* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neurotransmitter Parameter Definitions
Single-unit recording wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Electrophysiology wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Vesicular monoamine transporter wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Long-term depression wikipedia , lookup
Signal transduction wikipedia , lookup
End-plate potential wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Aging brain wikipedia , lookup
Nervous system network models wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Synaptogenesis wikipedia , lookup
Spike-and-wave wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuroeconomics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Synaptic gating wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Biology of depression wikipedia , lookup
Chemical synapse wikipedia , lookup
Norepinephrine wikipedia , lookup
Neurotransmitter wikipedia , lookup
Molecular neuroscience wikipedia , lookup
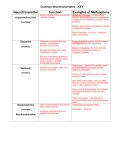
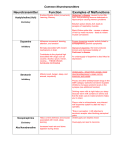
Neurotransmitter Parameter Definitions 5-Hydroxyindoleacetic acid (5-HIAA) is a major metabolite of serotonin, generated via a two step process, involving monoamine oxidase A (MAO-A) and aldehyde dehydrogenase. Measurement of 5HIAA in combination with serotonin may offer insight into mechanisms underlying various clinical symptoms. The ratio of serotonin to 5-HIAA may be used to evaluate serotonin turnover and monoamine oxidase activity. Abnormal levels of 5-HIAA have been associated with depression, suicidal behaviors, aggression, chronic psychotropic medication use, and Parkinson's Disease. Agmatine is an inhibitory neurotransmitter that can block the action of glutamate on the NMDA receptor. This mechanism is important in preventing the harmful effects of excess glutamate. Research has shown that agmatine can act to help with swelling which can help protect from chronic neuropathic pain. Downloadable PDF: Agmatine Pathway DOPAC: After neuronal dopamine is released it is inactivated primarily via reuptake mechanisms that remove it from the synapse and the extraneuronal space and return it to the presynaptic dopaminergic neuron or adjacent noradrenergic neurons. Some of the enzymes that degrade dopamine are only found in specific regions of the body. As such some dopamine metabolites are only produced in specific tissues. Understanding how and where these enzymes function can provide valuable insight about how dopamine is functioning in specific regions of the body. In order to understand these functions one must first realize Monoamine oxidase (MAO) is an enzyme present within the cytoplasm of neurons that breaks down dopamine to DOPAL. DOPAL in turn is very rapidly converted to DOPAC by a second cytoplasmic enzyme aldehyde dehydrogenase (AD). Because both of these enzymes are primarily found inside neurons, DOPAC levels are dependent on the amount of cytoplasmic dopamine. Combined measurements of DOPAC and dopamine have been used to assess the activity of dopaminergic neurons. This combination provides additional information than either parameter alone because a large portion of DOPAC is formed from dopamine without ever being released to the synaptic cleft. This suggests that DOPAC may be more closely related to the presynaptic dopamine levels while dopamine and similarly HVA levels, another important metabolite of dopamine that is formed outside of the neuron via the actions of catechols-O-methyltransferase (COMT), are related to the rate of neuron signaling. Said another way, extracellular DOPAC is related to the amount of dopamine made and stored in the presynaptic neuron while extracellular dopamine levels are related to the rate of dopamine released via the depolarization of dopamine neurons. Downloadable PDF:Catecholamine Pathway Dopamine is an excitatory and inhibitory neurotransmitter, depending on the dopaminergic receptor it binds to. It is derived from the amino acid tyrosine. Dopamine is the precursor to norepinephrine and epinephrine, which are all catecholamines. The function of dopamine is diverse but plays a large role in the pleasure/reward pathway (addiction and thrills), memory, and motor control. Dopamine, like norepinephrine and epinephrine, is stored in vesicles in the axon terminal. Dopamine plays a significant role in the cardiovascular, renal, hormonal, and central nervous systems. The dopaminergic neurons have dendrites that extend into various regions of the brain, controlling different functions through the stimulation of adrenergic and dopaminergic receptors (D1 D5). Common symptoms with low dopamine levels are loss of motor control, addictions, cravings, compulsions, and loss of satisfaction. When dopamine levels are elevated symptoms may manifest in the form of anxiety or hyperactivity. Some therapies utilize L-DOPA for parkinsonian symptoms which can also cause elevations in dopamine. Downloadable PDF: Catecholamine Pathway Epinephrine also known as adrenaline, is an excitatory neurotransmitter and hormone essential for lipolysis, which is a process in which the body metabolizes fat. Epinephrine is derived from the amine norepinephrine. As a neurotransmitter, epinephrine regulates attentiveness and mental focus. Epinephrine is synthesized from norepinephrine.As a hormone, epinephrine is secreted along with norepinephrine principally by the medulla of the adrenal gland. Heightened secretion can occur in response to fear or anger and will result in increased heart rate and the hydrolysis of glycogen to glucose. This reaction, referred to as the "fight or flight" response, prepares the body for strenuous activity. Epinephrine is used medicinally as a stimulant in cardiac arrest, as a vasoconstrictor in shock, as a bronchodilator and antispasmodic in bronchial asthma, and anaphylaxis. Commonly, epinephrine levels will be low due to adrenal fatigue (a pattern in which the adrenal output is suppressed due to chronic stress). Therefore, symptoms can be presented as fatigue with low epinephrine levels. Low levels of epinephrine can also contribute to weight gain and poor concentration. Elevated levels of epinephrine can be factors contributing to restlessness, anxiety, sleep problems, or acute stress. Downloadable PDF: Catecholamine Pathway GABA is a true neurotransmitter and is the major inhibitory neurotransmitter of the brain, occurring in 3040% of all synapses. GABA is second only to glutamate, the brain's major excitatory neurotransmitter. The GABA concentration in the brain is 200-1000 times greater than that of the monoamines or acetylcholine. The primary function of GABA is to prevent overstimulation. It does so by compensating for glutamate activity.; When GABA activates its receptor it causes negative ions to flow into the cell preventing depolarization. Glutamate can depolarize the cell and form an action potential by causing positive ions to flow into the cell when it activates its receptors. Overall, GABA regulates the activity of glutamate by preventing depolarization of the cell, therefore, preventing overstimulation. Downloadable PDF: GABA Pathway Glutamate is the major excitatory neurotransmitter in the brain which is necessary for memory and learning. In fact, it is believed that 70% of the fast excitatory CNS synapses utilize glutamate as a transmitter. Excitatory neurotransmitters increase the activity of signal-receiving neurons and play a major role in controlling brain function. Glutamate exerts its effects on cells, in part, through three types of receptors that, when activated, allow the flow of positively charged ions into the cell. These include the ionotropic receptors: kianate, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and Nmethyl-D-aspartate (NMDA) receptors. There are also series metabotropic glutamate receptors that do not directly manipulate an ion channel.; Of the ionotropic receptors, the N-methyl-D-aspartate (NMDA) receptor plays a particularly important role in controlling the brain's ability to adapt to environmental and genetic influences which is important for learning and memory. An event or process that dramatically increases the activity of glutamate often induces the death of neurons. Such a scenario is believed to take place in e.g. ischemia, trauma, hypoxia, hypoglycemia, and hepatic encephalopathy. More mild but chronic malfunctioning of glutamatergic systems may be involved in many neurodegenerative diseases such as Huntington´s disease, Parkinson´s disease, Alzheimer´s disease, vascular dementia, amyotrophic lateral sclerosis, AIDS-neurodegeneration, Tourette´s syndrome, and Korsakoff syndrome.It is unlikely that a disturbance of glutamate homeostasis is the sole initiator of these neurodegenerative diseases, but rather that excitotoxicity plays a pivotal executive role in events triggered by other processes such as energy deficits that facilitate the neurotoxic potential of endogenous glutamate. Downloadable PDF: GABA Pathway Glutamine is an amino acid which acts as a precursor to glutamate. Glutamine aids in the maintenance of gut barrier function, intestinal cell proliferation and differentiation. Glutamine supplementation is commonly utilized to help repair the mucosal lining of the gut which can help with food sensitivities and other stomach/ intestinal issues. High levels may be a sign of inhibitory/excitatory imbalances in the neurotransmitter system. Downloadable PDF: GABA Pathway Glycine is a principal inhibitory amino acid in the brainstem and spinal cord that regulates excitatory neurotransmission in much the same way as GABA. Glycine, much like GABA and taurine, can become elevated to compensate for elevations in excitatory neurotransmitters, primarily, glutamate and aspartic acid. This non-essential amino acid is common in protein-based foods, and can be synthesized metabolically from a number of different amino acids, including serine and threonine. Curiously, glycine is a necessary cofactor in the activation of the glutamate receptor, NMDA. It seems paradoxical that a primarily inhibitory amino acid facilitates the activation of an excitatory receptor. It has been postulated that glycine’ s inhibitory and excitatory actions are part of the many checks and balances the body has for regulating neurotransmission. Downloadable PDF:Glycine Pathway Histamine is an excitatory neurotransmitter involved in the sleep/wake cycle and inflammatory response. Depending on the receptor histamine activates a wide array of biological actions can occur. For instance, one receptor helps regulate the sleep/wake cycle whereas another receptor helps regulates norepinephrine, serotonin, and acetylcholine release. There are also other receptors that may be activated to induce inflammatory response, which is commonly associated with the exposure to an allergen. Interestingly, histamine containing neurons have been found to have a pacemaker function within the brain. The firing rates of these neurons correlate positively with brain activity levels and displays distinct day-night rhythms. Within the posterior region of the hypothalamus, there are a large number of neurons that synthesize and utilize histamine. These neurons provide the stimulation that maintains or modulates activity in many other regions of the brain. Histamine, like the other biogenic amines (serotonin, dopamine, norepinephrine, epinephrine, and PEA) is stored in presynaptic vesicles and is released into the synapse. Also like other amine neurotransmitters, histamine binds to transmembrane G-protein coupled receptors on the post-synaptic neurons to exert its function. Downloadable PDF: Histamine Pathway Norepinephrine is an excitatory neurotransmitter that is important for attention and focus. Norepinephrine is synthesized from dopamine by means of the enzyme dopamine beta-hydroxylase, with oxygen, copper, and vitamin C as co-factors. Dopamine is synthesized in the cytoplasm, but norepinephrine is synthesized in the neurotransmitter storage vesicles.; Cells that use norepinephrine for formation of epinephrine use SAMe as a methyl group donor. Levels of epinephrine in the CNS are only about 10% of the levels of norepinephrine. The noradrenergic system is most active when an individual is awake, which is important for focused attention. Elevated norepinephrine activity seems to be a contributor to anxiousness. Also, brain norepinephrine turnover is increased in conditions of stress. Interestingly, benzodiazepines, the primary anxiolytic drugs, decrease firing of norepinephrine neurons. This may also help explain the reasoning for benzodiazepine use to induce sleep. Norepinephrine acts as an excitatory neurotransmitter and modulates neuron voltage potentials to favor glutamate activity and neurotransmitter firing. Downloadable PDF: Catecholamine Pathway Beta-phenylethylamine (PEA) is an excitatory neurotransmitter derived from the amino acid phenylalanine.Studies have found that PEA promotes energy and elevates mood. PEA also functions as a synaptic neuromodulator inhibiting the reuptake of dopamine and norepinephrine. Studies have discovered that patients with depression have decreased PEA levels while increased levels have been found in patients with psychopathic symptoms. It has also been implicated in headaches and the antidepressant effects of exercise.; One of the biochemical abnormalities resulting from phenylketonuria, the absence of the enzyme that helps to synthesize phenylalanine into tyrosine, is an increased production of PEA. This can cause an elevated level of PEA in the urine. Since PEA is lipid soluble and readily crosses the blood-brain-barrier, the administration of PEA or of its precursor, phenylalanine, has been found to improve outcome with some antidepressants. Also, supplementation to manipulate PEA can help increase focus and attention. PEA acts as an excitatory neurotransmitter and modulates neuron voltage potentials to favor glutamate activity and neurotransmitter firing. Downloadable PDF: Catecholamine Pathway Serotonin is an inhibitory neurotransmitter synthesized by enzymes that act on tryptophan and/or 5-HTP. Serotonin is stored in presynaptic vesicles and released to transmit electrochemical signals across the synapse. Extensive research has been conducted surrounding serotonin and acts as a target for symptoms like low mood, compulsions, anxiousness, and headaches. Serotonin acts, in most cases, as an inhibitory neurotransmitter and, like GABA, modulates neuron voltage potentials to inhibit glutamate activity and neurotransmitter firing. Serotonin neurons have large numbers of axons and are important in integrating neural circuits. This also provides an explanation for serotonin's role in so many health concerns. Downloadable PDF: Serotonin Pathway Taurine is an inhibitory neurotransmitter involved in neuromodulatory and neuroprotective actions. Supplementing with taurine can have a specific effect on GABA function.There are two primary ways in which taurine affects GABA.; First, it can inhibit GABA transaminase, an enzyme that metabolizes GABA. This allows GABA to stay in the synaptic cleft longer to bind to the postsynaptic receptor. Second, taurine can bind to the GABAAreceptor mimicking the effects of GABA. By helping GABA function, taurine is an important neuromodulator for prevention of excitoxicity. Excitability occurs when glutamate binds to its receptor, in this case, the NMDA receptor. Once glutamate activates the NMDA receptor there is an increase in intracellular Ca++ causing depolarization or cell excitability. With glutamate release, there is also simultaneous GABA and taurine release. When the inhibitory neurotransmitters, GABA and taurine, activate the GABAA receptor, the result is an increase in intracellular Cl- ions. This results in hyperpolarization which reduces cell excitability. Thus, the overall effect of taurine supplementation is to support GABA function. The relevance of GABA support is to prevent overstimulation due to high levels of excitatory amino acids. Therefore, taurine and GABA constitute an important protective mechanism against excessive excitatory amino acids. Similarly, taurine is increased in response to the exposure of free radicals elucidating its neuroprotective actions. Exposure to free radicals increases glutamate excretion, further potentiation NMDA receptor activation. Taurine modulates this effect to prevent cell excitability by keeping the cell hyperpolarized. The supplementation of taurine can help alleviate some excitability issues associated with elevated excitatory amino acids as well as play a role in regulating the effect of free radicals. Tyramine (4-hydroxy-phenethylamine) is a naturally occurring monoamine compound formed by the enzymatic decarboxylation of the aromatic amino acid tyrosine. The enzyme monoamine oxidase is responsible for the breakdown of tyramine. When this metabolic pathway is compromised by monoamine oxidase inhibitors (MAOIs), tyramine levels can become elevated and cause the release of neurotransmitters, such as dopamine, norepinephrine, and epinephrine, potentially leading to an increase in blood pressure. Increased decarboxylation of tyrosine will also increase tyramine and decrease substrates available for catecholamine (Epinephrine, Norephinephrine, Dopamine) synthesis. Dietary intake of tyramine has also been associated with cluster headaches and migraines, forcing many to restrict foods containing tyramine such as fish, chocolate, alcohol, and fermented foods including cheese, processed meat, and sauerkraut. Tryptamine is a trace amine formed from tryptophan decarboxylase. Tryptophan is also the substrate for tryptophan hydroxylase, the rate-limiting step in the synthesis of serotonin. The activity of these two enzymes acting on a common substrate will affect neurotransmission by altering tryptamine and serotonin levels. Tryptamine also occurs naturally in fermented foods, and similar to tyramine, consumption by patients treated with monoamine oxidase inhibitors can precipitate hypertensive crisis. Alterations in tryptamine levels are an indicator for dysfunction within tryptophan pathways, and therefore, serotoninrelated systems and altered tryptamine levels have been reported in psychiatric conditions, including depression and schizophrenia. High tryptamine may also indicate an increased shunting of tryptophan away from serotonin synthesis. Inflammation due to illness, a factor known to affect tryptophan metabolism, as well as normal sleep, decreases tryptamine levels. Tryptamine is normally metabolized by monoamine oxidase, the same enzyme that metabolizes central nervous system monoamines. Alterations in the activity of monoamine oxidase will result in changes in tryptamine levels. this insight can provide important information regarding the biochemical underpinnings of central nervous system imbalances, monoamine turnover, and the enzymes, cofactors, and other constituents of the monoamine metabolic pathway. Creatinine is a normalizing parameter used to calculate neurotransmitter levels. Creatinine is produced at a constant rate through the kidneys. Therefore, by using creatinine as the constant factor, spot urinary measurements can be performed with out having to factor in the patient's hydration state, possible renal disorders or diuretic substances that may have been used.