* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Projections from the superior temporal sulcus to the agranular frontal
Clinical neurochemistry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Affective neuroscience wikipedia , lookup
Development of the nervous system wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Environmental enrichment wikipedia , lookup
Emotional lateralization wikipedia , lookup
Visual search wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Mirror neuron wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Neural coding wikipedia , lookup
Embodied language processing wikipedia , lookup
Human brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Cortical cooling wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroplasticity wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroesthetics wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Time perception wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Cerebral cortex wikipedia , lookup
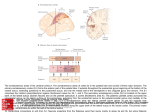
European Journal of Neuroscience, Vol. 14, pp. 1035±1040, 2001 ã Federation of European Neuroscience Societies SHORT COMMUNICATION Projections from the superior temporal sulcus to the agranular frontal cortex in the macaque Giuseppe Luppino, Roberta Calzavara, Stefano Rozzi and Massimo Matelli Istituto di Fisiologia Umana, UniversitaÁ di Parma, Via Volturno, 39, I-43100 Parma, Italy Keywords: motion information, motor cortex, temporal cortex Abstract The aim of this study was to investigate the organization of the projections from the superior temporal sulcus (STS) to the various areas forming the agranular frontal cortex. Injections of retrograde neuronal tracers were made in the various agranular areas, in nine macaque monkeys. The results showed that two rostral premotor areas, F6 (pre-SMA) and F7, and the ventrorostral part of area F2 (F2vr) are targets of projections from the upper bank of the STS (uSTS). F6 and the dorsorostral part of F7 (supplementary eye ®eld, SEF) are targets of projections from the rostral part of the uSTS, corresponding to the socalled `superior temporal polysensory area' (STP). In contrast, the ventral part of area F7 (not including the SEF) and F2vr are targets of afferents from the caudal part of the uSTS. Ventral F7 is the target of weak afferents from the caudalmost and dorsalmost part of the uSTS (area 7a), whilst F2vr is the target of projections from a relatively more rostral and ventral sector of the uSTS, close to the fundus of the sulcus. This sector should correspond to area MST. In conclusion, F6 and SEF receive high order information from STP, whereas ventral F7 and F2vr receive information from areas of the dorsal visual stream. Introduction The upper bank of the superior temporal sulcus (uSTS) can be functionally subdivided into a caudal and a rostral sector. The caudal sector is considered to be part of the so-called `dorsal visual stream' and is largely occupied by the middle superior temporal area (MST), which extends over about the ventral half of the bank (Desimone & Ungerleider, 1986). Area MST is a major target of the adjacent middle temporal area (MT; Ungerleider & Desimone, 1986b) and appears to play an important role in motion perception (Komatsu & Wurtz, 1988; Andersen et al., 1997). The dorsalmost part of the caudal uSTS, referred to as the `posterior parietal' area (PP) by Desimone & Ungerleider (1986), corresponds to a visually responsive region considered by some authors to be part of area 7a of the inferior parietal lobule (Andersen et al., 1990; see Felleman & Van Essen, 1991). The rostral sector of the uSTS, including also the corresponding part of the fundus, is generally referred to as the `superior temporal polysensory area' (STP) and is a site of convergent projections from somatosensory, auditory and visual areas of both the dorsal and the ventral visual stream (Bruce et al., 1981; Felleman & Van Essen, 1991). Functional data showed that neurons in this area have somatosensory, auditory or visual receptive ®elds and that many visually responsive neurons have very complex properties (Bruce et al., 1981; Perrett et al., 1989; see also Carey et al., 1997). Previous data showed that a major target of the uSTS, in the frontal lobe, is the prefrontal cortex (Seltzer & Pandya, 1989), although there is also some evidence that the uSTS may also project directly to the agranular frontal cortex (Seltzer & Pandya, 1989; Boussaoud et al., Correspondence: Professor Giuseppe Luppino, as above. E-mail: [email protected] Received 20 February 2001, revised 18 June 2001, accepted 9 August 2001 1990; Maioli et al., 1998). Recent evidence, however, showed that the agranular frontal cortex is formed by a multiplicity of independent areas with different anatomical connections and different functional roles in motor control (Rizzolatti et al., 1998; Luppino & Rizzolatti, 2000). Thus, the issue addressed in the present study was to identify the agranular areas which are targets of projections from the uSTS and to de®ne also the areas, within the superior temporal sulcus (STS), which are the origin of these projections. Preliminary data have been presented elsewhere in abstract form (Luppino et al., 2000). Materials and methods All experimental procedures used in the present study were approved by the Veterinarian Animal Care and Use Committee of the University of Parma and complied with the European law on the care and use of laboratory animals. In nine macaque monkeys (six Macaca nemestrina and three Macaca fascicularis) injections of retrograde tracers were made in all the various agranular frontal areas, de®ned according to Matelli et al. (1985, 1991). Data from Case 4 have already been presented elsewhere (Luppino et al., 1993). In this study, they have been re-analysed and presented together with the other cases in order to obtain a comprehensive view of the projections from the STS to all the various premotor areas. In all monkeys but two (Cases 2 and 4), the injection sites were chosen by using sulci and dimples as landmarks. Each animal was anaesthetized with ketamine hydrochloride (15 mg/kg i.m.). Under aseptic conditions, the bone was removed from over the appropriate cortical region and the dura opened. In Cases 2 and 4 the location of the injection sites was selected on the basis of chronic electrophysiological experiments (see Luppino et al., 1993; Matelli et al., 1036 G. Luppino et al. TABLE 1. Monkey species, localization of the cortical injections and tracers employed in the experiments Monkey Species Hemisphere Area Tracer (%) Amount (mL) Case 2 M. Fascicularis Case 4 M. Fascicularis Case 10 Case 11 M. Nemestrina M. Nemestrina Case 12 M. Nemestrina Case 13 M. Fascicularis Case 14 Case 18 M. Nemestrina M. Nemestrina Case 20 M. Nemestrina Left Right Right Left Left Right Right Left Left Left Left Left Left Left Left Left F7-SEF F3 F6 F6 F2d F2vr F7±not SEF F2d F4 F7±not SEF F5 F1 F5 F2vr F4 F1 FB (3) WGA-HRP (4) FB (3) DY (2) FB (3) FB (3) DY (2) DY (2) FB (3) FB (3) DY (2) TB (5) FB (3) CTB-gold (0.5) FB 3) CTB-gold (0.5) 1 8 1 1 1 1 2 1 1 1 1 2 1 1 1 1 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 0.2 0.08 0.2 0.2 0.2 0.35 0.2 0.15; 1 3 0.2 0.2 0.2 0.2 0.2 0.2; 1 3 0.3 1 0.2 1 SEF, supplementary eye ®eld; F2vr, ventrorostral part of F2; F2d, dimple region of F2. 1998). Once the appropriate cortical site was chosen, ¯uorescent tracers (Fast Blue, FB, 3% in distilled water, True Blue, TB, 5% in distilled water, Diamidino Yellow, DY, 2% in 0.2 M phosphate buffer at pH 7.2; EMS-POLYLOY GmbH, Gross-Umstadt, Germany), wheatgerm agglutinin, peroxidase-conjugated (WGA-HRP; 4% in distilled water, Sigma, St Louis, MO, USA), or cholera toxin B subunit, gold-conjugated (CTB-gold, 0.5% in distilled water; LIST, Campbell, CA, USA) was slowly pressure-injected through a glass micropipette (tip diameter 50±100 mm) attached to a 1-mL Hamilton microsyringe (Reno, NV, USA). Table 1 summarizes the locations of injections, the injected tracers and their amounts. The tracers injected into the cortical convexity were delivered 1.5 mm below the cortical surface, whilst those injected in the posterior bank of the arcuate sulcus or in the areas of the mesial wall of the hemisphere were delivered at various depths from the cortical crown. After the injections had been made, the dural ¯ap was sutured, the bone replaced and the super®cial tissues sutured in layers. After appropriate survival periods (12±14 days following ¯uorescent tracer injections, 7 days following injection of CTB-gold and 2 days following injections of WGA-HRP), each animal was anaesthetized with ketamine hydrochloride (15 mg/kg i.m.) followed by an i.v. lethal injection of sodium thiopental and perfused through the left cardiac ventricle with saline, and then with one of the following procedures: (i) 4% paraformaldehyde, 10% sucrose plus 1.5% dimethyl sulfoxide (Cases 2 and 4); (ii) 4% paraformaldehyde, 5% glycerol (Case 10); or (iii) 3.5% paraformaldehyde, 5% glycerol (Cases 11±14, 18 and 20). All solutions were prepared in phosphate buffer 0.1 M pH 7.4. Brains were then blocked coronally, removed from the skull and placed for one day in cold sucrose buffer (Cases 2 and 4) or in 10% buffered glycerol for 3 days and 20% glycerol for 4 days (Cases 10±14, 18 and 20). Finally, they were cut on a freezing microtome in a coronal plane at 60 mm. In all monkeys, at least one section of each ®ve was mounted, air-dried and quickly coverslipped for ¯uorescence microscopy. In Case 2, one section of each ®ve was processed for WGA-HRP histochemistry with tetramethylbenzidine as chromogen (Mesulam, 1982). In Cases 18 and 20, in one section of each ®ve, CTB-gold was revealed by the silver intensi®cation protocol described by Kritzer & Goldman-Rakic (1995). In all cases the extent of the injection sites was evaluated and their location was attributed to cytoarchitectonic areas by using adjacent sections stained with the Nissl method. The injection sites presented in this study were all restricted within the limits of a single cytoarchitectonic area. Figure 1 (upper part) summarizes the cytoarchitectonic location of all the injection sites, plotted onto a dorsolateral and a mesial view of a single hemisphere. Retrogradely labelled neurons were plotted in each section every 600 mm. 2-D reconstructions of the distribution of cortical labelling were obtained according to the procedure described by Matelli et al. (1998). The 2-D reconstructions of the STS were obtained by aligning the unfolded sections through the sulcus at the fundus, in its rostral part, and at the midpoint of the ¯oor, in its caudal part. Results The results showed that only the two rostral premotor areas, F6 (preSMA) and F7, plus the ventrorostral part of area F2 (F2vr) were found to be targets of projections from the STS (Fig. 1, upper part, ®lled circles). Area F6 was injected in two cases. In Case 4l (injection site shown in Fig. 1, lower part), DY-labelled cells were located in the fundus of the sulcus, slightly extending also in the dorsal bank, in correspondence of the rostral end of the ¯oor and therefore in area STP (Fig. 2A). Very similar results were observed following FB injection in Case 4r (not shown). In both cases the labelling observed within the STS was relatively weak. Area F7 was injected in three cases. In Case 2l, FB was injected in the dorsal part of F7 corresponding to the supplementary eye ®eld [F7-supplementary eye ®eld (SEF), Fig. 1, lower part], as de®ned following extensive physiological mapping. In Cases 11r (injection site shown in Fig. 1, lower part) and 13l, tracers injections involved the ventral and caudal parts of F7 (F7±not SEF), respectively. Following F7-SEF injection, the labelling in the STS was much stronger than that observed in F6 injections. Almost all the labelling was located in the rostral two-thirds of the uSTS, in correspondence with the fundus, but also extending in the whole extent of the dorsal bank and therefore occupying a large part of area STP (Fig. 2B). Within this region, labelled cells were relatively more concentrated in two separate sectors: a caudal one, coinciding with the sector labelled following injections in F6, and a more rostral one. ã 2001 Federation of European Neuroscience Societies, European Journal of Neuroscience, 14, 1035±1040 STS projections to the motor cortex 1037 much weaker and located in the caudalmost and dorsalmost part of uSTS. It also extended to the adjacent parietal convexity. Whilst in Case 11r DY (Fig. 2C) the labelling was almost restricted to the caudalmost and dorsalmost part of the uSTS, in Case 13l (not shown) most of the labelling was found in the cortical convexity of the inferior parietal lobule. The territory labelled following both these cases appears to very likely correspond to area 7a (Andersen et al., 1990; Felleman & Van Essen, 1991). Area F2 was injected in four cases. Labelling within the uSTS was, however, observed only following injections in F2vr (Cases 18l and 11r FB), but not following injections near the superior precentral dimple (F2d). The labelled cortical sector was markedly different from those labelled following F6 and F7 injections. The results of Case 18l are shown in Fig. 2D (injection site shown in Fig. 1, lower part). Relatively dense labelling was concentrated in the caudal and ventral part of the uSTS, at a level caudal to the end of the lateral ®ssure. This labelling extended, ventrally, up to the border with the ¯oor, slightly invading the dorsalmost part of the ¯oor itself. The location of this labelling corresponds to that of area MST, as described by Desimone & Ungerleider (1986). Similar results were also observed using a different retrograde tracer (FB, Case 11r, not shown). Also in this case the labelling was relatively dense and its location appeared to coincide with that of Case 18l, although slightly shifted in a more caudal position. Sparse labelling was also observed in the caudalmost and dorsalmost part of uSTS. Discussion FIG. 1. (Upper part) Schematic view of the location of the injection sites plotted onto a dorsolateral and a mesial view of a single hemisphere. Numbers inside the circles refer to the injected cases (see Table 1). Dashed lines mark the cytoarchitectonic borders of the motor areas according to Matelli et al. (1985, 1991). Filled circles indicate injection sites which produced labelling within the STS or in adjacent parietal cortex. (Lower part) Coronal sections from the representative cases illustrated in Fig. 2, showing location and extent of injection sites with respect to cytoarchitectonics. AI, inferior arcuate sulcus; AS, superior arcuate sulcus; C, central sulcus; CC, corpus callosum; Cg, cingulated sulcus; L, lateral sulcus; IP, intraparietal sulcus; P, principal sulcus. Scale bar, 5 mm. In contrast with the results of F7-SEF injection, the labelling observed following injections in other parts of area (F7±not SEF) was The present data indicate that the projections from STS to the motor cortex are restricted to the two rostral premotor areas F6 and F7, plus F2vr. In particular, areas F6 and F7±not SEF receive afferents from rostral regions of the uSTS, whereas F7±not SEF and F2vr are targets of more caudal regions of the uSTS. The differential pattern of connectivity of different sectors of areas F2 (F2vr vs. F2d) and F7 (F7-SEF vs. F7±not SEF) is in line with previous observations on differential parietal connections of these two frontal areas (Matelli et al., 1998) as well as with evidence on their differential functional properties (see Rizzolatti et al., 1998). In spite of these hodological and functional differences, so far no clear cytoarchitectonic evidence has been provided in favour of a further subdivision of F7 and F2. Thus, the possibility that the different sectors of F7 and F2 represent separate areas or represent different modules within a given architectonic area needs further studies using architectonic techniques other than Nissl method. The rostral part of the uSTS corresponds to a cortical region, termed `superior temporal polysensory' area (STP; Bruce et al., 1981). According to Seltzer & Pandya (1978) and Cusick et al. (1995) area STP is architectonically not homogeneous. Functional studies, however, in which the distribution of multimodal STP neurons was correlated with architectonics, did not provide evidence that architectonic subdivisions may correspond to different functional entities (see, e.g. Bayliss et al., 1987). Hodological data indicate that STP is target of projections from somatosensory, auditory and visual areas. In particular, this region appears to be a privileged site of convergent inputs from visual areas belonging to both the dorsal and ventral visual streams (Boussaoud et al., 1990; Baizer et al., 1991; see also Felleman & Van Essen, 1991). Based on connectional differences Felleman & Van Essen (1991) proposed a subdivision of STP in a rostral (STPa) and a caudal (STPc) region. Although the limits of these two STP subdivisions cannot be set with certainty, it could be of some interest to note that, in our F7-SEF injection, ã 2001 Federation of European Neuroscience Societies, European Journal of Neuroscience, 14, 1035±1040 1038 G. Luppino et al. ã 2001 Federation of European Neuroscience Societies, European Journal of Neuroscience, 14, 1035±1040 STS projections to the motor cortex separate clusters of labelled cells were observed in the rostral and the caudal part of STP (possibly corresponding to STPa and STPc, respectively), whereas following F6 injections only the caudal part (possibly STPc) was labelled. Rostral and a caudal spots of labelling in STP were also found by Huerta & Kaas (1990) following SEF injection. Several electrophysiological studies have shown that neurons in area STP have complex sensory properties. Firstly, although STP neurons are predominantly purely visual, a signi®cant proportion of them have also somatosensory and/or auditory responses (Bruce et al., 1981; Bayliss et al., 1987). Secondly, visually responsive neurons usually have complex functional properties (for a review of the data, see, e.g. Carey et al., 1997). It has been proposed that area STP is involved in visuomotor functions and in integration of information within and across modalities (Bruce et al., 1981; Bayliss et al., 1987). Furthermore, according to the studies of Perrett and colleagues (Perrett et al., 1989; see also Carey et al., 1997) complex visual neurons in STP could be responsible for coding the visual appearance of the body, for coding particular types of body motion, for coding movements which are not consequence of the monkey's own action or for coding body movements as goal-directed actions. This highorder information, which can be of crucial importance in the social behaviour of primates, can be conveyed to the cortical motor system through the posterior parietal cortex or through a relay in the ventral dorsolateral prefrontal cortex (Seltzer & Pandya, 1989). Present data indicate that STP out¯ow has also a direct access to area F6 and the SEF. Area F6 (pre-SMA) is a rostral premotor area mainly concerned with the control of arm movements. Functional properties of F6 suggest that this area plays a key role in selection and preparation of movements and in learning and organizing complex movement sequences (for a review of these data, see Tanji et al., 1996; Rizzolatti et al., 1998; Hikosaka et al., 1999). The SEF is an electrically excitable oculomotor ®eld (Schlag & Schlag-Rey, 1987), located in the dorsal part of area F7. The functional properties of the SEF suggest that this area plays a more complex role in the organizazion of eye movements than that of the frontal eye ®eld. SEF is not necessary for producing accurate visually guided saccades, but it appears to play an important role in learning conditional oculomotor responses, in encoding saccades relative to an object centred frame of reference, or in monitoring the consequences of eye movements as part of a brain's supervisory control system (see Stuphorn et al., 2000). The notion that F6 and the SEF play a role in the control of arm or eye movements at a high hierarchical level is supported by data on the cortical connections of these two areas. Both these areas are target of very weak parietal input but, in contrast, they receive rich projections from cingulate area 24c, as well as from the dorsal and ventral parts of the dorsolateral prefrontal cortex (Huerta & Kaas, 1990; Luppino et al., 1993). Therefore, area F6 and the SEF are the only motor areas in which cognitive and motivational information may be integrated and conveyed to other arm representations, with the exclusion of that of F1 (in the case of F6), or to the frontal eye ®eld (in the case of the SEF). The projections from STP to F6 and the 1039 SEF, shown in this study, provide further support in favour of their peculiar functional role in motor control. Two areas occupy the caudal part of the uSTS; area MST, lying in the ventral half of the bank, and a dorsal and caudal cortical sector called area PP by Desimone & Ungerleider (1986), but included by Andersen et al. (1990) within the architectonic limits of area 7a. Area MST is target of projections from area MT (Maunsell & Van Essen, 1983; Ungerleider & Desimone, 1986b) and plays an important role in visual motion processing (Komatsu & Wurtz, 1988; see also Andersen et al., 1997). In particular, MST neurons appear to be more selective for motion patterns that occur with self motion (see Andersen et al., 1997) and, by combining visual and vestibular signals, they are able to enhance self-movement detection and disambiguate optic ¯ow that results from either self-movement or the movements of large objects near the observer (Duffy, 1998). Thus, MST appears to be very important for navigation using motion cues (see Andersen et al., 1997). The labelling observed in the caudal uSTS, following injections in F7±not SEF and in F2vr, was spatially segregated. Whilst F7±not SEF is a target of area 7a, F2vr is most probably a target of area MST as de®ned by Desimone & Ungerleider (1986). According to these authors, MST occupies `most of the bottom half of the upper bank of the caudal STS and a small portion of the adjacent ¯oor'. Area MST is myeloarchitectonically not homogeneous, with a densely myelinated zone (DMZ) occupying its dorsalmost part, at the border with area PP or area 7a (Desimone & Ungerleider, 1986; Ungerleider & Desimone, 1986a; Andersen et al., 1990). Our material was not stained for myelin, so that it was not possible to correlate the location of the labelling observed following F2vr injections with that of DMZ. However, if one compares the location of the labelling observed in the present study (see Fig. 2D, sections 150 and 152) with the location of MST, as shown by previous authors in coronal sections (see, e.g., Ungerleider & Desimone, 1986a; Boussaoud et al., 1990), it seems quite reasonable to attribute the labelling to this area. If this is accepted, one should conclude that F2vr is the only premotor area that receives a direct input from an area speci®cally devoted to motion perception. This ®nding supports the recent evidence that F2vr is hodologically and functionally independent of F2d (see Caminiti et al., 1996; Rizzolatti et al., 1998; Luppino & Rizzolatti, 2000). In contrast with F2d, F2vr is the target of visual or somatosensory and visual areas of the superior parietal lobule (MIP and V6A), is the target of a minor but consistent projection from the dorsal part of DLPF and contains visually responsive neurons. Thus, F2vr appears to be involved in planning and controlling arm movements on the basis of visual and somatosensory information. This information could be used for the visual guidance of the arm trajectory when, for example, reaching towards moving objects or reaching objects during self-movements of the body. Acknowledgements Work supported by MURST, CNR and HSPO. FIG. 2. Distribution of retrogradely labelled neurons in the STS following injections in areas (A) F6, (B) supplementary eye ®eld (F7-SEF), (C) F7±not SEF and (D)ventrorostral part of area F2 (F2vr). For each case, in the left part of the ®gure a view of the hemisphere shows the location of the injection sites and the cytoarchitectonic borders of the injected area. The middle part of the ®gure shows the unfolded view of the STS and the distribution of labelled neurons. Each dot corresponds to one neuron. The right part of the ®gure illustrates selected coronal sections showing the distribution of the labelled neurons within the STS. The levels of the sections are also reported on the unfolded view of the STS and on the view of the brain. Abbreviations as in Fig. 1. ã 2001 Federation of European Neuroscience Societies, European Journal of Neuroscience, 14, 1035±1040 1040 G. Luppino et al. Abbreviations CTB-gold, cholera toxin B subunit, gold-conjugated; DY, Diamidino Yellow; F2d, precentral dimple part of area F2; F2vr, ventrorostral part of area F2; FB, Fast Blue; MST, middle superior temporal area; MT, middle temporal area; PP, posterior parietal; SEF, supplementary eye ®eld; STP, superior temporal polysensory area; STS, superior temporal sulcus; TB, True Blue; uSTS, upper bank of the superior temporal sulcus; WGA-HRP, wheatgerm agglutinin, peroxidase-conjugated. References Andersen, R.A., Asanuma, C., Essick, G. & Siegel, R.M. (1990) Corticocortical connections of anatomically and physiologically de®ned subdivisions within the inferior parietal lobule. J. Comp. Neurol., 296, 65± 113. Andersen, R.A., Snyder, L.H., Bradley, D.C. & Xing, J. (1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci., 20, 303±330. Baizer, J.S., Ungerleider, L.G. & Desimone, R. (1991) Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci., 11, 168±190. Bayliss, G.C., Rolls, E.T. & Leonard, C.M. (1987) Functional subdivisions of the temporal lobe neocortex. J. Neurosci., 7, 330±342. Boussaoud, D., Ungerleider, L. & Desimone, R. (1990) Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol., 296, 462±495. Bruce, C., Desimone, R. & Gross, C.G. (1981) Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol., 46, 369±384. Caminiti, R., Ferraina, S. & Johnson, P.B. (1996) The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cerebral Cortex, 6, 319±328. Carey, D.P., Perrett, D.I. & Oram, M.W. (1997) Functional anatomy of human motor cortical areas. In Boller, F. & Grafman, J. (eds), Handbook of Neuropsychology, Vol. 11. Elsevier, Amsterdam, pp. 111±129. Cusick, C.G., Seltzer, B., Cola, M. & Griggs, E. (1995) Chemoarchitectonics and corticocortical terminations within the superior temporal sulcus of the rhesus monkey: evidence for subdivisions of superior temporal polysensory cortex. J. Comp. Neurol., 360, 513±535. Desimone, R. & Ungerleider, L.G. (1986) Multiple visual areas in the caudal superior temporal sulcus of the macaque. J. Comp. Neurol., 248, 164±189. Duffy, C.J. (1998) MST neurons respond to optic ¯ow and translational movement. J. Neurophysiol., 80, 1816±1827. Felleman, D.J. & Van Essen, D.C. (1991) Distributed hierarchical processing in primate cerebral cortex. Cerebral Cortex, 1, 1±47. Hikosaka, O., Sakai, K., Nakahara, H., Lu, X., Miyachi, S., Nakamura, K. & Rand, M.K. (1999) Neural mechanisms for learning of sequential procedures. In Gazzaniga, M.S. (ed.), The New Cognitive Neurosciences. MIT Press, Cambridge, USA, pp. 553±572. Huerta, M.F. & Kaas, J.H. (1990) Supplementary eye ®eld as de®ned by intracortical microstimulation: connections in Macaques. J. Comp. Neurol., 293, 299±330. Komatsu, H. & Wurtz, R.H. (1988) Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J. Neurophysiol., 60, 580±603. Kritzer, M.F. & Goldman-Rakic, P.S. (1995) Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J. Comp. Neurol., 359, 131±143. Luppino, G., Calzavara, R., Rozzi, S. & Matelli, M. (2000) Projections from the superior temporal sulcus to prefrontal recipient premotor areas in the macaque. Soc. Neurosci. Abstr., 26, 1712. Luppino, G., Matelli, M., Camarda, R. & Rizzolatti, G. (1993) Corticocortical connections of area F3 (SMA proper) and area F6 (pre-SMA) in the macaque monkey. J. Comp. Neurol., 338, 114±140. Luppino, G. & Rizzolatti, G. (2000) The organization of the frontal motor cortex. News. Physiol. Sci., 15, 219±224. Maioli, M.G., Squatrito, S., SamolskyDekel, B.G. & Sanseverino, E.R. (1998) Corticocortical connections between frontal periarcuate regions and visual areas of the superior temporal sulcus and the adjoining inferior parietal lobule in the macaque monkey. Brain Res., 789, 118±125. Matelli, M., Govoni, P., Galletti, C., Kutz, D.F. & Luppino, G. (1998) Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J. Comp. Neurol., 402, 327±352. Matelli, M., Luppino, G. & Rizzolatti, G. (1985) Patterns of cytochrome oxidase activity in the frontal agranular cortex of macaque monkey. Behav. Brain Res., 18, 125±137. Matelli, M., Luppino, G. & Rizzolatti, G. (1991) Architecture of superior and mesial area 6 and of the adjacent cingulate cortex. J. Comp. Neurol., 311, 445±462. Maunsell, J.H.R. & Van Essen, D.C. (1983) The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci., 3, 2563±2586. Mesulam, M.-M. (1982) Principles of horseradish peroxidase neurohistochemistry and their applications for tracing neural pathways. In Mesulam, M.-M. (ed.), Tracing Neural Connections with Horseradish Peroxidase. Wiley, Chichester, pp. 1±152. Perrett, D.I., Harries, M.V., Bevan, R., Thomas, S., Benson, P.J., Mistlin, A.J., Chitty, A.J., Hietanen, J.K. & Ortega, J.E. (1989) Framework of analysis for the neural representation of animate objects and actions. J. Exp. Biol., 146, 87±113. Rizzolatti, G., Luppino, G. & Matelli, M. (1998) The organization of the cortical motor system: new concepts. Electroencephalogr. Clin. Neurophysiol., 106, 283±296. Schlag, J. & Schlag-Rey, M. (1987) Evidence for a supplementary eye ®eld. J. Neurophysiol., 57, 179±200. Seltzer, B. & Pandya, D.N. (1978) Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res., 149, 1±24. Seltzer, B. & Pandya, D.N. (1989) Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J. Comp. Neurol., 281, 97±113. Stuphorn, V., Taylor, T.L. & Schall, J.D. (2000) Performance monitoring by the supplementary eye ®eld. Nature, 408, 857±860. Tanji, J., Shima, K. & Mushiake, H. (1996) Multiple cortical motor areas and temporal sequencing of movements. Cognitive Brain Res., 5, 117±122. Ungerleider, L.G. & Desimone, R. (1986a) Projections to the superior temporal sulcus from the central and peripheral representations of V1 and V2. J. Comp. Neurol., 248, 147±163. Ungerleider, L.G. & Desimone, R. (1986b) Cortical projections of visual area MT in the macaque. J. Comp. Neurol., 248, 190±222. ã 2001 Federation of European Neuroscience Societies, European Journal of Neuroscience, 14, 1035±1040