* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download afaf-el-ansary-king-saud-university-saudi
Brain Rules wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuroplasticity wikipedia , lookup
Long-term depression wikipedia , lookup
Selfish brain theory wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Nervous system network models wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neurogenomics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychology wikipedia , lookup
Subventricular zone wikipedia , lookup
Synaptogenesis wikipedia , lookup
History of neuroimaging wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Aging brain wikipedia , lookup
NMDA receptor wikipedia , lookup
Optogenetics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Circumventricular organs wikipedia , lookup
Heritability of autism wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Haemodynamic response wikipedia , lookup
Autism spectrum wikipedia , lookup
Neuroanatomy wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Glutamate receptor wikipedia , lookup
Molecular neuroscience wikipedia , lookup
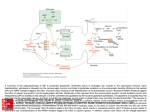
Dysregulated redox balance associated with glutamate excitotoxicity in autism spectrum disorders Prof. Afaf El-Ansary Department of Biochemistry Science College, King Saud University [email protected] Background Autism spectrum disorders (ASD) are characterized by three core behavioral domains: social deficits, impaired communication, and repetitive behaviors. Glutamate excitotoxicity has been found in various preclinical models of this disorder. Inefficient detoxification system leads to oxidative stress, gut dysbiosis, and immune dysfunction has been also accepted as etiological mechanism of autism. This work is an attempt to understand the relationship between glutamate excitotoxicity and impaired detoxification as two mechanisms recently related to autism. Central Nervous System (CNS) • Brain & Spinal Cord Peripheral Nervous System (PNS) • Afferent (sensory) Nerves – Carry sensory information to the CNS • Efferent (motor) Nerves – Transmit information to muscles or glands Cells of the Nervous System Neurons • Signal integration/generation; direct control of skeletal muscle (motor axons) Supporting Cells (Glia cells) • Astrocytes (CNS – blood brain barrier) • Oligodendrocytes (CNS – myelination) • Schwann cells (PNS – myelination) • Microglia (activated astrocytes) Cellular Events in Neurodevelopment Events: Division Migration Differentiation Neurogenesis Formation of synapses Myelination Apoptosis Active throughout childhood & adolescence Why is the Brain Particularly Vulnerable to Injury? Neurons are post-mitotic cells High dependence on oxygen • Little anaerobic capacity • Brief hypoxia/anoxia-neuron cell death Dependence on glucose • Sole energy source (no glycolysis) • High metabolic rate Many substances go directly to the brain via inhalation Blood Supply to the Brain Blood-brain Barrier The BBB consists of around 500 miles of blood vessels throughout the brain, all packed with endothelial cells that are highly selective about what gets through to the brain. With few exceptions, only fat-soluble small molecules penetrate - alcohol, caffeine, and nicotine are among them. BBB can be broken down by Hypertension: high blood pressure opens the BBB Hyperosmolarity: high concentration of solutes can open the BBB. Infection: exposure to infectious agents can open the BBB. Trauma, Ischemia, Inflammation, Pressure: injury to the brain can open the BBB. Development: the BBB is not fully formed at birth. Glutamate Synapses Excitatory synapse of brain Required to generate action potentials Both AMPA and NMDA receptors are critical for normal brain function NMDA-high permeability Ca++ Glutamate/glutamine cycle It is well known that glial cells, mainly astrocytes surround glutamatergic synapses, and express glutamate transporters and the glutamate-metabolizing enzyme, glutamine synthetase (GS). Glutamate is transported into glial cells and amidated by GS to the non-toxic amino acid glutamine. Glutamine is then released by glial cells and taken up by neurons, where it is hydrolyzed by glutaminase to form glutamate again, completing the glutamate/glutamine cycle. Appropriate clearance of synaptic glutamate is required for the normal function of brain excitatory synapses and hence for prevention of neurotoxicity recorded in patients with autism. Brain oxidative stress The central nervous system presents high vulnerability to free radical damage due to its elevated oxidative metabolic rate and enriched content of unsaturated lipids, as well as to its elevated rate of free radical generation derived from neurotransmitters metabolism, and poor radical scavenging mechanisms. It is also hypothesized that autistic patients as poor detoxifiers have reduced ability to eliminate mercury. Higher levels of mercury were recently found to be associated with social impairment and severity of autism. ANTROPOGENIC EMISSIONS NATURAL EMISSIONS oVolcanoes oRock erosion oFires oCoal power plants oCement production Methylation Plankton OTHER POTENTIAL SOURCES oFolk medicines oCosmetics oAmalgams oVaccines Excitotoxicity-Glutamate Mediated Cell Death Glutamate induces a delayed cell death in neurons This cell death requires extracellular calcium and is blocked by antagonists of NMDA receptors Action potentials are initiated in the nerve axon after glutamate excitatory activation of receptors in the neuron's dendrites and cell body. Hypothesis: Prolonged or inappropriate activation of NMDA receptors underlies glutamate excitotoxicity of neurons . Methodology 20 male patients with autism age of 8 ± 4 were enrolled through the Autism Research and Treatment (ART) Center clinic. The diagnosis of ASD was confirmed in all subjects using the Autism Diagnostic Interview-Revised (ADI-R) criteria, the Autism Diagnostic Observation Schedule-Generic (ADOS-G) criteria and the Developmental, Dimensional and Diagnostic Interview (3DI). Another age and sex matching 30 as a control group. Samples collection After overnight fast, 10 ml blood samples were collected from autistic and control groups in test tubes containing anticoagulant. Tubes were centrifuged at 1000 rpm at room temperature for 15 minutes, plasma was obtained and deep freezed (at -80°C) and RBCs were kept at -20°C until analysis time. Biochemical assays: Glutamate, glutamine, thioredoxins I&III, Thioredoxin reductase, peroxidoxins, glutathione status and glutathione-stransferase were measured in plasma samples of autistic children compared to controls using ELISA kits. Mercury was measured using atomic absorption. 1. Independent t-test • Independent t-test to compare between the 2 studied groups. • Results were expressed as means ± S.D. Statistical comparisons were performed with independent t-tests using (SPSS). • Significance were assigned at the level of P < 0.05. 2. ROC analysis Area under the curve, cutoff values, and degree of specificity and sensitivity were calculated. Table 1: Table 1 Mean ± SD of the measured chemicals in plasma or red blood cells of patients with autism compared with age-matched controls Parameter Group N Mean ± S.D. Percent Change Control 20 111.91 ± 4.51 100.00 Autistic 20 152.80 ± 6.47 136.54 Control 20 241.82 ± 12.93 100.00 Autistic 20 111.34 ± 5.69 46.04 Control 20 0.46 ± 0.03 100.00 Autistic 20 1.37 ± 0.06 296.18 Glutamate Dehydrogenase Control (GLDH) (U/l) Autistic 20 1.71 ± 0.47 100.00 20 0.93 ± 0.36 54.22 Control 20 44.71 ± 7.43 100.00 Autistic 20 74.70 ± 9.04 167.09 Control 20 1.83 ± 0.52 100.00 Autistic 20 3.31 ± 1.11 180.87 Control 20 19.58 ± 4.76 100.00 Autistic 20 34.56 ± 8.32 176.55 Control 20 24.30 ± 2.69 100.00 Autistic 20 43.05 ± 5.86 177.16 Control 20 26.07 ± 5.03 100.00 Autistic 20 8.03 ± 2.46 30.79 Glutathione S Transferase Control activity (µmol/min/ml) Autistic 20 0.69 ± 0.20 100.00 20 0.41 ± 0.12 59.26 Control 20 4.64 ± 0.68 100.00 Autistic 20 6.93 ± 0.74 149.40 Glutamic (µmol/l) Glutamine (µmol/l) Glutamic / Glutamine Ratio Thioredoxin 1 (ng/ml) Thioredoxin reductase (mU/ml) Peroxiredoxin 1 (ng/ml) Peroxiredoxin 3 (ng/ml) GSH /GSSG Mercury (µg/L) P value 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 Higher Hg can be easily related to of GSH/GSSG status. It is well known that sulfhydrylcontaining enzymes are inhibited by MeHg. With particular toxicity induced known that its directly interacts group of GSH, formation of an HgCH3 complex emphasis on the by MeHg, it is mercury atom with the thiol leading to the execretable GS- A mechanism links dysregulated redox status to glutamate excitotoxicity could be easily proposed. Simply, in the brain, ion gradients across neural membranes is important for resting membrane and this usually maintained by ATP-dependent ion pumps, such as a Na+/K+ pump. ATP depletion induces impairment in the repolarization of neural membranes after a depolarizing stimulus. Defective repolarization can relieve a voltage dependent Mg+ block of NMDA channels, leading to persistent receptor activation by endogenous glutamate. This hypothesis is derived from an in vitro experiment showing that the inhibition of energy metabolism makes glutamate neurotoxic at concentrations that ordinarily show no toxicity. Calcium entry via NMDA receptors can trigger neuronal cell death Table 2: ROC-Curve of all parameters in autistic groups AUC Best Cutoff value Sensitivi ty % Specifici ty % 1.000 131.110 100.0 % 100.0 % 1.000 169.800 100.0 % 100.0 % Glutamic / Glutamine Ratio 1.000 0.906 100.0 % 100.0 % Glutamate Dehydrogenase (GLDH) (U/l) 0.915 1.575 100.0 % 75.0 % Thioredoxin 1 level (ng/ml) 0.993 57.400 100.0 % 95.0 % Thioredoxinredu ctase activity (mU/ml) 0.894 2.000 80.0 % 75.0 % 0.977 27.250 95.0 % 100.0 % 1.000 33.000 100.0 % 100.0 % GSH / GSSG 1.000 16.340 100.0 % 100.0 % Glutathione S Transferase activity (µmol/min/ml) 0.900 0.505 85.0 % 95.0 % Mercury (µg/L) 0.988 5.997 95.0 % 100.0 % Glutamic (µmol/l) Glutamine (µmol/l) Peroxiredoxin 1 level (ng/ml) Peroxiredoxin 3 level (ng/ml) Table 3: Multiple Regression using Stepwise method for Glutamic (µmol/l)as a dependent variable Predictor Variable Beta P value Glutamic (µmol/l) 0.006 0.001 Glutamine (µmol/l) -0.005 0.001 Thioredoxin reductase activity (mU/ml) 0.013 0.038 GSH / GSSG -0.003 0.018 Model Adjusted R2 F value P value 0.995 1938.823 0.001 Table 4: Multiple Regression using Stepwise method for Glutamine (µmol/l)as a dependent variable Predictor Variable Glutamine (µmol/l) Glutamic / Glutamine Ratio Peroxiredoxin 1 level (ng/ml) Beta P value 0.302 0.001 92.604 0.001 -0.273 0.011 Model Adjusted R2 F value P value 0.964 352.514 0.001 Table 5: Multiple Regression using Stepwise method for Glutamic / Glutamine Ratioas a dependent variable Predictor Variable Glutamic (µmol/l) Glutamic / Glutamine Ratio Thioredoxin 1 level (ng/ml) Beta P value 0.969 0.001 -177.627 0.001 -0.274 0.044 Model Adjusted R2 square 0.990 F value P value 1243.754 0.001 Based on this, the excitotoxicity suggested in the present study as etiological factor in autism can be related to the impaired Prx I and III, Trx, Trxreductase , GSH/GSSG, and GST as markers of impaired detoxification mechanisms. This could find support in the study of Leveille et al (2009)who reported that excitotoxic insults can render the neurons more vulnerable to peroxidoxin hyperoxidation through the oxidation of cystine to cysteine. AUTISM CONCLUSION Mercury as neurotoxic metal could inhibit glutamate transporters resulting in raising the levels of extracellular glutamate by inhibiting antioxidant enzymes and glutathione. Mercury aggravates free radical generation and lipid peroxidation. Majority of children with autism exhibited high Hg, mitochondrial dysfunction, and oxidative stress, conditions that magnify glutamate excitotoxicity. This should call attention to glutamate signaling being a major mechanism in damage to the autistic brain. Stimulation of glutamate transporters could be a good strategy to treat this disorder.