* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Do superior colliculus projection zones in the inferior pulvinar
Brain Rules wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neurophilosophy wikipedia , lookup
Development of the nervous system wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Environmental enrichment wikipedia , lookup
Central pattern generator wikipedia , lookup
Human brain wikipedia , lookup
Cortical cooling wikipedia , lookup
Neural coding wikipedia , lookup
Time perception wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Mirror neuron wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroesthetics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Hypothalamus wikipedia , lookup
Optogenetics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Circumventricular organs wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
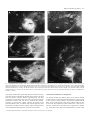
© European Neuroscience Association European Journal of Neuroscience, Vol. 11, pp. 469–480, 1999 Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Iwona Stepniewska, Hui-Xin Qi and Jon H. Kaas Department of Psychology, Wilson Hall, Vanderbilt University, Nashville, TN 37240 USA Keywords: ascending projection, extrastriate cortex, lateral geniculate nucleus, monkey, posterior thalamus Abstract In order to determine the relationship of superior colliculus inputs to thalamic neurons projecting to the middle temporal visual area (MT), injections of wheat germ agglutinin conjugated with horseradish peroxidase were placed in the superior colliculus of three owl monkeys, with injections of Fast Blue in the MT. The locations of labelled terminals and neurons in the posterior thalamus were related to four architectonically distinct nuclei of the inferior pulvinar (Stepniewska & Kaas, Vis. Neurosci. 14, pp.1043– 1060, 1997). Fast Blue injections in the MT labelled neurons largely in the medial nucleus of the inferior pulvinar. A few labelled neurons were found in the adjoining central medial nucleus of the inferior pulvinar, as well as in the lateral pulvinar and the dorsal lateral geniculate nucleus. Superior colliculus inputs were most dense in the posterior and medial nuclei of the inferior pulvinar. There were sparser inputs to the central lateral nucleus of the inferior pulvinar, locations in the lateral and medial pulvinar, and the dorsal lateral geniculate nucleus. The results indicate that the medial nucleus of the inferior pulvinar, the major projection zone to the MT, does not receive a significant input from the superior colliculus. Introduction Traditionally, the pulvinar complex of the dorsal thalamus has been divided into the inferior, lateral, medial and, sometimes, anterior divisions (see Kaas & Huerta, 1988). Although all of these large divisions probably have functionally significant subdivisions, the most compelling evidence has been for the inferior pulvinar, where several nuclei or subnuclei have been distinguished on the basis of architectonic distinctions and differences in connections. Nearly 30 years ago, Mathers (1971) reported that the superior colliculus projections were unevenly distributed in the inferior pulvinar with a concentration of inputs in a medioposterior region distinguished as a ‘posterior’ nucleus. Soon thereafter, Spatz & Tigges (1973) described projections from the middle temporal visual area (MT), as most dense in a medial portion of the inferior pulvinar, just lateral to the ‘posterior’ nucleus of Mathers (1971). Next, Lin et al. (1974) found that injections of the newly available retrograde tracer, horseradish peroxidase, into the MT labelled the same medial zone of the inferior pulvinar. In subsequent studies, Lin & Kaas (1979, 1980) concluded that the boundaries of these nuclei in the inferior pulvinar could be distinguished in myelin preparations by encapsulating fibre bands. A posterior nucleus of the inferior pulvinar (which they labelled IPp), corresponded to the posterior nucleus of Mathers (1971), and a central nucleus (their IPc), received inputs from the superior colliculus and projected to more rostral and more caudal divisions of visual cortex, respectively. A medial nucleus (their IPm) was described as devoid of superior colliculus inputs, while projecting densely to the MT. Thus, no part of the inferior pulvinar complex provided a significant relay of visual information from the superior colliculus to the MT. Recently, there have been reasons to question the basic proposal Correspondence: Dr Jon H. Kaas, as above. E-mail: [email protected] Received 20 May 1998, revised 7 September 1998, accepted 10 September 1998 that three nuclei subdivide the inferior pulvinar, with none of the three providing a significant relay of superior colliculus information to the MT. First, re-examinations of the architecture of the inferior pulvinar, using a range of current histochemical techniques, have revealed further subdivisions. In our recent re-interpretation of the organization of the inferior pulvinar (the original IPc) has been divided into central medial and central lateral nuclei or subnuclei (which we refer to as PICM and PICL) while the medial and posterior nuclei of the inferior pulvinar (PIm and PIp) are retained, resulting in four quite distinguishable structures (Stepniewska & Kaas, 1997). Gutierrez et al. (1995) had previously used similar histochemical procedures to divide the PIc into three subdivisions, a ‘PIc’ corresponding to our PICM, a ‘PIL’ largely corresponding to our PICL, and a narrow, lateral shell. Both Gutierrez et al. (1995) and an earlier study of Cusick et al. (1993) provided valuable information on the histochemical characteristics of the PIm and PIp. These new interpretations of how the inferior pulvinar is subdivided raise new questions about how these redefined subdivisions relate to inputs from the superior colliculus and to cortical targets, such as the MT. While the evidence across primate species is rather compelling for the conclusion that one of these subdivisions, the PIm, projects preferentially to the MT (see Cusick et al., 1993 for review), the PIm is in a location that could correspond to the site of dense inputs from the superior colliculus (see Gutierrez et al., 1995; for review). Cusick et al. (1993) noted that histochemical features of the PIm, such as dense staining for cytochrome oxidase (CO) and parvalbumin, and light staining for calbindin, are characteristics of the relay nuclei of the thalamus, such as the ventroposterior nucleus. Thus, the histochemical features suggest that the PIm could function as a relay nucleus by receiving inputs from the superior colliculus and projecting to the MT. Another reason to consider the possibility that the superior colliculus relays to the MT through the inferior pulvinar is the evidence that 470 I. Stepniewska et al. the MT can be quite responsive to visual stimuli after lesions of striate cortex. Rodman et al. (1989) found that many neurons in the MT of macaque monkeys were responsive to visual stimuli after removal of striate cortex, and that some neurons even retained at least some of their normal response properties, such as selectivity for direction of movement. Furthermore, lesions of the superior colliculus, in conjunction with striate cortex lesions, abolished responsiveness to visual stimuli (Rodman et al., 1990). The most reasonable interpretation of these findings is that the superior colliculus relays visual information to the MT via projections to the pulvinar, and that this relay adequately activates the MT in the absence of primary visual area (striate cortex, V1). As the target of the most dense inputs from the superior colliculus, the inferior pulvinar would be the likely source of the relay. In the present study, we sought to resolve some of the questions concerning possible relay of superior colliculus information to the MT by studying pulvinar connections with the superior colliculus and with the MT in the same animals. In addition, we used histochemical procedures to define the currently proposed subdivisions of the inferior pulvinar. Finally, we studied owl monkeys, the primate from which the evidence that the inferior pulvinar does not provide a significant relay of visual input from the superior colliculus to the MT was originally gathered, so that early and present results could be directly compared. Materials and methods Connections of the pulvinar were studied in three adult owl monkeys (Aotus trivirgatus) by placing injections of tracers into the superior colliculus and the MT under aseptic conditions. Results were related to recently described architectonic subdivisions of the inferior pulvinar (Stepniewska & Kaas, 1997). Surgery and injections Monkeys were anaesthetized for surgery with intramuscular injection of a mixture of ketamine hydrochloride (30 mg/kg) and xylazine hydrochloride (1–2 mg/kg), supplemented as needed to maintain a surgical level of anaesthesia or replaced with 2% isoflurane as an inhalation anaesthetic. The head was fixed in a stereotaxic apparatus, and a portion of posterior parietal and visual cortex was exposed during aseptic surgery. In each case, a low-impedance microelectrode was lowered through posterior parietal cortex to the superior colliculus, using stereotaxic measurements from the brain sections of four owl monkeys processed for other studies. After recordings of visually evoked multineuron activity were used to establish the location of the superior colliculus, several additional electrode penetrations were made to determine its approximate rostral, caudal and lateral boundaries. Next, a bevelled Hamilton microsyringe, with a microelectrode glued to the needle, was filled with a solution of 2% wheat germ agglutinin conjugated with horseradish peroxidase (WGA-HRP) in saline. The microsyringe was then lowered to the previously determined level of the superior colliculus and adjusted until adequate visually evoked responses were recorded from the attached microelectrode. Then, µ 0.1 µL of tracer solution was injected, and the microsyringe was removed after 15–20 min. Next, the fluorescent tracer, Fast Blue (FB) was pressure-injected into the MT by using a glass micropipette attached to a Hamilton syringe. Approximately 0.2–0.4 µL of 2% FB in saline was injected in the location of the MT, which was estimated by its position relative to the caudal tip of the superior temporal sulcus (Allman & Kaas, 1971). The micropipette was removed after 15–20 min. The exposed cortex was covered with gelatin film, the skull opening sealed with an artificial bone flap made of dental cement, and the skin sutured. Animals were carefully monitored during recovery from anaesthesia, and they received antibiotics and analgesics. After 4–5 days, the animals were deeply anaesthetized and perfused through the heart with saline followed by 2% paraformaldehyde in saline as a fixative, followed by 10% sucrose in fixative. The brain was removed and cortex was separated from the brainstem, and manually flattened between glass slides. The cortex and brain stem were stored in 30% sucrose in the refrigerator overnight. Histology and anatomical analysis Twelve to 24 h after perfusion, the cortex and brainstem (including the thalamus) were cut into 40–50-µm sections on a freezing microtome. A block of flattened cortex containing the MT and other visual areas was cut parallel to the surface, and divided into three series of sections. One series was mounted on glass slides and coverslipped without further processing so that neurons labelled with FB could be located. Another series was processed for myelin according to the procedure of Gallyas (1979), and the third series was processed for CO following the procedures of Wong-Riley (1979). Both the myelin and CO procedures allowed us to identify the MT as a darker oval in the processed sections (see Krubitzer & Kaas, 1990) and to locate the injection sites relative to the MT boundaries. The brainstem through the midbrain and thalamus was cut in the coronal plane and divided into five separate series of every fifth section. One series was mounted and coverslipped without further processing so that neurons labelled by retrogradely transported FB could be visualized. Another series of sections was histochemically processed to reveal WGA-HRP according to the procedures of Gibson et al. (1984). The remaining sections were processed to reveal subdivisions of the inferior pulvinar (see Stepniewska & Kaas, 1997). One series was treated histochemically for acetylcholinesterase (AChE) using the protocol of Geneser-Jensen & Blackstad (1971), and another for CO (Wong-Riley, 1979). The last series was processed immunocytochemically for the calcium-binding protein, CalbindinD28K (Cb) (Celio, 1990). Brainstem sections were drawn through a drawing tube with a Wild macroscope, and regions of WGA-HRP label were located under darkfield illumination. The distributions of neurons labelled with FB were charted with a fluorescent microscope. Sections processed for AChE, CO, or Cb were drawn at the same magnification, and boundaries of pulvinar subdivisions were outlined. These boundaries were then superimposed on the drawings of labelled neurons and terminals using adjacent brain sections. A surface view of the superior colliculus was reconstructed from serial sections, and the region labelled by each injection was determined relative to visual hemifield coordinates superimposed from standardized retinotopic maps of the superior colliculus of owl monkeys (Lane et al., 1973). The injection sites of FB were localized within the architectonic boundaries of the MT that were determined by aligning drawings of the injection sites and CO-and myelin-based boundaries. Results Injections of WGA-HRP were placed in the superior colliculus of the same three owl monkeys in which FB had been placed in the MT. The resulting patterns of labelled neurons from the FB injection and terminals from the WGA-HRP injections were related to the architecture of the pulvinar complex and the adjoining dorsal lateral geniculate nucleus (LGNd). The results indicate that there is little overlap in the pulvinar between superior colliculus inputs and neurons © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 Superior colliculus projections 471 FIG. 1. Darkfield photomicrographs of the coronal sections through the pulvinar in Case 97-53. Top panel: adjacent sections through the inferior pulvinar (A) showing the distribution of anterogradely labelled superior colliculus terminals and (B) stained for CO. Note that CO-dark PIm is free of label, and laterally adjacent CO-lighter PICM is full of labelled terminals. Bottom panel: sections from the different levels of the inferior pulvinar with anterogradely labelled terminals distributed in (C) the PIp, (D) the PIp and PICM with a visible fusion zone below the PIm and (E) the PICM and PL. Note the label of fibres coursing through the PIm in C and D. In E, the label in the PL is patchy and much sparser than in the PICM. The thin black line marks the region of labelled fibres. Scale bar 5 500 µm. projecting to the MT. More specifically, the PIm constitutes the major projection nucleus to the MT, but the PIm receives little or no input from the superior colliculus. Results supporting these and other conclusions are presented in three parts. First, we briefly present the architectonic evidence that was used to subdivide the pulvinar. Secondly, we describe the superior colliculus projections to the posterior thalamus. Next, we indicate where neurons projecting from the pulvinar to the MT are located. Finally, superior colliculus inputs to the LGNd and LGNd projections to the MT are described. Architectonic subdivisions of the pulvinar Our material revealed four distinct regions of the inferior pulvinar complex. We have recently described the architecture of these regions in owl monkeys, squirrel monkeys and macaques (Stepniewska & Kaas, 1997), and the present results closely conform to this previous account. The most significant of these subdivisions is PIm, since it has been considered to be the main source of projections to the MT (e.g. Lin & Kaas, 1980). PIm was dark and patchy in CO and AChE, © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 472 I. Stepniewska et al. © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 Superior colliculus projections 473 and light in Cb preparations. Examples of the appearance of PIm in CO sections are shown relative to distributions of terminals labelled by WGA-HRP in Fig. 1 (A and B). More medially, the posterior nucleus, PIp, was dark for Cb, and moderate for CO and AChE. More laterally, the medial central nucleus, PICM, was dark for Cb and light for AChE and CO. Most laterally, the lateral central nucleus, PICL, was dark for AChE and moderate for CO and Cb. Overall, the differences in appearance between the four nuclei were obvious, and there was no chance of misidentification. Most importantly, the newly proposed subdivision, PICM, was distinct from PIm. Projections of the superior colliculus Projections of the superior colliculus to the pulvinar complex were studied after large injections of WGA-HRP into the superior colliculus of three owl monkeys. The full extent of the injections varied, however, with the largest injection occurring in Case 97-53, where the superficial layers were densely labelled throughout all but the most rostral fringe of the superior colliculus (Fig. 2A). In addition, the deep layers of the superior colliculus and part of the inferior colliculus were labelled in this case. A somewhat smaller zone of dense label was found in Case 96-89 (Fig. 3A). While the densely labelled zone included all but the most rostral part of the medial superior colliculus, less of the rostrolateral superior colliculus was included. Yet, projections would involve large parts of the representations of both the upper and lower visual quadrants in this case. Here, only the superficial layers of the superior colliculus were densely labelled. In the third case, 96-82 (Fig. 4A), the dense label from the injection covered much of the lateral part of the superior colliculus representing the upper visual quadrant, and only lighter, diffuse label was found in the medial part of the superior colliculus. Projections in this case were probably restricted to those from the representation of the upper visual quadrant. The overall pattern of projections from the superior colliculus to the pulvinar was demonstrated most effectively in Case 97-53, which had the largest injection site (Fig. 2A). In this case, transported label reflecting terminations (Fig. 2C) was exceedingly dense throughout most of the PICM and PIp (Fig. 1A and C–E). Regions of moderate to light concentrations of label occurred in the PICL and in the lateral pulvinar (PL) (Fig. 1E). Label in the medial pulvinar (PM) was sparse and scattered. Thus, most of the label was in the PICM and PIp of the inferior pulvinar. The zones of label in the two nuclei appeared to fuse ventrally in the caudal thalamus (see section 92, Fig. 1D), suggesting either the course of entering fibres of passage, or that the PIm does not extend to the ventral margin of the pulvinar in this location. The zones of label in the PICM and PIp also extended dorsally past the brachium of the superior colliculus, the traditional dorsal boundary of the inferior pulvinar, in agreement with the compelling architectonic evidence that both of these nuclei extend dorsally past the brachium (see Stepniewska & Kaas, 1997). Most of the PIm and even the PICL were free of label. The sparse label that was observed in parts of the PIm could easily reflect preterminal fibres coursing to the PICM. The transported label in the pulvinar was less extensively distributed in Case 96-89 (Fig. 3), possibly because the dense injection core did not involve the rostral superior colliculus representing the central 5° of vision. The injection core was also superficial, with only limited involvement of the deeper layers. Again, the label was most dense in the PICM and PIp, but the label was concentrated ventrally, and it did not extend dorsally across the brachium of the superior colliculus. This observation is consistent with the premise that central vision is represented dorsally in these two nuclei. Some label was apparent in the PICM and in the caudal part of the PL; the PIm was devoid of label, as was the entire medial pulvinar. In Case 96-82, the injection core was confined to the lateral half of the colliculus, and the dense label was in the superficial layers (Fig. 4A). The transported label in the pulvinar complex was largely confined to the PICM and PIp, with nearly all of the label in the portions of these nuclei under the brachium of the superior colliculus (Fig. 4C). In both nuclei, the label was mostly caudal, and more medial caudally and more lateral rostrally. The results suggest that the lower quadrant of the visual hemifield is represented across the more caudal aspects of the PICM and PIp. A region of quite sparse label was found in the medial part of the PICL, possibly reflecting label in fibres terminating in the PICM. No label was noted in other parts of the PICL, PL or PM. Pulvinar projections to the MT In all three monkeys, the injections of FB were located within the MT as defined architectonically in sections cut parallel to the brain surface. These sections allowed the MT to be outlined as an oval of cortex that was more densely stained for myelin and CO. In Case 97-53 (Fig. 2) the FB injection was placed centrally in the MT in a location representing paracentral vision slightly into the lower quadrant (Allman & Kaas, 1971). Most of the retrogradely labelled neurons in the pulvinar complex were in the PIm, with labelled neurons in both portions over and under the brachium of the superior colliculus. A few labelled neurons were in the PICL and possibly upper PICM, and several foci of labelled neurons were in the PL. No labelled neurons were found in the PM. Results from Case 96-89 (Fig. 3), with an injection in the lateral MT, devoted to paracentral vision of the upper quadrant, were similar, in that most of the labelled neurons were in the PIm, a few were in the PICM and PICL, and several foci of labelled neurons were in the PL. Unexpectedly, some labelled neurons were in the PM, and a few were in the PIp. The labelled neurons in the PIm were largely below the brachium of the superior colliculus and they were more caudal than rostral in the nucleus. Labelled neurons were also most densely distributed in the PIm of the third case, 96-82 (Fig. 4), where the injection was placed in the rostral MT, which is devoted to peripheral vision. However, in this case neurons labelled with FB were located mainly in the upper portion of the PIm, above the brachium of superior colliculus. Other labelled neurons were in the PICL, and a few were in the PICM, PL and PM. The LGN: superior colliculus inputs and projections to the MT In two of three cases (97-53 and 96-89) superior colliculus injections produced label in the LGNd, as well as the pregeniculate (PG) or FIG. 2. The pattern of afferent and efferent pulvinar projections, originating from the superior colliculus and sent to the MT in Case 97-53. (A) The WGA-HRP injection site in the superior colliculus is shown in coronal sections from posterior (26) to anterior (68). The effective injection site of dense label is dark while the diffusion zone is hatched. (B) The FB injection site in the MT reconstructed from the sections of flattened cortex. (C) The distribution of anterogradely labelled terminals after the superior colliculus injection (small dots) and retrogradely labelled neurons after the MT injection (large dots), shown on coronal thalamic sections from posterior (80) to anterior (122). The boundaries of pulvinar subdivisions are marked with thin lines. Central (PIc), central lateral (PICL), central medial (PICM), medial (PIm) and posterior (PIp) divisions of the inferior pulvinar are indicated. © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 474 I. Stepniewska et al. FIG. 3. The pattern of pulvinar connections originating in the superior colliculus and sent to the MT in Case 96-89. Other conventions as in Fig. 2. Superior colliculus projections 475 FIG. 4. The pattern of pulvinar connections originating in the superior colliculus and sent to the MT in Case 96-82. Other conventions as in Fig. 2. 476 I. Stepniewska et al. ventral lateral geniculate nucleus (Figs 2, 3, 5 and 6). Because of the large injection site in Case 97-53, the label was much more extensive than in Case 96-89. However, in both cases the terminals of axons coming from the superior colliculus were unevenly distributed, aggregating in patches in the intercalated or koniocellular (K) LGN layers (Hendry & Casagrande, 1996). The main focus of label was always in the K1 (or S) layer (see Kaas et al., 1978) that lies next to the optic tract. Smaller patches of label were found in layers K2, between magnocellular (M) layers, and K3, between the M and parvocellular (P) layers. In Case 97-53, the label was most extensive at the most caudal LGN level, extending through its central level, and the anterior LGNd was almost free of label (Fig. 5). Injections in the MT in Case 97-53 labelled a few neurons in the K layers or on the borders of M layers of the LGN (Fig. 5). The terminals labelled in K layers after superior colliculus injections partly overlapped with retrogradely labelled neurons, suggesting that these connections form a pathway through which visual information from the retina reaches area the MT. These neurons, however, were not numerous, and some of them may not receive superior colliculus inputs. In Case 96-89 (Fig. 6) the anterogradely labelled axonal terminals were more restricted than in Case 97-53 and were found only in the most caudal portions of the K1 and K2 LGN layers. At the same level, few neurons labelled after the MT injections were found at the border of the magnocellular layer receiving input from the contralateral eye (Mc) and K2 layers (Fig. 6, section 147), and they did not overlap with labelled superior colliculus terminals. We did not find neurons labelled after the MT injections in Case 96-82. Injections in the superior colliculus also labelled the PG. With WGA-HRP, both anterogradely labelled terminals and retrogradely labelled neurons were found, showing that connections between the superior colliculus and the pregeniculate are reciprocal. The label was very extensive in Case 97-53 (Fig. 5), covering almost the whole extent of the PG. In Case 96-89 only scattered anterogradely labelled terminals were found (Fig. 6). Discussion The present results demonstrate that the superior colliculus projects most densely to the PIp and PICM nuclei of the inferior pulvinar complex, and that the PIm nucleus, situated between them, provides the main pulvinar projection to the MT. Thus, the PIm does not relay superior colliculus information to the MT. The superior colliculus also projects to other parts of the pulvinar complex, as well as the LGNd. Some of these pulvinar regions and a few cells of the LGNd project to the MT, providing sparse, but direct, pathways from the superior colliculus to the MT. Subdivisions of the pulvinar Modern histochemical and immunocytochemical procedures clearly reveal four distinct regions in the territory of the traditional inferior pulvinar. Two of these subdivisions, the posterior (PIp) and medial (PIm) nuclei of the inferior pulvinar, correspond to those previously described by Lin & Kaas (1979, 1980). However, their third central division (IPc) contains obvious medial and lateral subdivisions, which we have termed PICM and PICL, to indicate that they were derived from the original PIc (Stepniewska & Kaas, 1997). These subdivisions are apparent in both New and Old World monkeys, and they were first recognized by others (Gutierrez et al., 1995). Somewhat different terminologies have been applied for the region of the original ‘PIc’ in a manner that has been inconsistent across species (see Stepniewska & Kaas, 1997, for review). The present terminology avoids the confusion of using the earlier term of ‘PIc’ in new and inconsistent ways. Whether the subdivisions of the inferior pulvinar should be considered as separate nuclei or subnuclei (layers) is another issue (see Gutierrez et al., 1995), but we refer to the subdivisions as nuclei simply for convenience. The PL and PM undoubtedly contain subdivisions as well, but none have been clearly demarcated, and quite possibly there are further subdivisions within the inferior pulvinar. The important point here is that four subdivisions of the inferior pulvinar are now obvious, and they can be related to superior colliculus inputs and to the locations of neurons projecting to the MT (for a schematic description of different proposed subdivisions of the inferior pulvinar in owl, squirrel and macaque monkeys, see fig. 16 of Stepniewska & Kaas, 1997). Projections of the PIm and other parts of the pulvinar to the MT In each of the three cases with MT injections, most of the labelled neurons were in PIm. In these cases, the PIm was identified as calbindin-poor and AChE- and CO-rich. Thus, there was no doubt about which nucleus provided the major projection to the MT. The PIm, of course, has not been identified by such criteria in previous studies of thalamic projections to the MT, but it has been clear that the projections have been either from the PIm or a very similar region of the thalamus. In the original study of pulvinar projections to the MT in primates, the medial location of the projection zone in the inferior pulvinar was stressed, and the suggestion was made that this medial group of cells form a separate nucleus that projects to the MT (Lin et al., 1974). Later, these projecting neurons were related to a medial subdivision of the inferior pulvinar, the PIm, that was apparent in brain sections stained for myelin, since encapsulating fibre bands separated the PIm from the smaller PIp on its posterior-medial border and a larger PIc on its lateral border (Lin & Kaas, 1979). By comparing brain sections processed for myelin with those processed for Cb, AChE or CO in the same owl monkeys, we showed that the encapsulated PIm of owl monkeys is Cb-poor, AChE-rich and CO-rich (Stepniewska & Kaas, 1997). The PIm has also been distinguished by Cb, AChE and CO procedures in squirrel monkeys and macaque monkeys (Cusick et al., 1993; Gutierrez et al., 1995; Stepniewska & Kaas, 1997). In squirrel monkeys, the PIm is the region of the pulvinar that is the most densely interconnected with the MT (Spatz & Tigges, 1973; Cusick et al., 1993), and the PIm region has been shown to have dense MT connections in macaque monkeys (Standage & Benevento, 1983; Ungerleider et al., 1984; however, see Maunsell & Van Essen, 1983), marmosets (Dick et al., 1991) and galagos (Wall et al., 1982). Thus, it seems clear that the major connections of the MT with the pulvinar are with the PIm. Our MT injections also labelled some neurons in the PICM, PICL and PIp. In previous studies horseradish peroxidase injections in the MT resulted in few or no labelled neurons in the PIc and PIp (Lin & Kaas, 1980), but FB may be more sensitive as a retrogradely transported marker. Another possibility is that terminals just outside the MT took up some of the FB, but there was no indication of that possibility from our examinations of the injection sites. However, the PIc and PIp do project to the cortex surrounding the MT (Lin & Kaas, 1979; Steele et al., 1991; Cusick et al., 1993). We conclude that the PIp and PIc probably project to the MT, but this projection is sparse and possibly variable across species and individuals. Injections of FB also labelled foci of neurons in the PL. In previous studies in squirrel monkeys, injections involving the MT, as well as cortex caudal to the MT, labelled neurons in the PL (Cusick et al., 1993), and the MT clearly projects to the PL in macaques (Ungerleider et al., 1984), squirrel monkeys (Spatz & Tigges, 1973) and galagos © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 Superior colliculus projections 477 FIG. 5. The pattern of LGN connections originating in the superior colliculus and sent to the MT in Case 97-53, shown in a series of coronal sections arranged from posterior (128) to anterior (164). Anterogradely labelled terminals after superior colliculus injections are marked with small dots, and retrogradely labelled neurons after the MT injections are marked with large dots. The LGNd layers are marked with thin lines. The koniocellular, magnocellular and parvocellular layers are designated K, M, and P, respectively. The ‘c’ layers are those receiving input from the contralateral eye, the ‘i’ layers are those receiving input from the ipsilateral eye. In the PG, the WGA-HRP anterogradely labelled terminals overlapped with the WGA-HRP retrogradely labelled neurons. For clarity the retrogradely labelled neurons are not marked. © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 478 I. Stepniewska et al. FIG. 6. The pattern of LGN connections originating in the superior colliculus in Case 96-89 shown on coronal sections arranged from posterior (142) to anterior (152). Other conventions as in Fig. 5. (Wall et al., 1982). Since corticothalamic connections are expected to be reciprocal, it is likely that the PL projects to the MT in all these primates. The projection neurons in owl monkeys were scattered, and not numerous. The separate foci may relate to proposed subdivisions of PL (Stepniewska & Kaas, 1997; Beck & Kaas, 1998; see also Wong-Riley, 1977), but these results do not provide clear evidence for such subdivisions. Superior colliculus inputs to the pulvinar Our results provide compelling evidence that the major projections from the superior colliculus to the pulvinar complex are to the PIp, PICM and PICL, much as Lin & Kaas (1979) had described earlier. By injecting tracers into the superior colliculus and the MT of the same monkeys, we were able to demonstrate that the superior colliculus projections were very sparse in the zone where dense MT projections originate in the inferior pulvinar, and our architectonic analysis in these monkeys allowed the four nuclei of the inferior pulvinar to be reliably identified. We conclude that PIm does not provide a relay of superior colliculus visual information to the MT, at least in owl monkeys. The importance of resolving this issue lies in the considerable uncertainty over the possibility of a major relay of superior colliculus inputs from the inferior pulvinar to the MT. The superior colliculus inputs have been described as densely distributed to both lateral and medial sectors of the inferior pulvinar, allowing for the possibility that there is considerable overlap, rather than complete segregation, in the inputs and the projections to the MT. Indeed, Cusick et al. (1993) have noted that the dense CO and parvalbumin staining and calbindin-poor nature of the PIm are highly suggestive of a primary sensory relay nucleus, such as the ventroposterior nucleus or the lateral geniculate nucleus (LGN). They also noted that the tectorecipient zones in the inferior pulvinar of macaque monkeys (Benevento & Fallon, 1975; Harting et al., 1980; Benevento & Standage, 1983) and squirrel monkeys (Mathers, 1971), based on location, seem likely to include the PIm. Furthermore, Raczkoski & Diamond (1980) have previously concluded that the tectorecipient zone of galagos projects to the temporal lobe, ‘probably’ the MT. While evidence for such a relay in these other primates needs to be directly examined, since primate taxa could differ, there is no compelling evidence for this relay in any primate. Rather, the approximation of the two projection zones, together with little evidence for how the superior colliculus inputs relate to the chemoarchitecture of the inferior pulvinar, allowed several possible interpretations of connection patterns. The present results suggest that we reconsider the sources of activation of the MT in the absence of V1. Rodman et al. (1989) reported that considerable responsiveness to visual stimuli remained for MT neurons after striate cortex removal. Furthermore, this responsiveness disappeared after superior colliculus lesions in such monkeys (Rodman et al., 1990). These results suggested the need for a direct tectopulvinar pathway that is capable of activating MT © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 Superior colliculus projections 479 neurons, and preserving considerable visual functions in the absence of V1. Such a pathway has also been suggested for the preservation of visual abilities in brain-damaged patients that demonstrate aspects of preserved vision known as blindsight (see e.g. Cowey & Stoerig, 1991). The results of Rodman et al. (1989) seem to differ from those of Maunsell et al. (1990) who reported that blocking LGN activity in macaques ‘essentially eliminated’ the evoked activity of MT neurons. Kaas & Krubitzer (1992) found that striate cortex lesions deactivated MT neurons in owl monkeys. The reasons for these seemingly different results remain unclear, but the present results suggest that any role for the superior colliculus in MT activity does not involve a direct tecto-pulvinar–MT relay. While some overlap of sparse inputs from the superior colliculus with neurons in the pulvinar may occur, especially in the PL, these connections would seem to be too few projecting to the MT to substitute for the striate cortex inputs. Instead, the substantial projections from the PIc and PIp to cortical areas that are interconnected with the MT are more likely to play this role. Our results suggest that the main thalamic inputs from the superficial layers of the superior colliculus are to the inferior pulvinar. Labelled terminals were found outside the inferior pulvinar in the PL in only one of our two cases with superficial injections. However, more foci of labelled terminals were apparent in the PL of our case with the largest injection, that involved both superficial and deep layers. Additional foci were in the PM. In both squirrel monkeys (Harting et al., 1978; Huerta & Harting, 1983) and macaques (Harting et al., 1980), the superior colliculus clearly projects to the PL, although these projections are not always obvious (Partlow et al., 1977). Some label was apparent in the PM after injections in the superior colliculus in both squirrel and macaque monkeys (Harting et al., 1978, 1980). The PM is usually considered a target of only the deeper layers of the superior colliculus (see Kaas & Huerta, 1988). Since the MT injections labelled neurons in the PL that were occasionally overlapped by superior colliculus inputs, a pathway does exist for the direct activation of MT neurons via a tecto-pulvinarpathway connection. However, this pathway would probably have only weak effects on the MT because of the sparseness of the superior colliculus inputs and the limited numbers of PL neurons projecting to the MT. A tecto-geniculo-cortical path to the MT Our superior colliculus injections also revealed projections to the LGNd. These inputs are known to originate from the superficial grey layer and terminate most densely on the interlaminar zones and the ventrally located superficial ‘S’ layers (Harting et al., 1978, 1980), and are likely to activate the koniocellular or small cell relay in the LGNd that projects to superficial layers of striate cortex (Casagrande & Kaas, 1994; Casagrande, 1994). Our MT injections labelled a few large neurons in the LGNd that were distributed near, or in, the interlaminar zones, and these neurons are possibly activated by superior colliculus inputs. Although the vast majority of LGN neurons in primates project to V1, a few have been shown to project to the secondary visual area (prestriate cortex, V2) (Bullier & Kennedy, 1983), dorsolateral visual cortex (Lysakowski et al., 1988; Cowey & Stoerig, 1989), the dorsomedial visual area (Beck & Kaas, 1998), the MT (Fries, 1981), and even inferior temporal cortex (HernandezGonzalez et al., 1994; however, see Sorenson & Rodman, 1996). Projections from the LGNd to the MT might be expected from early evidence that the MT projects to the LGNd (Lin & Kaas, 1977; Graham et al., 1979; however, see Ungerleider et al., 1984) and the evidence that connections between visual structures are usually reciprocal (see Felleman & Van Essen, 1991). Such extrastriate projections of the LGNd have been postulated as important in mediating aspects of vision in extrastriate cortex after lesions of V1 (Cowey & Stoerig, 1989), but the ones to the MT constitute a very sparse and probably ineffective source of direct activation for MT neurons after lesions of V1 (Rodman et al., 1989). Acknowledgements We thank Judy Ives and Laura Trice for technical assistance. Helpful comments in the manuscript were provided by Pam Beck, Troy Hackett, Fabrizio Strata and Hilary Taub. The research was supported by NEI Grant EY 02686. Abbreviations AChE, acetylcholinesterase; c, (suffix) contralateral; Cb, Calbindin-D28K; CO, cytochrome oxidase; FB, Fast Blue; i, (suffix) ipsilateral; K, koniocellular layer of the LGN; LGN, lateral geniculate nucleus; LGNd, dorsal LGN; M, magnocellular (layer); MT, middle temporal visual area; P, parvocellular (layer); PIm, medial nucleus of the inferior pulvinar; PIc, original nomenclature, largely corresponds to PICM here; PIL, original nomenclature, largely corresponds to PICL here; PICM, central medial nucleus of the inferior pulvinar; PICL, central lateral nucleus of the inferior pulvinar; PIp, posterior nucleus of the inferior pulvinar; PG, pregeniculate nucleus; PL, lateral pulvinar; PM, medial pulvinar; V1, primary visual area (striate cortex); WGA-HRP, wheat germ agglutinin conjugated to horseradish peroxidase. References Allman, J.M. & Kaas, J.H. (1971) A representation of the visual field in the posterior third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res., 3, 85–105. Beck, P.D. & Kaas, J.H. (1998) Thalamic connections with the dorsomedial visual cortical area (DM). J. Comp. Neurol., 396, 381–398. Benevento, L.A. & Fallon, J.H. (1975) The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). J. Comp. Neurol., 160, 339–362. Benevento, L.A. & Standage, G.P. (1983) The organization of projections of the retinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J. Comp. Neurol., 217, 307–336. Bullier, J. & Kennedy, H. (1983) Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkeys. Exp. Brain Res., 53, 168–171. Casagrande, V.A. (1994) A third parallel visual pathway to primate area V1. Trends Neurosci., 17, 305–310. Casagrande, V.A. & Kaas, J.H. (1994) The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In Peters, A., Rockland, K. (eds), Cerebral Cortex, Vol. 10, Primary Visual Cortex in Primates. Plenum Press, New York, pp. 201–259. Celio, M.R. (1990) Calbindin D28K and parvalbumin in the rat nervous system. Neuroscience, 35, 375–475. Cowey, A. & Stoerig, P. (1989) Projection patterns of surviving neurons in the dorsal lateral geniculate nucleus following discrete lesions of striate cortex implications for residual vision. Exp. Brain Res., 75, 631–638. Cowey, A. & Stoerig, P. (1991) The neurobiology of blindsight. Trends Neurosci., 14, 140–145. Cusick, C.G., Scripter, J.L., Darensbourg, J.G. & Weber, J.T. (1993) Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual area, MT and DLr. J. Comp. Neurol., 336, 1–30. Dick, A., Kaske, A. & Crutzfeldt, O.D. (1991) Topographical and topological organization of the thalamocortical projection to the striate and prestriate cortex in the marmoset (Callithrix jacchus). Exp. Brain Res., 844, 233–253. Felleman, D.J. & Van Essen, D.C. (1991) Distributed hierachical processing in primate cerebral cortex. Cerebral Cortex, 1, 1–47.‘ Fries, W. (1981) The projections from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey. Proc. R. Soc. Lond. B. Biol. Sci., 213, 73–80. Gallyas, F. (1979) Silver staining of myelin by means of physical development. Neurol. Res., 1, 203–209. © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480 480 I. Stepniewska et al. Geneser-Jensen, F.A. & Blackstad, T.W. (1971) Distributed of acetylcholinesterase in the hippocampal region of the guinea pig. 1. Entorhinal area, parasubiculum, and presubiculum. Z. Zellforsch. Mikrosk. Anat., 114, 460–481. Gibson, A.R., Hansma, D.I., Houk, J.C. & Robinson, F.R. (1984) A sensitive low artifact TMB procedure for the demonstartion of WGA-HRP in the CNS. Brain Res., 298, 235–241. Graham, J., Lin, C.-S. & Kaas, J.H. (1979) Subcortical projections of six visual cortical areas in owl monkey, Aotus trivirgatus. J. Comp. Neurol., 187, 557–580. Gutierrez, C., Yaun, A. & Cusick, C.G. (1995) Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J. Comp. Neurol., 363, 545–562. Harting, J.K., Casagrande, V.A. & Weber, J.T. (1978) The projections of the primate superior colliculus upon the dorsal lateral geniculate nucleus: autoradiographic demonstration of interlaminar distribution of tectogeniculate axons. Brain Res., 150, 593–599. Harting, J.K., Huerta, M., Frankfurter, A.J., Strominger, N.L. & Royce, G.J. (1980) Ascending pathways from the monkey superior colliculus. An autoradiographic analysis. J. Comp. Neurol., 192, 853–882. Hendry, S.H.C. & Casagrande, V.A. (1996) A common pattern for a third visual channel in the primate LGN. Soc. Neurosci. Abstr., 22, 1605. Hernandez-Gonzalez, A., Cavada, C. & Reinoso-Suarez, F. (1994) The lateral geniculate nucleus projects to the inferior temporal cortex in the macaque monkey. Neuroreport, 5, 2693–2696. Huerta, M.F. & Harting, J.K. (1983) Sublamination within the superficial gray layer of the squirrel monkey: an analysis of the tectopulvinar projection using anterograde and retrograde transport methods. Brain Res., 261, 119–126. Kaas, J.H. & Huerta, M.F. (1988) Subcortical visual system of primates. In Steklis, H.P. (ed.). Comparative Primate Biology, Vol., 4; Neurosciences. Alan R. Liss, Inc., New York, pp. 327–391. Kaas, J.H., Huerta, M.F., Weber, J.T. & Harting, J.K. (1978) Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J. Comp. Neurol., 182, 517–554. Kaas, J.H. & Krubitzer, L.A. (1992) Area 17 lesions deactivate area MT in owl monkeys. Visual Neurosci., 9, 399–408. Krubitzer, L.A. & Kaas, J.H. (1990) Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Visual Neurosci., 5, 165–204. Lane, R.H., Allman, J.M., Kaas, J.H. & Miezin, F.M. (1973) The visuotopic organization of the superior colliculus of the owl monkey (Aotus trivirgatus) and the bush baby (Galago senegalensis). Brain, 60, 335–349. Lin, C.-S. & Kaas, J.H. (1977) Projections from cortical visual areas 17, 18, and MT onto the dorsal lateral geniculate nucleus in owl monkeys. J. Comp. Neurol., 173, 457–474. Lin, C.-S. & Kaas, J.H. (1979) The inferior pulvinar complex in owl monkeys: architectonic subdivisions and patterns of input from the superior colliculus and subdivisons of visual cortex. J. Comp. Neurol., 187, 655–678. Lin, C.-S. & Kaas, J.H. (1980) Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of visual cortex. Neuroscience, 5, 2219–2228. Lin, C.-S., Wagor, E. & Kaas, J.H. (1974) Projections from the pulvinar to the middle temporal visual area (MT) in the owl monkey (Aotus trivirgatus). Brain Res., 76, 145–149. Lysakowski, A., Standage, G.P. & Benevento, L.A. (1988) An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Exp. Brain Res., 69, 651–661. Mathers, L.H. (1971) Tectal projections to the posterior thalamus of the squirrel monkey. Brain Res., 35, 295–298. Maunsell, J.H.R., Nealey, T.A. & Depriest, D.D. (1990) Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J. Neurosci., 10, 3322–3334. Maunsell, J.H.R. & Van Essen, D.C. (1983) The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci., 3, 2563–2586. Partlow, G.D., Colonnier, M. & Szabo, J. (1977) Thalamic projections of the superior colliculus in the rhesus monkey, Macaca mulatta. A light and electron microscopic study. J. Comp. Neurol., 171, 285–318. Raczkoski, D. & Diamond, I.T. (1980) Cortical connections of the pulvinar nucleus in galago. J. Comp. Neurol., 193, 1–40. Rodman, H.R., Gross, C.G. & Albright, T.D. (1989) Afferent basis of visual response properties in area MT of the macaque. 1. Effects of striate cortex removal. J. Neurosci., 9, 2033–2050. Rodman, H.R., Gross, C.G. & Albright, T.D. (1990) Afferent basis of visual response properties in area MT of the macaque: 1. Effects of superior colliculus removal J. Neurosci., 10, 1154–1164. Sorenson, K.M. & Rodman, H.R. (1996) The lateral geniculate does not project to area TE in infant and adult macaques. Neurosci. Lett., 217, 5–8. Spatz, W.B. & Tigges, J. (1973) Studies on the visual area MT in primates. II. Projection fibers to subcortical structures. Brain Res., 61, 374–378. Standage, G.P. & Benevento, L.A. (1983) The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Res., 262, 288–294. Steele, G.E., Weller, R.E. & Kaas, J.H. (1991) A comparative study of the subcortical connecions of the dorsolateral area (V4) in primates. Soc. Neurosci. Abstr, 17, 845. Stepniewska, I. & Kaas, J.H. (1997) Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci., 14, 1043–1060. Ungerleider, L.G., Desimone, R., Galkin, T.W. & Mishkin, M. (1984) Subcortical projections of area MT in the macaque. J. Comp. Neurol., 223, 368–386. Wall, J.T., Symonds, L.L. & Kaas, J.H. (1982) Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J. Comp. Neurol., 211, 193–214. Wong-Riley, M.T.T. (1977) Connections between the pulvinar nucleus and the prestriate cortex in the squirrel monkey as revealed by peroxidase histochemistry and autoradiography. Brain Res., 134, 249–267. Wong-Riley, M.T.T. (1979) Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res., 171, 11–29. © 1999 European Neuroscience Association, European Journal of Neuroscience, 11, 469–480