* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Horizontal Interactions in Cat Striate Cortex: 1. Anatomical Substrate
Holonomic brain theory wikipedia , lookup
Affective neuroscience wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Metastability in the brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Development of the nervous system wikipedia , lookup
Time perception wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Apical dendrite wikipedia , lookup
Optogenetics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptic gating wikipedia , lookup
Environmental enrichment wikipedia , lookup
Aging brain wikipedia , lookup
Human brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuroanatomy wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroesthetics wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Superior colliculus wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
0 European Neuroscience Association O953-81&/90 $3.00
European Journal of Neuroscience, Vol. 2, pp. 344-357
Horizontal Interactions in Cat Striate Cortex:
1. Anatomical Substrate and Postnatal Development
H. J. Luhmann’, W. Singer2, and L. Martinez-Millan3
‘Universitat Koln, Physiologisches Institut, Albertus-Magnus-Platz,D-5000 Koln 41, FRG
2Max-Planck-lnstitutfur Hirnforschung, Deutschordenstr. 46, D-6000 Frankfurt a.M. 71, FRG
3Department of Anatomy, Faculty of Medicine, Lejona (Bilbao), Spain
Key words: visual cortex, intrinsic circuitry, development, deprivation
Abstract
The system of tangential connections was studied in area 17 of normally reared (NR), binocularly deprived
(BD) and dark-reared (DR) kittens and adult cats. Connections were labelled antero- and retrogradely by
intracortical micro-injections of several fluorescent markers and horseradish peroxidase conjugated with
wheat-germ agglutinin (WGA-HRP). In 5-day-old kittens tangential connections consist of homogeneously
distributed fibres extending maximally over 2.7 mm. Around postnatal day (pnd) ten these connections start
to express the patchy pattern characteristic of the adult. Retrogradely stained somata and anterogradely
labelled terminals become organized in individual 300 to 350 pm wide clusters with a centre-to-centre spacing
of about 500 pm. During the first three postnatal weeks the horizontal connections increase their span to up
to 10.5 mm and the spacing between individual patches increases to about 700 prn. Over the following
4 weeks these projections become reduced in length and number. In adult NR cats, tangential connections
span a distance of up to 3 mm and form a lattice of 200 - 500 pm wide clusters, which have an average
centre-to-centre spacing of 1050 pm. Tangential connections originate and terminate in all cortical laminae
except layer I and they are organized in register. The distances spanned are largest in supragranular,
intermediate in infragranular and shortest in granular layers. In BD and DR cats older than 10 weeks, the
length of intracortical tangential fibres becomes reduced to the same extent as in NR animals, but individual
clusters are less numerous. The authors conclude that the lattice-like structure of lateral connections evolves
independently of visual experience, and that the selectivity of interactions results from pruning of initially
exuberant connections. It is suggested that this pruning process is dependent on activity and influenced by
visual experience.
Introduction
It remains undetermined how the sensory input from subcortical
structures is transformed within the neocortex to produce the highly
specific functional response properties of individual neurons. Intrinsic
lateral interactions have been proposed to be involved in the generation
of receptive field properties, such as large and elongated excitatory
regions, inhibitory side-bands and end-zones, multiple widely separated
discharge areas and finally, the selectivity for stimulus orientation and
direction of movement (for review see Gilbert, 1985; Mitchison and
Crick, 1982; Sillito, 1984; Singer, 1985a,b; Swindale, 1982; Wiesel
and Gilbert, 1983). Most of these properties undergo characteristic
alterations during the first postnatal weeks and are highly sensitive to
manipulations of early visual experience (for review see Blakemore,
1977; Frtgnac and Imbert, 1984). In the following series of reports
the authors present anatomical and physiological data on the postnatal
development of projections within cat striate cortex and discuss their
relation to other columnar systems and their functions in specific
neocortical operations.
Correspondence to: W . Singer, as above
Received I 1 April 1989, revised 5 December 1989, accepted I 1 December 1989
Qualitative observations made in some of the animals included in
this study have been reported previously (Luhmann et al., 1986) and
part of the present results have been presented in abstract form
(Luhmann et al., 1987).
Materials and methods
Animals and rearing procedures
This study is based on 39 cats, which were raised under different
conditions of visual experience and were analysed at different postnatal
ages (Table 1). Most animals were bred in the cat colony of the institute,
some were obtained from a commercial SPF breeding colony.
The postnatal development of horizontal intrinsic connections in
normally reared (NR) animals was investigated in 2 1 kittens between
5 and 118 days of age (NROI -NR21) and in seven adult cats, which
were older than 150 days (NR22-NR28). The effect of binocular
deprivation (BD) was studied in six animals aged 35 to 145 days
Development of lateral connections in cat area 17 345
TABLE
I . Experimental animals tabulated in 3 different blocks according to the
rearing conditions described in the text
Animal
NROI
NR02
NR03
NR04*
NR05
NR 06*
NR 07
NR 08
NR 09
NR 10
NR I I *
NR 12*
NR 13*
NR 14"
NR 15
NR 16*
NR 17
NR 18
NR 19*
NR 20
NR 21"
NR 22
NR 23
NR 24*
NR 25
NR 26*
NR 27*
NR 28*
Age at
injection
[pndl
3
3
3
8
9
15
17
18
18
21
22
23
26
36
40
42
1:14,r:43
1:14,r:50
59
1:60,r:62
115
adult
adult
adult
adult
adult
adult
adult
33
41
Age at
perfusion
[pndl
5
5
5
10
I1
17
20
21
21
22
24
25
28
39
42
44
46
53
61
64
118
adult
adult
adult
adult
adult
adult
adult
35
43
106
133
138
145
adult
adult
BD 01*
BD02*
BD 03*
BD04
BD 05
BD 06
BD 07*
BD 08*
131
135
142
adult
adult
DR01
DR 02
DR 03
73
69
80
76
1: 108,r: 110 112
104
+3
+3
+2
+2
+2
+2
+2
+
+
No. of injections
X
dye
Left hemisphere
Right hemisphere
1 xRBs
F
S
F
T
T
T
F
T
T
S
T
T
T
F
T
T
S
S
T
S
S
F
F
T
T
T
I x H R P , IXRBs
1 xRBs
1xHRP
I xHRP
1xHRP
1 XRBs, 1 XFB
1 xRBs
1 xHRP
1 xHRP
I XHRP
1x H R P
I xHRP
1xHRP
2xHRP
2xHRP
I xRBs I DY
I xRBs
2XHRP
1 xRBs
I xHRP
IxRBs
IxRBs
IXHRP
2xHRP
2xHRP
-
2xHRP
1x H R P
1x H R P
2 xHRP
1 XHRP
1 XDY
I XDY
2 2xHRP
2 2xHRP
1xFG
1 XHRP
1 xRBs
IXHRP
IxHRP
IxHRP
IxHRP
IxHRP
lxHRP
IXDY
IXRBs
IXHRP
lxHRP
IXHRP
IXHRP
IxHRP
lxDY
2xHRP
2xHRP
lxDY
IxFG
2xHRP
IxHRP
lxDY
IXHRP
2xHRP
IxHRP
2xHRP
2xHRP
1 xHRP
T IXHRP
T
T
T
T
S
S
S
T
T
T
IxHRP
IXHRP
2xHRP
IxHRP
2xHRP
lxFG
2xHRP
2xHRP
S
F
S
T
T
F
F
F
T
T
T
T
F
T
T
S
F
T
S
F
T
T
T
T
T
T
T
T
T
T
S
S
T
T
F IXDY
S
S IxRBsIDY F
T
F IxHRP
Animals labelled with (*) were investigated in a previous study (Luhmann et al.,
1986) and NR cats marked with (") were kept in the dark between injection
and perfusion. The age at injection and perfusion is given for each animal in
postnatal days (pnd). For adult cats the survival time after injection is given
in additional days. Columns 4 and 5 indicate the number of injections, the kind
of injected dye and the cutting plane for the left and right hemipshere,
respectively. Abbrevations: NR, normally reared; BD, binocularly deprived;
DR, dark-reared; I , left hemisphere; r, right hemisphere; F, frontal; S, sagittal;
T, tangential. For other abbreviations see text.
(BW1 -BD06) and in two adult cats (BD07-BW8). In kittens of this
experimental group, at the beginning of the second postnatal week the
eyelids of both eyes were sutured closed under ketamine hydrochloride
(30 mg/kg) and xylazine hydrochloride (15 mglkg) anaesthesia. The
suture was examined daily and immediately re-closed in case of lidopening. Before natural eye opening, three animals selected for dark-
room rearing (DR) were transferred with the whole litter into a
darkroom (DROI -DR03). For intracortical injections, these DR cats
were deeply anaesthetized in the dark, wore black lenses during the
preparation, and were brought back into the dark room before they
woke up.
lntracortical injections of retro- and anterograde anatomical
markers
For surgery, the animals were anaesthetized with a mixture of 30 mg/kg
ketamine hydrochloride and 15 mglkg xylazine hydrochloride i.m.
When the same marker was applied in both hemispheres, the injections
were restricted to nonhomotopic regions to prevent confusion with
transcallosal labelling. All injections were made with a stereotaxically
mounted 0.5 or 1 .O p1 Hamilton syringe with a conical canula tip. Care
was taken to centre the injection in the superficial layers at a depth
of approximately 0.5 mm by inserting only the canula tip into the tissue.
The depth and centre of the injection site could be verified in frontal
and parasagittal sections after histological processing. Occasionally in
very young kittens, a contamination of the middle laminae could not
be avoided due to diffusion of WGA-HRP. WGA-HRP (Sigma, type
VI) was diluted in 0.5 M NaCl, injected in concentrations of 3%
(100 nl) and 6% (50 nl), and produced foci of local labelling of
2 -4 mm in diameter. The following fluorescent dyes were used: fast
blue (FB) (Bentivoglio ef al., 1980), fluoro gold (FG) (Schmued and
Fallon, 1986), diamidino yellow dihydrochloride (DY) (Keizer ef al.,
1983) and rhodamine-conjugated latex beads (RBs) (Katz et al., 1984).
FB was dissolved at a concentration of 5 % in distilled water, DY at
a concentration of 2% in distilled water and FG was dissolved at a
concentration of 3 % in 0.2 M phosphate buffer (PB) and injected in
amounts of 500 nl. RBs were applied via Hamilton syringe in amounts
of 100 nl. In most cases the injection was made perpendicular to the
cortical surface in the posterior-medial part of the posterolateral gyrus.
Each tracer was applied in three to four small doses within 5 - 10 min
and afterwards the syringe needle was left in place for at least 15 min
to reduce the spread of the marker along the needle track during
retraction of the syringe (Alheid et al., 1982).
The influence of an activity-dependent pinocytotic uptake and/or
transport of WGA-HRP (see Harrison et al., 1984; Jankowska, 1985;
Singer et al., 1977) and DY was studied in two NR cats (NR14, NR2 I ;
Table 1). These animals were kept in the darkroom between the
injection and perfusion.
Perfusion and tissue processing
After 2 or 3 days of survival animals that received only WGA-HRP
injections and whose visual cortex was to be prepared for tangential
sectioning (see Table l), were anaesthetized with ketamine hydrochloride and xylazine hydrochloride, and killed with an overdose
injection of pentobarbitone sodium (Nembutal@ ) i.p. (300 mg/kg).
They were perfused transcardially with saline, followed by a fixative
containing 1 % glutaraldehyde and 0.5% paraformaldehyde in 0.1 M
PB. The visual cortex was removed, unfolded and flat mounted
(Freeman et al., 1987; Olavarria and van Sluyters, 1985; Tootell and
Silverman, 1985). After postfixation for 4-6 hours in 6% paraformaldehyde in 0.1 M PB the tissue was infiltrated with 30% sucrose in
0.1 M PB over night. According to Tootell et al. (1982) distortion
during flat-mounting and sectioning amounts to less than 3% and thus
this technique was adopted to investigate the horizontal organization
of intracortical connections.
Animals which received injections of a long-term fluorescent tracer
were anaesthetized and killed in the same way, but after a survival
346 Development of lateral connections in cat area 17
time of 3 -39 days. To avoid glutaraldehyde-induced background
fluorescence, these cats were perfused with saline, followed by 4 or
6% paraformaldehyde in 0.1 M PB and 20% sucrose in 0.1 M PB.
A block of visual cortex was removed and infiltrated with 30% sucrose
in 0.1 M PB for about 12 h. Frontal or parasagittal sections containing
WGA-HRP or fluorescent dyes were cut at 50-60 pm on a freezing
microtome. The flat-mounted visual cortex was sectioned tangentially
at 50- 150 pm on a cryotome (see Table 1). Sections containing WGAHRP were reacted for HPR using a slightly modified version of the
tetramethyl benzidine method of Mesulam (1978). Some of the sections
were either Nissl-stained or reacted for cytochrome oxidase (WongRiley, 1979). Sections were mounted on gelatine-coated slides, airdried and coverslipped with Eukitt@ . Sections containing fluorescent
dyes were mounted on gelatinized slides, air-dried and coverslipped
with UV-inert@ . Some of these were stained for Nissl substance or
reacted for cytochrome oxidase.
Image analysis
Nonfluorescent sections were examined with a Wild binocular and a
Zeiss photo-microscope In. Tangential sections of WGA-HRP injected
striate cortex were further analysed with a digital image processing
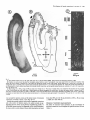
system (Fig. 1).
The magnified image of the original section was digitized (Fig. 1A)
and then band-pass filtered (3 - 3 1 cycles/mm) to enhance the contrast,
especially around the diffusely labelled injection site (Fig. 1B). This
processed image was used to determine the average spacing of the
patches by measuring the luminance distributions along 10 to 26 parallel
vectors, that were positioned rostro-caudally across the zone of patchy
labelling. The average of the Fourier spectra was plotted in a calibrated
coordinate system (Fig. 1C; see also Fig. 2 in Luhmann et a/., 1986).
The reciprocal of the peak value of this spatial frequency distribution
was taken as the average patch period. In addition the band-pass filtered
picture served as a basis for graphical reconstructions of the spatial
organization of horizontal connections (Fig. 1D). Sections containing
fluorescent markers were examined with a Zeiss fluorescence
microscope equipped with four different combinations of
excitatiodbarrier filters: 365/397, 400 -440/470, 450 -490/520, and
5 10-560/590 nm. The distribution of retrogradely stained fluorescent
cells was analysed from montages or photographs taken with TRI-X
pan 400 ASA or from enlarged plots that were produced with a Hewlett
Packard X-Y recorder coupled to the microscope stage. Nonfluorescent
sections were photographed with Ilford 25 or 50 ASA film.
The boundaries between visual areas were determined in frontal
sections according to the maps of Tusa ef a/.(1981), the histological
criteria of Otsuka and Hassler (1962) and the cytochrome oxidase
pattern (Price, 1985). The laminar boundaries were identified according
to the criteria of Otsuka and Hassler (1962), Garey (197 I ) and Innocenti
et a / . (1986). No such precise distinctions were made in the very young
kittens, because ongoing cell migration precludes subdividing supragranular layers (Shatz and Luskin, 1986). Likewise it was difficult to
identify particular laminae and areal boundaries in tangential sections
of flat mounts. Here the authors relied on the locations of sulci, which
were still visible despite flat mounting, and on comparisons between
the serial order of the sections and cortical thickness.
Results
Control for activity-dependent transport of WGA-HRP and DY
Two NR animals (NR14 and NR21) were kept in the dark between
injection and perfusion to assess any influence of activity-dependent
t
caud.
-
1 mm
FIG.1, Illustration of the computer assisted qualitative and quantitative analysis
of tangential sections obtained from WGA-HRP-injected flat-mounted striate
cortex.
(A) Digitized tangential section through supragranular layers of area 17 with
WGA-HRP injection (asterisk).
(B) Same section as in A, but bandpass-filtered (3-31 cycles/mm). Note the
discontinuous distribution of label within the diffusion zone.
(C) The patch period was determined by measuring the average Fourier
spectrum along 10 parallel vectors (white bars) in the rostro-caudal axis. The
Fourier spectrum below panel C reveals a peak at 1.15 cycles/mm corresponding
to a patch spacing of about 870 pm.
(D) Schematic illustration of the spatial distribution of labelled patches (black)
and of the size of the diffusion zone (dotted area) derived from bandpass-filtered
sections.
uptake and/or transport of WGA-HRP or DY on the visualization of
lateral intrinsic connections. In both animals and for both tracers, the
pattern of tangential projections showed no significant differences when
compared to NR animals of similar age, which were kept in normal
environment between injection and perfusion (see Fig. 2 for NR21 and
next paragraph for further description).
Laminar and columnar organization
Intracortical injections of WGA-HRP or fluorescent dyes revealed a
characteristic pattern of tangential intrinsic projections. The basic
organization of these connections such as their laminar specificity were
independent of the animals' age and will be described first. The results
from parasagittal and frontal sections will be described separately
because there were systematic differences between the organization
of connections in the frontal and sagittal planes.
In WGA-HRP labelled parasagittal sections, clearly separated clusters
of anterogradely labelled terminals and retrogradely stained neurons
were present in all laminae except layer I (Fig. 2). In both kittens and
adults, patches in different laminae were organized in register
perpendicular to the cortical surface. This is exemplified in the photo-
Development of lateral connections in cat area 17 347
FIG.2. Nissl-stained (A) and corresponding TMB-reacted (B) parasagittal section
of primary visual cortex from a 17-week-old cat reared normally (NWI), which
had received an intracortical injection of WCA-HRP and was visually deprived
in the darkroom until the time of perfusion. At a distance of I .5 to 2.7 mm
from the injection centre four individual clusters (arrows in B) of retrogradely
stained pyramidal cells are distinguishable. These clusters are in register with
respect to the trajectories of cortical columns. Two additional patches in laminae
I11 and IV with a diameter of about 400 gm emerge out of the diffusion zone
(white arrows in B). Scale bar and the dorsal (D) and caudal (C) coordinate
given in part B refer to part A and B. The sections have been cut at about 1.8 nun
lateral to the midline.
micrograph of a parasagittal section through the WGA-HRP injected
striate cortex of a 17-week-old cat (NR21) (Fig. 2B). Four clearly
separated patches are distinguishable in layers I1 to VI (black arrows
in Fig. 2B). These clusters are arranged in a 0.5 mm wide by 2.8 mm
long slab, oriented nearly perpendicular to the cortical lamination.
Individual clusters had diameters between 200 and 500 pM and
comprised 10-30 retrogradely labelled neurons. Most of these cells
could be identified as pyramidal because of the triangular shape of the
soma and the clearly detectable apical dendrite. In the parasagittal
section that is shown in Figure 2B, two additional 400 pm wide zones
of label are distinguishable in layers 111 and IV. These protrude out
of the injection site and extend up to 1.3 mm from the injection centre
(white arrows in Fig. 2B). The maximal distance of labelled clusters
from the injection centre varied between cortical layers. In the mature
visual cortex this distance was in the range of about 3 mm in supragranular layers, 1-2 mm in lamina IV and 2.5 mm in infragranular
layers.
There was a similar relationship between lateral extent and laminar
position of intrinsic connections in kitten striate cortex. A typical
example is documented for a 10-day-old kitten in Figure 3. Four to
five overlapping, 300 to 350 pm wide clusters are located in laminae
I1 and I11 rostral to the injection site. Their average spacing is about
500 pm and their maximal distance from the injection centre is 3 mm
(Fig. 3B-E). A dense continuous band of retrogradely labelled cells
extends caudally within layer I1 for more than 6 mm from the injection
centre (Fig. 3E-G). All neurons in this band whose filling allowed
for morphological classification were pyramidal cells (Fig. 3F, G).
In layer IV only a single 400 pm wide patch is visible 1.4 mm rostral
to the injection centre (see white arrow in Fig. 3E). This patch is in
register with one of the supragranular clusters (black arrow in Fig. 3E).
In lamina VI, a second, relatively weakly labelled continuous band
of retrogradely stained neurons and anterogradely labelled terminals
extends 2.5 mm from the injection site (see black triangle in Fig. 3E).
In contrast to parasagittal sections, single frontal sections of kitten
and adult area 17 revealed a less clear patchy organization of longrange lateral connections. Graphical and photographical reconstructions
of frontal sections indicated that intrinsic projections tend to be more
continuous along the medio-lateral than the rostro-caudal axis. The
longest intracortical connections along the medio-lateral axis were
observed in the visual cortex of a 2 1-day-old kitten (NR08) following
one injection of RBs into the supragranular layers and the upper part
of layer IV (Fig. 4). Most of the retrogradely labelled cells could be
clearly identified as pyramidal (see white arrows in Fig. 4C). They
form a uniform band in layers II/III, which extends throughout the
entire medial bank of area 17 down to the monocular segment, up to
a distance of 10.5 mm from the injection site (Fig. 4B). In infragranular
layers another continuous band of labelled cells extends up to 4 mm
from the injection centre. Also in this frontal section the lateral spread
of labelled profiles in layer IV is less than in other laminae and does
not exceed 2.5 mm.
As exemplified in Figure 5, a very similar pattern of tangential
connections was observed with injections of DY. Again there was no
clear indication of a patchy distribution of retrogradely labelled cells
along the medio-lateral axis. DY-stained cells were distributed in two
uniformly labelled bands in supra- and infragranular layers. In
agreement with established connectivity schemes (Symonds and Rosenquist, 1984a, b), numerous clusters of retrogradely labelled neurons
were also observed in adjacent visual cortical areas (Figs 4B, 5).
Postnatal development
During early postnatal development the tangential intracortical
connections undergo drastic changes in organization and total extent.
The youngest animals in this study received injections of WGA-HRP
and RBs at pnd three and were examined when 5 days old
(NROI -NR03; Table 1). At this early developmental stage, a clear
lattice-like pattern could not be demonstrated and retrogradely labelled
neurons were found up to 2.7 mm from the injection site (Fig. 6).
At pnd ten the extent of horizontal intrinsic connections had increased
to a maximum of about 6 mm from the injection site and at this stage
there was a clear indication of a patchy distribution with a centre-tocentre distance of approximately 500 pm (Fig. 7A). In a 24-day-old
kitten (NRI 1) 200-300 pm wide patches were found up to 5 mm from
the centre of the injection site and were occasionally organized in mediolaterally running bands of about 200 pm width and up to 3 mm length
(Fig. 7B). The largest spatial spread of retrogradely stained neurons
and the greatest number of labelled clusters were observed in 2- to
4-week-old kittens. At this age, patches were organized in 6-8 rows
348 Development of lateral connections in cat area 17
FIG. 3. Parasagittal sections through the right striate cortex of a 10-day-old kitten (NR04). which received one intracortical injection of WGA-HRP (asterisk
in A and E). In the Nissl-stained section A. the cortical lamination is indicated on the right and the locations of the TMB-reacted sections E and F are indicated
by the rectangles. Caudal to the injection site retrogradely stained cells are organized in a uniformly labelled band in layer 11 (B,E), which extends up to 6 m m
from the injection centre (E, F, G ) . A system of clustered neurons and axon terminals is present rostral to the injection and consists of several individual patches
in layers IUIII (black arrows in E) and one cluster in lamina IV (white arrow in E). In these clusters. retrogradely labelled pyramidal cells are clearly recognizable
(C, D). About 6 mm from the injection centre, retrogradely stained pyramidal neurons can be identified by their triangular soma and distinct apical dendrite
( G ) . The coordinates in A, which indicate the dorsal (D),ventral (V), caudal (C) and rostral (R) direction refer to all sections. Scale bars in B-D and F-G
= 100 pn; and in A and E = 1 mm.
around the injection site with the largest spread occurring rostrocaudally.
In cats older than 4 weeks, both the spatial spread of tangential label
and the number of patches decreased to reach adult levels at about
8 weeks of age (Fig. 7C). In adult striate cortex, labelled clusters of
200-500 pm in diameter are in general arranged in only one o r two
Development of lateral connections in cat area 17 349
FIG.4.
(A) Nissl-stained frontal section of the right visual cortex of a 21-day-old kitten (NROE), which received an intracortical injection of RBs.
(B) Graphical reconstruction of the pattern of retrogradely stained neurons in area 17 and adjacent visual areas (areal borders are marked by triangles). Dots
indicate the relative density of labelled cells. The broken line indicates the border with the white matter, and the injection site is marked by an arrowhead. In
supragranular layers, pyramidal cells are present in the monocular segment of area 17, 10.5 mm from the injection site Lamina IV and the infragranular layers
are characterized by a much smaller lateral spread of horizontal intrinsic connections, and retrogradely stained neurons are restricted to 2 . 5 and 4 mm, respectively,
from the injection site.
(C) Photomontage of a 1300 pm long and 300 pm wide sector through area 17. The sector is located about 2 mm central from the injection site (see rectangle
in Fig. B) and includes the whole grey matter. Labelled neurons are present throughout all cortical layers, but show the highest density in laminae I1 and VI,
where they are uniformly distributed in a 200 pm wide band. In most cases individual neurons can be clearly identified as pyramidal (white arrows). The laminar
boundaries were determined from the Nissl-stained section shown in A. Sections are taken approximately 5 mm posterior. The scale bar of 1 mm refers to both
A and B.
rows around the injection centre and the lateral extent of horizontal
connections is limited t o about 3 mm (Figs 7D-F).
Besides the postnatal reduction in the extent of tangential connections
and in the number of labelled clusters, the authors noticed an agedependent increase in the spacing between neighbouring patches. In
WGA-HRP injected sections cut tangentially to the cortical lamination,
the mean centre-to-centre distance between individual patches increased
from about 500 pm in the 10-day-old kitten to 1050 f 89 pm (mean
f SEM) in 5 adult NR cats.
influence of restricted visual experience
T h e effect of restricted visual experience on the development of
horizontal connections was investigated in eight BD and three DR cats
(Table 1).
350 Development of lateral connections in cat area 17
FIG.5. Photomontage of a frontal section through the right striate cortex of a 20-day-old kitten (NR07). injected with DY in the superficial layers. Retrogradely
stained neurons in area 17 are organized in two uniformly labelled bands in supra- and infragranular laminae. Neurons are labelled at distances of 3 and 6 mm
ventral of the injection site (white arrows). The montage on the left shows a 1500 p n long and 130 pm wide sector (white rectangle), located about 1.5 mm
ventral from the injection (arrowhead). The section is taken approximately 2.5 mm posterior.
Up to an age of 5 weeks there were no systematic differences between
visually deprived and normally reared kittens. This is shown in
Figure 8A for a 35-day-old BD kitten in which the distribution of
patches is comparable to that of NR animals of approximately the same
age (see Fig. 7B). As in NR cats, clusters of transported label had
diameters between 200 and 500 pm. They were organized in a
characteristic patchy pattern around the injection site, were often fused
to medio-laterally running bands of 2 mm length and spread up to a
distance of 5 mm from the injection site. With increasing age the
number and spatial spread of patches became reduced with a similar
time course as in NR animals but the reduction was more pronounced.
In a 43-day-old BD kitten the maximal lateral extent of horizontal
connections was still 2.5 mm but patches covered less than half the
cortical area that was covered in a BD kitten 8 days younger (Fig.8A,
B). In BD cats older than 2 months (Fig. 8C, D) the number of labelled
clusters was further reduced and the circular organization in one or
two rows around the injection site, characteristic of NR animals
(Fig. 7D-F), was no longer apparent. There was no significant
difference between mature NR and BD cats in the maximal lateral extent
of intrinsic connections, which in both groups was 2.5-3.0 mm. In
Development of lateral connections in cat area 17 351
1 mm
-’*
I
/
r 0 s t r . v
ventr.
FIG. 6. Reconstruction of a parasagittal section through the striate cortex of
a 5-day-old kitten (NR02) injected with RBs when 3 days old. The arrow
indicates the injection site and the dots represent retrogradely labelled neurons.
The section is taken approximately 1 rnm lateral to the midline.
DR cats between 10 and 16 weeks of age, tangential connections were
organized in a pattern similar to BD cats. Compared to NR adults,
the number of labelled clusters was reduced but the lateral spread of
label was alike in both groups and restricted to 3 mm. In the deprived
cats our quantitative measurements of interpatch distance revealed a
distinct patch-period for the 35-day-old kitten BDOl (770 pm), the
43-day-old kitten BD02 (lo00 pm) and the 15-week-old cat B W 3
(1 100 pm). The Fourier spectra in BD or DR cats older than 15 weeks
lacked a clear peak, because of the scarcity of patches.
Discussion
Limitations in the use of neuroanatomical tracers following
extracellular injections
Before interpreting results the authors wish to address several
methological restrictions of the applied tracing techniques (see also
Innocenti er al., 1986). Investigations of the influence of activity on
uptake and/or transport of WGA-HRP and DY in two cats (NR14 and
NR21), which were kept in the dark between injection and perfusion,
indicate that visual stimulation has no detectable effect on the
distribution of these tracers. This makes it unlikely that the differences
observed in BD and DR cats were solely due to changes in the level
of neuronal activity. Another potential source of error is the possibility
that WGA-HRP is transported trans-synaptically in both the anterograde
(Fabian and Coulter, 1985; Gerfen et al., 1982; Itaya and van Hoessen,
1982) and retrograde direction (Gerfen et a / . , 1982; Harrison er a / . ,
1984; Jankowska, 1985). To control for this possibility the authors
included RBs as tracers, because the relatively large, 20-200 nm,
particle size renders trans-synaptic transport very unlikely (Katz er a / .,
1984). Comparison between the projection pattern obtained with the
different tracers revealed no detectable difference, suggesting that
transneuronal transport had not influenced our results.
Another problem is related to the possibility that our tracer injections
invaded white matter and labelled axons ascending to and descending
from cortex. From histological verification of the injection sites the
authors can confirm that in kittens and adult cats the needle invaded
only supragranular layers and did not reach the white matter. Still,
contamination could have occurred by wide-spread diffusion. This is
unlikely, however, for two reasons: first, intact axons are unlikely to
incorporate the tracers used in this study. Second, the latex beads that
the authors used as control tracers diffuse very little and did not reach
white matter. But even if the authors had labelled axons in white matter
this would not invalidate their main conclusions, which are based on
the distribution of retrogradely labelled cells. It is true that corticipetal
axons bifurcate extensively as they enter the cortex and these branches
course obliquely within cortex until they reach their target zone. Thus,
orthograde labelling of these afferents could have given rise to clusters
of terminal labelling. However, even if one considers developmental
changes in the laminar termination pattern of afferents to cortex, the
observed layer-specific spread of label cannot easily be related to
corticipetal projection systems. Even more difficult to explain by white
matter contamination is the distribution of retrogradely labelled cells
that were clustered within the patches of terminal labelling. Corticifugal
axons tend to leave cortex in a rather straight course that is
perpendicular to the cortical lamination. It is thus difficult to see how
a focal contamination of white matter could have led to the labelling
of cell clusters that are spaced periodically, are in precise register with
orthogradely labelled axon terminals and are located in cortical regions
remote from the injection site. These same arguments also render it
unlikely that the spatial distribution of transported label in cortex was
due to corticipetal and corticifugal axons labelled en passanr within
cortex. Therefore we propose, that most of the tangential spread of
label was actually due to transport in the network of intrinsic cortical
connections.
Another possible source of error is that injections may have been
located in different layers at different ages due to variations in cortical
thickness. The authors consider this possibility unlikely for two reasons:
first, there are no indications from their material that the depth of the
injection varied systematically with age. Second, the inevitable
variations in the laminar position of the injections showed no consistent
relationship to the spatial distribution of retro- and anterogradely
labelled profiles.
Finally, there is the possibility of a systematic and age-dependent
variation in the size of the effective uptake zone. This source of artifact
is notoriously difficult to control for. However, this would imply that
the uptake zone increases over the first postnatal weeks and then
decreases again whereby the decrease would have to be experiencedependent. The authors consider this unlikely. Moreover, the size of
the effective uptake zone is in all likelihood different for different
tracers, but there was no indication that different tracers produced
different staining patterns. Thus, variations in the size of the effective
uptake zone appeared to have little influence on the staining patterns
evaluated in this study.
Organization of horizontal intrinsic connections
Horizontal intrinsic connections in adult cat striate cortex can be
characterized by the following properties: (i) in the rostro-caudal
direction tangential projections are organized in 200-500 pm wide
patches, which consist of anterogradely labelled terminals and retrogradely stained neurons of predominantly pyramidal type; (ii) these
clusters are prominent in all cortical layers except layer I and show
a columnar organization; (iii) the mean centre-to-centre distance
352 Development of lateral connections in cat area 17
0 24
pnd
c
0
adult
0
adult
adult
FIG.7. Postnatal development of clustered horizontal connections in primary visual cortex of NR cats. Drawings are based on bandpass filtered sections through
superficial layers (see Fig. 1). Black lines indicate cracks caused by tissue processing during flat-mounting. The age of each animal at perfusion is given in
postnatal days (pnd). Cats older than 150 days are classed as adult. The coordinates below each panel indicate the rostra1 (R) and medial (M) direction and scale
bars equal 1 mm for A-F. Drawings are taken from flat-mounted area 17 of the following animals: (A) NR04; (B) NRII; (C) NR19; (D) NR24; (E) NR26:
and (F) NR28 (see Table 1).
between adjacent patches measured in the rostro-caudal direction is
1050 pn f 89 p n SEM; (iv) in the medio-lateral axis horizontal
connections show a less pronounced patchiness and retrogradely stained
neurons are organized in beaded bands with continuously decreasing
cell density from the injection site to the furthest lateral extent; (v) the
tangential spread of intrinsic connections differs between the cortical
layers and reaches up to 3 mm in supragranular laminae, less than 2 mm
in layer IV and between 2 and 3 mm in infragranular laminae; and
(vi) anterogradely labelled terminal fields and clusters of retrogradely
stained neurons tend to be superimposed indicating reciprocity of
connections between the patches and the injection site.
These data confirm most of the observations. which have been
Development of lateral connections in cat area 17 353
BD pnd 35
BD pnd 43
R
t
U
BD pnd 133
BD adult
0
FIG.8. The development of horizontal intrinsic connections in the primary visual cortex of cats binocularly deprived by lid-suture from the second postnatal
week. Drawings are based on the method shown in Figure 1 and represent the supragranular staining pattern after an injection of WGA-HRP into area 17 of
animals BDOl (A), BD02 (B), BD04 (C) and BD08 (D). For further explanations, see Figure 7. Scale bar below each panel indicates I mm.
reported previously in mammalian visual cortex using extracellular
injections of horseradish peroxidase (HRP). In area 17 of the rat
(Burkhalter, 1989), the ferret (Rockland, 1985b), the tree shrew
(Rockland et al., 1982), and the squirrel and macaque monkey
(Rockland and Lund, 1983), far-reaching lateral connections are mainly
located in layers IIIIII, and with the exception of the rat (Burkhalter,
1989), are periodically organized. Tangential axons originate
predominantly or exclusively from pyramidal neurons (Rockland,
1985a) and they extend 1.5-3.0 mm from the centre of the injection
site. In lamina IV lateral connections are much shorter than in upper
and lower layers and are restricted to about 1 mm in extent (Fitzpatrick
et al., 1985). The authors’ results on the columnar organization and
layer-specific extent of horizontal projections are in agreement with
recent observations by LeVay (1988) in cat area 18. Using the same
tracers as in our study, LeVay demonstrated lateral connections
extending up to 4 mm from the injection site. Pyramidal and/or spinal
stellate cells were retrogradely labelled in 10- 15 patches of about
500 pn in diameter through all cortical layers, with the densest labelling
in layer 111.
In cat primary visual cortex, tangential intrinsic projections have been
investigated extensively by staining neurons intracellularly with HRP
and reconstructing their axonal and dendritic pattern (Gilbert and
Wiesel, 1983; Kisvarday et al., 1986; Martin and Whitteridge, 1984).
According to these studies, the maximal lateral spread of intracellularly
filled axons of spiny stellate and pyramidal cells is between 4 and 6 mm
and 100- 150 pm wide tufts of terminal arbors show a centre-to-centre
distance of about 1 mm. The authors’ measurements on the average
spacing between neighbouring clusters in five adult NR cats
(NR23 -NR27) correspond to data obtained from intracellularly stained
cells, but the authors noticed a relatively large variation between
different animals (810- 1330 am). These interindividual differences
probably reflect variations in the arrangement of cortical maps, as
already demonstrated for the system of orientation columns within cat
area 17 (Lowel et al., 1988).
Discussion has centred around the question of whether intracortical
tangential connections are inhibitory or excitatory. Long-range
inhibitory interactions can be mediated either directly via basket cells
with horizontal axons (Marin-Padilla, 1969; Martin et al., 1983;
Somogyi et al., 1983), or indirectly via long pyramidal cell axons,
which contact inhibitory interneurons (Matsubara et al., 1985, 1987;
354 Development of lateral connections in cat area 17
Silva and Connors, 1986). The results of this and of previous studies
(Blasdel et al., 1985; LeVay, 1988; Luhmann et a / . , 1986; Rockland,
1985a,b; Rockland and Lund, 1983; Rockland et al., 1982) indicate
that the large majority of retrogradely labelled cells are pyramidal,
excluding a major contribution of basket cells to tangential interactions.
The second hypothesis of indirect inhibitory interactions via
interneurons also seems rather unlikely, because 80-90% of the
terminals of long-range lateral axons originating from layers II/III
pyramidal cells form asymmetric, Graey type I synapses with dendritic
spines of other pyramidal or spiny stellate cells (Gabbott et a l . , 1987;
Kisvirday et al., 1986; LeVay, 1988). Finally current source-density
analysis of responses to intracortical microstimulation indicates that
long-range tangential interactions are mediated by excitatory projections
terminating on apical dendrites of pyramidal cells (Luhmann et al.,
1990b).
Correlation with other columnar systems
In both kitten and adult cat striate cortex, the authors noticed a distinct
patchy organization of lateral connections, with an average spacing
of 1050 pm. This value is in the range of the space constants of other
columnar systems in adult cat area 17 (Hubel and Wiesel, 1962, 1963),
suggesting a possible relationship between intrinsic horizontal
projections and other columnar systems. Both, the system of ocular
dominance bands with a periodicity of 650-870 pm (Anderson et al.,
1988; Liiwel et al., 1988; Lowel and Singer, 1987; Shatz et al., 1977)
and the system of orientation columns with a periodicity of
1ooO- 1100 pm (Albus, 1975; Albus and Sieber, 1984; Lowel ef al.,
1987; Singer, 1981; Singer er al., 1981) are candidates. Furthermore
it has been demonstrated, that iso-orientation domains consist of
elongated slabs that tend to be parallel to the frontal plane (Lowel er a / .,
1987). This is reminiscent of the present finding that horizontal
connections extend continuously in the frontal and discontinuously in
the sagittal plane. Additional support for a functional relationship
between orientation columns and lateral intrinsic projections comes
from the authors physiological data in kitten striate cortex on stimulusspecific responses outside the classical receptive field (Luhmann et al.,
1990c), and from cross-correlation analysis in cat area 17. Ts’o et a / .
(1986) were able to demonstrate horizontal excitatory interactions up
to 3 mm between layer II/III pyramidal cells with similar orientation
preference and recently Gray et al. (1989) and Gray and Singer (1989)
have shown that orientation columns as far apart as 7 mm can
synchronize their respective oscillatory responses if they have similar
Orientation preferences, suggesting selective coupling between columns
with the same preference. A recent double labelling study in cat visual
cortex by Gilbert and Wiesel (1989) also indicates that horizontal
intrinsic projections connect columns of similar orientation selectivity,
supporting the hypothesis of a functional specificity of long-range lateral
connections.
Ontogenetic and experience-dependent reorganization
The authors’ results suggest that the characteristic arrangement and
wide-spread distribution of horizontal connections develop in two
sequential phases, mainly in the first two postnatal months. In an early,
experience-independentphase tangential fibres show a marked increase
in their length from a maximum of 2.7 mm at pnd five to a maximum
of approximately 10 mm in the third postnatal week. A patchy
distribution of horizontal connections was already present at pnd ten,
but could not be demonstrated in 5-day-old kittens. In a second phase
that coincides with the critical period for experience-dependent pruning
of ocular dominance and orientation columns (for review see FrCgnac
and Imbert, 1984), lateral projections undergo a reduction in their
maximal extent and in the number of clusters. Selective cell death of
neurons with wide-spread axon collaterals and/or withdrawal of longrange axons have been proposed as mechanisms for the pruning of
juvenile exuberant connections (for review see Cowan et al., 1984;
Innocenti, 1984; Stanfield, 1984). The authors did not carry out detailed
statistical analysis to investigate the role of these processes. Data
obtained from two NR cats (NR17 and NR18; Table l), which had
received one intracortical injection of a long-lasting marker at pnd 14
and then survived for an additional 32 and 39 days, respectively, were
compatible with either hypothesis and did not allow the authors to
distinguish between them. However, recent observations by Callaway
and Katz (1989) indicate that the adult pattern is achieved between the
third and sixth postnatal week by specific elimination of inappropriately
projecting axonal collaterals.
A pattern of postnatal development similar to that described in this
study for intracortical projections within area 17, has been observed
for intrinsic connections in kitten area 18 (Price, 1986) and for corticocortical projections from area 17 to area 18 (Price and Blakemore,
1985). Both projection systems show an immature, non-patchy
distribution in very young kittens, but during the second postnatal week
the intrinsic projections and the cortico-cortical association pathway
in area 18 become restricted to discrete, dense clusters, predominantly
located in superficial layers (Price, 1986; Price and Blakemore, 1985).
Comparable ontogenetic changes also occur during the postnatal
development of other neocortical systems, such as the callosal
connections (Innocenti, 1981; Innocenti and Clarke, 1984; Ivy and
Killackey, 1982).
Besides a reduction in the extent of horizontal intrinsic connections,
the authors also noticed a continuous change in the centre-to-centre
spacing of neighbouring clusters. The mean patch period increased
continuously from about 500 pm in the 10-day-old kitten to a mean
of 1050 pm in the adult. A similar maturation pattern has been observed
for the projection from area 17 to 18, where the periodicity of the
connections increased from 560 pm in a 20-day-old NR kitten to 630
and 720 pm in two 28-day-old BD animals (Price and Blakemore,
1985). Reasons for this increase in spacing could be selective loss of
patches and/or postnatal growth of the cortical surface. In adult cats
individual cortical areas are about twice as large as in kittens (see Rose
and Goodfellow, 1973), suggesting that the postnatal increase in the
periodicity of patchy intrinsic connections might be related to a growth
of the visual cortex.
Earlier observations from Katz and Wiesel (1987) in kitten striate
cortex indicate that horizontal collaterals of intracellularly injected layer
II/III pyramidal cells are restricted to about 1 mm in extent and that
clustered tangential connections do not appear before pnd 50. These
observations are in conflict with the authors’ present findings and with
earlier studies in kitten primary and secondary visual cortex. Meyer
and Ferres-Torres (1984) have found that the postnatal maturation of
some nonpyramidal neurons in cat visual cortex is also characterized
by an initial overproduction and subsequent reduction of axonal
collaterals and Price (1986) demonstrated in kitten area 18 that
intracortical connections already become patchy in the second postnatal
week. The authors suggest that these conflicting results are due to
methodological differences. The absence of patchy axonal arborizations
in the data of Katz and Wiesel (1987) might be due to the fact that
the slices were cut in the coronal plane, while tangential projections
tend to be patchy mainly in the sagittal plane. Furthermore, our data
in young kittens indicate that the percentage of cells with lateral
connections of more than 5 mm is relatively small (see Figs 3F, 4B,
5 ) , reducing the probability to stain these cells intracellularly. An
Development of lateral connections in cat area 17 355
additional possibility is that individual pyramidal cells continue to
generate new terminal branches within certain patches while at the same
time retracting collaterals from other previously innervated patches.
Such a process would be compatible with the results of Katz and Wiesel
(1987) and with the authors’ present observations. Furthermore, Katz
and Callaway (1989) recently reported that intrinsic connections in
striate cortex of 8-day-old kittens extend laterally up to 4 mm and that
an early, crude clustering is already present during the second postnatal
week.
Preliminary data from the authors’ laboratory using the complementary technique of postmortem axonal tracing with the lipophilic dye
DiI (Godement et al., 1987) also support the hypothesis that lateral
intrinsic connections are initially wide-spread and subsequently become
reduced to the mature pattern (unpublished observations). Finally, the
results of two related electrophysiological investigations (Luhmann
et al., 1990a,b) also suggest developmental changes in the intrinsic
circuitry as they have been found in this anatomical study.
The role of activity in axonal pruning
Long-range lateral projections within cat visual cortex undergo a
substantial modification in a phase of postnatal development during
which cortical functions are influenced by visual experience (for review
see Blakemore, 1977; Fregnac and Imbert, 1984). The authors’ data
indicate that visual experience does play a role in the pruning of
intracortical projections. Visual deprivation by binocular lid-suture or
dark-rearing had no effect on the maximal lateral extent of tangential
projections, but it reduced the number of labelled clusters. The authors
propose that under normal rearing conditions neuronal activity
influences the pruning of tangential connections by selectively
stabilizing certain subsets of the initially exuberant connections, a
process that has been shown to occur in numerous other neuronal
systems (for review see Easter et al., 1985; Fawcett and O’Leary, 1985;
Frost and Innocenti, 1986; Schmidt and Tieman, 1985). In the mature
cortex, lateral connections appear t o be selective and related to the
spatial organization of functional columns. The maturation of the
topological organization of these columnar systems is influenced by
neuronal activity (for review see Blakemore, 1977; Fregnac and Imbert,
1984). Thus, it would seem appropriate that the development of
connections assuring specific intercolumnar interactions could also be
influenced by neuronal activity. This would allow for the selection of
connections according to functional criteria.
Acknowledgements
We wish to thank Helga Duckstein, Alexa Franke, Ines Galin and Monika Sum
for their excellent technical assistance, Margitt Ehms-Sommer, Hedwig Thomas,
and Conny Steffens for photographic service, Renate Ruhl for assistance with
the graphics and Gisela Knott and Gabriele Trauten-Luhmann for editorial
assistance. We are grateful to Dr P. Somogyi for helpful discussions and to
Drs Carla Shatz and Joan Dann for critically reading the manuscript. Rhodaminelabelled latex microspheres were kindly provided by Dr L. C. Katz and Fluoro
Gold by Drs L. C. Schmued and J. H. Fallon. This work is part of the PhD
thesis of H. J . Luhmann that was presented to the University of Bremen.
Abbreviations
FB
FG
BD
DR
DY
fast blue
fluoro gold
binocularly deprived
dark-reared
diamidino Yellow dihydrochloride
HRP
NR
PB
Pnd
RBs
SEM
WGA-HRP
horseradish peroxidase
normally reared
phosphate buffer
postnatal day
rhodamine-conjugated latex beads
standard error of the mean
wheat germ agglutinin-conjugated horseradish peroxidase
References
Albus, K. (1975) A quantitative study of the projection area of the central and
the paracentral visual field in area 17 of the cat. 11. The spatial organization
of the orientation domain. Exp. Brain Res. 24: 181 -202.
Albus, K. and Sieber, B. (1984) On the spatial arrangement of iso-orientation
bands in the cat’s visual areas 17 and 18-a C-14 deoxyglucose study. Exp.
Brain Res. 56: 384-388.
Alheid, G. F., Edwards, S. B., Kitai, S. T., Park, M. R., and Switzer, R. G.
(1982) Methods for delivering tracers. In: Heimer, L., and Robards, M. J.
(eds) Neuroanatomical tract-tracing methods pp. 91 - 116. Plenum Press,
New York.
Anderson, P. A., Olavarria, J., and van Sluyters, R. C. (1988) The Overall
Pattern of Ocular Dominance Bands in Cat Visual Cortex. J. Neurosci. 8:
2183 -2200.
Bentivoglio, M., Kuypers, H. G. J. M., Catsman-Berrevoets, C. E., Loewe,
H., and Dann, 0. (1980) Two new fluorescent retrograde neuronal tracers
which are transported over long distances. Neurosci. Lett. 18: 25-30.
Blakemore, C. (1977) Genetic instructions and developmental plasticity in the
kitten’s visual cortex. Phil. Trans. Roy. SOC. (Lond.) B 278: 425-434.
Blasdel, G. G., Lund, J. S., and Fitzpatrick, D. (1985) Intrinsic connections
of macaque striate cortex: axonal projections of cells outside lamina 4c. J.
Neurosci. 5: 3350-3369.
Burkhalter, A. (1989) Intrinsic connections of rat primary visual cortex: laminar
organization of axonal projections. J. Comp. Neurol. 279: 171 - 186.
Callaway, E. M., and Katz, L. C. (1989) Development of clustered horizontal
connections in cat striate cortex: roles of process elimination and cell death.
Soc. Neurosci. Abstr. 15: 3.
Cowan, W. M., Fawcett, J. W., and O’Leary,D. D. M. (1984) Regressive
events in neurogenesis. Science 225: 1258- 1265.
Easter, S. S., Purves, D., Rakic, P., and Spitzer, N . C. (1985) The changing
view of neuronal specificity. Science 230: 507 -51 1 .
Fabian, R. H., and Coulter, J.D. (1985) Transneuronal transport of lectins.
Brain Res. 344: 41 -48.
Fawcett, J. W., and O’Leary, D. D. M. (1985) The role of electrical activity
in the formation of topographic maps in the nervous system. Trends Neurosci.
8: 201 -206.
Fitzpatrick, D., Lund, J. S., and Blasdel, G. G. (1985) Intrinsic connections
of macaque striate cortex: afferent and efferent connections of lamina 4c.
J. Neurosci. 5: 3329-3349.
Freeman, B., Lowel, S., and Singer, W. (1987) Deoxyglucose mapping in the
cat visual cortex following carotid artery injection and cortical flat-mounting.
J. Neurosci. Meth. 20: 115- 129.
FrCgnac. Y.,and Imbert, M. (1984) Development of neuronal selectivity in
primary visual cortex of cat. Physiol. Rev. 64: 325-434.
and Innocenti, G. M. (1986) Effects of sensory experience on
Frost, D. 0..
the development of visual callosal connections. In: Lepore, F., Ptiti, M.,
and Jasper, H. H. (eds) Two hemispheres-one brain: functions of the corpus
callosum pp. 255-266. Alan R. Liss, New York.
Gabbott, P. L. A,, Martin, K. A. C., and Whitteridge, D. (1987) Connections
between pyramidal neurons in layer 5 of cat visual cortex (area 17). J. Comp.
Neurol. 259: 364-381.
Garey, L. J . (1971) A light and electron microscopic study of the visual cortex
of the cat and monkey. Proc. Roy. SOC.(Lond.) B 179: 21 -40.
Gerfen, C. R., O’Leary, D. D. M., and Cowan, W. M. (1982) A note on the
transneuronal transport of wheat germ agglutinin-conjugated horseradish
peroxidase in the avian and rodent visual system. Exp. Brain Res. 48:
443-448.
Gilbert, C. D. (1985) Horizontal integration in the neocortex. Trends Neurosci.
8: 160-165.
Gilbert, C. D. and Wiesel, T. N. (1983) Clustered intrinsic connections in cat
visual cortex. J. Neurosci. 3: 11 16- 1133.
Gilbert, C. D. and Wiesel, T. N. (1989) Columnar specificity of intrinsic
horizontal and corticocortical connections in cat visual cortex. J. Neurosci.
356 Development of lateral connections in cat area 17
9: 2432-2442.
Godement, P., Vanselow, J . , Thanos, S., and Bonhoeffer. F. (1987) A study
in developing visual systems with a new method of staining neurones and
their processes in fixed tissue. Development 101: 697-713.
Gray, C. M., Konig, P., Engel, A. K., and Singer, W. (1989) Oscillatory
responses in cat visual cortex exhibit inter-columnar synchronization which
reflects global stimulus properties. Nature 338: 334-337.
Gray, C. M. and Singer, W. (1989) Stimulus-specific neuronal oscillations in
orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. (USA) 86:
1698-1702.
Harrison, P. J., Hultborn, H., Jankowska, E., Katz, R., Storai, B., and
Zytnicki, D. (1984) Labelling of interneurones by retrograde trans-synaptic
transport of horseradish peroxidase from motoneurones in rats and cats.
Neurosci. Lett. 45: 15-19.
Hubel, D. H. and Wiesel, T. N. (1962) Receptive fields, binocular interaction
and functional architecture in the cat’s visual cortex. J. Physiol. 160:
106-154.
Hubel, D. H. and Wiesel, T. N. (1963) Shape and arrangement of columns
in the cat’s striate cortex. J. Physiol. 165: 559-568.
Innocenti, G. M. (1981) Growth and reshaping of axons in the establishment
of visual callosal connections. Science 212: 824-827.
Innocenti, G. M. (1984) Role of axon elimination in the development of visual
cortex. In: Stone, J., Dreher, B., and Rapaport, D. H. (eds) Development
of visual pathways in mammals pp. 243-253. Alan R. Liss, New York.
Innocenti, G. M. and Clarke, S. (1984) Bilateral transitory projection to visual
areas from auditory cortex in kittens. Dev. Brain Res. 14: 143- 148.
Innocenti, G. M., Clarke, S., and Kraftsik, R. (1986) Interchange of callosal
and association projections in the developing visual cortex. J . Neurosci. 6:
1384- 1409.
Itaya, S. K. and van Hoessen, G. W. (1982) WGA-HRP as a transneuronal
marker in the visual pathways of monkey and rat. Brain Res. 236: 199-204.
Ivy, G. 0. and Killackey, H. P. (1982) Ontogenetic changes in the projections
of neocortical neurons. J. Neurosci. 2: 735 -743.
Jankowska, E. (1985) Further indications for enhancement of retrograde
transneuronal transport of WGA-HRP by synaptic activity. Brain Res. 341:
403-408.
Katz, L. C., Burkhalter, A,, and Dreyer, W. J . (1984) Fluorescent latex
microspheres as a retrograde neuronal marker for in vivo and in v i m studies
of visual cortex. Nature 310: 498-500.
Katz, L. C. and Callaway, E. M. (1989) Development of clustered horizontal
connections in cat striate cortex: progressive and regressive events. SOC.
Neurosci. Abstr. 15: 2.
Katz, L. C. and Wiesel, T.N. (1987) Postnatal development of intrinsic axonal
arbors of pyramidal neurons in cat striate cortex. Soc. Neurosci. Abstr. 13:
1025.
Keizer, K., Kuypers, H. G. J. M., Huisman, A. M., and Dann, 0. (1983)
Diamidino yellow dihydrochloride (DY-2 HC 1); a new fluorescent retrograde
neuronal tracer, which migrates only very slowly out of the cell. Exp. Brain
Res. 51: 179-191.
Kisvhrday, Z. F., Martin, K. A. C., Freund, T. F., Maglkzky, Z.,
Whitteridge, D., and Somogyi, P. (1986) Synaptic targets of HRP-filled layer
111 pyramidal cells in the cat striate cortex. Exp. Brain Res. 64:541 -552.
LeVay, S. (1988) Patchy intrinsic projections in visual cortex, area 18, of the
cat: morphological and immunocytochemical evidence for an excitatory
function. J. Comp. Neurol. 269: 265-274.
Lowel, S., Freeman, B., and Singer, W. (1987) Topographic organization of
the orientation column system in large flat-mounts of the cat visual cortex:
a 2-deoxyglucose study. J. Comp. Neurol. 255: 401 -415.
Lowel, S. and Singer, W. (1987) The pattern of ocular dominance columns
in flat-mounts of the cat visual cortex. Exp. Brain Res. 68: 661 -666.
Lowel, S., Bischof, H.-J., Leutenecker, B., and Singer, W. (1988) Topographic
relations between ocular dominance and orientation columns in the cat striate
cortex. Exp. Brain Res. 71: 33-46.
Luhmann, H. J., Martiinez-Millan, L., and Singer, W. (1986) Development
of horizontal intrinsic connections in cat striate cortex. Exp. Brain Res. 63:
443-448.
Luhmann, H. J., Greuel, J. M., and Singer, W. (1987) Postnatal development,
structure and possible function of long-range horizontal intrinsic connections
in cat area 17. Soc. Neurosci. Abstr. 13: 3.
Luhmann, H. J., Greuel, J . M., and Singer, W. (1990a) Horizontal interactions
in cat striate cortex. 11. A current source-density analysis. Europ. J. Neurosci.
2: 358-368.
Luhmann, H. J., Greuel, J . M . , and Singer, W. (199Ob) Horizontal interactions
in cat striate cortex: 111. Ectopic receptive fields and transient exuberance
of tangential interactions. Europ. J. Neurosci. 2: 369-377.
Marin-Padilla, M. (1969) Origin of the pericellular baskets of the pyramidal
cells of the human motor cortex: a Golgi study. Brain Res. 14: 633-646.
Martin, K. A. C. and Whitteridge, D. (1984) Form, function and intracortical
projections of spiny neurones in the striate visual cortex of the cat. J. Physiol.
353: 463-504.
Martin, K. A. C., Somogyi. P., and Whitteridge, D. (1983) Physiological and
morphological properties of identified basket cells in cat’s visual cortex. Exp.
Brain Res. 50: 193-200.
Matsubara, J. A,, Cynader, M. S., Swindale, N. V., and Stryker, M. P. (1985)
Intrinsic projections within visual cortex: evidence for orientation-specific
local connections. Proc. Natl. Acad. Sci. (USA) 82: 935-939.
Matsubara, J. A,, Cynader, M. S., and Swindale, N. V. (1987) Anatomical
properties and physiological correlates of the intrinsic connections in cat area
18. J. Neurosci. 7: 1428-1446.
Mesulam, M. M. (1978) Tetramethyl benzidine for horseradish peroxidase
neurohistochemistry : a non-carcinogenic blue reaction product with superior
sensitivity for visualizing neural afferents and efferents. J. Histochem.
Cytochem. 26: 106- 117.
Meyer, G. and Ferres-Torres, R. (1984) Postnatal development of nonpyramidal
neurons in the visual cortex of the cat. J. Comp. Neurol. 228: 226-244.
Mitchison, G . and Crick. F. (1982) Long axons within the striate cortex: their
distribution, orientation and patterns of connection. Proc. Natl. Acad. Sci.
(USA) 79: 3661 -3665.
Olavarria. J. and van Sluyters, R.C. (1985) Unfolding and flattening the cortex
of gyrencephalic brains. J. Neurosci. Meth. 15: 191 -202.
Otsuka, R. and Hassler, R. (1962) Uber Aufbau und Gliederung der corticalen
Sehsphare bei der Katze. Arch. Psychiat. Nervenkrh. 203: 212-234.
Price, D. J. (1985) Patterns of cytochrome oxidase activity in areas 17, 18 and
19 of the visual cortex of cats and kittens. Exp. Brain Res. 58: 125- 133.
Price, D. J . (1986) The postnatal development of clustered intrinsic connections
in area 18 of the visual cortex in kittens. Dev. Brain Res. 24: 31 -38.
Price, D. J. and Blakemore, C. (1985) The postnatal development of the
association projection from visual cortical area 17 to area 18 in the cat. J.
Neurosci. 5: 2443-2452.
Rockland, K. S. (1985a) A reticular pattern of intrinsic Connections in primate
area V2 (area 18). J. Comp. Neurol. 235: 467-478.
Rockland, K. S. (1985b) Anatomical organization of primary visual cortex (area
17) in the ferret. J. Comp. Neurol. 241: 225-236.
Rockland, K. S., Lund, J. S., and Humphrey, A. L. (1982) Anatomical banding
of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J. Comp.
Neurol. 209; 41 -58.
Rockland, K. S. and Lund. J . S. (1983) Intrinsic laminar lattice connections
in primate visual cortex. J . Comp. Neurol. 216: 303-318.
Rose, G . H. and Goodfellow, E. F. (1973) A stereotaxic atlas for the kitten
brain. Brain Inform. Serv.. Brain Res Inst., Univ. California, Los Angeles.
Schmidt, J . T. and Tieman, S. B. (1985) Eye-specific segregation of optic
afferents in mammals, fish, and frogs: the role of activity. Cell. and Molec.
Neurobiol. 5: 5-34.
Schmued, L. C. and Fallon, J. H. (1986) Fluoro-gold: a new fluorescent
retrograde axonal tracer with numerous unique properties. Brain Res. 377:
147- 154.
Shatz, C. J . . Lindstrom, S. and Wiesel, T. N. (1977) The distribution of
afferents representing the right and left eyes in the cat’s visual cortex. Brain
Res. 131: 103-116.
Shatz, C.J. and Luskin, M. B. (1986) The relationship between the geniculocortical afferents and their cortical target cells during development of the
cat’s primary visual cortex. J. Neurosci. 6: 3655 -3668.
Sillito, A.M. (1984) Functional considerations of the operation of GABAergic
inhibitory processes in the visual cortex. In: Jones, E.G. and Peters, A. (eds)
Cerebral Cortex, Vol. 2, pp.91- 117. Plenum Press, New York. London.
Silva, L.R. and Connors, B.W. (1986) Spatial distribution of intrinsic cortical
neurons that excite or inhibit layer 2/3 pyramidal cells: a physiological study
of neocortex in v i m . SOC.Neurosci. Abstr. 12: 1435.
Singer, W. (1981) Topographic organization of orientation columns in the cat
visual cortex. Exp. Brain Res. 44: 431 -436.
Singer, W. (1985a) Hebbian modification of synaptic transmission as a common
mechanism in experience-dependentmaturation of cortical functions. In: Levy,
W.B., Anderson, J.A., and Lehmkuhle, S. (eds) Synaptic Modification,
Neuron Selectivity, and Nervous System Orientation pp. 35 -64. Lawrence
Development of lateral connections in cat area 17 357
Erlbaum Assn., Hillsdale, New Jersey, London.
Singer, W. (1985b) Activity-dependentself-organizationof the mammalian visual
cortex. In: Rose, D. and Dobson, V.G. (eds) Models of the Visual Cortex
pp. 123- 136. John Wiley & Sons, Chichester, New York,Brisbane, Toronto,
Singapore.
Singer, W., Hollander, H., and Vanegas, H. (1977) Decreased peroxidase
labeling of lateral geniculate neurons following deafferentation. Brain Res.
130: 133-137.
Singer, W., Freeman, B., and Rauschecker, J . (1981) Restriction of visual
experience to a single orientation affects the organization of orientation
columns in cat visual cortex. Exp. Brain Res. 41: 199-215.
Somogyi, P., Kisvirday, Z . F . , Martin, K.A.C., and Whitteridge, D. (1983)
Synaptic connections of morphologically identified and physiologically
characterized large basket cells in the striate cortex of cat. Neurosci. 10:
261 -294.
Stanfield, B.B. (1984) Postnatal reorganization of cortical projections: the role
of collateral elimination. Trends Neurosci. 7: 37-41.
Swindale, N.V. (1982) An enlarged view of intracortical connectivity. Nature
300: 313-324.
Symonds, L.L. and Rosenquist, A.C. (1984a) Corticocortical connections among
visual areas in the cat. J. Comp. Neurol. 229: 1-38.
Symonds, L.L. and Rosenquist, A.C. (1984b) Laminar origins of visual corticocortical connections in the cat. J. Comp. Neurol. 229: 39-47.
Tootell, R.B.H. and Silverman, M.S. (1985) Two methods for flat-mounting
cortical tissue. J. Neurosci. Meth. 15: 177-190.
Tootell, R.B.H., Silverman, M.S., Switkes, E., and de Valois, R.L. (1982)
Deoxyglucose analysis of retinotopic organization in primate striate cortex.
Science 218: 902-904.
Ts'o, D.Y., Gilbert, C.D., and Wiesel, T.N. (1986) Relationship between
horizontal interactions and functional architecture in cat striate cortex as
revealed by cross-correlation analysis. J. Neurosci. 6: 1160- 1170.
Tusa, R.J., Palmer, L.A., and Rosenquist. A.C. (1981) Multiple cortical visual
areas: visual field topography in the cat. In: Woolsey, C.N. (ed.), Cortical
Sensory Organization pp. 1-31, Humana Press, Clifton, NJ.
Wiesel, T.N. and Gilbert, C.D. (1983) The Sharpey-Schafer lecture.
Morphological basis of visual cortical function. Quart. J. Exp. Physiol. 68:
525-543.
Wong-Riley, M. (1979) Changes in the visual system of monocularly sutured
or enucleated cats demonstrable with cytochrome oxidase histochemistry.
Brain Res. 171: 11 -28.