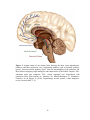
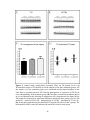
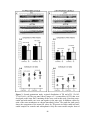
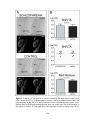
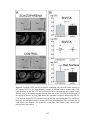
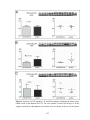
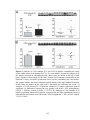
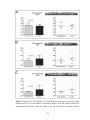
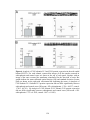
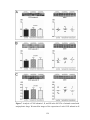
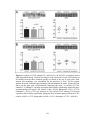
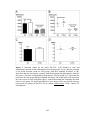
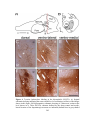
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download neuropathology of dopamine systems in schizophrenia
Artificial general intelligence wikipedia , lookup
Neural oscillation wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Human brain wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Development of the nervous system wikipedia , lookup
History of neuroimaging wikipedia , lookup
Human multitasking wikipedia , lookup
Neuropsychology wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Time perception wikipedia , lookup
Biology of depression wikipedia , lookup
Haemodynamic response wikipedia , lookup
Brain Rules wikipedia , lookup
Nervous system network models wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroplasticity wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neurogenomics wikipedia , lookup
Aging brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Basal ganglia wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Irving Gottesman wikipedia , lookup
Metastability in the brain wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Sluggish schizophrenia wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
NEUROPATHOLOGY OF DOPAMINE SYSTEMS IN SCHIZOPHRENIA: REGIONAL DOPAMINE PATHOLOGIES IN THE SUBSTANTIA NIGRA/VENTRAL TEGMENTAL AREA COMPLEX by MATTHEW W. RICE MIGUEL MELENDEZ-FERRO, COMMITTEE CO-CHAIR EMMA PEREZ-COSTAS, COMMITTEE CO-CHAIR FRANKLIN R. AMTHOR EDWIN W. COOK III LINDA OVERSTREET-WADICHE ROSALINDA C. ROBERTS DIANE C. TUCKER A DISSERTATION Submitted to the graduate school faculty of The University of Alabama at Birmingham, in partial fulfillment of the requirements for the degree of Doctor of Philosophy BIRMINGHAM, ALABAMA 2014 NEUROPATHOLOGY OF DOPAMINE SYSTEMS IN SCHIZOPHRENIA: REGIONAL DOPAMINE PATHOLOGIES IN THE SUBSTANTIA NIGRA/VENTRAL TEGMENTAL AREA COMPLEX MATTHEW W. RICE BEHAVIORAL NEUROSCIENCE GRADUATE PROGRAM ABSTRACT Dopaminergic neurotransmission anomalies are a well-established aspect of schizophrenia, a devastating mental disorder that affects approximately 1% of the world population. The substantia nigra/ventral tegmental area (SN/VTA), which extends from diencephalic to mesencephalic territories, provides the largest dopaminergic input to the brain. The SN/VTA contains subpopulations of dopaminergic neurons that project preferentially through different pathways, providing dopamine input to brain regions where dopaminergic anomalies have been reported in schizophrenia. We hypothesized that anomalies in the synthesis of tyrosine hydroxylase (TH), the rate-limiting enzyme for the production of dopamine, could contribute to the dopaminergic anomalies observed in this disorder. We first tested the expression of TH mRNA and protein in schizophrenia and matched control cases. In our schizophrenia cases we found significant deficits in TH protein expression that were driven by the rostral SN/VTA, which preferentially provides dopaminergic input to cortical areas. Next, we assessed if metabolic anomalies in the SN/VTA could contribute to this deficit by examining cytochrome c oxidase (COX) activity and subunit composition. We found a decrease in specific key subunits for the assembly and function of the enzyme. Interestingly, these deficits were not accompanied by alterations in COX activity. These findings can be explained by the fact that measurements of COX activity in postmortem tissue provided only a “snapshot” of the ii basal activity at time of death, while changes in subunit protein expression are related to long-term regulation of the enzyme, affecting the “reserve capacity” of the mitochondria to respond to higher energy demands. Finally, we performed neuronal counts of dopaminergic and total number of neurons, as well as a qualitative analysis of TH expression in the SN/VTA of schizophrenia and matched controls using immunohistochemistry. We found that, while neuronal counts were unaltered, TH protein expression was markedly reduced within rostral areas of the SN/VTA in schizophrenia. These studies support that presynaptic dopaminergic deficits are concentrated in cell populations that preferentially project through the mesolimbic and mesocortical pathways. Thus, our results indicate that presynaptic dopaminergic anomalies contribute to the hypodopaminergia of schizophrenia neuropathology, which is directly related to the negative symptomology and cognitive impairments of this disorder. Keywords: cytochrome c oxidase, immunohistochemistry, mesocortical pathway, mesolimbic pathway, metabolism, tyrosine hydroxylase iii ACKNOWLEDGEMENTS First, I wish to express my gratitude and appreciation to my mentors, Drs. Emma Perez-Costas and Miguel Melendez-Ferro for their patience, guidance, and support throughout my graduate career. I would further like to acknowledge the members of my dissertation committee, Drs. Franklin Amthor, Edwin Cook, Linda Overstreet-Wadiche, Rosalinda Roberts, and Diane Tucker for their comments and critical evaluations. Furthermore, I am grateful for the remaining members of the laboratory, Kristen Smith, Lesley McCollum, and Joy Roche for their assistance and support. Last but not least, I want to thank the members of my family and my friends who were always willing to provide encouragement. Thank you all. iv TABLE OF CONTENTS Page ABSTRACT ........................................................................................................................ ii ACKNOWLEDGEMENTS ............................................................................................... iv LIST OF TABLES ............................................................................................................ vii LIST OF FIGURES ........................................................................................................... ix INTRODUCTION ...............................................................................................................1 The Mesodiencephalic Dopaminergic System.........................................................1 Neuroanatomy ..............................................................................................2 Efferent Projections of the SN/VTA ............................................................3 Afferent Projections to the SN/VTA............................................................7 Dopamine .....................................................................................................9 Developmental Origins of the Mesodiencephalic Dopaminergic System .............14 Connecting the Dopaminergic System.......................................................18 Schizophrenia .........................................................................................................22 Schizophrenia Pathology ...........................................................................23 Dopamine Neurotransmission in Schizophrenia ........................................26 Metabolic Anomalies in Schizophrenia .....................................................30 DOPAMINE PATHOLOGY IN SCHIZOPHRENIA: ANALYSIS OF TOTAL AND PHOSPHORYLATED TYROSINE HYDROXYLASE IN THE SUBSTANTIA NIGRA ............................................................................................46 AN ACCURATE METHOD FOR THE QUANTIFICATION OF CYTOCHROME C OXIDASE IN TISSUE SECTIONS .................................................88 ASSESSMENT OF CYTOCHROME C OXIDASE DYSFUNCTION IN THE SUBSTANTIA NIGRA/VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA ....113 MAPPING DOPAMINERGIC DEFICIENCIES IN THE SUBSTANTIA NIGRA/ VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA............................................162 v DISCUSSION ..................................................................................................................211 LIST OF GENERAL REFERENCES .............................................................................225 APPENDIX: IRB APPROVAL FORM...........................................................................262 vi LIST OF TABLES Table Page DOPAMINE PATHOLOGY IN SCHIZOPHRENIA: ANALYSIS OF TOTAL AND PHOSPHORYLATED TYROSINE HYDROXYLASE IN THE SUBSTANTIA NIGRA 1 Pilot study: demographic table and clinical data in schizophrenia and controls .........81 2 Demographic table and clinical data second postmortem study ..................................82 AN ACCURATE METHOD FOR THE QUANTIFICATION OF CYTOCHROME C OXIDASE IN TISSUE SECTIONS 1 Final concentrations of pure COX in standards and detailed chart for their preparation .................................................................................................................108 ASSESSMENT OF CYTOCHROME C OXIDASE DYSFUNCTION IN THE SUBSTANTIA NIGRA/VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA 1 Demographic and clinical data of the cases used for the study .................................148 2 Effect of demographic and sample variables on COX activity and COX subunit expression ..................................................................................................................149 MAPPING DOPAMINERGIC DEFICIENCIES IN THE SUBSTANTIA NIGRA/ VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA 1 Demographic and clinical data of the cases used for the study .................................198 2 Neuronal counts obtained as the average of the neuronal counts of two different control cases ...............................................................................................................199 vii 3 Effect of demographic variables (i.e. age, gender,race) on neuronal counts .............200 4 Effect of sample quality variables (i.e. postmortem interval, brain pH) on neuronal counts ..........................................................................................................201 viii LIST OF FIGURES Figure Page INTRODUCTION 1 Organization of nuclei within the mesodiencephalic dopamine system ......................35 2 Sagittal image of the human brain showing the three main dopaminergic pathways and their projection sites ..............................................................................36 3 Schematic of the afferent and efferent connections of the SN/VTA ...........................37 4 In vivo biosynthesis of dopamine .................................................................................38 5 Diagram of a dopaminergic synapse ............................................................................39 6 Primary pathways of dopamine catabolism .................................................................40 7 Development of the mesodiencephalic dopaminergic system .....................................41 8 Progression of axons from the developing mesodiencephalic dopaminergic complex ........................................................................................................................42 9 Schematic of the electron transport chain of the mitochondria ...................................43 10 Three-dimensional representation of cytochrome c oxidase .......................................44 11 Model for cytochrome c oxidase (COX) assembly in cultured human cells ...............45 DOPAMINE PATHOLOGY IN SCHIZOPHRENIA: ANALYSIS OF TOTAL AND PHOSPHORYLATED TYROSINE HYDROXYLASE IN THE SUBSTANTIA NIGRA 1 Pilot study: TH mRNA and protein levels in schizophrenia and control cases ...........83 2 Animal study: antipsychotic treatment effect on TH protein levels ............................85 3 Second postmortem study: regional distribution of TH and pTH................................86 ix AN ACCURATE METHOD FOR THE QUANTIFICATION OF CYTOCHROME C OXIDASE IN TISSUE SECTIONS 1 Preparation of standards and Image J ........................................................................109 2 Relation between micrograms of COX, optical density and units of activity ...........110 3 Creation of a standard curve in Image J.....................................................................111 4 Measurement of COX activity in brain sections ........................................................112 ASSESSMENT OF CYTOCHROME C OXIDASE DYSFUNCTION IN THE SUBSTANTIA NIGRA/VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA 1 Analysis of COX activity in samples containing the entire rostro-caudal extent of the human SN/VTA .........................................................................................................150 2 Analysis of COX activity in samples containing only the mid-caudal regions of the human SN/VTA .........................................................................................................152 3 Analysis of COX subunits I, II, and III in samples containing the entire rostro-caudal extent of the human SN/VTA ....................................................................................153 4 Analysis of COX subunits IV-I and IV-II in samples containing the entire rostrocaudal extent of the human SN/VTA .........................................................................155 5 Analysis of COX subunits I, II, and III protein expression in the mid-caudal human SN/VTA .....................................................................................................................156 6 Analysis of COX subunits IV-I and IV-II protein expression in the mid-caudal human SN/VTA .....................................................................................................................158 7 Analysis of COX subunits I, II, and III in the SN/VTA of animals treated with antipsychotic drugs ....................................................................................................159 x 8 Analysis of COX subunits IV-I and IV-II in the SN/VTA of animals treated with antipsychotic drugs ....................................................................................................161 MAPPING DOPAMINERGIC DEFICIENCIES IN THE SUBSTANTIA NIGRA/ VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA 1 Neuronal counts for the entire SN/VTA ....................................................................202 2 Neuronal counts in the diencephalic (rostral) SN/VTA .............................................203 3 Neuronal counts in the mesencephalic (mid-caudal) SN/VTA..................................204 4 Tyrosine hydroxylase labeling in the diencephalic SN/VTA ....................................205 5 Tyrosine hydroxylase labeling of cell and processes in the diencephalic SN/VTA .....................................................................................................................207 6 Tyrosine hydroxylase labeling in the mesencephalic SN/VTA .................................209 DISCUSSION 1 Measurement of oxygen consumption rate from isolated neonatal rat ventricular myocytes ....................................................................................................................224 xi INTRODUCTION The Mesodiencephalic Dopaminergic System The substantia nigra/ventral tegmental area (SN/VTA) is a cellular complex located within the mesencephalon that contains the largest group of dopaminergic neurons in the brain (Fallon et al., 1978a,b; Gibb and Lees, 1991; Gaspar et al., 1992; Gibb, 1992; McRitchie et al., 1995; Hardman et al., 1996; see also as reviews van Domburg and ten Donkelaar, 1991; Haber and Fudge, 1997). The SN/VTA, along with the retrorubral area (RRA), are collectively known as the mesodiencephalic dopaminergic (mdDA) complex. The mdDA complex contains 400,000-600,000 neurons in the human brain (Van den Heuvel and Pasterkamp, 2008) and is composed of a diverse group of dopaminergic subpopulations with unique developmental origins (Puelles and Verney, 1998). The mdDA complex is also the origin of several major dopaminergic pathways in the brain (i.e. nigrostriatal, mesolimbic, and mesocortical pathways) and is an important complex in regard to movement, learning, cognition, goal-driven behaviors, emotion, and attention (see as reviews DeLong and Georgopoulos, 1981; Horvitz, 2000; Floresco and Magyar, 2006; Berridge, 2007; Cousins et al., 2009; Roze et al., 2010; Subramaniam and Snyder, 2011). 1 Neuroanatomy The “substantia nigra” or “black substance” derives its name from the strong black/brown pigmentation that accumulates in the cytoplasm of mesodiencephalic dopaminergic neurons. This pigmentation is due to the presence of neuromelanin, which is a byproduct of the metabolism of dopamine, giving the SN its distinct dark color (Double et al., 2000; Zecca et al., 2008). Within this mesodiencephalic dopaminergic complex, the VTA is located medial to the SN, with both nuclei primarily rostral to the RRA [Figure 1]. Based on morphology, chemical characterization, and cellular organization, the SN can be divided in two distinct subareas: the pars reticulata (SNr) and the pars compacta (SNc) (see as a review Bjorklund and Dunnett, 2007). The SNr is located in the most ventro-lateral area of the SN and is composed primarily of GABAergic neurons that project to the thalamus, superior colliculus, reticular formation, and SNc (Hopkins and Niessen, 1976; Beckstead et al., 1979; Menke et al., 2010) [Figure 1]. The SNc is located medial and dorsal to the SNr and has a denser cell population, consisting primarily of dopaminergic neurons (Yelnik et al., 1987; Hardman et al., 2002; see as a review Haber and Gdowski, 2004) [Figure 1]. The SNc can be further divided in a “dorsal tier” and a “ventral tier,” which will be described in detail in subsequent paragraphs. Projections from the SNc merge with dopaminergic axonal processes from the VTA forming a continuum of dopamine input, which also includes projections from neurons of the retrorubral area and the hypothalamus (Beckstead et al., 1979; Veazey et al., 1982; Loughlin and Fallon, 1984; Sanchez-Gonzalez et al., 2005; Ferreira et al., 2008) [Figure 2]. This continuum is responsible for the dopaminergic efferent projections to the striatum (nigrostriatal pathway), limbic regions (mesolimbic 2 pathway), frontal and prefrontal cortex (mesocortical pathway), hypothalamus (tuberoinfundibular pathway) and thalamus (Porrino and Goldman-Rakic, 1982; Veazey et al., 1982; Sadikot and Parent, 1990; Gaspar et al., 1992; Sanchez-Gonzalez et al., 2005; Menke et al., 2010) [Figure 2]. Efferent Projections of the SN/VTA The striatum, the target region for the dopaminergic projections of the nigrostriatal and mesolimbic pathways, is the principal receptive region of the basal ganglia, which are a group of functionally related brain areas that include the striatum, SN, globus pallidus, and subthalamic nucleus. In the brain of primates and other higher mammals, the striatum can be divided into dorsal and ventral regions. The functional role of the dorsal striatum has long been associated with the control of movement (see as reviews DeLong and Georgopoulos, 1981; Obeso et al., 2008; Cousins et al., 2009; Roze et al., 2010; Subramaniam and Snyder, 2011). More recently, the dorsal striatum has been associated with cognition and emotion, as well as being connected to psychiatric and psychological disorders (Middleton and Strick, 1994; Roberts et al., 1996, 2005; see also as reviews Dunlop and Nemeroff, 2007; Wanat et al., 2009; Perez-Costas et al., 2010). The dorsal striatum includes the caudate nucleus and putamen, which in higher mammals are separated by the internal capsule, while the ventral striatum includes the nucleus accumbens and the olfactory tubercle (see as a review Nieuwenhuys et al., 2007). The distinction between these two areas of the striatum, dorsal and ventral, becomes important when discussing the synaptic connections of the mdDA complex. 3 Two distinct cytoarchitectonic components can be distinguished in the dorsal striatum. The first is referred to as the “matrix” and constitutes the majority of the dorsal striatum; the second is the “striosomes” (also known as “patches”) that lie within the matrix (Graybiel and Ragsdale, 1983; Donoghue and Herkenham, 1986; Bolam et al., 1988; Ragsdale and Graybiel, 1988; Hirsch et al., 1989; Graybiel, 1990; Gerfen, 1992; Kubota and Kawaguchi, 1993; Holt et al., 1997; Roberts and Knickman, 2002; see as a review Nieuwenhuys et al., 2007). These two distinct compartments receive preferential projections, with limbic regions synapsing primarily onto the striosomes, while motor and somatosensory cortex predominantly synapse onto the matrix (Graybiel and Ragsdale, 1983; Donoghue and Herkenham, 1986; Ragsdale and Graybiel, 1988; Graybiel, 1990; Gerfen, 1992; Kubota and Kawaguchi, 1993; see as reviews Crittenden and Graybiel, 2011; Buot and Yelnik, 2012). The dorsal striatum has also been shown to receive topographically organized connections from the SN/VTA, which constitute the nigrostriatal pathway (Fallon et al., 1978a,b; Beckstead et al., 1979; Kubota et al., 1986; Gaspar et al., 1992; McRitchie et al., 1995; see also as reviews Iversen, 1984; Haber and Fudge, 1997; Joel and Weiner, 2000) [Figure 2, see also Figure 3]. Projections of the nigrostriatal pathway have a medio-lateral (i.e. the medial SNc sends projections to the medial caudate and putamen) and a rostrocaudal (i.e. the rostral SNc projects to the rostral caudate and putamen) organization (Carpenter and Peter, 1972; Szabo, 1979, 1980; see as a review Haber and Fudge, 1997). The majority of the connections of the nigrostriatal pathway arise from neurons of the ventral portion of the SNc (Fallon and Moore, 1978a,b; Fallon et al., 1978b; McRitchie et al., 1995; Haber and Fudge, 1997; Joel and Weiner, 2000; Halliday, 2004). This area of the SNc is known as the “ventral 4 tier” and is located primarily in the mid to caudal portions of the substantia nigra (Fallon and Moore, 1978a,b; Haber and Fudge, 1997; Joel and Weiner, 2000) [Figure 1C]. The “dorsal tier” of the SNc extends from diencephalic to mesencephalic territories, and is located primarily within the rostral to mid portions of the SN/VTA complex (Crosby and Woodburne, 1943; see as reviews Haber and Fudge, 1997; Marin et al., 1998; Joel and Weiner, 2000; Augustine et al., 2008) [Figure 1C]. Projections of the dorsal tier of the SNc merge medially with projections of the VTA dopaminergic neurons that are located at the same rostrocaudal level, forming a common dopaminergic output (see as reviews Haber and Gdowski, 2004; Nieuwenhuys et al., 2007). This dorsal tier is responsible for the two remaining dopaminergic projections that arise from the SN/VTA, the mesolimbic and mesocortical pathways (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004). The mesolimbic pathway [Figure 2] sends dopaminergic projections to the nucleus accumbens (ventral striatum), amygdala, hippocampus, and the bed nucleus of the stria terminalis (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Aggleton et al., 1980; Mehler, 1980; Norita and Kawamura, 1980; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004; see as a review Nieuwenhuys et al., 2007). The dopaminergic connections of the mesolimbic pathway that terminate onto the nucleus accumbens are commonly referred to as the “reward pathway.” Increased activity in the mesolimbic pathway, which would yield increased dopamine output, has been demonstrated during times of reward, adverse stimuli, and addiction (see also as reviews Schultz, 2002; Wise, 2002; Gonzales et al, 2004; Laviolette, 2007). Due to its 5 connections with the amygdala and hippocampus, the mesolimbic pathway is also associated with learning, emotion, motivation, and salience/novelty seeking behavior (Ballard et al., 2011; see also as reviews Di Chiara, 1998, Horvitz, 2000; Berridge, 2007). The mesocortical pathway [Figure 2] is the third major dopaminergic pathway originating from the SN/VTA, and is responsible for the large number of dopaminergic terminals found within the cortex (Porrino and Goldman-Rakic, 1982; Veazey et al., 1982; Sadikot and Parent, 1990; Gaspar et al., 1992; Sanchez-Gonzalez et al., 2005; Menke et al., 2010). Dopaminergic projections of the mesocortical pathway innervate several regions of the cortex, although the majority of these projections proceed to the frontal cortex, innervating the orbital, medial, and dorso-lateral prefrontal cortex (see as a review Haber and Gdowski, 2004). As opposed to the nigrostriatal pathway, axonal processes of the mesocortical pathway are diffusely arranged, in such manner that a single neuron of the SN/VTA is capable of projecting to several different areas of the cortex (Gaspar et al., 1992). Due to its strong projection to the frontal cortex, the dopaminergic mesocortical pathway is involved in a variety of aspects of higher cognition such as communication, executive function, planning, learning, and memory (Weinberger and Berman, 1988; see as a review Floresco and Magyar, 2006). In summary, it has been shown that the SN/VTA dopaminergic projections are highly heterogeneous with specialized subgroups of dopaminergic neurons providing dopamine input to different brain regions, using different pathways. The nigrostriatal pathway that projects to the dorsal striatum originates from the ventral tier of the SNc, which is located in the mid to caudal portions of the substantia nigra pars compacta (Fallon and Moore, 1978a,b; Haber and Fudge, 1997; Joel and Weiner, 2000). Rostral to 6 mid regions of the SNc contain most of what is known as the dorsal tier (Fallon and Moore, 1978a,b; Haber and Fudge, 1997; Joel and Weiner, 2000) and along with the VTA, projections from this area preferentially synapse onto the cortex, ventral striatum, and limbic regions. These projections constitute the mesocortical and mesolimbic pathways (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and GoldmanRakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004). Afferent Projections to the SN/VTA Dopaminergic neurons of the SN/VTA receive modulatory input from other nuclei of the basal ganglia as well as from other brain regions [Figure 3]. Neurons from the prefrontal cortex and subthalamic nucleus send excitatory glutamatergic and cholinergic afferents that synapse onto the SN/VTA (Lavoie and Parent, 1994; Charara et al., 1996; Frankle et al., 2006). There are also inhibitory GABAergic projections, and these projections account for at least 50-70% of the afferents that synapse onto the dopaminergic neurons of the substantia nigra (Bolam and Smith, 1990). The majority of these GABAergic projections originate from nuclei within the basal ganglia, including the dorsal striatum (Grofova and Rinvik, 1970; Somogyi et al., 1981; Bolam and Smith, 1990), the external segment of the globus pallidus (GPe) (Grofova, 1975, Smith and Bolam, 1990), and the substantia nigra pars reticulata (Grace and Bunney, 1979, 1985; Nitsch and Riesengerg, 1988; Hajos and Greenfield, 1993, 1994; Tepper et al., 1995; Celada et al., 1999; Mailly et al., 2003; see as a review Tepper and Lee, 2007). Additional GABAergic input arises from areas outside the basal ganglia such as the 7 superior colliculus, lateral habenula, and central nucleus of the amygdala (Gao et al., 1996; Coizet et al., 2003; Comoli et al., 2003). The location of GABAergic neurons in the dorsal striatum determines the preferential target of their projections in the substantia nigra. Projections from neurons located in the matrix synapse primarily onto the GABAergic neurons of the SNr, while projections from neurons located in the striosomes terminate primarily onto the dopaminergic neurons of the SNc (Gerfen, 1985; Gerfen et al., 1987). In contrast to striatal afferents, GABAergic projections from GPe neurons have been shown to synapse onto both GABAergic and dopaminergic neurons of the SN without preference (Bolam and Smith, 1990). Inhibitory input from the SNr onto the SNc arises from local collaterals of SNr GABAergic projection neurons, rather than locally projecting interneurons (Matsuda et al., 1987; Tepper et al., 1995, 2002; Mailly et al., 2003; see as a review Tepper and Lee, 2007). When stimulated, these SNr GABAergic neurons inhibit the activity of dopaminergic neurons located within the SNc (Hajos and Greenfield, 1994). Additionally, it has been shown that SNr inhibitory input has a greater influence in the firing characteristics of the dopaminergic neurons of the SNc than inhibitory input originating from either the GPe or striatum (see as review Tepper and Lee, 2007). For example, when the GABAergic projections of the GPe or striatum are stimulated, an increase in the firing of dopaminergic neurons of the SNc is observed. This increase in dopaminergic neuronal firing is due to the inhibition of the GABAergic neurons of the SNr, which leads to a disinhibition of the dopaminergic neurons of the SNc. This connection has a greater impact than the direct inhibition of the SNc dopaminergic neurons by the striatal and pallidal afferents (Grace and Bunney, 1985; Lee et al., 2004). 8 In brief, the excitatory and inhibitory afferents described above regulate the firing of dopaminergic neurons located in the SN/VTA, modulating the release of dopamine. Dopamine Dopamine is an organic chemical messenger and a member of the catecholamine and phenethylamine families. In vivo, the synthesis of dopamine is carried out through a series of enzymatic reactions [Figure 4], beginning with the non-essential amino acid tyrosine, which is converted to L-3,4-dihydroxyphenylalanine (L-DOPA) by the enzyme tyrosine hydroxylase (Pickel et al., 1975; Lindgren et al., 2001; Dunkley et al., 2004; see as a review Miyake et al., 2011). L-DOPA is then converted to dopamine by the enzyme DOPA decarboxylase, also known as aromatic L-amino acid decarboxylase (see as reviews Elsworth and Roth, 1997; Miyake et al., 2011). Dopamine can be further metabolized into the neurotransmitters noradrenaline (norepinephrine), by the enzyme dopamine β-hydroxylase (DBH), and adrenaline (epinephrine) by the enzyme phenylethanolamine N-methyltransferase (Hartman et al., 1972; Vincent, 1988; Bailhache and Balthazart, 1993). Importantly, DBH is not expressed in the mdDA complex, ensuring that neurons are dopaminergic rather than noradrenergic or adrenergic (Hartman et al., 1972; Vincent, 1988; Bailhache and Balthazart, 1993). Tyrosine hydroxylase (TH) is the rate-limiting enzyme for the production of dopamine, and measurements of this enzyme are often used in human post-mortem and animal studies in order to determine the capability of neurons to produce dopamine (Pickel et al., 1975; Lindgren et al., 2001; Dunkley et al., 2004). Among mammalian species the number of TH isoforms synthesized differ, with a single isoform identified in 9 rodents and non-primates, two in non-human primates, and four TH isoforms in humans. All these different isoforms are the result of alternative mRNA splicing from a single gene (Le Bourdelles et al., 1991; Haycock, 2002; Gordon et al., 2009). This TH gene encodes for tyrosine hydroxylase and it has been shown that the regulation of the production of this enzyme is unique within the mdDA complex, such that, TH mRNA expression levels do not necessarily correspond to TH protein levels (Wong and Tank, 2007; Chen et al., 2008; Tank et al., 2008; Perez-Costas et al., 2012). This is due to the posttranscriptional regulation mechanism that stabilizes TH mRNA in neurons of the mdDA complex (Tank et al., 2008; Lenartowski and Goc, 2011). Tank et al. (2008) postulated that Poly(C)-binding proteins bind to TH mRNA, which results in the stabilization of the mRNA molecule and an increased efficacy of its translation into protein. It is also important to note that TH becomes active when it is phosphorylated, with Ser40 being the most physiologically active phosphorylation site in the SN/VTA (Lindgren et al., 2001; Dunkley et al., 2004; Nakashima et al., 2009). The advantages of using TH as a marker for dopaminergic neurons instead of measuring dopamine directly include: 1) Dopamine has been demonstrated to degrade at a faster rate than TH, thus limiting the usefulness of directly studying dopamine in post-mortem cases (Blank et al., 1979), 2) TH is located throughout the cytoplasm of the neurons, as opposed to dopamine which is sequestered into vesicles, thus the detection of TH allows for the visualization of the entire cell (Pickel et al., 1975; 1980), and 3) the analysis of TH levels allows for the study of the ability of the cell to produce dopamine, rather than its ability to store dopamine. 10 Once synthesized, dopamine is transported by vesicular monoamine transporter 2 (VMAT2) from the cytosol into specialized storage vesicles located primarily at axon terminals (Eiden et al., 2004; Sun et al., 2012; Hu et al., 2013) [Figure 5]. In addition, dopamine can also be released from the dendrites where it can be stored both in vesicles and within the endoplasmic reticulum (Geffen et al., 1976; Kerwin and Pycock, 1978; Araneda and Bustos, 1989; Cragg and Greenfield, 1997; Patel et al., 2009). Upon the arrival of an action potential, dopamine is released into the synaptic cleft where it may bind to one of two families of dopamine receptors, D1 or D2 [Figure 5]. The D1-like family of dopamine receptors includes the D1 and D5 dopamine receptor subtypes, and is coupled to the G protein Gsα (Nestler et al., 1990; Tepper et al., 1997; Chien et al., 2010). When activated, D1-like receptors activate the adenylate cyclase enzyme, resulting in an increase of cyclic adenosine monophosphate (cAMP) production (Neves et al., 2002; see as a review Jose et al., 2003). The D2-like family of dopamine receptors include the D2(short), D2(long), D3, and D4 dopamine receptor subtypes, and are coupled to the G protein Gi/oα. Activation of Gi/oα results in the inhibition of adenylate cyclase and a reduction in the amount of cAMP produced (Nestler et al., 1990; Tepper et al., 1997; Neves et al., 2002; Chien et al., 2010). In addition to the postsynaptic dopamine receptors, there are also presynaptic receptors (autoreceptors), that have been identified as D2(short) dopamine receptors, and extrasynaptic receptors (Meador-Woodruff et al., 1991; Cooper and Stanford, 2001; Blasi et al., 2009; see as reviews De Mei et al., 2009; Fuxe et al., 2012). Dopamine autoreceptors are located in the vicinity of synapses throughout the structure of the neuron, including the soma, dendrites, and axon terminals (Meador-Woodruff et al., 11 1991; Cragg and Greenfield, 1997; see as reviews Elsworth and Roth, 1997; Adell and Artigas, 2004) [Figure 5]. When activated, autoreceptors located in the soma and dendrites function to slow the rate of firing of the dopaminergic neurons, whereas autoreceptors located in terminals inhibit the release and/or synthesis of dopamine (see as reviews Elsworth and Roth, 1997; Adell and Artigas, 2004). Supporting the existence of functional differences among subpopulations of the mdDA complex, it has been shown that neurons located within ventral portions of the SNc express high levels of dopamine transporter (DAT) and D2 receptor mRNA, while neurons contributing to the mesocortical and mesolimbic pathways express low levels of DAT and D2 receptor mRNA (Hurd et al., 1994). Another mechanism regulating the firing of dopaminergic neurons is through reuptake of dopamine by either the high-affinity DAT or low-affinity norepinephrine transporter (NET) (Moron et al., 2002; see as a review Torres et al., 2003). These transporters serve to recycle dopamine that has been released into the synaptic cleft by pumping extracellular dopamine back into the presynaptic neuron [Figure 5]. The activities of dopamine and norepinephrine transporters increase the intracellular concentration of dopamine within the presynaptic neuron, and are critical in terminating neurotransmission and maintaining transmitter homeostasis (see as reviews Elsworth and Roth, 1997; Torres et al., 2003). In some dopaminergic synapses the rate of dopamine release supersedes the actions of DAT, resulting in the formation of a “cloud” of dopamine. This dopaminergic “cloud” can activate neurons outside the synaptic cleft via extrasynaptic receptors in a process called “volume transmission” (Rice and Cragg, 2008; see as a review Fuxe et al., 2012). 12 Catabolism of dopamine [Figures 5, 6] is mediated by a set of enzymes consisting of catechol-O-methyltransferase (COMT), monoamine oxidase (MAO), aldose reductase (ALD), aldehyde dehydrogenase (ALD-D), and alcohol dehydrogenase (ADH) (see as reviews Kopin, 1985; Elsworth and Roth, 1997; Eisenhofer et al., 2004). These enzymes act in concert to form a number of intermediate metabolites [Figure 6] including 3,4dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylacetaldehyde (DOPAL), 3,4dihydroxyphenylethanol (DOPET), 3-Methoxytyramine (3-MT), 3-methoxy-4- hydroxyphenylethanol (MOPET; also known as homovanillyl alcohol) and homovanillic acid (HVA) (see as reviews Kopin, 1985; Elsworth and Roth, 1997; Eisenhofer et al., 2004). These metabolic pathways can also result in the formation of neuromelanin within the neuronal somas of the mdDA neurons, the accumulation of which results in the characteristic dark pigmentation of the SN/VTA (Double et al., 2000; Zecca et al., 2008). In most areas of the brain, dopamine is metabolized by MAO into DOPAC after the uptake of dopamine by dopamine transporters from the synaptic cleft. However, in the prefrontal cortex DATs are only present in restricted areas, while low-affinity NETs are pervasive throughout the entire region (Lewis et al., 2001; Moron et al., 2002). Additionally, COMT mRNA and protein are more strongly expressed in cortical areas than in subcortical areas such as the striatum (Matsumoto et al., 2003; see as a review Mannisto et al., 1999). This results in an increased catabolism of dopamine by COMT into 3-MT, which is then further catabolized by MAO into HVA. Dopamine that is not fully metabolized can be repackaged into vesicles for future release (Michael et al., 1987; Ungless, 2004). 13 Developmental Origins of the Mesodiencephalic Dopaminergic System According to the prosomeric model of brain development, the early vertebrate brain is a segmented structure that consists of a continuous sequence of transverse divisions within the neural tube (see as reviews Puelles, 2001; 2009; Puelles and Rubenstein, 2003). These divisions are known as “neuromeres” [Figure 7] and from rostral to caudal include a large forebrain structure (i.e. secondary prosencephalon) comprising the telencephalon, hypothalamus, and developing eyes; the diencephalic neuromeres (i.e. prosomeres 1-3); the mesomere (i.e. the developing mesencephalon); the rhombencephalon consisting of eleven rhombomeres; and spinal myelomeres (Bergquist, 1932; Bergquist and Kallen, 1953; Lauter et al., 2013; see as reviews Rubenstein et al., 1994; Puelles and Rubenstein, 2003; Puelles, 2009; Martinez-Ferre and Martinez, 2012). MdDA progenitor cells originate specifically within the mesomere and the diencephalic prosomeres 1-3 (Puelles and Verney, 1998; see as reviews Smits et al, 2006; Smidt and Burbach, 2007). Along with the anterior-posterior organization of the neuromeres, there is also a dorsoventral organization of the neural tube consisting of the roof, alar, basal, and floor plates (see as a review Smits et al., 2006) [Figure 7]. Molecular signaling from the roof plate and floor plate divide the developing brain into dorsal and ventral components, and these same molecular signals influence the development and differentiation of the mdDA neurons (Lin and Rosenthal, 2003; Placzek and Briscoe, 2005; see as reviews Smits et al., 2006; Van den Heuvel and Pasterkamp, 2008). During development, the neuronal progenitors of the mesodiencephalic dopaminergic complex are situated in areas of the neural tube that develop into the mesencephalon and caudal diencephalon (see as a review Jacobs et al., 2006; Smits et al., 14 2006; Smidt and Burbach, 2007; Van den Heuvel and Pasterkamp, 2008; Hegarty et al., 2013) [Figure 7]. The diverse neurodevelopmental origins of the retrorubral area, ventral tegmental area, and substantia nigra result in a heterogeneous collection of THexpressing neuronal subpopulations that differ in regard to their molecular profiles, electrophysiological properties, genetic expression, neurochemical characterization, and susceptibility to disease (Gibb and Lees, 1991; Chung et al., 2005a,b; Greene et al., 2005; Van den Heuvel and Pasterkamp, 2008). A number of molecular signals are involved in the differentiation and final positioning of mdDA neurons. Sonic hedgehog (Shh) is a glycoprotein that is secreted by the floor plate of the neural tube and is essential for the development of the ventral portion of the mesencephalon and diencephalon, as well as for the induction and differentiation of dopaminergic neurons (Hynes et al., 1995a,b; Hynes and Rosenthal, 1999; Placzek and Briscoe, 2005). In fact, ectopic expression of dopaminergic neurons can be achieved by overexpression of Gli-1, a downstream effector of Shh (Hynes et al., 1997). Fibroblast growth factor 8 (Fgf8) is secreted from the isthmus (the region between the rhombencephalon and mesencephalon), and is responsible for confining the differentiation of mdDA neurons to ventral portions of the neural tube (Hynes and Rosenthal, 1999; Lin and Rosenthal, 2003). In addition, many other transcription factors are required for the correct development and maturation of mdDA neurons. Otx2 is required for neuronal specification of mdDA progenitors (Puelles et al., 2003, 2004; Vernay et al., 2005), Msx1 and Ngn2 are needed for neuronal differentiation (Andersson et al, 2006a,b; Kele et al., 2006), Lmx1b participates in the maintenance of mature mdDA neurons (Smidt et al., 2000), En1/2 are required for both the generation and survival of 15 mature neurons (Simon et al., 2001; Alberi et al., 2004), Nurr1 is essential for the transcriptional activation of TH (Zetterstrom et al., 1997; Saucedo-Cardenas et al., 1998; Wallen et al., 1999, 2001; Smits et al., 2003), and Pitx3 is involved in the development and survival of mdDA neurons (Hwang et al., 2003; Nunes et al., 2003; van den Munckhof et al., 2003; Smidt et al., 2004; Maxwell et al., 2005). Pitx3 is a bicoid-related, homeodomain-containing transcription factor that is expressed by all TH-positive neurons of the mdDA complex (see as a review Hegarty et al., 2013). However, the absence of Pitx3 expression only affects the development of the dopaminergic neurons that contribute to the nigrostriatal pathway (i.e. ventral tier), while those dopaminergic neurons contributing to the mesocortical and mesolimbic pathways remain unaffected (Hwang et al, 2003; Nunes et al., 2003; van den Munckhof et al., 2003; Smidt et al., 2004a,b). It is believed that impaired Pitx-3-dependent functions within these neurons contribute directly to this susceptibility, although the exact mechanism is not fully understood (Luk et al., 2013). In Parkinson’s disease the neurons of the nigrostriatal pathway are most heavily impacted (see as a review Feve, 2012), and it has been demonstrated that those neurons that express Pitx3 are more susceptible to this neuronal death pathology (Fuchs et al., 2009; Bergman et al., 2010; Luk et al., 2013). The difference in the susceptibility of dopaminergic neurons to the absence of Pitx3 further supports the existence of different dopaminergic subpopulations within the mdDA complex. The exact origin site of the different nuclei of the mdDA complex within the developing brain has not been fully elucidated. It has been demonstrated that different subgroups of dopaminergic neurons of the SN and VTA originate from several locations 16 within the neural tube, from the most caudal limit of the developing mesencephalon (i.e. the isthmus) to the most rostral part of prosomere 3 within the developing diencephalon (Puelles and Verney, 1998; see as reviews Smits et al, 2006; Smidt and Burbach, 2007). The retrorubral area is believed to originate entirely from neuronal progenitors that are present only in the developing mesencephalon, or mesomere (Puelles and Verney, 1998). Smits et al. (2006) postulated that in the mouse there might be as many as eight different subsets or groups of neurons that constitute the developing mdDA complex. The identification of these eight subgroups is based on their location within the anteriorposterior axis (mesomere and prosomeres 1-3) and on their place of origin within the dorsoventral territories of the neural tube (i.e. floor plate or basal plate). For the developing human brain, Puelles and Verney (1998) have shown that there is a plurisegmental origin for the SN/VTA mdDA neurons, which only later fuse into one complex. Further evidence of the existence of distinct mdDA subpopulations comes from electrophysiological, genetic, and neurochemical studies. For example, unique firing characteristics have been reported between the dopaminergic populations of the substantia nigra and ventral tegmental area in rodents (Grimm et al., 2004; Korotkova et al., 2004; Thuret et al., 2004; Chung et al., 2005a,b; Greene et al., 2005). Also, it has been demonstrated that there are subpopulations of the mdDA complex that present different genetic profiles, which may help explain the different susceptibility of dopaminergic neurons in Parkinson’s disease (Chung et al., 2005a; Greene et al., 2005). Van den Heuvel and Pasterkamp (2008) state that the axon guidance receptor “deleted in colorectal cancer” (DCC) is strongly expressed in dopaminergic neurons of the ventral portion of the SNc, while it is almost absent in the dorsal SNc and VTA. In contrast, 17 dopaminergic neurons of the dorsal tier of the SNc and VTA express the calcium binding protein calbindin, while dopaminergic neurons of the ventral tier of the SNc do not (Gibb and Lees, 1991; Haber et al., 1995; Nemoto et al., 1999; see as a review Smith and Kieval, 2000; Cebrian and Prensa, 2010; Luk et al., 2013). In summary, the mdDA progenitors of the developing brain arise from areas stretching from the most caudal part of the mesomere to the most rostral diencephalic prosomere. Despite the fact that these dopaminergic subpopulations later coalesce to form the mdDA complex and share a common TH+ phenotype, these subpopulations differ in many aspects. In short, it is important to realize that, regardless of their spatial proximity and shared dopaminergic nature, the different developmental origins and molecular “make-up” of these subpopulations have important implications for the functioning of the mdDA system as a whole. Connecting the Dopaminergic System The dopaminergic neurons of the mdDA complex start to send out their projections shortly after acquiring their dopaminergic phenotype. These projections initially proceed dorsally from their mesodiencephalic origin, but are soon redirected towards a more rostral direction by chemical signals of the midbrain-hindbrain boundary (Nakamura et al., 2000; Gates et al., 2004; Yamauchi et al., 2009) [Figure 8]. Rostrallydirected projections proceed through the ventral portion of the developing brain towards the diencephalon and telencephalon (Nakamura et al., 2000; Gates et al., 2004) and form two large bundles of axons called the medial forebrain bundles (MFBs). The MFBs proceed to striatal and cortical areas, forming the basis of the major dopaminergic 18 pathways of the mdDA complex (Specht et al., 1981a; Verney, 1999; Zhao et al., 2004) [Figure 8]. Synaptic connections of the nigrostriatal pathway form earlier than connections of the remaining two pathways, with the most mature neurons of the mdDA complex synapsing onto the most mature neurons of the striatum (Specht et al., 1981b; Voorn et al., 1988). Connections between the mdDA complex and cortex are established later, partially due to molecular signals originating from unidentified regions of the developing cortex and striatum that result in a “waiting period” for the development of dopaminergic cortical connections (Hemmendinger et al., 1981, Gates et al., 2004; see as a review Van den Heuvel and Pasterkamp, 2008). These molecular signals eventually dissipate or are overcome by more powerful attractant signals, with mdDA fibers only synapsing onto differentiated layers of the developing cortex (Verney et al., 1982; Berger et al., 1985; see as a review Van den Heuvel and Pasterkamp, 2008). After these initial connections are established, axonal pruning becomes a critical step in the final maturation of the mdDA pathways. Pruning within the nigrostriatal, mesolimbic, and mesocortical pathways is necessary to remove excess synaptic contacts that would otherwise disrupt the correct functioning of the mdDA system (Hu et al., 2004; Lou and O’Leary, 2005). It is important to note that all the nuclei of the mdDA complex (i.e. RFF, SN, and VTA) provide projections that will innervate the developing striatum and cortex (Lindvall and Bjorklund, 1974; Fallon and Moore, 1978b; Roffler-Tarlov and Graybiel, 1984; see as a review Van den Heuvel and Pasterkamp, 2008). Studies on the development of these pathways have provided further evidence supporting the existence of distinct subpopulations within each of the nuclei of the mdDA 19 complex. For example, it has been demonstrated that two distinct regions of the VTA give rise to projections that terminate onto the PFC and nucleus accumbens in rodents (Fallon, 1981; Swanson, 1982; Margolis et al., 2006). Interestingly, in paired box 6 (PAX6) knockout mice the majority of the mdDA projections that form the MFBs do not fasciculate properly, although there is a small subset of axons whose path to the forebrain is not disrupted (Vitalis et al., 2000). In addition, Eells et al. (2002) demonstrated that nuclear receptor related-1 (Nurr1) heterozygous mice have significantly reduced levels of dopamine and of the dopamine metabolite DOPAC in the projection areas of the mesolimbic and mesocortical pathways (e.g. nucleus accumbens and prefrontal cortex, respectively), whereas no significant change in dopamine or DOPAC was observed within the striatum. Finally, in the developing mdDA complex of the rat brain, areas corresponding to both the SN and VTA express mRNA for the axon guidance factor robo1 receptor, while robo2 receptor mRNA is expressed only within the SN (Marillat et al., 2002; Lin et al., 2005). All these findings support that distinct neuronal subpopulations exist within the mdDA complex, which respond differently to pathwayfinding cues during development. Molecules transiently expressed during development act as chemoattractants and chemorepellents and contribute to the formation of the dopaminergic pathways, ensuring proper axon guidance (see as a review Prasad and Pasterkamp, 2009). The generally rostral orientation of mdDA projections is determined by chemorepellent molecules expressed along the midbrain-hindbrain boundary of the developing brain, in conjunction with chemoattractant molecules expressed in the forebrain (Nakamura et al., 2000; Gates et al., 2004; Yamauchi et al., 2009) [Figure 8]. However, the chemical signals that guide 20 the initial dorsal movement of the dopaminergic fibers have yet to be discovered (see as a review Van den Heuvel and Pasterkamp, 2008). Chemoattractant and chemorepellent factors act in concert to guide the developing dopaminergic axons into the MFBs and subsequently into the striatum and cortex (see as a review Van den Heuvel and Pasterkamp, 2008) [Figure 8]. Within the developing striatum and cortex, chemorepellent molecules act to halt the rostral projections of a subset of the axons, resulting in the differentiation of the nigrostriatal and mesolimbic/mesocortical pathways (Gates et al., 2004; see as reviews Van den Heuvel, 2008; Prestoz et al., 2012). One of these guidance molecules, Ephrin, which serves as the ligand to the Ephrin receptor (Maisonpierre et al., 1993; Yue et al. 1999; Willson et al., 2006), has been shown to play a direct role in the determination of the synaptic connections of the axons proceeding from the mdDA complex. Ephrin-A5 is a member of the ephrin ligand family, and is found primarily within the ventral midbrain. Studies have demonstrated that the degree to which developing axons of the mdDA complex respond to ephrin-A5 correlates with their final target. That is, low-responding ephrin-A5 mdDA axons connect primarily to the ventral striatum, while high-responding ephrin-A5 mdDA axons are repelled towards more dorsal areas of the striatum. MdDA axons that do not respond in any way to ephrin-A5 proceed to cortical regions (Deschamps et al., 2009; see as a review Prestoz et al., 2012). Other specific chemoattractant and chemorepellent molecules as well as their receptors have been identified and include netrins/DCC (Livesey and Hunt, 1997; Vitalis et al., 2000; Lin et al., 2005), semaphorins/plexins/neuropilins (Kawano et al., 2003; Hernandez-Montiel et al., 2008), and Robo/Slits (Dugan et al., 2011; see as a review Hegarty et al., 2013). Many of these chemical signals can act as both attractants and 21 repellents in the developing brain, depending on the neurochemical characteristics of the growth cone (Kennedy et al., 1994; Colamarino and Tessier-Lavigne, 1995; VarelaEchavarria et al., 1997; Alcantara et al., 2000; Zhou et al., 2008; see as a review Masuda and Shiga, 2005). In fact, midbrain neurons from rodent models of Parkinson’s disease and from individuals who suffer from Parkinson’s disease react differently from their respective controls when exposed to axon guidance cues, demonstrating again the heterogeneity of the mesodiencephalic dopaminergic system (Grunblatt et al., 2001, 2004; Miller et al., 2004; Hauser et al., 2005). Schizophrenia Estimates indicate that as much as 1% of the world population may be afflicted with schizophrenia, with this mental illness producing a “marked social or occupational dysfunction” (DSM-IV-TR, 2000). In the United States there are approximately 3.2 million individuals that suffer from this disorder, incurring an estimated cost of $62.7 billion in 2002 (Wu et al., 2005). In the clinic, patients are diagnosed with schizophrenia based on the presence of symptoms that are commonly grouped into three distinct categories: positive symptoms, negative symptoms, and cognitive impairments. Positive symptoms “appear to reflect an excess or distortion of normal functions” (DSM-IV-TR, 2000). Manifestations of these symptoms include hallucinations, disorganized speech, and delusions, which normally appear in early adulthood. Negative symptoms “appear to reflect a diminution or loss of normal function” (DSM-IV-TR, 2000; Stauss et al., 2013). Avolition, affective flattening, and anhedonia are all examples of the negative symptomatology of schizophrenia. Cognitive impairments found in schizophrenia include 22 deficits in communication, executive function, and memory (Pietrzak et al., 2009). None of these symptoms are exclusive of schizophrenia, and different aspects of the disease (e.g. psychotic behavior, anhedonia, cognitive deficits, etc.) can be found in other mental disorders such as paranoid personality disorder, bipolar disorder, major depression, and delusional disorders (DSM-IV-TR, 2000). In addition, the clinical diagnosis of schizophrenia allows for the presence of a wide array of symptoms (DSM-IV-TR, 2000). Schizophrenia Pathology Numerous studies have demonstrated that genetic inheritance is a major underlying factor in the development of schizophrenia (Kallmann, 1946; Sullivan et al., 2003; see as reviews Tsuang, 2000; Shih et al., 2004; Sullivan, 2005). Genetic inheritance of schizophrenia does not depend on a single gene, rather it is due to a medley of genes acting in concert (see as a review Harrison and Weinberger, 2005), supporting the concept of schizophrenia as a heterogenetic disorder. A non-exhaustive list of genes shown to be altered in schizophrenia include Dystrobrevin-binding protein 1, Neuregulin 1, D-amino acid oxidase activator, disrupted in schizophrenia 1, Proline dehydrogenase, COMT, and D2 and D4 receptor genes (Fan et al., 2003; Kirov et al., 2004; Corvin et al., 2007; Liu et al., 2007; Pletnikov et al., 2008). Additionally, these genetic anomalies differ within the schizophrenia population, such that not all of these genes are affected in all individuals diagnosed with this disorder (Gardner et al., 2006; Pletnikov et al., 2008; see as a review Harrison and Weinberger, 2005). However, studies have consistently found that genes involved in dopamine production, transmission, and metabolism are altered in schizophrenia (Wiesel et al., 1987; Cleghorn et al., 1989; Wiesel, 1992; 23 Sommer et al., 1993; Arinami et al., 1997; Akil et al., 2003; Prabakaran et al., 2004; see as a review Harrison and Weinberger, 2005). One of the most replicated genetic anomalies found in schizophrenia is that of COMT, which is involved in monoamine catabolism [Figures 5, 6]. In addition, anomalies in the expression of the dopamine D2 receptor and dopamine D4 receptor genes have also been demonstrated, highlighting the importance of abnormal dopamine function in the pathology of this illness (Sommer et al., 1993; Arinami et al., 1997; Akil et al., 2003; see as a review Harrison and Weinberger, 2005). Another important contributor to the elevated risk of developing schizophrenia is environmental factors. These include season and geographic location of birth, substance abuse by the mother, infection and illness, malnutrition during pregnancy, and obstetric complications (Mortensen et al., 1999; Willinger, 2001; Henquet et al., 2005; Schwarcz and Hunter, 2007; see as reviews Leask, 2004; Clarke et al., 2006; Jenkins, 2013). More important than a specific environmental factor is the fact that all these environmental conditions result in some degree of stress being placed on the individual. Tsuang (2000) remarked on the possibility that it might be that these stressful events affect genome expression, contributing to the development of schizophrenia. The examination of the gross neuroanatomy of brains from individuals that suffered from schizophrenia yields few differences when compared to non-psychiatric controls. The only consistently reported changes are mild enlargements of the lateral and third ventricles, and decreased cerebral volume (Suddath et al., 1990; Van Horn and McManus, 1992; Goldman et al., 2008, 2009; Rimol et al., 2010; Schultz et al., 2010; see also as reviews Harrison, 1999; Shenton et al., 2001). This increase in ventricular size 24 and reduction in cerebral volume is believed to stem from a reduction in cortical neuronal size and/or neuropil without neuronal death (Garey et al., 1998; Pierri et al., 2001). These gross neuroanatomical changes have also been shown to be present in drug-naïve patients, indicating that these anomalies are intrinsic to the disorder rather than the result of antipsychotic medications (Bertolino et al., 1998; Cahn et al., 2002; Chua et al. 2007). Differences between the brains of individuals with schizophrenia and brains from non-psychiatric individuals are more apparent at the microscopic level. There are subtle changes in many areas of the brain including the basal ganglia, prefrontal cortex, thalamus, and limbic regions (Woo et al., 2008; Sodhi et al., 2011; Kimoto et al., 2014; see as reviews Harrison, 1999; Powers, 1999; DeLisi et al., 2006; Perez-Costas, 2010; Haukvik et al., 2013). In addition, these anomalies affect several neurotransmitter systems including the dopaminergic, serotonergic, cholinergic, glutamatergic, and GABAergic systems (Gonzalez-Maeso et al., 2008; Lewis et al., 2008; Roberts et al., 2009; Perez-Costas et al., 2012; see also as reviews Carlsson et al., 1999; Tandon, 1999; Kristiansen et al., 2006, 2007; Raedler et al., 2007; Stone et al., 2007; Howes and Kapur, 2009; Perez-Costas et al., 2010; Noetzel et al., 2012; de Bartolomeis et al., 2013; Eggers, 2013). Examples of this include hyperdopaminergia in subcortical regions and hypodopaminergia in cortical regions (Akil et al., 1999; see also as reviews Davis et al., 1991; Howes and Kapur, 2009; Perez-Costas et al., 2010; Howes et al., 2012; Kuepper et al., 2012; Laruelle, 2013), alterations in cholinergic receptor density and binding in the striatum and frontal cortex respectively (Tandon, 1999), changes in GABA receptors, glutamic acid decarboxylase (GAD) mRNA and protein levels, and GABA transporter-1 mRNA levels throughout the brain (Simpson et al., 1989; Impagnatiello et al., 1998; 25 Guidotti et al., 2000; Volk et al., 2001; Fatemi et al., 2005; Bullock et al., 2008; Thompson et al., 2009; see also as reviews Guidotti et al., 2000; Costa et al., 2004; Lisman et al., 2008; Costa et al., 2009; Daskalakis and George, 2009), as well as NMDA receptor hypofunction (see as reviews Jentsch and Roth, 1999; Olney et al., 1999; Kristiansen et al., 2006, 2007; Stone et al., 2007; Lisman et al., 2008; Gordon, 2010; McCullumsmith et al., 2012). Dopamine Neurotransmission in Schizophrenia The involvement of dopamine pathology in schizophrenia was first elucidated by the observation that phenothiazines (a group of drugs that decrease dopaminergic neurotransmission) could be used to treat psychotic symptoms, while amphetamines (drugs that increase dopaminergic neurotransmission) could produce a state resembling psychosis (Woodward et al., 2011; see as a review Ohlow and Moosmann, 2011). Experimentally, the seminal work of Carlsson and Lindqvist (1963) revealed that antipsychotic medication, specifically chlorpromazine and haloperidol, increased the accumulation of catecholamine (e.g. dopamine) metabolites in in the brains of mice. It was speculated that these increased metabolites were the result of a compensatory increase in the activation of monoaminergic neurons due to a proposed blocking of monoamine receptors by chlorpromazine and haloperidol. In addition, earlier work had demonstrated that the antipsychotic reserpine blocked the reuptake of dopamine into presynaptic neurons (Carlsson et al., 1957). Further studies on antipsychotic medications revealed that their effectiveness could be directly linked to their ability to bind to dopamine receptors (Seeman and Lee, 1975; Seeman et al., 1976; Creese et al., 1976; see 26 also as a review Frankle and Laruelle, 2002). Taking this information into account, version I of the dopamine hypothesis of schizophrenia was formulated in the 1970s. This early version stated that schizophrenia was caused by an excess of dopaminergic transmission throughout the brain (see as a review Howes and Kapur, 2009). However, studies in subsequent years yielded information on the contrary. For instance, positron emission tomography and functional magnetic resonance imaging studies revealed a decrease in dopaminergic activity in the frontal cortex of individuals with schizophrenia (Hazlett et al., 2000; Riehemann et al., 2001). Also, studies examining the dopamine metabolite HVA found reduced levels of this metabolite in cerebrospinal fluid, which is indicative of decreased dopamine levels in cortical regions (Davidson and Davis, 1988; see as a review Davis et al., 1991). Taking this new information into account, Davis et al. (1991) postulated the version II of the dopamine hypothesis of schizophrenia. Version II of the dopamine hypothesis of schizophrenia states that hyperdopaminergia is associated with subcortical structures, in particular the striatum, while hypodopaminergia is associated with cortical areas such as the prefrontal cortex (see as reviews Davis et al., 1991; Howes and Kapur, 2009). Furthermore, version II states that the subcortical hyperdopaminergia is the root of psychotic symptoms, while negative symptoms and cognitive deficits could be linked to the cortical hypodopaminergia (see as reviews Davis et al., 1991; Howes and Kapur, 2009). This hypothesis is supported by studies showing an increase in dopamine neurotransmission in the striatum (Kegeles et al., 2010; see also as reviews Toda and Abi-Dargham, 2007; Meyer-Lindenberg, 2010), and a decrease in dopaminergic axonal length and overall activation in the prefrontal cortex of individuals with schizophrenia (Akil et al., 1999, see 27 also as reviews Toda and Abi-Dargham, 2007; Meyer-Lindenberg, 2010). Also supporting this hypothesis is the fact that all antipsychotic medications act to block dopamine receptors, and that these medications normally relieve only psychotic symptoms, which are associated with the hyperdopaminergia observed in subcortical areas. An increase in subcortical dopamine release was also observed when healthy individuals were subjected to an amphetamine challenge, resulting in a state that resembled psychosis (Klawans and Margolin, 1975; Flaum and Schultz, 1996; Woodward et al., 2011). In addition, it has been reported that individuals with schizophrenia release a comparatively greater amount of dopamine than non-psychiatric controls when administered an amphetamine challenge, reflecting the presence of increased basal levels of subcortical dopamine (Abi-Dargham et al., 1998). This increase in dopamine release has been shown to be present in schizophrenia patients who are experiencing an episode of clinical deterioration, but not in clinically stable patients (see as a review Laruelle, 2000). Recent findings demonstrate that altered dopamine transmission is not a result of medication, but rather an intrinsic feature of the disease (see as a review Perez-Costas et al., 2010). Additionally, altered dopamine transmission predates the onset of prodromal psychotic symptoms and may be a risk factor that could be used as a predictor for the development of schizophrenia (Howes et al., 2009; see also as reviews Howes et al., 2007; Perez-Costas et al., 2010). Disturbances within the nigrostriatal, mesolimbic, and mesocortical dopaminergic pathways are also correlated with the phenotypic symptomology of schizophrenia. Through projections to the dorsal striatum, the nigrostriatal pathway is involved in the alleviation of psychosis in schizophrenia with the use of antipsychotic medications 28 (Howes et al., 2009). The nigrostriatal pathway is also associated with tardive dyskinesia, a potential side effect of the administration of first generation antipsychotic medications (see as reviews Newman-Tancredi and Kleven, 2010; Jafari et al., 2012). The mesolimbic pathway proceeds from the SN/VTA and projects to areas of the brain that are associated with emotion and motivation, e.g. nucleus accumbens and amygdala (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004), and disruptions within this pathway are associated with emotional and motivational disturbances. This type of pathology is present in schizophrenia, and is grouped under the category of negative symptomology (DSM-IV-TR, 2000). Anomalies in the mesocortical dopaminergic pathway are most closely related to the cognitive impairments, e.g. deficits in communication, executive function, and memory, which also take place in schizophrenia (Weinberger and Berman, 1988; see as a review Floresco and Magyar, 2006). The reason for this association is due to the fact that the mesocortical pathway contains the projections that arise from the mesodiencephalic dopaminergic neurons of the SN/VTA and proceed to the cortex, particularly the frontal cortex (see as a review Haber and Gdowski, 2004). Since the frontal cortex, and more specifically the prefrontal cortex, is closely associated with these aspects of human functioning (e.g. cognition, etc.), disruptions in this pathway could lead to deficits commonly seen in schizophrenia. In fact, it has been demonstrated that individuals who carry the Val variant of the gene that encodes for COMT present a four-fold increase in activity of the enzyme, and thus an increase in the rate of dopamine catabolism within the cortex. This COMT variant and its subsequent effects have been connected with deficits in executive function (Egan et al., 29 2001). Furthermore, low levels of homovanillic acid, which are indicative of decreased dopamine activity in the prefrontal cortex, correlate with poor performances on working memory tasks (see as a review Toda and Abi-Dargham, 2007). In summary, the nigrostriatal pathway is most closely associated with positive symptoms, the mesolimbic pathway is involved in negative symptomology, and the mesocortical pathway is closely related to the cognitive impairments that have been described in schizophrenia. Metabolic Anomalies in Schizophrenia Changes in cellular metabolism have also been implicated in the pathology of schizophrenia, and previous studies have reported anomalies in the metabolic rate of cortical and subcortical regions of brains from individuals with schizophrenia when compared to non-psychiatric controls (Wiesel et al., 1987; Cleghorn et al., 1989; Wiesel, 1992; Prabakaran et al., 2004). One possible explanation for these observed differences is the existence of anomalies in mitochondrial function. In support of this possibility, mitochondrial density and mitochondrial morphological anomalies have been observed in both anterior limbic cortex and the caudate nucleus in postmortem schizophrenia brain tissue (Uranova and Aganova, 1989; Kung and Roberts, 1999; Somerville et al., 2011a,b). In addition, Prabakaran et al. (2004) reported that transcriptional alterations of genes involved in glucose, fatty acid, and oxidative phosphorylation metabolism, as well as genes involved in oxidative stress were present in approximately 90% of the postmortem brain tissue collected from schizophrenia patients. The most well documented abnormalities of metabolism in schizophrenia revolve around the study of perturbations in oxidative phosphorylation. This pathway is studied 30 extensively because of its role in the production of adenosine triphosphate (ATP), which is used to power the metabolism of cells (see as a review Campbell et al., 2006). Reductions in ATP concentrations have been observed in the frontal lobe of schizophrenia subjects, which implies either a reduced number or dysfunctional mitochondria (Volz et al., 2000). The production of ATP is dependent on a proton gradient that is established across the inner mitochondrial membrane by the electron transport chain (ETC) [Figure 9]. The ETC is composed of four enzymes that are located in the inner membrane of the mitochondria consisting of NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome bc1 (complex III), and cytochrome c oxidase (complex IV) (Saddar et al., 2008; see as a review Manatt and Chandra, 2011), with each enzyme performing a specific task along the chain. The proton gradient established by the ETC is then used to power ATP synthase, resulting in the synthesis of ATP from surrounding ADP and inorganic phosphate (see as reviews Michel et al, 1998; Campbell et al., 2006; Rezin et al., 2009) [Figure 9]. It has been shown that anomalies within the individual complexes can be sufficient to disrupt cellular metabolism (Barksdale et al., 2010; Lee et al., 2013), although disruptions in one complex of the ETC does not necessarily affect other complexes downstream (Prince et al., 1997; Barksdale et al., 2010). Cytochrome c oxidase (complex IV or COX) is one of the most relevant enzymes of the ETC, and has been referred to as the rate-limiting enzyme of ATP production (Li et al., 2006). Studies on the structure of COX using x-ray crystallography have revealed that this enzyme is composed of 13 subunits [Figure 10]. Three of these subunits (COX I, II, and III) are encoded by the mitochondrial genome, while the remaining subunits (COX 31 IV, Va, Vb, VIa, VIb, VIc, VIIa, VIIb, VIIc, and VIII) originate from nuclear DNA, requiring the bidirectional communication of the two genomes for the coordinated synthesis of the different subunits (Tsukihara et al., 1996, Nijtmans et al., 1998). Mitochondrial-encoded subunits constitute the “catalytic core” of the enzyme, which is responsible for substrate binding, electron transfer, oxidation, and eventual expulsion of H+ ions from the matrix into the intermembrane space (see as a review Taanman, 1997). Of the nuclear-encoded subunits, COX IV is the most functionally relevant due to its roles in electron transfer and protection of the catalytic core from reactive oxygen species (Nijtman et al., 1998; Li et al., 2006). In addition, COX IV plays an important role in the assembly of the enzyme. A model for the assembly of COX has been proposed that consists of two assembly intermediates. The first intermediate is formed by the binding of COX I to COX IV, along with the insertion of heme A and heme A3 groups. This is followed by the insertion of the other two subunits of the catalytic core (COX II, COX III), together with COX Va, COX Vb, COX VII a or b, COX VIIc, and COX VIII to form the second intermediate. The addition of COX VIa and COX VII a or b finishes the formation of the complete COX holoenzyme, which will then form a dimer resulting in the active form of COX (Nijtmans et al., 1998; Li et al., 2006; see as a review Fontanesi et al., 2006) [Figure 11]. Several studies have shown that within the schizophrenia population COX activity is differently affected depending on the region of the brain being tested. For example, reduced activity has been noted within the frontal cortex, temporal cortex, and the caudate nucleus (Cavelier et al., 1995; Maurer et al., 2001), with an increase in activity within the putamen (Prince et al., 1999), and no changes in COX activity in the nucleus 32 accumbens, globus pallidus, thalamus, cerebellum, or mesencephalon (Prince et al., 1999; Maurer et al., 2001). Although antipsychotic medications have been reported to cause increases (Prince et al. 1997; 1998) and decreases (Maurer and Moller, 1997) in COX activity, the majority of studies have established that COX activity is not affected by antipsychotic medication (Burkhardt et al., 1993; Whatley et al., 1998; Balijepalli et al., 1999; 2001; Streck et al., 2007). These findings suggest that reduced COX activity is intrinsic to the pathology of schizophrenia. COX subunits have not been extensively studied in schizophrenia, with only one paper reporting increased levels of COX-II mRNA in the frontal cortex (Whatley et al., 1996). Mitochondrial DNA (mtDNA) deletions have also been suggested to play a role in the COX anomalies observed in schizophrenia (see as a review Kato, 2001), although no significant changes have been found in the number of mtDNA deletions within the hippocampus, thalamus, inferior temporal and superior temporal gyrus, cingulate gyrus, caudate nucleus, as well as prefrontal, occipital, and parietal cortices of individuals with schizophrenia, when compared to non-psychiatric controls (Cavelier et al., 1995; Lindholm et al., 1997; Kakiuchi et al., 2005; Sabunciyan et al., 2007). Despite of the fact that the SN/VTA houses the majority of the dopaminergic neurons of the brain, this brain region is the least studied when it comes to the pathology of schizophrenia. To this end, the present study was conducted to determine the contribution of regional anomalies in the SN/VTA to the pathology of this disorder. Specifically, our study of the pathology of the SN/VTA in schizophrenia was focused in three main aspects: 1) Study of regional differences of TH mRNA and protein expression in the SN/VTA in schizophrenia compared to non-psychiatric controls. 2) Assessment of 33 metabolic anomalies linked to COX dysfunction and their contribution to the pathology of the SN/VTA in schizophrenia. This part of the study required the development of an improved method for the measurement of COX activity, which was then used for the assessment of COX activity in the SN/VTA. A second aspect of the analysis of COX dysfunction was to assess if alterations in the expression of critical subunits for the functioning and assembly of the COX enzyme could contribute to schizophrenia pathology in the SN/VTA. 3) Taking into account our findings on TH protein expression anomalies in the SN/VTA in schizophrenia, we wanted to assess if TH pathology could be linked to a significant reduction in the number of neurons in the SN/VTA (i.e. reduction of the total number of neurons or specifically dopaminergic neurons). To test this, we performed stereological counts of dopaminergic and total number of neurons in the SN/VTA of schizophrenia and control cases. As an alternative hypothesis, we also wanted to test the possibility of a reduction of TH protein expression without significant neuronal loss, and the possibility that reductions in TH expression could be assigned to specific neuronal groups within the SN/VTA. To test this, we performed a qualitative analysis of TH protein expression to pinpoint the specific areas and subcellular location of TH deficits within the SN/VTA. 34 Figure 1. Organization of nuclei within the mesodiencephalic dopaminergic system. (A) Sagittal view of the location of the substantia nigra within the human brain. (B) Coronal slice of the human brain showing the location of the substantia nigra. (C) Coronal section through the human brainstem immunohistochemically stained for tyrosine hydroxylase showing the distribution of dopaminergic nuclei at the intermediate level of the substantia nigra. The retrorubral area is located caudal to this section. (D) Coronal section of the rat brainstem immunostained for tyrosine hydroxylase showing the distribution of the dopaminergic cell groups at the intermediate level of the substantia nigra. The retrorubral area is located caudal to this section. 3n: third cranial nerve, Aq: cerebral aqueduct, cp: cerebral peduncle, PAG: periaqueductal gray, PBP: parabrachial pigmented nucleus, PN: paranigral nucleus, R: red nucleus, RLi: rostral linear nucleus, SNC: substantia nigra pars compacta SND: dorsal tier of the substantia nigra pars compacta, SNL: substantia nigra pars lateralis, SNM: substantia nigra pars medialis, SNR: substantia nigra pars reticulata, SNV: ventral tier of the substantia nigra pars compacta, VTA: ventral tegmental area. Figures A & B reproduced with permission from Warner, J. J. (2001). Atlas of neuroanatomy: With systems orgaization and case correlations. (Oxford: ButterworthHeinemann). Figure C reproduced with permission from Reyes, S., Fu, Y., Double, K., Thompson, L., Kirik, D., Paxinos, G., & Halliday, G. M. (2012). GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J Comp Neurol, 520(12), 2591-2607. Figure D from Drs. Miguel Melendez-Ferro and Emma PerezCostas (unpublished). 35 Figure 2. Sagittal image of the human brain showing the three main dopaminergic pathways and their projections sites: Nigrostriatal pathway (red), mesolimbic pathway (green), and mesocortical pathway (blue). Note the intermingling of the pathways and their initial overlapping origin among the individual nuclei of the mdDA complex. SNc: substantia nigra pars compacta, VTA: ventral tegmental area. Reproduced with permission from Arias-Carrion, O., Stamelou, M., Murillo-Rodriguez, E., MenendezGonzalez, M., & Poppel, E. (2010). Dopaminergic reward system: a short integrative review. Int Arch Med, 3, 24. 36 Figure 3. Schematic of the afferent and efferent connections of the SN/VTA. Pathways colored in red indicate GABAergic connections; pathways in green indicate glutamatergic connections. C: caudate nucleus, Ctx: cortex, DA: dopamine, Enk: enkephalin, GPe: globus pallidus external segment, GPi: globus pallidus internal segment, NStrP: nigrostriatal pathway, P: putamen, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, SP: substance P, STN: subthalamic nucleus, StrNP: striatonigral projections, VA: ventral anterior thalamic nucleus, VLa: anterior part of the ventral lateral thalamic nucleus. D1-like family of dopamine receptors and D2-like family of dopamine receptors are indicated by empty and filled small rectangles, respectively. The efferent projection of the SNc to the striatum (nigrostriatal pathway; NStrP) is shown in black. Reproduced with permission from Nieuwenhuys R., Voogd J., and van Huijzen C. (2007). Telencephalon: Basal Ganglia. In: The Human Central Nervous System: A Synopsis and Atlas, Fourth Edition (New York: Springer), 427-489. 37 Figure 4. In vivo biosynthesis of dopamine. Reproduced with permission from Purves, D., Augustine, G. J., Fitzpatrick, D., et al. (2001). Neuroscience, 2nd edition. (Sunderland: Sinauer Associates). 38 Figure 5. Diagram of a dopaminergic synapse. The presynaptic terminal is located at the top and the postsynaptic neuron is located at the bottom of the image. COMT: catecholO-methyltransferase, DOPA: 3,4-dihydroxyphenylalanine, DOPAC: 3,4dihydroxyphenylacetic acid, D1R: D1-like family of dopamine receptors, D2R: D2-like family of dopamine receptors, HVA: homovanillic acid, MAO: monoamine oxidase, VMAT2: vesicular monoamine transporter 2. 39 Figure 6. Primary pathways of dopamine catabolism. Enzymes (in bold italics) are ADH: alcohol dehydrogenase, ALD-D: aldehyde dehydrogenase, ALR: aldehyde reductase, COMT: catechol-O-methyltransferase, and MAO monoamine oxidase. Cofactors (in parenthesis) are NAD+: nicotinamide adenine dinucleotide, NADP+: nicotinamide adenine dinucleotide phosphate, O2: molecular oxygen, and SAM: S-adenosyl-Lmethionine. Metabolites include 3-MT: 3-Methoxytyramine, DOPAC: 3,4dihydroxyphenylacetic acid, DOPAL: 3,4-dihydroxyphenylacetaldehyde, DOPET: 3,4dihydroxyphenylethanol, HVA: homovanillic acid, and MOPET: 3-methoxy-4hydroxyphenylethanol. Starred enzymes indicate pathways that have been shown to be altered in schizophrenia pathology. Underlined metabolites indicated the most common dopamine metabolite of the striatum (DOPAC) and the cortex (HVA). Reproduced with permission from Elsworth, J. D., & Roth, R. H. (1997). Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol, 144(1), 4-9. 40 Figure 7. Development of the mesodiencephalic dopaminergic system. Top: Sagittal view of an archetypical mammalian brain during development. Dopaminergic neuronal precursors originate in ventral portions of the mesomere and prosomeres 1-3 (area shown in red). The isthmus is indicated in blue. Bottom: Cross-section through the developing mesencephalon at the level indicated by the dashed line. Dopaminergic neuronal precursors originate from the basal and floor plates of the developing brain. AP: alar plate, Aq: cerebral aqueduct, BP: basal plate, FP: floor plate, IZ: intermediate zone, M: mesomere, MZ: marginal zone, P1: prosomere 1, P2: prosomere 2, P3: prosomere 3, RD: rostral diencephalon, Rhom: rhombencephalon, SNc: substantia nigra pars compacta, Tele: telencephalon, VTA: ventral tegmental area, VZ: ventricular zone. 41 Figure 8. Progression of axons from the developing mesodiencephalic dopaminergic complex. (a) Chemorepulsive signals from caudal areas of the developing brain direct axons towards a dorsal direction. (b) Dorsal progression of dopaminergic axons is halted, and axons are directed towards a more rostral direction. (c) Axon guidance cues from the thalamus result in the development of the medial forebrain bundles (MFBs). (d) Dopaminergic axons of the nigrostriatal and mesolimbic/mesocortical pathways begin to differentiate due to guidance cues emanating from the striatum and cortex, respectively. (e) Guidance cues from the cortex help to establish the mesolimbic and mesocortical pathways, while having a chemorepulsive effect on axons of the nigrostriatal pathway. (f) Chemoattractants within the striatum help to finalize the synaptic connections of the nigrostriatal pathway. OB: olfactory bulb, DM: dorsal midbrain, MFB: medial forebrain bundles, SNc: substantia nigra pars compacta, VTA: ventral tegmental area. Reproduced with permission from Van den Heuvel, D. M., & Pasterkamp, R. J. (2008). Getting connected in the dopamine system. Prog Neurobiol, 85(1), 75-93. 42 Figure 9. Schematic of the electron transport chain of the mitochondria. Image showing flow and direction of electrons (e-) and hydrogen ions (H+). ADP: adenosine diphosphate, ATP: adenosine triphosphate, Cyt c: cytochrome c, Pi: inorganic phosphate, ROS: reactive oxygen species, UQ: ubiquinone. Reproduced with permission from Al Ghouleh, I., Khoo, N. K., Knaus, U. G., Griendling, K. K., Touyz, R. M., Thannickal, V. J., Barchowsky, A., Nauseef, W. M., Kelley, E. E., Bauer, P. M., Darley-Usmar, V., Shiva, S., Cifuentes-Pagano, E., Freeman, B. A., Gladwin, M. T., & Pagano, P. J. (2011). Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med, 51(7), 1271-1288. 43 Figure 10. Three-dimensional representation of cytochrome c oxidase. The flux of H+ from the matrix to the intermembrane space is indicated by the red arrow. COX I, II, and III (circled) constitute the catalytic core of the enzyme. COX IV (boxed) is fundamental for the assembly and short-term regulation of the enzyme. Reproduced with permission from Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., & Yoshikawa, S. (1996). The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science, 272(5265), 1136-1144. 44 Figure 11. Model for cytochrome c oxidase (COX) assembly in cultured human cells. Note the particular importance of COX IV, as well as COX II, in the initial assembly of the enzyme. S1: stage 1, S2: stage 2, S3: stage 3, S4: stage 4. Reproduced with permission from Nijtmans, L. G., Taanman, J. W., Muijsers, A. O., Speijer, D., & Van den Bogert, C. (1998). Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem, 254(2), 389-394. 45 DOPAMINE PATHOLOGY IN SCHIZOPHRENIA: ANALYSIS OF TOTAL AND PHOSPHORYLATED TYROSINE HYDROXYLASE IN THE SUBSTANTIA NIGRA by Emma Perez-Costas, Miguel Melendez-Ferro, Matthew W. Rice, Robert R. Conley, & Rosalinda C. Roberts. Frontiers in Psychiatry Copyright 2012 by Frontiers Used with permission Format adapted and errata corrected for dissertation 46 Abstract Introduction: Despite the importance of dopamine neurotransmission in schizophrenia, very few studies have addressed anomalies in the mesencephalic dopaminergic neurons of the substantia nigra/ventral tegmental area (SN/VTA). Tyrosine hydroxylase (TH) is the rate-limiting enzyme for the production of dopamine, and a possible contributor to the anomalies in the dopaminergic neurotransmission observed in schizophrenia. Objectives: In this study, we had three objectives: (1) Compare TH expression (mRNA and protein) in the SN/VTA of schizophrenia and control postmortem samples. (2) Assess the effect of antipsychotic medications on the expression of TH in the SN/VTA. (3) Examine possible regional differences in TH expression anomalies within the SN/VTA. Methods: To achieve these objectives three independent studies were conducted: (1) A pilot study to compare TH mRNA and TH protein levels in the SN/VTA of postmortem samples from schizophrenia and controls. (2) A chronic treatment study was performed in rodents to assess the effect of antipsychotic medications in TH protein levels in the SN/VTA. (3) A second postmortem study was performed to assess TH and phosphorylated TH protein levels in two types of samples: schizophrenia and control samples containing the entire rostro-caudal extent of the SN/VTA, and schizophrenia and control samples containing only mid-caudal regions of the SN/VTA. Results and Conclusions: Our studies showed impairment in the dopaminergic system in schizophrenia that could be mainly (or exclusively) located in the rostral region of the SN/VTA. Our studies also showed that TH protein levels were significantly abnormal in schizophrenia, while mRNA expression levels were not affected, indicating that TH pathology in this region may occur posttranscriptionally. 47 Lastly, our antipsychotic animal treatment study showed that TH protein levels were not significantly affected by antipsychotic treatment, indicating that these anomalies are an intrinsic pathology rather than a treatment effect. Keywords: neuropsychiatric disorders, postmortem studies, human brain, rat brain, western-blot 48 Introduction Tyrosine hydroxylase (TH) is a crucial enzyme in the production of dopamine and other catecholamines. In the substantia nigra/ventral tegmental area (SN/VTA), dopamine is the final product of cells that express TH and these cells are the largest source of dopamine in the brain. Studies of TH in the SN/VTA of schizophrenia subjects are almost anecdotal, with just one study that addressed enzymatic activity (Toru et al., 1988), and two studies that addressed mRNA levels (Ichinose et al., 1994; Mueller et al., 2004). However, no studies have assessed TH protein levels in the SN/VTA in schizophrenia. In addition, recent studies on the synthesis of TH have shown that the regulation of the production of TH is very different in the SN/VTA than in other catecholaminergic neurons (Wong and Tank, 2007; Chen et al., 2008; Tank et al., 2008). These studies also showed that posttranscriptional regulations have a crucial role in the synthesis of TH in the SN/VTA (Chen et al., 2008; Tank et al., 2008). Posttranscriptional modulation occurs after the production of mRNA is completed, and includes the stabilization of the mRNA, the attachment to polyribosomes, and the regulation of the efficacy of translation into protein (Tank et al., 2008; Lenartowski and Goc, 2011). For all these reasons, we believe that is necessary to study TH protein levels in the SN/VTA in schizophrenia, to assess the possibility of pathologies that could be located in posttranscriptional steps in the synthesis of TH, rather than at the transcription level. In addition to its production, TH protein activity in the SN/VTA is modulated by the phosphorylation of certain serine sites in the first forty amino acids of the N-terminus of the TH protein (Haycock, 1990; Nakashima et al., 2009). Of these phosphorylation sites, serine 40 has been shown to be the most important in the regulation of TH activity 49 in the brain (Nakashima et al., 2009). The phosphorylation of this site is a complex process that includes proper conservation of the amino acid residues flanking this serine residue, and phosphorylation of this site produces the disinhibition of the catalytic subunit of TH, thus allowing the production of dopamine (Nakashima et al., 2009). Genetic association studies in schizophrenia have produced somewhat inconclusive results (Meloni et al., 1995; Wei et al., 1995; Jonsson et al., 1996, 1998; Thibaut et al., 1997; Burgert et al., 1998; Ishiguro et al., 1998; Kunugi et al., 1998; Kurumaji et al., 2001; Ota et al., 2001; Chao and Richardson, 2002; Pae et al., 2003; Hoogendoorn et al., 2005; Jacewicz et al., 2008; Talkowski et al., 2008; Andreou et al., 2009). However, some of these studies have shown consistent data in different cohorts of patients, indicating an association of certain mutations in the first intron of the TH gene with a higher risk of schizophrenia (Meloni et al., 1995; Wei et al., 1995; Kurumaji et al., 2001; Pae et al., 2003; Jacewicz et al., 2008). Interestingly this first intron of the TH gene has been postulated to be a modulatory region for the synthesis of TH (Pae et al., 2003; Jacewicz et al., 2008). Previous studies assessing anomalies of the dopaminergic system in schizophrenia have heavily focused their attention on different aspects of the dopamine neurotransmission at the postsynaptic level, and studies of molecules involved in the transport and degradation of dopamine (see as reviews, Abi-Dargham et al., 2010; PerezCostas et al., 2010; Tost et al., 2010). Another important aspect of the dopaminergic neurotransmission, which originates in neurons in the SN/VTA, is the topographical organization of the dopamine producing cells in this complex, which defines the preferential terminal target of their projections (e.g. the mesolimbic, mesocortical or 50 nigro-striatal pathways; Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Haber and Gdowski, 2004; Halliday, 2004). Some studies have found anomalies in TH in the terminals of the nigro-striatal pathway in schizophrenia (Roberts et al., 2009) and in the terminal fields of the mesocortical pathway (Akil et al., 1999; 2000), but none of theses studies have assessed the cells of origin of the dopaminergic inputs to these brain regions. This brought our attention to the importance of assessing regional anomalies in TH synthesis within the SN/VTA in schizophrenia. Materials and Methods Ethics statement All the human brain samples used in this study were obtained from the Maryland Brain Collection (Maryland Psychiatric Research Center, University of Maryland School of Medicine) with permission from the Maryland Brain Collection Steering Committee. All experimental procedures were also approved by the University of Alabama at Birmingham Institutional Review Board (protocol # N110505002) and in accordance with The Code of Ethics of the World Medical Association. All the animal experiments were conducted at the University of Maryland following a protocol approved by the Institutional Animal Care and Use Committee (protocol #0705010), in accordance with National Institutes of Health guidelines regarding the care and use of animals for experimental procedures. 51 Postmortem human brain samples For all the postmortem human cases used in this study, two independent senior psychiatrists established DSM-IV diagnoses based on the review of all available medical records and the data obtained from the Structured Clinical Interview for DSM-IIIR (Spitzer et al., 1992) and DSM-IV Axis I disorders with the next of kin. Control cases had enough information from next of kin and medical records to discard any major neurological or neuropsychiatric disorders. Two types of samples were used in this study: samples containing the entire rostro-caudal extent of the SN/VTA (pilot study and second postmortem study), and samples containing only mid-caudal SN/VTA (second postmortem study). In both cases samples were frozen on dry ice and stored at -800C until use. Samples were sectioned on a cryostat at -200C obtaining five parallel series of 16 μm thick sections. Four parallel series were collected on superfrost slides, while the fifth series was collected in a tube for protein extraction. In all cases, series #1 was stained with thionin (Nissl stain) to assess possible morphological anomalies. Only cases that presented good preservation of the SN/VTA region were included in the study. Rostral and caudal limits for the SN/VTA were determined using the Paxinos and Huang (1995) brainstem atlas. For samples containing only mid-caudal regions the rostral limit was set at the starting point of the substantia nigra compacta pars ventralis as defined by McRitchie et al. (1995). Two different sets of experiments were performed with human brain samples: a) a pilot study in which TH mRNA and TH protein levels were assessed in samples from the same cases (see Table 1); b) a second postmortem study of total TH protein levels and 52 phosphorylated TH levels in samples containing the entire extent or only mid-caudal regions of the SN/VTA (see Table 2). Animal samples A total of 27 adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used in this study to test the effect of chronic treatment with antipsychotic drugs on TH protein levels. Animals were allowed to adapt to the animal room for 1 week prior to starting any treatment, and water consumption was monitored to calculate initial treatment doses. Animals were then randomly assigned to one of the 3 treatment groups (n=9 per group): haloperidol (1.5 mg/kg/day), olanzapine (6 mg/kg/day), or control. Antipsychotics were administered for 3 weeks in drinking water, adjusting the doses to body weight and daily liquid consumption as previously described in Perez-Costas et al. (2008). At endpoint, animals were decapitated, and trunk blood was collected to monitor plasma drug levels. Brains were immediately removed from the skull, frozen in dry ice, and stored at -800C until use. Four parallel series at the level of the SN/VTA were collected on superfrost slides, while the 5th series was collected in a tube for protein extraction. For all animals, series #1 was stained with thionin to assess possible morphological anomalies. No morphological anomalies were found in any of the samples used. In situ hybridization In situ hybridization was performed for the postmortem SN/VTA samples included in the pilot study (Table 1). Slides containing sections at the level of the rostral, 53 medial and caudal SN/VTA were selected from each case to perform this technique. To select the sections, series #1 (stained with thionin) was used to accurately identify matching rostral, medial and caudal areas of the SN/VTA for each case. A 48-mer antisense oligonucleotide probe that hybridizes to nucleotides 32-79 of the human TH mRNA sequence was used. This sequence is common to all three mRNA transcript variants identified for the human TH (GeneBank accession numbers: NM_199292; NM_000360; NM_199293). Probe labeling, hybridization, and post-hybridization rinses, were performed as described in Gao et al. (1998). Briefly, oligonucleotide probes were labeled with 35 S-dATP at the 3’-end using terminal transferase. The sections were hybridized overnight at 37°C with an hybridization buffer containing 50% deionized formamide, 4X sodium saline citrate (SSC), Denhardt’s solution, 10X dextran sulfate, yeast tRNA (250 μg/ml), single strand salmon DNA (500 μg/ml) and 100mM dithiothreitol (DTT). After hybridization, the sections were sequentially rinsed in 1X SSC containing 10 mM DTT at 550C, and 1X SSC at room temperature. Finally, the sections were gradually dehydrated in ethanol, air dried and autoradiograms were generated by exposing Kodak BioMax MR films (Kodak, Rochester, NY) along with [ 14C] microscale standards (Amersham Biosciences, Little Chalfont, UK) for 14 days. Films were developed using Kodak D-19 developer and fixer, scanned at 600 dpi, and optical density was measured using Image Pro-Plus 6.2 software (Media Cybernetics, Bethesda, MD). Western-blot Western-blot assays were performed for human postmortem SN/VTA protein samples (pilot study and second postmortem study; see Tables 1 and 2), and for rat SN/VTA 54 protein samples (animal study). In all cases the protein extraction procedure and sample handling were identical. The only exceptions were the antibodies used for the detection of TH. For the human postmortem studies a mouse monoclonal anti-TH antibody (SigmaAldrich, St. Louis, MO, USA) diluted 1:10,000 was used, while for the animal study a mouse monoclonal anti-TH antibody (Millipore, Billerica, MA, USA) diluted 1:20,000 was used. In addition, a rabbit polyclonal anti-phospho-Ser40 TH antibody (PhosphoSolutions, Aurora, CO, USA) diluted 1:4,000 was used in human samples (second postmortem study) for the detection of phosphorylated TH (pTH). Finally, reblots for actin were performed in all the western-blot experiments (human and rat) using a mouse monoclonal anti-actin antibody (Millipore) diluted 1: 40,000. Antibodies specificity In this work, we used two different types of antibodies to detect TH, one type to detect total content of tyrosine hydroxylase, and another type to detect the active (phosphorylated) form of this enzyme. In addition, an antibody against actin was used as an internal control for the accuracy of the experiments performed (e.g. protein loading and tissue preservation control). For the detection of total tyrosine hydroxylase, two different antibodies were used: For the human studies, a mouse monoclonal anti-TH manufactured by Sigma-Aldrich (clone TH-16, catalog # T2928) was used. As per information provided in the data sheet by the manufacturer, this antibody is raised against purified rat tyrosine hydroxylase and recognizes an epitope in the N-terminal region between aminoacids 40-152 of human TH. For the rat study, we used an anti-TH antibody manufactured by Millipore (clone 55 2/40/15, catalog # MAB5280). As per information provided by the manufacturer, this antibody is raised against purified tyrosine hydroxylase from a rat pheochromocytoma and its specificity is routinely assessed by western-blot by the manufacturer. For the detection of phosphorylated (active) tyrosine hydroxylase, a rabbit polyclonal anti-phospho-ser40 antibody by Phosphosolutions (catalog# p1580-40) was used. As per information provided by the manufacturer, this antibody is raised against a phosphopeptide corresponding to aminoacid residues surrounding the phosphor-ser40 of rat tyrosine hydroxylase. The antibody is purified by affinity purification via sequential chromatography on phospho- and dephosphopeptide affinity columns. The antibody specificity was also tested by western-blot, and has been shown to recognize exclusively phosphorylated TH. For the detection of actin, a mouse monoclonal anti-actin antibody by Millipore (catalog# MAB1501, clone C4) was used. As per information provided by the manufacturer, this antibody is raised against purified chicken gizzard actin, and recognizes an epitope located at the N-terminal two thirds of the protein near aminoacids 50-70. This antibody reacts against actin from all vertebrates. Protein extraction Tissue collected for western-blot was diluted 1:5 in lysis buffer containing TrisHCl pH 8.0, sodium chloride, sodium dodecyl sulphate, disodium EDTA, and a protease inhibitor cocktail (Sigma-Aldrich; P8340). Samples were homogenized with a sonicator, and the homogenate was centrifuged at 13,000 rpm for 15 minutes at 40C. The 56 supernatant was recovered, and the total protein concentration was measured using a modified Lowry kit (Bio-Rad; 500-0113, 500-0114). Gel electrophoresis and western-blot Protein extracts with total protein concentrations of 30μg (rat), or 60μg (human), were loaded onto 10% polyacrylamide gels along with a molecular weight marker (BioRad, Hercules, CA; 161-0324). Proteins were resolved at 150 volts, and then transferred at 30 volts overnight onto PVDF membranes (Bio-Rad; 162-0174). The following day, the membranes were blocked in Tris-buffered saline (TBS) containing 5% non-fat powdered milk and 0.1% Tween-20 for 1 hour at room temperature. TH or pTH were detected by incubating the membranes with the appropriate primary antibodies (see above) diluted in TBS containing 1% non-fat powdered milk and 0.1% Tween-20 (TBSTm) for 21 hours at 40C. After rinsing in TBSTm, the membranes were incubated for 1 hour at room temperature with a goat anti-mouse alkaline phosphatase-coupled secondary antibody (Millipore; AP124A) diluted 1:15,000, or a goat anti-rabbit alkaline phosphatase-coupled secondary antibody (Vector Laboratories, Burlingame, CA; AP1000) diluted 1:1,000. The membranes were then rinsed in TBS, and the bands were visualized using a chemiluminescent system (Bio-Rad; 170-5018), exposing Kodak Biomax XAR films. In all cases, the membranes were reblotted and re-incubated with an antibody against actin (see details above). Films were scanned at 600 dpi using a flatbed scanner, and optical density was measured using Image Pro Plus 6.2 software (Media Cybernetics). 57 Statistical analysis All data sets (outcome measures and possible covariates) were assessed for normality using the Kolmogorov-Smirnov test. For this and subsequent statistical tests, categorical variables (i.e. gender and race) were assessed using a “dummy coding” scheme. Power analyses were performed entering the outcome measures (i.e. TH protein levels in schizophrenia and control groups) from the first postmortem study (pilot study) in G power 3.1 software (Faul et al. 2007). For subsequent postmortem studies (i.e. regional expression of TH and pTH protein) an “a priori” test to compute sample size for t-test comparison was used. For the animal study, an “a priori” test to compute sample size for one-way ANOVA was used. The effect of possible covariates in the outcome measures of interest (i.e. mRNA TH levels, TH protein, and pTH protein levels) for postmortem studies was assessed using a multiple regression model, in which the effect of the possible covariates was tested independently for each of the outcome measures of interest. The covariates included in the multiple regression analyses were carefully selected based on their possible influence on the expression of TH (mRNA or protein) or pTH (protein). The tested covariates included age, race, gender, postmortem interval (PMI), pH, and actin levels. PMI and pH are directly related with the quality of the tissue preservation (Stan et al., 2006), while actin levels are directly related with the accuracy on the processing of the samples (i.e. loading of the samples on the electrophoresis gels). The model of multiple regression analysis used also included the assessment of possible multicollinearity. For the animal study, the only covariate assessed was actin levels, and this was done by linear regression analysis. For postmortem human studies the statistical analysis of the outcome measures was performed by using the appropriate t-test (i.e. 58 Student’s t-test, or Welch’s t-test) for data that were normally distributed, or a nonparametric test (Mann-Whitney U-test), when one or both of the data sets were not normally distributed. One-way ANOVA was used in the case of animals treated with antipsychotics. The statistical analysis was performed using InStat 3.0 software (GraphPad Software Inc., La Jolla, CA). Results Demographic features Human postmortem cases were carefully selected for each study, trying to match as much as possible the cases in the control and schizophrenia groups by their age, gender, race, PMI and brain pH (see Tables 1 and 2). In addition, statistical analysis was performed for each of these demographic features to ensure that the control and schizophrenia groups were not significantly different. Pilot study Age, gender, race, and PMI were not normally distributed (Kolmogorov-Smirnov Normality test) for one or both groups, therefore a Mann-Whitney U-test was used to compare each of these demographic features between the control and schizophrenia groups (Age: U=13.00; P=0.79. Race: U=11.00; P=0.46. Gender: U=14.00; P=0.91; PMI: U=14.50; P>0.99). Brain pH data were normally distributed for both groups, therefore a standard unpaired t-test was performed (t(9)=0.72; P=0.49). In summary, all the demographic features were not significantly different between the control and schizophrenia groups for the pilot study (Table 1). 59 Regional expression of TH and pTH study This study was performed in two different types of samples that were analyzed independently: Cases containing all the rostro-caudal extent of the SN/VTA, and cases containing only the mid-caudal region of the SN/VTA. The demographic features for control and schizophrenia cases for each of these two types of samples were statistically analyzed. In rostro-caudal samples, age, PMI, and brain pH were normally distributed for the control and schizophrenia groups, therefore an unpaired t-test was performed (Age: t(12)=0.15; P=0.89. PMI: t(12)=0.40; P=0.70. Brain pH: t(12)=1.79; P=0.1). On the other hand, race and gender for the rostro-caudal samples were not normally distributed at least for one of the groups, therefore a Mann-Whitney U-test was performed (Race: U=18.00; P=0.39. Gender: U=22.00; P=0.79). In summary, no demographic features were significantly different between the control and schizophrenia groups for the cases containing all the rostro-caudal extent of the SN/VTA. In mid-caudal samples, age, PMI, and pH were normally distributed for the control and schizophrenia groups, therefore an unpaired t-test was performed (Age: t(12)=1.08; P=0.30. PMI: t(12)=0.50; P=0.63; Brain pH: t(12)=1.70; P=0.11). For mid-caudal samples race and gender were not normally distributed and a Mann-Whitney U-test was performed (Race: U=10.00; P=0.045. Gender: U=18.00; P=0.39). In summary, race was the only demographic feature that presented significant differences between the schizophrenia and control groups in midcaudal samples. This significant difference was further assessed (see below, second postmortem study results) as a possible predictor for the main outcome measures (TH and pTH protein levels; Table 2). 60 Pilot study: TH mRNA and protein expression in human postmortem schizophrenia and control cases TH mRNA and TH protein levels were tested in SN/VTA samples from the same cases (see Table 1). In this pilot study a total of 11 cases were used (n=5 controls; n=6 schizophrenia). TH mRNA levels Multiple regression analysis with the main outcome measure (TH mRNA levels) as the dependent variable, and the possible covariates as independent variables was used to test if the covariates (i.e. age, race, gender, PMI, pH) significantly affected the TH mRNA levels regardless of diagnoses (schizophrenia or control). The results of this test were non-significant (R2=0.38; P=0.70). After that, TH mRNA data in the schizophrenia and control groups were tested for normality using the Kolmogorov-Smirnov test. For both groups the TH mRNA data were normally distributed (Control group: KS=0.23; P>0.10. Schizophrenia group: KS=0.23; P>0.10). Since the data were normally distributed, an unpaired two-tailed t-test assuming equal variances was performed to test for differences in TH mRNA levels between the controls and schizophrenia groups, which yielded a non-significant result (t(9)=1.74; P=0.12). See also Figure 1A. TH protein levels Multiple regression analysis with TH protein as the main outcome measure (dependent variable) and age, race, gender, PMI, pH, and actin levels as possible covariates (independent variables) revealed a non-significant result (R2=0.60; P=0.52). 61 Kolmogorov-Smirnov normality test revealed that TH protein levels data in the control and schizophrenia groups were normally distributed (Control group: KS=0.21; P>0.10. Schizophrenia group: KS=0.29; P>0.10). An unpaired two-tailed t-test assuming unequal variances (Welch’s correction) was used since the standard deviations between the two groups were significantly different (F=72.75; P=0.001). The t-test with Welch’s correction revealed significant differences in TH protein levels between the control and schizophrenia groups (t(5)=2.96; P=0.031). See also Figure 1B. In summary, this pilot study showed that TH protein levels were significantly different between the schizophrenia and control groups, while TH mRNA levels did not differ between the two groups. These results prompted us to design new experiments to test: a) if chronic antipsychotic treatment could significantly affect the levels of TH protein (animal study), and b) if the differences observed in TH protein expression were regionally specific within the SN/VTA, and if these differences were also present in the active form of TH (pTH), which were tested in a subsequent postmortem human study. Animal study: Antipsychotic treatment effect on TH protein levels Power analysis For this study, an “a priori” power analysis was performed to determine the minimum adequate sample size for a one-way ANOVA with a minimum power of 0.80. Taking into account the results of the pilot study for TH protein levels in postmortem human tissue, we used the following assumption: if changes in TH protein levels can be explained as an effect of the antipsychotic medication, these changes have to be large to explain the differences observed in the pilot study between the control and schizophrenia 62 groups. For this reason, we modeled the study to expect a large effect size (0.80). With these assumptions, the power analysis determined that a minimum sample size of n= 21 (n=7 per group) was necessary to obtain the minimum power required. Since in our study the sample size was n=27 animals (n=9 per group), a post-hoc power analysis using the actual effect size obtained (f=0.64) revealed that the actual power of our study was 0.804 (critical F=3.40; α=0.05). TH protein levels in animals chronically treated with APDs The only covariate to be assessed for this study was actin protein levels, which is a measurement of the accuracy of the experiment. The effect of this possible covariate on TH protein levels was analyzed by linear regression, which revealed a non-significant result (r=0.15; P=0.44). The Kolmogorov-Smirnov test showed that TH protein levels were normally distributed for the three groups (Control group: KS=0.22; P>0.10; Haloperidol group: KS=0.17; P>0.10. Olanzapine group: KS=0.26; P=0.08). However, a Kruskal-Wallis non-parametric ANOVA was used, since a Bartlett’s test for homogeneity of variances showed very significant differences among the three groups (Bartlett statistic=10.37; P=0.006). The Kruskal-Wallis ANOVA revealed non-significant differences among the three groups for TH protein levels (KW=1.79; P=0.41). See also Figure 2. In summary, this study showed that chronic treatment with antipsychotic medications (typical or atypical) did not produce significant differences in TH protein levels when compared with untreated controls. 63 Second postmortem study: Regional expression of TH and phosphorylated TH Power analysis For this study, an “a priori” power analysis was performed to determine the minimum adequate sample size for a two-tailed t-test analysis with a minimum power of 0.80. To calculate this, data obtained from the TH protein levels in the pilot study were entered to calculate the effect size. This analysis yielded that a sample size of n=14 cases (schizophrenia n=8; control n=6) would produce an actual power of 0.83 (t critical =2.18; α=0.05). In mid-caudal samples, an outlier was present in the schizophrenia group (case SZ11). This case was detected as an outlier using a combination of different factors: first, the pH of this sample was highly basic (pH=7.80, see Table 2); second, TH levels were highly different from any other case in the schizophrenia or control groups, and a Grubb’s test was performed using Quick Calcs Online calculator (Graphpad Software Inc.). This test showed that case SZ11 was a significant outlier for TH levels (Z=2.94; P<0.05). For these reasons, this case was removed from the subsequent statistical analysis of mid-caudal TH and pTH expression. After removing this case for the analysis, a two-tailed t-test “compromise” power was calculated, entering the data obtained for TH protein levels in the pilot study to calculate the effect size. This analysis yielded a power of 0.80 (critical t=2.20; α=0.05) for the mid-caudal analysis with a sample size of n=13 (control n=6; schizophrenia n=7). TH protein levels in rostro-caudal SN/VTA samples Multiple regression analysis with TH protein as the main outcome measure (dependent variable) and age, race, gender, PMI, pH, and actin levels as possible 64 covariates (independent variables) revealed a non-significant result (R2=0.74; P=0.06). Kolmogorov-Smirnov normality test revealed that TH protein levels in the control and schizophrenia groups were normally distributed (Control group: KS=0.31; P=0.07. Schizophrenia group: KS=0.17; P>0.10). An unpaired two-tailed t-test assuming unequal variances (Welch’s correction) was used since the standard deviations between the two groups were significantly different (F=32.97; P=0.001). The t-test with Welch’s correction revealed significant differences in TH protein levels between the control and schizophrenia groups (t(7)=2.59; P=0.036). See also Figure 3A. TH protein levels in mid-caudal SN/VTA samples Multiple regression analysis with TH protein as the main outcome measure and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a nonsignificant result (R2=0.75; P=0.11). In addition, the multiple regression analysis showed that, even when race was detected as a significantly different demographic feature between the schizophrenia and control groups for mid-caudal cases (see demographic features analysis above), this was not a significant contributor (predictor) of TH levels outcome (t ratio=0.75; P=0.48). Kolmogorov-Smirnov Normality test revealed that TH protein levels were normally distributed for the control group but not for the schizophrenia group (Control group: KS=0.27; P>0.1. Schizophrenia group: KS=0.31; P=0.047). Since data were not normally distributed for one of the groups, a nonparametric Mann-Whitney U-test was used. This test revealed non-significant differences in TH protein levels between the control and schizophrenia groups (U=13.0; P=0.29). See also Figure 3A. 65 Phosphorylated TH protein levels in rostro-caudal SN/VTA samples Multiple regression analysis with pTH protein levels as the main outcome measure, and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a non-significant result (R2=0.51; P=0.39). Kolmogorov-Smirnov normality test revealed that pTH protein levels data in the control and schizophrenia groups were normally distributed (Control group: KS=0.27; P>0.10. Schizophrenia group: KS=0.27; P=0.09). An unpaired two-tailed t-test assuming unequal variances yielded no significant differences (t(12)=2.12; P=0.056), although the P value was low enough to indicate a trend towards significance. See also Figure 3B. Phosphorylated TH protein levels in mid-caudal SN/VTA samples Multiple regression analysis with pTH protein as the main outcome measure and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a nonsignificant result (R2=0.73; P=0.13). In addition, the multiple regression analysis showed that, even when race was detected as a significantly different demographic feature between the schizophrenia and control groups for mid-caudal cases (see demographic features analysis above), this was not a significant contributor (predictor) of pTH levels outcome (t ratio=0.36; P=0.73). Kolmogorov-Smirnov normality test revealed that pTH protein levels were normally distributed for the control group but not for the schizophrenia group (Control group: KS=0.24; P>0.1. Schizophrenia group: KS=0.35; P=0.01). Since data were not normally distributed for one of the groups, a non-parametric Mann-Whitney U-test was used. This test revealed non-significant differences in TH 66 protein levels between the control and schizophrenia groups (U=12.5; P=0.25). See also Figure 3B. In summary, this regional postmortem study showed that TH protein levels in samples containing the entire rostro-caudal extent of the SN/VTA were significantly different between the schizophrenia and control groups, while no significant differences were detected in samples that only contained mid-caudal regions. In addition, no significant differences were detected for pTH protein levels for samples containing either the entire rostro-caudal, or only mid-caudal regions of the SN/VTA. However, a trend towards significance (P=0.056) was observed for pTH levels in samples containing the entire rostro-caudal extent of the SN/VTA. Discussion As far as we are aware, this is the first study to analyze TH protein levels in the SN/VTA of schizophrenia compared to controls, and only two previous studies have assessed TH mRNA levels in schizophrenia (Ichinose et al., 1994; Mueller et al., 2004). Pilot study: TH mRNA and protein expression in human postmortem schizophrenia and control cases The most remarkable finding of our first postmortem study (pilot study) was the fact that in some of the schizophrenia cases (three out of the six cases studied) TH protein levels were profoundly altered, while in the same brain samples TH mRNA levels were similar to control cases. It was also remarkable the large variability presented in the schizophrenia group, with some cases presenting levels in the same range than controls, 67 while others presented very low TH protein levels. The regulation of the transcription and translation of the TH gene is a highly complex process that is also specific to the type of TH expressing cell (i.e. dopaminergic versus adrenergic or noradrenergic neurons). Tank and collaborators have shown that while in adrenergic and noradrenergic cell populations of the locus coeruleus and adrenal medulla TH is highly regulated at the transcription level, this type of regulation is minimal in the case of dopaminergic cells of the SN/VTA (Chen et al., 2008; Tan et al., 2008). In the locus coeruleus and adrenal medulla, most types of stress can induce short-term and long-term upregulation of TH mRNA transcription, that is well correlated with upregulation of TH protein levels (Alterio et al., 2001; Wong and Tank, 2007; Tank et al., 2008). This is also correlated with changes in transcription factors that are known to regulate the TH gene promoter (Wong and Tank, 2007; Tank et al., 2008). However, in the case of the TH expressing cells of the SN/VTA it has been shown that induction of the transcription for TH mRNA does not always lead to an upregulation of TH protein levels (Chen et al., 2008; Tan et al., 2008). The application of drugs that are known to affect the transcription rate of TH mRNA in the adrenal medulla and the locus coeruleus (Alterio et al., 2001; Wong and Tank, 2007; Tank et al., 2008), does not produce any effect in TH mRNA levels in the SN/VTA, while they produce an upregulation of TH protein levels (Chen et al., 2008; Tan et al., 2008). In midbrain slice explants containing the SN/VTA, these authors demonstrated that forskolin and cAMP analogs increase TH protein levels in a dose dependent manner, while TH mRNA levels are unchanged. These authors proposed that in the midbrain there is a cAMP-mediated induction of TH protein by a translational mechanism that includes the formation of binding complexes with Poly(C)-binding proteins, producing the 68 stabilization of TH mRNA, and/or increase of its association with polysomes, finally leading to increased translation (Tank et al., 2008). The only two previous studies addressing TH mRNA levels in schizophrenia reported opposite results. One of these studies (Ichinose et al., 1994) showed that, as in our study, TH mRNA levels in schizophrenia were unchanged compared to controls. A second study (Mueller et al., 2004) showed significantly elevated levels of TH mRNA in schizophrenia. Several factors can contribute to this difference in the results obtained, including differences in the cohort of patients as well as methodological differences. Animal study: Antipsychotic treatment effect on TH protein levels In our study, we also addressed the possibility that the observed changes in TH protein in the SN/VTA of schizophrenia patients could be an effect of antipsychotic drug treatments. Our results showed that chronic treatment with antipsychotic medication did not produce significant changes in TH protein expression in the SN/VTA of rodents. Supporting our findings, a previous study in rodents using a different atypical antipsychotic (clozapine) has shown similar results for the SN/VTA dopaminergic cells, with no changes in TH protein levels or TH immunolabeling (Tejedor-Real et al., 2003). In addition, it has been shown that chronic treatment with haloperidol does not produce changes in TH axonal density in the prefrontal and entorhinal cortex of monkeys, while lamina specific changes in TH axonal density are present in schizophrenia patients on antipsychotic medication (Akil et al., 1999, 2000). Two laboratories have reported changes in cell morphology and TH immunolabeling in the SN/VTA after chronic treatment with haloperidol (Levinson et al., 1998; Mazurek et al., 1998; Marchese et al., 69 2002). However, in these studies drugs were administered in very high doses. Mazurek and collaborators performed two studies using intraperitoneal injections of haloperidol once a day in doses ranging from 1 to 10/mg/kg/day (Levinson et al., 1998; Mazurek et al., 1998). The other group found changes in animals that were subcutaneously administered haloperidol twice a day at a dose of 1mg/kg (Marchese et al., 2002). The doses used by these investigators are between 10- and 100-fold the dose necessary to achieve receptor occupancies equivalent to therapeutic conditions (Kapur et al., 2003), which can lead to cellular toxicity. Finally, another chronic rodent study using haloperidol or combinations of haloperidol and quetiapine did not find any morphological anomalies in the dopaminergic neurons of the SN/VTA (Zhang et al., 2007). Second postmortem study: Regional expression of TH and phosphorylated TH Our second postmortem study addressed the regional expression of TH and pTH protein using two different types of samples: One set of cases contained the entire rostrocaudal extent of the SN/VTA, and another set contained only mid-caudal regions. Compared to controls, schizophrenia rostro-caudal samples presented significant differences in TH protein levels, while for pTH there was a trend towards significance. However, in the case of samples containing only mid-caudal regions there were no differences detected between schizophrenia and controls for TH or pTH protein levels. These results are especially interesting because there is a regional segregation on the projections from the SN/VTA complex to cortical and subcortical regions. The rostral to mid-regions of the SN/VTA complex contain the rostral part of the dorsal tier of the SNc and the adjacent VTA. Both of these neuronal groups project preferentially to the 70 prefrontal, entorhinal and piriform cortex, as well the ventral striatum and the amygdala (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004). The mid to caudal regions of the SN/VTA complex contain the remainder of the dorsal tier, as well as the entire ventral tier of the SNc that projects preferentially to the dorsal striatum (Fallon and Moore, 1978a,b; Fallon et al., 1978b; McRitchie et al., 1995; Haber and Fudge, 1997; Joel and Weiner, 2000; Halliday, 2004). Our findings indicate that the tyrosine hydroxylase anomalies are located preferentially (or exclusively) in the rostral areas of the SN/VTA complex. This finding suggests that TH protein anomalies could be preferentially located in the mesocortical and mesolimbic pathways. Supporting this, anomalies in TH immunolabeling have been found in specific layers of the prefrontal and entorhinal cortex in schizophrenia (Akil et al., 1999, 2000; Lewis and Gonzalez-Burgos, 2006). Although TH protein levels have not been assessed before, a previous study in a cohort of Japanese patients addressed TH enzymatic activity in the SN/VTA of schizophrenia compared to controls (Toru et al., 1988). TH enzymatic activity is linked with the portion of the TH protein that is active, which in our study we assessed by measuring the levels of pTH. In brain tissue, it has been shown that TH can be phosphorylated at several serine residues, including serine 19, 31, and 40, all located in the first 40 amino acids of the N-terminus (Haycock, 1990). Among these serine phosphorylation sites, serine 40 appears to be the most relevant for the activation of TH (Haycock, 1990; Nakashima et al., 2009), and the positively charged amino acids flanking this serine play an important role in the proper phosphorylation of this site 71 (Nakashima et al., 2009). This serine phosphorylation area is considered a modulatory region for the activity of the catalytic subunit of the TH protein, since phosphorylation in this area produces an enhancement of the activity of TH (Haycock, 1990; Nakashima et al., 2009). Our study of pTH levels in schizophrenia showed a trend toward a significant difference compared to controls in the samples containing the entire rostro-caudal extent of the SN/VTA, while no differences where observed for the samples containing midcaudal regions. Toru et al. (1988) in their work assessing TH enzymatic activity reported a significant increase in TH activity in the SN/VTA of schizophrenia samples. In our study, pTH levels presented a very heterogeneous profile: For the samples containing the entire rostro-caudal extent of the SN/VTA, pTH levels were lower or equal than in control samples, while for the cases containing only mid-caudal regions, pTH levels ranged from higher than controls to lower (see scatter plot graphs in Figure 3B). The discrepancy between our results and the previous study by Toru et al. (1988) may be due to several factors. This includes differences in the area selected for study (e.g. their dissection included only the substantia nigra, while we included in our study the substantia nigra and the VTA as a dopaminergic producing complex), differences in the cohort of patients, and the use of different techniques. Another interesting finding was that TH protein levels were very variable in schizophrenia, while they were rather constant in the control group, which suggests that the schizophrenia group has a large heterogeneity of TH expression anomalies. Based on previous studies concerning the regulation of TH expression, genetic studies of the TH gene in schizophrenia, and in our data, we hypothesize that different anomalies in the TH gene and modulatory elements in the translation of the protein may account for this 72 heterogeneity. A large number of genetic association studies for the TH gene have found very diverse results. The most consistent association was found for intron 1 of the TH gene (TH01 locus), an area that has been postulated as a modulatory element for TH transcription (Pae et al., 2003; Jacewicz et al., 2008). In the TH01 locus, certain alleles of a DNA microsatellite sequence repeat have been linked with a significant higher risk for schizophrenia. However, this association was found in some cohorts of patients (Meloni et al., 1995; Wei et al., 1995; Kurumaji et al., 2001; Pae et al., 2003; Jacewicz et al., 2008), but not in others (Burgert et al., 1998; Jonsson et al., 1998). In addition, TH protein synthesis is a highly regulated process, with epigenetic, transcriptional and posttranscriptional regulations (Lenartowski and Goc, 2011) that vary depending on the class of catecholaminergic neuron (i.e. dopaminergic versus adrenergic or noradrenergic; Tank et al., 2008). Our data strongly support that TH anomalies in schizophrenia occur at the posttranscriptional level, which may include anomalies in the stabilization of the mRNA, the proper attachment of mRNA to polysomes, and/or the efficacy of translation of the mRNA into protein. Conclusions of the study In summary, our study shows for the first time that an anomaly in TH protein synthesis is present in the SN/VTA in schizophrenia. Since this pathology is present in the protein, but not in the mRNA, it strongly suggests that the anomalies occur during posttranscriptional processes, but this will require further investigation to identify the exact processes that are affected. Finally, our study provides the first data indicating that within the SN/VTA, the anomalies in the synthesis of TH may be restricted to specific 73 dopaminergic neuronal populations, mostly located in the rostral regions of this complex. This suggests that anomalies in TH synthesis may be linked with pathologies in the mesocortical and/or mesolimbic dopamine pathways, but further studies will be needed to pinpoint the specific populations affected. Conflict of interest statement Dr. Robert R, Conley is currently a Distinguished Lilly Scholar at Eli Lilly and Co. However, his involvement in this work is independent from his current contractual relation with Eli Lilly and Co. None of the work reported here has been financially supported by Eli Lilly and Co., nor has this company had any role in the study design, in the collection, analysis and interpretation of data, writing of the report and submission of the paper. All the other authors have no conflict of interest to declare. Acknowledgements The authors wish to thank the staff of the Maryland Brain Collection, University of Maryland School of Medicine for their help to obtain the samples used in this study. We also wish to thank Dr. Xue-Min Gao for her assistance with the in situ hybridization experiments. This work was supported by the National Institutes of Health (USA), grant RO1MH066123 to Miguel Melendez-Ferro, Emma Perez-Costas, and Rosalinda C. Roberts. 74 References Abi-Dargham, A., Slifstein, M., Kegeles, L. and Laruelle, M. (2010) “Dopamine dysfuction in schizophrenia”, in Dopamine Handbook, eds. L.L. Iversen, S.D. Iversen, S.B. Dunnett, and A. Bjorklund (Oxford, UK: Oxford University Press), 511-519. Akil, M., Edgar, C.L., Pierri, J.N., Casali, S. and Lewis, D.A. (2000). Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol. Psychiatry 47, 361-370. Akil, M., Pierri, J.N., Whitehead, R.E., Edgar, C.L., Mohila, C., Sampson, A.R. and Lewis, D.A. (1999) .Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry 156, 1580-1589. Alterio, J., Mallet, J. and Biguet, N.F. (2001). Multiple complexes involved in tyrosine hydroxylase mRNA stability in rat adrenal medulla, after reserpine stimulation. Mol. Cel. Neurosci. 17, 179-189. Andreou, D., Saetre, P., Lundmark, P., Hansen, T., Timm, S., Melle, I., Djurovic, S., Andreassen, O.A., Werge, T., Hall, H., Agartz, I., Terenius, L., Jonsson, E.G. (2009). Tyrosine hydroxylase Val81Met polymorphism: lack of association with schizophrenia. Psychiatr. Genet.19, 273-274. Burgert, E., Crocq, M.A., Bausch, E., Macher, J.P. and Morris-Rosendahl, D.J. (1998). No association between the tyrosine hydroxylase microsatellite marker HUMTH01 and schizophrenia or bipolar I disorder. Psychiatr. Genet. 8 (2), 4548. Chao, H.M., and Richardson, M.A. (2002). .Aromatic amino acid hydroxylase genes and schizophrenia. Am. J. Med. Genet. 114, 626-630. Chen, X., Xu, L., Radcliffe, P., Sun, B. and Tank, A.W. (2008). Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Mol. Pharmacol. 73, 1816-1828. Fallon, J.H., Koziell, D.A. and Moore, R.Y. (1978a). Catecholamine innervation of the basal forebrain II: Amygdala, suprarhinal cortex and entorhinal cortex. J. Comp. Neurol. 180, 509-532. Fallon, J.H. and Moore, R.Y. (1978a). Catecholamine innervation of the basal forebrain III Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J. Comp. Neurol. 180, 533-544. 75 Fallon, J.H. and Moore, R.Y. (1978b). Catecholamine innervation of the basal forebrain IV: Topography of the dopamine projection to the basal forebrain and neostriatum. J. Comp. Neurol. 180, 545-580. Fallon, J.H., Riley, J.N. and Moore, R.Y. (1978b). Substantia nigra dopamine neurons: separate populations project to neostriatum and allocortex. Neurosci. Lett. 7, 157162. Faul, F., Erdfelder, E., Lang, A.G. and Buchner, A. (2007). G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175-191. Gao, X.M., Hashimoto, T. and Tamminga, C.A. (1998). Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 29 (1), 14-28. Gaspar, P., Stepniewska, I. and Kaas, J.H. (1992). Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J. Comp. Neurol. 325 (1), 1-21. Haber, S.N. and Fudge, J.L. (1997). The primate substantia nigra and VTA: integrative circuitry and function. Crit. Rev. Neurobiol. 11(4), 323-342. Haber, S.N. and Gdowski, M.J. (2004). “The basal ganglia”, in The Human Nervous System, eds. G. Paxinos and J.K. Mai (London, UK: Elsevier Academic Press), 676-738. Halliday, G. (2004). “Substantia nigra and locus coeruleus”, in The Human Nervous System, eds. G. Paxinos and J.K. Mai (London, UK: Elsevier Academic Press), 449-463. Haycock, J.W. (1990). Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J. Biol. Chem . 265 (20), 11682-11691. Hoogendoorn, M.L.C., Bakker, S.C., Schnack, H.G., Selten, J,P,C., Otten, H.G., Verduijn, W., van der Heijden, F.M., Pearson, P.L., Khan, R.S., Sinke, R.J. (2005). No association between 12 dopaminergic genes and schizophrenia in a large Dutch sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B (1), 6-9. Ichinose, H., Ohye, T., Fujita, K., Pantucek, F., Lange, K., Riederer, P. and Nagatsu, T. (1994) .Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J. Neural Transm. 8, 149-158. Ishiguro, H., Arinami, T., Saito, T., Akazawa, S., Enomoto, M., Mitushio, H., Fujishiro, H., Tada, K., Akimoto, Y, Mifune, H., Shiozuka, S., Hamaguchi, H., Toru, M., 76 Shibuya, H. (1998). Systematic search for variations in the tyrosine hydroxylase gene and their associations with schizophrenia, affective disorders, and alcoholism. Am J Med Genet 81, 388-396. Jacewicz, R., Galecki, P., Florkowski, A. and Berent, J. (2008). Association of the tyrosine hydroxylase gene polymorphism with schizophrenia in the population of central Poland. Psychiatr. Pol. 42 (4), 583-593. Joel, D. and Weiner, I. (2000). The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96 (3), 451-474. Jonsson, E., Brene, S., Geijer, T., Terenius, L., Tylec, A., Persson, M.L., Sedvall, G. (1996). A search for association between schizophrenia and dopamine-related alleles. Eur. Arch. Psychiatry Clin. Neurosci. 246, 297-304. Jonsson, E.G., Geijer, T., Gyllander, A., Terenius, L. and Sedvall, G.C. (1998). Failure to replicate an association between a rare allele of a tyrosine hydroxylase gene microsatellite and schizophrenia. Eur .Arch. Psychiatry Clin. Neurosci. 248, 6163. Kapur, S., Vanderspek, S.C., Brownlee, B.A. and Nobrega, J.N. (2003). Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J. Pharmacol. Exp. Ther. 305, 625-631. Kunugi, H., Kawada, Y., Hattori, M., Ueki, A., Otsuka, M., Nanko, S. (1998). Association study of structural mutations of the tyrosine hydroxylase gene with schizophrenia and Parkinson’s disease. Am J Med Genet 81, 131-133. Kurumaji, A., Kuroda, T., Yamada, K., Yoshikawa, T. and Toru, M. (2001). An association of the polymorphic repeat of tetranucleotide (TCAT) in the first intron of the human tyrosine hydroxylase gene with schizophrenia in a Japanese sample. J. Neural Transm. 108, 489-495. Lenartowski, R. and Goc, A. (2011). Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int. J. Dev. Neurosci. 29 (8), 873883. Levinson, A.J., Garside, S., Rosebush. P.I. and Mazurek, M.F. (1998). Haloperidol induces persistent down-regulation of tyrosine hydroxylase immunoreactivity in substantia nigra but no ventral tegmental area in the rat. Neuroscience 84 (1), 201-211. Lewis, D.A. and Gonzalez-Burgos, G. (2006). Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 12 (9), 1016-1022. 77 Marchese, G., Casu, M.A., Bartholini, F., Ruiu, S., Saba, P., Gessa, G.L. and Pani, L. (2002). Sub-chronic treatment with classical but not atypical antipsychotics produces morphological changes in rat nigro-striatal dopaminergic neurons directly related to “early onset” vacuous chewing. Eur. J. Neurosci. 15, 11871196. Mazurek, M.F., Savedia, S.M., Bobba, R.S., Garside, S. and Rosebush, P.I. (1998). Persistent loss of tyrosine hydroxylase immunoreactivity in the substantia nigra after neuroleptic withdrawal. J. Neurol. Neurosurg. Psychiatry 64, 799-801. McRitchie, D.A., Halliday, G.M. and Cartwright, H. (1995). Quantitative analysis of the variability of substantia nigra pigmented cell clusters in the human. Neuroscience 68 (2), 539-551. Meloni, R., Laurent, C., Campion, D., Hadjali, B.B., Thibaut, F., Dollfus, S., Petit, M., Samolyk, D., Martinez, M., Poirier, M.F. and Mallet, J. (1995). A rare allele of a microsatellite located in the tyrosine hydroxylase gene found in schizophrenic patients. C. R. Acad. Sci. Paris 318, 803-809. Mueller, H.T., Haroutunian, V., Davis, K.L. and Meador-Woodruff, J.H. (2004). Expression of the ionotropic glutamate receptor subunits and NMDA receptorassociated intracellular proteins in the substantia nigra in schizophrenia. Mol. Brain Res. 121, 60-69. Nakashima, A., Hayashi, N., Kaneko, Y.S., Mori, K., Sabban, E.L., Nagatsu, T. and Ota, A. (2009) .Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J. Neural Transm. 116, 1355-1362. Ota, M., Nakashima, A., Ikemoto, K., Nojima, S., Tanaka, M., Okuda, M., Koga, H., Mori, K., Kaneko, Y.S., Fujiwara, K., Yamamoto, H., Nagatsu, T., Ota, A. (2001). Exon 3 of tyrosine hydroxylase gene: lack of association with Japanese schizophrenic patients. Mol. Psychiatry 6, 315-319. Pae, C.U., Kim, J.J., Serretti, A., Lee, C.U., Lee, S.J., Lee, C. and Paik, I.H. (2003). VNTR polymorphism of tyrosine hydroxylase gene and schizophrenia in the Korean population. Neuropsychobiology 47 (3), 131-136. Paxinos, G. and Huang, X.F. (1995). Atlas of the human brainstem. San Diego: Academic Press. Perez-Costas, E., Guidetti, P., Melendez-Ferro, M., Kelley, J.J. and Roberts, R.C. (2008). Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J. Neural Transm. 115, 745-753. 78 Perez-Costas, E., Melendez-Ferro, M., Roberts, R.C. (2010). Basal ganglia pathology in schizophrenia: Dopamine connections and anomalies. J. Neurochem. 113(2), 287302. Porrino, L.J. and Goldman-Rakic, P.S. (1982). Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J. Comp. Neurol. 205 (1), 63-76. Roberts, R.C., Roche, J.K., Conley, R.R. and Lahti, A.C. (2009). Dopaminergic synapses in the caudate of subjects with schizophrenia: Relationship to treatment response. Synapse 63, 520-530. Spitzer, R.L., Williams, J.B., Gibbon, M. and First, M.B. (1992). The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Arch. Gen. Psychiatry 49 (8), 624-629. Stan, A.D., Ghose, S., Gao, X.M., Roberts, R.C., Lewis-Amezcua, K., Hatanpaa, K.J. and Tamminga, C. A. (2006). Human postmortem tissue: what quality markers matter? Brain Res. 1123 (1), 1-11. Talkowski, M.E., Kirov, G., Bamne, M., Georgieva, L., Torres, G., Mansour, H., Chowdari, K.W., Milanova, V., Wood, J., McClain, L., Prasad, K., Shirts, B., Zhang, J., O’Donovan, M.C., Owen, M.J., Devlin, B., Nimgaonkar, V.L. (2008). A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum. Mol. Genet. 17 (5), 747-758. Tank, A.W., Xu, L., Chen, X., Radcliffe, P. and Sterling, C.R. (2008). Posttranscriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann. N. Y. Acad. Sci. 1148, 238-248. Tejedor-Real, P., Biguet, N.F., Dumas, S. and Mallet J. (2003). Tyrosine hydroxylase mRNA and protein are down-regulated by chronic clozapine in both the mesocorticolimbic and the nigrostriatal systems. J. Neurosci. Res. 72, 105-115. Thibaut, F., Ribeyre, J.M., Dourmap, N., Meloni, R., Laurent, C., Campion, D., Menard, F.J., Dollfus, S., Mallet, J., Petit, M. (1997). Association of DNA polymorphism in the first intron of the tyrosine hydroxylase gene with disturbances of the catecholaminergic system in schizophrenia. Schizophr. Res. 23, 259-264. Toru, M., Watanabe, S., Shibuya, H., Nishikawa, T., Noda, K., Mitsushio, H., Ichikawa, H., Kurumaji, A., Takashima, M., Mataga, N. and Ogawa, A. (1988). Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr. Scand. 78 (2), 121-137. Tost, H., Hakimi, S. and Meyer-Lindenberg, A. (2010). “Dopamine dysfunction is schizophrenia: from genetic susceptibility to cognitive impairment”, in Dopamine 79 Handbook, eds. L.L. Iversen, S.D. Iversen, S.B. Dunnett and A. Bjorklund (Oxford, UK: Oxford University Press), 558-571. Wei, J., Ramchand, C.N. and Hemmings, G.P. (1995). Association of polymorphic VNTR region in the first intron of the human TH gene with disturbances of the catecholamine pathway in schizophrenia. Psychiatr. Genet . 5, 83-88. Wong, D.L. and Tank, A.W. (2007). Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress 10 (2), 121-130. Zhang, Y., Xu, H., He, J., Yang, D., Jiang, W., Li, X. and Li, X.M. (2007). Quetiapine reverses altered locomotor activity and tyrosine hydroxylase immunoreactivity in rat caudate putamen following long-term haloperidol treatment. Neurosci. Lett. 420, 66-71. 80 Table 1. Pilot study: demographic table and clinical data in schizophrenia and controls. Abbreviations: A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; C, Caucasian; COD, cause of death; DVT, deep vein thrombosis; F, female; M, male; MI, myocardial infarction; NOS, not otherwise specified; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; TD, tardive dyskinesia; un, unknown. 81 Table 2. Demographic table and clinical data second postmortem study. Abbreviations: A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; C, Caucasian; COD, cause of death; CUT, chronic undifferentiated type; DVT, deep vein thrombosis; F, female; M, male; MI, myocardial infarction; NOS, not otherwise specified; MVA, motor vehicle accident; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; TD, tardive dyskinesia; un, unknown. 82 Figure 1. Pilot study: TH mRNA and protein levels in schizophrenia and control cases. *P<0.05. (A) TH mRNA levels in control (n=5) and schizophrenia (n=6) cases. The top panel shows representative images of TH mRNA in situ hybridization in rostral (R), medial (M), and caudal (C) sections of the SN/VTA of a control case (C2), and a schizophrenia case (SZ2). Comparison of the mean values for TH mRNA OD is shown in 83 the bar graph (middle panel). Mean optical density (OD) values for TH mRNA levels in control and schizophrenia (SZ) cases did not differ significantly (P=0.12; OD mean±SD: control=0.187±0.027, schizophrenia=0.161±0.023). The bottom panel shows a scatter plot for the individual TH mRNA OD values. The horizontal bar in the scatter plot indicates the mean OD value for each group. Note the dense clustering of all the OD values for both groups. (B) TH protein levels in the same control and schizophrenia cases used for the mRNA study. The top panel shows representative images of the same western-blot membrane, first blotted for TH protein, and then reblotted for actin. This membrane contained all the control (C1-C5) and schizophrenia (SZ1-SZ6) cases used in this pilot study. Note the conspicuous difference in blot signal among the different samples in the TH western-blot image, while actin expression is uniform among all cases. The comparison of the means for TH protein levels in controls and schizophrenia is shown in the mid panel (bar graph). OD values for TH protein were significantly different (*) between the two groups (P=0.031; OD mean±SD: control= 0.912±0.046, schizophrenia= 0.433±0.393). The bottom panel shows a scatter plot for the individual TH protein OD values. The horizontal bar in the scatter plot indicates the mean OD value for each group. Note the dense clustering of the OD values for the control group, while in the schizophrenia group there is a large scattering of the data. However, note that in the lower TH expression cluster, two cases presented the same exact TH OD value and are represented in the scatter plot as a single circle. 84 Figure 2. Animal study: antipsychotic treatment effect on TH protein levels. (A) Western-blot images of TH protein for all the animals of the three treatment groups. All the samples (n=9 per treatment group) were incubated and developed together on the same film to accurately measure OD. Note the homogeneity of expression of TH for all cases independently of the treatment group. (B) The same western-blot membrane shown in A was reblotted for the detection of actin protein. (C) Comparison of the mean OD for TH protein levels showed no significant differences among the three groups (P=0.41; OD mean±SD: control=0.824±0.181, haloperidol=0.917±0.049, olanzapine=0.973±0.136). (D) Scatter plot graph showing the individual TH protein OD values for the 3 groups. The horizontal bar in the scatter plot indicates the mean OD value for each group. 85 Figure 3. Second postmortem study: regional distribution of TH and pTH. *P<0.05; # P>0.05<0.06. (A) The top panel shows images of 2 western-blots for TH that were developed on the same film, containing all the cases used in this study. Note that rostrocaudal and mid-caudal cases were run together in the same gels. In addition, reblots for actin of the same membranes are shown immediately below. The graph bar (mid panel) shows the comparison of the mean OD values for TH protein in rostro-caudal and midcaudal samples for controls and schizophrenia. Only the rostro-caudal samples showed 86 significant differences (*) between schizophrenia and control cases (P=0.036; OD mean±SD: control rostro-caudal=0.749±0.044, schizophrenia rostro-caudal=0.513±0.252; control mid-caudal=0.743±0.107, schizophrenia mid-caudal=0.712±0.261). The bottom panel shows a scatter plot for the individual TH OD data. The horizontal bar in the scatter plot indicates the mean OD value for each group. (B) The same membranes in A were reblotted for the detection of pTH (top panel). In addition, reblots for actin of the same membranes are shown immediately below. The graph bar in the mid panel shows the comparison of the mean OD values for pTH in rostro-caudal and mid-caudal samples. Even when there were no significant differences in pTH OD values between controls and schizophrenia in rostro-caudal samples, a trend (#) towards significance was observed (P=0.056; OD mean± SD: control rostro-caudal=0.405±0.120, schizophrenia rostrocaudal=0.203±0.208; control mid-caudal=0.421±0.168, schizophrenia midcaudal=0.419±0.246). For comparison, the dashed line on the top of each of the individual bars indicates the mean level of total TH for each group. The ratio of pTH versus total TH for each groups was as follows: control rostro-caudal=54.07%, schizophrenia rostro-caudal=39.57%; control mid-caudal=56.66%, schizophrenia midcaudal=58.85%. The bottom panel shows a scatter plot for the individual pTH OD data. The horizontal bar in the scatter plot indicates the mean OD value for each group. 87 AN ACCURATE METHOD FOR THE QUANTIFICATION OF CYTOCHROME C OXIDASE IN TISSUE SECTIONS by Miguel Melendez-Ferro, Matthew W. Rice, Rosalinda C. Roberts, & Emma Perez-Costas Journal of Neuroscience Methods Copyright 2013 by Elsevier Used with permission Format adapted and errata corrected for dissertation 88 Abstract Cytochrome oxidase (COX) is the enzyme that constitutes the last step of the mitochondrial electron transport chain for the production of ATP. Measurement of COX activity can be achieved by histochemistry, thus providing information about the metabolic status of the brain. Brain regions with high metabolism will present high COX activity in histochemistry assays and vice versa. Using histochemistry versus biochemistry to assess COX activity presents the advantage of providing a map of the differences in metabolism in discrete brain regions. Moreover, COX histochemistry allows quantifying the activity of a particular brain region, by converting units of optical density into units of activity. In the present work we have devised a methodology that allows not only quantifying differences in COX activity between groups, but also quantifying the amount of COX present in brain tissue sections, by directly relating optical density (OD) measurements to cytochrome C oxidase concentration, something that traditionally is achieved by the use of western blot. For this purpose we created a set of standards of known concentration of COX that were affixed to a nitrocellulose membrane, and this membrane was incubated together with the tissue sections in which COX activity was assessed. A standard curve was created using a gradient of different concentrations of purified bovine heart cytochrome oxidase (from 2 micrograms to 0.1 micrograms in intervals of 0.25 micrograms). This standard curve allowed us to detect changes in optical density as low as 5%, and relate these OD differences with known concentrations of cytochrome C oxidase. Keywords: Optical density; histochemistry; human brain; substantia nigra; ATP; electron transport chain; image analysis 89 Introduction Metabolism has the ultimate goal of producing the energy necessary to maintain all the body functions, and this energy is stored in the form of ATP. The brain is one of the most metabolically demanding organs, requiring high levels of ATP production to maintain its functions, and different brain regions utilize varying amounts of ATP depending on their metabolic status (Hevner and Wong-Riley, 1991). Within the same brain region these differences in metabolic activity can be found at the cellular and subcellular levels (Hevner and Wong-Riley, 1989; 1991; Wong-Riley, 2012). ATP is synthesized in mitochondria by oxidative phosphorylation, a process that takes place at the inner mitochondrial membrane with participation of the electron transport chain. The electron transport chain is composed of a series of enzymes or complexes, of which cytochrome C oxidase (COX or Complex IV) is the terminal enzyme. Cytochrome C oxidase catalyzes the oxidation of its substrate cytochrome C, and the transfer of electrons for the reduction of molecular oxygen. This ultimately produces the electrochemical proton gradient that culminates in the production of ATP, by pumping protons from the matrix to the cytosolic side of the inner mitochondrial membrane (see as a review Wong-Riley, 2012). Measurement of the metabolic activity of a particular brain region can be reliably achieved by measuring COX activity by histochemistry. Metabolically active brain regions (e.g. with high firing rates) show increased COX histochemistry ratios compared with other regions in which neuronal activity is lower (Hevner and Wong-Riley, 1989; 1991). The advantage of COX histochemistry is that it can provide an accurate mapping 90 of the differences in metabolism in a certain brain region, something that cannot be achieved by the biochemical measurement of COX (see Hevner et al., 1993). The histochemical assay for the detection of COX activity in tissue has been used for decades, however very variable methods have been applied for the conversion of optical density measurements to enzyme amount or to enzymatic activity. When standards have been used, the most commonly reported technique to create these consisted of the preparation of homogenates from tissue that was known to contain a high concentration of COX (i.e. heart tissue; Hess and Pope, 1953), or homogenates from tissue of the same brain area to be studied (Gonzalez-Lima and Garrosa, 1991; Gonzalez-Lima and Jones, 1994). These homogenates are shaped in blocks and sliced using various methodologies. Then, these standards are used to directly compare tissue samples, or their OD values are compared to spectrophotometric measurements of COX activity for the same standards, so they can be transformed into “units of activity” (Gonzalez-Lima and Cada, 1994; Gonzalez-Lima and Jones, 1994). However, none of these methods is able to provide exact measurements of COX expression in the tissue sample, since the concentration of COX in the standards is unknown. In the present study we show a technique that allows obtaining optical density (OD) measurements of COX histochemistry, and directly convert those to measurements of COX expression in tissue, to then compare COX activity among samples. Our technique has enough resolution to accurately detect changes in COX as low as 5% between samples, in the lower end of the scale. In addition, we show how to analyze the optical data measurements and convert directly to micrograms of COX present in the tissue using NIH Image J software. 91 Materials and Methods Brain tissue samples We chose the human substantia nigra/ventral tegmental area (SN/VTA) to test this technique due to our long-term interest in studying SN/VTA pathology in neuropsychiatric and neurological disorders (Perez-Costas et al., 2010, 2012). However, this technique would be equally useful to assess COX expression and activity in other brain areas. Postmortem human SN/VTA tissue samples (University of Alabama at Birmingham, IRB protocol # N110505002) were obtained from the Alabama Brain Collection (ABC, University of Alabama at Birmingham). These samples were from a 69 year old Caucasian male without any neuropsychiatric or neurological disorder (i.e. control case), with a brain pH of 6.10, and a postmortem interval of 15 hours. The brainstem containing the SN/VTA was dissected immediately upon reception of the brain and frozen in dry ice and stored in a -800C freezer. The frozen block containing the entire rostro-caudal extent of the SN/VTA was sectioned coronally in five parallel series on a cryostat obtaining 14 micron-thick sections. Four of the series were collected on charged slides, while the fifth series was collected in a vial for protein determination. All the series were preserved at -800C until use. The first series was stained with thionin (Nissl stain) to assess proper morphological preservation. Using this series for landmark determination, several slides containing the SN/VTA from a parallel series were randomly chosen for COX histochemistry. 92 Preparation of standards Cytochrome C oxidase from bovine heart (Sigma-Aldrich, St. Louis, MO, catalog #C5499) was used to create the standards. The concentration of COX in the vial was 5 mg/ml, and this purified COX had 20 units of activity per mg of enzyme. To prepare the standards it was necessary to determine the highest reasonable concentration of COX to be expected in brain tissue. This was necessary to create an array of standards spanning the high and low ends of COX expression in the brain. We used some assumptions to do this calculation: Since COX is a very abundant protein in brain tissue we assumed that it could represent at least 1% of the total protein content in the brain. Then, we used series #5 to calculate total protein concentration in this brain area, using a spectrophotometer (Bio-Rad SmartSpec Plus, Bio-Rad, Hercules, CA) and the DC protein assay (Bio-Rad, catalog #500-0113 and #500-0114). Since we knew the number of sections collected per series, we were able to estimate the total protein concentration in a single 14 micron-thick section of the SN/VTA, dividing the total protein concentration of series #5 (13,970µg) by the number of sections contained in a series (n=127). Our calculations yielded a total protein concentration of 110µg per section. Using our assumption that COX could represent as much as 1% of the total protein content in the tissue, we estimated that each SN/VTA section could contain 1.1 µg of COX. Since we wanted to guarantee that none of the COX present in the brain could be out of range of our standards, we set the highest concentration of our standards as 2 µg of pure COX. From this highest concentration we created an array of 16 standards (8 of them ranging from 2µg to 0.25µg in decreasing steps of 0.25µg, and 8 additional standards ranging from 0.1µg to 0.03µg, in decreasing steps of 0.01µg). 93 Once the concentrations of pure COX to be used as standards were determined, the next step was to design a methodology that would allow us to reliably obtain optical density measurements of known concentrations of pure COX. To achieve this objective, we used a dot-blot apparatus (Bio-Dot SF microfiltration apparatus, Bio-Rad, catalog #170-6542) to affix the desired concentrations of pure COX to a nitrocellulose membrane. Table 1 presents the concentrations of COX that were used to create the standards in the nitrocellulose membranes. To create the standards, two stock solutions of bovine heart COX were prepared in Tris-Buffered saline (TBS, Bio-Rad, catalog #170-6435). A 1:500 dilution stock (0.01µg/µl of COX) was prepared to create the 2.0 to 0.10µg standards. A second dilution (1:10,000; 0.0005µg/µl of COX) was prepared to create the 0.09 to 0.03µg standards. From these stock solutions the appropriate volumes were taken to create the standards by further diluting the stocks in TBS (Table 1). Following the instructions on the manufacturer’s manual, the dot-blot apparatus was assembled using the optional slot-blot insert. This allowed applying the samples in rectangular wells instead of the traditional round-shaped wells. Within the apparatus, filter paper (Bio-Dot SF, Bio-Rad, catalog #162-0161) and the nitrocellulose membrane (Bio-Rad, catalog #162-0117) were sandwiched between the top and the bottom parts of the dot-blot apparatus. After proper assemblage, the system was connected to an in-house vacuum system using a vacuum trap. The nitrocellulose membrane was rinsed 2 times using 100µl of TBS per well. For each rinse vacuum was applied to force the TBS through the membrane. Then, 200µl per well of freshly prepared dilutions of the pure COX in TBS (table 1) were applied (from most concentrated to most diluted) to two rows 94 of the dot-blot apparatus. The unused wells were filled with 200µl of TBS. To affix the COX complex to the membrane, vacuum was applied, and immediately after this, 2 consecutive rinses with TBS were applied using the same vacuum method. These steps allowed the immobilization of COX complex into the nitrocellulose membrane and thus, obtaining an easy to handle and durable support for the standards. Care was taken to uniformly load the wells of the dot-blot apparatus to avoid within-well side-to-side gradients of COX. After this process was completed, the membrane was placed in TBS to be immediately incubated for COX histochemistry together with the sections to be tested for COX activity. Preparation of negative controls These controls serve the purpose of monitoring the specificity of the COX reaction by inactivating the activity of the enzyme prior to the histochemical reaction using sodium azide as the inactivating agent (Seligman et al, 1968). Negative controls were prepared by randomly selecting some of the SN/VTA sections from the same case and pretreating them to inactivate the COX enzyme present in the tissue. The control sections were removed from the -800C freezer and immediately fan-dried for 25 minutes at room temperature (RT). Sections were immersed in 4% paraformaldehyde in 0.1M phosphate buffer (PB) pH 7.4 for 60 minutes at RT. After five rinses in PB (5 minute each), the slides were incubated in a 10mM sodium azide solution in PB for 17 hours at RT to inactivate COX. After this, sections were rinsed in PB (5 times, 5 minute each) and they were ready to be incubated together with the standards and the sections to be assayed for COX activity. 95 COX histochemistry assay We used a previously published COX histochemistry assay that produces reliable and consistent results in frozen brain samples (Divac et al., 1995; Poeggeler et al., 1998). Prior to starting the COX assay, SN/VTA sections were removed from the -800C freezer and fan-dried as described for the negative controls (see above). After that, these sections, together with the negative controls and COX standards were placed in the same cuvette for incubation. The incubation medium contained the following components: 22.4 mg cytochrome C (Sigma-Aldrich, catalog #C3131), 115.23 mg diaminobenzidine (DAB, Sigma-Aldrich, catalog #D5637), 4.5 g sucrose (Sigma-Aldrich, catalog #S0389), 12.51 ml of a 1% nickel ammonium sulfate hexahydrate (Aldrich, catalog #A1827) solution, all mixed in 100 ml of 0.1M Hepes buffer (Sigma-Aldrich, catalog #H3375), pH 7.4. This solution was prepared in an aluminum foil-covered beaker under constant stirring adding the cytochrome C, DAB and the sucrose to the Hepes buffer first, and then adding the nickel ammonium sulfate solution dropwise under constant stirring. The sections and the standards were incubated with this solution for 2 hours in an incubator at 370C in the dark. The COX enzymatic reaction was terminated by immersing the sections and standards in 4% paraformaldehyde in PB for 1 hour at RT. After that, sections and standards were rinsed in PB (4 times, 5 minute each). Sections were then dehydrated in ethanol, cleared in xylene and coverslipped with Eukitt (Electron Microscopy Sciences, catalog #15322). The nitrocellulose membrane containing the standards was rinsed in distilled water and dried at RT between two sheets of filter paper. 96 Image acquisition and analysis Images from SN/VTA sections and standards were acquired using a flatbed scanner at a resolution of 600 dpi and 16 bits grayscale. For optical density analysis all images were saved as uncompressed TIFF files, and then imported into NIH Image J software version 1.46r (http://rsbweb.nih.gov/ij/). In Image J, prior to measuring optical density (OD), background was subtracted for all images first (process>subtract background), using a “rolling ball radius” of 25 pixels for light background. After this, images were inverted to negative (edit>invert) in order to obtain OD values. A standard curve was created by measuring the OD values for the standards in Image J and then inputting the known values of COX protein concentration in micrograms (table 1) for each standard. To obtain the OD values for each standard, a rectangular selection tool was used to define the “region of interest” (ROI). Once all the OD values for the standards were obtained, Image J calibration tool (image>measure>calibrate) was used to input manually the COX microgram values for each standard versus their OD values. The appropriate type of function (equation) that relates OD versus COX micrograms was selected (i.e. exponential), and the standard curve was plotted, bringing the standard curve graph and the goodness of fit of the curve into view. Once the calibration curve was created, images for analysis were opened in image J, and OD values for ROIs were measured. Rectangular ROIs were used to select several areas within the section, and using the measurement tools (analyze>tools>ROI manager, and then, analyze>measure), OD values were measured and immediately converted by the software to micrograms of COX. Afterwards, micrograms of COX were converted manually to COX activity units, using the information provided by the 97 manufacturer regarding the COX activity units present in the COX used for our standards (i.e. 20 activity units per microgram of COX). Statistical analysis Graphpad Prism 5.04 software (Graphpad Inc., San Diego, CA, USA) was used to perform a regression analysis of optical density values versus COX concentration loaded for the standards. A paired t-test was also performed for the analysis of the interpolated values of COX amount obtained with Image J. Results and Discussion In the present work we tested a set of 16 different concentrations of pure COX for the creation of our standards (from 2 µg as the highest value to 0.03 µg as the lowest, see Table 1). Our tests showed that in the low end of the curve, the limit of resolution for reliable measurement of COX optical density values was 0.1 µg of COX, since below this value the measurement of OD levels using Image J software became unreliable for reproducibility. Thus, a set of 9 different COX concentrations ranging from 2 to 0.1 µg was the optimal range to build our standard curve to assess COX expression in brain tissue sections (Figure 1), which was enough to cover the whole range of COX amount present in our brain tissue sections. Traditionally, COX standards were created using brain homogenates sectioned at different thicknesses that were incubated in the same conditions as the brain sections to be analyzed, thus allowing a quantitative analysis of COX activity (Gonzalez-Lima and Cada, 1994; Gonzalez-Lima and Jones, 1994). Since sample preparation can significantly influence enzymatic activity of COX (Hevner et al., 98 1993) the use of pure COX enzyme that is affixed to an inert matrix such as nitrocellulose (thus requiring a minimal amount of manipulation) can eliminate those issues. However, in our method it is important to avoid gradients in the distribution of COX within the well when creating the standards. The uniformity in the amount of COX in the well allows the use of different voxel sizes for the measurement of COX amount in the standards. In our case, the use of different voxel size within the same sample gave identical results. Our COX histochemical experiments show that the concentration of our standards and their corresponding OD values fit well a linear relation (R2 value=0.929 Figure 2A). Since the amount of histochemical reaction for COX is mainly dependent on the amount of enzyme (Hevner and Wong-Riley, 1989), a linear relation is usually predicted for the histochemical reaction of standards, as it occurs for the spectrophotometric measurement of COX activity (Hevner et al., 1993). However, with our experimental conditions, and for the range of COX enzyme contained in our standards, the mathematical analysis of our results reveals that the predicted relation between the amount of COX in the standards and their OD values also fits an exponential curve as revealed by the R2 value (see figures 2B and 3). For the range of our standards, an analysis of the interpolated values of COX amount obtained from the two curves using Image J revealed no significant differences (paired t-test assuming equal variances; n=27 values per curve; P=0.3516, df=26). For the two curves, the interpolated data were normally distributed, as revealed by the Kolmogorov-Smirnov normality test (KS=0.15, P>0.10). Finally, a point by point assessment of the interpolated values revealed a high correlation between the two curves (r=0.9662, P<0.0001). Differences in turnover of individual enzymes may account for the exponential COX reaction observed for our standards in our experimental 99 conditions. Total COX activity is equal to the product of COX amount and COX turnover (Hevner and Wong-Riley, 1989). Although a minor role for enzyme turnover has been postulated (Hevner and Wong-Riley, 1989), this cannot be discarded when we consider a reaction taking place in a biological material (see the differences between ROI #5 and ROI #8 in figure 4). Several agents have been shown to inhibit COX activity. Among these are chemical fixatives (Chalmers and Edgerton, 1989), as well as sodium azide (Seligman et al., 1968). In our experiments, we used a combination of both methods to ensure a complete abolition of COX activity in our controls (see above). Our negative controls performed this way showed no residual activity of COX in sections of the SN/VTA (not shown). The use of COX OD measurements together with standards created using known amounts of enzyme allows the quantification of the amount of enzyme present in brain sections (Figure 4). Our methodology allows for the detection of differences as low as 5% in COX concentration, and hence activity, in the lower end of the standard scale. This is important because subtle differences in COX amount in different subregions can thus be quantified, since there is a close relation between COX OD measured by histochemistry in sections, and the amount of enzyme present in those sections (Hevner and Wong-Riley, 1989). As it can be seen in figure 4, different ROIs within the SN/VTA show different amounts of COX in micrograms (compare the value of micrograms of COX for adjacent ROIs # 4 and 5, with ROI #8 located in the cerebellar peduncle), which indicates the existence of differences in the metabolic status of different subnuclei and between different regions (Hevner and Wong-Riley, 1989). The fact that these 100 differences can be measured with our methodology allows sampling in brain subregions to detect differences in COX activity that otherwise would be masked by the use of biochemical assays for its measurement (Hevner et al., 1993). In addition, with our methodology the amount of COX present in the tissue is easily converted to units of activity using the information provided by the manufacturer, and does not require the use of any biochemical assay. Our methodology employs metal enhancement to increase the contrast between gray and white matter in the histochemical reaction, something that is not achieved using DAB alone (Divac et al., 1995). The use of metal enhancement can negatively affect the COX reaction by making regions with low amount of COX appear stained qualitatively equal to regions with high COX activity, due to the saturation of the system (GonzalezLima and Cada, 1994; Gonzalez-Lima and Jones, 1994). Our quantitative analysis shows that this is not the case for our samples, since different ROIs within the same section present different amounts of COX OD (compare ROI #5 and ROI #4 with ROI #8; Figure 4), which allows quantifying COX amount and activity in different subregions of the SN/VTA. Quantification of proteins is important, since the expression of a particular protein in the brain can reflect a pathological status in a disease (see e.g. Perez-Costas et al., 2012). Proteins are usually quantified by their immunological detection in western blot techniques. However, western blots can mask the subtle differences in protein expression in a particular neuronal subpopulation in a brain region, and at the same time does not provide the anatomical distribution of that protein in the tissue. The use of a method that can give an accurate measurement of protein quantity and activity and at the same time 101 provides a map of the expression of that protein is desirable, especially if regional differences are expected or observed (Hevner and Wong-Riley, 1993). That said, there is a series of assumptions that have to be taken into account when quantifying OD with histochemistry for COX. This type of analysis works with the assumption that the entire COX present in the tissue is functional, thus the differences observed by histochemistry are based on amount of enzyme. In the case of a structural deficiency of COX that impairs the functioning of the complex, histochemistry will underestimate the amount of COX present in the tissue, and it will only determine the amount of functional COX present based on its ability to produce a reaction product that is measurable by OD. Another important parameter to take into account when quantifying enzymatic activity is the fact that the enzyme has to be active for the reaction to take place. The method of preservation of the tissue has to ensure that the full activity of the enzyme is kept. Our samples were all fresh-frozen human postmortem brain samples, not subjected to chemical fixation. Chemical fixation has been shown to diminish the enzymatic activity of COX at various degrees and a reduction of up to 10.5% has been reported, with some neurons being more affected than others (Chalmers and Edgerton, 1989). Although some authors have described a small decrease of COX activity after chemical fixation of the sections (Gonzalez-Lima and Jones, 1994), this small reduction in activity can mask subtle differences in COX activity within and between samples, especially if differential effects are produced. Fixation is usually utilized to avoid detachment of sections from slides through the histochemical procedure (Gonzalez-Lima and Jones, 1994). In our case, our samples were all frozen, and no detachment of sections from the slides occurred. In our protocol fixation was only used as the last step of the 102 histochemical reaction to stop the enzymatic activity of COX. Moreover, freezing of the samples has been shown not to have an effect in COX activity (Hevner at al., 1993). A variety of other methods have been used to assess cell metabolism. These include studying the expression of mRNA levels for specific subunits of the COX enzyme (Vila et al., 1997; Perier et al., 2000; Bacci et al., 2002; Rolland et al., 2007), assessing the activity of the enzyme succinate dehydrogenase that participates both in the electron transport chain and in the tricarboxylic acid cycle (Bertoni-Freddari et al., 2001; Bubber et al., 2011), the measurement of 2-deoxyglucose levels (see e.g. Porrino et al., 1987; Mitchell et al., 1989, 1992), and the analysis of c-fos expression by immunohistochemistry (see e.g. Joh and Weiser, 1993; Schulte et al., 2006; Reyes and Mitrofanis, 2008). Among these methods, the measurement of COX subunit I mRNA levels has been shown to be a very reliable marker that correlates well with cytoplasmic COX activity, although it requires the use of radioactive mRNA probes and dedicated laboratory space. Succinate dehydrogenase assays provide a good measurement of general metabolic status, but do not assess specifically the health of the electron transport chain. On the other hand, 2-deoxyglucose measurements have been used reliably to measure short term changes in metabolism, but COX measurements are more reliable for the assessment of long term metabolic changes (Vila et al., 1997). Finally, c-fos immunohistochemistry has been used to assess neuronal activity. However, the use of cfos as a metabolic marker presents several problems (Dragunow and Faull, 1989), and is only reliable for the study of acute metabolic changes (Hoffman et al., 1993; Hoffman and Lyo, 2002). 103 Conclusions Here we have devised a method to accurately measure the amount of COX enzyme present in brain tissue sections, using standards of known COX concentration. The amount of COX can be easily converted into activity units without the need of complex biochemical assays. Our methodology can detect differences as low as 5% in COX amount. This is useful to analyze and compare the relative abundance of COX between different regions within a brain, or between subregions within a brain region. Acknowledgments The authors wish to thank the Alabama Brain Collection (University of Alabama at Birmingham) for providing the human brain tissue used in this work. This work was supported by NIMH RO1 grant MH066123 to MMF, EPC and RCR. 104 References Bacci JJ, Kerkerian-Le Goff L, Salin P. Effects of intralaminar thalamic nuclei lesion on glutamic acid decarboxylase (GAD65 and GAD67) and cytochrome oxidase subunit I mRNA expression in the basal ganglia of the rat. Eur. J. Neurosci., 2002;15:1918-28. Bertoni-Freddari C, Fattoretti P, Casoli T, Di Stefano G, Solazzi M, Gracciotti N, Pompei P. Mapping of mitochondrial metabolic competence by cytochrome oxidase and succinic dehydrogenase cytochemistry. J. Histochem. Cytochem., 2001;49:11912. Bubber P, Hartounian V, Gibson GE, Blass JP. Abnormalities in the tricarboxylic acid (TCA) cycle in the brains of schizophrenia patients. Eur. Neuropsychopharmacol., 2011;21:254-60. Chalmers GR, Edgerton VR. Marked and variable inhibition by chemical fixation of cytochrome oxidase and succinate dehydrogenase in single motoneurons. J. Histochem. Cytochem., 1989;37:899-901. Divac I, Mojsilovic-Petrovic J, Lopez-Figueroa MO, Petrovic-Minic B, Moller M. Improved contrast in histochemical detection of cytochrome oxidase: metallic ions protocol. J. Neurosci. Methods, 1995;56:105-13. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods, 1989;29:261-5. Gonzalez-Lima F, Cada A. Cytochrome oxidase activity in the auditory system of the mouse: a qualitative and quantitative histochemical study. Neuroscience, 1994;63:559-78. Gonzalez-Lima F, Jones D. Quantitative mapping of cytochrome oxidase activity in the central auditory system of the gerbil: a study with calibrated activity standards and metal-intensified histochemistry. Brain Res., 1994;660:34-49. Hess HH, Pope A. Ultramicrospectrophotometric determination of cytochrome oxidase for quantitative histochemistry. J. Biol. Chem., 1953;204:295-306. Hevner RF, Liu S, Wong-Riley MTT. An optimized method for determining cytochrome oxidase activity in brain tissue homogenates. J. Neurosci. Methods, 1993;50:30919. Hevner RF, Wong-Riley MTT. Brain cytochrome oxidase: purification, antibody production, and immunohistochemical/histochemical correlations in the CNS. J. Neurosci., 1989;9:3884-98. 105 Hevner RF, Wong-Riley MTT. Neuronal expression of nuclear and mitochondrial genes for cytochrome oxidase (CO) subunits analyzed by in situ hybridization: comparison with CO activity and protein. J. Neurosci., 1991;11:1942-58. Hevner RF, Wong-Riley MTT. An optimized method for determining cytochrome oxidase activity in brain tissue homogenates. J. Neurosci. Methods, 1993;50:30919. Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all 'fos-ed out'? J. Neuroendocrinol., 2002;14:259-68. Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol., 1993;14:173-213. Joh TH, Weiser M. Early and late molecular events occurring following neuronal degeneration in the dopamine system. Adv. Neurol., 1993;60:316-20. Mitchell IJ, Boyce S, Sambrook MA, Crossman AR. A 2-deoxyglucose study of the effects of dopamine agonists on the parkinsonian primate brain. Implications for the neural mechanisms that mediate dopamine agonist-induced dyskinesia. Brain, 1992;115:809-24. Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6tetrahydropyridine. Neuroscience, 1989;32:213-26. Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front. Psychiatry 3:31. doi:10.3389/fpsyt.2012.00031. Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J. Neurochem., 2010;113:287-302. Perier C, Vila M, Feger J, Agid Y, Hirsch EC. Functional activity of zona incerta neurons is altered after nigrostriatal denervation in hemiparkinsonian rats. Exp. Neurol., 2000;162:215-24. Poeggeler B, Rassoulpour A, Guidetti P, Wu HQ, Schwarcz R. Dopaminergic control of kynurenate levels and N-Methyl-D-Aspartate toxicity in the developing rat striatum. Dev. Neurosci., 1998;20:146-53. 106 Porrino LJ, Burns RS, Crane AM, Palombo E, Kopin IJ, Sokoloff L. Local cerebral metabolic effects of L-dopa therapy in 1-methyl-4-phenyl-1,2,3,6tetrahydropyridine-induced parkinsonism in monkeys. Proc. Natl. Acad. Sci. USA, 1987;84:5995-9. Reyes S, Mitrofanis J. Patterns of FOS expression in the spinal cord and periaqueductal grey matter of 6OHDA-lesioned rats. Int. J. Neurosci., 2008;118:1053-79. Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, François C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain, 2007;130:265-75. Schulte T, Brecht S, Herdegen T, Illert M, Mehdorn HM, Hamel W. Induction of immediate early gene expression by high-frequency stimulation of the subthalamic nucleus in rats. Neuroscience, 2006;138:1377-85. Seligman AM, Karnowsky MJ, Wasserkrug HL, Hanker JS. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J. Cell Biol., 1968;38:1-14. Vila M, Levy R, Herrero MT, Ruberg M, Faucheux B, Obeso JA, Agid Y, Hirsch EC. Consequences of nigrostriatal denervation on the functioning of the basal ganglia in human and nonhuman primates: an in situ hybridization study of cytochrome oxidase subunit I mRNA. J. Neurosci., 1997;17:765-73. Wong-Riley MTT. Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. In Kadenbach B, editor. Mitochondrial oxidative phosphorylation, Advances in Experimental Medicine and Biology. Springer: New York, 2012;748:283-304. 107 Table 1. Final concentrations of pure COX in standards and detailed chart for their preparation. 108 Figure 1. Preparation of standards and Image J. After opening Image J (A), the scanned image of the nitrocellulose membrane containing the COX standards is imported into the software, and background is subtracted. B) Scanned image of the nitrocellulose membrane after background subtraction. The numbers indicate the amount of pure COX loaded for each standard, in micrograms. C) Image of the standards after it has been inverted in Image J, ready to be sampled. Note that bands of 0.09 to 0.03 micrograms of COX are also visible, but they are not reliably detected by the software. D) The ROI manager records the location of each measurement within the image, thus allowing having all the ROIs visible in the image. 109 Figure 2. Relation between micrograms of COX, optical density and units of activity. In our experimental conditions and for the values of the standards, the analysis of the best fit equation for the relation between micrograms of COX loaded in the standards and OD values (as a surrogate of the reaction product) showed that both a linear (A) and an exponential (B) curve provided a good fit for the data, as shown by the R2 values. For both graphs, the equivalence of COX micrograms to COX activity units is shown in the right Y axis. 110 Figure 3. Creation of a standard curve in Image J. A) OD measurements are obtained for each standard (see box). B) Using the calibration tools of Image J, the appropriate function (equation) is selected (i.e. exponential). The OD values for each standard obtained in A are inputted in the table together with the amount of COX that was loaded for each standard, in micrograms. C) The curve is then plotted and ready to start measuring COX activity in the brain tissue samples. 111 Figure 4. Measurement of COX activity in brain sections. A) A digitized image of COX histochemistry in a section through the human brainstem containing the SN/VTA and the red nucleus (RN) is opened in Image J. This image has already been processed for background subtraction and inverted. A total of eight ROIs were randomly selected, 1-5 in the SN/VTA, 6 and 7 in the RN, and 8 in the cerebellar peduncle (CP), a tissue area that presented little to no COX activity. B) The ROI manager records the location of each ROI selected for measurement. For informative purposes we have noted the number of the ROI next to each location. C) The results of the measurements of OD in the 8 ROIs are converted directly to micrograms of COX (boxed area). Note the big difference in COX amount in micrograms between ROI #5 and ROI #8, calculated by Image J. This can also be inferred qualitatively by the difference in “brightness” between those two areas. Other data in the results include the area of the ROIs, maximum and minimum pixel intensity, and integrated density (IntDen), which is the result of multiplying the area by the mean intensity. D: dorsal; L: lateral; M: medial; V: ventral. 112 ASSESSMENT OF CYTOCHROME C OXIDASE DYSFUNCTION IN THE SUBSTANTIA NIGRA/ VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA by Matthew W. Rice, Kristen L. Smith, Rosalinda C. Roberts, Emma Perez-Costas, & Miguel Melendez-Ferro PLOS ONE Copyright 2014 by PLOS ONE Used with permission Format adapted and errata corrected for dissertation 113 Abstract Perturbations in metabolism are a well-documented but complex facet of schizophrenia pathology. Optimal cellular performance requires the proper functioning of the electron transport chain, which is constituted by four enzymes located within the inner membrane of mitochondria. These enzymes create a proton gradient that is used to power the enzyme ATP synthase, producing ATP, which is crucial for the maintenance of cellular functioning. Anomalies in a single enzyme of the electron transport chain are sufficient to cause disruption of cellular metabolism. The last of these complexes is the cytochrome c oxidase (COX) enzyme, which is composed of thirteen different subunits. COX is a major site for oxidative phosphorylation, and anomalies in this enzyme are one of the most frequent causes of mitochondrial pathology. The objective of the present report was to assess if metabolic anomalies linked to COX dysfunction may contribute to substantia nigra/ventral tegmental area (SN/VTA) pathology in schizophrenia. We tested COX activity in postmortem SN/VTA from schizophrenia and non-psychiatric controls. We also tested the protein expression of key subunits for the assembly and activity of the enzyme, and the effect of antipsychotic medication on subunit expression. COX activity was not significantly different between schizophrenia and non-psychiatric controls. However, we found significant decreases in the expression of subunits II and IV-I of COX in schizophrenia. Interestingly, these decreases were observed in samples containing the entire rostro-caudal extent of the SN/VTA, while no significant differences were observed for samples containing only mid-caudal regions of the SN/VTA. Finally, rats chronically treated with antipsychotic drugs did not show significant changes in COX subunit expression. These findings suggest that COX subunit expression may be 114 compromised in specific sub-regions of the SN/VTA (i.e. rostral regions), which may lead to a faulty assembly of the enzyme and a greater vulnerability to metabolic insult. 115 Introduction Schizophrenia is a devastating mental illness that affects approximately 1% of the world population [1]. Currently, most studies on schizophrenia concentrate on the study of pathologies of either neuronal circuitry or molecular mechanisms at the cellular and subcellular levels. This includes the assessment of cellular metabolism and mitochondrial function. One of the first studies that implicated mitochondrial dysfunction in the pathology of schizophrenia was performed by Takahashi and Ogushi [2], which revealed reduced aerobic glycolysis in schizophrenia post-mortem brain tissue. Since then, perturbations in metabolism have become a well-documented, if complex, facet of schizophrenia pathology. This is also supported by studies showing changes in mitochondrial density and increased mitochondrial morphological anomalies in several brain regions, including the prefrontal and limbic cortex, the striatum and the substantia nigra [3-8]. Some of the most thoroughly studied metabolic anomalies in schizophrenia are related to disruptions in oxidative phosphorylation. The brain is a high energy-demanding organ, which obtains the majority of its energy from oxidative phosphorylation [9-11] and disruptions of this pathway could account for some of the metabolic anomalies observed in schizophrenia. As an example, decreased concentrations of ATP have been observed in the frontal lobe of schizophrenia subjects [12], which is indicative of a deficit in oxidative phosphorylation. The synthesis of ATP requires proper functioning of the electron transport chain (ETC), which consists of a series of four enzyme complexes located within the inner membrane of the mitochondria [13,14]. These enzymes transfer electrons between electron donors and acceptors, establishing a proton gradient that is 116 ultimately used to power the enzyme ATP synthase [15-18]. Adequate production of ATP is crucial for neuronal plasticity, intracellular signaling, calcium buffering, and neurotransmission [19-23]. Anomalies in any single individual complex of the ETC can be sufficient to cause a disruption in cellular metabolism [24, 25]. However, the activity of a given complex is not contingent on the proper functioning of the other complexes [24, 26]. In schizophrenia, anomalies have been reported in individual components of the ETC, including complexes I, III, and IV [14, 27-34]. Complex IV or cytochrome c oxidase (COX) is the terminal enzyme of the ETC, and its role is to catalyze the oxidation of cytochrome c, transferring electrons to molecular oxygen in order to produce a molecule of H2O [35-37]. COX has been recognized as a major regulation site for oxidative phosphorylation, and anomalies in this enzyme are some of the most frequent causes of mitochondrial pathology [38-40]. X-ray crystallography has shown that COX is composed of 13 subunits, three of which (COX I, II, and III) are encoded by the mitochondrial genome, with the remaining subunits (COX IV, Va, Vb, VIa, VIb, VIc, VIIa, VIIb, VIIc, and VIII) encoded by the nuclear DNA [41-42]. Mitochondrial DNAencoded subunits are commonly known as the “catalytic core” of the enzyme due to their role in electron transfer and proton expulsion [43]. Mutations within subunits of the catalytic core result in disruptions of both structure and function of the entire COX enzyme [40, 44-47]. Among the nuclear DNA-encoded subunits, COX subunit IV is especially important for the assembly and proper functioning of the enzyme [37]. COX subunit IV is the largest of the nuclear DNA-encoded subunits and may be involved in the modulation of electron transfer between components of the catalytic core [36-37]. 117 Suppression of subunit IV has been linked to a reduced function in overall COX activity and an increased susceptibility to apoptosis [36-37]. In schizophrenia COX activity is differently affected depending on the region of the brain studied. Reduced COX activity has been reported in the frontal and temporal cortex, as well as in the caudate nucleus [27, 30], while an increase in activity was observed in the putamen [31], and no changes in activity have been reported in brain tissue from the nucleus accumbens, globus pallidus, thalamus, cerebellum, or mesencephalon [27, 31]. On the other hand, Whatley et al. [48] did not find changes in COX activity within the frontal cortex, adding to the complexity of the pathology of COX within the schizophrenia brain. Although some studies have reported increases [26, 31] and decreases [49] in COX activity due to the use of antipsychotic medication, the majority of studies have concluded that COX activity is not affected by antipsychotic drugs [50-54]. Taken together, these findings suggest that COX anomalies are likely an intrinsic feature of schizophrenia. The substantia nigra/ventral tegmental area complex (SN/VTA) contains the largest group of dopaminergic neurons in the brain, and is the origin for several major dopamine pathways [55-56]. Dopaminergic anomalies are a well-known component of schizophrenia pathology [57], and previous findings from our laboratory have shown significant reductions in tyrosine hydroxylase (TH) protein levels within rostral regions of the SN/VTA in schizophrenia [58]. The aim of this study was to assess if metabolic anomalies linked to COX dysfunction might contribute to SN/VTA pathology in schizophrenia. To accomplish this we tested for differences in COX activity within the SN/VTA in human postmortem 118 samples from schizophrenia subjects compared to non-psychiatric controls. In addition, we tested the hypothesis that alterations in the subunit composition of COX could contribute to schizophrenia pathology in the SN/VTA. To test this we analyzed protein expression for all the COX subunits that constitute the catalytic core, as well as for subunit IV, which is critical for the assembly and function of the enzyme. Materials and Methods Ethics statement Human postmortem brain samples used in this study were obtained from the Maryland Brain Collection (Maryland Psychiatric Research Center, University of Maryland School of Medicine) with permission from the Maryland Brain Collection Steering Committee. The Maryland Brain Collection obtains written consent from the next-of-kin for the use in research of all human brain samples collected. This brain collection has full approval from the Institutional Review Board (IRB) at the University of Maryland School of Medicine (DHMH protocol # 07-23). In addition, all postmortem human brain experimental procedures were conducted following a protocol approved by the University of Alabama at Birmingham Institutional Review Board (protocol # N110505002), and in accordance with the principles expressed in the Declaration of Helsinki. No animals were handled, treated or sacrificed for this study. However, brain protein extracts from adult rats from a stock maintained in our laboratory were used. In strict accordance with the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals, the samples used in this study are considered cadaveric tissues, 119 and were not prepared or collected specifically for this study. This protein stock was the remnant from a previously published study [58] performed by some of the authors of the present work at the University of Maryland School of Medicine, and on that previous study, all research was conducted following an approved IACUC protocol (IACUC protocol # 0705010). All animal housing, care, and experimental procedures were done in strict accordance with National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures. Postmortem human brain samples All human brain samples were obtained from the Maryland Brain Collection (Maryland Psychiatric Research Center, University of Maryland School of Medicine). For all postmortem human cases, two independent psychiatrists established DSM-IV diagnoses based on the review of available medical and autopsy records, and data obtained from the Structured Clinical Interview for DSM-IIIR [59] and DSM-IV Axis I disorders with the next-of-kin. Table 1 contains demographic data, as well as information on antipsychotic use, cause of death, and other relevant parameters for the samples used in this study. Human brain samples were dissected in two different ways: 1) samples that contained the entire rostro-caudal extent of the SN/VTA (n=15; Table 1) or 2) samples that contained only the mid-caudal regions of the SN/VTA (n=14; Table 1). Cases from these two different dissections were analyzed separately due to the heterogeneous nature of the subnuclei within the SN/VTA. All samples were frozen in dry ice and stored at -80°C prior to sectioning. Using a cryostat, five parallel (i.e. adjacent) series of 16 µm 120 thick sections were obtained in the coronal plane following the longitudinal axis of the brainstem. Sample blocks were trimmed to reduce the presence of cell groups not related to our study. All samples were sectioned in their entirety. Four series were collected on charged slides, while the fifth series was collected in a vial for protein extraction. Series #1 was stained with thionin to assess proper morphological preservation, and only those cases that presented good preservation of the SN/VTA were included in the study. Exclusion criteria included the presence of extensive autolysis of cells, cell shrinkage and poor morphological preservation. These criteria were equally applied to select the schizophrenia and control cases included in the study. Rostral and caudal boundaries for samples containing the entire extent of the SN/VTA were determined using the Paxinos and Huang [60] atlas of the human brainstem. Mid-Caudal SN/VTA samples were defined as those transversally dissected caudal to the transition between the red nucleus parvocellularis and magnocellularis. Animal brain protein samples A stock of protein extracts maintained in the laboratory, which was obtained from rats previously treated with antipsychotics [58], was used to assess the effects of antipsychotic medication on COX subunit protein expression. Treatment procedures for these animals have been previously described in detail in [58]. Briefly, animals were randomly assigned to one of three treatment groups (n=9 per group) consisting of haloperidol (1.5mg/kg/day), olanzapine (6mg/kg/day), or control (i.e. water). Antipsychotics were administered for three weeks in drinking water, adjusting the doses to body weight and daily water consumption as described in Perez-Costas et al. [61]. 121 Animals were euthanized and brains were immediately removed from the skull, frozen in dry ice, and stored at -80°C. Brains were sectioned on a cryostat at -20°C in the coronal plane, and 16m thick sections through the SN/VTA were collected in five parallel series. Sample blocks were trimmed to reduce the presence of cell groups not related to our study. Four series were collected on charged slides, while the fifth series was collected in a vial for protein extraction. Series #1 was stained with thionin to assess morphology and general quality of the tissue collected. No morphological anomalies were found in any of the samples used in this study. Cytochrome c oxidase histochemistry in human SN/VTA Slides containing human SN/VTA sections were removed from -80°C storage and fan-dried for 25 minutes at room temperature. Sections were then incubated in the dark for two hours at 37°C in the COX incubation medium, which consisted in a solution of 22.4 mg cytochrome c (Sigma-Aldrich, St. Louis, MO, USA; C3131), 115.23 mg diaminobenzidine (DAB, Sigma-Aldrich, D5637), 4.5 g sucrose (Sigma-Aldrich, S0389), 12.51 ml of a 1% nickel ammonium sulfate solution (Sigma-Aldrich, A1827), all mixed in 100 ml of 0.1M Hepes buffer (Sigma-Aldrich, H3375) pH 7.4 [62, 63]. To terminate the COX enzymatic reaction, sections were immersed in 4% paraformaldehyde in 0.1M phosphate buffer (PB) pH 7.4 for one hour at room temperature. Sections were then rinsed in PB, dehydrated in ethanol, cleared in xylene, and coverslipped using Eukitt (Electron Microscopy Science, Hatfield, PA, USA; 15322). Negative controls were done in adjacent tissue sections by prior immersion in a solution of 4% paraformaldehyde in 0.1M PB pH 7.4 for one hour at room temperature, followed by rinses in PB, and then 122 immersion in a solution of 10mM sodium azide in PB for 17 hours at room temperature. The aim of the negative controls is to subtract from the analysis the presence of any background not specifically due to COX activity (e.g. possible random precipitation of DAB or nickel ammonium sulfate). Thus, the prior treatment with paraformaldehyde and sodium azide was used to irreversibly inactivate COX in the negative control sections. After inactivating the COX enzyme in the negative control, these sections were incubated simultaneously with the samples in the COX incubation medium. In order to more accurately assess the differences in COX activity among our groups, we applied a novel methodology developed in our laboratory [64]. Briefly, cytochrome c oxidase from bovine heart (Sigma-Aldrich, C5499) was diluted in TrisBuffered-Saline (TBS) to create a series of standards that contained a known quantity of pure COX. Each of these known standards was loaded onto a nitrocellulose membrane, using vacuum and a slot-blot microfiltration apparatus (Bio-Rad, Hercules, CA, USA; 170-6542). For all COX histochemical assays, negative controls and COX standards were incubated together with sections to be analyzed for COX activity. Protein extracts for COX subunit analysis in human and rat SN/VTA Human and rat brain protein extracts were obtained by sonication of tissue in a cold lysis buffer mixture (1:5 w:v) containing 50mM Tris pH 8.0, 5mM EDTA, 150mM NaCl, 1% SDS, and 10l/ml of a protease inhibitor cocktail (Sigma-Aldrich; P8340). Homogenates were then centrifuged at 15000g for 15 minutes at 4°C, the supernatant was collected, and total protein concentration was measured using a modified Lowry technique (Bio-Rad, DC Protein Assay; 500-0113 and 500-0114). Known concentrations 123 of bovine serum albumin were used as standards. Aliquots of both rat and human SN/VTA protein extracts were stored at -80°C until use. Gel electrophoresis and western-blot Western-blot assays were used to test for differences in COX subunit protein expression in both animal and human SN/VTA samples. For gel electrophoresis, samples were diluted 1:1 in loading buffer (62.5mM Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 0.01% bromophenol blue, and 5% β-mercaptoethanol) and heated to 95°C in a dry bath for 5 minutes before loading on a 4-20% polyacrylamide gradient gel (Lonza, Basel, Switzerland; 58505). For rat samples, 25g of SN/VTA total protein extract were loaded in each lane, while for human samples 60g of SN/VTA total protein extract were loaded. In addition, a molecular weight marker (Lonza; 50550) was loaded for molecular weight reference. Proteins were resolved using a constant 150V current and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad; 162-0714) under a constant current of 30V for 21 hours at 4°C. For western-blot assays, membranes were initially rinsed in 0.05M TBS, followed by blocking in a solution of 5% non-fat dry milk (Bio-Rad; 170-6404) in TBS containing 0.1% Tween 20 (TBS-T) for one hour at room temperature. Membranes were then incubated with the appropriate primary antibody at the concentrations listed below, in a solution of 1% non-fat dry milk in TBS-T for 19 hours at 4°C. Following that, membranes were rinsed in a solution of 1% non-fat dry milk in TBS-T prior to incubation with the appropriate secondary antibody at the concentrations listed below, in a solution of 1% non-fat dry milk in TBS-T for one hour at room temperature. After incubation with 124 the secondary antibody, membranes were rinsed first with TBS-T and then with TBS. Membranes were developed using an Immun-Star alkaline phosphatase substrate chemiluminescence kit (Bio-Rad; 170-5018), exposing Kodak Biomax XAR films (Kodak, Rochester, NY, USA; 166-0760). For each subunit tested a minimum of two separate western-blot experiments were carried out. For human SN/VTA, rostro-caudal and mid-caudal samples were run in the same experiment, which required the simultaneous incubation and development of 3 western-blot membranes per experiment. Three western-blot membranes were also necessary to accommodate all rat samples in a single experiment. Antibodies The following primary antibodies were used for western-blot assays: mouse monoclonal anti-COX subunit I (MitoSciences, Eugene, OR, USA; ab14705, diluted 1:1000 for rat samples), rabbit polyclonal anti-COX subunit I (Abcam, Cambridge, England; ab90668, diluted 1:1000 for human samples), mouse monoclonal anti-COX subunit II (MitoSciences; ab110258, diluted 1:1000 for human samples), goat polyclonal anti-COX subunit II (Santa Cruz Biotechnology, Dallas, TX, USA; sc-23984, diluted 1:1000 for rat samples), rabbit polyclonal anti-COX subunit III (Mitosciences; ab138956, diluted 1:2000 for rat samples and 1:1000 for human samples), rabbit polyclonal antiCOX subunit IV-I (Sigma-Aldrich; HPA002485, diluted 1:2000 for rat and human samples), rabbit polyclonal anti-COX subunit IV-II (Sigma-Aldrich; SAB4503384, diluted 1:1000 for rat samples), and rabbit polyclonal anti-COX subunit IV-II (SigmaAldrich, HPA029307, diluted 1:2000 for human samples). For all western-blot 125 experiments membranes were reblotted for actin using a mouse monoclonal anti-actin antibody (Millipore, Billerica, MA, USA; MAB1501, diluted 1:40,000 for both rat and human samples). All primary antibodies used in the study were previously tested for specificity by the manufacturers and produced consistent bands at the expected molecular weight. Secondary antibodies used included alkaline phosphatase-conjugated goat antimouse (Millipore; AP124A, diluted 1:15,000), alkaline phosphatase-conjugated goat antirabbit (Vector Laboratories, Burlingame, CA, USA; AP-1000, diluted 1:1000), and alkaline phosphatase-conjugated donkey anti-goat (Millipore, AP180A, diluted 1:15,000). Image acquisition and analysis Complete series of slides containing SN/VTA sections that were assessed using COX histochemistry, membranes containing COX standards, and western-blot films, were digitized using a flatbed scanner (Epson Perfection V750-M Pro; Seiko Epson Suwa, Japan), and saved at a resolution of 600 dpi as uncompressed TIFF files. Images of sections assessed by COX histochemistry were acquired as 16 bit grayscale, while images of western blot films were obtained as 8 bit grayscale. NIH Image J software (version 1.46r; http://rsbweb.nih.gov/ij/) was used to measure optical density (OD) for all experiments. For COX activity measurements, masks defining the region of interest were created by delineating the SN/VTA on the corresponding scanned Nissl-stained section within each case. A standard curve was created using OD values from the COX standards 126 [64]. In addition, as an internal control for the validity of the assay, COX activity was also measured in the red nucleus within the same section, since no studies have reported changes in COX activity for the red nucleus in schizophrenia. Using a random number generator (random.org), twelve slides (for rostro-caudal cases) or eight slides (for midcaudal cases) representing different sub-regions of the SN/VTA were randomly chosen for analysis. For each section measured, using Image J rectangle tool, five nonoverlapping boxes of 0.19 x 0.19 cm were randomly placed within the mask delineating the region of interest (i.e. SN/VTA or red nucleus). For western-blot analysis of human and rat samples, an OD standard curve was created using a step calibration tablet of known calibrated OD (Stouffer Industries Inc., Mishawaka, IN, USA; T2120, series #130501). After calibration, OD measurements were obtained using Image J rectangle tool to delineate each band. In all cases, measurements of digitized images were performed by two different researchers blind to diagnosis or treatment group. Each researcher performed three measurements for each sample assessed. Measures obtained from the same experiment were averaged for analysis. Statistical analysis Schizophrenia and non-psychiatric control cases were matched for demographic variables (age, gender, race), and sample variables (postmortem interval, brain pH). We used multiple regression to assess the possible influence of demographic and sample variables on our outcome measures (i.e. COX activity and subunits expression) independent of disease status. Demographic variables (i.e. age, gender, race) and sample 127 variables (i.e. postmortem interval and pH) where tested in separated multiple regression analyses. For the analysis of demographic variables a dummy coding was used to numerically interpret gender and race. For protein measures, actin values were standardized by dividing the observed actin value of each case by the average actin value of each individual film. COX subunit protein measures were then normalized over actin by dividing the observed “raw” calibrated OD value of each measure by its respective standardized actin calibrated OD value. Human postmortem samples containing the entire rostro-caudal extent of the SN/VTA and those containing only mid-caudal regions were analyzed separately. In both cases, outcome measures obtained from schizophrenia and non-psychiatric control samples were assessed using unpaired Student’s t-test or Mann-Whitney (non-parametric) tests after assessing normality and the possible lognormal distribution of the data. Since COX activity has been previously shown to best fit an exponential curve [64], all COX activity data were transformed to their common logarithm prior to analysis. For COX subunit expression, the data were assessed using the following scheme: Data sets were assessed for normality, and when samples presented significant deviations from normality, the data were transformed to their common logarithms in order to test if they were a good fit for a lognormal distribution. If the lack of normality persisted after logarithmic transformation, the original (untransformed) data were tested for the presence of outliers and used for analysis. One-way ANOVA was used to assess the outcome measures on protein samples from animals treated with antipsychotic medications. Assumptions of the parametric tests were addressed in all analyses, including normal distribution of the residuals in the dependent variable, homogeneity of variance among 128 the groups, and independence of errors. The presence of outliers was determined using the “Robust regression and OUtlier Removal” (ROUT) test for outlier detection [65], with the coefficient Q set to 1%. When detected, outliers were removed from the analysis and graphical representations, but are still present in the western-blot images. ShapiroWilk and Kolmogorov-Smirnov normality tests were used to assess normality in all data sets. Significant deviations from normality, and the presence of outliers, when present, are reported in the results section. The experimental design was done considering each subunit outcome independent (i.e. not corrected for multiple comparisons). It has been shown that specific subunits of the COX enzyme can be independently regulated and targeted by specific pharmacological treatments [70-74]. For example, mutations in subunit I [74] as well as specific decreases in the expression of subunit II [72] or subunit III [73] affect the activity of the complex without altering significantly the expression of the other subunits of the complex [70-74]. On the other hand, it has been shown that multiple comparison corrections reduce the risk of type I errors, but greatly increase the risk of type II errors, that is, not detecting relevant differences due to the reduction of power produced by the correction [66, 67]. However, due to the lack of agreement in the field of statistics on the use (or not) of corrections for multiple comparisons [67] data were also analyzed using multiple t-test correction (Holm-Sidak correction with alpha 0.05). Thus, results using independent t-test (i.e. uncorrected) as well as corrected for multiple comparisons, are both reported (see results). For the multiple corrections approach, the COX subunits that form the catalytic core (i.e. subunits I, II, III) were analyzed together. Subunits IV-I and IV-II were also analyzed using this correction. Subunits I, II and III constitute the 129 catalytic core of the enzyme, are encoded by the mitochondrial genome, and their transcription and translation occur within the mitochondria, while subunits IV-I and IV-II are encoded by the nuclear genome and their synthesis occurs in the cytoplasm [43, 6869]. Thus, mitochondrial and nuclear subunits were analyzed separately. Mean and standard deviation data are reported in the figure legends. Statistical analysis and graphical representation of the results obtained were done using GraphPad Prism 6.0 software (La Jolla, CA, USA). Results and Discussion Anomalies in metabolism are a well-established aspect of schizophrenia pathology. Reported anomalies include decreased overall metabolic activity in the cortex, changes in mitochondrial density within the caudate nucleus and putamen, and reductions in ATP concentration within the frontal lobe [3-7, 12]. Despite the potential role of metabolic anomalies in the pathology of the dopamine system in schizophrenia, few studies have addressed the metabolic status of the SN/VTA in this disorder. Interestingly, there have been reports of mitochondrial hyperplasia within presynaptic neurons that synapse onto dopamine neurons in the substantia nigra compacta [8]. In the present study we assessed the metabolic health of the SN/VTA complex by quantifying COX activity, and by measuring protein expression for key subunits of the COX enzyme. Activity measures provided a “snapshot” of the functional status of the SN/VTA, while assessing the protein expression of key subunits of the COX enzyme allowed us to infer information about the subunit composition of the enzyme in the SN/VTA in schizophrenia. 130 Demographic and sample features Postmortem human samples selected for this study were carefully matched between schizophrenia and non-psychiatric control groups for 1) demographic (age, gender, race) and 2) sample variables (brain pH and PMI) (see Table 1). In addition, multiple regression was used to assess if 1) demographic or 2) sample variables, had a significant contribution to our dependent measures. The effect of these demographic and sample variables was tested independently for COX activity, as well as for the expression of each COX subunit assessed in our study (i.e. COX subunits I, II, III, IV-1, IV-II). In all cases, multiple regression analysis showed that these variables did not have significant predictive value. Table 2 summarizes the results obtained from the multiple regression tests performed independently for the rostro-caudal and mid-caudal SN/VTA cases, and reports the exact p value and adjusted R2 for each independently tested outcome measure (see Table 2). In addition we did not find any significant colinearity among the demographic or sample variables. Cytochrome c oxidase activity in schizophrenia versus non-psychiatric controls COX activity was measured independently for samples containing the entire rostro-caudal extent of the SN/VTA and those containing only mid-caudal regions. For both types of dissections COX activity was also measured for the red nucleus for each case as an internal control for the validity of the assay (see methods). As stated in methods, COX activity has been shown to best fit an exponential curve [64], thus, all OD data for COX activity were transformed to their common logarithm [y=-1*log (y)], prior to analysis. COX activity is shown in all graphs as the logarithm of OD units, and the 131 mean value reflects the geometrical mean of the lognormal distribution. After logarithmic transformation, all COX activity data were normally distributed (Shapiro-Wilk and Kolmogorov-Smirnov tests in all cases p>0.1), which further supports the exponential curve fit of COX activity. COX activity in Rostro-Caudal samples COX activity measurements for the entire rostro-caudal extent of the SN/VTA were assessed using unpaired t-test to compare the geometric means of the schizophrenia and control groups. This analysis yielded non-significant differences in COX activity between schizophrenia and controls for the rostro-caudal SN/VTA (p=0.6001, t=0.5373, df=13). The geometric mean for schizophrenia group was 1.389 (95% CI: 1.212, 1.592) and for the control group was 1.328 (95% CI: 1.130, 1.561) [Figure 1]. For the red nucleus, unpaired t-test revealed non-significant differences for COX activity (p=0.2011, t=1.347, df=13). The geometric mean for the red nucleus for the schizophrenia group was 1.513 (95% CI: 1.426, 1.605) and for the control group was 1.409 (95% CI: 1.255, 1.581) [Figure 1]. In addition, analysis of COX activity just for the rostral region of the SN/VTA in these same cases also yielded non-significant differences in activity (unpaired t-test p=0.5816, t=0.5652, df=13). For this sub-analysis of the rostral region, COX activity was measured at 4 different rostro-caudal levels within the rostral region. The geometric mean for COX activity in the rostral SN/VTA for the schizophrenia group was 1.435 (95% CI: 1.259, 1.635) and for the control group was 1.340 (95% CI: 1.060, 1.695). 132 COX activity in Mid-Caudal samples In samples containing only mid-caudal regions of the SN/VTA unpaired t-test revealed non-significant differences between schizophrenia and control cases (p=0.8737, t= 0.1624, df= 11) [Figure 2]. The geometric mean for the schizophrenia group was 1.083 (95% CI: 0.9102, 1.290) and for the control group was 1.076 (95% CI: 0.9021, 1.285). For the red nucleus, unpaired t-test revealed non-significant differences for COX activity (p=0.5145, t=0.7011, df=5). The geometric mean for the red nucleus for the schizophrenia group was 1.252 (95% CI: 0.8047, 1.947) and for the control group was 1.127 (95% CI: 0.8010, 1.585) [Figure 2]. Anomalies of COX activity in schizophrenia have been studied in various regions of the brain including frontal and temporal cortex, caudate nucleus, putamen, nucleus accumbens, globus pallidus, thalamus, and cerebellum [27, 30, 31, 48]. These studies revealed that COX activity is differently affected depending on the region examined. In the present study we tested for differences in COX activity in two different types of samples from the SN/VTA (i.e. rostro-caudal and mid-caudal). Our analysis of COX activity did not yield any significant differences for either region of the SN/VTA. This is in agreement with a previous study by Prince et al. [31] that also found no changes in COX activity within the mesencephalon. An alternative explanation for our findings is that metabolic pathology, and more specifically anomalies in COX activity, may affect discrete subpopulations of dopaminergic neurons within the SN/VTA. 133 Cytochrome c oxidase subunit analysis in the SN/VTA Subunit composition of the COX enzyme was assessed in schizophrenia and nonpsychiatric control cases, as well as in animals chronically treated with antipsychotic medications, by measuring protein expression. Subunits I, II, and III of the COX enzyme were selected for study because these mitochondrial DNA-encoded subunits constitute the catalytic core of the enzyme. In addition, COX subunit IV, which is encoded by nuclear DNA, was selected for study due to its essential role in the assembly of the enzyme and in respiratory function [37]. COX subunit IV has two isoforms (i.e. COX IVI and COX IV-II) in the human, rat, and mouse [36], thus, protein expression of both isoforms was assessed in our study. COX subunits in Rostro-Caudal SN/VTA samples COX subunit I expression did not differ significantly between schizophrenia and non-psychiatric control samples (unpaired t-test, p=0.6895, t= 0.4086, df= 13) [Figure 3A]. Interestingly, COX subunit II was significantly decreased in schizophrenia subjects versus non-psychiatric controls (unpaired t-test with Welch’s correction, p=0.0332, adjusted t= 2.716, adjusted df=6.305). Welch’s correction was used to address the significant differences in variance between the two groups (F-test p=0.0007). Two outliers were identified in the schizophrenia group (SZ1 and SZ6). Notably, the mean expression of COX subunit II in schizophrenia compared to the control group presented a 42.77% reduction [Figure 3B]. COX subunit III did not present significant differences between the two groups (unpaired t-test with Welch’s correction, p=0.0925, adjusted t= 1.884, adjusted df= 8.92). Welch’s correction was used to address the significant 134 differences in variance between the two groups (F-test p=0.0267). One outlier (C2) was identified for COX subunit III in the control group [Figure 3C]. COX subunit IV-I expression data were not normally distributed for the schizophrenia group (Shapiro-Wilk, p=0.0018; Kolmogorov-Smirnov p=0.0141), and one outlier was identified in the schizophrenia group (SZ6). There were significant differences in COX subunit IV-I expression between schizophrenia and non-psychiatric controls (Mann-Whitney test, p=0.048, U= 9) [Figure 4A]. However, no significant differences were observed between the two groups for COX subunit IV-II (unpaired t-test, p= 0.9234, t= 0.0981, df= 13) [Figure 4B]. Multiple t-test Holm-Sidak correction for the analysis of subunits I, II, III (i.e. catalytic core) produced similar results to the independent analysis of these subunits, yielding significant differences in the expression of subunit II between the schizophrenia and control samples (p=0.0291, t= 2.5082, df=11), while subunits I and III did not present significant differences between the two groups (subunit I: p=0.6904, t= 0.4074, df=13; subunit III: p=0.1218, t= 1.6649, df=12). For subunits IV-I and IV-II (i.e. nuclear genome encoded) Holm-Sidak corrected t-test yielded non-significant differences between schizophrenia and control groups (subunit IV-I: p=0.9212, t= 0.0386, df=12; subunit IVII: p=0.0291, t= 0.1008, df=13). COX subunits in Mid-Caudal SN/VTA samples COX subunit I expression did not differ significantly between schizophrenia and control groups for mid-caudal SN/VTA samples (unpaired t-test, p=0.6736, t= 0.4327, df=11) [Figure 5A]. Despite the significant differences found in rostro-caudal SN/VTA 135 samples for COX subunit II protein expression, in mid-caudal samples no significant differences were found between the two groups (unpaired t-test, p=0.2093, t=1.334, df=11) [Figure 5B]. For COX subunit III, an outlier was detected in the schizophrenia group (SZ14), and no significant differences were found between the two groups for this subunit (unpaired t-test with Welch’s correction, p=0.2057, t=1.443, df=5). Welch’s correction was used to address the significant differences in variance between the two groups (F-test p=0.0013). [Figure 5C]. No significant differences were found for either of the two isoforms of COX subunit IV (subunit IV-I, unpaired t-test, p=0.0963, t=1.818, df= 11; subunit IV-II unpaired t-test, p=0.3249, t=1.031, df=11) [Figure 6A-B]. Multiple t-test Holm-Sidak correction also yielded non-significant results for the analysis of the catalytic core subunits (subunit I: p=0.6742, t= 0.4318, df=11; subunit II: p=0.2096, t= 1.3327, df=11; subunit III: p=0.1796, t= 1.4429, df=10), as well as for the nuclear genome encoded subunits (subunit IV-I: p=0.0960, t= 1.8203, df=11; subunit IVII: p=0.3232, t= 1.0343, df=11). Effect of antipsychotic medication on COX subunit expression Since all schizophrenia samples included in our study were obtained from individuals that were medicated with antipsychotic drugs at time of death, we tested in rats if antipsychotic treatment could have an impact in the expression of the COX subunits included in our study. All rat samples included the entire rostro-caudal extent of the SN/VTA. One-way analysis of variance (ANOVA) was used to compare the expression of each subunit among the 3 treatment groups. We did not find any statistically significant 136 differences among the three treatment groups for the expression of any of the COX subunits tested. COX subunit I [one-way ANOVA p= 0.5971, F (2,24)= 0.5269] [Figure 7A], COX subunit II [one-way ANOVA p=0.2668, F (2,24)= 1.397] [Figure 7B], COX subunit III [one-way ANOVA p= 0.6903, F (2, 24)= 0.3764] [Figure 7C], COX subunit IV-I [one-way ANOVA p= 0.8636, F(2,24)= 0.1475] [Figure 8A], and COX subunit IVII [one-way ANOVA p= 0.1403, F (2,24)= 2.134] [Figure 8B]. These data support that antipsychotic drugs do not affect COX subunits expression in the SN/VTA. Unlike COX activity, little research has been conducted on the subunit composition of cytochrome c oxidase in regard to schizophrenia pathology. Furthermore, to the authors’ knowledge, no studies have examined how antipsychotic medication may affect the individual subunits of this enzyme. For this study we concentrated our efforts on examining COX subunits that contribute directly to electron transfer, proton pumping, and enzyme stability. To this end, we studied COX subunits I, II, and III (i.e. the “catalytic core”) and the two isoforms of COX subunit IV (i.e. COX IV-I and COX IVII). Our study revealed that COX subunit II presented robust significant reductions (i.e. regardless of the type of analysis used) of its expression in schizophrenia when the entire rostro-caudal SN/VTA region was present. In addition, a significant reduction of subunit IV-I was also found for SN/VTA containing the entire SN/VTA in the independent analysis, but not when the Holm-Sidak correction for multiple t-test comparisons was applied. All the other subunits assessed (i.e. subunits I, III and IV-II) did not yield any significant differences between the two groups for samples containing the entire SN/VTA. For samples containing only mid-caudal SN/VTA regions, none of the subunits assessed presented significant differences between schizophrenia and non-psychiatric 137 controls. Even though all the samples used in the study were trimmed to reduce the presence of other cell groups, we cannot discard small contributions of surrounding cell groups to our results. Although we did not find significant changes in COX activity, the alterations in the expression of key subunits of the COX enzyme could still have important functional implications. It has been demonstrated that mitochondria have a “reserve capacity” of energy production that is critical in determining cellular metabolic response to insults such as metabolic or xenobiotic stress (e.g. oxidative stress and hypoxia) [75-76]. It is highly plausible that disruptions in subunit composition could affect the proper assembly of the COX enzyme, thus lowering the reserve capacity of mitochondria, and making them more vulnerable to insult. In other words, a non-optimally assembled COX enzyme may be able to maintain proper basal COX activity and ATP production, but may have an impaired capacity to respond to higher energy demands. Supporting this idea, it has been shown that specific mutations in subunits of the catalytic core produce severe impairment of mitochondrial function [44-47]. Subunits II and IV of the COX enzyme are crucial for the proper functioning of the COX complex as a whole [42, 45-46]. COX subunit II is responsible for the binding of cytochrome c and the subsequent electron transfer to subunit I of the COX enzyme [43]. Interestingly, anomalies in COX subunit II mRNA expression have been previously reported in the frontal cortex in schizophrenia without significant changes in COX activity [45]. In addition, reduced expression of COX subunit II has been shown to correlate with a higher susceptibility to neuronal loss, and with an increased susceptibility to kainic acid-induced epilepsy in mice deficient in DNA mismatch repair [77]. COX 138 subunit IV is encoded by nuclear DNA and is not a component of the catalytic core, although several studies support that subunit IV is necessary for proper electron transfer and enzyme stability [36-37, 42]. In addition, subunit IV has a crucial role in the correct assembly of the COX enzyme, and the binding of subunit IV to subunit I is the first step for the assembly of the entire COX complex [15, 37, 42]. Furthermore, suppression of COX subunit IV expression has been shown to increase cell susceptibility to apoptosis [37]. It is important to note that subunits encoded by both the mitochondria and nuclear genomes were affected in our study. As far as we are aware, mutations in the genes encoding these subunits have not been reported in schizophrenia. Deficiencies in the expression of these subunits in the SN/VTA in schizophrenia could be due to a faulty transcription or translation, although anomalies in mRNA expression for subunit II have been previously reported in the cortex for this disorder [48]. Another interesting finding of our study is that significant decreases in protein expression for subunits II and IV-I of the COX enzyme were found only in schizophrenia samples that contained the entire rostro-caudal extent of the SN/VTA. These findings suggest that anomalies in the expression of key subunits of the COX enzyme could affect specifically rostral regions of the SN/VTA, which is in strong agreement with our previous report on deficits in tyrosine hydroxylase (i.e. the rate limiting enzyme for the production of dopamine) in the same rostral region of the SN/VTA [58]. This holds important neuroanatomical and clinical significance since rostral areas of the SN/VTA mainly contain dopaminergic neurons that contribute to the mesolimbic and mesocortical pathways [56, 78-81]. Interestingly, anomalies in the expression of tyrosine hydroxylase [82] and subunit II of the COX enzyme [48] have also been reported in the prefrontal and 139 frontal cortex in schizophrenia, which are some of the main projection areas for the neurons of the rostral SN/VTA. Decreases in the expression of these subunits could produce a faulty stoichiometry in the assembly of the COX enzyme, leading to a greater vulnerability to metabolic insults. Additional studies will be needed to fully elucidate the meaning of these findings, including if the anomalies observed in subunits II and IV-I of the COX enzyme occur at the level of transcription or translation, and if other components of the metabolic machinery may be compromised within the rostral SN/VTA. Acknowledgements The authors wish to thank the staff of the Maryland Brain Collection, University of Maryland School of Medicine for their help in obtaining the samples used in this study. Drs. Emma Perez-Costas and Miguel Melendez-Ferro contributed equally to this work. 140 References 1. American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders-IV Text Revision. Washington, DC: American Psychiatric Press. 2. Takahashi Y, Ogushi T (1953) On biochemical studies of schizophrenia. I. An enzymological study on brain tissue and serum of schizophrenic patients; choline esterase. Folia Psychiatr Neurol Jpn 6: 244-261. 3. Uranova NA, Aganova E A (1989) Ultrastructure of the synapses of the anterior limbic cortex in schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova 89: 56-59. 4. Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, et al. (2001) Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 55: 597–610. 5. Kung L, Roberts RC (1999) Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse 31: 67–75. 6. Somerville SM, Lahti AC, Conley RR, Roberts RC (2011) Mitochondria in the striatum of subjects with schizophrenia: relationship to treatment response. Synapse 65: 215–224. 7. Somerville SM, Conley RR, Roberts RC (2012) Striatal mitochondria in subjects with chronic undifferentiated vs. chronic paranoid schizophrenia. Synapse 66: 29–41. 8. Kolomeets NS, Uranova NA (1997) Synaptic contacts in schizophrenia: study with immunocytochemical identification of dopaminergic neurons. Zh Nevrol Psikhiatr Im S S Korsakova 97: 39–43. 9. Orth M, Schapira AH (2001) Mitochondria and degenerative disorders. Am J Med Genet 106: 27–36. 10. Rollins B, Martin M V, Sequeira PA, Moon EA, Morgan LZ, et al. (2009) Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One 4: e4913. 11. Hall CN, Klein-Flugge MC, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32: 8940–8951. 12. Volz HR, Riehemann S, Maurer I, Smesny S, Sommer M, et al. (2000) Reduced phosphodiesters and high-energy phosphates in the frontal lobe of schizophrenic patients: a (31)P chemical shift spectroscopic-imaging study. Biol Psychiatry 47: 954–961. 141 13. Saddar S, Dienhart MK, Stuart RA (2008) The F1F0-ATP synthase complex influences the assembly state of the cytochrome bc1-cytochrome oxidase supercomplex and its association with the TIM23 machinery. J Biol Chem 283: 6677–6686. 14. Manatt M, Chandra S (2011) The effects of mitochondrial dysfunction in schizophrenia. J Med Genet Genomics. 3: 84-94. 15. Fontanesi F, Soto IC, Horn D, Barrientos A (2006) Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol 291: C1129–C1147. 16. Fukui H, Moraes CT (2008) The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci 31: 251–256. 17. Michel H, Behr J, Harrenga A, Kannt A (1998) Cytochrome c oxidase: structure and spectroscopy. Annu Rev Biophys Biomol Struct 27: 329–356. 18. Campbell N. A., Williamson B., and Heyden R. J. (2006). Biology: Exploring Life. Boston: Pearson Prentice Hall. 19. Babcock DF, Hille B (1998) Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol 8: 398–404. 20. Brodin L, Bakeeva L, Shupliakov O (1999) Presynaptic mitochondria and the temporal pattern of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354: 365–372. 21. Mattson MP, Culmsee C, Yu Z, Camandola S (2000) Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem 74: 443–456. 22. Li Z, Okamoto K-I, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119: 873–887. 23. Verstreken P, Ly CV, Venken KJT, Koh T-W, Zhou Y, et al. (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47: 365–378. 24. Barksdale KA, Perez-Costas E, Gandy JC, Melendez-Ferro M, Roberts RC, et al. (2010) Mitochondrial viability in mouse and human postmortem brain. FASEB J 24: 3590–3599. 142 25. Lee Y, Oh SB, Park HR, Kim HS, Kim MS, et al. (2013) Selective impairment on the proliferation of neural progenitor cells by oxidative phosphorylation disruption. Neurosci Lett 535: 134-139. 26. Prince JA, Yassin MS, Oreland L (1997) Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther 280: 261–267. 27. Maurer I, Zierz S, Moller H (2001) Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res 48: 125–136. 28. Rosenfeld M, Brenner-Lavie H, Ari SG-B, Kavushansky A, Ben-Shachar D (2011) Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry 69: 980–988. 29. Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, et al. (2012) Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 13: 293–307. 30. Cavelier L, Jazin EE, Eriksson I, Prince J, Bave U, et al. (1995) Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics 29: 217–224. 31. Prince JA, Blennow K, Gottfries CG, Karlsson I, Oreland L (1999) Mitochondrial function is differentially altered in the basal ganglia of chronic schizophrenics. Neuropsychopharmacology 21: 372–379. 32. Ben-Shachar D and Karry R (2008) Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One 3 (11): e3676. 33. Ben-Shachar D (2009) The interplay between mitochondrial complex I, dopamine and Sp1 in schizophrenia. J Neural Transm 116: 1383- 1398. 34. Rosenfeld M, Brenner-Lavie H, Ari SG, Kavushansky A, Ben-Shachar D (2011) Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psych 69: 980- 988. 35. Verkhovsky MI, Jasaitis A, Verkhovskaya ML, Morgan JE, Wikstrom M (1999) Proton translocation by cytochrome c oxidase. Nature 400: 480–483. 36. Huttemann M, Kadenbach B, Grossman LI (2001) Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267: 111–123. 37. Li Y, Park J-S, Deng J-H, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38: 283–291. 143 38. Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E (2000) Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic Biol Med 29: 211–221. 39. Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, et al. (2002) Cytochrome oxidase in health and disease. Gene 286: 53–63. 40. Shoubridge EA (2001) Cytochrome c oxidase deficiency. Am J Ned Genet 106:4652. 41. Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, et al. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272: 1136–1144. 42. Nijtmans LG, Taanman JW, Muijsers AO, Speijer D, Van den Bogert C (1998) Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem 254: 389– 394. 43. Taanman JW (1997) Human cytochrome c oxidase: structure, function, and deficiency. J Bioenerg Biomembr 29: 151–163. 44. Bruno C, Martinuzzi A, Tang Y, Andreu AL, Pallotti F, et al. (1999) A stop-codon mutation in the human mtDNA cytochrome c oxidase I gene disrupts the functional structure of complex IV. Am J Hum Genet 65: 611–620. 45. Clark KM, Taylor RW, Johnson MA, Chinnery PF, Chrzanowska-Lightowlers ZM, et al. (1999) An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am J Hum Genet 64: 1330–1339. 46. Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, et al. (1999) A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am J Hum Genet 65: 1030–1039. 47. Tiranti V, Corona P, Greco M, Taanman JW, Carrara F, et al. (2000) A novel frameshift mutation of the mtDNA COIII gene leads to impaired assembly of cytochrome c oxidase in a patient affected by Leigh-like syndrome. Hum Mol Genet 9: 2733–2742. 48. Whatley SA, Curti D, Marchbanks RM (1996) Mitochondrial involvement in schizophrenia and other functional psychoses. Neurochem Res 21: 995–1004. 49. Maurer I, Moller HJ (1997) Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem 174: 255–259. 144 50. Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker WD (1993) Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol 33: 512– 517. 51. Whatley SA, Curti D, Das Gupta F, Ferrier IN, Jones S, et al. (1998) Superoxide, neuroleptics and the ubiquinone and cytochrome b5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry 3: 227–237. 52. Balijepalli S, Boyd MR, Ravindranath V (1999) Inhibition of mitochondrial complex I by haloperidol: the role of thiol oxidation. Neuropharmacology 38: 567–577. 53. Balijepalli S, Kenchappa RS, Boyd MR, Ravindranath V (2001) Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int 38: 425–435 54. Streck EL, Rezin GT, Barbosa LM, Assis LC, Grandi E, et al. (2007) Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedebergs Arch Pharmacol 376: 127–133. 55. van Domburg PH, ten Donkelaar HJ (1991) The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol 121: 1–132. 56. Haber SN, Fudge JL (1997) The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol 11: 323–342. 57. Perez-Costas E, Melendez-Ferro M, Roberts RC (2010) Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem 113: 287–302. 58. Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC (2012) Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front psychiatry 3: 31. 59. Spitzer RL, Williams JB, Gibbon M, First MB (1992) The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry 49: 624–629. 60. Paxinos G, Huang XF (1995) Atlas of the human brainstem. San Diego: Academic Press. 61. Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC (2008) Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm 115: 745–753. 145 62. Divac I, Mojsilovic-Petrovic J, Lopez-Figueroa MO, Petrovic-Minic B, Moller M (1995) Improved contrast in histochemical detection of cytochrome oxidase: metallic ions protocol. J Neurosci Methods 56: 105–113. 63. Poeggeler B, Rassoulpour A, Guidetti P, Wu HQ, Schwarcz R (1998) Dopaminergic control of kynurenate levels and N-methyl-D-aspartate toxicity in the developing rat striatum. Dev Neurosci 20: 146–153. 64. Melendez-Ferro M, Rice MW, Roberts RC, Perez-Costas E (2013) An accurate method for the quantification of cytochrome C oxidase in tissue sections. J Neurosci Methods 214: 156–162. 65. Motulski HJ, Brown RE (2006) Detecting outliers when fitting data with nonlinear regression- a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7:123. 66. Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1: 43-46. 67. Motulsky HJ (2010) Intuitive biostatistics: A nonmathematical guide to statistical thinking. New York: Oxford University Press. 331-342 pp. 68. Pel HJ, Grivell LA (1994) Protein synthesis in mitochondria. Mol Biol Rep 19: 183194. 69. Richman TR, Davies SM, Shearwood AM, Ermer JA, Scott LH, et al. (2014) A bifunctional protein regulates mitochondrial protein synthesis. Nuclei Acids Res 42: 5483-5494. 70. Gattermann N, Retzlaff S, Wang YL, Hofhaus G, Heinisch J, et al. (1997) Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood 90: 4961-4972. 71. You KR, Wen J, Lee ST, Kim DG (2002) Cytochrome c oxidase subunit III: a molecular marker for N-(4-hydroxyphenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem 277: 3870-3877. 72. Abril J, de Heredia ML, Gonzalez L, Cleries R, Nadal M, et al. (2008) Altered expression of 12S/MT-RNR1, MTCO2/COX2, and MT-ATP6 mitochondrial genes in prostate cancer. Prostate 68: 1086-1096. 73. Ivanova MM, Luken KH, Zimmer AS, Lenzo FL, Smith RJ, et al. (2011) Tamoxifen increases nuclear respiratory factor 1 transcription by activating estrogen receptor beta and AP-1 recruitment to adjacent promoter binding sites. FASEB J 25: 14021416. 146 74. Arnold SR, Sun Q, Sun CQ, Richards JC, O’Hearn S, et al. (2013) A inherited heteroplasmic mutation in mitochondrial gene COI in a patient with prostate cancer alters reactive oxygen, reactive nitrogen and proliferation. Biomed Res Int (Epub ahead of print) doi: 10.1155/2013/239257. 75. Perez J, Hill BG, Benavides GA, Dranka BP, Darley-Usmar VM (2010) Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem J 428: 255–267. 76. Mitchell T, Chacko B, Ballinger SW, Bailey SM, Zhang J, et al. (2013) Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans 41: 127–133. 77. Francisconi S, Codenotti M, Ferrari Toninelli G, Uberti D, Memo M (2006) Mitochondrial dysfunction and increased sensitivity to excitotoxicity in mice deficient in DNA mismatch repair. J Neurochem 98: 223-233. 78. Crosby EC, Woodburne RT (1943) The nuclear pattern of the non-tectal portions of the midbrain and isthmus in primates. J Comp Neurol 78: 441-482. 79. Marin O, Smeets WJ, Gonzalez A (1998) Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci 21: 487– 494. 80. Joel D, Weiner I (2000) The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96: 451–474. 81. Augustine JR (2008) The brain stem. In: Human Neuroanatomy. London: Academic Press. pp. 49-68. 82. Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, et al. (1999) Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156: 1580–1589. 147 Table 1. Demographic and clinical data of the cases used for the study. Abbreviations: A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; COD, cause of death; CUT, chronic undifferentiated type; DVT, deep vein thrombosis; F, female; M, male; MI, myocardial infarction; NOS, not otherwise specified; MVA, motor vehicle accident; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; TD, tardive dyskinesia; un, unknown; UNK, unknown medication at time of death; W, white. 148 Table 2. Effect of demographic and sample variables on COX activity and COX subunit expression. Note that experiments were run independently for each outcome measure, thus, independent multiple regression tests were run for each outcome measure. Analyses were carried independently for rostro-caudal and mid-caudal samples. Demographic and sample variables were tested in separated tests. Abbreviations: R2a, adjusted R squared. pH, brain pH. PMI, postmortem interval. 149 Figure 1. Analysis of COX activity in samples containing the entire rostro-caudal extent of the human SN/VTA. A) Representative images of thionin-stained sections and COX histochemistry in the SN/VTA and red nucleus from schizophrenia and control cases. Dashed lines in the thionin-stained sections show the masks used for the delineation of the region of interest. B) Top panel shows the logarithm of optical density (log OD) for 150 COX activity in the SN/VTA (geometric mean with bar representing the 95% confidence interval). The mid panel shows a scatter plot of the individual data. The horizontal bar in each scatter plot indicates the geometric mean OD. The bottom panel shows COX activity in the red nucleus. 151 Figure 2. Analysis COX activity in samples containing only the mid-caudal regions of the human SN/VTA. A) Representative images of thionin-stained sections and COX histochemistry in the SN/VTA and red nucleus from schizophrenia and control cases. Dashed lines in the thionin-stained sections show the masks used for the delineation of the region of interest. B) Top panel shows the logarithm of optical density (log OD) for COX activity in the SN/VTA (geometric mean with bar representing the 95% confidence interval). The mid panel shows a scatter plot of the individual data. The horizontal bar in each scatter plot indicates the geometric mean OD. The bottom panel shows COX activity in the red nucleus. 152 Figure 3. Analysis of COX subunits I, II, and III in samples containing the entire rostrocaudal extent of the human SN/VTA. For each subunit, western blot images of all the samples assessed in schizophrenia and control cases are shown at the top of each panel, 153 together with an image of the same western blot membrane reincubated for the detection of actin. Outliers are indicated with a red box surrounding the case number. Bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX Subunit I expression. Subunit I protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 0.6182 ± 0.07451; control: 0.6014 ± 0.08532). B) Analysis of COX Subunit II expression. There were significant (*) differences for subunit II expression between schizophrenia and controls (OD mean ± SD: schizophrenia: 0.21719 ± 0.02887; control: 0.4751± 0.1954). C) Analysis of COX Subunit III expression. Subunit III protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 0.8153 ± 0.3878; control: 0.5388 ± 0.1281). 154 Figure 4. Analysis of COX subunits IV-I and IV-II in samples containing the entire rostro-caudal extent of the human SN/VTA. For each subunit, western blot images of all the samples assessed in schizophrenia and control cases are shown at the top of each panel, together with an image of the same western blot membrane reincubated for the detection of actin. An outlier is indicated with a red box surrounding the case number. Bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX Subunit IV-I expression. Subunit IV-I protein expression showed significant (*) differences between the two groups (OD mean ± SD: schizophrenia: 0.43541 ± 0.20501; control: 0.84416 ± 0.56772). B) Analysis of COX Subunit IV-II protein expression. Subunit IV-II protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 1.340 ± 0.2261; control: 1.354 ± 0.3104). 155 Figure 5. Analysis of COX subunits I, II, and III protein expression in the mid-caudal human SN/VTA. For each subunit, western blot images of all the samples assessed in schizophrenia and control cases are shown at the top of each panel, together with an 156 image of the same western blot membrane reincubated for the detection of actin. Outliers are indicated with a red box surrounding the case number. Bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX Subunit I. Subunit I protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 0.6355 ± 0.1680; control: 0.5998 ± 0.1213). B) Analysis of COX Subunit II. Subunit II protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 0.9332 ± 0.3837; control: 1.352 ± 0.7247). C) Analysis of COX Subunit III. Subunit III protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 0.5228 ± 0.08916; control: 0.8408 ± 0.5324). 157 Figure 6. Analysis of COX subunits IV-I and IV-II protein expression in the mid-caudal human SN/VTA. For each subunit, western blot images of all the samples assessed in schizophrenia and control cases are shown at the top of each panel, together with an image of the same western blot membrane reincubated for the detection of actin. Bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX Subunit IV-I. Subunit IV-I protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 1.336 ± 0.4237; control: 1.789 ± 0.4741). B) Analysis of COX Subunit IV-II. Subunit IV-II protein expression did not differ significantly between schizophrenia and control cases (OD mean ± SD: schizophrenia: 1.279 ± 0.3895; control: 1.097 ± 0.1950). 158 Figure 7. Analysis of COX subunits I, II, and III in the SN/VTA of animals treated with antipsychotic drugs. Western blot images of the expression of each COX subunit in all 159 animals from the three treatment groups are shown at the top of each panel. Each western blot membrane was reincubated for the detection of actin. Bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX subunit I. Subunit I protein expression did not differ significantly among the three treatment groups (OD mean ± SD: control: 0.4753 ± 0.06210; haloperidol: 0.5145 ± 0.1158; olanzapine: 0.4731 ± 0.1025). B) Analysis of COX subunit II. Subunit II protein expression did not differ significantly among the three treatment groups (OD mean ± SD: control: 0.4740 ± 0.1118; haloperidol: 0.5535 ± 0.1312; olanzapine: 0.5564 ± 0.1121). C) Analysis of COX subunit III. Subunit III protein expression did not differ significantly among the three treatment groups (OD mean ± SD: control: 1.480 ± 0.4543; haloperidol: 1.609 ± 0.2989; olanzapine: 1.455 ± 0.4438). 160 Figure 8. Analysis of COX subunits IV-I and IV-II in the SN/VTA of animals treated with antipsychotic drugs. Western blot images of the expression of each COX subunit in all animals from the three treatment groups are shown at the top of each panel. Each western blot membrane was reincubated for the detection of actin. The bar graphs indicate the mean calibrated optical density (OD) and standard deviation. Scatter plots are shown on the right with a horizontal bar indicating the mean. A) Analysis of COX subunit IV-I. Subunit IV-I protein expression did not differ significantly among the three treatment groups (OD mean ± SD: control: 1.084 ± 0.2609; haloperidol: 1.021 ± 0.3270; olanzapine: 1.098 ± 0.3559). B) Analysis of COX subunit IV-II. Subunit IV-II protein expression did not differ significantly among the three treatment groups (OD mean ± SD: control: 0.6829 ± 0.1375; haloperidol: 0.8100 ± 0.1611; olanzapine: 0.7551 ± 0.08140). 161 MAPPING DOPAMINERGIC DEFICIENCIES IN THE SUBSTANTIA NIGRA/ VENTRAL TEGMENTAL AREA IN SCHIZOPHRENIA by Matthew W. Rice, Rosalinda C. Roberts, Miguel Melendez-Ferro, & Emma Perez-Costas Under Review in Brain Structure and Function Format adapted and errata corrected for dissertation 162 Abstract Previous work from our laboratory showed deficits in tyrosine hydroxylase protein expression within the substantia nigra/ventral tegmental area (SN/VTA) in schizophrenia. However, little is known about the nature and specific location of these deficits within the SN/VTA. The present study had two aims: 1) test if tyrosine hydroxylase deficits could be explained as the result of neuronal loss; 2) assess if deficits in tyrosine hydroxylase are sub-region specific within the SN/VTA, and thus, could affect specific dopaminergic pathways. To achieve these objectives: 1) we obtained estimates of the number of dopaminergic neurons, total number of neurons and their ratio in matched SN/VTA schizophrenia and control samples; 2) we performed a qualitative assessment in SN/VTA schizophrenia and control matched samples that were processed simultaneously for tyrosine hydroxylase immunohistochemistry. We did not find any significant differences in the total number of neurons, dopaminergic neurons, or their ratio. Our qualitative study of TH expression showed a conspicuous decrease in labeling of neuronal processes and cell bodies within the SN/VTA, which was sub-region specific. Dorsal diencephalic dopaminergic populations of the SN/VTA presented the most conspicuous decrease in TH labeling. These data support the existence of pathway-specific dopaminergic deficits that would affect the dopamine input to the cortex without significant neuronal loss. Interestingly, these findings support earlier reports of decreases in tyrosine hydroxylase labeling in the target areas for this dopaminergic input in the prefrontal and entorhinal cortex. Finally, our 163 findings support that tyrosine hydroxylase deficits could contribute to the hypodopaminergic state observed in cortical areas in schizophrenia. 164 Introduction In schizophrenia, anomalies within the dopaminergic system are an intrinsic pathological feature of the illness (see as reviews van Os and Kapur 2009; Abi-Dargham et al. 2010; Perez-Costas et al. 2010; Tost et al. 2010). In addition, the effectiveness of antipsychotic medication is directly linked to their action on the dopaminergic system (Carlsson et al. 1957; Carlsson and Lindqvist 1963; Seeman and Lee 1975; Seeman et al. 1976; Creese et al. 1976; see also as reviews Frankle and Laruelle 2002; Howes and Kapur 2009; Perez-Costas et al. 2010). Despite the importance of dopaminergic pathologies in schizophrenia, only a few studies have examined the substantia nigra/ventral tegmental area (Bogerts et al. 1983; Ichinose et al.1994; Mueller et al. 2004; Perez-Costas et al. 2012; Howes et al. 2013; Williams et al. 2013), which is the most prominent source for dopamine in the brain. The substantia nigra/ventral tegmental area (SN/VTA) complex is a heterogeneous collection of dopaminergic cell groups that extends from diencephalic to mesencephalic territories within the brain of rodents, non-human primates, and humans (Puelles and Verney 1998; see as reviews Smits et al. 2006; Smidt and Burbach 2007; Smits et al. 2013). The SN/VTA contains the largest number of dopaminergic neurons in the brain (see as reviews van Domburg and ten Donkelaar 1991; Haber and Fudge 1997; Nieuwenhuys et al. 2008), with average estimates ranging in most studies from approximately 250,000-440,000 dopaminergic neurons within the complex (Bogerts et al. 1983; Hirsch et al. 1988; van Domburg and ten Donkelaar 1991; Damier et al. 1999a; Kubis et al. 2000). 165 The human SN/VTA presents high complexity and is composed of several dopaminergic neuronal subpopulations that differ in their neurodevelopmental origin (Zecevic and Verney 1995; Puelles and Verney 1998; Verney et al. 2001), genetic and neurochemical profile (Haber et al. 1995; Grimm et al. 2004; Thuret et al. 2004; Chung et al. 2005; Luk et al. 2013), projection sites (van Domburg and ten Donkelaar 1991; Damier et al. 1999a; Nieuwenhuys et al. 2008), and susceptibility to disease (Gibb and Lees 1991; Damier et al. 1999b; Hauser et al. 2005; Fuchs et al. 2009; Bergman et al. 2010). In fact, developmental studies of the human brain have clearly demonstrated the existence of well-defined rostro-caudal (i.e. anterior-posterior) sub-regions within the SN/VTA, which contain subgroups of dopaminergic cells with different genetic profiles and developmental schedule (Puelles and Verney 1998; Verney et al. 2001; van den Heuvel and Pasterkamp 2008; Nelander et al. 2009). For example, TH-expressing dopaminergic neurons located in the caudal sub-region (i.e. immature mesencephalon) of the SN/VTA are already detected by the 6th gestational week, while in the rostral SN/VTA sub-region (i.e. immature diencephalon or prosomeres 1-3 of the neural tube) these cells are not clearly observed until the 10th gestational week (Zecevic and Verney, 1995; Verney, 1999; Puelles and Verney 1998; Verney et al. 2001). In the adult brain, this diencephalic sub-region includes approximately all SN/VTA neuronal populations located rostral to the exit of the 3rd cranial nerve and to the transition between the parvocellular and magnocellular parts of the red nucleus (see for anatomical details van Domburg and ten Donkelaar 1991; Damier et al. 1999a; Nieuwenhuys et al. 2008). In addition, detailed neuropathological studies in Parkinson’s disease have shown that diencephalic SN/VTA dopaminergic neurons are significantly more resilient to neuronal 166 death than the mesencephalic ones (Damier et al. 1999b), thus supporting the validity of using developmental criteria to define SN/VTA sub-regions for neuropathological studies in the adult brain. Detailed studies on the projection targets of the dopaminergic neurons of the SN/VTA have also demonstrated the existence of neuronal subgroups within the complex that preferentially project to cortical or subcortical regions (see as reviews van Domburg and ten Donkelaar 1991; Haber and Gdowski 2004; Nieuwenhuys et al. 2008). For example, dorsal neuronal groups of the SN/VTA in primates preferentially provide dopaminergic input to cortical areas through the mesocortical pathway, although they also partially contribute to the mesolimbic pathway (Gaspar et al. 1992; Haber and Fudge 1997; see also as reviews Haber and Godowski 2004; Nieuwenhuys et al. 2008). Interestingly, dopaminergic deficits in these pathways have been associated with the hypodopaminergic state observed in individuals diagnosed with schizophrenia (Akil et al. 1999, 2000; Finlay 2001; Seamans and Yang 2004; Toda and Abi-Dargham 2007), and are most closely related to the cognitive deficits that are a core feature of the illness (Finlay 2001; Seamans and Yang 2004; Tanaka 2006; Toda and Abi-Dargham 2007). Ventrally-located neurons of the SN/VTA complex preferentially project to subcortical regions such as the dorsal and ventral striatum through the nigrostriatal, and in part, through the mesolimbic pathway (see as reviews Haber and Godowski 2004; Nieuwenhuys et al. 2008). Disturbances of dopamine neurotransmission within the nigrostriatal pathway in schizophrenia are associated with a hyperdopaminergic state, and are most closely linked to the positive symptoms (e.g. psychosis) seen in this illness 167 (Seeman 1987; Davis et al. 1991; Toda and Abi-Dargham 2007; Howes and Kapur 2009; Meyer-Lindenberg 2010). Dopamine plays a key role in the modulation of cognitive processes in the prefrontal cortex, and it is known that low dopamine levels reduce working memory performance in humans (Seamans and Yang 2004; Floresco and Magyar 2006; Tanaka 2006). Several different molecular pathologies that produce, as a net result, a deficit of dopaminergic neurotransmission in the prefrontal cortex have been identified in schizophrenia subjects (Akil et al. 1999; Seamans and Yang 2004; Guillin et al. 2007; Tan et al. 2007; Raznahan et al. 2011; Perez-Costas et al. 2012a; Kaalund et al. 2013; Lopez-Garcia et al. 2013). Among these pathologies, work from our laboratory has revealed deficits in TH protein expression in the SN/VTA in schizophrenia subjects, although these deficits were only observed in cases that contained the rostral (diencephalic) region of the SN/VTA complex (Perez-Costas et al. 2012a), which indicates a possible sub-region specific deficit within the complex. In this study we sought to elucidate if these TH deficits could be related to overall and/or subregionspecific reductions in neuronal number, by examining both total and dopaminergic neuronal counts in schizophrenia subjects compared to non-psychiatric controls. In addition, we performed a qualitative assessment on the specific location of these TH expression deficits. 168 Materials and Methods Ethics statement All human brain samples were obtained from the Maryland Brain Collection (Maryland Psychiatric Research Center, University of Maryland School of Medicine) with approval from the Maryland Brain Collection Steering Committee. All experimental procedures were approved by the University of Alabama at Birmingham Institutional Review Board (protocols #N110505002 and #N131015007) and were in accordance with The Code of Ethics of the World Medical Association. Postmortem human brain samples For all postmortem human cases, two independent psychiatrists established DSMIV diagnoses based on the review of available medical records and data obtained from the Structured Clinical Interview for DSM-IIIR (Spitzer et al., 1992) and DSM-IV Axis I disorders with the next-of-kin. Non-psychiatric control cases had enough information from medical records and the next-of-kin to discard any major neurological or neuropsychiatric disorders. Table 1 contains demographic data, as well as information on the use of antipsychotic treatment, cause of death, and other relevant parameters for the samples used in this study. All postmortem SN/VTA samples were dissected as described by Damier et al (1999a) and preserved in 4% paraformaldehyde prepared in 0.1M phosphate buffer pH 7.4 (PB), at 4°C. Prior to sectioning, the tissue was thoroughly rinsed in PB, and immersed in a solution containing 30% sucrose in PB at 4°C, for a minimum of two weeks. Tissue samples were frozen using dry ice and sectioned on a freezing microtome. 169 Eight adjacent (i.e. parallel) series were obtained perpendicular to the longitudinal axis of the brainstem at a thickness of 50m. All samples were sectioned in this manner to allow for replication of the experiments, and to maintain backup series for future studies. All series were equivalent and contained sections of the entire rostro-caudal extent of the SN/VTA. Series #1 and 2 were collected in PB containing 0.02% sodium azide and stored at 4°C until use. Series #3 through 8 were collected in a cryoprotectant solution prepared as described in Watson et al. (1986) and stored at -20°C. For all cases, series #1 was stained using thionin (FD Neurotechnologies, MD, USA; PS101-01) to assess for any morphological anomalies related to poor preservation of the tissue, and only those cases that presented good preservation of the SN/VTA were included in the study. Exclusion criteria were used in the same manner for schizophrenia and control samples as previously described (Perez-Costas et al, 2012a). Immunohistochemistry Complete series immunohistochemistry. preserved in cryoprotectant solution were used for Sections were rinsed multiple times in 0.01M phosphate buffered saline (PBS) at room temperature (RT) to remove the cryoprotectant solution. Sections were then transferred to citrate buffer (Vector Laboratories, Burlingame, CA, USA; H-3300) for ten minutes at RT, prior to a 30 minute citrate pretreatment at 80°C. Following pretreatment, sections were rinsed in citrate buffer at RT and transferred to PBS. After that, endogenous peroxidase was blocked with 5% H202 in PBS for 30 minutes at RT, followed by several rinses in PBS. Sections were then incubated with 10% 170 normal horse serum (NHS; Vector Laboratories, S2000) in PBS containing 0.5% Triton X-100 (PBS-T) for 1 hour at RT. Immediately after, sections were incubated for 66 hours at 4°C with an anti-TH antibody (Millipore, Billerica, MA, USA; MAB318, diluted 1:5000) prepared in a solution containing 3% NHS in PBS-T. Next, sections were rinsed in PBS and incubated with a biotinylated horse anti-mouse secondary antibody (Vector Laboratories, BA-2001, diluted 1:400) in a solution containing 3% NHS in PBS-T for 45 minutes at RT. After this, sections were rinsed in PBS and incubated with a peroxidasecoupled avidin-biotin-complex (Vector Laboratories PK6100, diluted 1:100) for 45 minutes at RT. Finally, sections were rinsed in PBS and developed using a 3,3'diaminobenzidine peroxidase kit (Vector Laboratories, SK4100). A negative control, incubated in a solution lacking the primary antibody and consisting of 3% NHS in PBST, was also performed to ensure specificity of the secondary antibody. In addition, the specificity of the primary antibody has been previously determined by the manufacturer. For each case, a minimum of two complete series representing the entire rostrocaudal extent of the SN/VTA were immunolabeled for TH. One of these series was immunolabeled for TH without counterstaining, while the other series was counterstained using thionin (FD Neurotechnologies) after immunolabeling. In all cases, sections were dehydrated in ethanol, cleared in xylene and coverslipped using Eukitt (Electron Microscopy Sciences, Hatfield, PA, USA; 15322). Schizophrenia and matched control cases were processed together for immunohistochemistry, carefully maintaining the exact same incubation and developing conditions across cases. 171 Photography and image processing Images were obtained using a Nikon Eclipse 50i microscope equipped with a Nikon DS-Fi1 color digital camera ((Nikon, Tokyo, Japan). Images were saved as uncompressed TIFF files at a 2560x1920 resolution. Adjustments of brightness/contrast were done using the same parameters for all images. These adjustments as well as photomontage and lettering of figure plates were done using Corel DRAW X5 (Corel Corporation, Ottawa, Canada). Neuronal counts and mapping of TH deficits Oversampling test for study design A pilot study was conducted to determine the optimal number of sections, and the number of counting frames to be analyzed from each section. A complete series (approximately 30-32 sections) from each of two control cases (C3 and C6) were used to perform this preliminary assessment. Each section was photographed using a 10X objective and setting randomly a fixed point in the Z axis within the 50μm thickness of the section. Ten non-overlapping images were taken from each section to cover the entire SN/VTA complex within the section. Each image was considered a “counting frame” with the bottom and right margins of the image as the forbidden lines of the counting frame. Neurons were selected for counting only if the entire cell body and the nucleus were clearly defined in the image. Neurons touching the right and bottom borders of the frame were excluded from counting. A “complete series estimate” (CSE) for the number of total and dopaminergic neurons was obtained as the mean of the counts of all the neurons (dopaminergic or total) for one entire series for the two cases. The neuronal 172 counts accuracy was calculated as the “percentage of variation” from the CSE using the following formula: % of variation= |[(Partial count estimate – CSE)/CSE] x 100| A summary of the results of this oversampling test is reported in Table 2 (see also results). Neuronal counts in schizophrenia and control cases Following the oversampling test, the optimal counting scheme was set as follows: 1) For each case, using a complete SN/VTA series labeled for TH and counterstained with thionin, the rostral and caudal boundaries of the SN/VTA were determined using the Paxinos and Huang (1995) atlas of the human brainstem as well as the neuroanatomical study of van Domburg and ten Donkelaar (1991). The boundary between the diencephalic (i.e. rostral) and mesencephalic (i.e. mid-caudal) sub-regions of the SN/VTA was defined as the transition between the parvocellular and magnocellular parts of the red nucleus, at the level of the exit of the oculomotor nerve (see e.g. Damier et al. 1999a). 2) The first section to be counted was selected randomly within 1200μm of the rostral boundary of the SN/VTA using a random number generator (i.e. random.org). 3) From this randomly chosen rostral starting point, every third section of the series, and up to the caudal limit of the SN/VTA, was selected for neuronal counts (i.e. approximately 10-11 sections per case). 4) A random focal point in the Z axis was chosen within the 50μm thickness of the section. 5) Using the 10X objective, five images representing 5 randomly placed and nonoverlapping “counting frames” were taken for each section within the SN/VTA. 6) The 173 “cell counter” plug-in of NIH Image J software version 1.46r (http://rsbweb.nih.gov/ij/) was used for the quantification of dopaminergic and non-dopaminergic neuronal counts. To avoid investigator bias on data collection, all cases were coded, and the researcher performing the neuronal counts was blind for the case diagnoses. In addition, our experimental design prevented any risk of double-counting neurons since each section selected for neuronal counts was 1200μm apart (i.e. one series was used for counts out of 8 series collected, and from this series, 1 out of every 3 sections was counted). Dopaminergic neurons were identified by the presence of TH immunolabeling, and non-dopaminergic neurons identified by their purple-blue thionin staining and lack of TH immunostaining. Qualitative mapping of dopaminergic deficits Three schizophrenia cases and their most closely matched control cases were used for this qualitative analysis. Two entire SN/VTA series per case labeled for TH were used for this assessment. For this part of the study we selected schizophrenia cases SZ1, SZ3 and SZ6, for which we previously assessed TH protein expression by western-blot in the opposite hemisphere (Perez-Costas et al. 2012; cases SZ1, SZ4, SZ6). The matched controls cases were C2, C5 and C6, from which C6 was also included in the previous protein expression study (i.e. case C4 in Perez-Costas et al. 2012). 174 Statistical analysis of neuronal counts Effect of demographic and sample quality variables on outcome measures Schizophrenia and non-psychiatric control cases were matched as much as possible for demographic (i.e. age, gender, race), and sample (i.e. postmortem interval, brain pH) variables. We used multiple regression to assess the possible influence of demographic and sample variables on our outcome measures (i.e. cell counts) independent of disease status. Demographic and sample variables (i.e. postmortem interval and pH) were tested in separated analyses. For the analysis of demographic variables a “dummy coding” was used to numerically interpret gender and race. The effect of demographic and sample variables was assessed independently for each brain region/sub-region (i.e. entire SN/VTA, diencephalic region, mesencephalic region). However, the effect of these variables on all cell counts of a given region/sub-region was assessed in the same analysis, correcting for multiple comparisons, and the adjusted R 2 is reported to eliminate the add-on effect of testing for multiple outcome variables. Neuronal counts in schizophrenia versus controls All data collected on neuronal counts were assessed for the presence of significant deviations from normality. In addition, the presence of outliers was determined using the “Robust regression and OUtlier Removal” (ROUT) test for outlier detection (Motulski and Brown 2006), with the coefficient Q set to 1%. Unpaired t-tests were corrected for multiple comparisons using the False Discovery Rate (FDR) with Q set at 1% (Motulsky 2010). Multiple comparisons corrections were applied for all parameters tested in the same specific region/sub-region (e.g. total number of neurons, dopamine neurons and 175 ratio in the diencephalic SN/VTA were treated as multiple comparisons). Statistical analysis and graphical representation of the results obtained were done using GraphPad Prism 6.0 software (Graphpad Software Inc., La Jolla, CA, USA). Results Oversampling test for study design In order to obtain accurate estimates of the number of dopaminergic and total neurons, we performed an oversampling study using two control cases (C3 and C6), in which neuronal counts were performed for the entire rostro-caudal extent of the SN/VTA. A summary of the results obtained is shown in Table 2. From this assessment we concluded that we can obtain highly accurate cell count estimates by starting at a random section within the most rostral 1200µm of the SN/VTA, and counting cells on every 3rd section. We also showed that reducing the number of counting frames to one-half (i.e. 5 randomly selected frames counted per section) did not have a significant impact on the accuracy of the counts. Demographic and sample features The effect of demographic (i.e. age, gender, race) and sample (i.e. brain pH and postmortem interval) variables on neuronal counts (i.e. total neurons and dopamine neurons) was assessed by performing independent multiple regression analyses for these two types of variables. Data obtained are summarized in Tables 3 and 4. None of these variables had any significant effect on cell counts (total or dopamine neurons) for any of the regions/sub-regions (i.e. rostro-caudal, rostral, mid-caudal SN/VTA) analyzed. 176 Dopaminergic and total neuronal counts Rostral and caudal limits of the SN/VTA were defined as described in previous neuroanatomical studies and atlases that used the same sectioning orientation used in the present study (van Domburg and ten Donkelaar 1991; Paxinos and Huang 1995; Damier et al. 1999a; Nieuwenhuys et al. 2008). The rostral sub-region was defined as the diencephalic territories of the SN/VTA, while mid-caudal sub-region consisted in the mesencephalic territories of the complex. These subdivisions were included in the study design (see methods), and were based on previous developmental studies showing clearly demarcated diencephalic and mesencephalic SN/VTA sub-regions in the human (Zecevic and Verney 1995; Puelles and Verney 1998; Verney et al. 2001), and conspicuous differences in the response to pathological processes of these two sub-regions (Gibb and Lees 1991; Damier et al. 1999b; Hauser et al. 2005). Neuronal counts in the entire SN/VTA We obtained estimates of the total number of neurons and dopaminergic neurons for the entire SN/VTA. Unpaired t-test corrected for multiple comparisons were used to compare these measurements in schizophrenia versus controls. There were no significant differences in the total number of neurons (schizophrenia=603,160±165,990; control=656,537±128,964; p=0.5272, t=0.6529, df=11), number of dopaminergic neurons (schizophrenia=218,528±70,563; control=249,161±44,353; p=0.3997, t=0.8760, df=11), or the ratio of dopaminergic/total neurons (schizophrenia=0.3610±0.0735; control=0.3819±0.0328; p=0.5099, t=0.6811, df=11) [Figure 1]. 177 Neuronal counts in the diencephalic SN/VTA Unpaired t-test corrected for multiple comparisons yielded non-significant differences in the total number of neurons (schizophrenia=224,064±28,903; control=202,519±61,180; p=0.4479, t=0.787, df=11), number of dopaminergic neurons (schizophrenia=70,640±25,495; control=70,512±22,552; p=0.9925, t=0.0096, df=11), or the ratio of dopaminergic/total neurons (schizophrenia=0.3198±0.1168; control=0.3554±0.0748; p=0.5189, t=0.6664, df=11) in the diencephalic (rostral) subregion of the SN/VTA [Figure 2]. Neuronal counts in the mesencephalic SN/VTA Unpaired t-test corrected for multiple comparisons for the mesencephalic (midcaudal) sub-region also yielded non-significant differences in the total number of neurons (schizophrenia=379,096±174,111; control=454,018±93,937; p=0.3445, t=0.9876, df=11), number of dopaminergic neurons (schizophrenia=147,888±52,813; control=178,649±50,190; p=0.3051, t=1.0757, df=11), or the ratio of dopaminergic/total neurons (schizophrenia=0.4165±0.1389; control=0.3898±0.0410; p=0.6354, t=0.4876, df=11) [Figure 3]. Qualitative mapping of dopaminergic deficits in the SN/VTA There is great variability in the sectioning orientation and nomenclature of subdivisions of the human SN/VTA in anatomical studies, creating great complexity for comparative assessments of neuropathology. In the present study all samples were sectioned in a plane perpendicular to the main axis of the brainstem, which is the same 178 sectioning orientation previously used by van Domburg and ten Donkelaar (1991), Damier et al (1999a) and Nieuwenhuys et al (2008) detailed anatomical studies of the human SN/VTA. For the qualitative assessment of TH expression we also followed the major sub-regions within the SN/VTA (i.e. diencephalic versus mesencephalic subregions) previously used for the neuronal counts. In addition, we also took into account the dorso-ventral and medio-lateral location of the dopaminergic cell groups, defining three sub-areas for our qualitative assessment: dorsal SN/VTA, ventro-lateral SN/VTA, and ventro-medial SN/VTA [Figures 4-6]. Diencephalic SN/VTA The dorsal area of the diencephalic SN/VTA included the nuclei identified by van Domburg and ten Donkelaar (1991) as the pars lateralis of the substantia nigra, the anterolateral nucleus of the substantia nigra compacta, and the diencephalic part of the parabrachial nucleus pigmentosus. The ventrolateral area corresponded with the anterointermediate nucleus of the substantia nigra compacta, while the ventro-medial area included the anteromedial nucleus of the substantia nigra compacta and the diencephalic part of the paranigral nucleus of van Domburg and ten Donkelaar (1991) [Figures 4A-B]. The diencephalic sub-region of the SN/VTA presented an overall conspicuous reduction of TH labeling in schizophrenia compared to matched controls [Figure 4]. Interestingly, there was also a clear dorso-ventral pattern of TH deficits in schizophrenia compared to controls [Figures 4-5]. In schizophrenia, dorsal areas of the SN/VTA showed the lowest TH labeling, while ventral areas (ventro-lateral and ventro-medial), although also presenting clearly decreased labeling, consistently showed stronger TH 179 expression than dorsal SN/VTA dopaminergic neuronal groups [Figures 4 and 5]. Immunolabeling for TH was clearly decreased in cell bodies and also in neuronal processes [Figure 5]. Mesencephalic SN/VTA The dorsal area of the mesencephalic SN/VTA included the postero-superior and postero-lateral subnuclei of the substantia nigra compacta, as well as the mesencephalic part of the parabrachial nucleus pigmentosus of van Domburg and Donkelaar (1991), while the ventrolateral and ventromedial areas corresponded with the posteromedial nucleus of the substantia nigra compacta and the mesencephalic part of the paranigral nucleus, respectively. In the mesencephalic region of the SN/VTA differences in TH labeling between schizophrenia and controls were subtle [Figure 6]. Dorsal SN/VTA presented moderate to subtle reductions in overall labeling [Figures 6C-D, F-G]. Ventrolateral and ventromedial SN/VTA areas did not present clear differences in TH immunolabeling between schizophrenia and their matched control cases [Figures 6C, 6E, 6F, 6H]. Discussion The present study shows evidence of conspicuous sub-region specific deficits in TH protein expression within the diencephalic sub-region of the SN/VTA, without neuronal loss. Neuropathological changes in the SN/VTA, and more specifically, in the dopaminergic cell groups of the SN/VTA in schizophrenia subjects have been scarcely 180 studied. An early report by Bogerts et al. (1983) reported deficits in volume and neuronal size in specific subnuclei of the SN/VTA, while a recent work has reported increases in the volume of dopaminergic cells as well as glial anomalies in the mesencephalic portion of the SN/VTA (Williams et al. 2013). However, most of the few postmortem studies performed have focused on the expression of tyrosine hydroxylase (Ichinose et al. 1994; Mueller et al. 2004; Perez-Costas et al. 2012a; Howes et al. 2013), which is the ratelimiting enzyme for the production of dopamine. Tyrosine hydroxylase expression studies have reported different results, from increases in TH mRNA (Mueller et al. 2004) and TH protein immunolabeling (Howes et al. 2013), to no changes in TH mRNA (Ichinose et al. 1994; Perez-Costas et al. 2012a), and deficits in TH protein expression (Perez-Costas et al. 2012a). The differences in the data obtained in these studies could be due to several factors including the heterogeneity of the disease (see as reviews Keshavan et al. 2011; Nasrallah et al. 2011), methodological differences in the detection of TH mRNA and protein expression, and of special importance, differences in the sampling area within the SN/VTA. Our results will be discussed taking into account developmental and neuroanatomical considerations and addressing potential functional implications. Neuronal counts Neuronal count estimates obtained in the present study for total neurons as well as dopaminergic neurons, in schizophrenia and control samples, were comparable to previous reports in which estimates were obtained from the entire SN/VTA (Bogerts et al. 1983; Hirsch et al, 1988; van Domburg and ten Donkelaar 1991; Damier et al. 1999a; Rudow et al. 2008). Our analysis of the effect of demographic variables on the number of 181 dopaminergic neurons showed no significant effect of any of these variables, including age. While progressive cell loss related to normal aging has been reported in the SN/VTA (Fearnley and Lees 1991; Kubis et al. 2000; Rudow et al. 2008), most of the cases included in the present study were in the “middle age” group, ranging from 40-65 years of age, and only two cases were above this age (see Table 1). Previous studies assessing total and dopaminergic neuronal cell number in the entire SN/VTA have shown similar results for dopaminergic neuronal estimates in their “middle age” groups (Kubis et al. 2000; Rudow et al. 2008). It is worth noting that although Rudow et al. (2008) reported significant reductions in neuronal number with age in their study, their differences were reported by comparing individuals with an average age for their groups of 19.9±1.21 (young) vs 50.1±5.01 (middle age) vs 87.4±7.87 (elderly), thus large gaps of age (e.g. > 17 years between the “young” and “middle age”, and >20 years between the “middle age” and “elderly”) were present among these groups. Our comparison of neuronal counts in the schizophrenia and control cases did not reveal significant differences in the total number of neurons or dopaminergic neurons, both, when the entire SN/VTA was analyzed as a whole, and when the diencephalic and mesencephalic sub-regions were assessed independently. These results confirm the early report by Bogerts et al. (1983) who also found a lack of significant differences in neuronal number in schizophrenia subjects compared to controls in the SN/VTA. Qualitative mapping of TH immunolabeling The qualitative mapping of TH labeling in the SN/VTA was done in three of the schizophrenia cases and their most closely matched control cases (see methods). In this 182 qualitative assessment we found conspicuous deficits in TH immunolabeling in diencephalic (rostral) sub-region, while TH deficits in the mesencephalic sub-region were weak or not found (see Figure 4-5 versus Figure 6). These data are in accordance with our previous western-blot study, in which we found that TH protein deficits in schizophrenia cases were only present in protein homogenates that contained the rostral sub-region of the SN/VTA and could not be explained as an effect of antipsychotic medication (Perez-Costas et al. 2012a). In addition, in the present study we found that TH deficits affected very conspicuously not only the cell bodies, but also the neuronal processes of these dopaminergic neurons (see detail images in Figure 5), which suggests that these deficits should also be present in the target areas of these projections. Interestingly, Akil et al. (1999, 2000) have reported layer-specific deficits in dopaminergic processes in the prefrontal and entorhinal cortex of schizophrenia subjects. In earlier neuroanatomical studies of dopaminergic afferents of the human SN/VTA, it was already described that approximately the rostral third of the SN/VTA preferentially provided dopaminergic input to cortical areas (see as a review van Domburg and ten Donkelaar 1991). Supporting this idea, detailed developmental studies have shown that in the human embryonic brain, the rostral (i.e. diencephalic) dopaminergic neurons of the SN/VTA differentiate later, and that there is a caudo-rostral gradient of maturation for these cells and their projections (Zecevic and Verney 1995; Verney 1999; Verney et al. 2001). The dopaminergic SN/VTA neurons of the mesencephalic sub-region are clearly observed at gestational week 6, and their projections reach subcortical areas such as the dorsal striatum by the 7th-8th week of embryonic development. Meanwhile the dopaminergic neurons of the diencephalic subregion are not clearly observed until the 10- 183 11th week (Zecevic and Verney 1995; Verney 1999; Puelles and Verney 1998; Verney et al. 2001). Dopaminergic projections reach cortical regions by week 13th (Zecevic and Verney 1995; Verney 1999; Verney et al. 2001), correlating with the maturation of the diencephalic SN/VTA neurons. Developmental and neuropathological studies on the primate SN/VTA support the existence of fundamental differences between the diencephalic and mesencephalic SN/VTA dopaminergic neurons. In the adult brain, dopaminergic neurons of these two sub-regions present different neurochemical profiles (Haber et al. 1995; Grimm et al. 2004; Thuret et al. 2004; Chung et al. 2005; Luk et al. 2013) and susceptibility to pathology (Gibb and Lees 1991; Damier et al. 1999b; Hauser et al. 2005; Fuchs et al. 2009; Bergman et al. 2010). It has been shown that these neuronal populations are segregated from early on in the development of the embryonic human brain (Zecevic and Verney 1995; Verney 1999; Puelles and Verney 1998; Verney et al. 2001). Gene expression boundaries between the diencephalic and mesencephalic sub-regions are developed very early on, thus separating the immature dopaminergic cell precursors of both sub-regions, which are exposed to different neurochemical factors (Smits et al. 2006; Smidt and Burbach 2007; Smits et al. 2013). This argues in favor of the existence of diencephalic-specific deficits of TH protein expression in the SN/VTA of schizophrenia subjects. Also supporting the existence of fundamental differences between diencephalic and mesencephalic sub-regions, the detailed study by Damier et al (1999b) showed that dopaminergic SN/VTA neurons located in the mesencephalic sub-region degenerate at much earlier stages in Parkinson’s disease than diencephalic SN/VTA neurons. 184 Taking into account all the evidence presented above, we hypothesize that TH deficits in the SN/VTA of schizophrenia subjects will preferentially (or perhaps exclusively) affect dopaminergic cortical projections of the mesocortical and mesolimbic pathways, thus connecting the present findings with the hypodopaminergic state observed in the cortex in schizophrenia patients (Finlay 2001; Remington et al. 2011), and with postmortem studies showing dopaminergic deficits in the cortex (Akil et al. 1999; 2000). Although Howes et al. (2013) have reported increases in TH labeling, a careful review of their study shows that there are fundamental differences in their sampling methods and postmortem interval, as well as in the area sampled. While in our study we have mapped the entire SN/VTA, Howes et al. (2013) performed an analysis of an area located at the level of the superior colliculus, limiting their study to a small portion of the mesencephalic SN/VTA sub-region. In addition, in the supplementary data section of Howes et al. (2013) it is reported that their time from death to sample preservation ranged from 24 to 126 hours for the schizophrenia group, and between 24 and 68 hours for the control group, while in the present study all cases were below 32 hours from death to preservation (see Table 1). In our qualitative assessment of the mesencephalic SN/VTA, we only found subtle differences in TH immunolabeling restricted to the dorsal portion of the mesencephalic SN/VTA between schizophrenia and control subjects, while ventrolateral and ventromedial areas did not present any differences (see Figure 6). The lack of conspicuous differences in mesencephalic SN/VTA is also in agreement with our previous quantitative study showing no significant differences in TH protein expression in this sub-region (Perez-Costas et al. 2012a). 185 Another feature clearly observed in our qualitative assessment of the rostral SN/VTA of the schizophrenia samples was the presence of a dorso-ventral pattern of TH deficits, in which the dorsal region was the most affected (see Figure 4). It has been consistently reported that dopaminergic neurons located in the “dorsal tier” of the SN/VTA (see as a review Haber and Godowski 2004), which approximately correspond with the dorsal sub-area of the present study, preferentially project to cortical areas (Fallon and Loughlin 1987; Gaspar et al. 1992; Haber et al. 1995; Haber and Godowski 2004; Nieuwenhuys et al. 2008), further supporting a direct link between the tyrosine hydroxylase deficits reported here and the reports by Akil et al. (1999, 2000) on TH deficits in specific layers of the entorhinal and prefrontal cortex. It is important to note that for most of the schizophrenia cases included in this study we have previously assessed TH mRNA and protein expression in the opposite hemisphere (i.e. SZ2-SZ6 of the present study were cases SZ1 and SZ3-SZ6 of PerezCostas et al. 2012a), in which we found TH protein deficits without a decrease in TH mRNA expression. Those data suggested that TH deficits in schizophrenia must occur after mRNA transcripts are successfully produced. This is also supported by a preliminary study in which we found that deficits in TH protein expression in schizophrenia significantly correlate with deficits in poly-c-binding protein 4, which is involved in the stabilization of TH mRNA for its translation into protein (Perez-Costas et al. 2012b). In addition, we have found deficits in the expression of specific subunits of cytochrome c oxidase that are also linked to the diencephalic sub-region of the SN/VTA in schizophrenia (Rice et al. 2014), which may also contribute to a lack of proper TH protein synthesis. 186 In summary, the present study confirms our previous findings of dopaminergic deficits in the SN/VTA in schizophrenia subjects (Perez-Costas et al. 2012a), mapping these deficits to the rostral SN/VTA, and supporting the existence of pathway-specific deficits in dopaminergic neurotransmission that would preferentially or exclusively affect cortical areas. In addition, we show here that these deficits cannot be attributed to significant neuronal loss but rather to a deficit in TH protein synthesis. Dopamine is essential for the proper performance of tasks controlled by the prefrontal cortex, which include working memory, behavioral flexibility and attention (Goldman-Rakic 1996; Mizoguchi et al. 2009; Winter et al. 2009). Future studies should further address the mechanisms involved in these dopaminergic deficits, and the relation of these deficits with specific symptoms observed in the clinic in schizophrenia. Acknowledgements The authors wish to thank the Maryland Brain Collection, University of Maryland School of Medicine for providing the samples used in this study. This work was supported by the National Institutes of Health (USA) grant RO1MH066123 awarded to MMF, EPC and RCR. None of the authors have any conflict of interest to disclose. Ethical Standards The manuscript does not contain clinical studies or patient data. Conflict of Interest The authors do not have any conflict of interest to report. 187 References Abi-Dargham A, Silstein M, Kegeles L and Laruelle M (2010) Dopamine dysfunction in schizophrenia. In: Iversen LL, Iversen SD, Dunnett SB and Bjorklund A (eds) Dopamine Handbook. Oxford University Press, Oxford, pp 511-519 Akil M., Edgar C. L., Pierri J. N., Casali S. and Lewis D. A. (2000) Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry, 47, 361-370. Akil M., Pierri J. N., Whitehead R. E., Edgar C. L., Mohila C., Sampson A. R. and Lewis D. A. (1999) Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry, 156, 1580-1589. Bergman O., Hakansson A., Westberg L., Nordenstrom K., Carmine Belin A., Sydow O., Olson L., Holmberg B., Eriksson E. and Nissbrandt H. (2010) PITX3 polymorphism is associated with early onset Parkinson's disease. Neurobiol Aging, 31, 114-117. Bogerts B., Hantsch J. and Herzer M. (1983) A morphometric study of the dopaminecontaining cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry, 18, 951-969. Carlsson A., Lindqvist M. and Magnusson T. (1957) 3,4-Dihydroxyphenylalanine and 5hydroxytryptophan as reserpine antagonists. Nature, 180, 1200. Carlsson A. and Lindqvist M. (1963) Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh), 20, 140-144. Chung C. Y., Seo H., Sonntag K. C., Brooks A., Lin L. and Isacson O. (2005) Cell typespecific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet, 14, 1709-1725. 188 Creese I., Burt D. R. and Snyder S. H. (1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science, 192, 481483. Damier P., Hirsch E. C., Agid Y. and Graybiel A. M. (1999a) The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain, 122, 1421-1436. Damier P., Hirsch E. C., Agid Y. and Graybiel A. M. (1999b) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain, 122, 1437-1448. Davis K. L., Kahn R. S., Ko G. and Davidson M. (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry, 148, 1474-1486. Fallon J. H. and Loughlin S. E. (1987) Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia, in Cerebral Cortex, (Jones E.G. and Peters A., eds), pp. 41-109. Plenum Press, New York. Fearnley J. M. and Lees A. J. (1991) Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain, 114, 2283-2301. Finlay J. M. (2001) Mesoprefrontal dopamine neurons and schizophrenia: role of developmental abnormalities. Schizophr Bull, 27, 431-442. Floresco S. B. and Magyar O. (2006) Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl), 188, 567-585. Frankle W. G. and Laruelle M. (2002) Neuroreceptor imaging in psychiatric disorders. Ann Nucl Med, 16, 437-446. 189 Fuchs J., Mueller J. C., Lichtner P., Schulte C., Munz M., Berg D., Wullner U., Illig T., Sharma M. and Gasser T. (2009) The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging, 30, 731-738. Gaspar P., Stepniewska I. and Kaas J. H. (1992) Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol, 325, 1-21. Gibb W. R. and Lees A. J. (1991) Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry, 54, 388-396. Goldman-Rakic PS. (1996) Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA, 93, 13473-13480. Grimm J., Mueller A., Hefti F. and Rosenthal A. (2004) Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci USA, 101, 13891-13896. Grunblatt E., Mandel S., Maor G. and Youdim M. B. (2001) Gene expression analysis in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice model of Parkinson's disease using cDNA microarray: effect of R-apomorphine. J Neurochem, 78, 1-12. Guillin O., Abi-Dargham A, Laurelle M. (2007) Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol, 78, 1-39. Haber S. N. and Fudge J. L. (1997) The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol, 11, 323-342. Haber S. N. and Gdowski M. J. (2004) The basal ganglia, in The Human Nervous System, (Paxinos G. and Mai J. K., eds), pp. 676-738. Elsevier Academic Press, London. 190 Haber S. N., Ryoo H., Cox C. and Lu W. (1995) Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol, 362, 400-410. Hauser M. A., Li Y. J., Xu H., Noureddine M. A., Shao Y. S., Gullans S. R., Scherzer C. R., Jensen R. V., McLaurin A. C., Gibson J. R., Scott B. L., Jewett R. M., Stenger J. E., Schmechel D. E., Hulette C. M. and Vance J. M. (2005) Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Arch Neurol, 62, 917-921. Hirsch E., Graybiel A. M., Agid A. M. (1988) Melanized dopaminergic neurons are differentially susceptible to degradation in Parkinson’s disease. Nature, 334, 345-348. Howes O. D. and Kapur S. (2009) The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull, 35, 549-562. Howes O. D., Williams M., Ibrahim K., Leung G., Egerton A., McGuire P. K. and Turkheimer F. (2013) Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain, 136, 3242-3251. Ichinose H., Ohye T., Fujita K., Pantucek F., Lange K., Riederer P., Nagatsu T. (1994) Quantification of mRNA of tyrosine hydroxylase and aromatic L-aminoacid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J Neural Transm Park Dis Dement Sect, 8, 149-158. Kaalund S.S, Newburn E. N., Ye T., Tao R., Li C., Deep-Soboslay A., Herman M. M., Hyde T. M., Winberger D. R., Lipska B. K., Kleinman J. E. (2013) Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia Mol Psychiatry, 10.1038/mp.2013.165 [Epub ahead of print] 191 Keshavan M. S., Nasrallah H. A. and Tandon R. (2011) Schizophrenia, "Just the Facts" 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophr Res, 127, 3-13. Kubis N., Faucheux B. A., Ransmayr G., Damier P., Duyckaerts C., Henin D., Forette B., Le Charpentier Y., Hauw J. J., Agid Y. and Hirsch E. C. (2000) Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain, 123, 366-373. Lopez-Garcia P., Young Espinoza L., Molero Santos P., Marin J., Ortuno SanchezPedreno F. (2013) Impact of COMT genotype on cognition in schizophrenia spectrum patients and their relatives. Psychiatry Res, 208, 118-124. Luk K. C., Rymar V. V., van den Munckhof P., Nicolau S., Steriade C., Bifsha P., Drouin J. and Sadikot A. F. (2013) The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. J Neurochem, 125, 932-943. Meyer-Lindenberg A. (2010) Imaging genetics of schizophrenia. Dialogues Clin Neurosci, 12, 449-456. Mizoguchi K., Shoji H., Tanaka Y., Maruyama W. and Tabira T. (2009) Age-related spatial working memory impairment is caused by prefrontal cortical dopaminergic dysfunction in rats. Neuroscience, 162, 1192-1201. Motulsky H. J. (2010) Intuitive biostatistics: A nonmathematical guide to statistical thinking. New York: Oxford University Press. Motulsky H. J. and Brown R. E. (2006) Detecting outliers when fitting data with nonlinear regression-a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics, 7, 123. 192 Mueller H. T., Haroutunian V., Davis K. L., Meador-Woodruff J.H. (2004) Expression of ionotropic gluatamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res, 121, 60-69. Nasrallah H., Tandon R. and Keshavan M. (2011) Beyond the facts in schizophrenia: closing the gaps in diagnosis, pathophysiology, and treatment. Epidemiol Psychiatr Sci, 20, 317-327. Nelander J., Hebsgaard J. B. and Parmar M. (2009) Organization of the human embryonic ventral mesencephalon. Gene Expr Patterns, 9, 555-561. Nieuwenhuys R., Voogd J. and van Huijzen C. (2008) Topography of spinal cord, brain stem and cerebellum, in The Human Central Nervous System, (Nieuwenhuys R., Voogd J. and van Huijzen C., eds), pp. 177-246. Springer-Verlag, Berlin. Paxinos G. and Huang X.F. (1995) Atlas of the human brainstem. Academic Press, San Diego. Perez-Costas E., Melendez-Ferro M. and Roberts R. C. (2010) Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem, 113, 287-302. Perez-Costas E., Melendez-Ferro M., Rice M. W., Conley R. R. and Roberts R. C. (2012a) Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry, 3, 31. Perez-Costas E., Rodriguez-Pallares J., Roberts R. C., Labandeira-Garcia J. L. and Melendez-Ferro M. (2012b) Poly-c-binding proteins in schizophrenia: a possible mechanism for tyrosine hydroxylase pathology. Program No. 452.11. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. Online. 193 Puelles L. and Verney C. (1998) Early neuromeric distribution of tyrosine-hydroxylaseimmunoreactive neurons in human embryos. J Comp Neurol, 394, 283-308. Raznahan A., Greenstein D., Lee Y., Long R., Clasen L., Gochman P., Addington A., Giedd J. N., Rapoport J. L., Gogtay N. (2011) Catechol-o-methyl transferase (COMT) val158met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. Neuroimage, 57, 1517-1523. Remington G., Agid O. and Foussias G. (2011) Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev Neurother, 11, 589-607. Rudow G., O'Brien R., Savonenko A. V., Resnick S. M., Zonderman A. B., Pletnikova O., Marsh L., Dawson T. M., Crain B. J., West M. J. and Troncoso J. C. (2008) Morphometry of the human substantia nigra in ageing and Parkinson's disease. Acta Neuropathol, 115, 461-470. Seamans J.K. and Yang CR. (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol, 74, 1-58. Seeman P. and Lee T. (1975) Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science, 188, 1217-1219. Seeman P., Lee T., Chau-Wong M. and Wong K. (1976) Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature, 261, 717-719. Seeman P. (1987) Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse, 1, 133-152. Smidt M. P. and Burbach J. P. (2007) How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci, 8, 21-32. 194 Smits S. M., Burbach J. P. and Smidt M. P. (2006) Developmental origin and fate of meso-diencephalic dopamine neurons. Prog Neurobiol, 78, 1-16. Smits S. M., von Oerthel L., Hoekstra E. J., Burbach J. P. and Smidt MP. (2013) Molecular marker differences relate to developmental position and subsets of mesodiencephalic dopaminergic neurons. PLoS One, 8(10):e76037. Spitzer R. L., Williams J. B., Gibbon M. and First M. B. (1992) The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry, 49, 624-629. Tan H.Y., Callicott J.H. and Weinberger D. R. (2007) Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex, 17, 171-181. Tanaka S. (2006) Dopaminergic control of working memory and its relevance to schizophrenia: A circuit dynamics perspective. Neuroscience, 139, 153-171. Toda M. and Abi-Dargham A. (2007) Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep, 9, 329-336. Tost H, Hakimi S, Meyer-Lindenberg A (2010) Dopamine dysfunction in schizophrenia: from genetic susceptibility to cognitive impairment. In Iversen L L, Iversen SD, Dunnett SB, Bjorklund A. (eds) Dopamine Handbook. Oxford University Press, Oxford, pp 558571. Thuret S., Bhatt L., O'Leary D. D. and Simon H. H. (2004) Identification and developmental analysis of genes expressed by dopaminergic neurons of the substantia nigra pars compacta. Mol Cell Neurosci, 25, 394-405. 195 van den Heuvel D. M. and Pasterkamp R. J. (2008) Getting connected in the dopamine system. Prog Neurobiol, 85, 75-93. van Domburg P. H. and ten Donkelaar H. J. (1991) The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol, 121, 1-132. van Os J, Kapur S (2009) Schizophrenia. Lancet: 374, 635-645. Verney C. (1999) Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc Res Tech, 46, 24-47. Verney C., Zecevic N. and Puelles L. (2001) Structure of longitudinal brain zones that provide the origin for the substantia nigra and ventral tegmental area in human embryos, as revealed by cytoarchitecture and tyrosine hydroxylase, calretinin, calbindin and GABA immunoreactions. J Comp Neurol, 429, 22-44. Watson R. E., Jr., Wiegand S. J., Clough R. W. and Hoffman G. E. (1986) Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides, 7, 155-159. Williams M. R., Galvin K., O'Sullivan B., Macdonald C. D., Ching E. W., Turkheimer F., Howes O. D., Pearce R. K., Hirsch S. R. and Maier M. (2013) Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci. In press. Winter S., Dieckmann M. and Schwabe K. (2009) Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behav Brain Res 198, 206213. Zecevic N. and Verney C. (1995) Development of the catecholamine neurons in human 196 embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol, 351, 509-35. 197 Table 1. Demographic and clinical data of the cases used for the study Schizophrenia samples (n=6) Case # Age (years) race gender COD PMI (hours) pH SZ subtype APD type Chlorpromazine + Clozapine + Fluphenazine + Quetiapine (T + A) SZ1 45 W M ASCVD 31 6.78 CUT SZ2 53 W M ASCVD 14 6.01 Undifferentiated Quetiapine (A) 46 W F DVT 24 6.18 NOS Clozapine (A) SZ4 54 W M Suicide (bleeding) 19 6.58 NOS Quetiapine (A) SZ5 83 W M Electrocution 18 6.98 NOS Quetiapine (A) SZ6 42 AA F ASCVD 13 5.86 Chronic paranoid Fluphenazine + Quetiapine (T + A) Mean 53.80 19.83 6.40 SD 15.04 6.74 0.45 COD PMI (hours) pH SZ3 Control samples (n=7) Case # Age (years) race C1 49 AA M Cardiac arrest 19 6.76 C2 45 W M Cardiac arrest 18 6.97 C3 51 AA M ASCVD 19 C4 63 W M ASCVD 11 C5 37 W F DVT 10 6.49 C6 62 W M Cardiac arrest 19 6.48 C7 67 AA M ASCVD 17 6.68 Mean 53.43 16.14 6.75 SD 10.92 3.93 0.25 gender 7.18 6.71 Table 1. Demographic and clinical data of the cases used for the study. Abbreviations: A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; COD, cause of death; CUT, chronic undifferentiated type; DVT, deep vein thrombosis; F, female; M, male; NOS, not otherwise specified; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; W, white. 198 Table 2: Oversampling data summary Number of Number sections per of Estimated total series used counting neurons for counts frames per section All sections 10 635,240 All sections 5 635,568 1/3rd 10 651,624 rd 1/3 5 638,832 % variation from CSE Estimated dopamine neurons % variation from CSE 0 0.05% 2.58% 0.56% 361,464 357,248 370,416 354,048 0 1.17% 2.48% 2.05% Table 2. Neuronal counts obtained as the average of the neuronal counts of two different control cases. Neuronal count estimates of the SN/VTA (both hemispheres) were performed by counting neurons in a entire series. Each counting frame corresponds to the area of the SN/VTA fitting in an image taken with the 10X magnification objective. Abbreviations: CSE, complete series estimate; 1/3rd, one out of each 3 sections. 199 Table 3: Effect of demographic variables on neuronal counts OUTCOME MEASURES ENTIRE SN/VTA DIENCEPHALIC SN/VTA MESENCEPHALIC SN/VTA Total neurons p=0.2141 R2a=0.1697 p=0.5490 R2a=-0.0665 p=0.4145 / 2 R a=0.0138 / Dopaminergic neurons p=0.2283 R2a=0.1561 p=0.1520 R2a=0.2377 p=0.4597 / 2 R a=-0.0145 / Table 3. Effect of demographic variables (i.e. age, gender,race) on neuronal counts. These analyses were done testing the effect of these variables on cell counts regardless of disease status. For all analyses n=13, df=9. None of the analyses yielded significant results. Abbreviations: R2a= adjusted R2 for multiple comparisons. 200 Table 4: Effect of sample quality variables on neuronal counts OUTCOME MEASURES ENTIRE SN/VTA DIENCEPHALIC SN/VTA MESENCEPHALIC SN/VTA Total neurons p=0.2055 R2a=0.1256 p=0.8659 R2a=-0.1659 p=0.1947 / 2 R a=0.1349 / Dopaminergic neurons p=0.1710 R2a=0.1571 p=0.9619 R2a=-0.1907 p=0.1423 / 2 R a=-0.1875 / Table 4. Effect of sample quality variables (i.e. postmortem interval, brain pH) on neuronal counts. These analyses were done testing the effect of these variables on cell counts regardless of disease status. For all analyses n=13, df=10. None of the analyses yielded significant results. Abbreviations: R2a= adjusted R2 for multiple comparisons. 201 Figure 1. Neuronal counts for the entire SN/VTA. A-B) Number of total and dopaminergic neurons in the schizophrenia and control groups. In (A), data are presented as the mean neuronal count for each group with their standard deviation. In (B), individual data for each subject is plotted, with the horizontal bar indicating the mean for the group. C-D) Ratio of dopaminergic/total neurons. The bar graph in (C) represents the mean ratio for each group and their standard deviations. The scatter plot in (D) represents the ratio values for each individual subject, with the horizontal bar indicating the mean ratio for each group. No significant differences were observed for any of these measures between the two groups for the SN/VTA as a whole. Abbreviations: CTRL: control; SZ: schizophrenia 202 Figure 2. Neuronal counts in the diencephalic (rostral) SN/VTA. A-B) Number of total and dopaminergic neurons in the schizophrenia and control groups for the diencephalic sub-region of the SN/VTA. In (A), data are presented as the mean neuronal count for each group with their standard deviation. In (B), individual data for each subject is plotted, with the horizontal bar indicating the mean for the group. C-D) Ratio of dopaminergic/total neurons. The bar graph in (C) represents the mean ratio for each group and their standard deviations. The scatter plot in (D) represents the ratio values for each individual subject, with the horizontal bar indicating the mean ratio for each group. No significant differences were observed for any of these measures between the two groups in the diencephalic SN/VTA. Abbreviations: CTRL: control; SZ: schizophrenia 203 Figure 3. Neuronal counts in the mesencephalic (mid-caudal) SN/VTA. A-B) Number of total and dopaminergic neurons in the schizophrenia and control groups for the mesencephalic sub-region of the SN/VTA. In (A), data are presented as the mean neuronal count for each group with their standard deviation. In (B), individual data for each subject is plotted, with the horizontal bar indicating the mean for the group. C-D) Ratio of dopaminergic/total neurons. The bar graph in (C) represents the mean ratio for each group and their standard deviations. The scatter plot in (D) represents the ratio values for each individual subject, with the horizontal bar indicating the mean ratio for each group. No significant differences were observed for any of these measures between the two groups in the mesencephalic SN/VTA. Abbreviations: CTRL: control; SZ: schizophrenia 204 Figure 4. Tyrosine hydroxylase labeling in the diencephalic SN/VTA. A) Sagittal schematic drawing indicating the rostro-caudal level of sectioning (red line) of the images shown in this figure. B) Representative schematic drawing of a transverse section at the level shown in A. Three SN/VTA subareas defined by the dorso-ventral and mediolateral location of the dopaminergic neurons are indicated (dashed lines in gray-shaded 205 area). There was a conspicuous qualitative difference in overall immunolabeling between matched schizophrenia (C-E) and control (F-H) samples in the diencephalic sub-region of the SN/VTA. Note also that within this sub-region, the dorsal area of the SN/VTA presented the weakest immunolabeling in the schizophrenia samples (C), while was very intensely labeled in the controls (F). Ventro-lateral areas of the SN/VTA presented also notably weaker immunolabeling in schizophrenia samples (D) compared to the controls (G). Differences in labeling were less apparent when comparing the ventro-medial area of matched schizophrenia (E) and control samples (H). The white asterisks indicate a similar level within the section for comparison of TH labeling between the schizophrenia and control samples. Coordinates indicate the orientation of the sections: C: caudal; D: dorsal; L: lateral; R: rostral. Dashed lines in D, G indicate the approximate transition between the dorsal (d) and ventro-lateral (vl) regions of the SN/VTA. Abbreviations: cp: cerebral peduncle; CTRL: control; d: dorsal SN/VTA; III: oculomotor nerve exit; m: mammillary body; pg: periaqueductal grey; RN: red nucleus; SN/VTA: Substantia nigra/ventral tegmental area; SZ: schizophrenia; Thal: thalamus; vl: ventro-lateral SN/VTA; vm: ventro-medial SN/VTA. Calibration bar: 1 mm in C-H. 206 Figure 5. Tyrosine hydroxylase labeling of cell and processes in the diencephalic SN/VTA. A, D) Dorsal subarea of the diencephalic SN/VTA: In schizophrenia (A, A’) dopaminergic cell bodies presented the typical neuromelanin pigmentation (n), but TH labeling was very weak. Dopaminergic processes also presented weak labeling for TH (white arrowheads). The matched control (D, D’) displayed very intense immunostaining for TH both in cell bodies and processes. B, E) The ventro-lateral subarea of the diencephalic SN/VTA also presented conspicuous deficits in TH immunolabeling in schizophrenia (B, B’) compared with a matched control (E, E’). Note also that there is a gradient of TH immunolabeling deficit, with the areas close to the transition between the 207 ventro-lateral (vl) and dorsal (d) subareas presenting even weaker TH labeling in the schizophrenia (B) but not in the control (E). Boxed areas in A, D, B, E are shown at higher magnification in A’, D’, B’, E’, respectively. C, F) The ventro-medial subarea of the SN/VTA presented scarce dopaminergic neurons (arrows) sparsely distributed among a dense net of thin TH labeled processes both in the schizophrenia (C) and control (F) samples. The differences in TH immunolabeling between schizophrenia and matched controls for this ventro-medial subarea were more subtle, displaying overall moderate qualitative deficits of TH labeling in their dense net of thin dopaminergic processes, and no clear qualitative reductions of labeling in dopaminergic cell bodies. The white asterisks indicate a similar level within the section for comparison of TH labeling between the schizophrenia and control samples. Coordinates indicate the orientation of the sections: D: dorsal; L: lateral. Abbreviations: CTRL: control; SZ: schizophrenia. Calibration bar: 250 µm in A-F. 208 Figure 6. Tyrosine hydroxylase labeling in the mesencephalic SN/VTA. A) Sagittal schematic drawing indicating the rostro-caudal level of sectioning (red line) of the images shown in this figure. B) Representative schematic drawing of a transverse section at the level shown in A. Three SN/VTA subareas defined by the dorso-ventral and mediolateral location of the dopaminergic neurons are indicated (dashed lines in gray-shaded 209 area). C, F) These images show the overall pattern of TH labeling in the mesencephalic sub-region of matched schizophrenia (C) and control (F) samples. In these low magnification images the differences in labeling between schizophrenia and controls were very subtle. D, G) At higher magnification, in the dorsal (d) subarea there were moderate-to-subtle deficits in labeling in schizophrenia (D) compared with matched control (G) samples. E, H) In the ventro-lateral (vl) subarea there were no obvious differences between schizophrenia (E) and matched control (H) samples. Coordinates indicate the orientation of the sections: C: caudal; D: dorsal; L: lateral; R: rostral. The white asterisks in C and F indicate a similar level within the section for comparison of TH labeling between the schizophrenia and control samples Dashed lines in C, F indicate the approximate transition between the dorsal (d) and ventro-lateral (vl) regions of the SN/VTA. The areas marked with dashed boxes in C and F are shown at high magnification in D, E, G, H. Arrowheads in D-E and G-H indicate some examples of TH immunolabeling in thin processes. Abbreviations: cp: cerebral peduncle; CTRL: control; d: dorsal SN/VTA; III: oculomotor nerve exit; m: mammillary body; pg: periaqueductal grey; RN: red nucleus; SN/VTA: Substantia nigra/ventral tegmental area; SZ: schizophrenia; Thal: thalamus; vl: ventro-lateral SN/VTA; vm: ventro-medial SN/VTA. Calibration bars: 1 mm in C, F; 500 µm in D, E, G, H. 210 DISCUSSION Anomalies in the dopaminergic system are a core feature of schizophrenia, and a large body of data has been generated concerning pathologies associated with the main cortical and subcortical areas that receive dopaminergic input (Akil et al., 1999, 2000; see also as reviews Davis et al., 1991; Harrison, 1999; Powers, 1999; DeLisi et al., 2006; Howes and Kapur, 2009; Perez-Costas et al., 2010; Howes et al., 2012; Kuepper et al., 2012; Haukvik et al., 2013; Laruelle, 2013). Surprisingly, when the present work was started, few studies had addressed possible pathologies associated with the region of origin of the dopaminergic innervation (Bogerts et al., 1983; Toru et al., 1988; Ichinose et al., 1994; Mueller et al., 2004). Even though dopaminergic cell groups are present in all main brain areas, the main source of dopamine for the cortical and subcortical areas affected in schizophrenia is the substantia nigra/ventral tegmental area (SN/VTA), which stretches from diencephalic to mesencephalic regions within the human brain (Puelles and Verney, 1998; Damier et al. 1999a; Verney, 1999; Nieuwenhuys et al. 2008). The SN/VTA has a complex embryonic development, and since early development there are fundamental differences between diencephalic (i.e. rostral) and mesencephalic (i.e. midcaudal) SN/VTA dopaminergic neurons (Zecevic and Verney, 1995; Verney, 1999; Puelles and Verney 1998; Verney et al. 2001). Although all dopaminergic neurons of the SN/VTA contribute to some degree to all main pathways (i.e. mesocortical, mesolimbic, and nigrostriatal), neurons located in the diencephalic sub-region of the SN/VTA preferentially provide dopamine to cortical areas, while mesencephalic neurons preferentially project to subcortical brain areas (van Domburg and ten Donkelaar, 1991; 211 see as reviews Haber and Gdowski, 2004; Halliday, 2004; Nieuwenhuys et al., 2007). Interestingly, morphological studies often divide the SN/VTA in a so-called “dorsal tier,” in which cells are primarily located in the rostral, diencephalic region of the SN/VTA, and a “ventral tier” that starts more caudally and is most prevalent in the mid-caudal, mesencephalic region of SN/VTA (Crosby and Woodburne, 1943; see as reviews Haber and Fudge, 1997; Marin et al., 1998; Joel and Weiner, 2000; Augustine et al., 2008). In summary, the SN/VTA presents great complexity from early on in development, and different subsets of dopaminergic neurons within the complex preferentially provide input to different brain areas. In addition, studies in Parkinson’s disease have shown that there are significant differences in the resilience to Parkinson’s pathology for different sub-regions of the SN/VTA complex (Damier et al 1999b; see as a review Feve, 2012). The overall goal of this dissertation was to assess the existence of pathologies in dopaminergic neurons of the SN/VTA that could affect the dopaminergic input to particular brain areas in schizophrenia. Specifically, our study focused on three main aspects: 1) The examination of regional differences of TH mRNA and protein expression in the SN/VTA in schizophrenia compared to non-psychiatric controls. 2) Assessment of metabolic anomalies linked to COX dysfunction and their contribution to the pathology of the SN/VTA in this disorder. A second aspect of the analysis of COX dysfunction was to assess if alterations in the expression of critical subunits for the assembly and functioning of the COX enzyme could contribute to pathology in the SN/VTA in schizophrenia. 3) Taking into account our findings on TH protein expression anomalies in the SN/VTA in schizophrenia, we wanted to assess if TH pathology could be linked to a significant reduction in the number of neurons in the SN/VTA (i.e. a reduction in the 212 total number of neurons, or specifically in dopaminergic neurons). As an alternative hypothesis, we also wanted to test the possibility of a reduction in TH protein expression without significant neuronal loss, as well as the possibility that the reduction in TH expression could be assigned to specific neuronal groups within the SN/VTA. 1) Study of regional differences of TH mRNA and protein expression in the SN/VTA in schizophrenia compared to non-psychiatric controls Our first study (Perez-Costas et al., 2012) revealed significant TH protein expression deficits in schizophrenia, without alterations in TH mRNA expression. This was found by analyzing TH mRNA and protein expression in the same schizophrenia and non-psychiatric control cases. We also found that TH protein deficits in the schizophrenia samples were only present if protein expression was assessed for the entire SN/VTA complex, while the analysis of TH protein expression for the mesencephalic sub-region of the SN/VTA did not yield any significant differences between schizophrenia and nonpsychiatric control samples. These findings supported the existence of a TH protein expression pathology that preferentially affected the diencephalic sub-region (i.e. rostral portion) of the SN/VTA. Taking into account that the diencephalic SN/VTA preferentially provides dopaminergic input to cortical areas, our study supports earlier work showing TH deficits in the entorhinal and prefrontal cortex in postmortem human brains from schizophrenia subjects (Akil et al, 1999, 2000). These data are also in agreement with the current hypothesis of dopamine pathology in schizophrenia, which includes a hypodopaminergic state in cortical areas (see as a review Davis et al., 1991). This led us to hypothesize that TH pathology may be concentrated in the dopamine cell 213 populations that preferentially project through the mesolimbic and mesocortical pathways. To ensure that these findings were not a result of the use of antipsychotic medication by the subject, TH protein levels were also assessed in animals treated with both typical (Haloperidol) and atypical (Olanzapine) antipsychotics. These antipsychotics were administered in doses that matched effective clinical dosing in humans, based upon dopamine receptor occupancy (Perez-Costas et al., 2008). The result of this analysis showed that antipsychotics do not affect TH protein levels in the SN/VTA complex, supporting that the TH anomalies observed in the SN/VTA of schizophrenia brains is intrinsic to the pathology of the illness. 2) Assessment of metabolic anomalies in the SN/VTA in schizophrenia A second aspect of our study of the pathology of the SN/VTA in schizophrenia was to assess if metabolic anomalies could contribute to the dopaminergic deficits identified in the first part of our study. In schizophrenia, metabolic anomalies are a welldocumented aspect of the pathology of the disorder (Wiesel et al., 1987; Cleghorn et al., 1989; Wiesel, 1992; Prabakaran et al., 2004). We decided to examine the activity of cytochrome c oxidase (COX) within the SN/VTA complex as an indicator of metabolic health. The reason behind choosing COX activity as a marker of metabolic health was three-fold. First, COX is one of the major regulation sites of oxidative phosphorylation that leads to the production of ATP (Li et al., 2006). Second, regional anomalies in COX activity, both increases and decreases, have been reported in schizophrenia (Cavelier et al., 1995; Prince et al., 1999; Maurer et al., 2001), although no studies have specifically 214 examined COX activity within the SN/VTA complex. Finally, it has been shown that the activity of the COX enzyme is not affected by antipsychotic medication (Burkhardt et al., 1993; Whatley et al., 1998; Balijepalli et al., 1999; 2001; Streck et al., 2007). At the time that our study was planned, the available methodologies for the detection of COX activity in tissue sections were not sensitive enough for our measures, nor did they allow for the quantity of COX enzyme in the tissue to be realized. Thus, we developed an improved methodology for the measurement of COX activity (MelendezFerro et al., 2013). This new methodology involved the application of a known amount of pure COX enzyme onto a nitrocellulose membrane, which was then incubated alongside the tissue sections. Not only did this improve the accuracy of our measures (being able to detect changes as small as 5%, compared to 20% with previous methods), but also allowed for the determination of the amount of COX enzyme present in the tissue. Utilizing this methodology, we tested for differences in COX activity within the SN/VTA between schizophrenia and non-psychiatric control cases (Rice et al., 2014). Our study revealed that COX activity did not differ significantly between the two groups. This lack of difference in COX activity was observed for the entire SN/VTA, as well as when the diencephalic (rostral) and mesencephalic (mid-caudal) sub-regions were analyzed independently. However, it should kept in mind that the analysis of COX activity in postmortem human brain sections only provides a “snapshot” of metabolic health at time of death. Thus, to assess if long-term COX function is compromised in schizophrenia, we also examined protein expression of key subunits for the functioning and assembly of the COX enzyme. 215 Of the subunits chosen for analysis, subunits I, II and III of the COX enzyme constitute the “catalytic core”, and are encoded exclusively by the mitochondrial genome. These enzymes are responsible for substrate binding, electron transfer, and oxidation that ultimately lead to the expulsion of H+ ions, creating the proton gradient necessary for the production of ATP (see as a review Taanman, 1997). Subunit IV of the COX enzyme is encoded by the nuclear genome and is critical for the proper assembly of the complex, protects the catalytic core from reactive oxygen species, and aids in electron transfer (Li et al., 2006; Nijtman et al., 1998) [see figure 11 in Introduction]. Additionally, there are two isoforms of subunit IV in the mouse, rat, and primate, which are referred to as subunits IV-1 and IV-2 (Huttemann et al., 2001). Western-blot analysis of COX subunits within the SN/VTA revealed significant decreases in the expression of subunits II and IV-1 in schizophrenia cases compared to non-psychiatric controls. Furthermore, these decreases were observed only in those schizophrenia cases that contained the entire rostral-caudal extent of the SN/VTA complex. In cases that contained only the mesencephalic sub-region (i.e. mid-caudal portion) of the SN/VTA, no significant changes were observed in any COX subunit analyzed. Importantly, we also tested the effect of antipsychotic medications on the expression of the different COX subunits. Our analysis revealed that neither firstgeneration/typical (Haloperidol) nor second-generation/atypical (Olanzapine) antipsychotics administered in clinically relevant doses had any significant effect on protein expression for any of the COX subunits analyzed in this study. Our data indicate that the observed deficits in subunits II and IV-1 are driven by the diencephalic (i.e. 216 rostral) sub-region of the SN/VTA complex, and that these anomalies in COX subunit expression are intrinsic to the pathology of schizophrenia. Subunit II of the COX enzyme is responsible for the binding of the cytochrome c substrate and subsequent electron transfer to subunit I within the complex (see as a review Taanman, 1997). Furthermore, reductions in subunit II expression are associated with a higher susceptibility to neuronal loss (Francisconi et al., 2006). Subunit IV, as mentioned previously, is critical for the proper assembly of the COX enzyme. In fact, the binding of subunit IV to subunit I forms the first intermediary step in the assembly of the enzyme (Nijtmans et al., 1998; Fontanesi et al., 2006; Li et al., 2006). Additionally, subunit IV has a role in the modulation of the enzyme kinetics, so that the ATP/ADP ratio is maintained (Napiwotzki and Kadenbach, 1998). Finally, the suppression of subunit IV has been linked to an overall reduction of COX activity and an increased susceptibility to apoptosis (Li et al., 2006). In summary, our findings indicate that COX activity within the SN/VTA does not significantly differ between schizophrenia and non-psychiatric controls. However, the expression of specific subunits encoded by both mitochondrial (i.e. subunit II) and nuclear (i.e. subunit IV-I) genomes is significantly decreased, with these deficits linked to the rostral sub-region of the SN/VTA complex. The explanation of how subunit composition of COX is perturbed without affecting activity may lie in the innate functioning of the mitochondria. In physiologically “normal” conditions, the mitochondria function well below their maximum capacity, and the difference between basal activity and maximum respiratory capacity is known as “reserve capacity” (Perez et al., 2010; see as a review Mitchell et al., 2013) [Figure 1]. We hypothesize that in 217 schizophrenia this reserve capacity could be decreased in the SN/VTA, thus making mitochondria more vulnerable to insult. In other words, a non-optimally assembled COX enzyme may be able to maintain proper basal COX activity and ATP production, but have an impaired capacity to respond to higher energy demands. Supporting this idea, it has been shown that specific mutations in subunits of the catalytic core produce severe impairment of mitochondrial function (Bruno et al, 1999; Clark et al, 1999; Rahman et al, 1999; Tiranti et al., 2000) 3) Assessing changes in the number of neurons, and mapping of dopaminergic deficits in the SN/VTA This part of the study was designed to test if the TH deficits identified in our previous study (Perez-Costas et al, 2012) could be linked to alterations in neuron number and/or changes in TH immunolabeling within sub-regions of the SN/VTA complex. This was accomplished, in part, by performing neuronal counts to test if there was a significant difference in the total number of neurons, in the number of dopaminergic neurons, and in the ratio of dopaminergic neurons to total neurons in the SN/VTA between schizophrenia and non-psychiatric control cases. Neuronal counts were analyzed independently for the diencephalic and mesencephalic sub-regions, and also for the entire rostro-caudal extent of the SN/VTA complex. The comparison of neuronal counts between schizophrenia and non-psychiatric control cases did not reveal significant differences in the total number of neurons, number of dopaminergic neurons, or the ratio between the two. This was true when the entire SN/VTA was analyzed as a whole, and also when the diencephalic and mesencephalic 218 sub-regions were assessed independently. These results confirm an earlier report by Bogerts et al. (1983) that also found a lack of significant differences in neuronal number in schizophrenia subjects compared to non-psychiatric controls in the SN/VTA. These data support that the dopaminergic deficits previously reported by our group (PerezCostas et al., 2012) cannot be explained by a reduction in the number of neurons. Our qualitative immunohistochemical study of TH expression showed conspicuous deficits in TH immunolabeling in the diencephalic (rostral) sub-region, while TH deficits in the mesencephalic (mid-caudal) sub-region were weak or not found. These data are in accordance with our previous western-blot study, in which we found that TH protein deficits in schizophrenia cases were only present in protein homogenates that contained the rostral sub-region of the SN/VTA (Perez-Costas et al. 2012). In addition, we found that TH deficits affected not only the cell bodies, but also the neuronal processes of these dopaminergic neurons, which suggests that these deficits could also be present in the target areas of these projections. Interestingly, Akil et al. (1999, 2000) have reported layer-specific deficits in dopaminergic processes in the prefrontal and entorhinal cortices of schizophrenia subjects, which are target areas for the dopaminergic neurons of the diencephalic sub-region of the SN/VTA (van Domburg and ten Donkelaar, 1991; see as reviews Haber and Gdowski, 2004; Nieuwenhuys et al., 2008). In summary, the present study shows evidence of conspicuous sub-region specific deficits in TH protein expression within the diencephalic portion of the SN/VTA, without neuronal loss. Taking this evidence into account, we hypothesize that TH deficits in the SN/VTA of schizophrenia subjects will preferentially (or perhaps exclusively) affect dopaminergic cortical projections of the mesocortical and mesolimbic pathways. This 219 establishes a connection between our present findings and the hypodopaminergic state observed in the cortex in schizophrenia patients (Finlay 2001; Remington et al. 2011), and with postmortem human brain studies showing dopaminergic deficits in the cortex (Akil et al. 1999; 2000). The lack of differences in the mesencephalic SN/VTA is also in agreement with our previous quantitative study showing no significant differences in TH protein expression in this sub-region (Perez-Costas et al. 2012). Future studies should further address the mechanisms involved in these dopaminergic deficits, and the relation of these deficits with specific symptoms observed in the clinic in schizophrenia. Conclusions The primary goal of this work was to assess if dopaminergic impairments described previously in target areas for dopamine input in schizophrenia (e.g. the striatum and prefrontal cortex) could also be present in the neurons of origin for those dopaminergic inputs, which are located in the substantia nigra/ventral tegmental area. From this work we drew the following conclusions: Examination of TH protein and mRNA revealed that deficits in tyrosine hydroxylase protein expression (but not mRNA) are present in the SN/VTA complex. This examination also suggested that TH deficits were concentrated in the diencephalic (i.e. rostral) sub-region of the SN/VTA, which contains cell populations that preferentially contribute to the mesolimbic and mesocortical pathways. 220 The preferential presence of this TH protein deficit in the diencephalic sub-region of the SN/VTA was confirmed by our immunohistochemical study of TH expression in schizophrenia and matched non-psychiatric control cases. This study also revealed that these deficits occurred without significant neuronal loss, and that decreases in TH immunolabeling affected the cell bodies and processes. This linked our findings with previous studies reporting decreased TH immunolabeling in the prefrontal and entorhinal cortex, which are target areas for the dopaminergic projections of the diencephalic sub-region of the SN/VTA. Finally, we found deficits in key subunits of the cytochrome c oxidase complex (i.e. subunits II and IV-1), which are involved in long-term regulation and assembly of the enzyme. These deficits were also linked to the rostral sub-region of the SN/VTA. Overall, the results of the present work show the existence of sub-region specific deficits in dopamine synthesis in the SN/VTA in schizophrenia, which mostly affect cortical dopaminergic input. In addition, metabolic anomalies related to deficits in the assembly and/or long-term regulation of the COX enzyme may also contribute to these deficits. These findings provide a framework for the understanding of pathway-specific presynaptic alterations within the dopaminergic system in schizophrenia pathology. Importantly, our findings can be directly applied to the current view of the dopamine pathology of schizophrenia. In fact, version II of the dopamine hypothesis of schizophrenia states that there is a hypodopaminergic state within cortical regions (see as 221 a review Davis et al., 1991). It is plausible that our observed presynaptic reductions in TH protein expression and increase in metabolic vulnerability within the rostral SN/VTA contribute to the hypodopaminergic state observed in schizophrenia pathology. Additionally, this can be related back to the phenotypic symptoms observed in the clinic that are used for the diagnosis of schizophrenia. Specifically, perturbations within the mesolimbic and mesocortical pathways are most closely associated with the negative symptomology and cognitive impairments of schizophrenia, respectively (see as a review Howes and Kapur, 2009). Our findings can also have relevance for other neuropsychiatric disorders, since cortical hypodopaminergia is not exclusive an exclusive feature of schizophrenia. Several other neuropsychiatric disorders including attention-deficit/hyperactivity disorder, bipolar disorder, and major depression have also been hypothesized to present deficits in cortical dopaminergic neurotransmission (Rossetti et al., 1993; Berk et al., 2007; see as reviews Russell, 2002; Nestler and Carlezon, 2006; Prince, 2008; Yadid and Friedman, 2008; Gowrishankar et al., 2013). Dopaminergic deficits have also been postulated to play a role in addiction, likely through abnormalities of dopamine signaling within the mesolimbic pathway, and also in perturbations in working memory and attention, which are most closely related to anomalies of the dopaminergic mesocortical pathway (Egan et al., 2001; see as reviews Toda and Abi-Dargham, 2007; Li and Gao, 2011; Gowrishankar et al., 2013). In fact, it is now recognized that many neuropsychiatric disorders share a common pathological background. Recently, the National Institute of Mental Health (NIMH) has proposed a strategic plan referred to as The Research Domain Criteria project (RDoC), which aims to “define basic dimensions of functioning … cutting across 222 disorders as traditionally defined.” (NIMH, 2014). Essentially, the goal of the NIMH RDoC strategy is to analyze aspects of the pathologies of neuropsychiatric illness regardless of psychiatric diagnosis in order to discover shared abnormalities among the neuropsychiatric disorders currently diagnosed in the clinic. This new strategy is proposed as a research framework that will eventually be able to produce better diagnostic and treatment tools for the clinic. Taking into account our data and the existence of cortical dopaminergic anomalies in several neuropsychiatric disorders, it would be plausible that deficits in tyrosine hydroxylase cross current diagnosis categories. In this scenario, using the RDoC approach could lead to the development of treatments for TH deficits that would be symptom-specific rather than disorder-specific In summary, the work presented here contributes to the understanding of localized, presynaptic alterations in the dopamine pathology of schizophrenia, which can be related directly to schizophrenia symptomology. Furthermore, as stated above, the findings of this dissertation may be applicable to other neuropsychiatric disorders, in which deficits of cortical dopamine neurotransmission have been observed. 223 Figure 1. Measurement of oxygen consumption rate from isolated neonatal rat ventricular myocytes. OCR: oxygen consumption rate. Reproduced with permission, from Hill, B. G., Dranka, B. P., Zou, L., Chatham, J. C., & Darley-Usmar, V. M. (2009). Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J, 424(1), 99-107. © the Biochemical Society. 224 LIST OF GENERAL REFERENCES Abi-Dargham, A., Gil, R., Krystal, J., Baldwin, R. M., Seibyl, J. P., Bowers, M., van Dyck, C. H., Charney, D. S., Innis, R. B., & Laruelle, M. (1998). Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry, 155(6), 761-767. Adell, A., & Artigas, F. (2004). The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev, 28(4), 415-431. Aggleton, J. P., Burton, M. J., & Passingham, R. E. (1980). Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res, 190(2), 347-368. Akil, M., Kolachana, B. S., Rothmond, D. A., Hyde, T. M., Weinberger, D. R., & Kleinman, J. E. (2003). Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci, 23(6), 2008-2013. Akil, M., Pierri, J. N., Whitehead, R. E., Edgar, C. L., Mohila, C., Sampson, A. R., & Lewis, D. A. (1999). Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry, 156(10), 15801589. Alberi, L., Sgado, P., & Simon, H. H. (2004). Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development, 131(13), 3229-3236. Alcantara, S., Ruiz, M., De Castro, F., Soriano, E., & Sotelo, C. (2000). Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development, 127(7), 1359-1372. Al Ghouleh, I., Khoo, N. K., Knaus, U. G., Griendling, K. K., Touyz, R. M., Thannickal, V. J., Barchowsky, A., Nauseef, W. M., Kelley, E. E., Bauer, P. M., DarleyUsmar, V., Shiva, S., Cifuentes-Pagano, E., Freeman, B. A., Gladwin, M. T., & Pagano, P. J. (2011). Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med, 51(7), 1271-1288. 225 American Psychiatric Association (APA) (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision (DSM-IV-TR) (Washington DC: American Psychiatric Association). Andersson, E., Jensen, J. B., Parmar, M., Guillemot, F., & Bjorklund, A. (2006). Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development, 133(3), 507-516. Andersson, E., Tryggvason, U., Deng, Q., Friling, S., Alekseenko, Z., Robert, B., Perlmann, T., & Ericson, J. (2006). Identification of intrinsic determinants of midbrain dopamine neurons. Cell, 124(2), 393-405. Araneda, R., & Bustos, G. (1989). Modulation of dendritic release of dopamine by Nmethyl-D-aspartate receptors in rat substantia nigra. J Neurochem, 52(3), 962970. Arias-Carrion, O., Stamelou, M., Murillo-Rodriguez, E., Menendez-Gonzalez, M., & Poppel, E. (2010). Dopaminergic reward system: a short integrative review. Int Arch Med, 3, 24. Arinami, T., Gao, M., Hamaguchi, H., & Toru, M. (1997). A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet, 6(4), 577-582. Augustine J. R. (2008). The brain stem. In: Human Neuroanatom. (London, UK: Elsevier Academic Press), 49-68. Bailhache, T., & Balthazart, J. (1993). The catecholaminergic system of the quail brain: immunocytochemical studies of dopamine beta-hydroxylase and tyrosine hydroxylase. J Comp Neurol, 329(2), 230-256. Balijepalli, S., Boyd, M. R., & Ravindranath, V. (1999). Inhibition of mitochondrial complex I by haloperidol: the role of thiol oxidation. Neuropharmacology, 38(4), 567-577. Balijepalli, S., Kenchappa, R. S., Boyd, M. R., & Ravindranath, V. (2001). Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int, 38(5), 425-435. Ballard, I. C., Murty, V. P., Carter, R. M., MacInnes, J. J., Huettel, S. A., & Adcock, R. A. (2011). Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci, 31(28), 10340-10346. Barksdale, K. A., Perez-Costas, E., Gandy, J. C., Melendez-Ferro, M., Roberts, R. C., & Bijur, G. N. (2010). Mitochondrial viability in mouse and human postmortem brain. FASEB J, 24(9), 3590-3599. 226 Beckstead, R. M., Domesick, V. B., & Nauta, W. J. (1979). Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res, 175(2), 191-217. Berger, B., Verney, C., Alvarez, C., Vigny, A., & Helle, K. B. (1985). New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analyses. Neuroscience, 15(4), 983-998. Bergman, O., Hakansson, A., Westberg, L., Nordenstrom, K., Carmine Belin, A., Sydow, O., Olson, L., Holmberg, B., Ericksson, E., & Nissbrandt, H. (2010). PITX3 polymorphism is associated with early onset Parkinson's disease. Neurobiol Aging, 31(1), 114-117. Bergquist H. (1932). Zur Morphologie des zwischenhirns bei niederen Wirbel Tieren. Acta Zoologica. 13(1-2), 57-303. Bergquist, H., & Kallen, B. (1953). On the development of neuromeres to migration areas in the vertebrate cerebral tube. Acta Anat (Basel), 18(1), 65-73. Berk, M., Dodd, S., Kauer-Santanna, M., Malhi, G. S., Bourin, M., Kapczinski, F., & Normal, T. (2007). Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Suppl. 434, 41-49. Berridge, K. C. (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl), 191(3), 391-431. Bertolino, A., Callicott, J. H., Elman, I., Mattay, V. S., Tedeschi, G., Frank, J. A., Breier, A., & Weinberger, D. R. (1998). Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry, 43(9), 641-648. Bjorklund, A., & Dunnett, S. B. (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci, 30(5), 194-202. Blank, C. L., Sasa, S., Isernhagen, R., Meyerson, L. R., Wassil, D., Wong, P., Modak, A. T., & Stavinoha, W. B. (1979). Levels of norepinephrine and dopamine in mouse brain regions following microwave inactivation--rapid post-mortem degradation of striatal dopamine in decapitated animals. J Neurochem, 33(1), 213-219. Blasi, G., Lo Bianco, L., Taurisano, P., Gelao, B., Romano, R., Fazio, L., Papazacharias, A., Di Giorgio, A., Caforio, G., Rampino, A., Masellis, R., Papp, A., Ursini, G., Sinibaldi, L., Popolizio, T., Sadee, W., & Bertolino, A. (2009). Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci, 29(47), 14812-14819. 227 Bogerts B., Hantsch J. and Herzer M. (1983). A morphometric study of the dopaminecontaining cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry, 18, 951-969. Bolam, J. P., Izzo, P. N., & Graybiel, A. M. (1988). Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: a combined Golgi and immunocytochemical study in cat and ferret. Neuroscience, 24(3), 853-875. Bolam, J. P., & Smith, Y. (1990). The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res, 529(1-2), 57-78. Bruno C, Martinuzzi A, Tang Y, Andreu AL, Pallotti F, et al. (1999). A stop-codon mutation in the human mtDNA cytochrome c oxidase I gene disrupts the functional structure of complex IV. Am J Hum Genet 65: 611–620. Bullock, W. M., Cardon, K., Bustillo, J., Roberts, R. C., & Perrone-Bizzozero, N. I. (2008). Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry, 165(12), 1594-1603. Buot, A., & Yelnik, J. (2012). Functional anatomy of the basal ganglia: limbic aspects. Rev Neurol (Paris), 168(8-9), 569-575. Burkhardt, C., Kelly, J. P., Lim, Y. H., Filley, C. M., & Parker, W. D., Jr. (1993). Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol, 33(5), 512-517. Cahn, W., Hulshoff Pol, H. E., Bongers, M., Schnack, H. G., Mandl, R. C., Van Haren, N. E., Durston, S., Koning, H., Van Der Linden, J. A., & Kahn, R. S. (2002). Brain morphology in antipsychotic-naive schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl, 43, s66-72. Campbell N. A., Williamson B., and Heyden R. J. (2006). Biology: Exploring Life. (Boston: Pearson Prentice Hall). Carlsson, A., & Lindqvist, M. (1963). Effect of chlorpromazine and haloperidol on formation of 3 methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh), 20, 140-144. Carlsson, A., Lindqvist, M., & Magnusson, T. (1957). 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature, 180(4596), 1200. Carlsson, A., Waters, N., & Carlsson, M. L. (1999). Neurotransmitter interactions in schizophrenia--therapeutic implications. Biol Psychiatry, 46(10), 1388-1395. 228 Carpenter, M. B., & Peter, P. (1972). Nigrostriatal and nigrothalamic fibers in the rhesus monkey. J Comp Neurol, 144(1), 93-115. Cavelier, L., Jazin, E. E., Eriksson, I., Prince, J., Bave, U., Oreland, L., & Gyllensten, U. (1995). Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics, 29(1), 217-224. Cebrian, C., & Prensa, L. (2010). Basal ganglia and thalamic input from neurons located within the ventral tier cell cluster region of the substantia nigra pars compacta in the rat. J Comp Neurol, 518(8), 1283-1300. Celada, P., Paladini, C. A., & Tepper, J. M. (1999). GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience, 89(3), 813-825. Charara, A., Smith, Y., & Parent, A. (1996). Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol, 364(2), 254-266. Chen, X., Xu, L., Radcliffe, P., Sun, B., & Tank, A. W. (2008). Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Mol Pharmacol, 73(6), 1816-1828. Chien, E. Y., Liu, W., Zhao, Q., Katritch, V., Han, G. W., Hanson, M. A., Shi, L., Newman, A. H., Javitch, J. A., Cherezov, V., & Stevens, R. C. (2010). Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science, 330(6007), 1091-1095. Chua, S. E., Cheung, C., Cheung, V., Tsang, J. T., Chen, E. Y., Wong, J. C., Cheung, J. P., Yip, L., Tai, K. S., Suckling, J., & McAlonan, G. M. (2007). Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res, 89(1-3), 12-21. Chung, C. Y., Seo, H., Sonntag, K. C., Brooks, A., Lin, L., & Isacson, O. (2005). Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet, 14(13), 1709-1725. Chung, S., Hedlund, E., Hwang, M., Kim, D. W., Shin, B. S., Hwang, D. Y., Kang, U. J., Isacson, O., & Kim, K. S. (2005). The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci, 28(2), 241-252. 229 Clarke, M. C., Harley, M., & Cannon, M. (2006). The role of obstetric events in schizophrenia. Schizophr Bull, 32(1), 3-8. Clark KM, Taylor RW, Johnson MA, Chinnery PF, Chrzanowska-Lightowlers ZM, et al. (1999). An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am J Hum Genet 64: 1330–1339. Cleghorn, J. M., Garnett, E. S., Nahmias, C., Firnau, G., Brown, G. M., Kaplan, R., Szechtman, H., & Szechtman, B. (1989). Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res, 28(2), 119133. Coizet, V., Comoli, E., Westby, G. W., & Redgrave, P. (2003). Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci, 17(1), 28-40. Colamarino, S. A., & Tessier-Lavigne, M. (1995). The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell, 81(4), 621-629. Comoli, E., Coizet, V., Boyes, J., Bolam, J. P., Canteras, N. S., Quirk, R. H., Overton, P. G., & Redgrave, P. (2003). A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci, 6(9), 974-980. Cooper, A. J., & Stanford, I. M. (2001). Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology, 41(1), 62-71. Corvin, A., Donohoe, G., McGhee, K., Murphy, K., Kenny, N., Schwaiger, S., Nangle, J. M., Morris, D., & Gill, M. (2007). D-amino acid oxidase (DAO) genotype and mood symptomatology in schizophrenia. Neurosci Lett, 426(2), 97-100. Costa, E., Chen, Y., Dong, E., Grayson, D. R., Kundakovic, M., Maloku, E., Ruzicka, W., Satta, R., Veldic, M., Zhubi, A., & Guidotti, A. (2009). GABAergic promoter hypermethylation as a model to study the neurochemistry of schizophrenia vulnerability. Expert Rev Neurother, 9(1), 87-98. Costa, E., Davis, J. M., Dong, E., Grayson, D. R., Guidotti, A., Tremolizzo, L., & Veldic, M. (2004). A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol, 16(1-2), 1-23. Cousins, D. A., Butts, K., & Young, A. H. (2009). The role of dopamine in bipolar disorder. Bipolar Disord, 11(8), 787-806. 230 Cragg, S. J., & Greenfield, S. A. (1997). Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci, 17(15), 5738-5746. Creese, I., Burt, D. R., & Snyder, S. H. (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science, 192(4238), 481-483. Crittenden, J. R., & Graybiel, A. M. (2011). Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat, 5, 59. Crosby E. C. and Woodburne R. T. (1943). The nuclear pattern of the non-tectal portions of the midbrain and isthmus in primates. 78(3), 441-482. Damier P., Hirsch E. C., Agid Y. and Graybiel A. M. (1999a). The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain, 122, 1421-1436. Damier P., Hirsch E. C., Agid Y. and Graybiel A. M. (1999b). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain, 122, 1437-1448. Daskalakis, Z. J., & George, T. P. (2009). Clozapine, GABA(B), and the treatment of resistant schizophrenia. Clin Pharmacol Ther, 86(4), 442-446. Davidson, M., & Davis, K. L. (1988). A comparison of plasma homovanillic acid concentrations in schizophrenic patients and normal controls. Arch Gen Psychiatry, 45(6), 561-563. Davis, K. L., Kahn, R. S., Ko, G., & Davidson, M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry, 148(11), 1474-1486. de Bartolomeis, A., Buonaguro, E. F., & Iasevoli, F. (2013). Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology (Berl), 225(1), 1-19. De Mei, C., Ramos, M., Iitaka, C., & Borrelli, E. (2009). Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol, 9(1), 53-58. DeLisi, L. E., Szulc, K. U., Bertisch, H. C., Majcher, M., & Brown, K. (2006). Understanding structural brain changes in schizophrenia. Dialogues Clin Neurosci, 8(1), 71-78. 231 DeLong M.R., and Georgopoulos A.P. (1981). Motor functions of the basal ganglia. In: Brookhart J. M., Mountcastle V. B., and Brooks V. B. (eds) Handbook of Physiology, sect 1: The nervous system, vol 2: Motor control, part 2. (Bethesda: American Physiological Society), 1017-1061. Deschamps, C., Faideau, M., Jaber, M., Gaillard, A., & Prestoz, L. (2009). Expression of ephrinA5 during development and potential involvement in the guidance of the mesostriatal pathway. Exp Neurol, 219(2), 466-480. Di Chiara, G. (1998). A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol, 12(1), 54-67. Donoghue, J. P., & Herkenham, M. (1986). Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res, 365(2), 397-403. Double, K. L., Zecca, L., Costi, P., Mauer, M., Griesinger, C., Ito, S., Ben-Shachar, D., Bringmann, G., Fariello, R. G., Riederer, P., & Gerlach, M. (2000). Structural characteristics of human substantia nigra neuromelanin and synthetic dopamine melanins. J Neurochem, 75(6), 2583-2589. Dugan, J. P., Stratton, A., Riley, H. P., Farmer, W. T., & Mastick, G. S. (2011). Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals. Mol Cell Neurosci, 46(1), 347-356. Dunkley, P. R., Bobrovskaya, L., Graham, M. E., von Nagy-Felsobuki, E. I., & Dickson, P. W. (2004). Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem, 91(5), 1025-1043. Dunlop, B. W., & Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry, 64(3), 327-337. Eells, J. B., Lipska, B. K., Yeung, S. K., Misler, J. A., & Nikodem, V. M. (2002). Nurr1null heterozygous mice have reduced mesolimbic and mesocortical dopamine levels and increased stress-induced locomotor activity. Behav Brain Res, 136(1), 267-275. Egan, M. F., Goldberg, T. E., Kolachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., Goldman, D., & Weinberger, D. R. (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A, 98(12), 6917-6922. Eggers, A. E. (2013). A serotonin hypothesis of schizophrenia. Med Hypotheses, 80(6), 791-794. 232 Eiden, L. E., Schafer, M. K., Weihe, E., & Schutz, B. (2004). The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch, 447(5), 636-640. Eisenhofer, G., Kopin, I. J., & Goldstein, D. S. (2004). Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev, 56(3), 331-349. Elsworth, J. D., & Roth, R. H. (1997). Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol, 144(1), 4-9. Fallon, J. H. (1981). Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci, 1(12), 1361-1368. Fallon, J. H., Koziell, D. A., & Moore, R. Y. (1978). Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol, 180(3), 509-532. Fallon, J. H., & Moore, R. Y. (1978). Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol, 180(3), 545-580. Fallon, J. H., & Moore, R. Y. (1978). Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol, 180(3), 533-544. Fallon, J. H., Riley, J. N., & Moore, R. Y. (1978). Substantia nigra dopamine neurons: separate populations project to neostriatum and allocortex. Neurosci Lett, 7(2-3), 157-162. Fan, J. B., Ma, J., Zhang, C. S., Tang, J. X., Gu, N. F., Feng, G. Y., St Clair, D., & He, L. (2003). A family-based association study of T1945C polymorphism in the proline dehydrogenase gene and schizophrenia in the Chinese population. Neurosci Lett, 338(3), 252-254. Fatemi, S. H., Stary, J. M., Earle, J. A., Araghi-Niknam, M., & Eagan, E. (2005). GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res, 72(2-3), 109-122. Ferreira, J. G., Del-Fava, F., Hasue, R. H., & Shammah-Lagnado, S. J. (2008). Organization of ventral tegmental area projections to the ventral tegmental areanigral complex in the rat. Neuroscience, 153(1), 196-213. 233 Feve, A. P. (2012). Current status of tyrosine hydroxylase in management of Parkinson's disease. CNS Neurol Disord Drug Targets, 11(4), 450-455. Finlay J. M. (2001). Mesoprefrontal dopamine neurons and schizophrenia: role of developmental abnormalities. Schizophr Bull, 27, 431-442. Flaum, M., & Schultz, S. K. (1996). When does amphetamine-induced psychosis become schizophrenia? Am J Psychiatry, 153(6), 812-815. Floresco, S. B., & Magyar, O. (2006). Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl), 188(4), 567585. Fontanesi, F., Soto, I. C., Horn, D., & Barrientos, A. (2006). Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol, 291(6), C1129-1147. Francisconi, S., Codenotti, M., Ferrari Toninelli, G., Uberti, D., & Memo, M. (2006). Mitochondrial dysfunction and increased sensitivity to excitotoxicity in mice deficient in DNA mismatch repair. J Neurochem, 98(1), 223-233. Frankle, W. G., & Laruelle, M. (2002). Neuroreceptor imaging in psychiatric disorders. Ann Nucl Med, 16(7), 437-446. Frankle, W. G., Laruelle, M., & Haber, S. N. (2006). Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology, 31(8), 1627-1636. Fuchs, J., Mueller, J. C., Lichtner, P., Schulte, C., Munz, M., Berg, D., Wullner, U., Illig, T., Sharma, M., & Gasser, T. (2009). The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging, 30(5), 731-738. Fuxe, K., Borroto-Escuela, D. O., Romero-Fernandez, W., Diaz-Cabiale, Z., Rivera, A., Ferraro, L., Tanganelli, S., Tarakanov, A. O., Garriga, P., Narvaez, J. A., Ciruela, F., Guescini, M., & Agnati, L. F. (2012). Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Front Physiol, 3, 136. Gao, D. M., Hoffman, D., & Benabid, A. L. (1996). Simultaneous recording of spontaneous activities and nociceptive responses from neurons in the pars compacta of substantia nigra and in the lateral habenula. Eur J Neurosci, 8(7), 1474-1478. Gardner, M., Gonzalez-Neira, A., Lao, O., Calafell, F., Bertranpetit, J., & Comas, D. (2006). Extreme population differences across Neuregulin 1 gene, with implications for association studies. Mol Psychiatry, 11(1), 66-75. 234 Garey, L. J., Ong, W. Y., Patel, T. S., Kanani, M., Davis, A., Mortimer, A. M., Barnes, T. R., & Hirsch, S. R. (1998). Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry, 65(4), 446453. Gaspar, P., Stepniewska, I., & Kaas, J. H. (1992). Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol, 325(1), 1-21. Gates, M. A., Coupe, V. M., Torres, E. M., Fricker-Gates, R. A., & Dunnett, S. B. (2004). Spatially and temporally restricted chemoattractive and chemorepulsive cues direct the formation of the nigro-striatal circuit. Eur J Neurosci, 19(4), 831-844. Geffen, L. B., Jessell, T. M., Cuello, A. C., & Iversen, L. L. (1976). Release of dopamine from dendrites in rat substantia nigra. Nature, 260(5548), 258-260. Gerfen, C. R. (1985). The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol, 236(4), 454-476. Gerfen, C. R. (1992). The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci, 15, 285-320. Gerfen, C. R., Herkenham, M., & Thibault, J. (1987). The neostriatal mosaic: II. Patchand matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci, 7(12), 3915-3934. Gibb, W. R. (1992). Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Res, 581(2), 283291. Gibb, W. R., & Lees, A. J. (1991). Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry, 54(5), 388-396. Goldman, A. L., Pezawas, L., Mattay, V. S., Fischl, B., Verchinski, B. A., Chen, Q., Weinberger, D. R., & Meyer-Lindenberg, A. (2009). Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry, 66(5), 467-477. Goldman, A. L., Pezawas, L., Mattay, V. S., Fischl, B., Verchinski, B. A., Zoltick, B., Weinberger, D. R., & Meyer-Lindenberg, A. (2008). Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry, 63(5), 475-483. 235 Gonzales, R. A., Job, M. O., & Doyon, W. M. (2004). The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther, 103(2), 121-146. Gonzalez-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., Lopez-Gimenez, J. F., Zhou, M., Okawa, Y., Callado, L. F., Milligan, G., Gingrich, J. A., Filizola, M., Meana, J. J., & Sealfon, S. C. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature, 452(7183), 93-97. Gordon, J. A. (2010). Testing the glutamate hypothesis of schizophrenia. Nat Neurosci, 13(1), 2-4. Gordon, S. L., Bobrovskaya, L., Dunkley, P. R., & Dickson, P. W. (2009). Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: Isoform 2 is not phosphorylated at Ser35. Biochim Biophys Acta, 1793(12), 1860-1867. Grace, A. A., & Bunney, B. S. (1979). Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol, 59(3-4), 211-218. Grace, A. A., & Bunney, B. S. (1985). Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res, 333(2), 271-284. Graybiel, A. M. (1990). Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci, 13(7), 244-254. Graybiel, A. M., and Ragsdale, C. W., Jr. (1983). Biochemical anatomy of the striatum. In: Emson PC (ed) Chemical neuroanatomy. (New York: Raven Press), 427-504. Greene, J. G., Dingledine, R., & Greenamyre, J. T. (2005). Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis, 18(1), 19-31. Grimm, J., Mueller, A., Hefti, F., & Rosenthal, A. (2004). Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci U S A, 101(38), 1389113896. Gowrishankar, R., Maureen, K. H., & Blakely, R. D. (2013). Good riddance to dopamine: Roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem Int, S0197-0186(13), [Epub ahead of print]. Grofova, I. (1975). The identification of striatal and pallidal neurons projecting to substantia nigra. An experimental study by means of retrograde axonal transport of horseradish peroxidase. Brain Res, 91(2), 286-291. 236 Grofova, I., & Rinvik, E. (1970). An experimental electron microscopic study on the striatonigral projection in the cat. Exp Brain Res, 11(3), 249-262. Grunblatt, E., Mandel, S., Jacob-Hirsch, J., Zeligson, S., Amariglo, N., Rechavi, G., Li, J., Ravid, R., Roggendorf, W., Riederer, P., & Youdim, M. B. (2004). Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm, 111(12), 1543-1573. Grunblatt, E., Mandel, S., Maor, G., & Youdim, M. B. (2001). Gene expression analysis in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice model of Parkinson's disease using cDNA microarray: effect of R-apomorphine. J Neurochem, 78(1), 1-12. Guidotti, A., Auta, J., Davis, J. M., Di-Giorgi-Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., Uzunov, D., & Costa, E. (2000). Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry, 57(11), 1061-1069. Guidotti, A., Pesold, C., & Costa, E. (2000). New neurochemical markers for psychosis: a working hypothesis of their operation. Neurochem Res, 25(9-10), 1207-1218. Haber, S. N., & Fudge, J. L. (1997). The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol, 11(4), 323-342. Haber, S. N., Ryoo, H., Cox, C., & Lu, W. (1995). Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol, 362(3), 400-410. Haber S. N., and Gdowski M. J. (2004). The Basal Ganglia. In: Paxinos G., and Mai J. K. (eds) The Human Nervous System. (London, UK: Elsevier Academic Press), 676738. Hajos, M., & Greenfield, S. A. (1993). Topographic heterogeneity of substantia nigra neurons: diversity in intrinsic membrane properties and synaptic inputs. Neuroscience, 55(4), 919-934. Hajos, M., & Greenfield, S. A. (1994). Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules within the substantia nigra. Brain Res, 660(2), 216-224. 237 Halliday G. (2004). Substantia nigra and locus coeruleus. In: Paxinos G., and Mai J. K. (eds) The Human Nervous System. (London, UK: Elsevier Academic Press), 449463. Hardman, C. D., Henderson, J. M., Finkelstein, D. I., Horne, M. K., Paxinos, G., & Halliday, G. M. (2002). Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol, 445(3), 238-255. Hardman, C. D., McRitchie, D. A., Halliday, G. M., Cartwright, H. R., & Morris, J. G. (1996). Substantia nigra pars reticulata neurons in Parkinson's disease. Neurodegeneration, 5(1), 49-55. Harrison, P. J. (1999). The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain, 122 ( Pt 4), 593-624. Harrison, P. J., & Weinberger, D. R. (2005). Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry, 10(1), 40-68; image 45. Hartman, B. K., Zide, D., & Udenfriend, S. (1972). The use of dopamine -hydroxylase as a marker for the central noradrenergic nervous system in rat brain. Proc Natl Acad Sci U S A, 69(9), 2722-2726. Haukvik, U. K., Hartberg, C. B., & Agartz, I. (2013). Schizophrenia--what does structural MRI show? Tidsskr Nor Laegeforen, 133(8), 850-853. Hauser, M. A., Li, Y. J., Xu, H., Noureddine, M. A., Shao, Y. S., Gullans, S. R., Scherzer, C. R., Jensen, R. V., McLaurin, A. C., Gibson, J. R., Scott, B. L., Jewett, R. M., Stenger, J. E., Schmechel, D. E., Hulette, C. M., & Vance, J. M. (2005). Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Arch Neurol, 62(6), 917-921. Haycock, J. W. (2002). Species differences in the expression of multiple tyrosine hydroxylase protein isoforms. J Neurochem, 81(5), 947-953. Hazlett, E. A., Buchsbaum, M. S., Jeu, L. A., Nenadic, I., Fleischman, M. B., Shihabuddin, L., Haznedar, M. M., & Harvey, P. D. (2000). Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res, 43(1), 33-46. Hegarty, S. V., Sullivan, A. M., & O'Keeffe, G. W. (2013). Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol, 379(2), 123-138. 238 Hemmendinger, L. M., Garber, B. B., Hoffmann, P. C., & Heller, A. (1981). Target neuron-specific process formation by embryonic mesencephalic dopamine neurons in vitro. Proc Natl Acad Sci U S A, 78(2), 1264-1268. Henquet, C., Murray, R., Linszen, D., & van Os, J. (2005). The environment and schizophrenia: the role of cannabis use. Schizophr Bull, 31(3), 608-612. Hernandez-Montiel, H. L., Tamariz, E., Sandoval-Minero, M. T., & Varela-Echavarria, A. (2008). Semaphorins 3A, 3C, and 3F in mesencephalic dopaminergic axon pathfinding. J Comp Neurol, 506(3), 387-397. Hill, B. G., Dranka, B. P., Zou, L., Chatham, J. C., & Darley-Usmar, V. M. (2009). Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J, 424, 99-107. Hirsch, E. C., Graybiel, A. M., Hersh, L. B., Duyckaerts, C., & Agid, Y. (1989). Striosomes and extrastriosomal matrix contain different amounts of immunoreactive choline acetyltransferase in the human striatum. Neurosci Lett, 96(2), 145-150. Holt, D. J., Graybiel, A. M., & Saper, C. B. (1997). Neurochemical architecture of the human striatum. J Comp Neurol, 384(1), 1-25. Hopkins, D. A., & Niessen, L. W. (1976). Substantia nigra projections to the reticular formation, superior colliculus and central gray in the rat, cat and monkey. Neurosci Lett, 2(5), 253-259. Horvitz, J. C. (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience, 96(4), 651-656. Howes, O. D., Kambeitz, J., Kim, E., Stahl, D., Slifstein, M., Abi-Dargham, A., & Kapur, S. (2012). The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry, 69(8), 776-786. Howes, O. D., & Kapur, S. (2009). The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull, 35(3), 549-562. Howes, O. D., Montgomery, A. J., Asselin, M. C., Murray, R. M., Grasby, P. M., & McGuire, P. K. (2007). Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl, 51, s13-18. Howes, O. D., Montgomery, A. J., Asselin, M. C., Murray, R. M., Valli, I., Tabraham, P., Bramon-Bosch, E., Valmaggia, L., Johns, L., Bromme, M., McGuire, P. K., & Grasby, P. M. (2009). Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry, 66(1), 13-20. 239 Hu, G., Henke, A., Karpowicz, R. J., Jr., Sonders, M. S., Farrimond, F., Edwards, R., Sulzer, D., & Sames, D. (2013). New Fluorescent Substrate Enables Quantitative and High-Throughput Examination of Vesicular Monoamine Transporter 2 (VMAT2). ACS Chem Biol. Hu, Z., Cooper, M., Crockett, D. P., & Zhou, R. (2004). Differentiation of the midbrain dopaminergic pathways during mouse development. J Comp Neurol, 476(3), 301311. Hurd, Y. L., Pristupa, Z. B., Herman, M. M., Niznik, H. B., & Kleinman, J. E. (1994). The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience, 63(2), 357-362. Huttemann, M., Kadenbach, B., & Grossman, L. I. (2001). Mammalian subunit IV isoforms of cytochrome c oxidase. Gene, 267(1), 111-123. Hwang, D. Y., Ardayfio, P., Kang, U. J., Semina, E. V., & Kim, K. S. (2003). Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res, 114(2), 123-131. Hynes, M., Porter, J. A., Chiang, C., Chang, D., Tessier-Lavigne, M., Beachy, P. A., & Rosenthal, A. (1995). Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron, 15(1), 35-44. Hynes, M., Poulsen, K., Tessier-Lavigne, M., & Rosenthal, A. (1995). Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell, 80(1), 95-101. Hynes, M., & Rosenthal, A. (1999). Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol, 9(1), 26-36. Hynes, M., Stone, D. M., Dowd, M., Pitts-Meek, S., Goddard, A., Gurney, A., & Rosenthal, A. (1997). Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron, 19(1), 15-26. Ichinose, H., Ohye, T., Fujita, K., Pantucek, F., Lange, K., Riederer, P. and Nagatsu, T. (1994). Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J. Neural Transm. 8, 149-158. Impagnatiello, F., Guidotti, A. R., Pesold, C., Dwivedi, Y., Caruncho, H., Pisu, M. G., Uzunov, D. P., Smalheiser, N. R., Davis, J. M., Pandey, G. N., Pappas, G. D., Tueting, P., Sharma, R. P., & Costa, E. (1998). A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A, 95(26), 15718-15723. 240 Iversen, S. D. (1984). Behavioural effects of manipulation of basal ganglia neurotransmitters. Ciba Found Symp, 107, 183-200. Jacobs, F. M., Smits, S. M., Hornman, K. J., Burbach, J. P., & Smidt, M. P. (2006). Strategies to unravel molecular codes essential for the development of mesodiencephalic dopaminergic neurons. J Physiol, 575(Pt 2), 397-402. Jafari, S., Fernandez-Enright, F., & Huang, X. F. (2012). Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J Neurochem, 120(3), 371-384. Jenkins, T. A. (2013). Perinatal complications and schizophrenia: involvement of the immune system. Front Neurosci, 7, 110. Jentsch, J. D., & Roth, R. H. (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology, 20(3), 201-225. Joel, D., & Weiner, I. (2000). The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience, 96(3), 451-474. Jose, P. A., Eisner, G. M., & Felder, R. A. (2003). Regulation of blood pressure by dopamine receptors. Nephron Physiol, 95(2), p19-27. Kakiuchi, C., Ishiwata, M., Kametani, M., Nelson, C., Iwamoto, K., & Kato, T. (2005). Quantitative analysis of mitochondrial DNA deletions in the brains of patients with bipolar disorder and schizophrenia. Int J Neuropsychopharmacol, 8(4), 515522. Kallmann, F. J. (1946). The genetic theory of schizophrenia; an analysis of 691 schizophrenic twin index families. Am J Psychiatry, 103(3), 309-322. Kato, T. (2001). The other, forgotten genome: mitochondrial DNA and mental disorders. Mol Psychiatry, 6(6), 625-633. Kawano, H., Horie, M., Honma, S., Kawamura, K., Takeuchi, K., & Kimura, S. (2003). Aberrant trajectory of ascending dopaminergic pathway in mice lacking Nkx2.1. Exp Neurol, 182(1), 103-112. Kegeles, L. S., Abi-Dargham, A., Frankle, W. G., Gil, R., Cooper, T. B., Slifstein, M., Hwang, D. R., Huang, Y., Haber, S. N., & Laruelle, M. (2010). Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry, 67(3), 231-239. 241 Kele, J., Simplicio, N., Ferri, A. L., Mira, H., Guillemot, F., Arenas, E., & Ang, S. L. (2006). Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development, 133(3), 495-505. Kennedy, T. E., Serafini, T., de la Torre, J. R., & Tessier-Lavigne, M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell, 78(3), 425-435. Kerwin, R., & Pycock, C. (1978). Specific stimulation of [3H]-dopamine release from dendrites of rat substantia nigra by glycine [proceedings]. Br J Pharmacol, 64(3), 428P. Kimoto, S., Bazmi, H. H., & Lewis, D. A. (2014). Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: Contribution of altered regulated by Zif268. Am J Psychiatry, [Epub ahead of print]. Kirov, G., Ivanov, D., Williams, N. M., Preece, A., Nikolov, I., Milev, R., Koleva, S., Dimitrova, A., Toncheva, D., O’Donovan, M. C., & Owen, M. J. (2004). Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry, 55(10), 971-975. Klawans, H. L., & Margolin, D. I. (1975). Amphetamine-induced dopaminergic hypersensitivity in guinea pigs. Implications in psychosis and human movement disorders. Arch Gen Psychiatry, 32(6), 725-732. Kopin, I. J. (1985). Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev, 37(4), 333-364. Korotkova, T. M., Ponomarenko, A. A., Brown, R. E., & Haas, H. L. (2004). Functional diversity of ventral midbrain dopamine and GABAergic neurons. Mol Neurobiol, 29(3), 243-259. Kristiansen, L. V., Beneyto, M., Haroutunian, V., & Meador-Woodruff, J. H. (2006). Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry, 11(8), 737-747, 705. Kristiansen, L. V., Huerta, I., Beneyto, M., & Meador-Woodruff, J. H. (2007). NMDA receptors and schizophrenia. Curr Opin Pharmacol, 7(1), 48-55. Kubota, Y., Inagaki, S., & Kito, S. (1986). Innervation of substance P neurons by catecholaminergic terminals in the neostriatum. Brain Res, 375(1), 163-167. 242 Kubota, Y., & Kawaguchi, Y. (1993). Spatial distributions of chemically identified intrinsic neurons in relation to patch and matrix compartments of rat neostriatum. J Comp Neurol, 332(4), 499-513. Kuepper, R., Skinbjerg, M., & Abi-Dargham, A. (2012). The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. Handb Exp Pharmacol(212), 1-26. Kung, L., & Roberts, R. C. (1999). Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse, 31(1), 67-75. Laruelle, M. (2000). Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab, 20(3), 423451. Laruelle, M. (2013). The second revision of the dopamine theory of schizophrenia: implications for treatment and drug development. Biol Psychiatry, 74(2), 80-81. Lauter, G., Soll, I., & Hauptmann, G. (2013). Molecular characterization of prosomeric and intraprosomeric subdivisions of the embryonic zebrafish diencephalon. J Comp Neurol, 521(5), 1093-1118. Laviolette, S. R. (2007). Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull, 33(4), 971-981. Lavoie, B., & Parent, A. (1994). Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol, 344(2), 232-241. Le Bourdelles, B., Horellou, P., Le Caer, J. P., Denefle, P., Latta, M., Haavik, J., Guibert, B., Mayaux, J. F., & Mallet, J. (1991). Phosphorylation of human recombinant tyrosine hydroxylase isoforms 1 and 2: an additional phosphorylated residue in isoform 2, generated through alternative splicing. J Biol Chem, 266(26), 1712417130. Leask S. J. (2004). Environmental influences in schizophrenia: The known and the unknown. Adv. Psychiatr. Treat. 10, 323-330. Lee, C. R., Abercrombie, E. D., & Tepper, J. M. (2004). Pallidal control of substantia nigra dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine levels. Neuroscience, 129(2), 481-489. Lee, Y., Oh, S. B., Park, H. R., Kim, H. S., Kim, M. S., & Lee, J. (2013). Selective impairment on the proliferation of neural progenitor cells by oxidative phosphorylation disruption. Neurosci Lett, 535, 134-139. 243 Lenartowski, R., & Goc, A. (2011). Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int J Dev Neurosci, 29(8), 873-883. Lewis, D. A., Cho, R. Y., Carter, C. S., Eklund, K., Forster, S., Kelly, M. A., & Montrose, D. (2008). Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry, 165(12), 1585-1593. Lewis, D. A., Melchitzky, D. S., Sesack, S. R., Whitehead, R. E., Auh, S., & Sampson, A. (2001). Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol, 432(1), 119136. Li, Y. C., & Gao, W. J. (2011). GSK-3β Activity and Hyperdopamine-dependent Behaviors. Neurosci Biobehav Rev, 35(3), 645-654. Li, Y., Park, J. S., Deng, J. H., & Bai, Y. (2006). Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr, 38(5-6), 283-291. Lin, J. C., & Rosenthal, A. (2003). Molecular mechanisms controlling the development of dopaminergic neurons. Semin Cell Dev Biol, 14(3), 175-180. Lin, L., Rao, Y., & Isacson, O. (2005). Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol Cell Neurosci, 28(3), 547555. Lindgren, N., Xu, Z. Q., Herrera-Marschitz, M., Haycock, J., Hokfelt, T., & Fisone, G. (2001). Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur J Neurosci, 13(4), 773-780. Lindholm, E., Cavelier, L., Howell, W. M., Eriksson, I., Jalonen, P., Adolfsson, R., Blackwood, D. H., Muir, W. J., Brookes, A. J., Gyllensten, U. & Jazin, E. E. (1997). Mitochondrial sequence variants in patients with schizophrenia. Eur J Hum Genet, 5(6), 406-412. Lindvall, O., & Bjorklund, A. (1974). The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl, 412, 1-48. Lisman, J. E., Coyle, J. T., Green, R. W., Javitt, D. C., Benes, F. M., Heckers, S., & Grace, A. A. (2008). Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci, 31(5), 234-242. Liu, Y. L., Fann, C. S., Liu, C. M., Chang, C. C., Yang, W. C., Hung, S. I., Hsieh, M. H., Liu, C. C., Tsuang, M. M., Wu, J. Y., Jou, Y. S., Faraone, S. V., Tsuang, M. T., 244 Chen, W. J., & Hwu, H. G. (2007). More evidence supports the association of PPP3CC with schizophrenia. Mol Psychiatry, 12(10), 966-974. Livesey, F. J., & Hunt, S. P. (1997). Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci, 8(6), 417-429. Loughlin, S. E., & Fallon, J. H. (1984). Substantia nigra and ventral tegmental area projections to cortex: topography and collateralization. Neuroscience, 11(2), 425435. Luk, K. C., Rymar, V. V., van den Munckhof, P., Nicolau, S., Steriade, C., Bifsha, P., Drouin, J., & Sadikot, A. F. (2013). The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. J Neurochem, 125(6), 932-943. Luo, L., & O'Leary, D. D. (2005). Axon retraction and degeneration in development and disease. Annu Rev Neurosci, 28, 127-156. Mailly, P., Charpier, S., Menetrey, A., & Deniau, J. M. (2003). Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci, 23(12), 5247-5257. Maisonpierre, P. C., Barrezueta, N. X., & Yancopoulos, G. D. (1993). Ehk-1 and Ehk-2: two novel members of the Eph receptor-like tyrosine kinase family with distinctive structures and neuronal expression. Oncogene, 8(12), 3277-3288. Manatt M., and Chandra S. (2011) The effects of mitochondrial dysfunction in schizophrenia. Journal of medical genetics and genomics. 3(5), 84-94. Mannisto, P. T., & Kaakkola, S. (1999). Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev, 51(4), 593-628. Margolis, E. B., Lock, H., Chefer, V. I., Shippenberg, T. S., Hjelmstad, G. O., & Fields, H. L. (2006). Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A, 103(8), 2938-2942. Marillat, V., Cases, O., Nguyen-Ba-Charvet, K. T., Tessier-Lavigne, M., Sotelo, C., & Chedotal, A. (2002). Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol, 442(2), 130-155. Marin, O., Smeets, W. J., & Gonzalez, A. (1998). Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci, 21(11), 487-494. 245 Martinez-Ferre, A., & Martinez, S. (2012). Molecular regionalization of the diencephalon. Front Neurosci, 6, 73. Masuda, T., & Shiga, T. (2005). Chemorepulsion and cell adhesion molecules in patterning initial trajectories of sensory axons. Neurosci Res, 51(4), 337-347. Matsuda, Y., Fujimura, K., & Yoshida, S. (1987). Two types of neurons in the substantia nigra pars compacta studied in a slice preparation. Neurosci Res, 5(2), 172-179. Matsumoto, M., Weickert, C. S., Akil, M., Lipska, B. K., Hyde, T. M., Herman, M. M., Kleinman, J. E., & Weinberger, D. R. (2003). Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience, 116(1), 127-137. Maurer, I., & Moller, H. J. (1997). Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem, 174(1-2), 255-259. Maurer, I., Zierz, S., & Moller, H. (2001). Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res, 48(1), 125-136. Maxwell, S. L., Ho, H. Y., Kuehner, E., Zhao, S., & Li, M. (2005). Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol, 282(2), 467-479. McCullumsmith, R. E., Hammond, J., Funk, A., & Meador-Woodruff, J. H. (2012). Recent advances in targeting the ionotropic glutamate receptors in treating schizophrenia. Curr Pharm Biotechnol, 13(8), 1535-1542. McRitchie, D. A., Halliday, G. M., & Cartwright, H. (1995). Quantitative analysis of the variability of substantia nigra pigmented cell clusters in the human. Neuroscience, 68(2), 539-551. Meador-Woodruff, J. H., Mansour, A., Healy, D. J., Kuehn, R., Zhou, Q. Y., Bunzow, J. R., Akil, H., Civelli, O., & Watson, S. J., Jr. (1991). Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology, 5(4), 231-242. Mehler, W. R. (1980). Subcortical afferent connections of the amygdala in the monkey. J Comp Neurol, 190(4), 733-762. Melendez-Ferro, M., Rice, M. W., Roberts, R. C., & Perez-Costas, E. (2013). An accurate method for the quantification of cytochrome C oxidase in tissue sections. J Neurosci Methods, 214(2), 156-162. 246 Menke, R. A., Jbabdi, S., Miller, K. L., Matthews, P. M., & Zarei, M. (2010). Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. Neuroimage, 52(4), 1175-1180. Meyer-Lindenberg, A. (2010). Imaging genetics of schizophrenia. Dialogues Clin Neurosci, 12(4), 449-456. Michael, A. C., Ikeda, M., & Justice, J. B., Jr. (1987). Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Res, 421(1-2), 325-335. Michel, H., Behr, J., Harrenga, A., & Kannt, A. (1998). Cytochrome c oxidase: structure and spectroscopy. Annu Rev Biophys Biomol Struct, 27, 329-356. Middleton, F. A., & Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science, 266(5184), 458-461. Miller, R. M., Callahan, L. M., Casaceli, C., Chen, L., Kiser, G. L., Chui, B., KaysserKranich, T. M., Sendera, T. J., Palaniappan, C., & Federoff, H. J. (2004). Dysregulation of gene expression in the 1-methyl-4-phenyl-1,2,3,6tetrahydropyridine-lesioned mouse substantia nigra. J Neurosci, 24(34), 74457454. Mitchell, T., Chacko, B., Ballinger, S. W., Bailey, S. M., Zhang, J., & Darley-Usmar, V. (2013). Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans, 41(1), 127-133. Miyake, N., Thompson, J., Skinbjerg, M., & Abi-Dargham, A. (2011). Presynaptic dopamine in schizophrenia. CNS Neurosci Ther, 17(2), 104-109. Moron, J. A., Brockington, A., Wise, R. A., Rocha, B. A., & Hope, B. T. (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci, 22(2), 389-395. Mortensen, P. B., Pedersen, C. B., Westergaard, T., Wohlfahrt, J., Ewald, H., Mors, O., Andersen, P. K., & Melbye, M. (1999). Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med, 340(8), 603-608. Mueller, H.T., Haroutunian, V., Davis, K.L. and Meador-Woodruff, J.H. (2004). Expression of the ionotropic glutamate receptor subunits and NMDA receptorassociated intracellular proteins in the substantia nigra in schizophrenia. Mol. Brain Res. 121, 60-69. 247 Nakamura, S., Ito, Y., Shirasaki, R., & Murakami, F. (2000). Local directional cues control growth polarity of dopaminergic axons along the rostrocaudal axis. J Neurosci, 20(11), 4112-4119. Nakashima, A., Hayashi, N., Kaneko, Y. S., Mori, K., Sabban, E. L., Nagatsu, T., & Ota, A. (2009). Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J Neural Transm, 116(11), 1355-1362. Napiwotzki, J., & Kadenbach, B. (1998). Extramitochondrial ATP/ADP-ratios regulate cytochrome c oxidase activity via binding to the cytosolic domain of subunit IV. Biol Chem, 379(3), 335-339. National Institute of Mental Health. (2014). Research Domain Criteria (RDoC). Retrieved from: http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml Nemoto, C., Hida, T., & Arai, R. (1999). Calretinin and calbindin-D28k in dopaminergic neurons of the rat midbrain: a triple-labeling immunohistochemical study. Brain Res, 846(1), 129-136. Nestler, E. J., & Carlezon. W. A. Jr. (2006). The mesolimbic dopamine reward circuit in depression.. Biol Psychiatry, 59(12), 1151-1159. Nestler, E. J., Terwilliger, R. Z., Walker, J. R., Sevarino, K. A., & Duman, R. S. (1990). Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem, 55(3), 1079-1082. Neves, S. R., Ram, P. T., & Iyengar, R. (2002). G protein pathways. Science, 296(5573), 1636-1639. Newman-Tancredi A., and Kleven M. S. (2010). Pharmacology of “atypicality” of antipsychotic drugs: status and perspectives. Arch. Psychiatry & Psychotherapy. 4, 5-11. Nieuwenhuys R., Voogd J., and van Huijzen C. (2007). Telencephalon: Basal Ganglia. In: The Human Central Nervous System: A Synopsis and Atlas, Fourth Edition (New York: Springer), 427-489. Nieuwenhuys R., Voogd J. and van Huijzen C. (2008). The Human Central Nervous System: Topography of spinal cord, brain stem and cerebellum (Nieuwenhuys R., Voogd J. and van Huijzen C., eds), (Verlin: Springer-Verlag), 177-246. Nijtmans, L. G., Taanman, J. W., Muijsers, A. O., Speijer, D., & Van den Bogert, C. (1998). Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem, 254(2), 389-394. 248 Nitsch, C., & Riesenberg, R. (1988). Immunocytochemical demonstration of GABAergic synaptic connections in rat substantia nigra after different lesions of the striatonigral projection. Brain Res, 461(1), 127-142. Noetzel, M. J., Jones, C. K., & Conn, P. J. (2012). Emerging approaches for treatment of schizophrenia: modulation of glutamatergic signaling. Discov Med, 14(78), 335343. Norita, M., & Kawamura, K. (1980). Subcortical afferents to the monkey amygdala: an HRP study. Brain Res, 190(1), 225-230. Nunes, I., Tovmasian, L. T., Silva, R. M., Burke, R. E., & Goff, S. P. (2003). Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A, 100(7), 4245-4250. Obeso, J. A., Rodriguez-Oroz, M. C., Benitez-Temino, B., Blesa, F. J., Guridi, J., Marin, C., & Rodriguez, M. (2008). Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord, 23 Suppl 3, S548559. Ohlow, M. J., & Moosmann, B. (2011). Phenothiazine: the seven lives of pharmacology's first lead structure. Drug Discov Today, 16(3-4), 119-131. Olney, J. W., Newcomer, J. W., & Farber, N. B. (1999). NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res, 33(6), 523-533. Patel, J. C., Witkovsky, P., Avshalumov, M. V., & Rice, M. E. (2009). Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci, 29(20), 6568-6579. Perez, J., Hill, B. G., Benavides, G. A., Dranka, B. P., & Darley-Usmar, V. M. (2010). Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem J, 428(2), 255-267. Perez-Costas, E., Guidetti, P., Melendez-Ferro, M., Kelley, J. J., & Roberts, R. C. (2008). Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm, 115(5), 745-753. Perez-Costas, E., Melendez-Ferro, M., Rice, M. W., Conley, R. R., & Roberts, R. C. (2012). Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry, 3, 31. Perez-Costas, E., Melendez-Ferro, M., & Roberts, R. C. (2010). Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem, 113(2), 287-302. 249 Pickel, V. M., Joh, T. H., Field, P. M., Becker, C. G., & Reis, D. J. (1975). Cellular localization of tyrosine hydroxylase by immunohistochemistry. J Histochem Cytochem, 23(1), 1-12. Pickel, V. M., Specht, L. A., Sumal, K. K., Joh, T. H., Reis, D. J., & Hervonen, A. (1980). Immunocytochemical localization of tyrosine hydroxylase in the human fetal nervous system. J Comp Neurol, 194(2), 465-474. Pierri, J. N., Volk, C. L., Auh, S., Sampson, A., & Lewis, D. A. (2001). Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry, 58(5), 466-473. Pietrzak, R. H., Snyder, P. J., Jackson, C. E., Olver, J., Norman, T., Piskulic, D., & Maruff, P. (2009). Stability of cognitive impairment in chronic schizophrenia over brief and intermediate re-test intervals. Hum Psychopharmacol, 24(2), 113-121. Placzek, M., & Briscoe, J. (2005). The floor plate: multiple cells, multiple signals. Nat Rev Neurosci, 6(3), 230-240. Pletnikov, M. V., Ayhan, Y., Nikolskaia, O., Xu, Y., Ovanesov, M. V., Huang, H., Mori, S., Moran, T. H., & Ross, C. A. (2008). Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry, 13(2), 173-186, 115. Porrino, L. J., & Goldman-Rakic, P. S. (1982). Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol, 205(1), 63-76. Powers, R. E. (1999). The neuropathology of schizophrenia. J Neuropathol Exp Neurol, 58(7), 679-690. Prabakaran, S., Swatton, J. E., Ryan, M. M., Huffaker, S. J., Huang, J. T., Griffin, J. L., Wayland, M., Freeman, T., Dudbridge, F., Lilley, K. S., Karp, N. A., Hester, S., Tkachev, D., Mimmack, M. L., Yolken, R. H., Webster, M. J., Torrey, E. F., & Bahn, S. (2004). Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry, 9(7), 684697, 643. Prasad, A. A., & Pasterkamp, R. J. (2009). Axon guidance in the dopamine system. Adv Exp Med Biol, 651, 91-100. Prestoz, L., Jaber, M., & Gaillard, A. (2012). Dopaminergic axon guidance: which makes what? Front Cell Neurosci, 6, 32. Prince, J. (2008). Catecholamine Dysfunction in Attention-Deficit/Hyperactivity Disorder: An Update. J Clin Psychopharmacol, 28(3 Suppl 2), S39-45. 250 Prince, J. A., Blennow, K., Gottfries, C. G., Karlsson, I., & Oreland, L. (1999). Mitochondrial function is differentially altered in the basal ganglia of chronic schizophrenics. Neuropsychopharmacology, 21(3), 372-379. Prince, J. A., Yassin, M. S., & Oreland, L. (1997). Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther, 280(1), 261-267. Prince, J. A., Yassin, M. S., & Oreland, L. (1998). A histochemical demonstration of altered cytochrome oxidase activity in the rat brain by neuroleptics. Eur Neuropsychopharmacol, 8(1), 1-6. Puelles, L. (2009). Forebrain development: Prosomere model. Developmental Neurobiology. 95-99. Puelles, E., Acampora, D., Lacroix, E., Signore, M., Annino, A., Tuorto, F., Filosa, S., Corte, G., Wurst, W., Ang, S. L., & Simeone, A. (2003). Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat Neurosci, 6(5), 453-460. Puelles, E., Annino, A., Tuorto, F., Usiello, A., Acampora, D., Czerny, T., Brodski, C., Ang, S. L., Wurst, W., & Simeone, A. (2004). Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development, 131(9), 2037-2048. Puelles, L. (2001). Brain segmentation and forebrain development in amniotes. Brain Res Bull, 55(6), 695-710. Puelles, L., & Rubenstein, J. L. (2003). Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci, 26(9), 469-476. Puelles, L., & Verney, C. (1998). Early neuromeric distribution of tyrosine-hydroxylaseimmunoreactive neurons in human embryos. J Comp Neurol, 394(3), 283-308. Purves, D., Augustine, G. J., Fitzpatrick, D., et al. (2001). Neuroscience, 2nd edition. (Sunderland: Sinauer Associates). Raedler, T. J., Bymaster, F. P., Tandon, R., Copolov, D., & Dean, B. (2007). Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry, 12(3), 232-246. Ragsdale, C. W., Jr., & Graybiel, A. M. (1988). Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol, 269(4), 506-522. Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, et al. (1999). A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am J Hum Genet 65: 1030–1039. 251 Remington G., Agid O. and Foussias G. (2011). Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev Neurother, 11, 589-607. Reyes, S., Fu, Y., Double, K., Thompson, L., Kirik, D., Paxinos, G., & Halliday, G. M. (2012). GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J Comp Neurol, 520(12), 2591-2607. Rezin, G. T., Amboni, G., Zugno, A. I., Quevedo, J., & Streck, E. L. (2009). Mitochondrial dysfunction and psychiatric disorders. Neurochem Res, 34(6), 1021-1029. Rice, M. E., & Cragg, S. J. (2008). Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev, 58(2), 303313. Rice, M. W., Smith, K. L., Roberts, R. C., Perez-Costas, E., & Melendez-Ferro, M. (2014). Assessment of cytochrome c oxidase dysfunction in the substantia nigra/ventral tegmental area in schizophrenia. PLOS ONE. [Epub ahead of print]. Riehemann, S., Volz, H. P., Stutzer, P., Smesny, S., Gaser, C., & Sauer, H. (2001). Hypofrontality in neuroleptic-naive schizophrenic patients during the Wisconsin Card Sorting Test--a fMRI study. Eur Arch Psychiatry Clin Neurosci, 251(2), 6671. Rimol, L. M., Hartberg, C. B., Nesvag, R., Fennema-Notestine, C., Hagler, D. J., Jr., Pung, C. J., Jennings, R. G., Haukvik, U. K., Lange, E., Nakstad, P. H., Melle, I., Andreassen, O. A., Dale, A. M., & Agartz, I. (2010). Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry, 68(1), 41-50. Roberts, R. C., Conley, R., Kung, L., Peretti, F. J., & Chute, D. J. (1996). Reduced striatal spine size in schizophrenia: a postmortem ultrastructural study. Neuroreport, 7(6), 1214-1218. Roberts, R. C., & Knickman, J. K. (2002). The ultrastructural organization of the patch matrix compartments in the human striatum. J Comp Neurl, 452(2), 128-138. Roberts, R. C., Roche, J. K., & Conley, R. R. (2005). Synaptic differences in the postmortem striatum of subjects with schizophrenia: a stereological ultrastructural analysis. Synapse, 56(4), 185-197. Roberts, R. C., Roche, J. K., Conley, R. R., & Lahti, A. C. (2009). Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse, 63(6), 520-530. 252 Roffler-Tarlov, S., & Graybiel, A. M. (1984). Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature, 307(5946), 62-66. Rossetti, Z. L., Lai, M., Hmaidan, Y., & Gessa, G. L. (1993). Depletion of mesolimbic dopamine during behavioral despair: Partial reversal by chronic imipramine. Eur J Pharmacol, 242(3), 313-315. Roze, E., Bonnet, C., Betuing, S., & Caboche, J. (2010). Huntington's disease. Adv Exp Med Biol, 685, 45-63. Rubenstein, J. L., Martinez, S., Shimamura, K., & Puelles, L. (1994). The embryonic vertebrate forebrain: the prosomeric model. Science, 266(5185), 578-580. Russell, V. A. (2002). Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 130(1-2), 191-196. Sabunciyan, S., Kirches, E., Krause, G., Bogerts, B., Mawrin, C., Llenos, I. C., & Weis, S. (2007). Quantification of total mitochondrial DNA and mitochondrial common deletion in the frontal cortex of patients with schizophrenia and bipolar disorder. J Neural Transm, 114(5), 665-674. Saddar, S., Dienhart, M. K., & Stuart, R. A. (2008). The F1F0-ATP synthase complex influences the assembly state of the cytochrome bc1-cytochrome oxidase supercomplex and its association with the TIM23 machinery. J Biol Chem, 283(11), 6677-6686. Sadikot, A. F., & Parent, A. (1990). The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience, 36(2), 431447. Sanchez-Gonzalez, M. A., Garcia-Cabezas, M. A., Rico, B., & Cavada, C. (2005). The primate thalamus is a key target for brain dopamine. J Neurosci, 25(26), 60766083. Saucedo-Cardenas, O., Quintana-Hau, J. D., Le, W. D., Smidt, M. P., Cox, J. J., De Mayo, F., Burbach, J. P., & Conneely, O. M. (1998). Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A, 95(7), 4013-4018. Schultz, C. C., Koch, K., Wagner, G., Roebel, M., Schachtzabel, C., Gaser, C., Nenadic, I., Reichenbach, J. R., Sauer, H., & Schlosser, R. G. (2010). Reduced cortical thickness in first episode schizophrenia. Schizophr Res, 116(2-3), 204-209. 253 Schultz, W. (2002). Getting formal with dopamine and reward. Neuron, 36(2), 241-263. Schwarcz, R., & Hunter, C. A. (2007). Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull, 33(3), 652-653. Seeman, P., & Lee, T. (1975). Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science, 188(4194), 12171219. Seeman, P., Lee, T., Chau-Wong, M., & Wong, K. (1976). Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature, 261(5562), 717-719. Shenton, M. E., Dickey, C. C., Frumin, M., & McCarley, R. W. (2001). A review of MRI findings in schizophrenia. Schizophr Res, 49(1-2), 1-52. Shih, R. A., Belmonte, P. L., & Zandi, P. P. (2004). A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatry, 16(4), 260-283. Simon, H. H., Saueressig, H., Wurst, W., Goulding, M. D., & O'Leary, D. D. (2001). Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J Neurosci, 21(9), 3126-3134. Simpson, M. D., Slater, P., Deakin, J. F., Royston, M. C., & Skan, W. J. (1989). Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett, 107(13), 211-215. Smidt, M. P., Asbreuk, C. H., Cox, J. J., Chen, H., Johnson, R. L., & Burbach, J. P. (2000). A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci, 3(4), 337-341. Smidt, M. P., & Burbach, J. P. (2007). How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci, 8(1), 21-32. Smidt, M. P., Smits, S. M., Bouwmeester, H., Hamers, F. P., van der Linden, A. J., Hellemons, A. J., Graw, J., & Burbach, J. P. (2004). Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development, 131(5), 1145-1155. Smidt, M. P., Smits, S. M., & Burbach, J. P. (2004). Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res, 318(1), 35-43. Smith, Y., & Bolam, J. P. (1990). The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol, 296(1), 47-64. 254 Smith, Y., & Kieval, J. Z. (2000). Anatomy of the dopamine system in the basal ganglia. Trends Neurosci, 23(10 Suppl), S28-33. Smits, S. M., Burbach, J. P., & Smidt, M. P. (2006). Developmental origin and fate of meso-diencephalic dopamine neurons. Prog Neurobiol, 78(1), 1-16. Smits, S. M., Ponnio, T., Conneely, O. M., Burbach, J. P., & Smidt, M. P. (2003). Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci, 18(7), 1731-1738. Sodhi, M. S., Simmons, M., McCullumsmith, R., Haroutunian, V., & Meador-Woodruff, J. H. (2011). Glutamatergic gene expression is specifically reduced in thalamocortical projecting relay neurons in schizophrenia. Biol Psychiatry, 70(7), 646-654. Somerville, S. M., Conley, R. R., & Roberts, R. C. (2011). Striatal mitochondria in subjects with chronic undifferentiated vs. chronic paranoid schizophrenia. Synapse, 66(1), 29-41. Somerville, S. M., Lahti, A. C., Conley, R. R., & Roberts, R. C. (2011). Mitochondria in the striatum of subjects with schizophrenia: relationship to treatment response. Synapse, 65(3), 215-224. Sommer, S. S., Lind, T. J., Heston, L. L., & Sobell, J. L. (1993). Dopamine D4 receptor variants in unrelated schizophrenic cases and controls. Am J Med Genet, 48(2), 90-93. Somogyi, P., Bolam, J. P., Totterdell, S., & Smith, A. D. (1981). Monosynaptic input from the nucleus accumbens--ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res, 217(2), 245-263. Specht, L. A., Pickel, V. M., Joh, T. H., & Reis, D. J. (1981). Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. J Comp Neurol, 199(2), 255-276. Specht, L. A., Pickel, V. M., Joh, T. H., & Reis, D. J. (1981). Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. I. Early ontogeny. J Comp Neurol, 199(2), 233-253. Stone, J. M., Morrison, P. D., & Pilowsky, L. S. (2007). Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol, 21(4), 440-452. Strauss, G. P., Horan, W. P., Kirkpartirck, B., Fischer, B. A., Keller, W. R., Miski, P., Buchanan, R. W., Green, M. F., & Carpenter W. T. (2013). Deconstructing negative symptoms of schizophrenia: Avolition-apathy and diminished expression 255 clusters predict clinical presentation and functional outcome. J Psychiatr Res, 47(6), 783-790. Streck, E. L., Rezin, G. T., Barbosa, L. M., Assis, L. C., Grandi, E., & Quevedo, J. (2007). Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedebergs Arch Pharmacol, 376(1-2), 127-133. Subramaniam, S., & Snyder, S. H. (2011). Huntington's disease is a disorder of the corpus striatum: focus on Rhes (Ras homologue enriched in the striatum). Neuropharmacology, 60(7-8), 1187-1192. Suddath, R. L., Christison, G. W., Torrey, E. F., Casanova, M. F., & Weinberger, D. R. (1990). Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med, 322(12), 789-794. Sullivan, P. F. (2005). The genetics of schizophrenia. PLoS Med, 2(7), e212. Sullivan, P. F., Kendler, K. S., & Neale, M. C. (2003). Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry, 60(12), 1187-1192. Sun, J., Xu, J., Cairns, N. J., Perlmutter, J. S., & Mach, R. H. (2012). Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One, 7(11), e49483. Swanson, L. W. (1982). The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull, 9(1-6), 321-353. Szabo, J. (1979). Strionigral and nigrostriatal connections. Anatomical studies. Appl Neurophysiol, 42(1-2), 9-12. Szabo, J. (1980). Organization of the ascending striatal afferents in monkeys. J Comp Neurol, 189(2), 307-321. Taanman, J. W. (1997). Human cytochrome c oxidase: structure, function, and deficiency. J Bioenerg Biomembr, 29(2), 151-163. Tandon, R. (1999). Cholinergic aspects of schizophrenia. Br J Psychiatry Suppl(37), 711. Tank, A. W., Xu, L., Chen, X., Radcliffe, P., & Sterling, C. R. (2008). Posttranscriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann N Y Acad Sci, 1148, 238-248. 256 Tepper J. M., Celada P., Iribe Y., and Paladini C. (2002). Afferent control of nigral dopaminergic neurons: The role of GABAergic inputs. IN: Graybiel A. et al. (Eds.), The Basal Ganglia VI. (New York: Kluwer Academic/Plenum Publishers), 641-651. Tepper, J. M., & Lee, C. R. (2007). GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res, 160, 189-208. Tepper, J. M., Martin, L. P., & Anderson, D. R. (1995). GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci, 15(4), 3092-3103. Tepper, J. M., Sun, B. C., Martin, L. P., & Creese, I. (1997). Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo. J Neurosci, 17(7), 2519-2530. Thompson, M., Weickert, C. S., Wyatt, E., & Webster, M. J. (2009). Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res, 43(11), 970-977. Thuret, S., Bhatt, L., O'Leary, D. D., & Simon, H. H. (2004). Identification and developmental analysis of genes expressed by dopaminergic neurons of the substantia nigra pars compacta. Mol Cell Neurosci, 25(3), 394-405. Tiranti V, Corona P, Greco M, Taanman JW, Carrara F, et al. (2000). A novel frameshift mutation of the mtDNA COIII gene leads to impaired assembly of cytochrome c oxidase in a patient affected by Leigh-like syndrome. Hum Mol Genet 9: 2733– 2742. Toda, M., & Abi-Dargham, A. (2007). Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep, 9(4), 329-336. Torres, G. E., Gainetdinov, R. R., & Caron, M. G. (2003). Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci, 4(1), 13-25. Toru, M., Watanabe, S., Shibuya, H., Nishikawa, T., Noda, K., Mitsushio, H., Ichikawa, H., Kurumaji, A., Takashima, M., Mataga, N. and Ogawa, A. (1988). Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr. Scand. 78 (2), 121-137. Tsuang, M. (2000). Schizophrenia: genes and environment. Biol Psychiatry, 47(3), 210220. Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., & Yoshikawa, S. (1996). The whole structure of 257 the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science, 272(5265), 11361144. Ungless, M. A. (2004). Dopamine: the salient issue. Trends Neurosci, 27(12), 702-706. Uranova, N. A., & Aganova, E. A. (1989). [Ultrastructure of the synapses of the anterior limbic cortex in schizophrenia]. Zh Nevropatol Psikhiatr Im S S Korsakova, 89(7), 56-59. Van den Heuvel, D. M., & Pasterkamp, R. J. (2008). Getting connected in the dopamine system. Prog Neurobiol, 85(1), 75-93. van den Munckhof, P., Luk, K. C., Ste-Marie, L., Montgomery, J., Blanchet, P. J., Sadikot, A. F., & Drouin, J. (2003). Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development, 130(11), 2535-2542. van Domburg, P. H., & ten Donkelaar, H. J. (1991). The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol, 121, 1-132. Van Horn, J. D., & McManus, I. C. (1992). Ventricular enlargement in schizophrenia. A meta-analysis of studies of the ventricle:brain ratio (VBR). Br J Psychiatry, 160, 687-697. Varela-Echavarria, A., Tucker, A., Puschel, A. W., & Guthrie, S. (1997). Motor axon subpopulations respond differentially to the chemorepellents netrin-1 and semaphorin D. Neuron, 18(2), 193-207. Veazey, R. B., Amaral, D. G., & Cowan, W. M. (1982). The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II. Efferent connections. J Comp Neurol, 207(2), 135-156. Vernay, B., Koch, M., Vaccarino, F., Briscoe, J., Simeone, A., Kageyama, R., & Ang, S. L. (2005). Otx2 regulates subtype specification and neurogenesis in the midbrain. J Neurosci, 25(19), 4856-4867. Verney, C. (1999). Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc Res Tech, 46(1), 24-47. Verney, C., Berger, B., Adrien, J., Vigny, A., & Gay, M. (1982). Development of the dopaminergic innervation of the rat cerebral cortex. A light microscopic immunocytochemical study using anti-tyrosine hydroxylase antibodies. Brain Res, 281(1), 41-52. 258 Verney C., Zecevic N. and Puelles L. (2001). Structure of longitudinal brain zones that provide the origin for the substantia nigra and ventral tegmental area in human embryos, as revealed by cytoarchitecture and tyrosine hydroxylase, calretinin, calbindin and GABA immunoreactions. J Comp Neurol, 429, 22-44. Vincent, S. R. (1988). Distributions of tyrosine hydroxylase-, dopamine-betahydroxylase-, and phenylethanolamine-N-methyltransferase-immunoreactive neurons in the brain of the hamster (Mesocricetus auratus). J Comp Neurol, 268(4), 584-599. Vitalis, T., Cases, O., Engelkamp, D., Verney, C., & Price, D. J. (2000). Defect of tyrosine hydroxylase-immunoreactive neurons in the brains of mice lacking the transcription factor Pax6. J Neurosci, 20(17), 6501-6516. Volk, D., Austin, M., Pierri, J., Sampson, A., & Lewis, D. (2001). GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry, 158(2), 256-265. Volz, H. R., Riehemann, S., Maurer, I., Smesny, S., Sommer, M., Rzanny, R., Holstein, W., Czekalla, J., & Sauer, H. (2000). Reduced phosphodiesters and high-energy phosphates in the frontal lobe of schizophrenic patients: a (31)P chemical shift spectroscopic-imaging study. Biol Psychiatry, 47(11), 954-961. Voorn, P., Kalsbeek, A., Jorritsma-Byham, B., & Groenewegen, H. J. (1988). The preand postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience, 25(3), 857-887. Wallen, A., Zetterstrom, R. H., Solomin, L., Arvidsson, M., Olson, L., & Perlmann, T. (1999). Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res, 253(2), 737-746. Wallen, A. A., Castro, D. S., Zetterstrom, R. H., Karlen, M., Olson, L., Ericson, J., & Perlmann, T. (2001). Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci, 18(6), 649-663. Wanat, M. J., Willuhn, I., Clark, J. J., & Phillips, P. E. (2009). Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev, 2(2), 195-213. Warner, J. J. (2001). Atlas of neuroanatomy: With systems orgaization and case correlations. (Oxford: Butterworth-Heinemann). Weinberger, D. R., & Berman, K. F. (1988). Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull, 14(2), 157-168. 259 Whatley, S. A., Curti, D., Das Gupta, F., Ferrier, I. N., Jones, S., Taylor, C., & Marchbanks, R. M. (1998). Superoxide, neuroleptics and the ubiquinone and cytochrome b5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry, 3(3), 227-237. Whatley, S. A., Curti, D., & Marchbanks, R. M. (1996). Mitochondrial involvement in schizophrenia and other functional psychoses. Neurochem Res, 21(9), 995-1004. Wiesel, F. A. (1992). Glucose metabolism in psychiatric disorders: how can we facilitate comparisons among studies? J Neural Transm Suppl, 37, 1-18. Wiesel, F. A., Wik, G., Sjogren, I., Blomqvist, G., Greitz, T., & Stone-Elander, S. (1987). Regional brain glucose metabolism in drug free schizophrenic patients and clinical correlates. Acta Psychiatr Scand, 76(6), 628-641. Willinger, U., Heiden, A. M., Meszaros, K., Formann, A. K., & Aschauer, H. N. (2001). Neurodevelopmental schizophrenia: obstetric complications, birth weight, premorbid social withdrawal and learning disabilities. Neuropsychobiology, 43(3), 163-169. Willson, C. A., Foster, R. D., Onifer, S. M., Whittemore, S. R., & Miranda, J. D. (2006). EphB3 receptor and ligand expression in the adult rat brain. J Mol Histol, 37(8-9), 369-380. Wise, R. A. (2002). Brain reward circuitry: insights from unsensed incentives. Neuron, 36(2), 229-240. Wong, D. L., & Tank, A. W. (2007). Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress, 10(2), 121-130. Woo, T. U., Shrestha, K., Lamb, D., Minns, M. M., Benes, F. M. (2008). N-methyl-Daspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biol Psychiatry, 64(9), 803-809. Woodward, N. D., Cowan, R. L., Park, S., Ansari, M. S., Baldwin, R. M., Li, R., Doop, M., Kessler, R. M., & Zald, D. H. (2011). Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry, 168(4), 418-426. Wu, E. Q., Birnbaum, H. G., Shi, L., Ball, D. E., Kessler, R. C., Moulis, M., & Aggarwal, J. (2005). The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry, 66(9), 1122-1129. Yadid G., & Friedman, A. (2008). Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res, 172, 265-285. 260 Yamauchi, K., Mizushima, S., Tamada, A., Yamamoto, N., Takashima, S., & Murakami, F. (2009). FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J Neurosci, 29(13), 4044-4055. Yelnik, J., Francois, C., Percheron, G., & Heyner, S. (1987). Golgi study of the primate substantia nigra. I. Quantitative morphology and typology of nigral neurons. J Comp Neurol, 265(4), 455-472. Yue, Y., Widmer, D. A., Halladay, A. K., Cerretti, D. P., Wagner, G. C., Dreyer, J. L., & Zhou, R. (1999). Specification of distinct dopaminergic neural pathways: roles of the Eph family receptor EphB1 and ligand ephrin-B2. J Neurosci, 19(6), 20902101. Zecca, L., Wilms, H., Geick, S., Claasen, J. H., Brandenburg, L. O., Holzknecht, C., Panizza, M. L., Zucca, F. A., Deuschl, G., Sievers, J., & Lucius, R. (2008). Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease. Acta Neuropathol, 116(1), 47-55. Zecevic N. and Verney C. (1995). Development of the catecholamine neurons in human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol, 351, 509-35. Zetterstrom, R. H., Solomin, L., Jansson, L., Hoffer, B. J., Olson, L., & Perlmann, T. (1997). Dopamine neuron agenesis in Nurr1-deficient mice. Science, 276(5310), 248-250. Zhao, S., Maxwell, S., Jimenez-Beristain, A., Vives, J., Kuehner, E., Zhao, J., O’Brien, C., de Felipe, C., Semina, E., & Li, M. (2004). Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci, 19(5), 1133-1140. Zhou, Y., Gunput, R. A., & Pasterkamp, R. J. (2008). Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci, 33(4), 161-170. 261 APPENDIX IRB APPROVAL FORM 262