* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A functional magnetic resonance study
Brain morphometry wikipedia , lookup
Cortical cooling wikipedia , lookup
Embodied language processing wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Human multitasking wikipedia , lookup
Persistent vegetative state wikipedia , lookup
Executive functions wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Neurolinguistics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuroesthetics wikipedia , lookup
Limbic system wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Nervous system network models wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroplasticity wikipedia , lookup
Time perception wikipedia , lookup
Human brain wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Metastability in the brain wikipedia , lookup
Affective neuroscience wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neurophilosophy wikipedia , lookup
History of neuroimaging wikipedia , lookup
Emotional lateralization wikipedia , lookup
Aging brain wikipedia , lookup
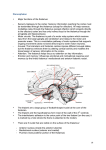
Abnormal functional connectivity with mood regulating circuit in unmedicated individual with major depression: a resting-state functional magnetic resonance study PENG Daih-uia, FANG Yi-rua, XU Yi-fengb,c, SHEN Tingb, ZHANG Jiea, HUANG Jiaa, LIU Jund, LIU Shu-yonge and JIANG Kai-dab a Division of Mood Disorder, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, China (PENG Daih-ui, FANG Yi-ru, ZHANG Jie, HUANG Jia) b Clinic Medical Center, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, China (XU Yi-feng, SHEN Ting, JIANG Kai-da) c Huashan Hospital, Medical School of Colleague, Fudan University, Shanghai 200032, China (XU Yi-feng) d Department of Medical Imaging, Shanghai Pu Tuo People’s Hospital 200062, Shanghai, China (LIU Jun) e Department of Medical Imaging, Taian People’s hospital, Shangdong 271021, PR China (LIU Shu-yong) Correspondence to: Dr. JIANG Kai-da, Clinic Medical Center, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, China (Tel: 86-21-34289888 ext. 3528. Fax: 86-21-64387986. Email: jiangkaida66@126. com); Dr. FANG Yi-ru, Division of Mood Disorder, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, China (Tel: 86-21-34289888 ext. 3529. Fax: 86-21-64387986. Email: [email protected]) This work was partly supported by the grant (114119a5500) from Science and Technology Commission of Shanghai municipality. Key words: depression; anterior cingulate cortex; thalamus; functional connectivity; functional magnetic resonance imaging Background Reports on mood regulating circuit (MRC) indicated different activities between depressed patients and healthy controls. The functional networks based on MRC have not been described in major depression disorder (MDD). Both the anterior cingulate cortex (ACC) and thalamus are all the key regions of MRC. This study was to investigate the two functional networks related to ACC and thalamus in MDD. Methods Sixteen patients with MDD on first episode which never got any medication and sixteen matched health controls were scanned by 3.0 T functional magnetic resonance imaging (fRMI) during resting-state. The pregenual anterior cingulate cortex (pgACC) was used as seed region to construct the functional network by cortex section. The thalamus was used as seed region to construct the functional network by limbic section. Paired-t tests between-groups were performed for the seed-target correlations based on the individual fisher z-transformed correlation maps by SPM2. Results Depressed subjects exhibited significantly greater functional connectivity (FC) between pgACC and the parahippocampus gyrus in one cluster (size 923) including left parahippocampus gyrus (-21,-49,7), left parietal lobe (-3,-46,52) and left frontal lobe (-27,-46,28). The one cluster (size 962) of increased FC on thalamus network overlapped the precuneus near to right parietal lobe (9,-52,46) and right cingulate gyrus (15,-43,43) in health controls. Conclusions Abnormal functional networks exist in earlier manifestation of MDD related to MRC by both cortex and limbic sections. The increased functional connectivity of pgACC and decreased functional connectivity of thalamus is mainly involved in bias mood processing and cognition. The major depression disorder (MDD) shows the bias negative mood processing, cognition deficits and physical symptoms. Abnormal brain function is one mechanism of the depressed symptoms, which are likely to be present in functional connectivities (FCs) between brain regions, rather than within brain regions.1,2 These brain regions should involve in negative emotion, cognition and physical symptoms.3,4,5 The emotion processing is dependent on a distributed neuronal network.6 The mood regulating circuit (MRC) should be composed of a putative prefrontal-amygdalar-pallidostriatal-mediothalamic.7 Bias mood processing is related with abnormal MRC in depression.8,9 The decreased correlations between the anterior cingulate cortex (ACC) and thalamus are consistent with the hypothesis that decreased cortical regulation of limbic activation in response to negative mood stimuli may be present in depression.9,10 Both ACC and thalamus are all the key regions of MRC. The pregenual anterior cingulate cortex (pgACC) has always been focused on same as the subgenual ACC, which is involved in abnormal mood.11 Through its connections with the emotion processing regions, including the hypothalamus, middle thalamus (MTHAL), and other limbic structures, it regulates the relationship of depressed emotion, autonomic and visceral function.12 Some researches showed ACC increased its contributions in resting-state FCs in MDD.13,14 Other researches showed thalamus decreased its spontaneous activity in MDD during resting-state.15,16 The thalamus has been found to be abnormal structure and function, related to the visceral function of depression.4,5 The MRC is related with the cognition network and default mode network (DMN).17,18 Previous studies characterized the MRC as a homogenous network, but few examined the differential contributions of MRC in MDD. The previous method to construct the MRC is to analyze the relationship among specific regions of interest (ROI) by emotion-activation experiment task or resting state.9,10 The functional networks based on MRC have not been described across global brain in MDD to date. In functionally related regions of the brain during resting state, low frequency fluctuations (LFFs) (< 0.08 Hz) are synchronous and exhibit high temporal coherence, defined as resting state functional connectivity (RSFC) 19,20. By RSFC analysis based on global brain signal, it’s benefit to explore the potential implications of MRC in MDD. In this study, the objective was to investigate the two functional networks from both cortex and limbic sections of MRC in MDD. The hypothesis is that the two functional networks should alter in early manifestation of MDD. Specifically, (1) the FC in MDD has the enhance tendency from seed pgACC of cortex; (2) the FC in MDD will be decreasing from seed thalamus of limbic region. In order to test the hypothesis, the RSFC analysis was performed across whole brain, which measured the correlations of spontaneous LFF signal between seed region and global brain voxels. Then to investigate the differences of the two functional networks between medication-naïve patients with MDD on first episode and healthy control subjects. METHODS MDD patients were recruited from the outpatient clinic at Huashan Hospital and Shanghai mental health centre. Healthy subjects matched for age, sex, education level were recruited via advertisement. Inclusive criteria for depressed subjects were: ages ranged from 25 to 50 years, satisfy DSM-IV diagnosis criteria of MDD, first episode, medication-naïve, Hamilton rating scale for depression 24 (24-HAMD)21 score>20, Hamilton anxiety scale 14 (14-HAMA) score<7. Exclusion criteria for depressed patients were: meeting criteria of any current or past Axis I disorder of diagnostic and statistical manual of mental disorders IV (DSM-IV) such as schizophrenia, schizoaffective disorder, bipolar disorder or an anxiety disorder as a primary diagnosis, acutely suicidal, homicidal or requiring inpatient treatment, meeting criteria for substance dependence within the past year, except caffeine or nicotine, serious medical or neurological illness, current pregnancy or breastfeeding, and metallic implants or other contraindications to MRI. Inclusive criteria for healthy subjects were: ages ranged from 25 to 50 years, no history of psychiatric illness or substance abuse or dependence, no family history of major psychiatric or neurological illness in first degree relatives, and no serious medical or neurological illness. Exclusion criteria for healthy subjects were: pregnant or breastfeeding, and metallic implants or other contraindication to MRI. Sixteen depressed patients and sixteen healthy subjects were recruited. All participants were right-handed. All subjects signed an informed consent form approved by the Investigational Review Board (IRB) at Shanghai mental health centre, and were paid 200 yuans for their participation. There were not significant differences between the two groups in age, gender distribution and education level. The clinic status of all subjects is showed in table 1. Fuctional MRI Acquisition Structural images and functional images were acquired on a 3.0 T General Electric Signa scanner (USA) by using a standard General Electric whole-head coil. T1 weighed structural images with a horizontal axis acquired using the SE sequence in a level position with parameters: repeat time (TR) = 500 ms, echo time (TE) = 14ms, flip angle = 15°, 5.0 mm thickness, no interval. Whole-brain functional images acquired using multislice echo planar imaging (EPI) sequence with parameters: TR = 3000 ms, TE = 30 ms, flip angle = 90°, 5.0 mm thickness, no interval, field of vision (FOV) = 240 mm × 240 mm, matrix size is 64 × 64, spatial resolution is 3.75 × 3.75× 5.0. The 3 D reconstruction by rapid interference phase gradient echo flip recovery (FSPGRIR) pulse sequence (T1 weighed) scanning, with parameters: TR = 6.4 ms, TE = 1.6 ms, TI = 40 ms, 1.0 mm thickness, no interval, FOV = 240 mm × 240 mm, bandwidth = 31.25, 256 × 256 × 1.0 spatial resolution, including the anatomical images with 146 layers. Each brain volume was comprised of 22 axial slices parallel to anterior cortex - posterior cortex. The functional run contained 104 volumes. The fMRI instruction: lie and remain motionless, to keep your eyes closed and relax as possibly. The scanning time of resting state: 5 min and 12 secs. Data preprocessing The first 4 time points of the resting state were discarded because of the magnetization equilibrium of the initial MRI signal leaving 100 time points. The data were firstly preprocessed (motion correction, slice timing, realignment, spatial normalization to the standard MNI space and re-sampled at 3mm3) using SPM2 (www.fil.ion.ucl.ac.uk/spm). The data were spatially smoothed using a 6-mm FWHM Gaussian kernel. After these, a low-pass frequency filter (0.01 < f < 0.08 Hz) was applied to reduce physiological high frequency noise using the 3d bandpass program of AFNI (http://www.afni.nimh.nih.gov/).22 To reduce the effects of confounding factors, six motion parameters, mean time series of white matter, cerebrospinal fluid and all voxels in global brain and linear drift were extracted and removed from the data by linear regression using SPM2(www.fil.ion.ucl.ac.uk/spm).23,24 Seed region selection Two seeds were chosen from cortex and limbic regions of MRC separately, the pgACC and thalamus. The two gray matter structures are potential to emotion processing and relative symptoms in MDD.16,25 The pgACC [12 41 13] was chosen based on the literature25. The seed was near to the subgeneual ACC reported in the literatures (Fig.1) 9,10 . The thalamus [−7.5 −17.5 5.5] was identified based on decreased spontaneous activity brain area in MDD according to the primary paper16. Seed region analysis and test of functional correlations Extraction of the time series of the seed regions (pgACC and thalamus) was generated using SPM2 (www.fil.ion.ucl.ac.uk/spm). The blood-oxygen-level-dependent (BOLD) time series of the voxels within each seed were averaged to generate the reference time series for this seed region. A correlation map was produced by computing the correlation coefficients (Pearson's r) between the reference time series and the time series of every voxel in the global brain. After application of Fisher’s r-to-z transform {z = 0.5 Ln [(1 + r)/(1 − r)]}, which yielded variates that were approximately normally distributed26, fisher z maps were combined across subjects by using a random-effects analysis (within group t-test for the two groups, separately). After the seeds were applied to test the connectivity analysis, paired t-test between-groups was calculated for the seed-target correlations based on the individual fisher z-transformed correlation maps as described above. The paired t values between two groups were converted to equally fisher z scores and thresholded by P < 0.05. RESULTS Using pgACC (12,41,13) and thalamus (−7.5,−17.5,5.5) as the two seed regions shown in Fig. 1, we determined significant differences in mean correlation coefficients between depressed and control subjects for each network. The locations of seed regions were circle-coded by seed origin (Fig. 1). The regions of altered FCs in depression were identified in pgACC network maps and thalamus network maps separately (Fig. 2 and Fig.3). Depressed subjects exhibited significantly greater FCs between pgACC and the parahippocampus gyrus in one cluster (size 923) including left sub-lobar (-21,-49,7), left parietal lobe (-27,-46,28) and left frontal lobe (-3,-46,52) (Fig. 2 and Table 2). The FCs analysis on thalamus network showed increased connectivity in health control compared with depressed subjects in one cluster (size 962) overlapping the right precuneus near to parietal lobe(9,-52,46) and right cingulate gyrus (15,-43,43) (Fig. 3 and Table 2). There were no clusters that showed significantly greater FCs in the health control compared to depressed subjects in pgACC network (Table 2). And there were also no clusters that showed significantly greater FCs in depressed subjects compared to the health control in thalamus network (Table 2). DISCUSSION In this study, one finding showed increased FCs in depressed subjects between pgACC with the left parahippocampus gyrus, parietal lobe and frontal lobe. Another finding showed decreased FCs in depressed subjects between thalamus with right precuneus and right cingulate gyrus. The earlier two studies on MRC showed decreased correlations between cortex such as ACC and limbic regions such as MTHAL. Decreased connectivity was used to insist their hypothesis that decreased cortical regulation of limbic activation in response to negative mood stimuli may be present.9,10 However, different analysis procedures focus on different research objects. In previous two studies, the functional connectivity was constructed by analyzing the defined regions of interests (ROI) in pgACC, dorsomedial thalamus (DMTHAL), pallidostriatum (PST) and amygdale (AMYG). It’s to explore the regulation relationship between brain areas by ROI-ROI analysis. In this study, the analysis of seed-target correlations was based on the global brain signals. It’s benefit to explore the physical implication of global FCs.19,22 The findings from seed pgACC in this study were consistent with recent studies, in which ACC increased its contributions in resting-state FCs in MDD.13,14 Tryptophan depletion in patients with remitted depression, as precursor for 5-hydroxytryptamine (5-HT), resulted in change of metabolism in several regions including ACC.27 The potential biochemical issue on depression is that abnormal serotonergic (5-HT) nerve system should be responsible for bias mood processing, which could regulate the interaction between ACC and other brain areas.28 These studies suggest ACC might be related with bias mood on MDD.13 Recent mapping studies support individual has variability in connections between brain nodes,29 which can indicate pathological implications.30 The finding of increasing FCs between pgACC and left parahippocampus might implicate episodic memory processing and partly visceral monitoring, which are compromised of cognition symptoms in depression.12,18 Some studies reported participants with elevated depression symptoms showed weaker activation in parietal regions by cognitive control test.31 Depressive patients decreased activity in parietal region as prefrontal cortex, and anterior cingulated gyrus et al by face-profession pairs test.32 These studies showed the coherence of function between ACC and parietal lobe, frontal lob. Thus, increasing FCs between pgACC and parietal lobe, frontal lobe in this study should be related with the cognitive dysfunction on MDD. However, how this increasing alteration occurs is not clear. It might involve early developmental changes related with structure and biochemical paradigms especially for depressive “trait”.33 The results from seed thalamus supported some previous studies, in which it showed abnormal structure and function of thalamus.4,5 In this study, thalamus was chose as seed region based on a latest interesting finding, which showed significantly lower regional homogeneity in the left thalamus in MDD than health control.16 The decreased hemodynamic responses indicated abnormal function of local neural activity.15,16 In this study, the findings indicated decreased FCs in depressed subjects between thalamus and the right precuneus and right cingulate gyrus in health control compared with depressed subjects. The decreased spontaneous activity within thalamus should be responsible for the decreasing FCs to other areas. Thalamus was involved in the serotonergic (5-HT) mechanism of depression.34 The altered functional network might implicate somatic symptoms of depression, such as characterized insomnia, lack of appetite and lower libido.4,5 In this study, the decreased FCs between thalamus with right precuneus and cingulate gyrus are not agreement with some prior literatures. One earlier study identified over connectivity in thalamus related to depression by independent component analysis (ICA).13 Another study showed there was not significantly altered FCs between precuneus and thalamus in depression patients.35 Actually, there are some different findings if post analysis is different. The differences of patients sample were also confounding factors between medicine- naïve and ever taking medicine subjects. Precuneus plays an important role in executive-function to attentional targets. The FCs of thalamus to precuneus and cingulate gyrus have implicated cognition symptoms due to bias mood processing, such as the focus of self-relevant thought,36 declining episodic memory and executive function, 31 et al. In this study, two seeds were separately used to construct the functional networks from cortex and limbic regions of MRC. The patients sample is first episode, and never got any psychotropic medicine, which is ideal to explore the intrinsic character related with depression. However, the present findings may be limited by the small sample size of participants. The strait or trait signatures related with disease mechanism could not be investigated by this cross section study. Future studies need larger number sample to indicate the issue by following up. In summary, the present results confirm altered functional network related with MRC in MDD. The increased functional connectivity of pgACC and decreased functional connectivity of thalamus is mainly involved in bias mood processing and cognition. The two networks would be combined to explore the abnormal functional signatures in depression. The present findings may be limited by the small sample size of participants. Actually, the fMRI also received the influence of age. In the future, the larger sample on different age level needs to be explored. Additionally, the following up study would be necessarily to explore the implications of abnormal FCs in MDD. ACKNOWLEDGEMENTS The authors thank Yuan Zhou for their valuable suggestions on the data analysis (Center for Social and Economic Behavior, Institute of Psychology, Chinese Academy of Sciences, Beijing, PR China). The authors thank Yi Jin, Jianyin Qiu and Chunbo Li for their suggestions on manuscript (Shanghai Mental Health Center). REFERENCES 1. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003;65:193-207. 2. Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry 2010;67:161-167. 3. Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K, Suzuki S, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord 2010;122:76-85. 4. Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp 2009;30:2617-2627. 5. Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 2010;50:347-356. 6. Damasio AR. Neuropsychology. Towards a neuropathology of emotion and mood. Nature 1997;386:769-770. 7. Anand A, Charney DS. 1999. Abnormality of catecholamines and pathophysiology of bipolar disorder. In: Soares JC, Gershon S, editors. Bipolar Disorder: Basic Mechanisms and Therapeutic Implications. New York: Marcel Dekker, 59–94. 8. Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med 1998;49:341-361. 9. Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study.Biol Psychiatry 2005;57:1079-1088. 10. Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res: neuroimaging 2009;171:189-198. 11. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4:215-222. 12. Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 2003;460:425-449. 13. Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007;62:429-437. 14. Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus.Proc Natl Acad Sci USA 2010;107:11020-11025. 15. Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry 2005;62:397-408. 16. Peng D, Jiang K, Fang Y, Xu Y, Long X, Zang Y. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chinese Medical Journal 2011;124:369-373. 17. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201-215. 18. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003;100:253-258. 19. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 1998;7:119-132. 20. Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. Am J Neuroradiol 2000;21:1397-1401. 21. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278-296. 22. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537-541. 23. Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord 2010 121:220–230. 24. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673-9678. 25. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8:1057-1061. 26. Press WH, Teukolsky SA, Vetterling WT, Flannery BP (Eds). 1992. Numerical Recipes in C, Second Ed. Cambridge University Press, UK. 27. Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 2004;61:765-773. 28. Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828-834. 29. Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol 2008;6:e159. 30. Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 2009;29:1860-1873. 31. Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional study.Neuroscience 2010;167:97-103. magnetic resonance imaging 32. Werner NS, Meindl T, Materne J, Engel RR, Huber D, Riedel M, et al. Functional MRI study of memory-related brain regions in patients with depressive disorder. J Affect Disord 2009;119:124-131. 33. Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol Psychiatry 2009;50:307-316. 34. Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, et al. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry 2004;61:556-563. 35. Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 2009;63:754-761. 36. Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression.Soc Cogn Affect Neurosci 2009;4:313-327. Table 1. Demographic and Clinical characteristics Depressed Patients (n = 16) Healthy Subjects (n = 16) t P 33.445.77 9/7 33.255.09 9/7 0.09 0.92 -0.27 15.482.69 15.723.03 33.135.74 3.081.30 32.26 31.192.95 2.631.96 8.18 5.620.72 3.310.87 HAMD: Hamilton Depression Rating Scale. HAMA: Hamilton Anxiety Scale. 0.78 Age (years) Gender (male/female) Education level (years) Age on set (years) Duration of illness (months) 24-item HAMD Score 14-item HAMA Score 0.00 0.00 Table 2. Significant Clusters in the Paired t-Tests Comparing Functional Connectivity of ACC and Thalamus in MDD subjects versus Controls Cluster Location (BA) Primary Peak Location Cluster Size T -Score 923 3.97 pgACC MDD versus Controls parahippocampus gyrus -21 -49 7 Parietal Prefrontal -3 -27 -64 -46 52 28 3.59 3.33 Controls versus MDD No significant cluster Thalamus MDD versus Controls No significant cluster Controls versus MDD Parietal 9 15 -52 -43 46 43 962 4.89 3.92 cingulate gyrus Listed are the Talairach coordinates (MNI), voxel number (Cluster size), and significance level for primary peak of each region. Height and extent thresholds of P < 0.05 were used to determine significant clusters. Abbreviations: MDD, major depression disorder. BA, Broadmann’s Area. pgACC, pregenual anterior cingulate cortex Figure 1. Location of seed regions. Red open circle corresponds to a seed region in the pgACC [12 41 13], and blue open circle corresponds to a seed region in the thalamus [−7.5 −17.5 5.5] Figure 2. Comparison of connectivity maps for depressed and control subjects across the FCs of the pgACC. X, Y and Z are the Talairach coordinates (MNI) of primary peak in significantly different clusters. The color bar indicates that images were thresholded at P < 0.05 Figure 3. Comparison of connectivity maps for depressed and control subjects across the FCs of the thalamus. X, Y and Z are the Talairach coordinates (MNI) of primary peak in significantly different clusters. The color bar indicates that images were thresholded at P < 0.05.