* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Research Presentation Slides - Emory University School of Medicine
Brain morphometry wikipedia , lookup
History of neuroimaging wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neuropsychology wikipedia , lookup
Nervous system network models wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroplasticity wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Biology of depression wikipedia , lookup
Synaptic gating wikipedia , lookup
Externalizing disorders wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Perivascular space wikipedia , lookup
Neuroeconomics wikipedia , lookup
National Institute of Neurological Disorders and Stroke wikipedia , lookup
Haemodynamic response wikipedia , lookup
Aging brain wikipedia , lookup
Limbic system wikipedia , lookup
Neuroinformatics wikipedia , lookup
Abnormal psychology wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
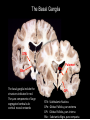
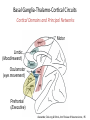
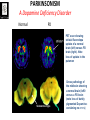
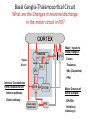
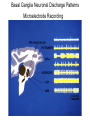
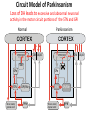
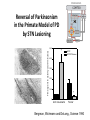
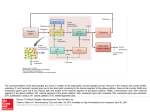
Circuit Disorders of the Basal Ganglia: Parkinson’s Disease Pathophysiology and Surgical Treatments Mahlon R. DeLong M.D. W. P. Timmie Professor of Neurology Emory University School of Medicine The Basal Ganglia STN SNc The basal ganglia include the structures indicated in red. They are components of large segregated corBcal-‐sub-‐ corBcal neural networks GPe Putamen GPi STN: Subthalamic Nucleus GPe: Globus Pallidus, pars externa GPi: Globus Pallidus, pars internae SNc: SubstanBa Nigra, pars compacta Basal Ganglia Circuits • For decades the basal ganglia were viewed as playing a role in movement by selecBng from compeBng commands from diverse corBcal areas sent to the input regions of the basal ganglia, which provided a pathway for funneling this command to the motor cortex, thus leading to movement. • The prevailing schema was summarized by Kemp and Powell in the 1970s. Basal Ganglia and Cerebellar Systems: Kemp and Powell-‐Circa 1970 “Funneling of Cor-cal Commands to the Motor Cortex” Kemp and Powell, ca. 1970 Segregated Circuit Hypothesis Physiologic studies carried out by DeLong and colleagues led to a major revision of the basal ganglia circuitry, whereby the sub-‐nuclei of the basal ganglia are viewed as components of a distributed family of segregated corBcal-‐ subcorBcal networks with separate domains involving four major funcBons: Motor (bodily movements and posture)) Oculomotor (eye movement) Prefrontal (execuBve/cogniBve) Limbic (emoBonal/reward) Basal Ganglia-Thalamocortical Circuits Segregated Parallel Pathways Alexander, DeLong & Strick, Ann Review of Neuroscience, ‘85 Basal Ganglia-‐Thalamo-‐CorBcal Circuits Cor-cal Domains and Principal Networks Motor Limbic (Mood/reward) Oculomotor (eye movement) Prefrontal (Executive) Alexander, DeLong & Strick, Ann Review of Neuroscience, ‘85 Segregated Circuit Hypothesis • The revised anatomic/physiologic framework provided the basis for an understanding of the role of these structures in mulBple broadly segregated funcBonal domains as well as the presence of disturbances of movement as well as of cogniBon, mood and behavior in clinical disorders involving the basal ganglia, such as Parkinson’s disease. • Parkinson’s is now recognized as one of a number of “Circuit Disorders” Circuit Disorders of the Basal Ganglia • MulBple neurologic and psychiatric disorders may be viewed as resulBng from abnormaliBes of neuronal acBvity within specific anatomically and funcBonally defined neuronal networks. – Many of these disorders involve the family of corBcal-‐basal ganglia thalamocorBcal circuits • The signs and symptoms of these disorders it appears result from signature abnormal neuronal acBvity within individual networks, which can be modulated by various clinical approaches, including drugs as well as surgical ablaBon or deep brain sBmulaBon (DBS) Basal Ganglia Circuit Disorders • Movement Disorders (Motor circuit) – Parkinson’s Disease (PD) – Dystonia – Hemiballismus – HunBngton’s chorea (HD) • Neuropsychiatric Disorders (Limbic circuit) – Toure]e’s Syndrome (TS) – Obsessive Compulsive Disorder (OCD) – Depression Basal Ganglia-Thalamocortical Circuits Associated Clinical Disorders PD, HD, TS DYSTONIA PD, HD PD, HD TS, OCD, DEPRESSION Wichmann and Delong, Neuron (2006) Parkinson’s Disease • Cardinal motor features (Parkinsonism) – Akinesia/Bradykinesia • (paucity and slowness of movement) – Tremor at rest – Muscular rigidity • Non-‐motor features – Depression/ anxiety – autonomic dysfuncBon – sleep disorders – cogniBve impairment – Anosmia (loss of sense of smell) Parkinson’s Disease Historical Aspects • Parkinson’s disease, a progressive neurologic disorder, characterized by tremor, rigidity and slowness of movement (parkinsonism) was shown in the 1960’s to result from loss of the neurotransmi]er dopamine (DA) within the basal ganglia. • Parkinsonism was subsequently found to respond dramaBcally to oral administraBon of levodopa, its precursor of DA. • Unfortunately levodopa replacement is oben associated with a number of significant side effects aber five or more years of treatment and there remains a great need for more effecBve treatments for both the movement and other aspects of the disease. PARKINSONISM A Dopamine Deficiency Disorder Normal Normal PD Parkinson’s disease PET scan showing striatal fluorodopa uptake of a normal brain (left) versus PD brain (right). Note loss of uptake in the putamen Substantia nigra Gross pathology of the midbrain showing a normal brain (left0 versus a PD brain (note loss of darkly pigmented Dopaminecontaining neurons) Brooks 1993 Marsden 1994 Lang & Lozano 1998 Basal Ganglia-‐ThalamocorBcal Circuit What are the Changes in neuronal discharge in the motor circuit in PD? STRIATUM D2 “HyperDirect” Indirect GPe D1 SNc Direct CM VA/VL Major Inputs to Basal Ganglia: Cortex Thalamus SNc (Dopamine) PPN Intrinsic Connections from striatum to GPi STN GPi/SNr Indirect pathway Direct pathway Brain stem/ Spinal cord PPN Major Outputs of Basal Ganglia: GPi/SNr Inhibitory (GABAergic) Basal Ganglia Neuronal Discharge Patterns Microelectrode Recording Parkinson’s Disease Pathophysiology • Physiologic studies using single cell microelectrode recording carried out in the 1980s and 90s in a primate model of Parkinson’s disease, revealed clear disturbances in neuronal acBvity in the sub-‐nuclei of the basal ganglia, including increased discharge rate, as well as abnormal pa]ern of discharge including increased bursBng abnormal oscillaBons. • The observed changes in neuronal acBvity in the subnuclei of the basal ganglia was consistent with the prevailing circuit model of PD, which postulated abnormally increased (inhibitory) output from the basal ganglia (GPi) resulBng from excessive excitatory drive from the STN. Circuit Model of Parkinsonism Loss of DA leads to excessive and abnormal neuronal acBvity in the motor circuit porBons of the STN and GPi STRIATUM GPe SNc STN GPi/SNr PPN Brain stem/ Spinal cord Parkinsonism CM VA/VL STRIATUM GPe SNc X Normal STN GPi/SNr PPN Brain stem/ Spinal cord CM VA/VL Primate (MPTP) Parkinsonism Changes in Discharge Pa8erns in Basal Ganglia Nuclei (representa-ve microelectrode recordings) SNr 4 6 6 6 6 8 8 8 10 12 14 10 12 14 Data segment 2 4 Data segment 2 4 Data segment 2 4 10 12 14 8 10 12 14 16 16 16 16 18 18 18 18 20 20 20 400 600 time (ms) 800 0 1000 2 2 4 4 6 6 8 8 10 12 14 18 20 20 800 1000 0 0 1000 14 18 400 600 time (ms) 800 12 16 200 400 600 time (ms) 10 16 0 200 20 200 400 600 time (ms) 800 400 600 time (ms) 800 1000 200 400 600 time (ms) 800 1000 0 200 400 600 time (ms) 800 1000 2 2 4 4 6 6 8 10 12 14 8 10 12 14 16 16 18 18 20 200 0 1000 Data segment 200 Data segment Data segment GPi 2 0 PD STN Data segment Normal Data segment GPe 0 20 200 400 600 time (ms) 800 1000 Discharge rate decreased increased increased increased Pattern Synchronization oscill. bursts increased oscill. bursts increased oscill. bursts increased oscill. bursts increased Rate Changes in Primate Models of PD Oscillatory STN AcBvity in the Primate MPTP Model of PD Bergman et al., J Neurophysiology, 1994 TesSng the Hypothesis InacSvaSon of the STN in the Primate Model of Parkinsonism In order to test the circuit model of PD, the STN was lesioned in the primate model of PD Direct neurotoxin lesioning of the STN immediately abolished the cardinal features of parkinsonism (bradykinesia, tremor and rigidity) in the contralateral limbs and led to increased overall moBlity. These and subsequent studies of lesioning of the GPi demonstrated the key role of abnormal discharge in the sensorimotor porBons of the STN and the GPi in the pathophysiology of PD. Reversal of Parkinsonism in the Primate Model of PD by STN Lesioning Time spent in activity (s/30 minutes) 500 * MPTP MPTP/STN-lesion 400 300 200 100 * 0 Arm movements Bergman, Tremor Bergman et al., Science 249:1436 (1990) Baron et al. Jand Neuroscience 592 (2002) Wichmann DeLong.,22:Science 1990) The Renaissance in FuncSon Surgery • The striking aboliBon of parkinsonism with STN lesioning, combined with the earlier advances in our understanding of the network abnormaliBes underlying parkinsonism, provided a clear raBonal for surgical lesioning of the motor circuit, including pallidotomy (lesioning of GPi). • These discoveries contributed greatly to the renaissance in funcBonal surgery for PD and related disorders in the 1990’s, including pallidotomy and subthalamotomy. • High-‐frequency Deep Brain SBmulaBon (DBS) of the STN, which was introduced subsequently as a less invasive, reversible and adjustable surgical approach, is currently the treatment of choice for advanced PD, with over 100,000 surgeries performed worldwide. • Although many quesBons remain unanswered about the mechanism of acBon of these surgical approaches, in general, whereas lesioning acts directly to block abnormal network acBvity, DBS appears to act by overriding and replacing abnormal acBvity in the network.