* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Galanin-like peptide: a key player in the homeostatic regulation of
Artificial general intelligence wikipedia , lookup
Neural oscillation wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neural coding wikipedia , lookup
Environmental enrichment wikipedia , lookup
Multielectrode array wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Mirror neuron wikipedia , lookup
Development of the nervous system wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Central pattern generator wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Synaptogenesis wikipedia , lookup
Selfish brain theory wikipedia , lookup
Axon guidance wikipedia , lookup
Aging brain wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Channelrhodopsin wikipedia , lookup
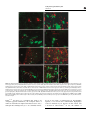
International Journal of Obesity (2011) 35, 619–628 & 2011 Macmillan Publishers Limited All rights reserved 0307-0565/11 www.nature.com/ijo REVIEW Galanin-like peptide: a key player in the homeostatic regulation of feeding and energy metabolism? S Shioda1, H Kageyama1, F Takenoya1,2and K Shiba1 1 Department of Anatomy, Showa University School of Medicine, Tokyo, Japan and 2Department of Physical Education, Hoshi University School of Pharmacy and Pharmaceutical Science, Tokyo, Japan The hypothalamus has a critical role in the regulation of feeding behavior, energy metabolism and reproduction. Galanin-like peptide (GALP), a novel 60 amino-acid peptide with a nonamidated C-terminus, was first discovered in porcine hypothalamus. GALP is mainly produced in the hypothalamic arcuate nucleus and is involved in the regulation of feeding behavior and energy metabolism, with GALP-containing neurons forming networks with several feeding-regulating peptide-containing neurons. The effects of GALP on food intake and body weight are complex. In rats, the central effect of GALP is to first stimulate and then reduce food intake, whereas in mice, GALP has an anorectic function. Furthermore, GALP regulates plasma luteinizing hormone levels through activation of gonadotropin-releasing hormone-producing neurons, suggesting that it is also involved in the reproductive system. This review summarizes the research on these topics and discusses current evidence regarding the function of GALP, particularly in relation to feeding and energy metabolism. We also discuss the effects of GALP activity on food intake, body weight and locomotor activity after intranasal infusion, a clinically viable mode of delivery. International Journal of Obesity (2011) 35, 619–628; doi:10.1038/ijo.2010.202; published online 12 October 2010 Keywords: galanin-like peptide; feeding; energy metabolism; reproduction; clinical implication Introduction Galanin, which was originally isolated from porcine upper small intestine, is a 29 amino-acid orexigenic peptide (30 in humans).1 Galanin has a widespread distribution in various brain regions such as the bed nucleus of the stria terminalis, amygdala, hippocampus, locus coeruleus, hypothalamus containing the preoptic suprachiasmatic nuclei, the supraoptic, the hypothalamic periventricular, the paraventricular (PVH) and the arcuate nucleus (ARC).2 In addition, galanin is also present in the sensory dorsal root ganglion cells, interneurons in the spinal cord and sympathetic neurons innervating the islet of Langerhans in the pancreas.3,4 The galanin receptors are a member of the G-protein-coupled receptor family and have three known subtypes, GALR1, GALR2 and GALR3. On the basis of studies using pharmacological reagents, and the GALR1 and GALR2 knockout and galanin-overexpressing transgenic animals, galanin has been implicated in stimulating feeding behavior, cognitive performance and mood through the central mechanisms.5,6 Correspondence: Dr S Shioda, Department of Anatomy, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan. E-mail: [email protected] Received 24 September 2009; revised 2 May 2010; accepted 30 July 2010; published online 12 October 2010 In 1999, galanin-like peptide (GALP) was isolated from the porcine hypothalamus by Ohtaki et al.7 on the basis of its ability to bind and activate galanin receptors in vitro. GALP is a novel 60 amino-acid peptide, the amino-acid residues (9th-21st) of which are identical with the biologically active N-terminal (1st-13th) portion of galanin,7 and its cDNA has been cloned from pig,7 rat,7,8 mouse,9,10 monkey11 and human.7 GALP can be detected in the general circulation, with a high concentration being found in the blood. Fasting for 48 h, but not for 24 h decreases both plasma GALP levels and GALP influx into the brain.12 To date, GALP has been shown to act as an agonist at all three galanin receptor subtypes in vitro, although it shows preference for GALR2 and GALR3, as compared with GALR1.7,13 There is a considerable body of evidence linking GALP to the regulation of food intake, energy metabolism and reproduction,13–19 as well as fluid intake.20,21 Furthermore, recent reports have suggested that GALP has a role in conditions such as epilepsy, Alzheimer’s disease22 and diabetes,22–24 in addition to serving a vasoactive function.25 Here, we review a number of lines of investigation that have advanced our understanding of GALP systems in the hypothalamic nuclei, including localization and regulatory studies, and suggest possible future research that may help to clarify the precise functional significance of GALP. Feeding and energy metabolism by GALP S Shioda et al 620 GALP and its receptors GALP was originally discovered as an endogenous ligand for galanin receptors in the porcine hypothalamus.7,11,26 The GALP gene comprises six exons with a structural organization similar to that of galanin (Figure 1).7,11 GALP is encoded by a distinct gene located on chromosome 19 in humans, chromosome 7 in mice and chromosome 1 in rat. GALP (1st-60th) is cleaved from the precursor peptide preproGALP, which consists of 115–120 amino acids depending on the species, with GALP residues 9–21 being homologous to residues 1–13 of galanin (Figure 2). The second region of GALP (14th-60th) is dissimilar to any other known peptides and may represent the binding portion for a GALP-specific receptor. Although the existence of a putative receptor for endogenous GALP has been suggested,27 the molecular identity of this receptor remains unknown. The members of the galanin peptide family were initially described as high-affinity agonists for both GALR1 and GALR2, with GALP binding preferentially to GALR2.7 In a later study, binding of GALP was observed at all three human galanin receptor subtypes expressed in human neuroblastoma cells, with the highest affinity being observed for GALR3, followed by GALR2; the putative fragment GALP (3rd-32nd) was also more effective than mature GALP (1st-60th) at displacing 125I-galanin.13 In situ hybridization mapping studies have shown that all three galanin receptor (GALR1–3) transcripts are present throughout the hypothalamus, including the PVH, dorsomedial nucleus (DMH), lateral hypothalamus (LHA) and periventricular nucleus, although each subtype exhibits a unique profile in terms of its abundance and distribution. In addition, GALR1 is expressed in the supraoptic nucleus and nucleus tractus solitarius.28,29 When GALP is injected centrally, GALR2-expressing neurons in the PVH do not show c-Fos expression, whereas GALR1-expressing neurons in the supraoptic nucleus display strong c-Fos expression. Thus, there are differences in the distribution of neurons activated by GALP and those expressing its receptor. These results imply the existence of an unidentified galanin receptor that may be specific for GALP. The receptor responsible for the anorectic function of GALP is currently unknown. GALP reduces food intake and body weight to the same degree in GALR1- and GALR2-deficient mice when compared with wild-type mice,30 and GALR2-deficient mice do not show abnormal Figure 1 Gene structure of GALP in mouse. GALP is located on the chromosome 7. GALP gene consists of six exons. Amino acid is represented by one letter code. EX, exon; bp, base pair; aa, amino acid. Figure 2 The primary structure of galanin and GALP. The gray area indicates a common amino acid sequence in both galanin and GALP among different organisms. International Journal of Obesity Feeding and energy metabolism by GALP S Shioda et al 621 feeding behavior.15 Furthermore, the GALR2/3 agonist (AR-M1896) has no effect on feeding or body weight in either the rat or the mouse.19 Therefore, more studies are required to determine the receptor mediating the anorectic actions of GALP but, as this peptide can act differently in rodents when compared with galanin, a new/unidentified GALP-specific receptor is likely to be involved. Distribution and localization of GALP-containing neurons Galanin is widely distributed in the brain, but GALP is found only in neurons in the hypothalamic ARC, the median eminence and the posterior pituitary gland. Using in situ hybridization, GALP mRNA has been shown to be distributed within the periventricular regions of the ARC,26,31,32 as well as in the median eminence26,31 and posterior pituitary gland of the rat.21 Larm et al.32 have reported that GALP mRNA is highly expressed in the periventricular region of the ARC, gradually increasing between postnatal days 8 and 14, before markedly increasing between days 14 and 40, which represent the weaning and pubertal period in rats.33 The expression of GALP may therefore be associated with developmental changes linked to weaning, feeding and maturation of reproductive function.33 Subsequently, based on immunohistochemical analysis using an anti-GALP monoclonal antibody, GALP-positive neuronal cell bodies have been demonstrated in the ARC, being particularly dense in its medial posterior section and in the posterior pituitary.34–36 In addition, GALP-positive cell bodies are distributed in the median eminence and in infundibular stalk, but are absent from other hypothalamic nuclei and brain loci.9,35 GALP-containing neurons in the ARC are reported to be different from the leptin receptor-expressing neurons that express neuropeptide Y (NPY)/agouti-related protein and galanin.32,36–38 GALP has been shown to be coexpressed with a-melanocyte-stimulating hormone, which is derived from pro-opiomelanocortin in neurons in the ARC,36 with orexin-1 receptor immunoreactivity also being observed in a few GALP-positive neurons.38 We have previously reported that 3–12% of GALP-positive cells contain a-melanocyte-stimulating hormone-like immunoreactivity.36 In spite of these findings, there are no other neuropeptides or transmitters that are colocalized with GALP in the ARC, suggesting that it is a unique peptide. GALP-positive fibers in the rat ARC project to several hypothalamic nuclei. There are at least two major proposed GALP neural pathways, one in which GALP-containing neurons project from the ARC to the PVH and the other in which they project to the medial preoptic area, the bed nucleus of the stria terminalis and the lateral septal nucleus (Figure 3). GALP-positive cell bodies are present from the rostral to the caudal part of the ARC, with fibers projecting to PVH, bed nucleus of the stria terminalis, medial preoptic area and lateral septal nucleus.35,39 In addition, GALP-positive fibers are found in the LHA near the fornix.39 With regard to the target for GALP-containing neurons, morphological studies have shown that GALP-positive fibers appear to have direct contact with orexin- and melaninconcentrating hormone (MCH)-containing neurons in the LHA.39 In addition, we have found that GALP-positive nerve fibers appear to make direct contact with tyrosine hydroxylase-containing neurons in the ARC,40 suggesting that GALP may interact with dopaminergic neurons in this region. At the ultrastructural level, GALP-containing neurons in the ARC receive synapses from immunonegative axon terminals that are symmetrical in the case of synapses on the perikarya, and both asymmetric and symmetric for dendritic processes.41 The axon terminals of these Figure 3 Distribution of GALP-producing neurons in the brain. GALP-positive cell bodies are located in the ARC and posterior pituitary gland. GALP-positive fibers project from the ARC to other part of the hypothalamus. International Journal of Obesity Feeding and energy metabolism by GALP S Shioda et al 622 GALP-positive neurons also often make synapses, all of which are symmetric, on immunonegative dendrites.41 Synapses are also found between axon terminals and perikarya, as well as dendrites, of GALP-positive neurons.41 GALP-positive axon terminals have been found to make synapses on orexin-positive cell bodies and dendrites, but not on MCH-positive neurons in the LHA.42 Further, in vitro studies have suggested additional targets for GALP in the hypothalamus, with activation of growth hormone-releasing hormone-containing neurons isolated from the ARC, this being reflected by increased cytosolic Ca2 þ levels.43 In electrophysiological studies of ARC neurons in hypothalamic slices, GALP was shown to inhibit excitatory and inhibitory postsynaptic currents in a similar way to galanin, whereas the two peptides differentially affected the intrinsic membrane properties, with galanin inducing hyperpolarization of the resting membrane potential and GALP having no effect.44 Galanin also suppressed spontaneous firing in the ARC, whereas GALP produced a mixture of suppression and enhancement of firing, and appeared to antagonize the galanin effects.44 Regulation of GALP neurons Morphological studies have shown that GALP-containing neurons in the ARC may be a target for the orexigenic neuropeptides, NPY and orexin. Orexin-containing neurons have been reported to make contact with GALP-positive neurons in the ARC, with 9% of these neurons expressing orexin-1 receptor protein.38 Furthermore, NPY-positive fibers project to GALP-containing neurons in the ARC and PVH.36 These morphological studies suggest a potential role for GALP in feeding and energy metabolism. GALP may exert this effect through the galanin receptors GALR1–3, and is a potential target for NPY and/or orexin. It has previously been shown that 485% of GALPcontaining neurons express the leptin receptor.35 Fasting decreases both the number of GALP-expressing neurons26 and the expression of GALP mRNA.45 Leptin administration restores GALP-expressing cells in fasted rats26 and ob/ob mice.9 It also induces a significant increase in GALP mRNA levels in the hypothalamus, and GALP mRNA levels are reduced in the hypothalamus of Zucker obese rats and db/db and ob/ob obese mice,10 with GALP mRNA expression in ob/ob mice being reversed by leptin replacement.10 These findings clearly show that leptin positively regulates GALPrelated neuronal activity in the hypothalamus. Fraley et al.23 have also demonstrated that GALP-containing neurons are responsive to changes in circulating concentrations of insulin using streptozotocin-induced diabetic rats and central injection of insulin into the fasted rat. On the basis of studies using castrated, ovariectomized, adrenalectomized and growth hormone-deficient rats, neither gonadal or adrenal steroids nor growth hormone appear to be involved in the expression of GALP mRNA in International Journal of Obesity the pituicytes of the posterior pituitary.46 Thyroidectomy in rats leads to a reduction in GALP mRNA expression, as compared with the intact control, with thyroxine replacement normalizing this expression.46 The expression of GALP mRNA is increased in the posterior pituitary of lactating rats, whereas GALP mRNA expression in the hypothalamic ARC is not affected by lactation.46 These findings suggest that GALP mRNA in the pituicytes is controlled by thyroid hormone, and that GALP mRNA is physiologically associated with the activation of oxytocin- and vasopressin-secreting neurons during lactation.46 Catecholaminergic neurons in the A1/C1 region in the medulla oblongata are glucose responsive and project to the ARC,47 and A1/C1 efferents to the hypothalamus are involved in the glucostatic regulation of GALP mRNA, feeding behavior and LH secretion.47 Neuronal network of GALP neurons Central administration of GALP activates neurons in various regions of the rat brain (Figure 4). However, different doses of GALP lead to different patterns of GALP-induced c-Fos expression. Injection of GALP into the lateral ventricle activates several brain regions, including the hypothalamic ARC, medial preoptic area, supraoptic nucleus, DMH, periventricular nucleus and LHA, as well as the nucleus tractus solitarius of the medulla.48 Furthermore, the same paradigm activates astrocytes but not microglia.48 In contrast, infusion of GALP into the third ventricle induces c-Fos expression in the caudal preoptic area, ARC, median eminence and horizontal limb of the diagonal band of Broca.49 However, it has not yet been determined what kind of neurons are activated by GALP, and further studies are required to reveal the physiological significance of the effect of GALP on both neurons and astrocytes. NPY- and orexin-containing axon terminals lie in close apposition with GALP-containing neurons in the ARC.36,38 Using a double-labeling in situ hybridization method, Cunningham et al.50 have demonstrated that GALPcontaining neurons in the macaque express both NPY Y1 and serotonin 2C receptor. In an in vitro study of GALPtreated hypothalamic explants, GALP-induced hyperphagia appeared to be mediated through an increase in NPY release and a decrease in cocaine- and amphetamine-regulated transcript (CART) release.26 However, GALP may not be the target of neurons that contain NPY and/or pro-opiomelanocortin, as these neurons do not respond to GALP in vitro.43 Orexigenic effect of GALP on feeding behavior and energy metabolism The intracerebroventricular (i.c.v.) infusion of GALP into the rat lateral ventricle increases cumulative food intake in the first 1–2 h.48,49,51 The potency of GALP is 10 times higher than that of galanin in terms of the stimulation of food Feeding and energy metabolism by GALP S Shioda et al 623 Figure 4 Dual immunofluorescence photomicrographs of neural interaction between GALP and several neuropeptides. (a) NPY-immunoreactive fibers(green) are in close apposition (arrow) with GALP-immunoreactive neurons (red) in the ARC. (b) Orexin-immunoreactive fibers (red) are in close apposed (arrow) to GALP-like immunoreactive neurons (green) in the ARC. (c) GALP-immunoreactive fibers (red) are in direct contact (arrow) with orexin-immunoreactive neurons (green) in the LH. (d) GALP-immunoreactive fibers (red) make direct contact (arrow) on MCH-immunoreactive neurons (green) in the LH. (e) aMelanocyte-stimulating hormoneimmunoreactive neurons (green) are colocalized with GALP (red) (arrow head). (f) GALP-immunoreactive neurons (green) in the ARC express orexin type-1 receptor (orexin1-R) (red). Yellow color indicates the colocalized GALP and orexin1-R (arrow head). (g) GALP-immunoreactive fibers (red) are closely apposed (arrow) to tyrosine hydroxylase (TH)-like immunoreactive neurons (green) in the ARC. (h) GALP-immunoreactive fibers (red) are closely apposed (arrow) to green fluorescent protein (GFP)-positive immunoreactive neurons (green) in the medial preoptic area of the transgenic (Tg) rat that expresses GFP driven by a LHRH promoter (LHRHGFP Tg rat). (i) GALP-positive terminals (red) in direct contact (arrow) with GFP-positive dendritic processes (green) in the LHRH-GFP Tg rat. 3V, the third ventricle. Scale bar is 20 mm. intake,49,52 and thus it is considered that GALP is an orexigenic peptide. Furthermore, the orexigenic effects of GALP are intensified in high-fat-diet-induced obese rats,53 although these findings have yet to be confirmed in mice. In an in vitro study of GALP-treated rat hypothalamic explants, it was shown that GALP-induced hyperphagia could be mediated by an increase in NPY release and a decrease in CART release.51 In vivo, the number of International Journal of Obesity Feeding and energy metabolism by GALP S Shioda et al 624 pro-opiomelanocortin mRNA-expressing cells in the ARC of the ob/ob mouse is reduced after chronic GALP injection.54 NPY release in the DMH has been proposed to have a role in mediating the effects of GALP on feeding regulation,55 although another study reported no such effect.56 Furthermore, inhibition of endogenous NPY or NPY receptors suppresses GALP-induced acute orexigenic effect in rats.55 These findings suggest that GALP promotes feeding behavior through suppression of the anorexigenic pro-opiomelanocortin system and NPY release from the DMH. Our recent experimental data suggest that the orexigenic actions of GALP are mediated by orexin in the LHA, based on the electromicroscopic observation that GALP-positive axon terminal appears to have made synapse with orexinpositive perikaryon and dendritic process in the rat LHA.39,42 Furthermore, i.c.v. injection of GALP in the rat induces expression of c-Fos in orexin-positive neurons in the LHA, and blocking the actions of orexin with an antiorexin antibody inhibits GALP-induced hyperphagia.42 However, injection of GALP into the LHA fails to stimulate feeding.55,56 One possible explanation is that the distribution of orexin is different within the LHA, which is long between rostral and caudal part.57 Therefore, it is not yet known whether a position for infusions is suitable. Further study is required to address the issue whether GALP has a direct effect on orexin-containing neurons in the LHA. GALP-containing fibers also make direct contact with MCH-containing neurons in the LHA.39 However, in contrast to orexin, i.c.v. injection of GALP fails to induce c-Fos expression in MCH neurons.42 Thus, the contribution of MCH to the orexigenic actions of GALP remains unknown. Our findings, together with those of others, suggest that GALP stimulates feeding through the release of orexin in the LHA and/or NPY in the DMH of rats. Nonetheless, the relationship between the action of GALP on these two neuropeptides needs to be clarified in further studies. Direct microinjection of GALP into the medial preoptic area or PVH also promotes food intake,56 suggesting the possibility that other nuclei such as the medial preoptic area and PVH may have roles in the regulation of feeding behavior. The receptor responsible for the orexigenic effect of GALP in rats is likely to be GALR1, as it has been shown to be expressed in the LHA and DMH,28 and in vivo studies using agonists of GALR1–3 have demonstrated that activation of GALR1 stimulates feeding behavior.29 There is no evidence to support a role for GALR2 or GALR3 in orexigenic function in rats.19,29,51 It is possible that the GALR1 mediates the transient orexigenic effects of GALP through actions on orexinand NPY-containing neurons in the LHA and DMH, respectively. Recently, it was reported that high-fat diet-fed GALPdeficient mice gain less body weight compared with wildtype animals.58 Moreover, GALP-deficient mice do not change the balance of energy intake and expenditure under normal nutritional conditions,58 suggesting that GALP may regulate energy balance to imposed nutritional circumstances.58 International Journal of Obesity Anorexigenic effect of GALP on feeding behavior and energy metabolism In experiments with rat and mouse, both body weight gain and food intake were reduced at 24 h after injection of GALP.52,59 In genetically obese rodents, such as the ob/ob, db/db mice and Zucker rats (fa/fa), both gene and/or peptide expression of GALP is undetectable in the ARC9,10,60,61 as well as in hypoleptinemic diabetic animals, and in rats that are acutely or chronically deprived of food.23,61 Although some studies using rat, but not mouse, have indicated a transient increase in food intake after i.c.v. injection of GALP, it is possible that decrease in GALP level in the ARC causes obesity. Thus, it is possible that GALP has a physiologically important role in anorexigenic action.18,47 Interestingly, GALP can be detected in the general circulation, and fasting decreases both plasma levels and GALP influx into the brain.12 These findings suggest that GALP conveys a satiety signal from peripheral organs to brain, although there are few studies mentioning the presence of GALP-producing cells in the peripheral organs. Central and/ or peripheral infusion of leptin restores to normal level of the expression of GALP mRNA in the ARC of fasted rats and leptin-deficient obese ob/ob mice.9,10,26 Leptin directly upregulates GALP neuronal activity in the hypothalamus, as more than 85% of GALP neurons express leptin receptors in the ARC of rats and macaques.23,35 However, the effect of elevated endogenous leptin on GALP expression in the normal animals is less clear. Although rats fed a diet high in either saturated or polyunsaturated fats become hyperleptinemic, GALP mRNA is only increased in the ARC of the obese rat fed a high polyunsaturated fat diet 62. This result indicated that apart from leptin, GALP mRNA expression level might be affected by various forms of dietary fat. Although the injection of GALP into the lateral ventricle results in a decrease in food intake in the first hour after infusion in mice, this is not the case for rats.59 Krasnow et al.59 speculate that the observed recovery of food intake and body weight are attributable to the activation of a compensatory neural feeding pathway, driving the animals to eat in an attempt to restore body weight back to normal. Although the i.c.v. injection of GALP into ob/ob mice initially decreases both food intake and body weight gain,54 chronic GALP injection, results in a sustained decrease in body weight and an increase in core body temperature, despite the recovery of food intake.54 In a pair-fed study, chronic GALP administration produced a greater decrease in body weight than that which occurred in the control.54 Taken together, these findings lead us to theorized that GALP-positive neurons in the ARC are a target for leptin, and that GALP could be used as a replacement for leptin. Core body temperature increased in a dose-dependent manner, and this increase was maintained for 6–8 h after injection of GALP.52 Cytokines such as interleukin-1 (IL-1) and IL-6 are important mediators of inflammation that induce fever by the release of prostaglandins in the Feeding and energy metabolism by GALP S Shioda et al 625 hypothalamus. Central administration of GALP induced c-Fos expression in astrocytes or neurons in the peri-third ventricle. Astrocytes express cyclooxygenase, which is a ratelimiting enzyme for the biosynthesis of prostaglandins.48 Pretreatment of cyclooxygenase inhibitor completely blocks GALP-induced thermogenesis.52 Moreover, the studies using IL-1a, IL-1b or IL-1 type-I receptor-deficient mice demonstrates that GALP-induced thermogenesis, body weight loss and anorexic actions are mediated by IL-1 type-I receptor.63 Table 1 Leptin reduces food intake and body weight, but increases the core body temperature in rodents, partly through IL-1 system.64 These findings suggest that proinflammatory cytokines mediate the thermogenesis by GALP.18,65 Brown adipose tissue is recognized as the primary site of thermogenesis, and potentially functions to regulate body weight in rodents.66 GALP treatment has been shown to increase both uncoupling protein-1 mRNA and protein expression in brown adipose tissue thermogenesis in the Distribution and physiological function of GALP Constituent amino acid residues Receptor Localization (cell body) Physiological action Leptin injection Insulin injection Effects of GALP Reference 60 GALR1 (IC50 ¼ 4.3 nM* or 77 nM**) GALR2 (IC50 ¼ 0.24 nM* or 28 nM**) GALR3 (IC50 ¼ 10 nM**) Specific receptor ? The arcuate nucleus, the median eminence (rat) The posterior pituitary gland Increase in LH secretion (rat, mouse, monkey) Increase in testosterone secretion (mouse) Suppression of TSH secretion (rat) Increase followed by decrease in food intake (rat) Suppression of food intake (mouse) Suppression of body weight Increase in heat production Suppression of locomotor activity (mouse) Induction of taste aversion (mouse) Increase in the gene expression of GALP Increase in the gene expression of GALP Ohtaki et al.7 *Ohtaki et al.7, **Lang et al.13 *Ohtaki et al.7, **Lang et al.13 **Lang et al.13 Juréus et al.26, Kerr et al.31, Larm and Gundlach32; Takatsu et al.35 Shen et al.21, Kerr et al.31, Fujiwara et al.34 Seth et al.8, Cunningham et al.50, Krasnow et al.59 Krasnow et al.59 Seth et al.51 Matsumoto et al.49, Lawrence et al.52, Krasnow et al.59 Krasnow et al.59 Lawrence et al.48, Krasnow et al.59 Lawrence et al.48, Lawrence et al.52, Hansen et al.54 Krasnow et al.59 Krasnow et al.59 Fraley et al.23, Juréus et al.26 Fraley et al.23 Abbreviations: GALP, galanin-like peptide; IC50, half maximal inhibitory concentration; LH, luteinizing hormone; TSH, Thyroid-stimulating hormone. *Correspond to reference 7. **Correspond to reference 13. Figure 5 Schematic illustration of the putative appetite-regulating neuronal circuit involving GALP in the hypothalamus. GALP neurons modulate body weight, feeding intake, thermogenesis and reproduction through several neuropeptide and hormones producing neurons. ?, Unknown; 3V, the third ventricle; Arc, arcuate hypothalamic nucleus; Leptin-R, leptin receptor; LH, lateral hypothalamic area; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NTS, the nucleus of the solitary tract; orexin1-R, orexin type-1 receptor; þ , physiological activation; , physiological suppression; VMH, ventromedial hypothalamic nucleus. International Journal of Obesity Feeding and energy metabolism by GALP S Shioda et al 626 ob/ob mouse.54 These findings suggest that GALP may partly mediate energy metabolism through thermogenesis by long-term activation of the sympathetic nervous system. Polysaccharide was reported to induce GALP mRNA expression in the rat posterior pituitary, suggesting that GALP may also be involved in exacerbated thermogenesis in response to inflammatory stress.67 Thus, GALP can have both an anorectic and a catabolic action. The hypothalamo–pituitary–thyroid axis and activation of uncoupling protein-1 by way of sympathetic nervous system stimulation are known to influence thermogenesis and basal metabolic rate.68,69 The PVH, which is associated with the regulation of food ingestion and contains numerous thyrotropin-releasing-hormone-containing neurons,70 acts as the central hypothalamic regulator of the hypothalamo–pituitary– thyroid axis. Similar to other orexigenic peptides such as NPY71 and agouti-related protein,72 administration of GALP into the PVH decreases the level of plasma thyroid-simulating hormone within 10 min.51 In other words, orexigenic peptides exhibit an anabolic action by the suppression of thyroid hormone release. However, GALP has no effect on the release of thyroid-simulating hormone directly from dispersed rat pituitary cells. Although GALP inhibits the release of thyrotropin-releasing hormone from hypothalamic explants in vitro, GALP-positive nerve terminals are associated with few thyrotropin-releasing hormone neurons in the medial parvocellular subdivision of the PVH.70 These findings suggest that, although GALP has an anabolic action during an acute phase, it may influence the hypothalamo–pituitary–thyroid axis through an indirect mechanism to suppress circulating thyroid-simulating hormone levels. Recently, our group has been investigating the intranasal delivery of GALP into the brain in order to determine the potential clinical efficacy based on anorexigenic effect of GALP. Intranasal infusion provided a 40-to 100-fold improvement in targeting brain versus peripheral tissue compared with intravenous infusion.73 This study indicates that intranasal administration is an effective route for the delivery of GALP into the brain.73 We have also been studying the physiological effects of intranasal infusion of GALP on body weight, food intake, water intake and locomotor activity in mice. Intranasal infusion of GALP significantly reduced body weight over the course of a week. However, food and water intake, as well as locomotor activity, remained unchanged (Shiba et al., manuscript in preparation). Our results strongly suggest that intranasal administration of GALP will be useful for obese people to control obesity and life-style-related diseases in the future. However, the authors emphasize that the mechanism of body weight loss by intranasal administration of GALP remains to be clarified. Conclusion GALP has dichotomous actions on energy balance in rat (transient hyperphagia followed by reduction of food intake International Journal of Obesity and body weight), in contrast to acting as a reducing agent in both feeding behavior and body weight loss in mouse. Transient orexigenic actions of GALP are mediated through NPY and orexin pathway in the rat. The long-term anorectic and thermogenesis of GALP are mediated through proinflammatory pathway in rodents. The findings of the studies reviewed here highlight the fact that GALP is involved in numerous physiological processes, including feeding behavior and energy metabolism (Table 1), through neuronal networks in the hypothalamus (Figure 5). Further elucidation of the functions of GALP is important for the discovery of therapeutic drugs, and for the treatment and prevention of obesity and related disorders. Conflict of interest The authors declare no conflict of interest. Acknowledgements We thank Dr Tetsuya Ohtaki of the Takeda Pharmaceutical Company Ltd. (Japan) and Ms Sachi Kato for their support in completing this work. We are also grateful to Dr Randeep Rakwal (Showa University) for his help in reading the manuscript. This study was supported, in part, by the High-Technology Research Center Project from the Ministry of Education, Sports, Science and Technology (Japan). References 1 Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin – a novel biologically active peptide from porcine intestine. FEBS Lett 1983; 164: 124–128. 2 Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides 1985; 6: 509–546. 3 Melander T, Hokfelt T, Rokaeus A, Fahrenkrug J, Tatemoto K, Mutt V. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res 1985; 239: 253–270. 4 Skofitsch G, Jacobowitz DM. Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res Bull 1985; 15: 191–195. 5 Lundstrom L, Elmquist A, Bartfai T, Langel U. Galanin and its receptors in neurological disorders. Neuromolecular Med 2005; 7: 157–180. 6 Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci 2008; 65: 1796–1805. 7 Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M et al. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem 1999; 274: 37041–37045. 8 Seth A, Stanley S, Jethwa P, Gardiner J, Ghatei M, Bloom S. Galanin-like peptide stimulates the release of gonadotropinreleasing hormone in vitro and may mediate the effects of leptin on the hypothalamo-pituitary-gonadal axis. Endocrinology 2004; 145: 743–750. Feeding and energy metabolism by GALP S Shioda et al 627 9 Juréus A, Cunningham MJ, Li D, Johnson LL, Krasnow SM, Teklemichael DN et al. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology 2001; 142: 5140–5144. 10 Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology 2003; 144: 2634–2643. 11 Cunningham MJ, Scarlett JM, Steiner RA. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 2002; 143: 755–763. 12 Kastin AJ, Akerstrom V, Hackler L. Food deprivation decreases blood galanin-like peptide and its rapid entry into the brain. Neuroendocrinology 2001; 74: 423–432. 13 Lang R, Berger A, Santic R, Geisberger R, Hermann A, Herzog H et al. Pharmacological and functional characterization of galaninlike peptide fragments as potent galanin receptor agonists. Neuropeptides 2005; 39: 179–184. 14 Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 2007; 86: 175–182. 15 Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends Endocrinol Metab 2004; 15: 215–221. 16 Gundlach AL. Galanin/GALP and galanin receptors: role in central control of feeding, body weight/obesity and reproduction? Eur J Pharmacol 2002; 440: 255–268. 17 Kageyama H, Takenoya F, Kita T, Hori T, Guan JL, Shioda S. Galanin-like peptide in the brain: effects on feeding, energy metabolism and reproduction. Regul Pept 2005; 126: 21–26. 18 Lawrence CB. Galanin-like peptide modulates energy balance by affecting inflammatory mediators? Physiol Behav 2009; 97: 515–519. 19 Man PS, Lawrence CB. Galanin-like peptide: a role in the homeostatic regulation of energy balance? Neuropharmacology 2008; 55: 1–7. 20 Kawasaki M, Saito J, Hashimoto H, Suzuki H, Otsubo H, Fujihara H et al. Induction of the galanin-like peptide gene expression in the posterior pituitary gland after acute osmotic stimulus in rats. Neurosci Lett 2007; 419: 125–130. 21 Shen J, Larm JA, Gundlach AL. Galanin-like peptide mRNA in neural lobe of rat pituitary. Increased expression after osmotic stimulation suggests a role for galanin-like peptide in neuronglial interactions and/or neurosecretion. Neuroendocrinology 2001; 73: 2–11. 22 Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther 2007; 115: 177–207. 23 Fraley GS, Scarlett JM, Shimada I, Teklemichael DN, Acohido BV, Clifton DK et al. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes 2004; 53: 1237–1242. 24 Stoyanovitch AG, Johnson MA, Clifton DK, Steiner RA, Fraley GS. Galanin-like peptide rescues reproductive function in the diabetic rat. Diabetes 2005; 54: 2471–2476. 25 Schmidhuber SM, Santic R, Tam CW, Bauer JW, Kofler B, Brain SD. Galanin-like peptides exert potent vasoactive functions in vivo. J Invest Dermatol 2007; 127: 716–721. 26 Juréus A, Cunningham MJ, McClain ME, Clifton DK, Steiner RA. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology 2000; 141: 2703–2706. 27 Cunningham MJ. Galanin-like peptide as a link between metabolism and reproduction. J Neuroendocrinol 2004; 16: 717–723. 28 Mitchell V, Habert-Ortoli E, Epelbaum J, Aubert JP, Beauvillain JC. Semiquantitative distribution of galanin-receptor (GAL-R1) mRNA-containing cells in the male rat hypothalamus. Neuroendocrinology 1997; 66: 160–172. 29 Wang S, Ghibaudi L, Hashemi T, He C, Strader C, Bayne M et al. The GalR2 galanin receptor mediates galanin-induced jejunal contraction, but not feeding behavior, in the rat: differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett 1998; 434: 277–282. 30 Krasnow SM, Hohmann JG, Gragerov A, Clifton DK, Steiner RA. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology 2004; 79: 268–277. 31 Kerr NC, Holmes FE, Wynick D. Galanin-like peptide (GALP) is expressed in rat hypothalamus and pituitary, but not in DRG. Neuroreport 2000; 11: 3909–3913. 32 Larm JA, Gundlach AL. Galanin-like peptide (GALP) mRNA expression is restricted to arcuate nucleus of hypothalamus in adult male rat brain. Neuroendocrinology 2000; 72: 67–71. 33 Kawagoe R, Yamamoto Y, Kubo K, Dobashi K, Asayama K, Ueta Y et al. Postnatal development of galanin-like peptide mRNA expression in rat hypothalamus. Regul Pept 2008; 145: 133–140. 34 Fujiwara K, Adachi S, Usui K, Maruyama M, Matsumoto H, Ohtaki T et al. Immunocytochemical localization of a galanin-like peptide (GALP) in pituicytes of the rat posterior pituitary gland. Neurosci Lett 2002; 317: 65–68. 35 Takatsu Y, Matsumoto H, Ohtaki T, Kumano S, Kitada C, Onda H et al. Distribution of galanin-like peptide in the rat brain. Endocrinology 2001; 142: 1626–1634. 36 Takenoya F, Funahashi H, Matsumoto H, Ohtaki T, Katoh S, Kageyama H et al. Galanin-like peptide is co-localized with alphamelanocyte stimulating hormone but not with neuropeptide Y in the rat brain. Neurosci Lett 2002; 331: 119–122. 37 Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 1998; 18: 559–572. 38 Takenoya F, Aihara K, Funahashi H, Matsumoto H, Ohtaki T, Tsurugano S et al. Galanin-like peptide is target for regulation by orexin in the rat hypothalamus. Neurosci Lett 2003; 340: 209–212. 39 Takenoya F, Hirayama M, Kageyama H, Funahashi H, Kita T, Matsumoto H et al. Neuronal interactions between galaninlike-peptide- and orexin- or melanin-concentrating hormonecontaining neurons. Regul Pept 2005; 126: 79–83. 40 Kageyama H, Takenoya F, Hori Y, Yoshida T, Shioda S. Morphological interaction between galanin-like peptide- and dopaminecontaining neurons in the rat arcuate nucleus. Regul Pept 2008; 145: 165–168. 41 Guan JL, Kageyama H, Wang QP, Takenoya F, Kita T, Matsumoto H et al. Electron microscopy examination of galanin-like peptide (GALP)-containing neurons in the rat hypothalamus. Regul Pept 2005; 126: 73–78. 42 Kageyama H, Kita T, Toshinai K, Guan JL, Date Y, Takenoya F et al. Galanin-like peptide promotes feeding behaviour via activation of orexinergic neurones in the rat lateral hypothalamus. J Neuroendocrinol 2006; 18: 33–41. 43 Kuramochi M, Kohno D, Onaka T, Kato S, Yada T. Galanin-like peptide and ghrelin increase cytosolic Ca2+ in neurons containing growth hormone-releasing hormone in the arcuate nucleus. Regul Pept 2005; 126: 85–89. 44 Dong Y, Tyszkiewicz JP, Fong TM. Galanin and galanin-like peptide differentially modulate neuronal activities in rat arcuate nucleus neurons. J Neurophysiol 2006; 95: 3228–3234. 45 Mitchell V, Bouret S, Howard AD, Beauvillain JC. Expression of the galanin receptor subtype Gal-R2 mRNA in the rat hypothalamus. J Chem Neuroanat 1999; 16: 265–277. 46 Cunningham MJ, Krasnow SM, Gevers EF, Chen P, Thompson CK, Robinson IC et al. Regulation of galanin-like peptide gene expression by pituitary hormones and their downstream targets. J Neuroendocrinol 2004; 16: 10–18. 47 Fraley GS. Immunolesions of glucoresponsive projections to the arcuate nucleus alter glucoprivic-induced alterations in food intake, luteinizing hormone secretion, and GALP mRNA, International Journal of Obesity Feeding and energy metabolism by GALP S Shioda et al 628 48 49 50 51 52 53 54 55 56 57 58 59 60 but not sex behavior in adult male rats. Neuroendocrinology 2006; 83: 97–105. Lawrence CB, Williams T, Luckman SM. Intracerebroventricular galanin-like peptide induces different brain activation compared with galanin. Endocrinology 2003; 144: 3977–3984. Matsumoto Y, Watanabe T, Adachi Y, Itoh T, Ohtaki T, Onda H et al. Galanin-like peptide stimulates food intake in the rat. Neurosci Lett 2002; 322: 67–69. Cunningham MJ, Shahab M, Grove KL, Scarlett JM, Plant TM, Cameron JL et al. Galanin-like peptide as a possible link between metabolism and reproduction in the macaque. J Clin Endocrinol Metab 2004; 89: 1760–1766. Seth A, Stanley S, Dhillo W, Murphy K, Ghatei M, Bloom S. Effects of galanin-like peptide on food intake and the hypothalamo-pituitary-thyroid axis. Neuroendocrinology 2003; 77: 125–131. Lawrence CB, Baudoin FM, Luckman SM. Centrally administered galanin-like peptide modifies food intake in the rat: a comparison with galanin. J Neuroendocrinol 2002; 14: 853–860. Tan HM, Gundlach AL, Morris MJ. Exaggerated feeding response to central galanin-like peptide administration in diet-induced obese rats. Neuropeptides 2005; 39: 333–336. Hansen KR, Krasnow SM, Nolan MA, Fraley GS, Baumgartner JW, Clifton DK et al. Activation of the sympathetic nervous system by galanin-like peptide-a possible link between leptin and metabolism. Endocrinology 2003; 144: 4709–4717. Kuramochi M, Onaka T, Kohno D, Kato S, Yada T. Galanin-like peptide stimulates food intake via activation of neuropeptide Y neurons in the hypothalamic dorsomedial nucleus of the rat. Endocrinology 2006; 147: 1744–1752. Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, Ghatei MA et al. Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol 2006; 18: 742–747. Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res 1999; 827: 243–260. Dungan Lemko HM, Clifton DK, Steiner RA, Fraley GS. Altered response to metabolic challenges in mice with genetically targeted deletions of galanin-like peptide. Am J Physiol Endocrinol Metab 2008; 295: E605–E612. Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology 2003; 144: 813–822. Saito J, Ozaki Y, Kawasaki M, Ohnishi H, Okimoto N, Nakamura T et al. Galanin-like peptide gene expression in the hypothalamus International Journal of Obesity 61 62 63 64 65 66 67 68 69 70 71 72 73 and posterior pituitary of the obese fa/fa rat. Peptides 2004; 25: 967–974. Shen J, Gundlach AL. Galanin-like peptide mRNA alterations in arcuate nucleus and neural lobe of streptozotocin-diabetic and obese zucker rats. Further evidence for leptin-dependent and independent regulation. Neuroendocrinology 2004; 79: 327–337. Dziedzic B, Szemraj J, Bartkowiak J, Walczewska A. Various dietary fats differentially change the gene expression of neuropeptides involved in body weight regulation in rats. J Neuroendocrinol 2007; 19: 364–373. Man PS, Lawrence CB. Interleukin-1 mediates the anorexic and febrile actions of galanin-like peptide. Endocrinology 2008; 149: 5791–5802. Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA 1999; 96: 7047–7052. Saito J, Ozaki Y, Kawasaki M, Ohnishi H, Okimoto N, Nakamura T et al. Induction of galanin-like peptide gene expression in the arcuate nucleus of the rat after acute but not chronic inflammatory stress. Brain Res Mol Brain Res 2005; 133: 233–241. Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J 1990; 4: 2890–2898. Saito J, Ozaki Y, Ohnishi H, Nakamura T, Ueta Y. Induction of galanin-like peptide gene expression in the rat posterior pituitary gland during endotoxin shock and adjuvant arthritis. Brain Res Mol Brain Res 2003; 113: 124–132. Freake HC, Oppenheimer JH. Thermogenesis and thyroid function. Annu Rev Nutr 1995; 15: 263–291. Yoshitomi H, Yamazaki K, Abe S, Tanaka I. Differential regulation of mouse uncoupling proteins among brown adipose tissue, white adipose tissue, and skeletal muscle in chronic beta 3 adrenergic receptor agonist treatment. Biochem Biophys Res Commun 1998; 253: 85–91. Wittmann G, Sarkar S, Hrabovszky E, Liposits Z, Lechan RM, Fekete C. Galanin- but not galanin-like peptide-containing axon terminals innervate hypophysiotropic TRH-synthesizing neurons in the hypothalamic paraventricular nucleus. Brain Res 2004; 1002: 43–50. Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol 1991; 260: R321–R327. Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG et al. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes 2001; 50: 248–254. Nonaka N, Farr SA, Kageyama H, Shioda S, Banks WA. Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J Pharmacol Exp Ther 2008; 325: 513–519.