* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download studying neurogenesis in cephalopods - UMR BOREA
Blood–brain barrier wikipedia , lookup
Optogenetics wikipedia , lookup
Human brain wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroethology wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Selfish brain theory wikipedia , lookup
Neuroesthetics wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Aging brain wikipedia , lookup
Neurolinguistics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroinformatics wikipedia , lookup
Brain morphometry wikipedia , lookup
Neuroplasticity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Haemodynamic response wikipedia , lookup
Circumventricular organs wikipedia , lookup
Synaptogenesis wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
History of neuroimaging wikipedia , lookup
Brain Rules wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroregeneration wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuropsychology wikipedia , lookup
Nervous system network models wikipedia , lookup
Neural engineering wikipedia , lookup
Axon guidance wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
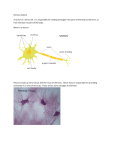
STUDYING NEUROGENESIS IN CEPHALOPODS: WHY AND HOW ? Sébastien Baratte1, Sandra Navet, Aude Andouche & Laure Bonnaud Equipe Neurogenèse des Céphalopodes Muséum National d’Histoire Naturelle, DMPA BOME, UMR CNRS 5178, 55 rue Buffon, 75005 Paris [email protected] INTRODUCTION CephBase CephBase The nervous system of cephalopods exhibits numerous sensorial and structural innovations among molluscs. Their developed central nervous system (ganglia fused into a brain) has been used as a comparative model to vertebrates (Young, 1971, 1974, 1976; Messenger, 1979; Hochner et al., 2003) and giant axons have long been an important material for neurocytology, electrophysiology and biophysics. Intense efforts have been conducted to understand physiological function of the brain and giant axons but comparatively nothing is known about the molecular pathways underlying their development. Similarly, the diversity of cephalopod nervous systems indicates a high flexibility and adaptability, which makes them a relevant biological material for evolutionary studies. Nevertheless, neither their development nor the mechanisms that could have led to the emergence of these derived traits have been studied. For example, the process of neural bilaterality establisment remains unknown in these species without neural cord. CephBase CephBase DEVELOPMENT & NERVOUS SYSTEM in Sepia officinalis Cephalopods present a direct development, there is no veliger stage and no metamorphosis as in other molluscs. The nervous system is established before hatching but changes occur from juvenile to “adult”, mostly regarding the relative proportions of brain parts. Neurogenesis has to be described in S. officinalis and this first requires the description of embryonic neural territories Stage 18 (apical view) Optic ganglion Stage 21 (apical view) Adult Stage 24 (ventral view) Peripheral Nervous System Cerebral ganglion Central Nervous System brain eye Brachial & pedal ganglia visceral ganglion arms funnel stellate ganglion the 2 stellate ganglia (s.g.) are peripheral relays of the brain chromatophore fibres pass through the s.g. without any connection Embryonic neural territories in S. officinalis are deduced here from that of Octopus vulgaris and Sepiotheutis lessoniana (squid) embryos. In S. officinalis, fecundation is stage 0 and hatching occurs at stage 30. Main organogenetic changes occur between stage 15 and 24. a: arm; e: eye; fp: funnel pouch; ft: funnel tube; g: gill; ma: mantle; me: mantle edge; mo: mouth; ssa: shell sac aperture; sse: shell sac edge; st: statocyste. Scale bar: 500µm. in stellar nerves, giant fibers are made of fused axons of 3rdorder neurons. Synapses with 2nd order neurons from the brain are located in the s.g. the brain is made of fused ganglia (visceral, cerebral, pedal and optic) NEURAL SPECIFICITIES RAISE NEUROGENETIC and EVOLUTIONNARY QUESTIONS Giant axons Stellate ganglion gill stellar nerves dissection α-tubulin immunostaining How do stellate ganglia develop ? as a result of what molecular mechanisms ? How do they eventually get connected to the brain ? May they provide information about the CNS history within Lophotrochozoa ? [a] Stellate ganglia in cephalopods exhibit various degrees of complexity and of axon fusion, depending of life habit: [a] and [b] are pelagic and able of rapid muscular contractions, [c] is benthic and is a poor swimmer. [b] Chromatophores [c] [b] [a] CephBase Loligo Sepia (squid) giant fibers Octopus giant axons Chromatophores are neuromuscular structures allowing rapid color changes typical axons How do axons fuse ? Which genetic or molecular differences could explain the functional adaptations of stellate ganglion throughout the evolution of cephalopods ? How the chromatophore pattern is controlled? [a] What controls axon guidance throughout stellate ganglia from brain to chromatophores ? How bilateral pattern is set up without any central nervous axis ? [b] LOOKING FOR HOMOLOGOUS GENES AND GENE EXPRESSION Among genes known to participate to the nervous system formation in Metazoans few have been identified in the Mollusca yet. According to our problematic (see questions above), we focus on genes involved in neuronal determination neuronal specification axonal growth during embryonic development. Our aim is to characterise the genes, to determine their expression patterns and identify their function by extinction and/or surexpression. Engrailed,… Pax6,… In vertebrates, engrailed is involved in organizing the mid-hindbrain boundary or in correctly guiding retinal axons to the optic tectum. Pax-6 is a master gene in eye development. In cephalopods, it is expressed in eyes, olfactory organs, brain and arms. We did not find evidence of an engrailed role in the Sepia brain formation. However, it seems required for sensitive structures development: for the retina and lids formation in eyes, in arms Stage 17 Stage 20 ov e ma Stage 25 ma i le r ligand receptor(s) semaphorins plexins neuropilins e netrins DCC ephrins ephrin receptors ap4 vl a4 arm 4 at stage 25 [ Nk1 / Nk2 / Nk6 ] dl ap5 line of suckers ? Attraction and/or repulsion of growth cone Stage 25 e Neural network in a fin Neuroectodermic determination and neuronal specification the NK family e C …and other candidate genes under identification Baratte et al. 2007- Gen. Gene Evol Stage 25 PERSPECTIVES By providing a better understanding of the molecular mechanisms underlying the development of cephalopod nervous structures, this project will improve the knowledge of the nervous system evolution among Lophotrochozoans. The nervous peculiarities of cephalopods among molluscs offer a unique opportunity to investigate how genes produce diversity in neural organisation. Beside traditional invertebrate models (Drosophila, C. elegans) belonging to ecdysozoan, lophotrochozoan models are essential for the comparative study of molecular mechanisms and pattern formation as representative of the bilaterian diversity. Median line establishment slit robo ACKNOWLEDGMENTS We are grateful to Muriel Jager for providing us anti-α-tubulin antibody, to Ludovic Dickel and Christelle Alves from the Station Biologique Luc/Mer [University of Caen] for providing biological material. We thank Madeleine Martin for technical help.