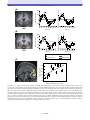
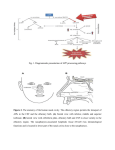
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Olfactory System - Tim J. van Hartevelt
Neural coding wikipedia , lookup
Biology of depression wikipedia , lookup
Neuroanatomy wikipedia , lookup
Limbic system wikipedia , lookup
Emotional lateralization wikipedia , lookup
Neuroesthetics wikipedia , lookup
Development of the nervous system wikipedia , lookup
Environmental enrichment wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Affective neuroscience wikipedia , lookup
Subventricular zone wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Time perception wikipedia , lookup
Human brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Cortical cooling wikipedia , lookup
Apical dendrite wikipedia , lookup
Aging brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptic gating wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Sensory cue wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
C H A P T E R 34 The Olfactory System Tim J van Hartevelt 1, 2, Morten L Kringelbach 1, 2, 3 1 2 University of Oxford, Department of Psychiatry, Warneford Hospital, Oxford, UK, Aarhus University, Centre for Functionally Integrative Neuroscience (CFIN), Denmark, 3 Nuffield Department of Surgery, John Radcliffe Hospital, Oxford, UK O U T L I N E Introduction 1219 Rostral, “Olfactory” Entorhinal Cortex 1229 The Olfactory System 1220 Olfactory Mucosa Location Structure 1221 1221 1221 Accessory Olfactory Cortical Areas Centrifugal Projections to the Olfactory Bulb Intracortical Associational Fiber System 1229 1230 1230 The Vomeronasal Organ 1224 Olfactory Bulb 1224 Primary Olfactory Cortex 1225 Cortical Structure and Projections of the Olfactory Bulb 1225 Anterior Olfactory Nucleus 1225 Olfactory Tubercle 1226 Piriform Cortex Anterior Cortical Nucleus of the Amygdala and Periamygdaloid Cortex (PAC) 1226 Human Imaging of Olfactory Sensory Activity Olfaction as a Multimodal System Olfactory Dysfunction and Anhedonia 1230 1230 1231 1231 1231 1232 1233 1235 1227 INTRODUCTION In order to survive both as individuals and as a species, we need to eat and procreate. Our sensory systems have developed to allow us to identify, evaluate, and predict stimuli in the environment such that we make sensible decisions about them (Kringelbach and Stein, 2010). Olfaction, or as it is more commonly known, the sense of smell, is of great importance for both food selection and reproduction, especially when taken together with the sense of taste (gustation). In fact, taste and smell are so closely linked that when most people talk about The Human Nervous System, Third Edition DOI: 10.1016/B978-0-12-374236-0.10034-3 Olfactory Projections Beyond the Primary Olfactory Cortex Olfactory Projections to the Amygdala and Hippocampus Olfactory Projections to the Hypothalamus Olfactory Projections to the Striatum, Pallidum, and Thalamus Olfactory Projections to Neocortical Areas the taste of something, they are usually referring to the multimodal experience of taste and smell. Studies on, amongst others, single-cell organisms indicate that the combined chemical sense of taste and smell is possibly the oldest of the senses and the most universally employed (Kovács, 2004; Hoover, 2010). Taste and smell has since diverged such that gustation is foremost a proximal sense allowing us to identify nearby stimuli, while more distal stimuli can be identified by olfaction. We and other animals rely strongly on olfaction to locate and identify food sources. Most animals also rely heavily on olfaction for mate selection and to 1219 Copyright Ó 2012 Elsevier Inc. All rights reserved. 1220 34. THE OLFACTORY SYSTEM identify their offspring. In addition, animals use olfaction to navigate not only to food sources, but also to their homes or in some cases to their birth places to lay eggs (e.g. sea turtles and salmon). Further evidence for the importance of olfaction can be found in the size of the olfactory organ and system in a vast majority of species. For example, dogs have about 100 times more olfactory receptor cells compared to humans. Some researchers have argued that compared to other animals, humans depend less on olfactory functions and more on other senses such as vision and hearing (Jacobs, 2009). Such arguments have some truth to them but are difficult to uphold given the evidence for how we rely heavily on our sense of smell to identify spoiled or toxic food, or our ability to identify sources of potential danger such as fire or gas leaks. The function of olfaction with regard to food selection is not, however, limited to identifying potentially dangerous food sources. Deficits in olfactory function can sometimes lead to malnutrition (Warner et al., 1986). The lack of olfaction is a strong predictor of anhedonia, the lack of pleasure in eating or smelling and tasting food, and as such may contribute to the malnutrition. In addition to the function of smell in food selection, olfaction is also important in sex. Although humans do not use olfaction for mate selection to the extent of other animals such as dogs, olfaction is still related to sex drive. Studies using smell for attractiveness ratings show that men and women are susceptible to pheromones and rate the opposite sex as more or less attractive, depending on their hormonal state and the hormonal state of the opposite sex (Mostafa et al., 2011). Smells from family members are readily identified as such (and generally rated as less attractive), which may indicate a mechanism to select genetically different partners and avoid inbreeding. Mothers are able to recognize the smell of their newborn babies (and vice versa) after prior exposure of as little as one hour (Porter, 1998). Furthermore, olfactory dysfunction is associated with apathy, depression, and a lower quality of life (Smeets et al., 2009; Cramer et al., 2010). These observations combined with the fact that olfactory neurons project directly to the amygdala and hippocampus without a thalamic relay, suggest a prominent role for olfaction in mediating hedonic experience. In addition to its role in food, sex, and pleasure, olfactory cues have a remarkable ability to evoke extremely vivid memories, as noted for example by Proust in his novel À la recherche du temps perdu. Taken together, the weight of the evidence shows that olfaction is a very important sensory system in humans which needs to be fully functioning to support not only our survival but very much our sense of well-being in general. This chapter outlines the human olfactory system, beginning with the peripheral receptor neurons in the nasal cavity and the first relay in the olfactory bulb. The projections of the olfactory bulb to the primary olfactory cortical areas are described using evidence from macaque monkeys, and the comparable olfactory areas in the human brain are also described. Finally, olfactory projections to the hypothalamus, thalamus, and frontal cortex are discussed, based on experimental work in monkeys and in particular using new evidence from functional imaging studies in humans. THE OLFACTORY SYSTEM Similar to other sensory systems, olfactory information must be transmitted from peripheral structures (the olfactory epithelium) to more central structures (the olfactory bulb and cortex), integrated to detect and discriminate specific stimuli, and then transferred to other parts of the brain in order to reach sensory awareness and affect behavior. Similar to other sensory systems, the olfactory system is able to identify objects by its emitted odorants and locate the source. However, the olfactory system is different from the other sensory systems in three fundamental ways. Firstly, as mentioned before, olfaction is the only sensory modality that is directly connected into the cerebral hemisphere (in a sense, the telencephalon developed in relation to olfactory input). Possibly because of this phylogenetic relationship, olfactory sensory activity is transferred directly from the olfactory bulb to the olfactory cortex, without a thalamic relay. Although there is a subsequent projection from the olfactory cortex to the mediodorsal thalamic nucleus and from there to the posterior orbitofrontal/agranular insular cortex, this transthalamic pathway is less substantial (in terms of the number of neurons involved) than a direct, monosynaptic projection from the olfactory cortex to the same orbitofrontal/insular areas. The transthalamic projection is therefore not essential for relay of sensory information to the neocortex. Secondly, the neural integration and analysis of olfactory stimuli may not involve a topographic organization beyond the olfactory bulb. Olfactory stimuli are not intrinsically ordered along spatial axes, like vision and somatic sensation, or along a frequency axis, like audition. Although an organization has been found in the projection from the olfactory epithelium to the olfactory bulb (e.g. Mombaerts, et al., 1996), there is little if any solid evidence for a topographic organization from the bulb to the olfactory cortex. While it is possible that a more complex organization may eventually be discovered, on the present evidence it is more likely that spatio-temporal patterns across large regions of the olfactory cortex may be the critical factor in detecting and discriminating different odors. VI. SYSTEMS 1221 OLFACTORY MUCOSA Thirdly, olfactory receptor neurons exhibit significant turnover throughout life. Olfactory neurons have a short life span averaging approximately 30–60 days. They are constantly replaced by mitotic division of the basal stem cell population in the olfactory epithelium. The olfactory receptors are the only neurons that are inserted in the surface epithelium of the body and are, therefore, directly exposed to the environment. It is likely that this makes them more vulnerable to insult and necessitates their regenerative capacity. Although other receptor-related cells also have a turnover, such as the taste buds, these are not true neurons that make synapses within the central nervous system. As noted in the introduction, humans are generally considered “microsmatic,” with a relatively poorly developed olfactory system compared to that of “macrosmatic” mammals. Indeed, the structure and lamination of the olfactory bulb and primary olfactory cortex (POC) are not as well-defined in humans as in rodents and carnivores. Furthermore, the olfactory structures certainly do not make up as large a fraction of the forebrain in humans as in rats and cats. However, almost all of the major olfactory structures found in rats are also present in humans, and in absolute terms the human structures are far from rudimentary. For example, the volume of the olfactory bulb in a young adult human is reported to be 50–60 mm3 (per side) (Turetsky et al., 2000), while it is only 15–20 mm3 in rats (Hinds and McNelly, 1977). Similarly, the primary olfactory cortical areas such as the anterior olfactory nucleus (AON), piriform cortex, and periamygdaloid cortex are readily recognizable around the junction of the frontal and temporal lobes of human brains. What is more, humans are able to discriminate between odors differing by only one carbon atom (Laska and Teubner, 1999). The vomeronasal organ is another interesting potential difference between humans and other animals. Although some researchers have argued that humans do not possess a vomeronasal organ and an accessory olfactory bulb, which represent the peripheral receptors and first relay of the “accessory olfactory system” in other animals, this structure has been found in humans (Jacob et al., 2000). What is not clear is whether its functions are intact in humans. Substantial debate has been raised over this matter and on balance the evidence suggests that this system is inactive in humans (Mast and Samuelsen, 2009; Frasnelli et al., 2011). The major axonal target of the accessory olfactory bulb in rodents, the medial amygdaloid nucleus, is present in adult humans, but may have come to mediate non-olfactory functions. It should be noted that the accessory olfactory system is anatomically and functionally distinct from the main olfactory system, and has been implicated in perception of odors important for species-specific functions, including aspects of reproduction (Wysocki and Meredith, 1987). OLFACTORY MUCOSA Location The peripheral olfactory receptor neurons in the humans are situated within the olfactory epithelium, in the posterodorsal recess of the nasal cavity (e.g. see Lovell et al., 1982; Jafek, 1983; Nakashima et al., 1984) (see Figure 34.1). This specialized epithelium occupies an area of approximately 1 cm2 in each nasal cavity (covering the cribriform plate of the ethmoid bone), located in the dorsal posterior recess on the dorsal aspects of the nasal vault, the septum, and the superior turbinate. It is, however, important to note that olfactory epithelium is replaced by respiratory epithelium during aging, meaning that the surface area of olfactory epithelium decreases considerably. The remainder of the nasal cavity (60 cm2 per side) is occupied by respiratory epithelium, which serves the major function of conditioning the air that passes through the nose and at the end of the nasal cavity, air is 31–34 degrees Celsius and has a Relative Humidity of 90–95% to match the conditions in the lungs (Elad et al., 2008). The inaccessibility of the olfactory epithelium serves an important role in protecting the receptor neurons, which are the only sensory neurons that are directly exposed to the external environment in the surface epithelium of the body. Although the access of odorants through the nostrils is seemingly straightforward, a phenomenon called the nasal cycle influences the intake of air into the nasal cavity by diluting and restricting the left and right nasal airways in a switching manner. The switching occurs periodically with (large) individual differences. This non-conscious mechanism allows for odorant molecules with different sorption quality (i.e. whether molecules are readily absorbed by the nasal mucosa or not) to have an optimal possibility for binding to olfactory mucosa when inhaled (e.g. see Sobel et al., 1999; Gottfried, 2009). Structure The human olfactory epithelium is considerably thicker than the respiratory epithelium (70 versus 45 mm), and is generally described as containing bipolar receptor neurons, supporting or sustentacular cells, and basal cells. In addition, microvillar cells have been found near the surface, and Bowman’s glands extend deep to the epithelium into the lamina propria. The epithelium is pseudostratified, and both the bipolar receptor cells and the sustentacular cells span the full thickness. Cilia or microvillae extend from the apical surface of the cells into a layer of mucus that covers the epithelium and separates it from the air of the nasal cavity (Figure 34.2). The Bowman’s glands are tubuloalveolar in nature, with ducts extending through the epithelium to the VI. SYSTEMS 1222 34. THE OLFACTORY SYSTEM (A) (B) FIGURE 34.1 Schematic representation of olfactory system neuroanatomy. (A) Odors are delivered to the olfactory epithelium both orthonasally and retronasally. Receptors in the olfactory epithelium project to the olfactory bulb, which in turn sends signals to primary olfactory cortical areas. (B) Olfactory receptor neurons merge with identical receptor neurons and connect to mitral (M) and tufted (T) cells in the glomerular layer. Mitral and tufted cells are modulated by granule cells (G) from the granular layer. Mitral and tufted cells project to both the anterior olfactory nucleus located in the olfactory bulb as well as to primary olfactory cortical areas through the lateral olfactory tract. (A) modified from Lafreniere and Mann (2009) and (B) modified from Duda (2009). surface. In humans, they apparently secrete a serous fluid that contributes to the mucous layer overlying the epithelium (Jafek, 1983). Because of the number of these glands and their restricted distribution in the olfactory region, it has been suggested that the secretion of Bowman’s glands may play a role in olfactory transduction, but this has not been well established. The bipolar receptor neurons have a superficial “dendrite” that extends to the surface of the epithelium, and a thin (0.2–0.3 mm) axon which runs into the lamina propria. The axons then group together into small bundles or “fila” and run through the cribriform plate to the olfactory bulb. The cell bodies of the receptors occupy a broad band in the middle of the olfactory epithelium, deep to the nuclei of the supporting cells and superficial to the basal cells. Counts of the receptors in humans indicate that their density is about 30 000 per mm2, or 6 million per nose (Moran et al., 1982a). As in other mammals, the human receptor neurons and their processes show selective immunoreactivity for the olfactory marker protein (Nakashima et al., 1985). Evidence from rats and other animals indicates that the transmitter used by the receptor neurons is glutamate (Berkowicz, et al., 1994; Aroniadou-Anderjasska et al., 1997). Superficially, the receptor dendrite terminates in the knoblike olfactory vesicle, which protrudes above the surface of the epithelium. Ten to 30 non-motile cilia arise from basal bodies in each olfactory vesicle and protrude into the mucous layer (Jafek, 1983). The cilia generally have the typical 9 pairs þ2 microtubule structure, without dynein arms between the microtubule pairs. The ciliary membranes carry molecular odorant receptors, which mediate sensory transduction from odorant molecules to neural signals. There are believed to be about 950 odorant receptors, each of which binds a specific chemical moiety. The receptors are coded for by the largest known gene family in mammals (Buck and Axel, 1991; Glusman et al., 2000). In humans, odorant receptor genes are distributed over at least 16 chromosomes, but as many as 63–70% of them are reported to have accumulated mutations that make them dysfunctional pseudogenes (Gaillard et al., 2004). Studies in rodents have indicated that olfactory receptor neurons (ORN) are organized into four zones within the olfactory epithelium, which express different groups of molecular odorant receptors (Ressler et al., 1993; Vassar et al., 1993; Mori et al., 1999). Within each zone, neurons expressing specific odorant receptors are intermingled with neurons expressing other receptors. The microvillar cells are flask-shaped cells in the superficial zone of the epithelium with a rapidly tapering neck that ends in a tuft of microvilli which extends out into the mucous layer (Moran et al., 1982b; VI. SYSTEMS 1223 OLFACTORY MUCOSA Food Sensory input Decoding Evaluation - Expectation - Experience Decision - Selection Environment Body Brain OM Smell OB Sensory cortices PIR The orbitofrontal cortex Agranular insula/ posterior OFC Medial OFC lateral OFC Evaluation leading to change Thalamus Taste Cingulate cortex INS/OP mid anterior OFC Touch Nucleus Accumbens S1..S2 Correlates of hedonic experience V1..V4 IT Vision A1..A2 Hearing Reinforcer identity medial OFC Multi-modal representations Reward value representations Hypothalamus Amygdala Ventral Pallidum Monitoring / learning / memory Cortical structures Hippocampus Sub-cortical structures Brainstem Receptors Autonomic Gastro-intestinal tract / liver / pancreas / muscle / adipose FIGURE 34.2 Schematic representation of the sensory pathways involved in food intake. The figure summarizes the interactions with environment to procure suitable food sources with a special focus on olfaction. Potential food sources are identified on the basis of the sensory input, which through the appropriate receptors are relayed to the orbitofrontal cortex (only one hemisphere shown), where processing is taking place of evaluation, expectation, experience as well as decision and selection. Here the input is processed in the primary sensory cortices via the thalamus (except for olfaction) and made available for pattern-association between primary (e.g. taste) and secondary (e.g. visual) reinforcers. Stimulus sensory identities are then processed for multimodal perceptual integration in the posterior orbitofrontal cortex. Hedonic reward value is represented in more anterior parts of orbitofrontal cortex, from where it can then be used to influence subsequent behavior (in lateral parts of the anterior orbitofrontal cortex with connections to anterior cingulate cortex), stored for valence learning/memory (in medial parts of the anterior orbitofrontal cortex) and made available for subjective hedonic experience (in mid-anterior orbitofrontal cortex). There are multiple modulatory brain-loops with other important structures such as the nucleus accumbens, ventral pallidum, hippocampus, amygdala, and hypothalamus, as well as modulation with autonomic input from the gut (Batterham et al., 2007). Abbreviations: V1, V2, V4, primary and secondary visual areas; SS, somatosensory cortex (3,1,2); A1.A2, auditory cortex; INS/OP, insular cortex/frontal operculum; IT, inferior temporal visual cortex; PIR, piriform cortex; OB, olfactory bulb; OFC, orbitofrontal cortex. Modified from Kringelbach and Stein (2010). Jafek, 1983). Although the neuronal nature of these cells is not established, they have a thin, axon-like process that extends toward the deepest layer of the epithelium toward the olfactory bulb. The supporting cells in the olfactory epithelium are columnar cells, with a microvillous border at their apex. Unlike similar cells in the adjacent respiratory epithelium, those in the olfactory epithelium do not show morphological specializations characteristic of mucous secretion. The function of the supporting cells is not clear but several functions have been suggested, including secretion of a non-mucous substance, phagocytotic removal of substances from the mucous, or provision of glia-like support for the receptor neurons (e.g. Jafek, 1983). The basal cells are situated in the deepest zone of the olfactory epithelium. As in other vertebrates, the basal cells in humans are apparently stem cells, undergoing mitotic division and differentiation into receptor neurons lost to turnover or injury (Moran et al., 1982a). Such replacement cannot keep up with degeneration over a human lifetime, however. In adults substantial areas of the olfactory epithelium are found to have degenerated or been replaced with respiratory epithelium (Nakashima et al., 1984, 1985; Morrison and Costanzo, 1992). In VI. SYSTEMS 1224 34. THE OLFACTORY SYSTEM addition, contrary to olfactory epithelium in (young) adults, olfactory epithelium in elderly is found to be thinner than respiratory epithelium. THE VOMERONASAL ORGAN The vomeronasal organ (also known as Jacobson’s organ) is located in the anterior third of the epithelium of the nasal septum at the base of the nasal cavity. Prevalence studies of the vomeronasal organ in humans vary widely from 25% to 100%. Moreover, repeated observation shows us that the vomeronasal organ changes in visibility from being absent to being well defined and vice versa (Frasnelli et al., 2011). The vomeronasal organ is believed to be involved in species-specific behavior, such as reproduction, in many animals. The observation of synchronization of the menstrual cycle in women living together suggested a similar function of the vomeronasal system in humans. However, recent evidence refutes this hypothesis. The human vomeronasal organ contains very few neurons. Most cells express keratin proteins, specific markers for epithelial cells and no cells in fact express olfactory marker protein (Mast and Samuelsen, 2009). Additionally, no evidence has been found for a neural connection between the vomeronasal organ and the accessory olfactory bulb, a specific region of the olfactory bulb. In fact, the presence of an accessory olfactory bulb in humans is doubtful. Studies investigating the role of the vomeronasal organ in pheromone detection have shown that androstadienone is in fact detected by the olfactory epithelium and not the vomeronasal organ. Subjects with functional occlusion of the vomeronasal organ or without a visible vomeronasal organ showed no difference in perception or detection of androstadienone compared to controls. Further investigation using PET imaging confirmed that the vomeronasal organ in humans has no function. The notion that the human vomeronasal organ is not functional does not imply humans are unable to detect pheromones or social chemical signals. Detection of pheromones could be achieved through the olfactory epithelium in a similar way as in the olfactory system in mice (Mast and Samuelsen, 2009). OLFACTORY BULB In most primates, including humans, the olfactory bulb is pulled forward from its point of attachment to the cerebral hemisphere, remaining connected by a relatively long olfactory stalk or peduncle. The olfactory bulb in young adult humans is 50–60 mm3 (per side) (Turetsky et al., 2000). The intrinsic structural features of the human olfactory bulb are similar to those in other species, but they are somewhat less sharply defined. As in all mammals, in humans the bulb has a concentric laminar structure with distinct neuronal somata and synaptic neuropil separated into layers. Although our understanding of the synaptic organization of the olfactory bulb depends on observations made in rats and other animals (see Macrides et al., 1985; Scott and Harrison, 1987; Mori et al., 1999 for reviews), it is very likely that the human bulb has the same basic organization. The olfactory nerve fibers ramify in the most superficial layer (olfactory nerve layer), after passing through the cribriform plate from the nasal cavity. Where the nerves first reach the olfactory bulb, rostrally and ventrally, the olfactory nerve layer is thick. It becomes progressively thinner in the more caudal and dorsal part of the bulb. Deep to the olfactory nerve layer, the fibers terminate in the characteristic glomerular formations both on the primary dendritic tufts of the “mitral” and “tufted” relay cells and on other cell types. In rats, the transmitter used at this synapse has been shown to be glutamate, which acts on both NMDA and nonNMDA receptors (Aroniadou-Anderjaska et al., 1997). In rodents, the organization of the olfactory epithelium into zones appears to be more or less preserved in the olfactory bulb, based on the distribution of zone-specific molecular markers (Mori et al., 1999). Within each zone in the epithelium, axons from the several thousand receptor neurons that express a given molecular odorant receptor converge onto one or two glomeruli in the corresponding zone of the olfactory bulb (Ressler et al., 1994; Vassar et al., 1994). In humans the ratio from olfactory receptor neurons to glomeruli is 16:1 with a total of approximately 5500 glomeruli (Maresh et al., 2008). This suggests that the glomeruli are the unit structures for olfactory discrimination (e.g. Mori et al., 1999). That is, the initial distinction between different odors is based on hard-wired connections between specific receptor neurons and specific glomeruli. Studies with 2-deoxyglucose as a functional imaging method have also shown that specific odorants activate a small, consistent portion of the olfactory bulb, again suggesting that initial olfactory discrimination depends on a spatial code in the olfactory bulb (e.g. Johnson and Leon, 2000; Xu et al., 2000). The synaptic neuropil of the glomeruli is outlined by somata of small “periglomerular cells”. These interneurons send dendrites and axons into adjacent glomeruli and receive synapses from the olfactory nerves. Like the granule cells in the deeper part of the bulb, the periglomerular cell dendrites form “reciprocal” or bidirectional synapses with mitral and tufted dendrites. In humans as in other animals, most (at least) of the periglomerular cells are GABAergic and also contain one of the calcium-regulating proteins, parvalbumin or calbindin (Kosaka et al., 1987; Ohm et al., 1990, 1991). In addition, many are dopaminergic (Smith et al., 1991). In rats VI. SYSTEMS PRIMARY OLFACTORY CORTEX it has been shown that GABA and dopamine colocalize within periglomerular cells (Kosaka et al., 1985; Gall et al., 1987). There are also a number of superficial “short axon cells” in the glomerular region. Deep to the glomeruli is the external plexiform layer, with dendrites of mitral, tufted, and granule cells. The tufted cell somata are also scattered through this layer, but the mitral cell somata are located in a thin layer at the border between the external plexiform layer and the deeper granule cell layer. One or occasionally two “primary” dendrites of the mitral and tufted cells arise from the superficial aspect of the somata and extend through the external plexiform layer to the glomeruli. Several “secondary” dendrites ramify in the external plexiform layer. The small granule cells are by far the most numerous neuron type in the olfactory bulb and the granule cell layer makes up approximately half of the volume of the bulb (Bhatnagar et al., 1987). These interneurons lack an axon and instead send a superficial “dendritic” process into the external plexiform layer, where they form reciprocal synapses with mitral and tufted cell dendrites. Other dendrites ramify within the granule cell layer and are solely post-synaptic. There are also short axon cells in the granule cell layer, which synapse on the granule cells. Studies in rats and other animals indicate that the mitral to granule cell component of the reciprocal synapse uses glutamate, acting on both NMDA and non-NMDA receptors (Chen et al., 2000). The granule to mitral cell component of the synapse is GABAergic, acting on GABA-A receptors. The reciprocal synapses appear to allow for spatial and temporal modulation of activity in the relay cells, both on a local (at individual synapses) and on a more global level (through conduction along granule and mitral/tufted cell dendrites). It has been reported in non-human primates, including chimpanzees, that the somata and dendrites of many cells in the olfactory bulb may be ensheathed by compact myelin (Tigges and Tigges, 1980). Since many, at least, of these cells have presynaptic dendrites, it is possible that the myelin will affect conduction between different segments of the neuron. In humans, approximately 5500 glomeruli and 40 000 mitral cells have been counted in young adults (Maresh et al., 2008; Meisami et al., 1998). There is a progressive decrease with age in the number and structural integrity of the glomeruli, and in the thickness of the glomerular/ periglomerular layer (Bhatnagar et al., 1987; Meisami et al., 1998; although see Maresh et al., 2008). In olfactory bulbs from very old individuals (>90 years old) less than 30% of the glomeruli and mitral cells were recognized. Together with the degeneration of the olfactory epithelium mentioned above, this presumably is related to the decline in olfactory function with age (e.g. Murphy et al., 2000). 1225 PRIMARY OLFACTORY CORTEX Cortical Structure and Projections of the Olfactory Bulb The axons of mitral and tufted cells run through the granule cell layer and emerge from the caudolateral aspect of the olfactory bulb to form the lateral olfactory tract (LOT). This tract forms the bulk of the olfactory peduncle in humans and other primates. Although a “medial olfactory tract” has been described in the past, a large body of experimental studies in several mammalian species, including primates, has shown that all of the axons from the olfactory bulb pass through the LOT. There is no medial olfactory tract in mammals. The LOT can be visualized readily in human brain sections stained for fibers or myelin. It runs just deep to the pial surface, from the olfactory peduncle onto the posterior ventral surface of the frontal lobe and then laterally around the junction between the frontal and temporal lobes (the limen insulae) and onto the anteromedial part of the temporal lobe. Most of the primary olfactory cortical (POC) areas have a relatively simple structure, with a broad plexiform layer composed of dendrites of neurons in deeper layers (I), a well-defined, compact layer of pyramidal-like cell somata (II) and a deeper layer(s) of pyramidal and non-pyramidal cells (III and higher). The full efferent projection of the olfactory bulb can only be mapped with experimental methods, so the description of this projection is based on data from non-human primates (Heimer et al., 1977; Turner et al., 1978; Carmichael et al., 1994). As in lower animals, axons from the olfactory bulb in monkeys run caudally through the LOT and give off collaterals that extend medially, laterally, and deep to the tract to contact dendrites in the superficial part of layer I of the olfactory cortex. In addition, in primates a few axons from the olfactory bulb are found in and deep to layer II. In contrast to the olfactory bulb, there is very little evidence for a spatial organization in the olfactory cortex that could support a spatial code. That is, small areas of the olfactory bulb project to virtually the entire olfactory cortex, and small areas of the cortex receive fibers from virtually the entire olfactory bulb (e.g. Haberly and Price, 1977; Luskin and Price, 1982; Haberly 1985). There may be a more “fine-grain” organization within this (e.g. Ojima et al., 1984), but it has not yet been recognized. Anterior Olfactory Nucleus The anterior olfactory nucleus (AON) is the most rostral of the primary olfactory structures. In primates, including humans, there is a “bulbar” part of this nucleus located in the rostral part of the olfactory VI. SYSTEMS 1226 34. THE OLFACTORY SYSTEM peduncle, including several groups of pyramidal-like cells in the caudolateral part of the olfactory bulb. As in other olfactory cortical areas, axons of the mitral and tufted cells synapse on the distal segments of dendrites that extend into the plexiform layer around the neuron clusters. More caudal “medial” and “lateral” subdivisions of the AON are situated on either side of the LOT where it joins the orbitofrontal cortex, at the posterior end of the olfactory peduncle. In monkeys and other animals, “external” and “dorsal” subdivisions are also recognizable, but these are not readily distinguishable in humans. As described earlier, olfactory perception can be influenced by alternating air intake through the nostrils. Recently, Kikuta and colleagues (Kikuta et al., 2010) found that rodents can localize the source of an odor by comparing the inputs to the left and right nostril. This is achieved by neurons in the AON pars externa, which showed different response levels to ipsi- versus contranostril odor stimulation. Although humans do exhibit a similar mechanism of source location in the auditory system (Chapter 36), whether this is also true for olfaction is unknown. The lack of readily distinguishable subdivision of the AON does make generalization of animal models problematic. Olfactory Tubercle More caudally on the ventral surface of the frontal lobe, the LOT runs at the junction of the olfactory tubercle (OTu), medially, and the piriform cortex, dorsal and laterally (Figures 34.3, 34.4, 34.5). The OTu is a prominent structure in rodents and birds with a well-developed laminar structure similar to that in other olfactory cortical areas. It is much less distinct in primates, but still has a laminar arrangement of cell bodies and afferent fibers. In all mammals, collaterals of axons in the LOT leave the medial side of the tract and run through the superficial part of layer I of the OTu to contact apical dendrites of cells with somata in layers II and III. Although this laminar structure is clearly cortical, other features of the OTu resemble the underlying corpus striatum, and it has often been included with the nucleus accumbens (NAcc) in the “ventral striatum” (Chapter 20). For example, the OTu has a high concentration of acetylcholinesterase, apparently related to intrinsic cholinergic neurons, and it receives a prominent dopaminergic input from the ventral midbrain. Its major output is to the “ventral pallidum”, which is similar to and dorsally continuous with the globus pallidus. The primary neurons of the OTu closely resemble the “medium spiny” cells of other parts of the striatum in their dendritic structure and appearance. Between and deep to these cells are clusters of small neurons that constitute the islands of Calleja. In monkeys, ventral pallidal elements are distributed somewhat loosely within the deep part of the OTu rostrally, although more distinct components of the ventral pallidum are found further caudally and dorsally. Both in humans and non-human primates the OTu is less readily identifiable due to the small size of the basal forebrain bulge, which houses the OTu (Wesson and Wilson, 2011). In human imaging studies the OTu has been identified between the uncus and the medial forebrain bundle. Furthermore, besides the direct inputs from the olfactory bulb, the OTu also receives minor input from the olfactory amygdala (Martinez-Marcos, 2009). PIRIFORM CORTEX The piriform cortex is the largest and most distinctive olfactory cortical area. The area was previously referred to as the prepiriform or prepyriform cortex, apparently because it is partially situated rostral to the “pyriform lobule” in carnivores. This designation is not descriptive in most animals, however, and the suffix “pre” has been dropped. Furthermore, the piriform lobule in turn received its name thanks to its pear-shaped anatomy. In both monkeys and humans, the piriform cortex is situated deep and lateral to the LOT, from the caudolateral aspect of the frontal lobe, around the limen insulae, to the rostral dorsomedial aspect of the temporal lobe. The piriform cortex is characterized by a densely packed layer II composed of moderately large pyramidal cell somata, and a less dense layer III of slightly larger pyramidal cells and other neurons. Layer II is found throughout the piriform cortex but layer III is only well developed in the caudal part of the cortex. In monkeys, layer II is thick and prominent, but in humans layer II is relatively thin and the boundaries of the cortex are not as distinct. Deep to the piriform cortex, a ventral continuation of the claustrum forms the endopiriform nucleus. There are little data on this nucleus in primates, but in rodents it is closely interconnected with the overlying piriform cortex. Axon collaterals leave the deep and lateral aspects of the LOT and terminate primarily on apical dendrites in the superficial part of layer I. In monkeys, although not in rodents or carnivores, a few fibers from the olfactory bulb also ramify in deeper layers. As discussed below, the piriform cortex gives rise to a substantial association fiber system that extends throughout all parts of the POC. Evidence from anatomical, physiological, and functional differences suggests that the piriform cortex can actually be divided into two different sections, the anterior piriform cortex (APC) and the posterior piriform cortex (PPC) (Gottfried, 2010). However, input from the olfactory bulb does not appear to exhibit spatial VI. SYSTEMS 1227 PIRIFORM CORTEX RS 10m 24b 11l 14r 32 25 Visual 12m 12l 13b 14c 13m 13l 13a 47/12r 11m Visceral 11l 10r 46 24b 13m 14r Olfaction IOS 9 Somato sensory 12o PrCO Iam Ial AON Iapm Iai Iapl G 45 13l TOS 47/12l 13b 32m 14c 47/12m 13a 9 24b 11l 14c 25 13m 13l 13a Iapm Olfaction Visceral Gustation 10o 12r 11m 9 11l 14r 32 25 13m 13a 12o 11m 46 24b 13m 14r Gustation 25 45 13l 14c Lateral hypothalamus vlPAG Visual 47/12l 13b 32m Visceral 11l 10r Somato sensory PrCO Iam Ial AON Iapm Iai Iapl G Olfaction Medial and lateral hypothalamus dlPAG 13l 47/12r 9 Visual 12m 12l 13b 14c Dorsal hypothalamus lPAG Gustation Visceral 10o 10m 10m Ial Iai Somato sensory 12o PrCO Iam Ial AON Iapm Iai Iapl G Olfaction 24b Iam AON Visual 12m 12l 13b Somato sensory 47/12s 12r 14r 32 Visual LOS 10o 11m HR Gustation 25 10m 10o MOS 12r 11m 9 FMS OS 10m 10o Somato sensory 47/12m 13a 47/12s Iam AON Iapm Olfaction Ial Iai Gustation Visceral 10o 10m 47/12r 9 11m 11l 10r 46 24b 13m 14r 25 45 13l 14c 47/12s Iam AON Iapm Olfaction Medial and lateral hypothalamus dlPAG Somato sensory 47/12m 13a Dorsal hypothalamus lPAG Visual 47/12l 13b 32m Visceral Iai Ial Gustation Lateral hypothalamus vlPAG FIGURE 34.3 Olfaction and comparative networks in the orbitofrontal cortex of macaques and humans. Left-hand images show areas on the medial and orbitofrontal surfaces, a cortico-cortical network and projections from the primary olfactory cortices in macaque monkeys (Öngür and Price, 2000). Right-hand images show areas on the medial and orbitofrontal surfaces, a cortico-cortical network and projections from the primary olfactory cortices in humans (Kringelbach and Rolls, 2004). patterning across the piriform cortex. Additionally, contrary to the olfactory bulb, the piriform cortex has not been shown to have a spatial organization. Anterior Cortical Nucleus of the Amygdala and Periamygdaloid Cortex (PAC) Caudal and lateral to the piriform cortex the axons from the olfactory bulb continue into several small areas on the medial surface of the amygdala. The anterior cortical amygdaloid nucleus is directly caudal to the piriform cortex and is characterized by a relatively loosely packed layer II and even more diffuse layer III. The periamygdaloid cortex is a larger area located ventrolateral to the piriform cortex. It is markedly heterogeneous, and in monkeys and humans can be divided into five subdivisions, PACO, PAC1, PAC2, PAC3, and PACS, based on architectonic differences VI. SYSTEMS 1228 34. THE OLFACTORY SYSTEM (A) Signal change (%) 0.8 * 0.4 0 -0.4 APC 0 4 8 12 16 20 24 26 Time (s) (B) 0 4 8 12 16 20 24 26 Time (s) * Signal change (%) 0.8 0.4 0 -0.4 -0.8 PPC 0 4 8 12 16 20 24 26 Time (s) 0 4 8 12 16 20 24 26 Time (s) Different group Different quality Same group Similar quality (C) 6.5 Signal change (%) 0.8 0.6 0.4 0.2 0 0.0 -0.2 -2 1 -1 0 Subjective pleasantness of smell FIGURE 34.4 Olfactory processing in humans measured with neuroimaging. A dissociation has been found between the anterior and posterior parts of the piriform cortex with the anterior part encoding difference between group of odors but not their perceptual quality, while the posterior part encodes perceptual quality but not between group of odors (Gottfried, 2010). (A) Specifically, the presentation of odorants from the same functional group leads to significantly reduced activity in the anterior piriform cortex (circled in red), as shown in the left-most plot, while the right-most graph shows no significant differences for perceptual quality. (B) This is different from the presentation of odorants containing similar perceptual qualities which leads to reduced activity in the posterior piriform cortex (circled in red). Here, the left-most plot shows no effect for functional group, while the right-most graph shows a significant difference between perceptual qualities. Following this perceptual processing, the affective valence is processed in the orbitofrontal cortex. (C) Region of the medial orbitofrontal (circled in white) and medial prefrontal cortices correlate significantly with the subjective pleasantness ratings of odors, as demonstrated by the correlation between signal change and pleasantness ratings (shown on the right). (Rolls et al., 2003). VI. SYSTEMS ACCESSORY OLFACTORY CORTICAL AREAS FIGURE 34.5 Representations of odor Odour object Stimulus A Identification and categorization of odor object N1 Stimulus B N2 N1 N2 0 1 2 3 4 5 Time point Stimulus A Stimulus B 0 1,1 1,1 1 0,3 4,2 2 1,4 3,3 3 3,2 2,2 4 3,4 3,1 5 1,2 0.3 2 Third dimension Time point 4,3 Citral Geraniol 0.3 1 0.6 B 0.9 0 0.6 1.2 –1 2 4 2 1.2 0.9 Number of spikes of neuron 2 1229 1 0 1 0 Second dimension –1 First dimension –1 3 object in the brain. Odor objects are difficult to decode in terms of their temporal and spatial properties. Here we describe a potential model for how this information might be encoded in brain networks. The top image show the spatio-temporal patterns in the olfactory system for two different hypothetical odor stimuli depicted in blue and red. This firing pattern is divided into five time bins. Visualization of the network activity for these two different stimuli results in two different neural trajectories (shown in the lower left plot). The points in the trajectory hold information about which stimulus was present, as well as when it was present, thus including both temporal and spatial information (Buonomano and Maass, 2009). Compare this with real data taken from recordings of 87 projection neurons from the locust antennal lobe (right-most plot). These projection neurons from the locust were recorded during multiple presentations of two odors (citral and geraniol). This three-dimensional plot (reduced from the 87 vectors) reveals that each odor produces a different trajectory, and thus different spatiotemporal patterns of activity. The numbers along the trajectory indicate time points (seconds), and the point marked B indicates the resting state of the neuronal population. 2 1 0 1 2 3 4 Number of spikes of neuron 1 Valence of odor object (Price et al., 1987; Amaral et al., 1992; Carmichael et al., 1994). All but the most caudal of these (PACS) receive input from the olfactory bulb in monkeys, although the layer of axons is thick only in PACO, and is quite thin in PAC1, PAC2, and PAC3. superficial lamina in layer I in which the fibers end. EOl is characterized by a distinct but thin layer II, which is broken up into cell islands, as in other parts of the entorhinal cortex. The deeper layers are thicker and more complex than in other olfactory cortical areas, but less well developed than in other regions of the entorhinal cortex. Rostral, “Olfactory” Entorhinal Cortex In rodents and carnivores, a large fraction of the entorhinal cortex receives fibers directly from the olfactory bulb (e.g. Price, 1973; Boeijinga and Van Groen, 1984). In monkeys, however, olfactory bulb input is limited to a small “olfactory” zone at the rostral edge of the entorhinal cortex (Amaral et al., 1987; Carmichael et al., 1994). Even in this region, the olfactory projection is relatively slight, both in density, and in the thickness of the ACCESSORY OLFACTORY CORTICAL AREAS Although humans lack an accessory olfactory bulb, the major target of the accessory olfactory tract in other animals, the medial amygdaloid nucleus, is present in humans. The medial nucleus is situated immediately VI. SYSTEMS 1230 34. THE OLFACTORY SYSTEM caudal to the anterior cortical amygdaloid nucleus, but has a slightly denser cellular layer II. In rodents, this nucleus has been shown to be involved in mating behavior and a variety of other, related functions. Presumably, the medial amygdaloid nucleus may have similar functions in humans, but these may not be as strongly modulated by olfactory stimuli. Centrifugal Projections to the Olfactory Bulb All of the olfactory cortical areas except the OTu send fibers back to the olfactory bulb (Haberly, 1985; Price, 1987; Carmichael et al., 1994). These fibers arise from cells in layers II and III of the cortex and end primarily in the granule cell layer of the olfactory bulb. The projection from the anterior olfactory nucleus and (in monkeys) the anterior part of the piriform cortex is bilateral, with fibers crossing in the anterior commissure. In addition, a thin “external” part of the anterior olfactory nucleus just deep to the most rostral part of the LOT projects solely or (in monkeys) primarily to the contralateral olfactory bulb. There is also a substantial fiber projection from the nucleus of the diagonal band to the olfactory bulb (Mesulam et al., 1983; Carmichael and Price, 1994). Although this fiber system is partially cholinergic, only about 10% of the cells stain for acetylcholinesterase (Mesulam et al., 1983). In rats, other cells in the nucleus that project to the olfactory bulb have been shown to be GABAergic (Zaborsky et al., 1985). Intracortical Associational Fiber System Experimental studies in many mammals, including monkeys, have indicated that the primary olfactory cortex is organized in a very different way than other primary sensory cortices. In particular, there is an extensive system of intracortical connections with the POC (e.g. Price, 1973; Luskin and Price, 1983; Haberly, 1985; Carmichael et al., 1994; Johnson et al., 2000). This system suggests that the olfactory cortex functions as a correlative region, comparable to higher-order “association” cortex in other sensory systems. The greatest number of association fibers arises in the piriform cortex, but fibers originate in all of the olfactory cortical areas except the OTu. The fibers are distributed throughout the olfactory cortex and to adjacent orbitofrontal and agranular insular areas. In rats, at least, individual neurons in the piriform cortex have axons that extend into all or most of these areas (Johnson et al., 2000). Within the olfactory cortex itself, the association fibers terminate primarily in the deep part of layer I and in layer III. This laminar pattern of termination is complementary to the termination of the fibers from the olfactory bulb in the superficial part of layer I. In the tangential dimension of the cortex, there is a broad and relatively complex organization within the association fibers, which has been best defined in rats. While cortical regions near the LOT project to other parts of the olfactory cortex near the tract, regions distant to the tract tend to project to regions at the edges of the olfactory cortex (Luskin and Price, 1983; Carmichael et al., 1994). The association projections from the AON and the anterior part of the piriform cortex also extend to the contralateral olfactory cortex, crossing in the anterior commissure (Luskin and Price, 1983; Carmichael and Price, 1994). The laminar and areal patterns of termination in the contralateral cortex are approximately the same as on the ipsilateral side. Presumably, the association system interacts with the sensory activity being input to the cortex from the olfactory bulb to support olfactory discrimination. In contrast to the olfactory bulb, neither of these fiber systems has the sort of detailed topographic organization that would be expected if odors were represented in a spatial code. It is possible that cells related to different odors are dispersed in many parts of the cortex, and appropriate bulbar and association fibers synapse on them in a selective manner. It may be more likely, however, that olfactory discrimination depends on a system of spatio-temporal patterning across the cortex, in which adjustments in synaptic strength are built up from sensory experience. OLFACTORY PROJECTIONS BEYOND THE PRIMARY OLFACTORY CORTEX Olfactory information is transmitted from the POC to several other parts of the forebrain, including the orbitofrontal cortex, amygdala, hippocampus, ventral striatum, hypothalamus, and mediodorsal thalamus (Price, 1987; Russchen et al., 1987; Carmichael et al., 1994), areas that are associated with affective learning and memory. Although these connections have been best studied in rodents, there are also some experimental data from monkeys, and functional imaging studies have recently provided data on humans. Olfactory Projections to the Amygdala and Hippocampus Both the amygdala and hippocampus have often been considered to be closely associated with olfaction, especially in lower animals. In primates, these limbic structures have become dominated by other sensory inputs, especially vision, but they still have direct olfactory connections. These arise primarily in the VI. SYSTEMS OLFACTORY PROJECTIONS BEYOND THE PRIMARY OLFACTORY CORTEX periamygdaloid cortex and the olfactory part of entorhinal cortex, which projects both to deep amygdaloid nuclei and to several parts of the hippocampus (Price et al., 1987; Jolkkonen et al., 2001; Chapter 24). Olfactory Projections to the Hypothalamus In rats, both electrophysiological recordings and axonal tracer experiments indicate that there are olfactory inputs to several parts of the hypothalamus. The most direct projection arises from cells in the deepest layer of the piriform cortex and other olfactory cortical areas. Although the fibers run through the full rostrocaudal extent of the hypothalamus, in the medial forebrain bundle, they terminate predominantly in the caudal half of the lateral hypothalamic area. Axons from the anterior cortical nucleus and medial nucleus of the amygdala end in more rostral and medial parts of the hypothalamus. The olfactory inputs to the hypothalamus have not been as well defined in monkeys. Experiments with retrograde tracer injections in the lateral hypothalamus, however, label neurons in most of the olfactory cortical areas, as well as in related orbitofrontal/insular cortical areas (Öngür et al., 1998). Electrophysiological responses to olfactory stimuli have also been recorded in the lateral hypothalamic area in monkeys (Tazawa et al., 1987; Karadi et al., 1989). Olfactory Projections to the Striatum, Pallidum, and Thalamus Along with the amygdala, the olfactory cortex projects to the ventral part of the striatum, including the NAcc and the OTu. The olfactory projections are largely restricted to the OTu and the caudal, ventrolateral part of the NAcc (Price, 1973; Luskin and Price, 1983; Fuller et al., 1986). These areas project out to the ventral pallidum, a continuation of the globus pallidus ventral to the anterior commissure. The major output of the ventral pallidum, in turn, is to the mediodorsal thalamic nucleus. As with the dorsal striato-pallidal-thalamic system, the inputs to the ventral striatum are glutamatergic, but the striato-pallidal and pallido-thalamic projections are GABAergic (Fuller et al., 1986; Kuroda and Price, 1991). As in other mammals, both electrophysiological recording and axonal tracing in monkeys also indicate excitatory olfactory input to the mediodorsal thalamic nucleus (MD) from the olfactory cortex (Yarita et al., 1980; Russchen et al., 1987). The neurons that project to MD are primarily located in the deep layers of the piriform cortex and other olfactory cortical areas. There are relatively small numbers of these neurons, and it 1231 appears that they may relay convergent activity from a relatively large portion of the olfactory cortex. Within MD in monkeys, fibers from small areas of the olfactory cortex or the amygdala end in small “patches”, suggesting that inputs from restricted regions converge onto a few thalamic neurons (Russchen et al., 1987; Ray and Price, 1993). Similarly, olfactory-responsive units are restricted to a relatively small region of the medial, magnocellular part of MD. Compared to the hypothalamus, the olfactory-related units in MD appear to be relatively broadly tuned and respond to many different odors (Yarita et al., 1980). In addition to MD, anatomical and electrophysiological studies in both rats and monkeys indicate that there is an olfactory projection to the anteroventral portion of the submedial thalamic nucleus (SM; Price and Slotnick, 1987; Russchen et al., 1987). SM is separated from MD only by the internal medullary lamina, and it may represent a portion of the same nucleus functionally. In rats, the projection to SM arises in a relatively restricted zone at the junction of the piriform cortex and OTu (Price and Slotnick, 1987). The portion of medial MD that receives olfactory input is reciprocally connected with several areas in the posterior orbitofrontal cortex and rostral agranular insular cortex (Russchen et al., 1987). The cortical connections of SM are to a more restricted area near the junction of the olfactory peduncle and the frontal lobe (especially area 13a). It might be presumed, therefore, that MD would relay olfactory information to the orbitofrontal cortex. As discussed below, however, these same cortical areas receive more numerous, monosynaptic projections directly from the POC. These corticocortical projections appear to be better suited to relay detailed sensory information. It is likely, therefore, that the trans-thalamic projection does not represent a sensory relay as such. Olfactory Projections to Neocortical Areas In the 1970s and 1980s Takagi and colleagues reported that odorant stimuli or electrical stimulation of the olfactory bulb could evoke neuronal responses in the orbitofrontal cortex (Tanabe et al., 1975a, 1975b; Yarita et al., 1980; Takagi, 1986). Two areas in the lateral and central parts of the posterior orbitofrontal cortex were identified. In the lateral area the units were relatively specific, responding to only one or two odorants, while in the more central area units responded to several odorants. These studies suggested that the sensory information reached the orbitofrontal cortex from the POC through the hypothalamus and thalamus. There is now clear anatomical evidence in monkeys and other animals, however, that the principal pathway is directly from the POC to the orbitofrontal cortex. VI. SYSTEMS 1232 34. THE OLFACTORY SYSTEM Injections of anterograde axonal tracers in the piriform cortex of monkeys label axons in several areas of the agranular insula/posterior orbitofrontal cortex (areas Iam, Iapm, Iai, Ial, 13a, 13m, and 14c; Carmichael et al., 1994). All of these cortical areas are agranular or dysgranular, and the areas nearest to the POC have sometimes been referred to as periallocortex. The projection is different from more usual sensory inputs to cortex that are relayed through the thalamus, because the label is heaviest in layer I. Retrograde axonal tracer injections into the same agranular insula/posterior orbitofrontal areas label neurons in many parts of the POC, including the AON, piriform cortex, anterior cortical amygdaloid nucleus, PAC, and olfactory part of the entorhinal cortex. The labeled cells are located in both layer II and layer III. Electrical stimulation of the olfactory bulb also evokes unit and field potential responses in most of the areas where there is anatomical evidence of olfactory input (Carmichael et al., 1994). The projections from the POC are largely reciprocated by fibers from agranular insula/posterior orbitofrontal areas. Anterograde axonal tracer injections into several of the agranular insular areas label axons in the rostral parts of the POC (Carmichael et al., 1994). These include the OTu, which is the only olfactory cortical area that does not project to the orbitofrontal cortex. The agranular insula/orbitofrontal cortical areas that receive olfactory input interact with other areas on the orbital surface through cortico-cortical connections to integrate olfactory sensory information with other sensory modalities (Carmichael and Price, 1995, 1996). Taste information reaches the orbitofrontal cortex through the thalamic gustatory relay and the primary gustatory cortex (Chapter 33). The orbitofrontal cortex is the first place where olfactory information and taste information converge, so it presumably underlies the sensation of flavor, which depends on both of the primary modalities. In addition, there are corticocortical somatosensory and visual inputs to the orbitofrontal cortex from the parietal and inferior temporal cortex. The somatosensory inputs appear to be related to the hand and mouth (Carmichael and Price, 1995). The cortico-cortical connections between orbitofrontal areas form an “orbitofrontal network” that appears to function in the integration and analysis of food-related sensory information (Carmichael and Price, 1996). Recordings in the orbitofrontal cortex in monkeys show neuronal responses to food and food-related stimuli, including visual stimuli that are associated with food (Rolls, 2000). Importantly, the responses reflect the affective or reward significance of the stimuli as well as their sensory properties. For example, the neuronal response to a particular visual stimulus (e.g. a triangle) will change markedly if the association of the stimulus with a food reward changed. In addition, if the animal is fed to satiety with a food stimulus, the neuronal response to that food will decrease. HUMAN IMAGING OF OLFACTORY SENSORY ACTIVITY Several studies have used functional imaging methods to identify the human cortical areas activated by olfactory stimuli (see Zald and Pardo, 2000). Odorant-induced responses were first obtained in the region of primary cortex by Zatorre et al. (1992), and have been confirmed by subsequent reports from the same research group (Small et al., 1997; Dade et al., 1998). Other studies have failed to find substantial activation of primary olfactory areas, however (e.g. Zald and Pardo, 1997; Yousem et al., 1997; Sobel et al., 1998). Several factors may explain the inconsistency. Technical factors such as the “susceptability” artefact related to nearby bone and air sinuses make it difficult to image this region with fMRI. Neuronal responses to odorants in the piriform cortex are also rapidly adapting and may be coded for by temporal or spatial patterns of activity instead of response amplitude. Further, sniffrelated activity may mask odorant-related activity (Sobel et al., 1998). When these are taken into account odorant-related activation can be visualized in the POC (Sobel et al., 2000). Olfactory-related activity has consistently been detected in the orbitofrontal cortex (e.g. Zatorre et al., 1992; Small et al., 1997; Zald and Pardo 1997; Sobel et al., 2000; Royet et al., 2001; Rolls et al., 2003). All of these studies found an area of activation in the central orbitofrontal cortex, in some cases through the rostral to caudal extent (Sobel et al., 2000). It has also been demonstrated that there was a correlation between the subjective pleasantness ratings of the odors with activity of a medial region of the rostral orbitofrontal cortex (Anderson et al., 2003; Rolls et al., 2003). In contrast, a correlation between the subjective unpleasantness ratings of odors was found in regions of the left and more lateral orbitofrontal cortex (Anderson et al., 2003; Rolls et al., 2003). It is not yet possible to identify these areas in terms of the architectonic subdivisions of the orbitofrontal cortex, but they appear to correspond to the “orbitofrontal network” defined in monkeys (see above; Carmichael and Price, 1996). Zald and Pardo (1997) also identified an area in the lateral orbitofrontal/anterior insular cortex with activity following stimulation with aversive odors; the region of the amygdala was also activated to the same odors. Imaging studies have shown activation in the piriform cortex during olfactory learning and memory tasks as well as olfactory tasks related to motivational and VI. SYSTEMS HUMAN IMAGING OF OLFACTORY SENSORY ACTIVITY cognitive states. This indicates a higher or more elaborate function of the piriform cortex than solely the function of a relay to olfactory cortical areas beyond the POC (Gottfried, 2010). A more recent fMRI study has found activity in the central posterior orbitofrontal cortex related to the detection of discrepant olfactory events (Sabri et al., 2005). Although this study also found activation in the subgenual cingulate cortex, this activity may be attributed to selective attention. These areas were only activated if there was no attention paid to the olfactory stimuli. However, when attention was being paid to the olfactory stimulation, activation was found in a small part of the right and lateral OFC region. The OFC has been shown to be activated during a multitude of tasks, ranging from odor discrimination learning to multisensory integration. In addition, patients with orbitofrontal lesions (e.g. head trauma) have difficulties with odor identification, memory, and discrimination while having little difficulties with odor detection. More specifically, a case-study showed that an injury in the right OFC led to anosmia while activation in the left OFC and bilateral piriform cortex still allowed for odor-evoked autonomic responses to unpleasant smells. This lateralization of olfactory functioning finds support from an early PET study by Zatorre and colleagues (1992), who found greater activation in right OFC compared to the contralateral homologous OFC after odorant stimulation. Meanwhile olfaction areas in the temporal lobes were symmetrically active. Although some have suggested that the right OFC is necessary for conscious olfactory processing (Li et al., 2010), lesion studies in patients with medically refractory epilepsy showed a similar impairment on all olfactory tasks after unilateral temporal lobectomy, leaving detection thresholds intact (Eskenazi et al., 1983). This would seem to indicate a more complex system with regard to olfactory tasks. In favor of a lateralization of olfactory function is an MRI study measuring gray matter loss using voxelbased morphometry in people suffering from anosmia (Bitter et al., 2010). This study showed a greater atrophy on the right side. This controlled study furthermore showed that anosmia leads to reduced gray matter in the anterior cingulate cortex, the middle cingulate cortex, the dorsolateral prefrontal cortex, the subcallosal gyrus, and the NAcc. Further analysis in this study showed volume loss in the right piriform cortex the right insular cortex and the right OFC. Direct comparison of these results with fMRI data from the same participants showed an overlap of areas activated in healthy participants with gray matter loss areas in anosmic participants. It has long been thought that orthonasal and retronasal olfactory stimulation are similar to each other. 1233 Reported activation in piriform cortex, insula, OFC, hippocampus and entorhinal cortex after retronasal stimulation by Cerf-Ducastel and Murphy (2001) contributed to this belief, as the reported areas are similar to those active after orthonasal olfactory stimulation. No direct comparisons were made, however, between the two different types of olfactory stimulation. More recently it has been found that there are fundamental differences in both odor perception as well as neural activation. According to Small and colleagues (2005) the insula, opercula, thalamus, hippocampus, amygdala/piriform, and caudolateral OFC show greater activation after orthonasal stimulation whereas the perigenual cingulate, posterior cingulate, medial OFC, and superior temporal gyrus extending into the temporal operculum show greater activation after retronasal olfactory stimulation. A very important note is that this difference was only found in food related odors. Non-food odors did not result in a similar dissociation. This finding supports one of the first theories of orthonasal versus retronasal differences coined by Rozin (1982), stating that there are different behavioral consequences depending on the two types of information. In short, orthonasal stimulation represents information from odor sources in the environment ranging from animals to plants to fire. Retronasal stimulation signals information from odor sources in the oral cavity, which is generally an object that has been previously selected as food. Olfaction as a Multimodal System Food selection is one of the most important functions of olfaction, and there are close connection between olfaction and gustation. Ultrasound imaging has shown that retronasal stimulation (compared with orthonasal stimulation) increases the speed of swallowing (Welge-Lüssen et al., 2009). However, only food-related odors were used in this study, so whether this is specifically related to food intake or also to non-food-related odors requires further investigation. The central orbitofrontal cortical region that responds to odorants also is active by taste stimuli (Small et al., 1999). This corresponds well with the anatomical evidence in monkeys that cortico-cortical interconnections within the orbitofrontal cortex relate olfactory and gustatory systems. The responses to simultaneous, matched taste and olfactory stimuli appear to be quantitatively different from both unimodal stimuli alone, suggesting that flavor is not a simple convergence of its component senses (Small et al., 1997). As suggested by recordings in monkeys (see above), the olfaction- and taste-related responses in the orbitofrontal cortex also depend on hedonic properties of the stimuli. For example, fMRI responses to food-related olfactory VI. SYSTEMS 1234 34. THE OLFACTORY SYSTEM stimuli show specific decreases after feeding to satiety with that food (O’Doherty et al., 2000). Also, in addition to areas with activity following either olfactory stimulation or taste stimulation, such as parts of the orbitofrontal cortex, amygdala, insular cortex, and anterior cingulate cortex, some parts of the orbitofrontal cortex are active only when both taste and olfactory stimuli were combined (De Araujo et al., 2003). Taken together, the evidence indicates that the orbitofrontal cortex must be considered as a key node in the brain networks available for the analysis of food and food-related stimuli. More generally, it has been proposed that the orbitofrontal cortex plays a central role for the analysis of reward and hedonic processing (Kringelbach, 2005) (see Figure 34.6). (A) This view is further supported by how olfactory dysfunction has been shown to influence malnutrition (Mesholam et al., 1998; Lafreniere and Mann, 2009). Both Parkinson disease and Alzheimer disease are accompanied by malnutrition and weight loss without clear evidence for changes in metabolism (Warner et al., 1986; Korczyn and Gurevich, 2009). Also, the loss of smell (and taste) in elderly is believed to negatively influence appetite and pleasure of food intake, which in turn lead to malnutrition. Besides the gustatory system, the trigeminal system (see Chapter 31) also plays a prominent role in olfaction. The free nerve endings of the trigeminal nerve are stimulated by a majority of odorants. Additionally, the trigeminal nerve is also stimulated by odorless vapors (B) Monitoring / learning / memory 10o anterior 47/12r 11m 11l 13m 14r lateral Reward value representations Evaluations leading to change lateral Correlates of hedonic experience Increase in complexity medial anterior 45 13l 47/12l 13b posterior medial 14c Multi-modal representations 46 47/12m 13a 47/12s Iam am m AON posterior Iapm Ia Iap Iai Ial Primary sensory cortices Reinforcer identity Olfaction Somatosensory Autonomic Visual Auditory Gustatory FIGURE 34.6 General model of interaction between sensory and hedonic processing in the OFC. The proposed model shows the interactions between sensory and hedonic systems in the orbitofrontal cortex using as an example one hemisphere of the orbitofrontal cortex (Kringelbach, 2004). Information is flowing from bottom to top on the figure. Sensory information arrives from the periphery to the primary sensory cortices, where the stimulus identity is decoded into stable cortical representations. This information is then conveyed for further multimodal integration in brain structures in the posterior parts of the orbitofrontal cortex. The reward value of the reinforcer is assigned in more anterior parts of the orbitofrontal cortex from where it can then be used to influence subsequent behavior (in lateral parts of the anterior orbitofrontal cortex with connections to anterior cingulate cortex), stored for learning/memory (in medial parts of the anterior orbitofrontal cortex) and made available for subjective hedonic experience (in mid-anterior orbitofrontal cortex). The reward value and the subjective hedonic experience can be modulated by hunger and other internal states. In addition, there is important reciprocal information flowing between the various regions of the orbitofrontal cortex and other brain regions involved in hedonic processing. VI. SYSTEMS REFERENCES such as carbon dioxide, which induces a sensation of pain (Kobal, 1985; Hummel et al., 2003). The stimulation of the trigeminal system by odorants is known to influence the perception of odorants. Animal studies have shown that, whereas trigeminal stimuli have an inhibitory effect on olfactory afferent nerves, blockage of trigeminal activity enhances activity in, for example, the olfactory bulb (Stone et al., 1968; Brand, 2006). It is furthermore believed that pungency, as tested with carbon dioxide, can diminish or suppress certain odors. However, olfactory sensitivity for both purely olfactory as well as olfactory/trigeminal odorants increases after previous trigeminal stimulation. The effect of trigeminal activity on olfactory perception is believed to be both central as well as peripheral, e.g. interaction may take place in parts of the thalamus and fibers of the trigeminal nerve innervate the olfactory epithelium (Brand, 2006). Contrary to the olfactory system, the trigeminal system has a limited amount of sensations (e.g. pain, temperature, humidity) compared to the vast amount of odors detected by the olfactory system and is more involved in protective reflexes than the olfactory system. So, although most odorants stimulate both systems, they are separate systems and can indeed be affected separately, e.g. most patients with Parkinson disease suffer from olfactory dysfunction while the trigeminal system appears to be unaffected (Barz et al., 1997). Olfactory Dysfunction and Anhedonia There is a vast amount of literature on olfactory dysfunction, ranging from nasal fractures and head trauma to toxic exposure, depression, and neurodegenerative disorders. Head trauma can cause olfactory dysfunction by severing the neurons from the olfactory epithelium to the olfactory bulb through the cribriform plate, where scar tissue is subsequently blocking the growth of new neurons. Olfactory dysfunction due to neurodegenerative disorders could perhaps tell us more about the nature of the olfactory system. Although the literature on olfactory dysfunction in for example Parkinson disease or Alzheimer disease kept growing steadily for the past 30 years, the exact underlying mechanisms for olfactory dysfunction in these disorders is still largely unknown (Hawkes, 2003; Murphy et al., 2005; Doty, 2007). Whereas both apathy and olfactory dysfunction are common symptoms in Parkinson disease, Cramer and colleagues (2010) found a correlation between olfactory impairment and apathy where non-apathetic patients performed better on olfactory tasks. Also, humans suffering from olfactory dysfunction are found to report a lower quality of life, in particular with respect to enjoying food and drinks, socializing, and intimate relationships (Frasnelli and 1235 Hummel, 2005; Smeets et al., 2009). Furthermore, the olfactory bulb is significantly reduced in patients suffering from acute major depression (Negoias et al., 2010). These studies, however, only show a correlation between olfactory dysfunction and anhedonia. A wellknown and often-used model for depression in rats is the creation of anhedonia through olfactory bulbectomy (Song and Leonard, 2005; Romeas et al., 2009). Peripherally induced loss of olfaction does, however, not induce the same behavioral changes. In other words, the induction of anhedonia relies not solely on olfaction, but is caused by the disruption of a more complex system, the cortical–hippocampal–amygdala circuit. Olfactory bulbectomy in rats also means that input from the vomeronasal organ is removed, which is important for the behavior of rats. In humans, Bitter and colleagues (2010) showed that a loss of the sense of smell leads to loss of gray matter cortical areas, including the OFC, medial prefrontal cortex, anterior cingulate cortex, insular cortex and NAcc, associated with pleasure or hedonic experience (Berridge and Kringelbach, 2008; Kringelbach and Berridge, 2010). While the differences in olfactory functioning (especially the influence of the vomeronasal organ) in rats make it difficult to extrapolate the results to humans, converging evidence from imaging and lesioning studies in humans do seem to indicate a major role of the olfactory system in anhedonia, which should be further investigated in the coming years. References Alheid GF, Heimer L, Switzer RC: Basal Ganglia. In Paxinos G, editor: “The Human Nervous System” San Diego, (1990; update to Basal ganglia chapter, current edition), Academic Press, pp 483– 582. Amaral DG, Insausti R: Hippocampal formation. In Paxinos G, editor: “The Human Nervous System” San Diego, (1990; update to Hippocampal formation chapter, current edition), Academic Press, pp 711–755. Amaral DG, Insausti R, Cowan WM: The entorhinal cortex of the monkey. I. Cytoarchitectonic organization, J Comp Neurol 264: 326–355, 1987. Amaral DG, Price JL, Pitkanen A, Carmichael ST: Anatomical organization of the primate amygdaloid complex. In Aggleton JP, editor: The Amygdala, New York, 1992, Wiley-Liss, pp 1–66. Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N: Dissociated neural representations of intensity and valence in human olfaction, Nature Neuroscience 6:196–202, 2003. Aroniadou-Anderjaska V, Ennis M, Shipley MT: Glomerular synaptic responses to olfactory nerve input in rat olfactory bulb slices, Neuroscience 79:425–434, 1997. Barz S, Hummel T, Pauli E, Majer M, Lang CJ, Kobal G: Chemosensory event-related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson’s disease, Neurology 49:1424–1431, 1997. VI. SYSTEMS 1236 34. THE OLFACTORY SYSTEM Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC: PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans, Nature 450:106–109, 2007. Berkowicz DA, Trombley PQ, Shepherd GM: Evidence for glutamate as the olfactory receptor cell neurotransmitter, J Neurophysiol 71:2557–2561, 1994. Berridge K, Kringelbach M: Affective neuroscience of pleasure: reward in humans and animals, Psychopharmacology 199:457–480, 2008. Bhatnagar KP, Kennedy RC, Baron G, Greenberg RA: Number of mitral cells and the bulb volume in the aging human olfactory bulb a quantitative morphological study, Anat Rec 218:73–87, 1987. Bitter T, Gudziol H, Burmeister HP, Mentzel H-J, Guntinas-Lichius O, Gaser C: Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses bjq028, 2010. Boeijinga PH, Van Groen T: Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. II. Physiological studies, Exp Brain Res 57:40–48, 1984. Brand G: Olfactory/trigeminal interactions in nasal chemoreception, Neuroscience & Biobehavioral Reviews 30:908–917, 2006. Buck L, Axel R: A novel multigene family may encode odorant receptors a molecular basis for odor recognition, Cell 65:175–187, 1991. Buonomano DV, Maass W: State-dependent computations: spatiotemporal processing in cortical networks, Nat Rev Neurosci 10:113–125, 2009. Carmichael ST, Clugnet M-F, Price JL: Central olfactory connections in the macaque monkey, J Comp Neurol 346:403–434, 1994. Carmichael ST, Price JL: Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey, J Comp Neurol 346:366–402, 1994. Carmichael ST, Price JL: Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys, J Comp Neurol 363:642–664, 1995. Carmichael ST, Price JL: Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys, J Comp Neurol 371:179–207, 1996. Cerf-Ducastel B, Murphy C: fMRI activation in response to odorants orally delivered in aqueous solutions, Chem Senses 26:625–637, 2001. Chen WR, Xiong W, Shepherd GM: Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses, Neuron 25:625–633, 2000. Cramer CK, Friedman JH, Amick MM: Olfaction and apathy in Parkinson’s disease, Parkinsonism Relat Disord 16:124–126, 2010. Dade LA, Jones-Gotman M, Zatorre RJ, Evans AC: Human brain function during odor encoding and recognition, A PET activation study, Ann NY Acad Sci 855:572–574, 1998. De Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N: Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain, European Journal of Neuroscience 18:2059–2068, 2003. Doty RL: Olfaction in Parkinson’s disease, Parkinsonism Relat Disord 13 3(Suppl):S225–228, 2007. Duda JE: Olfactory system pathology as a model of Lewy neurodegenerative disease, Journal of the Neurological Sciences 289:49–54, 2009. Elad D, Wolf M, Keck T: Air-conditioning in the human nasal cavity, Respir Physiol Neurobiol 163(1–3):121–127, 2008. Eskenazi B, Cain WS, Novelly RA, Friend KB: Olfactory functioning in temporal lobectomy patients, Neuropsychologia 21:365–374, 1983. Frasnelli J, Hummel T: Olfactory dysfunction and daily life, Eur Arch Otorhinolaryngol 262:231–235, 2005. Frasnelli J, Lundström JN, Boyle JA, Katsarkas A, Jones-Gotman M: The vomeronasal organ is not involved in the perception of endogenous odors, Human Brain Mapping 32:450–460, 2011. Fuller TA, Russchen RT, Price JL: Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striato-pallidal region, J Comp Neurol 256:317–338, 1986. Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW: Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb, J Comp Neurol 266:307–318, 1987. Glusman G, Bahar A, Sharon D, Pilpel Y, White J, Lancet D: The olfactory receptor gene superfamily data mining, classification, and nomenclature Mamm, Genome 11:1016–1023, 2000. Gottfried JA: Olfaction: When Nostrils Compete, Current Biology 19:R862–R864, 2009. Gottfried JA: Central mechanisms of odour object perception, Nat Rev Neurosci 11:628–641, 2010. Haberly LB: Neuronal circuitry in olfactory cortex anatomy and functional implications, Chem Senses 10:219–238, 1985. Haberly LB, Price JL: The axonal projection pattern of the mitral and tutfed cells of the olfactory bulb in the rat, Brain Res 129:152–157, 1977. Hawkes CH: Olfaction in neurodegenerative disorder, Movement Disorders 18:364–372, 2003. Heimer L, Van Hoesen GW, Rosene DL: The olfactory pathways and the anterior perforated substance in the primate brain. Int J Neurol 12:42–52, 1977. Hinds JW, McNelly NA: Aging of the rat olfactory bulb growth and atrophy of constituent layers and changes in size and number of mitral cells, J Comp Neurol 72:345–367, 1977. Hoover KC: Smell with inspiration: The evolutionary significance of olfaction, American Journal of Physical Anthropology 143:63–74, 2010. Hummel T, Mohammadian P, Marchl R, Kobal G, Lötsch J: Pain in the trigeminal system: irritation of the nasal mucosa using short- and long-lasting stimuli, International Journal of Psychophysiology 47: 147–158, 2003. Jacob S, Zelano B, Gungor A, Abbott D, Naclerio R, McClintock MK: Location and gross morphology of the nasopalatine duct in human adults, Arch Otolaryngol Head Neck Surg 126:741–748, 2000. Jacobs GH: Evolution of colour vision in mammals, Philosophical Transactions of the Royal Society B: Biological Sciences 364:2957–2967, 2009. Jafek B: Ultrastructure of human nasal mucosa, Laryngoscope 93: 1576–1599, 1983. Johnson BA, Leon M: Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration, J Comp Neurol 422:496–509, 2000. Johnson DM, Illig KR, Behan M, Haberly LB: New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems, J Neurosci 20:6974–6982, 2000. Jolkkonen E, Miettinen R, Pitkanen A: Projections from the amygdalopiriform transition area to the amygdaloid complex a PHA-l study in rat, J Comp Neurol 432:440–465, 2001. Karadi Z, Oomura Y, Nishino H, Aou S: Olfactory coding in the monkey lateral hypothalamus behavioral and neurochemical properties of odor-responding neurons, Physiol Behav 45:1249–1257, 1989. Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, Mori K: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources, Proc Natl Acad Sci 107: 12363–12368, 2010. Kobal G: Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation, Pain 22:151–163, 1985. VI. SYSTEMS REFERENCES Korczyn AD, Gurevich T: Parkinson’s disease: Before the motor symptoms and beyond, Journal of the Neurological Sciences 289:2–6, 2009. Kosaka T, Hataguchi Y, Hama K, Nagatsu I, Wu JY: Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb possible coexistence of gamma-aminobutyric acid (GABA) and dopamine, Brain Res 343:166–171, 1985. Kosaka T, Kosaka K, Heizmann CW, Nagatsu I, Wu JY, Yanaihara N, Hama K: An aspect of the organization of the GABAergic system in the rat main olfactory bulb laminar distribution of immunohistochemically defined subpopulations of GABAergic neurons, Brain Res 411:373–378, 1987. Kovács T: Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders, Ageing Research Reviews 3:215–232, 2004. Kringelbach ML: Food for thought: hedonic experience beyond homeostasis in the human brain, Neuroscience 126:807–819, 2004. Kringelbach ML, Rolls ET: The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology, Prog Neurobiol 72:341–372, 2004. Kringelbach ML: The orbitofrontal cortex: linking reward to hedonic experience, Nature Reviews Neuroscience 6:691–702, 2005. Kringelbach ML, Berridge KC: The functional neuroanatomy of pleasure and happiness, Discov Med 9:579–587, 2010. Kringelbach ML, Stein A: Cortical mechanisms of human eating, Forum Nutr 63:164–175, 2010. Kuroda M, Price JL: Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat, J Comp Neurol 303:513–533, 1991. Lafreniere D, Mann N: Anosmia: Loss of smell in the elderly, Otolaryngologic Clinics of North America 42:123–131, 2009. Laska M, Teubner P: Olfactory discrimination ability of human subjects for ten pairs of enantiomers, Chem Senses 24:161–170, 1999. Li W, Lopez L, Osher J, Howard JD, Parrish TB, Gottfried JA: Right orbitofrontal cortex mediates conscious olfactory perception, Psychological Science 21:1454–1463, 2010. Lovell MA, Jafek BW, Moran DT, Rowley JC: Biopsy of human olfactory mucosa. An instrument and a technique, Arch Otolaryngol (Chicago) 108:247–249, 1982. Luskin MB, Price JL: The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques, J Comp Neurol 209:249–263, 1982. Luskin MB, Price JL: The topographic organization of association fibers in the olfactory system in the rat, including centrifugal fibers to the olfactory bulb, J Comp Neurol 216:264–291, 1983. Macrides F, Schonfeld TA, Marchand JE, Clancy AN: Evidence for morphologically, neurochemically and functionally heterogeneous classes of mitral and tufted cells in the olfactory bulb, Chem Senses 10:175–218, 1985. Maresh A, Rodriguez Gil D, Whitman MC, Greer CA: Principles of Glomerular Organization in the Human Olfactory Bulb – Implications for Odor Processing, PLoS One 3(7):e2640, 2008. Martinez-Marcos A: On the organization of olfactory and vomeronasal cortices, Progress in Neurobiology 87:21–30, 2009. Mast TG, Samuelsen CL: Human Pheromone Detection by the Vomeronasal Organ: Unnecessary for Mate Selection? Chemical Senses 34:529–531, 2009. Meisami E, Mikhail L, Baim D, Bhatnagar KP: Human olfactory bulb aging of glomeruli and mitral cells and a search for the accessory olfactory bulb, Ann N Y Acad Sci 855:708–715, 1998. Mesholam RI, Moberg PJ, Mahr RN, Doty RL: Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases, Arch Neurol 55:84–90, 1998. 1237 Mesulam M-M, Mufson EJ, Levey AI, Wainer BH: Cholinergic innervation of the cortex by the basal forebrain Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey, J Comp Neurol 214:170–197, 1983. Moran DT, Rowley JC III, Jafek BW, Lowell MA: The fine structure of the olfactory mucosa in man, J Neurocytol 11:721–746, 1982a. Moran DT, Rowley JC III, Jafek BW: Electron microscopy of human olfactory epithelium reveals a new cell type The microvillar cell, Brain Res 253:39–46, 1982b. Mori K, Nagao H, Yoshihara Y: The olfactory bulb Coding and processing of odor molecule information, Science 286:711–715, 1999. Morrison EE, Costanzo RM: Morphology of olfactory epithelium in humans and other vertebrates, Microsc Res Tech 23:49–61, 1992. Mostafa T, Khouly GE, Hassan A: Pheromones in sex and reproduction: Do they have a role in humans? J Adv Res doi:10.1016/j. jare.2011.03.003, 2011. Murphy C, Cerf-Ducastel B, Calhoun-Haney R, Gilbert PE, Ferdon S: ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer’s disease, Chem Senses 30 1(Suppl):i170–171, 2005. Murphy C, Morgan CD, Geisler MW, Wetter S, Covington JW, Madowitz MD, Nordin S, Polich JM: Olfactory event-related potentials and aging normative data, Int J Psychophysiol 36:133–145, 2000. Nakashima T, Kimmelman CP, Snow JB Jr: Structure of human fetal and adult olfactory neuroepithelium, Arch Otolaryngol (Chicago) 110:641–646, 1984. Nakashima T, Kimmelman CP, Snow JB Jr: Vomeronasal organs and nerves of Jacobson in the human fetus, Acta Oto-laryngol 99: 266–271, 1985. Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T: Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression, Neuroscience 169:415–421, 2010. O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G: Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex, Neuroreport 11:893–897, 2000. Ohm TG, Muller H, Ulfig N, Braak E: Glutamic-acid-decarboxylaseand parvalbumin-like-immunoreactive structures in the olfactory bulb of the human adult, J Comp Neurol 291:1–8, 1990. Ohm TG, Muller H, Braak E: Calbindin-D-28k-like immunoreactive structures in the olfactory bulb and anterior olfactory nucleus of the human adult distribution and cell typology–partial complementarity with parvalbumin, Neuroscience 42:823–840, 1991. Ojima H, Mori K, Kishi K: The trajectory of mitral cell axons in the rabbit olfactory cortex revealed by intracellular HRP injection, J Comp Neurol 230:77–87, 1984. Öngür D, An Z, Price JL: Prefrontal cortical projections to the hypothalamus in macaque monkeys, J Comp Neurol 401:480–505, 1998. Öngür D, Price JL: The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans, Cereb Cortex 10:206–219, 2000. Porter R: Olfaction and human kin recognition, Genetica 104:259–263, 1998. Price JL, Slotnick BM: Dual olfactory representation in the rat thalamus: an anatomical and electrophysiological study, J Comp Neurol 215:63–67, 1987. Price JL, Russchen FT, Amaral DG: The limbic region. II The amygdaloid complex. In Bjorklund A, Hokfelt T, Swanson LW, editors: Handbook of Chemical Neuroanatomy, vol 5, Integrated Systems of the CNS. Part I, Amsterdam, 1987, Elsevier Science Publishers, pp 279–388. VI. SYSTEMS 1238 34. THE OLFACTORY SYSTEM Price JL: An autoradiographic study of complimentary laminar patterns of termination of afferent fibers to the olfactory cortex, J Comp Neurol 150:87–108, 1973. Price JL: The central olfactory and accessory olfactory systems. In Finger TE, Silver WL, editors: Neurobiology of Taste and Smell, New York, 1987, John Wiley and Sons, pp 179–204. Ray JP, Price JL: The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys, J Comp Neurol 337:1–31, 1993. Ressler KJ, Sullivan SL, Buck LB: A zonal organization of odorant receptor gene expression in the olfactory epithelium, Cell 73:597–609, 1993. Ressler KJ, Sullivan SL, Buck LB: Information coding in the olfactory system evidence for a stereotyped and highly organized epitope map in the olfactory bulb, Cell 79:1245–1255, 1994. Rolls ET: The orbitofrontal cortex and reward, Cereb Cortex 10: 284–294, 2000. Rolls ET, Kringelbach ML, de Araujo IE: Different representations of pleasant and unpleasant odours in the human brain, Eur J Neurosci 18:695–703, 2003. Romeas T, Morissette M-C, Mnie-Filali O, Piñeyro G, Boye S: Simultaneous anhedonia and exaggerated locomotor activation in an animal model of depression, Psychopharmacology 205:293–303, 2009. Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, Costes N, Holley A: Functional neuroanatomy of different olfactory judgments, Neuroimage 13:506–519, 2001. Rozin P: “Taste-smell confusions” and the duality of the olfactory sense, Percept Psychophys 31:397–401, 1982. Russchen FT, Amaral DG, Price JL: The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis, J Comp Neurol 256:175–210, 1987. Sabri M, Radnovich AJ, Li TQ, Kareken DA: Neural correlates of olfactory change detection, NeuroImage 25:969–974, 2005. Scott JW, Harrison TA: The olfactory bulb anatomy and physiology. In Finger TE, Silver WL, editors: Neurobiology of Taste and Smell, New York, 1987, John Wiley and Sons, pp 151–178. Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC: Flavor processing more than the sum of its parts, Neuroreport 8:3913–3917, 1997. Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Frey S, Petrides M: Human cortical gustatory areas A review of functional neuroimaging data, Neuroreport 10:7–14, 1999. Small DM, Gerber JC, Mak YE, Hummel T: Differential Neural Responses Evoked by Orthonasal versus Retronasal Odorant Perception in Humans, Neuron 47:593–605, 2005. Smeets MAM, Veldhuizen MG, Galle S, Gouweloos J, de Haan A-M JA, Vernooij J, Visscher F, Kroeze JHA: Sense of smell disorder and healthrelated quality of life, Rehabilitation Psychology 54:404–412, 2009. Smith RL, Baker H, Kolstad K, Spencer DD, Greer CA: Localization of tyrosine hydroxylase and olfactory marker protein immunoreactivities in the human and macaque olfactory bulb, Brain Res 548:140–148, 1991. Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JDE: Sniffing and smelling separate subsystems in the human olfactory cortex, Nature 392:282–286, 1998. Sobel N, Khan RM, Saltman A, Sullivan EV, Gabrieli JD: The world smells different to each nostril, Nature 402:35, 1999. Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JDE: Time course of odorant-induced activation in the human primary olfactory cortex, J Neurophysiol 83:537–551, 2000. Song C, Leonard BE: The olfactory bulbectomised rat as a model of depression, Neurosci Biobehav Rev 29:627–647, 2005. Stone H, Williams B, Carregal EJA: The role of the trigeminal nerve in olfaction, Experimental Neurology 21:11–19, 1968. Takagi SF: Studies on the olfactory nervous system of the old world monkey, Prog Neurobiol 27:195–249, 1986. Tanabe T, Yarita H, Iino M, Ooshima Y, Takagi SF: An olfactory projection area in orbitofrontal cortex of the monkey, J Neurophysiol 38:1269–1283, 1975a. Tanabe T, Iino M, Takagi SF: Discrimination of odors in the olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey, J Neurophysiol 38:1284–1296, 1975b. Tazawa Y, Onoda N, Takagi SF: Olfactory input to the lateral hypothalamus of the old world monkey, Neurosci Res 4:357–375, 1987. Tigges M, Tigges J: Distribution and morphology of myelinated perikarya and dendrites in the olfactory bulb of primates, J Neurocytol 9:825–834, 1980. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE: Reduced olfactory bulb volume in patients with schizophrenia, Am J Psychiatry 157:828–830, 2000. Turner BH, Gupta KC, Mishkin M: The location and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta, J Comp Neurol 177:381–396, 1978. Vassar R, Ngai J, Axel R: Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium, Cell 74:309–318, 1993. Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R: Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium, Cell 79:981–991, 1994. Warner MD, Peabody CA, Flattery JJ, Tinklenberg JR: Olfactory deficits and Alzheimer’s disease, Biol Psychiatry 21:116–118, 1986. Welge-Lüssen A, Ebnöther M, Wolfensberger M, Hummel T: Swallowing Is Differentially Influenced by Retronasal Compared with Orthonasal Stimulation in Combination with Gustatory Stimuli, Chemical Senses 34:499–502, 2009. Wesson DW, Wilson DA: Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neuroscience & Biobehavioral Reviews 35:655–668, 2011. Wysocki C, Meredith M: The vomeronasal system. In Finger TE, Silver WL, editors: Neurobiology of Taste and Smell, New York, 1987, John Wiley and Sons, pp 125–150. Xu F, Greer CA, Shepherd GM: Odor maps in the olfactory bulb, J Comp Neurol 422:489–495, 2000. Yarita H, Iino M, Tanabe T, Kogure S, Takagi SF: A transthalamic olfactory pathway to the orbitofrontal cortex in the monkey, J Neurophysiol 43:69–85, 1980. Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M, Geckle RJ, Suskind D, Bullmore ET, Brammer MJ, Doty RL: Functional MR imaging during odor stimulation preliminary data, Radiology 204:833–838, 1997. Zaborsky L, Carlsen J, Brashear HR, Heimer L: Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band, J Comp Neurol 243:488–509, 1985. Zald DH, Pardo JV: Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation, Proc Natl Acad Sci. 94:4119–4124, 1997. Zald DH, Pardo JV: Functional neuroimaging of the olfactory system in humans, Int J Psychophysiol 36:165–181, 2000. Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E: Functional localization and lateralization of human olfactory cortex, Nature 360:339–340, 1992. VI. SYSTEMS