* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neuronal and microglial cathepsins in aging and age
Selfish brain theory wikipedia , lookup
Human brain wikipedia , lookup
Neural oscillation wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Brain morphometry wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Brain Rules wikipedia , lookup
Subventricular zone wikipedia , lookup
History of neuroimaging wikipedia , lookup
Multielectrode array wikipedia , lookup
Neuropsychology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Synaptic gating wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroplasticity wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Nervous system network models wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neurogenomics wikipedia , lookup
Environmental enrichment wikipedia , lookup
Alzheimer's disease wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Metastability in the brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
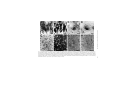
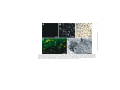
Ageing Research Reviews 2 (2003) 367–381 Neuronal and microglial cathepsins in aging and age-related diseases Hiroshi Nakanishi∗ Laboratory of Oral Aging Science, Faculty of Dental Sciences, Kyushu University, Fukuoka 812-8582, Japan Received 20 March 2003; accepted 25 March 2003 Abstract It has been long believed that cathepsins compensate for each other because of their overlapping substrate specificities. However, there is increasing evidence that disturbance of the normal balance of their enzymatic activities is the first insult in brain aging and age-related diseases. The imbalance of cathepsins may further cause age-related neuropathological changes such as accumulation of autophagic vacuoles and the formation of ceroid-lipofuscin leading to neuronal dysfunction and damage. Leakage of cathepsins due to the fragility of lysosomal membranes during aging also contributes to neurodegeneration. Furthermore, the deficiency of cathepsin D has been recently revealed to provoke a novel type of lysosomal storage disease associated with massive neurodegeneration. In these animals, microglia are activated to initiate inflammatory and cytotoxic responses by binding and phagocytosis of storage neurons. Activated microglia also release some members of cathepsins to induce neuronal death by degrading extracellular matrix proteins. Thus the microglial activation possibly through sensing neuronal storage may also be an important causative factor for neurodegeneration in lysosomal storage diseases and age-related diseases such as Alzheimer’s disease. This review describes the pathological roles of neuronal and microglial cathepsins in brain aging and age-related diseases. © 2003 Published by Elsevier Ireland Ltd. Keywords: Cathepsin; Neuron; Microglia; Endosomal/lysosomal system; Ceroid-lipofuscin; Aging; Alzheimer’s disease; Lysosomal storage disease 1. Introduction A group of proteases in the endosomal/lysosomal proteolytic system is called cathepsin which is derived from the Greek term meaning “to digest” (Willstätter and Bamann, 1929). ∗ Tel.: +81-92-642-6413; fax: +81-92-642-6415. E-mail address: [email protected] (H. Nakanishi). 1568-1637/$ – see front matter © 2003 Published by Elsevier Ireland Ltd. doi:10.1016/S1568-1637(03)00027-8 368 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 Cathepsins are synthesized on membrane-bound ribosomes as N-glycosylated precursors and are transferred into the endoplasmic reticulum and later into the Golgi complex. During transport to the Golgi complex, pro-cathepsins acquire modification of their carbohydrate moieties, which includes the formation of the mannose 6-phosphate (M6P) residues. Following the binding to M6P-specific receptors (MPRs), the enzyme–receptor complexes exit the trans-Golgi network in clathrin-coated vesicles and are transported to the late endosomes (Kornfeld, 1992). Upon fusion with the late endosomes, the dissociation of ligands occurs. The delivery of pro-cathepsins to lysosomes is accompanied by a series of proteolytic cleavages into their mature forms. In addition to a M6P-dependent targeting system, cathepsins can be also targeted to the lysosomes in a M6P-independent mechanism (Glickman and Kornfeld, 1993). Although the primary function of cathepsins is to degrade proteins by bulk proteolysis in lysosomes, recent results from cathepsin gene knockouts have revealed that cathepsins carry out their specific functions by limited proteolysis of proteins (Saftig et al., 1995; Turk et al., 2000; Reinheckel et al., 2001). It is likely that limited proteolysis is exerted by cathepsins in less acidic intracellular compartments such as early and late endosomes. There is increasing evidence that disturbance of normal balance and extralysosomal localization of cathepsins contribute to neurodegeneration in Alzheimer’s disease, stroke, and lysosomal storage diseases (Cataldo et al., 1990, 1991; Nakanishi et al., 1994, 1997; Nixon, 2000; Koike et al., 2000; Yamashima, 2000). Furthermore, the dysfunction of endosomal/lysosomal system in neurons is closely associated with an activation of microglia which could initiate an inflammatory response to provoke a neurodegeneration (Nakanishi, 2003b). Activated microglia also release some members of cathepsins to induce neuronal death through degradation of extracellular matrix proteins (Nakanishi, 2003a, 2003b). Thus, this review will focus on the pathological roles of neuronal and microglial cathepsins in brain aging and age-related diseases. 2. Cathepsins and brain aging 2.1. Cathepsin alterations during brain aging Alterations in the concentration and localization of cathepsins in the central nervous system (CNS) have been reported in normal aged brain (Nakamura et al., 1989). We have reported that the levels of cathepsins D and E were significantly increased during normal aging process (Fig. 1A and B) (Nakanishi et al., 1994, 1997). The enzymatic activity of cathepsin B was also increased significantly in the neostriatum of the aged rat but is relatively unchanged in other regions from 2 to 28 months. By contrast, the enzymatic activity of cathepsin L was decreased significantly (approximately 90%) in all brain regions of aged rats (Nakanishi et al., 1994). There were also marked age-related changes in cellular localization and enzymatic activities of cathepsins in peripheral neurons. Cathepsins D, B and L were widely and evenly distributed throughout the cytoplasm as coarse intracytoplasmic granules, whereas they were localized at focal cytoplasmic sites in trigeminal ganglion neurons of aged rats (Fig. 1C and D). The enzymatic activities of cathepsins B and L were decreased significantly (approximately 50%) in aged rats (Amano et al., 1995). Lynch’s group has been motivated H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 369 Fig. 1. Age-related changes of cathepsins in CNS and trigeminal ganglion neurons. Staining of cathepsin D in the hippocampal CA1 neurons of 2-month-old (A) and 30-month-old (B) rats, respectively. Staining of cathepsin D in the trigeminal ganglion neuron of 2-month-old (C) and 30-month-old (D) rats, respectively. Immunoreactivities of cathepsin D (E) and their colocalization with autofluorescent lipopigments (F) in cortical neurons of 30-month-old rat. Colocalization of cathepsin D-immunoreactivities and autofluorescent lipopigments was indicated by arrowheads. Double-staining immunohistochemistry of cathepsin E (G) and carboxy-terminal fragments of APP (APP643–695 ) (H) in the brainstem neurons of 30-month-old rat. Colocalization of immunoreactivities of cathepsin E and APP643–695 was indicated by arrowheads. Scale bars: 10 m (A–D), 50 m (E and F), 100 m (G and H). 370 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 by these observations and examined effects of long-term treatment of cysteine protease inhibitors on the hippocampal slice cultures. N-CBZ-l-phenylalanyl-l-alanine-diazomethyl ketone, a selective inhibitor of cathepsins B and L, induced increased cathepsin D levels, the accumulation of lysosome-related dense bodies and hyperphospholylated fragments, and the formation of meganeurites in the perykarya of CA1 neurons in cultured hippocampal slices (Bednarski and Lynch, 1996; Bednarski et al., 1997). These observations strongly suggest that disturbance of the normal balance of enzymatic activity of cathepsins causes age-related neuropathological changes. However, lysosome-related dense bodies induced by N-CBZ-l-phenylalanyl-l-alanine-diazomethyl ketone did not emit autofluorescence, indicating that they do not contain ceroid-lipofuscin, a major type of residual body observed in various types of neurons during the aging process (Bednarski et al., 1997). This contrasts with the observation that intraventricular application of leupeptin, a cystein protease inhibitor, or chloroquine, a general lysosomal inhibitor, in young rats induced the formation of lysosme-associated granular aggregates that closely resemble ceroid-lipofuscin (Ivy et al., 1984). Upregulation of the endosomal/lysosomal system has also been demonstrated in affected neurons of Alzheimer’s disease brain (Nixon and Cataldo, 1993, 1995; Nixon, 2000). In the senile plaques, the enzymatically active cathepsin D, as well as cathepsins B and L, are present extracellularly at high levels (Bernstein et al., 1989; Cataldo and Nixom, 1990; Cataldo et al., 1991). Furthermore, secondary lysosomes and residual bodies are markedly increased in affected neurons of Alzheimer’s disease brain (Cataldo et al., 1990; Nixon and Cataldo, 1993). The activation of the endosomal/lysosomal system has been suggested to be closely associated with increased production of amyloid  (A) peptides in sporadic Alzheimer’s disease (Nixon, 2000). 2.2. Amyloid precursor protein (APP) metabolism It is well known that APP is metabolized through two different pathways, secretory and endosomal/lysosomal pathways. In the secretory pathway, -site APP cleavage enzyme (BACE), a novel transmembrane aspartic protease, has been recently identified as -secretase (Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999). On the other hand, cathepsins D and E are considered to influence A peptides generation within the endosomal/lysosomal system because they have -secretase activity (Grüninger-Leitch et al., 2000). BACE was also found in early endosomes (Vassar et al., 1999). We have found that cathepsins D and E are colocalized with ceroid-lipofuscin (Fig. 1E and F) and carboxy-terminal fragments of APP (Fig. 1G and H) in CNS neurons of aged rats (Nakanishi et al., 1997). Immunoblot analysis revealed that the molecular sizes of accumulated carboxy-terminal APP fragments were 19 and 22 kDa, suggesting that they might contain an intact A peptide domain and therefore be amyloidogenic. Mathews et al. (2002) have recently reported that overexpression of MPRs which selectively redirect lysosomal hydrolases to early endosomes increases the generation of A peptides. It is also known that MRPs are more highly expressed in Alzheimer’s disease brain (Nixon, 2000). These observations strongly suggest that the upregulation of early endosomes as a consequence of increased expression of MRPs are closely associated with enhanced A peptides production and secretion in Alzheimer’s disease. Although Saftig et al. (1996) excluded the possible H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 371 involvement of cathepsin D in amyloidogenic processing of APP by utilizing cathepsin D-deficient mice, age-associated factors such as increased expression and endosomal localization may be required for cathepsin D to work as -secretase (Mathews et al., 2002). 2.3. Lysosomal membrane impermeability Besides functions inside the endosomal/lysosomal system, there is evidence that some members of cathepsins are also involved in extracellular proteolysis resulting in pathological conditions. Leakage of cathepsins into the cytoplasm is often achieved by the endocytosis of oxidizable substrates that destabilize the lysosomal membranes through lipid peroxidation. It has been proposed that the increased level of cytosolic cathepsin D in the aged rat brain is due to the age-dependent increase in the fragility of the lysosomal membrane (Matsu and Green, 1987; Nakamura et al., 1989). We have previously reported that subcellular fractionation showed that the amounts of cathepsins D and E in the soluble fraction of the aged rat brain were markedly increased as compared with those of the young rat brain, suggesting that these enzymes leaked into the cytoplasm. More recently, leakage of cathepsin D into the cytoplasm of neurons in aged rats is demonstrated by immunoelectron microscopy (Jung et al., 1999). In senile plauques of Alzheimer’s disease brain, lysosomal hydrolases such as cathepsin D distribute in an abnormal extracellular location (Cataldo et al., 1991). In vitro, cathepsin D and -hexosaminidase are increased in the soluble cytosolic compartment of the cells following treatment with A peptides (Yang et al., 1998). Some lysosomal cysteine proteases such as cathepsins B, H and L are relatively unstable after leakage from lysosomes, whereas others such as cathepsins D, E, and S are stable even at neutral pH (Bednarski and Lynch, 1996; Turk et al., 2000). Therefore, these enzymes are capable of degrading cytoskeletal proteins and bioactive peptides in the cytoplasm of neurons. Furthermore, there is increasing evidence that some members of cathepsins are directly involved in apoptosis. Cathepsin D has been first reported to play as a final executioner of apoptosis induced by interferon-␥, Fas/APO-1, TNF-␣ (Deiss et al., 1996), chemotherapeutic agents such as adriamycin and etoposide (Wu et al., 1998), and serum deprivation (Shibata et al., 1998; Isahara et al., 1999). Cathepsin B has been also implicated in the activation of the proinflammatory caspases 1 and 11 (Vancompernolle et al., 1998; Schotte et al., 1998), and the cleavage of Bcl-2 family member Bid (Stoka et al., 2001) which may lead to cytochrome c release from the mitochondria and subsequent caspase activation (Guicciardi et al., 2000). Taken together, the abnormal localization of cathepsins resulting from the disintegration of lysosomal as well as plasma membranes may contribute to the pathogenesis of age-related neuronal dysfunction and Alzheimer’s disease. 2.4. Upregulation of endosomal/lysosomal system in microglia In aged rat and Alzheimer’s disease brains, microglia are known to express activation markers such as major histocompatibility complex (MHC) class II molecules, lysosomal membrane marker ED1, and the leucocyte common antigen and CD4 (Perry et al., 1993; Togo et al., 2000). Although the microglial reaction has long been considered as a secondary event following neuronal damage and death, there is increasing evidence that the activation of microglia is closely associated with brain aging and the pathogenesis of Alzheimer’s 372 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 disease. A peptides have been reported to induce neuronal death indirectly by activating microglia to produce inflammatory mediators such as nitric oxide (NO), cytokines, and reactive oxygen intermediates (Meda et al., 1995; El Khoury et al., 1996). Several lines of evidence suggest that activated microglia secrete cathepsins to induce neuronal death. In response to lipopolysaccharide (LPS), there was a substantial increase in cathepsin S activity secreted from both macrophages and microglia (Petanceska et al., 1996). This may suggest that cathepsin S plays a role in degenerative disorders because cathepsin S degrades components of extracellular matrix proteins even at neutral pH. Cathepsin B was also secreted from immortalized murine microglial cell line, BV-2 cells, as the heavy chain form in addition to the proform upon stimulation with LPS (Ryan et al., 1995). More recently, it has been demonstrated that secreted cathepsin B is a major causative factor of microglia-induced neuronal apoptosis (Kingham and Pocock, 2001). We have shown that some cathepsins are closely linked with the proteolytic processing of exogenous antigens and invariant chain of MHC class II molecules through the endosomal/lysosomal system of microglia (Nishioku et al., 2002). Although the precise implication of exogenous antigen presentation in the CNS is not fully understood, inflammatory cytokines secreted from activated helper T cells and microglia activated through their interaction may contribute to the tissue damage and/or repair. Therefore, increased levels of cathepsins in activated microglia distributed especially in the white matter of aged rat (Nakanishi et al., 1994) and Alzheimer’s disease brain (Bernstein and Wiederanders, 1994; Yoshiyama et al., 2000) may have some pathological significance. 2.5. Degradation of Aβ peptides in lysosomes Cathepsin D has been suggested to be responsible for the intracellular clearance of A peptides in human and rat brains (Hamazaki, 1996; McDermott and Gibson, 1996). A peptides are taken up predominantly by microglia via class A scavenger receptors and class B scavenger receptor type I (Paresce et al., 1996; Husemann et al., 2001). Then A peptides are accumulated and degraded in the lysosomes of microglia (Paresce et al., 1997). Importantly, pepstatin A has been reported to inhibit the degradation of A peptides in microglia (Kakimura et al., 2002). These observations strongly suggest that phagocytosed A peptides are mainly degraded by cathepsin D in lysosomes of microglia. It is also noteworthy that immunization with A peptides has been demonstrated to reduce A peptides in transgenic mice with A plaques (Schenk et al., 1999). Thus, the phagocytosis and subsequent degradation of A peptides by microglia may play a pivotal role in a strategy for the immunotherapy of Alzheimer’s disease. 3. Cathepsins and lysosomal storage diseases 3.1. Cathepsin D-deficient mice To gain more insight into functions of cathepsin D, mice deficient in cathepsin D were generated by gene targeting (Saftig et al., 1995). The homozygous mutant mice developed a progressive atrophy of the intestinal mucosa and a profound destruction of lymphoid tissues. H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 373 Although the mutant mice could develop normally during the first 2 weeks, they stopped thriving in the third week, decreased in weight, and then died between 25 and 27 days. At the cellular level, however, lysosomal bulk proteolysis was maintained in these mice. It was concluded that the essential functions of cathepsin D depend on limited proteolysis of biologically active proteins such as growth factors rather than on bulk degradation of proteins in lysosomes. During the course of our studies on morphological as well as functional changes in CNS neurons of cathepsin D-deficient mice, we noticed that these animals showed neurological phenotypes such as seizures and blindness near the terminal stage. The most striking feature found in the CNS was a profound storage of autophagosome/autolysosome-like bodies with part of the cytoplasm, granular osmiophilic deposits, and fingerprint profiles (Koike et al., 2000). Almost all neurons especially in the cerebral cortex and thalamus contained large autofluorescent bodies, indicating the accumulation of ceroid-lipofuscin in the lysosomal structures (Fig. 2A and B). These ceroid-lipofuscin loaded lysosome contained subunit c of mitochondrial F1 F0 -ATP synthase, a common storage material of neuronal ceroid lipofusinosis (NCL) except for the infantile form of NCL. Interestingly, however, the protein and activity levels of tripeptidyl peptidase I, whose deficiency causes late-infantile NCL, were increased in cathepsin D-deficient mouse brain (Koike et al., 2000). These observations strongly suggest that the loss of cathepsin D activity causes a novel type of lysosomal storage disease associated with massive neurodegeneration. 3.2. Activated microglia-induced inflammation in lysosomal storage diseases We have observed a marked accumulation of morphologically transformed microglia exhibiting expanded and round cell bodies with a few thick processes especially in the cerebral cortex and thalamus near terminal stage of cathepsin D-deficient mice (Fig. 2C). The morphological transformation of microglia may be caused by binding and uptake of neurons laden with large autofluorescent bodies (Fig. 2D and E). Furthermore, there is a prominent expression of inducible nitric oxide synthase (iNOS) in both morphologically transformed microglia and peripheral macrophages in cathepsin D-deficient mice (Nakanishi et al., 2001). NO and the superoxide anion, which is generated in mitochondria, react rapidly to form a peroxinitrite anion. This, in turn, generates highly toxic hydroxyl radicals and hydrogen peroxide. Although NO is synthesized from l-arginine by NOS, iNOS is thought to be the isoform that produces the large quantities of NO that can result in tissue damage or death. To directly address the possible involvement of NO in tissue damage and neuronal death in cathepsin D-deficient mice, we examined effects of l-NG -nitro-arginine methylester (lNAME), a potent competitive NOS inhibitor and S-methylisothiourea hemisulfate (SMT), an iNOS inhibitor. Chronic treatment with l-NAME or SMT significantly decreased the total number of terminal dUTP nick-end labeling (TUNEL)-positive cells counted in the thalamus of cathepsin D-deficient mice (Nakanishi et al., 2001). In the course of these experiments, we unexpectedly found that chronic treatment with l-NAME or SMT markedly ameliorated a severe hemorrhage-necrotic appearance of the small intestine and atrophic changes of the ileal mucosa of cathepsin D-deficient mice. Therefore, the activated microglia/macrophageinduced inflammatory response is considered to be a major causative factor for pathological changes in the CNS and the small intestine of cathepsin D-deficient mice. 374 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 Fig. 2. Accumulation of ceroid-lipofuscin in CNS neurons of 24-day-old cathepsin D-deficient mice and phagocytosis by microglia. Autofluorescent lipopigments accumulated in thalamic neurons of the wild-type littermate (A) and cathepsin D-deficient mouse (B), respectively. (C) Accumulation of activated microglia stained with F4/80 in the thalamus of cathepsin D-deficient mouse. (D) Engulfment of neurons that were laden with large autofluorescent bodies (orange) by microglia labeled by F4/80 (green) in the thalamus of cathepsin D-deficient mouse. (E) Electron micrograph of microglia (m) attached to neuron (n) laden with autophagosome–autolysosome-like bodies in the cerebral cortex of cathepsin D-deficient mouse. Scale bars: 30 m (A and B), 20 m (C and D), 4.0 m (E). H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 375 Our hypothesis for a mechanism underlying activation of microglia and subsequent massive neuronal death in the CNS caused by cathepsin D-deficiency is summarized in Fig. 3. When cathepsin D activity is deficient, hydrophobic proteolipids such as subunit c of mitochondrial F1 F0 -ATP synthase are first accumulated in lysosomes. These storage materials further facilitate the formation of ceroid-lipofuscin especially in lysosomes of CNS neurons leading to neuronal dysfunction and damage. By binding and phagocytosis of these storage neurons, microglia are activated to produce NO through iNOS. Microglia may be also activated and/or primed through their own accumulation of proteolipids due to cathepsin Ddeficiency. Finally, the sustained and high levels of NO released from activated microglia initiate an intensive inflammatory response in the CNS leading to secondary neuronal damage. Bone marrow transplantation (BMT) has emerged as a potential treatment for some types of lysosomal storage diseases. It has been shown that bone-marrow-derived monocytes/macrophages can cross the blood–brain barrier and become perivascular macrophages and microglia (Eglitis and Mezey, 1997; Ono et al., 1999; Priller et al., 2001). The secretion of lysosomal enzymes from microglia/macrophages derived from hematopoietic donor cells and uptake by surrounding brain cells via M6P receptor-mediated endocytosis are proposed as the basis for BMT therapy for lysosomal storage diseases. However, it has been recently reported that microglia/macrophages secrete lysosomal enzymes such as arylsulphatase A and cathepsin D, which are incompetent for M6P receptor-dependent uptake by brain cells due to the lack of M6P residues (Muschol et al., 2002). Furthermore, BMT has been reported to extend the lifespan and ameliorate neurologic symptoms of Sandhoff disease mice without a clear reduction of neuronal GM2 ganglyoside storage (Norflus et al., 1998; Wada et al., 2000). Furthermore, the involvement of activated microglia in the pathogenesis of lysosomal storage diseases has been also suggested in other mouse models; metachromatic leukodystrophy (Hess et al., 1996), Sandhoff disease (Wada et al., 2000), Niemann-Pick disease type C (German et al., 2002), and mucopolysaccharidoses I and IIIB (Ohmi et al., 2003). Taken together, it is likely that the pathogenesis of these diseases is dominated by activated microglia-induced inflammation rather than lysosomal storage due to the deficiency of lysosomal enzymes. Furthermore, infiltration of Blood-born microglial precursors after BMT may improve neuronal pathology by a mechanism other than supplying the missing enzymes. 3.3. White Swedish Landscape sheep Tyynelä et al. (2000) have shown a novel form of NCL, congenital ovine NCL (CONCL) arising from a naturally occurring cathepsin D mutation. CONCL was observed in a flock of White Swedish Landscape sheep maintained on an experimental farm in Northern Sweden. In CONCL lambs, a single nucleotide mutation in the cathepsin D gene is substituted by a conserved active-site aspartate by asparagines at position 295 (Asp → Asn) resulting in a catalytically inactive protein. These animals show some phenotypes similar to those of cathepsin D-deficient mice such as neuronal death in the cerebral cortex and neuronal storage of autofluorescent granules. The activity of tripeptidyl peptidase I was also elevated. However, there are several differences between CONCL lambs and cathepsin D-deficient mice. Unlike cathepsin D-deficient mice, the newborn CONCL lambs do not exhibit any pathological changes in the lymphoid tissues and ileal mucosa. On the other hand, CONCL 376 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 Fig. 3. Microglial activation and neuronal death in CNS neurons of cathepsin D-deficient mice. Neuronal storage of hydrophobic storage materials such as subunit c of mitochondrial F1 F0 -ATP synthase is the primary insult due to cathepsin D-deficiency. The accumulation of these proteolipids facilitate the formation of neuronal ceroid-lipofuscin leading to neuronal dysfunction and damage. Microglia are activated by opposing these storage neurons and/or their own accumulation of proteolipids to produce NO through iNOS. Microglial NO may initiate an intensive inflammatory response in the CNS leading to secondary massive neurodegeneration. H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 377 lambs have a severe brain atrophy which is never observed in cathepsin D-deficient mice. Theses discrepancies may be due to the species difference, the maturity of the CNS, and/or the gestation periods. 3.4. Phenotypes of cathepsin B- and cathepsin L-deficient mice Cathepsin B-deficient mice show no obvious phenotype (Deussing et al., 1998), but a reduction in premature intrapancreatic trypsinogen activation (Halangk et al., 2000) and increased resistance to TNF-␣-mediated hepatocyte apoptosis (Guicciardi et al., 2000) were observed under experimental conditions. Cathepsin L-deficient mice also present with a reduction in CD4+ T cells (Nakagawa et al., 1998) and recurrent hair loss (Roth et al., 2000). More recently, however, it has been reported that combined deficiency of cathepsins B and L is lethal during the second to fourth week of life. These double-mutant mice reveal massive apoptosis of select neurons in the cerebral cortex and the cerebellar Purkinje and granule cell layers resulting in a brain atrophy (Felbor et al., 2002), suggesting that cathepsins B and L are essential for maturation and integrity of the postnatal CNS and that they compensate for each other. In the double-mutant mice, neurodegeneration is preceded by an accumulation of inclusions characterized by electron-dense, often membrane-bound bodies of varying size and shape occasionally containing lamellae. Furthermore, the double-mutant mice show normal activities of both pamitoyl protein thioesterase and tripeptidyl peptidase I, and no clear accumulation of subunit c and ceroid-lipofuscin. Thus, phenotypes of mice deficient for both cathepsins B and L is ultrastructurally and biochemically distinct from those seen in cathepsin D-deficient mice and classical NCLs. 4. Conclusions Cathepsins are now recognized to have more complex functions than simply being garbage disposers, and their imbalance during aging and age-related diseases may provoke deleterious effects on CNS neurons. The growing understanding of consequences of their age-related changes in neurons and microglia could contribute to the development of therapeutic interventions in massive neurodegeneration associated with age-related diseases such as Alzheimer’s disease and NCLs. Acknowledgements This work was supported by a Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. References Amano, T., Nakanishi, H., Kondo, T., Tanaka, T., Oka, M., Yamamoto, K., 1995. Age-related changes in cellular localization and enzymatic activities of cathepsins B, L and D in the rat trigeminal ganglion neuron. Mech. Ageing Dev. 83, 133–141. 378 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 Bednarski, E., Lynch, G., 1996. Cytosolic proteolysis of by cathepsin D in hippocampus following suppression of cathepsins B and L. J. Neurochem. 67, 1846–1855. Bednarski, E., Ribak, E.C., Lynch, G., 1997. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J. Neurosci. 17, 4006–4021. Bernstein, H.G., Brusziz, S., Schmidt, D., Wiederanders, B., Dorn, A., 1989. Immunodetection of cathepsin D in neuritic plaques found in brains of patients with dementia of Alzheimer type. J. Hirnforsch. 30, 613–618. Bernstein, H.G., Wiederanders, B., 1994. An immunohistochemical study of cathepsin E in Alzheimer-type dementia brains. Brain Res. 667, 287–290. Cataldo, A.M., Nixom, R.A., 1990. Enzymatically active lysosomal proteinases are associated with amyloid deposits in Alzheimer brain. Proc. Natl. Acad. Sci. U.S.A. 87, 3861–3865. Cataldo, A.M., Hamilton, D.J., Nixon, R.A., 1990. Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res. 640, 68–80. Cataldo, A.M., Paskevich, P.A., Kominami, E., Nixon, R.A., 1991. Lysosomal hydrorases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 88, 10998–11002. Deiss, L.P., Galinka, H., Berissi, H., Cohen, O., Kimchi, A., 1996. Cathepsin D protease mediates programmed cell death induced by interferon-␥, Fas/APO-1 and TNF-␣. EMBO J. 15, 3861–3870. Deussing, J., Roth, W., Saftig, P., Teters, C., Ploegh, H.L., Villadangos, J.A., 1998. Cathepsins B and D are dispensable for major histocompatibility complex II-mediated antigen presentation. Proc. Natl. Acad. Sci. U.S.A. 95, 4516–4521. Eglitis, M.A., Mezey, É., 1997. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. U.S.A. 94, 4080–4085. El Khoury, J., Hickman, S.E., Thomas, C.A., Cao, L., Silverstein, S.C., Loike, J.D., 1996. Scavenger receptor-mediated adhesion of microglia to -amyloid fibrils. Nature 382, 716–719. Felbor, U., Kessler, B., Mothes, W., Goebel, H.H., Ploegh, H.L., Bronson, R.T., Olsen, B.R., 2002. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. U.S.A. 12, 7883–7888. German, D.C., Liang, C.L., Song, T., Yazdani, U., Xie, C., Dietschy, J.M., 2002. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience 109, 437–450. Glickman, J.N., Kornfeld, S., 1993. Mannose 6-phosphate-independent targeting of lysosomal enzymes in I-cell disease B lymphoblasts. J. Cell Biol. 123, 99–108. Guicciardi, M.E., Deussing, J., Miyoshi, H., Bronk, S.F., Svingen, P.A., Peters, C., Kaufmann, S.H., Gores, G.J., 2000. Cathepsin B contributes to TNF-␣-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137. Grüninger-Leitch, F., Berndt, P., Langen, H., Nelboeck, P., Döbeli, H., 2000. Identification of -secretase-like activity using a mass spectrometry-based assay system. Nat. Biotech. 18, 66–70. Halangk, W., Lerch, M.M., Brandt-Nedelev, B., Roth, W., Ruthenbuerger, M., Reinheckel, T., Domschke, W., Lippert, H., Peters, C., Deussing, J., 2000. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 106, 773–781. Hamazaki, H., 1996. Cathepsin D is involved in the clearance of Alzheimer’s -amyloid protein. FEBS Lett. 396, 139–142. Hess, B., Saftig, P., Hartmann, D., Coenen, R., Lüllmann-Rauch, R., Goebel, H.H., Evers, M., von Figura, K., D’Hooge, R., Nagels, G., De Deyn, P., Peters, C., Gieselmann, V., 1996. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc. Natl. Acad. Sci. U.S.A. 93, 14821–14826. Husemann, J., Loike, J.D., Kodama, T., Silverstein, S.C., 2001. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrilar -amyloid. J. Neuroimmunol. 114, 142–150. Isahara, K., Ohsawa, Y., Kanamori, S., Shibata, M., Waguri, S., Sato, N., Gotow, T., Watanabe, T., Momoi, T., Urase, K., Kominami, E., Uchiyama, Y., 1999. Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases. Neuroscience 91, 233–249. Ivy, G.O., Schottler, F., Wenzel, J., Baudry, M., Lynch, G., 1984. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science 226, 985–987. Jung, H., Lee, E.Y., Lee, S.I., 1999. Age-related changes in ultrastructural features of cathepsin B- and D-containing neurons in rat cerebral cortex. Brain Res. 844, 43–54. Kakimura, J., Kitamura, Y., Takata, K., Umeki, M., Suzuki, S., Shibagaki, K., Taniguchi, T., Nomura, Y., Gebicke-Haerter, P.J., Smith, M.A., Perry, G., Shimohama, S., 2002. Microglial activation and amyloid- clearance induced by exogenous heat-shock proteins. FASEB J. 16, 601–603. H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 379 Kingham, P.J., Pocock, J.M., 2001. Microglial apoptosis induced by chromogranin A is mediated by mitochondrial depolarization and the permeability transition but not by cytochrome c release. J. Neurochem. 74, 1452–1462. Koike, M., Nakanishi, H., Saftig, P., Ezaki, J., Isahara, K., Ohsawa, Y., Schulz-Schaeffer, W., Watanabe, T., Waguri, S., Kametaka, S., Shibata, M., Yamamoto, K., Kominami, E., Peters, C., von Figura, K., Uchiyama, Y., 2000. Cathepsin D deficiency induces lysosomal storage with ceroid-lipofuscin in mouse CNS neurons. J. Neurosci. 20, 6898–6906. Kornfeld, S., 1992. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Ann. Rev. Biochem. 61, 307–330. Mathews, P.M., Guerra, C.B., Jiang, Y., Grbovic, O.M., Kato, B.H., Schmidt, S.D., Dinakar, R., Mercken, M., Hille-Rehfeld, A., Rohrer, J., Mehta, P., Cataldo, A.M., Nixon, R.A., 2002. Alzheimer’s disease-related overexpression of cation-dependent mannose 6-phosphate receptor increases A secretion. Role for altered lysosomal hydrolases distribution in -amyloidogenesis. J. Biol. Chem. 277, 5299–5307. Matsu, A., Green, G.D., 1987. Age-related increase in a cathepsin D like proteinase that degrades brain microtubule-associated proteins. Biochemistry 26, 8083–8086. McDermott, J.R., Gibson, A.M., 1996. Degradation of Alzheimer’s -amyloid protein by human cathepsin D. NeurorReport 7, 2163–2166. Meda, L., Cassatella, M.A., Szendre, G.I., Otvos Jr., L., Baron, P., Villalba, M., Ferrari, D., Rossi, F., 1995. Activation of microglial cells by -amyloid protein and interferon-␥. Nature 74, 647–650. Muschol, N., Matzner, U., Tiede, S., Gieselmann, V., Ullrich, K., Braulke, T., 2002. Secretion of phosphomannosyl-deficient arylsulphatase A and cathepsin D from isolated human macrophages. Biochem. J. 368, 845–853. Nakagawa, T., Roth, W., Wong, P., Nelson, A., Farr, A., Deussing, J., Villadangos, J.A., Ploegh, H., Peters, C., Rudensky, A.Y., 1998. Cathepsin L: critical role in Ii degradation and CD4T cell selection in the thymus. Science 280, 450–453. Nakamura, Y., Takeda, M., Suzuki, H., Morita, H., Tada, K., Hariguchi, S., Nishimura, R., 1989. Lysosome instability in aged rat brain. Neurosci. Lett. 97, 215–220. Nakanishi, H., 2003a. Microglial proteases: strategic targets for neuroprotective agents. Curr. Neuropharmacol. 1, 99–108. Nakanishi, H., 2003b. Microglial functions and proteases. Mol. Neurobiol. 27, 163–176. Nakanishi, H., Tominaga, K., Amano, T., Hirotsu, I., Inoue, T., Yamamoto, K., 1994. Age-related changes in activities and localizations of cathepsins D, E, B, and L in the rat brain tissues. Exp. Neurol. 125, 1–10. Nakanishi, H., Amano, T., Sastradipura, D.F., Yoshimine, Y., Tsukuba, T., Tanabe, K., Hirotsu, I., Ohono, T., Yamamoto, K., 1997. Increased expression of cathepsins E and D in neurons of the aged rat brain and their colocalization with lipofuscin and carboxy-terminal fragments of Alzheimer amyloid precursor protein. J. Neurochem. 68, 739–749. Nakanishi, H., Zhang, J., Koike, M., Nishioku, T., Okamoto, Y., Kominami, E., von Figura, K., Peters, C., Yamamoto, K., Uchiyama, Y., 2001. Involvement of nitric oxide released from microglia/macrophages in pathological changes of cathepsin D-deficient mice. J. Neurosci. 21, 7526–7533. Nishioku, T., Hashimoto, K., Yamashita, K., Liou, S.-Y., Katsunuma, N., Peters, C., von Figura, K., Saftig, P., Yamamoto, K., Nakanishi, H., 2002. Involvement of cathepsin E in exogenous antigen processing in primary cultured murine microglia. J. Biol. Chem. 277, 4816–4822. Nixon, R.A., 2000. A “protease activation cascade” in the pathogenesis of Alzheimer’s disease. Ann. N.Y. Acad. Sci. 924, 117–131. Nixon, R.A., Cataldo, A.M., 1993. The lysosomal system in neuronal cell death: a review. Ann. N.Y. Acad. Sci. 679, 87–109. Nixon, R.A., Cataldo, A.M., 1995. The endosomal–lysosomal system of neurons: new roles. Trends Neurosci. 18, 489–496. Norflus, F., Tiff, C.J., McDonald, M.P., Goldstein, G., Crawley, J.N., Hoffmann, A., Sandhoff, K., Suzuki, K., Proia, R.L., 1998. Bone marrow transplantation prolongs life span and ameliorates neurologic manifestation in Sandhoff disease mice. J. Clin. Invest. 101, 1881–1888. Ohmi, K., Greenberg, D.S., Rajavel, K.S., Ryazantsev, S., Li, H.H., Neufeld, E.F., 2003. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. U.S.A. 100, 1902–1907. Ono, K., Takii, T., Onozaki, K., Ikawa, M., Okabe, M., Sawada, M., 1999. Migration of exogenous immature hematopoietic cells into adult mouse brain parenchyma under GFP-expressing bone marrow chimera. Biochem. Biophys. Res. Commun. 262, 610–614. 380 H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 Paresce, D.M., Ghosh, R.N., Maxfield, F.R., 1996. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid -protein via scavenger receptor. Neuron 17, 553–565. Paresce, D.M., Chung, H., Maxfield, F.R., 1997. Slow degradation of aggregates of Alzheimer’s disease amyloid -protein by microglial cells. J. Biol. Chem. 46, 29390–29397. Petanceska, S., Canoll, P., Devi, A.L., 1996. Expression of rat cathepsin S in phagocytic cells. J. Biol. Chem. 271, 4403–4409. Perry, V.H., Matyszak, M.K., Fearn, S., 1993. Altered antigen expression of microglia in the aged rodent CNS. Glia 7, 60–67. Priller, J., Flügel, A., Wehner, T., Boentert, M., Hass, C.A., Prinz, M., Fernández-Klett, F., Prass, K., Bechmann, D., De Boer, B.A., Frotscher, M., Kreuzberg, G.W., Persons, D.A., Dirnagl, U., 2001. Targeting gene-modifies hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engrafment. Nat. Med. 7, 1356–1361. Reinheckel, T., Deussing, J., Roth, W., Peters, C., 2001. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 382, 735–741. Roth, W., Deussing, J., Botchkarev, V.A., Pauly-evers, M., Saftig, P., Hafner, A., Schmidt, P., Schmahl, W., Scherer, J., Anton-Lamprecht, I., von Figura, K., Paus, R., Peters, C., 2000. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 14, 2075–2086. Ryan, R.E., Sloane, B.F., Sameni, M., Wood, P.L., 1995. Microglial cathepsin B: an immunological examination of cellular and secreted species. J. Neurochem. 65, 1035–1045. Saftig, P., Hetman, M., Schmahl, W., Weber, K., Heine, L., Mossmann, H., Köster, A., Hess, B., Evers, M., von Figura, K., Peters, C., 1995. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 14, 3599–3608. Saftig, P., Peters, C., von Figura, K., Craessaerts, K., Van Leuven, F., De Stroop, B., 1996. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J. Biol. Chem. 271, 27241–27244. Schotte, P., Van Criekinge, W., Van de Craen, M., Van Loo, G., Desmedt, M., Grooten, J., Cornelissen, M., De Ridder, L., Vandekerckhove, J., Fier, W., Vandenabeele, P., Beyaert, R., 1998. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251, 379–387. Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Le, M., Lio, Z., Lieberburg, I., Motter, R., Mutter, L., Soriano, F., Shopp, G., Vasquez, N., Vendevert, C., Walker, S., Wogulis, M., Yednock, T., Games, D., Seubert, P., 1999. Immunization with amyloid- attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177. Shibata, M., Kanamori, S., Isahara, K., Ohsawa, Y., Konishi, A., Kametaka, S., Watanabe, T., Ebisu, S., Ishido, K., Kominami, E., Uchiyama, Y., 1998. Participation of cathepsin B and D in apoptosis of PC12 cells following serum deprivation. Biochem. Biophys. Res. Commun. 251, 199–203. Sinha, S., Anderson, J.P., Barbour, R., Basi, G.S., Caccavello, R., Davis, D., Doan, M., Dovey, H.F., Frigon, N., Hong, J., Jacobson-Croak, K., Jewett, N., Schenk, D., Seubert, P., Suomensaari, S.M., Wang, S., Walker, D., Zhao, J., McConlogue, L., Jhon, V., 1999. Purification and cloning of amyloid precursor protein -secretase from human brain. Nature 402, 537–540. Stoka, V., Turk, B., Schendel, S.L., Kim, T.-H., Cirman, T., Snipas, S.J., Ellerby, L.M., Bredesen, D., Freeze, H., Abrahamson, M., Brömme, D., Krajewski, S., Reed, J.C., Yin, X.-M., Turk, V., Salvesen, G.S., 2001. Lysosomal protease pathways to apoptosis. Cleavage of Bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276, 3149–3157. Togo, T., Akiyama, H., Kondo, H., Ikeda, K., Kato, M., Iseki, E., Kosaka, K., 2000. Expression of CD40 in the brain of Alzheimer’s disease and other neurological diseases. Brain Res. 885, 117–121. Turk, B., Turk, D., Turk, V., 2000. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta 1477, 98–111. Tyynelä, J., Sohar, I., Sleat, D.E., Gin, R.M., Donnelly, R.J., Baumann, M., Haltia, M., Lobel, P., 2000. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 12, 2786–2792. Vancompernolle, K., Van Herreweghe, F., Pynaert, G., Van der Craen, M., De Vos, K., Totty, N., Sterling, A., Fiers, W., Vandenabeele, P., Grooten, J., 1998. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 438, 150–158. H. Nakanishi / Ageing Research Reviews 2 (2003) 367–381 381 Vassar, R., Bennett, B.D., Babu-Khan, S., Kahn, S., Mendiaz, E.A., Denis, P., Teplow, D.B., Ross, S., Amarante, P., Loeloff, R., Luo, Y., Fischer, S., Fuller, J., Edenson, S., Lile, J., Jarosinski, M.A., Biere, A.L., Curran, E., Burgess, T., Louis, J.C., Collins, F., Treanor, J., Pogers, G., Citron, M., 1999. -Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741. Wada, R., Tifft, C.J., Proia, R.L., 2000. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc. Natl. Acad. Sci. U.S.A. 97, 10954–10959. Willstätter, R., Bamann, E., 1929. Über die Proteased der Magen-schleimahaut. Erste Abhandlung über die Enzyme der Leukozyten. Hoppe-Sseylers Z. Physiol. Chem. 180, 127–143. Wu, G.S., Saftig, P., Peters, C., El-Deiry, W.S., 1998. Potential role for cathepsion D in p53-dependent tumor suppression and chemosensitiivity. Oncogene 16, 2177–2183. Yamashima, T., 2000. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62, 273–295. Yan, R., Bienkowski, M.J., Shuck, M.E., Miao, H., Tory, M.C., Pauley, A.M., Brashier, J.R., Stratman, N.C., Mathews, W.R., Buhl, A.E., Carter, D.B., Tomasselli, A.G., Parodi, L.A., Heinrikson, R.L., Gurney, M.E., 1999. Membrane-anchored aspartyl protease with Alzheimer’s disease -secretase activity. Nature 402, 533– 537. Yang, A.J., Chandswangbhuvana, D., Margol, L., Glabe, C.G., 1998. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid A1–42 pathogenesis. J. Neurosci. Res. 52, 691–698. Yoshiyama, Y., Arai, K., Oli, T., Hattori, T., 2000. Expression of invariant chain and pro-cathepsin L in Alzheimer’s brain. Neurosci. Lett. 290, 125–128.