* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cell Density in the Border Zone Around Old Small Human Brain
Activity-dependent plasticity wikipedia , lookup
Intracranial pressure wikipedia , lookup
Emotional lateralization wikipedia , lookup
Persistent vegetative state wikipedia , lookup
Selfish brain theory wikipedia , lookup
Brain Rules wikipedia , lookup
Neuroeconomics wikipedia , lookup
Optogenetics wikipedia , lookup
Single-unit recording wikipedia , lookup
Multielectrode array wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Synaptic gating wikipedia , lookup
Subventricular zone wikipedia , lookup
Lateralization of brain function wikipedia , lookup
Development of the nervous system wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Human brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Cortical cooling wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
History of neuroimaging wikipedia , lookup
Nervous system network models wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychology wikipedia , lookup
Dual consciousness wikipedia , lookup
Aging brain wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuroanatomy wikipedia , lookup
1129
Cell Density in the Border Zone Around Old Small Human
Brain Infarcts
M.
NEDERGAARD, M . D . ,
S. VORSTRUP, M . D . ,
AND J. ASTRUP,
M.D.
SUMMARY Nine brain autopsy cases of small old cerebral infarcts were selected for neuropathological
studies. Eight of the patients had cortical infarcts, in two cases with extension into the striate body. In one
case the infarct involved the striate body only. The density of neurons and glial cells was measured in the
coronal and the horizontal planes at various distances from the margin of the infarct. Corresponding
counting points in the contralateral hemisphere served as control.
On light microscopy, the infarcted cortex was irregularly shaped, but on serial sections the bulging parts
appeared to be cut off from the Infarcted tissue ("pseudo-infarct islands"). The zone of transition from
infarcted to normal brain tissue was less than a few mm wide. In one patient, tomographic measurements of
the cerebral blood flow (CBF) and a CT scan could be compared with the neuropathological findings. In this
patient, CBF in the surroundings of the infarct was decreased despite a normal neuronal density. The study
supports the traditional view held by pathologists that a sharp transition exists between infarcted and
normal brain tissue and suggests that the hypoperfUsion zone surrounding the region of complete infarction
may be due to mechanisms other than selective loss of neurons.
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
Stroke Vol 17, No 6, 1986
A CHRONIC BRAIN INFARCT appears sharply demarcated on the CT scan. In contrast, three-dimensional imaging of the cerebral blood flow (CBF) by
xenon-133 inhalation1 shows a reduced flow in the
region of infarction and, in most cases, a large zone of
hypoperfusion around the infarct.2'3 the cause of this
hypoperfusion remains uncertain. The tissue is not ischemic in terms of a low perfusion pressure and poor
collateral filling, because in most cases the hypoperfusion is resistant to surgical revascularization.3 A state
of incomplete infarction, i.e. a selective neuronal cell
necrosis with otherwise intact glial tissue and a normal
CT appearance, has been suggested. 43 This explanation has found support from experimental studies,6"8
but in a recent study of large old infarcts in humans, we
found no evidence of selective neuronal death in the
surroundings of the infarct.9 This previous neuropathological study showed that the infarcts were sharply
demarcated from the normally structured brain. As
large infarcts represent complete necrosis of the territory of a major intracerebral artery, the sharp demarcation could represent the transition from one vascular
territory to the other.
We therefore felt it was necessary to study small
infarcts located within the territory of a major artery.
The aim was to ascertain whether evidence could be
found of selective neuronal cell death in the surroundings of minor infarcts in explanation of the hypoperfusion observed in the chronic phase.
Material and Methods
Nine brain autopsy cases of minor chronic infarcts
were selected for the study. Only cases with a single
ischemic infarct and good clinical recovery were seFrom the Institute of Neuropathology, University of Copenhagen,
and the Departments of Neurology and Neurosurgery, State University
Hospital, Rigshospitalet, Copenhagen, Denmark.
Address correspondence to: Dr. Maiken Nedergaard, Institute of
Neuropathology, University of Copenhagen, 11 Frederik V's Vej, DK2100 Copenhagen, Denmark.
Received October 23, 1985, revision # 2 accepted June 9, 1986.
lected. Eight patients died a non-cerebral death without clinical evidence of recent cerebral ischemia. One
patient died after a brain stem infarct. The patients
were selected among the total number of neuroautopsy
cases performed at the Institute of Neuropathology of
Rigshospitalet from 1979 to 1984.
After immersion fixation in formalin for at least two
weeks, the cerebral hemispheres were separated from
the brain stem and cerebellum by transection through
the midbrain. The hemispheres were cut coronally at 1
cm intervals. Cerebellum and brain stem were cut horizontally. Routine slices were taken from the frontal
and occipital lobes, the striate body, mesencephalon,
pons and medulla.
The brain from patient No. 9, in whom neuropathology could be compared with both the CT scan and the
xenon-133 tomogram, was cut into horizontal sections
to allow this comparison. The sections were placed
parallel with the plane through the inferior aspect of
the frontal lobe and the groove between the pons and
medulla oblongata. A brain-cutting box of plexiglass
was constructed for horizontal sectioning of the brain.
The brain was on a support plate which could be lifted
to adjust the plane of section according to the planes
indicated by the CT and flow measurement. A movable frame mounted with 10 knives (trimming blade,
cat. no. 02.055.00.000 Feather) allowed sectioning of
the entire brain in 10 mm thick slices.
The coronal slices from patients 1-8 and the horizontal slices from patient No. 9 were cut into blocks at
the level where the macroscopic lesion was largest. For
the estimations in the horizontal direction in patients
1-8 at least 3 blocks rostral and 3 blocks caudal to the
infarct were taken. The blocks were embedded in paraffin, and 7 /xm sections were stained by the method of
Kluver-Barrera. At least 3 counting points adjacent to
the infarct on each side were marked for cell density
measurements. Corresponding points on the contralateral hemisphere were selected for control measurements. Slides were prepared at the marked positions,
numbered in arbitrary sequence, and the cells were
STROKE
1130
VOL 17, No
6, NOVEMBER-DECEMBER
1986
TABLE 1 Summary of Age, Sex, and Major Diseases, Cause of Death, Localization of the Infarct in Main Artery Territory, Cortical and/or
Striate Involvement, Artherosclerosis, Finding of Arterial Occlusions, Size of Cortical Surface of the Infarct, Time-lapse between Stroke and
Death, and Clinical Recovery in Patients No. 1-9
Hypertension
Patient
Age
Sex
1
88
M
2
56
M
—
3
84
M
4
51
5
Diabetes
Other
diseases
Cause of death
Artery
^ ^ ^
territory
Cortical Striate
Artherosclerosis
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
Prostatic
cancer
General
deterioration
(remained conscious)
PCA
+
Severe
—
Prostatic
cancer
Acute myocardial
infarction
MCA
+
Moderate
-
-
—
Acute pulmonary
embolus
MCA
+
Moderate
M
-
-
Pulmonary
sarcoidosis
Acute respiratory
failure
MCA
+
Mild
48
M
+
+
—
Acute myocardial
failure
MCA
+
Mild
6
69
M
Stenosis of
the mitral
valve
Postoperative
cardiogenic
failure
MCA
+
+
Mild
7
76
F
Cardiac
failure
Not found
died during
hospitalization
MCA
-
+
Severe
8
19
M
—
Drowned
MCA
+
+
9
47
M
—
Brain stem
infarction
MCA
+
+
-
—
Severe
artery.
counted blindly by one of us (MN). Only nucleolated
cells with Nissl substance were defined as neurons.
The number of glial cells was found by subtracting the
neuronal cell count from the total cell count. Endothelial cells were not counted. The number of histologically intact neurons and glial cells was counted in
columns with a width of 0.40 mm measured perpendicularly to the cortical surface by means of an ocular
grid and an object micrometer. The total number of
neurons and glial cells in one column and the thickness
of the cortex at each point were measured by moving
the grid for the base to the top from every counting
point. The diameter of neurons and glial cells was
determined as the average of 40 cells in each patient by
a computerized analyser (Leitz TAS plus). The thickness of the sections was determined as the thickness of
folds. The cell numbers were corrected for section
thickness and cell diameter by means of Abercromie's
formula.10 The intra-observer agreement for counting
procedures was found by counting the same column of
cortex 10 times. Expressed as the mean and standard
deviation, values of 1566 ± 102 were found for the
total cell count and 488 ± 16 for the neuron count.
The borderline of the infarct was defined as the
outermost point with a total loss of neurons. The zone
of transition between infarcted and non-infarcted cortex was defined as the distance between zero and normal neuron count. The width of this transition zone
was measured in a radial direction from the rim of the
infarct.
After sectioning, photographs of the cut surface of
the tissue blocks before (in fixed state) and after histological preparation were taken. The lengths of 10 tis-
sue blocks and corresponding sections were compared
in each patient. The linear shrinkage of 29% ± 3%
(mean and standard variation) produced during the
histological preparation was taken into account in all
length and density measurements.
The patient's data are summarized in table 1, but
more details on patient 9 will be given. This patient
was a 47 year old man, who had been treated for
hypertension for 8 years. 23 months prior to death he
suffered an attack of right-sided weakness and dyscoordination with hemiparesthesia. Five months later
he had an episode of a day or two with motor aphasia.
CT performed after the latter episode showed a small
infarct in the left parieto-occipital region. Aortocervical arteriography revealed occlusion of the left internal
carotid artery. Measurement of CBF by xenon-133
inhalation and tomography showed extensive areas
with decreased flow in the left hemisphere. An extracranial-intracranial (EC-IC) anastomosis was performed. Postoperatively the clinical condition was
unchanged with persisting weakness of the right extremities and slight aphasia. Angiography 5 months
after shunting showed a patent but narrow anastomosis
with sparse filling of the MCA-territory, and the regional CBF was unchanged (fig. 5). Two months later
the patient suddenly experienced nausea, vertigo, and
vomiting, followed by weakness of both lower limbs
and the right arm and inability to talk. He died after 15
hours. This stroke was clinically regarded as an insult
in the brain stem.
Autopsy showed a small infarct in the posterior part
of the left MCA territory and a small infarct of older
age in the anterior part of the left caudate nucleus. In
CELL DENSITY AROUND SMALL BRAIN WFARCTS/Nedergaard et al
TABLE 1
Artery
occlusion
—
(Continued)
Size
(cm2)
Age of
infarct
Clinical
recovery
1.8
Older than
3 months
Infarct
unnoticed
4.4
3 months
Gradually
improving
Infarct
unnoticed
1.2
2.1
—
2.1
3 years
Improving
rapidly
—
0.8
9 years
Improving
rapidly
—
2.8
2 years
Gradually
improving
1 year
Very rapidly
improving
Improving
rapidly
—
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
MCA
ICA
1.4
+ 3.1
1.7
18 months
Infarct
unnoticed
addition, several small areas of encephalomalacia
were found in the pons, only one of recent date.
Atherosclerosis of the intracerebral vessels was pronounced, but no occlusions were found. The vessels on
the neck were not dissected.
Results
General Comments on the Cases
All the patients had a good clinical recovery following the infarct, and in three patients the ischemic episode had passed without admission to hospital. Only
two had permanent arterial occlusion treated in one
with an EC-IC bypass. Two patients had hypertension
and two had diabetes mellitus. All infarcts were less
than 5 cm2 measured at the cortical surface. Five were
located in the cortex only. One patient had a cortical
infarct and a smaller infarct in the caudate nucleus, two
had infarcts in the striate body and adjacent cortex, and
one had an infarct in the striate body only (table 1). In
all cases the infarcts were macroscopically sharply demarcated from the surrounding tissue.
Histological Findings
The areas of infarction were composed of small cavities traversed by fine trabeculae with only a few remaining cells. Lymphocytes grossly infiltrated the
area, but some hypertrophic macfophages were seen.
Several blood vessels traversed the spaces. A subpial
margin of cortical tissue with intensive gliosis was
often preserved. In all cases cortical as well as striatal
infarcts were sharply demarcated from the normal tissue. The zone of transition was less than a few cells in
thickness in nearly all cases. In 3 out of the 25 sections
cutting the zone of transition between the infarct and
the bordering tissue disclosed small rounded islands of
infarcted tissue. The distance between the islands and
1131
the infarct never exceeded more than a few mm. Serial
sections revealed that these islands were "periinsulas"
since, they could be traced as extensions from the
irregular bulging margin of the infarct. The astrocytes
in the surrounding tissue were both hypertrophic and
hyperplastic. No fibrosis was observed in this area, but
many astrocytic fibers were present. Irrespective of
their location or of their combination with deep infarcts
as in patients No. 6 and 7, the cortical infarcts appeared as described above. In patient No. 8 two separate parts of the cortex lying superjacent to the striate
infarct showed cortical necrosis with preservation of a
few neurons in the tissue necrosis. As seen from figure
1 the cortical thickness was reduced, but the injury was
clearly recognizable on CT despite relative preservation of the intervening white matter.
Quantitative Findings
The neuron counts were in accordance with the
histological findings, as the density of the neurons was
restored a few mm from the border of the infarct in all
cases (fig. 2). A tendency to gliosis was evident in
cases 1 and 6 (fig. 3). In no instance was a peri-infarct
zone with a reduced neuron density observed.
Patient No. 9 deserves a specific comment, as the
neuropathological findings could be compared with
the CT scan and measurement of the CBF (fig. 4). The
finding of a small infarct in the left parieto-occipital
region on the CT scan was confirmed by the neuropathological examination; the infarct appeared as a
single irregular cavity measuring 1.2-1.4 cm in diameter and containing clear fluid. It was located in the
posterior part of the MCA territory. On light microscopy it was as sharply demarcated as described above
for the cortical infarcts. The infarct in the caudate
nucleus was evident by an enlargement of the frontal
horn. CBF tomography showed a low flow in major
parts of the left hemisphere extending beyond the regions of the small cortical infarct. The flow in the periinfarct areas was reduced to 60% of the values in the
symmetrical situated regions in the opposite hemisphere.
Discussion
In the present neuropathological study of minor
chronic cerebral infarcts the transition from infarcted
to normal tissue was abrupt. "Islands" of infarcted
tissue were occasionally observed within a few mm
from the infarct. On serial sections these islahds appeared to be "pseudo-infarct islands", since they could
be traced to the infarct as ramifications from the bulging margin of the infarct. The results are in accordance
with the generally held view among pathologists, and
they are identical with our previous observations in
major chronic infarcts. 9 '"
Estimation of a few percent loss of neurons in human material is difficult. The reduced quality innate in
any autopsy material leaves the possibility that we
have overlooked a minor reduction in the neuron density in the surroundings of the infarct. Furthermore, cellular injury caused by alterations not detectable by
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
VJl
1986
6, NOVEMBER-DECEMBER
VOL 17, No
STROKE
CELL DENSITY AROUND SMALL BRAIN MFARCTS/Nedergaard et al
NIURON DENSITY
1133
NEURON DENSITY
.1.1.
•f-W'
y - . •- -:
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
0
IHFABCTXD
HEM1SPRERJ
X
OPPOSITE
HEMISPHERE
FIGURE 2. Neuron density in patients 1-8. Relationship between the neuronal density and the distance to the margin of the
infarct as measured in the frontal and the horizontal plane. The infarct is indicated on the x-axis by its zero neuron density.
Points on both sides of the two zero values are neuron densities at increasing distances from the infarct margin with the frontal
or upper point marked first on the x-axis. The counting points were projected on the outer surface of the hemisphere and the
distance along the outer surface measured on photo of the brain slices. X-axis intervals in cm, y-axis intervals in 10 neurons per
0.001 mm3. 0-0 neuron density in the hemisphere with infarct. x-x neuron density in the opposite hemisphere.
light microscopy could also explain the state of low
function. In addition, the measurements of cell density
would tend to be overestimated in case of collapse of
the tissue in the peri-infarct zone. Our findings are in
agreement with a study by Torvik and Svindland.12 In
16 stroke cases studied by them in the early phase after
onset all had only narrow zones with scattered necrotic
neurons. One patient had a 2 cm broad zone, but only
in one out of 5 sections. Recently, Metteretal 13 studied
a 69 year old man with multiple brain infarcts who died
8 days after being examined by positron emission tomography and (F-18)-fluorodeoxyglucose. Metabolic
abnormalities were greater than structural changes in
size and extent, and hypometabolism was found in
areas with normal neuron density. Lassen et al' 4 found
extensive areas of incomplete infarction in two cases
with deep infarcts on the CT scan and proximal occlusion of the MCA in the acute phase. These patients
died of a new stroke in the opposite hemisphere 3 and
34 months respectively after the first incident. Proximal occlusion of the MCA with only deep infarction
causes a severe decrease in perfusion in the corresponding cortex." The cortical tissue covering the
deep infarct may be considered as an extended infarct
border, and in cases with limited collateral capacity
this may represent a unique state in which incomplete
infarction can be recognized in large areas of the human brain.
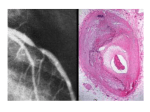
FIGURE 1. Coronal brain slide and horizontal CT scansfrompatient No. 8. A major infarct in the striate body is observed (*).
The superjacent cortex shows necrosis (arrows) with relative structural preservation of the intervening white matter. The
necrotic parts of the cortex show a marked reduction in cortical thickness. The CT scan (bottom) showed the striate infarct and a
decreased density in the areas corresponding to the cortical necrosis.
STROKE
1134
CUAL CELL DENSITY
VOL
17, No 6, NOVEMBER-DECEMBER
1986
GLLU. CEIL DENSITY
HORIZONTAL
•
VV
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
t
*',
<
\
1
Li
X INFARCTED
HDM5PHEKE
*-i\^
0 OPPOSITE
HUUSPUEKE
FIOURE 3. Glial cell density in patients 1-8. Relationship between glial cell density (y-axis), and the distance to the infarct
margin (x-axis). X-axis intervals in cm, y-axis intervals in 10 glial cells per 0.001 mm3. 0-0 glial cell density in the hemisphere
with infarct. x-x glial cell density in the opposite hemisphere.
Observations in baboons with permanent MCA occlusion are in agreement with our finding of an abrupt
infarct margin in the human brain. Symon and Brierly
found that 3 year-old infarcts in the primate brain were
sharply demarcated, although short lengths of sclerosis
with calcified neurons were sometimes observed in
cortex lying adjacent to the infarct.16 Similarly, in the
macaque monkey, permanent MCA occlusion caused
total necrosis with relatively sharp margins. In contrast, monkeys that underwent moderate to shortterm
ischemia (30 min to four hours) showed multiple lesions with incomplete tissue destruction and with predominant loss of neurons.17
In cats, experimental ischemia has not confirmed
that cerebral infarcts display an abrupt transition between necrotic and normal tissue. Garcia et al found
"red neurons" in the marginally perfused border areas
18 hours after MCA occlusion.18 Strong et al noted
shrunken neurons with dark nuclei a few hours after
MCA occlusion.7 In cats with MCA occlusion of 8
weeks' duration, Mies et al6 observed reduced neuronal density in a wide peri-infarct zone. Neuronal density was depressed in areas with normal or near-normal
levels of CBF. Ongoing studies of focal ischemia in
rats in our laboratory indicate that selective neuronal
injury takes place in a wide cortical peri-infarct zone.8
Obviously there is a discrepancy between the results
observed in humans and baboons and those observed in
smaller experimental animals.
Patients with minor stroke show a small infarct on
CT, but on measurement of the CBF in the chronic
phase a large low-flow area corresponding to the infarct and peri-infarct regions is a common finding. In
most cases, this flow defect cannot be corrected by an
EC-IC bypass.3 This indicates that the reduction of
CBF is not caused by a restriction in flow with a reduced perfusion pressure, suggesting that the flow defect is non-ischemic representing more probably regional low function and metabolism. Patient No. 9 was
such a case. Despite a patent EC-IC bypass he had an
unchanged flow pattern with decreased flow in the
infarct and peri-infarct areas. These low flow areas
appeared normal on CT and at the subsequent postmortem examination. This suggests that the peri-infarct
low flow areas, although of normal structure, probably
have a reduced function and metabolism. Deactivation
of the cortex due to undercutting of the fibers which
pass through the infarcted area could be one reason for
1135
CELL DENSITY AROUND SMALL BRAIN INFARCTS/Nedergaard et al
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
STROKE
1136
OM 5
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
the reduced flow. The deactivated neurons survive and
remain light microscopically normal — a phenomenon
well known from transplantation studies in which the
graft remains structurally normal despite "no sprouting".19 In addition, the integrity of brain function may
be compromised by damage to remote cortical areas.
The disturbance of function in the surroundings of an
infarct, as caused by direct disconnection of neuronal
pathways or by disturbed integrity, may be termed
peri-infarct diaschisis.
Our histopathological studies support the concept of
a sharp transition from infarcted to structurally normal
(although not functionally normal) brain tissue in man.
This sharp transition may be viewed in connection with
the so called flow thresholds in ischemia, which have
been discussed in association with the concept of the
ischemic penumbra.20 In acute ischemia there appears
to be a flow threshold of failure of the synaptic transmission and a distinctly lower flow threshold of energy
failure with ATP depletion and ion-pump failure causing transmembrane leak of ions and membrane depolarization. The term penumbra describes the zone of
intermediate flow between these two thresholds. It appears that the state of energy failure (as well as the state
of synaptic transmission failure) is sharply demarcated
already in the acute state: it is either present or not,
depending on whether blood flow is below or above
the threshold value. Assuming a similar flow threshold
of infarction, which may be identical with the flow
threshold of energy failure,21 the sharp demarcation of
the infarct can be understood. The infarct may well be
of an irregular shape depending on local vaso-architecture, but at the microscopic level a sharp demarcation
should be expected, reflecting the position of a critical
lowflow value in the acute state. In our view, the sharp
VOL 17, No
6, NOVEMBER-DECEMBER
1986
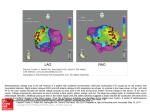
FIGURE 4. Comparative horizontal slides of
(A) CBF, (B) CT, brain section and (C) the
corresponding neuronal densities, placed 5 cm
above the orbito-meatal (OM) plane in patient
No. 9. Left side on the patient is on the left on
the figure, and right side of the patient is on the
right side of the figure. (A) top far left — the
CBF study was performed 3 months after an
EC-IC bypass and 4 months prior to death.
CBF tomography showed a reduced flow in
extensive parts of the left hemisphere. (B) bottom left — the minor cerebral infarct was identified on the CT scan and on the brain section.
Despite the large low flow areas no ischemic
cell damage was found outside the infarct. (C)
immediate left — cell count showed normal
neuron density at all points outside the infarcted cavity. Only counting points placed on the
surface of the sulci are marked on the graph,
since projection of the profound points will
overlap each other. X-axis intervals in cm, yaxis intervals in 10 neurons per 0.001 mm3. 00 neuron density in the left hemisphere, x-x
neuron density in the right hemisphere.
demarcation of the ischemic infarcts is in accordance
with the concept of the ischemic penumbra.
References
1. Stolcely EM, Sveinsdottir E, Lassen NA, Rommer P. A single
photon dynamic computer assisted tomograph (DCAT) for imaging
brain function in multiple cross sections. J Comput Assist Tomogr
4: 230-240, 1980
2. Vorstrup S, Hemmingsen R, Henriksen L, Lindewald H, Engell
HC, Lassen NA: Regional cerebral blood flow in patients with
transient ischemic attacks studied by Xenon-133 inhalation and
emission tomography. Stroke 14: 903-910, 1983
3. Vorstrup S, Lassen NA, Henriksen L, Haase J, Lauritzen M, Boysen G, Paulson O: CBF before and after extxacranial-intracranial
bypass surgery in patients with ischemic cerebrovascular disease
studied with 133Xe-inhalation tomography. Stroke 16: 616-623,
1985
4. Lassen NA: Incomplete cerebral infarction — focal incomplete
ischemic tissue necroses not leading to emollition. Stroke 13:
522-523, 1982
5. Lassen NA, Vorstrup S: Ischemic penumbra results in incomplete
infarction. Stroke 15: 755-758, 1984
6. Mies G, Auer LM, Ebhardt G, Traupe H, Heiss WD: Flow and
neuronal density in tissue surrounding chronic infarction. Stroke
14: 22-27, 1983
7. Strong AJ, Tomlinson BE, Venables GS, Gibson G, Hardy JA: The
cortical ischaemic penumbra associated with occlusion of the middle cerebral artery in the cat: 2. Studies of histopathology water
content, and in vitro neurotransmitter uptake. J Cereb Blood Flow
Metabol 3: 97-108, 1983
8. NedergaardM, DiemerNH: Sporadic neuronal necrosis in the periinfarct zone following MCA occlusion in the rat. Acta Ncurol
Scand 73: 187, 1985
9. Nedergaard M, Astrup J, Klinken L: Cell density and cortex thickness in the border surrounding old infact in the human brain. Stroke
15: 1033-39, 1984
10. Abercrombie M: Estimation of nuclear population from microtome
sections. Anat Rec 94: 239, 1946
11. Nedergaard M, Astrup J, Klinken L: No evidence of incomplete
infarction in the surroundings of larger and smaller chronic infarcts
in the MCA territory in man. Acta Neural Scand 70: 133, 1984
CELL DENSITY AROUND SMALL BRAIN INFARCTS/Nedergaard et al
12. Torvik A, Svindland A: Is there nerve cell loss in the surroundings
of brain infarcts? The penumbra zone. Acta Neurol Scand, in press
13. MetterEJ.MazziottaJC, Itabashi HH, MankovichNJ, PhelpsME,
Kuhl DE: Comparison of glucose metabolism, x-ray CT, and postmortem data in a patient with multiple cerebral infarcts. Neurology
35: 1695-1701, 1985
14. Lassen NA, Olsen TS, H0jgaard K, Skriver E: Incomplete infarction: a CT-negative irreversible ischemic brain lesion. J Cereb
Blood Flow Metab 3: 602-603, 1983
15. Skyh0j Olsen T, Larsen B, Herning M, Slcriver EB, Lassen NA:
Blood flow and vascular reactivity in collateral perfused brain
tissue. Evidence of an ischemic penumbra in patients with acute
stroke. Stroke 14: 332-342, 1983
16. Symon L, Brierley J: Morphological changes in cerebral blood
vessels in chronic infarction: flow correlation obtained by the hy-
17.
18.
19.
20.
21.
1137
drogen clearance method. The cerebral vessel wall, edited by J
Cervos-Navarro et al: pp 165-174, 1976
DeGirolami U, Crowell RM, Marcoux FW: Selective necrosis and
total necrosis in focal cerebral ischemia. Neuropathologic observations on experimental middle cerebral artery occlusion in the macaque monkey. J Neuropathol Exp Neurol 43: 57-71, 1984
Garcia JH, Lossinsky AS, Kauffman FC, Conger KA: Neuronal
ischemic injury: light microscopy, ultrastrucrure and biochemistry.
Acta Neuropathol 43: 85-95, 1978
Sunde N, Zimmer J: Transplantation of central nervous tissue. Acta
Neurol Scand 63: 323-335, 1981
Astrup J, Siesjo BK, Symon L: Thresholds in cerebral ischemia —
the ischemic penumbra. Stroke 12: 723-725, 1981
Astrup J: Energy-requinng cell functions in the ischemic brain. J
Neurosurg 56: pp 482-497, 1982
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
Restenosis and Occlusion After Carotid Surgery Assessed by
Duplex Scanning and Digital Subtraction Angiography
VERA ZBORNIKOVA, M . D . , P H . D . , * JOHAN ELFSTROM, M . D . , P H . D . , ! CLAES LASSVIK, M . D . , P H . D . , $
INGEGERD JOHANSSON, M . D . , § JAN-EDVIN OLSSON, M . D . , P H . D . , * AND U L F BJORNLERT, M.D.H
SUMMARY In a study of 140 patients operated upon with 143 carotid endarterectomies (mean follow-up
time 5.2 ± 2.3 years, range 1 month — 9.3 years), vessel morphology was examined with duplex scanning in
113 patients and with digital subtraction angiography (DSA) in 82 patients. The operative mortality was
1.4%; persisting stroke morbidity 3.6% and the combined operative mortality/morbidity 5%. During the
follow-up time a further 20 patients (14.5%) died, 13 had new strokes and 14 new TIAs. By life table
analysis, the annual rate of stroke including the operative period was 2.7% (1.7% on the operated side and
1.0% on the non-operated side). Fourteen new occlusions (12%) of the operated carotid artery was found
and restenosis (>50%) in 13 patients (11.2%). Progression of the atherosclerotic disease in the contralaterai
non-operated carotid artery was found in 41 patients (37%) including 3 new occlusions. Agreement DSA/
duplex was 88% on the operated side and 92% on the non-operated side. New strokes or TIAs on the
operated side were more common in patients with occlusions or restenosis (p < 0.05), whereas no symptoms
were referable to occlusions on the non-operated side. Risk factor analysis revealed an increased risk of
atherosclerotic progression on the non-operated side in smokers and those with two or more risk factors.
The risk of restenosis in the operated carotid artery was higher in females (p < 0.025).
Stroke Vol 17, No 6, 1986
MORPHOLOGICAL
AND
HAEMODYNAMIC
CHANGES in extracranial arteries following endarterectomy have not been extensively investigated, since
serial angiography examinations involve a certain
risk.' • 2 The rate of restenosis > 50% of diameter reduction/occlusion has previously been investigated predominantly in patients with recurrent neurological
symptoms and is reported to amount to 1-5%. w Using
continuous wave Doppler, the incidence of restenosis
> 5 0 % and occlusion has been reported to be 36% after
a mean observation time of 6 years. 6 A combination of
From the Departments of Neurology,* Surgery,t Clinical Physiology, t Diagnostic Radiology,§ University Hospital, Linkoping, and the
Department of Diagnostic Radiology,11 Motala Hospital, Motala,
Sweden.
This study was supported by grants from County Council of OstergotIand, Mutual Group Life Insurance Company, Stockholm, Tore Nilsson's Foundation, and Lion's Foundation.
Address correspondence to: VeraZbomikova, M.D., Ph.D., Department of Neurology, University Hospital, S-581 85 Linkoping, Sweden.
Received January 28, 1986, revision # 1 accepted July 7, 1986.
2-D image and pulsed Doppler, so-called duplex, offers the advantage of providing both anatomical and
haemodynamic information ahd makes possible the
detection of stenosis less than 50%.7> 8 The validity of
this method has also been demonstrated in patients
after endarterectomy in comparison with postoperative
angiogram.9 With this technique, the incidence of restenosis >50%/occlusion has been reported to be
19%10 after a mean observation time of 16 months.
A new semi-invasive method, digital subtraction angiography (DSA), has gained wide use during recent
years and is still under evaluation although hitherto the
reported results are less accurate compared to conventional angiography."" 13
The aim of the present study was to evaluate the
frequency of restenosis or occlusion after carotid endarterectomy (CE), the possible correlation between
morphological changes and recurrent symptoms and
the possible influence of vascular risk factors on these
events.
There are few published investigations on the natural course of asymptomatic carotid artery lesions.14
Cell density in the border zone around old small human brain infarcts.
M Nedergaard, S Vorstrup and J Astrup
Stroke. 1986;17:1129-1137
doi: 10.1161/01.STR.17.6.1129
Downloaded from http://stroke.ahajournals.org/ by guest on June 17, 2017
Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1986 American Heart Association, Inc. All rights reserved.
Print ISSN: 0039-2499. Online ISSN: 1524-4628
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://stroke.ahajournals.org/content/17/6/1129
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office.
Once the online version of the published article for which permission is being requested is located, click Request
Permissions in the middle column of the Web page under Services. Further information about this process is
available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Stroke is online at:
http://stroke.ahajournals.org//subscriptions/