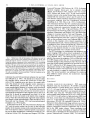
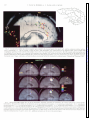
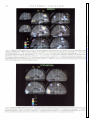
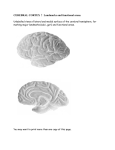
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Role of the Human Anterior Cingulate Cortex in the Control of
Neuroscience and intelligence wikipedia , lookup
Synaptic gating wikipedia , lookup
Dual consciousness wikipedia , lookup
Metastability in the brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neurophilosophy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Biology of depression wikipedia , lookup
Neurolinguistics wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Human multitasking wikipedia , lookup
Neurocomputational speech processing wikipedia , lookup
Broca's area wikipedia , lookup
Executive functions wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroplasticity wikipedia , lookup
Human brain wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Time perception wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Embodied language processing wikipedia , lookup
Affective neuroscience wikipedia , lookup
Aging brain wikipedia , lookup
Motor cortex wikipedia , lookup
Cerebral cortex wikipedia , lookup
Posterior cingulate wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Emotional lateralization wikipedia , lookup
JOURNALOF NEUROPHYSIOLOGY Vol. 70, No. 2, August 1993. Printed in U.S.A. Role of the Human Anterior Cingulate Cortex in the Control of Oculomotor, Manual, and Speech Responses: A Positron Emission Tomography Sudy TOMAS PAUS, Montreal Neurological SUMMARY AND MICHAEL PETRIDES, Institute, McGill ALAN C. EVANS, University, Montreal, CONCLUSIONS INTRODUCTION Current advances in neuroimaging techniques, and in particular the possibility of measuring changes in regional cerebral blood flow (r CBF) by means of positron emission tomography (PET) in human volunteers, have led to an increased interest in the role of the cingulate cortex (Fig. 1) in the control of human behavior. A number of interpretations have been proposed to account for changes in rCBF observed in this region during the performance of various cognitive and motor tasks. One of the first attempts to explain such changes was the notion of the “anterior attention system” (Posner and Petersen 1990; Posner et al. 1988). It was suggested that the anterior cingulate cortex (ACC) may be involved in target detection (Posner and Petersen 1990) and activated when “attention to action” is required ERNST MEYER (Posner et al. 1988). Furthermore, it was stressed that “although attention for action seems to imply motor acts, internal selections involved in detecting or noting an event may be sufficient to involve attention in this sense” (Posner et al. 1988). Other investigators also pointed out that the high “attentional” demands and/ or “response selection” processes may underlie significant changes in rCBF in the ACC obtained during the performance of the Stroop test (Pardo et al. 1990) and voluntary generation of motor responses (Frith et al. 199 1). In the present study, we set out to test a somewhat different view based on the demonstrated close relationship between the ACC, the motor system, and the prefrontal cortex in the monkey (see below). We propose that the ACC is a cortical region where a cognitive / motor “command”, coming from a different cortical region (e.g., prefrontal cortex), is being modulated and “funneled” to the motor system. We further propose that this modulation takes place within distinct, motor output-specific subregions of the ACC, thus emphasizing the “motor” character of this region. The prediction that rCBF changes in the ACC will map onto distinct subregions, depending on the output modality, was based on the results of several recent neurophysiological and neuroanatomic studies. These studies provide evidence for the existence of a somatotopic organization of the connections between the ACC and the primary motor cortex (Dum and Strick 199 1; Morecraft and Van Hoesen 1992; Muakkassa and Strick 1979) and between the ACC and the spinal cord (Dum and Strick 199 1). Such connectivity explains the fact that movements can be elicited by electrical stimulation of the ACC. Classical stimulation studies (Hughes and Mazurowski 1962; Showers 1959) and a more recent microstimulation study (Luppino et al. 199 1) showed that there is a systematic relationship between the site of stimulation and the part of the body that moves. In other words, a somatotopic organization of the region was demonstrated consistent with its known anatomic connections. It should be noted at this point that the ACC is not a morphologically homogeneous region. It comprises at least two cytoarchitectonic areas, Brodmann’s areas 24 and 32. In the monkey, area 32 represents the rostralmost part of the ACC, located rostra1 to the genu of the corpus callosum (Barbas and Pandya 1989). Area 24 of the monkey ACC can be further subdivided into ventral 0022-3077/93 $2.00 Copyright 0 1993 The American Physiological Society 453 Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 I. Two experiments were aimed at investigating the functional organization of the human anterior cingulate cortex (ACC) in relation to higher-order motor control. 2. The ‘50-labeled H,O bolus method was used to measure relative changes of regional cerebral blood flow (rCBF) in 18 healthy human subjects as they performed oculomotor, manual, or speech tasks. 3. Task-specific rCBF changes were obtained in distinct subregions of the ACC, depending on the output system employed. The oculomotor and the manual task-related foci were found in the rostra1 and caudal regions of the ACC, respectively, whereas the speech foci were localized within two cingulate subregions, the intermediate dorsal and the rostra1 ACC. 4. In the manual tasks, two groups of activation foci could be distinguished, one just behind and the other just in front of the vertical plane traversing the anterior commissure. 5. The above pattern of r CBF changes was observed only if there was concomitant activation within the lateral prefrontal cortex (except for the posterior group of foci obtained in the manual tasks). 6. The localization of output-specific rCBF changes within the human ACC is consistent with the known somatotopic organization of the cingulate cortex in the monkey. 7. It is tentatively proposed that the ACC participates in motor control by facilitating the execution of the appropriate responses and/ or suppressing the execution of the inappropriate ones. Such a modulatory effect would be of particular importance when behavior has to be modified in new and challenging situations. AND Quebec H3A 2B4, Canada T. PAUS, M. PETRIDES, A. C. EVANS, (subareas 24a and 24b) and dorsal (subareas 24c and 24d) tiers ( Matelli et al. 199 1). The dorsal tier is buried within the cingulate sulcus, whereas the ventral tier occupies the cortex of the cingulate gyrus just above the corpus callosum. The dorsal tier of the cingulate cortex represents a more differentiated, transitional form of cortex having some morphological features in common with neocortical areas 4 and 6 (Matelli et al. 199 1). The border between this transitional portion of area 24 and true neocortex (area 6) runs along the dorsal bank of the cingulate sulcus close to its dorsal lip. It should be emphasized here that it is in the dorsal tier of area 24, buried in the depth of the cingulate sulcus, where the somatotopically organized motor regions were found in both neuroanatomic ( Dum and Strick 199 1; Morecraft and Van Hoesen 1992) and neurophysiological ( Luppino et al. 199 1) studies. At the behavioral level, the close interaction between the ACC and the motor system can be seen in the following studies. In the monkey, lesions to the rostralmost part ofthe ACC were shown to reduce condition-specific vocal output, but not to affect spontaneous vocalization emitted in a social group ( Aitken 198 1; Kirzinger and Jurgens 1982; Mac- E. MEYER Lean and Newman 1988; Sutton et al. 1974). In humans, bilateral cingulate lesions give rise to akinetic mutism (Barris and Schuman 1953; Jurgens and Von Cramon 1982; Nielsen and Jacobs 195 1). In the nonvocalization domain, lesions to the medial frontal lobe, which often included both the neocortex (area 6 and 8) and the ACC, were found to impair voluntary suppression of arm and eye movements triggered from the contralateral hemispace (Goldberg et al. I98 1; Paus et al. 199 1). Furthermore, a close interaction between the ACC and the lateral prefrontal cortex, which should not be surprising in the light of an extensive reciprocal connectivity between the two structures (Pandya et al. 198 1; Vogt and Pandya 1987), was documented in the following studies. Increased glucose metabolism (Matsunami and Kubota 1983) and task-related changes in neuronal activity (Niki and Watanabe 1976) have been observed within the ACC in monkeys performing a delayed-response task that is known to depend critically on the prefrontal cortex (see Goldman-Rakic 1987). Lesions to the cingulate cortex impair acquisition of the delayed-alternation task, whereas performance of this task is unaffected when learned preoperatively (Pribram et al. 1962 ). Thus the involvement of the ACC in the control of behavior may depend critically on a close interaction with both the prefrontal cortex and the motor system. The PET technique, in combination with magnetic resonance imaging (MRI), allows direct localization of rCBF changes that are thought to reflect changes in neuronal activity ( Raichle 1987). The following two questions were asked in the present investigation. First, will the task-specific rCBF changes within the ACC be accompanied by concomitant changes in the prefrontal cortex? Second, will the exact site of task-related modulation of neuronal activity in the ACC (reflected by rCBF changes) follow a somatotopic organization similar to that suggested by the monkey studies? The first question was explored by means of sensorimotor tasks designed to engage the prefrontal cortex differentially. The second question was addressed by employing three different output systems, the oculomotor, manual, and speech systems. METHODS Experiment 1 BLOOD-FLOW MEASUREMENT. PET scans were obtained using the Scanditronix PC-2048B tomograph, which produces 15 image planes at an intrinsic resolution of 5 X 5 X 6.5 mm (Evans et al. 199 1b). The distribution of normalized cerebral blood flow was measured during each 60-s PET scan using the 150-labeled H,O bolus method with averaged image subtraction (Fox et al. 1985b; Raichle et al. 1983). For each subject, a high-resolution MRI study (63 slices, 2 mm thick) was obtained from a Philips Gyroscan ( 1.5 T) and resliced for co-registration with the PET data using a PIXAR 3-D computer (Evans et al. 1991a). Interactive three-dimensional image software was used to establish an orthogonal coordinate frame on the basis of the anterior commissure-posterior commissure line as identified in the MRI image volume. These coordinates were used to apply a linear resampling of matched MRI-PET data sets into a standardized three-dimensional coordinate system (Talairach and Tournoux 1988). To overcome reCEREBRAL Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 FG 1. Medial view of the left hemisphere of the human (lop) and the monkey (bot!om) brain. Three reference lines are depicted: AC-PC, line passing through the anterior and posterior commissures; VCA, line traversing the anterior commissure in the vertical plane; VCP, line passing through the posterior commissure. Botromphomgruph: arrow indicates the position of the genu of the arcuate sulcus projected onto the medial surface. Note that an uninterrupted single cingulate sulcus is present only in -60% of cases in the human brain; 25% of cases show a double parallel type (One et al. 1990). The human brain depicted in this figure is of the latter type. CS, cingulate sulcus; PCS, paracingulate sulcus (Smith 1907). The scale is indicated by the horizontal line ( I cm) that is adjacent to the human and the monkey brains. AND HIJMAN ANTEKIOR Eight right-handed normal subjects ( 1 female, 7 male; 20-28 yr of age) participated in this experiment. Each subject underwent seven 60-s PET scans within a single session. Written informed consent was obtained in all experiments in accordance with guidelines approved by the Ethics Committee of the Montreal Neurological Institute and the Declaration of Human Rights, Helsinki, 1975. ATE COR?‘EX 355 one of three words in response to the specific word heard. The auditory stimuli were three verbs (take, join, find), and the responses were three pronouns (them, her, him). The stimuli were presented through a pair of headphones. The latency and accuracy of the verbal responses were measured bv means of a voice trigger. ” In the overpracticed versions of the above tasks, the association between stimuli and responses had been established in a training session administered the day before scanning (900 trials per task). In the reversal versions, the same stimuli and responses were used as in the overpracticed tasks, but the subjects were assigned a new combination of stimulus-response associations. The sets of stimuli used in the reversal and overpracticed tasks, respectively, were counterbalanced across subjects. In the baseline scan, an asterisk was flashed in the center of the screen. Scanning during the overpracticed version of each task was always followed by scanning in the corresponding reversal task. The order of the oculomotor, manual, and speech tasks was counterbalanced across the subjects with the baseline scan following either the first, second, or third pair of the sensorimotor tasks. The stimulus-response rate was constant in all tasks. stimuli being presented every 2 s, with 60 stimuli presented in total ( 30 stimuli during the actual scanning). SU BJ ECTS. PARADIGMS. There were three pairs of sensorimotor tasks that diKered in terms of output modality. Each pair comprised an overpracticed and a reversal version of the task. In the baseline scan, the subjects were not required to execute any responses other than fixating the center of the screen. &%MZU/ tcrsL~. In the manual task, one of three response keys had to be pressed according to the particular visual stimulus shown. Stimuli cuing the responses (simple geometric forms such as a cross or a circle) were presented for 200 ms inside an empty circle (0.5” diam) that was displayed permanently in the center of the screen. The three response keys were arranged in a row; the subjects responded by pressing the keys with the second, third, or fourth finger of their right hand. The latency and accuracy of responses were recorded. OC*ZI/OIMOZOI’ZLLS~.In the oculomotor task, the subjects were asked to make a direction-specitic saccade, depending on the particular visual stimulus presented. Eye movements were directed toward one of three squares (0.3” X 0.3”) arranged one above the other with a between-target distance of 1.3O. The targets were displayed permanently within the right hemitield at a distance of 5” from the center of the screen. Stimuli cuing the responses (simple geometric forms such as a square or a triangle) were presented for 200 ms inside an empty circle (0.5” diam) that was displayed permanently in the center of the screen. The latency and accuracy of oculomotor responses were recorded using a Pupil/Cornea1 Reflection Tracker by ISCAN. $XYY/Z USLL In the speech task, the subjects had to say BEHAVIORAL E~puim en f 2 In this experiment, we used a different paradigm that emphasized suppression of externally triggered motor programs and minimized the cognitive operations of the tasks employed in experiment 1. CEREBRAL. BLOOD-FLOW MEASIJRfiMEN’I‘. In this experiment, acquisition and analysis of the cerebral blood flow data were identical to those used in experiment 1. St JBJ ECI‘S. Nine right-handed subjects (4 female, 5 male: 19-30 yr of age) underwent seven 60-s PET scans in a single session. BEII1AVIORAl. PARADIGMS. There were three pairs of tasks that differed in terms of output modality (oculomotor, manual, and speech). Each pair comprised a prostimulus and an antistimulus version of the task. The baseline scan did not involve any sensory stimulation or the execution of any kind of response. During all scans, the subjects were blindfolded, except for the two oculomotor tasks. :2~~rrz~/ Z&-S. In the manual task, the subjects were asked to keep two levers pressed down using the second and third fingers of their right hand. The pressure required corresponded approximately to a force of 2 N. A tactile stimulus (light touch) was delivered to the palmar side of either of the two fingers by a solenoid moving up and down (duration of 100 ms). The subjects were required to lift either the finger that was touched (prostimulus task) or the other one (antistimulus task). O~z&~rnoto~ faslis. In the oculomotor tasks, the subjects were required to make a saccade either towJard (prostimulus task) or awav from (antistimulus task) a visual stimulus presented with& the right or left hemitield (Hallett 1978 ). Each trial started with presentation of a fixation stimulus (empty circle, 0.5” diam) in the center of the computer screen. The fixation stimulus remained on the screen for 300 ms and was followed by a 200-ms gap ( blank screen) Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 sidual anatomic variability persisting after the stereotactic standardization, PET images were smoothed with an 1% mm Hanning filter. The PET data were normalized for global cerebral blood flow and the mean state-dependent change image volume obtained. For statistical analysis, the mean state-dependent change volume was converted to a t-statistic volume by dividing each voxel by the mean SD in normalized cerebral blood flow for all intracerebral voxels (Worsley et al. 1992 ). Individual MRI images were subjected to the same averaging procedure such that composite stereotactic image volumes, 128 X 128 X 80 voxels in extent and sampled at - 1.5 mm in each dimension, dere obtained for both t-statistic and MRI volumes (Evans et al. 1992). Anatomic and functional images were merged to allow direct localization of f-statistic peaks on the MRI images. These peaks were identified by an automatic peakdetection algorithm. The peak distribution was then searched for significant signals using change-distribution analysis and Z- score thresholding (Fox et al. 1988). A threshold for reporting a peak as significant was set at z value of 2.17 (P < 0.03). Peaks with z value equal to or higher than the threshold value are reported throughout the present study. These peaks are referred to as either rCBF changes or activation foci. CINGlJi 456 T. PAUS, M. PETRIDES, q A. C. EVANS, AND E. MEYER q q Overpractised 1000 400 t * Pro-stimulus Anti-stimulus r 300 200 0 50 s t5 r 20 10 s 5 O Speech Manual Oculomotor Speech Manual Oculomotor FIG. 2. Histograms illustrate the mean response latency for each of the 3 tasks, the mean of the within-subject response variability, and the mean error rate (left panel: experiment 1; rightpanel: experiment 2). The means are based on data obtained in the 60 trials of each task administered during the positron emission tomography (PET) session. Bars on the histograms: mean + SE. In experiment 2, because of a technical failure, oculomotor data were obtained in only 6 subjects. The statistical significance of the differences is based on a l-tailed paired t test with Bonferroni correction (*P < 0.05, **P < 0.01). between the offset of the fixation and the onset ofthe peripheral stimulus. The gap was used to facilitate the execution of saccades (Fischer and Breitmeyer 1987). The peripheral stimulus (filled square, 0.3 by 0.3 ‘, duration of 200 ms) was displayed 5’ to the left or the right of the fixation stimulus. Speechtasks.In the speech tasks, the subjects heard two letters (A or L) through a pair of headphones. In the prostimulus version of the task, the subjects were asked to respond by saying the letter that immediately follows in the alphabetic sequence ( A-“B”, L-“M”). In the antistimulus task, they were required to respond in the opposite way by saying “M” when they heard the letter A and “B” for the letter L. The average rate of stimulus presentation was the same for all tasks. The interstimulus interval was held constant ( 1.2 s) in the prostimulus versions of each task, but it varied pseudorandomly ( 1.O, 1.2, or 1.4 s) in the antistimulus versions. There were 60 stimuli presented in each task. The side of the visual stimulus (left or right hemifield), the location of the tactile stimulus (2nd or 3rd finger), and the type of letter (A or L) alternated regularly in the prostimulus versions, but were presented in a pseudorandom order in the antistimulus version of each task. The predictability of the stimuli in the prostimulus tasks was intended to reduce the task requirements to those of a simple alternation of the two possible responses. Scanning in the prostimulus version of each task was always followed by scanning in the corresponding antistimulus one. The order of the oculomotor, manual, and speech tasks was counterbalanced across all subjects. The baseline scan followed either the first, second or third antistimulus task. The latency and accuracy of motor responses were measured. Eye movements were recorded by means of a Pupil/Cornea1 Reflection Tracker (ISCAN) and the verbal responses with a voice trigger device. Experiment 3 This experiment is a replication of the speech part of experiment 1. The procedure and the tasks (reversal and overpracticed) were identical to those used in the original study (see above). The only difference was the baseline scan. In contrast to experiment 1, where the subject was required in the baseline condition to fixate the center of the screen, in this experiment the baseline procedure was identical to that used in experiment 2, i.e., the eyes were closed. Eight right-handed normal subjects ( 5 female, 3 male; 1924 yr of age) participated in the experiment. Each subject underwent six 60-s PET scans within a single session. Three Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 100 HUMAN TABLE 1. ANTERIOR CINGULATE CORTEX 457 Stereotaxic coordinates of activation foci obtained in the reversal minus overpracticed subtraction Region Brodmann’s Area x Y Z t Z 30 25 32 17 27 18 -54 -50 -54 13 24 20 51 29 -3 39 40 31 3.1 3.0 3.8 3.1 2.8 3.0 4.2 3.4 2.9 2.4 2.3 2.9 2.4 2.2 2.3 3.2 2.6 2.2 13 8 -42 -47 -2 30 49 47 39 -6 3.3 3.0 4.3 4.0 3.2 2.5 2.3 3.3 3.0 2.4 58 22 22 6 20 49 3.7 3.9 4.3 2.2 2.3 2.6 Letter Code A. Ocukomotor subtraction Right inferior frontal sulcus Left inferior frontal sulcus Left anterior cingulate (rostral) Right anterior cingulate (dorsal) Right anterior cingulate (rostral) Left inferior frontal gyrus Right intraparietal sulcus Left intraparietal sulcus Posterior cingulate or precuneus (midline) 46/45 9145 24/32 32/8/6 32 47 7 7 23/3 1 38 -43 -3 5 7 -36 36 -39 0 A B B. Manual subtraction inferior frontal sulcus anterior ci ngulate (caudal) intraparietal sulcus intraparietal sulcus putamen 9144 24/32 7 7 -47 -15 46 -35 -28 C. Speech subtraction Left frontal Left inferior Left anterior pole frontal sulcus cingulate (dorsal) 10 9145 3218 The anatomic regions and Brodmann’s areas listed in this and following statistical values are reported for each activation focus. of the six scans were unrelated to this study. Only activation foci obtained on the medial wall of the frontal lobe are reported here (see Table 7 and Figs. 3 and 5 ). RESULTS Experiment 1 Behavioral data obtained during the performance of the overpracticed and the reversal versions of each task are shown in Fig. 2. Task-specific changes in rCBF were identified as the difference in rCBF between two scanning conditions. Two types of subtractions were carried out. First, rCBF obtained in the baseline scan was subtracted from that obtained either in the overpracticed or the reversal version of each task. These subtractions will be referred to as the baseline subtractions. Second, in the between-tasks subtractions, rCBF obtained in the over-practiced task was subtracted from that obtained in the reversal version of each task. The coordinates of all activation foci (with the above-threshold value of z) obtained in these subtractions are given in Tables l-3. On the convexity of the frontal lobe, the reversal minus overpracticed subtraction yielded a significant activation focus within the left inferior frontal sulcus for each output modality. The activation focus obtained from the manual subtraction was caudal to that obtained from the oculomotor and the speech subtractions. For the oculomotor output modality, changes in rCBF within the inferior frontal sulcus were bilateral. On the medial wall of the frontal lobe, the same between-tasks subtraction yielded significant changes in r CBF within the left anterior cingulate region for each output modality. The most anterior focus was located just above the genu of the corpus callosum (Fig. 6, focus A). This was observed in the oculomotor subtraction. The most -39 -42 -1 tables are based on the atlas by Talairach and Tournoux D (1988). Both 1 and z posterior focus, buried in the depth of the cingulate sulcus [ see also Talairach and Tournoux ( 1988 ), coronal section taken at y = +8 mm depicted in Fig. 761, was detected in the manual subtraction (Fig. 4, focus C). In the speech subtraction, the activation focus (Fig. 5, focus D) was found further dorsally in the vicinity of the paracingulate sulcus. Even though the rCBF changes encroach on the cortex of the medial frontal gyrus (area 8 ), a close relationship of the maximum of this focus to the paracingulate sulcus can be seen in Fig. 5 (inset). In the oculomotor subtraction, changes in r CBF were also detected within the right ACC. One of these oculomotor foci was located in the rostral ACC (as was the focus in the left hemisphere), whereas the second one was found further dorsally in the vicinity of the paracingulate sulcus. The intermediate dorsal location of the latter focus was similar to that obtained in the speech subtraction. In the reversal minus baseline subtraction, a similar pattern of rCBF changes was obtained within the left anterior cingulate region. However, some differences with regard to the precise localization of the activation foci in comparison to those obtained in the between-tasks subtractions should be noted. Thus the activation focus obtained in the manual subtraction was located more closely to the midline (X = -4 mm) (Fig. 4, focus G), the rCBF changes yielded in the speech subtraction extended into the cortex ventral to the paracingulate sulcus (Fig. 5, focus H) with the maximum of the activation again very close the this sulcus (Fig. 5, inset), and, in the oculomotor subtraction, two foci were found adjacent to the genu of the corpus callosum (Fig. 6, foci E and F) . No significant r CBF changes were detected within the intermediate dorsal ACC in the latter subtraction. None of the foci observed within the inferior frontal sulcus in the reversal minus overpracticed subtraction (see above) were now seen. By contrast, changes in rCBF could be clearly Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 Left Left Right Left Left 458 TABLE T. PAUS, M. PETRIDES, 2. A. C. EVANS, AND E. MEYER Stereotaxic coordinatesof activation foci obtained in the reversalminusbaselinesubtraction Region Brodmann’s Area Left precentral sulcus Right precentral sulcus Left anterior cingulate (rostral) Left anterior cingulate (rostral) Left superior parietal lobule Right intraparietal sulcus (caudal) Left intraparietal sulcus Right calcarine sulcus Left middle occipital gyrus Left lingual gyrus 6 6 24132 24 7 7 7 17 18 18 z t z 5 3 29 32 -61 -54 -44 -76 -83 -83 44 48 22 12 49 40 44 11 22 -11 4.6 3.2 3.9 3.8 6.1 4.5 5.1 4.7 3.8 5.7 3.0 2.2 2.6 2.5 4.0 3.0 3.4 3.1 2.6 3.8 -28 -37 -30 -21 49 49 44 15 -2 7.6 3.9 3.4 3.3 3.8 5.4 2.9 2.5 2.4 2.7 15 -23 -33 -13 49 3 11 0 4.8 7.7 6.5 5.3 2.2 3.6 3.0 2.4 Y X Letter Code A. Oculomotor subtraction -23 24 -8 -9 -19 35 -35 4 -21 -8 E F B. Manual subtraction 4 3216 7 -36 -4 43 1 -13 5 G C. Speech subtraction Left anterior cingulate (dorsal) Right superior temporal sulcus Left superior temporal sulcus Left superior temporal sulcus 32/8 22 22 22 -4 59 -54 -58 identified in the primary motor regions. There was bilateral activation within the depth of the precentral sulcus in the oculomotor task. For the manual task, the left central region was activated. TABLE 3. H In the over-practiced minus baseline subtraction, a different pattern of blood flow changes was observed within the medial wall of the frontal lobe. In both oculomotor and speech subtractions, significant changes of rCBF were Stereotaxic coordinatesof activation foci obtained in the overpracticedminus baselinesubtraction Region Brodmann’s Area Left precentral sulcus Left superior frontal sulcus (caudai) Left medial frontal gyrus Left superior parietal lobule Right superior parietal lobule Right calcarine sulcus Left superior/middle occipital gyrus Left lingual gyrus Left thalamus or corpus callosum 6 6 6 7 7 17 18/19 18 Left postcentral gyrus Left central sulcus Left anterior cingulate (caudal) Right calcarine sulcus Left lingual gyrus Left substantia nigra Left cingulum or corpus callosum 1 4 24132 17 18 Y Z t Z -1 -2 -4 -56 -57 -74 -88 -81 -26 45 51 60 53 53 12 20 -8 20 3.7 3.3 2.9 5.5 3.0 5.3 4.6 6.9 3.1 2.8 2.5 2.2 4.1 2.3 3.9 3.4 5.1 2.3 -21 -25 3 -71 -64 -23 30 33 51 39 11 0 -2 12 3.2 6.9 4.1 3.7 3.4 3.8 3.2 2.5 5.3 3.2 2.9 2.7 2.9 2.5 -11 -4 -2 -25 -19 -76 33 22 57 3 8 11 5.9 4.4 3.9 9.7 8.6 4.0 3.5 2.6 2.3 5.8 5.1 2.4 x A. Oculomotor subtraction -36 -20 -12 -24 25 4 -21 -11 -4 B. Manual subtraction -51 -38 -8 7 -11 -16 -7 C. Speech subtraction Left central sulcus Right precentral gyrus Left medial frontal gyrus (SMA) Right superior temporal sulcus Left superior temporal gyrus Right calcarine sulcus SMA, supplementary motor area. 4 4 6 22 22 17 -51 62 -4 58 -56 3 Letter Code Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 Left central sulcus Left anterior cingulate (caudal) Right intraparietal sulcus (rostral) Right thalamus or corpus callosum Left substantia nigra HUMAN TABLE 4. ANTERIOR CINGULATE CORTEX 459 Stereotaxic coordinatesof activation foci obtainedin the antistimulusminusprostimulussubtraction Region Brodmann’s Area x Y z t Z Letter Code 8 10 -6 -64 -59 -81 -18 51 42 51 49 54 5 -6 3.5 3.4 3.2 3.7 3.9 3.4 5.0 2.4 2.4 2.2 2.6 2.7 2.4 3.5 J 10 49 -1 1 18 10 22 -64 -54 -68 38 24 49 49 0 45 38 45 51 62 3.0 2.7 3.6 3.2 3.4 2.9 3.2 3.0 3.5 3.3 2.4 2.2 2.8 2.6 2.7 2.3 2.6 2.4 2.8 2.7 48 39 -59 -68 -83 24 24 51 31 45 2.5 2.1 2.0 2.0 1.8 3.0 2.6 2.4 2.4 2.2 A. Oculomotor subtraction Right anterior cingulate (caudal) Right anterior cingulate (caudal) Left anterior cingulate (caudal) or precental sulcus Right superior parietal lobule Precuneus (midline) Right calcarine sulcus Left substantia nigra 24132 32 2416 7 7 17 12 1 -15 17 0 7 -9 B. Manual subtraction It middle frontal gyrus It mediai frontal gyrus precentral sulcus nt precentral sulcus It inferior frontal gyrus anterior cingulate (caudal) It anterior cingulate (dorsal) It superior parietal lobule it precuneus nt precuneus/superior parietal lobule 9 9 6 6 45147 32 32 7 7 7 50 8 -39 39 34 -5 1 24 1 8 L C. Speech subtraction Right frontal pole/rostra1 middle frontal gyrus Left frontal pole/rostra1 middle front. g. Left inferior parietal lobule Left precuneus Precuneus/cuneus (midline) 9/10 9/10 7 7 7/19 found within the caudal and dorsal region of the left medial frontal gyrus ( medial area 6). The activation focus obtained from the manual subtraction was localized within the caudal left ACC (Fig. 4, focus I), as was the case in the reversal minus overpracticed subtraction. Changes of rCBF were also seen in the primary motor regions in all output modalities. Experiment 2 The behavioral findings obtained for all subjects are shown in Fig. 2. In the prostimulus version of the oculomotor task, because of the predictability of the stimulus side, the saccades appeared to be paced by the offset of the fixation rather than the onset of the peripheral stimulus. The latency was 206 t 85 (SD) ms when measured from the offset of the fixation and 6 t 85 ms from the onset of the peripheral stimulus. The latter value is the one presented in Fig. 2. Task-specific changes in rCBF were identified as the difference in r CBF between two scanning conditions. As in experiment 1, in the baseline subtractions the baseline scan was subtracted from either the prostimulus or the antistimulus scan. In the between-tasks subtractions, normalized subtractions are given in Tables 4-6. On the convexity of the frontal lobe, the antistimulus minus prostimulus subtractions yielded the following pattern of rCBF changes. In the oculomotor subtraction, there were no activation foci. In the speech subtraction, activation was seen bilaterally in the frontal pole. In the manual 31 -32 -42 -4 0 subtraction, activation foci were found within the caudal part of the right middle frontal gyrus, the left and right precentral sulcus, and the right inferior frontal gyrus. On the medial wall of the frontal lobe, significant changes in rCBF were observed in the oculomotor and manual subtractions, but not in the speech one (see below for reanalyzed data). In the oculomotor task, one activation focus was found at the border between the caudal and the intermediate portion of the anterior cingulate region. The other activation focus (Fig. 6, focus J) was located more dorsally but also more laterally from the midline and could be classified as falling within either the dorsal part of the caudal ACC or the most ventral part of the neocortical portion of the supplementary motor area (SMA). In the manual subtraction, two activation foci were observed within the anterior cingulate region, one located in its caudal portion in the left hemisphere (Fig. 4, focus L) and one located within the intermediate dorsal portion of the ACC, close to the mid*. line. In the antistimulus minus baseline subtractions, significant rCBF changes were seen in the following regions of the lateral frontal cortex: the primary motor regions (oculomotor and manual subtractions), the inferior frontal gyrus (manual and speech subtractions), and the caudalmost portion of the middle frontal gyrus (manual subtraction). On the medial wall of the frontal lobe, an activation focus was detected in the caudal portion of the left anterior cingulate region in the manual subtraction (Fig. 4, focus N). The activation focus obtained in the oculomotor subtraction was located at the border between the dorsal portion of the caudal ACC and the ventral part of the neocortical SMA (Fig. 6, focus AJ). In the prostimulus minus baseline subtractions, only the Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 Rig Rig Lef Rig Rig Lef Rig Rig Rig Rig T, PAUS, M. PETRIDES, 460 TABLE 5. A. C. EVANS, AND E. MEYER Stereotaxic coordinatesqf activation foci obtained in the antistimulus minus basehe subtraction Region Brodmann’s Area x Y t z Letter Code A. Ocuk~motor subtraction Right precentral sulcus/superior frontal sulcus Left anterior cingulate (caudal) Right intraparietal sulcus Left intraparietal sulcus Right calcarine sulcus Right inferior occipital lobule Left middle occipital lobule Right inferior temporal gyrus (caudal) Right inferior temporal sulcus (caudal) 6 3216 7 7 17 19 18 37 22 25 -9 27 -20 3 40 -28 47 52 -2 -59 -59 -85 -69 -85 -61 -52 53 53 47 48 6 -5 15 -11 9 4.8 5.4 5.6 4.8 9.4 3.8 3.7 3.7 3.6 2.9 3.2 3.3 2.9 5.4 2.3 2.3 2.3 2.2 -19 -2 -4 22 -44 -54 -30 -19 -18 51 56 48 -5 40 56 13 -2 -5 9.6 4.4 7.0 3.3 3.8 3.3 5.5 3.4 4.2 5.9 2.8 4.3 2.2 2.4 2.2 3.4 2.3 2.7 24 22 20 -21 -23 -2 -30 -8 -5 0 0 -3 -9 -11 3.6 3.2 3.0 5.9 5.4 4.5 4.9 2.6 2.3 2.2 4.4 4.0 3.3 3-7 I M B. Manual subtraction 4 6 24/6 47 7 7 42 22 -42 24 -5 38 38 -12 -54 -36 0 N C. Speech subtract ion Right inferior frontal gyrus Left inferior frontal gyrus Left inferior frontal gyrus Left superior temporal gyrus Right superior temporal sulcus Right middle temporal gyrus Tectum (midline) 47 47 47 22 21122 21 primary motor regions were activated on the lateral convexity of the frontal lobe (oculomotor and manual subtractions). In the speech subtraction, two activation foci were observed within the right inferior frontal gyrus. On the medial wall of the frontal lobe, significant changes of r CBF were observed in the oculomotor and manual subtractions. The activation focus obtained in the oculomotor subtraction was localized quite dorsally within the caudal part of the right medial frontal gyrus. The localization of rCBF changes observed in the manual subtraction was the same as that of the antistimulus minus baseline subtraction, i.e., the caudal part of the ACC (Fig. 4, focus 0). In the speech antistimulus task, a clear difference in the performance of individual subjects was noticed: four subjects passed the speech antistimulus task without making any etiors (“passed” group), whereas the remaining five subjects made 10% errors on average (“failed” group). The PET data were then reanalyzed for each group separately. The task subtraction yielded a significant r CBF change within the dorsal intermediate ACC in the passed group only ( Fig. 5, focus P) . Furthermore, the prostimulus minus baseline subtraction performed in the failed group yielded significant r CBF changes not only in the medial frontal gyrus (SMA) but also in the intermediate ACC (Fig. 5, focus Q). DISCUSSION An overview of the r CBF changes observed on the medial wall of the fron tal lobes in all three experiments is provided 39 -34 -29 -60 64 59 0 in Fig. 3 and Table 7. Three main points emerge from this summary of the data. First, most of the activation foci obtained in the reversal and/or antistimulus scans were located in the portion of the medial wall that extends between and includes the cingulate and paracingulate sulci. This region has been referred to as the paralimbic zone by Sanides ( 1964) (see Fig. 3, inset) because it comprises cortex of a transitional type that shares both limbic and neocortical morphological features. This region includes Brodmann’s area 32 and the dorsalmost part of area 24. This finding is consistent with the localization of the cingulate motor areas in the dorsal tier of area 24 of the monkey cingulate cortex (Dum and Strick 199 1; Luppino et al. 199 1). Second, somatotopic organization of the activation foci could be seen in that the “manual” foci were located in the caudalmost portion of the ACC (around the vertical plane traversing the anterior commissure (VCA), the “oculomotor” foci obtained in experiment 1 were located in the rostral part of the ACC (close to the genu of the corpus callosum), and the “speech” foci were found in the middle portion of the ACC just anterior to the manual foci. In the speech subtractions (experiments 1 and 3 ), additional activation was obtained also in the rostralmost portion of the ACC, corresponding most likely to the face representation. Thus the distinct localization of r CBF changes within the human ACC is again consistent with the neuroanatomic and neurophysiological findings in the monkey, as alluded to in the introduction and corroborated below. Third, activation foci found almost exclusively in the Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 Left central sulcus Right middle frontal gyrus (caudal) Left anterior cingulate (caudal) Right inferior frontal gyrus Right intraparietal sulcus Left superior parietal lobule Left superior temporal gyrus Left temporal insula Nucleus ruber (midline) HUMAN ANTERIOR CINGULATE CORTEX 461 A. Oculomotor subtraction Right precentral sulcus Left precentral gyrus Right medial frontal gyrus Right intraparietal sulcus Right calcarine sulcus Left lingual gyrus Left middle occipital gyrus Right lingual gyrus 6 416 6 17 18 19 18 34 -44 3 27 1 -11 -27 24 -2 -6 -2 -54 -88 -83 -90 -76 48 44 60 45 8 -6 17 -8 3.4 3.3 3.7 3.7 7.5 5.7 3.7 4.8 2.4 2.3 2.6 2.6 5.0 3.8 2.6 3.3 48 49 11 9 15 -3 9.8 5.9 5.8 3.7 3.7 4.2 6.0 3.7 3.7 2.4 2.4 2.7 -5 5 5 2 -9 -11 3 3.2 2.9 7.0 6.7 5.1 4.0 3.7 2.6 2.3 5.6 5.3 4.1 3.2 3.0 B. IManual subtraction 4 2416 22 22 22 -43 -5 -50 63 58 0 -23 -7 -26 -37 -33 -18 0 C. Speech subtraction Right inferior frontal gyrus Right inferior frontal gyrus Left superior temporal gyrus Right superior temporal gyrus Right middle temporal gyrus Right tectum Right thalamus 47 45147 22 22 21 38 28 -56 62 60 7 3 over-practiced and / or prostimulus scans were located more dorsally in the caudal part of the medial frontal gyrus ( Brodmann’s area 6). Localization of these oculomotor and “speech/ face” foci is in agreement with the results of other PET studies (eye movements: Fox et al. 1985a; speech: Petersen et al. 1988 ) . It also demonstrates that both the speech/ face and eye representations, located in the neocortical portion of the SMA, overlap to a great extent. Before discussing in greater detail the above points, we would like to comment on some results that might be viewed as incongruent with the above interpretation of somatotopy. First, there is a difference between the rCBF changes observed in the reversal (experiment 1) and the antistimulus (experiment 2) oculomotor tasks (see Fig. 3). It is possible that the lack of significant activation of the rostra1 ACC during the performance of the antistimulus task, which was observed in this region in the reversal task, is related to the absence of concomitant activation of the prefrontal cortex. The caudal localization of the rCBF changes in the antistimulus subtractions might be related to either involvement of the supplementary eye field (compare the localization of the foci obtained in these subtractions to those found in the overpracticed and prostimulus ones) or to the tendency of subjects to make a simultaneous head movement’ when required to move the eyes away from a peripheral stimulus. Luppino and collaborators ( 199 1 ), using microstimulation in the awake monkey, elicited neck and upper-trunk movements within the caudal ’ In a pilot study run after this experiment, both subjective reports from subjects and electromyographic recording from neck muscles in the PET setting provided evidence of an increase in neck muscle tension related to the gaze response required in the antistimulus task. 22 18 -18 -23 -4 -30 -16 portion of the ACC, where forelimb movements were also elicited. A second set of results that might be considered incongruent with the somatotopic organization of the human ACC is the involvement of the intermediate, speechrelated portion of the ACC in the tasks requiring no overt speech output ( see Table 7 B, activation foci K, V, and IV). These changes in rCBF, observed in this region during the manual (antistimulus, focus IV) and oculomotor (reversal, focus Vand antistimulus, focus K) tasks, may be related to covert speech. This explanation is supported by the fact that subjects noted the occurrence of covert speech more frequently during the more difficult reversal or antistimulus tasks. Furthermore, in the same subtractions, the “primary” activation foci were found in the expected regions, i.e., the caudal and rostra1 ACC in the case of the manual and oculomotor subtractions, respectively. In addition, the “secondary” foci (K, I/, W) were not yielded by the respective “baseline” subtractions, whereas that was the case for the primary foci. Zatorre et al. ( 1992) observed activation of Broca’s area during the phonological analysis of words heard, without any overt verbal responses. This strongly suggests that “speech-related” regions could be activated without actual motor output. It should also be pointed out that an alternative interpretation of the data presented in this communication might be that there are multiple regions on the medial wall of the frontal lobe where different output modalities are represented. Thus the intermediate ACC (area 32) may be viewed as a distinct region containing eye, hand, and speech representations and involved in cognitive aspects of motor control. The rostra1 ACC would then constitute an incomplete map containing only eye and face representations; no significant r CBF changes were found here in the manual Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 Left central ‘sulcus Left anterior cingulate (caudal) Left superior temporal gyrus Right superior temporal gyrus Right superior temporal gyrus Nucleus ruber (midline) T. PAUS, M. PETRIDES, A. C. EVANS, AND E. MEYER I MANUAL FIG. 4. Merged PET-MRI sagittal slices of the left hemisphere, depicting activation foci obtained in the manual subtractions. individual foci. Distances from midline are in millimeters; minus sign indicates left hemisphere. C’: reversal minus ovcrpracticed ( 15). minus prostimulus (- 5). G: reversal minus baseline (-4). I: overpracticed minus baseline (-8). N: antistimulus minus baseline (-5). minus baseline (-5). Note that with the Scanditronix PC-2048 tomograph and the PET data analysis used in this study, a point source would be detected as a focus with full width at half maximum (FWHM) of 18 mm. Therefore, the spatial extent of the activation foci N necessarily imply an involvement of the whole underlying cortex. For that reason, we also present the position of the maximal activation respective subtractions (foci N and 0. insrls). In L. additional activation can be seen in the parietal region. Letters identify L: antistimulus 0: prostimulus of radioactivity and 0 need not obtained in the Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 FIG. 3. Activation foci within the anterior cingulate region yielded by the manual (green), the speech (red ). and the oculomotor (yellow) subtracr . . ._-. tions m experiments l-3. Ihe toc~ located m either the left or nght hemisphere were superimposed on the sagittal section OI me averagea MKI (experiment 1) passing 2.7 mm to the left ofthe midline. Bottom k$ corner: letters identify the individual foci. Both the cingulate and paracingulate sulci are highlighted by grey squares. Inset: (reprinted with permission from Sanides 1964) architectural zones and principal sulci of the medial wall of the human frontal lobe (cm, sulcus callosomarginahs; Pro, proisocortex; PlZd, dorsal paralimbic zone; FmZ, frontomotor zone; PmZ, paramotor zone; FpZ, frontopolar zone). Images, schematics, and point locations are presented within the standardized coordinate space (Talairach and Tournoux 1988). HUMAN TABLE 7. Letter Code ANTERIOR CINGULATE CORTEX 463 Stereotaxic coordinatesofactivationfici obtainedon the medial wall of thefiontal lobesin three PET experiments Y X z z t Subtraction Output Modality Brodmann’s Area Interpretation A. Rostra1 anterior cingulate cortex A B E F x Y z* -3 -8 -9 -4 -9 32 27 29 32 30 34 34 20 29 22 12 17 13 22 3.8 2.8 3.9 3.8 3.4 3.5 3.8 2.9 2.2 2.6 2.5 2.0 1.5 2.0 REV-OVR REV-OVR REV-BASE REV-BASE REV-OVR REV-BASE REV-OVR Oculomotor Oculomotor Oculomotor Oculomotor Speech Speech Speech 24132 32 24132 24 24 24 24 Face Face Face 3218 32 32 24132 32 32 32 32 3218 32 32 Speech/vocalization Speech/vocalization Speech/vocalization Speech/vocalization Speech/vocalization Speech/vocalization Speech/vocalization Speech/vocalization Covert speech ? Covert speech ? Covert speech ? 24132 3216 24132 32 24123 24123 24132 3216 Arm Arm Arm Arm Arm Arm Head movement ? Head/SEF ? Eye Eye Eye Eye B. Intermediate anterior cingulate cortex D H P -1 -4 0 8 Q 3 5 -1 5 10 12 18 20 13 20 17 22 10 49 49 44 39 44 36 48 38 51 38 42 4.3 4.8 2.8 4.0 3.9 3.5 4.0 3.3 3.1 3.2 3.4 2.6 2.2 2.7 2.8 2.1 1.9 2.2 1.8 2.4 2.6 2.4 REV-OVR REV-BASE ANT-PRO PRO-BASE REV-OVR REV-OVR REV-BASE REV-BASE REV-OVR ANT-PRO ANT-PRO Speech Speech Speech passed Speech failed Speech Speech Speech Speech Oculomotor Manual Oculomotor C. Caudal anterior cingulate cortex c G L N 0 J M -15 8 5 3 -4 -8 -5 -5 -5 12 -- 9 10 -4 -7 8 -2 49 49 39 45 48 49 51 53 3.0 3.9 4.1 2.9 7.0 5.9 3.5 5.4 2.3 2.9 3.2 2.3 4.3 3.7 2.4 3.2 REV-OVR REV-BASE OVR-BASE ANT-PRO ANT-BASE PRO-BASE ANT-PRO ANT-BASE Manual Manual Manual Manual Manual Manual Oculomotor Oculomotor D. Medial frontal gyrus (supplementary motor area) 2 3 4* -12 -4 3 -4 -2 -2 -1 -1 5* 6 5 4 1 60 57 60 60 58 62 2.9 3.9 3.7 5.2 4.7 3.3 2.2 2.3 2.6 3.1 2.6 1.8 OVR-BASE OVR-BASE PRO-BASE OVR-BASE REV-BASE PRO-BASE Oculomotor Speech Oculomotor Speech Speech Speech failed 6 6 6 6 6 6 SEF Speech/vocal/face SEF Speech/vocal/face Speech/vocal/face Speech/vocal/face Experiment 1: reversal (REV), overpracticed (OVR), and baseline (Base) scans. Experiment 2: antistimulus (ANT), prostimulus (PRO), and baseline (Base) scans. Experiment 3 (replication of experiment 1): REV, OVR, and Base scans. SEF, Supplementary Eye Field. * Activation foci obtained in experiment 3 are marked with an asterisk. ? tentative interpretation of the given activation focus. subtractions. Finally, the caudal portion of the medial frontal cortex contains representations for all the output modalities explored. However, it should be stressed that the neocortical portion of the SMA and the more ventrally located caudal ACC probably do not constitute one homogeneous region. The dorsal group of foci was found to be activated mainly during the performance of the overpracticed and the prostimulus tasks (oculomotor or speech), whereas the ventral group was activated mainly during the reversal and the antistimulus tasks (manual or oculomotor, the latter only in experiment 2; see above). Changes in rCBF in the caudal ACC yielded by both reversal (antistimulus) and overpracticed (prostimulus) manual subtractions may be related to the existence of the rostra1 and the caudal arm representations within the ACC (see below). The following discussion will concentrate on two issues. First, the localization of the rCBF changes observed within the human ACC will be examined in the context of findings obtained in neuroanatomic and neurophysiological studies. Second, some functional will be outlined. considerations regarding the ACC Localization As already pointed out, the medial wall of the primate frontal lobe can be subdivided along the dorsoventral axis into three general types of cortex. The dorsalmost region is of the true neocortical type and comprises Brodmann’s areas 6, 8, and 9 (in a caudorostral direction). The ventralmost part (the cortex of the cingulate gyrus, i.e., areas 24a and 24b) lies above the corpus callosum and comprises cortex of the limbic type. Between these two regions lies a transitional type of cortex comprising Brodmann’s area 32 and the dorsalmost part of area 24. Most of the activation foci observed in all three experiments were found in this portion of the ACC. MANUAL SYSTEM. Changes of rCBF obtained in the manual subtractions in both experiments 1 and 2 were localized Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 R* S* T* u* V w K 22 15 T. PAUS, M. PETRIDES, vco A. C. EVANS, UIA AND E. MEYER SPEECH _ . . . . -. .-...-- . . . _.. FIG. 6. Merged PET-MRI sagittal shces of the left or the rtght hcmrsphcrc, dcptctmg acttvatton foci obtamed m the oculomotor subtractions. identify individual foci. Distances from midline are in millimeters; minus sign indicates left hemisphere. .4 : reversal minus overpracticed (-3). reversal minus baseline (-8 and -9 for Eand F, respectively). J: antistimulus minus prostimulus ( 12). M: antistimulus minus baseline (-9). and M, a parietal and occipital activation foci can also be seen. Caudally located foci (J and 1211)were obtained in Experiment 2. Letters Eand F: In E, F, Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 FIG. 5. Merg identify the ind tvidual foci. Distances from the mtdline arc in milhmeters. wtth the mmus sign tndicattng tb-Ic IJ~ 1L11 hc 18zm&phere. D: reversal minus overpraticed, experiment 1 (~ 1 ). H: reversal minus basehne, experiment 1 ( -4) 1’. antrrtrmulus mrnuz prostrmu lus, passed subgroup (.\- = 0, y = 10,F 44). Q: prostrmulus minus baseline, fakd subgroup ( I ~ 7. 1’ = 12, 2 = 39). R and S: reversal menus overpractic- ed-1 ewneriment - .~-. 3 (3 and 5 for R and S, respecttvely). Tand C’: reversal minus baseline. experiment 3 (~ 1 and 7 for Tand I’, respecttvely). I: reversal minus overt aracticed. experiment 1I(-4). Y: reversal minus baseline. experiment 1 ( -9). L: reversal minus overpracticcd. experiment 3 (7). In\<>/\: posttton ofthe I,.~,,,...~. n~v;fl~t activation for foci II and H located in the ctctnity of the paracingulate sulcus. In scctrons Q and 1’. tn additron to the focus In the anterior cingulate reg.-.., . .._ .._VC rinn the nenr cortical portion of the supplementary motor area was also activated. HUMAN ANTERIOR CORTEX 465 al. ( 1980). However, neither in our study nor in those studies could simultaneous activation of both neocortical and cingulate arm representations be demonstrated. Such a finding would provide the most direct evidence for the existence of multiple, distinct arm representations on the medial wall of the human frontal lobe. Precisely such a pattern of r CBF changes has recently been found in a PET experiment involving painful stimulation of the left forearm (Coghill et al. 1992) .2 In this study, the painful (48°C) minus control (35 “C) subtraction yielded three distinct activation foci on the medial wall of the frontal lobe. These foci were located close to the coronal plane defined by the VCA (Y coordinates: + 1, -4, and -6 mm, respectively), one above the other (2 coordinates: +42, +54, and +66 mm above the anterior commissure-posterior commissure plane, respectively) (R. C. Coghill, personal communication). Thus the manual foci observed in the reversal and antistimulus tasks were located in between the most ventral focus and the intermediate one obtained in the “pain” study. The dorsalmost arm representation activated in the “pain” study did not seem to be involved in our study. In experiment I, the oculomotor OCULOMOTOR SYSTEM. task subtraction yielded activation foci within the most anterior part of the ACC, just above the genu of the corpus callosum. Connections between the frontal eye field (FEF), representing a primary cortical region for the control of eye movements3 and the cingulate cortex have not yet been systematically explored. Nevertheless, in studies in which retrograde tracers were injected either into the dorsocaudal portion of Brodmann’s area 8 (Barbas 1988; Barbas and Mesulam 198 1; Barbas and Pandya 1989) or into physiologically defined FEF (Huerta et al. 1987), labeled neurons were identified within the rostra1 portion of the ACC, in front of the genu of the corpus callosum. Furthermore, this region is also known to send efferents to the supplementary eye fields (Luppino et al. 1990) and to both the superior colliculus (Leichnetz et al. 198 1) and the paramedian pontine reticular formation (Leichnetz et al. 1984). Thus the neuroanatomic findings suggest that the rostralmost part of the ACC (located around the genu of the corpus callosum) may be concerned with oculomotor control, and as such they are consistent with the localization of the oculomotor foci observed in experiment 1. SPEECH SYSTEM. In the classical study by Penfield and Welch ( 195 1) , electrical stimulation of the medial surface of the frontal lobe in epileptic patients yielded vocalization (or speech arrest) not only from the neocortical portion of 2 Changes in rCBF observed on the medial wall of the frontal lobes during the painful stimulation could be related to the necessity of suppressing a withdrawal response induced by the nociceptive stimulus. 3 Primary cortical areas involved in oculomotor control in humans could be revealed by the baseline subtractions (see methods). In both experiments, oculomotor baseline subtractions yielded rCBF changes in the depth of the precentral sulcus or the caudalmost part of the superior frontal gyrus. These findings are in agreement with what has been identified as the human FEF by Penfield and Rasmussen ( 1952) and Fox et al. ( 1985a). In nonhuman primates, the FEF is found along the anterior bank of the arcuate sulcus, where it overlaps the posterior border of Brodmann’s area 8 with area 6 (Stanton et al. 1989). Interestingly, rCBF changes were found in the precentral sulcus not only in the oculomotor baseline subtractions but also in the manual between-tasks subtraction (experiment 2). This finding agrees well with the fact that the FEF is adjacent to the hand premotor region ( Rizzolati et al. 1988). Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 within the dorsal portion of the caudal ACC. In the rostrocaudal direction, the foci were localized from 10 mm in front of the VCA (focus L) to 7 mm behind the VCA (focus 0). Along the dorsoventral axis, the foci were mapped onto the region beginning ventrally at the level of the cingulate sulcus (Fig. 4, focus I) and extending dorsally to the level defined by the caudalmost part of the paracingulate sulcus (Fig. 4, foci G and 0, see also Fig. 3). The localization of this “hand’‘-related area is consistent with the results of classical stimulation studies carried out in epileptic patients (Penfield and Jasper 1954; Penfield and Rasmussen 1952; Penfield and Welch 195 1; Talairach and Bancaud 1966). In those studies, movements could be elicited not only from the medial frontal gyrus (medial area 6) but also from the dorsal bank of the cingulate sulcus. Classical studies in nonhuman primates confirmed these findings (Penfield and Welch 195 1; Woolsey et al. 1952). After these pioneering studies, the concept of the SMA as a functionally homogeneous motor region containing one arm representation and occupying primarily the medial portion of neocortical area 6 gradually evolved (see, e.g., Wiesendanger 1986). Recent neurophysiological and neuroanatomic studies have, however, provided ample evidence for the existence of multiple arm representations on the medial wall of the monkey frontal lobe, located both dorsally within medial area 6 and ventrally within the limits of the cingulate sulcus (but without encroaching upon the cortex adjacent to the corpus callosum, i.e., the cingulate gyrus). For instance, projections to the cervical spinal cord and the arm area of the primary motor cortex have been shown to originate not only from the medial portion of area 6, but also from three distinct subregions of the monkey cingulate cortex (Dum and Strick 199 1). One group of projecting neurons has been found in the region of area 24c just rostra1 to the coronal plane defined by the genu of the arcuate sulcus (see Fig. 1). The other two subregions are located caudal to this plane. One extends for a considerable rostrocaudal distance (8 mm) within the dorsal bank of the cingulate sulcus, and the other, smaller, region is located on the ventral bank of the sulcus (Dum and Strick 199 1). By means of cortical microstimulation in the awake monkey, Luppino et al. ( 199 1) elicited arm movements (mostly contralateral movements restricted to a single joint) from the caudal and rostra1 medial area 6, as well as from two subregions of the ACC located within the cingulate sulcus. A low-threshold cingulate subregion was located caudal to the coronal plane defined above (subarea 24d), and a high-threshold subregion was found rostra1 to this plane (subarea 24~). In another study, Shima et al. ( 199 1) recorded unit activity within the cingulate cortex of monkeys performing either externally triggered or self-paced wrist movements. Two distinct arm representations within the cingulate sulcus ( 1 rostra1 and 1 caudal to the coronal plane defined by the genu of the arcuate sulcus) were again demonstrated. Thus, in the context of the monkey studies reviewed above, we believe that the localization of the activation foci obtained in our manual subtractions corresponds to the ventral, i.e., cingulate, arm representation. In contrast, activation of the neocortical portion of the SMA, which most likely corresponds to the dorsal arm representation found in the monkey, has been demonstrated in PET studies by Colebatch et al. ( 199 1). Fox et al. ( 1985a). and Roland et CINGULATE 466 T. PAUS, M. PETRIDES, A. C. EVANS, AND E. MEYER Functional considerations Besides confirming the prediction related to the somatotopic organization of the ACC, our results emphasize the importance of concomitant activation of the prefrontal cortex for such a somatotopy to emerge. This finding underscores the fact that the flow of information between, and local computations within, anatomically interconnected and functionally related cortical areas represent critical factors underlying the emergence of a specific pattern of r CBF changes. This might be due to the fact that function-related changes in metabolic activity occur at the level of nerve terminals (synaptic events) rather than cell bodies (Sokoloff 1991). The data obtained in experiment 1 provide a clear example of a specific activation pattern. In the oculomotor reversal minus overpracticed subtraction, concomitant activation was observed in the caudal part of the intraparietal sulcus, the rostra1 portion of the inferior frontal sulcus, and the rostra1 part of the ACC. By contrast, in the manual task subtraction, the rostra1 intraparietal sulcus, the caudal inferior frontal sulcus, and the caudal ACC were coactivated. This difference in the pattern of activated brain regions depending on the output system used is consistent with the organization of corticocortical connections, not only between the primary motor regions and the ACC (see above) but also between the intraparietal sulcus and the lateral frontal cortex. The rostralmost portion of the intraparietal sulcus, which is related to the somatosensory system, is linked to more caudal frontal areas, whereas the more posterior (visuomotor) intraparietal sulcus is connected to more rostra1 frontal areas (Petrides and Pandya 1984). The speech subtraction revealed rCBF changes within the left inferior frontal sulcus, the left frontal pole (area lo), the intermediate ACC (area 32), and the rostra1 ACC (area 24). Area 32 is known to be closely interconnected with both the prefrontal cortex and also the auditory association cortex4 ( Barbas 1988 ) . The dense connections with the auditory cortex suggest that the intermediate ACC (area 32) may play a more specific role in the control of vocalization and speech (e.g., a feedback-type modulation). On the other hand, the connections with the prefrontal cortex might also imply a close functional relationship between the two regions. In contrast, the close relationship between the rostra1 ACC (area 24) and the face representation of the primary motor cortex points to a more basic role of this region in motor control. This view is supported by a recent neurophysiological investigation of the monkey ACC showing that unit activity recorded from the rostra1 portion of area 24 is related not only to vocalization but also to orofacial movements, such as jaw opening or tongue protrusion (West and Larson 1992). In the overpracticed and prostimulus tasks, the motor responses were executed in a more automatic way. A different pattern of rCBF changes within the frontal cortex now resulted. In addition to the primary motor cortical areas, activation foci were obtained in the SMA or the inferior frontal gyrus. This was the case in the oculomotor (SMA, experiments 1 and 2) and the speech (SMA, experiments 1 and 3; inferior frontal gyrus, experiment 2) subtractions. By contrast, in the manual overpracticed (or prostimulus) minus baseline subtraction, task-specific activation remained localized to the dorsal portion of the caudal ACC. The neocortical portion of the SMA that was found to be activated in previous studies requiring finger movements (Colebatch et al. 199 1; Fox et al. 1985a; Roland et al. 1980) was probably not involved in this study. Two groups of activation foci could be distinguished, however, within the caudal cingulate region. One of those was located just behind and the other just in front of the vertical plane traversing the anterior commissure. The posterior group was obtained in the baseline subtractions (experiment 2), whereas the two most anterior foci were found in the between-tasks subtractions ( experiments 1 and 2) ( see Fig. 3 ) . These two groups of manual foci may represent two functionally distinct subregions within the caudal ACC. This suggestion would be consistent with the fact that there are clear differences between the rostra1 and the caudal arm representations of the monkey cingulate cortex. The stimu4 In both experiments 1 and 2, the speech baseline subtractions yielded rCBF changes within the superior temporal gurus and/or sulcus. Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 the SMA, but also from the dorsal bank of the cingulate sulcus. The speech between-tasks subtraction in experiment 1 revealed a significant change in rCBF, the maximum of which was localized within the paracingulate sulcus (see Fig. 5, focus D). This is even more apparent in the baseline subtraction using the same active condition (reversal minus baseline) (see Fig. 5, focus H) and in the replication study (Fig. 5, foci R-U). In experiment 2, a significant rCBF change within this region was obtained in the passed (antistimulus minus prostimulus subtraction) and failed (prostimulus minus baseline) group. Thus most of the activation observed in relation to speech was localized between the cingulate and paracingulate sulci and extended in the rostrocaudal direction from 22 mm (focus D) to 10 mm (focus p)’ in front of the VCA ( see Fig. 3). The relative position of hand- and speech-related foci, the latter being located rostra1 to the former, is consistent with the results of the PET study by Frith et al. ( 199 1) and, to some extent, with the stimulation study by Fried et al. ( 199 1; see, e.g., patient 12). Work with nonhuman primates has demonstrated that vocalization can be elicited by electrical stimulation of the rostra1 portion of the ACC [ for review, see Sutton and Jurgens ( 1988) and Vogt and Barbas ( 1988)]. This region comprises area 32, the rostra1 portion of area 24, and the subcallosal area 25. Neurons in this physiologically defined vocalization region project directly to the periaqueductal grey, a pontomesencephalic phonation area ( Muller-Preuss and Jurgens 1976). The rostralmost part of area 24 is linked to the face representation of the primary motor cortex (Jurgens 1976; Morecraft and Van Hoesen 1992; Muakkassa and Strick 1979). Reciprocal connections exist between areas 24 and 32 (Vogt and Barbas 1988). As pointed out above, area 32 in the human brain is not limited to the rostra1 part of the ACC but extends caudally, occupying an intermediate position between the dorsal tier of area 24 and the neocortical areas 6,8, and 9. The intermediate group of the speech-related activation foci obtained in our study is most likely located within area 32, whereas the rostra1 group ( foci X-Z ), found in the same speech subtractions, appears to be located in the rostralmost part of area 24. HUMAN ANTERIOR CORTEX 467 in facilitation of the execution of the appropriate responses and/or suppression of the inappropriate ones. We thank the staff of the McConnell Brain Imaging Center for technical assistance, and Drs. A. Gjedde, G. Luppino, and B. Milner for comments on the manuscript. This work was supported by the McDonnell-Pew Program in Cognitive Neuroscience and by the Medical Research Council (Canada) Special Project SP-30 Grant (coordinator: Dr. A. Gjedde). Address for reprint requests: T. Paus, Montreal Neurological Institute, 3801 University St., Montreal, Quebec H3A 2B4, Canada. Received 15 September 1992; accepted in final form 17 March 1993. REFERENCES AITKEN, P. G. Cortical control of conditioned and spontaneous vocal behavior in rhesus monkeys. Brain Lang. 13: 17 I- 184, 198 1. ALEXANDER,G. E., CRUTCHER, M. D., AND DELONG, M. R.Basalganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. In: Progress in Brain Research. The Prefrontal Cortex. Its Structure, Function and Pathology, edited by H. B. M. Uylings, C. G. Van Eden, J. P. C. de Bruin, M. A. Corner, and M. G. P. Feenstra. Amsterdam: Elsevier, 1990, vol. 85, p. 119-146. BARBAS, H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Clomp. Neural. 276: 313-342, 1988. BARBAS, H. AND MESULAM, M.-M. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J. Comp. Neural. 200: 407431, 1981. BARBAS, H. AND PANDYA, D. N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Clomp. Neural. 286: 353375, 1989. BARRIS, R. W. AND SCHUMAN, H. R. Bilateral anterior cingulate gyrus lesions. Syndrome of the anterior cingulate gyri. Neurology 3: 44-52, 1953. BERMANJ. F., RANDOLPH,C.,GOLD, J., HOLT, D., JONES, D.W., GOLDBERG, T. E., CARSON, R. E., HERSCOWITCH, P., AND WEINBERGER, D. R. Physiological activation of frontal lobe studied with positron emission tomography and oxygen-15 water during working memory tasks (Abstract). J. Cereb. Blood Flow Metab. Suppl. 2: 85 1, 199 1. BRAAK, H. A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109: 2 19-233, 1976. BRODMANN, K. Vergieichende Lokalisationslehre der Groshirnrinde. Leipzig: Barth, 1909. COGHILL, R. C., TALBOT, J., EVANS, A., GJEDDE, A., MEYER, E., DUNCAN, G. H., AND BUSHNELL, M. C. Human cerebral processing of noxious and innocuous stimuli. Sot. Neurosci. Abstr. 18: 386, 1992. COLEBATCH, J. G., DEIBER, M.-P., PASSINGHAM, R. E., FRISTON, K. J., AND FRACKOWIAK, R. S. J. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J. Neurophysiol. 65: 1392-1401, 1991. CORBETTA, M., MIEZEN, F. M., DOBMEYER, S., SHULMAN, G. L., AND PETERSEN, S. E. Selective and divided attention during visual discrimination of shape, color, and speed: functional anatomy by positron emission tomography. J. Neurosci. 11: 2383-2402, 199 1. DEIBER, M.-P., PASSINGHAM, R. E., COLEBATCH, J. G., FRISTON, IS. J., NIXON, P. D., AND FRACKOWIAK, R. S. J. Cortical areas and the selection of movement: a study with positron emission tomography. Exp. Brain Res. 84: 393-402, 199 1. DUM, R. P. AND STRICK, P. L. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11: 667-689, 199 1. ECONOMO, C. AND KOSKINAS, G. N. Die Cytoarchitektonik der fiirnrinde des erwachsenen Menschen. Berlin: Springer-Verlag, 1925. EVANS, A. C., MARRETT, S., NEELIN, P., COLLINS, L., WORSLEY, K., DAI, W., MILOT, S., MEYER, E., AND BUB, D. Anatomical mapping of functional activation in stereotactic coordinate space. Neurolmage 1: 43-53, 1992. EVANS, A. C., MARRETT, S., TORRESCORZO, J., Ku, S., AND COLLINS, L. MRI-PET correlative analysis using a volume of interest (VOI) atlas. J. Cereb. Blood Flow Metab. 1 1: 69-78, 199 la. EVANS, A. C., THOMPSON, C. J., MARRETT, S., MEYER, E., AND MAZZA, M. Performance characteristics of the PC-2048: a new 15-slice encodedcrystal PET scanner for neurological studies. IEEE Trans. Med. Imaging 10: 90-98, 1991b. Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 lation threshold for eliciting movement is lower in the cauda1 arm representation than in the rostra1 one (Luppino et al. 199 1). Similarly, unit activity recorded from the caudal arm region is not as complex as that recorded from the rostra1 one (Shima et al. 199 I). The connectivity of these two cingulate subregions is consistent with the above differences. The caudal arm representation [area 6c (Dum and Strick 199 1) or area 24d (Matelli et al. 199 1 )] sends dense projections to the spinal cord, the primary motor cortex, and the caudal part of the SMA (medial area 6aa). On the other hand, the projections of rostra1 area 24c target mainly the rostra1 portion of the SMA (medial area 6ap), with limited direct access to the primary motor cortex and the spinal cord (Dum and Strick 199 1; Luppino et al. 1990). Furthermore, a region containing gigantopyramidal neurons, which resemble the Betz pyramidal cells of the primary motor cortex, was found in the depth of the human cingulate sulcus just behind the VCA (Braak 1976). The posterior of the two arm representations described in the caudal portion of the ACC is probably more closely related to the motor apparatus, whereas the anterior one is rather involved in some aspects of higher-order motor control. The results obtained in this study support the notion of a multiplicity of motor regions on the medial wall of the frontal lobe. These regions are organized in a distinct way in terms of 1) the output system involved (somatotopy) and 2) the specific type of motor control exercised. The somatotopic organization reflects the existence of separate skeletal and oculomotor channels linking different levels of the motor system (Alexander et al. 1990). The specific functional contribution of a given region is probably related to its connectivity with nonmotor regions of the brain. A shift in the activation pattern can be brought about by changing the task requirements. In the present study, the human ACC was activated mainly when the subject was forced to choose from a set of competing responses, rather than relying on well-established stimulus-response associations (see also Berman et al. 199 1; Corbetta et al. 199 1; Deiber et al. 199 1; Frith et al. 199 1; Pardo et al. 1990; Raichle et al. 199 1). Animal data concur with this notion in that both electrophysiological correlates of a specific behavior and the effect of lesions to the ACC diminish as the animal becomes overtrained (Gabriel 1990; Pribram et al. 1962). Two factors may be critical for task-specific activation of the ACC. First, there are the particular cognitive requirements related to the selection of the appropriate motor program. We believe that the specific computations underlying such selection are carried out within the lateral prefrontal cortex (Petrides 1989) and thence communicated to the ACC. The concomitant activation within the lateral prefrontal cortex and the ACC observed in this and other (see above) PET studies is consistent with this view. Second, there is the challenge brought about by the necessity to modify behavior in new and unpredictable situations. This may activate nonspecific modulatory systems (e.g., the mesocortical dopaminergic system), which target, among other regions, the ACC. We speculate that, at the level of the ACC, subcortical nonspecific systems modulate the processing of cognitive information coming from the lateral prefrontal cortex. In this fashion, the ACC may contribute to the funneling of cognitive commands to the motor structures. At the operational level, such a process would result CINGULATE T. PAUS, M. PETRIDES, 468 A. C. EVANS, FISCHER,B. AND BREITMEYER, B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologiu 25: 73-83, 1987. Fox, P. T., Fox, J. M., RAICHLE, M. E., AND BURDE, R. M. The role of cerebral cortex in the generation of voluntary saccades: a positron emission tomographic study. J. Neurophysiol. 54: 348-369, 1985a. Fox, P. T., MINTUN, M. A., REIMAN, E. M., AND RAICHLE, M. E. Enhanced detection of focal brain responses using intersubject averaging and distribution analysis of subtracted PET images. J. Cereb. Blood Flow Metab. 8: 642-653, 1988. Fox, P. T., PERLMUTTER, J. S., AND RAICHLE, M. E. A stereotactic method of anatomical localization for positron emission tomography. J. Comput. Assist. Tomogr. 9: 141-153, 1985b. FRIED, I., KATZ, A., MCCARTHY,G.,S~SS,K.,WILLIAMSON, P., SPENCER, S. S., AND SPENCER,D. D. Functional organization of human supplementary motor cortex studied by electrical stimulation. J. Neurosci. 11: 3656-3666, 199 1. 450: 11 l-123, 1988. MORECRA~, R. J. AND VAN HOESEN, G. W. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23~. J. Comp. Neural. 322: 47 l-489, 1992. MUAKKASSA, K. F. AND STRICK, P. L. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized “premotor” areas. Brain Res. 177: 176-182, 1979. E. MEYER MULLER-PREUSS, P. AND JURGENS, U. Projections from the “cingular” vocalization area in the squirrel monkey. Brain Res. 103: 29-43, 1976. NIELSEN, J. M. AND JACOBS,L. L. Bilateral lesions of the anterior cingulate gyri. Report of case. Bull. Los Angel. Neural. Sot. 16: 23 l-234, 195 1. NW, H. AND WATANABE, M. Cingulate unit activity and delayed response. Brain Res. 110: 38 l-386, 1976. ONO, M., KUBIK, S., AND ABERNATHEY, C. D. Atlas of the Cerebral S&i. Stuttgart, FRG: Thieme, 1990. PANDYA, D. N., VAN HOESEN, G. W., AND MESULAM, M.-M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp. Brain Res. 42: 3 19-330, 198 1. PARDO, J. V., PARDO, P. J., JANER, K. W., AND RAICHLE, M. E. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acud. Sci. USA 87: 256-259, 1990. PAUS, T., KALINA, M., PATOCKOVA, L., ANGEROVA,Y.,CERNY, R., MECIR, P., BAUER, J., AND KRABEC, P. Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain 114: 205 l-2067, 199 1. PENFIELD, W. AND JASPER,H. Epilepsy and the Functional Anatomy of the Human Bruin. Boston, MA: Little, Brown, 1954. PENFIELD, W. AND RASMUSSEN, T. The Cerebral Cortex ofMan. A Clinical Study of Localization ofFunction. New York: Macmillan, 1952. PENFIELD, W. AND WELCH, K. The supplementary motor area of the cerebral cortex. A clinical and experimental study. Arch. Neural. Psychiatr. 66: 289-317, 1951. PETERSEN,~. E.,Fox,P.T., POSNER,M.I., MINTUN, M., AND RAICHLE, M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nuture Lond. 33 1: 585-589, 1988. PETRIDES, M. Frontal lobes and memory. In: Handbook of Neuropsychology, edited by F. Boller and J. Grafman. Amsterdam: Elsevier, 1989, vol. 3, p. 75-90. PETRIDES, M. AND PANDYA, D. N. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J. Comp. Neural. 228: 105-l 16, 1984. POSNER, M. I. AND PETERSEN, S. E. The attention system of the human brain. Annu. Rev. Neurosci. 13: 25-42, 1990. POSNER,M.I., PETERSEN,~. E.,Fox,P.T., ANDRAICHLE, M.E.Localization of cognitive operations in the human brain. Science Wash. DC240: 1627-1631, 1988. PRIBRAM, K.H., WILSON, W. A., JR., ANDCONNORS, J.Effectsoflesions of the medial forebrain on alternation behavior of rhesus monkeys. Exp. Neural. 6: 36-47, 1962. RAICHLE, M. E. Circulatory and metabolic correlates of brain function in normal humans. In: Hundbook of Physiology. The Nervous System. Higher Functions of the Brain. Bethesda, MD: Am. Physiol. Sot., 1987, sect. 1, vol. V, p. 643-674. RAICHLE, M. E., FIEZ, J., VIDEEN, T. O., Fox, P. T., PARDO, J. V., AND PETERSEN, S. E. Practice-related changes in human brain functional anatomy. Sot. Neurosci. Abstr. 17: 2 1, 199 1. RAICHLE, M.E., MARTIN, W.R. W., HERSCOVITCH, P., MINTUN, M.A., AND MARKHAM, J. Brain blood flow measured with intravenous H 150 II. Implementation and validation. J. Nucl. Med. 24: 790-798, 1983. RIZZOLATI, G., CAMARDA, R., FOGASSI, L., GENTILUCCI, M., LUPPINO, G., AND MATELLI, M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp. Brain Res. 7 1: 49 l-507, 1988. ROLAND, P.E., LARSEN, B., LASSEN,N. A., ANDSKINHOJ, E.Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 43: 118- 136, 1980. SANIDES, F. Structure and function of the human frontal lobe. Neuropsychologia 2: 209-2 19, 1964. SARKISSOV, S. A., FILIMONOFF, I. N., KONONOWA, E. P., PREOBRASCHENSKAJA,I. S., AND KUKUEW, L. A. Atlas of the Cytoarchitectonics of the Human Cerebral Cortex. Moscow: Medzig, 1955. SHIMA, K.. AYA. K.. MUSHIAKE. H.. INASE, M_, AJEAW~,H, .&ND7]4NJJ,A Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J. Neurophysiol. 65: 188-202, 1991. SHOWERS,M. J. C. The cingulate gyrus: additional motor area and cortical autonomic regulator. J. Comp. Neural. 112: 23 l-287, 1959. SMITH, G. E. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol. 41: 237-254, 1907. Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017 FRITH, C. D., FRISTON, K., LIDDLE, P. F., AND FRACKOWIAK, R. S. J. Willed action and the prefrontal cortex in man: a study with PET. Proc. R. Sot. Land. Ser. B Biol. Sci. 244: 24 l-246, 199 1. GABRIEL, M. Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. In: Progress in Brain Research: The Prefrontal Cortex. Its Structure, Function and Pathology, edited by H. B. M. Uylings, C. G. Van Eden, J. P. C. de Bruin, M. A. Corner, and M. G. P. Feenstra. Amsterdam: Elsevier, 1990, vol. 85, p. 465-48 1. GOLDBERG,G., MAYER, N. H., AND TOGLIA, J. U. Medial frontal cortex infarction and the alien hand sign. Arch. Neural. 38: 683-686, 198 1. GOLDMAN-RAKIC, P. S. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook ofphysiology. The Nervous System. Higher Functions of the Brain. Bethesda, MD: Am. Physiol. Sot., 1987, sect. 1, vol V, p. 373-4 17. HALLETT, P. E. Primary and secondary saccades to goals defined by instructions. Vision Res. 18: 1279-1296, 1978. HUERTA, M. F., KRUBITZER, L. A., AND KAAS, J. H. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J. Comp. Neural. 265: 332-36 1, 1987. HUGHES, J. R. AND MAZUROWSKI, J. A. Studies of the supracallosal mesial cortex of unaesthetized, conscious mammals. II. Monkey. A. Movements elicited by electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 14: 477-485, 1962. JURGENS,U. Projections from the cortical larynx area in the squirrel monkey. Exp. Brain Res. 25: 401-4 11, 1976. JURGENS,U. AND VON CRAMON, D. On the role of the anterior cingulate cortex in phonation: a case report. Brain Lang. 15: 234-248, 1982. KIRZINGER, A. AND JURGENS, U. Cortical lesion effects and vocalization in the squirrel monkey. Brain Res. 233: 299-3 15, 1982. LEICHNETZ,G. R., SMITH, D.J., ANDSPENCER, R.F.Corticalprojections to the paramedian tegmental and basilar pons in the monkey. J. Comp. Neural. 228: 388-408, 1984. LEICHNETZ,G. R., SPENCER,R.F., HARDY,S.G. P., ANDASTRUC, J.The prefrontal corticotectal projection in the monkey: an anterograde and retrograde horseradish peroxidase study. Neuroscience 6: 1023- 104 1, 1981. LUPPINO,G.,MATELLI, M., CAMARDA, R.M., GALLESE, V., AND RIZZOLATTI, G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J. Comp. Neural. 3 11: 463-482, 199 1. LUPPINO,G.,MATELLI, M., ANDRIZZOLATTI, G.Cortico-corticalconnections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp. Brain Res. 82: 2 14-2 18, 1990. MATELLI, M., LUPPINO,G.,ANDRIZZOLATTI, G. Architectureofsuperior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J. Comp. Neural. 3 11: 445-462, 199 1. MATSUNAMI, K. AND KUBOTA, K. Radioactive deoxyglucose uptake in the prefrontal cortex during a delayed response task of the monkey. Neurosci. Lett. 36: 329-333, 1983. MACLEANP_ I’3 AND NEWM~, -J- I)_ RDJP ofm~d~~~~fi~~~~r~~~~~ .CZX= tex in production of the isolation call of squirrel monkeys. Brain Res. AND HUMAN ANTERIOR SOKOLOFF, L. General discussion. In: Exploring Brain Functional Anatomy with Positron Tomography, edited by D. J. Chadwick and J. Whelan. New York: Wiley, 199 1, p. 43-65. STANTON,G. B., DENG,S.-Y.,GOLDBERG, M.E., ANDMCMULLEN,N. T. Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. J. Comp. Neurol. 282: 415-427, 1989. SUTTON, D. AND JURGENS,U. Neural control of vocalization. In: Comparative Primate Biology. Neurosciences, edited by H. D. Steklis and J. Erwin. New York: Liss, 1988, vol. 4, p. 625-647. SUTTON, D., LARSON& AND LINDEMAN, R.C.Neocorticaland limbic lesion effects on primate phonation. Brain Res. 7 1: 6 l-75, 1974. TALAIRACH, J. AND BANCAUD, J. The supplementary motor area in man. Int. J. Neurol. 5: 330-347, 1966. TALAIRACH, J. AND TOURNOUX, P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: an Approach to Cerebral Imaging. Stuttgart, FRG: Thieme, 1988. VOGT, B. A. AND BARBAS, H. Structure and connections of the cingulate vocalization region in the rhesus monkey. In: The Physiological Control of Mammalian Vocalization, edited by J. D. Newman. New York: Plenum, 1988, p. 203-225. CINGULATE CORTEX 469 VOGT, B. A. AND PANDYA, D. N. Cingulate cortex of the rhesus monkey. II. Cortical afferents. J. Comp. Neurol. 262: 27 l-289, 1987. WEST, R. AND LARSON, C. R. Neurons of the anterior cingulate motor area are related to vocalization and other oromotor behaviors. Sot. Neurosci. Abstr. 18: 1410, 1992. WIESENDANGER,M. Recent developments in studies of the supplementary motor area in primates. Rev. Physiol. Biochem. Pharmacol. 18: l-59, 1986. WOOLSEY, C. N., SETTLAGE, P. H., MEYER, D. R., SENCER,W., PINTO HAMUY, T., AND TRAVIS, A. M. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 30: 238-264, 1952. WORSLEY& J., EVANS, A.C., MARRETT,S.,ANDNEELIN, P.Determining the number of statistically significant areas of activation in subtracted activation studies from PET. J. Cereb. Blood Flow kfetab. 12: 900-918, 1992. ZATORRE, R.J., EVANS, A.C., MEYER, E., ANDGJEDDE, A.Lateralization of phonetic and pitch discrimination in speech processing. Science Wash. DC 256: 846-849, 1992. Downloaded from http://jn.physiology.org/ by 10.220.33.4 on April 29, 2017