* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Basal ganglia discharge abnormalities in Parkinson`s disease
Neural coding wikipedia , lookup
Biology of depression wikipedia , lookup
Perivascular space wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuroeconomics wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Nervous system network models wikipedia , lookup
Environmental enrichment wikipedia , lookup
Aging brain wikipedia , lookup
Embodied language processing wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Muscle memory wikipedia , lookup
Central pattern generator wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Neuroplasticity wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Spike-and-wave wikipedia , lookup
Neural oscillation wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Synaptic gating wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Parkinson's disease wikipedia , lookup
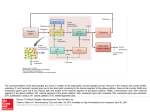
J Neural Transm (2006) [Suppl] 70: 21–25 # Springer-Verlag 2006 Basal ganglia discharge abnormalities in Parkinson’s disease T. Wichmann1;2 and M. R. DeLong1 1 2 Department of Neurology, and Yerkes National Primate Center, Emory University, Atlanta, GA, USA Summary. In the traditional model of the pathophysiology of parkinsonism, parkinsonian motor signs are viewed as the result of changes in discharge rates in the basal ganglia. However, not all experimental findings can be explained by rate changes alone, and changes in discharge patterns in these nuclei are increasingly emphasized as pathophysiologically important, including changes in burst discharges, in synchrony, and in oscillatory activity. This brief review highlights the pathophysiologic relevance of these rate and pattern changes in the pathophysiology of parkinsonism. Introduction In early Parkinson’s disease selective degeneration of a small number of dopaminergic cells in the lateral portion of the substantia nigra pars compacta (SNc) leads to the signs of parkinsonism. Both the behavioral specificity and the seemingly disproportionate magnitude of the effect of such a small lesion in the midbrain are artifacts of the anatomy of the basal ganglia. The functional specificity arises from the fact that the basal ganglia are topographically organized, and that the early degenerative process in Parkinson’s disease affects predominately those SNc neurons that project to the motor portion of the striatum (the putamen) with relative sparing of other striatal areas. The magnitude of the behavior- al effect of SNc lesions is explained by the fact that the basal ganglia are major components of circuits involving specific regions of the cerebral cortex and thalamus (Alexander et al., 1986) so that loss of dopamine in the motor portion of the striatum (the putamen) exerts strong effects on all other elements of the ‘motor’ circuit, including motor areas of the extrastriatal basal ganglia, and movementrelated areas in thalamus and precentral motor fields. Thus, although pathologically a localized problem (at least initially), Parkinson’s disease is pathophysiologically a disorder engaging the entire motor circuit. We here review the changes in neuronal activity that occur in this circuit in Parkinson’s disease. Changes in discharge rate Inputs from cortical sensory-motor areas reach the basal ganglia through the putamen and the subthalamic nucleus (STN), while motor portions of the internal pallidal segment (GPi) and the substantia nigra pars reticulata (SNr) serve as output stations of the basal ganglia, projecting to the ventral anterior and ventrolateral nuclei of the thalamus. Information is conveyed between putamen and GPi=SNr through a monosynaptic GABAergic projection (‘direct’ pathway), and a polysynaptic (‘indirect’) pathway which involves the external pallidal segment (GPe) and STN. 22 T. Wichmann and M. R. DeLong Tonic GABAergic output from neurons in GPi=SNr is thought to inhibit their projection targets in thalamus, thereby reducing cortical activation. Dopamine, released from terminals of the nigrostriatal projection, is thought to facilitate transmission along the direct pathway, and to reduce transmission along the indirect pathway. These dual dopamine actions lead to a net reduction of inhibitory basal ganglia output, which may facilitate cortical activity, and eventually movement (Wichmann and DeLong, 2003). Traditional models of the pathophysiology of parkinsonism are strongly influenced by this anatomic arrangement (reviewed in Wichmann and DeLong, 2003; Albin et al., 1989), which suggests that the overall amount of movement is in some way inversely related to the magnitude of basal ganglia output. According to the scheme mentioned above, loss of striatal dopamine within the motor circuit would result in increased STN activity, and increased basal ganglia output, reduced thalamocortical activity and the development of parkinsonian motor signs, such as akinesia or bradykinesia. Conversely, dopamine-induced dyskinesias have been postulated to result from decreased basal ganglia output. The prediction that the discharge rates of neurons in the basal ganglia output nuclei and in the STN are increased in Parkinson’s disease has been generally confirmed in animal models of Parkinson’s disease, and is also supported by recording studies in humans undergoing functional neurosurgery as treatment for Parkinson’s disease. Moreover, decreases in discharge in GPi have been found in animals with dyskinesias induced by administration of dopaminergic drugs. It is noteworthy, however, that the predicted rate changes have not been found in all studies of parkinsonian subjects. More importantly, in a recent monkey study designed to test the rate-based model, we showed that increased STN and GPi rates (in this case produced by ibotenic acid lesions of GPe) are not per se responsible for parkinsonian signs (Soares et al., 2004). Despite rate changes similar to those in parkinsonian animals, the GPe-lesioned animals showed only minor motor impairments. Lesion studies have also demonstrated problems with rate-based models of parkinsonian pathophysiology. For example, based on the model, pallidal lesions would be expected to result in involuntary movements, but, in fact, have little effect on normal motor behavior. Similarly, thalamic inactivation, although predicted to induce parkinsonism, does not. Pattern changes Given that changes in discharge rate of basal ganglia neurons cannot fully explain the generation of parkinsonism or the production of drug-induced dyskinesias, changes in the activity patterns of basal ganglia neurons have been intensely studied. Although we will discuss here different types of pattern changes separately, all of them tend to occur in parallel, and may result from the same underlying mechanism(s). Burst discharges One of the firing properties that has been studied in detail is the incidence of burst discharges. Such burst discharges are a normal feature of basal ganglia discharge, and the timing and ‘strength’ of bursting may represent aspects of external events or behavior, probably representing the increased synchronization of cortical inputs to the subthalamic nucleus or striatum (Magill et al., 2000). In parkinsonism, the incidence of bursts is increased (e.g., Soares et al., 2004), most often in the context of synchronized oscillatory activity (see below). In part, this may occur because of an enhanced interaction between cortical areas and basal ganglia areas, particularly the STN, but other pathologic phenomena may also play a role. For instance, in dopamine-free co-cultures of STN and GPe cells, synchronized bursting Basal ganglia discharge abnormalities in Parkinson’s disease develops because of network properties (Plenz and Kitai, 1999). Prominent bursts in STN discharge have been shown to occur as rebound phenomena in response to prolonged or synchronized inhibitory GPe inputs to the STN (e.g., Bevan et al., 2002). Although less well studied, rebound bursts are also known to occur in other basal ganglia areas. Changes in inhibitory inputs to these areas may increase rebound bursting. Synchrony A second important phenomenon affecting the firing properties of basal ganglia cells in parkinsonism are changes in the synchrony of firing between neighboring neurons. Under physiologic conditions, the firing of neighboring basal ganglia neurons is largely uncorrelated (Bergman et al., 1994; Wilson et al., 2004). In the dopamine-depleted state, however, increased synchrony is observed in the STN (Bergman et al., 1994; Levy et al., 2002), in the pallidum (e.g., Heimer et al., 2002), in the striatum (between tonically active neurons, most likely corresponding to cholinergic interneurons) (Raz et al., 2001), and in frontal cortical areas (Goldberg et al., 2002). The link between synchrony and dopamine depletion is most clearly revealed by the fact that treatment with dopaminergic agents rapidly reduces the interneuronal synchronization observed in parkinsonism in the monkey pallidum (Heimer et al., 2002) and in the human STN (Levy et al., 2002). However, the mechanisms by which dopamine exerts these effects remain unclear. It has been proposed that dopamine loss in the striatum may trigger enhanced electrotonic coupling between neighboring striatal cells (see, e.g., Onn and Grace, 2000), or activity changes in interneurons or axon collaterals (Guzman et al., 2003). In the STN, synchrony is more likely to be the result of synchronous (or divergent) inputs from external sources rather than locally generated (Wilson et al., 2004). The potential synchro- 23 nizing effects of local dopamine loss in GPe, GPi, or SNr, has not been explored in detail. Oscillations A third major change in the discharge patterns of basal ganglia neurons in parkinsonism is that dopamine loss enhances the tendency of neurons in the basal ganglia-thalamocortical circuitry to discharge in an oscillatory pattern (Soares et al., 2004; Bergman et al., 1994). These oscillatory changes are rapidly reversed by systemic treatment with dopaminergic agents (Levy et al., 2002), and are therefore likely to represent a direct effect of dopamine deficiency. Such oscillations are mostly confined to the extrastriatal basal ganglia, and characteristically occur in the alpha- and beta frequency bands. For instance, in parkinsonian monkeys and patients, oscillatory bursting typically emerges in both the 3–8 Hz band, and a power spectral band around 10 Hz (Bergman et al., 1994; Levy et al., 2000). Local field potential (LFP) recordings through implanted deep brain stimulation (DBS) electrodes in GPi and STN of untreated parkinsonian patients have shown similar oscillatory activity in the 10–30 Hz range, likely reflecting synchronized oscillatory neuronal spiking (Levy et al., 2002; Brown, 2003). Given the relation between the basal ganglia, thalamus and cortex, it is not surprising that pathologic oscillatory activity in the ‘antikinetic’ 10–30 Hz band has also been observed in areas of cortex that are related to the basal ganglia in parkinsonian patients and animals (Goldberg et al., 2002). MEG studies have also identified an oscillatory network with pathologic coupling at 10 Hz in patients with parkinsonian tremor, which included multiple cortical motor areas, as well as diencephalic and cerebellar areas (Timmermann et al., 2003). Global engagement of the basal gangliathalamocortical circuits in synchronized oscillatory bursts may severely disrupt processing 24 T. Wichmann and M. R. DeLong at all levels of the circuitry. This may affect cortical activities such as event-related modulation of beta-band oscillations, or functions such as motor planning or sequence learning. In support of this concept, 10-Hz stimulation of the STN area has been shown to exacerbate akinesia (Timmermann et al., 2004). It has been speculated that DBS may act to desynchronize neurons in the basal ganglia (Brown et al., 2004). A recent study in MPTP-treated primates demonstrated that high-frequency cortical stimulation may also have antiparkinsonian effects, perhaps by the same mechanism (Drouot et al., 2004). Although it is tempting to speculate that parkinsonian tremor may directly result from synchronized oscillatory bursting in the basal ganglia, studies of the correlation or coherence between tremor and basal ganglia oscillations have not been conclusive, perhaps resulting from the fact that different limbs of parkinsonian patients may engage in tremor of different frequencies (Bergman et al., 1998), making it difficult to identify a ‘causative’ basal ganglia oscillation for a given tremor movement. Although often associated with tremor (Levy et al., 2002), synchronized oscillations do not always result in tremor (Soares et al., 2004; Heimer et al., 2002). Additional anatomic or physiologic factors (poorly defined at this moment) may be necessary for tremor to occur. It remains unclear at this point whether clinical symptoms such as tremor, akinesia or bradykinesia result from the impairment of specific cortical regions or motor loop sub-circuits (e.g., those centered on the supplementary motor area, motor cortex or other pre-central motor fields) or from incomplete functional compensation through less affected areas of frontal cortex. Conclusion Earlier models of the pathophysiology of Parkinson’s disease that emphasized the importance of changes in discharge rates in the basal ganglia of parkinsonian subjects, have been supplanted by a more complex scheme in which pattern changes take center stage. Rate and pattern changes are not, however, mutually exclusive. In fact, some of the rate changes are probably explained by the emerging pattern abnormalities, such as bursts in discharge. It appears that these rate and pattern changes in the basal ganglia-thalamocortical pathways actively disrupt cortical processing. The strongest argument in favor of this view is the fact that neurosurgical interventions which eliminate the disordered activity in the basal ganglia either through chronic electrical stimulation or lesions result in immediate improvements of parkinsonian motor signs, and in a relative ‘normalization’ of activity in cortical motor areas (as judged by functional imaging). References Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375 Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9: 357–381 Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520 Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A (1998) Physiology of MPTP tremor. Mov Disord 13 [Suppl 3]: 29–34 Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ (2002) Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci 25: 525–531 Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18: 357–363 Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V (2004) Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol 188: 480–490 Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, Dauguet J, Lefaucheur JP, Dolle F, Conde F, Bottlaender M, Peschanski M, Keravel Y, Basal ganglia discharge abnormalities in Parkinson’s disease Hantraye P, Palfi S (2004) Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron 44: 769–778 Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H (2002) Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J Neurosci 22: 4639–4653 Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J (2003) Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci 23: 8931–8940 Heimer G, Bar-Gad I, Goldberg JA, Bergman H (2002) Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J Neurosci 22: 7850–7855 Levy R, Hutchison WD, Lozano AM, Dostrovsky JO (2000) High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20: 7766–7775 Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO (2002) Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease [see comment]. Brain 125: 1196–1209 Magill PJ, Bolam JP, Bevan MD (2000) Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J Neurosci 20: 820–833 Onn SP, Grace AA (2000) Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci 20: 2332–2345 25 Plenz D, Kitai S (1999) A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400: 677–682 Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H (2001) Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci 21: RC128 Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T (2004) Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPTP-induced parkinsonism and lesions of the external pallidal segment. J Neurosci 24: 6417–6426 Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A (2003) The cerebral oscillatory network of parkinsonian resting tremor. Brain 126: 199–212 Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A (2004) Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson’s disease. Mov Disord 19: 1328–1333 Wichmann T, DeLong MR (2003) Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol 91: 9–18 Wilson CL, Puntis M, Lacey MG (2004) Overwhelmingly asynchronous firing of rat subthalamic nucleus neurones in brain slices provides little evidence for intrinsic interconnectivity. Neuroscience 123: 187–200 Author’s address: M. R. DeLong, MD, Department of Neurology, Emory University, Suite 6000, Woodruff Memorial Research Building, 1639 Pierce Drive, Atlanta, GA 30322, USA, e-mail: [email protected]