* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hippocampal mechanisms for the context-dependent retrieval of episodes 2005 Special issue
Neural modeling fields wikipedia , lookup
Executive functions wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Environmental enrichment wikipedia , lookup
Memory consolidation wikipedia , lookup
Theta model wikipedia , lookup
Limbic system wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Spatial memory wikipedia , lookup
Convolutional neural network wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neural coding wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Sparse distributed memory wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neural oscillation wikipedia , lookup
Optogenetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Biological neuron model wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Apical dendrite wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Metastability in the brain wikipedia , lookup
Recurrent neural network wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Synaptic gating wikipedia , lookup























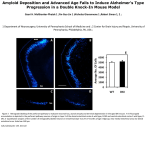

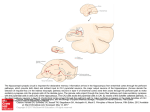
![Theorem [On Solving Certain Recurrence Relations]](http://s1.studyres.com/store/data/007280551_1-3bb8d8030868e68365c06eee5c5aa8c8-150x150.png)


