* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neuroanatomical correlates of the near response: voluntary
Functional magnetic resonance imaging wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neurolinguistics wikipedia , lookup
Executive functions wikipedia , lookup
History of neuroimaging wikipedia , lookup
Optogenetics wikipedia , lookup
Environmental enrichment wikipedia , lookup
Embodied language processing wikipedia , lookup
Neurophilosophy wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Visual search wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Cortical cooling wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Affective neuroscience wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Process tracing wikipedia , lookup
Dual consciousness wikipedia , lookup
Neuroeconomics wikipedia , lookup
Human brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Visual extinction wikipedia , lookup
Aging brain wikipedia , lookup
Visual memory wikipedia , lookup
Time perception wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Visual servoing wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Emotional lateralization wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroesthetics wikipedia , lookup
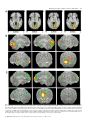
European Journal of Neuroscience, Vol. 12, pp. 311±321, 2000 ã European Neuroscience Association Neuroanatomical correlates of the near response: voluntary modulation of accommodation/vergence in the human visual system Hans O. Richter,1,2,3,* Joel T. Lee,3 and Jose V. Pardo3,4 1 Brain Sciences Center, Veterans Affairs Medical Center, Minneapolis, MN 55417, USA Department of Physiology, University of Minnesota, Minneapolis, MN 55455, USA 3 Cognitive Neuroimaging Unit, Psychiatry Service, Veterans Affairs Medical Center, Minneapolis, MN 55417, USA 4 Division of Neuroscience Research, Department of Psychiatry, University of Minnesota, Minneapolis, MN 55455, USA 2 Keywords: attention, brain blood ¯ow, brain mapping, frontal eye ®eld, saccade, smooth pursuit Abstract This study identi®es brain regions participating in the execution of eye movements for voluntary positive accommodation (VPA) during open-loop vergence conditions. Neuronal activity was estimated by measurement of changes in regional cerebral blood ¯ow (rCBF) with positron emission tomography and 15O-water. Thirteen naive volunteers viewed a checkerboard pattern with their dominant right eye, while a lens interrupted the line of gaze during alternate 1.5 s intervals. Three counterbalanced tasks required central ®xation and viewing of a stationary checkerboard pattern: (i) through a 0.0 diopter (D) lens; (ii) through a ±5.0-D lens while avoiding volitional accommodation and permitting blur; and (iii) through a ±5.0-D lens while maintaining maximal focus. The latter required large-amplitude, high-frequency VPA. As an additional control, seven of the subjects viewed passively a digitally blurred checkerboard through a 0.0-D lens as above. Optometric measurements con®rmed normal visual acuity and ability to perform the focusing task (VPA). Large-amplitude saccadic eye movements, veri®ed absent by electro-oculography, were inhibited by central ®xation. Image averaging across subjects demonstrated multifocal changes in rCBF during VPA: striate and extrastriate visual cortices; superior temporal cortices; and cerebellar cortex and vermis. Decreases in rCBF occurred in the lateral intraparietal area, prefrontal and frontal and/or supplementary eye ®elds. Analysis of regions of interest in the visual cortex showed systematic and appropriate task dependence of rCBF. Activations may re¯ect sensorimotor processing along the re¯ex arc of the accommodation system, while deactivations may indicate inhibition of systems participating in visual search. Introduction The principal stimulus triggering accommodative responses (AR) under typical viewing conditions is blur. Blur provides ambiguous information by lacking directionality in focusing power, i.e. no direct information about the over- or under-focusing of a target. Yet, the visual system is exquisitely sensitive to dioptric blur (Legge et al., 1987). Secondary cues (e.g. chromatic aberration) must be processed in parallel within a short time period and combined with the early computations of blur. Full posturing of the AR upon a target requires at least 350 ms following the detection of blur (Toates, 1972). During this time, computations include preliminary target identi®cation and the initiation of AR. The process by which the dioptric error becomes transformed into a motor command for accommodation remains largely unknown. The motor effector consists of ciliary muscles regulating both pupillary diameter and the curvature of the eye-lens. Autonomic parasympathetic ®bres from the Edinger±Westphal nucleus innervate the ciliary Correspondence: Dr J. V. Pardo, Cognitive Neuroimaging Unit (11P), VAMC, One Veterans Drive, Minneapolis, MN 55417, USA. E-mail: [email protected] *Present address: PET Centrum, Uppsala Universitet, UAS 75185, Uppsala and Department of Ophthalmology, Karolinska Institute, Huddinge Hospital, Huddinge, Sweden. Received 9 April 1999, revised 31 August 1999, accepted 14 September 1999 muscle (Fig. 1A). However, the eye does not adjust focus based upon a simple interaction between the blur signal and autonomic tonus. For example, stimulus sources can produce variation in the AR independent of retinal contrast (Westheimer, 1957; Provine & Enoch, 1975; Malmstrom & Randle, 1976; Ciuffreda & Kruger, 1988; McLin & Schor, 1988; McLin et al., 1988; Takeda et al., 1990; Richter & FranzeÂn, 1994). Structural correlates to these `higherorder' effects upon the AR re¯ex remain unmapped in humans. In sum, cortical processes preceding and monitoring the ciliary motor command signal are poorly understood (Fig. 1B). Several literature reports detail rapid learning and execution of volitional increases in dioptric strength of the crystalline eye lens, or voluntary positive accommodation (VPA, Campbell & Westheimer, 1960; Provine & Enoch, 1975; Ciuffreda & Kruger, 1988; McLin & Shor, 1988). Campbell & Westheimer (1960) measured VPA up to 3.0 D using a continuous recording optometer. Provine & Enoch (1975) successfully trained subjects to generate 9.0 D VPA to nullify the effects of a ±9.0-D contact lens. Ciuffreda & Kruger (1988) quanti®ed and compared re¯exive accommodation and VPA; both exhibited similar rates of change (diopters per second). The experiments presented here test the hypothesis that VPA movements of the eye involve neural circuits which can be visualized in healthy humans with positron emission tomography (PET) neuroimaging. Speci®cally, the present aim was to identify brain 312 H. O. Richter et al. FIG. 1. (A) Human dioptric focusing system and efferent pathways from the autonomic nervous system (ANS) to the ciliary muscle. The major innervation to the ciliary muscle is parasympathetic and follows the pathway shown by the thick solid lines. The parasympathetic pathway originates in the Edinger±Westphal nucleus and courses with the third nerve, where the ®bres travel to and synapse in the ciliary ganglion. The majority of the postganglionic parasympathetic ®bres travel to the ciliary muscle via the short ciliary nerves, but some of them (double asterisk) also travel with the long ciliary nerves. There is also evidence for a direct pathway of uncertain functional signi®cance (single asterisk) to the internal eye structures from the Edinger±Westphal nucleus. The sympathetic supply to the ciliary muscle (thin solid lines) originates in the diencephalon and travels down the spinal cord to the lower cervical and upper thoracic segments, to synapse in the spinociliary centre of Budge in the intermediolateral tract of the cord. From there, second-order nerves leave the cord by the last cervical and ®rst thoracic ventral roots; these preganglionic ®bres run up the cervical chain to synapse in the cervical ganglion. The third-order ®bres continue up the sympathetic carotid plexus and enter the orbit, either with the ®rst division of the trigeminal nerve (following the nasociliary division) or independently, where they join the long and short ciliary nerves, in the latter instance passing through the ciliary ganglion without synapse. Note that the connections between these pathways and the cerebral cortex are unspeci®ed. C8, cervical vertebra 8; T1, thoracic vertebra 1; T2, thoracic vertebra 2. (reproduced with permission from Kauffman, 1992). (B) Schematic model of accommodative processing during monocular viewing. When an image of a visual target on the retina is blurred, ~ 500 ms elapse between the detection of the blurred target and the complete AR to the target. During this time, neural circuits probably in the cerebral cortex and cerebellum compute the location of the intended target, decide whether to focus upon the intended target, and initiate the AR. Iterative computations tune the AR based upon initial visual sensory cues, feedback and comparison with the internal representation of the target. Open loop vergence in the viewing eye is inhibited. During visual accommodation top± down, cognitive-perceptual processing occurs within the accommodative system. activity with the process of accommodation (and secondary re¯ex vergence), not with the degree or precision of accommodation. Changes in neuronal activity, indexed by changes in regional cerebral blood ¯ow (rCBF), were measured across tasks with PET and the bolus 15O-water method. Three control conditions were used to evaluate the effects of blurring and contrast adaptation. The changes in rCBF were averaged after anatomical standardization across subjects. The resultant images identify putative networks involved in VPA and provide testable hypotheses for future research. Some ®ndings from this study have been presented previously in abstracts (Pardo et al., 1995; Richter et al., 1995). Materials and methods Subjects Thirteen naive healthy subjects (seven females and six males; mean age of 27 years, SD 66; range 20±43; all but one right-handed) participated in the PET study. Optometric measurements con®rmed normal visual acuity and ability to perform the focusing task (VPA). The volunteers gave written informed consent according to guidelines of the Institutional Review Board and the Radioactive Drug Research Committee. Behavioural paradigm and task analysis of VPA Four counterbalanced conditions provide contrasts between oculomotor processes during VPA: (i) stable (steady-state) visual accommodation through a 0.0-D glass lens (No-blur; Fig. 2A); (ii) inhibition of accommodation to blur induced optically by a ±5.0-D glass lens (Opt-blur; Fig. 2B and D): the subjects see alternately a sharp or a blurred image; (iii) VPA requiring large amplitude (~ ± 5 D), high-frequency (0.3 Hz), voluntary positive accommodation in the viewing eye in response to a retinal image defocused by a ±5.0-D lens (VPA; Fig. 2C) ± the subjects see mostly a sharp image, and the occluded eye shows synergistic accommodative/vergence movements (Fig. 2E); and (iv) passive viewing through a 0.0-D lens a checkerboard pattern blurred digitally to ~ ±5.0 D (Dig-blur) ± the subjects see a blurred image at all times. The resultant dioptric stimulus levels were as follows: 2.5 D for the No-blur condition; 7.5 D for the Optblur viewing condition; 2.5±7.5 D for the VPA condition; and 2.5 D for the Dig-blur condition. The subjects' non-dominant eye (i.e. left eye) was patched. They ®xated monocularly with the dominant eye upon a cross in the centre of a black and white monochromatic checkerboard pattern displayed on a video monitor 40 cm away (described below). In Opt-blur and VPA, the checkerboard pattern was blurred by manually placing a ± 5.0-D lens in front of the single naked (or corrected) eye for 1.5 s followed by removing the lens for 1.5 s. An electronic timer signalled the experimenter to change the lens position every 2 s. The same presentation procedure was followed for No-blur and Dig-blur except for the substitution of a 0.0-D lens. During VPA, the blurred image was brought into focus following each lens insertion by VPA. Instructions can bias the AR to sine wave gratings (Ciuffreda & Hokoda, 1985). The instructions used here were adapted from Stark & Atchison (1994): No-blur, `look at the checkerboard naturally ± the Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 PET study of human voluntary visual accommodation 313 same as you would when viewing a book or a sign at the same distance ± and maintain ®xation'; Opt-blur and Dig-blur, `look at the checkerboard ± make no effort to focus on it when the lens is placed in front of the eye ± let the checkerboard remain blurry and maintain ®xation'; and VPA, `look at the checkerboard and after each repeated defocus condition, carefully focus on the checkerboard so that it is maximally sharp and clear at all times ± maintain ®xation.' conditions, one of the subjects studied with PET underwent detailed measurement of eye movements in the Eye Movement Laboratory of the Department of Ophthalmology, Karolinska Institute (courtesy of Dr Han Ying). Eye movements under the different task conditions were measured using an infrared eye-tracker (Ober-2, Permobil, Sweden). Eye movement recording The black and white checkerboard (~ 1 cycle per degree) subtended 10.74° and contained a central ®xation cross (1.5°) to inhibit saccadic eye movements and to disable drifts in smooth pursuit. The display monitor was stationary, and there was no change in proximal stimulus for the conditions. The VPA responses occurred under dim lighting conditions. Space average luminance was 37.5 cd/m2 for the video screen and 1.0 cd/m2 for the surround (Quantum Instrument, Photometer LX, Garden City, NJ, USA). The digitally blurred checkerboard pattern was designed to appear subjectively similar to the optically blurred checkerboard (i.e. when viewed without accommodative effort through a ±5.0-D lens). Digital blurring was achieved by applying to the image of the checkerboard with ®xation mark a two-dimensional Gaussian ®lter with full-width at half-maximum (FWHM) of 2.8 mm (i.e. 4 pixel radius; pixel size 0.35 3 0.35 mm) in Adobe Photoshop V 4.0 (Adobe, San JoseÂ, CA, USA). During scanning, the absence of large amplitude saccades away from ®xation was monitored visually and with electro-oculograms (Coulbourn S75-01 Bioampli®er, Allentown, PA, USA; saccade detection sensitivity of ~ 5°). To ensure that the tasks elicited the expected eye movements during VPA and during the control Visual stimuli and effects of dioptric defocus PET scanning and image processing PET scans were acquired on an ECAT 953B camera (Siemens, Knoxville, TN, USA) in two-dimensional mode without correction for scatter or decay. Images contain 31 axial slices spaced ~ 3.5 mm apart. Subjects had an intravenous line in a forearm vein. Their head position was stabilized with a vacuum-moulded pillow (Olympic Vac Pac Medical, Seattle, WA, USA). Production and delivery of H215O were automated (Palmer et al., 1995). Approximately 1480± 1850 MBq (40±50 mCi) of H215O in 10 cc of normal saline was injected as a bolus over 10 s (Herscovitch et al., 1983). Tasks began ~ 15 s before tracer injection. Ten minutes elapsed between scans to allow decay. Changes in normalized tissue activity, integrated over 60 s after arrival of radioactivity into the brain, were used as an estimate of changes in rCBF. Data acquired with a zoom factor of 1.25 were reconstructed with ECAT software using ®ltered back- FIG. 2. Experimental design for neuroimaging of monocular VPA. (A) The No-blur condition involved maintaining ®xation while a 0.0-D lens was intermittently placed in front of the dominant eye. (B) The Opt-blur condition required the subject to avoid focusing upon the checkerboard whenever a ± 5.0-D lens intermittently interrupted the line of sight. The AR is inhibited volitionally and the retinal image remains blurred. (C) The condition of voluntary positive accommodation (VPA) required the subject to maintain maximal focus upon the checkerboard whenever the ±5.0-D lens interrupted the line of sight. High-frequency (0.3 Hz), large-amplitude (~ +4.5 D), voluntary AR must occur for successful performance. A compatible distribution of negative AR results after the removal of the ±5.0-D-lens, after each successful VPA response. One additional control (not shown) included passive viewing of a digitally blurred checkerboard (Dig-blur). (D) Typical amplitudes of horizontal eye movements during Opt-blur. Note the absence of large-amplitude, synkinetic vergence eye movements of the occluded left eye (contrast with E below) and stable ®xation of right eye. RE, right eye position; LE, left eye position. Downward de¯ection denotes eye movement to the right. (E) Typical amplitudes of horizontal eye movements during VPA to a ±5.0-D blurred checkerboard. Co-activation of vergence in the left (occluded eye) during VPA is obvious (mean 6 SD, 8.26 6 1.19). Although most vergence in the ®xating right eye is inhibited, small order conjunctional divergence movements to the right can be observed (mean 6 SD, 0.74 6 0.27). LE, RE, and eye movement conventions as in D. Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 314 H. O. Richter et al. projection with a Hann ®lter of 0.4 cycles/pixel. The ®nal ®ltered image resolution was 11 mm FWHM. Software developed by Minoshima and colleagues (Minoshima et al., 1992; 1993; 1994) normalized global activity within each scan, realigned scans within a study session, estimated the intercommissural line from image ®ducials, and transformed stereotactically the data using linear warping. ANALYSETM (BIR, Mayo, Rochester, MN, USA) permitted further image processing and display. pupillary constriction/dilatation. Blur-driven accommodation signals in¯uence vergence output, and similarly, disparity-driven vergence signals in¯uence accommodation output. We chose to study blurdriven accommodation with vergence disparity under open-loop conditions (i.e. monocular viewing), thereby, maximally stressing the accommodation subsystem and leaving the vergence system devoid of sensory input. Signi®cance threshold selection To explore the functional anatomy of voluntary accommodation processes across the whole brain, analyses of paired image subtraction were completed for the following three contrasts: (i) VPA minus No-blur; (ii) VPA minus Opt-blur; and (iii) VPA minus Dig-blur. Two scans were obtained from each subject for these three conditions (No-blur, Opt-blur, VPA). Seven of the subjects also contributed one scan of Dig-blur. Task order was counterbalanced whenever possible across subjects. Comparison of No-blur and Opt-blur did not reveal signi®cant omnibus differences (i.e. 0 pixels above a P < 0.001 threshold, with 89 pixels expected by chance). Therefore, this image subtraction was not signi®cantly different from image noise and was not further analysed. Comparing the VPA with control conditions, No-blur or Opt-blur, showed signi®cant differences (VPA minus No-blur had 187 pixels above a P < 0.001 threshold, 88 pixels expected from noise; VPA minus Opt-blur had 793 pixels, 88 pixels expected). Likewise, VPA differed signi®cantly from Dig-blur (149 pixels observed, 96 expected). Hence, high-frequency (0.3 Hz), large-amplitude (~ +4.5 D) monocular accommodation to a blurred checkerboard pattern produces signi®cant changes in rCBF when compared with all control conditions (No-blur, Opt-blur and Dig-blur). These activations along with their stereotactic coordinates appear in order of decreasing magnitude in Table 1a±f. Figure 3 displays activations and deactivations in the contrast between VPA and No-blur. ROI analysis Occipital activation during VPA Because the quality of the sensory representation plays a critical role in accommodative processing, unsubtracted or subtracted rCBF (DrCBF) in the visual cortex was assessed across the different scan conditions. Repeated-measures ANOVA was performed on individual activity in spherical ROIs in calcarine (~ 8 mm radius) or occipital (~ 16 mm radius) cortex. Bilateral ROIs were centred on the Talairach coordinates (+17, ±94, ±7) in BA 17 or (+17, ±87, 0) including BA 18 and 19. Additional spherical ROIs (~ 8 mm radius) were centred on the coordinates of peak subtracted rCBF (DrCBF) resulting from VPA minus either No-blur or Opt-blur. The Talairach coordinates that guided these ROIs appear in Table 1a±d. Pearson product moment correlations were next calculated to provide a common metric for displaying the relation between activity in the different ROIs. By cancelling sources of variance unrelated to VPA, the between-task DrCBF matrix shows a commonality in how structures become activated or deactivated during the production of VPA (for details of methodology, see Zald et al., 1998b). A distributed set of activation foci in occipital visual cortex (BA 17 and 18) appeared during VPA in comparison with all control conditions (see Table 1) Visual regions included the posterior portion of the calcarine cortex subserving the fovea, lingual and fusiform gyri, and cuneus. The visual responses show left hemispheric dominance. A signi®cance threshold of Z = 3.2 was used. This threshold is consistent with the reported literature given the methods, sample size and image resolution employed in this study. Non-parametric bootstrap analysis of intrasubject contrasts between ECR scans suggests that, on average, one false positive arises by chance in an averaged image (Zald et al., 1998a). Study design and contrasts Results Eye movements Generally, subjects found the VPA task easy to perform. Visual monitoring and electro-oculograms during the PET scans con®rmed ongoing ®xation. Outside the scanner, higher sensitivity measurements in a typical subject using an infrared eye-tracker demonstrated, during VPA, the expected synergistic vergence eye movements (accommodative/vergence) of the occluded eye (LE) with concomitant small amplitude (0.25±1°) eye movements in the viewing eye (RE; Fig. 2E). No large amplitude eye movements occurred in the control conditions; eye position was stable and task appropriate (Fig. 2D; Ditchburn, 1973). The accommodative and vergence subsystems are tightly cross-coupled (Mays & Gamlin, 1995); an AR is normally followed by vergence movements of the eyes and Overall comparisons across viewing conditions ANOVA This analysis was restricted to the principal conditions of interest (No-blur, Opt-blur and VPA), each with 13 subjects. The repeatedmeasures ANOVA of average rCBF in the calcarine ROI (sphere, ~ 8 mm radius; Fig. 4A) in a 3 3 2 (scan conditions 3 hemisphere) design revealed a signi®cant main effect of scan condition (F2,24 = 5.7, P = 0.009) and a signi®cant scan-condition±hemisphere interaction (F2,24 = 4.0, P = 0.03). The hemisphere main effect was not signi®cant (F1,12 = 0.2, P = 0.7). Post hoc testing with two-tailed ttests with Bonferroni correction for multiple comparisons shows that the hemisphere activity during VPA was signi®cantly greater than during No-blur (t12 = 3.6, P = 0.02) and Opt-blur (t12 = 3.6, P = 0.02, see Fig. 4B). Figure 4C displays rCBF for the BA 17 region of interest (ROI, averaged across hemispheres) for each subject and for each condition. Overall, averaged rCBF was greater during VPA than during Opt-blur or No-blur. Contrasting visual responses between Opt-blur and Dig-blur Contrast adaptation may confound the contrast between VPA versus No-blur or Opt-blur. In No-blur, the subject sees a sharp and unchanging checkerboard that can result in contrast adaptation. In Opt-blur, the subject sees alternately blurred and sharp checkerboards, respectively. In VPA, the subject initially sees the same stimuli as in No-blur, but then sees transients that could spare contrast adaptation. However, contrasting No-blur and Opt-blur did not produce signi®cant changes in rCBF (see above). Also, the worst case scenario for contrast adaptation, Dig-blur, did not produce visual Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 PET study of human voluntary visual accommodation 315 FIG. 3. Cerebral activation and deactivation during voluntary positive monocular accommodation (VPA). Regions activated by the VPA response (VPA minus Noblur comparison) are shown in A (horizontal slices) and B (surface rendering of activations along lateral, medial, frontal and posterior aspect of each hemisphere). Activation during VPA compared with No-blur. Threshold for these images set at Z = 2.1 to display optimally the pattern of activity; note that signi®cance threshold was set at Z = 3.2. Hotter colours denote greater Z scores; white set to maximum in each image. Panels show that VPA is associated with posterior activations in occiput and cerebellum. The visual hemispheric responses appear asymmetric. (C) (No-blur±VPA). Deactivation during VPA, condition VPA, compared with condition No-blur. VPA, when compared with this control condition, results in deactivations in anterior structures, prefrontal cortices and medial frontal cortex at the cingulate sulcus. Activation in many of these regions has been reported during eye movements (see Discussion). Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 316 H. O. Richter et al. rCBF responses any different from those arising in Opt-blur (unpaired t-test: for left hemisphere ROI, t18 = 0.4, P = 0.7; for right hemisphere ROI, t18 = 0.34, P = 0.7). Inter-hemispheric occipital correlations Data from the occipital ROI (~ 16 mm radius) were used to measure the functional connectivity between homologous visual regions across hemispheres. A signi®cant correlation was obtained during No-blur (r = 0.54, P = 0.056; Fig. 5A) and during VPA (r = 0.62, P = 0.02; Fig. 5B). The visual activations arising in the contrast between VPA and Opt-blur (see Table 1c; Fig. 6) were markedly intercorrelated (r = 0.86, P = 0.0001), suggesting functional interactions (Zald et al., 1998b). Hemispheric asymmetries Figure 3 suggests dominance of the left hemisphere during VPA. The ANOVA using the small ROI shown in Fig. 4A showed no main effect of hemisphere. ANOVA using the larger sphere (~ 16 mm radius, Fig. 4A) extending into the occipital activation foci of Fig. 3 demonstrates a main effect of hemisphere (F1,24 = 11, P = 0.003) with greater activation in the left hemisphere. These asymmetries in occipital rCBF appear even at the level of individual subjects and even during No-blur (see Figs 5 and 7). The degree of left hemisphere dominance for nine subjects during No-blur averaged 8% (range 4± 11%, SD 2.7), whereas the right dominance for the remaining four subjects averaged 4% (range 3±6%, SD 1.0). Temporal activation during VPA Activity in the right temporal region (BA 22) was greater during VPA than during Opt-blur (Table 1c, region 3). In the comparison between VPA and No-blur, the same area was activated, but failed to reach conservative levels of signi®cance (Z = 2.8). Right BA 22 had signi®cant (P < 0.05) functional connectivity with several regions that surfaced in the contrast between VPA and Opt-blur: right BA 17; left BA 18; thalamus; and right basal ganglia (see Table 1c). Cerebellar activation during VPA Increases in cerebellar rCBF arose consistently across all the comparisons between VPA and control conditions. These localized TABLE 1. Brain regions implicated in voluntary positive accommodation (VPA) Talairach coordinates Foci x (a) Regions activated by the VPA response (VPA minus No-blur comparison) 1. Right striate visual cortex (BA 17) 19 2. Anterior lobe of vermis 8 3. Left visual cortex (BA 17) ±10 4. Anterior lobe of vermis 3 5. Left visual cortex (BA 18) ±6 (b) Regions deactivated by the VPA response (comparison of No-blur with VPA) 1. Left superior frontal gyrus (BA 10) ±15 2. Right frontal lobule (BA 6) 26 3. Left/right posterior cingulate ±3 4. Left parietal lobule (BA 39/40) ±35 (c) Regions activated by VPA, as shown by the VPA minus Opt-blur comparison 1. Right primary visual cortex (BA 17) 17 2. Left visual cortex (BA 18) ±17 3. Right lateral temporal sulcus (BA 22) 60 4. Thalamus (midbrain) ±1 5. Right lenticular nucleus (basal ganglia) 33 6. Right lateral cerebellum 26 (d) Regions deactivated by the VPA response (comparison of Opt-blur with VPA) 1. Left superior frontal gyrus (BA 10)* ±15 (e) Regions activated by the VPA response (VPA minus Dig-blur comparison) 1. Right lateral cerebellum 12 2. Right lateral cerebellum 33 3. Left insula ±33 4. Left primary visual cortex (BA 17) ±3 5. Right primary visual cortex (BA 17) 8 6. Left visual cortex (BA 18) ±33 7. Left lateral cerebellum ±1 (f) Regions deactivated by the VPA response (Dig-blur minus VPA comparison) 1. Left superior frontal gyrus (BA10) ±8 2. Left lateral cerebellum ±44 3. Left cingulate sulcus (BA 32) ±6 4. Left lateral cerebellum ±26 5. Right cingulate sulcus (BA 24/32) 1 6. Right frontal lobule (BA 8) 17 7. Left post central gyrus (BA 1/2) ±57 8. Left frontal lobule (BA 8) ±26 Magnitude of peak activation (Z-score) y z ±89 ±67 ±76 ±69 ±85 ±7 ±2 14 ±16 20 4.35 3.94 3.54 3.22 3.20 62 ±4 ±55 ±46 25 45 29 32 ±5.09 ±3.85 ±3.48 ±3.40 ±94 ±80 5 ±8 ±10 ±73 ±7 ±9 4 0 ±2 ±27 5.16 4.29 3.44 3.40 3.32 3.22 62 25 ±4.27 ±85 ±87 21 ±91 ±91 ±76 ±69 ±9 ±9 2 7 ±2 0 ±34 3.95 3.87 3.58 3.50 3.40 3.35 3.22 64 ±67 50 ±82 35 19 ±22 26 18 ±38 14 ±32 ±4 43 40 47 ±6.15 ±4.14 ±3.83 ±3.77 ±3.63 ±3.24 ±3.24 ±3.22 Response magnitudes and locations in coordinates of the atlas of Talairach & Tournoux (1988). x, y and z (in mm) correspond to right±left, anterior±posterior and superior±inferior dimensions of the brain. Right, anterior and superior sides have positive coordinates. BA refers to approximate Brodmann areas. Foci at the brain margin are labelled by an asterisk. Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 PET study of human voluntary visual accommodation 317 both to the vermis (e.g. Table 1a, regions 2 and 4) and to the cerebellar cortex (e.g. Table 1c, region 6). Insular activation during VPA The only insular activation surfaced in the contrast between VPA minus Dig-blur (Table 1e, region 3). The insula was also weakly activated in the contrast between VPA and Opt-blur (not shown). Of note, both of these control conditions (i.e. Opt-blur and Dig-blur) do not require accurate posturing of the ciliary muscle to perform the task. Frontal and parietal deactivation during VPA The activity of the right premotor area BA 6 (frontal eye ®eld, FEF) was signi®cantly reduced during monocular VPA responses in the comparison involving VPA and No-blur (Table 1b, region 2). This decrease was highly correlated (r2 = 0.65, P < 0.001) with a concomitant activity decrease in the left parietal lobule BA 39/40 (Table 1b, region 4; Fig. 8). Left BA 10 demonstrated deactivation in the contrasts VPA minus No-blur; VPA minus Opt-blur; and VPA minus Dig-blur (Table 1b, region 1, 1d, and 1f, region 1). The contrast VPA minus Dig-blur revealed deactivations in the left cingulate sulcus (BA 32) and in bilateral BA 8 (Table 1f, regions 3, 5, 6 and 8). No changes localized to that region of the right anterior cingulate cortex critical to the anterior attention system (Pardo et al., 1990). No parietal activation was observed during VPA minus any of the controls. A posterior cingulate deactivation mapped to BA 31 in VPA minus No-blur (Table 1b, region 3). Also, left BA 40/39 (supramarginal gyrus; inferior parietal) was deactivated in VPA minus No-blur. This response appeared functionally connected to the BA 6 deactivation in VPA minus No-blur (Fig. 8). Discussion To the best extent possible, the major source of activation in the present study relates to VPA, and to a lesser extent, synkinetic vergence movements. Activations may overall re¯ect sensorimotor processing along the re¯ex arc of the accommodation system, while deactivations may indicate inhibition of systems participating in the visual search. However, the activity of some `near±far' neurons in the central nervous system (neurons which are activated by visual accommodative-vergence eye-movements) may still process both vergence and accommodation information regardless of whether or not these movements arise during normal viewing conditions, during blur-driven monocular viewing (open-loop vergence; present study), or during disparity-driven viewing (open-loop accommodation; Zhang & Gamlin, 1998). Occipital activity during VPA FIG. 4. (A) Horizontal and sagittal sections of Talairach brain showing placement of occipital and calcarine ROI (centred on x, y, z = +17, ±87, +1 and +17, ±94, ±7, respectively). (B) Group mean unsubtracted activity (rCBF) within bilateral ~ 8 mm diameter ROI centred on calcarine sulcus (BA 17) during controls (No-blur and Opt-blur) and during VPA. Data are mean 6 SEM. Paired t-test, *P < 0.05, **P < 0.01. (C) Mean individual unsubtracted activity (rCBF) within bilateral ~ 8 mm diameter sphere ROI centred on calcarine sulcus (BA 17) during two controls and during VPA. The blurring by ±5.0 D (~ 20/200 or 0.1 in visual acuity, Stein et al., 1987) relative to No-blur resulted in ~ 4% change in calcarine rCBF. Demer et al. (1993) reported that optical blur corresponding to visual acuity reductions from 20/20 to ~ 20/200 reduced relative glucose metabolism in the primary visual cortex by ~ 8%. However, the blur in Demer (1993) was constant rather than the ON/OFF mode used in the current study; this may explain the lower value of the present study. BA 19 was noticeably less affected than BA 17 and 18 by the dioptric manipulations in both studies. The visual activations in BA 17 from the contrast between VPA and Opt-blur (Table 1c) were expected and resulted in part from increased retinal contrast during successful VPA. The increased Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 318 H. O. Richter et al. FIG. 5. Test of hemispheric coupling of individual unsubtracted activity (rCBF) using the occipital ~ 16 mm spherical ROI during one control (No-blur) and during VPA. FIG. 6. Individual DrCBF increases from voluntary positive accommodation minus an optically blurred reference state (i.e. VPA±Opt-blur) in right primary visual cortex (BA 17) coupled with simultaneous increases in left visual cortex BA 18. The Talairach co-ordinates (x, y, z) for these ROI are listed in Table 1c (regions 1 and 2). visual activation during VPA compared with No-blur (Table 1a; Figs 3 and 4) cannot be explained so readily. Even if the subjects were completely accurate in their accommodation (an unrealistic view), no activation during VPA over that in No-blur would be expected based upon considerations of retinal contrast. Degradation of the retinal image by dioptric defocus decreases the amplitude of the component in visual evoked potentials dependent upon contrast (FranzeÂn et al., 1994). In No-blur, the visual target was always in focus. Visual cortical cells rapidly decrease their ®ring rate in response to decreasing frequency of stimulation (Fox & Raichle, 1984). The visual activation arising during VPA versus No-blur could theoretically occur from contrast adaptation in No-blur. However, VPA and Opt-blur should not differ in the extent of contrast adaptation. Dig-blur, a worst case scenario for contrast adaptation, did not produce signi®cant visual activity when compared with Opt-blur. Thus, contrast adaptation effects appear minimal in the present protocol. The occipital activation observed during VPA (Table 1a and Fig. 3A and B) may arise from a variety of sources. (i) Hardwired, local re-entrance within microcircuitry analogous to servo-mechan- FIG. 7. Test of the degree to hemispheric symmetry of unsubtracted activity (rCBF) within ~ 16 mm diameter ROI centred on the occipital lobule in both hemispheres during one control (No-blur) and during VPA. Talairach coordinates: x, y, z = +17, ±87, +1). Data are mean 6 SEM. n = 13. Paired ttest, *P < 0.05, **P < 0.01. isms. Even-error and odd-error focusing signals may form at an early stage within the visual cortex; the occipital activation reported here may in part be a consequence of ascending (`intended value') and descending streams (`actual value') of sensory information (Ullman, 1995). (ii) Cognitive `top±down' processing (e.g. Fulton, 1928; Kosslyn et al., 1993; Motter, 1993; Heinze et al., 1994). (iii) Feedforward gain control by eye position signals on activity of striate neurons (Trotter & Celebrini, 1999). Although the present experiment did not entail switching the viewing eye, the hemisphere laterality effects seen in the current study are consistent with past reports. Demer et al. (1988) used a strobe light ¯ashing at 8 Hz while the subjects wore their distant refractive correction during a monocular viewing condition. They reported that the hemisphere contralateral to the viewing eye was more active (5±20% difference) in both amblyopes and emmetropes. Also, Hung et al. (1990) quanti®ed cerebral activity with SPECT during monocular stimulation of either eye and reported contralateral dominance for each eye (cf. Imamura et al., 1997). Temporal activity during VPA Both the VPA versus No-blur and VPA versus Opt-blur image subtractions showed regions of activation in the right or left superior temporal regions. To some extent, these differences relate to the Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 PET study of human voluntary visual accommodation 319 PET study (Richter et al., 1998), however, adjacent BA 6 coordinates were also deactivated bilaterally during the execution of monocular negative voluntary accommodation. In sum, the ocular near response could interact functionally with the ®xation system to inhibit visual search for the purpose of providing a clear foveal image. Evidence in favour of this hypothesis was forwarded by Ohtsuka & Sato (1996). They presented electrophysiological and anatomical results linking omnipause activity (Munoz & Wurtz, 1993) with accommodation activity within the rostral superior colliculus (see also Sawa & Ohtsuka, 1994). Insular activation during VPA The insular activation in VPA versus Dig-blur (and to a lesser extent VPA versus Opt-blur) occurred under control conditions with the least recruitment of the ciliary muscle. Insular cortices play an important role in multimodal sensory integration and in autonomic processing (Augustine, 1996). Parietal deactivation during VPA choice of threshold. However, inhibition of re¯ex accommodation may have occurred in Opt-blur, possibly contributing to the change in temporal activity seen in Opt-blur. Individual components of the near triad have been dissected in paralysed anaesthetized cats using the microelectrode stimulation technique. Accommodative/vergence processing in the cat is known to occur within the lateral suprasylvian sulcus (LS) and middle suprasylvian sulcus (MSS), both extrastriate parietotemporal regions with homology to macaque V5 (Bando & Toda, 1991; Yoshizawa et al., 1991). Furthermore, Jampel (1960) found ARs, in addition to vergence eye movements and pupillary constriction, evoked by electrical stimulation of the cerebral cortex surrounding the superior temporal sulcus (STS) in the macaque temporal lobe. The parietal rCBF decreases are interpreted, likewise, as a disengagement of the cortical system responsible for covert and overt visual search. Speci®cally, the left parietal lobule (BA 39/40) was functionally coupled with rCBF decreases occurring within the right FEF (BA 6) during the cortical processing of VPA. Ample evidence suggests that the inferior parietal lobule (IPL) of primates plays a role in visually guided saccadic eye movements (reviewed recently in Petit & Haxby, 1999). Lesions to the IPL in humans often produce de®cits in saccadic eye movements (Pierrot-Deseilligny et al., 1995). In a parallel PET study (Richter et al., 1998), adjacent BA 39 (and BA 6) coordinates were also deactivated during the execution of monocular negative voluntary accommodation. IPL and BA 39 posterior parietal neurons in the macaque are known to carry premotor spatial signals relevant to changes in gaze within three-dimensional space relative to the plane of ®xation (Sakata et al., 1980; Gnadt & Mays, 1995). However, the present VPA paradigm did not require any overt three-dimensional spatial shifts in posturing of the ARs. The posterior parietal association cortices are also critical for directed attention, visual-spatial analysis and vigilance in the contralateral hemisphere (Mountcastle et al., 1977). Frontal deactivations during VPA Cerebellar involvement in VPA The frontal rCBF decreases are interpreted principally as a disengagement of the systems specialized for visual search during cortical processing of accommodation/vergence eye movements. Multiple prefrontal cortical ®elds involve sensorimotor processing for eye movements: frontal eye ®elds (FEF); supplementary motor area (SMA); supplementary eye ®elds (SEF); and several adjacent prefrontal regions (Mitz & Godschalk, 1989). Many of these regions have been visualized during neuroimaging studies of oculomotor tasks (Fox et al., 1985; Paus et al., 1993; Petit et al., 1993; Lang et al., 1994; O'Driscoll et al., 1995; Petit & Haxby, 1999). Several of these regions also activate during covert visual orienting of attention (Corbetta et al., 1998; Gitelman et al., 1999). During VPA, the neural systems involved in accommodative/vergence and visual search appear partly dissociable; prefrontal systems participating in visual search (e.g. orienting of visuospatial attention, voluntary saccades and smooth pursuit) deactivate in the presence of synkinetic accommodative/vergence movements in the occluded eye and small amplitude eye movements in the ®xating eye. De®nitive dissection will require simultaneous high-resolution recording of eye movements while scanning during VPA and visual search. In a parallel Several activations localized to the vermis and cerebellar cortex. The cerebellum is interconnected with many regions showing changes in activity during VPA (Bando et al., 1984; Kawasaki et al., 1993). The cerebellum receives a direct projection from the superior temporal sulcus (or lateral suprasylvian area) via the pontine nuclei. Cerebellar efferents project to the pretectum and parasympathetic oculomotor neurons. Recent reports document monosynaptic connections between FEF, IPL and the dentate nucleus, as well as between the dorsolateral prefrontal cortex (e.g. BA 46), globus pallidus, thalamus and dentate (Lynch et al., 1994; Middleton & Strick, 1994). The cerebellum is implicated in human accommodation. Kawasaki et al. (1993) described a patient with a cerebellar lesion who presented with `dif®culties at focusing on both near and far.' The patient had signi®cantly increased accommodation and relaxation times bilaterally. Gamlin & Clark (1995) studied in alert-behaving rhesus monkeys the single unit activity in the nucleus reticularis tegmenti, a precerebellar nucleus. Cells displayed bursts of activity during far-to-near viewing. Many had a tonic ®ring rate which increased as a function of increases in accommodation as well as convergence. Interestingly, the cells were often in close proximity to FIG. 8. Individual DrCBF decreases from VPA minus No-blur in right frontal BA 6 coupled with simultaneous decreases in left parietal BA 39/40. The Talairach co-ordinates (x, y, z) for these ROI are listed in Table 1b (regions 2 and 4). Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 320 H. O. Richter et al. neurons with saccade-related ®ring. The functional role of the vermal activation in the present study may relate uniquely to the processing of VPA (Ohtsuka & Sawa, 1997). The vermal area (lobule VII) receives information from visually responsive neurons including nucleus reticularis tegmenti (Schmahmann, 1996). Zhang & Gamlin (1998) found in a detailed study single units in the cerebellar interposed nucleus, connecting to the nucleus reticularis tegmenti, activity related to vergence and accommodation irrespective of whether or not these eye movements were elicited by blur or disparity cues. Current PET techniques would be insensitive to the detection of activity in such a small volume. Conclusion Our data show that VPA recruits circuits dissociable, in part, from those participating in other oculomotor tasks. Many of the recruited regions process visual information during ®xation steady-state accommodation, as they are also active during both No-blur and Opt-blur. The analysis of ROI in the visual cortex showed systematic and appropriate task dependence on rCBF in the great majority of the subjects. This gives credence to the validity of the present measures and to the approach used here. These data identify an extensive network of cortical and cerebellar regions involved in VPA. Overall, VPA is associated with rCBF increases in posterior structures (occipital, cerebellar and temporal regions), and with rCBF decreases in frontal and parietal regions. Many of these structures were previously implicated in re¯exive computations of accommodation from lesion studies and electrophysiological experiments in monkeys and cats (Maekawa & Ohtsuka, 1993). An interesting observation is the dynamic and reciprocal functional connectivity during VPA between the accommodation and visual search/visual attention systems. The saccade and accommodation systems share premotor circuitry based upon the direct connections between accommodation units in the superior colliculus and omnipaus neurons in the saccade system (Ohtsuka & Sato, 1996). However, functional linkage between these oculomotor systems is only now emerging and has relied on histological studies. The absence of activation in the anterior attention system and the deactivation in regions supporting covert orienting of visuospatial attention (and overt eye movements) suggests that these networks are dissociable from those mediating VPA. Acknowledgements We thank our study participants for their cooperation and patience; Dr Satoshi Minoshima (University of Michigan) for providing software for PET data analyses; Dr Michael Kuskowski for statistical consultation; Dr Richard Robb (Mayo) for providing ANALYSETM software; Chris Fy and the Eye Clinic (VAMC, Minneapolis, MN, USA) for performing optometric evaluations; Priscilla Weber for editing; Dr Ying Han for eye movement recordings with the Ober-2 Eye-Movement System; and two anonymous reviewers for their important comments. This work was supported by the Department of Veterans Affairs, USA, and the Karolinska Institute/University of Minnesota Exchange Program. Dr Richter was a Postdoctoral Fellow supported in part by the Fulbright Commission, the Swedish Institute, the Swedish Crown. Abbreviations AR, accommodative response; Dig-blur, digitally blurred checkerboard; DrCBF, subtracted regional cerebral blood ¯ow; FEF, frontal eye ®eld; FWHM, full-width at half-maximum; IPL, inferior parietal lobule; No-blur, normal (0.0 D `blurred') checkerboard; Opt-blur, ±5.0-D blurring of checkerboard; PET, positron emission tomography; rCBF, regional cerebral blood ¯ow; ROI, region of interest; SEF, supplementary eye ®elds; SMA, supplementary motor area; VPA, ±5.0-D blurring of checkerboard and VPA; VPA, voluntary positive accommodation. References Augustine, J.R. (1996) Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev., 22, 229±244. Bando, T., Yamamoto, N. & Tsukahara, N. (1984) Cortical neurons related to lens accommodation in posterior lateral suprasylvian area in cats. J. Neurophysiol., 52, 879±891. Bando, T. & Toda, H. (1991) Cerebral cortical and brainstem areas related to the central control of lens accommodation in cat and monkey. Comp. Biochem. Physiol., 98, 229±237. Campbell, F.W. & Westheimer, G. (1960) Dynamics of accommodation responses of the human eye. J. Physiol., 151, 285±295. Ciuffreda, K.J. & Hokoda, S.L. (1985) Effect of instruction set and higher level control on the accommodative response spatial frequency pro®le. Ophthalmic Physiol. Opt., 5, 221±223. Ciuffreda, K.J. & Kruger, D.B. (1988) Dynamics of human voluntary accommodation. Am. J. Optom. Physiol. Opt., 65, 365±370. Corbetta, M., Akbudak, E., Conturo, T., Snyder, A., Ollinger, J., Drury, H., Linenweber, M., Petersen, S., Raichle, M., Van Essen, D. & Shulman, G. (1998) A common network of functional areas for attention and eye movements. Neuron, 21, 761±773. Demer, J.L., Noorden, G.K., Volkow, N.D. & Gould, K.L. (1988) Imaging of cerebral blood ¯ow and metabolism in amblyopia by positron emission tomography. Am. J. Ophthal., 105, 337±347. Demer, J. (1993) Positron emission tomographic studies of cortical function in human amblyopia. Neurosci. Biobehav. Rev., 17, 469±476. Ditchburn, R.W. (1973) Eye Movements and Visual Perception. Clarendon Press, Oxford, UK. Fox, P.T. & Raichle, M.E. (1984) Stimulus rate dependence of regional cerebral blood ¯ow in human striate cortex, demonstrated by positron emission tomography. J. Neurophysiol., 51, 1109±1120. Fox, P.T., Fox, J.M., Raichle, M.E. & Burde, R.M. (1985) The role of cerebral cortex in the generation of voluntary saccades: a positron emission tomographic study. J. Neurophysiol., 54, 348±369. FranzeÂn, O., Lennerstrand, G. & Richter, H. (1994) Brain potential correlates of supraliminal contrast functions and defocus. Int. J. Human Computer Interact., 6, 155±176. Fulton, J.F. (1928) Observations upon the vascularity of the human occipital lobe during visual activity. Brain, 51, 310±320. Gamlin, P.D. & Clarke, R.J. (1995) Single-unit activity in the primate nucleus reticularis tegmenti pontis related to vergence and ocular accommodation. J. Neurophysiol., 73, 2115±2119. Gitelman, D.R., Nobre, A.C., Parrish, T.B., LaBar, K.S., Kim, Y.H., Meyer, J.R. & Mesulam, M.M. (1999) A large-scale distributed network for covert spatial attention. Brain, 122, 1093±1106. Gnadt, J.W. & Mays, L.E. (1995) Neurons in monkey parietal area LIP are tuned for eye-movement parameters in 3D space. J. Neurophysiol., 73, 280± 297. Heinze, H.J., Mangun, G.R., Burchert, W., Hinrichs, H., Scholz, M., Munte, T.F., GoÈs, A., Scher, M., Johannes, S., Hundeshagen, H., Gazzaniga, M.S. & Hillyard, S.A. (1994) Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature, 372, 543±546. Herscovitch, P., Markham, J. & Raichle, M.E. (1983) Brain blood ¯ow measured with intravenous H215O. I. Theory and error analysis. J. Nucl. Med., 24, 782±789. Hung, G.K., Stahl, T. & Powell, A. (1990) Neural activity following monocular accommodation using SPECT. Med. Sci. Res., 18, 865±868. Imamura, K., Richter, H., Fischer, H., Lennerstrand, G., FranzeÂn, O., Rydberg, A., Andersson, J., Schneider, H., Onoe, H., Watanabe, Y. & LaÊngstroÈm, B. (1997) Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neurosci. Lett., 225, 173±176. Jampel, R.S. (1960) Convergence, divergence, pupillary reactions and accommodation of the eyes from faradic stimulation of the macaque brain. J. Comp. Neurol., 115, 371±399. Kauffman, P. (1992) Accommodation and presbyopia: neuromuscular and biophysical aspects. In Hart, W. (ed.), Adlers' Physiology of the Eye, 9th edn. Mosby Year Book, Mosby, St Louis, p. 397. Kawasaki, T., Kiyosawa, M., Fujino, T. & Tokoro, T. (1993) Slow accommodation release with a cerebellar lesion. Brit. J. Ophthalmol., 77, 678. Kosslyn, S.M., Alpert, N.M., Thompson, W.L., Maljkovic, V., Weise, S.B., Chabris, C.F., Hamilton, S.E., Rauch, S.L. & Buonanno, F. (1993) Visual mental imagery activates topographically organized visual cortex: PET investigations. J. Cognit. Neurosci., 5, 263±287. Lang, W., Petit, L., HoÈllinger, P., Pietrzyk, U., Tzourio, N., Mazoyer, B. & Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321 PET study of human voluntary visual accommodation Berthoz, A. (1994) A positron emission tomography study of oculomotor imagery. Neuroreport, 5, 921±924. Legge, G.E., Mullen, K.T., Woo, G.C. & Campbell, F.W. (1987) Tolerance to visual defocus. J. Opt. Soc. Am., 4, 1594±1598. Lynch, J.C., Hoover, J.E. & Strick, P.L. (1994) Input to the frontal eye ®eld from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp. Brain Res., 100, 181±186. Maekawa, H. & Ohtsuka, K. (1993) Afferent and efferent connections of the cortical accommodation area in the cat. Neurosci. Res., 17, 315±323. Malmstrom, F.V. & Randle, R.J. (1976) Effects of visual imagery on the accommodative response. Percept. Psychophys., 19, 450±453. Mays, L.E. & Gamlin, P.D.R. (1995) Neuronal circuitry controlling the near response. Curr. Opin. Neurobiol., 5, 763±768. McLin, L.N. & Schor, C.M. (1988) Voluntary effort as a stimulus to accommodation and vergence. Invest. Ophthalmol. Vis. Sci., 29, 1739±1746. McLin, L.N., Schor, C.M. & Kruger, P.B. (1988) Changing size (looming) as a stimulus to accommodation and vergence. Vision Res., 28, 883±898. Middleton, F.A. & Strick, P.C. (1994) Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science, 266, 458± 461. Minoshima, S., Berger, K., Lee, K. & Mintun, M. (1992) An automated method for rotational correction and centering of three-dimensional functional brain images. J. Nucl. Med., 33, 1579±1585. Minoshima, S., Koeppe, R., Mintun, M., Berger, K., Taylor, S. & Frey, K. (1993) Automated detection of the intercommissural line for stereotactic localization of functional brain images. J. Nucl. Med., 34, 322±329. Minoshima, S., Koeppe, K.L., Frey, K. & Kuhl, D. (1994) Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J. Nucl. Med., 35, 1528±1537. Mitz, A.R. & Godschalk, M. (1989) Eye-movement representation in the frontal lobe of rhesus monkeys. Neurosci. Lett., 106, 157±162. Motter, B.C. (1993) Focal attention produces spatially selective processing in visual cortical areas V1, V2 and V4 in the presence of competing stimuli. J. Neurophysiol., 70, 909±919. Mountcastle, V.B., Talbot, W.H. & Yin, T.C.T. (1977) Parietal lobe mechanisms for directed visual attention. J. Neurophysiol., 40, 362±389. Munoz, D.P. & Wurtz, R.H. (1993) Fixation cells in monkey superior colliculus I. Characteristics of cell discharge. J. Neurophysiol., 70, 559±575. O'Driscoll, G.A., Alpert, N.M., Matthysse, S.W., Levy, D.L., Rauch, S.L. & Holzman, P.S. (1995) Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc. Natl Acad. Sci. USA, 92, 925±929. Ohtsuka, K. & Sato, A. (1996) Descending projections from the cortical accommodation area in cat. Inv. Ophthalmol. Vis. Sci., 37, 1429±1436. Ohtsuka, K. & Sawa, M. (1997) Frequency characteristics of accommodation in a patient with agenesis of the posterior vermis and normal subjects. Br. J. Ophthalmol., 81, 476±480. Palmer, B.M., Sajjad, M. & Rottenberg, D.A. (1995) An automated [15O]H2O production and injection system for PET imaging. Nucl. Med. Biol., 22, 241±249. Pardo, J.V., Pardo, P.J., Janer, K.W. & Raichle, M.E. (1990) The anterior cingulate cortex mediates processing selection in the Stroop attentional con¯ict paradigm. Proc. Natl Acad. Sci. USA, 87, 256±259. Pardo, J.V., Richter, H. & Lee, J.T. (1995) Anatomy of human voluntary visual accommodation. Hum. Brain Mapp., 1 (S1), 58. Paus, T., Petrides, M., Evans, A. & Meyer, E. (1993) Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J. Neurophysiol., 70, 453±469. Petit, L., Orssaud, C., Tzourio, N., Salamon, G., Mazoyer, B. & Berthoz, A. (1993) PET study of voluntary saccadic eye movements in humans: basal 321 ganglia-thalamocortical system and cingulate cortex involvement. J. Neurophysiol., 69, 1009±1017. Petit, L. & Haxby, J.V. (1999) Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J. Neurophysiol., 81, 463±471. Pierrot-Deseilligny, C., Rivaud, S., Gaymard, B., MuÈri, R.M. & Vermersch, A. (1995) I. Cortical control of saccades. Ann. Neurol., 37, 557±567. Provine, R.R. & Enoch, J.M. (1975) On voluntary ocular accommodation. Percept. Psychophys., 17, 209±212. Richter, H. & FranzeÂn, O. (1994) Velocity percepts of apparent laser speckle motion modulated by voluntary changes of visual accommodation: realtime, in-vivo measurements of the accommodative response. Behav. Brain Res., 62, 93±102. Richter, H., Lee, J.T. & Pardo, J. (1995) Central correlates of voluntary visual accommodation in humans measured with 15O-water and PET. Invest. Ophthalmol. Vis. Sci., 36, 459. Richter, H., Abdi, S., Han, Y., Lennerstrand, G., FranzeÂn, O., Anderssson, J., Pardo, J.V., Schneider, H. & LaÊngstroÈm, B. (1998) Higher order control processes and plasticity in neural CNS circuitry subserving negative voluntary accommodation. Invest. Ophthalmol. Vis. Sci., 39, 1047. Sawa, M. & Ohtsuka, K. (1994) Lens accommodation evoked by microstimulation of the superior colliculus in the cat. Vision Res., 34, 975±981. Sakata, H., Shibutani, H. & Kawano, K. (1980) Spatial properties of visual ®xation neurons in posterior parietal association cortex of the monkey. J. Neurophysiol., 43, 1654±1672. Schmahmann, J.D. (1996) From movement to thought: anatomical substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp., 4, 174±198. Stark, L.R. & Atchison, D.A. (1994) Subject instructions and the methods of target presentation in the accommodation research. Invest. Ophthalmol. Vis. Sci., 35, 528±537. Stein, H.A., Slatt, B.J. & Stein, R.M. (1987) Ophthalmic Terminology, 2nd edn. C.V. Mosby Company, Toronto. Takeda, T., Iida, T. & Fukui, Y. (1990) Eye accommodation evoked by apparent distances. Opt. Vis. Sci., 67, 450±455. Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain. Theime Medical Publishers, New York. Toates, F.M. (1972) Accommodative function of the human eye. Physiol. Rev., 52, 828±863. Trotter, Y. & Celebrini, S. (1999) Gaze direction controls response gain in primary visual-cortex neurons. Nature, 18, 239±242. Ullman, S. (1995) Sequence seeking and counter streams: a computational model for bidirectional information ¯ow in the visual cortex. Cerebral Cortex, 1, 1±11. Westheimer, G. (1957) Accommodation measurements in empty ®elds. J. Opt. Soc. Am., 47, 714±718. Yoshizawa, T., Takagi, M., Toda, H., Shimizu, H., Norita, M., Hirano, T., Abe, H., Iwata, K. & Bando, T. (1991) A projection of the lens accommodation-related area to the pupillo-constrictor area in the posteromedial lateral suprasylvian area in cats. Jap. J. Ophthalmol., 35, 107±118. Zald, D.H., Lee, J.T., Fluegel, K. & Pardo, J.V. (1998a) Aversive gustatory stimulation activates limbic circuits in humans. Brain, 121, 1143±1154. Zald, D.H., Donndelinger, M.J. & Pardo, J.V. (1998b) Elucidating dynamic brain interactions with across-subjects correlational analysis of PET data: the functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J. Cereb. Blood Flow Metab., 18, 896±905. Zhang, H. & Gamlin, P.D.R. (1998) Neurons in the posterior interposed nucleus of the cerebellum related to vergence and accommodation. I. Steady-state characteristics. J. Neurophysiol., 79, 1255±1269. Ó 2000 European Neuroscience Association, European Journal of Neuroscience, 12, 311±321