* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neuronal networks for induced `40 Hz` rhythms
Cognitive neuroscience of music wikipedia , lookup
Recurrent neural network wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Long-term depression wikipedia , lookup
Signal transduction wikipedia , lookup
Executive functions wikipedia , lookup
Circadian rhythm wikipedia , lookup
Haemodynamic response wikipedia , lookup
Aging brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Limbic system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Central pattern generator wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuroplasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuroregeneration wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neurotransmitter wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Synaptic gating wikipedia , lookup
Nervous system network models wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Chemical synapse wikipedia , lookup
Neural oscillation wikipedia , lookup
Neural binding wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
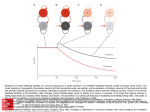
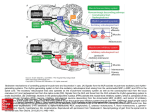
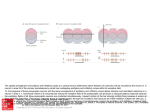
R V. Cornilleau-P6r& E and C. Gielen – Processing of optic flow 36 Buizza, A. et al. (1980) Exp. Brain Res. 39, 165-176 37 Nakayama, K. (1981) VisionRes. 21, 1475-1482 38 Dijkstra,T.M.H. et al. (1995) VisionRes. 35, 453-462 39 Van den Berg, A.V. and Collewijn, H. (1986) Vision Res. 26, 1209–1222 Acknowledgements 40 Ungerleider,L.G. and Mishkin, M. (1982) in Analysis of Visual Behavior (Ingle, D.J., Goodale,M.A. and Mansfield,R.J.W., eds), This work was pp. 549–586, MIT Press supportedby Esprit 41 Maunsell, J.H.R. and Van Essen, D.C. (1983) J. Neurosci.3, ProjectMucorrc2 2563-2586 6615. We thank 42 Koenderink, J.J. and Van Doom, A.J. (1975) Optica Acta 22, 773-791 TjeerdDijkstra, 43 Saito, H. et al. (1986) J. Neurosci. 6, 145-157 [acques Droulez, 44 Lagae, L. et aZ. (1994) /. NeurophysioL71, 1597-1626 FranckBrernmer 45 Duffy, C.J. and Wurtz, R.H. (1991) ]. NeurophysioL 65, 1329-1345 and WernerGraf for valuable 46 Motter, B.C. and Mountcastle, V.B. (1981) 1. Neccrosci.1, 3–26 47 Orban, G.A.et al. (1992) Proc.NatlAcad. Sci. USA89, 2595–2599 comments on the 48 Allman,J., Miezin,F. and McGuinness,E. (1985) Perception14, 105–126 manusm”pt. V I 49 Maunsell, J.H.R. and Van Essen, D.C. (1983) J. Neurophysiol. 49, 1148-1167 50 Roy, J.P. and Wurtz, R.H. (1990) Nature 348, 160-162 51 Bradley, D.C., Qian, N. and Andersen,R.A. (1995) Nature 373, 609-611 52 Andersen, R.A. (1987) in Handbook of Physiology (Section 1, Vol. 5) (Brookhart, J.M. and Mountcastle, V.B., eds), PP. 483-518, AmericanPhysiologicalSociety 53 Komatsu, K.and Wurtz, R.H. (1988) ]. NeurophysioL60, 580-603 54 Sakata, H., Shibutani, H. and Kawano, K. (1980) J. Neurophysiol.43, 1654-1672 55 Kawano, K., Sasaki, M. and Yamashita, M. (1984) J. Neurophysiol.51, 340-351 56 Thier, P. and Erickson, R.G. (1992) Eur.J. Neurosci. 4, 539-553 57 Berman, N., Blakemore, C. and Cynader, M. (1975) J. PhysioL 246, 595-615 58 Hoffmann, K.P. (1982) in Functional Basis of Ocular Motility Disorder (Lennerstrand, G., Zee, D.S. and Keller, E.L., eds), pp. 303–311, Pergamon 59 Grasse,K.L. (1994) VisionRes. 34, 1673-1689 Neuronal networks for induced ’40 Hz’ rhythms John G.R. Jefferys, Roger D. Traub and Miles A. Whittington A fast,coherentEEG rhythm,calleda gamma or a ’40Hz’ rhythm,hasbeen implicatedboth in higherbrainfunctions,suchasthe‘binding’of featuresthat are detectedby sensorycorticesinto perceivedobjects,and in lower levelprocesses, suchas the phasecodingof neuronalactivity. Computer simulationsof severalparts of the brain suggestthat gamma rhythms can be generatedby poolsof excitatoryneurones,networksof inhibitoryneurones,or networksof both excitatoryand inhibitoryneurones.The strongestexperimentalevidencefor rhythm generators J.G.R.Jeff2rysis hasbeen shownfor: (1) neocorticaland thalamicneuronesthat are intrinsic’40Hz’ oscillators, at the Dept of although synchronystill requiresnetwork mechanisms; and (2) hippocampaland neocortical Physiology,The networksof mutually inhibitory interneuronesthat generatecollective40Hz rhythms when MedicalSchool, Unive7si@of excitedtonically. Birmingham, Trends Neurosci. (1996) 19, 202-208 Birmingham,UX B1527T. In humans the auditory response includes brief AST, GAMMA RHYTHMS have been implicated in MilesA. Whittbcgtonis at higher cognitive function. They are also known as ‘40Hz transientresponses’’9’20,which increasewhen the the Neuronal ‘40 Hz’rhythms, but actually range from 30 to 100Hz subjectpaysattention, andwhich disappearwith lossof Repetitiveauditory NetworksGroup, and might vary in frequency during a response. The consciousnessduringanaesthesia21. DeptofPhysiology 20-100 Hzrangeweconsiderhereoverlapswith the beta stimulation at -40 Hz generates a large ’40 Hz steadyand Biophysics, band (15–30Hz)of the EEG,but wewillignorethe finer state response’zz.Recordingsof brain magnetic activity StMary’sHospital points of EEG classification. The natural history and (magnetoencephalogramsor MEGs)in humans suggest during MedicalSchool, functionalrolesof synchronousgammaoscillationshave that gammarhythms can be verywidespread23, Imperial College, been reviewedrecentlyl-3,and sowillbe consideredonly both waking and dream states. Other MEG measurements in humans suggestthat gamma rhythms might London, briefly. Gamma rhythms occur in humans and other mam- be organizedto sweepacrossthe whole brain, perhaps UK W2 lPG. RogerD. Traub is at mals following sensory stimuli, often in brief runs. providing ‘temporal binding...into a single cognitive the IBMResearch ‘Inducedrhythms’at 50-60 Hzwerefirstdescribedin the experience’24. Division,T.!. olfactorybulbby Adrian4,andhavesincebeenidentified Neuronalfiring auditorycorWatson Research in the olfactory cortexs, visualcortex3’&9, somatosensory cortexlz and motor cortex13-15. Single-unit recordings in vivo have revealed much Center,Yorktown tex10’11, Heights,NY 10598, Gammaoscillationsalsooccur in the hippocampuslc’17, aboutthe eventsor featuresto which neuronesrespond. USA,and the Dept wherethe linkwithexternalsensorystimuliis lessdirect, Individualneuronesdonot detecttheirpreferredsensory of Neurology, but might stillexistin the formof multimodalinputsre- featuresin isolation,but formpartof neuronalnetworks Columbia ceivedfrom higher-ordersensorycortices.Hippocampal whoseemergentpropertiesdefinethe feature-detection Universi~,New gamma rhythms tend to occur during the theta band propertiesof the corticalcolumn. In the visualsystem,it York,NY 10032, (4-12 Hz)of the EEG,whichis a prominentfeatureof the usedto be thought that successivehierarchiesof neurespeciallyduringexploration. ones encoded progressivelymore-complex features of USA. hippocampusin vivo1618, F 202 TINS Vol. 19, No. 5, 1996 Copyright @ 1996, Elsevier Science Ltd. All rights reserved. 0166- 2236/96/S15.00 PII: S0166-2236(96)1 OO23-O REVIEW J.G.R. Jefferys et cd. – Neuronal networks for induced ‘40 Hz’ rhythms A c B Recurrent inhibition Mutual excitation D Intrinsic oscillator in network Mutual inhibition Fig. 2. Simplified representationsto illustrate the essentialfeaturesof severalmechanismsproposed ta be involved in the generation of gamma oscillations. In each case, E (excitatory) and / (inhibitory) representnetworks of neuronesthat are mutually connected,the continuouslines indicate the key connectionsfor their respectivemechanisms,and the dot-dash arrow indicatesthe ffow of specificinformation through the network.(A) i//ustratesthe recurrent inhibitory loop model proposedby Freemanet al.s Computertheoreticalanalysishas subsequentlyshown that mutual excitationisrequired,but that mutua/ inhibition isnot. (B) showsa simi/armode/in whichthe time de/aysa/ong the axonscouplinggroupsof excitatoryneuronesplay a keyro/e33,and whichalsoreceivescontributionsfrom recurrent inhibition. (C) proposesthat neuroneswith intrinsic-osci//atorpropertiescan imposetheir own rhythm on the synaptic networkin which theyare embedded34-37. (D) representsour own mode/of gamma oscillationsin the hippocampus,in which interneuronesare tonica//yexcited(thin arrows)so that they will fire at a rate >40 Hz. Thedivergentinhibitory connectionsbetween theseneuronesresult in synchronizedinhibition acrossthe population. When this decays,the neuraneswill discharaedue to the tanic excitationthat drivesthe rhvthm38imDosinoo rhvthm of about 40 Hz. Notice . ,./, that in its simplestform (D) separatesthe role of the oscillator(or clock)and the processor. sites, or to technical differences(gammarhythms can occur in brief bursts with a considerable jitter in the frequency”, so any correlation could conceivably be smudgedout when measurementsare averagedduring 0.5s runs of an EEG, or 20 cycles at 40 Hz; Ref. 28). However,the reasons for these discrepanciesremain unresolved. Coherent rhythms might have other functions. One idea is that they providea timing referencefor a neural code that depends on the phase relationship of individual neurones with the reference oscillation. The stronger the excitation to an individualneurone, the earlierin the cycle it willfire.Thus neuronesthat fire at similarphasesin the rhythm will have receivedsimilar intensifies of input which might be used,for example, to lock their outputstogether for a more effectivesummation.Thishypothesiswasproposedfortheta rhythms in the hippocampus30,and also, more recently, for gamma rhythms31. The role of gammarhythmsis unknown.They might be central to our cognitivefunction, be fundamentalto the neural code, have some entirely different role, or simplybean epiphenomenon32with no deepmeaning. We believethat one key step to resolvingthese issuesis to understandthe cellularandnetworkmechanismsthat generate gamma rhythms, and to developpharmacologicaltools that willallowus to probetheirrolesin vivo. What drivesthegammarhythm? The original models for binding and segmentation introduced the idea that neurones that oscillated togetheralsoworkedtogethe~s,reflectingthat synchronization is a more important factor than a narrow bandwidth327.Although single episodes of neuronal synchronization might occur by chance, repeatedsynchronizationis much lesslikelyto do so. Atthe cellular 204 TINS VOI. 19, NO. 5,1996 levelrepeatedsynchronizationcould promote temporal summation at active synapses. Severaltheories exist for the generation of gamma oscillations in variouspartsof the brain, which all need furtherexperimentaltesting.It is possible that gamma oscillations ariseby differentmechanismsin different parts of the brain, and that severalmechanismscan combine in individual regions. Figure 2 shows highly simplifiedrepresentationsof some of the components of these differentmechanisms,whichwewill consider below in roughly chronological order. Feedback loops between excitatory and inhibitory neurones (The main regionsimplicatedinclude: the olfactory bulb, the piriform cortex, the entorhinal cortex and the primary visual cortex.) Freemanand colleaguesdevelopeda model for induced rhythms in several olfactorystructures,which proposed that synchronous oscillation is generated by a feedback loop between excitatory and inhibitory neuroness.Theyproposedthat some mutual connectivity was also requiredwithinthe poolsof both excitato~ andinhibitory neuronesto stabiiizethe oscillations. Ermentrout39has shown that mutual excitation amongst the excitatory neurones is necessaryfor stable oscillations to be generated by a recurrent inhibitory loop. However, our recent simulationssuggestthat other conditions might sufficefor stable oscillations (R.D.Traub, unpublished observations). Freemanet al.s predictedthat inhibitory cells should lag behind the excitatory cells by a quarter of a cycle (6.5 ms at 40Hz). Experimental support came from single-unitand EEGrecordingsin vivofromthe olfactory bulb, anteriorolfactorynucleus,prepiriformcortex and entorhinalcortexs.The signalsfell into two groups:one set firedin phasewith the gamma EEG,and one either led or laggedthe gamma EEGby a quarter of a cycle. Unfortunatelythese measurementscannot identifythe types of neurones in each group. In contrast, hippocampalinterneuronesrecordedduringthe gammaEEG fire in phasewith pyramidalcells”. This is predictedby our inhibitory networkmodel (see below), both when isolatedfrom the excitatory network38,and when connected with pyramidalcells (R.D. Traub et aZ.,unpublished observations). Why the hippocampal and the (superficiallysimilar)olfactorycortical circuitryshould differ remains unclear5. Wilson and Bowermade similar models of the piriform cortex33and the primaryvisualcortex32.The geometric structure of these models differed, but the essential idea in both was that the amplitudeand the frequency of coherent 30-60Hz oscillations, elicited by afferent volleys, were determined (or ‘tuned’) by a fast-feedbackinhibitory loop (Fig. 2A). Essentially, if the stimulus is appropriate (not too strong), enough activityin the recurrentexcitatoryconnectionsbetween pyramidalcells persists after the recurrent inhibition J.G.R. Jefferys et al.– wanes in order to re-excite the pyramidal-cellpopulation. In the case of the piriform-cortex model, they showed that the time constant of inhibition ‘tuned’ the frequency of the gamma rhythm, so that longer time spent open for the chloride channels resultedin slowerrhythms (and also a loss of power). In their model of the primaryvisual cortex Wilson and Bower32note, in passing, that local mutual inhibition betweenthe interneurones‘improvedfrequency locking and produced auto- and cross-correlations with more pronouncedoscillatorycharacteristics’.This differs from the central role of similar connections in the generationof gammarhythmsin the hippocampus, where they were both necessaryand sufficient38,40. In the visual-cortex model, horizontal pyramidal cell axons wereessential for long-range(>1mm) crosscorrelations32.These had zero phase lag as long as the excitatory postsynaptic potentials (EPSPS)that they generatedwerenot too strong. StrongerEPSPSresultin phaselagsconsistentwith delaysin axonal conduction, while weakerEPSPSwere reminiscent of other kinds of looselycoupledoscillators.In both the visual-cortexand the piriform-cortex versions of this model, gamma rhythms arose from interactions between networksof excitatory neurones, could dependon the conduction velocitiesof intrinsic cortical connections (Fig.2B), and were tuned by the time constants of excitatory and inhibitory synapses.We are not awareof any attempts to dissectthese complexinteractionsexperimentally;in particular,an investigationof the effectsof conduction delayson cortical oscillations wouldbe instructive. Intrinsic oscillations in individual neurones (The main regions implicated include the thalamus and the neocortex; seeFig.2C.) Neuronesin many parts of the brain have the intrinsic capacity to oscillate at about 40 Hz. Space does not permit an exhaustive reviewof intrinsic oscillators;here we outline one or two relevant cases. For example, severaltypes of neurones in the thaiamocortical system such as the reticular34 and intralaminar35neurones do so. In the neocortex itself, examplesof intrinsic cellularoscillators include: sparselyspiny, layer-4 neurones3b,about 20% of longaxon projection neurones in layers 5 and 6 (Ref. 37), and ‘chattering cells’ (cells that fire brief trains of action potentials at 200 Hz about 40times a second), which were recently reported in vivo41. Slice studies revealed that oscillations of 40Hz in sparselyspiny neurones in the frontal cortex are generatedby persistent,voltage-dependentNa+currentsand delayed voltage-dependentrectifier currents3b.Other frontal-cortexneuronesuse fast persistentNa+currents, leak and slow non-inactivating K+currentsto generate oscillations of 4–20 Hz (Ref. 42). Various models suggest that similarmechanisms can generateoscillations of 40 Hz (Ref.43). At least some cortical neuroneswith intrinsic oscillator mechanismsproject to contralateral areas, and to the thalamus, providingroutes for longrangesynchronizationof these oscillations37.The existence of cells with intrinsic oscillations at -40 Hz does not in itself explain the synchronizationof local populations of neurones, but it is likely to pace population rhythms when the neurones are suitably coupled by chemical or electrical synapsesor both44. A REVEW Neuronal networks for induced ‘40 Hz’ rhythms Isolated interneurone / B Interneurone network 50 mV 50 ms Fig. 3. Inhibitory neuronal networks generate gamma oscillations. (A) Computersimulationof the briskexcitationof an iso/atedinhibitory interneurone by an injection of current to mimic the activation of metabotropic glutamate (mG/u) receptors.(B) The same inhibitory interneuroneas part of a network of inhibitory neuronescoupledby fast, GABA,-mediatedinhibitory postsynapticpatentiak (/f2Ps), Its responseto mCJureceptoractivationis now sculptedinto an osci//ation of 33 Hz by synchronized/P5Psgeneratedby the inhibitory netwark. based on experiments and computer simulations on the hippocampal slice (Fig. lB,C). Essentially, when networksof inhibitory neurones are tonically excited, they tend to entrain each other into rhythmic firing throughtheir mutualinhibitoryconnections. Figure3A showsa computersimulationof the effect of a depolarizing current in an isolated interneurone. The rapid dischargebecomes organizedinto a rhythmic pattern of -40Hz whenthe interneuroneis synapticallycoupled to a networkof similarlyactivatedinterneurones (Fig. 3B). In effect the rhythm is sculpted from the tonic discharge by synchronous inhibitory postsynaptic potentials (IPSPS).The experimental evidence for this model is that synchronous IPSPSat frequenciesin the gamma band occur in hippocampal and neocortical slices where all monotropicglutamate-receptor containing synapses are blocked. In these experiments, interneuronesare excited by the activation of metabotropic glutamate (mGlu) receptors. The experimental blockadeof fast EPSPSexcludesmodelsthat dependon these (Fig. 2A–C). We are not aware of hippocampal neuronesthat oscillate preferentiallyat 40 Hz,and our computer simulations show that such intrinsic oscillators are not necessary for gamma oscillations in a neuronal network. In this model, what is necessaryis tonic excitationof the interneurones(forexample,from Networks of inhibitory neurones (The main regions implicated include the hippo- metabotropic or NMDAreceptors). Fast EPSPSmight campus and the parietal neocortex; see Fig. 2D.) We be superimposedon the tonic excitation, but they are haverecentlyproposeda newmodelof gammarhythms not required,as shown by the original experiments38. TZNSVOL 19, No. 5,1996 205 REVIEW J.G.R. Jefferys et al. - Ne.renal networks for induced ‘40 Hz’ rhythms tonic excitation of the interneurones (which is the case experimen1.0 200 PA 200 PA c tally in the hippocampus and at o least part of the neocortex). The 100 ms 100 ms ~ 0.5 recurrentinhibition model appears k o to be stabilizedby mutual connecso tions within the population of 2 1 I 1 1 1 I inhibitory neurones32(and also by -0.5 20 40 60 20 40 60 mutuallyexcitatoryneurones39),but t(ms) t(ins) this is very different from the central role that such connections play in the inhibitory network model. Thisnewmodelpredictsthat both B 50 excitatory and inhibitory neurones fire in phase with the gamma a rhythm, becauseboth typesof neurone are clocked by the same popu-7 &40 lation IPSPS.This is the case in the > : hippocampus in vivo16,but appar0 ently not in the olfactory bulb and related areaswhere a phase lag was predictedas a resultof the reciprocal .-:30 activity in the recurrentexcitatory– inhibitory loops. Interestingly, the b olfactorybulb and anteriorolfactory nucleus have a peak in power at 20 oscillation frequencies of around 1,~, 75 Hz, comparedwith 40-50Hz for the hippocampus,and couldusedif0 30 35 10 15 20 25 5 0 ferent mechanisms. Lateral entoIPSC decay constant, TD(ins) rhinal cortex and prepiriformcortex have peaks in power at both these Fig. 4. The frequency of oscillation in the inhibitory neuronal network is a function of the decay constant of the frequencies. inhibitory postsynaptic current (IPSC). (A) shows autocorrelationsof voltage-clamprecordingsfrom inhibitory Inhibitory network mechanisms interneuronesin stratum oriensmade during an application of glutamate in the presenceof drugs to blockianotropic glutamate receptors.(a) Priorto additian of 20~~ pentobarbital, the netwarkoscillatedat 22.7ms [44 Hz; IPSCdecay might also function in the neoconstant (TJ was 9.1 i 0.4ms], which is faster than pyramidal cells which have a T. of 22.4 *0.8ms. (b) After cortex. Metabotropic glutamate equilibration with pentobarbital the period slowedto 44.5 ms (22 Hz; TDreached>30ms). (B) Measurementsmadeof agonistselicitedgammaoscillations both networkfrequencyand T. (opencircles)during the wash-inof 2pMpentobarbital reveala closerelationship,which when the monotropic glutamate matchesthat predictedby computersimulations(filled diamonds).More recentcomputersimulationsmatch the non- receptors were blocked pharmacolinearity found at lower frequenciesand the upper and lower limits to the synchronousnetworkoscillations,fo//owing logically, much as they did in the an increasein the connectivityof the simulatednetwork40. Figure adapted, with permission,from Ref.38. hippocampus38. This meansthat the cortical inhibitory networkcan susThe frequency of the oscillation is controlled, in part, tain gamma oscillations,but we cannot exclude other by the time constant of the fast GABAA-mediated IPSP. parallel mechanisms. As mentioned above, the neoComputersimulationshavepredicted,and experiments cortex contains neurones that are intrinsic oscillators have confirmed, that drugsthat slow the decay of the at -40 Hz. We predict that inhibitory neurones that IPSP(forexample,barbiturates),alsoslowthe frequency oscillatewilltend to stabilizethe gammarhythm of the of the oscillation(Fig.4). (In 1950, Adrianmadea similar inhibitory network. At least some intrinsic oscillator observationon the olfactorybulb when he noted that neurones are inhibitory. Golomb, Wang and Rinze14’made simulations that frequenciesof inducedoscillationsin the olfactorybulb differed between urethane-induced and barbiturate- showthat mutualinhibition can entraina network,proinduced anesthesia, at around 50 and 15 Hz respec- vided that the individualneurones, when uncoupled, tively.) In a more extensiveexploration of the control oscillate with a short period relative to the inhibitory of these synchronous, inhibitory gamma oscillations, timecourse. In the case of gamma oscillations in the we findthat they can existovera rangeof 20-70 Hz,and hippocampus,these conditions are met when TGABAis that they desynchronize outside of this range40.The in the range 8–13 ms, and there is sufficient tonic oscillations speed up, in both experiment and simu- ‘drive’to the interneurones (Figs3 and 4). This model lation, with an increasedexcitatory drive,a shortened was developedfor the reticular nucleus of the thalainhibitory postsynapticcurrent (IPSC)decay constant mus, which participatesin the generation of synchro(.~A,A),or a decreasedIPSC amplitude.The recordings nous 7–12Hz ‘spindle’ discharges and 3 Hz absenceIn both cases,the low threshold also show that at least two classesof interneuronepar- seizuredischarges4s’4G. ticipate in this activity:fast spikingcellsin strataoriens (T), voltage-dependentCa2+currentplaysa crucialrole, generating rebound excitation when the IPSPSdecay. and pyramidale40. This inhibitory networkmodel resemblesthe recur- Computer models showed that full synchronization rent inhibitory loop mechanism (above), in that both dependson a sufficientlyslowinhibitorypotentialcomare sensitive to IPSPdecay constants. It differsin that paredwiththe excitationcomponent(the low-threshold it doesnot requireintact fast EPSPS,and it doesrequire Ca2+spike)4547.In the case of spindledischargesthis is Aa b o 0 5 2 0al 4= z = 8 0 00 ~o 0 206 TINSVOL 19, NO. 5,1996 REVIEW J.G.R. Jefferys et al. - Neuronal networks for induced ‘40 Hz’ rhythms providedby fast GABAA-mediated IPSPS,and in the case coherence providedby this kind of mechanism is too of 3 Hzabsence-likeseizures,by slow GABA~-mediated weakto providefor reliablebinding, in that it appears IPSPS.In practice,in the diencephalicslice,the thalamic in simulations only when multiple trials are used32. reticular nucleus alone does not produce spindle dis- Scaling to largernetwprksor the presence of intrinsic charges. Coherent oscillations also require excitatory oscillators, or both, might circumvent this problem. synaptic input from thalamocortical-projection neur- Alternatively(or additionally)oscillations in different one545,48< Without the projection neuronesthe network partsof the cortex couldresonatethroughthe thalamotends to generate ‘clusters’, in which only part of the cortical loop3s(but see Ref. 1). We have a long way to population participatesin the oscillation, which then go before we can identify how these long-rangelinks has a frequencythat is an integer multiple (usuallyx 2 are made and broken, and understandingthe mechaor x 3) of the mean firing rate of individual neur- nisms of local networkoscillations is a key step in this ones45.This is perhapseasiestto imagine in the case of direction. We startedthis reviewdescribingthe association of two mutually inhibitory neurones with rebound excitation; as long as the IPSPSlast long enough to de- fast rhythms with higher cortical functions. Evidence inactivatethe T current,then stimulatingone will lead from MEG recordings suggests that impairments of to a persistentsequenceof alternatingdischargesin the these rhythms could be involvedin brain diseasesuch two neurones.Asyet there is no experimentalevidence as Alzheimerlsdisease23.The relationship of gamma rhythms with selective attention has prompted ideas as to whether clusteringexists in thalamic tissue. Inhibitory networksynchronization differsbetween that disruptionof its mechanism could play a role in “ lg. Understandingthe cellular and netthalamus and hippocampus.In gamma oscillations in schizophrema the hippocampus,the ‘rebound excitation’ is not due work mechanisms that generategamma rhythms proto a Ca2+current, but rather to the sustained inward vides a starting point for thinking how they could be current that is turned on by activating mGlu recep- manipulatedtherapeutically. torsqg.This causes inhibitory neurones to dischargeas soon as the IPSPhas decayed sufficiently. More work Selectedreferences is needed to find out whether this mechanism applies 1 Gray, C.M. (1994) J. CompuL Neurosci. 1, 11-38 218-226 2 Engel, A.K. et aL (1992) TrendsNeurosci. 1S, in other regions that can generate gamma rhythms, 3 Singer, W. and Gray, C.M. (1995) Annu. Rev. Neurosci. 18, such as olfactory structures and neocortex. Some of 555-586 the mechanisms outlined above could well coexist in 4 Adrian, E.D. (1950) Electroencephalogr. Clin. Neurophysiol.2, 377-388 particular parts of the brain: inhibitory network and 5 Eeckman, F.H.and Freeman,W.J. (1990) BrainRes.528,238-244 intrinsic mechanismscould combine in the neocortex; 6 Gray, C.M. et al. (1989) Nature 338, 334-337 and excitatory networks might be essential for the re7 Engel, A.K. et al. (1991) Science 252, 1177-1179 current inhibition loop to work39.Ourmuch improved 8 Engel, A.K., Konig, P. and Singer, W. (1991) Proc.Natl Acad. Sci. USA88, 91369140 understandingof networkoscillatorshas startedto make 9 Freeman,W.J. and van Dijk,B.W. (1987) BrainRes.422,267-276 their mechanisms amenable to direct experimental 10 Madler, C. et aL (1991) Br. 1.Anaestlr.66, 81-87 testing. 11 Keller,L et aL (1990) Clin.Hectroencephakgr. 21, 88-92 Functionalconsequences of rhythmicinhibition Both theorysoand experiment38r51-53 show that inhibitory neurones are very effective at determining when a pyramidalcell will fire. Ourproposalis that the inhibitory networkreceivesa steadyor slowexcitatory drivewhich makesit oscillate,providinga clock which determines when pyramidalcells can fire, if they receive suprathreshold, excitatory afferent inputs. The fact that the inhibitory network can, by itself, sustain a rhythm in the gamma frequency range without a requirement for fast EPSPS,separatesthe synchronizing control or ‘clock’ from the specific neuronal processingof information ‘the Central ProcessingUnit’. A possibleimplication is this: if firing of pyramidalneurones is constrainedto occur at particulartimes imposed by a 40Hz interneuronal network clock, then brain regions expressing40 Hz might not use rate-encoding of information, but rather might encode information through selection of which pyramidalcells fire at all. Furtherwork also is requiredto find out whether this provides the means for associating or binding attributes of specificobjects, for controlling summationand potentiation, or for a phase code for neural signaling. If the role of gamma rhythms is indeed to mediate binding, then mechanisms must exist for the selective coupling of areas involved in processingcommon entities. One ideais that reciprocalexcitatoryconnections can do this, although there seemsto be a constraint in that the conduction delaymust be less than one third of the period of the rhythm54.Others argue that the 12 Bouyer, J.J. et aZ. (1987) Neuroscience22, 863-869 13 Ptimtscheller, G., Flotzinger, D. and Neuper, C. (1994) Electroencephalogr.Clin. Neurophysiol.90, 456-460 14 Murthy, V.N. and Fetz, E.E. (1992) Proc. Natl Acad.Sci.USA89, 5670-5674 15 Sanes,J.N. and Donoghue, J.P. (1993) Proc. Natl Acad.Sci. USA 90, 4470-4474 16 Bragin, A. et al. (1995)]. Neurosci.15, 47-60 17 Stumpf, C. (1965) Electroencephalogr. CZin. NeurophysioL 18, 477-486 18 Soltesz,L and Deschi2nes,M. (1993) J NeurophysioL70, 97-116 19 Tiitinen, H. et al. (1993) Nature 364, 59-60 20 Pantev, C. et al. (1991) Proc.Natl Acad. Sci. USA88, 8996-9000 21 Kulli,J. and Koch, C. (1991) TrendsNeurosci. 14, 6-10 22 Galambos, R., Makeig, S. and Talmachoff, P.J. (1981) Proc. Natl Acad. Sci. USA78, 2643-2647 23 Ribary,U. et aL (1991) Proc.NatZAcad.Sci. USA88,11037-11041 24 LlinAs,R. and Ribary, U. (1993) Proc. NatZAcad. Sci. USA90, 2078-2081 25 von der Malsburg, C. and Schneider, W. (1986) BioZ.Cybem. 54, 29-40 26 Gray, C.M. et al. (1992) Visual Neurosci. 8, 337-347 27 Engel, A.K., Konig, P. and Schillen, T.B. (1992) Cur’r.BioL2, 33%334 28 Young, M.P., Tanaka, K. and Yamane, S. (1992) f. Neurophysiol. 67, 1464-1474 29 Tovee, M.J. and Rolls, E.T. (1992) NeuroReport3, 369-372 30 O’Keefe,J. and Recce, M.L. (1993) Hippocampus 3, 317-330 31 Hopfield,J.J. (1995) Nature 376, 33-36 32 Wilson, M.A.and Bower,J.M. (1991) NeuralComput.3,498-509 33 Wilson, M. and Bower,J.M. (1992) J. NeurophysioL67, 981-995 34 Pinault, D. and Desch@nes,M. (1992) Neuroscience51,245-258 35 Steriade, M., Curro Dossi, R. and Contreras, D. (1993) Neuroscience 56, 1-9 36 Llinfis,R.R., Grace, A.A.and Yarom, Y. (1991) Proc. Natl Acad. Sci. USA88, 897–901 37 Nuilez,A., Amzica, F. and Steriade,M. (1992) Neuroscience 51, 7-1o 38 Whittington, M.A., Traub, R.D. and Jefferys, J.G.R. (1995) Nature 373, 612-615 TINS Vol. 19, ~0, S, 1996 Acknowledgements This workwas supportedby the Wellcome Trustand IBM. We thank GyorgyBuzsaki, Bard Errrrentrout, Charles Grayand John Rinzelfor helpful discussions duringthe preparationof this manuscript. 207 REVIEW J.G.R. Jefferys et al.– Neuronal networks for induced ‘40 Hz’ rhythms 39 Ermentrout, G.B. (1995) in The Handbook of Brain Theory and NeuraZNetworks (Arbib,M.A., cd.), pp. 732–738, MIT Press 40 Traub, R.D. et aL 1. I%ysiol. (in press) 41 McCormick, D.A., Gray, C.M. and Wang, Z. (1993) Soc. Neurosci.Abstr. 19, 359 42 Gutfreund, Y., Yarom, Y. and Segev, I. (1995) ~.Physiol. 483, 621-640 43 Wang, X-J. (1993) NeuroReport5, 221-224 44 Llinfis,R.R. (1988) Science 242, 1654-1664 45 Golomb, D., Wang, X-J. and Rinzel,J. (1994) J. NeurophysioL 72, 1109-1126 46 Wang, X-J. and Rinzel,J. (1993) Neuroscience53, 899-904 47 Destexhe, A. et d. (1994) J. NeurophysioL72, 803-818 48 Von Krosigk,M., Bal, T. and McCormick, D.A. (1993) Science 261, 361-364 49 McBain, C.J., DiChiara, T.J. and Kauer,J.A. (1994) J. Neurosci. 14, 4433-4445 50 Lytton, W.W. and Sejnowski, T.J. (1991) J. Neuro@rysioL66, 1059-1078 51 Buhl, E.H., Halasy, K. and Somogyi, P. (1994) Nature 368, 823-828 52 Lacaille,J.C. et aL (1987) J. Neurosci. 7, 1979-1993 53 Knowles, W.D. and Schwartzkroin, P.A. (1981) J. Neurosci. 1, 318-322 54 Konig, P., Engel, A.K. and Singer, W. (1995) Proc. Natl Acad. Sci. USA92, 29&294 BOOK REVIEW Methods in Enzymology.Vol. 255- Small GTPases and Their Regulators,Part A: Ras Family; Vol. 256- Small GTPasesand Their Regulators, Part B: Rho Family edited by W.E. Balch,CJ Der andA. Hall,AcademicPress,1995.$99.00 (xxxi + 548 pages) ISBFJO /2 /82/56 O (Vol.255); $80.00 (xxix+ 40/ pages)ISBNO 12 182/57 9 (Vo/.256) The Rasand Rho proteinsare membersof a large superfamilyof small GTPasesthat are activateduponGTP bindingand return to an ‘off’ state when GTP is cleavedto GDP + Pi as a result of their intrinsic GTPase property. The Ras proteins encoded by viral Rasgenesdifferfrom those encodedby cellulargenesby a few amino acids.These point mutationsimpair their GTPaseactivityandthereforeinterferewith’ their normal shut-off mechanism,making them constitutivelyactive. The different interconversionstatesof the GTPases(whichhasmadethem popularly known as ‘molecularswitches’)regulate many intracellularsignalingpathways. Although both the Rasand Rho family of GTPasesbelongto a classof proteinsthat end with the sequenceCXXXX, they are functionallydistinct.Rasproteins regulate cell growth and differentiationby providinga linkbetweengrowth factor receptors and gene expression’,whereas Rho proteins regulate the assemblyof focal adhesionsand cell movement.AlthoughRho proteins belong to the GTPase superfamily,they are mainlyinvolvedin the regulation of actin cytoskeletalorganization2’3. Volumes 255 and 256 of Methods in Enzymologyare dedicatedto the biology and biochemistryof Ras-and Rho-related proteins, respectively, and are divided into four sections.Both volumesare categorized in a similar fashion. The first sectiondescribesthe methodsusedfor the cloning and purification of recombinant Ras or Rho proteins from bacteria,yeast and the baculovirus–insect-cell system.In this section, emphasisis placed on the post-translationalmodificationsof the Ras proteins,which makesthem hydrophobic 208 TINSVoL 19, No. S, 1996 mightbe. The lackof detailedintroduction in some of the chapters might make it diftlcult to follow for students or those enteringthe area of signaltransductionfor the first time, and a few articleshave not been appropriately assignedto the relevant chapters. In some articles the authors have failed to emphasize the importance of post-translational differences (such as glycosylation,phosphorylation andfarnesylation)for the functionof Ras-related proteins when expressing them in different systems (for example, E. coli,yeast and insectcells).This distinction isimportantandcouldhavebeenindicated. The importance of selecting the correct system or cell line has been ignored in the chapters that describe protein–protein interactions,which could lead to bona fide protein-protein interactions being missed due to weak expressionor lackof interactingproteins.In addition, misfolding of proteins (for example, in E. coli, yeast, Sf9 cells or mammaliancell lines)or failure of certain post-translationaleventsin the target proteins might lead to incorrect conclusions. The antisenseapproachthat hasbeenused to inhibit Ras function is convincing,but control experiments are missingor have not been describedhere4. Overall, these books are a most useful and valuable resource to everyone involved in the field of protein research. They will certainlyserveasguidancebooks and many of the techniques described might remain central to the field of signal transductionin the future. and allows them to become attached to the plasmamembrane.The importanceof this modificationiswell addressed. The second section deals mainly with the cyclic processof interconversionbetween the GTP (’on’) state and the GDP (’off’) state of Rasproteins.The technical details required to monitor the GTPbindingproperty both at an in vivo(in situ) level and at an in vitro level have been adequatelycovered. The third section describes the various approachestaken to identifythe proteinprotein interactionsbetween components of the Ras-relatedsignaltransductionpathway, for which a rangeof techniqueshas beenwidelyused,from classicaltechniques (such as metabolic labelingand immunoprecipitation)to more recentmolecularapproaches(suchasthe two-hybrid system). The final section describesthe various fascinatingapproachesthat havebeenused to monitor the biologicalactivity of Ras genes, which include oocyte and mammalian microinjection assays, fibroblast complementationassaysandthe screening of phagepeptidelibrariesfor SH3 Iigands. Both of these volumes have several strengths:the readableand concisecollecTilat A.RJzvi tion of chaptersare written by some of Llept of Cc//Biology,Neurobiologyand the leadersin the field of signaltransducAnatomy, Universityof Cincinnati Medical tion, the logical organization of inforCenter,231 8ethesdaAvenue,Cincinnati, mation makes it quick to access inforOH 45267-0521, USA. mation related to specific Ras or Rho genes,and many of the figuresare generReferences ally convincingand well done, particularly 1 Barbacid, M. (1987) Annu. Rev.Biochern. the photomicrographsin the section ‘the 56, 779-827 2 Burridge, K. et aL (1988) Annu. Rev. Cell role of Rho proteins in cellularfunction’, BioL 4, 487-525 which are of excellentquality. 3 Vincent, S., Jeanteur, P. and Fort, P. However, there are a few areaswhere (1992) Mol.CeKBioZ.12, 3138-3148 4 Gura, T. (1995) Science 270, 575-577 these books are not as strong as they Copyright 01996, Elsevier Science Ltd. All rights reserved. 0166- 2236/96/$15.00 PII: S0166-2236(96)60012-5