) that is time-locked to stimulus appearance (Wurtz and
Goldberg, 1971; Sparks, 1975; Mohler and Wurtz, 1976). Visuomotor neurons in the
intermediate SC also discharge a second burst (motor response) to drive the saccade
(Mohler and Wurtz, 1976; Sparks, 1978) and these visuomotor neurons project directly to
the saccade premotor circuit in the brainstem reticular formation (Rodgers et al., 2006).
Despite the detailed understanding of the circuit, we do not understand how the visual
response in the SC is transformed into the motor command to guide behavior.
The quality and properties of incoming sensory signals affect the neural
computations underlying the visuomotor transformation (Sparks, 1986). For short-latency
express saccades (Fischer and Boch, 1983; Fischer and Weber, 1993), it appears that
there is a single burst from visuomotor neurons in the SC that triggers the saccade
(Edelman and Keller, 1996; Dorris et al., 1997; Sparks et al., 2000), which is suggestive
67
of a direct visuomotor transformation with minimal processing. More commonly
however, during regular latency saccades, the visual and motor bursts in the SC are
distinctly separate responses (Mohler and Wurtz, 1976). A question that arises is how do
the properties of a visual stimulus change the visual response in the SC and how do these
changes subsequently influence saccade behavior, if at all? Previous studies have shown
that the timing and magnitude of the visual responses in the SC are modulated by contrast
(Li and Basso, 2008). Furthermore, changes to the SC visual response have also been
shown to correlate with saccadic reaction time (SRT) (Dorris et al., 2002; Fecteau et al.,
2004; Bell et al., 2006; White et al., 2009).
We hypothesize that the early visual response (i.e. the initial phasic burst of action
potentials) in the SC plays a key role in the development of the motor command that will
drive regular latency saccades. To test this hypothesis we manipulate target luminance as
a means of modulating the timing and magnitude of the visual response in the SC and
identify how changes to these signal properties link to saccade behavior. We show that
despite the fact that visual and motor responses are temporally separate during regular
saccades, modulations to the earliest part of the visual response carry through the
visuomotor transformation to influence saccade latency and metrics.
3.3 Materials and Methods
3.3.1 Animal preparation
All animal care and experimental procedures were in accordance with the
Canadian Council on Animal Care policies on use of laboratory animals and approved by
Queen’s University Animal Care Committee. Two male monkeys (Macaca mulatta,
68
W:age 6 yrs, 8 kg; and, Q:7yrs, 12 kg) were used in these studies. A detailed description
of the surgical techniques used to prepare animals for neuronal recording from the SC
and eye movement recordings in our laboratory have been described previously (Marino
et al., 2008). Briefly, both animals underwent surgery under aseptic conditions for the
insertion of eye coils, a stainless steel head holder, and a recording chamber that was
mounted on the skull using stainless steel bone screws and dental acrylic. The recording
chamber was oriented towards the SC at an angle of 38° posterior from vertical in the
mid-sagital plane. Monkeys were given at least 4 weeks to recover prior to onset of
behavioral training.
3.3.2 Experimental tasks and behavioral stimuli
Behavioral paradigms and visual displays were under the control of two Dell 8100
computers running UNIX-based real-time data control and presentation systems (Rex
6.1) (Hays et al., 1982). Monkeys were seated in a primate chair with their heads
restrained for the duration of an experiment (2-4 hours). They faced a display cathode ray
tube monitor that provided an unobstructed view of the central visual area 60°(horizontal)
x 50°(vertical). Monkeys were required to perform several visually-guided saccade tasks
(Fig. 3.1A,B). Experiments were performed in darkness with individual trials lasting ~1-2
seconds depending on the variability of fixation duration and SRT. Each trial required the
monkey to generate a single saccade from the central fixation point (FP) to a peripheral
visual target (T). At the start of each trial, the screen turned black and after a period of
250 ms a circular grayscale FP of constant luminance (0.25° diameter spot, 3.5cd/m2)
appeared at the center of the screen against a black background (~0.0001 cd/m2). Fixation
69
A
DELAY TASK
Visual Target
Baseline Epoch
Saccade
Epoch
PSVR
Saccade
Baseline Epoch
B
FP
FP
T
EYE
T
EYE
500 - 800ms 500 - 800ms
SRT
500 - 800ms
Saccade
Onset
TARGET ALIGNED RESPONSE
VISUAL-MOTOR
VISUAL
NEURON
NEURON
Firing Rate (spikes/s) Firing Rate (spikes/s)
C
E
Saccade
Epoch
PSVR
Target
Epoch Epoch
GAP TASK
Visual
Baseline
SRT
Saccade
Onset
SACCADE ALIGNED RESPONSE
Target
Epoch
Saccade SaccadePSVR
Baseline Epoch Epoch
D
600
600
300
300
0
0
F
600
300
0
200ms
post-saccadic
visual
response
600
300
150
0
0
150
Time from Target Onset (ms)
150
0
150
Time from Saccade Onset (ms)
Figure 3.1. Schematic representation of temporal events in the delay (A) and gap (B)
tasks for the fixation point (FP), target (T) and eye position (EYE). Vertical gray bars
denote key analysis epochs used to classify responses and neurons. C-F. Rasters
and spike density functions of a representative V (C,D) and VM (E,F) neuron for
target-aligned (C,E) and saccade-aligned (D,F) responses to the optimal target
location in the delay task. A post-saccadic visual response can be clearly seen in both
the V and VM examples.
70
of the FP was required for a variable period (500 - 800 ms) until either a small circular
0.25º grayscale T appeared (delay task), or the FP was extinguished (gap task). During
the inter-trial interval (800-1500 ms duration), the display screen was diffusely
illuminated to prevent dark adaptation.
The delay task (Fig. 3.1A) was used to dissociate visual and saccade related
activity. In this task, the monkeys were required to continue fixation of the FP for an
additional 500-800 ms after T appearance. Only after FP disappearance was the monkey
allowed to initiate a saccade to the T. In the delay task, the target was presented at one
location only: at the center of each neuron’s visual response field. See supplementary
figure 3.S1 for additional details regarding the characterization of visual response fields.
The gap task (Fig. 3.1B) served to reduce the inhibition due to active visual
fixation and thereby making the oculomotor system more responsive to visual inputs
(Dorris and Munoz, 1995; Paré and Munoz, 1996; Dorris et al., 1997; Machado and
Rafal, 2000; Krauzlis, 2003). In this task, a 200 ms period of darkness (gap) was inserted
between FP disappearance and T appearance. The monkey was required to initiate a
saccade to the T after its appearance. In the gap task, targets were presented with equal
probability at one of two possible locations: the center of each neuron’s visual response
field and at a location opposite the horizontal and vertical meridians. The gap and delay
tasks were presented as separate blocks of trials.
Target luminance was manipulated systematically using seven randomly
interleaved luminance levels (0.001 cd/m2, 0.005 cd/m2, 0.044 cd/m2, 0.4 cd/m2, 3.5
cd/m2, 17.5 cd/m2, and 42.5 cd/m2). Luminance was measured with an optometer (UDT
instruments, model S471) that was positioned directly against the monitor screen and
71
centered on the stimulus (T or FP). Each correct trial was rewarded with a drop of water.
A computer controlled window ensured eye position remained within 2.5º of the FP and
5º of the T during correct trials in all tasks.
3.3.3 Recording Techniques
Extracellular recording was performed with tungsten microelectrodes (0.5-5 MΩ
impedance, Frederick Haer) inserted through guide tubes (23 gauge) that were anchored
in delrin grids with 1 mm hole separations inside the recording chamber (Crist C.F. et al.,
1988). Electrodes were advanced with a Narishige microdrive (MO95) to the dorsal
surface of the SC, distinguished by large increases in background activity following each
saccade or change in visual stimuli. The electrode was then slowly lowered into the SC to
record from individual visually responsive neurons.
3.3.4 Data Collection
Neural waveforms (800 μsec duration, sampled at 40 kHz, filtered (150 Hz low
frequency cut off, 9 kHz high frequency cut off) and horizontal and vertical eye position
(sampled at 1 kHz , magnetic search coil technique (Robinson, 1963)) were recorded in
real time with Plexon data acquisition hardware (Plexon Inc.). Accurate isolation and
sorting of individual neurons was performed offline (Offline Sorter 2.5, Plexon Inc.).
72
3.3.5 Neuron classification
In order to characterize the activity of individual neurons across stimulus
conditions, trains of action potentials averaged across identical correct trials were
convolved into spike density functions using a Gaussian kernel (σ = 5ms) for each spike
(Richmond et al., 1987). Spike density functions were aligned on target appearance when
analyzing visual responses (Fig. 3.1C,E) and saccade onset when analyzing motor
responses (Fig. 3.1D,F). Neurons were classified as visual-only (V, Fig. 3.1C,D) or
visuomotor (VM, Fig. 3.1E,F) based on the presence or absence of saccade activity
during the delay task (Fig. 3.1A,D,F) using the brightest target luminance. Visual and
saccade activity was defined relative to a baseline. Visual baseline activity (Fig. 3.1A)
was calculated as the average discharge from all correct trials during the 100 ms prior to
T appearance. Saccade baseline activity was calculated from the average discharge on all
correct trials from 100 to 50 ms prior to the onset of the saccade in the delay task (Fig.
3.1A). A significant visual response was defined as an increase in target-aligned activity
greater than 50 spikes/s above the visual baseline during the target epoch (50-150ms
following target presentation). A significant motor response was defined as an increase in
saccade aligned activity greater than 50 spikes/s above both the target baseline and
saccade baseline during the saccade epoch (+/- 10 ms from saccade).V neurons we
describe were located within 1000 μm of the dorsal SC surface as measured from the
microdrive. All VM neurons recorded were located below V neurons (McPeek and
Keller, 2002) and were recorded within 2500 μm of the dorsal SC surface. A subset of the
V and VM cells discharged a short burst of action potentials 50-80 ms after saccade onset
that was distinctly separate from the motor burst. This subset of V and VM neurons were
73
classified as containing a post-saccadic visual response (Fig. 3.1D,F)(Li and Basso,
2008). A significant post-saccadic visual response was classified based on a distinct peak
of activity aligned on saccade onset that was greater than 50 spikes/s above the visual
baseline activity during the post-saccadic visual response epoch (50-100ms following
saccade onset).
3.3.6 Behavioral Analyses
Data were analyzed offline with custom Matlab (Matlab 7.4, Mathworks Inc)
software. The start and end of saccades were determined from velocity and acceleration
template matching criteria; verified offline by the experimenter, and corrected when
necessary. Because visual response onset latency (VROL, see below) changed with target
luminance, anticipatory saccades (saccades with SRTs less than the luminance specific
afferent visual delays; see below) were removed based on the calculated VROL.
Anticipatory and express saccades were removed from analysis to ensure that visual and
motor neural responses were temporally separated (i.e., target and saccade epochs Fig.
3.1A,B.). These were defined as all saccades with SRTs less than 50 ms after the mean
luminance specific VROL calculated in the delay task. This ensured a minimum of 50 ms
temporal separation between the onset of the visual response and the onset of the saccade
movement.
Error rates were calculated from the trials in which the target was presented. Early
aborted trials in which the monkey failed to fixate or maintain fixation of the FP at the
beginning of the trial prior to target appearance were eliminated from further analysis
because they were not representative of active task participation. Error rates were
74
computed from those trials that were initiated by the monkey (by actively fixating the FP
at the start of a trial and then holding fixation until the target appeared). Errors included:
1) anticipation errors (saccades made after the target appearance, but before the visual
response reached the SC; see RESULTS); 2) saccade errors (saccades initiated after
target was perceived that landed outside the target window and were therefore incorrect);
3) delay task timing errors (saccades initiated to the target after target appearance but
before FP disappearance); 4) trials in which no saccade was made. Percent error rate was
compared and analyzed using a z test for proportions.
Saccade endpoint accuracy was defined as the Euclidean distance in degrees
between the saccade endpoint (mean eye position during the first 10 ms of fixation
following the saccade) and the target location. If a small corrective saccade occurred (less
than 1% of trials), the endpoint of the initial saccade, landing within the target window,
was used.
3.3.7 Neuronal Analyses
The effects of luminance on visual response properties was determined from
changes in the initial phasic burst of the visual response including: VROL, the peak
magnitude of the target related discharge, the time from the VROL to the peak of the
target related discharge (representing the growth time in which the target related
discharge increased towards its peak), and the decay rate (slope) of the initial visual
response. Changes to the timing and peak magnitude of the visual response are important
for saccadic visuomotor transformations because these changes have been found
previously to correlate with saccadic reaction time (VROL: Bell et al., 2006; Peak
magnitude: Dorris et al., 2002; Fecteau et al., 2004, Bell et al. 2004). The time required to
75
reach the peak discharge (relative to the VROL) is also important because it reflects the
rate in which neural activity is increasing or accumulating within the saccadic system.
Finally, changes in the rate of decay or shutdown of the visual response relative to the
peak are important because this should reflect when and how this response is shaped and
or decreased to influence downstream structures. Likewise, the effects of target
luminance on the motor response were determined from changes in the peak of the motor
response and the ascending slope (slope was calculated in lieu of the time to the motor
peak as the precise onset of motor related activity could not be dissociated accurately
from motor preparation and/or sustained visual activity).
VROL was determined from a running non-parametric Rank Sum test of a trialby-trial spike density function aligned to the appearance of the visual target (Poisson-like
exponential growth/decay function resembling a postsynaptic potential):
R(t) = [1 - exp (-t/ τg)]·[exp (-t/ τd)]
Where R(t) defines the rate as a function time, growth constant (τg) = 1ms; decay
constant (τd) = 20ms (Thompson et al., 1996). The Poisson-like exponential
growth/decay function is critical for the calculation of signal onset latencies because the
kernel only temporally smoothes neural activity later in time in order to preserve accurate
onset times (Thompson et al., 1996). VROL was determined by the onset of stable
statistical significance (p<0.05) between the mean activity during the visual baseline and
a moving temporal window (1 ms resolution) within the target epoch (Fig. 3.1A). The
peak visual response was calculated independently in both the delay and gap tasks. The
magnitude and timing of the peak visual response was calculated at each target luminance
76
from the maximum of the trial averaged spike density function (Gaussian kernel) aligned
to the appearance of the target in the target epoch (Fig. 3.1A). We chose a 5ms pulse
width as this value allowed for a smooth continuous function to be generated with
minimal temporal smoothing of neural activity. The time to the peak was calculated as
the time from T appearance to the peak response. The decay rate of the visual response
was calculated from the slope of a linear regression fitted to the target aligned trial
averaged spike density function (Gaussian kernel; σ = 5ms) from the time of the visual
peak until 25ms after the peak.
The effects of target luminance on the motor response were similarly
characterized by the changes in the peak and ascending slope of the saccade related
discharge (aligned to saccade onset). The peak magnitude of the saccade burst was
calculated at each target luminance from the trial averaged spike density function
(Gaussian kernel; σ = 5ms) aligned to saccade onset in the saccade epoch (Fig. 3.1A,B).
The ascending slope of the motor related burst was calculated from a linear regression
over the saccade aligned trial averaged spike density function (Gaussian kernel). This
slope was calculated over the last 25 ms prior to the onset of the saccade in order to
isolate saccade related activity. All calculations made on the neural response from each
neuron were verified visually during offline analysis. All statistical comparisons were
calculated with repeated measures ANOVA with post hoc Bonferroni corrected pairwise
comparisons unless otherwise stated.
3.4 Results
77
3.4.1 Target Luminance Modulates Saccade Behavior
We recorded saccade behavior while monitoring activity from single SC neurons
in the delay and gap tasks (Fig. 3.1). All express and short latency saccades were
removed to ensure all visual and motor responses were temporally separate in the gap
task (See Methods for details). Consistent with our previous study (Marino and Munoz,
2009), target luminance modulated SRT, peak velocity, endpoint error, and overall error
rate across both the gap and delay tasks across a range of target eccentricities (range: 3.6º
- 27.9º) defined by each neuron's response field optimal (Fig. 3.2). In the delay task, there
was a main effect of target luminance from 0.001 cd/m2 to 0.044 cd/m2 such that mean
SRT decreased significantly (P<0.01). For luminance values above 0.044 cd/m2, mean
SRT remained constant (Fig. 3.2A). In the gap task, there was also a main effect of target
luminance on SRT (F(6,366) = 136.5, P<0.01): SRT decreased with luminance increases
up to 0.044 cd/m2 (P<0.01).
There was a main effect of luminance on saccade velocity in both the gap
(F(6,366) = 48.8, P<0.01) and delay tasks (F(6,396) = 42.4, P<0.01). Saccades to the
dimmest target luminance had the lowest mean peak velocity (P<0.01) (Fig. 3.2B). Peak
saccade velocity increased in both the gap and delay tasks from target luminance ranges
from 0.001 cd/m2 to 0.044 cd/m2 (P<0.01).
There was also a main effect of luminance on saccade endpoint accuracy in both
the gap (F(6,366) = 48.8, P<0.01) and delay tasks (F(6,396) = 42.4, P<0.01). Saccade
endpoint accuracy significantly improved as target luminance increased from 0.001 cd/m2
to 0.044 cd/m2 in both the gap and delay tasks velocity (P<0.01) (Fig. 3.2C).
78
A
B
400
Delay Task
530
Gap Task
Mean SRT (ms)
Peak Velocity (deg/s)
350
300
250
200
490
470
3.5
4
04
0.4
3.5 17.5 42.5
0.4
3.5 17.5 42.5
0.
1
D
4
0.
00
04
0.
3.5 17.5 42.5
0.
4
5
00
1
0.
0.
0.4
00
5
430
00
40
30
Error Rate (%)
3
2.5
20
10
2
04
4
0.
00
0.
Target Luminance (cd/m 2 )
5
0
3.5 17.5 42.5
1
0.4
00
04
4
0.
5
00
0.
0.
00
1
1.5
0.
Endpoint Error From Target (deg)
510
450
150
C
550
Figure 3.2. Effect of target luminance on mean SRT (A), mean peak velocity (B),
mean endpoint error (Euclidian distance of saccade endpoint from target in degrees)
(C) and percent error rate (D) (+/- standard error) for the gap (black) and delay tasks
(gray).
79
Finally, the percent error rate decreased significantly with increasing target
luminance from 0.001 cd/m2 to 0.044 cd/m2 (z test for proportions P<0.01) across both
the gap and delay tasks (Fig. 3.2D). This error rate was significantly below chance levels
across all target luminance conditions, indicating that all targets were above visual
detection thresholds.
3.4.2 Target Luminance Modulates SC Visual Activity
We recorded from 65 V and 64 VM neurons in the delay task. Of this group, 46 V
and 64 VM single neurons were also recorded in the gap task (Table 3.1). Variations in
target luminance led to systematic modulations in the visual responses of V and VM
neurons. Figure 3.3 illustrates the population activity from all V and VM neurons
recorded in the delay and gap tasks aligned on target appearance (left column) and
saccade onset (right column). Increasing target luminance decreased the timing (VROL
and time of peak), while increasing the magnitude of the visual response. Furthermore,
increasing luminance also increased the steepness of the rise and fall of the initial phasic
component of the visual response.
A second visual response was observed in some V and VM neurons that occurred
~50-80ms after the onset of the saccade to the visual target (Fig. 3.3, right column) that
has been described previously (Li and Basso, 2008). Like the initial visual response
following the appearance of the target, the timing and peak magnitude of this postsaccadic visual response scaled with target luminance. This post-saccadic visual response
was only observed when the visual target was present within a neuron’s response field at
the time of saccade initiation.
80
VISUAL DELAY TASK
TARGET ALIGNED RESPONSE
SACCADE ALIGNED RESPONSE
VISUAL
Firing Rate (spikes/s)
200
100
100
Firing Rate (spikes/s)
VISUAL-MOTOR
0
200
B
VISUAL
Firing Rate (spikes/s)
100
150
n=65
200
250 -100
0.001
0.005
0.044
0.4
3.5
17.5
42.5
post-saccadic
visual response
-50
0
50
100
150
-50
0
50
100
150
200
100
50
100
150
n=64
200
250 -100
GAP TASK
C
200
post-saccadic
visual response
200
100
100
0
Firing Rate (spikes/s)
50
100
0
VISUAL-MOTOR
200
A
200
D
50
100
150
n=46
200
250
-50
0
50
100
150
200
100
100
0
-100
n=64
50
100
150
200
250
Time from Target Appearance (ms)
-100
-50
0
50
100
150
Time from Saccade Appearance (ms)
Figure 3.3. Population spike density functions (Gaussian kernel σ = 5ms) aligned on
target onset (left column) and saccade onset (right column) for all V (A,C) and VM
(B,D) neurons recorded in the delay (A,B) and gap tasks (C,D) with seven randomly
interleaved target luminance levels. Line colours denote target luminance (black:
0.001 cd/m2, pink: 0.005 cd/m2, cyan: 0.044 cd/m2, navy blue: 0.4 cd/m2, green: 3.5
cd/m2, yellow: 17.5 cd/m2, red: 42.5 cd/m2).
81
Table 3.1: Neuron breakdown by monkey, task and subtype.
Cell Type
Delay
task
V
VM
Gap task
V
VM
Monkey
W
Monkey
Q
26/32
33/41
30/33
17/23
16/18
30/42
24/28
17/22
82
Figure 3.4 illustrates how different components of the phasic visual response
changed across the luminance manipulation. Visual response properties of the V and VM
neurons were similarly modulated by luminance and no significant differences between
these populations were found (See Supplementary Fig. 3.S2). Therefore, we collapsed
across V and VM populations. At the dimmest target luminance, VROL was only
measurable from 61% of the neurons recorded in the gap task and 40% of the neurons
recorded in the delay task. As a result, repeated measures ANOVAs for VROL and
growth rate were not performed on data from the dimmest luminance unless otherwise
stated. Main effects with target luminance were found in both tasks for all the visual
response signal properties examined (VROL: (gap) F(5,545) = 930, P < 0.01, (delay)
F(5,640) = 1076 P <0.01, Peak Time: (gap) F(6,654) = 1095, P < 0.01, (delay) F(6,768) =
989, P < 0.01, Growth Rate: (gap) F(5,545) = 18.2, P < 0.01, (delay) F(5,640) = 21.1, P <
0.01, Peak Magnitude: (gap) F(6,654) = 102.9, P < 0.01, (delay) F(6,768) = 144.3, P <
0.01, and Decay Rate (gap) F(6,654) = 37.5, P < 0.01, (delay) F(6,768) = 78.2, P < 0.01).
The VROL and peak time decreased significantly across all target luminance
ranges tested in the gap and delay tasks (P<0.01) (Fig. 3.4A). The growth time of the
visual response was also faster as luminance increased between 0.005 cd/m2 and 42.5
cd/m2 (Fig. 3.4B).
There were also significant changes in the peak magnitude of the visual response
(Fig. 3.4C). As target luminance increased, the peak firing rate of the visual response
increased up to 0.044 cd/m2 in the gap task and up to 0.4 cd/m2 in the delay task. In the
delay task an additional increase was observed between 0.4 and 42.5 cd/m2 (P<0.01).
83
B
150
30
Peak Time
130
Peak Visual Response
Magnitude (spikes/s)
VROL
20
90
15
70
C 275
Growth Time
25
110
50
35
Time From ROL to Peak
of Visual Response (ms)
DelayTask
GapTask
01 05 44 0.4 3.5 17.5 42.5
0.0 0.0 0.0
225
175
10
D
0
01 5 44 0.4 3.5 17.5 42.5
0.0 0.00 0.0
Decay Rate
(Slope)
Slope of Visual Response
Decay
Time From Target Onset
(ms)
A 170
-3
Peak Magnitude
125
-5
75
-7
25
01 05 44 0.4 3.5 17.5 42.5
0.0 0.0 0.0
Target Luminance (cd/m2 )
100
VROL
Decay Rate
80
0.8
Median r2
Cumulative Percent
E
60
40
20
0
01 05 44 0.4 3.5 17.5 42.5
0.0 0.0 0.0
Target Luminance (cd/m2 )
0.6
0.4
0.2
0
-1
-0.5
0
0.5
Correlation Coefficient (r)
1
Figure 3.4. Effects of target luminance on the signal properties of the visual sensory
response in the delay (gray points and lines) and gap (black points and lines) tasks
collapsed across all V and VM neurons. See supplementary Fig. 2 for separation of V
and VM neurons. A. Mean VROL (solid line) and peak time (dotted line) of the visual
sensory response. B. Mean growth time (time from the VROL to the peak) of the visual
response. C. Mean peak magnitude of the visual response. D. The slope of the decay
rate of the initial visual response burst. E. Cumulative distributions of correlation
coefficients and median r2 values (inset) for correlations between the peak magnitude
of the visual response and both the VROL and decay rate in the gap task. Filled circles
in the cumulative plots denote statistically significant correlations (within neuron). Color
denotes the neural signal property that was correlated: VROL (blue), decay rate (red).
Median r2 values denote how much variance was accounted for by each correlation.
84
Finally, the decay rate of the initial burst of the visual response was modulated by
target luminance (Fig. 3.4D). As luminance increased, the rate of this decay became
faster leading to a steeper downward slope of the signal up to 0.044 cd/m2 in the gap task,
and up to 0.4 cd/m2 in the delay task. A further decrease was observed between 0.4 and
42.5cd/m2 in the delay task. (P<0.01).
3.4.3 Relationships Between Visual Response Properties
In order to assess how the luminance modulated properties of the visual response
were interrelated (i.e., how the beginning and end of the visual response was related to
the peak), we correlated peak magnitude with VROL and decay rate (Fig. 3.4E) (median
r2 values were used to compensate for distribution skew). We found that both the VROL
and decay rate were significantly negatively correlated with the peak magnitude of the
visual response (median r2: VROL:0.63, decay rate:0.57). Thus, the later the visual
signal arrives in the SC, the weaker its magnitude and the shallower its decay rate.
3.4.4 Linking Visual Response Properties to Saccade Behavior
We have shown that target luminance modulated both saccade behavior (Fig. 3.2)
and the properties of the visual response in the SC (Figs. 3.3, 3.4). In order to link
modulations of neural activity to behavior, we correlated the properties of the neural
response of V and VM cells to saccade behavior. These correlations assessed the
influence of the initial phasic visual response on the sensorimotor transformation that
determined the timing and metrics of the resulting eye movement. All correlations were
performed with data collected in the gap task when movement initiation was a direct
85
consequence of target appearance. The left column of Fig. 3.5 illustrates the cumulative
distributions of correlation coefficients between visual response property (VROL, growth
time to the visual peak, peak magnitude of the visual response and the decay rate of the
initial visual response) and saccade behavior (SRT: Fig. 3.5A; peak saccade velocity: Fig.
3.5B; and saccade endpoint accuracy error: Fig. 3.5C) across all seven luminance levels
when possible (one neuron was excluded because it possessed fewer than 3 data points
for correlation). Filled circles denote significant correlations. All visual response
properties analyzed were significantly correlated with saccade behavior (Fig. 3.5, right
panels). Significant main effects were found between the r2 values of the visual response
properties and SRT (F(4,436) = 37.5, P < 0.01, Fig. 3.5A), peak saccade velocity
(F(4,436) = 14.9, P < 0.01, Fig. 3.5B), and saccade endpoint error (F(4,436) = 21.9, P <
0.01, Fig. 3.5C). The strongest correlations with saccade behavior were VROL and the
peak magnitude of the visual response. These strong correlations suggest that VROL and
peak magnitude of the visual response influence the latency, velocity, and accuracy of the
ensuing saccade. The growth and decay rate of the visual response had weaker
correlations, but they were still significantly correlated with saccade behavior. There was
no difference in the correlation strength between growth time and decay rate (Fig. 3.5,
right column). There were no significant differences in the correlation strength of
identical visual response properties with each behavioral measure (Fig. 3.5 A,B,C right
panels).
Overall, although some visual response signal properties correlated better to
saccade behavior than others, significant correlations were found between SRT, saccade
velocity and saccade accuracy and all visual signal properties measured. This suggests
86
VROL
Growth Time
Peak Magnitude (inverse)
Decay Rate
80
60
0.6
0.4
40
0.2
20
0
-1
-0.5
0
0.5
Correlation Coefficient (r)
0
1
0.8
Peak Saccade Velocity
80
0.6
Median r2
Cumulative Percent
B 100
60
0.4
40
0.2
20
0
-1
C 100
-0.5
0
0.5
Correlation Coefficient (r)
0
1
0.8
Saccade Endpoint Error (Accuracy)
80
0.6
Median r2
Cumulative Percent
0.8
SRT
Median r2
Cumulative Percent
A 100
60
0.4
40
0.2
20
0
-1
-0.5
0
0.5
Correlation Coefficient (r)
1
0
Figure 3.5. Cumulative distributions of correlation coefficients (left column) and median
r2 values (right column) for each behavioral and visual sensory neural variable
measured. Filled circles in the cumulative plots denote statistically significant
correlations (within neuron). Color denotes the neural signal property that was
correlated: VROL (blue), growth time (time from VROL to peak) (green), additive
inverse peak magnitude (negative correlations were plotted in order to compare with
the positively correlated VROL and peak magnitude values) (black) and decay rate
(pink). Each neural signal property was correlated independently to SRT (A), peak
saccade velocity (B), and saccade endpoint error (C). Median r2 values denote how
much variance was accounted for by each correlation.
87
that the visual response not only influenced when the saccade was initiated, but it also
carried through the visuomotor transformation to influence the metrics of the saccade.
3.4.5 Target Luminance Modulates the Saccade Motor Response
In order to identify elements of the motor response that varied with target
luminance, we analyzed the peak magnitude (Fig. 3.6A) of the motor response at saccade
onset and the rate of growth in discharge (Fig. 3.6B) of the motor response (i.e., slope)
during a pre-saccade epoch (from 25ms before the onset of the movement to the onset of
the movement). We analyzed the slope instead of absolute growth rate of the motor
response because in the gap task it was not possible to identify the precise onset of the
motor response following the visual response. Figure 3.6 shows the peak and slope of the
increase in the motor response for VM neurons across the seven luminance levels tested.
There was a main effect of motor peak with target luminance (F(6,378) = 15.3, P < 0.01),
however, the motor burst was only significantly modulated at the dimmest target intensity
rate (P<0.01) (Fig. 3.6A). This weak modulation of motor burst magnitude at the
dimmest target luminance was likely related to the reduced accuracy of the saccade
endpoint this luminance (Fig. 3.2C). Therefore, saccades did not always fall within the
center of each neuron’s motor response field, and this resulted in reduced SC motor
discharge (Stanford and Sparks, 1994; Marino et al., 2008). There was also a main effect
of the growth rate of the motor response (F(6,378) = 3.56, P < 0.02). The slope of the
motor burst was only significantly different between the dimmest and brightest target
luminance (P<0.01) (Fig. 3.6B). Thus, although modulations of the motor response were
88
225
200
Peak Magnitude
150
0.
00
1
0.
00
5
0.
04
4
125
4.2
Growth Rate
(Slope)
3.7
3.2
2.7
2.2
1.7
1.2
0.4 3.5 17.5 42.5
0.
00
1
0.
00
5
0.
04
4
175
Slope of Motor Response
B
250
Motor Response at Saccade Onset
(spikes/s)
A
Target Luminance (cd/m2)
0.4 3.5 17.5 42.5
Target Luminance (cd/m2)
Figure 3.6. Effects of target luminance on the properties of the saccade motor
response in the gap task collapsed across all VM neurons. A. Magnitude of the motor
burst at saccade onset. B. Mean growth rate (slope) of the motor response (-25ms
before saccade onset to saccade onset). Error bars denote standard error.
89
correlated with target luminance (Fig. 3.6), these correlations were weaker than those
computed with the visual response (Fig. 3.5).
3.4.6 Linking Motor Response Properties to Saccade Behavior
It has been previously shown that the timing of the saccadic motor burst in the SC
almost perfectly correlates with saccade occurrence (Sparks, 1978). We extend this
important finding by examining how luminance related changes in the peak magnitude
and slope of the motor burst correlate with the saccade behavior. Figure 3.7 shows the
cumulative distribution of correlation coefficients between the peak magnitude and
growth rate (slope) of the motor response with saccade behavior (SRT, saccade velocity,
and saccade accuracy). Both the peak and growth rate of the motor response showed
significant correlation with saccade behavior; however the peak magnitude of the motor
response was significantly more correlated with all saccade behaviors than its slope
(growth rate) (t tests; SRT: t =-2.59, d.f. =126, P < 0.012; Peak Velocity: t =-2.45, d.f. =
126, P < 0.016; Saccade Endpoint Error: t = -3.01, d.f. = 126, P < 0.01) (Fig 3.7. right
column).
3.5 Discussion
Here, we have linked specific components of the visual response in the SC to
saccade initiation. Target luminance modulated multiple components of the neural
response in the SC, which then correlated to the resultant saccadic behavior. Of these
modulations, the peak magnitude and onset time of the visual response correlated most
90
Growth Rate (Slope)
Peak Magnitude
80
0.6
Median r2
60
0.4
40
0.2
20
0
-1
-0.5
0
0.5
Correlation Coefficient (r)
Peak Saccade Velocity
0
1
0.8
80
0.6
60
Median r2
Cumulative Percent
B 100
0.4
40
0.2
20
0
-1
C 100
Cumulative Percent
0.8
SRT
-0.5
0
Correlation Coefficient (r)
0.5
0
1
Saccade Endpoint Error (Accuracy)
0.8
80
0.6
60
Median r2
Cumulative Percent
A 100
0.4
40
0.2
20
0
-1
-0.5
0
Correlation Coefficient (r)
0.5
1
0
Figure 3.7. Cumulative distributions of correlation coefficients (left column) and median
r2 values (right column) between each behavioral and saccade motor neural variable
measured (VM neurons only). Filled circles in the cumulative plots denote statistically
significant correlations (within neuron). Color denotes the neural signal property that
was correlated: growth rate slope (gray), and magnitude of the motor burst at saccade
onset (black). Each neural signal property is correlated independently to SRT (A),
peak saccade velocity (B), and saccade endpoint error (C). Median r2 values denote
how much variance was accounted for by each correlation. Error bars denote standard
error.
91
strongly to saccadic reaction time, and metrics (peak velocity and accuracy). These
correlations between the earliest part of the visual response and the timing and metrics of
the saccade indicate that the properties of the visual response carry through into the
neural computations that determine how fast the eyes move and how accurately they
align the fovea with a visual stimulus. We also found significant correlations between the
peak magnitude and growth rate of the motor response with variations in saccade
behavior as a function of target luminance. These data indicate that elements of the
earliest part of the visual response may play a crucial role in the sensory-motor
transformation that underlie visually guided saccades and influence behavior. This is
crucial because these signal properties arrive in the SC at latencies that approach
minimum afferent delays and precede the actual motor behavior by up to hundreds of
milliseconds. This suggests that the sensory-motor transformation that takes place
between the visual and motor responses in the SC is being influenced by the timing and
magnitude of the earliest part of the visual response.
3.5.1 Relation to Previous Work
Previous studies have examined the effects of target luminance or contrast on
visual activity within the SC. Contrast responses were initially described in the
superficial SC of anaesthetized cats (Bisti and Sireteanu, 1976), but have also recently
been found across the SC of humans with functional magnetic resonance imaging
(Schneider and Kastner, 2005) and in awake monkeys using single cell
electrophysiological recording (Bell et al., 2006; Li and Basso, 2008). However, the
conclusions from these studies were limited because the luminance and contrast
92
modulated changes of the visual and motor responses were never systematically tested or
linked to behavior. Specifically, Bisti and Siureteanu (1976) reported changes in firing
rate and Schneider and Kastner (2005) reported changes in fMRI BOLD activation in the
visual response in the SC, but neither of these studies correlated these changes with
saccade behavior. Likewise, both Bell et al. 2006 and Li and Basso 2008 report changes
in the VROL with luminance contrast; however, Bell and colleagues (2006) only used
two luminance levels and did not test different luminance levels within the same neurons,
while Li and Basso (2008) never correlated the observed changes in VROL or peak
magnitude to behavior. Here we tested a systematic range of target luminance levels
within the same neurons and determined how changes to the visual and motor response
link with saccade latency and metrics.
3.5.2 Stages of Visuomotor Processing
The luminance driven modulations of the visual response in the SC are
propagated directly into premotor circuit controlling saccade production. Specifically, the
visual and motor responses from VM neurons are aligned spatially (Marino et al., 2008)
and these neurons project via the predorsal bundle to the saccade generating circuit in the
brainstem (Moschovakis et al., 1990; Moschovakis et al., 1996; Kato et al., 2006;
Rodgers et al., 2006). Thus, luminance based modulations of visual signals are likely
used by both oculomotor (Edelman and Keller, 1996; Dorris et al., 1997; Sparks et al.,
2000) and head premotor circuits (Corneil et al., 2004; Corneil et al., 2007; Corneil et al.,
2008) to guide visually triggered orienting.
93
In our study, luminance affected the timing (VROL, peak time) and shape of the
visual response (peak magnitude, growth time, and decay rate). At brighter target
luminance values, the sensory input influenced visual processing more rapidly via the
quicker arrival, faster growth, and increased magnitude of the visual response. The
underlying neural mechanisms that generated these modulations in the visual response
must occur early in visual processing and then propagate through stages to influence the
motor response because they were observed within both V and VM neuron populations
within the SC. It is presumed that V neuron subtypes are located more superficially in the
SC than VM neuron subtypes (McPeek and Keller, 2002), however, only saccade related
neurons in the SC have been histologically linked to specific anatomical layers
(Moschovakis et al., 1988; Ma et al., 1991). We did not find any significant difference in
the timing of the VROL between V and VM populations (Supplementary Fig. 3.S2).
This suggests that simple luminance channels relay visual sensory information rapidly
across both the superficial and intermediate layers of the SC. However, there was a
tendency for the visual peak to increase at a faster rate in the V relative to the VM
neurons (Supplementary Fig. 3.S2). Multiple interpretations could explain this temporal
asynchrony. One possibility is that it could result from processing stages between the
superficial and intermediate SC. The superficial SC receives input from early stages of
visual processing (i.e., the retina and primary visual cortex) (Robinson and McClurkin,
1989; Sherman, 2007). Visual activity might reach its peak more slowly in the
intermediate SC because it is filtered through additional connections from the superficial
SC and additional extrastriate visual cortical regions (Kunzle et al., 1976; Fries, 1984;
Cusick, 1988; Lock et al., 2003). Alternatively, enhanced inhibition could cause the
94
initial visual burst to terminate earlier in the superficial SC thereby shifting the time of
the peak earlier relative to intermediate SC. Finally, there was a tendency for the peak
magnitude of the visual response to be larger in VM neurons relative to V neurons. If this
trend is real, it indicates that the visual sensory signal could be amplified as it passes
through the stages of processing prior to the VM neurons that influence resulting
saccades.
3.5.3 Evidence for Visual Inhibitory Feedback
We observed a steepening slope in the decay of the initial visual response with
increasing luminance (Figs. 3.3 left column, 4D). This could indicate that a neural
mechanism exists whereby the strength of the initial visual response determined the
magnitude of the suppression of the later part of the same response. This suppression
could play a role in terminating the initial phasic component of the visual response and
may provide a mechanism for controlling express saccade generation (fast saccades with
SRTs that approach minimum conduction delays) that might otherwise be triggered if the
visual response grows large enough to cross a neural threshold and trigger a saccade
(Edelman and Keller, 1996; Dorris et al., 1997).
It is unclear whether this observed suppression in the visual response could result
from local inhibitory circuitry within the SC (Isa and Hall, 2009) or if it is relayed from
upstream structures. One possible upstream candidate is the visual sector of Thalamic
Reticular Neurons (TRNs), which have been shown to receive excitatory inputs from the
LGN and project an inhibitory signal back to the LGN (Crabtree and Killackey, 1989;
Conley and Diamond, 1990; Harting et al., 1991; Uhlrich et al., 2003; Fitzgibbon et al.,
95
2007). Interconnections between the SC and TRNs have been previously identified
(Wilson et al., 1995; Vaccaro and Mitrofanis, 1996; Kolmac and Mitrofanis, 1998; Jones,
2007), so that it is at least possible that visual activity in the SC could influence
inhibitory TRN feedback and vice versa to influence the underlying visuomotor
transformation. This would suggest a new role for the SC in controlling visually guided
saccades. Future studies that assess the relationships between visual responses in the SC
and TRN activation will be important to address these questions.
3.5.4 Implications for Modelling of the Visual System
The modulations of the visual response that we reported with changes in target
luminance suggest a built-in mechanism that could be used to bias signals that influence
both visual processing and visuomotor transformations. For example, when multiple
visual stimuli are competing for foveation within the saccadic system, this mechanism
could influence the neural competition between individual visual responses via changes
to their timing, magnitude, or shape. These changes in visual processing could therefore
influence the order in which individual stimuli are selected for upcoming saccades. Such
selective enhancement or inhibition of salient features in the visual system are
hypothesized to be a key feature for influencing saccades during natural viewing (Itti and
Koch, 2001; Berg et al., 2009). The SC is an excellent locus for these spatial interactions
to occur because it contains a topographic sensorimotor map for saccades (Robinson,
1972; Marino et al., 2008) where multiple sensory and goal driven signals compete to
influence saccade production (Dorris et al., 2007). The differences in VROL, peak, and
slope that were modulated by luminance could therefore impact the spatial competition of
96
bottom-up signals on the SC map and influence 1) which stimuli become saccade targets,
2) the order that visual signals are chosen for saccade targets (i.e., which are selected
first, second etc), 3) and which stimuli are ignored. Incorporating these signal properties
into winner-take-all spatial competition models (Kopecz, 1995; Itti and Koch, 2001;
Trappenberg et al., 2001) could significantly improve the models’ capacity for predicting
saccade selection. Because natural vision in primates commonly involves making
visually guided saccades to targets of varying luminance, future studies of the visual or
saccade system will benefit from a better understanding of how luminance impacts both
sensory processing and motor behavior that these results provide.
97
Chapter 4: The Timing and Magnitude of Visual and Preparatory Responses in the
Superior Colliculus Influences Express Saccade Latency and Prevalence
98
4.1 Abstract
Express saccades represent the fastest possible visually-triggered saccadic eye
movement with latencies that approach minimum sensory-motor conduction delays. In
the primate superior colliculus (SC, a midbrain orienting structure), two of the neural
signals that influence the probability of generating an express saccade are pre-target
saccade preparatory activity and the transient visual response. Here we use target
luminance as a tool to alter the timing and magnitude of the visual response which we
hypothesized would significantly influence express saccade timing and prevalence. We
recorded from visually responsive and preparatory build-up neurons in the SC of
monkeys performing the gap saccade task while target luminance was systematically
varied between 0.001 cd/m2 to 42.5 cd/m2. Express saccade timing and prevalence was
determined independently from each luminance specific visual response. Target
luminance influenced both the timing of and proportion of express saccades that were
produced. At luminance levels below 0.044 cd/m2, express saccades were generated at
significantly longer latencies (130-160ms) than traditionally reported. Increasing target
luminance (up to 3.5 cd/m2) increased and then decreased the proportion of express
saccades forming an inverted U-shaped function. The increase in the proportion of
express saccades correlated strongly with large increases in the magnitude of the visual
response. The subsequent reduction in express saccades at luminance levels above
3.5cd/m2, was not accounted for by modulations to the visual-peak or accumulated buildup alone. This suggests that other neural parameters likely play an important role in
influencing whether an express or longer latency regular saccade is generated.
99
4.2 Introduction
The ability to react as quickly as possible can have important consequences that
can range from saving a shot on goal and winning the big game to successfully evading
some physical attack or threat. Such timely responses first require that a sensory signal
be detected and/or processed by the nervous system before an appropriate action can be
performed. In the visual system, this detection and/or processing often involves a rapid
movement of the eyes (saccade) so that a critical visual object or threat can be oriented
and analyzed. Express saccades reflect the fastest visually triggered saccades in primates
with latencies that approach the minimum afferent and efferent conduction delays
between the retina and the extra-ocular muscles (Fischer and Boch, 1983; Fischer and
Weber, 1993; Paré and Munoz, 1996; Dorris et al., 1997). Here, the neural processing
times that would otherwise be necessary to make higher order decisions about the
meaning of stimuli or how to respond to them are bypassed. Thus, express saccades
represent a "visual grasp reflex" (Hess et al., 1946) whereby responses to abruptly
appearing visual stimuli are transformed directly into saccadic orienting responses
(Edelman and Keller, 1996; Dorris et al., 1997; Sparks et al., 2000; Corneil et al., 2004).
Historically, express saccades have been defined by behavioral criteria that was
based on the observation of multiple modes within saccadic reaction time (SRT)
distributions (Fischer and Boch, 1983; Fischer and Ramsperger, 1984). These studies
described the earliest and fastest mode as express saccades (typically ~70-90ms in
monkeys) and the slower later mode as regular saccades (above 120 ms) (Fischer and
Boch, 1983; Fischer and Ramsperger, 1984). These temporal ranges for express and
regular saccade epochs are often used to define express and regular saccade ranges in
100
many studies of saccadic latency. However, because anticipatory movements and
bimodality in SRT distributions are not always clearly separable, and express saccade
ranges have been shown to vary between species, laboratories, and individuals, it is
sometimes inaccurate or inappropriate to infer whether express saccades were produced
from the analysis of latency distributions alone. Because of this problem, it has been
proposed that the temporal range of express saccade latencies should be determined by
the precise timing of the visual response within visual and oculomotor structures and not
by an arbitrary or absolute latency range(s) (Bell et al., 2006). This method ensures that
all saccades are visually triggered (i.e., not anticipated and initiated prior to the visual
response) and express saccade latencies are defined by ranges that are constrained by
neural conduction/transduction delays.
Although some of the neural mechanisms underlying the generation of express
saccades have been identified (Edelman and Keller, 1996; Kirschfeld et al., 1996; Dorris
et al., 1997; Sparks et al., 2000; Hamm et al., 2010), several key details remain poorly
understood. Here, we used stimulus intensity as a tool for altering the timing and
magnitude of visual responses in the SC and examined the corresponding effects on the
latency and probability of evoking an express saccade. Several variables have been
identified previously that influence the probability of generating an express saccade
including: 1) imposing a temporal gap between the disappearance of the fixation point
and the appearance of a visual target (Saslow, 1967; Fischer and Ramsperger, 1984;
Reuter-Lorenz et al., 1991; Rohrer and Sparks, 1993; Paré and Munoz, 1996; Shafiq et
al., 1998); 2) abruptly presenting only a limited number (1 or 2) of nearby target stimuli
at a time (McPeek and Schiller, 1994; Weber and Fischer, 1994; Dorris and Munoz,
101
1998); 3) maximizing the spatial and temporal predictability of when or where the visual
target can appear (Rohrer and Sparks, 1993; Paré and Munoz, 1996; Dorris and Munoz,
1998); and 4) repetitively overtraining subjects on specific visually guided saccade tasks
(Kowler, 1990; Paré and Munoz, 1996). Here we add to this understanding by examining
how stimulus driven modulations to the timing and magnitude of the visual response
affect the timing and probability of producing express saccades.
The superior colliculus (SC) is an oculomotor control structure in the midbrain
that integrates sensory, motor, and cognitive signals related to visual orienting (Hall and
Moschovakis, 2003). The SC is also a critical structure in the generation of express
saccades because: 1) it receives visual input directly and indirectly from the retina along
the retino-tectal and renino-geniculo-tectal pathways (Fries, 1984; Cusick, 1988;
Robinson and McClurkin, 1989; Lock et al., 2003) ; 2) it projects directly to the saccadic
brainstem burst generator to drive saccades (Rodgers et al., 2006); and 3) when lesioned,
express saccades are abolished (Schiller et al., 1987).
During a regular latency saccade, high frequency bursts of action potentials
related to visual and motor responses can be observed as temporally separate events in
the intermediate layers of the SC (SCi) (Wurtz and Goldberg, 1972b; Sparks and Mays,
1980). However, during an express saccade, these visual and motor responses occur
simultaneously (Edelman and Keller, 1996; Dorris et al., 1997; Dorris and Munoz, 1998;
Sparks et al., 2000), thereby producing a single indistinguishable burst of action
potentials that can be of higher frequency than the distinct and temporally separate visual
and motor bursts that are observed during regular saccades. Because the motor response
to move the eyes is time locked to the visual response during an express saccade, and the
102
timing of the visual response has been shown to change with stimulus luminance (Bell et
al., 2006; Li and Basso, 2008; Marino et al., 2011), we hypothesize that express saccade
latencies are not fixed to a specific temporal range, but are dependent on stimulus
specific visual response onset latencies. Consequently, we also hypothesize that express
saccades may be present within single mode SRT distributions whenever a saccade is
triggered with a latency that temporally overlaps or merges with the visual response.
Increases in pre-target preparation responses in the SCi during the gap period also
influence express saccade generation (Dorris et al., 1997; Dorris and Munoz, 1998). This
low frequency pre-target activity (<100Hz) starts ~100ms in to the gap period and is
predictive of where or when a visual target will appear (Dorris and Munoz, 1998; Basso
and Wurtz, 2002). In particular, increases in this pre-target activity correlate with an
increase in the likelihood of producing an express saccade (Dorris et al., 1997). Because
decreased target luminance delays the onset latency of SC visual responses (Bell et al.,
2006; Li and Basso, 2008; Marino et al., 2011), the amount of time available for pretarget activity to accumulate in the gap task (prior to the arrival of the visual response)
will increase. Thus target luminance modulates both the magnitude of the visual transient
as well as the amount of pre-target preparatory activity in the SCi prior to when the visual
transient arrives.
These observations have led to the development of a threshold based accumulator
model for express saccades in the SCi (Fig. 4.1) (Edelman and Keller, 1996; Dorris and
Munoz, 1998). In this model, an express saccade is triggered when the visual response is
significantly large enough to cross a neural threshold (Edelman and Keller, 1996; Dorris
and Munoz, 1998). When combined with pre-target activity, the magnitude of the visual
103
Pre-existing Express Saccade Model
(Dorris et. al J. Neurosci. 1997, Edelman and Keller J.Neurophys 1996)
SRT
Regular
VROL
Target Onset
FP Offset
Neuronal Activation
Saccade Trigger Threshold
Express
Neural Signal in SCi neurons
Figure 4.1. A pre-existing model of express saccade generation based on neural
trigger thresholds in the SCi. In this model, an express saccade is triggered when the
combined visual response and build-up activity (red curve) cross a neural threshold
(dotted grey line) to trigger an express saccade (Edelman and Keller 1996; Dorris and
Munoz 1998). When this combined response does not cross threshold, a regular
latency saccade can be triggered at a later time by a separate and distinct motor
response. (grey line).
104
response is increased thereby making it more likely to cross threshold and trigger an
express saccade (Dorris and Munoz, 1998). Without sufficient pre-target activity, it
should be unlikely for a visual response to cross this saccade threshold. Based on this
model, both the magnitude of the visual response and the amount of pre-target activity
should combine to determine whether an express saccade is triggered. Here, we test this
model explicitly by examining how changes to the magnitude of the visual response and
the amount of pre-target activity interact to influence express saccade production. We
hypothesize that express saccades can be generated toward dim targets because weaker
visual responses can be compensated for by increased pre-target activity that accumulates
when the visual response is delayed.
4.3 Methods
4.3.1 Animal preparation
All animal care and experimental procedures were in accordance with the
Canadian Council on Animal Care policies on use of laboratory animals and approved by
Queen’s University Animal Care Committee. The surgical techniques required to prepare
animals for neuronal and eye movement recordings in our laboratory have been described
previously (Marino et al., 2008). In brief, two male monkeys (Macaca mulata) were
trained to perform several oculomotor tasks. Both animals underwent surgery under
aseptic conditions for the insertion of bilateral eye coils, a stainless steel head holder, and
a recording chamber that was mounted on the skull using stainless steel bone screws and
dental acrylic. The recording chamber was oriented towards the SC at an angle of 38°
105
posterior of vertical in the mid-sagittal plane. Monkeys were given at least 2 weeks to
recover prior to resuming of behavioral training.
4.3.2 Experimental tasks and behavioral stimuli
All behavioral tasks, data collection and recording techniques have been
described previously (Marino et al., 2011). In brief, monkeys were seated in a primate
chair with their heads restrained for the duration of an experiment (2-4 hours). They
faced a display cathode ray tube monitor that provided an unobstructed view of the
central visual area 50° x 60°. Extracellular recording was performed with tungsten
microelectrodes (0.5-5 MΩ impedance, Frederick Haer) inserted through guide tubes (23
gauge) that were anchored in delrin grids with 1 mm hole separations inside the recording
chamber (Crist C.F. et al., 1988). Electrodes were advanced with a microdrive into the
intermediate layers of the SC where we proceeded to isolate single neurons.
For this study, each monkey was required to perform several visually-guided
saccade tasks (Fig. 4.2A,B). Each trial required the monkey to generate a single saccade
from the central fixation point (FP) to a peripheral visual target (T). At the start of each
trial, the screen turned black and after a period of 250 ms a circular grayscale FP of
constant luminance (0.25° diameter spot, 3.5cd/m2) appeared at the center of the screen
against a black background (~0.0001 cd/m2). Fixation of the FP was required for a
variable period (500 - 800 ms) until either a small circular 0.25º grayscale T appeared
(delay task), or the FP was extinguished (gap task). During the inter-trial interval (8001500 ms duration), the display screen was diffusely illuminated to prevent dark
adaptation.
106
DELAY TASK
A
Visual Target
Baseline Epoch
Saccade
Epoch
Saccade
Baseline
GAP TASK
B
Visual
Baseline
Saccade
Preparatory Epoch
Epoch Target
Epoch
FP
FP
T
T
EYE
EYE
500 - 800ms 500 - 800ms
500 - 800ms
Saccade
Onset
D 600
Activity (Spikes/s)
Visual Response
Activity (Spikes/s)
DELAY TASK
(No Build-Up)
C 600
SRT
400
200
0
400
200
0
200
400
Time From Target Appearance (ms)
Activity (Spikes/s)
400
200
0
400
200
0
200
400
Time From Target Appearance (ms)
SRT
Saccade
Onset
Saccade Response
400
200
0
400
200
0
200
400
Time From Saccade Onset (ms)
F
600
Activity (Spikes/s)
GAP TASK
(Build-Up Neuron)
E
200ms
600
400
200
0
400
200
0
200
400
Time From Saccade Onset (ms)
Figure 4.2. Schematic representation of temporal events in the delay (A) and gap (B)
tasks for the fixation point (FP), target (T) and eye position (EYE). Vertical gray bars
denote key analysis epochs used to classify responses and neurons. C-F. Rasters
and spike density functions of a representative visual-motor burst neuron (VMBN) and
a build-up neuron (BUN) recorded during saccades toward the optimal target location.
C,D. A VMBN recorded in the delay task exhibiting separate visual (C, target aligned)
and saccade (D, saccade aligned) related responses. E,F. A VM-BUN neuron
recorded in the gap task exhibiting pre-target build-up activity during the gap period.
107
The delay task (Fig. 4.2A) was used to temporally dissociated neural activity that
was related to either the appearance of the target or the saccadic motor response. In the
delay task, the monkeys were required to continue fixation of the FP for an additional
500-800 ms after T appearance. Only after FP disappearance was the monkey allowed to
initiate a saccade to the T. The gap task (Fig. 4.2B) was used to elicit pre-target
preparatory activity as well as make the visual system behave more reflexively by
reducing SRT and facilitating the production of express saccades. (Edelman and Keller,
1996; Dorris et al., 1997; Sparks et al., 2000). In the gap task, the monkey was required
to make a saccade to a visual target immediately after its appearance. In the gap task a
200 ms period of darkness (gap) was inserted into each trial between FP disappearance
and T appearance (Saslow, 1967). During the 200ms gap, the monkey was required to
continue central fixation until a target appeared either in the optimal or opposite location
of each recorded neuron's visual response field (see below). Once the target appeared,
the monkey was required to initiate a saccade to it within 1000ms of its appearance.
Fixation point luminance was held constant at 3.5 cd/m2 and seven distinct target
luminances (0.001 cd/m2, 0.005 cd/m2, 0.044 cd/m2, 0.4 cd/m2, 3.5 cd/m2, 17.5 cd/m2,
and 42.5 cd/m2) were randomly interleaved within each block of trials. Luminance was
measured with an optometer (UDT instruments, model S471) that was positioned directly
against the screen of the monitor and centered on the stimulus. After each correct trial the
monkey was rewarded with fruit juice or water.
108
4.3.3 Averaged spike density functions and neuron classification
In order to group and characterize the activity of individual neurons, trains of action
potentials averaged across all correct trials were convolved into spike density functions
using either a Poisson or a Gaussian kernel (σ = 5ms) for each spike depending on
whether activity related to neural onset times (Poisson kernel) or peak responses
(Gaussian kernel) was being analyzed. Here a Poisson-like exponential growth/decay
function was used that resembled a postsynaptic potential:
R(t) = [1 - exp (-t/ τg)]·[exp (-t/ τd)]
Where R(t) defines the rate as a function of time, growth constant (τg) = 1ms;
decay constant (τd) = 20ms (Thompson et al., 1996). The Poisson-like exponential
growth/decay function is critical for the calculation of signal onset latencies because the
kernel only temporally smoothes neural activity later in time in order to preserve accurate
onset times (Thompson et al., 1996). Spike density functions were aligned on target
appearance when analyzing visual responses or pre-target preparatory activity and
saccade onset when analyzing motor responses. Neural activity from repeated trials with
the same target location were summed together and averaged to produce spike density
functions of neural activity.
Neurons were classified based on their target or saccade related responses in the
delay task. Visual and saccade related activity was classified relative to two specific
baseline epoch periods (Fig.4.2A). Visual baseline activity was calculated as the average
discharge from all correct trials during the last 100 ms of active central fixation prior to
target appearance. Saccade baseline activity was calculated from the average discharge
109
from all correct trials 100-50ms prior to the onset of the saccade. (Fig.4.2A). A
significant visual response was defined as an increase of target aligned activity (Gaussian
kernel) greater than 50 spikes/s above the visual baseline during the target epoch (50150ms after target appearance) (Fig. 4.2A). A motor response (Gaussian kernel) was
defined as a significant increase activity in the saccade epoch (+/- 10 ms around the onset
of the saccade) that was greater than 50 spikes/s above both the visual and saccade
baselines. Saccade activity was required to exceed the saccade baseline (in addition to the
visual baseline) in order to ensure that any sustained tonic visual activity related to the
continued presence of the target in the delay task would not be misclassified as motor
related saccade activity.
We recorded 78 saccadic motor burst neurons from 2 monkeys (see Table 4.1).
73/78 neurons had both visual and motor responses and 5/78 neurons had only motor
responses when the target was placed within the optimal response location in the visual
field. This optimal location was defined by the spatial target location that elicited the
highest frequency burst of action potentials related to the appearance of the T (visualmotor neurons) or the initiation of a saccade toward the T (motor-only neurons).
4.3.4 Classification of build-up neurons.
A subset of the visual-motor and motor-only burst neurons were sub-classified
based on whether or not they exhibited significant pre-target preparatory activity in the
gap task after the disappearance of the FP but prior to the onset of the visual response
(build-up neurons: BUNs)(Munoz and Wurtz, 1995a). BUNs were classified based on a
significant increase in low frequency activity during the preparatory epoch (10 ms after
110
Monkey (age, weight) VMBN
BUN BUN
(No Build-Up) VM M
W (6 years, 8 kg)
41
1
0
Q (7 years, 12 kg)
22
9
5
Total Neurons
63
10
5
Table 4.1: SCi neuron breakdown by monkey and subtype. VMBN: Visual-Motor burst
neuron with no build-up activity. BUN: A motor burst neuron with build-up activity that
may (BUN-VM) or may not (BUN-M) also exhibit a visual response to the appearance of
the target.
111
target appearance to 40 ms after target appearance) relative to the visual baseline epoch
(last 100ms of active fixation prior to the disappearance of the fixation point) (Fig. 4.2B).
The activity in the preparatory epoch had to be at least 15 spikes/s greater than the
activity in the visual baseline epoch and significant by a non-parametric Rank Sum test (p
<0.05). Based on this criteria, there were 63 visual-motor burst neurons with no build-up
(VMBNs) and 15 build-up neurons (BUNs, of which 10 were visual-motor neurons and 5
were motor-only neurons).
4.3.5 Behavioral Analyses
Data were analyzed offline with custom Matlab (Matlab 7.4, Mathworks Inc)
software. The start and end of saccades were determined from velocity and acceleration
template matching criteria; verified offline by the experimenter, and corrected when
necessary. Because visual response onset latency (VROL, see below) changed with target
luminance, anticipatory saccades (saccades with SRTs less than the luminance specific
afferent visual delays; see below) were removed based on each calculated VROL.
Error rates were calculated from the trials in which the target appeared. Early aborted
trials in which the monkey failed to fixate or maintain fixation of the FP at the beginning
of the trial prior to target appearance were eliminated from further analysis because they
were not representative of active task participation. Error rates were computed from
those trials that were initiated by the monkey (by actively fixating the FP at the start of a
trial and then holding fixation until the target appeared). Errors included: 1) anticipation
errors (saccades made after the target appearance, but before the visual response reached
the SC; see RESULTS); 2) saccade errors (saccades initiated after target was perceived
112
that landed outside the target window and were therefore incorrect); 3) delay task timing
errors (saccades initiated to the target after target appearance but before FP
disappearance); 4) trials in which no saccade was made. For a detailed analysis of error
rates see Chapter 3.
To determine the shortest SRT for visually-triggered saccades, we analyzed SRT
histograms (5ms bins) aligned on target appearance when two equally possible target
locations were presented within blocks of gap saccade trials. The distribution of SRTs
for correct saccades made into the target window was compared to the SRT distribution
for direction errors: i.e., saccades made incorrectly to the opposite target location. The
value of each pair of correct and incorrect bins was then compared at every target
luminance presented. Anticipatory saccades occurred with equal probability to either of
the two target locations (50% predictable), whereas the earliest visually-guided
movements were biased heavily toward the correct target location. The first bin in both
distributions where the monkey made significantly more correct trials than direction
errors determined the end of the anticipatory saccade period and denoted the minimum
time required for visual information from target appearance to propagate through the
visual system to influence saccade initiation (Fischer and Boch, 1983; Edelman and
Keller, 1996; Paré and Munoz, 1996; Dorris et al., 1997). Bin sizes between correct and
direction error saccades were compared using running binary sign tests. If fewer than 10
saccades were represented within the first consecutive and statistically greater SRT bin,
additional bins were included until the total number of saccades represented in the bins
exceeded 10 counts in order to compensate for the smaller sample sizes present at some
target intensities.
113
4.3.6 Neuronal Analyses
The effects of luminance on visual response properties was determined from
changes in the timing (visual response onset latency: VROL) and peak magnitude of the
initial phasic burst of the visual response as these properties were found to be highly
correlated to saccade behavior (Marino et al., 2011). VROL was determined
independently for each neuron at each target luminance from a running non-parametric
Rank Sum test of a trial-by-trial spike density function (Poisson kernel) aligned to the
appearance of the visual target. VROL was defined as the onset of stable statistical
significance (p<0.05) between the mean activity during the visual baseline and a moving
temporal window (1 ms resolution) within the target epoch (Fig. 4.2A). The peak visual
response was calculated independently in both the delay and gap tasks and was measured
separately for both express and regular latency saccades. The magnitude and timing of
the peak visual response was calculated at each target luminance from the maximum of
the trial averaged spike density function (Gaussian kernel) aligned to the appearance of
the target in the target epoch (Fig. 4.2A). We chose a 5ms pulse width as this value
allowed for a smooth continuous function to be generated with minimal temporal
smoothing of neural activity. The time to the peak was calculated as the time from T
appearance to the peak response.
The effects of luminance on the accumulated amount of preparatory build-up
activity was determined from the summated area under the curve of the corresponding
spike density function from target appearance until 10 ms prior to the luminance specific
VROL (VROL occurred earlier with increasing luminance) in the gap task. This epoch
114
enabled differences in the accumulated build-up to be robustly compared across each
luminance without any contamination from the visual response. Both the VROL and
accumulated preparatory build-up were calculated using the Poisson function instead of a
Gaussian function to ensure that each luminance specific VROL was not artificially
shifted earlier in time. The amounts of accumulated build-up that were calculated at each
luminance level were compared to each other using a z test for proportions. All statistical
comparisons were calculated with repeated measures ANOVA with post hoc Bonferroni
corrected pair-wise comparisons unless stated otherwise.
4.3.7 Calculation of Express Saccade Ranges
Express saccade ranges were defined independently at each target luminance
value from the timing of the corresponding VROL and shortest visually triggered SRTs.
We defined express saccades as visually triggered saccades where the visual response is
temporally merged with the motor response within VMBNs in the SCi. The mean time
difference between the VROL and the shortest SRT bin was 16.02 ms +/- 2.6 ms.
Therefore we defined the express saccade range as a 30 ms epoch beginning 15 ms after
each luminance specific VROL in the gap task. We chose a conservative 30ms epoch in
order to ensure that only express saccades were included
4.3.8 Expanded Express Saccade Model
It was proposed previously that express saccades are generated when the transient
visual response and preparatory build-up activity sum together to cross a neural threshold
and trigger a saccade that temporally merges the visual and saccadic motor responses
115
(Edelman and Keller, 1996; Dorris et al., 1997). In order to test this model, we examined
how changes to the combined peak visual response and accumulated build-up activity at
each luminance level affected the proportion of express saccades that were produced.
The individual contributions of the peak visual response and cumulative build-up activity
were normalized and the proportion of express saccades produced was modelled as a
linear combination of each signal:
w1(Vpeak) + w2(BUacc) = % Express Saccades Produced
Where w1 and w2 are the strengths of the proportional weights of the normalized
visual peak response (Vpeak) and accumulated pre-target build-up activity (BUacc). Values
of w1 and w2 were calculated that most closely matched the measured proportions of
express saccades that were produced at each luminance level. This enabled the model to
predict the relative importance of changes to the peak magnitude of the visual response
relative to the accumulated build-up activity in influencing the likelihood of producing an
express saccade.
4.4 Results
4.4.1 Target Luminance Modulates Express Saccade Latency
We recorded saccade behavior while recording from single saccade related burst
neurons in the SCi of two monkeys performing the gap task (See Table 1 for a detailed
breakdown). In order to assess the dependencies of express saccade latency ranges on
external stimulus properties, we examined how the timing of neural visual responses and
the shortest visually triggered SRTs were modulated by target luminance. Figure 4.3A
illustrates the effect of changing target luminance on the underlying SRT distribution and
116
A
150
2
0.001 cd/m
350
Onset of Correct Visually
Triggerred Saccades
B
Timing of Visual Response
and Saccade Behavior
160
Time of Visual Peak
Time of First Correct SRT BIN
Visual Response Onset Latency
0
150
0
2 350
0.005 cd/m
Time (ms)
140
120
100
0
150
2
17.5 cd/m
0
350
140
120
120
100
D
80
60
60
80
120
140
160
60
Monkey W
Monkey Q
Mean
12
8
0.
0.
0
1
4
00
0
0
100
200
300
SRT (ms) (Target Directions Collapsed)
% Express Saccades
Total Trials
0
2
42.5 cd/m
0
350
100
80
Visual Response Onset Latency (ms)
20 Proportion of Express Saccades
16
0
150
100
slope = 0.9996
r = 0.996
slope = 1.1970
r=0.997
Time of Visual Peak (ms)
2
3.5 cd/m
140
4
0
350
160
5
0
150
160
04
2
0.4 cd/m
0
350
00
150
VROL versus First Correct SRT
VROL versus Time of Visual Peak
0.
Regular
0.4 3.5 17.5 42.5
Target Luminance (cd/m2)
C
Express
0
60
0.
00
1
0.
00
5
0.
04
4
2
0.044 cd/m
Time of First Correct SRT BIN (ms)
0
150
Activity (Spikes/s)
SRT Behavior and Visual-Motor Neuron Population
(No Build-up Poisson Kernal)
80
0
350
0.4 3.5 17.5 42.5
Target Luminance (cd/m2)
Figure 4.3. Effects of target luminance on express saccades in the gap task. (A), Grey bars denote a
histogram of SRTs for correct saccades (above the zero line) and direction error saccades (below the
zero line) in the gap task across 7 different luminance levels (column is organized from top to bottom in
ascending luminance). The colored lines denote the population activity of VMBNs for regular latency
(thick line) and express latency saccades (thin line) across each luminance. (B) Temporal modulation
of visual response properties (time of response onset and peak) and the earliest visually triggered
saccade latency (first histogram bin when saccade performance exceeds chance). The onset and
peak of the visual response are denoted by solid and dotted grey lines respectively. The time of the
first correct SRT bin is denoted by a solid black line. (C) Linear correlations between VROL and the
peak time of the visual response (gray data points and line) and VROL and the earliest SRT response
time (black data points and line). (D). The proportion of express saccades produced by monkey W
(black line) and Q (grey line) as determined by the timing of the visual response properties and earliest
visually triggered SRT latencies. The dotted grey line denotes the mean proportion of express
saccades.
117
the corresponding visual response of VMBNs neurons. As target luminance decreased
toward detection threshold, the VROL and peak of the visual response in VMBNs in the
SCi occurred significantly later in time (Fig. 4.3A, Colored lines). Furthermore, SRT
behavior was also affected such that as luminance decreased, the earliest time for a
visually-triggered saccade (non-anticipatory) also occurred significantly later in time
(Fig. 4.3A, histogram: vertical dotted lines denote bin in SRT distribution where
performance exceeds anticipatory chance, Binary sign test p<0.05) . Figure 4.3B
summarizes the temporal changes to the visual and earliest SRT response: as target
luminance increased, the neural (VROL and time of visual peak) and behavioral response
decreased in latency (VROL: (gap) F(5,545) = 930, P < 0.01, Peak Time: (gap) F(6,654)
= 1095, P < 0.01). The mean time between the VROL and the earliest visually triggered
SRT bin was 16ms +/- 3ms (standard error). The time of earliest visually-triggered SRT
bin and the peak of the visual response were nearly identical (Bonferroni corrected pairwise comparisons p > 0.05) at all but the 2 dimmest luminance levels (Bonferroni
corrected pair-wise comparisons p < 0.05). This suggests that at all but the dimmest target
luminance levels, a visually triggered saccade cannot be launched until the visual
response reaches its peak level. The timing of both the neural visual response and fastest
visually triggered SRT changed with target luminance, suggesting that the corresponding
express saccade (saccades with merged visual and motor responses in the SCi) ranges
were not fixed but instead were altered by luminance. The maximum difference between
the fastest and slowest mean VROL, visual response peak time and earliest visually
triggered SRT was VROL: 75.5ms +/- 3.4 (standard error), Peak Time: 89ms +/- 2.4
(standard error), SRT: 75 ms (difference of bins), which suggests that express saccade
118
ranges can be altered by up to 75 ms. Furthermore, VROL is linearly related to and
highly correlated with both the peak time of the visual response (gray line: slope = 1.197,
r = 0.997 p<0.05) and the earliest SRT response time (black line: slope = 0.9996, r =
0.996) (Fig. 4.3C).
4.4.2 Target Luminance Modulates SRT Bimodality
Express saccades were first defined by the earliest distinct mode present within a
multimodal SRT distribution (Fischer and Boch, 1983; Fischer, 1986). However, the
SRT distributions in Fig. 4.3A only demonstrate clear bimodality at the brightest
luminance level employed. This clear bimodality gradually merges into a single mode as
luminance levels decrease toward detection threshold. However, if an express saccade is
not defined by bimodality but by the temporal merging of visual and saccade motor
responses in the SCi, then the poorly separated or single modes within the dimmer SRT
distributions still contain a proportion of express saccades. In summary: 1) The
separation between regular and express saccade modes can be altered by target
luminance; 2) Express saccades can still be present within some single mode SRT
distributions and 3) Whenever express saccades are present within single mode SRT
distributions, their latency ranges cannot be clearly determined without knowledge of the
timing of the visual response in the oculomotor circuitry.
4.4.3 Merging of Sensory and Motor Bursts During Express Saccades
During an express saccade, the visual and motor responses of SC VMBNs are
temporally coincident. In order to examine how the merged visuomotor response differed
119
from temporally separated visual responses during regular saccades, we compared the
target aligned response of VMBNs during express (Fig. 4.3A thin colored lines) and
regular (Fig. 4.3A thick colored lines) saccades. When the target aligned responses of this
population were compared, express saccades showed a significantly increased target
aligned response both before the visual response (increased pre-target preparatory buildup activity) and during the visual response. This increased visual response during express
saccades has been previously shown to be related to the merging of the visual and motor
responses (Edelman and Keller, 1996; Dorris et al., 1997; Sparks et al., 2000) as well as
the summation of the visual response with the increased pre-target build-up activity
(Basso and Wurtz, 1998; Dorris and Munoz, 1998). Furthermore this pre-target build-up
activity is correlated with a reduction in SRT and an increased likelihood of producing an
express saccade (Dorris et al., 1997; Dorris and Munoz, 1998).
4.4.4 Target Luminance Modulates the Likelihood of Producing an Express
Saccade
In order to determine how target luminance modulated the likelihood of
producing an express saccade, we calculated the proportion of express saccades produced
within the 30 ms express saccade epoch (as determined by the timing of the VROL and
time of the earliest visually triggered SRT bin, see Methods). Figure 4.3C describes the
percent express saccades produced at each luminance level from each monkey. For each
monkey, the percent express saccades formed an inverted U-shaped function whereby the
percent of express saccades increased from 0.001 cd/m2 to 3.5 cd/m2 (z test for
120
proportions z=4.85, p<0.01) and then decreased from 3.5 cd/m2 to 42.5cd/m2 (z test for
proportions z=6.88, p<0.01).
4.4.5 Target Luminance Modulates Peak Visual Response and Accumulated
Build-up in the gap task
Because the amount of pre-target build-up activity (Dorris et al., 1997; Dorris and
Munoz, 1998) as well as the magnitude of the visual response (Marino et al., 2011) in the
SCi have been shown previously to influence SRT and express saccade production, we
examined how each of these neural properties were modulated by target luminance in the
gap task. Figure 4.4A illustrates the effect of target luminance on the target aligned visual
response on the population of VMBNs recorded. Here all neurons with significant
increases in pre-target preparatory build-up activity (BUNs) were removed in order to
isolate the effects of luminance on the magnitude of the visual response alone. There was
a main effect of luminance on the peak of the visual response (Peak Magnitude: (gap)
F(6,654) = 102.9, P < 0.01). As luminance was increased from 0.001 cd/m2 to 42.5 cd/m2
the onset of the visual response occurred later and had a lower peak (Fig. 4.4A). Figure
4.4C illustrates the mean peak visual response (measured from each VMBN without
build-up) at each luminance level. As luminance increased from 0.001 to 0.4 cd/m2, the
mean peak visual response increased (Bonferroni corrected pair-wise comparisons p <
0.05). Above 0.4 cd/m2 the trend of the peak visual response increased, however these
increases were not significant (p>0.05). In summary, the peak visual response increased
with increasing target luminance.
121
160
42.5
17.5
3.5
0.4
0.044
0.005
0.001
120
1500
Target Luminance
00
3.5 17.5 42.5
0.
4
04
0.4
0.
1
00
0.
00
0.
14
(cd/m 2 )
Linear Model Combining
Visual Response Magnitude and Build-up
F
0.4
3.5 17.5 42.5
Target Luminance (cd/m 2 )
0.12
Optimal Parameter Weights
Behavior
Model
12
0.06
10
8
3.5
0
17.5 42.5
-u
p
0.4
ild
0.
04
4
00
5
Target Luminance (cd/m 2 )
Bu
0.
0.
00
1
6
Pe
ak
% Express Saccades
E
5
75
4
125
Cumulative Build-up
04
175
n=14 neurons
0
-200 -150-100 -50 0 50 100 150 200 250
Time From Target Onset (ms)
6500
5
D
225
25
80
0.
Peak Visual Response
Peak Visual Response
(Spikes/s)
C
0
150
Time From Target Onset
(ms)
40
n=64 neurons
0
-200 -150-100 -50 0 50 100 150 200 250
Time From Target Onset (ms)
275
0
-200
00
50
Cumulative Build-Up
Luminance Specific Cut-off Times
100
0.
100
Build-up Neurons (Motor + Visual Motor)
Gaussian Kernel
200
Population Response
(Spikes/Sec)
150
B
1
Population Response
(Spikes/s)
200
Visual-Motor Neurons (No Build-up)
Gaussian Kernel
Population Response
(Spikes/s)
250
Cumulative Build-up (Area Under Curve)
(Spikes/s x ms)
A
Figure 4.4. Population spike density functions (Gaussian kernel σ = 5ms) for all VMBNs (A)
and all BUNs (B) aligned on target appearance in the gap task. Colored lines denote the
population response at each target luminance level (red: 42.5 cd/m2, orange 17.5 cd/m2,
green 3.5 cd/m2, dark blue 0.4 cd/m2, cyan 0.044 cd/m2, pink 0.005 cd/m2, black 0.001
cd/m2). The inset figure in (B) denotes mean build-up activity averaged across all luminance
levels up to the time of each luminance specific VROL. C,D Mean peak visual response (C)
and cumulative build-up activity (D) for each luminance level. E. Best model fit (linear
combination of peak visual response and cumulative build-up: red line) to the mean proportion
of express saccades (grey line) produced across luminance levels. F. The calculated weights
for each of the normalized neural parameters (peak visual response and cumulative build-up)
that best fit the behavioral data.
122
Figure 4.4B illustrates the effect of target luminance on the target aligned
preparatory build-up response of all BUNs (See Methods). Because stimulus luminance
modulated when the visual response arrived in the SCi, the accumulation time for pretarget build-up activity was also changed. When the onset of the visual response
occurred later for dimmer target stimuli, the amount of pre-target build-up activity
increased (Fig. 4.4B inset). Figure 4.4D illustrates the effect of target luminance on the
cumulative build-up activity within our population of BUNs. As target luminance
increased, both the cumulative build-up and the VROL decreased. In summary, the
cumulative build-up decreased with increasing luminance levels due to the earlier visual
response onset latency which allowed less time for the build-up to accumulate.
4.4.6 Predicting the Relative Influence of the Visual Response and Build-up
Activity for Express Saccade Production
Previous studies have suggested that both the peak visual response and
accumulated build-up activity influence whether an express saccade is generated.
However, it is unknown whether one of these parameters is significantly more influential
than the other for producing an express saccade. In order to determine this, we fit a linear
model to the data that calculated a weighted combination for each neural parameter (peak
and build-up) that best fits the calculated likelihood of producing an express saccade at
each of the 7 target luminance levels tested (See Methods). The individual weights of the
visual peak and accumulated build-up parameters provide evidence as to which parameter
most strongly influenced express saccade production.
123
Figure 4.4E illustrates the best model fit to the calculated express saccade
likelihood. Because of the complex relationship between express saccade likelihood and
target luminance whereby percent express saccades increase and then decrease with
increasing luminance, the linear combination of the peak visual response and cumulative
build-up (which only changed in one direction) could not account for decreases in
express saccade likelihood when target luminance was above 3.5 cd/m2. This suggests
that additional neural parameters are also required to determine whether an express
saccade will be produced. The best fit of the model resulted in parameter weights that
were 0.06 for changes in accumulated build-up and 0.10 for changes in the peak visual
response (Fig. 4.4F). This indicates that changes to the peak visual response have a
stronger influence on whether an express saccade will be generated. Although our model
predicted that the accumulated build-up activity was less influential than the peak visual
response for triggering an express saccade, its overall weight was only reduced by 40%
relative to the peak visual response. This indicates that the accumulated build-up still
plays an important and influential role in express saccade generation.
4.5 Discussion
Here, we have shown that the latency and likelihood of producing express
saccades was not fixed but depended on the external properties of the stimulus.
Specifically, target luminance modulated both the timing of visual responses and the
amount of accumulated preparatory pre-target build-up activity in the SCi, which together
influenced express saccade latency and likelihood. Altered stimulus luminance has been
previously shown to affect both retinal transduction times (Lennie, 1981; Barbur et al.,
124
1998) as well as the timing and magnitude of visual responses in the SC (Bell et al.,
2006; Li and Basso, 2008; Marino et al., 2011). As a consequence, at dimmer luminance
levels (< 0.044 cd/m2) a small proportion of express saccades were generated at
significantly slower latencies (130-160 ms) than traditionally reported. Based on our
observations, we propose an expanded definition for express saccades that does not
require bimodality in the underlying SRT distribution. When target luminance was
decreased, the separation between regular and express saccade modes within the SRT
distributions decreased until only one mode was discernable. Because we defined express
saccades as being temporally linked to the timing of the visual response in the SCi, and
this visual responses was delayed at dimmer luminance levels, we observed later express
saccades even when no bimodality was evident in the SRT distribution (i.e., at luminance
levels below 0.044 cd/m2, Fig. 4.3A). This result suggests that the neural mechanisms
underlying express saccade generation are highly sensitive to stimulus properties like
luminance. Therefore, express saccades can only be fully dissociated from regular
saccades when the timing of the visual response through visual and oculomotor nuclei is
known. This issue is especially relevant for clinical behavioral studies of saccades where
the specific temporal parameters of the visual response are not measured or taken into
account when analyzing the occurrence of express or shorter latency saccades (Fischer,
1986; Biscaldi et al., 1996; Carpenter, 2001; Dickov and Morrison, 2006).
4.5.1 Neural Correlates of Express Saccades in the SCi
It has been shown previously that the amount of pre-target activity in SCi buildup neurons correlates with saccade latency and their discharge predicts express saccade
125
occurrence (Dorris et al., 1997). Furthermore, this pre-target preparatory activity is
modulated by top-down (TD) factors such as target predictability (Basso and Wurtz,
1997; Basso and Wurtz, 1998; Dorris and Munoz, 1998) and the expected value of
saccadic goals (Milstein and Dorris, 2007). In our study, pre-target activity was varied by
changing when the visual response arrived in the SCi, thus impacting the time available
for pre-target activity to accumulate. We observed a reciprocal relationship between the
timing of the visual response and the accumulated amount of preparatory pre-target buildup activity present in the SCi prior to saccades. Specifically, visual responses to dimmer
stimuli were delayed and therefore provided additional time for build-up activity to
accumulate. This increased build-up activity on trials with reduced target luminance may
have helped to facilitate those express saccades that we observed.
Altering target luminance non-linearly influenced the proportion of express
saccades that were produced such that they increased up to 3.5 cd/m2 but then decreased
up to 42.5 cd/m2. The increase in the proportion of express saccades up to 3.5cd/m2
correlated strongly with large early increases in the magnitude of the visual response,
which suggested that in this range, luminance most strongly influences whether an
express saccade was produced. The subsequent reduction in the proportion of express
saccades at luminance levels above 3.5cd/m2, however, could not be fully accounted for
by modulations to the visual-peak or accumulated build-up alone. This suggests that other
neural mechanisms also play a role in influencing whether an express or regular latency
saccade is generated.
126
4.5.2 Top-Down and Bottom-Up Influences on Express Saccades
Express saccades represent a fast and direct sensory to motor transformation
where visual stimuli are "grasped reflexively" by the eyes (Hess et al., 1946; Paré and
Munoz, 1996; Edelman and Keller, 1998; Sparks et al., 2000). The neural mechanisms
underlying their generation appear to function differently than regular latency saccades
where sensory and motor responses are temporally separated by a neural decision process
(Carpenter, 2004; Marino et al., 2011). One important factor that has been shown to
affect express saccades is extensive overtraining or practice on a visually guided saccade
task. As training progresses, the percentage of express saccades increases (Kowler, 1990;
Paré and Munoz, 1996). Many of the neural mechanisms underlying express saccades
have been shown to be influenced by both goal-driven (top-down) and stimulus-driven
(bottom-up) processes. One TD influence is temporal or spatial target predictability. As
the spatial location of the target is more certain, saccade latencies decrease and the
proportion of express saccades increase (Rohrer and Sparks, 1993; Paré and Munoz,
1996). These behavioral changes have been shown to be correlated with increases in TD
pre-target preparatory build-up signals in the SCi (Dorris and Munoz, 1998).
Several bottom-up (BU) factors have also been shown to influence express
saccades. For example, when multiple distant visual targets abruptly appear during visual
search tasks where an oddball target is presented amongst distractors, express saccades
are almost never made (McPeek and Schiller, 1994; Weber and Fischer, 1994)).
However, express saccades can be elicited during scanning tasks when multiple stable
objects are present, but an abrupt appearance of a single target at an anticipated location
is still required (Sommer, 1994; Sommer, 1997). When multiple targets appear abruptly,
127
express saccades tend to only be made if: 1) a temporal asynchrony is introduced to the
time the targets appear (Schiller et al., 2004), or 2) if only 2 targets are presented within
close spatial proximity (i.e. within 45° of visual angle from each other)(Edelman and
Keller, 1996). When visual targets are presented in close proximity, the corresponding
activity on the SC sensory-motor map for nearby visual targets will likely overlap
(Anderson et al., 1998; Marino et al., 2008) forming a single hill of activity on the SC
map rather than multiple competing ones.
Another BU factor influencing express saccades is the retinal location of the
target. When targets are presented within 2° from fixation a dead zone for express
saccades has been reported (Weber et al., 1992). In this region of the SC map
corresponding to the target also likely overlaps with the rostral pole which has been
shown to be involved in smooth pursuit (Krauzlis et al., 2000) and visual fixation (Munoz
and Wurtz, 1993a; Munoz and Wurtz, 1993b). Our results add to this set of BU criteria
by demonstrating how the proportion of express saccades depend on the intensity of the
target stimulus, which influences the balance between the timing and magnitude of the
visual response as well as the amount of pre-target build-up activity that accumulates.
Here, we have demonstrated that BU target luminance is another important factor
that influences the express saccade generating mechanism by influencing when and how
often express saccades are made. This study further extends a previous study by Weber
and colleagues (Weber et al., 1991) that did not show any effect of luminance contrast on
the proportion of express saccades generated. However as we have discussed, without
insight into the corresponding BU modulations to the visual response, it is difficult to
interpret Weber and colleagues negative result. This highlights why it is important to first
128
understand how altered stimulus properties affects the visual response before predictions
or explanations can be made as to how it impacts express saccade occurrence.
4.5.3 Expanding Express Saccade Models
Previous models of express saccade generation have hypothesized that an express
saccade is generated when the visual response is sufficiently large enough to exceed a
neural threshold and generate a saccade (Edelman and Keller, 1996). This idea was
further developed by Dorris and colleagues (Dorris et al., 1997; Dorris and Munoz,
1998), who suggested that it was the combination of both pre-target preparatory activity
and the visual response in the SCi that additively must cross a neural threshold to trigger
the saccade (Fig. 4.1). Based on our results, we propose a further extension of this model
whereby faster express saccades generated to high luminance stimuli are more strongly
influenced by larger visual responses. For low luminance stimuli that approach detection
thresholds, longer latency express saccades can still be generated despite a significantly
reduced visual response because of the additional accumulation of pre-target build-up
activity (Fig. 4.5).
4.5.4 Other Potential Neural Properties Influencing Express Saccade
Production
In our data, the combined modulations of the peak visual response (bottom-up)
and accumulated build-up activity (top-down) could only account for increases in the
proportion of express saccades with increasing target luminance up to 3.5 cd/m2. Above
this luminance level, changes to the peak or build-up activity could not account for the
129
VROL (Dim)
VROL (Bright)
Target Onset
Neuronal Activation
Expanded Express Saccade Model
Higher proportion Express
SRT
Lower proportion Express
Saccade Trigger Threshold
Delayed VROL increases time for
pre-target preparatory acitivity
Figure 4.5. Our proposed extension of the pre-existing express saccade model whereby
bright stimuli trigger early express saccades (red line) and dim stimuli trigger later
express saccades (orange line). In this model early express saccades are more
prevalent because bright stimuli elicit larger visual responses. A reduced proportion of
late express saccades can still be triggered despite a significantly reduced visual
response because of the extra build-up activity that can accumulate.
130
decrease in express saccades with increasing target luminance that we observed. This
result indicates that there are additional neural mechanisms that modulated express
saccade occurance to visual targets brighter than 3.5 cd/m2. One possible mechanism that
may also be effecting the express saccade mechanism is the fixation related activity
located in the rostral pole of the SCi (Munoz and Wurtz, 1993a). This fixation related
activity aids in anchoring gaze on a visual target and inhibits saccades (Munoz and
Wurtz, 1993b; Krauzlis, 2005). It has been shown that during the gap, fixation related
activity decreases (Dorris and Munoz, 1995; Everling et al., 1999) and this decrease covaries with mean SRT when the gap duration is varied (Dorris and Munoz, 1995).
However when the gap duration remains constant, the rate of decrease of fixation activity
is largely invariant and does not correlate with inter-trial variations in SRT (Dorris et al.,
1997) and is not altered during express saccades (Dorris et al., 1997). This evidence
suggests that decreases in fixation activity can only influence SRT (either directly or
indirectly) when external stimulus properties (related to the timing of the visual response
relative to the disappearance of the fixation point) are altered. Thus, like the accumulated
build-up, changes to the timing of the visual response effects the amount of fixation
activity present when the visual response is triggered in the SC. As luminance increases,
the visual response occurs earlier and allows less time for fixation related activity to
decrease and disinhibit the rest of the SC.
Another possible neural mechanism that may be influencing the express saccades
is the area or size of the visual response within the SCi map. We have shown previously
that the size of this population response or point image (McIlwain, 1986) changes with
luminance (Marino et al., 2007). Furthermore, we used a neural field model of lateral
131
interactions within the SCi to predict that changes to the size of visual point images
significantly affected SRT such that increases in point image area decreased and then
increased SRT (Marino et al., 2011). This is because increases in point image size will
decrease SRT until the point image grows beyond the hypothesized spatial extent of local
excitation and into regions of distal inhibition in the SC which slows SRT. We therefore
hypothesize that the reduction in the proportion of express saccades above 3.5 cd/m2
could result from larger visual response point images in the SCi that may actually inhibit
the express saccade mechanism. Further study of the relationship between the spatial
extent of point images and their influence on saccades is needed to address this
possibility.
Finally, a recent EEG study in humans has shown evidence for cortical network
involvement in express saccade generation (Hamm et al., 2010). This study indicated that
a distributed posterior cortical network helps prepare the saccade system to respond more
rapidly to influence express saccades. It is possible that the contribution of these cortical
areas could also be modulated by target luminance levels and account for the reduction in
express saccades that we observed above 3.5cd/m2.
4.5.5 Conclusions
Express saccades represent the fastest and most direct sensory to motor
transformation in the visual system. The neural mechanism underlying their generation
does not function at a fixed temporal latency but is strongly linked to the timing of the
visual response which can be modified by the external properties of the stimulus.
Furthermore, the likelihood of producing an express saccade is also not fixed but is
132
strongly influenced by such stimulus properties. These results highlight several important
links between bottom-up visual response properties and top-down preparatory responses
during direct sensory to motor transformations within an express saccade.
133
Chapter 5: Spatial Interactions in the Superior Colliculus Predict Saccade Behavior
in a Neural Field Model
134
5.1 Abstract
During natural vision, eye movements are dynamically controlled by the
combinations of goal-related top-down (TD) and stimulus-related bottom-up (BU) neural
signals that map onto objects or locations of interest in the visual world. In primates, both
BU and TD signals converge in many areas of the brain including the intermediate layers
of the superior colliculus (SCi), a midbrain structure that contains a retinotopically coded
map for saccades. How TD and BU signals combine or interact within the SCi map to
influence saccades remains poorly understood and actively debated. It has been proposed
that winner-take-all competition between these signals occurs dynamically within this
map to determine the next location for gaze. Here, we examine how TD and BU signals
interact spatially within an artificial 2-dimensional dynamic winner-take-all neural field
model of the SCi to influence saccadic reaction time (SRT). We measured point images
(spatially organized population activity on the SC map) physiologically to inform the TD
and BU model parameters. In this model, TD and BU signals interacted nonlinearly
within the SCi map to influence SRT via changes to the: 1) spatial size or extent of
individual signals, 2) peak magnitude of individual signals, 3) total number of competing
signals, and 4) the total spatial separation between signals in the visual field. This model
reproduces previous behavioral studies of TD and BU influences on SRT, and it accounts
for multiple inconsistencies between them. This is achieved by demonstrating how under
different experimental conditions, the spatial interactions of TD and BU signals can lead
to either increases or decreases in SRT. Our results suggest that dynamic winner-take-all
modeling with local excitation and distal inhibition in two dimensions accurately reflects
135
both the physiological activity within the SCi map and the behavioral changes in SRT
that result from BU and TD manipulations.
136
5.2 Introduction
The spatial organization of the external world is represented in neural maps
throughout the visual system (Hubel and Wiesel, 1969; Cynader and Berman, 1972;
Kaas, 1997; Hall and Moschovakis, 2003). For primates, these maps are especially
important because the highest visual acuity is limited to the fovea where only 1-2° of
visual angle are represented (Perry and Cowey, 1985b). Because the fovea can only be at
one place at any time, simultaneously occurring relevant or interesting objects in the
visual field must compete for foveation. Two neural processes that influence saccades are
stimulus triggered bottom-up (BU) and goal directed top-down (TD) (Itti et al., 2005;
Fecteau and Munoz, 2006). BU processes reflect the exogeneous properties of external
visual stimuli and affect neural responses. TD processes involve endogeneous, internallydriven cognitive mechanisms and include attention and prediction that reflect the
relevancy of task-driven goals. In the oculomotor system, how TD and BU signals are
combined in neural maps to influence saccade processing is poorly understood. The goal
of this paper is to develop a two dimensional neural field model of the intermediate layers
of the superior colliculus (SCi) that is constrained by new physiological data and capable
of explaining how TD and BU processes interact and compete to account for observed
saccade behavior.
The SCi is the ideal place in which to model these relationships because it is a
critical structure in the guidance of saccadic eye movements (Schiller et al., 1980;
Schiller et al., 1987). The SCi contains a map that has been described physiologically
(Robinson, 1972) and defined mathematically (Ottes et al., 1986; Van Gisbergen et al.,
137
1987). The SCi also serves as a sensorimotor integration node where sensory (Goldberg
and Wurtz, 1972b; Meredith and Stein, 1985), saccadic preparation (Basso and Wurtz,
1998; Dorris and Munoz, 1998), and saccadic motor signals (Sparks, 1978; Sparks and
Mays, 1980; Munoz and Wurtz, 1995a) converge onto individual neurons that are
spatially aligned within the visual field (Marino et al., 2008) and project directly to the
premotor circuitry in the brainstem to drive saccades (Moschovakis et al., 1988; Scudder
et al., 1996; Rodgers et al., 2006).
Recent evidence suggests that lateral interactions contribute to the spatial
interactions of activity within the SCi map. These interactions can result in local regions
of excitation and surrounding regions of remote inhibition on the SCi map (Fig. 5.1)
(Dorris et al., 2007). When multiple BU and TD signals arrive in the SCi, physiological
evidence has suggested that a single saccadic goal is selected via a winner-take-all
mechanism (Munoz and Istvan, 1998; Trappenberg et al., 2001; McPeek and Keller,
2002; Munoz and Fecteau, 2002; Dorris et al., 2007). Neural field models of the
oculomotor system employing such lateral interactions (Amari, 1977) have been shown
to accurately simulate saccadic behavior (Wang et al., 2002) across several task
conditions (Kopecz and Schoner, 1995; Kopecz, 1995). When specifically applied to
describe the activity within the SC, this type of model has been able to successfully
account for an even larger variety of saccadic behaviors, including: 1) differences in SRT
due to endogeneously and exogeneously triggered saccades (Trappenberg et al., 2001;
Trappenberg, 2008); 2) saccade trajectory variations produced by focal SCi lesions
(Badler and Keller, 2002); and 3) competing visual stimuli (Arai and Keller, 2005).
138
Uniform Lateral Interaction Profile
(across any 1 dimensional cross section)
C
Right
SC
Weights between
fully connected nodes
Network Activation
2-Dimensional Network Architecture
Left S
0
Region of
Local Excitation
+
0
Region of
Distal Inhibition
at
iol
ed
M
-
s
de
No
al
er
audal
oc
Rostr
s
+
-
Distance Between
Neural Field Nodes (mm)
Node
Figure 5.1. (left panel). Architecture of the 2-dimensional neurofield model (developed
from Trappenberg et. al 2001). (right panel). Schematic of the Gaussian shaped
spatial profile of local excitation and distal inhibition centered at each neural node
within the model.
139
The model presented here extends an earlier model (Trappenberg. et. al 2001) by
addressing several limitations of the previous one-dimensional architecture that was not
reflective of the two-dimensional neural map represented within the SC (Robinson,
1972). Furthermore, the spatial size of the TD and BU input parameters in this previous
model were only roughly estimated from individual neural response fields (Munoz and
Wurtz, 1995a; Munoz and Wurtz, 1995b). A more accurate estimate of these spatial
parameters should reflect the population activity or “point image” (McIlwain, 1986)
(spatially organized regions of localized activity on the SC map). This is critical because
individual response fields show significant variation in both size and magnitude, whereas
point images remain approximately constant across the SC map for all target locations
and saccade vectors (Munoz and Wurtz, 1995b; Anderson et al., 1998; Marino et al.,
2008).
The neural mechanisms that underlie how TD and BU processes influence
saccades are poorly understood, yet they have a profound effect on the timing or “mental
chronometry” (Posner, 2005) of the sensory to motor transformations that are computed
by the visual and oculomotor systems. Previous reaction time studies on the effects of TD
target predictability and BU stimulus intensity and numbers of stimuli show many
apparent inconsistencies and contradictions that seem to indicate that the underlying
neural mechanisms are highly complex. However, we demonstrate here that lateral
interactions within a winner-take-all model of the SCi can account for these many
different results via the simple spatial properties and locations of the underlying TD and
BU point images (Fig. 5.2).
140
Width of Point Image
A
Width of
local excitation
-
+
Activity Smaller Than
Network Excitatory Width
Width of
local excitation
-
Activity Larger Than
Netork Excitatory Width
Network
Activation
Lateral
Interaction 0
-
+
B
+
+
-
0
Distance Between Multiple Point Images
+
-
Distal Spatial Activation
Results in Competative Inhibition
Proximal Spatial Activation
Results in Excitatory Summation
Network
Activation
Lateral
Interaction 0
-
-
+
0
Figure 5.2. Schematics of spatial competition and lateral interaction that can occur from
individual (A) or multiple (B) activation signals within the retinotopic, topographical SCi
map as predicted by our model. A. lateral interaction could theoretically result from
individual hills of activity corresponding to spatially specific TD or BU signals depending
on whether they are wider (1A right panel) or narrower (1A left panel) than the underlying
excitatory interaction profile in the SCi map. Spatial signals that are wider than the width
of the excitatory area will result in lateral inhibition across the input signal. This lateral
inhibition is predicted to result in longer SRT relative to spatial signals whose width does
not exceed the width of local excitation. B. When input signals (either TD or BU) are
distant, dynamic winner-take-all mechanisms must resolve the resulting saccade vector
by suppressing competing TD and BU signals. In the case where these competing signals
are distant, the corresponding spatial signals on the SCi map are distant and spatially
distinct. In this case strong lateral inhibition suppresses each competing signal until only
one remains. However, in the case where these competing signals are close together,
they could additively combine together into a larger area of excitation in the SCi map. We
predict that the summation of nearby signals should reduce SRT relative to more distant
signals because of the more global area of excitation that results on SCi the map.
141
To improve the physiological accuracy of the model, we measure the point
images of BU sensory responses and pre-target TD preparatory responses in the SCi and
used them to constrain the input parameters in the model. Then, we model how the spatial
interactions between TD or BU signals can influence SRT. We test how these interactions
can occur both within individual signals at a single location (i.e., via changes in
magnitude and width) (Fig. 5.2A), or between multiple signals at different locations in
the map (Fig. 5.2B). Specifically, we assess how SRT is influenced by the: 1) area of
tissue activated (Fig. 5.2A), 2) peak magnitude of the input signal, 3) distance between
input signals (Fig. 5.2B), and 4) overall number of competing TD or BU signals. When
only one TD or BU input signal is present within the model, we hypothesize that signals
that extend beyond the area of local excitation in the SC map will trigger saccades more
slowly than narrower input signals (Fig. 5.2A). This may result because signals with
wider activation than the excitatory interaction region could simultaneously activate both
local excitatory and remote inhibitory connections. This should increase accumulation
time to saccadic threshold leading to an increase in SRT. When two or more input signals
are present we hypothesize that distal signals will mutually inhibit each other and result
in slower SRTs, while simultaneously occurring proximal signals will combine their
point-images (activity on the SC map) and lead to faster SRT (Fig. 5.2B).
5.3 Methods
5.3.1 Animal preparation
All animal care and experimental procedures were in accordance with the
Canadian Council on Animal Care policies on use of laboratory animals and approved by
142
Queen’s University Animal Care Committee. Four male monkeys (Macaca mulatta) were
used in these studies. A detailed description of the surgical techniques used to prepare
animals for neuronal and eye movement recordings in our laboratory have been described
previously (Marino et al., 2008).
5.3.2 Experimental tasks and behavioral stimuli
Neurophysiological experiments were designed to measure BU and TD point
images in the SCi. Behavioral paradigms and visual displays were under the control of a
UNIX-based real-time data control and presentation systems (Rex 6.1) (Hays et al.,
1982). The display screen spanned ± 35º of the central visual field. Luminance was
measured with a hand held photometer (Minolta CS-100) that was positioned at the same
location as the monkey (86cm away from the display screen). Monkeys were required to
perform several interleaved visually-guided saccade tasks including the delay and gap
tasks (e.g., Fig. 5.3A,B), as well as the step task and memory-guided task (paradigm and
data not shown). Experiments were performed in total darkness with individual trials
lasting ~1-2 seconds. Each trial required the monkey to generate a single saccade from
the central fixation point (FP) to a peripheral visual target (T). During the inter-trial
interval (800-1500 ms duration), the display screen was diffusely illuminated to prevent
dark adaptation. At the start of each trial, the screen turned black and, after a period of
250 ms, a red FP (0.3 cd/m2) appeared at the center of the screen against a black
background. Following FP appearance, the monkeys had 1000 ms to fixate the FP before
the trial was aborted as an error. Central fixation was then maintained for a variable
period (500 - 800 ms) until the T (5 cd/m2) appeared.
143
A
1
FP
T
Delay Task
2
B
3 4
FP
T
EYE
EYE
500-800ms 500-800ms SRT
C
Activity (Spikes/s)
500-800ms 200ms SRT
Visual - Motor Neuron (Delay Task)
2
3 4
1
600
600
300
300
0
-400
D
600
Activity (Spikes/s)
Gap Task
5
6
300
0
-400
0
-400
0
400
Time From Target Appearance (ms)
5
6
0
400
Time From Saccade Onset (ms)
Buildup Neuron (Gap Task)
600
300
0
0
400
400 -400
0
Time From Saccade Onset (ms)
Time From Target Appearance (ms)
Figure 5.3. B-C Schematics of spatial competition and lateral interaction that can occur
from individual (A) or multiple (B) activation signals within the retinotopic, topographical
SCi map as predicted by our model. A. lateral interaction could theoretically result from
individual hills of activity corresponding to spatially specific TD or BU signals depending
on whether they are wider (1A right panel) or narrower (1A left panel) than the underlying
excitatory interaction profile in the SCi map. Spatial signals that are wider than the width
of the excitatory area will result in lateral inhibition across the input signal. This lateral
inhibition is predicted to result in longer SRT relative to spatial signals whose width does
not exceed the width of local excitation. B. When input signals (either TD or BU) are
distant, dynamic winner-take-all mechanisms must resolve the resulting saccade vector
by suppressing competing TD and BU signals. In the case where these competing signals
are distant, the corresponding spatial signals on the SCi map are distant and spatially
distinct. In this case strong lateral inhibition suppresses each competing signal until only
one remains. However, in the case where these competing signals are close together,
they could additively combine together into a larger area of excitation in the SCi map. We
predict that the summation of nearby signals should reduce SRT relative to more distant
signals because of the more global area of excitation that results on SCi the map.
144
The delay task (Fig. 5.3A) was used to dissociate visual and saccade related
activity. In this task, the monkeys were required to continue fixation of the FP for an
additional 500-800ms after T appearance. Only after FP disappearance was the monkey
allowed to initiate a saccade to the T. The trial was scored as an error and analyzed
separately if fixation was broken before FP disappearance, or if the monkey did not
complete a saccade to the target location within a 2º window (of visual arc) around the T,
or if the monkey failed to initiate a saccade within 1000ms of FP disappearance. The gap
task (Fig. 5.3B) was used to minimize the inhibition resulting from active visual fixation
(Dorris and Munoz, 1995; Paré and Munoz, 1996; Machado and Rafal, 2000; Krauzlis,
2003). In the gap task, a 200 ms period of darkness (gap) was inserted between FP
disappearance and T appearance (Saslow, 1967). The monkey was required to continue
central fixation until T appearance and then initiate a saccade to the T within 1000ms of
its appearance. Tasks were randomly interleaved within two separate blocks of trials. In
the first block, targets were presented at one of two possible locations: at the center of
each neuron’s response field (see below), or opposite the horizontal and vertical
meridians. In the second block, the targets were presented at 10º left or right of center
along the horizontal meridian. Each correct trial was rewarded with a drop of fruit juice
or water. The duration of all correct trials ranged from approximately 1 to 2 seconds
depending on the variability of fixation duration and SRT.
5.3.3 Neural Classification
All techniques for extracellular neuronal recording and data collection were
described previously (Dorris et al., 2007). We recorded 113 single neurons from 4
145
monkeys (see Table 5.1). Most neurons (106/113) had both visual and motor responses
and the remaining 7 neurons exhibited only motor responses when the target was
centered in the neuron's response fields. The neurons were sub-classified based on
whether or not they exhibited significant pre-target preparatory activity during the gap
period in the gap task (Munoz and Wurtz, 1995a; Dorris et al., 1997). Based on this
criteria, there were 71 visual-motor burst neurons with no build-up (VMBNs) and 42
build-up neurons (BUNs, of which 35 were visual-motor neurons and 7 were motor-only
neurons).
In order to characterize the activity of individual neurons, trains of action
potentials averaged across all correct trials were convolved into spike density functions
using a gaussian kernel (σ = 5ms) for each spike (Richmond et al., 1987). Spike density
functions were aligned on target appearance when analyzing visual responses (Fig.
5.3C,D left panels) and saccade onset when analyzing motor responses (Fig. 5.3C,D right
panels). VMBNs and BUNs were classified based on their discharge in the delay and gap
tasks (Fig. 5.3A,B). VMBNs (e.g., Fig. 5.3C) exhibited a significant increase in discharge
related to both the appearance of a visual target (visual response) and the initiation of the
saccade (motor response) but exhibited no increase in saccadic preparatory activity
(build-up) in the gap task. A visual response was defined as a significant increase in
target-aligned activity greater than 50 spikes/s above a visual baseline (last 100ms of
active fixation before T appearance; Fig. 5.3A, epoch 1) during the target epoch (50150ms after T appearance; Fig. 5.3A, epoch 2). A motor response was defined as a
significant increase in saccade aligned activity that was greater than 50 spikes/s above
both the visual and saccade (100 to 50 ms prior to the onset of the saccade; Fig. 5.3A,
146
Monkey (age, weight)
F (9 years, 7.2 kg)
H (6 years, 7.5 kg)
O (6 years, 11.6 kg)
R (8 years, 10 kg)
Total Neurons
VMBN
(No Build-Up)
40
21
10
0
71
BUN
VM
18
7
9
1
35
BUN
M
4
1
2
0
7
Table 5.1: SCi Neuron Breakdown by Monkey and Subtype. VMBN: Visual -Motor burst neuron with no
build-up activity. BUN: A motor burst neuron with build -up activity that may (BUN -VM) or may not
(BUN-M) also exhibit a visual response to the appearance of the target.
147
epoch 3) baselines during the saccade epoch (+/- 10 ms around the onset of the saccade;
Fig 5.3A, epoch 4). Saccade activity was required to exceed the saccade baseline in order
to ensure that any sustained tonic visual activity related to the continued presence of the
target was not misclassified as motor-related.
BUNs (e.g., Fig. 5.3D) exhibited a significant increase in saccadic preparatory
activity (build-up) in the gap task irrespective of any significant visual or motor response
in the delay task. Preparatory build-up activity involved a significant increase in
discharge between the visual baseline (last 100ms of active visual fixation in the gap task;
Fig. 5.3B, epoch 5) and the gap epoch (50ms before to 50m after T appearance in the gap
task; Fig. 5.3B, epoch 6). The gap epoch ended prior to the onset of the earliest visual
responses for all neurons recorded. All significant increases between epochs were
determined by a Wilcoxin rank-sum test, p<0.05.
5.3.4 Calculation of Neural Population Point Images on the SC Map
We analyzed the population activity or “point images” (McIlwain, 1975;
McIlwain, 1986) of BU visual, TD motor preparation, and saccadic-motor activity across
the SC during presentation of targets at 10° left and right. The position of each neuron on
the map was determined from the saccadic vector within the motor response field that
yielded the maximal motor discharge of action potentials at movement onset (Marino et
al., 2008). This was determined online by systematically testing the motor response to
multiple saccade vectors across each cell's motor response field. To visualize spatial
properties within the SC map, visual field coordinates were transformed into SC
148
coordinates using previously described transformations (Ottes et al., 1986; Van
Gisbergen et al., 1987):
R 2 2 AR cos A 2
u Bu ln
A
R sin
v Bv arctan
R sin A
(1)
(2)
Where u denotes the anatomical distance from the rostral pole (mm) along the horizontal
position axis, v is the distance (mm) along the vertical axis, R is the retinal eccentricy,
and Ф is the meridional direction of the target (deg). The constants are Bu = 1.4 mm; Bv =
1.8 mm/rad; and A = 3°. The spatial extent of each point image was calculated from a
cubic spline (de Boore, 1978) that interpolated between the cell locations on the SC map.
Population point image activity was calculated by plotting all activity ipsilateral to the
target stimulus within the left SC and all activity contralateral to the target stimulus
within the right SC. Neurons were mirrored across the horizontal meridian to improve
the spatial resolution of the fitted surface by exploiting the underlying symmetry within
the map. This assumption of symmetry was based upon no observed vertical biases
(above or below the horizontal meridian) in visuomotor responses between the upper and
lower visual fields when plotted on the SC map beyond 2° eccentricity (van Opstal and
van Gisbergen, 1989; Marino et al., 2008).
The BU visual response was calculated from the peak of the isolated visual
response recorded from VMBNs in the delay task (peak response occurred 102 ms after
target appearance, Fig, 5.4A left panel, Fig. 5.5A). The TD preparatory response was
calculated from the maximum build-up activity of BUNs in the gap task that occurred
149
300
A
Temporal Characteristics of Population Activity
Visual-Motor Neurons Without Build-up (Delay Task)
a
300
Saccade Into
RF
Saccade out
of RF
Activity
(Spikes/s)
Activity
(Spikes/s)
Target In RF
Target out of RF
0
0
0
-400
400
-400 Time From Target Appearance (ms)
B
0
400
Time From Saccade Onset (ms)
Build-up Neurons Only (Gap Task)
bc
400
Activity
(Spikes/s)
Activity
(Spikes/s)
400
N = 59 neurons
0
0
0
-400 Time From Target Appearance (ms)400 -400
d
N = 42 neurons
0
400
Time From Saccade Onset (ms)
Figure 5.4. Population spike density functions of VMBNs in the delay task (A) and
BUNs in the gap task (B) aligned on target appearance (left column) and saccade onset
(right column). Dashed gray lines denote the times of the peak visual response in the
delay task (time a), preparatory build-up response in the gap task (time b), combined
visual and preparatory response in the gap task (time c), and motor saccadic response
in the gap task (time c) that are plotted spatially on the SCi map and analyzed
separately in Figure 5.
150
Peak = 151 Spikes/s
FWHM = 1.4mm
1mm
Vertical Dimension [mm]
N = 113
150
1mm
Horizontal Dimension [mm]
0
D
Vertical Dimension [mm]
150
N = 42
1mm
Horizontal Dimension [mm]
0
Peak = 53 Spikes/s
FWHM = 1.53 mm (R), 1.79 mm (L)
1mm
Task)
Visual-Motor + Buildup Neurons (Gap
150
N = 113
1mm
Horizontal Dimension [mm]
0
Peak = 151 Spikes/s
FWHM = 1.66 mm
100 Spikes/s
100 Spikes/s
Peak = 38 Spikes/s
FWHM = 2.07 mm
Preparatory Response
0
Buildup Neurons (Gap Task)
100 Spikes/s
1mm
Horizontal Dimension [mm]
B
Vertical Dimension [mm]
Vertical Dimension [mm]
N = 71
Visual-Motor + Buildup Neurons (Gap Task)
Preparatory and Visual Response
C
150
100 Spikes/s
Visual Response
Visual-Motor Neurons (Delay Task)
Saccade Response
A
1mm
Peak = 134 Spikes/s
Half Peak Width = 1.73 mm
1mm
Figure 5.5. Spatial point images of BU visual, TD preparatory, and saccadic motor activity
in the SCi map. A (top panel), Isolated visual response of VMBNs in the delay task for a
10° leftward target stimulus. A (bottom panel) Cross section of the visual response (above)
from along the horizontal meridian. B (top panel), Isolated pre-target preparatory
responses of BUNs in the gap task where the target could appear with equal probability
10° left or right of central fixation. B (bottom panel) Cross section of the preparatory
responses (above) from along the horizontal meridian. C (top panel), Combined visual and
preparatory response of combined BUNs and VMBNs in the gap task for a 10° leftward
target stimulus immediately prior to the suppression of the preparatory activity at 10° right.
C (bottom panel) Cross section of the visual and preparatory response (above) from along
the horizontal meridian. D (top panel), Motor response of MBNs, VMBNs and BUNs in the
gap task for a 10° leftward saccade at movement onset. D (bottom panel) Cross section of
the saccadic motor response (above) from along the horizontal meridian. A-D Dashed
gray lines denote full width of point image at half of maximum activity (FWHM). Dashed
black lines denote interpolated area of rostral SC where no neurons were recorded.
151
immediately prior to the onset of the visual response (which occurred 55 ms following
target presentation, Fig. 5.3D, Fig. 5.4B, Fig. 5.5B). The combined TD and BU response
was calculated from all combined VMBNs and BUNs in the gap task immediately after
the onset of the visual response, but prior to the extinction of the preparatory activity that
had accumulated at the other possible target location (90 ms following target
presentation, Fig. 5.5C). The saccadic motor burst was calculated from all combined
VMBNs and BUNs in the gap task at saccade onset. The size of the TD preparatory, BU
visual and motor point images were determined independently using both the half width
of the peak activity falling along the horizontal meridian (Fig. 5.5A-D lower panels) and
from a fitted 3-dimensional Gausian surface. In some cases these independently
calculated values were slightly different due to some vertical and horizontal asymetry
(Fig. 5.5A-D upper panels)
5.3.5 2-Dimensional Continuous Attractor Neural Field Model
We modeled the neural activity represented within the SCi temporally (relative to
stimulus and saccadic events) and spatially (across the SC map) during saccade
preparation and execution. We interpret the input signals in the model to represent the
physiological activity in the SCi that results from TD preparation/prediction signals and
BU visual responses combining. We implemented a two dimensional fully connected
neural field model where the individual weights between connected nodes were
dependent on their respective distances across the network and the instantaneous amount
of activity within the network. We used a spatial interaction profile that exploited the
local excitation and distal inhibition relationships measured within the retinotopic SC
map (Dorris et al., 2007) that were described and modeled previously (Trappenberg et al.,
152
2001). In all simulations, the onset of spatially distinct external input signals (Fig. 5.6A)
resulted in competitive interactions across the network, which ultimately stabilized via
dynamic winner take all behavior into a single hill of activity that remained stable even
after the external input signals were removed (Fig. 5.6C). The resolution of multiple
signals in the network to a single hill of activity caused a nonlinear rise to threshold and
demonstrates the importance of special interactions within the SC (Fig. 5.6D).
5.3.6 External Inputs
Three different types of external inputs were used to represent the TD and BU
signals that we recorded physiologically. The BU input signal reflected the responses
produced by external visual stimuli. Two different kinds of TD inputs were used to
reflect internally generated saccade related signals that could occur across both SCi. One
TD signal (Fig. 5.6A top panel) represented the spatial and temporal prediction signals in
SCi BUNs that was present prior to the appearance of a saccade target stimulus (Fig.
5.3D, 5.4B, 5.5B) in the gap task (Dorris et al., 1997; Dorris and Munoz, 1998). Another
TD signal (Fig. 5.6A bottom panel) represented an internally generated linear saccade
decision signal similar to that proposed in the LATER model (Linear Accumulation to
Threshold with Ergodic Rate) (Carpenter and Williams, 1995; Reddi and Carpenter,
2000) that influenced saccade selection based on the requirements of the task and the
accumulated TD and BU information available (Schall, 1995; Hanes and Schall, 1996;
McPeek and Keller, 2002). This signal differed from preparatory activity because it
reflected the internally generated decision that the appropriate visual stimuli was present
and should be foveated (Carpenter, 2004). The inclusion of this saccade decision signal
153
B
A
Spatial Input Activation
of External Signals (I ext)
Node A
Pretarget TD
Preparatory Input
C
100%
Resulting Stabilized
Network State (r out)
Network
Activation
Initial Network
State
Node B
Node A
0%
BU Visual Input
Node B
Post target TD
Saccade Decision Input
D
Temporal profile of Local Network Activity
(at Nodes A and B)
100%
Accumulation to
Saccade Threshold
saccade threshold
at Node B (winning location)
at Node A (competing location)
0
Time (t) From Onset of External Input Signal
Figure 5.6. Implementation and functionality of the 2-dimensional neurofield model. A.
Gaussian shaped inputs (representing spatially defined TD and BU signals) are initially
fed into the model. B. The initial network state prior to onset of the TD and BU input
signals. C. Stable hill of activity maintained in the model after dynamic winner-take-all
competition has resolved the input signals. The accumulation of this activity represents
the rise to threshold of the saccadic motor command in BUNs that do not possess a
visual response. D. Activation of a two neural nodes located at the direct center of the
resulting saccadic motor command (node A, black line) and competing location (node B,
gray line) in B and C (white circles).
154
was important in order to incorporate inputs from other visual or oculomotor brain
regions that help to select the saccade target based on internally motivated task related
goals. This enabled the model to choose between multiple TD or BU driven saccadic
goals and not always choose the strongest or most salient BU sensory input signal.
Both the TD and BU external input signals were localized to the retinotopic
positions of the simulated visual stimuli and saccade locations. The temporal dynamics
of these external input signals were set to increase linearly to their maximum activation
over a fixed interval. The BU visual and TD preparatory signals were defined to increase
faster than the TD saccade decision signal. This made intuitive sense because the BU
visual and TD preparation signals are processed quickly once the target stimuli appeared,
while the TD goal driven movement signal should be slower because it reflects a more
cortically driven decision process (See Discussion). This balance ensured that lateral
interactions between the BU and TD signals significantly impacted SRT, but the TD
motor saccadic decision signal influenced which input signal would be selected for the
saccade.
We imposed a linear increasing time course for the external TD preparatory and
BU visual input signals:
(3)
Where I is the maximum amplitude is a constant and ton was the time of signal onset
and
was the time constant for the signal buildup (
155
= 173 ms). The TD
saccadic decision signal that we employed was similar to that proposed in the linear
LATER (Linear Approach to Threshold with Ergodic Rate) model (Carpenter and
Williams, 1995; Reddi et al., 2003; Carpenter, 2004), however in our implementation it
was a separate input signal that influenced the accumulation within the model and did not
reflect the direct output of the model. Like the TD preparatory and BU visual signals, the
TD saccadic decision signal increased linearly; however, it followed a slower rate of rise
and was delayed relative to the pre-target TD preparation and BU visual response to
ensure that there was enough time for lateral interaction to occur between these other
input signals. The TD saccadic decision signal was defined by:
(4)
Where t1 = 104 ms and
= 1041 ms. Although the profile and magnitude of the local
excitation remained constant, the magnitude of the lateral inhibition was dependent on
the instantaneous amount of excitation within the model at any time. This insured that
competing input signals were able to accumulate some activity within the model before
the competing lateral inhibition shut them down. This assumption was based on recent
physiological evidence in mouse SC showing local inhibitory circuitry within the SCi that
followed a slower time course relative to local excitation (Phongphanee et al., 2008).
In simulations 3 and 4 we increased the numbers of BU visual distractors
(simulation 3) or TD goal-related potential saccade locations (simulation 4) from 1 to 2 to
4 to 6 to 8. In these simulations, each time the number of TD or BU signals was
156
increased, the magnitude of the BU or TD signals was correspondingly decreased by 5%.
This successive decrease incorporated the observed decrease in the visual response in the
SC with increasing numbers of BU visual distractors (Basso and Wurtz, 1998; McPeek
and Keller, 2002), as well as the decrease in TD preparatory activity with increasing
numbers of potential saccade target locations (Basso and Wurtz, 1998).
5.3.7 Model Architecture
The interactions between TD and BU signals affected the time required by the
model to accumulate to a single stable hill of activity (Fig. 5.6 C,D). Because the height
of the TD and BU inputs were initially changing linearly over time, the total resulting
input (I) at each node location at any time (t) is given by:
I(t)in = I(t)TD + I(t)BU
(5)
Where all types of external TD or BU input have a Gaussian spatial shape such that the
value of input at node location k is given by:
(6)
Here l is the location of the center of the input signal, Δx is the resolution of the model
(2π divided by the number of neural nodes), and aTD,BU is either a constant (when
constant magnitude of BU inputs are assumed) or dependant on σ (when constant volume
of BU inputs are assumed, simulation 1 width manipulation only). The width of each of
the TD inputs and BU inputs were derived from the corresponding point images on the
SC (Fig. 5.5).
157
The internal dynamics of the model were described previously (Trappenberg et
al., 2001) and only changes from this 2001 implementation are described below. We
utilized a 2 dimensional grid of 101 x101 identical neural nodes with identical local
excitatory and distal inhibitory interaction profiles at each node defined on a negatively
shifted Gaussian. This grid is continuous such that nodes located at the edge wrap around
their connections to the opposite side. A uniformly distributed interaction profile was
used over the surface of the entire model because it was previously reported that visual
and motor point images in the SC map were symmetrical and their size remained
approximately constant and uniform across eccentricity (Marino et al., 2008).
Accumulation to saccade threshold was plotted from the activity of the single neural node
located at the center of the resulting dynamic winner-take-all activation (Fig. 5.6D). This
point represented the simulated activity of a motor neuron in the SCi whose receptive
field was likewise centered at the resulting saccade location (Marino et al., 2008).
5.3.8 Model Parameters
The time course necessary for the model to accumulate to saccade threshold
determined the simulated SRT. Thus all temporal parameters and time constants (
and
) were set in order for the model to approximate the appropriate behavioral
ranges of saccadic latencies reported in humans and monkeys (Boch et al., 1984; Fischer,
1986; Basso and Wurtz, 1998; Marino and Munoz, 2009). Furthermore, the magnitude of
TD and BU input signals in the model were likewise mapped to approximate equivalent
signals in the frequencies of neural action potentials in VMBNs and BUNs in the SCi
(Sparks and Hartwich-Young, 1989; Munoz and Wurtz, 1995a; Marino et al., 2008). We
utilized a width of 0.5 mm as the standard deviation of the Gaussian shaped excitatory
158
interaction profile in order to maintain accuracy and consistency with previous
neurophysiological studies (Munoz and Wurtz, 1995a; Dorris et al., 2007). The maximal
magnitude of distal inhibition was set to balance equally with the maximal magnitude of
the local excitation to ensure that the parameter region of the model would resolve to a
stable memory state, that maintained the activity of the winner-take-all point image after
all external inputs were removed (Kopecz and Schoner, 1995; Kopecz, 1995). We used
an overall scale factor of 13 and an additional afferent delay of 50 ms to convert
simulation cycle times to the rate of experimentally observed reaction times.
5.3.9 Model simulations
Multiple simulations were run utilizing BU and TD external inputs that were
based on VMBN and BUN activity measured within the first 100ms of visual target
appearance (Fig. 5.5). All simulations were set up and run from the theoretical time
epoch when both BU and TD signals were simultaneously present within the SCi map
(Fig. 5.4B epoch b). Within each simulation, the external TD and BU signals increased
linearly to their maximum activation and then were removed once network activity had
stabilized. The maintenance of stable network activation after removal of the external
signals demonstrated that our model utilized a parameter region characterized by
balanced excitation and inhibition that has been argued previously to be of particular
interest for describing the saccadic systems (Kopecz and Schoner, 1995; Kopecz, 1995).
After onset of the TD and BU signals, the internal network dynamics resulted in winnertake-all competition between each external signal. Eventually after several network
iterations, a stable region of activity out competed all others and formed at the network
location coding the chosen saccade vector. The non-linear accumulation of activity at the
159
center of this resultant stable bubble reflected the rise to threshold of the neural saccade
signal. We chose an activation level of 80% (consistent with Trappenberg et. al 2001) of
the peak of the final stable activity to represent the saccadic threshold trigger line as all
other competing saccadic target locations were eliminated at this threshold level.
5.4 Results
5.4.1 Spatial Properties of Visual, Preparatory, and Motor Point Images on
the SC
We required neurophysiological data to constrain the model's parameters and
ensure that it more accurately reflected the spatial patterns of activity in the primate SCi.
Figure 5.5 illustrates point image population activity in the monkey SCi for a BU visual
response (Fig. 5.5A), a TD preparatory response (Fig. 5.5B), a mixed BU and TD
response (Fig. 5.5C), and a saccadic motor response (Fig. 5.5D). The peak of the BU
visual response (Fig. 5.5A) was calculated in the delay task (epoch 2, Fig. 5.3A) when the
visual response was dissociated temporally from saccadic motor activity. In the spatially
organized population of neurons we recorded, the peak visual response occurred 102 ms
after target appearance and resulted in a visual point image with a peak of 151 spikes/s.
The width of each point image population response was calculated independently using 2
separate methods. First, in order to avoid any smoothing errors that could potentially
have resulted from symmetrically mirroring activity above and below the horizontal
meridian (see Methods), we calculated the activity occurring directly along the horizontal
meridian (Fig. 5.5A lower panel). From this activity profile along the horizontal
meridian we calculated the width of the activity at half of the peak. This width was 1.4
160
mm. Second, we also calculated the width from the full 2-dimensional point image by
fitting a Gaussian surface (Euler method) to the data and calculating the standard
deviation (sigma). The sigma for the visual response in Fig. 5.5A was 0.53 mm (mean
residual -0.9).
We measured the peak of the TD pre-target preparatory activity during the gap
task, in the epoch immediately prior to the arrival of the target aligned visual response
(epoch 6, Fig. 5.3B), which is when the peak preparatory activity occurred (Fig. 5.3D,
5.3B). The maximal preparatory response occurred 55 ms after target appearance and
resulted in two point images, one on each side of the SC that were centered at the location
of the 2 equally probable potential target locations (10° left or right, Fig. 5.5B). The
maximum value of the TD preparatory activity was 53 spikes/s. The mean full width at
half maximum calculated along the horizontal meridian was 1.53 mm (right) and 1.79
mm (left) (Fig. 5.5B lower panel). The Gaussian fitted sigma for these two TD responses
were 1.35 mm (mean residual -0.97) (right) and 1.45 mm (mean residual -1.05) (left).
In the gap task, we recorded a brief period when both BU visual and TD
preparatory responses were present within the right SCi and an isolated TD preparatory
response was simultaneously present in the left SCi (Figs. 5.4B, 5.5C). This temporal
overlap occurred because at the time of the earliest part of the visual response the
preparatory activity in the opposite SC had not yet been eliminated. Thus, although the
TD preparatory spatial prediction signal (opposite to the target) preceded the onset of the
BU visual response, it persisted long enough to compete and/or interact with it. The
existence of this physiological epoch is critically important for our model simulations
161
because it justifies the simultaneous input of TD and BU signals into the model in order
to study how their competitive spatial interactions influenced SRT. Within the
population of neurons recorded, the maximum combined BU and TD responses occurred
in the gap task 90 ms after target appearance. This resulted in two point images, one on
each side of the SC that were centered at the location of the target (Fig. 5.5C, right SC)
and the equally likely potential location where the target could have, but did not, appear
(Fig. 5.5C, left SC). Here, the peak of the BU visual activity was 134 spikes/s and the
peak of the TD preparatory activity was 38 spikes/s. The peak TD preparatory activity
illustrated in Fig. 5.5C was reduced relative to Fig. 5.5B because this combined BU and
TD population point image included VMBNs that did not exhibit TD preparatory activity.
The mean full width at half maximum calculated along the horizontal meridian (Fig. 5.5C
lower panel) was 1.73 mm for the BU visual response and 2.07 mm for the TD
preparatory response. The Gaussian fitted sigma for these were 0.67 mm (mean residual
-0.12, right BU visual) and 1.19 mm (mean residual -0.35, left TD preparatory).
Finally, we also measured the height and width of the peak of the saccadic motor
burst from saccade aligned activity in the gap task (Fig. 5.5D). The peak of this saccadic
motor burst was 218 spikes/s. The full width at half maximum calculated along the
horizontal meridian (Fig. 5.5D lower panel) was 1.66 mm. The Gaussian fitted width
was σ=0.54 mm (mean residual 1.7).
5.4.2 Simulation 1. Spatial Interactions Within Single Input Signals
Recent evidence has shown that the magnitude of visual responses of individual
SC neurons are modulated by stimulus properties such as contrast (Li and Basso, 2008)
162
and luminance (Marino et. al., 2011). This suggests that the corresponding magnitude of
these visual response point images were also changing. It is not known, however, whether
the width of the point images on the SC map were also affected by luminance or
contrast. SRTs have been shown to decrease to a minimum with increasing target
luminance (Boch et al., 1984; Doma and Hallett, 1988; Jaskowski and Sobieralska, 2004)
or contrast (Carpenter, 2004; Ludwig et al., 2004; White et al., 2006). However our
recent study (Marino and Munoz, 2009) suggested that SRT initially decreased with
increasing target luminance but then increased again at brighter luminance levels. Here
we assess how changes to the height (magnitude) or width of point images (Fig. 5.2A) in
the model can explain these previous results.
Effects of Input Signal Magnitude: To assess the effect of input signal magnitude
on saccadic latency in the model, we varied the peak magnitude of the input signal across
7 different values that ranged from 65 to 375 spikes/s (Fig. 5.7B) while keeping the width
of the signal constant at σ=0.6mm. These ranges were used to approximate the BU
visual responses that were reported within SCi neurons when luminance or contrast was
manipulated (i.e., from below 50 spikes/s to more than 350 spikes/s), (Li and Basso,
2008; Marino et al., 2011).
When the magnitude of the input was increased (Fig. 5.7 A-C), the time required
by the model to reach saccadic threshold (simulated SRT) decreased (Fig. 5.7C) in a
manner similar to previous behavioral results (Boch et al., 1984; Doma and Hallett, 1988;
Carpenter, 2004; Jaskowski and Sobieralska, 2004; Ludwig et al., 2004; White et al.,
163
D
Effects of Input Signal Magnitude
(Width Constant)
Effects of Input Signal Width
(Magnitude Constant)
2mm
0
Accumulation to
Saccade Threshold
100%
80%
250
0
E
Time to Reach Saccade Threshold
Saccade Trigger Threshold
100%
Accumulation to
Saccade Threshold
B
Activity (Spikes/s)
2mm
C
Time of Saccade Threshold Crossing
80% Saccade Trigger Threshold
300
200
100
50
100 150 200 250 300 350 400
Input Magnitude (Spikes/s simulated)
Input Width
(gaussian sigma mm)
0.4
0.6
0.9
1.2
1.5
1.8
2.2
0%
100
150
200
250
Time from Simulated Input Onset (ms)
F Time of Intersection With Saccade Threshold
Simulated SRT (ms)
(Magnitude Constant)
400
250
Time to Reach Saccade Threshold
Input Magnitude
(spikes/sec)
65
100
125
150
225
275
375
0%
50
150
250
350
450
Time from Simulated Input Onset (ms)
Simulated SRT (ms)
Activity (Spikes/s)
700
325
300
275
Increased Inhibition
Standard Inhibition
Simulated SRT (ms)
(Volume Constant)
A
600
500
250
400
225
300
200
200
175
0.2
0.6
1.0
1.4
1.8
Width of Input (mm simulated)
100
2.2
Figure 5.7. Simulations of the effects of magnitude (A-C) and width (D-F) of a single
input signal on modeled SRT. A,D. Examples of strong (375 spikes/s, A:left panel) and
weak (65 spikes/s, A: right panel) input signal magnitudes, and wide (σ=1.5mm, D:left
panel) and narrow (σ=0.4mm spikes/s, D:right panel) input signal widths modeled. B,E.
Accumulation to saccade threshold of a neural node centered at the spatial dynamic
winner-take-all network location coding the resulting saccade over a range of signal
input magnitudes (B) and widths (E). C,F. Modeled SRT for each input signal
magnitude (C) and width (F) tested based on saccadic threshold crossing time. Solid
and dotted black lines denote effects of signal width (with constant magnitude) on SRT
(F). Dotted black line denotes the effect of increasing global inhibition relative to the
solid black line. Gray data points in F denote effects of changing width while keeping
volume of activation constant (i.e. signal magnitude decreases with increasing width to
maintain constant volume).
164
2006). At low magnitude (65 spikes/s), simulated SRT was 383 ms, whereas at high
magnitude (375 spikes/s), simulated SRT decreased to 133 ms.
Effects of Input Signal Width: To date, no study has examined whether altered
stimulus properties can affect the size of BU visual point images in the SC, therefore we
examined how input signal size influenced SRT in our model (Fig. 5.2A). This is an
important simulation because we hypothesized that increases in activation area could
cause both decreases or increases in SRT depending on the area of the SC map activated.
This possible relationship between the size of SCi point images and SRT could explain
our previous finding that SRT can decrease or increase with increasing luminance
(Marino and Munoz, 2009).
To assess the effect of signal width on saccade latency independently, we
manipulated the width of the Gaussian shaped input while keeping the peak magnitude
constant (150 spikes/s, a physiological range that matched the peak of our isolated visual
response point image in Fig. 5.5A). We systematically altered the width (σ) of the input
from 0.4 mm to 2.1 mm (Fig. 5.7 D-F) in order to insure that we did not exceed the upper
or lower width range of our recorded BU or TD point images by more than 70% (see Fig.
5.5). We did not alter the width beyond these values because larger or smaller values
would probably be too far removed from realistic physiological ranges in the SCi (range:
from 0.54 mm for the motor burst to 1.45 mm for the preparatory build up). When the
width was increased from 0.4 mm to 2.1 mm, simulated SRT decreased from 230 ms
(σ=0.4mm) to 189 ms (σ=1.2 mm) and then increased again up to 200 ms (σ=2.1 mm,
black line in Fig. 5.7E,F). This resulted in a u-shaped SRT function when width was
165
increased and the magnitude of the input signal was kept constant. The initial decrease in
SRT that we observed resulted from increases in the width dependant volume of the input
signal. However as the width of the signal increased beyond the excitatory width of the
interaction profile within our model (Fig. 5.2A right panel), the surrounding inhibition
became activated and caused an 11 ms increase in SRT when the width was increased
from 1.2 to 2.1 mm. When the external inhibition was increased by 20% (achieved by
globally shifting the interaction profile more negatively, see Methods), the SRT increase
with increasing signal width was 30ms. Although this relationship follows our initial
prediction, the increase in SRT did not occur for all signals that were wider than the
excitatory interaction profile. In our model, we only observed this increase in SRT when
the input width was between 240%-278% above that of the excitatory interaction profile.
By changing the width (while keeping the magnitude of the signal constant), we made the
assumption that the overall volume of the signals were changing.
Constant Volume Hypothesis: It is possible that the magnitude of point images
does not remain constant but that the volume of the overall activity (regardless of its
width or magnitude) might remain constant across different conditions. If this is the case,
a constant volume of neural activity would require normalization between the width and
the peak. Thus any increase in signal width would result in a corresponding decrease in
its peak magnitude to ensure that the overall activity (volume) integrated over the SCi
map remained constant.
In order to examine this constant volume assumption, we manipulated signal
width without the assumption of constant magnitude. In these simulations SRT increased
166
approximately linearly with increasing signal width from 124 ms (σ=0.4mm) to 653 ms
(σ=2.1 mm) (Fig. 5.7F solid gray line). Because the constant volume condition resulted
in decreases in peak magnitude with each increase in signal width, the observed increases
in SRT were most strongly influenced by these corresponding decreases in peak
magnitude.
Simulation 1 Summary: Our model predicted that any changes to the magnitude or
the width of point images within the SCi map should influence SRT. As the magnitude
of the input signal was increased, SRT decreased. As the width of the input signal was
increased, SRT either increased or decreased then increased, respectively depending on
whether a constant magnitude or constant volume signal was used. Based on these
results, the decrease of SRT with increasing target luminance (Boch et al., 1984; Doma
and Hallett, 1988; Jaskowski and Sobieralska, 2004) or contrast (Carpenter, 2004;
Ludwig et al., 2004; White et al., 2006) previously reported likely results from increases
to the magnitude (Li and Basso, 2008; Marino et al., 2011), of the peak visual response in
the SC. The model also predicted that changes to the spatial width of input signals also
affected SRT such that increasing the spatial width decreased SRT until it was wide
enough to extend beyond the region of local excitation and into inhibitory regions of the
map (Fig. 5.2A). The decreases followed by increases in SRT with increasing luminance
that we previously reported (Marino and Munoz, 2009) could be explained by increases
to the width of visual point images in the SCi.
167
5.4.3 Simulation 2. Effects of Spatial Signal Distance
It has been reported previously that saccades to two targets elicit a greater
proportion of short-latency express saccades when the targets are closer together (i.e.,
located within 45° of each other) (Edelman and Keller, 1998). This result suggests that
SRT can be reduced when competing point images are close enough together to overlap.
In our model, the distance between point images similarly determines if they will overlap
and summate or compete over a larger distance. We examined how the spatial distance
between TD or BU related saccade signals in the model interacted to influence SRT.
Figure 5.8 shows how SRT was influenced by the spatial distance between 2 distinct
input signals of equal size. When the magnitude of the input signals matched the
magnitude of the isolated visual response point image (150 spikes/s, σ = 0.6mm Fig.
5.5A), SRT increased with increasing distance between the nodes up to 1.2-2.4 mm (Fig.
5.8B,C). Further increases in distance had no effect on SRT. In order to determine
whether this effect of distance was dependant on the strength or magnitude of the 2
competing signals, we also examined weak magnitude (65 spikes/s, which approximated
the lower frequency top-down preparatory signals that we recorded) and strong
magnitude (375 spikes/s, which approximate the highest frequency discharge of
visuomotor burst neurons that we recorded of which 23% had a peak visual response
above 350 spikes/s in the delay task) signals independently. As the magnitude of the 2
competing signals decreased, the influence of distance between the signals increased in
the simulations (Fig. 5.8B,C). Specifically, at an input magnitude of 65 spikes/s, the
increase in SRT between pairs of signals located 0.5mm apart and 2.4 mm apart was
162ms (0.5 mm: 237 ms, 2.4 mm: 399 ms, Fig. 5.8C light gray circles). At an input
168
A
Effects of Distance Between 2 Input Signals
2mm
0
B
250
Time to Reach Saccade Threshold
100%
Accumulation to
Saccade Threshold
Activity (Spikes/s)
Saccade Trigger Threshold
80%
Input Signal Distance
(mm)
0.5
0.59
0.71
0.89
1.2
2.4
6
0%
50
100
150
200
250
300
Time from Simulated Input Onset (ms)
C
Time of Saccade Threshold Crossing
Simulated SRT (ms)
400
300
Weak signal strength (65 spikes/sec)
Medium signal strength(150 spikes/sec)
Strong signal strength(375 spikes/sec)
200
100
0
2
4
6
Input Signal Distance (mm simulated)
Figure 5.8. Simulations of the effects of distance between two single input signals on
modeled SRT. A. Examples of distant (6 mm, A:left panel) and nearby (0.89 mm, A:
right panel) input signal distances. B. Accumulation to saccade threshold of a neural
node centered at the spatial dynamic winner-take-all network location coding the
resulting saccade over a range of signal input distances. C. Modeled SRT for each
input signal distance (weak magnitude: black line, 65 spikes/s, medium magnitude: dark
gray line, 150 spikes/s, and strong magnitude: light gray line, 375 spikes/s) tested
based on saccadic threshold crossing time.
169
magnitude of 375 spikes/s the increase in SRT between pairs of signals located 0.5 mm
apart and 2.4 mm apart was only 41 ms (0.5 mm: 109 ms, 2.4 mm: 150 ms, Fig 5.8C
light black circles).
Simulation 2 Summary: The model predicted that overlapping point images (both
TD and BU) reduced SRT compared to non-overlapping point images because
overlapping point images summated to reduce the time to accumulate to saccade
threshold (Fig. 5.2B). Therefore the distance between visual stimuli or potential saccade
target locations in the visual field significantly influenced SRT. Furthermore, the
influence of spatial distance between input signals on SRT was strongest for weaker
signals as these require more time to accumulate to saccade threshold and were thus
more susceptible to be influenced by such spatial interactions. This result supports a
previous study that showed increases in short latency saccades when multiple target
stimuli were presented within 45° of each other (The approximate angular width of a BU
visual point image in the SC) (Anderson et al., 1998; Edelman and Keller, 1998; Marino
et al., 2008).
5.4.4 Simulation 3. The Effects of the Number of competing BU Signals
During visual search experiments, a target must compete with distractor stimuli
that are simultaneously present on the retina and compete for foveation by the saccadic
system (Schall and Thompson, 1999). Previous studies of visual search have
demonstrated that the addition of distractors increases SRT compared to when only one
target stimulus is present (McPeek and Schiller, 1994; McPeek and Keller, 2001; Arai et
al., 2004). Further increasing the number of distractors, however, has been shown to both
170
increase and decrease SRT. Increases in SRT with increasing distractors ("set-size
effect") has been suggested to result from increases in the time needed to find a target
among increasing numbers of distractors (Carrasco and Yeshurun, 1998; Shen and Pare,
2006; Balan et al., 2008; Cohen et al., 2009). However other visual search experiments
have instead shown that increasing numbers of distractors can also decrease SRT without
sacrificing accuracy (McPeek et al., 1999; Arai et al., 2004). This reverse of the "set-size
effect" has been hypothesized to be related to a BU grouping process that shifts attention
to the target more quickly when distractor densities are greater (Bravo and Nakayama,
1992).
In order to determine whether these differing effects of distractor number on SRT
could be explained by spatial competition within our model, we examined the effects of
1, 2, 4, 6, or 8 competing BU signals. We utilized magnitude and width profiles for the
target and distractor stimuli that matched those of the isolated visual response point
image in the SCi that we recorded in the delay task in order maximize physiological
accuracy (Fig. 5.3A, Fig. 5.5A). In the simulations, the BU signals were placed at equally
spaced locations around a circle that was centered at the midpoint of the model. This
midpoint represented the center of the foveal region in the SCi, located between the
rostral poles of each colliculus (Robinson, 1972; Munoz and Wurtz, 1993a; Krauzlis,
2003). Even numbers of signals were mirrored across the vertical meridian to ensure
symmetry and equal weighting between the left and right SC (Fig. 5.9A,D). Because the
simple distance between input signals alone was shown to affect our simulated SRTs
significantly (Fig 5.8), we examined the effects of multiple BU signals at two
independent distances across the left and right SC: far (6 mm diameter circle) and near
171
Effects of Number of Competing Exogeneous Signals
A
2mm
0
B
0
Number of
Inputs
1
2
4
6
8
0%
100
150
200
250
300
Time from Simulated Input Onset (ms)
Time of Saccade Threshold Crossing
E
F
280
260
240
220
1
2
4
6
Number of Input Signals
250
80% Saccade Trigger Threshold
Number of
Inputs
1
2
4
6
8
0%
100
150
200
250
300
Time from Simulated Input Onset (ms)
Time of Saccade Threshold Crossing
300
280
260
240
220
200
8
Activity (Spikes/s)
Time to Reach Saccade Threshold
100%
Simulated SRT (ms)
300
Simulated SRT (ms)
2mm
250
Saccade Trigger Threshold
200
0
(Near)
Accumulation to
Saccade Threshold
Accumulation to
Saccade Threshold
80%
Activity (Spikes/s)
Time to Reach Saccade Threshold
100%
C
D
(Far)
0
1
2
4
6
Number of Input Signals
8
Figure 5.9. Simulations of the effects of number of competing BU inputs both nearby
(A-C) and far (D-F) from central fixation on modeled SRT. A,D. Examples of individual
(A,D:left panel) and multiple (six inputs, A,D: right panel) BU input signals at distant (3
mm from central fixation, A:left panel) and nearby (1 mm from central fixation, D:right
panel) locations within the network. B,E. Accumulation to saccade threshold of a neural
node centered at the spatial dynamic winner-take-all network location coding the
resulting saccade over a range of signal numbers for distant (B) and nearby (E) signals.
C,F. Modeled SRT for each number of input signals for distant (C) and nearby (F)
targets.
172
(0.67 mm diameter). The far distance ensured that each BU signal remained spatially
distinct when up to 8 signals were simultaneously presented (Fig. 5.9A). The near
distance ensured that the multiple BU signals would be close enough together to have
overlapping point images of activity that would interact via summation (Fig. 5.9D).
When distant BU inputs were used, simulated SRT increased linearly with increasing
numbers of competing targets from 206 ms (1 input) to 295ms (8 inputs) (Fig. 5.9B,C).
In contrast, when nearby BU inputs were used (Fig. 5.9 E,F), simulated SRT increased
linearly with increasing numbers of competing targets from 1 (206 ms) to 2 (221 ms) to 4
(240 ms) targets, but then decreased from 4 to 6 (230 ms) to 8 (216 ms) targets. The
initial increase in SRT up to 4 signals, resulted from increased competition from
relatively few targets; however, when sufficiently large numbers of BU signals were
present, the activity from each signal summated together to increase network excitability
over a larger area of the model and this resulted in an overall reduction of SRT.
Simulation 3 Summary: The model predicted that increasing the number of visual
stimuli increased SRT when the corresponding point images in the SC were spatially
separate, but decreased SRT when the point images overlapped (Fig. 5.2B). This suggests
that different "set-size effects" can result, depending upon the distance between the point
images of the target and multiple visual distractors. This simple result can explain the
apparent discrepancies between classical "set-size effects" (where SRT increases with
increasing numbers of distractors) (Carrasco and Yeshurun, 1998; Balan et al., 2008;
Cohen et al., 2009) and other studies which show decreases in SRT (with corresponding
increases in saccade accuracy) with increasing numbers of visual distractors (McPeek et
al., 1999; Arai et al., 2004). It should be acknowledged, however, that this simulation
173
utilized identical BU point images to reflect both the target and the distractor stimuli. In
many visual search tasks the visual features and/or saliency of the target is different from
the distractors. In these situations the BU point images for the target and distractors
would also be of different sizes which would also influence the SRT predicted by the
model. The addition of these parameters could enable the model to also account for
additional differences in SRT due to salient target pop-out within dense fields of
distractors.
5.4.5 Simulation 4. Effects of number of competing TD Signals
In addition to the competition that can occur between multiple BU stimuli on the
retina, multiple TD signals can also be present in the SCi simultaneously (when
foreknowledge of task related goals exists) to influence SRT and chosen endpoint of the
resulting saccade (Basso and Wurtz, 1998; Dorris and Munoz, 1998; Story and Carpenter,
2009) (Fig. 5.5B,C). The effects of TD spatial target probability on SRT have been
studied previously and many seemingly contradictory results have been reported.
Specifically, decreasing spatial target predictability has been shown to influence SRT in
many different ways including: no change (Kveraga et al., 2002; Kveraga and Hughes,
2005), increases (Basso and Wurtz, 1998; Story and Carpenter, 2009), decreases
(Lawrence et al., 2008), and U-shaped increases and then decreases (Marino and Munoz,
2009). Although these results appear to be in conflict, we show how each of them can be
reproduced by altering the spatial properties of the underlying TD and BU signals (size,
magnitude, distance apart, and number of signals).
174
Figure 5.5C demonstrated that both BU and TD signals were present in the SCi
map simultaneously and we recreated this epoch to explore how the spatial competition
between these different signals influenced SRT. We set up simulations with 1, 2, 4, 6, or
8 TD input signals and set the magnitude and width of these signals to match the
corresponding point images that we recorded in the SCi (Fig. 5.5 B,C). In addition to the
TD signals, we also included one BU signal (with magnitude and width matched to the
visual response point image in the delay task Fig. 5.5A) to mimic the single resulting
visual input generated by target appearance. All simulations were tested independently at
two distances: far (6 mm diameter circle) and near (0.67 mm diameter circle). The far
distance (6 mm diameter) ensured that each BU and TD signals were spatially distinct
when up to 8 were simultaneously presented (Fig. 5.2B left, 5.10A)and the near distance
(0.67 mm) ensured that these signals were close enough together to have overlapping
activity (Fig. 5.2B right, 5.10D).
Results were generally similar to simulation 3. When distant TD inputs were used,
simulated SRT increased approximately linearly with increasing numbers of competing
TD signals from 203 ms (1 TD input) to 293ms (8 TD inputs) (Fig. 5.10A-C). However,
when nearby TD inputs were used, simulated SRT decreased approximately linearly with
increasing numbers of competing targets from 203 ms (1 TD input) to 139ms (8 TD
inputs) (Fig. 5.10D-F). Because the TD signals were wider than the BU signals (σ=0.6
mm vs σ=1.42 mm; see Fig. 5.5), their activity summated together with as few as 2
signals (in contrast to the 6 signals required for the BU condition in simulation 3) to more
easily increase network excitability over a larger area and thereby reduce SRT.
175
Effects of Number of Competing Endogeneous Signals
A
2mm
0
B
2mm
250
0
Saccade Trigger Threshold
80%
(Near)
E
0%
50
100
150
200
250
300
Time from Simulated Input Onset (ms)
Time of Saccade Threshold Crossing
0%
F
Simulated SRT (ms)
Simulated SRT (ms)
Number of
Endogeneous
Inputs
1
2
4
6
8
50
100
150
200
250
300
Time from Simulated Input Onset (ms)
Time of Saccade Threshold Crossing
300
300
260
220
180
140
100
250
Saccade Trigger Threshold
80%
Number of
Endogeneous
Inputs
1
2
4
6
8
Activity (Spikes/s)
Time to Reach Saccade Threshold
100%
Accumulation to
Saccade Threshold
Accumulation to
Saccade Threshold
Activity (Spikes/s)
Time to Reach Saccade Threshold
100%
C
D
(Far)
0
1
2
4
6
Number of Input Signals
260
220
180
140
100
8
0
1
2
4
6
Number of Input Signals
8
Figure 5.10. Simulations of the effects of number of competing TD inputs on modeled
SRT for signals located nearby (A-C) and far (D-F) from central fixation (1 BU signal
representing the appearance of the visual target was always presented at the resulting
saccade location). A,D. Examples of individual (A,D:left panel) and multiple (six inputs,
A,D: right panel) BU input signals at distant (3 mm from central fixation, A:left panel)
and nearby (1 mm from central fixation, D:right panel) locations within the network. B,E.
Accumulation to saccade threshold of a neural node centered at the spatial dynamic
winner-take-all network location coding the resulting saccade over a range of signal
numbers for distant (B) and nearby (E) TD signals. C,F. Modeled SRT for each number
of TD input signals for distant (C) and nearby (F) targets.
176
Simulation 4 Summary: The model predicted that increasing the number of TD
pre-target signals could increase or decrease SRT depending on the amount of overlap of
their point images in the SCi map. Specifically, when the number of nearby TD potential
target locations increased, the corresponding point images overlapped leading to reduced
SRT via excitatory summation. In contrast, when the number of distant TD potential
target locations increased, the corresponding point images remained separate leading to
increased SRT via lateral inhibitory competition (Fig. 5.2B) .This mechanism can explain
the complex results of previous studies that have shown no change (Kveraga et al., 2002;
Kveraga and Hughes, 2005), increases (Basso and Wurtz, 1998; Story and Carpenter,
2009)), decreases (Lawrence et al., 2008), or increases followed by decreases in SRT
(Marino and Munoz, 2009) with increasing numbers of potential saccade target locations.
Each of these different results can be explained by this model via the spatial interactions
(i.e. magnitude, width and the relative amount of overlap) of their underlying point
images.
5.5 Discussion
Here, we presented a new two dimensional neural field model of the SCi that can
explain many previous complex and contradictory results regarding how BU or TD
signals converge to influence SRT via the spatial interactions of their point images. We
demonstrated that the spatial properties (magnitude, width, distance apart, or number of
competing signals) of the underlying TD or BU signals on the SCi map influenced SRT.
We presented new neurophysiological data, recorded from neurons in the SCi that were
used to calculate point image size and constrain the model parameters. These
177
physiological point images represented isolated BU visual responses, TD preparatory
activity, and saccadic motor bursts that were spatially organized within the SCi map. BU
visual response point images had greater magnitude and a narrower width relative to TD
preparatory point images. During the initial part of the visual response to an appearing
saccade target stimulus, TD preparatory activity can persist briefly within the SCi map
wherever visual targets were expected to appear. This indicates that winner-take-all
competition does take place between TD and BU signals within the SCi map. Our model
demonstrates how during this period of overlap, TD and BU signals compete to
determine where the next saccade will be directed. The model made novel and
experimentally supported predictions about the properties and spatial interactions of TD
and BU signals in the SCi.
The model demonstrated several simple principles that govern how spatial signals
(BU or TD) interact within the SCi map to influence SRT: For individual input signals
(BU or TD), increasing the peak magnitude decreased SRT, while increasing the width
decreased SRT until it spread beyond local excitatory distances into inhibitory ranges,
which then caused SRT to increase (Fig. 5.2A). Increasing the number of spatially
separate input signals (TD or BU) increased SRT when the point images were spatially
distant in the map and decreased SRT when these point images were close enough to
overlap and summate (Fig. 5.2B). Thus the model demonstrates how the spatial
interactions of individual or multiple TD and BU point images can influence visually
guided saccade behavior.
178
5.5.1 An Expansion of Linear Saccade Accumulator Models
Linear accumulator models remain a popular and useful framework for describing
the neural processes underlying saccade initiation. One such model (LATER: Linear
Approach to Threshold with Ergodic Rate) assumes that a saccadic decision signal
increases linearly until a neural threshold is crossed and a saccade is triggered to a
specific location (Carpenter and Williams, 1995; Hanes and Schall, 1996; Munoz and
Schall, 2003; Carpenter, 2004). Neural correlates of this accumulation signal have been
identified within neurons located within both the FEF (Hanes and Schall, 1996; Everling
and Munoz, 2000), and the SCi (Dorris et al., 1997; Dorris and Munoz, 1998; Paré and
Hanes, 2003). Previously, this model has been used successfully to describe a simplified
neural framework that can explain a wide range of SRT variations including: speed
versus accuracy trade-offs (Reddi and Carpenter, 2000), contrast and probability
(Carpenter, 2004), task switching (Sinha et al., 2006), reading (Carpenter and McDonald,
2007), and the gap effect (Story and Carpenter, 2009). However, the limitations of
LATER are evident whenever the effects of TD or BU experimental manipulations on
SRT are non-linearly related such as the complex increases and decreases in SRT we
have explained here via lateral interactions. In the current study, we used linear input
signals to represent each individual TD and BU signal within the model. In particular, a
TD saccade decision signal was required by the model in order to reinforce the specific
BU sensory or TD task-related signal that represented the appropriate saccade target goal.
This ensured that the correct visual stimulus was chosen over the visual distractors or
other potential target locations. This TD saccadic decision signal is conceptually very
similar to the decision signal described by the LATER model. By incorporating a linear
179
accumulating decision signal within our neural field, we utilized an underlying neural
mechanism that was conceptually similar to LATER, however, we extended its predictive
power into the non-linear domain. This enabled our model to be robust enough to explain
the non-linear and apparently contradictory behavioral results from previous studies of
TD and BU influences on SRT. This additional explanatory and predictive power was
achieved via the spatial interactions of competing point images that resulted from the
lateral interactions that are hypothesized to be present within the SCi map (Munoz and
Istvan, 1998; Munoz and Fecteau, 2002; Dorris et al., 2007).
5.5.2 Oculomotor Violations of Sensorimotor Transformation Laws
Simple laws govern the basic effects of BU and TD processes on motor response
latencies across sensory modalities. Hick’s law states that response latencies increase as a
log function of the number of possible choice response alternatives (Hick, 1952). Here,
we have modeled several seemingly contradictory studies that have shown agreement
with (Basso and Wurtz, 1998; Lee et al., 2005; Thiem et al., 2008) and violations of
(Kveraga et al., 2002; Kveraga and Hughes, 2005; Lawrence et al., 2008; Marino and
Munoz, 2009) Hick’s law. Our simulations have demonstrated that the degree of overlap
between potential saccade target locations within the SCi map can lead to both agreement
with (separate point images compete and increase SRT) and violations of (overlapping
point images summate and reduce SRT) Hick’s law in the oculomotor system.
Pieron’s law mathametically describes a hyperbolic decay function between
stimulus intensity and response latency such that reaction time logarithmically decreases
with increasing stimulus intensity regardless of the sensory modality (Pieron, 1952).
180
Manipulating BU target luminance decreased and then increased SRT with increasing BU
target luminance in violation of this law (Marino and Munoz, 2009). The model revealed
that the spatial interactions of individual BU visual signals within the SC map can
produce violations of Pieron’s law whenever the width of the BU point image increases
with increasing stimulus intensity until it is large enough to cause SRT to increase. Thus,
the interactions of BU and TD point images in the SC map resulting from local excitation
and distal surrounding inhibition predict a potential neural mechanism that can account
for violations of Hick's and Pieron's laws within the oculomotor system. These
mechanisms may be unique within the visuo-saccadic modality and thus may not
translate across other sensory modalities or non oculomotor motor responses. Future
research will be required to test these assumptions.
5.5.3 Intrinsic versus extrinsic sources of inhibition in the SC
Previous neural field models of the SC have assumed the existence of lateral
inhibition that is intrinsic to the local SC circuit that results in winner-take-all behavior
(van Opstal and van Gisbergen, 1989; Massone and Khoshaba, 1995; Grossberg et al.,
1997; Bozis and Moschovakis, 1998; Arai et al., 1999; Trappenberg et al., 2001; Badler
and Keller, 2002). This assumption was initially supported by evidence from an in vivo
electrical stimulation study (Munoz and Istvan, 1998) and an in vitro pharmacological
study (Meredith and Ramoa, 1998) that showed evidence of long-range inhibitory
connections in the SC. However, later evidence from in vitro SC slices (parasagital or
coronal) only showed evidence for very restricted short range inhibition (Lee and Hall,
2006). Furthermore, an in vivo microinjection study in the SC (cholinergic agonist
181
nicotine ) did not produce long range inhibitory effects on saccade performance
(Watanabe et al., 2005). This led Arai and Keller (2005) to eliminate all intrinsic lateral
inhibition from their model and instead it relied entirely on extrinsic lateral inhibition that
was input into their model. This external inhibition was assumed to originate from the
substantia nigra pars reticulata, a basal ganglia output structure that projects GABAergic
inhibition into the SCi (Hikosaka, 2007). More recently, studies have provided evidence
for long-range lateral interactions within the SCi. Firstly from an in vivo SC study that
simultaneously presented proximal and distal visual targets and distractors to awake
behaving monkeys performing visually driven saccades (Dorris et al., 2007). This study
demonstrated spatially dependent lateral interactions (local excitation and distal
inhibition) between targets and distractors, however, this study could not dissociate
between intrinsic and extrinsic sources of lateral inhibition. A recent in vitro study of
novel horizontal slice preparations of mouse SCi (which preserved the lateral circuitry
across SCi map) has demonstrated long range lateral inhibitory and excitatory synaptic
connections that were intrinsic to the SCi (Phongphanee et al., 2008). Based on this
evidence, we assume the existence of at least some of intrinsic lateral inhibition in the SC
circuitry that can result in the winner-take-all behavior that we have modeled.
The lateral interactions in our model were a simplified combination of both the
intrinsic (originating within the SCi) and extrinsic inhibitory signals. We acknowledge
that the interactions between these two types of inhibition are likely more complex as the
relative levels of each could be modulated by differing amounts of dynamic BU and TD
related inhibition from multiple external structures including the basal ganglia in addition
to the activity dependent inhibition from within the SC itself. However, such interactions
182
(wherever they are), can be utilized within an organized neural field to explain the
different and apparently contradictory behavioral findings previously reported. This
highlights the potential power and versatility of such simplified principles of lateral
competition within a neural winner-take-all mechanism.
5.5.4 Conclusions
The spatial interactions between point images (i.e. size, shape, location and overlap
of TD and BU signals) in the SCi are the critical factors that govern the latency of the
visuomotor transformations that underlie visually guided saccades. By exploiting these
spatial relationships within a 2-dimensional neural field that resembles the underlying
physiological map, we accurately reproduce changes in SRT that result from TD and BU
experimental manipulations.
183
Chapter 6: General Discussion
184
This thesis has provided multiple novel insights into how the oculomotor system
transforms visual stimuli into saccade targets. This was achieved by presenting four
individual research studies of visuomotor transformations in the superior colliculus of
non-human primates. Each of these studies followed a logical progression from the
sensory input to the motor output. First the spatial alignment of visual and motor
response fields were described. Next the stimulus driven modulation of visual sensory
responses were examined and used to guide the development of a neural network model
of the SC capable of simulating both sensory input and motor output during visually
guided saccades. The general hypothesis and major findings of each research project are
summarized below within the context of each of the three main objectives of this thesis.
6.1 Summary of Objectives and Major Findings
The first objective was to assess the correspondence between visual and
motor maps within the SCi. In chapter 2 I described the relationships between these
maps both in visual field space and within the logarithmically organized physiological SC
map. I recorded from neurons in the SCi that discharged both visual and motor responses
and compared their response fields in simple visual coordinates as well as within a
mathematical representation of the SC map (Van Gisbergen et al., 1987). This study
revealed that the visual and motor maps in the SCi are well aligned. Furthermore visual
response fields tended to be smaller and completely encapsulated within motor response
fields. When examined as a population on the SCi map, these responses were
approximately the same size no matter where they were located (i.e. rostral or caudal). In
185
contrast, these responses increased in size with increasing eccentricity when described in
untransformed visual field coordinates. Together these results demonstrate a critical link
between the visual input and motor output systems in the nervous system during visually
guided saccades. Furthermore, the computational consequences of transforming response
fields onto the logarithmic SC map result in over representing foveal regions while
compressing para-foveal regions in order to guarantee that equally sized areas of neural
tissue are always recruited across within the SC.
The second objective was to examine how external stimulus properties can
modulate visual processing in the SC and to determine how these modulations
influence saccade behavior. In chapter 3 and 4 I recorded from neurons in the SCs and
SCi while monkeys performed visually guided saccades to targets of varying luminance.
This BU manipulation of luminance modulated may properties of the visual response as
well as influenced the saccade behavior. These studies revealed how luminance can alter
multiple properties of the visual response including the peak magnitude, timing, as well
as the growth and decay rate of the sensory signal. These modulations to the sensory
response were also shown to be important to the underlying neural mechanisms
computing the sensory to motor transformation since they influenced multiple aspects of
saccade behavior (including SRT and movement metrics). In chapter 4 I demonstrated
that these luminance induced modulations to the visual response also influenced the
latency and prevalence of express saccades which were always generated at latencies that
approached the minimum neural conduction delays. Here any luminance modulated
change to the visual response directly influenced the motor command that was relayed to
the brainstem burst generator (PPRF) to move the eyes because during an express
186
saccade these two responses merged into one (Edelman and Keller, 1996; Dorris and
Munoz, 1998). The changes in express saccade latency I observed were correlated with
the luminance modulated changes to the arrival time of the visual sensory response in the
SC. The changes to express saccade prevalence were partially correlated with luminance
modulated changes in visual response magnitude as well as the accumulated preparatory
build-up activity. Thus modulations to visual sensory responses strongly influence both
regular and express saccade behavior in a robust and reliable fashion.
The third objective was to apply our findings to the development of a neural
network model capable of simulating visuomotor transformations within the SC. In
Chapter 5 I described a two dimensional neural field model that simulated SRTs to BU
visual and TD predicted/anticipated targets. This model simulated the sensorimotor
transformation underlying saccades as a non-linear accumulation to threshold
mechanism. The parameters used to define the size and shape of the TD and BU input
signals were based on neural data recorded from the SCi in order to make them as
physiologically realistic as possible. The local excitatory and distal inhibitory lateral
interactions employed within this SC model exploited evidence from recent experimental
studies that observed this mechanism physiologically within the SCi (Dorris et al., 2007;
Phongphanee et al., 2008). In the model, these lateral interactions affected SRT due to the
resulting competition or excitatory summation between individual or multiple TD and
BU signals depending on their size or relative position within the SC map. Our
simulations demonstrated that these lateral interactions could account for many
previously published contradictory findings of how TD and BU manipulations can
influence SRT (See chapter 5 for details). These simulations predicted how SRT could
187
either decrease or increase depending on the size, distance apart or overlap between BU
or TD signals on the two dimensional SCi map. Thus, the model predicted that the
spatial interactions of input signals that represent expected (TD) or actual stimuli (BU)
are critical determinants of the overall latency required by the oculomotor system to
compute saccadic sensorimotor transformations.
6.2 Unanswered Questions
Although this thesis has contributed insight into some of the building blocks
underlying sensorimotor transformations for saccades, many unanswered questions still
remain. One particularly relevant question is how does the initial part of the phasic
visual response contribute to a saccade accumulation signal during regular latency
saccades if at all? This remains especially puzzling because the burst of action potentials
related to a visual sensory response is temporally separated and distinct from the
subsequent motor response burst during regular latency saccades (See Chapter 3). It is
still unclear how this signal accumulates to a saccade triggering threshold between the
visual and motor responses as is assumed in many popular linear (Carpenter and
Williams, 1995; Hanes and Schall, 1996; Munoz and Schall, 2003; Carpenter, 2004) and
non-linear (Arai et al., 1994; Kopecz and Schoner, 1995; Trappenberg et al., 2001; Arai
and Keller, 2005) accumulator models of saccades (including the model presented in
Chapter 5).
Some physiological evidence for an accumulating saccade signal has been
previously observed in both the SCi (Paré and Hanes, 2003) and FEF. (Hanes and Schall,
1996) Although these observations provide some physiological validation for
188
accumulator model assumptions, this evidence is limited as the underlying data was
recorded during a countermanding saccade task where subjects initiated or aborted
saccades to a target depending on the appearance of a stop stimulus. Relative to the
simple gap, step, and delayed saccade tasks employed in this thesis, the countermanding
task produces longer and more variable SRTs and does not any evoke express saccades.
This happens because during the countermanding task, stop and go signals are
unpredictably mixed and additional neural voluntary control mechanisms need to be used
to perform the task correctly (Chikazoe et al., 2009). These additional neural control
mechanisms are not required during straightforward saccades to visual targets in classic
gap, step and delayed saccade tasks. Thus some evidence does exists for an accumulating
signal in the SC sub-serving sensorimotor transformations; however, compelling
evidence from simple visually guided saccade tasks (like those recorded and modeled in
this thesis) has still not yet been demonstrated.
The findings in chapter 3 suggest that in V and VM populations, the simple signal
properties of the earliest part of the visual response are strongly predictive of the resulting
saccadic behavior. This result is puzzling given that accumulator models assume that the
saccade signal should progressively increase in influence between the sensory input and
motor output. This might indicate that accumulator models are a more accurate
depiction of neural activity rising to threshold during express saccades when the
accumulating saccadic decision signal and the early part of the visual response are
merged into the same signal (Edelman and Keller, 1996; Dorris and Munoz, 1998). For
example during an express saccade, the response onset latency, rate of rise and peak of
the visual response could be directly mapped on to the motor response that drives the eye
189
movement. Thus additional physiological evidence is necessary to determine exactly how
the visual response accumulates to trigger the motor response as well as to confirm the
physiological accuracy of accumulator models.
Another fundamental question underlying visuomotor transformations is where in
the nervous system does the saccadic motor command originate? As the BU sensory and
TD goal related information is received and processed across the sensory and motor areas
of the saccade circuit (Fig. 1.2A), what nucleus or structure initially decides that the
accumulated TD and BU information is sufficient to warrant that a saccade be
performed? Furthermore does a single nucleus or structure initially generate this motor
response or is it simultaneously generated over multiple distributed regions? Several
oculomotor structures have been identified (in addition to the SC) that exhibit activity
related to both visual-sensory and saccadic-motor responses. Two important cortical
areas that (like the SC) also possess signals related to visual input and motor output are
the lateral intraparietal area (LIP) and Frontal Eye Fields (FEF). Projections from LIP to
the (SCi) have been shown to be involved in sensory-motor transformations as well as
attentional processing for saccades (Anderson et al., 1998; Colby and Goldberg, 1999;
Glimcher, 2001). The frontal eye fields (FEF) are strongly interconnected with parietal
visual areas (Schall, 1997; Schall and Thompson, 1999) as well as multiple frontal areas
including the supplementary eye fields (SEF), dorsolateral prefrontal cortex (DLPFC),
SCi, and also the basal ganglia. In fact, it is not usual to find neurons with similar sensory
and motor responses across all three regions (Pare and Wurtz, 2001; Wurtz et al., 2001a;
Munoz and Schall, 2003). The similarities between the FEF and SC are especially
noteworthy as the both appear to act like centralized hubs that connect with several
190
cortical and sub-cortical areas. The numerous distributed connections between the SCi,
FEF, and LIP indicate that these structures form an important subsystem within the
oculomotor circuit. Perhaps a more detailed understanding of the simultaneous
cooperation and synchronization of the sensory and motor signals within all these
structures is necessary in order to more fully understand how saccadic accumulation
occurs and where the motor response originates. Thus determining where or how the
saccadic decision threshold is determined in which structure and where the saccadic
motor burst originates remains a fundamental question that may provide critical insight
into how visuomotor transformations are computed in the brain.
6.3 Future Directions
The observations and conclusions presented in this thesis have inspired many
additional questions and hypotheses for future research. Furthermore, the neurofield
model presented in chapter 5 has also proposed simple testable hypotheses regarding how
neural activity may interact spatially within the SCi map during visually guided saccades.
The following discussion describes a few critical hypotheses and experiments that could
further develop some of the key ideas presented in this thesis.
6.3.1 Testing the Neural Field model of the SCi
The neural field model presented in chapter 5 makes several testable predictions
about to how TD and BU signals could interact spatially across the SCi map to influence
saccade behavior. One specific prediction of the model was that increasing BU stimulus
intensity could modulate the area of visual response fields of SCi neurons. As this type of
spatial exploration of SCi responses has never been systematically studied, these results
191
could provide novel insight into how sensory stimuli interact within networks of neural
tissue to influence behavior. A simple experiment to test this hypothesis involves a small
variant of the response field mapping task described in chapter 3. This task involved
displaying a pseudo random sequence of flashes (100ms in duration, 150ms apart) across
a large portion of the visual field while the monkey was fixating at the centre of the
screen. In chapter 3, the luminance of the flashes was always kept constant; however, if
each neuron's visual response field was examined across a range of luminance values,
then the model's predicted changes to the area of the visual response could be assessed.
A positive finding in this experiment would provide evidence that the model accurately
predicts novel spatial interactions within in the SCi map.
6.3.2 Is Preparatory Activity Preceding Express Saccades TD or BU?
The production of unintended express saccades are becoming increasingly
important clinically because they are emerging as a sensitive biomarker for abnormality
in several neurological and psychiatric conditions such as dyslexia (Biscaldi et al., 1996),
ADHD (Munoz et al., 2003) and PD (Chan et al., 2005). The emergence of such clinical
relevance highlights why it is important to gain a more detailed understanding of the
neural mechanisms underlying express saccade generation.
In the laboratory, gap tasks (like the one used to demonstrate the luminance
dependence of express saccades in chapter 4) are extremely effective at manipulating
excitability in pre-motor circuits and producing express saccades (Edelman and Keller,
1996; Dorris and Munoz, 1998; Sparks et al., 2000). This is because the extinguishment
of the fixation point prior to the appearance of the target has been shown to both reduce
fixation activity at the rostral pole (Dorris and Munoz, 1995) as well as facilitate the pre192
target activation of saccade neurons (Dorris et al., 1997; Basso and Wurtz, 1998; Dorris
and Munoz, 1998; Dorris et al., 2007). However, the gap task incorporates both TD
(spatial and temporal target predictability) and BU (fixation disengagement resulting
from the disappearance of the FP) components that cannot be readily separated from one
another. This means that at the level of the SCi, the relative TD or BU contributions to
changes in caudal pre-target build-up activity and rostral fixation activity cannot be
determined. Likewise at the behavioral level, it is also uncertain how much each of the
TD or BU components are individually contributing or interacting to influence the gap
effect (reduction in overall SRT) and/or express saccade production. Thus the standard
gap task is an inadequate tool for dissociating how TD and BU signals independently
influence the neural mechanisms sub-serving express saccades and the gap effect.
A simple task that may be able to dissociate TD prediction from BU FP offset
effects is a novel variant of the gap task whereby the luminance of the FP is manipulated
during the gap period. By systematically increasing or decreasing the luminance of the
FP during the gap while keeping the TD prediction signal constant (temporal or spatial
predictability of the target) warning and visual offset effects could be dissociated. For
example, if increases in low frequency caudal preparatory build-up activity is primarily
related to a TD prediction signal, then increases in build-up should be observed whether
the FP becomes brighter, dimmer, or disappears entirely. However, if this caudal
preparatory build-up activity is primarily related to the BU disappearance of the FP, then
increases in FP luminance during the gap should not significantly increase build-up
activity during the gap. Finally, how would the activity of fixation neurons be affected?
Do they always maintain the same firing rate during active fixation of a stimulus or do
193
they change depending on FP luminance (i.e. the intensity of the visual input)? Also, is
this fixation activity also influenced in some way by the TD temporal or spatial target
prediction during the gap?
If successful, this experiment could provide insight into whether modulations to
caudal preparation and rostral fixation signals in the SCi are driven by primarily TD or
BU mechanisms. If the majority of the gap effect results from BU sensory processing and
not higher level TD cognitive signals then this could indicate that express saccades are
primarily a low level sensory driven response. This could provide clinically relevant
insight into which brain areas (early sensory or higher level cognitive) are most critically
involved in express saccade generation and potentially why these direct visual to motor
transformations are such an important biomarker in the clinic.
6.3.3 Moving Towards Understanding Visuomotor Transformations During
Real World Free Viewing
The ultimate goal of saccade research is to understand the neural circuitry
controlling visuomotor transformations during normal unrestricted free viewing in natural
environments. Such orienting behaviors are critical for a multitude of common tasks
(such as reading, shopping or driving a car) and are so basic to daily life that they are
typically unconsciously performed and taken for granted. The study of saccades in the
laboratory has typically involved highly controlled and artificial saccade tasks that are
performed within simplistic and artificial visual environments. However, these type of
examinations impose many artificial constraints that may not reflect how the nervous
system or saccade behavior operates in naturalistic viewing situations. Typical
methodology (including those employed within this thesis) involves the use of small
194
numbers of over trained animals performing simple stereotypical saccade tasks within an
artificial and visually impoverished environment.
This thesis has described multiple observations and methodologies that could
assist in the development and interpretation of future free viewing experiments. In
general, these insights suggest that the investigation of visuomotor transformations
during natural free viewing should involve an examination of the spatiotemporal
interactions of BU and TD signals within critical saccade related structures like the SC
(See fig 1.2). This thesis has described multiple methods for spatially mapping visual and
motor responses. In chapter 2 I described a step task that mapped the spatial extent of
visual and motor response fields in two dimensions. In chapter 3 and 4 I utilized a
different visual response mapping task that rapidly measured visual response fields
accurately and at a high spatial resolution. Such methods of mapping the spatial extent of
sensory and motor responses may be an important component for constraining any
analysis of subsequent TD or BU signal interactions during free viewing experiments.
However the spatial aspects of these signals are not the only important parameters
governing visuomotor transformations. In chapter 3 I demonstrated that BU visual
responses are variable and can be altered by external visual stimulus properties. This
observation may also offer critical insight into the interpretation of visual responses
during natural viewing since these might be altered dramatically in magnitude and timing
when visual stimuli change dynamically over time on the retina. Taken together, these
results demonstrate that BU sensory and TD goal related signals can be described
multidimensionality within the maps that are used by networks of neural tissue. Finally,
in chapter 2 I described one method by which multiple sites can be simultaneously
195
recorded across the SC. Such simultaneous multi recording methodologies will likely be
critical for future research seeking to unravel how the SC and other structures function at
the network level rather than the classic but limited single neuron perspective.
The movement toward studying free viewing is still a very complex undertaking
that can be greatly simplified by the use of theoretical frameworks from which to design
experiments and interpret data. One potentially critical framework emerges from the use
of computational models that simulate and predict how TD and BU signals influence
visuomotor transformations to guide saccades. The neural network model presented in
chapter 5 is one of many such computational models that can be used to create testable
predictions about the underlying network of dynamic activity across visual and motor
maps. Such models are demonstrating themselves to be an invaluable and critical partner
for advancing our knowledge of the neural mechanisms within the nervous system.
196
Reference List
Amari S (1977) Dynamics of pattern formation in lateral-inhibition type neural fields.
Biol Cybern 27:77-87.
Andersen RA, Snyder LH, Bradley DC, Xing J (1997) Multimodal representation of
space in the posterior parietal cortex and its use in planning movements. Annu Rev
Neurosci 20:303-330.
Anderson RW, Keller EL, Gandhi NJ, Das S (1998) Two-dimensional saccade-related
population activity in superior colliculus in monkey. J Neurophysiol 80:798-817.
Arai K, Keller EL, Edelman JA (1994) Two-dimensional neural network model of the
primate saccadic system. Neural Netw 7:1115-1135.
Arai K, Keller EL (2005) A model of the saccade-generating system that accounts for
trajectory variations produced by competing visual stimuli. Biol Cybern 92:21-37.
Arai K, McPeek RM, Keller EL (2004) Properties of saccadic responses in monkey when
multiple competing visual stimuli are present. J Neurophysiol 91:890-900.
Arai K, Das S, Keller EL, Aiyoshi E (1999) A distributed model of the saccade system:
Simulations of temporally perturbed saccades using position and velocity feedback.
Neural Netw 12:1359-1375.
Badler JB, Keller EL (2002) Decoding of a motor command vector from distributed
activity in superior colliculus. Biol Cybern 86:179-189.
Balan PF, Oristaglio J, Schneider DM, Gottlieb J (2008) Neuronal correlates of the setsize effect in monkey lateral intraparietal area. PLoS Biol 6:e158.
Barbur JL, Wolf J, Lennie P (1998) Visual processing levels revealed by response
latencies to changes in different visual attributes. Proc Biol Sci 265:2321-2325.
Basso MA, Wurtz RH (2002) Neuronal activity in substantia nigra pars reticulata during
target selection. J Neurosci 22:1883-1894.
Basso MA, Wurtz RH (1998) Modulation of neuronal activity in superior colliculus by
changes in target probability. J Neurosci 18:7519-7534.
Basso MA, Wurtz RH (1997) Modulation of neuronal activity by target uncertainty.
Nature 389:66-69.
Batschelet E (1981) Circular statistics in biology. New York: Academic Press.
197
Bell AH, Meredith MA, Van Opstal AJ, Munoz DP (2006) Stimulus intensity modifies
saccadic reaction time and visual response latency in the superior colliculus. Exp Brain
Res .
Berg DJ, Boehnke SE, Marino RA, Munoz DP, Itti L (2009) Free viewing of dynamic
stimuli by humans and monkeys. J Vis 9:19.1-1915.
Biscaldi M, Fischer B, Stuhr V (1996) Human express saccade makers are impaired at
suppressing visually evoked saccades. J Neurophysiol 76:199-214.
Bisti S, Sireteanu RC (1976) Sensitivity to spatial frequency and contrast of visual cells
in the cat superior colliculus. Vision Res 16:247-251.
Boch R, Fischer B, Ramsperger E (1984) Express-saccades of the monkey: Reaction
times versus intensity, size, duration, and eccentricity of their targets. Exp Brain Res
55:223-231.
Bozis A, Moschovakis AK (1998) Neural network simulations of the primate oculomotor
system. III. an one-dimensional, one-directional model of the superior colliculus. Biol
Cybern 79:215-230.
Bravo MJ, Nakayama K (1992) The role of attention in different visual-search tasks.
Percept Psychophys 51:465-472.
Buttner-Ennever JA, Horn AK, Henn V, Cohen B (1999) Projections from the superior
colliculus motor map to omnipause neurons in monkey. J Comp Neurol 413:55-67.
Buttner-Ennever JA, Cohen B, Pause M, Fries W (1988) Raphe nucleus of the pons
containing omnipause neurons of the oculomotor system in the monkey, and its
homologue in man. J Comp Neurol 267:307-321.
Carpenter RH (2004) Contrast, probability, and saccadic latency; evidence for
independence of detection and decision. Curr Biol 14:1576-1580.
Carpenter RH (2001) Express saccades: Is bimodality a result of the order of stimulus
presentation? Vision Res 41:1145-1151.
Carpenter RH, McDonald SA (2007) LATER predicts saccade latency distributions in
reading. Exp Brain Res 177:176-183.
Carpenter RH, Williams ML (1995) Neural computation of log likelihood in control of
saccadic eye movements. Nature 377:59-62.
Carrasco M, Yeshurun Y (1998) The contribution of covert attention to the set-size and
eccentricity effects in visual search. Journal of Experimental Psychology: Human
Perception and Performance 24:673-692.
198
Chambers JM, Prescott TJ (2010) Response times for visually guided saccades in persons
with parkinson's disease: A meta-analytic review. Neuropsychologia 48:887-899.
Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP (2005) Deficits in saccadic eyemovement control in parkinson's disease. Neuropsychologia 43:784-796.
Chaparro A, Stromeyer CF,3rd, Huang EP, Kronauer RE, Eskew RT,Jr (1993) Colour is
what the eye sees best. Nature 361:348-350.
Chevalier G, Vacher S, Deniau JM (1984) Inhibitory nigral influence on tectospinal
neurons, a possible implication of basal ganglia in orienting behavior. Exp Brain Res
53:320-326.
Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009)
Preparation to inhibit a response complements response inhibition during performance of
a stop-signal task. J Neurosci 29:15870-15877.
Cohen JY, Heitz RP, Woodman GF, Schall JD (2009) Neural basis of the set-size effect
in frontal eye field: Timing of attention during visual search. J Neurophysiol 101:16991704.
Colby CL, Goldberg ME (1999) Space and attention in parietal cortex. Annu Rev
Neurosci 22:319-349.
Conley M, Diamond IT (1990) Organization of the visual sector of the thalamic reticular
nucleus in galago. Eur J Neurosci 2:211-226.
Corneil BD, Munoz DP, Olivier E (2007) Priming of head premotor circuits during
oculomotor preparation. J Neurophysiol 97:701-714.
Corneil BD, Olivier E, Munoz DP (2004) Visual responses on neck muscles reveal
selective gating that prevents express saccades. Neuron 42:831-841.
Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL (2008) Neuromuscular
consequences of reflexive covert orienting. Nat Neurosci 11:13-15.
Crabtree JW, Killackey HP (1989) The topographic organization and axis of projection
within the visual sector of the rabbit's thalamic reticular nucleus. Eur J Neurosci 1:94109.
Crawford JD, Cadera W, Vilis T (1991) Generation of torsional and vertical eye position
signals by the interstitial nucleus of cajal. Science 252:1551-1553.
Crist C.F., Yamasaki DSG, Komatsu H, Wurtz RH (1988) A grid system and a
microsyringe for single cell recording. J Neurosci Methods 26:117-122.
199
Cusick CG (1988) Anatomical organization of the superior colliculus in monkeys:
Corticotectal pathways for visual and visuomotor functions. Prog Brain Res 75:1-15.
Cynader M, Berman N (1972) Receptive-field organization of monkey superior
colliculus. J Neurophysiol 35:187-201.
de Boore C (1978) A practical guide to splines. Applied Mathematical Sciences 27:392.
Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C (2006) Conscious,
preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10:204211.
Dickov LA, Morrison JD (2006) Effects of uncertainty and target displacement on the
latency of express saccades in man. Vision Res 46:2505-2512.
Doma H, Hallett PE (1988) Dependence of saccadic eye-movements on stimulus
luminance, and an effect of task. Vision Res 28:915-924.
Dorris MC, Munoz DP (1998) Saccadic probability influences motor preparation signals
and time to saccadic initiation. J Neurosci 18:7015-7026.
Dorris MC, Munoz DP (1995) A neural correlate for the gap effect on saccadic reaction
times in monkey. J Neurophysiol 73:2558-2562.
Dorris MC, Olivier E, Munoz DP (2007) Competitive integration of visual and
preparatory signals in the superior colliculus during saccadic programming. J Neurosci
27:5053-5062.
Dorris MC, Pare M, Munoz DP (1997) Neuronal activity in monkey superior colliculus
related to the initiation of saccadic eye movements. J Neurosci 17:8566-8579.
Dorris MC, Klein RM, Everling S, Munoz DP (2002) Contribution of the primate
superior colliculus to inhibition of return. J Cogn Neurosci 14:1256-1263.
Doubell TP, Skaliora I, Baron J, King AJ (2003) Functional connectivity between the
superficial and deeper layers of the superior colliculus: An anatomical substrate for
sensorimotor integration. J Neurosci 23:6596-6607.
D'Zmura M, Lennie P, Tiana C (1997) Color search and visual field segregation. Percept
Psychophys 59:381-388.
Edelman JA, Keller EL (1998) Dependence on target configuration of express saccaderelated activity in the primate superior colliculus. J Neurophysiol 80:1407-1426.
Edelman JA, Keller EL (1996) Activity of visuomotor burst neurons in the superior
colliculus accompanying express saccades. J Neurophysiol 76:908-926.
200
Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with
pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387-400.
Everling S, Dorris MC, Klein RM, Munoz DP (1999) Role of primate superior colliculus
in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19:27402754.
Fecteau JH, Munoz DP (2006) Salience, relevance, and firing: A priority map for target
selection. Trends Cogn Sci 10:382-390.
Fecteau JH, Bell AH, Munoz DP (2004) Neural correlates of the automatic and goaldriven biases in orienting spatial attention. J Neurophysiol 92:1728-1737.
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate
cerebral cortex. Cereb Cortex 1:1-47.
Fischer B (1986) Express saccades in man and monkey. Prog Brain Res 64:155-160.
Fischer B, Weber H (1993) Express saccades and visual attention. Behav Brain Sci
16:553-610.
Fischer B, Ramsperger E (1984) Human express saccades: Extremely short reaction times
of goal directed eye movements. Exp Brain Res 57:191-195.
Fischer B, Boch R (1983) Saccadic eye movements after extremely short reaction times
in the monkey. Brain Res 260:21-26.
Fischer B, Boch R, Ramsperger E (1984) Express-saccades of the monkey: Effect of
daily training on probability of occurrence and reaction time. Exp Brain Res 55:232-242.
Fitzgibbon T, Szmajda BA, Martin PR (2007) First order connections of the visual sector
of the thalamic reticular nucleus in marmoset monkeys (callithrix jacchus). Vis Neurosci
24:857-874.
Ford KA, Everling S (2009) Neural activity in primate caudate nucleus associated with
pro- and antisaccades. J Neurophysiol 102:2334-2341.
Fries W (1984) Cortical projections to the superior colliculus in the macaque monkey: A
retrograde study using horseradish peroxidase. J Comp Neurol 230:55-76.
Gancarz G, Grossberg S (1999) A neural model of saccadic eye movement control
explains task-specific adaptation. Vision Res 39:3123-3143.
Gandhi NJ, Keller EL (1997) Spatial distribution and discharge characteristics of superior
colliculus neurons antidromically activated from the omnipause region in monkey. J
Neurophysiol 78:2221-2225.
201
Glimcher PW (2001) Making choices: The neurophysiology of visual-saccadic decision
making. Trends Neurosci 24:654-659.
Goldberg ME, Wurtz RH (1972a) Activity of superior colliculus in behaving monkey. I.
visual receptive fields of single neurons. J Neurophysiol 35:542-559.
Goldberg ME, Wurtz RH (1972b) Activity of superior colliculus in behaving monkey. I.
visual receptive fields of single neurons. J Neurophysiol 35:542-559.
Grantyn A, Berthoz A (1985) Burst activity of identified tecto-reticulo-spinal neurons in
the alert cat. Exp Brain Res 57:417-421.
Grantyn A, Grantyn R (1982) Axonal patterns and sites of termination of cat superior
colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res 46:243-256.
Grossberg S, Roberts K, Aguilar M, Bullock D (1997) A neural model of multimodal
adaptive saccadic eye movement control by superior colliculus. J Neurosci 17:9706-9725.
Guitton D (1992a) Eye movements. (Carpenter RHS, ed), pp244-276. London:
MacMillian.
Guitton D (1992b) Control of eye-head coordination during orienting gaze shifts. Trends
Neurosci 15:174-179.
Guitton D, Munoz DP (1991) Control of orienting gaze shifts by the tectoreticulospinal
system in the head-free cat. I. identification, localization, and effects of behavior on
sensory responses. J Neurophysiol 66:1605-1623.
Guitton D, Bergeron A, Choi WY, Matsuo S (2003) On the feedback control of orienting
gaze shifts made with eye and head movements. Prog Brain Res 142:55-68.
Hall WC, Moschovakis AK (2003) The superior colliculus: New approaches for studying
sensorimotor integration.
Hamm JP, Dyckman KA, Ethridge LE, McDowell JE, Clementz BA (2010) Preparatory
activations across a distributed cortical network determine production of express saccades
in humans. J Neurosci 30:7350-7357.
Handel A, Glimcher PW (2000) Contextual modulation of substantia nigra pars reticulata
neurons. J Neurophysiol 83:3042-3048.
Handel A, Glimcher PW (1999) Quantitative analysis of substantia nigra pars reticulata
activity during a visually guided saccade task. J Neurophysiol 82:3458-3475.
Hanes DP, Wurtz RH (2001) Interaction of the frontal eye field and superior colliculus
for saccade generation. J Neurophysiol 85:804-815.
202
Hanes DP, Schall JD (1996) Neural control of voluntary movement initiation. Science
274:427-430.
Harting JK, Van Lieshout DP, Feig S (1991) Connectional studies of the primate lateral
geniculate nucleus: Distribution of axons arising from the thalamic reticular nucleus of
galago crassicaudatus. J Comp Neurol 310:411-427.
Hayhoe M, Ballard D (2005) Eye movements in natural behavior. Trends Cogn Sci
9:188-194.
Hays A, Richmond B, Optican L (1982) A UNIX-based multiple process system for realtime data acquisition and control. WESCON Conf Proc 2:1-10.
Hepp K, Henn V (1983) Spatio-temporal recoding of rapid eye movement signals in the
monkey paramedian pontine reticular formation (PPRF). Exp Brain Res 52:105-120.
Hepp K, Henn V, Jaeger J (1982) Eye movement related neurons in the cerebellar nuclei
of the alert monkey. Exp Brain Res 45:253-264.
Hess WR, Bürgi S, Bucher V (1946) Motorische funktion des tektal- und
tegmentalgebietes (motor functions of tectal and tegmental areas). Mschr Psychiat Neurol
112:1-52.
Hick WE (1952) On the rate of gain of information. Q J Exp Psychol 4:11-26.
Hikosaka O (2007) GABAergic output of the basal ganglia. Prog Brain Res 160:209-226.
Hikosaka O (1989) Role of basal ganglia in saccades. Rev Neurol (Paris) 145:580-586.
Hikosaka O, Wurtz RH (1983a) Visual and oculomotor functions of monkey substantia
nigra pars reticulata. III. memory-contingent visual and saccade responses. J
Neurophysiol 49:1268-1284.
Hikosaka O, Wurtz RH (1983b) Visual and oculomotor functions of monkey substantia
nigra pars reticulata. I. relation of visual and auditory responses to saccades. J
Neurophysiol 49:1230-1253.
Hikosaka O, Wurtz RH (1983c) Visual and oculomotor functions of monkey substantia
nigra pars reticulata. II. visual responses related to fixation of gaze. J Neurophysiol
49:1254-1267.
Hikosaka O, Nakamura K, Nakahara H (2006) Basal ganglia orient eyes to reward. J
Neurophysiol 95:567-584.
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of
purposive saccadic eye movements. Physiol Rev 80:953-978.
203
Hikosaka O, Sakamoto M, Usui S (1989) Functional properties of monkey caudate
neurons. I. activities related to saccadic eye movements. J Neurophysiol 61:780-798.
Hubel DH, Wiesel TN (1969) Anatomical demonstration of columns in the monkey
striate cortex. Nature 221:747-750.
Isa T (2002) Intrinsic processing in the mammalian superior colliculus. Curr Opin
Neurobiol 12:668-677.
Isa T, Hall WC (2009) Exploring the superior colliculus in vitro. J Neurophysiol
102:2581-2593.
Itti L, Koch C (2001) Computational modelling of visual attention. Nat Rev Neurosci
2:194-203.
Itti L, Rees G, Tsotsos JK (2005) Neurobiology of attention. San Diego, CA: Elsevier.
Jaskowski P, Sobieralska K (2004) Effect of stimulus intensity on manual and saccadic
reaction time. Percept Psychophys 66:535-544.
Jayaraman A, Batton RR,3rd, Carpenter MB (1977) Nigrotectal projections in the
monkey: An autoradiographic study. Brain Res 135:147-152.
Jiang H, Stein BE, McHaffie JG (2003) Opposing basal ganglia processes shape midbrain
visuomotor activity bilaterally. Nature 423:982-986.
Johnston K, Everling S (2008) Neurophysiology and neuroanatomy of reflexive and
voluntary saccades in non-human primates. Brain Cogn 68:271-283.
Jones EG (2007) In: The thalamus pp1270-1271.Cambridge University Press.
Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for
measurement of eye position: An improved method. Vision Res 20:535-538.
Kaas JH (1997) Topographic maps are fundamental to sensory processing. Brain Res
Bull 44:107-112.
Kamogawa H, Ohki Y, Shimazu H, Suzuki I, Yamashita M (1996) Inhibitory input to
pause neurons from pontine burst neuron area in the cat. Neurosci Lett 203:163-166.
Kaneda K, Isa K, Yanagawa Y, Isa T (2008) Nigral inhibition of GABAergic neurons in
mouse superior colliculus. J Neurosci 28:11071-11078.
Kato R, Grantyn A, Dalezios Y, Moschovakis AK (2006) The local loop of the saccadic
system closes downstream of the superior colliculus. Neuroscience 143:319-337.
204
Kirschfeld K, Feiler R, Wolf-Oberhollenzer F (1996) Cortical oscillations and the origin
of express saccades. Proc Biol Sci 263:459-468.
Kolmac CI, Mitrofanis J (1998) Patterns of brainstem projection to the thalamic reticular
nucleus. J Comp Neurol 396:531-543.
Kopecz K (1995) Saccadic reaction times in gap/overlap paradigms: A model based on
integration of intentional and visual information on neural, dynamic fields. Vision Res
35:2911-2925.
Kopecz K, Schoner G (1995) Saccadic motor planning by integrating visual information
and pre-information on neural dynamic fields. Biol Cybern 73:49-60.
Kowler E (1990) The role of visual and cognitive processes in the control of eye
movement. Rev Oculomot Res 4:1-70.
Krauzlis RJ (2005) The control of voluntary eye movements: New perspectives.
Neuroscientist 11:124-137.
Krauzlis RJ (2003) Neuronal activity in the rostral superior colliculus related to the
initiation of pursuit and saccadic eye movements. J Neurosci 23:4333-4344.
Krauzlis RJ, Basso MA, Wurtz RH (2000) Discharge properties of neurons in the rostral
superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol
84:876-891.
Kunzle H, Akert K, Wurtz RH (1976) Projection of area 8 (frontal eye field) to superior
colliculus in the monkey. an autoradiographic study. Brain Res 117:487-492.
Kveraga K, Hughes HC (2005) Effects of stimulus-response uncertainty on saccades to
near-threshold targets. Exp Brain Res 162:401-405.
Kveraga K, Boucher L, Hughes HC (2002) Saccades operate in violation of hick's law.
Exp Brain Res 146:307-314.
Land M, Mennie N, Rusted J (1999) The roles of vision and eye movements in the
control of activities of daily living. Perception 28:1311-1328.
Land MF (2009) Vision, eye movements, and natural behavior. Vis Neurosci 26:51-62.
Lawrence BM, St John A, Abrams RA, Snyder LH (2008) An anti-hick's effect in
monkey and human saccade reaction times. J Vis 8:26.1-26.7.
Lee C, Rohrer WH, Sparks DL (1988) Population coding of saccadic eye movements by
neurons in the superior colliculus. Nature 332:357-360.
205
Lee KM, Keller EL, Heinen SJ (2005) Properties of saccades generated as a choice
response. Exp Brain Res 162:278-286.
Lee P, Hall WC (2006) An in vitro study of horizontal connections in the intermediate
layer of the superior colliculus. J Neurosci 26:4763-4768.
Leigh RJ, Zee DS (2006) The neurology of eye movements. Philadelphia: Davis
Company.
Leigh RJ, Zee DS (1999) The neurology of eye movements. Philadelphia, PA.: F.A.
Davis Company.
Lennie P (1981) The physiological basis of variations in visual latency. Vision Res
21:815-824.
Li X, Basso MA (2008) Preparing to move increases the sensitivity of superior colliculus
neurons. J Neurosci 28:4561-4577.
Liversedge SP, Findlay JM (2000) Saccadic eye movements and cognition. Trends Cogn
Sci 4:6-14.
Lock TM, Baizer JS, Bender DB (2003) Distribution of corticotectal cells in macaque.
Exp Brain Res 151:455-470.
Ludwig CJ, Gilchrist ID, McSorley E (2004) The influence of spatial frequency and
contrast on saccade latencies. Vision Res 44:2597-2604.
Ma TP, Graybiel AM, Wurtz RH (1991) Location of saccade-related neurons in the
macaque superior colliculus. Exp Brain Res 85:21-35.
Machado L, Rafal RD (2000) Strategic control over saccadic eye movements: Studies of
the fixation offset effect. Percept Psychophys 62:1236-1242.
Marino RA, Trappenberg TP, Levy R, Munoz DP (2007)
Target luminance modulates spatial visual receptive field sizes in the superior colliculus
as predicted by a neural field model. Soc Neurosci Abstr 719.11:.
Marino RA, Levy R, Boehnke S, White, BJ, Itti L, Munoz DP (2011) Linking visual
response properties in the superior colliculus to saccade behavior. Eur J Neurosci (In
Revision).
206
Marino RA, Munoz DP (2009) The effects of bottom-up target luminance and top-down
spatial target predictability on saccadic reaction times. Exp Brain Res 197:321-335.
Marino RA, Rodgers CK, Levy R, Munoz DP (2008) Spatial relationships of visuomotor
transformations in the superior colliculus map. J Neurophysiol 100:2564-2576.
Massone LLE, Khoshaba T (1995) Local dynamic interactions in the collicular motor
map: A neural network model. Network: Comput Neural Syst 6:1-18.
McIlwain JT (1986) Point images in the visual system: New interest in an old idea. TINS
9:354.
McIlwain JT (1975) Visual receptive fields and their images in superior colliculus of the
cat. J Neurophysiol 38:219-230.
McPeek RM, Keller EL (2002) Saccade target selection in the superior colliculus during
a visual search task. J Neurophysiol 88:2019-2034.
McPeek RM, Keller EL (2001) Short-term priming, concurrent processing, and saccade
curvature during a target selection task in the monkey. Vision Res 41:785-800.
McPeek RM, Schiller PH (1994) The effects of visual scene composition on the latency
of saccadic eye movements of the rhesus monkey. Vision Res 34:2293-2305.
McPeek RM, Maljkovic V, Nakayama K (1999) Saccades require focal attention and are
facilitated by a short-term memory system. Vision Res 39:1555-1566.
Meredith MA, Ramoa AS (1998) Intrinsic circuitry of the superior colliculus:
Pharmacophysiological identification of horizontally oriented inhibitory interneurons. J
Neurophysiol 79:1597-1602.
Meredith MA, Stein BE (1985) Descending efferents from the superior colliculus relay
integrated multisensory information. Science 227:657-659.
Milstein DM, Dorris MC (2007) The influence of expected value on saccadic preparation.
J Neurosci 27:4810-4818.
Mohler CW, Wurtz RH (1976) Organization of monkey superior colliculus: Intermediate
layer cells discharging before eye movements. J Neurophysiol 39:722-744.
Moschovakis AK, Gregoriou GG, Savaki HE (2001) Functional imaging of the primate
superior colliculus during saccades to visual targets. Nat Neurosci 4:1026-1031.
Moschovakis AK, Scudder CA, Highstein SM (1996) The microscopic anatomy and
physiology of the mammalian saccadic system. Prog Neurobiol 50:133-254.
207
Moschovakis AK, Scudder CA, Highstein SM (1990) A structural basis for hering's law:
Projections to extraocular motoneurons. Science 248:1118-1119.
Moschovakis AK, Karabelas AB, Highstein SM (1988) Structure-function relationships
in the primate superior colliculus. II. morphological identity of presaccadic neurons. J
Neurophysiol 60:263-302.
Munoz DP, Schall JD (2003) Concurrent, distributed control of saccade initiation in the
frontal eye field and superior colliculus. (Hall WC, Moschovakis A, eds), pp55. Boca
Raton: CRC Press.
Munoz DP (2002) Commentary: Saccadic eye movements: Overview of neural circuitry.
Prog Brain Res 140:89-96.
Munoz DP, Fecteau JH (2002) Vying for dominance: Dynamic interactions control visual
fixation and saccadic initiation in the superior colliculus. Prog Brain Res 140:3-19.
Munoz DP, Istvan PJ (1998) Lateral inhibitory interactions in the intermediate layers of
the monkey superior colliculus. J Neurophysiol 79:1193-1209.
Munoz DP, Wurtz RH (1995a) Saccade-related activity in monkey superior colliculus. I.
characteristics of burst and buildup cells. J Neurophysiol 73:2313-2333.
Munoz DP, Wurtz RH (1995b) Saccade-related activity in monkey superior colliculus. II.
spread of activity during saccades. J Neurophysiol 73:2334-2348.
Munoz DP, Wurtz RH (1993a) Fixation cells in monkey superior colliculus. I.
characteristics of cell discharge. J Neurophysiol 70:559-575.
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus. II. reversible
activation and deactivation. J Neurophysiol 70:576-589.
Munoz DP, Guitton D (1991) Control of orienting gaze shifts by the tectoreticulospinal
system in the head-free cat. II. sustained discharges during motor preparation and
fixation. J Neurophysiol 66:1624-1641.
Munoz DP, Guitton D, Pelisson D (1991) Control of orienting gaze shifts by the
tectoreticulospinal system in the head-free cat. III. spatiotemporal characteristics of
phasic motor discharges. J Neurophysiol 66:1642-1666.
Munoz DP, Armstrong IT, Hampton KA, Moore KD (2003) Altered control of visual
fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J
Neurophysiol 90:503-514.
Munoz DP, Dorris MC, Paré M, Everling S (2000) On your mark, get set: Brainstem
circuitry underlying saccadic initiation. Can J Physiol Pharmacol 78:934-944.
208
Nakahara H, Nakamura K, Hikosaka O (2006a) Extended LATER model can account for
trial-by-trial variability of both pre- and post-processes. Neural Netw 19:1027-1046.
Nakahara H, Morita K, Wurtz RH, Optican LM (2006b) Saccade-related spread of
activity across superior colliculus may arise from asymmetry of internal connections. J
Neurophysiol 96:765-774.
Olivier E, Grantyn A, Chat M, Berthoz A (1993) The control of slow orienting eye
movements by tectoreticulospinal neurons in the cat: Behavior, discharge patterns and
underlying connections. Exp Brain Res 93:435-449.
Optican LM (1995) A field theory of saccade generation: Temporal-to-spatial transform
in the superior colliculus. Vision Res 35:3313-3320.
Ottes FP, Van Gisbergen JA, Eggermont JJ (1986) Visuomotor fields of the superior
colliculus: A quantitative model. Vision Res 26:857-873.
Pare M, Wurtz RH (2001) Progression in neuronal processing for saccadic eye
movements from parietal cortex area lip to superior colliculus. J Neurophysiol 85:25452562.
Paré M, Hanes DP (2003) Controlled movement processing: Superior colliculus activity
associated with countermanded saccades. J Neurosci 23:6480-6489.
Paré M, Munoz DP (1996) Saccadic reaction time in the monkey: Advanced preparation
of oculomotor programs is primarily responsible for express saccade occurrence. J
Neurophysiol 76:3666-3681.
Peltsch A, Hoffman A, Armstrong I, Pari G, Munoz DP (2008) Saccadic impairments in
huntington's disease. Exp Brain Res 186:457-469.
Perry VH, Cowey A (1985a) The ganglion cell and cone distributions in the monkey's
retina: Implications for central magnification factors. Vision Res 25:1795-1810.
Perry VH, Cowey A (1985b) The ganglion cell and cone distributions in the monkey's
retina: Implications for central magnification factors. Vision Res 25:1795-1810.
Phongphanee P, Marino RA, Kaneda K, Yanagawa Y, Munoz DP, Isa T (2008) The
lateral interaction in the superficial and intermediate layers of the mouse superior
colliculus slice. Soc Neurosci Abstr .
Pieron H (1952) The sensations: Their functions, processes and mechanisms. London:
Frederick Muller Ltd.
Port NL, Sommer MA, Wurtz RH (2000) Multielectrode evidence for spreading activity
across the superior colliculus movement map. J Neurophysiol 84:344-357.
209
Posner MI (2005) Timing the brain: Mental chronometry as a tool in neuroscience. PLoS
Biol 3:e51.
Raybourn MS, Keller EL (1977) Colliculoreticular organization in primate oculomotor
system. J Neurophysiol 40:861-878.
Rayner K (1998) Eye movements in reading and information processing: 20 years of
research. Psychol Bull 124:372-422.
Reddi BA, Carpenter RH (2000) The influence of urgency on decision time. Nat
Neurosci 3:827-830.
Reddi BA, Asrress KN, Carpenter RH (2003) Accuracy, information, and response time
in a saccadic decision task. J Neurophysiol 90:3538-3546.
Reuter-Lorenz PA, Hughes HC, Fendrich R (1991) The reduction of saccadic latency by
prior offset of the fixation point: An analysis of the gap effect. Percept Psychophys
49:167-175.
Richmond BJ, Optican LM, Podell M, Spitzer H (1987) Temporal encoding of twodimensional patterns by single units in primate inferior temporal cortex. I. response
characteristics. J Neurophysiol 57:132-146.
Robinson DA (1972) Eye movements evoked by collicular stimulation in the alert
monkey. Vision Res 12:1795-1808.
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in
a magnetic field. IEEE Trans Biomed Eng 10:137-145.
Robinson DA, Fuchs AF (1969) Eye movements evoked by stimulation of frontal eye
fields. J Neurophysiol 32:637-648.
Robinson DL, McClurkin JW (1989) The visual superior colliculus and pulvinar. Rev
Oculomot Res 3:337-360.
Rodgers CK, Munoz DP, Scott SH, Pare M (2006) Discharge properties of monkey
tectoreticular neurons. J Neurophysiol 95:3502-3511.
Rohrer WH, Sparks DL (1993) Express saccades: The effects of spatial and temporal
uncertainty. Vision Res 33:2447-2460.
Saslow MG (1967) Effects of components of displacement-step stimuli upon latency for
saccadic eye movement. J Opt Soc Am 57:1024-1029.
Schall JD (1997) Visuomotor areas of the frontal lobe. In: Cerebral cortex (Peters RA,
Kaas JH, eds), pp527-638. New York: Plenum.
210
Schall JD (1995) Neural basis of saccade target selection. Rev Neurosci 6:63-85.
Schall JD, Thompson KG (1999) Neural selection and control of visually guided eye
movements. Annu Rev Neurosci 22:241-259.
Schiller PH, Malpeli JG (1977) Properties and tectal projections of monkey retinal
ganglion cells. J Neurophysiol 40:428-445.
Schiller PH, Koerner F (1971) Discharge characteristics of single units in superior
colliculus of the alert rhesus monkey. J Neurophysiol 34:920-936.
Schiller PH, Haushofer J, Kendall G (2004) An examination of the variables that affect
express saccade generation. Vis Neurosci 21:119-127.
Schiller PH, Sandell JH, Maunsell JH (1987) The effect of frontal eye field and superior
colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57:10331049.
Schiller PH, True SD, Conway JL (1980) Deficits in eye movements following frontal
eye-field and superior colliculus ablations. J Neurophysiol 44:1175-1189.
Schiller PH, Malpeli JG, Schein SJ (1979) Composition of geniculostriate input ot
superior colliculus of the rhesus monkey. J Neurophysiol 42:1124-1133.
Schiller PH, Stryker M, Cynader M, Berman N (1974) Response characteristics of single
cells in the monkey superior colliculus following ablation or cooling of visual cortex. J
Neurophysiol 37:181-194.
Schneider KA, Kastner S (2005) Visual responses of the human superior colliculus: A
high-resolution functional magnetic resonance imaging study. J Neurophysiol 94:24912503.
Scudder CA, Kaneko CS, Fuchs AF (2002) The brainstem burst generator for saccadic
eye movements: A modern synthesis. Exp Brain Res 142:439-462.
Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM (1996) Anatomy and
physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. I.
descending projections from the mesencephalon. J Neurophysiol 76:332-352.
Shafiq R, Stuart GW, Sandbach J, Maruff P, Currie J (1998) The gap effect and express
saccades in the auditory modality. Exp Brain Res 118:221-229.
Shen K, Pare M (2006) Guidance of eye movements during visual conjunction search:
Local and global contextual effects on target discriminability. J Neurophysiol 95:28452855.
211
Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17:417422.
Shires J, Joshi S, Basso MA (2010) Shedding new light on the role of the basal gangliasuperior colliculus pathway in eye movements. Curr Opin Neurobiol .
Short SJ, Enderle JD (2001) A model of the internal control system within the superior
colliculus. Biomed Sci Instrum 37:349-354.
Sinha N, Brown JT, Carpenter RH (2006) Task switching as a two-stage decision
process. J Neurophysiol 95:3146-3153.
Soetedjo R, Kaneko CR, Fuchs AF (2002) Evidence against a moving hill in the superior
colliculus during saccadic eye movements in the monkey. J Neurophysiol 87:2778-2789.
Sommer MA (1997) The spatial relationship between scanning saccades and express
saccades. Vision Res 37:2745-2756.
Sommer MA (1994) Express saccades elicited during visual scan in the monkey. Vision
Res 34:2023-2038.
Sparks D, Rohrer WH, Zhang Y (2000) The role of the superior colliculus in saccade
initiation: A study of express saccades and the gap effect. Vision Res 40:2763-2777.
Sparks DL (2002) The brainstem control of saccadic eye movements. Nat Rev Neurosci
3:952-964.
Sparks DL (1986) Translation of sensory signals into commands for control of saccadic
eye movements: Role of primate superior colliculus. Physiol Rev 66:118-171.
Sparks DL (1978) Functional properties of neurons in the monkey superior colliculus:
Coupling of neuronal activity and saccade onset. Brain Res 156:1-16.
Sparks DL (1975) Response properties of eye movement-related neurons in the monkey
superior colliculus. Brain Res 90:147-152.
Sparks DL, Hartwich-Young R (1989) The deep layers of the superior colliculus. Rev
Oculomot Res 3:213-255.
Sparks DL, Mays LE (1980) Movement fields of saccade-related burst neurons in the
monkey superior colliculus. Brain Res 190:39-50.
Sparks DL, Holland R, Guthrie BL (1976) Size and distribution of movement fields in the
monkey superior colliculus. Brain Res 113:21-34.
212
Stanford TR, Sparks DL (1994) Systematic errors for saccades to remembered targets:
Evidence for a dissociation between saccade metrics and activity in the superior
colliculus. Vision Res 34:93-106.
Stanford TR, Freedman EG, Sparks DL (1996) Site and parameters of microstimulation:
Evidence for independent effects on the properties of saccades evoked from the primate
superior colliculus. J Neurophysiol 76:3360-3381.
Story GW, Carpenter RH (2009) Dual LATER-unit model predicts saccadic reaction time
distributions in gap, step and appearance tasks. Exp Brain Res 193:287-296.
Talbot SA, Masson ME (1941) Physiological studies on neuronal mechanisms of visual
localization and discrimination. Am J Ophthalmol 1255–1263.
Thiem PD, Hill JA, Lee KM, Keller EL (2008) Behavioral properties of saccades
generated as a choice response. Exp Brain Res 186:355-364.
Thompson KG, Hanes DP, Bichot NP, Schall JD (1996) Perceptual and motor processing
stages identified in the activity of macaque frontal eye field neurons during visual search.
J Neurophysiol 76:4040-4055.
Trappenberg TP (2008) Decision making and population decoding with strongly
inhibitory neural field models. In: Computational modelling in behavioural neuroscience:
Closing the gap between neurophysiology and behaviour (Heinke D, Mavritsaki E, eds),
London: Psychology Press.
Trappenberg TP, Dorris MC, Munoz DP, Klein RM (2001) A model of saccade initiation
based on the competitive integration of exogenous and endogenous signals in the superior
colliculus. J Cogn Neurosci 13:256-271.
Treue S, Martinez Trujillo JC (1999) Feature-based attention influences motion
processing gain in macaque visual cortex. Nature 399:575-579.
Uhlrich DJ, Manning KA, Feig SL (2003) Laminar and cellular targets of individual
thalamic reticular nucleus axons in the lateral geniculate nucleus in the prosimian primate
galago. J Comp Neurol 458:128-143.
Vaccaro TM, Mitrofanis J (1996) Reticular thalamic region in the rabbit: Organisation of
efferents to the superior colliculus. J Comp Neurol 369:209-219.
Van Essen DC (2004) Organization of visual areas in macaque and human cerebral
cortex. In: The visual neurosciences (Chalupa L, Werner JS, eds), pp507-521.MIT Press.
Van Essen DC, Anderson CH, Felleman DJ (1992) Information processing in the primate
visual system: An integrated systems perspective. Science 255:419-423.
213
Van Gisbergen JA, Van Opstal AJ, Tax AA (1987 May) Collicular ensemble coding of
saccades based on vector summation. Neuroscience 21:541-55.
Van Gisbergen JA, Van Opstal AJ, Tax AA (1987) Collicular ensemble coding of
saccades based on vector summation. Neuroscience 21:541-555.
Van Opstal AJ, Munoz DP (2004) Auditory-visual interactions subserving primate gaze
orienting. In: Handbook of multisensory processes (Calvert GA, Spence C, Stein BE,
eds), pp373-394. Cambridge, MA.: MIT Press.
van Opstal AJ, van Gisbergen JAM (1989) Scatter in the metrics of saccades and
properties of the collicular motor map. Vision Res 29:1183-1196.
Wang X, Jin J, Jabri M (2002) Neural network models for the gaze shift system in the
superior colliculus and cerebellum. Neural Netw 15:811-832.
Watanabe M, Kobayashi Y, Inoue Y, Isa T (2005) Effects of local nicotinic activation of
the superior colliculus on saccades in monkeys. J Neurophysiol 93:519-534.
Weber H, Fischer B (1994) Differential effects of non-target stimuli on the occurrence of
express saccades in man. Vision Res 34:1883-1891.
Weber H, Latanov A, Fischer B (1993) Context dependent amplitude modulations of
express and regular saccades in man and monkey. Exp Brain Res 93:335-344.
Weber H, Aiple F, Fischer B, Latanov A (1992) Dead zone for express saccades. Exp
Brain Res 89:214-222.
Weber H, Fischer B, Bach M, Aiple F (1991) Occurrence of express saccades under
isoluminance and low contrast luminance conditions. Vis Neurosci 7:505-510.
White BJ, Kerzel D, Gegenfurtner KR (2006) Visually guided movements to color
targets. Exp Brain Res 175:110-126.
White BJ, Boehnke SE, Marino RA, Itti L, Munoz DP (2009) Color-related signals in the
primate superior colliculus. J Neurosci 29:12159-12166.
Wilson JR, Hendrickson AE, Sherk H, Tigges J (1995) Sources of subcortical afferents to
the macaque's dorsal lateral geniculate nucleus. Anat Rec 242:566-574.
Winograd-Gurvich CT, Georgiou-Karistianis N, Evans A, Millist L, Bradshaw JL,
Churchyard A, Chiu E, White OB (2003) Hypometric primary saccades and increased
variability in visually-guided saccades in huntington's disease. Neuropsychologia
41:1683-1692.
Wurtz RH, Goldberg ME (1989) The neurobiology of saccadic eye movements. 3:.
214
Wurtz RH (2008) Neuronal mechanisms of visual stability. Vision Res 48:2070-2089.
Wurtz RH, Optican LM (1994) Superior colliculus cell types and models of saccade
generation. Curr Opin Neurobiol 4:857-861.
Wurtz RH, Goldberg ME (1972a) Activity of superior colliculus in behaving monkey. 3.
cells discharging before eye movements. J Neurophysiol 35:575-586.
Wurtz RH, Goldberg ME (1972b) Activity of superior colliculus in behaving monkey. 3.
cells discharging before eye movements. J Neurophysiol 35:575-586.
Wurtz RH, Goldberg ME (1971) Superior colliculus cell responses related to eye
movements in awake monkeys. Science 171:82-84.
Wurtz RH, Sommer MA, Pare M, Ferraina S (2001a) Signal transformations from
cerebral cortex to superior colliculus for the generation of saccades. Vision Res 41:33993412.
Wurtz RH, Sommer MA, Pare M, Ferraina S (2001b) Signal transformations from
cerebral cortex to superior colliculus for the generation of saccades. Vision Res 41:33993412.
Yarbus AL (1967) Eye movements and vision. New York: Plenum Press.
Yarbus AL (1961) Eye movements during the examination of complicated objects.
Biofizika 6(2):52-56.
215
Appendix: Supplementary Analysis and Figures
216
3.A1 Supplemental Experimental Procedures for Chapter 3
3.A1.1 Characterization of Visual Response Field
We mapped the spatial visual response field of each neuron over the entire
viewable area of the display screen (60º x 50º). The visual response field reflected the
specific area of the visual display where visual stimuli could reliably elicit a visual
response. A response field mapping paradigm was used to map visual response fields.
Flashes of white light 100 ms (42.5 cd/m2), separated by 150 ms were presented in
pseudorandom order to 182 locations (Supplementary Fig. 3.S1A,B). No two subsequent
flashes within 10° eccentricity of the center were presented within 10° of each other and
no two subsequent flashes beyond 10° eccentricity of the center appeared within 20° of
each other. This ensured that no two targets ever appeared within the average response
field of a VM SCi neuron (Marino et al., 2008). The maximum visual response within the
response field was determined using a cubic smoothing spline interpolation algorithm
(Supplementary Fig. 3.S1C). In all subsequent experiments, stimuli were only presented
at this optimal location or the mirror location at a position opposite the horizontal and
vertical meridians.
217
A
B
VISUAL STIMULATION TASK
FP
T
EYE
50°
100ms
C
150ms
60°
Firing Rate (spikes/s)
D
200
Firing Rate (spikes/s)
100
100
0
Firing Rate (spikes/s)
E
150 100 50 0 50 100 150
Time from Target Onset (ms)
300
200
100
150 100 50 0 50 100 150
Time from Target Onset (ms)
Supplementary Figure 3.S1. A. Schematic representation of temporal events in the
visual response field mapping task for the fixation point (FP), target (T) and eye
position. B. 182 target locations (black dots) presented at 24 different directions and
6-10 different eccentricities used to map the visual response fields. C. Visual response
field for the representative visually responsive neuron shown in D and E. D,E
Target-aligned rasters and spike density functions of a representative visually
responsive neuron to locations outside (D) and inside its visual response field (E).
218
3.A2 Supplemental Results for Chapter 3
3.A2.1 Comparison of the visual response between V and VM neurons
Across the populations of V and VM neurons (collapsed over the 4 brightest
luminance levels where the most robust visual response was elicited) in the gap and delay
tasks, there was a trend for VM neurons to have a later VROL (1-1.5ms, Supplementary
Fig. 3.S2A), a faster growth time (1.6 ms, Supplementary Fig. 3.S2C) and a greater peak
magnitude (15-36 spikes/sec, Supplementary Fig. 3.S2D), however these differences did
not achieve statistical significance (t tests P > 0.05). No differences were found in the
decay rate between V and VM neurons (Supplementary Fig. 3.S2E).
When the properties of the visual response were compared across tasks
(Supplementary Fig. 3.S2), it was found that in the VM population only, the VROL in the
gap occurred earlier than the delay (t test, P < 0.05), and the rate of rise occurred faster in
the delay than in the gap (t test, P < 0.05). The reduced VROL in the VM neurons of the
gap relative to the delay has been shown previously (Bell et al., 2006) and likely resulted
from fixation offset effects during the gap (Dorris and Munoz, 1995).
219
D elay
VROL
A
Gap
Peak Magnitude
B
300
200
*
*
15
VM
V
-5
-4
-3
VM
V
VM
V
10
-6
VM
25
Time from ROL to Peak
(ms)
V
Slope of Visual Response Decay
C
Decay Rate
(Slope)
D
V
Growth Time
20
150
VM
V
VM
V
50
VM
VROL
(ms)
60
250
VM
*
70
V
*
P e a k M a g n itu d e
(s p ik e s /s )
80
Supplementary Figure 3.S2. Effects of neuron V and VM subtype on visual sensory
response properties recorded at the brightest luminance (42 cd/m2) in the delay (gray)
and gap (black) tasks. A. VROL, B. Peak Magnitude, C. Growth Time, D. Decay Rate.
Asterisks denote statistical significance (t test, P <0.05). All error bars denote standard
error.
220